UNITED
STATES
SECURITIES AND EXCHANGE
COMMISSION
WASHINGTON,
D.C. 20549
FORM
10-K
þ
ANNUAL REPORT UNDER SECTION 13 OR
15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
For the
fiscal year ended October 31, 2009
|
|
MERA
PHARMACEUTICALS, INC.
|
|
|
|
(Exact
name of registrant as specified in charter)
|
|
|
DELAWARE
|
|
033-23460
|
|
04-3683628
|
|
(State
or other jurisdiction
of
incorporation)
|
|
(Commission
File
Number)
|
|
(IRS
Employer
Identification
No.)
|
|
|
73-4460
QUEEN KA'AHUMANU HIGHWAY, SUITE 110, KAILUA-KONA, HAWAII,
96740
|
|
|
|
(Address
of principal executive offices)
|
|
|
|
(808)
326-9301
|
|
|
|
(Registrant’s
Telephone Number, including Area Code)
|
|
Securities
registered under Section 12(b) of the Act:
|
Title
of each class
|
|
Name
of each exchange on which registered
|
|
|
|
|
Securities
registered under Section 12(g) of the Exchange Act: NONE
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities Act. Yes
o
No
þ
Indicate
by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Act. Yes
o
No
þ
Indicate
by check mark whether the registrant (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was required
to file such reports), and (2) has been subject to such filing requirements for
the past 90 days. Yes
þ
No
o
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Web site, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405
of this chapter) during the preceding 12 months (or for such shorter period that
the registrant was required to submit and post such files). Yes
o
No
o
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K (§229.405 of this chapter) is not contained herein, and will not
be contained, to the best of registrant’s knowledge, in definitive proxy or
information statements incorporated by reference in Part III of this Form 10-K
or any amendment to this Form 10-K.
þ
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer, or a smaller reporting
company.
|
Large
accelerated filer
|
¨
|
Accelerated
filer
|
¨
|
|
Non-accelerated
filer
(Do
not check if a smaller reporting company)
|
¨
|
Smaller
reporting company
|
þ
|
Indicate
by check mark whether the registrant is a shell company (as defined in
Rule 12b-2 of the Exchange Act) Yes
o
No
þ
Mera
Pharmaceuticals' revenues for its most recent fiscal year:
$599,091.
The
aggregate market value of the common equity held by non-affiliates computed by
reference to the price at which the stock was sold, or the average bid and asked
prices of such stock as reported on the NASD Pink Sheets, as of October 31,
2009 is $2,738,847.
The
number of shares outstanding of common equity, as of October 31, 2009, was
547,769,515
shares
of Common Stock, $0.0001 par value.
DOCUMENTS
INCORPORATED BY REFERENCE
None
MERA
PHARMACEUTICALS, INC.
TABLE
OF CONTENTS
|
|
|
|
Page
|
|
|
|
|
|
|
|
|
1
|
|
|
|
|
7
|
|
|
|
|
15
|
|
|
|
|
15
|
|
|
|
|
15
|
|
|
|
|
|
|
|
|
16
|
|
|
|
|
17
|
|
|
|
|
19
|
|
|
|
|
19
|
|
|
|
|
20
|
|
|
|
|
20
|
|
|
|
|
|
|
|
|
21
|
|
|
|
|
22
|
|
|
|
|
23
|
|
|
|
|
24
|
|
|
|
|
24
|
|
|
|
|
|
|
|
|
25
|
|
|
|
26
|
|
|
|
F-1
|
|
|
|
F-7
|
PART I
THIS
ANNUAL REPORT ON FORM 10-KSB CONTAINS CERTAIN FORWARD-LOOKING STATEMENTS WITHIN
THE MEANING OF SECTION 21E OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED,
INCLUDING STATEMENTS THAT INDICATE WHAT WE "BELIEVE", "EXPECT" AND "ANTICIPATE"
OR SIMILAR EXPRESSIONS. THESE STATEMENTS INVOLVE KNOWN AND UNKNOWN RISKS,
UNCERTAINTIES AND OTHER FACTORS WHICH MAY CAUSE OUR ACTUAL RESULTS, PERFORMANCE
OR ACHIEVEMENTS TO DIFFER MATERIALLY FROM THOSE EXPRESSED OR IMPLIED BY SUCH
FORWARD-LOOKING STATEMENTS. SUCH FACTORS INCLUDE, AMONG OTHERS, THE INFORMATION
CONTAINED UNDER THE CAPTION "PART II, ITEM 6. MANAGEMENT'S DISCUSSION AND
ANALYSIS OF FINANCIAL CONDITIONS AND RESULTS OF OPERATIONS" AND ELSEWHERE IN
THIS ANNUAL REPORT. YOU SHOULD NOT PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING
STATEMENTS, WHICH REFLECT OUR MANAGEMENT'S ANALYSIS ONLY AS OF THE DATE OF THIS
ANNUAL REPORT. WE UNDERTAKE NO OBLIGATION TO PUBLICLY RELEASE THE RESULTS OF ANY
REVISION OF THESE FORWARD-LOOKING STATEMENTS. YOU ARE STRONGLY URGED TO READ THE
INFORMATION SET FORTH UNDER THE CAPTION "PART II, ITEM 6, MANAGEMENT'S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITIONS AND RESULTS OF OPERATION - RISK
FACTORS" FOR A MORE DETAILED DESCRIPTION OF THESE SIGNIFICANT RISKS AND
UNCERTAINTIES.
HISTORY
Mera
Pharmaceuticals, Inc. is the successor issuer to Aquasearch, Inc. (the
"Predecessor"), which was incorporated in Colorado in 1987 for the purpose of
developing useful products from aquatic microorganisms and making their
production economically feasible. On July 25, 2002, the Predecessor merged
with and into Mera Pharmaceuticals, Inc., a Delaware corporation formed in
June 2002 for the purpose of changing the corporation's name to Mera
Pharmaceuticals, Inc. and changing its state of incorporation from Colorado to
Delaware (the "Reincorporation Merger"). Mera Pharmaceuticals continues the
operations and business of the Predecessor. Each share of the Predecessor's
common stock outstanding at the time of the Reincorporation Merger was exchanged
for one share of common stock of Mera. Following the Reincorporation Merger, the
former stockholders of the Predecessor continued to own 100% of the Company's
issued and outstanding capital stock.
On
September 16, 2002, Aqua RM Co., Inc., a privately-held, non-operating
Delaware corporation established specifically for the purpose of facilitating
the Predecessor's reorganization under Chapter 11 of the U.S. Bankruptcy Code
("Aqua RM"), merged with and into the Company (the "Reorganization Merger"). The
Company was the surviving corporation of the Reorganization Merger. Each share
of Aqua RM common stock outstanding at the time of the Reincorporation Merger
was exchanged for 100 shares of the Company's common stock. Following the
Reorganization Merger, former Aqua RM stockholders held approximately 68% of the
Company's common stock. The Reorganization Merger was the last material event in
the fulfillment of the Company's Chapter 11 Plan of Reorganization.
OVERVIEW
OF THE COMPANY'S BUSINESS
The
Company develops and commercializes natural products derived principally from
microalgae using our patented photobioreactor technology known as the Mera
Growth Module ("MGM").
Microalgae
are a diverse group of microscopic plants estimated to consist of more than
30,000 species. They have a wide range of physiological and biochemical
characteristics. Microalgae produce many different substances and bioactive
compounds that have existing and potential applications in a variety of
commercial areas, including human nutrition, pharmaceuticals, and other high
value commodities such as bio-diesel.
The major
challenge to commercial exploitation of microalgae has been the availability of
photobioreactors large enough to achieve commercial production levels at an
economic cost. A photobioreactor is a fermentation system that is used to grow
photosynthetic organisms. At more than 6,000 gallons (25,000 liters), our
proprietary MGM is one of the largest photobioreactors in existence and one of
the few photobioreactors used for commercial production of
microalgae.
The MGM
incorporates a very high level of computerized process control, resulting in a
higher degree of reproducible performance at high efficiency levels. This
increased reliability is due in large measure to the use of turbulence to
control the exposure of the algae to light and nutrients at a frequency that
improves yields. Mera owns the basic patent for use of turbulence in this way.
Our patents, proprietary process controls and the very low cost of constructing
the MGM make the MGM very advanced, cost-effective and scalable.
The
Company has used its advantage in photobioreactor technology in the production
and marketing of its first commercial product, ASTAFACTOR®), a nutraceutical and
source of natural astaxanthin. Natural astaxanthin, a carotenoid found in many
species of fish and seafood (it gives wild salmon its distinctive color), has
long been recognized as a valuable nutritional supplement. The Company's
development of the MGM has enabled it to produce astaxanthin for commercial
distribution cost and introduce SALMON ESSENTIALS™), a proprietary combination
of astaxanthin and Omega-3 fatty acids.
The
Company's business strategy is to exploit its leading position in microalgae
cultivation technology to expand the sales of ASTAFACTOR®) AND SALMON
ESSENTIALS™ while preserving margins. We also hope to develop and introduce
additional microalgae-based nutritional products to the marketplace in the
future.
We face
significant potential competition for ASTAFACTOR®). We expect that other
nutraceutical products that the Company may launch in the future will face
meaningful competition as well, although at present we are not aware of a
product that combines the same ingredients as SALMON ESSENTIALS™ in a single
product.
We do not
believe that any commercial entity has developed a photobioreactor that matches
the MGM's combination of large size, low cost and level of process control or
sustained performance for a wide variety of aquatic species, although a number
of other companies are developing closed environment production systems for
marine micro-organisms. We believe that competition in each of these areas may
increase significantly over time as alternatives to the Company's patented
technology are developed. For more information on our competition, see "Risk
Factors; Risks Related to Our Industry.”
Mera
Pharmaceuticals' patents and intellectual property include issued patents
relating to the MGM and general processes for cultivating microalgae in
photobioreactors. The Company believes that intellectual property relative to
aquatic organism biotechnology will become much more important, challenging and
complex in the future.
Government
regulation and product testing are strong factors in the markets for the
products we have developed and produced. Our products are subject to regulation
by the U.S. Food and Drug Administration or similar agencies in foreign
countries and may require extensive testing for safety and efficacy before being
released for sale.
The
Company currently manufactures its products at a five-acre research, development
and production facility at the Hawaii Ocean Science and Technology Business Park
in Kailua-Kona, Hawaii. The facility is ideally located for research and
development and the commercial production of microalgae. We have access to large
volumes of deep ocean water (used for temperature control) in a stable tropical
climate with plentiful sunlight, conditions that are well suited to microalgae
cultivation. Although Hawaii's distance from many markets increases certain
costs of operation, on balance there are few locations, domestic or
international, that are as well suited to our cultivation
processes.
In
addition to the production of our own products, the Company is also seeking new
research collaborations with other enterprises to demonstrate the economic
feasibility of producing valuable substances that they have identified in
microalgae. Such collaborations will be sought to expand the applications of
Mera's technology. We will also explore licensing opportunities for the
technology where that makes economic sense.
RATIONALE
FOR MICROALGAE AS A SOURCE OF COMMERCIAL PRODUCTS
Microalgae
represent approximately half of all plant species. Many of their characteristics
make them attractive for commercial production.
|
|
·
|
Fewer
than 5,000 out of the estimated total of 30,000 species are believed to
have been cultivated in the
laboratory
|
|
|
·
|
Fewer
than 1,000 species are believed to have been carefully investigated for
new substances
|
|
|
·
|
Fewer
than 10 species have been cultivated at commercial
scale
|
|
2)
|
DEMONSTRATED
SOURCE OF NEW SUBSTANCES
|
|
|
·
|
Several
hundred new bioactive substances have been discovered in the small number
of microalgae that have been researched to
date
|
|
3)
|
SOURCE
OF VALUABLE SUBSTANCES
|
|
|
·
|
Many
molecules derived from microalgae are already known to be valuable for use
as enzymes, pigments, vitamins, nutraceuticals, pharmaceuticals and the
like
|
|
|
·
|
Bioactive
compounds extracted from microalgae have substantial potential value as
pharmaceuticals
|
|
|
·
|
Growth
rates for microalgae species range from about 1 to 10 divisions per
day
|
|
|
·
|
Growth
rates for these plants are, in general, faster than any other
plants
|
|
5)
|
LOW
COST OF RAW MATERIALS
|
|
|
·
|
Water,
sunlight, fertilizer and carbon dioxide, the principal raw materials used
in cultivation, are plentiful and
economical
|
THE
COMPANY'S MGM TECHNOLOGY
FEATURES OF MGM TECHNOLOGY.
The key features of MGM technology are sterility, size (25,000 liters) and
enhanced control over virtually all environmental factors affecting growth rates
and metabolic activity, such as temperature, pH, nutrient mix and distribution,
light, pests and contaminants.
This
combination of size and control has been the goal of international research
efforts for the past several decades. The MGM has achieved that goal. Although
it is among the largest photobioreactors ever operated, the MGM's patented
technology allows a far greater degree of control of the growth environment than
has been possible in systems previously.
PROCESS CONTROL SYSTEMS - THE KEY TO
REPRODUCIBLE PERFORMANCE.
In order to take greatest advantage of the MGM
technology, we have developed proprietary, computerized process control systems
for the MGM that make it possible to conduct the following operations
automatically:
|
|
·
|
monitoring
of key production variables at intervals more frequent than one
minute;
|
|
|
·
|
data
archiving for comprehensive analysis of
system
|
|
|
·
|
performance;
automated control of all operations performed more than once
a
|
|
|
·
|
day
(both a process control improvement and labor cost
saving);
|
|
|
·
|
immediate
alarm system for any system component not operating within parameters;
and
|
|
|
·
|
automated
maintenance for hundreds of system components, reducing failures and
preventing contamination.
|
Increasing
control over processes has produced several benefits. Product quality and
consistency have gone up, the scale at which processes are controllable has
increased and the amount of capital and labor required to accomplish a given
amount of production has decreased. This combination of effects translates into
enhanced efficiency, which translates into lower cost per unit of production and
higher margins.
COMPETITIVE PRODUCTION
SYSTEMS.
We are not aware of any closed system photobioreactor that
compares favorably with the MGM.
There are
other systems that cultivate microalgae at larger than experimental scale.
However, we believe that the advantages of the MGM over these other systems
include size, versatility, cost-effectiveness and higher yields. We believe that
an important advantage of the MGM over any competing technology is the ability
to achieve a high degree of control over all critical environmental factors for
microalgae, except those species that proliferate under the most extreme
conditions. As a result, it can be used in efficiently cultivating hundreds,
even thousands, of microalgal species at commercial scale. We do not believe any
other large scale system has such flexibility and versatility, which are
important factors in the development of new products from a variety of
microalgal species.
PRODUCTS
FROM MICROALGAE
DESCRIPTION AND PROPERTIES.
Astaxanthin is a red-orange, carotenoid pigment. It is closely related to other
well-known carotenoids, such as beta-carotene, lutein and
zeaxanthin.
All of
these molecules are antioxidants, substances shown by research to protect
health, but astaxanthin is among the strongest. Some studies indicate that it is
ten times more potent than beta-carotene, and more than 500 times more potent
than vitamin E - another well known and commonly used antioxidant.
Astaxanthin
is one of the main pigments in aquatic animals. It gives the flesh of salmon its
characteristic color, for example. Yet, it is far more than a pigment.
Astaxanthin has been shown to perform many essential biological functions,
including:
|
|
·
|
protecting
against the harmful effects of UV
light;
|
|
|
·
|
enhancing
the immune response;
|
|
|
·
|
protecting
against the oxidation of essential polyunsaturated fatty
acids;
|
|
|
·
|
stimulating
pro-vitamin A activity and vision;
|
|
|
·
|
improving
reproductive capacity; and assisting in
communication.
|
In
species like salmon or shrimp, astaxanthin is essential to normal growth and
survival and has been attributed vitamin-like properties. Some of these unique
properties are also effective in mammals. Studies in human and animal models
suggest that astaxanthin may substantially improve human health by virtue of its
antioxidant properties, protecting vision, reducing inflammation (recently shown
to be a major factor in heart attacks) slowing neurodegenerative diseases and
preventing certain cancers.
THE ASTAFACTOR® MARKET.
We
believe that the market for ASTAFACTOR® is likely to expand over the next few
years. There is growing evidence in the scientific and medical literature that
astaxanthin contributes meaningfully to the general well-being of humans.
Although we face competition in this market, we believe that our technology will
give us significant cost and quality advantages over our competitors in our
effort to capture a significant share of this growing market.
Sales of
ASTAFACTOR(R) began in Hawaii in March, 2000. Our experience has shown that
effectively promoting retail sales of astaxanthin requires longer format
advertising than can be readily used in typical retail advertising. The Company
has maintained its focus in the Hawaiian market, which offers revenue potential
to help support the cost of broader retail distribution.
During
2004 the Company introduced an extension of its ASTAFACTOR®) line, Salmon
Essentials™. This product offers a unique combination of astaxanthin and Omega-3
fatty acids, the two most important nutritional components available from wild
salmon, which is widely recognized as one of nature's most healthful foods. This
product offers all of the health benefits associated with ASTAFACTOR®) and adds
to them the significant benefits of including Omega-3 fatty acids in your diet,
such as improved cardiac health. Omega-3s also help reduce inflammation, adding
to that important benefit of SALMON ESSENTIALS™.
We began
marketing SALMON ESSENTIALS™ during 2004, launching the product initially
through our Hawaiian retail distribution system. We have also made SALMON
ESSENTIALS™ available through our web site. The Company is contemplating a
number of strategies to expand the distribution of what we believe is a product
with significant potential.
|
(3)
|
PRODUCT
LINE EXPANSION
|
The
Company is currently evaluating product line extensions for its ASTAFACTOR® and
Kona Sea Salt™ products and is also contemplating exploring additional
microalgae sources for development of nutraceutical products.
We
believe that many more nutraceutical products could be developed from
microalgae, but they remain unexamined and unexploited because there has been no
feasible way to grow them at a large enough scale. The MGM opens a path to this
untapped resource by combining effective, reproducible control with commercial
scale production.
DEPENDENCE
ON KEY CUSTOMERS
Our
business depends on key relationships in Hawaii. Sales to our largest customer,
Longs Drugs, owned by CVS, continues to represent the largest, but
decreasing percentage of our product sales. Sales to various domestic
distributors have been slowly increasing as have internet sales.
OUR
STRATEGIES
Our
objective is to sustain Mera Pharmaceuticals' leadership in microalgae
cultivation technology and to identify, optimize and directly commercialize
high-value microalgae products. We have several strategies to achieve these
goals.
1) INCREASE SALES OF
NUTRACEUTICAL PRODUCTS.
The Company intends to increase sales of its
current products, ASTAFACTOR®, SALMON ESSENTIALS™ and KONE SEA SALT™, by
expanding distribution through a variety of sales channels. It appears that
awareness of the benefits of our products is growing in the marketplace, and
once the benefits of our products are more fully understood by the general
public, we will focus on entering national domestic retail chains with the
potential for delivering high sales volumes at attractive margins and efficient
distribution.
2) EXPAND STRATEGIC
ALLIANCES.
We intend to develop relationships with other companies who
have products that will benefit from utilizing our technology under license. We
believe that there is significant potential revenue associated with the
licensing of our technology to other companies or in performing contract work
for them utilizing our facilities or expanded facilities if
justified.
MANUFACTURING
Our Kona
facility has sufficient capacity to meet our current demand for
products. Ability to expand our production is sufficient to meet our
projected needs for the foreseeable future. Further expansion of the Kona
facility is feasible, if demand for our products justifies doing
so.
RESEARCH
AND DEVELOPMENT COSTS
Research
and development costs include salaries, development materials, plant and
equipment depreciation and costs associated with operating our five-acre
research and development/production facility. During the last fiscal year, the
Company has spent $324,383 versus $299,696 in the previous year on research and
development activities.
MARKETING
AND SALES
The
Company's marketing strategy for its products may vary depending on the specific
product being sold and its target market, but that strategy is generally built
on two fundamental tenets:
1. CREATE ALLIANCES WITH
EFFICIENT, HIGH-QUALITY DISTRIBUTORS IN KEY U.S. REGIONAL AND INTERNATIONAL
MARKETS AND SUPPORT THOSE DISTRIBUTORS WITH EFFECTIVE ADVERTISING AND PROMOTION
TO THE CONSUMER.
The Company currently markets ASTAFACTOR®, SALMON
ESSENTIALS™ and KONA SEA SALT™ to mass retail outlets in Hawaii through
distribution arrangements with established companies. We are also expanding our
efforts to distribute outside of Hawaii.
2. SELL ASTAFACTOR®,
SALMONESSENTIALS™ AND KONA SEA SALT™ DIRECTLY TO THE CONSUMER.
This
approach allows us to reach consumers throughout the domestic market at the most
attractive margins, since it eliminates distributor and retailer profits.
Web-based and specialty media promotion are also used to reinforce our marketing
efforts and product visibility to all consumers. We are building a customer data
base that will enhance our ability to reach regular customers with new products
as they are developed and come to market.
COMPETITION
The
Company believes that its proprietary technology and process control systems and
software give it a significant advantage relative to its competitors. However,
there are a number of companies that are engaged in efforts to develop
microalgae-based products that compete with ours, either directly or
indirectly.
We
believe that our original MGM technology was the first closed-system,
process-controlled photobioreactor to be operated at commercial scales larger
than 2,750 gallons (10,000 liters). We can now operate at a scale more than
twice that (25,000 liters). We are aware of only three other companies in the
world - Biotechna of Australia, Algatechnologies of Israel and AstaReal of
Hawaii - that claim to possess proprietary photobioreactor
technology.
COMPETITORS
FOR ASTAFACTOR®) There are various other producers of astaxanthin that compete
with ASTAFACTOR®, our nutraceutical astaxanthin product. Competition also comes
from non-producers who acquire raw materials from the producers for distribution
in the nutraceuticals market.
Potential
competitors include producers of the synthetic material as well. However, to our
knowledge neither BASF, a large German chemical company, nor Hoffman-LaRoche, a
large Swiss pharmaceutical company, both large global producers of synthetic
astaxanthin, has indicated an interest in this market.
Furthermore,
we believe that consumers of nutraceuticals prefer products from natural sources
to those from synthetic sources. We are aware of other companies that are
interested in or are actually marketing nutraceutical astaxanthin which use a
fermentation process. We believe our production process has cost or quality
advantages or both over those other companies.
PATENTS,
LICENSES AND PROPRIETARY TECHNOLOGY
We rely
upon a combination of patents, copyrights, trade secrets, know-how, continuing
technological innovation and licensing opportunities to develop and maintain our
competitive position. Our future prospects depend in part on our ability to
obtain patent protection for our products and processes. We need to preserve our
copyrights, trademarks and trade secrets. We also need to operate without
infringing the proprietary rights of third parties.
PATENTS.
We have been awarded
or have filed applications for patents relating to various processes, including,
but not limited to, the process and apparatus for the production of
photosynthetic microbes and the method of control of microorganism growth
processes. These patents are active in the United States and potentially other
countries. The original duration of these patents varied from fifteen to twenty
years from the date of filing or issuance, and the Company's current patents
will be active for five to nine years, provided the maintenance fees associated
with such patents are timely paid. The Company reassesses the value of each
patent it holds at the time maintenance fees are due, and in cases where
maintaining a patent is judged to be of no significant strategic value, we do
not renew the patent.
Other
companies may have filed and in the future are likely to file patent
applications that are similar or identical to ours. To determine the priority of
inventions, we may have to participate in interference proceedings declared by
the United States Patent and Trademark Office. Such proceedings could result in
substantial costs to us. We cannot ensure that any such third-party patent
application will not have priority over ours. Additionally, the laws of certain
foreign countries may not protect our patent and other intellectual property
rights to the same extent as the laws of the United States.
Our
future prospects also depend in part on our neither infringing on patents or
proprietary rights of third parties nor breaching any licenses that may relate
to our technologies and products. We cannot guarantee that we will not infringe
on the patents, licenses or other proprietary rights of third parties. We have
not conducted an exhaustive patent search, and we cannot ensure that patents do
not exist or could not be filed that would have a material adverse effect on our
ability to develop and market our products. There are many United States and
foreign patents and patent applications in our area of interest.
We
attempt to control the disclosure and use of our proprietary technology,
know-how and trade secrets under agreements with the parties involved. However,
we cannot ensure that others will honor all confidentiality agreements. We
cannot prevent others from independently developing similar or superior
technology, nor can we prevent disputes that could arise concerning the
ownership of intellectual property.
TRADEMARKS AND SERVICE MARKS.
The following trademarks and service marks have been registered or are claimed
marks that the Company has not registered but as to which it believes it has
established a common law right of use and as to which it has no information to
the contrary. The registered trademark on ASTAFACTOR® is valid through
March 2012, and the following other claimed marks that the Company believes
it has established a common law right of use are valid for the standard period
of duration, as provided in applicable common law:
SALMONESSENTIALS™
AQUAXAN®
MERA
PHARMACEUTICALS™
MERA
GROWTH MODULE ™ (MGM)
MERA
PROCESS CONTROL SYSTEM™ (MPCS)
MERA
REMOTE DATA WEB ACCESS™ (RDWA)
DRUGS
FROM THE SEA™
KONA SEA
SALT™
GOVERNMENT
REGULATION AND PRODUCT TESTING
Our
current and potential products and our manufacturing and research activities are
or may become subject to varying degrees of regulation by many government
authorities in the United States and other countries. Such regulatory
authorities could include the State of Hawaii Department of Health or
Agriculture Department, the FDA, and comparable authorities in foreign
countries. Each existing or potential microalgae product intended for human use
that we develop or market, either directly or through licensees or strategic
partners, may present unique regulatory problems and risks. Relevant regulations
depend on product type, use and method of manufacture. The FDA regulates, in
varying degrees and in different ways, dietary supplements, other food products,
medical devices and pharmaceutical products. Regulations govern manufacture,
testing, exporting, labeling and advertising.
Any
products we develop for use in human nutrition, or cosmetics could require that
we develop and adhere to Good Manufacturing Practices ("GMP"), as suggested by
the FDA, European standards and any other applicable standards mandated by
federal, state, local or foreign laws, regulations and policies. Our current
cultivation and processing facilities and procedures are not yet required to
comply with GMP or ISO standards, although our contract extraction and
encapsulation facilities must meet GMP standards, and they do. We believe we are
prepared to meet these requirements more broadly when necessary.
The
Company currently distributes ASTAFACTOR®, SALMON ESSENTIALS™ and KONE SEA SALT™
in the United States, and in certain foreign countries. We also hope to expand
our distribution internationally. Regulatory approval requirements vary by
country. We believe the approval process for our products in most countries will
come under their "natural" status and that it will be approved relatively
quickly; however, we can provide no assurances in this regard.
EMPLOYEES.
As of
October 31, 2009, the Company had five (5) full-time employees and
various part time help as needed. We consider relations with our employees to be
good. None of our employees is covered by a collective bargaining
agreement.
YOU
SHOULD CAREFULLY CONSIDER THE RISKS DESCRIBED BELOW, IN ADDITION TO ALL OTHER
INFORMATION INCLUDED IN THIS REPORT, BEFORE YOU DECIDE TO INVEST IN OUR COMMON
STOCK. IF ANY OF THE FOLLOWING RISKS OCCURS, OUR BUSINESS, FINANCIAL CONDITION
OR RESULTS OF OPERATIONS COULD BE MATERIALLY ADVERSELY AFFECTED. THE TRADING
PRICE OF OUR COMMON STOCK COULD DECLINE DUE TO ANY OF THE FOLLOWING RISKS OR
OTHERS NOT YET IDENTIFIED BY MANAGEMENT, AND YOU COULD LOSE ALL OR PART OF YOUR
INVESTMENT.
RISKS
RELATED TO OUR BUSINESS
WE
HAVE INCURRED SUBSTANTIAL OPERATING LOSSES AND EXPECT TO INCUR FUTURE LOSSES.
OUR FUTURE FINANCIAL RESULTS ARE UNCERTAIN, AND WE MAY NEVER BECOME A PROFITABLE
COMPANY.
From
September 16, 2002 (the date that we completed our reorganization
proceedings and adopted "fresh-start accounting") through October 31, 2009
we had an accumulated deficit of $8,111,859. Our losses to date are primarily
due to the costs of research and development, and the general and administrative
costs associated with our operations. We expect to continue to incur smaller
operating losses through at least fiscal year 2010. We expect to have
quarter-to-quarter and year-to-year fluctuations in revenues and expenses. As a
result, our losses may increase in the future, even if we achieve our revenue
goals, and some of those losses could be significant.
Should we
achieve profitability, we may be unable to sustain or increase profitability on
a quarterly or annual basis. Many factors could affect our ability to achieve
and maintain profitability, including:
|
|
·
|
our
ability to complete successfully the commercialization and production cost
optimization of our products;
|
|
|
·
|
our
ability to manage production costs and yield issues associated with
increased production of our
products;
|
|
|
·
|
the
progress of our research and development programs for developing other
microalgal products;
|
|
|
·
|
the
time and costs associated with obtaining regulatory approvals for our
products;
|
|
|
·
|
our
ability to protect our proprietary rights, or the expense of doing
so;
|
|
|
·
|
the
costs of filing, maintaining, protecting and enforcing our
patents;
|
|
|
·
|
competing
technological and market
developments;
|
|
|
·
|
changes
in our pricing policies or the pricing policies of our
competitors;
|
|
|
·
|
the
costs of commercializing and marketing our existing and potential
products; and
|
|
|
·
|
the
inability to achieve a level of sales of our products necessary to
generate sufficient revenues to cover research, development and operating
costs.
|
If our
revenues grow more slowly than we anticipate or if our operating expenses exceed
our expectations and cannot be reduced, our losses could continue beyond our
present expectations, and we may never become a profitable company.
WE
MAY BE UNABLE TO RAISE ADDITIONAL CAPITAL OR GENERATE THE CAPITAL NECESSARY
TO
SUPPORT OUR PLANNED LEVEL OF RESEARCH AND DEVELOPMENT ACTIVITIES AND TO
MANUFACTURE
AND MARKET OUR PRODUCTS.
We will
require expenditures to support our research and development activities and to
manufacture and market our products. Over the next twelve months, we project
expenditures of approximately $600,000 in operating capital, not including any
capital expenditures that may be necessary or desirable. Many factors will
determine our future capital requirements, including:
|
|
·
|
market
acceptance of our products;
|
|
|
·
|
our
ability to manufacture our products cost-effectively in quantities needed
to sustain growing sales of our ASTAFACTOR® and KONA SEA SALT™ line of
products;
|
|
|
·
|
the
extent and progress of our research and development
programs;
|
|
|
·
|
the
time and costs of obtaining regulatory clearances for some of our
products;
|
|
|
·
|
the
costs of filing, maintaining, protecting and enforcing patent
claims;
|
|
|
·
|
the
need to address competing technological and market
developments;
|
|
|
·
|
the
cost of developing and/or operating production facilities for our existing
and potential products; and
|
|
|
·
|
the
costs of commercializing our
products.
|
Revenue
from product sales and other sources pay some of our operating costs, but to
date that revenue has not been sufficient to cover our operating costs fully. We
are seeking investment from various sources to help sustain our operations until
we can increase revenues to the point that they can sustain our operations
indefinitely. However, additional financing may not be available on favorable
terms, if at all. If we do not have adequate funds, we may have to curtail
operations significantly. In addition, we may have to enter into unfavorable
agreements that could force us to relinquish certain technology or product
rights, including patent and other intellectual property rights. If we cannot
raise enough capital, then we may have to curtail production, limit our product
development activities, reduce marketing activities or delay other plans
intended to increase revenue and help us achieve profitability.
IF
WE CANNOT OVERCOME THE CHALLENGES OF PRODUCING MICROALGAE ON A COMMERCIAL SCALE,
WE MAY NOT ACHIEVE ECONOMIC PRODUCTION COSTS.
To be
successful, we must produce products at acceptable costs while ensuring that the
quantity and quality of our products comply with contractual and regulatory
requirements and regulations. Many factors complicate the production of
microalgal products, and they could limit production at any time. These
include:
|
|
·
|
microbial
contamination;
|
|
|
·
|
variability
in production cycle times due to technical, environmental and biological
factors; and
|
|
|
·
|
losses
of final product due to inefficient
processing.
|
We
currently have sufficient inventory to meet the foreseeable requirements of our
existing customers. However, we are engaged in efforts to increase sales in
order to achieve profitability, potentially beyond the capacity of our existing
facility to produce. We have prepared to meet that increased demand, should it
occur, by acquiring additional space at our Kona, Hawaii facility.
IF
THE DEMAND FOR NATURAL ASTAXANTHIN OR OUR OTHER PRODUCTS EXCEEEDS OUR CURRENT
PRODUCTION CAPABILITIES, AND IF WE ARE UNABLE TO EXPAND OUR PRODUCTION CAPACITY
IN A TIMELY MANNER, WE MAY EXPERIENCE SIGNIFICANT FINANCIAL, TECHNICAL AND
COMMERCIAL CHALLENGES.
The
capacity of our existing production facility in Hawaii is sufficient to
meet
current demand
for our products. However, demand for our natural astaxanthin and other products
may eventually exceed the current capacity of our Hawaiian production facility.
To address this capacity question, we have initiated efforts to increase our
production efficiency and to prepare for expansion of our Hawaiian facility, if
needed. However, our efforts are focused on generating a level of sales that
would make it difficult to meet our total demand from our Hawaiian facility,
especially if we develop additional products. We believe that our inventory plus
our existing production capacity is sufficient to meet demand for the
foreseeable future. In the event that sales increase to a level that we cannot
meet with our existing capacity, we have planned for the expansion of our
Hawaiian facility. If we are unable to expand our current production facility,
we may be unable to meet demand for product and could lose the opportunity to
increase our revenues. We could also lose customers, both current and potential,
who may not do business with us absent an assurance of the ability to deliver
product in sufficient quantities.
OUR
CUSTOMER BASE IS CONCENTRATED AMONG RELATIVELY FEW CUSTOMERS, AND THE LOSS OF
ANY OF THESE CUSTOMERS WOULD MATERIALLY ADVERSELY AFFECT OUR
REVENUES.
Our
business currently depends on key distribution relationships in Hawaii.
These customers
currently purchase approximately 30% of the natural products we sell,
with the remainder being sold to smaller retail accounts, directly to consumers
or internationally. If we lose one or more of these customers, or if they do not
continue buying our products at the current and anticipated purchase levels,
then our revenues could decrease. In addition, the loss of one or more of these
customers may adversely affect our reputation, and we could have difficulty
attracting new customers as a result.
IF
WE FAIL TO ATTRACT AND RETAIN KEY PERSONNEL WE WILL BE UNABLE TO EXECUTE OUR
BUSINESS PLAN, AND OUR BUSINESS WILL SUFFER.
Our
success depends on the continued efforts of the principal members of our
management team. The Company presently has the key members of that team that it
needs to retain to execute its plans fully. Success in doing so cannot be
assured.
OUR
BUSINESS WILL NOT OPERATE EFFICIENTLY AND OUR RESULTS OF OPERATIONS WILL BE
NEGATIVELY AFFECTED IF WE ARE UNABLE TO MANAGE OUR GROWTH
EFFECTIVELY.
Until
2002 we focused almost exclusively on product research and technology
development. As we moved toward commercial production of microalgal products we
had to initiate or expand many activities, including outsourcing, customer
relations, engineering, construction, recruiting and training. During this
transition, the size of our organization increased rapidly. During the Company's
reorganization, the size of our organization decreased again. We expanded our
employee base nominally during fiscal year 2009 and expect to rationally expand
during fiscal year 2010 in both sales staff and production assuming revenues
expand with increased demand for our products. If revenues do not increase, we
may not have the financial resources needed to sustain operations.
We expect
demands on our financial and management control systems to increase this year.
If we fail to upgrade our financial and management control systems, or if we
encounter difficulties during upgrades of these systems, then we may not be able
to manage our human and financial resources effectively. Such ineffectiveness
could make it difficult to retain or attract employees and could directly or
indirectly create unnecessary expenses or lead to incorrect decisions by
management.
AS
WE EXPAND OUR PRODUCT LINE AND ATTEMPT TO PENETRATE ADDITIONAL MARKETS, WE MAY
FACE SIGNIFICANT CHALLENGES TO SUCCESS.
We are
exploring expanding our product lines. The success of our
product lines will
depend on our ability to implement our marketing strategy and comply with the
standards of Good Manufacturing Practice, or GMP, as and when applicable. We
believe the prospects for nutraceutical products will depend, in the short
term, on product quality and education of consumers regarding its benefits. Our
ability to penetrate new markets for our natural astaxanthin products will, we
believe, depend strongly on regulatory approval in several major markets within
and outside the United States. We expect the success of our products to depend
primarily on our ability to develop and market these new products.
We cannot
assure successful development of any potential products, nor can we guarantee
market acceptance of any of our products, existing or future. We have limited
marketing experience in nutraceutical markets. We have eight years of experience
in electronic marketing and direct retail sales. We cannot assure you that we or
our consultants or contractors will be successful in our marketing efforts, nor
can we prevent them from competing with us or assisting our competitors. If we
are unable to develop or commercialize any of our product lines successfully,
then our revenues will fail to grow.
OUR
PRODUCTS AND PRODUCTION ACTIVITIES ARE SUBJECT TO GOVERNMENT REGULATION AND
ACTION, WHICH ARE SUBJECT TO CHANGE.
We are
affected by changes in or the imposition of governmental regulations and
actions, including: (i)
new laws, regulations and judicial decisions related to the production,
marketing and sale of nutraceutical products, (ii) changes in the United States
Food and Drug Administration and foreign regulatory approval processes that may
delay or prevent the approval of new products and result in lost market
opportunity, (iii) new laws, regulations and judicial decisions affecting
pricing or marketing of our products and (iv) changes in the tax laws relating
to Mera Pharmaceuticals' operations.
WE
MAY BE UNABLE TO PROTECT OR ENFORCE OUR INTELLECTUAL PROPERTY RIGHTS
ADEQUATELY,
AND OUR EFFORTS TO DO SO COULD BE TIME CONSUMING AND EXPENSIVE AND COULD DIVERT
MANAGEMENT ATTENTION FROM EXECUTING OUR BUSINESS STRATEGY.
We regard
the protection of our patents, copyrights, trade secrets and know-how
(collectively
intellectual property) as critical to our success. We rely on a combination of
patent, copyright and trade secret laws and contractual restrictions to protect
our intellectual property and maintain our competitive position. Our future
prospects depend in part on our ability to protect our intellectual property
while operating without infringing the intellectual property rights of third
parties.
We may be
unable to develop any additional patentable technologies. We cannot be certain
that any patents issued to us or available to us through a license arrangement
will establish the means to produce or provide us with any competitive advantage
for any product or products. Third parties could challenge our patents or could
obtain patents that have a material adverse effect on our ability to do business
efficiently and effectively.
The
patent positions of nutraceutical, pharmaceutical, biopharmaceutical and
biotechnology companies, including ours, are generally uncertain and involve
complex legal and factual questions. Patent law continues to evolve in the scope
of claims in the technology area in which we operate. Therefore, the degree of
future protection for our proprietary rights is uncertain. We cannot guarantee
that others will not independently develop similar or alternative technologies.
Other parties may duplicate our technologies, or, if patents are issued to us,
they may design around those patented technologies. Other parties may have filed
or could file patent applications that are similar or identical to some of ours.
These patent applications could have priority over ours. To determine the
priority of inventions, we may have to participate in interference proceedings
declared by the United States Patent and Trademark Office, which could be very
costly. In addition, the laws of some foreign countries may not protect our
patents and other intellectual property rights to the same extent as the laws of
the United States.
We could
incur substantial costs in litigation if we need to defend ourselves against
patent infringement claims brought by third parties, or if we choose to initiate
claims against others. We have in the past, and we may in the future, be
required to dedicate significant management time and financial resources to
prosecute or defend infringement actions. In addition, a finding of
non-infringement or declaration of invalidity of our patents could reduce or
eliminate the exclusivity of our proprietary technology. Present and potential
collaborators may terminate or decide not to enter into relationships with us if
our intellectual property position is weakened. In addition, a finding of
non-infringement or declaration of invalidity of our patents could reduce our
ability to obtain future financing.
There
could be significant litigation in our industry regarding patent and other
intellectual property rights. For example, third parties may bring infringement
or other claims against us for using intellectual property that we internally
developed or license from third parties. In addition, although nondisclosure
agreements generally control the disclosure and use of our proprietary
technology, know-how and trade secrets, we cannot guarantee that all
confidentiality agreements will be honored or that our proprietary technology,
know-how and trade secrets will not be disseminated, or that any party
responsible for doing so will be able to compensate us adequately for such
loss.
We may
not prevail in the prosecution or defense of any action, nor can we predict
whether third parties will license necessary intellectual property rights to us
on commercially acceptable terms, if at all. Any of these outcomes could be very
costly and could diminish our ability to develop and commercialize future
products.
OUR
PRODUCTION CAPABILITY IS HIGHLY DEPENDENT ON ENVIRONMENTAL AND CLIMATIC FACTORS
BEYOND OUR CONTROL.
All of
our current production capacity is located at a single facility in Kona, Hawaii.
We currently have an ample inventory to meet our foreseeable demand, but any
future event that causes a long-term disruption in production at our facility
could significantly impair our ability to meet customer demand. These events
could include fires, volcanic eruptions, earthquakes, tidal waves, hurricanes or
other natural disasters. In addition, consistent sunlight, high ambient
temperatures and an ample supply of fresh water are necessary for microalgal
growth. If we experience any significant or unusual change in climate, or should
our water supplies be threatened by microbial contamination we cannot control,
there could be an adverse impact on our production. If we cease production for
any significant period, the success of our business would be threatened from a
resulting loss of customers, revenues and valuable employees.
CURRENCY
FLUCTUATIONS AND DIFFERENT STANDARDS, REGULATIONS AND LAWS RELATING TO
INTERNATIONAL OPERATIONS MAY ADVERSELY AFFECT OUR RESULTS OF
OPERATIONS.
We expect
to sell our products on a global scale due to projected international market
demand. International business is generally more difficult than domestic
business and can create additional costs and delays not associated with business
conducted solely within the United States. Factors related to doing business
internationally that could impact us include: foreign government controls and
regulations, economic conditions, currency fluctuations, duties and taxes,
political and economic instability or unrest, imposition of or increases in
tariffs, disruptions or delays in shipments and other trade restrictions. These
factors, among others, can all lead to interference with or increased costs of
operation and the ability to sell products in international markets. If any such
factors were to render the conduct of business in a particular country
undesirable or impracticable, there could be a material adverse effect on our
business, our financial condition and the results of operations. There can be no
assurance that our products or marketing efforts will be successful in foreign
markets.
In
addition, fluctuations in currency exchange rates could make our products more
expensive in some countries, resulting in the loss of customers in those
markets.
WE
MAY BE SUBJECT TO PRODUCT LIABILITY LAWSUITS, AND OUR INSURANCE MAY BE
INADEQUATE TO COVER DAMAGES.
Clinical
trials or marketing of any of our current or potential products may expose us to
liability claims arising from the use of these products. Even the most thorough
of clinical trials could fail to detect a significant side effect associated
with long-term use of a product, and it is possible that liabilities will arise
even after our products receive any required regulatory approvals. Even if such
claims are not well-founded, defending them will be very costly and consume
substantial management attention and energy. We cannot ensure that our current
product liability insurance, together with indemnification rights under our
existing or future licenses and collaborative arrangements, will be adequate to
protect us against any claims and resulting liabilities. As we expand our
business, we may be unable to obtain additional insurance on commercially
reasonable terms. We could suffer harm to our financial condition and our
reputation if a product liability claim or recall exceeds the limits of our
insurance coverage.
BECAUSE
OUR PRINCIPAL STOCKHOLDERS HAVE SIGNIFICANT CONTROL OF OUR MANAGEMENT AND
AFFAIRS, THESE STOCKHOLDERS MAY BE ABLE TO CONTROL US AND ALSO PREVENT
POTENTIALLY BENEFICIAL ACQUISITIONS OF OUR COMPANY BY OTHERS.
As of
October 31, 2009, our current directors and executive officers and family
and related entities, as a group, beneficially owned approximately 100,100,000
of the approximately 548,000,000 shares of our common stock outstanding as of
October 31, 2009. As a result, our officers and directors may be able to
exert considerable influence over the actions of the Board of Directors and
matters requiring approval of our stockholders. This concentration of ownership
could delay or prevent a change in control and may adversely affect the ability
of other stockholders to adopt a position in opposition to these directors and
officers. Our principal stockholders may have interests that differ from our
other stockholders, particularly in the context of potentially beneficial
acquisitions of our Company by others, and they may legitimately vote as
stockholders in a manner that protects their interests.
RISKS
RELATED TO OUR INDUSTRY
IF
WE FAIL TO COMPETE EFFECTIVELY AGAINST LARGER, MORE ESTABLISHED COMPANIES
WITH
GREATER RESOURCES, OUR BUSINESS WOULD SUFFER.
Competition
in the nutraceutical market is intense. Factors affecting competition
include financial resources, research and development capabilities and
manufacturing and marketing experience and resources.
Our
nutraceutical astaxanthin products will compete directly with the products of
several companies that sell a similar nutraceutical product. At least three
companies that we are aware of have a product like ours. We expect that our
nutraceutical astaxanthin products will compete on the basis of product quality,
price, and efficiencies derived from our intellectual property and an effective
marketing strategy. However, if our competitors develop a proprietary position
that inhibits our ability to compete, or if our marketing strategy is not
successful, then our revenues may not increase.
There are
various companies using microalgae cultivation technology processes that compete
with our processes. We are aware of two U.S. companies, Martek of Maryland and
Omega-Tech of Colorado, that produce commercial quantities of microalgae using
modified fermentation processes. We are also aware of one company, Cell Tech of
Oregon, which harvests microalgae from natural environmental sources. There are
three other companies in the world - Biotechna of Australia, AlgaTechnologies of
Israel and AstaReal of Hawaii - that claim to possess proprietary
photobioreactor technology and use it for commercial purposes. While there are
many other photobioreactors in operation besides those, to our knowledge, they
are all operated by universities or research institutes and are not used for
commercial purposes. It is possible that competing photobioreactor technologies
that could adversely affect our perceived technical and competitive advantages
already exist or may emerge in the future.
We also
anticipate that the competition to develop microalgal-based products other than
natural astaxanthin will increase. We expect competitors to include major
pharmaceutical, food processing, chemical and specialized biotechnology
companies. Many of these companies will have financial, technical and marketing
resources significantly greater than ours. There are also other emerging marine
biotechnology companies that could form collaborations with large established
companies to support research, development and commercialization of products
that may compete with our current and future products. Also, academic
institutions, governmental agencies and other public and private research
organizations are conducting research activities and seeking patent protection
for microalgal products and may commercialize products that compete with ours on
their own or through joint ventures. In addition, there may be technologies we
are unaware of, or technologies that may be developed in the future, that could
adversely affect our perceived technical and competitive advantage.
INCREASED
COMPETITION MAY SIGNIFICANTLY REDUCE THE MARKET PRICE OF NATURAL
ASTAXANTHIN.
Astaxanthin
can be produced either naturally from Haematococcus pluvialis, as we do, from
yeast by fermentation, as Igene Biotechnology, Inc. does, or through synthesis
of chemical compounds. We are not aware that synthetic astaxanthin is approved
for direct human consumption in any jurisdiction, although the FDA approved the
Hoffman-LaRoche, Ltd. synthetic astaxanthin product as a food additive in fish
feed in 1995. The Igene yeast-based product is also not approved for regular
human consumption. We believe that the cost of producing synthetic astaxanthin
is significantly lower than that for natural astaxanthin. We are not able to
determine how production costs for the yeast-based product compares with ours.
If we succeed in commercializing natural astaxanthin to the extent we project,
producers of yeast-based and synthetic astaxanthin may increase their efforts to
obtain approval of their product for human consumption. Studies have shown that
natural astaxanthin is more effective than synthetic astaxanthin when used with
various fish and shellfish populations. However, we have not determined that to
be the case in human applications. The introduction of yeast-based or synthetic
astaxanthin into the human nutraceutical marketplace could adversely affect the
price at which we sell our product and the market share that we can obtain.
While we believe that there are substantial hurdles to the approval of
yeast-based and synthetic astaxanthin for human consumption in the U.S. and
other major markets, we cannot be certain that such approval will not occur. A
single producer, Hoffman-LaRoche, Inc., currently dominates the synthetic
astaxanthin market. Hoffman-LaRoche has maintained the market price of its
synthetic astaxanthin, which is derived from petrochemicals, at approximately
$1,800 - $2,500 per kilogram. That is below the price at which we would be able
to sell astaxanthin in comparable form.
IF
WE ARE UNABLE TO COMPLY WITH GOVERNMENT REGULATION OF OUR PRODUCTS AND
PRODUCTION
ACTIVITIES, WE MAY BE FORCED TO DISCONTINUE PRODUCTION OF CURRENT OR FUTURE
PRODUCTS.
We are
subject to federal, state, local and foreign laws and regulations governing our
products and production activities. This makes us vulnerable to: (i) the
imposition of new laws, regulations and judicial decisions related to
pharmaceutical and nutraceutical products, (ii) changes in the United States
Food and Drug Administration and foreign regulatory approval processes that may
delay or prevent the approval of new products and result in lost market
opportunity, (iii) delays in the receipt of or the inability to obtain required
approvals, (iv) new laws, regulations and judicial decisions affecting pricing
or marketing of pharmaceutical and nutraceutical products, (v) the suspension or
revocation of the authority necessary for manufacture, marketing or sale of our
products, (vi) the imposition of additional or different regulatory
requirements, such as those affecting labeling, (vii) seizure or recall of
products, and (viii) the failure to obtain, the imposition of limitations on the
use of, or the loss of patent and other intellectual property rights. While we
do not consider our products to be "herbal" supplements (i.e., products that are
made from drying and grinding entire plant parts), increased regulatory scrutiny
of herbal products as the result of health issues (e.g., with ephedra) may also
lead to more stringent regulation of our products.
Each
existing or potential product that we develop, produce, market or license
presents unique regulatory problems and risks. Relevant regulations depend on
product type, use and method of manufacture. The FDA regulates, in varying
degrees and in different ways, dietary supplements, other food products, medical
devices and pharmaceutical products. Regulations govern manufacture, testing,
exportation and labeling, while the Federal Trade Commission (FTC) regulates
advertising.
We are or
may become subject to other federal, state and foreign laws, regulations and
policies with respect to labeling of products, importation of organisms and
occupational safety, among others. Federal, state and foreign laws, regulations
and policies are always subject to change and depend heavily on administrative
policies and interpretations. We cannot ensure that any of our products will
satisfy applicable regulatory requirements. Changes could occur in federal,
state and foreign laws, regulations and policies and, particularly with respect
to the FDA or other such regulatory bodies, such changes could be retroactive.
Such changes could have a material adverse effect on our business, financial
condition, results of operations and relationships with corporate
partners.
Nutraceutical
products that we develop will be viewed as human dietary supplements. The FDA
requires pre-market clearance in the United States, as do other countries where
these nutraceutical products are marketed, if they are intended for human
consumption. The process of obtaining FDA clearance for either a food additive
or a human dietary supplement can be expensive and time consuming, although
significantly less expensive than the process for obtaining clearances for a new
pharmaceutical. With natural products such as ours there is often only a brief
and inexpensive waiting period before marketing of a nutraceutical can begin.
Extensive information is required on the toxicity of the additive, including
carcinogenicity studies and other animal testing. FDA clearance to market
dietary supplements is obtained by notifying the FDA in writing of the intention
to market a certain product and providing supporting documentation regarding
toxicity. If the FDA does not object within a specified period of time, approval
is deemed granted.
Mera's
corporate predecessor received FDA clearance for the ASTAFACTOR® in early 2000.
We similarly notified the FDA of our intention to market Salmon Essentials™ in
2004, without objection. While we believe that the natural products on which we
are focused will be subject to few objections in this approval process, we
cannot ensure that any of our future products, on which we may have expended
substantial development effort, will be cleared by the FDA on a timely basis, if
at all.
The
ASTAFACTOR® AND SALMON ESSENTIALS™ our nutraceutical astaxanthin products are
being distributed internationally already and are likely to be distributed in
others. Regulatory approvals in foreign markets vary by country. We believe the
approval process for these products will generally come under their "natural
product" status and be approved relatively quickly. However, we can provide no
assurances in this regard.
THE
PRICE OF OUR COMMON STOCK MAY BE HIGHLY VOLATILE.
The
trading price of our common stock has been, and is likely to continue to be,
highly volatile. We could be subject to wide fluctuations in price in response
to various factors, many of which are beyond our control,
including:
|
|
·
|
announcements
of technological innovations or new commercial products by us or our
competitors;
|
|
|
·
|
developments
concerning proprietary rights, including patents, by us or our
competitors;
|
|
|
·
|
publicity
regarding actual or potential benefits or drawbacks relating to products
under development by us or our
competitors;
|
|
|
·
|
conditions
or trends in the life sciences, nutraceutical or pharmaceutical
markets;
|
|
|
·
|
changes
in the market valuations of biotechnology and life sciences companies in
general; and
|
|
|
·
|
general
regulatory developments affecting our products in both the United States
and foreign countries.
|
In
addition, technology companies have experienced extreme price and volume
fluctuations that have often been unrelated or disproportionate to the operating
performance of those companies. There has been particular volatility in the
market prices of securities of life science companies. These broad market and
industry factors may seriously harm the market price of our common stock,
regardless of our operating performance.
RISKS
RELATED TO THE SECURITIES MARKETS AND OUR COMMON STOCK
OUR
COMMON STOCK IS TRADED ON THE PINK-SHEET MARKET, WHICH MAY MAKE THE STOCK MORE
DIFFICULT TO TRADE ON THE OPEN MARKET.
Our
common stock is currently traded in the PINK-SHEET market. PINK-SHEET markets
are generally more difficult to trade than those on the Nasdaq National Market,
the Nasdaq Small Cap Market or the major stock exchanges. Since the initial
public offering of our common stock in January 1989, the average daily
trading volume of our common stock has been relatively low. We cannot ensure
that a more active public trading market will ever develop for our common stock.
In addition, accurate price quotations can be difficult to obtain and price
volatility is common for companies whose securities trade on the
PINK-SHEETS.
THE
SALE OF A SUBSTANTIAL NUMBER OF SHARES OF OUR COMMON STOCK BY STOCKHOLDERS COULD
CAUSE THE MARKET PRICE OF OUR COMMON STOCK TO DECLINE.
As of
October 31, 2009 we had 547,759,915 shares of common stock outstanding. Of
these shares, approximately 193,000,000 have either been registered under the
Securities Act of 1933, as amended (the "Securities Act"), are freely tradable
without volume limitations under Rule 144 of the Securities Act or are
exempt from registration under 11 U.S.C. 1145 as a result of the reorganization
of our predecessor issuer, Aquasearch, Inc.
We cannot
predict the effect, if any, that sales of shares of our common stock or the
availability of these shares being offered for sale will have on prevailing
market prices. However, substantial amounts of our common stock were sold in the
public market, to the point where raising capital has become extremely
difficult.
We will
need additional funding for capital expenditures and operating capital. If we
raise additional funds by selling equity securities, the share ownership of our
existing investors would be diluted. In addition, new equity purchasers may
obtain rights, preferences or privileges that are superior to those of our
existing stockholders and most likely demand a major reverse stock split in
order to clean up the capital structure. This would allow for
potential additional add on investments.
THE
ABILITY OF OUR BOARD OF DIRECTORS TO ISSUE PREFERRED STOCK COULD ADVERSELY
AFFECT THE INTERESTS OF OUR STOCKHOLDERS.
Our
Certificate of Incorporation authorizes the issuance of up to 10,000 shares of
"blank check" preferred stock, of which only slightly more than 1,000 have been
issued to date. Our Board of Directors has the power to determine all
designations, rights, preferences, privileges and restrictions of this preferred
stock. In addition, our Board of Directors is not required to obtain stockholder
approval to issue preferred stock with dividend, liquidation, conversion, voting
or other rights that could adversely affect the voting power or other rights of
the holders of our common stock. The Board of Directors could issue the
preferred stock in order to raise needed capital, or to discourage, delay or
prevent a change in control of our Company, even if a change of control would be
beneficial to our stockholders.
Our
research, development and production facilities are located in the Hawaii Ocean
Science and Technology (HOST) Business Park in Kailua-Kona, Hawaii. Our facility
currently consists of approximately five acres containing a number of MGMs,
finishing ponds, a processing facility, several laboratories, administrative
offices and additional space for production and research and development. All
our products are currently produced at this facility.
We
currently occupy this facility on a long term lease basis of 30 years with rents
increases renegotiated every five (5) years.
|
ITEM 3.
|
LEGAL
PROCEEDINGS.
|
The
Company is not currently a party to any pending legal proceedings, nor is any of
its property the subject of a pending legal proceeding.
|
ITEM 4.
|
SUBMISSION
OF MATTERS TO A VOTE OF SECURITY
HOLDERS.
|
No
matters were submitted to matters to a vote of security holders during the
fourth quarter of our 2009 fiscal year.
PART II
|
ITEM 5.
|
MARKET
FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER
PURCHASES OF EQUITY SECURITIES.
|
MARKET
FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Our
common stock is traded in the over-the-counter market on the NASD Pink Sheets.
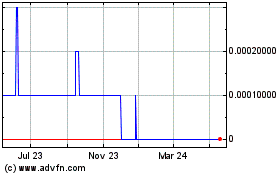
Symbol: "MRPI"). The following table shows the last sale price of the Company’s
stock sale as of October 31, 2009. as reported by financial reporting
services. This quotation is believed to represent inter-dealer quotations
without adjustment for retail mark-up, mark-down or commissions, and may not
represent actual transactions.
|
|
|
|
|
PERIOD
|
|
CLOSING
PRICE
|
|
FISCAL
2009
|
|
$ 0.005
|
As of
October 31, 2009, we had approximately 1,246 record holders of our
547,769,915 outstanding shares of common stock.
SECURITIES
AUTHORIZED FOR ISSUANCE UNDER EQUITY COMPENSATION PLANS
In
November 2004 the Board of Directors rescinded the previously adopted 2003
Stock Option Plan and adopted in its place the 2004 Stock Option Plan. That plan
authorizes the issuance of options to purchase up to 60,000,000 shares of the
Company's common stock. The Board of Directors granted options to purchase
approximately, 48,000,000 shares of stock, with vesting credit having been given
for past service to the company dating back to the effective date of our
reorganization. As of October 31, 2009, 12,430,000 of the options had
vested.
DIVIDEND
POLICY
We have
never paid cash dividends on our capital stock. We currently intend to retain
all available funds to operate and expand our business. We do not anticipate
paying any cash dividends in the foreseeable future.
RECENT
SALES OF UNREGISTERED SECURITIES
In
March 2005 the Company issued a total of 700,000 shares of common stock to
consultants in compensation for their services. These issuances were exempt from
registration under the Securities Act of 1933, as amended, pursuant to
Section 4(2).
In
March 2005 the Company issued 2,614,370 shares of its common stock to
Aquasearch Investment Partners, a general partnership, in exchange for
cancellation of a debt in the amount of $26,143.70 owed to it under a factoring
agreement previously entered into, or an effective price of $0.01 per share.
Gregory F. Kowal, the chairman of the Company's board of directors, is a general
partner of Aquasearch Investment Partners. This issuance was exempt from
registration under the Securities Act of 1933, as amended, pursuant to
Section 4(2).
In
March 2005 the Company issued 400,000 shares of stock to Anthony E.
Applebaum, the Company's principal accounting and financial officer, in exchange
for his forgiveness of the amount of $4,000 owed to him for services rendered,
or an effective per share price of $0.01. This issuance was exempt from
registration under the Securities Act of 1933, as amended, pursuant to
Section 4(2).
In
March 2005 the Company issued 87,298, 1,354,028 and 1,582,771 to Gregory F.
Kowal, Daniel P. Beharry and Kenneth Crowder, respectively, in exchange for
their forgiveness of debts in the amount of $872.98, $13,540.28 and $15,827.71
owed to them by the Company for expenses incurred by them on the Company's
behalf. The effective per share price of these issuances was $0.01 per share.
Messrs. Kowal, Beharry and Crowder were all members of Mera's board of
directors. These issuances were exempt from registration under the Securities
Act of 1933, as amended, pursuant to Section 4(2).
In
March 2005 the Company issued 10,000,000 shares of its common stock to an
investor in exchange for investment of $100,000, a per share price of $0.01per
share. This issuance was exempt from registration under the Securities Act of
1933, as amended, pursuant to Section 4(2).
In
March and April 2005 the Company issued 6,810,770 shares to a director
in exchange for total investment of $68,107.70, a per share price of $0.01. This
issuance was exempt from registration under the Securities Act of 1933, as
amended, pursuant to Section 4(2).
In
September and October 2005, the Company issued promissory notes to a
director of the Company in the amount of $10,000 in exchange for loans totaling
that amount made by the director to the Company for use as working
capital.
In
September and October 2005, the Company issued promissory notes to a
director of the Company in the amount of $25,400 in exchange for loans totaling
that amount made by the director to the Company for use as working
capital.
In
December 2005 the Company issued 11,538,462 shares of common stock to
investors in exchange for total investment of $150,000, a per share price of
$0.013. This issuance was exempt from registration under the securities act of
1933, as amended, pursuant to Section 4(2)
In
August 2008 the Company issued 5,000,000 shares of its common stock to an
investor in exchange for investment of $25,000, at a per share price of $0.005
per share. This issuance was exempt from registration under the Securities Act
of 1933, as amended pursuant to Section 4(2).
In
September 2008 the Company issued 30,000,000 shares of common stock to a
director of the Company in exchange for investment of $150,000 at a per share
price of $0.005 per share. This issuance was exempt from registration under the
Securities Act of 1933, as amended pursuant to Section 4(2).
In
October 2008, the Company issued 2,400,000 shares to a director in exchange
for his forgiveness of debt in the amount of a debt in the amount of $12,000.00
for past services rendered on the Company’s behalf. The effective price of the
share issuance was $0.005 per share. These issuances were exempt from
registration under the Securities Act of 1933, as amended, pursuant to
Section 4(2).
|
ITEM 7.
|
MANAGEMENT'S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATION
|
OVERVIEW
Since
inception, our primary operating activities have consisted of basic research and
development and production process development, recruiting personnel, purchasing
operating assets and raising capital. From September 16, 2002, the
effective date of our reorganization, through October 31, 2009, we had an
accumulated deficit of $8,111,859. Our losses to date have resulted
primarily from costs incurred in research and development and from general and
administrative expenses associated with operations. We expect to continue to
incur smaller operating losses for the 2010 fiscal year. We expect to have
quarter-to-quarter and year-to-year fluctuations in revenues, expenses and
losses, some of which could be significant.
We have a
limited operating history. An assessment of our prospects should include the
technology risks, market risks, expenses and other difficulties frequently
encountered by early-stage operating companies, and particularly companies
attempting to enter competitive industries with significant technology risks and
barriers to entry. We have attempted to address these risks by, among other
things, hiring and retaining highly qualified persons and expanding our product
offering while increasing our efforts to expand sales and improving our
production efficiencies and minimizing our overhead. However, our best efforts
cannot guarantee that we will overcome these risks in a timely manner, if at
all.
CRITICAL
ACCOUNTING POLICIES
This
discussion and analysis of the Company's financial condition and results of
operations is based upon our financial statements, which have been prepared in
accordance with accounting principles generally accepted in the United States.
The preparation of these financial statements requires the Company to make
estimates and judgments that affect the reported amounts of assets, liabilities,
revenue and expenses and related disclosure of contingent assets and
liabilities. On an on-going basis, the Company evaluates its estimates. The
Company bases its estimates on historical experience and on other assumptions
that it believes to be reasonable under the circumstances, the results of which
form the basis for making judgments about the carrying values of assets and
liabilities that are not readily apparent from other sources. Actual results may
differ from these estimates under different assumptions or
conditions.
CASH
AND CASH EQUIVALENTS
The
Company considers all highly liquid debt securities purchased with original or
remaining maturities of three months or less to be cash
equivalents.
INVENTORIES
Inventories
are stated at the lower of cost or market. The Company determines cost on a
first-in, first-out basis. On an ongoing basis, the company tests its inventory
for obsolescence.
REVENUE
RECOGNITION
Product
revenue is recognized upon shipment to customers. Contract services revenue is
recognized as services are performed on a cost reimbursement basis.
PLANT
AND EQUIPMENT
Plant and
equipment are stated at cost. Depreciation is provided on the straight-line
method over the estimated useful lives of the assets.
IMPAIRMENT
OF LONG-LIVED ASSETS AND LONG-LIVED ASSETS TO BE DISPOSED OF
In
August 2001, the Financial Accounting Standards Board ("FASB") issued
Statement of Financial Accounting Standards ("SFAS") No. 144 "Accounting
for the Impairment or Disposal of Long-Lived Assets" which supersedes both SFAS
No. 121, "Accounting for the Impairment of Long-Lived Assets and for
Long-Lived Assets to Be Disposed Of" and the accounting and reporting provisions
of Accounting
Practice
Bulletin ("APB") Opinion No. 30, "Reporting the Results of Operations -
Reporting the Effects of Disposal of a Segment of a Business, and Extraordinary,
Unusual and Infrequently Occurring Events and Transactions," for the disposal of
a segment of a business (as previously defined in that opinion).
This
statement establishes the accounting model for long-lived assets to be disposed
of by sale and applies to all long-lived assets, including discontinued
operations. This statement requires those long-lived assets be measured at the
lower of carrying amount or fair value less cost to sell, whether reported in
continuing operations or discontinued operations. Therefore, discontinued
operations will no longer be measured at net realizable value or include amounts
for operating losses that have not yet occurred. The Company adopted SFAS
No. 144 in the fiscal year ending October 31, 2002.
SFAS
No. 144 retains the fundamental provisions of SFAS No. 121 for
recognizing and measuring impairment losses on long-lived assets held for use
and long-lived assets to be disposed of by sale, while also resolving
significant implementation issues associated with SFAS No. 121. The Company
adopted SFAS No. 144 in its evaluation of the fair value of certain assets
in connection with the adoption of fresh-start accounting.
STOCK
ISSUED FOR SERVICES
The value
of stock issued for services is based on management's estimate of the fair value
of the Company's stock at the date of issue or the fair value of the services
received, whichever is more reliably measurable.
RESEARCH
AND DEVELOPMENT
Research
and Development costs are expensed as incurred.
INCOME
TAXES
The
Company uses the asset and liability method of accounting for income taxes as
required by SFAS No. 109 "Accounting for Income Taxes". SFAS No. 109
requires the recognition of deferred tax assets and liabilities for the expected
future tax consequences of temporary differences between the carrying amounts
and the tax basis of certain assets and liabilities. Since its inception, the
Company has incurred net operating losses. Accordingly, no provision has been
made for income taxes.
FRESH
START ACCOUNTING
On
September 16, 2002, the Company adopted "fresh start" accounting as a
result of the completion of bankruptcy proceedings. Accordingly, all assets and
liabilities were restated to reflect their respective fair values as of that
date.
GOODWILL
AND OTHER INTANGIBLE ASSETS
The
Company accounts for intangible assets in accordance with SFAS 142. Generally,
intangible assets with indefinite lives, and goodwill, are no longer amortized;
they are carried at lower of cost or market and subject to annual impairment
evaluation, or interim impairment evaluation if an interim triggering event
occurs, using a new fair market value method. Intangible assets with finite
lives are amortized over those lives, with no stipulated maximum, and an
impairment test is performed only when a triggering event occurs. Such assets
are amortized on a straight-line basis over the estimated useful life of the
asset. Intangible assets are reviewed for impairment whenever events or changes
in circumstances indicate that the carrying amount may not be recoverable. If
the fair value is less than the carrying amount of the asset, an impairment loss
is then recognized.
DISCLOSURES
ABOUT FAIR VALUE OF FINANCIAL INSTRUMENTS
The
carrying amounts of the Company's financial instruments, including cash,
accounts receivable, notes receivable, accounts payable and notes payable are
deemed to approximate fair value due to their short-term nature.
RESULTS
OF OPERATIONS
The
following discussion and analysis provides information that the Company's
management believes is relevant to an assessment and understanding of our
results of operations and financial condition. This discussion should be read in
conjunction with the financial statements and footnotes, which appear elsewhere
in this prospectus.
RESULTS
OF OPERATIONS FOR THE FISCAL YEAR ENDED OCTOBER 31, 2009 COMPARED TO THE FISCAL
YEAR ENDED OCTOBER 31, 2008
REVENUES.
During the years
ended October 31, 2009 and 2008, product and technical services revenue
totaled $599,091 and $609,984 respectively, resulting in a decrease of 1.8%.
During fiscal 2009, this revenue was generated principally through direct sales
and a technical service contract which expired during June 2009. A small portion
of sales were attributed to international distribution of our products and some
sales of raw materials.
EXPENSES.
Overall operating
expenses were $688,276 in 2009 compared with $642,294 in fiscal 2008, an
increase of 7.25%. This increase resulted principally from continued efforts to
expand research and development of potential new products which will expand
revenue in the future as new products are deemed acceptable for human
consumption
COST OF PRODUCTS SOLD.
Cost of
products sold include manufacturing and production costs associated with
ASTAFACTOR®), SALMON ESSENTIALS™®, KONA SEA SALT™® as well as the cost of sales
of raw materials and certain other products. Expenses decreased in the
categories of cost of products sold, due principally to better production
methods and inventory management. Cost of product sold in 2009 was
$28,021 versus $38,935 in 2008, a decline of 28%. It is expected that
the cost of products sold will increase during 2010 as we expand our sales going
forward.
RESEARCH AND DEVELOPMENT
COSTS.
Research and development costs include salaries, research supplies
and materials and other expenses related to product development, exclusive of
those costs for which the company is reimbursed. Research and development costs
for the year ended October 31, 2009 were $324,383 as compared to $299,696
for fiscal 2008. This represents an 8.3% increase due to a technical service
agreement that the company was engaged in during fiscal year
2009. This contract ended in June 2009 and additional research and
development costs associated with new product development.
GENERAL AND ADMINISTRATIVE
EXPENSES.
General and administrative expenses consist principally of
salaries, fees for professional services and promotional and marketing expenses
related to our various product lines. General and administrative expenses for
the fiscal year 2009 were $363,893 versus $342,598 in 2008, an increase of 6.3%.
This increase was due to the company's hiring of sales personnel and staff in a
continuing effort to increase sales for future growth.
INTEREST EXPENSE.
For the
years ended October 31, 2009 and 2008, interest expense was $5,033 and
$10,140 respectively. The amount of interest expense incurred in any particular
period varies with the amount of debt outstanding and the rate of interest
payable on that debt. The total amount of debt decreased in 2009 due to less
short term borrowing. It is expected that interest expenses will be comparable
in 2010 as the Company’s cash position becomes more stable.
PROVISION FOR EXCESS AND OBSOLETE
INVENTORY.
There were no changes to the provisions during the fiscal year
ended October 31, 2009.
LIQUIDITY
AND CAPITAL RESOURCES
We have
financed our operations until now through public and private sales of debt and
equity securities and debt instruments, together with revenues from product
sales, contract work and royalties. During the years ended October 31, 2009
and 2008, we did not raise any additional funding versus $175,000 of net
proceeds from the sale of shares of common stock and/or the issuance of debt in
private placement transactions.
|
ITEM 8.
|
FINANCIAL
STATEMENTS AND SUPPLEMENTARY DATA.
|
Audited
balance sheet as of October 31, 2009 and the related statements of
operations, cash flows and stockholders' equity (deficit) for the years ended
October 31, 2009 and 2008, together with related notes and the report of
Jewett, Schwartz & Associates, our independent auditors, appear on pages F-1
through F-17 of this Report.
|
ITEM 9.
|
CHANGES
IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL
DISCLOSURE
|
There
were no disagreements with accountants on accounting and financial disclosure
during fiscal 2009 or 2008.
|
ITEM 9A.
|
CONTROLS
AND PROCEDURES.
|
Items 307
and 308 of Regulation S-B.
Evaluation
of Disclosure Controls and Procedures. Our Chief Executive
Officer and Chief
Financial Officer have evaluated the effectiveness of our disclosure controls
and procedures (as such term is defined in Rules 13a-15 (e) and 15d-15(e) under
the Securities Exchange Act of 1934, as amended (the "Exchange Act")) as of the
end of the period covered by this report. Based on such evaluation, such
officers have concluded that, as of such date, our disclosure controls and
procedures are effective in alerting them on a timely basis to material
information relating to Mera Pharmaceuticals required to be included in our
reports filed or submitted under the Exchange Act.
Changes
in Internal Control over Financial Reporting. There were no significant changes
in our internal control over financial reporting that have materially affected
or are reasonably likely to materially affect, our internal control over
financial reporting.
|
ITEM 9B.
|
OTHER
INFORMATION.
|
None.
PART III
|
ITEM 10.
|
DIRECTORS,
EXECUTIVE OFFICERS AND CORPORATE
GOVERNANCE.
|
DIRECTORS
AND EXECUTIVE OFFICERS
The
following table sets forth certain information regarding our current directors
and executive officers.
|
NAME
|
|
AGE
|
|
POSITION
|
|
Gregory
F. Kowal
|
|
61
|
|
Chief
Executive Officer (1), and Director
|
|
Kenneth
I. Crowder
|
|
71
|
|
Director,
Chief Operating Officer(1)
|
|
Russell
M. Yamamoto
|
|
58
|
|
Director
(1)
|
|
Michael
F. Corcoran PH.D
|
|
60
|
|
Director
(1)
|
|
Melanie
K. Kelekolio
|
|
42
|
|
Corporate
Secretary(1) Vice President and Operations
Manager(1)
|
———————
|
1.
|
As
of January 3, 2006, Mr. Kowal assumed the role of the Company's
chief executive officer in addition to his role as chairman of the board
of directors.
|
GREGORY
F. KOWAL, CHIEF EXECUTIVE OFFICER AND DIRECTOR, has served as a director of our
Company since June 2002. He assumed the role of CEO on January 3,
2006. Mr. Kowal is co-founder of First Honolulu Securities, Inc. and has
been continuously associated with it since 1979. He currently is Chairman of the
Board and Portfolio Manager of First Honolulu Asset management. He
was also President of Hawaii Tsunami Pro Soccer Incorporated. Prior to founding
First Honolulu, he was associated with a large west coast regional brokerage
firm from 1973 until 1979. Mr. Kowal received his BSBA from Roosevelt
University (Chicago, IL) in 1972. His area of concentration included management
and finance.
KENNETH
I. CROWDER, CHIEF OPERATING OFFICER AND DIRECTOR, has served as a director of
the Company since September 16, 2003. Mr. Crowder is the founder and
Chief Executive officer of Concordia Finance, which participates in the
financing of big rig (Class 8) trucks. Prior to that, he spent more than
two decades as an engineer for Northrup Corporation, working on a number of
products. He began his engineering career with the U.S. Naval Ordnance Lab in
Corona, California. Mr. Crowder received a BA in physics from University of
California Riverside in 1960 and a Masters in physics from California State
University at Long Beach in 1966.
RUSSELL
M. YAMAMOTO, DIRECTOR, joined the Company’s Board of Directors in July, 2008.
Mr. Yamamoto is the President and CEO of RMY Construction, Inc. which he
founded in 1984. He has worked on various municipal projects which range from
athletic fields to major sewer and waterline rehabilitation projects statewide
in Hawaii. Mr. Yamamoto is also involved in numerous charitable
organizations which include Charities of Hawaii, American Heart Association,
Palama Settlement, American Diabetes Association, Cancer Research Center of
Hawaii, the University of Hawaii Foundation and HHSAA. He is a 1973 graduate
from the University of Hawaii with a Bachelor of Business Administration
degree.
MICHAEL
F. CORCORAN, PH.D. DIRECTOR joined the Company’s Board of Directors in
July 2008. Dr. Corcoran founded Gull Rock Services, which provides
consulting and fundraising services to non-profits in 1989 and national Data
Solutions, LLC, a mailing list management company in 2002. He also served as CEO
of a large non-profit conservation organization from 1984 through 1993. He
received a doctorate in zoology from Duke University in 1981 and also studied
oceanography at the University of Hawaii.
MELANIE
K.KELEKOLIO, CORPORATE SECRETARY, VICE PRESIDENT AND OPERATIONS MANAGER, joined
the company in May 1999 as a Research Associate and became the Team Leader
of Production in 2001. Ms. Kelekolio has served as Corporate Secretary and
Operations Manager since July, 2008. She has been instrumental in the
optimization of our cultivation and scale up processes used to produce the
Haematococcus algae and astaxanthin products, as well as Research and
Development projects now in the works. Ms. Kelekolio has over 17 years of
experience in the aquaculture field.
|
ITEM 11.
|
EXECUTIVE
COMPENSATION
|
SUMMARY
COMPENSATION
The
following table sets forth the information, on an accrual basis, with respect to
the compensation of our executive officers for the three fiscal years ended
October 31, 2009.
|
|
|
|
|
|
|
|
|
|
|
NAME
AND PRINCIPAL POSITION
|
|
YEAR
ENDED OCTOBER 31,
|
|
SALARY
($)
|
|
RESTRICTED
STOCK AWARD(S) ($)
|
|
Gregory
F. Kowal, CEO
|
|
2009
|
|
$
|
0
|
|
$
|
0
|
|
Gregory
F. Kowal, CEO
|
|
2008
|
|
$
|
0
|
|
$
|
0
|
|
Gregory
F. Kowal, CEO
|
|
2007
|
|
$
|
0
|
|
$
|
0
|
OPTION
GRANTS IN FISCAL 2009
No
options were granted to Executive officers in fiscal year 2008.
STOCK
OPTIONS EXERCISED DURING FISCAL 2008
No stock
options were exercised by the named executive officers of the Company during
fiscal 2009.
FISCAL
YEAR-END OPTION VALUES
The total
value of executive and officer’s unexercised vested options as of
October 31,
2009, was
$62,150.
LTIP
AWARDS DURING FISCAL YEAR
We did
not make any long-term incentive plan awards to any executive officers or
directors during the fiscal year ended October 31, 2009.
DIRECTOR
COMPENSATION
Our
directors do not receive compensation for services they provide as directors,
although they are reimbursed their expenses for attendance at board meetings and
those otherwise incurred in connection with their duties. We do not provide
additional compensation for committee participation or special assignments of
the Board of Directors.
Our
outside directors, Messrs. Kowal and Crowder, were each granted options on
December 2004 to purchase 5,000,000 shares of the Company's common stock in
respect of their service as board members. The exercise price of those options
was $0.01 per share. None of the options has been exercised.
EMPLOYMENT
CONTRACTS
We
currently do not have employment contracts with our named executive
officers.
|
ITEM 12.
|
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED
STOCKHOLDER MATTERS.
|
The
following table provides information known to us about the beneficial ownership
of our common stock as of January 6, 2010 for: (1) each person, entity or group
that is known by us to beneficially own five percent or more of our common
stock; (2) each of our directors (and former directors, as applicable); (3) each
of our named executive officers (and former officers, as applicable) as defined
in Item 402(a)(2) of Regulation S-B; and (4) our directors and executive
officers as a group. To the best of our knowledge, each stockholder identified
below has voting and investment power with respect to all shares of common stock
shown, unless community property laws or footnotes to this table are
applicable.
|
|
|
|
|
|
|
|
|
DIRECTORS
AND OFFICERS (1)
|
|
NATURE
OF
BENEFICIAL
OWNERSHIP
|
|
NUMBER
OF SHARES
BENEFICIALLY
OWNED
|
|
PERCENTAGE
OF SHARES
BENEFICIALLY
OWNED (1)
|
|
|
|
|
|
|
|
|
|
Kenneth
Crowder
|
|
|
|
|
|
|
|
c/o
Mera Pharmaceuticals, Inc.
|
|
|
|
|
|
|
|
73-4460
Queen Ka'ahumanu
Highway,
Suite 110
|
|
Direct
and Indirect
|
|
6,302,379(2)
|
|
1.15%
|
|
Kailua-Kona,
Hawaii 96740
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael
F. Corcoran PH.D.
|
|
|
|
|
|
|
|
c/o
Mera Pharmaceuticals, Inc.
|
|
|
|
|
|
|
|
73-4460
Queen Ka'ahumanu
Highway,
Suite 110
|
|
Direct
and Indirect
|
|
5,250,154(3)
|
|
.096%
|
|
Kailua-Kona,
HI 96740
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gregory
F. Kowal
|
|
|
|
|
|
|
|
First
Honolulu Securities
|
|
Direct
and Indirect
|
|
29,754,502(4)
|
|
5.431%
|
|
900
Fort Street Suite 950
|
|
|
|
|
|
|
|
Honolulu,
Hawaii 96813
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Russell
M. Yamamoto
|
|
Direct
and Indirect
|
|
63,298,370(5)
|
|
11.556%
|
|
c/o
Mera Pharamaceuticals,
|
|
|
|
|
|
|
|
Inc.
|
|
|
|
|
|
|
|
73-4460
Queen Ka’ahumanu
|
|
|
|
|
|
|
|
Highway,
Suite 110
|
|
|
|
|
|
|
|
Kailua-Kona,
HI 96740
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All
directors and executive
officers
(4)
|
|
Direct
and Indirect
|
|
104,605,405
|
|
19.10%
|
———————
|
1.
|
Applicable percentage
of beneficial ownership is based on shares outstanding as of
January 5, 2010. Beneficial ownership is determined in accordance
with rules and regulations of the Securities and Exchange Commission. In
computing the number of shares beneficially owned by a person and
the percentage ownership of that person, shares of common stock
subject to options or warrants held by that person that are currently
exercisable or exercisable within 60 days the date of this report are
deemed outstanding, but are not deemed outstanding for computing
the percentage of any other
person.
|
|
2.
|
This
amount includes 3,125,000 shares that Mr. Crowder has the right to
acquire within sixty days from the date of this report on Form 10-KSB
by exercising stock options.
|
|
3.
|
This
amount includes 2,400,000 shares held by Dr. Corcoran’s Company (directly
or indirectly), plus 73,000 shares that Dr. Corcoran has acquired in the
open market prior to becoming a
director.
|
|
4.
|
These
shares held indirectly are in the name of Aquasearch Investment Partners,
of which Mr. Kowal is a general partner. This figure also includes
warrants to acquire 1,660,000 shares of common stock which were issued to
Aquasearch Investment Partners and Gregory F. Kowal and 4,375,000 shares
that Mr. Kowal has the right to acquire within sixty days of this
report on Form 10-KSB by exercising stock
options.
|
|
5.
|
This
amount includes 7,692,308 shares of RMY Corporation of which
Mr. Yamamoto is the founder and principal shareholder. This figure
also includes 53,606,062 shares held in the name of his wife and other
immediate family members.
|
|
ITEM 13.
|
CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR
INDEPENDENCE.
|
In
March 2005 the Company issued 2,614,370 shares of its common stock to
Aquasearch Investment Partners, a general partnership, in exchange for
cancellation of a debt in the amount of $26,143.70 owed to it under a factoring
agreement previously entered into, or an effective price of $0.01 per share.
Gregory F. Kowal, the chairman of the Company's board of directors, is a general
partner of Aquasearch Investment Partners. This issuance was exempt from
registration under the Securities Act of 1933, as amended, pursuant to
Section 4(2).
In
March 2005 the Company issued 87,298, 1,354,028 and 1,582,771 to Gregory F.
Kowal, Daniel P. Beharry and Kenneth Crowder, respectively, in exchange for
their forgiveness of debts in the amount of $872.98, $13,540.28 and $15,827.71
owed to them by the Company for expenses incurred by them on the Company's
behalf. The effective per share price of these issuances was $0.01 per share.
Messrs. Kowal, Beharry and Crowder are all members of Mera's board of
directors. These issuances were exempt from registration under the Securities
Act of 1933, as amended, pursuant to Section 4(2).
In
March and April 2005 the Company issued 6,810,770 shares to a director
in exchange for total investment of $68,107.70, a per share price of $0.01. This
issuance was exempt from registration under the Securities Act of 1933, as
amended, pursuant to Section 4(2).
In
September and October 2005, the Company issued promissory notes to a
director of the Company in the amount of $10,000 in exchange for loans totaling
that amount made by the director to the Company for use as working
capital.
In
September and October 2005, the Company issued promissory notes to a
director of the Company in the amount of $25,400 in exchange for loans totaling
that amount made by the director to the Company for use as working
capital.
In
October 2008, the Company issued 2,400,000 shares of its common stock to
KMG marketing Group, a corporate entity founded by Dr. Corcoran in exchange for
cancellation of a debt in the amount of $12,000 owed for expenses for web site
development services at a effective price of $0.005 per share. These issuances
were exempt from registration under the Securities Act of 1933, as amended,
pursuant to Section 4(2).
|
ITEM 14.
|
PRINCIPAL
ACCOUNTANT FEES AND SERVICES.
|
The following is a summary of the fees billed to us by Jewett, Schwartz,
Wolfe & Associates ("JSWA") for professional services rendered for the
fiscal years ended October 31, 2009 and 2008:
|
Fee Category
|
|
Fiscal
200
9
|
|
|
Fiscal
2008
|
|
|
|
|
|
|
|
|
|
|
Audit
Fees
|
|
$
|
30,000
|
|
|
$
|
30,000
|
|
|
Audit
Related Fees
|
|
$
|
0
|
|
|
$
|
0
|
|
|
Tax
Fees
|
|
$
|
0
|
|
|
$
|
0
|
|
|
All
Other Fees
|
|
$
|
0
|
|
|
$
|
0
|
|
|
Total
Fees
|
|
$
|
30,000
|
|
|
$
|
30,000
|
|
|
|
|
|
|
|
|
|
|
|
Audit fees consisted of fees billed for professional services rendered or
the audit of the
Company's consolidated financial
statements included in our annual reports
on Form 10-K and Form 10-KSB for the
years ended October 31, 2009 and 2008, respectively, and or reviews of the
consolidated financial statements included in the Company's quarterly reports on
Form 10-Q and Form 10-QSB during fiscal 2009
and 2008, respectively.
PART IV
INDEX OF
EXHIBITS
|
Exhibit
List
|
|
Description
|
|
31.1
|
|
Certification
of the Chief Executive Officer pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002 (18 U.S.C. Section 350)
|
|
31.2
|
|
Certification
of the Principal Accounting Officer pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002 (18 U.S.C. Section 350)
|
|
32.1
32.2
|
|
Certification
of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350 (as
adopted pursuant to Section 906 of the Sarbanes-Oxley Act of
2002)
Certification
of the Principal Accounting Officer pursuant to 18 U.S.C. Section
1350 (as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of
2002)
|
SIGNATURES
In
accordance with the requirements of the Securities Act, the registrant certifies
that it has reasonable grounds to believe that it meets all the requirements of
filing on Form 10-KSB/B and authorized this Form 10-KSB/B to be signed
on its behalf by the undersigned, in the City of Honolulu, State of Hawaii, on
January 27, 2010.
|
|
MERA
PHARMACEUTICALS, INC.
|
|
|
|
|
|
|
|
|
By:
|
/s/
G
regory
F.
K
owal
|
|
|
|
|
Gregory
F. Kowal
|
|
|
|
|
Chief
Executive Officer
|
|
In
accordance with the requirements of the Securities Act, this Registration
Statement on Form 10-KSB was signed by the following persons in the
capacities and on the dates stated:
|
|
|
|
|
|
|
SIGNATURE
|
|
TITLE
|
|
DATE
|
|
|
|
|
|
|
|
/s/
G
regory
F.
K
owal
|
|
Chief
Executive Officer, and Director
|
|
January
27, 2010
|
|
Gregory
F. Kowal
|
|
|
|
|
|
|
|
|
|
|
|
/s/
A
nthony
A
pplebaum
|
|
Principal
Financial and Accounting Officer
|
|
January
27, 2010
|
|
Anthony
Applebaum
|
|
|
|
|
|
|
|
|
|
|
|
/s/
K
enneth
I.
C
rowder
|
|
Director
|
|
January 27,
2010
|
|
Kenneth
I. Crowder
|
|
|
|
|
|
|
|
|
|
|
|
/s/
M
elanie
K
elekolio
|
|
Secretary
|
|
January 27,
2010
|
|
Melanie
Kelekolio
|
|
|
|
|
FINANCIAL STATEMENTS
MERA
PHARMACEUTICALS, INC.
TABLE
OF CONTENTS
|
|
|
|
F – 2
|
|
|
|
|
|
|
|
|
|
|
|
F – 3
|
|
|
|
|
|
|
|
|
|
|
|
F – 4
|
|
|
|
|
|
|
|
|
|
|
|
F – 5
|
|
|
|
|
|
|
|
|
|
|
|
F – 6
|
|
|
|
|
|
|
|
|
|
|
|
F –
7
|
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
To the
Board of Directors and Shareholders of
Mera
Pharmaceuticals, Inc.
We have
audited the accompanying balance sheets of Mera Pharmaceuticals, Inc. as of
October 31, 2009 and 2008 and the related statements of operations, changes in
shareholders' equity`, and cash flows for the years ended October 31, 2009 and
2008. These financial statements are the responsibility of the
Company's management. Our responsibility is to express an opinion on
these financial statements based on our audits.
We
conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require
that we plan and perform the audit to obtain reasonable assurance about whether
the financial statements are free of material misstatement. An audit includes
examining, on a test basis, evidence supporting the amounts and disclosures in
the financial statements. An audit also includes assessing the
accounting principles used and significant estimates made by management, as well
as evaluating the overall financial statement presentation. We
believe that our audit provides a reasonable basis for our opinion.
In our
opinion, the financial statements referred to above present fairly, in all
material respects, the financial position of Mera Pharmaceuticals, Inc. as of
October 31, 2009 and 2008 and the results of its operations and its cash flows
for the years then ended 2009 and 2008 in conformity with accounting principles
generally
accepted in the United States of America.
These
accompanying financial statements referred to above have been prepared assuming
that the Company will continue as a going concern. As more fully described in
Note 2, the Company’s need to seek new sources or methods of financing or
revenue to pursue its business strategy, raise substantial doubt about the
Company’s ability to continue as a going concern. Management’s plans
as to these matters are also described in Note 2. The financial
statements do not include any adjustments that might result from the outcome of
this uncertainty.
JEWETT,
SCHWARTZ, WOLFE & ASSOCIATES
Hollywood,
Florida
January
26, 2010
MERA
PHARMACEUTICALS, INC.
BALANCE SHEET
S
|
|
|
October
31,
2009
|
|
|
October
31,
2008
|
|
|
ASSETS
|
|
|
|
|
|
|
|
Current
assets:
|
|
|
|
|
|
|
|
Cash
and cash equivalents
|
|
$
|
3,135
|
|
|
$
|
19,288
|
|
|
Marketable
equitable securities
|
|
|
—
|
|
|
|
34,400
|
|
|
Accounts
receivable
|
|
|
6,656
|
|
|
|
11,007
|
|
|
Tax
credit receivable
|
|
|
31,229
|
|
|
|
31,713
|
|
|
Other
current assets
|
|
|
15,994
|
|
|
|
16,867
|
|
|
Total
current assets
|
|
|
57,014
|
|
|
|
113,275
|
|
|
|
|
|
|
|
|
|
|
|
|
Plant
and equipment, net
|
|
|
200,482
|
|
|
|
281,439
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
Assets
|
|
$
|
257,496
|
|
|
$
|
394,714
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES
AND STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
|
|
Current
liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts
payable and accrued expenses
|
|
$
|
314,212
|
|
|
|
283,590
|
|
|
Notes
payable - related parties
|
|
|
51,936
|
|
|
|
51,936
|
|
|
Deferred
revenue
|
|
|
30,450
|
|
|
|
—
|
|
|
Other
cUrrent liabilities
|
|
|
—
|
|
|
|
5,412
|
|
|
Total
current liabilities
|
|
|
396,598
|
|
|
|
340,938
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments
and contingencies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’
equity (deficit):
|
|
|
|
|
|
|
|
|
|
Convertible
preferred stock, $.0001 par value, 10,000 shares authorized, 80 Series A
shares issued and outstanding and 974 Series B shares issued and
outstanding
|
|
|
2
|
|
|
|
2
|
|
|
Common
stock, $.0001 par value: 750,000,000 shares authorized, 547,769,915 shares
issued and outstanding
|
|
|
54,777
|
|
|
|
54,777
|
|
|
Additional
paid-in capital
|
|
|
7,920,003
|
|
|
|
7,920,003
|
|
|
Treasury
stock at cost
|
|
|
(2,025
|
)
|
|
|
(2,025
|
)
|
|
Accumulated
deficit
|
|
|
(8,111,859
|
)
|
|
|
(7,918,981
|
)
|
|
Total
stockholders’ equity (deficit)
|
|
|
(139,102
|
)
|
|
|
53,776
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
Liabilities and Stockholders’ Equity (Deficit)
|
|
$
|
257,496
|
|
|
$
|
394,714
|
|
See the
accompanying notes to the financial statements
MERA
PHARMACEUTICALS, INC.
STATEMENTS OF OPERATIONS
|
|
|
Twelve
Months Ended
October 31,
2009
|
|
|
Twelve
Months Ended
October 31,
2008
|
|
|
NET
SALES
|
|
$
|
599,091
|
|
|
$
|
609,984
|
|
|
Cost
of goods sold
|
|
|
28,021
|
|
|
|
38,935
|
|
|
|
|
|
|
|
|
|
|
|
|
GROSS
PROFIT
|
|
|
571,070
|
|
|
|
571,049
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating
Expenses
|
|
|
|
|
|
|
|
|
|
Selling
and administrative expenses
|
|
|
363,893
|
|
|
|
342,597
|
|
|
Research
and development costs
|
|
|
324,383
|
|
|
|
301,566
|
|
|
Depreciation
and amortization
|
|
|
84,646
|
|
|
|
220,912
|
|
|
Impairment
loss
|
|
|
20,413
|
|
|
|
1,684,737
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
operating expenses
|
|
|
793,335
|
|
|
|
2,549,812
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating
loss
|
|
|
(222,265
|
)
|
|
|
(1,978,763
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Other
income (expense)
|
|
|
|
|
|
|
|
|
|
Interest
income
|
|
|
2,445
|
|
|
|
607
|
|
|
Realized
gain
|
|
|
746
|
|
|
|
214
|
|
|
Other
income
|
|
|
—
|
|
|
|
11,013
|
|
|
Interest
expense
|
|
|
(5,033
|
)
|
|
|
(10,140
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total
other income (expense)
|
|
|
(1,842
|
)
|
|
|
1,694
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss before tax provision
|
|
|
(224,107
|
)
|
|
|
(1,977,069
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Refundable
tax credit
|
|
|
31,229
|
|
|
|
31,713
|
|
|
|
|
|
|
|
|
|
|
|
|
NET
LOSS
|
|
$
|
(192,878
|
)
|
|
$
|
(1,945,356
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss per common share
|
|
$
|
(0.0004
|
)
|
|
$
|
(0.0038
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
average shares outstanding
|
|
|
547,769,915
|
|
|
|
516,830,024
|
|
MERA
PHARMACEUTICALS, INC.
STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
Treasury
|
|
|
|
Stockholders'
|
|
|
|
|
Convertible
Preferred
|
|
Common
|
|
Paid
- In
|
|
Stock At
|
|
Accumulated
|
|
Equity
|
|
|
|
|
Series
A Shares
|
|
Series
B Shares
|
|
Amount
|
|
Shares
|
|
Amount
|
|
Capital
|
|
Cost
|
|
(Deficit)
|
|
(Deficit
)
|
|
|
Balance
at October 31, 2007
|
|
|
80
|
|
|
974
|
|
$
|
2
|
|
|
510,369,915
|
|
$
|
51,037
|
|
$
|
7,736,743
|
|
$
|
—
|
|
$
|
(5,973,625
|
)
|
$
|
1,814,157
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance
of common shares at $0.005 per share
|
|
|
|
|
|
|
|
|
|
|
|
5,000,000
|
|
|
500
|
|
|
24,500
|
|
|
|
|
|
|
|
|
25,000
|
|
|
Issuance
of common shares at $0.005 per share
|
|
|
|
|
|
|
|
|
|
|
|
30,000,000
|
|
|
3,000
|
|
|
147,000
|
|
|
|
|
|
|
|
|
150,000
|
|
|
Issuance
of common shares at $0.005 per share
|
|
|
|
|
|
|
|
|
|
|
|
2,400,000
|
|
|
240
|
|
|
11,760
|
|
|
|
|
|
|
|
|
12,000
|
|
|
Acquisition
of treasury shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(2,025
|
)
|
|
|
|
|
(2,025
|
)
|
|
Loss
for the year ended October 31, 2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1,945,356
|
)
|
|
(1,945,356
|
)
|
|
Balance
at October 31, 2008
|
|
|
80
|
|
|
974
|
|
$
|
2
|
|
|
547,769,915
|
|
$
|
54,777
|
|
$
|
7,920,003
|
|
$
|
(2,025
|
)
|
$
|
(7,918,981
|
)
|
$
|
53,776
|
|
|
Loss
for the year ended October 31, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(192,878
|
)
|
|
(192,878
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance
at October 31, 2008
|
|
|
80
|
|
|
974
|
|
$
|
2
|
|
|
547,769,915
|
|
$
|
54,777
|
|
$
|
7,920,003
|
|
$
|
(2,025
|
)
|
$
|
(8,111,859
|
)
|
$
|
(139,102
|
)
|
See the
accomanying notes to the financial statements
MERA
PHARMACEUTICALS, INC.
STATEMENTS OF CASH FLOWS
|
|
|
|
|
|
|
|
|
|
|
Twelve
Months Ended
October 31,
2009
|
|
|
Twelve
Months Ended
October 31,
2008
|
|
|
Cash
Flows from Operating Activities:
|
|
|
|
|
|
|
|
Net
loss
|
|
$
|
(192,878
|
)
|
|
$
|
(1,945,356
|
)
|
|
Adjustments
to reconcile net loss to net cash used in operating
activities:
|
|
|
|
|
|
|
|
|
|
Accumulated
depreciation and amortization
|
|
|
84,646
|
|
|
|
220,912
|
|
|
Common
stock issued in satisfaction of accounts payable
|
|
|
—
|
|
|
|
12,000
|
|
|
Impairment
of fixed assets
|
|
|
20,413
|
|
|
|
1,684,737
|
|
|
Changes
in operating Assets and Liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts
receivable
|
|
|
4,351
|
|
|
|
(2,533
|
)
|
|
Other
current assets
|
|
|
1,357
|
|
|
|
(30,761
|
)
|
|
Accounts
payable and accured expenses
|
|
|
25,210
|
|
|
|
(57,590
|
)
|
|
Deferred
revenue and other current liabilities
|
|
|
30,450
|
|
|
|
—
|
|
|
Net
cash used by operating activities
|
|
|
(26,451
|
)
|
|
|
(118,591
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash
Flows from Investing Activities:
|
|
|
|
|
|
|
|
|
|
Purchases
of fixed assets
|
|
|
(24,102
|
)
|
|
|
—
|
|
|
Marketable
security
|
|
|
34,400
|
|
|
|
(34,400
|
)
|
|
Net
cash used by investing activities
|
|
|
10,298
|
|
|
|
(34,400
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash
Flows from Financing Activities
|
|
|
|
|
|
|
|
|
|
Proceeds
from issuance of common stock
|
|
|
—
|
|
|
|
175,000
|
|
|
Proceeds
from notes payable
|
|
|
—
|
|
|
|
162,910
|
|
|
Payment
of notes payable
|
|
|
—
|
|
|
|
(176,310
|
)
|
|
Purchase
of treasury stock
|
|
|
—
|
|
|
|
(2,025
|
)
|
|
Net
cash provided by financing activities
|
|
|
—
|
|
|
|
159,575
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
increase (decrease) in cash and cash equivalents
|
|
|
(16,153
|
)
|
|
|
6,584
|
|
|
Cash
and cash equivalents, beginning of the period
|
|
|
19,288
|
|
|
|
12,704
|
|
|
Cash
and cash equivalents, end of the period
|
|
$
|
3,135
|
|
|
$
|
19,288
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
paid for taxes
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Cash
paid for interest
|
|
$
|
—
|
|
|
$
|
6,028
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental
cash flow information
|
|
|
|
|
|
|
|
|
|
Non
cash financing activities
|
|
|
|
|
|
|
|
|
|
Common
stock issued in satisfaction of accounts payable
|
|
$
|
—
|
|
|
$
|
12,000
|
|
See the
accompanying notes to the financial statements
MERA
PHARMACEUTICALS, INC.
NOTES TO THE FINANCIAL STATEMENTS
1.
SUMMARY OF ORGANIZATION AND SIGNIFICANT ACCOUNTING POLICIES
Organization
- Mera
Pharmaceuticals, Inc. (the “Company” or “Mera”), primarily develops and
commercializes natural products from microalgae using its proprietary,
large-scale photobioreactor technology. The Company's operations are located in
Kailua-Kona, Hawaii.
Microalgae
are a diverse group of microscopic plants comprising an estimated 30,000 species
that display a wide range of physiological and biochemical characteristics. Many
of these organisms are known to contain valuable substances that have identified
and potential commercial applications in such fields as animal and human
nutrition, food colorings, cosmetics, diagnostic products, pharmaceuticals,
research grade chemicals, pigments and dyes. Microalgae grow ten times faster
than the fastest growing land-based crops and represent a largely unexploited
and renewable natural resource with a biodiversity comparable to that of
land-based plants.
The
Company has devoted most of its efforts since inception to research and
development, and was considered a development stage company until fiscal
2001. Since 2001 the Company has with various degrees of success
attempted to enter the retail market for its line of nutraceutical
products. 2009 proved no exception with mixed results due to the lack
of available marketing funds and staffing
Cash and Cash
Equivalents
- The Company considers all highly liquid debt securities
purchased with original or remaining maturities of three months or less to be
cash equivalents. The carrying value of cash equivalents approximates fair
value.
Fair Value of Financial
Instruments
- The carrying amounts of cash and cash equivalents, accounts
receivable, accounts payable and accrued expenses approximate fair market value
because of the short maturity of those instruments. Notes payable
approximate fair value.
Accounts Receivable
-
The Company performs ongoing credit evaluations of customers, and generally does
not require collateral. Allowances are maintained for potential credit losses
and returns and such losses have been within management’s
expectations.
Credit Risk
- It is
the Company’s practice to place its cash equivalents in either high quality
money market securities or to invest in short term corporate
bonds. Certain amounts of such funds might not be insured by the
Federal Deposit Insurance Corporation.
However, the Company
considers its credit risk associated with cash and cash equivalents to be
minimal.
Inventories
-
Inventories are stated at the lower of cost (which approximates first-in,
first-out) or market. At October 31, 2009, inventories consisted of
$742,736 of work in process, $133,767 of finished goods and $19,338 of raw
materials. Management has recorded a full valuation allowance for
obsolete and excess inventory totaling $895,841.
Revenue Recognition
-
Product revenue is recognized upon shipment to customers. Contract services
revenue is recognized as services are performed on a cost reimbursement basis.
Royalties are recognized upon receipt. The Company has adopted Securities and
Exchange Commission’s (“SEC”) Staff Accounting Bulletin (“SAB”) No. 104, which
provides guidance on the recognition, presentation and disclosure of revenue in
financial statements.
Plant and Equipment,
net
- Plant and equipment are stated at cost less accumulated
depreciation. Depreciation is recorded principally using the straight-line
method, based on the estimated useful lives of the assets (property and plant,
10-40 years; machinery and equipment, 3-10 years). When applicable, leasehold
improvements and capital leases are amortized over the lives of respective
leases, or the service lives of the improvements, whichever is
less.
MERA
PHARMACEUTICALS, INC.
NOTES
TO THE FINANCIAL STATEMENTS
Expenditures
for renewals and improvements that significantly extend the useful life of an
asset are capitalized. The costs of software with an expected life of
more than one year, and used in the business operations are capitalized and
amortized over their expected useful lives. Expenditures for
maintenance and repairs are charged to operations when incurred. When
assets are sold or retired, the cost of the asset and the related accumulated
depreciation are removed from the accounts and any gain or loss is recognized at
such time.
Impairment of Long Lived
Assets and Long Lived Assets to be Disposed Of
- Statement of Financial
Accounting Standards (“SFAS”) No. 144 “Accounting for the Impairment or Disposal
of Long-Lived Assets” establishes the accounting model for long-lived assets to
be disposed of by sale and applies to all long-lived assets, including
discontinued operations. This statement requires those long-lived assets be
measured at the lower of carrying amount or fair value less cost to sell,
whether reported in continuing operations or discontinued operations. Therefore,
discontinued operations will no longer be measured at net realizable value or
include amounts for operating losses that have not yet occurred.
Intangible Assets
-
The Company accounts for intangible assets in accordance with SFAS 142.
Generally, intangible assets with indefinite lives, and goodwill, are no longer
amortized; they are carried at lower of cost or market and subject to annual
impairment evaluation, or interim impairment evaluation if an interim triggering
event occurs, using a new fair market value method. Intangible assets
with finite lives are amortized over those lives, with no stipulated maximum,
and an impairment test is performed only when a triggering event
occurs. Such assets are amortized on a straight-line basis over the
estimated useful life of the asset. Intangible assets are reviewed for
impairment whenever events or changes in circumstances indicate that the
carrying amount may not be recoverable. If the fair value is less than the
carrying amount of the asset, an impairment loss is then
recognized.
Stock Issued For
Services
- The value of stock issued for services is based on
management's estimate of the fair value of the Company's stock at the date of
issue or the fair value of the services received, whichever is more reliably
measurable.
Preferred Stock
- The
Company has authorized 10,000 shares of "blank check" preferred stock, with such
designations, rights, preferences, privileges and restrictions to be determined
by the Company's Board of Directors. As of October 31, 2009, 1,054 shares of
preferred stock were issued and outstanding.
Research and Development
Costs
- Generally accepted accounting principles state that costs that
provide no discernible future benefits, or allocating costs on the basis of
association with revenues or among several accounting periods that serve no
useful purpose, should be charged to expense in the period
occurred. SFAS No. 2 “Accounting for Research and Development Costs”
requires that certain costs be charged to current operations including, but not
limited to: salaries and benefits; contract labor; consulting and
professional fees; depreciation; repairs and maintenance on operational assets
used in the production of prototypes; testing and modifying product and service
capabilities and design; and, other similar costs.
Income Taxes
- The
Company uses the asset and liability method of accounting for income taxes as
required by SFAS No. 109 “Accounting for Income Taxes”. SFAS No. 109 requires
the recognition of deferred tax assets and liabilities for the expected future
tax consequences of temporary differences between the carrying amounts and the
tax basis of certain assets and liabilities. Since its inception, the Company
has incurred net operating losses. Accordingly, no provision has been made for
income taxes. Statutory taxes not based on income are included in general and
administrative expenses.
MERA
PHARMACEUTICALS, INC.
NOTES
TO THE FINANCIAL STATEMENTS
Loss Per Share
- The
Company computed basic and diluted loss per share amounts for October 31, 2009
and 2008 pursuant to the SFAS No. 128, “Earnings per Share.” The
assumed effects of the exercise of outstanding stock options, warrants, and
conversion of notes were anti-dilutive and, accordingly, dilutive per share
amounts have not been presented in the accompanying statements of
operations.
Use of Estimates
-
The preparation of financial statements in conformity with generally accepted
accounting principles requires management to make estimates and assumptions that
affect the reported amounts of assets and liabilities, and disclosure of
contingent assets and liabilities, at the date of these financial statements,
and the reported amounts of revenues and expenses during the reporting period.
Actual results could differ from those estimates.
Recent Authoritative
Pronouncements
Determining
Whether Instruments Granted in Share-Based Payment Transactions Are
Participating Securities
In
June 2008, the FASB issued FSP Emerging Issues Task Force (“EITF”) Issue
No. 03-6-1, “Determining Whether Instruments Granted in Share-Based Payment
Transactions Are Participating Securities.” The FSP addresses whether
instruments granted in share-based payment transactions are participating
securities prior to vesting and, therefore, need to be included in the earnings
allocation in computing earnings per share under the two-class method. The FSP
affects entities that accrue dividends on share-based payment awards during the
awards’ service period when the dividends do not need to be returned if the
employees forfeit the award. This FSP is effective for fiscal years beginning
after December 15, 2008. The Company is currently assessing the impact of
FSP EITF 03-6-1 on its financial position and results of
operations.
Determining
Whether an Instrument (or an Embedded Feature) Is Indexed to an entity's Own
Stock
In June
2008, the FASB ratified EITF Issue No. 07-5, "Determining Whether an Instrument
(or an Embedded Feature) Is Indexed to an Entity's Own Stock" (EITF
07-5). EITF 07-5 provides that an entity should use a two step
approach to evaluate whether an equity-linked financial instrument (or embedded
feature) is indexed to its own stock, including evaluating the instrument's
contingent exercise and settlement provisions. It also clarifies on
the impact of foreign currency denominated strike prices and market-based
employee stock option valuation instruments on the evaluation. EITF
07-5 is effective for fiscal years beginning after December 15,
2008. The Company is currently assessing the impact of EITF 07-5 on
its financial position and results of operations.
Accounting
for Convertible Debt Instruments That May Be Settled in Cash upon Conversion
(Including Partial Cash Settlement)
In
May 2008, the FASB issued FSP Accounting Principles Board (“APB”) Opinion
No. 14-1, “Accounting for Convertible Debt Instruments That May Be
Settled in Cash upon Conversion (Including Partial Cash Settlement).” The FSP
clarifies the accounting for convertible debt instruments that may be settled in
cash (including partial cash settlement) upon conversion. The FSP
requires issuers to account separately for the liability and equity components
of certain convertible debt instruments in a manner that reflects the issuer's
nonconvertible debt (unsecured debt) borrowing rate when interest cost is
recognized. The FSP requires bifurcation of a component of the debt,
classification of that component in equity and the accretion of the resulting
discount on the debt to be recognized as part of interest expense in our
condensed statement of operations. The FSP requires retrospective
application to the terms of instruments as they existed for all periods
presented. The FSP is effective for fiscal years beginning after
December 15, 2008 and early adoption is not permitted. The Company is
currently evaluating the potential impact of FSP APB 14-1 upon its financial
statements.
MERA
PHARMACEUTICALS, INC.
NOTES
TO THE FINANCIAL STATEMENTS
The
Hierarchy of Generally Accepted Accounting Principles
In May
2008, the FASB issued SFAS No. 162, "The Hierarchy of Generally Accepted
Accounting Principles" (SFAS No. 162). SFAS No. 162 identifies the
sources of accounting principles and the framework for selecting the principles
used in the preparation of financial statements. SFAS No. 162 is
effective 60 days following the SEC's approval of the Public Company Accounting
Oversight Board amendments to AU Section 411, "The Meaning of Present Fairly in
Conformity with Generally Accepted Accounting Principles". The
implementation of this standard will not have a material impact on the Company's
financial position and results of operations.
Determination
of the Useful Life of Intangible Assets
In April
2008, the Financial Accounting Standards Board (“FASB”) issued FASB Staff
Position on Financial Accounting Standard (“FSP FAS”) No. 142-3, “Determination
of the Useful Life of Intangible Assets”, which amends the factors that should
be considered in developing renewal or extension assumptions used to determine
the useful life of intangible assets under SFAS No. 142 “Goodwill and Other
Intangible Assets”. The intent of this FSP is to improve the consistency
between the useful life of a recognized intangible asset under SFAS No. 142 and
the period of the expected cash flows used to measure the fair value of the
asset under SFAS No. 141 (revised 2007) “Business Combinations” and other U.S.
generally accepted accounting principles. The Company is
currently evaluating the potential impact of FSP FAS No. 142-3 on its financial
statements.
Disclosure
about Derivative Instruments and Hedging Activities
In March
2008, the FASB issued SFAS No. 161, “Disclosure about Derivative Instruments and
Hedging Activities, an amendment of SFAS No. 133”, (SFAS No. 161). This
statement requires that objectives for using derivative instruments be disclosed
in terms of underlying risk and accounting designation. The Company is required
to adopt SFAS No. 161 on January 1, 2009. The Company is currently evaluating
the potential impact of SFAS No. 161 on the Company’s financial
statements.
Delay
in Effective Date
In
February 2008, the FASB issued FSP FAS No. 157-2, “Effective Date of FASB
Statement No. 157”. This FSP delays the effective date of SFAS No. 157 for
all nonfinancial assets and nonfinancial liabilities, except those that are
recognized or disclosed at fair value on a recurring basis (at least annually)
to fiscal years beginning after November 15, 2008, and interim periods
within those fiscal years. The impact of adoption was not material to the
Company’s financial condition or results of operations.
Business
Combinations
In
December 2007, the FASB issued SFAS No. 141(R) “Business Combinations” (SFAS
141(R)). This Statement replaces the original SFAS No.
141. This Statement retains the fundamental requirements in SFAS
No. 141 that the acquisition method of accounting (which SFAS No. 141
called the purchase method) be used for all business combinations and for an
acquirer to be identified for each business combination. The objective of SFAS
No. 141(R) is to improve the relevance, and comparability of the information
that a reporting entity provides in its financial reports about a business
combination and its effects. To accomplish that, SFAS No. 141(R) establishes
principles and requirements for how the acquirer:
MERA
PHARMACEUTICALS, INC.
NOTES
TO THE FINANCIAL STATEMENTS
a.
Recognizes
and measures in its financial statements the identifiable assets acquired, the
liabilities assumed, and any noncontrolling interest in the
acquiree.
b.
Recognizes
and measures the goodwill acquired in the business combination or a gain from a
bargain purchase.
c.
Determines
what information to disclose to enable users of the financial statements to
evaluate the nature and financial effects of the business
combination.
This
Statement applies prospectively to business combinations for which the
acquisition date is on or after the beginning of the first annual reporting
period beginning on or after December 15, 2008 and may not be applied before
that date. The Company is unable at this time to determine the effect that its
adoption of SFAS No. 141(R) will have on its results of operations and financial
condition.
Noncontrolling
Interests in Consolidated Financial Statements—an amendment of ARB No.
51
In
December 2007, the FASB issued SFAS No. 160 “Noncontrolling Interests in
Consolidated Financial Statements – an amendment of ARB No. 51” (SFAS No.
160). This Statement amends the original Accounting Review Board
(ARB) No. 51 “Consolidated Financial Statements” to establish accounting and
reporting standards for the noncontrolling interest in a subsidiary and for the
deconsolidation of a subsidiary. It clarifies that a noncontrolling interest in
a subsidiary is an ownership interest in the consolidated entity that should be
reported as equity in the consolidated financial statements. This Statement is
effective for fiscal years and interim periods within those fiscal years,
beginning on or after December 15, 2008 and may not be applied before that
date. The Company is unable at this time to determine the effect that
its adoption of SFAS No. 160 will have on its results of operations and
financial condition.
Fair
Value Option for Financial Assets and Financial Liabilities
In
February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for
Financial Assets and Financial Liabilities – Including an amendment of SFAS No.
115” (SFAS No. 159), which became effective for the Company on February 1, 2008,
permits companies to choose to measure many financial instruments and certain
other items at fair value and report unrealized gains and losses in earnings.
Such accounting is optional and is generally to be applied instrument by
instrument. The election, of this fair-value option did not have a material
effect on its financial condition, results of operations, cash flows or
disclosures.
Fair
Value Measurements
In
September 2006, the FASB issued SFAS No. 157, "Fair Value Measurements" (SFAS
No. 157). SFAS No. 157 provides guidance for using fair value to measure assets
and liabilities. SFAS No. 157 addresses the requests from investors for expanded
disclosure about the extent to which companies’ measure assets and liabilities
at fair value, the information used to measure fair value and the effect of fair
value measurements on earnings. SFAS No. 157 applies whenever other standards
require (or permit) assets or liabilities to be measured at fair value, and does
not expand the use of fair value in any new circumstances. SFAS No. 157 is
effective for financial statements issued for fiscal years beginning after
November 15, 2007 and was adopted by the Company in the first quarter of fiscal
year 2008. The adoption of SFAS No. 157 did not have a material effect on its
results of operations and financial condition.
MERA
PHARMACEUTICALS, INC.
NOTES
TO THE FINANCIAL STATEMENTS
Accounting
Changes and Error Corrections
In May
2005, the FASB issued SFAS No. 154, "Accounting Changes and Error Corrections"
(SFAS No. 154), which replaces Accounting Principles Board (APB) Opinion No. 20,
"Accounting Changes," and SFAS No. 3, "Reporting Accounting Changes in Interim
Financial Statements - An Amendment of APB Opinion No. 28”. SFAS No. 154
provides guidance on the accounting for and reporting of accounting changes and
error corrections, and it establishes retrospective application, or the latest
practicable date, as the required method for reporting a change in accounting
principle and the reporting of a correction of an error. SFAS No. 154 is
effective for accounting changes and corrections of errors made in fiscal years
beginning after December 15, 2005. The Company adopted SFAS No. 154 in the first
quarter of fiscal year 2007 and it did not have a material impact on its results
of operations and financial condition.
2.
GOING CONCERN
These
financial statements have been prepared assuming that the Company will continue
as a going concern. The Company has operating and liquidity concerns,
has incurred an accumulated deficit of approximately $8.1 million through the
year ended October 31, 2009. The Company anticipates that future
revenue will be sufficient to cover certain operating expenditures, and, in the
interim, will continue to pursue additional capital investment. However, there
can be no assurance that the Company will be able to successfully acquire the
necessary capital to continue their on-going development efforts and bring
products to the commercial market. These factors, among others, create an
uncertainty about the Company’s ability to continue as a going
concern.
3.
RELATED PARTY TRANSACTIONS
In
December 2003 the Board agreed to pay one of its members a commission of 4% of
sales made to a Hawaiian distributor. The commission amount to be paid is based
on sales consummated retroactive to May 2003 and approximately $11,600 was
payable under this agreement as of October 31, 2009.
4.
INCOME TAXES
The
Company provides for income taxes in accordance with SFAS No. 109 using an asset
and liability based approach. Deferred income tax assets and liabilities are
recorded to reflect the tax consequences on future years of temporary
differences of revenue and expense items for financial statement and income tax
purposes.
Since its
formation the Company has incurred net operating losses. As of October 31, 2009,
the Company had a net operating loss carryforward of $20,000,000 available to
offset future taxable income for federal and state income tax
purposes.
SFAS No.
109 requires the Company to recognize income tax benefits for loss carryforwards
that have not previously been recorded. The tax benefits recognized must be
reduced by a valuation allowance if it is more likely than not that loss
carryforwards will expire before the Company is able to realize their benefit,
or that future deductibility is uncertain. For financial statement purposes, the
deferred tax asset for loss carryforwards has been fully offset by a valuation
allowance since it is uncertain whether any future benefit will be
realized. An increase in the valuation allowance of $80,000 occurred
as of October 31, 2009.
MERA
PHARMACEUTICALS, INC.
NOTES
TO THE FINANCIAL STATEMENTS
The provision (benefit) for income
taxes from continued operations for the years ended October 31, 2009 and 2008
consist of the following:
|
|
|
October
31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
Current:
|
|
|
|
|
|
|
|
Federal
|
|
$
|
-
|
|
|
$
|
-
|
|
|
State
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
-
|
|
|
|
-
|
|
|
Increase
in valuation allowance
|
|
|
(80,000
|
)
|
|
|
(62,000
|
)
|
|
Benefit
of operating loss carryforward
|
|
|
80,000
|
|
|
|
62,000
|
|
|
Provision
(benefit) for income taxes, net
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
Deferred
income taxes result from temporary differences in the recognition of income and
expenses for the financial reporting purposes and for tax purposes. The tax
effect of these temporary differences representing deferred tax asset and
liabilities result principally from the following:
|
|
|
October
31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
Net
operating loss carry-
forwards
expiring after the year 2009
|
|
$
|
8,300,000
|
|
|
$
|
8,200,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred income tax
asset
|
|
$
|
8,300,000
|
|
|
$
|
8,200,000
|
|
|
|
|
|
|
|
|
|
|
|
The net
deferred tax assets and liabilities are comprised of the following:
|
|
|
October
31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
Deferred
tax assets
|
|
|
|
|
|
|
|
Current
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Non-current
|
|
|
8,300,000
|
|
|
|
8,200,000
|
|
|
|
|
|
|
|
|
|
8,200,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Less
valuation allowance
|
|
|
(8,300,000
|
)
|
|
|
(8,200,000
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net deferred income tax
asset
|
|
$
|
-
|
|
|
$
|
-
|
|
MERA
PHARMACEUTICALS, INC.
NOTES
TO THE FINANCIAL STATEMENTS
The
Company has the following carryforwards available at October 31,
2008:
|
Operating
losses
|
|
Amount
|
Expires
|
|
|
|
|
8,600,000
|
2012
- 2019
|
|
4,000,000
|
2022
|
|
2,600,000
|
2023
|
|
1,900,000
|
2024
|
|
1,200,000
|
2025
|
|
930,000
|
2026
|
|
420,000
|
2027
|
|
150,000
|
2028
|
|
190,000
|
2029
|
The
Company is a Qualified High Tech Business (“QHTB”) in the State of Hawaii. QHTBs
qualify for certain refundable state tax credits as they relate to research and
development activities (“QHTB tax credit refunds”). For the year ended October
31, 2009, the Company has approximately $32,000 in QHTB tax credit refunds
receivable.
6.
NOTES PAYABLE - RELATED PARTIES
Notes
payable – related parties consists of the following as of October 31,
2009:
|
Unsecured
demand notes payable – shareholder notes bearing an annual interest rate
of 10% due on March 31, 2004. Notes are currently past maturity; however
no demand for payment has been made.
|
|
$
|
41,936
|
|
|
Unsecured
demand notes payable – shareholder notes bearing an annual interest rate
of 8% due on various dates through March 26, 2006. Notes are currently
past maturity; however no demand for payment
has been
made.
|
|
|
10,000
|
|
|
Total
notes payable, related parties
|
|
$
|
51,936
|
|
Total
interest expense on notes payable – related parties was $4,992 and $10,140 for
the years ended October 31, 2009 and 2008, respectively.
7.
COMMON STOCK, PREFERRED STOCK AND COMMON STOCK PURCHASE WARRANTS
In August
2008 the Company issued a total of 5,000,000 shares of common stock to a private
investor at a price of $0.005 per share. This issuance was exempt
from registration under the Securities Act of 1933, as amended, pursuant to
Section 4(2).
MERA
PHARMACEUTICALS, INC.
NOTES
TO THE FINANCIAL STATEMENTS
In
September 2008 the Company issued a total of 30,000,000 shares of common stock
to a private investor at a price of $0.005 per share. This issuance
was exempt from registration under the Securities Act of 1933, as amended,
pursuant to Section 4(2).
In August
2008 the Company issued a total of 2,400,000 shares of common stock to a private
investor at a price of $0.005 per share. This issuance was exempt
from registration under the Securities Act of 1933, as amended, pursuant to
Section 4(2).
The
following table summarizes warrant activity through October 31,
2009:
|
|
|
Warrants
|
|
|
Price
|
|
Term
|
|
Balance,
October 31, 2007
|
|
|
2,080,000
|
|
|
|
0.05
|
|
5
Years
|
|
Expired
warrants - 7/2008
|
|
|
(1,460,600
|
)
|
|
|
0.05
|
|
5
Years
|
|
Expired
warrants - 10/2008
|
|
|
(619,400
|
)
|
|
|
0.05
|
|
5
Years
|
|
Balance,
October 31, 2009
|
|
|
-
|
|
|
|
|
|
|
8.
STOCK BASED COMPENSATION
On
November 7, 2004 the Board of Directors adopted the 2004 Stock Option Plan,
authorizing issuance of options on up to 60 million shares of the Company’s
common stock. In December of 2004, the Board approved issuance of
options to purchase approximately 48,000,000 shares of its common stock to
existing officers, directors and employees, subject to shareholder approval of
the plan. In May 2005, such approval was received. The fair value of the options
on the grant date was $480,000 calculated using the Black-Scholes Option Pricing
Model. As of October 31, 2009, approximately 12,400,000 were deemed vested based
on length of service with the Company since the date the Company’s Plan of
Reorganization was approved. Terminated employees who have elected not to
exercise options have forfeited their options. Approximately 36,000,000 total
options have been forfeited since the adoption of the Stock Option
Plan.
The
Company applies Accounting Principles Board Opinion No. 25,
“
Accounting for Stock Issued
to Employees,” in accounting for stock-based employee compensation arrangements
whereby no compensation cost related to stock options is deducted in determining
net income or loss. However, compensation cost for stock option grants to the
Company’s employees would still be determined pursuant to SFAS No. 123,
“Accounting for Stock-Based Compensation,” and the corresponding decrease in net
income disclosed. As no options were issued during the fiscal year ended October
31, 2009, no such computation is included herewith.
MERA
PHARMACEUTICALS, INC.
NOTES
TO THE FINANCIAL STATEMENTS
The
following table summarizes the transactions of the Company’s stock options for
the two-year period ended October 31, 2009:
|
|
|
Number
of Shares
|
|
|
Exercise
Price
|
|
|
Options
outstanding, November 1, 2005
|
|
|
48,426,800
|
|
|
$
|
-
|
|
|
Options
granted
|
|
|
-
|
|
|
|
0.010
|
|
|
Options
exercised
|
|
|
(60,750
|
)
|
|
|
0.010
|
|
|
Options
forfeited
|
|
|
(35,936,050
|
)
|
|
|
0.010
|
|
|
Options
outstanding, October 31, 2009
|
|
|
12,430,000
|
|
|
$
|
0.010
|
|
Options
to purchase 12,430,000 shares were exercisable at October 31, 2009. No options
were offered or exercised during the year ending October 31, 2009.
9.
LONG-LIVED ASSETS
In
accordance with SFAS No. 144, “Accounting for the Impairment or Disposal of
Long-lived Assets,” the Company assesses the impairment of long-lived assets
annually or when events or changes in circumstances indicate that the carrying
value may not be recoverable. Recoverability is measured by comparing the
carrying amount of an asset to expected future net cash flows generated by the
asset. If the carrying amount of an asset exceeds its estimated undiscounted
future cash flows, the carrying amount is compared to its fair value and an
impairment charge is recognized to the extent of the difference. Factors that
the Company considers important which could individually or in combination
trigger an impairment review include the following: (1) significant
underperformance relative to expected historical or projected future operating
results; (2) significant changes in the manner of the Company’s use of the
acquired assets or the strategy for the Company’s overall business; and (3)
significant adverse change in the extent or manner in which a long-lived asset
is being used or in its physical condition. On a quarterly basis, the Company
assesses whether events or changes in circumstances occur that potentially
indicate that the carrying value of long-lived assets may not be recoverable.
The primary factor that could impact the outcome of an impairment evaluation is
the estimate of future cash flows expected to be generated by the asset being
evaluated. Considerable management judgment is necessary to estimate the cash
flows. Accordingly, if actual results fall short of such estimates,
significant future impairments could result. In fiscal 2008, the Company
recorded an asset impairment charge of $1,684,737 related to production and
research equipment that has deteriorated and is in disrepair. No asset
impairment charges were recorded in fiscal year 2009.
10.
MARKETABLE SECURITIES
The
Company’s marketable securities are reported at fair value with the related
unrealized gains and losses included in accumulated other comprehensive income
(loss), a component of shareholders’ equity, net of any tax effect. Realized
gains or losses on the sale of marketable securities are determined using the
specific-identification method. The Company evaluates its investments
periodically for possible other-than-temporary impairment by reviewing factors
such as the length of time and extent to which fair value has been below cost
basis, the financial condition of the issuer and the Company’s ability and
intent to hold the investment for a period of time which may be sufficient for
anticipated recovery of market value. The Company records an impairment charge
to the extent that the carrying value of our available for sale securities
exceeds the estimated fair market value of the securities and the decline in
value is determined to be other-than-temporary. The Company did not record any
impairment charges related to other-than temporary decline in value of its
marketable securities for the years ending October 31, 2009. As of
October 31, 2009, the Company’s held no marketable securities. All marketable
securities are classified as available-for-sale securities.
MERA
PHARMACEUTICALS, INC.
NOTES
TO THE FINANCIAL STATEMENTS
The
following table summarizes the Company’s marketable security investments as of
October 31, 2009:
|
|
|
|
|
|
Net
Unrealized
|
|
|
Estimated
Fair
|
|
|
Marketable
securities:
|
|
Cost
|
|
|
Gains
(Losses)
|
|
|
Market
Value
|
|
|
Corporate
bonds
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total available-for-sale investments
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
11.
PROPERTY, PLANT AND EQUIPMENT, NET
Property,
Plant and Equipment is as follows:
|
|
|
October
31, 2009
|
|
|
Plant
|
|
$
|
274,102
|
|
|
Equipment
|
|
|
-
|
|
|
|
|
|
274,102
|
|
|
Accumulated
depreciation
|
|
|
(73,620
|
)
|
|
Total
|
|
$
|
200,482
|
|
12.
INTANGIBLE ASSETS
The
Company issued detachable stock purchase warrants with an aggregate fair market
value of $165,600 that expired in 2008. During the year ended October
31, 2004, 3,440,000 warrants were cancelled with an aggregate value of $103,000.
For the year ended October 31, 2009 the Company recognized no amortization
expense.
13.
SUBSEQUENT EVENTS
On
November 2, 2009 the Company entered into an agreement with an entity created
and controlled by certain members of its Board of Directors. The agreement
involves the purchase by such entity of Bulk Kona Deep Sea Salt from unsold
inventory at a price of $7.25 per kilogram. The Company estimates its direct
cost for this material is approximately $5.00 per kilogram. The
purpose of this transaction is, in the absence of any other funding sources, to
provide cash flow needed to maintain and grow operations so that the Company is
able to produce enough Kona Deep Sea Salt to market and sell outside of
Hawaii. This program will end once the Company is able to attain
positive cash flow sufficient to sustain such operations. Under this agreement,
the Company shall have the right of first refusal to repurchase some or the
entire product purchased by the related entity at a price of $8.50 per Kilogram
if certain conditions are met.
Mera Pharmaceuticals (CE) (USOTC:MRPI)
Historical Stock Chart
From Dec 2024 to Jan 2025
Mera Pharmaceuticals (CE) (USOTC:MRPI)
Historical Stock Chart
From Jan 2024 to Jan 2025