Use these links to rapidly review the document
TABLE OF CONTENTS
Table of Contents
Filed pursuant to Rule 424(b)(5)
Registration Statement No. 333-213430
PROSPECTUS SUPPLEMENT
(to Prospectus dated September 12, 2016)
2,800,000 Shares

Common Stock
We are offering 2,800,000 shares of our common stock. Our common stock is listed on The NASDAQ Global Market under the symbol "ZYNE." On January 18,
2017, the last reported sale price per share of our common stock was $22.46 per share.
We are an "emerging growth company" as defined under the federal securities laws and, as such, have elected to comply with certain reduced public company reporting
requirements.
Investing in our common stock involves a high degree of risk. Please see "Risk Factors" beginning on page S-10 of this prospectus supplement and in the documents
incorporated by reference into this prospectus supplement.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful
or complete. Any representation to the contrary is a criminal offense.
|
|
|
|
|
|
|
|
|
|
|
PER SHARE
|
|
TOTAL
|
|
|
Public offering price
|
|
$
|
18.00
|
|
$
|
50,400,000
|
|
|
Underwriting discounts and commissions
(1)
|
|
$
|
1.08
|
|
$
|
3,024,000
|
|
|
Proceeds to us, before expenses
|
|
$
|
16.92
|
|
$
|
47,376,000
|
|
-
(1)
See
"Underwriting" for a description of compensation payable to the underwriters.
Delivery
of the shares of common stock in this offering is expected to be made on or about January 24, 2017. We have granted the underwriters an option for a period of 30 days to
purchase up to 420,000 additional shares of our common stock. If the underwriters exercise this option in full, the total underwriting discounts and commissions payable by us will be $3,477,600 and
the total proceeds to us, before expenses, will be $54,482,400.
Joint Book-Running Managers
|
|
|
|
|
Jefferies
|
|
Piper Jaffray
|
Lead Manager
|
Cantor Fitzgerald & Co.
|
Co-Managers
|
|
Oppenheimer & Co.
|
|
Roth Capital Partners
|
Prospectus Supplement dated January 19, 2017
Table of Contents
Table of Contents
Table of Contents
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying base prospectus are part of a registration statement that we filed with the Securities and Exchange Commission,
or the SEC, using a "shelf" registration process. The prospectus supplement describes the specific terms of this offering and also adds to and updates the information contained in the accompanying
base prospectus and the documents incorporated by reference into this prospectus supplement and the accompanying base prospectus. The accompanying base prospectus gives more general information, some
of which may not apply to this offering. If there is a difference between the information contained in this prospectus supplement and the information contained in the accompanying base prospectus or
any document incorporated by reference having an earlier date than the date of this prospectus supplement, you should rely on the information in this prospectus supplement. Generally, when we refer to
the prospectus, we are referring to this prospectus supplement and the accompanying base prospectus combined, together with the documents incorporated by reference herein or therein.
You
should rely only on the information contained or incorporated by reference in this prospectus supplement, in the accompanying base prospectus, or contained in any free writing prospectus with
respect to this offering filed by us with the SEC, including the additional information under the captions "Incorporation of Certain Information by Reference" and "Where You Can Find More Information"
in this prospectus supplement. We have not, and the underwriters have not, authorized anyone to provide you with different information. You should assume that the information appearing in this
prospectus supplement or the accompanying base prospectus and the documents incorporated by reference herein or therein, and in any free writing prospectus that we have authorized for use in
connection with this offering, is accurate only as of the date of those respective documents. Our business, financial condition, results of operations and prospects may have changed since those dates.
We
are offering to sell, and seeking offers to buy, shares of common stock only in jurisdictions where offers and sales are permitted. The distribution of this prospectus supplement and the
accompanying base prospectus and the offering of the common stock in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus
supplement and the accompanying base prospectus must inform themselves about, and observe any restrictions relating to, the offering of the common stock and the distribution of this prospectus
supplement and the accompanying base prospectus outside the United States. We are not, and the underwriters are not, making an offer to sell these securities in any jurisdiction where the offer or
sale is not permitted. This prospectus supplement and the accompanying base prospectus do not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to
buy, any securities offered by this prospectus supplement and the accompanying base prospectus by any person in any jurisdiction in which it is unlawful for such person to make such an offer or
solicitation.
Unless
the context otherwise requires or as otherwise expressly stated, references in this prospectus supplement to the "Company," "Zynerba," "we," "us," "our" and similar terms refer to Zynerba
Pharmaceuticals, Inc. and its subsidiary, Zynerba Pharmaceuticals Pty Ltd, unless we state otherwise or the context indicates otherwise.
S-1
Table of Contents
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Any statements in this prospectus supplement, the base prospectus, or contained in any free writing prospectus with respect to this offering filed by us with
the SEC, and the information incorporated herein and therein by reference relating to future financial or business performance, conditions or strategies and other financial and business matters,
including expectations regarding future revenues and operating expenses, are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements that
are not descriptions of historical facts are forward-looking statements and are based on management's estimates, assumptions, and projections that are subject to risks and uncertainties. These
statements can generally be identified by the use of forward-looking words such as "believe," "expect," "intend," "may," "will," "should," "anticipate," "estimate" or similar terminology. Although we
believe that the expectations reflected in our forward-looking statements are reasonable as of the date we make them, actual results could differ materially from those currently anticipated due to a
number of factors, including risks relating to:
-
§
-
our estimates regarding expenses, future revenue, capital requirements and timing and
availability of and the need for additional financing;
-
§
-
the success and timing of our preclinical studies and clinical trials;
-
§
-
the potential results of preclinical studies and clinical trials for ZYN002 and ZYN001;
-
§
-
our dependence on third parties in the conduct of our preclinical studies and clinical
trials;
-
§
-
the difficulties and expenses associated with obtaining and maintaining regulatory
approval of ZYN002 and ZYN001;
-
§
-
our plans and ability to develop and commercialize ZYN002 and ZYN001;
-
§
-
the successful development of our commercialization capabilities, including sales and
marketing capabilities;
-
§
-
the size and growth of the potential markets for ZYN002 and ZYN001, the rate and degree
of market acceptance of ZYN002 and ZYN001 and our ability to serve those markets;
-
§
-
legal and regulatory developments in the United States and foreign countries;
-
§
-
the success of competing therapies and products that are or become available;
-
§
-
our ability to limit our exposure under product liability lawsuits;
-
§
-
our use of the proceeds from our initial public offering, or IPO, our current
"at-the-market," or ATM, offering and this offering;
-
§
-
our ability to obtain and maintain intellectual property protection for ZYN002 and
ZYN001;
-
§
-
recently enacted and future legislation regarding the healthcare system;
-
§
-
our ability to obtain and maintain third-party manufacturing for our product candidates
on commercially reasonable terms;
-
§
-
the performance of third parties upon which we depend, including third-party contract
research organizations and third-party manufacturers; and
-
§
-
our ability to recruit or retain key scientific or management personnel or to retain our
executive officers.
Further
information on the factors and risks that could affect our business, financial condition and results of operations are set forth in this prospectus supplement under "Risk Factors" and in our
filings with the SEC, which are available at http://www.sec.gov. Any forward-looking statement speaks only as of the date on which it is made, and we undertake no obligation to update any
forward-looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events. New factors emerge from time to time,
and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors,
may cause actual results to differ materially from those
S-2
Table of Contents
contained
in any forward-looking statements. Except as required by law, we undertake no obligation to publicly revise our forward-looking statements to reflect events or circumstances that arise after
the date of this prospectus supplement, the date of the accompanying base prospectus, the date of any free writing prospectus, or the date of documents incorporated by reference herein or therein.
S-3
Table of Contents
PROSPECTUS SUPPLEMENT SUMMARY
This summary description about us, our business and this offering highlights selected information contained elsewhere in this prospectus
supplement or incorporated in this prospectus supplement by reference. This summary does not contain all of the information you should consider before deciding to invest in our common stock. You
should carefully read this entire prospectus supplement, the accompanying base prospectus and any free writing prospectus with respect to this offering filed by us with the SEC, including each of the
documents incorporated herein or therein by reference, before making an investment decision. Investors should carefully consider the information set forth under "Risk Factors" on page S-10 and
in the documents incorporated by reference into this prospectus supplement or the accompanying base prospectus.
Company Overview
We are a clinical stage specialty pharmaceutical company dedicated to developing and commercializing innovative transdermal synthetic cannabinoid treatments
for patients with high unmet needs. We are evaluating two patent protected product candidates, ZYN002 and ZYN001, in five indications. We are studying ZYN002 in adult patients with refractory
epileptic focal seizures (formerly known as complex partial seizures) and osteoarthritis, or OA, and in children with fragile X syndrome, or FXS. We intend to study ZYN001 in patients with
fibromyalgia and peripheral neuropathic pain. We believe these product candidates will provide new treatment options for patients, as well as additional treatment options for patients not currently
receiving adequate relief from current treatment regimens. In 2016, we completed a Phase 1 program for ZYN002 in which it was demonstrated to be safe and well tolerated, which consisted of two
Phase 1 clinical trials for ZYN002 in healthy volunteers and patients with epilepsy and a third Phase 1 clinical trial to evaluate different concentrations of cannabidiol, or CBD, in the
ZYN002 gel in healthy volunteers. We initiated a Phase 2 clinical trial for ZYN002 in adult patients with refractory epileptic focal seizures in June 2016, a Phase 2 clinical trial for
ZYN002 in patients with OA in August 2016 and a Phase 2 clinical trial for ZYN002 in patients with FXS in December 2016. We expect to initiate Phase 1 clinical trials for ZYN001 in the
first half of 2017.
Cannabinoids
are a class of compounds derived from
Cannabis
plants. The two primary cannabinoids contained in
Cannabis
are CBD and
D
9-tetrahydrocannabinol, or THC. Clinical and preclinical data suggest that CBD has positive
effects on treating epilepsy, arthritis and FXS, and THC has positive effects on treating pain. We believe ZYN002 may potentially offer first-line therapies to patients suffering from epilepsy, OA,
and FXS, and ZYN001 may potentially offer first-line therapies to patients suffering from fibromyalgia and peripheral neuropathic pain.
ZYN002
ZYN002 is the first and only synthetic CBD formulated as a permeation-enhanced gel for transdermal delivery, and is patent-protected through 2030. CBD is the
primary non-psychoactive component of
Cannabis
. In preclinical animal studies, ZYN002's permeation enhancer increased delivery of CBD through the layers
of the skin and into the circulatory system. These preclinical studies suggest increased bioavailability, consistent plasma levels and the avoidance of first-pass liver metabolism. In addition, an in
vitro study published in
Cannabis and Cannabinoid Research
in April 2016 demonstrated that CBD is degraded to THC in an acidic environment such as the
stomach. We believe such degradation may lead to increased psychoactive effects if CBD is delivered orally and may be avoided with the transdermal delivery of ZYN002, which maintains CBD in a neutral
pH. ZYN002, which is being developed as a clear gel with once- or twice-daily dosing, is targeting treatment of epilepsy, OA and FXS, which collectively affect millions of patients using treatments
that currently comprise a multi-billion dollar market. We have been granted orphan drug designation from the U.S. Food and Drug Administration, or FDA, for ZYN002 for the treatment of FXS.
S-4
Table of Contents
Phase 1 Clinical Trials
In June 2016, we completed two Phase 1 clinical trials for ZYN002 in healthy volunteers and patients with epilepsy. The first Phase 1 single
rising dose clinical trial for ZYN002 in healthy human subjects and in patients with epilepsy evaluated the tolerability and pharmacokinetic, or PK, profile of ZYN002. Results from this clinical trial
demonstrated that ZYN002 was safe and well-tolerated at all tested dose levels and the incidence of adverse events associated with ZYN002 was similar to placebo for both healthy subjects and epilepsy
patients. The second Phase 1 clinical trial was a randomized, double-blind, placebo controlled multiple rising dose clinical trial for ZYN002 in twenty-four healthy volunteers and twelve
patients with epilepsy to evaluate the PK profile, pharmacodynamics, or PD, and tolerability of multiple doses (200, 250, and 500 mg) of ZYN002. Each volunteer and patient received seven days of
either ZYN002 or placebo. Results from this clinical trial demonstrated that ZYN002 was safe and well-tolerated at all dose levels. The twice daily dosing provided a more
favorable PK profile with comparable results between healthy volunteers and epilepsy patients. Transdermal application of ZYN002 was very well tolerated with minimal skin erythema. Skin dryness at the
application site was common for both ZYN002 and placebo gel. Overall, the incidence of adverse events associated with ZYN002 was similar to placebo in both healthy volunteers and adult epilepsy
patients. There were no reports of somnolence or fatigue and a very low incidence of gastrointestinal events was observed. There were no serious adverse events or discontinuations for healthy
volunteers and epilepsy patients receiving ZYN002. One healthy volunteer receiving placebo gel developed a serious adverse event suspected to be a catheter infection and was discontinued from the
study. In addition, healthy volunteers and epilepsy patients had no drug related changes in performance on the Trail Making Test, a test of visual attention, psychomotor ability, and task switching; a
divided attention task; and the Paced Auditory Serial Addition Task, or PASAT, a test that measures working memory and focused attention. These results indicate that ZYN002 did not produce impairment
in critical areas of cognitive functioning often impacted by central nervous system drugs. No changes in mood symptoms as accessed by the Inventory of Depression and Anxiety Symptoms, or IDAS, and the
Positive and Negative Affect Schedule, or PANAS, were observed for ZYN002 suggesting that ZYN002 is not associated with declines in psychological health.
In
July 2016, we completed a third Phase 1 clinical trial for ZYN002 which was randomized, double-blind and placebo controlled in 42 healthy volunteers. The volunteers received a range of CBD
doses from 395 mg to 504 mg daily in 2.5% and 4.2% ZYN002 formulations for fourteen days. Results from this clinical trial demonstrated that ZYN002 was very well tolerated with minimal skin
erythema. CBD plasma concentrations were dose dependent and did not fluctuate at steady state. The 4.2% formulation demonstrated a comparable PK and tolerability profile to the 2.5% concentration and
was easier to use due to the lower volume. There were no serious adverse events or discontinuations from this clinical trial.
Overall,
in the Phase 1 program, ZYN002 was demonstrated to be safe and well tolerated, provided a favorable CBD PK profile, and no THC was detected in plasma or urine.
Phase 2 Clinical Trials
In June 2016, we initiated a Phase 2 randomized, multi-center, multi-dose clinical trial designed to evaluate the efficacy and safety of ZYN002 in adult
patients with refractory epileptic focal seizures, which we refer to as the STAR 1 (
S
ynthetic
T
ransdermal
Cann
a
bidiol for the T
r
eatment of Epilepsy) trial. Patients will be screened in the trial and will be
followed for 8 weeks during the baseline phase. After the baseline phase, approximately 180 patients will be randomized (1:1:1) to receive one of two doses of CBD gel (195 mg or 97.5 mg
CDB in ZYN002 4.2%) or placebo gel every 12 hours for 12 weeks. The primary endpoint of the study is median reduction in seizure frequency per 28-day period comparing baseline with the
maintenance period via a daily seizure diary. In August 2016, we announced that the first patients were randomized and dosed in STAR 1. We expect to report preliminary topline results for STAR 1 in
July/August 2017. In November 2016, we announced that we initiated a 12-month open-label extension clinical trial (STAR 2) for patients who successfully complete the STAR 1 trial.
S-5
Table of Contents
In
August 2016, we initiated a Phase 2 randomized, multi-center, multi-dose clinical trial designed to evaluate the efficacy and safety of ZYN002 in adult patients with knee pain due to OA,
which we refer to as the STOP (
S
ynthetic
T
ransdermal
Cannabidi
o
l for the Treatment of Knee
P
ain due to Osteoarthritis) trial. Patients will be screened in the
trial and will be followed for two weeks during a baseline phase, which includes a one-week washout period. After completion of the baseline phase, approximately 300 patients will be randomized
(1:1:1) to receive one of two doses of CBD gel (250 mg or 125 mg CBD in ZYN002 4.2%) or placebo gel every 12 hours for 12 weeks. The primary endpoint of the study is the change from
baseline in the weekly mean of the 24-hour average worst pain score. In September 2016, we announced that the first patients were randomized and dosed in the STOP trial. We expect to report topline
results for STOP in July/August 2017.
In
December 2016, we initiated an exploratory Phase 2 clinical trial designed to evaluate the safety and efficacy of ZYN002 in children with FXS, which we refer to as the FAB-C (Treatment of
F
ragile X
Syndrome
A
nxiety and
B
ehavioral Challenges with
C
BD) trial. Approximately 16 patients between the ages of
8-17 years will be enrolled in the clinical trial and will be followed for six weeks
during a titration period, where they will receive a daily dose of CBD gel (50 mg CBD in ZYN002 4.2%) that may be titrated up to an elevated daily dose of CBD gel (250 mg CBD in ZYN002 4.2%). After
completion of the titration period, patients will receive one of three daily maintenance doses of CBD gel (250 mg, 100 mg or 50mg CBD in ZYN002 4.2%) for an additional six weeks. The primary endpoint
of the study is the changes in anxiety, depression and mood as measured by the Anxiety, Depression, and Mood Scale, or ADAMS, scale, a validated patient reported outcomes questionnaire. We expect to
report topline results for FAB-C at the end of the first half of 2017.
ZYN001
ZYN001 is a pro-drug of THC that enables effective transdermal delivery via a patch and is patent-protected through 2031. A pro-drug is a drug administered in
an inactive or less active form and designed to enable more effective delivery, which is then converted into an active form through a normal metabolic process. In addition, we expect that ZYN001 will
be classified by the FDA as a new chemical entity. We are currently working with a development partner, LTS LOHMANN Therapie-Systeme AG, or LTS, to optimize the formulation of ZYN001 into a state of
the art drug-adhesive matrix transdermal patch.
In
our preclinical animal studies, ZYN001 demonstrated effective skin permeation with sustained delivery and rapid conversion of ZYN001 to THC. These preclinical studies suggest increased
bioavailability, consistent plasma levels and the avoidance of first-pass liver metabolism. In addition, preclinical testing has shown no genotoxicity findings and safety pharmacology findings
consistent with those seen with THC. ZYN001 is targeting two pain indications, fibromyalgia and peripheral neuropathic pain, which collectively represent multi-billion dollar markets.
We
plan to evaluate the tolerability and PK profile of ZYN001 in a Phase 1 single rising dose clinical trial in healthy human subjects in the first half of 2017. Subsequent to the single rising
dose clinical trials, we intend to conduct a Phase 1 multiple rising dose clinical trial to examine the tolerability, PK and PD of multiple doses of ZYN001 in healthy human subjects and in
patients with fibromyalgia.
S-6
Table of Contents
Key Development Programs and Expected Timelines
Our key development programs and expected timelines for the development of ZYN002 and ZYN001 are shown in the table below:

Financial Information
We have never been profitable and have incurred net losses since inception. Our net losses were $16.5 million for the nine months ended
September 30, 2016. We expect to incur losses for the foreseeable future, and we expect these losses to increase as we continue our development of, and seek regulatory approvals for, our
product candidates. Because of the numerous risks and uncertainties associated with product development, we are unable to predict the timing or amount of increased expenses or when, or if, we will be
able to achieve or maintain profitability.
In
September 2016, we entered into an Open Market Sales Agreement, or Sales Agreement, with Jefferies LLC, or Jefferies, pursuant to which, as of December 31, 2016, we sold and issued
794,906 shares of our common stock in the open market at a weighted average selling price of $13.39 per share, for net proceeds of $10.0 million. Of the 794,906 shares sold pursuant to the
Sales Agreement, as of September 30, 2016, we sold and issued 428,359 shares of our common stock in the open market at a weighted-average selling price of $13.16, for net proceeds of
$5.3 million, and from October 1, 2016 through November 16, 2016, we sold and issued 366,547 shares of our common stock in the open market at a weighted average selling price of
$13.66 per share, for net proceeds of $4.7 million.
Recent Developments
Financial Results as of December 31, 2016
Based on information currently available, we estimate that as of December 31, 2016 cash and cash equivalents were approximately $31.0 million.
Our cash and cash equivalents includes approximately $4.7 million of net proceeds from our at the market offering with Jefferies for sales occurring from October 1, 2016 through
November 16, 2016, the last sale under the at the market offering program.
These
estimates are preliminary and actual results may differ from these estimates due to the completion of our closing procedures with respect to the fiscal year ended December 31, 2016, final
adjustments and other developments that may arise between now and the time the financial results for the 2016 fiscal year are finalized.
As such, these estimates should not be viewed as a substitute for our full audited financial statements prepared in accordance with U.S. generally accepted accounting principles. These expected
S-7
Table of Contents
results
could change materially and are not necessarily indicative of the results to be achieved for our 2016 fiscal year or any future period. As a result of the foregoing considerations and the
other limitations described herein, investors are cautioned not to place undue reliance on this preliminary financial information. We do not undertake any obligation to publicly update or revise this
estimate, except as required by law.
Corporate Information
Our principal executive offices are located at 80 W. Lancaster Avenue, Suite 300, Devon, PA 19333 and our telephone number is (484) 581-7505. Our
website address is http://www.zynerba.com. The information contained in, or that can be accessed through, our website is not part of this prospectus supplement. We make available free of charge on our
website our annual, quarterly and current reports, including amendments to such reports, as soon as reasonably practicable after we electronically file such material with, or furnish such material to,
the SEC.
Implications of Being an Emerging Growth Company
We are an "emerging growth company," as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. We will remain an emerging growth company
until the earliest of (1) the beginning of the first fiscal year following the fifth anniversary of our initial public offering, or January 1, 2021, (2) the beginning of the first
fiscal year after our annual gross revenue is $1.0 billion or more, (3) the date on which we
have, during the previous three-year period, issued more than $1.0 billion in non-convertible debt securities and (4) as of the end of any fiscal year in which the market value of our
common stock held by non-affiliates exceeded $700 million as of the end of the second quarter of that fiscal year.
For
as long as we remain an "emerging growth company," we may take advantage of certain exemptions from various reporting requirements that are applicable to public companies that are not "emerging
growth companies" including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations
regarding executive compensation and financial statements in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote to approve executive
compensation and shareholder approval of any golden parachute payments not previously approved. We will take advantage of these reporting exemptions until we are no longer an "emerging growth
company."
The
JOBS Act provides that an "emerging growth company" can take advantage of an extended transition period for complying with new or revised accounting standards. We have irrevocably elected not to
avail ourselves of this exemption and, therefore, we are subject to the same new or revised accounting standards as other public companies that are not "emerging growth companies."
S-8
Table of Contents
THE OFFERING
|
|
|
|
|
Common stock to be offered by us
|
|
2,800,000 shares
|
|
Common stock to be outstanding immediately following this offering
|
|
12,794,825 shares (or 13,214,825 shares if the underwriters' option described below is exercised in full)
|
|
Option to purchase additional shares from us
|
|
We have granted the underwriters an option for 30 days from the date of this prospectus supplement to purchase up to
420,000 additional shares of our common stock.
|
|
Use of proceeds
|
|
We intend to use the net proceeds from this offering to continue to fund the clinical development of ZYN002 and ZYN001, for
research and development, and for general corporate purposes, which may include capital expenditures and funding our working capital needs. See "Use of Proceeds" on page S-13.
|
|
Risk Factors
|
|
Investing in our common stock involves a high degree of risk. See "Risk Factors" and other information included in this
prospectus supplement, the accompanying base prospectus and the documents incorporated by reference in this prospectus supplement and the accompanying base prospectus for a discussion of factors you should carefully consider before deciding to invest
in shares of our common stock.
|
|
NASDAQ Global Market symbol
|
|
ZYNE
|
The
number of shares of common stock outstanding immediately following this offering set forth above is based on 9,994,825 shares of our common stock outstanding as of December 31, 2016, and
excludes:
-
§
-
1,808,493 shares of our common stock issuable upon the exercise of stock options
outstanding as of January 17, 2017 at a weighted-average exercise price of $10.22 per share; and
-
§
-
1,195,150 shares of our common stock available for future issuance as of
January 17, 2017 under our Amended and Restated 2014 Omnibus Incentive Compensation Plan, or the 2014 Plan.
Unless
otherwise indicated, all information in this prospectus supplement assumes no exercise of the outstanding options described above and no exercise by the underwriters of their option to purchase
additional shares of our common stock.
S-9
Table of Contents
RISK FACTORS
An investment in our common stock involves a high degree of risk. Our business, financial condition and results of operations could be
materially and adversely affected by any of these risks. If any of these risks occur, the value of our common stock may decline and you may lose all or part of your investment. Before investing in our
common stock, you should consider carefully the risk factors set forth in this prospectus supplement, the accompanying base prospectus and contained in any free writing prospectus with respect to this
offering filed by us with the SEC, along with the risk factors described in "Item 1A. Risk Factors" in our Annual Report on Form 10-K for the year ended December 31, 2015, as
updated by other filings we make with the SEC incorporated by reference into this prospectus supplement.
Risks Related to This Offering
Our management team may invest or spend the proceeds of this offering in ways with which you may not agree or
in ways which may not yield a significant return.
We currently intend to use the net proceeds from this offering to continue to fund the clinical development of ZYN002 and ZYN001, for research and development,
and for general corporate purposes, which may include capital expenditures and funding our working capital needs. However, we have not determined the specific allocation of the net proceeds among
these potential uses. Our management will have broad discretion over the use and investment of the net proceeds of this offering, and, accordingly, investors in this offering will need to rely upon
the judgment of our management with respect to the use of proceeds, with only limited information concerning our specific intentions. These proceeds could be applied in ways that do not improve our
operating results or increase the value of your investment. Please see the section entitled "Use of Proceeds" on page S-13 of this prospectus supplement for further information.
If you purchase our common stock in this offering, you will experience immediate and substantial dilution in
your investment.
Since the public offering price per share of our common stock is substantially higher than the net tangible book value per share of our common stock, you will
suffer immediate and substantial dilution in the net tangible book value of the common stock you purchase in this offering. As a result, investors purchasing shares of common stock in this offering
will incur immediate dilution of approximately $11.53 per share, based upon the public offering price of $18.00 per share and our pro forma net tangible book value as of September 30, 2016,
after giving effect to this offering. For a further description of the dilution that you will experience immediately after this offering, see the section in this prospectus supplement entitled
"Dilution" on page S-15.
Issuances of shares of common stock or securities convertible into or exercisable for shares of common stock
following this offering, as well as the exercise of options, will dilute your ownership interests and may adversely affect the future market price of our common stock.
As a development stage company we will need additional capital to fund the development and commercialization of our product candidates. We may seek additional
capital through a combination of private and public equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements, which may cause your ownership interest to be
diluted. In addition, as of December 31, 2016, there were approximately 1.8 million options to purchase shares of our common stock outstanding. If these securities are exercised, you may
incur further dilution. Moreover, to the extent that we issue additional options to purchase, or securities convertible into or exchangeable for, shares of our common stock in the future and those
options or other securities are exercised, converted or exchanged, you may experience further dilution.
S-10
Table of Contents
A substantial number of shares may be sold in the market following this offering, which may depress the
market price for our common stock.
Sales of a substantial number of shares of our common stock in the public market following this offering could cause the market price of our common stock to
decline. Upon completion of this offering, based on our shares outstanding as of December 31, 2016, we will have 12,794,825 shares of common stock outstanding, assuming no exercise of the
underwriters' option to purchase additional shares of common stock. Of these shares, 610,543 shares are subject to a contractual lock-up with the underwriters for this offering for a period of
90 days following this offering. These shares can be sold, subject to any applicable volume limitations under federal securities laws, after the earlier of the expiration of, or release from,
the 90-day lock-up period. The balance of our outstanding shares of common stock, including any shares purchased in this offering, may be resold into the public market immediately without restriction,
unless these shares are owned or purchased by "affiliates" as that term is defined in Rule 144 under the Securities Act of 1933, as amended, or the Securities Act.
As
of December 31, 2016, there were approximately 1.7 million shares subject to outstanding options under the 2014 Plan, all of which shares we have registered under the Securities Act
on a registration statement on Form S-8. These shares can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements
described above, to the extent applicable.
The market price and trading volume of our stock may be volatile.
The trading price of our common stock has been, and may continue to be, volatile and could be subject to wide fluctuations in response to various factors, some
of which are beyond our control. In addition, the trading volume of our common stock may fluctuate and cause significant price variations to occur. In addition to the factors discussed in this "Risk
Factors" section and elsewhere in this prospectus, these factors include:
-
§
-
the success of competitive products;
-
§
-
regulatory actions with respect to our product candidates or our competitors' products
and product candidates;
-
§
-
actual or anticipated changes in our growth rate relative to our competitors;
-
§
-
announcements by us or our competitors of significant acquisitions, strategic
partnerships, joint ventures, collaborations or capital commitments;
-
§
-
results of clinical trials of ZYN002, ZYN001 or product candidates of our competitors;
-
§
-
regulatory or legal developments in the United States and other countries;
-
§
-
developments or disputes concerning patent applications, issued patents or other
proprietary rights;
-
§
-
the recruitment or departure of key personnel;
-
§
-
the level of expenses related to our preclinical and clinical development programs;
-
§
-
the results of our efforts to in-license or acquire additional product candidates or
products;
-
§
-
actual or anticipated changes in estimates as to financial results, development timelines
or recommendations by securities analysts;
-
§
-
variations in our financial results or those of companies that are perceived to be
similar to us;
-
§
-
fluctuations in the valuation of companies perceived by investors to be comparable to us;
-
§
-
share price and volume fluctuations attributable to inconsistent trading volume levels of
our common stock;
-
§
-
announcement or expectation of additional financing efforts;
-
§
-
sales of our common stock by us, our insiders or our other stockholders;
-
§
-
changes in the structure of healthcare payment systems;
-
§
-
market conditions in the pharmaceutical sector; and
S-11
Table of Contents
-
§
-
general economic, industry and market conditions.
These
broad market and industry factors may decrease the market price of our common stock, regardless of our actual operating performance.
The
stock market in general has, from time to time, experienced extreme price and volume fluctuations, including in recent months. In addition, in the past, following periods of volatility in the
overall market and decreases in the market price of a company's securities, securities class action litigation has often been instituted against these companies. This litigation, if instituted against
us, could result in substantial costs and a diversion of our management's attention and resources.
S-12
Table of Contents
USE OF PROCEEDS
We estimate that the net proceeds to us from our issuance and sale of shares of our common stock in this offering will be approximately $47.2 million,
or approximately $54.3 million if the underwriters exercise in full their option to purchase up to 420,000 additional shares of common stock, after deducting estimated underwriting discounts
and commissions and estimated offering expenses payable by us.
We
intend to use the net proceeds from this offering payable to us to continue to fund the clinical development of ZYN002 and ZYN001, for research and development, and for general corporate purposes,
which may include capital expenditures and funding our working capital needs.
The
expected use of the net proceeds from this offering represents our intentions based upon our current plans and business conditions, which could change in the future as our plans and business
conditions evolve. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including our ability to gain access to additional financing, the relative
success and cost of our research, preclinical and clinical development programs and whether we are able to enter into future licensing or collaboration arrangements. As a result, our management will
have broad discretion in the application of the net proceeds from this offering, and investors will be relying on the judgment of our management regarding the application of the net proceeds from this
offering. Pending their ultimate use, we intend to invest the net proceeds from this offering in short- and intermediate-term, interest-bearing obligations, investment-grade instruments, certificates
of deposit or direct or guaranteed obligations of the U.S. government.
S-13
Table of Contents
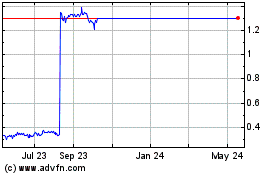
PRICE RANGE OF OUR COMMON STOCK
Our common stock has been listed on The NASDAQ Global Market under the symbol "ZYNE" since our IPO on August 5, 2015. Prior to that time, there was no
public market for our common stock. The following table sets forth the high and low sale prices per share for our common stock on The NASDAQ Global Market for the periods indicated:
|
|
|
|
|
|
|
|
|
|
|
High
|
|
Low
|
|
|
2017
|
|
|
|
|
|
|
|
|
First Quarter (through January 18, 2017)
|
|
$
|
23.07
|
|
$
|
15.80
|
|
|
2016
|
|
|
|
|
|
|
|
|
First Quarter
|
|
$
|
21.56
|
|
$
|
4.64
|
|
|
Second Quarter
|
|
$
|
11.68
|
|
$
|
6.02
|
|
|
Third Quarter
|
|
$
|
14.44
|
|
$
|
6.62
|
|
|
Fourth Quarter
|
|
$
|
16.91
|
|
$
|
10.56
|
|
|
2015
|
|
|
|
|
|
|
|
|
Third Quarter (since August 5, 2015)
|
|
$
|
43.00
|
|
$
|
12.00
|
|
|
Fourth Quarter
|
|
$
|
18.25
|
|
$
|
9.33
|
|
On
January 18, 2017, the closing price of our common stock as reported by The NASDAQ Global Market was $22.46 per share. As of January 18, 2017, there were approximately 48 holders of
record of our common stock. This does not include the number of persons whose stock is in nominee or "street name" accounts through brokers.
DIVIDEND POLICY
We have never declared or paid any cash dividends on our capital stock and do not anticipate paying any cash dividends in the foreseeable future. We expect to
retain available cash to finance ongoing operations and the potential growth of our business. Any future determination to pay dividends on our common stock will be at the discretion of our board of
directors and will depend upon, among other factors, our results of operations, financial condition, capital requirements, business prospects and other factors our board of directors may deem
relevant, and subject to the restrictions contained in any future financing instruments.
S-14
Table of Contents
DILUTION
If you invest in our common stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the public
offering price per share of our common stock and the as adjusted net tangible book value per share of our common stock upon closing of this offering. Net tangible book value per share of our common
stock is determined at any date by subtracting our total liabilities from the amount of our total tangible assets (total assets less intangible assets) and dividing the difference by the number of
shares of our common stock deemed to be outstanding at that date.
Our
historical net tangible book value as of September 30, 2016 was $30.9 million, or $3.21 per share, based on 9,628,278 shares of common stock outstanding as of September 30,
2016. Our pro forma net tangible book value as of September 30, 2016 was $35.6 million, or $3.56 per share, based on 9,628,278 shares of common stock outstanding as of
September 30, 2016, and giving effect to our sale of 366,547 shares of common stock for $4.7 million in net proceeds under our ATM offering between October 1, 2016 and
November 16, 2016, or the ATM Shares.
After
giving effect to the sale of 2,800,000 shares of common stock in this offering at the public offering price of $18.00 per share, after deducting underwriting discounts and commissions and
estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of September 30, 2016, would have been $82.8 million, or $6.47 per share. This amount
represents an immediate increase in net tangible book value of $2.91 per share of our common stock to existing stockholders and an immediate dilution in net tangible book value of $11.53 per share of
our common stock to new investors purchasing shares of common stock in this offering.
The
following table illustrates this per share dilution to new investors purchasing shares of common stock in this offering:
|
|
|
|
|
|
|
|
|
|
Public offering price per share
|
|
|
|
|
$
|
18.00
|
|
|
Historical net tangible book value per share as of September 30, 2016
|
|
$
|
3.21
|
|
|
|
|
|
Pro forma increase in net tangible book value per share attributable to sales of the ATM Shares
|
|
$
|
0.35
|
|
|
|
|
|
Pro forma net tangible book value per share as of September 30, 2016 as a result of sales of the ATM Shares
|
|
$
|
3.56
|
|
|
|
|
|
Increase in net tangible book value per share attributable to new investors purchasing shares in this offering
|
|
|
2.91
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pro forma as adjusted net tangible book value per share after this offering
|
|
|
|
|
|
6.47
|
|
|
|
|
|
|
|
|
|
|
|
Dilution per share to new investors purchasing common stock in this offering
|
|
|
|
|
$
|
11.53
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
If
the underwriters exercise their option to purchase additional shares in full, at the public offering price of $18.00 per share, the pro forma as adjusted net tangible book value will increase to
$6.80 per share, representing an immediate increase in net tangible book value to existing stockholders of $3.24 per share and immediate dilution in net tangible book value of $11.20 per share to new
investors.
The
foregoing table and calculations are based on 9,628,278 shares of our common stock outstanding as of September 30, 2016, and
excludes:
-
§
-
1,808,493 shares of our common stock issuable upon the exercise of stock options
outstanding as of January 17, 2017 at a weighted-average exercise price of $10.22 per share;
S-15
Table of Contents
-
§
-
1,195,150 shares of our common stock available for future issuance as of
January 17, 2017 under the 2014 Plan; and
-
§
-
with respect to the historical net tangible book value, 366,547 shares of our common
stock sold under our ATM offering between October 1, 2016 and November 16, 2016.
To
the extent that any options are exercised, new options are issued under our equity incentive plans or we otherwise issue additional shares of common stock in the future at a price less than the
public offering price, there may be further dilution to new investors purchasing common stock in this offering.
S-16
Table of Contents
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS
The following summary describes certain material U.S. federal income tax consequences of the acquisition, ownership and disposition of our common stock
acquired in this offering by Non-U.S. Holders (as defined below). This discussion does not address all aspects of U.S. federal income tax and does not deal with foreign, state or local tax
consequences that may be relevant to Non-U.S. Holders in light of their particular circumstances, nor does it address U.S. federal tax consequences other than income tax. Special rules different from
those described below may apply to certain Non-U.S. Holders that are subject to special treatment under the United States Internal Revenue Code of 1986, as amended, or the Code, such as financial
institutions, insurance companies, tax-exempt organizations, broker-dealers and traders in securities, U.S. expatriates, "controlled foreign corporations," "passive foreign investment companies,"
corporations that accumulate earnings to avoid U.S. federal income tax, persons that hold our common stock as part of a "straddle," "hedge," "conversion transaction," "synthetic security" or
integrated investment or other risk reduction strategy, persons subject to the alternative minimum tax or Medicare contribution tax, partnerships and other pass-through entities, and investors in such
pass-through entities. Such Non-U.S. Holders are urged to consult their tax advisors to determine the
U.S. federal, state, local and other tax consequences that may be relevant to them. Furthermore, the discussion below is based upon the provisions of the Code, and Treasury regulations, rulings and
judicial decisions thereunder as of the date hereof, and such authorities may be repealed, revoked or modified, perhaps retroactively, so as to result in U.S. federal income tax consequences different
from those discussed below. We have not requested a ruling from the U.S. Internal Revenue Service, or the IRS, with respect to the statements made and the conclusions reached in the following summary,
and there can be no assurance that the IRS will agree with such statements and conclusions. This discussion assumes that the Non-U.S. Holder holds our common stock as a "capital asset" within the
meaning of Section 1221 of the Code (generally, property held for investment).
The
following summary is for general information only and is not tax advice. Persons considering the purchase of our common stock pursuant to this offering should consult their tax advisors concerning
the U.S. federal income tax consequences of acquiring, owning and disposing of our common stock in light of their particular situations as well as any consequences arising under the laws of any other
taxing jurisdiction, including any state, local or foreign tax consequences.
For
the purposes of this discussion, a "Non-U.S. Holder" is, for U.S. federal income tax purposes, a beneficial owner of common stock that is neither a U.S. Holder, a partnership (or other entity
treated as a partnership for U.S. federal income tax purposes regardless of its place of organization or formation), nor an entity that is treated as a disregarded entity for U.S. federal income tax
purposes (regardless of its place of organization or formation). A "U.S. Holder" means a beneficial owner of our common stock that is for U.S. federal income tax purposes (a) an individual who
is a citizen or resident of the United States, (b) a corporation or other entity treated as a corporation created or organized in or under the laws of the United States, any state thereof or
the District of Columbia, (c) an estate the income of which is subject to U.S. federal income taxation regardless of its source or (d) a trust if it (1) is subject to the primary
supervision of a court within the United States and one or more U.S. persons (within the meaning of Section 7701(a)(30) of the Code) have the authority to control all substantial decisions of
the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person for U.S. federal income tax purposes.
Distributions
Distributions, if any, made on our common stock will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or
accumulated earnings and profits. Distributions in excess of our current and accumulated earnings and profits will first be treated as a return of capital to the extent of
S-17
Table of Contents
a
Non-U.S. Holder's adjusted tax basis in the common stock and thereafter as capital gain from the sale or exchange of such common stock as described below under "— Gain on Disposition of
Our Common Stock."
Dividends
paid to a Non-U.S. Holder of our common stock generally will be subject to withholding tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. To obtain
a reduced rate of withholding under a treaty, a Non-U.S. Holder generally will be required to provide us with a properly executed applicable IRS Form W-8, or other appropriate form, certifying
the Non-U.S. Holder's entitlement to benefits under that treaty. In the case of a Non-U.S. Holder that is an entity, Treasury Regulations and the relevant tax treaty provide rules to determine
whether, for purposes of determining the applicability of a tax treaty, dividends will be treated as paid to the entity or to those holding an interest in that entity. If a Non-U.S. Holder holds stock
through a financial institution or other agent acting on the holder's behalf, the holder will be required to provide appropriate documentation to such agent. The holder's agent will then be required
to provide certification to us or our paying agent, either directly or through other intermediaries. If a Non-U.S. Holder is eligible for a reduced rate of U.S. federal withholding tax under an income
tax treaty, such Non-U.S. Holder may be able to obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim with the IRS.
We
generally are not required to withhold tax on dividends paid to a Non-U.S. Holder that are effectively connected with the Non-U.S. Holder's conduct of a trade or business within the United States
(and, if required by an applicable income tax treaty, are attributable to a permanent establishment that such holder maintains in the United States) if a properly executed IRS Form W-8ECI,
stating that the dividends are so connected, is furnished to us (or, if stock is held through a financial institution or other agent, to such agent). In general, such effectively connected dividends
will be subject to U.S. federal income tax on a net income basis at the regular graduated rates, unless a specific treaty exemption applies. A corporate Non-U.S. Holder receiving effectively connected
dividends may also be subject to an additional "branch profits tax," which is imposed, under certain circumstances, at a rate of 30% (or such lower rate as may be specified by an applicable treaty) on
the corporate Non-U.S. Holder's effectively connected earnings and profits, subject to certain adjustments.
Gain on Disposition of Our Common Stock
Subject to the discussion below regarding backup withholding and FATCA, a Non-U.S. Holder generally will not be subject to U.S. federal income tax with respect
to gain realized on a sale or other disposition of our common stock unless (a) the gain is effectively connected with a trade or business of such holder in the United States (and, if required
by an applicable income tax treaty, is attributable to a permanent establishment that such holder maintains in the United States), (b) the Non-U.S. Holder is a nonresident alien individual and
is present in the United States for 183 or more days in the taxable year of the disposition and certain other conditions are met, or (c) we are or have been a "United States real property
holding corporation", or USRPHC, within the meaning of Code Section 897(c)(2) at any time within the shorter of the five-year period
preceding such disposition or such holder's holding period. In general, we would be a USRPHC if U.S. real property interests comprised (by fair market value) at least half of our business assets. We
believe that we are not, and do not anticipate becoming, a USRPHC. Because the determination of whether we are a USRPHC depends on the fair market value of our U.S. real property interests relative to
the fair market value of our other business assets and our non-U.S. real property interests, however, there can be no assurance we are not a USRPHC or will not become one in the future. Even if we are
treated as a USRPHC, gain realized by a Non-U.S. Holder on a disposition of our common stock will not be subject to U.S. federal income tax so long as (1) the Non-U.S. Holder owned, directly,
indirectly and constructively, no more than five percent of our common stock at all times within the shorter of (i) the five-year period preceding the disposition or (ii) the holder's
holding period and (2) our common stock is regularly traded on an established securities market. If the foregoing exception does not apply, and if we are or were to become a USRPHC, a purchaser
may be required to withhold 15% of the proceeds payable to a Non-U.S. Holder from a sale or other taxable disposition of our common stock and such
S-18
Table of Contents
Non-U.S.
Holder generally will be taxed on its net gain derived from the disposition at the graduated U.S. federal income tax rates applicable to United States persons (as defined in the Code).
A
Non-U.S. Holder described in (a) above, will generally be required to pay tax on the net gain derived from the sale at regular graduated U.S. federal income tax rates (unless a specific
treaty exemption applies), and corporate Non-U.S. Holders described in (a) above may be subject to the additional branch profits tax at a 30% rate or such lower rate as may be specified by an
applicable income tax treaty. An individual Non-U.S. Holder described in (b) above, will be required to pay a flat 30% tax on the gain derived from the sale, which gain may be offset by U.S.
source capital losses (even though the individual is not considered a resident of the United States).
Information Reporting Requirements and Backup Withholding
Generally, payors must report information to the IRS with respect to any dividends paid including the amount of any such dividends, the name and address of the
recipient, and the amount, if any, of tax withheld. A similar report is sent to the holder to whom any such dividends are paid. Pursuant to tax treaties or certain other agreements, the IRS may make
its reports available to tax authorities in the recipient's country of residence.
Dividends
paid by us (or our paying agents) to a Non-U.S. Holder may also be subject to U.S. backup withholding. U.S. backup withholding generally will not apply to a Non-U.S. Holder who provides a
properly executed applicable IRS Form W-8 (provided that the payor does not have actual knowledge or reason to know that such holder is a U.S. person) or otherwise establishes an exemption.
Under
current U.S. federal income tax law, U.S. information reporting and backup withholding requirements generally will apply to the proceeds of a disposition of our common stock effected by or
through a U.S. office of any broker, U.S. or foreign, except that information reporting and such requirements may be avoided if the holder provides a properly executed applicable IRS Form W-8
or otherwise meets documentary evidence requirements for establishing Non-U.S. Holder status or otherwise establishes an exemption. Generally, U.S. information reporting and backup withholding
requirements will not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is effected outside the United States through a non-U.S. office of a non-U.S. broker.
Information reporting and backup withholding requirements may, however, apply to a payment of disposition proceeds if the broker has actual knowledge, or reason to know, that the holder is, in fact, a
U.S. person. For information reporting purposes, certain brokers with substantial U.S. ownership or operations will generally be treated in a manner similar to U.S. brokers.
Backup
withholding is not an additional tax. Any amounts of tax withheld under the backup withholding rules may be allowed as a refund or credit against the tax liability of persons subject to backup
withholding, provided that the required information is timely furnished to the IRS.
FATCA
The Foreign Account Tax Compliance Act, or FATCA, imposes a 30% withholding tax on certain types of payments made to "foreign financial institutions" and other
specified non-U.S. entities unless certain due diligence, reporting, withholding and certification requirements are satisfied.
The
Treasury Department and the IRS have issued final regulations under FATCA. As a general matter, FATCA imposes a 30% withholding tax on dividends on, and gross proceeds from the sale or other
disposition of, our common stock if paid to a foreign entity unless:
-
§
-
the foreign entity is a "foreign financial institution" that undertakes specified due
diligence, reporting, withholding and certification obligations or, in the case of a foreign financial institution that is a resident in a jurisdiction that has entered into an intergovernmental
agreement to implement FATCA, the entity complies with the diligence and reporting requirements of such an agreement;
S-19
Table of Contents
-
§
-
the foreign entity is not a "foreign financial institution" and identifies certain of its
U.S. investors; or
-
§
-
the foreign entity otherwise is exempted under FATCA.
An
intergovernmental agreement between the United States and an applicable non-U.S. government may modify these rules. Withholding is required with respect to dividends on our common stock and for
dispositions that occur on or after January 1, 2019, with respect to gross proceeds from a sale or other disposition of our common stock.
If
withholding is imposed under FATCA on a payment related to our common stock, a beneficial owner that is not a foreign financial institution and that otherwise would not be subject to withholding
(or that otherwise would be entitled to a reduced rate of withholding) generally may obtain a refund from the IRS by filing a U.S. federal income tax return (which may entail significant
administrative burden). Prospective investors should consult their tax advisors regarding the effect of FATCA in their particular circumstances.
EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS TAX ADVISOR REGARDING THE TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF
ANY PROPOSED CHANGE IN APPLICABLE LAW.
S-20
Table of Contents
UNDERWRITING
Subject to the terms and conditions set forth in the underwriting agreement, dated January 19, 2017, among us and Jefferies LLC and Piper
Jaffray & Co., as the representatives of the underwriters named below and the joint book-running managers for this offering, we have agreed to sell to the underwriters, and each of the
underwriters has agreed, severally and not jointly, to purchase from us, the respective number of shares of common stock shown opposite its name below:
|
|
|
|
|
UNDERWRITER
|
|
NUMBER OF
SHARES
|
|
Jefferies LLC
|
|
1,120,000
|
|
Piper Jaffray & Co.
|
|
770,000
|
|
Cantor Fitzgerald & Co.
|
|
490,000
|
|
Oppenheimer & Co. Inc.
|
|
210,000
|
|
Roth Capital Partners, LLC
|
|
210,000
|
|
|
|
|
|
Total
|
|
2,800,000
|
|
|
|
|
|
|
|
|
|
|
|
|
The
underwriting agreement provides that the obligations of the several underwriters are subject to certain conditions precedent such as the receipt by the underwriters of officers' certificates and
legal opinions and approval of certain legal matters by their counsel. The underwriting agreement provides that the underwriters will
purchase all of the shares of common stock if any of them are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the nondefaulting underwriters
may be increased or the underwriting agreement may be terminated. We have agreed to indemnify the underwriters and certain of their controlling persons against certain liabilities, including
liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make in respect of those liabilities.
The
underwriters have advised us that, following the completion of this offering, they currently intend to make a market in the common stock as permitted by applicable laws and regulations. However,
the underwriters are not obligated to do so, and the underwriters may discontinue any market-making activities at any time without notice in their sole discretion. Accordingly, no assurance can be
given as to the liquidity of the trading market for the common stock, that you will be able to sell any of the common stock held by you at a particular time or that the prices that you receive when
you sell will be favorable.
The
underwriters are offering the shares of common stock subject to their acceptance of the shares of common stock from us and subject to prior sale. The underwriters reserve the right to withdraw,
cancel or modify offers to the public and to reject orders in whole or in part. In addition, the underwriters have advised us that they do not intend to confirm sales to any account over which they
exercise discretionary authority.
Commissions and Expenses
The underwriters have advised us that they propose to offer the shares of common stock to the public at the public offering price set forth on the cover page
of this prospectus and to certain dealers, which may include the underwriters, at that price less a concession not in excess of $0.648 per share of common stock. After the offering, the public
offering price, concession and reallowance to dealers may be reduced by the representatives. No such reduction will change the amount of proceeds to be received by us as set forth on the cover page of
this prospectus.
S-21
Table of Contents
The
following table shows the public offering price, the underwriting discounts and commissions that we are to pay the underwriters and the proceeds, before expenses, to us in connection with this
offering. Such amounts are shown assuming both no exercise and full exercise of the underwriters' option to purchase additional shares.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PER SHARE
|
|
TOTAL
|
|
|
|
WITHOUT
OPTION TO
PURCHASE
ADDITIONAL
SHARES
|
|
WITH
OPTION TO
PURCHASE
ADDITIONAL
SHARES
|
|
WITHOUT
OPTION TO
PURCHASE
ADDITIONAL
SHARES
|
|
WITH
OPTION TO
PURCHASE
ADDITIONAL
SHARES
|
|
|
Public offering price
|
|
$
|
18.00
|
|
$
|
18.00
|
|
$
|
50,400,000
|
|
$
|
57,960,000
|
|
|
Underwriting discounts and commissions paid by us
|
|
$
|
1.08
|
|
$
|
1.08
|
|
$
|
3,024,000
|
|
$
|
3,477,600
|
|
|
Proceeds to us, before expenses
|
|
$
|
16.92
|
|
$
|
16.92
|
|
$
|
47,376,000
|
|
$
|
54,482,400
|
|
We
estimate expenses payable by us in connection with this offering, other than the underwriting discounts and commissions referred to above, will be approximately $165,000. We have agreed to pay the
filing fees incident to, and the fees and disbursements of counsel for the underwriters in connection with, any required review by the Financial Industry Regulatory Authority, Inc., or FINRA,
in connection with this offering in an amount not to exceed $20,000, and to reimburse the underwriters for certain other expenses, as set forth in the underwriting agreement.
Listing
Our common stock is listed on The NASDAQ Global Market under the trading symbol "ZYNE."
Option to Purchase Additional Shares
We have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase, from time to time, in whole or in
part, up to an aggregate of 420,000 shares from us at the public offering price set forth on the cover page of this prospectus, less underwriting discounts and commissions. If the underwriters
exercise this option, each underwriter will be obligated, subject to specified conditions, to purchase a number of additional shares proportionate to that underwriter's initial purchase commitment as
indicated in the table above.
No Sales of Similar Securities
We and our officers and directors have agreed, subject to specified exceptions, not to directly or
indirectly:
-
§
-
sell, offer to sell, contract to sell or lend, pledge, hypothecate or grant any security
interest in, transfer, effect any short sale or establish or increase a "put equivalent position" or liquidate or decrease "call equivalent position," each within the meaning of Rule 16a-l(h)
under the Securities Exchange Act of 1934, as amended, or the Exchange Act, or otherwise dispose of any shares of common stock, options or warrants to acquire shares of common stock, or securities
exchangeable or exercisable for or convertible into shares of common stock currently or hereafter owned either of record or beneficially, or
-
§
-
engage in any swap, hedge or similar arrangement or agreement that transfers, in whole or
in part, the economic risk of ownership of any shares of common stock, options, or warrants, regardless of whether any such transaction is to be settled in securities, in cash or otherwise, or
S-22
Table of Contents
-
§
-
make any demand for, or exercise any right with respect to, the registration under the
Securities Act of the offer and sale of any shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock, or cause to be filed a registration
statement, prospectus or prospectus supplement (or an amendment or supplement thereto) with respect to any such registration, or
-
§
-
publicly announce an intention to do any of the foregoing for a period of 90 days
after the date of this prospectus without the prior written consent of Jefferies LLC and Piper Jaffray & Co.
This
restriction terminates after the close of trading of the common stock on and including the 90th day after the date of this prospectus.
Jefferies LLC
and Piper Jaffray & Co. may, in their sole discretion and at any time or from time to time before the termination of the 90-day period, release all or a portion of the
securities subject to lock-up agreements.
Stabilization
The underwriters have advised us that they, pursuant to Regulation M under the Exchange Act and certain persons participating in the offering may engage
in short sale transactions, stabilizing transactions, syndicate covering transactions or the imposition of penalty bids in connection with this offering. These activities may have the effect of
stabilizing or maintaining the market price of the common stock at a level above that which might otherwise prevail in the open market. Establishing short sales positions may involve either "covered"
short sales or "naked" short sales.
"Covered"
short sales are sales made in an amount not greater than the underwriters' option to purchase additional shares of our common stock in this offering. The underwriters may close out any
covered short position by either exercising their option to purchase additional shares of our common stock or purchasing shares of our common stock in the open market. In determining the source of
shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they
may purchase shares through the option to purchase additional shares.
"Naked"
short sales are sales in excess of the option to purchase additional shares of our common stock. The underwriters must close out any naked short position by purchasing shares in the open
market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares of our common stock in the open market
after pricing that could adversely affect investors who purchase in this offering.
A
stabilizing bid is a bid for the purchase of shares of common stock on behalf of the underwriters for the purpose of fixing or maintaining the price of the common stock. A syndicate covering
transaction is the bid for or the purchase of shares of common stock on behalf of the underwriters to reduce a short position incurred by the underwriters in connection with the offering. Similar to
other purchase transactions, the underwriter's purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common
stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open
market. A penalty bid is an arrangement permitting the underwriters to reclaim the selling concession otherwise accruing to a syndicate member in connection with the offering if the common stock
originally sold by such syndicate member are purchased in a syndicate covering transaction and therefore have not been effectively placed by such syndicate member.
Neither
we, nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common
stock. The underwriters are not obligated to engage in these activities and, if commenced, any of the activities may be discontinued at any time.
S-23
Table of Contents
The
underwriters may also engage in passive market making transactions in our common stock on the NASDAQ Global Select Market in accordance with Rule 103 of Regulation M during a period
before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion of distribution. A passive market maker must display its bid at a price
not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker's bid, that bid must then be lowered when specified purchase
limits are exceeded.
Electronic Distribution
A prospectus in electronic format may be made available by e-mail or on the web sites or through online services maintained by one or more of the underwriters
or their affiliates. In those cases, prospective investors may view offering terms online and may be allowed to place orders online. The underwriters may agree with us to allocate a specific number of
shares of common stock for sale to online brokerage account holders. Any such allocation for online distributions will be made by the underwriters on the same basis as other allocations. Other than
the prospectus in electronic format, the information on the underwriters' web sites and any information contained in any other web site maintained by any of the underwriters is not part of this
prospectus supplement or the accompanying base prospectus, has not been approved and/or endorsed by us or the underwriters and should not be relied upon by investors.
Other Activities and Relationships
The underwriters and certain of their affiliates are full service financial institutions engaged in various activities, which may include securities trading,
commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriters and certain of
their affiliates have, from time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services for us and our affiliates, for which they
received or will receive customary fees and expenses.
In
the ordinary course of their various business activities, the underwriters and certain of their affiliates may make or hold a broad array of investments and actively trade debt and equity
securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities
activities may involve securities and/or instruments issued by us and our affiliates. If the underwriters or their respective affiliates have a lending relationship with us, they routinely hedge their
credit exposure to us consistent with their customary risk management policies. The underwriters and their respective affiliates may hedge such exposure by entering into transactions which consist of
either the purchase of credit default swaps or the creation of short positions in our securities or the securities of our affiliates, including potentially the common stock offered hereby. Any such
short positions could adversely affect future trading prices of the common stock offered hereby. The underwriters and certain of their respective affiliates may also communicate independent investment
recommendations, market color or trading ideas and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that
they acquire, long and/or short positions in such securities and instruments.
Disclaimers about Non-U.S. Jurisdictions
European Economic Area
In relation to each member state of the European Economic Area which has implemented the Prospectus Directive (each, a Relevant Member State), an offer to the
public of any common shares which are the subject of the offering contemplated by this prospectus supplement and the accompanying base prospectus may not be made in that Relevant Member State except
that an offer to the public in that Relevant Member State
of any common shares may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
-
(a)
-
to
any legal entity which is a "qualified investor" as defined in the Prospectus Directive;
S-24
Table of Contents
-
(b)
-
to
fewer than 100 or, if the Relevant Member State has implemented the relevant provisions of Directive 2010/73/EU, or the 2010 PD Amending Directive, 150 natural or
legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the underwriters or the
underwriters nominated by us for any such offer; or
-
(c)
-
in
any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of common shares shall require us or any of
the underwriters to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
For
the purposes of this provision, the expression an "offer common shares to the public" in relation to the common shares in any Relevant Member State means the communication in any form and by any
means of sufficient information on the terms of the offer and the common shares to be offered so as to enable an investor to decide to purchase or subscribe to the common shares, as the same may be
varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State and the expression "Prospectus Directive" means Directive 2003/71/EC (and
amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in the Relevant Member State.
United Kingdom
This prospectus is only being distributed to, and is only directed at, persons in the United Kingdom that are qualified investors within the meaning of
Article 2(1)(e) of the Prospectus Directive that are also (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial
Promotion) Order 2005, as amended (the "Order") and/or (ii) high net worth entities falling within Article 49(2)(a) to (d) of the Order and other persons to whom it may lawfully
be communicated (each such person being referred to as a "relevant person").
This
prospectus and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by recipients to any other persons in the United Kingdom.
Any person in the United Kingdom that is not a relevant person should not act or rely on this document or any of its contents.
Australia
This prospectus supplement and the accompanying base prospectus are not a disclosure document for the purposes of Australia's Corporations Act 2001 (Cth) of
Australia, or Corporations Act, has not been lodged with the Australian Securities & Investments Commission and is only directed to the categories of exempt persons set out below. Accordingly,
if you receive this prospectus supplement and the accompanying base prospectus in Australia:
You
confirm and warrant that you are either:
-
(a)
-
a
"sophisticated investor" under section 708(8)(a) or (b) of the Corporations Act;
-
(b)
-
a
"sophisticated investor" under section 708(8)(c) or (d) of the Corporations Act and that you have provided an accountant's certificate to the Company
which complies with the requirements of section 708(8)(c)(i) or (ii) of the Corporations Act and related regulations before the offer has been made;
-
(c)
-
a
person associated with the Company under Section 708(12) of the Corporations Act; or
-
(d)
-
a
"professional investor" within the meaning of section 708(11)(a) or (b) of the Corporations Act.
To
the extent that you are unable to confirm or warrant that you are an exempt sophisticated investor, associated person or professional investor under the Corporations Act any offer made to you under
this prospectus supplement and the accompanying base prospectus is void and incapable of acceptance.
S-25
Table of Contents
You
warrant and agree that you will not offer any of the securities issued to you pursuant to this prospectus supplement and the accompanying base prospectus for resale in Australia within
12 months of those securities being issued unless any such resale offer is exempt from the requirement to issue a disclosure document under section 708 of the Corporations Act.
Hong Kong
No securities have been offered or sold, and no securities may be offered or sold, in Hong Kong, by means of any document, other than to persons whose ordinary
business is to buy or sell shares or debentures, whether as principal or agent; or to "professional investors" as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong ("SFO") and
any rules made under that Ordinance; or in other circumstances which do not result in the document being a "prospectus" as defined in the Companies Ordinance (Cap. 32) of Hong Kong ("CO") or
which do not constitute an offer or invitation to the public for the purpose of the CO or the SFO. No document, invitation or advertisement relating to the securities has been issued or may be issued
or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read
by, the public of Hong Kong (except if permitted under the securities laws of Hong Kong) other than with respect to securities which are or are intended to be disposed of only to persons outside Hong
Kong or only to "professional investors" as defined in the SFO and any rules made under that Ordinance.
This
prospectus supplement and the accompanying base prospectus have not been registered with the Registrar of Companies in Hong Kong. Accordingly, this prospectus supplement and the accompanying base
prospectus may not be issued, circulated or distributed in Hong Kong, and the securities may not be offered for subscription to members of the public in Hong Kong. Each person acquiring the securities
will be required, and is deemed by the acquisition of the securities, to confirm that he is aware of the restriction on offers of the securities described in this prospectus and the relevant offering
documents and that he is not acquiring, and has not been offered any securities in circumstances that contravene any such restrictions.
Israel
This document does not constitute a prospectus under the Israeli Securities Law, 5728-1968, and has not been filed with or approved by the Israel Securities
Authority. In Israel, this prospectus is being distributed only to, and any offer of the shares of our common stock is directed only at, (i) a limited number of persons in accordance with
Israeli Securities Law and (ii) investors listed in the first addendum, or the Addendum, to the Israeli Securities Law, consisting primarily of joint investment in trust funds, provident funds,
insurance companies, banks, portfolio managers, investment advisors, members of the Tel Aviv Stock Exchange, underwriters, venture capital funds, entities with equity in excess of NIS
50 million and "qualified individuals," each as defined in the Addendum (as it may be amended from time to time), collectively referred to as qualified investors (in each case, purchasing for
their own account or, where permitted under the Addendum, for the accounts of their clients who are investors listed in the Addendum). Qualified investors are required to submit written confirmation
that they fall within the scope of the Addendum, are aware of the meaning of the same and agree to it.
Japan
The offering has not been and will not be registered under the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948 of Japan, as
amended), or FIEL, and the initial purchaser will not offer or sell any securities, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term as used herein
means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to, or for
the benefit of, any resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the FIEL and any other applicable laws, regulations and
ministerial guidelines of Japan.
S-26
Table of Contents
Singapore
This prospectus supplement and the accompanying base prospectus have not been and will not be lodged or registered as a prospectus with the Monetary Authority
of Singapore. Accordingly, this prospectus supplement and the accompanying base prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or
purchase, of the securities may not be circulated or distributed, nor may the securities be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or
indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the "SFA"),
(ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275, of the SFA,
or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where
the securities are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
-
(a)
-
a
corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire
share capital of which is owned by one or more individuals, each of whom is an accredited investor; or
-
(b)
-
a
trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an
accredited investor,
securities
(as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries' rights and interest (howsoever described) in that trust shall not be transferred within six months
after that corporation or that trust has acquired the securities pursuant to an offer made under Section 275 of the SFA except:
-
(a)
-
to
an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in
Section 275(1A) or Section 276(4)(i)(B) of the SFA;
-
(b)
-
where
no consideration is or will be given for the transfer;
-
(c)
-
where
the transfer is by operation of law;
-
(d)
-
as
specified in Section 276(7) of the SFA; or
-
(e)
-
as
specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore.
Switzerland
The common shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or SIX, or on any other stock exchange or
regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of
Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility
in Switzerland. Neither this prospectus supplement and the accompanying base prospectus nor any other offering or marketing material relating to the common shares or this offering may be publicly
distributed or otherwise made publicly available in Switzerland.
Neither
this document nor any other offering or marketing material relating to this offering, the Company or the common shares has been or will be filed with or approved by any Swiss regulatory
authority. In particular, this document will not be filed with, and the offer of common shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of
common shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes, or CISA. The investor protection afforded to acquirers of interests in collective
investment schemes under the CISA does not extend to acquirers of common shares.
S-27
Table of Contents
Canada
Resale Restrictions
The distribution of shares in Canada is being made only in the provinces of Ontario, Québec, Alberta and British Columbia on a private placement
basis exempt from the requirement that we prepare and file a prospectus with the securities regulatory authorities in each province where trades of these securities are made. Any resale of the
shares in Canada must be made under applicable securities laws which may vary depending on the relevant jurisdiction, and which may require resales to be made under available statutory exemptions or
under a discretionary exemption granted by the applicable Canadian securities regulatory authority. Purchasers are advised to seek legal advice prior to any resale of the securities.
Representations of Canadian Purchasers
By purchasing shares in Canada and accepting delivery of a purchase confirmation, a purchaser is representing to us and the dealer from whom the purchase
confirmation is received that:
-
(a)
-
the
purchaser is entitled under applicable provincial securities laws to purchase the shares without the benefit of a prospectus qualified under those securities
laws as it is an "accredited investor" as defined under National Instrument 45-106 — Prospectus Exemptions,
-
(b)
-
the
purchaser is a "permitted client" as defined in National Instrument 31-103 — Registration Requirements, Exemptions and Ongoing
Registrant Obligations,
-
(c)
-
where
required by law, the purchaser is purchasing as principal and not as agent, and
-
(d)
-
the
purchaser has reviewed the text above under Resale Restrictions.
Conflicts of Interest
Canadian purchasers are hereby notified that certain of the underwriters are relying on the exemption set out in section 3A.3 or 3A.4, if applicable, of
National Instrument 33-105 — Underwriting Conflicts from having to provide certain conflict of interest disclosure in this document.
Statutory Rights of Action
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if the offering memorandum
(including any amendment thereto) such as this document contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit
prescribed by the securities legislation of the purchaser's province or territory. The purchaser of these securities in Canada should refer to any applicable provisions of the securities legislation
of the purchaser's province or territory for particulars of these rights or consult with a legal advisor.
Enforcement of Legal Rights
All of our directors and officers as well as the experts named herein may be located outside of Canada and, as a result, it may not be possible for Canadian
purchasers to effect service of process within Canada upon us or those persons. All or a substantial portion of our assets and the assets of those persons may be located outside of Canada and, as a
result, it may not be possible to satisfy a judgment against us or those persons in Canada or to enforce a judgment obtained in Canadian courts against us or those persons outside of Canada.
Taxation and Eligibility for Investment
Canadian purchasers of shares should consult their own legal and tax advisors with respect to the tax consequences of an investment in the shares in their
particular circumstances and about the eligibility of the shares for investment by the purchaser under relevant Canadian legislation.
S-28
Table of Contents
LEGAL MATTERS
The legal validity of the securities offered by this prospectus supplement will be passed upon for us by Hogan Lovells US LLP, Philadelphia,
Pennsylvania. Wilmer Cutler Pickering Hale and Dorr LLP, New York, New York, is acting as counsel for the underwriters in connection with this offering.
EXPERTS
The consolidated financial statements of Zynerba Pharmaceuticals, Inc. and subsidiary (formerly AllTranz, Inc.) as of December 31, 2015
and 2014 and for each of the years in the three-year period
ended December 31, 2015, have been incorporated by reference herein in reliance upon the report of KPMG LLP, independent registered public accounting firm, incorporated by reference
herein, and upon the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus supplement is part of a registration statement we filed with the SEC. This prospectus supplement does not contain all of the information set
forth in the registration statement and the exhibits to the registration statement. For further information with respect to us and the securities we are offering under this prospectus supplement, we
refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement. You should rely only on the information contained in this prospectus supplement or
incorporated by reference. We have not authorized anyone else to provide you with different information. We are not making an offer of these securities in any state where the offer is not permitted.
You should not assume that the information in this prospectus supplement is accurate as of any date other than the date on the front page of this prospectus supplement, regardless of the time of
delivery of this prospectus supplement or any sale of the securities offered by this prospectus supplement.
We
are currently subject to the reporting requirements of the Exchange Act, and in accordance therewith file periodic reports, proxy statements and other information with the SEC. You may read and
copy any such reports, proxy statements and other information at the SEC's Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further
information on the operation of the public reference room. Our SEC filings are also available to you on the SEC's website at
http://www.sec.gov
and in the
"Investor Relations" section of our website at
http://www.zynerba.com.
Our website and the information contained on that site, or connected to that site,
are not incorporated into and are not a part of this prospectus supplement.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
We incorporate information into this prospectus supplement by reference, which means that we disclose important information to you by referring you to another
document filed separately with the SEC. The information incorporated by reference is deemed to be part of this prospectus supplement, except to the extent superseded by information contained in this
prospectus supplement. This prospectus supplement incorporates by reference the documents set forth below, the file number for each of which is 001-37526, that have been previously filed with the SEC
(other than, in each case, documents or information deemed to have been "furnished" and not "filed" in accordance with SEC
rules):
-
§
-
our Annual Report on Form 10-K for the year ended December 31, 2015, filed
with the SEC on March 14, 2016 (including the portions of our proxy statement for our 2016 annual meeting of stockholders incorporated by reference therein);
S-29
Table of Contents
-
§
-
our Quarterly Reports on Form 10-Q for the fiscal quarters ended March 31,
2016, June 30, 2016 and September 30, 2016, filed with the SEC on May 12, 2016, August 11, 2016 and November 14, 2016, respectively;
-
§
-
our Current Reports on Form 8-K (other than portions thereof furnished under
Item 2.02 or Item 7.01 of Form 8-K and exhibits accompanying such reports that are related to such items, unless specifically stated to the contrary) filed with the SEC on
June 1, 2016, August 16, 2016, September 1, 2016, October 13, 2016, October 17, 2016, October 19, 2016, November 16, 2016, December 15, 2016,
January 6, 2017 and January 18, 2017; and
-
§
-
the description of our common stock contained in our registration statement on
Form 8-A filed under the Exchange Act on July 30, 2015, including any amendment or report filed for the purpose of updating such description.
In
addition, all documents that we file with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus supplement and before the termination
of the offering of our securities shall be deemed incorporated by reference into this prospectus supplement and to be a part of this prospectus supplement from the respective dates of filing such
documents; provided, however, that we are not incorporating any information furnished under Item 2.02 or Item 7.01 of any Current Report on Form 8-K.
Any
statement contained in a document incorporated by reference in this prospectus supplement shall be deemed to be modified or superseded for purposes of this prospectus supplement to the extent that
a statement contained in this prospectus supplement or in any other subsequently filed document that also is or is deemed to be incorporated by reference in this prospectus supplement modifies or
supersedes such statement. Any statement so
modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus supplement.
You
may obtain copies of any of these filings by contacting us at the address and telephone number indicated below or by contacting the SEC as described below under the section entitled "Where You Can
Find More Information." Documents incorporated by reference are available from us without charge, excluding all exhibits unless an exhibit has been specifically incorporated by reference into this
prospectus supplement, by requesting them in writing or by telephone at:
Zynerba
Pharmaceuticals, Inc.
Attention: Corporate Secretary
80 W. Lancaster Avenue, Suite 300
Devon, PA 19333
(484) 581-7505
S-30
PROSPECTUS

$150,000,000
Common Stock
Preferred Stock
Debt Securities
Warrants
Purchase Contracts
Units
Subscription Rights
We may from time to time offer up to $150,000,000 in total of:
-
•
-
shares of our common stock, par value $0.001 per share;
-
•
-
shares of our preferred stock, par value $0.001 per share;
-
•
-
debt securities consisting of debentures, notes or other evidences of indebtedness;
-
•
-
warrants to purchase shares of common stock, preferred stock and/or debt securities;
-
•
-
purchase contracts to purchase common stock or preferred stock;
-
•
-
units consisting of a combination of the foregoing securities;
-
•
-
subscription rights to purchase any of the foregoing securities; or
-
•
-
any combination of these securities.
We
may offer common stock, preferred stock, debt securities, warrants, purchase contracts, units or subscription rights separately or together, in separate series, in amounts, at prices
and on terms to be set forth in one or more supplements to this prospectus. The preferred stock, debt securities, warrants, or subscription rights we may offer may be convertible into or exercisable
or exchangeable for common or preferred stock or other securities of ours or equity securities of one or more other entities. We refer to our common stock, preferred stock, debt securities, warrants,
purchase contracts, units and subscription rights collectively as the "securities." When we decide to issue securities, we will provide you with the specific terms and the public offering price of the
securities in prospectus supplements. In the case of shares of preferred stock, these terms will include, as applicable, the specific title and stated value, and any dividend, liquidation, redemption,
conversion, voting and other rights. You should read this prospectus and any applicable prospectus supplement carefully before you invest. This prospectus may not be used to offer or sell securities
unless accompanied by a prospectus supplement.
We
may sell these securities to or through underwriters and also to other purchasers or through agents. We will set forth the names of any underwriters or agents in an accompanying
prospectus supplement, if applicable.
Our
common stock is traded on The NASDAQ Global Market, or NASDAQ, under the symbol "ZYNE." None of the other securities are currently publicly traded. On August 30, 2016, the
last reported sale price per share of our common stock was $8.98 per share.
We are an "emerging growth company" as defined under the federal securities laws and, as such, have elected to comply with certain reduced public company reporting
requirements.
You should read carefully this prospectus, the documents incorporated by reference in this prospectus and any prospectus supplement before you
invest. Investing in our common stock involves risks. Please see "Risk Factors" on page 5 for more information.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these
securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is September 12, 2016
TABLE OF CONTENTS
|
|
|
|
|
|
|
|
Page
|
|
|
About this Prospectus
|
|
|
ii
|
|
|
Cautionary Note Regarding Forward-Looking Statements
|
|
|
1
|
|
|
About the Company
|
|
|
2
|
|
|
Risk Factors
|
|
|
5
|
|
|
Use of Proceeds
|
|
|
5
|
|
|
Ratio of Earnings to Fixed Charges
|
|
|
6
|
|
|
Plan of Distribution
|
|
|
6
|
|
|
Description of Our Capital Stock
|
|
|
8
|
|
|
Description of Debt Securities
|
|
|
11
|
|
|
Description of Warrants
|
|
|
15
|
|
|
Description of Purchase Contracts
|
|
|
16
|
|
|
Description of Units
|
|
|
17
|
|
|
Description of Subscription Rights
|
|
|
17
|
|
|
Legal Matters
|
|
|
17
|
|
|
Experts
|
|
|
17
|
|
|
Incorporation of Certain Information by Reference
|
|
|
17
|
|
|
Where You Can Find More Information
|
|
|
18
|
|
i
ABOUT THIS PROSPECTUS
This prospectus is a part of a registration statement that we filed with the Securities and Exchange Commission, or the SEC, using a "shelf"
registration process. Under this shelf registration process, we may offer to sell any of the securities, or any combination of the securities, described in this prospectus, in each case in one or more
offerings, up to a total dollar amount of $150,000,000.
This
prospectus provides you with a general description of the securities we may offer. Each time we sell securities pursuant to this prospectus, we will provide a prospectus supplement
or free writing prospectus that will contain specific information about the terms of that offering. The prospectus supplement or free writing prospectus may also add, update or change information
contained in this prospectus. Therefore, if there is any inconsistency between the information in this prospectus and the prospectus supplement, you should rely on the information in the prospectus
supplement. You should read both this prospectus and any prospectus supplement together with the additional information described under the headings "Where You Can Find More Information" and
"Incorporation of Certain Information by Reference," and any free writing prospectus that we may prepare and distribute.
The
registration statement that contains this prospectus, including the exhibits to the registration statement and the information incorporated by reference, contains additional
information about the securities offered under this prospectus. That registration statement can be read at the SEC website or at the SEC offices mentioned below under the heading "Where You Can Find
More Information."
You
should rely only on the information provided in this prospectus and in any prospectus supplement, including the information incorporated by reference. We have not authorized anyone
to provide you with different information.
Neither
this prospectus nor any accompanying prospectus supplement constitutes an offer to sell or the solicitation of an offer to buy any securities other than the registered securities
to which they relate, nor do this prospectus or any prospectus supplement constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is
unlawful to make such offer or solicitation in such jurisdiction.
You
should not assume that the information in this prospectus or any supplement to this prospectus is accurate at any date other than the date indicated on the cover page of these
documents or that any information we have incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference.
Unless
the context otherwise requires or as otherwise expressly stated, references in this prospectus to the "Company," "Zynerba," "we," "us," "our" and similar terms refer to Zynerba
Pharmaceuticals, Inc. and its subsidiary, Zynerba Pharmaceuticals Pty Ltd, unless we state otherwise or the context indicates otherwise.
ii
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Any statements in this prospectus, any accompanying prospectus supplement and the information incorporated herein and therein by reference
relating to future financial or business performance, conditions or strategies and other financial and business matters, including expectations regarding future revenues and operating expenses, are
forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements that are not descriptions of historical facts are forward-looking statements and
are based on management's estimates, assumptions, and projections that are subject to risks and uncertainties. These statements can generally be identified by the use of forward-looking words such as
"believe," "expect," "intend," "may," "will," "should," "anticipate," "estimate" or similar terminology. Although we believe that the expectations reflected in our forward-looking statements are
reasonable as of the date we make them, actual results could differ materially from those currently anticipated due to a number of factors, including risks relating to:
-
•
-
our estimates regarding expenses, future revenue, capital requirements and timing and availability of and the need for additional financing;
-
•
-
the success and timing of our preclinical studies and clinical trials;
-
•
-
the potential results of preclinical studies and clinical trials for ZYN002 and ZYN001;
-
•
-
our dependence on third parties in the conduct of our preclinical studies and clinical trials;
-
•
-
the difficulties and expenses associated with obtaining and maintaining regulatory approval of ZYN002 and ZYN001;
-
•
-
our plans and ability to develop and commercialize ZYN002 and ZYN001;
-
•
-
the successful development of our commercialization capabilities, including sales and marketing capabilities;
-
•
-
the size and growth of the potential markets for ZYN002 and ZYN001, the rate and degree of market acceptance of ZYN002 and ZYN001 and our
ability to serve those markets;
-
•
-
legal and regulatory developments in the United States and foreign countries;
-
•
-
the success of competing therapies and products that are or become available;
-
•
-
our exposure to additional scrutiny as a public company;
-
•
-
our ability to limit our exposure under product liability lawsuits;
-
•
-
our use of the proceeds from our initial public offering, or IPO, and any offering pursuant to this prospectus and any accompanying prospectus
supplement;
-
•
-
our ability to obtain and maintain intellectual property protection for ZYN002 and ZYN001;
-
•
-
recently enacted and future legislation regarding the healthcare system;
-
•
-
the performance of third parties upon which we depend, including third-party contract research organizations and third-party manufacturers; and
-
•
-
our ability to recruit or retain key scientific or management personnel or to retain our executive officers.
Further
information on the factors and risks that could affect our business, financial condition and results of operations are set forth in this prospectus under "Risk Factors" and in
our filings with the SEC, which are available at http://www.sec.gov. Any forward-looking statement speaks only as of the date on which it is made, and we undertake no obligation to update any
forward-looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the
1
occurrence
of unanticipated events. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on
our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. Except as required by
law, we undertake no obligation to publicly revise our forward-looking statements to reflect events or circumstances that arise after the date of this prospectus or the date of documents incorporated
by reference in this prospectus.
ABOUT THE COMPANY
Company Overview
We are a clinical stage specialty pharmaceutical company dedicated to developing and commercializing innovative transdermal synthetic
cannabinoid treatments for patients with high unmet needs. We are evaluating two patent protected product candidates, ZYN002 and ZYN001, in five indications. We are studying ZYN002 in adult patients
with refractory focal seizures and osteoarthritis, or OA, and intend to study ZYN002 in patients with fragile X syndrome, or FXS. We intend to study ZYN001 in patients with fibromyalgia and peripheral
neuropathic pain. We believe these product candidates will provide new treatment options for patients, as well as additional treatment options for patients not currently receiving adequate relief from
current treatment regimens.
In June 2016, we completed two Phase 1 clinical trials for ZYN002 in healthy volunteers and patients with epilepsy, initiated a third Phase 1 clinical trial to evaluate different
concentrations of ZYN002 in healthy volunteers and initiated a Phase 2 clinical trial for ZYN002 in adult patients with refractory focal seizures. We announced the randomization and dosing of
the first patients in this Phase 2 study on August 1, 2016. In August 2016 we initiated a Phase 2 clinical trial for ZYN002 in patients with OA. We expect to initiate a
Phase 2 clinical trial for ZYN002 in patients with FXS in the second half of 2016. We expect to initiate Phase 1 clinical trials for ZYN001 in the second half of 2016.
Cannabinoids
are a class of compounds derived from
Cannabis
plants. The two primary cannabinoids contained in
Cannabis
are cannabidiol, or CBD, and
9-tetrahydrocannabinol, or THC. Clinical and preclinical data suggest that CBD has positive effects on treating
refractory epilepsy, arthritis and FXS, and THC has positive effects on treating pain. In addition to ZYN002 and ZYN001 potentially offering first-line therapies to patients suffering from OA, FXS,
fibromyalgia and peripheral neuropathic pain, we believe ZYN002 may provide a complementary treatment for patients suffering from epilepsy who are refractory to their current treatment regimens.
ZYN002
is the first and only synthetic CBD formulated as a permeation enhanced gel for transdermal delivery, and is patent-protected through 2030. CBD is the primary non-psychoactive
component of
Cannabis
. In preclinical animal studies, ZYN002's permeation enhancer increased delivery of CBD through the layers of the skin and into the
circulatory system. These preclinical studies suggest increased bioavailability, consistent plasma levels and the avoidance of first pass liver metabolism. In addition, an in vitro study published in
Cannabis and Cannabinoid
Research
in April 2016 demonstrated that CBD is degraded to THC in an acidic environment such as the stomach. We believe such
degradation may lead to increased psychoactive effects and may be avoided with the transdermal delivery of ZYN002, which maintains CBD in a neutral pH. ZYN002, which is being developed as a clear gel
with once or twice daily dosing, is targeting treatment of refractory epilepsy, OA and FXS, which collectively affect millions of patients using treatments that currently comprise a multi-billion
dollar market. We have been granted orphan drug designation from the U.S. Food and Drug Administration, or FDA, for ZYN002 for the treatment of FXS.
ZYN001
is a pro-drug of THC that enables effective transdermal delivery via a patch and is patent-protected through 2031. A pro-drug is a drug administered in an inactive or less active
form and designed to enable more effective delivery, which is then converted into an active form through a
2
normal
metabolic process. In addition, we expect that ZYN001 will be classified by the FDA as a new chemical entity. In our preclinical animal studies, ZYN001 demonstrated effective skin permeation
with sustained delivery and rapid conversion of ZYN001 to THC. These preclinical studies suggest increased bioavailability, consistent plasma levels and the avoidance of first pass liver metabolism.
In addition, preclinical testing conducted has shown no genotoxicity findings and safety pharmacology findings
consistent with those seen with THC. ZYN001 is targeting two pain indications, fibromyalgia and peripheral neuropathic pain.
In
June 2016, we completed two Phase 1 clinical trials for ZYN002 in healthy volunteers and patients with epilepsy. The first Phase 1 single rising dose clinical trial for
ZYN002 in healthy human subjects and in patients with refractory epilepsy evaluated the tolerability and pharmacokinetic, or PK, profile of ZYN002. Results from this clinical trial demonstrated that
ZYN002 was safe and well-tolerated at all tested dose levels and the incidence of adverse events associated with ZYN002 was similar to placebo for both healthy subjects and epilepsy patients. The
second Phase 1 clinical trial was a randomized, double-blind, placebo controlled multiple rising dose clinical trial for ZYN002 in twenty-four healthy volunteers and twelve patients with
epilepsy to evaluate the PK profile, pharmacodynamics, or PD, and tolerability of multiple doses (200, 250, and 500 mg) of ZYN002. Each volunteer and patient received seven days of either ZYN002 or
placebo. Top line results from this clinical trial demonstrated that ZYN002 was safe and well-tolerated at all dose levels. The twice daily dosing provided a more favorable PK profile with comparable
results between healthy volunteers and epilepsy patients. Transdermal application of ZYN002 was very well tolerated with minimal skin erythema. Skin dryness at the application site was common for both
ZYN002 and placebo gel. Overall, the incidence of adverse events associated with ZYN002 was similar to placebo in both healthy volunteers and adult epilepsy patients. There were no reports of
somnolence or fatigue and a very low incidence of gastrointestinal events was observed. There were no serious adverse events or discontinuations for healthy volunteers and epilepsy patients receiving
ZYN002. One healthy volunteer receiving placebo gel developed a serious adverse event suspected to be a catheter infection and was discontinued from the study. In addition, healthy volunteers and
epilepsy patients had no drug related changes in performance on the Trail Making Test, a test of visual attention, psychomotor ability, and task switching; a divided attention task; and the Paced
Auditory Serial Addition Task, or PASAT, a test that measures working memory and focused attention. These results indicate that ZYN002 did not produce impairment in critical areas of cognitive
functioning often impacted by central nervous system drugs. No changes in mood symptoms as accessed by the Inventory of Depression and Anxiety Symptoms, or IDAS, and the Positive and Negative Affect
Schedule, or PANAS were observed for ZYN002 suggesting that ZYN002 is not associated with declines in psychological health.
In
June 2016, we initiated a third Phase 1 clinical trial for ZYN002 which was randomized, double-blind and placebo controlled in 42 healthy volunteers. The volunteers received a
range of ZYN002 doses from 395 mg to 504 mg daily in 2.5% and 4.2% ZYN002 formulations for fourteen days. Results from this clinical trial demonstrated that ZYN002 was very well tolerated with minimal
skin erythema. The 4.2% formulation demonstrated a comparable PK and tolerability profile to the 2.5% concentration and was easier to use due to the lower volume. There were no serious adverse events
or discontinuations from this clinical trial.
In
the Phase 1 studies, ZYN002 was safe and well tolerated, provided a favorable CBD PK profile, and no THC was detected in plasma or urine.
In
June 2016, we initiated a Phase 2 randomized, multi-center, multi-dose clinical trial designed to evaluate the efficacy and safety of ZYN002 in adult patients with refractory
focal seizures, which we refer to as the STAR 1 (Synthetic Transdermal Cannabidiol for the Treatment of Epilepsy) trial. Approximately 180 patients will be enrolled in the trial and will be followed
for 8 weeks during the
baseline phase. After the baseline phase, patients are randomized (1:1:1) to receive one of two doses (390 mg or 195 mg daily) of ZYN002 or placebo for 12 weeks. The primary endpoint of the
study is
3
median
percentage change in seizure frequency at 12 weeks. On August 1, 2016, we announced that the first patients were randomized and dosed in STAR 1, and we expect to report
preliminary top line results in the first half of 2017.
In
August 2016, we initiated a Phase 2 randomized, multi-center, multi-dose clinical trial designed to evaluate the efficacy and safety of ZYN002 in adult patients with knee pain
due to OA, which we refer to as the STOP 1 (Synthetic Transdermal Cannabidiol for the Treatment of Knee Pain due to Osteoarthritis) trial. Up to 300 patients will be enrolled in the clinical trial and
will be followed for two weeks during a baseline phase, which includes a one-week washout period. After completion of the baseline phase, patients will be randomized (1:1:1) to receive either 250 mg
of ZYN002 4.2% CBD gel every 12 hours, 125 mg of ZYN002 4.2% CBD gel every 12 hours or placebo gel every 12 hours for 12 weeks. The primary endpoint of the study is the
change from baseline in the weekly mean of the 24-hour average worst pain score. We expect to report topline results in the first half of 2017.
We
plan to evaluate the tolerability and PK profile of ZYN001 in a Phase 1 single rising dose clinical trial in healthy human subjects in the second half of 2016. Subsequent to
the single rising dose clinical trials, we intend to conduct a Phase 1 multiple rising dose clinical trial to examine the tolerability, PK and PD of multiple doses of ZYN001 in healthy human
subjects and in patients with fibromyalgia.
Key Development Programs and Expected Timelines
Our key development programs and expected timelines for the development of ZYN002 and ZYN001 are shown in the table below:
|
|
|
|
|
|
|
|
|
|
|
Product Candidate
|
|
Target Indication
|
|
Delivery Method
|
|
Current
Development
Status
|
|
Expected Next Steps
|
|
ZYN002
|
|
Refractory Epilepsy
|
|
Permeation enhanced Gel
|
|
Phase 2
|
|
1H17: TopLine Results
|
|
|
|
Osteoarthritis
|
|
|
|
|
|
1H17: TopLine Results
|
|
|
|
Fragile X Syndrome
|
|
|
|
|
|
2H16: Initiate Phase 2
|
|
ZYN001
|
|
Fibromyalgia
|
|
Transdermal Patch
|
|
Preclinical
|
|
2H16: Initiate Phase 1
|
|
|
|
Peripheral Neuropathic Pain
|
|
|
|
|
|
1H17: Initiate Phase 2
|
We
have never been profitable and have incurred net losses since inception. Our net losses were $10.5 million for the six months ended June 30, 2016. We expect to incur
losses for the foreseeable future, and we expect these losses to increase as we continue our development of, and seek regulatory approvals for, our product candidates. Because of the numerous risks
and uncertainties associated with product development, we are unable to predict the timing or amount of increased expenses or when, or if, we will be able to achieve or maintain profitability.
Corporate Information
Our principal executive offices are located at 80 W. Lancaster Avenue, Suite 300, Devon, PA 19333 and our telephone number is
(484) 581-7505. Our website address is
http://www.zynerba.com
. The information contained in, or that can be accessed through, our website is not
part of this prospectus. We make available free of charge on our website our annual, quarterly and current reports, including amendments to such reports, as soon as reasonably practicable after we
electronically file such material with, or furnish such material to, the SEC.
Implications of Being an Emerging Growth Company
We are an "emerging growth company," as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. We will remain an emerging
growth company until the earliest of (1) the beginning of the first fiscal year following the fifth anniversary of our initial public offering, or
4
January 1,
2021, (2) the beginning of the first fiscal year after our annual gross revenue is $1.0 billion or more, (3) the date on which we have, during the previous
three-year period, issued more than $1.0 billion in non-convertible debt securities and (4) as of the end of any fiscal year in which the market value of our common stock held by
non-affiliates exceeded $700 million as of the end of the second quarter of that fiscal year.
For
as long as we remain an "emerging growth company," we may take advantage of certain exemptions from various reporting requirements that are applicable to public companies that are
not "emerging growth companies" including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced
disclosure obligations regarding executive compensation and financial statements in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory
vote to approve executive compensation and shareholder approval of any golden parachute payments not previously approved. We will take advantage of these reporting exemptions until we are no longer an
"emerging growth company."
The
JOBS Act provides that an "emerging growth company" can take advantage of an extended transition period for complying with new or revised accounting standards. We have irrevocably
elected not to avail ourselves of this exemption and, therefore, we are subject to the same new or revised accounting standards as other public companies that are not "emerging growth companies."
RISK FACTORS
Investing in our securities involves a high degree of risk. You should carefully consider and evaluate all of the information contained in this
prospectus, any accompanying prospectus supplement, and in the documents we incorporate by reference in this prospectus before you decide to purchase our securities. In particular, you should
carefully consider and evaluate the risks and uncertainties described in "Part I—Item 1A. Risk Factors" of our most recent Form 10-K, as updated by the additional
risks and uncertainties set forth in other filings we make with the SEC or any accompanying prospectus supplement. Any of the risks and uncertainties set forth therein could materially and adversely
affect our business, results of operations and financial condition, which in turn could materially and adversely affect the trading price or value of our securities. As a result, you could lose all or
part of your investment.
USE OF PROCEEDS
Unless otherwise indicated in a prospectus supplement, we anticipate that the net proceeds from our sale of any securities will be used to fund
the development of our clinical programs, for other research and development activities and for general corporate purposes, which may include capital expenditures and funding our working capital
needs. We expect from time to time to evaluate the acquisition of businesses, products and technologies for which a portion of the net proceeds may be used, although we currently are not planning or
negotiating any such transactions. Pending such uses, we may invest the net proceeds in investment grade interest-bearing securities.
The
amounts actually expended for each purpose may vary significantly depending upon numerous factors, including the amount and timing of the proceeds from the offering and progress with
our clinical development programs. Expenditures will also depend upon the establishment of collaborative arrangements with other companies, the availability of additional financing and other factors.
Investors will be relying on the judgment of our management regarding the application of the proceeds of any sale of securities.
5
RATIO OF EARNINGS TO FIXED CHARGES
The following table sets forth our ratio of earnings to fixed charges and the ratio of our combined fixed charges and preferred dividends to
earnings for each of the periods indicated. The following table is qualified by the more detailed information appearing in the computation table set forth in Exhibit 12.1 to the registration
statement of which this prospectus is part and the historical financial statements, including the notes to those financial statements, incorporated by reference in this prospectus.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
Six Months
Ended
June 30,
|
|
|
(Amounts shown are in thousands, except for ratios)
|
|
2013
|
|
2014
|
|
2015
|
|
2016
|
|
|
Ratio of earnings to fixed charges
|
|
|
N/A
|
|
|
N/A
|
|
|
N/A
|
|
|
N/A
|
|
|
Ratio of combined fixed charges and preferred dividends to earnings
|
|
|
N/A
|
|
|
N/A
|
|
|
N/A
|
|
|
N/A
|
|
|
Deficiency of earnings available to cover fixed charges
|
|
$
|
(655
|
)
|
$
|
(5,691
|
)
|
$
|
(12,547
|
)
|
$
|
(10,554
|
)
|
|
Deficiency of earnings available to cover combined fixed charges and preferred stock dividends
|
|
$
|
(744
|
)
|
$
|
(5,739
|
)
|
$
|
(12,547
|
)
|
$
|
(10,554
|
)
|
For
purposes of computing the ratio of earnings to fixed charges and the ratio of our combined fixed charges and preferred dividends to earnings, earnings consist of income (loss) from
continuing operations before income taxes plus fixed charges. Fixed charges consist of interest expense and an estimate of the interest within rental expense. We did not record earnings for any of the
years ended December 31, 2015, 2014 and 2013 or the six months ended June 30, 2016. Accordingly, our earnings were insufficient to cover fixed charges for such periods and we are unable
to disclose a ratio of earnings to fixed charges for such periods.
PLAN OF DISTRIBUTION
We may sell the securities being offered by this prospectus separately or together:
-
•
-
directly to purchasers;
-
•
-
through agents;
-
•
-
to or through underwriters;
-
•
-
through dealers;
-
•
-
through a block trade in which the broker or dealer engaged to handle the block trade will attempt to sell the securities as agent, but may
position and resell a portion of the block as principal to facilitate the transaction; or
-
•
-
through a combination of any of these methods of sale.
In
addition, we may issue the securities being offered by this prospectus as a dividend or distribution.
We
may effect the distribution of the securities from time to time in one or more transactions:
-
•
-
at a fixed price or prices, which may be changed from time to time;
-
•
-
at market prices prevailing at the times of sale;
-
•
-
at prices related to prevailing market prices; or
-
•
-
at negotiated prices.
6
For
example, we may engage in at-the-market offerings into an existing trading market in accordance with Rule 415(a)(4) under the Securities Act. We may also sell securities
through a rights offering, forward contracts or similar arrangements. In any distribution of subscription rights to stockholders, if all of the underlying securities are not subscribed for, we may
then sell the unsubscribed securities directly to third parties or may engage the services of one or more underwriters, dealers or agents, including standby underwriters, to sell the unsubscribed
securities to third parties.
The
securities issued and sold under this prospectus will have no established trading market, other than our common stock, which is listed on NASDAQ. Any shares of our common stock sold
pursuant to this prospectus will be eligible for listing and trading on the NASDAQ, subject to official notice of issuance. Any underwriters to whom securities are sold by us for public offering and
sale may make a market in the securities, but the underwriters will not be obligated to do so and may discontinue any market
making at any time without notice. The securities, other than our common stock, may or may not be listed on a national securities exchange or other trading market.
We
will set forth in a prospectus supplement:
-
•
-
the terms of any underwriting or other agreement that we reach relating to sales under this prospectus;
-
•
-
the method of distribution of the securities;
-
•
-
the names of any agents, underwriters or dealers, including any managing underwriters, used in the offering of securities;
-
•
-
the terms of any direct sales, including the terms of any bidding or auction process, or the terms of any other transactions;
-
•
-
the compensation payable to agents, underwriters and dealers, which may be in the form of discounts, concessions or commissions;
-
•
-
any activities that may be undertaken by agents, underwriters and dealers to stabilize, maintain or otherwise affect the price of the
securities; and
-
•
-
any indemnification and contribution obligations owing to agents, underwriters and dealers.
If
we sell directly to institutional investors or others, they may be deemed to be underwriters within the meaning of the Securities Act with respect to any resale of the securities.
Unless otherwise indicated in a prospectus supplement, if we sell through an agent, such agent will be acting on a best efforts basis for the period of its appointment. Any agent may be deemed to be
an "underwriter" of the securities as that term is defined in the Securities Act. If a dealer is used in the sale of the securities, we or an underwriter will sell securities to the dealer, as
principal. The dealer may resell the securities to the public at varying prices to be determined by the dealer at the time of resale.
We
may authorize agents, underwriters and dealers to solicit offers by certain institutional investors to purchase offered securities under contracts providing for payment and delivery
on a future date specified in a prospectus supplement. The prospectus supplement will also describe the public offering price for the securities and the commission payable for solicitation of these
delayed delivery contracts. Delayed delivery contracts will contain definite fixed price and quantity terms. The obligations of a purchase under these delayed delivery contracts will be subject to
only two conditions:
-
•
-
that the institution's purchase of the securities at the time of delivery of the securities is not prohibited under the law of any jurisdiction
to which the institution is subject; and
-
•
-
that we shall have sold to the underwriters the total principal amount of the offered securities, less the principal amount covered by the
delayed contracts.
7
To
the extent permitted by and in accordance with Regulation M under the Securities Exchange Act of 1934, as amended, or the Exchange Act, in connection with an offering an
underwriter may engage in over-allotments, stabilizing transactions, short covering transactions and penalty bids. Over-allotments involve sales in excess of the offering size, which creates a short
position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. Short covering transactions involve purchases of
the securities in the open market after the
distribution is completed to cover short positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the dealer are purchased
in a covering transaction to cover short positions. Those activities may cause the price of the securities to be higher than it would be otherwise. If commenced, the underwriters may discontinue any
of the activities at any time.
To
the extent permitted by and in accordance with Regulation M under the Exchange Act, any underwriters who are qualified market makers on NASDAQ may engage in passive market
making transactions in the securities on NASDAQ during the business day prior to the pricing of an offering, before the commencement of offers or sales of the securities. Passive market makers must
comply with applicable volume and price limitations and must be identified as passive market makers. In general, a passive market maker must display its bid at a price not in excess of the highest
independent bid for such security; if all independent bids are lowered below the passive market maker's bid, however, the passive market maker's bid must then be lowered when certain purchase limits
are exceeded.
The
specific terms of any lock-up provisions in respect of any given offering will be described in the applicable prospectus supplement.
In
compliance with the guidelines of the Financial Industry Regulatory Authority, Inc., or FINRA, the maximum consideration or discount to be received by any FINRA member or
independent broker-dealer may not exceed 8% of the aggregate proceeds of the offering.
The
underwriters, dealers and agents may engage in transactions with us, or perform services for us, in the ordinary course of business for which they receive compensation.
No
securities may be sold under this prospectus without delivery, in paper format, in electronic format on the Internet, or both, of the applicable prospectus supplement describing the
method and terms of the offering.
DESCRIPTION OF OUR CAPITAL STOCK
The following, together with the additional information we include in the applicable prospectus supplement, describes the common stock and
preferred stock that we may offer under this prospectus, including the material provisions of our sixth amended and restated certificate of incorporation, or our charter, and our amended and restated
bylaws, or our bylaws, and certain provisions of the Delaware General Corporation Law, or the DGCL. Because the following is only a summary, it does not contain all of the information that may be
important to you. For a complete description, you should refer to our charter and our bylaws, copies of which have been filed as exhibits
to the registration statement of which this prospectus is a part, as well as the relevant provisions of the DGCL.
General
Our charter authorizes us to issue up to 210,000,000 shares, 200,000,000 of which are designated as common stock with a par value of $0.001 per
share and 10,000,000 of are designated as preferred stock with a par value of $0.001 per share.
8
Common Stock
As of August 30, 2016, there were 9,199,919 shares of our common stock issued and outstanding and options to purchase 1,732,399 shares of
our common stock outstanding.
Each share of our common stock is entitled to one vote in each matter submitted to a vote at a meeting of stockholders including in all
elections for directors; stockholders are not entitled to cumulative voting in the election for directors. Our stockholders may vote either in person or by proxy. Certain matters identified in our
charter and our bylaws, including amending certain provisions of our charter, such as the provisions relating to director liability, amending our bylaws, removing directors without cause or changing
our exclusive forum provision, require the approval of 66
2
/
3
% of our issued and outstanding common stock. Our directors shall be elected by a plurality of votes cast. All other
questions shall be decided by a majority of votes cast.
Our Board of Directors may authorize, and we may make, distributions to our common stockholders, subject to any restriction in our charter and
to those limitations prescribed by law. However, we have never paid cash dividends on our common stock or any other securities. We anticipate that we will retain all of our future earnings, if any,
for use in the expansion and operation of our business and do not anticipate paying cash dividends in the foreseeable future.
Holders of our common stock have no preemptive rights and have no other rights to subscribe for additional securities under Delaware law. Nor
does our common stock have any conversion rights or rights of redemption (or, if any such rights have been granted in relation to our common stock, any such rights have been waived). Upon liquidation,
all holders of our common stock are entitled to participate pro rata in our assets available for distribution, subject to the rights of any class of preferred stock then outstanding.
Preferred Stock
Under our charter, our board of directors has the authority, subject to limitations prescribed by Delaware law, to issue preferred stock in one
or more series, to establish from time to time the number of shares to be included in each series and to fix the designation, powers, preferences and rights of the shares of each series and any of its
qualifications, limitations and restrictions. Our board of directors also can increase or decrease the number of shares of any series, but not below the number of shares of that series then
outstanding.
Our
board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the
common stock. The issuance of preferred stock could delay, defer or prevent a change in control of our company and may adversely affect the market price of our common stock and the voting and other
rights of the holders of common stock. We have no current plan to issue any shares of preferred stock.
9
Delaware Anti-Takeover Law and Provisions of Our Certificate of Incorporation and Bylaws
We are subject to Section 203 of the DGCL. Subject to certain exceptions, Section 203 prevents a publicly held Delaware
corporation from engaging in a "business combination" with any "interested stockholder" for three years following the date that the person became an interested stockholder, unless prior to the date of
the transaction the interested stockholder attained such status with the approval of our board of directors or unless the business combination is approved in a prescribed manner. A "business
combination" includes, among other things, a merger or
consolidation involving us and the "interested stockholder" and the sale of 10% or more of our assets. In general, an "interested stockholder" is any entity or person beneficially owning 15% or more
of our outstanding voting stock and any entity or person affiliated with or controlling or controlled by such entity or person.
Our charter and bylaws contain provisions that may delay or discourage transactions involving an actual or potential change of control or change
in our management, and could adversely affect the price of our common stock. Among other things, these provisions of our charter and bylaws:
-
•
-
permit our board of directors to issue up to 10 million shares of preferred stock, with any rights, preferences and privileges as it may
designate;
-
•
-
provide that all vacancies on our board of directors, including as a result of newly created directorships, may, except as otherwise required
by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum;
-
•
-
require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be
taken by written consent;
-
•
-
provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at
a meeting of stockholders must provide advance notice in writing, and also specify requirements as to the form and content of a stockholder's notice;
-
•
-
provide that the authorized number of directors may be changed only by the resolution of our board of directors;
-
•
-
provide that special meetings of our stockholders may be called only by the board of directors or by such person or persons requested by a
majority of the board of directors to call such meetings;
-
•
-
provide that the Court of Chancery of the State of Delaware is the exclusive forum in which we and our directors may be sued by our
stockholders; and
-
•
-
provide that our certificate of incorporation and bylaws can only be amended to remove or revise the anti-takeover measures discussed above
upon consent of 66
2
/
3
% of the outstanding capital stock.
Our common stock is listed on the NASDAQ Global Market under the symbol "ZYNE."
The transfer agent and registrar for our common stock is American Stock Transfer and Trust Company, LLC.
10
DESCRIPTION OF DEBT SECURITIES
This section describes the general terms and provisions of the debt securities that we may offer under this prospectus, any of which may be
issued as convertible or exchangeable debt securities. We will set forth the particular terms of the debt securities we offer in a prospectus supplement. The extent, if any, to which the following
general provisions apply to particular debt securities will be described in the applicable prospectus supplement. The following description of general terms relating to the debt securities and the
indenture under which the debt securities will be issued are summaries only and therefore are not complete. You should read the indenture and the prospectus supplement regarding any particular
issuance of debt securities.
We
will issue any debt under an indenture to be entered into between us and the trustee identified in the applicable prospectus supplement. The terms of the debt securities will include
those stated in the indenture and those made part of the indenture by reference to the Trust Indenture Act of 1939, as in effect on the date of the indenture. We have filed or will file a copy
of the form of indenture as an exhibit to the registration statement in which this prospectus is included. The indenture will be subject to and governed by the terms of the Trust Indenture Act of
1939.
We
may offer under this prospectus up to an aggregate principal amount of $150,000,000 in debt securities, or if debt securities are issued at a discount, or in a foreign currency,
foreign currency units or composite currency, the principal amount as may be sold for an aggregate initial public offering price of up to $150,000,000. Unless otherwise specified in the applicable
prospectus supplement, the
debt securities will represent direct, unsecured obligations of the Company and will rank equally with all of our other unsecured indebtedness.
The
following statements relating to the debt securities and the indenture are summaries, qualified in their entirety by reference to the detailed provisions of the indenture and the
final form indenture as may be filed with a future prospectus supplement.
The
prospectus supplement will set forth, to the extent required, the following terms of the debt securities in respect of which the prospectus supplement is
delivered:
-
•
-
the title of the series;
-
•
-
the aggregate principal amount;
-
•
-
the issue price or prices, expressed as a percentage of the aggregate principal amount of the debt securities;
-
•
-
any limit on the aggregate principal amount;
-
•
-
the date or dates on which principal is payable;
-
•
-
the interest rate or rates (which may be fixed or variable) or, if applicable, the method used to determine such rate or rates;
-
•
-
the date or dates from which interest, if any, will be payable and any regular record date for the interest payable;
-
•
-
the place or places where principal and, if applicable, premium and interest, is payable;
-
•
-
the terms and conditions upon which we may, or the holders may require us to, redeem or repurchase the debt securities;
-
•
-
the denominations in which such debt securities may be issuable, if other than denominations of $1,000 or any integral multiple of that number;
-
•
-
whether the debt securities are to be issuable in the form of certificated securities (as described below) or global securities (as described
below);
11
-
•
-
the portion of principal amount that will be payable upon declaration of acceleration of the maturity date if other than the principal amount
of the debt securities;
-
•
-
the currency of denomination;
-
•
-
the designation of the currency, currencies or currency units in which payment of principal and, if applicable, premium and interest, will be
made;
-
•
-
if payments of principal and, if applicable, premium or interest, on the debt securities are to be made in one or more currencies or currency
units other than the currency of denomination, the manner in which the exchange rate with respect to such payments will be determined;
-
•
-
if amounts of principal and, if applicable, premium and interest may be determined by reference to an index based on a currency or currencies
or by reference to a commodity, commodity index, stock exchange index or financial index, then the manner in which such amounts will be determined;
-
•
-
the provisions, if any, relating to any collateral provided for such debt securities;
-
•
-
any addition to or change in the covenants and/or the acceleration provisions described in this prospectus or in the indenture;
-
•
-
any events of default, if not otherwise described below under "Defaults and Notice";
-
•
-
the terms and conditions, if any, for conversion into or exchange for shares of our common stock or preferred stock;
-
•
-
any depositaries, interest rate calculation agents, exchange rate calculation agents or other agents; and
-
•
-
the terms and conditions, if any, upon which the debt securities shall be subordinated in right of payment to other indebtedness of the
Company.
We
may issue discount debt securities that provide for an amount less than the stated principal amount to be due and payable upon acceleration of the maturity of such debt securities in
accordance with the terms of the indenture. We may also issue debt securities in bearer form, with or without coupons. If we issue discount debt securities or debt securities in bearer form, we will
describe material U.S. federal income tax considerations and other material special considerations which apply to these debt securities in the applicable prospectus supplement. We may issue debt
securities denominated in or payable in a foreign currency or currencies or a foreign currency unit or units. If we do, we will describe the restrictions, elections, and general tax considerations
relating to the debt securities and the foreign currency or currencies or foreign currency unit or units in the applicable prospectus supplement.
Exchange and/or Conversion Rights
We may issue debt securities which can be exchanged for or converted into shares of our common stock or preferred stock. If we do, we will
describe the terms of exchange or conversion in the prospectus supplement relating to these debt securities.
Transfer and Exchange
We may issue debt securities that will be represented by either:
-
•
-
"book-entry securities," which means that there will be one or more global securities registered in the name of a depositary or a nominee of a
depositary; or
12
-
•
-
"certificated securities," which means that they will be represented by a certificate issued in definitive registered form.
We
will specify in the prospectus supplement applicable to a particular offering whether the debt securities offered will be book-entry or certificated securities.
Certificated Debt Securities
If you hold certificated debt securities issued under an indenture, you may transfer or exchange such debt securities in accordance with the
terms of the indenture. You will not be charged a service charge for any transfer or exchange of certificated debt securities but may be required to pay an amount sufficient to cover any tax or other
governmental charge payable in connection with such transfer or exchange.
Global Securities
The debt securities of a series may be issued in the form of one or more global securities that will be deposited with a depositary or its
nominees identified in the prospectus supplement relating to the debt securities. In such a case, one or more global securities will be issued in a denomination or aggregate denominations equal to the
portion of the aggregate principal amount of outstanding debt securities of the series to be represented by such global security or securities.
Unless
and until it is exchanged in whole or in part for debt securities in definitive registered form, a global security may not be registered for transfer or exchange except as a whole
by the depositary for such global security to a nominee of the depositary and except in the circumstances described in the prospectus supplement relating to the debt securities. The specific terms of
the depositary arrangement with respect to a series of debt securities will be described in the prospectus supplement relating to such series.
Protection in the Event of Change of Control
Any provision in an indenture that governs our debt securities covered by this prospectus that includes any covenant or other provision
providing for a put or increased interest or otherwise that would afford holders of our debt securities additional protection in the event of a recapitalization transaction, a change of control of the
Company, or a highly leveraged transaction will be described in the applicable prospectus supplement.
Covenants
Unless otherwise indicated in this prospectus or the applicable prospectus supplement, our debt securities may not have the benefit of any
covenant that limits or restricts our business or operations, the pledging of our assets or the incurrence by us of indebtedness. We will describe in the applicable prospectus supplement any material
covenants in respect of a series of debt securities.
Consolidation, Merger and Sale of Assets
We may agree in any indenture that governs the debt securities of any series covered by this prospectus that we will not consolidate with or
merge into any other person or convey, transfer, sell or lease our properties and assets substantially as an entirety to any person, unless such person and such proposed transaction meets various
criteria, which we will describe in detail in the applicable prospectus supplement.
13
Defaults and Notice
The debt securities of any series will contain events of default to be specified in the applicable prospectus supplement, which may include,
without limitation:
-
•
-
failure to pay the principal of, or premium or make-whole amount, if any, on any debt security of such series when due and payable (whether at
maturity, by call for redemption, through any mandatory sinking fund, by redemption at the option of the holder, by declaration or acceleration or otherwise);
-
•
-
failure to make a payment of any interest on any debt security of such series when due;
-
•
-
failure to perform or observe any other covenants or agreements in the indenture with respect to the debt securities of such series;
-
•
-
certain events relating to our bankruptcy, insolvency or reorganization; and
-
•
-
certain cross defaults, if and as applicable.
If
an event of default with respect to debt securities of any series shall occur and be continuing, we may agree that the trustee or the holders of at least 25% in aggregate principal
amount of the then outstanding debt securities of such series may declare the principal amount (or, if the debt securities of such series are issued at an original issue discount, such portion of the
principal amount as may be specified in the terms of the debt securities of such series) of all debt securities of such series or such other amount or amounts as the debt securities or supplemental
indenture with respect to such series may provide, to be due and payable immediately. Any provisions pertaining to events of default and any remedies associated therewith will be described in the
applicable prospectus supplement.
Any
indenture that governs our debt securities covered by this prospectus may require that the trustee under such indenture shall, within 90 days after the occurrence of a
default, give to holders of debt securities of any series notice of all uncured defaults with respect to such series known to it. However, in the case of a default that results from the failure to
make any payment of the principal of, premium or make-whole amount, if any, or interest on the debt securities of any series, or in the payment of any mandatory sinking fund installment with respect
to debt securities of such series, if any, the trustee may withhold such notice if it in good faith determines that the withholding of such notice is in the interest of the holders of debt securities
of such series. Any terms and provisions relating to the foregoing types of provisions will be described in further detail in the applicable prospectus supplement.
Any
indenture that governs our debt securities covered by this prospectus will contain a provision entitling the trustee to be indemnified by holders of debt securities before proceeding
to exercise any trust or power under the indenture at the request of such holders. Any such indenture may provide that the holders of at least a majority in aggregate principal amount of the then
outstanding debt securities of any series may direct the time, method and place of conducting any proceedings for any remedy available to the trustee for such series, or of exercising any trust or
power conferred upon the trustee with respect to the debt securities of such series. However, the trustee under any such indenture may decline to follow any such direction if, among other reasons, the
trustee determines in good faith that the actions or proceedings as directed may not lawfully be taken, would involve the trustee in personal liability or would be unduly prejudicial to the holders of
the debt securities of such series not joining in such direction.
Any
indenture that governs our debt securities covered by this prospectus may endow the holders of such debt securities to institute a proceeding with respect to such indenture, subject
to certain conditions, which will be specified in the applicable prospectus supplement and which may include, that the holders of at least a majority in aggregate principal amount of the debt
securities of such series then outstanding make a written request upon the trustee to exercise its power under the indenture,
14
indemnify
the trustee and afford the trustee reasonable opportunity to act. Even so, such holders may have an absolute right to receipt of the principal, premium or make-whole amount, if any, and
interest when due, with respect to such debt securities, to require conversion or exchange of debt securities if such indenture provides for convertibility or exchangeability at the option of the
holder and to institute suit for the enforcement of such rights. Any terms and provisions relating to the foregoing types of provisions will be described in further detail in the applicable prospectus
supplement.
Modification of the Indenture
We and the trustee may modify any indenture that governs our debt securities of any series covered by this prospectus with or without the
consent of the holders of such debt securities, under certain circumstances to be described in a prospectus supplement.
Investors
may hold their interests in a global security directly through DTC if they are DTC participants, or indirectly through organizations that are DTC participants. Except in the
limited circumstances described below, holders of debt securities represented by interests in a global security will not be entitled to receive their debt securities in fully registered certificated
form.
Defeasance; Satisfaction and Discharge
The prospectus supplement will outline the conditions under which we may elect to have certain of our obligations under the indenture discharged
and under which the indenture obligations will be deemed to be satisfied.
Regarding the Trustee
We will identify the trustee and any relationship that we may have with such trustee, with respect to any series of debt securities, in the
prospectus supplement relating to the applicable debt securities. You should note that if the trustee becomes a creditor of Zynerba Pharmaceuticals, Inc., the indenture and the Trust Indenture
Act of 1939 limit the rights of the trustee to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim, as security or otherwise. The trustee
and its affiliates may engage in, and will be permitted to continue to engage in, other transactions with us and our affiliates. If, however, the trustee acquires any "conflicting interest" within the
meaning of the Trust Indenture Act of 1939, it must eliminate such conflict or resign.
Governing Law
The law governing the indenture and the debt securities will be identified in the prospectus supplement relating to the applicable indenture and
debt securities.
DESCRIPTION OF WARRANTS
The following, together with the additional information we may include in the applicable prospectus supplement, summarizes the material terms
and provisions of the warrants that we may offer under this prospectus and the related warrant agreements and warrant certificates. While the terms summarized below will apply generally to any
warrants we may offer, we will describe the particular terms of any series of warrants in more detail in the applicable prospectus supplement.
As
of the date of this prospectus, there are no outstanding warrants.
We
may issue warrants for the purchase of common stock, debt securities, preferred stock or any combination of the foregoing securities. Warrants may be issued independently or together
with our securities offered by any prospectus supplement. Series of warrants may be issued under a separate
warrant agreement. The applicable prospectus supplement will describe the terms of the warrants offered, including but not limited to the following:
-
•
-
the number of warrants offered;
15
-
•
-
the price or prices at which the warrants will be issued;
-
•
-
the currency or currencies in which the prices of the warrants may be payable;
-
•
-
securities for which the warrants are exercisable;
-
•
-
whether the warrants will be issued with any other securities and, if so, the amount and terms of these securities;
-
•
-
the amount of securities purchasable upon exercise of each warrant and the price at which and the currency or currencies in which the
securities may be purchased upon such exercise, and the events or conditions under which the amount of securities may be subject to adjustment;
-
•
-
the date on which the right to exercise such warrants shall commence and the date on which such right shall expire;
-
•
-
the circumstances, if any, which will cause the warrants to be deemed to be automatically exercised;
-
•
-
the minimum or maximum amount of such warrants, if any, that may be exercised at any one time;
-
•
-
any material risk factors relating to such warrants; and
-
•
-
any other material terms of such warrants.
Prior
to the exercise of any warrants, holders of such warrants will not have any rights of holders of the securities purchasable upon such exercise, including the right to receive
payments of dividends, or the right to vote such underlying securities.
Prospective
purchasers of warrants should be aware that special United States federal income tax, accounting and other considerations may be applicable to instruments such as warrants.
The applicable prospectus supplement will describe such considerations, to the extent they are material, as they apply generally to purchasers of such warrants.
DESCRIPTION OF PURCHASE CONTRACTS
We may issue purchase contracts, including contracts obligating holders to purchase from or sell to us, and obligating us to sell to or purchase
from the holders, a specified number of shares of our common stock or preferred stock at a future date or dates, which we refer to in this prospectus as purchase contracts. The price per share of
common stock or preferred stock and the number of shares of each may be fixed at the time the purchase contracts are issued or may be determined by reference to a specific formula set forth in the
purchase contracts. The purchase contracts may be issued separately or as part of units, consisting of one or more purchase contracts and beneficial interests in debt securities or any other
securities described in the applicable prospectus supplement or any combination of the foregoing, securing the holders' obligations to purchase the common stock or preferred stock under the purchase
contracts.
The
purchase contracts may require us to make periodic payments to the holders of the units or vice versa, and these payments may be unsecured or prefunded on some basis. The purchase
contracts may require holders to secure their obligations under those contracts in a specified manner, including pledging their interest in another purchase contract.
The
applicable prospectus supplement will describe the terms of the purchase contracts, including, if applicable, collateral or depositary arrangements.
16
DESCRIPTION OF UNITS
The following, together with the additional information we may include in the applicable prospectus supplement, summarizes the material terms
and provisions of the units that we may offer under this prospectus. While the terms summarized below will apply generally to any units we may offer, we will describe the particular terms of any
series of units in more detail in the applicable prospectus supplement.
We
may, from time to time, issue units comprised of one or more of the other securities that may be offered under this prospectus, in any combination. Each unit will be issued so that
the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each included security. The unit
agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately at any time, or at any time before a specified date.
Any
applicable prospectus supplement will describe:
-
•
-
the material terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may
be held or transferred separately;
-
•
-
any material provisions relating to the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the
units; and
-
•
-
any material provisions of the governing unit agreement that differ from those described above.
DESCRIPTION OF SUBSCRIPTION RIGHTS
As specified in any applicable prospectus supplement, we may issue subscription rights consisting of one or more debt securities, shares of
preferred stock, shares of common stock or any combination of such securities.
LEGAL MATTERS
The legal validity of the securities offered by this prospectus will be passed upon for us by Hogan Lovells US LLP, Philadelphia,
Pennsylvania.
EXPERTS
The consolidated financial statements of Zynerba Pharmaceuticals, Inc. and subsidiary (formerly AllTranz, Inc.) as of
December 31, 2015 and 2014 and for each of the years in the three-year period ended December 31, 2015, have been incorporated by reference herein in reliance upon the report of
KPMG LLP, independent registered public accounting firm, incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
We incorporate information into this prospectus by reference, which means that we disclose important information to you by referring you to
another document filed separately with the SEC. The information incorporated by reference is deemed to be part of this prospectus, except to the extent superseded by information contained in this
prospectus. This prospectus incorporates by reference the documents set forth below, the file number for each of which is 001-37526, that have been previously
17
filed
with the SEC (other than, in each case, documents or information deemed to have been "furnished" and not "filed" in accordance with SEC rules):
-
•
-
our Annual Report on Form 10-K for the year ended December 31, 2015, filed with the SEC on March 14, 2016 (including the
portions of our proxy statement for our 2016 annual meeting of stockholders incorporated by reference therein);
-
•
-
our Quarterly Reports on Form 10-Q for the fiscal quarters ended March 31, 2016 and June 30, 2016, filed with the SEC on
May 12, 2016 and August 11, 2016;
-
•
-
our Current Reports on Form 8-K (other than portions thereof furnished under Item 2.02 or Item 7.01 of Form 8-K and
exhibits accompanying such reports that are related to such items, unless specifically stated to the contrary) filed with the SEC on June 1, 2016, and August 16, 2016; and
-
•
-
the description of our common stock contained in our registration statement on Form 8-A filed under the Exchange Act on July 30,
2015, including any amendment or report filed for the purpose of updating such description.
In
addition, all documents that we file with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of the initial registration statement of
which this prospectus is a part and prior to the effectiveness of such registration statement and all such documents that we file with the SEC after the date of this prospectus and before the
termination of the offering of our securities shall be deemed incorporated by reference into this prospectus and to be a part of this prospectus from the respective dates of filing such documents.
Any
statement contained in a document incorporated by reference in this prospectus shall be deemed to be modified or superseded for purposes of this prospectus to the extent that a
statement contained in this prospectus or in any other subsequently filed document that also is or is deemed to be incorporated by reference in this prospectus modifies or supersedes such statement.
Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
You
may obtain copies of any of these filings by contacting us at the address and telephone number indicated below or by contacting the SEC as described below under the section entitled
"Where You Can Find More Information." Documents incorporated by reference are available from us without
charge, excluding all exhibits unless an exhibit has been specifically incorporated by reference into this prospectus, by requesting them in writing or by telephone at:
Zynerba
Pharmaceuticals, Inc.
Attention: Corporate Secretary
80 W. Lancaster Avenue, Suite 300
Devon, PA 19333
(484) 581-7505
WHERE YOU CAN FIND MORE INFORMATION
We are currently subject to the reporting requirements of the Exchange Act, and in accordance therewith file periodic reports, proxy statements
and other information with the SEC. You may read and copy any such reports, proxy statements and other information at the SEC's Public Reference Room at 100 F Street, N.E., Washington, D.C.
20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference room. Our SEC filings are also available to you on the SEC's website at
http://www.sec.gov
and in the "Investor Relations" section of our website at
http://www.zynerba.com
. Our
website and the information contained on that site, or connected to that site, are not incorporated into and are not a part of this prospectus.
18
Table of Contents
2,800,000 Shares

Common Stock
PROSPECTUS SUPPLEMENT
Joint Book-Running Managers
|
|
|
|
|
Jefferies
|
|
Piper Jaffray
|
Lead Manager
|
Cantor Fitzgerald & Co.
|
Co-Managers
|
|
Oppenheimer & Co.
|
|
Roth Capital Partners
|
January 19, 2017
Zynerba Pharmaceuticals (NASDAQ:ZYNE)
Historical Stock Chart
From Mar 2024 to Apr 2024
Zynerba Pharmaceuticals (NASDAQ:ZYNE)
Historical Stock Chart
From Apr 2023 to Apr 2024