SECURITIES
AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN
PRIVATE ISSUER PURSUANT TO RULE 13A-16 OR 15D-16
UNDER THE SECURITIES
EXCHANGE ACT OF 1934
October, 2024
Commission File Number 1-15182
DR.
REDDY’S LABORATORIES LIMITED
(Translation of registrant’s name into English)
8-2-337, Road No. 3, Banjara Hills
Hyderabad, Telangana 500 034, India
+91-40-49002900
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form
20-F x
Form 40-F ¨
Indicate by check mark if the registrant is submitting the Form 6-K
in paper as permitted by Regulation S-T Rule 101(b)(1): ______
Note: Regulation S-T Rule 101(b)(1) only permits the submission
in paper of a Form 6-K if submitted solely to provide an attached annual report to security holders.
Indicate by check mark if the registrant is submitting the Form 6-K
in paper as permitted by Regulation S-T Rule 101(b)(7): ______
Note: Regulation S-T Rule 101(b)(7) only
permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer
must furnish and make public under the laws of the jurisdiction in which the registrant is incorporated, domiciled or legally organized
(the registrant’s “home country”), or under the rules of the home country exchange on which the registrant’s securities
are traded, as long as the report or other document is not a press release, is not required to be and has not been distributed to the
registrant’s security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other
Commission filing on EDGAR.
Indicate by check mark whether by furnishing the
information contained in this Form, the registrant is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b)
under the Securities Exchange Act of 1934.
Yes
¨
No x
If “Yes” is marked, indicate below the file number assigned
to registrant in connection with Rule 12g3-2(b): 82-________.
EXHIBITS
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
DR. REDDY’S LABORATORIES LIMITED
(Registrant) |
| |
|
|
|
| Date: October 2, 2024 |
By: |
/s/ K Randhir Singh |
| |
|
Name: |
K Randhir Singh |
| |
|
Title: |
Company Secretary |
Exhibit 99.1
 |
Dr. Reddy’s Laboratories Ltd.
8-2-337, Road No. 3, Banjara Hills,
Hyderabad - 500 034, Telangana,
India.
CIN : L85195TG1984PLC004507
Tel : +91 40 4900 2900
Fax : +91 40 4900 2999
Email : mail@drreddys.com
www.drreddys.com |
October 2, 2024
National Stock Exchange of India Ltd. (Stock Code: DRREDDY-EQ)
BSE Limited (Stock Code: 500124)
New York Stock Exchange Inc. (Stock Code: RDY)
NSE IFSC Ltd. (Stock Code: DRREDDY)
Dear Sir/Madam,
Sub: Press Release
Please find enclosed a Press Release on “Dr.
Reddy’s signs voluntary licensing agreement with Gilead Sciences to manufacture and commercialise Lenacapavir in India and other
countries”.
This is for your information and record.
Thanking you.
Yours faithfully,
For Dr. Reddy’s Laboratories Limited

K Randhir Singh
Company Secretary, Compliance Officer &
Head-CSR
Encl: As above

| |
CONTACT |
| DR. REDDY'S LABORATORIES LTD. |
Investor relationS |
Media relationS |
| 8-2-337, Road No. 3, Banjara Hills, |
RICHA PERIWAL |
USHA IYER |
| Hyderabad - 500034. Telangana, India. |
richaperiwal@drreddys.com |
ushaiyer@drreddys.com |
Dr. Reddy’s signs voluntary licensing
agreement with Gilead Sciences to manufacture and commercialise Lenacapavir in India and other countries
Hyderabad India; October 2, 2024 –
Dr. Reddy’s Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY; along with its subsidiaries together referred
to as “Dr. Reddy’s”), today announced that it has entered into a royalty-free non-exclusive voluntary licensing agreement
with Gilead Sciences Ireland UC for the manufacture and commercialisation of the drug, Lenacapavir, in India and 120 other countries.
Lenacapavir is a United States Food and Drug Administration
(USFDA) approved drug indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in heavily treatment-experienced
adults with multidrug resistant HIV-1 infection failing their current antiretroviral regimen due to resistance, intolerance, or safety
considerations. Additionally, Lenacapavir is currently under investigation for the prevention of HIV (PrEP) which is yet to be approved
globally.
Gilead Sciences first launched Lenacapavir under
the brand name Sunlenca® in the United States and Europe markets in the year 2022. It is a first-in-class HIV-1 capsid inhibitor.
Dr. Reddy’s has been granted a non-exclusive
voluntary licence to manufacture Lenacapavir and market it in 120 countries, for the current approved indication of HIV treatment in heavily
treatment-experienced (HTE) adults with multi-drug resistant HIV. Dr. Reddy's will be responsible for technology transfer at its manufacturing
site, conducting bioequivalence/clinical studies, product registration and launch in the agreed markets. Additionally, the agreement grants
licence to Dr. Reddy’s to manufacture and commercialise lenacapavir for the indication of prevention of HIV (PrEP) in 120 countries,
if approved.
Deepak Sapra, Chief Executive Officer- API
and Services, Dr. Reddy’s Laboratories Ltd., said: "Lenacapavir marks an important milestone for Dr. Reddy’s in patient
access and affordability for pre and post exposure treatment of HIV. The collaboration with Gilead will help us make this latest treatment
option available to patients in 120 primarily low- and lower- middle income countries, including in India. Many of these countries have
a very high disease burden of HIV. This is an important endeavour in our journey to create impact on 1.5 billion patients by 2030.”
About Lenacapavir:
Lenacapavir is approved in multiple countries
for the treatment of adults with multi-drug resistant HIV in combination with other antiretrovirals. The use of lenacapavir for HIV prevention
is investigational and the safety and efficacy of lenacapavir for this use have not been established.
The multi-stage mechanism of action of lenacapavir
is distinguishable from other currently approved classes of antiviral agents. While most antivirals act on just one stage of viral replication,
lenacapavir is designed to inhibit HIV at multiple stages of its lifecycle and has no known cross resistance exhibited in vitro to other
existing drug classes.
Lenacapavir
is being evaluated as a long-acting option in multiple ongoing and planned early and late-stage clinical studies in Gilead's HIV prevention
and treatment research program. Lenacapavir is being developed as a foundation for potential future HIV therapies with the goal of offering
both long-acting oral and injectable options with several dosing frequencies, in combination or as a mono agent, that help address individual
needs and preferences of people and communities affected by HIV1.
About Dr. Reddy’s: Dr. Reddy’s
Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY) is a global pharmaceutical company headquartered in Hyderabad,
India. Established in 1984, we are committed to providing access to affordable and innovative medicines. Driven by our purpose of ‘Good
Health Can’t Wait’, we offer a portfolio of products and services including APIs, generics, branded generics, biosimilars
and OTC. Our major therapeutic areas of focus are gastrointestinal, cardiovascular, diabetology, oncology, pain management and dermatology.
Our major markets include – USA, India, Russia & CIS countries, China, Brazil and Europe. As a company with a history of deep
science that has led to several industry firsts, we continue to plan ahead and invest in businesses of the future. As an early adopter
of sustainability and ESG actions, we released our first Sustainability Report in 2004. Our current ESG goals aim to set the bar high
in environmental stewardship; access and affordability for patients; diversity; and governance. For more information, log on to: www.drreddys.com.
Disclaimer: This press release may include statements
of future expectations and other forward-looking statements that are based on the management’s current views and assumptions and
involve known or unknown risks and uncertainties that could cause actual results, performance or events to differ materially from those
expressed or implied in such statements. In addition to statements which are forward-looking by reason of context, the words "may",
"will", "should", "expects", "plans", "intends", "anticipates", "believes",
"estimates", "predicts", "potential", or "continue" and similar expressions identify forward-looking
statements. Actual results, performance or events may differ materially from those in such statements due to without limitation, (i) general
economic conditions such as performance of financial markets, credit defaults , currency exchange rates, interest rates, persistency levels
and frequency / severity of insured loss events, (ii) mortality and morbidity levels and trends, (iii) changing levels of competition
and general competitive factors, (iv) changes in laws and regulations and in the policies of central banks and/or governments, (v) the
impact of acquisitions or reorganization, including related integration issues, and (vi) the susceptibility of our industry and the markets
addressed by our, and our customers’, products and services to economic downturns as a result of natural disasters, epidemics, pandemics
or other widespread illness, including coronavirus (or COVID-19), and (vii) other risks and uncertainties identified in our public filings
with the Securities and Exchange Commission, including those listed under the "Risk Factors" and "Forward-Looking Statements"
sections of our Annual Report on Form 20-F for the year ended March 31, 2024. The company assumes no obligation to update any information
contained herein.
| 1 |
https://www.gilead.com/news/news-details/2024/gileads-twiceyearly-lenacapavir-for-hiv-prevention-reduced-hiv-infections-by-96-and-demonstrated-superiority-to-daily-truvada |
Dr Reddys Laboratories (NYSE:RDY)
Historical Stock Chart
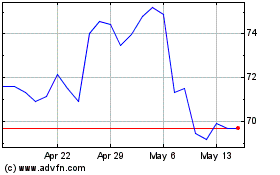
From Sep 2024 to Oct 2024
Dr Reddys Laboratories (NYSE:RDY)
Historical Stock Chart
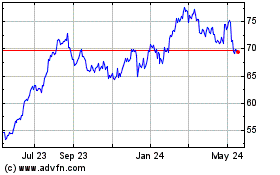
From Oct 2023 to Oct 2024