GlaxoSmithKline Notes Positive Interim Data for Combined HIV/Tuberculosis Treatment
March 05 2018 - 11:56AM
Dow Jones News
By Adam Clark
GlaxoSmithKline PLC (GSK.LN) said on Monday that its
majority-owned ViiV Healthcare joint venture has reported positive
interim results from a study of dolutegravir, a treatment for
adults with both HIV and tuberculosis.
GSK said the ViiV phase IIIb study of dolutegravir showed the
treatment was effective and well-tolerated in patients already
receiving rifampin medication for tuberculosis.
John Pottage, chief scientific and medical officer of ViiV,
said, "Tuberculosis remains the leading cause of death among people
living with HIV, accounting for around one in three AIDS-related
deaths. Concurrent treatment of TB and HIV remains a challenge as
it is compounded by drug interactions, overlapping toxicities and
immune reconstitution inflammatory syndrome."
GSK has a 78% stake in ViiV, while Shionogi & Co. Ltd.
(4507.TO) and Pfizer Inc. (PFE) have minority holdings.
Shares in GlaxoSmithKline closed up 18.80 pence, or 1.5%, at
1308.80 pence on Monday.
Write to Adam Clark at adam.clark@dowjones.com
(END) Dow Jones Newswires
March 05, 2018 12:41 ET (17:41 GMT)
Copyright (c) 2018 Dow Jones & Company, Inc.
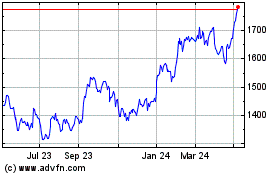
Gsk (LSE:GSK)
Historical Stock Chart
From Apr 2024 to May 2024
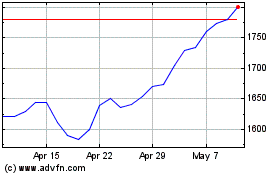
Gsk (LSE:GSK)
Historical Stock Chart
From May 2023 to May 2024