Acadia Pharmaceuticals Announces Exclusive License Agreement with Saniona for SAN711
November 26 2024 - 3:05PM
Business Wire
- SAN711 is a first-in-class, highly selective
GABAA-α3 positive allosteric modulator
- Targeting initiation of Phase 2 study of
SAN711 for the treatment of essential tremor in 2026
Acadia Pharmaceuticals Inc. (Nasdaq: ACAD) today announced it
has entered into an exclusive worldwide license agreement with
Saniona (OMX: SANION) for the development and commercialization of
SAN711, a first-in-class, highly selective GABAA-α3 positive
allosteric modulator. The first indication the Company plans to
pursue is development of SAN711 for essential tremor, a
neurological condition that includes shaking or trembling movements
in one or more parts of the body. Acadia is planning to initiate a
Phase 2 study of SAN711 in essential tremor in 2026.
"Licensing SAN711 to expand our pipeline underscores our
unwavering commitment to delivering innovative therapies for
patients with central nervous system disorders," said Catherine
Owen Adams, Chief Executive Officer. "Essential tremor is a
condition that has not seen innovation in treatment for decades,
creating a compelling opportunity to address a long-overlooked
need. Our work with SAN711 will draw on our deep expertise in
developing and commercializing cutting-edge treatments for
neurological disorders."
Under the terms of the License Agreement, Saniona will receive
US $28 million upfront plus potential milestone payments of up to
US $582 million. In addition, Saniona is eligible to receive tiered
royalties of mid-single digits to low double digits on net sales of
commercial products that may result from development of SAN711. The
potential milestone payments to Saniona consist of up to US $147
million subject to achievement of development and commercial
milestones related to potential first and second indications, and
up to US $435 million subject to achievement of thresholds of
annual net sales of SAN711 worldwide. Acadia will lead further
clinical development, regulatory submissions, and global
commercialization efforts for SAN711 while also providing financial
support for Saniona’s ongoing Phase 1 study and preparations for
Phase 2.
About Acadia Pharmaceuticals
Acadia is advancing breakthroughs in neuroscience to elevate
life. Since our founding we have been working at the forefront of
healthcare to bring vital solutions to people who need them most.
We developed and commercialized the first and only FDA-approved
drug to treat hallucinations and delusions associated with
Parkinson’s disease psychosis and the first and only approved drug
in the United States and Canada for the treatment of Rett syndrome.
Our clinical-stage development efforts are focused on Prader-Willi
syndrome, Alzheimer’s disease psychosis and multiple other programs
targeting neuropsychiatric symptoms in central nervous system
disorders. For more information, visit us at Acadia.com and follow
us on LinkedIn and X.
Forward-Looking Statements
This press release contains forward-looking statements within
the meaning of the Private Securities Litigation Reform Act of
1995. Forward-looking statements include all statements other than
statements of historical fact and can be identified by terms such
as “intends,” “may,” “will,” “should,” “could,” “would,” “expects,”
“plans,” “anticipates,” “believes,” “estimates,” “projects,”
“predicts,” “potential” and similar expressions (including the
negative thereof) intended to identify forward-looking statements.
Forward-looking statements contained in this press release,
include, but are not limited to, statements about: (i.) the timing
of the commencement of a Phase 2 clinical trial evaluating SAN711;
(ii.) the likelihood of success of such clinical trial; (iii.) the
prospects for FDA approval of SAN711 for essential tremor; and
(iv.) the success of any efforts to commercialize SAN711.
Forward-looking statements are subject to known and unknown risks,
uncertainties, assumptions and other factors that may cause our
actual results, performance or achievements to differ materially
and adversely from those anticipated or implied by our
forward-looking statements. Such risks, uncertainties, assumptions
and other factors include, but are not limited to: the timing,
enrollment and results of ongoing and future clinical trials and
our ability to continue to stay in compliance with applicable laws
and regulations. Given these risks and uncertainties, you should
not place undue reliance on these forward-looking statements. For a
discussion of these and other risks, uncertainties, assumptions and
other factors that may cause our actual results, performance or
achievements to differ, please refer to our quarterly report on
Form 10-Q for the period ended September 30, 2024 filed with the
Securities and Exchange Commission on November 7, 2024, as well as
our subsequent filings with the Securities and Exchange Commission
from time to time. The forward-looking statements contained herein
are made as of the date hereof, and we undertake no obligation to
update them after this date, except as required by law.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20241126842206/en/
Investor Contact: Acadia Pharmaceuticals Inc. Al Kildani (858)
261-2872 ir@acadia-pharm.com
Media Contact: Acadia Pharmaceuticals Inc. Deb Kazenelson (818)
395-3043 media@acadia-pharm.com
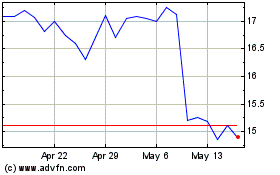
Acadia Pharmaceuticals (NASDAQ:ACAD)
Historical Stock Chart
From Jan 2025 to Feb 2025
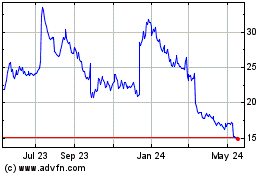
Acadia Pharmaceuticals (NASDAQ:ACAD)
Historical Stock Chart
From Feb 2024 to Feb 2025