As filed with the
Securities and Exchange Commission on November 18, 2024.
Registration Statement No. 333-
UNITED STATES
SECURITIES AND EXCHANGE
COMMISSION
Washington, D.C.
20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES
ACT OF 1933
Applied DNA Sciences, Inc.
(Exact name of Registrant
as specified in its charter)
| Delaware |
|
7380 |
|
59-2262718 |
(State
or other jurisdiction of
incorporation
or organization) |
|
(Primary
Standard Industrial
Classification
Code Number) |
|
(I.R.S.
Employer
Identification
Number) |
50 Health Sciences
Drive
Stony Brook, New
York 11790
631-240-8800
(Address, including
zip code, and telephone number, including area code, of Registrant’s principal executive offices)
James A. Hayward,
Ph.D., Sc.D.
Chairman, Chief Executive Officer and President
Applied DNA Sciences, Inc.
50 Health Sciences Drive
Stony Brook, New York 11790
631-240-8801
(Name, address,
including zip code, and telephone number, including area code, of agent for service)
Copies to:
Merrill M. Kraines
Todd Kornfeld
McDermott Will &
Emery LLP
One Vanderbilt Avenue
New York, New York
10017-3852
(212)
547-5616
Approximate date of commencement of
proposed sale to the public:
From time to time after this Registration
Statement becomes effective.
If
any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under
the Securities Act of 1933, check the following box. x
If
this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please
check the following box and list the Securities Act registration statement number of the earlier effective registration statement
for the same offering. ¨
If
this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and
list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If
this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and
list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check
mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or
an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller
reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer |
|
¨ |
|
Accelerated filer |
|
¨ |
| |
|
|
|
| Non-accelerated filer |
|
x |
|
Smaller reporting company |
|
x |
| |
|
|
|
| |
|
|
|
Emerging growth company |
|
¨ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ¨
The Registrant
hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant
shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance
with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on
such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The
information contained in this prospectus is not complete and may be changed. The selling stockholders named in this prospectus may not
sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus
is not an offer to sell these securities and we are not soliciting offers to buy these securities in any jurisdiction where such offer
or sale is not permitted.
| PRELIMINARY
PROSPECTUS |
|
SUBJECT
TO COMPLETION |
|
DATED
NOVEMBER 18, 2024 |
Up to 20,312,500
shares of Common Stock underlying the Series C Warrants
Up to 20,312,500
shares of Common Stock underlying the Series D Warrants
Up to 1,015,625
shares of Common Stock underlying the Placement Agent Warrants

Applied
DNA Sciences, Inc.
This prospectus relates
to the resale from time to time, by the selling stockholders (the “Selling Stockholders”) identified in this prospectus under
the caption “Selling Stockholders,” of up to an aggregate of 41,640,625 shares of common stock, par value $0.001 per share
(the “Common Stock”), which the selling stockholders (the “Selling Stockholders”) may acquire upon the exercise
of outstanding warrants, consisting of (i) 20,312,500 Series C Warrants (the “Series C Warrants”), (ii) 20,312,500
Series D Warrants (“Series D Warrants”, and, together with the Series C Warrants, the “Series Warrants”)
and (iii) 1,015,625 Placement Agent Warrants (“Placement Agent Warrants”, and together with the Series Warrants,
the “Private Placement Warrants”).
We issued the Series Warrants
to the Selling Stockholders in a private placement concurrent with a registered direct offering (the “Offering”) of 19,247,498
shares of Common Stock and pre-funded warrants (the “Pre-Funded Warrants”) to purchase 1,065,002 shares of Common Stock.
We issued the Placement Agent Warrants to the Selling Stockholders pursuant to that certain engagement letter dated August 23, 2024,
by and between the Company and Craig-Hallum Capital Group LLC (“Craig Hallum”).
The exercisability
of the Private Placement Warrants will be available only upon receipt of such stockholder approval (the “Warrant Stockholder Approval”)
as may be required by the applicable rules and regulations of The Nasdaq Stock Market LLC. Each Series C Warrant has an exercise
price of $0.32 per share of Common Stock, will become exercisable upon the first trading day (the “Stockholder Approval Date”)
following the Company’s notice to warrantholders of Warrant Stockholder Approval, and will expire on the five-year anniversary
of the Stockholder Approval Date. Each Series D Warrant has an exercise price of $0.32 per share of Common Stock, will become exercisable
upon the Stockholder Approval Date, and will expire on the 18-month anniversary of the Stockholder Approval Date. Each Placement Agent
warrant has an exerice price of $0.32, will become exercisable upon the Stockholder Approval date and will expire on October 30,
2029.
The closing of the
issuance and sale of the Private Placement Warrants, Common Stock and Pre-Funded Warrants was consummated on October 31, 2024.
The Selling Stockholders
of the securities and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their securities
covered hereby on the principal trading market or any other stock exchange, market or trading facility on which the securities are traded
or in private transactions. These sales may be at fixed or negotiated prices. See “Plan of Distribution” in this prospectus
for more information. We will not receive any proceeds from the resale or other disposition of the Common Stock by the Selling Stockholders.
However, we will receive the proceeds of any cash exercise of the Private Placement Warrants. See “Use of Proceeds” beginning
on page 13 and “Plan of Distribution” beginning on page 17 of this prospectus for more information.
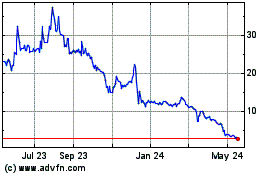
Our Common Stock is listed on Nasdaq under the symbol “APDN.”
On November 15, 2024, the last reported sale price of our Common Stock was $0.16 per share.
We are a “smaller
reporting company,” as defined under the federal securities laws and, as such, have elected to comply with certain reduced reporting
requirements for this prospectus and may elect to do so in future filings. See the section titled “Implications of Being a Smaller
Reporting Company.”
Investing
in our securities involves a high degree of risk. See “Risk Factors”
beginning on page 8 of this prospectus and under similar headings in the other documents that are incorporated by reference into
this prospectus for a discussion of information that should be considered in connection with an investment in our securities.
Neither the Securities
and Exchange Commission (“SEC”) nor any state securities commission has approved or disapproved of these securities or passed
upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense. The securities are not being
offered in any jurisdiction where the offer is not permitted.
The date of this prospectus
is , 2024.
TABLE OF CONTENTS
Prospectus
ABOUT
THIS PROSPECTUS
The
information contained in this prospectus is not complete and may be changed. You should rely only on the information provided in or incorporated
by reference in this prospectus, or in a related free writing prospectus, or documents to which we otherwise refer you. We have not authorized
anyone else to provide you with different information.
We have not authorized
any dealer, agent or other person to give any information or to make any representation other than those contained or incorporated by
reference in this prospectus or any related free writing prospectus. You must not rely upon any information or representation not contained
or incorporated by reference in this prospectus or any related free writing prospectus. This prospectus and any related free writing
prospectus, if any, do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the registered
securities to which they relate, nor do this prospectus and any related free writing prospectus, if any, constitute an offer to sell
or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation
in such jurisdiction. You should not assume that the information contained in this prospectus and any related free writing prospectus,
if any, is accurate on any date subsequent to the date set forth on the front of such document or that any information we have incorporated
by reference is correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus and
any related free writing prospectus is delivered or securities are sold on a later date.
We have not done anything
that would permit this offering or possession or distribution of this prospectus or any free writing prospectus in any jurisdiction where
action for that purpose is required, other than in the United States. You are required to inform yourself about and to observe any restrictions
relating as to this offering and the distribution of this prospectus and any such free writing prospectus outside the United States.
We further note that
the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated
by reference in this prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for the
purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant
to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations,
warranties and covenants should not be relied on as accurately representing the current state of our affairs.
The SEC allows us
to “incorporate by reference” into this prospectus the information in documents we file with it, which means that we can
disclose important information to you by referring you to those documents. The information incorporated by reference is considered to
be a part of this prospectus, and information that we file later with the SEC will automatically update and supersede this information.
Any statement contained in any document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified
or superseded for purposes of this prospectus to the extent that a statement contained in or omitted from this prospectus or any accompanying
prospectus supplement, or in any other subsequently filed document which also is or is deemed to be incorporated by reference herein,
modifies or supersedes such statement. Any such statement so modified or superseded shall not be deemed, except as so modified or superseded,
to constitute a part of this prospectus.
PROSPECTUS
SUMMARY
This summary highlights
certain information about us, this offering and information appearing elsewhere in this prospectus and in the documents we incorporate
by reference in this prospectus. This summary is not complete and does not contain all of the information that you should consider before
investing in our securities. After you carefully read this summary, to fully understand our Company and this offering and its consequences
to you, you should read this entire prospectus and any related free writing prospectus authorized by us, including the information referred
to under the heading “Risk Factors” in this prospectus beginning on page 8, and any related free writing prospectus,
as well as the other documents that we incorporate by reference into this prospectus, including our financial statements and the notes
to those financial statements, which are incorporated herein by reference from our Annual
Report on Form 10-K for the year ended September 30, 2023, filed December 7, 2023, as amended
on January 26, 2024, and our Quarterly
Reports on Form 10-Q for the three month periods ended December 31, 2023, filed on February 8, 2024, March 31,
2024, filed on May 10,
2024 and June 30, 2024, filed on August 8,
2024, respectively. Please read “Where You Can Find More Information” on page 23 of this prospectus.
In this prospectus,
unless context requires otherwise, references to “we,” “us,” “our,” or “the Company”
refer to Applied DNA Sciences, Inc., a Delaware corporation and its consolidated subsidiaries. Our trademarks currently used in
the United States include Applied DNA Sciences®, SigNature® molecular tags, SigNature® T molecular tags, fiberTyping®,
SigNify®, Beacon®, CertainT®, LineaDNA®, Linea RNAPTM, Linea™ COVID-19 Diagnostic Assay Kit, safeCircle®
COVID-19 testing and TR8TM pharmacogenetic testing. We do not intend our use or display of other companies’
trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies. All trademarks,
service marks and trade names included in this prospectus are the property of the respective owners.
Applied DNA Sciences, Inc.
Introduction
We are a biotechnology
company developing and commercializing technologies to produce and detect deoxyribonucleic acid (“DNA”) and ribonucleic acid
(“RNA”). Using polymerase chain reaction (“PCR”) to enable the production and detection of DNA and RNA, we currently
operate in three primary business markets: (i) the enzymatic manufacture of synthetic DNA for use in the production of nucleic acid-based
therapeutics (including biologics and drugs), as well as the development and sale of a proprietary RNA polymerase (“RNAP”)
for use in the production of messenger RNA (“mRNA”) therapeutics (“Therapeutic DNA Production Services”); (ii) the
detection of DNA and RNA in molecular diagnostics and genetic testing services (“MDx Testing Services”); and (iii) the
manufacture and detection of DNA for industrial supply chains and security services (“DNA Tagging and Security Products and Services”).
Our current growth
strategy is to primarily focus our resources on the further development, commercialization, and customer adoption of our Therapeutic
DNA Production Services, including the expansion of our contract development and manufacturing operation (“CDMO”) for the
manufacture of synthetic DNA for use in the production of nucleic acid-based therapies.
We will continue to
update our business strategy and monitor the use of our resources regarding our various business segments. The Company’s management
is currently engaged in a strategic review of the Company’s business segments that may result in the closure or divestiture of
the Company’s MDx Testing Services and/or DNA Tagging and Security Products and Services, as well as workforce reductions and potential
management changes. The terms and structure of any possible closure or divestiture have not been determined or agreed to by the Company’s
Board of Directors. Although the purpose of any closure or divestiture would be to reduce the Company’s expenses and effectuate
cost savings, it is possible that there may be restructuring costs. We expect that based on available opportunities and our beliefs regarding
future opportunities, we will continue to modify and refine our business strategy.
Therapeutic DNA
Production Services
Through our LineaRX, Inc.
subsidiary we are developing and commercializing our Linea DNA and Linea IVT platforms for the manufacture of synthetic DNA and proprietary
enzymes for use in the production of nucleic acid-based therapeutics.
Linea DNA Platform
Our Linea DNA platform
is our core enabling technology, and enables the rapid, efficient, and large-scale cell-free manufacture of high-fidelity DNA sequences
for use in the manufacturing of a broad range of nucleic acid-based therapeutics. The Linea DNA platform enzymatically produces a linear
form of DNA we call “LineaDNA” that is an alternative to plasmid-based DNA manufacturing technologies that have supplied
the DNA used in biotherapeutics for the past 40 years.
As of the third quarter
of calendar year 2024, there were 4,099 gene, cell and RNA therapies in development from preclinical through pre-registration stages,
almost all of which use DNA in their manufacturing process. (Source: ASGCT Gene, Cell & RNA Therapy Landscape: Q3 2024 Quarterly
Report ). Due to what we believe are the Linea DNA platform’s numerous advantages over legacy nucleic acid-based therapeutic
manufacturing platforms, we believe this large number of therapies under development represents a substantial market opportunity for
the Linea DNA platform to supplant legacy manufacturing methods in the manufacture of nucleic acid-based therapies although no assurance
can be given that we will be successful in exploiting this market opportunity.
We believe our Linea
DNA platform holds several important advantages over existing cell-based plasmid DNA manufacturing platforms. Plasmid-based DNA manufacturing
is based on the complex, costly and time-consuming biological process of amplifying DNA in living bacterial cells. Once amplified, the
DNA must be separated from the living cells and other process contaminants via multiple rounds of purification, adding further complexity
and costs. Unlike plasmid-based DNA manufacturing, the Linea DNA platform does not require living cells and instead amplifies DNA via
the enzymatic process of PCR. The Linea DNA platform is simple and can rapidly produce very large quantities of DNA without the need
for complex purification steps.
We believe the key
advantages of the Linea DNA platform include:
| |
· |
Speed –
Production of Linea DNA can be measured in terms of hours, not days and weeks as is the case with plasmid-based DNA manufacturing
platforms. |
| |
· |
Scalability
– Linea DNA production takes place on efficient bench-top instruments, allowing for rapid scalability in a minimal footprint. |
| |
|
|
| |
· |
Purity –
DNA produced via PCR is pure, resulting in only large quantities of only the target DNA sequence. Unwanted DNA sequences such as
the plasmid backbone and antibiotic resistance genes, inherent to plasmid DNA, are not present in Linea DNA. |
| |
|
|
| |
· |
Simplicity
– The production of Linea DNA is streamlined relative to plasmid-based DNA production. Linea DNA requires only four primary
ingredients, does not require living cells or complex fermentation systems and does not require multiple rounds of purification. |
| |
|
|
| |
· |
Flexibility
– DNA produced via the Linea DNA platform can be easily chemically modified to suit specific customer applications. In addition,
the Linea DNA platform can produce a wide range of complex DNA sequences that are difficult to produce via plasmid-based DNA production
platforms. These complex sequences include inverted terminal repeats and long homopolymers such as polyadenylation sequences (poly
(A) tail) important for gene therapy and mRNA therapies, respectively. |
Preclinical studies
conducted by the Company have shown that Linea DNA is substitutable for plasmid DNA in numerous nucleic acid-based therapies, including:
| |
· |
DNA templates
to produce RNA, including mRNA therapeutics; |
| |
· |
adoptive
cell therapy (CAR-T) manufacturing; and |
| |
· |
homology-directed
repair (HDR)-mediated gene editing. |
Further, we believe
that Linea DNA is also substitutable for plasmid DNA in the following nucleic acid-based therapies:
| |
· |
viral vector
manufacturing for in vivo and ex vivo gene editing; |
| |
· |
clustered
regularly interspaced short palindromic repeats-mediated gene therapy; and |
| |
· |
non-viral
gene therapy. |
Linea IVT Platform
The number of mRNA
therapies under development is growing at a rapid rate, thanks in part to the success of the mRNA COVID-19 vaccines. mRNA therapeutics
are produced via a process called in vitro transcription (“IVT”) that requires DNA as a starting material. As of
the third quarter of calendar 2024, there were over 450 mRNA therapies under development, with the majority of these therapies (67%)
in the preclinical stage (Source: ASGCT Gene, Cell & RNA Therapy Landscape: Q2 2024 Quarterly Report). The Company believes
that the mRNA market is in a nascent stage that represents a large growth opportunity for the Company via the production and supply of
DNA critical starting materials and RNAP to produce mRNA therapies.
In August 2022,
the Company launched DNA IVT templates manufactured via its Linea DNA platform that have resulted in evaluations of the Company’s
IVT templates by numerous therapeutic developers and CDMOs in the United States and the Asia-Pacific. In addition, the Company’s
IVT templates are under late-stage evaluations by two therapeutic developers and one CDMO for use as templates for the production of
mRNA intended for clinical use. However there can be no assurance that related contracts will be entered into. In response to this demand,
the continued growth of the mRNA therapeutic market, and the unique abilities of the Linea DNA platform, the Company acquired Spindle
in July 2023 to potentially increase its mRNA-related total addressable market (“TAM”).
Through our acquisition
of Spindle, we launched our Linea IVT platform in July 2023, which combines Spindle’s proprietary high-performance RNAP, now
marketed by the Company as Linea RNAP, with our enzymatically produced Linea DNA IVT templates. We believe the Linea IVT platform enables
our customers to make better mRNA, faster. Based on data generated by the Company, we believe the integrated Linea IVT platform offers
the following advantages over conventional mRNA production to therapy developers and manufacturers:
| |
· |
The prevention
or reduction of double stranded RNA (“dsRNA”) contamination resulting in higher target mRNA yields with the potential
to reduce downstream processing steps. dsRNA is a problematic immunogenic byproduct produced during conventional mRNA manufacture; |
| |
· |
delivery
of IVT templates in as little as 14 days for milligram scale and 30 days for gram scale; |
| |
· |
reduced mRNA
manufacturing complexities; and |
| |
· |
potentially
enabling mRNA manufactures to produce mRNA drug substance in less than 45 days. |
According to the Company’s
internal modeling, the ability to sell both Linea DNA IVT templates and Linea RNAP under the Linea IVT platform potentially increases
the Company’s mRNA-related TAM by approximately 3-5x as compared to selling Linea DNA IVT templates alone, while also providing
a more competitive offering to the mRNA manufacturing market. Currently, Linea RNAP is produced for the Company under an ISO 13485 quality
system by Alphazyme, LLC a third-party CDMO located in the United States. The Company recently completed manufacturing process development
work on its Linea RNAP to increase the production scale of the enzyme and reduce unit costs.
Manufacturing Scale-up
The Company plans
to offer several quality grades of Linea DNA, each of which will have different permitted uses.
| Quality
Grade |
Permitted
Use |
Company
Status |
| GLP |
Research
and pre-clinical discovery |
Currently
available |
| GMP
for Starting Materials |
DNA
critical starting materials for the production of mRNA therapies |
Planned
availability in Q4 of CY2024 |
| GMP |
DNA
biologic, drug substance and/or drug product |
Planned
availability second half of CY 2025 (1) |
| |
(1) |
Dependent
on the availability of future financing. |
|
| |
|
|
|
|
We are currently manufacturing
Linea DNA pursuant to Good Laboratory Practices (“GLP”) and, are in the final stages of creating a fit for purpose manufacturing
facility within our current Stony Brook, NY laboratory space capable of producing Linea DNA IVT templates under Good Manufacturing Practices
(“GMP”) suitable for use as a critical starting material for clinical and commercial mRNA therapeutics, with an anticipated
completion date in the fourth quarter of calendar year 2024 (“GMP Site 1”). We also plan to offer additional capacity for
Linea DNA IVT templates as well as capacity for Linea DNA materials manufactured under GMP suitable for use as, or incorporation into,
a biologic, drug substance and/or drug product, with availability expected during the second half of calendar year 2025, dependent upon
the availability of future funding (“GMP Site 2”). GMP is a quality standard used globally and by the U.S. Food and Drug
Administration (“FDA”) to ensure pharmaceutical quality. Drug substances are the pharmaceutically active components of drug
products.
Segment Business
Strategy
Our business strategy
for our Therapeutic DNA Production Services is to capitalize upon the rapid growth of mRNA therapies in the near term via our planned
near term future availability of Linea DNA IVT templates manufactured under GMP at our GMP Site 1, while at the same time laying the
basis for additional clinical and commercial applications of Linea DNA with our future planned availability of Linea DNA manufactured
under GMP suitable for use as, or incorporation into, a biologic, drug substance and/or drug product at planned GMP Site 2. Planned GMP
Site 2 may also be used for additional Linea DNA IVT template manufacturing if customer demand exceeds capacity of GMP Site 1. Our current
plan is: (i) through our Linea IVT platform and planned near term future GMP manufacturing capabilities for IVT templates at GMP
Site 1 to secure commercial-scale supply contracts with clinical and commercial mRNA and/or self-amplifying mRNA (“sa-RNA”)
manufacturers for Linea DNA IVT templates and/or Linea RNAP as critical starting materials; (ii) to utilize our current GLP production
capacity for non-IVT template applications to secure supply and/or development contracts with pre-clinical therapy developers that use
DNA in their therapy manufacturing, and (iii) upon our development of our planned future Linea DNA production under GMP suitable
for use as, or incorporation into, a biologic, drug substance and/or drug product at planned GMP Site 2, to convert existing and new
Linea DNA customers into large-scale supply contracts to supply Linea DNA for clinical and commercial use as, or incorporation into,
a biologic, drug substance and/or drug product in a wide range of nucleic acid therapies. Until we complete our GMP Site 1 to produce
DNA critical starting materials (DNA IVT templates) for mRNA manufacturing, we will not be able to realize significant revenues from
this business. We estimate the remaining capital expenditure (“CAPEX”) costs to creating GMP Site 1 will be less than $0.30
million. If we were to expand our facilities to enable GMP production of Linea DNA for use as, or incorporation, into a biologic, drug
substance and/or drug product as planned for GMP Site 2, the additional CAPEX may be up to approximately $10 million which would require
additional funding. We are currently building GMP Site 1 within our existing laboratory space. We anticipate that a GMP Site 2 would
require us to acquire additional space.
MDx Testing
Services
Through Applied DNA
Clinical Labs, LLC (“ADCL”), we leverage our expertise in DNA and RNA detection via PCR to provide and develop clinical molecular
diagnostics and genetic (collectively “MDx”) Testing Services. ADCL is a New York State Department of Health (“NYSDOH”)
clinical laboratory improvement amendments (CLIA)-certified laboratory which is currently permitted for virology and genetics (molecular).
In providing MDx Testing Services, ADCL employs its own or third-party molecular diagnostic tests.
We have successfully
internally validated our pharmacogenomics testing services (the “PGx Testing Services”). Our PGx Testing Services utilizes
a 120-target PGx panel test to evaluate the unique genotype of a specific patient to help guide the patient’s healthcare provider
in making individual drug therapy decisions. Our PGx Testing Services are designed to interrogate DNA targets on over 33 genes and provide
genotyping information relevant to certain cardiac, mental health, oncology, and pain management drug therapies.
On June 12, 2024
we received full approval from NYSDOH for our PGx Testing Services. Recently published studies show that population-scale PGx enabled
medication management can significantly reduce overall population healthcare costs, reduce adverse drug events, and increase overall
population wellbeing. These benefits can result in significant cost savings to large entities and self-insured employers, the latter
accounting for approximately 65% of all U.S. employers in 2022.We plan to leverage our PGx Testing Services to provide PGx testing services
to large entities, self-insured employers and healthcare providers, as well as concierge healthcare providers.
On September 11,
2024, we announced that ADCL has launched an expansion of its clinical testing services for the detection of Mpox (formerly monkeypox)
to include testing for both Mpox Clade I and Clade II. The launch of the expanded Mpox testing service comes after ADCL’s interaction
with relevant regulatory bodies, including the New York State Department of Health (NYSDOH) and the U.S. Food and Drug Administration
(FDA).The Company believes that ADCL will be able to support New York and other states’ response to the threat of Mpox. ADCL’s
Linea Mpox Virus 1.0 Assay was previously approved as a laboratory-developed test for the detection of Mpox Clade II by NYSDOH in September 2022.
In August 2024, ADCL conducted additional validation testing showing the Assay can also detect the genetic sequence of Mpox Clade
I, which is the subject of the World Health Organization’s (WHO) August 14, 2024 declaration of a public health emergency
of international concern. ADCL will provide the testing service from its CLEP/CLIA molecular diagnostics laboratory in Stony Brook, N.Y.
There can be no assurance that we will be able to generate revenue and and profits from Mpox testing.
Historically, the
majority of our revenue attributable to our MDx Testing Services has been derived from our safeCircle® COVID-19 testing solutions,
for which testing demand has significantly declined commencing in our fiscal third quarter of 2023, resulting in substantially reduced
revenues. We expect future demand for COVID-19 testing to continue to be reduced and we may terminate COVID-19 testing services in the
future.
DNA Tagging and
Security Products and Services
By leveraging our
expertise in both the manufacture and detection of DNA via PCR, our DNA Tagging and Security Products and Services allow our customers
to use non-biologic DNA tags manufactured on our Linea DNA platform to mark objects in a unique manner and then identify these objects
by detecting the absence or presence of the DNA tag. The Company’s core DNA Tagging and Security Products and Services, which are
marketed collectively as a platform under the trademark CertainT®, include:
| |
· |
SigNature®
Molecular Tags, which are short non-biologic DNA taggants produced by the Company’s Linea DNA platform, provide a methodology
to authenticate goods within large and complex supply chains with a focus on cotton, nutraceuticals and other products. |
| |
· |
SigNify®
portable DNA readers and SigNify consumable reagent test kits provide definitive real-time authentication of the Company’s
DNA tags in the field. |
| |
· |
fiberTyping®
and other product genotyping services use PCR-based DNA detection to determine a cotton species or cultivar, via a product’s
naturally occurring DNA sequence for the purposes of product provenance authentication. |
| |
· |
Isotopic
analysis testing services, provided in partnership with third-party labs, use cotton’s carbon, hydrogen and oxygen elements
to indicate origin of its fiber through finished goods. |
To date, our largest
commercial application for our DNA Tagging and Security Products and Services is in the tracking and provenance authentication of cotton.
The Uyghur Forced
Labor Prevention Act (“UFLPA”) signed into law on December 23, 2021 establishes that any goods mined, produced, or manufactured
wholly or in part in the Xinjiang Uyghur Autonomous Region (“XUAR”) of the People’s Republic of China are not entitled
to entry to the United States. On June 17, 2022, the UFLPA additionally listed DNA tagging and isotopic analysis as evidence that
importers may use to potentially prove that a good did not originate in XUAR. Recently, in July of 2024, the Company announced a
multi-year commercialization agreement for its CertainT platform with Indus Group, a multinational apparel/textile manufacturing and
sourcing company.
Our business plan
is to leverage consumer and governmental awareness for product traceability to expand our existing partnerships and seek new partnerships
for our DNA Tagging and Security Products and Services with a focus on cotton.
Recent Developments
Nasdaq Minimum Bid Price Requirement
Deficiency Notification
On November 12,
2024, the Company received written notice (the “Notification Letter”) from the Listing Qualifications Department of The Nasdaq
Stock Market LLC (“Nasdaq”) notifying the Company that it is not in compliance with the minimum bid price requirements set
forth in Nasdaq Listing Rule 5550(a)(2) for continued listing on The Nasdaq Capital Market. Nasdaq Listing Rule 5550(a)(2) requires
listed securities to maintain a minimum bid price of $1.00 per share, and Nasdaq Listing Rule 5810(c)(3)(A) provides that a
failure to meet the minimum bid price requirement exists if the deficiency continues for a period of thirty (30) consecutive business
days (collectively, the “Bid Price Rule”). Based on the closing bid price of the Company’s Common Stock for the thirty-one
(31) consecutive business days from September 27, 2024 to November 11, 2024, the Company no longer meets the requirements of
the Bid Price Rule.
The Notification Letter
does not impact the Company’s listing on The Nasdaq Capital Market at this time. The Notification Letter states that the Company
has 180 calendar days, or until May 12, 2025, to regain compliance with the Bid Price Rule. To regain compliance, the bid price
of the Company’s Common Stock must have a closing bid price of at least $1.00 per share for a minimum of ten (10) consecutive
business days, with a longer period potentially required by the staff of Nasdaq (the “Staff”). If the Company does not regain
compliance with the Bid Price Rule by May 12, 2025, the Company may be eligible for an additional 180 calendar day compliance
period. To qualify, the Company would be required to meet the continued listing requirement for market value of publicly held shares
and all other initial listing standards for The Nasdaq Capital Market, with the exception of the Bid Price Rule, and would need to provide
written notice of its intention to cure the deficiency during the second compliance period, by effecting a reverse stock split, if necessary,
no later than ten (10) business days prior to May 12, 2025.
However, if it appears
to the Staff that the Company will not be able to cure the deficiency, or if the Company is otherwise not eligible, Nasdaq would notify
the Company that its securities would be subject to delisting. In the event of such a notification, the Company may appeal the Staff’s
determination to delist its securities, but there can be no assurance the Staff would grant the Company’s request for continued
listing.
Pursuant to the securities
purchase agreement entered into in connection with the Offering (the “Purchase Agreement”), the Company is required to effect
a reverse stock split of its outstanding shares of Common Stock if, at any time after the Stockholder Approval Date, it is not in compliance
with Nasdaq’s Bid Price Rule and has received a deficiency letter from the Listing Qualifications Department of The Nasdaq
Stock Market LLC (the “Reverse Stock Split”). The Company must effect the Reverse Stock Split within 30 days of the Stockholder
Approval Date; provided that if within such 30 day period the Company regains compliance with the Bid Price Rule, the Company shall have
no obligation to effect the Reverse Stock Split. The Company intends to implement a reverse stock split of its outstanding securities
to regain compliance with the Bid Price Rule and to comply with the provisions of the Purchase Agreement.
Amendment to Series A Warrant
As previously disclosed
on our current report on Form 8-K filed on May 29, 2024 we closed on such date a public offering (the “May 2024
Offering”) of common stock and warrants, including 9,230,769 series A common stock purchase warrants (“May 2024 Series A
Warrants”) and 9,230,769 series B common stock purchase warrants (“May 2024 Series B Warrants”, and, with
the May 2024 Series A Warrants, the “May 2024 Series Warrants”), with Craig-Hallum and Laidlaw &
Company (UK) Ltd. (“Laidlaw”) as placement agents. As part of the May 2024 Offering, the Company entered into a
Placement Agency Agreement, dated May 28, 2024, with Craig-Hallum and Laidlaw (the “May 2024 Placement Agency Agreement”).
Subject to certain
exceptions, the Series A Warrants provide for an adjustment to the exercise price and number of shares underlying the Series A
Warrants upon the Company’s issuance of Common Stock or Common Stock equivalents at a price per share that is less than the exercise
price of the Series A Warrants (the “Price Reset Mechanism”).
On October 30,
2024, the Company and certain holders of the May 2024 Series A Warrants entered into an amendment to such holders’ May 2024
Series A Warrants (the “Warrant Amendment”), pursuant to which the Price Reset Mechanism became subject to a floor equal
to $0.20.
In connection with
the Offering, the Price Reset Mechanism in the May 2024 Series A Warrants was triggered, which resulted in the number of shares
of Common Stock issuable upon exercise of the May 2024 Series A Warrants increasing from 9,230,769 to 91,890,698. The exercise
price of the May 2024 Series A Warrants was adjusted from $1.99 per share to $0.20 per share with respect to the May 2024
Series A Warrants amended by the Warrant Amendment, and to $0.19 with respect to the May 2024 Series A Warrants not amended
by the Warrant Amendment.
Company Information
We are a Delaware
corporation, which was initially formed in 1983 under the laws of the State of Florida as Datalink Systems, Inc. In 1998, we reincorporated
in the State of Nevada, and in 2002, we changed our name to our current name, Applied DNA Sciences, Inc. On December 17, 2008,
we reincorporated from the State of Nevada to the State of Delaware.
Our corporate headquarters
are located at the Long Island High Technology Incubator at Stony Brook University in Stony Brook, New York, where we have established
laboratories for the manufacture and detection of nucleic acids (DNA and mRNA) to support our various business units. In addition, this
location also houses our NYSDOH CLEP-permitted, Clinical Laboratory Improvement Amendments (“CLIA”)-certified clinical laboratory
where we perform MDx Testing Services. The mailing address of our corporate headquarters is 50 Health Sciences Drive, Stony Brook, New
York 11790, and our telephone number is (631) 240-8800.
Implications of Being a Smaller Reporting
Company
We are a “smaller
reporting company” as defined in the Securities Exchange Act of 1934, as amended, or the Exchange Act, and have elected to take
advantage of certain of the scaled disclosures available to smaller reporting companies. We will continue to be a “smaller reporting
company” until we have $250 million or more in public float (based on our Common Stock) measured as of the last business day
of our most recently completed second fiscal quarter or, in the event we have no public float (based on our Common Stock) or a public
float (based on our Common Stock) that is less than $700 million, annual revenues of $100 million or more during the most recently
completed fiscal year.
We may choose to take
advantage of some, but not all, of these exemptions. We have taken advantage of reduced reporting requirements in this prospectus. Accordingly,
the information contained herein may be different from the information you receive from other public companies in which you hold stock.
THE
OFFERING
| Common
Stock offered by us |
41,640,625
shares of Common Stock issuable upon exercise of the Private Placement Warrants (subject to Warrant
Stockholder Approval). |
| Use
of proceeds |
We
will not receive any proceeds from the Common Stock offered by the Selling Stockholders under this prospectus. However, we will receive
the proceeds of any cash exercise of the Private Placement Warrants. We intend to use the net proceeds from any cash exercise of
the Private Placement Warrants for further development of our Therapeutic DNA Production Services, as well as for working capital
and general corporate purposes. See “Use of Proceeds.” |
| Market
for Common Stock |
Our Common Stock is listed on The Nasdaq Capital Market under the symbol
“APDN.” On November 15, 2024, the last reported sale price of our Common Stock was $0.16 per share. |
| Risk
Factors |
See
“Risk Factors” beginning on page 8 and the other information included in this prospectus
for a discussion of factors you should carefully consider before investing in our securities. |
The
number of shares of our Common Stock to be outstanding after this offering is based on the 50,896,710
shares of our Common Stock outstanding as of November 15, 2024, and excludes the following:
| · |
108,176 shares
of Common Stock issuable upon exercise of options outstanding as of November 15, 2024, with a weighted average exercise price
of $185.48 per share; |
| · |
96,083,181
shares of Common Stock issuable upon exercise of warrants outstanding as of November 15, 2024, with a weighted average exercise
price of $0.43 per share (which includes an aggregate of 94,147,750 May 2024 Series A Warrants (reflecting the the Price
Reset Mechanism being triggered by the Offering) and May 2024 Series B Warrants, of which 2,257,052 have an alternative
cashless exercise mechanism representing the right to receive 3 shares of Common Stock for each warrant, which, if exercised, would
result in 6,771,156 shares of Common Stock being issued); |
| · |
41,640,625
shares of Common Stock issuable upon exercise of the Private Placement Warrants, whose exercise
is subject to Warrant Stockholder Approval, of which 20,312,500 have an alternative cashless exercise mechanism representing the
right to receive 1 share of Common Stock for each warrant; and |
| · |
269,069
shares of Common Stock reserved for future grant or issuance as of November 15, 2024, under our equity incentive plan. |
Unless otherwise indicated,
this prospectus reflects and assumes no exercise of outstanding options and warrants.
RISK
FACTORS
Investing in our
securities involves a high degree of risk. In addition to the other information included or incorporated by reference in this prospectus,
you should carefully consider the risks described below and in the section titled “Risk Factors” in our Annual Report on
Form 10-K for our most recent fiscal year filed with the SEC, subsequent Quarterly Reports on Form 10-Q, any amendment or updates
thereto reflected in subsequent filings with the SEC, and in other reports we file with the SEC that are incorporated by reference herein,
before making an investment decision. The following risks are presented as of the date of this prospectus and we expect that these will
be updated from time to time in our periodic and current reports filed with the SEC, which will be incorporated herein by reference.
Please refer to these subsequent reports for additional information relating to the risks associated with investing in our securities.
The risks and uncertainties
described therein and below could materially adversely affect our business, operating results and financial condition, as well as cause
the value of our securities to decline. You may lose all or part of your investment as a result. You should also refer to the other information
contained in this prospectus, or incorporated by reference, including our financial statements and the notes to those statements, and
the information set forth under the caption “Special Note Regarding Forward-Looking Statements.” Our actual results could
differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks mentioned
below. Forward-looking statements included in this prospectus are based on information available to us on the date hereof, and all forward-looking
statements in documents incorporated by reference are based on information available to us as of the date of such documents. We disclaim
any intent to update any forward-looking statements. The risks described below and contained in our Annual Report on Form 10-K,
Quarterly Reports on Form 10-Q and in our other periodic reports are not the only ones that we face. Additional risks not presently
known to us or that we currently deem immaterial may also adversely affect our business operations.
Risks Related to our Business:
We may not successfully implement
our business strategies, including achieving our growth objectives.
We may not be able
to fully implement our business strategies or realize, in whole or in part within the expected time frames, the anticipated benefits
of our various growth or other initiatives. Our various business strategies and initiatives, including our growth, operational and management
initiatives and the development in particular of our Therapeutic DNA Production Services, are subject to business, economic and competitive
uncertainties and contingencies, many of which are beyond our control. The execution of our business strategy and our financial performance
will continue to depend in significant part our ability to obtain sufficient financing and on our executive management team and other
key management personnel, our ability to identify and complete suitable acquisitions, our executive management team’s ability to
execute new operational initiatives, and certain matters outside of our control. In addition, we may incur certain costs as we pursue
our growth, operational and management initiatives, and we may not meet anticipated implementation timetables or stay within budgeted
costs. As these initiatives are undertaken, we may not fully achieve our expected efficiency improvements or growth rates, or these initiatives
could adversely impact our customer retention, supplier relationships or operations. Also, our business strategies may change from time
to time in light of our ability to implement our business initiatives, competitive pressures, economic uncertainties or developments,
or other factors.
We may modify and refine our business
strategy, including possible divestures or closings.
Our management is
currently engaged in a strategic review of the Company’s business segments that may result in the divestiture or closure of the
Company’s MDx Testing Services and/or DNA Tagging and Security Products and Services, as well as workforce reductions and potential
management changes. The terms and structure of any possible divestiture or closure, including financial terms, have not been determined
or approved by our Board of Directors. Although the purpose of any divestiture or closure would be to reduce our expenses and effectuate
cost savings, it is possible that there may be related restructuring costs and the benefits of any divesture or closing may be less than
anticipated. The initial cash received from any divestiture, if any, may be limited, although the terms of a divesture may include
future royalties, earn-outs or similar terms, any of which could fail to be earned or received.
Stockholders may suffer substantial
dilution if certain provisions in the May 2024 Series Warrants are utilized.
On May 29, 2024,
we closed on such date the May 2024 Offering, which included the May 2024 Series A Warrants and May 2024 Series B
Warrants, pursuant to the May 2024 Placement Agency Agreement.
If the May 2024
Series B Warrants are exercised by way of an alternative cashless exercise, such exercising holder will receive three times the
number of shares of Common Stock they would receive in a cash exercise for each May 2024 Series B Warrant they exercise, without
any cash payment to us. In addition, the Series A Warrants and Series B Warrants each include a provision that
resets their exercise price in the event of a reverse split of our Common Stock, to a price equal to the lesser of (i) the then
exercise price and (ii) lowest volume weighted average price (VWAP) during the period commencing five trading days immediately preceding
and the five trading days commencing on the date we effect a reverse stock split in the future with a proportionate adjustment to the
number of shares underlying the applicable warrant,
In addition, and subject
to certain exemptions, if we sell, enter into an agreement to sell, or grant any option to purchase, or sell, enter into an agreement
to sell, or grant any right to reprice (excluding Exempt Issuances, as defined in the May 2024 Placement Agency Agreement), or otherwise
dispose of or issue (or announce any offer, sale, grant or any option to purchase or other disposition) any shares of common stock, at
an effective price per share less than the exercise price of the May 2024 Series A Warrants then in effect, the exercise price
of the May 2024 Series A Warrants will be reduced to the lower of such price or (i) $0.20 with respect to the May 2024
Series A Warrants as amended by the Warrant Amendment or (ii) the lowest volume weighted average price (VWAP) during the five
consecutive trading days immediately following such dilutive issuance or announcement thereof with respect to the the remaining May 2024
Series A Warrants not amended by the Warrant Amendment. The number of shares issuable upon exercise after the aforementioned mechanism
has been triggered will be proportionately adjusted such that the aggregate exercise price will remain unchanged.
In connection with
the Offering, the Price Reset Mechanism in the May 2024 Series A Warrants was triggered, which resulted in the number of shares
of Common Stock issuable upon exercise of the May 2024 Series A Warrants increasing from 9,230,769 to 91,890,698. The exercise
price of the May 2024 Series A Warrants was adjusted from $1.99 per share to $0.20 per share with respect to the May 2024
Series A Warrants amended by the Warrant Amendment, and to $0.19 with respect to the May 2024 Series A Warrants not amended
by the Warrant Amendment.
If any of the above provisions in the May 2024
Series Warrants are further utilized, our stockholders may suffer substantial dilution.
Stockholders may suffer substantial
dilution if certain provisions in the Series D Warrants are utilized.
If the Series D
Warrants are exercised by way of an alternative cashless exercise, assuming receipt of Warrant Stockholder Approval, such exercising
holder will receive one share of Common Stock for each share of Common Stock they would receive in a cash exercise for each May 2024
Series D Warrant they exercise, without any cash payment to us.
In addition, the Series D
Warrants include a provision that resets their exercise price in the event of a reverse split of our Common Stock, to a price equal to
the lesser of (i) the then exercise price and (ii) lowest volume weighted average price (VWAP) during the period commencing
five trading days immediately preceding and the five trading days commencing on the date we effect a reverse stock split in the future
with a proportionate adjustment to the number of shares underlying the Series D Warrants, subject to a floor of $0.0634.
If any of the above provisions in the Series Warrants
are utilized, our stockholders may suffer substantial dilution.
The exercisability
of the Private Placement Warrants is contingent upon us obtaining Warrant Stockholder Approval. If we do not obtain such Warrant Stockholder
Approval, the Series Warrants may never become exercisable.
The Private Placement
Warrants are not immediately exercisable, as their exercisability is contingent upon us obtaining Warrant Stockholder Approval. The Series Warrants
will become exercisable upon the Stockholder Approval Date and will expire on the five-year anniversary of such date with respect to
the Series C Warrants, and on the eighteen-month anniversary of such date with respect to the Series D Warrants. The Placement
Agent Warrants will become exercisable upon the Stockholder Approval Date and will expire on October 30, 2029. While we intend to
promptly seek Warrant Stockholder Approval for these mechanisms, there is no guarantee that it will ever be obtained. In the event that
we cannot obtain Warrant Stockholder Approval, the Series Warrants may never become exercisable. If we are unable to obtain
the Warrant Stockholder Approval, the Series Warrants will have no value.
We have agreed to
hold a special meeting of shareholders (which may also be at the annual meeting of shareholders) at the earliest practicable date after
the date hereof, but in no event later than ninety days after the closing of the offering, in order to obtain Warrant Stockholder Approval.
There is no guarantee we will be able to hold a special meeting within this timeframe, or at all. If we do not obtain Warrant Stockholder
Approval at the first meeting, we are obligated to call a meeting every ninety days thereafter to seek Warrant Stockholder Approval until
the earlier of the date on which Stockholder Approval is obtained or the Series Warrants are no longer outstanding.
There are a
large number of shares of Common Stock underlying our outstanding options and warrants and the sale of these shares may depress the market
price of our Common Stock and cause immediate and substantial dilution to our existing stockholders.
As
of November 15, 2024, we had 50,896,710 shares of Common Stock issued and outstanding, outstanding
options to purchase 108,176 shares of Common Stock, outstanding warrants to purchase 96,083,181 shares of Common Stock, and 269,069 shares
available for grant under our equity incentive plan. The issuance of shares upon exercise of our outstanding options and warrants will
cause immediate and substantial dilution to our stockholders and any sale thereof may depress the market price of our Common Stock.
There is substantial doubt relating
to our ability to continue as a going concern.
We have recurring
net losses, which have resulted in an accumulated deficit of $306,376,012 as of June 30, 2024. We have incurred a net loss of $3,774,563
for the nine months ended June 30, 2024. At June 30, 2024, we had cash and cash equivalents of $10,442,131. We have concluded
that these factors raise substantial doubt about our ability to continue as a going concern for one year from the issuance of the June 30,
2024 financial statements. We will continue to seek to raise additional working capital through public equity, private equity or debt
financings. If we fail to raise additional working capital, or do so on commercially unfavorable terms, it would materially and adversely
affect our business, prospects, financial condition and results of operations, and we may be unable to continue as a going concern. If
we seek additional financing to fund our business activities in the future and there remains substantial doubt about our ability to continue
as a going concern, investors or other financing sources may be unwilling to provide additional funding to us on commercially reasonable
terms, if at all.
There can be no assurance that that
a commercial demand for our Linea™ Mpox Virus Assay and/or mpox testing services will develop.
On September 11,
2024 the Company announced that after interactions with relevant regulatory bodies, including NYSDOH and U.S. FDA, it was launching clinical
testing services for both mpox clade I and clade II utilizing the Linea mpox Virus Assay (the “Assay”) in New York State
and in states that recognize New York’s CLEP/CLIA certification. To date, the Company has not performed clinical testing for mpox
clade I or clade II. Future commercial demand for the Assay and/or associated mpox testing services is based upon the unknown and unpredictable
future path of the mpox public health emergency. Currently, mpox disease prevalence (both clade I and clade II) is extremely low the
United States, resulting in minimal demand for clinical mpox testing. It is unknown whether a future commercial demand for the Assay
will develop.
We have received
written notice from Nasdaq that we are not in compliance with Nasdaq’s minimum bid price requirements and if we are unable to regain
compliance with Nasdaq continued listing standards, which may require effecting a reverse stock split of our Common Stock, we could be
delisted from Nasdaq, which would negatively impact our business, our ability to raise capital, and the market price and liquidity of
our Common Stock.
On November 12,
2024, the Company received the Notification Letter from the Listing Qualifications Department of Nasdaq notifying the Company that, because
the closing bid price for its Common Stock has been below $1.00 per share for 30 consecutive business days, it no longer complies with
the Bid Price Rule for continued listing on The Nasdaq Stock Market LLC. There is no assurance that we will be able to regain compliance
with the Bid Price Rule. The Notification Letter had no immediate effect on the listing of the Company’s Common Stock on The Nasdaq
Stock Market LLC. The Company has been provided an initial compliance period of 180 calendar days, or until May 12, 2025, to
regain compliance with the Bid Price Rule. During the compliance period, the Company’s shares of Common Stock will continue to
be listed and traded on The Nasdaq Stock Market LLC. To regain compliance, the closing bid price of the Company’s Common Stock
must meet or exceed $1.00 per share for a minimum of ten consecutive business days during the 180-day compliance period, with a longer
period potentially required by the Staff.
If our Common Stock
is delisted by Nasdaq, our Common Stock may be eligible for quotation on an over-the-counter quotation system or on the pink sheets but
will lack the market efficiencies associated with Nasdaq. Upon any such delisting, our Common Stock would become subject to the regulations
of the SEC relating to the market for penny stocks. A penny stock is any equity security not traded on a national securities exchange
that has a market price of less than $5.00 per share. The regulations applicable to penny stocks may severely affect the market liquidity
for our Common Stock and could limit the ability of stockholders to sell securities in the secondary market. In such a case, an investor
may find it more difficult to dispose of or obtain accurate quotations as to the market value of our Common Stock, and there can be no
assurance that our Common Stock will be eligible for trading or quotation on any alternative exchanges or markets.
Delisting from Nasdaq
could adversely affect our ability to raise additional financing through public or private sales of equity securities, would significantly
affect the ability of investors to trade our securities and would negatively affect the value and liquidity of our Common Stock. Delisting
could also have other negative results, including the potential loss of confidence by employees and customers, the loss of institutional
investor interest and fewer business development opportunities.
Pursuant to the Purchase
Agreement, the Company is required to effect a reverse stock split of its outstanding shares of Common Stock if, at any time after the
Stockholder Approval Date, it is not incompliance with Nasdaq’s Bid Price Rule and has received a deficiency letter from the
Listing Qualifications Department of The Nasdaq Stock Market LLC (the “Reverse Stock Split”). The Company must effect the
Reverse Stock Split within 30 days of the Stockholder Approval Date; provided that if within such 30 day period the Company regains compliance
with the Bid Price Rule, the Company shall have no obligation to effect the Reverse Stock Split. The Company intends to implement a reverse
stock split of its outstanding securities to regain compliance with the Bid Price Rule and to comply with the provisions of the
Purchase Agreement.
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and
the documents that we incorporate herein by reference contain forward-looking statements concerning our business, operations and
financial performance and condition, as well as our plans, objectives and expectations for our business operations and financial performance
and condition. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements.
In some cases, you can identify forward-looking statements by terminology such as “can”, “may”, “could”,
“should”, “assume”, “forecasts”, “believe”, “designed to”, “will”,
“expect”, “plan”, “anticipate”, “estimate”, “potential”, “position”,
“predicts”, “strategy”, “guidance”, “intend”, “budget”, “seek”,
“project” or “continue”, or the negative thereof or other comparable terminology regarding beliefs, plans, expectations
or intentions regarding the future. You should read statements that contain these words carefully because they:
| |
· |
discuss our
future expectations; |
| |
· |
contain projections
of our future results of operations or of our financial condition; and |
| |
· |
state other
“forward-looking” information. |
We believe it is important
to communicate our expectations. However, forward-looking statements are based on our current expectations, assumptions, estimates and
projections about our business and our industry and are subject to known and unknown risks, uncertainties and other factors. Accordingly,
our actual results and the timing of certain events may differ materially from those expressed or implied in such forward-looking statements
due to a variety of factors and risks, including, but not limited to, those set forth under “Risk Factors” in this prospectus,
and the following factors and risks:
| |
· |
our expectations
of future revenues, expenditures, capital or other funding requirements; |
| |
· |
the adequacy
of our cash and working capital to fund present and planned operations and growth; |
| |
· |
the substantial
doubt relating to our ability to continue as a going concern; |
| |
· |
our need
for additional financing which may in turn require the issuance of additional shares of Common Stock, preferred stock or other debt
or equity securities (including convertible securities) which would dilute the ownership held by stockholders; |
| |
· |
our business
strategy and the timing of our expansion plans, including the development of new production facilities for our Therapeutic DNA Production
Services; |
| |
· |
demand for
Therapeutic DNA Production Services; |
| |
· |
demand for
DNA Tagging Services; |
| |
· |
demand for
MDx Testing Services; |
| |
· |
our expectations
concerning existing or potential development and license agreements for third-party collaborations or joint ventures; |
| |
· |
regulatory
approval and compliance for our Therapeutic DNA Production Services, upon which our business strategy is substantially dependent; |
| |
· |
whether we
are able to achieve the benefits expected from the acquisition of Spindle; |
| |
· |
the effect
of governmental regulations generally; |
| |
· |
our expectations
of when regulatory submissions may be filed or when regulatory approvals may be received; |
| |
· |
our expectations
concerning product candidates for our technologies; |
| |
· |
our expectations
concerning potential restructuring of our business model; |
| |
· |
our expectations
of when or if we will become profitable; |
| |
· |
our current
non-compliance with Nasdaq's Bid Price Rule, which in the absence of a reverse split, may lead to delisting, potentially negatively
impacting our business, our ability to raise capital, and the market price and liquidity of our Common Stock; |
| |
· |
the risk
that our LDTs may become subject to additional regulatory requirements due to FDA rulemaking activity, and that compliance with such
requirements may be expensive and time-consuming, resulting in significant or unanticipated delay; and |
| |
· |
unknown future
market demand for the Linea Mpox Virus 1.0 Assay and associated mpox testing services. |
Any or all of our
forward-looking statements may turn out to be wrong. They may be affected by inaccurate assumptions that we might make or by known or
unknown risks and uncertainties. Actual outcomes and results may differ materially from what is expressed or implied in our forward-looking
statements. Among the factors that could affect future results are:
| |
· |
the inherent
uncertainties of product development based on our new and as yet not fully proven technologies; |
| |
· |
the risks
and uncertainties regarding the actual effect on humans of seemingly safe and efficacious formulations and treatments when tested
clinically; |
| |
· |
formulations
and treatments that utilize our Therapeutic DNA Production Services; |
| |
· |
the inherent
uncertainties associated with clinical trials of product candidates, including product candidates that utilize our Therapeutic DNA
Production Services; |
| |
· |
the inherent
uncertainties associated with the process of obtaining regulatory clearance or approval to market product candidates, including product
candidates that utilize our Therapeutic DNA Production Services; |
| |
· |
the inherent
uncertainties associated with commercialization of products that have received regulatory clearance or approval, including products
that utilize our Therapeutic DNA Production Services; |
| |
· |
the inherent
uncertainties associated with commercialization of our PGx Testing Services; |
| |
· |
economic
and industry conditions generally and in our specific markets; |
| |
· |
the volatility
of, and decline in, our stock price; and |
| |
· |
our ability
to obtain the necessary financing to fund our operations and effect our strategic development plan. |
All forward-looking
statements and risk factors included in this prospectus are made as of the date hereof, and all forward-looking statements and risk factors
included in documents incorporated herein by reference are made as of their original date, in each case based on information available
to us as of the date hereof, or in the case of documents incorporated by reference, the original date of any such document, and we assume
no obligations to update any forward-looking statement or risk factor, unless we are required to do so by law. If we do update one or
more forward-looking statements, no inference should be drawn that we will make updates with respect to other forward-looking statements
or that we will make any further updates to those forward-looking statements at any future time.
Forward-looking statements
may include our plans and objectives for future operations, including plans and objectives relating to our products and our future economic
performance, projections, business strategy and timing and likelihood of success. Assumptions relating to the foregoing involve judgments
with respect to, among other things, future economic, competitive and market conditions, future business decisions, demand for our products
and services, and the time and money required to successfully complete development and commercialization of our technologies, all of
which are difficult or impossible to predict accurately and many of which are beyond our control.
Any of the assumptions
underlying the forward-looking statements contained in this prospectus could prove inaccurate and, therefore, we cannot assure you that
any of the results or events contemplated in any of such forward-looking statements will be realized. Based on the significant uncertainties
inherent in these forward-looking statements, the inclusion of any such statement should not be regarded as a representation or as a
guarantee by us that our objectives or plans will be achieved, and we caution you against relying on any of the forward looking-statements
contained herein.
MARKET, INDUSTRY
AND OTHER DATA
Market
data and certain industry data and forecasts used throughout this prospectus were obtained from sources we believe to be reliable, including
market research databases, publicly available information, reports of governmental agencies and industry publications and surveys. We
have relied on certain data from third-party sources, including internal surveys, industry forecasts and market research, which we believe
to be reliable based on our management’s knowledge of the industry. Forecasts are particularly likely to be inaccurate, especially
over long periods of time. In addition, we do not necessarily know what assumptions regarding general economic growth were used in preparing
the third-party forecasts we cite. Statements as to our market position are based on the most currently available data. While we are
not aware of any misstatements regarding the industry data presented in this prospectus and the documents incorporated by reference into
this prospectus, our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed
under the heading “Risk Factors” in this prospectus and the documents incorporated by reference into this prospectus.
USE
OF PROCEEDS
We will not receive
any proceeds from the sale of the shares of Common Stock by the Selling Stockholders. However, we will receive proceeds from the exercise
of the Private Placement Warrants by the Selling Stockholders to the extent they are exercised for cash. We estimate that the maximum
proceeds that we may receive from the exercise of the Private Placement Warrants, assuming all the Private Placement Warrants are exercised
at their exercise price of $0.32, will be $13,325,000. We do not know, however, whether any of the Private Placement Warrants will be
exercised for cash or, if any of the Private Placement Warrants are exercised for cash, when they will be exercised. It is possible that
the Private Placement Warrants will expire and never be exercised.
There are circumstances
under which the Private Placement Warrants may be exercised on a cashless basis, including pursuant to the alternative cashless exercise
mechanism of the Series D Warrants. In these circumstances, even if the Private Placement Warrants are exercised, we may not receive
any proceeds, or the proceeds that we do receive may be significantly less than what we might expect. We intend to use the aggregate
net proceeds from the exercise of the Private Placement Warrants for the further development of our Therapeutic DNA Production Services,
as well as for general corporate purposes, including working capital. The actual allocation of proceeds realized from the exercise of
these Private Placement Warrants will depend upon the amount and timing of such exercises, our operating revenues and cash position at
such time and our working capital requirements. The Selling Stockholders will pay any expenses incurred by the Selling Stockholders for
brokerage, accounting, tax or legal services or any other expenses incurred by the Selling Stockholders in disposing of their shares
of Common Stock. We will bear all other costs, fees and expenses incurred in effecting the registration of the shares covered by this
prospectus, including, without limitation, all registration fees and fees and expenses of our counsel and our accountants.
MARKET
PRICE OF OUR COMMON STOCK AND RELATED STOCKHOLDER MATTERS
Market Information
Our Common Stock is
listed on The Nasdaq Capital Market under the symbol “APDN.” A description of our Common Stock is set forth under the heading
“Description of Capital Stock” beginning on page 19 of this prospectus.
The last reported sale price for our Common Stock on November 15,
2024 was $0.16 per share.
Holders
As of November 15, 2024, we had 375 record holders of our Common
Stock and no preferred stock issued and outstanding. The number of record holders was determined from the records of our transfer
agent and does not include beneficial owners of Common Stock whose shares are held in the names of various security brokers, dealers,
and registered clearing agencies. The transfer agent of our Common Stock is Equiniti Trust Company, LLC. The transfer agent and registrar’s
address is 90 Park Avenue, New York, NY 10016.
Dividend Policy
The Company has never
previously declared or paid any cash dividends on its Common Stock. We currently intend to retain earnings and profits, if any, to support
our business strategy and do not intend to pay any cash dividends within the foreseeable future. Any future determination to pay cash
dividends will be at the sole discretion of our Board of Directors and will depend upon the financial condition of the Company, its operating
results, capital requirements, general business conditions and any other factors that our board of directors deems relevant.
PRIVATE
PLACEMENT OF WARRANTS
Concurrently with the sale of shares of Common Stock and Pre-Funded Warrants, we also issued and sold to the Selling Stockholders the
Private Warrants to purchase an aggregate of up to 41,640,625 shares of our Common Stock, consisting of (i) 20,312,500 Series C
Warrants, (ii) 20,312,500 Series D Warrants and (iii) 1,015,625 Placement Agent Warrants.
The exercisability
of the Private Placement Warrants will be available only upon receipt of Warrant Stockholder Approval. Each Series C Warrant has
an exercise price of $0.32 per share of Common Stock, will become exercisable upon the Stockholder Approval Date, and will expire on
the five-year anniversary of the Stockholder Approval Date. Each Series D Warrant has an exercise price of $0.32 per share of Common
Stock, will become exercisable upon the Stockholder Approval Date, and will expire on the 18-month anniversary of the Stockholder Approval
Date. Each Placement Agent warrant has an exerice price of $0.32, will become exercisable upon the Stockholder Approval date and will
expire on October 30, 2029.
The Private Placement
Warrants are exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and,
at any time a registration statement registering the issuance of the Common Stock underlying the Private Placement Warrants under the
Securities Act of 1933, as amended (the “Securities Act”) is effective and available for the issuance of such shares, or
an exemption from registration under the Securities Act is available for the issuance of such shares, by payment in full in immediately
available funds for the number of Common Stock purchased upon such exercise. If at the time of exercise there is no effective registration
statement registering, or the prospectus contained therein is not available for the issuance of the Common Stock underlying the Private
Placement Warrants, then the Private Placement Warrants may also be exercised, in whole or in part, at such time by means of a cashless
exercise, in which case the holder would receive upon such exercise the net number of common shares determined according to the formula
set forth in the Private Placement Warrants.
Under the alternate
cashless exercise option of the Series D Warrants, the holder of a Series D Warrant has the right to receive an aggregate number
of shares equal to the product of (x) the aggregate number of shares of Common Stock that would be issuable upon a cash exercise
of the Series D Warrant and (y) 1.0. In addition, the Series D Warrants include a provision that resets their exercise
price in the event of a reverse split of our Common Stock, to a price equal to the lesser of (i) the then exercise price and (ii) lowest
volume weighted average price (VWAP) during the period commencing five trading days immediately preceding and the five trading days commencing
on the date we effect a reverse stock split in the future with a proportionate adjustment to the number of shares underlying the Series D
Warrants, subject to a floor of $0.0634.
Subject to limited
exceptions, a holder of Private Placement Warrants does not have the right to exercise any portion of its Private Placement Warrants
if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the purchaser prior to
issuance of the Private Placement Warrants, 9.99%) of the number of shares of our Common Stock outstanding immediately after giving effect
to such exercise. A holder may increase or decrease the beneficial ownership limitation up to 9.99%, provided, however, that any increase
in the beneficial ownership limitation shall not be effective until 61 days following notice of such change to the Company.
Pursuant to the Purchase
Agreement, within 20 calendar days from the date of the Purchase Agreement, we agreed to file a registration statement on Form S-1
providing for the resale of the shares issuable upon exercise of the Private Placement Warrants. We agreed to use commercially reasonable
efforts to cause such registration statement to become effective within 50 calendar days following the closing date of the Purchase Agreement
(or 90 calendar days following the closing date of the Purchase Agreement in the event that the SEC requires the Company to include its
audited year-end financial statements for the fiscal year ended September 30, 2024 in such registration statement) and to keep such
registration statement effective at all times until no Selling Stockholder owns any Private Placement Warrants or shares of Common Stock
issuable upon exercise thereof.
We also agreed to
hold a special meeting of stockholders to obtain the Warrant Stockholder Approval no later than 90 days after the closing of the Offering
(the “Special Meeting”). If the Company does not obtain Warrant Stockholder Approval at the first meeting, the Company is
obligated to call a meeting every ninety days thereafter to seek Warrant Stockholder Approval until the earlier of the date on which
Warrant Stockholder Approval is obtained or the Series Warrants are no longer outstanding. The Company has agreed to file a preliminary
proxy statement with respect to obtaining Warrant Stockholder Approval at the Special Meeting within 20 days following the closing date
of the Purchase Agreement.
In the event of any
fundamental transaction, as described in the Private Placement Warrants and generally including any merger with or into another entity,
sale of all or substantially all of the Company’s assets, tender offer or exchange offer, or reclassification of the shares of
Common Stock, subject to certain exceptions, then upon any subsequent exercise of a Private Placement Warrant, the holder has the right
to receive as alternative consideration, for each share of Common Stock that would have been issuable upon such exercise immediately
prior to the occurrence of such fundamental transaction, the number of shares of Common Stock of the successor or acquiring corporation
of the Company, if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction
by a holder of the number of shares of Common Stock for which the Private Placement Warrant is exercisable immediately prior to such
event. Notwithstanding the foregoing, in the event of a fundamental transaction, the holders of the Private Placement Warrants have the
right to require the Company or a successor entity to purchase the Private Placement Warrants for cash in the amount of the Black Scholes
Value (as defined in the Private Placement Warrants) of the unexercised portion of the Private Placement Warrants concurrently with or
within 30 days following the consummation of a fundamental transaction. However, in the event of a fundamental transaction which is not
in the Company’s control or in which the consideration payable consists of equity securities of a successor entity that is quoted
or listed on a nationally recognized securities exchange, the holders of the Private Placement Warrants will only be entitled to receive
from the Company or its successor entity, as of the date of consummation of such fundamental transaction the same type or form of consideration
(and in the same proportion), at the Black Scholes Value of the unexercised portion of the Private Placement Warrants that is being offered
and paid to the holders of Common Stock in connection with the fundamental transaction, whether that consideration is in the form of
cash, stock or any combination of cash and stock, or whether the holders of Common Stock are given the choice to receive alternative
forms of consideration in connection with the fundamental transaction.
SELLING
STOCKHOLDERS
The shares of Common
Stock being offered by the Selling Stockholders are those issuable to the Selling Stockholders, upon exercise of the Private Placement
Warrants. For additional information regarding the issuances of the Private Placement Warrants, see “Private Placement of Warrants”
above. We are registering the shares of Common Stock underlying the Private Placement Warrants in order to permit the Selling Stockholders
to offer the shares for resale from time to time. In addition to the ownership of the shares of Common Stock and the Private Placement
Warrants, the Selling Stockholders have had material relationships with us within the past three years.
On May 28, 2024,
the Company entered into the May Placement Agency Agreement with Craig-Hallum and Laidlaw (collectively, the “May Placement
Agents”) pursuant to which the May Placement Agents agreed to serve as the co-placement agents, on a “reasonable best
efforts” basis, in connection with the May 2024 Offering of 9,230,769 units (the “Units”), with each
Unit consisting of either (A) one share of the Company’s Common Stock, one May 2024 Series A Warrant
and one May 2024 Series B Warrant or (B) one pre-funded warrant (each, a “May 2024 Pre-Funded Warrant”)
to purchase one share of Common Stock and one May 2024 Series A Warrant and one May 2024
Series B Warrant. In connection with the May 2024 Offering, the Company also issued placement agent warrants to purchase up
to 461,538 shares of Common Stock. The May 2024 Offering closed on May 29, 2024. The purchase price of each Unit
was $1.30, except for Units which include May 2024 Pre-Funded Warrants, which had a purchase price of $1.2999.
The Company received
net proceeds from the May Offering, after deducting placement agent fees and other offering expenses payable by the Company, of
approximately $10.5 million. Each of the Selling Stockholders participated in the May 2024 Offering.
On August 8,
2022, the Company completed a best efforts public offering (the “August 2022 Offering”) with respect to the issuance
and sale of: (i) 2,820,000 of shares of the Company’s Common Stock, (ii) 3,000,000 series A common stock purchase warrants,
each to purchase one share of Common Stock, (iii) 3,000,000 series B common stock purchase warrants, each to one share of Common
Stock, and (iv) 180,000 pre-funded common stock purchase warrants, each to purchase one share of Common Stock. The Company received
net proceeds of approximately $11.1 million from the August 2022 Offering, after deducting the estimated offering expenses payable
by the Company, including placement agent fees. Three of the Selling Stockholders, Sabby Volatility Warrant Master Fund, Ltd.,
Anson Master Funds and Michael Bigger, participated in the August 2022 Offering.
The table below lists
the Selling Stockholders and other information regarding the beneficial ownership of the shares of Common Stock by each of the Selling
Stockholders. The second column lists the number of shares of Common Stock beneficially owned by each Selling Stockholders, based on
its ownership of the shares of Common Stock, Priavte Placement Warrants, and other warrants, as of November 15, 2024, assuming exercise
of the warrants held by the Selling Stockholders on that date, without regard to any limitations on exercises. The third and fourth columns
assume the sale of all of the shares offered by the Selling Stockholders pursuant to this prospectus. The third column lists the shares
of Common Stock underlying the Private Placement Warrants offered by this prospectus by the Selling Stockholders.
In accordance with
the terms of the Purchase Agreement with the Selling Stockholders, this prospectus generally covers the resale of the maximum number
of shares of Common Stock issuable upon exercise of the Private Placement Warrants.
Under the terms of
the Private Placement Warrants, and under the terms of certain other warrants held by the Selling Stockholders, a Selling Stockholder
may not exercise the warrants to the extent such exercise would cause such Selling Stockholder, together with its affiliates and attribution
parties, to beneficially own a number of shares of Common Stock which would exceed 4.99% or 9.99%, as applicable, of our then outstanding
Common Stock following such exercise, excluding for purposes of such determination shares of Common Stock issuable upon exercise of such
warrants which have not been exercised. The number of shares in the table below does not reflect the Private Placement Warrants Beneficial
Ownership Limitation. The Selling Stockholder may sell all, some or none of their shares in this offering. See “Plan of Distribution.”
| Name
of Selling Stockholders | |
Shares
Owned
prior to
Offering | | |
Shares
Offered
by this
Prospectus | | |
Shares
Owned
after
Offering | | |
Percentage of
Shares
Beneficially
Owned
after
Offering
(1) | |
| Altium
Growth Fund, LP (2) | |
| 28,548,579 | (3) | |
| 7,500,000 | (4) | |
| 21,048,579 | | |
| 31.38 | % |
| Anson
Master Funds (5) | |
| 27,455,769 | (6) | |
| 7,500,000 | (7) | |
| 19,955,769 | | |
| 29.60 | % |
| Michael
Bigger (8) | |
| 27,223,289 | (9) | |
| 7,500,000 | (10) | |
| 19,723,289 | | |
| 29.49 | % |
| L1
Capital Global Opportunities Master Fund (11) | |
| 24,805,128 | (12) | |
| 6,250,000 | (13) | |
| 18,555,128 | | |
| 27.98 | % |
| Sabby
Volatility Warrant Master Fund, Ltd. (14) | |
| 26,971,005 | (15) | |
| 7,500,000 | (16) | |
| 19,471,005 | | |
| 29.45 | % |
| S.H.N.
Finnaical Investments Ltd (17) | |
| 23,330,127 | (18) | |
| 4,375,000 | (19) | |
| 18,955,127 | | |
| 28.69 | % |
| Craig-Hallum
Capital Group LLC (20) | |
| 1,384,855 | (21) | |
| 1,015,625 | (22) | |
| 369,230 | | |
| * | |
*Less than one percent.
| (1) |
Percentages
are based on 50,896,710 shares of Common Stock outstanding as of November 15, 2024. |
| (2) |
The securities
are directly held as of November 6, 2024, by Altium Growth Fund, LP (“Altium”), and may be deemed to be beneficially
owned by Jacob Gottlieb, who exercises investment and voting control over the securities. The address of Altium is c/o Altium Capital
Management, LP, 152 West 57th Street, 20th Floor, New York, NY 10019. |
| (3) |
Consists
of (i) 4,877,375 shares of Common Stock, (ii) Series C Warrants to purchase up to 3,750,000 shares of Common Stock,
(iii) Series D Warrants to purchase up to 3,750,000 shares of Common Stock, (iv) warrants to purchase up to 15,846,791
shares of Common Stock, and (v) Pre-Funded Warrants to purchase up to 324,413 shares of Common Stock. |
| (4) |
Represents
Common Stock underlying (i) 3,750,000 Series C Warrants and (ii) 3,750,000 Series D Warrants to purchase an aggregate
of up to 7,500,000 shares of Common Stock. |
| (5) |
The securities
are directly held as of November 6, 2024, by (i) Anson East Master Fund LP (“Anson East”) and (ii) Anson
Investments Master Fund LP (“Anson Investments”, and, collectively with Anson East, the “Anson Master Funds”).
Anson Advisors Inc and Anson Funds Management LP, the Co-Investment Advisers of the Anson Master Funds, hold voting and dispositive
power over the shares of Common Stock held by the Anson Master Funds. Tony Moore is the managing member of Anson Management GP LLC,
which is the general partner of Anson Funds Management LP. Moez Kassam and Amin Nathoo are directors of Anson Advisors Inc. Mr. Moore,
Mr. Kassam and Mr. Nathoo each disclaim beneficial ownership of these shares of Common Stock except to the extent of their
pecuniary interest therein. The principal business address of the Anson Master Funds is Maples Corporate Services Limited, PO Box
309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands. |
| (6) |
Consists
of (i) 3,425,587 shares of Common Stock, (ii) Series C Warrants to purchase up to 3,750,000 shares of Common Stock,
(iii) Series D Warrants to purchase up to 3,750,000 shares of Common Stock, (iv) warrants to purchase up to 16,205,769
shares of Common Stock, and (v) Pre-Funded Warrants to purchase up to 324,413 shares of Common Stock. |
| (7) |
Represents
Common Stock underlying (i) 3,750,000 Series C Warrants and (ii) 3,750,000 Series D Warrants to purchase an aggregate
of up to 7,500,000 shares of Common Stock. |
| (8) |
The securities
are directly held as of November 6, 2024, by (i) Bigger Capital Fund, LP (“Bigger”) and (ii) District
2 Capital Fund LP (“District 2”), and may be deemed to be beneficially owned by Michael Bigger, who exercises investment
and voting control over the securities. The address of Bigger is 11700 W. Charleston Blvd. 170-659, Las Vegas, NV 89135, and the
address of District 2 is 14 Wall Street, Huntington, NY 11743. |
| (9) |
Consists
of (i) 3,750,000 shares of Common Stock, (ii) Series C Warrants to purchase up to 3,750,000 shares of Common Stock,
(iii) Series D Warrants to purchase up to 3,750,000 shares of Common Stock and (iv) warrants to purchase up to 15,973,289
shares of Common Stock. |
| (10) |
Represents
Common Stock underlying (i) 3,750,000 Series C Warrants and (ii) 3,750,000 Series D Warrants to purchase an aggregate
of up to 3,750,000 shares of Common Stock. |
| (11) |
The securities
are directly held as of November 6, 2024, by L1 Capital Global Opportunities Master Fund (“L1”), and may be deemed
to be beneficially owned by David Feldman and Joel Arber, who exercise investment and voting control over the securities. The address
of L1 is 161A Shedden Road, 1 Artillery Court, PO Box 10085, Grand Cayman KY1-1001, Cayman Islands. |
| (12) |
Consists
of (i) 3,125,000 shares of Common Stock, (ii) Series C Warrants to purchase up to 3,125,000 shares of Common Stock,
(iii) Series D Warrants to purchase up to 3,125,000 shares of Common Stock and (iv) warrants to purchase up to 15,430,128
shares of Common Stock. |
| (13) |
Represents
Common Stock underlying (i) 3,125,000 Series C Warrants and (ii) 3,125,000 Series D Warrants to purchase an aggregate
of up to 6,250,000 shares of Common Stock. |
| (14) |
The securities
are directly held as of November 7, 2024, by Sabby Volatility Warrant Master Fund, Ltd. (“Sabby”). Sabby Management,
LLC is the investment manager of Sabby and shares voting and investment power with respect to these shares in this capacity. As manager
of Sabby Management, LLC, Hal Mintz also shares voting and investment power on behalf of Sabby. Each of Sabby Management, LLC and
Hal Mintz disclaims beneficial ownership over the securities listed except to the extent of their pecuniary interest therein. The
address of Sabby is c/o Sabby Management, LLC, 7012 Fisher Island Dr., Miami Beach, FL 33109. |
| (15) |
Consists
of (i) 4,259,627 shares of Common Stock, (ii) Series C Warrants to purchase up to 3,750,000 shares of Common Stock,
(iii) Series D Warrants to purchase up to 3,750,000 shares of Common Stock and (iv) warrants to purchase up to 15,211,378
shares of Common Stock. |
| (16) |
Represents
Common Stock underlying (i) 3,750,000 Series C Warrants and (ii) 3,750,000 Series D Warrants to purchase an aggregate
of up to 7,500,000 shares of Common Stock. |
| (17) |
The securities
are directly held as of November 6, 2024, by S.H.N. Financial Investments Ltd. (“SHN”), and may be deemed to be
beneficially owned by Nir Shamir and Hadar Shamir who exercise investment and voting control over the securities. The address of
SHN is Arik Einstein 3, Herzliya, Israel. |
| (18) |
Consists
of (i) 3,775,000 shares of Common Stock, (ii) Series C Warrants to purchase up to 2,187,500 shares of Common Stock,
(iii) Series D Warrants to purchase up to 2,187,500 shares of Common Stock and (iv) warrants to purchase up to 15,180,127
shares of Common Stock. |
| (19) |
Represents
Common Stock underlying (i) 2,187,500 Series C Warrants and (ii) 2,187,500 Series D Warrants to purchase an aggregate
of up to 4,375,000 shares of Common Stock. |
| (20) |
The securities
are directly held as of November 14, 2024, by (i) John Lipman and (ii) Craig-Hallum Capital Group LLC, and may be
deemed to be beneficially owned by both Craig-Hallum and Mr. Lipman, as a managing partner of Craig-Hallum who exercises investment
and voting control over the securities. The address of both Mr. Lipman and Craig-Hallum is c/o Craig-Hallum Capital Group LLC,
222 South Ninth Street, Suite 350, Minneapolis, MN 55402. |
| (21) |
Consists
of (i) Placement Agent Warrants to purchase up to 1,015,625 shares of Common Stock and (ii) warrants to purchase up to
369,230 shares of Common Stock. |
| (22) |
Represents
Common Stock underlying 1,015,625 Placement Agent Warrants to purchase an aggregate of up to 1,015,625 shares of Common Stock. |
PLAN
OF DISTRIBUTION
Each Selling Stockholder
of the securities and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their securities
covered hereby on the principal trading market or any other stock exchange, market or trading facility on which the securities are traded
or in private transactions. These sales may be at fixed or negotiated prices. A Selling Stockholder may use any one or more of the following
methods when selling securities:
| |
· |
|
ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| |
· |
|
block
trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block
as principal to facilitate the transaction; |
| |
· |
|
purchases
by a broker-dealer as principal and resale by the broker-dealer for its account; |
| |
· |
|
an
exchange distribution in accordance with the rules of the applicable exchange; |
| |
· |
|
privately
negotiated transactions; |
| |
· |
|
settlement
of short sales; |
| |
· |
|
in
transactions through broker-dealers that agree with the Selling Stockholders to sell a specified number of such securities at a stipulated
price per security; |
| |
· |
|
through
the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| |
· |
|
a
combination of any such methods of sale; or |
| |
· |
|
any
other method permitted pursuant to applicable law. |
The Selling Stockholders
may also sell securities under Rule 144 or any other exemption from registration under the Securities Act, if available, rather
than under this prospectus.
Broker-dealers engaged
by the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or
discounts from the Selling Stockholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser)
in amounts to be negotiated, but, except as set forth in a supplement to this Prospectus, in the case of an agency transaction not in
excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup
or markdown in compliance with FINRA Rule 2121.
If
at the time of any offering made under this prospectus a member of FINRA participating in the offering has a “conflict of interest”
as defined in FINRA Rule 5121 (“Rule 5121”), that offering will be conducted in accordance with the relevant provisions
of Rule 5121.
Our Common Stock is
listed the Nasdaq Capital Market under the symbol “APDN.”
In connection with
the sale of the securities or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers or
other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume.
The Selling Stockholders may also sell securities short and deliver these securities to close out their short positions, or loan or pledge
the securities to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option or other
transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the delivery
to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer or
other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Stockholders
and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters” within the
meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents
and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts under the
Securities Act. Each Selling Stockholder has informed the Company that it does not have any written or oral agreement or understanding,
directly or indirectly, with any person to distribute the securities.
The Company is required
to pay certain fees and expenses incurred by the Company incident to the registration of the securities. The Company has agreed to indemnify
the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
We agreed to keep
this prospectus effective until the earlier of (i) the date on which the securities may be resold by the Selling Stockholders without
registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for the
Company to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of
similar effect or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act
or any other rule of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if
required under applicable state securities laws. In addition, in certain states, the resale securities covered hereby may not be sold
unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification
requirement is available and is complied with.
Under applicable rules and
regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in
market making activities with respect to the Common Stock for the applicable restricted period, as defined in Regulation M, prior to
the commencement of the distribution. In addition, the Selling Stockholders will be subject to applicable provisions of the Exchange
Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the Common
Stock by the Selling Stockholders or any other person. We will make copies of this prospectus available to the Selling Stockholders and
have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including
by compliance with Rule 172 under the Securities Act).
DESCRIPTION
OF CAPITAL STOCK
The following description
of our Common Stock and Private Placement Warrants summarizes the material terms and provisions of the securities that we may issue in
connection with this offering. It may not contain all the information that is important to you. For the complete terms of our Common
Stock, please refer to our Certificate of Incorporation and our by-laws (“By-Laws”), which are filed as exhibits to the registration
statement which includes this prospectus. See “Where You Can Find More Information.” The Delaware General Corporation Law
(“DGCL”) may also affect the terms of these securities. The summary below is qualified in its entirety by reference to our
Certificate of Incorporation and By-Laws, each as in effect at the time of any offering of securities under this prospectus.
As of November 15,
2024, our authorized capital stock consists of 200,000,000 shares of Common Stock, par value $0.001 per share, of which 50,896,710 shares
were issued and outstanding, and 10,000,000 shares of preferred stock, par value $0.001 per share, of which no shares were issued and
outstanding. In addition, as of November 15, 2024, there were 108,176 shares of Common Stock issuable upon exercise of options outstanding,
96,083,181 shares of Common Stock issuable upon exercise of warrants outstanding, and 269,069 shares of Common Stock reserved for future
grant or issuance. The authorized and unissued shares of Common Stock and preferred stock are available for issuance without further
action by our stockholders.
Common Stock
Each stockholder of
our Common Stock is entitled to one vote for each share issued and outstanding held on all matters to be voted upon by the stockholders.
Our shares of Common Stock have no preemptive, conversion, or redemption rights. The rights, preferences, and privileges of the holders
of Common Stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock.
Upon the sale of substantially all of our stock or assets or dissolution, liquidation or winding up, and after all liquidation preferences
payable to any series of preferred stock entitled thereto have been satisfied, our remaining assets shall be distributed to all holders
of Common Stock and any similarly situated stockholders who are not entitled to any liquidation preference or, if there be an insufficient
amount to pay all such stockholders, then ratably among such holders. All of our issued and outstanding shares of Common Stock are fully
paid and non-assessable. The holders of shares of our Common Stock will be entitled to such cash dividends as may be declared from time
to time by our board of directors from funds available therefor.
Our Common Stock is
listed the Nasdaq Capital Market under the symbol “APDN.” The transfer agent and registrar for our Common Stock is Equiniti
Trust Company, LLC. The transfer agent and registrar’s address is 90 Park Avenue, New York, NY 10016.
Private Placement Warrants
Duration and Exercise
Price
Each Private Placement
Warrant has an exercise price of $0.32 per share of Common Stock and will become exercisable upon the Stockholder Approval Date. The
Series C Warrants will expire on the five-year anniversary of the Stockholder Approval Date, the Series D Warrants will expire
on the 18-month anniversary of the Stockholder Approval Date and the Placement Agent Warrants will expire on October 30, 2029.
Exercisability
The Private Placement
Warrants are exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and,
at any time a registration statement registering the issuance of the Common Stock underlying the Private Placement Warrants under the
Securities Act is effective and available for the issuance of such shares, or an exemption from registration under the Securities Act
is available for the issuance of such shares, by payment in full in immediately available funds for the number of Common Stock purchased
upon such exercise. Subject to limited exceptions, a holder of Private Placement Warrants will not have the right to exercise any portion
of its Private Placement Warrants if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the
election of the purchaser prior to issuance of the Private Placement Warrants, 9.99%) of the number of shares of our Common Stock outstanding
immediately after giving effect to such exercise. A holder may increase or decrease the beneficial ownership limitation up to 9.99%,
provided, however, that any increase in the beneficial ownership limitation shall not be effective until 61 days following notice of
such change to the Company.
Cashless Exercise
If at the time of
exercise there is no effective registration statement registering, or the prospectus contained therein is not available for the issuance
of the Common Stock underlying the Private Placement Warrants, then the Private Placement Warrants may also be exercised, in whole or
in part, at such time by means of a cashless exercise, in which case the holder would receive upon such exercise the net number of common
shares determined according to the formula set forth in the Private Placement Warrant.
Under the alternate
cashless exercise option of the Series D Warrants, the holder of a Series D Warrant, has the right to receive an aggregate
number of shares equal to the product of (x) the aggregate number of shares of Common Stock that would be issuable upon a cash exercise
of the Series D Warrant and (y) 1.0.
Reverse Split
The Series D
Warrants include a provision that resets their exercise price in the event of a reverse split of our Common Stock, to a price equal to
the lesser of (i) the then exercise price and (ii) lowest volume weighted average price (VWAP) during the period commencing
five trading days immediately preceding and the five trading days commencing on the date we effect a reverse stock split in the future
with a proportionate adjustment to the number of shares underlying the Series D Warrants, subject to a floor of $0.0634.
Fundamental Transactions
In
the event of any fundamental transaction, as described in the Private Placement Warrants and generally including any merger with or into
another entity, sale of all or substantially all of the Company’s assets, tender offer or exchange offer, or reclassification of
the shares of Common Stock, subject to certain exceptions, then upon any subsequent exercise of a Private Placement Warrant, the holder
will have the right to receive as alternative consideration, for each share of Common Stock that would have been issuable upon such exercise
immediately prior to the occurrence of such fundamental transaction, the number of shares of Common Stock of the successor or acquiring
corporation of the Company, if it is the surviving corporation, and any additional consideration receivable upon or as a result of such
transaction by a holder of the number of shares of Common Stock for which the Private Placement Warrant is exercisable immediately prior
to such event. Notwithstanding the foregoing, in the event of a fundamental transaction, the holders of the Private Placement Warrants
have the right to require the Company or a successor entity to purchase the Private Placement Warrants for cash in the amount of the
Black Scholes Value (as defined in the Private Placement Warrants) of the unexercised portion of the Private Placement Warrants concurrently
with or within 30 days following the consummation of a fundamental transaction. However, in the event of a fundamental transaction which
is not in the Company’s control or in which the consideration payable consists of equity securities of a successor entity that
is quoted or listed on a nationally recognized securities exchange, the holders of the Private Placement Warrants will only be entitled
to receive from the Company or its successor entity, as of the date of consummation of such fundamental transaction the same type or
form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of the Private Placement Warrants
that is being offered and paid to the holders of Common Stock in connection with the fundamental transaction, whether that consideration
is in the form of cash, stock or any combination of cash and stock, or whether the holders of Common Stock are given the choice to receive
alternative forms of consideration in connection with the fundamental transaction.
Transferability
In
accordance with its terms and subject to applicable laws, a Private Placement Warrant may be transferred at the option of the holder
upon surrender of the Private Placement Warrant to us together with the appropriate instruments of transfer and payment of funds sufficient
to pay any transfer taxes (if applicable).
Fractional
Shares
No
fractional shares of Common Stock will be issued upon the exercise of the Private Placement Warrants. Rather, the number of shares of
Common Stock to be issued will, at our election, either be rounded up to the nearest whole number or we will pay a cash adjustment in
respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Trading Market
There
is no established trading market for the Private Placement Warrants, and we do not expect a market to develop. We do not intend to apply
for a listing for the Private Placement Warrants on any securities exchange or other nationally recognized trading system. Without an
active trading market, the liquidity of the Private Placement Warrant will be limited.
Rights
as a Shareholder
Except as otherwise
provided in the Private Placement Warrants or by virtue of the holders’ ownership of shares of Common Stock, the holders of Private
Placement Warrants do not have the rights or privileges of holders of our shares of Common Stock, including any voting rights, until
such Private Placement Warrant holders exercise their warrants.
Possible Anti-Takeover
Effects of Delaware Law and our Certificate of Incorporation and By-Laws
Our Certificate of
Incorporation contains provisions that could make it more difficult to acquire control of our company by means of a tender offer, open
market purchases, a proxy contest or otherwise. A description of these provisions is set forth below.
Anti-Takeover Effects
of Delaware Law
Companies incorporated
in Delaware are subject to the provisions of Section 203 of the DGCL, or Section 203, unless the corporation has “opted
out” of these provisions with an express provision in its original certificate of incorporation or an express provision in its
certificate of incorporation or by-laws resulting from a stockholders’ amendment approved by at least a majority of the outstanding
voting shares. We have opted out of Section 203 with an express provision in our Certificate of Incorporation. Therefore, the anti-takeover
effects of Section 203 do not apply to us.
In general, Section 203
prohibits a publicly-held Delaware corporation from engaging in a “business combination” with an “interested stockholder”
for a three-year period following the time that this stockholder becomes an interested stockholder, unless the business combination is
approved in a prescribed manner. A “business combination” includes, among other things, a merger, asset or stock sale or
other transaction resulting in a financial benefit to the interested stockholder. An “interested stockholder” is a person
who, together with affiliates and associates, owns, or did own within three years prior to the determination of interested stockholder
status, 15% or more of the corporation’s voting stock.
Under Section 203,
a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions:
before the stockholder became interested, the board of directors approved either the business combination or the transaction which resulted
in the stockholder becoming an interested stockholder; upon consummation of the transaction which resulted in the stockholder becoming
an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time
the transaction commenced, excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors
and also officers, and employee stock plans, in some instances; or at or after the time the stockholder became interested, the business
combination was approved by the board of directors of the corporation and authorized at an annual or special meeting of the stockholders
by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder.
Election and Removal
of Directors
Directors will be
elected by a plurality of the voting power of the shares present in person or represented by proxy at the stockholders meeting and entitled
to vote on the election of directors. Our Certificate of Incorporation does not provide for a classified board of directors or for cumulative
voting in the election of directors. Under Article VIII of the Certificate of Incorporation and Section 3.13 of the By-Laws,
directors may be removed by the stockholders of the Company only for cause, and in such case only by the affirmative vote of the holders
of at least a majority of the voting power of the issued and outstanding shares of capital stock of the Company then entitled to vote
in the election of directors. On December 21, 2015, the Court of Chancery of the State of Delaware invalidated as a matter of law
certain provisions of the certificate of incorporation and bylaws of VAALCO Energy, Inc. (“VAALCO”), a Delaware corporation,
that permitted the removal of VAALCO’s directors by its stockholders only for cause. In In re VAALCO Energy, Inc.
Stockholder Litigation, Consol. C.A. No. 11775-VCL (Del. Ch. Dec. 21, 2015), the Court ruled from the bench to hold
that, in the absence of a classified board of directors or cumulative voting, VAALCO’s “only for-cause” director removal
provisions conflict with Section 141(k) of the DGCL and are therefore invalid. Because the Company’s Certificate of Incorporation
and By-Laws contain similar “only for-cause” director removal provisions and the Company does not have a classified board
of directors or cumulative voting, the Company will not attempt to enforce the foregoing “only for-cause” director removal
provision in light of the recent VAALCO decision.
Size of Board and
Vacancies
The authorized number
of directors may be determined by the Board of Directors, provided the board shall consist of at least one (1) member. No decrease
in the number of directors constituting the board shall shorten the term of any incumbent director.
Vacancies occurring
on our Board of Directors for any reason and newly created directorships resulting from an increase in the authorized number of directors
may be filled only by a vote of a majority of the remaining members of the Board of Directors, although less than a quorum, or by a sole
remaining director, at any meeting of the Board of Directors.
Amendment
The Certificate of
Incorporation may be amended in the manner prescribed by the DGCL. The Board of Directors is authorized to adopt, amend, alter or repeal
the By-Laws by the affirmative vote of at least a majority of the Board of Directors then in office. No amendment to the Certificate
of Incorporation or the By-Laws may adversely affect any indemnification right or protection of any director, officer, employee or other
agent existing at the time of such amendment, repeal or adoption of an inconsistent provision for or in respect of any act, omission
or other matter occurring, or any action or proceeding accruing or arising prior to such amendment, repeal or adoption of an inconsistent
provision.
Authorized but
Unissued Shares of Common Stock and of Preferred Stock
We believe that the
availability of the “Blank Check” preferred stock under our Certificate of Incorporation provides us with flexibility in
addressing corporate issues that may arise. The Board of Directors has the power, subject to applicable law, to issue series of preferred
stock that could, depending on the terms of the series, impede the completion of a merger, tender offer or other takeover attempt that
some, or a majority, of the stockholders might believe to be in their best interests or in which stockholders might receive a premium
for their stock over the then prevailing market price of the stock. Our Board of Directors may issue preferred stock with voting rights
or conversion rights that, if exercised, could adversely affect the voting power of the holders of Common Stock.
The authorized shares
of preferred stock, as well as shares of Common Stock, will be available for issuance without further action by our stockholders, unless
action is required by applicable law or the rules of any stock exchange on which our securities may be listed. Having these authorized
shares available for issuance allows us to issue shares without the expense and delay of a special stockholders’ meeting. We may
use additional shares for a variety of purposes, including future public offerings to raise additional capital, to fund acquisitions
and as employee compensation. The existence of authorized but unissued shares of Common Stock and preferred stock could render more difficult
or discourage an attempt to obtain control of our company by means of a proxy contest, tender offer, merger or otherwise. The above provisions
may deter a hostile takeover or delay a change in control or management of our company.
Advance Notice
Procedure
Our By-Laws provide
an advance notice procedure for stockholders to nominate director candidates for election or to bring business before an annual meeting
of stockholders. Only persons nominated by, or at the direction of, our Board of Directors or by a stockholder of record who has given
proper and timely notice to our secretary prior to the meeting at which such stockholder is entitled to vote and appears, will be eligible
for election as a director. In addition, any proposed business other than the nomination of persons for election to our Board of Directors
must constitute a proper matter for stockholder action pursuant to a proper notice of meeting delivered to us. For notice to be timely,
it must generally be delivered to our secretary not less than 90 nor more than 120 calendar days prior to the first anniversary of the
previous year’s annual meeting (or if the date of the annual meeting is more than 30 calendar days before or more than 60 calendar
days after the anniversary date of the previous year’s annual meeting, not earlier than the 120th calendar day prior to such meeting
and not later than either the 90th calendar day prior to such meeting or the 10th calendar day after public disclosure of the date of
such meeting is first made by us). These advance notice provisions may have the effect of precluding the conduct of certain business
at a meeting if the proper procedures are not followed or may discourage or deter a potential acquirer from conducting a solicitation
of proxies to elect its own slate of directors or otherwise attempt to obtain control of us.
Special Meetings of Stockholders
Our By-Laws provide
that special meetings of stockholders may be called only by the Chairman of the Board, the Chief Executive Officer, or the Board of Directors
pursuant to a resolution adopted by a majority of the board.
LEGAL
MATTERS
The validity of the
issuance of our securities offered in this prospectus will be passed upon for us by McDermott Will & Emery LLP, New York, New
York.
EXPERTS
Marcum LLP, independent
registered public accounting firm, has audited our consolidated financial statements at September 30, 2023 and 2022. We have incorporated
by reference into this prospectus and in the registration statement our financial statements in reliance on Marcum LLP’s
report, which includes an explanatory paragraph as to the Company’s ability to continue as a going conern, given on their authority
as experts in accounting and auditing.
WHERE
YOU CAN FIND ADDITIONAL INFORMATION
We have filed with
the SEC a registration statement on Form S-1 under the Securities Act with respect to the securities offered by this prospectus.
This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration
statement, as permitted by the rules and regulations of the SEC. For further information with respect to us and our securities,
we refer you to the registration statement, including the exhibits filed as a part of the registration statement. Statements contained
in this prospectus concerning the contents of any contract or any other document are not necessarily complete. If a contract or document
has been filed as an exhibit to the registration statement, please see the copy of the contract or document that has been filed. Each
statement in this prospectus relating to a contract or document filed as an exhibit is qualified in all respects by the filed exhibit.
The SEC also maintains an Internet website that contains the registration statement of which this prospectus forms a part, as well as
the exhibits thereto. These documents, along with future reports, proxy statements and other information about us, are available at the
SEC’s website, www.sec.gov.
We are subject to
the information and reporting requirements of the Exchange Act, and, in accordance with this law, file periodic reports and other information
with the SEC. These periodic reports and other information are available at the SEC’s website, www.sec.gov. We also maintain a
website at http://www.adnas.com. You may access these materials free of charge as soon as reasonably practicable after they are electronically
filed with, or furnished to, the SEC. Information contained on our website is not a part of this prospectus, and the inclusion of our
website address in this prospectus is an inactive textual reference only.
MATERIAL
CHANGES
None.
Incorporation
of Certain Information by Reference.
We have elected to
incorporate certain information by reference into this prospectus. By incorporating by reference, we can disclose important information
to you by referring you to other documents we have filed or will file with the SEC. The information incorporated by reference is deemed
to be part of this prospectus, except for information incorporated by reference that is superseded by information contained in this prospectus.
This means that you must look at all of the SEC filings that we incorporate by reference to determine if any statements in the prospectus
or any document previously incorporated by reference have been modified or superseded. This prospectus incorporates by reference the
documents listed below and any future information filed (rather than furnished) with the SEC under Sections 13(a), 13(c), 14, or
15(d) of the Exchange Act between the date of this prospectus and the termination of this offering, provided, however, that we are
not incorporating any information furnished under Item 2.02 or Item 7.01 of any current report on Form 8-K:
| |
· |
|
Our Annual Report on Form 10-K for the fiscal year September 30, 2023 filed with the SEC on December 7, 2023, as amended on January 26, 2024. |
| |
|
|
|
| |
· |
|
Our Quarterly Report on Form 10-Q for the three-month periods ended December 31, 2023, filed with the SEC on February 8, 2024, March 31, 2024, filed with the SEC on May 10, 2024, and June 30, 2024, filed with the SEC on August 8, 2024, respectively; |
| |
· |
|
Our Current Reports on Form 8-K (other than portions thereof furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits accompanying such reports that are related to such items) filed with the SEC on November 7, 2023, December 6, 2023, January 5, 2024, January 31, 2024, February 1, 2024, April 16, 2024, April 19, 2024, April 22, 2024, as amended on April 23, 2024, May 16, 2024, May 29, 2024, June 18, 2024, June 28, 2024, July 15, 2024, August 2, 2024, September 18, 2024, September 30, 2024, October 31, 2024, November 7, 2024 and November 13, 2024; and |
Upon written or oral
request, we will provide without charge to each person, including any beneficial owner, to whom a copy of the prospectus is delivered
a copy of the documents incorporated by reference in this prospectus (other than exhibits to such documents unless such exhibits are
specifically incorporated by reference in this prospectus). You may request a copy of these filings, at no cost, by writing or telephoning
us at the following address: Applied DNA Sciences, Inc., 50 Health Sciences Drive, Stony Brook, New York 11790, c/o Investor Relations,
telephone: 631-240-8800. You may also access these documents on our website at www.adnas.com.
Information on our website, including subsections,
pages, or other subdivisions of our website, or any website linked to by content on our website, is not part of this prospectus and you
should not rely on that information unless that information is also in this prospectus or incorporated by reference in this prospectus.
Up to 20,312,500
shares of Common Stock underlying the Series C Warrants
Up to 20,312,500
shares of Common Stock underlying the Series D Warrants
Up to 1,015,625
shares of Common Stock underlying the Placement Agent Warrants

Applied DNA Sciences, Inc.
PRELIMINARY PROSPECTUS
,
2024
PART II
INFORMATION NOT
REQUIRED IN THE PROSPECTUS
| Item 13. |
Other Expenses of Issuance and
Distribution |
The following table
sets forth the expenses to be incurred in connection with the offering described in this Registration Statement. All amounts are estimates
except the SEC’s registration fee.
| | |
Amount to be Paid | |
| SEC
Registration Fee | |
$ | 1,179 | |
| Printing
expenses | |
$ | 10,000 | |
| Legal
fees and expenses | |
$ | 75,000 | |
| Accounting
fees and expenses | |
$ | 7,000 | |
| Transfer
agent and registrar fees | |
$ | 5,000 | |
| Miscellaneous
expenses | |
$ | 2,000 | |
| Total | |
$ | 100,179 | |
| Item 14. |
Indemnification of Directors and
Officers |
Section 145 of
the Delaware General Corporation Law empowers a corporation to indemnify its directors and officers and to purchase insurance with respect
to liability arising out of their capacity or status as directors and officers, provided that the person acted in good faith and in a
manner the person reasonably believed to be in our best interests, and, with respect to any criminal action, had no reasonable cause
to believe the person’s actions were unlawful. The Delaware General Corporation Law further provides that the indemnification permitted
thereunder shall not be deemed exclusive of any other rights to which the directors and officers may be entitled under the corporation’s
bylaws, any agreement, a vote of stockholders or otherwise. The amended and restated certificate of incorporation of the registrant provides
for the indemnification of the registrant’s directors and officers to the fullest extent permitted under the Delaware General Corporation
Law. In addition, the amended and restated bylaws of the registrant require the registrant to fully indemnify any person who was or is
a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (whether civil, criminal,
administrative or investigative) by reason of the fact that such person is or was a director or officer of the registrant, or is or was
a director or officer of the registrant serving at the registrant’s request as a director, officer, employee or agent of another
corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorney’s fees), judgments, fines
and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, to
the fullest extent permitted by applicable law.
Section 102(b)(7) of
the Delaware General Corporation Law permits a corporation to provide in its certificate of incorporation that a director of the corporation
shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director,
except (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders; (ii) for acts or
omissions not in good faith or which involve intentional misconduct or a knowing violation of law; (iii) for payments of unlawful
dividends or unlawful stock repurchases or redemptions; or (iv) for any transaction from which the director derived an improper
personal benefit. The registrant’s amended and restated certificate of incorporation provides that the registrant’s directors
shall not be personally liable to it or its stockholders for monetary damages for breach of fiduciary duty as a director and that if
the Delaware General Corporation Law is amended to authorize corporate action further eliminating or limiting the personal liability
of directors, then the liability of the registrant’s directors shall be eliminated or limited to the fullest extent permitted by
the Delaware General Corporation Law, as so amended.
As permitted by the
Delaware General Corporation Law, the registrant intends to enter into separate indemnification agreements with each of the registrant’s
directors and certain of the registrant’s officers which require the registrant, among other things, to indemnify them against
certain liabilities which may arise by reason of their status as directors, officers or certain other employees.
The registrant has
obtained and maintains insurance policies under which its directors and officers are insured, within the limits and subject to the limitations
of those policies, against certain expenses in connection with the defense of, and certain liabilities which might be imposed as a result
of, actions, suits or proceedings to which they are parties by reason of being or having been directors or officers. The coverage provided
by these policies may apply whether or not the registrant would have the power to indemnify such person against such liability under
the provisions of the Delaware General Corporation Law.
These indemnification
provisions and the indemnification agreements the registrant intends to enter into between the registrant and the registrant’s
officers and directors may be sufficiently broad to permit indemnification of the registrant’s officers and directors for liabilities
(including reimbursement of expenses incurred) arising under the Securities Act of 1933, as amended.
| Item 15. |
Recent Sales of Unregistered Securities |
February 2022
Offering
On February 21,
2022, concurrently with a registered direct offering of 1,496,400 shares of our Common Stock and/or pre-funded warrants (the “February 2022
Pre-Funded Warrants”) to an institutional investor (the “February 2022 Purchaser”), we issued to the February 2022
Purchaser in a private placement (together with the registered direct offering, the “February 2022 Offerings”) unregistered
warrants (“February 2022 Common Warrants”) to purchase up to 1,496,400 shares of Common Stock. Each February 2022
Common Warrant has an exercise price of $2.84 per share, is exercisable six months from the date of issuance and will expire five years
from the initial exercise date.
Roth Capital Partners,
LLC (the “February 2022 Placement Agent”) acted as the exclusive placement agent for the February 2022 Offerings,
pursuant to a placement agency agreement (the “February 2022 Placement Agreement”), dated February 21, 2022, by
and between the Company and the February 2022 Placement Agent.
The closing of the
February 2022 Offerings took place on February 24, 2022. The February 2022 Common Warrants and shares of Common Stock
issuable upon exercise thereof were issued in reliance on the exemptions from registration provided by Section 4(a)(2) under
the Securities Act and Regulation D promulgated thereunder for transactions not involving a public offering.
The net proceeds from
the February 2022 Offerings were approximately $3.7 million. Net proceeds are what we received after paying the placement agent’s
fees and other expenses of the offering. The net proceeds exclude the proceeds, if any, from the exercise of the February 2022 Pre-Funded
Warrants and the February 2022 Common Warrants sold in the February 2022 Offerings.
Each of the above
securities were not registered under the Securities Act and were issued in reliance on the exemptions from registration provided by Section 4(a)(2) under
the Securities Act and Regulation D promulgated thereunder, for transactions not involving a public offering. The Company filed
a registration statement for the resale of the February 2022 Common Warrants on July 19, 2022, and the registration statement
was declared effective on July 27, 2022.
January 2024
Offering
On
January 31, 2024, the Company entered into a Placement Agreement (the “January 2024 Placement Agreement”) with
Maxim Group (“Maxim”) pursuant to which Maxim agreed to serve as the sole placement agent, on a “reasonable best efforts”
basis, in connection with the an offering (the “January 2024 Offering”) of 3,228,056 shares of the Company’s
Common Stock and pre-funded warrants to purchase up to 2,416,005 shares of Common Stock, and in a concurrent private placement,
unregistered common warrants (the “January 2024 Common Warrants”) to purchase up to 41,640,625 shares of Common Stock.
Also on January 31, 2024, in connection with the Offering, the Company entered into purchase agreements with the purchasers in the
January 2024 Offering (the “January 2024 Purchase Agreements”).
The
January 2024 Offering closed on February 2, 2024. The Company received gross proceeds from the January 2024 Offering,
before deducting placement agent fees and other estimated offering expenses payable by the Company, of approximately $3.4 million.
Each
of the above securities were not registered under the Securities Act and were issued in reliance on the exemptions from registration
provided by Section 4(a)(2) under the Securities Act and Regulation D promulgated thereunder, for transactions not involving
a public offering.
Pursuant
to the January 2024 Purchase Agreements, within 45 calendar days from the date of the January 2024 Purchase Agreements,
the Company agreed to file a registration statement on Form S-3 (or other appropriate form if the Company is not then S-3 eligible)
providing for the resale by the Purchasers of the Shares issuable upon exercise of the Private Common Warrants. The Company agreed to
use commercially reasonable efforts to cause such registration statement to become effective within 90 days following the closing
date of the Purchase Agreements and to keep such registration statement effective at all times until no Purchaser owns any January 2024
Common Warrants or shares of Common Stock issuable upon exercise thereof. The Company filed the registration statement for this transaction
on March 12, 2024, and the registration statement was declared effective on March 20, 2024.
October 2024 Offering
On October 30,
2024, the Company entered into the Purchase Agreement with certain institutional investors, pursuant to which the Company agreed to issue
and sell in the Offering (i) 19,247,498 shares Company’s Common Stock and Pre-Funded Warrants to purchase up to 1,065,002
shares of Common Stock, and (ii) Series C Warrants to purchase up to 20,312,500 shares of Common Stock, Series D Warrants
to purchase up to 20,312,500 shares of Common Stock and (iii) Placement Agent Warrants to purchase up to 1,015,625.
The Company received
gross proceeds from the Offering, before deducting placement agent fees and other estimated offering expenses payable by the Company,
of approximately $6.5 million.
Each of the above
securities were not registered under the Securities Act and were issued in reliance on the exemptions from registration provided by Section 4(a)(2) under
the Securities Act and Regulation D promulgated thereunder, for transactions not involving a public offering.
Pursuant to the Purchase
Agreement, within 20 calendar days from the date of the Purchase Agreement, the Company agreed to file a registration statement on Form S-1
providing for the resale by the purchasers in the Offering of the shares of Common Stock issuable upon exercise of the Private Placement
Warrants. The Company agreed to use commercially reasonable efforts to cause such registration statement to become effective within 50
calendar days following the closing date of the Purchase Agreement (or 90 calendar days following the closing date of the Purchase Agreement
in the event that the Commission requires the Company to include its audited year-end financial statements for the fiscal year ended
September 30, 2024 in such registration statement) and to keep such registration statement effective at all times until no Purchaser
owns any Private Placement Warrants or shares of Common Stock issuable upon exercise thereof.
| Item 16. |
Exhibit and Financial Statement
Schedules |
(a) Exhibits.
The exhibit index
attached hereto is incorporated herein by reference.
(b) Financial Statement Schedules.
Schedules have been
omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
The undersigned registrant
hereby undertakes:
(1) To file,
during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include
any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect
in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective
amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities
offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range
may be reflected in the form of prospectus filed with the U.S. Securities and Exchange Commission pursuant to Rule 424(b) if,
in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set
forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include
any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material
change to such information in the registration statement; provided, however, that paragraphs (1)(i), (1)(ii) and (1)(iii) above
do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed
with or furnished to the Securities and Exchange Commission by the registrant pursuant to Section 13 or Section 15(d) of
the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement.
(2) That, for
the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a
new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed
to be the initial bona fide offering thereof.
(3) To remove
from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination
of the offering.
(4) That, for
the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as
part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than
prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the
date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that
is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement
or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first
use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement
or made in any such document immediately prior to such date of first use.
(5) That for
the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution
of securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant
to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities
are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to
the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary
prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free
writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned
registrant;
(iii) The portion
of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its
securities provided by or on behalf of the undersigned registrant; and
(iv) Any other
communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(6) Insofar as
indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons
of the registrant pursuant to any charter provision, by law or otherwise, the registrant has been advised that in the opinion of the
U.S. Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and
is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than payment by the registrant
of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action,
suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the
registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate
jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be
governed by the final adjudication of such issue.
(7) The undersigned
registrant hereby undertakes that:
(i) For purposes
of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this
registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or
(4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared
effective; and
(ii) For the
purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus
shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at
that time shall be deemed to be the initial bona fide offering thereof.
EXHIBIT INDEX
| Exhibit |
|
|
|
Incorporated by Reference |
|
Filed or
Furnished |
| Number |
|
Description |
|
Form |
|
Exhibit |
|
File No. |
|
Date Filed |
|
Herewith |
| 2.1*† |
|
Share Purchase Agreement, dated July 12, 2023, by and among Spindle Acquisition Corp., Spindle Biotech Inc., the persons listed on Schedule 1.1 therein, Lai Him Chung and Applied DNA Sciences, Inc. |
|
8-K |
|
2.1 |
|
001-36745 |
|
7/13/2023 |
|
|
| 3.1 |
|
Conformed version of Certificate of Incorporation of Applied DNA Sciences, Inc., as most recently amended by the Sixth Certificate of Amendment, effective Thursday, April 25, 2024 |
|
S-1/A |
|
3.1 |
|
333-278890 |
|
05/24/2024 |
|
|
| 3.2 |
|
Conformed version of By-Laws, as amended by the Certificate of Amendment to the By-Laws, effective November 7, 2024. |
|
|
|
|
|
|
|
|
|
Filed |
| 4.1 |
|
Description of Securities |
|
10-K |
|
4.1 |
|
001-36745 |
|
12/9/2021 |
|
|
| 4.2 |
|
Form of Purchase Warrant |
|
8-K |
|
4.1 |
|
001-36745 |
|
12/20/2017 |
|
|
| 4.3 |
|
Common Stock Purchase Warrant |
|
8-K |
|
4.1 |
|
001-36745 |
|
12/21/2018 |
|
|
| 4.4 |
|
Form of common warrant certificate (included in the Warrant Agreement, dated November 15, 2019) |
|
8-K |
|
4.2 |
|
001-36745 |
|
11/18/2019 |
|
|
| 4.5 |
|
Form of Indenture |
|
S-3 |
|
4.1 |
|
333-238557 |
|
05/21/2020 |
|
|
| 4.6 |
|
Form of Common Stock Purchase Warrant |
|
8-K |
|
10.3 |
|
001-36745 |
|
10/14/2020 |
|
|
| 4.7 |
|
Form of Pre-Funded Common Stock Purchase Warrant |
|
8-K |
|
4.1 |
|
001-36745 |
|
2/23/2022 |
|
|
| 4.8 |
|
Form of Common Stock Purchase Warrant |
|
8-K |
|
4.2 |
|
001-36745 |
|
2/23/2022 |
|
|
| 4.9 |
|
Form of Series A Warrant |
|
8-K |
|
4.1 |
|
001-36745 |
|
8/9/2022 |
|
|
| 4.10 |
|
Form of Series B Warrant |
|
8-K |
|
4.2 |
|
001-36745 |
|
8/9/2022 |
|
|
| 4.11 |
|
Form of Prefunded Warrant |
|
8-K |
|
4.3 |
|
001-36745 |
|
8/9/2022 |
|
|
| 4.12 |
|
Form of Pre-Funded Warrant. |
|
8-K |
|
4.1 |
|
001-36745 |
|
02/01/2024 |
|
|
| 4.13 |
|
Form of Private Common Warrant. |
|
8-K |
|
4.2 |
|
001-36745 |
|
02/01/2024 |
|
|
| 4.14 |
|
Form of Pre-Funded Warrant |
|
8-K |
|
4.4 |
|
001-36745 |
|
05/29/2024 |
|
|
| 4.15 |
|
Form of Series A Warrant |
|
8-K |
|
4.2 |
|
001-36745 |
|
05/29/2024 |
|
|
| 4.16 |
|
Form of Series B Warrant |
|
8-K |
|
4.3 |
|
001-36745 |
|
05/29/2024 |
|
|
| 4.17 |
|
Form of Placement Agent Warrant |
|
8-K |
|
4.1 |
|
001-36745 |
|
05/29/2024 |
|
|
| 4.18 |
|
Form of Pre-Funded Warrant |
|
8-K |
|
4.1 |
|
001-36745 |
|
10/30/2024 |
|
|
| 4.19 |
|
Form of Series C Common Stock Purchase Warrant |
|
8-K |
|
4.2 |
|
001-36745 |
|
10/30/2024 |
|
|
| 4.20 |
|
Form of Series D Common Stock Purchase Warrant |
|
8-K |
|
4.3 |
|
001-36745 |
|
10/30/2024 |
|
|
| 4.21 |
|
Form of Placement Agent Warrant |
|
8-K |
|
4.4 |
|
001-36745 |
|
10/30/2024 |
|
|
| 5.1 |
|
Opinion of McDermott Will & Emery LLP |
|
|
|
|
|
|
|
|
|
Filed |
| 10.1† |
|
Form of employee stock option agreement under the Applied DNA Sciences, Inc. 2005 Incentive Stock Plan |
|
10-Q |
|
4.1 |
|
002-90539 |
|
05/15/2012 |
|
|
| 10.2† |
|
Applied DNA Sciences, Inc. 2005 Incentive Stock Plan, as amended and restated |
|
DEF 14A |
|
Appendix A |
|
001-36745 |
|
04/04/2019 |
|
|
| 10.3† |
|
Form of employee stock option agreement under the Applied DNA Sciences, Inc. 2005 Incentive Stock Plan, as amended |
|
10-K |
|
10.1 |
|
001-36745 |
|
12/14/2015 |
|
|
| 10.4† |
|
Applied DNA Sciences, Inc. 2020 Equity Incentive Plan |
|
DEF 14A |
|
Appendix A |
|
001-36745 |
|
08/03/2020 |
|
|
| 10.5† |
|
Applied DNA Sciences, Inc. 2020 Equity Incentive Plan Stock Option Grant Notice and Award Agreement |
|
S-8 |
|
10.3 |
|
333-249365 |
|
10/07/2020 |
|
|
| 10.6† |
|
Employment Agreement, dated July 1, 2016, between James A. Hayward and Applied DNA Sciences, Inc. |
|
8-K |
|
10.1 |
|
001-36745 |
|
8/2/2016 |
|
|
| 10.7† |
|
Form of Indemnification Agreement dated as of September 7, 2012, by and between Applied DNA Sciences, Inc. and each of its directors and executive officers |
|
8-K |
|
10.1 |
|
002-90539 |
|
9/13/2012 |
|
|
| 10.8 |
|
Warrant Agreement, dated November 20, 2014, between Applied DNA Sciences, Inc. and American Stock Transfer & Trust Company, LLC as warrant agent |
|
8-K |
|
4.1 |
|
001-36745 |
|
11/20/2014 |
|
|
| 10.9 |
|
First Amendment to Warrant Agreement dated April 1, 2015 between Applied DNA Sciences, Inc. and American Stock Transfer & Trust Company, LLC as warrant agent |
|
8-K |
|
4.1 |
|
001-36745 |
|
4/1/2015 |
|
|
| 10.10 |
|
Second Amendment to Warrant Agreement dated November 2, 2016 |
|
8-K |
|
10.4 |
|
001-36745 |
|
11/2/2016 |
|
|
| 10.11 |
|
Registration Rights Agreement dated November 2, 2016 |
|
8-K |
|
10.3 |
|
001-36745 |
|
11/2/2016 |
|
|
| 10.12* |
|
License Agreement with Himatsingka America, Inc. dated June 23, 2017 |
|
10-Q |
|
10.1 |
|
001-36745 |
|
8/10/2017 |
|
|
| 10.13 |
|
Placement Agency Agreement by and between Applied DNA Sciences, Inc. and Maxim Group LLC, dated December 20, 2017. |
|
8-K |
|
10.1 |
|
001-36745 |
|
12/20/2017 |
|
|
| 10.14 |
|
Registration Rights Agreement, dated November 29, 2018 |
|
8-K |
|
10.2 |
|
001-36745 |
|
12/6/2018 |
|
|
| 10.15 |
|
Securities Purchase Agreement, dated November 29, 2018 |
|
8-K |
|
10.3 |
|
001-36745 |
|
12/6/2018 |
|
|
| 10.16 |
|
Registration Rights Agreement, dated August 31, 2018 |
|
8-K/A |
|
10.2 |
|
001-36745 |
|
12/10/2018 |
|
|
| 10.17 |
|
Securities Purchase Agreement, dated August 31, 2018 |
|
10-K |
|
10.45 |
|
001-36745 |
|
12/18/2018 |
|
|
| 10.18+ |
|
Patent and Know-How License and Cooperation Agreement, dated March 28, 2019, between the Company, APDN (B.V.I.), Inc., and ETCH BioTrace S.A. |
|
10-Q |
|
10.10 |
|
001-36745 |
|
5/9/2019 |
|
|
| 10.19 |
|
Registration Rights Agreement, dated July 16, 2019 by and among Applied DNA Sciences, Inc. and the investor named on the signature page thereof. |
|
8-K |
|
10.2 |
|
001-36745 |
|
07/17/2019 |
|
|
| 10.20 |
|
Securities Purchase Agreement, dated July 16, 2019 by and among Applied DNA Sciences, Inc. and the investor named on the signature page thereof. |
|
8-K |
|
10.3 |
|
001-36745 |
|
07/17/2019 |
|
|
| 10.21 |
|
Asset Purchase Agreement, dated July 29, 2019 by and between LineaRX, Inc. and Vitatex Inc. |
|
8-K |
|
10.1 |
|
001-36745 |
|
8/12/2019 |
|
|
| 10.22 |
|
Form of Subscription Agreement between investors and Applied DNA Sciences, Inc., dated August 22, 2019. |
|
8-K |
|
10.1 |
|
001-36745 |
|
8/26/2019 |
|
|
| 10.23 |
|
Underwriting Agreement entered into by and between Applied DNA Sciences, Inc. and Maxim Group LLC, as Representative of the Underwriters listed in Schedule I hereto, dated November 13, 2019. |
|
8-K |
|
1.1 |
|
001-36745 |
|
11/14/2019 |
|
|
| 10.24 |
|
Warrant Agreement, dated November 15, 2019, between Applied DNA Sciences, Inc. and American Stock Transfer & Trust Company, LLC |
|
8-K |
|
4.1 |
|
001-36745 |
|
11/18/2019 |
|
|
| 10.25† |
|
Consulting Agreement, dated as of December 12, 2019, by and between Applied DNA Sciences, Inc. and Meadow Hill Place, LLC |
|
10-Q |
|
10.1 |
|
001-36745 |
|
08/06/2020 |
|
|
| 10.26 |
|
Agreement of Lease dated June 14, 2013, between Applied DNA Sciences, Inc. and Long Island High Technology Incubator, Inc. |
|
10-Q |
|
10.2 |
|
002-90539 |
|
8/13/2013 |
|
|
| 10.27 |
|
Agreement of Lease, dated November 1, 2015, by and between Applied DNA Sciences, Inc. and Long Island High Technology Incubator, Inc. |
|
10-Q |
|
10.2 |
|
001-36745 |
|
08/06/2020 |
|
|
| 10.28 |
|
Option Exercise Notice, dated December 3, 2015, Pursuant to Lease dated June 14, 2013, between Applied DNA Sciences, Inc. and Long Island High Technology Incubator, Inc. |
|
10-Q |
|
10.2 |
|
001-36745 |
|
05/12/2016 |
|
|
| 10.29 |
|
Temporary Lease Extension Agreement, dated August 9, 2019, by and between Applied DNA Sciences, Inc. and Long Island High Technology Incubator, Inc. |
|
10-Q |
|
10.3 |
|
001-36745 |
|
08/06/2020 |
|
|
| 10.30 |
|
Amendment to Leases, dated November 4, 2019, by and between Long Island High Technology Incubator, Inc. and Applied DNA Sciences, Inc. |
|
10-Q |
|
10.4 |
|
001-36745 |
|
08/06/2020 |
|
|
| 10.31 |
|
Amendment to Leases, dated January 17, 2020, by and between Long Island High Technology Incubator, Inc. and Applied DNA Sciences, Inc. |
|
10-Q |
|
10.5 |
|
001-36745 |
|
08/06/2020 |
|
|
| 10.32 |
|
Registration Rights Agreement, dated October 7, 2020, by and between Applied DNA Sciences, Inc. and Dillon Hill Capital, LLC. |
|
8-K |
|
10.4 |
|
001-36745 |
|
10/14/2020 |
|
|
| 10.33 |
|
Registration Rights Agreement, dated October 7, 2020, by and between Applied DNA Sciences, Inc. and Dillon Hill Investment Company LLC. |
|
8-K |
|
10.5 |
|
001-36745 |
|
10/14/2020 |
|
|
| 10.34+ |
|
Joint Development Agreement, dated September 11, 2018, between LineaRx, Inc., Takis S.R.L. and Evvivax S.R.L., as amended by that First Amendment, dated February 3, 2020 |
|
10-K |
|
10.46 |
|
001-36745 |
|
12/17/2020 |
|
|
| 10.35 |
|
Animal Clinical Trial Agreement, dated September 14, 2020, between Applied DNA Sciences, Inc., Evvivax S.R.L. and Veterinary Oncology Services, PLLC |
|
10-K |
|
10.47 |
|
001-36745 |
|
12/17/2020 |
|
|
| 10.36 |
|
Letter Agreement dated March 2, 2021, by and between the Company and Dr. James Hayward |
|
8-K |
|
10.1 |
|
001-36745 |
|
3/4/2021 |
|
|
| 10.37 |
|
Office Lease Renewal Letter Agreement, dated February 1, 2022, by and between Long Island High Technology Incubator, Inc. and Applied DNA Sciences, Inc. |
|
10-K |
|
10.43 |
|
001 36745 |
|
12/14/2022 |
|
|
| 10.38 |
|
Laboratory Lease Renewal Letter Agreement, dated February 1, 2022, by and between Long Island High Technology Incubator, Inc. and Applied DNA Sciences, Inc. |
|
10-K |
|
10.44 |
|
001 36745 |
|
12/14/2022 |
|
|
| 10.39+ |
|
Contract Number T212206, dated August 3, 2021, by and between The City University of New York and Applied DNA Clinical Labs, LLC. |
|
10-K |
|
10.45 |
|
001 36745 |
|
12/14/2022 |
|
|
| 10.40+ |
|
First Amendment to Contract No. T212206, dated December 16, 2021, by and between The City University of New York and Applied DNA Clinical Labs, LLC. |
|
10-K |
|
10.46 |
|
001 36745 |
|
12/14/2022 |
|
|
| 10.41+ |
|
Second Amendment to Contract No. T212206, dated July 19, 2022, by and between The City University of New York and Applied DNA Clinical Labs, LLC. |
|
10-K |
|
10.47 |
|
001 36745 |
|
12/14/2022 |
|
|
| 10.42 |
|
Equity Distribution Agreement, dated November 7, 2023, by and between Applied DNA Sciences, Inc. and Maxim Group LLC |
|
8-K |
|
10.1 |
|
001-36745 |
|
11/7/2023 |
|
|
| 10.43† |
|
Letter Agreement, dated January 4, 2024, by and between Applied DNA Sciences, Inc. and James A. Hayward. |
|
8-K |
|
10.1 |
|
001-36745 |
|
1/5/2024 |
|
|
| 10.44† |
|
Letter Agreement, dated January 4, 2024, by and between Applied DNA Sciences, Inc. and Judith Murrah. |
|
8-K |
|
10.2 |
|
001-36745 |
|
1/5/2024 |
|
|
| 10.45 |
|
Amended and Restated Lease Agreement, dated February 24, 2023, by and between Long Island High Technology Incubator, Inc. and Applied DNA Sciences, Inc. (Office Lease). |
|
8-K |
|
10.1 |
|
001-36745 |
|
02/28/2023 |
|
|
| 10.46 |
|
Amended and Restated Lease Agreement, dated February 24, 2023, by and between Long Island High Technology Incubator, Inc. and Applied DNA Sciences, Inc. (Laboratory Lease). |
|
8-K |
|
10.2 |
|
001-36745 |
|
02/28/2023 |
|
|
| 10.47 |
|
Lease Renewal Agreement dated January 10, 2024 (Laboratory Lease). |
|
10-Q |
|
10.3 |
|
001-36745 |
|
02/08/2024 |
|
|
| 10.48 |
|
Placement Agency Agreement by and between Applied DNA Sciences, Inc. and Maxim Group LLC, dated January 31, 2024. |
|
8-K |
|
10.1 |
|
001-36745 |
|
02/01/2024 |
|
|
| 10.49 |
|
Form of Securities Purchase Agreement, dated January 31, 2024, by and between Applied DNA Sciences, Inc. and the parties thereto. |
|
8-K |
|
10.2 |
|
001-36745 |
|
02/01/2024 |
|
|
| 10.50 |
|
Form of Purchase Warrant Amendment |
|
8-K |
|
10.1 |
|
001-36745 |
|
04/19/2024 |
|
|
| 10.51 |
|
Form of Book-Entry Warrant Amendment |
|
8-K |
|
10.2 |
|
001-36745 |
|
04/19/2024 |
|
|
| 10.50 |
|
Form of Placement Agency Agreement by and between Applied DNA Sciences, Inc. Craig-Hallum Capital Group LLC and Laidlaw & Company (UK) Ltd. |
|
8-K |
|
10.1 |
|
001-36745 |
|
05/29/2024 |
|
|
| 10.51 |
|
Form of Securities Purchase Agreement, dated October 30, 2024, by and between Applied DNA Sciences, Inc. and the parties thereto. |
|
8-K |
|
10.1 |
|
001-36745 |
|
10/30/2024 |
|
|
| 10.52 |
|
Form of Warrant Amendment |
|
8-K |
|
10.2 |
|
001-36745 |
|
10/30/2024 |
|
|
| 10.53 |
|
Waiver of Negative Covenant |
|
8-K |
|
10.3 |
|
001-36745 |
|
10/30/2024 |
|
|
| 14.1 |
|
Code of Business Conduct and Ethics. |
|
10-K |
|
14.1 |
|
001-36745 |
|
12/14/2022 |
|
|
| 21.1 |
|
Subsidiaries of Applied DNA Sciences, Inc. |
|
10-K |
|
21.1 |
|
001-36745 |
|
12/07/2023 |
|
|
| † |
Indicates
a management contract or any compensatory plan, contract or arrangement. |
| * |
A request
for confidentiality has been granted for certain portions of the indicated document. Confidential portions have been omitted and
filed separately with the SEC as required by Rule 24b-2 promulgated under the Exchange Act. |
| + |
Portions
of this exhibit have been omitted because the information is both not material and is the type that the Company treats as private
or confidential. The omissions have been indicated by bracketed asterisks (“[***]”). |
SIGNATURES
Pursuant to the requirements
of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the
undersigned, thereunto duly authorized, in the Town of Stony Brook, State of New York, on the 18th day of November, 2024.
| APPLIED DNA SCIENCES, INC. |
|
| |
|
|
| By: |
/s/
James A. Hayward |
|
| |
James
A. Hayward |
|
| |
President
and Chief Executive Officer |
|
POWER
OF ATTORNEY
KNOW ALL MEN BY THESE
PRESENTS, that each officer and director of Applied DNA Sciences, Inc. whose signature appears below constitutes and appoints Beth
M. Jantzen his or her true and lawful attorney-in-fact and agent, with full power of substitution and revocation, for him or her and
in his or her name, place and stead, in any and all capacities, to execute any or all amendments including any post-effective amendments
and supplements to this Registration Statement, and any additional Registration Statement filed pursuant to Rule 462(b), and to
file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting
unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary
to be done, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said
attorney-in-fact and agent, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
****
Pursuant to the requirements
of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities and
on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
/s/
James A. Hayward
James A. Hayward |
|
Chief Executive Officer, President and
Chairman of the Board of Directors
(Principal Executive Officer) |
|
November 18, 2024 |
| |
|
|
/s/
Beth Jantzen
Beth Jantzen |
|
Chief Financial Officer
(Principal Financial Officer and Principal Accounting Officer) |
|
November 18, 2024 |
| |
|
|
/s/
Robert B. Catell
Robert B. Catell |
|
Director |
|
November 18, 2024 |
| |
|
|
/s/
Joseph D. Ceccoli
Joseph D. Ceccoli |
|
Director |
|
November 18, 2024 |
| |
|
|
/s/
Sanford R. Simon
Sanford R. Simon |
|
Director |
|
November 18, 2024 |
| |
|
|
/s/
Yacov A. Shamash
Yacov A. Shamash |
|
Director |
|
November 18, 2024 |
| |
|
|
|
|
/s/
Elizabeth M. Schmalz Shaheen
Elizabeth M. Schmalz
Shaheen |
|
Director |
|
November 18, 2024 |
Exhibit 3.2
BYLAWS
OF
APPLIED DNA SCIENCES, INC.
TABLE OF CONTENTS
| |
|
|
Page |
| |
|
|
|
| ARTICLE I |
|
CORPORATE OFFICES |
1 |
| 1.1 |
|
REGISTERED OFFICE |
1 |
| 1.2 |
|
OTHER OFFICES |
1 |
| ARTICLE II |
|
MEETINGS OF STOCKHOLDERS |
1 |
| 2.1 |
|
PLACE OF MEETINGS |
1 |
| 2.2 |
|
ANNUAL MEETING |
1 |
| 2.3 |
|
SPECIAL MEETING |
1 |
| 2.4 |
|
NOTICE OF STOCKHOLDERS’ MEETINGS |
2 |
| 2.5 |
|
MANNER OF GIVING NOTICE; AFFIDAVIT OF NOTICE |
3 |
| 2.6 |
|
QUORUM |
3 |
| 2.7 |
|
ADJOURNED MEETING; NOTICE |
3 |
| 2.8 |
|
ADMINISTRATION OF THE MEETING |
3 |
| 2.9 |
|
VOTING |
4 |
| 2.10 |
|
STOCKHOLDER ACTION BY WRITTEN CONSENT WITHOUT A MEETING |
5 |
| 2.11 |
|
RECORD DATE FOR STOCKHOLDER NOTICE; VOTING; GIVING CONSENTS |
5 |
| 2.12 |
|
PROXIES |
5 |
| 2.13 |
|
LIST OF STOCKHOLDERS ENTITLED TO VOTE |
6 |
| 2.14 |
|
NOTICE OF STOCKHOLDER BUSINESS AND NOMINATIONS |
6 |
| 2.15 |
|
SUBMISSION OF QUESTIONNAIRE, REPRESENTATION AND AGREEMENT |
11 |
| ARTICLE III |
|
DIRECTORS |
11 |
| 3.1 |
|
POWERS |
11 |
| 3.2 |
|
NUMBER OF DIRECTORS |
11 |
| 3.3 |
|
ELECTION, QUALIFICATION AND TERM OF OFFICE OF DIRECTORS |
11 |
| 3.4 |
|
RESIGNATION AND VACANCIES |
12 |
| 3.5 |
|
PLACE OF MEETINGS; MEETINGS BY TELEPHONE |
12 |
| 3.6 |
|
REGULAR MEETINGS |
12 |
| 3.7 |
|
SPECIAL MEETINGS; NOTICE |
12 |
| 3.8 |
|
QUORUM |
13 |
| 3.9 |
|
WAIVER OF NOTICE |
13 |
| 3.10 |
|
BOARD ACTION BY WRITTEN CONSENT WITHOUT A MEETING |
13 |
| 3.11 |
|
ADJOURNED MEETING; NOTICE |
14 |
| 3.12 |
|
FEES AND COMPENSATION OF DIRECTORS |
14 |
| 3.13 |
|
REMOVAL OF DIRECTORS |
14 |
| ARTICLE IV |
|
COMMITTEES |
14 |
| 4.1 |
|
COMMITTEES OF DIRECTORS |
14 |
| 4.2 |
|
COMMITTEE MINUTES |
14 |
| 4.3 |
|
MEETINGS AND ACTION OF COMMITTEES |
15 |
| ARTICLE V |
|
OFFICERS |
15 |
| 5.1 |
|
OFFICERS |
15 |
| 5.2 |
|
APPOINTMENT OF OFFICERS |
16 |
| 5.3 |
|
SUBORDINATE OFFICERS |
16 |
| 5.4 |
|
REMOVAL AND RESIGNATION OF OFFICERS |
16 |
| 5.5 |
|
VACANCIES IN OFFICES |
16 |
| 5.6 |
|
REPRESENTATION OF SHARES OF OTHER CORPORATIONS |
16 |
| 5.7 |
|
AUTHORITY AND DUTIES OF OFFICERS |
17 |
| ARTICLE VI |
|
RECORDS AND REPORTS |
17 |
| 6.1 |
|
MAINTENANCE AND INSPECTION OF RECORDS |
17 |
| 6.2 |
|
INSPECTION BY DIRECTORS |
17 |
| ARTICLE VII |
|
GENERAL MATTERS |
17 |
| 7.1 |
|
CHECKS; DRAFTS; EVIDENCES OF INDEBTEDNESS |
17 |
| 7.2 |
|
EXECUTION OF CORPORATE CONTRACTS AND INSTRUMENTS |
17 |
| 7.3 |
|
STOCK CERTIFICATES; PARTLY PAID SHARES |
18 |
| 7.4 |
|
SPECIAL DESIGNATION ON CERTIFICATES |
18 |
| 7.5 |
|
LOST CERTIFICATES |
18 |
| 7.6 |
|
DIVIDENDS |
19 |
| 7.7 |
|
FISCAL YEAR |
19 |
| 7.8 |
|
SEAL |
19 |
| 7.9 |
|
TRANSFER OF STOCK |
19 |
| 7.10 |
|
STOCK TRANSFER AGREEMENTS |
19 |
| 7.11 |
|
REGISTERED STOCKHOLDERS |
19 |
| 7.12 |
|
WAIVER OF NOTICE |
20 |
| ARTICLE VIII |
|
NOTICE BY ELECTRONIC TRANSMISSION |
20 |
| 8.1 |
|
NOTICE BY ELECTRONIC TRANSMISSION |
20 |
| 8.2 |
|
DEFINITION OF ELECTRONIC TRANSMISSION |
21 |
| 8.3 |
|
INAPPLICABILITY |
21 |
| ARTICLE IX |
|
INDEMNIFICATION OF DIRECTORS AND OFFICERS |
21 |
| 9.1 |
|
POWER TO INDEMNIFY IN ACTIONS, SUITS OR PROCEEDINGS OTHER THAN THOSE BY OR IN THE RIGHT OF THE CORPORATION |
21 |
| 9.2 |
|
POWER TO INDEMNIFY IN ACTIONS, SUITS OR PROCEEDINGS BY OR IN THE RIGHT OF THE CORPORATION |
22 |
| 9.3 |
|
AUTHORIZATION OF INDEMNIFICATION |
22 |
| 9.4 |
|
GOOD FAITH DEFINED |
23 |
| 9.5 |
|
INDEMNIFICATION BY A COURT |
23 |
| 9.6 |
|
EXPENSES PAYABLE IN ADVANCE |
23 |
| 9.7 |
|
NONEXCLUSIVITY OF INDEMNIFICATION AND ADVANCEMENT OF EXPENSES |
24 |
| 9.8 |
|
INSURANCE |
24 |
| 9.9 |
|
CERTAIN DEFINITIONS |
24 |
| 9.10 |
|
SURVIVAL OF INDEMNIFICATION AND ADVANCEMENT OF EXPENSES |
25 |
| 9.11 |
|
LIMITATION ON INDEMNIFICATION |
25 |
| 9.12 |
|
INDEMNIFICATION OF EMPLOYEES AND AGENTS |
25 |
| 9.13 |
|
EFFECT OF AMENDMENT OR REPEAL |
25 |
| ARTICLE X |
|
MISCELLANEOUS |
26 |
| 10.1 |
|
PROVISIONS OF CERTIFICATE GOVERN |
26 |
| 10.2 |
|
CONSTRUCTION; DEFINITIONS |
26 |
| 10.3 |
|
SEVERABILITY |
26 |
| 10.4 |
|
AMENDMENT |
26 |
BYLAWS
OF
APPLIED DNA SCIENCES, INC.
ARTICLE I
CORPORATE OFFICES
1.1 REGISTERED
OFFICE.
The registered office of Applied DNA Sciences, Inc.
(the “Company”) shall be fixed in the Company’s certificate of incorporation, as the same may be amended
and/or restated from time to time (as so amended and/or restated, the “Certificate”).
1.2 OTHER
OFFICES.
The Company’s Board of Directors (the “Board”)
may at any time establish other offices at any place or places where the Company is qualified to do business.
ARTICLE II
MEETINGS OF STOCKHOLDERS
2.1 PLACE
OF MEETINGS.
Meetings of stockholders shall be held at any place
within or outside the State of Delaware as designated by the Board. The Board may, in its sole discretion, determine that a
meeting of stockholders shall not be held at any place, but may instead be held solely by means of remote communication as authorized
by Section 211(a)(2) of the Delaware General Corporation Law (the “DGCL”). In the absence
of any such designation or determination, stockholders’ meetings shall be held at the Company’s principal place of business.
2.2 ANNUAL
MEETING.
The annual meeting of stockholders shall be held
each year on a date and at a time designated by the Board. At the annual meeting, directors shall be elected and any other
proper business may be transacted.
2.3 SPECIAL
MEETING.
Unless otherwise required by law or the Certificate,
special meetings of the stockholders may be called at any time, for any purpose or purposes, only by (i) the Chairman of the Board,
(ii) the Chief Executive Officer or (iii) by the Board acting pursuant to a resolution adopted by a majority of the Board.
2.4 NOTICE
OF STOCKHOLDERS’ MEETINGS.
All notices of meetings of stockholders shall be
sent or otherwise given in accordance with either Section 2.5 or Section 8.1 of these bylaws not less than ten
(10) nor more than sixty (60) days before the date of the meeting to each stockholder entitled to vote at such meeting, except
as otherwise required by applicable law. The notice shall specify the place, if any, date and hour of the meeting, the means
of remote communication, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at such meeting,
and, in the case of a special meeting, the purposes for which the meeting is called. Any previously scheduled meeting of stockholders
may be postponed, and, unless the Certificate provides otherwise, any special meeting of the stockholders may be cancelled by resolution
duly adopted by a majority of the Board members then in office upon public notice given prior to the date previously scheduled for such
meeting of stockholders.
Whenever notice is required to be given, under
the DGCL, the Certificate or these bylaws, to any person with whom communication is unlawful, the giving of such notice to such person
shall not be required and there shall be no duty to apply to any governmental authority or agency for a license or permit to give such
notice to such person. Any action or meeting which shall be taken or held without notice to any such person with whom communication
is unlawful shall have the same force and effect as if such notice had been duly given. In the event that the action taken
by the corporation is such as to require the filing of a certificate with the Secretary of State of Delaware, the certificate shall state,
if such is the fact and if notice is required, that notice was given to all persons entitled to receive notice except such persons with
whom communication is unlawful.
Whenever notice is required to be given, under
any provision of the DGCL, the Certificate or these bylaws, to any stockholder to whom (A) notice of two (2) consecutive annual
meetings or (B) all, and at least two (2), payments (if sent by first-class mail) of dividends or interest on securities during a
12-month period, have been mailed addressed to such person at such person’s address as shown on the records of the corporation and
have been returned undeliverable, the giving of such notice to such person shall not be required. Any action or meeting which
shall be taken or held without notice to such person shall have the same force and effect as if such notice had been duly given. If
any such person shall deliver to the corporation a written notice setting forth such person’s then current address, the requirement
that notice be given to such person shall be reinstated. In the event that the action taken by the corporation is such as to
require the filing of a certificate with the Secretary of State of Delaware, the certificate need not state that notice was not given
to persons to whom notice was not required to be given pursuant to Section 230(b) of the DGCL.
The exception in subsection (A) of
the above paragraph to the requirement that notice be given shall not be applicable to any notice returned as undeliverable if the notice
was given by electronic transmission.
2.5 MANNER
OF GIVING NOTICE; AFFIDAVIT OF NOTICE.
Notice of any meeting of stockholders shall be
given:
(A) if
mailed, when deposited in the United States mail, postage prepaid, directed to the stockholder at such stockholder’s address as
it appears on the Company’s records;
(B) if
electronically transmitted, as provided in Section 8.1 of these bylaws; or
(C) otherwise,
when delivered.
An affidavit of the secretary or an assistant secretary
of the Company or of the transfer agent or any other agent of the Company that the notice has been given shall, in the absence of fraud,
be prima facie evidence of the facts stated therein.
Notice may be waived in accordance with Section 7.12
of these bylaws.
2.6 QUORUM.
The holders of a majority of the stock issued and
outstanding and entitled to vote, present in person or represented by proxy, shall constitute a quorum for the transaction of business
at all meetings of the stockholders. If, however, such quorum is not present or represented at any meeting of the stockholders,
then either (i) the chairperson of the meeting or (ii) the stockholders entitled to vote at the meeting, present in person or
represented by proxy, shall have power to adjourn the meeting from time to time, without notice other than announcement at the meeting,
until a quorum is present or represented. At such adjourned meeting at which a quorum is present or represented, any business
may be transacted that might have been transacted at the meeting as originally noticed.
2.7 ADJOURNED
MEETING; NOTICE.
When a meeting is adjourned to another time or
place, unless these bylaws otherwise require, notice need not be given of the adjourned meeting if the time, place if any thereof, and
the means of remote communications if any by which stockholders and proxy holders may be deemed to be present in person and vote at such
adjourned meeting are announced at the meeting at which the adjournment is taken. At the continuation of the adjourned meeting,
the Company may transact any business that was permitted to have been transacted at the original meeting. If the adjournment
is for more than 30 days, or if after the adjournment a new record date is fixed for the adjourned meeting, a notice of the adjourned
meeting shall be given to each stockholder of record entitled to vote at the meeting in accordance with the provisions of Section 2.4
and Section 2.5 of these bylaws.
2.8 ADMINISTRATION
OF THE MEETING.
Meetings of stockholders shall be presided over
by the Chairman of the Board, or in the absence of the Chairman of the Board, the Chief Executive Officer of the Company. If
both the Chairman of the Board and the Chief Executive Officer will not be present at a meeting of stockholders, such meeting shall be
presided over by such chairman as the Board shall appoint, or, in the event that the Board shall fail to make such appointment, any officer
of the Company appointed by the Board. The secretary of the meeting shall be the secretary of the Company, or, in the absence
of the secretary of the Company, such person as the chairman of the meeting appoints.
The Board shall, in advance of any meeting of stockholders,
appoint one (1) or more inspector(s), who may include individual(s) who serve the Company in other capacities, including without
limitation as officers, employees or agents, to act at the meeting of stockholders and make a written report thereof. The Board
may designate one (1) or more persons as alternate inspector(s) to replace any inspector who fails to act. If no
inspector or alternate has been appointed or is able to act at a meeting of stockholders, the chairman of the meeting shall appoint one
(1) or more inspector(s) to act at the meeting. Each inspector, before discharging his or her duties, shall take
and sign an oath to faithfully execute the duties of inspector with strict impartiality and according to the best of his or her ability. The
inspector(s) or alternate(s) shall have the duties prescribed pursuant to Section 231 of the DGCL and other applicable
law.
The Board shall be entitled to make such rules or
regulations for the conduct of meetings of stockholders as it shall deem necessary, appropriate or convenient. Subject to such
rules and regulations, if any, the chairman of the meeting shall have the right and authority to prescribe such rules, regulations
and procedures and to do all acts as, in the judgment of such chairman, are necessary, appropriate or convenient for the proper conduct
of the meeting, including without limitation establishing an agenda of business of the meeting, rules or regulations to maintain
order, restrictions on entry to the meeting after the time fixed for commencement thereof and the fixing of the date and time of the opening
and closing of the polls for each matter upon which the stockholders will vote at a meeting (and shall announce such at the meeting).
2.9 VOTING.
The stockholders entitled to vote at any meeting
of stockholders shall be determined in accordance with the provisions of Section 2.11 of these bylaws, subject to Section 217
(relating to voting rights of fiduciaries, pledgors and joint owners of stock) and Section 218 (relating to voting trusts and other
voting agreements) of the DGCL.
Except as otherwise provided in the provisions
of Section 213 of the DGCL (relating to the fixing of a date for determination of stockholders of record), each stockholder shall
be entitled to that number of votes for each share of capital stock held by such stockholder as set forth in the Certificate, or in the
case of shares of Preferred Stock, by resolution of the Board.
In all matters, other than the election of directors
and except as otherwise required by law, the Certificate or these bylaws, the affirmative vote of a majority of the voting power of the
shares present or represented by proxy at the meeting and entitled to vote on the subject matter shall be the act of the stockholders. Directors
shall be elected by a plurality of the voting power of the shares present in person or represented by proxy at the meeting and entitled
to vote on the election of directors.
The stockholders of the Company shall not have
the right to cumulate their votes for the election of directors of the Company.
2.10 STOCKHOLDER
ACTION BY WRITTEN CONSENT WITHOUT A MEETING.
Subject to the rights of the holders of the shares
of any series of Preferred Stock or any other class of stock or series thereof having a preference over the Common Stock as to dividend
or liquidation rights, any action required or permitted to be taken by the stockholders of the Company must be effected at a duly called
annual or special meeting of stockholders of the Company and may not be effected by any consent in writing by such stockholders.
2.11 RECORD
DATE FOR STOCKHOLDER NOTICE; VOTING; GIVING CONSENTS.
In order that the Company may determine the stockholders
entitled to notice of or to vote at any meeting of stockholders or any adjournment thereof, or entitled to receive payment of any dividend
or other distribution or allotment of any rights, or entitled to exercise any rights in respect of any change, conversion or exchange
of stock or for the purpose of any other lawful action, the Board may fix, in advance, a record date, which record date shall not precede
the date on which the resolution fixing the record date is adopted and which shall not be more than sixty (60) nor less than ten
(10) days before the date of such meeting, nor more than sixty (60) days prior to any other such action.
If the Board does not fix a record date in accordance
with these bylaws and applicable law:
(A) The
record date for determining stockholders entitled to notice of or to vote at a meeting of stockholders shall be at the close of business
on the day next preceding the day on which notice is given, or, if notice is waived, at the close of business on the day next preceding
the day on which the meeting is held.
(B) The
record date for determining stockholders for any other purpose shall be at the close of business on the day on which the Board adopts
the resolution relating thereto.
A determination of stockholders of record entitled
to notice of or to vote at a meeting of stockholders shall apply to any adjournment of the meeting; provided, however,
that the Board may fix a new record date for the adjourned meeting.
2.12 PROXIES.
Each stockholder entitled to vote at a meeting
of stockholders may authorize another person or persons to act for such stockholder by proxy authorized by an instrument in writing or
by a transmission permitted by law and filed with the secretary of the Company, but no such proxy shall be voted or acted upon after three
(3) years from its date, unless the proxy provides for a longer period. A stockholder may also authorize another person
or persons to act for him, her or it as proxy in the manner(s) provided under Section 212(c) of the DGCL or as otherwise
provided under Delaware law. The revocability of a proxy that states on its face that it is irrevocable shall be governed by
the provisions of Section 212 of the DGCL.
2.13 LIST
OF STOCKHOLDERS ENTITLED TO VOTE.
The officer who has charge of the stock ledger
of the Company shall prepare and make, at least ten (10) days before every meeting of stockholders, a complete list of the stockholders
entitled to vote at the meeting, arranged in alphabetical order, and showing the address of each stockholder and the number of shares
registered in the name of each stockholder. The Company shall not be required to include electronic mail addresses or other
electronic contact information on such list. Such list shall be open to the examination of any stockholder, for any purpose
germane to the meeting for a period of at least ten (10) days prior to the meeting: (a) on a reasonably accessible electronic
network, provided that the information required to gain access to such list is provided with the notice of the meeting or (b) during
ordinary business hours, at the Company’s principal place of business.
In the event that the Company determines to make
the list available on an electronic network, the Company may take reasonable steps to ensure that such information is available only to
stockholders of the Company. If the meeting is to be held at a place, then the list shall be produced and kept at the time
and place of the meeting during the whole time thereof, and may be inspected by any stockholder who is present. If the meeting
is to be held solely by means of remote communication, then the list shall also be open to the examination of any stockholder during the
whole time of the meeting on a reasonably accessible electronic network, and the information required to access such list shall be provided
with the notice of the meeting. Such list shall presumptively determine the identity of the stockholders entitled to vote at
the meeting and the number of shares held by each of them.
2.14 NOTICE
OF STOCKHOLDER BUSINESS AND NOMINATIONS.
(A) Annual
Meetings of Stockholders.
(1) Nominations
of persons for election to the Board and the proposal of business to be considered by the stockholders may be made at an annual meeting
of stockholders only (a) pursuant to the Company’s notice of meeting (or any supplement thereto), (b) by or at the direction
of the Board, or (c) by any stockholder of the Company who (i) was a stockholder of record of the Company at the time the notice
provided for in this Section 2.14 is delivered to the Secretary of the Company, (ii) shall be entitled to vote at such
meeting, and (iii) complies with the notice procedures set forth in this Section 2.14 as to such nomination or business. Clause
(c) shall be the exclusive means for a stockholder to (x) submit business (other than matters properly brought under Rule 14a-8
(or any successor thereto) under the Securities Exchange Act and set forth in the Company’s notice of meeting) or (y) make
nominations before an annual meeting of stockholders.
(2) Without
qualification, for nominations or any other business to be properly brought before an annual meeting by a stockholder pursuant to Section 2.14(A)(1)(c),
the stockholder, in addition to any other applicable requirements, must have given timely notice thereof in writing to the Secretary of
the Company and any such proposed business must constitute a proper matter for stockholder action. To be timely, a stockholder’s
notice must be delivered to the Secretary of the Company at the principal executive offices of the Company not later than the close of
business on the ninetieth (90th) day nor earlier than the close of business on the one hundred twentieth (120th) day prior to the first
anniversary of the preceding year’s annual meeting (provided, however, that in the event that the date of the annual meeting is
more than thirty days before or more than sixty (60) days after such anniversary date, notice by the stockholder must be so delivered
not earlier than the close of business on the one hundred twentieth (120th) day prior to such annual meeting and not later than the close
of business on the later of the ninetieth (90th) day prior to such annual meeting or the tenth (10th) day following the day on which public
announcement of the date of such meeting is first made by the Company). In no event shall the public announcement of an adjournment
or postponement of the annual meeting of stockholders commence a new time period (or extend any time period) for the giving of a stockholder’s
notice as described above. To be in proper form, a stockholder’s notice to the Secretary (whether pursuant to this Section 2.14(A)(2) or
Section 2.14(B)) shall set forth:
(a) as
to each person, if any, whom the stockholder proposes to nominate for election as a director (i) all information relating to such
person that is required to be disclosed in solicitations of proxies for election of directors in an election contest, or is otherwise
required, in each case pursuant to and in accordance with Section 14 of the Exchange Act and the rules and regulations promulgated
thereunder, (ii) such person’s written consent to being named in the proxy statement as a nominee and to serving as a director
if elected, (iii) a description of all direct and indirect compensation and other material monetary agreements, arrangements and
understandings during the past three years, and any other material relationships, between or among such stockholder and beneficial owner,
if any, and their respective affiliates and associates, or others acting in concert therewith, on the one hand, and each proposed nominee,
and his or her respective affiliates and associates, or others acting in concert therewith, on the other hand, including, without limitation
all information that would be required to be disclosed pursuant to Rule 404 promulgated under Regulation S-K if the stockholder making
the nomination and any beneficial owner on whose behalf the nomination is made, if any, or any affiliate or associate thereof or person
acting in concert therewith, were the “registrant” for purposes of such rule and the nominee were a director or executive
officer of such registrant; and (iv) with respect to each nominee for election or reelection to the Board, include a completed and
signed questionnaire, representation and agreement required by Section 2.15;
(b) if
the notice relates to any business (other than the nomination of persons for election as directors) that the stockholder proposes to
bring before the meeting, (i) a brief description of the business desired to be brought before the annual meeting, (ii) the
reasons for conducting such business at the annual meeting, (iii) the text of the proposal or business (including the text of any
resolutions proposed for consideration and in the event that such business includes a proposal to amend these bylaws, the language of
the proposed amendment), (iv) any material interest in such business of such stockholder and the beneficial owner, if any, on whose
behalf the proposal is made, and (v) a description of all agreements, arrangements and understandings between such stockholder and
beneficial owner, if any, and any other person or persons (including their names) in connection with the proposal of such business by
such stockholder; and
(c) as
to the stockholder giving the notice and the beneficial owner, if any, on whose behalf the nomination or proposal is made (i) the
name and address of such stockholder, as they appear on the Company’s books, and of such beneficial owner, if any, (ii)(A) the
class or series and number of shares of capital stock of the Company that are, directly or indirectly, owned beneficially and of record
by such stockholder and by such beneficial owner, (B) any option, warrant, convertible security, stock appreciation right, or similar
right with an exercise or conversion privilege or a settlement payment or mechanism at a price related to any class or series of capital
stock of the Company, whether or not such instrument or right shall be subject to settlement in the underlying class or series of capital
stock of the Company or otherwise (a “Derivative Instrument”) directly or indirectly owned beneficially by such
stockholder and by such beneficial owner, if any, and any other direct or indirect opportunity held or owned beneficially by such stockholder
and by such beneficial owner, if any, to profit or share in any profit derived from any increase or decrease in the value of shares of
the Company, (C) any proxy, contract, arrangement, understanding, or relationship pursuant to which such stockholder or beneficial
owner, if any, has a right to vote any shares of any security of the Company, (D) any short interest in any security of the Company
(for purposes of this Section 2.14, a person shall be deemed to have a short interest in a security if such person directly
or indirectly, through a contract, arrangement, understanding, relationship or otherwise, has the opportunity to profit or share in any
profit derived from any decrease in the value of the subject security), (E) any right to dividends on the shares of capital stock
of the Company owned beneficially by such stockholder or such beneficial owner, if any, which right is separated or separable from the
underlying shares, (F) any proportionate interest in shares of capital stock of the Company or Derivative Instrument held, directly
or indirectly, by a general or limited partnership in which such stockholder or such beneficial owner, if any, is a general partner or
with respect to which such stockholder or such beneficial owner, if any, directly or indirectly, beneficially owns an interest in a general
partner, and (G) any performance-related fees (other than an asset-based fee) to which such stockholder or such beneficial owner,
if any, is entitled to based on any increase or decrease in the value of shares of the Company or Derivative Instruments, if any, in each
case with respect to the information required to be included in the notice pursuant to (A) through (G) above, as of the date
of such notice and including, without limitation, any such interests held by members of such stockholder’s or such beneficial owner’s
immediate family sharing the same household (which information shall be supplemented by such stockholder and such beneficial owner, if
any, (y) not later than 10 days after the record date for the annual meeting to disclose such ownership as of the record date and
(z) 10 days before the annual meeting date, (iii) any other information relating to such stockholder and beneficial owner, if
any, that would be required to be disclosed in a proxy statement or other filings required to be made in connection with solicitation
of proxies for election of directors in a contested election pursuant to Section 14 of the Exchange Act and the rules and regulations
promulgated thereunder, (iv) a representation that the stockholder is a holder of record of stock of the Company entitled to vote
at such meeting and intends to appear in person or by proxy at the meeting to propose such business or nomination, and (v) a representation
whether the stockholder or the beneficial owner, if any, intends or is part of a group that intends (a) to deliver a proxy statement
and/or form of proxy to holders of at least the percentage of the Company’s outstanding capital stock required to approve or adopt
the proposal or elect the nominee or (b) otherwise to solicit proxies from stockholders in support of such proposal or nomination.
The Company may require any proposed nominee to furnish such other
information as it may reasonably require (i) to determine the eligibility of such proposed nominee to serve as a director of the
Company, including with respect to qualifications established by any committee of the Board (ii) to determine whether such nominee
qualifies as an “independent director” or “audit committee financial expert” under applicable law, securities
exchange rule or regulation, or any publicly-disclosed corporate governance guideline or committee charter of the Company; and (iii) that
could be material to a reasonable stockholder’s understanding of the independence and qualifications, or lack thereof, of such nominee.
(3) Notwithstanding
anything in the second sentence of Section 2.14(A)(2) to the contrary, in the event that the number of directors to be
elected to the Board at an annual meeting is increased and there is no public announcement by the Company naming all of the nominees for
director or specifying the size of the increased Board at least one hundred (100) days prior to the first anniversary of the preceding
year’s annual meeting, a stockholder’s notice required by this Section 2.14 shall also be considered timely, but
only with respect to nominees for any new positions created by such increase, if it shall be delivered to the Secretary of the Company
at the principal executive offices of the Company not later than the close of business on the tenth (10th) day following the day on which
such public announcement is first made by the Company.
(B) Special
Meetings of Stockholders.
Only such business shall be conducted at a special
meeting of stockholders as shall have been brought before the meeting pursuant to the Company’s notice of meeting. Nominations
of persons for election to the Board may be made at a special meeting of stockholders at which directors are to be elected pursuant to
the Company’s notice of meeting (1) by or at the direction of the Board or (2) provided that the Board has determined
that the directors shall be elected at such meeting, by any stockholder of the Company who is a stockholder of record at the time the
notice provided for in this Section 2.14 is delivered to the Secretary of the Company and at the time of the special meeting,
who is entitled to vote at the meeting and upon such election, and who complies with the notice procedures set forth in this Section 2.14. In
the event the Company calls a special meeting of stockholders for the purpose of electing one or more directors to the Board, any such
stockholder entitled to vote in such election of directors may nominate a person or persons (as the case may be) for election to such
position(s) as specified in the Company’s notice of meeting, if the stockholder’s notice in the same form as required
by Section 2.14(A)(2) with respect to any nomination (including the completed and signed questionnaire, representation
and agreement required by Section 2.15) shall be delivered to the Secretary at the principal executive offices of the Company
not earlier than the close of business on the one hundred twentieth (120th) day prior to such special meeting and not later than the close
of business on the later of the ninetieth (90th) day prior to such special meeting or the tenth (10th) day following the day on which
public announcement is first made of the date of the special meeting and of the nominees proposed by the Board to be elected at such meeting. In
no event shall the public announcement of an adjournment or postponement of a special meeting commence a new time period (or extend any
time period) for the giving of a stockholder’s notice as described above.
(C) General.
(1) Subject
to Section 3.13, only such persons who are nominated in accordance with the procedures set forth in this Section 2.14
shall be eligible to be elected at an annual or special meeting of stockholders of the Company to serve as directors and only such business
shall be conducted at a meeting of stockholders as shall have been brought before the meeting in accordance with the procedures set forth
in this Section 2.14. Except as otherwise provided by law, the Certificate of Incorporation of the Company, as
amended (the “Certificate of Incorporation”) or these bylaws, the Chairman of the meeting shall have the power
and duty (a) to determine whether a nomination or any business proposed to be brought before the meeting was made or proposed, as
the case may be, in accordance with the procedures set forth in this Section 2.14 and (b) if any proposed nomination
or business was not made or proposed in compliance with this Section 2.14, to declare that such nomination shall be disregarded
or that such proposed business shall not be transacted. Notwithstanding the foregoing provisions of this Section 2.14,
unless otherwise required by law, if the stockholder (or a qualified representative of the stockholder) does not appear at the annual
or special meeting of stockholders of the Company to present a nomination or proposed business, such nomination shall be disregarded and
such proposed business shall not be transacted, notwithstanding that proxies in respect of such vote may have been received by the Company. For
purposes of this Section 2.14, to be considered a qualified representative of the stockholder, a person must be authorized
by a writing executed by such stockholder or an electronic transmission delivered by such stockholder to act for such stockholder as proxy
at the meeting of stockholders and such person must produce such writing or electronic transmission, or a reliable reproduction of the
writing or electronic transmission, at the meeting of the stockholders.
(2) For
purpose of this Section 2.14, “public announcement” shall include disclosure in a press release reported by the
Dow Jones News Service, Associated Press, or comparable national news service or in a document publicly filed by the Company with the
Securities and Exchange Commission pursuant to Section 13, 14, or 15(d) of the Exchange Act and the rules and regulations
promulgated thereunder.
(3) Nothing
in this Section 2.14, shall be deemed to affect any rights (a) of stockholders to request inclusion of proposals or nominations
in the Company’s proxy statement pursuant to Rule 14a-8 (or any successor thereto) promulgated under the Exchange Act or (b) of
the holders of any series of Preferred Stock to nominate and elect directors pursuant to and to the extent provided in any applicable
provisions of the Certificate of Incorporation.
2.15 SUBMISSION
OF QUESTIONNAIRE, REPRESENTATION AND AGREEMENT.
To be eligible to be a nominee for election or
reelection as a director of the Company, a person must deliver (in accordance with the time periods prescribed for delivery of notice
under Section 2.14 of these bylaws) to the Secretary at the principal executive offices of the Company a written questionnaire
with respect to the background and qualification of such person and the background of any other person or entity on whose behalf the nomination
is being made (which questionnaire shall be provided by the Secretary upon written request) and a written representation and agreement
(in the form provided by the Secretary upon written request) that such person (A) is not and will not become a party to (1) any
agreement, arrangement or understanding with, and has not given any commitment or assurance to, any person or entity as to how such person,
if elected as a director of the Company, will act or vote on any issue or question (a “Voting Commitment”) that
has not been disclosed to the Company or (2) any Voting Commitment that could limit or interfere with such person’s ability
to comply, if elected as a director of the Company, with such person’s fiduciary duties under applicable law, (B) is not and
will not become a party to any agreement, arrangement or understanding with any person or entity other than the Company with respect to
any direct or indirect compensation, reimbursement or indemnification in connection with service or action as a director that has not
been disclosed therein, and (C) in such person’s individual capacity and on behalf of any person or entity on whose behalf
the nomination is being made, would be in compliance, if elected as a director of the Company, and will comply with all applicable publicly
disclosed corporate governance, conflict of interest, confidentiality and stock trading policies and guidelines of the Company.
ARTICLE III
DIRECTORS
3.1 POWERS.
Subject to the provisions of the DGCL and any limitations
in the Certificate, the business and affairs of the Company shall be managed and all corporate powers shall be exercised by or under the
direction of the Board.
3.2 NUMBER
OF DIRECTORS.
The Board shall consist of one or more members,
each of whom shall be a natural person. The authorized number of directors shall be determined from time to time by resolution of the
Board, provided the Board shall consist of at least one (1) member. No reduction of the authorized number of directors
shall have the effect of removing any director before that director’s term of office expires.
3.3 ELECTION,
QUALIFICATION AND TERM OF OFFICE OF DIRECTORS.
Directors need not be stockholders unless so required
by the Certificate or these bylaws. The Certificate or these bylaws may prescribe other qualifications for directors. Each
director, including a director elected to fill a vacancy, shall hold office until such director’s successor is elected and qualified
or until such director’s earlier death, resignation or removal.
3.4 RESIGNATION
AND VACANCIES.
Any director may resign at any time upon written
notice or by electronic transmission to the Company.
Vacancies occurring on the Board of Directors for
any reason and newly created directorships, resulting from an increase in the authorized number of directors may be filled only by a vote
of a majority of the remaining members of the Board of Directors, although less than a quorum, or by a sole remaining director, at any
meeting of the Board of Directors. A person so elected by the Board of Directors to fill a vacancy or newly created directorship
shall hold office until the next annual meeting of stockholders and until his or her successor shall be duly elected and qualified.
3.5 PLACE
OF MEETINGS; MEETINGS BY TELEPHONE.
The Board may hold meetings, both regular and special,
either within or outside the State of Delaware.
Unless otherwise restricted by the Certificate
or these bylaws, members of the Board, or any committee designated by the Board, may participate in a meeting of the Board, or any committee,
by means of conference telephone or other communications equipment by means of which all persons participating in the meeting can hear
each other, and such participation in a meeting shall constitute presence in person at the meeting.
3.6 REGULAR
MEETINGS.
Regular meetings of the Board may be held without
notice at such time and at such place as shall from time to time be determined by the Board.
3.7 SPECIAL
MEETINGS; NOTICE.
Special meetings of the Board for any purpose or
purposes may be called at any time by the Chairman of the Board, the Chief Executive Officer, at any time when there is no Chief Executive
Officer, the President, or a majority of the authorized number of directors. The person(s) authorized to call special
meetings of the Board may fix the place and time of the meeting.
Notice of the time and place of special meetings
shall be:
(A) delivered
personally by hand, by courier or by telephone;
(B) sent
by United States first-class mail, postage prepaid;
(C) sent
by facsimile; or
(D) sent
by electronic mail,
directed to each director at that director’s address, telephone
number, facsimile number or electronic mail address, as the case may be, as shown on the Company’s records.
If the notice is (i) delivered personally
by hand, by courier or by telephone, (ii) sent by facsimile or (iii) sent by electronic mail, it shall be delivered or sent
at least 24 hours before the time of the holding of the meeting. If the notice is sent by United States mail, it shall be deposited
in the United States mail at least four (4) days before the time of the holding of the meeting. Any oral notice may be
communicated either to the director or to a person at the office of the director who the person giving notice has reason to believe will
promptly communicate such notice to the director. The notice need not specify the place of the meeting if the meeting is to
be held at the Company’s principal executive office nor the purpose of the meeting.
3.8 QUORUM.
Except as otherwise required by law or the Certificate,
at all meetings of the Board, a majority of the authorized number of directors (as determined pursuant to Section 3.2 of these bylaws)
shall constitute a quorum for the transaction of business, except to adjourn as provided in Section 3.11 of these bylaws. A
meeting at which a quorum is initially present may continue to transact business notwithstanding the withdrawal of directors, if any action
taken is approved by at least a majority of the required quorum for that meeting. The vote of a majority of the directors present
at any meeting at which a quorum is present shall be the act of the Board, except as may be otherwise specifically provided by statute,
the Certificate or these bylaws.
3.9 WAIVER
OF NOTICE.
Whenever notice is required to be given under any
provisions of the DGCL, the Certificate or these bylaws, a written waiver thereof, signed by the person entitled to notice, or a waiver
by electronic transmission by the person entitled to notice, whether before or after the time stated therein, shall be deemed equivalent
to notice. Attendance of a person at a meeting shall constitute a waiver of notice of such meeting, except when the person
attends a meeting solely for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because
the meeting is not lawfully called or convened. Neither the business to be transacted at, nor the purpose of, any regular or
special meeting of the directors, or members of a committee of directors, need be specified in any written waiver of notice or any waiver
by electronic transmission unless so required by the Certificate or these bylaws.
3.10 BOARD
ACTION BY WRITTEN CONSENT WITHOUT A MEETING.
Unless otherwise restricted by the Certificate
or these bylaws, any action required or permitted to be taken at any meeting of the Board, or of any committee thereof, may be taken without
a meeting if all members of the Board or committee, as the case may be, consent thereto in writing or by electronic transmission and the
writing or writings or electronic transmission or transmissions are filed with the minutes of proceedings of the Board or committee. Such
filing shall be in paper form if the minutes are maintained in paper form and shall be in electronic form if the minutes are maintained
in electronic form.
3.11 ADJOURNED
MEETING; NOTICE.
If a quorum is not present at any meeting of the
Board, then a majority of the directors present thereat may adjourn the meeting from time to time, without notice other than announcement
at the meeting, until a quorum is present.
3.12 FEES
AND COMPENSATION OF DIRECTORS.
Unless otherwise restricted by the Certificate
or these bylaws, the Board shall have the authority to fix the compensation of directors.
3.13 REMOVAL
OF DIRECTORS.
Any director may be removed from the Board of Directors
by the stockholders of the Company only for cause, and in such case only by the affirmative vote of the holders of at least a majority
of the voting power of the issued and outstanding shares of capital stock of the Company then entitled to vote in the election of directors.
ARTICLE IV
COMMITTEES
4.1 COMMITTEES
OF DIRECTORS.
The Board may designate one or more committees,
each committee to consist of one or more of the directors of the Company. The Board may designate one or more directors as
alternate members of any committee, who may replace any absent or disqualified member at any meeting of the committee. In the
absence or disqualification of a member of a committee, the member or members thereof present at any meeting and not disqualified from
voting, whether or not such member or members constitute a quorum, may unanimously appoint another member of the Board to act at the meeting
in the place of any such absent or disqualified member. Any such committee, to the extent provided in the resolution of the
Board or in these bylaws, shall have and may exercise such lawfully delegable powers and duties as the Board may confer.
4.2 COMMITTEE
MINUTES.
Each committee shall keep regular minutes of its
meetings and report to the Board when required.
4.3 MEETINGS
AND ACTION OF COMMITTEES.
Meetings and actions of committees shall be governed
by, and held and taken in accordance with, the provisions of:
(A) Section 3.5
(relating to place of meetings and meetings by telephone);
(B) Section 3.6
(relating to regular meetings);
(C) Section 3.7
(relating to special meetings and notice);
(D) Section 3.8
(relating to quorum);
(E) Section 3.9
(relating to waiver of notice);
(F) Section 3.10
(relating to action without a meeting); and
(G) Section 3.11
(relating to adjournment and notice of adjournment)
of these bylaws, with such changes in the context of those bylaws as
are necessary to substitute the committee and its members for the Board and its members.
Notwithstanding the foregoing:
(i) the
time of regular meetings of committees may be determined either by resolution of the Board or by resolution of the committee;
(ii) special
meetings of committees may also be called by resolution of the Board or by resolution of the committee; and
(iii) notice
of special meetings of committees shall also be given to all alternate members, who shall have the right to attend all meetings of the
committee. The Board may adopt rules for the government of any committee not inconsistent with the provisions of these bylaws.
ARTICLE V
OFFICERS
5.1 OFFICERS.
The officers of the Company shall be a President
and a Secretary. The Company may also have, at the discretion of the Board, a Chairman of the Board, a vice chairman of the
Board, a Chief Executive Officer, a Chief Financial Officer or Treasurer, one or more vice presidents, one or more assistant vice presidents,
one or more assistant treasurers, one or more assistant secretaries, and any such other officers as may be appointed in accordance with
the provisions of these bylaws.
Any number of offices may be held by the same person.
5.2 APPOINTMENT
OF OFFICERS.
The Board shall appoint the officers of the Company,
except such officers as may be appointed in accordance with the provisions of Section 5.3 of these bylaws, subject to the
rights, if any, of an officer under any contract of employment. Each officer shall hold office until his or her successor is
elected and qualified or until his or her earlier resignation or removal. A failure to elect officers shall not dissolve or
otherwise affect the Company.
5.3 SUBORDINATE
OFFICERS.
The Board may appoint, or empower the Chief Executive
Officer or, in the absence of a Chief Executive Officer, the President of the Company to appoint, such other officers and agents as the
business of the Company may require. Each of such officers and agents shall hold office for such period, have such authority,
and perform such duties as are provided in these bylaws or as the Board may from time to time determine.
5.4 REMOVAL
AND RESIGNATION OF OFFICERS.
Any officer may be removed, either with or without
cause, by an affirmative vote of the majority of the Board at any regular or special meeting of the Board or, except in the case of an
officer appointed by the Board, by any officer upon whom such power of removal may be conferred by the Board.
Any officer may resign at any time by giving written
notice to the Company. Any resignation shall take effect at the date of the receipt of that notice or at any later time specified
in that notice. Unless otherwise specified in the notice of resignation, the acceptance of the resignation shall not be necessary
to make it effective. Any resignation is without prejudice to the rights, if any, of the Company under any contract to which
the officer is a party.
5.5 VACANCIES
IN OFFICES.
Any vacancy occurring in any office of the Company
may only be filled by the Board or as provided in Section 5.3 of these bylaws.
5.6 REPRESENTATION
OF SHARES OF OTHER CORPORATIONS.
The Chairman of the Board or the Chief Executive
Officer, or any other person authorized by the Board, the Chairman of the Board or the Chief Executive Officer, is authorized to vote,
represent, and exercise on behalf of this Company all rights incident to any and all shares or other equity interests of any other Company
or entity standing in the name of this Company. The authority granted herein may be exercised either by such person directly
or by any other person authorized to do so by proxy or power of attorney duly executed by such person having the authority.
5.7 AUTHORITY
AND DUTIES OF OFFICERS.
In addition to the foregoing authority and duties,
all officers of the Company shall respectively have such authority and perform such duties in the management of the business of the Company
as may be designated from time to time by the Board.
ARTICLE VI
RECORDS AND REPORTS
6.1 MAINTENANCE
AND INSPECTION OF RECORDS.
The Company shall, either at its principal executive
office or at such place or places as designated by the Board, keep a record of its stockholders, listing their names and addresses and
the number and class of shares held by each stockholder, a copy of these bylaws, as may be amended to date, minute books, accounting books
and other records.
Any such records maintained by the Company may
be kept on, or by means of, or be in the form of, any information storage device or method, provided that the records so kept can be converted
into clearly legible paper form within a reasonable time. The Company shall so convert any records so kept upon the request
of any person entitled to inspect such records pursuant to the provisions of the DGCL. When records are kept in such manner,
a clearly legible paper form produced from or by means of the information storage device or method shall be admissible in evidence, and
accepted for all other purposes, to the same extent as an original paper form accurately portrays the record.
6.2 INSPECTION
BY DIRECTORS.
Any director shall have the right to examine the
Company’s stock ledger, a list of its stockholders, and its other books and records for a purpose reasonably related to his or her
position as a director.
ARTICLE VII
GENERAL MATTERS
7.1 CHECKS;
DRAFTS; EVIDENCES OF INDEBTEDNESS.
From time to time, the Board shall determine by
resolution which person or persons may sign or endorse all checks, drafts, other orders for payment of money, notes or other evidences
of indebtedness that are issued in the name of or payable to the Company, and only the persons so authorized shall sign or endorse those
instruments.
7.2 EXECUTION
OF CORPORATE CONTRACTS AND INSTRUMENTS.
Except as otherwise provided in these bylaws, the
Board, or any officers of the Company authorized thereby, may authorize any officer or officers, or agent or agents, to enter into any
contract or execute any instrument in the name of and on behalf of the Company; such authority may be general or confined to specific
instances.
7.3 STOCK
CERTIFICATES; PARTLY PAID SHARES.
The shares of the Company shall be represented
by certificates, provided that the Board may provide by resolution or resolutions that some or all of any or all classes or series of
its stock shall be uncertificated shares. Any such resolution shall not apply to shares represented by a certificate until
such certificate is surrendered to the Company. Every holder of stock represented by certificates and upon request every holder
of uncertificated shares shall be entitled to have a certificate signed by, or in the name of the Company by the Chairman of the Board
or a vice-chairman of the Board, or the President or vice-president, and by the Treasurer or an assistant treasurer, or the Secretary
or an assistant secretary of the Company representing the number of shares registered in certificate form. Any or all of the
signatures on the certificate may be a facsimile. In case any officer, transfer agent or registrar who has signed or whose
facsimile signature has been placed upon a certificate has ceased to be such officer, transfer agent or registrar before such certificate
is issued, it may be issued by the Company with the same effect as if he or she were such officer, transfer agent or registrar at the
date of issue.
7.4 SPECIAL
DESIGNATION ON CERTIFICATES.
If the Company is authorized to issue more than
one class of stock or more than one series of any class, then the powers, designations, preferences, and relative, participating, optional
or other special rights of each class of stock or series thereof and the qualifications, limitations or restrictions of such preferences
and/or rights shall be set forth in full or summarized on the face or back of the certificate that the Company shall issue to represent
such class or series of stock; provided, however, that, except as otherwise provided in Section 202 of
the DGCL, in lieu of the foregoing requirements there may be set forth on the face or back of the certificate that the Company shall issue
to represent such class or series of stock a statement that the Company will furnish without charge to each stockholder who so requests
the powers, designations, preferences, and relative, participating, optional or other special rights of each class of stock or series
thereof and the qualifications, limitations or restrictions of such preferences and/or rights.
7.5 LOST
CERTIFICATES.
Except as provided in this Section 7.5, no
new certificates for shares shall be issued to replace a previously issued certificate unless the latter is surrendered to the Company
and cancelled at the same time. The Company may issue a new certificate of stock or uncertificated shares in the place of any
certificate theretofore issued by it, alleged to have been lost, stolen or destroyed, and the Company may require the owner of the lost,
stolen or destroyed certificate, or such owner’s legal representative, to give the Company a bond sufficient to indemnify it against
any claim that may be made against it on account of the alleged loss, theft or destruction of any such certificate or the issuance of
such new certificate or uncertificated shares.
7.6 DIVIDENDS.
The Board, subject to any restrictions contained
in either (a) the DGCL or (b) the Certificate, may declare and pay dividends upon the shares of its capital stock. Dividends
may be paid in cash, in property, or in shares of the Company’s capital stock.
The Board may set apart out of any of the funds
of the Company available for dividends a reserve or reserves for any proper purpose and may abolish any such reserve.
7.7 FISCAL
YEAR.
The fiscal year of the Company shall be fixed by
resolution of the Board and may be changed by the Board.
7.8 SEAL.
The Company may adopt a corporate seal, which shall
be adopted and which may be altered by the Board. The Company may use the corporate seal by causing it or a facsimile thereof
to be impressed or affixed or in any other manner reproduced.
7.9 TRANSFER
OF STOCK.
Transfers of stock shall be made only upon the
transfer books of the Company kept at an office of the Company or by transfer agents designated to transfer shares of the stock of the
Company. Except where a certificate is issued in accordance with Section 7.5 of these bylaws, an outstanding certificate
for the number of shares involved shall be surrendered for cancellation before a new certificate is issued therefore. Upon
surrender to the Company or the transfer agent of the Company of a certificate for shares duly endorsed or accompanied by proper evidence
of succession, assignation or authority to transfer, it shall be the duty of the Company to issue a new certificate to the person entitled
thereto, cancel the old certificate, and record the transaction in its books.
7.10 STOCK
TRANSFER AGREEMENTS.
The Company shall have power to enter into and
perform any agreement with any number of stockholders of any one or more classes or series of stock of the Company to restrict the transfer
of shares of stock of the Company of any one or more classes or series owned by such stockholders in any manner not prohibited by the
DGCL.
7.11 REGISTERED
STOCKHOLDERS.
The Company:
(A) shall
be entitled to recognize the exclusive right of a person registered on its books as the owner of shares to receive dividends and to vote
as such owner; and
(B) shall
not be bound to recognize any equitable or other claim to or interest in such share or shares on the part of another person, whether or
not it shall have express or other notice thereof, except as otherwise provided by the laws of Delaware.
7.12 WAIVER
OF NOTICE.
Whenever notice is required to be given under any
provision of the DGCL, the Certificate or these bylaws, a written waiver, signed by the person entitled to notice, or a waiver by electronic
transmission by the person entitled to notice, whether before or after the time of the event for which notice is to be given, shall be
deemed equivalent to notice. Attendance of a person at a meeting shall constitute a waiver of notice of such meeting, except
when the person attends a meeting solely for the express purpose of objecting at the beginning of the meeting, to the transaction of any
business because the meeting is not lawfully called or convened. Neither the business to be transacted at, nor the purpose
of, any regular or special meeting of the stockholders need be specified in any written waiver of notice or any waiver by electronic transmission
unless so required by the Certificate or these bylaws.
ARTICLE VIII
NOTICE BY ELECTRONIC TRANSMISSION
8.1 NOTICE
BY ELECTRONIC TRANSMISSION.
Without limiting the manner by which notice otherwise
may be given effectively to stockholders pursuant to the DGCL, the Certificate or these bylaws, any notice to stockholders given by the
Company under any provision of the DGCL, the Certificate or these bylaws shall be effective if given by a form of electronic transmission
consented to by the stockholder to whom the notice is given. Any such consent shall be revocable by the stockholder by written
notice to the Company. Any such consent shall be deemed revoked if:
(A) the
Company is unable to deliver by electronic transmission two consecutive notices given by the Company in accordance with such consent;
and
(B) such
inability becomes known to the secretary or an assistant secretary of the Company or to the transfer agent, or other person responsible
for the giving of notice.
However, the inadvertent failure to treat such inability as a revocation
shall not invalidate any meeting or other action.
Any notice given pursuant to the preceding paragraph
shall be deemed given:
(i) if
by facsimile telecommunication, when directed to a number at which the stockholder has consented to receive notice;
(ii) if
by electronic mail, when directed to an electronic mail address at which the stockholder has consented to receive notice;
(iii) if
by a posting on an electronic network together with separate notice to the stockholder of such specific posting, upon the later of (A) such
posting and (B) the giving of such separate notice; and
(iv)
if by any other form of electronic transmission, when directed to the
stockholder.
An affidavit of the secretary or an assistant secretary
or of the transfer agent or other agent of the Company that the notice has been given by a form of electronic transmission shall, in the
absence of fraud, be prima facie evidence of the facts stated therein.
8.2 DEFINITION
OF ELECTRONIC TRANSMISSION.
An “electronic transmission” means
any form of communication, including without limitation an email communication, not directly involving the physical transmission of paper,
that creates a record that may be retained, retrieved, and reviewed by a recipient thereof, and that may be directly reproduced in paper
form by such a recipient through an automated process.
8.3 INAPPLICABILITY.
Notice by a form of electronic transmission shall
not apply to Section 164 (relating to failure to pay for stock; remedies), Section 296 (relating to adjudication of claims;
appeal), Section 311 (relating to revocation of voluntary dissolution), Section 312 (relating to renewal, revival, extension
and restoration of certificate of incorporation) or Section 324 (relating to attachment of shares of stock or any option, right or
interest therein) of the DGCL.
ARTICLE IX
INDEMNIFICATION OF DIRECTORS AND OFFICERS
9.1 POWER
TO INDEMNIFY IN ACTIONS, SUITS OR PROCEEDINGS OTHER THAN THOSE BY OR IN THE RIGHT OF THE CORPORATION.
Subject to Section 9.3 of these bylaws,
the Company shall indemnify, to the fullest extent permitted by the DGCL, as now or hereafter in effect, any person who was or is a party
or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative
or investigative (other than an action by or in the right of the Company) by reason of the fact that such person (or the legal representative
of such person) is or was a director or officer of the Company or any predecessor of the Company, or is or was a director or officer of
the Company serving at the request of the Company as a director or officer, employee or agent of another Company, partnership, joint venture,
trust, employee benefit plan or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid
in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted
in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the Company, and, with
respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. The
termination of any action, suit or proceeding by judgment, order, settlement, conviction, or upon a plea of nolo contendere or
its equivalent, shall not, of itself, create a presumption that the person did not act in good faith and in a manner which such person
reasonably believed to be in or not opposed to the best interests of the Company, and, with respect to any criminal action or proceeding,
had reasonable cause to believe that such person’s conduct was unlawful.
9.2 POWER
TO INDEMNIFY IN ACTIONS, SUITS OR PROCEEDINGS BY OR IN THE RIGHT OF THE CORPORATION.
Subject to Section 9.3 of these bylaws,
the Company shall indemnify, to the fullest extent permitted by the DGCL, as now or hereafter in effect, any person who was or is a party
or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the Company to procure
a judgment in its favor by reason of the fact that such person (or the legal representative of such person) is or was a director or officer
of the Company or any predecessor of the Company, or is or was a director or officer of the Company serving at the request of the Company
as a director, officer, employee or agent of another Company, partnership, joint venture, trust, employee benefit plan or other enterprise
against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement
of such action or suit if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the
best interests of the Company; except that no indemnification shall be made in respect of any claim, issue or matter as to which such
person shall have been adjudged to be liable to the Company unless and only to the extent that the Court of Chancery or the court in which
such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all the circumstances
of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court
shall deem proper.
9.3 AUTHORIZATION
OF INDEMNIFICATION.
Any indemnification under this Article IX
(unless ordered by a court) shall be made by the Company only as authorized in the specific case upon a determination that indemnification
of the director or officer is proper in the circumstances because such person has met the applicable standard of conduct set forth in
Section 9.1 or Section 9.2 of these bylaws, as the case may be. Such determination shall be made, with
respect to a person who is either a director or officer at the time of such determination or a former director or officer, (i) by
a majority vote of the directors who are not parties to such action, suit or proceeding, even though less than a quorum, or (ii) by
a committee of such directors designated by a majority vote of such directors, even though less than a quorum, or (iii) if there
are no such directors, or if such directors so direct, by independent legal counsel in a written opinion or (iv) by the stockholders
(but only if a majority of the directors who are not parties to such action, suit or proceeding, if they constitute a quorum of the board
of directors, presents the issue of entitlement to indemnification to the stockholders for their determination). To the extent,
however, that a present or former director or officer of the Company has been successful on the merits or otherwise in defense of any
action, suit or proceeding described above, or in defense of any claim, issue or matter therein, such person shall be indemnified against
expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection therewith, without the necessity
of authorization in the specific case.
9.4 GOOD
FAITH DEFINED.
For purposes of any determination under Section 9.3
of these bylaws, to the fullest extent permitted by applicable law, a person shall be deemed to have acted in good faith and in a manner
such person reasonably believed to be in or not opposed to the best interests of the Company, or, with respect to any criminal action
or proceeding, to have had no reasonable cause to believe such person’s conduct was unlawful, if such person’s action is based
on the records or books of account of the Company or another enterprise, or on information supplied to such person by the officers of
the Company or another enterprise in the course of their duties, or on the advice of legal counsel for the Company or another enterprise
or on information or records given or reports made to the Company or another enterprise by an independent certified public accountant
or by an appraiser or other expert selected with reasonable care by the Company or another enterprise. The term “another
enterprise” as used in this Section 9.4 shall mean any other Company or any partnership, joint venture, trust, employee benefit
plan or other enterprise of which such person is or was serving at the request of the Company as a director, officer, employee or agent. The
provisions of this Section 9.4 shall not be deemed to be exclusive or to limit in any way the circumstances in which a person may
be deemed to have met the applicable standard of conduct set forth in Section 9.1 or Section 9.2 of these bylaws,
as the case may be.
9.5 INDEMNIFICATION
BY A COURT.
Notwithstanding any contrary determination in the
specific case under Section 9.3 of this Article IX, and notwithstanding the absence of any determination thereunder,
any director or officer may apply to the Court of Chancery in the State of Delaware for indemnification to the extent otherwise permissible
under Section 9.1 and Section 9.2 of these bylaws. The basis of such indemnification by a court shall
be a determination by such court that indemnification of the director or officer is proper in the circumstances because such person has
met the applicable standards of conduct set forth in Section 9.1 or Section 9.2 of these bylaws, as the case may
be. Neither a contrary determination in the specific case under Section 9.3 of these bylaws nor the absence of any determination
thereunder shall be a defense to such application or create a presumption that the director or officer seeking indemnification has not
met any applicable standard of conduct. Notice of any application for indemnification pursuant to this Section 9.5
shall be given to the Company promptly upon the filing of such application. If successful, in whole or in part, the director
or officer seeking indemnification shall also be entitled to be paid the expense of prosecuting such application.
9.6 EXPENSES
PAYABLE IN ADVANCE.
To the fullest extent not prohibited by the DGCL,
or by any other applicable law, expenses incurred by a person who is or was a director or officer in defending any civil, criminal, administrative
or investigative action, suit or proceeding shall be paid by the Company in advance of the final disposition of such action, suit or proceeding;
provided, however, that if the DGCL requires, an advance of expenses incurred by any person in his or her
capacity as a director or officer (and not in any other capacity) shall be made only upon receipt of an undertaking by or on behalf of
such person to repay such amount if it shall ultimately be determined that such person is not entitled to be indemnified by the Company
as authorized in this Article IX.
9.7 NONEXCLUSIVITY
OF INDEMNIFICATION AND ADVANCEMENT OF EXPENSES.
The indemnification and advancement of expenses
provided by or granted pursuant to this Article IX shall not be deemed exclusive of any other rights to which those seeking
indemnification or advancement of expenses may be entitled under the Certificate, any bylaw, agreement, vote of stockholders or disinterested
directors or otherwise, both as to action in such person’s official capacity and as to action in another capacity while holding
such office, it being the policy of the Company that indemnification of the persons specified in Section 9.1 and Section 9.2
of these bylaws shall be made to the fullest extent permitted by law. The provisions of this Article IX shall not
be deemed to preclude the indemnification of any person who is not specified in Section 9.1 or Section 9.2 of
these bylaws but whom the Company has the power or obligation to indemnify under the provisions of the DGCL, or otherwise. The
Company is specifically authorized to enter into individual contracts with any or all of its directors, officers, employees or agents
respecting indemnification and advances, to the fullest extent not prohibited by the DGCL, or by any other applicable law.
9.8 INSURANCE.
To the fullest extent permitted by the DGCL or
any other applicable law, the Company may purchase and maintain insurance on behalf of any person who is or was a director, officer, employee
or agent of the Company, or is or was a director, officer, employee or agent of the Company serving at the request of the Company as a
director, officer, employee or agent of another Company, partnership, joint venture, trust, employee benefit plan or other enterprise
against any liability asserted against such person and incurred by such person in any such capacity, or arising out of such person’s
status as such, whether or not the Company would have the power or the obligation to indemnify such person against such liability under
the provisions of this Article IX.
9.9 CERTAIN
DEFINITIONS.
For purposes of this Article IX, references
to “the Company” shall include, in addition to the resulting Company, any constituent Company (including any constituent of
a constituent) absorbed in a consolidation or merger which, if its separate existence had continued, would have had power and authority
to indemnify its directors or officers, so that any person who is or was a director or officer of such constituent Company, or is or was
a director or officer of such constituent Company serving at the request of such constituent Company as a director, officer, employee
or agent of another Company, partnership, joint venture, trust, employee benefit plan or other enterprise, shall stand in the same position
under the provisions of this Article IX with respect to the resulting or surviving Company as such person would have with
respect to such constituent Company if its separate existence had continued. For purposes of this Article IX, references
to “fines” shall include any excise taxes assessed on a person with respect to an employee benefit plan; and references to
“serving at the request of the Company” shall include any service as a director, officer, employee or agent of the Company
which imposes duties on, or involves services by, such director or officer with respect to an employee benefit plan, its participants
or beneficiaries; and a person who acted in good faith and in a manner such person reasonably believed to be in the interest of the participants
and beneficiaries of an employee benefit plan shall be deemed to have acted in a manner “not opposed to the best interests of the
Company” as referred to in this Article IX.
9.10 SURVIVAL
OF INDEMNIFICATION AND ADVANCEMENT OF EXPENSES.
The rights to indemnification and advancement of
expenses conferred by this Article IX shall continue as to a person who has ceased to be a director or officer and shall inure
to the benefit of the heirs, executors, administrators and other personal and legal representatives of such a person.
9.11 LIMITATION
ON INDEMNIFICATION.
Notwithstanding anything contained in this Article IX
to the contrary, except for proceedings to enforce rights to indemnification (which shall be governed by Section 9.5 of these
bylaws), the Company shall not be obligated to indemnify any director or officer in connection with a proceeding (or part thereof) initiated
by such person unless such proceeding (or part thereof) was authorized or consented to by the board of directors of the Company.
9.12 INDEMNIFICATION
OF EMPLOYEES AND AGENTS.
The Company may, to the extent authorized from
time to time by the board of directors, provide rights to indemnification and to the advancement of expenses to employees and agents of
the Company similar to those conferred in this Article IX to directors and officers of the Company.
9.13 EFFECT
OF AMENDMENT OR REPEAL.
Neither any amendment or repeal of any Section of
this Article IX, nor the adoption of any provision of the Certificate or the bylaws inconsistent with this Article IX,
shall adversely affect any right or protection of any director, officer, employee or other agent established pursuant to this Article IX
existing at the time of such amendment, repeal or adoption of an inconsistent provision, including without limitation by eliminating or
reducing the effect of this Article IX, for or in respect of any act, omission or other matter occurring, or any action or
proceeding accruing or arising (or that, but for this Article IX, would accrue or arise), prior to such amendment, repeal
or adoption of an inconsistent provision.
ARTICLE X
MISCELLANEOUS
10.1 PROVISIONS
OF CERTIFICATE GOVERN.
In the event of any inconsistency between the terms
of these bylaws and the Certificate, the terms of the Certificate will govern.
10.2 CONSTRUCTION;
DEFINITIONS.
Unless the context requires otherwise, the general
provisions, rules of construction, and definitions in the DGCL shall govern the construction of these bylaws. Without
limiting the generality of this provision, the singular number includes the plural, the plural number includes the singular, and the term
“person” includes both a corporation and a natural person.
10.3 SEVERABILITY.
In the event that any bylaw or the application
thereof becomes or is declared by a court of competent jurisdiction to be illegal, void or unenforceable, the remaining bylaws will continue
in full force and effect.
10.4 AMENDMENT.
In furtherance and not in limitation of the powers
conferred by statute, the Board of Directors is expressly authorized to adopt, amend, alter or repeal these bylaws. The affirmative
vote of at least a majority of the Board of Directors then in office shall be required in order for the Board of Directors to adopt, amend,
alter or repeal these bylaws. No bylaw hereafter legally amended, altered or repealed shall invalidate any prior act of the
directors or officers of the Company that would have been valid if such bylaw had not been amended, altered or repealed.
CERTIFICATE OF AMENDMENT TO THE
BYLAWS
OF
APPLIED DNA SCIENCES, INC.
FIRST: The
Bylaws (“Bylaws”) of Applied DNA Sciences, Inc., a Delaware corporation (the “Corporation”)
have been amended as follows:
Section 2.6 of Article II of the Bylaws shall be amended
and restated in its entirety to state as follows:
“2.6 QUORUM.
The holders of one-third of the stock issued and outstanding and entitled
to vote, present in person or represented by proxy, shall constitute a quorum for the transaction of business at all meetings of the stockholders. If,
however, such quorum is not present or represented at any meeting of the stockholders, then either (i) the chairperson of the meeting
(ii) the Chief Executive Officer of the Company or (iii) the stockholders entitled to vote at the meeting, present in person
or represented by proxy, shall have power to adjourn the meeting from time to time, without notice other than announcement at the meeting,
until a quorum is present or represented. At such adjourned meeting at which a quorum is present or represented, any business
may be transacted that might have been transacted at the meeting as originally noticed.”
SECOND: The
foregoing amendment has been duly adopted on or approximately on the date hereof in accordance with applicable provisions of the Bylaws.
IN
WITNESS WHEREOF, the Corporation has caused this Certificate of Amendment of the Bylaws to be signed by its Chief Executive
Officer, on Thursday, November 7, 2024.
[Signature Page Follows]
| APPLIED DNA SCIENCES, INC. |
|
| |
|
| By: |
/s/ James A. Hayward |
|
| |
|
| Name: |
James A. Hayward |
|
| Title: |
President and Chief Executive Officer |
|
[Signature Page to Certificate of Amendment]
Exhibit 5.1

November 18, 2024
Applied DNA Sciences, Inc.
50 Health Sciences Drive
Stony Brook, NY 11790
Re: Registration of Warrant Shares
Ladies and Gentlemen:
Reference is made to the filing by Applied DNA Sciences, Inc.,
a Delaware corporation (the “Company”), with the United States Securities and Exchange Commission (the “SEC”)
pursuant to the Securities Act of 1933, as amended (the “Securities Act”), of the Company’s registration
statement on Form S-1 (the “Registration Statement”), filed on November 18, 2024, which
includes a prospectus (the “Prospectus”).
We are rendering this opinion in connection with the filing by the
Company with the SEC of the Registration Statement relating to the offering by selling stockholders of up to 41,640,625 shares
of the Company’s common stock, par value $0.001 per share (the “Shares”). The Shares will be issued upon
the exercise of certain warrants of the Company, consisting of (i) 20,312,500 Series C Warrants (the “Series C
Warrants”), (ii) 20,312,500 Series D Warrants (the “Series D Warrants”) and (iii) 1,015,625
Placement Agent Warrants (the “Placement Agent Warrants”, and together with the Series C Warrants and the
Series D Warrants, the “Warrants”). The Warrants were issued pursuant to (i) that certain securities
purchase agreement, dated October 30, 2024, by and between the Company and the purchasers listed thereto (the “Securities
Purchase Agreement”) and (ii) that certain engagement letter, dated August 23, 2024, by and between the Company
and the Craig-Hallum Capital Group LLC (the “Engagement Letter”). The exercise of the Warrants is contingent
upon the Company’s receipt of stockholder approval, in accordance with Nasdaq Listing Rule 5635(d) (“Stockholder
Approval”).
We understand that the Shares to be issued upon the exercise of the
Warrants are to be offered and sold in the manner set forth in the Prospectus. This opinion letter is furnished to you at your request
to enable you to fulfill the requirements of Item 601(b)(5) of Regulation S-K in connection with the Registration Statement.
We have acted as your counsel in connection with the preparation of
the Registration Statement. We are familiar with the proceedings taken by the board of directors of the Company (the “Board”)
in connection with the authorization, issuance and sale of the Shares. We have examined all such documents as we considered necessary
to enable us to render this opinion, including, but not limited to: (i) the Registration Statement, (ii) the Warrants, (iii) the
Securities Purchase Agreement, (iv) the Engagement Letter, (v) the Company’s certificate of incorporation, as amended
to date, (vi) the Company’s bylaws, as amended to date, (vii) certain resolutions of the Board and the pricing committee
thereof, and (viii) such other corporate records and instruments, and such laws and regulations as we have deemed necessary for purposes
of rendering the opinions set forth herein.
In our examination, we have assumed the legal capacity of all natural
persons, the genuineness of all signatures, the authenticity of all documents submitted to us as originals, the conformity to original
documents of all documents submitted to us as certified, conformed, photostatic or facsimile copies, the authenticity of all documents
submitted to us as certified, conformed, photostatic or facsimile copies and the authenticity of the originals of such certified, conformed,
photostatic or facsimile copies. In addition, we have assumed that the Shares will be offered in the manner and on the terms identified
or referred to in the Prospectus. As to any facts material to the opinions expressed herein, which were not independently established
or verified, we have relied upon statements and representations of officers and other representatives of the Company and others.
We express no opinion herein as to the law of any state or jurisdiction
other than the laws of the State of New York and the General Corporation Law of the State of Delaware.
Based upon the foregoing, and subject to the assumptions, limitations
and qualifications stated herein, we are of the opinion that the Shares have been duly authorized and, when issued and delivered by the
Company subsequent to Stockholder Approval in accordance with the terms of the Warrants and upon receipt by the Company of the consideration
therefor provided therein, will be validly issued, fully paid and non-assessable.
We assume no obligation to supplement this opinion if any applicable
law changes after the date hereof or if we become aware of any fact that might change the opinion expressed herein after the date hereof.
We hereby consent to the filing of this opinion as a part of the Registration Statement and to the reference of our firm under the caption
“Legal Matters” in the Prospectus. In giving such consent, we do not hereby admit that we are in the category of persons whose
consent is required under Section 7 of the Securities Act or the rules and regulations of the SEC.
| |
Very truly yours, |
| |
|
| |
/s/ McDermott Will and Emery LLP |
| |
McDermott Will and Emery LLP |
 |
One Vanderbilt Avenue New
York NY 10017-3852 Tel +1 212 547 5400 Fax +1 212 547 5444
US practice conducted through McDermott
Will & Emery LLP. |
Exhibit 23.1
Independent
Registered Public Accounting Firm’s Consent
We consent to the incorporation by reference in
this Registration Statement of Applied DNA Sciences, Inc. on Form S-1 of our report, which includes an explanatory paragraph as to the
Company’s ability to continue as a going concern, dated December 7, 2023, with respect to our audits of the consolidated financial
statements of Applied DNA Sciences, Inc, as of September 30, 2023 and 2022 and for each of the two years in the period ended September
30, 2023 appearing in the Annual Report on Form 10-K of Applied DNA Sciences, Inc. for the year ended September 30, 2023. We also consent
to the reference to our firm under the heading “Experts” in the Prospectus, which is part of this Registration Statement.
/s/ Marcum llp
Marcum llp
Melville, NY
November 18, 2024
Exhibit 107
Calculation of Filing Fee Tables
Form S-1
(Form Type)
APPLIED DNA SCIENCES, INC.
(Exact Name of Registrant as Specified in its Charter)
| |
|
|
|
|
|
|
|
|
| |
|
Security
Type |
|
Security
Class
Title |
|
Fee
Calculation
or Carry
Forward
Rule |
|
Amount
Registered (1) |
|
Proposed
Maximum
Offering
Price Per
Share(2) |
|
Maximum
Aggregate
Offering
Price |
|
Fee
Rate |
|
Amount of
Registration
Fee |
|
| |
|
|
|
|
|
|
|
|
| Fees to Be Paid |
|
Equity |
|
Common Stock, par value $0.001 per share |
|
457(c) |
|
41,640,625(3) |
|
$0.185 |
|
$7,703,515.63 |
|
0.0001531 |
|
$ |
1,179.41 |
|
| |
|
|
|
|
|
| |
|
Total Offering Amounts |
|
|
|
$7,703,515.63 |
|
|
|
$ |
1,179.41 |
|
| |
|
|
|
|
|
| |
|
Total Fees Previously Paid |
|
|
|
|
|
|
|
$ |
0 |
|
| |
|
|
|
|
|
| |
|
Total Fee Offsets |
|
|
|
|
|
|
|
$ |
0 |
|
| |
|
|
|
|
|
| |
|
Net Fee Due |
|
|
|
|
|
|
|
$ |
1,179.41 |
|
| (1) |
Pursuant to Rule 416 under the Securities Act, there is also being registered hereby such indeterminate number of additional shares of our common stock, par value $0.001 per share (the “Common Stock”) as may be issued or issuable because of stock splits, stock dividends stock distributions, and similar transactions. |
| (2) |
Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(c) under the Securities Act of 1933, as amended. The proposed maximum offering price per share and proposed maximum aggregate offering price are based upon the average of the high $0.19 and low $0.18 sale prices of our Common Stock on November 13, 2024, as reported on The Nasdaq Capital Market. |
| (3) |
Comprised of shares issuable upon the exercise of an aggregate of 41,640,625 warrants, which includes (i) 20,312,500 Series C Warrants to purchase shares of our Common Stock, (ii) 20,312,500 Series D Warrants to purchase shares of our Common Stock and (iii) 1,015,625 Placement Agent Warrants to purchase shares of our Common Stock. |
Applied DNA Sciences (NASDAQ:APDN)
Historical Stock Chart
From Oct 2024 to Nov 2024
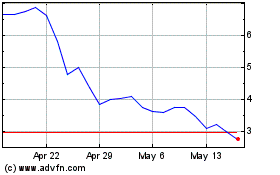
Applied DNA Sciences (NASDAQ:APDN)
Historical Stock Chart
From Nov 2023 to Nov 2024