Filed Pursuant to Rule 424(b)(5)
Registration No. 333-261638
PROSPECTUS SUPPLEMENT
(To Prospectus dated December 23, 2021)
Up to $10,000,000

Common Stock
We have entered into an open
market sales agreement (the “Sales Agreement”) with Leerink Partners LLC (“Leerink Partners”), dated as of December 14,
2021, relating to the sale of shares of our common stock, $0.0001 par value per share, offered by this prospectus supplement and the accompanying
prospectus. In accordance with the terms of the Sales Agreement, pursuant to this prospectus supplement and the accompanying prospectus,
we may offer and sell shares of our common stock having an aggregate offering price of up to $10,000,000 from time to time through or
to Leerink Partners, acting as our agent.
Our common stock is listed
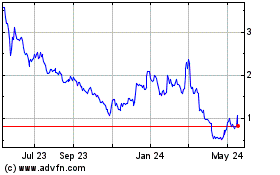
on The Nasdaq Capital Market under the symbol “EYEN”. On May 15, 2024, the last reported sale price of our common stock
on The Nasdaq Capital Market was $1.06 per share.
Sales of the common stock,
if any, under this prospectus supplement may be made in sales deemed to be an “at the market offering” as defined in Rule 415(a)(4) promulgated
under the Securities Act of 1933, as amended (the “Securities Act”). Leerink Partners is not required to sell any specific
amount of securities, but will act as our sales agent using commercially reasonable efforts consistent with its normal trading and sales
practices, on mutually agreed terms between Leerink Partners and us. There is no arrangement for funds to be received in any escrow, trust
or similar arrangement.
The compensation to Leerink
Partners for sales of our common stock will be 3.0% of the gross sales price per share sold. See “Plan of Distribution” beginning
on page S-10 of this prospectus supplement for additional information regarding the compensation to be paid to Leerink Partners.
In connection with the sale of the common stock on our behalf, Leerink Partners will be deemed to be an “underwriter” within
the meaning of the Securities Act and the compensation of Leerink Partners will be deemed to be underwriting commissions. We have also
agreed to provide indemnification and contribution to Leerink Partners with respect to certain liabilities, including liabilities under
the Securities Act.
We are a “smaller reporting company”
under applicable Securities and Exchange Commission rules and are subject to reduced public company reporting requirements for this
prospectus supplement and future filings.
INVESTING IN OUR COMMON
STOCK INVOLVES A HIGH DEGREE OF RISK. YOU SHOULD REVIEW CAREFULLY THE RISKS AND UNCERTAINTIES DESCRIBED UNDER THE HEADING “RISK
FACTORS” ON PAGE S-5 OF THIS PROSPECTUS SUPPLEMENT, AND UNDER SIMILAR HEADINGS IN THE DOCUMENTS THAT ARE INCORPORATED BY
REFERENCE INTO THIS PROSPECTUS SUPPLEMENT.
Neither the Securities
and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus
supplement or the accompanying prospectus are truthful or complete. Any representation to the contrary is a criminal offense.
Leerink Partners
The date of this prospectus supplement is May 16,
2024
TABLE OF CONTENTS
PROSPECTUS
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is in two parts.
The first part is this prospectus supplement, which describes the terms of this offering of common stock from time to time and also adds
to and updates information contained in the accompanying prospectus and the documents incorporated by reference into this prospectus supplement
and the accompanying prospectus. The second part, the accompanying prospectus dated December 23, 2021, including the documents incorporated
by reference therein, provides more general information that may not relate to this offering. Generally, when we refer to this prospectus,
we are referring to both parts of this document combined. To the extent there is a conflict between the information contained in this
prospectus supplement, on the one hand, and the information contained in the accompanying prospectus or in any document incorporated by
reference that was filed with the Securities and Exchange Commission (the “SEC”), before the date of this prospectus supplement,
on the other hand, you should rely on the information in this prospectus supplement. If any statement in one of these documents is inconsistent
with a statement in another document having a later date (for example, a document incorporated by reference in the accompanying prospectus),
the statement in the document having the later date modifies or supersedes the earlier statement.
Before buying any of the common
stock that we are offering, we urge you to carefully read this prospectus supplement, the accompanying prospectus and all of the information
incorporated by reference herein and therein, as well as the additional information described under the heading “Incorporation of
Certain Documents by Reference.” These documents contain important information that you should consider when making your investment
decision.
We have not, and Leerink Partners
has not, authorized anyone to provide you with information that is different from or in addition to the information contained in this
prospectus supplement and the accompanying prospectus and in any related free writing prospectus filed by us with the SEC. Accordingly,
neither we nor Leerink Partners takes any responsibility for, or can provide any assurance as to the reliability of, any information that
others may give. Neither we nor Leerink Partners is making offers to sell or seeking offers to buy shares of our common stock in any jurisdiction
where the offer or sale is not permitted. You should assume that the information appearing in this prospectus supplement, the accompanying
prospectus, the documents incorporated by reference herein and therein and any related free writing prospectus is accurate only as of
the respective dates of such documents, regardless of the time of delivery of this prospectus supplement or any sale of the common stock
offered hereby. Our business, financial condition, results of operations and prospects may have changed materially since those dates.
We further note that the representations,
warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference in
this prospectus supplement or the accompanying prospectus were made solely for the benefit of the parties to such agreement, including,
in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation,
warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly,
such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
Unless the context otherwise
requires, “Eyenovia,” “the Company,” “we,” “us,” “our” and similar terms refer
to Eyenovia, Inc. and our subsidiaries.
INDUSTRY AND MARKET DATA
We obtained the industry,
statistical and market data in this prospectus supplement and the accompanying prospectus from our own internal estimates and research,
as well as from industry and general publications and research, surveys and studies conducted by third parties. In presenting this information,
we have made assumptions based on such data and other similar sources, and on our knowledge of, and our experience to date in, the potential
markets for our product candidates. Although we believe the data from these third-party sources is reliable and are responsible for the
accuracy of such data, we have not independently verified any third-party information. The industry in which we operate is subject to
a high degree of uncertainty and risk due to a variety of factors, including those described in the section entitled “Risk Factors”.
These and other factors could cause results to differ materially from those expressed in the estimates made by third parties and by us.
PROSPECTUS SUPPLEMENT SUMMARY
The following is a summary
of what we believe to be the most important aspects of our business and the offering of our common stock under this prospectus supplement
and the accompanying prospectus. We urge you to read this entire prospectus supplement and the accompanying prospectus, including the
more detailed consolidated financial statements, notes to the consolidated financial statements and other information incorporated by
reference from our other filings with the SEC. Investing in our securities involves risks. Therefore, carefully consider the risk factors
set forth in our most recent annual and quarterly filings with the SEC, as well as other information in this prospectus supplement, the
accompanying prospectus and the documents incorporated by reference herein or therein, before purchasing our common stock. Each of the risk
factors could adversely affect our business, operating results and financial condition, as well as adversely affect the value of an investment
in our common stock.
Overview
We are an ophthalmic technology
company focused on the late-stage development of MicroPine in the multi-billion dollar pediatric progressive myopia market while commercializing
Mydcombi™ (tropicamide and phenylephrine HCL ophthalmic spray) for inducing mydriasis for routine diagnostic procedures and in conditions
where short term pupil dilation is desired, and clobetasol propionate ophthalmic suspension, for the treatment of post-operative pain
and inflammation following ocular surgery. We are also developing the Optejet® delivery system both for use in combination with our
own drug-device therapeutic programs and for out-licensing for use in combination with therapeutics for additional indications. Our aim
is to improve the delivery of topical ophthalmic medication through the ergonomic design of the Optejet which facilitates ease-of-use
and delivery of more physiologically appropriate medication volume, with the goal to reduce side effects and improve tolerability, and
introduce digital health technology to improve therapy compliance and ultimately medical outcomes.
The ergonomic and functional
design of the Optejet allows for horizontal drug delivery and eliminates the need to tilt the head back or the manual dexterity to squeeze
a bottle, to administer medications. Drug is delivered in a microscopic array of droplets faster than the blink reflex to help ensure
instillation success. The precise delivery of a low-volume columnar spray by the Optejet device minimizes contamination risk with a non-protruding
nozzle and self-closing shutter. In clinical trials, the Optejet has demonstrated that its targeted delivery achieves a high rate of successful
administration, with 98% of sprays being accurately delivered upon first attempt compared to the established rate reported with traditional
eye drops of ~ 50%.
A more physiologically appropriate
volume of medication in the range of seven to nine microliters is delivered by the Optejet, which is approximately one-fifth of the 35
to 50 microliter dose typically delivered in a single eye drop. Lower volume of medication exposes the ocular surface to less active ingredient
and preservatives, potentially reducing ocular stress and surface damage and improving tolerability. The lower volume also minimizes the
potential for drug to enter systemic circulation, with the goal of avoiding some common side effects that are related to overdosing of
the eye.
We are developing versions
of the Optejet with on-board digital technology that records the date and time of each use. These data may be used to provide reminders
via Bluetooth to smart devices and to allow healthcare practitioners to monitor usage. This information can then be used by practitioners
and health care systems to measure treatment compliance and improve medical decision making. In this way, the Optejet could serve as an
extension of the physician’s office by providing information that is not currently possible to collect except through the use of
diaries.
Our drug-device product line
includes Mydcombi (tropicamide and phenylephrine HCL ophthalmic spray) and therapeutic programs MicroPine (atropine ophthalmic spray)
and MicroLine (pilocarpine ophthalmic spray). MicroPine is our first-in-class topical therapy for the treatment of progressive myopia,
a disease associated with pathologic axial elongation of the eye and sclero-retinal stretching. In the United States, myopia is estimated
to affect approximately 25 million children, with up to five million considered to be at high risk for progressive myopia. In February
2019, the U.S. Food and Drug Administration (“FDA”) accepted our Investigational New Drug application (“IND”) to initiate the CHAPERONE study to reduce the progression of myopia in children. The first patient was
enrolled in the CHAPERONE study in June 2019.
On October 9, 2020, we
entered into a license agreement with Bausch + Lomb (“B+L”), pursuant to which B+L had the rights to develop and
commercialize MicroPine in the United States and Canada (the “Bausch License Agreement”). Under the terms of the Bausch License Agreement, we received an upfront
payment of $10.0 million and were eligible to receive up to a total of $35.0 million in additional payments, based on the
achievement of certain regulatory and launch-based milestones. B+L also agreed to pay royalties to Eyenovia on a tiered basis
(ranging from mid-single digit to mid-teen percentages) on gross profits from sales of MicroPine in the United States and Canada,
subject to certain adjustments. Under the terms of the Bausch License Agreement, B+L assumed sponsorship of the IND as well as
ownership and the costs related to the ongoing CHAPERONE study.
On January 12, 2024, we entered
into an agreement with B+L to repatriate our rights to MicroPine and take control of the CHAPERONE study. In this agreement, we agreed
to pay B+L $2.0 million in cash up front. In addition, we have issued $3.0 million in common stock upon successful transfer of the regulatory
documents and study elements to us, which occurred in April 2024. We also agreed to pay B+L a 2% royalty on net sales once MicroPine is
commercialized in the United States, assuming receipt of regulatory approvals. We believe that this arrangement is in our and our shareholders’
best interests, as it may substantially increase the value of the asset through potential improvements in the conduct of the study, including
a planned interim analysis of the data in late 2024.
We have also
successfully expanded our manufacturing capabilities through a partnership with Coastline International, Inc. located in Tijuana,
Mexico, as well as the construction of our new manufacturing facility in Reno, Nevada and the construction of our own fill and
finish facility in Redwood City, California. We have received FDA clearance for using both Coastline International, Inc. and our
Redwood City facility for the production of Mydcombi cartridges, and FDA clearance for using our Reno facility for the production of
technical elements such as the base unit for the Optejet device.
MicroLine is our investigational
pharmacologic treatment for presbyopia, a non-preventable, age-related hardening of the lens, which causes the gradual loss of the eye’s
ability to focus on near objects and impairs near visual acuity. There are two FDA-approved treatments for presbyopia which use pilocarpine,
the same drug used in our investigational product. We have completed two Phase III studies using our Optejet® device. In these studies,
patients reported high satisfaction with using the device and a strong preference over using an eye dropper bottle. We released positive
top-line results from VISION-2 in the fourth quarter of 2022. We are planning to meet with the FDA in mid-2024 to discuss a transition
of the product into our new Gen-2 Optejet device, which has a significantly lower cost to manufacture than the first generation device.
Mydcombi is the only FDA-approved
fixed combination of the two leading mydriatic agents, tropicamide and phenylephrine in the United States and our first FDA-approved product.
As an ophthalmic spray delivered with Optejet technology, Mydcombi may present a number of benefits for ophthalmic surgical centers, optometric
and ophthalmic offices and patients. Those benefits may include improved cost-effectiveness in centers that employ single-use bottles
for mydriasis, more efficient use of office time and resources, and an overall improved doctor-patient experience. We have begun the commercialization
of Mydcombi, with the first commercial sale of the product occurring on August 3, 2023 as part of a targeted launch, and are planning
to expand our launch with the onboarding of ten sales representatives in early 2024. We received FDA approval for our primary Mydcombi
manufacturing facility in February 2024, which we believe will allow us to expand and continue to build our manufacturing operations.
On August 10, 2020, we
entered into a license agreement with Arctic Vision pursuant to which Arctic Vision may develop and commercialize MicroPine,
MicroLine and Mydcombi in Greater China (mainland China, Hong Kong, Macau and Taiwan) and South Korea (the “Arctic Vision License Agreement”). Under the terms of the
Arctic Vision License Agreement, as amended, we received an upfront payment of $4.25 million before any payments to Senju. In
addition, we may receive up to a total of $37.7 million in additional payments, based on various development and regulatory
milestones, including the initiation of clinical research and approvals in Greater China and South Korea, and development costs.
Arctic Vision also will purchase its supply of MicroPine, MicroLine and Mydcombi from Eyenovia or, for such products not supplied by
Eyenovia, pay a mid-single digit percentage royalty on net sales of such products, subject to certain adjustments. We will pay
between 30 and 40 percent of such payments, royalties, or net proceeds of such supply to Senju pursuant to the Senju License
Agreement.
We are in active discussions
with manufacturers of existing and late-stage ophthalmic medications to explore whether development with the Optejet technology can solve
unmet medical and business needs. Some of those business needs could include extension of exclusivity under the Optejet patents, improvement
in a drug’s tolerability profile, or potential improvement in treatment compliance.
On August 15, 2023, we
entered into a license agreement (the “Formosa License”) with Formosa Pharmaceuticals, Inc. (“Formosa”),
whereby we acquired the exclusive U.S. rights to commercialize any product related to a novel formulation of clobetasol propionate
ophthalmic suspension 0.05% (the “Licensed Product”), which was approved by the FDA, for post-operative inflammation and
pain after ocular surgery, on March 4, 2024. The Formosa License will remain in effect for ten years from the date of the first
commercial sale of a Licensed Product, unless earlier terminated.
We paid Formosa an
upfront payment in an aggregate amount of $2.0 million which consisted of (a) cash in the amount of $1.0 million and (b) 487,805
shares of common stock valued at $1.0 million. We also capitalized $122,945 of transaction costs in connection with the Formosa
License. In addition, we must pay Formosa up to $4.0 million upon the achievement of certain development milestones and up to $80
million upon the achievement of certain sales milestones. The trigger for the initial $2.0 million development milestone payments
was FDA approval of the Licensed Product and the effective date of the acceptance by the Company of the transfer and assignment of
the FDA approval occurred on March 11, 2024. Based on the achievement of this milestone, we paid Formosa the aggregate amount of
$2.0 million, consisting of (a) cash in the amount of $1.0 million on April 26, 2024 and (b) 613,496 shares of common stock valued
at $1.0 million on May 2, 2024. The remaining $2.0 million development milestone will be triggered on the earlier of twelve months
after FDA approval of the Licensed Product or six months following the first commercial sale of the Licensed Product.
Corporate Information
We were organized as a corporation
under the laws of the State of Florida on March 12, 2014 under the name “PGP Holdings V, Inc.” On May 5, 2014, we changed
our name to Eyenovia, Inc. On October 6, 2014, we reincorporated in the State of Delaware by merging into Eyenovia, Inc., a Delaware corporation.
Our principal executive office is located at 295 Madison Avenue, Suite 2400, New York, NY 10017, and our telephone number is (833) 393-6684.
We maintain a website at http://www.eyenovia.com, to which we regularly post copies of our press releases as well as additional information
about us. The information contained on, or that can be accessed through, our website is not a part of this prospectus supplement or the
accompanying prospectus. We have included our website address in this prospectus supplement solely as an inactive textual reference.
All brand names or trademarks
appearing in this prospectus supplement and the accompanying prospectus are the property of their respective holders. Use or display
by us of other parties’ trademarks, trade dress, or products in this prospectus supplement and the accompanying prospectus is not
intended to, and does not, imply a relationship with, or endorsements or sponsorship of, us by the trademark or trade dress owners.
THE OFFERING
| Shares of common stock offered by us: |
Shares of our common stock having an aggregate offering price of up to $10,000,000. |
| |
|
| Shares of common stock to be outstanding immediately after this offering: |
Up to 63,304,724 shares, assuming sales of 9,433,962 shares of common
stock are made in this offering at an offering price of $1.06 per share, the
last reported sale price of shares of our common stock on The Nasdaq Capital Market on May
15, 2024. The actual number of shares that may be issued will vary depending
on the sales price under this offering. |
| |
|
| Manner of offering: |
“At the market offering” that may be made from time to time on The Nasdaq Capital Market or other existing trading market for shares of our common stock through or to, Leerink Partners. See the section entitled “Plan of Distribution” on page S-10 of this prospectus supplement. |
| |
|
| Use of proceeds: |
We intend to use the net proceeds of this offering for working capital
and general corporate purposes. Proceeds may also be used to repay amounts outstanding under the Loan and Security Agreement with
Avenue Capital Management II, L.P. and related entities (together, “Avenue”). See the section titled “Use of Proceeds”
on page S-8 of this prospectus supplement. |
| |
|
| Risk factors: |
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page S-5 of this prospectus supplement and the other information included in, or incorporated by reference into, this prospectus supplement and the accompanying prospectus for a discussion of certain factors you should carefully consider before deciding to invest in shares of our common stock. |
| |
|
| The Nasdaq Capital Market symbol: |
EYEN |
The number of shares of common
stock outstanding immediately after this offering is based on 53,870,762 shares of common stock outstanding as of March 31, 2024 on a pro forma
basis, which includes 47,386,349 shares outstanding as of March 31, 2024 (actual); 347,794 shares of common stock issued since March 31, 2024
pursuant to our at-the-market offering facility; 3,223,726 shares of common stock issued in a registered direct offering in April 2024 (the “Registered Direct Offering”); 613,496 shares
issued to Formosa in April 2024 pursuant to the Formosa License; and 2,299,397 shares issued to B+L in May 2024 pursuant to our agreement
with B+L.
This number excludes:
| |
· |
6,022,877 shares of our common stock underlying outstanding options to purchase common stock under
our 2014 Equity Incentive Plan, as amended (the “2014 Plan”) and our Amended and Restated 2018 Omnibus Stock Incentive
Plan (the “2018 Plan”) with a weighted average exercise price of $3.19 per share; |
| |
· |
241,764 shares of our common stock issuable in connection with restricted
stock units under the 2014 Plan and the 2018 Plan; |
| |
· |
1,337,909 shares of our common stock reserved for future issuance under the 2014 Plan and the 2018
Plan; |
| |
|
|
| |
· |
2,327,747 shares of our common stock issuable upon conversion of outstanding
convertible notes; and |
| |
· |
warrants to purchase 10,926,554 shares of our common stock, with a weighted average exercise price of $2.28 per share. |
Unless otherwise noted, the
information in this prospectus supplement reflects and assumes no exercise of outstanding options and warrants.
RISK FACTORS
Investing in our securities
involves a high degree of risk. You should consider carefully the risks and uncertainties, as well as other information, in this prospectus
supplement and the accompanying prospectus and the documents incorporated by reference herein and therein, including the risks described
under “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2023, as amended by Amendment No. 1
thereto, and as updated by any other document that we subsequently file with the SEC and that is incorporated by reference into this prospectus
supplement, and any free writing prospectus that we have authorized for use in connection with this offering. The risks set forth in this
prospectus supplement and the accompanying prospectus and incorporated by reference herein and therein are those which we believe are
the material risks that we face. These risks are not the only ones facing us and there may be additional matters that we are unaware of
or that we currently consider immaterial. The occurrence of any of such risks may materially and adversely affect our business, financial
condition, results of operations and future prospects. In such an event, the market price of our common stock could decline, and you could
lose part or all of your investment.
Risks Related to this Offering
You may experience immediate and substantial
dilution.
The offering price per share
being offered may exceed the net tangible book value per share of our common stock outstanding prior to this offering. Assuming that an
aggregate of 9,433,962 shares of our common stock are sold under this prospectus supplement at a price of $1.06
per share, the last reported sale price of our common stock on The Nasdaq Capital Market on May 15, 2024, the aggregate gross proceeds
will be approximately $10.0 million. After deducting commissions and estimated aggregate offering expenses payable by us, you will
experience immediate dilution of $0.94 per share, representing the difference between our pro forma as adjusted net tangible book value
per share as of March 31, 2024, after giving effect to the pro forma adjustments further described in “Dilution” and the sale
of shares in this offering at the assumed offering price. Shares issuable pursuant to the exercise of outstanding stock options and warrants, restricted stock units, new awards pursuant to the 2014 Plan or 2018 Plan, or upon the conversion of convertible notes payable, may result in further
dilution of your investment. See the section entitled “Dilution” below for a more detailed illustration of the dilution you
would incur if you participate in this offering.
You may experience future dilution as a
result of future equity offerings.
In order to raise additional
capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our
common stock at prices that may not be the same as the price per share paid by any investor in this offering. We may sell shares or other
securities in any other offering at a price per share that is less than the price per share paid by an investor in this offering, and
investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share
at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions
may be higher or lower than the price per share paid by investors in this offering.
We may be required to raise additional capital
by issuing new securities with terms or rights superior to those of our existing securityholders, which could adversely affect your investment
in our company, the market price of shares of our common stock and our business.
We might require additional
financing to fund future operations, including our research and development, manufacturing and any possible sales and marketing activities.
We may not be able to obtain financing on favorable terms, if at all. If we raise additional funds by issuing equity securities, the percentage
ownership of our then current stockholders will be reduced, and the holders of the new equity securities may have rights superior to those
of our then existing securityholders, which could adversely affect the market price of our common stock and the voting power of shares
of our common stock. If we raise additional funds by issuing debt securities, the holders of these debt securities would similarly have
some rights senior to those of our then existing securityholders, and the terms of these debt securities could impose restrictions on
operations and create a significant interest expense for us which could have a materially adverse effect on our business.
Sales of our common stock in this offering,
or the perception that such sales may occur, could cause the market price of our common stock to fall.
We may issue and sell shares
of our common stock for aggregate gross proceeds of up to $10,000,000 from time to time in connection with this offering. The actual number
of shares of common stock that may be issued and sold in this offering, as well as the timing of any such sales, will depend on a number
of factors, including, among others, the prices at which any shares are actually sold in this offering (which may be influenced by market
conditions, the trading price of our common stock and other factors) and our determinations as to the appropriate timing, sources and
amounts of funding we need. The issuance and sale from time to time of these new shares of common stock, or the mere fact that we are
able to issue and sell these shares in this offering, could cause the market price of our common stock to decline.
It is not possible to predict the actual
number of shares of common stock we will sell under the Sales Agreement, or the gross proceeds resulting from those sales.
Subject to certain limitations
in the Sales Agreement and compliance with applicable law, we have the discretion to deliver a placement notice to Leerink Partners at
any time throughout the term of the Sales Agreement. The number of shares that are sold through Leerink Partners after the delivery of
a placement notice will fluctuate based on a number of factors, including the market price of our common stock during the sales period,
the limits we set with Leerink Partners in any applicable placement notice, and the demand for our common stock during the sales period.
Because the price per share will fluctuate during this offering, it is not currently possible to predict the number of shares that will
be sold or the gross proceeds to be raised in connection with those sales.
The common stock offered hereby will be
sold in “at-the-market offerings” and investors who buy shares at different times will likely pay different prices.
Investors who purchase shares
in this offering at different times will likely pay different prices, and accordingly may experience different levels of dilution and
different outcomes in their investment results. We will have discretion, subject to market demand, to vary the timing, prices and number
of shares sold in this offering. Investors may experience a decline in the value of the shares they purchase in this offering as a result
of sales made at prices lower than the prices they paid.
We have broad discretion in the use of the
net proceeds from this offering and may not use them effectively.
Our management will have
broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our
business, financial condition or results of operations or enhance the value of our common stock. See “Use of Proceeds” on
page S-8 of this prospectus supplement for a description of our proposed use of proceeds from this offering.
The failure by our management
to apply these funds effectively could result in financial losses that could harm our business, cause the price of our common stock to
decline and delay the development of our product candidates. Pending their use, we may invest the net proceeds from this offering in a
manner that does not produce income or that loses value.
If securities or industry analysts do not
publish research or reports about our business, or publish negative reports about our business, our stock price and trading volume could
decline.
The trading market for our
common stock will be influenced by the research and reports that securities or industry analysts publish about us or our business. We
do not have any control over these analysts. If one or more of the analysts who cover us downgrade our stock or change their opinion of
our stock in a negative manner, our stock price would likely decline. If one or more of these analysts cease coverage of our company or
fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our stock price or trading
volume to decline.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement,
the accompanying prospectus, the documents we have filed with the SEC that are incorporated by reference herein and therein and any free
writing prospectus that we have authorized for use in connection with this offering contain “forward-looking statements” within
the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Securities
Exchange Act of 1934, as amended (the “Exchange Act”). These statements relate to future events or to our future operating
or financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance
or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking
statements. Forward-looking statements may include, but are not limited to, statements about:
| · | our need to raise additional money to fund our operations for the next twelve months as a going concern; |
| · | our estimates regarding expenses, future revenue, timing of any future revenue, capital requirements and needs for additional financing; |
| · | our expectations related to the use of proceeds from our financings; |
| · | risks of our and our licensees’ clinical trials including, but not limited to, the costs, design, initiation and enrollment,
timing, progress and results of such trials; |
| · | the timing and our or our licensees’ ability to submit applications for, obtain and maintain regulatory approval for Mydcombi,
clobetasol propionate and our product candidates; |
| · | the production and commercialization of Mydcombi and clobetasol propionate; |
| · | reliance on third parties to develop and commercialize Mydcombi, clobetasol propionate and certain of our product candidates; |
| · | our and our partners’ ability to timely develop, implement and maintain manufacturing, commercialization and marketing capabilities
and strategies for Mydcombi, clobetasol propionate and certain of our product candidates; |
| · | our estimates regarding the potential market opportunities for Mydcombi, clobetasol propionate and our product candidates; |
| · | the potential advantages of Mydcombi, clobetasol propionate and our product candidates and platform technology and potential revenues
from licensing transactions; |
| · | the rate and degree of market acceptance and clinical utility of Mydcombi, clobetasol propionate and our product candidates; |
| · | our intellectual property position; |
| · | our ability to identify additional products, product candidates or technologies with significant commercial potential that are consistent
with our commercial objectives; |
| · | our ability to attract and retain key personnel; |
| · | the impact of government laws and regulations; |
| · | our competitive position; |
| · | developments relating to our competitors and our industry; |
| · | our ability to maintain and establish collaborations; |
| · | general or regional economic conditions; |
| · | changes in U.S. GAAP; and |
| · | changes in the legal, regulatory and legislative environments in the markets in which we operate, and the impact of these changes
on our ability to obtain regulatory approval for our products. |
In some cases, you can identify
forward-looking statements by terms such as “anticipate,” “believe,” “can,” “continue,”
“could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,”
“potential,” “project,” “seek,” “should,” “target,” “will,” “would”
and similar expressions or variations intended to identify forward- looking statements. These statements reflect our current views with
respect to future events, are based on assumptions and are subject to risks and uncertainties. Given these uncertainties, you should not
place undue reliance on these forward-looking statements. We discuss in greater detail, and incorporate by reference into this prospectus
supplement and the accompanying prospectus in their entirety, many of these risks under the headings “Risk Factors” on page
S-5 of this prospectus supplement, and in our Annual Report on Form 10-K for the year ended December 31, 2023, as amended by Amendment No. 1 thereto and as updated by our subsequent filings under the Exchange Act, which are incorporated herein by reference, as may be updated
or superseded by the risks and uncertainties described under similar headings in the other documents that are filed after the date hereof
and incorporated by reference into this prospectus supplement. Also, these forward-looking statements represent our estimates and assumptions
only as of the date of the document containing the applicable statement.
You should read this prospectus
supplement, the accompanying prospectus, the documents we have filed with the SEC that are incorporated by reference herein and therein
and any free writing prospectus that we have authorized for use in connection with this offering completely and with the understanding
that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in the
foregoing documents by these cautionary statements.
Unless required by law, we
undertake no obligation to update or revise any forward-looking statements to reflect new information or future events or developments.
Thus, you should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking
statements.
USE OF PROCEEDS
We may issue and sell up to
$10,000,000 of our common stock from time to time. Because there is no minimum offering amount required as a condition to close this offering,
the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time. There can be no assurance
that we will sell any shares under or fully utilize the Sales Agreement with Leerink Partners as a source of financing.
We intend to use the net proceeds
from this offering, if any, for working capital and general corporate purposes. Proceeds may also be used to repay amounts outstanding under the Loan and Security Agreement with Avenue. The Avenue loan bears interest
at an annual rate equal to the greater of (a) 7.0% and (b) the prime rate as reported in The Wall Street Journal plus 4.45%. The maturity
date is November 1, 2025.
As of the date of this prospectus
supplement, we cannot specify with certainty any of the particular uses of the proceeds, if any, from this offering. Accordingly, we will
retain broad discretion over the use of any such proceeds. Pending the use of the net proceeds, if any, from this offering as described
above, we intend to invest the net proceeds in investment-grade, interest-bearing instruments.
DILUTION
If you invest in our common
stock in this offering, your ownership interest will be diluted to the extent of the difference between the offering price per share paid
by the purchasers in this offering and our pro forma as adjusted net tangible book value per share immediately after this offering.
Our historical net tangible
book deficit as of March 31, 2024 was approximately $(4.3) million, or $(0.09) per share of common stock. We calculate tangible book value
per share by dividing our total tangible assets, less total liabilities, by the number of shares of our common stock outstanding as of
March 31, 2024.
Our pro forma net
tangible book deficit as of March 31, 2024 was approximately
$(2.1) million, or
$(0.04) per share of common stock. We calculate pro forma net tangible
book deficit per share by dividing (a) the total of our historical net tangible book value, the net proceeds received after March 31,
2024 from our at-the-market offering and the net proceeds received in the Registered Direct Offering, by (b) the sum of (i) the
total number of shares of our common stock outstanding as of March 31, 2024, (ii) the number of shares of our common stock issued
after March 31, 2024 in our at-the-market offering, (iii) the number of shares issued in the Registered Direct Offering, (iv) the
number of shares issued to Formosa in April 2024 pursuant to the Formosa License and (v) the number of shares issued to B+L in April
2024 pursuant to our agreement with B+L.
We present dilution on a
pro forma as adjusted basis to give effect to the sale by us of shares of our common stock in this offering in the aggregate amount
of $10,000,000, at an assumed public offering price of $1.06 per share of common stock, the last reported sale price of our common
stock on The Nasdaq Capital Market on May 15, 2024, after deducting commissions and estimated offering expenses payable by us. This
offering represents an immediate increase in net tangible book value to existing stockholders of $0.16 per share of common stock and
immediate dilution in net tangible book value to purchasers of shares of common stock in this offering of $0.94 per share of common
stock. The following table illustrates this dilution per share of common stock:
| Assumed public offering price per share | |
| | | |
$ | 1.06 | |
| Historical net tangible book deficit per share as of March 31, 2024 | |
$ | (0.09 | ) | |
| | |
| Pro forma increase in net tangible book value per share as of March 31, 2024 | |
| 0.05 | | |
| | |
| Pro forma net tangible book deficit per share | |
| (0.04 | ) | |
| | |
| Increase in pro forma net tangible book value per share attributable to this offering | |
| 0.16 | | |
| | |
| Pro forma as adjusted tangible book value per share, after giving effect to this offering | |
| | | |
| 0.12 | |
| Dilution per share to investors in this offering | |
| | | |
$ | 0.94 | |
The number of shares of common
stock outstanding immediately after this offering is based on 53,870,762 shares of common stock outstanding as of March 31, 2024 on a pro forma
basis, which includes 47,386,349 shares outstanding as of March 31, 2024 (actual); 347,794 shares of common stock issued since March 31, 2024
pursuant to our at-the-market offering facility; 3,223,726 shares of common stock issued in the Registered Direct Offering; 613,496 shares
issued to Formosa in April 2024 pursuant to the Formosa License; and 2,299,397 shares issued to B+L in April 2024 pursuant to our agreement
with B+L.
This number excludes:
| |
· |
6,022,877 shares of our common stock underlying outstanding options to purchase common Stock under
the 2014 Plan and the 2018 Plan with a weighted average exercise price of $3.19 per share; |
| |
· |
241,764 shares of our common stock issuable in connection with restricted
stock units under the 2014 Plan and the 2018 Plan; |
| |
· |
1,337,909 shares of our common stock reserved for future issuance under the 2014 Plan and the 2018
Plan; |
| |
|
|
| |
· |
2,327,747 shares of our common stock issuable upon conversion of outstanding
convertible notes; and |
| |
· |
warrants to purchase 10,926,554 shares of our common stock, with a weighted average exercise price of $2.28 per share. |
Unless otherwise noted, the information in this prospectus supplement
reflects and assumes no exercise of outstanding options and warrants.
To the extent that any
of these options or warrants are exercised, new options are issued under our 2014 Plan or 2018 Plan, shares are issued in satisfaction of the restricted stock units, or convertible notes payable are converted, or we issue additional shares of
common stock, warrants or other equity securities in the future, there may be further dilution to investors participating in this
offering.
In addition, we may choose
to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current
or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities,
the issuance of these securities may result in further dilution to our shareholders.
PLAN OF DISTRIBUTION
We have entered into a Sales
Agreement with Leerink Partners under which we may issue and sell shares of common stock having an aggregate offering price of up to $50,000,000
from time to time through Leerink Partners as our sales agent. As of the date of this prospectus, an aggregate of $14,450,163 of shares
of common stock have been sold pursuant to the Sales Agreement, and up to $10,000,000 of shares of common stock may be sold pursuant to
this prospectus supplement and the accompanying prospectus. Sales of shares of common stock, if any, under this prospectus supplement
will be made at market prices by any method that is deemed to be an “at-the-market” offering, as defined in Rule 415
under the Securities Act, including sales made directly on the Nasdaq Capital Market or any other trading market for our common stock.
If authorized by us in writing, Leerink Partners may purchase shares of our common stock as principal.
Leerink Partners will offer
shares of our common stock subject to the terms and conditions of the Sales Agreement on a daily basis or as otherwise agreed upon by
us and Leerink Partners. We will designate the maximum amount of common stock to be sold through Leerink Partners on a daily basis or
otherwise determine such maximum amount together with Leerink Partners. Subject to the terms and conditions of the Sales Agreement, Leerink
Partners will use its commercially reasonable efforts consistent with its normal trading and sales practices to sell on our behalf all
of the shares of common stock requested to be sold by us. We may instruct Leerink Partners not to sell shares of common stock if the sales
cannot be effected at or above the price designated by us in any such instruction. Leerink Partners or we may suspend the offering of
shares of our common stock being made through Leerink Partners under the Sales Agreement upon proper notice to the other party. Leerink
Partners and we each have the right, by giving written notice as specified in the Sales Agreement, to terminate the Sales Agreement in
each party’s sole discretion at any time. The offering of shares of our common stock pursuant to the Sales Agreement will otherwise
terminate upon the termination of the Sales Agreement as provided therein.
The aggregate compensation
payable to Leerink Partners as sales agent will be an amount equal to 3.0% of the gross proceeds of any shares sold through it pursuant
to the Sales Agreement. We previously reimbursed Leerink Partners $75,000 of Leerink Partners’ actual outside legal expenses incurred
by Leerink Partners in connection with this offering. We have also agreed to reimburse Leerink Partners for certain ongoing fees of its
legal counsel. We estimate that the total expenses of the offering payable by us, excluding commissions payable to Leerink Partners under
the Sales Agreement, will be approximately $75,000.
Leerink Partners will provide
written confirmation to us following the close of trading on the Nasdaq Capital Market on each day in which shares of common stock are
sold through it as sales agent under the Sales Agreement. Each confirmation will include the number of shares of common stock sold through
it as sales agent on that day, the volume weighted average price of the shares of common stock sold, the percentage of the daily
trading volume and the net proceeds to us.
Settlement for sales of shares
of common stock will occur, unless the parties agree otherwise, on the second business day that is also a trading day following the date
on which any sales were made in return for payment of the net proceeds to us. Pursuant to recent amendments to Rule 15c6-1 of the Exchange
Act, settlement for any securities offered under this prospectus on or after May 28, 2024, may occur on the first business day that is
also a trading day following the date on which any sales were made in return for payment of the net proceeds to us. There is no arrangement
for funds to be received in an escrow, trust or similar arrangement. There is no arrangement for funds to be received in an escrow, trust
or similar arrangement.
We will report at least quarterly
the number of shares of common stock sold through Leerink Partners under the Sales Agreement, the net proceeds to us and the compensation
paid by us to Leerink Partners in connection with the sales of shares of common stock during the relevant period.
In connection with the sales
of shares of common stock on our behalf, Leerink Partners may be deemed to be an “underwriter” within the meaning of the Securities
Act, and the compensation paid to Leerink Partners may be deemed to be underwriting commissions or discounts. We have agreed in the Sales
Agreement to provide indemnification and contribution to Leerink Partners against certain liabilities, including liabilities under the
Securities Act. As sales agent, Leerink Partners will not engage in any transactions that stabilize our common stock.
LEGAL MATTERS
The validity of the common
stock offered by this prospectus supplement will be passed upon by Covington & Burling LLP, Boston, Massachusetts. Paul Hastings LLP,
New York, New York, is representing Leerink Partners in connection with the offering.
EXPERTS
The financial statements
of Eyenovia, Inc. as of December 31, 2023 and 2022 and for each of the two years in the period ended December 31, 2023, have been audited
by Marcum LLP, independent registered public accounting firm, as stated in their report, which includes an explanatory paragraph as to
the Company’s ability to continue as a going concern, which is incorporated herein by reference. Such financial statements of Eyenovia,
Inc. are incorporated in this prospectus by reference in reliance on the report of such firm given upon their authority as experts in
accounting and auditing.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC allows us to “incorporate
by reference” information from other documents that we file with it, which means that we can disclose important information to you
by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus supplement.
Information in this prospectus supplement supersedes information incorporated by reference that we filed with the SEC prior to the date
of this prospectus supplement, while information that we file later with the SEC will automatically update and supersede the information
in this prospectus supplement. We incorporate by reference into this prospectus supplement and the accompanying prospectus the information
or documents listed below that we have filed with the SEC (Commission File No. 001-38365):
The SEC file number for each
of the documents listed above is 001-38365.
In addition, any future filings
(other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such
items unless such Form 8-K expressly provides to the contrary) made with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the
Exchange Act, prior to the termination of this offering, will be deemed incorporated by reference into this prospectus supplement.
Any statement contained in
this prospectus supplement or in a document incorporated or deemed to be incorporated by reference into this prospectus supplement will
be deemed to be modified or superseded for purposes of this prospectus supplement to the extent that a statement contained in this prospectus
supplement or other subsequently filed document that also is or is deemed to be incorporated by reference into this prospectus supplement
modifies or supersedes the statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded,
to constitute a part of this prospectus supplement.
We will furnish without charge
to you, on written or oral request, a copy of any filing or report incorporated by reference, including exhibits to the document. You
should direct any requests for documents to Eyenovia, Inc., 295 Madison Avenue, Suite 2400, New York, NY 10017, (833) 393-6684,
Attention: Corporate Secretary.
PROSPECTUS

$100,000,000
COMMON
STOCK
PREFERRED STOCK
DEBT SECURITIES
WARRANTS
RIGHTS
UNITS
This prospectus
will allow us to issue, from time to time at prices and on terms to be determined at or prior to the time of the offering, up to $100,000,000
of any combination of the securities described in this prospectus, either individually or in units. We may also offer common stock, or
preferred stock upon conversion of or exchange for the debt securities; common stock upon conversion of or exchange for preferred stock;
common stock, preferred stock or debt securities upon the exercise of warrants or rights.
This prospectus
describes the general terms of these securities and the general manner in which these securities will be offered. We will provide you
with the specific terms of any offering in one or more supplements to this prospectus. The prospectus supplements will also describe the
specific manner in which these securities will be offered and may also supplement, update or amend information contained in this document.
You should read this prospectus and any prospectus supplement, as well as any documents incorporated by reference into this prospectus
or any prospectus supplement, carefully before you invest.
Our securities
may be sold directly by us to you, through agents designated from time to time or to or through underwriters or dealers. For additional
information on the methods of sale, you should refer to the section entitled “Plan of Distribution” in this prospectus and
in the applicable prospectus supplement. If any underwriters or agents are involved in the sale of our securities with respect to which
this prospectus is being delivered, the names of such underwriters or agents and any applicable fees, commissions or discounts and over-
allotment options will be set forth in a prospectus supplement. The price to the public of such securities and the net proceeds that we
expect to receive from such sale will also be set forth in a prospectus supplement.
Our common stock
is listed on The Nasdaq Capital Market under the symbol “EYEN.” On December 10, 2021, the last reported sale price of our
common stock was $3.76 per share. The applicable prospectus supplement will contain information, where applicable, as to any other listing,
if any, on The Nasdaq Capital Market or any securities market or other securities exchange of the securities covered by the prospectus
supplement. Prospective purchasers of our securities are urged to obtain current information as to the market prices of our securities,
where applicable.
We
are an “emerging growth company” and a “smaller reporting company” under applicable Securities and Exchange Commission
rules and are subject to reduced public company reporting requirements for this prospectus and future filings.
Investing
in our securities involves a high degree of risk. See “Risk Factors” included in any accompanying prospectus supplement and
in the documents incorporated by reference in this prospectus for a discussion of the factors you should carefully consider before deciding
to purchase these securities.
Neither the
Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if
this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus
is December 23, 2021.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus
is part of a registration statement that we filed with the U.S. Securities and Exchange Commission (the “SEC”), utilizing
a “shelf” registration process. Under this shelf registration process, we may offer shares of our common stock, various series
of debt securities or preferred stock, and warrants or rights to purchase any such securities, either individually or in units, in one
or more offerings, with a total value of up to $100,000,000. This prospectus provides you with a general description of the securities
we may offer. Each time we offer a type or series of securities under this prospectus, we will provide a prospectus supplement that will
contain specific information about the terms of that offering.
This
prospectus does not contain all of the information included in the registration statement. For a more complete understanding of the
offering of the securities, you should refer to the registration statement, including its exhibits. The prospectus supplement may
also add, update or change information contained or incorporated by reference in this prospectus. However, no prospectus supplement
will offer a security that is not registered and described in this prospectus at the time of its effectiveness. This prospectus,
together with the applicable prospectus supplements and the documents incorporated by reference into this prospectus, includes all
material information relating to the offering of securities under this prospectus. You should carefully read this prospectus, the
applicable prospectus supplement, the information and documents incorporated herein by reference and the additional information
under the heading “Where You Can Find More Information” before making an investment decision.
You should rely
only on the information we have provided or incorporated by reference in this prospectus or any prospectus supplement. We have not authorized
anyone to provide you with information different from that contained or incorporated by reference in this prospectus. No dealer, salesperson
or other person is authorized to give any information or to represent anything not contained or incorporated by reference in this prospectus.
You must not rely on any unauthorized information or representation. This prospectus is an offer to sell only the securities offered hereby,
but only under circumstances and in jurisdictions where it is lawful to do so. You should assume that the information in this prospectus
or any prospectus supplement is accurate only as of the date on the front of the document and that any information we have incorporated
herein by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this
prospectus or any sale of a security.
We
further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any
document that is incorporated by reference in the prospectus were made solely for the benefit of the parties to such agreement,
including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a
representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the
date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the
current state of our affairs.
This prospectus
may not be used to consummate sales of our securities, unless it is accompanied by a prospectus supplement. To the extent there are inconsistencies
between any prospectus supplement, this prospectus and any documents incorporated by reference, the document with the most recent date
will control.
Unless the context
otherwise requires, “Eyenovia,” “EYEN,” “the Company,” “we,” “us,” “our”
and similar terms refer to Eyenovia, Inc. and our subsidiaries.
PROSPECTUS SUMMARY
The following
is a summary of what we believe to be the most important aspects of our business and the offering of our securities under this prospectus.
We urge you to read this entire prospectus, including the more detailed consolidated financial statements, notes to the consolidated financial
statements and other information incorporated by reference from our other filings with the SEC or included in any applicable prospectus
supplement. Investing in our securities involves risks. Therefore, carefully consider the risk factors set forth in any prospectus supplements
and in our most recent annual and quarterly filings with the SEC, as well as other information in this prospectus and any prospectus supplements
and the documents incorporated by reference herein or therein, before purchasing our securities. Each of the risk factors could adversely
affect our business, operating results and financial condition, as well as adversely affect the value of an investment in our securities.
About Eyenovia, Inc.
We are a clinical
stage ophthalmic biopharmaceutical company developing a pipeline of advanced therapeutics based on our proprietary microdose array print
(MAP™) therapeutics. We aim to achieve clinical microdosing of next-generation formulations of well-established ophthalmic pharmaceutical
agents using our high-precision targeted ocular delivery system, branded the Optejet® which has the potential to replace conventional
eye dropper delivery and improve safety, tolerability, patient compliance and topical delivery success for ophthalmic eye treatments.
In the clinic, the Optejet has demonstrated the ability to horizontally deliver ophthalmic medication with a success rate significantly
higher than that of traditional eye drops (~ 90% vs. ~ 50%). Our technology is designed to achieve single-digit μl-volume physiologic
drug delivery with up to a 75% reduction in ocular drug and preservative topical dosing and has demonstrated significant improvement in
the therapeutic index in drugs used for mydriasis and IOP lowering through three Phase II and Phase III trials. Conventional eye formulations
lack high-precision micro-volume delivery and expose the ocular surface to approximately 300% more medication and preservatives than are
physiologically indicated leading to clinically recognized ocular and non-ocular side effects. Using the Optejet, we are developing the
next generation of smart ophthalmic therapeutics which target new indications or new combinations where there are currently no comparable
drug therapies approved by the U.S. Food and Drug Administration, (the “FDA”). Our microdose therapeutics follow the FDA-designated
pharmaceutical registration and regulatory process. Consistent with the recent FDA reclassification of MydCombi, we believe that most
of our product candidates are, or will be, classified by the FDA as drug- led combination products.
Our pipeline is
currently focused on the late-stage development of novel, potential first-in-class therapeutic indications for over an estimated five
million potential patients with progressive myopia in the United States and over an estimated one hundred million potential patients with
age-related near vision impairment, or presbyopia — indications where there is tremendous unmet need and no known existing FDA-approved
therapies. We are also developing the first microdose fixed combination ophthalmic pharmaceutical for mydriasis to address the estimated
over 100 million annual comprehensive eye exams with pupil dilation.
MicroPine
is our investigational first-in-class topical therapy for the treatment of progressive myopia, a back-of-the-eye ocular disease
associated with pathologic axial elongation and sclero-retinal stretching. In the United States, myopia is estimated to affect
approximately 25 million children, with up to five million considered to be at risk for high myopia. In February 2019, the FDA
accepted our investigational new drug application, or IND, to initiate a Phase III registration trial of MicroPine (the CHAPERONE
study) to reduce the progression of myopia in children. We enrolled the first patient in the CHAPERONE study in June 2019. Due to
the COVID-19 pandemic, we experienced delays in trial enrollment and initiation as a result of reduced clinical trial activities and
operations at investigator sites during the first half of 2020. However, we have since been able to resume enrollment in the
CHAPERONE study at a slower pace than initially planned.
On
October 9, 2020, we entered into a License Agreement (the “Bausch License Agreement”) with a subsidiary of Bausch Health
Companies Inc. (“Bausch Health”) pursuant to which Bausch Health may develop and commercialize MicroPine in the United States
and Canada. Under the terms of the Bausch License Agreement, we received an upfront payment of $10.0 million and we may receive up to
a total of $35.0 million in additional payments, based on the achievement of certain regulatory and launch-based milestones. Bausch Health
also will pay us royalties on a tiered basis (ranging from mid-single digit to mid-teen percentages) on gross profits from sales of MicroPine
in the United States and Canada, subject to certain adjustments. Under the terms of the Bausch License Agreement, Bausch Health is in
the process of assuming oversight for, and has assumed the costs related to the ongoing CHAPERONE study.
MicroLine
is our investigational pharmacologic treatment for presbyopia. Presbyopia is a non- preventable, age-related hardening of the lens,
which causes the gradual loss of the eye’s ability to focus at near and impairs near visual acuity. There currently are no
known FDA-approved drugs for the improvement of near vision in patients with presbyopia, although other companies have related
therapies in their pipeline. We have two planned Phase III VISION trials for MicroLine, and initiated the first of these trials in
December 2020. On May 25, 2021, we announced positive topline data from the Phase III VISION-1 study evaluating MicroLine for the
temporary improvement of near vision in adults with presbyopia. The study achieved its primary endpoint and the Company recently
initiated VISION -2, a second Phase III registration study. VISION-2, is a double-masked, placebo-controlled, cross-over superiority
trial designed to enroll 120 patients randomized between 2% pilocarpine and placebo cohorts. Topline data from VISION-2 is
anticipated in mid-2022. These studies will serve as the basis for a planned New Drug Application (NDA) submission to FDA. VISION-1
results will be presented at a future ophthalmic-focused medical meeting.
On
August 10, 2020, we entered into a License Agreement (the “Arctic Vision License Agreement”) with Arctic Vision (Hong
Kong) Limited (“Arctic Vision”), pursuant to which Arctic Vision may develop and commercialize MicroPine and MicroLine
in Greater China (mainland China, Hong Kong, Macau and Taiwan) and South Korea. Under the terms of the Arctic Vision License
Agreement, we received an upfront payment of $4.0 million before any payments to Senju Pharmaceutical Co., Ltd.
(“Senju”). In addition, we may receive up to a total of $43.75 million in additional payments, based on various
development and regulatory milestones, including the initiation of clinical research and approvals in Greater China and South Korea,
and development costs. Milestone revenue includes $2.0 million related to the MicroStat product resulting from Amendment 1 to the
Arctic Vision License Agreement between the Company and Arctic Vision which was executed on September 14, 2021. Arctic Vision also
will purchase its supply of MicroPine, MicroLine and MicroStat from us or, for such products not supplied by us, pay us a mid-single
digit percentage royalty on net sales of such products, subject to certain adjustments. We will pay a mid- double digit percentage
of such payments, royalties, or net proceeds of such supply to Senju pursuant to the Exclusive License Agreement with Senju dated
March 8, 2015, as amended by the License Amendment dated April 8, 2020, and a Letter Agreement dated August 10, 2020 (the
“Senju License Agreement”).
The
Senju License Agreement was amended further by the License Amendment 2, effective September 14, 2021 (the “Amendment
2”). The Amendment 2 excludes Greater China and South Korea from the territory in which Senju was granted an exclusive
royalty-bearing license from the Company. In consideration for this exclusion, and upon and after the execution of Amendment 1 with
Arctic Vision, the Company must make payments to Senju based on non-royalty license revenue and sales revenue, including a one-time
upfront payment of $250,000 which represented an inducement to Senju to approve Amendment 1 of the Arctic Vision License Agreement
related to the MicroStat Product. This upfront payment to Senju was in addition to and separate from the previously established 40%
payment on milestone revenue.
MydCombi™
(or MicroStat) is our fixed combination formulation of phenylephrine-tropicamide for mydriasis, designed to be a novel approach for the
estimated over one hundred million office-based comprehensive and diabetic eye exams performed every year in the United States. We have
completed two Phase III trials for MydCombi and announced positive results from these studies, known as MIST-1 and MIST-2. In March 2021,
the FDA accepted our NDA, for MydCombi for use to achieve mydriasis in routine diagnostic procedures and in conditions where short-term
pupil dilation is desired. On October 25, 2021, the Company announced the reclassification of the Company’s proprietary, first-in-class
combination microdose formulation of tropicamide and phenylephrine for in-office pupil dilation, MydCombi, as a drug- device combination
product by the FDA in a Complete Response Letter (“CRL”) received on October 22, 2021, following a change in the agency’s
legal interpretation of its authorities imposed by a recent court ruling. The Company is preparing the necessary documents for expedited
resubmission of the new drug application for MydCombi in response to the CRL.
We have not received
U.S. marketing approval for any product candidate and we have therefore not generated any revenues from product sales.
Additional Information
For additional
information related to our business and operations, please refer to the reports incorporated herein by reference, including our Annual Report on Form 10-K for the year ended December 31, 2020, as described under the caption “Incorporation of Documents by Reference”
on page 27 of this prospectus.
Impact of COVID-19
In March 2020,
the World Health Organization declared COVID-19 a global pandemic and recommended containment and mitigation measures worldwide. The COVID-19
pandemic has been evolving, and to date has led to the implementation of various responses, including government-imposed quarantines,
travel restrictions and other public health safety measures.
Management continues
to closely monitor the impact of the COVID-19 pandemic on all aspects of the business, including how it will impact operations and the
operations of customers, vendors, and business partners. Due to the COVID-19 pandemic, we have experienced delays in trial enrollment
and initiation as a result of reduced clinical trial activities and operations at investigator sites. However, we have since been able
to resume operations largely as they were prior to the pandemic. The extent to which COVID-19 impacts the future business, results of
operation and financial condition will depend on future developments, which are highly uncertain and cannot be predicted with confidence
at this time, such as the continued duration of the outbreak, new information that may emerge concerning the severity or other strains
of COVID-19 or the effectiveness of actions to contain COVID-19 or treat its impact, among others. If we or any of the third parties with
which we engage, however, were to experience shutdowns or other business disruptions, the ability to conduct our business in the manner
and on the timelines presently planned could be materially and negatively affected, which could have a material adverse impact on business,
results of operation and financial condition. The estimates of the impact on the Company’s business may change based on new information
that may emerge concerning COVID-19 and the actions to contain it or treat its impact and the economic impact on local, regional, national,
and international markets.
Our Corporate Information
We
were organized as a corporation under the laws of the State of Florida on March 12, 2014 under the name “PGP Holdings V,
Inc.” On May 5, 2014, we changed our name to Eyenovia, Inc. On October 6, 2014, we reincorporated in the State of Delaware by
merging into Eyenovia, Inc., a Delaware corporation. Our principal executive office is located at 295 Madison Avenue, Suite 2400,
New York, NY 10017, and our telephone number is 917-289-1117. We maintain a website at http://www.eyenovia.com, to which we
regularly post copies of our press releases as well as additional information about us. The information contained on, or that can be
accessed through, our website is not a part of this prospectus. We have included our website address in this prospectus solely as an
inactive textual reference.
All brand names
or trademarks appearing in this prospectus are the property of their respective holders. Use or display by us of other parties’
trademarks, trade dress, or products in this prospectus is not intended to, and does not, imply a relationship with, or endorsements or
sponsorship of, us by the trademark or trade dress owners.
Implications of Being an Emerging
Growth Company
As
a company with less than $1.07 billion in revenue during our last fiscal year, we qualify as an “emerging growth
company” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). For so long as we remain
an emerging growth company, we are permitted and intend to rely on exemptions from specified disclosure requirements that are
applicable to other public companies that are not emerging growth companies. These exemptions include:
| · | being permitted to provide only two years of audited financial
statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s
Discussion and Analysis of Financial Condition and Results of Operations” disclosure; |
| · | not being required to comply with the auditor attestation requirements
in the assessment of our internal control over financial reporting; |
| · | not being required to comply with any requirement that may be
adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s
report providing additional information about the audit and the financial statements; |
| · | reduced disclosure obligations regarding executive compensation;
and |
| · | exemptions from the requirements of holding a nonbinding advisory
vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. |
We
may take advantage of these provisions through 2023 or such earlier time that we are no longer an emerging growth company. We would
cease to be an emerging growth company if we have more than $1.07 billion in annual revenues, have more than $700 million in market
value of our capital stock held by non-affiliates, or issue more than $1.0 billion of non-convertible debt over a three-year period.
We may choose to take advantage of some, but not all, of the available exemptions. We have taken advantage of some reduced reporting
burdens in this prospectus and the documents incorporated by reference into this prospectus. Accordingly, the information contained
herein may be different than the information you receive from other public companies in which you hold stock.
In addition, the
JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised
accounting standards. This provision allows an emerging growth company to delay the adoption of some accounting standards until those
standards would otherwise apply to private companies. We have elected to avail ourselves of this exemption from new or revised accounting
standards and, therefore, will not be subject to the same new or revised accounting standards as other public companies that are not emerging
growth companies.
We
are also a “smaller reporting company” as defined under the Securities Act of 1933, as amended and the Securities
Exchange Act of 1934, as amended. We may continue to be a smaller reporting company so long as either (i) the market value of shares
of our common stock held by non-affiliates is less than $250 million or (ii) our annual revenue was less than $100 million during
the most recently completed fiscal year and the market value of shares of our common stock held by non-affiliates is less than $700
million. If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on
exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller
reporting company, we may choose to present only the two most recent fiscal years of audited financial statements in our Annual
Report on Form 10-K and have reduced disclosure obligations regarding executive compensation, and, similar to emerging growth
companies, if we are a smaller reporting company under the requirements of (ii) above, we would not be required to obtain an
attestation report on internal control over financial reporting issued by our independent registered public accounting firm.
Offerings Under This Prospectus
Under
this prospectus, we may offer shares of our common stock, various series of debt securities and/or preferred stock, or warrants or
rights to purchase any of such securities, either individually or in units, with a total value of up to $100,000,000, from time to
time at prices and on terms to be determined by market conditions at the time of the offering. This prospectus provides you with a
general description of the securities we may offer. Each time we offer a type or series of securities under this prospectus, we will
provide a prospectus supplement that will describe the specific amounts, prices and other important terms of the securities,
including, to the extent applicable:
| · | designation or classification; |
| · | aggregate principal amount or aggregate offering price; |
| · | maturity, if applicable; |
| · | rates and times of payment of interest or dividends, if any; |
| · | redemption, conversion or sinking fund terms, if any; |
| · | voting or other rights, if any; and |
| · | conversion or exercise prices, if any. |
The prospectus
supplement also may add, update or change information contained in this prospectus or in documents we have incorporated by reference into
this prospectus. However, no prospectus supplement will fundamentally change the terms that are set forth in this prospectus or offer
a security that is not registered and described in this prospectus at the time of its effectiveness.
We may sell the
securities directly to investors or to or through agents, underwriters or dealers. We, and our agents or underwriters, reserve the right
to accept or reject all or part of any proposed purchase of securities. If we offer securities through agents or underwriters, we will
include in the applicable prospectus supplement:
| · | the names of those agents or underwriters; |
| · | applicable fees, discounts and commissions to be paid to them; |
| · | details regarding over-allotment options, if any; and |
This
prospectus may not be used to consummate a sale of any securities unless it is accompanied by a prospectus supplement.
RISK FACTORS
Investing in our
securities involves significant risk. The prospectus supplement applicable to each offering of our securities will contain a discussion
of the risks applicable to an investment in Eyenovia. Prior to making a decision about investing in our securities, you should carefully
consider the specific factors discussed under the heading “Risk Factors” in the applicable prospectus supplement, together
with all of the other information contained or incorporated by reference in the prospectus supplement or appearing or incorporated by
reference in this prospectus. You should also consider the risks, uncertainties and assumptions discussed under the heading “Risk
Factors” included in our most recent Annual Report on Form 10-K, as revised or supplemented by our subsequent Quarterly Reports
on Form 10-Q or our Current Reports on Form 8-K that we have filed with the SEC, all of which are incorporated herein by reference, and
which may be amended, supplemented or superseded from time to time by other reports we file with the SEC in the future. The risks and
uncertainties we have described are not the only ones we face. Additional risks and uncertainties not presently known to us or that we
currently deem immaterial may also affect our operations. The occurrence of any of these risks might cause you to lose all or part of
your investment in the offered securities.
SPECIAL NOTE
REGARDING FORWARD-LOOKING STATEMENTS
This prospectus
and the documents incorporated by reference in this prospectus include forward-looking statements within the meaning of Section 27A of
the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as
amended (the “Exchange Act”), that relate to future events or our future financial performance and involve known and unknown
risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially
from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Words
such as, but not limited to, “believe,” “expect,” “anticipate,” “estimate,” “intend,”
“may,” “plan,” “potential,” “predict,” “project,” “targets,” “likely,”
“will,” “would,” “could,” “should,” “continue,” and similar expressions or
phrases, or the negative of those expressions or phrases, are intended to identify forward-looking statements, although not all forward-looking
statements contain these identifying words. Although we believe that we have a reasonable basis for each forward- looking statement contained
in this prospectus and incorporated by reference in this prospectus, we caution you that these statements are based on our projections
of the future that are subject to known and unknown risks and uncertainties and other factors that may cause our actual results, level
of activity, performance or achievements expressed or implied by these forward-looking statements, to differ. The sections in our periodic
reports, including our Annual Report on Form 10-K for the fiscal year ended December 31, 2020, entitled “Business,” “Risk
Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” as supplemented
by our subsequent Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K, as well as other sections in this prospectus and
the other documents or reports incorporated by reference in this prospectus, discuss some of the factors that could contribute to these
differences.
You should understand
that the following important factors, in addition to those discussed in our periodic reports filed with the SEC under the Exchange Act
could affect our future results and could cause those results to differ materially from those expressed in such forward-looking statements:
| · | our need to raise additional money to fund our operations for
the next twelve months as a going concern; |
| · | our estimates regarding expenses, future revenue, timing of
any future revenue, capital requirements and needs for additional financing; |
| · | impacts of and uncertainty related to COVID-19; |
| · | fluctuations in our financial results and stock price, particularly
given market conditions and the potential economic impact of COVID-19; |
| · | our expectations related to the use of proceeds from our financings; |
| · | risks of our and our licensees’ clinical trials including,
but not limited to, the costs, design, initiation and enrollment (which could be adversely impacted by COVID-19 and resulting social
distancing), timing, progress and results of such trials; |
| · | the timing and our or our licensees’ ability to submit
applications for, obtain and maintain regulatory approval for our product candidates; |
| · | reliance on third parties to develop and commercialize certain
of our product candidates; |
| · | our and our partners’ ability to timely develop, implement
and maintain manufacturing, commercialization and marketing capabilities and strategies for certain of our product candidates; |
| · | our estimates regarding the potential market opportunity for
our product candidates; |
| · | the potential advantages of our product candidates and platform
technology and potential revenues from licensing transactions; |
| · | the rate and degree of market acceptance and clinical utility
of our product candidates; |
| · | our intellectual property position; |
| · | our ability to identify additional products, product candidates
or technologies with significant commercial potential that are consistent with our commercial objectives; |
| · | our ability to attract and retain key personnel; |
| · | the impact of government laws and regulations; |
| · | our competitive position; |
| · | developments relating to our competitors and our industry; |
| · | our ability to maintain and establish collaborations; |
| · | general or regional economic conditions; |
| · | changes in U.S. GAAP; and |
| · | changes in the legal, regulatory and legislative environments
in the markets in which we operate, and the impact of these changes on our ability to obtain regulatory approval for our products. |
We may
not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue
reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations
disclosed in the forward-looking statements we make. We have included important cautionary statements in this prospectus and in the documents
incorporated by reference in this prospectus, particularly in the “Risk Factors” section, that we believe could cause actual
results or events to differ materially from the forward-looking statements that we make. For a summary of such factors, please refer
to the section entitled “Risk Factors” in this prospectus, as updated and supplemented by the discussion of risks and uncertainties
under “Risk Factors” contained in any supplements to this prospectus and in our most recent Annual Report on Form 10-K, as
revised or supplemented by our subsequent Quarterly Reports on Form 10-Q or our Current Reports on Form 8-K, as well as any amendments
thereto, as filed with the SEC and which are incorporated herein by reference. The information contained in this document is believed
to be current as of the date of this document. We do not intend to update any of the forward-looking statements after the date of this
document to conform these statements to actual results or to changes in our expectations, except as required by law.
In light of these
assumptions, risks and uncertainties, the results and events discussed in the forward- looking statements contained in this prospectus
or in any document incorporated herein by reference might not occur. Investors are cautioned not to place undue reliance on the forward-looking
statements, which speak only as of the date of this prospectus or the date of the document incorporated by reference in this prospectus.
We are not under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements, whether
as a result of new information, future events or otherwise. All subsequent forward-looking statements attributable to us or to any person
acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section.
USE OF PROCEEDS
Unless otherwise
indicated in the applicable prospectus supplement, we intend to use any net proceeds from the sale of securities under this prospectus
for general corporate purposes, including, but not limited to, clinical trials, research and development activities, working capital,
capital expenditures, acquisitions, should we choose to pursue any, and collaborations. We have not determined the amounts we plan to
spend on any of the areas listed above or the timing of these expenditures. As a result, our management will have broad discretion to
allocate the net proceeds, if any, we receive in connection with securities offered pursuant to this prospectus for any purpose. Pending
application of the net proceeds as described above, we may initially invest the net proceeds in short-term, investment-grade or interest-bearing
securities.
PLAN OF DISTRIBUTION
We may offer securities
under this prospectus from time to time pursuant to underwritten public offerings, negotiated transactions, block trades or a combination
of these methods. We may sell the securities (1) through underwriters or dealers, (2) through agents or (3) directly to one or more purchasers,
or through a combination of such methods. We may distribute the securities from time to time in one or more transactions at:
| · | a fixed price or prices, which may be changed from time to time; |
| · | market prices prevailing at the time of sale; |
| · | prices related to the prevailing market prices; or |
We may directly
solicit offers to purchase the securities being offered by this prospectus. We may also designate agents to solicit offers to purchase
the securities from time to time, and may enter into arrangements for “at-the-market,” equity line or similar transactions.
We will name in a prospectus supplement any underwriter or agent involved in the offer or sale of the securities.
If we utilize a
dealer in the sale of the securities being offered by this prospectus, we will sell the securities to the dealer, as principal. The dealer
may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale.
If
we utilize an underwriter in the sale of the securities being offered by this prospectus, we will execute an underwriting agreement
with the underwriter at the time of sale, and we will provide the name of any underwriter in the prospectus supplement which the
underwriter will use to make resales of the securities to the public. In connection with the sale of the securities, we, or the
purchasers of the securities for whom the underwriter may act as agent, may compensate the underwriter in the form of underwriting
discounts or commissions. The underwriter may sell the securities to or through dealers, and the underwriter may compensate those
dealers in the form of discounts, concessions or commissions.
With
respect to underwritten public offerings, negotiated transactions and block trades, we will provide in the applicable prospectus
supplement information regarding any compensation we pay to underwriters, dealers or agents in connection with the offering of the
securities, and any discounts, concessions or commissions allowed by underwriters to participating dealers. Underwriters, dealers
and agents participating in the distribution of the securities may be deemed to be underwriters within the meaning of the Securities
Act, and any discounts and commissions received by them and any profit realized by them on resale of the securities may be deemed to
be underwriting discounts and commissions. We may enter into agreements to indemnify underwriters, dealers and agents against civil
liabilities, including liabilities under the Securities Act, or to contribute to payments they may be required to make in respect
thereof.
If
so indicated in the applicable prospectus supplement, we will authorize underwriters, dealers or other persons acting as our agents
to solicit offers by certain institutions to purchase securities from us pursuant to delayed delivery contracts providing for
payment and delivery on the date stated in each applicable prospectus supplement. Each contract will be for an amount not less than,
and the aggregate amount of securities sold pursuant to such contracts shall not be less nor more than, the respective amounts
stated in each applicable prospectus supplement. Institutions with whom the contracts, when authorized, may be made include
commercial and savings banks, insurance companies, pension funds, investment companies, educational and charitable institutions and
other institutions, but shall in all cases be subject to our approval. Delayed delivery contracts will not be subject to any
conditions except that:
| · | the purchase by an institution of the securities covered under
that contract shall not at the time of delivery be prohibited under the laws of the jurisdiction to which that institution is subject;
and |
| · | if the securities are also being sold to underwriters acting
as principals for their own account, the underwriters shall have purchased such securities not sold for delayed delivery. The underwriters
and other persons acting as our agents will not have any responsibility in respect of the validity or performance of delayed delivery
contracts. |
One or more firms,
referred to as “remarketing firms,” may also offer or sell the securities, if a prospectus supplement so indicates, in connection
with a remarketing arrangement upon their purchase. Remarketing firms will act as principals for their own accounts or as our agents.
These remarketing firms will offer or sell the securities in accordance with the terms of the securities. Each prospectus supplement will
identify and describe any remarketing firm and the terms of its agreement, if any, with us and will describe the remarketing firm’s
compensation. Remarketing firms may be deemed to be underwriters in connection with the securities they remarket. Remarketing firms may
be entitled under agreements that may be entered into with us to indemnification by us against certain civil liabilities, including liabilities
under the Securities Act, and may be customers of, engage in transactions with or perform services for us in the ordinary course of business.
Certain
underwriters may use this prospectus and any accompanying prospectus supplement for offers and sales related to market-making
transactions in the securities. These underwriters may act as principal or agent in these transactions, and the sales will be made
at prices related to prevailing market prices at the time of sale. Any underwriters involved in the sale of the securities may
qualify as “underwriters” within the meaning of Section 2(a)(11) of the Securities Act. In addition, the
underwriters’ commissions, discounts or concessions may qualify as underwriters’ compensation under the Securities Act
and the rules of the Financial Industry Regulatory Authority, Inc.
Shares of our common
stock sold pursuant to the registration statement of which this prospectus is a part will be authorized for listing and trading on The
Nasdaq Capital Market. The applicable prospectus supplement will contain information, where applicable, as to any other listing, if any,
on The Nasdaq Capital Market or any securities market or other securities exchange of the securities covered by the prospectus supplement.
Underwriters may make a market in our common stock, but will not be obligated to do so and may discontinue any market making at any time
without notice. We can make no assurance as to the liquidity of or the existence, development or maintenance of trading markets for any
of the securities.
In
order to facilitate the offering of the securities, certain persons participating in the offering may engage in transactions that
stabilize, maintain or otherwise affect the price of the securities. This may include over- allotments or short sales of the
securities, which involve the sale by persons participating in the offering of more securities than we sold to them. In these
circumstances, these persons would cover such over- allotments or short positions by making purchases in the open market or by
exercising their over-allotment option. In addition, these persons may stabilize or maintain the price of the securities by bidding
for or purchasing the applicable security in the open market or by imposing penalty bids, whereby selling concessions allowed to
dealers participating in the offering may be reclaimed if the securities sold by them are repurchased in connection with
stabilization transactions. The effect of these transactions may be to stabilize or maintain the market price of the securities at a
level above that which might otherwise prevail in the open market. These transactions may be discontinued at any time.
The underwriters,
dealers and agents may engage in other transactions with us, or perform other services for us, in the ordinary course of their business.
DESCRIPTION
OF COMMON STOCK
We are authorized
to issue 90,000,000 shares of common stock, par value $0.0001 per share. As of December 10, 2021, we had 28,407,257 shares of common stock
outstanding and approximately 35 stockholders of record.
The
following summary of certain provisions of our common stock does not purport to be complete. You should refer to the section of this
prospectus entitled “Certain Provisions of Delaware Law and of the Company’s Certificate of Incorporation and
Bylaws” and our third amended and restated certificate of incorporation, as amended (our “Certificate of
Incorporation”), and our amended and restated bylaws (our “Bylaws”), both of which are included as exhibits to the
registration statement of which this prospectus is a part. The summary below is also qualified by provisions of applicable law.
General
We
are authorized to issue one class of common stock. Holders of our common stock are entitled to one vote for each share of common
stock held of record for the election of directors and on all matters submitted to a vote of stockholders, except matters that
relate only to one or more of the series of our preferred stock, and no holder has cumulative voting rights. Accordingly, the
holders of a majority of the shares of common stock entitled to vote in any election of directors can elect all of the directors
standing for election, if they so choose. Subject to preferences that may be applicable to any then outstanding preferred stock,
holders of our common stock are entitled to receive dividends ratably, if any, as may be declared by our board of directors out of
legally available funds, subject to any preferential dividend rights of any preferred stock then outstanding. Upon our dissolution,
liquidation or winding up, holders of our common stock are entitled to share ratably in our net assets legally available after the
payment of all our debts and other liabilities, subject to the preferential rights of any preferred stock then outstanding. Holders
of our common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of
holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of
preferred stock that are currently designated and issued or that we may designate and issue in the future. Except as described under
“Certain Provisions of Delaware Law and of the Company’s Certificate of Incorporation and Bylaws — Anti-Takeover
Provisions” below, a majority vote
of the holders of
common stock is generally required to take action under our Certificate of Incorporation and our Bylaws.
Stock Options and Warrants
As of December
10, 2021, we had outstanding options to purchase 4,387,051 shares of our common stock at a weighted average price of $3.88 per share under
our 2014 Equity Incentive Plan and 2018 Omnibus Stock Incentive Plan. All of our stock options expire 10 years after their grant date.
As
of December 10, 2021, we had outstanding warrants to purchase 1,217,715 shares of our common stock at exercise prices ranging from $2.47
to $4.76 per share. 1,125,831 warrants will expire on March 24, 2025, and 91,884 warrants will expire on May 6, 2031.
Transfer Agent and Registrar
The
transfer agent and registrar for our common stock is American Stock Transfer & Trust Company, LLC, with offices at 6201 15th
Avenue, Brooklyn, New York 11219.
Stock Exchange Listing
Our common stock is listed
for quotation on The Nasdaq Capital Market under the symbol “EYEN.”
DESCRIPTION
OF PREFERRED STOCK
The following description
of our preferred stock and the description of the terms of any particular series of preferred stock that we choose to issue hereunder
are not complete. These descriptions are qualified in their entirety by reference to our Certificate of Incorporation and the certificate
of designation relating to any series of preferred stock issued by us. The rights, preferences, privileges and restrictions of the preferred
stock of each series will be fixed by the certificate of designation relating to that series.
We
are authorized, without action by the stockholders, to designate and issue up to an aggregate of 6,000,000 shares of preferred
stock, par value $0.0001 per share. As of the date of this prospectus, no shares of our preferred stock were outstanding or
designated. The following summary of certain provisions of our preferred stock does not purport to be complete. You should refer to
our Certificate of Incorporation and our Bylaws, both of which are included as exhibits to the registration statement of which this
prospectus is a part. The summary below is also qualified by provisions of applicable law.
General
Our board of directors
can designate the rights, preferences and privileges of the shares of each series and any of its qualifications, limitations or restrictions.
Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the
voting power or other rights of the holders of common stock. The issuance of preferred stock, while providing flexibility in connection
with possible future financings and acquisitions and other corporate purposes could, under certain circumstances, have the effect of restricting
dividends on our common stock, diluting the voting power of our common stock, impairing the liquidation rights of our common stock, or
delaying, deferring or preventing a change in control of our Company, which might harm the market price of our common stock. See also
“Certain Provisions of Delaware Law and of the Company’s Certificate of Incorporation and Bylaws — Anti- Takeover Provisions.”
Our board of directors will make any determination to issue such shares based on its judgment as to our Company’s best interests
and the best interests of our stockholders.
If we offer a specific
series of preferred stock under this prospectus, we will describe the terms of the preferred stock in the prospectus supplement for such
offering and will file a copy of the amended and restated certificate establishing the terms of the preferred stock with the SEC. To the
extent required, this description will include:
| · | the maximum number of shares; |
| · | the designation of the shares; |
| · | the annual dividend rate, if any, whether the dividend rate is fixed or variable, the date or dates
on which dividends will accrue, the dividend payment dates, and whether dividends will be cumulative; |
| · | the price and the terms and conditions for redemption, if any,
including redemption at the option of F-star or at the option of the holders, including the time period for redemption, and any accumulated
dividends or premiums; |
| · | the liquidation preference, if any, and any accumulated dividends
upon the liquidation, dissolution or winding up of our affairs; |
| · | any sinking fund or similar provision, and, if so, the terms
and provisions relating to the purpose and operation of the fund; |
| · | the terms and conditions, if any, for conversion or exchange
of shares of any other class or classes of our capital stock or any series of any other class or classes, or of any other series of the
same class, or any other securities or assets, including the price or the rate of conversion or exchange and the method, if any, of adjustment; |
| · | any or all other preferences and relative, participating, optional
or other special rights, privileges or qualifications, limitations or restrictions; and |
| · | any preferred stock issued will be fully paid and nonassessable upon issuance. |
Transfer Agent and Registrar
The transfer agent
and registrar for any series of preferred stock that is designated by our board of directors will be set forth in the applicable prospectus
supplement.
DESCRIPTION
OF DEBT SECURITIES
The following description,
together with the additional information we include in any applicable prospectus supplements, summarizes the material terms and provisions
of the debt securities that we may offer under this prospectus. While the terms we have summarized below will apply generally to any future
debt securities we may offer pursuant to this prospectus, we will describe the particular terms of any debt securities that we may offer
in more detail in the applicable prospectus supplement. If we so indicate in a prospectus supplement, the terms of any debt securities
offered under such prospectus supplement may differ from the terms we describe below, and to the extent the terms set forth in a prospectus
supplement differ from the terms described below, the terms set forth in the prospectus supplement shall control.
We may sell from
time to time, in one or more offerings under this prospectus, debt securities, which may be senior or subordinated. We will issue any
such senior debt securities under a senior indenture that we will enter into with a trustee to be named in the senior indenture. We will
issue any such subordinated debt securities under a subordinated indenture, which we will enter into with a trustee to be named in the
subordinated indenture. We have filed forms of these documents as exhibits to the registration statement, of which this prospectus is
a part. We use the term “indentures” to refer to either the senior indenture or the subordinated indenture, as applicable.
The indentures will be qualified under the Trust Indenture Act of 1939, as in effect on the date of the indenture. We use the term “debenture
trustee” to refer to either the trustee under the senior indenture or the trustee under the subordinated indenture, as applicable.
The following summaries
of material provisions of the senior debt securities, the subordinated debt securities and the indentures are subject to, and qualified
in their entirety by reference to, all the provisions of the indenture applicable to a particular series of debt securities.
General
Each indenture
provides that debt securities may be issued from time to time in one or more series and may be denominated and payable in foreign currencies
or units based on or relating to foreign currencies. Neither indenture limits the amount of debt securities that may be issued thereunder,
and each indenture provides that the specific terms of any series of debt securities shall be set forth in, or determined pursuant to,
an authorizing resolution and/or a supplemental indenture, if any, relating to such series.
We will describe in each
prospectus supplement the following terms relating to a series of debt securities:
| · | the title or designation; |
| · | the aggregate principal amount and any limit on the amount that may be issued; |
| · | the currency or units based on or relating to currencies in
which debt securities of such series are denominated and the currency or units in which principal or interest or both will or may be
payable; |
| · | whether we will issue the series of debt securities in global
form, the terms of any global securities and who the depositary will be; |
| · | the maturity date and the date or dates on which principal will be payable; |
| · | the interest rate, which may be fixed or variable, or the method
for determining the rate and the date interest will begin to accrue, the date or dates interest will be payable and the record dates
for interest payment dates or the method for determining such dates; |
| · | whether or not the debt securities will be secured or unsecured, and the terms of any secured debt; |
| · | the terms of the subordination of any series of subordinated debt; |
| · | the place or places where payments will be payable; |
| · | our right, if any, to defer payment of interest and the maximum length of any such deferral period; |
| · | the date, if any, after which, and the price at which, we may,
at our option, redeem the series of debt securities pursuant to any optional redemption provisions; |
| · | the date, if any, on which, and the price at which we are obligated,
pursuant to any mandatory sinking fund provisions or otherwise, to redeem, or at the holder’s option to purchase, the series of
debt securities; |
| · | whether the indenture will restrict our ability to pay dividends,
or will require us to maintain any asset ratios or reserves; |
| · | whether we will be restricted from incurring any additional indebtedness; |
| · | a discussion of any material or special U.S. federal income
tax considerations applicable to a series of debt securities; |
| · | the denominations in which we will issue the series of debt securities, if other than denominations of $1,000 and any integral
multiple thereof; and |
| · | any other specific terms, preferences, rights or limitations of, or restrictions on, the debt securities. |
We may issue debt securities
that provide for an amount less than their stated principal amount to be due and payable upon declaration of acceleration of their maturity
pursuant to the terms of the indenture. We will provide you with information on the federal income tax considerations and other special
considerations applicable to any of these debt securities in the applicable prospectus supplement.
Conversion or Exchange Rights
We will set forth
in the prospectus supplement the terms, if any, on which a series of debt securities may be convertible into or exchangeable for our common
stock or our other securities. We will include provisions as to whether conversion or exchange is mandatory, at the option of the holder
or at our option. We may include provisions pursuant to which the number of shares of our common stock or our other securities that the
holders of the series of debt securities receive would be subject to adjustment.
Consolidation, Merger or Sale;
No Protection in Event of a Change of Control or Highly Leveraged Transaction
The indentures do not
contain any covenant that restricts our ability to merge or consolidate, or sell, convey, transfer or otherwise dispose of all or substantially
all of our assets. However, any successor to or acquirer of such assets must assume all of our obligations under the indentures or the
debt securities, as appropriate.
Unless we state
otherwise in the applicable prospectus supplement, the debt securities will not contain any provisions that may afford holders of the
debt securities protection in the event we have a change of control or in the event of a highly leveraged transaction (whether or not
such transaction results in a change of control), which could adversely affect holders of debt securities.
Events of Default Under the Indenture
The following
are events of default under the indentures with respect to any series of debt securities that we may issue:
| · | if we fail to pay interest when due and our failure continues
for 90 days and the time for payment has not been extended or deferred; |
| · | if we fail to pay the principal, or premium, if any, when due
and the time for payment has not been extended or delayed; |
| · | if we fail to observe or perform any other covenant set forth
in the debt securities of such series or the applicable indentures, other than a covenant specifically relating to and for the benefit
of holders of another series of debt securities, and our failure continues for 90 days after we receive written notice from the debenture
trustee or holders of not less than a majority in aggregate principal amount of the outstanding debt securities of the applicable series;
and |
| · | if specified events of bankruptcy, insolvency or reorganization occur as to us. |
No
event of default with respect to a particular series of debt securities (except as to certain events of bankruptcy, insolvency or
reorganization) necessarily constitutes an event of default with respect to any other series of debt securities. The occurrence of
an event of default may constitute an event of default under any bank credit agreements we may have in existence from time to time.
In addition, the occurrence of certain events of default or an acceleration under the indenture may constitute an event of default
under certain of our other indebtedness outstanding from time to time.
If an event of
default with respect to debt securities of any series at the time outstanding occurs and is continuing, then the trustee or the holders
of not less than a majority in principal amount of the outstanding debt securities of that series may, by a notice in writing to us (and
to the debenture trustee if given by the holders), declare to be due and payable immediately the principal (or, if the debt securities
of that series are discount securities, that portion of the principal amount as may be specified in the terms of that series) of and premium
and accrued and unpaid interest, if any, on all debt securities of that series. Before a judgment or decree for payment of the money due
has been obtained with respect to debt securities of any series, the holders of a majority in principal amount of the outstanding debt
securities of that series (or, at a meeting of holders of such series at which a quorum is present, the holders of a majority in principal
amount of the debt securities of such series represented at such meeting) may rescind and annul the acceleration if all events of default,
other than the non-payment of accelerated principal, premium, if any, and interest, if any, with respect to debt securities of that series,
have been cured or waived as provided in the applicable indenture (including payments or deposits in respect of principal, premium or
interest that had become due other than as a result of such acceleration). We refer you to the prospectus supplement relating to any series
of debt securities that are discount securities for the particular provisions relating to acceleration of a portion of the principal amount
of such discount securities upon the occurrence of an event of default.
Subject to the
terms of the indentures, if an event of default under an indenture shall occur and be continuing, the debenture trustee will be under
no obligation to exercise any of its rights or powers under such indenture at the request or direction of any of the holders of the applicable
series of debt securities, unless such holders have offered the debenture trustee reasonable indemnity. The holders of a majority in principal
amount of the outstanding debt securities of any series will have the right to direct the time, method and place of conducting any proceeding
for any remedy available to the debenture trustee, or exercising any trust or power conferred on the debenture trustee, with respect to
the debt securities of that series, provided that:
| · | the direction so given by the holder is not in conflict with
any law or the applicable indenture; and |
| · | subject to its duties under the Trust Indenture Act, the debenture
trustee need not take any action that might involve it in personal liability or might be unduly prejudicial to the holders not involved
in the proceeding. |
A holder of the
debt securities of any series will only have the right to institute a proceeding under the indentures or to appoint a receiver or trustee,
or to seek other remedies if:
| · | the holder previously has given written notice to the debenture
trustee of a continuing event of default with respect to that series; |
| · | the holders of at least a majority in aggregate principal amount
of the outstanding debt securities of that series have made written request, and such holders have offered reasonable indemnity to the
debenture trustee to institute the proceeding as trustee; and |
| · | the debenture trustee does not institute the proceeding and
does not receive from the holders of a majority in aggregate principal amount of the outstanding debt securities of that series (or at
a meeting of holders of such series at which a quorum is present, the holders of a majority in principal amount of the debt securities
of such series represented at such meeting) other conflicting directions within 60 days after the notice, request and offer. |
These limitations do
not apply to a suit instituted by a holder of debt securities if we default in the payment of the principal, premium, if any, or interest
on, the debt securities.
We will periodically
file statements with the applicable debenture trustee regarding our compliance with specified covenants in the applicable indenture.
Modification of Indenture; Waiver
The debenture trustee
and we may change the applicable indenture without the consent of any holders with respect to specific matters, including:
| · | to fix any ambiguity, defect or inconsistency in the indenture;
and |
| · | to change anything that does not materially adversely affect
the interests of any holder of debt securities of any series issued pursuant to such indenture. |
In addition, under
the indentures, the rights of holders of a series of debt securities may be changed by us and the debenture trustee with the written consent
of the holders of at least a majority in aggregate principal amount of the outstanding debt securities of each series (or, at a meeting
of holders of such series at which a quorum is present, the holders of a majority in principal amount of the debt securities of such series
represented at such meeting) that is affected. However, the debenture trustee and we may make the following changes only with the consent
of each holder of any outstanding debt securities affected:
| · | extending the fixed maturity of the series of debt securities; |
| · | reducing the principal amount, reducing the rate of or extending
the time of payment of interest, or any premium payable upon the redemption of any debt securities; |
| · | reducing the principal amount of discount securities payable upon acceleration of maturity; |
| · | making the principal of or premium or interest on any debt security
payable in currency other than that stated in the debt security; or |
| · | reducing the percentage of debt securities, the holders of which
are required to consent to any amendment or waiver. |
Except for certain
specified provisions, the holders of at least a majority in principal amount of the outstanding debt securities of any series (or, at
a meeting of holders of such series at which a quorum is present, the holders of a majority in principal amount of the debt securities
of such series represented at such meeting) may on behalf of the holders of all debt securities of that series waive our compliance with
provisions of the indenture. The holders of a majority in principal amount of the outstanding debt securities of any series may on behalf
of the holders of all the debt securities of such series waive any past default under the indenture with respect to that series and its
consequences, except a default in the payment of the principal of, premium or any interest on any debt security of that series or in respect
of a covenant or provision, which cannot be modified or amended without the consent of the holder of each outstanding debt security of
the series affected; provided, however, that the holders of a majority in principal amount of the outstanding
debt securities of
any series may rescind an acceleration and its consequences, including any related payment default that resulted from the acceleration.
Discharge
Each indenture provides
that we can elect to be discharged from our obligations with respect to one or more series of debt securities, except for obligations
to:
| · | register the transfer or exchange of debt securities of the series; |
| · | replace stolen, lost or mutilated debt securities of the series; |
| · | maintain paying agencies; |
| · | hold monies for payment in trust; |
| · | compensate and indemnify the trustee; and |
| · | appoint any successor trustee. |
In order to exercise
our rights to be discharged with respect to a series, we must deposit with the trustee money or government obligations sufficient to pay
all the principal of, the premium, if any, and interest on, the debt securities of the series on the dates payments are due.
Form, Exchange, and Transfer
We
will issue the debt securities of each series only in fully registered form without coupons and, unless we otherwise specify in the
applicable prospectus supplement, in denominations of $1,000 and any integral multiple thereof. The indentures provide that we may
issue debt securities of a series in temporary or permanent global form and as book-entry securities that will be deposited with, or
on behalf of, The Depository Trust Company or another depositary named by us and identified in a prospectus supplement with respect
to that series.
At the option
of the holder, subject to the terms of the indentures and the limitations applicable to global securities described in the applicable
prospectus supplement, the holder of the debt securities of any series can exchange the debt securities for other debt securities of the
same series, in any authorized denomination and of like tenor and aggregate principal amount.
Subject
to the terms of the indentures and the limitations applicable to global securities set forth in the applicable prospectus
supplement, holders of the debt securities may present the debt securities for exchange or for registration of transfer, duly
endorsed or with the form of transfer endorsed thereon duly executed if so required by us or the security registrar, at the office
of the security registrar or at the office of any transfer agent designated by us for this purpose. Unless otherwise provided in the
debt securities that the holder presents for transfer or exchange or in the applicable indenture, we will make no service charge for
any registration of transfer or exchange, but we may require payment of any taxes or other governmental charges.
We
will name in the applicable prospectus supplement the security registrar, and any transfer agent in addition to the security
registrar, that we initially designate for any debt securities. We may at any time designate additional transfer agents or rescind
the designation of any transfer agent or approve a change in the office through which any transfer agent acts, except that we will
be required to maintain a transfer agent in each place of payment for the debt securities of each series.
If we elect to redeem the
debt securities of any series, we will not be required to:
| · | issue, register the transfer of, or exchange any debt securities
of that series during a period beginning at the opening of business 15 days before the day of mailing of a notice of redemption of any
debt securities that may be selected for redemption and ending at the close of business on the day of the mailing; or |
| · | register the transfer of or exchange any debt securities so
selected for redemption, in whole or in part, except the unredeemed portion of any debt securities we are redeeming in part. |
Information Concerning the Debenture
Trustee
The debenture trustee,
other than during the occurrence and continuance of an event of default under the applicable indenture, undertakes to perform only those
duties as are specifically set forth in the applicable indenture. Upon an event of default under an indenture, the debenture trustee under
such indenture must use the same degree of care as a prudent person would exercise or use in the conduct of his or her own affairs. Subject
to this provision, the debenture trustee is under no obligation to exercise any of the powers given it by the indentures at the request
of any holder of debt securities unless it is offered reasonable security and indemnity against the costs, expenses and liabilities that
it might incur.
Payment and Paying Agents
Unless we otherwise
indicate in the applicable prospectus supplement, we will make payment of the interest on any debt securities on any interest payment
date to the person in whose name the debt securities, or one or more predecessor securities, are registered at the close of business on
the regular record date for the interest.
We will pay principal
of and any premium and interest on the debt securities of a particular series at the office of the paying agents designated by us, except
that unless we otherwise indicate in the applicable prospectus supplement, will we make interest payments by check which we will mail
to the holder. Unless we otherwise indicate in a prospectus supplement, we will designate the corporate trust office of the debenture
trustee in the City of New York as our sole paying agent for payments with respect to debt securities of each series. We will name in
the applicable prospectus supplement any other paying agents that we initially designate for the debt securities of a particular series.
We will maintain a paying agent in each place of payment for the debt securities of a particular series.
All money we pay to
a paying agent or the debenture trustee for the payment of the principal of or any premium or interest on any debt securities which remains
unclaimed at the end of two years after such principal, premium or interest has become due and payable will be repaid to us, and the holder
of the security thereafter may look only to us for payment thereof.
Governing Law
The indentures
and the debt securities will be governed by and construed in accordance with the laws of the State of New York, except to the extent that
the Trust Indenture Act is applicable.
Subordination of Subordinated
Debt Securities
Our
obligations pursuant to any subordinated debt securities will be unsecured and will be subordinate and junior in priority of payment to
certain of our other indebtedness to the extent described in a prospectus supplement. The subordinated indenture does not limit the amount
of senior indebtedness we may incur.
It
also does not limit us from issuing any other secured or unsecured debt.
DESCRIPTION OF WARRANTS
General
We may issue warrants
to purchase shares of our common stock, preferred stock and/or debt securities in one or more series together with other securities or
separately, as described in the applicable prospectus supplement.
Below
is a description of certain general terms and provisions of the warrants that we may offer. Particular terms of the warrants will be
described in the warrant agreements and the prospectus supplement relating to the warrants.
The applicable
prospectus supplement will contain, where applicable, the following terms of and other information relating to the warrants:
| · | the specific designation and aggregate number of, and the price at which we will issue, the warrants; |
| · | the currency or currency units in which the offering price, if any, and the exercise price are payable; |
| · | the designation, amount and terms of the securities purchasable upon exercise of the warrants; |
| · | if applicable, the exercise price for shares of our common stock
and the number of shares of common stock to be received upon exercise of the warrants; |
| · | if applicable, the exercise price for shares of our preferred
stock, the number of shares of preferred stock to be received upon exercise, and a description of that series of our preferred stock; |
| · | if applicable, the exercise price for our debt securities, the
amount of debt securities to be received upon exercise, and a description of that series of debt securities; |
| · | the date on which the right to exercise the warrants will begin
and the date on which that right will expire or, if you may not continuously exercise the warrants throughout that period, the specific
date or dates on which you may exercise the warrants; |
| · | whether the warrants will be issued in fully registered form
or bearer form, in definitive or global form or in any combination of these forms, although, in any case, the form of a warrant included
in a unit will correspond to the form of the unit and of any security included in that unit; |
| · | any applicable material U.S. federal income tax consequences; |
| · | the identity of the warrant agent for the warrants and of any
other depositaries, execution or paying agents, transfer agents, registrars or other agents; |
| · | the proposed listing, if any, of the warrants or any securities
purchasable upon exercise of the warrants on any securities exchange; |
| · | if applicable, the date from and after which the warrants and
the common stock, preferred stock and/or debt securities will be separately transferable; |
| · | if applicable, the minimum or maximum amount of the warrants
that may be exercised at any one time; |
| · | information with respect to book-entry procedures, if any; |
| · | the anti-dilution provisions of the warrants, if any; |
| · | any redemption or call provisions; |
| · | whether the warrants may be sold separately or with other securities as parts of units; and |
| · | any additional terms of the warrants, including terms, procedures
and limitations relating to the exchange and exercise of the warrants. |
Transfer Agent and Registrar
The transfer agent
and registrar for any warrants will be set forth in the applicable prospectus supplement.
DESCRIPTION OF UNITS
The following description,
together with the additional information that we include in any applicable prospectus supplements summarizes the material terms and provisions
of the units that we may offer under this prospectus. While the terms we have summarized below will apply generally to any units that
we may offer under this prospectus, we will describe the particular terms of any series of units in more detail in the applicable prospectus
supplement. The terms of any units offered under a prospectus supplement may differ from the terms described below.
We will incorporate
by reference from reports that we file with the SEC, the form of unit agreement that describes the terms of the series of units we are
offering, and any supplemental agreements, before the issuance of the related series of units. The following summaries of material terms
and provisions of the units are subject to, and qualified in their entirety by reference to, all the provisions of the unit agreement
and any supplemental agreements applicable to a particular series of units. We urge you to read the applicable prospectus supplements
related to the particular series of units that we may offer under this prospectus, as well as any related free writing prospectuses and
the complete unit agreement and any supplemental agreements that contain the terms of the units.
General
We may issue units
consisting of common stock, preferred stock, one or more debt securities, warrants or rights for the purchase of common stock, preferred
stock and/or debt securities in one or more series, in any combination. Each unit will be issued so that the holder of the unit is also
the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each
security included in the unit. The unit agreement under which a unit is issued may provide that the securities included in the unit may
not be held or transferred separately, at any time or at any time before a specified date.
We will describe in
the applicable prospectus supplement the terms of the series of units being offered, including:
| · | the designation and terms of the units and of the securities
comprising the units, including whether and under what circumstances those securities may be held or transferred separately; |
| · | any provisions of the governing unit agreement that differ from those described below; and |
| · | any provisions for the issuance, payment, settlement, transfer
or exchange of the units or of the securities comprising the units. |
The provisions
described in this section, as well as those set forth in any prospectus supplement or as described under “Description of Common
Stock,” “Description of Preferred Stock,” “Description of Debt Securities,” “Description of Warrants,”
and “Description of Rights” will apply to each unit, as applicable, and to any common stock, preferred stock, debt security,
warrant or right included in each unit, as applicable.
Unit Agent
The name and address
of the unit agent, if any, for any units we offer will be set forth in the applicable prospectus supplement.
Issuance in Series
We may issue units in
such amounts and in such numerous distinct series as we determine.
Enforceability of Rights by Holders
of Units
Each unit agent
will act solely as our agent under the applicable unit agreement and will not assume any obligation or relationship of agency or trust
with any holder of any unit. A single bank or trust company may act as unit agent for more than one series of units. A unit agent will
have no duty or responsibility in case of any default by us under the applicable unit agreement or unit, including any duty or responsibility
to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a unit may, without the consent of the related
unit agent or the holder of any other unit, enforce by appropriate legal action its rights as holder under any security included in the
unit.
DESCRIPTION OF RIGHTS
General
We
may issue rights to our stockholders to purchase shares of our common stock, preferred stock or the other securities described in
this prospectus. We may offer rights separately or together with one or more additional rights, debt securities, preferred stock,
common stock or warrants, or any combination of those securities in the form of units, as described in the applicable prospectus
supplement. Each series of rights will be issued under a separate rights agreement to be entered into between us and a bank or trust
company, as rights agent. The rights agent will act solely as our agent in connection with the certificates relating to the rights
of the series of certificates and will not assume any obligation or relationship of agency or trust for or with any holders of
rights certificates or beneficial owners of rights. The following description sets forth certain general terms and provisions of the
rights to which any prospectus supplement may relate. The particular terms of the rights to which any prospectus supplement may
relate and the extent, if any, to which the general provisions may apply to the rights so offered will be described in the
applicable prospectus supplement. To the extent that any particular terms of the rights, rights agreement or rights certificates
described in a prospectus supplement differ from any of the terms described below, then the terms described below will be deemed to
have been superseded by that prospectus supplement. We encourage you to read the applicable rights agreement and rights certificate
for additional information before you decide whether to purchase any of our rights. We will provide in a prospectus supplement the
following terms of the rights being issued:
| · | the date of determining the stockholders entitled to the rights distribution; |
| · | the aggregate number of shares of common stock, preferred stock
or other securities purchasable upon exercise of the rights; |
| · | the aggregate number of rights issued; |
| · | whether the rights are transferrable and the date, if any, on
and after which the rights may be separately transferred; |
| · | the date on which the right to exercise the rights will commence,
and the date on which the right to exercise the rights will expire; |
| · | the method by which holders of rights will be entitled to exercise; |
| · | the conditions to the completion of the offering, if any; |
| · | the withdrawal, termination and cancellation rights, if any; |
| · | whether there are any backstop or standby purchaser or purchasers
and the terms of their commitment, if any; |
| · | whether stockholders are entitled to oversubscription rights, if any; |
| · | any applicable material U.S. federal income tax considerations; and |
| · | any other terms of the rights, including terms, procedures and
limitations relating to the distribution, exchange and exercise of the rights, as applicable. |
Each right will
entitle the holder of rights to purchase for cash the principal amount of shares of common stock, preferred stock or other securities
at the exercise price provided in the applicable prospectus supplement. Rights may be exercised at any time up to the close of business
on the expiration date for the rights provided in the applicable prospectus supplement.
Holders
may exercise rights as described in the applicable prospectus supplement. Upon receipt of payment and the rights certificate
properly completed and duly executed at the corporate trust office of the rights agent or any other office indicated in the
prospectus supplement, we will, as soon as practicable, forward the shares of common stock, preferred stock or other securities, as
applicable, purchasable upon exercise of the rights. If less than all of the rights issued in any rights offering are exercised, we
may offer any unsubscribed securities directly to persons other than stockholders, to or through agents, underwriters or dealers or
through a combination of such methods, including pursuant to standby arrangements, as described in the applicable prospectus
supplement.
Rights Agent
The rights agent for
any rights we offer will be set forth in the applicable prospectus supplement.
CERTAIN
PROVISIONS OF DELAWARE LAW AND OF THE COMPANY’S CERTIFICATE OF INCORPORATION AND BYLAWS
Anti-Takeover Provisions
The provisions of Delaware
law, our Certificate of Incorporation and Bylaws may have the effect of delaying, deferring or discouraging another person from acquiring
control of our company.
Delaware Law
We are subject
to Section 203 of the Delaware General Corporation Law (“DGCL”). Subject to certain exceptions, Section 203 prevents a publicly
held Delaware corporation from engaging in a “business combination” with any “interested stockholder” for three
years following the date that the person became an interested stockholder, unless the interested stockholder attained such status with
the approval of our board of directors or unless the business combination is approved in a prescribed manner. A “business combination”
includes, among other things, a merger or consolidation involving us and the “interested stockholder” and the sale of more
than 10% of our assets. In general, an “interested stockholder” is any entity or person beneficially owning 15% or more of
our outstanding voting stock and any entity or person affiliated with or controlling or controlled by such entity or person.
Board of Directors
Our Certificate
of Incorporation and Bylaws provide that any or all of the directors may be removed from office at any time, but only for cause and only
by the affirmative vote of holders of a majority of the voting power of all then outstanding shares of capital stock of the Corporation
entitled to vote generally in the election of directors, voting together as a single class; however, whenever the holders of one or more
series of the Preferred Stock shall have the right, voting separately by class or series, to elect one or more directors, the term of
office, the filling of vacancies, the removal from office and other features of such directorships shall be governed by the terms of such
series of the Preferred Stock. Under our Certificate of Incorporation and Bylaws, any vacancy on our board of directors, including a vacancy
resulting from an enlargement of our board of directors, may be filled only by vote of a majority of our directors then in office. Furthermore,
our Certificate of Incorporation provides that the authorized number of directors may be changed only by the resolution of our board of
directors, subject to the rights of any holders of preferred stock to elect directors. The classification of our board of directors and
the limitations on the ability of our stockholders to remove directors, change the authorized number of directors and fill vacancies could
make it more difficult
for a third party to acquire, or discourage a third party from seeking to acquire, control of our company.
Authorized but Unissued Shares
The authorized
but unissued shares of common stock and preferred stock are available for future issuance without stockholder approval, subject to any
limitations imposed by the listing standards of any exchange on which our shares are listed. These additional shares may be used for a
variety of corporate finance transactions, acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved
common stock and preferred stock could make more difficult or discourage an attempt to obtain control of us by means of a proxy contest,
tender offer, merger or otherwise.
Special Meeting of Stockholders;
Advance Notice Requirements for Stockholder Proposals and Director Nominations
Special meetings of
our stockholders can be called only by the chairman of the Board of Directors, the President, Chief Executive Officer or such other persons
designated by the Board of Directors. In addition, stockholders must provide advance notice to nominate persons for election to our Board
of Directors or submit proposals for consideration at stockholder meetings.
Amendment of By-laws
The
General Corporation Law of the State of Delaware provides generally that the affirmative vote of a majority of the shares entitled
to vote on any matter is required to amend a corporation’s certificate of incorporation or by-laws, unless a
corporation’s certificate of incorporation or by-laws, as the case may be, require a greater percentage. Our by-laws may be
amended or repealed by a majority vote of our board of directors or the affirmative vote of the holders of at least a majority of
the voting power of all then outstanding shares of capital stock of the Corporation entitled to vote generally in the election of
directors, voting together as a single class, provided however that that no by-laws adopted by the stockholders may invalidate any
prior act of the board of directors that would have been valid if such by-laws had not been adopted.
Limitation of Liability and Indemnification
We
are incorporated under the laws of the State of Delaware. Section 145 of the DGCL provides that a Delaware corporation may indemnify
any persons who are, or are threatened to be made, parties to any threatened, pending, or completed action, suit, or proceeding,
whether civil, criminal, administrative, or investigative (other than an action by or in the right of such corporation), by reason
of the fact that such person was an officer, director, employee, or agent of such corporation, or is or was serving at the request
of such person as an officer, director, employee, or agent of another corporation or enterprise. The indemnity may include expenses
(including attorneys’ fees), judgments, fines, and amounts paid in settlement actually and reasonably incurred by such person
in connection with such action, suit, or proceeding, provided that such person acted in good faith and in a manner he or she
reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or
proceeding, had no reasonable cause to believe that his or her conduct was illegal. A Delaware corporation may indemnify any person
who is, or is threatened to be made, a party to any threatened, pending, or completed action or suit by or in the right of the
corporation by reason of the fact that such person was a director, officer, employee, or agent of such corporation, or is or was
serving at the request of such corporation as a director, officer, employee, or agent of another corporation or enterprise. The
indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with
the defense or settlement of such action or suit provided such person acted in good faith and in a manner he or she reasonably
believed to be in or not opposed to the corporation’s best interests, except that no indemnification is permitted without
judicial approval if the officer or director is adjudged to be liable to the corporation. Where an officer or director is successful
on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him or her against the
expenses which such officer or director has actually and reasonably incurred. Our Certificate of Incorporation and Bylaws provide
for the indemnification of our directors and officers to the fullest extent permitted under the DGCL.
Section
102(b)(7) of the DGCL permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not
be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director, except
for liability for any:
| · | breach of a director’s duty of loyalty to the corporation or its stockholders; |
| · | act or omission not in good faith or that involves intentional
misconduct or a knowing violation of law; |
| · | unlawful payment of dividends, stock purchase or redemption of shares; or |
| · | transaction from which the director derives an improper personal benefit. |
Our
Certificate of Incorporation provides that we will indemnify and hold harmless each person who is or was made a party or is
threatened to be made a party to or is otherwise involved in any threatened pending or completed action, suit or proceeding, whether
civil, criminal, administrative or investigative (a “proceeding”) by reason of the fact that he or she is or was a
director or officer of the Corporation or, while a director or officer of the Corporation, is or was serving at the request of the
Corporation as a director, officer, general partner, manager, managing member, employee or agent of another corporation or of a
partnership, joint venture, limited liability company, trust, other enterprise or nonprofit entity, including service with respect
to an employee benefit plan (an “indemnitee”), whether the basis of such proceeding is alleged action in an official
capacity as a director, officer, employee or agent, or in any other capacity while serving as a director, officer, employee or
agent, against all liability and loss suffered and expenses (including, without limitation, attorneys’ fees, judgments, fines,
ERISA excise taxes and penalties and amounts paid in settlement) reasonably incurred by such indemnitee in connection with such
proceeding. Our Certificate of Incorporation includes a provision providing for the limitation of liability to the maximum extent
permitted under the DGCL. Expenses incurred by any officer or director in defending any proceeding in advance of its final
disposition shall be paid by us upon delivery to us of an undertaking by or on behalf of such director or officer, to repay all
amounts advanced if it should ultimately be determined that such director or officer is not entitled to be indemnified by us.
Section 174 of
the DGCL provides, among other things, that a director, who willfully or negligently approves of an unlawful payment of dividends or an
unlawful stock purchase or redemption, may be held liable for such actions. A director who was either absent when the unlawful actions
were approved, or dissented at the time, may avoid liability by causing his or her dissent to such actions to be entered on the books
containing minutes of the meetings of the board of directors at the time such action occurred or immediately after such absent director
receives notice of the unlawful acts.
We maintain a
directors’ and officers’ liability insurance policy. The policy insures directors and officers against unindemnified losses
arising from certain wrongful acts in their capacities as directors and officers and reimburses us for those losses for which we have
lawfully indemnified the directors and officers. The policy contains various exclusions.
The foregoing
discussion of our Certificate of Incorporation, Bylaws, indemnification agreements, indemnity agreement, and Delaware law is not intended
to be exhaustive and is qualified in its entirety by our Certificate of Incorporation, Bylaws, indemnification agreements and by applicable
law.
Insofar as indemnification
for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing
provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed
in the Securities Act and is, therefore, unenforceable.
Provisions of our
Certificate of Incorporation on Choice of Forum
Unless we consent
to the selection of an alternative forum, our Certificate of Incorporation provides that the Court of Chancery of the State of Delaware,
or the Court of Chancery, will be, to the fullest extent permitted by law, the sole and exclusive forum for any derivative action or proceeding
brought on our behalf; any action asserting a claim of breach of fiduciary duty owed by any of our directors, officers or other employees
or agent to the Company or our stockholders; any action asserting a claim against us arising pursuant to the DGCL, or our Certificate
of Incorporation or Bylaws; any action to enforce or determine the validity of our Certificate of Incorporation or Bylaws; or any action
asserting a claim against us that is governed by the internal affairs doctrine. Since the choice of forum provisions are only applicable
to “the fullest extent permitted by law”, as provided in our Certificate of Incorporation, the provisions do not designate
the Court of Chancery as the exclusive forum for any derivative action or other claim for which the applicable statute creates exclusive
jurisdiction in another forum. As such, the choice of forum provisions do not apply to any actions arising under the Securities Act or
the Exchange Act.
We
believe the choice of forum provisions in our Certificate of Incorporation may benefit us by providing increased consistency in the
application of Delaware law, where permitted, by chancellors and judges particularly experienced in resolving corporate disputes,
efficient administration of cases on a more expedited schedule relative to other forums and protection against the burdens of
multi-forum litigation. However, the provision may have the effect of discouraging lawsuits against our directors, officers,
employees, and agents as it may limit any stockholder’s ability to bring a claim in a judicial forum that such stockholder
finds favorable for disputes with us or our directors, officers, employees, or agents and result in increased costs for stockholders
to bring a claim. The enforceability of similar choice of forum provisions in other companies’ certificates of incorporation
has been challenged in legal proceedings, and it is possible that, in connection with any applicable action brought against us, a
court could find the choice of forum provisions contained in our Certificate of Incorporation to be inapplicable or unenforceable in
such action.
LEGAL MATTERS
Mintz, Levin,
Cohn, Ferris, Glovsky and Popeo, P.C., Boston, Massachusetts, will pass upon the validity of the issuance of the securities to be offered
by this prospectus.
EXPERTS
The
financial statements of Eyenovia, Inc. as of December 31, 2020 and 2019 and for each of the two years in the period ended December
31, 2020, have been audited by Marcum LLP, independent registered public accounting firm, as stated in their report, which includes
an explanatory paragraph as to the Company’s ability to continue as a going concern, which is incorporated herein by
reference. Such financial statements of Eyenovia, Inc. are incorporated in this prospectus by reference in reliance on the report of
such firm given upon their authority as experts in accounting and auditing.
WHERE YOU
CAN FIND MORE INFORMATION
We are subject
to the reporting requirements of the Exchange Act, and file annual, quarterly and current reports, proxy statements and other information
with the SEC. SEC filings are available at the SEC’s web site at http://www.sec.gov. This prospectus is only part of a registration
statement on Form S-3 that we have filed with the SEC under the Securities Act and therefore omits certain information contained in the
registration statement. We have also filed exhibits and schedules with the registration statement that are excluded from this prospectus,
and you should refer to the applicable exhibit or schedule for a complete description of any statement referring to any contract or other
document.
We
also maintain a website at http://www.eyenovia.com, through which you can access our SEC filings. The information set forth
on our website is not part of this prospectus.
INCORPORATION
OF DOCUMENTS BY REFERENCE
The SEC allows
us to “incorporate by reference” information that we file with them. Incorporation by reference allows us to disclose important
information to you by referring you to those other documents. The information incorporated by reference is an important part of this prospectus,
and information that we file later with the SEC will automatically update and supersede this information. We filed a registration statement
on Form S-3 under the Securities Act, with the SEC with respect to the securities we may offer pursuant to this prospectus. This prospectus
omits certain information contained in the registration statement, as permitted by the SEC. You should refer to the registration statement,
including the exhibits, for further information about us and the securities we may offer pursuant to this prospectus. Statements in this
prospectus regarding the provisions of certain documents filed with, or incorporated by reference in, the registration statement are not
necessarily complete and each statement is qualified in all respects by that reference. Copies of all or any part of the registration
statement, including the documents incorporated by reference or the exhibits, may be accessed on the SEC website as noted above in “Where
You Can Find More Information.” The documents we are incorporating by reference are:
| · | our Current Reports on Form 8-K that we filed with the SEC on
February 3, 2021, March 2, 2021, March 15, 2021, April 1, 2021, May 5, 2021, May 10, 2021, May 14, 2021, May 25, 2021, June 15, 2021,
June 16, 2021, June 21, 2021, August 11, 2021, September 15, 2021, October 25, 2021, November 5, 2021, November 10, 2021, December 3, 2021, and December 6, 2021 (except for the information furnished under Items 2.02 or 7.01 and the exhibits furnished thereto); |
| · | all reports and other documents subsequently filed by us pursuant
to Sections 13(a), 13(c), 14 and 15(d) of the Exchange Act after the date of this prospectus and prior to the termination or completion
of the offering of securities under this prospectus shall be deemed to be incorporated by reference in this prospectus and to be a part
hereof from the date of filing such reports and other documents. |
The SEC file number for
each of the documents listed above is 001-38365.
In addition, all
reports and other documents filed by us pursuant to the Exchange Act after the date of the initial registration statement and prior to
effectiveness of the registration statement shall be deemed to be incorporated by reference into this prospectus.
Any statement
contained in this prospectus or in a document incorporated or deemed to be incorporated by reference into this prospectus will be deemed
to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus or any other
subsequently filed document that is deemed to be incorporated by reference into this prospectus modifies or supersedes the statement.
Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
You may request,
orally or in writing, a copy of any or all of the documents incorporated herein by reference. These documents will be provided to you
at no cost, by contacting:
Eyenovia,
Inc.
295 Madison
Avenue
Suite 2400
New
York, NY 10017
Attn: Corporate Secretary.
(917) 289-1117
You may also access
these documents on our website, http://www.eyenovia.com. The information contained on, or that can be accessed through, our website
is not a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
You should rely
only on information contained in, or incorporated by reference into, this prospectus and any prospectus supplement. We have not authorized
anyone to provide you with information different from that contained in this prospectus or incorporated by reference in this prospectus.
We are not making offers to sell the securities in any jurisdiction in which such an offer or solicitation is not authorized or in which
the person making such offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make such offer or solicitation.
Up to $10,000,000

Common Stock
PROSPECTUS SUPPLEMENT
Leerink Partners
May 16, 2024
Eyenovia (NASDAQ:EYEN)
Historical Stock Chart
From May 2024 to Jun 2024
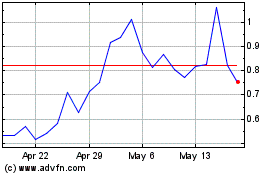
Eyenovia (NASDAQ:EYEN)
Historical Stock Chart
From Jun 2023 to Jun 2024