GLUCOTRACK
ANNOUNCES EXPANSION OF ITS CONTINUOUS GLUCOSE MONITORING TECHNOLOGY
TO EPIDURAL GLUCOSE MONITORING
Expansion offers
the potential for continuous, discreet and simplified epidural
glucose monitoring solutions for patients with Painful Diabetic
Neuropathy
Rutherford, NJ, April 16, 2024 --
InvestorsHub NewsWire -- Glucotrack, Inc. (Nasdaq:
GCTK) ("Glucotrack" or the "Company"), a
medical device company focused on the design, development, and
commercialization of novel technologies for people with diabetes,
announced that it is expanding its glucose monitoring technology to
include measuring glucose in the epidural space. A continuous
glucose monitoring system that takes readings in the spinal
epidural space may be integrated with existing treatments for
patients with Painful Diabetic Neuropathy (PDN).
PDN is a progressive neurological
disorder that affects approximately one-fifth of the more than 38
million Americans with with diabetes, equating to more than 7
million individuals.1,2
Its symptoms include pain and numbness
in the feet, legs, and hands which can significantly impact
patients' quality of life and functional
ability.3
Recently, Spinal Cord Stimulation
(SCS) technology has been indicated as a treatment option providing
significant long-term pain relief to these
patients.4
A spinal cord stimulator is an
implanted device, with electrodes placed in the epidural space,
that sends low levels of electricity directly into the spinal cord
to relieve pain.5
Glucotrack's sensor has the potential
to be integrated with existing SCS devices to measure epidural
glucose in patients with PDN who are undergoing SCS treatment.
Combining SCS and continuous glucose monitoring (CGM) could provide
several possible advantages, such as simplifying device management
for those patients.
Glucotrack has successfully completed
preclinical animal testing in an acute setting. Building on the success of the acute
studies, the Company has now initiated a long-term animal study to
assess sustained epidural glucose monitoring performance. This is
the second application of Glucotrack's technology for implantable
continuous glucose monitoring, in addition to its development of a
long-term Continuous Blood Glucose Monitoring (CBGM)
system.
The preclinical testing compared the
Glucotrack sensor against blood glucose and a commercially
available subcutaneous CGM in an acute large animal model while
varying blood glucose levels for several hours. The results
demonstrated the Glucotrack epidural glucose values closely tracked
both the blood glucose and subcutaneous CGM values. The study was
completed with no adverse effect on the animals. A second acute
study successfully confirmed the repeatability of these
results.
"We have always been committed to
developing a portfolio of innovative glucose monitoring
technologies to offer patients with diabetes more choice; today's
announcement underscores this commitment." said Paul V. Goode, PhD,
CEO of Glucotrack. "We are excited to pioneer the epidural glucose
monitoring space which we believe holds meaningful strategic
potential. By making disease management more intuitive and less
intrusive, Glucotrack is looking beyond traditional approaches to
improve the quality of life for millions of people with
diabetes."
For more information about Glucotrack,
visit glucotrack.com.
# # #
About Glucotrack, Inc.
Glucotrack, Inc. (NASDAQ: GCTK) is focused on the design,
development, and commercialization of novel technologies for people
with diabetes. The Company is currently developing a long-term
implantable continuous blood glucose monitoring system for people
living with diabetes.
Glucotrack's CBGM is a long-term,
implantable system that continually measures blood glucose levels
with a sensor longevity of 2+ years, no on-body wearable component
and with minimal calibration. For more information, please
visit http://www.glucotrack.com.
Forward-Looking Statements
This news release contains forward-looking statements within the
meaning of the Private Securities Litigation Reform Act of 1995.
Statements contained in this news release that are not statements
of historical fact may be deemed to be forward-looking statements.
Without limiting the generality of the foregoing, words such as
"believe", "expect", "plan" and "will" are intended to identify
forward-looking statements. Such forward-looking statements are
based on the beliefs of management, as well as assumptions made by,
and information currently available to, management. These
statements relate only to events as of the date on which the
statements are made, and Glucotrack undertakes no obligation to
publicly update any forward-looking statements, whether as a result
of new information, future events or otherwise, except as required
by law. All of the forward-looking statements made in this press
release are qualified by these cautionary statements, and there can
be no assurance that the actual results anticipated by the
Glucotrack will be realized or, even if substantially realized,
that they will have the expected consequences to or effects on us
or our business or operations. Readers are cautioned that certain
important factors may affect Glucotrack's actual results and could
cause such results to differ materially from any forward-looking
statements that may be made in this news release. Factors that may
affect Glucotrack's results include, but are not limited to, the
ability of Glucotrack to raise additional capital to finance its
operations (whether through public or private equity offerings,
debt financings, strategic collaborations or otherwise); risks
relating to the receipt (and timing) of regulatory approvals
(including U.S. Food and Drug Administration approval); risks
relating to enrollment of patients in, and the conduct of, clinical
trials; risks relating to Glucotrack's current and future
distribution agreements; risks relating to its ability to hire and
retain qualified personnel, including sales and distribution
personnel; and the additional risk factors described in
Glucotrack's filings with the U.S. Securities and Exchange
Commission (the "SEC"), including its Annual Report on Form 10-K
for the year ended December 31, 2023 as filed with the SEC on March
28, 2023.
Contacts:
Investor Relations:
investors@glucotrack.com
Media:
GlucotrackPR@icrinc.com
1 Abbott?CA, Malik?RA,
van Ross?ER, Kulkarni?J, Boulton?AJ. Prevalence and characteristics
of painful diabetic neuropathy in a large community-based diabetic
population in the U.K. Diabetes Care 2011;34:2220-2224.
2 Centers for Disease Control and
Prevention. (2022). National Diabetes Statistics Report website.
https://www.cdc.gov/diabetes/data/statistics-report/index.html
3 Schmader KE. Epidemiology and impact on quality of
life of postherpetic neuralgia and painful diabetic
neuropathy.
Clin J Pain. 2002;18(6):350-354.
doi:10.1097/00002508-200211000-00002.
4 Erika A. Petersen, Thomas G. Stauss,
James A. Scowcroft, Elizabeth S. Brooks, Judith L. White, Shawn M.
Sills, Kasra Amirdelfan, Maged N. Guirguis, Jijun Xu, Cong Yu, Ali
Nairizi, Denis G. Patterson, Kostandinos C. Tsoulfas, Michael J.
Creamer, Vincent Galan, Richard H. Bundschu, Neel D. Mehta, Dawood
Sayed, Shivanand P. Lad, David J. DiBenedetto, Khalid A. Sethi,
Johnathan H. Goree, Matthew T. Bennett, Nathan J. Harrison, Atef F.
Israel, Paul Chang, Paul W. Wu, Charles E. Argoff, Christian E.
Nasr, Rod S. Taylor, David L. Caraway, Nagy A. Mekhail; Durability
of High-Frequency 10-kHz Spinal Cord Stimulation for Patients With
Painful Diabetic Neuropathy Refractory to Conventional Treatments:
12-Month Results From a Randomized Controlled Trial. Diabetes Care
5 January 2022; 45 (1): e3-e6. https://doi.org/10.2337/dc21-1813
5 Dydyk
AM, Tadi P. Spinal Cord Stimulator Implant. [Updated 2023 Jul 3].
In: StatPearls [Internet]. Treasure Island (FL): StatPearls
Publishing; 2024 Jan-. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK555994/
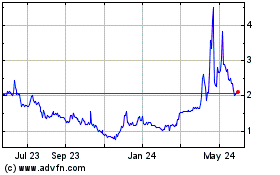
GlucoTrack (NASDAQ:GCTK)
Historical Stock Chart
From Nov 2024 to Dec 2024
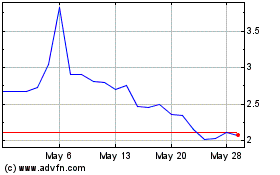
GlucoTrack (NASDAQ:GCTK)
Historical Stock Chart
From Dec 2023 to Dec 2024