| PROSPECTUS |
|
Filed Pursuant to
Rule 424(b)(3)
Registration No.
333-276882 |

MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
Warrants to Purchase 1,205,255 Ordinary Shares
This prospectus relates to the resale, from time
to time, by the Selling Shareholders (as defined herein) named herein or their permitted assigns, of up to 1,205,255 ordinary shares,
par value $0.0005 per share, of the Company exercisable at $2.9869 per share (the “Warrant Shares”), issuable upon exercise
of warrants (the “Warrants”). The Warrants and the Warrant Shares were sold in a private placement pursuant to an exemption
from the registration requirements of the Securities Act of 1933, as amended (the “Securities Act”), provided in Section
4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder.
We are not selling any ordinary shares under
this prospectus and will not receive any proceeds from the sale of the ordinary shares by the Selling Shareholders. To the extent the
Warrants are exercised for cash, if at all, we will receive the exercise price of such Warrants. We cannot, however, predict when or
if the Warrants will be exercised and it is possible that the Warrants may expire or never be exercised, or they will be exercised cashlessly,
in accordance with the terms of the Warrants, in which case we will not receive any cash proceeds. The Selling Shareholders will bear
all commissions and discounts, if any, attributable to the sale of the ordinary shares underlying the Warrants. We will bear all costs,
expenses and fees in connection with the registration of the ordinary shares issuable to the selling shareholders upon the exercise of
the Warrants.
The Selling Shareholders may sell the Warrant
Shares included in this prospectus in a number of different ways and at varying prices. We provide more information about how the Selling
Shareholders may sell the shares in the section entitled “Plan of Distribution.” Each Selling Shareholder is an “underwriter”
within the meaning of Section 2(a)(11) of the Securities Act of 1933, as amended, or the Securities Act.
The Selling Shareholders will pay all brokerage
fees and commissions and similar expenses in connection with the offer and sale of the Warrant Shares by the Selling Shareholders pursuant
to this prospectus. We will pay the expenses (except brokerage fees and commissions and similar expenses) incurred to register under
the Securities Act the offer and sale of the Warrant Shares included in this prospectus by the Selling Shareholders. See “Plan
of Distribution.”
We may amend or supplement this prospectus supplement
from time to time by filing amendments or supplements as required. You should read the entire base prospectus and prospectus supplement
and any amendments or supplements carefully before you make your investment decision.
We have utilized the home country rule exemption
in relation to the placement and elected to be exempt from the Nasdaq Marketplace Rule 5635(d), and notified Nasdaq of our decision to
exercise such exemption. Please see “Implications of Being a Foreign Private Issuer” in this prospectus supplement.
We are an “emerging growth company”
and a “foreign private issuer,” each as defined under the federal securities laws, and, as such, we will be subject to reduced
public company reporting requirements for this prospectus supplement and future filings. See “Prospectus Supplement Summary - Implications
of Being an Emerging Growth Company” and “Prospectus Supplement Summary - Implications of Being a Foreign Private
Issuer.”
Our ordinary shares are listed on the Nasdaq
Capital Market under the symbol “MHUA.” On February 22, 2024, the last reported sale price of our ordinary shares on the
Nasdaq Stock Market was $0.719.
Investing in our securities involves a high
degree of risk. Before making an investment decision, please read the information under the heading “Risk Factors” beginning
on page 19 of this prospectus and the risk factors set forth in our most recent annual report on Form 20-F, as amended and filed with
the SEC on August 29, 2023 (the “2022 Annual Report”).
Unless otherwise stated, as used in this prospectus
supplement, “Meihua,” refers to Meihua International Medical Technologies Co., Ltd., 美华国际医疗科技有限公司,
a Cayman Islands exempted company, and depending on the context, “we,” “us,” “our company,” “our,”
“the Company” and “Meihua International” refer to Meihua International Medical Technologies Co., Ltd., 美华国际医疗科技有限公司,
a Cayman Islands exempted company, its subsidiaries, Kang Fu International Medical Co., Limited (“Kang Fu International Medical”),
Yangzhou Huada Medical Device Co., Ltd. (“Yangzhou Huada”), Jiangsu Yada Technology Group Co., Ltd. (“Jiangsu Yada”),
Jiangsu Huadong Medical Device Industrial Co., Ltd. (“Jiangsu Huadong”), Yangzhou Guanghui Medical Technology Co., Ltd. (“Guanghui”),
Hainan Guoxie Technology Group Co., Ltd. (“Hainan Guoxie”) and Hainan Ruiying Technology Co., Ltd. (“Hainan Ruiying”).
Guanghui was dissolved on June 1, 2023. See “Prospectus Supplement Summary — Company Overview.”
Meihua
is a holding company which was incorporated under the laws of the Cayman Islands on November 10, 2020. Meihua’s direct subsidiary
is Kang Fu International Medical, a Hong Kong company. Kang Fu International Medical was incorporated on October 13, 2015 by four shareholders,
Yongjun Liu, Yin Liu, Ace Capital Limited and King Tai International Holding Limited. On November 22, 2019, Yongjun Liu acquired 9,300,000
shares in Kang Fu International Medical from Ace Capital Limited and 4,500,000 shares in Kang Fu International Medical from King Tai
International Holding Limited, respectively. Upon consummation of such share transfer, Yongjun Liu and Yin Liu constituted all of the
shareholders of Kang Fu International Medical, holding 100% of the shares of Kang Fu International Medical. On December 21, 2020, Meihua
in turn acquired 41,400,000 shares (69% of the outstanding shares) from Yongjun Liu and 18,600,000 shares (31% of the outstanding shares)
from Yin Liu, respectively, resulting in Kang Fu International Medical becoming Meihua’s wholly owned subsidiary. In exchange
for the acquisition on Kang Fu, Meihua issued a total of 15,935,000 Ordinary Shares to Mr. and Mrs. Liu, who in turn transferred their
shares to Bright Accomplish Limited, a holding company for which they are the sole shareholders, on December 21, 2020. Bright Accomplish
Limited is Meihua’s controlling shareholder, holding approximately 63.54% of Meihua’s Ordinary Shares as of the date of this
prospectus supplement. As such, we are a “controlled company” under Nasdaq Listing Rules 5615(c) and are allowed
to follow certain exemptions afforded to a “controlled company” under the Nasdaq Listing Rules. However, we do not intend
to avail ourselves of such corporate governance exemptions. See “Risks Related to Doing Business in China — We are a “controlled
company” within the meaning of the Nasdaq listing rules and may follow certain exemptions from certain corporate governance requirements
that could adversely affect our public shareholders” at page 42 of this prospectus.
Meihua is not a Chinese
operating company, but a Cayman Islands holding company with operations conducted by its subsidiaries located in mainland China. Meihua
owns 100% of Kang Fu International Medical. Kang Fu International Medical owns 100% of Yangzhou Huada and 55% of Hainan Guoxie. Yangzhou
Huada owns 100% of Jiangsu Yada. Jiangsu Yada, in turn, owns 100% of Jiangsu Huadong. Jiangsu Huadong, in turn, owns 100% of the equity
interests of Guanghui, which was dissolved on June 1, 2023. Jiangsu Huadong owns 51% of
Hainan Ruiying.
The structure of cash
flows within our organization, and a summary of the applicable regulations, is as follows:
| 1. | Our
equity structure is a direct holding structure, pursuant to which the overseas entity listed in the U.S., Meihua International Medical
Technologies Co., Ltd. (“Meihua” or Meihua International”), directly controls Yangzhou Huada Medical Device Co., Ltd
(“Yangzhou Huada”) (the “WFOE”) and other domestic operating entities which are directly owned through the Hong
Kong company, Kang Fu International Medical Co., Limited (“Kang Fu International Medical”). |
| 2. |
Within our direct holding
structure, the cross-border transfer of funds within our corporate group is legal and compliant with the laws and regulations of
the PRC. After foreign investors’ funds enter Meihua International at the close of securities offerings, the funds can be directly
transferred to Kang Fu International Medical, and then transferred to subordinate operating entities through the WFOE. |
While we don’t
presently intend to issue dividends to our shareholders, if the Company distributes dividends to our shareholders in the future, we will
transfer the dividends to Kang Fu in accordance with the laws and regulations of the PRC, and then Kang Fu will transfer the dividends
to Meihua International and the dividends will then be distributed from Meihua International to all shareholders respectively in proportion
to the shares they hold, regardless of whether the shareholders are U.S. investors or investors in other countries or regions.
| 3. |
Cash transfers occurred
among the Company and its subsidiaries are fully eliminated in the consolidated financial statements and summarized as below: |
| | |
From
January 1, 2023 to December 31, 2023 (in
US$) | |
| Inter-company cash transfers: | |
Meihua
(Cayman) | | |
Kang Fu
International
Medical
(HK) | | |
PRC
subsidiaries | |
| Cash transferred from Kang Fu International Medical to PRC subsidiaries (1) | |
| - | | |
$ | (1,000,000 | ) | |
$ | 1,000,000 | |
| (1) |
Kang Fu International Medical
transferred $1,000,000 to Yangzhou Huada, one of the PRC subsidiaries, as working capital loan. |
| | |
For
the year ended December 31, 2022 (in
US$) | |
| Inter-company cash transfers: | |
Meihua
(Cayman) | | |
Kang Fu
International
Medical
(HK) | | |
PRC
subsidiaries | |
| Cash transferred from Meihua to Kang Fu International
Medical (1) | |
$ | (26,010,150 | ) | |
$ | 26,010,150 | | |
| - | |
| Cash transferred from Kang Fu International Medical
to Meihua (2) | |
$ | 390 | | |
$ | (390 | ) | |
| - | |
| Cash transferred from Kang Fu International Medical to PRC subsidiaries
(3) | |
| - | | |
$ | (20,389,970 | ) | |
$ | 20,389,970 | |
| Cash transferred from PRC subsidiaries to Kang Fu International
Medical (4) | |
| - | | |
$ | 130,000 | | |
$ | (130,000 | ) |
| (1) |
Meihua transferred $26,010,150
to Kang Fu International Medical as a working capital loan. |
| (2) |
Kang Fu International Medical
transferred $390 to Meihua for repayment of a working capital loan. |
| (3) |
Kang Fu International Medical
contributed $20,389,970 to PRC subsidiaries as a capital contribution.* |
| (4) |
Yangzhou Huada, one of
the PRC subsidiaries, transferred $130,000 to Kang Fu International Medical as a working capital loan. |
| * |
On February 18, 2022, the
Company closed its initial public offering of Ordinary Shares and received approximately US$35 million. In March and April 2022,
the Company transferred approximately US$26.0 million to Kang Fu International Medical for working capital purpose then Kang Fu International
Medical made capital injections aggregating approximately US$20.4 million to PRC subsidiaries - Yangzhou Huada and Hainan Guoxie. |
| | |
For
the year ended December 31, 2021 (in
US$) | |
| Inter-company cash transfers: | |
Meihua
(Cayman) | | |
Kang Fu
International
Medical
(HK) | | |
PRC
subsidiaries | |
| Cash transferred from Kang Fu International Medical
to PRC subsidiaries (5) | |
| - | | |
$ | (46,297 | ) | |
$ | 46,297 | |
| Cash transferred from PRC subsidiaries to Kang Fu International
Medical (6) | |
| - | | |
$ | 768,042 | | |
$ | (768,042 | ) |
| (5) |
Kang Fu International Medical
transferred $46,297 to PRC subsidiaries for repayment of a working capital loan. |
| (6) |
Yangzhou Huada, one of
the PRC subsidiaries, transferred $768,042 to Kang Fu International Medical as a working capital loan. |
| | |
For
the year ended December 31, 2020 (in
US$) | |
| Inter-company cash transfers: | |
Meihua
(Cayman) | | |
Kang Fu
International
Medical
(HK) | | |
PRC
subsidiaries | |
| Cash transferred from PRC subsidiaries to Kang Fu
International Medical (7) | |
| - | | |
$ | 499,998 | | |
$ | (499,998 | ) |
| (7) |
Yangzhou Huada, one of
the PRC subsidiaries, transferred $499,998 to Kang Fu International Medical as a working capital loan. |
| 4. |
As of the date of this
prospectus supplement, no dividends or other distributions have been made to date from our subsidiaries to our holding company nor
have we or any of our subsidiaries ever paid dividends or made distributions to U.S. investors to date. For the foreseeable future,
the Company intends to use the earnings for research and development, to develop new products and to expand its production capacity.
As a result, we do not expect to pay any cash dividends for the near term. |
Our PRC subsidiaries’
ability to distribute dividends is based upon their distributable earnings. Current PRC regulations permit our PRC subsidiaries to pay
dividends to their respective shareholders only out of their accumulated profits, if any, determined in accordance with PRC accounting
standards and regulations. In addition, each of our PRC subsidiaries is required to set aside at least 10% of its after-tax profits each
year, if any, to fund a statutory reserve until such reserve reaches 50% of each of their registered capitals. These reserves are not
distributable as cash dividends.
To address persistent
capital outflows and the RMB’s depreciation against the U.S. dollar in the fourth quarter of 2016, the People’s Bank of China
and the State Administration of Foreign Exchange, or SAFE, have implemented a series of capital control measures in the subsequent months,
including stricter vetting procedures for China-based companies to remit foreign currency for overseas acquisitions, dividend payments
and shareholder loan repayments. The PRC government may continue to strengthen its capital controls and our PRC subsidiaries’ dividends
and other distributions may be subject to tightened scrutiny in the future. The PRC government also imposes controls on the conversion
of RMB into foreign currencies and the remittance of currencies out of the PRC. Therefore, we may experience difficulties in completing
the administrative procedures necessary to obtain and remit foreign currency for the payment of dividends from our profits, if any. Furthermore,
if our subsidiaries in the PRC incur debt on their own in the future, the instruments governing the debt may restrict their ability to
pay dividends or make other payments.
In addition, the Enterprise
Income Tax Law and its implementation rules provide that a withholding tax at a rate of 10% will be applicable to dividends payable by
Chinese companies to non-PRC-resident enterprises unless reduced under treaties or arrangements between the PRC central government and
the governments of other countries or regions where the non-PRC resident enterprises are tax resident. Pursuant to the tax agreement
between Mainland China and the Hong Kong Special Administrative Region, the withholding tax rate in respect to the payment of dividends
by a PRC enterprise to a Hong Kong enterprise may be reduced to 5% from a standard rate of 10%. However, if the relevant tax authorities
determine that our transactions or arrangements are for the primary purpose of enjoying a favorable tax treatment, the relevant tax authorities
may adjust the favorable withholding tax in the future. Accordingly, there is no assurance that the reduced 5% withholding rate will
apply to dividends received by our Hong Kong subsidiary from our PRC subsidiaries. This withholding tax will reduce the amount of dividends
we may receive from our PRC subsidiaries.
Meihua faces various
legal and operational risks and uncertainties as a company with its principal subsidiaries based in and primarily operating in China.
Most of Meihua’s subsidiaries operations are conducted in the PRC, and are governed by PRC laws, rules and regulations. Because
the laws, rules and regulations are relatively new and quickly evolving, and because of the limited number of published decisions and
the non-precedential nature of these decisions, and because the laws, rules and regulations often give the relevant regulator certain
discretion in how to enforce them, the interpretation and enforcement of these laws, rules and regulations involve uncertainties
in practice. As a result, the application, interpretation, and enforcement of new and existing laws and regulations are often uncertain.
In addition, these laws and regulations may be interpreted and applied inconsistently by different agencies or authorities, and inconsistently
with our current policies and practices. In particular, recent statements and regulatory actions by China’s government, such as
those related to data security or anti-monopoly concerns, as well as the ability of Public Company Accounting Oversight Board, or the
PCAOB, to inspect our auditors, may impact our Company’s ability to conduct our business, accept foreign investments, or be listed
on a U.S. or other foreign stock exchange. See “Risk Factors – Risks Related to Doing Business in China” beginning
at page 36 of this prospectus.
On December 28, 2021,
the Cyberspace Administration of China (the “CAC”) and 12 other relevant PRC government authorities published the amended
Cybersecurity Review Measures, which came into effect on February 15, 2022. The Cybersecurity Review Measures provide that a “network
platform operator” that possesses personal information of more than one million users and seeks a listing in a foreign country
must apply for a cybersecurity review. Further, the relevant PRC governmental authorities may initiate a cybersecurity review against
any company if they determine certain network products, services, or data processing activities of such company affect or may affect
national security. As of the date of this prospectus supplement, Meihua and its PRC subsidiaries have not been involved in any investigations
on cybersecurity review initiated by any PRC regulatory authority, nor has any of them received any inquiry, notice or sanction.
As of the date of this
prospectus supplement and according to the currently in-effect PRC laws and regulations,
we do not believe that we and our PRC subsidiaries are subject to the cybersecurity review, reporting or other permission requirements
by the CAC under the applicable PRC cybersecurity laws and regulations with respect to the offering of our securities or the business
operations of our PRC Subsidiaries, because neither we nor any of our PRC Subsidiaries qualifies as a critical information infrastructure
operator or has conducted any data processing activities that affect or may affect national security or holds personal information of
more than one million users.
In addition, we also
believe that, as of the date of this prospectus supplement, we (1) are not required to obtain permissions from any PRC authorities to
operate or issue our Ordinary Shares to foreign investors, (2) are not subject to permission requirements from the China Securities Regulatory
Commission, or the CSRC, the CAC or any other entity that is required to approve our PRC subsidiaries’ operations, and (3) have
not received or otherwise been denied such permissions by any PRC authorities. Nevertheless, the General Office of the Central Committee
of the Communist Party of China and the General Office of the State Council jointly issued the “Opinions on Severely Cracking Down
on Illegal Securities Activities According to Law,” or the Opinions, which were made available to the public on July 6, 2021. The
Opinions emphasized the need to strengthen the administration over illegal securities activities, and the need to strengthen the supervision
over overseas listings by Chinese companies. Given the current PRC regulatory environment, it is uncertain when and whether we or our
PRC subsidiaries, will be required to obtain permission from the PRC government to continue to list on U.S. exchanges in the future,
and even when such permission is obtained, whether it will be denied or rescinded. We have been closely monitoring regulatory developments
in China regarding any necessary approvals from the CSRC or other PRC governmental authorities required for overseas listings. As of
the date of this prospectus supplement, we have not received any inquiry, notice, warning, sanctions or regulatory objection to this
offering from the CSRC or other PRC governmental authorities. However, there remains significant uncertainty as to the enactment, interpretation
and implementation of regulatory requirements related to overseas securities offerings and other capital markets activities.
On February 17, 2023,
the CSRC promulgated Trial Administrative Measures of the Overseas Securities Offering and Listing by Domestic Companies, or the Trial
Measures, and five relevant supporting guidelines, together as the New Overseas Listing Rules, which became effective on March 31, 2023.
According to the New Overseas Listing Rules, PRC domestic companies that seek to offer and list securities in overseas markets, either
by direct or indirect means, are required to complete the filing procedure with the CSRC and report relevant information. In addition,
an overseas-listed company must also submit the filing with respect to its follow-on offerings, issuance of convertible corporate bonds
and exchangeable bonds, and other equivalent offering activities, within the time frame specified by the Trial Measures. The New Overseas
Listing Rules set forth the regulatory filing requirements for both direct and indirect overseas listings and clarify the determination
criteria for indirect overseas listing in overseas markets. For more detailed information, see “Risk Factors — Risks Related
to Doing Business in China — Potential Chinese governmental and regulatory interference could significantly limit or completely
hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline
or be worthless” in the at page 37 of this prospectus and “Risk Factors — Risk Related to Doing Business in China
— If the Chinese government chooses to exert more oversight and control over offerings that are conducted overseas and/or foreign
investment in China-based issuers, such action could significantly limit or completely hinder our ability to offer or continue to offer
securities to investors outside of China and, as a result, cause the value of such securities to significantly decline or be worthless”
at page 36 of this prospectus. The Warrants were sold to the Selling Shareholders in the previous private placement and we have filed
with the CSRC within three business days after the completion of the previous placement as required under the New Overseas Listing Rules.
As we are not selling any ordinary shares or other equivalent securities under this prospectus, and this offering is conducted by the
Selling Shareholders, rather than by us, this offering is not subject to the filing requirement under the New Overseas Listing Rules..
We may be subject to orders to rectify, warnings and fines if we fail to comply with the requirements under the Trial Measures for any
future overseas securities offering or listing, or if the CSRC disagrees with our view on the applicability of the New Overseas Listing
Rules to this offering by the Selling Shareholder. Failure to comply with the filing requirements may result in an order of rectification,
a warning and fines up to RMB10 million to the non-compliant domestic companies, and the directly responsible persons of the companies
will be warned and fined between RMB500,000 and RMB5 million. Furthermore, if the controlling shareholder and the actual controller of
the non-compliant companies organize or instigate the breach, they will be fined between RMB1 million and RMB10 million. In addition
to the above filing requirements, the New Overseas Listing Rules also requires an issuer to report to the CSRC within three business
days after occurrence of any the following events: (i) a change of control; (ii) its being subject to investigation or sanctions by any
overseas securities regulators or overseas authorities; (iii) its change of listing status or listing segment; (iv) voluntary or mandatory
delisting; and (v) material change of its principal business operations to the extent that it ceases to be subject to the filing requirements
of the Trial Measures.
As a result of the legal and operational risks
associated with us being based in and having our operations in China, such risks could result in a material change in our operations
and/or the value of our securities and could significantly limit or completely hinder our ability to offer or continue to offer securities
to investors and cause the value of such securities to significantly decline or be worthless. See “Risk Factors – Risks Related
to Doing Business in China” beginning at page 36 of this prospectus.
In addition, our Ordinary
Shares may be delisted from Nasdaq or prohibited from being traded over the counter under the Holding Foreign Companies Accountable Act
(“HFCA Act”) and related regulations if the PCAOB is unable to inspect our auditor for two consecutive years beginning in
2022. Our auditor is subject to inspection by the PCAOB on a regular basis and has not been subject to the determinations announced by
the PCAOB on December 16, 2021. If trading in our Ordinary Shares is prohibited under the HFCA Act in the future because the PCAOB determines
that it cannot inspect or fully investigate our auditor at such future time, the Nasdaq Stock Market may determine to delist our Ordinary
Shares. On December 29, 2022, legislation entitled “Consolidated Appropriations Act, 2023” (the “Consolidated Appropriations
Act”) was signed into law by President Biden, which contained a provision to amend the HFCA Act by requiring the U.S. Securities
and Exchange Commission, or the SEC, to prohibit an issuer’s securities from trading on any U.S. stock exchanges if its auditor
is not subject to PCAOB inspections for two consecutive years instead of three, thus reducing the time period for triggering the delisting
of our Company and the prohibition of trading in our securities if the PCAOB is unable to inspect our accounting firm at such future
time. On December 15, 2022, the PCAOB Board determined that the PCAOB was able to secure complete access to inspect and investigate registered
public accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous determinations to the contrary.
However, should PRC authorities obstruct or otherwise fail to facilitate the PCAOB’s access in the future, the PCAOB Board will
consider the need to issue a new determination. Our securities may be delisted or prohibited from trading if the PCAOB determines that
it cannot inspect or investigate completely our auditor under the Holding Foreign Companies Accountable Act. Our auditor, Kreit &
Chiu CPA LLP, is headquartered in New York, NY with its office at 733 Third Avenue, Floor 16, #1014, New York, NY 10017, and is subject
to inspection by the PCAOB on a regular basis. See “Risk Factors — Risks Related to Doing Business in China — Although
the audit report included in this report was issued by U.S. auditors who are currently inspected by the PCAOB, if it is later determined
that the PCAOB is unable to inspect or investigate our auditor completely, investors would be deprived of the benefits of such inspection
and our Ordinary Shares may be delisted or prohibited from trading” at page 41 of this prospectus
As a medical device
manufacturing and sales company, we are subject to extensive government regulation and supervision in the PRC. Pursuant to PRC laws,
we must obtain production license for Class II and III disposable medical devices and production filing for Class I disposable medical
device, operation license for Class III disposable medical devices and operation filing for Class II disposable medical devices, and
filing or registration certificates for certain Class I, II or Class III disposable medical devices. As of the date of this prospectus
supplement, we are current on all licenses and certificates and have obtained Class I, II and III disposable medical device qualifications
in the PRC.
However, if we fail
to timely renew our medical device licenses or registration certificates, it could adversely affect our reputation, financial conditions
and results of operations. See “Risk Factors — Risks Related to Our Business and Industry — If we fail to timely
renew our medical device licenses or registration certificates, it could adversely affect our reputation, financial conditions, and results
of operations” at page 34 of this prospectus.
Neither the U.S.
Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon
the adequacy or accuracy of this prospectus supplement or the accompanying base prospectus. Any representation to the contrary is a criminal
offense.
The date of this prospectus is February 27, 2024
TABLE OF CONTENTS
ABOUT
THIS PROSPECTUS
This prospectus is part of a resale registration
statement on Form F-1. The information contained in this prospectus is accurate only as of the date on the front cover of this prospectus,
regardless of the time of delivery of this prospectus or any sale of our ordinary shares. Our business, financial condition, results
of operations and prospects may have changed since that date. You should also read and consider the information in the documents to which
we have referred you under the caption “Where You Can Find More Information” in this prospectus.
For investors outside the United States: We have
not done anything that would permit a public offering of the securities or possession or distribution of this prospectus in any jurisdiction
where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession
of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities and the distribution
of this prospectus outside of the United States. You are required to inform yourselves about and to observe any restrictions relating
to this offering and the distribution of this prospectus outside of the United States.
You should rely only on the information contained
in or incorporated by reference in this prospectus and in any free writing prospectus prepared by or on behalf of us. We have not authorized
anyone to provide you with information different from, or in addition to, that contained in or incorporated by reference in this prospectus
or any related free writing prospectus. This prospectus is an offer to sell only the securities offered hereby but only under circumstances
and in jurisdictions where it is lawful to do so. The information contained in or incorporated by reference in this prospectus is current
only as of its date. Our business, financial condition, results of operations and prospects may have changed since that date.
We are not offering to sell or seeking offers
to purchase these securities in any jurisdiction where the offer or sale is not permitted. We have not done anything that would permit
this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than
in the United States. Persons outside the United States who come into possession of this prospectus and any free writing prospectus related
to this offering in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions relating
to this offering and the distribution of this prospectus and any such free writing prospectus applicable to that jurisdiction.
Unless otherwise indicated, information contained
in this prospectus concerning our industry and the markets in which we operate or plan to operate, including our general expectations
and market position, market opportunity and market share, is based on information from our own management estimates and research, as
well as from industry and general publications and research, surveys and studies conducted by third parties. Management estimates are
derived from publicly available information, our knowledge of our industry, and assumptions based on such information and knowledge which
we believe to be reasonable. Our management estimates have not been verified by any independent source, and we have not independently
verified any third-party information. In addition, assumptions and estimates of our company’s and our industry’s future performance
are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in the section
entitled “Risk Factors” beginning on page 19. These and other factors could cause our future performance to differ materially
from our assumptions and estimates. See “Cautionary Note Regarding Forward-Looking Statements” on page iv below.
Unless otherwise stated, as used in this prospectus
supplement, “Meihua,” refers to Meihua International Medical Technologies Co., Ltd., 美华国际医疗科技有限公司,
a Cayman Islands exempted company, and depending on the context, “we,” “us,” “our company,” “our,”
“the Company” and “Meihua International” refer to Meihua International Medical Technologies Co., Ltd., 美华国际医疗科技有限公司,
a Cayman Islands exempted company, its subsidiaries, Kang Fu International Medical Co., Limited (“Kang Fu International Medical”),
Yangzhou Huada Medical Device Co., Ltd. (“Yangzhou Huada”), Jiangsu Yada Technology Group Co., Ltd. (“Jiangsu Yada”),
Jiangsu Huadong Medical Device Industrial Co., Ltd. (“Jiangsu Huadong”), Yangzhou Guanghui Medical Technology Co., Ltd. (“Guanghui”),
Hainan Guoxie Technology Group Co., Ltd. (“Hainan Guoxie”) and Hainan Ruiying Technology Co., Ltd. (“Hainan Ruiying”).
Guanghui was dissolved on June 1, 2023.
“PRC” or “China” refers
to the People’s Republic of China, excluding, for the purpose of this prospectus supplement, Taiwan, Hong Kong, and Macau, “RMB”
or “Renminbi” refers to the legal currency of China and “$” or “U.S. dollars” refers to the legal
currency of the United States.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement
on Form F-1 under the Securities Act relating to this offering of our ordinary shares. This prospectus does not contain all of the
information contained in the registration statement. The rules and regulations of the SEC allow us to omit certain information from this
prospectus that is included in the registration statement. Statements made in this prospectus concerning the contents of any contract,
agreement or other document are summaries of all material information about the documents summarized, but are not complete descriptions
of all terms of these documents. If we filed any of these documents as an exhibit to the registration statement, you may read the document
itself for a complete description of its terms.
We are subject to the reporting requirements
of the Securities Exchange Act of 1934, as amended (the “Exchange
Act”). In accordance with these requirements, we file reports and other information as a foreign private issuer with the
SEC. Those reports or other information may be inspected without charge at the locations described below. As a foreign private issuer,
we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors
and principal stockholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the
Exchange Act. In addition, we are not required under the Exchange Act to file quarterly reports on Form 10-Q or current reports
on Form 8-K with the SEC, or to file our annual report as promptly as U.S. companies whose securities are registered under the Exchange
Act. However, we are required to file with the SEC, within four months after the end of each fiscal year, or such applicable time as
required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent registered public accounting
firm, and will submit to the SEC, on Form 6-K, unaudited quarterly financial information for the first three quarters of each fiscal
year.
You may read and copy the registration statement,
including the related exhibits and schedules, and any document we file with the SEC without charge at the SEC’s public reference
room at 100 F Street, N.E., Room 1580, Washington, DC 20549. You may also obtain copies of the documents at prescribed
rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Room 1580, Washington, DC 20549. Please
call the SEC at 1-800-SEC-0330 for further information on the public reference room. The SEC also maintains an Internet website that
contains reports and other information regarding issuers that file electronically with the SEC. Our filings with the SEC are also available
to the public through the SEC’s website at http://www.sec.gov.
We maintain a corporate website at http://www.meihuamed.com.
Our filings with the SEC, and exhibits incorporated in and amendments to those reports, are available free of charge on our website as
soon as reasonably practicable after they are filed with, or furnished to, the SEC. Information contained on, or that can be accessed
through, our website does not constitute a part of this prospectus. We have included our website address in this prospectus solely as
an inactive textual reference.
CAUTIONARY NOTE REGARDING
FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements.
These statements are based on our management’s current beliefs, expectations and assumptions about future events, conditions and
results and on information currently available to us. Discussions containing these forward-looking statements may be found, among other
places, in the “Business Overview” and “Risk Factors” section in this prospectus as well as other documents we
have filed with the SEC, including our most recent annual report on Form 20-F, as well as any amendments thereto (the “2022 Annual
Report”).
In some cases, you can identify forward-looking
statements by terminology such as “may,” “will,” “should,” “could,” “would,”
“predicts,” “potential,” “continue,” “expects,” “anticipates,” “future,”
“intends,” “plans,” “believes,” “estimates,” as well as statements in the future tense
or the negative or plural of those terms, and similar expressions intended to identify statements about the future, although not all
forward- looking statements contain these words. These statements involve known and unknown risks, uncertainties and other factors that
may cause our actual results, levels of activity, performance, or achievements to be materially different from the information expressed
or implied by these forward-looking statements.
Any statements in this prospectus supplement,
the accompanying prospectus or the documents incorporated by reference herein and therein about our expectations, beliefs, plans, objectives,
assumptions or future events or performance are not historical facts and are forward-looking statements. Within the meaning of Section
27A of the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), these
forward- looking statements include, without limitation, statements regarding:
| |
● |
our future business development, financial condition
and results of operations; |
| |
|
|
| |
● |
expected changes in our revenues, costs or expenditures;
|
| |
|
|
| |
● |
our estimates regarding revenues, cash flows, capital
requirements and our need for additional financing; |
| |
|
|
| |
● |
our expectations regarding demand for and market acceptance
of our services; |
| |
|
|
| |
● |
competition in our industry; and |
| |
|
|
| |
● |
government policies and regulations relating to our
industry. |
The accuracy of these forward-looking statements
depends upon a number of known and unknown risks and events. Many factors could cause our actual results to differ materially from those
expressed or implied in our forward-looking statements. Consequently, you should not place undue reliance on these forward-looking statements.
In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors,
may cause actual results to differ materially from those contained in any forward-looking statements.
The forward-looking statements speak only as
of the date on which they are made; and, except as required by law we undertake no obligation to update any forward-looking statement
to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events.
You should refer to “Risk Factors”
in this prospectus for a discussion of important factors that may cause our actual results to differ materially from those expressed
or implied by our forward-looking statements. Given these risks, uncertainties and other factors, many of which are beyond our control,
we cannot assure you that the forward-looking statements in this prospectus supplement, the accompanying prospectus or the documents
incorporated by reference herein and therein will prove to be accurate, and you should not place undue reliance on these forward-looking
statements. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant
uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any
other person that we will achieve our objectives and plans in any specified time frame, or at all.
You should read this prospectus completely and
with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking
statements by these cautionary statements.
This prospectus may contain market data and industry
forecasts that were obtained from industry publications. These data involve a number of assumptions and limitations, and you are cautioned
not to give undue weight to such estimates. While we believe the market position, market opportunity and market size information included
in this prospectus, the accompanying prospectus and the documents incorporated by reference herein and therein is generally reliable,
such information is inherently imprecise.
PROSPECTUS SUMMARY
This summary highlights certain information
about us, this offering and selected information contained elsewhere in or incorporated by reference into this prospectus supplement,
and does not contain all of the information that you need to consider in making your investment decision. For a more complete understanding
of our business and this offering, you should carefully read this entire prospectus supplement and the accompanying prospectus, including
our historical financial statements and the notes thereto, which are incorporated herein by reference. You should read “Risk Factors”
beginning on page 19 of this prospectus for more information about important risks that you should consider before making a decision
to invest in our securities.
Company Overview
Meihua International
is a Cayman Islands exempted company incorporated on November 10, 2020. Kang Fu International is our wholly owned subsidiary formed in
Hong Kong on October 13, 2015. We operate our business through our operating subsidiaries in China, namely 1) Yangzhou Huada, a wholly
foreign owned subsidiary of Kang Fu International Medical, formed on December 24, 2001, located in Yangzhou, Jiangsu Province, PRC; 2)
Jiangsu Yada, a wholly owned subsidiary of Yangzhou Huada, formed on December 5, 1991, located in Yangzhou, Jiangsu Province, PRC; 3)
Jiangsu Huadong, a wholly owned subsidiary of Jiangsu Yada, formed on November 18, 2000, located in Yangzhou, Jiangsu Province, PRC,
4) Hainan Guoxie Technology Group Co., Ltd., or Hainan Guoxie, of which Kang Fu owns 55% shares, formed on October 7, 2021, located in
Qionghai City, Hainan Province, and 5) Hainan Ruiying Technology Co., Ltd., or Hainan Ruiying, of which Jiangsu Huadong owns 51% shares,
form on October 25, 2023, located in Hainan Free Trade Port Boao Hope City, Hainan Province.
Through our operating
subsidiaries in the PRC, Yangzhou Huada, Jiangsu Yada, Jiangsu Huadong, Hainan Guoxie, and Hainan Ruiying, we are mainly specialized
in the research, development, manufacturing and sales of Class I, Class II and Class III disposable medical devices both domestically
and internationally.
Pursuant to the Regulations
on the Supervision and Administration of Medical Devices promulgated on January 4, 2000, which is effective on June 1, 2014 and amended
by the State Council on May 4, 2017, medical devices are classified into the following three categories based on the degree of risk.
| Class |
|
Standard
(per PRC National Medical Device Management regulations) |
| I |
|
Class
I medical devices shall refer to those devices with low level of risks and whose safety and effectiveness can be ensured through
routine administration. |
| II |
|
Class
II medical devices shall refer to those devices with moderate risks that must be strictly controlled and regulated to ensure their
safety and effectiveness. |
| III |
|
Class
III medical devices shall refer to those devices with relatively high risks that must be strictly controlled and regulated through
special measures to ensure their safety and effectiveness. |
We provide our customers
with one-stop solution for a variety of safety and quality disposable medical devices. The safety and quality of disposable medical devices
are always our core values. We attribute our success to our sustainable and organic growth driven by our capacity expansion based on
market demand, our deep understanding of our target end markets and our sound relationships with our customers, distributors, independent
sales agents, and suppliers.
Our Revenue
Model
We generate revenues
through 1) manufacturing and sales of Class I, II, III disposable medical devices under our own brands, 2) resales of Class I, II, III
disposable medical devices sourced by us from other manufacturers. For the fiscal years ended December 31, 2022, 2021, and 2020, we recognized
$103,346,341, $104,037,710, and $89,061,010, respectively, in revenues, of which our own brand sales accounted for 48.88%, 46.19%, and
49.94%, respectively, and the resales of sourced disposable medical devices from other manufactures accounted for 51.12%, 53.81%, and
50.06%, respectively.
Our disposable medical
devices reach end users both domestically and internationally. For the fiscal years ended December 31, 2022, 2021, and 2020, our total
sales to domestic direct end users customers and domestic distributor customers accounted for 99.52%, 85.78%, and 81.90% of our revenues,
respectively. For the fiscal years ended December 31, 2022, 2021, and 2020, our sales to overseas distributing customers accounted for
0.48%, 14.22%, and 18.10%, respectively, of our revenues.
We sell disposable medical
devices through our direct sales force and distributors. For the fiscal years ended December 31, 2022, 2021, and 2020, our sales through
direct sale channels accounted for 9.16%, 9.13%, and10.59%, respectively, of our revenues, and our sales through distributors accounted
for 90.84%, 90.87%, and 89.41%, respectively, of our revenues, of which domestic distributors accounted for 90.36%, 76.65%, and 71.31%,
respectively, and exporting distributors accounted for 0.48%,14.22%, and 18.10%, respectively, of our revenues.
Our Products
Our products are sold
throughout the PRC. Internationally, our products are exported to more than 30 countries, including Europe, North America, South America,
Asia, Africa and Oceania.
Our current product
portfolio (consisting of both self-manufactured and out-sourced products) includes: 1) Class I disposable medical devices, such as, disposable
medical X-ray films, medical dry films, dry laser imagers, gauze bandages, examination gloves, pharmaceutical packaging materials and
containers, low-density polyethylene (LDPE) bottles for eye drops, high-density polyethylene (HDPE) bottles for tablets, etc.; 2) Class
II disposable medical devices, such as, disposable full anesthesia kits, medical brush, woman’s examination kits, urethral catheterization
kits, gynecological examination kits, endotracheal intubation, medical masks, anal bags, and suction connecting tube, etc.; and 3) Class
III disposable medical devices, such as disposable infusion pumps, anesthesia puncture kits, electronic pumps, etc.
As a medical device
manufacturing and sales company, we are subject to extensive government regulation and supervision in the PRC. Pursuant to PRC laws,
we must obtain production licenses for Class II and III disposable medical devices, an operation license for Class III disposable medical
devices, and filing or registration certificates for certain Class I, II or Class III disposable medical devices. As of the date of this
prospectus supplement, we are current on all licenses and certificates and have obtained Class I, II and III disposable medical device
qualifications in the PRC. In addition, we maintain a robust quality assurance system. We have received international “CE”
certification and ISO 13485 system certification. We have also registered with the FDA (registration number: 3006554788, 3007912861)
for more than 30 products as of the date hereof, including but not limited to ID bracelets, surgical tapes, elastic and adhesive bandages,
which are all FDA Class I products.
Our operating subsidiaries
in the PRC focus on the manufacturing and sales of disposable medical devices as follows:
Yangzhou Huada
Yangzhou Huada mainly
manufactures and sells Class I disposable medical devices, such as disposable pharmaceutical packaging materials and containers using
LDPE for eye drops and high-density polyethylene (“HDPE”) bottles for tablets, as well as disposable plastic baby bottles,
NB/PSN rubber covers and 8.2mL folded spoons for tools and containers, etc.
Additionally, Yangzhou
Huada is also engaged in the resales of Class I and II disposable medical devices sourced from other manufacturers when we provide one-stop
shopping experience to our customers.
Jiangsu Yada
Jiangsu Yada mainly
manufactures and sells both domestically and internationally 1) Class I disposable medical devices, such as medical dry imaging films;
and 2) Class II disposable medical devices, such as disposable woman’s examination kits, suction connecting tubes, and Class II
6866 medical polymer materials and products (including but not limited to transfusion equipment and pipelines, endotracheal intubation
for respiratory anesthesia or ventilation), etc.
In addition to above,
Jiangsu Yada is also engaged in the domestic and international resales of 1) Class I and Class II disposable medical devices sourced
from other manufacturers when we provide one-stop shopping experience to our customers.
Jiangsu Huadong
Jiangsu Huadong mainly
manufactures and sales both domestically and internationally 1) Class I medical devices, such as medical x-ray films, multi-functional
self-extracting X-ray film machines, dry films for medical use, gauze bandages, examination gloves, etc.; 2) Class II medical devices,
such as disposable full anesthesia kits, urethral catheterization kits, gynecological examination kits, endotracheal intubation, medical
masks, and various tubes, etc.; and 3) Class III medical devices, such as disposable infusion pumps, anesthesia puncture kits, electronic
pumps, etc.
In addition to above,
Jiangsu Huadong is also engaged in the domestic and international resales of Class I, II and III medical devices sourced from other manufacturers
when we provide one-stop shopping experience to our customers.
Hainan Guoxie
Leveraging the geographical
and policy advantages of Hainan Province, Hainan Guoxie aims to become a manufacturer of advanced and high-tech medical equipment in
Hainan Province. As of the date of this prospectus supplement, Hainan Guoxie is under construction.
Hainan Ruiying
Hainan Ruiying is a
trading and import-export company, aiming to introduce cutting-edge patented pharmaceuticals, medical technologies, and civilian household
equipment from renowned markets like the United States and Japan to China. The core focus of Hainan Ruiying will be in acting as a portal
and facilitating the introduction of new medical technology, devices, and equipment to the Chinese market, including state-of-the-art
medical devices like hearing aids, ultrasonic earwax cleaners, and advanced blood glucose meters. As of the date of this prospectus supplement,
the Company has not engaged in any business operations.
Our Holding Company
Structure
The Registered Notes
and Conversion Shares offered pursuant to this prospectus supplement are the securities of Meihua International, an exempted company
which was incorporated under the laws of the Cayman Islands on November 10, 2020 by our shareholder Yongjun Liu. Meihua’s direct
subsidiary is Kang Fu International Medical, a Hong Kong company. Kang Fu International Medical was incorporated on October 13, 2015
by four shareholders, Yongjun Liu, Yin Liu, Ace Capital Limited and King Tai International Holding Limited. On November 22, 2019, Yongjun
Liu acquired 9,300,000 shares in Kang Fu International Medical from Ace Capital Limited and 4,500,000 shares in Kang Fu International
Medical from King Tai International Holding Limited, respectively. Upon consummation of the share transfer, Yongjun Liu and Yin Liu constituted
all of the shareholders of Kang Fu International Medical, holding 100% shares of Kang Fu International Medical. On December 21, 2020,
Meihua in turn acquired 41,400,000 shares (69% of the outstanding shares) from Yongjun Liu and 18,600,000 shares (31% of the outstanding
shares) from Yin Liu, respectively, resulting in Kang Fu International Medical becoming Meihua International’s wholly owned
subsidiary. In exchange for the acquisition on Kang Fu, Meihua issued a total of 15,935,000 Ordinary Shares to Mr. and Mrs. Liu, who
in turn transferred their shares to Bright Accomplish Limited, a holding company for which they are the sole shareholders, on December
21, 2020. Bright Accomplish Limited is Meihua International’s controlling shareholder, holding approximately 63.54% of Meihua International’s
Ordinary Shares as of the date of this prospectus supplement.
Meihua International
is not a Chinese operating company, but a Cayman Islands holding company with operations conducted by its subsidiaries located in mainland
China. Meihua International operates its business through its indirect subsidiaries in China. Below is a list of Meihua International’s
operating subsidiaries:
| |
● |
Yangzhou Huada Medical
Device Co., Ltd., or Yangzhou Huada: a subsidiary wholly owned by Kang Fu International Medical and established in Yangzhou, Jiangsu
Province, PRC on December 24, 2001 with a registered capital of $602,400, which manufactures and sells Class I disposable medical
devices under our own brands, and distributes Class I and Class II disposable medical devices sourced from other manufacturers, to
our domestic customers. Specifically, Yangzhou Huada mainly focuses on the manufacturing, sales and distributions of non-bottled
products, such as brushes, ID bracelets for domestic sales. |
| |
● |
Jiangsu Yada Technology
Group Co., Ltd., or Jiangsu Yada: a subsidiary wholly owned by Yangzhou Huada and established in Yangzhou, Jiangsu Province, PRC
on December 5, 1991 with a registered capital of RMB51,390,000, which manufactures and sells Class I and Class II disposable medical
devices under our own brands, and distributes Class I and Class II disposable medical devices sourced from other manufacturers, to
our domestic and overseas customers. Specifically, Jiangsu Yada mainly focuses on overseas sales. |
| |
● |
Jiangsu Huadong Medical
Device Industrial Co., Ltd., or Jiangsu Huadong: a subsidiary wholly owned by Jiangsu Yada and established in Yangzhou, Jiangsu Province,
PRC on November 18, 2000 with a registered capital of RMB50,000,000, which manufactures and sells Class I, II and III disposable
medical devices under our own brands, and distributes Class I, II and III disposable medical devices sourced from other manufacturers,
to our domestic and overseas customers. Specifically, Jiangsu Huadong mainly focuses on the manufacturing, sales and distributions
of polyethylene bottled products, such as eye drop bottles and tablet bottles. |
| |
● |
Hainan Guoxie Technology
Group Co., Ltd., or Hainan Guoxie: a subsidiary of which 55% of registered capital (subscribed but unpaid registered capital) was
acquired by Kang Fu International Medical from an individual Qin Wang with nil consideration on July 6, 2022, in order to conduct
local business activities in Hainan. Hainan Guoxie was established in Qionghai, Hainan Province, China on October 7, 2021 with a
registered capital of RMB100,000,000. |
| |
● |
Hainan Ruiying Technology
Co., Ltd, or Hainan Ruiying: a subsidiary of which 51% of registered capital (subscribed but unpaid registered capital) is owned
by Jiangsu Huadong and established in Qionghai City, Hainan Province, China on October 25, 2023 with registered capital of RMB10,000,000
for purposes of serving as a trading and import-export company with a focus on facilitating the introduction of new medical technology,
devices and equipment. |
Meihua International
owns 100% of Kang Fu International Medical. Kang Fu International Medical owns 100% of Yangzhou Huada and 55% of Hainan Guoxie. Yangzhou
Huada owns 100% of Jiangsu Yada. Jiangsu Yada, in turn, owns 100% of Jiangsu Huadong. Jiangsu Huadong owns 51% of Hainan Ruiying. The
following diagram illustrates the Company’s corporate structure as of the date of this prospectus supplement, including Meihua
International’s principal subsidiary and their respective principal subsidiaries.

Key Information Related
to Doing Business in China
Risks and Uncertainties Related to Doing
Business in China
Meihua faces various legal and operational risks
and uncertainties as a company which its principal subsidiaries based in and primarily operating in China. Most of Meihua’s subsidiaries
operations are conducted in the PRC, and are governed by PRC laws, rules, and regulations. Because PRC laws, rules, and regulations are
relatively new and quickly evolving, and because of the limited number of published decisions and the non-precedential nature of these
decisions, and because the laws, rules and regulations often give the relevant regulator certain discretion in how to enforce them,
the interpretation and enforcement of these laws, rules and regulations involve uncertainties and can be inconsistent and unpredictable.
The PRC government has the power to exercise significant oversight and discretion over the conduct of our business, and the regulations
to which we are subject may change rapidly and with little notice to us or our shareholders. As a result, the application, interpretation,
and enforcement of new and existing laws and regulations in the PRC are often uncertain. In addition, these laws and regulations may
be interpreted and applied inconsistently by different agencies or authorities, and inconsistently with our current policies and practices.
See “Risk Factors — Risks Related
to Doing Business in China — Because all of our operations are in China, our business is subject to the complex and rapidly
evolving laws and regulations there. The Chinese government may exercise significant oversight and discretion over the conduct of our
business and may intervene in or influence our operations at any time, which could result in a material change in our operations and/or
the value of our Ordinary Shares,” and “— PRC’s economic, political and social conditions, as well
as changes in any government policies, laws and regulations, could have a material adverse effect on our business,” as set
forth at page 39 of this prospectus.
The PRC government has significant oversight
and discretion over the conduct of our business, and may intervene in or influence our operations through adopting and enforcing rules and
regulatory requirements. For example, in recent years the PRC government, has enhanced regulation in areas such as anti-monopoly, anti-unfair
competition, cybersecurity and data privacy. See “Risk Factors — Risks Related to Our Business and Industry —
If the Chinese government chooses to exert more oversight and control over offerings that are conducted overseas and/or foreign investment
in China-based issuers, such action could significantly limit or completely hinder our ability to offer or continue to offer securities
to investors outside of China and, as a result, cause the value of such securities to significantly decline or be worthless”;
and “— Uncertainties with respect to the PRC legal system could adversely affect us,” as set forth at page
39 of this prospectus.
In addition, our Ordinary Shares may be delisted
from Nasdaq or prohibited from being traded over-the-counter under the Holding Foreign Companies Accountable Act if the PCAOB is unable
to inspect our auditor for two consecutive years. Our auditor has been inspected by the PCAOB on a regular basis and it is not subject
to the determinations announced by the PCAOB on December 16, 2021. If trading in our Ordinary Shares is prohibited under the Holding
Foreign Companies Accountable Act in the future because the PCAOB determines that it cannot inspect or fully investigate our auditor
at such future time, the Nasdaq Stock Market may determine to delist our Ordinary Shares. On June 22, 2021, the U.S. Senate passed the
Accelerating Holding Foreign Companies Accountable Act and on December 29, 2022, a legislation entitled “Consolidated Appropriations
Act, 2023” (the “Consolidated Appropriations Act”) was signed into law by President Biden, which contained, among other
things, an identical provision to Accelerating Holding Foreign Companies Accountable Act and reduces the number of consecutive non-inspection
years required for triggering the listing and trading prohibitions from three years to two years,, thus reducing the time period before
our securities may be prohibited from trading or delisted. On December 15, 2022, the PCAOB Board determined that the PCAOB was able to
secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong and
voted to vacate its previous determinations to the contrary. However, should PRC authorities obstruct or otherwise fail to facilitate
the PCAOB’s access in the future, the PCAOB Board will consider the need to issue a new determination. Our securities may be delisted
or prohibited from trading if the PCAOB determines that it cannot inspect or investigate completely our auditor under the Holding Foreign
Companies Accountable Act. Our auditor, Kreit & Chiu CPA LLP, is headquartered in New York, NY with its office at 733 Third Avenue,
Floor 16, #1014, New York, NY 10017, and has been inspected by the PCAOB on a regular basis. See “Risk Factors — Risks Related
to Doing Business in China — Although the audit report included in this report was issued by U.S. auditors who are currently
inspected by the PCAOB, if it is later determined that the PCAOB is unable to inspect or investigate our auditor completely, investors
would be deprived of the benefits of such inspection and our Ordinary Shares may be delisted or prohibited from trading,” as
set forth at page 41 of this prospectus.
Permissions and Approvals Required to be Obtained from PRC Authorities
for our Business Operations
As a medical device manufacturing and sales company,
we are subject to extensive government regulation and supervision in the PRC. Pursuant to PRC laws, we must obtain production license
for Class II and III disposable medical devices and production filing for Class I disposable medical device, operation license for Class
III disposable medical devices and operation filing for Class II disposable medical devices, and filing or registration certificates
for certain Class I, II or Class III disposable medical devices. As of the date of this prospectus supplement, we are current on all
licenses and certificates and have obtained Class I, II and III disposable medical device qualifications in the PRC.
However, if we fail to timely renew our medical
device licenses or registration certificates, it could adversely affect our reputation, financial conditions and results of operations.
See “Risk Factors — Risks Related to Our Business and Industry — If we fail to timely renew our medical device
licenses or registration certificates, it could adversely affect our reputation, financial conditions and results of operations,”
as set forth at page 34 of this prospectus.
Permissions and Approvals Required to be Obtained from PRC Authorities
for our Securities Offerings
As of the date of this prospectus supplement,
we believe that we and our PRC subsidiaries, (1) are not required to obtain permissions from any PRC authorities to operate or issue
our Ordinary Shares to foreign investors, (2) are not subject to permission requirements from the China Securities Regulatory Commission
(the “CSRC”), the Cyberspace Administration of China (the “CAC”) or any other entity that is required to approve
our PRC subsidiaries’ operations, and (3) have not received or were denied such permissions by any PRC authorities. Nevertheless,
the General Office of the Central Committee of the Communist Party of China and the General Office of the State Council jointly issued
the “Opinions on Severely Cracking Down on Illegal Securities Activities According to Law,” or the Opinions, which were made
available to the public on July 6, 2021. The Opinions emphasized the need to strengthen the administration over illegal securities activities,
and the need to strengthen the supervision over overseas listings by Chinese companies. Given the current PRC regulatory environment,
it is uncertain when and whether we or our PRC subsidiaries, will be required to obtain permission from the PRC government to continue
to list on U.S. exchanges in the future, and even when such permission is obtained, whether it will be denied or rescinded. We have been
closely monitoring regulatory developments in China regarding any necessary approvals from the CSRC or other PRC governmental authorities
required for overseas listings. As of the date of this report, we have not received any inquiry, notice, warning, sanctions or regulatory
objection to this offering from the CSRC or other PRC governmental authorities. However, there remains significant uncertainty as to
the enactment, interpretation and implementation of regulatory requirements related to overseas securities offerings and other capital
markets activities.
On February 17, 2023, the CSRC promulgated Trial
Administrative Measures of the Overseas Securities Offering and Listing by Domestic Companies, or the Trial Measures, and relevant five
supporting guidelines, together as the New Overseas Listing Rules, which became effective on March 31, 2023. According to the New Overseas
Listing Rules, PRC domestic companies that seek to offer and list securities in overseas markets, either in direct or indirect means,
are required to complete the filing procedure with the CSRC and report relevant information. In addition, an overseas-listed company
must also submit the filing with respect to its follow-on offerings, issuance of convertible corporate bonds and exchangeable bonds,
and other equivalent offering activities, within the time frame specified by the Trial Measures. The New Overseas Listing Rules laid
out the regulatory filing requirements for both direct and indirect overseas listings and clarify the determination criteria for indirect
overseas listing in overseas markets. For more detailed information, see “Item 3. Key Information—D. Risk Factors—Risks
Related to Doing Business in China—Potential Chinese governmental and regulatory interference could significantly limit or completely
hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline
or be worthless.” Pursuant to the New Overseas Listing Rules, (i) in connection with our previous issuance of securities to foreign
investors, neither we, nor our PRC subsidiaries are required to obtain any permissions or approvals from the CSRC, and (ii) should we
decide to issue additional equity or equity-linked securities for listing overseas in the future, we are not required to obtain any permissions
or approvals from any PRC government authorities, except for the requisite filing with the CSRC in connection with such issuance. Failure
to comply with the filing requirements may result in an order of rectification, a warning and fines up to RMB10 million to the non-compliant
domestic companies, and the directly responsible persons of the companies will be warned and fined between RMB500,000 and RMB5 million.
Furthermore, if the controlling shareholder and the actual controller of the non-compliant companies organizes or instigates the breach,
they will be fined between RMB1 million and RMB10 million. In addition to above filing requirements, the Filings Rules also requires
an issuer to report to the CSRC within three business days after occurrence of any the following events: (i) its change of control; (ii)
its being subject to investigation or sanctions by any overseas securities regulators or overseas authorities; (iii) its change of listing
status or listing segment; (iv) voluntary or mandatory delisting; and (v) material change of its principal business operations to the
extent that it ceases to be subject to the filing requirements of the Trial Measures.
Securities Purchase Agreement with the Selling
Shareholders
On December 27, 2023, the Company entered
into a securities purchase agreement (the “SPA”) with Anson Investment Master Fund LP and Anson East Master Fund LP
(together, the “Investors”), pursuant to which the Company agreed to issue, from time to time, up to $50,500,0000 in the
Company’s securities (the “Offering”), consisting of convertible notes, issuable at a 7.0% original issue discount
(the “Convertible Notes”), and accompanying ordinary share purchase warrants (the “Warrants”) with five-year
terms and exercisable for a number of the Company’s ordinary shares, par value $0.0005 per share (the “Ordinary Shares”),
equal to 50% of the number obtained from dividing each Convertible Note’s principal amount by the applicable VWAP (as defined in
the SPA), subject to adjustment pursuant and a 4.99% beneficial ownership limitation. Pursuant to the SPA, the Company agreed to issue
to the Investors at the initial closing of the Offering (the “First Closing”) $6,000,000 in Notes, convertible at the lower
of (i) $2.738 per share (or 110% of the VWAP of the Ordinary Shares on December 27, 2023) or (ii) a price per share equal to 95% of the
lowest VWAP of the Ordinary Shares during the seven (7)-trading day period immediately preceding the applicable conversion date, subject
to certain adjustments and a 4.99% beneficial ownership limitation, and Warrants exercisable for up to an aggregate of 1,205,255 ordinary
shares, at an exercise price of $2.9869 per share (or 120% of the VWAP of the Ordinary Shares on December 27, 2023). The Convertible
Notes do not bear interest except upon the occurrence of an event of default thereunder, have 364-day maturity dates, must be redeemed
by the Company at a premium in the event of (i) a Subsequent Financing (as defined in the SPA), (ii) a Change of Control (as defined
in the SPA) and (iii) certain equity conditions listed therein. The Company also has the option to redeem the Notes in the event that
the Company deems it in its best interest to do so, such as if it believes an event of default under the Notes is imminent. The Notes
contain certain other covenants and events of default customary for similar transactions.
The First Closing occurred on January
2, 2024. After the First Closing, and subject to the satisfaction of certain additional conditions, including an Investor holding an
outstanding Convertible Note with a principal amount below $500,000, additional tranches of funding may occur pursuant to the SPA (each,
an “Additional Closing”). In conjunction with each Additional Closing, Investors will receive an additional Convertible Note
containing substantially the same terms as the initial Convertible Notes issued, convertible into Ordinary Shares at 110% of the VWAP
of the Ordinary Shares on the trading day immediately preceding such Additional Closing and subject to adjustment, and a Warrant exercisable
for Ordinary Shares equal to 50% of the number obtained from dividing the principal amount of the Note by the VWAP on the trading day
immediately prior to such Additional Closing, and such Warrant will be exercisable for 120% of the VWAP of the Ordinary Shares on the
trading day immediately preceding such Additional Closing.
In
addition, pursuant to the SPA and subject to certain exceptions, the Company (i) granted the Investors the right to participate in a
Subsequent Financing (as defined in the SPA) until 12 months from the date on which no Convertible Notes or Warrants are outstanding,
(ii) agreed not to issue or announce the issuance or proposed issuance of any Ordinary Shares or Ordinary Share Equivalents (as defined
in the SPA) or file any registration statement with respect thereto during (x) the 60-day period beginning on the date of the SPA and
(y) the 60-day period commencing on each Additional Closing and (iii) agreed not to enter into a Variable Rate Transaction (as defined
in the SPA), or conduct a dilutive issuance (unless Investor approval is received) until such time that no Convertible Notes or Warrants
are outstanding. In the event of a Subsequent Financing, the Investor has the right to demand the Company use 30% of such proceeds to
repay their outstanding Convertible Notes at a 105% premium and on a pro rata basis. Additionally, the Company agreed not to effect a
reverse split or reclassification of its capital stock within 12 months from the date of the SPA without the Investors’ prior consent,
and subject to certain exceptions, and not to incur any additional indebtedness over $500,000 without the Investors’
prior consent, which consent is not to be unreasonably withheld. The SPA contains certain other representations and warranties,
covenants and indemnities customary for similar transactions.1
The Convertible Note and the Ordinary
Shares underlying the Notes are being issued pursuant to an effective shelf registration statement on Form F-3, as amended (File No.
333-27419), initially filed with the U.S. Securities and Exchange Commission (“SEC”) on August 24, 2023 and declared effective
on September 29, 2023. On January 2, 2024, the Company filed a prospectus supplement pursuant to Rule 424(b)(5) of the Securities Act,
registering the Convertible Notes issued at the First Closing and the Ordinary Shares underlying such Convertible Notes.
Registration Rights Agreement
Concurrently with the entry into the SPA and
pursuant to its terms, the Company entered into a registration rights agreement (the “RRA”) with the Investors, pursuant
to which the Company agreed to register the resale of the Ordinary Shares issuable upon exercise of the Warrants issued at the First
Closing (the “Warrant Shares”) on a registration statement on Form F-3 or Form F-1, in the event Form F-3 is not available
then available to the Company, (the “Registration Statement”) with the SEC. In addition, in accordance with the terms of
the RRA, the Company will be obligated to register any other Notes, Warrants and Ordinary Shares underlying such future issuances of
Notes or Warrants that may be sold and issued to the Investors in accordance with the terms of the SPA.
The Company agreed to use its best efforts to
cause the Registration Statement registering the Warrant Shares to be declared effective by the SEC within sixty (60) calendar days (or,
in the event of a “full” review by the SEC, within 90 calendar days) after the date of the RRA, and to use its best efforts
to keep such Registration Statement continuously effective until the earliest of (i) the date on which the securities may be resold by
the Selling Stockholders (as defined therein) without registration and without regard to any volume or manner-of-sale limitations by
reason of Rule 144 of the Securities Act (“Rule 144”), without the requirement for the Company to be in compliance with the
current public information under Rule 144 or any other rule of similar effect or (ii) the date on which all of the securities have been
sold pursuant to this prospectus or Rule 144 or any other rule of similar effect.
Placement Agent
On December 27, 2023, the Company also entered
into a placement agent agreement (the “PAA”) with Maxim Group LLC (“Maxim” or “Placement Agent”),
to engage Maxim as its exclusive Placement Agent for the Offering. Maxim acted as the sole Placement Agent for the Offering. At closing
of the Offering, on January 2, 2024, in accordance with the terms of the PAA, the Company paid the Placement Agent an aggregate cash
fee equal to 7.0% of the gross proceeds raised in the Offering, plus up to $50,000 for all travel and other out-of-pocket expenses, including
the reasonable and accounted fees and expenses of legal counsel to the Placement Agent.
Summary of Risk Factors
Investing in our securities involves significant
risks. Before making any investment in our securities, in addition to reviewing the summary risk factors set forth below, you should
carefully consider all of the information in our 2022 Annual Report, any amendment or update thereto reflected in subsequent filings
with the SEC, including in our annual reports on Form 20-F, any amendments thereto, and all other information contained in this prospectus,
and as updated by our subsequent filings under the Exchange Act. These risks are discussed more fully under “Item 3. Key Information—D.
Risk Factors” in our 2022 Annual Report and in the section titled “Risk Factors” beginning on page 19 of this prospectus.
Risks Related to Our Business and Industry
(for a more detailed discussion of the summary risk factors below, see “Risk Factors – Risks Related to Our Business and
Industry” beginning at page 24 below)
Risks and uncertainties related to our business
include, but are not limited to, the following:
| |
● |
in connection with the
initial public offering of our Ordinary Shares in February 2022, we entered into certain agreements with a Hong Kong entity pursuant
to which we believe we were defrauded of in excess of $10 million; because we failed to disclose such agreements at the time of the
initial public offering, regardless of the arguably fraudulent nature of such agreements, we may be subject to potential liabilities
or litigation exposure as a result of such agreements; |
| |
● |
our operating history may
not be indicative of our future growth or financial results and we may not be able to sustain our historical growth rates; |
| |
● |
any failure to maintain
effective quality control over our products and services could materially adversely affect our business; |
| |
● |
due to the nature of our
business, we may experience or be exposed to significant liability claims or complaints from customers, doctors, patients or hospitals,
litigation and regulatory investigations and proceedings, such as claims arising in relation to medical device safety, or adverse
publicity involving our products, which could adversely affect our financial condition and results of operations; |
| |
● |
a significant interruption
in the operations of our third-party suppliers and other business partners could potentially disrupt our operations; |
| |
● |
while we utilize numerous
suppliers, we do not have long term contracts with any of those suppliers and they could reduce order quantities or terminate their
sales to us at any time; |
| |
● |
we are dependent on our
top customers. If we fail to acquire new customers or retain existing customers in a cost-effective manner, our business, financial
condition and results of operations may be materially and adversely affected; |
| |
● |
if we fail to provide a
one-stop solution to our customers, we may lose customers, which would cause our financial conditions and results of operations to
be adversely affected; |
| |
● |
the continuing development
of our products depends upon our maintaining strong working relationships with our customers, distributors and independent sales
agents; |
| |
● |
if we are unable to collect
account receivables from our customers, our results of operations and cash flows could be adversely affected; |
| |
● |
we may be subject to intellectual
property infringement claims, which may be expensive to defend and may disrupt our business and operations; |
| |
● |
changes in U.S. and international
trade policies, particularly with regard to China, may adversely impact our business and operating results; |
| |
● |
we have no business liability
or disruption insurance, which could expose us to significant costs and business disruption; |
| |
● |
pandemics and epidemics,
natural disasters, terrorist activities, political unrest, and other outbreaks could disrupt our delivery and operations, which could
materially and adversely affect our business, financial condition, and results of operations; |
| |
● |
failure to keep up with
the changes in domestic industry policies or standards could have a material and adverse effect on our reputation, financial condition
and results of operations; |
| |
● |
if we fail to timely renew
our medical device licenses or registration certificates, it could adversely affect our reputation, financial conditions and results
of operations; |
Risks Related to Our Corporate Structure
(for a more detailed discussion of the summary risk factors below, see “Risk Factors—Risks Related to Our Corporate Structure”
beginning at page 34 below)
We
are also subject to risks and uncertainties related to our corporate structure, including, but not limited to, the following:
| |
● |
our directors and officers
currently own an aggregate of 63.54% of the total voting power of our outstanding Ordinary Shares; |
| |
● |
you may face difficulties
in protecting your interests, and your ability to protect your rights through U.S. courts may be limited because we are incorporated
under Cayman Islands law; |
| |
● |
recently introduced economic
substance legislation of the Cayman Islands may impact the Company or its operations; |
Risks Related to Doing Business in China
(for a more detailed discussion of the summary risk factors set forth below, see “Risk Factors—Risks Related to Doing Business
in China” beginning at page 36 below)
We
face certain risks and uncertainties related to doing business in China in general, including, but not limited to, the following:
| |
● |
because all of our operations
are in China, our business is subject to the complex and rapidly evolving laws and regulations there. The Chinese government may
exercise significant oversight and discretion over the conduct of our business and may intervene in or influence our operations at
any time, which could result in a material change in our operations and/or the value of our Ordinary Shares; |
| |
● |
if the Chinese government
chooses to exert more oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers,
such action could significantly limit or completely hinder our ability to offer or continue to offer securities to investors outside
of China and, as a result, cause the value of such securities to significantly decline or be worthless; |
| |
● |
potential Chinese governmental
and regulatory interference could significantly limit or completely hinder our ability to offer or continue to offer securities to
investors and cause the value of such securities to significantly decline or be worthless; |
| |
● |
changes in China’s
economic, political or social conditions or government policies could have a material adverse effect on our business and operations; |
| |
● |
we may rely on dividends
and other distributions on equity paid by our PRC subsidiaries to fund any cash and financing requirements we may have, and any limitation
on the ability of our PRC subsidiaries to make payments to us could have a material and adverse effect on our ability to conduct
our business; |
| |
● |
PRC regulation of loans
to and direct investment in PRC entities by offshore holding companies and governmental control of currency conversion may delay
us from using the proceeds of future securities offerings to make loans or additional capital contributions to our PRC subsidiaries,
which could materially and adversely affect our liquidity and our ability to fund and expand our business; |
| |
● |
although the audit report
included in the 2022 Annual Report was issued by U.S. auditors who are currently subject to inspection by the PCAOB, if it is later
determined that the PCAOB is unable to inspect or investigate our auditor completely, investors would be deprived of the benefits
of such inspection and our Ordinary Shares may be delisted or prohibited from trading; |
| |
● |
any failure to comply with
PRC regulations regarding cybersecurity and data protection may subject us to fines and other legal or administrative sanctions,
claims or legal proceedings; |
| |
● |
U.S. regulatory bodies
may be limited in their ability to conduct investigations or inspections of our operations in the PRC; |
| |
● |
if we are classified as
a PRC resident enterprise for PRC income tax purposes, such classification could result in unfavorable tax consequences to us and
our non-PRC shareholders; |
| |
● |
we face uncertainty with
respect to indirect transfers of equity interests in PRC resident enterprises by their non-PRC holding companies; |
Risks Related to Our Ordinary Shares (for
a more detailed discussion of the summary risk factors set forth below, see “Risks Related to Our Ordinary Shares” beginning
at page 48 below)
In
addition to the risks described above, we are subject to general risks and uncertainties related to our Ordinary Shares and the trading
market, including, but not limited to, the following:
| |
● |
the market price for our
Ordinary Shares may be volatile |
| |
● |
because
we do not expect to pay dividends in the foreseeable future, you must rely on price appreciation of our Ordinary Shares in order
to obtain return on your investment; |
| |
● |
substantial future sales
or perceived potential sales of Ordinary Shares in the public market could cause the price of our Ordinary Shares to decline; |
| |
● |
we may need additional
capital and may sell additional Ordinary Shares or other equity securities or incur indebtedness, which could result in additional
dilution to our shareholders or increase our debt service obligations; |
| |
● |
certain existing shareholders
have substantial influence over our company and their interests may not be aligned with the interests of our other shareholders; |
| |
● |
we are an emerging growth
company within the meaning of the Securities Act and may take advantage of certain reduced reporting requirements; and |
| |
● |
we may lose our foreign
private issuer status in the future, which could result in significant additional costs and expenses. |
Cash Flows
Through Our Company and Dividend Payments
The
structure of cash flows within our organization, and a summary of the applicable regulations, is as follows:
| 1. |
Our equity structure is
a direct holding structure, pursuant to which the overseas entity listed in the U.S., Meihua International Medical Technologies Co.,
Ltd., directly controls Yangzhou Huada and other domestic operating entities which are directly owned through the Hong Kong company,
Kang Fu. |
| 2. |
Within our direct holding
structure, the cross-border transfer of funds within our corporate group is legal and compliant with the laws and regulations of
the PRC. After foreign investors’ funds enter Meihua International at the close of securities offerings, the funds can be directly
transferred to Kang Fu, and then transferred to subordinate operating entities through the WFOE. |
As
of the date of this prospectus supplement, we have not made any dividends or distributions to any U.S. investors. While we don’t
presently intend to issue dividends to our shareholders, if the Company distributes dividends to our shareholders in the future, we will
transfer the dividends to Kang Fu in accordance with the laws and regulations of the PRC, and then Kang Fu will transfer the dividends
to Meihua International and the dividends will then be distributed from Meihua International to all shareholders respectively in proportion
to the shares they hold, regardless of whether the shareholders are U.S. investors or investors in other countries or regions.
| 3. |
In the reporting periods
presented in for the year ended December 31, 2022, no cash and other asset transfers occurred among the Company and its subsidiaries
other than as summarized as below: |
| |
|
For
the year ended December 31, 2022
(in US$) |
|
| Inter-company
cash transfers: |
|
Meihua
(Cayman) |
|
|
Kang
Fu
International
Medical
(HK) |
|
|
PRC
subsidiaries |
|
| Cash transferred from Meihua to
Kang Fu International Medical (1) |
|
$ |
(26,010,150 |
) |
|
$ |
26,010,150 |
|
|
|
- |
|
| Cash transferred from Kang Fu International
Medical to Meihua (2) |
|
$ |
390 |
|
|
$ |
(390 |
) |
|
|
- |
|
| Cash transferred from Kang Fu International
Medical to PRC subsidiaries (3) |
|
|
- |
|
|
$ |
(20,389,970 |
) |
|
$ |
20,389,970 |
|
| Cash transferred from PRC subsidiaries to Kang Fu
International Medical (4) |
|
|
- |
|
|
$ |
130,000 |
|
|
$ |
(130,000 |
) |
| (1) |
Meihua transferred $26,010,150 to Kang Fu International
Medical as a working capital loan. |
| (2) |
Kang Fu International Medical transferred $390 to Meihua
for repayment of a working capital loan. |
| (3) |
Kang Fu International Medical contributed $20,389,970
to PRC subsidiaries as a capital contribution.* |
| (4) |
Yangzhou Huada, one of the PRC subsidiaries, transferred
$130,000 to Kang Fu International Medical as a working capital loan. |
| * |
On February 18, 2022, the
Company closed its initial public offering of Ordinary Shares and received approximately US$35 million. In March and April 2022,
the Company transferred approximately US$26.0 million to Kang Fu International Medical for working capital purpose then Kang Fu International
Medical made capital injection in aggregated of approximately US$20.4 million to PRC subsidiaries - Yangzhou Huada and Hainan Guoxie. |
| |
|
For
the year ended December 31, 2021
(in US$) |
|
| Inter-company
cash transfers: |
|
Meihua
(Cayman) |
|
|
Kang
Fu
International
Medical
(HK) |
|
|
PRC
subsidiaries |
|
| Cash transferred from Kang Fu International
Medical to PRC subsidiaries (5) |
|
|
- |
|
|
$ |
(46,297 |
) |
|
$ |
46,297 |
|
| Cash transferred from PRC subsidiaries to Kang
Fu International Medical (6) |
|
|
- |
|
|
$ |
768,042 |
|
|
$ |
(768,042 |
) |
| (5) |
Kang Fu International Medical transferred $46,297 to
PRC subsidiaries for repayment of a working capital loan. |
| (6) |
Yangzhou Huada, one of the PRC subsidiaries, transferred
$768,042 to Kang Fu International Medical as a working capital loan. |
| |
|
For
the year ended December 31, 2020
(in US$) |
|
| Inter-company
cash transfers: |
|
Meihua
(Cayman) |
|
|
Kang
Fu
International
Medical
(HK) |
|
|
PRC
subsidiaries |
|
| Cash transferred from PRC subsidiaries
to Kang Fu International Medical (7) |
|
|
- |
|
|
$ |
499,998 |
|
|
$ |
(499,998 |
) |
| (7) |
Yangzhou Huada, one of
the PRC subsidiaries, transferred $499,998 to Kang Fu International Medical as a working capital loan. |
| 4. |
No dividends or distributions
of a subsidiary have been made to the Company for the years ended December 31, 2020, 2021 and 2022. For the foreseeable future, the
Company intends to use the earnings for research and development, to develop new products and to expand its production capacity.
As a result, we do not expect to pay any cash dividends for the near term. |
Our
PRC subsidiaries’ ability to distribute dividends is based upon their distributable earnings. Current PRC regulations permit our
PRC subsidiaries to pay dividends to their respective shareholders only out of their accumulated profits, if any, determined in accordance
with PRC accounting standards and regulations. In addition, each of our PRC subsidiaries is required to set aside at least 10% of its
after-tax profits each year, if any, to fund a statutory reserve until such reserve reaches 50% of each of their registered capital.
These reserves are not distributable as cash dividends.
According
to the PRC Company Law and Foreign Investment Law, each of our PRC subsidiaries, as a foreign invested enterprise, or FIE, are required
to draw 10% of its after-tax profits each year, if any, to fund a common reserve, which may stop drawing its after-tax profits if the
aggregate balance of the common reserve has already accounted for over 50% of its registered capital. These reserves are not distributable
as cash dividends. Furthermore, under the EIT Law, which became effective in January 2008, the maximum tax rate for the withholding tax
imposed on dividend payments from PRC foreign invested companies to their overseas investors that are not regarded as “resident”
for tax purposes is 20%. The rate was reduced to 10% under the Implementing Regulations for the EIT Law issued by the State Council.
However, a lower withholding tax rate might be applied if there is a tax treaty between China and the jurisdiction of the foreign holding
companies, such as tax rate of 5% in the case of Hong Kong companies that holds at least 25% of the equity interests in the foreign-invested
enterprise, and certain requirements specified by PRC tax authorities are satisfied.
To
address persistent capital outflows and the RMB’s depreciation against the U.S. dollar in the fourth quarter of 2016, the People’s
Bank of China and the State Administration of Foreign Exchange, or SAFE, implemented a series of capital control measures in the subsequent
months, including stricter vetting procedures for China-based companies to remit foreign currency for overseas acquisitions, dividend
payments and shareholder loan repayments. The PRC government may continue to strengthen its capital controls and our PRC subsidiaries’
dividends and other distributions may be subject to tightened scrutiny in the future. The PRC government also imposes controls on the
conversion of RMB into foreign currencies and the remittance of currencies out of the PRC. Therefore, should we wish to distribute dividends
to our shareholders, we may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign
currency for the payment of dividends from our profits, if any. Furthermore, if our subsidiaries in the PRC incur debt on their own in
the future, the instruments governing such debt may restrict their ability to pay dividends or make other payments.
In
addition, the Enterprise Income Tax Law and its implementation rules provide that a withholding tax at a rate of 10% will be applicable
to dividends payable by Chinese companies to non-PRC-resident enterprises unless reduced under treaties or arrangements between the PRC
central government and the governments of other countries or regions where the non-PRC resident enterprises are tax resident. Pursuant
to the tax agreement between Mainland China and the Hong Kong Special Administrative Region, the withholding tax rate in respect to the
payment of dividends by a PRC enterprise to a Hong Kong enterprise may be reduced to 5% from a standard rate of 10%. However, if the
relevant tax authorities determine that our transactions or arrangements are for the primary purpose of enjoying a favorable tax treatment,
the relevant tax authorities may adjust the favorable withholding tax in the future. Accordingly, there is no assurance that the reduced
5% withholding rate will apply to dividends received by our Hong Kong subsidiary from our PRC subsidiaries. This withholding tax will
reduce the amount of dividends we may receive from our PRC subsidiaries.
Selected
Condensed Consolidating Financial Schedule
In
the tables below, we provide you with historical selected financial data for the years ended December 31, 2022 and 2021, which
have been derived from our consolidated financial statements for those years. Historical results are not necessarily indicative
of the results that may be expected for any future period.
Condensed
Consolidating Schedule — Statement of Operations
| | |
For
the Year Ended December 31, 2022 | |
| | |
Meihua
(Cayman) | | |
Kang
Fu
International
Medical
(HK) | | |
PRC
subsidiaries | | |
Eliminations | | |
Consolidated
Total | |
| Revenues | |
$ | - | | |
$ | - | | |
$ | 103,346,341 | | |
$ | - | | |
$ | 103,346,341 | |
| Cost | |
$ | - | | |
$ | - | | |
$ | 65,247,864 | | |
$ | - | | |
$ | 65,247,864 | |
| Gross
profit | |
$ | - | | |
$ | - | | |
$ | 38,098,477 | | |
$ | - | | |
$ | 38,098,477 | |
| Income
(loss) from operations | |
$ | (2,757,913 | ) | |
$ | (4,046,265 | ) | |
$ | 17,606,140 | | |
$ | - | | |
$ | 10,801,962 | |
| Income
before consolidation | |
$ | 8,994,612 | | |
$ | 13,481,369 | | |
$ | - | | |
$ | (22,475,981 | ) | |
$ | - | |
| Net
income | |
$ | 6,242,969 | | |
$ | 8,994,611 | | |
$ | 13,416,105 | | |
$ | (22,475,981 | ) | |
$ | 6,177,704 | |
| | |
For
the Year Ended December 31, 2021 | |
| | |
Meihua
(Cayman) | | |
Kang
Fu
International
Medical
(HK) | | |
PRC
subsidiaries | | |
Eliminations | | |
Consolidated
Total | |
| Revenues | |
$ | - | | |
$ | - | | |
$ | 104,037,710 | | |
$ | - | | |
$ | 104,037,710 | |
| Cost | |
$ | - | | |
$ | - | | |
$ | 64,232,469 | | |
$ | - | | |
$ | 64,232,469 | |
| Gross
profit | |
$ | - | | |
$ | - | | |
$ | 39,805,241 | | |
$ | - | | |
$ | 39,805,241 | |
| Income
(loss) from operations | |
$ | (99,738 | ) | |
$ | (16,387 | ) | |
$ | 26,377,079 | | |
$ | - | | |
$ | 26,260,954 | |
| Income
before consolidation | |
$ | 21,049,317 | | |
$ | 19,812,052 | | |
$ | - | | |
$ | (40,861,369 | ) | |
$ | - | |
| Net
income | |
$ | 20,949,579 | | |
$ | 21,049,317 | | |
$ | 19,812,052 | | |
$ | (40,861,369 | ) | |
$ | 20,949,579 | |
Condensed
Consolidating Schedule — Balance Sheet
| | |
As
of December 31, 2022 | |
| | |
Meihua
(Cayman) | | |
Kang
Fu
International
Medical
(HK) | | |
PRC
subsidiaries | | |
Eliminations | | |
Consolidated
Total | |
| Cash
and restricted cash | |
$ | 6,038,630 | | |
$ | 1,611,955 | | |
$ | 19,086,115 | | |
$ | - | | |
$ | 26,736,700 | |
| Total
current assets | |
$ | 33,625,172 | | |
$ | 52,461,542 | | |
$ | 120,694,223 | | |
$ | (71,461,782 | ) | |
$ | 135,319,155 | |
| Investments
in subsidiaries | |
$ | 104,497,765 | | |
$ | 108,984,522 | | |
$ | - | | |
$ | (213,482,287 | ) | |
$ | - | |
| Total
non-current assets | |
$ | 104,497,765 | | |
$ | 108,984,522 | | |
$ | 28,258,424 | | |
$ | (213,482,287 | ) | |
$ | 28,258,424 | |
| Total
assets | |
$ | 138,122,937 | | |
$ | 161,446,064 | | |
$ | 148,952,647 | | |
$ | (284,944,069 | ) | |
$ | 163,577,579 | |
| Total
liabilities | |
$ | - | | |
$ | 1,274 | | |
$ | 24,897,225 | | |
$ | - | | |
$ | 24,898,499 | |
| Total
shareholders’ equity | |
$ | 138,122,937 | | |
$ | 161,444,790 | | |
$ | 124,055,422 | | |
$ | (284,944,069 | ) | |
$ | 138,679,080 | |
| Total
liabilities and shareholders’ equity | |
$ | 138,122,937 | | |
$ | 161,446,064 | | |
$ | 148,952,647 | | |
$ | (284,944,069 | ) | |
$ | 163,577,579 | |
| |
|
As
of December 31, 2021 |
|
| |
|
Meihua
(Cayman) |
|
|
Kang
Fu
International Medical
(HK) |
|
|
PRC
subsidiaries |
|
|
Eliminations |
|
|
Consolidated
Total |
|
| Cash
and restricted cash |
|
$ |
- |
|
|
$ |
129,037 |
|
|
$ |
8,020,239 |
|
|
$ |
- |
|
|
$ |
8,149,276 |
|
| Total
current assets |
|
$ |
1,616,971 |
|
|
$ |
(5,839,105 |
) |
|
$ |
88,203,305 |
|
|
$ |
13,295,179 |
|
|
$ |
97,276,350 |
|
| Investments
in subsidiaries |
|
$ |
106,251,369 |
|
|
$ |
106,092,745 |
|
|
$ |
- |
|
|
$ |
(212,344,114 |
) |
|
$ |
- |
|
| Total
non-current assets |
|
$ |
106,251,369 |
|
|
$ |
106,092,745 |
|
|
$ |
30,776,439 |
|
|
$ |
(203,539,522 |
) |
|
$ |
39,581,031 |
|
| Total
assets |
|
$ |
107,868,340 |
|
|
$ |
100,253,640 |
|
|
$ |
118,979,744 |
|
|
$ |
(190,244,343 |
) |
|
$ |
136,857,381 |
|
| Total
liabilities |
|
$ |
99,738 |
|
|
$ |
443,265 |
|
|
$ |
28,545,776 |
|
|
$ |
- |
|
|
$ |
29,088,779 |
|
| Total
shareholders’ equity |
|
$ |
107,768,602 |
|
|
$ |
99,810,375 |
|
|
$ |
90,433,968 |
|
|
$ |
(190,244,343 |
) |
|
$ |
107,768,602 |
|
| Total
liabilities and shareholders’ equity |
|
$ |
107,868,340 |
|
|
$ |
100,253,640 |
|
|
$ |
118,979,744 |
|
|
$ |
(190,244,343 |
) |
|
$ |
136,857,381 |
|
Condensed
Consolidating Schedule — Statement of Cash Flows
| |
|
For
the Year Ended December 31, 2022 |
|
| |
|
Meihua
(Cayman) |
|
|
Kang
Fu
International
Medical (HK) |
|
|
PRC
subsidiaries |
|
|
Eliminations |
|
|
Consolidated
Total |
|
| Net
cash (used in) provided by operating activities |
|
$ |
(28,820,953 |
) |
|
$ |
21,906,025 |
|
|
$ |
(2,248,110 |
) |
|
$ |
- |
|
|
$ |
(9,163,038 |
) |
| Net
cash used in investing activities |
|
$ |
- |
|
|
$ |
(20,165,956 |
) |
|
$ |
(8,620,292 |
) |
|
$ |
20,165,956 |
|
|
$ |
(8,620,292 |
) |
| Net
cash provided by (used in) financing activities |
|
$ |
34,859,583 |
|
|
$ |
(257,150 |
) |
|
$ |
22,809,023 |
|
|
$ |
(20,165,956 |
) |
|
$ |
37,245,500 |
|
| |
|
For
the Year Ended December 31, 2021 |
|
| |
|
Meihua
(Cayman) |
|
|
Kang
Fu International Medical (HK) |
|
|
PRC
subsidiaries |
|
|
Eliminations |
|
|
Consolidated
Total |
|
| Net
cash provided by (used in) operating activities |
|
$ |
- |
|
|
$ |
604,599 |
|
|
$ |
(659,262 |
) |
|
$ |
- |
|
|
$ |
(54,663 |
) |
| Net
cash used in investing activities |
|
$ |
- |
|
|
$ |
- |
|
|
$ |
(833,817 |
) |
|
$ |
- |
|
|
$ |
(833,817 |
) |
| Net
cash (used in) provided by financing activities |
|
$ |
- |
|
|
$ |
(817,571 |
) |
|
$ |
2,677,805 |
|
|
$ |
- |
|
|
$ |
1,860,234 |
|
Corporate
Information
Our
principal executive offices are located at 88 Tongda Road, Touqiao Town, Guangling District, Yangzhou, 225000 People’s Republic
of China, and our phone number is +86-0514-89800199. We maintain corporate websites at http://meihuamed.com and http://ir.meihuamed.com.
The information contained in, or accessible from, our website or any other website does not constitute a part of this prospectus supplement.
Our agent for service of process in the United States is Dorsey & Whitney LLP, 51 West 52nd Street, New York, New York
10016 and its phone number is (212) 415-9200.
Implications
of Being an Emerging Growth Company
As
a company with less than US$1.235 billion in revenue for our last fiscal year, we qualify as an “emerging growth company”
pursuant to the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of specified
reduced reporting and other requirements compared to those that are otherwise applicable generally to public companies. These provisions
include, but are not limited to:
| |
● |
being permitted to present
only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial
Condition and Results of Operations in our SEC filings; |
| |
● |
not being required to comply
with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; |
| |
● |
reduced disclosure obligations
regarding executive compensation in periodic reports, proxy statements and registration statements; and |
| |
● |
exemptions from the requirements
of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously
approved. |
The
JOBS Act also provides that an emerging growth company does not need to comply with any new or revised financial accounting standards
until such date that a private company is otherwise required to comply with such new or revised accounting standards. We have elected
to use the extended transition period under the JOBS Act. Accordingly, our financial statements may not be comparable to the financial
statements of public companies that comply with such new or revised accounting standards.
We
will remain an emerging growth company until the earliest of (a) the last day of the fiscal year during which we have total annual
gross revenues of at least US$1.235 billion; (b) the last day of our fiscal year following the fifth anniversary of the completion
of this offering; (c) the date on which we have, during the preceding three-year period, issued more than US$1.0 billion in
non-convertible debt; or (d) the date on which we are deemed to be a “large accelerated filer” under the Exchange Act,
which would occur as of the end of our fiscal year if the market value of our Ordinary Shares that are held by non-affiliates exceeds
US$700 million as of the last business day of our most recently completed second fiscal quarter. Once we cease to be an emerging
growth company, we will not be entitled to the exemptions provided in the JOBS Act discussed above.
Implications
of Being a Foreign Private Issuer
We
are incorporated under the laws of the Cayman Islands, and more than 50 percent of our outstanding voting securities are not directly
or indirectly held by residents of the United States. Therefore, we are a “foreign private issuer,” as defined in Rule 405
under the Securities Act and Rule 3b-4(c) under the Exchange Act. As a result, we are not subject to the same requirements as U.S. domestic
issuers. Under the Exchange Act, we will be subject to reporting obligations that, to some extent, are more lenient and less frequent
than those of U.S. domestic reporting companies. For example:
| |
● |
we are not required to
provide as many Exchange Act reports or provide periodic and current reports as frequently, as a domestic public company; |
| |
● |
for interim reporting,
we are permitted to comply solely with our home country requirements, which are less rigorous than the rules that apply to domestic
public companies; |
| |
● |
we are not required to
provide the same level of disclosure on certain issues, such as executive compensation; |
| |
● |
we are exempt from provisions
of Regulation FD aimed at preventing issuers from making selective disclosures of material information; |
| |
● |
we are not required to
comply with the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security
registered under the Exchange Act; and |
| |
● |
we are not required to
comply with Section 16 of the Exchange Act requiring insiders to file public reports of their share ownership and trading activities
and establishing insider liability for profits realized from any “short-swing” trading transaction. |
As
an exempted company with limited liability incorporated under the laws of the Cayman Islands that is listed on Nasdaq, the Company is
subject to Nasdaq corporate governance listing standards. Under Nasdaq rules, a foreign private issuer may, in general, follow its home
country corporate governance practices in lieu of some of the Nasdaq corporate governance requirements. Pursuant to the home country
rule exemption set forth under Nasdaq Listing Rule 5615(a)(3)(A), a foreign private issuer may follow its home country practice in lieu
of the requirements of the Nasdaq Marketplace Rule 5600 series so long as the issuer discloses in its annual report filed with the SEC
each requirement of Rule 5600 that it does not follow and describes the home country practice followed in lieu of such requirement.
As
such, we have exercised the home country rule exemption and elected to be exempt from the Nasdaq Marketplace Rule 5635(d) and notified
Nasdaq of our decision to exercise such exemption. Nasdaq Marketplace Rule 5635(d) sets forth the circumstances under which shareholder
approval is required prior to an issuance of securities, other than in a public offering, equal to 20% or more of the voting power outstanding
at a price less than the lower of: (x) the Nasdaq Official Closing Price (as reflected on Nasdaq.com) immediately preceding the signing
of the binding agreement; or (y) the average Nasdaq Official Closing Price of the Ordinary Shares (as reflected on Nasdaq.com) for the
five trading days immediately preceding the signing of the binding agreement. We have notified Nasdaq that we have elected to be exempt
from the Nasdaq Marketplace Rule 5635(d). In addition, we will disclose the home country rule exemption of Nasdaq Marketplace Rule 5635(d)
in our annual report on Form 20-F for the fiscal year ending December 31, 2023.
Ogier,
our Cayman Islands counsel, has provided a letter to the Nasdaq Stock Market certifying that under Cayman Islands law, we are not required
to comply with above-mentioned requirements.
THE
OFFERING
| Ordinary Shares Offered: |
|
1,205,255 ordinary shares issuable
upon exercise of outstanding warrants |
| |
|
|
| Ordinary Shares outstanding before this offering: |
|
24,234,673 |
| |
|
|
| Ordinary Shares outstanding after this offering (1): |
|
25,439,928 |
| |
|
|
| Use of Proceeds |
|
While we may receive proceeds
from the Selling Shareholders’ exercise of the Warrants (which may, in certain circumstances, be exercised cashlessly), we
will not receive any proceeds from the sale of the Ordinary Shares underlying the Warrants offered in this prospectus. For further
information, see the “Use of Proceeds” section below. |
| |
|
|
| Risk Factors |
|
See “Risk Factors” beginning
on page 19 of this prospectus as well as other information included in this prospectus and our Annual Report on Form 20-F/A, as filed
with the SEC on August 29, 2023, for a discussion of certain factors you should carefully consider before investing in our securities. |
| |
|
|
| Nasdaq Global Market Symbol |
|
MHUA |
| (1) | The
number of shares outstanding following completion of the Offering do not include shares issuable
upon conversion of the Convertible Notes held by the Selling Shareholders. The $6,000,000
in Convertible Notes sold to the Selling Shareholders in a primary offering on Form F-3 are
convertible from time to time at the lower of (i) $2.738 per share or (ii) a price per share
equal to 95% of the lowest VWAP of the Ordinary Shares during the seven trading day period
immediately preceding the applicable conversion date, subject to certain adjustments and
a 4.99% beneficial ownership limitation. |
RISK
FACTORS
Investment
in any securities offered pursuant to this prospectus supplement and the accompanying prospectus involves risks. You should carefully
consider the risk factors described below and in our 2022 Annual Report and the accompanying prospectus, any amendment or update thereto
reflected in subsequent filings with the SEC, including in our annual reports on Form 20-F, and all other information contained or incorporated
by reference in this prospectus, as updated by our subsequent filings under the Exchange Act. The risks and uncertainties we have described
are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may
also affect our operations. The occurrence of any of these risks might cause you to lose all or part of your investment in the offered
securities.
Risks
Related to this Offering
Our
recent entry into a Securities Purchase Agreement with the Selling Shareholders for the sale of up to $50.5 million in Convertible Notes
and Warrants, of which we have sold $6.0 million to date, along with any subsequent conversion and/or exercise thereof, may depress the
price of our Ordinary Shares and encourage short sales by third parties, which could further depress the price of our Ordinary Shares.
To
the extent that the Selling Shareholders opt to purchase additional Convertible Notes and Warrants from the Company, for up to an additional
$44,500,000 in convertible notes and warrants, there could be substantial additional dilution and additional pressure placed on the market
price of our Ordinary Shares in the event such Convertible Notes are converted. The risk of dilution from such issuances of Ordinary
Shares may cause shareholders to sell our Ordinary Shares held by them, which could further contribute to any decline in the price of
our Ordinary Shares. Any downward pressure on the price of our Ordinary Shares caused by the sale or potential sale of such Ordinary
Shares could encourage short sales by third parties. In a short sale, a prospective seller borrows shares from a stockholder or broker
and sells the borrowed shares. The prospective seller hopes that the share price will decline, at which time the seller can purchase
shares at a lower price for delivery back to the lender. The seller profits when the share price declines because it is purchasing shares
at a price lower than the sale price of the borrowed shares. Such sales could place downward pressure on the price of our Ordinary Shares
by increasing the number of Ordinary Shares being sold, which could further contribute to any decline in the market price of our Ordinary
Shares. In addition, such price depression could also result in our failure to meet the Nasdaq minimum bid price requirement set forth
in Nasdaq Listing Rule 5450(a)(1). If the closing bid price of our Ordinary Shares falls below $1.00 per Ordinary Share for 30 consecutive
trading days, we cannot assure you that we will regain compliance within the timeframes set by Nasdaq, and, although we would need to
take such actions to maintain listing, such as completing a reverse stock split, we cannot assure you that our Ordinary Shares would
not be delisted from the Nasdaq.
Raising
additional capital and the sale of additional Ordinary Shares or other equity securities could result in dilution to our shareholders,
while the incurrence of debt may impose restrictions on our operations.
We
may require additional cash resources due to certain future developments, including any investments or acquisitions we may decide to
pursue. If our cash resources are insufficient to satisfy our cash requirements, we may seek to sell equity or debt securities or obtain
a credit facility in addition to or outside of the Convertible Note and Warrant offering. Such sale of equity securities would result
in dilution to our shareholders. The incurrence of indebtedness would result in increased debt service obligations and could require
us to agree to operating and financing covenants that would restrict our operations. Furthermore, the issuance of additional securities
by us, whether equity or debt, or the possibility of such issuance, may cause the market price of our Ordinary Shares to decline and
existing shareholders may not agree with our financing plans or the terms of such financings.
Future
sales of our Ordinary Shares, whether by us or our shareholders, could cause our share price to decline.
If
our existing shareholders sell, or indicate an intent to sell, substantial amounts of our Ordinary Shares on the public market, the trading
price of Ordinary Shares could decline significantly. Similarly, the perception in the public market that our shareholders might sell
Ordinary Shares could also depress the market price of our Ordinary Shares. A decline in the price of our Ordinary Shares might impede
our ability to raise capital through the issuance of additional Ordinary Shares or other equity securities. In addition, the issuance
and sale by us of additional Ordinary Shares or securities convertible into or exercisable for Ordinary Shares, or the perception that
we will issue such securities, could reduce the trading price for our Ordinary Shares as well as make future sales of equity securities
by us less attractive or not feasible.
Securities
analysts may not cover our Ordinary Shares and this may have a negative impact on the market price of our Ordinary Shares.
The
trading market for our Ordinary Shares will depend, in part, on the research and reports that securities or industry analysts publish
about us or our business. We do not have any control over independent analysts. We do not currently have and may never obtain research
coverage by independent securities and industry analysts. If no independent securities or industry analysts commence coverage of us,
the trading price for our Ordinary Shares would be negatively impacted. If we obtain independent securities or industry analyst coverage
and if one or more of the analysts who covers us downgrades our Ordinary Shares, changes their opinion of our shares or publishes inaccurate
or unfavorable research about our business, our share price would likely decline. If one or more of these analysts ceases coverage of
us or fails to publish reports on us regularly, demand for our Ordinary Shares could decrease and we could lose visibility in the financial
markets, which could cause our share price and trading volume to decline.
Techniques
employed by short sellers may drive down the market price of our Ordinary Shares.
Short
selling is the practice of selling securities that the seller does not own but rather has borrowed from a third party with the intention
of buying identical securities back at a later date to return to the lender. The short seller hopes to profit from a decline in the value
of the securities between the sale of the borrowed securities and the purchase of the replacement shares, as the short seller expects
to pay less in that purchase than it received in the sale. As it is in the short seller’s interest for the price of the security
to decline, many short sellers publish, or arrange for the publication of, negative opinions regarding the relevant issuer and its business
prospects in order to create negative market momentum and generate profits for themselves after selling a security short. These short
attacks have, in the past, led to selling of shares in the market.
Public
companies listed in the United States that have a substantial majority of their operations in China have at times been the subject of
short selling. Much of the scrutiny and negative publicity has centered on allegations of a lack of effective internal control over financial
reporting resulting in financial and accounting irregularities and mistakes, inadequate corporate governance policies or a lack of adherence
thereto and, in many cases, allegations of fraud. As a result, many of these companies are now conducting internal and external investigations
into the allegations and, in the interim, are subject to shareholder lawsuits and/or SEC enforcement actions.
We
may in the future be the subject of unfavorable allegations made by short sellers. Any such allegations may be followed by periods of
instability in the market price of our Ordinary Shares and negative publicity. If and when we become the subject of any unfavorable allegations,
whether such allegations are proven to be true or untrue, we could have to expend a significant amount of resources to investigate such
allegations and/or defend ourselves. While we would strongly defend against any such short seller attacks, we may be constrained in the
manner in which we can proceed against the relevant short seller by principles of freedom of speech, applicable federal or state law
or issues of commercial confidentiality. Such a situation could be costly and time-consuming and could distract our management from growing
our business. Even if such allegations are ultimately proven to be groundless, allegations against us could severely impact our business
operations and shareholder’s equity, and the value of any investment in our Ordinary Shares could be greatly reduced or rendered
worthless.
Management
will have broad discretion as to the use of the proceeds from the exercise of any Warrants by the Selling Shareholders, and uses may
not improve our financial condition or market value.
Because
we have not designated the amount of net proceeds from the exercise of any Warrants by the Selling Shareholders to be used for any particular
purpose, our management will have broad discretion as to the application of such proceeds. Our management may use the proceeds for working
capital and general corporate purposes that may not improve our financial condition or advance our business objectives.
Risks
and Uncertainties Related to Doing Business in China
Meihua
faces various legal and operational risks and uncertainties as a company which its principal subsidiaries based in and primarily operating
in China. Most of Meihua’s subsidiaries operations are conducted in the PRC, and are governed by PRC laws, rules, and regulations.
Because PRC laws, rules, and regulations are relatively new and quickly evolving, and because of the limited number of published decisions
and the non-precedential nature of these decisions, and because the laws, rules and regulations often give the relevant regulator
certain discretion in how to enforce them, the interpretation and enforcement of these laws, rules and regulations involve uncertainties
and can be inconsistent and unpredictable. The PRC government has the power to exercise significant oversight and discretion over the
conduct of our business, and the regulations to which we are subject may change rapidly and with little notice to us or our shareholders.
As a result, the application, interpretation, and enforcement of new and existing laws and regulations in the PRC are often uncertain.
In addition, these laws and regulations may be interpreted and applied inconsistently by different agencies or authorities, and inconsistently
with our current policies and practices.
See
“Risk Factors — Risks Related to Doing Business in China — Because all of our operations are in China, our business
is subject to the complex and rapidly evolving laws and regulations there. The Chinese government may exercise significant oversight
and discretion over the conduct of our business and may intervene in or influence our operations at any time, which could result in a
material change in our operations and/or the value of our Ordinary Shares.” And “— PRC’s economic, political
and social conditions, as well as changes in any government policies, laws and regulations, could have a material adverse effect on our
business.”
The
PRC government has significant oversight and discretion over the conduct of our business, and may intervene in or influence our operations
through adopting and enforcing rules and regulatory requirements. For example, in recent years the PRC government, has enhanced
regulation in areas such as anti-monopoly, anti-unfair competition, cybersecurity and data privacy. See “Risk Factors — Risks
Related to Our Business and Industry — If the Chinese government chooses to exert more oversight and control over offerings that
are conducted overseas and/or foreign investment in China-based issuers, such action could significantly limit or completely hinder our
ability to offer or continue to offer securities to investors outside of China and, as a result, cause the value of such securities to
significantly decline or be worthless.”; and “— Uncertainties with respect to the PRC legal system could adversely
affect us.”
In
addition, our Ordinary Shares may be delisted from Nasdaq or prohibited from being traded
over-the-counter under the Holding Foreign Companies Accountable Act if the PCAOB is unable
to inspect our auditor for two consecutive years. Our auditor has been inspected by the PCAOB
on a regular basis and it is not subject to the determinations announced by the PCAOB on
December 16, 2021. If trading in our Ordinary Shares is prohibited under the Holding Foreign
Companies Accountable Act in the future because the PCAOB determines that it cannot inspect
or fully investigate our auditor at such future time, the Nasdaq Stock Market may determine
to delist our Ordinary Shares. On June 22, 2021, the U.S. Senate passed the Accelerating
Holding Foreign Companies Accountable Act and on December 29, 2022, a legislation entitled
“Consolidated Appropriations Act, 2023” (the “Consolidated Appropriations
Act”) was signed into law by President Biden, which contained, among other things,
an identical provision to Accelerating Holding Foreign Companies Accountable Act and reduces
the number of consecutive non-inspection years required for triggering the listing and trading
prohibitions from three years to two years,, thus reducing the time period before our securities
may be prohibited from trading or delisted. On December 15, 2022, the PCAOB Board determined
that the PCAOB was able to secure complete access to inspect and investigate registered public
accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous
determinations to the contrary. However, should PRC authorities obstruct or otherwise fail
to facilitate the PCAOB’s access in the future, the PCAOB Board will consider the need
to issue a new determination. Our securities may be delisted or prohibited from trading if
the PCAOB determines that it cannot inspect or investigate completely our auditor under the
Holding Foreign Companies Accountable Act. Our auditor, Kreit & Chiu CPA LLP, is headquartered
in New York, NY with its office at 733 Third Avenue, Floor 16, #1014, New York, NY 10017,
and has been inspected by the PCAOB on a regular basis. See “Risk Factors — Risks
Related to Doing Business in China — Although the audit report included in this report
was issued by U.S. auditors who are currently inspected by the PCAOB, if it is later determined
that the PCAOB is unable to inspect or investigate our auditor completely, investors would
be deprived of the benefits of such inspection and our Ordinary Shares may be delisted or
prohibited from trading.”
Permissions
and Approvals Required to be Obtained from PRC Authorities for our Business Operations
As
a medical device manufacturing and sales company, we are subject to extensive government regulation and supervision in the PRC. Pursuant
to PRC laws, we must obtain production license for Class II and III disposable medical devices and production filing for Class I disposable
medical device, operation license for Class III disposable medical devices and operation filing for Class II disposable medical devices,
and filing or registration certificates for certain Class I, II or Class III disposable medical devices. As of the date of this prospectus,
we are current on all licenses and certificates and have obtained Class I, II and III disposable medical device qualifications in the
PRC.
However,
if we fail to timely renew our medical device licenses or registration certificates, it could adversely affect our reputation, financial
conditions and results of operations. See “Risk Factors — Risks Related to Our Business and Industry — If we fail to
timely renew our medical device licenses or registration certificates, it could adversely affect our reputation, financial conditions
and results of operations.”
Permissions
and Approvals Required to be Obtained from PRC Authorities for our Securities Offerings
As
of the date of this prospectus, we believe that we and our PRC subsidiaries, (1) are not required to obtain permissions from any PRC
authorities to operate or issue our Ordinary Shares to foreign investors, (2) are not subject to permission requirements from the China
Securities Regulatory Commission (the “CSRC”), the Cyberspace Administration of China (the “CAC”) or any other
entity that is required to approve our PRC subsidiaries’ operations, and (3) have not received or were denied such permissions
by any PRC authorities. Nevertheless, the General Office of the Central Committee of the Communist Party of China and the General Office
of the State Council jointly issued the “Opinions on Severely Cracking Down on Illegal Securities Activities According to Law,”
or the Opinions, which were made available to the public on July 6, 2021. The Opinions emphasized the need to strengthen the administration
over illegal securities activities, and the need to strengthen the supervision over overseas listings by Chinese companies. Given the
current PRC regulatory environment, it is uncertain when and whether we or our PRC subsidiaries, will be required to obtain permission
from the PRC government to continue to list on U.S. exchanges in the future, and even when such permission is obtained, whether it will
be denied or rescinded. We have been closely monitoring regulatory developments in China regarding any necessary approvals from the CSRC
or other PRC governmental authorities required for overseas listings. As of the date of this report, we have not received any inquiry,
notice, warning, sanctions or regulatory objection to this offering from the CSRC or other PRC governmental authorities. However, there
remains significant uncertainty as to the enactment, interpretation and implementation of regulatory requirements related to overseas
securities offerings and other capital markets activities.
On
February 17, 2023, the CSRC promulgated Trial Administrative Measures of the Overseas Securities Offering and Listing by Domestic Companies,
or the Trial Measures, and relevant five supporting guidelines, together as the New Overseas Listing Rules, which became effective on
March 31, 2023. According to the New Overseas Listing Rules, PRC domestic companies that seek to offer and list securities in overseas
markets, either in direct or indirect means, are required to complete the filing procedure with the CSRC and report relevant information.
In addition, an overseas-listed company must also submit the filing with respect to its follow-on offerings, issuance of convertible
corporate bonds and exchangeable bonds, and other equivalent offering activities, within the time frame specified by the Trial Measures.
The New Overseas Listing Rules laid out the regulatory filing requirements for both direct and indirect overseas listings and clarify
the determination criteria for indirect overseas listing in overseas markets. For more detailed information, see “Item 3. Key Information—D.
Risk Factors—Risks Related to Doing Business in China—Potential Chinese governmental and regulatory interference could significantly
limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to
significantly decline or be worthless.” Pursuant to the New Overseas Listing Rules, (i) in connection with our previous issuance
of securities to foreign investors, neither we, nor our PRC subsidiaries are required to obtain any permissions or approvals from the
CSRC, and (ii) should we decide to issue additional equity or equity-linked securities for listing overseas in the future, we are not
required to obtain any permissions or approvals from any PRC government authorities, except for the requisite filing with the CSRC in
connection with such issuance. Failure to comply with the filing requirements may result in an order of rectification, a warning and
fines up to RMB10 million to the non-compliant domestic companies, and the directly responsible persons of the companies will be warned
and fined between RMB500,000 and RMB5 million. Furthermore, if the controlling shareholder and the actual controller of the non-compliant
companies organizes or instigates the breach, they will be fined between RMB1 million and RMB10 million. In addition to above filing
requirements, the Filings Rules also requires an issuer to report to the CSRC within three business days after occurrence of any the
following events: (i) its change of control; (ii) its being subject to investigation or sanctions by any overseas securities regulators
or overseas authorities; (iii) its change of listing status or listing segment; (iv) voluntary or mandatory delisting; and (v) material
change of its principal business operations to the extent that it ceases to be subject to the filing requirements of the Trial Measures.
Risks
Related to Our Business and Industry
In
connection with the initial public offering of our Ordinary Shares in February 2022, we entered into certain agreements with a Hong Kong
entity pursuant to which we believe we were defrauded of in excess of $10 million; because we failed to disclose such agreements at the
time of the initial public offering, regardless of the arguably fraudulent nature of such agreements, we may be subject to potential
liabilities or litigation exposure as a result of such agreements.
The
Company closed its initial public offering (the “IPO”) of its ordinary shares (the “Ordinary Shares”) on February
18, 2022, through which the Company raised $39.4 million through the sale of 3,940,000 Ordinary Shares at a purchase price of $10.00
per share. During the roadshow leading up to the IPO, a Hong Kong investment company named Tai He International Group Limited (“Tai
He”) was referred to the Company by Shengang Securities Company Limited (“Shengang”), the Company’s co-underwriter
based in the People’s Republic of China (“PRC”). Tai He, through negotiations with Shengang, asserted that it would
participate in the IPO as an investor. As a development stage PRC medical device company, the Company’s management had no experience
in the U.S. capital markets and relied on its investment bankers and underwriters in providing guidance and advice concerning its U.S.
public offering. Accordingly, the Company was unaware of the actual motives of Tai He. Based on the Company’s trust in Shengang
and given the circumstances that the Company was unable to make further verification, the Company hastily entered into a series of agreements
with Tai He (the “Tai He Agreements”).
Pursuant
to the Tai He Agreements, Tai He agreed to invest a minimum of $35 million in the IPO subject to the Company making a $7.0 million refundable
deposit (the “Refundable Deposit”) and advancing a $3.0 million service fee for investor relations and other services (together,
the “Services”) payable to Tai He. The Company, its affiliates and individual shareholders paid the $7.0 million Refundable
Deposit and $3.0 million service fee to Tai He in several installments from January 27, 2022 to March 11, 2022. After March 11, 2022,
the Company has made no other payments to, nor had any direct interaction with, Tai He, and, in actuality, the Company never directly
communicated with Tai He during the negotiations, only communicating about Tai He and the Tai He Agreements through Shengang.
After
the IPO, through the Company’s internal risk control process, the Company learned that Tai He does not appear to be an investor
in the Company and, when the Company attempted to inquire about it, Tai He refused to speak with the Company. Based on the facts as investigated
and understood by the Company, the Company believes that it was defrauded by Tai He and that Tai He never actually invested $35 million
in the IPO. In fact, the Company learned that Tai He made no investments in the Company at all, and Tai He has no involvement in the
Company’s IPO matters. In addition, the Refundable Deposit has not been repaid to the Company and no Services have been provided
to the Company by Tai He. As a result, the Company believes that the Tai He Agreements may be deemed a fraud perpetrated by Tai He and
Shengang against the Company by Tai He taking advantage of asymmetric information, including the Company’s lack of knowledge and
understanding of the U.S. markets, IPO rules and processes, and the trust of the Company.
After
consultation with professionals, the Company is now aware that the Tai He Agreements were required to be disclosed to the public and
the Company believes that the agreements themselves are fraudulent and non-enforceable, and therefore the Company is disclosing the Tai
He Agreements to meet the compliance requirements of the Securities and Exchange Commission (“SEC”). Further, the Company
acknowledges that it had improperly relied on the advice, direction and counsel of Shengang when entering into the Tai He Agreements.
The Company is now proactively working to terminate the Tai He Agreements and recover the monies already paid to Tai He by the Company.
Due to the uncertainty of collection, the Company has written off approximately $4.8 million deposit and fully expensed $2.3 million
service fee paid by the Company to Tai He in the year ended December 31, 2022. The Company and its executive management team intends
to cooperate with the SEC, as well as any other relevant authorities, to ensure that its investors’ interests are protected while
such matters are resolved. Nonetheless, the existence of the Tai He Agreements and the Company’s failure to disclose them could
expose the Company potential shareholder litigation and/or SEC enforcement or other regulatory action.
Our
operating history may not be indicative of our future growth or financial results and we may not be able to sustain our historical growth
rates.
Our
operating history may not be indicative of our future growth or financial results. There is no assurance that we will be able to grow
our revenues in future periods. Our growth rates may decline for any number of possible reasons, and some of them are beyond our control,
including decreasing customer demand, increasing competition, declining growth of the medical device industry in general, emergence of
alternative business models, or changes in government policies or general economic conditions. We will continue to expand our sales network
and product offerings to bring greater convenience to our customers and to increase our customer base and number of transactions. However,
the execution of our expansion plan is subject to uncertainty and the total number of items sold and number of transacting customers
may not grow at the rate we expect for the reasons stated above. If our growth rates decline, investors’ perceptions of our business
and prospects may be adversely affected and the market price of our Ordinary Shares could decline.
Failure
to maintain the quality and safety of our products could have a material and adverse effect on our reputation, financial condition and
results of operations.
The
quality and safety of our products, whether self-manufactured or outsourced, are critical to our success. As a medical device manufacturer
with a history of over 30 years, and a management team with more than 40 years of industry experience, quality and safety are maintained
as our core value. Our medical devices are directly used on the human body and are essential to human health. We pay close attention
to quality control, monitoring each step in the process from procurement to production and from warehouse to delivery. Maintaining consistent
product quality depends significantly on the effectiveness of our quality control system, which in turn depends on a number of factors,
including but not limited to the design of our quality control system, employee training to ensure that our employees adhere to and implement
our quality control policies and procedures and the effectiveness of monitoring of any potential violation(s) of our quality control
policies and procedures. There can be no assurance that our quality control system will always prove to be effective.
In
addition, the quality of the products or services provided by our suppliers or business partners is subject to factors beyond our control,
including the effectiveness and the efficiency of their quality control system, among others. There can be no assurance that our suppliers
or business partners will always be able to adopt appropriate quality control systems and meet our stringent quality control requirements
in respect of the products or services they provide. Any failure of our suppliers or business partners to provide satisfactory products
or services could harm our reputation and adversely impact our operations. In addition, we may be unable to receive sufficient compensation
from suppliers and business partners for the losses caused by them.
As
of the date of this report, we are unaware of any material accidents related to the quality or safety of our products.
Any
failure to maintain effective quality control over our products and services could materially adversely affect our business.
The
quality of our services and products is critical to the success of our business, and such quality to a large extent depends on the effectiveness
of our quality control system. We have developed a rigorous quality control system that enables us to monitor each stage of the production
process.
However,
despite our quality control management system, we cannot eliminate all risks of errors, defects or failures. We may fail to detect or
cure defects as a result of a number of factors, many of which are outside our control, including:
| |
● |
technical or mechanical
malfunctions in the production process; |
| |
● |
human error or malfeasance
by our quality control personnel; |
| |
● |
tampering by third parties;
and |
| |
● |
defective raw materials
or equipment. |
Failure
to detect quality defects in our products could result in patient injury, customer dissatisfaction, or other problems that could seriously
harm our reputation and business, expose us to liability, and adversely affect our revenue and profitability.
In
2018, our PRC subsidiaries Jiangsu Yada and Jiangsu Huadong were fined an immaterial amount for noncompliance with certain local laws
and regulations, which non-compliance was cured by us in the same year.
For
the fiscal year of 2022 and as of the date of report, we are not aware of any material investigations, prosecutions, disputes, claims
or other proceedings relating to quality or quality control issues, nor has the Company received any notices of, been punished for, nor
can we foresee any punishment being made by any related government authorities of the PRC as of the date of this report.
Due
to the nature of our business, we may experience or be exposed to significant liability claims or complaints from customers, doctors,
patients or hospitals, litigation and regulatory investigations and proceedings, such as claims arising in relation to medical device
safety, or adverse publicity involving our products, which could adversely affect our financial condition and results of operations.
We
face an inherent risk of liability claims or complaints related to the use of our products by our customers, doctors, patients and hospitals.
We take those complaints and claims seriously and endeavor to reduce such complaints by implementing various remedial measures. Nevertheless,
we cannot assure you that we can successfully prevent or address all such complaints.
Any
complaints or claims against us, even if meritless and unsuccessful, may divert management’s attention and other resources from
our business and adversely affect our business and operations. Such complaints or claims may cause customers to lose confidence in us
and our brand, which may adversely affect our business and results of operations. Furthermore, negative publicity including but not limited
to negative online reviews on social media and crowd-sourced review platforms, industry findings or media reports related to medical
device quality and safety, public health concerns, illness, injuries, whether or not accurate, and whether or not concerning our products,
can adversely affect our business, results of operations and reputation.
We
face potential liability, expenses for legal claims and exposure to other harm due to the nature of our business. For example, customers
could assert legal claims against us in connection with personal injuries or illness related to the use of medical devices we sell. The
PRC government, media outlets and public advocacy groups have been increasingly focused on customer protection in recent years. Selling
of defective products may expose us to liabilities associated with customer protection laws. We may be deemed responsible for compensating
customer losses even if personal injuries or illness are not directly caused by us. Such liability could arise through the actions of
our suppliers or business partners. Thus, we may be held liable if our suppliers or other business partners fail to comply with applicable
product quality and safety related rules and regulations. In such a situation, though we can ask the responsible parties to indemnify
us for such liability, even if we receive full indemnification our reputation could still be adversely affected.
We
may face additional exposure to claims and lawsuits. These claims could divert management time and attention away from our business and
result in significant costs to investigate and defend, regardless of the merits of the claims. In some instances, we may elect or be
forced to pay substantial damages if we are unsuccessful in our efforts to defend against these claims, which could harm our business,
financial condition and results of operations. In addition, our directors, management and employees may from time to time be subject
to litigation and regulatory investigations and proceedings or otherwise face potential liability and expense in relation to medical
device quality and safety, commercial, labor, employment, securities or other matters, which could adversely affect our reputation and
results of operations.
As
of the date of this report, we are not aware of any notices, warnings, investigations, prosecutions, disputes, claims or other proceedings
related to customer rights protection having been brought against us, nor have we been punished or fined or can we foresee any punishment
or fines to be made by any government authorities of the PRC or any other jurisdiction.
We
face the risk of fluctuations in the cost, availability and quality of our raw materials, which could adversely affect our results of
operations.
The
cost, availability and quality of our principal raw materials, such as rubber, chemical PE, polyethylene, polypropylene, nylon, non-woven
fabrics and other bulk commodities, are important to our operations. After years of development, we have established long-term cooperative
relationships with numerous raw material suppliers under a positive price negotiation and adjustment mechanism. However, if the cost
of raw materials increases due to policy changes, large market price fluctuations or any other reasons, our business and results of operations
could be adversely affected.
Lack
of availability of raw materials, whether due to shortages in supply, delays or interruptions in processing, failure of timely deliver
materials or otherwise, could interrupt our operations and adversely affect our financial results.
Defective
raw materials or raw materials with quality issues could subject us to product liability claims or legal actions, which could adversely
affect our financial conditions and results of operations.
A
significant interruption in the operations of our third-party suppliers and other business partners could potentially disrupt our operations.
We
have limited control over the operations of our third-party suppliers and other business partners and any significant interruption in
their operations may have an adverse impact on our operations. For example, a significant interruption in the operations of our supplier’s
manufacturing facilities could cause delay or termination of shipment of the raw materials to us, which may cause delay or termination
of shipment of ordered products to our customers, resulting in damage to our customer relationships. If we could not solve the impact
of the interruptions of operations of our third-party suppliers, our business operations and financial results may be materially and
adversely affected.
Although
we believe that we could establish alternate sources from other suppliers for most of our raw materials, any delay in locating and establishing
relationships with other sources could result in shortages or back orders for such raw materials. There can be no assurance that such
replacement suppliers will provide the raw materials that are needed by us in the quantities that we request or at the prices that we
are willing to pay. Any shortage in quantities or increase in prices could adversely affect our financial conditions and results of operations.
As
the relevant land plots are of allocated land use right, we may be required to pay the land granting fees to convert such allocated land
use rights to granted land use rights.
Allocated
land use rights may only be held by Chinese state-owned enterprises, government authorities and public entities or used for certain prescribed
purposes - for example, military installations and infrastructure projects. Allocated lands may be requisitioned by the state at any
time and without compensation. An allocated land use right may be converted to a granted land use rights by the payment of land
granting fees to the land authority. Therefore, we might be required to convert the land use rights of the relevant land plots from “allocated”
to “granted” by payment of the land granting fees.
While
we utilize numerous suppliers, we do not have long term contracts with any of those suppliers and they could reduce order quantities
or terminate their sales to us at any time.
While
we utilize numerous suppliers, we do not have long term contracts with any of those suppliers. As a result, at any time, our suppliers
could reduce the quantities of products they sell to us or cease selling products to us altogether. Such reductions or terminations could
have a material adverse impact on our revenues, profits and financial condition.
Overall
tightening of the labor market, increases in labor costs or any possible labor unrest may adversely affect our business and results of
operations.
Our
business requires a substantial number of personnel. Any failure to retain and maintain stable and dedicated workforce by us may lead
to disruption to our business operations. Although we have not experienced any labor shortages to date, we have observed an overall tightening
and increasingly competitive labor market. We have experienced, and expect to continue to experience, increases in labor costs due to
increases in salary, social benefits and employee headcount. We compete with other companies in our industry and other labor-intensive
industries for labor, and we may not be able to offer competitive remuneration and benefits compared to them. If we are unable to manage
and control our labor costs, our business, financial condition and results of operations may be materially and adversely affected.
We
are dependent on our top customers. If we fail to acquire new customers or retain existing customers in a cost-effective manner, our
business, financial condition and results of operations may be materially and adversely affected.
Maintaining
existing customers and developing new customers are always essential to our success. Although we are not heavily dependent on one or
two customers, we are still dependent on our top customers. For the years ended December 31, 2022, 2021 and 2020, our top five customers
contributed approximately 56.53%, 56.96%, and 53.47%, respectively, to our revenue.
Our
ability to cost-effectively attract new customers and retain existing customers, especially our top customers, is crucial to driving
net revenues growth and achieving profitability. We have invested significantly in branding, sales and marketing to acquire and retain
customers since our inception. For example, we attend domestic and international expos and exhibitions in marketing our products and
attracting new customers. We also expect to continue to invest significantly to acquire new customers and retain existing ones, especially
our top customers. There can be no assurance that new customers will stay with us, or the net revenues from new customers we acquire
will ultimately exceed the cost of acquiring those customers. In addition, if our existing customers, especially our existing top customers
no longer find our products appealing, or if our competitors offer more attractive products, prices, discounts or better customer services,
our existing customers may lose interest in us, decrease their orders or even stop ordering from us. If we are unable to retain our existing
customers, especially our top customers or to acquire new customers in a cost-effective manner, our revenues may decrease and our results
of operations will be adversely affected.
If
we are unable to build and maintain sufficient sales and distribution network to meet increasing demand of our products, our ability
to execute on our business plan as outlined in this report will be impaired.
We
sell our products through our direct sales force and distribution channel. As of the date of this report, we have 81 employees in our
sales department and 5,000 independent sales agents, 3,004 distributors for domestic sales and 324 exporting distributors for overseas
sales. Each of the aforementioned 324 exporting distributors may sell medical devices to at least three overseas customers. We have also
established a cooperative network with more than 525 hospitals through our direct sales network. For the years ended December 31, 2022,
2021, and 2020, our direct sales force contributed 9.16%, 9.13%, and 10.59% a, respectively, to our revenues, and distributors contributed
90.84%, 90.87%, and 89.41%, respectively, to our revenues.
Although
our sales and distribution satisfy our existing business needs, they might be insufficient to meet demand for our products as we continue
to grow our business, which could result in harm to our sales and business operations, financial condition and results of operations.
To mitigate such risk, we intent to invest our internally generated cash from operations and capital to be raised to add additional teams
to our direct sales force, expand our geographic reach with new distribution channels into other provinces within China and overseas.
If our planned efforts to expand our direct sales force and distribution channels are not effective, our ability to execute on our business
plan and to realize continued growth with be impaired.
If
we fail to provide a one-stop solution to our customers, we may lose customers, which would cause our financial conditions and results
of operations to be adversely affected.
We
sell our own brand of products and the products of other brands. For the fiscal year ended December 31, 2022, 2021, and 2020, we recognized
total revenues of $103,346,341, $104,037,710, and $89,061,010, respectively, of which our own brand sales accounted for 48.88%, 46.19%,
and 49.94%, respectively, and the resales of sourced disposable medical devices from other manufacturers accounted for 51.12%, 53.81%,
and 50.06%, respectively.
When
we receive an order containing products out of our product portfolio, we may procure such products per the specific order requirements
from other manufacturers and provide our customers with a one-stop shopping experience. Lack of availability of these products from other
manufacturers, whether due to shortages in supply, delays or interruptions in processing, failure of timely delivery or otherwise, could
interrupt our operations and adversely affect our financial results.
Our
industry is intensely competitive. We may face competition from, and we may be unable to compete successfully against, new entrants and
established companies with greater resources.
The
medical device industry is intensely competitive and includes thousands of companies both domestically and internationally. As more medical
device companies seek to outsource more of the design, prototyping and manufacturing of their products, we will face increasing competitive
pressures to grow our business in order to maintain our competitive position and we may encounter competition from, and lose customers
to, other companies with design, technological and manufacturing capabilities similar to ours. Some of our potential competitors may
have greater name recognition, greater operating revenues, larger customer bases, longer customer relationships and greater financial,
technical, personnel and marketing resources than we have. If we are unsuccessful competing with our competitors for our existing and
prospective customers’ business, our financial conditions and results of operation may be adversely affected.
Furthermore,
increased competition may reduce our market share and profitability and require us to increase our sales and marketing efforts and capital
commitment in the future, which could negatively affect our results of operations or force us to incur further losses. Although we continually
aim to grow our customer base, there is no assurance that we will be able to continue to do so in the future against current or future
competitors, and such competitive pressures may have a material adverse effect on our business, financial condition and results of operations.
The
continuing development of our products depends upon our maintaining strong working relationships with our customers, distributors and
independent sales agents.
The
research, development, marketing and sales of our current products and potential new and improved products or future product indications
for which we receive regulatory clearance or approval depend upon our maintaining working relationships with our customers, distributors
and independent sales agents. See “Our Research and Development” below. We rely on those professionals to provide
us with considerable knowledge and experience regarding the research, development, marketing and sales of our products. Distributors
and independent sales agents assist us in marketing and sales, as well as collecting customers’ feedbacks and advice related to
our products. Researchers at hospital and medical institution customers keep us informed of their latest requirements and R&D results.
If we cannot maintain our strong working relationships with these professionals and continue to receive their advice and input, the development,
improvement and marketing of our products could suffer, which could have a material adverse effect on our business, financial condition
and results of operations.
Technological
change may adversely affect sales of our products and may cause our products to become obsolete.
The
medical device market is characterized by extensive research and development and rapid technological change. Technological progress or
new developments in our industry could adversely affect sales of our products. Our products could be rendered obsolete because of future
innovations by our competitors or others, which would have a material adverse effect on our business, financial condition and results
of operations.
Consolidation
in the medical device industry could have an adverse effect on our revenue and results of operations.
Many
medical device companies are consolidating to create new companies with greater market power. As the medical device industry consolidates,
competition to provide goods and services to industry participants will become more intense. These industry participants may try to use
their market power to negotiate price concessions or reductions for our products. If we reduce our prices because of consolidation in
the healthcare industry, our revenue would decrease, which could have a material adverse effect on our business, financial condition
and results of operations.
If
we fail to identify, acquire and develop other products, we may be unable to grow our business.
As
a significant part of our growth strategy, we intend to develop and commercialize additional products through our research and development
program or by acquiring additional technologies and patents from third parties. The success of this strategy depends upon our ability
to identify, select and acquire the technologies and patents on terms that are acceptable to us.
Any
patents and technology we identify or acquire may require additional development efforts prior to commercial manufacturing and sale,
including approval or clearance by the applicable regulatory authorities. All products are prone to the risks of failure inherent in
medical device product development, including the possibility that the product will not be shown to be sufficiently safe and effective
for approval or clearance by regulatory authorities. In addition, we cannot assure you that any such products that are approved or cleared
will be manufactured or produced economically, successfully commercialized or widely accepted in the marketplace.
If
we are unable to develop suitable potential products through internal research programs or by obtaining patents or technologies from
third parties, it could have a material adverse effect on our business, financial condition and results of operations.
If
we are not able to implement our strategies to achieve our business objectives, our business operations and financial performance will
be adversely affected.
Our
business plan and growth strategy are based on currently prevailing circumstances and the assumption that certain circumstances will
or will not occur, as well as the inherent risks and uncertainties involved in various stages of development. However, there is no assurance
that we will be successful in implementing our strategies or that our strategies, even if implemented, will lead to the successful achievement
of our objectives. If we are not able to successfully implement our strategies, our business operations and financial performance will
be adversely affected.
We
are dependent upon certain key executives and highly qualified managers and we cannot assure their retention.
Our
success depends, in part, upon the continued services of key members of our management team. Our executives’ and managers’
knowledge of the market, our business and our company represents a key strength of our business, which cannot be easily replicated. The
success of our business strategy and our future growth also depends on our ability to attract, train, retain and motivate skilled managerial,
sales, administration, development and operating personnel.
Although
we have developed a mature production system, a stable and reliable sales network and marketing team, a complete after-sales service
system, and a research and development process and team that keeps us abreast of market needs, and also developed complete management
systems, including personnel management and welfare systems, raw material procurement and supply systems, warehousing systems, safe production
and manufacturing procedures and systems, capital utilization management systems, sales and after-sales service systems, quality assessment,
review and inspection systems, labor safety security system, accountability system for violations of laws and disciplines, and a comprehensive
information feedback system, which ensure the normal business development of the company, if we loss any of our key personnel, there
can be no assurance that our existing personnel will be adequate or qualified to carry out our strategy, or that we will be able to hire
or retain experienced, qualified employees to carry out our strategy. The loss of one or more of our key management or operating personnel,
or the failure to attract and retain additional key personnel, could have a material adverse effect on our business, financial condition
and results of operations.
If
we fail to adopt new technologies to evolving customer needs or emerging industry standards, our business may be materially and adversely
affected.
To
remain competitive, we must continue to stay abreast of the constantly evolving industry trends and enhance and improve our technology
accordingly. Our success will depend, in part, on our ability to identify, develop or acquire leading technologies useful in our business.
There can be no assurance that we will be able to use new technologies effectively or meet customer’s requirements. If we are unable
to adapt in a cost-effective and timely manner in response to changing market conditions or customer preferences, whether for technical,
legal, financial or other reasons, our business may be materially and adversely affected.
Changes to our payment terms with both
customers and suppliers may materially adversely affect our operating cash flows.
We may experience significant pressure from our
suppliers to reduce the number of days our accounts payable remain outstanding. At the same time, we may experience pressure from our
customers to extend the number of days before paying our accounts receivable. Any failure to manage our accounts payable and accounts
receivable may have a material adverse effect on our business, financial condition and results of operations.
If we are unable to collect account receivables
from our customers, our results of operations and cash flows could be adversely affected.
Our business depends on our ability to successfully
obtain payment from customers of the amounts they owe us for products sold. As of December 31, 2022 and 2021, our accounts receivable
balance amounted to $68,945,792 and $67,101,297, respectively. This growth in receivables reflects, in part, our growth in sales. If
we are unable to timely collect our accounts receivable on a timely and consistent basis, however, our cash flows and access to operating
capital could be adversely affected.
If we fail to manage our inventory effectively,
our operations and financial condition may be materially and adversely affected.
If we fail to manage our inventory effectively,
we may be subject to a heightened risk of inventory obsolescence, a decline in inventory value, and significant inventory write-downs
or write-offs. As of December 31, 2022 and 2021, our inventory balance amounted to $1,122,038 and $1,251,393, respectively.
Economic recessions could have a significant,
adverse impact on our business.
Our revenues are generated from sales of medical
devices both domestically and internationally and we anticipate that revenues from such sales will continue to represent a substantial
portion of our total revenues in the near future. Our sales and earnings can also be affected by changes in the general economy.
The medical device industry historically has
experienced cyclical fluctuations in financial results due to economic recession, downturns in business cycles of our customers, interest
rate fluctuations, and other economic factors beyond our control. Deterioration in the economic environment subjects our business to
various risks, which may have a material and adverse impact on our operating results and cause us to not reach our long-term growth goals.
For example, a downturn in the economy could directly affect the discretionary spending power of our customers and in turn, depress the
number of orders for our products.
We may be subject to intellectual property
infringement claims, which may be expensive to defend and may disrupt our business and operations.
We cannot be certain that our operations or any
aspects of our business do not or will not infringe upon or otherwise violate intellectual property rights held by third parties. For
instance, our subsidiary, Jiangsu Huadong was historically a third defendant in an intellectual property infringement case, under which
certain products purchased by Jiangsu Huadong involved patent infringement. As of the date of this report, such case has been settled
and Jiangsu Huadong has permanently stopped purchasing and selling the infringing products and destroyed the products in stock as requested
by court’s decision. In the future, however, we may be subject to other legal proceedings and claims relating to the intellectual
property rights of others. Such future proceedings could also involve existing intellectual property of which we are not aware and on
which our products may inadvertently infringe. We cannot assure you that holders of intellectual property purportedly relating to some
aspect of our technology or business, if any such holders exist, would not bring such intellectual property claims or enforcement actions
against us in China or any other jurisdiction. If we are found to have violated the intellectual property rights of others, we may be
subject to liability for our infringement activities or may be prohibited from using such intellectual property going forward, and, in
addition, we may incur licensing fees or be forced to develop alternatives of our own. In addition, we may incur significant expenses,
and may be forced to divert management’s time and other resources from our business and operations to defend against these infringement
claims, regardless of their merits. Successful infringement or licensing claims made against us may result in significant monetary liabilities
and may materially disrupt our business and operations by restricting or prohibiting our use of the intellectual property in question,
and our business, financial position and results of operations could be materially and adversely affected.
Further, the application and interpretation of
China’s patent laws and the procedures and standards for granting patents in China are still evolving and uncertain, and we cannot
assure you that PRC courts or regulatory authorities would agree with our analysis or interpretation concerning their implementation.
We may not be able to prevent others from
the unauthorized use of our intellectual property, which could materially harm our business and competitive position.
We rely on a combination of trademark, fair trade
practice, patent, copyright and trade secret protection laws in China, as well as confidentiality procedures and contractual provisions,
to protect our intellectual property rights. We enter into confidentiality agreements with our employees that include terms identifying
all employee-developed intellectual property as service inventions belonging to the Company. In addition, we are carful to remain current
in our annual patent fee payments. We regard our trademark, patents, know-how, proprietary technologies, and similar intellectual property
as critical to our success. We may become an attractive target to intellectual property attacks in the future with the increasing recognition
of our brand. Any of our intellectual property rights could be challenged, invalidated, circumvented or misappropriated, or such intellectual
property may not be sufficient to provide us with competitive advantages. In addition, there can be no assurance that (i) all of
our intellectual property rights will be adequately protected, or (ii) our intellectual property rights will not be challenged by
third parties or found by a judicial authority to be invalid or unenforceable. Intellectual property protection may not be sufficient
in China. Confidentiality agreements may be breached by counterparties, and there may not be adequate remedies available to us for any
such breach. Accordingly, we may not be able to effectively protect our intellectual property rights or to enforce our contractual rights
in China. In addition, policing any unauthorized use of our intellectual property is difficult, time-consuming and costly and the steps
we have taken may be inadequate to prevent the misappropriation of our intellectual property. In the event that we must resort to litigation
to enforce our intellectual property rights, such litigation could result in substantial costs and a diversion of our managerial and
financial resources. We can provide no assurance that we will prevail in such litigation. In addition, our trade secrets may be leaked
or otherwise become available to, or be independently discovered by, our competitors. Any failure in protecting or enforcing our intellectual
property rights could have a material adverse effect on our business, financial condition and results of operations.
Changes in U.S. and international trade
policies, particularly with regard to China, may adversely impact our business and operating results.
The U.S. government has recently made statements
and taken certain actions that may lead to potential changes to U.S. and international trade policies, including imposing tariffs affecting
certain products manufactured in China. It is unknown whether and to what extent new tariffs (or other new laws or regulations) will
be adopted, or the effect that any such actions would have on us or our industry and customers. Although cross-border business currently
contributes a small portion of our business, as we continue to sell our products internationally in the future, any unfavorable government
policies on international trade, such as capital controls or tariffs, may affect the demand for our products and services, impact the
competitive position of our products or prevent us from being able to sell products in certain countries. If any new tariffs, legislation
and/or regulations are implemented, or if existing trade agreements are renegotiated or, in particular, if the U.S. government takes
retaliatory trade actions due to the recent U.S.-China trade tension, such changes could have an adverse effect on our business, financial
condition, results of operations.
We have no business liability or disruption
insurance, which could expose us to significant costs and business disruption.
The insurance industry in China is still at an
early stage of development, and insurance companies in China currently offer limited business-related insurance products. We do not have
any business liability or disruption insurance to cover our operations. We have determined that the costs of insuring for these risks
and the difficulties associated with acquiring such insurance on commercially reasonable terms make it impractical for us to have such
insurance. Any uninsured risks may result in substantial costs and the diversion of resources, which could adversely affect our results
of operations and financial condition.
We may incur liabilities that are not covered
by insurance.
While we seek to maintain appropriate levels
of insurance, not all claims are insurable and we may experience major incidents of a nature that are not covered by insurance. We maintain
accident insurance for some of our high risk employees, such as electricians. We also provide social security insurance including pension,
medical insurance, unemployment insurance, maternity insurance, on-the-job injury insurance and housing fund plans through a PRC government-mandated
benefit contribution plan for our employees. We do not carry any key-man life insurance, product liability and professional liability
insurance. Even if we were to purchase these kinds of insurance, the insurance may not fully protect us from the financial impact of
defending against product liability or professional liability claims. We have not purchased any property insurance or business interruption
insurance. We have determined that the costs of insuring for related risks and the difficulties associated with acquiring such insurance
on commercially reasonable terms make it impractical. We consider our insurance coverage to be sufficient for our business operations
in China. We maintain an amount of insurance protection that we believe is adequate, but there can be no assurance that such insurance
will continue to be available on acceptable terms or that our insurance coverage will be sufficient or effective under all circumstances
and against all liabilities to which we may be subject. If we were to incur substantial losses or liabilities due to fire, explosions,
floods, other natural disasters or accidents or business interruption, our results of operations could be materially and adversely affected.
We could, for example, be subject to substantial claims for damages upon the occurrence of several events within one calendar year. In
addition, our insurance costs may increase over time in response to any negative development in our claims history or due to material
price increases in the insurance market in general.
Pandemics and epidemics, natural disasters,
terrorist activities, political unrest, and other outbreaks could disrupt our delivery and operations, which could materially and adversely
affect our business, financial condition, and results of operations.
Global pandemics, epidemics in China or elsewhere
in the world, or fear of spread of contagious diseases, such as Ebola virus disease (EVD), coronavirus disease 2019 (COVID-19), Middle
East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), H1N1 flu, H7N9 flu, and avian flu, as well as hurricanes,
earthquakes, tsunamis, or other natural disasters could disrupt our business operations, reduce or restrict our supply of products and
services, incur significant costs to protect our employees and facilities, or result in regional or global economic distress, which may
materially and adversely affect our business, financial condition, and results of operations. Actual or threatened war, terrorist activities,
political unrest, civil strife, and other geopolitical uncertainty could have a similar adverse effect on our business, financial condition,
and results of operations. Any one or more of these events may impede our production and delivery efforts and adversely affect our sales
results, or even for a prolonged period of time, which could materially and adversely affect our business, financial condition, and results
of operations.
We are also vulnerable to natural disasters and
other calamities. We cannot assure you that we are adequately protected from the effects of fire, floods, typhoons, earthquakes, power
loss, telecommunications failures, break-ins, war, riots, terrorist attacks, or similar events. Any of the foregoing events may give
rise to interruptions, damage to our property, delays in production, breakdowns, system failures, technology platform failures, or internet
failures, which could cause the loss or corruption of data or malfunctions of our manufacturing facility as well as adversely affect
our business, financial condition, and results of operations.
Our international sales are subject to
a variety of risks that could adversely affect our profitability and operating results.
We sell disposable medical devices internationally.
For the fiscal years ended December 31, 2022, 2021, and 2020, our international sales accounted for 0.48%, 14.22%, and 18.10%, respectively,
of our revenues. Although we take measures to minimize risks inherent to our international sales, the following risks may have a negative
effect on our profitability and operating results, impair the performance of our foreign sales or otherwise disrupt our business:
| ● | fluctuations
in the value of currencies could cause exchange rates to change and impact our profitability; |
| ● | greater
difficulty in collecting accounts receivable and longer payment cycles, which can be more
common in our international sales, could adversely impact our operating results over a particular
fiscal period; and |
| ● | changes
in foreign regulations, export duties, taxation and limitations on imports or exports could
increase our operational costs, impose fines or restrictions on our ability to carry on our
business or expand our international sales. |
We are subject to a variety of environmental
laws that could be costly for us to comply with, and we could incur liability if we fail to comply with such laws or if we are responsible
for releases of contaminants to the environment.
Our operating subsidiaries are all located in
PRC. The manufacturing of our products will generate wastewater, exhaust gas, solid waste and equipment noise. Chinese laws impose various
environmental controls on the management, handling, generation, manufacturing, transportation, storage, use and disposal of wastewater,
exhaust gas, solid waste, equipment noise and other materials used or generated in the manufacturing of our products. If we fail to comply
with any present or future environmental laws, we could be subject to fines, corrective action, other liabilities or the suspension of
production.
We pay great attention to environmental protection
and governance and have formulated a systematic environmental protection management system in the treatment of our wastewater, exhaust
gas, solid waste and equipment noise in accordance with national requirements. However, changes in environmental laws may result in costly
compliance requirements or otherwise subject us to future liabilities. To the extent these changes affect our customers and require changes
to their demand in medical devices, our customers may have a reduced need for our products, and, as a result, our revenue could adversely
affect.
In addition, with the implementation of the fund-raising
investment project, our pollutant emissions will increase, resulting in the increase of the environmental protection spending and the
difficulty of environmental protection management, which could have an adverse effect on our financial conditions and results of operations.
As of the date of this report, the Company is
not aware of any investigations, prosecutions, disputes, claims or other proceedings in respect of environmental protection, nor has
the Company been punished or can foresee any punishment to be made by any environmental administration authorities of the PRC.
Failure to keep up with the changes in
domestic industry policies or standards could have a material and adverse effect on our reputation, financial condition and results of
operations.
As a medical device manufacturer, the products
we manufacture and sell are closely related to human health, which is subject to strict supervision by relevant Chinese authorities.
The related national government authorities have issued a series of regulatory guidelines and industry policies to ensure the healthy
development of the industry. In recent years, as China further deepens the reform of its medical and health system, relevant government
departments have successively implemented a series of regulations and policies regarding industry standards, bidding, price formation
mechanisms, circulation systems and other related fields, which have brought wide and profound impact on the livelihood and development
of pharmaceutical companies.
In April 2016, the General Office of the State
Council issued the Notice on Key Tasks for Deepening the Reform of the Pharmaceutical and Healthcare System in 2016, proposing to actively
encourage the implementation of the “two-invoice system” in pilot cities for comprehensive reform of public hospitals. In
December 2016, the Medical Reform Office of the State Council promulgated the Opinions on the Implementation of the “Two-Invoice
System” in Drug Procurement by Public Medical Institutions (for Trial Implementation), which means that the “Two Invoice
System” has been officially launched and will be further promoted nationwide. Under the “two-invoice system,” invoices
are issued once when pharmaceutical products are sold from manufacturers to wholesalers; and then, invoices are issued again when wholesalers
resell the products to hospitals. The aim is to shorten circulation links and reduce hospital procurement costs. Under the “two-invoice
system,” consumable products manufacturers with advantages of brand and economies of scale could increase their coverage of terminals.
At the same time, the “two-invoice system” also presents consumable products manufacturers with higher requirements for the
construction and optimization of marketing channels. Manufacturers will need to grow their marketing teams, expand sales networks and
improve refined service capabilities.
The deepening of the reform of the domestic pharmaceutical
industry and the strengthening of supervision may affect our operations and profitability in the domestic market. If we fail to adapt
to the profound changes in industry policies in a timely manner, it could materially and adversely affect our business, financial condition
and results of operations.
We depend on our professional technology
research and development talents and we cannot assure their retention.
Our success partly depends upon the retention
of our professional technology research and development talents (“R&D Talents”). As a high-tech enterprise in the medical
device industry, we have a professional R&D Talents team comprised of 30 employees as of the date of this report, who have expertise
in polymer materials, medicine, molds, and mechanical automation and possess high-level professional technology expertise and profound
industry experience. Among those R&D Talents, seven have a bachelor’s degree and the other 23 have received an associate degree.
There can be no assurance that our existing R&D Talents will be adequate or qualified to carry out our strategy, or that we will
be able to hire or retain new R&D Talents to carry out our strategy. The loss of one or more of our R&D Talents, or the failure
to attract and retain additional R&D Talents, could have a material adverse effect on our business, financial condition and results
of operations.
In addition, if we fail to establish a competitive
incentive mechanism in terms of career prospects, salary, benefits and working environment, we may face the risk of instability in the
scientific research team, which could adversely affect our long-term development.
Loss of certain procurement bids could
have a material and adverse effect on our reputation, financial conditions and results of operations.
According to the Notice of the Ministry of Health
on Further Strengthening the Management of Centralized Procurement of Medical Devices (Wei Gui Cai Fa [2007] No. 208), centralized procurement
of medical equipment within the large medical equipment management list is required. For other medical equipment and consumables, the
provincial health administrative department shall study and formulate a centralized procurement catalog at the provincial and prefecture
level based on actual conditions. In recent years, China has actively promoted a provincial-level bidding platform for medical devices
with reference to the drug procurement bidding platform. At present, some provinces and cities have conducted a unified bid for some
types of medical devices. According to the Notice of the Office of the National Healthcare Security Administration on Centralized Pharmaceutical
Purchasing and Price Management in 2023 (Yi Bao Ban Han [2023] No. 13) and the Notice on Key Tasks for Deepening the Reform of the Pharmaceutical
and Healthcare System in 2022 (Guo Ban Fa [2022] No. 14),it is a strategy to continually promote the volume-based centralized
procurement for medical devices in a long-term period.
Under the centralized procurement model, the
price information becomes more transparent and open, which will put greater downward pressure on the winning bid price of the product.
If we lose a bid to our competitors due to the disadvantage of product price or other reason, we will lose some hospital customers. If
the local procurement platform does not solicit a supplementary bid for a long time or reopen the bid, it could have a material and adverse
effect on our reputation, financial conditions and results of operations.
If our employees or customers are involved
in improper medical device sales transactions, it could adversely affect our reputation, financial conditions and results of operations.
We have established and improved our internal
control system against unfair business practices, to prevent, minimize and eliminate employees and customers improper behaviors in the
medical device sales transactions, including unauthorized rebates. There can be no assurance that our existing internal control system
will be adequate to prevent, minimize and eliminate such improper transactions, that we will be able to effectively implement our internal
control polices, or that we will be able to perfect our internal control system to eliminate such improper transactions. If any individual
employees or downstream customers have improper business practices in the purchase and sale of medical devices, we may be identified
by the relevant regulatory authorities as a violator of relevant laws and regulations and thus included in the blacklist of commercial
records, which could adversely affect our reputation, financial conditions and results of operations.
If we fail to timely renew our medical
device licenses or registration certificates, it could adversely affect our reputation, financial conditions and results of operations.
As a medical device manufacturer with all of
our operating subsidiaries located in PRC, all of our manufacturing and sales activities must comply with relevant Chinese laws and regulations.
Pursuant to the Measures for the Administration of Registration of Medical Devices promulgated on June 27, 2014and effective on October
1, 2014, as amended from time to time and currently amended as Measures for the Administration of Registration and Recordation of Medical
Devices effective on October 1, 2021, Class I medical devices are subject to recordation administration with Class II and Class III medical
devices subject to registration administration. We are in the business of manufacturing and sales of Class I, II and III medical devices.
If we fail to timely record or register our medical devices, our financial conditions and results of operations will be adversely affected.
As of the date of this report, we are current
in the recording and/or registration of all of our medical devices.
Risks Related to Our Corporate Structure
Our directors and officers currently own an
aggregate of 63.54% of the total voting power of our outstanding Ordinary Shares.
Currently, our directors and officers collectively
own an aggregate of 63.54% of the total voting power of our outstanding Ordinary Shares. These beneficial owners could have significant
influence on determining the outcome of any corporate transaction or other matter submitted to the shareholders for approval, including
mergers, consolidations, the election of directors and other significant corporate actions. In cases where their interests are aligned
and they vote together, these beneficial owners will also have the power to prevent or cause a change in control. Without the consent
of some or all of these shareholders, we may be prevented from entering into transactions that could be beneficial to us or
our minority shareholders. The interests of these beneficial owners may differ from the interests of our other shareholders. The concentration
in the ownership of our Ordinary Shares may cause a material decline in the value of our Ordinary Shares.
You may face difficulties in protecting your
interests, and your ability to protect your rights through U.S. courts may be limited because we are incorporated under Cayman Islands
law.
We are an exempted company incorporated under
the laws of the Cayman Islands. Our corporate affairs are governed by our amended and restated memorandum and articles of association,
the Companies Act (As Revised) of the Cayman Islands (the “Companies Act”) and the common law of the Cayman Islands. The
rights of shareholders to take action against the directors, actions by minority shareholders and the fiduciary duties of our directors
to us under Cayman Islands law are to a large extent governed by the common law of the Cayman Islands. The common law of the Cayman Islands
is derived in part from comparatively limited judicial precedent in the Cayman Islands as well as from the common law of England, the
decisions of whose courts are of persuasive authority, but are not binding, on a court in the Cayman Islands. The rights of our shareholders
and the fiduciary duties of our directors under Cayman Islands law are not as clearly established as they would be under statutes or
judicial precedent in some jurisdictions in the United States. In particular, the Cayman Islands has a different body of securities laws
than the United States. Some U.S. states, such as Delaware, have more fully developed and judicially interpreted bodies of corporate
law than the Cayman Islands. In addition, Cayman Islands companies may not have standing to initiate a shareholder derivative action
in a federal court of the United States. There is no statutory recognition in the Cayman Islands of judgments obtained in the United
States, although the courts of the Cayman Islands will in certain circumstances recognize and enforce a non-penal judgment of a foreign
court of competent jurisdiction without retrial on the merits.
Shareholders of Cayman Islands exempted companies
like us have no general rights under Cayman Islands law to inspect corporate records (other than the memorandum and articles of association
and any special resolutions passed by such companies, and the register of mortgages and charges of such companies) or to obtain copies
of lists of shareholders of these companies. Our directors have discretion under our articles of association to determine whether or
not, and under what conditions, our corporate records may be inspected by our shareholders, but are not obliged to make them available
to our shareholders. This may make it more difficult for you to obtain the information needed to establish any facts necessary for a
shareholder motion or to solicit proxies from other shareholders in connection with a proxy contest.
Certain corporate governance practices in the
Cayman Islands, which is our home country, differ significantly from requirements for companies incorporated in other jurisdictions such
as the United States. To the extent we choose to follow home country practice with respect to corporate governance matters, our shareholders
may be afforded less protection than they otherwise would under rules and regulations applicable to U.S. domestic issuers.
As a result of all of the above, our public shareholders
may have more difficulty in protecting their interests in the face of actions taken by management, members of the board of directors
or controlling shareholders than they would as public shareholders of a company incorporated in the United States.
You may be unable to present proposals
before annual general meetings or extraordinary general meetings not called by shareholders.
Cayman Islands law provides shareholders with
only limited rights to convene a general meeting and does not provide shareholders with any right to put any proposal before a general
meeting. However, these rights may be provided in a company’s articles of association. Our articles of association allow our shareholders
holding shares representing in aggregate not less than ten percent of our voting share capital in issue, to convene a general meeting
of our shareholders, in which case our directors are obliged to call such meeting. Advance notice of at least seven days is required
for the convening of our general meetings. A quorum required for a meeting of shareholders consists of at least one shareholder present
or by proxy, representing a majority of the paid up voting share capital in the Company.
Recently introduced economic substance
legislation of the Cayman Islands may impact the Company or its operations
The Cayman Islands, together with several other
non-European Union jurisdictions, have recently introduced legislation aimed at addressing concerns raised by the Council of the European
Union as to offshore structures engaged in certain activities which attract profits without real economic activity. Effective January
1, 2019, the International Tax Co-operation (Economic Substance) Act, 2018 (the “Substance Law”) and issued Regulations and
Guidance Notes came into force in the Cayman Islands introducing certain economic substance requirements for “relevant entities”
which are engaged in certain “relevant activities,” which in the case of exempted companies incorporated before January 1,
2019, will apply in respect of fiscal years commencing July 1, 2019, onwards. A “relevant entity” includes an exempted company
incorporated in the Cayman Islands; however, it does not include an entity that is tax resident outside the Cayman Islands. Accordingly,
for so long as the Company is a tax resident outside the Cayman Islands, it is not required to satisfy the economic substance test under
the Substance Law. Although it is presently anticipated that the Substance Law will have little material impact on the Company or its
operations, as the legislation is new and remains subject to further clarification and interpretation it is not currently possible to
ascertain the precise impact of these legislative changes on the Company.
Certain judgments obtained against us by
our shareholders may not be enforceable.
We are a Cayman Islands company and substantially
all of our assets are located in mainland China. All of our current business operations are conducted in mainland China.
In addition, all of our current officers, including
Yongjun Liu, Xin Wang, Lianzhang Zhao, Xiaoming E, Huijuan Zhao and Wenzhang Jia are nationals and residents of the PRC. Substantially
all of the assets of these persons are located in the PRC. As a result, it may be difficult or impossible for you to bring an action
against us or against these individuals in the United States in the event that you believe that your rights have been infringed under
the U.S. federal securities laws or otherwise. Even if you are successful in bringing an action of this kind, the laws of the Cayman
Islands and of the PRC may render you unable to enforce a judgment against our assets or the assets of our directors and officers.
Risks Related to Doing Business in China
Because all of our operations are in China,
our business is subject to the complex and rapidly evolving laws and regulations there. The Chinese government may exercise significant
oversight and discretion over the conduct of our business and may intervene in or influence our operations at any time, which could result
in a material change in our operations and/or the value of our Ordinary Shares.
As a business operating in China, we are subject
to the laws and regulations of the PRC, which can be complex and evolve rapidly. The PRC government has the power to exercise significant
oversight and discretion over the conduct of our business, and the regulations to which we are subject may change rapidly and with little
notice to us or our shareholders. As a result, the application, interpretation, and enforcement of new and existing laws and regulations
in the PRC are often uncertain. In addition, these laws and regulations may be interpreted and applied inconsistently by different agencies
or authorities, and inconsistently with our current policies and practices. New laws, regulations, and other government directives in
the PRC may also be costly to comply with, and such compliance or any associated inquiries or investigations or any other government
actions may:
| ● | Delay
or impede our development, |
| ● | Result
in negative publicity or increase our operating costs, |
| ● | Require
significant management time and attention, and |
| ● | Subject
us to remedies, administrative penalties and even criminal liabilities that may harm our
business, including fines assessed for our current or historical operations, or demands or
orders that we modify or even cease our business practices. |
The promulgation of new laws or regulations,
or the new interpretation of existing laws and regulations, in each case that restrict or otherwise unfavorably impact the ability or
manner in which we conduct our business and could require us to change certain aspects of our business to ensure compliance, which could
decrease demand for our products, reduce revenues, increase costs, require us to obtain more licenses, permits, approvals or certificates,
or subject us to additional liabilities. To the extent any new or more stringent measures are required to be implemented, our business,
financial condition and results of operations could be adversely affected as well as materially decrease the value of our Ordinary Shares.
If the Chinese government chooses to exert
more oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers, such action could
significantly limit or completely hinder our ability to offer or continue to offer securities to investors outside of China and, as a
result, cause the value of such securities to significantly decline or be worthless.
Recent statements by the Chinese government have
indicated an intent to exert more oversight and control over offerings that are conducted outside of China and/or foreign investments
in China based issuers. The PRC has recently promulgated new rules that require companies collecting or holding large amounts of data
to undergo a cybersecurity review prior to listing in foreign countries, a move that will significantly tighten oversight over China-based
internet giants. The Measures for Cybersecurity Review (2021 version) was promulgated on December 28, 2021 and became effective on February
15, 2022. These measures specify that any “online platform operators” controlling the personal information of more than one
million users which seek to list on a foreign stock exchange are subject to prior cybersecurity review.
As our business belongs to the medical instrument
industry in China, and our business does not involve the collection of user data or involve any other type of restricted industry, our
business is generally outside the scope of the Measures for Cybersecurity Review. Based on the advice of counsel and our understanding
of currently applicable PRC laws and regulations, our recent registered initial public offering in the U.S. was not subject to the review
or prior approval of the CAC or the CSRC. Uncertainties still exist, however, due to the possibility that laws, regulations or policies
in the PRC could change or rapidly evolve in the future. Any future action by the PRC government expanding the categories of industries
and companies whose foreign securities offerings are subject to review by the CSRC or the CAC could significantly limit or completely
hinder our ability to offer or continue to offer securities to investors and could cause the value of such securities to significantly
decline or be worthless.
Potential Chinese governmental and regulatory
interference could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause
the value of such securities to significantly decline or be worthless.
On July 6, 2021, the General Office of the Central
Committee of the Communist Party of China and the General Office of the State Council jointly issued the “Opinions on Severely
Cracking Down on Illegal Securities Activities According to Law,” or the Opinions. The Opinions emphasized the need to strengthen
the administration over illegal securities activities, and the need to strengthen the supervision over overseas listings by Chinese companies.
Effective measures, such as promoting the construction of relevant regulatory systems will be taken to deal with the risks and incidents
of China-concept overseas listed companies, and cybersecurity and data privacy protection requirements and similar matters.
In addition, an overseas offering and listing
is prohibited under any of the following circumstances: (1) if the intended securities offering and listing is specifically prohibited
by national laws and regulations and relevant provisions; (2) if the intended securities offering and listing may constitute a threat
to or endangers national security as reviewed and determined by competent authorities under the State Council in accordance with law;
(3) if there are material ownership disputes over the equity, major assets, and core technology, etc. of the issuer; (4) if, in the past
three years, the domestic enterprise or its controlling shareholders or actual controllers have committed corruption, bribery, embezzlement,
misappropriation of property, or other criminal offenses disruptive to the order of the socialist market economy, or are currently under
judicial investigation for suspicion of criminal offenses, or are under investigation for suspicion of major violations; (5) if, in past
three years, directors, supervisors, or senior executives have been subject to administrative punishments for severe violations, or are
currently under judicial investigation for suspicion of criminal offenses, or are under investigation for suspicion of major violations;
(6) other circumstances as prescribed by the State Council. The Administration Provisions defines the legal liabilities of breaches such
as failure in fulfilling filing obligations or fraudulent filing conducts, imposing a fine between RMB 1 million and RMB 10 million,
and in cases of severe violations, a parallel order to suspend relevant business or halt operation for rectification, revoke relevant
business permits or operational license.
On February 17, 2023, the CSRC promulgated the
Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Companies, or the Trial Measures, and five supporting
guidelines, which came into effect on March 31, 2023. The Trial Measures and its supporting guidelines reiterate the basic principles
of the Draft Rules Regarding Overseas Listing and impose substantially the same requirements for the overseas securities offering and
listing by domestic enterprises. Pursuant to the Trial Measures, domestic companies that seek to offer or list securities overseas, both
directly and indirectly, shall complete the filing procedures with the CSRC pursuant to the requirements of the Trial Measures within
three working days following its submission of initial public offerings or listing application, or its completion of follow-on offering
in the same overseas market where it has listed (including issuance of any corporate convertible bonds, exchangeable bonds and other
equity-linked securities, but excluding the offering for employees incentive, dividend distribution by shares and share split). If a
domestic company fails to complete the required filing procedures or conceals any material fact or falsifies any major content in its
filing documents, such domestic company may be subject to administrative penalties, such as orders to rectify, warnings, fines, and its
controlling shareholders, actual controllers, the person directly in charge and other directly liable persons may also be subject to
administrative penalties, such as warnings and fines.
According to the Notice on the Administrative
Arrangements for the Filing of the Overseas Securities Offering and Listing by Domestic Companies from the CSRC, or the CSRC Notice,
which was promulgated on February 17, 2023 and became effective on the same day, the domestic companies that have already been listed
overseas before the effective date of the Trial Measures (i.e. March 31, 2023) shall be deemed as existing issuers (the “Existing
Issuers”). Existing Issuers are not required to complete the filing procedures immediately, and they shall be required to file
with the CSRC for any subsequent offerings. Further, according to the CSRC Notice, domestic companies obtained approval from overseas
regulatory authorities or securities exchanges (for example, the effectiveness of a registration statement for offering and listing in
the U.S. has been approved) for their indirect overseas offering and listing prior to March 31, 2023 but have not yet completed their
indirect overseas issuance and listing, are granted a six-month transition period from March 31, 2023 to September 30, 2023. Those issuers
that complete their indirect overseas offering and listing within such six-month period are deemed as Existing Issuers and are not required
to file with the CSRC for their indirect overseas offerings and listings. Within such six-month transition period, however, if such domestic
companies fail to complete their indirect overseas issuance and listing, they shall complete the filing procedures with the CSRC.
However, since the Trial Measures were newly
promulgated, their interpretation, application and enforcement remain unclear. If the filing procedure with the CSRC under the Trial
Measures is required for any subsequent offerings, listing or any other capital raising activities, which may subject us to additional
compliance requirements in the future, we cannot assure you that we will be able to get the clearance of filing procedures under the
Trial Measures on a timely basis, or at all. If we do not complete any required record-filing or if we incorrectly conclude that record-filing
is not required or if the CSRC or other regulatory agencies promulgate new rules, explanations or interpretations requiring that we obtain
their prior approvals or record-filing for any follow-on offering, we may be unable to obtain such approvals and record-filing which
could significantly limit or completely hinder our ability to offer or continue to offer securities to our investors.
Furthermore, the PRC government authorities may
strengthen oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers like us.
Such actions taken by the PRC government authorities may intervene or influence our operations at any time, which are beyond our control.
Therefore, any such action may adversely affect our operations and significantly limit or hinder our ability to offer or continue to
offer securities and reduce the value of such securities.
As of the date of this report, we and our PRC
subsidiaries have not been involved in any investigations on cybersecurity review initiated by the Cyber Administration of China or related
governmental regulatory authorities, and have not received any requirements to obtain permissions from any PRC authorities to issue our
Ordinary Shares to foreign investors or were denied such permissions by any PRC authorities. However, given the current PRC regulatory
environment, it is uncertain when and whether we or our PRC subsidiaries, will be required to obtain permission from the PRC government
to list on U.S. exchanges in the future, and even when such permission is obtained, whether it will be denied or rescinded.
We have been closely monitoring regulatory developments
in China regarding any necessary approvals from the CSRC or other PRC governmental authorities required for overseas listings. As of
the date of this report, except for the potential uncertainties disclosed above, we have not received any inquiry, notice, warning, sanctions
or regulatory objection from the CSRC or other PRC governmental authorities. However, there remains significant uncertainty as to the
enactment, interpretation and implementation of regulatory requirements related to overseas securities offerings and other capital markets
activities.
Changes in China’s economic, political
or social conditions or government policies could have a material adverse effect on our business and operations.
Substantially all of our assets and operations
are located in the PRC. Accordingly, our business, financial condition, results of operations and prospects may be influenced to a significant
degree by political, economic and social conditions in the PRC generally. The Chinese economy differs from the economies of most developed
countries in many respects, including the level of government involvement, development, growth rate, control of foreign exchange and
allocation of resources. Although the Chinese government has implemented measures emphasizing the utilization of market forces for economic
reform, the reduction of state ownership of productive assets, and the establishment of improved corporate governance in business enterprises,
a substantial portion of productive assets in the PRC is still owned by the government. In addition, the Chinese government continues
to play a significant role in regulating industry development by imposing industrial policies. The Chinese government also exercises
significant control over the PRC’s economic growth through allocating resources, controlling payment of foreign currency-denominated
obligations, setting monetary policy and providing preferential treatment to particular industries or companies.
While the Chinese economy has experienced significant
growth over past decades, growth has been uneven, both geographically and among various sectors of the economy. Any adverse changes in
economic conditions in the PRC, in the policies of the Chinese government or in the laws and regulations in the PRC could have a material
adverse effect on the overall economic growth of the PRC. Such developments could adversely affect our business and operating results,
lead to a reduction in demand for our services and adversely affect our competitive position. The Chinese government has implemented
various measures to encourage economic growth and guide the allocation of resources. Some of these measures may benefit the overall Chinese
economy but may nonetheless have a negative effect on us. For example, our financial condition and results of operations may be adversely
affected by government control over capital investments or changes in tax regulations. In addition, in the past the Chinese government
has implemented certain measures, including interest rate adjustment, to control the pace of economic growth. These measures may cause
decreased economic activity in the PRC, which may adversely affect our business and operating results.
Non-compliance with labor-related laws
and regulations of the PRC may have an adverse impact on our financial condition and results of operation.
We have been subject to stricter regulatory requirements
in terms of entering into labor contracts with our employees and paying various statutory employee benefits, including pensions, housing
fund, medical insurance, work-related injury insurance, unemployment insurance and childbearing insurance to designated government agencies
for the benefit of our employees. Pursuant to the PRC Labor Contract Law, or the Labor Contract Law, that became effective in January
2008 and its implementing rules that became effective in September 2008 and was amended in July 2013, employers are subject to stricter
requirements in terms of signing labor contracts, minimum wages, paying remuneration, determining the term of employees’ probation
and unilaterally terminating labor contracts. In the event that we decide to terminate some of our employees or otherwise change our
employment or labor practices, the Labor Contract Law and its implementation rules may limit our ability to effect those changes in a
desirable or cost-effective manner, which could adversely affect our business and results of operations. We believe our current practice
complies with the Labor Contract Law and its amendments. However, the relevant governmental authorities may take a different view and
impose fines on us.
As the interpretation and implementation of labor-related
laws and regulations are still evolving, we cannot assure you that our employment practice does not and will not violate labor-related
laws and regulations in China, which may subject us to labor disputes or government investigations. If we are deemed to have violated
relevant labor laws and regulations, we could be required to provide additional compensation to our employees and our business, financial
condition and results of operations could be materially and adversely affected.
The PRC’s economic, political and
social conditions, as well as changes in any government policies, laws and regulations, could have a material adverse effect on our business.
All of our operations are located in the PRC
and substantially of our net revenues are derived from customers where the contracting entity is located in the PRC. Accordingly, our
business, financial condition, results of operations, prospects and certain transactions we may undertake may be subject, to a significant
extent, to economic, political and legal developments in the PRC.
The PRC’s economy differs from the economies
of most developed countries in many respects, including the amount of government involvement, level of development, growth rate, control
of foreign exchange and allocation of resources. Although the PRC’s economy has been transitioning from a planned economy to a
more market-oriented economy since the late 1970s, the PRC government continues to play a significant role in regulating industry development
by imposing industrial policies. The PRC government also exercises significant control over the PRC’s economic growth through allocating
resources, controlling the incurrence and payment of foreign currency-denominated obligations, setting monetary policy and providing
preferential treatment to particular industries or companies. Changes in any of these policies, laws and regulations could adversely
affect the economy in the PRC and could have a material adverse effect on our business.
The PRC government has implemented various measures
to encourage foreign investment and sustainable economic growth and to guide the allocation of financial and other resources. However,
we cannot assure you that the PRC government will not repeal or alter these measures or introduce new measures that will have a negative
effect on us. PRC’s social and political conditions may change and become unstable. Any sudden changes to PRC’s political
system or the occurrence of widespread social unrest could have a material adverse effect on our business and results of operations.
Uncertainties with respect to the PRC legal
system could adversely affect us.
The PRC legal system is a civil law system based
on written statutes. Unlike the common law system, prior court decisions under the civil law system may be cited for reference but have
limited precedential value.
In 1979, the PRC government began to promulgate
a comprehensive system of laws and regulations governing economic matters generally. The overall effect of legislation over the past
three decades has significantly enhanced the protections afforded to various forms of foreign investments in the PRC. However, the PRC
has not developed a fully integrated legal system, and recently enacted laws and regulations may not sufficiently cover all aspects of
economic activities in the PRC. In particular, the interpretation and enforcement of these laws and regulations involve uncertainties.
Since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory provisions and
contractual terms, it may be difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection
we enjoy. These uncertainties may affect our judgment on the relevance of legal requirements and our ability to enforce our contractual
rights or tort claims. In addition, these regulatory uncertainties may be exploited through unmerited or frivolous legal actions or threats
in attempts to extract payments or benefits from us.
Furthermore, the PRC legal system is based in
part on government policies and internal rules, some of which are not published on a timely basis or at all and may have a retroactive
effect. As a result, we may not be aware of our violation of any of these policies and rules until sometime after the violation. In addition,
any administrative and court proceedings in the PRC may be protracted, resulting in substantial costs and diversion of resources and
management attention.
You may experience difficulties in effecting
service of legal process, enforcing foreign judgments or bringing actions in the PRC against us or our management named in the report
based on foreign laws.
We conduct substantially all of our operations
in the PRC, and substantially all of our assets are located in the PRC. In addition, all of our officers and directors are PRC nationals
and reside within PRC. As a result, it may be difficult for our shareholders to effect service of process upon us or our directors and
officers inside the PRC. In addition, the PRC does not have treaties providing for the reciprocal recognition and enforcement of judgments
of courts with the Cayman Islands and many other countries and regions. Therefore, recognition and enforcement in the PRC of judgments
of a court in any of these non-PRC jurisdictions in relation to any matter not subject to a binding arbitration provision may be difficult
or impossible.
We may rely on dividends and other distributions
on equity paid by our PRC subsidiaries to fund any cash and financing requirements we may have, and any limitation on the ability of
our PRC subsidiaries to make payments to us could have a material and adverse effect on our ability to conduct our business.
We rely principally on dividends and other distributions
on equity from our PRC subsidiaries for our cash requirements, including for services of any debt we may incur.
Our PRC subsidiaries’ ability to distribute
dividends is based upon their distributable earnings. Current PRC regulations permit our PRC subsidiaries to pay dividends to their respective
shareholders only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In
addition, each of our PRC subsidiaries, as a Foreign Invested Enterprise, or FIE, are required to draw 10% of its after-tax profits each
year, if any, to fund a common reserve, which may stop drawing its after-tax profits if the aggregate balance of the common reserve has
already accounted for over 50 percent of its registered capital. These reserves are not distributable as cash dividends. If our PRC subsidiaries
incur debt on their own behalf in the future, the instruments governing the debt may restrict their ability to pay dividends or make
other payments to us. Any limitation on the ability of our PRC subsidiaries to distribute dividends or other payments to their respective
shareholders could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our
business, pay dividends or otherwise fund and conduct our business.
In addition, the Enterprise Income Tax Law and
its implementation rules provide that a withholding tax rate of up to 10% will be applicable to dividends payable by Chinese companies
to non-PRC-resident enterprises unless otherwise exempted or reduced according to treaties or arrangements between the PRC central government
and governments of other countries or regions where the non-PRC resident enterprises are incorporated.
PRC regulation of loans to and direct investment
in PRC entities by offshore holding companies and governmental control of currency conversion may delay us from using the proceeds of
future securities offerings to make loans or additional capital contributions to our PRC subsidiaries, which could materially and adversely
affect our liquidity and our ability to fund and expand our business.
Any funds we transfer to our PRC subsidiaries,
either as a shareholder loan or as an increase in registered capital, are subject to approval by or registration with relevant governmental
authorities in the PRC. According to the relevant PRC regulations on foreign-invested enterprises, or FIEs, in the PRC, capital contributions
to our PRC subsidiaries are subject to the approval of or filing with the Ministry of Commerce, or MOFCOM or its local branches and registration
with a local bank authorized by the State Administration of Foreign Exchange, or SAFE. In addition, (i) a foreign loan of less one year
duration procured by our PRC subsidiaries is required to be registered with SAFE or its local branches and (ii) a foreign loan of one
year duration or more procured by our PRC subsidiaries is required to be applied to the NDRC in advance for undergoing recordation registration
formalities. Any medium or long-term loan to be provided by us to our PRC operating subsidiaries, must be registered with the NDRC and
the SAFE or its local branches. We may not be able to complete such registrations on a timely basis, with respect to future capital contributions
or foreign loans by us to our PRC subsidiaries. If we fail to complete such registrations, our ability to use the proceeds of any future
securities offerings and to capitalize our PRC operations may be negatively affected, which could adversely affect our liquidity and
our ability to fund and expand our business.
On March 30, 2015, the SAFE promulgated the Circular
on Reforming the Management Approach Regarding the Foreign Exchange Capital Settlement of Foreign-Invested Enterprises, or SAFE Circular
19, which took effect as of June 1, 2015. SAFE Circular 19 launched a nationwide reform of the administration of the settlement of the
foreign exchange capitals of FIEs and allows FIEs to settle their foreign exchange capital at their discretion, but continues to prohibit
FIEs from using the Renminbi fund converted from their foreign exchange capital for expenditure beyond their business scopes, providing
entrusted loans or repaying loans between nonfinancial enterprises. The SAFE issued the Circular on Reforming and Regulating Policies
on the Control over Foreign Exchange Settlement of Capital Accounts, or SAFE Circular 16, effective in June 2016. Pursuant to SAFE Circular
16, enterprises registered in China may also convert their foreign debts from foreign currency to Renminbi on a self-discretionary basis.
SAFE Circular 16 provides an integrated standard for conversion of foreign exchange under capital account items (including but not limited
to foreign currency capital and foreign debts) on a self-discretionary basis which applies to all enterprises registered in China. SAFE
Circular 16 reiterates the principle that Renminbi converted from foreign currency-denominated capital of a company may not be directly
or indirectly used for purposes beyond its business scope or prohibited by PRC laws or regulations, while such converted Renminbi shall
not be provided as loans to its non-affiliated entities. On October 23, 2019, the SAFE issued the Notice of the State Administration
of Foreign Exchange on Further Facilitating Cross-border Trade and Investment, or the SAFE Circular 28, which, among other things, expanded
the use of foreign exchange capital to domestic equity investment area. The foreign-invested enterprises, whose business scope do not
include equity investment, are allowed to lawfully make domestic equity investments by using their capital on the premise without violation
to prevailing special administrative measures for access of foreign investments (negative list) and the authenticity and compliance with
the regulations of domestic investment projects. As this circular is relatively new, there remains uncertainty as to its interpretation
and application and any other future foreign exchange related rules. Violations of these Circulars could result in severe monetary or
other penalties. SAFE Circular 19, SAFE Circular 16 and SAFE Circular 28 may significantly limit our ability to use Renminbi converted
from the net proceeds of future securities offerings to fund our PRC subsidiaries, to invest in or acquire any other PRC companies through
our PRC subsidiaries, which may adversely affect our business, financial condition and results of operations.
Although the audit report included in this
prospectus was issued by U.S. auditors who are currently inspected by the PCAOB, if it is later determined that the PCAOB is unable to
inspect or investigate our auditor completely, investors would be deprived of the benefits of such inspection and our Ordinary Shares
may be delisted or prohibited from trading.
The audit report included in this prospectus
for our 2022 and 2021 fiscal year was issued by Kreit & Chiu CPA LLP (“KC”), a U.S.-based accounting firm that is registered
with the PCAOB and can be inspected by the PCAOB. The audit report included in this prospectus for our 2020 fiscal year was issued by
Briggs & Veselka Co. (“B&V”), which was also a U.S.-based accounting firm that was registered with the PCAOB and
can be inspected by the PCAOB. We have no intention of engaging any auditor not based in the U.S. and not subject to regular inspection
by the PCAOB. As an auditor of companies that are registered with the SEC and publicly traded in the United States and a firm registered
with the PCAOB, KC is required under the laws of the United States to undergo regular inspections by the PCAOB to assess its compliance
with the laws of the United States and professional standards. If we were to engage a different auditor at any time in the future, we
would engage an auditor that is U.S.-based and subject to full PCAOB inspection with all materials related to the audit of our financial
statements accessible to the PCAOB. There is no guarantee, however, that any future auditor engaged by the Company would remain subject
to full PCAOB inspection during the entire term of our engagement. In such case, we will engage a new qualified and fully inspected auditor,
which may result in us delaying or restating our financial statements.
The PCAOB was unable to conduct inspections in
the PRC without the approval of Chinese government authorities. If it is later determined that the PCAOB is unable to inspect or investigate
our auditor completely, investors may be deprived of the benefits of such inspection. Any audit reports not issued by auditors that are
completely inspected by the PCAOB, or a lack of PCAOB inspections of audit work undertaken in the PRC that prevents the PCAOB from regularly
evaluating our auditors’ audits and their quality control procedures, could result in a lack of assurance that our financial statements
and disclosures are adequate and accurate.
As part of a continued regulatory focus in the
U. S. on access to audit and other information currently protected by national law, in particular PRC’s, in June 2019, a bipartisan
group of lawmakers introduced bills in both houses of the U.S. Congress which, if passed, would require the SEC to maintain a list of
issuers for which PCAOB was not able to inspect or investigate the audit work performed by a foreign public accounting firm completely.
The proposed Ensuring Quality Information and Transparency for Abroad-Based Listings on our Exchanges (“EQUITABLE”) Act prescribes
increased disclosure requirements for these issuers and, beginning in 2025, the delisting from U.S. national securities exchanges such
as the Nasdaq of issuers included on the SEC’s list for three consecutive years. It is unclear if this proposed legislation will
be enacted. Furthermore, there have been recent deliberations within the U.S. government regarding potentially limiting or restricting
China-based companies from accessing U.S. capital markets. On May 20, 2020, the U.S. Senate passed the Holding Foreign Companies Accountable
Act (the “HFCAA”), which includes requirements for the SEC to identify issuers whose audit work is performed by auditors
that the PCAOB is unable to inspect or investigate completely because of a restriction imposed by a non-U.S. authority in the auditor’s
local jurisdiction. The U.S. House of Representatives passed the HFCAA on December 2, 2020, and the HFCAA was signed into law on December
18, 2020. Additionally, in July 2020, the U.S. President’s Working Group on Financial Markets issued recommendations for actions
that can be taken by the executive branch, the SEC, the PCAOB or other federal agencies and department with respect to Chinese companies
listed on U.S. stock exchanges and their audit firms, in an effort to protect investors in the United States. In response, on November
23, 2020, the SEC issued guidance highlighting certain risks (and their implications to U.S. investors) associated with investments in
China-based issuers and summarizing enhanced disclosures the SEC recommends China-based issuers make regarding such risks. On December
2, 2021, the SEC adopted final rules relating to the implementation of certain disclosure and documentation requirements of the HFCAA.
We will be required to comply with these rules if the SEC identifies us as having a “non-inspection” year (as defined in
the interim final rules) under a process to be subsequently established by the SEC. The SEC is assessing how to implement other requirements
of the HFCAA, including the listing and trading prohibition requirements described above. Under the HFCAA, our securities may be prohibited
from trading on the Nasdaq or other U.S. stock exchanges if our auditor is not able to be inspected by the PCAOB for three consecutive
years, and this ultimately could result in our Ordinary Shares being delisted. Furthermore, on June 22, 2021, the U.S. Senate passed
the Accelerating Holding Foreign Companies Accountable Act (“AHFCAA”), which, if enacted, would amend the HFCAA and require
the SEC to prohibit an issuer’s securities from trading on any U.S. stock exchanges if its auditor is not able to be inspected
by the PCAOB for two consecutive years instead of three. On September 22, 2021, the PCAOB adopted a final rule implementing the HFCAA,
which provides a framework for the PCAOB to use when determining, as contemplated under the HFCAA, whether PCAOB is unable to inspect
or investigate completely registered public accounting firms located in a foreign jurisdiction because of a position taken by one or
more authorities in that jurisdiction. On December 16, 2021, the PCAOB issued a Determination Report which found that the PCAOB is unable
to inspect or investigate completely registered public accounting firms headquartered in: (1) mainland China of the People’s Republic
of China, because of a position taken by one or more authorities in mainland China; and (2) Hong Kong, a Special Administrative Region
and dependency of the PRC, because of a position taken by one or more authorities in Hong Kong. On December 23, 2022, the AHFCAA was
enacted, which amended the HFCAA by requiring the SEC to prohibit an issuer’s securities from trading on any U.S. stock exchanges
if its auditor is not subject to PCAOB inspections for two consecutive years instead of three, thus reducing the time period for triggering
the prohibition on trading. On August 26, 2022, the China Securities Regulatory Commission (the “CSRC”), the Ministry of
Finance of the PRC (the “MOF”), and the PCAOB signed a Statement of Protocol (the “Protocol”), governing inspections
and investigations of audit firms based in mainland China and Hong Kong, taking the first step toward opening access for the PCAOB to
inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong. Pursuant to the fact sheet
with respect to the Protocol disclosed by the SEC, the PCAOB shall have independent discretion to select any issuer audits for inspection
or investigation and has the unfettered ability to transfer information to the SEC. On December 15, 2022, the PCAOB determined that the
PCAOB was able to secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China
and Hong Kong and voted to vacate its previous determinations to the contrary. However, should PRC authorities obstruct or otherwise
fail to facilitate the PCAOB’s access in the future, the PCAOB will consider the need to issue a new determination.
Our securities may be delisted under the HFCAA
if the PCAOB is unable to inspect auditors with presence in China for three consecutive years. The delisting of our securities, or the
threat of their being delisted, may materially and adversely affect the value of your investment. Additionally, the inability of the
PCAOB to conduct inspections deprives our investors of the benefits of such inspections.
The above recent developments may have added
uncertainties to our ability to continue to list on Nasdaq or to offer our securities and we cannot assure you whether Nasdaq or other
regulatory authorities would apply additional and more stringent criteria to us since we are an emerging growth company and substantially
all of our operations are conducted in China. The SEC may propose additional rules or guidance that could impact us if our auditor is
not subject to PCAOB inspection.
Moreover, on December 29, 2022, legislation entitled
“Consolidated Appropriations Act, 2023” (the “Consolidated Appropriations Act”) was signed into law by President
Biden. The Consolidated Appropriations Act contained, among other things, an identical provision to the HFCAA, which reduces the number
of consecutive non-inspection years required for triggering the prohibitions under the HFCAA from three years to two.
If our Ordinary Shares are unable to be listed
on another securities exchange by then, such a delisting would substantially impair your ability to sell or purchase our Ordinary Shares
when you wish to do so, and the risk and uncertainty associated with a potential delisting would have a negative impact on the price
of our Ordinary Shares.
We are a “controlled
company” within the meaning of the Nasdaq listing rules, and may follow certain exemptions from certain corporate governance requirements
that could adversely affect our public shareholders.
Our largest shareholder, Mr. Yongjun Liu, owns
more than a majority of the voting power of our outstanding Ordinary Shares. Under the Nasdaq listing rules, a company of which more
than 50% of the voting power is held by an individual, group, or another company is a “controlled company” and is permitted
to phase in its compliance with the independent committee requirements. Although we do not intend to rely on the “controlled company”
exemptions under the Nasdaq listing rules even if we are deemed a “controlled company,” we could elect to rely on these exemptions
in the future. If we were to elect to rely on the “controlled company” exemptions, a majority of the members of our board
of directors might not be independent directors and our nominating and corporate governance and compensation committees might not consist
entirely of independent directors. Accordingly, if we rely on the exemptions, during the period we remain a controlled company and during
any transition period following a time when we are no longer a controlled company, you would not have the same protections afforded to
shareholders of companies that are subject to all of the corporate governance requirements of Nasdaq.
Fluctuations in exchange rates could have
a material and adverse effect on our results of operations and the value of your investment.
The value of the Renminbi against the U.S. dollar
and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions and the foreign
exchange policy adopted by the PRC government. It is difficult to predict how long such appreciation of RMB against the U.S. dollar may
last and when and how the relationship between the RMB and the U.S. dollar may change again. All of our revenues and substantially
all of our costs are denominated in Renminbi. We rely on dividends paid by our operating subsidiaries in China for our cash needs. Any
significant revaluation of Renminbi may materially and adversely affect our results of operations and financial position reported in
Renminbi when translated into U.S. dollars, and the value of, and any dividends payable on, the Ordinary Shares in U.S. dollars. To the
extent that we need to convert U.S. dollars into Renminbi for our operations, appreciation of the Renminbi against the U.S. dollar would
have an adverse effect on the Renminbi amount we would receive. Conversely, if we decide to convert our Renminbi into U.S. dollars for
the purpose of making payments for dividends on our Ordinary Shares or for other business purposes, appreciation of the U.S. dollar against
the Renminbi would have a negative effect on the U.S. dollar amount.
Governmental control of currency conversion
may limit our ability to utilize our revenues effectively and affect the value of your investment.
The PRC government imposes controls on the convertibility
of the Renminbi into foreign currencies and, in certain cases, the remittance of currency out of China. We receive substantially all
of our revenues in Renminbi. Under our current corporate structure, we primarily rely on dividend payments from our PRC subsidiaries
to fund any cash and financing requirements we may have. Under existing PRC foreign exchange regulations, payments of current account
items, including profit distributions, interest payments and trade and service-related foreign exchange transactions, can be made in
foreign currencies without prior approval of SAFE by complying with certain procedural requirements. Specifically, under the existing
exchange restrictions, without prior approval of SAFE, cash generated from the operations of our PRC subsidiaries in China may be used
to pay dividends to our company. However, approval from or registration with appropriate government authorities is required, in principle,
where RMB is to be converted into foreign currency and remitted out of China to pay capital expenses such as the repayment of loans denominated
in foreign currencies. As a result, we need to obtain SAFE approval to use cash generated from the operations of our PRC subsidiaries
to pay off their respective debt in a currency other than Renminbi owed to entities outside China, or to make other capital expenditure
payments outside China in a currency other than Renminbi. The PRC government may at its discretion restrict access to foreign currencies
for current account transactions in the future. If the foreign exchange control system prevents us from obtaining sufficient foreign
currencies to satisfy our foreign currency demands, we may not be able to pay dividends in foreign currencies to our shareholders, including
holders of the Ordinary Shares.
Any failure to comply with PRC regulations
regarding cybersecurity and data protection may subject us to fines and other legal or administrative sanctions, claims or legal proceedings.
The PRC Criminal Law, as amended by its Amendment
7 (effective on February 28, 2009) and Amendment 9 (effective on November 1, 2015), prohibits institutions, companies and their employees
from selling or otherwise illegally disclosing a citizen’s personal information obtained during the course of performing duties
or providing services, or obtaining such information through theft or other illegal means. On November 7, 2016, the Standing Committee
of the National People’s Congress of the PRC issued the Cyber Security Law of the PRC, or Cyber Security Law, which became effective
on June 1, 2017. Pursuant to the Cyber Security Law, network operators must not collect users’ personal information without their
consent and may only collect users’ personal information necessary to the provision of services. Providers are also obliged to
provide security maintenance for their products and services and shall comply with provisions regarding the protection of personal information
as stipulated under the relevant laws and regulations. The Civil Code of the PRC (issued by the National People’s Congress of the
PRC on May 28, 2020 and effective from January 1, 2021) provides the main legal basis for privacy and personal information infringement
claims under PRC civil law.
PRC regulators, including the Cyberspace Administration
of China (the “CAC”), the Ministry of Industry and Information Technology and the Ministry of Public Security, have been
increasingly focused on regulation in areas of data security and data protection. The PRC regulatory requirements regarding cybersecurity
are constantly evolving. For example, various PRC regulatory bodies, including the CAC, the Ministry of Public Security and the State
Administration for Market Regulation (“SAMR”), have enforced data privacy and protection laws and regulations with varying
and evolving standards and interpretations.
In April 2020, the PRC government promulgated
the 2020 Cybersecurity Review Measures, which came into effect on June 1, 2020. In July 2021, the CAC and other related authorities released
a draft amendment to the 2020 Cybersecurity Review Measures for public comments. On December 28, 2021, the PRC government promulgated
the 2022 Cybersecurity Review Measures, which came into effect and replaced the 2020 Cybersecurity Review Measures on February 15, 2022.
According to the 2022 Cybersecurity Review Measures, (i) critical information infrastructure operators that purchase network products
and services and internet platform operators that conduct data processing activities shall be subject to cybersecurity review in accordance
with the 2022 Cybersecurity Review Measures if such activities affect or may affect national security; and (ii) internet platform operators
holding personal information of more than one million users and seeking to have their securities list on a stock exchange in a foreign
country shall file for cybersecurity review with the Cybersecurity Review Office. Under the Regulation on Protecting the Security of
Critical Information Infrastructure promulgated by the State Council on July 30, 2021, effective September 1, 2021, “critical information
infrastructure” is defined as important network facilities and information systems in important industries and fields, such as
public telecommunication and information services, energy, transportation, water conservancy, finance, public services, e-government
and national defense, science, technology and industry, as well as other important network facilities and information systems that, in
case of destruction, loss of function or leak of data, may severely damage national security, the national economy and the people’s
livelihood and public interests. As of the date of this prospectus, neither we nor any of our PRC Subsidiaries has been informed by any
PRC governmental authority that we or any of our PRC Subsidiaries is a “critical information infrastructure operator.”
On November 14, 2021, the CAC released the Administrative
Regulations on the Management of Network Data Security (Draft for comments) or the Draft Administrative Regulation. Under the Draft Administrative
Regulation, (i) data processors, i.e., individuals and organizations who can decide on the purpose and method of their data processing
activities at their own discretion, that process personal information of more than one million individuals shall apply for cybersecurity
review before listing in a foreign country; (ii) foreign- listed data processors shall carry out annual data security evaluation and
submit the evaluation report to the municipal cyberspace administration authority; and (iii) where the data processor undergoes merger,
reorganization and subdivision that involves important data and personal information of more than one million individuals, the recipient
of the data shall report the transaction to the in-charge authority at the municipal level.
According to the currently in-effect PRC laws
and regulations, we believe that neither we nor any of our PRC Subsidiaries is subject to the cybersecurity review, reporting or other
permission requirements by the CAC under the applicable PRC cybersecurity laws and regulations with respect to the offering of our securities
or the business operations of our PRC Subsidiaries, because neither we nor any of our PRC Subsidiaries qualifies as a critical information
infrastructure operator or has conducted any data processing activities that affect or may affect national security or holds personal
information of more than one million users. Additionally, as of the date of this prospectus, neither we nor any of our PRC Subsidiaries
has been required by any PRC governmental authority to apply for cybersecurity review, nor have we or any of our PRC Subsidiaries received
any inquiry, notice, warning, sanction in such respect or been denied permission from any PRC regulatory authority to list on U.S. exchanges.
However, as PRC governmental authorities have significant discretion in interpreting and implementing statutory provisions and there
remains significant uncertainty in the interpretation and enforcement of relevant PRC cybersecurity laws and regulations if the PRC regulatory
authorities take a position contrary to ours, we cannot assure you that we or any of our PRC Subsidiaries will not be deemed to be subject
to PRC cybersecurity review requirements under the 2022 Cybersecurity Review Measures or the Draft Administrative Regulations (if enacted),
nor can we assure you that we or our PRC Subsidiaries would be able to pass such review. If we or any of our PRC Subsidiaries fails to
receive any requisite permission or approval from the CAC for future offerings or the business operations of our PRC Subsidiaries, or
the waiver for such permission or approval, in a timely manner, or at all, or inadvertently concludes that such permission or approval
is not required, or if applicable laws, regulations or interpretations change and obligate us to obtain such permission or approvals
in the future, we or our PRC Subsidiaries may be subject to fines, suspension of business, website closure, revocation of business licenses
or other penalties, as well as reputational damage or legal proceedings or actions against us, which may have a material adverse effect
on our business, financial condition or results of operations. In addition, we could become subject to enhanced cybersecurity review
or investigations launched by PRC regulators in the future pursuant to new laws, regulations or policies.
As of the date of this prospectus, we have not
been involved in any investigations or become subject to a cybersecurity review initiated by the CAC, and we have not received any inquiry,
notice, warning, sanctions in such respect or any regulatory objections with respect to our business operations or in connection with
future offerings from the CAC. However, as the Cybersecurity Review Measures and the Draft Administrative Regulation were newly issued,
there remain uncertainties as to how it would be interpreted and enforced, and to what extent it may affect us.
Certain PRC regulations may make it more
difficult for us to pursue growth through acquisitions.
Among other things, the Regulations on Mergers
and Acquisitions of Domestic Enterprises by Foreign Investors (“M&A Rules”) and Anti-Monopoly Law of the People’s
Republic of China promulgated by the Standing Committee of the NPC which became effective in 2008 and amended in 2022 (“Anti-Monopoly
Law”), established additional procedures and requirements that could make merger and acquisition activities by foreign investors
more time-consuming and complex. Such regulation requires, among other things, that State Administration for Market Regulation (“SAMR”)
be notified in advance of any change-of-control transaction in which a foreign investor acquires control of a PRC domestic enterprise
or a foreign company with substantial PRC operations, if certain thresholds under the Provisions of the State Council on the Standard
for Declaration of Concentration of Business Operators, issued by the State Council in 2008 and amended in 2018, are triggered. Moreover,
the Anti-Monopoly Law requires that transactions which involve the national security, the examination on the national security shall
also be conducted according to the relevant provisions of the State. In addition, PRC Measures for the Security Review of Foreign Investment
which became effective in January 2021 require acquisitions by foreign investors of PRC companies engaged in military-related or certain
other industries that are crucial to national security be subject to security review before consummation of any such acquisition. We
may pursue potential strategic acquisitions that are complementary to our business and operations.
Complying with the requirements of these regulations
to complete such transactions could be time-consuming, and any required approval processes, including obtaining approval or clearance
from the MOFCOM, may delay or inhibit our ability to complete such transactions, which could affect our ability to expand our business
or maintain our market share.
PRC regulations relating to the establishment
of offshore special purpose companies by PRC residents may subject our PRC resident beneficial owners or our PRC subsidiaries to liability
or penalties, limit our ability to inject capital into our PRC subsidiaries, limit our PRC subsidiaries’ ability to increase their
registered capital or distribute profits to us, or may otherwise adversely affect us.
In July 2014, SAFE promulgated the Circular on
Relevant Issues Concerning Foreign Exchange Control on Domestic Residents’ Offshore Investment and Financing and Roundtrip Investment
Through Special Purpose Vehicles, or SAFE Circular 37, to replace the Notice on Relevant Issues Concerning Foreign Exchange Administration
for Domestic Residents’ Financing and Roundtrip Investment Through Offshore Special Purpose Vehicles, or SAFE Circular 75, which
ceased to be effective upon the promulgation of SAFE Circular 37. SAFE Circular 37 requires PRC residents (including PRC individuals
and PRC corporate entities) to register with SAFE or its local branches in connection with their direct or indirect offshore investment
activities. SAFE Circular 37 is applicable to our shareholders who are PRC residents and may be applicable to any offshore acquisitions
that we make in the future.
Under SAFE Circular 37, PRC residents who make,
or have prior to the implementation of SAFE Circular 37 made, direct or indirect investments in offshore special purpose vehicles, or
SPVs, will be required to register such investments with SAFE or its local branches. In addition, any PRC resident who is a direct or
indirect shareholder of an SPV is required to update its filed registration with the local branch of SAFE with respect to that SPV, to
reflect any material change. Moreover, any subsidiary of such SPV in China is required to urge the PRC resident shareholders to update
their registration with the local branch of SAFE. If any PRC shareholder of such SPV fails to make the required registration or to update
the previously filed registration, the subsidiary of such SPV in China may be prohibited from distributing its profits or the proceeds
from any capital reduction, share transfer or liquidation to the SPV, and the SPV may also be prohibited from making additional capital
contributions into its subsidiary in China. On February 13, 2015, the SAFE promulgated a Notice on Further Simplifying and Improving
Foreign Exchange Administration Policy on Direct Investment, or SAFE Notice 13, which became effective on June 1, 2015. Under SAFE
Notice 13, applications for foreign exchange registration of inbound foreign direct investments and outbound overseas direct investments,
including those required under SAFE Circular 37, will be filed with qualified banks instead of SAFE. The qualified banks will directly
examine the applications and accept registrations under the supervision of SAFE.
Certain of our shareholders that we are aware
of are subject to SAFE regulations, and we expect all of these shareholders will have completed all necessary registrations with the
local SAFE branch or qualified banks as required by SAFE Circular 37. We cannot assure you, however, that all of these shareholders will
continue to make required filings or updates in a timely manner, or at all. We can provide no assurance that we are or will in the future
continue to be informed of identities of all PRC residents holding direct or indirect interest in our company. Any failure or inability
by such shareholders to comply with SAFE regulations may subject us to fines or legal sanctions, such as restrictions on our cross-border
investment activities or our PRC subsidiaries’ ability to distribute dividends to, or obtain foreign exchange-denominated loans
from, our company or prevent us from making distributions or paying dividends. As a result, our business operations and our ability to
make distributions to you could be materially and adversely affected.
Furthermore, as these foreign exchange regulations
are still relatively new and their interpretation and implementation have been constantly evolving, it is unclear how these regulations,
and any future regulation concerning offshore or cross-border transactions, will be interpreted, amended and implemented by the relevant
government authorities. For example, we may be subject to a more stringent review and approval process with respect to our foreign exchange
activities, such as remittance of dividends and foreign-currency-denominated borrowings, which may adversely affect our financial condition
and results of operations. In addition, if we decide to acquire a PRC domestic company, we cannot assure you that we or the owners of
such company, as the case may be, will be able to obtain the necessary approvals or complete the necessary filings and registrations
required by the foreign exchange regulations. This may restrict our ability to implement our acquisition strategy and could adversely
affect our business and prospects.
As of the date of this disclosure, to our knowledge
these PRC resident shareholders have applied for foreign exchange registration under the SAFE Circular 37 and other related
rules. Although they are in the process of making foreign exchange registration, they may still face the above said possible fines in
accordance with the PRC Laws.
Failure to make adequate contributions
to various employee benefit plans and withhold individual income tax on employees’ salaries as required by PRC regulations may
subject us to penalties.
Companies operating in the PRC are required to
participate in various government-mandated employee benefit contribution plans, including certain social insurance, housing funds and
other welfare-oriented payment obligations, and contribute to the plans in amounts equal to certain percentages of salaries, including
bonuses and allowances, of our employees up to a maximum amount specified by the local government from time to time at locations where
we operate our businesses. The requirement of employee benefit contribution plans has not been implemented consistently by the local
governments in the PRC given the different levels of economic development in different locations. Companies operating in the PRC are
also required to withhold individual income tax on employees’ salaries based on the actual salary of each employee upon payment.
We may be subject to late fees and fines in relation to the underpaid employee benefits and under-withheld individual income tax, our
financial condition and results of operations may be adversely affected.
Any failure to comply with PRC regulations
regarding the registration requirements for employee stock incentive plans may subject the PRC plan participants or us to fines and other
legal or administrative sanctions.
Pursuant to the Notices on Issues Concerning
the Foreign Exchange Administration for Domestic Individuals Participating in Stock Incentive Plan of Overseas Publicly-Listed Company,
promulgated by SAFE in 2012, or SAFE Notices No. 7, PRC citizens and non-PRC citizens who reside in China for a continuous period
of no less than one year who participate in any stock incentive plan of an overseas publicly listed company offered to the director,
supervisor, senior management and other employees of, and any individual who has labor relationship with its domestic affiliated entities
are required to register with SAFE through a domestic qualified agent, which could be a PRC subsidiary of such overseas listed company,
and complete certain other procedures. In addition, an overseas entrusted institution must be retained to handle matters in connection
with the exercise or sale of stock options and the purchase or sale of shares and interests. We and our directors, executive officers
and other employees who are PRC citizens or who reside in the PRC for a continuous period of no less than one year and who have been
granted stock options are subject to these regulations. Failure to complete the SAFE registrations for our employee incentive plans may
subject them to fines and legal sanctions and may also limit our ability to contribute additional capital into our PRC subsidiaries and
limit our PRC subsidiaries’ ability to distribute dividends to us. We also face regulatory uncertainties that could restrict our
ability to adopt additional incentive plans for our directors, executive officers and employees under PRC law.
In addition, the State Administration of Taxation,
or SAT, has issued certain circulars concerning employee stock options and restricted shares. Under these circulars, our employees working
in the PRC who exercise stock options or are granted restricted shares will be subject to PRC individual income tax. Our PRC subsidiaries
have obligations to file documents related to employee stock options or restricted shares with relevant tax authorities and to withhold
individual income taxes of those employees who exercise their share options or are granted with restricted shares. If our employees fail
to pay or we fail to withhold their income taxes according to relevant laws and regulations, we may face sanctions imposed by the tax
authorities or other PRC governmental authorities.
U.S. regulatory bodies may be limited in
their ability to conduct investigations or inspections of our operations in the PRC.
Any disclosure of documents or information located
in the PRC by foreign agencies may be subject to jurisdiction constraints and must comply with the PRC’s state secrecy laws, which
broadly define the scope of “state secrets” to include matters involving economic interests and technologies. There is no
guarantee that requests from U.S. federal or state regulators or agencies to investigate or inspect our operations will be honored by
us, by entities who provide services to us or with whom we associate, without violating PRC legal requirements, especially as those entities
are located in the PRC. Furthermore, under the current PRC laws, an on-site inspection of our facilities by any of these regulators may
be limited or prohibited.
If we are classified as a PRC resident
enterprise for PRC income tax purposes, such classification could result in unfavorable tax consequences to us and our non-PRC shareholders.
Under the PRC Enterprise Income Tax Law and its
implementation rules, an enterprise established outside of the PRC with its “de facto management body” within
the PRC is considered a “resident enterprise” and will be subject to the enterprise income tax on its global income at the
rate of 25%. The implementation rules define the term “de facto management body” as the body that exercises full
and substantial control and overall management over the business, productions, personnel, accounts and properties of an enterprise. In
2009, the State Administration of Taxation, or SAT, issued a circular, known as SAT Circular 82, which provides certain specific criteria
for determining whether the “de facto management body” of a PRC-controlled enterprise that is incorporated offshore
is located in China. Although this circular applies only to offshore enterprises controlled by PRC enterprises or PRC enterprise groups,
not those controlled by PRC individuals or foreigners, the criteria set forth in the circular may reflect the SAT’s general position
on how the “de facto management body” text should be applied in determining the tax resident status of all offshore
enterprises. According to SAT Circular 82, an offshore incorporated enterprise controlled by a PRC enterprise or a PRC enterprise group
will be regarded as a PRC tax resident by virtue of having its “de facto management body” in China, and will
be subject to PRC enterprise income tax on its global income only if all of the following conditions are met: (i) the primary location
of the day-to-day operational management is in the PRC; (ii) decisions relating to the enterprise’s financial and human resource
matters are made or are subject to approval by organizations or personnel in the PRC; (iii) the enterprise’s primary assets,
accounting books and records, company seals, and board of directors and shareholder resolutions are located or maintained in the PRC;
and (iv) at least 50% of voting board members or senior executives habitually reside in the PRC.
We believe our company is not a PRC resident
enterprise for PRC tax purposes. However, the tax resident status of an enterprise is subject to determination by the PRC tax authorities
and uncertainties remain with respect to the interpretation of the term “de facto management body.” If the PRC
tax authorities determine that our company is a PRC resident enterprise for enterprise income tax purposes, we would be subject to PRC
enterprise income on our worldwide income at the rate of 25%. Furthermore, we would be required to withhold a 10% tax from dividends
we pay to our shareholders that are non-resident enterprises. In addition, non-resident enterprise shareholders may be subject to PRC
tax on gains realized on the sale or other disposition of the Ordinary Shares, if such income is treated as sourced from within the PRC.
Furthermore, if we are deemed a PRC resident enterprise, dividends paid to our non-PRC individual shareholders and any gain realized
on the transfer of the Ordinary Shares by such shareholders may be subject to PRC tax at a rate of 20% (which, in the case of dividends,
may be withheld at source by us). These rates may be reduced by an applicable tax treaty, but it is unclear whether non-PRC shareholders
of our company would be able to claim the benefits of any tax treaties between their country of tax residence and the PRC in the event
that we are treated as a PRC resident enterprise. Any such tax may reduce the returns on your investment in our Ordinary Shares.
We face uncertainty with respect to indirect
transfers of equity interests in PRC resident enterprises by their non-PRC holding companies.
On February 3, 2015, the SAT issued the
Public Notice Regarding Certain Corporate Income Tax Matters on Indirect Transfer of Properties by Non-Tax Resident Enterprises, or SAT
Bulletin 7. SAT Bulletin 7 extends its tax jurisdiction to transactions involving the transfer of taxable assets through offshore transfer
of a foreign intermediate holding company. In addition, SAT Bulletin 7 has introduced safe harbors for internal group restructurings
and the purchase and sale of equity through a public securities market. SAT Bulletin 7 also brings challenges to both foreign transferor
and transferee (or other person who is obligated to pay for the transfer) of taxable assets, as such persons need to determine whether
their transactions are subject to these rules and whether any withholding obligation applies.
On October 17, 2017, the SAT issued the
Announcement of the State Administration of Taxation on Issues Concerning the Withholding of Non-resident Enterprise Income Tax at Source,
or SAT Bulletin 37, which came into effect on December 1, 2017. The SAT Bulletin 37 further clarifies the practice and procedure
of the withholding of non-resident enterprise income tax.
Where a non-resident enterprise transfers taxable
assets indirectly by disposing of the equity interests of an overseas holding company, which is an “Indirect Transfer,” the
non-resident enterprise as either transferor or transferee, or the PRC entity that directly owns the taxable assets, may report such
Indirect Transfer to the relevant tax authority. Using a “substance over form” principle, the PRC tax authority may disregard
the existence of the overseas holding company if it lacks a reasonable commercial purpose and was established for the purpose of reducing,
avoiding or deferring PRC tax. As a result, gains derived from such Indirect Transfer may be subject to PRC enterprise income tax, and
the transferee or other person who pays for the transfer is obligated to withhold the applicable taxes currently at a rate of 10% for
the transfer of equity interests in a PRC resident enterprise. Both the transferor and the transferee may be subject to penalties under
PRC tax laws if the transferee fails to withhold the taxes and the transferor fails to pay the taxes.
We face uncertainties as to the reporting and
other implications of certain past and future transactions where PRC taxable assets are involved, such as offshore restructuring, sale
of the shares in our offshore subsidiaries and investments. Our company may be subject to filing obligations or taxed if our company
is transferor in such transactions and may be subject to withholding obligations if our company is transferee in such transactions, under
SAT Bulletin 7 and/or SAT Bulletin 37. For transfer of shares in our company by investors who are non-PRC resident enterprises, our PRC
subsidiaries may be requested to assist in the filing under SAT Bulletin 7 and/or SAT Bulletin 37. As a result, we may be required to
expend valuable resources to comply with SAT Bulletin 7 and/or SAT Bulletin 37 or to request the relevant transferors from whom we purchase
taxable assets to comply with these circulars, or to establish that our company should not be taxed under these circulars, which may
have a material adverse effect on our financial condition and results of operations.
Risks Related to Our Ordinary Shares
The market price for our Ordinary Shares
may be volatile.
The trading prices of our Ordinary Shares are
likely to be volatile and could fluctuate widely due to factors beyond our control. This may happen because of broad market and industry
factors, like the performance and fluctuation in the market prices or the underperformance or deteriorating financial results of internet
or other companies based in China that have listed their securities in the United States in recent years. The securities of some of these
companies have experienced significant volatility since their initial public offerings, including, in some cases, substantial price declines
in their trading prices. The trading performances of other Chinese companies’ securities after their offerings, including internet
and e-commerce companies, may affect the attitudes of investors toward Chinese companies listed in the United States, which consequently
may impact the trading performance of our Ordinary Shares, regardless of our actual operating performance. In addition, any negative
news or perceptions about inadequate corporate governance practices or fraudulent accounting, corporate structure or other matters of
other Chinese companies may also negatively affect the attitudes of investors towards Chinese companies in general, including us, regardless
of whether we have conducted any inappropriate activities. In addition, securities markets may from time to time experience significant
price and volume fluctuations that are not related to our operating performance, such as the large decline in share prices in the United States,
China and other jurisdictions in late 2008, early 2009 and the second half of 2011, which may have a material adverse effect on the market
price of our Ordinary Shares.
In addition to the above factors, the price and
trading volume of our Ordinary Shares may be highly volatile due to multiple factors, including the following:
| |
● |
regulatory
developments affecting us, our consumers, or our industry; |
| |
● |
conditions
in the medical supplies business and the public perception of the legitimacy and ethics of certain business practices of our competitors
or other market players within the industry; |
| |
● |
announcements
of studies and reports relating to the quality of our product and service offerings or those of our competitors; |
| |
● |
changes
in the economic performance or market valuations of other medical supplies businesses; |
| |
● |
actual
or anticipated fluctuations in our quarterly results of operations and changes or revisions of our expected results; |
| |
● |
changes
in financial estimates by securities research analysts; |
| |
● |
announcements
by us or our competitors of new product and service offerings, acquisitions, strategic relationships, joint ventures or capital commitments; |
| |
● |
additions
to or departures of our senior management; |
| |
● |
detrimental
negative publicity about us, our management or our industry; |
| |
● |
fluctuations
of exchange rates between the Renminbi and the U.S. dollar; |
| |
● |
release
or expiry of lock-up or other transfer restrictions on our outstanding Ordinary Shares; and |
| |
● |
sales
or perceived potential sales of additional Ordinary Shares. |
The trading market for our Ordinary Shares will
depend in part on the research and reports that securities or industry analysts publish about us or our business. If research analysts
do not establish and maintain adequate research coverage or if one or more of the analysts who cover us downgrade shares or publish inaccurate
or unfavorable research about our business, the market price for our Ordinary Shares would likely decline. If one or more of these analysts
cease coverage of our company or fail to publish reports on us regularly, we could lose visibility in the financial markets, which, in
turn, could cause the market price or trading volume for our Ordinary Shares to decline.
If securities or industry analysts do not
publish research or reports about our business, or if they adversely change their recommendations regarding our Ordinary Shares, the
market price for our Ordinary Shares and trading volume could decline.
The trading market for our Ordinary Shares are
influenced by research or reports that industry or securities analysts publish about our business. If industry or securities analysts
decide to cover us and in the future downgrade our Ordinary Shares, the market price for our Ordinary Shares would likely decline. If
one or more of these analysts cease to cover us or fail to regularly publish reports on us, we could lose visibility in the financial
markets, which in turn could cause the market price or trading volume for our Ordinary Shares to decline.
Because we do not expect to pay dividends
in the foreseeable future, you must rely on price appreciation of our Ordinary Shares for return on your investment.
We currently intend to retain most, if not all,
of our available funds and any future earnings to fund the development and growth of our business. As a result, we do not expect to pay
any cash dividends in the foreseeable future. Therefore, you should not rely on an investment in our Ordinary Shares as a source for
any future dividend income.
Our board of directors has discretion as to whether
to distribute dividends, subject to certain restrictions under Cayman Islands law, namely that our company may only pay dividends out
of profits or share premium; provided that in no circumstances may a dividend be paid if this would result in our company being unable
to pay its debts as they fall due in the ordinary course of business. In addition, our shareholders may by ordinary resolution declare
a dividend, but no dividend may exceed the amount recommended by our board of directors. Even if our board of directors decides to declare
and pay dividends, the timing, amount and form of future dividends, if any, will depend on, among other things, our future results of
operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiaries,
our financial condition, contractual restrictions and other factors deemed relevant by our board of directors. Accordingly, the return
on your investment in our Ordinary Shares will likely depend entirely upon any future price appreciation of our Ordinary Shares. There
is no guarantee that our Ordinary Shares will appreciate in value or even maintain the price at which you purchased our Ordinary Shares.
You may not realize a return on your investment in our Ordinary Shares and you may even lose your entire investment in our Ordinary
Shares.
Substantial future sales or perceived potential
sales of Ordinary Shares in the public market could cause the price of our Ordinary Shares to decline.
Sales of Ordinary Shares in the public market,
or the perception that these sales could occur, could cause the market price of our Ordinary Shares to decline. As of the date of this
report, we have 24,234,973 Ordinary Shares outstanding. All of the 3,940,000 Ordinary Shares sold in our recent initial public
offering are freely transferable without restriction or additional registration under the Securities Act of 1933, as amended, or the
Securities Act, and we recently sold $6,000,0000 in 7% OID convertible notes (“Convertible Notes”) in a primary offering
pursuant to a takedown from our shelf registration statement on Form F-3, which Notes will be convertible from time to time at the lower
of $2.738 per share or 95% of the lowest VWAP over the seven trading days prior to conversion. All of our executive officers and directors
and shareholders holding at least ten percent of our Ordinary Shares are subject to a six month lock-up agreement that was entered into
in conjunction with the Convertible Notes offering. Ordinary Shares subject to these lock-up agreements become eligible for sale in the
public market upon expiration of these lock-up agreements, subject to volume and other restrictions as applicable under Rules 144
and 701 under the Securities Act. To the extent shares issued upon conversion of the Convertible Notes, as well as exercise of the
Warrants and sale of the Warrant Shares being registered herein, as well as any shares held by our executive officers, directors or significant
shareholders, are sold into the market, the market price of our Ordinary Shares could decline. Moreover, the perceived risk of this
potential dilution could cause shareholders to attempt to sell their shares and investors to short our Ordinary Shares. These sales also
may make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem reasonable
or appropriate. For further information about our recent offering of Convertible Notes, see “Our recent entry into a Securities
Purchase Agreement with the Selling Shareholders for the sale of up to $50.5 million in Convertible Notes and Warrants, of which we have
sold $6.0 million to date, along with any subsequent conversion and/or exercise thereof, may depress the price of our Ordinary Shares
and encourage short sales by third parties, which could further depress the price of our Ordinary Shares,” below.
We may need additional capital and may
sell additional Ordinary Shares or other equity securities or incur indebtedness, which could result in additional dilution to our shareholders
or increase our debt service obligations.
We may require additional cash resources due
to changed business conditions or other future developments, including any investments or acquisitions we may decide to pursue. If our
cash resources are insufficient to satisfy our cash requirements, we may seek to sell additional equity or debt securities or obtain
a credit facility. The sale of additional equity securities or equity-linked debt securities could result in additional dilution to our
shareholders. The incurrence of indebtedness would result in debt service obligations and could result in operating and financing covenants
that would restrict our operations. We cannot assure you that financing will be available in amounts or terms acceptable to us, if at all.
Certain existing shareholders have substantial
influence over our company and their interests may not be aligned with the interests of our other shareholders.
Our directors and officers collectively own an
aggregate of 63.54% of the total voting power of our outstanding Ordinary Shares. As a result, they have substantial influence over our
business, including significant corporate actions such as mergers, consolidations, election of directors and other significant corporate
actions.
They may take actions that are not in the best
interest of us or our other shareholders. This concentration of ownership may discourage, delay or prevent a change in control of our
company, which could deprive our shareholders of an opportunity to receive a premium for their shares as part of a sale of our company
and may reduce the price of our Ordinary Shares. These actions may be taken even if they are opposed by our other shareholders. In addition,
the significant concentration of share ownership may adversely affect the trading price of our Ordinary Shares due to investors’
perception that conflicts of interest may exist or arise.
We are an emerging growth company within
the meaning of the Securities Act and may take advantage of certain reduced reporting requirements.
We are an “emerging growth company,”
as defined in the JOBS Act, and we may take advantage of certain exemptions from various requirements applicable to other public companies
that are not emerging growth companies including, most significantly, not being required to comply with the auditor attestation requirements
of Section 404 of the Sarbanes-Oxley Act of 2002 so long as we are an emerging growth company. As a result, if we elect not to comply
with such auditor attestation requirements, our investors may not have access to certain information they may deem important.
In addition, under the JOBS Act, emerging growth
companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies.
We have elected to avail ourselves of an exemption that allows us to delay adopting new or revised accounting standards until such time
as those standards apply to private companies. As a result, we will not be subject to the same new or revised accounting standards as
other public companies that comply with the public company effective dates. We have also elected to take advantage of certain of the
reduced disclosure obligations and may elect to take advantage of other reduced reporting requirements in future filings. As a result
of these elections, the information that we provide to our stockholders may be different than you might receive from other public reporting
companies.
We are a foreign private issuer within
the meaning of the rules under the Exchange Act and, as such, we are exempt from certain provisions applicable to U.S. domestic public
companies.
Because we qualify as a foreign private issuer
under the Exchange Act, we are exempt from certain provisions of the securities rules and regulations in the United States that
are applicable to U.S. domestic issuers, including:
| |
● |
the rules
under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q or current reports on Form 8-K; |
| |
● |
the sections
of the Exchange Act regulating the solicitation of proxies, consents, or authorizations in respect of a security registered under
the Exchange Act; |
| |
● |
the sections
of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders
who profit from trades made in a short period of time; and |
| |
● |
the selective
disclosure rules by issuers of material nonpublic information under Regulation FD. |
We are required to file an annual report on Form 20-F
within four months of the end of each fiscal year. In addition, we intend to publish our financial results on a semi-annual basis in
the form of press releases, distributed pursuant to the rules and regulations of the Nasdaq Global Market. Press releases relating to
financial results and material events will also be furnished to the SEC on Form 6-K. However, the information we are required to
file with or furnish to the SEC will be less extensive and less timely compared to that required to be filed with the SEC by U.S. domestic
issuers. As a result, you may not be afforded the same protections or information that would be made available to you were you investing
in a U.S. domestic issuer.
As a company incorporated in the Cayman Islands,
we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from the
Nasdaq Global Market corporate governance requirements; these practices may afford less protection to shareholders than they would enjoy
if we complied fully with the Nasdaq Global Market corporate governance requirements. However, presently, we do not have any immediate
plans to rely on home country practice with respect to our corporate governance.
We may lose our foreign private issuer
status in the future, which could result in significant additional costs and expenses.
As discussed above, we are a foreign private
issuer and, therefore, we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange
Act. The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently
completed second fiscal quarter. We would lose our foreign private issuer status if, for example, more than 50% of our Ordinary Shares
are directly or indirectly held by residents of the U.S. and we fail to meet additional requirements necessary to maintain our foreign
private issuer status. In the future, if we lose our foreign private issuer status as of the last date of our second fiscal quarter,
we would be required to file with the SEC periodic reports and registration statements on U.S. domestic issuer forms beginning on the
following January 1, which are more detailed and extensive than the forms available to us as a foreign private issuer. In such case,
we would also have to comply with U.S. federal proxy requirements, and our officers, directors and principal shareholders would
become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. In addition, we would
lose our ability to rely upon exemptions from certain corporate governance requirements under the Nasdaq Global Market listing rules.
As a U.S. listed public company that is not a foreign private issuer, we would incur significant additional legal, accounting and
other expenses that we do not presently incur as a foreign private issuer listed on a U.S. securities exchange.
If we fail to establish and maintain proper
internal financial reporting controls, our ability to produce accurate financial statements or comply with applicable regulations could
be impaired.
Prior to our initial public offering in February
of 2022, we were a private company with limited accounting personnel and other resources with which to address our internal controls
and procedures. Since then, we have been in a continuing process to develop, establish, and put in place a system to maintain internal
controls and procedures that will allow our management to report on, and our independent registered public accounting firm to attest
to, our internal controls over financial reporting if and when required to do so under Section 404 of the Sarbanes-Oxley Act of 2002.
Although our independent registered public accounting firm is not required to attest to the effectiveness of our internal control over
financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act until the date we are no longer an emerging growth company,
our management will be required to report on our internal controls over financial reporting under Section 404.
As of December 31, 2022, our management assessed
the effectiveness of our internal control over financial reporting. The material weaknesses relate to the fact that the Company does
not have accounting personnel with sufficient knowledge of U.S. GAAP and SEC reporting procedures. Management concluded that as of December
31, 2022, our internal control over financial reporting were ineffective.
In order to address and resolve the foregoing
material weakness, we have implemented measures designed to improve our internal control over financial reporting to remediate this material
weakness, including hiring Shanghai Bluehill Advisory Co., Ltd. as our consultant and who has the requisite training and experience in
the preparation of financial statements in compliance with applicable SEC requirements. With the assistance of our consultant, we are
currently in compliance with U.S. GAAP and SEC reporting requirements. In addition to hiring an outside consultant, we also plan to take
remedial measures including (i) hiring more qualified accounting personnel with relevant U.S. GAAP and SEC reporting experience and qualifications
to strengthen the financial reporting function and to set up a financial and system control framework; (ii) implementing regular and
continuous U.S. GAAP accounting and financial reporting training programs for our accounting and financial reporting personnel; and (iii)
setting up an internal audit function as well as engaging an external consulting firm to assist us with assessment of Sarbanes-Oxley
compliance requirements and improvement of overall internal control.
The implementation of these measures may not
fully address the material weaknesses in our internal control over financial reporting, and we cannot conclude that they have been fully
remedied. Our failure to correct theses material weaknesses or our failure to discover and address any other material weaknesses could
result in inaccuracies in our financial statements and could also impair our ability to comply with applicable financial reporting requirements
and related regulatory filings on a timely basis. As a result, our business, financial condition, results of operations and prospects,
as well as the trading price of our Ordinary Shares, may be materially and adversely affected. Moreover, ineffective internal control
over financial reporting significantly hinders our ability to prevent fraud. In addition, once we cease to be an “emerging growth
company” as such term is defined in the JOBS Act, our independent registered public accounting firm must attest to and report on
the effectiveness of our internal control over financial reporting. Our management may conclude that our internal control over financial
reporting is not effective. Moreover, even if our management concludes that our internal control over financial reporting is effective,
our independent registered public accounting firm, after conducting its own independent testing, may issue a report that is qualified
if it is not satisfied with our internal controls or the level at which our controls are documented, designed, operated or reviewed,
or if it interprets the relevant requirements differently from us. In addition, as a public company, our reporting obligations may place
a significant strain on our management, operational and financial resources and systems for the foreseeable future. We may be unable
to timely complete our evaluation testing and any required remediation.
DESCRIPTION OF THE SECURITIES THAT WE ARE OFFERING
Warrants and Warrant Shares
General
On December 27, 2023, we entered into a Securities
Purchase Agreement (the “Securities Purchase Agreement”) with the Selling Shareholders pursuant to which we agreed to issue
to the Investors up to $50,500,000 in senior convertible promissory notes and accompanying warrants exercisable for a number of Ordinary
Shares equal to 50% of the number obtained from dividing each such note’s principal amount by the applicable VWAP (as defined in
the Securities Purchase Agreement). On the same date, we agreed to sell to such Selling Shareholders $6,000,000 of senior convertible
notes (the “Convertible Notes”), which were offered and sold in a primary offering registered pursuant to a shelf registration
statement on Form F-3, and five-year warrants (the “Warrants”) to purchase up to 1,205,255 Ordinary Shares at an exercise
price of $2.9869 per share, subject to certain adjustments, which Warrants were sold concurrently through a private placement. The Convertible
Notes were sold with a 7% original issue discount (“OID”), or $420,000. As such, the Selling Shareholders paid for the Convertible
Notes and Warrants by delivering $5,580,000, before deducting fees and other offering expenses payable by the Company, in cash consideration
to the Company.
The following description relates to the particular
material terms of the Warrants being registered herein.
The Warrants are immediately exercisable upon
issuance (the “Initial Exercise Date”) at an exercise price of $2.9869 per share, subject to customary adjustments thereunder,
and will expire on the fifth (5th) anniversary of the Initial Exercise Date. The Warrants are exercisable on a cashless basis
if there is no effective registration statement registering the Warrant Shares for resale at the time of exercise.
The Warrants and the Warrant Shares being registered
herein were initially sold pursuant to an exemption from the registration requirements of the Securities Act provided in Section 4(a)(2)
of the Securities Act and/or Regulation D promulgated thereunder. Accordingly, the Selling Shareholders may exercise such Warrants and
sell the Ordinary Shares underlying such Warrants only pursuant to an effective registration statement under the Securities Act covering
the resale of such shares, an exemption under Rule 144 under the Securities Act, or another applicable exemption under the Securities
Act.
For a complete description of the documents entered
into in relation to such concurrent private placement, you should review a copy of the form of Warrant and form of Registration Rights
Agreement, which are included as exhibits to the Company’s Current Report on Form 6-K, filed with the SEC on December 28, 2023.
Concurrent Primary Offering
Concurrently with the private placement of the
Warrants and Warrant Shares we are registering herein, we simultaneously sold $6,000,000 in 7% OID Convertible Notes to the Selling Shareholders.
The Convertible Notes, which were sold pursuant to a prospectus supplement filed on Form F-3, are described below.
Maturity Date
The Convertible Notes will mature 364 days after
the date of issuance.
Interest
The Convertible Notes bear no interest unless
an event of default thereon has occurred. From and after the occurrence of any such event of default, the aggregate unconverted and then
outstanding principal amount of the Convertible Notes shall be paid at the rate of ten percent (10%) per annum. See “Events
of Default” below.
Conversion
The Convertible Notes are convertible at any
time after the issuance, at the option of the Investors, into our Ordinary Shares at the lower of (i) $2.738 per share, (ii) 95% of the
lowest VWAP of the Ordinary Shares during the seven (7)-trading day period immediately preceding the applicable conversion date, in each
case subject to adjustment as provided therein.
Certain Adjustments
The conversion price is subject to a standard
adjustments in the event of any stock split, stock dividend, stock combination, recapitalization or other similar transaction.
Mandatory Redemption
If, at any time while the Convertible Notes are
outstanding the Company carries out one or more Subsequent Financings, the Investors have the right to require us to first use up to
30% of the gross proceeds of such Subsequent Financing to redeem all or a portion of the Registered Notes, on a pro rata basis, for an
amount in cash equal to the outstanding principal amount of the Registered Notes, plus all accrued but unpaid interest, plus all liquidated
damages, if any, and any other amounts, if any, multiplied by 1.05.
If, at any time while the Convertible Notes are
outstanding, a Change of Control occurs, the Investors will have the right to require us to redeem all of the Convertible Notes, plus
all accrued but unpaid interest, plus all liquidated damages, if any, and any other amounts, if any, then owing to the Investors in respect
of the Convertible Notes.
Negative Covenants
We will be subject to certain customary affirmative
and negative covenants regarding the incurrence of certain indebtedness, the existence of liens, the repayment of indebtedness, the payment
of cash in respect of dividends, distributions or redemptions, and the transfer of assets, among other matters.
Events of Default
The Convertible Notes include certain customary
and other events of default, including, among other things, failure to maintain an effective registration statement to allow the Investors
to resell the Ordinary Shares, whether such shares are issued pursuant to the Convertible Notes, the Warrants, or any Additional Notes
or Additional Warrants that may be subsequently sold to the Investors, and maintain our Nasdaq listing.
In connection with such an event of default,
the aggregate unconverted and then outstanding principal amount of the Convertible Notes are required to be paid at the rate of ten percent
(10%) per annum, plus any interest or penalties that may be then owed.
Change of Control
In connection with a Change of Control, the Investors
have the right to require us to redeem all of the Convertible Notes, plus all accrued but unpaid interest, plus all liquidated damages,
if any, and any other amounts, if any, then owing to the Investors in respect of the Convertible Notes for an amount in cash equal to
the total amount owed to the investors multiplied by 1.05.
Conversion Limitation
Each Investor will not have the right to convert
any portion of the Convertible Notes, to the extent that, after giving effect to such conversion, the Investors (together with certain
of its related parties) would beneficially own in excess of 4.99% of our Ordinary Shares outstanding immediately after giving effect
to such conversion. The Investors may from time to time increase this limit to 9.99%, provided that any such increase will not be effective
until the 61st day after delivery of a notice to us of such increase.
Related Transaction Agreements
In connection with the
concurrent private placement of Warrants contemplated by the Securities Purchase Agreement, on December 27, 2023, we and the Selling
Shareholders entered into a Registration Rights Agreement (the “Registration Right Agreement”) pursuant to which we agreed
to file with the SEC an additional registration statement (the “Registration Statement”) to register the Ordinary Shares
underlying the Warrants and any additional notes or additional warrants purchased by the Investors in any Additional Closings (as defined
in the Securities Purchase Agreement) within 60 calendar days following the date of the Registration Rights Agreement. In addition, on
December 27, 2023, the Company entered into a placement agent agreement (“Placement Agent Agreement”) pursuant to which Maxim
Group LLC (“Maxim”) was engaged to act as exclusive placement agent in the offering and would be entitled to an aggregate
cash fee of 7.0% of the gross proceeds of the offering, plus up to $50,000 for all travel and other out-of-pocket expenses, including
reasonable and accounted for fees and expenses of Maxim’s legal counsel.
The above summaries
of certain terms and provisions of the Convertible Notes, the Warrants, the Securities Purchase Agreement, the Registration Rights Agreement
and Placement Agent Agreement are not complete and are subject to, and qualified in its entirety by, the provisions of the forms of such
documents, which have been filed as exhibits to the Company’s Current Report on Form 6-K on December 28, 2023. You should review
the copies of such documents before you invest in our securities.
USE OF PROCEEDS
We will not receive any proceeds from the sale
of the Warrant Shares by the Selling Shareholders. However, we may receive funds upon the Selling Shareholders’ exercise of the
Warrants. In the event we receive funds from the Selling Shareholder’s exercise of the Warrants, pursuant to the Securities Purchase
Agreement, we have agreed to use the net proceeds to fund our ongoing general corporate and working capital needs. However, the amount
and timing of our actual expenditures will depend on numerous factors, including our business strategy and development efforts. Pending
these uses, the proceeds will be invested in short-term bank deposits. We may also use a portion of the net proceeds for the acquisitions
of businesses, products, technologies, or licenses that are complementary to our business, although we have no present commitments or
agreements to do so.
PRINCIPAL AND SELLING SHAREHOLDERS
The table below sets forth certain information
regarding the beneficial ownership of our ordinary shares as of the date of this prospectus, by:
| ● | each of
our directors and executive officers; |
| ● | all of
our executive officers and directors as a group; |
| ● | each person
known to us to beneficially own more than five percent of our ordinary shares; and |
| ● | the selling
stockholders. |
The ordinary shares being offered by the Selling
Shareholders are those issuable to the Selling Shareholders pursuant to the terms of certain of the Company’s warrants sold to
the Selling Shareholders pursuant to the Securities Purchase Agreement. For additional information regarding the issuances of the Warrants,
see “Private Placement of Warrants” above. We are registering the ordinary shares in order to permit the Selling Shareholders
to offer the shares for resale from time to time. Except for the sale of the Convertible Notes (sold in a primary offering described
in “Concurrent Primary Offering” above) and the accompanying Warrants being described herein, the Selling Shareholders have
not had any material relationship with us within the past three years.
The table below lists the Selling Shareholders
and other information regarding the beneficial ownership of our ordinary shares by each of the Selling Shareholders. The second column
lists the number of ordinary shares beneficially owned by each Selling Shareholder, based on its ownership of the Convertible Notes and
accompanying Warrants, as of February 22, 2024, assuming the conversion of the Convertible Notes and accompanying Warrants held by the
Selling Shareholders on that date, without regard to any limitations on conversion.
The third column lists the ordinary shares being
offered by this prospectus by the Selling Shareholders.
In accordance with the terms of a registration
rights agreement with the Selling Shareholders, this prospectus covers the resale of 1,205,255 ordinary shares issuable upon exercise
of the Warrants, The fourth column assumes the sale of all of the shares offered by the Selling Shareholders pursuant to this prospectus.
Under the terms of the Convertible Notes and
Warrants held by the Selling Shareholders, a Selling Shareholder may not be issued shares under the notes or accompanying warrants to
the extent such issuance would cause such Selling Shareholder, together with its affiliates and attribution parties, to beneficially
own a number of ordinary shares which would exceed 4.99% of our then outstanding ordinary shares following such conversion. The number
of shares in the second column does not reflect this limitation. The selling shareholders may sell all, some or none of their shares
in this offering. See “Plan of Distribution.”
| Name
of Shareholder | |
Number
of
Shares
Owned Prior
to Offering | | |
Maximum
Number of
Shares to be
Offered for
the Account
of the Selling
Shareholder | | |
Number
of
Shares
Owned After
the Offering** | | |
%
Owned
After the
Offering** | |
| Selling
Shareholders | |
| | |
| | |
| | |
| |
| Anson East Master
Fund LP(1) | |
| 679,328 | | |
| 241,051 | | |
| 438,277 | | |
| 1.75 | % |
| Anson Investment Master Fund
LP(2) | |
| 2,173,514 | | |
| 964,204 | | |
| 1,209,310 | | |
| 4.82 | % |
| Current
Executive Officers and Directors | |
| | | |
| | | |
| | | |
| | |
| Yonjun Liu(3) | |
| 15,935,000 | | |
| - | | |
| 15,935,000 | | |
| 60.62 | % |
| Xin Wang | |
| - | | |
| - | | |
| - | | |
| - | % |
| Yulin Wang | |
| - | | |
| - | | |
| - | | |
| - | % |
| Xiaoming E | |
| - | | |
| - | | |
| - | | |
| - | % |
| Huijuan Zhao(4) | |
| 740 | | |
| - | | |
| 740 | | |
| - | % |
| Wenzhang Jia(5) | |
| 1,866 | | |
| - | | |
| 1,866 | | |
| - | % |
| All Current
Officers and Directors | |
| 15,936,906 | | |
| - | | |
| 15,936,606 | | |
| 60.62 | % |
| ≥ 5%
Beneficial Owners | |
| - | | |
| - | | |
| - | | |
| - | % |
| * |
The percentage of ownership before the offering
is calculated based on 25,077,467 shares outstanding as of February 22, 2024. The percentage of ownership after the offering assumes
the issuance of all of the shares issuable upon exercise of the Warrants that are offered for resale hereby, and the sale by such
selling stockholder of all of the shares offered for resale hereby. |
| ** |
Assumes all Warrant Shares have been sold in the Offering
and assumes that any shares issuable upon conversion of the Convertible Notes will be subject to a 4.99% ownership limitation, as
set forth in the Securities Purchase Agreement. |
| (1). |
Consists
of (i) 438,277 shares issuable upon conversion of the Convertible Notes, assuming a fixed conversion price of $2.738 per share, and
(ii) 241,051 shares issuable upon exercise of the Warrants held by Anson East Master Fund LP (“AEMF”). Anson Advisors
Inc and Anson Funds Management LP, the Co-Investment Advisers of AEMF, hold voting and dispositive power over the ordinary shares
held by AEMF. Tony Moore is the managing member of Anson Management GP LLC, which is the general partner of Anson Funds Management
LP. Moez Kassam and Amin Nathoo are directors of Anson Advisors Inc. Mr. Moore, Mr. Kassam and Mr. Nathoo each disclaim beneficial
ownership of these ordinary shares except to the extent of their pecuniary interest therein. The principal business address of AEMF
is Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands. |
| (2) |
Consists
of (i) 1,209,310 shares issuable upon conversion of the convertible Notes, assuming a fixed conversion price of $2.738 per share,
and (ii) 964,204 shares issuable upon exercise of the Warrants held by Anson Investments Master Fund LP (“AIMF”). Anson
Advisors Inc and Anson Funds Management LP, the Co-Investment Advisers of AIMF, hold voting and dispositive power over the ordinary
shares held by AIMF. Tony Moore is the managing member of Anson Management GP LLC, which is the general partner of Anson Funds Management
LP. Moez Kassam and Amin Nathoo are directors of Anson Advisors Inc. Mr. Moore, Mr. Kassam and Mr. Nathoo each disclaim beneficial
ownership of these ordinary shares except to the extent of their pecuniary interest therein. The principal business address of AIMF
is Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands. |
| (3) | Mr. Liu holds these shares through Bright
Accomplish Limited, a holding company controlled jointly by Mr. Liu and his wife, Yin Liu. |
| (4) | Represents options to purchase 1,886 Ordinary
Shares of the Company, at an exercise price of $5.30 per share. The options vest in equal
monthly installments over Mr. Jia’s initial year of service and are exercisable for
a term of ten years. |
| (5) | Represents options to purchase 740 Ordinary
Shares of the Company, at an exercise price of $13.51 per share. The options vest in equal
monthly installments over Ms. Zhao’s initial year of service and are exercisable for
a term of ten years. |
As
used in this table, “beneficial ownership” means the sole or shared power to vote, or to direct the voting of, a security,
or the sole or shared investment power with respect to a security (i.e., the power to dispose of, or to direct the disposition of, a
security). In addition, for purposes of this table, a person is deemed, as of any date, to have “beneficial ownership” of
any security that such person has the right to acquire within 60 days after such date.
The persons named above have full voting and
investment power with respect to the shares indicated. Under the rules of the Securities and Exchange Commission, a person (or group
of persons) is deemed to be a “beneficial owner” of a security if he or she, directly or indirectly, has or shares the power
to vote or to direct the voting of such security, or the power to dispose of or to direct the disposition of such security. Accordingly,
more than one person may be deemed to be a beneficial owner of the same security. A person is also deemed to be a beneficial owner of
any security, which that person has the right to acquire within 60 days, such as options or warrants to purchase our Ordinary Shares.
Major Shareholders
Other than as set forth above, there are no beneficial
owners of 5% or more of our voting securities. The company is not directly or indirectly owned or controlled by another corporation(s)
or by any foreign government. There are no arrangements, known to us, the operation of which may at a subsequent date result in a change
in control of the company.
CORPORATE HISTORY AND STRUCTURE
History and Development of the Company
Meihua is a holding company incorporated under
the laws of the Cayman Islands on November 10, 2020 by our shareholder Yongjun Liu. Meihua’s direct subsidiary is Kang Fu International
Medical, a Hong Kong company. Kang Fu International Medical was incorporated on October 13, 2015 by four shareholders, Yongjun Liu, Yin
Liu, Ace Capital Limited and King Tai International Holding Limited. On November 22, 2019, Yongjun Liu acquired 9,300,000 shares from
Ace Capital Limited and 4,500,000 shares from King Tai International Holding Limited, respectively. Upon consummation of such share transfer,
Yongjun Liu and Yin Liu constituted all of the shareholders of Kang Fu International Medical, holding 100% shares of Kang Fu International
Medical. On December 21, 2020, Meihua in turn acquired 41,400,000 shares (or 69%) from Yongjun Liu and 18,600,000 shares (or 31%) from
Yin Liu, respectively, resulting in Kang Fu International Medical becoming Meihua’s wholly owned subsidiary. In exchange for
the acquisition on Kang Fu, Meihua issued a total of 15,935,000 Ordinary Shares to Mr. and Mrs. Liu, who in turn transferred their shares
to their wholly-owned holding company, Bright Accomplish Limited, on December 21, 2020. Bright Accomplish Limited is Meihua’s controlling
shareholder, holding approximately 66.56% of Meihua’s Ordinary Shares as of the date of this report.
Meihua is not a Chinese operating company
but a Cayman Islands holding company with operations conducted by its subsidiaries located in mainland China. Meihua operates its
business through its indirect subsidiaries in China. Below is a list of Meihua’s operating subsidiaries in China:
| |
● |
Yangzhou
Huada Medical Device Co., Ltd., or Yangzhou Huada: a subsidiary wholly owned by Kang Fu International Medical and established in
Yangzhou, Jiangsu Province, PRC on December 24, 2001 with a registered capital of $602,400, which manufactures and sells Class I
disposable medical devices under our own brands, and distributes Class I and Class II disposable medical devices sourced from other
manufacturers, to our domestic customers. Specifically, Yangzhou Huada mainly focuses on the manufacturing, sales and distributions
of non-bottled products, such as brushes, ID bracelets for domestic sales. |
| |
● |
Jiangsu
Yada Technology Group Co., Ltd., or Jiangsu Yada: a subsidiary wholly owned by Yangzhou Huada and established in Yangzhou, Jiangsu
Province, PRC on December 5, 1991 with a registered capital of RMB51,390,000, which manufactures and sells Class I and Class II disposable
medical devices under our own brands, and distributes Class I and Class II disposable medical devices sourced from other manufacturers,
to our domestic and overseas customers. Specifically, Jiangsu Yada mainly focuses on overseas sales. |
| |
● |
Jiangsu
Huadong Medical Device Industrial Co., Ltd., or Jiangsu Huadong: a subsidiary wholly owned by Jiangsu Yada and established in Yangzhou,
Jiangsu Province, PRC on November 18, 2000 with a registered capital of RMB50,000,000, which manufactures and sells Class I, II and
III disposable medical devices under our own brands, and distributes Class I, II and III disposable medical devices sourced from
other manufacturers, to our domestic and overseas customers. Specifically, Jiangsu Huadong mainly focuses on the manufacturing, sales
and distributions of polyethylene bottled products, such as eye drop bottles and tablet bottles. |
| |
● |
Yangzhou
Guanghui Medical Technology Co., Ltd., or Guanghui: a subsidiary wholly owned by Jiangsu Huadong was established in Yangzhou, China
on December 22, 2020 with a registered capital of RMB1,000,000, to mainly manufactures and sells Class II disposable medical devices.
Guanghui has not conduct real business. |
| |
● |
Hainan
Guoxie Technology Group Co., Ltd., or Hainan Guoxie: a subsidiary of which 55% of registered capital (subscribed but unpaid registered
capital) was acquired by Kang Fu International Medical from an individual Qin Wang with nil consideration on July 6, 2022, in order
to conduct local business activities in Hainan. Hainan Guoxie was established in Qionghai, Hainan Province, China on October 7, 2021
with a registered capital of RMB100,000,000. Further disclosure will be made in 6-K regarding the new controlling subsidiary. |
Meihua owns 100% of Kang Fu International
Medical. Kang Fu International Medical owns 100% of Yangzhou Huada and 55% of Hainan Guoxie. Yangzhou Huada owns 100% of Jiangsu Yada.
Jiangsu Yada, in turn, owns 100% of Jiangsu Huadong. Jiangsu Huadong, in turn, owns 100% of the equity interests of Guanghui. The following
diagram illustrates our corporate structure as of the date of this report, including our principal subsidiary and their respective principal
subsidiaries.

The structure of cash flows within our organization,
and a summary of the applicable regulations, is as follows:
| |
1. |
Our
equity structure is a direct holding structure, pursuant to which the overseas entity listed in the U.S., Meihua International Medical
Technologies Co., Ltd. (“Meihua International”), directly controls Yangzhou Huada Medical Device Co., Ltd (“Yangzhou
Huada”) (the “WFOE”) and other domestic operating entities which are directly owned through the Hong Kong company,
Kang Fu International Medical Co., Limited (“Kang Fu”). |
| |
2. |
Within
our direct holding structure, the cross-border transfer of funds within our corporate group is legal and compliant with the laws
and regulations of the PRC. After foreign investors’ funds enter Meihua International at the close of securities offerings,
the funds can be directly transferred to Kang Fu, and then transferred to subordinate operating entities through the WFOE. |
If the Company intends to distribute dividends,
the Company will transfer the dividends to Kang Fu in accordance with the laws and regulations of the PRC, and then Kang Fu will transfer
the dividends to Meihua International, and the dividends will be distributed from Meihua International to all shareholders respectively
in proportion to the shares they hold, regardless of whether the shareholders are U.S. investors or investors in other countries or regions.
| |
3. |
In
the reporting periods presented herein, no cash and other asset transfers have occurred among the Company and its subsidiaries
are as summarized as below: |
| | |
For
the year ended December 31, 2022 (in
US$) | |
| Inter-company cash transfers: | |
Meihua
(Cayman) | | |
Kang Fu
International
Medical
(HK) | | |
PRC
subsidiaries | |
| Cash transferred from Meihua to Kang Fu International Medical (1) | |
$ | (26,010,150 | ) | |
$ | 26,010,150 | | |
| - | |
| Cash transferred from Kang Fu International Medical to Meihua (2) | |
$ | 390 | | |
$ | (390 | ) | |
| - | |
| Cash transferred from Kang Fu International Medical to PRC subsidiaries (3) | |
| - | | |
$ | (20,389,970 | ) | |
$ | 20,389,970 | |
| Cash transferred from PRC subsidiaries to Kang Fu International Medical (4) | |
| - | | |
$ | 130,000 | | |
$ | (130,000 | ) |
| (1) |
Meihua transferred $26,010,150 to Kang Fu International
Medical as a working capital loan. |
| (2) |
Kang Fu International Medical transferred $390 to
Meihua for repayment of a working capital loan. |
| (3) |
Kang Fu International Medical contributed $20,389,970
to PRC subsidiaries as a capital contribution.* |
| (4) |
Yangzhou Huada, one of the PRC subsidiaries, transferred
$130,000 to Kang Fu International Medical as a working capital loan. |
| |
* |
On February 18, 2022,
the Company closed its initial public offering of Ordinary Shares and received approximately US$35 million. In March and April 2022,
the Company transferred approximately US$26.0 million to Kang Fu International Medical for working capital purpose then Kang Fu International
Medical made capital injection in aggregated of approximately US$20.4 million to PRC subsidiaries - Yangzhou Huada and Hainan Guoxie. |
| | |
For
the year ended December 31, 2021 (in
US$) | |
| Inter-company cash transfers: | |
Meihua
(Cayman) | | |
Kang Fu
International
Medical
(HK) | | |
PRC
subsidiaries | |
| Cash transferred from Kang Fu International Medical to PRC subsidiaries (5) | |
| - | | |
$ | (46,297 | ) | |
$ | 46,297 | |
| Cash transferred from PRC subsidiaries to Kang Fu International Medical (6) | |
| - | | |
$ | 768,042 | | |
$ | (768,042 | ) |
| (5) |
Kang Fu International Medical transferred $46,297
to PRC subsidiaries for repayment of a working capital loan. |
| (6) |
Yangzhou Huada, one of the PRC subsidiaries, transferred
$768,042 to Kang Fu International Medical as a working capital loan. |
| | |
For
the year ended December 31, 2020 (in
US$) | |
| Inter-company cash transfers: | |
Meihua
(Cayman) | | |
Kang Fu
International
Medical
(HK) | | |
PRC
subsidiaries | |
| Cash transferred from PRC subsidiaries to Kang Fu International Medical (7) | |
| - | | |
$ | 499,998 | | |
$ | (499,998 | ) |
| (7) |
Yangzhou Huada, one of the PRC subsidiaries, transferred
$499,998 to Kang Fu International Medical as a working capital loan. |
| |
4. |
No dividends or distributions
of a subsidiary have been made to the Company for the years ended December 31, 2020, 2021 and 2022. For the foreseeable future, the
Company intends to use the earnings for research and development, to develop new products and to expand its production capacity.
As a result, we do not expect to pay any cash dividends for the near term. |
Our PRC subsidiaries’ ability to distribute
dividends is based upon their distributable earnings. Current PRC regulations permit our PRC subsidiaries to pay dividends to their respective
shareholders only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In
addition, each of our PRC subsidiaries is required to set aside at least 10% of its after-tax profits each year, if any, to fund a statutory
reserve until such reserve reaches 50% of each of their registered capitals. These reserves are not distributable as cash dividends.
See “Regulations Relating to Dividend Distributions” for more information.
To address persistent capital outflows and
the RMB’s depreciation against the U.S. dollar in the fourth quarter of 2016, the People’s Bank of China and the State Administration
of Foreign Exchange, or SAFE, have implemented a series of capital control measures in the subsequent months, including stricter vetting
procedures for China-based companies to remit foreign currency for overseas acquisitions, dividend payments and shareholder loan repayments.
The PRC government may continue to strengthen its capital controls and our PRC subsidiaries’ dividends and other distributions
may be subject to tightened scrutiny in the future. The PRC government also imposes controls on the conversion of RMB into foreign currencies
and the remittance of currencies out of the PRC. Therefore, we may experience difficulties in completing the administrative procedures
necessary to obtain and remit foreign currency for the payment of dividends from our profits, if any. Furthermore, if our subsidiaries
in the PRC incur debt on their own in the future, the instruments governing the debt may restrict their ability to pay dividends or make
other payments.
In addition, the Enterprise Income Tax Law
and its implementation rules provide that a withholding tax at a rate of 10% will be applicable to dividends payable by Chinese companies
to non-PRC-resident enterprises unless reduced under treaties or arrangements between the PRC central government and the governments
of other countries or regions where the non-PRC resident enterprises are tax resident. Pursuant to the tax agreement between Mainland
China and the Hong Kong Special Administrative Region, the withholding tax rate in respect to the payment of dividends by a PRC enterprise
to a Hong Kong enterprise may be reduced to 5% from a standard rate of 10%. However, if the relevant tax authorities determine that our
transactions or arrangements are for the primary purpose of enjoying a favorable tax treatment, the relevant tax authorities may adjust
the favorable withholding tax in the future. Accordingly, there is no assurance that the reduced 5% withholding rate will apply to dividends
received by our Hong Kong subsidiary from our PRC subsidiaries. This withholding tax will reduce the amount of dividends we may receive
from our PRC subsidiaries.
Recent Developments
Convertible Note and Warrant Offering
On December 27, 2023, the Company entered
into a securities purchase agreement (the “SPA”) with certain institutional investors (the “Investors”), pursuant
to which the Company agreed to issue, from time to time, up to $50,500,0000 in the Company’s securities (the “Offering”),
consisting of convertible notes, issuable at a 7.0% original issue discount (the “Notes”), and accompanying ordinary share
purchase warrants (the “Warrants”) with five-year terms and exercisable for a number of the Company’s ordinary shares,
par value $0.0005 per share (the “Ordinary Shares”), equal to 50% of the number obtained from dividing each Note’s
principal amount by the applicable VWAP (as defined in the SPA), subject to adjustment pursuant and a 4.99% beneficial ownership limitation.
Pursuant to the SPA, the Company agreed to issue to the Investors at the initial closing of the Offering (the “First Closing”)
$6,000,000 in Notes, convertible at the lower of (i) $2.738 per share (or 110% of the VWAP of the Ordinary Shares on December 27, 2023)
or (ii) a price per share equal to 95% of the lowest VWAP of the Ordinary Shares during the seven (7)-trading day period immediately
preceding the applicable conversion date, subject to certain adjustments and a 4.99% beneficial ownership limitation, and Warrants exercisable
for up to an aggregate of 1,205,255 ordinary shares, at an exercise price of $2.9869 per share (or 120% of the VWAP of the Ordinary Shares
on December 27, 2023). The Notes do not bear interest except upon the occurrence of an event of default thereunder, have 364-day maturity
dates, must be redeemed by the Company at a premium in the event of (i) a Subsequent Financing (as defined in the SPA), (ii) a Change
of Control (as defined in the SPA) and (iii) certain equity conditions listed therein. The Company also has the option to redeem the
Notes in the event that the Company deems it in its best interest to do so, such as if it believes an event of default under the Notes
is imminent. The Notes contain certain other covenants and events of default customary for similar transactions. The First Closing occurred
on January 2, 2024. Any additional closings are not expected to occur until there remains $500,000 or less outstanding on the issued
Notes.
Completion of the Initial Public Offering
(“IPO”)
On February 18, 2022, we closed our initial
public offering (“IPO”) of 3,940,000 Ordinary Shares, par value US$0.0005 per share at a public offering price of $10.00
per share, and our Ordinary Shares started to trade on the Nasdaq Global Market under the ticker symbol “MHUA” on February
16, 2022. We sold 3,600,000 shares pursuant to our underwriters’ firm commitment, together with an additional 340,000 shares sold
pursuant to the underwriters’ partial exercise of their over-allotment option.
The total net proceeds to the Company from
the IPO, after deducting discounts, expense allowance, and expenses, were approximately $34,576,000. At the closing of the IPO, the Company
deposited $500,000 from the gross proceeds from the IPO into an escrow account established by Wilmington Trust, National Association,
as escrow agent, for purpose of covering potential legal actions against Prime Number Capital LLC, as a representative of the Underwriters,
pursuant to an indemnification escrow agreement.
As a result of certain developments related
to the IPO, the Company may face certain legal liabilities and exposure. Specifically, during the roadshow leading up to the IPO, a Hong
Kong investment company named Tai He was referred to the Company by Shengang, the Company’s co-underwriter based in the PRC. Tai
He, through negotiations with Shengang, asserted that it would participate in the IPO as an investor. As a development stage PRC medical
device company, the Company’s management had no experience in the U.S. capital markets and relied on its investment bankers and
underwriters in providing guidance and advice concerning its U.S. public offering. Accordingly, the Company was unaware of the actual
motives of Tai He. Based on the Company’s trust in Shengang and given the circumstances that the Company was unable to make further
verification, the Company hastily entered into the Tai He Agreements.
Pursuant to the Tai He Agreements, Tai He
agreed to invest a minimum of $35 million in the IPO subject to the Company making a $7.0 million refundable deposit and advancing a
$3.0 million service fee for investor relations and other services (together, the “Services”) payable to Tai He. The Company,
its affiliates and individual shareholders paid the $7.0 million Refundable Deposit and $3.0 million service fee to Tai He in several
installments from January 27, 2022 to March 11, 2022. After March 11, 2022, the Company has made no other payments to, nor had any direct
interaction with, Tai He, and, in actuality, the Company never directly communicated with Tai He during the negotiations, only communicating
about Tai He and the Tai He Agreements through Shengang.
After the IPO, through the Company’s
internal risk control process, the Company learned that Tai He does not appear to be an investor in the Company and, when the Company
attempted to inquire about it, Tai He refused to speak with the Company. Based on the facts as investigated and understood by the Company,
the Company believes that it was defrauded by Tai He and that Tai He never actually invested $35 million in the IPO. In fact, the Company
learned that Tai He made no investments in the Company at all, and Tai He has no involvement in the Company’s IPO matters. In addition,
the Refundable Deposit has not been repaid to the Company and no Services have been provided to the Company by Tai He. As a result, the
Company believes that the Tai He Agreements may be deemed a fraud perpetrated by Tai He and Shengang against the Company by Tai He taking
advantage of asymmetric information, including the Company’s lack of knowledge and understanding of the US markets, IPO rules and
processes, and the trust of the Company.
After consultation with professionals, the
Company is now aware that the Tai He Agreements were required to be disclosed to the public and the Company believes that the agreements
themselves are fraudulent and non-enforceable, and therefore the Company is disclosing the Tai He Agreements to meet the compliance requirements
of the SEC. Further, the Company acknowledges that it had improperly relied on the advice, direction and counsel of Shengang when entering
into the Tai He Agreements. The Company is now proactively working to terminate the Tai He Agreements and recover the monies already
paid to Tai He by the Company. Due to uncertainty of collection, the Company has written off approximately $4.8 million deposit and fully
expensed $2.3 million service fee paid by the Company to Tai He in the year ended December 31, 2022. The Company and its executive management
team intends to cooperate with the SEC, as well as any other relevant authorities, to ensure that its investors’ interests are
protected while such matters are resolved.
Corporate Information
Our principal executive offices are located
at 88 Tongda Road, Touqiao Town, Guangling District, Yangzhou, PRC. Our telephone number at this address is +86-0514-89800199. Our registered
office in the Cayman Islands is currently located at the office of Sertus Incorporations (Cayman) Limited, Sertus Chambers, Governors
Square, Suite #5-204, 23 Lime Tree Bay Avenue, P.O. Box 2547, Grand Cayman, KY1-1104, Cayman Islands, which may be changed from time
to time at the discretion of directors. Our agent for service of process in the United States is Dorsey & Whitney LLP,
51 West 52nd Street, New York, New York 10019
BUSINESS
Overview
Meihua International is a Cayman Islands exempted
company incorporated on November 10, 2020. Kang Fu International is our wholly owned subsidiary formed in Hong Kong on October 13, 2015.
We operate our business through our operating subsidiaries in China, namely 1) Yangzhou Huada, a wholly foreign owned subsidiary of Kang
Fu International Medical, formed on December 24, 2001, located in Yangzhou, Jiangsu Province, PRC; 2) Jiangsu Yada, a wholly owned subsidiary
of Yangzhou Huada, formed on December 5, 1991, located in Yangzhou, Jiangsu Province, PRC; 3) Jiangsu Huadong, a wholly owned subsidiary
of Jiangsu Yada, formed on November 18, 2000, located in Yangzhou, Jiangsu Province, PRC; and 4) Guanghui, a wholly owned subsidiary
of Jiangsu Huadong, formed December 22, 2020.
Through our operating subsidiaries in the
PRC, Yangzhou Huada, Jiangsu Yada and Jiangsu Huadong, we are mainly specialized in the research, development, manufacturing and sales
of Class I, Class II and Class III disposable medical devices both domestically and internationally.
Pursuant to the Regulations on the Supervision
and Administration of Medical Devices promulgated on January 4, 2000, which is effective on June 1, 2014 and amended by the State Council
on May 4, 2017, medical devices are classified into the following three categories based on the degree of risk.
| Class |
|
Standard
(per PRC National Medical Device Management regulations) |
| I |
|
Class
I medical devices shall refer to those devices with low level of risks and whose safety and effectiveness can be ensured through
routine administration. |
| II |
|
Class
II medical devices shall refer to those devices with moderate risks that must be strictly controlled and regulated to ensure their
safety and effectiveness. |
| III |
|
Class
III medical devices shall refer to those devices with relatively high risks that must be strictly controlled and regulated through
special measures to ensure their safety and effectiveness. |
We provide our customers with one-stop solution
for a variety of safety and quality disposable medical devices. The safety and quality of disposable medical devices are always our core
values. We attribute our success to our sustainable and organic growth driven by our capacity expansion based on market demand, our deep
understanding of our target end markets and our sound relationships with our customers, distributors, independent sales agents, and suppliers.
Our Revenue Model
We generate revenues through 1) manufacturing
and sales of Class I, II, III disposable medical devices under our own brands, 2) resales of Class I, II, III disposable medical devices
sourced by us from other manufacturers. For the fiscal years ended December 31, 2022, 2021, and 2020, we recognized $103,346,341, $104,037,710,
and $89,061,010, respectively, in revenues, of which our own brand sales accounted for 48.88%, 46.19%, and 49.94%, respectively, and
the resales of sourced disposable medical devices from other manufactures accounted for 51.12%, 53.81%, and 50.06%, respectively.
Our disposable medical devices reach end users
both domestically and internationally. For the fiscal years ended December 31, 2022, 2021, and 2020, our total sales to domestic direct
end users customers and domestic distributor customers accounted for 99.52%, 85.78%, and 81.90% of our revenues, respectively. For the
fiscal years ended December 31, 2022, 2021, and 2020, our sales to overseas distributing customers accounted for 0.48%, 14.22%, and 18.10%,
respectively, of our revenues.
We sell disposable medical devices through
our direct sales force and distributors. For the fiscal years ended December 31, 2022, 2021, and 2020, our sales through direct sale
channels accounted for 9.16%, 9.13%, and10.59%, respectively, of our revenues, and our sales through distributors accounted for 90.84%,
90.87%, and 89.41%, respectively, of our revenues, of which domestic distributors accounted for 90.36%, 76.65%, and 71.31%, respectively,
and exporting distributors accounted for 0.48%,14.22%, and 18.10%, respectively, of our revenues.
Our Products
Our products cover all regions of PRC. Internationally,
our products are exported to more than 30 countries, including Europe, North America, South America, Asia, Africa, and Oceania.
Our current product portfolio (consisting
of both self-manufactured and out-sourced products) includes: 1) Class I disposable medical devices, such as, disposable medical X-ray
films, medical dry films, dry laser imagers, gauze bandages, examination gloves, pharmaceutical packaging materials and containers, low-density
polyethylene (LDPE) bottles for eye drops, high-density polyethylene (HDPE) bottles for tablets, etc.; 2) Class II disposable medical
devices, such as, disposable full anesthesia kits, medical brush, woman’s examination kits, urethral catheterization kits, gynecological
examination kits, endotracheal intubation, medical masks, anal bags, and suction connecting tube, etc.; and 3) Class III disposable medical
devices, such as disposable infusion pumps, anesthesia puncture kits, electronic pumps, etc.
As of the date of this report, we have a total
of 1,063 products in our product portfolio, including 937 products for domestic sales and 126 products for overseas sales.
Our top 20 products for the fiscal years ended
December 31, 2022 and 2021 were as follows:
| Class
Category |
|
Product
Name |
|
Image |
|
Own
brand /
source from
other |
|
Use |
|
%
of
Sales in
2022 |
|
|
%
of
Sales in
2021 |
|
| Class
II |
|
Disposable
ID bracelet |
|
 |
|
Own
Brand |
|
Identify
patients |
|
|
14.40 |
% |
|
|
13.07 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class II |
|
Disposable
woman’s examination kits |
|
 |
|
Own
Brand |
|
Gynecological
examination |
|
|
8.39 |
% |
|
|
5.55 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class II |
|
Disposable
medical brush |
|
 |
|
Own
Brand |
|
Clean
the test tube or plastic pipe |
|
|
8.14 |
% |
|
|
7.16 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class II |
|
Medical
kit |
|
 |
|
source
from other |
|
For
operation |
|
|
4.83 |
% |
|
|
3.86 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class II |
|
Medical
sterile dressing surgical kits |
|
 |
|
Own
brand |
|
Use
before operation |
|
|
4.56 |
% |
|
|
3.81 |
% |
| Class
Category |
|
Product
Name |
|
Image |
|
Own
brand /
source from
other |
|
Use |
|
%
of
Sales in
2021 |
|
|
%
of
Sales in
2020 |
|
| Class
II |
|
Medical
Brush |
|
 |
|
source
from other |
|
Hand
wash before Operation |
|
|
3.96 |
% |
|
|
4.10 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class
I |
|
High-density
polyethylene (HDPE) bottles for tablets |
|
 |
|
Own
brand |
|
Tablet
bottles |
|
|
3.13 |
% |
|
|
2.48 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class
II |
|
Medical
catheter |
|
 |
|
source
from other |
|
Use
for catheterization |
|
|
2.83 |
% |
|
|
3.46 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class
II |
|
Uterine
tissue suction tube |
|
 |
|
Own
brand |
|
Uterine
tissue sampling |
|
|
2.78 |
% |
|
|
3.44 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other |
|
Disposable
plastic milk bottle |
|
 |
|
Own
brand |
|
Use
for feeding |
|
|
2.34 |
% |
|
|
1.49 |
% |
| Class
Category |
|
Product
Name |
|
Image |
|
Own
brand /
source from
other |
|
Use |
|
%
of
Sales in
2021 |
|
|
%
of
Sales in
2020 |
|
| Class
I |
|
Low-density
polyethylene (LDPE) bottles for eye drops |
|
 |
|
Own
brand |
|
Eyedrop
bottle |
|
|
2.30 |
% |
|
|
2.78 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class
II |
|
Disposable
Gynecological sampler |
|
 |
|
Own
brand |
|
Getting
samples during gynecological examination |
|
|
1.92 |
% |
|
|
2.31 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class
II |
|
Disposable
medical brush (type B1) |
|
 |
|
Own
brand |
|
Hand
wash before operation |
|
|
1.48 |
% |
|
|
1.24 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Class
II |
|
Disposable
anal bag |
|
 |
|
Own
brand |
|
For
the rectal colon or ileum anal stoma postoperative patients and patients with urinary incontinence to collect feces and other feces
care |
|
|
1.44 |
% |
|
|
1.20 |
% |
As a medical device manufacturing and sales
company, we are subject to extensive government regulation and supervision in the PRC. Pursuant to PRC laws, we must obtain production
license for Class II and III disposable medical devices, operation license for Class III disposable medical devices, and filing or registration
certificates for certain Class I, II or Class III disposable medical devices. As of the date of this report, we are current on all licenses
and certificates and have obtained Class I, II and III disposable medical device qualifications in the PRC. Meanwhile, we have established
a sound quality assurance system. We have received international “CE” certification and ISO 13485 system certification. We
have also registered with the FDA (registration number: 3006554788) for over 20 products as of the date hereof, including but not limited
to ID bracelets, surgical tapes, elastic and adhesive bandages, which are all FDA Class I products.
Our operating subsidiaries in the PRC focus
on the manufacturing and sales of the disposable medical devices as follows:
Yangzhou Huada
Yangzhou Huada mainly manufactures and sells
Class I disposable medical devices, such as disposable pharmaceutical packaging materials and containers using LDPE for eye drops and
high-density polyethylene (“HDPE”) bottles for tablets, as well as disposable plastic baby bottles, NB/PSN rubber covers
and 8.2mL folded spoons for tools and containers, etc.
Additionally, Yangzhou Huada is also engaged
in the resales of Class I and II disposable medical devices sourced from other manufacturers when we provide one-stop shopping experience
to our customers.
As of the date of this report, Yangzhou Huada
has no manufacturing activities for Class II and III disposable medical devices and its sales are limited to our domestic customers.
Jiangsu Yada
Jiangsu Yada mainly manufactures and sells
both domestically and internationally 1) Class I disposable medical devices, such as medical dry imaging films; and 2) Class II disposable
medical devices, such as disposable woman’s examination kits, suction connecting tubes, and Class II 6866 medical polymer materials
and products (including but not limited to transfusion equipment and pipelines, endotracheal intubation for respiratory anesthesia or
ventilation), etc.
In addition to above, Jiangsu Yada is also
engaged in the domestic and international resales of 1) Class I and Class II disposable medical devices sourced from other manufacturers
when we provide one-stop shopping experience to our customers.
As of the date of this report, Jiangsu Yada
has no manufacturing and sales activities for Class III disposable medical devices.
Jiangsu Huadong
Jiangsu Huadong mainly manufactures and sales
both domestically and internationally 1) Class I medical devices, such as medical x-ray films, multi-functional self-extracting X-ray
film machines, dry films for medical use, gauze bandages, examination gloves, etc.; 2) Class II medical devices, such as disposable full
anesthesia kits, urethral catheterization kits, gynecological examination kits, endotracheal intubation, medical masks, and various tubes,
etc.; and 3) Class III medical devices, such as disposable infusion pumps, anesthesia puncture kits, electronic pumps, etc.
In addition to above, Jiangsu Huadong is also
engaged in the domestic and international resales of Class I, II and III medical devices sourced from other manufacturers when we provide
one-stop shopping experience to our customers.
COVID-19 Pandemic Products
With the impact of the coronavirus disease
2019 (COVID-19) outbreak commencing in the first half of 2020, demand in products related to virus prevention and control surged worldwide.
Although these products were not our mainstream products previously, we witnessed order surges and demand outstripped supply for several
months starting in February 2020. For the fiscal year ended December 31, 2020, orders for masks and gloves were valued at $10.1 million
and $2.4 million, respectively.
As of the date of this report, although the
current market demand for certain virus prevention products is not as strong as that in 2020 in China due to the ongoing recovery from
the COVID-19, awareness of protection against COVID-19 and other similarly situated droplet transmission diseases is to some extent rooted
among people in China, resulting in continuing high demand among people for virus prevention and control products compared to previous
years. Internationally, except for China, other countries are still in high demand of certain virus prevention products for the prevention
and control of COVID-19. According to a COVID-19 vaccines update released by World Health Organization in December 2020, there are three
COVID-19 vaccines for which certain national regulatory authorities have authorized the use. None have yet received WHO EUL/PQ authorization.
The impact of COVID-19 vaccines on the pandemic will depend on several factors, such as the effectiveness of the vaccines; how quickly
they are approved, manufactured, and delivered; and how many people get vaccinated. Most scientists anticipate that, like most other
vaccines, COVID-19 vaccines will not be 100% effective. (Source: https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines?adgroupsurvey={adgroupsurvey}&gclid=CjwKCAiA_eb-BRB2EiwAGBnXXrbZVR1ojK9i13YVEq7Hg5YBAULf8ii7IRf8kxKBu3fQQVpzJ8iD9hoCtHUQAvD_BwE).
As a result, we believe certain virus prevention products will still be in high demand due to the uncertainty surrounding the future
severity of COVID-19 and the awareness of the general public towards the virus prevention. To anticipate and adapt to this new normal
phase, the Company was building a 2,550 square meter of new factory for expanding the production scale of exporting products, located
in Jiangsu Yada’s factory plant. The Company received government approval on December 22, 2020 and has started construction. The
Company has completed construction during the current year, with a total cost of approximately $1.2 million. The source of funds for
the new factory comes mainly from bank loans. The Company has placed an order for 12 production lines, which will be installed in the
new factory when construction is complete. The new production lines have an estimated cost of $3.71 million and will be used to produce
medical and civil non-woven products for outbreak prevention, including masks, protective clothing and testing papers. The annual production
capacity of the new production lines includes approximately 45 million masks, 2.7 million insulation suits, 1.5 million protection suits,
90 million testing papers, 0.6 million Minimally invasive high-value consumables. Starting from February 2020, we began to gradually
add to the production line for masks and increase the production of masks. However, as the sales of masks is declining, our eight mask
production lines have been shut down in the past year. Sales of masks for the year ended December 31, 2022, 2021, and 2020 were approximately
$0.53 million, $1.02 million, and $10.1 million, respectively.
As the epidemic gradually flattens out, the
needs for masks decreased. We have not generated material cost consumption on the production of masks and masks are not our main sales
product. Accordingly, the decrease of demand for masks in 2022 and 2021 not have a material adverse effect on our revenue.
With additional capacity and a broad spectrum
of product offerings, we are capable of providing tailored “one-stop” services to our customers, ranging from wound care,
surgical auxiliary supplies to disease prevention.
Our One-stop Service
Our operating subsidiaries are located in
Touqiao Town, Yangzhou City, Jiangsu Province, PRC (“Touqiao Town”), one of the four medical device centers in PRC. Touqiao
Town is conferred on “Hometown of Medical Device & Consumables in China” by China Medical Device Industry Association
and granted by the Jiangsu local government as one of the 25 nationally certified “Chinese Towns with Special Features” on
medical devices. Hundreds of disposable medical device manufacturers have their facilities or offices in Touqiao Town and provide a variety
of product offerings to more than 100 countries and areas, including United States, Europe, Africa, India, Brazil, etc. Lots of medical
device professionals, including R&D and technology professionals and thousands of independent sales agents are also based in Touqiao
Town, providing sufficient labor to local medical device companies.
Due to our unique geographical advantage,
we are capable of providing one-stop service to our customers. By placing one single order with us, our customers will receive all products
on their order even if there are more than 100 to 1000 different kinds of products on such order and some products are not in our product
portfolio. Upon receipt of such orders, we are able to quickly fulfill the orders by including our own brand products in addition to
qualified products sourced from other manufacturers in Touqiao Town.
Our one-stop service not only bring benefits
and conveniences to our customers, but also reduces our procurement costs, such as transportation fee and travel fee, and mitigates the
impact of raw material price fluctuations in the market, thus increasing our profit margins.
Our Customers
We have three types of customers, i) direct
end user customers, which includes hospitals, pharmacies, and medical institutions, ii) domestic distributor customers, which distribute
our products to end-user customers in China, and iii) export distributor customers, which distribute our products to end users customers
in North America, Asia, South America, Africa and Oceania. As of December 31, 2022, the company had a total of 3,853 customers, of which
525 are direct end user customers, 3,004 are domestic distributors customers, and 324 are export distributors customers. Our direct end
user customers, as well as substantially all of our domestic distributor customers and our export distributor customers, are based in
China.
We do not have long-term written sales agreements
with our customers. Each customer sale is typically governed by a brief purchase-order based sales agreement. The key terms of the sales
agreements (including those agreements with our top customers) include:
| |
● |
The product’s
name, type, quantity and price. |
| |
● |
Quality standard - medical
device qualifications, including business license, medical device production and operation licenses, medical device registration
certificates, inspection report, etc. Lack of one of the qualifications will result in termination of the agreements. |
| |
● |
Delivery method and
payment terms. Payments are typically due within 90 days after delivery. |
| |
● |
Breach of contract terms,
including refund and return of products. Purchasers are entitled to refunds and may return the product if the wrong product is delivered
or the product does not meet agreed quality standards. |
| |
● |
Shipping costs are typically
borne by the seller. |
| |
● |
Dispute solutions, including
bringing a lawsuit at the local People’s court if negotiations are unsuccessful. |
For the fiscal years ended December 31, 2022,
2021, and 2020, the revenues generated from our direct end user customers and domestic distributor customers amounted to $102,845,751,
$89,240,394, and $72,941,380, respectively, constituting approximately 99.52%, 85.78%, and 81.90%, respectively, of our total revenues,
with export distributor customers accounting for $500,590, $14,797,316, and $16,119,630, respectively, constituting approximately 0.48%,
14.22%, and 18.10%, respectively, of our total revenues.
As we provide our products to export distributor
customers based upon their regional coverage, we do not have country-specific information on end-users overseas. Substantially all end
users who acquire our products through domestic distributors are based in China. End users who acquire our products through licensed
export distributors have two types – foreign distributors from other countries and end users from other countries. For the fiscal
year ended December 31, 2022, our total sales to the top two customers accounted for 27.63% and 10.97% of our revenues, respectively.
The top two customers for the fiscal year ended December 31, 2022 were Customer A (a domestic distributor customer) and Customer C (a
domestic distributor customer). Our top two customers for the years ended December 31, 2021 and 2020 were the same for both years. They
were Customer A (a domestic distributor customer) and Customer B (an export distributor customer). For the year ended December 31, 2021,
our total sales to the top two customers accounted for 21.91% and 11.26% of our revenues, respectively. For the year ended December 31,
2020, our total sales to the top two customers accounted for 17.68% and 12.71% of our revenues, respectively.
Our Suppliers
We source our suppliers through multiple channels:
(i) through referrals from local medical device industry associations, (ii) through industry exhibitions/expos, (iii) through our distributors,
and (iv) through open bids.
Our suppliers are divided into two categories:
(1) those providing raw materials for the manufacturing of our products, and (2) those providing products for our resales.
Our raw and auxiliary materials include rubber,
chemical polyethylene (PE), polyethylene, polypropylene, nylon and non-woven fabrics, all of which are purchased from certified and qualified
suppliers in China. Our raw materials supply has been very stable for many years and are easily sourced due to our unique geographical
location.
We distribute products sourced from certain
suppliers when it comes to our one-stop service. We from time to time receive orders from our customers with a variety of products not
in our product portfolio. Through our suppliers, we are capable of accommodating our customers’ need and providing one-stop service
to our customers.
We do not have long-term written purchase
agreements with our suppliers. We do not consider any of our suppliers to be material to our business. As of the date of this report,
we have a total number of 71 suppliers. We can utilize any supplier we determine at our sole discretion. Although we can utilize any
supplier we determine, we believe that we established healthy and stable relationships with our significant suppliers through years of
cooperation. These significant suppliers, in the aggregate, accounted for over 25% of our raw material purchases for the year ended December
31, 2022, 2021, and 2020. For the year ended December 31, 2022, our three significant suppliers are Yangzhou Tianyi Medical Instrument
Co., Ltd., Yangzhou Xiaguang Medical Instrument Co., Ltd. and Yangzhou Jiangzhou Medical Instrument Co., Ltd. representing 11.93%, 7.58%
and 7.01% of our total purchase respectively. For the year ended December 31, 2021, our three significant suppliers are Yangzhou Tianyi
Medical Instrument Co., Ltd., Jiangsu Changfeng Medical Industry Co., Ltd. and Yangzhou Jiangzhou Medical Instrument Co., Ltd. representing
9.42%, 8.88% and 8.33% of our total purchase respectively. For the year ended December 31, 2020, our three significant suppliers are
Yangzhou Xiaguang Medical Instrument Co., Ltd., Yangzhou Jiangzhou Medical Instrument Co., Ltd. Ltd. and Yangzhou Tianyi Medical Instrument
Co., Ltd. representing 9.25%, 8.13% and 8.09% of our total purchase respectively. There are no minimum purchase requirements with any
of our suppliers, including these significant ones. Each supplier order is typically governed by a brief purchase-order based purchase
agreement. The key terms of the supplier purchase agreements (including those agreements with our significant suppliers) include:
| |
● |
The product’s
name, type, quantity and price. |
| |
● |
The supply cooperation
relationship of the parties. Some suppliers supply finished products for re-sale and others supply raw materials for manufacturing. |
| |
● |
Quality terms which
are typically expressed with reference to national or industry standards. |
| |
● |
Delivery method and
payment terms; typically, payment is due 90 days after delivery. Shipping costs are the responsibility of the supplier |
| |
● |
Breach of contract terms,
including refund and return of products, compensatory damages. If the supplier cannot deliver the product within the time agreed,
or if the products do not meet the stated quality standard, the supplier must compensate us for losses caused, including treble damages
if the products are defective or counterfeit. In the event we cannot pay timely, liquidated damages are due to the supplier. |
| |
● |
For some significant
supplier agreements, the breaching party is contractually required to pay 10% of the contract amount as liquidated damages if they
unilaterally terminate the agreement. If the supplier fails to deliver the products within the time agreed, the supplier is contractually
required to pay 5% of the contract amount on a daily basis for each and every date they delay delivery. |
Marketing and Sales
We market and sell our products through multiple
channels: (1) through direct sales force, including our own employees and independent sales agents, and (ii) through distribution network,
including our domestic and exporting distributors.
Direct Sales Force
Our Sales Team
As of the date of this report, we have a direct
sales team consisting of 81 employees. Our sales team provides us with direct access to our customers and is capable of addressing our
customers’ needs in a fast and efficient way. They also coordinate with our distributors and independent sales agents in marketing
and sales of our products.
The compensation package for our sales team
includes fixed base salaries and commissions of 0.5%-1.0% based on the revenues or collection they achieve. We provide our sales team
with regular training and internally developed systems to assist them in quickly becoming proficient and productive sales personnel.
Independent Sales Agents
As of the date of this report, we have a large
number of independent sales agents for the marketing and sales of our products in the Chinese market, covering all regions of the PRC.
Our independent sale agents market and sell our products in the regions where they are located.
We have no written sale agent agreements with
our independent sale agents and we are connected with them via oral agreements. Upon successful sales of our products to customers secured
by them, they settle their commission with our customers. We do not provide any commission or make any payments to them.
Our direct sales force has secured a total
of 525 domestic customers, including hospitals and medical institutions. We will continue to work with existing, and identify and secure
new, independent sales agents, to expand our customer base and enhance our brand recognition across China.
Distribution Network
As of the date of this report, we have 3,004
domestic distributors and 324 exporting distributors. Distributors usually purchase products from us at a lower price and then resell
our products to end customers both domestically and internationally at a comparatively higher price and earn the price difference.
Our domestic distributors cover 32 provincial-level
administrative regions of PRC for the resales of our products in the Chinese market. They market and distribute our products in the regions
where they locate and have secured approximately 5,884 domestic customers for us, including hospitals and medical institutions.
Our exporting distributors are limited to
our overseas sales. Each of our exporting distributor usually sells medical devices to at least three overseas customers. We therefore
estimate conservatively that the total number of established direct and indirect customer relationships established overseas through
our exporting distributors in Europe, North America, South America, Asia, Africa, and Oceania to be about 915.
Distributors must have related qualifications
in order to distribute our products. Upon our verification and approval by inspecting their qualification materials, such as business
license, disposable medical device operation license, and medical device exporting license, etc., and verifying their sales channels,
distribution capacity and business reputation, distributors are authorized to distribute our products to their domestic and overseas
customers.
We do not have long-term written agreements
with our distributors. Each distributor order is typically governed by a brief purchase-order based sales agreement. The key terms of
the distributor purchase agreements include:
| |
● |
The product’s
name, type, quantity and price. |
| |
● |
Quality standard - medical
device qualifications, including business license, medical device production and operation licenses, medical device registration
certificates, inspection report, etc. Lack of one of the qualifications will result in termination of the agreements. |
| |
● |
Delivery method and
payment terms; payment is typically due within 90 days after delivery Shipping costs re typically borne by us. |
| |
● |
Breach of contract terms,
including refund and return of products. Distributors are entitled to refunds and may return the product if the wrong product is
delivered or the product does not meet agreed quality standards. |
| |
● |
Dispute solutions, including
bringing a lawsuit at the local People’s court. |
Our distributors have secured a total of approximately
6,799 customers for us both domestically and internationally. We will continue to work with existing distributors, and identify and secure
new distributors, to expand our customer base and enhance our brand recognition both in PRC and abroad.
Our Research and Development (“R&D”)
We invest in R&D efforts that advance
our technology with the goal to expand new products and improve upon our existing product offerings. Our R&D expenses totaled approximately
$3.0 million, $2.7 million, and $2.5 million, for the years ended December 31, 2022, 2021, and 2020, respectively. R&D expenses mainly
consist of applicable personnel, design, sample testing and materials expenses. As of the date of this report, we had a total of 30 employees
in the R&D department. In the future, we expect R&D expenses to increase in absolute dollars as we continue to develop new products,
enhance existing products and technologies and perform activities related to obtaining additional regulatory approval.
We adhere to a market-oriented R&D approach
and actively cooperate with universities, hospitals, medical institutions, distributors and independent sales agents in sorting out our
R&D orientation based on the real market demand. Our market-oriented R&D approach includes the following:
| |
● |
Hospitals to Factories.
There is usually a technology department or scientific research department in every hospital in China. Hospitals conduct R&D
on innovative products due to their needs, but they normally have no manufacturing capabilities and qualifications. As a disposable
medical device manufacturer, to serve our customers better, we from time to time communicate with hospital personnel and keep ourselves
informed of their latest demands, including but not limited to acquiring the patents on their IP list required for the manufacturing
of certain products, and research, develop and manufacture such products that tailored to their needs. |
| |
● |
Universities to Factories.
A lot of universities and medical colleges have research centers, where they develop and patent certain R&D results. We from
time to time communicate with their research personnel and keep ourselves informed of their latest R&D and patents, and if needed
by our customers, purchase patents from them and research, develop and manufacture products with such patents. |
| |
● |
Customers’
Feedbacks from Distributors and Independent Sales Agents. Distributors and independent sales agents from time to time receive
feedbacks and proposals from end use customers and then pass on to us. Upon our internal evaluations on those feedbacks and proposals,
we may either research, develop and improve our products accordingly, or entrust university or college research centers for R&D.
Once we receive their R&D results, we may improve our products in accordance with their R&D results, including acquiring
patents from those centers for manufacturing of our products. |
By continuously upgrading and improving products
and technologies that tailed to our customers’ requirements, we have further strengthened our customer’s loyalty.
As of the date of this report, we have 27
registered patents. Faced with the ever-changing market demands, we continue to abandon and phase out unsuitable patents and technologies,
and simultaneously invest in acquiring new patents and technologies that tailored to our customer’s fast changing requirements.
We believe our ability to rapidly develop
innovative products is attributable to the dynamic product innovation process that we have implemented, the versatility and leveragability
of our core technology and the management philosophy behind that process. We have recruited and retained professionals with significant
experience in the development and improvement of medical devices. We have a pipeline of products in various stages of development that
are expected to provide additional commercial opportunities. Our research and development efforts are based at our operating subsidiaries
in PRC.
Industry and
Competition
The medical device industry is intensely competitive,
subject to rapid change and significantly affected by new product introductions and other market activities of industry participants.
We compete or plan to compete with manufacturers of disposable medical devices. Some of these competitors are large, well-capitalized
companies with significantly greater market share and resources than we have. As a consequence, they are able to spend more on product
development, marketing, sales and other product initiatives than we can. We also compete with smaller medical device companies that have
single products or a limited range of products. Some of our competitors have:
| |
● |
significantly greater
name recognition; |
| |
● |
broader or deeper relations
with healthcare professionals, customers and third-party payers; |
| |
● |
more established distribution
networks; |
| |
● |
additional lines of
products and the ability to offer rebates or bundle products to offer greater discounts or other incentives to gain a competitive
advantage; |
| |
● |
greater experience in
conducting research and development, manufacturing, clinical trials, marketing and obtaining regulatory clearance or approval for
products; and |
| |
● |
greater financial and
human resources for product development, sales and marketing and patent prosecution. |
The Company believes that it competes favorably
with respect to these factors, although there can be no assurance that the Company will be able to continue to compete successfully in
the future.
We believe the following companies may be
our competitors:
| |
● |
Shandong Weigao Group
Medical Polymer Co., Limited, is principally engaged in the research and development, production and sale of single-use medical devices.
The Group has a wide range of products, which includes: i) consumables (infusion sets, syringes, medical needles, blood bags, pre-filled
syringes, blood sampling products, and other consumables); ii) orthopedic materials and iii) blood purification consumables and equipment.
Currently, its sales are mainly conducted in the PRC market, but it is actively exploring opportunities in international markets,
with products having been exported to 30 countries and regions, including the United States, Germany, Romania, Australia and the
United Kingdom. (Source: http://en.weigaogroup.com/gfccontentEn/Enterpr.aspx) |
| |
● |
Jiangxi Hongda Medical
Equipment Group Ltd. specializes in manufacturing sterile medical devices for single use. Their products cover nine categories, such
as infusion sets, blood transfusion equipment, injection equipment, puncture sets, examination and assistant supplies, anesthesia
appliances, catheters, medical equipment, cardiovascular intervention, blood purification products, etc. It is a major supplier of
sterile medical equipment for a lot of countries, such as the United States, Europe, Africa, the Middle East and the Southeast Asia.
It is also one of biggest manufacturers to produce and process medical disposables, with nearly a quarter of the total market share
in China alone. (Source: http://en.jxhd.cn/comcontent_detail.html) |
| |
● |
Henan Tuoren Medical
Device Co., Ltd., founded in 2004, is a healthcare solutions provider, focusing on medical consumables and extending to electronic
medical devices, surgery devices and biomedical materials. It is dedicated to designing, developing and distributing medical devices
to its customers, covering 220 kinds of products with over 1880 specifications mainly in the field of anesthesia, pain management,
nursing, diagnostics, surgery, hemodialysis and intervention. Its products have been sold to more than 70 countries and regions around
the world, with international subsidiaries established in the United States and India. (Source: http://www.tuoren.com/en/index.php?s=/about/history.html) |
| |
● |
Allmed Medical Products
Co., Ltd., founded in 1992, is the largest OEM (original equipment manufacturer) manufacturer and exporter of wound care products
in China, providing a worldwide range of traditional wound care products, including gauze swabs, non-woven swabs, lap sponges, fluffy
bandages, abdominal pads, non-stick pads, adhesive bandages, elastic bandages, medical kits and disposable drapes, etc. Compared
with our products, their products are very limited. (Source: http://www.allmed-china.com/index.php?m=content&c=index&a=lists&catid=11) |
| |
● |
Jiangxi Sanxin Medtec
Co., Ltd., founded in 1997, is a listed company in China, focusing on researching and developing, manufacturing and marketing of
medical devices. It is the first listed company in the field of syringe and infusion. Their main products are “catheter
tubing series,” “blood purification series,” “syringe series” and “infusion & transfusion
series,” totally four series products with over 30 types of more than 1000 specifications. Its products are sold both domestically
and internationally, covering more than 30 provinces in China and more than 60 countries and regions nationwide. (Source: http://www.sanxin-med.com/category/Category/index/cid/295) |
| |
● |
Jiangxi 3L Products
Group Co., Ltd., founded in 1990, combines the scientific research, production and marketing of single use medicinal macromolecular
products and equipment, medical purification equipment sales, and maintenance and installation work into one. It established trade
abroad with more than 20 countries and regions. It also has branch offices in Hong Kong, South Africa, Russia, etc. In the past few
years, their surgical towels comprised of more than 90% of the domestic market share in China, and their combined sales have taken
over half of the nationwide total product needs in the domestic market in China. (Source: http://www2.3l.com.cn/web/2.htm) |
Our main competitors from other provinces
and cities are Shandong Weigao Group, Jiangxi Hongda Group, Henan Tuoren Group, and Hubei Allmed Co., Ltd. These companies are our competitors
as well as our partners. For example, Weigao Group in Shandong Province distributes disposable infusion pumps, medical kits, and other
products produced by our Huodong subsidiary, while our Huadong subsidiary distributes the detained needle products of Weigao Group in
our Yangzhou area; Tuoren Group distributes nitrile glove products of our Huadong subsidiary in Henan Province, while our Huadong subsidiary
distributes Tuoren’s medical guidewires and catheters in our Jiangsu Province. Another example is Hubei Allmed Co., Ltd., 80% of
whose products are exported with 20% are domestically distributed (in comparison to our 80% domestic sales and 20% indirect exporting
sales). The disposable stainless steel medical brushes it exports are exclusively supplied by our Yada subsidiary, so we regard company
not only our domestic client, but also our competitor in the international market. Because of the huge market in China and in the world,
there are tens of thousands of varieties of medical consumables. Not a single company in the world can dominate the entire market. While
competing in the market, we more often cooperate with each other. Under the guidance of industry associations and local governments,
we have formed an industry alliance to continuously exchange ideas with each other, discuss market development needs, and to build a
common development platform. It should be noted that in the face of the sudden outbreak of the epidemic last year and this year, we shared
information, supported each other with epidemic prevention materials, raw materials, and auxiliary materials, as well as production equipment,
thus achieving win-win cooperation with fruitful outcomes.
A demand for one-stop service for medical
consumables is an inevitable development in the medical consumables industry. There are several reasons why our company has achieved
steady development over its more than 30 years history. One of the most important reasons is our one-stop service system, through which
we can supply all disposable medical devices required by the client, combining products manufactured by us with products outsourced from
others but passed through our quality control scrutiny. Through market research, we have learned that our competitors are also developing
a one-stop service system and are in one of the following levels of progress: first, some companies have established a one-stop service
system; second, some companies have established a one-stop service system, but their system is underperforming and needs improvement;
and third, some companies are still undergoing development of a one-stop service system.
There are currently 385 manufacturers and
distributors of medical consumables where the company is located in Touqiao Town, which is known as the “Hometown of Medical Consumables
in China.” As a top player in the region, the Company’s revenues accounted for approximately 10% of the total revenues generated
from all medical device manufactures in Touqiao Town. Our total sales in 2020 were more than RMB600 million (approximately ($89 million),
with more than 10,000 product types. By comparison, our research indicates that the annual sales of the second- and third-ranking companies
in Touqiao Town are just over RMB100 million, while the annual sales of the fourth- and fifth-ranking companies are in the range of RMB80
million to RMB90 million, with a lesser variety of product types. Since our production scale and number of product types far surpass
those of other companies in Touqiao Town, we consider other local companies to be our partners more than our competitors. We supply each
other’s needs in terms of production, procurement, and distribution. At the same time, these local enterprises in Touqiao Town
are not able to provide customers with the same one-stop service that we provide due to their limited scale and product types. The total
output value of the top 20 companies in Touqiao Town, including our company, was about RMB2.3 billion in 2020. Our company’s output
value in 2020 was RMB610 million, accounting for about 26.5% of the total output value, while the other four next-largest companies in
Touqiao Town had a total output value of about RMB400 million in 2020, accounting for 17.4% of the total output value.
Competitive Strengths
We are dedicated to serving our customers.
We believe that the following strengths contribute to our success and are the differentiating factors that set us apart from our peers:
| |
● |
Cost-effective
methods to address customers’ significant needs with a variety of products. As of the date of this report, we have
a total of 1063 products in our product portfolio covering all Class I, II, and III disposable medical devices, including 937 products
for domestic sales and 126 products for overseas sales. Through sales of different products to our customers via our one-stop service,
we are able to cost-effectively address our client’s needs. |
| |
● |
Massive distribution
network of clients, distributors, and suppliers. Through both direct sales and our massive distribution network, our
products are sold to hospitals, pharmacies and medical institutions both domestically and internationally. As of the date of this
report, we have 81 employees in our sales department and 5,000 independent sales agents, 3,004 distributors for domestic sales and
324 exporting distributors for overseas sales. We not only have accumulated a substantial domestic customer base and forged strong
relationships with these customers, but also established good long-term cooperative relationships with well-known foreign medical
equipment brand companies, which extends our reach world-wide. We believe that these customers will continue to be a source of business
as well as a good referral source to new customers. |
| |
● |
Geographical
advantages allow us to provide one-stop service to our customers at reduced cost. Hospitals and other medical institutions
normally have lists of over a hundred or even a thousand of different kinds of disposable medical devices which they must procure
on a regular basis. Our PRC operating subsidiaries and primary operations are located in Touqiao Town, Yangzhou City, Jiangsu Province,
one of the four medical device centers in PRC. Dubbed the “Hometown of Medical Devices & Consumables in China,” Touqiao
Town hosts hundreds of disposable medical device manufacturers manufacturing all different kinds of products. In addition to our
own products, we are qualified to distribute products sourced from other manufacturers. As a result, our clients are able to receive
all required products by placing just a single order with us. When we receive an order from our hospital clients or distributors,
we are able to quickly fulfill the order by including our own branded products and qualified products sourced from other manufacturers
in Touqiao Town. There are also large numbers of medical device professionals, including research and development, or “R&D,”
and technology professionals, and thousands of independent sales agents based near our primary operations. We are therefore able
to procure high quality raw materials and products of other brands at a comparatively low price within a short period of time and
to obtain sufficient labor and support to our one-stop service and manufacturing. |
| |
● |
Economies of
scale and automation provide significant cost advantages. The scale of our production is regional-leading within
Yangtze River Delta region of China. The disposable medical devices we manufacture and sell are mainly low value-added products,
which, however, are largely consumed and in huge demand every day in hospitals, medical institutions, and other health related industry
entities. Through scaled production, we are able to increase our profits margin. In the procurement process, the production scale
reduces our procurement costs and mitigate the impact of raw material price fluctuations. In the manufacturing process, we retrofitted
equipment and introduced automation to improve production efficiency. At present, we have 12 purification plants covering a total
area of approximately 110,352 square feet (10,252 square meters). |
| |
● |
Leading competitive
position maintained by high quality standard systems. Quality and safety are always our core value. Applying information
acquired during our long-term business transactions with major medical institutions across China, we have developed a sophisticated
quality management system, as well as a strict and effective internal control standard system. All of our products, either self-manufactured
or sourced, fall within our quality control system subject to our quality inspection before delivery (See the section entitled “Quality
Control” in Business). |
| |
●
|
Market-driven
research and development allow for continual improvement and long-term client loyalty. As of the date of this
report, we have an R&D team of 78 people, accounting for 12.46% of our total employees. For the fiscal year ended December 31,
2022, we have invested a total of $2,962,904 in the products and technologies R&D. For the fiscal years ended December 31, 2021
and 2020, we have invested a total of $2,725,014 and $2,492,059 in the products and technologies R&D. We adhere to a market-oriented
R&D approach and actively cooperate with universities, hospitals, medical institutions, distributors and independent sales agents
in sorting out our R&D orientation based on the real market demand. We continuously upgrade and improve our products and technologies
to better suit our customers. |
| |
● |
Visionary and experienced management
team
Building a trusted brand and always doing
the right thing for people has been at the heart of our founding management team since day one. Our company culture, strategic vision
and operational execution are driven by our visionary founder, Yongjun Liu. Mr. Liu is a successful entrepreneur who has been engaged
in the medical device industry for more than 40 years and has accumulated extensive experiences and led his businesses to make remarkable
achievements. He has been awarded as Excellent Entrepreneur, Honest Entrepreneur Representative and Medical Device Industry Representative
on multiple occasions. At the same time, he is keen on public welfare undertakings. He has also sponsored a lot of splendid undertakings
such as road reconstruction in towns and villages, donations to the Red Cross Society, reconstruction of nursing homes, poverty alleviation
and aid for students. Our company culture mirrors our founder’s mission to empower and serve those who serve others. |
Quality Control
All of our products, either self-manufactured
or sourced, fall within our quality control system subject to our quality inspection before delivery. For sourced products, they must
first be shipped to us for quality inspection, upon passing inspection, be packaged, labeled and shipped to our customers.
Medical device and equipment are medical products
directly applied to the human body, which is closely related to the life and health of users. Quality and safety are always our core
value. Reliable, safe and stable product quality is an important driving factor for maintaining market competitiveness. Through long-term
business dealings with major hospitals and medical institutions across China, we believe that we have developed a sophisticated quality
control management system as well as a strict and effective internal control system in accordance with the requirements of Chinese laws
and regulations.
We prioritize product quality management and
are committed to strengthening the professional ethics and cultivating quality consciousness of our employees, forming a strict quality
management system, which we believe is in line with international standards.
Our rigorous quality control management programs
have earned us a number of quality-related manufacturing designations. Our manufacturing facilities are ISO 13485 compliant with ISO
13485:2016 edition certification achieved in 2020. In 2018, we achieved compliance with European Union’s CE certification, allowing
certain of our products (such as Disposable Amniotic Membrane Perforators, Disposable Medical Suction Connecting Tubes and Disposable
Gynecologic Samplers) to be CE marked. In April 2020, we renewed registration of certain products with the FDA, including ID bracelet,
surgical tapes, elastic, adhesive bandages, etc., allowing our products to enter U.S. market. We have more than 60 categories of products
passed the quality system inspections administered by the China Food and Drug Administration and local authorities in Yangzhou City.
We have annual quality targets, which are
distributed to our employees and all departments annually. We conduct monthly follow-ups and quarterly evaluations on execution of the
plans held to ensure that the annual quality targets are met.
However, despite our quality control management
system, we cannot eliminate all risks of errors, defects or failures. We may fail to detect or cure defects as a result of a number of
factors, many of which are outside our control, including:
| |
● |
technical or mechanical
malfunctions in the production process; |
| |
● |
human error or malfeasance
by our quality control personnel; |
| |
● |
tampering by third parties;
and |
| |
● |
defective raw materials
or equipment. |
Failure to detect quality defects in our products
could result in patient injury, customer dissatisfaction, or other problems that could seriously harm our reputation and business, expose
us to liability, and adversely affect our revenue and profitability.
In 2018, our PRC subsidiaries Jiangsu Yada
and Jiangsu Huadong got fined of immaterial amount for noncompliance with the local laws and regulations. Upon receipt of the fine notices,
we promptly reacted to the comments from the related local government, rectified the noncompliance situation, recalled all noncompliance
products and paid the fines in full. As a result, we took some measures to avoid future noncompliance.
Specific rectification measures and the impacts
on our products and business are as follows:
| No. |
|
Penalty
decision |
|
Misconduct
for penalized |
|
Regulatory
measures after
rectification |
|
Effect
on
products/business |
| 1 |
|
(Yangzhou)
Shi Yao Jian Xie Fa [2017] No. 48 / (Yangzhou) Shi Yao Jian Xie Fa [2018] No. 23 |
|
Medical
surgical film does not label the texture of the transparent plastic film/ providing fake registration product criterion to the Food
and Drug regulatory authority that supervises and inspects products |
|
Canceling
registration certificates and stopping producing such related products |
|
The
Company has canceled the relevant registration certificates and no longer produced such products. Because the output of these products
is minor, which occupies an extremely low proportion of the Company’s total products, terminating the production of this product
will not exert any adverse effect on the Company’s business. |
| |
|
|
|
|
|
|
|
|
| 2 |
|
(Yangzhou)
Shi Yao Jian Xie Fa [2017] No. 46 |
|
Production
of inspection gloves that fail to meet the compulsory standards [model specification: 7.5] (Production date of glossy powder: 20170108)
(The reason for the insufficient tension is that the rubber supplier has not followed the specifications standards during production) |
|
Enhancing
the random inspection of the supplier’s production specification standards when accepting outsourced materials, as well as
promoting the proportion of random inspections |
|
Such
administrative penalty has not adversely affected products and the Company’s business. |
| |
|
|
|
|
|
|
|
|
| 3 |
|
(Yangzhou)
Shi Yao Jian Xie Fa [2018] No. 16 |
|
“ultraviolet
absorbance” test of disposable infusion pumps has failed (The main reason that caused the product’s UV absorbance to
exceed the standard is the secondary vulcanization time and vulcanization temperature of the liquid storage bag has not met the specified
requirements. The liquid storage bag is an outsourcing material, and the original supplier does not strictly follow its production
process specifications during production, which shortens the secondary vulcanization time and vulcanization temperature, causing
UV absorbance to exceed the standard.) |
|
1.
Switch to another supplier of the liquid storage bag;
2. Improve the internal control standard requirements for the incoming inspection of liquid storage bags (the ultraviolet absorbance
index of the incoming inspection liquid storage bag is ≤ 0.2) |
|
Such
administrative penalty has not adversely affected products and the Company’s business. |
For the fiscal year of 2022 and as of the
date of this report, we are not aware of any investigations, prosecutions, disputes, claims or other proceedings in respect of quality
issues, nor has the Company been punished or can foresee any punishment to be made by any related government authorities of the PRC.
Class I, II, and III Medical Device Approval
Process in China
“Class I” medical devices in China
refers to sanitary and civilian products with extremely low risk, which do not need to be disinfected. To record this type of product
in the official catalogue, a recordation application must be submitted on the website of the National Medical Products Administration.
The application must clarify relevant information about product standard, the scope of use, production technologies, and instructions
for the use of material. The device may then be produced and distributed after obtaining the online approval of the National Medical
Products Administration. The application and approval process for Class I devices is as follows:
Schedule 1 Flow Chart of the Application
Procedures for Recordation of the Medical Devices of Class I
(statutory time limit: on-the-spot conclusion)
[Chart derived from regulations on the supervision
and administration of medical devices (Order No. 650 of the State Council of the PRC) and the Measures for the supervision and administration
of the operation of medical devices (Order No. 8 of the State Food and Drug Administration)].
“Class II” and “Class III”
medical devices in China refer to medical-grade products with higher risk. The application procedures for recordation of these two types
of products in China are as follows: First, the products are submitted for inspection with various materials. After the submitted materials
are reviewed by the experts to their satisfaction, the National Medical Products Administration will organize a team of experts to visit
the factory for on-site inspection and acceptance. After passing the on-site acceptance, the products with higher risk are required to
be clinically tested. Only after the clinical report and other application documents are submitted to the National Medical Products Administration,
which approves these materials and issues the product registration certificate, shall the enterprise be allowed to produce and distribute
the product. The application and approval process for Class II and III devices is as follows:

[Chart derived from regulations on the supervision
and administration of medical devices (Order No. 650 of the State Council of the PRC) and the Measures for the supervision and administration
of the operation of medical devices (Order No. 8 of the State Food and Drug Administration)].
The chart below summarizes the classification,
approval dates, and current approval terms of our top 20 products:
| No. |
|
Product
Name |
|
Remarks |
|
Date
of
approval |
|
Term
of validity |
|
Class |
| 1 |
|
Disposable ID bracelet |
|
By Yada, CE certified |
|
2020-12-17 |
|
2023-12-16 |
|
Class II |
| 2 |
|
Disposable medical brush |
|
|
|
|
|
|
|
Class II |
| 3 |
|
Masks |
|
By Huadong |
|
2021-2-26 |
|
2026-2-25 |
|
Class II |
| 4 |
|
Disposable woman’s examination kits |
|
By Huadong |
|
2021-2-25 |
|
2026-2-24 |
|
Class II |
| 5 |
|
Medical Brush |
|
Source from other (No registration certificate is
required) |
|
Class II |
|
|
|
|
| 6 |
|
Medical kit |
|
Source from other (No registration certificate is
required) |
|
Class II |
|
|
|
|
| 7 |
|
Medical sterile dressing surgical kits |
|
By Yada, CE certified |
|
2020-12-17 |
|
2023-12-16 |
|
Class II |
| 8 |
|
Medical catheter |
|
Source from other (No registration certificate is
required) |
|
Class II |
|
|
|
|
| 9 |
|
Uterine tissue suction tube |
|
By Yada, CE certified |
|
2020-12-17 |
|
2023-12-16 |
|
Class II |
| 10 |
|
Low-density polyethylene (LDPE) bottles for eye
drops |
|
By Huada, Long term |
|
Class I |
|
|
|
|
| 11 |
|
High-density polyethylene (HDPE) bottles for tablets |
|
By Huada, Long term |
|
Class I |
|
|
|
|
| 12 |
|
Disposable women’s examination kits |
|
Source from other (No registration certificate is
required) |
|
Class II |
|
|
|
|
| 13 |
|
Disposable Gynecological sampler |
|
By Yada, CE certified |
|
2020-12-17 |
|
2023-12-16 |
|
Class II |
| 14 |
|
Disposable medical brush (type B1) |
|
By Yada, CE certified |
|
2020-12-17 |
|
2023-12-16 |
|
Class II |
| 15 |
|
Disposable anal bag |
|
By Yada, CE certified |
|
2020-12-17 |
|
2023-12-16 |
|
Class II |
| 16 |
|
Inspection gloves |
|
By Yada |
|
2016-2-29 |
|
Long term |
|
Class I |
| 17 |
|
Disposable humidified nasal oxygen tube |
|
By Huadong |
|
2021-1-27 |
|
2026-1-26 |
|
Class II |
| 18 |
|
Disposable suction connecting tube |
|
By Huadong |
|
2021-3-9 |
|
2026-3-8 |
|
Class II |
| 19 |
|
Medical dressing |
|
By Huadong |
|
2018-4-18 |
|
2023-4-17 |
|
Class II |
| 20 |
|
Electronic pump |
|
By Huadong |
|
2021-3-9 |
|
2026-3-8 |
|
Class III |
Competitive Challenges
| |
● |
Our production
capacity for certain pandemic prevention products is limited. Our existing production capacity for certain products, consisting
primarily of some pandemic prevention-related products, is unable to meet the current market demand due to limitations on our funding,
production equipment, and facilities. As a result, our ability to expand our market share in this area is limited. |
| |
● |
To date, limited
access to capital has slowed our ability to gain additional market share. Our various product lines have developed rapidly
and are competitive in the market. However, expansion of our production capacity, deepening of our marketing network, and improvement
of our research and development efforts require sufficient capital investment. To date, lack of access to sufficient capital has
limited some projects with development potential and has tempered our further expansion of market share. The Company needs more capital
to expand its operations. |
| |
● |
We sell primarily
low value-added products, which limits our sales margins. The Company currently primarily manufactures and sells Class I
and Class II medical products, with a lesser proportion of Class III products. The medical device industry is highly competitive,
especially in respect of low value-added medical devices, which has low entry requirements subject to rapid change and significantly
affected by new product introductions and other activities of industry participants. We face potential competition from major medical
device companies worldwide, many of which have longer, more established operating histories, and significantly greater financial,
technical, marketing, sales, distribution, and other resources. As a result, we may find it difficult to compete with companies commercializing
high-end products and enjoying a higher profit margins per-product. |
Manufacturing
Our production is comprised of both in-house
manufacturing and outsourcing to third parties. Except for purchases according to the requirement of bid clients, all third party manufactures
shall ship products to us for our inspection first, and upon passing our inspection, we will label and assemble, and then ship them to
clients per orders.
In-house Manufacturing
All of our in-house production is located
at our facilities in Yangzhou, Jiangsu Province, PRC. At those facilities, we produce products and stock inventory of raw materials,
components and finished goods at our facilities pursuant to the market demand, orders we receive or plan to receive, our production plan
and capacity, procurement information from our direct sales force and our distributors. Our in-house per-order production model is as
follows:

Due to the nature of the products, all products
must be produced in the dust-free purification workshops and must be sterilized. This production process is subject to continuous review
and monitoring by the quality control team to ensure that finished products are of the highest quality and meet customer requirements
and ISO 13485 medical device quality management systems standard.
In order to the maintain product safety and
a high standard of product quality, we implement a strict set of quality control policies and inspection protocols. These policies and
protocols are enforced by our quality control team, senior management and officers along every step of the production to post-production
process.
Outsourcing
Our outsourcing of products, which to some
extent expands our production capacity, are produced by third party manufacturers by either 1) using qualified raw material suppliers
designated by us and completing the production in accordance with our standards, or 2) using their own selection of raw materials and
production standards in line with our quality control requirements.
Given our unique geographical location, we
are able to procure qualified raw materials, and locate qualified third party manufacturers and suppliers locally at a cost-effective
way such as lower price and save of transportation costs in a shorten period, thus realizing scale production, reducing our production
costs and increasing profit margin. By outsourcing some semi-finished product processing to the local consigned manufacturers in Touqiao
Town, we not only expand our production capacity and improve our production efficiency, but also reduce production costs while meeting
clients’ demands for products of various specifications.
Environmental
Due to the nature of the Company’s products,
the Company’s PRC subsidiaries do not generate industrial wastewater and wastes, the wastewater they generate is sanitary wastewater
which can be disposed directly into municipal pipelines. The generated corner wastes shall be cleaned and collected by the cleaning personnel
on time, and then transported to the municipal garbage disposal site for treatment by the local sanitation department. Solid wastes generated
during operation shall be collected and sent to relevant manufacturers for recycling. If new products are developed in the future and
environmental measures are needed according to law, the Company will take corresponding environmental protection measures according to
relevant laws and regulations. The waste discharge fees for fiscal years of 2022, 2021, and 2020 were $4,384, $4,890, and $5,009, respectively,
which has been paid in full.
The Company and its subsidiaries passed the
environmental inspection and evaluation by the Environmental Protection Bureau of Yangzhou Guangling District in 2020, which determined
that no waste, hazardous substances or wastewater were produced during manufacturing.
As of the date of this report, our waste discharge
is in compliance with the local laws and regulations and we are not aware of any warning, investigations, prosecutions, disputes, claims
or other proceedings in respect of environmental protection, nor have we been punished or can foresee any punishment to be made by any
government authorities of the PRC.
Intellectual Property
Our business is dependent on a combination
of trademarks, patents, copy rights, domain names, trade names, trade secrets and other proprietary rights in order to protect our intellectual
property rights. As of the date of this report, we have two (2) registered trademark, twenty-seven (27) registered patents and two (2)
copyrights in the PRC.
Trademarks
Set forth below is a detailed description
of our registered trademarks:
| Country |
|
Trademark |
|
Trademark
Registration No. |
|
Trademark
Name |
|
Trademark
Registration
Date |
|
Trademark
Classes |
|
Trademark
Owner |
|
Trademark
Term |
|
Trademark
Status |
|
| China |
|
 |
|
19576090 |
|
Hu
Jun |
|
08/28/2017 |
|
30 |
|
Jiangsu
Yada |
|
08/28/2017
to 08/27/2027 |
|
Registered |
|
| China |
|
 |
|
1415306 |
|
Yada |
|
06/28/2000 |
|
10 |
|
Jiangsu Yada
|
|
06/28/2020
to 6/27/2030 |
|
Registered |
|
| China |
|
 |
|
1421255 |
|
Yada |
|
07/14/2000 |
|
6 |
|
Jiangsu
Yada |
|
07/14/2020
to 07/13/2030 |
|
Registered |
|
Patents
China’s patent law stipulates that there
are three types of patent protection: invention patent, utility model patent and design patent.
| |
● |
A patent for invention
refers to a new technical solution for a product, a method or an improvement thereof. |
| |
● |
Utility model patent
refers to a new technical solution suitable for practical use, which is proposed for the shape, structure or combination of the product. |
| |
● |
Design patent refers
to the new design of product shape, pattern, color or their combination, which is aesthetic and suitable for industrial application. |
Set forth below is a detailed description
of our registered patents:
| Country |
|
Patent
No. |
|
Patent
Name |
|
Patent
Publication
Date |
|
Patent
Type |
|
Patent
Validity
Period |
|
Patent
Status |
|
| China |
|
ZL201310537304.7 |
|
Wound marginal cell crawling
promoting type temperature control swell-shrink type drainage tube |
|
09/09/2015 |
|
Invention |
|
20 years from the application
date (11/03/2013) |
|
Renewed and effective |
|
| China |
|
ZL201420092814.8 |
|
Nano-crystalline cellulose
dressing for treating extensive burns |
|
2/25/2015 |
|
Utility model |
|
10 years from the application
date (3/3/2014) |
|
Registered and effective |
|
| China |
|
ZL201821229644.8 |
|
Bone mineral density
instrument with good anti-falling performance |
|
7/5/2019 |
|
Utility model |
|
10 years from the application
date (8/1/2018) |
|
Registered and effective |
|
| China |
|
ZL2019 2235101 2.X |
|
Novel device for gynecological
diagnosis and treatment |
|
10/16/2020 |
|
Utility model |
|
10 years from the application
date (12/24/2019) |
|
Registered and effective |
|
| China |
|
ZL202020002206.9 |
|
Joint fixation frame
for orthopedic surgery |
|
10/23/2020 |
|
Utility model |
|
10 years from the application
date (01/01/2020) |
|
Registered and effective |
|
| China |
|
ZL202020017703.6 |
|
Wound debridement device
for emergency care |
|
10/16/2020 |
|
Utility model |
|
10 years from the application
date (01/06/2020) |
|
Registered and effective |
|
| Country |
|
Patent
No. |
|
Patent
Name |
|
Patent
Publication
Date |
|
Patent
Type |
|
Patent
Validity
Period |
|
Patent
Status |
|
| China |
|
ZL2013105373
27.8 |
|
Interrupted
thread clearance hole wall type injection needle |
|
11/4/2015 |
|
Invention |
|
20
years from the application date (11/3/2013) |
|
Registered and effective |
|
| China |
|
ZL201922473496.5 |
|
Sputum
suction tube preventing respiratory mucosa from being damaged |
|
10/20/2020 |
|
Utility
model |
|
10
years from the application date (12/31/2019) |
|
Registered and effective |
|
| China |
|
ZL202020129072.7 |
|
High-flow
oxygen supply mask drainage and medicine delivery mechanism in the department of respiratory medicine |
|
10/20/2020 |
|
Utility
model |
|
10
years from the application date (01/20/2020) |
|
Registered and effective |
|
| China |
|
ZL201922332612.1 |
|
Uterine
cavity sampler for gynecological reproductive clinics |
|
10/23/2020 |
|
Utility
model |
|
10
years from the application date (12/23/2019) |
|
Registered and effective |
|
| China |
|
ZL201922412254.5 |
|
Disposable
intubate package |
|
10/30/2020 |
|
Utility
model |
|
10
years from the application date (12/28/2019) |
|
Registered and effective |
|
| China |
|
ZL201921757111.1 |
|
Painless
anesthetic needle |
|
11/3/2020 |
|
Utility
model |
|
10
years from the application date (10/19/2019) |
|
Registered and effective |
|
| China |
|
ZL201910037322.6 |
|
Neurological
rehabilitation adjuvant therapy stimulation device |
|
10/23/2020 |
|
Invention |
|
20
years from the application date (01/15/2019) |
|
Registered and effective |
|
| China |
|
ZL202023332943.4 |
|
Glucometer |
|
10/15/2021 |
|
Utility
model |
|
10
years from the application date (12/31/2020) |
|
Registered and effective |
|
| China |
|
ZL202023296047.7 |
|
Portable
blood glucose meter |
|
1/7/2022 |
|
Utility
model |
|
10
years from the application date (12/31/2020) |
|
Registered and effective |
|
| China |
|
ZL202023343934.5 |
|
Bone
density tester |
|
1/14/2022 |
|
Utility
model |
|
10
years from the application date (12/31/2020) |
|
Registered and effective |
|
| China |
|
ZL202023296027.X |
|
Rotatable
densitometer |
|
1/18/2022 |
|
Utility
model |
|
10
years from the application date (12/31/2020) |
|
Registered and effective |
|
| China |
|
ZL202023290045.7 |
|
Surgical
stapler |
|
1/18/2022 |
|
Utility
model |
|
10
years from the application date (12/31/2020) |
|
Registered and effective |
|
| China |
|
ZL202023295973.2 |
|
New
cutting stapler |
|
3/29/2022 |
|
Utility
model |
|
10
years from the application date (12/31/2020) |
|
Registered and effective |
|
| China |
|
ZL202023313858.3 |
|
New
medical mask |
|
1/17/2022 |
|
Utility
model |
|
10
years from the application date (12/31/2020) |
|
Registered and effective |
|
| China |
|
ZL202023295982.1 |
|
New
warm and anti-fog masks |
|
3/29/2022 |
|
Utility
model |
|
10
years from the application date (12/31/2020) |
|
Registered and effective |
|
| Country |
|
Patent
No. |
|
Patent
Name |
|
Patent
Publication
Date |
|
Patent
Type |
|
Patent
Validity
Period |
|
Patent
Status |
|
| China |
|
ZL202023296016.1 |
|
Wrist
blood pressure meter |
|
3/29/2022 |
|
Utility
model |
|
10
years from the application date (12/31/2020) |
|
Registered and effective |
|
| China |
|
ZL202023342335.1 |
|
Portable
blood pressure meter |
|
1/14/2022 |
|
Utility
model |
|
10
years from the application date (12/31/2020) |
|
Registered and effective |
|
| China |
|
ZL202023313904.X |
|
Medical
identification wristband |
|
3/29/2022 |
|
Utility
model |
|
10
years from the application date (12/31/2020) |
|
Registered and effective |
|
| China |
|
ZL202023342334.7 |
|
Corrective
device to prevent myopia |
|
1/18/2022 |
|
Utility
model |
|
10
years from the application date (12/31/2020) |
|
Registered and effective |
|
| China |
|
2018212296448 |
|
Bone
densitometer with good anti-fall performance |
|
7/26/2019 |
|
Utility
model |
|
10
years from the application date
(8/1/2018) |
|
Registered and effective |
|
| China |
|
2014200928148 |
|
Nanocellulose
dressing for extensive burns |
|
2/25/2015 |
|
Utility
model |
|
10
years from the application date
(3/3/2014) |
|
Registered and effective |
|
Our currently registered patents do not relate
to our top 20 products, which are mature products. Instead, our registered patents represent the phased achievements of our R&D department,
which will serve as the basis for future research and the planned development of new products.
Currently, we have 27 registered and effective
patents, and we have not signed any royalty or licensing agreements with any third parties with respect to these patents. Currently,
however, we are working under a collaboration agreement to develop and register a patented product for production and sale. The patent
relates to a tracheal tube kit capable of pulverization and dosing by a tube wall fan spraying concurrently, and it is a utility model
patent issued in China with a validity period of ten years from May 29, 2019. A utility model patent is issued for a new technical scheme
suitable for practical use based on the shape, structure or combination of the products used. Moreover, to be suitable for a utility
model patent, an invention must possess novelty, creativity and practicability. We are authorized by the patent owner to develop a new
use for his patent and to file a medical device registration certificate for the developed product. Under our agreement with the patent
owner, once the product is in production and sales, we will distribute 25% of the profit after tax to him. Under the agreement, the patent
owner has the right to request an accounting of profits at his own expense. If the patent owner wishes to transfer the patent in the
future, he can only transfer it to us. For this collaboration agreement, we have completed the development of the new use of the patented
product, and we are in the process of applying for medical device registration certificate. Because the new product has not been put
into production and has not generated any sales yet, we have not distributed any profit to the patent owner and no other fees have been
paid or received under the agreement. The collaboration agreement and the profit-sharing arrangement are for a perpetual term. The collaboration
agreement may be terminated, however, in the event that we are unable to provide an accurate accounting of profits for the product to
the patent owner. In that case, all rights to the patent may be returned to the patent owner and we will be liable for damages in the
amount of an additional 10% of net profits received from the product.
Copyrights
Set forth below is a detailed description
of our registered copyrights:
| Country |
|
Copyright
No. |
|
Copyright
Name |
|
Copyright
Publication
Date |
|
Copyright
Type |
|
Copyright
Application
Date |
|
Copyright
Status |
|
| China |
|
2019SR0829585 |
|
Self-service printing
terminal control system software of image diagnostic film (V1.0) |
|
8/9/2019 |
|
software copyright |
|
6/13/2019 |
|
Registered and effective |
|
| China |
|
2019SR0813645 |
|
Intelligent Medical film
image printing output system software (V1.0) |
|
8/6/2019 |
|
software copyright |
|
6/20/2019 |
|
Registered and effective |
|
Insurance
We maintain Group Life Insurance for some
of our high-risk employees, such as electricians, plumbers and tooling operators. We also provide social security insurance including
pension, medical insurance, unemployment insurance, maternity insurance, on-the-job injury insurance and housing fund plans through a
PRC government-mandated benefit contribution plan for our employees. We do not carry any key-man life insurance, product liability and
professional liability insurance. Even if we purchase these kinds of insurance, the insurance may not fully protect us from the financial
impact of defending against product liability or professional liability claims. We have not purchased any property insurance or business
interruption insurance. We have determined that the costs of insuring for related risks and the difficulties associated with acquiring
such insurance on commercially reasonable terms make it impractical. We consider our insurance coverage to be sufficient for our business
operations in China.
Legal Proceedings
We may from time to time be subject to various
legal or administrative claims and proceedings arising in the ordinary course of business. Litigation or any other legal or administrative
proceeding, regardless of the outcome, is likely to result in substantial cost and diversion of our resources, including our management’s
time and attention.
With the exception of the following proceedings,
we are currently not a party to any material legal or administrative proceedings:
| ● | On
August 29, 2023, a legal proceeding, Zhu Cheng v. Jiangsu Yada Technology Group Co.,
Ltd., Yangzhou Huada Medical Equipment Co., Ltd., Jiangsu Huadong Medical Equipment Industrial
Co., Ltd., and Rehabilitation International Medical Co., Ltd. [Case No.: (2023) Su 1091 Minchu
No. 1779] , was filed with the Yangzhou Economic and Technological Development Zone People’s
Court. The materials of the company contract dispute case include a complaint, evidence,
notice of response and subpoena, among other things. Zhu Cheng’s lawsuit
requires Jiangsu Yada Technology Group Co., Ltd., Yangzhou Huada Medical Equipment Co., Ltd.,
Jiangsu Huadong Medical Equipment Industrial Co., Ltd., and Rehabilitation International
Medical Co., Ltd. to jointly and severally pay him 17 million yuan and from June 2022 Loss
of 1.5 times LPR from the 30th to the actual date of payment. The main basis for Zhu Cheng's
prosecution is the "Letter of Termination of Contract" signed by two foreign companies
(Xintai International Holdings Co., Ltd., Ace Capital Limited) and Jiangsu Yada Technology
Group Co., Ltd. on August 20, 2019. The "Contract Termination Agreement" mainly
stipulates that Jiangsu Yada Technology Group Co., Ltd. will compensate Xintai International
Holdings Co., Ltd. and Ace Capital Limited for the fees paid by companies that were listed
in South Korea. The case was heard for the first time on October 9, 2023, in the
Eighth Court of the Yangzhou Economic and Technological Development Zone People's Court.
The content of the hearing was the presentation and cross-examination of evidence by both
parties. The court required the plaintiff to provide registration information proving Xintai
International Holdings Co., Ltd. and Ace Capital Limited within one month. So far, the plaintiff
has not filed the required information with the court. The case is still ongoing and is expected
to be heard again sometime in March. To date, there has been no court decision or settlement
letter. |
Regulations
We operate our business in the PRC under a
legal regime consisting of the National People’s Congress, which is the country’s highest legislative body, the State Council,
which is the highest authority of the executive branch of the PRC central government, and several ministries and agencies under its authority,
including the State Administration of Foreign Exchange, or SAFE, the Ministry of Commerce, or MOFCOM, the National Development and Reform
Commission, or NDRC, the State Administration for Market Regulation, or SAMR, formerly known as the State Administration for Industry
and Commerce, or SAIC, the Ministry of Civil Affairs, or MCA, and their respective authorized local counterparts.
This section sets forth a summary of the most
significant rules and regulations that affect our business activities in the PRC.
Regulation Relating to Foreign Investment
All limited liability companies incorporated
and operating in the PRC are governed by the Company Law of the People’s Republic of China, or the Company Law, which
was amended and promulgated by the Standing Committee of the National People’s Congress on October 26, 2018. However, on December
24, 2021, the Standing Committee of the National People’s Congress issued the Company Law of the People’s Republic of China
(Draft for Comments) (the “Revised Company Law”), which is now open for public comments. The Revised Company Law further
stipulates the establishment and withdrawal of the company, the organizational structure and the capital system of the company, and strengthens
the responsibilities of shareholders and management personnel and Corporate Social Responsibility. Foreign invested projects must also
comply with the Company Law, with exceptions as specified in foreign investment laws.
The Foreign Investment Law of the People’s
Republic of China (the “Foreign Investment Law”) was adopted by the second meeting of the 13th National People’s Congress
on March 15, 2019, which became effective on January 1, 2020. On December 26, 2019, the State Council promulgated Regulation for Implementing
the Foreign Investment Law of the People’s Republic of China (the “Regulation”), which became effective on January
1, 2020.
The Foreign Investment Law and the Regulation
apply the administrative system of pre-establishment national treatment plus negative list to foreign investment and clarify the state
shall develop a catalogue of industries for encouraging foreign investment to specify the industries, fields, and regions where foreign
investors are encouraged and directed to invest. The MOFCOM and NDRC promulgated the Special Administrative Measures for the Access of
Foreign Investment (Negative List) (2021 Version) (the “2021 Negative List”) on December 27, 2021, which became effective
on January 1, 2022. The 2021 Negative List replaced the Special Administrative Measures for the Access of Foreign Investment (2020 Version)
and serves as the main basis for management and guidance for the MOFCOM to manage and supervise foreign investments. The restricted and
prohibited industry categories have shareholding requirements as prescribed in the 2021 Negative List. Those industries not set out on
the 2021 Negative List shall be classified as industries permitted for foreign investment. None of our businesses are on the 2021 Negative
List, nor on the 2020 Negative List. Therefore, the Company is able to conduct its business through its wholly owned PRC Subsidiaries
without being subject to restrictions imposed by the foreign investment laws and regulations of the PRC. In addition, the version of
the Catalogue of Industries for Encouraged Foreign Investment currently in force was amended in 2022 and became effective on January
1, 2023, which replaced the Catalogue of Industries for Encouraged Foreign Investment (2020 version).
Regulation Relating to Wholly Foreign-owned
Enterprises
The abovementioned Company Law of the People’s
Republic of China provides that companies established in the PRC may take the form of company of limited liability or company limited
by shares. Each company has the status of a legal person and owns its assets itself. Assets of a company may be used in full for the
company’s liability. The Company Law applies to foreign-invested companies unless relevant laws provide otherwise.
The Foreign Investment Law replaced Law of
the People’s Republic of China on Wholly Foreign-owned Enterprises. It stipulates that the PRC implements a system of pre-establishment
national treatment plus negative list for the administration of foreign investment. Foreign investors are not allowed to invest in fields
or sectors prohibited in the market access negative list for foreign investment. Foreign investors that intend to invest in the fields
subject to access restrictions stipulated in market access negative list for foreign investment shall satisfy the conditions stipulated
in such negative list. The PRC policies supporting enterprise development are equally applicable to foreign-invested enterprises. The
PRC does not impose expropriation on foreign investment. Under special circumstances, if it requires imposing expropriation on foreign
investment due to the need of public interest, expropriation shall be imposed according to legal procedures, and the foreign-invested
enterprises concerned shall receive fair and reasonable compensation. Foreign-invested enterprises can raise funds through public issuance
of stocks, corporate bonds and other securities in accordance with the law. Overall, The Foreign Investment Law establishes the clear
principle of applying national treatment to FIEs except those engaged in industries on the 2020 Negative List. Since our current and
planned business is not on the 2021 Negative List, to the best of our knowledge, it will not create any material adverse effect to our
Company’s business.
Regulations Relating to Intellectual Property
Copyright
China has adopted comprehensive legislation
governing intellectual property rights, including trademarks and copyrights. China is a signatory to the primary international conventions
on intellectual property rights and has been a member of the Agreement on Trade Related Aspects of Intellectual Property Rights since
its accession to the WTO in December 2001.
In September 1990, the SCNPC promulgated
the Copyright Law of the People’s Republic of China, effective in June 1991 and amended in 2001, 2010 and 2020 respectively. The
amended Copyright Law extends copyright protection to internet activities, products disseminated over the internet and software products.
In addition, there is a voluntary registration system administered by the Copyright Protection Centre of China.
In order to further implement the Computer
Software Protection Regulations, promulgated by the State Council in December 2001 and amended in 2011 and 2013 respectively, the National
Copyright Administration issued Computer Software Copyright Registration Procedures in February 2002, which specify detailed procedures
and requirements with respect to the registration of software copyrights.
Trademark
According to the Trademark Law of the People’s
Republic of China, promulgated by the SCNPC in August 1982, and amended in 1993, 2001, 2013 and 2019 respectively, the Trademark Office
of China National Intellectual Property Administration is responsible for the registration and administration of trademarks and is also
responsible for resolving trademark disputes in China. Registered trademarks are valid for ten years from the date the registration is
approved. A registrant may apply to renew a registration within twelve months before the expiration date of the registration. If the
registrant fails to apply in a timely manner, a grace period of six additional months may be granted. If the registrant fails to apply
before the grace period expires, the registered trademark shall be deregistered. Renewed registrations are valid for ten years. In April
2014, the State Council issued the revised Implementation of the Trademark Law, which specified the requirements of applying for trademark
registration and review.
Patent
According to the Patent Law of the People’s
Republic of China promulgated by the SCNPC in 1984 and amended in 1992, 2000, 2008 and 2020, respectively, a patentable invention or
a utility model must meet three criteria: novelty, inventiveness and practicability. A patent is valid for a twenty-year term for an
invention and a ten-year term for a utility model or design, starting from the application date.
Domain Names
In May 2012, the China Internet Network
Information Center issued the Implementing Rules for Domain Name Registration setting forth the detailed rules for registration of domain
names. In August 2017, China’s Ministry of Industry and Information Technology promulgated the Administrative Measures on Internet
Domain Names, or the Domain Name Measures. The Domain Name Measures regulate the registration of domain names, such as the top-level
domain name “.cn”.
Regulations on Offshore Parent Holding
Companies’ Direct Investment in and Loans to Their PRC Subsidiaries
An offshore company may invest equity in a
PRC company, which will become the PRC subsidiary of the offshore holding company after investment. Such equity investment is subject
to a series of laws and regulations generally applicable to any foreign-invested enterprise in China, all as amended from time to time,
and their respective implementing rules; the Administrative Provisions on Foreign Exchange in Domestic Direct Investment by Foreign Investors;
and the Notice of the State Administration on Foreign Exchange on Further Improving and Adjusting Foreign Exchange Administration Policies
for Direct Investment. Under the aforesaid laws and regulations, the increase of the registered capital of a foreign-invested enterprise
is subject to the prior approval by the original approval authority of its establishment. In addition, the increase of registered capital
and total investment amount shall both be registered with SAIC and SAFE. Shareholder loans made by offshore parent holding companies
to their PRC subsidiaries are regarded as foreign debts in China for regulatory purpose, which is subject to a number of PRC laws and
regulations, including the PRC Foreign Exchange Administration Regulations, the Interim Measures on Administration on Foreign Debts,
the Tentative Provisions on the Statistics Monitoring of Foreign Debts and its implementation rules, and the Administration Rules on
the Settlement, Sale and Payment of Foreign Exchange. Under these regulations, the shareholder loans made by offshore parent holding
companies to their PRC subsidiaries shall be registered with SAFE.
Regulations Relating to Foreign Exchange
Pursuant to the Foreign Exchange Administration
Regulations, as amended in August 2008, the RMB is freely convertible for current account items, including the distribution of dividends,
interest payments, trade and service-related foreign exchange transactions, but not for capital account items, such as direct investments,
loans, repatriation of investments and investments in securities outside the PRC, unless SAFE’s prior approval is obtained and
prior registration with SAFE is made. In May 2013 SAFE promulgated the Circular of the SAFE on Printing and Distributing the Administrative
Provision on Foreign Exchange in Domestic Direct Investment by Foreign Investors and Relevant Supporting Documents which provides
for and simplifies the operational steps and regulations on foreign exchange matters related to direct investment by foreign investors,
including foreign exchange registration, account opening and use, receipt and payment of funds, and settlement and sales of foreign exchange.
Pursuant to the Circular on Relevant
Issues concerning Foreign Exchange Administration of Overseas Investment and Financing and Return Investments Conducted by Domestic Residents
through Overseas Special Purpose Vehicles or the SAFE Circular 37, promulgated by SAFE and which became effective on July 4,
2014, (a) a PRC resident shall register with the local SAFE branch before he or she contributes assets or equity interests in an
overseas special purpose vehicle, or Overseas Special Purpose Vehicles (SPV), that is directly established or controlled by the PRC Resident
for the purpose of conducting investment or financing; and (b) following the initial registration, the PRC Resident is also required
to register with the local SAFE branch for any major change, in respect of the Overseas SPV, including, among other things, a change
of the Overseas SPV’s PRC Resident shareholder(s), name of the Overseas SPV, term of operation, or any increase or reduction of
the Overseas SPV’s registered capital, share transfer or swap, and merger or division. Pursuant to SAFE Circular 37, failure
to comply with these registration procedures may result in penalties.
Pursuant to the Circular of the State Administration
of Foreign Exchange on Further Simplifying and Improving the Direct Investment-related Foreign Exchange Administration Policies, or the
SAFE Notice 13, which was promulgated on February 13, 2015 and with effect from June 1, 2015, the foreign exchange registration under
domestic direct investment and the foreign exchange registration under overseas direct investment is directly reviewed and handled by
banks in accordance with the SAFE Notice 13, and the SAFE and its branches shall perform indirect regulation over the foreign exchange
registration via banks.
Regulations Relating to Dividend Distributions
According to the PRC Company Law and Foreign
Investment Law, each of our PRC subsidiaries, as a foreign invested enterprise, or FIE, are required to draw 10% of its after-tax profits
each year, if any, to fund a common reserve, which may stop drawing its after-tax profits if the aggregate balance of the common reserve
has already accounted for over 50% of its registered capital. These reserves are not distributable as cash dividends. Furthermore, under
the EIT Law, which became effective in January 2008, the maximum tax rate for the withholding tax imposed on dividend payments from PRC
foreign invested companies to their overseas investors that are not regarded as “resident” for tax purposes is 20%. The rate
was reduced to 10% under the Implementing Regulations for the EIT Law issued by the State Council. However, a lower withholding tax rate
might be applied if there is a tax treaty between China and the jurisdiction of the foreign holding companies, such as tax rate of 5%
in the case of Hong Kong companies that holds at least 25% of the equity interests in the foreign-invested enterprise, and certain requirements
specified by PRC tax authorities are satisfied.
Regulations Relating to Overseas Listings
On July 6, 2021, the General Office of the
Central Committee of the Communist Party of China and the General Office of the State Council jointly issued the “Opinions on Severely
Cracking Down on Illegal Securities Activities According to Law,” or the Opinions. The Opinions emphasized the need to strengthen
the administration over illegal securities activities, and the need to strengthen the supervision over overseas listings by Chinese companies.
Effective measures, such as promoting the construction of relevant regulatory systems will be taken to deal with the risks and incidents
of China-concept overseas listed companies, and cybersecurity and data privacy protection requirements and similar matters.
On December 24, 2021, the China Securities
Regulatory Commission, or the CSRC, issued Provisions of the State Council on the Administration of Overseas Securities Offering and
Listing by Domestic Companies (Draft for Comments) (the “Administration Provisions”), and the Measures for the Overseas Issuance
of Securities and Listing Record-Filings by Domestic Enterprises (Draft for Comments) (the “Measures”), which were published
for public comments only, for which the comment period expired on January 23, 2022.
The Administration Provisions and Measures
(collectively, the “Draft Rules Regarding Overseas Listing”) for overseas listings lay out specific requirements for filing
documents and include unified regulation management, strengthening regulatory coordination, and cross-border regulatory cooperation.
Domestic companies seeking to list abroad must carry out relevant security screening procedures if their businesses involve such supervision.
Companies endangering national security are among those off-limits for overseas listings.
On February 17, 2023, the CSRC promulgated
the Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Companies, or the Trial Measures, and five
supporting guidelines, which came into effect on March 31, 2023. The Trial Measures and its supporting guidelines, reiterate the basic
principles of the Draft Rules Regarding Overseas Listing and impose substantially the same requirements for the overseas securities offering
and listing by domestic enterprises. Pursuant to the Trial Measures, domestic companies that seek to offer or list securities overseas,
both directly and indirectly, shall complete filing procedures with the CSRC pursuant to the requirements of the Trial Measures within
three working days following its submission of initial public offerings or listing application. If a domestic company fails to complete
the required filing procedures or conceals any material fact or falsifies any major content in its filing documents, such domestic company
may be subject to administrative penalties, such as orders to rectify, warnings, fines, and its controlling shareholders, actual controllers,
the person directly in charge and other directly liable persons may also be subject to administrative penalties, such as warnings and
fines.
According to the Notice on the Administrative
Arrangements for the Filing of the Overseas Securities Offering and Listing by Domestic Companies from the CSRC, or the CSRC Notice,
which was promulgated on February 17, 2023 and became effective on the same day, the domestic companies that have already been listed
overseas before the effective date of the Overseas Listing Trial Measures (i.e. March 31, 2023) shall be deemed as existing issuers (the
“Existing Issuers”). Existing Issuers are not required to complete the filing procedures immediately, and they shall be required
to file with the CSRC for any subsequent offerings. Further, according to the CSRC Notice, domestic company obtained approval from overseas
regulatory authorities or securities exchanges (for example, the effectiveness of a registration statement for offering and listing in
the U.S. has been approved) for their indirect overseas offering and listing prior March 31, 2023 but have not yet completed their indirect
overseas issuance and listing, are granted a six-month transition period from March 31, 2023 to September 30, 2023. Those issuers that
complete their indirect overseas offering and listing within such six-month period are deemed as Existing Issuers and are not required
to file with the CSRC for their indirect overseas offerings and listings. Within such six-month transition period, however, if such domestic
companies fail to complete their indirect overseas issuance and listing, they shall complete the filing procedures with the CSRC.
The Opinions, the Trial Measures and any related
implementing rules to be enacted may subject us to additional compliance requirements in the future financial activities. See “Risk
Factors - Risks Relating to Doing Business in the PRC - The Chinese governmental and regulatory interference could significantly limit
or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly
decline or be worthless.”
In August 2006, six PRC regulatory authorities,
including the CSRC, jointly adopted the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or
the M&A Rules, amended in June 2009. The M&A Rules, among other things, require that if an overseas company established or controlled
by PRC companies or individuals, or PRC Citizens, intends to acquire equity interests or assets of any other PRC domestic company affiliated
with the PRC Citizens, such acquisition must be submitted to the MOFCOM for approval. The M&A Rules also require that an Overseas
SPV formed for overseas listing purposes and controlled directly or indirectly by the PRC Citizens shall obtain the approval of the CSRC
prior to overseas listing and trading of such Overseas SPV’s securities on an overseas stock exchange.
Based on current PRC laws and regulations,
our corporate structure and arrangements are not subject to the M&A Rules. However, there are substantial uncertainties as to how
the M&A Rules will be interpreted or implemented in the context of an overseas offering, and its opinions summarized above are subject
to any new laws, rules and regulations or detailed implementations and interpretations in any form relating to the M&A Rules.
Regulations Relating to Employment
The Labor Law of the People’s Republic
of China, or the Labor Law, which became effective in January 1995 and was amended in 2018, and the Employment Contract Law of the People’s
Republic of China, or the Employment Contract Law, effective in January 2008 and amended in 2012, require employers to provide written
contracts to their employees, restrict the use of temporary workers and aim to give employees long-term job security. Employers must
pay their employees’ wages equal to or above local minimum wage standards, establish labor safety and workplace sanitation systems,
comply with state labor rules and standards and provide employees with appropriate training on workplace safety. In September 2008, the
State Council promulgated the Implementing Regulations for the PRC Employment Contract Law which became effective immediately and interprets
and supplements the provisions of the Employment Contract Law.
Under the Labor Contract Law, an employer
shall limit the number of dispatched workers so that they do not exceed a certain percentage of its total number of workers. In January
2014, the MOHRSS issued the Interim Provisions on Labor Dispatching, which became effective in March 2014, pursuant to which it provides
that the number of dispatched workers used by an employer shall not exceed 10% of the total number of its employees.
The PRC governmental authorities have passed
a variety of laws and regulations regarding social insurance and housing funds from time to time, including, among others, the Social
Insurance Law of the People’s Republic of China, the Regulation of Insurance for Labor Injury, the Regulations of Insurance for
Unemployment, the Provisional Insurance Measures for Maternal Employees, the Interim Administrative Provisions on Registration of Social
Insurance and the Administrative Regulations on the Housing Provident Fund. Pursuant to these laws and regulations, enterprises in the
PRC shall provide their employees with welfare schemes covering pension insurance, unemployment insurance, maternity insurance, occupational
injury insurance and medical insurance, as well as housing fund and other welfare plans. Failure to comply with such laws and regulations
may result in various fines and legal sanctions and supplemental contributions to the local social insurance and housing fund regulatory
authorities.
Regulations Relating to Environmental Protection
Environmental Protection Law
The Environmental Protection Law of the PRC,
or the Environmental Protection Law, was promulgated and effective on December 26, 1989, and most recently amended on April 24, 2014.
This Environmental Protection Law has been formulated for the purpose of protecting and improving both the living environment and the
ecological environment, preventing and controlling pollution, other public hazards and safeguarding people’s health.
According to the provisions of the Environmental
Protection Law, in addition to other relevant laws and regulations of the PRC, the Ministry of Environmental Protection and its local
counterparts take charge of administering and supervising said environmental protection matters. Pursuant to the Environmental Protection
Law, the environmental impact statement on any construction project must assess the pollution that the project is likely to produce and
its impact on the environment, and stipulate preventive and curative measures; the statement shall be submitted to the competent administrative
department of environmental protection for approval. Installations for the prevention and control of pollution in construction projects
must be designed, built and commissioned together with the principal part of the project.
Permission to commence production at or utilize
any construction project shall not be granted until its installations for the prevention and control of pollution have been examined
and confirmed to meet applicable standards by the appropriate administrative department of environmental protection that examined and
approved the environmental impact statement. Installations for the prevention and control of pollution shall not be dismantled or left
idle without authorization. Where it is absolutely necessary to dismantle any such installation or leave it idle, prior approval shall
be obtained from the competent local administrative department of environmental protection.
The Environmental Protection Law makes
it clear that the legal liabilities of any violation of said law include warning, fine, rectification within a time limit, compulsory
cease operation, compulsory reinstallation of dismantled installations of the prevention and control of pollution or compulsory reinstallation
of those left idle, compulsory shutout or closedown, or even criminal punishment.
As of the date of this report, we are not
aware of any warning, investigations, prosecutions, disputes, claims or other proceedings in respect of environmental protection, nor
have we been punished or can foresee any punishment to be made by any government authorities of the PRC.
Order on Ecosystem by the Ministry of
Ecology and Environment 2019 Classification-based Management on Fixed Pollutant Source
Pursuant to the Catalog of Classified Management
of Pollutant Discharge Permits for Stationary Pollution Sources, which was promulgated by the Ministry of Ecology and Environment on
July 28, 2017 and most recently amended on December 20, 2019. The Ministry of Ecology and Environment implements a classification-based
management on the environmental impact assessment, or EIA, of pollutants according to pollutant amount and the impact of the pollutants
on the environment as below:
| |
● |
For those pollutant
discharge units with large amount of pollutants and significant environmental impacts, the key management on a pollutant discharge
permit is required; |
| |
|
|
| |
● |
For those pollutant
discharge units with small amount of pollutants and small environmental impacts, the simplified management on a pollutant discharge
permit is required; and |
| |
|
|
| |
● |
For those pollutant
discharge units with very small amount of pollutants and very small environmental impacts, the pollutant discharge registration form
is required. |
The medical device manufacturing is classified
as to fill in a Registration Form. Upon submission of all required documentation, we are registered under the new system by filling in
Pollution Source Registration Form.
Regulations Relating to Customer Rights
Protection
The PRC Customer Rights and Interests
Protection Law, or Customer Protection Law, as amended on October 25, 2013 and effective on March 15, 2014, sets out the obligations
of business operators and the rights and interests of the customers. Pursuant to this law, business operators must guarantee that the
commodities they sell satisfy the requirements for personal or property safety, provide customers with authentic information about the
commodities, and guarantee the quality, function, usage and term of validity of the commodities. Failure to comply with the Customer
Protection Law may subject business operators to civil liabilities such as refunding purchase prices, exchange of commodities, repairing,
ceasing damages, compensation, and restoring reputation, and even subject the business operators or the responsible individuals to criminal
penalties if business operators commit crimes by infringing the legitimate rights and interests of customers.
Regulations Relating to Tax in the PRC
Income Tax
The PRC Enterprise Income Tax Law was
promulgated in March 2007 and was most recently amended in December 2018. The PRC Enterprise Income Tax Law applies a uniform
25% enterprise income tax rate to both foreign-invested enterprises and domestic enterprises, except where tax incentives are granted
to special industries and projects. Under the PRC Enterprise Income Tax Law, an enterprise established outside China with “de
facto management bodies” within China is considered a “resident enterprise” for PRC enterprise income tax purposes
and is generally subject to a uniform 25% enterprise income tax rate on its worldwide income. Under the implementation regulations to
the PRC Enterprise Income Tax Law, a “de facto management body” is defined as the body that exercises full and
substantial control and overall management over the business, productions, personnel, accounts and properties of an enterprise.
In April 2009, the Ministry of Finance, or
MOF, and SAT jointly issued the Notice on Issues Concerning Process of Enterprise Income Tax in Enterprise Restructuring Business, or
the Circular 59. In December 2009, SAT issued the Notice on Strengthening Administration of Enterprise Income Tax for Share Transfers
by Non-PRC Resident Enterprises, or the Circular 698. Both Circular 59 and Circular 698 became effective retroactively as of January
2008. In March 2011, SAT issued the Notice on Several Issues Regarding the Income Tax of Non-PRC Resident Enterprises, or the
SAT Circular 24, effective in April 2011. By promulgating and implementing these circulars, the PRC tax authorities have enhanced their
scrutiny over the direct or indirect transfer of equity interests in a PRC resident enterprise by a non-resident enterprise.
In February 2015, SAT issued the Notice
on Certain Corporate Income Tax Matters on Indirect Transfer of Properties by Non-PRC Resident Enterprises, or the SAT Circular
7, to supersede existing provisions in relation to the indirect transfer as set forth in Circular 698, while the other provisions of
Circular 698 remain in force. SAT Circular 7 introduces a new tax regime that is significantly different from that under Circular 698.
SAT Circular 7 extends its tax jurisdiction to capture not only indirect transfers as set forth under Circular 698 but also transactions
involving transfer of immovable property in China and assets held under the establishment, and placement in China, of a foreign company
through the offshore transfer of a foreign intermediate holding company. SAT Circular 7 also addresses transfer of the equity interest
in a foreign intermediate holding company broadly. In addition, SAT Circular 7 provides clearer criteria than Circular 698 on how to
assess reasonable commercial purposes and introduces safe harbor scenarios applicable to internal group restructurings. However, it also
brings challenges to both the foreign transferor and transferee of the indirect transfer as they have to determine whether the transaction
should be subject to PRC tax and to file or withhold the PRC tax accordingly. In October 2017, SAT issued the Announcement on Issues
Relating to Withholding at Source of Income Tax of Non-resident Enterprises, or the SAT Circular 37, amended in June 2018. The SAT
Circular 37 superseded the Non-resident Enterprises Measures and SAT Circular 698 as a whole and partially amended some provisions in
SAT Circular 24 and SAT Circular 7. SAT Circular 37 purports to clarify certain issues in the implementation of the above regime, by
providing, among others, the definition of equity transfer income and tax basis, the foreign exchange rate to be used in the calculation
of withholding amount, and the date of occurrence of the withholding obligation. Specifically, SAT Circular 37 provides that where the
transfer income subject to withholding at source is derived by a non-PRC resident enterprise in installments, the installments may first
be treated as recovery of costs of previous investments. Upon recovery of all costs, the tax amount to be withheld must then be computed
and withheld.
Value-Added Tax
The PRC Provisional Regulations on Value-Added
Tax were promulgated by the State Council on December 13, 1993, which became effective on January 1, 1994 and were subsequently amended
from time to time. The Detailed Rules for the Implementation of the PRC Provisional Regulations on Value-Added Tax (2011 Revision) was
promulgated by the Ministry of Finance on December 25, 1993 and subsequently amended on December 15, 2008 and October 28, 2011. On November
19, 2017, the State Council promulgated the Decisions on Abolishing the PRC Provisional Regulations on Business Tax and Amending the
PRC Provisional Regulations on Value-Added Tax. Pursuant to these regulations, rules and decisions, all enterprises and individuals engaged
in sale of goods, provision of processing, repair, and replacement services, sales of services, intangible assets, real property, and
the importation of goods within the PRC territory are VAT taxpayers. On March 21, 2019, the Ministry of Finance, the SAT, and the General
Administration of Customs jointly issued the Announcement on Relevant Policies on Deepen the Reform of Value-Added Tax. Sales revenue
represents the invoiced value of goods, net of VAT. The VAT is based on gross sales price, starting from April 1, 2019, VAT rate was
lowered to 13%.
LAWS AND REGULATIONS RELATING TO MEDICAL
DEVICES
Regulation and Classification of Medical
Devices
Pursuant to the Regulations on the Supervision
and Administration of Medical Devices promulgated on January 4, 2000, effective on June 1, 2014, amended by the State Council on May
4, 2017 and now effective, and then amended on February 9, 2021 effective on June 1, 2021 (“Regulation on Supervision and Administration
of Medical Devices”), the Food and Drug Administration of the State Council shall be responsible for the national administration
and supervision of medical devices of the PRC and its local counterparts take charge of the local administration and supervision of medical
devices of the PRC.
Under this regulation, medical devices have
been classified into three categories based on the degree of risk. Class I medical devices shall refer to those devices with low level
of risks and whose safety and effectiveness can be ensured through routine administration. Class II medical devices shall refer to those
devices with moderate risks that must be strictly controlled and regulated to ensure their safety and effectiveness. Class III medical
devices shall refer to those devices with relatively high risks that must be strictly controlled and regulated through special measures
to ensure their safety and effectiveness.
The products we currently manufacture and
sell include Class I, II and III disposable medical devices.
Registration and Filings of Medical Devices
Pursuant to the Regulations on the Supervision
and Administration of Medical Devices and the Administrative Measures for the Registration of Medical Devices promulgated by CFDA on
July 30, 2014 and came into effect on October 1, 2014 (“the Supervision and Administration of Medical Devices” was amended
and came into effect on May 4, 2017; and which was then amended on February 9, 2021 and came into effect on June 1, 2021), Class I medical
devices are subject to filing administration, and Class II and Class III medical devices are subject to pre-approval registration administration.
A registration certificate for Class II and Class III medical devices are issued upon approval, which is valid for five years and may
be renewed six months prior to its expiration date.
Clinical trials are not required for the filing
of the Class I medical devices, but necessary for the registration of Class II and Class III medical device with certain exceptions.
As of the date of this report, we are current
on the registration and filing of medical devices.
Production License for Medical Devices
Pursuant to the Regulation on the Supervision
and Administration of Medical Devices promulgated on July 30, 2014 and came into effect on October 1, 2014, as amended in 2017 and came
into effect on May 4, 2017 (amended on February 9, 2021, came into effect on June 1, 2021), and the Administrative Measures on the Production
Supervision of Medical Devices promulgated on July 30, 2014 and came into effect on October 1, 2014, as amended in 2017 and came into
effect on November 11, 2017, manufacturers engaged in the manufacturing of Class I medical devices are subject to production filing administration
and receive production filing certificates upon satisfaction of filing requirements; while those engaged in the manufacturing of Class
II and Class III medical devices are subject to pre-approval licensing administration and receive medical device production licenses
upon receipt of approval for licensing. A medical device production license is valid for five years and may be renewed six months prior
to its expiration date.
In addition, a manufacturer of medical devices
shall satisfy the following conditions:
| |
(1) |
possessing production
sites, environmental conditions, production equipment and professional technicians that are suitable for such medical device produced; |
| |
(2) |
possessing organizations
or professional examination staff and examination equipment that carry out quality examination for such medical device produced; |
| |
(3) |
formulating a management
system which ensures the quality of such medical device; |
| |
(4) |
having capability of
after-sale services that is suitable for such medical device produced; (5) satisfying the requirements as prescribed in production
R&D and production technique documents. |
As of the date of this report, we are current
on the production filing and licensing of the medical devices.
Production and Quality Management of Medical
Devices
Pursuant to the Administrative Measures on
the Supervision of the Production of Medical Devices promulgated on December 29, 2014 and came into effect on March 1, 2015, as amended
in 2017 and came into effect on November 17, 2017, and the Standards on Production and Quality Management of Medical Devices promulgated
by the CFDA on December 29, 2014 and came into effect on March 1, 2015, an enterprise engaged in the production of medical devices shall
establish and effectively maintain a quality control system in accordance to the requirements of the Standards on Production and Quality
Management of Medical Devices. The enterprise engaged in the production of medical devices shall regularly conduct comprehensive self-inspection
on the operation of quality management system and submit this report to the local food and drug supervision and administration authorities
before the end of every year. The enterprise shall also establish its procurement control procedure and assess its suppliers by establishing
an examination system to ensure the purchased products are in compliance with the statutory requirements. The enterprise shall apply
risk management to the whole process of design and development, production, sales and after-sale services.
Pursuant to The Notice of Four Guidelines
including On-site Inspection Guidelines for the standards on Production and Quality Management of Medical Devices promulgated by the
CFDA on September 25, 2015 and came into effect on September 25, 2015, during the course of on-site verification of the registration
of medical devices and on-site inspection of production license t(including change production license), the inspection team shall, in
accordance with the guidelines, issue recommended conclusions for on-site inspections, which shall be divided into “Passed,”
“Failed” and “Reassessment after rectification.” During the supervision and inspection, if it is found that the
requirements of the key items or ordinary items that may have direct impact on product quality are not satisfied, the enterprise shall
suspend production and go through rectification. If it is found that the requirements of the ordinary items are not satisfied, and it
does not directly affect product quality, the enterprise shall rectify in a prescribed time. The regulatory authorities will examine
and verify the recommended conclusions and on-site inspection materials submitted by the inspection group and issue the final inspection
results.
The inspection team has conducted several
on-site inspections on our standards of production and quality management of medical devices during the track record period, the recommended
conclusions issued by the inspection team were “Passed” or “Rectification within the prescribed period.” The
matters with respect to “Rectification within the prescribed period” have been rectified within the prescribed period and
submitted to the inspection team.
According to the on-site inspections on our
standards of production and quality management conducted by competent authorities, we are in compliance with the requirements of the
standards on production and quality management of medical devices.
Good Clinical Practice for Medical Devices
On March 1, 2016, the CFDA and the National
Health and Family Planning Commission jointly promulgated the Good Clinical Practice for Medical Devices, which became effective as of
June 1, 2016. The regulation includes full procedures of clinical trial of medical devices, including, among others, the protocol design,
conduction, monitoring, verification, inspection, and data collection, recording, analysis and conclusion and reporting procedure of
a clinical trial.
For conducting clinical trials of medical
devices, an applicant shall organize to formulate scientific and reasonable clinical trial protocol based on the categories, risks and
intended use of the medical devices for the clinical study. The applicant shall be responsible for organizing to develop and revise of
the researcher’s manual, clinical trial protocol, informed consent form, case report form, relevant standard operating procedures
and other relevant documents, and shall be responsible for organizing necessary trainings for the clinical trials. The applicant shall
select the clinical trial institutions and its researchers from the qualified medical device clinical trial institutions according to
the characteristics of the medical devices to be used in the clinical study.
As an applicant for clinical trials of medical
devices, we are responsible for initiating, applying, organizing and monitoring such clinical trials, and shall be responsible for the
authenticity and reliability of the clinical trials.
Operation License for Medical Device
Pursuant to the Regulations on the Supervision
and Administration of Medical Devices and the Administrative Measures on the Operation Supervision of Medical Devices, promulgated on
July 30, 2014 and came into effect on October 1, 2014 and last amended on March 10, 2022, filing and licensing are not required for the
operation of Class I medical devices. Operators engaged in the operation of Class II medical devices are subject to filing administration
and will receive medical device operation filing certificate upon satisfaction of filing requirement, while operators engaged in the
operation of Class III medical devices are subject to pre-approval licensing administration and will receive medical device operation
license upon receipt of approval for licensing. A medical device operation license is valid for five years and may be renewed six months
prior to its expiration date.
To engage in business operations of medical
devices, the following requirements shall be met:
| |
1. |
Having a quality control
institution or staff corresponding to the business scope and scale, and the staff shall have relevant education or professional titles
certified by the state. |
| |
2. |
Having an operation
and storage premise corresponding to the business scope and scale. |
| |
3. |
Having storage conditions
corresponding to the business scope and scale; warehouses are not required if all storage is commissioned to other operators of medical
devices. |
| |
4. |
Having a quality control
system corresponding to the medical devices concerned. |
| |
5. |
Possessing the capability
of professional guidance, technical training and after-sale service corresponding to the medical devices it operates; or it has come
into an agreement on technical support with a relevant institution. |
An enterprise to be engaged in business operations
of Category III medical devices shall also have a computerized information management system compliant with quality standards to ensure
traceability of products. An enterprise to be engaged in business operations of Category I or Category II medical devices is encouraged
to set up such a system.
As of the date of this report, we are current
on the operation filing and licensing of the medical devices.
Special Procedures for Examination and
Approval of Innovative Medical Devices
On October 2017, the General Office of the
CPC Central Committee and the General Office of the State Council issued the Opinions on Deepening the Reform of the Evaluation and Approval
Systems and Encouraging Innovation on Drugs and Medical Devices, which aims to encourage the innovation for medical devices.
Pursuant to the Opinions, the priority review
and approval will be applicable to innovative medical devices supported by the National Science and Technology Major Projects and the
National Key R&D Program of China, and the clinical trials of which having been conducted by the National Clinical Research Center,
and approved by the management department of National Clinical Research Center. Pursuant to the Special Procedures for Examination and
Approval of Innovative Medical Devices which were promulgated by the NMPA on November 2, 2018 and came into effect on December 1, 2018,
special procedures shall be applicable to the examination and approval for medical devices in the following circumstances:
| |
(1) |
if the applicant legally
owns the invention patent of the core technology of the product through its technological innovation activities in the PRC, or legally
obtained the invention patent or the right of use thereof through transfer in the PRC, and that the interval between the date of
application for the special examination and approval of innovative medical devices to the date of authorized publication should not
exceed five years; or the patent administration department of the State Council has disclosed the application for the invention patent
of the core technology and the Patent Search and Consultation Center of the National Intellectual Property Administration of the
PRC has issued the patent search report setting out the novelty and innovation of the core technology solution of the product; |
| |
(2) |
the applicant has developed
the prototype product and completed the preliminary research under a true and controllable process that generated complete and traceable
data; |
| |
(3) |
the product has major
working mechanism or mechanism of action which is the first of its kind in the PRC, has fundamental improvement in product performance
or safety compared with similar products, is of an internationally leading standard in terms of techniques and has significant clinical
value. The Center for Medical Device Evaluation of the NMPA should give priority to the innovative medical devices in their technical
review upon receiving the registration application, after which the NMPA will give priority to the product in their administrative
approval. |
Advertisements of Medical Devices
Pursuant to the Regulations on Tentative Measures
for the Censorship of Advertisement for Drugs, Medical Devices, Dietary Supplements, Food Formula for Special Medical Purpose promulgated
by SAMR on December 24, 2019 and came into effect on March 1, 2020, the State Administration for Market Regulation is responsible for
organizing and guiding the review of advertisements for drugs, medical devices, health foods and formula foods for special medical purposes.
The administrations for market regulation and drug administrations (hereinafter referred to as the “advertisement review authorities”)
of all provinces, autonomous regions and centrally administered municipalities shall be responsible for the review of advertisements
for drugs, medical devices, health food and formula food for special medical purposes, and may entrust other administrative authorities
to implement review of advertisements pursuant to the law.
The validity period of the advertisement approval
number for drugs, medical devices, health food and formula food for special medical purposes shall be consistent with the shortest validity
period of the product registration certificate, filing certificate or production license. If no valid period is prescribed in the product
registration certificate, filing certificate or production license, the valid period of the advertisement approval number shall be two
years.
Advertisements for drugs, medical devices,
health food and formula food for special medical purposes shall be true and legitimate and shall not contain any false or misleading
contents. Advertisers shall be responsible for the veracity and legitimacy of the contents of advertisements for drugs, medical devices,
health food and formula food for special medical purposes.
National Medical Insurance Program
The national medical insurance program was
adopted pursuant to the Decision of the State Council on the Establishment of the Urban Employee Basic Medical Insurance Program issued
by the State Council on December 14, 1998, under which all employers in urban cities are required to enroll their employees in the Urban
Employee Basic Medical Insurance Program and the insurance premium is jointly contributed by the employers and employees. Pursuant to
the Opinions on the Establishment of the New Rural Cooperative Medical System forwarded by the General Office of the State Council on
January 16, 2003, China launched the New Rural Cooperative Medical System to provide medical insurance for rural residents in selected
areas which has since spread to the whole nation. The State Council promulgated the Guiding Opinions of the State Council about the Pilot
Urban Resident Basic Medical Insurance on July 10, 2007, under which urban residents of the pilot district, rather than urban employees,
may voluntarily join Urban Resident Basic Medical Insurance. In 2015, the PRC government announced the Outline for the Planning of the
National Medical and Health Service System (2015-2020) which aims to establish a basic medical and health care system that covers both
rural and urban citizens by 2020. On January 3, 2016, the State Council issued the Opinions on Integrating the Basic Medical Insurance
Systems for Urban and Rural Residents to integrate the Urban Resident Basic Medical Insurance and the New Rural Cooperative Medical System
and the establishment of a unified Basic Medical Insurance for Urban and Rural Residents, which will cover all urban and rural non-working
residents expect for rural migrant workers and persons in flexible employment arrangements who participate in the basic medical insurance
for urban employees.
With regard to reimbursement for medical devices
and diagnostic tests, the Notice of Opinion on the Diagnosis and Treatment Management, Scope and Payment Standards of Medical Service
Facilities Covered by the National Urban Employees Basic Medical Insurance Scheme (Lao She Bu Fa [1999] No. 22) prescribes the coverage
of diagnostic and treatment devices and diagnostic tests where part of the fees is paid through the basic medical insurance scheme. It
also includes a negative list that precludes certain devices and medical services from governmental reimbursement. Detailed reimbursement
coverage and rate for medical devices and medical services (including diagnostic tests and kits) are subject to each province’s
local policies.
Export Registration
Pursuant to the Regulation on the Supervision
and Administration of Medical Devices and the Measures for the Supervision and Administration of Medical Device Production promulgated
by the CFDA and amended on November 11, 2017 and March 10, 2022, CFDA, in accordance with the spirit of the Notice of Guo Ban Fa [94]
No. 66 of the State Council, conducts inspections of safety and legality of the exported products manufactured by domestic enterprises,
grants legitimate production license in China (if these products are sold within Chinese territory). In accordance with international
practice, the quality of exported medical devices is mainly supervised by the importing countries. However, some importing countries/regions
may require exporting enterprises to provide Medical Device Product Export Sales Certificates issued by the CFDA. Pursuant to Announcement
on Issuing the Provisions on the Administration of Medical Device Product Export Sales Certificates, promulgated by the CFDA and effective
on September 1, 2015, such exporting enterprises may apply to the provincial departments of the CFDA at the places where enterprises
are located for Medical Device Product Export Sales Certificates.
The premise of obtaining Medical Device Product
Export Sales Certificates is that the relevant production enterprises have obtained medical device product registration certificates
and production licenses or have undergone the formalities for recordation and production recordation of medical device products in China.
The valid period of Medical Device Product Export Sales Certificates, except being specified for one time use, shall not expire after
the earliest deadline of any certificate among various certificates submitted by the enterprise amid the application materials, and shall
be no longer than two years. Where the relevant materials submitted by an enterprise change, the enterprise shall report to the certificate
issuing department in a timely manner. Where the relevant materials change, or the Medical Device Product Export Sales Certificate still
needs to be used after its expiration, the enterprise shall apply for a new Medical Device Product Export Sales Certificate. Where the
CFDA find that any relevant enterprises fail to meet the requirements of relevant regulations on production, they shall downgrade the
credit ratings of such enterprises to lower levels; or, when any enterprises are considered failing to meet the requirements for issuance
of certificates anymore, or the relevant materials submitted by the enterprises change, the provincial CFDA departments shall notify
the relevant information in a timely manner.
Two-invoice System
According to the Notice of Publishing Opinions
on Implementing Two-invoice System in Drug Procurement Among Public Medical Institutions (For Trial Implementation) which was issued
on December 26, 2016, the “two-invoice system” refers to the system that requires one invoice to be issued from pharmaceutical
manufacturers to pharmaceutical distributors and the other invoice to be issued from pharmaceutical distributors to medical institutions.
The wholly owned or holding commercial company (only one commercial company is permitted in the whole country) or the domestic general
agent for overseas drugs (only one domestic agent is permitted in the whole country) established by a pharmaceutical manufacturer or
a group enterprise integrating science, industry and trade may be regarded as a manufacturer. The allocation of drugs between a pharmaceutical
distribution group enterprise and its wholly owned (holding) subsidiaries or among its wholly owned (holding) subsidiaries may not be
regarded as a process for which an invoice should be issued, but one invoice is allowed to be issued at most.
Currently, some provinces in the PRC have
formulated relevant rules and regulations to implement the “two-invoice system” in the field of medical consumables, for
instance, the Notice on the Sharing of Transparent Procurement Results of Medical Devices (Medical Consumables) across the Province promulgated
by the Fujian Provincial Medical Security Management Committee Office in July 2018, the Notice on Further Promoting the “Two Invoice
System” on Medicines and Medical Consumables issued by eight local government departments of Shaanxi Province including Deepen
Medical and Healthcare System Reform Leading Group Office of Shaanxi Province in July 2018, and the Opinions on Implementation of the
“Two Invoice System” in Medical Consumables Procurement by Public Medical Institutions in Anhui Province (for Trial Implementation)
issued by five local government departments of Anhui Province including Food and Drug Administration of Anhui Province in November 2017.
LAWS AND REGULATIONS RELATING TO LAND USE
Overview of relevant PRC Laws and Regulations
on Land Use Rights
Pursuant to relevant PRC land laws and stipulations,
there are two kinds of land in China: 1) collectively owned land, which is normally owned by the farmers or village for agricultural
use; and 2) state owned land which is sub-divided into allocated and granted land use rights. Allocated land are land rights granted
by the Chinese government to an entity for a particular purpose (e.g., research, military, medical etc.). These allocated rights are
inferior in that they must be used for the specified purpose and cannot be transferred, leased or mortgaged. Granted land, on the other
hand, is paid for and can be used for commercial and industrial purposes. These land use rights are the preferred land use rights for
foreign investors as they are freely transferable (subject normally to the land being developed, as undeveloped land cannot normally
be sold), leased and mortgaged. Land may be designated for commercial, industrial, residential or other purposes and may not be used
for any non-designated purpose. The land authorities may impose administrative sanctions, including fines, injunction orders or even
confiscation of the land use rights, for any breach of this provision. The term of land use rights varies depending on the designated
purpose. A land user may extend the term by entering into a contract to extend the term and pay an additional land grant fee to the land
authorities. Upon the execution of a land use rights grant contract and payment of the land grant fee, owners of land use rights will
be issued a State-owned land use certificate, which sets forth, among other things: (i) the nature (granted or allocated); (ii) designated
purpose; (iii) term of the land use rights; (iv) the location and area of the land; and (v) whether the land use rights are subject to
any security interest. This certificate is the primary evidence of legal and valid land use rights.
Overview of relevant PRC Laws and Regulations
on Buildings
It is required under the PRC law to obtain
relevant permits from different authorities before commencing the construction of a building. The required permits are, inter alia, a
State-owned Land Use Certificate, a Planning Permit of Land for Construction Use, a Planning Permit of Construction Project, and a Commencement
Permit of Construction Project (except for those projects where the construction investment is less than RMB 300,000 or the construction
area is less than 300 square meters). After the completion of construction, the owner shall also apply at relevant authorities for inspection
and acceptance of the construction project and then obtain a Certificate for Completion Acceptance of Construction Project as well as
a Title Certificate for Building. Further, pursuant to relevant PRC laws and regulations, the premises title certificate is the only
legal certificate by which the owner legally has the ownership in respect of the building and thereby exercises rights to possess, utilize,
profit from and dispose of the premises. Without such certificate, it is not permitted to transfer the premises.
According to the Urban and Rural Planning
Law of the PRC, if a rural construction planning permit is not obtained in accordance with the law or construction is not carried out
in accordance with the provisions of the rural construction planning permit, the township or town people’s government shall order
the construction to stop and make corrections within a time limit.
Not all the buildings of Jiangsu Yada and
Jiangsu Huadong attached on the land have appropriate title certificates. The buildings not granted title certificate are at a risk of
being dismantled or other administrative penalties if they are identified as illegal buildings due to the violation of the PRC Land Administration
Law, the PRC Law on Urban and Rural Planning, and other relevant laws and regulations.
Regulation and Classification of Land Allocation
According to the PRC Land Administration Law,
the State legally adopts the system of compensation for the use of land owned by the State, except where the State allocates the right
to use state-owned land within the bounds of the law; A construction project developer utilizing state-owned land shall generally obtain
the use right of state owned land through paid means such as granting for compensation. The following categories of land may be directly
allocated with the lawful approval of the people’s governments at or above the county level: (1) land for use by government institutions
or the military; (2) land for urban infrastructure or public welfare projects; (3) land for energy, transportation. and water conservancy
projects as well as other infrastructure projects supported by the government; and (4) other land as provided for by laws or administrative
regulations. In addition, according to the Provisions on the Economical and Intensive Use of Land (promulgated by Order No.61 of the
Ministry of Natural Resources on May 22, 2014 and amended in accordance with the Decision of the Ministry of Natural Resources on the
First Group of Repealed and Amended Departmental Rules adopted at the 2nd executive meeting of the Ministry of Natural Resources on July
16, 2019), except that land for military use, affordable housing, or other special purposes such as national security or public order
may be supplied without consideration by means of allocation, payment is required for land used for business purposes, including land
used for office space of state authorities, transportation, energy, or water conservancy and other infrastructure (industry), urban infrastructure
and various social undertakings; the land user and land prices for commercial use shall be determined by means of bidding, auction, or
listing. The acquisition and use of allocated land by enterprises shall comply with the special restrictions as prescribed by laws and
regulations.
Pursuant to Interim Regulations of the People’s
Republic of China Concerning the Assignment and Transfer of the Right to the Use of the State-owned Land in the Urban Areas, promulgated
by the State Council and amended on November 29, 2020, the allocated right to the use of the land may not be transferred, leased, or
mortgaged, with the exception of cases as specified in following cases and subject to the approval of the land administration departments
and the housing administration departments under the people’s governments at the municipal and county levels: (i) the land users
are companies, enterprises, or other economic organizations, or individuals; (ii) a certificate for the use of state-owned land had been
obtained; (iii) possessing legitimate certificates of property rights to the above-ground buildings and other attached objects; and (iv)
a contract for assigning the right to the use of land is signed in accordance with the regulations and the land user makes up for the
payment of the assignment fee to the local municipal or county people’s government or uses the proceeds resulting from the transfer,
lease or mortgage to pay the assignment fee. Any units or individuals that transfer, lease or mortgage the allocated right to the use
of the land without authorization shall have their illegal incomes thus secured confiscated by the land administration departments under
the people’s governments at the municipal and county levels and shall be fined in accordance with the seriousness of the case.
Property, Plants and Equipment
Our PRC headquarters, manufacturing facilities
and office spaces are located in Yangzhou, Jiangsu Province, PRC.
Land Use Rights We Obtained
We obtained the following land use rights
for the construction of our headquarters, manufacturing facilities and office spaces, which cover an aggregate lot area of approximately
383,172 square feet (equivalent to 35,597.84 square meters), with the breakdown of land use set forth in the table below:
| Land
User |
|
Land
Use Type |
|
Description/Use |
|
Location |
|
Lot
Area
(Square Meters) |
|
| Yangzhou Huada |
|
Allocation |
|
Industrial land |
|
Tongda Road, Touqiao Town, Hanjiang
District, Yangzhou |
|
|
6,700.24 |
|
| Jiangsu Yada |
|
Assignment |
|
Industrial land |
|
Xinqiao Village, Touqiao Town, Guangling District,
Yangzhou |
|
|
15,991.00 |
|
| Jiangsu Huadong |
|
Allocation |
|
Industrial land |
|
No.88 Tongda Road, Touqiao Town, Guangling District,
Yangzhou |
|
|
11,717.44 |
|
| Jiangsu Yada |
|
Allocation |
|
Industrial land |
|
Tongda Road, Touqiao Town, Hanjiang District, Yangzhou |
|
|
1,189.16 |
|
| Total |
|
|
|
|
|
|
|
|
35,597.84 |
|
Properties We Own
We own the premises of our headquarters, manufacturing
facilities and office spaces, which cover an aggregate building area of approximately 246,538 square feet (equivalent to 22,904.12 square
meters), with the breakdown of square footage set forth in the table below:
| Description/Use |
|
Owner |
|
Location |
|
Area
(Square Meters) |
| Manufacturing
facility |
|
Jiangsu Yada |
|
No.58 Yada Road,
Touqiao Town |
|
Land Lot Area 15,991.00/
Building Area 3,545.09 (Floors 1-4) |
| Manufacturing
facility |
|
Jiangsu Yada |
|
No.58 Yada Road,
Touqiao Town |
|
Land Lot Area 15,991.00/
Building Area 394.62 (Floor 1) |
| Manufacturing
facility |
|
Jiangsu Yada |
|
No.58 Yada Road,
Touqiao Town |
|
Land Lot Area 15,991.00/
Building Area 2,412.30 |
| Manufacturing
facility |
|
Jiangsu Yada |
|
No.58 Yada Road,
Touqiao Town |
|
Land Lot Area 15,991.00/
Building Area 428.79 (Floor 1) |
| Manufacturing
facility |
|
Yangzhou Huada |
|
No.1 East Tongda
Road, Touqiao Town |
|
Land Lot Area 6,700.24/
Building Area 2,109.77 (Floors 1-2) |
| Office
space & manufacturing facility |
|
Yangzhou Huada |
|
No.2,3,4 East Tongda
Road, Touqiao Town |
|
Land Lot Area 6,700.24
464.2 (Floor 1);1,224.45 (Floors 1-2);1,005.73 (Floor 1) |
| Manufacturing
facility |
|
Jiangsu
Yada |
|
No.1
Zhu Group, Xuzhuang, Datong Village, Touqiao Town |
|
3,023.2 |
| Manufacturing
facility |
|
Jiangsu Huadong |
|
No.88 Tongda Road,
Touqiao Town |
|
Land Lot Area 11717.44/
Building Area 3,709.93 (Floors 1-2) |
| Manufacturing
facility |
|
Jiangsu Huadong |
|
No.88 Tongda Road,
Touqiao Town |
|
Land
Lot Area 11717.44/ Building Area 4,586.04 (Floors 1-2) |
| Total |
|
|
|
|
|
22,904.12 |
Properties We Lease
In addition to the above-mentioned properties
that we own, we currently lease several properties in Yangzhou for an aggregate area of approximately 85,241 square feet (equivalent
to 7,919 square meters) from our PRC subsidiaries for processing shops and office space. All leases are subject to renewal upon approval
of the lessors, subject to the lessor receiving renewal requests at least three months in advance. Presently, the leases have been renewed
through 2024.
The breakdown of the leased properties is
as follows:
Lessor/Rental
Cost per month |
|
Lessee |
|
Location |
|
Area
(Square
Meter) |
|
Annual
Rent |
|
Term |
|
Use |
|
| Jiangsu
Huadong |
|
Yangzhou
Huada |
|
No.88
Tongda Road, Guangling District, Yangzhou |
|
670 |
|
$5,828
(RMB40,200.00) |
|
January
1, 2023 to December 31, 2024 |
|
Processing
Workshop |
|
| Jiangsu
Yada |
|
Yangzhou
Huada |
|
No.88
Tongda Road, Guangling District, Yangzhou |
|
20 |
|
$174
(RMB1,200.00) |
|
January
1, 2023 to December 31, 2024 |
|
Office |
|
| Jiangsu
Huadong |
|
Jiangsu
Yada |
|
No.88
Tongda Road, Guangling District, Yangzhou |
|
1,100 |
|
$9,569
(RMB66,000.00) |
|
January 1, 2023 to December 31, 2024 |
|
Processing
Workshop |
|
| Yangzhou
Huada |
|
Jiangsu
Huadong |
|
No.88
Tongda Road, Guangling District, Yangzhou |
|
4,804.15 |
|
$41,792
(RMB288,249.00) |
|
January
1, 2023 to December 31, 2024 |
|
Processing
Workshop |
|
| Jiangsu
Yada |
|
Jiangsu
Huadong |
|
No.88
Tongda Road, Guangling District, Yangzhou |
|
1,325.00 |
|
$11,526
(RMB79,500.00) |
|
January
1, 2023 to December 31, 2024 |
|
Office |
|
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITIONS AND RESULTS OF OPERATIONS
The following discussion of our financial
condition and results of operations is based upon and should be read in conjunction with our consolidated financial statements and their
related notes included in this prospectus. This Management’s Discussion and Analysis of Financial Conditions and Results of Operations
contains forward-looking statements. In evaluating our business, you should carefully consider the information provided under the caption
“Risk Factors” as well as “Corporate History and Structure” and “Business” included elsewhere in
this prospectus. We caution you that our businesses and financial performance of the Company are subject to substantial risks and uncertainties.
A. Operating Results
Business Overview
Meihua International Medical Technologies
Co., Ltd., through its operating subsidiaries, is mainly engaged in the manufacture, research and development and sales of Class I, II
and III medical devices. It has a history of more than 30 years and has multiple product categories, with more than 800 domestic products
and more than 120 export products. The main product lines include disposable infusion pumps, anesthesia puncture kits, electronic pumps,
full anesthesia kits, urethral catheterization kits, gynecological examination kits, endotracheal intubation, dressing applications and
various tubes. It is the leading enterprise in China’s medical consumables industry. The Company has received qualification to
manufacture and produce China’s first, second and third type of medical device consumables and, at the same time, the Company has
acquired FDA registration and the European Union’s CE certification. Relevant permissions have been obtained in major sales markets
to meet local regulatory requirements.
The Company’s distribution network covers
major global markets. Internationally, the Company mainly exports medical devices through exporting distributors. To date, the Company
has 334 exporting distributors responsible for distributing its products to end users in Europe, North America, Asia, South America,
Africa, and Oceania. In the Chinese market, the Company sells products under its own brand to customers all over the country. The Company’s
product permeation for mainland China has reached major medical institutions and pharmacies through some 3,159 distributors. At the same
time, the Company has established a cooperative network with more than 531 hospitals through its own direct sales channels.
Revenues decreased by $6.6 million, or approximately
12.1%, to $48.2 million in the six months ended June 30, 2023 from $54.8 million in the six months ended June 30, 2022. The decrease
was mainly due to a decline in demand for customer orders.
Net income increased by $0.5 million, or approximately
7.3%, to $7.0 million in the six months ended June 30, 2023 from $6.6 million in the six months ended June 30, 2022. The increase was
mainly due to decrease in operating expenses in the six months ended June 30, 2023.
Recent Developments
On August 24, 2023,
the Company filed with the U.S. Securities and Exchange Commission (the “SEC”) a registration statement on Form F-3 (the
“Form F-3”) under the Securities Act of 1933, as amended (the “Securities Act”), utilizing a “shelf”
registration process through which the company may offer and selling, from time to time, up to $100,000,000 of the Company’s ordinary
shares, par value $0.0005 per share, debt securities, warrants, rights, and units, or any combination thereof, together or separately
as described in the prospectus.
On January 2, 2024,
the Company sold $6,000,000 in convertible notes to two accredited investors in a primary offering pursuant to a “shelf take down”
off the Form F-3. Concurrently, the Company sold warrants to purchase 1,255,205 ordinary shares, which were sold pursuant to an exemption
from registration under Rule 506 of the Securities Act of 1933, as amended. The convertible note and warrant offering are described under
“Description of the Securities We Are Offering” at page 53 above.
Coronavirus (COVID-19) Update
On January 30, 2020, the World Health Organization
declared the outbreak of the corona-virus disease (COVID-19) a “Public Health Emergency of International Concern,” and on
March 11, 2020, the World Health Organization characterized the outbreak as a “pandemic.” Governments in affected countries
have imposed travel bans, quarantines and other emergency public health measures, which have caused material disruption to businesses
globally and result in an economic slowdown. These measures, though temporary in nature, may continue and increase depending on developments
in the COVID-19’s outbreak.
In fiscal year 2020, COVID-19 had a significant
impact on our business and results of operations as the sales volume of masks rose sharply, while at the same time sales of products
other than masks declined due to a decrease in market demand. In fiscal year 2021, as circumstances surrounding the COVID-19 epidemic
stabilized within the PRC, the market for masks was no longer in urgent shortage compared to the same period in 2020, and the production
of other epidemic prevention products resumed normal production levels. In general, with the precise control of the epidemic in the PRC,
our production and operations recovered smoothly, and the demand for other products has increased gradually.
After the initial outbreak of COVID-19, from
time to time, some instances of COVID-19 infections have emerged in various regions of China, including the infections caused by the
Omicron variants in 2022. For example, a wave of infections caused by the Omicron variants emerged in Shanghai in 2022, and a series
of restrictions and quarantines were implemented to contain the spread.
Many of the restrictive measures previously
adopted by the PRC governments at various levels to control the spread of the COVID-19 virus have been revoked or replaced with more
flexible measures since December 2022. While the revocation or replacement of the restrictive measures to contain the COVID-19 pandemic
could have a positive impact on our normal operations, the extent of the impact on the Company’s future financial results will
be dependent on future developments such as the length and severity of the crisis, the potential resurgence of the crisis, future government
actions in response to the crisis and the overall impact of the COVID-19 pandemic on the global economy and capital markets, among many
other factors, all of which remain highly uncertain and unpredictable. Given this uncertainty, the Company is currently unable to quantify
the expected impact of the COVID-19 pandemic on its future operations, financial condition, liquidity and results of operations if the
current situation continues.
In preparing these consolidated financial
statements, the Company has evaluated events and transactions for potential recognition or disclosure through six months ended June 30,
2023, the date the consolidated financial statements were available to be issued. No events require additional adjustment to or disclosure
in the consolidated financial statements.
Results of Operations for the Six Months Ended June 30, 2023
and 2022
The following table sets forth a summary of
our consolidated results of operations for the periods presented. This information should be read together with our consolidated financial
statements and related notes included elsewhere in this prospectus. The results of operations in any period are not necessarily indicative
of our future trends.
(Amounts expressed in U.S. dollars, except
share data and per share data, or otherwise noted)
For the Six months Ended June 30,
2023 and 2022
| | |
For the Six months
Ended June
30, | |
| | |
2023 | | |
2022 | |
| Revenues | |
| | |
| |
| Third party sales | |
$ | 48,178,325 | | |
$ | 54,803,181 | |
| Related party sales | |
| 11,751 | | |
| 29,666 | |
| Total revenues | |
| 48,190,076 | | |
| 54,832,847 | |
| Cost of revenues | |
| 31,019,347 | | |
| 33,941,115 | |
| | |
| | | |
| | |
| Gross profit | |
| 17,170,729 | | |
| 20,891,732 | |
| | |
| | | |
| | |
| Operating expenses | |
| | | |
| | |
| Selling | |
| 3,161,070 | | |
| 3,311,649 | |
| General and administrative | |
| 3,452,610 | | |
| 4,799,711 | |
| Research and development | |
| 1,460,376 | | |
| 1,642,204 | |
| Written-off Tai He deposit | |
| - | | |
| 2,469,466 | |
| Total operating expenses | |
| 8,074,056 | | |
| 12,223,030 | |
| | |
| | | |
| | |
| Income from operations | |
| 9,096,673 | | |
| 8,668,702 | |
| | |
| | | |
| | |
| Other (income) expense: | |
| | | |
| | |
| Interest expense | |
| 128,973 | | |
| 98,805 | |
| Interest income | |
| (361,532 | ) | |
| (19,725 | ) |
| Currency exchange (gain) loss | |
| 119,193 | | |
| (449,217 | ) |
| Other expense, net | |
| 114,298 | | |
| 50,180 | |
| Total other (income) expenses | |
| 932 | | |
| (319,957 | ) |
| | |
| | | |
| | |
| Income before income tax provision | |
| 9,095,741 | | |
| 8,988,659 | |
| Income taxes expense | |
| 2,064,212 | | |
| 2,433,772 | |
| Net income | |
$ | 7,031,529 | | |
$ | 6,554,887 | |
For the Six months Ended June 30, 2023
and 2022
Revenues
Revenues decreased by $6.6 million, or approximately
12.1%, to $48.2 million for the six months ended June 30, 2023 from $54.8 million in the six months ended June 30, 2022. The decrease
was mainly due to a decline in demand for customer orders.
Cost of revenues
Cost of revenues primarily include cost of
materials, direct labor costs, overhead, and other related incidental expenses that are directly attributable to the Company’s
principal operations. Cost of revenues decreased by $2.9 million, or approximately 8.6%, to $31.0 million in the six months ended June
30, 2023 from $33.9 million in the six months ended June 30, 2022. The decrease was generally in line with decrease in revenue except
some fixed cost such as lease expense and salary of administrative employees in production department.
Gross profit margin
The following table sets forth the overall
gross profit margin of the Company:
| | |
For the Six months
Ended June 30 | |
| | |
2023 | | |
2022 | |
| Revenues | |
$ | 48,190,076 | | |
$ | 54,832,847 | |
| Costs of revenues | |
| 31,019,347 | | |
| 33,941,115 | |
| Gross profit | |
$ | 17,170,729 | | |
$ | 20,891,732 | |
| Gross profit margin % | |
| 35.6 | % | |
| 38.1 | % |
Gross profit decreased by $3.7 million, or
approximately 17.8%, to $17.2 million in the six months ended June 30, 2023 from $20.9 million in the six months ended June 30, 2022.
Gross profit margin decreased from 38.1% in the six months ended June 30, 2022 to 35.6% in the six months ended June 30, 2023 due to
some fixed cost did not decrease proportionately with revenue.
Operating costs and expenses
Our operating costs and expenses consist of
selling expenses, general and administrative expenses and research and development expenses.
Selling
The following table sets forth a breakdown
of the selling expenses of the Company:
For the Six months Ended June 30,2023
and 2022
| | |
2023 | | |
2022 | |
| Transportation expenses | |
$ | 1,137,936 | | |
$ | 1,404,106 | |
| Salaries and benefits | |
| 698,086 | | |
| 787,279 | |
| Entertainment expenses | |
| 500,485 | | |
| 427,447 | |
| Conference expenses | |
| 493,850 | | |
| 415,750 | |
| Travel allowance | |
| 177,807 | | |
| 159,228 | |
| Auto expenses | |
| 118,629 | | |
| 101,031 | |
| Advertising expenses | |
| 8,275 | | |
| 7,603 | |
| Other expenses | |
| 26,002 | | |
| 9,205 | |
| Total | |
$ | 3,161,070 | | |
$ | 3,311,649 | |
Selling expenses decreased by $0.1 million,
or approximately 4.5%, to $3.2 million in the six months ended June 30, 2023 from $3.3 million in the six months ended June 30, 2022.
The decrease was mainly attributable to the combined effects of the followings:
| |
(a) |
Our conference expenses
increased by $78,100, or approximately 18.8% to $0.5 million in the six months ended June 30, 2023 from $0.4 million in the six months
ended June 30, 2022. Conference expenses are mainly related to the company’s market expansion, business development, business
negotiation, medical expo, and exhibition affairs. These expenditures helped the Company promote its products, develop markets and
channels, strengthen customer communication, and establish long-term and stable cooperative relations; |
| |
(b) |
Our transportation expenses
decreased by $0.3 million, or approximately 19.0%, to $1.1 million in the six months ended June 30, 2023 from $1.4 million in the
six months ended June 30, 2022. The reduction in business travel was due to a decline in demand for customer orders. |
| |
(c) |
Our salaries and benefits
expenses decreased by $89,193 or approximately 11.3%, to $0.7 million in the six months ended June 30, 2023 from $0.8 million in
the six months ended June 30, 2022. The decrease was due to a decrease in the salary and benefits of the sales team, which was in
line with revenue decrease. |
| |
(d) |
Our entertainment expenses
increased by $73,038, or approximately 17.1%, to $0.5 million in the six months ended June 30, 2023 from $0.4 million in the six
months ended June 30, 2022. The increase was mainly attributable to new customer acquisition efforts. |
General and administrative
General and administrative expenses primarily
consist of the following expenses:
For the Six months Ended June 30,
2023 and 2022
| | |
2023 | | |
2022 | |
| Salaries and benefits | |
$ | 738,077 | | |
$ | 600,682 | |
| Entertainment expenses | |
| 606,820 | | |
| 481,334 | |
| Conference fee | |
| 374,022 | | |
| 311,786 | |
| Auto expenses | |
| 113,810 | | |
| 83,363 | |
| Maintenance expenses | |
| 50,199 | | |
| 65,278 | |
| Depreciation expenses | |
| 45,951 | | |
| 16,793 | |
| Travel allowance | |
| 72,283 | | |
| 63,044 | |
| Office expenses | |
| 45,664 | | |
| 50,288 | |
| Surtax expenses | |
| 302,376 | | |
| 338,190 | |
| Amortization expenses | |
| 19,696 | | |
| 13,416 | |
| Rental expenses | |
| 7,299 | | |
| 7,805 | |
| Insurance expenses | |
| 118,000 | | |
| 50,336 | |
| Service expenses | |
| 726,302 | | |
| 2,496,058 | |
| Other expenses | |
| 232,111 | | |
| 221,338 | |
| Total | |
$ | 3,452,610 | | |
$ | 4,799,711 | |
General and administrative expenses decreased
by $1.3 million, or approximately 28.1%, to $3.5 million in the six months ended June 30, 2023 from $4.8 million in the six months ended
June 30, 2022. The decrease was primarily due to (a) service expenses decreasing by approximately $1.8 million from approximately $2.5
million in the six months ended June 30, 2022 to approximately $0.7 million in the six months ended June 30, 2023, offset by (b) salaries
and benefits increasing by approximately $137,000 from approximately $601,000 in the six months ended June 30, 2022 to approximately
$738,000 in the six months ended June 30, 2023, and (c) entertainment expenses increasing by $125,000 or 26.1% from approximately $481,000
in the six months ended June 30, 2022 to approximately $607,000 in the six months ended June 30, 2023
Research and development
The following table sets forth a breakdown
of the research and development expenses of the Company:
For the Six months Ended June 30,2023
and 2022
| |
|
2023 |
|
|
2022 |
|
| Sample manufacturing expenses |
|
$ |
676,283 |
|
|
$ |
850,532 |
|
| Salaries and benefits |
|
|
564,114 |
|
|
|
586,062 |
|
| Travel allowance |
|
|
58,886 |
|
|
|
64,201 |
|
| Depreciation expenses |
|
|
5,113 |
|
|
|
3,993 |
|
| Design expenses |
|
|
38,985 |
|
|
|
52,337 |
|
| Material expenses |
|
|
13,495 |
|
|
|
11,943 |
|
| Other expenses |
|
|
103,500 |
|
|
|
73,136 |
|
| Total |
|
$ |
1,460,376 |
|
|
$ |
1,642,204 |
|
The research and development expenses decreased
by $0.2 million, or approximately 11.1%, to $1.5 million in the six months ended June 30, 2023, from $1.6 million in the six months ended
June 30, 2022. The decrease was mainly due to decrease in sample manufacturing expenses.
Income from operations
As a result of the factors described above,
our income from operations increased by $0.4 million, or approximately 4.9%, to $9.1 million in the six months ended June 30, 2023 from
$8.7 million in the six months ended June 30, 2022.
Income tax expense
The provision for income taxes decreased by
$0.4 million, or approximately 15.2%, to $2.1 million in the six months ended June 30, 2023 from $2.4 million in the six months ended
June 30, 2022. The decrease was mainly due to the decrease of taxable income for the six months ended June 30, 2023.
Net income
As a result of the factors described above,
our net income increased by $0.5 million, or approximately 7.3%, to $7.0 million in the six months ended June 30, 2023 from $6.6 million
in the six months ended June 30, 2022.
Unrealized foreign currency translation
adjustment
The Company’s reporting currency is
the United States dollar (“US$”). The Company’s operations are primarily conducted through the PRC subsidiaries where
the local currency is the functional currency. The functional currency of Kang Fu HK is the Hong Kong dollar and the functional currency
of Huada, Yada, Huadong and Guanghui is the Renminbi (“RMB”). Results of operations and cash flows are translated at average
exchange rates during the period, assets and liabilities are translated at the unified exchange rate at the end of the period and equity
is translated at historical exchange rates. Translation adjustments resulting from the process of translating the financial statements
denominated in RMB into U.S. dollars are included in other comprehensive income. Our foreign currency translation loss in the six months
ended June 30, 2023 was $6.3 million, compared to the foreign currency translation loss of $6.1 million in the six months ended June
30, 2022. The change was primarily due to the exchange rate fluctuation of RMB against the U.S. dollars.
B. Liquidity and Capital Resources
Cash Flows and Working Capital
As of June 30, 2023 and 2022, we had cash
of $17.9 million and $34.7 million, respectively. We believe that our current cash, cash to be generated from our operations and access
to capital market will be sufficient to meet our working capital needs for at least the next twelve months. We do not have any amounts
committed to be provided by our related party. We are also not dependent upon future financing to meet our liquidity needs for the next
twelve months. In order to implement our growth strategies, we plan to expand our business. With additional capacity, and varied product
offerings, the Company will provide tailored “one-stop” services from wound care, to surgical auxiliary supplies, to disease
prevention. To do so, we may need more capital through equity financing to expand our production and meet market demands.
Substantially all of our operations are conducted
in China and all of our revenues, expense and cash are denominated in RMB. RMB is subject to the exchange control regulation in China,
and, as a result, we may have difficulty in distributing any dividends outside of China due to PRC exchange control regulations which
restrict the ability to convert RMB into U.S. Dollars.
Under applicable PRC regulations, foreign-invested
enterprises in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards
and regulations. In addition, a foreign-invested enterprise in China is required to set aside at least 10% of its after-tax profit every
year as its general reserves based on PRC accounting standards until the accumulative amount of such reserves reaches 50% of its registered
capital. These reserves can’t be distributed as cash dividends. The board of directors of a foreign-invested enterprise has the
discretion to allocate a portion of its after-tax profits to staff welfare and bonus funds, which can’t be distributed to equity
owners except liquidation. Under PRC law, RMB can be converted into U.S. Dollars under the company’s “current account”
(including dividends, trade and service-related foreign exchange transactions) rather than the “capital account” (including
foreign direct investments and loans, without the prior approval of the SAFE).
For retained earnings accrued after such date,
the board of directors will declare dividends after taking into account our operations, earnings, financial condition, the demand for
cash and availability and other relevant factors. Any declaration, payment and amount of dividends should be subject to our By-laws,
charter and applicable Chinese and U.S. state and federal laws and regulations, including the approval from the shareholders of each
subsidiary which intends to declare such dividends, if applicable.
We have limited financial obligations dominated
in U.S. dollars, thus the foreign currency restrictions and regulations in PRC on the dividends distribution will not have a material
impact on the liquidity, financial condition and results of operations of our Company.
Cash Flow Summary
For the Six months Ended June 30, 2023 and 2022
| | |
2023 | | |
2022 | |
| Net Cash Used in Operating Activities | |
$ | (5,424,569 | ) | |
$ | (7,798,816 | ) |
| | |
| | | |
| | |
| Net Cash Used in Investing Activities | |
| (3,866,151 | ) | |
| (459,163 | ) |
| | |
| | | |
| | |
| Net Cash Provided by Financing Activities | |
| 721,677 | | |
| 35,144,846 | |
| | |
| | | |
| | |
| Effect of Exchange Rate Changes on Cash | |
| (306,443 | ) | |
| (370,615 | ) |
| | |
| | | |
| | |
| Cash at Beginning of Year | |
| 26,736,700 | | |
| 8,149,276 | |
| | |
| | | |
| | |
| Cash at End of Year | |
$ | 17,861,214 | | |
$ | 34,665,528 | |
For the Six months Ended June 30, 2023 and 2022
Cash Flow in Operating Activities
Net cash used in operating activities was
$5.4 million in the six months ended June 30, 2023, primarily comprised of net income of approximately $7.0 million and adjusted for
non-cash items approximately $0.5 million, increase in accounts receivable of approximately $14.1 million, decrease of bank acceptance
receivable of approximately $2.8 million, decrease of accounts payable of approximately $1.6 million, increase in inventory of approximately
$0.6 million.
Net cash used in operating activities was
$7.8 million in the six months ended June 30, 2022, primarily comprised of net income of approximately $6.6 million and adjusted for
non-cash items approximately $2.4 million, decrease in accounts receivable of approximately $3.5 million, increase of bank acceptance
receivable of approximately $9.7 million, decrease of accounts payable of approximately $4.9 million, increase in prepayments and other
assets of approximately $5.3 million.
Cash Flow in Investing Activities
Net cash used in investing activities was
$3.9 million in the six months ended June 30, 2023. It consisted of purchases of property and equipment of $1.0 million, intangible assets
of $3.6 million, proceeds from disposal of fixed assets of $0.4 million, and proceeds from disposal of long-term investment of $0.4 million.
Net cash used in investing activities was
$0.5 million in the six months ended June 30, 2022. It consisted of purchases of property and equipment.
Cash Flow in Financing Activities
For the six months ended June 30, 2023, the
Company had net cash provided by financing activities of $0.7 million, which consisted of the proceeds from short-term bank loans of
$5.3 million, and repayments of short-term bank loans of $4.6 million.
For the six months ended June 30, 2022, the
Company had net cash provided by financing activities of $35.1 million, which consisted of the proceeds from short-term bank loans of
$3.5 million, and repayments of short-term bank loans of $2.9 million.
Off-Balance Sheet Arrangements
As of June 30, 2023 and December 31, 2022,
there were no off-balance sheet arrangements
C. Research and Development, Patents and Licenses, etc.
Please see “Business Overview—Intellectual
Property,” at page 84 above.
D. Trend Information
Other than as disclosed elsewhere in this
prospectus, we are not aware of any trends, uncertainties, demands, commitments or events for the six months ended June 30, 2023 that
are reasonably likely to have a material adverse effect on our net revenues, income, profitability, liquidity or capital resources, or
that would cause the disclosed financial information to be not necessarily indicative of future operating results or financial conditions.
E. Critical Accounting Estimates
Our discussion and analysis of our financial
condition and results of operations are based upon our unaudited condensed consolidated financial statements. These financial statements
are prepared in accordance with U.S. GAAP, which requires us to make estimates and assumptions that affect the reported amounts of our
assets and liabilities and revenue and expenses, to disclose contingent assets and liabilities on the date of the unaudited condensed
consolidated financial statements, and to disclose the reported amounts of revenue and expenses incurred during the financial reporting
period. The most significant estimates and assumptions include allowance for bad debts and the valuation of inventory. We continue to
evaluate these estimates and assumptions that we believe to be reasonable under the circumstances. We rely on these evaluations as the
basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Since
the use of estimates is an integral component of the financial reporting process, actual results could differ from those estimates. Some
of our accounting policies require higher degrees of judgment than others in their application. We believe critical accounting policies
as disclosed in this report reflect the more significant judgments and estimates used in preparation of our consolidated financial statements.
We believe there have been no material changes to our critical accounting policies and estimates.
Accounts
Receivable and Allowance for Doubtful Accounts
Accounts receivable represent trade receivables
and are recognized initially at fair value and subsequently adjusted for any allowance for doubtful accounts or impairment.
The Company records impairment losses for
accounts receivable based on assessments of the recoverability of the trade and other receivables and individual account analysis, including
the current creditworthiness and the past collection history of each debtor. Impairments arise when there is objective evidence indicating
that the balances may not be collectible. The identification of bad and doubtful debts, in particular of a loss event, requires the use
of judgment and estimates, which involve the estimates of specific losses on individual exposures, as well as a provision on historical
trends of collections. Based on management of customers’ credit and ongoing relationship, management makes conclusions whether
any balances outstanding at the end of the period will be deemed non-collectible on an individual basis and on aging analysis basis.
The provision is recorded against accounts receivables balances, with a corresponding charge recorded in the consolidated statements
of operations and comprehensive income. Delinquent account balances are written-off against the allowance for doubtful accounts after
management has determined that the likelihood of collection is not probable.
Inventories
Inventories are valued using the lower of
cost or net realizable value. Cost is principally determined using the weighted-average method. The Company records adjustments to inventory
for excess quantities, obsolescence, or impairment, when appropriate, to reflect inventory at net realizable value. These adjustments
are based upon a combination of factors including current sales volume, market conditions, lower of cost or market analysis and expected
realizable value of the inventory.
Years Ended December 31, 2022, 2021
and 2020
| |
|
For
the Year Ended December 31, |
|
| |
|
2022 |
|
|
2021 |
|
|
2020 |
|
| Revenues |
|
|
|
|
|
|
|
|
|
| Third party
sales |
|
$ |
103,317,145 |
|
|
$ |
103,461,809 |
|
|
$ |
88,244,403 |
|
| Related party sales |
|
|
29,196 |
|
|
|
575,901 |
|
|
|
816,607 |
|
| Total revenues |
|
|
103,346,341 |
|
|
|
104,037,710 |
|
|
|
89,061,010 |
|
| Cost of revenues |
|
|
65,247,864 |
|
|
|
64,232,469 |
|
|
|
51,900,823 |
|
| Gross profit |
|
|
38,098,477 |
|
|
|
39,805,241 |
|
|
|
37,160,187 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
| Selling |
|
|
7,109,524 |
|
|
|
6,457,801 |
|
|
|
6,624,332 |
|
| General and administrative |
|
|
12,468,551 |
|
|
|
4,361,472 |
|
|
|
4,577,570 |
|
| Research and development |
|
|
2,962,904 |
|
|
|
2,725,014 |
|
|
|
2,492,059 |
|
| Written-off Tai He deposit |
|
|
4,755,536 |
|
|
|
- |
|
|
|
- |
|
| Total operating costs and expenses |
|
|
27,296,515 |
|
|
|
13,544,287 |
|
|
|
13,693,961 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Income from operations |
|
|
10,801,962 |
|
|
|
26,260,954 |
|
|
|
23,466,226 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Other (income) expense: |
|
|
|
|
|
|
|
|
|
|
|
|
| Interest expense |
|
|
194,667 |
|
|
|
180,744 |
|
|
|
137,160 |
|
| Interest income |
|
|
(63,725 |
) |
|
|
(23,855 |
|
|
|
(36,583 |
) |
| Currency exchange gain |
|
|
(273,432 |
) |
|
|
(174,413 |
) |
|
|
(393,478 |
) |
| Other expense, net |
|
|
53,205 |
|
|
|
50,437 |
|
|
|
25,551 |
|
| Total other (income) expenses |
|
|
(89,285 |
) |
|
|
32,913 |
|
|
|
(267,350 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Income before income tax provision |
|
|
10,891,247 |
|
|
|
26,228,041 |
|
|
|
23,733,576 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Income taxes expense |
|
|
4,713,543 |
|
|
|
5,278,462 |
|
|
|
4,688,321 |
|
| Net income |
|
$ |
6,177,704 |
|
|
$ |
20,949,579 |
|
|
$ |
19,045,255 |
|
Year ended December 31, 2022 compared
to year ended December 31, 2021
Revenues
Revenues decreased by $691,369, or approximately
1%, to $103,346,341 for the year ended December 31, 2022 from $104,037,710 for the year ended December 31, 2021. The decrease was mainly
due to a decline in demand for customer orders.
Cost of revenues
Cost of revenues primarily include cost of
materials, direct labor costs, overhead, and other related incidental expenses that are directly attributable to the Company’s
principal operations. Cost of revenues increased by $1,015,395, or approximately 2%, to $65,247,864 for the year ended December 31, 2022
from $64,232,469 for the year ended December 31, 2021. The increase was mainly due to an increase in the price of materials.
Gross profit margin
The following table sets forth the overall
gross profit margin of the Company:
| | |
For the Year Ended
December,
31 | |
| | |
2022 | | |
2021 | |
| Revenues | |
$ | 103,346,341 | | |
$ | 104,037,710 | |
| Costs of revenues | |
| 65,247,864 | | |
| 64,232,469 | |
| Gross profit | |
$ | 38,098,477 | | |
$ | 39,805,241 | |
| Gross profit margin % | |
| 37 | % | |
| 38 | % |
Gross profit decreased by $1,706,764, or approximately
4%, to $38,098,477 for the year ended December 31, 2022 from $39,805,241 for the year ended December 31, 2021. Gross profit margin decreased
from 38% for the year ended December 31, 2021 to 37% for the year ended December 31, 2022 due to an increase in the cost of materials.
Operating costs and expenses
Our operating costs and expenses consist of
selling expenses, general and administrative expenses and research and development expenses.
Selling
The following table sets forth a breakdown
of the selling expenses of the Company:
Years Ended December 31, 2022 and
2021
| |
|
2022 |
|
|
2021 |
|
| Transportation expenses |
|
$ |
2,585,485 |
|
|
$ |
2,802,680 |
|
| Salaries and benefits |
|
|
1,468,247 |
|
|
|
1,287,436 |
|
| Entertainment expenses |
|
|
1,178,064 |
|
|
|
786,226 |
|
| Conference expenses |
|
|
1,125,353 |
|
|
|
774,298 |
|
| Travel allowance |
|
|
343,439 |
|
|
|
341,165 |
|
| Auto expenses |
|
|
243,706 |
|
|
|
168,832 |
|
| Advertising expenses |
|
|
95,432 |
|
|
|
21,498 |
|
| Other expenses |
|
|
69,798 |
|
|
|
275,666 |
|
| Total |
|
$ |
7,109,524 |
|
|
$ |
6,457,801 |
|
Selling expenses increased by $651,723, or
approximately 10%, to $7,109,524 for the year ended December 31, 2022 from $6,457,801 for the year ended December 31, 2021. The increase
was mainly attributable to the combined effects of the followings:
| |
(a) |
Our conference expenses
increased by $351,055 to $1,125,353 for the year ended December 31, 2022 from $774,298 for the year ended December 31, 2021. Conference
expenses are mainly related to the company’s market expansion, business development, business negotiation, medical expo, and
exhibition affairs. These expenditures helped the Company promote its products, develop markets and channels, strengthen customer
communication, and establish long-term and stable cooperative relations; |
| |
(b) |
Our transportation expenses
decreased by $217,195, or approximately 8%, to $2,585,485 for the year ended December 31, 2022 from $2,802,680 for the year ended
December 31, 2021. The reduction in business travel was impacted by COVID-19 related controls implemented by local governments
in fiscal year 2022; |
| |
(c) |
Our salaries and benefits
expenses increased by $180,811 or approximately 14%, to $1,468,247 for the year ended December 31, 2022 from $1,287,436 for the year
ended December 31, 2021. The increase was mainly attributable to the fact that the COVID-19 pandemic in China had been largely brought
under control and the local government cancelling the related social insurance relief policy. |
| |
(d) |
Our entertainment expenses
increased by $391,838, or approximately 50%, to $1,178,064 for the year ended December 31, 2022 from $786,226 for the year ended
December 31, 2021. The increase was mainly attributable to the Company’s business expansion and new customer acquisition. |
General and administrative
General and administrative expenses primarily
consist of the following expenses:
Years Ended December 31, 2022 and
2021
| |
|
2022 |
|
|
2021 |
|
| Salaries and benefits |
|
$ |
1,362,803 |
|
|
$ |
1,227,228 |
|
| Entertainment expenses |
|
|
1,230,556 |
|
|
|
816,196 |
|
| Conference fee |
|
|
846,374 |
|
|
|
510,267 |
|
| Auto expenses |
|
|
226,763 |
|
|
|
153,911 |
|
| Maintenance expenses |
|
|
129,425 |
|
|
|
126,650 |
|
| Depreciation expenses |
|
|
104,295 |
|
|
|
147,522 |
|
| Travel allowance |
|
|
131,872 |
|
|
|
118,113 |
|
| Office expenses |
|
|
102,417 |
|
|
|
94,686 |
|
| Surtax expenses |
|
|
642,748 |
|
|
|
655,679 |
|
| Amortization expenses |
|
|
22,196 |
|
|
|
26,951 |
|
| Rental expenses |
|
|
7,515 |
|
|
|
20,349 |
|
| Insurance expenses |
|
|
108,146 |
|
|
|
9,743 |
|
| Service expenses |
|
|
7,200,839 |
|
|
|
183,647 |
|
| Other expenses |
|
|
352,602 |
|
|
|
270,530 |
|
| Total |
|
$ |
12,468,551 |
|
|
$ |
4,361,472 |
|
General and administrative expenses increased
by $8,107,079, or approximately 186%, to $12,468,551 for the year ended December 31, 2022, from $4,361,472 for the year ended December
31, 2021. The increase was primarily due to (a) service expenses increasing by approximately $7.0 million from approximately $0.2 million
for the year ended December 31, 2021 to approximately $7.2 million for the year ended December 31, 2022, (b) entertainment expenses increasing
by $414,360 or 51% from approximately $0.8 million for the year ended December 31, 2021 to approximately $1.2 million for the year ended
December 31, 2022, (c) conference fee expenses increasing by approximately $0.3 million from approximately $0.5 million for the year
ended December 31, 2021 to approximately $0.8 million for the year ended December 31, 2022, and (d) salaries and benefits increasing
by approximately $0.1 million from approximately $1.2 million for the year ended December 31, 2021 to approximately $1.4 million for
the year ended December 31, 2022.
Research and Development
The following table sets forth a breakdown
of the research and development expenses of the Company:
Years Ended December 31, 2022 and
2021
| |
|
2022 |
|
|
2021 |
|
| Sample manufacturing expenses |
|
$ |
1,392,350 |
|
|
$ |
1,205,437 |
|
| Salaries and benefits |
|
|
1,161,696 |
|
|
|
1,093,717 |
|
| Travel allowance |
|
|
123,373 |
|
|
|
116,266 |
|
| Depreciation expenses |
|
|
8,309 |
|
|
|
8,161 |
|
| Design expenses |
|
|
98,068 |
|
|
|
110,265 |
|
| Material expenses |
|
|
27,371 |
|
|
|
46,103 |
|
| Other expenses |
|
|
151,737 |
|
|
|
145,065 |
|
| Total |
|
$ |
2,962,904 |
|
|
$ |
2,725,014 |
|
The research and development expenses increased
by $237,890, or approximately 9%, to $2,962,904 for the year ended December 31, 2022, from $2,725,014 for the year ended December 31,
2021. The increase was mainly due to the increase in salaries and benefits expenses which was mainly attributable to that the pandemic
in China had been brought under control and the local government had canceled the social insurance relief policy.
Income from operations
As a result of the factors described above,
our income from operations decreased by $15,458,992, or approximately 59%, to $10,801,962 for the year ended December 31, 2022 from $26,260,954
for the year ended December 31, 2021.
Income tax expense
The provision for income taxes decreased by
$564,919, or approximately 11%, to $4,713,543 for the year ended December 31, 2022, from $5,278,462 for the year ended December 31, 2021.
The decrease was mainly due to the decrease of profit before provision for income taxes in 2021.
Net income
As a result of the factors described above,
our net income decreased by $14,771,875, or approximately 71%, to $6,177,704 for the fiscal year ended December 31, 2022 from $20,949,579
for the fiscal year ended December 31, 2021.
Unrealized foreign currency translation
adjustment
The Company’s reporting currency is
the United States dollar (“US$”). The Company’s operations are primarily conducted through the PRC subsidiaries where
the local currency is the functional currency. The functional currency of Kang Fu HK is the Hong Kong dollar and the functional currency
of Huada, Yada, Huadong and Guanghui is the Renminbi (“RMB”). Results of operations and cash flows are translated at average
exchange rates during the period, assets and liabilities are translated at the unified exchange rate at the end of the period and equity
is translated at historical exchange rates. Translation adjustments resulting from the process of translating the financial statements
denominated in RMB into U.S. dollars are included in other comprehensive income. Our foreign currency translation loss for the fiscal
year ended December 31, 2022 was $9,155,028, compared to the foreign currency translation gain of $2,083,243 for the fiscal year ended
December 31, 2021. The change was primarily due to the exchange rate fluctuation of RMB against the U.S. dollars.
Year ended December 31, 2021 compared
to year ended December 31, 2020
Revenues
Revenues increased by $14,976,700, or approximately
17%, to $104,037,710 for the year ended December 31, 2021 from $89,061,010 for the year ended December 31, 2020. The increase was mainly
due to the Company’s business expansion, research and development of new products and development of new customers.
Cost of revenues
Cost of revenues primarily include cost of
materials, direct labors, overhead, and other related incidental expenses that are directly attributable to the Company’s principal
operations. Cost of revenue increased by $12,331,646, or approximately 24%, to $64,232,469 for the year ended December 31, 2021 from
$51,900,823 for the year ended December 31, 2020. The increase was mainly attributable to an overall increase in sales during fiscal
year 2021.
Gross profit margin
The following table sets forth the overall
gross profit margin of the Company:
| |
|
For
the Year Ended
December, 31 |
|
| |
|
2021 |
|
|
2020 |
|
| Revenues |
|
$ |
104,037,710 |
|
|
$ |
89,061,010 |
|
| Costs of revenues |
|
|
64,232,469 |
|
|
|
51,900,823 |
|
| Gross profit |
|
$ |
39,805,241 |
|
|
$ |
37,160,187 |
|
| Gross profit margin % |
|
|
38 |
% |
|
|
42 |
% |
Gross profit increased by $2,645,054, or approximately
7%, to $39,805,241 for the year ended December 31, 2021 from $37,160,187 for the year ended December 31, 2020. Gross profit margin decreased
from 42% for the year ended December 31, 2020 to 38% for the year ended December 31, 2021. The higher gross profit margin in fiscal 2020
was mainly attributable to much higher selling price and gross margin for medical masks as a result of COVID-19 in fiscal year 2020.
The price of medical mask fell back to normal level as a result of increased supply in fiscal 2021, which brought down the overall gross
profit margin.
Operating costs and expenses
Our operating costs and expenses consist of
selling expenses, general and administrative expenses and research and development expenses.
Selling
The following table sets forth a breakdown
of the selling expenses of the Company:
Years Ended December 31, 2021 and
2020
| | |
2021 | | |
2020 | |
| Transportation expenses | |
$ | 2,802,680 | | |
$ | 2,473,655 | |
| Salaries and benefits | |
| 1,287,436 | | |
| 1,110,441 | |
| Entertainment expenses | |
| 786,226 | | |
| 869,591 | |
| Conference expenses | |
| 774,298 | | |
| 805,832 | |
| Travel allowance | |
| 341,165 | | |
| 266,518 | |
| Auto expenses | |
| 168,832 | | |
| 191,860 | |
| Advertising expenses | |
| 21,498 | | |
| 53,770 | |
| Market research | |
| - | | |
| 579,357 | |
| Other expenses | |
| 275,666 | | |
| 273,308 | |
| Total | |
$ | 6,457,801 | | |
$ | 6,624,332 | |
Selling expenses decreased by $166,531, or
approximately 3%, to $6,457,801 for the year ended December 31, 2021 from $6,624,332 for the year ended December 31, 2020. The decrease
was mainly attributable to the combined effects of the followings:
| |
(a) |
Our market research
expenses decreased by $579,357 to nil for the year ended December 31, 2021 from $579,357 for the year ended December 31, 2020. The
decrease was mainly attributable to that the market research conducted in fiscal year 2020 were still valid in fiscal year 2021 and
therefore did not conduct additional market research in fiscal 2021. |
| |
(b) |
Our transportation expenses
increased by $329,025, or approximately 13%, to $2,802,680 for the year ended December 31, 2021 from $2,473,655 for the year ended
December 31, 2020. The increase was mainly attributable to the increased in sales in fiscal year 2021. |
| |
(c) |
Our salaries and benefits
expenses increased by $176,995 or approximately 16%, to $1,287,436 for the year ended December 31, 2021 from $1,110,441 for the year
ended December 31, 2020. The increase was mainly attributable to that the Covid-19 pandemic in China had been brought under control
and the local government had canceled the social insurance relief policy. |
| |
(d) |
Our entertainment expenses
decreased by $83,365, or approximately 10%, to $786,226 for the year ended December 31, 2021 from $869,591 for the year ended December
31, 2020. The decrease was mainly attributable to that Yangzhou City in PRC had been under lockdown for some period due to the epidemic
in fiscal 2021, so the relevant business entertainment expenses were reduced. |
General and administrative
General and administrative expenses primarily
consist of the following expenses:
Years Ended December 31, 2021 and
2020
| |
|
2021 |
|
|
2020 |
|
| Salaries and benefits |
|
$ |
1,227,228 |
|
|
$ |
1,062,041 |
|
| Entertainment expenses |
|
|
816,196 |
|
|
|
998,613 |
|
| Conference fee |
|
|
510,267 |
|
|
|
595,217 |
|
| Auto expense |
|
|
153,911 |
|
|
|
192,148 |
|
| Maintenance expenses |
|
|
126,650 |
|
|
|
153,896 |
|
| Depreciation expenses |
|
|
147,522 |
|
|
|
127,174 |
|
| Travel allowance |
|
|
118,113 |
|
|
|
80,179 |
|
| Office expenses |
|
|
94,686 |
|
|
|
115,204 |
|
| Surtax expenses |
|
|
655,679 |
|
|
|
557,522 |
|
| Amortization expenses |
|
|
26,951 |
|
|
|
26,195 |
|
| Rental expenses |
|
|
20,349 |
|
|
|
19,013 |
|
| Insurance expenses |
|
|
9,743 |
|
|
|
8,444 |
|
| Service expenses |
|
|
183,647 |
|
|
|
426,461 |
|
| Other expenses |
|
|
270,530 |
|
|
|
215,463 |
|
| Total |
|
$ |
4,361,472 |
|
|
$ |
4,577,570 |
|
General and administrative expenses decreased
by $216,098, or approximately 5%, to $4,361,472 for the year ended December 31, 2021, from $4,577,570 for the year ended December 31,
2020. The decrease was mainly due to a decrease of $0.2 million service expenses in fiscal year 2021.
Research and development
The following table sets forth a breakdown
of the research and development expenses of the Company:
Years Ended December 31, 2021 and
2020
| |
|
2021 |
|
|
2020 |
|
| Sample manufacturing expenses |
|
$ |
1,205,437 |
|
|
$ |
1,114,789 |
|
| Salaries and benefits |
|
|
1,093,717 |
|
|
|
931,569 |
|
| Travel allowance |
|
|
116,266 |
|
|
|
105,236 |
|
| Depreciation expenses |
|
|
8,161 |
|
|
|
5,642 |
|
| Design expenses |
|
|
110,265 |
|
|
|
125,199 |
|
| Material expenses |
|
|
46,103 |
|
|
|
50,869 |
|
| Other expenses |
|
|
145,065 |
|
|
|
158,755 |
|
| Total |
|
$ |
2,725,014 |
|
|
$ |
2,492,059 |
|
Research and development expenses increased
by $232,955, or approximately 9%, to $2,725,014 for the year ended December 31, 2021, from $2,492,059 for the year ended December 31,
2020. The increase was mainly due to the increase in salaries and benefits expenses which was mainly attributable to that the pandemic
in China had been brought under control and the local government had canceled the social insurance relief policy.
Income from operations
As a result of the factors described above,
our income from operations increased by $2,794,728, or approximately 12%, to $26,260,954 for the year ended December 31, 2021 from $23,466,226
for the year ended December 31, 2020.
Income tax expense
Provision for income taxes increased by $590,141,
or approximately 13%, to $5,278,462 for the year ended December 31, 2021, from $4,688,321 for the year ended December 31, 2020. The increase
was mainly due to the increase of profit before provision for income taxes in 2021.
Net income
As a result of the factors described above,
our net income increased by $1,904,324, or approximately 10%, to $20,949,579 for the fiscal year ended December 31, 2021 from $19,045,255
for the fiscal year ended December 31, 2020.
B. Liquidity and Capital Resources
Cash Flows and Working Capital
As of December 31, 2022 and 2021, we had cash
of $26,736,700, and $8,149,276, respectively. We believe that our current cash, cash to be generated from our operations and access to
capital market will be sufficient to meet our working capital needs for at least the next twelve months. We do not have any amounts committed
to be provided by our related party. We are also not dependent upon future financing to meet our liquidity needs for the next twelve
months. In order to implement our growth strategies, we plan to expand our business. With additional capacity, and varied product offerings,
the Company will provide tailored “one-stop” services from wound care, to surgical auxiliary supplies, to disease prevention.
To do so, we may need more capital through equity financing to expand our production and meet market demands.
Substantially all of our operations are conducted
in China and all of our revenues, expense and cash are denominated in RMB. RMB is subject to the exchange control regulation in China,
and, as a result, we may have difficulty in distributing any dividends outside of China due to PRC exchange control regulations which
restrict the ability to convert RMB into U.S. Dollars.
Under applicable PRC regulations, foreign-invested
enterprises in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards
and regulations. In addition, a foreign-invested enterprise in China is required to set aside at least 10% of its after-tax profit every
year as its general reserves based on PRC accounting standards until the accumulative amount of such reserves reaches 50% of its registered
capital. These reserves can’t be distributed as cash dividends. The board of directors of a foreign-invested enterprise has the
discretion to allocate a portion of its after-tax profits to staff welfare and bonus funds, which can’t be distributed to equity
owners except liquidation. Under PRC law, RMB can be converted into U.S. Dollars under the company’s “current account”
(including dividends, trade and service-related foreign exchange transactions) rather than the “capital account” (including
foreign direct investments and loans, without the prior approval of the SAFE).
For retained earnings accrued after such date,
the board of directors will declare dividends after taking into account our operations, earnings, financial condition, the demand for
cash and availability and other relevant factors. Any declaration, payment and amount of dividends should be subject to our By-laws,
charter and applicable Chinese and U.S. state and federal laws and regulations, including the approval from the shareholders of each
subsidiary which intends to declare such dividends, if applicable.
We have limited financial obligations dominated
in U.S. dollars, thus the foreign currency restrictions and regulations in PRC on the dividends distribution will not have a material
impact on the liquidity, financial condition and results of operations of our Company.
Cash Flow Summary
Years Ended December 31, 2022, 2021 and 2020
| | |
2022 | | |
2021 | | |
2020 | |
| Net Cash (Used in) Provided by Operating Activities | |
$ | (9,163,038 | ) | |
$ | (54,663 | ) | |
$ | 5,325,996 | |
| | |
| | | |
| | | |
| | |
| Net Cash Used in Investing Activities | |
| (8,620,292 | ) | |
| (833,817 | ) | |
| (16,094,404 | ) |
| | |
| | | |
| | | |
| | |
| Net Cash Provided by Financing Activities | |
| 37,245,500 | | |
| 1,860,234 | | |
| 1,706,750 | |
| | |
| | | |
| | | |
| | |
| Effect of Exchange Rate Changes on Cash | |
| (874,746 | ) | |
| (9,812 | ) | |
| 218,137 | |
| | |
| | | |
| | | |
| | |
| Cash at Beginning of Year | |
| 8,149,276 | | |
| 7,187,334 | | |
| 16,030,855 | |
| | |
| | | |
| | | |
| | |
| Cash at End of Year | |
$ | 26,736,700 | | |
$ | 8,149,276 | | |
$ | 7,187,334 | |
We had a balance of cash of $26,736,700, $8,149,276
and $7,187,334, respectively as of December 31, 2022, 2021, and 2020.
Years Ended December 31, 2022 and
2021
Cash Flow in Operating Activities
Net cash used in operating activities was
$9,163,038 for the year ended December 31, 2022, compared to net cash used in operating activities of $54,663 for the year ended December
31, 2021, representing a $9,108,375, or a 16,663% increase, in the net cash inflow used in operating activities. The increase in net
cash used in operating activities was primarily due to the following:
| a) | Change
in bank acceptance receivables was approximately $4.3 million net cash outflow for the year
ended December 31, 2022. For the year ended December 31, 2021, change in bank acceptance
receivables was approximately $6.5 million net cash outflow, which led to approximately $2.2
million decrease in net cash outflow from operating activities; |
| |
b) |
Change in accounts receivable
was approximately $7.1 million net cash outflow for the year ended December 31, 2022. For the year ended December 31, 2021, change
in accounts receivable was approximately $20.1 million net cash outflow, which led to approximately $12.9 million decrease in net
cash outflow from operating activities; |
| |
c) |
Change in accounts payables
was approximately $3.4 million net cash inflow for the year ended December 31, 2022. For the year ended December 31, 2021, change
in accounts payables was approximately $4.9 million net cash outflow, which led to approximately $8.3 million increase in net cash
inflow from operating activities; |
| |
|
|
| |
d) |
Change in taxes payable
was approximately $0.8 million net cash inflow for the year ended December 31, 2022. For the year ended December 31, 2021, change
in taxes payable was approximately $0.3 million net cash outflow, which let to approximately $1.1 million increase in net cash inflow
from operating activities; |
| |
e) |
Write-off of the Tai
He deposit resulted in approximately $4.8 million and $nil net cash outflow for the year ended December 31, 2022 and 2021, respectively,
which led to an approximately $4.8 million increase in net cash outflow from operating activities. During the IPO, the Company entered
into a series of agreements (the “Agreements”) with Tai He International Group Limited (“Tai He”), a Hong
Kong investment company. Pursuant to the Agreements, Tai He agreed to invest a minimum of $35 million in the IPO subject to the Company
making a $7.0 million refundable deposit and advancing a $3.0 million service fee for investor relations and other services payable
to Tai He. The Company paid an approximately $4.8 million deposit and $2.3 million service fee to Tai He in 2022. The Company’s
affiliates and individual shareholders paid the rest of the agreed amount. Later the Company learned that Tai He did not invest in
the IPO or provide services to the Company related to the IPO. The Company is now proactively working to terminate the Tai He Agreements
and recover the amount paid to Tai He by the Company. Due to the uncertainty of collection, the Company has written off the approximately
$4.8 million deposit and fully expensed the $2.3 million service fee paid by the Company to Tai He in the year ended December 31,
2022. |
| |
|
|
| |
f) |
Net cash used in operating
activities for the year ended December 31, 2022 consisted of net income of approximately $6.2 million, noncash adjustments of approximately
$5.1 million. Net cash used in operating activities for the year ended December 31, 2021 consisted of net income of approximately
$20.9 million, noncash adjustments of approximately $0.6 million |
Cash Flow in Investing Activities
Net cash used in investing activities was
$8,620,292 for the year ended December 31, 2022. It consisted of purchases of property and equipment of $2,698,729, long term investment
acquisition of $5,944,709 and proceeds from disposal of fixed assets of $23,146.
Net cash used in investing activities was
$833,817 for the year ended December 31, 2021. It consisted of purchases of property and equipment of $850,231 and proceeds from disposal
of fixed assets of $16,414.
Cash Flow in Financing Activities
For the year ended December 31, 2022, the
Company had net cash provided by financing activities of $37,245,500, which consisted of the proceeds from short-term bank loans of $7,579,135,
repayments of short-term bank loans of $6,241,641, proceeds from long-term bank loans of $743,052, capital contributed by non-controlling
shareholders of $635,310 and proceeds from IPO of $34,529,644.
For the year ended December 31, 2021, the
Company had net cash provided by financing activities of $1,860,234, which consisted of the proceeds from short-term bank loans of $6,510,820,
and repayments of short-term bank loans of $4,650,586.
Years Ended December 31, 2021 and
2020
Cash Flow in Operating Activities
Net cash used in operating activities was
$54,663 for the year ended December 31, 2021, compared to net cash generated from operating activities of $5,325,996 for the year ended
December 31, 2020, representing a $5,380,659, or a 101% decrease, in the net cash inflow generated from operating activities. The decrease
in net cash generated from operating activities was primarily due to the following:
| |
a) |
Change in bank acceptance
receivables was approximately $6.5 million net cash outflow for the year ended December 31, 2021. For the year ended December 31,
2020, change in bank acceptance receivables was approximately $5.8 million net cash outflow, which led to approximately $0.8 million
increase in net cash outflow from operating activities; |
| |
b) |
Change in accounts receivable
was approximately $20.1 million net cash outflow for the year ended December 31, 2021. For the year ended December 31, 2020, change
in accounts receivable was approximately $13.6 million net cash outflow, which led to approximately $6.5 million increase in net
cash outflow from operating activities; |
| |
c) |
Change in due from related
party was approximately $0.4 million net cash inflow for the year ended December 31, 2021. For the year ended December 31, 2020,
change in due from related party was approximately $0.2 million net cash outflow, which led to approximately $0.6 million increase
in net cash inflow from operating activities; |
| |
|
|
| |
d) |
Change in inventories
was approximately $0.1 million net cash inflow for the year ended December 31, 2021. For the year ended December 31, 2020, change
in inventory was approximately $0.2 million net cash outflow, which let to approximately $0.3 million decrease in net cash outflow
from operating activities; and |
| |
e) |
Change in deferred IPO
costs and other current assets was approximately $0.9 million net cash outflow for the year ended December 31, 2021. For the year
ended December 31, 2020, change in deferred IPO costs and other current assets was approximately $0.4 million net cash outflow, which
led to approximately $0.5 million increase in net cash outflow from operating activities. |
Cash Flow in Investing Activities
Net cash used in investing activities was
$833,817 for the year ended December 31, 2021. It consisted of purchases of property and equipment of $850,231 and proceeds from disposal
of fixed assets of $16,414.
Net cash used in investing activities was
$16,094,404 for the year ended December 31, 2020. It consisted of payments for long-term deposits for building of $12,311,347, purchases
of property and equipment of $3,808,259 and proceeds from disposal of fixed assets of $25,202.
Cash Flow in Financing Activities
For the year ended December 31, 2021, the
Company had net cash provided by financing activities of $1,860,234, which consisted of the proceeds from short-term bank loans of $6,510,820,
and repayments of short-term bank loans of $4,650,586.
For the year ended December 31, 2020, the
Company had net cash provided by financing activities of $1,706,750, which consisted of the proceeds from short-term bank loans of $4,359,665,
the proceeds from subscriptions receivable of $1,272,232, and repayments of short-term bank loans of $3,925,147.
Off-Balance Sheet Arrangements
As of December 31, 2022 and 2021, there were
no off-balance sheet arrangements.
C. Research and Development, Patents and Licenses, etc.
Please see “Business
Overview—Intellectual Property,” above.
D. Trend Information
Other than as disclosed elsewhere in this
prospectus, we are not aware of any trends, uncertainties, demands, commitments or events for the year ended December 31, 2022 that are
reasonably likely to have a material adverse effect on our net revenues, income, profitability, liquidity or capital resources, or that
would cause the disclosed financial information to be not necessarily indicative of future operating results or financial conditions.
E. Critical Accounting Estimates
Our discussion and analysis of our financial
condition and results of operations are based upon our consolidated financial statements. These financial statements are prepared in
accordance with U.S. GAAP, which requires us to make estimates and assumptions that affect the reported amounts of our assets and liabilities
and revenue and expenses, to disclose contingent assets and liabilities on the date of the consolidated financial statements, and to
disclose the reported amounts of revenue and expenses incurred during the financial reporting period. The most significant estimates
and assumptions include the collection of accounts receivable, the useful lives and impairment of our long-lived assets, and the provisions
for income taxes. We continue to evaluate these estimates and assumptions that we believe to be reasonable under the circumstances. We
rely on these evaluations as the basis for making judgments about the carrying values of assets and liabilities that are not readily
apparent from other sources. Since the use of estimates is an integral component of the financial reporting process, actual results could
differ from those estimates. Some of our accounting policies require higher degrees of judgment than others in their application. We
believe critical accounting policies as disclosed in this report reflect the more significant judgments and estimates used in preparation
of our consolidated financial statements. We believe there have been no material changes to our critical accounting policies and estimates.
A full list of our critical accounting estimates
is included in Note 2 of our consolidated financial statements included elsewhere in this report.
Use
of Estimates
The preparation of consolidated financial
statements in conformity with U.S. GAAP requires management to make certain estimates and assumptions that affect the amounts reported
and disclosed in the consolidated financial statements and related notes.
The most significant estimates and judgments
include allowance for bad debts, the valuation of inventory, useful life of property, plant and equipment and income taxes related to
realization of deferred tax assets and uncertain tax position. Actual amounts could differ from those estimates.
Fair
Value Measurement
Fair value is the price that would be received
from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company
considers the principal or most advantageous market in which it would transact, and it considers assumptions that market participants
would use when pricing the asset or liability.
The Company adopted the guidance of Accounting
Standards Codification (“ASC”) 820 for fair value measurements which clarifies the definition of fair value, prescribes methods
for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
| |
Level 1: |
Inputs
are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| |
|
|
| |
Level 2: |
Inputs
are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets
and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated
by observable market data. |
| |
|
|
| |
Level 3: |
Inputs
are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would
use in pricing the asset or liability based on the best available information. |
Cash
Cash consists of petty cash on hand and cash
held in banks, which are highly liquid and are unrestricted as to withdrawal or use.
Bank
Acceptance Receivables
Bank acceptance receivables are issued by
bank under the request of the Company’s customers, to pay for the purchased goods. The Company can choose to hold acceptance notes
until maturity and receive the face value payment from the bank, or sell (exchange) the acceptance notes at a discount to another party
willing to wait until maturity to receive the bank’s promised payment. The maturity date of the receivables is all within one year
of the original issuance date and carried at face value. The Company is not lending money, it just sells goods to the customers (customers
can pay the purchased goods by cash, accounts receivable or bank acceptance receivables). The receivables mature within one year and
are non-interest bearing. As bank acceptance receivables are issued by the banks, payments are guaranteed.
Accounts
Receivable and Allowance for Doubtful Accounts
Accounts receivable represent trade receivables
and are recognized initially at fair value and subsequently adjusted for any allowance for doubtful accounts or impairment.
The Company records impairment losses for
accounts receivable based on assessments of the recoverability of the trade and other receivables and individual account analysis, including
the current creditworthiness and the past collection history of each debtor. Impairments arise when there is objective evidence indicating
that the balances may not be collectible. The identification of bad and doubtful debts, in particular of a loss event, requires the use
of judgment and estimates, which involve the estimates of specific losses on individual exposures, as well as a provision on historical
trends of collections. Based on management of customers’ credit and ongoing relationship, management makes conclusions whether
any balances outstanding at the end of the period will be deemed non-collectible on an individual basis and on aging analysis basis.
The provision is recorded against accounts receivables balances, with a corresponding charge recorded in the consolidated statements
of operations and comprehensive income. Delinquent account balances are written-off against the allowance for doubtful accounts after
management has determined that the likelihood of collection is not probable.
Inventories
Inventories are valued using the lower of
cost or net realizable value. Cost is principally determined using the weighted-average method. The Company records adjustments to inventory
for excess quantities, obsolescence, or impairment, when appropriate, to reflect inventory at net realizable value. These adjustments
are based upon a combination of factors including current sales volume, market conditions, lower of cost or market analysis and expected
realizable value of the inventory.
DIRECTORS AND SENIOR MANAGEMENT
The following table sets forth certain information
regarding our directors and senior management, as well as employees upon whose work we are dependent, as of the date of this prospectus.
| Directors and Executive
Officers |
|
Age |
|
Position/Title |
| Yongjun Liu |
|
67 |
|
Chairman of the board of directors |
| Xin Wang |
|
35 |
|
Chief Executive Officer and Director |
| Lianzhang Zhao |
|
56 |
|
Chief Financial Officer |
| Xiaoming E |
|
62 |
|
Independent Director |
| Huijuan Zhao |
|
35 |
|
Independent Director |
| Wenzhang Jia |
|
58 |
|
Independent Director |
The following is a brief biography of each of
our executive officers and directors:
Yongjun Liu - Chairman of the board of
directors
Mr. Liu has been Chairman of the board of directors
since November 10, 2020, Chairman and General Manager of Kang Fu International Medical since October 2015, Chairman and General
Manager of Yangzhou Huada since December 2001, Chairman and General Manager of Jiangsu Huadong since November 2000, and Chairman and
General Manager of Jiangsu Yada since December 1990. In October 2015, Mr. Liu co-founded Kang Fu International Medical with several other
co-founders, In December 2001, November 2000 and December 1990, he founded Yangzhou Huada, Jiangsu Huadong and Jiangsu Yada, respectively.
From October 1998 to present, Mr. Liu has been
the Chairman of Yangzhou Medical Device Industry Association and Chamber of Commerce and Industry of Touqiao County. From November 2013
to present, Mr. Liu is the Deputy to The People’s Congress of Guangling district, Yangzhou City. In December 2018, Mr. Liu was
awarded the “Outstanding Entrepreneurs of Guangling District” in Yangzhou. Mr. Liu is a successful entrepreneur with over
40 years’ experience in the medical device industry. Mr. Liu is a successful entrepreneur with over 40 years of experience in the
medical device industry. He has been awarded as Excellent Entrepreneur, Honest Entrepreneur Representative and Medical Device Industry
Representative many times. Mr. Liu participates in philanthropic activities, including public welfare undertakings and has sponsored
various impactful undertakings, such as road reconstruction in towns and villages, donations to the Red Cross Society, reconstruction
of nursing homes, poverty alleviation, and aid for students.
As the founder of the Company and based on his
extensive experience in the medical device industry, we believe that Mr. Liu is qualified to serve as a director.
Xin Wang – Chief Executive Officer
and Director
Mr. Xin Wang has been our Chief Executive Officer
and Director since December 1, 2022. Prior to his appointment as CEO and director of MHUA, from 2021 to 2022, Mr. Wang was CEO of the
Jiangsu Yada. In addition, Mr. Wang served as Vice General Manager of Shanghai New Asia (Group) Co., Ltd., a pharmaceutical company,
where he led production management, market expansion, research and development of proprietary Chinese traditional medicines from 2020
to 2021. From 2018 to 2020, Mr. Wang held a key position at Panda Group, Inc., a franchise restaurant corporation based in California,
where he was in charge of large commercial projects related to expanding its business operations and upgrading its brand strategy in
the U.S. market. In addition, Mr. Wang previously worked as a financial analyst at Morgan Stanley and an area manager at Citibank, N.A.,
thus providing him insight into global investment banking and U.S. financial markets. Mr. Wang obtained his bachelor’s degree from
the University of California Santa Barbara, where he double majored in Financial Mathematics & Statistics Analysis and Computer Science.
He also obtained his master’s degree in Actuarial Science in 2012 from University of California Santa Barbara.
We believe that Mr. Wang is qualified to serve
as our CEO and a director because of his extensive knowledge and management experience in the area of the financial market and his experience
in the pharmaceutical industry.
Lianzhang Zhao– Chief Financial Officer
Mr. Lianzhang Zhao has been our Chief Financial
Officer since December 1, 2022. Prior to his appointment as CFO of MHUA, Mr. Lianzhang Zhao was a Senior Partner at Yangzhou Hanrui Accounting
Firm, a PRC accounting firm, from 2013 to 2022. Prior to that, from 2000 to 2013, Mr. Zhao served as the chief financial officer of Yangzhou
Guolian Garment Factory Co., which is a Sino-U.S. joint venture company in China. Mr. Zhao is a certified public accountant (“CPA”)
in China, allowing him to draw on his diverse financial accounting experience across multiple international markets to support MHUA’s
financial reporting matters. Mr. Zhao received his bachelor’s degree in Financial Accounting and Economic Management in 1990 from
Yangzhou University.
We believe that Mr. Zhao is qualified to serve
as our CFO because of his expertise as a CPA and his extensive knowledge and experience in the financial industry.
Xiaoming E – Independent Director
Mr. E has been a director of our Company since
February 18, 2022. From January 2010 to present, he has served as Chairman and General Manager of Jiangsu Changfeng Medical Industry
Co., Ltd. From May 2004 to present, he has been Vice Chairman of the Yangzhou Guangling District Medical Device Industry Association.
From July 1998 to May 2004, he served as Vice Chairman of Yangzhou Sanitary Product Association. He earned a college degree from Yangzhou
Education College in economic management in March 2004.
We believe that Mr. E is qualified to serve as
a director because of his experience in the medical device industry.
Huijuan Zhao – Independent Director
Ms. Huijuan Zhao has been a director of our Company
since December 1, 2022. Ms. Huijuan Zhao served as a member of the management team at Ping’An Bank, where she oversaw services
for high net wealth clients and risk control practices from 2019 to 2022. In her prior role as head of marketing and risk control operations
at two Jiangsu-based privately owned businesses operating in the business and financial service sectors, positions she held from 2013
to 2019, Ms. Zhao helped build out market expansion and risk control protocols and playbook and played critical supervisory and coaching
roles to the growth and expansion of both businesses. She holds a bachelor’s degree in Financial English from Suzhou University,
which she received in 2012.
We believe that Ms. Zhao is qualified to serve
as director because of her experience in risk control practice. We believe she can provide valuable guidance and oversight to the Company.
Wenzhang Jia – Independent Director
Mr. Wenzhang Jia has been a director of our Company,
and the chairman of our audit committee, since June 28, 2022. Mr. Jia is the chairman and chief financial officer of Jiangsu Xibei Electronic
Network Co., a company that produces and sells wire and cable, cable TV equipment and network communications equipment, a position he
has served in since February of 2000. Mr. Jia received his Bachelor’s degree in finance in 2004 from Yangzhou Institute of Commerce
and Industry and pursued post-graduate studies in business administration at Tsinghua University in 2007. Mr. Jia has been president
of the Yangzhou Electrical Appliance Industry Association since 2007.
As a result of Mr. Jia’s knowledge and
experience in business and finance, we believe Mr. Jia is qualified to serve as a director on our board of directors and will provide
us with valuable oversight and guidance.
Family Relationships
None of our directors or executive officers has
a family relationship as defined in Item 401 of Regulation S-K.
Controlled Company
Mr. Yongjun Liu, our chairman of the board of
directors, currently beneficially owns approximately 63.50% of the aggregate voting power of our outstanding Ordinary Shares, based on
the total shares outstanding as of the date of this prospectus and before exercise of the Warrant Shares being registered herein. As
a result, we are a “controlled company” within the meaning of the Nasdaq listing rules. As a controlled company, we are permitted
to elect to rely on certain exemptions from the obligations to comply with certain corporate governance requirements, including:
| |
● |
the requirement that a majority of the board of directors consist of
independent directors; |
| |
|
|
| |
● |
the requirement that our director nominees be selected or recommended
solely by independent directors; and |
| |
|
|
| |
● |
the requirement that we have a nominating and corporate governance
committee and a compensation committee that are composed entirely of independent directors with a written charter addressing the
purposes and responsibilities of the committees. |
Although we do not intend to rely on the controlled
company exemptions under the Nasdaq listing rules even if we are a controlled company, we could elect to rely on these exemptions in
the future, and if so, you would not have the same protection afforded to shareholders of companies that are subject to all of the corporate
governance requirements of Nasdaq.
Compensation
Set forth below is the compensation paid to our
executive officers and directors during the fiscal year ended December 31, 2023:
| Name | |
2023
Compensation | |
| Yongjun Liu | |
$ | 51,429 | |
| Xin Wang (2) | |
| 67,572 | |
| Lianzhang Zhao (2) | |
| 51,429 | |
| Xiaoming E | |
| 13,715 | |
| Huijuan Zhao (3) | |
| 42,857 | |
| Wenzhang Jia | |
| - | |
Board Practices
Term of Office
Our directors are not subject to a term of office
and hold office until their resignation, death, incapacity or occurrence of an event resulting in disqualification, or until their respective
successors have been duly elected and qualified or until removed from office by ordinary resolution of the Company in accordance with
our articles of association.
Employment Agreement with our Chief Executive
Officer and Chief Financial Officer
Effective December 1, 2022, the Company appointed
Mr. Xin Wang to serve as the Company’s CEO and a director and Mr. Lianzhang Zhao to serve as the Company’s CFO. In conjunction
with his appointment as CEO of MHUA, Mr. Wang entered into a three-year employment agreement with MHUA. In conjunction with his appointment
as CFO of MHUA, Mr. Zhao entered into an employment agreement with MHUA.
Directors Service Contracts
On February 18, 2022, we entered into Director
Service Agreements (the “Agreements”) with each of our independent directors, Xiaoming E, Xu Han (who resigned from his position
as an independent director since November 30, 2022) and Heung Ming Wong (whose service terminated on June 10, 2022). The Agreements each
feature a one-year term with annual renewals. Mr. E and Mr. Han would each receive a monthly stipend of ¥8,000 (approximately $1,265)
per month during their terms of service. Mr. Wong, who served as the Chairman of the Audit Committee of the board of directors, received
a monthly stipend of $2,500 per month during his term of service. In addition, Mr. Wong received options to purchase 1,000 Ordinary Shares
of the Company, at an exercise price of $8.90 per share, representing a value of $10,000, as determined by reference to the Company’s
IPO price. The options were to vest in equal monthly installments over Mr. Wong’s initial year of service and were exercisable
for a term of ten years. Upon annual renewals of his service agreement, Mr. Wong was to receive additional options valued at $10,000,
with the number of options granted to be determined with reference to the volume weighted average price for the Company’s Ordinary
Shares during the thirty (30) trading days preceding the annual renewal date. We entered into a similar agreement with Mr. Jia following
his appointment to the board of directors, and as chair of the Audit Committee, on June 28, 2022, pursuant to which Mr. Jia received
$10,000, or 1,886, of our Ordinary Shares, exercisable at $5.30 per share, which was the closing price of our Ordinary Shares on the
date of grant and which options vest in substantially equal monthly installments over a period of 12 months. Mr. Jia also receive a cash
fee of $2,500 per month during his term of service.
On December 1, 2022, in conjunction with her
appointment as a director, Ms. Zhao entered into a letter agreement with the Company outlining the terms of her service as a director.
Ms. Zhao, who serves as a member of audit committee and the chairwoman of the nominating committee, will receive a monthly stipend of
$2,500 per month during her term of service. In addition, Ms. Zhao will receive stock options to purchase 740 of the Company’s
Ordinary Shares, par value $0.0005 per share, at an exercise price of $13.51. The options will vest and become exercisable in equal monthly
installments over the course of Ms. Zhao’s initial year of service, that is over a 12 month period.
Committees of the Board
We have established and will maintain three committees
under the board of directors: an audit committee, a compensation committee, and a nominating and corporate governance committee. We have
adopted the charter for each of the three committees. Each committee’s members and functions are described below.
Audit Committee. Our audit committee
consists of Wenzhang Jia, Xiaoming E and Huijuan Zhao, and is chaired by Mr. Jia. Wenzhang Jia, Xiaoming E and Huijuan Zhao each satisfies
the “independence” requirements of Rule 5605(c)(2) of the Listing Rules of the Nasdaq Stock Market and meets the independence
standards under Rule 10A-3 under the Exchange Act. We have determined that Wenzhang Jia qualifies as an “audit committee
financial expert.” The audit committee will oversee our accounting and financial reporting processes and the audits of the financial
statements of our company. The audit committee is responsible for, among other things:
| ● | selecting
the independent registered public accounting firm and pre-approving all auditing and non-auditing
services permitted to be performed by the independent registered public accounting firm; |
| ● | reviewing
with the independent registered public accounting firm any audit problems or difficulties
and management’s response; |
| ● | reviewing
and approving all proposed related party transactions, as defined in Item 404 of Regulation S-K
under the Securities Act; |
| ● | discussing
the annual audited financial statements with management and the independent registered public
accounting firm; |
| ● | reviewing
major issues as to the adequacy of our internal controls and any special audit steps adopted
in light of material control deficiencies; |
| ● | annually
reviewing and reassessing the adequacy of our audit committee charter; |
| ● | meeting
separately and periodically with management and the independent registered public accounting
firm; and |
| ● | reporting
regularly to the board of directors. |
Compensation Committee. Our compensation
committee consists of Wenzhang Jia, Xiaoming E and Huijuan Zhao, and is chaired by Huijuan Zhao. The compensation committee will assist
the board of directors in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors
and executive officers. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated
upon. The compensation committee is responsible for, among other things:
| ● | reviewing
the total compensation package for our executive officers and making recommendations to the
board of directors with respect to it; |
| ● | reviewing
the compensation of our non-employee directors and making recommendations to the board of
directors with respect to it; and |
| ● | periodically
reviewing and approving any long-term incentive compensation or equity plans, programs or
similar arrangements, annual bonuses, and employee pension and welfare benefit plans. |
Nominating Committee. Our
nominating committee consists of Wenzhang Jia, Xiaoming E and Huijuan Zhao, and is chaired by Huijuan Zhao. The nominating committee
will assist the board of directors in selecting individuals qualified to become our directors and in determining the composition of the
board of directors and its committees. The nominating committee is responsible for, among other things:
| |
● |
recommending nominees to the board of directors for election or re-election
to the board of directors, or for appointment to fill any vacancy on the board of directors; |
| |
|
|
| |
● |
reviewing annually with the board of directors the current composition
of the board of directors with regards to characteristics such as independence, age, skills, experience and availability of service
to us; |
| |
|
|
| |
● |
selecting and recommending to the board of directors the names of directors
to serve as members of the audit committee and the compensation committee, as well as of the nominating committee itself; and |
| |
● |
monitoring compliance with our code of business conduct and ethics,
including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance. |
Duties of Directors
Under Cayman Islands law, our directors owe fiduciary
duties to our company, including a duty of loyalty, a duty to act honestly, and a duty to act in a manner they consider in good faith
to be in our best interests. Our directors must also exercise their powers only for a proper purpose. Our directors also owe to our company
a duty to exercise the skills they actually possess and such care and diligence that a reasonably prudent person would exercise in comparable
circumstances. In fulfilling their duty of care to us, our directors must ensure compliance with our memorandum and articles of association,
as amended and restated from time to time. We have the right to seek damages against any director who breaches a duty owed to us.
Terms of Directors
Our directors may be elected by resolution of
our board of directors, or by an ordinary resolution of our shareholders. Our directors are not subject to a term of office and hold
office until such time as they are removed from office by ordinary resolution of the shareholders or automatically vacated from office.
A director will automatically cease to be a director if, among other things, the director (i) becomes bankrupt or makes any arrangement
or composition with his creditors; (ii) dies; (iii) is found by our company to be or becomes of unsound mind; or (iv) resigns
his office by notice in writing to the company.
Code of Business Conduct and Ethics
Our board of directors has adopted a code of
business conduct and ethics that applies to our directors, officers and employees. A copy of this code is available on our website. We
intend to disclose on our website any amendments to the Code of Business Conduct and Ethics and any waivers of the Code of Business Conduct
and Ethics that apply to our principal executive officer, principal financial officer, principal accounting officer, controller, or persons
performing similar functions.
Employees
We had 617, 626 and 622 full-time employees as
of December 31, 2023, 2022 and 2021, respectively. The following table sets forth the number of full-time employees based on category
of employment and place of employment as of December 31, 2023:
| Function/Department | |
Yangzhou
Huada | | |
Jiangsu
Huadong | | |
Jiangsu
Yada | |
| Management | |
| 12 | | |
| 33 | | |
| 39 | |
| Sales and Marketing | |
| 9 | | |
| 36 | | |
| 36 | |
| Research and Development | |
| 0 | | |
| 37 | | |
| 37 | |
| Production | |
| 68 | | |
| 95 | | |
| 220 | |
| Subtotal Total | |
| 89 | | |
| 201 | | |
| 332 | |
| Total | |
| | | |
| 617 | | |
| | |
Our success depends on our ability to attract,
motivate, train and retain qualified personnel. We believe we offer our employees competitive compensation packages and an environment
that encourages self-development and, as a result, have generally been able to attract and retain qualified personnel and maintain a
stable core management team.
As required by laws and regulations in China,
we participate in various employee social security plans that are organized by municipal and provincial governments including, among
other things, pension, medical insurance, unemployment insurance, maternity insurance, on-the-job injury insurance and housing fund plans
through a PRC government-mandated benefit contribution plan. We are required under PRC law to make contributions to employee benefit
plans at specified percentages of the salaries, bonuses and certain allowances of our employees, up to a maximum amount specified by
the local government from time to time.
We believe we maintain a good working relationship
with our employees, and we have not experienced any material labor disputes. None of our employees is represented by a labor union.
Certain
Relationships and Related Party Transactions
Except for the transactions set forth below,
during our preceding three financial years up to the date of this prospectus, there have been no transactions or loans between the company
and (a) enterprises that directly or indirectly through one or more intermediaries, control or are controlled by, or are under common
control with, the company; (b) associates; (c) individuals owning, directly or indirectly, an interest in the voting power of the company
that gives them significant influence over the company, and close members of any such individual’s family; (d) key management personnel,
that is, those persons having authority and responsibility for planning, directing and controlling the activities of the company, including
directors and senior management of companies and close members of such individuals’ families; and (e) enterprises in which a substantial
interest in the voting power is owned, directly or indirectly, by any person described in (c) or (d) or over which such a person is able
to exercise significant influence.
Transaction with Yangzhou Meihua Import
and Export Co., Ltd.
We sell products at market price to Yangzhou
Meihua Import and Export Co., Ltd., an affiliate controlled by Kai Liu, the son of Yongjun Liu, our Chairman and shareholder. For the
fiscal years ended December 31, 2022 and 2021, the sales amount was $18,780 and $51,750, respectively, and as of December 31, 2022 and
December 31, 2021, the amount due from Yangzhou Meihua Import and Export Co., Ltd. both were nil. The main business of Yangzhou Meihua
Import and Export Co., Ltd. is the import and export of medical consumables, and the main types of products it purchases from the Company
are dressing wounds and wiping wounds, Yang Ke Tou (Suction tube), disposable urinary swab, disposable umbilical cord clamp, disposable
suction connection pipe, disposable medical gauze, medical urine cup, medical disinfection dressing surgical kit and round head steel
needles.
Transaction with Yangzhou Yada Powder Metallurgy
Co., Ltd.
We sell products at market price to Yangzhou
Yada Powder Metallurgy Co., Ltd., an affiliate controlled by Kai Liu, the son of Yongjun Liu, our Chairman and shareholder. For the fiscal
years ended December 31, 2022 and 2021, the sales amount was $nil and $347,737, respectively, and as of December 31, 2022 and December
31, 2021, the amount due from Yangzhou Yada Powder Metallurgy Co., Ltd. both were nil. The main business of Yangzhou Yada Powder Metallurgy
Co., Ltd. is gear and metal accessories processing, and the main types of products it purchases from the Company are One-way seat fittings,
Gear 2 accessories, Gear 3 accessories, Steel needle, Crank fittings, Universal joint seat fittings, Cylindrical pin, Axle sleeve, Axle
pin and Bevel gear fittings.
Transaction with Shanghai Xinya Pharmaceutical
Hanjiang Co., Ltd.
We sell products at market price to Shanghai
Xinya Pharmaceutical Hanjiang Co., Ltd., an affiliate controlled by Kai Liu, the son of Yongjun Liu, our Chairman and shareholder. For
the fiscal years ended December 31, 2022 and 2021, the sales amount was $10,416 and $176,414, respectively, and as of December 31, 2022
and December 31, 2021, the amount due from Xinya Pharmaceutical Hanjiang Co., Ltd. both were nil. The main business of Shanghai Xinya
Pharmacy Hanjiang Co., Ltd. is drug production and sales, and the main types of products it purchases from the Company are oral solid
medical high-density polyethylene bottles and disposable masks.
Share Exchange Agreement with Kang Fu International
Medical Co., Ltd. and its Shareholders
On December 21, 2020, we entered into a Share
Exchange Agreement with Kang Fu International Medical Co., Ltd. (“Kang Fu”) and its shareholders, Yongjun Liu (our Chairman
and Director) and Yin Liu (his wife). Under the Share Exchange Agreement, we issued a total of 15,933,000 Ordinary Shares to Mr. and
Mrs. Liu, and in turn acquired 41,400,000 shares (69%) of Kang Fu from Yongjun Liu and 18,600,000 shares (31%) of Kang Fu from Yin Liu,
respectively, resulting in Kang Fu becoming our wholly owned subsidiary. Mr. and Mrs. Liu subsequently transferred the 15,935,000 Ordinary
Shares received in this transaction to their holding company, Bright Accomplish Limited.
CHANGES IN REGISTRANT’S
CERTIFYING ACCOUNTANT
None.
DIVIDEND POLICY
We have not previously declared or paid cash
dividends and we have no plan to declare or pay any dividends in the near future on our Ordinary Shares. We currently intend to retain
most, if not all, of our available funds and any future earnings to operate and expand our business.
We are a holding company incorporated in the
Cayman Islands. We rely principally on dividends from our PRC subsidiaries for our cash requirements, including any payment of dividends
to our shareholders. PRC regulations may restrict the ability of our PRC subsidiaries to pay dividends to us. See “Risk Factors
— Risks Related to Doing Business in China — We may rely on dividends and other distributions on equity paid by our PRC subsidiaries
to fund any cash and financing requirements we may have, and any limitation on the ability of our PRC subsidiaries to make payments to
us could have a material and adverse effect on our ability to conduct our business.”
Our board of directors has discretion as to whether
to distribute dividends, subject to certain restrictions under Cayman Islands law, namely that our company may only pay dividends out
of profits or share premium, and provided always that in no circumstances may a dividend be paid if this would result in our company
being unable to pay its debts as they fall due in the ordinary course of business. In addition, our shareholders may by ordinary resolution
declare a dividend, but no dividend may exceed the amount recommended by our board of directors. Even if our board of directors decides
to pay dividends, the form, frequency, and amount will depend upon our future operations and earnings, capital requirements and surplus,
general financial condition, contractual restrictions and other factors that the board of directors may deem relevant. Please see the
section entitled “Taxation” beginning on page 144 of this prospectus supplement for information on the potential tax consequences
of any cash dividends declared.
DESCRIPTION OF SHARE CAPITAL
We are a Cayman Islands exempted company incorporated
with limited liability and our affairs are governed by our memorandum of association and articles of association, each as amended and
restated from time to time, and the Companies Act (Revised) of the Cayman Islands, which is referred to as the “Companies Act”
below, and the common law of the Cayman Islands.
As
of the date of this prospectus supplement, our Company’s authorized share capital is
US$50,000 divided into: (i) 80,000,000 Ordinary Shares, par value of US$0.0005 per share;
and (ii) 20,000,000 preferred shares, par value US$0.0005 per share. As of the date of this
prospectus supplement, 24,234,673 Ordinary Shares are issued and outstanding and no preferred
shares are issued and outstanding. All of our issued and outstanding Ordinary Shares are
fully paid.
In addition, we have issued Warrants to purchase
1,205,255 ordinary shares, which are being registered herein, and $6,000,000 of 7% OID Convertible Notes, which will convert, from time
to time, at the lower of $2.9869 per share or 95% of the lowest seven-day VWAP, thus causing additional shares to be issued into the
market upon each conversion of the Convertible Notes. The Convertible Notes were registered in a separate primary offering pursuant to
a take down from our effective “shelf” registration statement on Form F-3 (See the section entitled “Concurrent Primary
Offering” above).
Our Memorandum and Articles
The following are summaries of material provisions
of our amended and restated memorandum of association and amended and restated articles of association and of the Companies Act, insofar
as they relate to the material terms of our Ordinary Shares.
Objects of Our Company. Under
our amended and restated memorandum and amended and restated articles of association, the objects of our Company are unrestricted and
we have the full power and authority to carry out any object not prohibited by the law of the Cayman Islands.
Ordinary Shares. Our Ordinary
Shares are issued in registered form and are issued when registered in our register of members. Our shareholders who are non-residents
of the Cayman Islands may freely hold and vote their shares.
Dividends. The holders of
our Ordinary Shares are entitled to such dividends as may be declared by our board of directors. In addition, our shareholders may by
ordinary resolution declare a final dividend, but no dividend may exceed the amount recommended by our directors. Dividends may be declared
and paid out of our profits, realized or unrealized, or from any reserve set aside from profits which our board of directors determine
is no longer needed. Dividends may also be declared and paid out of share premium account or any other fund or account which can be authorized
for this purpose subject to the restrictions of the Companies Act, provided that in no circumstances may we pay a dividend if this would
result in our company being unable to pay its debts as they fall due in the ordinary course of business.
Voting Rights. Any action
required or permitted to be taken by the shareholders must be taken at a duly called and quorate annual or extraordinary general meeting
of the shareholders entitled to vote on such action, or in lieu of a general meeting, be effected by a resolution in writing. On a show
of hands each shareholder is entitled to one vote or, on a poll, each shareholder is entitled to one vote for each Ordinary Share, voting
together as a single class, on all matters that require a shareholder’s vote. Voting at any shareholders’ meeting is by show
of hands unless a poll is demanded. A poll may be demanded by the chairman of such meeting or one or more shareholders present in person
or by proxy entitled to vote and who together hold not less than 10 percent of the paid-up voting share capital for the Company.
A quorum required for a meeting of shareholders
consists of one or more shareholders present and holding at least a majority of the votes of the issued and outstanding voting shares
in our company. Shareholders may be present in person or by proxy or, if the shareholder is a legal entity, by its duly authorized representative.
Shareholders’ meetings may be convened by our board of directors on its own initiative or upon a request to the directors by shareholders
holding no less than 10 percent of our paid voting share capital. Advance notice of at least seven days is required for the convening
of our annual general shareholders’ meeting and any other general shareholders’ meeting.
An ordinary resolution to be passed at a meeting
by the shareholders requires the affirmative vote of a simple majority of the votes attaching to the Ordinary Shares cast at a meeting,
while a special resolution requires the affirmative vote of no less than two-thirds of the votes cast attaching to the outstanding Ordinary
Shares at a meeting. A special resolution will be required for important matters such as a change of name or making changes to our amended
and restated memorandum and articles of association. Holders of the Ordinary Shares may, among other things, divide or combine their
shares by ordinary resolution.
Election of directors. Directors
may be appointed by an ordinary resolution of our shareholder or by a resolution of the directors of the Company.
Meetings of directors. At
any meeting of directors, a quorum will be present if two directors are present, unless otherwise fixed by the directors. If there is
a sole director, that director shall be a quorum. A person who holds office as an alternate director shall be counted in the quorum.
A director who also acts as an alternate director shall be counted twice towards the quorum. An action that may be taken by the directors
at a meeting may also be taken by a resolution of directors consented to in writing by all of the directors.
Transfer of Ordinary Shares. Any
of our shareholders may transfer all or any of his or her Ordinary Shares by an instrument of transfer in the usual or common form or
any other form approved by our board of directors.
Our board of directors may, in its absolute discretion,
decline to register any transfer of any Ordinary Share whether or not it is fully paid up without assigning any reason for doing so.
If our directors refuse to register a transfer
they shall, within two months after the date on which the instrument of transfer was lodged, send to each of the transferor and the transferee
notice of such refusal.
The registration of transfers may be suspended
and the register closed at such times and for such periods as our board of directors may from time to time determine, provided, however,
that the registration of transfers shall not be suspended nor the register closed for more than 45 days in any year as our board may
determine.
Liquidation. On a return of
capital on winding up or otherwise (other than on conversion, redemption or purchase of shares), assets available for distribution among
the holders of Ordinary Shares shall be distributed among the holders of our shares on a pro rata basis. If our assets available for
distribution are insufficient to repay all of the paid-up capital, the assets will be distributed so that the losses are borne by our
shareholders proportionately.
Calls on Shares and Forfeiture of Shares. Our
board of directors may from time to time make calls upon shareholders for any amounts unpaid on their shares in a notice served to such
shareholders at least 14 days prior to the specified time and place of payment. The shares that have been called upon and remain unpaid
are subject to forfeiture.
Redemption of Shares. The
Companies Act and our amended and restated articles of association permit us to purchase, redeem or otherwise acquire our own shares,
subject to certain restrictions and requirements under the Companies Act, our amended and restated memorandum and articles of association
and any applicable requirements imposed from time to time by the Nasdaq, the Securities and Exchange Commission. In accordance with our
articles of association and provided the necessary shareholders or board approval have been obtained, we may issue shares on terms that
are subject to redemption, at our option or at the option of the holders of these shares, on such terms and in such manner, including
out of capital, as may be determined by our board of directors. Under the Companies Act, the repurchase of any share may be paid out
of our company’s profits, out of our share capital account or out of the proceeds of a fresh issue of shares made for the purpose
of such repurchase, or, subject to certain conditions, out of capital. If the repurchase proceeds are paid out of our Company’s
capital, our Company must, immediately following such payment, be able to pay its debts as they fall due in the ordinary course of business.
In addition, under the Companies Act, no such share may be repurchased (1) unless it is fully paid up, (2) if such repurchase would result
in there being no shares outstanding, and (3) unless the manner of purchase (if not so authorized under the amended and restated memorandum
and articles of association) has first been authorized by a resolution of our shareholders. In addition, under the Companies Act, our
Company may accept the surrender of any fully paid share for no consideration unless, as a result of the surrender, the surrender would
result in there being no shares outstanding (other than shares held as treasury shares).
Variations of Rights of Shares. The
rights attached to any class or series of shares (unless otherwise provided by the terms of issue of the shares of that class or series),
whether or not our company is being wound-up, may be varied with the consent in writing of the holders of two-thirds of the issued shares
of that class or series or with the sanction of a special resolution passed at a separate meeting of the holders of the shares of the
class or series. The rights conferred upon the holders of the shares of any class issued shall not, unless otherwise expressly provided
by the terms of issue of the shares of that class, be deemed to be varied by the creation or issue of further shares ranking pari
passu with such existing class of shares.
Changes in the number of shares we are
authorized to issue and those in issue. We may from time to time by resolution of shareholders in the requisite majorities:
| |
● |
increase or decrease the
authorized share capital of our Company; |
| |
● |
subdivide our authorized
and issued shares into a larger number of shares; and |
| |
● |
consolidate our authorized
and issued shares into a smaller number of shares. |
Issuance of Additional Shares. Our
amended and restated memorandum and articles of association authorizes our board of directors to issue additional Ordinary Shares from
time to time as our board of directors shall determine, to the extent of available authorized but unissued shares.
Inspection of Books and Records. Holders
of our Ordinary Shares will have no general right under Cayman Islands law to inspect or obtain copies of our list of shareholders or
our corporate records. However, we will provide our shareholders with annual audited financial statements. See “Where You Can Find
More Information.”
Preferred Shares
As at the date of this prospectus supplement,
we have not issued any preferred shares. Under the amended and restated articles of association, before any preferred shares of any series
are issued, our directors shall fix, by resolution of directors, the following provisions of such series:
| |
● |
the designation of such
series and the number of preferred shares to constitute such series; |
| |
● |
whether the shares of such
series shall have voting rights, in addition to any voting rights provided by Companies Act, and, if so, the terms of such voting
rights, which may be general or limited; |
| |
● |
the dividends, if any,
payable on such series, whether any such dividends shall be cumulative, and, if so, from what dates, the conditions and dates upon
which such dividends shall be payable, the preference or relation which such dividends shall bear to the dividends payable on any
shares of any other class of shares or any other series of preferred shares; |
| |
● |
whether the preferred shares
or such series shall be subject to redemption by the Company, and, if so, the times, prices and other conditions of such redemption; |
| |
● |
the amount or amounts payable
upon preferred shares of such series upon, and the rights of the holders of such series in, a voluntary or involuntary liquidation,
dissolution or winding up, or upon any distribution of the assets, of the Company; |
| |
● |
whether the preferred shares
of such series shall be subject to the operation of a retirement or sinking fund and, if so, the extent to and manner in which any
such retirement or sinking fund shall be applied to the purchase or redemption of the preferred shares of such series for retirement
or other corporate purposes and the terms and provisions relative to the operation of the retirement or sinking fund; |
| |
● |
whether the preferred shares
of such series shall be convertible into, or exchangeable for, shares of any other class of shares or any other series of preferred
shares or any other securities and, if so, the price or prices or the rate or rates of conversion or exchange and the method, if
any, of adjusting the same, and any other terms and conditions of conversion or exchange; |
| |
● |
the limitations and restrictions,
if any, to be effective while any preferred shares or such series are outstanding upon the payment of dividends or the making of
other distributions on, and upon the purchase, redemption or other acquisition by the Company of, the existing shares or shares of
any other class of shares or any other series of preferred shares; |
| |
● |
the conditions or restrictions,
if any, upon the creation of indebtedness of the Company or upon the issue of any additional shares, including additional shares
of such series or of any other class of shares or any other series of preferred shares; and |
| |
● |
any other powers, preferences
and relative, participating, optional and other special rights, and any qualifications, limitations and restrictions of any other
class of shares or any other series of preferred shares. |
Exempted Company
We are an exempted company incorporated with
limited liability under the Companies Act of the Cayman Islands. The Companies Act of the Cayman Islands distinguishes between ordinary
resident companies and exempted companies. Any company that is registered in the Cayman Islands but conducts business mainly outside
of the Cayman Islands may apply to be registered as an exempted company. The requirements for an exempted company are essentially the
same as for an ordinary company except that, for an exempted company that does not hold a license to carry on business in the Cayman
Islands:
| |
● |
an exempted company does
not have to file an annual return of its shareholders with the Registrar of Companies of the Cayman Islands; |
| |
● |
an exempted company’s
register of members is not required to be open to inspection; |
| |
● |
an exempted company does
not have to hold an annual general meeting; |
| |
● |
an exempted company is
prohibited from making any invitation to the public in the Cayman Islands to subscribe for any of its securities; |
| |
● |
an exempted company may
not issue negotiable or bearer shares; |
| |
● |
an exempted company may
obtain an undertaking against the imposition of any future taxation (such undertakings are usually given for 20 years in the
first instance); |
| |
● |
an exempted company may
register by way of continuation in another jurisdiction and be deregistered in the Cayman Islands; |
| |
● |
an exempted company may
register as an exempted limited duration company; and |
| |
● |
an exempted company may
register as a segregated portfolio company. |
“Limited liability” means that the
liability of each shareholder is limited to the amount unpaid by the shareholder on that shareholder’s shares of the company.
We are subject to reporting and other informational
requirements of the Exchange Act, as applicable to foreign private issuers. Except as we have elected to be exempt from the Nasdaq Marketplace
Rule 5635(d), as disclosed elsewhere in this prospectus supplement, we currently intend to comply with the Nasdaq Global Market rules
in lieu of following home country practice.
Differences in Corporate Law
The Companies Act is derived, to a large extent,
from the older Companies Acts of England but does not follow recent United Kingdom statutory enactments, and accordingly there are significant
differences between the Companies Act and the current Companies Act of England. In addition, the Companies Act differs from laws applicable
to United States corporations and their shareholders. Set forth below is a summary of certain significant differences between the provisions
of the Companies Act applicable to us and the laws applicable to companies incorporated in the United States and their shareholders.
Mergers and Similar Arrangements
The Companies Act permits mergers and consolidations
between Cayman Islands companies and between Cayman Islands companies and non-Cayman Islands companies. For these purposes, (a) “merger”
means the merging of two or more constituent companies and the vesting of their undertaking, property and liabilities in one of such
companies as the surviving company and (b) a “consolidation” means the combination of two or more constituent companies
into a combined company and the vesting of the undertaking, property and liabilities of such companies to the consolidated company. In
order to effect such a merger or consolidation, the directors of each constituent company must approve a written plan of merger or consolidation,
which must then be authorized by (a) a special resolution of the shareholders of each constituent company, and (b) such other
authorization, if any, as may be specified in such constituent company’s articles of association. The written plan of merger or
consolidation must be filed with the Registrar of Companies of the Cayman Islands together with a declaration as to the solvency of the
consolidated or surviving company, a list of the assets and liabilities of each constituent company and an undertaking that a copy of
the certificate of merger or consolidation will be given to the members and creditors of each constituent company and that notification
of the merger or consolidation will be published in the Cayman Islands Gazette. Court approval is not required for a merger or consolidation
which is effected in compliance with these statutory procedures.
A merger between a Cayman parent company and
its Cayman subsidiary or subsidiaries does not require authorization by a resolution of shareholders. For this purpose, a subsidiary
is a company of which at least 90% of the issued shares entitled to vote are owned by the parent company.
The consent of each holder of a fixed or floating
security interest of a constituent company is required unless this requirement is waived by a court in the Cayman Islands.
Except in certain limited circumstances, a shareholder
of a Cayman Islands constituent company who dissents from the merger or consolidation is entitled to payment of the fair value of his
or her shares (which, if not agreed between the parties, will be determined by the Cayman Islands court) upon dissenting from a merger
or consolidation, provided the dissenting shareholder complies strictly with the procedures set out in the Companies Act. The exercise
of such dissenter rights will preclude the exercise by the dissenting shareholder of any other rights to which he or she might otherwise
be entitled by virtue of holding shares, except for the right to seek relief on the grounds that the merger or consolidation is void
or unlawful.
Separate from the statutory provisions relating
to mergers and consolidations, the Companies Act also contains statutory provisions that facilitate the reconstruction and amalgamation
of companies by way of schemes of arrangement, provided that the arrangement is approved by seventy-five percent (75%) in value of the
shareholders or class of shareholders, as the case may be, that are present and voting either in person or by proxy at a meeting, or
meetings, convened for that purpose. The convening of the meetings and subsequently the arrangement must be sanctioned by the Grand Court
of the Cayman Islands. While a dissenting shareholder has the right to express to the court the view that the transaction ought not to
be approved, the court can be expected to approve the arrangement if it determines that:
| |
● |
the statutory
provisions as to the required majority vote have been met; |
| |
● |
the shareholders
have been fairly represented at the meeting in question; |
| |
● |
the arrangement
is such that may be reasonably approved by an intelligent and honest man of that class acting in respect of his interest; and |
| |
● |
the arrangement
is not one that would more properly be sanctioned under some other provision of the Companies Act. |
The Companies Act also contains a statutory power
of compulsory acquisition which may facilitate the “squeeze out” of dissentient minority shareholders upon a tender offer.
When a tender offer is made and accepted by holders of 90% of the shares affected within four months, the offeror may, within a two-month
period commencing on the expiration of such four-month period, require the holders of the remaining shares to transfer such shares to
the offeror on the terms of the offer. An objection can be made to the Grand Court of the Cayman Islands but this is unlikely to succeed
in the case of an offer which has been so approved unless there is evidence of fraud, bad faith or collusion.
If an arrangement and reconstruction by way of
scheme of arrangement is thus approved and sanctioned, or if a tender offer is made and accepted, in accordance with the foregoing statutory
procedures, a dissenting shareholder would have no rights comparable to appraisal rights, which would otherwise ordinarily be available
to dissenting shareholders of Delaware corporations, providing rights to receive payment in cash for the judicially determined value
of the shares.
Shareholders’ Suits
In principle, we will normally be the proper
plaintiff to sue for a wrong done to us as a company, and as a general rule a derivative action may not be brought by a minority shareholder.
However, based on English authorities, which would in all likelihood be of persuasive authority in the Cayman Islands, the Cayman Islands
court can be expected to apply and follow the common law principles (namely the rule in Foss v. Harbottle and the exceptions thereto,
which limits the circumstances in which a shareholder may bring a derivative action on behalf of the company or a personal action to
claim loss which is reflective of loss suffered by the company) which permit a minority shareholder to commence a class action against,
or derivative actions in the name of, a company to challenge the following:
| |
● |
a company
acts or proposes to act illegally or ultra vires and is therefore incapable of ratification by the shareholder; |
| |
● |
an irregularity
in the passing of a resolution which requires a qualified majority; |
| |
● |
an act
purporting to abridge or abolish the individual rights of a member; and |
| |
● |
an act
which constitutes a fraud on the minority where the wrongdoers are themselves in control of the company. |
In the case of a company (not being a bank) having
its share capital divided into shares, the Grand Court may, on the application of members holding not less than one fifth of the shares
of the company in issue, appoint an inspector to examine the affairs of the company and to report thereon in such manner as the Grand
Court shall direct.
Indemnification of Directors and Executive
Officers and Limitation of Liability
Cayman Islands law does not limit the extent
to which a company’s memorandum and articles of association may provide for indemnification of officers and directors, except to
the extent that any such provision may be held by the Cayman Islands courts to be contrary to public policy. Subject to the provisions
of the Companies Act and in the absence of fraud or willful default, the Company may indemnify against all expenses, including legal
fees, and against all judgments, fines and amounts paid in settlement and reasonably incurred in connection with legal, administrative
or investigative proceedings any person who: (a) is or was a party or is threatened to be made a party to any threatened, pending or
completed proceedings, whether civil, criminal, administrative or investigative, by reason of the fact that the person is or was a director,
managing director, agent, auditor, secretary and other officer for the time being of the Company; or (b) is or was, at the request of
the Company, serving as a director, managing director, agent, auditor, secretary and other officer for the time being of, or in any other
capacity is or was acting for, another company or a partnership, joint venture, trust or other enterprise.
Insofar as indemnification for liabilities arising
under the Securities Act may be permitted to our directors, officers or persons controlling us under the foregoing provisions, we have
been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is
therefore unenforceable.
Directors’ Fiduciary Duties
Under Delaware corporate law, a director of a
Delaware corporation has a fiduciary duty to the corporation and its shareholders. This duty has two components: the duty of care and
the duty of loyalty. The duty of care requires that a director act in good faith, with the care that an ordinarily prudent person would
exercise under similar circumstances. Under this duty, a director must inform himself of and disclose to shareholders, all material information
reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he or she reasonably
believes to be in the best interests of the corporation. He or she must not use his or her corporate position for personal gain or advantage.
This duty prohibits self-dealing by a director and mandates that the best interests of the corporation and its shareholders take precedence
over any interest possessed by a director, officer or controlling shareholder and not shared by the shareholders generally. In general,
actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken
was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary
duties. Should such evidence be presented concerning a transaction by a director, a director must prove the procedural fairness of the
transaction and that the transaction was of fair value to the corporation.
As a matter of Cayman Islands law, a director
of a Cayman Islands company is in the position of a fiduciary with respect to the company and therefore he owes the following duties
to the company—a duty to act in good faith in the best interests of the company, a duty not to make a personal profit based on
his or her position as director (unless the company permits him to do so), a duty not to put himself in a position where the interests
of the company conflict with his or her personal interest or his or her duty to a third party and a duty to exercise powers for the purpose
for which such powers were intended. A director of a Cayman Islands company owes to the company a duty to act with skill and care. It
was previously considered that a director need not exhibit in the performance of his or her duties a greater degree of skill than may
reasonably be expected from a person of his or her knowledge and experience. However, English and Commonwealth courts have moved towards
an objective standard with regard to the required skill and care and these authorities are likely to be followed in the Cayman Islands.
Shareholder Proposals
Under the Delaware General Corporation Law, a
shareholder has the right to put any proposal before the annual meeting of shareholders, provided it complies with the notice provisions
in the governing documents. The Delaware General Corporation Law does not provide shareholders an express right to put any proposal before
the annual meeting of shareholders, but in keeping with common law, Delaware corporations generally afford shareholders an opportunity
to make proposals and nominations provided that they comply with the notice provisions in the certificate of incorporation or bylaws.
A special meeting may be called by the board of directors or any other person authorized to do so in the governing documents, but shareholders
may be precluded from calling special meetings.
Cayman Islands law provides shareholders with
only limited rights to requisition a general meeting, and does not provide shareholders with any right to put any proposal before a general
meeting. However, these rights may be provided in a company’s articles of association. Our amended and restated memorandum and
articles of association provide that, on the requisition of any shareholders who hold not less than 10 percent of the paid up voting
share capital of the Company in respect to the matter for which the meeting is requested, our board of directors shall convene an extraordinary
general meeting and put the resolutions so requisitioned to a vote at such meeting. However, our amended and restated memorandum and
articles of association do not provide our shareholders with any right to put any proposals before annual general meetings or extraordinary
general meetings not called by such shareholders. As an exempted Cayman Islands company, we are not obliged by law to call shareholders’
annual general meetings.
Cumulative Voting
Under the Delaware General Corporation Law, cumulative
voting for elections of directors is not permitted unless the corporation’s certificate of incorporation specifically provides
for it. Cumulative voting potentially facilitates the representation of minority shareholders on a board of directors since it permits
the minority shareholder to cast all the votes to which the shareholder is entitled on a single director, which increases the shareholder’s
voting power with respect to electing such director. Cayman Islands law does not prohibit cumulative voting, but our amended and restated
articles of association do not provide for cumulative voting. As a result, our shareholders are not afforded any less protections or
rights on this issue than shareholders of a Delaware corporation.
Removal of Directors
Under the Delaware General Corporation Law, a
director of a corporation with a classified board may be removed only for cause with the approval of a majority of the outstanding shares
entitled to vote, unless the certificate of incorporation provides otherwise. Under our amended and restated memorandum and amended and
restated articles of association, any of our directors may be removed by ordinary resolution of our shareholders.
Transactions with Interested Shareholders
The Delaware General Corporation Law contains
a business combination statute applicable to Delaware public corporations whereby, unless the corporation has specifically elected not
to be governed by such statute by amendment to its certificate of incorporation or bylaws that is approved by its shareholders, it is
prohibited from engaging in certain business combinations with an “interested shareholder” for three years following the
date that such person becomes an interested shareholder. An interested shareholder generally is a person or a group who or which owns
or owned 15% or more of the target’s outstanding voting stock or who or which is an affiliate or associate of the corporation and
owned 15% or more of the corporation’s outstanding voting stock within the past three years. This has the effect of limiting the
ability of a potential acquirer to make a two-tiered bid for the target in which all shareholders would not be treated equally. The statute
does not apply if, among other things, prior to the date on which such shareholder becomes an interested shareholder, the board of directors
approves either the business combination or the transaction which resulted in the person becoming an interested shareholder. This encourages
any potential acquirer of a Delaware corporation to negotiate the terms of any acquisition transaction with the target’s board
of directors.
Cayman Islands law has no comparable statute.
As a result, we cannot avail ourselves of the types of protections afforded by the Delaware business combination statute. However, although
Cayman Islands law does not regulate transactions between a company and its significant shareholders, it does provide that such transactions
must be entered into bona fide in the best interests of the company and for a proper corporate purpose and not with the effect of constituting
a fraud on the minority shareholders.
Dissolution; Winding Up
Under the Delaware General Corporation Law, unless
the board of directors approves the proposal to dissolve, dissolution must be approved by shareholders holding 100% of the total voting
power of the corporation. Only if the dissolution is initiated by the board of directors may it be approved by a simple majority of the
corporation’s outstanding shares. Delaware law allows a Delaware corporation to include in its certificate of incorporation a supermajority
voting requirement in connection with dissolutions initiated by the board. Under Cayman Islands law, a company may be wound up by either
an order of the courts of the Cayman Islands or by a special resolution of its members or, if the company is unable to pay its debts
as they fall due, by an ordinary resolution of its members. The court has authority to order winding up in a number of specified circumstances
including where it is, in the opinion of the court, just and equitable to do so.
Variation of Rights of Shares
Under the Delaware General Corporation Law, a
corporation may vary the rights of a class of shares with the approval of a majority of the outstanding shares of such class, unless
the certificate of incorporation provides otherwise. Under our amended and restated memorandum and articles of association, if our share
capital is divided into more than one class of shares, the rights attached to any such class may, subject to any rights or restrictions
for the time being attached to any class, only be materially adversely varied with the consent in writing of the holders of two-thirds
of the issued shares of that class or with the sanction of a special resolution passed at a separate meeting of the holders of the shares
of that class.
Amendment of Governing Documents
Under the Delaware General Corporation Law, a
corporation’s certificate of incorporation may be amended only if adopted and declared advisable by the board of directors and
approved by a majority of the outstanding shares entitled to vote and the bylaws may be amended with the approval of a majority of the
outstanding shares entitled to vote and may, if so provided in the certificate of incorporation, also be amended by the board of directors.
Under the Companies Act, our amended and restated memorandum and articles of association may only be amended by special resolution of
our shareholders.
Rights of Non-Resident or Foreign Shareholders
There are no limitations imposed by our amended
and restated memorandum and amended and restated articles of association on the rights of non-resident or foreign shareholders to hold
or exercise voting rights on our shares. In addition, there are no provisions in our amended and restated memorandum and amended and
restated articles of association governing the ownership threshold above which shareholder ownership must be disclosed.
Directors’ Power to Issue Shares
Under our amended and restated memorandum and
amended and restated articles of association, our board of directors is empowered to issue or allot shares or grant options and warrants
with or without preferred, deferred, qualified or other special rights or restrictions.
Securities Outstanding
The following is a summary of our securities
outstanding as of the date of this prospectus supplement:
Ordinary Shares
The Company is authorized to issue 80,000,000
Ordinary Shares of $0.0005 par value and 20,000,000 preferred shares of $0.0005 par value. As of February 5, 2025 24,234,673 Ordinary
Shares were issued and outstanding and no preferred shares were issued and outstanding.
Option Grants
We have not granted any options to purchase our
Ordinary Shares.
Notes and Warrants
$6,000,000 of Registered Notes, convertible from
time to time into Ordinary Shares on the terms set forth in the Registered Notes, and Warrants to purchase 1,205,255 Ordinary Shares,
exercisable at $2.9869 per share, subject to adjustment, each as described elsewhere in this prospectus supplement.
Anti-money Laundering—Cayman Islands
In order to comply with legislation or regulations
aimed at the prevention of money laundering, we are required to adopt and maintain anti-money laundering procedures and may require subscribers
to provide evidence to verify their identity and source of funds. Where permitted, and subject to certain conditions, we may also delegate
the maintenance of our anti-money laundering procedures (including the acquisition of due diligence information) to a suitable person.
We reserve the right to request such information
as is necessary to verify the identity of a subscriber. In some cases, the directors may be satisfied that no further information
is required since an exemption applies under the Anti-Money Laundering Regulations (Revised) of the Cayman Islands, as amended and revised
from time to time (the “Regulations”). Depending on the circumstances of each application, a detailed verification of identity
might not be required where:
| |
● |
the subscriber
makes the payment for their investment from an account held in the subscriber’s name at a recognized financial institution;
or |
| |
● |
the subscriber
is regulated by a recognized regulatory authority and is based or incorporated in, or formed under the law of, a recognized jurisdiction;
or |
| |
● |
the application
is made through an intermediary which is regulated by a recognized regulatory authority and is based in or incorporated in, or formed
under the law of a recognized jurisdiction and an assurance is provided in relation to the procedures undertaken on the underlying
investors. |
For the purposes of these exceptions, recognition
of a financial institution, regulatory authority, or jurisdiction will be determined in accordance with the Regulations by reference
to those jurisdictions recognized by the Cayman Islands Monetary Authority as having equivalent anti-money laundering regulations.
In the event of delay or failure on the part
of the subscriber in producing any information required for verification purposes, we may refuse to accept the application, in which
case any funds received will be returned without interest to the account from which they were originally debited.
We also reserve the right to refuse to make any
redemption payment to a shareholder if our directors or officers suspect or are advised that the payment of redemption proceeds to such
shareholder might result in a breach of applicable anti-money laundering or other laws or regulations by any person in any relevant jurisdiction,
or if such refusal is considered necessary or appropriate to ensure our compliance with any such laws or regulations in any applicable
jurisdiction.
If any person resident in the Cayman Islands
knows or suspects or has reason for knowing or suspecting that another person is engaged in criminal conduct or is involved with terrorism
or terrorist property and the information for that knowledge or suspicion came to their attention in the course of their business in
the regulated sector, or other trade, profession, business or employment, the person will be required to report such knowledge or suspicion
to (i) a nominated officer (appointed in accordance with the Proceeds of Crime Act (Revised) of the Cayman Islands) or the Financial
Reporting Authority of the Cayman Islands, pursuant to the Proceeds of Crime Act (Revised), if the disclosure relates to criminal conduct
or money laundering or (ii) to a police constable or a nominated officer (pursuant to the Terrorism Act (Revised) of the Cayman
Islands) or the Financial Reporting Authority, pursuant to the Terrorism Act (Revised), if the disclosure relates to involvement with
terrorism or terrorist financing and terrorist property. Such a report shall not be treated as a breach of confidence or of any restriction
upon the disclosure of information imposed by any enactment or otherwise.
Data Protection in the Cayman Islands –
Privacy Notice
This privacy notice explains the manner in which
we collect, process, and maintain personal data about investors of the Company pursuant to the Data Protection Act (Revised) of the Cayman
Islands, as amended from time to time and any regulations, codes of practice, or orders promulgated pursuant thereto (the “DPA”).
We are committed to processing personal data
in accordance with the DPA. In our use of personal data, we will be characterized under the DPA as a “data controller,” whilst
certain of our service providers, affiliates, and delegates may act as “data processors” under the DPA. These service providers
may process personal information for their own lawful purposes in connection with services provided to us.
By virtue of your investment in the Company,
we and certain of our service providers may collect, record, store, transfer, and otherwise process personal data by which individuals
may be directly or indirectly identified.
Your personal data will be processed fairly and
for lawful purposes, including (a) where the processing is necessary for us to perform a contract to which you are a party or for
taking pre-contractual steps at your request, (b) where the processing is necessary for compliance with any legal, tax, or regulatory
obligation to which we are subject, or (c) where the processing is for the purposes of legitimate interests pursued by us or by
a service provider to whom the data are disclosed. As a data controller, we will only use your personal data for the purposes for which
we collected it. If we need to use your personal data for an unrelated purpose, we will contact you.
We anticipate that we will share your personal
data with our service providers for the purposes set out in this privacy notice. We may also share relevant personal data where it is
lawful to do so and necessary to comply with our contractual obligations or your instructions or where it is necessary or desirable to
do so in connection with any regulatory reporting obligations. In exceptional circumstances, we will share your personal data with regulatory,
prosecuting, and other governmental agencies or departments, and parties to litigation (whether pending or threatened), in any country
or territory including to any other person where we have a public or legal duty to do so (e.g. to assist with detecting and preventing
fraud, tax evasion, and financial crime or compliance with a court order).
Your personal data shall not be held by the Company
for longer than necessary with regard to the purposes of the data processing.
We will not sell your personal data. Any transfer
of personal data outside of the Cayman Islands shall be in accordance with the requirements of the DPA. Where necessary, we will ensure
that separate and appropriate legal agreements are put in place with the recipient of that data.
We will only transfer personal data in accordance
with the requirements of the DPA and will apply appropriate technical and organizational information security measures designed to protect
against unauthorized or unlawful processing of the personal data and against the accidental loss, destruction, or damage to the personal
data.
If you are a natural person, this will affect
you directly. If you are a corporate investor (including, for these purposes, legal arrangements such as trusts or exempted limited partnerships)
that provides us with personal data on individuals connected to you for any reason in relation to your investment into the Company, this
will be relevant for those individuals and you should inform such individuals of the content.
You have certain rights under the DPA, including
(a) the right to be informed as to how we collect and use your personal data (and this privacy notice fulfils our obligation in
this respect), (b) the right to obtain a copy of your personal data, (c) the right to require us to stop direct marketing,
(d) the right to have inaccurate or incomplete personal data corrected, (e) the right to withdraw your consent and require
us to stop processing or restrict the processing, or not begin the processing of your personal data, (f) the right to be notified
of a data breach (unless the breach is unlikely to be prejudicial), (g) the right to obtain information as to any countries or territories
outside the Cayman Islands to which we, whether directly or indirectly, transfer, intend to transfer, or wish to transfer your personal
data, general measures we take to ensure the security of personal data, and any information available to us as to the source of your
personal data, (h) the right to complain to the Office of the Ombudsman of the Cayman Islands, and (i) the right to require
us to delete your personal data in some limited circumstances.
If you consider that your personal data has not
been handled correctly, or you are not satisfied with our responses to any requests you have made regarding the use of your personal
data, you have the right to complain to the Cayman Islands’ Ombudsman. The Ombudsman can be contacted by calling +1 (345) 946-6283
or by emailing info@ombudsman.ky.
PLAN OF DISTRIBUTION
Each selling shareholder (each refers to as a
“Selling Shareholder,” and collectively refers to as the “Selling Shareholders”) of the securities of Meihua
International Medical Technologies Co., Ltd., an exempted company incorporated under the laws of the Cayman Islands (the “Company”),
and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their Company securities covered
hereby on the Nasdaq Global Market or any other stock exchange, market or trading facility on which such securities are traded or in
private transactions. These sales may be at fixed or negotiated prices. A Selling Shareholder may use any one or more of the following
methods when selling securities:
| ● | ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| ● | block trades
in which the broker-dealer will attempt to sell the securities as agent but may position
and resell a portion of the block as principal to facilitate the transaction; |
| ● | purchases
by a broker-dealer as principal and resale by the broker-dealer for its account; |
| ● | an exchange
distribution in accordance with the rules of the applicable exchange; |
| ● | privately
negotiated transactions; |
| ● | settlement
of short sales entered into after the effective date of the registration statement of which
this prospectus is a part; |
| ● | in transactions
through broker-dealers that agree with the Selling Shareholders to sell a specified number
of such securities at a stipulated price per security; |
| ● | through
the writing or settlement of options or other hedging transactions, whether through an options
exchange or otherwise; |
| ● | a combination
of any such methods of sale; or |
| ● | any other
method permitted pursuant to applicable law. |
The Selling Shareholders may also sell securities
under Rule 144 under the Securities Act of 1933, as amended (the “Securities Act”), if available, rather than under this
prospectus.
Broker-dealers engaged by the Selling Shareholders
may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Shareholders
(or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except
as set forth in a supplement to this Prospectus, in the case of an agency transaction not in excess of a customary brokerage commission
in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or markdown in compliance with FINRA 2121.
In connection with the sale of the securities
or interests therein, the Selling Shareholders may enter into hedging transactions with broker-dealers or other financial institutions,
which may in turn engage in short sales of the securities in the course of hedging the positions they assume. The Selling Shareholders
may also sell securities short and deliver these securities to close out their short positions, or loan or pledge the securities to broker-dealers
that in turn may sell these securities. The Selling Shareholders may also enter into option or other transactions with broker-dealers
or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other
financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution may
resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Shareholders and any broker-dealers
or agents that are involved in selling the securities may be deemed to be “underwriters” within the meaning of the Securities
Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale
of the securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Each Selling
Shareholder has informed the Company that it does not have any written or oral agreement or understanding, directly or indirectly, with
any person to distribute the securities. In no event shall any broker-dealer receive fees, commissions and markups which, in the aggregate,
would exceed eight percent (8%).
The Company is required to pay certain fees and
expenses incurred by the Company incident to the registration of the securities. The Company has agreed to indemnify the Selling Shareholders
against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
Because Selling Shareholders may be deemed to
be “underwriters” within the meaning of the Securities Act, they will be subject to the prospectus delivery requirements
of the Securities Act including Rule 172 thereunder. In addition, any securities covered by this prospectus which qualify for sale pursuant
to Rule 144 under the Securities Act may be sold under Rule 144 rather than under this prospectus. The Selling Shareholders have advised
us that there is no underwriter or coordinating broker acting in connection with the proposed sale of the resale securities by the Selling
Shareholders.
We agreed to keep this prospectus effective until
the earliest of (i) the date on which the securities may be resold by the Selling Shareholders without registration and without regard
to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for the Company to be in compliance with the
current public information under Rule 144 under the Securities Act or any other rule of similar effect or (ii) the date on which all
of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect.
The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state securities
laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered or qualified
for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under
the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market making activities
with respect to the ordinary for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution.
In addition, the Selling Shareholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder,
including Regulation M, which may limit the timing of purchases and sales of the Company’s securities by the Selling Shareholders
or any other person. We will make copies of this prospectus available to the Selling Shareholders and have informed them of the need
to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under
the Securities Act).
TAXATION
The following summary of the material Cayman
Islands, People’s Republic of China, and U.S. federal income tax consequences of an investment in our securities is based upon
laws and relevant interpretations thereof in effect as of the date of this prospectus supplement, all of which are subject to change.
This summary does not deal with all possible tax consequences relating to an investment in our securities, such as the tax consequences
under U.S. state and local tax laws or under the tax laws of jurisdictions other than the Cayman Islands, the People’s Republic
of China, and the United States.
Cayman Islands Taxation
The Cayman Islands currently
levies no taxes on individuals or corporations based upon profits, income, gains or appreciation and there is no taxation in the nature
of inheritance tax or estate duty. There are no other taxes likely to be material to investors levied by the government of the Cayman
Islands except for stamp duties which may be applicable on instruments executed in, or, after execution, brought within the jurisdiction
of the Cayman Islands. The Cayman Islands is a party to a double tax treaty entered with the United Kingdom in 2010 but is otherwise
not party to any double tax treaties which are applicable to any payments made to or by our company. There are no exchange control regulations
or currency restrictions in the Cayman Islands.
Payments of dividends
and capital in respect of Ordinary Shares will not be subject to taxation in the Cayman Islands and no withholding will be required under
Cayman Islands laws on the payment of a dividend or capital to any holder of Ordinary Shares, nor will gains derived from the disposal
of Ordinary Shares be subject to Cayman Islands income or corporation tax.
The Cayman Islands enacted the International
Tax Co-operation (Economic Substance) Act (Revised), which may be interpreted together with the Guidance Notes published from time to
time by the Cayman Islands Tax Information Authority. The Company is required to comply with the economic substance requirements from
July 1, 2019 and make an annual report in the Cayman Islands as to whether or not it is carrying on any relevant activities and, if it
is, it must satisfy an economic substance test.
People’s Republic
of China Taxation
Under the PRC EIT Law
and its implementation rules, an enterprise established outside the PRC with a “de facto management body” within the PRC
is considered a resident enterprise and will be subject to the enterprise income tax at the rate of 25% on its global income. The implementation
rules define the term “de facto management body” as the body that exercises full and substantial control over and overall
management of the business, production, personnel, accounts and properties of an enterprise. In April 2009, the SAT issued the Circular
of the SAT on Issues Relating to Identification of PRC-Controlled Overseas Registered Enterprises as Resident Enterprises in Accordance
With the De Facto Standards of Organizational Management, or SAT Circular 82, which provides certain specific criteria for determining
whether the “de facto management body” of a PRC-controlled enterprise that is incorporated offshore is located in China.
Although this circular only applies to offshore enterprises controlled by PRC enterprises or PRC enterprise groups, not those controlled
by PRC individuals or foreigners, the criteria set forth in the circular may reflect the SAT’s general position on how the “de
facto management body” test should be applied in determining the tax resident status of all offshore enterprises. According to
SAT Circular 82, an offshore incorporated enterprise controlled by a PRC enterprise or a PRC enterprise group will be regarded as a PRC
tax resident by virtue of having its “de facto management body” in the PRC only if all of the following conditions are met:
(i) the primary location of the day-to-day operational management is in the PRC; (ii) decisions relating to the enterprise’s financial
and human resource matters are made or are subject to approval by organizations or personnel in the PRC; (iii) the enterprise’s
primary assets, accounting books and records, company seals, and board and shareholder resolutions, are located or maintained in the
PRC; and (iv) at least 50% of voting board members or senior executives habitually reside in the PRC.
Further to SAT Circular
82, the SAT issued the SAT Bulletin 45, which took effect in September 2011, to provide more guidance on the implementation of SAT Circular
82. SAT Bulletin 45 provides for procedures and administration details of determination on resident status and administration on post-determination
matters. Our Company is a company incorporated outside the PRC. As a holding company, its sole asset is its share ownership of its direct
subsidiary, a Hong Kong company, and its key assets are located, and its records (including the resolutions of its board of directors
and the resolutions of its shareholders) are maintained, outside the PRC. As such, we do not believe that our Company meets all of the
conditions above or is a PRC resident enterprise for PRC tax purposes. However, the tax resident status of an enterprise is subject to
determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management
body.” There can be no assurance that the PRC government will ultimately take a view that is consistent with us. If the PRC tax
authorities determine that our Cayman Islands holding company is a PRC resident enterprise for PRC enterprise income tax purposes, a
number of unfavorable PRC tax consequences could follow. For example, a 10% withholding tax would be imposed on dividends we pay to our
non-PRC enterprise shareholders. In addition, nonresident enterprise shareholders may be subject to PRC tax on gains realized on the
sale or other disposition of Ordinary Shares, as if such income is treated as sourced from within the PRC. Furthermore, if we are deemed
a PRC resident enterprise, dividends paid to our non-PRC individual shareholders and any gain realized on the transfer of Ordinary Shares
by such shareholders may be subject to PRC tax at a rate of 20% (which, in the case of dividends, may be withheld at source by us). These
rates may be reduced by an applicable tax treaty, but it is unclear whether in practice non-PRC shareholders of our Company would be
able to obtain the benefits of any tax treaties between their country of tax residence and the PRC in the event that we are treated as
a PRC resident enterprise. See “Risk Factors — Risks Related to Doing Business in China — If we are classified as a
PRC resident enterprise for PRC income tax purposes, such classification could result in unfavorable tax consequences to us and our non-PRC
shareholders.”
Notwithstanding the
foregoing, our PRC subsidiaries, Jiangsu Huadong enjoyed preferential income tax rate of 15% until December 31, 2021, due to its treatment
as “National High-Tech Enterprises” in China. Prior to the expiration date of such treatment, it may submit applications
for renewal and continue enjoying the preferential income tax rate if granted.
United States
Federal Income Taxation Considerations
The following discussion
is a summary of United States federal income tax considerations generally applicable to the ownership and disposition of our Ordinary
Shares by a U.S. holder (as defined below) that acquires our Ordinary Shares and holds our Ordinary Shares as “capital
assets” (generally, property held for investment) under the United States Internal Revenue Code of 1986, as amended (the “Code”).
This discussion is based upon existing United States federal income tax law, which is subject to differing interpretations and may
be changed, possibly with retroactive effect. No ruling has been sought from the Internal Revenue Service (the “IRS”) with
respect to any United States federal income tax consequences described below, and there can be no assurance that the IRS or a court
will not take a contrary position. This discussion does not address all aspects of United States federal income taxation that may
be important to particular investors in light of their individual circumstances, including investors subject to special tax rules (for example,
certain financial institutions, insurance companies, broker-dealers, traders in securities that have elected the mark-to-market method
of accounting for their securities, partnerships and their partners, regulated investment companies, real estate investment trusts, and
tax-exempt organizations (including private foundations)), investors who are not U.S. holders, investors who own (directly, indirectly,
or constructively) 10% or more of our stock (by vote or value), investors that will hold their Ordinary Shares as part of a straddle,
hedge, conversion, constructive sale, or other integrated transaction for United States federal income tax purposes, investors required
to accelerate the recognition of any item of gross income with respect to our Ordinary Shares as a result of such income being recognized
on an applicable financial statement, or investors that have a functional currency other than the United States dollar, all of whom
may be subject to tax rules that differ significantly from those summarized below. In addition, this discussion does not discuss any
non-United States, alternative minimum tax, state, or local tax or any non-income tax (such as the U.S. federal gift or estate
tax) considerations, or the Medicare tax on net investment income. Each U.S. holder is urged to consult its tax advisor regarding
the United States federal, state, local, and non-United States income and other tax considerations of an investment in our
Ordinary Shares.
EACH U.S. HOLDER IS
URGED TO CONSULT ITS TAX ADVISOR REGARDING THE APPLICATION OF U.S. FEDERAL TAXATION TO ITS PARTICULAR CIRCUMSTANCES, AND THE STATE, LOCAL,
NON-U.S. AND OTHER TAX CONSIDERATIONS OF THE OWNERSHIP AND DISPOSITION OF THE ORDINARY SHARES.
General
For purposes of this
discussion, a “U.S. holder” is a beneficial owner of our Ordinary Shares that is, for United States federal income
tax purposes, (i) an individual who is a citizen or resident of the United States, (ii) a corporation (or other entity
treated as a corporation for United States federal income tax purposes) created in, or organized under the laws of, the United States
or any state thereof or the District of Columbia, (iii) an estate the income of which is subject to United States federal income
taxation regardless of its source, or (iv) a trust (A) the administration of which is subject to the primary supervision of
a United States court and which has one or more United States persons who have the authority to control all substantial decisions
of the trust or (B) that has otherwise elected to be treated as a United States person under the Code or applicable United States
Treasury regulations.
If a partnership (or other
entity or arrangement treated as a partnership for United States federal income tax purposes) is a beneficial owner of our Ordinary
Shares, the tax treatment of a partner in the partnership will generally depend upon the status of the partner and the activities of
the partnership. Partnerships holding our Ordinary Shares and partners in such partnerships are urged to consult their tax advisors as
to the particular United States federal income tax consequences of an investment in our Ordinary Shares.
Sale or Other
Disposition of Ordinary Shares
Subject to the PFIC
rules discussed below, a U.S. holder will generally recognize capital gain or loss upon the sale or other disposition of Ordinary
Shares in an amount equal to the difference between the amount realized upon the disposition and the U.S. holder’s adjusted
tax basis in such Ordinary Shares. Any capital gain or loss will be long-term if our Ordinary Shares have been held for more than one
year and will generally be United States source gain or loss for United States foreign tax credit purposes. Long-term capital
gain of individuals and other non-corporate U.S. holders is generally eligible for a reduced rate of taxation. The deductibility
of a capital loss may be subject to limitations.
In the event that we
are treated as a PRC “resident enterprise” under the Enterprise Income Tax Law and gain from the disposition of our Ordinary
Shares is subject to tax in the PRC, a U.S. holder that is eligible for the benefits of the income tax treaty between the United States
and the PRC may elect to treat the gain as PRC source income. If a U.S. holder is not eligible for the benefits of the income tax
treaty or fails to make the election to treat any gain as foreign source, then such U.S. holder may not be able to use the foreign
tax credit arising from any PRC tax imposed on the disposition of our Ordinary Shares unless such credit can be applied (subject to applicable
limitations) against U.S. federal income tax due on other income derived from foreign sources in the same income category (generally,
the passive category). U.S. holders are advised to consult their tax advisors regarding the tax consequences if a foreign tax is
imposed on a disposition of our Ordinary Shares, including the availability of the foreign tax credit under their particular circumstances
and the election to treat any gain as PRC source.
Passive Foreign
Investment Company Rules
If we are a PFIC for
any taxable year during which a U.S. holder holds our Ordinary Shares, and unless the U.S. holder makes a mark-to-market election
(as described below), the U.S. holder will generally be subject to special tax rules that have a penalizing effect, regardless
of whether we remain a PFIC, for subsequent taxable years, on (i) any excess distribution that we make to the U.S. holder (which
generally means any distribution paid during a taxable year to a U.S. holder that is greater than 125% of the average annual distributions
paid in the three preceding taxable years or, if shorter, the U.S. holder’s holding period for our Ordinary Shares), and (ii) any
gain realized on the sale or other disposition, including, under certain circumstances, a pledge, of Ordinary Shares. Under the PFIC
rules:
| |
● |
such excess
distribution and/or gain will be allocated ratably over the U.S. holder’s holding period for our Ordinary Shares; |
| |
● |
such amount
allocated to the current taxable year and any taxable years in the U.S. holder’s holding period prior to the first taxable
year in which we are a PFIC, or pre-PFIC year, will be taxable as ordinary income; |
| |
● |
such amount
allocated to each prior taxable year, other than a pre-PFIC year, will be subject to tax at the highest tax rate in effect for that
year; and |
| |
● |
an interest
charge generally applicable to underpayments of tax will be imposed on the tax attributable to each prior taxable year, other than
a pre-PFIC year. |
If we are a PFIC for
any taxable year during which a U.S. holder holds our Ordinary Shares and any of our non-United States subsidiaries is also
a PFIC, such U.S. holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC
for purposes of the application of these rules. U.S. holders are advised to consult their tax advisors regarding the application
of the PFIC rules to any of our subsidiaries.
As an alternative to
the foregoing rules, a U.S. holder of “marketable stock” in a PFIC may make a mark-to-market election with respect to
our Ordinary Shares, provided that our Ordinary Shares are regularly traded on the Nasdaq Global Market.
Because a mark-to-market
election cannot be made for any lower-tier PFICs that a PFIC may own, a U.S. holder who makes a mark-to-market election with respect
to our Ordinary Shares will generally continue to be subject to the general PFIC rules with respect to such U.S. holder’s
indirect interest in any investments held by us that are treated as an equity interest in a PFIC for United States federal income
tax purposes. If a mark-to-market election is made, the U.S. holder will generally (i) include as ordinary income for each
taxable year that we are a PFIC the excess, if any, of the fair market value of Ordinary Shares held at the end of the taxable year over
the adjusted tax basis of such Ordinary Shares and (ii) deduct as an ordinary loss the excess, if any, of the adjusted tax basis
of our Ordinary Shares over the fair market value of such Ordinary Shares held at the end of the taxable year, but only to the extent
of the net amount previously included in income as a result of the mark-to-market election. The U.S. holder’s adjusted tax
basis in our Ordinary Shares would be adjusted to reflect any income or loss resulting from the mark-to-market election. If a U.S. holder
makes an effective mark-to-market election, in each year that we are a PFIC any gain recognized upon the sale or other disposition of
our Ordinary Shares will be treated as ordinary income and loss will be treated as ordinary loss, but only to the extent of the net amount
previously included in income as a result of the mark-to-market election. If a U.S. holder makes a mark-to-market election it will
be effective for the taxable year for which the election is made and all subsequent taxable years unless our Ordinary Shares are no longer
regularly traded on a qualified exchange or the IRS consents to the revocation of the election.
If a U.S. holder
makes a mark-to-market election in respect of a PFIC and such corporation ceases to be a PFIC, the U.S. holder will not be required
to take into account the mark-to-market gain or loss described above during any period that such corporation is not a PFIC.
We do not intend to
provide information necessary for U.S. holders to make qualified electing fund elections, which, if available, would result in tax
treatment different from (and generally less adverse than) the general tax treatment for PFICs described above.
If a U.S. holder
owns our Ordinary Shares during any taxable year that we are a PFIC, such holder would generally be required to file an annual IRS Form 8621.
Each U.S. holder is advised to consult its tax advisors regarding the potential tax consequences to such holder if we are or become
a PFIC, including the possibility of making a mark-to-market election.
ENFORCEABILITY
OF CIVIL LIABILITIES
We are incorporated under the laws of the Cayman
Islands as an exempted company with limited liability. We incorporated in the Cayman Islands because of certain benefits associated with
being a Cayman Islands exempted company, such as political and economic stability, an effective judicial system, a favorable tax system,
the absence of foreign exchange control or currency restrictions and the availability of professional and support services. However,
the Cayman Islands have a less developed body of securities laws that provide significantly less protection to investors as compared
to the securities laws of the United States. In addition, Cayman Islands companies may not have standing to sue before the federal courts
of the United States.
Our constitutional documents do not contain provisions
requiring that disputes, including those arising under the securities laws of the United States, among us, our officers, directors, and
shareholders, be arbitrated.
Substantially all of our assets are located in
China. All of our directors and officers are nationals or residents of the PRC and all or
a substantial portion of their assets are located in the PRC. As a result, it may be difficult for investors to effect service
of process within the United States upon us or these five directors and officers, or to enforce against us or them judgments obtained
in United States courts, including judgments predicated upon the civil liability provisions of the securities laws of the United States
or any state in the United States. It may also be difficult for shareholder to enforce judgments obtained in U.S. courts based on the
civil liability provisions of the U.S. federal securities laws against us and our executive officers and directors. See “Risk Factors
— Risks Related to Doing Business in China — You may experience difficulties in effecting service of legal process,
enforcing foreign judgments or bringing actions in the PRC against us or our management named in the report based on foreign laws”
in this prospectus.
We have appointed Dorsey & Whitney LLP, 51
West 52nd Street, New York, NY 10016, as our agent to receive service of process with respect to any action brought against
us in the U.S. District Court for the Southern District of New York in connection with this offering under the federal securities laws
of the United States or the securities laws of any State in the United States or any action brought against us in the Supreme Court of
the State of New York in the County of New York in connection with this offering under the securities laws of the State of New York.
Ogier, our counsel with respect to the laws of
the Cayman Islands, and JunHe, LLP, our counsel with respect to PRC law, have advised us that there is uncertainty as to whether the
courts of the Cayman Islands or the PRC would (i) recognize or enforce judgments of United States courts obtained against us or
our directors or officers predicated upon the civil liability provisions of the securities laws of the United States or any state in
the United States or (ii) entertain original actions brought in each respective jurisdiction against us or our directors or officers
predicated upon the securities laws of the United States or any state in the United States.
Ogier has further advised us that there is currently
no statutory enforcement in the Cayman Islands of judgments obtained in the United States, although the courts of the Cayman Islands
will in certain circumstances recognize and enforce a foreign judgment, without any re-examination or re-litigation of matters adjudicated
upon, provided such judgment: (i) is given by a foreign court of competent jurisdiction; (ii) imposes on the judgment debtor
a liability to pay a liquidated sum for which the judgment has been given; (iii) is final; (iv) is not in respect of taxes,
a fine or a penalty; and (v) was not obtained by fraud, and (vi) is not of a kind the enforcement of which is contrary to natural
justice or public policy of the Cayman Islands. Subject to the above limitations, in appropriate circumstances, a Cayman Islands court
may give effect in the Cayman Islands o other kinds of final foreign judgments such as declaratory orders, orders for performance of
contracts and injunctions.
JunHe LLP has further advised us that the recognition
and enforcement of foreign judgments are provided for under the PRC Civil Procedure Law. PRC courts may recognize and enforce foreign
judgments in accordance with the requirements of the PRC Civil Procedure Law based either on treaties between China and the country where
the judgment is made or on reciprocity between jurisdictions. There are no treaties or other forms of reciprocity between China and the
United States for the mutual recognition and enforcement of court judgments. JunHe LLP has further advised us that under PRC law, PRC
courts will not enforce a foreign judgment against us or our officers and directors if the court decides that such judgment violates
the basic principles of PRC law or national sovereignty, security or public interest. See “Risk Factors — Risks Related to
Doing Business in China — You may experience difficulties in effecting service of legal process, enforcing foreign judgments
or bringing actions in the PRC against us or our management named in the report based on foreign laws.”
EXPENSES RELATED TO THE
OFFERING
| Financial Printing Fees |
|
$ |
- |
|
| SEC Filing Fees |
|
$ |
420 |
|
| FINRA Expenses |
|
$ |
- |
|
| Transfer Agent Fees |
|
$ |
- |
|
| Accounting Fees and Expenses |
|
$ |
20,000 |
|
| Legal Fees and Expenses |
|
$ |
50,000 |
|
| Miscellaneous Fees and Expenses |
|
$ |
- |
|
| Total |
|
$ |
70,420 |
|
LEGAL MATTERS
We are being represented by Dorsey & Whitney
LLP, New York, NY, with respect to certain legal matters of U.S. federal securities and New York State law. The validity of the ordinary
shares registered in this offering and certain other legal matters as to Cayman Islands law will be passed upon for us by Ogier, our
counsel as to Cayman Islands law. Legal matters as to PRC law will be passed upon for us by JunHe LLP. Dorsey LLP may rely upon Ogier
with respect to matters governed by Cayman Islands law and JunHe LLP with respect to matters governed by PRC law.
EXPERTS
The consolidated financial statements of Meihua
International Medical Technologies Co., Ltd. and subsidiaries for the year ended December 31, 2022 and 2021, as amended, have been
audited by Kreit & Chiu CPA LLP, an independent registered public accounting firm, as set forth in their reports thereon, and as
incorporated herein by reference. Such consolidated financial statements are included herein in reliance upon such report given on the
authority of such firm as experts in accounting and auditing. The office of Kreit & Chiu CPA LLP is located at 733 Third Avenue,
Floor 16, #1014, New York, NY 10017. The consolidated financial statements of Meihua International Medical Technologies Co., Ltd. and
subsidiaries appearing in our Annual Report on Form 20-F for the year ended December 31, 2020, as amended, have been audited
by Briggs & Veselka Co., an independent registered public accounting firm,
as set forth in their report thereon, and as incorporated herein by reference. Such consolidated financial statements are incorporated
herein by reference in reliance upon such reports given on the authority of such firm as experts in accounting and auditing. The office
of Briggs & Veselka Co. is located at Nine Greenway Plaza, Suite 1700, Houston,
TX 77046.
WHERE YOU CAN FIND MORE
INFORMATION
We have filed this registration statement on
Form F-1 with the Securities and Exchange Commission for purposes of registering certain ordinary shares issuable upon exercise of warrants
held by the Selling Shareholders named herein. This prospectus is part of that registration statement. In addition, for further information
with respect to us and the securities we are offering under this prospectus, we refer you to our other filings made with the SEC, as
well as the exhibits and schedules filed as a part of the registration statement. We have not authorized anyone to provide you with any
information other than that contained or incorporated by reference herein or any related free writing prospectus to which we have referred
you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information others may give you.
We have not authorized anyone to provide you with information different from that contained in this registration statement and the prospectus
contained therein or the information incorporated by reference herein or therein. We are not making an offer of these securities in any
state where the offer is not permitted. You should not assume that the information in this registration statement and prospectus is accurate
as of any date other than the date on the front page of this prospectus, regardless of the time of delivery of this prospectus supplement
or any sale of the securities offered by this prospectus supplement.
We are subject to the informational and reporting
requirements of the Exchange Act that are applicable to a foreign private issuer. Under the Exchange Act, we file annual reports on Form
20-F and other information with the SEC. The SEC maintains a website that contains reports, proxy and information statements and other
information regarding issuers, such as us, that file electronically with the SEC. The address of the SEC website is www.sec.gov.
We also furnish to the SEC under cover of Form
6-K material information required to be made public in our home country, filed with and made public by any stock exchange on which we
are listed or distributed by us to our shareholders. As a foreign private issuer, we are exempt from, among other things, the rules under
the Exchange Act prescribing the furnishing and content of proxy statements and our executive officers, directors and principal shareholders
are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we
will not be required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly
as U.S. companies whose securities are registered under the Exchange Act.
We also maintain a corporate website at http://ir.meihuamed.com/.
Information contained in, or that can be accessed through, our website is not a part of, and shall not be incorporated by reference into,
this prospectus or any documents incorporated by reference herein. We have included our website address in this prospectus supplement
solely as an inactive textual reference.
INDEX TO THE UNAUDITED
CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
UNAUDITED CONDENSED CONSOLIDATED
FINANCIAL STATEMENTS
TABLE OF CONTENTS
MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
UNAUDITED CONDENSED CONSOLIDATED
BALANCE SHEETS
As of June 30, 2023 and December 31, 2022
(US$, except share data or otherwise noted)
| | |
June
30, 2023 | | |
December
31, 2022 | |
| | |
| | |
| |
| Assets | |
| | |
| |
| Current Assets | |
| | |
| |
| Cash | |
$ | 17,861,214 | | |
$ | 26,736,700 | |
| Bank acceptance receivables | |
| 18,374,380 | | |
| 22,085,846 | |
| Accounts receivable | |
| 79,052,428 | | |
| 68,945,792 | |
| Inventories | |
| 1,647,146 | | |
| 1,122,038 | |
| Prepayment and other current assets | |
| 15,329,511 | | |
| 16,428,779 | |
| Total current assets | |
| 132,264,679 | | |
| 135,319,155 | |
| | |
| | | |
| | |
| Property, plant and equipment | |
| 8,617,192 | | |
| 8,758,047 | |
| Intangible assets | |
| 3,876,027 | | |
| 497,600 | |
| Investment | |
| 5,997,634 | | |
| 6,669,655 | |
| Other noncurrent assets | |
| 11,856,920 | | |
| 12,333,122 | |
| Total assets | |
$ | 162,612,452 | | |
$ | 163,577,579 | |
| | |
| | | |
| | |
| Liabilities and shareholders’ equity | |
| | | |
| | |
| Liabilities | |
| | | |
| | |
| Current liabilities | |
| | | |
| | |
| Short-term bank borrowings | |
$ | 7,171,128 | | |
$ | 6,089,428 | |
| Accounts payable | |
| 13,820,348 | | |
| 16,096,165 | |
| Taxes payable | |
| 1,451,855 | | |
| 1,131,276 | |
| Accrued expenses and other current liabilities | |
| 778,369 | | |
| 856,698 | |
| Total current liabilities | |
| 23,221,700 | | |
| 24,173,567 | |
| | |
| | | |
| | |
| Long term loan | |
| - | | |
| 724,932 | |
| Total liabilities | |
| 23,221,700 | | |
| 24,898,499 | |
| | |
| | | |
| | |
| Commitments and contingencies | |
| | | |
| | |
| Shareholders’ equity | |
| | | |
| | |
| Ordinary share, $0.0005 par value, 80,000,000 shares authorized, 23,940,000 and
23,940,000 shares issued and outstanding as of June 30, 2023 and December 31, 2022, respectively | |
| 11,970 | | |
| 11,970 | |
| Preferred share, $0.0005 par value, 20,000,000 shares authorized, no shares
issued and outstanding as of as of June 30, 2023 and December 31, 2022 | |
| - | | |
| - | |
| Additional paid-in capital | |
| 42,967,006 | | |
| 42,967,006 | |
| Statutory surplus reserves | |
| 15,665,860 | | |
| 15,665,860 | |
| Retained earnings | |
| 90,392,246 | | |
| 83,330,239 | |
| Accumulated other comprehensive income
(loss) | |
| (10,146,195 | ) | |
| (3,852,138 | ) |
| Total shareholders’ equity | |
| 138,890,887 | | |
| 138,122,937 | |
| Non-controlling interest | |
| 499,865 | | |
| 556,143 | |
| TOTAL EQUITY | |
| 139,390,752 | | |
| 138,679,080 | |
| | |
| | | |
| | |
| Total liabilities and
shareholders’ equity | |
$ | 162,612,452 | | |
$ | 163,577,579 | |
The accompanying notes
form an integral part of these consolidated financial statements.
MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
UNAUDITED CONDENSED CONSOLIDATED
STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
For the six months ended June 30, 2023 and
2022
(US$, except share data or otherwise noted)
| | |
For the Six months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| Revenues | |
| | |
| |
| Third party sales | |
$ | 48,178,325 | | |
$ | 54,803,181 | |
| Related party sales | |
| 11,751 | | |
| 29,666 | |
| Total revenues | |
| 48,190,076 | | |
| 54,832,847 | |
| Cost of revenues | |
| 31,019,347 | | |
| 33,941,115 | |
| | |
| | | |
| | |
| Gross profit | |
| 17,170,729 | | |
| 20,891,732 | |
| | |
| | | |
| | |
| Operating expenses | |
| | | |
| | |
| Selling | |
| 3,161,070 | | |
| 3,311,649 | |
| General and administrative | |
| 3,452,610 | | |
| 4,799,711 | |
| Research and development | |
| 1,460,376 | | |
| 1,642,204 | |
| Written-off Tai He deposit | |
| - | | |
| 2,469,466 | |
| Total operating expenses | |
| 8,074,056 | | |
| 12,223,030 | |
| | |
| | | |
| | |
| Income from operations | |
| 9,096,673 | | |
| 8,668,702 | |
| | |
| | | |
| | |
| Other (income) expense: | |
| | | |
| | |
| Interest expense | |
| 128,973 | | |
| 98,805 | |
| Interest income | |
| (361,532 | ) | |
| (19,725 | ) |
| Currency exchange gain | |
| 119,193 | | |
| (449,217 | ) |
| Other expense, net | |
| 114,298 | | |
| 50,180 | |
| Total other (income)
expenses | |
| 932 | | |
| (319,957 | ) |
| | |
| | | |
| | |
| Income before income tax provision | |
| 9,095,741 | | |
| 8,988,659 | |
| Income taxes expense | |
| 2,064,212 | | |
| 2,433,772 | |
| Net income | |
| 7,031,529 | | |
$ | 6,554,887 | |
| Net loss attributable to non-controlling
interests | |
| (30,478 | ) | |
| - | |
| Net income attributable to shareholders | |
| 7,062,007 | | |
| 6,554,887 | |
| | |
| | | |
| | |
| Foreign currency translation adjustment
– gain / (loss) | |
| (6,319,857 | ) | |
| (6,133,093 | ) |
| Comprehensive (loss)
income | |
$ | 711,672 | | |
$ | 421,794 | |
| Comprehensive loss attributable to non-controlling interests | |
| (56,278 | ) | |
| - | |
| Comprehensive (loss) income
attributable to shareholders | |
| 767,950 | | |
| 421,794 | |
| | |
| | | |
| | |
| Weighted average number of ordinary shares - basic and diluted | |
| 23,940,000 | | |
| 22,873,370 | |
| | |
| | | |
| | |
| Basic & diluted net income per ordinary
share | |
$ | 0.29 | | |
$ | 0.29 | |
The accompanying notes form an integral part
of these consolidated financial statements.
MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
UNAUDITED CONDENSED CONSOLIDATED
STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
For the six months ended June 30, 2023 and
2022
(US$, except share data)
| | |
Ordinary
shares | | |
Ordinary
shares
amount | | |
Additional
paid-in
capital | | |
Ordinary
shares
subscribed | | |
Statutory
surplus
reserves | | |
Retained
earnings | | |
Accumulated
other
comprehensive
income (loss) | | |
Non-
controlling
interests | | |
Total
Equity | |
| Balance
as of December 31, 2021 | |
| 20,000,000 | | |
$ | 10,000 | | |
$ | 9,716,484 | | |
$ | - | | |
$ | 15,178,467 | | |
$ | 77,574,663 | | |
$ | 5,288,988 | | |
| - | | |
$ | 107,768,602 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Ordinary
shares subscribed | |
| 3,940,000 | | |
| 1,970 | | |
| 33,748,358 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 33,750,328 | |
| Net
income | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 6,554,887 | | |
| - | | |
| - | | |
| 6,554,887 | |
| Currency
translation adjustment | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (6,133,093 | ) | |
| - | | |
| (6,133,093 | ) |
| Balance
as of June 30, 2022 | |
| 23,940,000 | | |
$ | 11,970 | | |
$ | 43,464,842 | | |
| - | | |
$ | 15,178,467 | | |
$ | 84,129,550 | | |
$ | (844,105 | ) | |
| - | | |
$ | 141,940,724 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
as of December 31, 2022 | |
| 23,940,000 | | |
$ | 11,970 | | |
$ | 42,967,006 | | |
| - | | |
$ | 15,665,860 | | |
$ | 83,330,239 | | |
$ | (3,852,138 | ) | |
| 556,143 | | |
$ | 138,679,080 | |
| Net
income | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 7,062,007 | | |
| - | | |
| (30,478 | ) | |
| 7,031,529 | |
| Currency
translation adjustment | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (6,294,057 | ) | |
| (25,800 | ) | |
| (6,319,857 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
as of June 30, 2023 | |
| 23,940,000 | | |
| 11,970 | | |
| 42,967,006 | | |
| - | | |
| 15,665,860 | | |
| 90,392,246 | | |
| (10,146,195 | ) | |
| 499,865 | | |
| 139,390,752 | |
The accompanying notes form an integral part
of these consolidated financial statements.
MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
UNAUDITED CONDENSED CONSOLIDATED
STATEMENTS OF CASH FLOWS
For the six months ended June 30, 2023 and
2022
(US$)
| | |
For
The Six Months Ended June
30, | |
| | |
2023 | | |
2022 | |
| Cash Flows from operating activities: | |
| | |
| |
| Net income | |
$ | 7,031,529 | | |
$ | 6,554,887 | |
| Adjustments for items not affecting cash: | |
| | | |
| | |
| Depreciation | |
| 283,484 | | |
| 239,597 | |
| Amortization | |
| 13,733 | | |
| 13,417 | |
| Net loss from disposal of property, plant and equipment | |
| 104,572 | | |
| - | |
| Written-off Tai He deposit | |
| - | | |
| 2,469,466 | |
| Deferred tax expenses (benefit) | |
| - | | |
| (294,567 | ) |
| Currency exchange (gain) loss | |
| 119,193 | | |
| - | |
| Loss from equity method investments | |
| 1,632 | | |
| - | |
| Gain from cost method investments | |
| (202 | ) | |
| - | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Bank acceptance receivables | |
| 2,755,706 | | |
| (9,730,622 | ) |
| Accounts receivable | |
| (14,101,576 | ) | |
| 3,472,261 | |
| Inventories | |
| (606,934 | ) | |
| (7,197 | ) |
| Prepayments and other assets | |
| 178,920 | | |
| (5,268,377 | ) |
| Due from related parties | |
| - | | |
| (33,523 | ) |
| Accounts payable | |
| (1,559,255 | ) | |
| (4,851,578 | ) |
| Taxes payable | |
| 393,343 | | |
| (285,556 | ) |
| Accrued expenses and other current liabilities | |
| (38,714 | ) | |
| (64,985 | ) |
| Advance from customers | |
| - | | |
| (12,039 | ) |
| Net cash used in operating activities | |
| (5,424,569 | ) | |
| (7,798,816 | ) |
| | |
| | | |
| | |
| Cash flows from investing activities: | |
| | | |
| | |
| Purchases of property, plant and equipment | |
| (1,001,925 | ) | |
| (459,163 | ) |
| Additions to intangible assets | |
| (3,581,058 | ) | |
| - | |
| Proceeds from disposal of property, plant and equipment | |
| 355,993 | | |
| - | |
| Proceeds from disposal of long-term investment | |
| 360,839 | | |
| - | |
| Net cash used in investing activities | |
| (3,866,151 | ) | |
| (459,163 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | |
| Proceeds from short-term bank borrowings | |
| 5,340,415 | | |
| 3,549,857 | |
| Repayments of short-term bank borrowings | |
| (4,618,738 | ) | |
| (2,932,491 | ) |
| Shareholder contribution | |
| - | | |
| 34,527,480 | |
| Net cash provided by financing activities | |
| 721,677 | | |
| 35,144,846 | |
| | |
| | | |
| | |
| Effect of foreign exchange rate changes | |
| (306,443 | ) | |
| (370,615 | ) |
| Net increase (decrease)
in cash | |
| (8,875,486 | ) | |
| 26,516,252 | |
| Cash, beginning of year | |
| 26,736,700 | | |
| 8,149,276 | |
| Cash, end of year | |
$ | 17,861,214 | | |
$ | 34,665,528 | |
| | |
| | | |
| | |
| SUPPLEMENTAL DISCLOSURE OF CASH FLOWS INFORMATION: | |
| | | |
| | |
| Cash paid during the period for: | |
| | | |
| | |
| Cash paid for income tax | |
$ | 2,313,417 | | |
$ | 2,956,643 | |
| Cash paid for interest | |
$ | 128,973 | | |
$ | 98,804 | |
| | |
| | | |
| | |
| Non-cash transactions | |
| | | |
| | |
| Shareholder contribution through deferred cost | |
| - | | |
| 1,277,152 | |
The accompanying notes form an integral part
of these consolidated financial statements.
MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
NOTES TO UNAUDITED CONDENSED
CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and principal activities
Principal Activities:
Meihua International Medical Technologies Co.,
Ltd. (“Meihua”) was incorporated on November 10, 2020 in the Cayman Islands. Meihua is a holding company with no operations.
Meihua produces and sells medical consumables through its wholly owned subsidiaries located in People’s Republic of China (“PRC”
or “China”). Below is Meihua’s organizational chart, as well as a description of the ownership structure.

Entity
Name | |
Registered
Location | |
Percentage
of
ownership | |
Date of
incorporation | |
Principal
activities |
| Meihua International Medical Technologies
Co., Ltd. (“Meihua”) | |
Cayman | |
Parent | |
November 10, 2020 | |
Investment holding |
| | |
| |
| |
| |
|
康复国际医疗有限公司
Kang Fu International Medical Co., Limited (“Kang Fu”) | |
Hong Kong | |
100% by Meihua | |
October 13, 2015 | |
Investment holding |
| | |
| |
| |
| |
|
扬州华达医疗器械有限公司
Yangzhou Huada Medical Equipment Co., Ltd. (“Huada”) | |
Yangzhou | |
100% by Kang Fu | |
December 24, 2001 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
江苏亚达科技集团有限公司
Jiangsu Yada Technology Group Co., Ltd. (“Yada”) | |
Yangzhou | |
100% by Huada | |
December 5, 1991 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
江苏华东医疗器械实业有限公司
Jiangsu Huadong Medical Device Industry Co., Ltd. (“Huadong”) | |
Yangzhou | |
100% by Yada | |
November 18, 2000 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
扬州光辉医疗科技有限公司*
Yangzhou Guanghui Medical Technology Co., Ltd. (“Guanghui”) | |
Yangzhou | |
100% by Huadong | |
December 22, 2020 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
海南国械医疗科技有限公司
Hainan GuoxieTechnology Group Co. Ltd. (“Hainan Guoxie”) | |
Hainan | |
55% by Kang Fu | |
October 07, 2021 | |
Medical Equipment Sales |
Kang Fu was incorporated on October 13, 2015
with a registered capital of HKD 53,911,815 ($6,911,771). Kang Fu is a holding company with no operations. The following operating entities
(Huada, Yada and Huadong) are all directly and indirectly 100% owned by Kang Fu for all the periods presented.
Huada is a subsidiary wholly owned by Kang Fu
and established in Yangzhou, China on December 24, 2001 with a registered capital of $17,193,021.
Yada is a subsidiary wholly owned by Huada and
was established in Yangzhou, China on December 5, 1991 with a registered capital of RMB51,390,000.
Huadong is a subsidiary wholly owned by Yada
and was established in Yangzhou, China on November 18, 2000 with a registered capital of RMB50,000,000.
Those three subsidiaries primarily manufacture
and sell Class I, II and III disposable medical devices under the Company’s own brands, and distribute Class I, II and III disposable
medical devices sourced from other manufacturers to our domestic and overseas customers.
Guanghui was a subsidiary wholly owned by Huadong
and was dissolved on June 1, 2023.
Hainan Guoxie is a subsidiary 55% owned by Kang
Fu and established in Hainan, China on October 07, 2021 with a registered capital of RMB100,000,000.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying unaudited condensed consolidated
financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S.
GAAP”) pursuant to the rules and regulations of the Securities Exchange Commission (“SEC”). The interim results of
operations are not necessarily indicative of results to be expected for any other interim period or for a full year. In the opinion of
management, all adjustments, consisting only of normal recurring adjustments, considered necessary for a fair presentation of its financial
position and operating results have been included. These financial statements should be read in conjunction with the Company’s
audited consolidated financial statements and the related notes thereto for the fiscal years ended December 31, 2022 and 2021.
Use of Estimates
The preparation of unaudited condensed consolidated
financial statements in conformity with U.S. GAAP requires management to make certain estimates and assumptions that affect the amounts
reported and disclosed in the consolidated financial statements and related notes.
The most significant estimates and judgments
include allowance for bad debts and the valuation of inventory. Actual amounts could differ from those estimates.
Non-controlling interests
Non-controlling interest represents the portion
of the net assets of subsidiaries attributable to interests that are not owned or controlled by the Company. The non-controlling interest
is presented in the consolidated balance sheets, separately from equity attributable to the shareholders of the Company. Non-controlling
interest’s operating results are presented on the face of the consolidated statements of income and comprehensive income as an
allocation of the total income for the year between non-controlling shareholders and the shareholders of the Company. As of June 30,
2023, non-controlling interests represent one non-controlling shareholders’ proportionate share of equity interests in Hainan Guoxie.
Functional Currency and Foreign Currency
Translation
The Company’s reporting currency is the
United States dollar (“US$”). The Company’s operations are principally conducted through the PRC subsidiaries where
the local currency is the functional currency. Therefore, the functional currency of Kang Fu is the Hong Kong dollar and the functional
currency of Huada, Yada, Huadong and Guanghui is the Renminbi (“RMB”).
Transactions denominated in currencies other
than the functional currencies are translated into the functional currency of the entity at the exchange rates prevailing on the transaction
dates. Monetary assets and liabilities denominated in currencies other than the applicable functional currency are translated into the
functional currency at the prevailing rates of exchange at the balance date. The resulting exchange differences are reported in the consolidated
statements of income and comprehensive income.
The assets and liabilities of the Company are
translated at the exchange spot rate at the balance sheet date, stockholders’ equity is translated at the historical rates and
the revenues and expenses are translated at the average exchange rates for the periods, except that the exchange rate used for translation
from Hong Kong dollar to US$ was 7.8000, a pegged rate determined by the linked exchange rate system in Hong Kong. This pegged rate was
used to translate Kang Fu’s balance sheets, income statement items and cash flow items for both the six months ended June 30, 2023
and 2022. The resulting translation adjustments are reported under other comprehensive income in the consolidated statements of income
and comprehensive income in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification
(“ASC”) 220, Comprehensive Income. The following are the exchange rates that were used in translating the Company’s
PRC subsidiaries’ financial statements into the consolidated financial statements:
| | |
June
30, 2023 | |
December 31, 2022 | |
June
30, 2022 |
| | |
| |
| |
|
| Period-end spot rate | |
US$1=RMB 7.2513 | |
US$1=RMB 6.8972 | |
US$1=RMB 6.6981 |
| | |
| |
| |
|
| Average rate | |
US$1=RMB 6.9283 | |
US$1=RMB 6.7290 | |
US$1=RMB 6.4791 |
Certain Risks and Concentration
The Company’s financial instruments that
potentially subject the Company to significant concentrations of credit risk consist primarily of cash and receivables. As of six months
ended June 30, 2023 and December 31, 2022 substantially all the Company’s cash were held in major financial institutions located
in Hong Kong and the PRC, which management considers to be of high credit quality.
For the six months ended June 30, 2023, two customers
accounted for approximately 18.18% and 10.01% of the Company’s total revenues. For the six months ended June 30, 2022, two customers
accounted for approximately 32.53% and 11.25% of the Company’s total revenues.
As of June 30, 2023, two customers accounted
for approximately 30.92% and 21.31% of the Company’s accounts receivable. As of December 31, 2022, two customers accounted for
approximately 27.73% and 13.14% respectively, of the Company’s accounts receivable.
For the six months ended June 30, 2023, one supplier
accounted for approximately 14.73% of the Company’s total purchases. As of six months ended June 30, 2022, there was no supplier
that individually represented greater than 10% of the total purchase of the Company’s total purchases.
Fair Value Measurement
Fair value is the price that would be received
from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company
considers the principal or most advantageous market in which it would transact, and it considers assumptions that market participants
would use when pricing the asset or liability.
The Company adopted the guidance of Accounting
Standards Codification (“ASC”) 820 for fair value measurements which clarifies the definition of fair value, prescribes methods
for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
| |
Level 1: |
Inputs
are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| |
|
|
| |
Level 2: |
Inputs
are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets
and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated
by observable market data. |
| |
|
|
| |
Level 3: |
Inputs
are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would
use in pricing the asset or liability based on the best available information. |
The Company’s financial instruments include
cash, accounts receivable, bank acceptance receivables, due from related parties, accounts payable, other liabilities and accrued expenses
and short-term bank borrowings. The carrying amounts approximate their fair values due to their short maturities as of June 30, 2023
and December 31, 2022.
The Company noted no transfers between levels
during any of the periods presented. The Company did not have any instruments that were measured at fair value on a recurring or non-recurring
basis as of June 30, 2023 and December 31, 2022.
Cash
Cash consists of petty cash on hand and cash
held in banks, which are highly liquid and are unrestricted as to withdrawal or use.
Bank Acceptance Receivables
Bank acceptance receivables are issued by bank
under the request of the Company’s customers, to pay for the purchased goods. The Company can choose to hold acceptance notes until
maturity and receive the face value payment from the bank, or sell (exchange) the acceptance notes at a discount to another party willing
to wait until maturity to receive the bank’s promised payment. The maturity date of the receivables is all within one year of the
original issuance date and carried at face value. The Company is not lending money, it just sells goods to the customers (customers can
pay the purchased goods by cash, accounts receivable or bank acceptance receivables). The receivables mature within one year, and are
non-interest bearing. As bank acceptance receivables are issued by the banks and payments are guaranteed. The Company has not discounted
any bank acceptances and there were no endorsed bank acceptances that are unmatured as of June 30, 2023. The Company collected approximately
$6.0 million as of August 31, 2023.
Accounts Receivable and Allowance for Doubtful
Accounts
Accounts receivable represent trade receivables
and are recognized initially at fair value and subsequently adjusted for any allowance for doubtful accounts or impairment.
The Company follows the guidance under ASC 326,
Financial Instruments – Credit Losses, Measurement of Credit Losses on Financial Instruments. The standard uses a new forward-looking
“methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information
to inform credit loss estimates.” The Company has adopted the loss rate methodology to estimate historical losses on accounts receivables.
The Company has adopted the aging methodology to estimate the credit losses on accounts receivables. The historical data is adjusted
to account for forecasted changes in the macroeconomic environment in order to calculate the current expected credit loss. The provision
is recorded against accounts receivables balances, with a corresponding charge recorded in the consolidated statements of operations
and comprehensive income.
The Company historically has not had material
bad debts in accounts receivable. There were no bad debt expenses related to accounts receivable for six months ended June 30, 2023 and
2022, and there was no provision for doubtful accounts as of June 30, 2023 and December 31, 2022.
Inventories
Inventories are valued using the lower of cost
or net realizable value. Cost is principally determined using the weighted-average method. Manufactured inventories included cost of
materials, labor and overhead expenses. The Company records adjustments to inventory for excess quantities, obsolescence, or impairment,
when appropriate, to reflect inventory at net realizable value. These adjustments are based upon a combination of factors including current
sales volume, market conditions, lower of cost or market analysis and expected realizable value of the inventory.
There were no write-downs recognized of inventories
as of June 30, 2023 and December 31, 2022.
Prepayment and other current assets
As of June 30, 2023 and December 31, 2022, prepayment
and other current assets were $15,329,511 and $16,428,779.
Prepayment and other assets primarily consist
of prepayments for land use right and property, refundable tax credits and receivables, security deposits made to customers, advances
to employees, which are presented net of allowance for doubtful accounts. These balances are unsecured and are reviewed periodically
to determine whether their carrying value has become impaired. The Company considers the balances to be impaired if the utilization or
refund of the balances becomes doubtful. The Company uses the aging method to estimate the allowance for uncollectible balances. The
allowance is also based on management’s best estimate of specific losses on individual exposures, as well as a provision on historical
trends of collections and utilizations. Actual amounts received or utilized may differ from management’s estimate of credit worthiness
and the economic environment. Delinquent account balances are written off against allowance for doubtful accounts after management has
determined that the likelihood of collection is not probable. The allowance for doubtful accounts amounted to nil as of June 30, 2023
and December 31, 2022.
Property, Plant and Equipment
Property, plant and equipment items are recorded
at their historic cost, less accumulated depreciation and impairment losses. The Company calculates depreciation using the straight-line
method, after consideration of the estimated residual values, over the following estimated useful lives:
| Category | |
Useful lives | |
Estimated
residual
value | |
| Buildings | |
20 years | |
| 10 | % |
| Machinery and Equipment | |
10 years | |
| 10 | % |
| Motor vehicles | |
5 years | |
| 10 | % |
| Electronic Equipment | |
5 years | |
| 10 | % |
| Office Equipment | |
3 years | |
| 10 | % |
| Inspection Equipment | |
5 years | |
| 10 | % |
Major improvements are capitalized and expenditures
for maintenance and repairs are expensed as incurred. Construction in progress represents property, plant and equipment under construction
or being installed. Costs include original cost, installation, construction and other direct costs. Interest expenses directly related
to construction in progress would be capitalized. Construction in progress is transferred to the appropriate fixed asset account and
depreciation commences when the asset has been substantially completed and placed in service.
Intangible Assets
Intangible assets are non-monetary assets without
physical substance. These items are initially measured at cost and subsequently carried at cost less any accumulated amortization and
impairment losses. Intangible assets with finite useful lives are amortized on a straight-line basis over their estimated useful lives.
Amortization of finite-lived intangible assets is computed using the straight-line method over the estimated useful lives, which is as
follows:
| Category | |
Useful lives |
| Land use rights | |
50 years |
| Patent | |
5 years |
| Trademark | |
10 years |
Impairment of Long-Lived Assets
The Company accounts for impairment of long-lived
assets in accordance with Accounting Standards Codification (“ASC”) 360, Property, Plant and Equipment. (“ASC
360”). Long-lived assets consist primarily of property, plant and equipment, and intangible assets. In accordance with ASC 360,
the Company evaluates the carrying value of long-lived assets when it determines a triggering event has occurred, or whenever events
or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. When indicators exist, recoverability
of assets is measured by a comparison of the carrying value of the asset group to the estimated undiscounted future net cash flows expected
to be generated by the asset. Examples of such triggering events include a significant disposal of a portion of such assets, and adverse
change in the market involving the business employing the related assets. If such assets are determined not to be recoverable, the Company
performs an analysis of the fair value of the asset group and will recognize an impairment loss when the fair value is less than the
carrying amounts of such assets. The fair value, based on reasonable and supportable assumptions and projections, require subjective
judgments. Depending on the assumptions and estimates used, the appraised fair value projected in the evaluation of long-lived assets
can vary within a range of outcomes. The Company considers the likelihood of possible outcomes in determining the best estimate for the
fair value of the assets. The Company did not record any impairment charges for the six months ended June 30, 2023 and 2022. There can
be no assurance that future events will not have impact on company revenue or financial position which could result in impairment in
the future.
Investment
In accordance with Financial Accounting Standards
Board (“FASB”) ASC 321, “Investment-Equity Securities,” the Company accounts for non-marketable securities on
a prospective basis. Equity investments that do not have readily determinable fair values and do not qualify for the net asset value
practical expedient are eligible for the measurement alternative.
On March 3, 2011, Yada invested in Yangzhou Juyuan
Guarantee Co., Ltd (“Juyuan”) and obtained 12% equity interest of Juyuan. For the Company’s passive and without significant
influence or control equity investment in private company which do not have readily determinable fair values, the Company has elected
the measurement alternative defined as cost, less impairment, plus or minus adjustments resulting from observable price changes in orderly
transactions for the identical or a similar investment of the Company. The investment is reviewed periodically to determine if its value
has been impaired and adjustments are recorded as necessary in profit or loss for the period. On January 5, 2023, majority shareholder
of Juyuan purchased 5% equity interest of Juyuan from Yada for a consideration of $360,839 (RMB 2.5 million).
Investments in entities in which the Company
can exercise significant influence but does not own a majority equity interest or control are accounted for using the equity method of
accounting in accordance with ASC 323, Investments-Equity Method and Joint Ventures (“ASC 323”). Under the equity method,
the Company initially records its investment at cost and the difference between the cost of the equity investee and the amount of the
underlying equity in the net assets of the equity investee is accounted for as if the investee were a consolidated subsidiary. The share
of earnings or losses of the investee are recognized in the consolidated statements of comprehensive loss. Equity method adjustments
include the Company’s proportionate share of investee income or loss, adjustments to recognize certain differences between the
Company’s carrying value and its equity in net assets of the investee at the date of investment, impairments, and other adjustments
required by the equity method. The Company assesses its equity investment for other-than-temporary impairment by considering factors
as well as all relevant and available information including, but not limited to, current economic and market conditions, the operating
performance of the investees including current earnings trends, the general market conditions in the investee’s industry or geographic
area, factors related to the investee’s ability to remain in business, such as the investee’s liquidity, debt ratios, and
cash burn rate and other company-specific information.
Investments in equity securities without readily
determinable fair values are measured at cost minus impairment adjusted by observable price changes in orderly transactions for the identical
or a similar investment of the same issuer. These investments are measured at fair value on a nonrecurring basis when there are events
or changes in circumstances that may have a significant adverse effect. An impairment loss is recognized in the consolidated statements
of comprehensive loss equal to the amount by which the carrying value exceeds the fair value of the investment. Prior to the adoption
of ASU 2016-01 on January 1, 2019, these investments were accounted for using the cost method of accounting, measured at cost less other-than-temporary
impairment.
On December 1, 2022, Huadong invested RMB 40
million into Jiangsu Zhongxiangxin International Science and Technology Innovation Park Co., Ltd. (“Zhongxiangxin”), and
obtained 25% ownership interest of Zhongxiangxin. Zhongxiangxin manufactures and sells medical materials in the PRC. The Company accounted
for the investments using equity method, because the Company has significant influence but does not own a majority equity interest or
otherwise control over the equity investee. Under the equity method, the Company adjusts the carrying amount of the investment and recognizes
investment income or loss for its share of the earnings or loss of the investee after the date of investment. When the Company’s
share of losses in the equity investee equals or exceeds its interest in the equity investee, the Company does not recognize further
losses, unless the Company has incurred obligations or made payments or guarantees on behalf of the equity investee. For the six months
ended June 30, 2023 and 2022, the investment loss from Zhongxiangxin was $1,632 and nil.
The Company continually reviews its investments
in equity investees to determine whether a decline in fair value below the carrying value is other-than-temporary. The primary factors
the Company considers in its determination include the financial condition, operating performance and the prospects of the equity investee;
other company specific information such as recent financing rounds; the geographic region, market and industry in which the equity investee
operates; and the length of time that the fair value of the investment is below its carrying value. If the decline in fair value is deemed
to be other-than-temporary, the carrying value of the equity investee is written down to fair value.
For the for the six months ended June 30, 2023
and 2022, no impairment indicators were identified of its investment in the private company was no impairment recorded.
Value-added Tax
Value-added taxes (“VAT”) collected
from customers relating to product sales and remitted to governmental authorities are presented on a net basis. VAT collected from customers
is excluded from revenue which is recorded in VAT payable. The Company is subject to a VAT rate of 13%. The VAT payable may be offset
by VAT paid by the Company on raw materials and other materials included in the cost of producing or acquiring its finished products.
Related Parties
Parties are considered to be related to the Company
if the parties, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with
the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of principal
owners of the Company and its management and other parties with which the Company may deal with if one party controls or can significantly
influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from
fully pursuing its own separate interests. The Company discloses all significant related party transactions.
Revenue Recognition
Based on the requirements of ASC Topic 606, the
Company recognizes revenue when control of the promised goods or services is transferred to the customers in an amount that reflects
the consideration the Company expects to be entitled to receive in exchange for those goods or services. The Company primarily sells
its products to hospitals and medical equipment companies. Revenue is recognized when the following 5-step revenue recognition criteria
are met:
| 1) | Identify
the contract with a customer |
| 2) | Identify
the performance obligations in the contract |
| 3) | Determine
the transaction price |
| 4) | Allocate
the transaction price |
| 5) | Recognize
revenue when or as the entity satisfies a performance obligation |
Revenue from product sales is recognized at the
point in time control of the products is transferred, generally upon customer receipt based upon the standard contract terms. Shipping
and handling activities are considered to be fulfillment activities rather than promised services and are not, therefore, considered
to be separate performance obligations. The Company’s sales terms provide no right of return outside of a standard quality policy
and returns are generally not significant. Payment terms for product sales are generally set at 90 to 180 days after customer acceptance
of the product.
Revenue Disaggregation
The Company’s disaggregated revenues are
represented by two categories which are type of goods and type of customers.
Type of Goods
| | |
For The Six Months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| | |
US$ | | |
US$ | |
| Self-Manufactured Products | |
| 23,435,544 | | |
| 27,046,663 | |
| Resales of Sourced Disposable Medical Devices
from Third Party Manufacturers | |
| 24,754,532 | | |
| 27,786,184 | |
| Total Revenue | |
| 48,190,076 | | |
| 54,832,847 | |
Type of Customers
| | |
For The Six Months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| | |
US$ | | |
US$ | |
| Direct sales | |
| 4,305,506 | | |
| 4,582,321 | |
| Distributors | |
| 43,884,570 | | |
| 50,250,526 | |
| Total Revenue | |
| 48,190,076 | | |
| 54,832,847 | |
Earnings per Ordinary Share
Earnings (loss) per ordinary share is calculated
in accordance with ASC 260, Earnings per Share. Basic earnings (loss) per ordinary share is computed by dividing the net income
(loss) attributable to shareholders of the Company by the weighted average number of ordinary shares outstanding during the year. Diluted
earnings per ordinary share is computed in accordance with the treasury stock method and based on the weighted average number of ordinary
shares and dilutive ordinary share equivalents. Dilutive ordinary share equivalents are excluded from the computation of diluted earnings
per ordinary share if their effects would be anti-dilutive. There is no ordinary share equivalent issued to date.
Comprehensive Income (Loss)
ASC 220, Comprehensive Income (“ASC 220”)
establishes rules for reporting and display of comprehensive income and its components. ASC 220 requires that unrealized gains and losses
on the Company’s foreign currency translation adjustments be included in comprehensive income (loss).
Advertising Costs
The Company’s advertising costs are expensed
as incurred. Advertising expenses are included in selling expenses in the accompanying consolidated statements of income and comprehensive
income. Advertising expenses were $8,275, and $7,603 for the six months ended June 30, 2023, and 2022, respectively.
Research and Development Costs
Research and development expenses are expensed
as incurred. Research and development expenses were $1,460,376 and $1,642,204 for the six months ended June 30, 2023, and 2022, respectively.
Income Tax
Current income taxes are provided on the basis
of net profit for financial reporting purposes, adjusted for income and expense items which are not assessable or deductible for income
tax purposes, in accordance with the regulations of the relevant tax jurisdictions.
Deferred income taxes are recognized for temporary
differences between the tax bases of assets and liabilities and their reported amounts in the consolidated financial statements, net
operating loss carry forwards and credits. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management,
it is more likely than not that some portion or all of the deferred tax assets will not be realized. Current income taxes are provided
in accordance with the laws of the relevant taxing authorities. Deferred tax assets and liabilities are measured using enacted rates
expected to apply to taxable income in which temporary differences are expected to be reversed or settled. The effect on deferred tax
assets and liabilities of changes in tax rates is recognized in the statement of comprehensive income in the period of the enactment
of the change.
The Company considers positive and negative evidence
when determining whether a portion or all of its deferred tax assets will more likely than not be realized. This assessment considers,
among other matters, the nature, frequency and severity of current and cumulative losses, forecasts of future profitability, the duration
of statutory carry-forward periods, its experience with tax attributes expiring unused, and its tax planning strategies. The ultimate
realization of deferred tax assets is dependent upon its ability to generate sufficient future taxable income within the carry-forward
periods provided for in the tax law and during the periods in which the temporary differences become deductible. When assessing the realization
of deferred tax assets, the Company has considered possible sources of taxable income including (i) future reversals of existing
taxable temporary differences, (ii) future taxable income exclusive of reversing temporary differences and carryforwards, (iii) future
taxable income arising from implementing tax planning strategies, and (iv) specific known trend of profits expected to be reflected
within the industry.
The Company recognizes a tax benefit associated
with an uncertain tax position when, in its judgment, it is more likely than not that the position will be sustained upon examination
by a taxing authority. For a tax position that meets the more-likely-than-not recognition threshold, the Company initially and subsequently
measures the tax benefit as the largest amount that the Company judges to have a greater than 50% likelihood of being realized upon ultimate
settlement with a taxing authority. The Company’s liability associated with unrecognized tax benefits is adjusted periodically
due to changing circumstances, such as the progress of tax audits, case law developments and new or emerging legislation. Such adjustments
are recognized entirely in the period in which they are identified. The Company’s effective tax rate includes the net impact of
changes in the liability for unrecognized tax benefits and subsequent adjustments as considered appropriate by management. The Company
classifies interest and penalties recognized on the liability for unrecognized tax benefits as income tax expense.
Segment Reporting
FASB ASC 280, “Segment Reporting,”
establishes standards for reporting information about operating segments on a basis consistent with the Company’s internal organizational
structure as well as information of the Company’s business segments, geographical areas, segments and major customers. The Company
uses the “management approach” in determining reportable operating segments. The management approach considers the internal
organization and reporting used by the Company’s chief operating decision maker for making operating decisions and assessing performance
as the source for determining the Company’s reportable segments. The chief operating decision maker is the Company’s president
and Chief Executive Officer (“CEO”). Management, including the chief operating decision maker, reviews operating results
of different products at revenue level with no allocation of operating costs. Consequently, based on management’s assessment, the
Company has determined that it has only one operating segment as defined by FASB ASC 280.
The Company has disclosed the type of revenue by government category
as follows.
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
US$ | | |
US$ | |
| Category | |
Produced | | |
Purchased | | |
Total | | |
Produced | | |
Purchased | | |
Total | |
| Class I | |
| 3,561,156 | | |
| 4,462,704 | | |
| 8,023,860 | | |
| 3,812,092 | | |
| 3,845,205 | | |
| 7,657,297 | |
| Class II | |
| 17,313,377 | | |
| 17,761,970 | | |
| 35,075,347 | | |
| 20,185,052 | | |
| 19,712,006 | | |
| 39,897,058 | |
| Class III | |
| 524,802 | | |
| 873,575 | | |
| 1,398,377 | | |
| 416,595 | | |
| 1,168,221 | | |
| 1,584,816 | |
| Others | |
| 2,036,209 | | |
| 1,656,283 | | |
| 3,692,492 | | |
| 2,632,924 | | |
| 3,060,752 | | |
| 5,693,676 | |
| Total | |
| 23,435,544 | | |
| 24,754,532 | | |
| 48,190,076 | | |
| 27,046,663 | | |
| 27,786,184 | | |
| 54,832,847 | |
Class I, II, and III medical devices are defined
by the National Medical Products Administration of China according to their risk levels under the Regulation on the Supervision and Administration
of Medical Devices (2021 Revision), Article 6 as follows:
| ● | “Class
I Medical Devices” means medical devices with low risks, whose safety and effectiveness
can be ensured through routine administration. |
| ● | “Class
II Medical Devices” means medical devices with moderate risks, which shall be strictly
controlled and administered to ensure their safety and effectiveness. |
| ● | “Class
III Medical Devices” means medical devices with relatively high risks, which shall
be strictly controlled and administered through special measures to ensure their safety and
effectiveness. |
Furthermore, the Company has disclosed revenue by major product type
included in each government category.
| | |
| |
June
30, 2023 | | |
June
30, 2022 | |
| Category | |
Products | |
US$ | | |
US$ | |
| Class I | |
Eye drops bottle | |
| 1,073,853 | | |
| 1,346,164 | |
| | |
Oral medicine bottle | |
| 1,830,363 | | |
| 2,189,018 | |
| | |
Anal bag | |
| 849,099 | | |
| 395,292 | |
| | |
Other Class I | |
| 4,270,545 | | |
| 3,726,823 | |
| Subtotal-Class I | |
| |
| 8,023,860 | | |
| 7,657,297 | |
| Class II | |
Masks | |
| 47,946 | | |
| 211,468 | |
| | |
Identification tape | |
| 5,494,306 | | |
| 7,218,564 | |
| | |
Disposable medical brush | |
| 4,481,601 | | |
| 4,606,634 | |
| | |
Gynecological inspection kits | |
| 3,022,727 | | |
| 5,807,398 | |
| | |
Surgical kit | |
| 2,206,201 | | |
| 13,546,908 | |
| | |
Medical brush | |
| 2,809,448 | | |
| 2,586,945 | |
| | |
Medical kit | |
| 983,584 | | |
| 883,977 | |
| | |
Other Class II | |
| 16,029,534 | | |
| 5,035,164 | |
| Subtotal-Class II | |
| |
| 35,075,347 | | |
| 39,897,058 | |
| Class III | |
Electronic pump | |
| 138,751 | | |
| 67,866 | |
| | |
Anesthesia puncture kit | |
| 229,616 | | |
| 205,218 | |
| | |
Disposable infusion pump | |
| 113,335 | | |
| 78,453 | |
| | |
Infusion pump | |
| 178,461 | | |
| 90,036 | |
| | |
Electronic infusion pump | |
| 330 | | |
| 43,397 | |
| | |
Laparoscopic trocar | |
| 38 | | |
| 94,337 | |
| | |
Other Class III | |
| 737,846 | | |
| 1,005,509 | |
| Subtotal-Class III | |
| |
| 1,398,377 | | |
| 1,584,816 | |
| Others | |
| |
| 3,692,492 | | |
| 5,693,676 | |
| Total | |
| |
| 48,190,076 | | |
| 54,832,847 | |
For the six months ended June 30, 2023, and 2022,
revenues and assets within PRC contributed over 99.1% of the Company’s total revenues and assets.
The Outbreak of COVID-19
On January 30, 2020, the World Health Organization
declared the outbreak of the coronavirus disease (COVID-19) a “Public Health Emergency of International Concern,” and on
March 11, 2020, the World Health Organization characterized the outbreak as a “pandemic.” COVID-19 has had a severe and negative
impact on the Chinese and the global economy and such impact persists as of the date of this annual report.
In fiscal year 2020, COVID-19 had a significant
impact on our business and results of operations as the sales volume of masks rose sharply while the sales of products other than masks
declined due to an overall decrease in market demand. In fiscal year 2021, with the stable control of the domestic epidemic in China,
the market of masks was no longer in urgent shortage compared to the same period in 2020, and the production of epidemic prevention products
resumed more normal production levels. In general, with the precise control of the epidemic in China, our production and operations have
recovered smoothly, and the demand for other products has increased gradually. After the initial outbreak of COVID-19, from time to time,
some instances of COVID-19 infections have emerged in various regions of China, including the infections caused by the Omicron variants
in 2022. For example, a wave of infections caused by the Omicron variants emerged in Shanghai in 2022, and a series of restrictions and
quarantines were implemented to contain the spread.
Many of the restrictive measures previously adopted
by the PRC governments at various levels to control the spread of the COVID-19 virus have been revoked or replaced with more flexible
measures since December 2022. While the revocation or replacement of the restrictive measures to contain the COVID-19 pandemic could
have a positive impact on our normal operations, the extent of the impact on the Company’s future financial results will be dependent
on future developments such as the length and severity of the crisis, the potential resurgence of the crisis, future government actions
in response to the crisis and the overall impact of the COVID-19 pandemic on the global economy and capital markets, among many other
factors, all of which remain highly uncertain and unpredictable. Given this uncertainty, the Company is currently unable to quantify
the expected impact of the COVID-19 pandemic on its future operations, financial condition, liquidity and results of operations if the
current situation continues.
Recently Issued Accounting Standards
In October 2021, the FASB issued ASU No. 2021-08,
“Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities from Contracts with Customers”
(“ASU 2021-08”). This ASU requires entities to apply Topic 606 to recognize and measure contract assets and contract liabilities
in a business combination. The amendments improve comparability after the business combination by providing consistent recognition and
measurement guidance for revenue contracts with customers acquired in a business combination and revenue contracts with customers not
acquired in a business combination. The amendments are effective for the Company beginning after December 15, 2023, and are applied prospectively
to business combinations that occur after the effective date. The Company does not expect the adoption of ASU 2021-04 will have a material
effect on the consolidated financial statements.
In June
2022, the FASB issued ASU 2022-03 Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject to Contractual
Sale Restrictions. The update clarifies that a contractual restriction on the sale of an equity security is not considered part of the
unit of account of the equity security and, therefore, is not considered in measuring fair value. The update also clarifies that an entity
cannot, as a separate unit of account, recognize and measure a contractual sale restriction. The update also requires certain additional
disclosures for equity securities subject to contractual sale restrictions. For public business entities, the amendments in this Update
are effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. For all other entities,
the amendments are effective for fiscal years beginning after December 15, 2024, and interim periods within those fiscal years. Early
adoption is permitted for both interim and annual financial statements that have not yet been issued or made available for issuance.
As an emerging growth company, the standard is effective for the Company for the year ended
December 31, 2025. The Company is in the process of evaluating the impact of the new guidance
on its consolidated financial statements.
3. Prepayments and other assets
Prepayments and other current assets consist
of the following:
| | |
June
30, 2023 | | |
December
31, 2022 | |
| | |
US$ | | |
US$ | |
| Other receivable | |
| 159,817 | | |
| 239,148 | |
| Prepaid tax | |
| - | | |
| 250,410 | |
| Prepaid for land use right (1) | |
| 15,169,694 | | |
| 15,948,501 | |
| Prepaid for property (2) | |
| 11,856,920 | | |
| 12,323,842 | |
| Total | |
| 27,186,431 | | |
| 28,761,901 | |
| Less: non-current portion | |
| (11,856,920 | ) | |
| (12,333,122 | ) |
| Prepayments and other current assets | |
| 15,329,511 | | |
| 16,428,779 | |
| (1) | On
October 22, 2018, the Company signed a land use right agreement with the government of Touqiao
Town, Yangzhou City and paid RMB 50 million ($6.9 million) and RMB 60 million ($8.27 million),
respectively, in 2018 and 2019 according to the agreement. As a result of COVID-19, the land
use right had not been transferred to the Company as scheduled. Both parties agreed to cancel
the transaction and the funds prepaid for land use right will be returned to the Company
before December 31, 2023. |
| (2) | On
April 20, 2020, the Company signed a factory building purchase agreement with Jiangsu Qionghua
Group Co., Ltd. and paid deposit of RMB 85 million ($11.72 million). As a result of COVID-19,
the factory building had not been completed as scheduled. Both parties agreed to cancel the
transaction and that the deposit for the building would be returned to the Company on or
before December 31, 2025, with such deposit accumulating interest at an annual interest rate
of 3.5%. |
4. Inventories
Inventories consist of the following:
| | |
June
30, 2023 | | |
December 31, 2021 | |
| | |
US$ | | |
US$ | |
| Raw material | |
| 493,143 | | |
| 177,474 | |
| Work-in-process | |
| 20,501 | | |
| 343,795 | |
| Finished goods | |
| 977,974 | | |
| 560,119 | |
| Goods in transit | |
| 95,814 | | |
| | |
| Low-value consumables | |
| 59,714 | | |
| 40,650 | |
| Total | |
| 1,647,146 | | |
| 1,122,038 | |
For the six months ended June 30, 2023 and 2022, there were no writes-down
of inventories.
5. Intangible Assets
Intangible assets consisted of the following:
| | |
June
30, 2023 | | |
December
31, 2022 | |
| | |
US$ | | |
US$ | |
| Land use rights | |
| 4,134,521 | | |
| 752,887 | |
| Patents | |
| 27,582 | | |
| 28,997 | |
| Software | |
| 12,113 | | |
| 9,424 | |
| Trademarks | |
| 115,836 | | |
| 121,789 | |
| Total | |
| 4,290,052 | | |
| 913,097 | |
| Less: accumulated amortization | |
| 414,025 | | |
| 415,497 | |
| Intangible assets, net | |
| 3,876,027 | | |
| 497,600 | |
Amortization expense was $13,733 and $13,417
for the six months ended June 30, 2023 and 2022, respectively. Hainan Guoxie spent $3.4 million in purchasing land use right for the
six months ended June 30, 2023 and the price was fully paid. The land will be used for manufacturing facility.
The following table sets forth the Company’s
amortization expenses for the twelve months ending December 31 of the following years:
| 2023 | |
$ | 41,870 | |
| 2024 | |
| 83,740 | |
| 2025 | |
| 83,740 | |
| 2026 | |
| 82,953 | |
| 2027 | |
| 82,690 | |
| Thereafter | |
| 3,501,034 | |
| | |
$ | 3,876,027 | |
As of June 30, 2023 and December 31, 2022, the
Company pledged land use rights to secure bank borrowings to the Company as disclosed in Note 7.
6. Investment
On March 3, 2011, Yada invested RMB 6 million
into Yangzhou Juyuan Guarantee Co., Ltd. (“Juyuan”) and obtained 12% equity interest of Juyuan. Juyuan mainly provides financing
guarantee services and relevant consulting services to customers. Juyuan has only one executive director and one supervisor. Neither
the executive director nor supervisor is related to Yada. Therefore, Yada has neither control nor significant influence over Juyuan.
For the Company’s passive and without significant influence or control equity investment in a private company which does not have
readily determinable fair values, the Company has elected the measurement alternative defined as cost, less impairment, plus or minus
adjustments resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer.
On January 5, 2023, majority shareholder of Juyuan purchased 5% equity interest of Juyuan from Yada for a consideration of $360,839(RMB
2.5 million). The carrying value of the investment amounted to approximately $0.5 million as of June 30, 2023.
On December 1, 2022, Huadong invested RMB40 million
into Zhongxiangxin, and obtained 25% ownership interest of Zhongxiangxin. Zhongxiangxin manufactures and sells medical materials in the
PRC. The Company accounted for the investments using the equity method, because the Company has significant influence but does not own
a majority equity interest or otherwise control over the equity investee. Under the equity method, the Company adjusts the carrying amount
of the investment and recognizes investment income or loss for its share of the earnings or loss of the investee after the date of investment.
When the Company’s share of losses in the equity investee equals or exceeds its interest in the equity investee, the Company does
not recognize further losses, unless the Company has incurred obligations or made payments or guarantees on behalf of the equity investee.
For the six months ended June 30, 2023 and 2022, the investment loss from Zhongxiangxin was $1,632 and $nil.
For the six months ended June 30, 2023 and 2022,
no impairment indicators were identified related to revaluation of its investment in the private company was recorded.
7. Bank Borrowings
Bank borrowings are working capital loans from
banks in China. Short-term bank borrowings as of June 30, 2023 consisted of the following:
| Lender | |
Company | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank of Communications | |
Huadong | |
| 3.55 | % | |
1/18/2023 | |
5/25/2024 | |
| 4,000,000 | | |
| 551,625 | |
| Bank of Communications | |
Huadong | |
| 3.50 | % | |
11/3/2022 | |
4/25/2024 | |
| 5,000,000 | | |
| 689,532 | |
| Agricultural Bank of China | |
Huadong | |
| 3.60 | % | |
8/12/2022 | |
7/12/2023 | |
| 9,000,000 | | |
| 1,241,157 | |
| Jiangsu Yangzhou Rural Commercial Bank | |
Huadong | |
| 3.95 | % | |
1/30/2023 | |
2/15/2024 | |
| 5,000,000 | | |
| 689,532 | |
| Bank of China | |
Huadong | |
| 3.80 | % | |
3/10/2023 | |
3/9/2024 | |
| 10,000,000 | | |
| 1,379,063 | |
| Agricultural Bank of China | |
Yada | |
| 3.60 | % | |
12/8/2022 | |
12/6/2023 | |
| 10,000,000 | | |
| 1,379,063 | |
| Industrial and Commercial Bank
of China* | |
Yada | |
| 3.45 | % | |
2/17/2022 | |
2/16/2024 | |
| 9,000,000 | | |
| 1,241,156 | |
| Total | |
| |
| | | |
| |
| |
| 52,000,000 | | |
| 7,171,128 | |
Short-term bank borrowings as of December 31, 2022 consisted of the
following:
| Lender | |
Company | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank of Communications | |
Huadong | |
| 3.55 | % | |
3/9/2022 | |
1/19/2023* | |
| 4,000,000 | | |
| 579,946 | |
| Agricultural Bank of China | |
Huadong | |
| 3.40 | % | |
12/8/2022 | |
12/7/2023* | |
| 9,000,000 | | |
| 1,304,877 | |
| Jiangsu Yangzhou Rural Commercial Bank | |
Huadong | |
| 3.95 | % | |
2/17/2022 | |
3/2/2023* | |
| 5,000,000 | | |
| 724,932 | |
| Bank of China | |
Huadong | |
| 3.55 | % | |
3/9/2022 | |
1/19/2023* | |
| 5,000,000 | | |
| 724,932 | |
| Agricultural Bank of China | |
Yada | |
| 3.60 | % | |
12/8/2022 | |
12/6/2023* | |
| 10,000,000 | | |
| 1,449,864 | |
| Industrial and Commercial Bank of China | |
Yada | |
| 3.70 | % | |
2/18/2022 | |
2/21/2023* | |
| 9,000,000 | | |
| 1,304,877 | |
| Total | |
| |
| | | |
| |
| |
| 42,000,000 | | |
| 6,089,428 | |
| * | These
loans were renewed upon maturity. |
Interest expense was $128,973, and $98,805 for
the six months ended June 30, 2023 and 2022, respectively.
The Company’s short-term bank borrowings
are pledged by the Company’s assets and guaranteed by the Company’s major shareholders Yongjun Liu, Yin Liu and its subsidiary
Yada.
The carrying values of the Company’s pledged assets to secure
short-term borrowings by the Company are as follows:
| | |
June
30, 2023 | | |
December 31,
2022 | |
| | |
US$ | | |
US$ | |
| Buildings, net | |
| 3,432,150 | | |
| 2,777,379 | |
| Land use right, net | |
| 90,322 | | |
| 96,416 | |
| Total | |
| 3,522,472 | | |
| 2,873,795 | |
8. Long-term bank loan
There was no long-term bank loan as of June 30, 2023.
Long-term bank borrowings as of December 31, 2022 consisted of the
following:
| Lender | |
Company | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank of Communications | |
Huadong | |
| 3.50 | % | |
11/3/2022 | |
4/25/2024 | |
| 5,000,000 | | |
| 724,932 | |
| Total | |
| |
| | | |
| |
| |
| 5,000,000 | | |
| 724,932 | |
On November 3, 2022, the Company signed a loan
agreement with Bank of Communications to obtain a two-year loan of RMB5 million ($724,932). The loan bears a floating interest rate of
a benchmark rate (3.50%). Huada mortgages the property and land for guaranteed repayment of the loan. The principal shall be repaid on
April 25, 2024. The balance was reclassified to short-term bank loan as of June 30, 2023.
9. Taxes Payable
Taxes payable consisted of the following:
| | |
June
30, 2023 | | |
December 31,
2022 | |
| | |
US$ | | |
US$ | |
| VAT payable | |
| 368,195 | | |
| 380,926 | |
| Income tax payable | |
| 1,012,775 | | |
| 690,824 | |
| Other tax payable | |
| 71,348 | | |
| 59,526 | |
| Total | |
| 1,452,318 | | |
| 1,131,276 | |
10. Income Taxes
Cayman Islands
Under the current laws of the Cayman Islands,
the Company is not subject to tax on income or capital gain. Additionally, upon payments of dividends to the shareholders, no Cayman
Islands withholding tax is imposed.
Hong Kong
Under the current Hong Kong Inland Revenue Ordinance,
the Company’s Hong Kong subsidiary, Kang Fu, is subject to 16.5% income tax on its taxable income generated from operations in
Hong Kong. On December 29, 2017, the Hong Kong government announced a two-tiered profit tax rate regime. Under the two-tiered tax rate
regime, the first HK$2.0 million earned in assessable profits will be subject to an 8.25% lower tax rate and the remaining taxable income
will continue to be taxed at the existing 16.5% tax rate. The two-tiered tax regime becomes effective from the assessment year of 2018
and 2019, which is on or after April 1, 2018. The application of the two-tiered rates is restricted to only one nominated enterprise
among connected entities. Kang Fu has been nominated by the Company as the entity to apply the two-tiered rates among the group for the
assessment years of 2023 and 2022.
PRC
Provisions for income tax are as follows:
| | |
June
30, 2023 | | |
June 30,
2022 | |
| | |
US$ | | |
US$ | |
| Provisions for current income tax | |
| 2,064,697 | | |
| 2,433,772 | |
| Provisions for deferred income tax | |
| - | | |
| - | |
| Total | |
| 2,064,697 | | |
| 2,433,772 | |
The following is a reconciliation of the Company’s
total income tax expense to the income before income taxes for the six months ended June 30, 2023 and 2022, respectively:
| | |
June
30, 2023 | | |
June 30,
2022 | |
| | |
US$ | | |
US$ | |
| Income before income tax provision | |
| 9,097,681 | | |
| 8,988,658 | |
| Tax at the PRC EIT tax rates | |
| 2,203,896 | | |
| 2,689,271 | |
| Change in valuation allowance | |
| 16,934 | | |
| - | |
| Tax effect of non-deductible expenses | |
| 208,961 | | |
| 157,007 | |
| Tax effect of R&D expenses additional
deduction* | |
| (365,094 | ) | |
| (412,506 | ) |
| Income tax expense | |
| 2,064,697 | | |
| 2,433,772 | |
| * | According
to PRC tax regulations, an additional of 100% of current year R&D expenses may be deducted
from tax income. |
Under the Enterprise Income Tax Law (“EIT
Law”), Foreign Investment Enterprises (“FIEs”) and domestic companies are subject to Enterprise Income Tax (“EIT”)
at a uniform rate of 25%.
Huadong was granted a High and New Technology
Enterprise (“HNTE”) certificate and received a preferential tax rate of 15% for a three-year validity period from November
30, 2016 and the HNTE certificate was renewed on December 22, 2022 with a three-year validity period. Thus, Huadong will remain eligible
for a 15% preferential tax rate from January 1, 2016 through December 31, 2025.
The EIT Law also provides that an enterprise
established under the laws of a foreign country or region but whose “de facto management body” is located in the PRC be treated
as a resident enterprise for PRC tax purposes and consequently be subject to the PRC income tax at the rate of 25% for its global income.
The Implementing Rules of the EIT Law define the location of the “de facto management body” as “the place where the
exercising, in substance, of the overall management and control of the production and business operation, personnel, accounting, properties,
etc., of a non-PRC company is located.” Based on a review of surrounding facts and circumstances, the Company does not believe
that it is likely that its entities registered outside of the PRC should be considered as resident enterprises for the PRC tax purposes.
The EIT Law also imposes a withholding income
tax on dividends distributed by a FIE to its immediate holding company outside of the PRC. As a result, Kang Fu, which is the parent
of Huada, Yada and Huadong, is therefore subject to a maximum withholding tax of 10% on dividends distributed by Huada, Yada and Huadong.
In accordance with accounting guidance, all undistributed earnings are presumed to be transferred to the parent company and are subject
to the withholding taxes. The presumption may be overcome if the Company has sufficient evidence to demonstrate that the undistributed
dividends will be re-invested and the remittance of the dividends will be postponed indefinitely. As of June 30, 2023, the Company has
determined that the undistributed earnings in Huada, Yada and Huadong will be re-invested into the subsidiary for the expansion of the
Company’s business in mainland China and hence the remittance of dividends will be postponed indefinitely.
Uncertain tax positions
The Company evaluates each uncertain tax position
(including the potential application of interest and penalties) based on the technical merits, and measure the unrecognized benefits
associated with the tax positions. As of June 30, 2023, and 2022, the Company did not have any significant unrecognized uncertain tax
positions.
11. Commitments and Contingencies
Operating lease
The Company has an operating lease to rent an
office space in Shanghai. The lease term is 12 months, with the option to renew annually. Rent expense was $5,856 and $5,856 and is included
in general and administrative expenses for the six months ended June 30, 2023 and 2022, respectively. The Company has renewed the same
operating lease with a term from January 1, 2023 to December 31, 2023, with all other lease terms remaining the same.
Other commitments
The Company did not have other significant commitments,
long-term obligations or guarantees as of June 30, 2023 and 2022.
Contingencies
The Company is subject to legal proceedings and
regulatory actions in the ordinary course of business. The results of such proceedings cannot be predicted with certainty, but the Company
does not anticipate that the final outcome arising out of any such matter will have a material adverse effect on our consolidated business,
financial position, cash flows or results of operations taken as a whole.
On February 4, 2022, Macias Gini & O’Connell,
LLP (“Plaintiff”) initiated a lawsuit in San Francisco Superior Court. Plaintiff, a certified public accounting firm based
in the U.S., was hired by Kang Fu International Medical Co, and subsequently by Meihua International Medical Technologies Co, Ltd. (collectively,
“Meihua”), to audit Meihua’s consolidated financial statements for the 2018 and 2019 calendar years. Plaintiff seeks
damages from Meihua for its alleged failure to pay for services rendered in the amount of $210,000, plus interest and attorneys’
fees. The case was dismissed with prejudice in August of 2023.
On August 29, 2023, Zhu Cheng initiated a lawsuit
against Yada, Huada, Huadong and Kang Fu in Yangzhou Economic Development Zone Court. Zhu Cheng seeks damages from the above entities
for service fee of approximately $2.3 million (RMB 17.0 million). The Company is preparing to file a motion to dismiss the case. No contingent
liability has been accrued since the Company has deemed the possibility of loss to be remote.
12. Statutory Surplus Reserves and Restricted Net Assets
Pursuant to laws applicable to entities incorporated
in the PRC, the Company is required to make appropriations to certain reserve funds, comprising the statutory surplus reserve and the
discretionary surplus reserve, based on after-tax net income determined in accordance with generally accepted accounting principles of
the PRC (“PRC GAAP”). Appropriations to the statutory surplus reserve are required to be at least 10% of the after-tax net
income determined in accordance with PRC GAAP until the reserve is equal to 50% of the entity’s registered capital. Appropriations
to the discretionary surplus reserve are made at the discretion of the Board of Directors. As of December 31, 2022 and June 30, 2023,
the Company did not have a discretionary surplus reserve. As of December 31, 2022, all of the Company’s PRC subsidiaries reserves
had reached 50% of their registered capital threshold and, as a result, the Company stopped being required to allocate after-tax profits
to this reserve.
As a result of these PRC laws and regulations
and the requirement that distributions by PRC entities can only be paid out of distributable profits computed in accordance with PRC
GAAP, the PRC entities are restricted from transferring a portion of their net assets to the Company. Amounts restricted include paid-in
capital and the statutory reserves of the Company’s PRC subsidiaries. The aggregate amounts of capital and statutory reserves restricted
which represented the amount of net assets of the relevant subsidiaries in the Company not available for distribution was $15,665,860
as of June 30, 2023 and December 31, 2022.
Under PRC laws and regulations, statutory surplus
reserves are restricted to set-off against losses, expansion of production and operation and increasing registered capital of the respective
company and are not distributable other than upon liquidation. The reserves are not allowed to be transferred to the Company in terms
of cash dividends, loans or advances, nor allowed for distribution except under liquidation.
13. Related Party Transactions and Balances
Related Parties:
| Name
of related parties |
|
Relationship
with the Company |
| Shanghai Xinya Pharmaceutical
Hanjiang Co., Ltd. |
|
An entity controlled by
Kai Liu, son of Yongjun Liu |
| Yangzhou Meihua Import
and Export Co., Ltd. |
|
An entity controlled by
Kai Liu, son of Yongjun Liu |
Related Party Sales
The Company sells products to its related parties
and the sales amount from related parties for the six months end 2023 and 2022 are as follows:
Sales:
| | |
For the Six Months ended
June 30, | |
| Name of related party | |
2023 | | |
2022 | |
| Yangzhou Meihua Import and Export Co., Ltd. | |
$ | 11,751 | | |
$ | 18,849 | |
| Shanghai Xinya Pharmaceutical Hanjiang Co., Ltd. | |
| - | | |
| 10,818 | |
| Total | |
$ | 11,751 | | |
$ | 29,667 | |
14. Subsequent Events
The Company has evaluated the impact of events
that have occurred subsequent to June 30, 2023, through the issuance date of the consolidated financial statements, and concluded that
no subsequent events have occurred that would require recognition in the consolidated financial statements or disclosure in the notes
to the consolidated financial statements.
INDEX
TO THE CONSOLIDATED FINANCIAL STATEMENTS
MEIHUA
INTERNATIONAL MEDICAL TECHNOLOGIES CO., LTD.
CONSOLIDATED
FINANCIAL STATEMENTS
AS
OF AND FOR THE YEARS ENDED
DECEMBER
31, 2022 AND 2021
TABLE
OF CONTENTS
Report
of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of
Meihua International Medical Technologies Co., Ltd.
Opinion on the Financial Statements
We have audited the accompanying consolidated
balance sheets of Meihua International Medical Technologies Co., Ltd. (the “Company”) as of December 31, 2022 and 2021, and
the related consolidated statements of income and comprehensive income, changes in shareholders’ equity, and cash flows for each
of the two years in the period ended December 31, 2022, and the related notes to the financial statements (collectively referred to as
the “financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects,
the consolidated financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows
for each of the two years in the period ended December 31, 2022, in conformity with accounting principles generally accepted in the United
States of America.
Basis for Opinion
These financial statements are the responsibility
of the entity’s management. Our responsibility is to express an opinion on the entity’s financial statements based on our
audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”)
and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the
standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged
to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding
of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the entity’s
internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to
assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that
respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial
statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well
as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Kreit & Chiu CPA LLP
(Formerly Paris, Kreit & Chiu CPA LLP)
We have served as the Company’s auditor since 2022.
New York, New York
April 14, 2023
REPORT OF INDEPENDENT REGISTERED
PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Meihua International Medical Technologies Co., Ltd
Opinion on the Financial Statements
We have audited the accompanying consolidated
balance sheet of Meihua International Medical Technologies Co., Ltd and subsidiaries (collectively, the “Company”) as of
December 31, 2020, and the related consolidated statements of income and comprehensive income, changes in shareholders’ equity,
and cash flows for the year then ended, and the related notes (collectively referred to as the financial statements). In our opinion,
the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and
the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted
in the United States of America.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our
audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are
required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and
regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the
standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged
to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding
of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s
internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess
the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures
that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the
financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management,
as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for
our opinion.
/s/ Briggs & Veselka Co.
Houston, Texas
June 4, 2021
We have served as the Company’s auditor since 2021.
MEIHUA
INTERNATIONAL MEDICAL TECHNOLOGIES CO., LTD.
CONSOLIDATED
BALANCE SHEETS
As
of December 31, 2022 and 2021
(US$,
except share data or otherwise noted)
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| Assets | |
| | |
| |
| Current Assets | |
| | |
| |
| Cash | |
$ | 26,736,700 | | |
$ | 8,149,276 | |
| Bank acceptance receivables | |
| 22,085,846 | | |
| 19,379,845 | |
| Accounts receivable | |
| 68,945,792 | | |
| 67,101,297 | |
| Inventories | |
| 1,122,038 | | |
| 1,251,393 | |
| Prepayment and other current assets | |
| 16,428,779 | | |
| 1,394,539 | |
| Total current assets | |
| 135,319,155 | | |
| 97,276,350 | |
| | |
| | | |
| | |
| Property, plant and equipment | |
| 8,758,047 | | |
| 7,477,744 | |
| Intangible assets | |
| 497,600 | | |
| 562,001 | |
| Investment | |
| 6,669,655 | | |
| 941,531 | |
| Other noncurrent assets | |
| 12,333,122 | | |
| - | |
| Deposits | |
| - | | |
| 30,599,755 | |
| Total assets | |
$ | 163,577,579 | | |
$ | 136,857,381 | |
| | |
| | | |
| | |
| Liabilities and shareholders’
equity | |
| | | |
| | |
| Liabilities | |
| | | |
| | |
| Current liabilities | |
| | | |
| | |
| Short-term bank borrowings | |
$ | 6,089,428 | | |
$ | 5,178,420 | |
| Accounts payable | |
| 16,096,165 | | |
| 20,981,041 | |
| Taxes payable | |
| 1,131,276 | | |
| 2,082,252 | |
| Accrued expenses and other current liabilities | |
| 856,698 | | |
| 847,066 | |
| Total current liabilities | |
| 24,173,567 | | |
| 29,088,779 | |
| | |
| | | |
| | |
| Long term loan | |
| 724,932 | | |
| - | |
| Total liabilities | |
| 24,898,499 | | |
| 29,088,779 | |
| | |
| | | |
| | |
| Commitments and contingencies | |
| | | |
| | |
| Shareholders’ equity | |
| | | |
| | |
| Ordinary share, $0.0005 par value, 80,000,000 shares authorized, 23,940,000
and 20,000,000 shares issued and outstanding as of December 31, 2022 and 2021, respectively | |
| 11,970 | | |
| 10,000 | |
| Preferred share, $0.0005 par value, 20,000,000 shares authorized, no shares
issued and outstanding as of December 31, 2022 and 2021 | |
| - | | |
| - | |
| Additional paid-in capital | |
| 42,967,006 | | |
| 9,716,484 | |
| Statutory surplus reserves | |
| 15,665,860 | | |
| 15,178,467 | |
| Retained earnings | |
| 83,330,239 | | |
| 77,574,663 | |
| Accumulated other comprehensive income (loss) | |
| (3,852,138 | ) | |
| 5,288,988 | |
| Total shareholders’ equity | |
| 138,122,937 | | |
| 107,768,602 | |
| Non-controlling interest | |
| 556,143 | | |
| - | |
| TOTAL EQUITY | |
| 138,679,080 | | |
| 107,768,602 | |
| | |
| | | |
| | |
| Total liabilities and shareholders’
equity | |
$ | 163,577,579 | | |
$ | 136,857,381 | |
The
accompanying notes form an integral part of these consolidated financial statements.
MEIHUA
INTERNATIONAL MEDICAL TECHNOLOGIES CO., LTD.
CONSOLIDATED
STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
For
the years ended December 31, 2022, 2021 and 2020
(US$,
except share data or otherwise noted)
| | |
For the Years Ended December 31, | |
| | |
2022 | | |
2021 | | |
2020 | |
| Revenues | |
| | |
| | |
| |
| Third party sales | |
$ | 103,317,145 | | |
$ | 103,461,809 | | |
$ | 88,244,403 | |
| Related party sales | |
| 29,196 | | |
| 575,901 | | |
| 816,607 | |
| Total revenues | |
| 103,346,341 | | |
| 104,037,710 | | |
| 89,061,010 | |
| Cost of revenues | |
| 65,247,864 | | |
| 64,232,469 | | |
| 51,900,823 | |
| | |
| | | |
| | | |
| | |
| Gross profit | |
| 38,098,477 | | |
| 39,805,241 | | |
| 37,160,187 | |
| | |
| | | |
| | | |
| | |
| Operating expenses | |
| | | |
| | | |
| | |
| Selling | |
| 7,109,524 | | |
| 6,457,801 | | |
| 6,624,332 | |
| General and administrative | |
| 12,468,551 | | |
| 4,361,472 | | |
| 4,577,570 | |
| Research and development | |
| 2,962,904 | | |
| 2,725,014 | | |
| 2,492,059 | |
| Written-off Tai He deposit | |
| 4,755,536 | | |
| - | | |
| - | |
| Total operating
expenses | |
| 27,296,515 | | |
| 13,544,287 | | |
| 13,693,961 | |
| | |
| | | |
| | | |
| | |
| Income from operations | |
| 10,801,962 | | |
| 26,260,954 | | |
| 23,466,226 | |
| | |
| | | |
| | | |
| | |
| Other (income) expense: | |
| | | |
| | | |
| | |
| Interest expense | |
| 194,667 | | |
| 180,744 | | |
| 137,160 | |
| Interest income | |
| (63,725 | ) | |
| (23,855 | ) | |
| (36,583 | ) |
| Currency exchange gain | |
| (273,432 | ) | |
| (174,413 | ) | |
| (393,478 | ) |
| Other expense, net | |
| 53,205 | | |
| 50,437 | | |
| 25,551 | |
| Total other
(income) expenses | |
| (89,285 | ) | |
| 32,913 | | |
| (267,350 | ) |
| | |
| | | |
| | | |
| | |
| Income before income tax provision | |
| 10,891,247 | | |
| 26,228,041 | | |
| 23,733,576 | |
| Income taxes
expense | |
| 4,713,543 | | |
| 5,278,462 | | |
| 4,688,321 | |
| Net income | |
| 6,177,704 | | |
$ | 20,949,579 | | |
$ | 19,045,255 | |
| Net loss attributable to non-controlling
interests | |
| (65,265 | ) | |
| - | | |
| - | |
| Net income attributable to shareholders | |
| 6,242,969 | | |
| 20,949,579 | | |
| 19,045,255 | |
| | |
| | | |
| | | |
| | |
| Foreign currency translation adjustment
– gain / (loss) | |
| (9,155,028 | ) | |
| 2,083,243 | | |
| 4,759,973 | |
| Comprehensive
(loss) income | |
$ | (2,977,324 | ) | |
$ | 23,032,822 | | |
$ | 23,805,228 | |
| Comprehensive loss attributable to non-controlling interests | |
| (79,167 | ) | |
| - | | |
| - | |
| Comprehensive
(loss) income attributable to shareholders | |
| (2,898,157 | ) | |
| 23,032,822 | | |
| 23,805,228 | |
| | |
| | | |
| | | |
| | |
| Weighted average number of ordinary shares - basic and
diluted | |
| 23,411,068 | | |
| 20,000,000 | | |
| 20,000,000 | |
| | |
| | | |
| | | |
| | |
| Basic & diluted net income per
ordinary share | |
$ | 0.27 | | |
$ | 1.05 | | |
$ | 0.95 | |
The
accompanying notes form an integral part of these consolidated financial statements.
MEIHUA
INTERNATIONAL MEDICAL TECHNOLOGIES CO., LTD.
CONSOLIDATED
STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
For
the years ended December 31, 2022, 2021 and 2020
(US$,
except share data)
| | |
Ordinary
shares | | |
Ordinary
shares
amount | | |
Additional
paid-in
capital | | |
Ordinary
shares
subscribed | | |
Statutory
surplus
reserves | | |
Retained
earnings | | |
Accumulated
other
comprehensive
income (loss) | | |
Non-
controlling
interests | | |
Total
Equity | |
| Balance as of December
31, 2019 | |
| 18,575,000 | | |
$ | 9,288 | | |
$ | 8,100,225 | | |
$ | 344,739 | | |
$ | 13,308,334 | | |
$ | 39,449,962 | | |
$ | (1,554,228 | ) | |
| - | | |
$ | 59,658,320 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| - | | |
| | |
| Share issuance | |
| 1,425,000 | | |
| 712 | | |
| 1,616,259 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,616,971 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| - | | |
| | |
| Ordinary shares subscribed | |
| - | | |
| - | | |
| - | | |
| (344,739 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| (344,739 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| - | | |
| | |
| Net income | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 19,045,255 | | |
| - | | |
| - | | |
| 19,045,255 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Appropriation of statutory
reserve | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,870,133 | | |
| (1,870,133 | ) | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Currency
translation adjustment | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 4,759,973 | | |
| - | | |
| 4,759,973 | |
| Balance as of December
31, 2020 | |
| 20,000,000 | | |
$ | 10,000 | | |
$ | 9,716,484 | | |
| - | | |
$ | 15,178,467 | | |
$ | 56,625,084 | | |
$ | 3,205,745 | | |
| - | | |
$ | 84,735,780 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net income | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 20,949,579 | | |
| - | | |
| - | | |
| 20,949,579 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Currency
translation adjustment | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 2,083,243 | | |
| - | | |
| 2,083,243 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance as of December 31, 2021 | |
| 20,000,000 | | |
$ | 10,000 | | |
$ | 9,716,484 | | |
| - | | |
$ | 15,178,467 | | |
$ | 77,574,663 | | |
$ | 5,288,988 | | |
| - | | |
$ | 107,768,602 | |
| Ordinary shares subscribed | |
| 3,940,000 | | |
| 1,970 | | |
| 33,250,522 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 33,252,492 | |
| Shareholders’ contribution | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 635,310 | | |
| 635,310 | |
| Net income | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 6,242,969 | | |
| | | |
| (65,265 | ) | |
| 6,177,704 | |
| Appropriation of statutory
reserve | |
| - | | |
| - | | |
| - | | |
| - | | |
| 487,393 | | |
| (487,393 | ) | |
| | | |
| | | |
| | |
| Currency
translation adjustment | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| | | |
| (9,141,126 | ) | |
| (13,902 | ) | |
| (9,155,028 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance as of December
31, 2022 | |
| 23,940,000 | | |
| 11,970 | | |
| 42,967,006 | | |
| - | | |
| 15,665,860 | | |
| 83,330,239 | | |
| (3,852,138 | ) | |
| 556,143 | | |
| 138,679,080 | |
The
accompanying notes form an integral part of these consolidated financial statements.
MEIHUA
INTERNATIONAL MEDICAL TECHNOLOGIES CO., LTD.
CONSOLIDATED
STATEMENTS OF CASH FLOWS
For
the years ended December 31, 2022, 2021 and 2020
(US$)
| | |
For the Year Ended December 31 | |
| | |
2022 | | |
2021 | | |
2020 | |
| Cash Flows from operating activities: | |
| | |
| | |
| |
| Net income | |
$ | 6,177,704 | | |
$ | 20,949,579 | | |
$ | 19,045,255 | |
| Adjustments for items not affecting cash: | |
| | | |
| | | |
| | |
| Depreciation | |
| 541,265 | | |
| 595,522 | | |
| 497,238 | |
| Amortization | |
| 22,196 | | |
| 26,951 | | |
| 26,195 | |
| Net loss from disposal of property, plant and equipment | |
| 25,023 | | |
| 15,281 | | |
| 2,852 | |
| Written-off Tai He deposit | |
| 4,755,536 | | |
| - | | |
| - | |
| Currency exchange (gain) loss | |
| (273,432 | ) | |
| - | | |
| - | |
| Changes in operating assets and liabilities: | |
| | | |
| | | |
| - | |
| Bank acceptance receivables | |
| (4,284,515 | ) | |
| (6,533,717 | ) | |
| (5,764,940 | ) |
| Accounts receivable | |
| (7,121,889 | ) | |
| (20,065,904 | ) | |
| (13,644,830 | ) |
| Inventories | |
| 35,027 | | |
| 105,121 | | |
| (219,787 | ) |
| Prepayments and other assets | |
| (4,932,145 | ) | |
| (861,799 | ) | |
| (367,249 | ) |
| Due from related parties | |
| - | | |
| 396,583 | | |
| (222,504 | ) |
| Accounts payable | |
| (3,371,275 | ) | |
| 4,908,971 | | |
| 5,341,710 | |
| Taxes payable | |
| (812,412 | ) | |
| 288,659 | | |
| 598,105 | |
| Accrued expenses and other current liabilities | |
| 75,879 | | |
| 120,090 | | |
| 102,749 | |
| Due to related parties | |
| - | | |
| - | | |
| (68,798 | ) |
| Net cash (used in) provided by operating activities | |
| (9,163,038 | ) | |
| (54,663 | ) | |
| 5,325,996 | |
| | |
| | | |
| | | |
| | |
| Cash flows from investing activities: | |
| | | |
| | | |
| | |
| Purchases of property, plant and equipment | |
| (2,698,729 | ) | |
| (850,231 | ) | |
| (3,808,259 | ) |
| Payments for long-term deposits for buildings | |
| - | | |
| - | | |
| (12,311,347 | ) |
| Long term investment | |
| (5,944,709 | ) | |
| - | | |
| - | |
| Proceeds from disposal of property, plant and equipment | |
| 23,146 | | |
| 16,414 | | |
| 25,202 | |
| Net cash used in investing activities | |
| (8,620,292 | ) | |
| (833,817 | ) | |
| (16,094,404 | ) |
| | |
| | | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | | |
| | |
| Proceeds from short-term bank borrowings | |
| 7,579,135 | | |
| 6,510,820 | | |
| 4,359,665 | |
| Proceeds from long-term bank loans | |
| 743,052 | | |
| - | | |
| - | |
| Capital contributed by non-controlling shareholders | |
| 635,310 | | |
| - | | |
| - | |
| Proceeds from initial public offering | |
| 34,529,644 | | |
| - | | |
| - | |
| Proceeds from issuance of shares | |
| 0 | | |
| - | | |
| 1,272,232 | |
| Repayments of short-term bank borrowings | |
| (6,241,641 | ) | |
| (4,650,586 | ) | |
| (3,925,147 | ) |
| Net cash provided by financing activities | |
| 37,245,500 | | |
| 1,860,234 | | |
| 1,706,750 | |
| | |
| | | |
| | | |
| | |
| Effect of foreign exchange rate changes | |
| (874,746 | ) | |
| (9,812 | ) | |
| 218,137 | |
| Net increase (decrease) in cash | |
| 18,587,424 | | |
| 961,942 | | |
| (8,843,521 | ) |
| Cash, beginning of year | |
| 8,149,276 | | |
| 7,187,334 | | |
| 16,030,855 | |
| Cash, end of year | |
$ | 26,736,700 | | |
$ | 8,149,276 | | |
$ | 7,187,334 | |
| | |
| | | |
| | | |
| | |
| SUPPLEMENTAL DISCLOSURE OF CASH FLOWS INFORMATION: | |
| | | |
| | | |
| | |
| Cash paid during the period for: | |
| | | |
| | | |
| | |
| Interest | |
$ | 194,667 | | |
$ | 180,744 | | |
$ | 137,160 | |
| Income taxes | |
$ | 5,703,288 | | |
$ | 5,042,816 | | |
$ | 4,362,169 | |
| | |
| | | |
| | | |
| | |
| Non-cash transactions | |
| | | |
| | | |
| | |
| Shareholder contribution through deferred cost | |
| 1,277,152 | | |
| | | |
| | |
The
accompanying notes form an integral part of these consolidated financial statements.
MEIHUA
INTERNATIONAL MEDICAL TECHNOLOGIES CO., LTD.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
1.
Organization and principal activities
Principal
Activities:
Meihua
International Medical Technologies Co., Ltd. (“Meihua”) was incorporated on November 10, 2020 in the Cayman Islands. It is
a holding company with no operations. Meihua produces and sells medical consumables through its wholly owned subsidiaries located in
People’s Republic of China (“PRC” or “China”). Below is Meihua’s organizational chart, as well as
a description of the ownership structure.
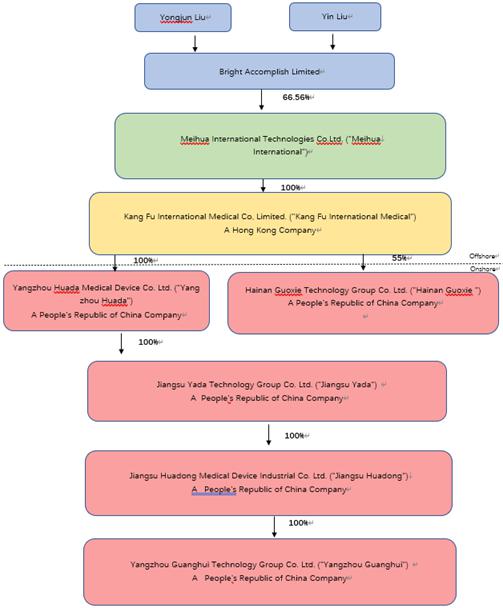
| Entity
Name |
|
Registered
Location |
|
Percentage
of ownership |
|
|
Date
of incorporation |
|
|
Principal
activities |
| Meihua
International Medical Technologies Co., Ltd. (“Meihua”) |
|
Cayman |
|
Parent |
|
|
November 10, 2020 |
|
|
Investment holding |
| |
|
|
|
|
|
|
|
|
|
|
康复国际医疗有限公司
Kang Fu International Medical Co., Limited (“Kang Fu”) |
|
Hong Kong |
|
100% by Meihua |
|
|
October 13, 2015 |
|
|
Investment holding |
| |
|
|
|
|
|
|
|
|
|
|
扬州华达医疗器械有限公司
Yangzhou Huada Medical Equipment Co., Ltd. (“Huada”) |
|
Yangzhou |
|
100% by Kang Fu |
|
|
December 24, 2001 |
|
|
Medical Equipment Sales |
| |
|
|
|
|
|
|
|
|
|
|
江苏亚达科技集团有限公司
Jiangsu Yada Technology Group Co., Ltd. (“Yada”) |
|
Yangzhou |
|
100% by Huada |
|
|
December 5, 1991 |
|
|
Medical Equipment Sales |
| |
|
|
|
|
|
|
|
|
|
|
江苏华东医疗器械实业有限公司
Jiangsu Huadong Medical Device Industry Co., Ltd. (“Huadong”) |
|
Yangzhou |
|
100% by Yada |
|
|
November 18, 2000 |
|
|
Medical Equipment Sales |
| |
|
|
|
|
|
|
|
|
|
|
扬州光辉医疗科技有限公司
Yangzhou Guanghui Medical Technology Co., Ltd. (“Guanghui”) |
|
Yangzhou |
|
100% by Huadong |
|
|
December 22, 2020 |
|
|
Medical Equipment Sales |
| |
|
|
|
|
|
|
|
|
|
|
海南国械医疗科技有限公司
Hainan GuoxieTechnology Group Co. Ltd. (“Hainan Guoxie”) |
|
Hainan |
|
55% by Kang Fu |
|
|
October 07,2021 |
|
|
Medical Equipment Sales |
Kang
Fu was incorporated on October 13, 2015 with a registered capital of HKD 53,911,815 ($6,911,771). Kang Fu is a holding company with no
operations. The following operating entities (Huada, Yada and Huadong) are all directly and indirectly 100% owned by Kang Fu for all
the periods presented.
Huada
is a subsidiary wholly owned by Kang Fu and established in Yangzhou, China on December 24, 2001 with a registered capital of $ 17,193,021.
Yada
is a subsidiary wholly owned by Huada and was established in Yangzhou, China on December 5, 1991 with a registered capital of RMB51,390,000.
Huadong
is a subsidiary wholly owned by Yada and was established in Yangzhou, China on November 18, 2000 with a registered capital of RMB50,000,000.
Those
three subsidiaries primarily manufacture and sell Class I, II and III disposable medical devices under the Company’s own brands,
and distribute Class I, II and III disposable medical devices sourced from other manufacturers to our domestic and overseas customers.
Guanghui
is a subsidiary wholly owned by Huadong and was established in Yangzhou, China on December 22, 2020 with a registered capital of RMB1,000,000.
Hainan
Guoxie is a subsidiary 55% owned by Kang Fu and established in Hainan, China on October 07, 2021 with a registered capital of RMB100,000,000.
Reorganization
and Share Issuance
On
November 10, 2020, Meihua was incorporated in the Cayman Islands and issued 50,000 ordinary shares at par value of $1.00 to Yongjun Liu.
On
December 21, 2020, Yongjun Liu surrendered 49,999 shares to the Company for cancellation. The Company subdivided each existing share
with a par value of $1.00 into 2,000 shares with a par value of $0.0005 par value and created a new class of preferred shares. Upon completion
of the share capital changes, the Company’s share capital includes 80,000,000 ordinary shares with a par value of $0.0005 per share
and 20,000,000 preferred shares with a par value of $0.0005 per share.
Also
on December 21, 2020, the Company engaged in a corporate reorganization to combine the controlled entities (now referred to as the subsidiaries)
into one legal corporation (the Company). The specific transactions related to this reorganization are outlined below. The Company entered
into a share exchange agreement with Kang Fu to issue an aggregate of 15,933,000 ordinary shares to Yongjun Liu and Yin Liu in exchange
for 100% ownership of Kang Fu, and allotted 2,640,000 ordinary shares at $0.0005 par value to three BVI companies held by founders of
the Company for no consideration. On December 22, 2020, Guanghui was incorporated for the purpose of foreign exchange registration under
the laws of the People’s Republic of China as there was no substantive business of Guanghui. The shares of Guanghui are owned by
13 natural persons (the “Guanghui Shareholders”) and on May 10, 2021, all of the Guanghui Shareholders agreed to transfer
all their shares held in Guanghui to Huadong (an indirect subsidiary of the Company) for no consideration. Through such transaction,
Guanghui then became a wholly owned subsidiary of Huadong.
On
December 18, 2020, Yongjun Liu and Yin Liu and other shareholders (collectively, the “Parties”) executed an Acting-in-Concert
Agreement. The major terms of this agreement are:
| |
●
|
The
Parties shall inform and discuss with each other and reach a consensus before exercising voting rights in the Company’s decision
making. |
| |
●
|
If
no consensus could be reached by the Parties, the decision made by Yongjun Liu and Yin Liu (who are a couple) prevails. |
As
a result of the Acting-in-Concert agreement, Yongjun Liu and Yin Liu together have the ultimate control of the Company.
The
Acting-in-Concert Agreement that establishes the common control between Meihua International and Kang Fu is treated as though it was
effective for all periods presented as during the years presented in these financial statements, the control of the entities has
never changed (always under the control of Yongjun Liu and Yin Liu who are a couple). Accordingly, the combination has been treated as
a corporate restructuring (reorganization) of entities under common control and thus the current capital structure has been retroactively
presented in prior periods as if such structure existed at that time and in accordance with ASC 805-50-45-5, the entities under common
control are presented on a combined basis for all periods to which such entities were under common control. Since all of the subsidiaries
were under common control for the entirety of the years ended December 31, 2020, the results of these subsidiaries are included in the
financial statements for all periods.
After
the restructuring, Meihua holds 100% ownership of Kang Fu and has 80,000,000 ordinary shares and 20,000,000 preferred shares authorized,
18,575,000 ordinary shares and nil preferred share issued and outstanding.
The
discussion and presentation of financial statements herein assumes the completion of the restructuring, which is accounted for retroactively
as if it occurred on January 1, 2020, and the equity has been restated to reflect the change as well.
On
December 22, 2020, the Company issued a total of 1,425,000 ordinary shares to three BVI companies with total consideration of $1,616,971
in a private offering. The consideration was received by Huada due to Meihua not having been incorporated at the time of payments received.
The timing of the payments received is listed below:
| | |
US$ | |
| December 2019 | |
| 344,739 | |
| January 2020 | |
| 359,885 | |
| April 2020 | |
| 508,814 | |
| September 2020 | |
| 403,533 | |
| Total | |
| 1,616,971 | |
2.
Summary of Significant Accounting Policies
Basis
of Presentation
The
consolidated financial statements include all accounts of Meihua and its subsidiaries (collectively, the “Company”) and have
been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
All inter-company transactions have been eliminated.
Use
of Estimates
The
preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make certain estimates and assumptions
that affect the amounts reported and disclosed in the consolidated financial statements and related notes.
The
most significant estimates and judgments include allowance for bad debts, the valuation of inventory, useful life of property, plant
and equipment and income taxes related to realization of deferred tax assets and uncertain tax position. Actual amounts could differ
from those estimates.
Non-controlling
interests
Non-controlling
interest represents the portion of the net assets of subsidiaries attributable to interests that are not owned or controlled by the Company.
The non-controlling interest is presented in the consolidated balance sheets, separately from equity attributable to the shareholders
of the Company. Non-controlling interest’s operating results are presented on the face of the consolidated statements of income
and comprehensive income as an allocation of the total income for the year between non-controlling shareholders and the shareholders
of the Company. As of December 31, 2022, non-controlling interests represent one non-controlling shareholders’ proportionate share
of equity interests in Hainan Guoxie.
Functional
Currency and Foreign Currency Translation
The
Company’s reporting currency is the United States dollar (“US$”). The Company’s operations are principally conducted
through the PRC subsidiaries where the local currency is the functional currency. Therefore, the functional currency of Kang Fu is the
Hong Kong dollar and the functional currency of Huada, Yada, Huadong and Guanghui is the Renminbi (“RMB”).
Transactions
denominated in currencies other than the functional currencies are translated into the functional currency of the entity at the exchange
rates prevailing on the transaction dates. Monetary assets and liabilities denominated in currencies other than the applicable functional
currency are translated into the functional currency at the prevailing rates of exchange at the balance date. The resulting exchange
differences are reported in the consolidated statements of income and comprehensive income.
The
assets and liabilities of the Company are translated at the exchange spot rate at the balance sheet date, stockholders’ equity
is translated at the historical rates and the revenues and expenses are translated at the average exchange rates for the periods. The
resulting translation adjustments are reported under other comprehensive income in the consolidated statements of income and comprehensive
income in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”)
220, Comprehensive Income. The following are the exchange rates that were used in translating the Company’s PRC subsidiaries’
financial statements into the consolidated financial statements:
| |
|
For
the Years Ended December 31 |
|
| |
|
2022 |
|
|
2021 |
|
|
2020 |
|
| Period
Ended spot |
|
|
US$1=RMB 6.8972 |
|
|
|
US$1=RMB
6.3726 |
|
|
|
US$1=RMB
6.5250 |
|
| Period
Average |
|
|
US$1=RMB
6.7290 |
|
|
|
US$1=RMB
6.4508 |
|
|
|
US$1=RMB
6.9042 |
|
The
exchanges rates used for translation from Hong Kong dollar to US$ was 7.8000, a pegged rate determined by the linked exchange rate system
in Hong Kong. This pegged rate was used to translate Kang Fu’s balance sheets, income statement items and cash flow items for both
the years ended December 31, 2022, 2021 and 2020.
Certain
Risks and Concentration
The
Company’s financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily
of cash and receivables. As of December 31, 2022 and 2021 substantially all the Company’s cash were held in major financial institutions
located in Hong Kong and the PRC, which management considers to be of high credit quality.
As
of December 31, 2022, two customers accounted for approximately 27.63% and 10.97% of the Company’s total revenues. As of December
31, 2021, two customers accounted for approximately 21.91% and 11.26% of the Company’s total revenues.
As
of December 31, 2022, two customers accounted for approximately 27.73% and 13.14% of the Company’s accounts receivable. As of December
31, 2021, three customers accounted for approximately 25.88%, 11.41% and 10.25%, respectively, of the Company’s accounts receivable.
For the year ended December 31, 2022, one supplier
accounted for approximately 11.93% of the Company’s total purchases There were no suppliers that individually represented greater
than 10% of the total purchase of the Group for the years ended December 31, 2021 and 2020.
Fair
Value Measurement
Fair
value is the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market
participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to
be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact, and it considers
assumptions that market participants would use when pricing the asset or liability.
The
Company adopted the guidance of Accounting Standards Codification (“ASC”) 820 for fair value measurements which clarifies
the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs
used in measuring fair value as follows:
| |
Level 1: |
Inputs
are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| |
|
|
| |
Level 2: |
Inputs
are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets
and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated
by observable market data. |
| |
|
|
| |
Level 3: |
Inputs
are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would
use in pricing the asset or liability based on the best available information. |
The
Company’s financial instruments include cash, accounts receivable, bank acceptance receivables, due from related parties, accounts
payable, other liabilities and accrued expenses and short-term bank borrowings. The carrying amounts approximate their fair values due
to their short maturities as of December 31, 2022 and 2021.
The
Company noted no transfers between levels during any of the periods presented. The Company did not have any instruments that were measured
at fair value on a recurring or non-recurring basis as of December 31, 2022 and 2021.
Cash
Cash
consists of petty cash on hand and cash held in banks, which are highly liquid and are unrestricted as to withdrawal or use.
Bank
Acceptance Receivables
Bank acceptance receivables are issued by bank
under the request of the Company’s customers, to pay for the purchased goods. The Company can choose to hold acceptance notes until
maturity and receive the face value payment from the bank, or sell (exchange) the acceptance notes at a discount to another party willing
to wait until maturity to receive the bank’s promised payment. The maturity date of the receivables is all within one year of the
original issuance date and carried at face value. The Company is not lending money, it just sells goods to the customers (customers can
pay the purchased goods by cash, accounts receivable or bank acceptance receivables). The receivables mature within one year, and are
non-interest bearing. As bank acceptance receivables are issued by the banks and payments are guaranteed. The Company has not discounted
any bank acceptances and there was no endorsed bank acceptances that are unmatured as of December 31, 2022. The Company collected approximately
$9.6 million as of March 31, 2023.
Accounts
Receivable and Allowance for Doubtful Accounts
Accounts
receivable represent trade receivables and are recognized initially at fair value and subsequently adjusted for any allowance for doubtful
accounts or impairment.
The
Company records impairment losses for accounts receivable based on assessments of the recoverability of the trade and other receivables
and individual account analysis, including the current creditworthiness and the past collection history of each debtor. Impairments arise
when there is objective evidence indicating that the balances may not be collectible. The identification of bad and doubtful debts, in
particular of a loss event, requires the use of judgment and estimates, which involve the estimates of specific losses on individual
exposures, as well as a provision on historical trends of collections. Based on management of customers’ credit and ongoing relationship,
management makes conclusions whether any balances outstanding at the end of the period will be deemed non-collectible on an individual
basis and on aging analysis basis. The provision is recorded against accounts receivables balances, with a corresponding charge recorded
in the consolidated statements of operations and comprehensive income. Delinquent account balances are written-off against the allowance
for doubtful accounts after management has determined that the likelihood of collection is not probable.
The
Company historically has not had material bad debts in accounts receivable. There were no bad debt expenses related to accounts receivable
for the years ended December 31, 2022, 2021 and 2020 and there was no provision for doubtful accounts as of December 31, 2022 and 2021.
Inventories
Inventories
are valued using the lower of cost or net realizable value. Cost is principally determined using the weighted-average method. Manufactured
inventories included cost of materials, labor and overhead expenses. The Company records adjustments to inventory for excess quantities,
obsolescence, or impairment, when appropriate, to reflect inventory at net realizable value. These adjustments are based upon a combination
of factors including current sales volume, market conditions, lower of cost or market analysis and expected realizable value of the inventory.
There
were no write-downs recognized of inventories as of December 31, 2022 and 2021.
Prepayment
and other current assets
As
of December 31, 2022 and 2021, prepayment and other current assets were $16,428,779 and $1,394,539.
Prepayment
and other assets primarily consist of deferred IPO costs, loans to third-parties, refundable tax credits and receivables, security deposits
made to customers, advances to employees and land use right receivable, which are presented net of allowance for doubtful accounts. These
balances are unsecured and are reviewed periodically to determine whether their carrying value has become impaired. The Company considers
the balances to be impaired if the utilization or refund of the balances becomes doubtful. The Company uses the aging method to estimate
the allowance for uncollectible balances. The allowance is also based on management’s best estimate of specific losses on individual
exposures, as well as a provision on historical trends of collections and utilizations. Actual amounts received or utilized may differ
from management’s estimate of credit worthiness and the economic environment. Delinquent account balances are written off against
allowance for doubtful accounts after management has determined that the likelihood of collection is not probable. The allowance for
doubtful accounts amounted to nil as of December 31, 2022 and 2021.
Deferred
IPO costs represent the incremental costs incurred for the Company’s initial public offering (“IPO”). These costs has
been charged against the gross proceeds of the IPO in 2022 (see Note 14). Deferred IPO costs primary include specific legal, audit
and professional consulting costs. As of December 31, 2022 and 2021, the deferred IPO costs were $nil and $1,277,152, respectively.
Property,
Plant and Equipment
Property,
plant and equipment items are recorded at their historic cost, less accumulated depreciation and impairment losses. The Company calculates
depreciation using the straight-line method, after consideration of the estimated residual values, over the following estimated useful
lives:
| Category | |
Useful lives | |
Estimated residual value | |
| Buildings | |
20 years | |
| 10 | % |
| Machinery and Equipment | |
10 years | |
| 10 | % |
| Motor vehicles | |
5 years | |
| 10 | % |
| Electronic Equipment | |
5 years | |
| 10 | % |
| Office Equipment | |
3 years | |
| 10 | % |
| Inspection Equipment | |
5 years | |
| 10 | % |
Major
improvements are capitalized and expenditures for maintenance and repairs are expensed as incurred. Construction in progress represents
property, plant and equipment under construction or being installed. Costs include original cost, installation, construction and other
direct costs. Interest expenses directly related to construction in progress would be capitalized. Construction in progress is transferred
to the appropriate fixed asset account and depreciation commences when the asset has been substantially completed and placed in service.
Intangible
Assets
Intangible
assets are non-monetary assets without physical substance. These items are initially measured at cost and subsequently carried at cost
less any accumulated amortization and impairment losses. Intangible assets with finite useful lives are amortized on a straight-line
basis over their estimated useful lives. Amortization of finite-lived intangible assets is computed using the straight-line method over
the estimated useful lives, which is as follows:
| Category | |
Useful lives |
| Land use rights | |
50 years |
| Patent | |
5 years |
| Trademark | |
10 years |
Impairment
of Long-Lived Assets
The
Company accounts for impairment of long-lived assets in accordance with Accounting Standards Codification (“ASC”) 360, Property,
Plant and Equipment. (“ASC 360”). Long-lived assets consist primarily of property, plant and equipment, and intangible
assets. In accordance with ASC 360, the Company evaluates the carrying value of long-lived assets when it determines a triggering event
has occurred, or whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. When
indicators exist, recoverability of assets is measured by a comparison of the carrying value of the asset group to the estimated undiscounted
future net cash flows expected to be generated by the asset. Examples of such triggering events include a significant disposal of a portion
of such assets, and adverse change in the market involving the business employing the related assets. If such assets are determined not
to be recoverable, the Company performs an analysis of the fair value of the asset group and will recognize an impairment loss when the
fair value is less than the carrying amounts of such assets. The fair value, based on reasonable and supportable assumptions and projections,
require subjective judgments. Depending on the assumptions and estimates used, the appraised fair value projected in the evaluation of
long-lived assets can vary within a range of outcomes. The Company considers the likelihood of possible outcomes in determining the best
estimate for the fair value of the assets. The Company did not record any impairment charges for the years ended December 31, 2022, 2021
and 2020. There can be no assurance that future events will not have impact on company revenue or financial position which could result
in impairment in the future.
Investment
In
accordance with Financial Accounting Standards Board (“FASB”) ASC 321, “Investment-Equity Securities,” the Company
accounts for non-marketable securities on a prospective basis. Equity investments that do not have readily determinable fair values and
do not qualify for the net asset value practical expedient are eligible for the measurement alternative.
On
March 3, 2011, Yada invested in Yangzhou Juyuan Guarantee Co., Ltd (“Juyuan”) and obtained 12% equity interest of Juyuan.
For the Company’s passive and without significant influence or control equity investment in private company which do not have readily
determinable fair values, the Company has elected the measurement alternative defined as cost, less impairment, plus or minus adjustments
resulting from observable price changes in orderly transactions for the identical or a similar investment of the Company. The investment
is reviewed periodically to determine if its value has been impaired and adjustments are recorded as necessary in profit or loss for
the period.
Investments
in entities in which the Company can exercise significant influence but does not own a majority equity interest or control are accounted
for using the equity method of accounting in accordance with ASC 323, Investments-Equity Method and Joint Ventures (“ASC 323”).
Under the equity method, the Company initially records its investment at cost and the difference between the cost of the equity investee
and the amount of the underlying equity in the net assets of the equity investee is accounted for as if the investee were a consolidated
subsidiary. The share of earnings or losses of the investee are recognized in the consolidated statements of comprehensive loss. Equity
method adjustments include the Company’s proportionate share of investee income or loss, adjustments to recognize certain differences
between the Company’s carrying value and its equity in net assets of the investee at the date of investment, impairments, and other
adjustments required by the equity method. The Company assesses its equity investment for other-than-temporary impairment by considering
factors as well as all relevant and available information including, but not limited to, current economic and market conditions, the
operating performance of the investees including current earnings trends, the general market conditions in the investee’s industry
or geographic area, factors related to the investee’s ability to remain in business, such as the investee’s liquidity, debt
ratios, and cash burn rate and other company-specific information.
Investments
in equity securities without readily determinable fair values are measured at cost minus impairment adjusted by observable price changes
in orderly transactions for the identical or a similar investment of the same issuer. These investments are measured at fair value on
a nonrecurring basis when there are events or changes in circumstances that may have a significant adverse effect. An impairment loss
is recognized in the consolidated statements of comprehensive loss equal to the amount by which the carrying value exceeds the fair value
of the investment. Prior to the adoption of ASU 2016-01 on January 1, 2019, these investments were accounted for using the cost method
of accounting, measured at cost less other-than-temporary impairment.
On
December 1, 2022, Huadong invested RMB 40 million into Jiangsu Zhongxiangxin International Science and Technology Innovation Park Co.,
Ltd. (“Zhongxiangxin”), and obtained 25% ownership interest of Zhongxiangxin. Zhongxiangxin manufactures and sells medical
materials in the PRC. The Company accounted for the investments using equity method, because the Company has significant influence but
does not own a majority equity interest or otherwise control over the equity investee. Under the equity method, the Company adjusts the
carrying amount of the investment and recognizes investment income or loss for its share of the earnings or loss of the investee after
the date of investment. When the Company’s share of losses in the equity investee equals or exceeds its interest in the equity
investee, the Company does not recognize further losses, unless the Company has incurred obligations or made payments or guarantees on
behalf of the equity investee. For the year ended December 31, 2022, the investment gain from Zhongxiangxin was $290.
The
Company continually reviews its investments in equity investees to determine whether a decline in fair value below the carrying value
is other-than-temporary. The primary factors the Company considers in its determination include the financial condition, operating performance
and the prospects of the equity investee; other company specific information such as recent financing rounds; the geographic region,
market and industry in which the equity investee operates; and the length of time that the fair value of the investment is below its
carrying value. If the decline in fair value is deemed to be other-than-temporary, the carrying value of the equity investee is written
down to fair value.
For
the years ended December 31, 2022, 2021 and 2020, no impairment indicators were identified and no loss related to revaluation of its
investment in the private company was recorded.
Value-added
Tax
Value-added
taxes (“VAT”) collected from customers relating to product sales and remitted to governmental authorities are presented on
a net basis. VAT collected from customers is excluded from revenue which is recorded in VAT payable. The Company is subject to a VAT
rate of 13%. The VAT payable may be offset by VAT paid by the Company on raw materials and other materials included in the cost of producing
or acquiring its finished products.
Related
Parties
Parties
are considered to be related to the Company if the parties, directly or indirectly, through one or more intermediaries, control, are
controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management,
members of the immediate families of principal owners of the Company and its management and other parties with which the Company may
deal with if one party controls or can significantly influence the management or operating policies of the other to an extent that one
of the transacting parties might be prevented from fully pursuing its own separate interests. The Company discloses all significant related
party transactions.
Revenue
Recognition
Effective
January 1, 2018, the Company adopted ASC Topic 606 using the modified retrospective adoption method. Based on the requirements of ASC
Topic 606, revenue is recognized when control of the promised goods or services is transferred to the customers in an amount that reflects
the consideration the Company expects to be entitled to receive in exchange for those goods or services. The Company primarily sells
its products to hospitals and medical equipment companies. Revenue is recognized when the following 5-step revenue recognition criteria
are met:
| |
1) |
Identify
the contract with a customer |
| |
2) |
Identify
the performance obligations in the contract |
| |
3) |
Determine
the transaction price |
| |
4) |
Allocate
the transaction price |
| |
5) |
Recognize
revenue when or as the entity satisfies a performance obligation |
Revenue
from product sales is recognized at the point in time control of the products is transferred, generally upon customer receipt based upon
the standard contract terms. Shipping and handling activities are considered to be fulfillment activities rather than promised services
and are not, therefore, considered to be separate performance obligations. The Company’s sales terms provide no right of return
outside of a standard quality policy and returns are generally not significant. Payment terms for product sales are generally set at
90 to 180 days after the consideration becomes due and payable.
Revenue
Disaggregation
The
Company’s disaggregated revenues are represented by two categories which are type of goods and type of customers.
Type
of Goods
| | |
For the year ended December 31, | |
| | |
2022 | | |
2021 | | |
2020 | |
| | |
US$ | | |
US$ | | |
US$ | |
| Self-Manufactured Products | |
| 50,514,976 | | |
| 48,059,165 | | |
| 44,473,076 | |
| Resales of Sourced Disposable Medical Devices from Third
Party Manufacturers | |
| 52,831,365 | | |
| 55,978,545 | | |
| 44,587,934 | |
| Total Revenue | |
| 103,346,341 | | |
| 104,037,710 | | |
| 89,061,010 | |
Type
of Customers
| | |
For the year ended December 31, | |
| | |
2022 | | |
2021 | | |
2020 | |
| | |
US$ | | |
US$ | | |
US$ | |
| Direct sales | |
| 9,465,644 | | |
| 9,499,748 | | |
| 9,430,082 | |
| Distributors | |
| 93,880,697 | | |
| 94,537,962 | | |
| 79,630,928 | |
| Total Revenue | |
| 103,346,341 | | |
| 104,037,710 | | |
| 89,061,010 | |
Earnings
per Ordinary Share
Earnings
(loss) per ordinary share is calculated in accordance with ASC 260, Earnings per Share. Basic earnings (loss) per ordinary share
is computed by dividing the net income (loss) attributable to shareholders of the Company by the weighted average number of ordinary
shares outstanding during the year. Diluted earnings per ordinary share is computed in accordance with the treasury stock method and
based on the weighted average number of ordinary shares and dilutive ordinary share equivalents. Dilutive ordinary share equivalents
are excluded from the computation of diluted earnings per ordinary share if their effects would be anti-dilutive. There is no ordinary
share equivalent issued to date.
Comprehensive
Income (Loss)
ASC
220, Comprehensive Income (“ASC 220”) establishes rules for reporting and display of comprehensive income and its components.
ASC 220 requires that unrealized gains and losses on the Company’s foreign currency translation adjustments be included in comprehensive
income (loss).
Advertising
Costs
The
Company’s advertising costs are expensed as incurred. Advertising expenses are included in selling expenses in the accompanying
consolidated statements of income and comprehensive income. Advertising expenses were $95,432, $21,498 and $53,770 for the years ended
December 31, 2022, 2021 and 2020, respectively.
Research
and Development Costs
Research
and development expenses are expensed as incurred. Research and development expenses were $2,962,904, $2,725,014 and $2,492,059 for the
years ended December 31, 2022, 2021 and 2020, respectively.
Income
Tax
Current
income taxes are provided on the basis of net profit for financial reporting purposes, adjusted for income and expense items which are
not assessable or deductible for income tax purposes, in accordance with the regulations of the relevant tax jurisdictions.
Deferred
income taxes are recognized for temporary differences between the tax bases of assets and liabilities and their reported amounts in the
consolidated financial statements, net operating loss carry forwards and credits. Deferred tax assets are reduced by a valuation allowance
when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized.
Current income taxes are provided in accordance with the laws of the relevant taxing authorities. Deferred tax assets and liabilities
are measured using enacted rates expected to apply to taxable income in which temporary differences are expected to be reversed or settled.
The effect on deferred tax assets and liabilities of changes in tax rates is recognized in the statement of comprehensive income in the
period of the enactment of the change.
The
Company considers positive and negative evidence when determining whether a portion or all of its deferred tax assets will more likely
than not be realized. This assessment considers, among other matters, the nature, frequency and severity of current and cumulative losses,
forecasts of future profitability, the duration of statutory carry-forward periods, its experience with tax attributes expiring unused,
and its tax planning strategies. The ultimate realization of deferred tax assets is dependent upon its ability to generate sufficient
future taxable income within the carry-forward periods provided for in the tax law and during the periods in which the temporary differences
become deductible. When assessing the realization of deferred tax assets, the Company has considered possible sources of taxable income
including (i) future reversals of existing taxable temporary differences, (ii) future taxable income exclusive of reversing
temporary differences and carryforwards, (iii) future taxable income arising from implementing tax planning strategies, and (iv) specific
known trend of profits expected to be reflected within the industry.
The
Company recognizes a tax benefit associated with an uncertain tax position when, in its judgment, it is more likely than not that the
position will be sustained upon examination by a taxing authority. For a tax position that meets the more-likely-than-not recognition
threshold, the Company initially and subsequently measures the tax benefit as the largest amount that the Company judges to have a greater
than 50% likelihood of being realized upon ultimate settlement with a taxing authority. The Company’s liability associated with
unrecognized tax benefits is adjusted periodically due to changing circumstances, such as the progress of tax audits, case law developments
and new or emerging legislation. Such adjustments are recognized entirely in the period in which they are identified. The Company’s
effective tax rate includes the net impact of changes in the liability for unrecognized tax benefits and subsequent adjustments as considered
appropriate by management. The Company classifies interest and penalties recognized on the liability for unrecognized tax benefits as
income tax expense.
Segment
Reporting
FASB
280, “Segment Reporting,” establishes standards for reporting information about operating segments on a basis consistent
with the Company’s internal organizational structure as well as information of the Company’s business segments, geographical
areas, segments and major customers. The Company uses the “management approach” in determining reportable operating segments.
The management approach considers the internal organization and reporting used by the Company’s chief operating decision maker
for making operating decisions and assessing performance as the source for determining the Company’s reportable segments. The chief
operating decision maker is the Company’s president and Chief Executive Officer (“CEO”). Management, including the
chief operating decision maker, reviews operating results of different products at revenue level with no allocation of operating costs.
Consequently, based on management’s assessment, the Company has determined that it has only one operating segment as defined by
FASB ASC 280.
The
Company has disclosed the type of revenue by government category as follows.
| | |
December
31, 2022 | | |
December
31, 2021 | | |
December
31, 2020 | |
| | |
US$ | | |
US$ | | |
US$ | |
| Category | |
Produced | | |
Purchased | | |
Total | | |
Produced | | |
Purchased | | |
Total | | |
Produced | | |
Purchased | | |
Total | |
| Class I | |
| 7,463,707 | | |
| 8,572,512 | | |
| 16,036,219 | | |
| 6,151,443 | | |
| 7,331,962 | | |
| 13,483,405 | | |
| 5,181,532 | | |
| 6,155,223 | | |
| 11,336,755 | |
| Class II | |
| 37,229,199 | | |
| 36,177,490 | | |
| 73,406,689 | | |
| 36,788,116 | | |
| 41,313,745 | | |
| 78,101,861 | | |
| 35,863,806 | | |
| 33,573,351 | | |
| 69,437,157 | |
| Class III | |
| 1,189,906 | | |
| 1,863,990 | | |
| 3,053,896 | | |
| 1,094,957 | | |
| 2,291,899 | | |
| 3,386,856 | | |
| 758,525 | | |
| 2,355,768 | | |
| 3,114,293 | |
| Others | |
| 4,632,164 | | |
| 6,217,373 | | |
| 10,849,537 | | |
| 4,024,649 | | |
| 5,040,939 | | |
| 9,065,588 | | |
| 2,669,213 | | |
| 2,503,592 | | |
| 5,172,805 | |
| Total | |
| 50,514,976 | | |
| 52,831,365 | | |
| 103,346,341 | | |
| 48,059,165 | | |
| 55,978,545 | | |
| 104,037,710 | | |
| 44,473,076 | | |
| 44,587,934 | | |
| 89,061,010 | |
Class
I, II, and III medical devices are defined by the National Medical Products Administration of China according to their risk levels under
the Regulation on the Supervision and Administration of Medical Devices (2021 Revision), Article 6 as follows:
| ● | “Class
I Medical Devices” means medical devices with low risks, whose safety and effectiveness
can be ensured through routine administration. |
| ● | “Class
II Medical Devices” means medical devices with moderate risks, which shall be strictly
controlled and administered to ensure their safety and effectiveness. |
| ● | “Class
III Medical Devices” means medical devices with relatively high risks, which shall
be strictly controlled and administered through special measures to ensure their safety and
effectiveness. |
Furthermore,
the Company has disclosed revenue by major product type included in each government category.
| | |
| |
December 31, | | |
December 31, | | |
December 31, | |
| | |
| |
2022 | | |
2021 | | |
2020 | |
| Category | |
Products | |
US$ | | |
US$ | | |
US$ | |
| Class I | |
Eye drops bottle | |
| 2,583,231 | | |
| 2,398,222 | | |
| 2,466,978 | |
| | |
Oral medicine bottle | |
| 3,965,345 | | |
| 3,263,135 | | |
| 2,200,569 | |
| | |
Anal bag | |
| 837,328 | | |
| 739,376 | | |
| 554,859 | |
| | |
Other Class I | |
| 8,650,315 | | |
| 7,082,672 | | |
| 6,114,349 | |
| Subtotal-Class I | |
| |
| 16,036,219 | | |
| 13,483,405 | | |
| 11,336,755 | |
| Class II | |
Masks | |
| 410,163 | | |
| 600,534 | | |
| 9,632,150 | |
| | |
Identification tape | |
| 12,262,269 | | |
| 15,049,686 | | |
| 11,617,668 | |
| | |
Disposable medical brush | |
| 8,337,650 | | |
| 8,493,760 | | |
| 6,353,649 | |
| | |
Gynecological inspection kits | |
| 7,571,089 | | |
| 8,752,617 | | |
| 4,924,689 | |
| | |
Surgical kit | |
| 4,830,930 | | |
| 4,754,769 | | |
| 3,383,215 | |
| | |
Medical brush | |
| 5,231,299 | | |
| 4,130,703 | | |
| 3,635,190 | |
| | |
Medical kit | |
| 4,066,663 | | |
| 5,037,054 | | |
| 3,429,371 | |
| | |
Other Class II | |
| 30,696,626 | | |
| 31,282,738 | | |
| 26,461,225 | |
| Subtotal-Class II | |
| |
| 73,406,689 | | |
| 78,101,861 | | |
| 69,437,157 | |
| Class III | |
Electronic pump | |
| 142,828 | | |
| 246,819 | | |
| 292,211 | |
| | |
Anesthesia puncture kit | |
| 539,615 | | |
| 430,288 | | |
| 438,047 | |
| | |
Disposable infusion pump | |
| 280,081 | | |
| 278,734 | | |
| 335,632 | |
| | |
Infusion pump | |
| 367,652 | | |
| 309,746 | | |
| 196,686 | |
| | |
Electronic infusion pump | |
| 44,812 | | |
| 291,725 | | |
| 185,030 | |
| | |
Laparoscopic trocar | |
| 139,284 | | |
| 219,901 | | |
| 134,585 | |
| | |
Other Class III | |
| 1,539,624 | | |
| 1,609,643 | | |
| 1,532,102 | |
| Subtotal-Class III | |
| |
| 3,053,896 | | |
| 3,386,856 | | |
| 3,114,293 | |
| Others | |
| |
| 10,849,537 | | |
| 9,065,588 | | |
| 5,172,805 | |
| Total | |
| |
| 103,346,341 | | |
| 104,037,710 | | |
| 89,061,010 | |
For
the years ended December 31, 2022, 2021 and 2020, revenues and assets within PRC contributed over 99.5% of the Company’s total
revenues and assets.
The
Outbreak of COVID-19
On
January 30, 2020, the World Health Organization declared the outbreak of the coronavirus disease (COVID-19) a “Public Health Emergency
of International Concern,” and on March 11, 2020, the World Health Organization characterized the outbreak as a “pandemic.”
COVID-19 has had a severe and negative impact on the Chinese and the global economy and such impact persists as of the date of this annual
report.
In
fiscal year 2020, COVID-19 had a significant impact on our business and results of operations as the sales volume of masks rose sharply
while the sales of products other than masks declined due to an overall decrease in market demand. In fiscal year 2021, with the stable
control of the domestic epidemic in China, the market of masks was no longer in urgent shortage compared to the same period in 2020,
and the production of epidemic prevention products resumed more normal production levels. In general, with the precise control of the
epidemic in China, our production and operations have recovered smoothly, and the demand for other products has increased gradually.
After the initial outbreak of COVID-19, from time to time, some instances of COVID-19 infections have emerged in various regions of China,
including the infections caused by the Omicron variants in 2022. For example, a wave of infections caused by the Omicron variants emerged
in Shanghai in 2022, and a series of restrictions and quarantines were implemented to contain the spread.
Many
of the restrictive measures previously adopted by the PRC governments at various levels to control the spread of the COVID-19 virus have
been revoked or replaced with more flexible measures since December 2022. While the revocation or replacement of the restrictive measures
to contain the COVID-19 pandemic could have a positive impact on our normal operations, the extent of the impact on the Company’s
future financial results will be dependent on future developments such as the length and severity of the crisis, the potential resurgence
of the crisis, future government actions in response to the crisis and the overall impact of the COVID-19 pandemic on the global economy
and capital markets, among many other factors, all of which remain highly uncertain and unpredictable. Given this uncertainty, the Company
is currently unable to quantify the expected impact of the COVID-19 pandemic on its future operations, financial condition, liquidity
and results of operations if the current situation continues.
Recently
adopted Accounting Standards
Effective
January 1, 2020, the Company adopted ASU 2016-13, Financial Instruments – Credit Losses (Topic 326), Measurement of Credit
Losses on Financial Instruments. The new standard replaces the ‘incurred loss methodology’ credit impairment model with a
new forward-looking “methodology that reflects expected credit losses and requires consideration of a broader range of reasonable
and supportable information to inform credit loss estimates.” In applying the new standard, the Company has adopted the loss rate
methodology to estimate historical losses on accounts receivables. The Company has adopted the aging methodology to estimate the credit
losses on accounts receivables. The historical data is adjusted to account for forecasted changes in the macroeconomic environment in
order to calculate the current expected credit loss. The Company’s adoption of ASC 326 did not result in a material change in the
carrying values of the Company’s financial assets on the transition date.
In
February 2016, FASB issued ASU No. 2016–02, Leases (Topic 842), ASC 842, and subsequently amended the guidance relating largely
to transition considerations under the standard in July 2018. The new guidance, which creates new accounting and reporting guidelines
for leasing arrangements, requires organizations that lease assets to recognize assets and liabilities on the balance sheet related to
the rights and obligations created by those leases, regardless of whether they are classified as finance or operating leases. Consistent
with current guidance, the recognition, measurement, and presentation of expenses and cash flows arising from a lease primarily will
depend on its classification as a finance or operating lease. The guidance also requires new disclosures to help financial statement
users better understand the amount, timing, and uncertainty of cash flows arising from leases. The new standard is effective for public
business entities for annual reporting periods beginning after December 15, 2018, including interim periods within that reporting period,
with early application permitted. In March 2019, the FASB issued ASU 2019-01, Leases (Topic 842) Codification Improvements,
which further clarifies the determination of fair value of the underlying asset by lessors that are not manufacturers or dealers and
modifies transition disclosure requirements for changes in accounting principles and other technical updates. The amendments in
ASU 2019-01 amend Topic 842 and the effective date of those amendments is for fiscal years beginning December 15, 2019, and interim periods
within those fiscal years for public business entities. For all other entities, ASC 842 is effective for annual periods beginning
after December 15, 2021. For the year ended December 31, 2022, the Company only had an operating lease of office space from January 1,
2022 to December 31, 2022, and the Company did not have any finance lease. The adoption of this guidance did not have a material impact
on the Company’s consolidated financial statements.
In
December 2019, the FASB issued ASU 2019-12, Simplifying the Accounting for Income Taxes, which removes specific exceptions to the general
principles in Topic 740 and to simplifies accounting for income taxes. The guidance is effective for public business entities for fiscal
years beginning after December 15, 2020 and for interim periods within those fiscal years. For all other entities, the amendments are
effective for fiscal years beginning after December 15, 2021, and interim periods within fiscal years beginning after December 15, 2022.
The adoption of this guidance did not have a material impact on the Company’s consolidated financial statements.
In
January 2020, the FASB issued ASU 2020-01, Investments-Equity Securities (Topic 321), Investments-Equity Method and Joint Ventures (Topic
323), and Derivatives and Hedging (Topic 815): Clarifying the Interactions between Topic 321, Topic 323, and Topic 815, which clarifies
the interaction of the accounting for equity investments under Topic 321 and investments accounted for under the equity method of accounting
in Topic 323 and the accounting for certain forward contracts and purchased options accounted for under Topic 815. The guidance is effective
for public business entities for fiscal years beginning after December 15, 2021 and for interim periods within those fiscal years. The
adoption of this guidance did not have a material impact on the Company’s consolidated financial statements.
Recently
Issued Accounting Standards
In
October 2021, the FASB issued ASU No. 2021-08, “‘Business Combinations (Topic 805): Accounting for Contract Assets and Contract
Liabilities from Contracts with Customers” (“ASU 2021-08”). This ASU requires entities to apply Topic 606 to recognize
and measure contract assets and contract liabilities in a business combination. The amendments improve comparability after the business
combination by providing consistent recognition and measurement guidance for revenue contracts with customers acquired in a business
combination and revenue contracts with customers not acquired in a business combination. The amendments are effective for the Company
beginning after December 15, 2023, and are applied prospectively to business combinations that occur after the effective date. The Company
does not expect the adoption of ASU 2021-04 will have a material effect on the consolidated financial statements.
3.
Prepayments and other assets
Prepayments
and other current assets consist of the following:
| | |
December 31,
2022 | | |
December 31,
2021 | |
| | |
US$ | | |
US$ | |
| Other
receivable | |
| 239,148 | | |
| 117,389 | |
| Prepaid
tax | |
| 250,410 | | |
| - | |
| Prepaid
for land use right (1) | |
| 15,948,501 | | |
| - | |
| Prepaid
for property (2) | |
| 12,323,842 | | |
| - | |
| Deferred
IPO cost | |
| - | | |
| 1,277,150 | |
| Total | |
| 28,761,901 | | |
| 1,394,539 | |
| Less:
non-current portion | |
| 12,333,122 | | |
| - | |
| Prepayments
and other current assets | |
| 16,428,779 | | |
| 1,394,539 | |
| (1) | On
October 22, 2018, the Company signed a land use right agreement with the government of Touqiao
Town, Yangzhou City and paid RMB 50 million ($7.25 million) and RMB 60 million ($8.70 million),
respectively, in 2018 and 2019 according to the agreement. As a result of COVID-19, the land
use right had not been transferred to the Company as scheduled. Both parties agreed to cancel
the transaction and the funds that were prepaid for land use right will be returned to the
Company before December 31, 2023. The balance was reclassified from deposits to prepayments
and other current assets. (see Note 8) |
| | |
| (2) | On
April 20, 2020, the Company signed a factory building purchase agreement with Jiangsu Qionghua
Group Co., Ltd. and paid deposit of RMB 85 million ($12.32 million). As a result of COVID-19,
the factory building had not been completed as scheduled. Both parties agreed to cancel the
transaction and that the deposit for the building would be returned to the Company on or
before December 31, 2025, with such deposit accumulating interest at an annual interest rate
of 3.5%. The balance was reclassified from deposits to prepayments and other current assets.
(see Note 8) |
4.
Inventories
Inventories
consist of the following:
| | |
December 31,
2022 | | |
December 31,
2021 | |
| | |
US$ | | |
US$ | |
| Raw
material | |
| 177,474 | | |
| 305,408 | |
| Work-in-process | |
| 343,795 | | |
| 224,694 | |
| Finished
goods | |
| 560,119 | | |
| 682,699 | |
| Low-value
consumables | |
| 40,650 | | |
| 38,592 | |
| Total | |
| 1,122,038 | | |
| 1,251,393 | |
For
the years ended December 31, 2022, 2021 and 2020, there were no writes-down of inventories.
5.
Property, Plant and Equipment
Property,
plant and equipment consisted of the following:
| | |
December 31,
2022 | | |
December 31,
2021 | |
| | |
US$ | | |
US$ | |
| Buildings | |
| 7,343,492 | | |
| 9,552,700 | |
| Machinery
and equipment | |
| 2,732,336 | | |
| 2,897,846 | |
| Motor
vehicles | |
| 678,112 | | |
| 574,006 | |
| Electronic
equipment | |
| 238,856 | | |
| 239,005 | |
| Office
equipment | |
| 43,413 | | |
| 46,987 | |
| Inspection
equipment | |
| 104,993 | | |
| 94,683 | |
| Construction
in progress | |
| 1,394,146 | | |
| 657,880 | |
| Total | |
| 12,535,348 | | |
| 14,063,107 | |
| Less:
accumulated depreciation | |
| 3,777,301 | | |
| 6,585,363 | |
| Property
and equipment, net | |
| 8,758,047 | | |
| 7,477,744 | |
Depreciation
expense was $541,265, $595,522 and $497,238 for the years ended December 31, 2022, 2021 and 2020, respectively.
As
of December 31, 2022 and 2021, the Company pledged buildings to secure bank borrowings to the Company as disclosed in Note 8.
6.
Intangible Assets
Intangible
assets consisted of the following:
| | |
December 31,
2022 | | |
December 31,
2021 | |
| | |
US$ | | |
US$ | |
| Land
use rights | |
| 752,887 | | |
| 814,865 | |
| Patents | |
| 28,997 | | |
| 31,384 | |
| Software | |
| 9,424 | | |
| 10,201 | |
| Trademarks | |
| 121,789 | | |
| 131,814 | |
| Total | |
| 913,097 | | |
| 988,264 | |
| Less:
accumulated amortization | |
| 415,497 | | |
| 426,263 | |
| Intangible
assets, net | |
| 497,600 | | |
| 562,001 | |
Amortization
expense was $22,196, $26,951 and $26,195 for the years ended December 31, 2022, 2021 and 2020, respectively.
The
following table sets forth the Company’s amortization expenses for the twelve months ending December 31 of the following years:
| 2023 | |
$ | 15,058 | |
| 2024 | |
| 15,058 | |
| 2025 | |
| 15,058 | |
| 2026 | |
| 15,058 | |
| 2027 | |
| 15,058 | |
| Thereafter | |
| 422,310 | |
| | |
$ | 497,600 | |
As
of December 31, 2022 and 2021, the Company pledged land use rights to secure bank borrowings to the Company as disclosed in Note 8.
7.
Investment
On
March 3, 2011, Yada invested RMB 6 million into Yangzhou Juyuan Guarantee Co., Ltd. (“Juyuan”) and obtained 12% equity interest
of Juyuan. Juyuan mainly provides financing guarantee services and relevant consulting services to customers. Juyuan has only one executive
director and one supervisor. Neither the executive director nor supervisor is related to Yada. Therefore, Yada has neither control nor
significant influence over Juyuan. For the Company’s passive and without significant influence or control equity investment in
a private company which does not have readily determinable fair values, the Company has elected the measurement alternative defined as
cost, less impairment, plus or minus adjustments resulting from observable price changes in orderly transactions for the identical or
a similar investment of the same issuer.
On
December 1, 2022, Huadong invested RMB 40 million into Zhongxiangxin, and obtained 25% ownership interest of Zhongxiangxin. Zhongxiangxin
manufactures and sells medical materials in the PRC. The Company accounted for the investments using the equity method, because the Company
has significant influence but does not own a majority equity interest or otherwise control over the equity investee. Under the equity
method, the Company adjusts the carrying amount of the investment and recognizes investment income or loss for its share of the earnings
or loss of the investee after the date of investment. When the Company’s share of losses in the equity investee equals or exceeds
its interest in the equity investee, the Company does not recognize further losses, unless the Company has incurred obligations or made
payments or guarantees on behalf of the equity investee. For the year ended December 31, 2022, the investment gain from Zhongxiangxin
was $290.
For
the years ended December 31, 2022, 2021 and 2020, no impairment indicators were identified and no loss related to revaluation of its
investment in the private company was recorded.
8.
Deposits
| | |
December 31,
2022 | | |
December 31,
2021 | |
| | |
US$ | | |
US$ | |
| Deposits
for land use right | |
| - | | |
| 17,261,400 | |
| Deposits
for buildings | |
| - | | |
| 13,338,355 | |
| Total | |
| - | | |
| 30,599,755 | |
On
October 22, 2018, the Company signed a land use right agreement with the government of Touqiao Town, Yangzhou City and paid RMB 50 million
($7.85 million) and RMB 60 million ($9.42 million), respectively, in 2018 and 2019 according to the agreement. This contract is terminated
and the payment is to be recovered. The balance was reclassified to prepayments and other current assets. (see Note 3)
On
April 20, 2020, the Company signed a factory building purchase agreement with Jiangsu Qionghua Group Co., Ltd. and paid deposit of RMB
85 million ($13.34 million). This contract is terminated and the payment is to be recovered. The balance was reclassified to prepayments
and other noncurrent assets. (see Note 3)
During the IPO, the Company entered into a series
of agreements with Tai He International Group Limited (“Tai He”), a Hong Kong investment company. Pursuant to the agreements,
Tai He agreed to invest a minimum of $35 million in the IPO subject to the Company making a $7.0 million refundable deposit and advancing
a $3.0 million service fee for investor relations and other services payable to Tai He. The Company paid approximately $4.8 million to
Tai He in 2022. The Company’s affiliates and individual shareholders paid the rest of the agreed amount. Later the Company learned
that Tai He did not invest in the IPO or provide any service to the Company. The Company is now proactively working to terminate the
Tai He Agreements and recover the amount paid to Tai He by the Company. Due to the uncertainty of collection, the Company has written
off the approximately $4.8 million deposit and fully expensed the $2.3 million service fee paid by the Company to Tai He in the year
ended December 31, 2022.
9.
Bank Borrowings
Bank
borrowings are working capital loans from banks in China. Short-term bank borrowings as of December 31, 2022 consisted of the following:
| Lender | |
Company | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank of Communications | |
Huadong | |
| 3.55 | % | |
3/9/2022 | |
1/19/2023 | |
| 4,000,000 | | |
| 579,946 | |
| Agricultural
Bank of China | |
Huadong | |
| 3.40 | % | |
12/8/2022 | |
12/7/2023 | |
| 9,000,000 | | |
| 1,304,877 | |
| Jiangsu
Yangzhou Rural Commercial Bank | |
Huadong | |
| 3.95 | % | |
2/17/2022 | |
3/2/2023* | |
| 5,000,000 | | |
| 724,932 | |
| Bank of China | |
Huadong | |
| 3.55 | % | |
3/9/2022 | |
1/19/2023* | |
| 5,000,000 | | |
| 724,932 | |
| Agricultural
Bank of China | |
Yada | |
| 3.60 | % | |
12/8/2022 | |
12/6/2023* | |
| 10,000,000 | | |
| 1,449,864 | |
| Industrial
and Commercial Bank of China | |
Yada | |
| 3.70 | % | |
2/18/2022 | |
2/21/2023* | |
| 9,000,000 | | |
| 1,304,877 | |
| Total | |
| |
| | | |
| |
| |
| 42,000,000 | | |
| 6,089,428 | |
| * |
These
loans were renewed upon maturity. |
Short-term
bank borrowings as of December 31, 2021 consisted of the following:
| Lender | |
Company | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank
of Communications | |
Huadong | |
3.70 | % | |
11/1/2021 | |
10/19/2022 | |
| 5,000,000 | | |
| 784,610 | |
| Agricultural
Bank of China | |
Huadong | |
3.70 | % | |
11/3/2021 | |
11/3/2022 | |
| 9,000,000 | | |
| 1,412,296 | |
| Jiangsu
Yangzhou Rural Commercial Bank | |
Huadong | |
3.95 | % | |
2/25/2021 | |
2/17/2022 | |
| 5,000,000 | | |
| 784,609 | |
| Bank of China | |
Huadong | |
3.80 | % | |
3/3/2021 | |
3/2/2022 | |
| 5,000,000 | | |
| 784,609 | |
| Industrial
and Commercial Bank of China | |
Yada | |
3.85 | % | |
2/19/2021 | |
2/18/2022 | |
| 9,000,000 | | |
| 1,412,296 | |
| Total | |
| |
| | |
| |
| |
| 33,000,000 | | |
| 5,178,420 | |
Interest
expense was $191,198, $180,744 and $137,160 for the years ended December 31, 2022, 2021 and 2020, respectively.
The
Company’s short-term bank borrowings are pledged by the Company’s assets and guaranteed by the Company’s major shareholders
Yongjun Liu, Yin Liu, Kai Liu and its subsidiary Yada.
The
carrying values of the Company’s pledged assets to secure short-term borrowings by the Company are as follows:
| | |
December 31,
2022 | | |
December 31,
2021 | |
| | |
US$ | | |
US$ | |
| Buildings,
net | |
| 2,777,379 | | |
| 2,499,131 | |
| Land
use right, net | |
| 96,416 | | |
| 294,582 | |
| Total | |
| 2,873,795 | | |
| 2,793,713 | |
10.
Long-term bank loan
| Lender | |
Company | | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank
of Communications | |
| Huadong | | |
| 3.50 | % | |
11/3/2022 | |
4/25/2024 | |
| 5,000,000 | | |
| 724,932 | |
| Total | |
| | | |
| | | |
| |
| |
| 5,000,000 | | |
| 724,932 | |
On
November 3, 2022, the Company signed a loan agreement with Bank of Communications to obtain a two-year loan of RMB 5 million ($724,932).
The loan bears a floating interest rate of a benchmark rate (3.50%). Huada mortgages the property and land for guaranteed repayment of
the loan. The principal shall be repaid on April 25, 2024.
Interest
expense was $3,468, nil and nil for the years ended December 31, 2022, 2021 and 2020, respectively.
11.
Taxes Payable
Taxes
payable consisted of the following:
| | |
December 31,
2022 | | |
December 31,
2021 | |
| | |
US$ | | |
US$ | |
| VAT
payable | |
| 380,926 | | |
| 488,443 | |
| Income
tax payable | |
| 690,824 | | |
| 1,521,767 | |
| Other
tax payable | |
| 59,526 | | |
| 72,042 | |
| Total | |
| 1,131,276 | | |
| 2,082,252 | |
12.
Income Taxes
Cayman
Islands
Under
the current laws of the Cayman Islands, the Company is not subject to tax on income or capital gain. Additionally, upon payments of dividends
to the shareholders, no Cayman Islands withholding tax is imposed.
Hong
Kong
Under
the current Hong Kong Inland Revenue Ordinance, the Company’s Hong Kong subsidiary, Kang Fu, is subject to 16.5% income tax on
its taxable income generated from operations in Hong Kong. On December 29, 2017, Hong Kong government announced a two-tiered profit tax
rate regime. Under the two-tiered tax rate regime, the first HK$2.0 million assessable profits will be subject to an 8.25% lower tax
rate and the remaining taxable income will continue to be taxed at the existing 16.5% tax rate. The two-tiered tax regime becomes effective
from the assessment year of 2018 and 2019, which is on or after April 1, 2018. The application of the two-tiered rates is restricted
to only one nominated enterprise among connected entities. Kang Fu is nominated by the Company as the entity to apply the two-tiered
rates among the group for the assessment years of 2022, 2021 and 2020.
PRC
Provisions
for income tax are as follows:
| | |
December 31,
2022 | | |
December 31,
2021 | | |
December 31,
2020 | |
| | |
US$ | | |
US$ | | |
US$ | |
| Provisions
for current income tax | |
| 4,713,543 | | |
| 5,278,462 | | |
| 4,688,321 | |
| Provisions
for deferred income tax | |
| - | | |
| - | | |
| - | |
| Total | |
| 4,713,543 | | |
| 5,278,462 | | |
| 4,688,321 | |
The
following is a reconciliation of the Company’s total income tax expense to the income before income taxes for the years ended December
31, 2022, 2021 and 2020, respectively:
| | |
2022 | | |
2021 | | |
2020 | |
| | |
US$ | | |
US$ | | |
US$ | |
| Income
before income tax provision | |
| 18,129,648 | | |
| 26,228,041 | | |
| 23,733,576 | |
| Tax
at the PRC EIT tax rates | |
| 3,758,113 | | |
| 5,605,035 | | |
| 4,724,933 | |
| Other | |
| 37,169 | | |
| (13,086 | ) | |
| (28,392 | ) |
| Tax
effect of non-deductible expenses | |
| 1,660,869 | | |
| 226,222 | | |
| 313,006 | |
| Tax
effect of R&D expenses additional deduction* | |
| (742,608 | ) | |
| (539,709 | ) | |
| (321,226 | ) |
| Income
tax expense | |
| 4,713,543 | | |
| 5,278,462 | | |
| 4,688,321 | |
| * |
According
to PRC tax regulations, an additional of 100% of current year R&D expenses may be deducted from tax income. |
Under
the Enterprise Income Tax Law (“EIT Law”), Foreign Investment Enterprises (“FIEs”) and domestic companies are
subject to Enterprise Income Tax (“EIT”) at a uniform rate of 25%.
Huadong
was entitled High and New Technology Enterprise (“HNTE”) certificate and enjoyed preferential tax rate of 15% for a three-year
validity period from November 30, 2016 and the HNTE certificate was renewed on November 22, 2019 with a three-year validity period. Thus,
Huadong is eligible for a 15% preferential tax rate from January 1, 2016 to December 31, 2021.
The
EIT Law also provides that an enterprise established under the laws of a foreign country or region but whose “de facto management
body” is located in the PRC be treated as a resident enterprise for PRC tax purposes and consequently be subject to the PRC income
tax at the rate of 25% for its global income. The Implementing Rules of the EIT Law define the location of the “de facto management
body” as “the place where the exercising, in substance, of the overall management and control of the production and business
operation, personnel, accounting, properties, etc., of a non-PRC company is located.” Based on a review of surrounding facts and
circumstances, the Company does not believe that it is likely that its entities registered outside of the PRC should be considered as
resident enterprises for the PRC tax purposes.
The
EIT Law also imposes a withholding income tax on dividends distributed by a FIE to its immediate holding company outside of the PRC.
Kang Fu, which is the parent of Huada, Yada and Huadong, is therefore subject to a maximum withholding tax of 10% on dividends distributed
by Huada, Yada and Huadong. In accordance with accounting guidance, all undistributed earnings are presumed to be transferred to the
parent company and are subject to the withholding taxes. The presumption may be overcome if the Company has sufficient evidence to demonstrate
that the undistributed dividends will be re-invested and the remittance of the dividends will be postponed indefinitely. As of December
31, 2022, the Company has determined that the undistributed earnings in Huada, Yada and Huadong will be re-invested into the subsidiary
for the expansion of the Company’s business in mainland China and hence the remittance of the dividends will be postponed indefinitely.
Uncertain
tax positions
The
Company evaluates each uncertain tax position (including the potential application of interest and penalties) based on the technical
merits, and measure the unrecognized benefits associated with the tax positions. As of December 31, 2022, 2021 and 2020, the Company
did not have any significant unrecognized uncertain tax positions.
13.
Commitments and Contingencies
Operating
lease
The
Company has an operating lease to rent an office space in Shanghai. The lease term is 12 months, with the option to renew annually. Rent
expense was $7,515, $7,839 and $7,324 and is included in general and administrative expenses for the years ended December 31, 2022, 2021
and 2020, respectively. The Company has renewed the same operating lease with a term from January 1, 2023 to December 31, 2023, with
all other lease contents remaining the same.
Other
commitments
The
Company did not have other significant commitments, long-term obligations, or guarantees as of December 31, 2022, 2021 and 2020.
Contingencies
The
Company is subject to legal proceedings and regulatory actions in the ordinary course of business. The results of such proceedings cannot
be predicted with certainty, but the Company does not anticipate that the final outcome arising out of any such matter will have a material
adverse effect on our consolidated business, financial position, cash flows or results of operations taken as a whole.
On February 4, 2022, Macias Gini & O’Connell,
LLP (“Plaintiff”) initiated a lawsuit in San Francisco Superior Court. Plaintiff, a certified public accounting firm based
in the U.S., was hired by Kang Fu International Medical Co, and subsequently by Meihua International Medical Technologies Co, Ltd. (collectively,
“Meihua”), to audit Meihua’s consolidated financial statements for the 2018 and 2019 calendar years. Plaintiff seeks
damages from Meihua for its alleged failure to pay for services rendered in the amount of $210,000, plus interest and attorneys’
fees. Although Meihua denies plaintiff’s allegations, on August 29, 2022, Meihua provided an initial settlement offer to Plaintiff.
To date, Plaintiff has not substantively responded. The Court has set and continued multiple case management conferences based on Plaintiff’s
requests for continuances while the parties attempt to informally resolve the matter. No contingent liability has been recognized in
relation to this matter since the outcome of the litigation is highly uncertain.
14.
Statutory Surplus Reserves and Restricted Net Assets
Pursuant
to laws applicable to entities incorporated in the PRC, the Company is required to make appropriations to certain reserve funds, comprising
the statutory surplus reserve and the discretionary surplus reserve, based on after-tax net income determined in accordance with generally
accepted accounting principles of the PRC (“PRC GAAP”). Appropriations to the statutory surplus reserve are required to be
at least 10% of the after-tax net income determined in accordance with PRC GAAP until the reserve is equal to 50% of the entity’s
registered capital. Appropriations to the discretionary surplus reserve are made at the discretion of the Board of Directors. And as
of December 31, 2022 and 2021, the Company did not have a discretionary surplus reserve. As of December 31, 2022, all of the Company’s
PRC subsidiaries reserves had reached 50% of their registered capital threshold and, as a result, the Company stopped being required
to allocate after-tax profits to this reserve.
As
a result of these PRC laws and regulations and the requirement that distributions by PRC entities can only be paid out of distributable
profits computed in accordance with PRC GAAP, the PRC entities are restricted from transferring a portion of their net assets to the
Company. Amounts restricted include paid-in capital and the statutory reserves of the Company’s PRC subsidiaries. The aggregate
amounts of capital and statutory reserves restricted which represented the amount of net assets of the relevant subsidiaries in the Company
not available for distribution was $15,665,860 and $15,178,467 as of December 31, 2022 and 2021, respectively.
Under
PRC laws and regulations, statutory surplus reserves are restricted to set-off against losses, expansion of production and operation
and increasing registered capital of the respective company and are not distributable other than upon liquidation. The reserves are not
allowed to be transferred to the Company in terms of cash dividends, loans or advances, nor allowed for distribution except under liquidation.
15.
Related Party Transactions and Balances
Related
Parties:
| Name
of related parties |
|
Relationship
with the Company |
| Yangzhou
Yada Powder Metallurgy Co., Ltd. |
|
An
entity controlled by Kai Liu, son of Yongjun Liu |
| Jiangsu
Zhida Pharmaceutical Co., Ltd. |
|
An
entity controlled by Kai Liu, son of Yongjun Liu |
| Shanghai
Xinya Pharmaceutical Hanjiang Co., Ltd. |
|
An
entity controlled by Kai Liu, son of Yongjun Liu |
| Yangzhou
Meihua Import and Export Co., Ltd. |
|
An
entity controlled by Kai Liu, son of Yongjun Liu |
Related
Party Sales
The
Company sells products to its related parties and the sales amount from related parties for 2022, 2021 and 2020 are as follows:
Sales:
| | |
For
the Year Ended
December 31, | |
| Name
of related party | |
2022 | | |
2021 | | |
2020 | |
| Yangzhou Meihua Import and Export
Co., Ltd. | |
$ | 18,780 | | |
$ | 51,750 | | |
$ | 71,885 | |
| Yangzhou Yada Powder Metallurgy Co., Ltd. | |
| - | | |
| 347,737 | | |
| 669,583 | |
| Shanghai Xinya Pharmaceutical Hanjiang Co.,
Ltd. | |
| 10,416 | | |
| 176,414 | | |
| 67,101 | |
| Jiangsu Zhida Pharmaceutical
Co., Ltd. | |
| - | | |
| - | | |
| 8,038 | |
| Total | |
$ | 29,196 | | |
$ | 575,901 | | |
$ | 816,607 | |
16.
Subsequent Events
The
Company has evaluated the impact of events that have occurred subsequent to December 31, 2022, through the date the consolidated financial
statements were available to be issued, and concluded that no subsequent events have occurred that would require recognition in the consolidated
financial statements or disclosure in the notes to the consolidated financial statements, except as follow:
Bank
borrowing
As
the date these consolidated financial statements were available to be issued, the Company has new bank borrowings in the amount of $2,609,755
(RMB 18 million) with interest rates ranging from 3.45-3.95% and has bank loan repayment of $2,609,755 (RMB 18 million).
New
bank borrowing
Subsequent
new bank borrowings consisted of the following:
| Lender | |
Company | | |
Rate | | |
Issuance
Date | |
Collateral/Security | |
Amount-RMB | | |
Amount-USD | |
| Jiangsu
Yangzhou Rural Commercial Bank | |
Huadong | | |
| 3.95 | % | |
1/30/2023 | |
Yada, Yongjun Liu | |
| 5,000,000 | | |
| 724,932 | |
| Industrial
and Commercial Bank of China | |
Yada | | |
| 3.45 | % | |
2/17/2023 | |
Properties of Yada | |
| 9,000,000 | | |
| 1,304,877 | |
| Bank
of Communications | |
Huadong | | |
| 3.50 | % | |
1/19/2023 | |
Yongjun Liu, Yin Liu,
Yada | |
| 4,000,000 | | |
| 579,946 | |
| Total | |
| | |
| | | |
| |
| |
| 18,000,000 | | |
| 2,609,755 | |
Repayment
Subsequent
repayments on bank borrowings consisted of the following:
| Lender | |
Company | | |
Rate | | |
Repayment
Date | |
Collateral/Security | |
Amount-RMB | | |
Amount-USD | |
| Jiangsu
Yangzhou Rural Commercial Bank | |
Huadong | | |
| 3.95 | % | |
1/31/2023 | |
Yada, Yongjun Liu | |
| 5,000,000 | | |
| 724,932 | |
| Bank
of Communications | |
Huadong | | |
| 3.55 | % | |
1/13/2023 | |
Yongjun Liu, Yin Liu, Yada | |
| 4,000,000 | | |
| 579,946 | |
| Industrial
and Commercial Bank of China | |
Yada | | |
| 3.70 | % | |
2/16/2023 | |
Properties of Yada | |
| 9,000,000 | | |
| 1,304,877 | |
| Total | |
| | |
| | | |
| |
| |
| 18,000,000 | | |
| 2,609,755 | |

MEIHUA INTERNATIONAL
MEDICAL TECHNOLOGIES CO., LTD.
1,205,255 Ordinary Shares Issuable upon
Exercise of Warrants
PROSPECTUS
February 27, 2024
Meihua International Med... (NASDAQ:MHUA)
Historical Stock Chart
From Dec 2024 to Jan 2025

Meihua International Med... (NASDAQ:MHUA)
Historical Stock Chart
From Jan 2024 to Jan 2025
