Notice of General Meeting
November 07 2024 - 3:30PM

November 7, 2024
Biodexa Pharmaceuticals
PLC(“Biodexa” or the “Company”)
Notice of General Meeting
Biodexa Pharmaceuticals PLC, (Nasdaq: BDRX), a
clinical stage biopharmaceutical company developing a pipeline of
innovative products for the treatment of diseases with unmet
medical needs announces a Notice of a General Meeting to be held at
the Company’s offices, 1 Caspian Point, Caspian Way, Cardiff, CF10
4DQ on 22 November 2024 at 13:00 GMT was posted to shareholders
yesterday.
The Board of Directors is proposing two
resolutions:
Ordinary Resolution
1. That,
subject to and conditional on the passing of Resolution 2, each of
the issued ordinary shares of £0.001 each in the capital of the
Company be subdivided and redesignated into one ordinary share of
£0.00005 each and 19 C deferred shares of £0.00005 each (such C
deferred shares having the rights and being subject to the
restrictions set out in the articles of association of the Company
adopted pursuant to Resolution 2). Special
Resolution 2. That,
subject to and conditional on the passing of Resolution 1, the
draft articles of association tabled at the meeting, initialled by
the Chairman, and available on the Company’s website,
www.biodexapharma.com and labelled the ‘New Articles’, be approved
and adopted as the new articles of association of the Company in
substitution for and to the entire exclusion of the Company’s
existing articles of association.
The purpose of the Resolutions is to lower the
par value of the ordinary shares and allow the Company to issue
ordinary shares above par value.
Neither the number of ordinary shares
outstanding nor the number of American Depositary Shares will
change as a result of passing of the Resolutions.
About Biodexa Pharmaceuticals PLC
Biodexa Pharmaceuticals PLC (listed on NASDAQ:
BDRX) is a clinical stage biopharmaceutical company developing a
pipeline of innovative products for the treatment of diseases with
unmet medical needs. The Company’s lead development programs
include eRapa, under development for Familial Adenomatous Polyposis
and Non-Muscle Invasive Blader Cancer; tolimidone, under
development for the treatment of type 1 diabetes; and MTX110, which
is being studied in aggressive rare/orphan brain cancer
indications.
eRapa is a proprietary oral tablet formulation
of rapamycin, also known as sirolimus. Rapamycin is an mTOR
(mammalian Target Of Rapamycin) inhibitor. mTOR has been shown to
have a significant role in the signalling pathway that regulates
cellular metabolism, growth and proliferation and is activated
during tumorigenesis.
Tolimidone is an orally delivered, potent and
selective inhibitor of Lyn kinase. Lyn is a member of the Src
family of protein tyrosine kinases, which is mainly expressed in
hematopoietic cells, in neural tissues, liver, and adipose tissue.
Tolimidone demonstrates glycaemic control via insulin sensitization
in animal models of diabetes and has the potential to become a
first in class blood glucose modulating agent.
MTX110 is a solubilized formulation of the
histone deacetylase (HDAC) inhibitor, panobinostat. This
proprietary formulation enables delivery of the product via
convection-enhanced delivery (CED) at chemotherapeutic doses
directly to the site of the tumor, by-passing the blood-brain
barrier and potentially avoiding systemic toxicity.
Biodexa is supported by three proprietary drug
delivery technologies focused on improving the bio-delivery and
bio-distribution of medicines. Biodexa’s headquarters and R&D
facility is in Cardiff, UK. For more information visit
www.biodexapharma.com.
Forward-Looking
StatementsCertain statements in this announcement may
constitute “forward-looking statements” within the meaning of
legislation in the United Kingdom and/or United States. Such
statements are made pursuant to the safe harbor provisions of the
Private Securities Litigation Reform Act of 1995 and are based on
management’s belief or interpretation. All statements contained in
this announcement that do not relate to matters of historical fact
should be considered forward-looking statements. In certain cases,
forward-looking statements can be identified by the use of words
such as “plans”, “expects” or “does not anticipate”, or “believes”,
or variations of such words and phrases or statements that certain
actions, events or results “may”, “could”, “would”, “might” or
“will be taken”, “occur” or “be achieved.” Forward-looking
statements and information are subject to various known and unknown
risks and uncertainties, many of which are beyond the ability of
the Company to control or predict, that may cause their actual
results, performance or achievements to be materially different
from those expressed or implied thereby, and are developed based on
assumptions about such risks, uncertainties and other factors set
out herein.
Reference should be made to those documents that
Biodexa shall file from time to time or announcements that may be
made by Biodexa in accordance with the rules and regulations
promulgated by the SEC, which contain and identify other important
factors that could cause actual results to differ materially from
those contained in any projections or forward-looking statements.
These forward-looking statements speak only as of the date of this
announcement. All subsequent written and oral forward-looking
statements by or concerning Biodexa are expressly qualified in
their entirety by the cautionary statements above. Except as may be
required under relevant laws in the United States, Biodexa does not
undertake any obligation to publicly update or revise any
forward-looking statements because of new information, future
events or events otherwise arising.
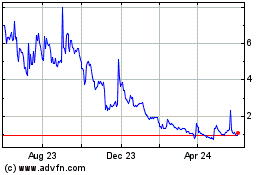
Biodexa Pharmaceuticals (NASDAQ:BDRX)
Historical Stock Chart
From Oct 2024 to Nov 2024
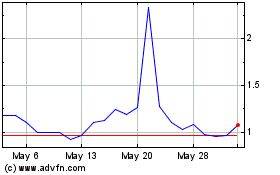
Biodexa Pharmaceuticals (NASDAQ:BDRX)
Historical Stock Chart
From Nov 2023 to Nov 2024