false 0001759138 0001759138 2024-11-18 2024-11-18
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
November 18, 2024
Date of Report (Date of earliest event reported)
CABALETTA BIO, INC.
(Exact name of Registrant as Specified in its Charter)
|
|
|
|
|
| Delaware |
|
001-39103 |
|
82-1685768 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
|
|
|
| 2929 Arch Street, Suite 600, Philadelphia, PA |
|
19104 |
| (Address of principal executive offices) |
|
(Zip Code) |
(267) 759-3100
(Registrant’s telephone number, including area code)
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of Each Class |
|
Trading
Symbol(s) |
|
Name of Each Exchange on Which Registered |
| Common Stock, par value $0.00001 per share |
|
CABA |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure |
On November 18, 2024, Cabaletta Bio, Inc. (“Cabaletta” or the “Company”) posted an investor presentation (the “Investor Presentation”) to the “News & Media” section of the Company’s website at www.cabalettabio.com. The Investor Presentation will be used in connection with a conference call and webcast today, November 18, 2024, at 8:00 a.m. ET, to review the clinical data presented at the American College of Rheumatology (ACR) Convergence 2024 conference (“ACR Convergence 2024”) and provide an update on the RESET clinical development program. A copy of the Investor Presentation is furnished herewith as Exhibit 99.1 to this Current Report on Form 8-K.
On November 18, 2024, the Company also issued a Press Release reporting new and updated clinical data on CABA-201 demonstrating the potential to achieve drug-free, compelling clinical responses based on eight patients dosed across the ongoing Phase 1/2 RESET-Myositis™, RESET-SLE™ and RESET-SSc™ clinical trials (the “Press Release”). A copy of the Press Release is furnished herewith as Exhibit 99.2 to this Current Report on Form 8-K.
The information contained in Item 7.01 of this Current Report on Form 8-K, including Exhibits 99.1 and 99.2 attached hereto, is being furnished and shall not be deemed to be “filed” for the purposes of Section 18 of the Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section and shall not be incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
On November 16, 2024, the Company presented translational updates from the RESET clinical trials at the ACR Convergence 2024 conference. A copy of the poster, which has been published to the “Technology - Posters & Presentations” section of the Company’s website is filed as Exhibit 99.3 to this Current Report on Form 8-K and is incorporated herein by reference.
On November 17, 2024, the Company presented a clinical update at the ACR Convergence 2024 conference. A copy of the slides, which has been published to the “News & Media” section of the Company’s website, is filed as Exhibit 99.4 to this Current Report on Form 8-K and is incorporated herein by reference.
On November 18, 2024, the Company issued the Press Release reporting new and updated clinical data on CABA-201 demonstrating the potential to achieve drug-free, compelling clinical responses based on eight patients dosed across the ongoing Phase 1/2 RESET-Myositis™, RESET-SLE™ and RESET-SSc™ clinical trials.
As of the data cut-off date of November 1, 2024, eight patients had been dosed with CABA-201 with sufficient follow-up to be evaluable across the RESET clinical development program. In the RESET-Myositis trial, one patient in the immune-mediated necrotizing myopathy (IMNM) cohort completed six months of follow-up and two patients, one in the IMNM cohort and one in the dermatomyositis (DM) cohort, each completed one month of follow-up. In the RESET-SLE trial, one patient in the non-renal systemic lupus erythematosus (SLE) cohort completed six months of follow-up, one patient in the lupus nephritis (LN) cohort completed four months of follow-up, and two patients in the non-renal SLE cohort each completed one month of follow-up. Translational assessments from the third patient in the non-renal SLE cohort were not available for inclusion at the time of the data cut-off. In the RESET-SSc trial, one patient in the severe skin cohort completed six weeks of follow-up.
Across these eight patients treated with CABA-201, patients were administered a one-time infusion of CABA-201 at 1 x 106 cells/kg, following a preconditioning regimen of fludarabine and cyclophosphamide. The primary endpoint of each trial is safety and tolerability within 28 days of infusion. Secondary endpoints include translational assessments and clinical outcomes.
Safety and Tolerability Profile: CABA-201 has shown a favorable risk-benefit profile in patients with active and refractory autoimmune disease
| |
• |
|
Through 28 days of follow-up, no evidence of cytokine release syndrome (CRS) of any grade was observed in five of the eight patients. Low-grade CRS (Grades 1-2) was observed in three patients, all of which recovered following standard care. Tocilizumab was not administered for any cases of CRS. |
| |
• |
|
No evidence of immune effector cell-associated neurotoxicity syndrome (ICANS) of any grade has been observed in any patient since reporting the initial safety data on the first LN patient in August 2024. This patient had acute inflammatory events shortly before CABA-201 treatment and demonstrated an abnormal, pro-inflammatory cytokine profile prior to infusion that continued after CABA-201 infusion, suggestive of a possible occult infection. |
Translational Assessments: CABA-201 induced consistent and complete B cell depletion, with early naïve B cell repopulation suggesting the potential to generate an immune system reset
| |
• |
|
CAR T cell expansion associated with CABA-201 reached its peak between day 8 and day 15. Translational assessments from the first patient in the LN cohort indicated a second peak at day 29. |
| |
• |
|
Complete B cell depletion was observed by day 22 after CABA-201 infusion. |
| |
• |
|
B cell repopulation occurred in the first two patients treated with CABA-201 as early as 8 weeks and exhibited a transitional naïve phenotype, reflecting the production of new B cells after deep systemic depletion. |
| |
• |
|
Two of the three patients with follow-up beyond three months demonstrated a reduction in disease-associated antibodies. Clinical responses in all three of these patients were observed independent of autoantibody levels. |
| |
• |
|
Vaccine and infectious pathogen antibodies remained generally stable. |
Clinical Outcomes: CABA-201 provided compelling signs of early efficacy, supporting the potential for drug-free clinical responses
| |
• |
|
Initial clinical responses in the RESET-Myositis trial were consistent with published data with response kinetics appearing to differ between myositis subtypes. |
| |
• |
|
The first known adult DM patient dosed with CAR T in the form of CABA-201 demonstrated an improvement in muscle strength to normal and a major total improvement score (TIS) response off all immunosuppressants at one month of follow-up. The Cutaneous Dermatomyositis Disease Area and Severity Index – Activity (CDASI-A) improved from 25 to 9. |
| |
• |
|
At six months of follow-up, the first IMNM patient demonstrated a continued and improved clinical response off immunosuppressants and without flares. At one month of follow-up, the second IMNM patient demonstrated a total improvement score consistent with the first IMNM patient at one month after CABA-201 infusion off immunosuppressants. |
| |
• |
|
All four patients in the RESET-SLE trial demonstrated clinical responses off immunosuppressants. |
| |
• |
|
All three patients in the non-renal SLE cohort demonstrated no clinical symptoms on SLEDAI-2K as of the latest follow-up and the first patient has completed a prednisone taper to discontinuation. |
| |
• |
|
The first patient in the LN cohort, who experienced the previously reported ICANS event, had a SLEDAI that improved from 22 at baseline to 8 at month four of follow-up. The patient’s proteinuria improved more than 90%, approaching normal levels, while off all immunosuppressants and with an ongoing prednisone taper. |
| |
• |
|
The first patient in the severe skin cohort in the RESET-SSc trial demonstrated early clinical improvements after discontinuation of disease-specific therapy. |
| |
• |
|
The modified Rodnan Skin Score of the first patient in the severe skin cohort improved from 42 at baseline (potential maximum of 51) to 36 at day 42, suggesting the potential emergence of a drug-free clinical response. |
Forward-Looking Statements
The information under this Item 8.01 contains “forward-looking statements” of Cabaletta Bio within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including without limitation, express or implied statements regarding: Cabaletta’s business plans and objectives as a whole; Cabaletta’s ability to realize its vision of launching the first curative targeted cell therapy designed specifically for patients with autoimmune diseases; Cabaletta’s ability to successfully complete research and further development and commercialization of its drug candidates in current or future indications, including the timing and results of Cabaletta’s clinical trials and its ability to conduct and complete clinical trials; expectation that clinical results will support CABA-201’s safety and activity profile; statements regarding the expectations of trial modifications and prophylactic measures, continued trial operations; statements regarding the timing of interactions with regulatory authorities, including such authorities’ review of safety information from Cabaletta’s ongoing clinical trials and potential registrational program designs for CABA-201; Cabaletta’s expectations around the potential success and therapeutic benefits of CABA-201, including its belief that CABA-201 has the potential to reset the immune system and result in compelling clinical responses without chronic therapy requirements in patients; the Company’s advancement of separate Phase 1/2 clinical trials of CABA-201 in patients with SLE, myositis, SSc and gMG and advancement of a RESET-PV trial, including updates related to status, safety data, efficiency of clinical trial design or otherwise; the clinical significance of the clinical data read-out at the ACR Convergence 2024 in November 2024 for patients with myositis, SLE and SSc treated with CABA-201; Cabaletta’s ability to increase enrollment from its rapidly expanding clinical network in the RESET clinical program in the United States and beyond and Cabaletta’s ability to leverage such growing clinical trial network to accelerate development of its therapy for patients.
Any forward-looking statements in this Item 8.01 are based on management’s current expectations and beliefs of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to: risks related to regulatory filings and potential clearance; the risk that signs of biologic activity or persistence may not inform long-term results; Cabaletta’s ability to demonstrate sufficient evidence of safety, efficacy and tolerability in its preclinical studies and clinical trials of CABA-201; the risk that the results observed with the similarly-designed construct employed in academic publications, including due to the dosing regimen, are not indicative of the results we seek to achieve with CABA-201; risks that modifications to trial design or approach may not have the intended benefits and that the trial design may need to be further modified; risks related to clinical trial site activation, delays in enrollment generally or enrollment rates that are lower than expected; delays related to assessment of clinical trial results; risks related to unexpected safety or efficacy data observed during clinical studies; risks related to volatile market and economic conditions and public health crises; Cabaletta’s ability to retain and recognize the intended incentives conferred by Orphan Drug Designation and Fast Track Designation or other designations for its product candidates, as applicable; risks related to Cabaletta’s ability to protect and maintain its intellectual property position; risks related to fostering and maintaining successful relationships with Cabaletta’s collaboration and manufacturing partners, including in light of recent legislation; uncertainties related to the initiation and conduct of studies and other development requirements for its product candidates; the risk that any one or more of Cabaletta’s product candidates will not be successfully developed and/or commercialized; and the risk that the initial or interim results of preclinical studies or clinical studies will not be predictive of future results in connection with future studies. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause Cabaletta’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in Cabaletta’s most recent annual report on Form 10-K as well as discussions of potential risks, uncertainties, and other important factors in Cabaletta’s other filings with the Securities and Exchange Commission. All information in this Item 8.01 is as of the date of this Current Report on Form 8-K, and the Company undertakes no duty to update this information unless required by law.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
|
|
|
| 99.1 |
|
Investor Presentation, dated November 18, 2024, furnished herewith. |
|
|
| 99.2 |
|
Press Release issued by the registrant on November 18, 2024, furnished herewith. |
|
|
| 99.3 |
|
Poster presentation from Cabaletta Bio, Inc., dated November 16, 2024 |
|
|
| 99.4 |
|
Slides from Cabaletta Bio, Inc.’s ACR Convergence 2024 Conference Presentation, dated November 17, 2024. |
|
|
| 104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL Document). |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned, hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
CABALETTA BIO, INC. |
|
|
|
|
| Date: November 18, 2024 |
|
|
|
By: |
|
/s/ Steven Nichtberger |
|
|
|
|
|
|
Steven Nichtberger, M.D. |
|
|
|
|
|
|
President and Chief Executive Officer |

Exhibit 99.1 CABA-201 Clinical and Translational Data from the RESET
Phase 1/2 Trials NOVEMBER 2024 © 2024 Cabaletta Bio. All rights reserved.

Disclaimer The following presentation, including any printed or
electronic copy of these slides, the talks given by the presenters, the information communicated during any delivery of the presentation and any question and answer session and any document or material distributed at or in connection with the
presentation (collectively, the “Presentation”) has been prepared by Cabaletta Bio, Inc. (“we,” “us,” “our,” “Cabaletta” or the “Company”) and is made for informational purposes
only. This Presentation does not purport to be a prospectus, to be complete or to contain all of the information you may desire. Statements contained herein are made as of the date of this Presentation unless stated otherwise, and this Presentation
shall not under any circumstances create an implication that the information contained herein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or
changes occurring after the date hereof. This Presentation may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to our business, operations, and financial
conditions, and include, but are not limited to, express or implied statements regarding our current beliefs, expectations and assumptions regarding: our business, future plans and strategies for our CAAR T and CARTA technologies; our ability to
grow our autoimmune-focused pipeline; the ability to capitalize on and potential benefits resulting from our research and translational insights; including those related to any similarly-designed constructs or dosing regimens; the anticipated market
opportunities for CABA-201 in patients with autoimmune diseases; our ability to successfully complete research and further development and commercialization of our candidates, including the timing and results of our clinical trials and our ability
to conduct and complete clinical trials; expectation that clinical results will support CABA-201’s safety and activity profile; statements regarding the expectations of trial modifications and prophylactic measures, continued trial operations;
statements regarding the timing of regulatory filings and interactions, including timing of such interactions, with regulatory authorities, including such authorities’ review of information from Cabaletta’s ongoing clinical trials and
potential registrational program designs for CABA-201; our business plans and objectives; our expectations around the potential success and therapeutic benefits of CABA-201, our advancement of separate Phase 1/2 clinical trials of CABA-201 in
patients with systemic lupus erythematosus (SLE), myositis, SSc, and generalized myasthenia gravis (gMG), and advancement of a RESET-PV trial, including the timing thereof, including our anticipated progress, timing of enrollment, expectations for
the efficiency of the clinical trial designs, updates related to status, safety data, or otherwise and the expected timing of the related data read-outs, and ability to leverage our experience in autoimmune cell therapy; the clinical significance of
the clinical data read-out at the ACR Convergence 2024 in November 2024 for patients with myositis, SLE and SSc treated with CABA-201; our planned initial clinical data read-out for patients with gMG treated with CABA-201 in the first half of 2025;
our ability to increase enrollment from our rapidly expanding clinical network in the RESET clinical program in the United States and beyond; our ability to activate clinical trial sites and pursue patient enrollment for the RESET-SLE trial in
Europe and leverage our recent CTA; the expectation that Cabaletta may improve outcomes for patients suffering from SLE, SSc, myositis, gMG, mucosal pemphigus vulgaris, or other autoimmune diseases; the ability of our clinical strategy to reduce
risk, maximize reach and accelerate timelines of our Phase 1/2 clinical trials of CABA-201; statements related to the differentiation of CAR T in the autoimmune setting; our ability to successfully complete our preclinical and clinical studies for
our product candidates, including our ability to enroll the requisite number of patients, dose each dosing cohort in the intended manner, and progress the trial; our ability to obtain and maintain regulatory approval of our product candidates,
including our expectations regarding the intended incentives conferred by and ability to retain Orphan Drug Designation and Fast Track Designations for our product candidates, as applicable; our ability to accelerate our pipeline and to develop
meaningful therapies for patients, including in collaboration with academic and industry partners and the ability to optimize such collaborations on our development programs; and our expectations regarding our use of capital and other financial
results, including our ability to fund operations into the first half of 2026. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,”
“intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Various risks, uncertainties and assumptions could cause actual
results to differ materially from those anticipated or implied in our forward-looking statements. Such risks and uncertainties include, but are not limited to, risks related to the success, cost, and timing of our product candidate development
activities and preclinical studies and clinical trials, risks related to our ability to demonstrate sufficient evidence of safety, efficacy and tolerability in our preclinical studies and clinical trials of CABA-201, DSG3-CAART and MuSK-CAART, the
risk that the results observed with the similarly-designed construct, including, but not limited to, due to dosing regimen, are not indicative of the results we seek to achieve with CABA-201, ethe risk that signs of biologic activity or persistence
may not inform long-term results, the risk that interim results do not always inform later results, the risk that piersistence observed with effective CD19-CAR T oncology studies in combination with lymphodepletion is not indicative of, or
applicable to, clinical responses in patients with mPV, risks related to clinical trial site activation or enrollment rates that are lower than expected, our ability to protect and maintain our intellectual property position, risks related to our
relationships with third parties, uncertainties related to regulatory agencies’ evaluation of regulatory filings and other information related to our product candidates, our ability to retain and recognize the intended incentives conferred by
any Orphan Drug Designations and Fast Track Designations, risks related to regulatory filings and potential clearance, the risk that any one or more of our product candidates will not be successfully developed and commercialized, the risk that the
results of preclinical studies or clinical studies will not be predictive of future results in connection with future studies, and risks related to volatile market and economic conditions and public health crises. New risks and uncertainties may
emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any
new information, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct.
Accordingly, you are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. For a discussion of these and
other risks and uncertainties, and other important factors, any of which could cause our actual results to differ materially from those contained in the forward-looking statements, see the section entitled Risk Factors in our most recent annual
report on Form 10-K, as well as discussions of potential risks, uncertainties, and other important factors in our other and subsequent filings with the Securities and Exchange Commission. Certain information contained in this Presentation relates to
or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this
Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. The Company is the owner of various trademarks, trade names
and service marks. Certain other trademarks, trade names and service marks appearing in this Presentation are the property of third parties. Solely for convenience, the trademarks and trade names in this Presentation are referred to without the
® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. 2

Today’s Agenda AGENDA TOPIC SPEAKER Steven Nichtberger, MD
CABA-201 Overview Chief Executive Officer Lessons from Oncology: Expanding CAR T Carl H. June, MD Director of the Center for Cellular Immunotherapies, Penn Medicine Cell Therapies into Autoimmunity CABA-201 Clinical and Translational Data David
Chang, MD, MPH, FACR TM Chief Medical Officer from the RESET Clinical Program Steven Nichtberger, MD Conclusions Chief Executive Officer Q&A 3
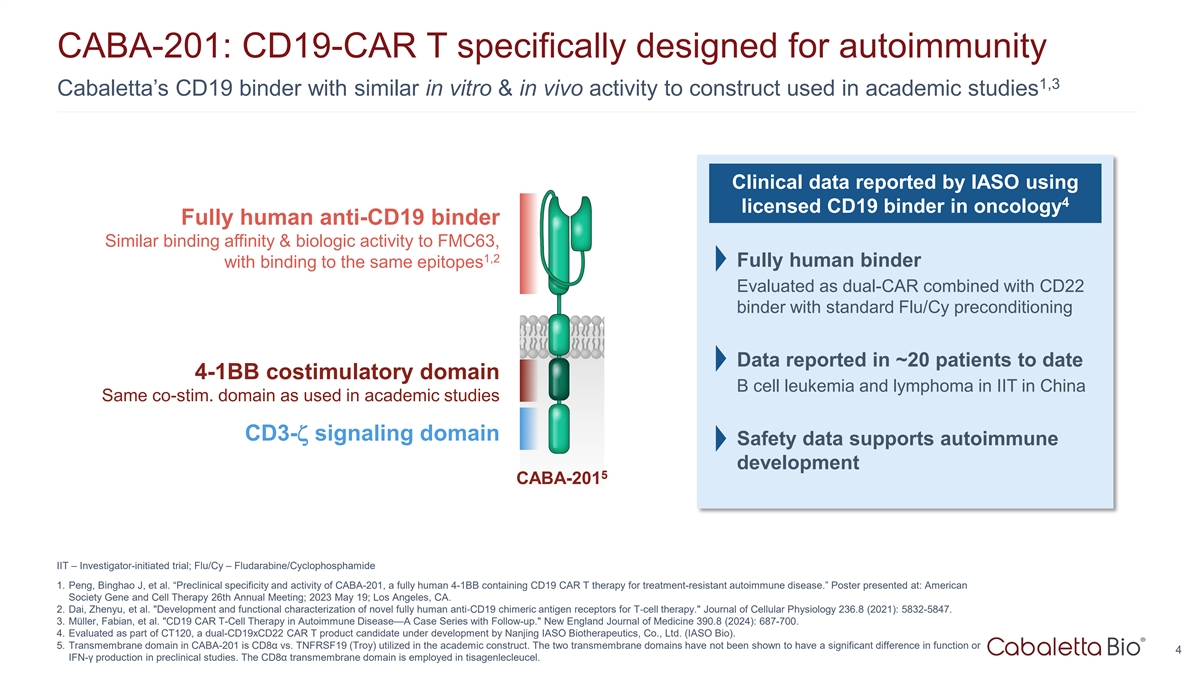
CABA-201: CD19-CAR T specifically designed for autoimmunity 1,3
Cabaletta’s CD19 binder with similar in vitro & in vivo activity to construct used in academic studies Clinical data reported by IASO using 4 licensed CD19 binder in oncology Fully human anti-CD19 binder Similar binding affinity &
biologic activity to FMC63, 1,2 with binding to the same epitopes Fully human binder Evaluated as dual-CAR combined with CD22 binder with standard Flu/Cy preconditioning Data reported in ~20 patients to date 4-1BB costimulatory domain B cell
leukemia and lymphoma in IIT in China Same co-stim. domain as used in academic studies CD3-ζ signaling domain Safety data supports autoimmune development 5 CABA-201 IIT – Investigator-initiated trial; Flu/Cy –
Fludarabine/Cyclophosphamide 1. Peng, Binghao J, et al. “Preclinical specificity and activity of CABA-201, a fully human 4-1BB containing CD19 CAR T therapy for treatment-resistant autoimmune disease.” Poster presented at: American
Society Gene and Cell Therapy 26th Annual Meeting; 2023 May 19; Los Angeles, CA. 2. Dai, Zhenyu, et al. Development and functional characterization of novel fully human anti‐ CD19 chimeric antigen receptors for T‐ cell therapy. Journal
of Cellular Physiology 236.8 (2021): 5832-5847. 3. Müller, Fabian, et al. CD19 CAR T-Cell Therapy in Autoimmune Disease—A Case Series with Follow-up. New England Journal of Medicine 390.8 (2024): 687-700. 4. Evaluated as part of CT120, a
dual-CD19xCD22 CAR T product candidate under development by Nanjing IASO Biotherapeutics, Co., Ltd. (IASO Bio). 5. Transmembrane domain in CABA-201 is CD8α vs. TNFRSF19 (Troy) utilized in the academic construct. The two transmembrane domains
have not been shown to have a significant difference in function or 4 IFN-γ production in preclinical studies. The CD8α transmembrane domain is employed in tisagenlecleucel.
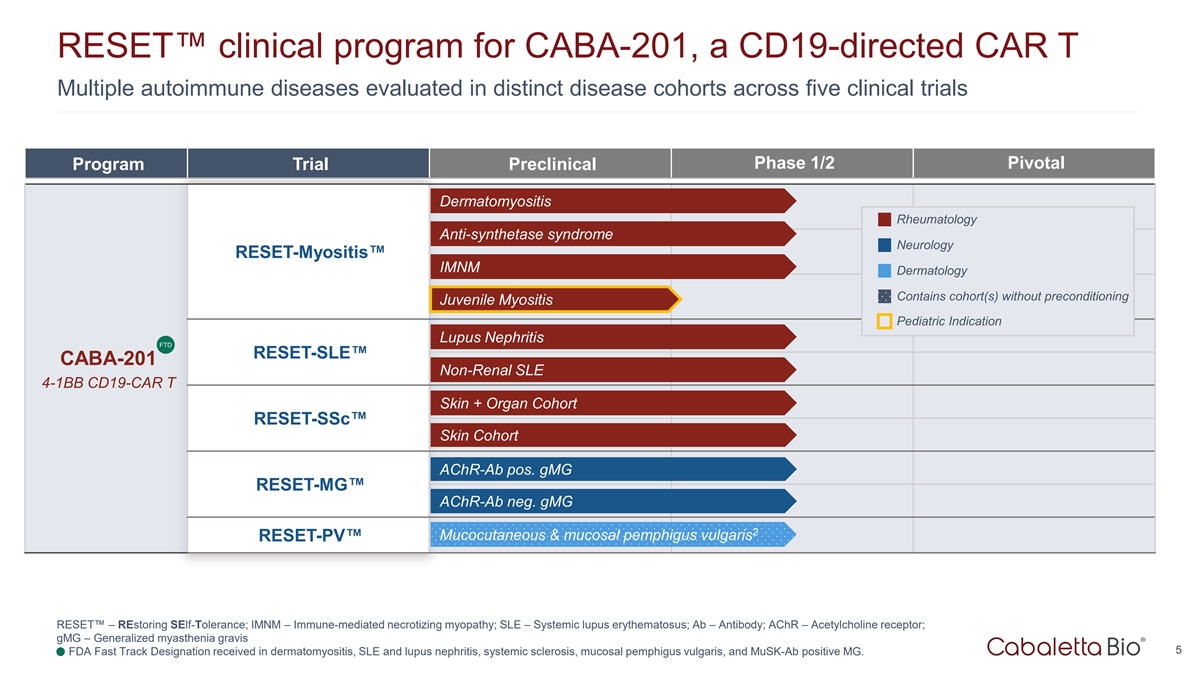
RESET clinical program for CABA-201, a CD19-directed CAR T Multiple
autoimmune diseases evaluated in distinct disease cohorts across five clinical trials Phase 1/2 Pivotal Program Trial Preclinical Dermatomyositis Rheumatology Anti-synthetase syndrome Neurology RESET-Myositis IMNM Dermatology Contains cohort(s)
without preconditioning Juvenile Myositis Pediatric Indication Lupus Nephritis FTD RESET-SLE CABA-201 Non-Renal SLE 4-1BB CD19-CAR T Skin + Organ Cohort RESET-SSc Skin Cohort AChR-Ab pos. gMG RESET-MG AChR-Ab neg. gMG 2 Mucocutaneous & mucosal
pemphigus vulgaris RESET-PV RESET – REstoring SElf-Tolerance; IMNM – Immune-mediated necrotizing myopathy; SLE – Systemic lupus erythematosus; Ab – Antibody; AChR – Acetylcholine receptor; gMG – Generalized
myasthenia gravis 5 1. FDA Fast Track Designation received in dermatomyositis, SLE and lupus nephritis, systemic sclerosis, mucosal pemphigus vulgaris, and MuSK-Ab positive MG.
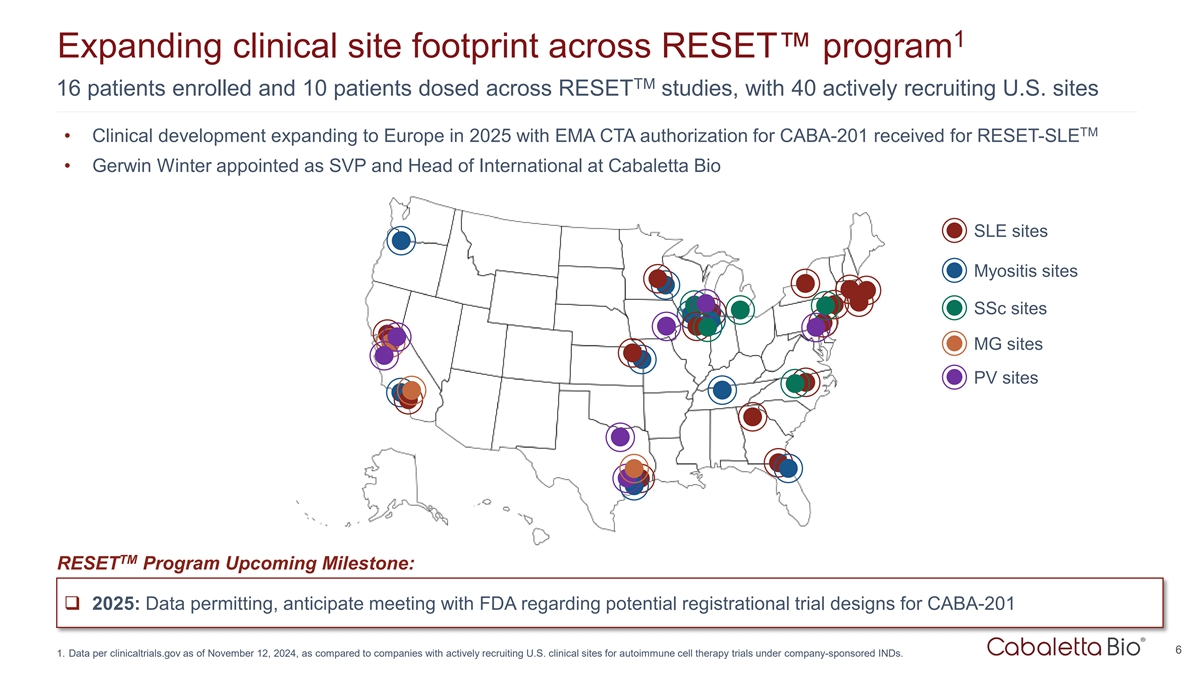
1 Expanding clinical site footprint across RESET program TM 16 patients
enrolled and 10 patients dosed across RESET studies, with 40 actively recruiting U.S. sites TM • Clinical development expanding to Europe in 2025 with EMA CTA authorization for CABA-201 received for RESET-SLE • Gerwin Winter appointed as
SVP and Head of International at Cabaletta Bio SLE sites Myositis sites SSc sites MG sites PV sites TM RESET Program Upcoming Milestone: q 2025: Data permitting, anticipate meeting with FDA regarding potential registrational trial designs for
CABA-201 6 1. Data per clinicaltrials.gov as of November 12, 2024, as compared to companies with actively recruiting U.S. clinical sites for autoimmune cell therapy trials under company-sponsored INDs.

Lessons from Oncology: Expanding CAR T Cell Therapies into Autoimmunity
7
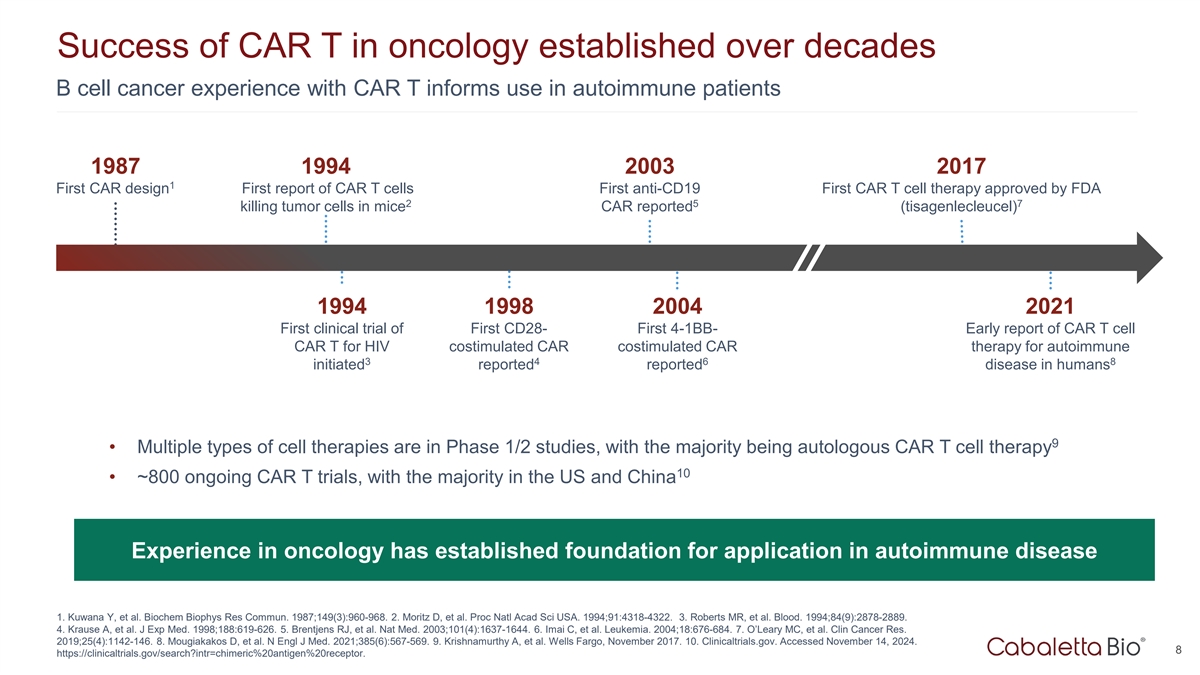
Success of CAR T in oncology established over decades B cell cancer
experience with CAR T informs use in autoimmune patients 1987 1994 2003 2017 1 First CAR design First report of CAR T cells First anti-CD19 First CAR T cell therapy approved by FDA 2 5 7 killing tumor cells in mice CAR reported (tisagenlecleucel)
1994 1998 2004 2021 First clinical trial of First CD28- First 4-1BB- Early report of CAR T cell CAR T for HIV costimulated CAR costimulated CAR therapy for autoimmune 3 4 6 8 initiated reported reported disease in humans 9 • Multiple types of
cell therapies are in Phase 1/2 studies, with the majority being autologous CAR T cell therapy 10 • ~800 ongoing CAR T trials, with the majority in the US and China Experience in oncology has established foundation for application in
autoimmune disease 1. Kuwana Y, et al. Biochem Biophys Res Commun. 1987;149(3):960-968. 2. Moritz D, et al. Proc Natl Acad Sci USA. 1994;91:4318-4322. 3. Roberts MR, et al. Blood. 1994;84(9):2878-2889. 4. Krause A, et al. J Exp Med.
1998;188:619-626. 5. Brentjens RJ, et al. Nat Med. 2003;101(4):1637-1644. 6. Imai C, et al. Leukemia. 2004;18:676-684. 7. O’Leary MC, et al. Clin Cancer Res. 2019;25(4):1142-146. 8. Mougiakakos D, et al. N Engl J Med. 2021;385(6):567-569. 9.
Krishnamurthy A, et al. Wells Fargo, November 2017. 10. Clinicaltrials.gov. Accessed November 14, 2024. 8 https://clinicaltrials.gov/search?intr=chimeric%20antigen%20receptor.
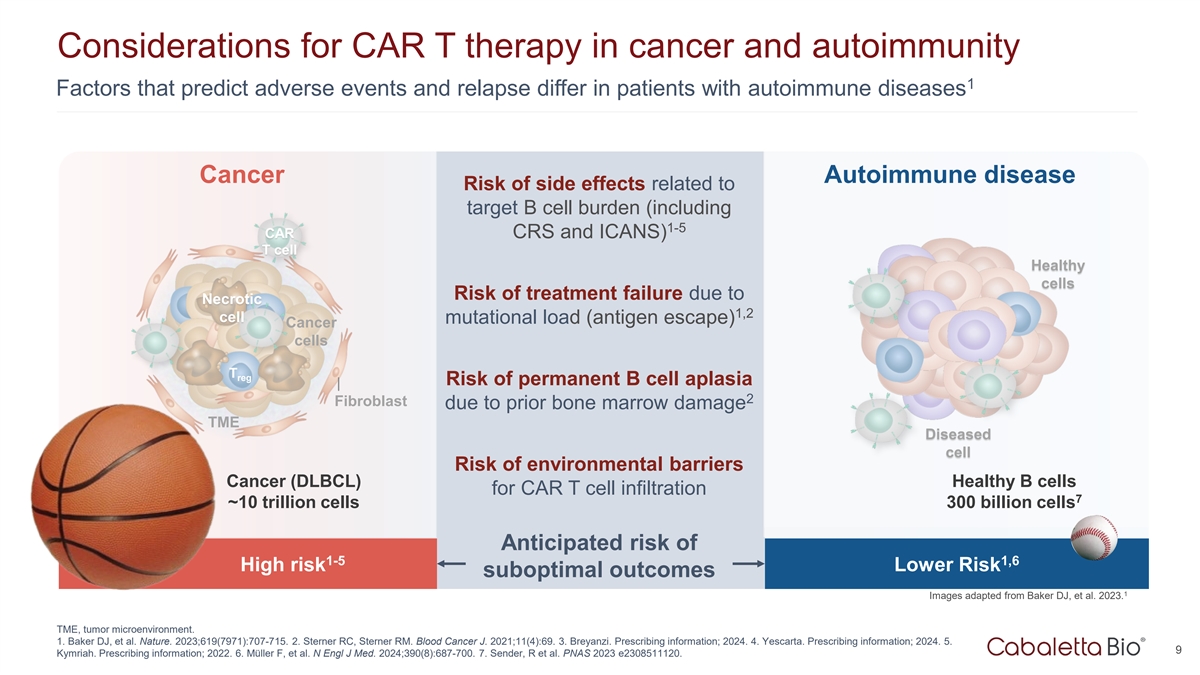
Considerations for CAR T therapy in cancer and autoimmunity 1 Factors
that predict adverse events and relapse differ in patients with autoimmune diseases Cancer Autoimmune disease Risk of side effects related to target B cell burden (including 1-5 CAR CRS and ICANS) T cell Healthy cells Risk of treatment failure due
to Necrotic 1,2 cell mutational load (antigen escape) Cancer cells T reg Risk of permanent B cell aplasia 2 Fibroblast due to prior bone marrow damage TME Diseased cell Risk of environmental barriers Cancer (DLBCL) Healthy B cells for CAR T cell
infiltration 7 ~10 trillion cells 300 billion cells Anticipated risk of 1-5 1,6 High risk Lower Risk suboptimal outcomes 1 Images adapted from Baker DJ, et al. 2023. TME, tumor microenvironment. 1. Baker DJ, et al. Nature. 2023;619(7971):707-715. 2.
Sterner RC, Sterner RM. Blood Cancer J. 2021;11(4):69. 3. Breyanzi. Prescribing information; 2024. 4. Yescarta. Prescribing information; 2024. 5. 9 Kymriah. Prescribing information; 2022. 6. Müller F, et al. N Engl J Med. 2024;390(8):687-700.
7. Sender, R et al. PNAS 2023 e2308511120.
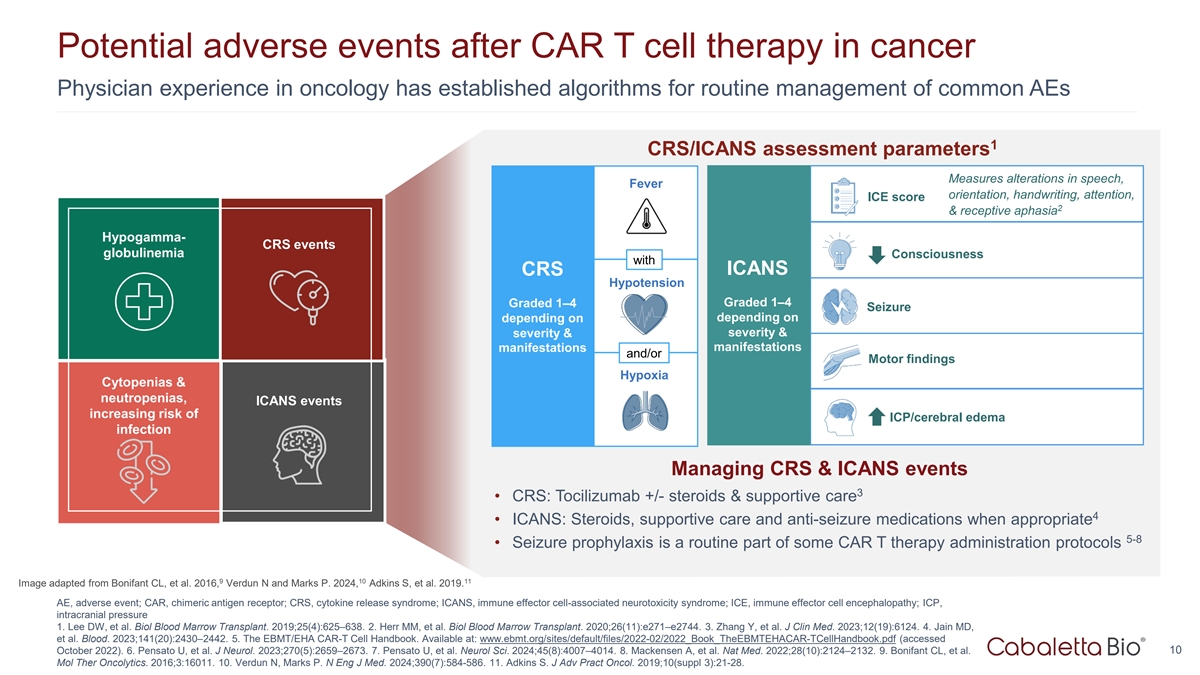
Potential adverse events after CAR T cell therapy in cancer Physician
experience in oncology has established algorithms for routine management of common AEs 1 CRS/ICANS assessment parameters Measures alterations in speech, Fever orientation, handwriting, attention, ICE score 2 & receptive aphasia Hypogamma- CRS
events globulinemia Consciousness with ICANS CRS Hypotension Graded 1–4 Graded 1–4 Seizure depending on depending on severity & severity & manifestations manifestations and/or Motor findings Hypoxia Cytopenias & neutropenias,
ICANS events increasing risk of ICP/cerebral edema infection Managing CRS & ICANS events 3 • CRS: Tocilizumab +/- steroids & supportive care 4 • ICANS: Steroids, supportive care and anti-seizure medications when appropriate 5-8
• Seizure prophylaxis is a routine part of some CAR T therapy administration protocols 9 10 11 Image adapted from Bonifant CL, et al. 2016, Verdun N and Marks P. 2024, Adkins S, et al. 2019. AE, adverse event; CAR, chimeric antigen receptor;
CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; ICE, immune effector cell encephalopathy; ICP, intracranial pressure 1. Lee DW, et al. Biol Blood Marrow Transplant. 2019;25(4):625–638. 2. Herr MM,
et al. Biol Blood Marrow Transplant. 2020;26(11):e271–e2744. 3. Zhang Y, et al. J Clin Med. 2023;12(19):6124. 4. Jain MD, et al. Blood. 2023;141(20):2430–2442. 5. The EBMT/EHA CAR-T Cell Handbook. Available at:
www.ebmt.org/sites/default/files/2022-02/2022_Book_TheEBMTEHACAR-TCellHandbook.pdf (accessed 10 October 2022). 6. Pensato U, et al. J Neurol. 2023;270(5):2659–2673. 7. Pensato U, et al. Neurol Sci. 2024;45(8):4007–4014. 8. Mackensen A,
et al. Nat Med. 2022;28(10):2124–2132. 9. Bonifant CL, et al. Mol Ther Oncolytics. 2016;3:16011. 10. Verdun N, Marks P. N Eng J Med. 2024;390(7):584-586. 11. Adkins S. J Adv Pract Oncol. 2019;10(suppl 3):21-28.

CABA-201 Clinical and Translational Data TM from the RESET Clinical
Program 11
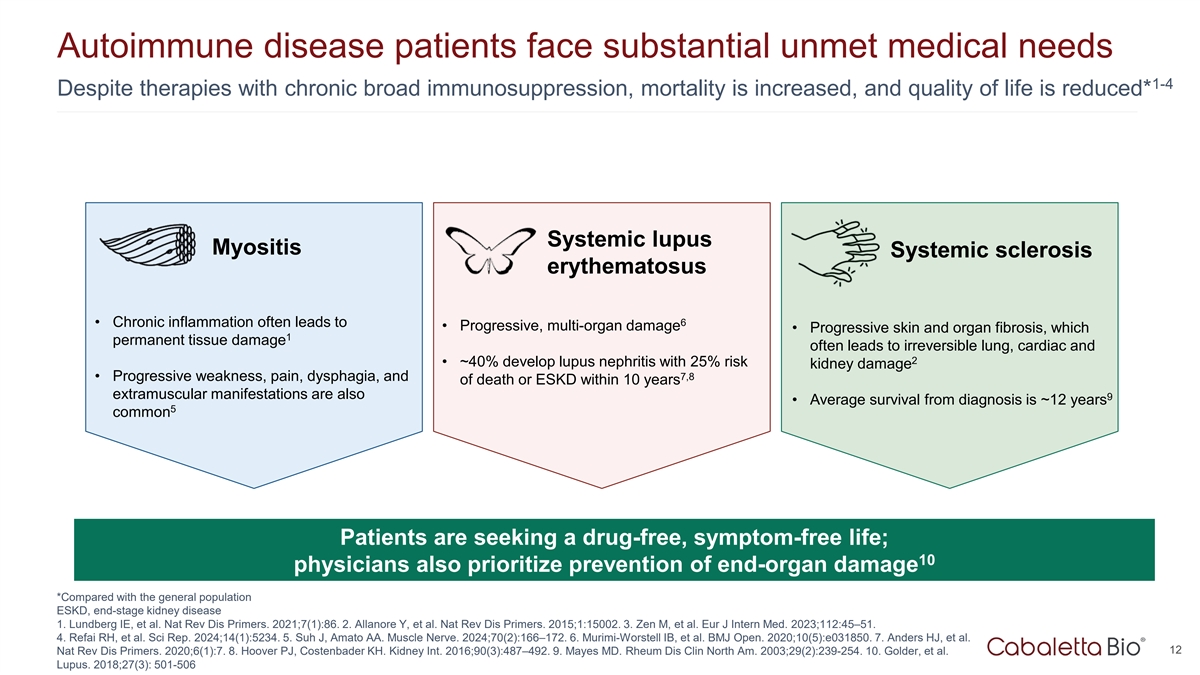
Autoimmune disease patients face substantial unmet medical needs 1-4
Despite therapies with chronic broad immunosuppression, mortality is increased, and quality of life is reduced* Systemic lupus Myositis Systemic sclerosis erythematosus 6 • Chronic inflammation often leads to • Progressive, multi-organ
damage • Progressive skin and organ fibrosis, which 1 permanent tissue damage often leads to irreversible lung, cardiac and 2 • ~40% develop lupus nephritis with 25% risk kidney damage 7,8 • Progressive weakness, pain, dysphagia,
and of death or ESKD within 10 years extramuscular manifestations are also 9 • Average survival from diagnosis is ~12 years 5 common Patients are seeking a drug-free, symptom-free life; 10 physicians also prioritize prevention of end-organ
damage *Compared with the general population ESKD, end-stage kidney disease 1. Lundberg IE, et al. Nat Rev Dis Primers. 2021;7(1):86. 2. Allanore Y, et al. Nat Rev Dis Primers. 2015;1:15002. 3. Zen M, et al. Eur J Intern Med. 2023;112:45–51.
4. Refai RH, et al. Sci Rep. 2024;14(1):5234. 5. Suh J, Amato AA. Muscle Nerve. 2024;70(2):166–172. 6. Murimi-Worstell IB, et al. BMJ Open. 2020;10(5):e031850. 7. Anders HJ, et al. 12 Nat Rev Dis Primers. 2020;6(1):7. 8. Hoover PJ, Costenbader
KH. Kidney Int. 2016;90(3):487–492. 9. Mayes MD. Rheum Dis Clin North Am. 2003;29(2):239-254. 10. Golder, et al. Lupus. 2018;27(3): 501-506
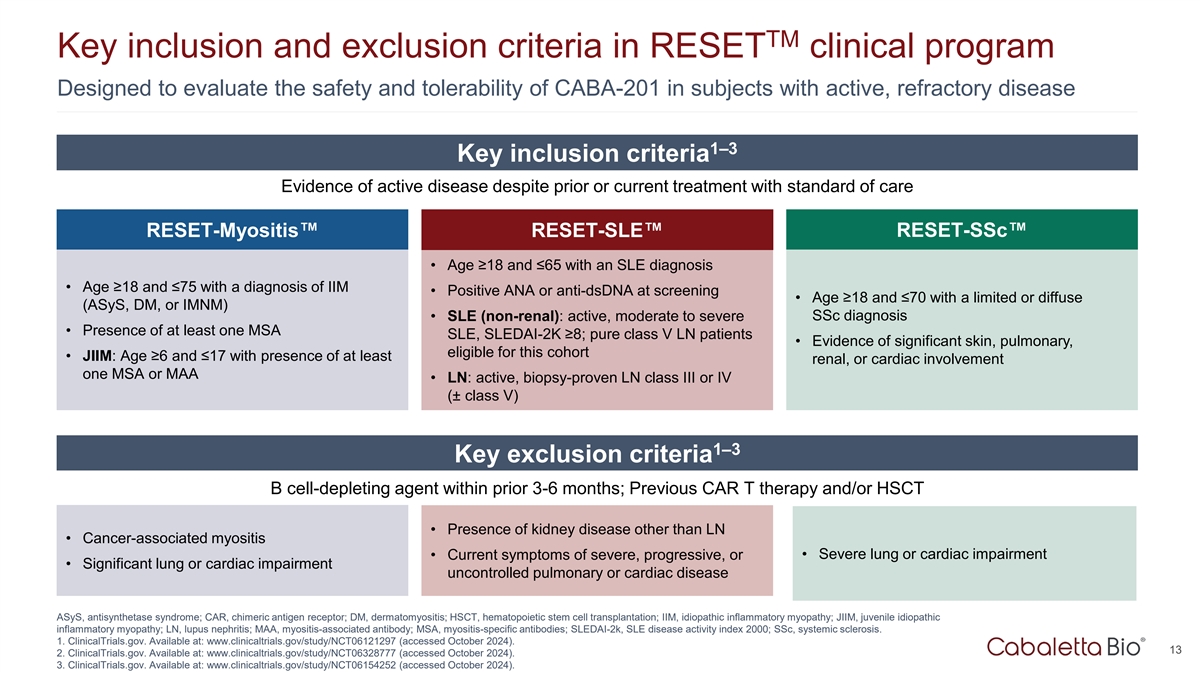
TM Key inclusion and exclusion criteria in RESET clinical program
Designed to evaluate the safety and tolerability of CABA-201 in subjects with active, refractory disease 1–3 Key inclusion criteria Evidence of active disease despite prior or current treatment with standard of care RESET-Myositis
RESET-SLE RESET-SSc • Age ≥18 and ≤65 with an SLE diagnosis • Age ≥18 and ≤75 with a diagnosis of IIM • Positive ANA or anti-dsDNA at screening • Age ≥18 and ≤70 with a limited or diffuse
(ASyS, DM, or IMNM) • SLE (non-renal): active, moderate to severe SSc diagnosis • Presence of at least one MSA SLE, SLEDAI-2K ≥8; pure class V LN patients • Evidence of significant skin, pulmonary, eligible for this cohort
• JIIM: Age ≥6 and ≤17 with presence of at least renal, or cardiac involvement one MSA or MAA • LN: active, biopsy-proven LN class III or IV (± class V) 1–3 Key exclusion criteria B cell-depleting agent within
prior 3-6 months; Previous CAR T therapy and/or HSCT • Presence of kidney disease other than LN • Cancer-associated myositis • Severe lung or cardiac impairment • Current symptoms of severe, progressive, or •
Significant lung or cardiac impairment uncontrolled pulmonary or cardiac disease ASyS, antisynthetase syndrome; CAR, chimeric antigen receptor; DM, dermatomyositis; HSCT, hematopoietic stem cell transplantation; IIM, idiopathic inflammatory
myopathy; JIIM, juvenile idiopathic inflammatory myopathy; LN, lupus nephritis; MAA, myositis-associated antibody; MSA, myositis-specific antibodies; SLEDAI-2k, SLE disease activity index 2000; SSc, systemic sclerosis. 1. ClinicalTrials.gov.
Available at: www.clinicaltrials.gov/study/NCT06121297 (accessed October 2024). 13 2. ClinicalTrials.gov. Available at: www.clinicaltrials.gov/study/NCT06328777 (accessed October 2024). 3. ClinicalTrials.gov. Available at:
www.clinicaltrials.gov/study/NCT06154252 (accessed October 2024).
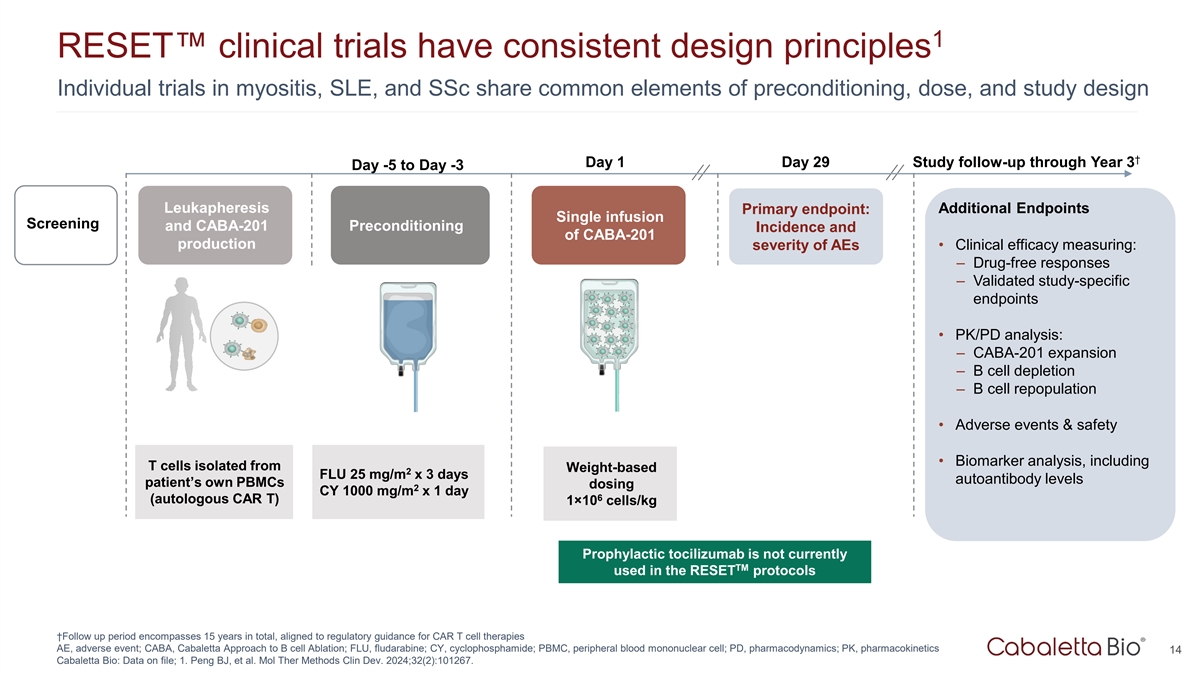
1 RESET clinical trials have consistent design principles Individual
trials in myositis, SLE, and SSc share common elements of preconditioning, dose, and study design † Day 1 Day 29 Study follow-up through Year 3 Day -5 to Day -3 Leukapheresis Primary endpoint: Additional Endpoints Single infusion Screening and
CABA-201 Preconditioning Incidence and of CABA-201 production severity of AEs • Clinical efficacy measuring: – Drug-free responses – Validated study-specific endpoints • PK/PD analysis: – CABA-201 expansion – B
cell depletion – B cell repopulation • Adverse events & safety • Biomarker analysis, including T cells isolated from Weight-based 2 FLU 25 mg/m x 3 days autoantibody levels patient’s own PBMCs dosing 2 CY 1000 mg/m x 1
day 6 (autologous CAR T) 1×10 cells/kg Prophylactic tocilizumab is not currently TM used in the RESET protocols †Follow up period encompasses 15 years in total, aligned to regulatory guidance for CAR T cell therapies AE, adverse event;
CABA, Cabaletta Approach to B cell Ablation; FLU, fludarabine; CY, cyclophosphamide; PBMC, peripheral blood mononuclear cell; PD, pharmacodynamics; PK, pharmacokinetics 14 Cabaletta Bio: Data on file; 1. Peng BJ, et al. Mol Ther Methods Clin Dev.
2024;32(2):101267.
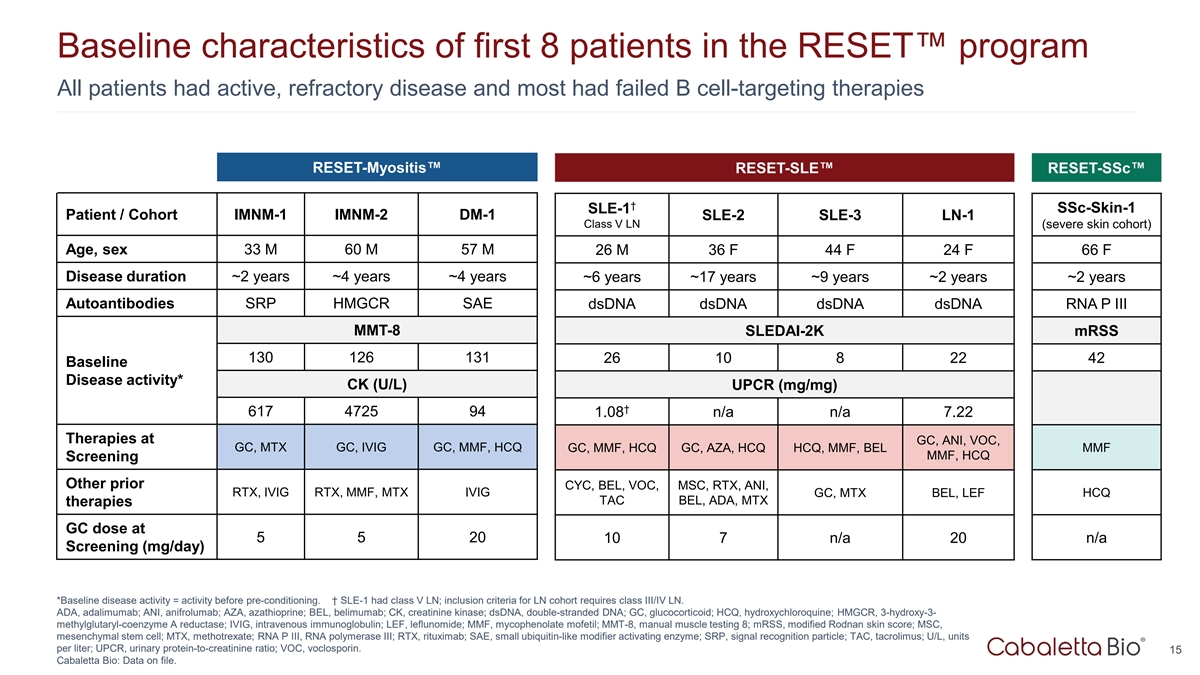
Baseline characteristics of first 8 patients in the RESET program All
patients had active, refractory disease and most had failed B cell-targeting therapies RESET-Myositis RESET-SLE RESET-SSc † SSc-Skin-1 SLE-1 Patient / Cohort IMNM-1 IMNM-2 DM-1 SLE-2 SLE-3 LN-1 Class V LN (severe skin cohort) Age, sex 33 M 60
M 57 M 26 M 36 F 44 F 24 F 66 F Disease duration ~2 years ~4 years ~4 years ~6 years ~17 years ~9 years ~2 years ~2 years Autoantibodies SRP HMGCR SAE dsDNA dsDNA dsDNA dsDNA RNA P III MMT-8 SLEDAI-2K mRSS 130 126 131 26 10 8 22 42 Baseline Disease
activity* CK (U/L) UPCR (mg/mg) † 617 4725 94 1.08 n/a n/a 7.22 Therapies at GC, ANI, VOC, GC, MTX GC, IVIG GC, MMF, HCQ GC, MMF, HCQ GC, AZA, HCQ HCQ, MMF, BEL MMF MMF, HCQ Screening Other prior CYC, BEL, VOC, MSC, RTX, ANI, RTX, IVIG RTX,
MMF, MTX IVIG GC, MTX BEL, LEF HCQ TAC BEL, ADA, MTX therapies GC dose at 5 5 20 n/a 10 7 n/a 20 Screening (mg/day) *Baseline disease activity = activity before pre-conditioning. † SLE-1 had class V LN; inclusion criteria for LN cohort
requires class III/IV LN. ADA, adalimumab; ANI, anifrolumab; AZA, azathioprine; BEL, belimumab; CK, creatinine kinase; dsDNA, double-stranded DNA; GC, glucocorticoid; HCQ, hydroxychloroquine; HMGCR, 3-hydroxy-3- methylglutaryl-coenzyme A reductase;
IVIG, intravenous immunoglobulin; LEF, leflunomide; MMF, mycophenolate mofetil; MMT-8, manual muscle testing 8; mRSS, modified Rodnan skin score; MSC, mesenchymal stem cell; MTX, methotrexate; RNA P III, RNA polymerase III; RTX, rituximab; SAE,
small ubiquitin-like modifier activating enzyme; SRP, signal recognition particle; TAC, tacrolimus; U/L, units per liter; UPCR, urinary protein-to-creatinine ratio; VOC, voclosporin. 15 Cabaletta Bio: Data on file.
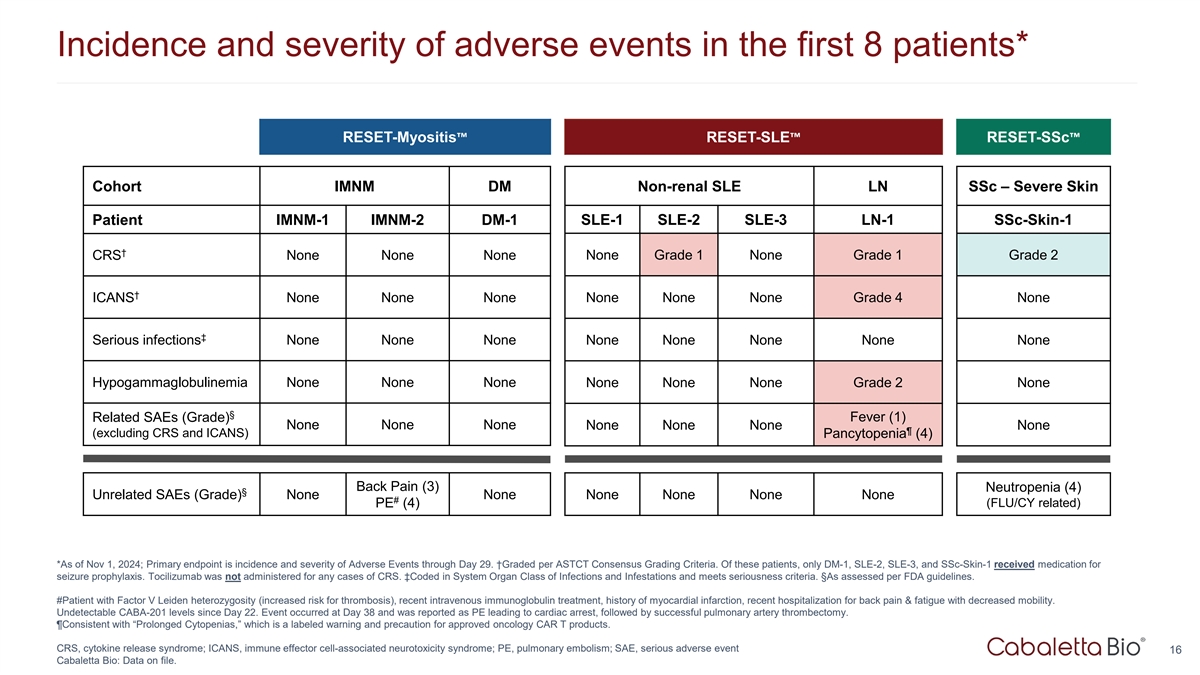
Incidence and severity of adverse events in the first 8 patients*
RESET-Myositis RESET-SLE RESET-SSc Cohort IMNM DM Non-renal SLE LN SSc – Severe Skin Patient IMNM-1 IMNM-2 DM-1 SLE-1 SLE-2 SLE-3 LN-1 SSc-Skin-1 † CRS None None None None Grade 1 None Grade 1 Grade 2 † ICANS None None None None
None None Grade 4 None ‡ Serious infections None None None None None None None None Hypogammaglobulinemia None None None None None None Grade 2 None § Fever (1) Related SAEs (Grade) None None None None None None None ¶ (excluding CRS
and ICANS) Pancytopenia (4) Back Pain (3) Neutropenia (4) § Unrelated SAEs (Grade) None None None None None None # PE (4) (FLU/CY related) *As of Nov 1, 2024; Primary endpoint is incidence and severity of Adverse Events through Day 29.
†Graded per ASTCT Consensus Grading Criteria. Of these patients, only DM-1, SLE-2, SLE-3, and SSc-Skin-1 received medication for seizure prophylaxis. Tocilizumab was not administered for any cases of CRS. ‡Coded in System Organ Class of
Infections and Infestations and meets seriousness criteria. §As assessed per FDA guidelines. #Patient with Factor V Leiden heterozygosity (increased risk for thrombosis), recent intravenous immunoglobulin treatment, history of myocardial
infarction, recent hospitalization for back pain & fatigue with decreased mobility. Undetectable CABA-201 levels since Day 22. Event occurred at Day 38 and was reported as PE leading to cardiac arrest, followed by successful pulmonary artery
thrombectomy. ¶Consistent with “Prolonged Cytopenias,” which is a labeled warning and precaution for approved oncology CAR T products. CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome;
PE, pulmonary embolism; SAE, serious adverse event 16 Cabaletta Bio: Data on file.
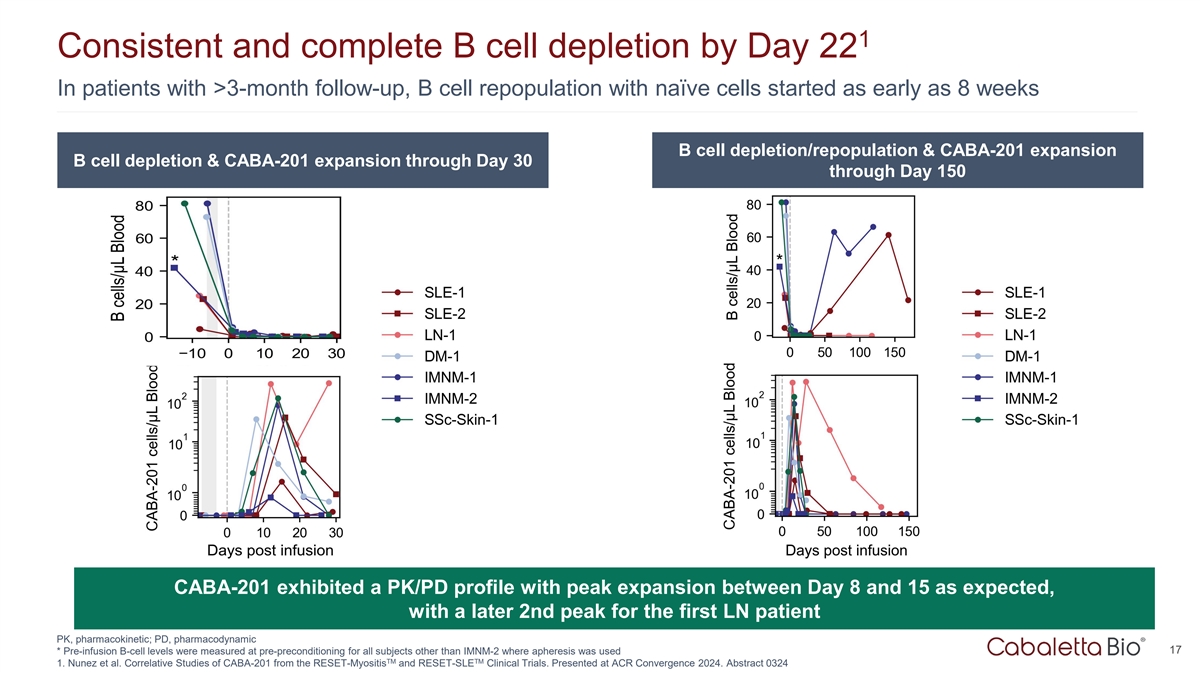
1 Consistent and complete B cell depletion by Day 22 In patients with
>3-month follow-up, B cell repopulation with naïve cells started as early as 8 weeks B cell depletion/repopulation & CABA-201 expansion B cell depletion & CABA-201 expansion through Day 30 through Day 150 CABA-201 exhibited a PK/PD
profile with peak expansion between Day 8 and 15 as expected, with a later 2nd peak for the first LN patient PK, pharmacokinetic; PD, pharmacodynamic 17 * Pre-infusion B-cell levels were measured at pre-preconditioning for all subjects other than
IMNM-2 where apheresis was used TM TM 1. Nunez et al. Correlative Studies of CABA-201 from the RESET-Myositis and RESET-SLE Clinical Trials. Presented at ACR Convergence 2024. Abstract 0324
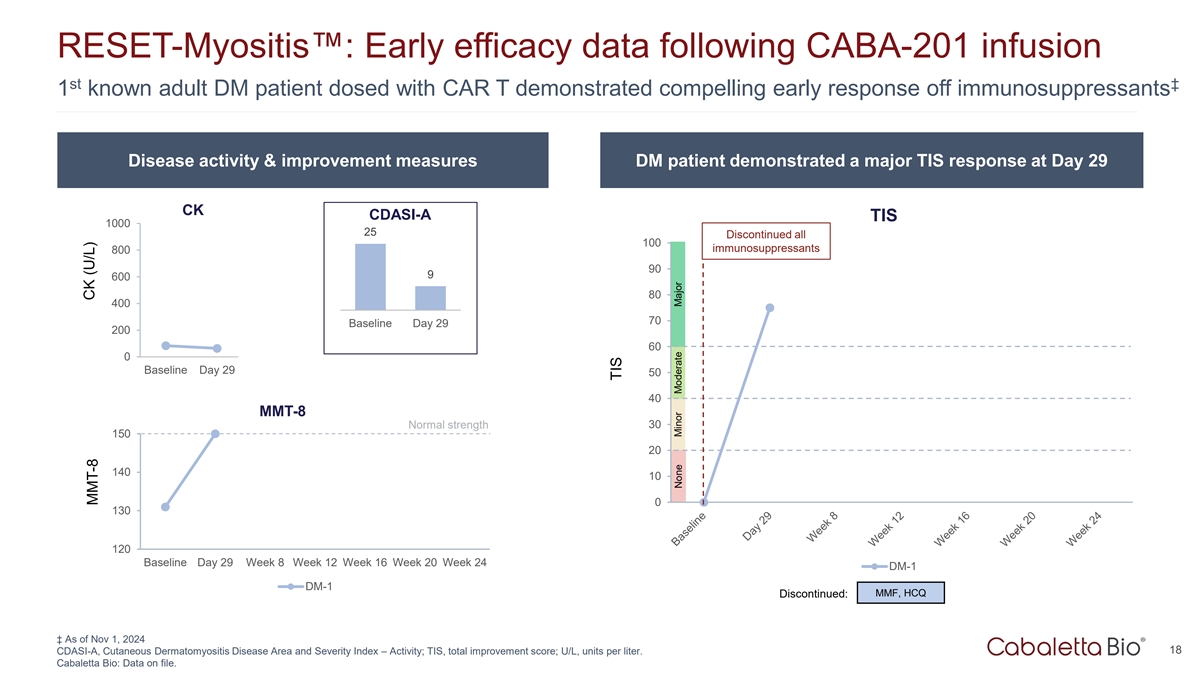
RESET-Myositis : Early efficacy data following CABA-201 infusion st
‡ 1 known adult DM patient dosed with CAR T demonstrated compelling early response off immunosuppressants Disease activity & improvement measures DM patient demonstrated a major TIS response at Day 29 CK CDASI-A TIS 1000 25 Discontinued
all 100 immunosuppressants 800 90 9 600 80 400 70 Baseline Day 29 200 DM-1 60 0 Baseline Day 29 Week 8 Week 12 Week 16 Week 20 Week 24 50 40 MMT-8 Normal strength 30 150 20 140 10 0 130 120 Baseline Day 29 Week 8 Week 12 Week 16 Week 20 Week 24 DM-1
DM-1 MMF, HCQ Discontinued: ‡ As of Nov 1, 2024 18 CDASI-A, Cutaneous Dermatomyositis Disease Area and Severity Index – Activity; TIS, total improvement score; U/L, units per liter. Cabaletta Bio: Data on file. CK (U/L) MMT-8 TIS None
Minor Moderate Major
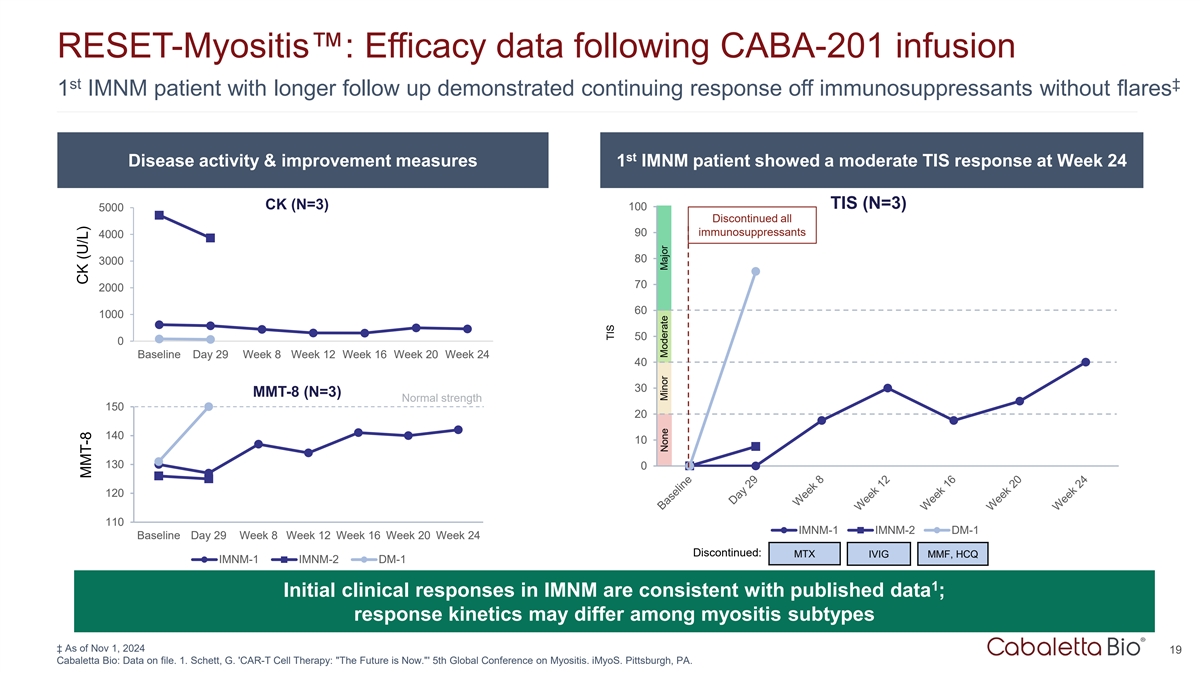
RESET-Myositis : Efficacy data following CABA-201 infusion st ‡ 1
IMNM patient with longer follow up demonstrated continuing response off immunosuppressants without flares st Disease activity & improvement measures 1 IMNM patient showed a moderate TIS response at Week 24 TIS (N=3) CK (N=3) 100 5000
Discontinued all 90 immunosuppressants 4000 80 3000 70 2000 60 1000 50 0 Baseline Day 29 Week 8 Week 12 Week 16 Week 20 Week 24 40 30 MMT-8 (N=3) Normal strength 150 20 140 10 130 0 120 110 IMNM-1 IMNM-2 DM-1 Baseline Day 29 Week 8 Week 12 Week 16
Week 20 Week 24 Discontinued: MTX IVIG MMF, HCQ IMNM-1 IMNM-2 DM-1 1 Initial clinical responses in IMNM are consistent with published data ; response kinetics may differ among myositis subtypes ‡ As of Nov 1, 2024 19 Cabaletta Bio: Data on
file. 1. Schett, G. 'CAR-T Cell Therapy: The Future is Now. ' 5th Global Conference on Myositis. iMyoS. Pittsburgh, PA. CK (U/L) MMT-8 TIS None Minor Moderate Major
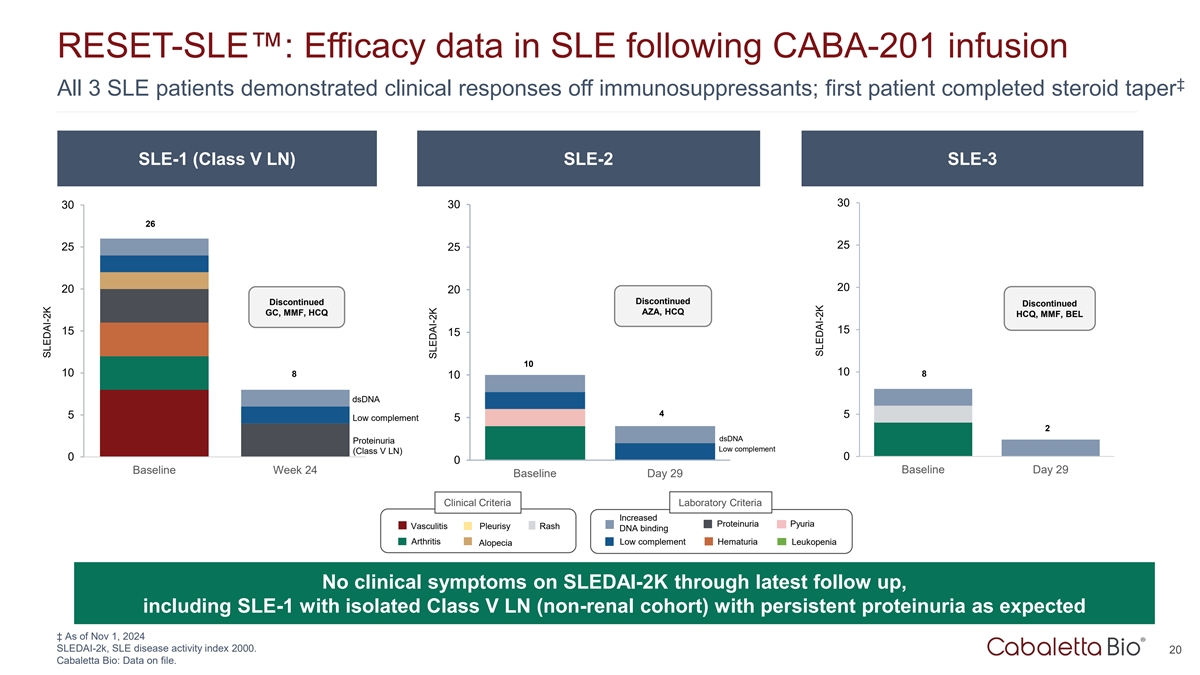
RESET-SLE : Efficacy data in SLE following CABA-201 infusion ‡
All 3 SLE patients demonstrated clinical responses off immunosuppressants; first patient completed steroid taper SLE-1 (Class V LN) SLE-2 SLE-3 30 30 30 26 25 25 25 20 20 20 Discontinued Discontinued Discontinued AZA, HCQ GC, MMF, HCQ HCQ, MMF, BEL
15 15 15 10 10 10 8 8 10 dsDNA 4 5 5 Low complement 5 2 dsDNA Proteinuria Low complement (Class V LN) 0 0 0 Baseline Week 24 Baseline Day 29 Baseline Day 29 Clinical Criteria Laboratory Criteria Increased Proteinuria Pyuria Vasculitis Pleurisy Rash
DNA binding Arthritis Low complement Hematuria Leukopenia Alopecia No clinical symptoms on SLEDAI-2K through latest follow up, including SLE-1 with isolated Class V LN (non-renal cohort) with persistent proteinuria as expected ‡ As of Nov 1,
2024 SLEDAI-2k, SLE disease activity index 2000. 20 Cabaletta Bio: Data on file. SLEDAI-2K SLEDAI-2K SLEDAI-2K
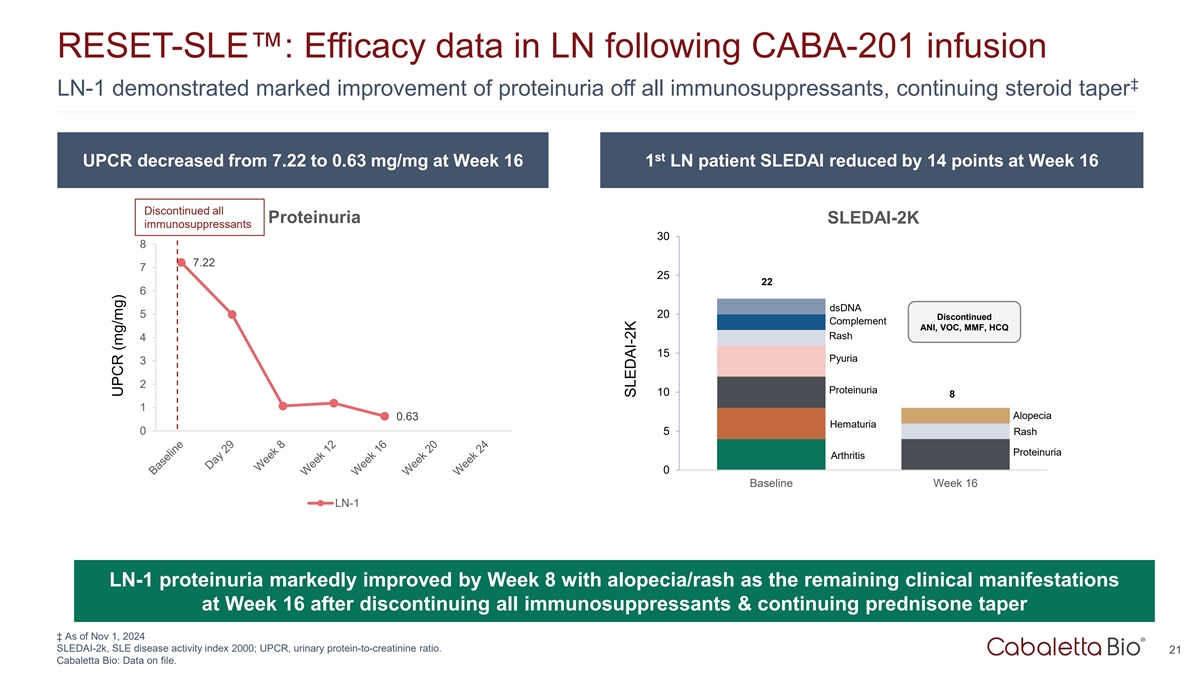
RESET-SLE : Efficacy data in LN following CABA-201 infusion ‡
LN-1 demonstrated marked improvement of proteinuria off all immunosuppressants, continuing steroid taper st UPCR decreased from 7.22 to 0.63 mg/mg at Week 16 1 LN patient SLEDAI reduced by 14 points at Week 16 Discontinued all Proteinuria SLEDAI-2K
immunosuppressants 30 8 7.22 7 25 22 6 dsDNA 5 20 Discontinued Complement ANI, VOC, MMF, HCQ Rash 4 15 Pyuria 3 2 Proteinuria 10 8 1 Alopecia 0.63 Hematuria 0 5 Rash Proteinuria Arthritis 0 Baseline Week 16 LN-1 LN-1 proteinuria markedly improved by
Week 8 with alopecia/rash as the remaining clinical manifestations at Week 16 after discontinuing all immunosuppressants & continuing prednisone taper ‡ As of Nov 1, 2024 SLEDAI-2k, SLE disease activity index 2000; UPCR, urinary
protein-to-creatinine ratio. 21 Cabaletta Bio: Data on file. UPCR (mg/mg) SLEDAI-2K
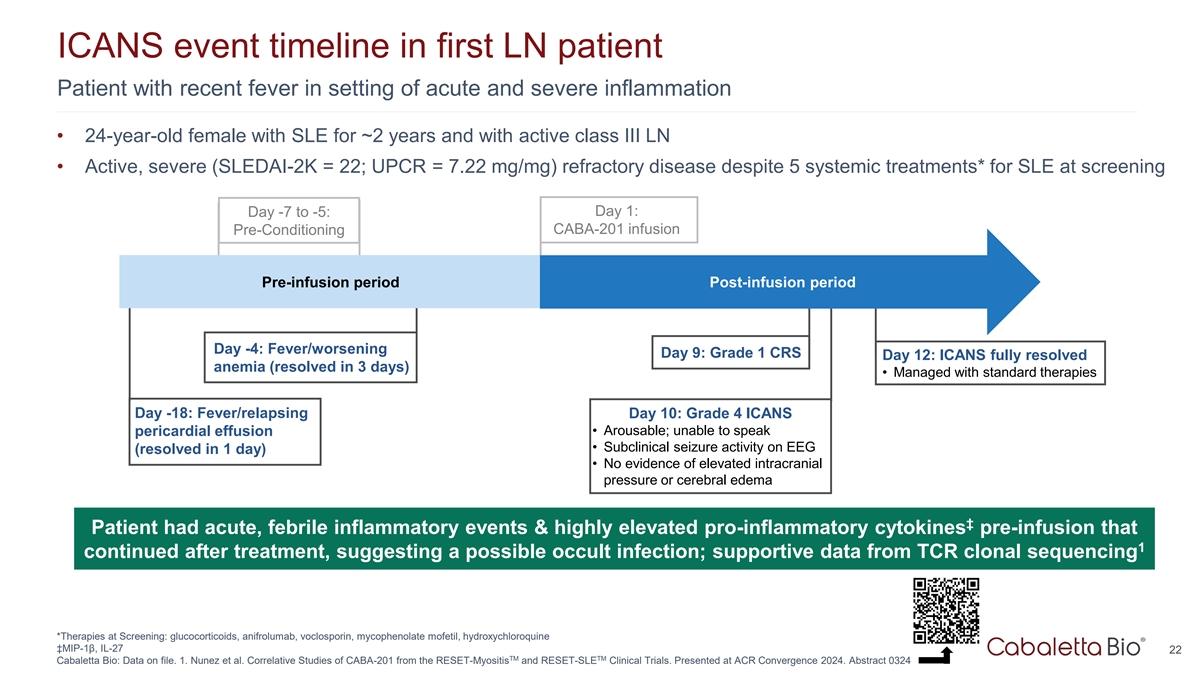
ICANS event timeline in first LN patient Patient with recent fever in
setting of acute and severe inflammation • 24-year-old female with SLE for ~2 years and with active class III LN • Active, severe (SLEDAI-2K = 22; UPCR = 7.22 mg/mg) refractory disease despite 5 systemic treatments* for SLE at screening
Day 1: Day -7 to -5: CABA-201 infusion Pre-Conditioning Pre-infusion period Post-infusion period Day -4: Fever/worsening Day 9: Grade 1 CRS Day 12: ICANS fully resolved anemia (resolved in 3 days) • Managed with standard therapies Day -18:
Fever/relapsing Day 10: Grade 4 ICANS • Arousable; unable to speak pericardial effusion • Subclinical seizure activity on EEG (resolved in 1 day) • No evidence of elevated intracranial pressure or cerebral edema ‡ Patient had
acute, febrile inflammatory events & highly elevated pro-inflammatory cytokines pre-infusion that 1 continued after treatment, suggesting a possible occult infection; supportive data from TCR clonal sequencing *Therapies at Screening:
glucocorticoids, anifrolumab, voclosporin, mycophenolate mofetil, hydroxychloroquine ‡MIP-1β, IL-27 22 TM TM Cabaletta Bio: Data on file. 1. Nunez et al. Correlative Studies of CABA-201 from the RESET-Myositis and RESET-SLE Clinical
Trials. Presented at ACR Convergence 2024. Abstract 0324
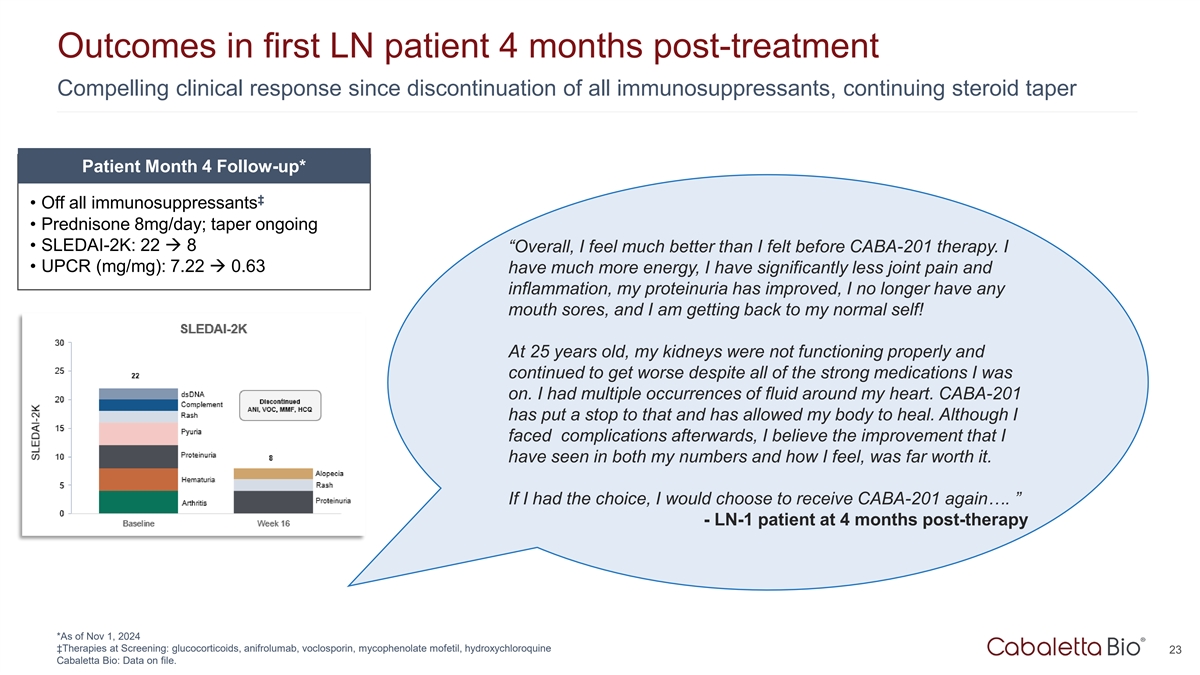
Outcomes in first LN patient 4 months post-treatment Compelling
clinical response since discontinuation of all immunosuppressants, continuing steroid taper Patient Month 4 Follow-up* ‡ • Off all immunosuppressants • Prednisone 8mg/day; taper ongoing • SLEDAI-2K: 22 à 8
“Overall, I feel much better than I felt before CABA-201 therapy. I • UPCR (mg/mg): 7.22 à 0.63 have much more energy, I have significantly less joint pain and inflammation, my proteinuria has improved, I no longer have any mouth
sores, and I am getting back to my normal self! At 25 years old, my kidneys were not functioning properly and continued to get worse despite all of the strong medications I was on. I had multiple occurrences of fluid around my heart. CABA-201 has
put a stop to that and has allowed my body to heal. Although I faced complications afterwards, I believe the improvement that I have seen in both my numbers and how I feel, was far worth it. If I had the choice, I would choose to receive CABA-201
again…. ” - LN-1 patient at 4 months post-therapy *As of Nov 1, 2024 ‡Therapies at Screening: glucocorticoids, anifrolumab, voclosporin, mycophenolate mofetil, hydroxychloroquine 23 Cabaletta Bio: Data on file.
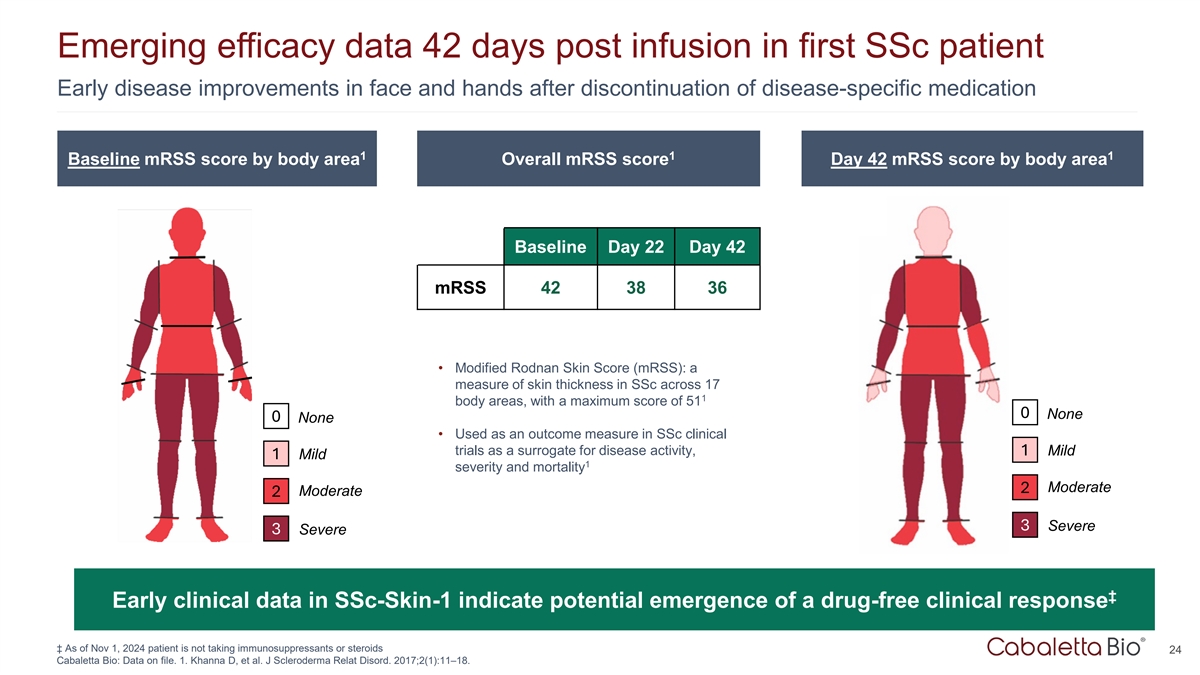
Emerging efficacy data 42 days post infusion in first SSc patient Early
disease improvements in face and hands after discontinuation of disease-specific medication 1 1 1 Baseline mRSS score by body area Overall mRSS score Day 42 mRSS score by body area Baseline Day 22 Day 42 mRSS 42 38 36 • Modified Rodnan Skin
Score (mRSS): a measure of skin thickness in SSc across 17 1 body areas, with a maximum score of 51 0 None 0 None • Used as an outcome measure in SSc clinical trials as a surrogate for disease activity, 1 Mild 1 Mild 1 severity and mortality
Moderate 2 Moderate 2 3 Severe 3 Severe ‡ Early clinical data in SSc-Skin-1 indicate potential emergence of a drug-free clinical response ‡ As of Nov 1, 2024 patient is not taking immunosuppressants or steroids 24 Cabaletta Bio: Data on
file. 1. Khanna D, et al. J Scleroderma Relat Disord. 2017;2(1):11–18.

Conclusions 25
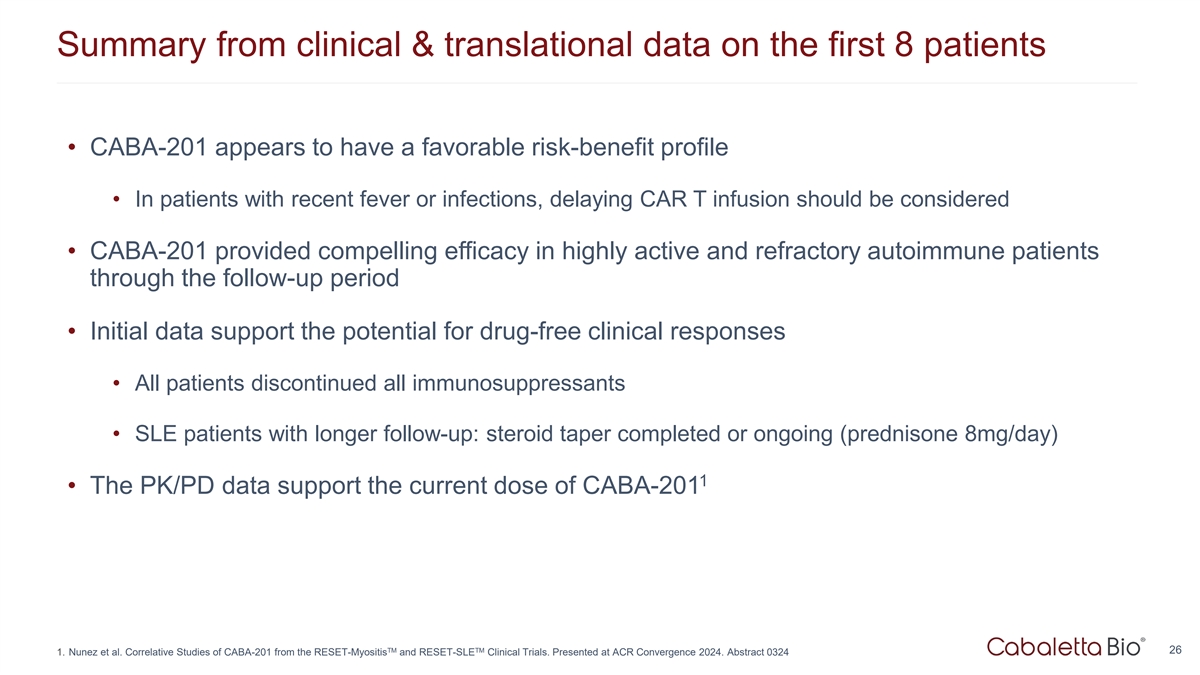
Summary from clinical & translational data on the first 8 patients
• CABA-201 appears to have a favorable risk-benefit profile • In patients with recent fever or infections, delaying CAR T infusion should be considered • CABA-201 provided compelling efficacy in highly active and refractory
autoimmune patients through the follow-up period • Initial data support the potential for drug-free clinical responses • All patients discontinued all immunosuppressants • SLE patients with longer follow-up: steroid taper completed
or ongoing (prednisone 8mg/day) 1 • The PK/PD data support the current dose of CABA-201 TM TM 26 1. Nunez et al. Correlative Studies of CABA-201 from the RESET-Myositis and RESET-SLE Clinical Trials. Presented at ACR Convergence 2024. Abstract
0324
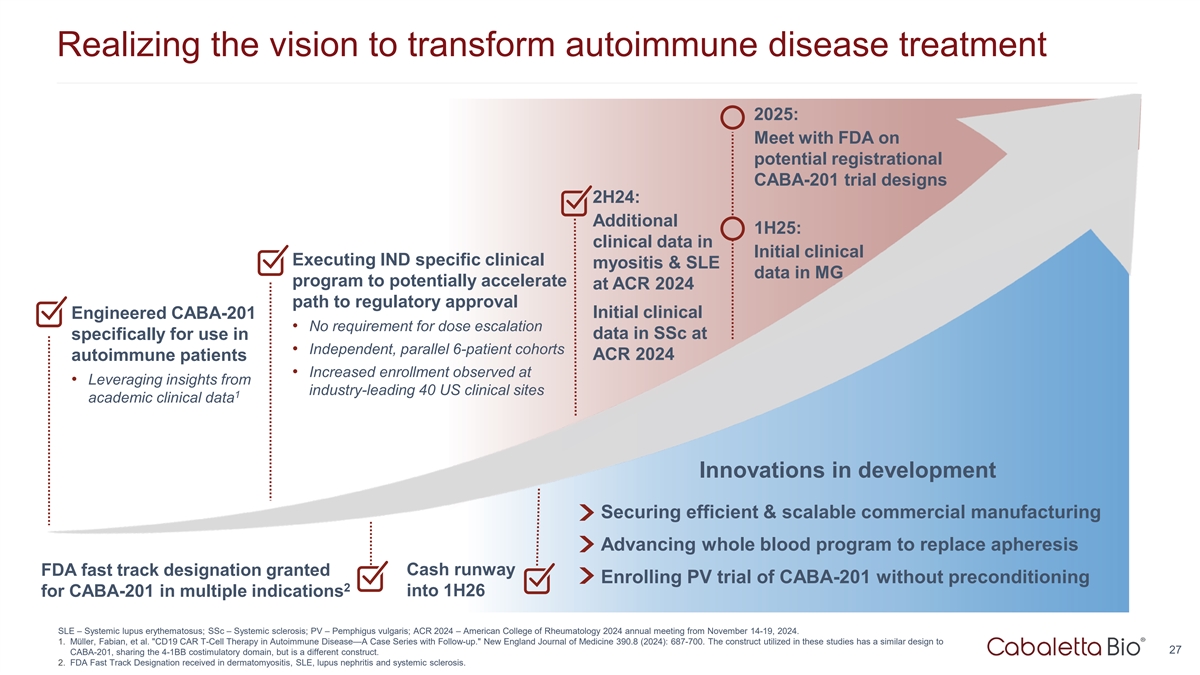
Realizing the vision to transform autoimmune disease treatment 2025:
Meet with FDA on potential registrational CABA-201 trial designs 2H24: Additional 1H25: clinical data in Initial clinical Executing IND specific clinical myositis & SLE data in MG program to potentially accelerate at ACR 2024 path to regulatory
approval Engineered CABA-201 Initial clinical • No requirement for dose escalation data in SSc at specifically for use in • Independent, parallel 6-patient cohorts ACR 2024 autoimmune patients • Increased enrollment observed at
• Leveraging insights from industry-leading 40 US clinical sites 1 academic clinical data Innovations in development Securing efficient & scalable commercial manufacturing Advancing whole blood program to replace apheresis Cash runway FDA
fast track designation granted Enrolling PV trial of CABA-201 without preconditioning 2 into 1H26 for CABA-201 in multiple indications SLE – Systemic lupus erythematosus; SSc – Systemic sclerosis; PV – Pemphigus vulgaris; ACR 2024
– American College of Rheumatology 2024 annual meeting from November 14-19, 2024. 1. Müller, Fabian, et al. CD19 CAR T-Cell Therapy in Autoimmune Disease—A Case Series with Follow-up. New England Journal of Medicine 390.8 (2024):
687-700. The construct utilized in these studies has a similar design to 27 CABA-201, sharing the 4-1BB costimulatory domain, but is a different construct. 2. FDA Fast Track Designation received in dermatomyositis, SLE, lupus nephritis and systemic
sclerosis.

Q&A 28
Exhibit 99.2

Cabaletta Bio Presents Positive Clinical Safety and Efficacy Data on
CABA-201 at ACR Convergence 2024
– CABA-201 safety
profile suggests favorable risk-benefit with no CRS or ICANS in the majority of patients; low-grade CRS in three of eight patients and one previously reported ICANS event –
– Compelling clinical responses observed in lupus and myositis patients with up to six months of
follow-up; first SSc patient demonstrated an emerging, drug-free clinical response –
– All eight patients with active, refractory autoimmune disease discontinued all immunosuppressants prior to CABA-201 infusion and through the follow-up period –
– Consistent and complete B cell depletion observed in all patients within the first month after
CABA-201 infusion; evidence of transitional naïve B cell repopulation as early as eight weeks in the first two patients –
– 40 U.S. clinical sites actively recruiting across the RESET™ clinical
development program, with 16 patients enrolled and 10 patients dosed with CABA-201 as of November 12, 2024 –
– Company to host live investor conference call and webcast today at 8:00 a.m. ET –
PHILADELPHIA, Nov. 18, 2024 – Cabaletta Bio, Inc. (Nasdaq: CABA), a clinical-stage biotechnology company focused on developing and launching the
first curative targeted cell therapies designed specifically for patients with autoimmune diseases, today announced new and updated clinical data on CABA-201 demonstrating the potential to achieve drug-free,
compelling clinical responses based on eight patients dosed across the ongoing Phase 1/2 RESET-Myositis™, RESET-SLE™ and RESET-SSc™ clinical trials. These data were presented in oral and poster presentations at the American College of Rheumatology (ACR)
Convergence 2024, which is being held at the Walter E. Washington Convention Center in Washington, D.C. from November 14-19, 2024. Presentation materials featured at ACR Convergence 2024 can be accessed on the
Company’s website here.
“The clinical data reported at ACR Convergence this weekend support the potential of the current dose of CABA-201 to provide immunosuppressant-free, compelling clinical responses in patients with active, refractory autoimmune disease. Data presented from the previously reported patient with lupus nephritis who
experienced ICANS and had acute inflammatory events shortly before CABA-201 treatment demonstrated an abnormal, pro-inflammatory cytokine profile prior to and after CABA-201 infusion, suggestive of an occult infection. As a result of these data, subjects in the RESET clinical program who develop a fever prior to scheduled infusion will wait a minimum of two weeks before
administration of CABA-201. Other than this patient with a second, later peak expansion, CABA-201 displayed a consistent PK and PD profile in all other patients,”
said David J. Chang, M.D., Chief Medical Officer of Cabaletta. “In addition to our promising clinical and translational data set from the RESET program, we believe our efficient clinical trial design, growing footprint of 40 actively recruiting
U.S. clinical sites and anticipated expansion into Europe in 2025 provide us with a differentiated opportunity to accelerate development of CABA-201 for patients. Data permitting, we anticipate meeting with
the FDA in 2025 to discuss potential registrational trial designs for CABA-201 that will allow us to bring the therapeutic potential of this investigational therapy closer to autoimmune patients.”
Cabaletta designed CABA-201, a 4-1BB-containing fully human CD19-CAR T cell investigational therapy, to deeply and transiently deplete CD19-positive B cells following a
one-time infusion that may enable a reset of the immune system with the potential for durable remission without chronic immunosuppressive therapies in patients with autoimmune diseases. Cabaletta is currently
evaluating CABA-201 in the RESET clinical development program across five company-sponsored clinical trials that each have disease-specific cohorts with six patients per cohort. All cohorts are evaluating the
same single, weight-based dose of CABA-201 at 1 x 106 cells/kg without a dose escalation requirement. Treatment with
CABA-201 in each clinical trial includes a preconditioning regimen of fludarabine and cyclophosphamide, consistent with the dosing regimen used in the third-party academic studies, except for the RESET-PV™ trial which is evaluating CABA-201 without preconditioning.
New and Updated Clinical Data Summary
As of the data cut-off date of November 1, 2024, eight patients had been dosed with CABA-201 with sufficient follow-up to be evaluable across the
RESET clinical development program. In the RESET-Myositis trial, one patient in the immune-mediated necrotizing myopathy (IMNM) cohort completed six months of follow-up and two patients, one in the IMNM cohort
and one in the dermatomyositis (DM) cohort, each completed one month of follow-up. In the RESET-SLE trial, one patient in the
non-renal systemic lupus erythematosus (SLE) cohort completed six months of follow-up, one patient in the lupus nephritis (LN) cohort completed four months of follow-up and two patients in the non-renal SLE cohort each completed one month of follow-up. Translational assessments from the third
patient in the non-renal SLE cohort were not available for inclusion at the time of the data cut-off. In the RESET-SSc trial, one patient in the severe skin cohort
completed six weeks of follow-up.
Across these eight patients treated with
CABA-201, patients were administered a one-time infusion of CABA-201 at 1 x
106 cells/kg, following a preconditioning regimen of fludarabine and cyclophosphamide. The primary endpoint of each trial is safety and tolerability within 28 days of infusion. Secondary
endpoints include translational assessments and clinical outcomes.
Safety and Tolerability Profile:
CABA-201 has shown a favorable risk-benefit profile in patients with active and refractory autoimmune disease
| |
• |
|
Through 28 days of follow-up, no evidence of cytokine release syndrome
(CRS) of any grade was observed in five of the eight patients. Low-grade CRS (Grades 1-2) was observed in three patients, all of which recovered following standard care.
Tocilizumab was not administered for any cases of CRS. |
| |
• |
|
No evidence of immune effector cell-associated neurotoxicity syndrome (ICANS) of any grade has been observed in
any patient since reporting the initial safety data on the first LN patient in August 2024. This patient had acute inflammatory events shortly before CABA-201 treatment and demonstrated an abnormal, pro-inflammatory cytokine profile prior to infusion that continued after CABA-201 infusion, suggestive of a possible occult infection. |
Translational Assessments: CABA-201 induced consistent and
complete B cell depletion, with early naïve B cell repopulation suggesting the potential to generate an immune system reset
| |
• |
|
CAR T cell expansion associated with CABA-201 reached its peak between
day 8 and day 15. Translational assessments from the first patient in the LN cohort indicated a second peak at day 29. |
| |
• |
|
Complete B cell depletion was observed by day 22 after CABA-201 infusion.
|
| |
• |
|
B cell repopulation occurred in the first two patients treated with
CABA-201 as early as 8 weeks and exhibited a transitional naïve phenotype, reflecting the production of new B cells after deep systemic depletion. |
| |
• |
|
Two of the three patients with follow-up beyond three months demonstrated
a reduction in disease-associated antibodies. Clinical responses in all three of these patients were observed independent of autoantibody levels. |
| |
• |
|
Vaccine and infectious pathogen antibodies remained generally stable. |
Clinical Outcomes: CABA-201 provided compelling signs of early efficacy, supporting the potential for
drug-free clinical responses
| |
• |
|
Initial clinical responses in the RESET-Myositis trial were consistent with published data with response kinetics
appearing to differ between myositis subtypes. |
| |
• |
|
The first known adult DM patient dosed with CAR T in the form of CABA-201
demonstrated an improvement in muscle strength to normal and a major total improvement score (TIS) response off all immunosuppressants at one month of follow-up. The Cutaneous Dermatomyositis Disease Area and
Severity Index – Activity (CDASI-A) improved from 25 to 9. |
| |
• |
|
At six months of follow-up, the first IMNM patient demonstrated a
continued and improved clinical response off immunosuppressants and without flares. At one month of follow-up, the second IMNM patient demonstrated a total improvement score consistent with the first IMNM
patient at one month after CABA-201 infusion off immunosuppressants. |
| |
• |
|
All four patients in the RESET-SLE trial demonstrated clinical responses
off immunosuppressants. |
| |
• |
|
All three patients in the non-renal SLE cohort demonstrated no clinical
symptoms on SLEDAI-2K as of the latest follow-up and the first patient has completed a prednisone taper to discontinuation. |
| |
• |
|
The first patient in the LN cohort, who experienced the previously reported ICANS event, had a SLEDAI that
improved from 22 at baseline to 8 at month four of follow-up. The patient’s proteinuria improved more than 90%, approaching normal levels, while off all immunosuppressants and with an ongoing prednisone
taper. |
| |
• |
|
The first patient in the severe skin cohort in the RESET-SSc trial demonstrated early clinical improvements after
discontinuation of disease-specific therapy. |
| |
• |
|
The modified Rodnan Skin Score of the first patient in the severe skin cohort improved from 42 at baseline
(potential maximum of 51) to 36 at day 42, suggesting the potential emergence of a drug-free clinical response. |
Investor Conference
Call and Webcast Information
Cabaletta will host a conference call and webcast today, November 18, 2024, at 8:00 a.m. ET to review the new and
updated clinical data presented at ACR Convergence 2024 and provide an update on the RESET clinical development program. A webcast of the live call can be accessed here or on the News and Events section of the Company’s website at
www.cabalettabio.com. An archived replay will be available on the Company’s website.
About the RESET-Myositis™ Trial
The RESET-Myositis™ trial is a Phase 1/2 open-label study of
CABA-201 in subjects with active idiopathic inflammatory myopathy (IIM, or myositis), including the subtypes of dermatomyositis (DM), anti-synthetase syndrome (ASyS), immune-mediated necrotizing myopathy
(IMNM) and juvenile myositis (JM), each evaluated in individual cohorts. Subjects will receive a one-time infusion of CABA-201 at a dose of 1 x 106 cells/kg, following a preconditioning regimen of fludarabine and cyclophosphamide. Key inclusion criteria for the DM, ASyS and IMNM cohorts include patients between ages 18 to 75 (inclusive),
evidence of active disease and disease activity despite prior or current treatment with standard of care treatments. Key exclusion criteria for the DM, ASyS and IMNM cohorts include cancer-associated myositis, significant lung or cardiac impairment,
treatment with a B cell depleting agent within the prior approximately six months or treatment with a biologic agent within the prior approximately three months.
About the RESET-SLE™ Trial
The RESET-SLE™ trial is a Phase 1/2 open-label study of CABA-201 in subjects with non-renal systemic lupus erythematosus (SLE) and lupus nephritis (LN), each evaluated in individual cohorts. Subjects will receive a one-time infusion of CABA-201 at a dose of 1 x 106 cells/kg, following a preconditioning regimen of fludarabine and
cyclophosphamide. Key inclusion criteria include patients between ages 18 to 65 (inclusive), evidence of active disease and disease activity despite prior or current treatment with standard of care treatments. Key exclusion criteria include
treatment with a B cell depleting agent within the prior approximately six months or treatment with a biologic agent within the prior approximately three months.
About the RESET-SSc™ Trial
The RESET-SSc™ trial is a Phase 1/2 open-label study of
CABA-201 in subjects with systemic sclerosis (SSc), including the subtypes of severe skin involvement and organ involvement regardless of skin involvement, each evaluated in individual cohorts. Subjects will
receive a one-time infusion of CABA-201 at a dose of 1 x 106 cells/kg, following a preconditioning regimen of
fludarabine and cyclophosphamide. Key inclusion criteria include patients between ages 18 and 70 (inclusive), evidence of significant skin, pulmonary, renal or cardiac involvement and significant organ involvement despite use of immunosuppressants.
Key exclusion criteria include a primary diagnosis of another rheumatic autoimmune disease, treatment with a B cell depleting agent within the prior approximately six months or treatment with a biologic agent within the prior approximately three
months.
About CABA-201
CABA-201 is a 4-1BB-containing fully
human CD19-CAR T cell investigational therapy for patients with autoimmune diseases where B cells contribute to the initiation and/or maintenance of disease. Following a
one-time infusion, CABA-201 is designed to transiently and completely deplete all CD19-positive cells. This approach has the potential to reset the immune system and
result in compelling clinical responses without chronic therapy requirements in patients. Cabaletta is currently evaluating CABA-201 in the RESET™
(REstoring SElf-Tolerance) clinical development program which includes multiple disease-specific, company-sponsored clinical trials across growing portfolios of autoimmune diseases in a broad range of therapeutic areas, including rheumatology,
neurology and dermatology.
About Cabaletta Bio
Cabaletta Bio (Nasdaq: CABA) is a clinical-stage biotechnology company focused on developing and launching the first curative targeted cell therapies designed
specifically for patients with autoimmune diseases. The CABA™ platform encompasses two complementary strategies which aim to advance the discovery and development of engineered T cell
therapies with the potential to become deep and durable, perhaps curative, treatments for a broad range of autoimmune diseases. The lead CARTA (Chimeric Antigen Receptor T cells for Autoimmunity) strategy is prioritizing the development of CABA-201, a 4-1BB-containing fully human CD19-CAR T cell investigational therapy. CABA-201 is currently being evaluated in the RESET™ (REstoring SElf-Tolerance) clinical development program spanning multiple therapeutic areas, including
rheumatology, neurology and dermatology. Cabaletta Bio’s headquarters and labs are located in Philadelphia, PA. For more information, please visit www.cabalettabio.com and connect with us on LinkedIn.
Forward-Looking Statements
This press release contains
“forward-looking statements” of Cabaletta Bio within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including without limitation, express or implied statements regarding: Cabaletta’s business plans
and objectives as a whole; Cabaletta’s ability to realize its vision of launching the first curative targeted cell therapy designed specifically for patients with autoimmune diseases; Cabaletta’s ability to successfully complete research
and further development and commercialization of its drug candidates in current or future indications, including the timing and results of Cabaletta’s clinical trials and its ability to conduct and complete clinical trials; expectation that
clinical results will support CABA-201’s safety and activity profile; statements regarding the expectations of trial modifications and prophylactic measures, continued trial operations; statements
regarding the timing of interactions with regulatory authorities, including such authorities’ review of safety information from Cabaletta’s ongoing clinical trials and potential registrational program designs for CABA-201; Cabaletta’s expectations around the potential success and therapeutic benefits of CABA-201, including its belief that
CABA-201 has the potential to reset the immune system and result in compelling clinical responses without chronic therapy requirements in patients; the Company’s advancement of separate Phase 1/2 clinical
trials of CABA-201 in patients with SLE, myositis, SSc and gMG and advancement of a RESET-PV trial, including updates related to status, safety data, efficiency of
clinical trial design or otherwise; the clinical significance of the clinical data read-out at the ACR Convergence 2024 in November 2024 for patients with myositis, SLE and SSc treated with CABA-201; Cabaletta’s ability to increase enrollment from its rapidly expanding clinical network in the RESET clinical program in the United States and beyond and Cabaletta’s ability to leverage such
growing clinical trial network to accelerate development of its therapy for patients.
Any forward-looking statements in this press release are based on
management’s current expectations and beliefs of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking
statements. These risks and uncertainties include, but are not limited to: risks related to regulatory filings and potential clearance; the risk that signs of biologic activity or persistence may not inform long-term results; Cabaletta’s
ability to demonstrate sufficient evidence of safety, efficacy and tolerability in its preclinical studies and clinical trials of CABA-201; the risk that the results observed with the similarly-designed
construct employed in academic publications, including due to the dosing regimen, are not indicative of the results we seek to achieve with CABA-201; risks that modifications to trial design or approach may
not have the intended benefits and that the trial design may need to be further modified; risks related to clinical trial site activation, delays in enrollment generally or enrollment rates that are
lower than expected; delays related to assessment of clinical trial results; risks related to unexpected safety or efficacy data observed during clinical studies; risks related to volatile market
and economic conditions and public health crises; Cabaletta’s ability to retain and recognize the intended incentives conferred by Orphan Drug Designation and Fast Track Designation or other designations for its product candidates, as
applicable; risks related to Cabaletta’s ability to protect and maintain its intellectual property position; risks related to fostering and maintaining successful relationships with Cabaletta’s collaboration and manufacturing partners,
including in light of recent legislation; uncertainties related to the initiation and conduct of studies and other development requirements for its product candidates; the risk that any one or more of Cabaletta’s product candidates will not be
successfully developed and/or commercialized; and the risk that the initial or interim results of preclinical studies or clinical studies will not be predictive of future results in connection with future studies. For a discussion of these and other
risks and uncertainties, and other important factors, any of which could cause Cabaletta’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in Cabaletta’s
most recent annual report on Form 10-K as well as discussions of potential risks, uncertainties, and other important factors in Cabaletta’s other filings with the Securities and Exchange Commission. All
information in this press release is as of the date of the release, and Cabaletta undertakes no duty to update this information unless required by law.
Contacts:
Anup Marda
Chief Financial Officer
investors@cabalettabio.com
William Gramig
Precision AQ
william.gramig@precisionaq.com
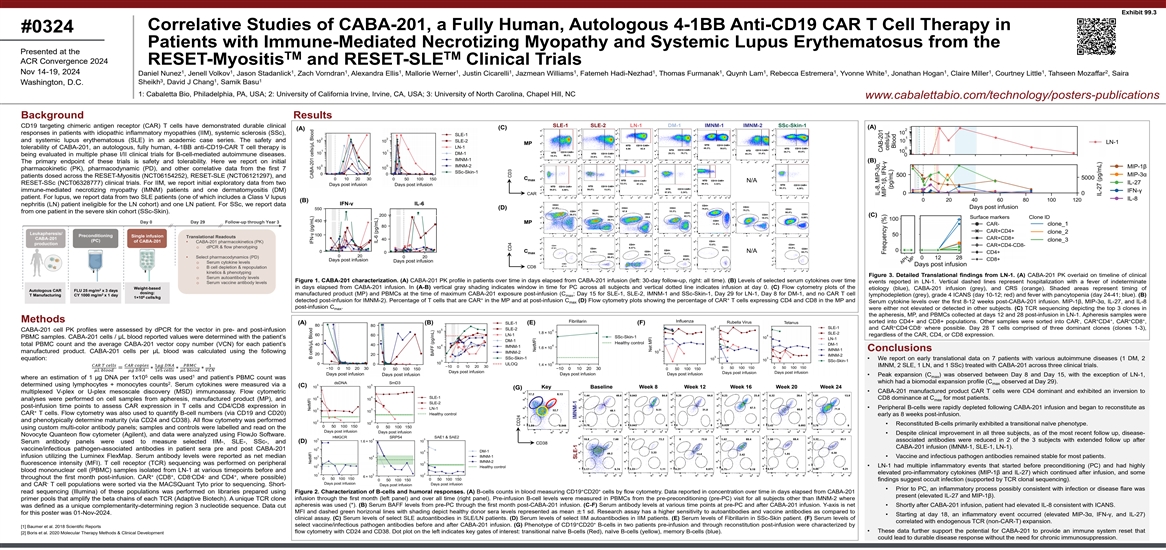
Exhibit 99.3 Correlative Studies of CABA-201, a Fully Human, Autologous
4-1BB Anti-CD19 CAR T Cell Therapy in #0324 Patients with Immune-Mediated Necrotizing Myopathy and Systemic Lupus Erythematosus from the Presented at the TM TM ACR Convergence 2024 RESET-Myositis and RESET-SLE Clinical Trials 1 1 1 1 1 1 1 1 1 1 1 1
1 1 1 1 2 Nov 14-19, 2024 Daniel Nunez , Jenell Volkov , Jason Stadanlick , Zach Vorndran , Alexandra Ellis , Mallorie Werner , Justin Cicarelli , Jazmean Williams , Fatemeh Hadi-Nezhad , Thomas Furmanak , Quynh Lam , Rebecca Estremera , Yvonne
White , Jonathan Hogan , Claire Miller , Courtney Little , Tahseen Mozaffar , Saira 3 1 1 Sheikh , David J Chang , Samik Basu Washington, D.C. 1: Cabaletta Bio, Philadelphia, PA, USA; 2: University of California Irvine, Irvine, CA, USA; 3:
University of North Carolina, Chapel Hill, NC www.cabalettabio.com/technology/posters-publications Background Results SLE-1 SLE-2 LN-1 DM-1 IMNM-1 IMNM-2 SSc-Skin-1 CD19 targeting chimeric antigen receptor (CAR) T cells have demonstrated durable
clinical (A) (C) (A) responses in patients with idiopathic inflammatory myopathies (IIM), systemic sclerosis (SSc), and systemic lupus erythematosus (SLE) in an academic case series. The safety and MP tolerability of CABA-201, an autologous, fully
human, 4-1BB anti-CD19-CAR T cell therapy is being evaluated in multiple phase I/II clinical trials for B-cell-mediated autoimmune diseases. (B) The primary endpoint of these trials is safety and tolerability. Here we report on initial
pharmacokinetic (PK), pharmacodynamic (PD), and other correlative data from the first 7 patients dosed across the RESET-Myositis (NCT06154252), RESET-SLE (NCT06121297), and C max N/A RESET-SSc (NCT06328777) clinical trials. For IIM, we report
initial exploratory data from two immune-mediated necrotizing myopathy (IMNM) patients and one dermatomyositis (DM) CAR patient. For lupus, we report data from two SLE patients (one of which includes a Class V lupus (B) IFN-γ IL-6 nephritis
(LN) patient ineligible for the LN cohort) and one LN patient. For SSc, we report data (D) from one patient in the severe skin cohort (SSc-Skin). (C) Surface markers Clone ID Day 0 Day 29 Follow-up through Year 3 MP Leukapheresis/ Preconditioning
Single infusion Translational Readouts CABA-201 (PC) of CABA-201 • CABA-201 pharmacokinetics (PK) production o dPCR & flow phenotyping C N/A max • Select pharmacodynamics (PD) o Serum cytokine levels CD8 o B cell depletion &
repopulation kinetics & phenotyping Figure 3. Detailed Translational findings from LN-1. (A) CABA-201 PK overlaid on timeline of clinical o Serum autoantibody levels Figure 1. CABA-201 characterization. (A) CABA-201 PK profile in patients over
time in days elapsed from CABA-201 infusion (left: 30-day follow-up, right: all time). (B) Levels of selected serum cytokines over time events reported in LN-1. Vertical dashed lines represent hospitalization with a fever of indeterminate o Serum
vaccine antibody levels in days elapsed from CABA-201 infusion. In (A-B) vertical gray shading indicates window in time for PC across all subjects and vertical dotted line indicates infusion at day 0. (C) Flow cytometry plots of the Weight-based
etiology (blue), CABA-201 infusion (grey), and CRS (orange). Shaded areas represent timing of 2 Autologous CAR FLU 25 mg/m x 3 days dosing: 2 manufactured product (MP) and PBMCs at the time of maximum CABA-201 exposure post-infusion (C ; Day 15 for
SLE-1, SLE-2, IMNM-1 and SSc-Skin-1, Day 29 for LN-1, Day 8 for DM-1, and no CAR T cell T Manufacturing CY 1000 mg/m x 1 day max lymphodepletion (grey), grade 4 ICANS (day 10-12; red) and fever with pancytopenia (day 24-41; blue). (B) 6 1×10
cells/kg + + detected post-infusion for IMNM-2). Percentage of T cells that are CAR in the MP and at post-infusion C (D) Flow cytometry plots showing the percentage of CAR T cells expressing CD4 and CD8 in the MP and Serum cytokine levels over the
first 8-12 weeks post-CABA-201 infusion. MIP-1β, MIP-3α, IL-27, and IL-8 max post-infusion C . max were either not elevated or detected in other subjects. (C) TCR sequencing depicting the top 3 clones in the apheresis, MP, and PBMCs
collected at days 12 and 28 post-infusion in LN-1. Apheresis samples were - + + + + Methods sorted into CD4+ and CD8+ populations. Other samples were sorted into CAR , CAR CD4 , CAR CD8 , (A) (B) (E) (F) + - - and CAR CD4 CD8 where possible. Day 28
T cells comprised of three dominant clones (clones 1-3), CABA-201 cell PK profiles were assessed by dPCR for the vector in pre- and post-infusion regardless of the CAR, CD4, or CD8 expression. PBMC samples. CABA-201 cells / μL blood reported
values were determined with the patient’s total PBMC count and the average CABA-201 vector copy number (VCN) for each patient’s Conclusions manufactured product. CABA-201 cells per μL blood was calculated using the following
equation: • We report on early translational data on 7 patients with various autoimmune diseases (1 DM, 2 ���� ����
������������������������
����������������1��������
����������������������������1 IMNM, 2 SLE, 1 LN, and
1 SSc) treated with CABA-201 across three clinical trials. =∗∗∗ ��������
����������������
������������1������
����������������������������
�������������������� • Peak expansion (C ) was observed between Day 8 and Day 15, with the exception of
LN-1, 5 1 max where an estimation of 1 μg DNA per 1x10 cells was used and patient’s PBMC count was which had a biomodal expansion profile (C observed at Day 29). 2 max determined using lymphocytes + monocytes counts . Serum cytokines were
measured via a (C) Key Baseline Week 8 Week 12 Week 16 Week 20 Week 24 (G) • CABA-201 manufactured product CAR T cells were CD4 dominant and exhibited an inversion to multiplexed V-plex or U-plex mesoscale discovery (MSD) immunoassay. Flow
cytometric CD8 dominance at C for most patients. analyses were performed on cell samples from apheresis, manufactured product (MP), and max post-infusion time points to assess CAR expression in T cells and CD4/CD8 expression in • Peripheral
B-cells were rapidly depleted following CABA-201 infusion and began to reconstitute as + CAR T cells. Flow cytometry was also used to quantify B-cell numbers (via CD19 and CD20) early as 8 weeks post-infusion. and phenotypically determine maturity
(via CD24 and CD38). All flow cytometry was performed • Reconstituted B-cells primarily exhibited a transitional naïve phenotype. using custom multi-color antibody panels; samples and controls were labelled and read on the • Despite
clinical improvement in all three subjects, as of the most recent follow up, disease- Novocyte Quanteon flow cytometer (Agilent), and data were analyzed using FlowJo Software. associated antibodies were reduced in 2 of the 3 subjects with extended
follow up after (D) Serum antibody panels were used to measure selected IIM-, SLE-, SSc-, and CD38 CABA-201 infusion (IMNM-1, SLE-1, LN-1). vaccine/infectious pathogen-associated antibodies in patient sera pre and post CABA-201 infusion utilizing
the Luminex FlexMap. Serum antibody levels were reported as net median • Vaccine and infectious pathogen antibodies remained stable for most patients. fluorescence intensity (MFI). T cell receptor (TCR) sequencing was performed on peripheral
• LN-1 had multiple inflammatory events that started before preconditioning (PC) and had highly blood mononuclear cell (PBMC) samples isolated from LN-1 at various timepoints before and elevated pro-inflammatory cytokines (MIP-1β and
IL-27) which continued after infusion, and some + + - - + throughout the first month post-infusion. CAR (CD8 , CD8 CD4 and CD4 , where possible) findings suggest occult infection (supported by TCR clonal sequencing). - and CAR T cell populations
were sorted via the MACSQuant Tyto prior to sequencing. Short- • Prior to PC, an inflammatory process possibly consistent with infection or disease flare was + + read sequencing (Illumina) of these populations was performed on libraries
prepared using Figure 2. Characterization of B-cells and humoral responses. (A) B-cells counts in blood measuring CD19 CD20 cells by flow cytometry. Data reported in concentration over time in days elapsed from CABA-201 present (elevated IL-27 and
MIP-1β). infusion through the first month (left panel) and over all time (right panel). Pre-infusion B-cell levels were measured in PBMCs from the pre-preconditioning (pre-PC) visit for all subjects other than IMNM-2 where primer pools that
amplify the beta chains of each TCR (Adaptive Biotech). A unique TCR clone apheresis was used (*). (B) Serum BAFF levels from pre-PC through the first month post-CABA-201 infusion. (C-F) Serum antibody levels at various time points at pre-PC and
after CABA-201 infusion. Y-axis is net • Shortly after CABA-201 infusion, patient had elevated IL-8 consistent with ICANS. was defined as a unique complementarity-determining region 3 nucleotide sequence. Data cut MFI and dashed green
horizonal lines with shading depict healthy donor sera levels represented as mean ±1 sd. Research assay has a higher sensitivity to autoantibodies and vaccine antibodies as compared to for this poster was 01-Nov-2024. • Starting at day
18, an inflammatory event occurred (elevated MIP-3α, IFN-γ, and IL-27) clinical assay. (C) Serum levels of select SLE autoantibodies in SLE/LN patients. (D) Serum levels of select IIM autoantibodies in IIM patients. (E) Serum levels of
Fibrillarin in SSc-Skin patient. (F) Serum levels of correlated with endogenous TCR (non-CAR-T) expansion. + + select vaccine/infectious pathogen antibodies before and after CABA-201 infusion. (G) Phenotype of CD19 CD20 B-cells in two patients
pre-infusion and through reconstitution post-infusion were characterized by [1] Baumer et al. 2018 Scientific Reports • These data further support the potential for CABA-201 to provide an immune system reset that flow cytometry with CD24 and
CD38. Dot plot on the left indicates key gates of interest: transitional naïve B-cells (Red), naïve B-cells (yellow), memory B-cells (blue). [2] Boris et al. 2020 Molecular Therapy Methods & Clinical Development could lead to durable
disease response without the need for chronic immunosuppression. CD4 CD3 CD24 IMNM-1 SLE-1
������������������������
������������������������

Safety and efficacy of CABA-201, a
fully human, autologous 4-1BB anti-CD19 CAR T cell therapy in patients with immune-mediated necrotizing myopathy and systemic lupus erythematosus from the RESET-Myositis™ and RESET-SLE™ clinical trials Saira Sheikh1; Tahseen Mozaffar2;
Vimal Derebail1; Natalie Grover1; Jonathan Hogan3; Courtney Little3; Yvonne White3; Claire Miller3; Rebecca Estremera3; Jenell Volkov3; Daniel Nunez3; Jason Stadanlick3; Mallorie Werner3; Zachary Vorndran3; Alexandra Ellis3; Jazmean Williams3;
Justin Cicarelli3; Quynh Lam3; Thomas Furmanak3; Chris Schmitt3; Fatemeh Nezhad3; Dan Thompson3; Samik Basu3 NOVEMBER 2024 Presenting author: David J. Chang3 1. University of North Carolina at Chapel Hill, Chapel Hill, NC; 2.
University of California, Irvine, Irvine, CA; 3. Cabaletta Bio, Philadelphia, PA Exhibit 99.4

Disclosures Author Disclosures David
J. Chang Employee: Cabaletta Bio Saira Sheikh Consultant: AstraZeneca; Biogen; Cabaletta Bio; GSK Tahseen Mozaffar Advisor or Review Panel Member, Grant/Research Support: Amicus; AnnJi; Argenx; Astellas Gene Therapy; AstraZeneca; Janssen; Sanofi;
Spark Therapeutics; UCB Advisor or Review Panel Member: Arvinas; AskBio; Horizon Therapeutics; Maze Therapeutics; Sarepta Grant/Research Support: Bristol-Myers Squibb (BMS); Cartesian Therapeutics; Grifols; ML-Bio; Valerion Vimal Derebail
Consultant: Amgen; Forma Therapeutics (NovoNordisk); iCell Gene; Novartis Natalie Grover Consultant: BMS Jonathan Hogan Courtney Little Yvonne White Claire Miller Rebecca Estremera Jenell Volkov Quynh Lam Thomas Furmanak Fatemeh Nezhad Dan Thompson
Daniel Nunez Jason Stadanlick Mallorie Werner Zachary Vorndran Alexandra Ellis Jazmean Williams Justin Cicarelli Chris Schmitt Samik Basu Employees: Cabaletta Bio Safety and efficacy of CABA-201• 17 NOV 2024
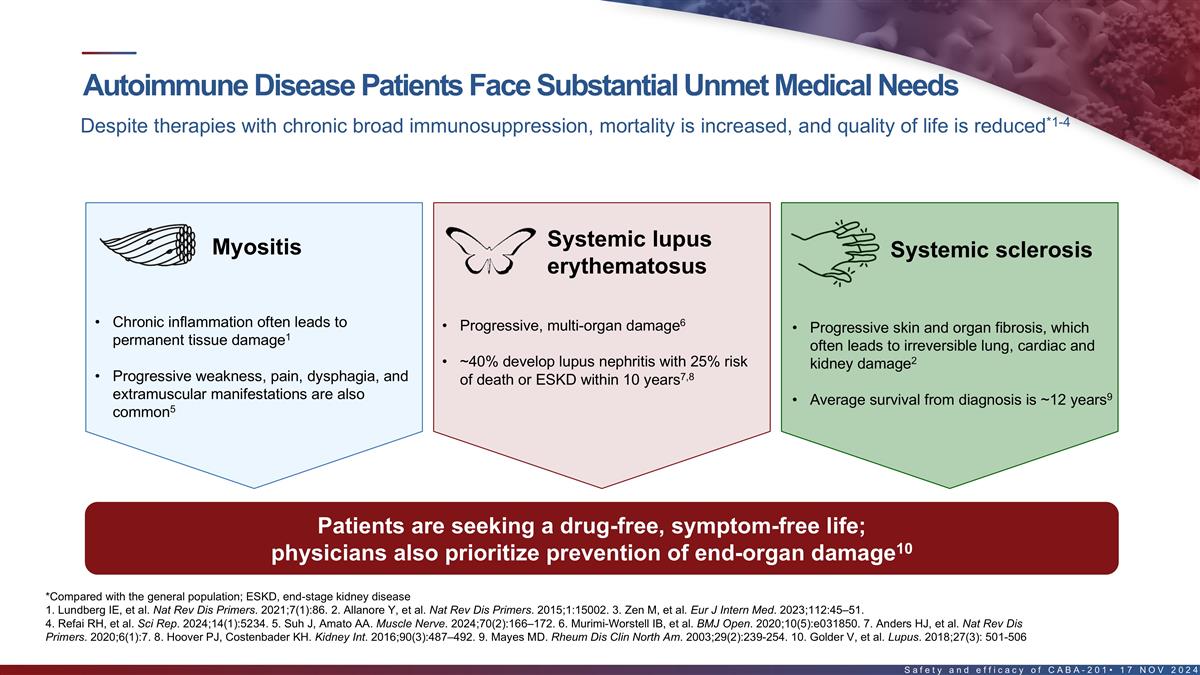
Autoimmune Disease Patients Face
Substantial Unmet Medical Needs Safety and efficacy of CABA-201• 17 NOV 2024 Myositis Chronic inflammation often leads to permanent tissue damage1 Progressive weakness, pain, dysphagia, and extramuscular manifestations are also common5
Systemic lupus erythematosus Systemic sclerosis Progressive, multi-organ damage6 ~40% develop lupus nephritis with 25% risk of death or ESKD within 10 years7,8 Progressive skin and organ fibrosis, which often leads to irreversible lung, cardiac and
kidney damage2 Average survival from diagnosis is ~12 years9 Safety and efficacy of CABA-201• 17 NOV 2024 Patients are seeking a drug-free, symptom-free life; physicians also prioritize prevention of end-organ damage10 Despite therapies with
chronic broad immunosuppression, mortality is increased, and quality of life is reduced*1-4 *Compared with the general population; ESKD, end-stage kidney disease 1. Lundberg IE, et al. Nat Rev Dis Primers. 2021;7(1):86. 2. Allanore Y, et al. Nat Rev
Dis Primers. 2015;1:15002. 3. Zen M, et al. Eur J Intern Med. 2023;112:45–51. 4. Refai RH, et al. Sci Rep. 2024;14(1):5234. 5. Suh J, Amato AA. Muscle Nerve. 2024;70(2):166–172. 6. Murimi-Worstell IB, et al. BMJ Open. 2020;10(5):e031850.
7. Anders HJ, et al. Nat Rev Dis Primers. 2020;6(1):7. 8. Hoover PJ, Costenbader KH. Kidney Int. 2016;90(3):487–492. 9. Mayes MD. Rheum Dis Clin North Am. 2003;29(2):239-254. 10. Golder V, et al. Lupus. 2018;27(3): 501-506
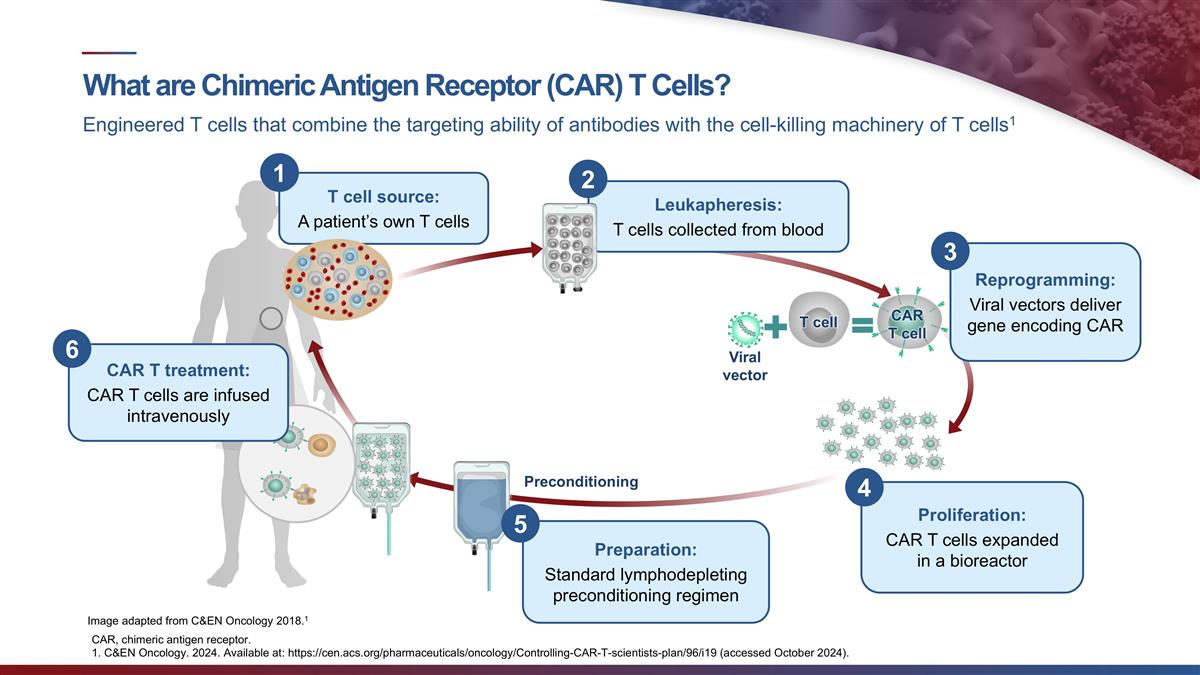
What are Chimeric Antigen Receptor
(CAR) T Cells? Safety and efficacy of CABA-201• 17 NOV 2024 Image adapted from C&EN Oncology 2018.1 Preparation: Standard lymphodepleting preconditioning regimen T cell source: A patient’s own T cells 1 Leukapheresis: T cells
collected from blood 2 Viral vector T cell Reprogramming: Viral vectors deliver gene encoding CAR 3 CAR T treatment: CAR T cells are infused intravenously 6 5 Preconditioning CAR T cell Proliferation: CAR T cells expanded in a bioreactor 4 CAR,
chimeric antigen receptor. 1. C&EN Oncology. 2024. Available at: https://cen.acs.org/pharmaceuticals/oncology/Controlling-CAR-T-scientists-plan/96/i19 (accessed October 2024). Engineered T cells that combine the targeting ability of antibodies
with the cell-killing machinery of T cells1
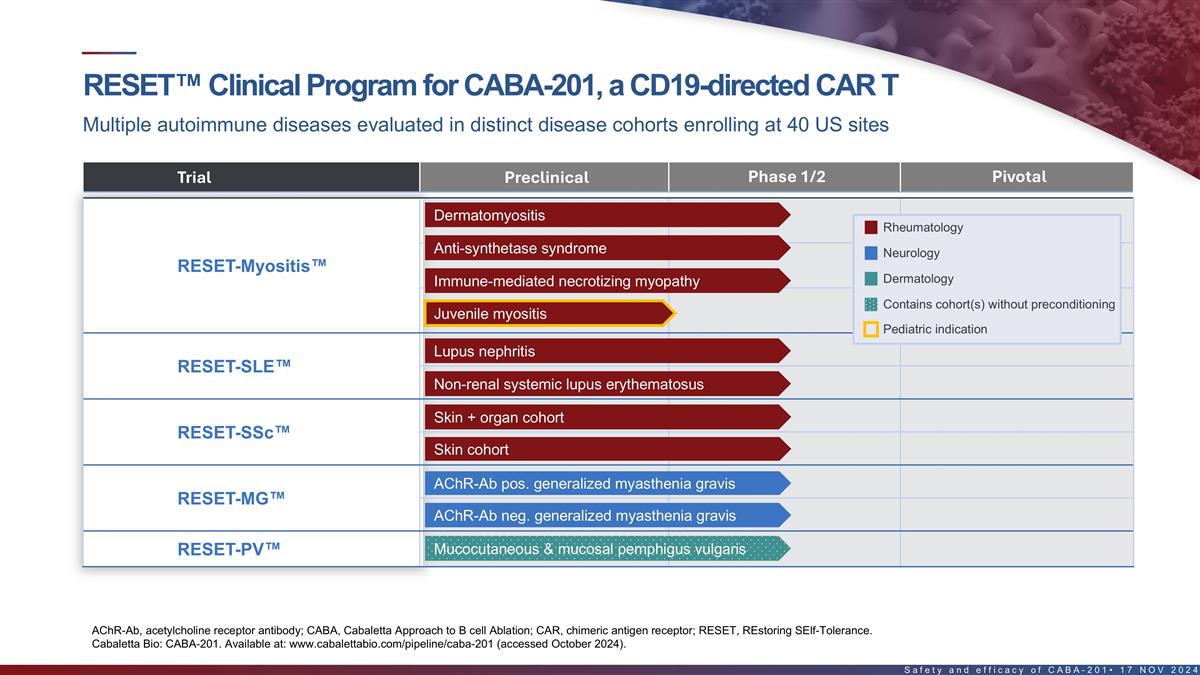
AChR-Ab, acetylcholine receptor
antibody; CABA, Cabaletta Approach to B cell Ablation; CAR, chimeric antigen receptor; RESET, REstoring SElf-Tolerance. Cabaletta Bio: CABA-201. Available at: www.cabalettabio.com/pipeline/caba-201 (accessed October 2024). Trial Preclinical Phase
1/2 Pivotal RESET-Myositis™ RESET-SLE™ RESET-SSc™ RESET-MG™ RESET-PV™ Dermatomyositis Anti-synthetase syndrome Immune-mediated necrotizing myopathy Lupus nephritis Non-renal systemic lupus erythematosus Skin + organ
cohort AChR-Ab neg. generalized myasthenia gravis AChR-Ab pos. generalized myasthenia gravis Skin cohort Mucocutaneous & mucosal pemphigus vulgaris Juvenile myositis Rheumatology Neurology Dermatology Contains cohort(s) without preconditioning
Pediatric indication RESET™ Clinical Program for CABA-201, a CD19-directed CAR T Safety and efficacy of CABA-201• 17 NOV 2024 Multiple autoimmune diseases evaluated in distinct disease cohorts enrolling at 40 US sites
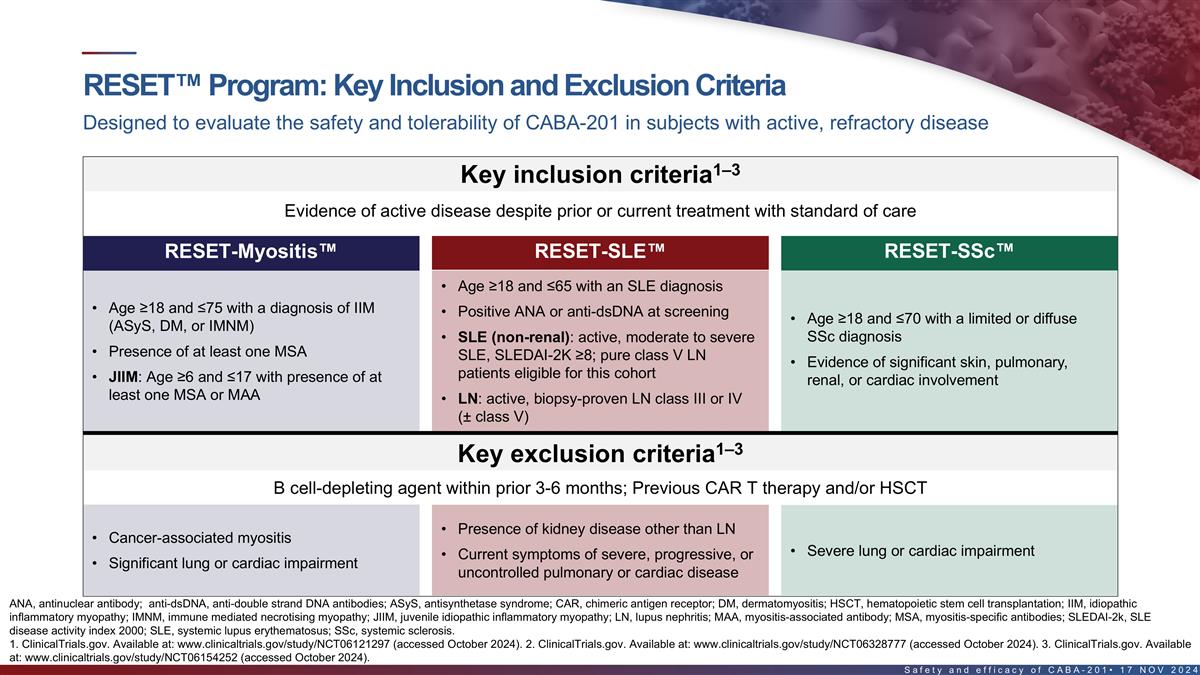
RESET™ Program: Key Inclusion
and Exclusion Criteria ANA, antinuclear antibody; anti-dsDNA, anti-double strand DNA antibodies; ASyS, antisynthetase syndrome; CAR, chimeric antigen receptor; DM, dermatomyositis; HSCT, hematopoietic stem cell transplantation; IIM, idiopathic
inflammatory myopathy; IMNM, immune mediated necrotising myopathy; JIIM, juvenile idiopathic inflammatory myopathy; LN, lupus nephritis; MAA, myositis-associated antibody; MSA, myositis-specific antibodies; SLEDAI-2k, SLE disease activity index
2000; SLE, systemic lupus erythematosus; SSc, systemic sclerosis. 1. ClinicalTrials.gov. Available at: www.clinicaltrials.gov/study/NCT06121297 (accessed October 2024). 2. ClinicalTrials.gov. Available at: www.clinicaltrials.gov/study/NCT06328777
(accessed October 2024). 3. ClinicalTrials.gov. Available at: www.clinicaltrials.gov/study/NCT06154252 (accessed October 2024). Designed to evaluate the safety and tolerability of CABA-201 in subjects with active, refractory disease
RESET-SLE™ Age ≥18 and ≤65 with an SLE diagnosis Positive ANA or anti-dsDNA at screening SLE (non-renal): active, moderate to severe SLE, SLEDAI-2K ≥8; pure class V LN patients eligible for this cohort LN: active,
biopsy-proven LN class III or IV (± class V) RESET-Myositis™ Age ≥18 and ≤75 with a diagnosis of IIM (ASyS, DM, or IMNM) Presence of at least one MSA JIIM: Age ≥6 and ≤17 with presence of at least one MSA or MAA
Age ≥18 and ≤70 with a limited or diffuse SSc diagnosis Evidence of significant skin, pulmonary, renal, or cardiac involvement RESET-SSc™ Evidence of active disease despite prior or current treatment with standard of care Key
inclusion criteria1–3 Key exclusion criteria1–3 B cell-depleting agent within prior 3-6 months; Previous CAR T therapy and/or HSCT Presence of kidney disease other than LN Current symptoms of severe, progressive, or uncontrolled
pulmonary or cardiac disease Cancer-associated myositis Significant lung or cardiac impairment Severe lung or cardiac impairment Safety and efficacy of CABA-201• 17 NOV 2024
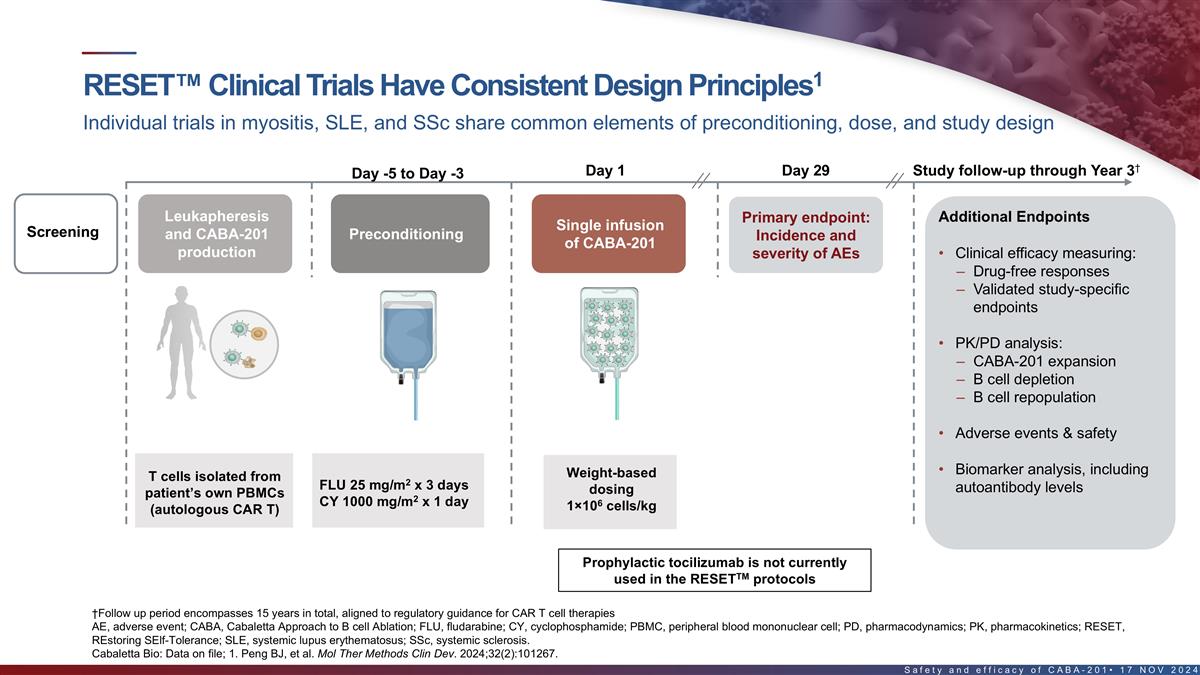
RESET™ Clinical Trials Have
Consistent Design Principles1 Leukapheresis and CABA-201 production Preconditioning Single infusion of CABA-201 Weight-based dosing 1×106 cells/kg Day 1 Primary endpoint: Incidence and severity of AEs T cells isolated from patient’s own
PBMCs (autologous CAR T) Day 29 Study follow-up through Year 3† Screening †Follow up period encompasses 15 years in total, aligned to regulatory guidance for CAR T cell therapies AE, adverse event; CABA, Cabaletta Approach to B cell
Ablation; FLU, fludarabine; CY, cyclophosphamide; PBMC, peripheral blood mononuclear cell; PD, pharmacodynamics; PK, pharmacokinetics; RESET, REstoring SElf-Tolerance; SLE, systemic lupus erythematosus; SSc, systemic sclerosis. Cabaletta Bio: Data
on file; 1. Peng BJ, et al. Mol Ther Methods Clin Dev. 2024;32(2):101267. Additional Endpoints Clinical efficacy measuring: Drug-free responses Validated study-specific endpoints PK/PD analysis: CABA-201 expansion B cell depletion B cell
repopulation Adverse events & safety Biomarker analysis, including autoantibody levels Safety and efficacy of CABA-201• 17 NOV 2024 Individual trials in myositis, SLE, and SSc share common elements of preconditioning, dose, and study
design Prophylactic tocilizumab is not currently used in the RESETTM protocols FLU 25 mg/m2 x 3 days CY 1000 mg/m2 x 1 day Day -5 to Day -3
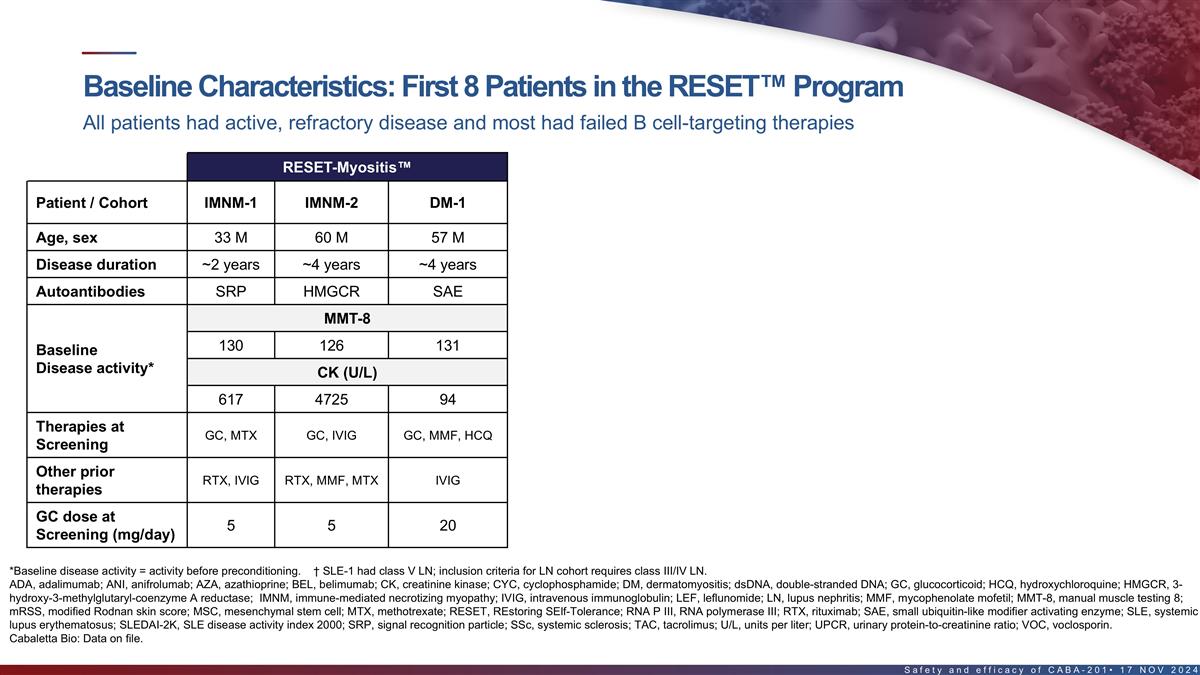
Baseline Characteristics: First 8
Patients in the RESET™ Program Safety and efficacy of CABA-201• 17 NOV 2024 All patients had active, refractory disease and most had failed B cell-targeting therapies RESET-Myositis™ RESET-Myositis Patient / Cohort IMNM-1 IMNM-2
DM-1 Age, sex 33 M 60 M 57 M Disease duration ~2 years ~4 years ~4 years Autoantibodies SRP HMGCR SAE Baseline Disease activity* MMT-8 130 126 131 CK (U/L) 617 4725 94 Therapies at Screening GC, MTX GC, IVIG GC, MMF, HCQ Other prior therapies RTX,
IVIG RTX, MMF, MTX IVIG GC dose at Screening (mg/day) 5 5 20 *Baseline disease activity = activity before preconditioning. † SLE-1 had class V LN; inclusion criteria for LN cohort requires class III/IV LN. ADA, adalimumab; ANI, anifrolumab;
AZA, azathioprine; BEL, belimumab; CK, creatinine kinase; CYC, cyclophosphamide; DM, dermatomyositis; dsDNA, double-stranded DNA; GC, glucocorticoid; HCQ, hydroxychloroquine; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; IMNM,
immune-mediated necrotizing myopathy; IVIG, intravenous immunoglobulin; LEF, leflunomide; LN, lupus nephritis; MMF, mycophenolate mofetil; MMT-8, manual muscle testing 8; mRSS, modified Rodnan skin score; MSC, mesenchymal stem cell; MTX,
methotrexate; RESET, REstoring SElf-Tolerance; RNA P III, RNA polymerase III; RTX, rituximab; SAE, small ubiquitin-like modifier activating enzyme; SLE, systemic lupus erythematosus; SLEDAI-2K, SLE disease activity index 2000; SRP, signal
recognition particle; SSc, systemic sclerosis; TAC, tacrolimus; U/L, units per liter; UPCR, urinary protein-to-creatinine ratio; VOC, voclosporin. Cabaletta Bio: Data on file.
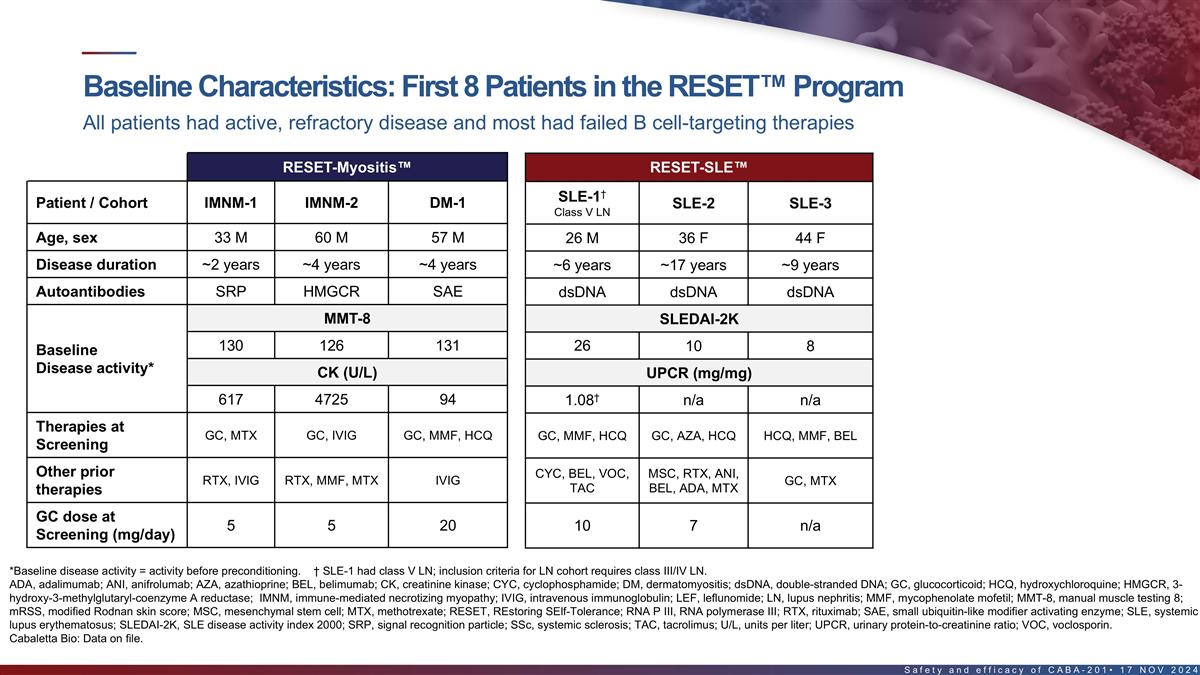
Baseline Characteristics: First 8
Patients in the RESET™ Program Safety and efficacy of CABA-201• 17 NOV 2024 RESET-Myositis™ RESET-Myositis Patient / Cohort IMNM-1 IMNM-2 DM-1 Age, sex 33 M 60 M 57 M Disease duration ~2 years ~4 years ~4 years Autoantibodies SRP
HMGCR SAE Baseline Disease activity* MMT-8 130 126 131 CK (U/L) 617 4725 94 Therapies at Screening GC, MTX GC, IVIG GC, MMF, HCQ Other prior therapies RTX, IVIG RTX, MMF, MTX IVIG GC dose at Screening (mg/day) 5 5 20 RESET-SLE™ SLE-1†
Class V LN SLE-2 SLE-3 26 M 36 F 44 F ~6 years ~17 years ~9 years dsDNA dsDNA dsDNA SLEDAI-2K 26 10 8 UPCR (mg/mg) 1.08† n/a n/a GC, MMF, HCQ GC, AZA, HCQ HCQ, MMF, BEL CYC, BEL, VOC, TAC MSC, RTX, ANI, BEL, ADA, MTX GC, MTX 10 7 n/a All
patients had active, refractory disease and most had failed B cell-targeting therapies *Baseline disease activity = activity before preconditioning. † SLE-1 had class V LN; inclusion criteria for LN cohort requires class III/IV LN. ADA,
adalimumab; ANI, anifrolumab; AZA, azathioprine; BEL, belimumab; CK, creatinine kinase; CYC, cyclophosphamide; DM, dermatomyositis; dsDNA, double-stranded DNA; GC, glucocorticoid; HCQ, hydroxychloroquine; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A
reductase; IMNM, immune-mediated necrotizing myopathy; IVIG, intravenous immunoglobulin; LEF, leflunomide; LN, lupus nephritis; MMF, mycophenolate mofetil; MMT-8, manual muscle testing 8; mRSS, modified Rodnan skin score; MSC, mesenchymal stem cell;
MTX, methotrexate; RESET, REstoring SElf-Tolerance; RNA P III, RNA polymerase III; RTX, rituximab; SAE, small ubiquitin-like modifier activating enzyme; SLE, systemic lupus erythematosus; SLEDAI-2K, SLE disease activity index 2000; SRP, signal
recognition particle; SSc, systemic sclerosis; TAC, tacrolimus; U/L, units per liter; UPCR, urinary protein-to-creatinine ratio; VOC, voclosporin. Cabaletta Bio: Data on file.
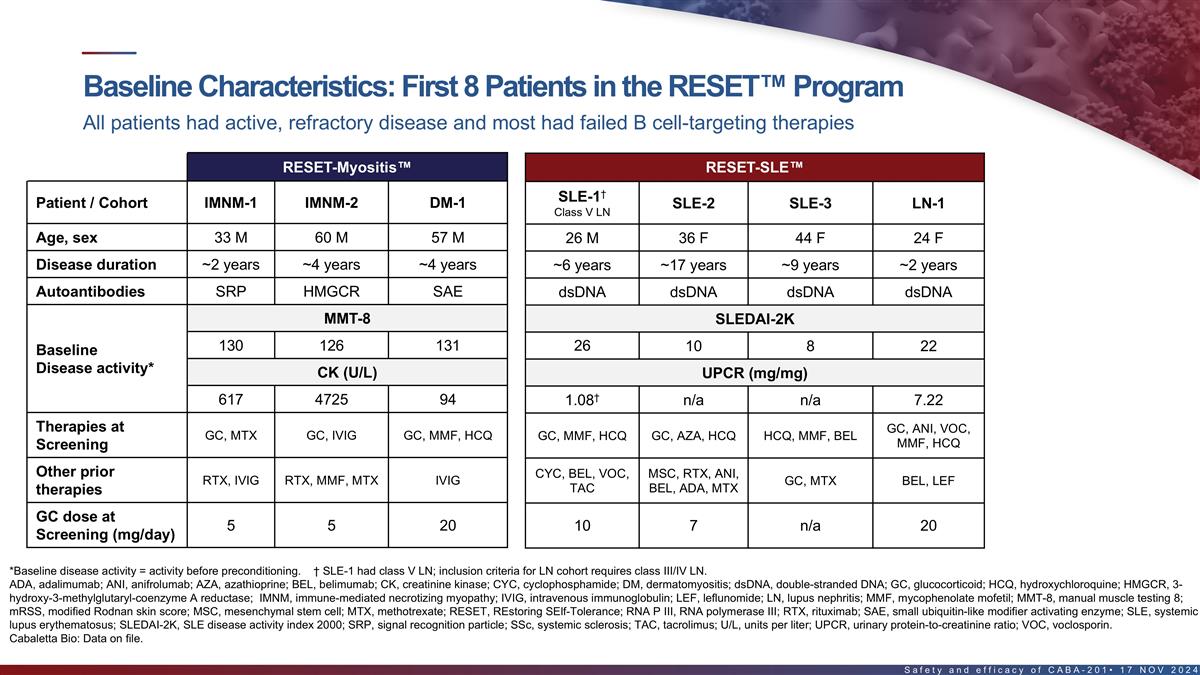
Baseline Characteristics: First 8
Patients in the RESET™ Program Safety and efficacy of CABA-201• 17 NOV 2024 RESET-Myositis™ RESET-Myositis Patient / Cohort IMNM-1 IMNM-2 DM-1 Age, sex 33 M 60 M 57 M Disease duration ~2 years ~4 years ~4 years Autoantibodies SRP
HMGCR SAE Baseline Disease activity* MMT-8 130 126 131 CK (U/L) 617 4725 94 Therapies at Screening GC, MTX GC, IVIG GC, MMF, HCQ Other prior therapies RTX, IVIG RTX, MMF, MTX IVIG GC dose at Screening (mg/day) 5 5 20 RESET-SLE™ SLE-1†
Class V LN SLE-2 SLE-3 LN-1 26 M 36 F 44 F 24 F ~6 years ~17 years ~9 years ~2 years dsDNA dsDNA dsDNA dsDNA SLEDAI-2K 26 10 8 22 UPCR (mg/mg) 1.08† n/a n/a 7.22 GC, MMF, HCQ GC, AZA, HCQ HCQ, MMF, BEL GC, ANI, VOC, MMF, HCQ CYC, BEL, VOC, TAC
MSC, RTX, ANI, BEL, ADA, MTX GC, MTX BEL, LEF 10 7 n/a 20 All patients had active, refractory disease and most had failed B cell-targeting therapies *Baseline disease activity = activity before preconditioning. † SLE-1 had class V LN;
inclusion criteria for LN cohort requires class III/IV LN. ADA, adalimumab; ANI, anifrolumab; AZA, azathioprine; BEL, belimumab; CK, creatinine kinase; CYC, cyclophosphamide; DM, dermatomyositis; dsDNA, double-stranded DNA; GC, glucocorticoid; HCQ,
hydroxychloroquine; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; IMNM, immune-mediated necrotizing myopathy; IVIG, intravenous immunoglobulin; LEF, leflunomide; LN, lupus nephritis; MMF, mycophenolate mofetil; MMT-8, manual muscle testing
8; mRSS, modified Rodnan skin score; MSC, mesenchymal stem cell; MTX, methotrexate; RESET, REstoring SElf-Tolerance; RNA P III, RNA polymerase III; RTX, rituximab; SAE, small ubiquitin-like modifier activating enzyme; SLE, systemic lupus
erythematosus; SLEDAI-2K, SLE disease activity index 2000; SRP, signal recognition particle; SSc, systemic sclerosis; TAC, tacrolimus; U/L, units per liter; UPCR, urinary protein-to-creatinine ratio; VOC, voclosporin. Cabaletta Bio: Data on file.
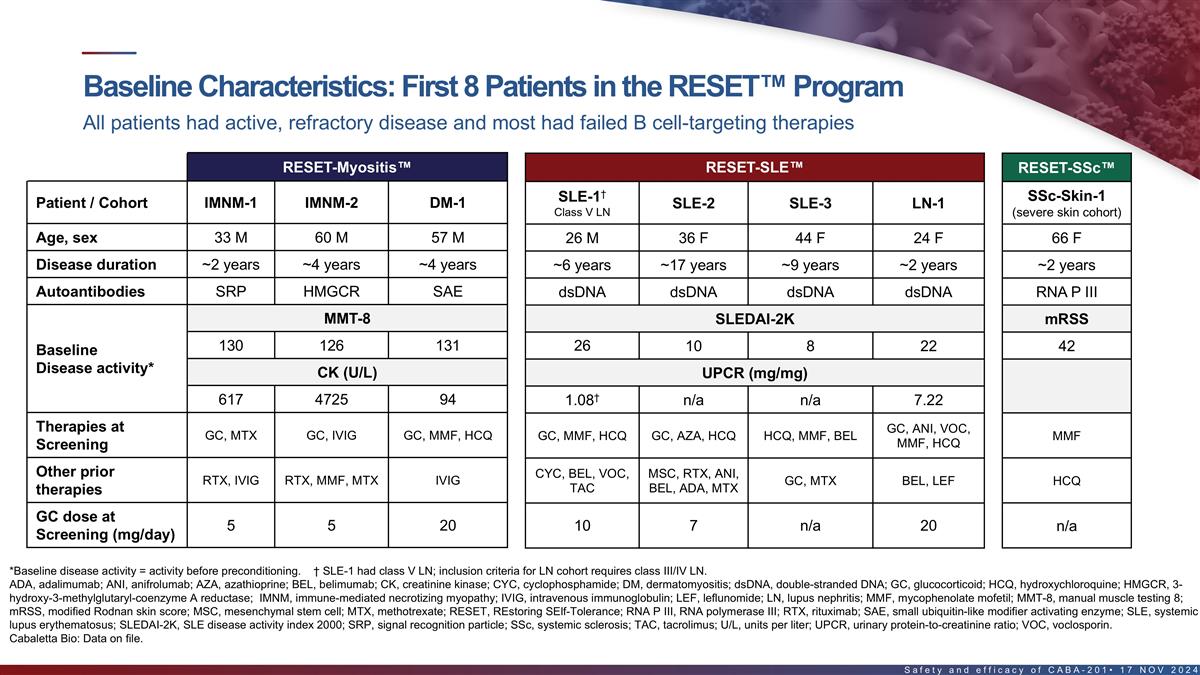
Baseline Characteristics: First 8
Patients in the RESET™ Program Safety and efficacy of CABA-201• 17 NOV 2024 RESET-Myositis™ RESET-Myositis Patient / Cohort IMNM-1 IMNM-2 DM-1 Age, sex 33 M 60 M 57 M Disease duration ~2 years ~4 years ~4 years Autoantibodies SRP
HMGCR SAE Baseline Disease activity* MMT-8 130 126 131 CK (U/L) 617 4725 94 Therapies at Screening GC, MTX GC, IVIG GC, MMF, HCQ Other prior therapies RTX, IVIG RTX, MMF, MTX IVIG GC dose at Screening (mg/day) 5 5 20 RESET-SLE™ SLE-1†
Class V LN SLE-2 SLE-3 LN-1 26 M 36 F 44 F 24 F ~6 years ~17 years ~9 years ~2 years dsDNA dsDNA dsDNA dsDNA SLEDAI-2K 26 10 8 22 UPCR (mg/mg) 1.08† n/a n/a 7.22 GC, MMF, HCQ GC, AZA, HCQ HCQ, MMF, BEL GC, ANI, VOC, MMF, HCQ CYC, BEL, VOC, TAC
MSC, RTX, ANI, BEL, ADA, MTX GC, MTX BEL, LEF 10 7 n/a 20 RESET-SSc™ SSc-Skin-1 (severe skin cohort) 66 F ~2 years RNA P III mRSS 42 MMF HCQ n/a All patients had active, refractory disease and most had failed B cell-targeting therapies
*Baseline disease activity = activity before preconditioning. † SLE-1 had class V LN; inclusion criteria for LN cohort requires class III/IV LN. ADA, adalimumab; ANI, anifrolumab; AZA, azathioprine; BEL, belimumab; CK, creatinine kinase; CYC,
cyclophosphamide; DM, dermatomyositis; dsDNA, double-stranded DNA; GC, glucocorticoid; HCQ, hydroxychloroquine; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; IMNM, immune-mediated necrotizing myopathy; IVIG, intravenous immunoglobulin;
LEF, leflunomide; LN, lupus nephritis; MMF, mycophenolate mofetil; MMT-8, manual muscle testing 8; mRSS, modified Rodnan skin score; MSC, mesenchymal stem cell; MTX, methotrexate; RESET, REstoring SElf-Tolerance; RNA P III, RNA polymerase III; RTX,
rituximab; SAE, small ubiquitin-like modifier activating enzyme; SLE, systemic lupus erythematosus; SLEDAI-2K, SLE disease activity index 2000; SRP, signal recognition particle; SSc, systemic sclerosis; TAC, tacrolimus; U/L, units per liter; UPCR,
urinary protein-to-creatinine ratio; VOC, voclosporin. Cabaletta Bio: Data on file.
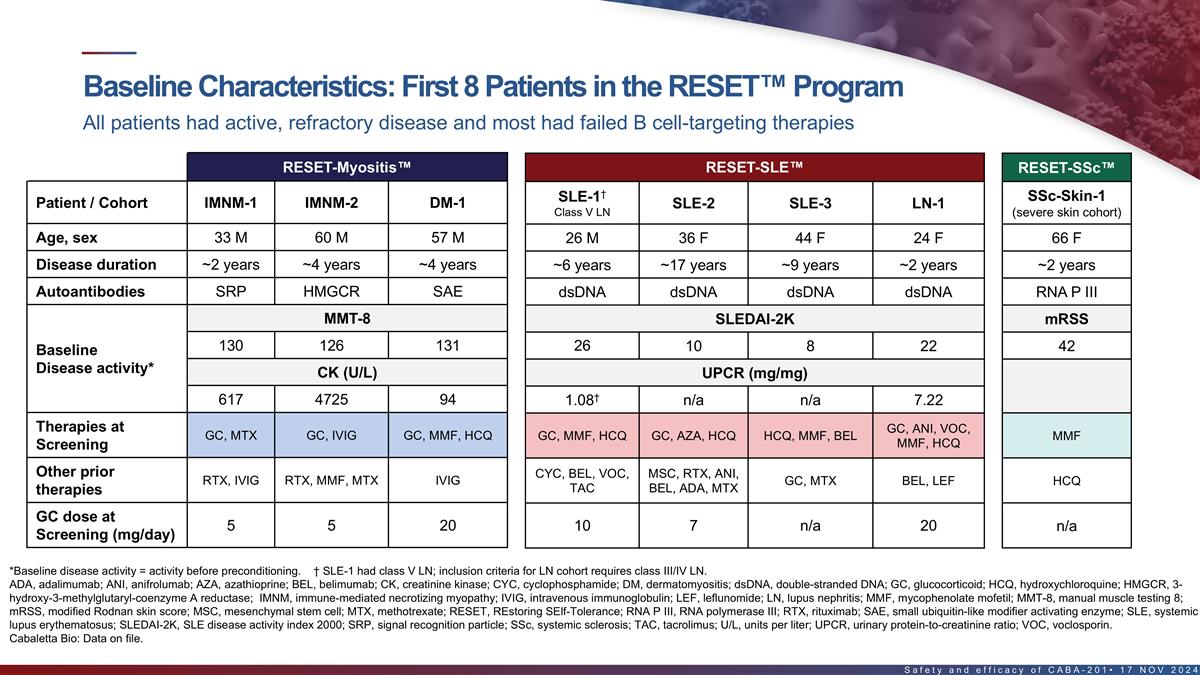
Baseline Characteristics: First 8
Patients in the RESET™ Program *Baseline disease activity = activity before preconditioning. † SLE-1 had class V LN; inclusion criteria for LN cohort requires class III/IV LN. ADA, adalimumab; ANI, anifrolumab; AZA, azathioprine; BEL,
belimumab; CK, creatinine kinase; CYC, cyclophosphamide; DM, dermatomyositis; dsDNA, double-stranded DNA; GC, glucocorticoid; HCQ, hydroxychloroquine; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; IMNM, immune-mediated necrotizing
myopathy; IVIG, intravenous immunoglobulin; LEF, leflunomide; LN, lupus nephritis; MMF, mycophenolate mofetil; MMT-8, manual muscle testing 8; mRSS, modified Rodnan skin score; MSC, mesenchymal stem cell; MTX, methotrexate; RESET, REstoring
SElf-Tolerance; RNA P III, RNA polymerase III; RTX, rituximab; SAE, small ubiquitin-like modifier activating enzyme; SLE, systemic lupus erythematosus; SLEDAI-2K, SLE disease activity index 2000; SRP, signal recognition particle; SSc, systemic
sclerosis; TAC, tacrolimus; U/L, units per liter; UPCR, urinary protein-to-creatinine ratio; VOC, voclosporin. Cabaletta Bio: Data on file. Safety and efficacy of CABA-201• 17 NOV 2024 RESET-Myositis™ RESET-Myositis Patient / Cohort
IMNM-1 IMNM-2 DM-1 Age, sex 33 M 60 M 57 M Disease duration ~2 years ~4 years ~4 years Autoantibodies SRP HMGCR SAE Baseline Disease activity* MMT-8 130 126 131 CK (U/L) 617 4725 94 Therapies at Screening GC, MTX GC, IVIG GC, MMF, HCQ Other prior
therapies RTX, IVIG RTX, MMF, MTX IVIG GC dose at Screening (mg/day) 5 5 20 RESET-SLE™ SLE-1† Class V LN SLE-2 SLE-3 LN-1 26 M 36 F 44 F 24 F ~6 years ~17 years ~9 years ~2 years dsDNA dsDNA dsDNA dsDNA SLEDAI-2K 26 10 8 22 UPCR (mg/mg)
1.08† n/a n/a 7.22 GC, MMF, HCQ GC, AZA, HCQ HCQ, MMF, BEL GC, ANI, VOC, MMF, HCQ CYC, BEL, VOC, TAC MSC, RTX, ANI, BEL, ADA, MTX GC, MTX BEL, LEF 10 7 n/a 20 RESET-SSc™ SSc-Skin-1 (severe skin cohort) 66 F ~2 years RNA P III mRSS 42 MMF
HCQ n/a All patients had active, refractory disease and most had failed B cell-targeting therapies
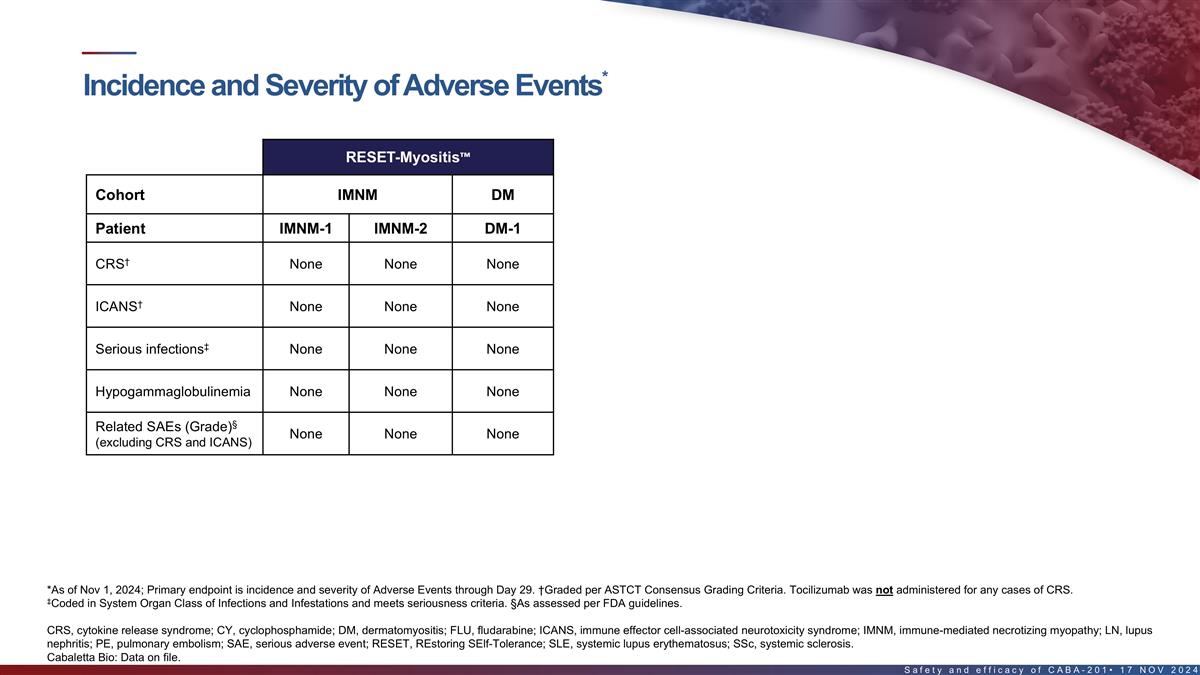
RESET-Myositis™ Cohort IMNM
DM Patient IMNM-1 IMNM-2 DM-1 CRS† None None None ICANS† None None None Serious infections‡ None None None Hypogammaglobulinemia None None None Related SAEs (Grade)§ (excluding CRS and ICANS) None None None Safety and efficacy
of CABA-201• 17 NOV 2024 Incidence and Severity of Adverse Events* *As of Nov 1, 2024; Primary endpoint is incidence and severity of Adverse Events through Day 29. †Graded per ASTCT Consensus Grading Criteria. Tocilizumab was not
administered for any cases of CRS. ‡Coded in System Organ Class of Infections and Infestations and meets seriousness criteria. §As assessed per FDA guidelines. CRS, cytokine release syndrome; CY, cyclophosphamide; DM, dermatomyositis;
FLU, fludarabine; ICANS, immune effector cell-associated neurotoxicity syndrome; IMNM, immune-mediated necrotizing myopathy; LN, lupus nephritis; PE, pulmonary embolism; SAE, serious adverse event; RESET, REstoring SElf-Tolerance; SLE, systemic
lupus erythematosus; SSc, systemic sclerosis. Cabaletta Bio: Data on file.
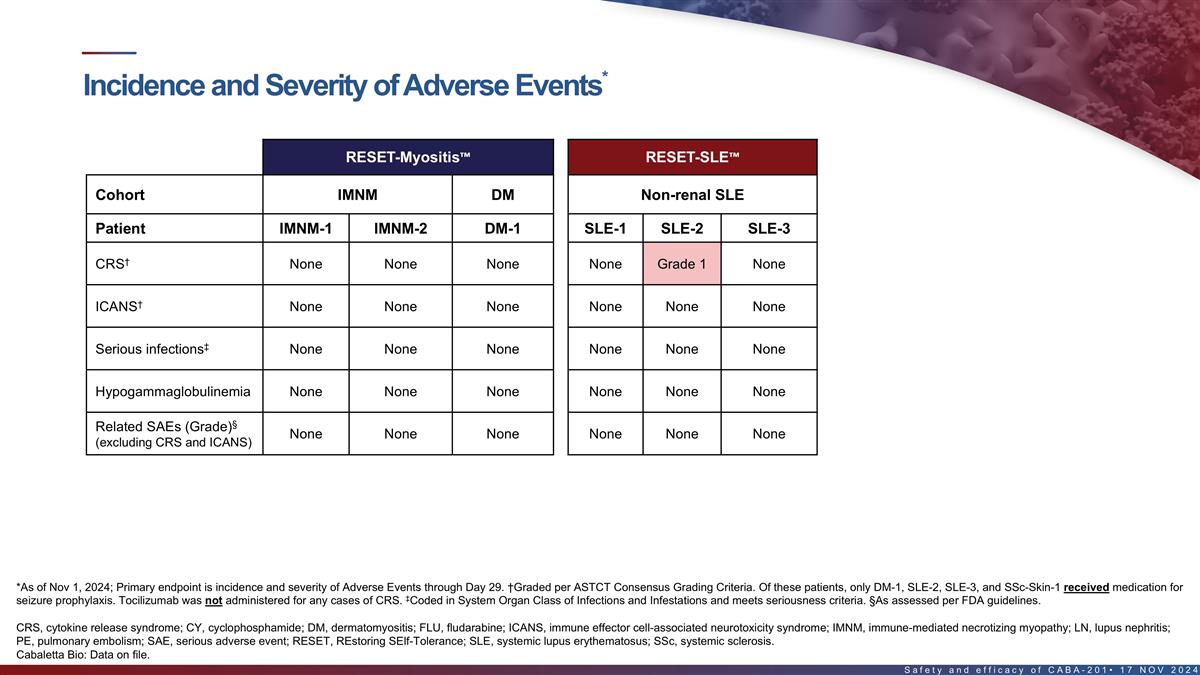
RESET-Myositis™ Cohort IMNM
DM Patient IMNM-1 IMNM-2 DM-1 CRS† None None None ICANS† None None None Serious infections‡ None None None Hypogammaglobulinemia None None None Related SAEs (Grade)§ (excluding CRS and ICANS) None None None RESET-SLE™
Non-renal SLE SLE-1 SLE-2 SLE-3 None Grade 1 None None None None None None None None None None None None None Safety and efficacy of CABA-201• 17 NOV 2024 Incidence and Severity of Adverse Events* *As of Nov 1, 2024; Primary endpoint is
incidence and severity of Adverse Events through Day 29. †Graded per ASTCT Consensus Grading Criteria. Of these patients, only DM-1, SLE-2, SLE-3, and SSc-Skin-1 received medication for seizure prophylaxis. Tocilizumab was not administered for
any cases of CRS. ‡Coded in System Organ Class of Infections and Infestations and meets seriousness criteria. §As assessed per FDA guidelines. CRS, cytokine release syndrome; CY, cyclophosphamide; DM, dermatomyositis; FLU, fludarabine;
ICANS, immune effector cell-associated neurotoxicity syndrome; IMNM, immune-mediated necrotizing myopathy; LN, lupus nephritis; PE, pulmonary embolism; SAE, serious adverse event; RESET, REstoring SElf-Tolerance; SLE, systemic lupus erythematosus;
SSc, systemic sclerosis. Cabaletta Bio: Data on file.
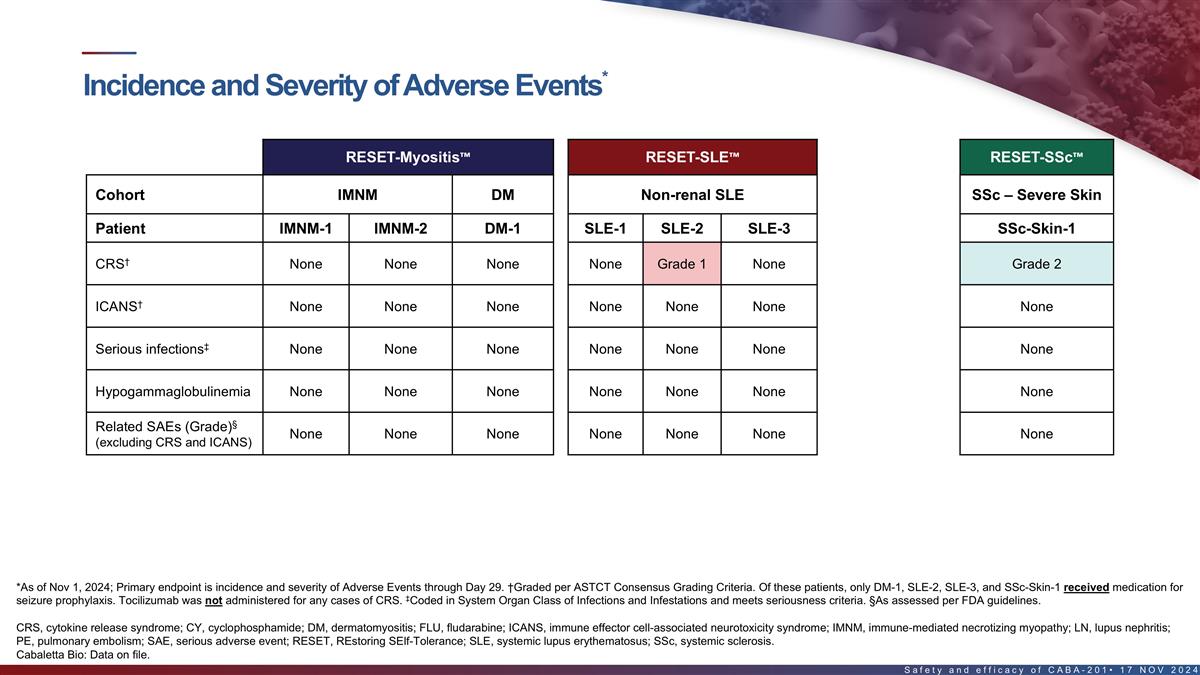
RESET-Myositis™ Cohort IMNM
DM Patient IMNM-1 IMNM-2 DM-1 CRS† None None None ICANS† None None None Serious infections‡ None None None Hypogammaglobulinemia None None None Related SAEs (Grade)§ (excluding CRS and ICANS) None None None RESET-SLE™
Non-renal SLE SLE-1 SLE-2 SLE-3 None Grade 1 None None None None None None None None None None None None None RESET-SSc™ SSc – Severe Skin SSc-Skin-1 Grade 2 None None None None Safety and efficacy of CABA-201• 17 NOV 2024
Incidence and Severity of Adverse Events* *As of Nov 1, 2024; Primary endpoint is incidence and severity of Adverse Events through Day 29. †Graded per ASTCT Consensus Grading Criteria. Of these patients, only DM-1, SLE-2, SLE-3, and SSc-Skin-1
received medication for seizure prophylaxis. Tocilizumab was not administered for any cases of CRS. ‡Coded in System Organ Class of Infections and Infestations and meets seriousness criteria. §As assessed per FDA guidelines. CRS, cytokine
release syndrome; CY, cyclophosphamide; DM, dermatomyositis; FLU, fludarabine; ICANS, immune effector cell-associated neurotoxicity syndrome; IMNM, immune-mediated necrotizing myopathy; LN, lupus nephritis; PE, pulmonary embolism; SAE, serious
adverse event; RESET, REstoring SElf-Tolerance; SLE, systemic lupus erythematosus; SSc, systemic sclerosis. Cabaletta Bio: Data on file.
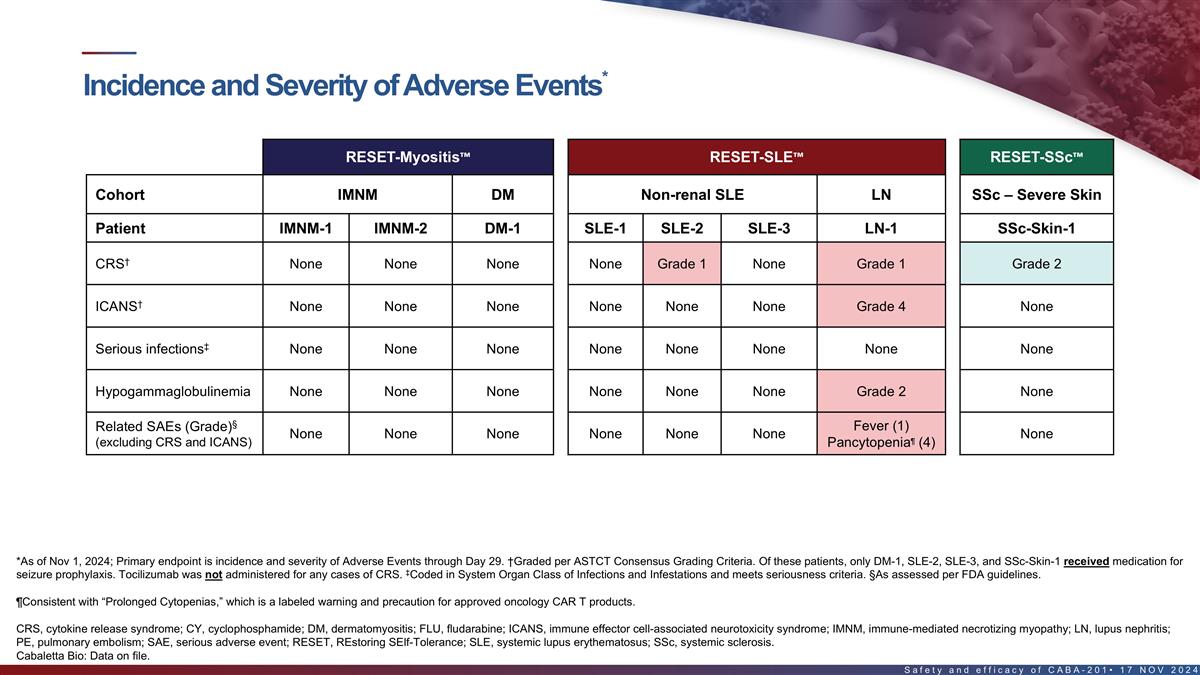
RESET-Myositis™ Cohort IMNM
DM Patient IMNM-1 IMNM-2 DM-1 CRS† None None None ICANS† None None None Serious infections‡ None None None Hypogammaglobulinemia None None None Related SAEs (Grade)§ (excluding CRS and ICANS) None None None RESET-SLE™
Non-renal SLE LN SLE-1 SLE-2 SLE-3 LN-1 None Grade 1 None Grade 1 None None None Grade 4 None None None None None None None Grade 2 None None None Fever (1) Pancytopenia¶ (4) RESET-SSc™ SSc – Severe Skin SSc-Skin-1 Grade 2 None None
None None Safety and efficacy of CABA-201• 17 NOV 2024 Incidence and Severity of Adverse Events* *As of Nov 1, 2024; Primary endpoint is incidence and severity of Adverse Events through Day 29. †Graded per ASTCT Consensus Grading
Criteria. Of these patients, only DM-1, SLE-2, SLE-3, and SSc-Skin-1 received medication for seizure prophylaxis. Tocilizumab was not administered for any cases of CRS. ‡Coded in System Organ Class of Infections and Infestations and meets
seriousness criteria. §As assessed per FDA guidelines. ¶Consistent with “Prolonged Cytopenias,” which is a labeled warning and precaution for approved oncology CAR T products. CRS, cytokine release syndrome; CY,
cyclophosphamide; DM, dermatomyositis; FLU, fludarabine; ICANS, immune effector cell-associated neurotoxicity syndrome; IMNM, immune-mediated necrotizing myopathy; LN, lupus nephritis; PE, pulmonary embolism; SAE, serious adverse event; RESET,
REstoring SElf-Tolerance; SLE, systemic lupus erythematosus; SSc, systemic sclerosis. Cabaletta Bio: Data on file.
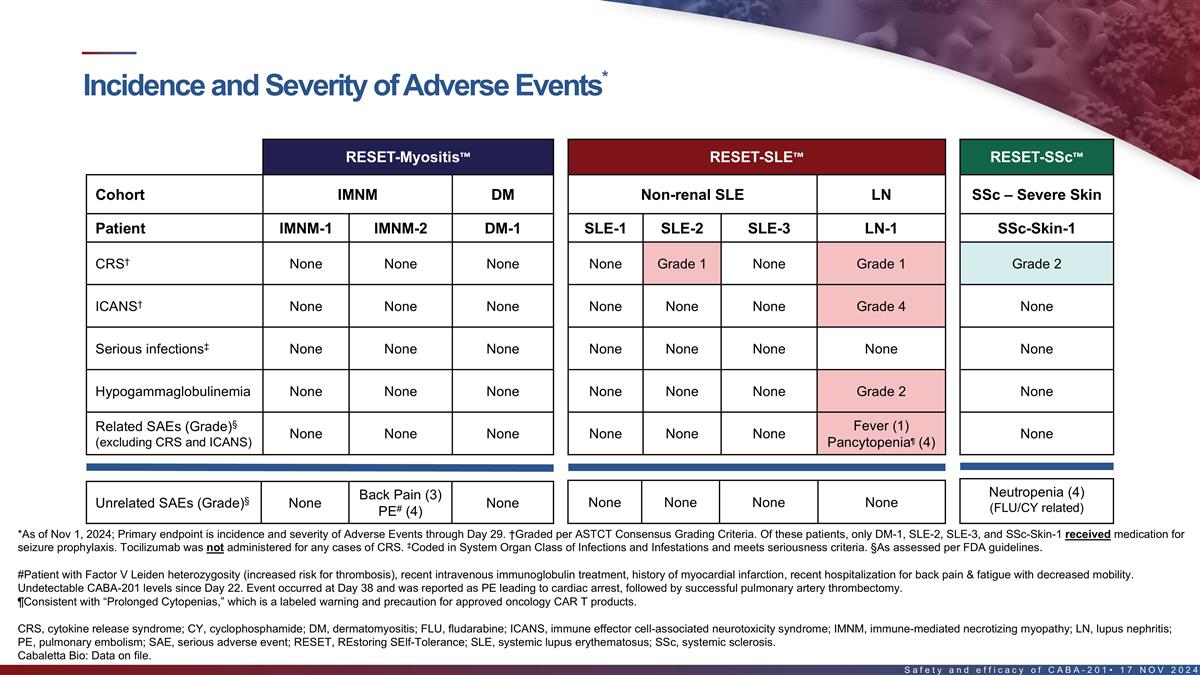
RESET-Myositis™ Cohort IMNM
DM Patient IMNM-1 IMNM-2 DM-1 CRS† None None None ICANS† None None None Serious infections‡ None None None Hypogammaglobulinemia None None None Related SAEs (Grade)§ (excluding CRS and ICANS) None None None RESET-SLE™
Non-renal SLE LN SLE-1 SLE-2 SLE-3 LN-1 None Grade 1 None Grade 1 None None None Grade 4 None None None None None None None Grade 2 None None None Fever (1) Pancytopenia¶ (4) RESET-SSc™ SSc – Severe Skin SSc-Skin-1 Grade 2 None None
None None Safety and efficacy of CABA-201• 17 NOV 2024 Incidence and Severity of Adverse Events* Unrelated SAEs (Grade)§ None Back Pain (3) PE# (4) None None None None None Neutropenia (4) (FLU/CY related) *As of Nov 1, 2024; Primary
endpoint is incidence and severity of Adverse Events through Day 29. †Graded per ASTCT Consensus Grading Criteria. Of these patients, only DM-1, SLE-2, SLE-3, and SSc-Skin-1 received medication for seizure prophylaxis. Tocilizumab was not
administered for any cases of CRS. ‡Coded in System Organ Class of Infections and Infestations and meets seriousness criteria. §As assessed per FDA guidelines. #Patient with Factor V Leiden heterozygosity (increased risk for thrombosis),
recent intravenous immunoglobulin treatment, history of myocardial infarction, recent hospitalization for back pain & fatigue with decreased mobility. Undetectable CABA-201 levels since Day 22. Event occurred at Day 38 and was reported as PE
leading to cardiac arrest, followed by successful pulmonary artery thrombectomy. ¶Consistent with “Prolonged Cytopenias,” which is a labeled warning and precaution for approved oncology CAR T products. CRS, cytokine release
syndrome; CY, cyclophosphamide; DM, dermatomyositis; FLU, fludarabine; ICANS, immune effector cell-associated neurotoxicity syndrome; IMNM, immune-mediated necrotizing myopathy; LN, lupus nephritis; PE, pulmonary embolism; SAE, serious adverse
event; RESET, REstoring SElf-Tolerance; SLE, systemic lupus erythematosus; SSc, systemic sclerosis. Cabaletta Bio: Data on file.
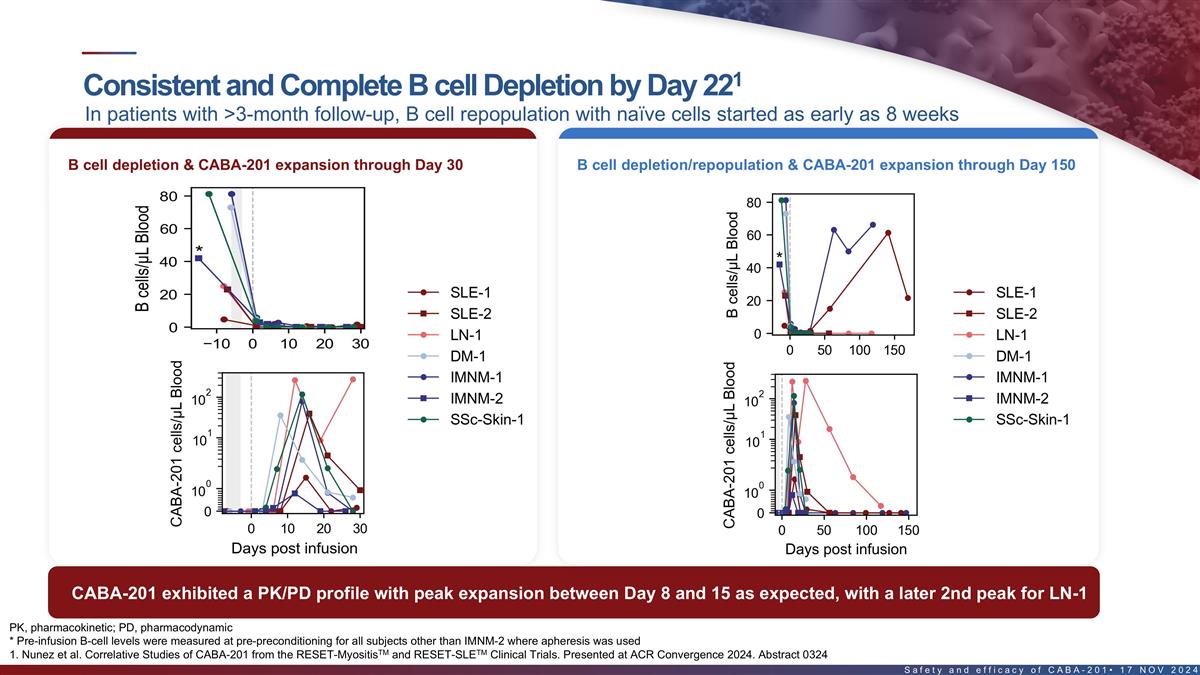
Consistent and Complete B cell
Depletion by Day 221 B cell depletion & CABA-201 expansion through Day 30 Safety and efficacy of CABA-201• 17 NOV 2024 PK, pharmacokinetic; PD, pharmacodynamic * Pre-infusion B-cell levels were measured at pre-preconditioning for all
subjects other than IMNM-2 where apheresis was used 1. Nunez et al. Correlative Studies of CABA-201 from the RESET-MyositisTM and RESET-SLETM Clinical Trials. Presented at ACR Convergence 2024. Abstract 0324 CABA-201 exhibited a PK/PD profile with
peak expansion between Day 8 and 15 as expected, with a later 2nd peak for LN-1 B cell depletion/repopulation & CABA-201 expansion through Day 150 In patients with >3-month follow-up, B cell repopulation with naïve cells started as early
as 8 weeks
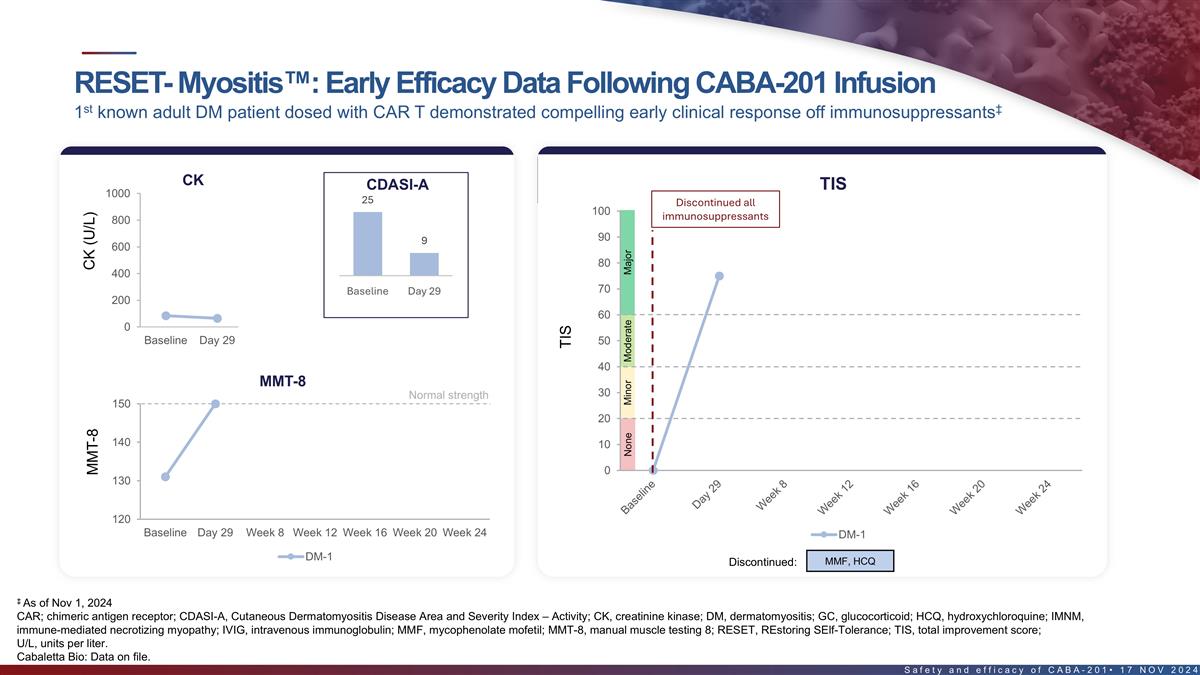
RESET- Myositis™: Early
Efficacy Data Following CABA-201 Infusion ‡ As of Nov 1, 2024 CAR; chimeric antigen receptor; CDASI-A, Cutaneous Dermatomyositis Disease Area and Severity Index – Activity; CK, creatinine kinase; DM, dermatomyositis; GC, glucocorticoid;
HCQ, hydroxychloroquine; IMNM, immune-mediated necrotizing myopathy; IVIG, intravenous immunoglobulin; MMF, mycophenolate mofetil; MMT-8, manual muscle testing 8; RESET, REstoring SElf-Tolerance; TIS, total improvement score; U/L, units per liter.
Cabaletta Bio: Data on file. Safety and efficacy of CABA-201• 17 NOV 2024 TIS Major Moderate Minor None CK (U/L) MMT-8 Normal strength CK DM-1 Discontinued all immunosuppressants MMF, HCQ Discontinued: 1st known adult DM patient dosed with CAR
T demonstrated compelling early clinical response off immunosuppressants‡
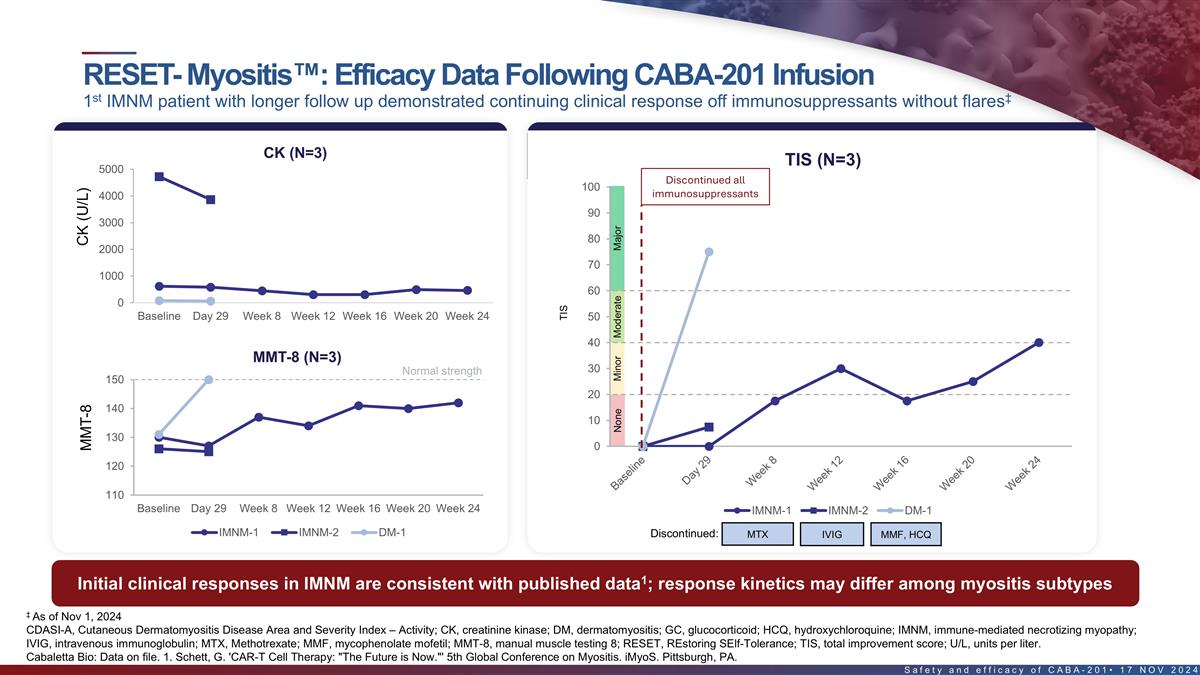
TIS Major Moderate Minor None
RESET- Myositis™: Efficacy Data Following CABA-201 Infusion ‡ As of Nov 1, 2024 CDASI-A, Cutaneous Dermatomyositis Disease Area and Severity Index – Activity; CK, creatinine kinase; DM, dermatomyositis; GC, glucocorticoid; HCQ,
hydroxychloroquine; IMNM, immune-mediated necrotizing myopathy; IVIG, intravenous immunoglobulin; MTX, Methotrexate; MMF, mycophenolate mofetil; MMT-8, manual muscle testing 8; RESET, REstoring SElf-Tolerance; TIS, total improvement score; U/L,
units per liter. Cabaletta Bio: Data on file. 1. Schett, G. 'CAR-T Cell Therapy: "The Future is Now."' 5th Global Conference on Myositis. iMyoS. Pittsburgh, PA. Safety and efficacy of CABA-201• 17 NOV 2024 1st IMNM patient with longer follow
up demonstrated continuing clinical response off immunosuppressants without flares‡ MTX IVIG MMF, HCQ CK (U/L) MMT-8 Normal strength CK (N=3) Discontinued all immunosuppressants Initial clinical responses in IMNM are consistent with
published data1; response kinetics may differ among myositis subtypes Discontinued:
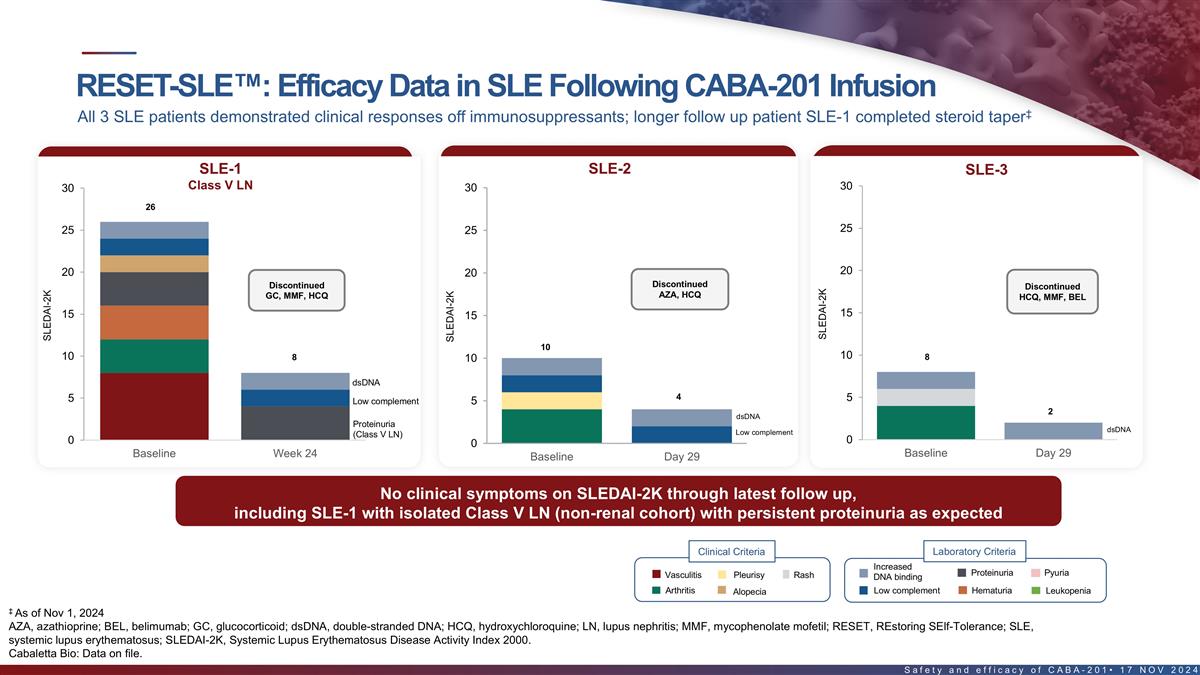
26 8 SLE-1 Class V LN 10 4 SLE-2 8
2 SLE-3 Discontinued GC, MMF, HCQ Discontinued AZA, HCQ Discontinued HCQ, MMF, BEL Increased DNA binding Low complement Proteinuria Hematuria Vasculitis Leukopenia Pleurisy Pyuria SLEDAI-2K SLEDAI-2K SLEDAI-2K RESET-SLE™: Efficacy Data in SLE
Following CABA-201 Infusion Safety and efficacy of CABA-201• 17 NOV 2024 No clinical symptoms on SLEDAI-2K through latest follow up, including SLE-1 with isolated Class V LN (non-renal cohort) with persistent proteinuria as expected ‡ As
of Nov 1, 2024 AZA, azathioprine; BEL, belimumab; GC, glucocorticoid; dsDNA, double-stranded DNA; HCQ, hydroxychloroquine; LN, lupus nephritis; MMF, mycophenolate mofetil; RESET, REstoring SElf-Tolerance; SLE, systemic lupus erythematosus;
SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000. Cabaletta Bio: Data on file. Proteinuria (Class V LN) Low complement dsDNA Low complement dsDNA dsDNA Alopecia Arthritis Rash Laboratory Criteria Clinical Criteria All 3 SLE
patients demonstrated clinical responses off immunosuppressants; longer follow up patient SLE-1 completed steroid taper‡
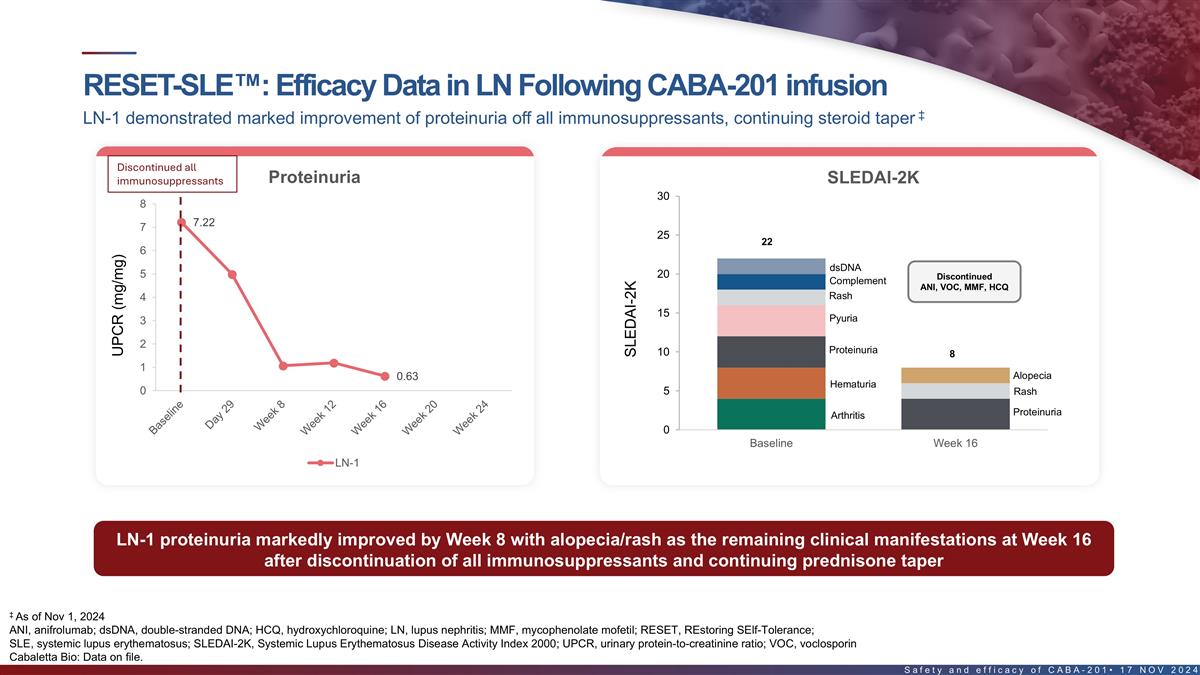
22 SLEDAI-2K 8 RESET-SLE™:
Efficacy Data in LN Following CABA-201 infusion Safety and efficacy of CABA-201• 17 NOV 2024 ‡ As of Nov 1, 2024 ANI, anifrolumab; dsDNA, double-stranded DNA; HCQ, hydroxychloroquine; LN, lupus nephritis; MMF, mycophenolate mofetil;
RESET, REstoring SElf-Tolerance; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; UPCR, urinary protein-to-creatinine ratio; VOC, voclosporin Cabaletta Bio: Data on file. Proteinuria markedly
improved in LN-1 off all 4 immunosuppressants by Week 8; SLE-1 with isolated Class V lupus nephritis (non-renal cohort) demonstrated resolution of all clinical symptoms with proteinuria persisting off immunosuppressants with prednisone tapered off.
LN-1 demonstrated marked improvement of proteinuria off all immunosuppressants, continuing steroid taper ‡ UPCR (mg/mg) Discontinued all immunosuppressants LN-1 proteinuria markedly improved by Week 8 with alopecia/rash as the remaining
clinical manifestations at Week 16 after discontinuation of all immunosuppressants and continuing prednisone taper Discontinued ANI, VOC, MMF, HCQ SLEDAI-2K dsDNA Complement Rash Pyuria Proteinuria Hematuria Arthritis Alopecia Rash
Proteinuria

Safety and efficacy of
CABA-201• 17 NOV 2024 Day -4: Fever/worsening anemia (resolved in 3 days) Day -7 to -5: Preconditioning Day -18: Fever/relapsing pericardial effusion (resolved in 1 day) LN-1 Patient : ICANS Event Timeline 24-year-old female with SLE for ~2
years and with active class III LN Active, severe (SLEDAI-2K = 22; UPCR = 7.22 mg/mg) refractory disease despite 5 systemic treatments* for SLE at screening Pre-infusion period Patient with recent fever in setting of acute and severe inflammation
*Therapies at Screening: glucocorticoids, anifrolumab, voclosporin, mycophenolate mofetil, hydroxychloroquine CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; LN, lupus nephritis; RESET, REstoring
SElf-Tolerance; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; UPCR, urinary protein-to-creatinine ratio Cabaletta Bio: Data on file. 1. Nunez et al. Correlative Studies of CABA-201 from the
RESET-MyositisTM and RESET-SLETM Clinical Trials. Presented at ACR Convergence 2024. Abstract 0324
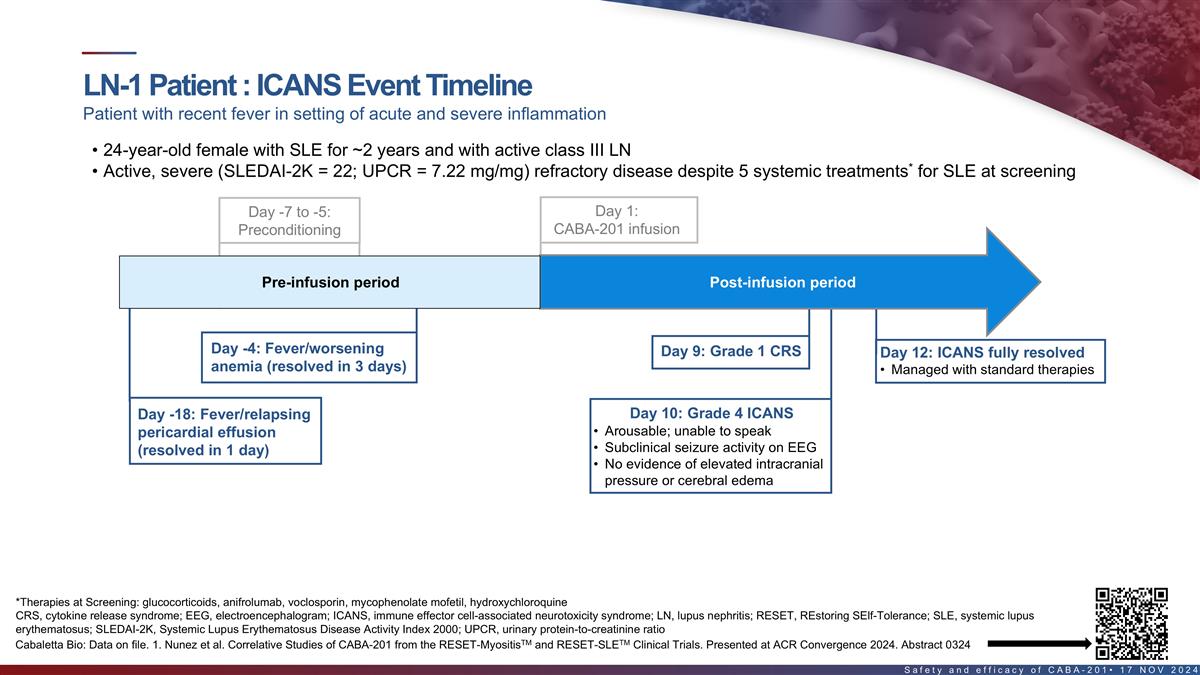
Safety and efficacy of
CABA-201• 17 NOV 2024 Day 1: CABA-201 infusion Day 9: Grade 1 CRS Day 10: Grade 4 ICANS Arousable; unable to speak Subclinical seizure activity on EEG No evidence of elevated intracranial pressure or cerebral edema Day 12: ICANS fully resolved
Managed with standard therapies Day -7 to -5: Preconditioning Day -18: Fever/relapsing pericardial effusion (resolved in 1 day) LN-1 Patient : ICANS Event Timeline 24-year-old female with SLE for ~2 years and with active class III LN Active, severe
(SLEDAI-2K = 22; UPCR = 7.22 mg/mg) refractory disease despite 5 systemic treatments* for SLE at screening Post-infusion period Patient with recent fever in setting of acute and severe inflammation *Therapies at Screening: glucocorticoids,
anifrolumab, voclosporin, mycophenolate mofetil, hydroxychloroquine CRS, cytokine release syndrome; EEG, electroencephalogram; ICANS, immune effector cell-associated neurotoxicity syndrome; LN, lupus nephritis; RESET, REstoring SElf-Tolerance; SLE,
systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; UPCR, urinary protein-to-creatinine ratio Cabaletta Bio: Data on file. 1. Nunez et al. Correlative Studies of CABA-201 from the RESET-MyositisTM and
RESET-SLETM Clinical Trials. Presented at ACR Convergence 2024. Abstract 0324 Pre-infusion period Day -4: Fever/worsening anemia (resolved in 3 days)
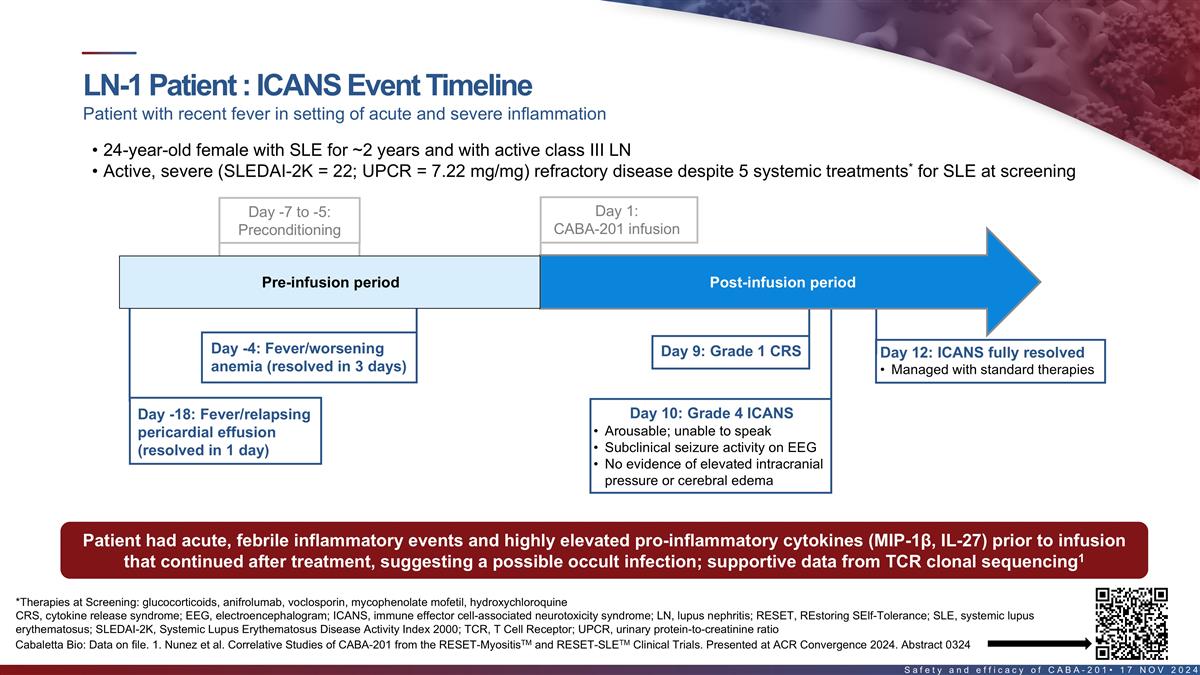
Safety and efficacy of
CABA-201• 17 NOV 2024 Day 1: CABA-201 infusion Day 9: Grade 1 CRS Day 10: Grade 4 ICANS Arousable; unable to speak Subclinical seizure activity on EEG No evidence of elevated intracranial pressure or cerebral edema Day 12: ICANS fully resolved
Managed with standard therapies Day -7 to -5: Preconditioning Day -18: Fever/relapsing pericardial effusion (resolved in 1 day) LN-1 Patient : ICANS Event Timeline 24-year-old female with SLE for ~2 years and with active class III LN Active, severe
(SLEDAI-2K = 22; UPCR = 7.22 mg/mg) refractory disease despite 5 systemic treatments* for SLE at screening Patient with recent fever in setting of acute and severe inflammation *Therapies at Screening: glucocorticoids, anifrolumab, voclosporin,
mycophenolate mofetil, hydroxychloroquine CRS, cytokine release syndrome; EEG, electroencephalogram; ICANS, immune effector cell-associated neurotoxicity syndrome; LN, lupus nephritis; RESET, REstoring SElf-Tolerance; SLE, systemic lupus
erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; TCR, T Cell Receptor; UPCR, urinary protein-to-creatinine ratio Cabaletta Bio: Data on file. 1. Nunez et al. Correlative Studies of CABA-201 from the
RESET-MyositisTM and RESET-SLETM Clinical Trials. Presented at ACR Convergence 2024. Abstract 0324 Patient had acute, febrile inflammatory events and highly elevated pro-inflammatory cytokines (MIP-1β, IL-27) prior to infusion that continued
after treatment, suggesting a possible occult infection; supportive data from TCR clonal sequencing1 Post-infusion period Pre-infusion period Day -4: Fever/worsening anemia (resolved in 3 days)
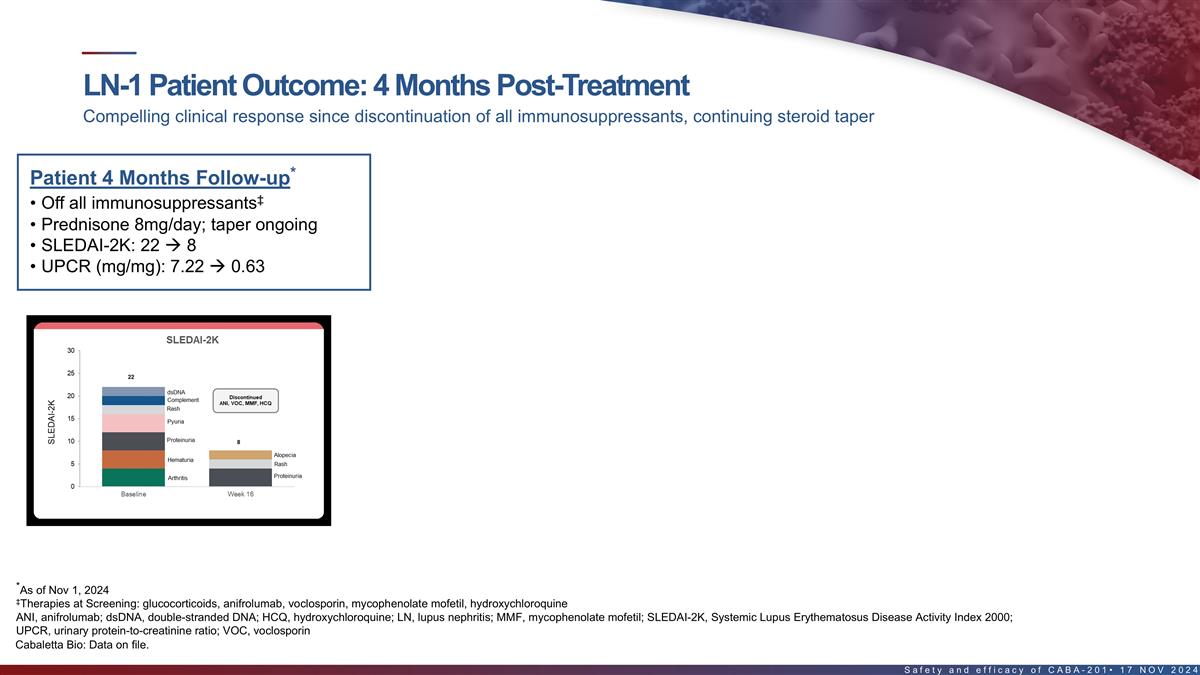
Safety and efficacy of
CABA-201• 17 NOV 2024 LN-1 Patient Outcome: 4 Months Post-Treatment Patient 4 Months Follow-up* Off all immunosuppressants‡ Prednisone 8mg/day; taper ongoing SLEDAI-2K: 22 à 8 UPCR (mg/mg): 7.22 à 0.63 Compelling clinical
response since discontinuation of all immunosuppressants, continuing steroid taper *As of Nov 1, 2024 ‡Therapies at Screening: glucocorticoids, anifrolumab, voclosporin, mycophenolate mofetil, hydroxychloroquine ANI, anifrolumab; dsDNA,
double-stranded DNA; HCQ, hydroxychloroquine; LN, lupus nephritis; MMF, mycophenolate mofetil; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; UPCR, urinary protein-to-creatinine ratio; VOC, voclosporin Cabaletta Bio: Data on
file.
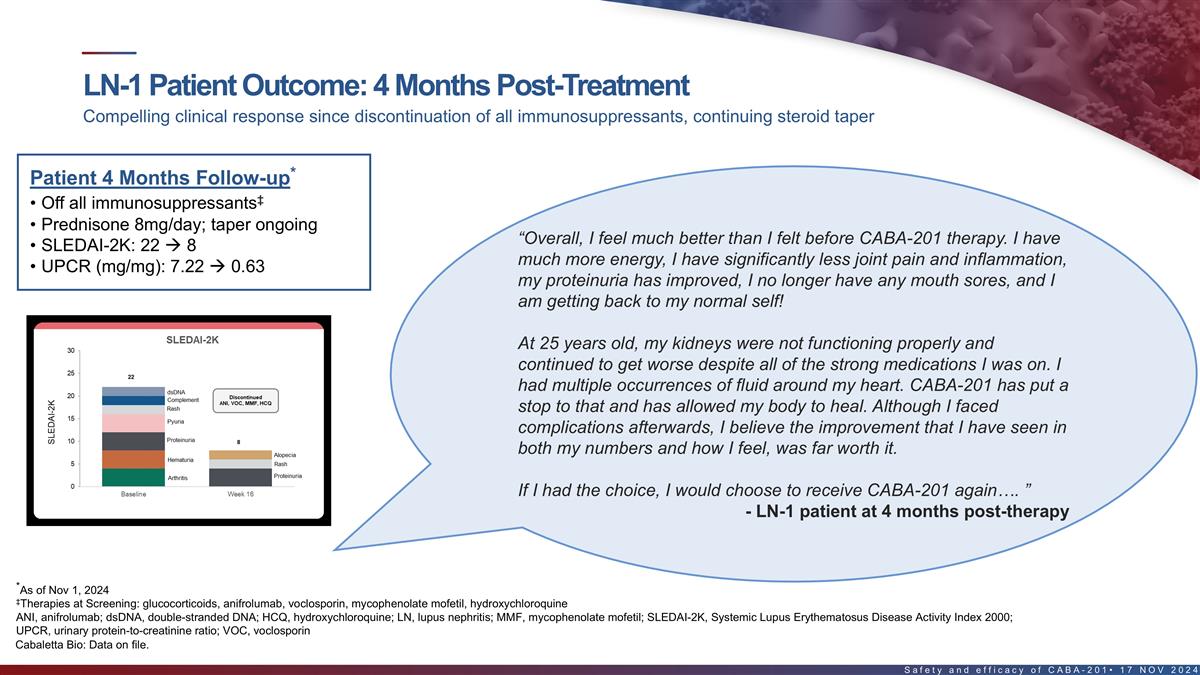
Safety and efficacy of
CABA-201• 17 NOV 2024 LN-1 Patient Outcome: 4 Months Post-Treatment “Overall, I feel much better than I felt before CABA-201 therapy. I have much more energy, I have significantly less joint pain and inflammation, my proteinuria has
improved, I no longer have any mouth sores, and I am getting back to my normal self! At 25 years old, my kidneys were not functioning properly and continued to get worse despite all of the strong medications I was on. I had multiple occurrences of
fluid around my heart. CABA-201 has put a stop to that and has allowed my body to heal. Although I faced complications afterwards, I believe the improvement that I have seen in both my numbers and how I feel, was far worth it. If I had the choice, I
would choose to receive CABA-201 again…. ” - LN-1 patient at 4 months post-therapy Patient 4 Months Follow-up* Off all immunosuppressants‡ Prednisone 8mg/day; taper ongoing SLEDAI-2K: 22 à 8 UPCR (mg/mg): 7.22 à 0.63
Compelling clinical response since discontinuation of all immunosuppressants, continuing steroid taper *As of Nov 1, 2024 ‡Therapies at Screening: glucocorticoids, anifrolumab, voclosporin, mycophenolate mofetil, hydroxychloroquine ANI,
anifrolumab; dsDNA, double-stranded DNA; HCQ, hydroxychloroquine; LN, lupus nephritis; MMF, mycophenolate mofetil; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; UPCR, urinary protein-to-creatinine ratio; VOC, voclosporin
Cabaletta Bio: Data on file.
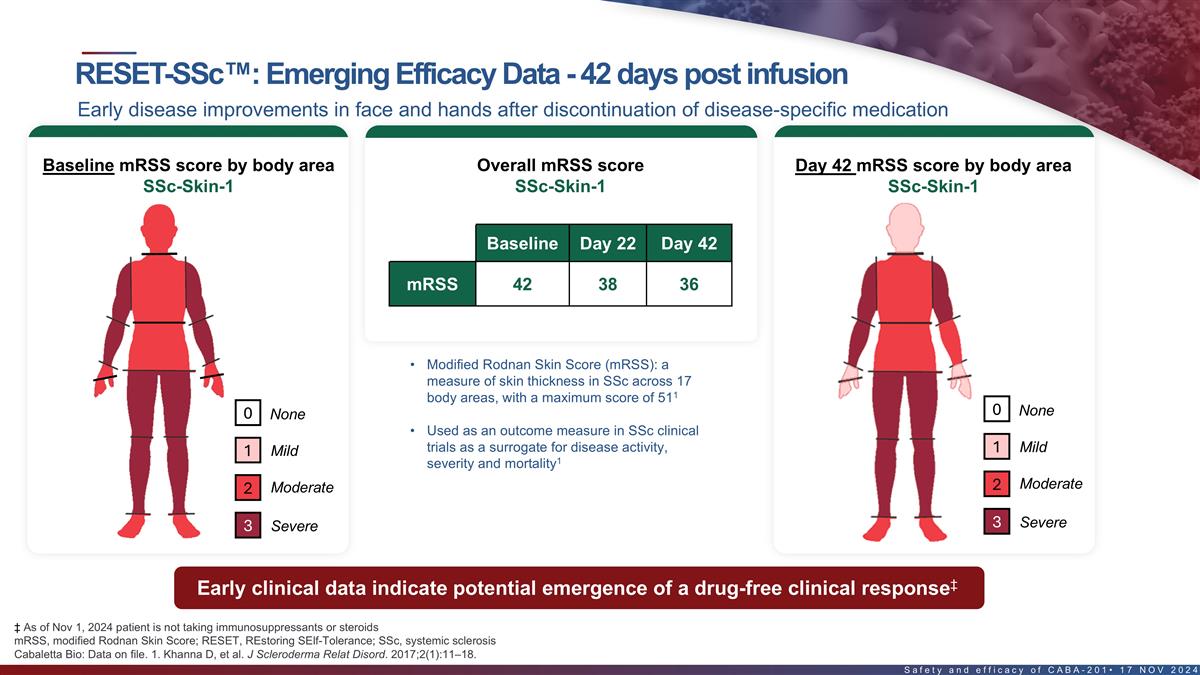
RESET-SSc™: Emerging Efficacy
Data - 42 days post infusion ‡ As of Nov 1, 2024 patient is not taking immunosuppressants or steroids mRSS, modified Rodnan Skin Score; RESET, REstoring SElf-Tolerance; SSc, systemic sclerosis Cabaletta Bio: Data on file. 1. Khanna D, et al. J
Scleroderma Relat Disord. 2017;2(1):11–18. Safety and efficacy of CABA-201• 17 NOV 2024 Early clinical data indicate potential emergence of a drug-free clinical response‡ Baseline Day 22 Day 42 mRSS 42 38 36 Overall mRSS score
SSc-Skin-1 Baseline mRSS score by body area SSc-Skin-1 0 1 2 3 None Mild Moderate Severe Modified Rodnan Skin Score (mRSS): a measure of skin thickness in SSc across 17 body areas, with a maximum score of 511 Used as an outcome measure in SSc
clinical trials as a surrogate for disease activity, severity and mortality1 0 1 2 3 None Mild Moderate Severe Day 42 mRSS score by body area SSc-Skin-1 Early disease improvements in face and hands after discontinuation of disease-specific
medication
Summary from Clinical and
Translational Data on the First 8 Patients Safety and efficacy of CABA-201• 17 NOV 2024 CABA-201 appears to have a favorable risk-benefit profile In patients with recent fever or infections, delaying CAR T infusion should be considered
CABA-201 provided compelling efficacy in highly active and refractory autoimmune patients through the follow-up period Initial data support the potential for drug-free clinical responses All patients discontinued all immunosuppressants SLE patients
with longer follow-up: steroid taper completed or ongoing (prednisone 8mg/day) The PK/PD data support the current dose of CABA-2011 CAR, chimeric antigen receptor; PK, pharmacokinetic; PD, pharmacodynamic; SLE, systemic lupus erythematosus 1. Nunez
et al. Correlative Studies of CABA-201 from the RESET-MyositisTM and RESET-SLETM Clinical Trials. Presented at ACR Convergence 2024. Abstract 0324

Patients and caregivers involved in
the RESET™ clinical program Site investigators and staff involved with these patients from the RESET™ clinical program Mayo Clinic, Rochester University of California, Davis University of California, Irvine University of Michigan
University of North Carolina Cabaletta Bio team Biostatistics Clinical Development Clinical Operations Computational Biology Manufacturing Medical Affairs Translational Medicine Acknowledgements Safety and efficacy of CABA-201• 17 NOV
2024
v3.24.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Cabaletta Bio (NASDAQ:CABA)
Historical Stock Chart
From Jan 2025 to Feb 2025
Cabaletta Bio (NASDAQ:CABA)
Historical Stock Chart
From Feb 2024 to Feb 2025