As filed with the Securities and Exchange Commission
on December 20, 2024
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM F-3
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
CASI Pharmaceuticals, Inc.
(Exact Name of Registrant as Specified in Its
Charter)
| Cayman
Islands |
|
Not
Applicable |
(State or other jurisdiction of
incorporation or organization) |
|
(I.R.S. Employer
Identification Number) |
1701-1702, China Central Office Tower 1
No. 81 Jianguo Road Chaoyang District
Beijing, 100025
People’s Republic of China
+86 (10) 6508 6063
(Address and telephone number of Registrant’s
principal executive offices)
Rui Zhang
VP of Finance and Operations
CASI Pharmaceuticals, Inc.
9620 Medical Center Drive, Suite 300
Rockville, MD 20850
240-864-2600
(Name, address and telephone number of agent
for service)
Copies of all communications, including
communications sent to agent for service, should be sent to:
Copies to:
Alexander R. McClean, Esq.
C. Christopher Murillo, Esq.
Harter Secrest & Emery LLP
1600 Bausch & Lomb Place
Rochester, NY 14604
Tel: (585) 232-6500
Fax: (585) 232-2152
Approximate
date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement.
If
only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check
the following box. ¨
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415
under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional
securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities
Act registration statement number of the earlier effective registration statement for the same offering. ¨
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box
and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If
this Form is a registration statement pursuant to General Instruction I.C. or a post-effective amendment thereto that shall become
effective upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ¨
If
this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.C. filed to register
additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following
box. ¨
Indicate by check mark whether the registrant is an emerging growth
company as defined in Rule 405 of the Securities Act of 1933.
Emerging
growth company ¨
If
an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant
has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided
pursuant to Section 7(a)(2)(B) of the Securities Act. ¨
† The term “new or revised financial
accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification
after April 5, 2012.
Pursuant to the provisions of Rule 429 under
the Securities Act, the prospectus contained in this registration statement also relates to the Registrant’s registration
statement on Form F-3 (File No. 333-279096). Upon effectiveness, this registration statement will also act as a post-effective
amendment to such earlier registration statement.
EXPLANATORY NOTE
On May 3, 2024, CASI
Pharmaceuticals, Inc., or the Company filed a registration statement, or the Prior Registration Statement, on Form F-3 (File
No. 333-279096) with the U.S. Securities and Exchange Commission, or the SEC, related to the offer and sale of up to an aggregate
of $50.0 million of any combination of the securities described in the prospectus relating to the Prior Registration Statement, or the
Prior Securities. The Prior Registration Statement was subsequently declared effective on May 10, 2024. Pursuant to Rule 429
under the Securities Act of 1933, as amended, or the Securities Act, this registration statement, which is a new registration statement,
combines the Prior Securities from the Prior Registration Statement, all of which remain unissued, with the additional securities registered
hereby for offer and sale by the Company, to enable the offer and sale of up to an aggregate of $200.0 million of the Company’s
ordinary shares, preferred shares, warrants, subscription rights, and/or units, from time to time in one or more offerings, pursuant
to a combined base prospectus. Pursuant to Rule 429 under the
Securities Act, this F-3 Registration Statement also constitutes a post-effective amendment to the Prior Registration Statement, and
such post-effective amendment shall hereafter become effective concurrently with the effectiveness of this F-3 Registration Statement
in accordance with Section 8(c) of the Securities Act.
Additionally, this registration
statement contains a Sales Agreement prospectus covering the offering, issuance and sale by the Company of up to $50.0 million of the Company’s
ordinary shares that may be issued and sold from time to time under an Open Market Sale AgreementSM the Company has entered
into with Jefferies LLC, as sales agent, or the Sales Agreement.
The combined base prospectus
relating to the offer and sale of up to $200.0 million of the Company’s ordinary shares, preferred shares, warrants, subscription
rights, and/or units immediately follows this explanatory note. The specific terms of any other securities to be offered pursuant to
the combined base prospectus will be specified in one or more prospectus supplements to the combined base prospectus.
The Sales Agreement prospectus
immediately follows the combined base prospectus. The $50.0 million of the Company’s ordinary shares that may be offered, issued
and sold from time to time under the Sales Agreement prospectus is included in the $200.0 million of securities that may be offered, issued
and sold by the Company under the combined base prospectus. Upon termination of the Sales Agreement, any portion of the $50.0 million
included in the Sales Agreement prospectus that is not sold pursuant to the Sales Agreement will be available for sale in other offerings
pursuant to the combined base prospectus and a corresponding prospectus supplement, and if no shares are sold under the Sales Agreement,
the full $200.0 million of securities may be sold in other offerings pursuant to the combined base prospectus and a corresponding prospectus
supplement.
The information in this prospectus
is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange
Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting offers to buy these securities
in any jurisdiction where such offer or sale is not permitted.
Subject to Completion, dated
December 20, 2024
PROSPECTUS
$200,000,000

Ordinary Shares
Preferred Shares
Warrants
Subscription Rights
Units
We may offer and sell from
time to time, in one or more offerings, up to an aggregate of $200.0 million of any combination of the securities described in this prospectus,
which are referred to as the securities. We may also offer securities as may be issuable upon conversion, redemption, repurchase, exchange
or exercise of the securities, including any applicable anti-dilution provisions.
We may offer and sell any
of the securities described in this prospectus in different series, at times, in amounts, at prices and on terms to be determined at
or prior to the time of each offering. This prospectus describes the general terms of these securities and the general manner in which
these securities will be offered. We will provide the specific terms of these securities and the specific manner in which these securities
will be offered in supplements to this prospectus. You should read this prospectus, the applicable prospectus supplement and any related
free writing prospectus, as well as any documents incorporated by reference, before you invest.
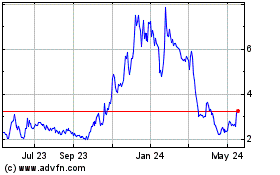
Our ordinary shares are traded
on The Nasdaq Capital Market under the symbol “CASI.” On December 18, 2024, the last sale price of our ordinary shares
as reported by The Nasdaq Capital Market was $2.86 per share. The applicable prospectus supplement will contain information, where applicable,
as to other listings, if any, on The Nasdaq Capital Market or other securities exchange of the securities covered by the applicable prospectus
supplement. Prospective purchasers of the securities are urged to obtain current information as to the market prices of the securities,
where applicable.
Investing in our securities involves a high
degree of risk. We may be subject to various legal and operational risks as a result of doing business in the PRC, risks relating to
our auditor, risks relating to cash and asset transfers among CASI and its subsidiaries, and risks relating to permission and filing
procedures required from the governmental authorities of the PRC with respect to the operation of our PRC subsidiaries and future offerings
in the United States. You should carefully review the risks and uncertainties described under the section titled “Risk Factors”
on page 8 of this prospectus and, if applicable, any risk factors described in any applicable prospectus supplement and in our filings
with the U.S. Securities and Exchange Commission, or SEC, that are incorporated by reference in this prospectus.
This prospectus may not be
used to consummate a sale of securities unless it is accompanied by a prospectus supplement.
The securities may be sold
directly by us to investors, through agents designated from time to time or to or through underwriters or dealers, on a continuous or
delayed basis. For additional information on the methods of sale, you should refer to the section titled “Plan of Distribution”
in this prospectus. If any agents or underwriters are involved in the sale of the securities with respect to which this prospectus is
being delivered, the names of such agents or underwriters will be set forth in a prospectus supplement. The price to the public of such
securities and the net proceeds we expect to receive from such sale will also be set forth in a prospectus supplement.
We are a “foreign private
issuer” as defined in Rule 405 under the Securities Act of 1933, as amended, and, as such, we have elected to comply with
certain reduced public company reporting requirements for this prospectus and future filings. Please see the section titled “Implications
of Being a Foreign Private Issuer” in this prospectus.
Neither the SEC nor any
state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete.
Any representation to the contrary is a criminal offense.
The date of this prospectus is
, 2024.
INTRODUCTORY COMMENTS
We are not a Chinese operating
company but a Cayman Islands holding company with business operations primarily conducted by our Chinese subsidiaries. This holding company
structure and our operation in China may involve risks. We currently conduct our business through the following consolidated subsidiaries:
CASI Pharmaceuticals (China) Co., Ltd., referred to as CASI China; CASI Pharmaceuticals (Wuxi) Co., Ltd., referred to as CASI
Wuxi; CASI Biopharmaceuticals (WUXI) Co., Ltd, referred to as CASI Biopharmaceuticals; and CASI Pharmaceuticals Co., Limited, referred
to as CASI Hong Kong.
Risks and
Uncertainties Relating to Doing Business in China
We face various risks and
uncertainties related to doing business in China. Our business operations are primarily conducted in China, and we are subject to complex
and evolving PRC laws and regulations. The PRC government’s significant authority in regulating our operations and its oversight
and control over offerings conducted overseas by, and foreign investment in, China-based issuers could significantly limit or completely
hinder our ability to offer or continue to offer securities to investors and cause the value of our securities to significantly decline
or become worthless. Implementation of industry-wide regulations, including data security or anti-monopoly related regulations, could
result in a material change in our operations and may cause the value of our securities to significantly decline or become worthless.
Risks and uncertainties arising from the legal system in China, including risks and uncertainties regarding the enforcement of laws and
quickly evolving rules and regulations in China, could result in a material adverse change in our operations and the value of our
ordinary shares. For example, China’s government has in recent years issued statements and regulatory actions to regulate certain
market players or to improve its supervision of the market in general, such as those related to data security or anti-monopoly concerns.
While we currently do not believe such regulatory actions have materially impacted our business operations, our ability to accept foreign
investments, or our ability to maintain listing with the Nasdaq Stock Market, there is no assurance that any new rules or regulations
promulgated in the future will not impose additional requirements on us. If any such rules or regulations are adopted, we may be
subject to more stringent regulatory scrutiny for our operation and financing efforts, which may in turn result in us incurring additional
compliance costs and expenses, delay our investment and financing activities, or otherwise impact our ability to conduct our business,
accept foreign investments, or list on a U.S. or other foreign exchange.
In addition to our existing
operations in China, we conduct clinical development of certain of our product candidates outside of China. In connection with these
efforts we have licensed the exclusive worldwide rights to certain product candidates, including CID-103, which rights are held by us
outside of China.
Risks Relating to Our Auditor
Our auditor, the independent
registered public accounting firm that issues the audit report contained in our annual report, as an auditor of companies that are traded
publicly in the United States and a firm registered with the Public Company Accounting Oversight Board, or PCAOB, is subject to laws
in the United States pursuant to which the PCAOB conducts regular inspections to assess its compliance with applicable professional standards.
Our auditor is located in mainland China, a jurisdiction where the PCAOB was historically unable to conduct inspections and investigations
completely before 2022. As a result, we and investors in CASI Pharmaceuticals, Inc., a Delaware corporation, our predecessor prior
to the redomicile merger, referred to as CASI Delaware were deprived of the benefits of such PCAOB inspections. Pursuant to the Holding
Foreign Companies Accountable Act, or the HFCAA, if the SEC determines that we have filed audit reports issued by a registered public
accounting firm that has not been subject to inspections by the PCAOB for two consecutive years, the SEC will prohibit our securities
from being traded on a national securities exchange or in the over-the-counter trading market in the United States.
On December 16, 2021,
the PCAOB issued a report to notify the SEC of its determination that the PCAOB was unable to inspect or investigate completely registered
public accounting firms headquartered in mainland China and Hong Kong and CASI Delaware’s auditor was subject to that determination.
In April 2022, the SEC conclusively listed CASI Delaware as a Commission-Identified Issuer under the HFCAA following the filing
of its annual report on Form 10-K for the fiscal year ended December 31, 2021. On December 15, 2022, the PCAOB issued
a report that vacated its December 16, 2021 determination and removed mainland China and Hong Kong from the list of jurisdictions
where it is unable to inspect or investigate completely registered public accounting firms. For this reason, we do not expect we will
be identified as a Commission-Identified Issuer under the HFCAA for the fiscal year ended December 31, 2024.
Each year in the future,
the PCAOB will determine whether it can inspect and investigate completely audit firms in mainland China and Hong Kong, among other jurisdictions.
If the PCAOB determines in the future that it no longer has full access to inspect and investigate completely accounting firms in mainland
China and Hong Kong and we use an accounting firm headquartered in one of these jurisdictions to issue an audit report on our financial
statements filed with the SEC, we would be identified as a Commission-Identified Issuer following the filing of the annual report for
the relevant fiscal year. In accordance with the HFCAA, our ordinary shares would be prohibited from being traded on a national securities
exchange or in the over-the-counter trading market in the United States if we are identified as a Commission-Identified Issuer for two
consecutive years in the future. If our ordinary shares are prohibited from trading in the United States, there is no certainty that
we will be able to list on a non-U.S. exchange or that a market for our ordinary shares will develop outside of the United States. A
prohibition of being able to trade in the United States would substantially impair your ability to sell or purchase our ordinary shares
when you wish to do so, and the risk and uncertainty associated with delisting would have a negative impact on the price of such shares.
Also, such a prohibition would significantly affect our ability to raise capital on terms acceptable to us, or at all, which would have
a material adverse impact on our business, financial condition, and prospects.
Cash and Asset Transfer
among CASI and its Subsidiaries
We provide funding to our
subsidiaries from time to time through capital contributions or loans, subject to satisfaction of applicable government registration
and approval requirements. For the year ended December 31, 2023, we provided funding of US$1.0 million through capital contributions
to CASI Hong Kong, our newly incorporated Hong Kong subsidiary.
Our subsidiaries may pay
dividends and make other distributions to us subject to satisfaction of applicable government filing and approval requirements. Such
dividend or other distributions may be subject to limitations and certain tax consequences, a discussion on which is set forth below.
For the year ended December 31, 2023, no dividends or other distributions were made by our subsidiaries.
We also pay service fees
to our PRC subsidiaries pursuant to certain sales support service agreements and research and development support service agreements.
For the year ended December 31 2023, we paid service fees of US$1.1 million to CASI China, one of our PRC subsidiaries. Under PRC
tax laws and regulations, the earnings of our subsidiaries under such agreements are subject to a statutory tax rate of 25%.
In the year ended December 31,
2023, no assets other than cash were transferred through our organization.
All cash transfers among
us and our subsidiaries have been eliminated in our consolidated statement of cash flows.
The existing PRC foreign
exchange regulations may limit our ability to initiate and complete cash transfers within our group. Approval from the State Administration
of Foreign Exchange, or SAFE, and the People’s Bank of China, or PBOC, may be required where RMB are to be converted into foreign
currencies, including U.S. dollars, and approval from SAFE and PBOC or their branches may be required where RMB are to be remitted out
of China.
We have never declared or
paid dividends on our ordinary shares or any other securities and we do not anticipate paying any dividends on our ordinary shares in
the foreseeable future. We may rely on dividends from our subsidiaries in China to pay dividend and other distributions on our ordinary
shares. PRC regulations may restrict the ability of our PRC subsidiaries to pay dividends to us. In addition to applicable foreign exchange
limitations, under the current regulatory regime in China, a PRC company may pay dividends only out of its accumulated profit, if any,
determined in accordance with PRC accounting standards and regulations, and is required to set aside as general reserves at least 10%
of its after-tax profit, until the cumulative amount of such reserves reaches 50% of its registered capital, prior to any dividend distribution.
In addition, a PRC company shall not distribute any profits in a given year until any losses from prior fiscal years have been offset.
Permission and Filing
Procedures Required from the PRC Authorities with respect to the Operations of Our PRC Subsidiaries and Future offering in the US
As the date hereof, our PRC
subsidiaries have obtained the requisite licenses and permits from the PRC government authorities that are material for our business
operations, including, among others, the Business License, the Drug Distribution License, the Drug Manufacturing Permit, the Clinical
Trial Application with the PRC National Medical Products Administration, or NMPA, and the notification filing for international collaborative
clinical trial or the application for international collaborative scientific research with the China Human Genetic Resources Administrative
Office, or HGRAO. We also work with our business partners which have obtained the requisite licenses and permits for their business collaboration
with us, including, among others, the Import Drug Registration for product(s) we promote and distribute in China. Given the uncertainties
of interpretation and implementation of relevant laws and regulations and the enforcement practices of the relevant government authorities,
we may be required to obtain additional permissions or approvals for our business operations.
As
the date hereof, we and our PRC subsidiaries (i) except for the requisite CSRC Filing(s) (as defined below), are not
required to obtain permissions from the China Securities Regulatory Commission, or the CSRC, (ii) are not required to go through
cybersecurity review by the Cyberspace Administration of China, or the CAC, and (iii) have not been asked to obtain or were denied
such permissions by applicable PRC authority. On July 7, 2022, the CAC published the Guidelines for Data Export Security Assessment
(《数据出境安全评估办法》),
or the Guidelines, which took effect on September 1, 2022. Pursuant to the Guidelines, the data processor who intends to transfer
certain important data or large volumes of personal information outside of China shall complete a prior CAC-led data outbound transfer
security assessment. For the data we accessed through or obtained from clinical trials, we have complied with the laws and regulations
then-in-effect, and completed the registration with HGRAO, but it is unclear if we will be required to go through the CAC-led or CAC-involved
security assessment or if the current HGRAO registration procedure will be changed in the future. We will closely monitor and review
any regulatory developments and comply with any new approval or license requirement when necessary. If (i) we have erroneously concluded
that such permissions or approvals are not required, or (ii) applicable laws, regulations, or interpretations change and we are
required to obtain such permissions or approvals in the future, we may have to expend significant time and costs to procure them. If
we are unable to do so, on commercially reasonable terms, in a timely manner or otherwise, we may become subject to sanctions imposed
by the PRC regulatory authorities, which could include fines and penalties, proceedings against us, and other forms of sanctions, and
our ability to conduct our business, invest into China as foreign investments or accept foreign investments, or be listed on a U.S. or
other overseas exchange may be restricted, and our business, reputation, financial condition, and results of operations may be materially
and adversely affected.
On
February 17, 2023, the CSRC released the Trial Administrative Measures of the Overseas Securities Offering and Listing by Domestic
Companies (《境内企业境外发行证券和上市管理试行办法》)
and five ancillary interpretive guidelines, collectively, the Overseas Listing Trial Measures, as amended, supplemented or otherwise
modified from time to time, which apply to overseas offerings and listing by PRC-based companies, or domestic companies, of equity shares,
depository receipts, corporate bonds convertible to equity shares, and other equity securities, and came into effect on March 31,
2023. According to the Overseas Listing Trial Measures, (1) domestic companies that seek to offer or list securities overseas, both
directly and indirectly, should fulfill the filing procedure and report relevant information to the CSRC, and if an overseas-listed PRC-based
issuer issues new securities in the same overseas market after the overseas offering and listing, it is also required to file with the
CSRC within three business days after the completion of the issuance, or the CSRC Filing; if a domestic company fails to complete the
filing procedure or conceals any material fact or falsifies any major content in its filing documents, such domestic company may be subject
to administrative penalties, such as order to rectify, warnings, fines, and its controlling shareholders, actual controllers, the person
directly in charge and other directly liable persons may also be subject to administrative penalties, such as warnings and fines; (2) if
a foreign-incorporated issuer meets both of the following conditions, its overseas offering and listing shall be determined as an indirect
overseas offering and listing by a domestic company of the PRC: (i) any of the total assets, net assets, revenues or profits of
the domestic operating entities of the issuer in the most recent accounting year accounts for more than 50% of the corresponding line
items in the issuer’s audited consolidated financial statements for the same period; and (ii) its major operational activities
are carried out in China or its main places of business are located in China, or the senior managers in charge of operation and management
of the issuer are mostly Chinese citizens or are domiciled in China; in addition to the aforementioned conditions, the determination
of an indirect overseas offering and listing by a domestic enterprise adheres to the principle of substance over form; and (3) where
a domestic company seeks to indirectly offer and list securities in an overseas market (including issuance of new securities after its
overseas offering and listing), the issuer shall designate a major domestic operating entity responsible for all filing procedures with
the CSRC.
Furthermore, in case any
of the following major events occurs after the overseas offering and listing, the issuer is also required to report the relevant information
to the CSRC within three business days of the occurrence and the announcement of the relevant events: (1) change of control; (2) the
foreign securities regulatory body or the relevant competent authority has taken such measures as investigation and punishment; (3) conversion
of listing status or listing board; and (4) voluntary of compulsory termination of listing. Where there is any material change in
the major business and operation of the issuer after overseas offering and listing, and such change does not fall within the scope of
filing, the issuer shall, within three business days of the occurrence of such change, submit a special report and a legal opinion issued
by a domestic law firm to the CSRC to explain the relevant situation.
As substantially all of our
operations are currently based in the PRC, our future offerings and major changes shall be subject to the filing procedures under the
Overseas Listing Trial Measures. We cannot assure you that we can meet such requirements, obtain the requisite permits from the relevant
government authorities, or complete such filing in a timely manner or at all. Any failure may significantly limit or completely hinder
our ability to continue to offer securities to investors and cause the value of our securities to significantly decline or be worthless.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is a part
of a registration statement that we filed with the U.S. Securities and Exchange Commission, or the SEC, using a “shelf” registration
process under the Securities Act of 1933, as amended, or the Securities Act. Under this shelf registration process, we may, from time
to time, sell up to an aggregate of $200.0 million of the securities described in this prospectus, either individually or in combination
with the other securities. This prospectus provides you with a general description of the securities that may be offered by us. Each
time we sell any type or series of securities, we will provide you a prospectus supplement accompanied by this prospectus. The prospectus
supplement will contain more specific information about the nature of the persons offering securities and the terms the securities being
offered at that time. We may also authorize one or more free writing prospectuses to be provided to you that may contain material information
relating to these offerings. The prospectus supplement and any related free writing prospectus that we may authorize to be provided to
you may also add, update or change any of the information contained in this prospectus or in the documents we have incorporated by reference
into this prospectus. To the extent that any statement that we make in a prospectus supplement is inconsistent with statements made in
this prospectus, the statements made in this prospectus will be deemed modified or superseded by those made in a prospectus supplement.
You should carefully read both this prospectus and the applicable prospectus supplement and any related free writing prospectus, together
with the additional information described under “Where You Can Find More Information” and “Information Incorporated
by Reference,” before buying any of the securities being offered.
To the extent there is a
conflict between the information contained in this prospectus, on the one hand, and the information contained in any prospectus supplement,
any free writing prospectus or in any document incorporated by reference in this prospectus, on the other hand, you should rely on the
information in this prospectus, provided that if any statement in one of these documents is inconsistent with a statement in another
document having a later date—for example, a prospectus supplement or a document incorporated by reference in this prospectus—the
statement in the document having the later date modifies or supersedes the earlier statement.
The information contained
in this prospectus, the applicable prospectus supplement, any applicable free writing prospectus or any document incorporated by reference
herein or therein is accurate only as of such documents’ respective dates, regardless of the time of delivery of this prospectus,
the applicable prospectus supplement, any applicable free writing prospectus or the documents incorporated by reference in this prospectus
or the sale of any securities. Our business, financial condition, results of operations and prospects may have changed materially since
those dates.
This prospectus may not be
used to consummate a sale of securities unless it is accompanied by a prospectus supplement.
Neither we, nor any agent,
underwriter or dealer has authorized any person to give any information or to make any representation other than those contained or incorporated
by reference in this prospectus, any applicable prospectus supplement or any related free writing prospectus prepared by or on behalf
of us or to which we have referred you. If anyone provides you with different or inconsistent information, you should not rely on it.
This prospectus, any applicable supplement to this prospectus or any related free writing prospectus does not constitute an offer to
sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor does this prospectus,
any applicable supplement to this prospectus or any related free writing prospectus constitute an offer to sell or the solicitation of
an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction.
You should not assume that
the information contained in this prospectus, any applicable prospectus supplement or any related free writing prospectus is accurate
on any date subsequent to the date set forth on the front of the document or that any information we have incorporated by reference is
correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus, any applicable prospectus
supplement or any related free writing prospectus is delivered, or securities are sold, on a later date.
This prospectus contains
summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for
complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred
to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this
prospectus is a part, and you may obtain copies of those documents as described below under the section entitled “Where You
Can Find More Information.”
For investors outside the
United States, neither we nor any underwriters, dealers or agents have taken any action that would permit the offering or possession
or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons
outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating
to, the offering of the securities described herein and the distribution of this prospectus outside the United States.
Throughout this prospectus,
references to the “Company,” “we,” “our,” “us,” “registrant,” “CASI”
or similar terms used in this prospectus refer to CASI Pharmaceuticals, Inc., an exempted company with limited liability under the
laws of the Cayman Islands, including its consolidated subsidiaries, unless the context otherwise indicates.
“PRC” or “China”
refers to the People’s Republic of China, excluding, for the purpose of this prospectus, Taiwan, Hong Kong and Macau, “RMB”
or “Renminbi” refers to the legal currency of China, and “$”, “US$” or “U.S. Dollars”
refers to the legal currency of the United States.
This prospectus may contain
translations of Renminbi amounts into U.S. dollars at specified rates solely for the convenience of the reader. We make no representation
that the Renminbi or U.S. dollar amounts referred to in this prospectus could have been or could be converted into U.S. dollars or Renminbi,
as the case may be, at any particular rate or at all.
INDUSTRY AND MARKET DATA
In this prospectus and the
documents incorporated by reference in this prospectus, we present industry data, information and statistics regarding the markets in
which the Company and its subsidiaries compete as well as publicly available information, industry and general publications and research
and studies conducted by third parties. This information is supplemented where necessary with the Company’s own internal estimates
and information obtained from discussions with its customers, taking into account publicly available information about other industry
participants and the Company’s management’s judgment where information is not publicly available.
Industry publications, research,
studies and forecasts generally state that the information they contain has been obtained from sources believed to be reliable, but that
the accuracy and completeness of such information is not guaranteed. Forecasts and other forward-looking information obtained from these
sources are subject to the same qualifications and uncertainties as the other forward-looking statements in this prospectus or any document
incorporated by reference into this prospectus. These forecasts and forward-looking information are subject to uncertainty and risk due
to a variety of factors, including those described under the section entitled “Risk Factors.” These and other factors
could cause results to differ materially from those expressed in any forecasts or estimates.
TRADEMARKS
AND TRADENAMES
This prospectus and the
information incorporated herein by reference include trademarks, service marks and trade names owned by us or other companies. All trademarks,
service marks and trade names included or incorporated by reference into this prospectus, any applicable prospectus supplement or any
related free writing prospectus are the property of their respective owners.
PROSPECTUS SUMMARY
This
summary highlights selected information contained elsewhere in this prospectus or incorporated by reference in this prospectus, and does
not contain all of the information that you need to consider in making your investment decision. You should carefully read the entire
prospectus, the applicable prospectus supplement and any related free writing prospectus, including the risks of investing in our securities
discussed under the section titled “Risk Factors” contained in this prospectus, the applicable prospectus supplement and
any related free writing prospectus, and under similar sections in the other documents that are incorporated by reference into this prospectus.
You should also carefully read the other information incorporated by reference into this prospectus, including our consolidated and condensed
consolidated financial statements, and the exhibits to the registration statement of which this prospectus is a part.
Overview
We are a biopharmaceutical
company focused on developing and commercializing innovative therapeutics and pharmaceutical products in China, the United States, and
throughout the world.
Holding Company Structure
CASI is not a Chinese operating
company but a Cayman Islands holding company with a significant portion of the business operations expected to be conducted by its Chinese
subsidiaries. This holding company structure and our operation in China may involve risks. We currently conduct our business through
the following consolidated subsidiaries:
| · | CASI
Pharmaceuticals (China) Co., Ltd., referred to as CASI China; |
| · | CASI
Pharmaceuticals (Wuxi) Co., Ltd., referred to as CASI Wuxi; |
| · | CASI
Biopharmaceuticals (WUXI) Co., Ltd, referred to as CASI Biopharmaceuticals; and |
| · | CASI
Pharmaceuticals Co., Limited, referred to as CASI Hong Kong. |
We do not
have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages
in leasing, hedging or product development services with us. The organizational chart of CASI as of December 18, 2024 is set forth
below:
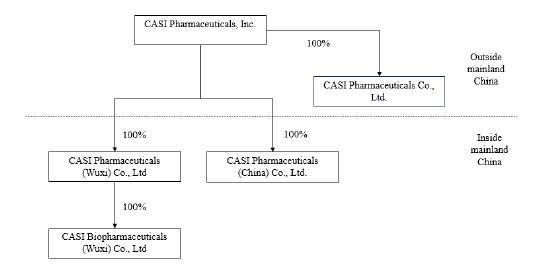
Note: Currently CASI Hong Kong has no meaningful operations.
In addition to our existing
operations in China, we conduct clinical development of certain of our product candidates outside of China. In connection with these
efforts we have licensed the exclusive worldwide rights to certain product candidates, including CID-103, which rights are held by us
outside of China.
Nasdaq Capital Market Listing; Redomiciliation
Our ordinary shares are traded
on The Nasdaq Capital Market under the symbol “CASI.” In March 2023, we completed a redomicile merger, with CASI surviving
the merger as the surviving company and successor issuer, and CASI’s ordinary shares continued trading on The Nasdaq Capital Market
under the symbol “CASI.” CASI is treated for U.S. federal income tax purposes as a U.S. corporation, including with respect
to any dividends paid by it, which dividends may be subject to U.S. withholding taxes.
Corporate Information
Our principal executive offices
are located at 1701-1702, China Central Office Tower 1, No. 81 Jianguo Road Chaoyang District, Beijing, 100025, People’s Republic
of China. Our telephone number at this address is +86 (10) 6508 6063. Our registered office in the Cayman Islands is located at
Maples Corporate Services Limited, P.O. Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands.
Our agent for service of
process in the United States is located at 9620 Medical Center Drive, Suite 300, Rockville, MD 20850, 240-864-2600.
Implications of Being a Foreign Private Issuer
As a “foreign private
issuer,” CASI is subject to different U.S. securities laws than domestic U.S. issuers. The rules governing the information
that CASI must disclose differ from those governing U.S. corporations pursuant to the Securities Exchange Act of 1934, as amended, or
the Exchange Act. Because we qualify as a foreign private issuer under the Exchange Act, we are exempt from certain provisions of the
securities rules and regulations in the United States that are applicable to U.S. domestic issuers, including:
| · | the
rules under the Exchange Act requiring the filing with the SEC of quarterly reports
on Form 10-Q or current reports on Form 8-K; |
| · | the
sections of the Exchange Act regulating the solicitation of proxies, consents, or authorizations
in respect of a security registered under the Exchange Act; |
| · | the
sections of the Exchange Act requiring insiders to file public reports of their stock ownership
and trading activities and liability for insiders who profit from trades made in a short
period of time; and |
| · | the
selective disclosure rules by issuers of material nonpublic information under Regulation
FD. |
We are required to file
an annual report on Form 20-F within four months of the end of each fiscal year. In addition, we intend to publish our results on
a quarterly basis as press releases, distributed pursuant to the rules and regulations of the Nasdaq Stock Market. Press releases
relating to financial results and material events will also be furnished to the SEC on Form 6-K. However, the information we are
required to file with or furnish to the SEC will be less extensive and less timely compared to that required to be filed with the SEC
by U.S. domestic issuers. As a result, you may not be afforded the same protections or information that would be made available to you
were you investing in a U.S. domestic issuer.
In addition, as a foreign
private issuer, CASI’s officers and directors and holders of more than 10% of the issued and outstanding ordinary shares are exempt
from the rules under the Exchange Act requiring insiders to report purchases and sales of ordinary shares as well as from Section 16
short swing profit reporting and liability. A company will lose its foreign private issuer status if more than 50% of its outstanding
voting securities are owned by U.S. residents and any of the following three circumstances applies: (i) the majority of its executive
officers or directors are U.S. citizens or residents, (ii) more than 50% of its assets are located in the United States or (iii) its
business is administered principally in the United States.
Risks Associated with our Business
Our business is subject to
numerous risks, as described under the heading “Risk Factors” contained in the applicable prospectus supplement and
in any free writing prospectuses we have authorized for use in connection with a specific offering, and under similar headings that are
incorporated by reference into this prospectus, including, without limitation, the further risks discussed below.
Our Recurring Operating
Losses have Raised Substantial Doubt Regarding Our Ability to Continue as a Going Concern.
Our recurring operating losses
raise substantial doubt about our ability to continue as a going concern. Since our inception in 1991, we have incurred significant losses
from operations and, as of June 30, 2024, had incurred an accumulated deficit of $677.3 million. For the six months ended June 30, 2024,
we had a net loss of $16.5 million and a cash outflow for operating activities of $18.1 million. As of June 30, 2024, we had net current
assets of $27.6 million. In addition, we had long term borrowing and non-current dividends payable in a total amount of $19.5 million,
which may become payable on demand if certain events of default occur (including with respect to a certain revenue threshold to be met
by CASI Wuxi in 2024, see Note 9 to our unaudited condensed consolidated financial statements for the six months ended June 30, 2024 included
elsewhere in this prospectus (the “Financial Statements”)). We also entered into an agreement with Precision Autoimmune Therapeutics
Co., Ltd., (“PAT”) and one of the investors of PAT, to purchase 19.8876% equity interest of PAT from the investor. The total
consideration is RMB 28.4 million plus interest and will be paid in three installments. We paid RMB 10.0 million in August 2024. The remaining
two installments will be paid by March 31, 2025 and December 31, 2025, respectively. Therefore, we will require additional liquidity to
continue our operations over the next 12 months. These factors raise substantial doubt about our ability to continue as a going concern
within a reasonable period of time, which is considered to be one year from the issuance date of the unaudited condensed consolidated
financial statements for the six months ended June 30, 2024. Our financial statements included into this prospectus do not include any
adjustments that might result if we are unable to continue as a going concern and, therefore, be required to realize our assets and discharge
our liabilities other than in the normal course of business, which could cause investors to suffer the loss of all or a substantial portion
of their investment. In order to have sufficient cash and cash equivalents to fund our operations in the future, we will need to raise
additional equity or debt capital and cannot provide any assurance that we will be successful in doing so. The perception of our ability
to continue as a going concern may make it more difficult for us to obtain financing for the continuation of our operations and could
result in the loss of confidence by investors, suppliers and employees.
Risks and Uncertainties Relating
to Doing Business in China
We face various risks and
uncertainties related to doing business in China. Our business operations are primarily conducted in China, and we are subject to complex
and evolving PRC laws and regulations. The PRC government’s significant authority in regulating our operations and its oversight
and control over offerings conducted overseas by, and foreign investment in, China-based issuers could significantly limit or completely
hinder our ability to offer or continue to offer securities to investors and cause the value of our securities to significantly decline
or become worthless. Implementation of industry-wide regulations, including data security or anti-monopoly related regulations, could
result in a material change in our operations and may cause the value of our securities to significantly decline or become worthless.
Risks and uncertainties arising from the legal system in China, including risks and uncertainties regarding the enforcement of laws and
quickly evolving rules and regulations in China, could result in a material adverse change in our operations and the value of our
ordinary shares. For example, China’s government has in recent years issued statements and regulatory actions to regulate certain
market players or to improve its supervision of the market in general, such as those related to data security or anti-monopoly concerns.
While we currently do not believe such regulatory actions have materially impacted our business operations, our ability to accept foreign
investments, or our ability to maintain listing with the Nasdaq Stock Market, there is no assurance that any new rules or regulations
promulgated in the future will not impose additional requirements on us. If any such rules or regulations are adopted, we may be
subject to more stringent regulatory scrutiny for our operation and financing efforts, which may in turn result in us incurring additional
compliance costs and expenses, delay our investment and financing activities, or otherwise impact our ability to conduct our business,
accept foreign investments, or list on a U.S. or other foreign exchange.
Risks Relating to Our Auditor
Our auditor, the independent
registered public accounting firm that issues the audit report contained in our annual report, as an auditor of companies that are traded
publicly in the United States and a firm registered with the Public Company Accounting Oversight Board, or PCAOB, is subject to laws
in the United States pursuant to which the PCAOB conducts regular inspections to assess its compliance with applicable professional standards.
Our auditor is located in mainland China, a jurisdiction where the PCAOB was historically unable to conduct inspections and investigations
completely before 2022. As a result, we and investors in CASI Pharmaceuticals, Inc., a Delaware corporation, our predecessor prior
to the redomicile merger, referred to as CASI Delaware were deprived of the benefits of such PCAOB inspections. Pursuant to the Holding
Foreign Companies Accountable Act, or the HFCAA, if the SEC determines that we have filed audit reports issued by a registered public
accounting firm that has not been subject to inspections by the PCAOB for two consecutive years, the SEC will prohibit our securities
from being traded on a national securities exchange or in the over-the-counter trading market in the United States.
On December 16, 2021,
the PCAOB issued a report to notify the SEC of its determination that the PCAOB was unable to inspect or investigate completely registered
public accounting firms headquartered in mainland China and Hong Kong and CASI Delaware’s auditor was subject to that determination.
In April 2022, the SEC conclusively listed CASI Delaware as a Commission-Identified Issuer under the HFCAA following the filing
of its annual report on Form 10-K for the fiscal year ended December 31, 2021. On December 15, 2022, the PCAOB issued
a report that vacated its December 16, 2021 determination and removed mainland China and Hong Kong from the list of jurisdictions
where it is unable to inspect or investigate completely registered public accounting firms. For this reason, we do not expect we will
be identified as a Commission-Identified Issuer under the HFCAA for the fiscal year ended December 31, 2024.
Each year in the future,
the PCAOB will determine whether it can inspect and investigate completely audit firms in mainland China and Hong Kong, among other jurisdictions.
If the PCAOB determines in the future that it no longer has full access to inspect and investigate completely accounting firms in mainland
China and Hong Kong and we use an accounting firm headquartered in one of these jurisdictions to issue an audit report on our financial
statements filed with the SEC, we would be identified as a Commission-Identified Issuer following the filing of the annual report for
the relevant fiscal year. In accordance with the HFCAA, our ordinary shares would be prohibited from being traded on a national securities
exchange or in the over-the-counter trading market in the United States if we are identified as a Commission-Identified Issuer for two
consecutive years in the future. If our ordinary shares are prohibited from trading in the United States, there is no certainty that
we will be able to list on a non-U.S. exchange or that a market for our ordinary shares will develop outside of the United States. A
prohibition of being able to trade in the United States would substantially impair your ability to sell or purchase our ordinary shares
when you wish to do so, and the risk and uncertainty associated with delisting would have a negative impact on the price of such shares.
Also, such a prohibition would significantly affect our ability to raise capital on terms acceptable to us, or at all, which would have
a material adverse impact on our business, financial condition, and prospects.
Cash and Asset Transfer among
the Company and its Subsidiaries
We provide funding to our
subsidiaries from time to time through capital contributions or loans, subject to satisfaction of applicable government registration
and approval requirements. For the year ended December 31, 2023, we provided funding of US$1.0 million through capital contributions
to CASI Hong Kong, our newly incorporated Hong Kong subsidiary.
Our subsidiaries may pay
dividends and make other distributions to us subject to satisfaction of applicable government filing and approval requirements. Such
dividend or other distributions may be subject to limitations and certain tax consequences, a discussion on which is set forth below.
For the year ended December 31, 2023, no dividends or other distributions were made by our subsidiaries.
We also pay service fees
to our PRC subsidiaries pursuant to certain sales support service agreements and research and development support service agreements.
For the year ended December 31 2023, we paid service fees of US$1.1 million to CASI China, one of our PRC subsidiaries. Under PRC
tax laws and regulations, the earnings of our subsidiaries under such agreements are subject to a statutory tax rate of 25%.
In the year ended December 31,
2023, no assets other than cash were transferred through our organization.
All cash transfers among
us and our subsidiaries have been eliminated in our consolidated statement of cash flows.
The existing PRC foreign
exchange regulations may limit our ability to initiate and complete cash transfers within our group. Approval from the State Administration
of Foreign Exchange, or SAFE, and the People’s Bank of China, or PBOC, may be required where RMB are to be converted into foreign
currencies, including U.S. dollars, and approval from SAFE and PBOC or their branches may be required where RMB are to be remitted out
of China.
We have never declared or
paid dividends on our ordinary shares or any other securities and we do not anticipate paying any dividends on our ordinary shares in
the foreseeable future. We may rely on dividends from our subsidiaries in China to pay dividend and other distributions on our ordinary
shares. PRC regulations may restrict the ability of our PRC subsidiaries to pay dividends to us. In addition to applicable foreign exchange
limitations, under the current regulatory regime in China, a PRC company may pay dividends only out of its accumulated profit, if any,
determined in accordance with PRC accounting standards and regulations, and is required to set aside as general reserves at least 10%
of its after-tax profit, until the cumulative amount of such reserves reaches 50% of its registered capital, prior to any dividend distribution.
In addition, a PRC company shall not distribute any profits in a given year until any losses from prior fiscal years have been offset.
Permission and Filing Procedures
Required from the PRC Authorities with Respect to the Operations of Our PRC Subsidiaries and Future Offering in the US
As the date hereof, our PRC
subsidiaries have obtained the requisite licenses and permits from the PRC government authorities that are material for our business
operations, including, among others, the Business License, the Drug Distribution License, the Drug Manufacturing Permit, the Clinical
Trial Application with the PRC National Medical Products Administration, or NMPA, and the notification filing for international collaborative
clinical trial or the application for international collaborative scientific research with the China Human Genetic Resources Administrative
Office, or HGRAO. We also work with our business partners which have obtained the requisite licenses and permits for their business collaboration
with us, including, among others, the Import Drug Registration for product(s) we promote and distribute in China. Given the uncertainties
of interpretation and implementation of relevant laws and regulations and the enforcement practices of the relevant government authorities,
we may be required to obtain additional permissions or approvals for our business operations.
As
the date hereof, we and our PRC subsidiaries (i) except for the requisite CSRC Filing(s) (as defined below), are not
required to obtain permissions from the China Securities Regulatory Commission, or the CSRC, (ii) are not required to go through
cybersecurity review by the Cyberspace Administration of China, or the CAC, and (iii) have not been asked to obtain or were denied
such permissions by applicable PRC authority. On July 7, 2022, the CAC published the Guidelines for Data Export Security Assessment
(《数据出境安全评估办法》),
or the Guidelines, which took effect on September 1, 2022. Pursuant to the Guidelines, the data processor who intends to transfer
certain important data or large volumes of personal information outside of China shall complete a prior CAC-led data outbound transfer
security assessment. For the data we accessed through or obtained from clinical trials, we have complied with the laws and regulations
then-in-effect, and completed the registration with HGRAO, but it is unclear if we will be required to go through the CAC-led or CAC-involved
security assessment or if the current HGRAO registration procedure will be changed in the future. We will closely monitor and review
any regulatory developments and comply with any new approval or license requirement when necessary. If (i) we have erroneously concluded
that such permissions or approvals are not required, or (ii) applicable laws, regulations, or interpretations change and we are
required to obtain such permissions or approvals in the future, we may have to expend significant time and costs to procure them. If
we are unable to do so, on commercially reasonable terms, in a timely manner or otherwise, we may become subject to sanctions imposed
by the PRC regulatory authorities, which could include fines and penalties, proceedings against us, and other forms of sanctions, and
our ability to conduct our business, invest into China as foreign investments or accept foreign investments, or be listed on a U.S. or
other overseas exchange may be restricted, and our business, reputation, financial condition, and results of operations may be materially
and adversely affected.
On
February 17, 2023, the CSRC released the Trial Administrative Measures of the Overseas Securities Offering and Listing by Domestic
Companies (《境内企业境外发行证券和上市管理试行办法》)
and five ancillary interpretive guidelines, collectively, the Overseas Listing Trial Measures, as amended, supplemented or otherwise
modified from time to time, which apply to overseas offerings and listing by PRC-based companies, or domestic companies, of equity shares,
depository receipts, corporate bonds convertible to equity shares, and other equity securities, and came into effect on March 31,
2023. According to the Overseas Listing Trial Measures, (1) domestic companies that seek to offer or list securities overseas, both
directly and indirectly, should fulfill the filing procedure and report relevant information to the CSRC, and if an overseas-listed PRC-based
issuer issues new securities in the same overseas market after the overseas offering and listing, it is also required to file with the
CSRC within three business days after the completion of the issuance, or the CSRC Filing; if a domestic company fails to complete the
filing procedure or conceals any material fact or falsifies any major content in its filing documents, such domestic company may be subject
to administrative penalties, such as order to rectify, warnings, fines, and its controlling shareholders, actual controllers, the person
directly in charge and other directly liable persons may also be subject to administrative penalties, such as warnings and fines; (2) if
a foreign-incorporated issuer meets both of the following conditions, its overseas offering and listing shall be determined as an indirect
overseas offering and listing by a domestic company of the PRC: (i) any of the total assets, net assets, revenues or profits of
the domestic operating entities of the issuer in the most recent accounting year accounts for more than 50% of the corresponding line
items in the issuer’s audited consolidated financial statements for the same period; and (ii) its major operational activities
are carried out in China or its main places of business are located in China, or the senior managers in charge of operation and management
of the issuer are mostly Chinese citizens or are domiciled in China; in addition to the aforementioned conditions, the determination
of an indirect overseas offering and listing by a domestic enterprise adheres to the principle of substance over form; and (3) where
a domestic company seeks to indirectly offer and list securities in an overseas market (including issuance of new securities after its
overseas offering and listing), the issuer shall designate a major domestic operating entity responsible for all filing procedures with
the CSRC.
Furthermore, in case any
of the following major events occurs after the overseas offering and listing, the issuer is also required to report the relevant information
to the CSRC within three business days of the occurrence and the announcement of the relevant events: (1) change of control; (2) the
foreign securities regulatory body or the relevant competent authority has taken such measures as investigation and punishment; (3) conversion
of listing status or listing board; and (4) voluntary of compulsory termination of listing. Where there is any material change in
the major business and operation of the issuer after overseas offering and listing, and such change does not fall within the scope of
filing, the issuer shall, within three business days of the occurrence of such change, submit a special report and a legal opinion issued
by a domestic law firm to the CSRC to explain the relevant situation.
As substantially all of our
operations are currently based in the PRC, our future offerings and major changes shall be subject to the filing procedures under the
Overseas Listing Trial Measures. We cannot assure you that we can meet such requirements, obtain the requisite permits from the relevant
government authorities, or complete such filing in a timely manner or at all. Any failure may significantly limit or completely hinder
our ability to continue to offer securities to investors and cause the value of our securities to significantly decline or be worthless.
Recent Developments
In December 2024, we received
a Termination Process Letter from Acrotech of a certain License Agreement (the “License Agreement”), dated September 17, 2014,
between Spectrum Pharmaceuticals, Inc. and us granting the exclusive rights to us to commercialize Evomela® in China, which was later
assigned to Acrotech on March 1, 2019. Acrotech alleged in such letter that we materially breached the License Agreement and failed to
cure such breach, and the License Agreement was therefore terminated. Pursuant to the License Agreement, we can continue to distribute
and sell Evomela® for a reasonable wind-down period not to exceed 24 months, so we do not expect any disruption to our current distribution
plan for Evomela® during such period.
On October 24, 2024,
we announced that the Center for Drug Evaluation of the NMPA has approved our Clinical Trial Application to proceed with a phase 1/2 study
of CID-103 in adult patients with chronic Immune Thrombocytopenia in China. This China study is part of the global study that was approved
by the U.S. Food and Drug Administration, or FDA, in May 2024.
In July 2024, with respect
to our previously announced dispute with Juventas, a PRC court issued an asset freezing order against Juventas in aid of the arbitration
proceedings we initiated at the Hong Kong International Arbitration Centre in connection to Juventas’s purported termination of
the parties’ agreements with respect to the commercialization of CNCT19 (the “Arbitration Proceeding”). In an order
dated July 15, 2024 and received by us on July 18, 2024, the First Intermediate People’s Court of Tianjin Municipality, where Juventas
is headquartered, granted our application to freeze Juventas’s assets while the Arbitration Proceeding is ongoing and decided to
freeze up to RMB 190 million in Juventas’s bank accounts or seize and freeze Juventas’s other assets of equivalent value (the
“Asset Freezing Order”). The Asset Freezing Order took effect upon issuance and the court has taken steps to enforce the Asset
Freezing Order.
In July 2024, we entered into
an agreement with PAT and one of the investors of PAT, to purchase 19.8876% equity interest of PAT from the investor. The total consideration
is RMB 28.4 million plus interest and will be paid in three installments. We paid RMB 10.0 million in August 2024. The remaining two installments
will be paid by March 31, 2025 and December 31, 2025, respectively.
On June 26, 2024, we
entered into Subscription Agreements and Subscription and Purchase Agreements with certain investors including Dr. Wei-Wu He, the
Chairman of the board of directors and Chief Executive Officer of the Company and his family trust. On July 15, 2024, the transaction
contemplated under such agreements closed, pursuant to which we issued 1,020,000 ordinary shares and Warrants to purchase 1,980,000 ordinary
shares to the investors for aggregate gross proceeds of approximately $15.0 million, before deducting placement agent fees and other private
placement expenses.
Our board of directors received
a preliminary non-binding proposal letter, or the Proposal Letter, dated June 21, 2024, from Dr. Wei-Wu He, Chairman of the
Board and CEO of the Company, to acquire the entire business operations of the Company in China and all license-in, distribution and related
rights in Asia (excluding Japan) related to all of our pipeline products, including but not limited to EVOMELA®, FOLOTYN®, CNCT19,
BI-1206, CB-5339,CID-103 and Thiotepa, for an aggregate purchase price of $40.0 million, which shall include assumption of up to $20.0
million of indebtedness of the Company, or the Proposed Transaction. On June 25, 2024, our board of directors formed a special committee
comprised solely of incumbent independent directors, or the Special Committee, to evaluate the transaction contemplated under the Proposal
Letter and such other strategic and business alternatives available to us in respect of the our business operations in China. As of the
date hereof, no decisions have been made by the Special Committee with respect to the Proposed Transaction or any alternative strategic
option that we may pursue.
Liquidity
and Capital Resources
The Financial Statements have
been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal
course of business. However, substantial doubt about our ability to continue as a going concern exists.
Since our inception in 1991,
we have incurred significant losses from operations and, as of June 30, 2024, had incurred an accumulated deficit of $677.3 million. For
the six months ended June 30, 2024, we had a net loss of $16.5 million and a cash outflow for operating activities of $18.1 million. As
of June 30, 2024, we had a net current asset of $27.6 million. In addition, we had long term borrowing and non-current dividends payable
in total amount of $19.5 million which may become payable on demand if certain events of default occur (including with respect to a certain
revenue threshold to be met by CASI Wuxi in 2024, see Note 9 to the Financial Statements). We also subsequently paid RMB 10.0 million
in August 2024, with two remaining installments to be paid by March 31, 2025 and December 31, 2025, respectively, for total consideration
of RMB 28.4 million plus interest related to investment in PAT. Therefore, we will require additional liquidity to continue our operations
over the next 12 months.
Historically, we have relied
principally on proceeds from equity financing and bank borrowings to finance our operations and business expansion. We have evaluated
plans to continue as a going concern which include, but are not limited to, (i) exploring opportunities for further equity financing (ii)
reducing discretionary capital and operating expenses (iii) negotiate with creditor to ease the credit terms (iv) obtaining additional
facilities from banks or other financial institutions. Notwithstanding this, we may be unable to access further equity or debt financing
when needed. As such, there can be no assurance that we will be able to obtain additional liquidity when needed or under acceptable terms,
if at all.
The unaudited interim condensed
consolidated financial statements do not include any adjustments to the carrying amounts and classification of assets, liabilities, and
reported expenses that may be necessary if we were unable to continue as a going concern.
RISK FACTORS
Investing
in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider carefully
the risks and uncertainties described under the section titled “Risk Factors” contained in the applicable prospectus
supplement and any related free writing prospectus, and discussed under the section titled
“Risk Factors” contained in our most recent annual report on Form 20-F and in our current reports on Form 6-K, as
well as any amendments thereto reflected in subsequent filings with the SEC, which are incorporated by reference into this prospectus
in their entirety, together with other information in this prospectus, the documents incorporated by reference and any free writing prospectus
that we may authorize for use in connection with a specific offering. See “Where You Can Find More Information.”
The
risks described in these documents are not the only ones we face, but those that we consider to be material. There may be other unknown
or unpredictable economic, business, competitive, regulatory or other factors that could have material adverse effects on our future
results. Past financial performance may not be a reliable indicator of future performance, and historical trends should not be used to
anticipate results or trends in future periods. If any of these risks actually occur, our business, financial condition, results of operations
or cash flow could be harmed. This could cause the trading price of our securities to decline, resulting in a loss of all or part of
your investment. Please also read carefully the section below titled “Special Note Regarding Forward-Looking Statements.”
SPECIAL NOTE REGARDING
FORWARD-LOOKING STATEMENTS
This
prospectus and the documents we have filed with the SEC that are incorporated by reference contain forward-looking statements within
the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the
Exchange Act. The statements contained in this prospectus or incorporated by reference herein that are not purely historical are forward-looking
statements. Our forward-looking statements include, but are not limited to, statements regarding our or our management’s expectations,
hopes, beliefs, intentions or strategies regarding the future. In addition, any statements that refer to projections, forecasts or other
characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements.
You can identify some of
these forward-looking statements by words or phrases such as “may,” “will,” “expect,” “anticipate,”
“aim,” “estimate,” “intend,” “plan,” “believe,” “is/are likely to,”
“potential,” “continue” or other similar expressions. These forward-looking statements include, among others,
statements regarding the timing of our commercial launch of products, clinical trials, our cash position and future expenses, and our
future revenues. We have based these forward-looking statements largely on our current expectations and projections about future events
that we believe may affect our financial condition, results of operations, business strategy and financial needs.
Actual
results could differ materially from those currently anticipated due to a number of factors, including: uncertainties related to the
Proposal Letter to acquire the Company’s business operations in China; the risk that we may be unable to continue as a going concern
as a result of our inability to raise sufficient capital for our operational needs; the possibility that we may be delisted from trading
on The Nasdaq Capital Market if we fail to satisfy applicable continued listing standards; the volatility in the market price of our
ordinary shares; the risk of substantial dilution of existing shareholders in future share issuances; the difficulty of executing our
business strategy on a global basis including China; our inability to enter into strategic partnerships for the development, commercialization,
manufacturing and distribution of our proposed product candidates or future candidates; legal or regulatory developments in China that
adversely affect our ability to operate in China; our lack of experience in manufacturing products and uncertainty about our resources
and capabilities to do so on a clinical or commercial scale; risks relating to the commercialization, if any, of our products and proposed
products (such as marketing, safety, regulatory, patent, product liability, supply, competition and other risks); our inability to predict
when or if our product candidates will be approved for marketing by the U.S. FDA, EMA, NMPA, or other regulatory authorities; our inability
to receive approval for renewal of license of our existing products; the risks relating to the need for additional capital and the uncertainty
of securing additional funding on favorable terms; the risks associated with our product candidates, and the risks associated with our
other early-stage products under development; the risk that result in preclinical and clinical models are not necessarily indicative
of clinical results; uncertainties relating to preclinical and clinical trials, including delays to the commencement of such trials;
our ability to protect our intellectual property rights; the lack of success in the clinical development of any of our products and our
dependence on third parties; the risks related to our dependence on Juventas to partner with us to co-market CNCT19; risks related to
the uncertainty in connection with the ongoing arbitration proceedings between us and Juventas with respect to Juventas’ purported
termination of certain CNCT19 license agreements; risks related to our dependence on Juventas to ensure the patent protection and prosecution
for CNCT19; risks related to the Company’s ongoing development of and regulatory application for CID-103 with respect to the treatment
of antibody-mediated rejection for organ transplant and autoimmune diseases and the license arrangements of CID-103; risks relating to
the interests of our largest shareholder and our Chairman and Chief Executive Officer that differ from our other shareholders; and risks
related to the success of a new manufacturing facility operated by CASI Wuxi. Such factors, among others, could have a material adverse
effect upon our business, results of operations and financial condition.
The
forward-looking statements contained in this prospectus are based on our current expectations and beliefs concerning future developments
and their potential effects on us. There can be no assurance that future developments affecting us will be those that we have anticipated.
These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or other assumptions
that may cause actual results or performance to be materially different from those expressed
or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to, those described in the
section titled “Risk Factors” and elsewhere in this prospectus. Should one or more of these risks or uncertainties
materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these
forward-looking statements. We discuss in greater detail many of these risks under the section titled “Risk Factors”
contained in the applicable prospectus supplement, in any free writing prospectuses we may authorize for use in connection with a specific
offering, and in our most recent annual report on Form 20-F, as well as any amendments thereto reflected in subsequent filings
with the SEC, as well as our reports on 6-F, which are incorporated by reference into this prospectus in their entirety. Also, these
forward-looking statements represent our estimates and assumptions only as of the date of the document containing the applicable statement.
Unless required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or future
events or developments. You should read this prospectus, any applicable prospectus supplement, together with the documents we have filed
with the SEC that are incorporated by reference and any free writing prospectus that we may authorize for use in connection with a specific
offering completely and with the understanding that our actual future results may be materially different from what we expect. We qualify
all of the forward-looking statements in the foregoing documents by these cautionary statements.
In
addition, statements that “we believe” and similar statements reflect our beliefs
and opinions on the relevant subject. These statements are based upon information available to us as of the date of this prospectus,
and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and
our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available
relevant information. These statements are inherently uncertain and you are cautioned not to unduly rely upon these statements.
OFFER STATISTICS AND EXPECTED
TIMETABLE
We may offer and sell ordinary
shares, preferred shares, warrants, subscription rights, and/or units, either individually or in combination with other securities, in
one or more offerings from time to time, with a total aggregate offering price of up to $200.0 million. The actual price of the securities
that we will offer pursuant to this prospectus will depend on a number of factors that may be relevant as of the time of offer. See “Plan
of Distribution.”
CAPITALIZATION
We intend to include information
about our capitalization and indebtedness in prospectus supplements.
USE OF PROCEEDS
Unless otherwise specified
in an applicable prospectus supplement, we intend to use the proceeds we receive from the sale of securities offered hereunder for general
corporate purposes, which may include working capital, capital expenditures, investments and the financing of possible acquisitions.
Additional information relating thereto may be set forth in any applicable prospectus supplement.
DESCRIPTION OF SECURITIES
The descriptions of the securities
contained in this prospectus, together with the applicable prospectus supplements, summarize the material terms and provisions of the
various types of securities that we may offer. We will describe in the applicable prospectus supplement the particular terms of any securities
offered by such prospectus supplement. If we so indicate in the applicable prospectus supplement, the terms of the securities may differ
from the terms we have summarized below.
We may sell from time to
time, in one or more offerings, ordinary shares, preferred shares, warrants, and units comprising any combination of these securities.
The total dollar amount of all securities that we may issue under this prospectus will not exceed $200.0 million.
DESCRIPTION
OF CAPITAL STOCK
The following is a summary
of the material terms of the ordinary shares of CASI. This summary is qualified by reference to the Amended and Restated Memorandum and
Articles of Association that is attached as Exhibit 1.1 to the Form 20-F filed with the SEC on March 28, 2024 and incorporated
herein by reference, referred to as the CASI Articles. You are encouraged to read the relevant provisions of the Companies Act (As Revised)
of the Cayman Islands, or the Companies Act, and the CASI Articles as they relate to the following summary.
CASI is authorized to issue
500,000,000 shares of a par value of US$0.0001 each. The directors of CASI are authorized to issue shares in one or more classes and
series and, with respect to each class or series, to determine the designations, powers, preferences, privileges and other rights, including
dividend rights, voting rights, conversion rights, terms of redemption and liquidation preferences, any or all of which may be greater
than the powers and rights associated with the ordinary shares, at such times and on such other terms as they think proper.
As of the close of business on December 18, 2024, CASI had 15,904,533
shares issued, among which 15,492,581 were outstanding shares, and 411,952 were treasury shares. CASI has no preferred shares issued
and outstanding.
General
All of CASI’s issued
and outstanding ordinary shares have been issued and credited as fully paid and non-assessable. CASI’s ordinary shares are issued
in registered form, and are issued when registered in CASI’s register of members. CASI’s shareholders who are non-residents
of the Cayman Islands may freely hold and transfer their ordinary shares.
Dividends
The holders of CASI’s
ordinary shares are entitled to such dividends as may be declared by CASI’s board of directors, subject to the Companies Act and
the CASI Articles, as amended and restated from time to time. In addition, CASI’s shareholders may declare dividends by ordinary
resolution, but no dividend shall exceed the amount recommended by CASI’s board of directors. Under Cayman Islands law, dividends
may be declared and paid only out of funds legally available therefor, namely out of either profit or share premium account, provided
that in no circumstances may CASI pay a dividend if this would result in CASI being unable to pay its debts as they fall due in the ordinary
course of business.
Register of Members
Under the Companies Act,
CASI must keep a register of members and there shall be entered therein:
| · | the
names and addresses of the members, together with a statement of the shares held by each
member, and such statement shall confirm (i) the amount paid or agreed to be considered
as paid, on the shares of each member, (ii) the number and category of shares held by
each member, and (iii) whether each relevant category of shares held by a member carries
voting rights under the CASI Articles, and if so, whether such voting rights are conditional; |
| · | the
date on which the name of any person was entered on the register as a member; and |
| · | the
date on which any person ceased to be a member. |
Under Cayman Islands law,
the register of members of CASI is prima facie evidence of the matters set out therein (i.e., the register of members will raise a presumption
of fact on the matters referred to above unless rebutted) and a member registered in the register of members shall be deemed as a matter
of Cayman Islands law to have legal title to the shares as set against its name in the register of members.
Voting Rights
Each holder of ordinary shares
is entitled to one vote for each share registered in his name on the register of members on all matters upon which the ordinary shares
are entitled to vote on a poll. Voting on any resolution put to the vote at any meeting of shareholders is required to be by way of a
poll and not on a show of hands. A poll shall be taken in such manner as the chairperson of the meeting directs, and the result of the
poll shall be deemed to be the resolution of the meeting.
A quorum required for a general
meeting of shareholders consists of one or more shareholders who hold shares which carry in aggregate not less than one-third of the
paid up voting share capital of CASI, present at the meeting. Although not required by the Companies Act or the CASI Articles, CASI expects
to hold shareholders’ meetings from time to time and such meetings may be convened by CASI’s board of directors on its own
initiative or upon a request to the directors by shareholders holding shares which carry in aggregate not less than one-third of all
votes attaching to CASI’s issued shares that carry the right to vote at general meetings. A general meeting may also be called
by the chairperson of the board of directors of CASI. Advance notice of at least seven calendar days is required for the convening of
CASI’s annual general meeting and other shareholders meetings.
An ordinary resolution to
be passed by the shareholders requires the affirmative vote of a simple majority of the votes which are cast by those shareholders who,
being entitled to do so, vote at a general meeting, while a special resolution requires the affirmative vote of not less than two-thirds
of the votes which are cast by those shareholders who, being entitled to do so, vote at a general meeting. Both ordinary resolutions
and special resolutions may also be passed by a unanimous written resolution signed by all the shareholders of CASI, as permitted by
the Companies Act and the CASI Articles. A special resolution will be required for important matters such as change of name or making
changes to the CASI Articles.
Transfer of Ordinary Shares
Subject to the restrictions
of CASI Articles, as applicable, any of CASI’s shareholders may transfer all or any of his or her ordinary shares by an instrument
of transfer in the usual or common form or any other form approved by CASI’s board of directors.
Subject to the Nasdaq listing
rules, CASI’s board of directors may, in its absolute discretion, decline to register any transfer of any ordinary share which
is not fully paid up or on which CASI has a lien. CASI’s directors may also decline to register any transfer of any ordinary share
unless:
| · | the
instrument of transfer is lodged with CASI, accompanied by the certificate for the ordinary
shares to which it relates and such other evidence as CASI’s board of directors may
reasonably require to show the right of the transferor to make the transfer; |
| · | the
instrument of transfer is in respect of only one class of ordinary shares; |
| · | the
instrument of transfer is properly stamped, if required; |
| · | in
the case of a transfer to joint holders, the number of joint holders to whom the ordinary
share is to be transferred does not exceed four; or |
| · | a
fee of such maximum sum as Nasdaq may determine to be payable, or such lesser sum as the
CASI’s board of directors may from time to time require, is paid to CASI in respect
thereof. |
If CASI’s directors
refuse to register a transfer they shall, within two calendar months after the date on which the instrument of transfer was lodged, send
to each of the transferor and the transferee notice of such refusal, including the relevant reason for such refusal. The registration
of transfers may, on 14 days’ notice being given by advertisement in such one or more newspapers or by electronic means, be suspended
and the register closed at such times and for such periods as CASI’s board of directors may from time to time determine; provided,
however, that the registration of transfers shall not be suspended and the register shall not be closed for more than 30 days in any
calendar year.
Liquidation
On a winding up of CASI,
if the assets available for distribution among its shareholders shall be more than sufficient to repay the whole of the share capital
at the commencement of the winding up, the surplus will be distributed among its shareholders in proportion to the par value of the shares
held by them at the commencement of the winding up, subject to a deduction from those shares in respect of which there are monies due,
of all monies payable to CASI for unpaid calls or otherwise. If CASI’s assets available for distribution are insufficient to repay
all of the paid-up capital, the assets will be distributed so that the losses are borne by its shareholders in proportion to the par
value of the shares held by them.
Calls on Ordinary Shares and Forfeiture
of Ordinary Shares
CASI’s board of directors
may from time to time make calls upon shareholders for any amounts unpaid on their ordinary shares in a notice served to such shareholders
at least 14 days prior to the specified time of payment. The ordinary shares that have been called upon and remain unpaid are subject
to forfeiture.
Redemption, Repurchase and Surrender of
Ordinary Shares
CASI may issue shares on
terms that are subject to redemption, at CASI’s option or at the option of the holders, on such terms and in such manner as may
be determined before the issue of such shares, by CASI’s board of directors or by an ordinary resolution of CASI’s shareholders.
CASI may also repurchase any of its shares provided that the manner and terms of such purchase have been approved by its board of directors
or by CASI’s shareholders by ordinary resolution or are otherwise authorized by the CASI Articles. Under the Companies Act, the
redemption or repurchase of any share may be paid out of CASI’s profits or out of the proceeds of a fresh issue of shares made
for the purpose of such redemption or repurchase, or out of capital (including share premium account and capital redemption reserve)
if CASI can, immediately following such payment, pay its debts as they fall due in the ordinary course of business. In addition, under
the Companies Act no such share may be redeemed or repurchased (a) unless it is fully paid up, (b) if such redemption or repurchase
would result in there being no shares outstanding, or (c) if the company has commenced liquidation. In addition, CASI may accept
the surrender of any fully paid share for no consideration.
Variations of Rights of Shares
Whenever the capital of CASI
is divided into different classes, the rights attached to any such class of shares may, subject to any rights or restrictions for the
time being attached to any class, be materially and adversely varied either with the written consent of the holders of at least two-thirds
of the issued shares of that class or with the sanction of a special resolution passed at a separate meeting of the holders of the shares
of that class. The rights conferred upon the holders of the shares of any class issued with preferred or other rights shall not, subject
to any rights or restrictions for the time being attached to the shares of that class, be deemed to be materially and adversely varied
by the creation or issue of further shares ranking pari passu with or subsequent to such existing class of shares or the redemption or
purchase of any shares of any class by CASI. The rights of the holders of shares shall not be deemed to be materially and adversely varied
by the creation or issue of shares with preferred or other rights including, without limitation, the creation of shares with enhanced
or weighted voting rights.
Inspection of Books and Records
Holders of CASI’s ordinary
shares have no general right under Cayman Islands law to inspect or obtain copies of CASI’s list of shareholders or its corporate
records (save for the CASI Articles, special resolutions and the register of mortgages and charges). However, CASI will provide its shareholders
with annual audited financial statements. See “Where You Can Find Additional Information.”
Changes in Capital
CASI may from time to time
by ordinary resolution:
| · | increase
its share capital by new shares of such amount as it thinks expedient; |
| · | consolidate
and divide all or any of its share capital into shares of a larger amount than its existing
shares; |
| · | sub-divide
its existing shares, or any of them into shares of a smaller amount that is fixed by the
CASI Articles; and |
| · | cancel
any shares which, at the date of the passing of the resolution, have not been taken or agreed
to be taken by any person and diminish the amount of its share capital by the amount of the
shares so cancelled. |
Subject to the Companies
Act and confirmation by the Grand Court of the Cayman Islands on an application by CASI for an order confirming such reduction, CASI
may by special resolution reduce its share capital and any capital redemption reserve in any manner authorized by the Companies Act.
Issuance of Additional Shares
The CASI Articles authorize
CASI’s board of directors to issue additional ordinary shares from time to time as its board of directors shall determine, to the
extent of available authorized but unissued shares.
The CASI Articles authorize
CASI’s board of directors to establish from time to time one or more series of preferred shares and to determine, with respect
to any series of preferred shares, the terms and rights of that series, including:
| · | the
designation of the series; |
| · | the
number of shares of the series; |
| · | the
dividend rights, dividend rates, conversion rights, voting rights; and |
| · | the
rights and terms of redemption and liquidation preferences. |
CASI’s board of directors
may issue preferred shares without action by its shareholders to the extent authorized but unissued. In addition, the issuance of preferred
shares may be used as an anti-takeover device without further action on the part of the shareholders. Issuance of these shares may dilute
the voting power of holders of ordinary shares.
Exempted Company
CASI is an exempted company
duly incorporated with limited liability under the Companies Act. The Companies Act distinguishes between ordinary resident companies
and exempted companies. Any company that is registered in the Cayman Islands but conducts business mainly outside of the Cayman Islands,
may apply to be registered as an exempted company. The requirements for an exempted company are essentially the same as for an ordinary
company except for certain exemptions and privileges, including (a) an exempted company does not have to file an annual return of
its shareholders with the Registrar of Companies, (b) an exempted company is not required to open its register of members for inspection,
(c) an exempted company does not have to hold an annual general meeting, (d) an exempted company may register by way of continuation
in another jurisdiction and be deregistered in the Cayman Islands, (e) an exempted company may obtain an undertaking against the
imposition of any future taxation (such undertakings are usually given for 30 years in the first instance), (f) an exempted company
may register as a limited duration company and (g) an exempted company may register as a segregated portfolio company.
“Limited liability”
means that the liability of each shareholder is limited to the amount unpaid by the shareholder on that shareholder’s shares of
the company (except in exceptional circumstances, such as fraud, the establishment of an agency relationship or an illegal or improper
purpose or other circumstances in which a court may be prepared to pierce or lift the corporate veil).
DESCRIPTION
OF WARRANTS
We may issue warrants to
purchase ordinary shares. Warrants may be issued independently or together with any other securities and may be attached to, or separate
from, such securities. Each series of warrants will be issued under a separate warrant agreement to be entered into between us and a
warrant agent. The warrant agent will act solely as our agent and will not assume any obligation or relationship of agency for or with
holders or beneficial owners of warrants. The terms of any warrants to be issued and a description of the material provisions of the
applicable warrant agreement will be set forth in the applicable prospectus supplement.
The applicable prospectus
supplement will describe the following terms of any warrants in respect of which this prospectus is being delivered:
| · | the
title of such warrants; |
| · | the
aggregate number of such warrants; |
| · | the
price or prices at which such warrants will be issued and exercised; |
| · | the
currency or currencies in which the price of such warrants will be payable; |
| · | the
securities purchasable upon exercise of such warrants; |
| · | the
date on which the right to exercise such warrants shall commence and the date on which such
right shall expire; |
| · | if
applicable, the minimum or maximum amount of such warrants which may be exercised at any
one time; |
| · | if
applicable, the designation and terms of the securities with which such warrants are issued
and the number of such warrants issued with each such security; |
| · | if
applicable, the date on and after which such warrants and the related securities will be
separately transferable; |
| · | information
with respect to book-entry procedures, if any; |
| · | any
material Cayman Islands and U.S. federal income tax consequences; |
| · | the
anti-dilution provisions of the warrants, if any; and |
| · | any
other terms of such warrants, including terms, procedures and limitations relating to the
exchange and exercise of such warrants. |
We and the warrant agent
may amend or supplement the warrant agreement for a series of warrants without the consent of the holders of the warrants issued thereunder
to effect changes that are not inconsistent with the provisions of the warrants and that do not materially and adversely affect the interests
of the holders of the warrants.
DESCRIPTION
OF SUBSCRIPTION RIGHTS
We may issue subscription
rights to purchase our ordinary shares or preferred shares. These subscription rights may be offered independently or together with any
other security offered hereby and may or may not be transferable by the shareholder receiving the subscription rights in such offering.
In connection with any offering of subscription rights, we may enter into a standby arrangement with one or more underwriters or other
purchasers pursuant to which the underwriters or other purchasers may be required to purchase any securities remaining unsubscribed for
after such offering.
The prospectus supplement
relating to any subscription rights we offer, if any, will, to the extent applicable, include specific terms relating to the offering,
including some or all of the following:
| · | the
price, if any, for the subscription rights; |
| · | the
exercise price payable for our ordinary shares or preferred shares upon the exercise of the
subscription rights; |
| · | the
number of subscription rights to be issued to each shareholder; |
| · | the
number and terms of our ordinary shares or preferred shares which may be purchased per each
subscription right; |
| · | the
extent to which the subscription rights are transferable; |
| · | any
other terms of the subscription rights, including the terms, procedures and limitations relating
to the exchange and exercise of the subscription rights; |
| · | the
date on which the right to exercise the subscription rights shall commence, and the date
on which the subscription rights shall expire; |
| · | the
extent to which the subscription rights may include an over-subscription privilege with respect
to unsubscribed securities or an over-allotment privilege to the extent the securities are
fully subscribed; and |
| · | if
applicable, the material terms of any standby underwriting or purchase arrangement which
may be entered into by us in connection with the offering of subscription rights. |
The description in the applicable prospectus
supplement of any subscription rights we offer will not necessarily be complete and will be qualified in its entirety by reference to
the applicable subscription rights certificate, which will be filed with the SEC if we offer subscription rights. We urge you to read
the applicable subscription rights certificate and any applicable prospectus supplement in their entirety.
DESCRIPTION
OF UNITS
We may issue units consisting
of some or all of the securities described above, in any combination, including ordinary shares, preferred shares and/or warrants. The
terms of these units will be set forth in a prospectus supplement. The description of the terms of these units in the related prospectus
supplement will not be complete. You should refer to the applicable form of unit and unit agreement for complete information with respect
to these units.
FORMS
OF SECURITIES
Each warrant, subscription
right and unit will be represented either by a certificate issued in definitive form to a particular investor or by one or more global
securities representing the entire issuance of securities. Certificated securities in definitive form and global securities will be issued
in registered form. Definitive securities name the investor or the investor’s nominee as the owner of the security, and in order
to transfer or exchange these securities or to receive payments other than interest or other interim payments, investor or the investor’s
nominee must physically deliver the securities to the trustee, registrar, paying agent or other agent, as applicable. Global securities
name a depositary or its nominee as the owner of the debt securities, warrants or units represented by these global securities. The depositary
maintains a computerized system that will reflect each investor's beneficial ownership of the securities through an account maintained
by the investor with its broker/dealer, bank, trust company or other representative, as we explain more fully below.
Registered Global Securities
We may issue the registered
warrants, subscription rights and units in the form of one or more fully registered global securities that will be deposited with a depositary
or its nominee identified in the applicable prospectus supplement and registered in the name of that depositary or nominee. In those
cases, one or more registered global securities will be issued in a denomination or aggregate denominations equal to the portion of the
aggregate principal or face amount of the securities to be represented by registered global securities. Unless and until it is exchanged
in whole for securities in definitive registered form, a registered global security may not be transferred except as a whole by and among
the depositary for the registered global security, the nominees of the depositary or any successors of the depositary or those nominees.
If not described below, any
specific terms of the depositary arrangement with respect to any securities to be represented by a registered global security will be
described in the prospectus supplement relating to those securities. We anticipate that the following provisions will apply to all depositary
arrangements.
Ownership of beneficial interests
in a registered global security will be limited to persons, called participants, that have accounts with the depositary or persons that
may hold interests through participants. Upon the issuance of a registered global security, the depositary will credit, on its book-entry
registration and transfer system, the participants' accounts with the respective principal or face amounts of the securities beneficially
owned by the participants. Any dealers, underwriters or agents participating in the distribution of the securities will designate the
accounts to be credited. Ownership of beneficial interests in a registered global security will be shown on, and the transfer of ownership
interests will be effected only through, records maintained by the depositary, with respect to interests of participants, and on the
records of participants, with respect to interests of persons holding through participants. The laws of some states may require that
some purchasers of securities take physical delivery of these securities in definitive form. These laws may impair such purchaser’s
ability to own, transfer or pledge beneficial interests in registered global securities.
So long as the depositary,
or its nominee, is the registered owner of a registered global security, that depositary or its nominee, as the case may be, will be
considered the sole owner or holder of the securities represented by the registered global security for all purposes under the applicable
warrant agreement subscription right agreement or unit agreement. Except as described below, owners of beneficial interests in a registered
global security will not be entitled to have the securities represented by the registered global security registered in their names,
will not receive or be entitled to receive physical delivery of the securities in definitive form and will not be considered the owners
or holders of the securities under the applicable warrant agreement, subscription right agreement or unit agreement. Accordingly, each
person owning a beneficial interest in a registered global security must rely on the procedures of the depositary for that registered
global security and, if that person is not a participant, on the procedures of the participant through which the person owns its interest,
to exercise any rights of a holder under the applicable warrant agreement subscription right agreement or unit agreement. We understand
that under existing industry practices, if we request any action of holders or if an owner of a beneficial interest in a registered global
security desires to give or take any action that a holder is entitled to give or take under the applicable warrant agreement subscription
right agreement or unit agreement, the depositary for the registered global security would authorize the participants holding the relevant
beneficial interests to give or take that action, and the participants would authorize beneficial owners owning through them to give
or take that action or would otherwise act upon the instructions of beneficial owners holding through them.
Any payments to holders with
respect to warrants, subscription rights or units, represented by a registered global security registered in the name of a depositary
or its nominee will be made to the depositary or its nominee, as the case may be, as the registered owner of the registered global security.
None of CASI, its affiliates, the warrant agents, the rights agents, the unit agents or any other agent of CASI, agent of the warrant
agents, rights agents or unit agents will have any responsibility or liability for any aspect of the records relating to payments made
on account of beneficial ownership interests in the registered global security or for maintaining, supervising or reviewing any records
relating to those beneficial ownership interests.
We expect that the depositary
for any of the securities represented by a registered global security, upon receipt of any payment of principal, premium, interest or
other distribution of underlying securities or other property to holders on that registered global security, will immediately credit
participants' accounts in amounts proportionate to their respective beneficial interests in that registered global security as shown
on the records of the depositary. We also expect that payments by participants to owners of beneficial interests in a registered global
security held through participants will be governed by standing customer instructions and customary practices, as is now the case with
the securities held for the accounts of customers in bearer form or registered in "street name," and will be the responsibility
of those participants.
If the depositary for any
of these securities represented by a registered global security is at any time unwilling or unable to continue as depositary or ceases
to be a clearing agency registered under the Exchange Act, and a successor depositary registered as a clearing agency under the Exchange
Act is not appointed by us within 90 days, we will issue securities in definitive form in exchange for the registered global security
that had been held by the depositary. Any securities issued in definitive form in exchange for a registered global security will be registered
in the name or names that the depositary gives to the relevant trustee, warrant agent, unit agent or other relevant agent of ours or
theirs. It is expected that the depositary's instructions will be based upon directions received by the depositary from participants
with respect to ownership of beneficial interests in the registered global security that had been held by the depositary.
PLAN OF DISTRIBUTION
We may sell the securities
offered by this prospectus from time to time in one or more transactions, including without limitation:
| · | through
underwriters, acting as an underwriting syndicate represented by managing underwriters or
by underwriters without a syndicate; |
| · | in
“at the market” offerings, within the meaning of Rule 415(a)(4) of
the Securities Act or into an existing trading market on an exchange or otherwise; |
| · | through
a combination of any of these methods; or |
| · | through
any other method permitted by applicable law and described in the applicable prospectus supplement. |
In addition, we may enter
into derivative or hedging transactions with third parties, or sell securities not covered by this prospectus to third parties in privately
negotiated transactions. In connection with such a transaction, the third parties may sell securities covered by and pursuant to this
prospectus and any accompanying prospectus supplement. If so, the third party may use securities borrowed from us or others to settle
such sales and may use securities received from us to close out any related short positions. We may also loan or pledge securities covered
by this prospectus and any accompanying prospectus supplement to third parties, who may sell the loaned securities or, in an event of
default in the case of a pledge, sell the pledged securities pursuant to this prospectus and any accompanying prospectus supplement.
The prospectus supplement
with respect to any offering of securities will include the following information:
| · | the
type and number or amount of securities being offered; |
| · | the
terms of the offering; |
| · | the
name or names of any underwriters, dealers, agents or direct purchasers; |
| · | the
name or names of any managing underwriter or underwriters; |
| · | the
purchase price or initial public offering price of the securities; |
| · | the
net proceeds from the sale of the securities; |
| · | any
delayed delivery arrangements; |
| · | any
underwriting discounts, commissions or agency fees and other items constituting underwriters’
or agents’ compensation; |
| · | any
options under which underwriters may purchase additional securities from us; |
| · | the
specific plan of distribution; |
| · | any
discounts or concessions allowed or reallowed or paid to dealers; |
| · | any
commissions paid to agents; and |
| · | any
securities exchange on which the securities may be listed. |
Any public offering price and any discounts or concessions allowed
or reallowed or paid to dealers may be changed from time to time.
Sale through Underwriters or Dealers
If underwriters are used
in the sale, the underwriters will acquire the securities for their own account. The underwriters may resell the securities from time
to time in one or more transactions, including:
| · | negotiated
transactions; |
| · | at
a fixed public offering price or prices, which may be changed; |
| · | at
market prices prevailing at the time of sale; |
| · | at
prices related to prevailing market prices; or |
Unless we inform you otherwise
in the applicable prospectus supplement, the obligations of the underwriters to purchase the securities will be subject to certain conditions,
and the underwriters will be obligated to purchase all of the offered securities if they purchase any of them. The underwriters may change
from time to time any initial public offering price and any discounts or concessions allowed or reallowed or paid to dealers.
During and after an offering
through underwriters, the underwriters may purchase and sell the securities in the open market. These transactions may include overallotment
and stabilizing transactions and purchases to cover syndicate short positions created in connection with the offering. The underwriters
may also impose a penalty bid, which means that selling concessions allowed to syndicate members or other broker-dealers for the offered
securities sold for their account may be reclaimed by the syndicate if the offered securities are repurchased by the syndicate in stabilizing
or covering transactions. These activities may stabilize, maintain or otherwise affect the market price of the offered securities, which
may be higher than the price that might otherwise prevail in the open market. If commenced, the underwriters may discontinue these activities
at any time.
Some or all of the securities
that we offer through this prospectus may be new issues of securities with no established trading market. Any underwriters to whom we
sell our securities for public offering and sale may make a market in those securities, but they will not be obligated to do so and they
may discontinue any market making at any time without notice. Accordingly, we cannot assure you of the liquidity of, or continued trading
markets for, any securities that we offer.
If dealers are used in the
sale of securities, we will sell the securities to them as principals. They may then resell those securities to the public at fixed prices
or at varying prices determined by the dealers at the time of resale. We will include in the applicable prospectus supplement the names
of the dealers and the terms of the transaction.
If agents are used in an
offering, the names of the agents and the terms of the agency will be specified in a prospectus supplement. Unless otherwise indicated
in a prospectus supplement, the agents will act on a best-efforts basis for the period of their appointment.
Dealers and agents named
in a prospectus supplement may be underwriters as defined in the Securities Act and any discounts or commissions they receive from us
and any profit on their resale of the securities may be treated as underwriting discounts and commissions under the Securities Act. We
will identify in the applicable prospectus supplement any underwriters, dealers or agents and will describe their compensation. We may
have agreements with the underwriters, dealers and agents to indemnify them against specified civil liabilities, including liabilities
under the Securities Act.
Underwriters, dealers or
agents and their associates may engage in other transactions with and perform other services for us in the ordinary course of business.
If so indicated in a prospectus
supplement, we will authorize underwriters or other persons acting as our agents to solicit offers by institutional investors to purchase
securities pursuant to contracts providing for payment and delivery on a future date. We may enter contracts with commercial and savings
banks, insurance companies, pension funds, investment companies, educational and charitable institutions and other institutional investors.
The obligations of any institutional investor will be subject to the condition that its purchase of the offered securities will not be
illegal at the time of delivery. The underwriters and other agents will not be responsible for the validity or performance of contracts.
Direct Sales and Sales through Agents
We may sell the securities
directly. In this case, no underwriters or agents would be involved. We may also sell the securities through agents designated by us
from time to time. In the applicable prospectus supplement, we will name any agent involved in the offer or sale of the offered securities,
and we will describe any commissions payable to the agent. Unless we inform you otherwise in the applicable prospectus supplement, any
agent will agree to use its reasonable best efforts to solicit purchases for the period of its appointment.
We may sell the securities
directly to institutional investors or others who may be deemed to be underwriters within the meaning of the Securities Act with respect
to any sale of those securities. We will describe the terms of any sales of these securities in the applicable prospectus supplement.
At the Market Offerings
We may also sell the securities
offered by any applicable prospectus supplement in “at the market offerings” within the meaning of Rule 415(a)(4) of
the Securities Act or into an existing trading market, on an exchange or otherwise.
Remarketing Arrangements
Securities may also be offered
and sold, if so indicated in the applicable prospectus supplement, in connection with a remarketing upon their purchase, in accordance
with a redemption or repayment pursuant to their terms, or otherwise, by one or more remarketing firms, acting as principals for their
own accounts or as agents for us. Any remarketing firm will be identified and the terms of its agreements, if any, with us and its compensation
will be described in the applicable prospectus supplement.
Delayed Delivery Contracts
If we so indicate in the
applicable prospectus supplement, we may authorize agents, underwriters or dealers to solicit offers from certain types of institutions
to purchase securities from us at the public offering price under delayed delivery contracts. These contracts would provide for payment
and delivery on a specified date in the future.
The contracts would be subject
only to those conditions described in the applicable prospectus supplement. The applicable prospectus supplement will describe the commission
payable for solicitation of those contracts.
General Information
We may have agreements with
the underwriters, dealers, agents and remarketing firms to indemnify them against certain civil liabilities, including liabilities under
the Securities Act, or to contribute with respect to payments that the underwriters, dealers, agents or remarketing firms may be required
to make. Underwriters, dealers, agents and remarketing firms may be customers of, engage in transactions with or perform services for
us in the ordinary course of their businesses.
ENFORCEABILITY OF CIVIL
LIABILITIES
CASI is incorporated in the
Cayman Islands because of certain benefits associated with being a Cayman Islands company, such as political and economic stability,
an effective judicial system, a favorable tax system, the absence of foreign exchange control or currency restrictions and the availability
of professional and support services. However, the Cayman Islands has a less developed body of securities laws as compared to the United
States and provides less protection for investors. In addition, Cayman Islands companies do not have standing to sue before the federal
courts of the United States.
A majority portion of CASI’s
assets are located outside the United States. In addition, a majority of CASI’s directors and officers are nationals or residents
of jurisdictions other than the United States and all or a majority portion of their assets are located outside the United States. As
a result, it may be difficult for investors to effect service of process within the United States upon CASI or these persons, or to bring
an action against CASI or against these persons in the United States, in the event that you believe that your rights have been infringed
under the securities laws of the United States or any state in the United States. It may also be difficult for you to enforce in U.S.
courts judgments obtained in U.S. courts based on the civil liability provisions of the U.S. federal securities laws against CASI and
its officers and directors.
Maples and Calder (Hong Kong)
LLP, our counsel as to Cayman Islands law, has advised us that there is uncertainty as to whether the courts of the Cayman Islands would
(1) recognize or enforce judgments of U.S. courts obtained against CASI or its directors or officers, predicated upon the civil
liability provisions of the securities laws of the United States or any state in the United States, or (2) entertain original actions
brought in the Cayman Islands against CASI or its directors or officers, predicated upon the securities laws of the United States or
any state in the United States.
Maples and Calder (Hong Kong)
LLP has informed us that although there is no statutory enforcement in the Cayman Islands of judgments obtained in the federal or state
courts of the United States (and the Cayman Islands are not a party to any treaties for the reciprocal enforcement or recognition of
such judgments), a judgment obtained in such jurisdiction will be recognized and enforced in the courts of the Cayman Islands at common
law, without any re-examination of the merits of the underlying dispute, by an action commenced on the foreign judgment debt in the Grand
Court of the Cayman Islands, provided such judgment (a) is given by a foreign court of competent jurisdiction, (b) imposes
on the judgment debtor a liability to pay a liquidated sum for which the judgment has been given, (c) is final, (d) is not
in respect of taxes, a fine or a penalty, and (e) was not obtained in a manner and is not of a kind the enforcement of which is
contrary to natural justice or the public policy of the Cayman Islands. However, the Cayman Islands courts are unlikely to enforce a
judgment obtained from the U.S. courts under civil liability provisions of the U.S. federal securities law if such judgment is determined
by the courts of the Cayman Islands to give rise to obligations to make payments that are penal or punitive in nature. Because such a
determination has not yet been made by a court of the Cayman Islands, it is uncertain whether such civil liability judgments from U.S.
courts would be enforceable in the Cayman Islands.
It is our understanding that
the PRC does not have treaties with the United States and many other countries providing for the reciprocal recognition and enforcement
of judgments of courts and that there is uncertainty as to whether the courts of the PRC would recognize or enforce judgments of United
States courts against CASI or the directors or officers of CASI predicated upon the civil liability provisions of the securities laws
of the United States or any state in the United States.
Additionally, it is our understanding
that it may be difficult for you to bring an original action against CASI or against its directors and officers who are nationals or
residents of countries other than the United States in a PRC court in the event that you believe that your rights have been infringed
under the U.S. federal securities laws, PRC laws, Cayman Islands laws or otherwise because we are incorporated under the laws of the
Cayman Islands and it may be difficult for U.S. shareholders, by virtue only of holding CASI ordinary shares, to establish a connection
to the PRC as required by the PRC Civil Procedures Law in order for a PRC court to have jurisdiction.
EXPENSES
The following is a statement
of expenses in connection with the distribution of the securities registered. All amounts shown are estimates except the SEC registration
fee. The estimates do not include expenses related to offerings of particular securities. Each prospectus supplement describing an offering
of securities will reflect the estimated expenses related to the offering of securities under that prospectus supplement.
| Expense | |
Estimated
Amount | |
| SEC registration fee | |
$ | 30,345 | (1) |
| FINRA filing fee | |
| 30,500 | |
| Printing expenses | |
| | (2) |
| Legal fees and expenses | |
| | (2) |
| Accounting fees and expenses | |
| | (2) |
| Miscellaneous costs | |
| | (2) |
| Total | |
$ | | (2) |
| (1) | Includes $7,380 that was previously
paid with respect to the offer and sale of up to an aggregate of $50.0 million in securities
described in the prospectus to the Prior Registration Statement on Form F-3 (Registration
No. 333-279096), which was declared effective by the SEC on May 10, 2024. |
| (2) | These
fees are calculated based on the number of issuances and amount of securities offered and
accordingly cannot be estimated at this time. |
TAXATION
Material income tax consequences
relating to the purchase, ownership and disposition of any of the securities offered by this prospectus will be set forth in the applicable
prospectus supplement relating to the offering of those securities.
LEGAL
MATTERS
Maples and Calder (Hong Kong)
LLP will pass upon the validity of the securities being registered hereby and certain other legal matters of Cayman Islands law in
connection with the registration of such securities. Harter Secrest & Emery LLP will pass upon certain matters of U.S. federal
and New York law for us in connection with the registration of certain securities being registered hereby. Additional legal matters may
be passed upon for us and any underwriter that we will name in the applicable prospectus supplement.
EXPERTS
The consolidated financial statements of CASI
Pharmaceuticals, Inc. as of December 31, 2023 and 2022, and for each of the years in the three-year period ended December 31,
2023, have been incorporated by reference herein in reliance upon the report of KPMG Huazhen LLP, independent registered public accounting
firm, incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE
INFORMATION
We are subject to the information
requirements of the Exchange Act that are applicable to foreign private issuers. Accordingly, we are required to file reports and other
information with the SEC, including annual reports on Form 20-F and disclosure furnished under cover of Form 6-K. The SEC maintains
a website (www.sec.gov) that contains reports and other information regarding issuers, such as us, that file electronically with
the SEC. We also maintain a website (www.casipharmaceuticals.com) from which you can access such reports and other information
free of charge as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information
contained on our website does not form a part of this prospectus.
As a foreign private issuer,
we are exempt under the Exchange Act from rules prescribing the furnishing and content of proxy statements, and our officers, directors
and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the
Exchange Act. In addition, we are not required under the Exchange Act to file periodic financial statements with the SEC as frequently
or as promptly as U.S. companies whose securities are registered under the Exchange Act.
We have filed with the SEC
a “shelf” registration statement (including amendments and exhibits to the registration statement) on Form F-3 under
the Securities Act. This prospectus, which is part of such registration statement, does not contain all of the information set forth
in the Registration Statement and the exhibits and schedules to the Registration Statement. We have omitted parts of the registration
statement in accordance with the rules and regulations of the SEC. For more detail about us and the securities offered by this prospectus,
you may examine the registration statement and the exhibits filed with it at the website provided in the previous paragraph. You should
rely only on the information contained in this prospectus, any applicable prospectus supplement, any free writing prospectus and the
documents incorporated by reference herein and therein. We have not authorized anyone else to provide you with different information.
We are not making an offer of these securities in any state where the offer is not permitted.
INCORPORATION BY REFERENCE
The SEC allows us to incorporate
by reference much of the information that we file with the SEC, which means that we can disclose important information to you by referring
you to those publicly available documents. The information that we incorporate by reference in this prospectus is considered to be part
of this prospectus. Because we are incorporating by reference future filings with the SEC, this prospectus is continually updated and
those future filings may modify or supersede some of the information included or incorporated by reference in this prospectus. This means
that you must look at all of the SEC filings that we incorporate by reference to determine if any of the statements in this prospectus
or in any document previously incorporated by reference have been modified or superseded.
This prospectus and any accompanying
prospectus supplement incorporate by reference the documents set forth below that have previously been filed with the SEC (other than
those documents or the portions of those documents that are “furnished” unless otherwise specified below):
| · | our reports on Form 6-K furnished with the SEC on April 8, 2024, June 27, 2024 (Film No. 241075375), June 27, 2024 (Film No. 241075377), July 8, 2024, July 19, 2024, September 12, 2024,
November 15, 2024, and December 16, 2024; and |
All reports and other documents
we subsequently file pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act, prior to the termination of this offering,
including all such documents we may file with the SEC after the date of the initial registration statement and prior to the effectiveness
of the registration statement, but excluding any information furnished to, rather than filed with, the SEC, will also be incorporated
by reference into this prospectus and deemed to be part of this prospectus from the date of the filing of such reports and documents.
We may incorporate by reference any reports on Form 6-K that we furnish to the SEC that we specifically identify in such form as
being incorporated by reference into this prospectus after the date hereof and prior to the completion or termination of the offering
of securities under this prospectus.
Any statement contained herein
or in a document, all or a portion of which is incorporated or deemed to be incorporated by reference herein, shall be deemed to be modified
or superseded for purposes of this prospectus to the extent that a statement contained herein or in any other subsequently filed document
which also is or is deemed to be incorporated by reference herein modifies or supersedes such statement. Any such statement so modified
or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus. Notwithstanding the
foregoing, no information is incorporated by reference in this prospectus or any prospectus supplement hereto where such information
under applicable forms and regulations of the SEC is not deemed to be “filed” under Section 18 of the Exchange Act or
otherwise subject to the liabilities of that section, unless the report or filing containing such information indicates that the information
therein is to be considered “filed” under the Exchange Act or is to be incorporated by reference in this prospectus or any
prospectus supplement hereto.
We will provide to each person,
including any beneficial owner, to whom a prospectus is delivered, without charge upon written or oral request, a copy of any or all
of the documents that are incorporated by reference into this prospectus but not delivered with the prospectus, including exhibits that
are specifically incorporated by reference into such documents. You may request a copy of such documents at no cost, by writing or telephoning
us at the following address or telephone number:
CASI Pharmaceuticals, Inc.
9620 Medical Center Drive, Suite 300
Rockville, MD 20850
240-864-2600
CASI PHARMACEUTICALS, INC.
Table of Contents
CASI Pharmaceuticals, Inc.
Unaudited Condensed Consolidated Balance Sheets
(In thousands, except share and per share data)
| | |
June 30, 2024 | | |
December 31,2023 | |
| ASSETS | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 9,533 | | |
$ | 17,083 | |
| Investment in equity securities, at fair value | |
| 3,194 | | |
| 1,675 | |
| Short term investments | |
| - | | |
| 12,011 | |
| Accounts receivable | |
| 6,781 | | |
| 9,551 | |
| Amounts due from related parties | |
| 963 | | |
| 587 | |
| Inventories | |
| 14,614 | | |
| 15,877 | |
| Prepaid expenses and other | |
| 2,111 | | |
| 2,560 | |
| Total current assets | |
| 37,196 | | |
| 59,344 | |
| | |
| | | |
| | |
| Long-term investments | |
| 1,773 | | |
| 1,686 | |
| Property, plant and equipment, net | |
| 8,496 | | |
| 9,241 | |
| Intangible assets, net | |
| 1,358 | | |
| 1,839 | |
| Right of use assets | |
| 1,756 | | |
| 2,392 | |
| Other assets | |
| 692 | | |
| 766 | |
| Total assets | |
$ | 51,271 | | |
$ | 75,268 | |
| | |
| | | |
| | |
| LIABILITIES AND SHAREHOLDERS’ EQUITY | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 792 | | |
$ | 4,438 | |
| Accrued and other current liabilities | |
| 8,791 | | |
| 12,288 | |
| Total current liabilities | |
| 9,583 | | |
| 16,726 | |
| | |
| | | |
| | |
| Long term borrowing | |
| 18,465 | | |
| 18,895 | |
| Other liabilities | |
| 14,202 | | |
| 15,482 | |
| Total liabilities | |
| 42,250 | | |
| 51,103 | |
| | |
| | | |
| | |
| Commitments and contingencies | |
| | | |
| | |
| | |
| | | |
| | |
| Shareholders’ equity: | |
| | | |
| | |
| Ordinary shares | |
| 1 | | |
| 1 | |
| Treasury shares | |
| (9,604 | ) | |
| (9,604 | ) |
| Subscription receivable | |
| (2,019 | ) | |
| — | |
| Additional paid-in capital | |
| 699,373 | | |
| 695,785 | |
| Accumulated other comprehensive loss | |
| (1,420 | ) | |
| (1,200 | ) |
| Accumulated deficit | |
| (677,310 | ) | |
| (660,817 | ) |
| Total shareholders’ equity | |
| 9,021 | | |
| 24,165 | |
| Total liabilities and shareholders’ equity | |
$ | 51,271 | | |
$ | 75,268 | |
The accompanying notes are
an integral part of these consolidated financial statements.
CASI Pharmaceuticals, Inc.
Unaudited Condensed Consolidated Statements of
Operations and Comprehensive Loss
(In thousands, except share and per share data)
| | |
Six Months Ended June 30 | |
| | |
2024 | | |
2023 | |
| Revenues | |
$ | 7,388 | | |
| 18,167 | |
| | |
| | | |
| | |
| Costs of revenues | |
| 3,515 | | |
| 7,364 | |
| | |
| | | |
| | |
| Gross profit | |
| 3,873 | | |
| 10,803 | |
| | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | |
| Research and development | |
| 3,730 | | |
| 5,148 | |
| General and administrative | |
| 10,755 | | |
| 13,446 | |
| Selling and marketing | |
| 8,161 | | |
| 8,782 | |
| Gain on disposal of intangible assets | |
| (500 | ) | |
| — | |
| Other operating income | |
| — | | |
| (1,306 | ) |
| Foreign exchange gain | |
| (30 | ) | |
| (15 | ) |
| Total operating expenses | |
| 22,116 | | |
| 26,055 | |
| | |
| | | |
| | |
| Loss from operations | |
| (18,243 | ) | |
| (15,252 | ) |
| | |
| | | |
| | |
| Non-operating income (expense): | |
| | | |
| | |
| Interest expense | |
| (382 | ) | |
| | |
| Interest income | |
| 266 | | |
| 341 | |
| Other income | |
| 176 | | |
| 40 | |
| Change in fair value of investments | |
| 1,690 | | |
| (1,078 | ) |
| Loss before income tax expense and share of net loss in an equity investee | |
| (16,493 | ) | |
| (15,949 | ) |
| Income tax benefit | |
| — | | |
| 80 | |
| Net loss before share of net loss in an equity investee | |
| (16,493 | ) | |
| (15,869 | ) |
| Share of net loss in an equity investee | |
| — | | |
| (32 | ) |
| Net loss | |
| (16,493 | ) | |
| (15,901 | ) |
| Less: Loss attributable to redeemable noncontrolling interest | |
| — | | |
| (1,260 | ) |
| Accretion to redeemable noncontrolling interest redemption value | |
| — | | |
| 1,607 | |
| Net loss attributable to CASI Pharmaceuticals, Inc. | |
$ | (16,493 | ) | |
| (16,248 | ) |
| | |
| | | |
| | |
| Net loss per share (basic and diluted) | |
$ | (1.23 | ) | |
| (1.22 | ) |
| Weighted average number of ordinary shares outstanding (basic and diluted) | |
| 13,450,694 | | |
| 13,341,897 | |
| | |
| | | |
| | |
| Comprehensive loss: | |
| | | |
| | |
| Net loss | |
$ | (16,493 | ) | |
| (15,901 | ) |
| Foreign currency translation adjustment | |
| (220 | ) | |
| (1,979 | ) |
| Total comprehensive loss | |
$ | (16,713 | ) | |
| (17,880 | ) |
| Less: Comprehensive loss attributable to redeemable noncontrolling interest | |
| — | | |
| (2,348 | ) |
| Comprehensive loss attributable to ordinary shareholders | |
$ | (16,713 | ) | |
| (15,532 | ) |
The accompanying notes are
an integral part of these consolidated financial statements.
CASI Pharmaceuticals, Inc.
Unaudited
Condensed Consolidated Statements of Shareholders’ Equity
(In thousands, except share data)
| | |
| | |
| | |
| | |
| | |
| | |
Accumulated | | |
| | |
| |
| | |
| | |
| | |
| | |
| | |
Additional | | |
Other | | |
| | |
| |
| | |
Ordinary
Share | | |
Treasury | | |
Subscription | | |
Paid-in | | |
Comprehensive | | |
Accumulated | | |
| |
| | |
Shares | | |
Amount | | |
Share | | |
Receivable | | |
Capital | | |
Loss | | |
Deficit | | |
Total | |
| Balance at December 31, 2023 | |
| 13,378,175 | | |
$ | 1 | | |
$ | (9,604 | ) | |
$ | — | | |
$ | 695,785 | | |
$ | (1,200 | ) | |
$ | (660,817 | ) | |
$ | 24,165 | |
| Issuance of
ordinary share for options exercised | |
| 1,060,949 | | |
| — | | |
| — | | |
| (2,019 | ) | |
| 2,048 | | |
| — | | |
| — | | |
| 29 | |
| Share-based
compensation expense, net of forfeitures | |
| — | | |
| — | | |
| — | | |
| — | | |
| 1,540 | | |
| — | | |
| — | | |
| 1,540 | |
| Foreign currency
translation adjustment | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (220 | ) | |
| — | | |
| (220 | ) |
| Net
loss attributable to CASI Pharmaceuticals, Inc. | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (16,493 | ) | |
| (16,493 | ) |
| Balance at June 30, 2024 | |
| 14,439,124 | | |
| 1 | | |
| (9,604 | ) | |
| (2,019 | ) | |
| 699,373 | | |
| (1,420 | ) | |
| (677,310 | ) | |
| 9,021 | |
| | |
| | |
| | |
| | |
| | |
| | |
Accumulated | | |
| | |
| |
| | |
| | |
| | |
| | |
| | |
Additional | | |
Other | | |
| | |
| |
| | |
Ordinary
Share | | |
Treasury | | |
Subscription | | |
Paid-in | | |
Comprehensive | | |
Accumulated | | |
| |
| | |
Shares | | |
Amount | | |
Share | | |
Receivable | | |
Capital | | |
Loss | | |
Deficit | | |
Total | |
| Balance at December 31, 2022 | |
| 13,457,625 | | |
$ | 1 | | |
$ | (9,330 | ) | |
$ | — | | |
$ | 691,766 | | |
$ | (703 | ) | |
$ | (637,160 | ) | |
$ | 44,574 | |
| Repurchase of ordinary share | |
| (136,118 | ) | |
| | | |
| (274 | ) | |
| — | | |
| (4 | ) | |
| — | | |
| — | | |
| (278 | ) |
| Issuance of
ordinary share for options exercised | |
| 56,668 | | |
| — | | |
| — | | |
| — | | |
| 109 | | |
| | | |
| — | | |
| 109 | |
| Share-based
compensation expense, net of forfeitures | |
| — | | |
| — | | |
| — | | |
| — | | |
| 4,960 | | |
| — | | |
| — | | |
| 4,960 | |
| Foreign currency
translation adjustment | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (891 | ) | |
| — | | |
| (891 | ) |
| Net
loss attributable to CASI Pharmaceuticals, Inc. | |
| — | | |
| — | | |
| — | | |
| — | | |
| (1,607 | ) | |
| — | | |
| (14,641 | ) | |
| (16,248 | ) |
| Balance at June 30, 2023 | |
| 13,378,175 | | |
| 1 | | |
| (9,604 | ) | |
| — | | |
| 695,224 | | |
| (1,594 | ) | |
| (651,801 | ) | |
| 32,226 | |
The accompanying notes are
an integral part of these consolidated financial statements.
CASI Pharmaceuticals, Inc.
Unaudited Condensed Consolidated Statements of
Cash Flows
(In thousands)
| | |
Six Months Ended | |
| | |
June 30, 2024 | | |
June 30, 2023 | |
| CASH FLOWS FROM OPERATING ACTIVITIES | |
| | | |
| | |
| Net cash used in operating activities | |
$ | (18,090 | ) | |
| (13,199 | ) |
| | |
| | | |
| | |
| CASH FLOWS FROM INVESTING ACTIVITIES | |
| | | |
| | |
| Proceeds from sale of an intangible asset | |
| 500 | | |
| — | |
| Purchases of property, plant and equipment | |
| (42 | ) | |
| (53 | ) |
| Purchases of intangible assets | |
| — | | |
| (36 | ) |
| Purchase of short term investments | |
| (1,407 | ) | |
| (23,580 | ) |
| Proceeds from sales or maturity of short term investments | |
| 13,294 | | |
| 7,077 | |
| Payments to taxing authorities in connection with cash withheld from a related person | |
| (632 | ) | |
| — | |
| Net cash provided by (used in) investing activities | |
$ | 11,713 | | |
| (16,592 | ) |
| | |
| | | |
| | |
| CASH FLOWS FROM FINANCING ACTIVITIES | |
| | | |
| | |
| Proceeds from exercise of share options | |
| — | | |
| 109 | |
| Repurchase of ordinary shares | |
| — | | |
| (278 | ) |
| Dividend payment to Wuxi Huicheng Yuanda Investment Partnership (Limited Partnership) (“Wuxi LP”) | |
| (721 | ) | |
| — | |
| Net cash used in financing activities | |
$ | (721 | ) | |
| (169 | ) |
| | |
| | | |
| | |
| Effect of exchange rate change on cash and cash equivalents | |
| (452 | ) | |
| (237 | ) |
| Net decrease in cash and cash equivalents | |
$ | (7,550 | ) | |
| (30,197 | ) |
| | |
| | | |
| | |
| Cash and cash equivalents at beginning of period | |
$ | 17,083 | | |
| 47,112 | |
| Cash and cash equivalents at end of period | |
$ | 9,533 | | |
| 16,915 | |
| | |
| | | |
| | |
| Supplemental disclosure of cash flow information: | |
| | | |
| | |
| Income taxes paid | |
| — | | |
| 1,807 | |
| | |
| | | |
| | |
| Non-cash investing and financing activities: | |
| | | |
| | |
| Payables related to property, plant and equipment | |
$ | 90 | | |
| 91 | |
| Settlement of subscription receivable by deducting payroll payable from a related person | |
$ | 29 | | |
| — | |
| Prepayment related to intangible asset | |
$ | 90 | | |
| 9 | |
The accompanying notes are
an integral part of these consolidated financial statements.
CASI Pharmaceuticals, Inc.
Notes to Unaudited Condensed Consolidated
Financial Statements
| 1. | DESCRIPTION OF BUSINESS |
Business Overview
On April 12, 2024, CASI
Pharmaceuticals, Inc. and its subsidiaries (“CASI” or “the Company”) submitted an Investigational New Drug
(“IND”) application to the Food and Drug Administration (“FDA”) for CID-103 to support a phase 1/2 study of CID-103
in adults with chronic Immune Thrombocytopenia (“ITP”). On May 13, 2024, the Company received a letter from FDA indicating
that study may proceed. CID-103 is a fully human IgG1 anti-CD38 monoclonal antibody recognizing a unique epitope that has demonstrated
encouraging preclinical efficacy and safety profile compared to other anti-CD38 monoclonal antibodies.
| 2. | LICENSE AND DISTRIBUTION AGREEMENTS |
China
Resources Pharmaceutical Commercial Group International Trading Co., Ltd. (“CRPCGIT”, previously known
as China Resources Guokang Pharmaceuticals Co., Ltd.)
In
February 2024, the Company further extended the exclusive distribution agreement with CRPCGIT for an additional three years,
the key terms of the agreement remains unchanged.
Juventas Cell Therapy Ltd. (“Juventas”)
On March 2, 2024, CASI
received a notice from Juventas, which purported to terminate the CNCT19 Agreements. CASI responded to Juventas’ purported termination
notice, noting that Juventas was not entitled to unilaterally terminate the CNCT19 Agreements and further demanding that Juventas cease
any conduct that may constitute further breach of the CNCT19 Agreements and execute a written undertaking regarding compliance with the
CNCT19 Agreements by March 13, 2024. Juventas did not comply with CASI’s demands. On March 20, 2024, CASI submitted a
Notice of Arbitration at the Hong Kong International Arbitration Centre (“HKIAC”) against Juventas pursuant to the CNCT19
Agreements’ dispute resolution clauses, claiming that Juventas’ purported termination was invalid and that Juventas breached
the CNCT19 Agreements and seeking, among other things, damages and injunctive reliefs. Together with the Notice of Arbitration, CASI also
submitted an application for the appointment of an emergency arbitrator, seeking emergency injunctive reliefs. On the same day, Juventas
also submitted a Notice of Arbitration at the HKIAC against CASI, alleging, among other things, that the CNCT19 Agreements were validly
terminated and that CASI breached the CNCT19 Agreements. The HKIAC has appointed an emergency arbitrator in accordance with CASI’s
application.
In an order dated July 15,
2024, and received by CASI on July 18, 2024, the First Intermediate People’s Court of Tianjin Municipality, where Juventas
is headquartered, granted CASI’s application to freeze Juventas’s assets while the Arbitration Proceeding is ongoing and decided
to freeze up to RMB 190 million in Juventas’s bank accounts or seize and freeze Juventas’s other assets of equivalent value
(the “Asset Freezing Order”). The Asset Freezing Order took effect upon issuance and the court has taken steps to enforce
the Asset Freezing Order.
The arbitration proceedings
are in their early stages and the Company cannot predict right now the outcome of either of these proceedings. If we do not prevail in
either of these proceedings completely or in part, or fail to reach a favorable settlement with Juventas, our plan with respect to the
commercialization of CNCT 19 may be delayed or otherwise adversely impacted, which may in turn result in adverse impacts on our results
of operations, financial condition and prospects.
| 3. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation
The
accompanying unaudited interim condensed consolidated financial statements were prepared in accordance with U.S. generally accepted
accounting principles (“U.S. GAAP”) for interim financial information. Accordingly, they do not include all of the information
and footnotes required by U.S. GAAP for annual financial statements. The interim financial statements should be read in conjunction with
the consolidated financial statements and related footnotes included in the Company’s annual report on Form 20-F for the year
ended December 31, 2023.
In the opinion of management,
all adjustments (which include normal recurring adjustments) necessary to present a fair statement of the financial position as of June 30,
2024, the results of operations and cash flows for the six months ended June 30, 2024 and 2023, have been made.
Liquidity and Capital Resources
The unaudited interim condensed
consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction
of liabilities in the normal course of business. However, substantial doubt about the Company’s ability to continue as a going concern
exists.
Since its inception in 1991,
the Company has incurred significant losses from operations and, as of June 30, 2024, had incurred an accumulated deficit of $677.3
million. For the six months ended June 30, 2024, the Company had a net loss of $16.5 million and a cash outflow for operating activities
of $18.1 million. As of June 30, 2024, the Company had a net current asset of $27.6 million. In addition, the Company has long term
borrowing and non-current dividends payable in total amount of $19.5 million which may become payable on demand if certain events of default
occur (including with respect to a certain revenue threshold to be met by CASI Wuxi in 2024, see Note 9). The Company also subsequently
paid RMB 10.0 million in August 2024, with two remaining installments to be paid by March 31, 2025 and December 31, 2025, respectively,
for total consideration of RMB 28.4 million plus interest related to investment in PAT (see Note 16). Therefore, the Company will require
additional liquidity to continue its operations over the next 12 months.
Historically,
the Company had relied principally on proceeds from equity financing and bank borrowings to finance its operations and business
expansion. The Company has evaluated plans to continue as a going concern which include, but are not limited to, (i) exploring opportunities
for further equity financing (ii) reducing discretionary capital and operating expenses (iii) negotiate with creditor to ease
the credit terms (iv) obtaining additional facilities from banks or other financial institutions. Notwithstanding this, the Company
may be unable to access further equity or debt financing when needed. As such, there can be no assurance that the Company will be able
to obtain additional liquidity when needed or under acceptable terms, if at all.
The unaudited interim condensed
consolidated financial statements do not include any adjustments to the carrying amounts and classification of assets, liabilities, and
reported expenses that may be necessary if the Company were unable to continue as a going concern.
Use of Estimates
The preparation of financial
statements and related disclosures in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the
reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements
and the reported amounts of revenues and expenses reported in those financial statements. Descriptions of the Company’s significant
accounting policies are discussed in the notes to consolidated financial statements in the Company’s Annual Report on Form 20-F
for the fiscal year ended December 31, 2023. Management evaluates the related estimates and assumptions on an ongoing basis using
historical experience and other factors, including the current economic environment, and makes adjustments when facts and circumstances
dictate. As future events and their effects cannot be determined with precision, actual results could differ significantly from those
estimates and assumptions. Significant changes, if any, in those estimates and assumptions resulting from continuing changes in the economic
environment will be reflected in the consolidated financial statements in future periods.
Recent
Accounting Pronouncements
There
were no accounting pronouncements that the Company adopted beginning January 1, 2024 that had a material impact on the Company’s
financial position, results of operations or cash flows.
| 4. | REVENUE AND ACCOUNTS RECEIVABLE |
The Company’s revenue
is primarily consisted of sales of EVOMELA® and FOLOTYN®. As of June 30, 2024, the Company had not
incurred, and therefore did not defer, any material costs to obtain or fulfill contracts. The Company did not have any contract assets
or contract liabilities as of June 30, 2024 and December 31, 2023.
CRPCGIT is the sole customer
of the Company's EVOMELA® product sales in China, and China National Medicines Corporation Ltd. (“CNMC”) is
the sole customer of the Company's FOLOTYN® product sales in China. As of June 30, 2024, and December 31, 2023,
accounts receivable from CRPCGIT represented approximately 93% and 100% of accounts receivable of the Company.
| 5. | INVESTMENT IN EQUITY SECURITIES, AT FAIR VALUE, SHORT -TERM INVESTMENTS AND LONG-TERM INVESTMENTS |
Investment in Equity Securities, at Fair Value
BioInvent International AB – ordinary shares
In October 2020, in conjunction
with its license agreement entered into with BioInvent International AB (“BioInvent”), a publicly traded company, CASI made
a $6.3 million investment (equivalent to SEK 53.8 million) to acquire 1.2 million new shares (after 25:1 reverse stock split) of BioInvent,
and 588,000 warrants, each warrant with a right to subscribe for 1 share (after 25:1 reverse stock split) in BioInvent within a period
of five years. In the second quarter of 2022, the Company sold 275,000 ordinary shares of BioInvent.
The fair value of the ordinary
shares was measured using its quoted market price, a Level 1 input (see Note 13). The Company recognized gains of $1.5 million and losses
of $1.3 million for the ordinary shares of BioInvent, respectively, for the six months ended June 30, 2024 and 2023. Gains (losses)
on the Company’s investments in equity securities are recognized as changes in fair value of investments in the consolidated statements
of operations and comprehensive loss.
The following table summarizes
the Company’s investments in equity securities with readily determinable fair value as of June 30, 2024, and December 31,
2023:
| | |
| | |
Gross | | |
| |
| (In thousands) | |
| | |
unrealized | | |
Aggregate fair | |
| As of June 30, 2024 | |
Cost | | |
losses | | |
value | |
| BioInvent - ordinary shares | |
$ | 4,337 | | |
$ | (1,143 | ) | |
$ | 3,194 | |
| | |
| | |
Gross | | |
| |
| (In thousands) | |
| | |
unrealized | | |
Aggregate fair | |
| As of December 31, 2023 | |
Cost | | |
losses | | |
value | |
| BioInvent - ordinary shares | |
$ | 4,337 | | |
$ | (2,662 | ) | |
$ | 1,675 | |
Short-term
Investments
The
Company’s short-term investments represent investments in financial instruments with a variable interest rate and term deposits
with original maturities of more than 90 days that are readily convertible to known amounts of cash. The Company elected the fair value
method at the date of initial recognition and carried these short-term investments subsequently at fair value. Changes in fair values
are reflected in the consolidated statements of operations and comprehensive loss.
| (In thousands) | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Aggregate cost basis | |
| — | | |
| 11,698 | |
| Gross unrealized gain | |
| — | | |
| 313 | |
| Aggregate fair value | |
$ | — | | |
$ | 12,011 | |
Long-term Investments
Long-term investments include
long term investments measured at fair value or measurement alternative, and an equity method investment.
Long-term investments measured at fair value
or measurement alternative
Long-term
investments measured at fair value or measurement alternative as of June 30, 2024, and December 31, 2023 consisted of
the following:
| | |
| | |
| | |
Gross | | |
| |
| | |
| | |
Gross | | |
unrealized | | |
| |
| As of June 30, 2024 | |
| | |
unrealized
| | |
losses (including | | |
Carrying | |
| (In thousands) | |
Cost | | |
gains | | |
impairment) | | |
amount | |
| Investments measured at fair value | |
| | | |
| | | |
| | | |
| | |
| Alesta Therapeutics B.V. - convertible loan | |
$ | 261 | | |
$ | 41 | | |
$ | — | | |
$ | 302 | |
| BioInvent International AB - warrants | |
| 656 | | |
| — | | |
| (570 | ) | |
| 86 | |
| Investments in equity securities using measurement alternative | |
| | | |
| | | |
| | | |
| | |
| Alesta Therapeutics B.V. - equity interests | |
| 2,250 | | |
| — | | |
| (865 | ) | |
| 1,385 | |
| Total | |
$ | 3,167 | | |
$ | 41 | | |
$ | (1435 | ) | |
$ | 1,773 | |
| | |
| | |
| | |
Gross | | |
| |
| | |
| | |
Gross | | |
unrealized | | |
| |
| As of December 31, 2023 | |
| | |
unrealized | | |
losses (including | | |
Carrying | |
| (In thousands) | |
Cost | | |
gains | | |
impairment) | | |
amount | |
| Investments measured at fair value | |
| | | |
| | | |
| | | |
| | |
| Alesta Therapeutics B.V. - convertible loan | |
$ | 261 | | |
$ | 35 | | |
$ | — | | |
$ | 296 | |
| BioInvent International AB - warrants | |
| 656 | | |
| — | | |
| (651 | ) | |
| 5 | |
| Investments in equity securities using measurement alternative | |
| | | |
| | | |
| | | |
| | |
| Alesta Therapeutics B.V. - equity interests | |
| 2,250 | | |
| — | | |
| (865 | ) | |
| 1,385 | |
| Total | |
$ | 3,167 | | |
$ | 35 | | |
$ | (1,516 | ) | |
$ | 1,686 | |
Equity method investment
Investment in Precision Autoimmune Therapeutics Co., Ltd., (“PAT”)
In May 2022, CASI China
entered into an agreement for the investment in PAT in the amount of RMB 20.0 million (approximately $3.0 million) in cash during PAT’s
first equity financing. CASI China paid all the consideration in June 2022. Upon consummation of such equity financing, CASI China
will hold 15% equity interests of PAT and will hold one of the three board seats. According to the agreement, CASI China assumes profit
or loss of PAT from the date when agreement was signed.
The investment is accounted
for under the equity method as CASI China does not control the investee but has the ability to exercise significant influence over the
operating and financial policies of the investee through its board representation. In 2023, due to PAT’s slow business progress
and delay of financing, the Company recognized an impairment of this investment of $2.0 million, and the balance of this investment is
zero as of June 30, 2024.
In
July 2024, the Company, a third-party shareholder of PAT, and PAT entered into a tri-party agreement, pursuant to which the Company
will purchase all of the third-party shareholder’s equity interest in PAT. The total consideration for this purchase consists
of (i) a principal of RMB 28.4 million (approximately $3.9 million) which equals to the original total consideration paid by this
shareholder, and (ii) interests calculated at a 6% non-compounding annual interest rate for the period between PAT’s receipt
of the investment principal and the Company’s return of the investment principal. The consideration will be paid by three installments.
The transaction is expected to complete by December 31, 2025, and upon the completion of the transaction, the Company will hold 40%
equity interests of PAT.
The
Company’s inventories consist of finished goods of EVOMELA® and FOLOTYN®. As of June 30,
2024 and December 31, 2023, the Company had balance of inventories in the aggregate of $14.6 million and $15.9 million, respectively.
No write down to the carrying amount of inventory have been recorded in the six months ended June 30, 2024 and 2023.
| 7. | PROPERTY, PLANT AND EQUIPMENT |
Property, plant and equipment
consist of the following:
| (In thousands) | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Leasehold improvements | |
$ | 7,026 | | |
$ | 7,190 | |
| Machinery and equipment | |
| 5,959 | | |
| 5,997 | |
| Office equipment and furniture | |
| 646 | | |
| 682 | |
| Construction in progress | |
| — | | |
| 23 | |
| Property, plant and equipment, gross | |
| 13,631 | | |
| 13,892 | |
| Accumulated depreciation | |
| (5,135 | ) | |
| (4,651 | ) |
| Property, plant and equipment, net | |
$ | 8,496 | | |
$ | 9,241 | |
| 8. | ACCRUED AND OTHER CURRENT LIABILITIES, AND OTHER LIABILITIES |
| | |
June 30, | | |
December 31, | |
| (In thousands) | |
2024 | | |
2023 | |
| Accrued and other current liabilities: | |
| | | |
| | |
| Payroll and welfare payable | |
$ | 2,396 | | |
$ | 3,124 | |
| Grants related to land use right | |
| 2,485 | | |
| 2,543 | |
| Payable to sales and marketing services | |
| 559 | | |
| 1,985 | |
| Payable to professional consulting service | |
| 421 | | |
| 1,023 | |
| Payable to clinical study service | |
| 551 | | |
| 1,014 | |
| Dividends and interest payable to Wuxi LP, current | |
| 1,475 | | |
| 753 | |
| Lease liabilities-current | |
| 429 | | |
| 728 | |
| Value-added tax and other tax payable | |
| 184 | | |
| 481 | |
| Payables related to property, plant and equipment | |
| 90 | | |
| 99 | |
| Other | |
| 201 | | |
| 538 | |
| | |
$ | 8,791 | | |
$ | 12,288 | |
| Other Liabilities | |
| | |
| |
| Profit-sharing liability to Juventas | |
| 11,821 | | |
| 11,821 | |
| Dividends payable to Wuxi LP, non current | |
$ | 1,000 | | |
$ | 2,045 | |
| Lease liabilities-noncurrent | |
| 1,381 | | |
| 1,616 | |
| | |
$ | 14,202 | | |
$ | 15,482 | |
| 9. | REDEEMABLE NONCONTROLLING INTEREST AND LONG TERM BORROWING |
Changes
in redeemable noncontrolling interest during the six months periods ended June 30, 2024 and 2023 are as follows:
| | |
Six Months Ended June 30, | |
| (In thousands) | |
2024 | | |
2023 | |
| Balance at beginning of period | |
$ | — | | |
$ | 22,358 | |
| Share of CASI Wuxi net loss | |
| — | | |
| (1,260 | ) |
| Accretion of redeemable noncontrolling interest | |
| — | | |
| 1,607 | |
| Foreign currency translation adjustment | |
| — | | |
| (1,088 | ) |
| Balance at end of period | |
$ | — | | |
$ | 21,617 | |
In December 2023, the
Company entered into a series of agreements, including a capital reduction agreement, a long term borrowing agreement, and four guarantee
agreements, with Wuxi LP, CASI China and CASI Wuxi, pursuant to which, (i) CASI Wuxi will reduce its registered capital and return
to Wuxi LP the investment principal made by Wuxi LP in CASI Wuxi in the amount of RMB134.2 million (equivalent to its original investment
of US$20 million, the “Investment Principal”), together with certain investment return in the amount of RMB26.2 million to
be paid in instalments (the “Dividends Payable”), and Wuxi LP shall cease to be a shareholder of CASI Wuxi, (ii) Wuxi
LP shall reinvest the Investment Principal into a three-year long term borrowing to CASI Wuxi (the “Long term borrowing”,
together with the Dividends Payable, the “Exchanged Liabilities”), which shall have a non-compounding annual interest rate
of 4.05% and can, from the beginning date of the Long term borrowing term till the six month anniversary after the maturity of the Long
term borrowing, be partially or fully converted into the equity interest of any subsidiaries of the Company at the conversion date fair
value, solely at Wuxi LP’s discretion, and (iii) each of the Company and CASI China will provide irrevocable joint and several
liability guarantees on the above-mentioned payment obligations. The term of the Long term borrowing will start on December 25, 2023
and end on December 31, 2026. The Dividends Payable shall be paid in four instalments in the amount of RMB 5.2 million, RMB 5.2 million,
RMB 7.9 million, and RMB 7.9 million in December 2023, June 2024, June 2025 and June 2026, respectively. As of June 30,
2024, the balance of the principal of the Long term borrowing is presented in “Long term borrowing” on the consolidated balance
sheet, the balance of remaining Dividends Payable and interests accrued for the Long term borrowing is included in “Accrued and
other current liabilities” and “Other liabilities” on the consolidated balance sheet. The provision related to the Long
term borrowing includes certain financial and non-financial covenants, including certain revenue threshold generated by CASI Wuxi for
each of the years from 2024 to 2028. If CASI Wuxi were to breach the covenants, the remaining balance of Dividends Payable and Long term
borrowing become payable on demand.
In June 2024, the Company
entered into securities purchase agreements with certain investors for a private investment in public equity financing (the “PIPE
Transaction”). The PIPE Transaction was led by Venrock Healthcare Capital Partners and Foresite Capital, with participation by Panacea
Venture and Dr. Wei-Wu He, the Chairman of the board of directors and Chief Executive Officer of the Company and his family trust.
In
the PIPE Transaction, the Company sold an aggregate of 1,020,000 ordinary shares, at a price of $5.00 per ordinary share, and in the case
of two investors, pre-funded warrants to purchase up to an aggregate of 1,980,000 ordinary shares at a per-share pre-funded exercise price
of $4.9999. Each pre-funded warrant has an exercise price of $0.0001 per share, and is exercisable immediately and remains exercisable
until exercised in full. The pre-funded warrants are not refundable and are subject to other terms and conditions, including certain ordinary
share ownership limitations. On July 15, 2024, the Company received the gross proceeds of $15.0 million, and paid the agents
fees of $0.9 million separately afterwards.
Net
loss per share (basic and diluted) was computed by dividing net loss attributable to ordinary shareholders, considering the accretions
to redemption value of the redeemable noncontrolling interest, by the weighted average number of shares of ordinary shares outstanding.
As of June 30, 2024 and 2023, outstanding share options totaling 2,703,136 and 4,010,592, respectively, were anti-dilutive,
and therefore, were not included in the computation of weighted average shares used in computing diluted loss per share.
The following table sets forth
the basic and diluted net loss per share computation and provides a reconciliation of the numerator and denominator for the periods presented:
| | |
Six Months Ended June 30, | |
| (In thousands, except share and per share data) | |
2024 | | |
2023 | |
| Numerator: | |
| | |
| |
| Net loss attributable to CASI Pharmaceuticals, Inc. | |
$ | (16,493 | ) | |
$ | (16,248 | ) |
| Denominator: | |
| | | |
| | |
| Weighted average number of ordinary share | |
| 13,450,694 | | |
| 13,341,897 | |
| Denominator for basic and diluted net loss per share calculation | |
| 13,450,694 | | |
| 13,341,897 | |
| Net loss per share | |
| | | |
| | |
| — Basic and diluted | |
$ | (1.23 | ) | |
$ | (1.22 | ) |
| 12. | SHARE-BASED COMPENSATION |
The Company has adopted various
share compensation plans for executive, management and staff of the Company, as well as outside directors and consultants.
The
share-based compensation expenses are recorded as components of research and development expense, sales and marketing expense,
and general and administrative expense, as follows:
| | |
Six Months Ended June 30, | |
| (In thousands) | |
2024 | | |
2023 | |
| Research and development | |
$ | 82 | | |
$ | 218 | |
| Sales and Marketing | |
| 134 | | |
| 143 | |
| General and administrative | |
| 1,324 | | |
| 4,599 | |
| Share-based compensation expense | |
$ | 1,540 | | |
$ | 4,960 | |
In July 2024, the 2024
Long-Term Incentive Plan (the “2024 Plan”) was approved by the Company’s board of directors. The maximum number of share
options that are available for grants shall not exceed 2,000,000 shares.
| 13. | FAIR VALUE MEASUREMENTS |
Financial
instruments of the Company primarily consist of cash and cash equivalents, investment in equity securities, short-term investments, accounts
receivable, receivable from a related party, term deposit, prepaid expenses and other, long-term investments, accounts payable, accrued
and other current liabilities, and long-term borrowing. As of June 30, 2024 and December 31, 2023, the carrying amount
of cash and cash equivalents, accounts receivable, amounts due from related parties, prepaid expenses and other, accounts payable and
accrued and other current liabilities are carried at cost which approximates their fair values due to the short-term nature of the instruments,
the carrying amount of long term borrowing approximates its fair value as interest rate is comparable to the prevailing interest rate
in the market.
Financial Assets and Liabilities Measured at Fair Value on a Recurring
Basis
The Company evaluates financial
assets and liabilities subject to fair value measurements on a recurring basis to determine the appropriate level at which to classify
them each reporting period. This determination requires the Company to make subjective judgments as to the significance of inputs used
in determining fair value and where such inputs lie within the hierarchy.
The
Company has an equity investment in the common stock of a publicly traded company. The Company’s investments in these equity securities
are carried at their estimated fair value, with changes in fair value reported in the consolidated statement of operations and comprehensive
loss each reporting period (see Note 5). The fair value of the common stock is based on quoted market price for the investees’
common stock, a Level 1 input.
The
Company has an equity investment in the warrants of a publicly traded company. The Company’s investment is carried at its estimated
fair value, with changes in fair value reported in the consolidated statement of operations and comprehensive loss each reporting period
(see Note 5). The fair value of the warrants was measured using observable market-based inputs other than quoted prices in active
markets for identical assets, level 2 inputs. The Company uses the Black-Scholes-Merton valuation model to estimate the fair value of
warrants. Option valuation models, including Black-Scholes-Merton, require the input of highly subjective assumptions, and changes in
the assumptions used can materially affect the fair value determination of a warrant.
The
Company has investments in the convertible debt of a private company. The Company’s investment is carried at its estimated fair
value, with changes in fair value reported in the consolidated statement of operations and comprehensive loss each reporting period (see
Note 5) using Level 3 input.
The
following tables present the Company’s financial assets accounted for at fair value on a recurring basis as of June 30,
2024 and December 31, 2023, by level within the fair value hierarchy:
| (In thousands) | |
Fair Value at | | |
| | |
| | |
| |
| Description | |
June 30, 2024 | | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Investment in equity securities, at fair value: | |
$ | 3,194 | | |
$ | 3,194 | | |
$ | — | | |
$ | — | |
| Long term investments: | |
| | | |
| | | |
| | | |
| | |
| Investment in warrants - Designated as investment measured at FVTPL | |
$ | 86 | | |
$ | — | | |
$ | 86 | | |
$ | — | |
| Investment in convertible loan - AFS | |
$ | 302 | | |
$ | — | | |
$ | — | | |
$ | 302 | |
| (In thousands) | |
Fair Value at | | |
| | |
| | |
| |
| Description | |
December 31, 2023 | | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Investment in equity securities, at fair value: | |
$ | 1,675 | | |
$ | 1,675 | | |
$ | — | | |
$ | — | |
| Short term investments: | |
$ | 12,011 | | |
$ | — | | |
$ | 12,011 | | |
$ | — | |
| Long term investments: | |
| | | |
| | | |
| | | |
| | |
| Investment in warrants - Designated as investment measured at FVTPL | |
$ | 5 | | |
$ | — | | |
$ | 5 | | |
$ | — | |
| Investment in convertible loan - AFS | |
$ | 296 | | |
$ | — | | |
$ | — | | |
$ | 296 | |
Financial Assets and Liabilities Measured at Fair Value on a Non-Recurring
Basis
The
Company measures equity investments without readily determinable fair values at its cost, minus impairment, if any, plus or minus changes
resulting from observable transactions of identical or similar securities of the same issuer.
Non-Financial Assets and Liabilities Measured
at Fair Value on a Recurring Basis
The
Company has no non-financial assets and liabilities that are measured at fair value on a recurring basis.
Non-Financial Assets and Liabilities Measured at Fair Value on a
Non-Recurring Basis
The Company had no non-financial
assets and liabilities that are measured at fair value on a non-recurring basis.
| 14. | RELATED PARTY TRANSACTIONS |
In
June 2024, Dr. Wei-Wu He, the Company’s Chairman of the board of directors and CEO, exercised 1,023,499 share options,
the exercise price was $2.0 million. In addition, the Company paid $0.6 million payroll tax resulting from this exercise gain on behalf
of him. As of June 30, 2024, total receivable due from Dr. Wei-Wu He was $2.6 million, which was subsequently paid off in December 2024.
In July 2024, the Company
closed the PIPE Transaction, in which Dr. Wei-Wu He and his family trust participated in this transaction. Dr. Wei-Wu He and
his family trust purchased 300,000 shares at the price of $5.0 per share for a total of $1.5 million.
| 15. | COMMITMENTS AND CONTINGENCIES |
In
conjunction with the Cleave assignment agreement entered into on July 18, 2023, the Company is responsible for certain milestone
and royalty payments. As of June 30, 2024, no milestones have been achieved.
In conjunction with the BioInvent
agreement entered into during 2020, the Company is responsible for certain milestone and royalty payments. As of June 30, 2024, no
milestones had been achieved.
In conjunction with the
Black Belt agreement entered into during 2019, the Company is responsible for certain milestone and royalty payments. In
June 2021, the Company achieved the First-Patient-In in the Phase 1 dose escalation and expansion study of CID-103, and made
$750,000 milestone payment in June 2021 and EUR250,000 ($305,000) in August 2021. As of June 30, 2024, no other
milestones had been achieved.
In
conjunction with the FOLOTYN® Assignment Agreement and Payment Agreement entered into in July 2023, in addition to
the $2 million upfront payment already paid in 2023, the Company is responsible for certain contingent Deferred Payments up to $10.0 million
to licensor and certain contingent payment up to $750,000 to Acrotech. As of June 30, 2024, none of these contingent payments
are considered probable based on the uncertainties of the successful renewal of the drug license in China.
The Company is subject in
the normal course of business to various legal proceedings in which claims for monetary or other damages may be asserted. Management does
not believe such legal proceedings, unless otherwise disclosed herein, are material.
In July 2024, with respect
to the Company’s previously announced dispute with Juventas, a PRC court issued an asset freezing order against Juventas in aid
of the arbitration proceedings CASI initiated at the Hong Kong International Arbitration Centre in connection to Juventas’s purported
termination of the parties’ agreements with respect to the commercialization of CNCT19 (the “Arbitration Proceeding”).
In an order dated July 15, 2024 and received by CASI on July 18, 2024, the First Intermediate People’s Court of Tianjin
Municipality, where Juventas is headquartered, granted CASI’s application to freeze Juventas’s assets while the Arbitration
Proceeding is ongoing and decided to freeze up to RMB 190 million in Juventas’s bank accounts or seize and freeze Juventas’s
other assets of equivalent value (the “Asset Freezing Order”). The Asset Freezing Order took effect upon issuance and the
court has taken steps to enforce the Asset Freezing Order.
In July 2024, the Company
entered into an agreement with PAT and one of the investors of PAT, to purchase 19.8876% equity interest of PAT from the investor. The
total consideration is RMB 28.4 million plus interest and will be paid in three installments. The Company has paid RMB 10.0 million in
August 2024. The remaining two installments will be paid by March 31, 2025 and December 31, 2025, respectively.
In December 2024, the
Company received a Termination Process Letter from Acrotech of certain License Agreement (the “License Agreement”), dated
September 17, 2014, between Spectrum Pharmaceuticals, Inc. and the Company granting the exclusive rights to the Company to commercialize
Evomela® in China, which was later assigned to Acrotech on March 1, 2019. Acrotech alleged in such letter that the
Company materially breached the License Agreement and failed to cure such breach, and the License Agreement was therefore terminated.
Pursuant to the License Agreement, the Company can continue to distribute and sell Evomela® for a reasonable wind-down
period not to exceed 24 months, so the Company does not expect any disruption to its current distribution plan for Evomela®
during such period.
$200,000,000

Ordinary Shares
Preferred Shares
Warrants
Subscription
Rights
Units
PROSPECTUS
,
2024
The information in this prospectus is not complete
and may be changed. We may not sell these securities or accept an offer to buy these securities until the registration statement filed
with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting
offers to buy these securities in any jurisdiction where such offer or sale is not permitted.
Subject to Completion, dated
December 20, 2024
PROSPECTUS

Up to $50,000,000
Ordinary Shares
We have entered into an Open Market Sale AgreementSM, or
Sales Agreement, with Jefferies LLC, or Jefferies, dated December 20, 2024, relating to our ordinary shares offered by this prospectus.
In accordance with the terms of the Sales Agreement, under this prospectus, we may offer and sell our ordinary shares having an aggregate
offering price of up to $50.0 million from time to time through Jefferies acting as our sales agent.
Our ordinary shares are traded on The Nasdaq Capital Market under the
symbol “CASI.” The last reported sale price of our ordinary shares on December 18, 2024 was $2.86 per share.
Sales of our ordinary shares,
if any, under this prospectus will be made by any method permitted that is deemed an “at the market offering” as defined
in Rule 415(a)(4) under the Securities Act of 1933, as amended, or the Securities Act, including sales made directly on or
through The Nasdaq Capital Market or any other existing trading market in the United States for our ordinary shares. Jefferies is not
required to sell any specific number or dollar amount of securities, but will act as our sales agent using commercially reasonable efforts
consistent with its normal trading and sales practices. There is no arrangement for funds to be received in any escrow, trust or similar
arrangement.
Jefferies will be entitled
to compensation at a commission rate of up to 3.0% of the gross sales price per ordinary share sold. In connection with the sale of the
ordinary shares on our behalf, Jefferies will be deemed to be an “underwriter” within the meaning of the Securities Act and
the compensation of Jefferies will be deemed to be underwriting commissions or discounts. We have also agreed to provide indemnification
and contribution to Jefferies with respect to certain liabilities, including liabilities under the Securities Act and the Exchange Act
of 1934, as amended, or the Exchange Act.
Investing in our securities
involves a high degree of risk. We may be subject to various legal and operational risks as a result of doing business in the PRC, risks
relating to our auditor, risks relating to cash and asset transfers among the Company and its subsidiaries, and risks relating to permission
and filing procedures required from the governmental authorities of the PRC with respect to the operation of our PRC subsidiaries and
future offerings in the United States. You should carefully review the risks and uncertainties described under the section titled “Risk
Factors” on page P-8 of this prospectus and, if applicable, any risk factors described in any applicable prospectus supplement
and in our filings with the U.S. Securities and Exchange Commission, or SEC, that are incorporated by reference in this prospectus.
Neither the SEC nor any
state securities commission has approved or disapproved of these securities, or determined if this prospectus is accurate or complete.
Any representation to the contrary is a criminal offense.
Jefferies
The date of this prospectus is ,
2024
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus relates to
the registration statement that we filed with the U.S. Securities and Exchange Commission, or SEC, using a “shelf” registration
process under the Securities Act of 1933, as amended, or the Securities Act. Under this shelf registration process, we may use this prospectus
to, from time to time, sell ordinary shares having an aggregate gross sales price of up to $50.0 million.
Before buying any of the
ordinary shares that we are offering, you should carefully read this prospectus and the documents incorporated by reference herein, as
well as the additional information described under the headings “Where You Can Find More Information” and “Information
Incorporated by Reference.” These documents contain important information that you should consider when making your investment
decision.
To the extent there is a
conflict between the information contained in this prospectus, on the one hand, and the information contained in any document incorporated
by reference in this prospectus, on the other hand, you should rely on the information in this prospectus, provided that if any statement
in one of these documents is inconsistent with a statement in another document having a later date—for example, a prospectus supplement
or a document incorporated by reference in this prospectus—the statement in the document having the later date modifies or supersedes
the earlier statement.
We
further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any
document that is incorporated by reference in this prospectus or any accompanying prospectus supplement were made solely for the benefit
of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements,
and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants
were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately
representing the current state of our affairs.
The information contained
in this prospectus or any document incorporated by reference herein is accurate only as of such documents’ respective dates, regardless
of the time of delivery of this prospectus, any applicable free writing prospectus or the documents incorporated by reference in this
prospectus or the sale of any securities. Our business, financial condition, results of operations and prospects may have changed materially
since those dates.
Neither we nor Jefferies
have authorized anyone to provide you with information that is different from that contained in this prospectus, any amendment or supplement
to this prospectus, or any free writing prospectus we may authorize to be delivered or made available to you. Neither we nor Jefferies
take responsibility for, or provide assurance as to the reliability of, any other information that others may give you. This prospectus
does not constitute an offer to sell or the solicitation of an offer to buy any securities other than the securities described in this
prospectus or an offer to sell or the solicitation of an offer to buy such securities in any circumstances in which such offer or solicitation
is unlawful.
For investors outside the
United States, neither we nor Jefferies have taken any action that would permit the offering or possession or distribution of this prospectus
in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who
come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities
described herein and the distribution of this prospectus outside the United States.
Throughout this prospectus,
references to the “Company,” “we,” “our,” “us,” “registrant,” “CASI”
or similar terms used in this prospectus refer to CASI Pharmaceuticals, Inc., an exempted company with limited liability under the
laws of the Cayman Islands, including its consolidated subsidiaries, unless the context otherwise indicates.
“PRC” or “China”
refers to the People’s Republic of China, excluding, for the purpose of this prospectus, Taiwan, Hong Kong and Macau, “RMB”
or “Renminbi” refers to the legal currency of China, and “$”, “US$” or “U.S. Dollars”
refers to the legal currency of the United States.
This prospectus may contain
translations of Renminbi amounts into U.S. dollars at specified rates solely for the convenience of the reader. We make no representation
that the Renminbi or U.S. dollar amounts referred to in this prospectus could have been or could be converted into U.S. dollars or Renminbi,
as the case may be, at any particular rate or at all.
This prospectus and the information
incorporated herein by reference include trademarks, service marks and trade names owned by us or other companies. All trademarks, service
marks and trade names included or incorporated by reference into this prospectus, any applicable prospectus supplement or any related
free writing prospectus are the property of their respective owners.
INDUSTRY AND MARKET DATA
In this prospectus and the
documents incorporated by reference in this prospectus, we present industry data, information and statistics regarding the markets in
which the Company and its subsidiaries compete as well as publicly available information, industry and general publications and research
and studies conducted by third parties. This information is supplemented where necessary with the Company’s own internal estimates
and information obtained from discussions with its customers, taking into account publicly available information about other industry
participants and the Company’s management’s judgment where information is not publicly available.
Industry publications, research,
studies and forecasts generally state that the information they contain has been obtained from sources believed to be reliable, but that
the accuracy and completeness of such information is not guaranteed. Forecasts and other forward-looking information obtained from these
sources are subject to the same qualifications and uncertainties as the other forward-looking statements in this prospectus or any document
incorporated by reference into this prospectus. These forecasts and forward-looking information are subject to uncertainty and risk due
to a variety of factors, including those described under the section entitled “Risk Factors.” These and other factors
could cause results to differ materially from those expressed in any forecasts or estimates.
TRADEMARKS
AND TRADENAMES
This prospectus and the
information incorporated herein by reference include trademarks, service marks and trade names owned by us or other companies. All trademarks,
service marks and trade names included or incorporated by reference into this prospectus, any applicable prospectus supplement or any
related free writing prospectus are the property of their respective owners.
PROSPECTUS SUMMARY
This
summary highlights selected information contained elsewhere in this prospectus or incorporated by reference in this prospectus, and does
not contain all of the information that you need to consider in making your investment decision. You should carefully read the entire
prospectus, the applicable prospectus supplement and any related free writing prospectus, including the risks of investing in our securities
discussed under the section titled “Risk Factors” contained in this prospectus, the applicable prospectus supplement and
any related free writing prospectus, and under similar sections in the other documents
that are incorporated by reference into this prospectus. You should also carefully read the other information incorporated by reference
into this prospectus, including our consolidated and condensed consolidated financial statements, and the exhibits to the registration
statement of which this prospectus is a part.
Overview
We are a biopharmaceutical
company focused on developing and commercializing innovative therapeutics and pharmaceutical products in China, the United States, and
throughout the world.
Holding Company Structure
CASI is not a Chinese operating
company but a Cayman Islands holding company with a significant portion of the business operations expected to be conducted by its Chinese
subsidiaries. This holding company structure and our operation in China may involve risks. We currently conduct our business through
the following consolidated subsidiaries:
| · | CASI
Pharmaceuticals (China) Co., Ltd., referred to as CASI China; |
| · | CASI
Pharmaceuticals (Wuxi) Co., Ltd., referred to as CASI Wuxi; |
| · | CASI
Biopharmaceuticals (WUXI) Co., Ltd, referred to as CASI Biopharmaceuticals; and |
| · | CASI
Pharmaceuticals Co., Limited, referred to as CASI Hong Kong. |
We do not
have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages
in leasing, hedging or product development services with us. The organizational chart of CASI as of December 18, 2024 is set forth
below:
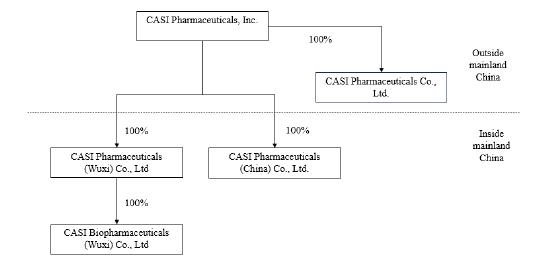
Note: Currently
CASI Hong Kong has no meaningful operations.
In addition to our existing
operations in China, we conduct clinical development of certain of our product candidates outside of China. In connection with these
efforts we have licensed the exclusive worldwide rights to certain product candidates, including CID-103, which rights are held by us
outside of China.
Nasdaq Capital Market Listing; Redomiciliation
Our ordinary shares are traded
on The Nasdaq Capital Market under the symbol “CASI.” In March 2023, we completed a redomicile merger, with CASI surviving
the merger as the surviving company and successor issuer, and CASI’s ordinary shares continued trading on The Nasdaq Capital Market
under the symbol “CASI.” CASI is treated for U.S. federal income tax purposes as a U.S. corporation, including with respect
to any dividends paid by it, which dividends may be subject to U.S. withholding taxes.
Corporate Information
Our principal executive offices
are located at 1701-1702, China Central Office Tower 1, No. 81 Jianguo Road Chaoyang District, Beijing, 100025, People’s Republic
of China. Our telephone number at this address is +86 (10) 6508 6063. Our registered office in the Cayman Islands is located at
Maples Corporate Services Limited, P.O. Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands.
Our agent for service of
process in the United States is located at 9620 Medical Center Drive, Suite 300, Rockville, MD 20850, 240-864-2600.
Implications of Being a Foreign Private Issuer
As a “foreign private
issuer,” CASI is subject to different U.S. securities laws than domestic U.S. issuers. The rules governing the information
that CASI must disclose differ from those governing U.S. corporations pursuant to the Securities Exchange Act of 1934, as amended, or
the Exchange Act. Because we qualify as a foreign private issuer under the Exchange Act, we are exempt from certain provisions of the
securities rules and regulations in the United States that are applicable to U.S. domestic issuers, including:
| · | the
rules under the Exchange Act requiring the filing with the SEC of quarterly reports
on Form 10-Q or current reports on Form 8-K; |
| · | the
sections of the Exchange Act regulating the solicitation of proxies, consents, or authorizations
in respect of a security registered under the Exchange Act; |
| · | the
sections of the Exchange Act requiring insiders to file public reports of their stock ownership
and trading activities and liability for insiders who profit from trades made in a short
period of time; and |
| · | the
selective disclosure rules by issuers of material nonpublic information under Regulation
FD. |
We are required to file an annual report on Form 20-F
within four months of the end of each fiscal year. In addition, we intend to publish our results on a quarterly basis as press releases,
distributed pursuant to the rules and regulations of the Nasdaq Stock Market. Press releases relating to financial results and material
events will also be furnished to the SEC on Form 6-K. However, the information we are required to file with or furnish to the SEC
will be less extensive and less timely compared to that required to be filed with the SEC by U.S. domestic issuers. As a result, you
may not be afforded the same protections or information that would be made available to you were you investing in a U.S. domestic issuer.
In addition, as a foreign
private issuer, CASI’s officers and directors and holders of more than 10% of the issued and outstanding ordinary shares are exempt
from the rules under the Exchange Act requiring insiders to report purchases and sales of ordinary shares as well as from Section 16
short swing profit reporting and liability. A company will lose its foreign private issuer status if more than 50% of its outstanding
voting securities are owned by U.S. residents and any of the following three circumstances applies: (i) the majority of its executive
officers or directors are U.S. citizens or residents, (ii) more than 50% of its assets are located in the United States or (iii) its
business is administered principally in the United States.
Risks Associated with our Business
Our business is subject to
numerous risks, as described under the heading “Risk Factors” contained in the applicable prospectus supplement and
in any free writing prospectuses we have authorized for use in connection with a specific offering, and under similar headings that are
incorporated by reference into this prospectus, including, without limitation, the further risks discussed below.
Risks and Uncertainties Relating
to Doing Business in China
We face various risks and
uncertainties related to doing business in China. Our business operations are primarily conducted in China, and we are subject to complex
and evolving PRC laws and regulations. The PRC government’s significant authority in regulating our operations and its oversight
and control over offerings conducted overseas by, and foreign investment in, China-based issuers could significantly limit or completely
hinder our ability to offer or continue to offer securities to investors and cause the value of our securities to significantly decline
or become worthless. Implementation of industry-wide regulations, including data security or anti-monopoly related regulations, could
result in a material change in our operations and may cause the value of our securities to significantly decline or become worthless.
Risks and uncertainties arising from the legal system in China, including risks and uncertainties regarding the enforcement of laws and
quickly evolving rules and regulations in China, could result in a material adverse change in our operations and the value of our
ordinary shares. For example, China’s government has in recent years issued statements and regulatory actions to regulate certain
market players or to improve its supervision of the market in general, such as those related to data security or anti-monopoly concerns.
While we currently do not believe such regulatory actions have materially impacted our business operations, our ability to accept foreign
investments, or our ability to maintain listing with the Nasdaq Stock Market, there is no assurance that any new rules or regulations
promulgated in the future will not impose additional requirements on us. If any such rules or regulations are adopted, we may be
subject to more stringent regulatory scrutiny for our operation and financing efforts, which may in turn result in us incurring additional
compliance costs and expenses, delay our investment and financing activities, or otherwise impact our ability to conduct our business,
accept foreign investments, or list on a U.S. or other foreign exchange.
Risks Relating to Our Auditor
Our auditor, the independent
registered public accounting firm that issues the audit report contained in our annual report, as an auditor of companies that are traded
publicly in the United States and a firm registered with the Public Company Accounting Oversight Board, or PCAOB, is subject to laws
in the United States pursuant to which the PCAOB conducts regular inspections to assess its compliance with applicable professional standards.
Our auditor is located in mainland China, a jurisdiction where the PCAOB was historically unable to conduct inspections and investigations
completely before 2022. As a result, we and investors in CASI Pharmaceuticals, Inc., a Delaware corporation, our predecessor prior
to the redomicile merger, referred to as CASI Delaware, were deprived of the benefits of such PCAOB inspections. Pursuant to the Holding
Foreign Companies Accountable Act, or the HFCAA, if the SEC determines that we have filed audit reports issued by a registered public
accounting firm that has not been subject to inspections by the PCAOB for two consecutive years, the SEC will prohibit our securities
from being traded on a national securities exchange or in the over-the-counter trading market in the United States.
On December 16, 2021,
the PCAOB issued a report to notify the SEC of its determination that the PCAOB was unable to inspect or investigate completely registered
public accounting firms headquartered in mainland China and Hong Kong and CASI Delaware’s auditor was subject to that determination.
In April 2022, the SEC conclusively listed CASI Delaware as a Commission-Identified Issuer under the HFCAA following the filing
of its annual report on Form 10-K for the fiscal year ended December 31, 2021. On December 15, 2022, the PCAOB issued
a report that vacated its December 16, 2021 determination and removed mainland China and Hong Kong from the list of jurisdictions
where it is unable to inspect or investigate completely registered public accounting firms. For this reason, we do not expect we will
be identified as a Commission-Identified Issuer under the HFCAA for the fiscal year ended December 31, 2024.
Each year in the future,
the PCAOB will determine whether it can inspect and investigate completely audit firms in mainland China and Hong Kong, among other jurisdictions.
If the PCAOB determines in the future that it no longer has full access to inspect and investigate completely accounting firms in mainland
China and Hong Kong and we use an accounting firm headquartered in one of these jurisdictions to issue an audit report on our financial
statements filed with the SEC, we would be identified as a Commission-Identified Issuer following the filing of the annual report for
the relevant fiscal year. In accordance with the HFCAA, our ordinary shares would be prohibited from being traded on a national securities
exchange or in the over-the-counter trading market in the United States if we are identified as a Commission-Identified Issuer for two
consecutive years in the future. If our ordinary shares are prohibited from trading in the United States, there is no certainty that
we will be able to list on a non-U.S. exchange or that a market for our ordinary shares will develop outside of the United States. A
prohibition of being able to trade in the United States would substantially impair your ability to sell or purchase our ordinary shares
when you wish to do so, and the risk and uncertainty associated with delisting would have a negative impact on the price of such shares.
Also, such a prohibition would significantly affect our ability to raise capital on terms acceptable to us, or at all, which would have
a material adverse impact on our business, financial condition, and prospects.
Cash and Asset Transfer among
the Company and its Subsidiaries
We provide funding to our
subsidiaries from time to time through capital contributions or loans, subject to satisfaction of applicable government registration
and approval requirements. For the year ended December 31, 2023, we provided funding of US$1.0 million through capital contributions
to CASI Hong Kong, our newly incorporated Hong Kong subsidiary.
Our subsidiaries may pay
dividends and make other distributions to us subject to satisfaction of applicable government filing and approval requirements. Such
dividend or other distributions may be subject to limitations and certain tax consequences, a discussion on which is set forth below.
For the year ended December 31, 2023, no dividends or other distributions were made by our subsidiaries.
We also pay service fees
to our PRC subsidiaries pursuant to certain sales support service agreements and research and development support service agreements.
For the year ended December 31, 2023, we paid service fees of US$1.1 million to CASI China, one of our PRC subsidiaries. Under PRC
tax laws and regulations, the earnings of our subsidiaries under such agreements are subject to a statutory tax rate of 25%.
In the year ended December 31,
2023, no assets other than cash were transferred through our organization.
All cash transfers among
us and our subsidiaries have been eliminated in our consolidated statement of cash flows.
The existing PRC foreign
exchange regulations may limit our ability to initiate and complete cash transfers within our group. Approval from the State Administration
of Foreign Exchange, or SAFE, and the People’s Bank of China, or PBOC, may be required where RMB are to be converted into foreign
currencies, including U.S. dollars, and approval from SAFE and PBOC or their branches may be required where RMB are to be remitted out
of China.
We have never declared or
paid dividends on our ordinary shares or any other securities and we do not anticipate paying any dividends on our ordinary shares in
the foreseeable future. We may rely on dividends from our subsidiaries in China to pay dividend and other distributions on our ordinary
shares. PRC regulations may restrict the ability of our PRC subsidiaries to pay dividends to us. In addition to applicable foreign exchange
limitations, under the current regulatory regime in China, a PRC company may pay dividends only out of its accumulated profit, if any,
determined in accordance with PRC accounting standards and regulations, and is required to set aside as general reserves at least 10%
of its after-tax profit, until the cumulative amount of such reserves reaches 50% of its registered capital, prior to any dividend distribution.
In addition, a PRC company shall not distribute any profits in a given year until any losses from prior fiscal years have been offset.
Permission and Filing Procedures
Required from the PRC Authorities with Respect to the Operations of Our PRC Subsidiaries and Future Offering in the US
As the date hereof, our PRC
subsidiaries have obtained the requisite licenses and permits from the PRC government authorities that are material for our business
operations, including, among others, the Business License, the Drug Distribution License, the Drug Manufacturing Permit, the Clinical
Trial Application with the PRC National Medical Products Administration, or NMPA, and the notification filing for international collaborative
clinical trial or the application for international collaborative scientific research with the China Human Genetic Resources Administrative
Office, or HGRAO. We also work with our business partners which have obtained the requisite licenses and permits for their business collaboration
with us, including, among others, the Import Drug Registration for product(s) we promote and distribute in China. Given the uncertainties
of interpretation and implementation of relevant laws and regulations and the enforcement practices of the relevant government authorities,
we may be required to obtain additional permissions or approvals for our business operations.
As
the date hereof, we and our PRC subsidiaries (i) except for the requisite CSRC Filing(s) (as defined below), are not
required to obtain permissions from the China Securities Regulatory Commission, or the CSRC, (ii) are not required to go through
cybersecurity review by the Cyberspace Administration of China, or the CAC, and (iii) have not been asked to obtain or were denied
such permissions by applicable PRC authority. On July 7, 2022, the CAC published the Guidelines for Data Export Security Assessment
(《数据出境安全评估办法》),
or the Guidelines, which took effect on September 1, 2022. Pursuant to the Guidelines, the data processor who intends to transfer
certain important data or large volumes of personal information outside of China shall complete a prior CAC-led data outbound transfer
security assessment. For the data we accessed through or obtained from clinical trials, we have complied with the laws and regulations
then-in-effect, and completed the registration with HGRAO, but it is unclear if we will be required to go through the CAC-led or CAC-involved
security assessment or if the current HGRAO registration procedure will be changed in the future. We will closely monitor and review
any regulatory developments and comply with any new approval or license requirement when necessary. If (i) we have erroneously concluded
that such permissions or approvals are not required, or (ii) applicable laws, regulations, or interpretations change and we are
required to obtain such permissions or approvals in the future, we may have to expend significant time and costs to procure them. If
we are unable to do so, on commercially reasonable terms, in a timely manner or otherwise, we may become subject to sanctions imposed
by the PRC regulatory authorities, which could include fines and penalties, proceedings against us, and other forms of sanctions, and
our ability to conduct our business, invest into China as foreign investments or accept foreign investments, or be listed on a U.S. or
other overseas exchange may be restricted, and our business, reputation, financial condition, and results of operations may be materially
and adversely affected.
On
February 17, 2023, the CSRC released the Trial Administrative Measures of the Overseas Securities Offering and Listing by Domestic
Companies (《境内企业境外发行证券和上市管理试行办法》)
and five ancillary interpretive guidelines, collectively, the Overseas Listing Trial Measures, as amended, supplemented or otherwise
modified from time to time, which apply to overseas offerings and listing by PRC-based companies, or domestic companies, of equity shares,
depository receipts, corporate bonds convertible to equity shares, and other equity securities, and came into effect on March 31,
2023. According to the Overseas Listing Trial Measures, (1) domestic companies that seek to offer or list securities overseas, both
directly and indirectly, should fulfill the filing procedure and report relevant information to the CSRC, and if an overseas-listed PRC-based
issuer issues new securities in the same overseas market after the overseas offering and listing, it is also required to file with the
CSRC within three business days after the completion of the issuance, or the CSRC Filing; if a domestic company fails to complete the
filing procedure or conceals any material fact or falsifies any major content in its filing documents, such domestic company may be subject
to administrative penalties, such as order to rectify, warnings, fines, and its controlling shareholders, actual controllers, the person
directly in charge and other directly liable persons may also be subject to administrative penalties, such as warnings and fines; (2) if
a foreign-incorporated issuer meets both of the following conditions, its overseas offering and listing shall be determined as an indirect
overseas offering and listing by a domestic company of the PRC: (i) any of the total assets, net assets, revenues or profits of
the domestic operating entities of the issuer in the most recent accounting year accounts for more than 50% of the corresponding line
items in the issuer’s audited consolidated financial statements for the same period; and (ii) its major operational activities
are carried out in China or its main places of business are located in China, or the senior managers in charge of operation and management
of the issuer are mostly Chinese citizens or are domiciled in China; in addition to the aforementioned conditions, the determination
of an indirect overseas offering and listing by a domestic enterprise adheres to the principle of substance over form; and (3) where
a domestic company seeks to indirectly offer and list securities in an overseas market (including issuance of new securities after its
overseas offering and listing), the issuer shall designate a major domestic operating entity responsible for all filing procedures with
the CSRC.
Furthermore, in case any
of the following major events occurs after the overseas offering and listing, the issuer is also required to report the relevant information
to the CSRC within three business days of the occurrence and the announcement of the relevant events: (1) change of control; (2) the
foreign securities regulatory body or the relevant competent authority has taken such measures as investigation and punishment; (3) conversion
of listing status or listing board; and (4) voluntary of compulsory termination of listing. Where there is any material change in
the major business and operation of the issuer after overseas offering and listing, and such change does not fall within the scope of
filing, the issuer shall, within three business days of the occurrence of such change, submit a special report and a legal opinion issued
by a domestic law firm to the CSRC to explain the relevant situation.
As substantially all of our
operations are currently based in the PRC, our future offerings and major changes shall be subject to the filing procedures under the
Overseas Listing Trial Measures. We cannot assure you that we can meet such requirements, obtain the requisite permits from the relevant
government authorities, or complete such filing in a timely manner or at all. Any failure may significantly limit or completely hinder
our ability to continue to offer securities to investors and cause the value of our securities to significantly decline or be worthless.
Recent Developments
In December 2024, we received
a Termination Process Letter from Acrotech of a certain License Agreement (the “License Agreement”), dated September 17, 2014,
between Spectrum Pharmaceuticals, Inc. and us granting the exclusive rights to us to commercialize Evomela® in China, which was later
assigned to Acrotech on March 1, 2019. Acrotech alleged in such letter that we materially breached the License Agreement and failed to
cure such breach, and the License Agreement was therefore terminated. Pursuant to the License Agreement, we can continue to distribute
and sell Evomela® for a reasonable wind-down period not to exceed 24 months, so we do not expect any disruption to our current distribution
plan for Evomela® during such period.
On October 24, 2024, we announced
that the Center for Drug Evaluation of the NMPA has approved our Clinical Trial Application to proceed with a phase 1/2 study of CID-103
in adult patients with chronic Immune Thrombocytopenia in China. This China study is part of the global study that was approved by the
U.S. Food and Drug Administration, or FDA, in May 2024.
In July 2024, with respect
to our previously announced dispute with Juventas, a PRC court issued an asset freezing order against Juventas in aid of the arbitration
proceedings we initiated at the Hong Kong International Arbitration Centre in connection to Juventas’s purported termination of
the parties’ agreements with respect to the commercialization of CNCT19 (the “Arbitration Proceeding”). In an order
dated July 15, 2024 and received by us on July 18, 2024, the First Intermediate People’s Court of Tianjin Municipality, where Juventas
is headquartered, granted our application to freeze Juventas’s assets while the Arbitration Proceeding is ongoing and decided to
freeze up to RMB 190 million in Juventas’s bank accounts or seize and freeze Juventas’s other assets of equivalent value (the
“Asset Freezing Order”). The Asset Freezing Order took effect upon issuance and the court has taken steps to enforce the Asset
Freezing Order.
In July 2024, we entered into
an agreement with PAT and one of the investors of PAT, to purchase 19.8876% equity interest of PAT from the investor. The total consideration
is RMB 28.4 million plus interest and will be paid in three installments. We paid RMB 10.0 million in August 2024. The remaining two installments
will be paid by March 31, 2025 and December 31, 2025, respectively.
On June 26, 2024, we entered
into Subscription Agreements and Subscription and Purchase Agreements with certain investors including Dr. Wei-Wu He, the Chairman of
the board of directors and Chief Executive Officer of the Company and his family trust. On July 15, 2024, the transaction contemplated
under such agreements closed, pursuant to which we issued 1,020,000 ordinary shares and Warrants to purchase 1,980,000 ordinary shares
to the investors for aggregate gross proceeds of approximately $15.0 million, before deducting placement agent fees and other private
placement expenses.
Our board of directors received
a preliminary non-binding proposal letter, or the Proposal Letter, dated June 21, 2024, from Dr. Wei-Wu He, Chairman of the Board and
CEO of the Company, to acquire the entire business operations of the Company in China and all license-in, distribution and related rights
in Asia (excluding Japan) related to all of our pipeline products, including but not limited to EVOMELA®, FOLOTYN®, CNCT19, BI-1206,
CB-5339,CID-103 and Thiotepa, for an aggregate purchase price of $40.0 million, which shall include assumption of up to $20.0 million
of indebtedness of the Company, or the Proposed Transaction. On June 25, 2024, our board of directors formed a special committee comprised
solely of incumbent independent directors, or the Special Committee, to evaluate the transaction contemplated under the Proposal Letter
and such other strategic and business alternatives available to us in respect of the our business operations in China. As of the date
hereof, no decisions have been made by the Special Committee with respect to the Proposed Transaction or any alternative strategic option
that we may pursue.
THE OFFERING
| Ordinary shares offered by us |
|
Ordinary shares, $0.0001 par value, having an aggregate offering price of up to $50.0 million. |
| |
|
|
| Ordinary shares to be outstanding immediately after
this offering |
|
Up to 32,943,574 ordinary shares, assuming a sales price of $2.86 per
share, which was the closing price on The Nasdaq Capital Market on December 18, 2024. The actual number of shares outstanding will
vary depending on the price which ordinary shares may be sold from time to time during this offering. |
| |
|
|
| Plan of Distribution |
|
“At the market offering” that may be made from time to time on the Nasdaq Capital Select
Market or other market for our ordinary shares in the United States through our sales agent, Jefferies LLC. See the section entitled
“Plan of Distribution” on page P-16 of this prospectus. |
| |
|
|
| Use of Proceeds |
|
We currently intend to use the net proceeds from the sale of the ordinary shares offered hereby to fund
the continued development and commercialization of our drug candidates and current products, including development of CID-103, and for
working capital, capital expenditures and general corporate purposes. See “Use of Proceeds” on page P-12 of this
prospectus. |
| |
|
|
| Risk Factors |
|
See “Risk Factors” beginning on page P-8 and other information included and incorporated
by reference in this prospectus for a discussion of factors that you should carefully consider before deciding to invest in the ordinary
shares. |
| |
|
|
| Nasdaq Capital Market symbol |
|
CASI |
The number of ordinary shares
to be outstanding after this offering is based on 15,461,057 ordinary shares outstanding as of September 30, 2024 and excludes:
| · | 3,390,727
ordinary shares issuable upon the exercise of options outstanding as of September 30,
2024 having a weighted-average exercise price of approximately $3.12 per share; |
| · | 1,980,000
ordinary shares issuable upon the exercise of warrants outstanding as of September 30,
2024 having a weighted-average exercise price of approximately $0.0001 per share; |
| · | 1,540,000
ordinary shares reserved for issuance pursuant to future awards under CASI’s 2024 Long-Term
Incentive Plan; and |
| · | 27,064
ordinary shares reserved for issuance pursuant to future awards under CASI Delaware’s
2021 Long-Term Incentive Plan. |
In addition, unless we specifically
state otherwise, all information in this prospectus assumes no exercise of outstanding options to purchase ordinary shares subsequent
to September 30, 2024.
RISK FACTORS
Investing
in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider
carefully the risks and uncertainties described under the section titled “Risk Factors” contained in the applicable
prospectus supplement and any related free writing prospectus, and discussed under the section titled “Risk Factors”
contained in our most recent annual report on Form 20-F for the year ended December 31, 2023, as filed with the SEC on March 28, 2024 and in our current reports on Form 6-K, as well as any amendments thereto reflected in subsequent filings with the
SEC, which are incorporated by reference into this prospectus in their entirety, together with other information in this prospectus,
the documents incorporated by reference and any free writing prospectus that we may authorize for use in connection with a specific offering.
See “Where You Can Find More Information.”
The
risks described in these documents are not the only ones we face, but those that we consider to be material. There may be other unknown
or unpredictable economic, business, competitive, regulatory or other factors that could have material adverse effects on our future
results. Past financial performance may not be a reliable indicator of future performance, and historical trends should not be used to
anticipate results or trends in future periods. If any of these risks actually occur, our business, financial condition, results of operations
or cash flow could be harmed. This could cause the trading price of our securities to decline,
resulting in a loss of all or part of your investment. Please also read carefully the section below titled “Special Note Regarding
Forward-Looking Statements.”
Risks Related to This Offering
Our Recurring Operating Losses have Raised
Substantial Doubt Regarding Our Ability to Continue as a Going Concern.
Our recurring operating losses
raise substantial doubt about our ability to continue as a going concern. Since our inception in 1991, we have incurred significant losses
from operations and, as of June 30, 2024, had incurred an accumulated deficit of $677.3 million. For the six months ended June 30, 2024,
we had a net loss of $16.5 million and a cash outflow for operating activities of $18.1 million. As of June 30, 2024, we had net current
assets of $27.6 million. In addition, we had long term borrowing and non-current dividends payable in a total amount of $19.5 million,
which may become payable on demand if certain events of default occur (including with respect to a certain revenue threshold to be met
by CASI Wuxi in 2024, see Note 9 to our unaudited condensed consolidated financial statements for the six months ended June 30, 2024
included elsewhere in this prospectus (the “Financial Statements”)). We also entered into an agreement with Precision Autoimmune
Therapeutics Co., Ltd., (“PAT”) and one of the investors of PAT, to purchase 19.8876% equity interest of PAT from the investor.
The total consideration is RMB 28.4 million plus interest and will be paid in three installments. We paid RMB 10.0 million in August
2024. The remaining two installments will be paid by March 31, 2025 and December 31, 2025, respectively. Therefore, we will require additional
liquidity to continue our operations over the next 12 months. These factors raise substantial doubt about our ability to continue as
a going concern within a reasonable period of time, which is considered to be one year from the issuance date of the unaudited condensed
consolidated financial statements for the six months ended June 30, 2024. Our financial statements included into this prospectus do not
include any adjustments that might result if we are unable to continue as a going concern and, therefore, be required to realize our
assets and discharge our liabilities other than in the normal course of business, which could cause investors to suffer the loss of all
or a substantial portion of their investment. In order to have sufficient cash and cash equivalents to fund our operations in the future,
we will need to raise additional equity or debt capital and cannot provide any assurance that we will be successful in doing so. The
perception of our ability to continue as a going concern may make it more difficult for us to obtain financing for the continuation of
our operations and could result in the loss of confidence by investors, suppliers and employees.
If you purchase our ordinary shares sold
in this offering, you may experience immediate and substantial dilution in the net tangible book value of your shares. In addition, we
may issue additional equity or convertible debt securities in the future, which may result in additional dilution to investors.
The price per ordinary share
being offered may be higher than the net tangible book value per share of our outstanding ordinary shares prior to this offering. Assuming
that an aggregate of 17,482,517 ordinary shares are sold at a price of $2.86 per share, the last reported sale price of our ordinary shares
on the Nasdaq Capital Select Market on December 18, 2024, for aggregate gross proceeds of approximately $50.0 million and
after deducting commissions and estimated offering expenses payable by us, new investors in this offering would incur immediate dilution
of $1.02 per share. For a more detailed discussion of the foregoing, see the section entitled “Dilution” below. To
the extent outstanding stock options or warrants are exercised, there will be further dilution to new investors. In addition, to the extent
we need to raise additional capital in the future and we issue additional ordinary shares or securities convertible or exchangeable for
our ordinary shares, our then existing stockholders may experience dilution and the new securities may have rights senior to those of
our ordinary shares offered in this offering.
We
have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our
management will have broad discretion in the application of the net proceeds from this offering, including for any of the purposes described
in the section entitled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision
to assess whether the net proceeds are being used appropriately. Because of the number and variability of factors that will determine
our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. Our management
might not apply our net proceeds in ways that ultimately increase the value of your investment. We currently intend to use the
net proceeds from the sale of the ordinary shares offered hereby to fund the continued development and commercialization of our drug
candidates and current products, including development of CID-103, and for working capital, capital expenditures and general corporate
purposes. The failure by our management to apply these funds effectively could harm our business.
Pending their use, we may invest the net proceeds from this offering in short-term, investment-grade, interest-bearing securities. These
investments may not yield a favorable return to our stockholders. If we do not invest or apply the net proceeds from this offering in
ways that enhance stockholder value, we may fail to achieve expected financial results, which could cause our stock price to decline.
Sales
of a substantial number of our ordinary shares in the public market, or the perception that such sales could occur, could depress the
market price of our ordinary shares.
Sales
of a substantial number of our ordinary shares in the public market, or the perception that such sales could occur, could depress the
market price of our ordinary shares and impair our ability to raise capital through the sale of additional equity securities. We cannot
predict the effect that future sales of our ordinary shares, including under the Sales Agreement, would have on the market price of our
ordinary shares.
The actual number of ordinary shares we
will sell under the Sales Agreement and the resulting gross proceeds are uncertain.
Subject to certain limitations
in the Sales Agreement and compliance with applicable law, we have the discretion to deliver a placement notice to Jefferies at any time
throughout the term of the Sales Agreement. The number of ordinary shares that are sold through Jefferies after we deliver a placement
notice will fluctuate based on the market price of our ordinary shares during the sales period and limits we set in the placement notice.
Because the price per share sold will fluctuate based on the market price of our ordinary shares during the sales period, it is not possible
to predict the number of our ordinary shares that will be ultimately sold or the resulting gross proceeds.
The ordinary shares offered in this offering
will be sold in “at the market offerings.” Investors who purchase our ordinary shares in this offering at different times
will likely pay different prices.
Investors who purchase our
ordinary shares in this offering at different times will likely pay different prices, and so may experience different outcomes in their
investment results. We will have discretion, subject to market demand, to vary the timing, prices and numbers of our ordinary shares
sold, and subject to certain limitations in the Sales Agreement, there is no minimum or maximum sales price. Investors may experience
a decline in the value of their ordinary shares and dilution as a result of sales made at prices lower than the prices they paid.
The price of our ordinary shares is and
may continue to be volatile and you may not be able to resell our securities at or above the price you paid.
The market price for our
ordinary shares is volatile and may fluctuate significantly in response to a number of factors, most of which we cannot control, such
as fluctuations in financial results, our ability to advance the development and commercialization of our drug candidates or changes
in securities analysts’ recommendations. In addition, our ordinary shares have been and may continue to be affected by limited
trading volume. Each of these factors, among others, could harm your investment in our ordinary shares and could result in you being
unable to resell the shares that you purchased at a price equal to or above the price you paid.
SPECIAL NOTE REGARDING
FORWARD-LOOKING STATEMENTS
This
prospectus and the documents we have filed with the SEC that are incorporated by reference contain forward-looking statements within
the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the
Exchange Act. The statements contained in this prospectus or incorporated by reference herein that are not purely historical are forward-looking
statements. Our forward-looking statements include, but are not limited to, statements regarding our or our management’s expectations,
hopes, beliefs, intentions or strategies regarding the future. In addition, any statements that refer to projections, forecasts or other
characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements.
You can identify some of
these forward-looking statements by words or phrases such as “may,” “will,” “expect,” “anticipate,”
“aim,” “estimate,” “intend,” “plan,” “believe,” “is/are likely to,”
“potential,” “continue” or other similar expressions. These forward-looking statements include, among others,
statements regarding the timing of our commercial launch of products, clinical trials, our cash position and future expenses, our future
revenues, and future financings, including any sale of our ordinary shares under the “at-the-market” program described herein.
We have based these forward-looking statements largely on our current expectations and projections about future events that we believe
may affect our financial condition, results of operations, business strategy and financial needs.
Actual
results could differ materially from those currently anticipated due to a number of factors, including: uncertainties related to the
Proposal Letter to acquire the Company’s business operations in China; the risk that we may be unable to continue as a going concern
as a result of our inability to raise sufficient capital for our operational needs; the possibility that we may be delisted from trading
on The Nasdaq Capital Market if we fail to satisfy applicable continued listing standards; the volatility in the market price of our
ordinary shares; the risk of substantial dilution of existing shareholders in future share issuances; the difficulty of executing our
business strategy on a global basis including China; our inability to enter into strategic partnerships for the development, commercialization,
manufacturing and distribution of our proposed product candidates or future candidates; legal or regulatory developments in China that
adversely affect our ability to operate in China; our lack of experience in manufacturing products and uncertainty about our resources
and capabilities to do so on a clinical or commercial scale; risks relating to the commercialization, if any, of our products and proposed
products (such as marketing, safety, regulatory, patent, product liability, supply, competition and other risks); our inability to predict
when or if our product candidates will be approved for marketing by the U.S. FDA, EMA, NMPA, or other regulatory authorities; our inability
to receive approval for renewal of license of our existing products; the risks relating to the need for additional capital and the uncertainty
of securing additional funding on favorable terms; the risks associated with our product candidates, and the risks associated with our
other early-stage products under development; the risk that preclinical and clinical models are not necessarily indicative of clinical
results; uncertainties relating to preclinical and clinical trials, including delays to the commencement of such trials; our ability
to protect our intellectual property rights; the lack of success in the clinical development of any of our products and our dependence
on third parties; the risks related to our dependence on Juventas to partner with us to co-market CNCT19; risks related to the uncertainty
in connection with the ongoing arbitration proceedings between us and Juventas with respect to Juventas’ purported termination
of certain CNCT19 license agreements; risks related to our dependence on Juventas to ensure the patent protection and prosecution for
CNCT19; risks related to the Company’s ongoing development of and regulatory application for CID-103 with respect to the treatment
of antibody-mediated rejection for organ transplant and autoimmune diseases and the license arrangements of CID-103; risks relating to
the interests of our largest shareholder and our Chairman and Chief Executive Officer that differ from our other shareholders; and risks
related to the success of a new manufacturing facility operated by CASI Wuxi. Such factors, among others, could have a material adverse
effect upon our business, results of operations and financial condition.
The
forward-looking statements contained in this prospectus are based on our current expectations and beliefs concerning future developments
and their potential effects on us. There can be no assurance that future developments affecting us will be those that we have anticipated.
These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or other assumptions
that may cause actual results or performance to be materially different from those expressed
or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to, those described in the
section titled “Risk Factors” and elsewhere in this prospectus. Should one or more of these risks or uncertainties
materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these
forward-looking statements. We discuss in greater detail many of these risks under the section titled “Risk Factors”
contained in the applicable prospectus supplement, in any free writing prospectuses we may authorize for use in connection with a specific
offering, and in our most recent annual report on Form 20-F, as well as any amendments thereto reflected in subsequent filings
with the SEC, as well as our reports on Form 6-K, which are incorporated by reference into this prospectus in their entirety. Also,
these forward-looking statements represent our estimates and assumptions only as of the date of the document containing the applicable
statement. Unless required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information
or future events or developments. You should read this prospectus, any applicable prospectus supplement, together with the documents
we have filed with the SEC that are incorporated by reference and any free writing prospectus that we may authorize for use in connection
with a specific offering completely and with the understanding that our actual future results may be materially different from what we
expect. We qualify all of the forward-looking statements in the foregoing documents by these cautionary statements.
In
addition, statements that “we believe” and similar statements reflect our beliefs
and opinions on the relevant subject. These statements are based upon information available to us as of the date of this prospectus,
and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and
our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available
relevant information. These statements are inherently uncertain and you are cautioned not to unduly rely upon these statements.
USE OF PROCEEDS
We may offer and sell our
ordinary shares having an aggregate offering price of up to $50.0 million from time to time through Jefferies. The amount of proceeds from
this offering will depend upon the number of ordinary shares sold and the market price at which they are sold and will be reduced by
commissions and other expenses of this offering. There can be no assurance that we will be able to sell any shares under or fully utilize
the Sales Agreement as a source of financing. Because there is no minimum offering amount required as a condition to close this offering,
the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time.
We currently intend to use
the net proceeds from this offering, if any, together with our cash and cash equivalents, primarily to fund the continued development
and commercialization of our drug candidates, including development of CID-103,and for working capital, capital expenditures and general
corporate purposes. Our expected use of the net proceeds from this offering represents our current intentions based on our present plans
and business condition, which could change as our plans and business conditions evolve. The amounts and timing of our actual use of the
net proceeds from this offering will vary depending on numerous factors, including our ability or desire to sell our ordinary shares
under the Sales Agreement. As a result, we cannot predict with certainty all of the particular uses for any net proceeds to be received
or the amounts that we will actually spend on the uses set forth above. Our board of directors and our management retain broad discretion
in the application of the net proceeds from this offering.
Pending these uses, we intend
to invest additional net proceeds, if any, in short- and intermediate-term interest-bearing financial instruments.
CAPITALIZATION
The following table sets
forth the capitalization of the Company as of September 30, 2024:
| · | on an as adjusted basis to give effect
to the sale of our ordinary shares in the aggregate amount of $50.0 million at an assumed public offering price of $2.86 per share,
which was the last reported sale price of our ordinary shares on The Nasdaq Capital Market on December 18, 2024, and after deducting
estimated commissions equal to 3% and estimated offering expenses in an amount equal to $503,500 payable by us. |
The
information below should be read in conjunction with, and is qualified in its entirety by, the audited consolidated financial
statements and schedules and notes thereto included in our annual report on Form 20-F for the financial year ended December 31, 2023, as incorporated by reference into this prospectus.
| |
|
As of September 30, 2024 |
|
| (In USD thousands, except share and per share data) |
|
Actual |
|
|
As Adjusted |
|
| Long term borrowing: |
|
$ |
19,123 |
|
|
$ |
19,123 |
|
| |
|
|
|
|
|
|
|
|
| Shareholder’s equity: |
|
|
|
|
|
|
|
|
| Ordinary shares, $0.0001 par value, 500,000,000 shares authorized, 15,461,057 shares issued and outstanding on an actual basis, and 28,151,412 shares issued and outstanding on an adjusted basis. |
|
$ |
2 |
|
|
$ |
3 |
|
| Treasury shares, at cost, 411,952 shares |
|
$ |
(9,604 |
) |
|
$ |
(9,604 |
) |
| Subscription receivable |
|
$ |
(1,975 |
) |
|
$ |
(1,975 |
) |
| Additional paid-in capital |
|
$ |
712,597 |
|
|
$ |
760,593 |
|
| Accumulated other comprehensive loss |
|
$ |
(1,468 |
) |
|
$ |
(1,468 |
) |
| Accumulated deficit |
|
$ |
(685,705 |
) |
|
$ |
(685,705 |
) |
| Total shareholders’ equity |
|
$ |
13,847 |
|
|
$ |
61,844 |
|
| Long term borrowing and total shareholders’ equity |
|
$ |
32,970 |
|
|
$ |
80,967 |
|
The information in the table
above is based on 15,461,057 ordinary shares outstanding as of September 30, 2024 and excludes:
| · | 3,390,727
ordinary shares issuable upon the exercise of options outstanding as of September 30,
2024 having a weighted-average exercise price of approximately $3.12 per share; |
| · | 1,980,000
ordinary shares issuable upon the exercise of warrants outstanding as of September 30,
2024 having a weighted-average exercise price of approximately $0.0001 per share; |
| · | 1,540,000
ordinary shares reserved for issuance pursuant to future awards under CASI’s 2024 Long-Term
Incentive Plan; and |
| · | 27,064
ordinary shares reserved for issuance pursuant to future awards under CASI Delaware’s
2021 Long-Term Incentive Plan. |
In addition, unless we specifically
state otherwise, all information in this prospectus assumes no exercise of outstanding options to purchase ordinary shares subsequent
to September 30, 2024.
DIVIDEND POLICY
We have never declared or
paid any cash dividends on our ordinary shares. We have no present plan to declare and pay any dividends on our ordinary shares in the
near future. We currently intend to retain most, if not all, of our available funds and any future earnings to operate and expand our
business. Any future determination to pay dividends will be at the discretion of our board of directors, subject to applicable laws,
and will depend on our financial condition, results of operations, capital requirements, general business conditions and other factors
that our board of directors considers relevant.
DILUTION
If you purchase any of our
ordinary shares, you will experience dilution to the extent of the difference between the offering price per ordinary share you pay in
this offering and the net tangible book value per ordinary share immediately after this offering.
Our net tangible book value as of September 30, 2024 was $12.7
million, or $0.82 per ordinary share based on 15,461,057 shares issued and outstanding. Net tangible book value per share represents our
total assets of $63.6 million, less our intangible assets, net of $1.1 million, less our total liabilities of $49.8 million, divided by
the aggregate number of our ordinary shares outstanding as of September 30, 2024. Dilution in net tangible book value per ordinary
share represents the difference between the amount per ordinary share paid by purchasers of ordinary shares in this offering and the as
adjusted net tangible book value per ordinary share of our ordinary shares immediately after giving effect to this offering.
After giving effect to the sale of our ordinary shares in the aggregate
amount of $50.0 million at an assumed offering price of $2.86 per ordinary share, the last reported sale price of our ordinary shares
on The Nasdaq Capital Market on December 18, 2024, and after deducting estimated offering commissions and other estimated offering
expenses payable by us, our as adjusted net tangible book value as of September 30, 2024 would have been $60.7 million, or approximately
$1.84 per ordinary share based on an aggregate of 32,943,574 ordinary shares issued and outstanding. This amount represents an immediate
increase in net tangible book value of $1.02 per ordinary share to existing shareholders as a result of this offering and immediate dilution
of approximately $1.02 per ordinary share to new investors purchasing our ordinary shares in this offering.
The following table illustrates
this dilution on a per ordinary share basis. The as adjusted information below is illustrative only and will adjust based on the actual
price to the public, the actual number of shares sold and other terms of the offering determined at the time our ordinary shares are
sold pursuant to this prospectus. The ordinary shares sold in this offering, if any, will be sold from time to time at various prices.
| Assumed public offering price per ordinary
share | |
|
| | |
$ |
2.86 |
|
| Net tangible book
value per ordinary share as of September 30, 2024 | |
$ | 0.82 | | |
|
|
|
| Increase
in net tangible book value per ordinary share attributable to this offering | |
$ | 1.02 | | |
|
|
|
| As-adjusted
net tangible book value per ordinary share after giving effect to this offering | |
| | | |
$ |
1.84 |
|
| Dilution
per ordinary share to new investors participating in this offering | |
| | | |
$ |
1.02 |
|
The ordinary shares sold in this offering, if any, will be sold from
time to time at various prices. Assuming all of the ordinary shares in an aggregate amount of $50.0 million are sold in this offering
at the assumed public offering price of $2.86 per share, a $1.00 increase in such offering price would increase our as adjusted net tangible
book value per share after this offering to $2.14 per share and dilution to new investors to $1.72 per share, after deducting commissions
and estimated offering expenses payable by us. A $1.00 decrease in the assumed public offering price of $2.86 per share would decrease
our as adjusted net tangible book value per share after this offering to $1.43 per share and dilution to new investors to $0.43 per share,
after deducting commissions and estimated offering expenses payable by us. The information discussed above is illustrative only and will
adjust based on the actual public offering price, the actual number of shares that we offer in this offering, and other terms of this
offering determined at the time of each offer and sale.
The above discussion and
table are based on 15,461,057 ordinary shares outstanding as of September 30, 2024 and excludes:
| · | 3,390,727
ordinary shares issuable upon the exercise of options outstanding as of September 30,
2024 having a weighted-average exercise price of approximately $3.12 per share; |
| · | 1,980,000
ordinary shares issuable upon the exercise of warrants outstanding as of September 30,
2024 having a weighted-average exercise price of approximately $0.0001 per share; |
| · | 1,540,000
ordinary shares reserved for issuance pursuant to future awards under CASI’s 2024 Long-Term
Incentive Plan; and |
| · | 27,064
ordinary shares reserved for issuance pursuant to future awards under CASI Delaware’s
2021 Long-Term Incentive Plan. |
PLAN OF DISTRIBUTION
We have entered into a Sales
Agreement with Jefferies, under which we may offer and sell our ordinary shares from time to time through Jefferies acting as agent.
Pursuant to this prospectus, we may offer and sell up to $50.0 million of our ordinary shares. Sales of our ordinary shares, if any, under
this prospectus will be made by any method that is deemed to be an “at the market offering” as defined in Rule 415(a)(4) under
the Securities Act.
Each time we wish to issue
and sell ordinary shares under the Sales Agreement, we will notify Jefferies of the number of shares to be issued, the dates on which
such sales are anticipated to be made, any limitation on the number of shares to be sold in any one day and any minimum price below which
sales may not be made. Once we have so instructed Jefferies, unless Jefferies declines to accept the terms of such notice, Jefferies
has agreed to use its commercially reasonable efforts consistent with its normal trading and sales practices to sell such shares up to
the amount specified on such terms. The obligations of Jefferies under the Sales Agreement to sell our ordinary shares are subject to
a number of conditions that we must meet.
The settlement of sales of
shares between us and Jefferies is generally anticipated to occur on the first trading day following the date on which the sale was made.
Sales of our ordinary shares as contemplated in this prospectus will be settled through the facilities of The Depository Trust Company
or by such other means as we and Jefferies may agree upon. There is no arrangement for funds to be received in an escrow, trust or similar
arrangement.
We will pay Jefferies a commission
up to 3.0% of the aggregate gross proceeds we receive from each sale of our ordinary shares. Because there is no minimum offering amount
required as a condition to close this offering, the actual total public offering amount, commissions and proceeds to us, if any, are
not determinable at this time. In addition, we have agreed to reimburse Jefferies for the fees and disbursements of its counsel, payable
upon execution of the Sales Agreement, in an amount not to exceed $250,000, in addition to certain ongoing disbursements of its legal
counsel, unless we and Jefferies otherwise agree. We estimate that the total expenses for the offering, excluding any commissions or
expense reimbursement payable to Jefferies under the terms of the Sales Agreement, will be approximately $253,500. The remaining sale
proceeds, after deducting any other transaction fees, will equal our net proceeds from the sale of such shares.
Jefferies will provide written
confirmation to us before the open on The Nasdaq Capital Market on the day following each day on which ordinary shares are sold under
the Sales Agreement. Each confirmation will include the number of shares sold on that day, the aggregate gross proceeds of such sales
and the proceeds to us.
In connection with the sale
of the ordinary shares on our behalf, Jefferies will be deemed to be an “underwriter” within the meaning of the Securities
Act, and the compensation of Jefferies will be deemed to be underwriting commissions or discounts. We have agreed to indemnify Jefferies
against certain civil liabilities, including liabilities under the Securities Act. We have also agreed to contribute to payments Jefferies
may be required to make in respect of such liabilities.
The offering of our ordinary
shares pursuant to the Sales Agreement will terminate as permitted therein.
This summary of the material
provisions of the Sales Agreement does not purport to be a complete statement of its terms and conditions. A copy of the Sales Agreement
is filed as an exhibit to the registration statement of which this prospectus forms a part.
Jefferies and its affiliates
may in the future provide various investment banking, commercial banking, financial advisory and other financial services for us and
our affiliates, for which services they may in the future receive customary fees. In the course of its business, Jefferies may actively
trade our securities for its own account or for the accounts of customers, and, accordingly, Jefferies may at any time hold long or short
positions in such securities.
A prospectus supplement and
the accompanying prospectus in electronic format may be made available on a website maintained by Jefferies, and Jefferies may distribute
the prospectus supplement and the accompanying prospectus electronically.
Jefferies LLC’s address
is 520 Madison Avenue, New York, New York 10022.
ENFORCEABILITY OF CIVIL
LIABILITIES
CASI is incorporated in the
Cayman Islands because of certain benefits associated with being a Cayman Islands company, such as political and economic stability,
an effective judicial system, a favorable tax system, the absence of foreign exchange control or currency restrictions and the availability
of professional and support services. However, the Cayman Islands has a less developed body of securities laws as compared to the United
States and provides less protection for investors. In addition, Cayman Islands companies do not have standing to sue before the federal
courts of the United States.
A majority portion of CASI’s
assets are located outside the United States. In addition, a majority of CASI’s directors and officers are nationals or residents
of jurisdictions other than the United States and all or a majority portion of their assets are located outside the United States. As
a result, it may be difficult for investors to effect service of process within the United States upon CASI or these persons, or to bring
an action against CASI or against these persons in the United States, in the event that you believe that your rights have been infringed
under the securities laws of the United States or any state in the United States. It may also be difficult for you to enforce in U.S.
courts judgments obtained in U.S. courts based on the civil liability provisions of the U.S. federal securities laws against CASI and
its officers and directors.
Maples and Calder (Hong Kong)
LLP, our counsel as to Cayman Islands law, has advised us that there is uncertainty as to whether the courts of the Cayman Islands would
(1) recognize or enforce judgments of U.S. courts obtained against CASI or its directors or officers, predicated upon the civil
liability provisions of the securities laws of the United States or any state in the United States, or (2) entertain original actions
brought in the Cayman Islands against CASI or its directors or officers, predicated upon the securities laws of the United States or
any state in the United States.
Maples and Calder (Hong Kong)
LLP has informed us that although there is no statutory enforcement in the Cayman Islands of judgments obtained in the federal or state
courts of the United States (and the Cayman Islands are not a party to any treaties for the reciprocal enforcement or recognition of
such judgments), a judgment obtained in such jurisdiction will be recognized and enforced in the courts of the Cayman Islands at common
law, without any re-examination of the merits of the underlying dispute, by an action commenced on the foreign judgment debt in the Grand
Court of the Cayman Islands, provided such judgment (a) is given by a foreign court of competent jurisdiction, (b) imposes
on the judgment debtor a liability to pay a liquidated sum for which the judgment has been given, (c) is final, (d) is not
in respect of taxes, a fine or a penalty, and (e) was not obtained in a manner and is not of a kind the enforcement of which is
contrary to natural justice or the public policy of the Cayman Islands. However, the Cayman Islands courts are unlikely to enforce a
judgment obtained from the U.S. courts under civil liability provisions of the U.S. federal securities law if such judgment is determined
by the courts of the Cayman Islands to give rise to obligations to make payments that are penal or punitive in nature. Because such a
determination has not yet been made by a court of the Cayman Islands, it is uncertain whether such civil liability judgments from U.S.
courts would be enforceable in the Cayman Islands.
It is our understanding that
the PRC does not have treaties with the United States and many other countries providing for the reciprocal recognition and enforcement
of judgments of courts and that there is uncertainty as to whether the courts of the PRC would recognize or enforce judgments of United
States courts against CASI or the directors or officers of CASI predicated upon the civil liability provisions of the securities laws
of the United States or any state in the United States.
Additionally, it is our understanding
that it may be difficult for you to bring an original action against CASI or against its directors and officers who are nationals or
residents of countries other than the United States in a PRC court in the event that you believe that your rights have been infringed
under the U.S. federal securities laws, PRC laws, Cayman Islands laws or otherwise because we are incorporated under the laws of the
Cayman Islands and it may be difficult for U.S. shareholders, by virtue only of holding CASI ordinary shares, to establish a connection
to the PRC as required by the PRC Civil Procedures Law in order for a PRC court to have jurisdiction.
TAXATION
Information regarding taxation
is set forth under the heading “Item 10.E. Taxation” in our annual report on Form 20-F for the financial year ended December 31, 2023, which is incorporated in this prospectus by reference, as updated by our subsequent filings under the Exchange
Act.
LEGAL MATTERS
Maples and Calder (Hong Kong)
LLP will pass upon the validity of the securities being registered hereby and certain other legal matters of Cayman Islands law in
connection with the registration of such securities. Harter Secrest & Emery LLP will pass upon certain matters of U.S. federal
and New York law for us in connection with the registration of certain securities being registered hereby. Jefferies LLC is represented
in connection with this offering by Cooley LLP, New York, New York.
EXPERTS
The consolidated financial
statements of CASI Pharmaceuticals, Inc. as of December 31, 2023 and 2022, and for each of the years in the three-year period
ended December 31, 2023, have been incorporated by reference herein in reliance upon the report of KPMG Huazhen LLP, independent
registered public accounting firm, incorporated by reference herein, and upon the authority of said firm as experts in accounting and
auditing.
WHERE YOU CAN FIND MORE
INFORMATION
We are subject to the information
requirements of the Exchange Act that are applicable to foreign private issuers. Accordingly, we are required to file reports and other
information with the SEC, including annual reports on Form 20-F and disclosure furnished under cover of Form 6-K. The SEC maintains
a website (www.sec.gov) that contains reports and other information regarding issuers, such as us, that file electronically with
the SEC. We also maintain a website (www.casipharmaceuticals.com) from which you can access such reports and other information
free of charge as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information
contained on our website does not form a part of this prospectus.
As a foreign private issuer,
we are exempt under the Exchange Act from rules prescribing the furnishing and content of proxy statements, and our officers, directors
and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the
Exchange Act. In addition, we are not required under the Exchange Act to file periodic financial statements with the SEC as frequently
or as promptly as U.S. companies whose securities are registered under the Exchange Act.
We have filed with the SEC
a “shelf” registration statement (including amendments and exhibits to the registration statement) on Form F-3 under
the Securities Act. This prospectus, which is part of such registration statement, does not contain all of the information set forth
in the Registration Statement and the exhibits and schedules to the Registration Statement. We have omitted parts of the registration
statement in accordance with the rules and regulations of the SEC. For more detail about us and the securities offered by this prospectus,
you may examine the registration statement and the exhibits filed with it at the website provided in the previous paragraph. You should
rely only on the information contained in this prospectus, any applicable prospectus supplement, any free writing prospectus and the
documents incorporated by reference herein and therein. We have not authorized anyone else to provide you with different information.
We are not making an offer of these securities in any state where the offer is not permitted.
INCORPORATION BY REFERENCE
The SEC allows us to incorporate
by reference much of the information that we file with the SEC, which means that we can disclose important information to you by referring
you to those publicly available documents. The information that we incorporate by reference in this prospectus is considered to be part
of this prospectus. Because we are incorporating by reference future filings with the SEC, this prospectus is continually updated and
those future filings may modify or supersede some of the information included or incorporated by reference in this prospectus. This means
that you must look at all of the SEC filings that we incorporate by reference to determine if any of the statements in this prospectus
or in any document previously incorporated by reference have been modified or superseded.
This prospectus and any accompanying
prospectus supplement incorporate by reference the documents set forth below that have previously been filed with the SEC (other than
those documents or the portions of those documents that are “furnished” unless otherwise specified below):
| · | our reports on Form 6-K furnished with the SEC on April 8, 2024, June 27, 2024 (Film No. 241075375), June 27, 2024 (Film No. 241075377), July 8, 2024, July 19, 2024, September 12, 2024,
November 15, 2024, and December 16, 2024; and |
All reports and other documents
we subsequently file pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act, prior to the termination of this offering,
including all such documents we may file with the SEC after the date of the initial registration statement and prior to the effectiveness
of the registration statement, but excluding any information furnished to, rather than filed with, the SEC, will also be incorporated
by reference into this prospectus and deemed to be part of this prospectus from the date of the filing of such reports and documents.
We may incorporate by reference any reports on Form 6-K that we furnish to the SEC that we specifically identify in such form as
being incorporated by reference into this prospectus after the date hereof and prior to the completion or termination of the offering
of securities under this prospectus.
Any statement contained herein
or in a document, all or a portion of which is incorporated or deemed to be incorporated by reference herein, shall be deemed to be modified
or superseded for purposes of this prospectus to the extent that a statement contained herein or in any other subsequently filed document
which also is or is deemed to be incorporated by reference herein modifies or supersedes such statement. Any such statement so modified
or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus. Notwithstanding the
foregoing, no information is incorporated by reference in this prospectus or any prospectus supplement hereto where such information
under applicable forms and regulations of the SEC is not deemed to be “filed” under Section 18 of the Exchange Act or
otherwise subject to the liabilities of that section, unless the report or filing containing such information indicates that the information
therein is to be considered “filed” under the Exchange Act or is to be incorporated by reference in this prospectus or any
prospectus supplement hereto.
We will provide to each
person, including any beneficial owner, to whom a prospectus is delivered, without charge upon written or oral request, a copy of any
or all of the documents that are incorporated by reference into this prospectus but not delivered with the prospectus, including exhibits
that are specifically incorporated by reference into such documents. You may request a copy of such documents at no cost, by writing
or telephoning us at the following address or telephone number:
CASI Pharmaceuticals, Inc.
9620 Medical Center Drive, Suite 300
Rockville, MD 20850
240-864-2600
EXPENSES
The following is a statement
of estimated expenses in connection with the issuance and distribution of the securities offered by this Sales Agreement prospectus,
all of which will be paid by us.
| Expense |
|
Estimated
Amount |
|
| Printing expenses |
|
|
7,500 |
|
| Legal fees and expenses |
|
|
395,000 |
|
| Accounting fees and expenses |
|
|
100,000 |
|
| Miscellaneous costs |
|
|
1,000 |
|
| Total |
|
$ |
503,500 |
|

Up to $50,000,000
Ordinary Shares
PROSPECTUS
Jefferies
,
2024
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 8. Indemnification of Directors and Officers
The laws of the Cayman Islands
do not limit the extent to which a company’s memorandum and articles of association may provide for indemnification of officers
and directors, except to the extent any such provision may be held by the Cayman Islands courts to be contrary to public policy, such
as to provide indemnification against willful default, willful neglect, civil fraud or the consequences of committing a crime.
The CASI Articles provide
that every director (including alternate director), secretary, assistant secretary, or other officer for the time being and from time
to time of CASI (but not including CASI’s auditors) and the personal representatives of the same (each an “Indemnified Person”)
shall be indemnified and secured harmless against all actions, proceedings, costs, charges, expenses, losses, damages or liabilities
incurred or sustained by such Indemnified Person, other than by reason of such Indemnified Person’s own dishonesty, willful default
or fraud, in or about the conduct of CASI’s business or affairs (including as a result of any mistake of judgment) or in the execution
or discharge of his or her duties, powers, authorities or discretions, including without prejudice to the generality of the foregoing,
any costs, expenses, losses or liabilities incurred by such Indemnified Person in defending (whether successfully or otherwise) any civil
proceedings concerning CASI or its affairs in any court whether in the Cayman Islands or elsewhere.
CASI will also enter into
indemnification agreements with its directors and officers under the laws of the Cayman Islands, pursuant to which we have agreed to
indemnify each such person and hold him or her harmless against expenses, judgments, fines and amounts payable under settlement agreements
in connection with any threatened, pending or completed action, suit or proceeding to which he has been made a party or in which he became
involved by reason of the fact that he or she is or was our director or officer. Except with respect to expenses to be reimbursed by
CASI in the event that the indemnified person has been successful on the merits or otherwise in defense of the action, suit or proceeding,
CASI’s obligations under the indemnification agreements are subject to certain customary restrictions and exceptions.
In addition, CASI will maintain
standard policies of insurance under which coverage is provided to its directors and officers against loss rising from claims made by
reason of breach of duty or other wrongful act, and to CASI with respect to payments which may be made by CASI to such directors and
officers pursuant to the above indemnification provision or otherwise as a matter of law.
Insofar as indemnification
for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling CASI pursuant to the
foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed
in the Securities Act and is theretofore unenforceable.
Item 9. Exhibits and Financial Statement Schedules
| * |
Filed herewith. |
| |
|
| ** |
To be filed, if necessary,
by amendment. |
Item 10. Undertakings
(a) The undersigned registrant hereby undertakes:
(1) To file, during any period
in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus
required by Section 10(a)(3) of the Securities Act;
(ii) To reflect in the prospectus
any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof)
which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding
the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed
that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the
form of prospectus filed with the Securities and Exchange Commission, or the Commission, pursuant to Rule 424(b) if, in the
aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the
“Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material
information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to
such information in the registration statement;
provided,
however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) of this section do not apply if the registration statement
is on Form S-3 or Form F-3 and the information required to be included in a post-effective amendment by those paragraphs is
contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or Section 15(d) of
the Exchange Act that are incorporated by reference in the registration statement, or, as
to a registration statement on Form F–3, is contained in a form of prospectus filed pursuant to Rule 424(b) that
is part of the registration statement.
(2) That, for the purpose of
determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement
relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona
fide offering thereof.
(3) To remove from registration
by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) To file a post-effective
amendment to the registration statement to include any financial statements required by Item 8.A of Form 20-F at the start of any
delayed offering or throughout a continuous offering. Financial statements and information otherwise required by Section 10(a)(3) of
the Securities Act need not be furnished, provided that the registrant includes in the prospectus, by means of a post-effective amendment,
financial statements required pursuant to this paragraph (a)(4) and other information necessary to ensure that all other information
in the prospectus is at least as current as the date of those financial statements. Notwithstanding the foregoing, with respect to registration
statements on Form F-3, a post-effective amendment need not be filed to include financial statements and information required by
Section 10(a)(3) of the Securities Act or Item 8.A of Form 20-F if such financial statements and information are contained
in periodic reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or Section 15(d) of
the Exchange Act that are incorporated by reference in the Form F-3.
(5) That, for the purpose of
determining liability under the Securities Act to any purchaser:
(A) Each prospectus filed by the
registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus
was deemed part of and included in the registration statement; and
(B) Each prospectus required to
be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating
to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by Section 10(a) of
the Securities Act shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of
prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the
prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such
date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement
to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide
offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the
registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus
that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede
or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made
in any such document immediately prior to such effective date.
(6) That, for the purpose of
determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, the
undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration
statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold
to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will
be considered to offer or sell such securities to such purchaser:
(A) Any preliminary prospectus
or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(B) Any free writing prospectus
relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(C) The portion of any other free
writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided
by or on behalf of the undersigned registrant; and
(D) Any other communication that
is an offer in the offering made by the undersigned registrant to the purchaser.
(b) The undersigned registrant hereby undertakes
that, for purposes of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant
to Section 13(a) or Section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit
plan’s annual report pursuant to Section 15(d) of the Exchange Act) that is incorporated by reference in the registration
statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities
at that time shall be deemed to be the initial bona fide offering thereof.
(c) Insofar as indemnification for liabilities
arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing
provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification
is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification
against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling
person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling
person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been
settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against
public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements
of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements
for filing on Form F-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto
duly authorized, in the city of Beijing, the Peoples Republic of China, on the 20th day of December, 2024.
| |
CASI Pharmaceuticals, Inc. |
|
| |
|
|
| By: |
/s/
Dr. Wei-Wu He |
|
| |
Name: |
Dr. Wei-Wu
He |
|
| |
Title: |
Chief Executive Officer
and Chairman of the Board of Directors |
|
Power of Attorney
KNOW ALL PERSONS BY THESE
PRESENTS, that each person whose signature appears below hereby constitutes and appoints Wei-Wu He and Daniel Lang, each acting alone,
as his or her true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for such person and in
his or her name, place and stead in any and all capacities, in connection with this registration statement, including to sign in the
name and on behalf of the undersigned, this registration statement and any and all amendments thereto, including post-effective amendments
and registrations filed pursuant to Rule 462 under the Securities Act of 1933, and to file the same, with all exhibits thereto,
and other documents in connection therewith, with the Securities and Exchange Commission, granting unto such attorneys-in-fact and agents,
each acting alone, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and
about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all
that said attorney-in-fact and agent, or her substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements
of the Securities Act of 1933, as amended, this Registration Statement has been signed by the following persons in the capacities and
on the dates indicated:
| Name |
Title |
Date |
| |
|
|
| /s/ Dr. Wei-Wu
He |
Chief Executive Officer
and Chairman of the Board |
December 20,
2024 |
| Dr. Wei-Wu He |
(Principal Executive
Officer) |
|
| |
|
|
| /s/ Daniel
Lang |
Chief Financial Officer
|
December 20,
2024 |
| Daniel
Lang |
(Principal Financial
and Accounting Officer) |
|
| |
|
|
| /s/ Y.
Alexander Wu |
Independent Director |
December 20,
2024 |
| Y. Alexander Wu |
|
|
| |
|
|
| /s/ Zhenbo Su |
Independent Director |
December 20,
2024 |
| Zhenbo Su |
|
|
| |
|
|
| /s/ Thomas
Folinsbee |
Independent Director |
December 20,
2024 |
| Thomas Folinsbee |
|
|
| |
|
|
| /s/ Xuebo Zeng
|
Independent Director |
December 20,
2024 |
| Xuebo Zeng |
|
|
AUTHORIZED REPRESENTATIVE
Pursuant to the requirement
of the Securities Act, the undersigned, the duly undersigned representative in the United States of CASI Pharmaceuticals, Inc.,
has signed this registration statement in the United States, on the 20th day of December, 2024.
| CASI Pharmaceuticals, Inc. |
|
| |
|
|
| By: |
/s/
Daniel Lang |
|
| |
Name: Daniel Lang |
|
| |
Title: Chief Financial
Officer |
|
Exhibit 1.2
OPEN
MARKET SALE AGREEMENTSM
December 20, 2024
JEFFERIES LLC
520 Madison Avenue
New York, New York 10022
Ladies and Gentlemen:
CASI Pharmaceuticals, Inc.,
an exempted company incorporated under the laws of the Cayman Islands (the “Company”), proposes, subject to the terms
and conditions stated herein, to issue and sell from time to time through Jefferies LLC, as sales agent and/or principal (the “Agent”),
ordinary shares of the Company, par value $0.0001 per share (the “Common Shares”), on the terms set forth in this agreement
(this “Agreement”).
Section 1. DEFINITIONS
(a) Certain
Definitions. For purposes of this Agreement, capitalized terms used herein and not otherwise defined shall have the following respective
meanings:
“Affiliate”
of a Person means another Person that directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under
common control with, such first- mentioned Person. The term “control” (including the terms “controlling,” “controlled
by” and “under common control with”) means the possession, direct or indirect, of the power to direct or cause the direction
of the management and policies of a Person, whether through the ownership of voting securities, by contract or otherwise.
“Agency Period”
means the period commencing on the date of this Agreement and expiring on the earliest to occur of (x) the date on which the Agent
shall have placed the Maximum Program Amount pursuant to this Agreement and (y) the date this Agreement is terminated pursuant to
Section 7.
“Commission”
means the U.S. Securities and Exchange Commission.
“Exchange Act”
means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the Commission thereunder.
“Floor Price”
means the minimum price set by the Company in the Issuance Notice below which the Agent shall not sell Shares during the applicable period
set forth in the Issuance Notice, which may be adjusted by the Company at any time during the period set forth in the Issuance Notice
by delivering written notice of such change to the Agent and which in no event shall be less than $1.00 without the prior written consent
of the Agent, which may be withheld in the Agent’s sole discretion.
SM
“Open Market Sale Agreement” is a service mark of Jefferies LLC
“Issuance Amount”
means the aggregate Sales Price of the Shares to be sold by the Agent pursuant to any Issuance Notice.
“Issuance Notice”
means a written notice delivered to the Agent by the Company in accordance with this Agreement in the form attached hereto as Exhibit A
that is executed by its Chief Executive Officer, President or Chief Financial Officer.
“Issuance Notice
Date” means any Trading Day during the Agency Period that an Issuance Notice is delivered pursuant to Section 3(b)(i).
“Issuance Price”
means the Sales Price less the Selling Commission.
“Maximum Program
Amount” means Common Shares with an aggregate Sales Price of the lesser of (a) the number or dollar amount of Common Shares
registered under the effective Registration Statement (defined below) pursuant to which the offering is being made, (b) the number
of authorized but unissued Common Shares (less Common Shares issuable upon exercise, conversion or exchange of any outstanding securities
of the Company or otherwise reserved from the Company’s authorized capital stock), (c) the number or dollar amount of Common
Shares permitted to be sold under Form F-3 (including General Instruction I.B.5 thereof, if applicable), or (d) the number or
dollar amount of Common Shares for which the Company has filed a Prospectus (defined below).
“Person”
means an individual or a corporation, partnership, limited liability company, trust, incorporated or unincorporated association, joint
venture, joint stock company, governmental authority or other entity of any kind.
“Principal Market”
means the Nasdaq Capital Market, or such other national securities exchange on which the Common Shares, including any Shares, are then
listed.
“PRC” means
the People’s Republic of China, which only for purposes hereof excludes Hong Kong, Macao and Taiwan.
“PRC Business Day”
means a day on which China Securities Regulatory Commission is open for general business in the PRC.
“Sales Price”
means the actual sale execution price of each Share placed by the Agent pursuant to this Agreement.
“Securities Act”
means the Securities Act of 1933, as amended, and the rules and regulations of the Commission thereunder.
“Selling Commission”
means three percent (3.0%) of the gross proceeds of Shares sold pursuant to this Agreement, or as otherwise agreed between the Company
and the Agent with respect to any Shares sold pursuant to this Agreement.
“Settlement Date”
means the first business day following each Trading Day during the period set forth in the Issuance Notice on which Shares are sold pursuant
to this Agreement, when the Company shall deliver to the Agent the amount of Shares sold on such Trading Day and the Agent shall deliver
to the Company the Issuance Price received on such sales.
“Shares”
means the Company’s Common Shares issued or issuable pursuant to this Agreement.
“Trading Day”
means any day on which the Principal Market is open for trading.
Section 2. REPRESENTATIONS
AND WARRANTIES OF THE COMPANY
The Company represents and
warrants to, and agrees with, the Agent that as of (1) the date of this Agreement, (2) each Issuance Notice Date, (3) each
Settlement Date, (4) each Triggering Event Date and (5) as of each Time of Sale (each of the times referenced above is referred
to herein as a “Representation Date”), except as may be disclosed in the Prospectus (including any documents incorporated
by reference therein and any supplements thereto) on or before a Representation Date:
(a) Registration
Statement. The Company has prepared and filed, or concurrently with the execution of this Agreement will file, with the Commission
a shelf registration statement on Form F-3 that contains a base prospectus (the “Base Prospectus”). Such registration
statement registers the issuance and sale by the Company of the Shares under the Securities Act. The Company may file one or more additional
registration statements from time to time that will contain a base prospectus and related prospectus or prospectus supplement, if applicable,
with respect to the Shares. Except where the context otherwise requires, such registration statement(s), including any information deemed
to be a part thereof pursuant to Rule 430B under the Securities Act, including all financial statements, exhibits and schedules thereto
and all documents incorporated or deemed to be incorporated therein by reference pursuant to Item 6 of Form F-3 under the Securities
Act as from time to time amended or supplemented, is herein referred to as the “Registration Statement,” and the prospectus
constituting a part of such registration statement(s), together with any prospectus supplement filed with the Commission pursuant to Rule 424(b) under
the Securities Act relating to a particular issuance of the Shares, including all documents incorporated or deemed to be incorporated
therein by reference pursuant to Item 6 of Form F-3 under the Securities Act, in each case, as from time to time amended or supplemented,
is referred to herein as the “Prospectus,” except that if any revised prospectus is provided to the Agent by the Company
for use in connection with the offering of the Shares that is not required to be filed by the Company pursuant to Rule 424(b) under
the Securities Act, the term “Prospectus” shall refer to such revised prospectus from and after the time it is first
provided to the Agent for such use. The Registration Statement at the time it originally became effective is herein called the “Original
Registration Statement.” As used in this Agreement, the terms “amendment” or “supplement” when applied
to the Registration Statement or the Prospectus shall be deemed to include the filing by the Company with the Commission of any document
under the Exchange Act after the date hereof that is or is deemed to be incorporated therein by reference.
All references in this Agreement
to financial statements and schedules and other information which is “contained,” “included” or “stated”
in the Registration Statement or the Prospectus (and all other references of like import) shall be deemed to mean and include all such
financial statements and schedules and other information which is or is deemed to be incorporated by reference in or otherwise deemed
under the Securities Act to be a part of or included in the Registration Statement or the Prospectus, as the case may be, as of any specified
date; and all references in this Agreement to amendments or supplements to the Registration Statement or the Prospectus shall be deemed
to mean and include, without limitation, the filing of any document under the Exchange Act which is or is deemed to be incorporated by
reference in or otherwise deemed under the Securities Act to be a part of or included in the Registration Statement or the Prospectus,
as the case may be, as of any specified date. The Company’s obligations under this Agreement to furnish, provide, deliver or make
available (and all other references of like import) copies of any report or statement shall be deemed satisfied if the same is filed with
the Commission through its Electronic Data Gathering, Analysis and Retrieval system (“EDGAR”).
At the time the Original Registration
Statement was or will be declared effective and at the time the Company’s most recent annual report on Form 20-F was filed
with the Commission, if later, the Company met the then-applicable requirements for use of Form F-3 under the Securities Act. During
the Agency Period, each time the Company files an annual report on Form 20-F the Company will meet the then-applicable requirements
for use of Form F-3 under the Securities Act.
(b) Compliance
with Registration Requirements. The Original Registration Statement and any Rule 462(b) Registration Statement have been
or will be declared effective by the Commission under the Securities Act. The Company has complied to the Commission’s satisfaction
with all requests of the Commission for additional or supplemental information, if any, with respect to the Registration Statement. No
stop order suspending the effectiveness of the Registration Statement or any Rule 462(b) Registration Statement is in effect
and no proceedings for such purpose have been instituted or are pending or, to the knowledge of the Company, are contemplated or threatened
by the Commission.
The Prospectus when filed
complied or will comply in all material respects with the Securities Act and, if filed with the Commission through EDGAR (except as may
be permitted by Regulation S-T under the Securities Act), was identical to the copy thereof delivered to the Agent for use in connection
with the issuance and sale of the Shares. Each of the Registration Statement, any Rule 462(b) Registration Statement and any
post-effective amendment thereto, at the time it became or becomes effective and at each Representation Date, complied and will comply
in all material respects with the Securities Act and did not and will not contain any untrue statement of a material fact or omit to state
a material fact required to be stated therein or necessary to make the statements therein not misleading. As of the date of this Agreement,
the Prospectus and any Free Writing Prospectus (as defined below) considered together (collectively, the “Time of Sale Information”)
did not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements therein, in
the light of the circumstances under which they were made, not misleading. The Prospectus, as amended or supplemented, as of its date
and at each Representation Date, did not and will not contain any untrue statement of a material fact or omit to state a material fact
necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading. The representations
and warranties set forth in the three immediately preceding sentences do not apply to statements in or omissions from the Registration
Statement, any Rule 462(b) Registration Statement, or any post-effective amendment thereto, or the Prospectus, or any amendments
or supplements thereto, made in reliance upon and in conformity with information relating to the Agent furnished to the Company in writing
by the Agent expressly for use therein, it being understood and agreed that the only such information furnished by the Agent to the Company
consists of the information described in Section 6 below. There are no contracts or other documents required to be described
in the Prospectus or to be filed as exhibits to the Registration Statement which have not been described or filed as required. The Registration
Statement and the offer and sale of the Shares as contemplated hereby meet the requirements of Rule 415 under the Securities Act
and comply in all material respects with said rule.
(c) Ineligible
Issuer Status. The Company is not an “ineligible issuer” in connection with the offering of the Shares pursuant to Rules 164,
405 and 433 under the Securities Act. Any Free Writing Prospectus that the Company is required to file pursuant to Rule 433(d) under
the Securities Act has been, or will be, filed with the Commission in accordance with the requirements of the Securities Act. Each Free
Writing Prospectus that the Company has filed, or is required to file, pursuant to Rule 433(d) under the Securities Act or that
was prepared by or on behalf of or used or referred to by the Company complies or will comply in all material respects with the requirements
of Rule 433 under the Securities Act including timely filing with the Commission or retention where required and legending, and each
such Free Writing Prospectus, as of its issue date and at all subsequent times through the completion of the issuance and sale of the
Shares did not, does not and will not include any information that conflicted, conflicts with or will conflict with the information contained
in the Registration Statement or the Prospectus, including any document incorporated by reference therein. Except for the Free Writing
Prospectuses, if any, and electronic road shows, if any, furnished to the Agent before first use, the Company has not prepared, used or
referred to, and will not, without the Agent’s prior consent, prepare, use or refer to, any Free Writing Prospectus.
(d) Incorporated
Documents. The documents incorporated or deemed to be incorporated by reference in the Registration Statement and the Prospectus,
at the time they were filed with the Commission, complied in all material respects with the requirements of the Exchange Act, as applicable,
and, when read together with the other information in the Prospectus, do not contain an untrue statement of a material fact or omit to
state a material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which
they were made, not misleading.
(e) Exchange
Act Compliance. The documents incorporated or deemed to be incorporated by reference in the Prospectus, at the time they were or hereafter
are filed with the Commission, and any Free Writing Prospectus or amendment or supplement thereto complied and will comply in all material
respects with the requirements of the Exchange Act, and, when read together with the other information in the Prospectus, at the time
the Registration Statement and any amendments thereto become effective and at each Time of Sale (as defined below), as the case may be,
will not contain an untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to
make the fact required to be stated therein or necessary to make the statements therein, in the light of the circumstances under which
they were made, not misleading.
(f) Foreign
Private Issuer. The Company is a “foreign private issuer” within the meaning of Rule 405 under the Securities Act.
(g) Statistical
and Market-Related Data. All statistical, demographic and market-related data included in the Registration Statement or the Prospectus
are based on or derived from sources that the Company believes, after reasonable inquiry, to be reliable and accurate. To the extent required,
the Company has obtained the written consent for the use of such data from such sources.
(h) Disclosure
Controls and Procedures; Deficiencies in or Changes to Internal Control Over Financial Reporting. The Company has established and
maintains disclosure controls and procedures (as defined in Rules 13a-15 and 15d-15 under the Exchange Act), which (i) are designed
to ensure that material information relating to the Company, including its consolidated subsidiaries, is made known to the Company’s
principal executive officer and its principal financial officer by others within those entities, particularly during the periods in which
the periodic reports required under the Exchange Act are being prepared; (ii) have been evaluated by management of the Company for
effectiveness as of the end of the Company’s most recent fiscal quarter; and (iii) are effective in all material respects to
perform the functions for which they were established. The Company’s internal controls over financial reporting were effective as
of the completion of the Company’s most recent fiscal year. Since the completion of the Company’s most recent fiscal year,
there have been no significant deficiencies or material weaknesses in the Company’s internal control over financial reporting (whether
or not remediated) and no change in the Company’s internal control over financial reporting that has materially affected, or is
reasonably likely to materially affect, the Company’s internal control over financial reporting. The Company is not aware of any
change in its internal control over financial reporting that has occurred during its most recent fiscal quarter that has materially affected,
or is reasonably likely to materially affect, the Company’s internal control over financial reporting, and is not aware of any fraud
that involves management or other employees of the Company who have a significant role in the Company’s internal reporting.
(i) This
Agreement. This Agreement has been duly authorized, executed and delivered by, and is a valid and binding agreement of, the Company,
enforceable in accordance with its terms, except as rights to indemnification hereunder may be limited by applicable law and except as
the enforcement hereof may be limited by bankruptcy, insolvency, reorganization, moratorium or other similar laws relating to or affecting
the rights and remedies of creditors or by general equitable principles.
(j) Authorization
of the Shares. The Shares have been duly authorized for issuance and sale pursuant to this Agreement and, when issued and delivered
by the Company against payment therefor pursuant to this Agreement, will be validly issued, fully paid and nonassessable, and the issuance
and sale of the Shares is not subject to any preemptive rights, rights of first refusal or other similar rights to subscribe for or purchase
the Shares.
(k) No
Applicable Registration or Other Similar Rights. There are no persons with registration or other similar rights to have any equity
or debt securities registered for sale under the Registration Statement or included in the offering contemplated by this Agreement, except
for such rights as have been duly waived.
(l) No
Material Adverse Change. Except as otherwise disclosed in the Registration Statement and the Prospectus, subsequent to the respective
dates as of which information is given in the Registration Statement and the Prospectus: (i) there has been no material adverse change,
or any development that could reasonably be expected to result in a material adverse change, in (A) the condition, financial or otherwise,
or in the earnings, business, properties, operations, operating results, assets, liabilities or prospects, whether or not arising from
transactions in the ordinary course of business, of the Company and its subsidiaries, considered as one entity or (B) the ability
of the Company to consummate the transactions contemplated by this Agreement or perform its obligations hereunder (any such change being
referred to herein as a “Material Adverse Change”); (ii) the Company and its subsidiaries, considered as one entity,
have not incurred any material liability or obligation, indirect, direct or contingent, including without limitation any losses or interference
with their business from fire, explosion, flood, earthquakes, accident or other calamity, whether or not covered by insurance, or from
any strike, labor dispute or court or governmental action, order or decree, that are material, individually or in the aggregate, to the
Company and its subsidiaries, considered as one entity, and have not entered into any material transactions not in the ordinary course
of business; and (iii) there has not been any material decrease in the capital stock or any material increase in any short-term or
long-term indebtedness of the Company or its subsidiaries and there has been no dividend or distribution of any kind declared, paid or
made by the Company or, except for dividends paid to the Company or other subsidiaries, by any of the Company’s subsidiaries on
any class of capital stock, or any repurchase or redemption by the Company or any of its subsidiaries of any class of capital stock.
(m) Independent
Accountants. KPMG Huazhen LLP, or such other accountants, which has (y) expressed its opinion with respect to the financial statements
(which term as used in this Agreement includes the related notes thereto) or (z) reviewed the financial statements, each as filed
with the Commission as a part of the Registration Statement and the Prospectus, is (i) an independent registered public accounting
firm as required by the Securities Act, the Exchange Act, and the rules of the Public Company Accounting Oversight Board (“PCAOB”),
(ii) in compliance with the applicable requirements relating to the qualification of accountants under Rule 2-01 of Regulation
S-X under the Securities Act and (iii) a registered public accounting firm as defined by the PCAOB whose registration has not been
suspended or revoked and who has not requested such registration to be withdrawn.
(n) Financial
Statements. The financial statements filed with the Commission as a part of the Registration Statement and included in the Prospectus
present fairly, in all material respects, the consolidated financial position of the Company and its subsidiaries as of the dates indicated
and the results of their operations, changes in shareholders’ equity and cash flows for the periods specified. Such financial statements
and supporting schedules have been prepared in conformity with generally accepted accounting principles as applied in the United States
applied on a consistent basis throughout the periods involved, except as may be expressly stated in the related notes thereto. The interactive
data in eXtensible Business Reporting Language included or incorporated by reference in the Registration Statement fairly presents the
information called for in all material respects and has been prepared in accordance with the Commission’s rules and guidelines
applicable thereto. No other financial statements or supporting schedules are required to be included in the Registration Statement or
the Prospectus. The financial data set forth in each of the Registration Statement and the Prospectus fairly present, in all material
respects, the information set forth therein on a basis consistent with that of the audited financial statements contained in the Registration
Statement and the Prospectus. All disclosures contained in the Registration Statement and the Prospectus that constitute non GAAP financial
measures (as defined by the rules and regulations under the Securities Act and the Exchange Act) comply with Regulation G under the
Exchange Act and Item 10 of Regulation S-K under the Securities Act, as applicable. To the Company’s knowledge, no person who has
been suspended or barred from being associated with a registered public accounting firm, or who has failed to comply with any sanction
pursuant to Rule 5300 promulgated by the PCAOB, has participated in or otherwise aided the preparation of, or audited, the financial
statements, supporting schedules or other financial data filed with or furnished to the Commission as a part of the Registration Statement
and the Prospectus.
(o) Company’s
Accounting System. The Company and each of its subsidiaries make and keep accurate books and records and maintain a system of internal
accounting controls sufficient to provide reasonable assurance that: (i) transactions are executed in accordance with management’s
general or specific authorization; (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity
with generally accepted accounting principles as applied in the United States and to maintain accountability for assets; (iii) access
to assets is permitted only in accordance with management’s general or specific authorization; (iv) the recorded accountability
for assets is compared with existing assets at reasonable intervals and appropriate action is taken with respect to any differences; and
(v) the interactive data in eXtensible Business Reporting Language included or incorporated by reference in the Registration Statement
and the Prospectus fairly presents the information called for in all material respects and is prepared in accordance with the Commission’s
rules and guidelines applicable thereto.
(p) Incorporation
and Good Standing of the Company. The Company has been duly incorporated and is validly existing as a corporation in good standing
under the laws of the jurisdiction of its incorporation and has the corporate power and authority to own, lease and operate its properties
and to conduct its business as described in the Registration Statement and the Prospectus and to enter into and perform its obligations
under this Agreement. The Company is duly qualified as a foreign corporation to transact business and is in good standing in each jurisdiction
in which such qualification is required, whether by reason of the ownership or leasing of property or the conduct of business, except
where the failure to so qualify or be in good standing, individually or in the aggregate, would not reasonably be expected to result in
a Material Adverse Change.
(q) Subsidiaries.
Each of the Company’s “subsidiaries” (for purposes of this Agreement, as defined in Rule 405 under the Securities
Act) has been duly incorporated or organized, as the case may be, and is validly existing as a corporation, partnership or limited liability
company, as applicable, in good standing under the laws of the jurisdiction of its incorporation or organization and has the power and
authority (corporate or other) to own, lease and operate its properties and to conduct its business as described in the Registration Statement
and the Prospectus. Each of the Company’s subsidiaries is duly qualified as a foreign corporation, partnership or limited liability
company, as applicable, to transact business and is in good standing in each jurisdiction in which such qualification is required, whether
by reason of the ownership or leasing of property or the conduct of business, except where the failure to so qualify or be in good standing,
individually or in the aggregate, would not reasonably be expected to result in a Material Adverse Change. All of the issued and outstanding
capital stock or other equity or ownership interests of each of the Company’s subsidiaries have been duly authorized and validly
issued, are fully paid and nonassessable and are owned by the Company, directly or through subsidiaries, free and clear of any security
interest, mortgage, pledge, lien, encumbrance or adverse claim. None of the outstanding capital stock or equity interest in any subsidiary
was issued in violation of preemptive or similar rights of any security holder of such subsidiary. The constitutive or organizational
documents of each of the subsidiaries comply in all material respects with the requirements of applicable laws of its jurisdiction of
incorporation or organization and are in full force and effect. The Company does not own or control, directly or indirectly, any corporation,
association or other entity other than the subsidiaries listed in Exhibit 8.1 to the Company’s most recent Annual Report on
Form 20-F.
(r) Capitalization
and Other Capital Stock Matters. The authorized, issued and outstanding capital stock of the Company is as set forth in the Registration
Statement and the Prospectus (other than for subsequent issuances, if any, pursuant to employee benefit plans or upon the exercise of
outstanding options or warrants, in each case described in the Registration Statement and the Prospectus). The Common Shares (including
the Shares) conform in all material respects to the description thereof contained in the Prospectus. All of the issued and outstanding
Common Shares have been duly authorized and validly issued, are fully paid and nonassessable and have been issued in compliance with all
federal and state securities laws. None of the outstanding Common Shares was issued in violation of any preemptive rights, rights of first
refusal or other similar rights to subscribe for or purchase securities of the Company. There are no authorized or outstanding options,
warrants, preemptive rights, rights of first refusal or other rights to purchase, or equity or debt securities convertible into or exchangeable
or exercisable for, any capital stock or other equity or ownership interests of the Company or any of its subsidiaries other than those
described in the Registration Statement and the Prospectus. The descriptions of the Company’s stock option, stock bonus and other
stock plans or arrangements, and the options or other rights granted thereunder, set forth in the Registration Statement and the Prospectus
accurately and fairly presents, in all material respects, the information required to be shown with respect to such plans, arrangements,
options and rights.
(s) Stock
Exchange Listing. The Common Shares are registered pursuant to Section 12(b) or 12(g) of the Exchange Act and are listed
on the Principal Market, and the Company has taken no action designed to, or which to its knowledge is likely to have the effect of, terminating
the registration of the Common Shares under the Exchange Act or delisting the Common Shares from the Principal Market, nor has the Company
received any notification that the Commission or the Principal Market is contemplating terminating such registration or listing. To the
Company’s knowledge, it is in compliance with all applicable listing requirements of the Principal Market.
(t) Non-Contravention
of Existing Instruments; No Further Authorizations or Approvals Required. Neither the Company nor any of its subsidiaries is in violation
of its charter or bylaws, partnership agreement or operating agreement or similar organizational documents, as applicable, or is in default
(or, with the giving of notice or lapse of time, would be in default) (“Default”) under any indenture, loan, credit
agreement, note, lease, license agreement, contract, franchise or other instrument (including, without limitation, any pledge agreement,
security agreement, mortgage or other instrument or agreement evidencing, guaranteeing, securing or relating to indebtedness) to which
the Company or any of its subsidiaries is a party or by which it or any of them may be bound, or to which any of their respective properties
or assets are subject (each, an “Existing Instrument”), except for such Defaults as would not reasonably be expected,
individually or in the aggregate, to result in a Material Adverse Change. The Company’s execution, delivery and performance of this
Agreement, consummation of the transactions contemplated hereby and by the Registration Statement and the Prospectus and the issuance
and sale of the Shares (including the use of proceeds from the sale of the Shares as described in the Registration Statement and the Prospectus
under the caption “Use of Proceeds”) (i) have been duly authorized by all necessary corporate action and will not result
in any violation of the provisions of the charter or bylaws, partnership agreement or operating agreement or similar organizational documents,
as applicable, of the Company or any subsidiary (ii) will not conflict with or constitute a breach of, or Default or a Debt Repayment
Triggering Event (as defined below) under, or result in the creation or imposition of any lien, charge or encumbrance upon any property
or assets of the Company or any of its subsidiaries pursuant to, or require the consent of any other party to, any Existing Instrument
and (iii) will not result in any violation of any law, administrative regulation or administrative or court decree applicable to
the Company or any of its subsidiaries, except in the case of (ii) and (iii), for any such conflict, breach, violation, Default,
Debt Repayment Triggering Event, lien, charge or encumbrance that would not, individually or in the aggregate, reasonably be expected
to result in a Material Adverse Change. No consent, approval, authorization or other order of, or registration or filing with, any court
or other governmental or regulatory authority or agency, is required for the Company’s execution, delivery and performance of this
Agreement and consummation of the transactions contemplated hereby and by the Registration Statement and the Prospectus, except such as
have been obtained or made by the Company and are in full force and effect under the Securities Act and such as may be required under
applicable state securities or blue sky laws or FINRA (as defined below). As used herein, a “Debt Repayment Triggering Event”
means any event or condition which gives, or with the giving of notice or lapse of time would give, the holder of any note, debenture
or other evidence of indebtedness (or any person acting on such holder’s behalf) the right to require the repurchase, redemption
or repayment of all or a portion of such indebtedness by the Company or any of its subsidiaries.
(u) No
Material Actions or Proceedings. Except as otherwise disclosed in the Prospectus, there is no action, suit, proceeding, inquiry or
investigation brought by or before any legal or governmental entity now pending or, to the knowledge of the Company, threatened, against
or affecting the Company or any of its subsidiaries, which could be expected, individually or in the aggregate, to result in a Material
Adverse Change or adversely affect the consummation of the transactions contemplated by this Agreement or any such action, suit or proceeding
is or would be material in the context of the sale of Shares. No material labor dispute with the employees of the Company or any of its
subsidiaries, or with the employees of any principal supplier, manufacturer, customer or contractor of the Company, exists or, to the
knowledge of the Company, is threatened or imminent.
(v) Intellectual
Property Rights. Except as otherwise disclosed in the Registration Statement or the Prospectus, the Company and its subsidiaries own,
or have obtained valid and enforceable non-exclusive licenses for, the inventions, patent applications, patents, trademarks, trade names,
service names, copyrights, trade secrets and other intellectual property described in the Registration Statement and the Prospectus as
being owned or licensed by them or which are necessary for the conduct of their respective businesses as currently conducted or as currently
proposed to be conducted (collectively, “Intellectual Property”) and to the Company’s knowledge, the conduct
of their respective businesses does not and will not infringe, misappropriate or otherwise conflict in any material respect with any such
rights of others. The Intellectual Property of the Company has not been adjudged by a court of competent jurisdiction to be invalid or
unenforceable, in whole or in part, and the Company is unaware of any facts which would form a reasonable basis for any such adjudication.
To the Company’s knowledge: (i) there are no third parties who have rights to any Intellectual Property, except for customary
reversionary rights of third-party licensors with respect to Intellectual Property that is disclosed in the Registration Statement and
the Prospectus as licensed to the Company or one or more of its subsidiaries; and (ii) there is no infringement by third parties
of any Intellectual Property. There is no pending or, to the Company’s knowledge, threatened action, suit, proceeding or claim by
others: (A) challenging the Company’s rights in or to any Intellectual Property, and the Company is unaware of any facts which
would form a reasonable basis for any such action, suit, proceeding or claim; (B) challenging the validity, enforceability or scope
of any Intellectual Property, and the Company is unaware of any facts which would form a reasonable basis for any such action, suit, proceeding
or claim; or (C) asserting that the Company or any of its subsidiaries infringes or otherwise violates, or would, upon the commercialization
of any product or service described in the Registration Statement or the Prospectus as under development, infringe or violate, any patent,
trademark, trade name, service name, copyright, trade secret or other proprietary rights of others, and the Company is unaware of any
facts which would form a reasonable basis for any such action, suit, proceeding or claim. The Company and its subsidiaries have complied
with the terms of each agreement pursuant to which Intellectual Property has been licensed to the Company or any subsidiary, and all such
agreements are in full force and effect, except in such cases as would not reasonably be expected, individually or in the aggregate, to
result in a Material Adverse Change. To the Company’s knowledge, there are no material defects in any of the patents or patent applications
included in the Intellectual Property. The Company and its subsidiaries have taken all reasonable steps to protect, maintain and safeguard
their Intellectual Property, except in such cases as would not reasonably be expected, individually or in the aggregate, to result in
a Material Adverse Change, including the execution of appropriate nondisclosure, confidentiality agreements and invention assignment agreements
and invention assignments with their employees, and no employee of the Company is in or has been in violation of any term of any employment
contract, patent disclosure agreement, invention assignment agreement, non-competition agreement, non-solicitation agreement, nondisclosure
agreement, or any restrictive covenant to or with a former employer where the basis of such violation relates to such employee’s
employment with the Company. To the Company’s knowledge, the duty of candor and good faith as required by the United States Patent
and Trademark Office during the prosecution of the United States patents and patent applications included in the Intellectual Property
have been complied with; and in all foreign offices having similar requirements, all such requirements have been complied with. To the
Company’s knowledge, none of the Company owned Intellectual Property or technology (including information technology and outsourced
arrangements) employed by the Company or its subsidiaries has been obtained or is being used by the Company or its subsidiary in violation
of any contractual obligation binding on the Company or its subsidiaries or any of their respective officers, directors or employees or
otherwise in violation of the rights of any persons. The product candidates described in the Registration Statement and the Prospectus
as under development by the Company or any subsidiary fall within the scope of the claims of one or more patents owned by, or exclusively
licensed to, the Company or any subsidiary.
(w) All
Necessary Permits, etc. Except as otherwise disclosed in the Prospectus, the Company and each subsidiary possess such valid and
current certificates, authorizations or permits required by state, federal or foreign regulatory agencies or bodies necessary for the
ownership or lease of its properties or to conduct their respective businesses as currently conducted and as described in the Registration
Statement or the Prospectus (“Permits”), or to permit all clinical studies and trials conducted by or on behalf of
the Company and its subsidiaries, including, without limitation, all necessary Food and Drug Administration of the U.S. Department of
Health and Human Services (the “FDA”) and applicable foreign regulatory agency approvals and registrations; and neither
the Company nor any of its subsidiaries is in violation of, or in default under, any of the Permits or has received, or has any reason
to believe that it will receive, any notice of proceedings relating to the revocation or modification of, or noncompliance with, any such
Permit that, if the subject of an unfavorable decision, ruling or finding, would reasonably be expected, individually or in the aggregate,
to result in a Material Adverse Change.
(x) Title
to Properties. The Company and each of its subsidiaries own no real property. Except as otherwise disclosed in the Prospectus, the
Company and its subsidiaries have good and marketable title to all of the personal property and other assets reflected as owned in the
financial statements referred to in Section 2(n) above or elsewhere in the Registration Statement or the Prospectus,
in each case free and clear of any security interests, mortgages, liens, encumbrances, equities, adverse claims and other defects, except
as would not reasonably be expected, individually or in the aggregate, to have a Material Adverse Change. The real property, improvements,
equipment and personal property held under lease by the Company or any of its subsidiaries are held under valid and enforceable leases,
with such exceptions as are not material and do not materially interfere with the use made or proposed to be made of such real property,
improvements, equipment or personal property by the Company or such subsidiary.
(y) Tax
Law Compliance. The Company and its subsidiaries have filed all necessary federal, state and foreign income and franchise tax returns
or have properly requested extensions thereof and have paid all taxes required to be paid by any of them and, if due and payable, any
related or similar assessment, fine or penalty levied against any of them except as may be being contested in good faith and by appropriate
proceedings. The Company has made adequate charges, accruals and reserves in the applicable financial statements referred to in Section 2(n) above
in respect of all federal, state and foreign income and franchise taxes for all periods as to which the tax liability of the Company or
any of its subsidiaries has not been finally determined.
(z) Company
Not an “Investment Company.” The Company is not, and will not be, either after receipt of payment for the Shares or after
the application of the proceeds therefrom as described under “Use of Proceeds” in the Registration Statement or the Prospectus,
required to register as an “investment company” under the Investment Company Act of 1940, as amended (the “Investment
Company Act”).
(aa) Insurance.
Except as otherwise disclosed in the Prospectus, each of the Company and its subsidiaries are insured by recognized, financially sound
and reputable institutions with policies in such amounts and with such deductibles and covering such risks as are generally deemed adequate
by the Company and customary for the Company’s and its subsidiaries’ businesses including, but not limited to, policies covering
real and personal property owned or leased by the Company and its subsidiaries against theft, damage, destruction, acts of vandalism and
earthquakes and policies covering the Company and its subsidiaries for product liability claims and clinical trial liability claims. The
Company has no reason to believe that it or any of its subsidiaries will not be able (i) to renew its existing insurance coverage
as and when such policies expire or (ii) to obtain comparable coverage from similar institutions as may be necessary or appropriate
to conduct its business as now conducted and at a cost that would not reasonably be expected to result in a Material Adverse Change. Neither
the Company nor any of its subsidiaries has been denied any insurance coverage which it has sought or for which it has applied.
(bb) No
Price Stabilization or Manipulation; Compliance with Regulation M. Neither the Company nor any of its subsidiaries has taken, directly
or indirectly, any action designed to or that might cause or result in stabilization or manipulation of the price of the Common Shares
or of any “reference security” (as defined in Rule 100 of Regulation M under the Exchange Act (“Regulation M”))
with respect to the Common Shares, whether to facilitate the sale or resale of the Shares or otherwise, and has taken no action which
would directly or indirectly violate Regulation M.
(cc) Related
Party Transactions. There are no business relationships or related-party transactions involving the Company or any of its subsidiaries
or any other person required to be described in the Registration Statement or the Prospectus which have not been described as required.
(dd) FINRA
Matters. All of the information provided to the Agent or to counsel for the Agent by the Company, its counsel, its officers and directors
and the holders of any securities (debt or equity) or options to acquire any securities of the Company in connection with the offering
of the Shares is true, complete, correct and compliant with Financial Industry Regulatory Authority, Inc.’s (“FINRA”)
rules and any letters, filings or other supplemental information provided to FINRA pursuant to FINRA Rules or NASD Conduct Rules is
true, complete and correct. Neither the Company nor any of its Affiliates directly, or indirectly through one or more intermediaries,
controls, or is controlled by, or is under common control with, or is a person associated with any member firm of FINRA.
(ee) No
Unlawful Contributions or Other Payments. Except as otherwise disclosed in the Prospectus, neither the Company nor any of its subsidiaries
nor, to the Company’s knowledge, any employee or agent of the Company or any subsidiary, has made any contribution or other payment
to any official of, or candidate for, any federal, state or foreign office in violation of any applicable law or of the character required
to be disclosed in the Registration Statement and the Prospectus.
(ff) Compliance
with Environmental Laws. Except as described in the Prospectus and except as would not reasonably be expected, individually or in
the aggregate, to result in a Material Adverse Change; (i) neither the Company nor any of its subsidiaries is in violation of any
federal, state, local or foreign statute, law, rule, regulation, ordinance, code, policy or rule of common law or any judicial or
administrative interpretation thereof, including any judicial or administrative order, consent, decree or judgment, relating to pollution
or protection of human health, the environment (including, without limitation, ambient air, surface water, groundwater, land surface or
subsurface strata) or wildlife, including, without limitation, laws and regulations relating to the release or threatened release of chemicals,
pollutants, contaminants, wastes, toxic substances, hazardous substances, petroleum or petroleum products (collectively, “Hazardous
Materials”) or to the manufacture, processing, distribution, use, treatment, storage, disposal, transport or handling of Hazardous
Materials (collectively, “Environmental Laws”), (ii) the Company and its subsidiaries have all permits, authorizations
and approvals required under any applicable Environmental Laws and are each in compliance with their requirements, (iii) there are
no pending or threatened administrative, regulatory or judicial actions, suits, demands, demand letters, claims, liens, notices of noncompliance
or violation, investigation or proceedings relating to any Environmental Law against the Company or any of its subsidiaries and (iv) to
the knowledge of the Company, there are no events or circumstances that might reasonably be expected to form the basis of an order for
clean-up or remediation, or an action, suit or proceeding by any private party or governmental body or agency, against or affecting the
Company or any of its subsidiaries relating to Hazardous Materials or any Environmental Laws.
(gg) ERISA
Compliance. Except as otherwise disclosed in the Prospectus, the Company and its subsidiaries and any “employee benefit plan”
(as defined under the Employee Retirement Income Security Act of 1974, as amended, and the regulations and published interpretations thereunder
(collectively, “ERISA”)) established or maintained by the Company, its subsidiaries or their “ERISA Affiliates”
(as defined below) are in compliance in all material respects with ERISA. “ERISA Affiliate” means, with respect to
the Company or any of its subsidiaries, any member of any group of organizations described in Sections 414(b), (c), (m) or (o) of
the Internal Revenue Code of 1986, as amended, and the regulations and published interpretations thereunder (the “Code”)
of which the Company or such subsidiary is a member. No “reportable event” (as defined under ERISA) has occurred or is reasonably
expected to occur with respect to any “employee benefit plan” established or maintained by the Company, its subsidiaries or
any of their ERISA Affiliates. Except as would not reasonably be expected, individually or in the aggregate, to result in a Material Adverse
Change, no “employee benefit plan” established or maintained by the Company, its subsidiaries or any of their ERISA Affiliates,
if such “employee benefit plan” were terminated, would have any “amount of unfunded benefit liabilities” (as defined
under ERISA). Neither the Company, its subsidiaries nor any of their ERISA Affiliates has incurred or reasonably expects to incur any
liability under (i) Title IV of ERISA with respect to termination of, or withdrawal from, any “employee benefit plan”
or (ii) Sections 412, 4971, 4975 or 4980B of the Code. Each “employee benefit plan” established or maintained by the
Company, its subsidiaries or any of their ERISA Affiliates that is intended to be qualified under Section 401(a) of the Code
is so qualified and nothing has occurred, whether by action or failure to act, which would cause the loss of such qualification.
(hh) Brokers.
Except as otherwise disclosed in the Prospectus, there is no broker, finder or other party that is entitled to receive from the Company
any brokerage or finder’s fee or other fee or commission as a result of any transactions contemplated by this Agreement.
(ii) No
Outstanding Loans or Other Extensions of Credit. The Company does not have any outstanding extension of credit, in the form of a personal
loan, to or for any director or executive officer (or equivalent thereof) of the Company except for such extensions of credit as are expressly
permitted by Section 13(k) of the Exchange Act.
(jj) Compliance
with Laws. The Company and its subsidiaries have been and are in compliance with all applicable laws, rules and regulations,
except where failure to be so in compliance would not be expected, individually or in the aggregate, to result in a Material Adverse Change.
(kk) Dividend
Restrictions. Except as disclosed in the Prospectus, no subsidiary of the Company is prohibited or restricted, directly or indirectly,
from paying dividends to the Company, or from making any other distribution with respect to such subsidiary’s equity securities
or from repaying to the Company or any other subsidiary of the Company any amounts that may from time to time become due under any loans
or advances to such subsidiary from the Company or from transferring any property or assets to the Company or to any other subsidiary.
(ll) Anti-Corruption
and Anti-Bribery Laws. Neither the Company nor any of its subsidiaries nor to the knowledge of the Company, any director, officer,
employee, agent, Affiliate or other person acting on behalf of the Company or any of its subsidiaries has, in the course of its actions
for, or on behalf of, the Company or any of its subsidiaries (i) used any corporate funds for any unlawful contribution, gift, entertainment
or other unlawful expenses relating to political activity; (ii) made or taken any act in furtherance of an offer, promise, or authorization
of any direct or indirect unlawful payment or benefit to any foreign or domestic government official or employee, including of any government-owned
or controlled entity or public international organization, or any political party, party official, or candidate for political office;
(iii) violated or is in violation of any provision of the U.S. Foreign Corrupt Practices Act of 1977, as amended (the “FCPA”),
the UK Bribery Act 2010, or any other applicable anti-bribery or anti-corruption law; or (iv) made, offered, authorized, requested,
or taken an act in furtherance of any unlawful bribe, rebate, payoff, influence payment, kickback or other unlawful payment or benefit.
The Company and its subsidiaries and, to the knowledge of the Company, the Company’s Affiliates have conducted their respective
businesses in compliance with the FCPA and other applicable anti-bribery or anti-corruption laws, and have instituted and maintain policies
and procedures designed to ensure, and which are reasonably expected to continue to ensure, continued compliance therewith.
(mm) Money
Laundering Laws. The operations of the Company and its subsidiaries are, and have been conducted at all times, in compliance with
applicable financial recordkeeping and reporting requirements of the Currency and Foreign Transactions Reporting Act of 1970, as amended,
the money laundering statutes of all applicable jurisdictions, the rules and regulations thereunder and any related or similar applicable
rules, regulations or guidelines, issued, administered or enforced by any governmental agency (collectively, the “Money Laundering
Laws”) and no action, suit or proceeding by or before any court or governmental agency, authority or body or any arbitrator
involving the Company or any of its subsidiaries with respect to the Money Laundering Laws is pending or, to the best knowledge of the
Company, threatened.
(nn) Clinical
Data and Regulatory Compliance. The preclinical tests and clinical trials, and other studies (collectively, “studies”)
that are described in, or the results of which are referred to in, the Registration Statement or the Prospectus were and, if still pending,
are being conducted in all material respects in accordance with the protocols, procedures and controls designed and approved for such
studies and with standard medical and scientific research procedures; each description of the results of such studies is accurate and
complete in all material respects and fairly presents the data derived from such studies, and the Company and its subsidiaries have no
knowledge of any other studies the results of which are inconsistent with, or otherwise call into question, the results described or referred
to in the Registration Statement or the Prospectus; the Company and its subsidiaries have made all necessary filings and obtained all
such approvals as may be required by the FDA or any committee thereof or from any other U.S. or foreign government or drug or medical
device regulatory agency, or health care facility Institutional Review Board (collectively, the “Regulatory Agencies”);
neither the Company nor any of its subsidiaries has received any notice of, or correspondence from, any Regulatory Agency requiring the
termination, suspension or modification of any clinical trials that are described or referred to in the Registration Statement or the
Prospectus; and the Company and its subsidiaries have each operated and currently are in compliance in all material respects with all
applicable rules, regulations and policies of the Regulatory Agencies.
(oo) Sanctions.
Neither the Company nor any of its subsidiaries, directors or officers, nor to the knowledge of the Company, after due inquiry, any employee,
agent, Affiliate or other person acting on behalf of the Company or any of its subsidiaries is currently the subject or the target of
any U.S. sanctions administered by the Office of Foreign Assets Control of the U.S. Department of the Treasury (“OFAC”)
or the U.S. Department of State, the United Nations Security Council, the European Union, His Majesty’s Treasury of the United Kingdom,
or other relevant sanctions authority (collectively, “Sanctions”); nor is the Company or any of its subsidiaries located,
organized or resident in a country or territory that is the subject or the target of Sanctions, including, without limitation, the Crimea
Region of Ukraine, the so-called Donetsk People’s Republic, the so-called Luhansk People’s Republic, Cuba, Iran, North
Korea, and Syria; and the Company will not directly or indirectly use the proceeds of this offering, or lend, contribute or otherwise
make available such proceeds to any subsidiary, or any joint venture partner or other person or entity, for the purpose of financing the
activities of or business with any person, or in any country or territory, that at the time of such financing, is the subject or the target
of Sanctions or in any other manner that will result in a violation by any person (including any person participating in the transaction
whether as underwriter, advisor, investor or otherwise) of applicable Sanctions. Since April 24, 2019, the Company and its subsidiaries
have not knowingly engaged in and are not now knowingly engaged in any dealings or transactions with any person, country, or territory
that at the time of the dealing or transaction is or was the subject or the target of Sanctions.
(pp) Sarbanes-Oxley.
The Company is in compliance, in all material respects, with all applicable provisions of the Sarbanes-Oxley Act of 2002 and the rules and
regulations promulgated thereunder.
(qq) Duties,
Transfer Taxes, Etc. No stamp or other issuance or transfer taxes or duties and no capital gains, income, withholding or other taxes
are payable by the Agent in the United States or any political subdivision or taxing authority thereof or therein in connection with the
execution, delivery or performance of this Agreement by the Company or the sale and delivery by the Company of the Shares. No transaction,
stamp, capital or other issuance, registration, transaction, transfer or withholding taxes or duties are payable in the PRC by or on behalf
of the Agent to any PRC taxing authority in connection with (i) the execution and delivery of this Agreement or (ii) the consummation
of the transactions contemplated hereunder, given that all of the above activities mentioned in clauses (i) and (ii) above shall
take place outside of the PRC.
(rr) Cybersecurity.
To its knowledge, the Company’s and its subsidiaries’ information technology assets and equipment, computers, systems, networks,
hardware, software, websites, applications, and databases (collectively, “IT Systems”) are adequate for, and operate
and perform in all material respects as required in connection with the operation of the business of the Company and its subsidiaries
as currently conducted, free and clear of all material bugs, errors, defects, Trojan horses, time bombs, malware and other corruptants.
The Company and its subsidiaries have implemented and maintained commercially reasonable physical, technical and administrative controls,
policies, procedures, and safeguards to maintain and protect their material confidential information and the integrity, continuous operation,
redundancy and security of all IT Systems and data, including “Personal Data,” used in connection with their businesses. “Personal
Data” means (i) a natural person’s name, street address, telephone number, e-mail address, photograph, social security
number or tax identification number, driver’s license number, passport number, credit card number, bank information, or customer
or account number; (ii) any information which would qualify as “personally identifying information” under the Federal
Trade Commission Act, as amended; (iii) “personal data” as defined by GDPR (as defined below); (iv) any information
which would qualify as “protected health information” under the Health Insurance Portability and Accountability Act of 1996,
as amended by the Health Information Technology for Economic and Clinical Health Act (collectively, “HIPAA”); (v) any
“personal information” as defined by the Cyber Security Law of the PRC and any laws, rules and regulations promulgated
by regulatory authorities of PRC concerning data protection (collectively, “Cyber Security Law”); and (vi) any
other piece of information that allows the identification of such natural person, or his or her family, or permits the collection or analysis
of any data related to an identified person’s health or sexual orientation. To the knowledge of the Company, there have been no
breaches, violations, outages or unauthorized uses of or accesses to same, except for those that have been remedied without material cost
or liability or the duty to notify any other person, nor any incidents under internal review or investigations relating to the same, that,
in each case, would reasonably be likely to result in a Material Adverse Change. The Company and its subsidiaries are presently in material
compliance with all applicable laws or statutes and all judgments, orders, rules and regulations of any court or arbitrator or governmental
or regulatory authority, internal policies and contractual obligations relating to the privacy and security of IT Systems and Personal
Data and to the protection of such IT Systems and Personal Data from unauthorized use, access, misappropriation or modification.
(ss) Compliance
with Data Privacy Laws. The Company and its subsidiaries are, and to the Company’s knowledge, at all prior times were, in material
compliance with all applicable state and federal data privacy and security laws and regulations, including without limitation HIPAA and
Cyber Security Law, and the Company and its subsidiaries have taken commercially reasonable actions to prepare to comply with, and since
May 25, 2018, have been and currently are in compliance with, the European Union General Data Protection Regulation (“GDPR”)
(EU 2016/679) (collectively, the “Privacy Laws”) in all material respects. To ensure compliance with the Privacy Laws,
the Company and its subsidiaries have in place, comply with, and take appropriate steps reasonably designed to ensure compliance in all
material respects with their policies and procedures relating to data privacy and security and the collection, storage, use, disclosure,
handling, and analysis of Personal Data (the “Policies”). The Company and its subsidiaries have at all times made all
disclosures to users or customers required by applicable laws and regulatory rules or requirements, and none of such disclosures
made or contained in any Policy have, to the knowledge of the Company, been inaccurate or in violation of any applicable laws and regulatory
rules or requirements in any material respect. The Company further certifies that neither it nor any subsidiary: (i) has received
notice of any actual or potential liability under or relating to, or actual or potential violation of, any of the Privacy Laws, and has
no knowledge of any event or condition that would reasonably be expected to result in any such notice; (ii) is currently conducting
or paying for, in whole or in part, any investigation, remediation, or other corrective action pursuant to any Privacy Law; or (iii) is
a party to any order, decree, or agreement that imposes any obligation or liability under any Privacy Law.
(tt) Forward-Looking
Statements. Each forward-looking statement (within the meaning of Section 27A of the Securities Act and Section 21E of the
Exchange Act) contained in the Registration Statement or the Prospectus was so included by the Company in good faith and with reasonable
basis after due consideration by the Company of the underlying assumptions, estimates and other applicable facts and circumstances.
(uu) Other
Underwriting Agreements. The Company is not a party to any agreement with an agent or underwriter for any other “at the market”
or continuous equity transaction.
(vv) Compliance
with Health Care Laws. The Company and its subsidiaries are, and at all times have been, in material compliance with all Health Care
Laws applicable to the Company. For purposes of this Agreement, “Health Care Laws” means: (i) the Federal Food,
Drug, and Cosmetic Act (21 U.S.C. Section 301 et seq.), the Public Health Service Act (42 U.S.C. Section 201 et seq.), and the
regulations promulgated thereunder; (ii) all applicable federal, state, local and foreign health care fraud and abuse laws, including,
without limitation, the Anti-Kickback Statute (42 U.S.C. Section 1320a-7b(b)), the Civil False Claims Act (31 U.S.C. Section 3729
et seq.), the criminal false statements law (42 U.S.C. Section 1320a-7b(a)), 18 U.S.C. Sections 286 and 287, the health care fraud
criminal provisions under HIPAA (42 U.S.C. Section 1320d et seq.), the Stark Law (42 U.S.C. Section 1395nn), the civil monetary
penalties law (42 U.S.C. Section 1320a-7a), the exclusion law (42 U.S.C. Section 1320a-7), the Physician Payments Sunshine Act
(42 U.S.C. Section 1320-7h), and applicable laws governing government funded or sponsored healthcare programs; (iii) HIPAA,
as amended by the Health Information Technology for Economic and Clinical Health Act (42 U.S.C. Section 17921 et seq.); (iv) the
Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010; (v) licensure,
quality, safety and accreditation requirements under applicable federal, state, local or foreign laws or regulatory bodies; (vi) the
PRC Drug Administration Law and the regulations promulgated thereunder, as well as all other local, state, federal, national, supranational
and foreign laws, relating to the regulation of the Company or its subsidiaries, and (vii) the directives and regulations promulgated
pursuant to such statutes and any state or non-U.S. counterpart thereof. Neither the Company nor any of its subsidiaries has received
written notice of any claim, action, suit, proceeding, hearing, enforcement, investigation, arbitration or other action from any court
or arbitrator or governmental or regulatory authority or third party alleging that any product operation or activity is in violation of
any Health Care Laws nor, to the Company’s knowledge, is any such claim, action, suit, proceeding, hearing, enforcement, investigation,
arbitration or other action threatened. The Company and its subsidiaries have filed, maintained or submitted all material reports, documents,
forms, notices, applications, records, claims, submissions and supplements or amendments as required by any Health Care Laws, and all
such reports, documents, forms, notices, applications, records, claims, submissions and supplements or amendments were complete and accurate
on the date filed in all material respects (or were corrected or supplemented by a subsequent submission). Neither the Company nor any
of its subsidiaries is a party to any corporate integrity agreements, monitoring agreements, consent decrees, settlement orders, or similar
agreements with or imposed by any governmental or regulatory authority. Additionally, neither the Company, any of its subsidiaries nor
any of their respective employees, officers, directors, or agents has been excluded, suspended or debarred from participation in any U.S.
federal health care program or human clinical research or, to the knowledge of the Company, is subject to a governmental inquiry, investigation,
proceeding, or other similar action that could reasonably be expected to result in debarment, suspension, or exclusion.
(ww) Payments
in Foreign Currency. Except as disclosed in the Registration Statement and the Prospectus, under current laws and regulations of the
Cayman Islands, Hong Kong and any political subdivision thereof, all dividends and other distributions declared and payable on the Shares
may be paid by the Company to the holder thereof in United States dollars that may be converted into foreign currency and may be freely
transferred out of the Cayman Islands and Hong Kong and all such payments made to holders thereof or therein who are non-residents of
the Cayman Islands or Hong Kong will not be subject to income, withholding or other taxes under laws and regulations of the Cayman Islands
or Hong Kong or any political subdivision or taxing authority thereof or therein and without the necessity of obtaining any governmental
authorization in the Cayman Islands and Hong Kong or any political subdivision or taxing authority thereof or therein. Except as disclosed
in the Registration Statement and the Prospectus, all dividends and other distributions declared and payable on the share capital of the
subsidiaries of the Company that are organized in the PRC may, under the current laws and regulations of the PRC and after full payment
of withholding tax, as applicable, be freely transferred out of the PRC and may be paid in United States dollars, provided that the remittance
of such dividends and other distributions outside of the PRC complies with the procedures required under the PRC laws and by the relevant
governmental agencies relating to foreign exchange, without other consent, approval, authorization or order of, or qualification with,
any court or governmental agency or body in such entity’s jurisdiction of incorporation or tax residence, and all such dividends
and other distributions will not be subject to other taxes under the laws and regulations of the PRC and are otherwise free and clear
of any other tax, withholding or deduction in the PRC, and without the necessity of obtaining any governmental authorization in the PRC.
(xx) CSRC
Filings. The Company is aware of and has been advised as to, the content of the Trial Administrative Measures of Overseas
Securities Offering and Listing by Domestic Companies (境内企业境外发行证券和上市管理试行办法)
promulgated by the China Securities Regulatory Commission (the “CSRC”) on February 17, 2023 and any official clarifications,
guidance, interpretations or implementation rules in connection with or related thereto (as amended, supplemented or otherwise modified
from time to time, the “CSRC Filing Rules”), including the relevant provisions thereof which purport to require that
the follow-on offering of shares of an issuer in the same overseas market where it has previously issued and listed securities shall be
filed with and reported to the CSRC within the prescribed timeframe. The issuance and sale of the Shares do not require the prior filing
with or approval of the CSRC, but will require filing with the CSRC within three (3) PRC Business Days after the completion of the
first sale of Shares hereunder and stating in the CSRC Filing Report (as defined below) the total number of securities to be issued under
the Registration Statement (“Aggregated Securities”), and reporting the overall offering information to the CSRC in
a consolidated manner after the completion of the sale of the Aggregated Securities
(yy) Compliance
with PRC Regulations on PRC Overseas Investment. The Company has taken all reasonable steps to comply with, and has made reasonable
efforts to ensure compliance by each of its shareholders, option holders, directors, officers and employees that is, or is directly or
indirectly owned or controlled by, a PRC resident or citizen with any applicable rules and regulations of the relevant PRC government
agencies (including but not limited to the Ministry of Commerce, the National Development and Reform Commission, the CSRC and the State
Administration of Foreign Exchange) relating to overseas investment by PRC residents and citizens (the “PRC Overseas Investment
Regulations”), including requesting each shareholder, option holder, director, officer and employee that is, or is directly
or indirectly owned or controlled by, a PRC resident or citizen to complete any registration and other procedures required under applicable
PRC Overseas Investment Regulations.
Any certificate signed by
any officer or representative of the Company or any of its subsidiaries and delivered to the Agent or counsel for the Agent in connection
with an issuance of Shares shall be deemed a representation and warranty by the Company to the Agent as to the matters covered thereby
on the date of such certificate.
The Company acknowledges that
the Agent and, for purposes of the opinions to be delivered pursuant to Section 4(o) hereof, counsel to the Company and
counsel to the Agent, will rely upon the accuracy and truthfulness of the foregoing representations and hereby consents to such reliance.
Section 3. ISSUANCE
AND SALE OF COMMON SHARES
(a) Sale
of Securities. On the basis of the representations, warranties and agreements herein contained, but subject to the terms and conditions
herein set forth, the Company and the Agent agree that the Company may from time to time seek to sell Shares through the Agent, acting
as sales agent, or directly to the Agent, acting as principal, as follows, with an aggregate Sales Price of up to the Maximum Program
Amount, based on and in accordance with Issuance Notices as the Company may deliver, during the Agency Period.
(b) Mechanics
of Issuances.
(i) Issuance
Notice. Upon the terms and subject to the conditions set forth herein, on any Trading Day during the Agency Period on which the conditions
set forth in Section 5(a) and Section 5(b) shall have been satisfied, the Company may exercise
its right to request an issuance of Shares by delivering to the Agent an Issuance Notice; provided, however, that (A) in no event
may the Company deliver an Issuance Notice to the extent that the sum of (x) the aggregate Sales Price of the requested Issuance
Amount, plus (y) the aggregate Sales Price of all Shares issued under all previous Issuance Notices effected pursuant to this Agreement,
would exceed the Maximum Program Amount; and (B) prior to delivery of any Issuance Notice, the period set forth for any previous
Issuance Notice shall have expired or been terminated. An Issuance Notice shall be considered delivered on the Trading Day that it is
received by email to the persons set forth in Schedule A hereto and confirmed by the Company by telephone (including a voicemail message
to the persons so identified), with the understanding that, with adequate prior written notice, the Agent may modify the list of such
persons from time to time.
(ii) Agent
Efforts. Upon the terms and subject to the conditions set forth in this Agreement, upon the receipt of an Issuance Notice, the Agent
will use its commercially reasonable efforts consistent with its normal sales and trading practices to place the Shares with respect to
which the Agent has agreed to act as sales agent, subject to, and in accordance with the information specified in, the Issuance Notice,
unless the sale of the Shares described therein has been suspended, cancelled or otherwise terminated in accordance with the terms of
this Agreement. For the avoidance of doubt, the parties to this Agreement may modify an Issuance Notice at any time provided they both
agree in writing to any such modification.
(iii) Method
of Offer and Sale. The Shares may be offered and sold (A) in negotiated transactions with the consent of the Company or (B) by
any other method permitted by law deemed to be an “at the market offering” as defined in Rule 415(a)(4) under
the Securities Act, including block transactions, sales made directly on the Principal Market or sales made into any other existing trading
market of the Common Shares. Nothing in this Agreement shall be deemed to require either party to agree to the method of offer and sale
specified in the preceding sentence, and (except as specified in clause (A) above) the method of placement of any Shares by the Agent
shall be at the Agent’s discretion.
(iv) Confirmation
to the Company. If acting as sales agent hereunder, the Agent will provide written confirmation to the Company no later than the opening
of the Trading Day next following the Trading Day on which it has placed Shares hereunder setting forth the number of shares sold on such
Trading Day, the corresponding Sales Price and the Issuance Price payable to the Company in respect thereof.
(v) Settlement.
Each issuance of Shares will be settled on the applicable Settlement Date for such issuance of Shares and, subject to the provisions of
Section 5, on or before each Settlement Date, the Company will, or will cause its transfer agent to, electronically transfer
the Shares being sold by crediting the Agent or its designee’s account at The Depository Trust Company through its Deposit/Withdrawal
At Custodian (DWAC) System, or by such other means of delivery as may be mutually agreed upon by the parties hereto and, upon receipt
of such Shares, which in all cases shall be freely tradable, transferable, registered shares in good deliverable form, the Agent will
deliver, by wire transfer of immediately available funds, the related Issuance Price in same day funds delivered to an account designated
by the Company prior to the Settlement Date. The Company may sell Shares to the Agent as principal at a price agreed upon at each relevant
time Shares are sold pursuant to this Agreement (each, a “Time of Sale”).
(vi) Suspension
or Termination of Sales. Consistent with standard market settlement practices, the Company or the Agent may, upon notice to the other
party hereto in writing or by telephone (confirmed immediately by verifiable email), suspend any sale of Shares, and the period set forth
in an Issuance Notice shall immediately terminate; provided, however, that (A) such suspension and termination shall not affect or
impair either party’s obligations with respect to any Shares placed or sold hereunder prior to the receipt of such notice; (B) if
the Company suspends or terminates any sale of Shares after the Agent confirms such sale to the Company, the Company shall still be obligated
to comply with Section 3(b)(v) with respect to such Shares; and (C) if the Company defaults in its obligation
to deliver Shares on a Settlement Date, the Company agrees that it will hold the Agent harmless against any loss, claim, damage or expense
(including, without limitation, penalties, interest and reasonable legal fees and expenses), as incurred, arising out of or in connection
with such default by the Company. The parties hereto acknowledge and agree that, in performing its obligations under this Agreement, the
Agent may borrow Common Shares from stock lenders in the event that the Company has not delivered Shares to settle sales as required by
subsection (v) above, and may use the Shares to settle or close out such borrowings. The Company agrees that no such notice shall
be effective against the Agent unless it is made to the persons identified in writing by the Agent pursuant to Section 3(b)(i).
(vii) No
Guarantee of Placement, Etc. The Company acknowledges and agrees that (A) there can be no assurance that the Agent will be successful
in placing Shares; (B) the Agent will incur no liability or obligation to the Company or any other Person if it does not sell Shares;
and (C) the Agent shall be under no obligation to purchase Shares on a principal basis pursuant to this Agreement, except as otherwise
specifically agreed by the Agent and the Company.
(viii) Material
Non-Public Information. Notwithstanding any other provision of this Agreement, the Company and the Agent agree that the Company shall
not deliver any Issuance Notice to the Agent, and the Agent shall not be obligated to place any Shares, during any period in which the
Company is in possession of material non-public information.
(c) Fees.
As compensation for services rendered, the Company shall pay to the Agent, on the applicable Settlement Date, the Selling Commission for
the applicable Issuance Amount (including with respect to any suspended or terminated sale pursuant to Section 3(b)(vi)) by
the Agent deducting the Selling Commission from the applicable Issuance Amount.
(d) Expenses.
The Company agrees to pay all costs, fees and expenses incurred in
connection with the performance of its obligations hereunder and in connection with the transactions contemplated hereby, including without
limitation (i) all expenses incident to the issuance and delivery of the Shares (including all printing and engraving costs); (ii) all
fees and expenses of the registrar and transfer agent of the Shares; (iii) all necessary issue, transfer and other stamp taxes in
connection with the issuance and sale of the Shares; (iv) all fees and expenses of the Company’s counsel, independent public
or certified public accountants and other advisors; (v) all costs and expenses incurred in connection with the preparation, printing,
filing, shipping and distribution of the Registration Statement (including financial statements, exhibits, schedules, consents and certificates
of experts), the Prospectus, any Free Writing Prospectus (as defined below) prepared by or on behalf of, used by, or referred to by the
Company, and all amendments and supplements thereto, and this Agreement; (vi) all filing fees, attorneys’ fees and expenses
incurred by the Company or the Agent in connection with qualifying or registering (or obtaining exemptions from the qualification or registration
of) all or any part of the Shares for offer and sale under the state securities or blue sky laws or the provincial securities laws of
Canada, and, if requested by the Agent, preparing and printing a “Blue Sky Survey” or memorandum and a “Canadian
wrapper”, and any supplements thereto, advising the Agent of such qualifications, registrations, determinations and exemptions;
(vii) the reasonable fees and disbursements of the Agent’s counsel, including the reasonable fees and expenses of counsel for
the Agent in connection with, FINRA review, if any, and approval of the Agent’s participation in the offering and distribution of
the Shares; (viii) the filing fees incident to FINRA review, if any; (ix) the costs and expenses of the Company relating to
investor presentations on any “road show” undertaken in connection with the marketing of the offering of the Shares, including,
without limitation, expenses associated with the preparation or dissemination of any electronic road show, expenses associated with the
production of road show slides and graphics, fees and expenses of any consultants engaged in connection with the road show presentations
with the prior approval of the Company, travel and lodging expenses of the representatives, employees and officers of the Company and
of the Agent and any such consultants, and the cost of any aircraft chartered in connection with the road show; and (x) the fees
and expenses associated with listing the Shares on the Principal Market. The fees and disbursements of Agent’s counsel pursuant
to subsections (vi) and (vii) above shall not exceed (A) $200,000 for its U.S. counsel in connection with the first Issuance Notice, provided such notice is delivered within thirty (30) days
of the effectiveness of the Original Registration Statement, and execution of this Agreement, (B) $50,000 for its PRC counsel in connection
with the first Issuance Notice, provided such notice is delivered within thirty (30) days of the effectiveness of the Original Registration
Statement, and execution of this Agreement, (C) $30,000 in connection with each Triggering Event Date (as defined below) involving the
filing of a Form 20-F on which the Company is required to provide a certificate pursuant to Section 4(o), and (D) $20,000 in connection
with each other Triggering Event Date on which the Company is required to provide a certificate pursuant to Section 4(o).
Section 4. ADDITIONAL
COVENANTS
The Company covenants and
agrees with the Agent as follows, in addition to any other covenants and agreements made elsewhere in this Agreement:
(a) Exchange
Act Compliance. During the Agency Period, the Company shall (i) file, on a timely basis, with the Commission all reports and
documents required to be filed under Section 13, 14 or 15 of the Exchange Act in the manner and within the time periods required
by the Exchange Act; and (ii) in the Company’s sole discretion, either (A) include in its quarterly reports or six-month
reports on Form 6-K and its annual reports on Form 20-F, a summary detailing, for the relevant reporting period, (1) the
number of Shares sold through the Agent pursuant to this Agreement and (2) the net proceeds received by the Company from such sales
or (B) prepare a prospectus supplement containing, or include in such other filing permitted by the Securities Act or Exchange Act
(each an “Interim Prospectus Supplement”), such summary information and, at least once a quarter and subject to this
Section 4, file such Interim Prospectus Supplement pursuant to Rule 424(b) under the Securities Act (and within
the time periods required by Rule 424(b) and Rule 430B under the Securities Act).
(b) Securities
Act Compliance. After the date of this Agreement, the Company shall promptly advise the Agent in writing (i) of the receipt of
any comments of, or requests for additional or supplemental information from, the Commission; (ii) of the time and date of any filing
of any post-effective amendment to the Registration Statement, any Rule 462(b) Registration Statement or any amendment or supplement
to the Prospectus, or any Free Writing Prospectus; (iii) of the time and date that any post-effective amendment to the Registration
Statement or any Rule 462(b) Registration Statement becomes effective; and (iv) of the issuance by the Commission of any
stop order suspending the effectiveness of the Registration Statement or any post-effective amendment thereto, any Rule 462(b) Registration
Statement or any amendment or supplement to the Prospectus or of any order preventing or suspending the use of any Free Writing Prospectus
or the Prospectus, or of any proceedings to remove, suspend or terminate from listing or quotation the Common Shares from any securities
exchange upon which they are listed for trading or included or designated for quotation, or of the threatening or initiation of any proceedings
for any of such purposes. If the Commission shall enter any such stop order at any time, the Company will use its reasonable best efforts
to obtain the lifting of such order as soon as practicable. Additionally, the Company agrees that it shall comply with the provisions
of Rule 424(b) and Rule 433, as applicable, under the Securities Act and will use its reasonable efforts to confirm that
any filings made by the Company under such Rule 424(b) or Rule 433 were received in a timely manner by the Commission.
(c) Amendments
and Supplements to the Prospectus and Other Securities Act Matters. If any event shall occur or condition exist as a result of which
it is necessary to amend or supplement the Prospectus so that the Prospectus does not include an untrue statement of a material fact or
omit to state a material fact necessary in order to make the statements therein, in the light of the circumstances when the Prospectus
is delivered to a purchaser, not misleading, or if in the opinion of the Agent or counsel for the Agent it is otherwise necessary to amend
or supplement the Prospectus to comply with applicable law, including the Securities Act, the Company agrees (subject to Section 4(d) and
Section 4(f)) to promptly prepare, file with the Commission and furnish at its own expense to the Agent, amendments or supplements
to the Prospectus so that the statements in the Prospectus as so amended or supplemented will not include an untrue statement of a material
fact or omit to state a material fact necessary in order to make the statements therein, in the light of the circumstances when the Prospectus
is delivered to a purchaser, to not be misleading or so that the Prospectus, as amended or supplemented, will comply with applicable law
including the Securities Act. Neither the Agent’s consent to, or delivery of, any such amendment or supplement shall constitute
a waiver of any of the Company’s obligations under Section 4(d) and Section 4(f). Notwithstanding the
foregoing, the Company shall not be required to file such amendment or supplement if there is no pending Issuance Notice and the Company
believes that it is in its best interests not to file such amendment or supplement.
(d) Agent’s
Review of Proposed Amendments and Supplements. Prior to amending or supplementing the Registration Statement (including any registration
statement filed under Rule 462(b) under the Securities Act) or the Prospectus (excluding any amendment or supplement through
incorporation of any report filed under the Exchange Act), the Company shall furnish to the Agent for review, a reasonable amount of time
prior to the proposed time of filing or use thereof, a copy of each such proposed amendment or supplement, insofar as such proposed amendment
or supplement relates to the transactions contemplated hereby, and the Company shall not file or use any such proposed amendment or supplement
without the Agent’s prior consent, and to file with the Commission within the applicable period specified in Rule 424(b) under
the Securities Act any prospectus required to be filed pursuant to such Rule.
(e) Use
of Free Writing Prospectus. Neither the Company nor the Agent has prepared, used, referred to or distributed, or will prepare, use,
refer to or distribute, without the other party’s prior written consent, any “written communication” that constitutes
a “free writing prospectus” as such terms are defined in Rule 405 under the Securities Act with respect to the offering
contemplated by this Agreement (any such free writing prospectus being referred to herein as a “Free Writing Prospectus”).
(f) Free
Writing Prospectuses. The Company shall furnish to the Agent for review, a reasonable amount of time prior to the proposed time of
filing or use thereof, a copy of each proposed free writing prospectus or any amendment or supplement thereto to be prepared by or on
behalf of, used by, or referred to by the Company and the Company shall not file, use or refer to any proposed free writing prospectus
or any amendment or supplement thereto without the Agent’s consent. The Company shall furnish to the Agent, without charge, as many
copies of any free writing prospectus prepared by or on behalf of, or used by the Company, as the Agent may reasonably request. If at
any time when a prospectus is required by the Securities Act (including, without limitation, pursuant to Rule 173(d)) to be delivered
in connection with sales of the Shares (but in any event if at any time through and including the date of this Agreement) there occurred
or occurs an event or development as a result of which any free writing prospectus prepared by or on behalf of, used by, or referred to
by the Company conflicted or would conflict with the information contained in the Registration Statement or included or would include
an untrue statement of a material fact or omitted or would omit to state a material fact necessary in order to make the statements therein,
in the light of the circumstances prevailing at that subsequent time, not misleading, the Company shall promptly amend or supplement such
free writing prospectus to eliminate or correct such conflict or so that the statements in such free writing prospectus as so amended
or supplemented will not include an untrue statement of a material fact or omit to state a material fact necessary in order to make the
statements therein, in the light of the circumstances prevailing at such subsequent time, not misleading, as the case may be; provided,
however, that prior to amending or supplementing any such free writing prospectus, the Company shall furnish to the Agent for review,
a reasonable amount of time prior to the proposed time of filing or use thereof, a copy of such proposed amended or supplemented free
writing prospectus and the Company shall not file, use or refer to any such amended or supplemented free writing prospectus without the
Agent’s consent, not to be unreasonably withheld, conditioned or delayed.
(g) Filing
of Agent Free Writing Prospectuses. The Company shall not take any action that would result in the Agent or the Company being required
to file with the Commission pursuant to Rule 433(d) under the Securities Act a free writing prospectus prepared by or on behalf
of the Agent that the Agent otherwise would not have been required to file thereunder.
(h) Copies
of Registration Statement and Prospectus. After the date of this Agreement through the last time that a prospectus is required by
the Securities Act (including, without limitation, pursuant to Rule 173(d)) to be delivered in connection with sales of the Shares,
the Company agrees to furnish the Agent with copies (which may be electronic copies) of the Registration Statement and each amendment
thereto, and with copies of the Prospectus and each amendment or supplement thereto in the form in which it is filed with the Commission
pursuant to the Securities Act or Rule 424(b) under the Securities Act, both in such quantities as the Agent may reasonably
request from time to time; and, if the delivery of a prospectus is required under the Securities Act or under the blue sky or securities
laws of any jurisdiction at any time on or prior to the applicable Settlement Date for any period set forth in an Issuance Notice in connection
with the offering or sale of the Shares and if at such time any event has occurred as a result of which the Prospectus as then amended
or supplemented would include an untrue statement of a material fact or omit to state any material fact necessary in order to make the
statements therein, in the light of the circumstances under which they were made when such Prospectus is delivered, not misleading, or,
if for any other reason it is necessary during such same period to amend or supplement the Prospectus or to file under the Exchange Act
any document incorporated by reference in the Prospectus in order to comply with the Securities Act or the Exchange Act, to notify the
Agent and to request that the Agent suspend offers to sell Shares (and, if so notified, the Agent shall cease such offers as soon as practicable);
and if the Company decides to amend or supplement the Registration Statement or the Prospectus as then amended or supplemented, to advise
the Agent promptly by telephone (with confirmation in writing) and to prepare and cause to be filed promptly with the Commission an amendment
or supplement to the Registration Statement or the Prospectus as then amended or supplemented that will correct such statement or omission
or effect such compliance; provided, however, that if during such same period the Agent is required to deliver a prospectus in respect
of transactions in the Shares, the Company shall promptly prepare and file with the Commission such an amendment or supplement.
(i) Blue
Sky Compliance. The Company shall cooperate with the Agent and counsel for the Agent to qualify or register the Shares for sale under
(or obtain exemptions from the application of) the state securities or blue sky laws or Canadian provincial securities laws of those jurisdictions
designated by the Agent, shall comply with such laws and shall continue such qualifications, registrations and exemptions in effect so
long as required for the distribution of the Shares. The Company shall not be required to qualify as a foreign corporation or to take
any action that would subject it to general service of process in any such jurisdiction where it is not presently qualified or where it
would be subject to taxation as a foreign corporation. The Company will advise the Agent promptly of the suspension of the qualification
or registration of (or any such exemption relating to) the Shares for offering, sale or trading in any jurisdiction or any initiation
or threat of any proceeding for any such purpose, and in the event of the issuance of any order suspending such qualification, registration
or exemption, the Company shall use its reasonable best efforts to obtain the withdrawal thereof as soon as practicable.
(j) Earnings
Statement. As soon as practicable, the Company will make generally available to its security holders and to the Agent an earnings
statement (which need not be audited) covering a period of at least twelve months beginning with the first fiscal quarter of the Company
occurring after the date of this Agreement which shall satisfy the provisions of Section 11(a) of the Securities Act and Rule 158
under the Securities Act.
(k) Listing;
Reservation of Shares. (a) The Company will use its reasonable best efforts to maintain the listing of the Shares on the Principal
Market; and (b) the Company will reserve and keep available at all times, free of preemptive rights, Shares for the purpose of enabling
the Company to satisfy its obligations under this Agreement.
(l) Transfer
Agent. The Company shall engage and maintain, at its expense, a registrar and transfer agent for the Shares.
(m) Due
Diligence. During the term of this Agreement, subject to the requirements and restrictions of the Provisions on Strengthening Confidentiality
and Archives Administration of Overseas Securities Offering and Listing by Domestic Companies (关于加强境内企业境外发行证券和上市相关保密和档案管理工作的规定),
the Company will reasonably cooperate with any reasonable due diligence review conducted by the Agent in connection with the transactions
contemplated hereby, including, without limitation, providing information and making available documents and senior corporate officers,
during normal business hours and at the Company’s principal offices, as the Agent may reasonably request from time to time.
(n) Representations
and Warranties. The Company acknowledges that each delivery of an Issuance Notice and each delivery of Shares on a Settlement Date
shall be deemed to be (i) an affirmation to the Agent that the representations and warranties of the Company contained in or made
pursuant to this Agreement are true and correct as of the date of such Issuance Notice or of such Settlement Date, as the case may be,
as though made at and as of each such date, except as may be disclosed in the Prospectus (including any documents incorporated by reference
therein and any supplements thereto); and (ii) an undertaking that the Company will advise the Agent if any of such representations
and warranties will not be true and correct as of the Settlement Date for the Shares relating to such Issuance Notice, as though made
at and as of each such date (except that such representations and warranties shall be deemed to relate to the Registration Statement and
the Prospectus as amended and supplemented relating to such Shares).
(o) Deliverables
at Triggering Event Dates; Certificates. The Company agrees that on or prior to the date of the first Issuance Notice and, during
the term of this Agreement after the date of the first Issuance Notice, upon:
(i) the
filing of the Prospectus or the amendment or supplement of any Registration Statement or Prospectus (other than a prospectus supplement
relating solely to an offering of securities other than the Shares or a prospectus filed pursuant to Section 4(a)(ii)(B)),
by means of a post-effective amendment, sticker or supplement, but not by means of incorporation of documents by reference into the Registration
Statement or Prospectus;
(ii) the
filing with the Commission of:
(A) an
annual report on Form 20-F; or
(B) a
report on Form 6-K containing quarterly or half-year consolidated financial information of the Company that is incorporated by reference
into the Registration Statement (including any Form 20-F/A or Form 6-K/A containing amended financial statements or a material
amendment to the previously filed annual report on Form 20-F or quarterly report or six-month report on Form 6-K incorporated
by reference into the Registration Statement), in each case, of the Company; or
(C) the
filing with the Commission of any other report on Form 6-K of the Company containing financial information incorporated by reference
into the Registration Statement that is material to the offering of securities of the Company in the Agent’s reasonable discretion;
(any such event, a “Triggering
Event Date”), the Company shall furnish the Agent (but in the case of clause (C) above only if the Agent reasonably determines
that the information contained in such report on Form 6-K of the Company that is incorporated by reference into the Registration
Statement is material) with a certificate as of the Triggering Event Date, in the form and substance satisfactory to the Agent and its
counsel, substantially similar to the form previously provided to the Agent and its counsel, modified, as necessary, to relate to the
Registration Statement and the Prospectus as amended or supplemented, (A) confirming that the representations and warranties of the
Company contained in this Agreement are true and correct, (B) confirming that the Company has performed all of its obligations hereunder
to be performed on or prior to the date of such certificate and as to the matters set forth in Section 5(a)(iii) hereof,
and (C) containing any other certification that the Agent shall reasonably request. The requirement to provide a certificate under
this Section 4(o) shall be waived for any Triggering Event Date occurring at a time when no Issuance Notice is pending
or a suspension is in effect, which waiver shall continue until the earlier to occur of the date the Company delivers instructions for
the sale of Shares hereunder (which for such calendar quarter shall be considered a Triggering Event Date) and the next occurring Triggering
Event Date. Notwithstanding the foregoing, if the Company subsequently decides to sell Shares following a Triggering Event Date when a
suspension was in effect and did not provide the Agent with a certificate under this Section 4(o), then before the Company
delivers the instructions for the sale of Shares or the Agent sells any Shares pursuant to such instructions, the Company shall provide
the Agent with a certificate in conformity with this Section 4(o) dated as of the date that the instructions for the
sale of Shares are issued.
(p) Legal
Opinions. On or prior to the date of the first Issuance Notice and on or prior to each Triggering Event Date with respect to which
the Company is obligated to deliver a certificate pursuant to Section 4(o) for which no waiver is applicable and excluding
the date of this Agreement, the Company shall cause to be delivered a negative assurances letter and the written legal opinion of Harter
Secrest & Emery LLP, U.S. counsel to the Company, the written legal opinion of Maples Group, Cayman Islands counsel to the Company,
Duan & Duan, PRC counsel to the Company, and Kilburn & Strode LLP, intellectual property counsel to the Company, each
dated the date of delivery, in form and substance reasonably satisfactory to Agent and its counsel, substantially similar to the form
previously provided to the Agent and its counsel, modified, as necessary, to relate to the Registration Statement and the Prospectus as
then amended or supplemented. In lieu of such opinions for subsequent periodic filings, in the discretion of the Agent, the Company may
furnish a reliance letter from such counsel to the Agent, permitting the Agent to rely on a previously delivered opinion letter, modified
as appropriate for any passage of time or Triggering Event Date (except that statements in such prior opinion shall be deemed to relate
to the Registration Statement and the Prospectus as amended or supplemented as of such Triggering Event Date). Notwithstanding the foregoing,
the Company shall be required to cause the delivery of such negative assurances letter and written legal opinions, or reliance letters
in lieu thereof, no more than once with each Form 20-F or Form 6-K that reports the Company’s quarterly financial information,
in each case, as filed by the Company with the SEC.
(q) Comfort
Letter. On or prior to the date of the first Issuance Notice and on or prior
to each Triggering Event Date with respect to which the Company is obligated to deliver a certificate pursuant to Section 4(o) for
which no waiver is applicable and excluding the date of this Agreement, the Company shall cause KPMG Huazhen LLP, the independent registered
public accounting firm who has audited the financial statements included or incorporated by reference in the Registration Statement, to
furnish the Agent a comfort letter, dated the date of delivery, in form and substance reasonably satisfactory to the Agent and its counsel,
substantially similar to the form previously provided to the Agent and its counsel; provided, however, that any such comfort letter will
only be required on the Triggering Event Date specified to the extent that it contains financial statements filed with the Commission
under the Exchange Act and incorporated or deemed to be incorporated by reference into a Prospectus. If requested by the Agent, the Company
shall also cause a comfort letter to be promptly furnished to the Agent in connection with any material transaction or event requiring
the filing of a report on Form 6-K containing material amended financial information of the Company, including the restatement of
the Company’s financial statements ; provided, that, such comfort letter shall not be required to be delivered to the Agent unless
and until the Company delivers an Issuance Notice that is effective on or after the date of occurrence of such material transaction or
event. The Company shall be required to furnish no more than one comfort letter hereunder per each filing of an annual report on Form 20-F
or a report filed on Form 6-K containing quarterly or half-year financial information.
(r) Secretary’s
Certificate. On or prior to the date of the first Issuance Notice and on or prior to each Triggering Event Date, the Company shall
furnish the Agent a certificate executed by the Secretary of the Company, signing in such capacity, dated the date of delivery (i) certifying
that attached thereto are true and complete copies of the resolutions duly adopted by the Board of Directors of the Company authorizing
the execution and delivery of this Agreement and the consummation of the transactions contemplated hereby (including, without limitation,
the issuance of the Shares pursuant to this Agreement), which authorization shall be in full force and effect on and as of the date of
such certificate, (ii) certifying and attesting to the office, incumbency, due authority and specimen signatures of each Person who
executed this Agreement for or on behalf of the Company, and (iii) containing any other certification that the Agent shall reasonably
request.
(s) Agent’s
Own Account; Clients’ Account. The Company consents to the Agent trading, in compliance with applicable law, in the Common Shares
for the Agent’s own account and for the account of its clients at the same time as sales of the Shares occur pursuant to this Agreement.
(t) Investment
Limitation. The Company shall not invest, or otherwise use the proceeds received by the Company from its sale of the Shares in such
a manner as would require the Company or any of its subsidiaries to register as an investment company under the Investment Company Act.
(u) Market
Activities. The Company will not take, directly or indirectly, any action designed to or that might be reasonably expected to cause
or result in stabilization or manipulation of the price of the Shares or any other reference security, whether to facilitate the sale
or resale of the Shares or otherwise, and the Company will, and shall cause each of its Affiliates to, comply with all applicable provisions
of Regulation M. If the limitations of Rule 102 of Regulation M (“Rule 102”) do not apply with respect to
the Shares or any other reference security pursuant to any exception set forth in Section (d) of Rule 102, then promptly
upon notice from the Agent (or, if later, at the time stated in the notice), the Company will, and shall cause each of its Affiliates
to, comply with Rule 102 as though such exception were not available but the other provisions of Rule 102 (as interpreted by
the Commission) did apply. The Company shall promptly notify the Agent if it no longer meets the requirements set forth in Section (d) of
Rule 102.
(v) Notice
of Other Sale. Without the written consent of the Agent, the Company will not, directly or indirectly, offer to sell, sell, contract
to sell, grant any option to sell or otherwise dispose of any Common Shares or securities convertible into or exchangeable for Common
Shares (other than Shares hereunder), warrants or any rights to purchase or acquire Common Shares, or effect a reverse stock split, recapitalization,
share consolidation, reclassification or similar transaction affecting the outstanding Common Shares, during the period beginning on the
third Trading Day immediately prior to the date on which any Issuance Notice is delivered to the Agent hereunder and ending on the third
Trading Day immediately following the Settlement Date with respect to Shares sold pursuant to such Issuance Notice; and will not directly
or indirectly enter into any other “at the market” or continuous equity transaction offer to sell, sell, contract to sell,
grant any option to sell or otherwise dispose of any Common Shares (other than the Shares offered pursuant to this Agreement) or securities
convertible into or exchangeable for Common Shares, warrants or any rights to purchase or acquire, Common Shares prior to the termination
of this Agreement; provided, however, that such restrictions will not be required in connection with the Company’s (i) issuance
or sale of Common Shares, options to purchase Common Shares or Common Shares issuable upon the exercise of options or other equity awards
pursuant to any employee or director share option, incentive or benefit plan, share purchase or ownership plan, long-term incentive plan,
dividend reinvestment plan, inducement award under Nasdaq rules or other compensation plan of the Company or its subsidiaries, as
in effect on the date of this Agreement, (ii) issuance or sale of Common Shares issuable upon exchange, conversion or redemption
of securities or the exercise or vesting of warrants, options or other equity awards outstanding at the date of this Agreement, (iii) issuance
or sale of securities pursuant to acquisitions, joint ventures, strategic alliances, or other strategic transactions, including without
limitation a preliminary non-binding proposal with respect to the acquisition of certain of the Company’s business operations in
China, the receipt of which was announced by the Company on June 26, 2024, and collaborations or arrangements involving research
and development or the sale or licensing of intellectual property, approved by a majority of the disinterested directors of the Company,
except for a transaction in which the Company is issuing securities primarily for the purpose of raising capital or to an entity whose
primary business is investing in securities (for avoidance of doubt, securities issued to a venture arm of a strategic investor shall
not be deemed issued to an entity whose primary business is investing in securities), provided, however, that the aggregate number of
shares of Common Shares, or securities convertible into or exercisable or exchangeable for shares of Common Shares, that the Company may
issue or agree to issue pursuant to this clause (iii) shall not exceed 10% of the total outstanding Common Shares of the Company
immediately following the issuance of Shares pursuant to this Agreement, and (iv) modification of any outstanding options, warrants
of any rights to purchase or acquire Common Shares.
(w) CSRC
Filings.
(i) The
Company undertakes to file or cause to be filed with the CSRC the requisite information and documents within three (3) PRC Business
Days after the completion of the first sale of Shares hereunder (the “CSRC Filing Report”) and provide or cause to
be provided to the CSRC any subsequent updates (together with the CSRC Filing Report and including any amendments, supplements and/or
modifications thereof, the “CSRC Filings”) in accordance with the CSRC Filing Rules. The Agent agrees to provide the
Company with reasonable and requisite assistance it may require in connection with the CSRC Filings, including without limitation to provide
such information relating to the Agent as may be necessary for the purposes of submitting such filings (including but not limited to a
letter of affirmation as stipulated in Appendix 2 of Regulatory Guidelines for the Application of Rules—Category 2 for Overseas
Issuance and Listing: Guidelines on the Content and Format of Filing Materials (《监管规则适用指引——境外发行上市类第
2 号: 备案材料内容和格式指引》)),
or may otherwise be required in order for the Company to satisfy the regulatory requirements in respect of the CSRC Filings.
(ii) The
Company acknowledges and undertakes that in connection with the CSRC Filings to be made to the CSRC for the offering of the Shares, it
and its directors shall:
(A) comply
with the requirements under the CSRC Filing Rules in the preparation and submission of the CSRC Filings;
(B) ensure
that all information and statements included in the CSRC Filings (including the CSRC Filing Report) are and will remain true, accurate
and complete, free from any false records or misleading statements, and that no material information or facts have been omitted or withheld;
(C) ensure
that (i) there are not and will not be any conflicting, inconsistent or materially different descriptions of facts contained in the
CSRC Filings, (ii) the CSRC Filings will not be difficult to understand due to any lack of clarity or logic in writing; (iii) the
CSRC Filings contain and will contain descriptions of all material events as required to be reported pursuant to the CSRC Filing Rules or
other applicable laws, regulations and rules; and (iv) the CSRC Filings and all other documents filed with the CSRC or issued by
or on behalf of the Company in connection with the offering of the Shares contemplated by this Agreement do not and will not contain any
statement or commentary that in any manner misrepresents or disparages laws, policies, business environment and judicial system of the
PRC;
(D) to
the extent the Agent is required to provide a letter of affirmation to the CSRC in connection with CSRC Filings under applicable laws,
provide the Agent with a written confirmation, in form and substance reasonably satisfactory to the Agent, duly signed by a director or
authorized representative of the Company, immediately before submission of the CSRC Filings, to confirm, among others, that (i) the
Company has complied with all relevant requirements under the applicable laws, regulations and regulatory requirements, including, without
limitation, the CSRC Filing Rules and the Provisions on Strengthening Confidentiality and Archives Administration of Overseas Securities
Offering and Listing by Domestic Companies (关于加强境内企业境外发行证券和上市相关保密和档案管理工作的规定)
issued by the CSRC, Ministry of Finance of the PRC, National Administration of State Secrets Protection of the PRC, and National Archives
Administration of the PRC on February 24, 2023, which became effective from March 31, 2023 (as amended, supplemented or otherwise
modified from time to time, the “CSRC Archive Rules”, together with the CSRC Filing Rules, the “CSRC Rules”),
and all relevant disclosure requirements in respect of the CSRC Filings pursuant to the CSRC Filing Rules; (ii) all information and
statements included in the CSRC Filings are and will remain true, accurate and complete, free from any false records or misleading statements,
and that no material information or facts have been omitted or withheld therefrom; (iii) the CSRC Filings and all other documents
filed with the CSRC or issued by or on behalf of the Company in connection with the offering of the Shares do not contain any statement
or commentary that in any manner misrepresents or disparages laws, policies, business environment and judicial system of the PRC; and
(iv) the Company is not aware that any of the circumstances in connection of the CSRC Filings set forth in Article 8 and 20
of the CSRC Filing Rules has occurred, and undertake to promptly notify the Agent if any of such circumstances occurs or is expected
to occur;
(E) to
the extent the Agent is required to provide a letter of affirmation to the CSRC in connection with CSRC Filings under applicable laws,
provide the Agent and its counsel with draft CSRC Filings (along with all relevant supporting documents in relation to the CSRC Filings)
in a timely manner for review and shall not make any amendment, supplement or modification to the final draft or substantially complete
draft of the CSRC Filings and (where applicable) the related PRC legal opinion unless prior approval from the Agent of any such amendment,
supplement or modification is obtained, and ensure that the Company has not submitted, any undertakings in connection with the Company
or the offering of the Shares to CSRC or other PRC regulators for the purposes of the offering of the Shares that the Agent is not aware
of; and
other
than the circumstance provided for in Sub-Section (v) above, to the extent any information in the CSRC Filings and, where
applicable, the related PRC legal opinion is related to the offering of the Shares contemplated by this Agreement, or the Agent, provide
the Agent with the draft that relates to the offering of the Shares contemplated by this Agreement, or the Agent, and any amendment, supplement
or modification thereto.
Section 5. CONDITIONS
TO DELIVERY OF ISSUANCE NOTICES AND TO SETTLEMENT
(a) Conditions
Precedent to the Right of the Company to Deliver an Issuance Notice and the Obligation of the Agent to Sell Shares. The right of the
Company to deliver an Issuance Notice hereunder is subject to the satisfaction, on the date of delivery of such Issuance Notice, and the
obligation of the Agent to use its commercially reasonable efforts to place Shares during the applicable period set forth in the Issuance
Notice is subject to the satisfaction, on each Trading Day during the applicable period set forth in the Issuance Notice, of each of the
following conditions:
(i) Accuracy
of the Company’s Representations and Warranties; Performance by the Company. The Company shall have delivered the certificate
required to be delivered pursuant to Section 4(o) on or before the date on which delivery of such certificate is required
pursuant to Section 4(o). The Company shall have performed, satisfied and complied with all covenants, agreements and conditions
required by this Agreement to be performed, satisfied or complied with by the Company at or prior to such date, including, but not limited
to, the covenants contained in Section 4(p), Section 4(q) and Section 4(r).
(ii) No
Injunction. No statute, rule, regulation, executive order, decree, ruling or injunction shall have been enacted, entered, promulgated
or endorsed by any court or governmental authority of competent jurisdiction or any self-regulatory organization having authority over
the matters contemplated hereby that prohibits or directly and materially adversely affects any of the transactions contemplated by this
Agreement, and no proceeding shall have been commenced that may have the effect of prohibiting or materially adversely affecting any of
the transactions contemplated by this Agreement.
(iii) Material
Adverse Changes. Except as disclosed in the Prospectus and the Time of Sale Information, (a) in the judgment of the Agent there
shall not have occurred any Material Adverse Change; and (b) there shall not have occurred any downgrading, nor shall any notice
have been given of any intended or potential downgrading or of any review for a possible change that does not indicate the direction of
the possible change, in the rating accorded any securities of the Company or any of its subsidiaries by any “nationally recognized
statistical rating organization” as such term is defined for purposes of Section 3(a)(62) of the Exchange Act.
(iv) No
Suspension of Trading in or Delisting of Common Shares; Other Events. The trading of the Common Shares (including without limitation
the Shares) shall not have been suspended by the Commission, the Principal Market or FINRA and the Common Shares (including without limitation
the Shares) shall have been approved for listing or quotation on and shall not have been delisted from the Principal Market or any of
its constituent markets. There shall not have occurred (and be continuing in the case of occurrences under clauses (i) and (ii) below)
any of the following: (i) trading or quotation in any of the Company’s securities shall have been suspended or limited by the
Commission or by the Principal Market or trading in securities generally on the Principal Market shall have been suspended or limited,
or minimum or maximum prices shall have been generally established on any of such stock exchanges by the Commission or the FINRA; (ii) a
general banking moratorium shall have been declared by any of federal or New York, authorities; or (iii) there shall have occurred
any outbreak or escalation of national or international hostilities or any crisis or calamity, or any change in the United States or international
financial markets, or any substantial change or development involving a prospective substantial change in United States’ or international
political, financial or economic conditions, as in the judgment of the Agent is material and adverse and makes it impracticable to market
the Shares in the manner and on the terms described in the Prospectus or to enforce contracts for the sale of securities.
(b) Documents
Required to be Delivered on each Issuance Notice Date. The Agent’s obligation to use its commercially reasonable efforts to
place Shares hereunder shall additionally be conditioned upon the delivery to the Agent on or before the Issuance Notice Date of a certificate
in form and substance reasonably satisfactory to the Agent, executed by the Chief Executive Officer, President or Chief Financial Officer
of the Company, to the effect that all conditions to the delivery of such Issuance Notice shall have been satisfied as at the date of
such certificate (which certificate shall not be required if the foregoing representations shall be set forth in the Issuance Notice).
(c) No
Misstatement or Material Omission. Agent shall not have advised the Company that the Registration Statement, the Prospectus or the
Time of Sale Information, or any amendment or supplement thereto, contains an untrue statement of fact that in the Agent’s reasonable
opinion is material, or omits to state a fact that in the Agent’s reasonable opinion is material and is required to be stated therein
or is necessary to make the statements therein not misleading.
(d) Agent
Counsel Legal Opinion. Agent shall have received from Cooley LLP, counsel for Agent, such opinion or opinions, on or before the date
on which the delivery of the Company counsel legal opinion is required pursuant to Section 4(p), with respect to such matters
as Agent may reasonably require, and the Company shall have furnished to such counsel such documents as they request for enabling them
to pass upon such matters.
Section 6. INDEMNIFICATION
AND CONTRIBUTION
(a) Indemnification
of the Agent. The Company agrees to indemnify and hold harmless the Agent, its officers and employees, and each person, if any, who
controls the Agent within the meaning of the Securities Act or the Exchange Act against any loss, claim, damage, liability or expense,
as incurred, to which the Agent or such officer, employee or controlling person may become subject, under the Securities Act, the Exchange
Act, other federal or state statutory law or regulation, the CSRC Rules, or the laws or regulations of foreign jurisdictions where Shares
have been offered or sold or at common law or otherwise (including in settlement of any litigation), insofar as such loss, claim, damage,
liability or expense (or actions in respect thereof as contemplated below) arises out of or is based upon (i) any untrue statement
or alleged untrue statement of a material fact contained in the Registration Statement, or any amendment thereto, including any information
deemed to be a part thereof pursuant to Rule 430B under the Securities Act, or the omission or alleged omission therefrom of a material
fact required to be stated therein or necessary to make the statements therein not misleading; (ii) any untrue statement or alleged
untrue statement of a material fact contained in any Free Writing Prospectus that the Company has used, referred to or filed, or is required
to file, pursuant to Rule 433(d) of the Securities Act or the Prospectus (or any amendment or supplement thereto), or the omission
or alleged omission therefrom of a material fact necessary in order to make the statements therein, in the light of the circumstances
under which they were made, not misleading; or (iii) any activities undertaken by the Agent in connection with the offering
of the Shares contemplated by this Agreement pursuant to all applicable laws, regulations and rules, including, without limitation, the
CSRC Rules, and to reimburse the Agent and each such officer, employee and controlling person for any and all expenses (including the
fees and disbursements of one counsel chosen by the Agent for each applicable jurisdiction) as such expenses are documented and reasonably
incurred by the Agent or such officer, employee or controlling person in connection with investigating, defending, settling, compromising
or paying any such loss, claim, damage, liability, expense or action; provided, however, that the foregoing indemnity agreement shall
not apply to any loss, claim, damage, liability or expense to the extent, but only to the extent, arising out of or based upon any untrue
statement or alleged untrue statement or omission or alleged omission made in reliance upon and in conformity with written information
furnished to the Company by the Agent expressly for use in the Registration Statement, any such Free Writing Prospectus or the Prospectus
(or any amendment or supplement thereto), it being understood and agreed that the only such information furnished by the Agent to the
Company consists of the information described in subsection (b) below. The indemnity agreement set forth in this Section 6(a) shall
be in addition to any liabilities that the Company may otherwise have.
(b) Indemnification
of the Company, its Directors and Officers. The Agent agrees to indemnify and hold harmless the Company, each of its directors, each
of its officers who signed the Registration Statement and each person, if any, who controls the Company within the meaning of the Securities
Act or the Exchange Act against any loss, claim, damage, liability or expense, as incurred, to which the Company or any such director,
officer or controlling person may become subject, under the Securities Act, the Exchange Act, or other federal or state statutory law
or regulation, or the laws or regulations of foreign jurisdictions where Shares have been offered or sold or at common law or otherwise
(including in settlement of any litigation), arising out of or based upon (i) any untrue statement or alleged untrue statement of
a material fact contained in the Registration Statement, or any amendment thereto, including any information deemed to be a part thereof
pursuant to Rule 430B under the Securities Act, or the omission or alleged omission therefrom of a material fact required to be stated
therein or necessary to make the statements therein not misleading; or (ii) any untrue statement or alleged untrue statement of a
material fact contained in any Free Writing Prospectus that the Company has used, referred to or filed, or is required to file, pursuant
to Rule 433(d) of the Securities Act or the Prospectus (or any amendment or supplement thereto), or the omission or alleged
omission therefrom of a material fact necessary in order to make the statements therein, in the light of the circumstances under which
they were made, not misleading; but, for each of (i) and (ii) above, only to the extent arising out of or based upon any untrue
statement or alleged untrue statement or omission or alleged omission made in reliance upon and in conformity with written information
furnished to the Company by the Agent expressly for use in the Registration Statement, any such Free Writing Prospectus or the Prospectus
(or any amendment or supplement thereto), it being understood and agreed that the only such information furnished by the Agent to the
Company consists of the information about the Agent set forth in the first sentence of the ninth paragraph under the caption “Plan
of Distribution” in the Prospectus, and to reimburse the Company and each such director, officer and controlling person for any
and all expenses (including the fees and disbursements of one counsel chosen by the Company) as such expenses are reasonably incurred
by the Company or such officer, director or controlling person in connection with investigating, defending, settling, compromising or
paying any such loss, claim, damage, liability, expense or action. The indemnity agreement set forth in this Section 6(b) shall
be in addition to any liabilities that the Agent or the Company may otherwise have.
(c) Notifications
and Other Indemnification Procedures. Promptly after receipt by an indemnified party under this Section 6 of notice
of the commencement of any action, such indemnified party will, if a claim in respect thereof is to be made against an indemnifying party
under this Section 6, notify the indemnifying party in writing of the commencement thereof, but the omission so to notify
the indemnifying party will not relieve it from any liability which it may have to any indemnified party for contribution or otherwise
than under the indemnity agreement contained in this Section 6 or to the extent it is not prejudiced as a proximate result
of such failure. In case any such action is brought against any indemnified party and such indemnified party seeks or intends to seek
indemnity from an indemnifying party, the indemnifying party will be entitled to participate in, and, to the extent that it shall elect,
jointly with all other indemnifying parties similarly notified, by written notice delivered to the indemnified party promptly after receiving
the aforesaid notice from such indemnified party, to assume the defense thereof with counsel reasonably satisfactory to such indemnified
party; provided, however, if the defendants in any such action include both the indemnified party and the indemnifying party and the indemnified
party shall have reasonably concluded that a conflict may arise between the positions of the indemnifying party and the indemnified party
in conducting the defense of any such action or that there may be legal defenses available to it and/or other indemnified parties which
are different from or additional to those available to the indemnifying party, the indemnified party or parties shall have the right to
select separate counsel to assume such legal defenses and to otherwise participate in the defense of such action on behalf of such indemnified
party or parties. Upon receipt of notice from the indemnifying party to such indemnified party of such indemnifying party’s election
so to assume the defense of such action and approval by the indemnified party of counsel, the indemnifying party will not be liable to
such indemnified party under this Section 6 for any legal or other expenses subsequently incurred by such indemnified party
in connection with the defense thereof unless (i) the indemnified party shall have employed separate counsel in accordance with the
proviso to the preceding sentence (it being understood, however, that the indemnifying party shall not be liable for the fees and expenses
of more than one separate counsel (together with local counsel), representing the indemnified parties who are parties to such action),
which counsel (together with any local counsel) for the indemnified parties shall be selected by the indemnified party (in the case of
counsel for the indemnified parties referred to in Section 6(a) and Section 6(b) above), (ii) the
indemnifying party shall not have employed counsel reasonably satisfactory to the indemnified party to represent the indemnified party
within a reasonable time after notice of commencement of the action or (iii) the indemnifying party has authorized in writing the
employment of counsel for the indemnified party at the expense of the indemnifying party, in each of which cases the fees and expenses
of counsel shall be at the expense of the indemnifying party and shall be paid as they are incurred.
(d) Settlements.
The indemnifying party under this Section 6 shall not be liable for any settlement of any proceeding effected without
its written consent, but if settled with such consent or if there be a final judgment for the plaintiff, the indemnifying party agrees
to indemnify the indemnified party against any loss, claim, damage, liability or expense by reason of such settlement or judgment. Notwithstanding
the foregoing sentence, if at any time an indemnified party shall have requested an indemnifying party to reimburse the indemnified party
for fees and expenses of counsel as contemplated by Section 6(c) hereof, the indemnifying party agrees that it shall
be liable for any settlement of any proceeding effected without its written consent if (i) such settlement is entered into more than
30 days after receipt by such indemnifying party of the aforesaid request; and (ii) such indemnifying party shall not have reimbursed
the indemnified party in accordance with such request prior to the date of such settlement. No indemnifying party shall, without the prior
written consent of the indemnified party, effect any settlement, compromise or consent to the entry of judgment in any pending or threatened
action, suit or proceeding in respect of which any indemnified party is or could have been a party and indemnity was or could have been
sought hereunder by such indemnified party, unless such settlement, compromise or consent includes an unconditional release of such indemnified
party from all liability on claims that are the subject matter of such action, suit or proceeding.
(e) Contribution.
If the indemnification provided for in this Section 6 is for any reason held to be unavailable to or otherwise insufficient
to hold harmless an indemnified party in respect of any losses, claims, damages, liabilities or expenses referred to therein, then each
indemnifying party shall contribute to the aggregate amount paid or payable by such indemnified party, as incurred, as a result of any
losses, claims, damages, liabilities or expenses referred to therein (i) in such proportion as is appropriate to reflect the relative
benefits received by the Company, on the one hand, and the Agent, on the other hand, from the offering of the Shares pursuant to this
Agreement; or (ii) if the allocation provided by clause (i) above is not permitted by applicable law, in such proportion as
is appropriate to reflect not only the relative benefits referred to in clause (i) above but also the relative fault of the Company,
on the one hand, and the Agent, on the other hand, in connection with the statements or omissions which resulted in such losses, claims,
damages, liabilities or expenses, as well as any other relevant equitable considerations. The relative benefits received by the Company,
on the one hand, and the Agent, on the other hand, in connection with the offering of the Shares pursuant to this Agreement shall be deemed
to be in the same respective proportions as the total gross proceeds from the offering of the Shares (before deducting expenses) received
by the Company bear to the total commissions received by the Agent. The relative fault of the Company, on the one hand, and the Agent,
on the other hand, shall be determined by reference to, among other things, whether any such untrue or alleged untrue statement of a material
fact or omission or alleged omission to state a material fact relates to information supplied by the Company, on the one hand, or the
Agent, on the other hand, and the parties’ relative intent, knowledge, access to information and opportunity to correct or prevent
such statement or omission.
The amount paid or payable
by a party as a result of the losses, claims, damages, liabilities and expenses referred to above shall be deemed to include, subject
to the limitations set forth in Section 6(c), any reasonable and documented legal or other fees or expenses reasonably
incurred by such party in connection with investigating or defending any action or claim. The provisions set forth in Section 6(c) with
respect to notice of commencement of any action shall apply if a claim for contribution is to be made under this Section 6(e);
provided, however, that no additional notice shall be required with respect to any action for which notice has been given under Section 6(c) for
purposes of indemnification.
The Company and the Agent
agree that it would not be just and equitable if contribution pursuant to this Section 6(e) were determined by pro
rata allocation or by any other method of allocation which does not take account of the equitable considerations referred to in this Section 6(e).
Notwithstanding the provisions
of this Section 6(e), the Agent shall not be required to contribute any amount in excess of the Selling Commission
received by the Agent in connection with the offering contemplated hereby. No person guilty of fraudulent misrepresentation (within the
meaning of Section 11(f) of the Securities Act) shall be entitled to contribution from any person who was not guilty of such
fraudulent misrepresentation. For purposes of this Section 6(e), each officer and employee of the Agent and each
person, if any, who controls the Agent within the meaning of the Securities Act or the Exchange Act shall have the same rights to contribution
as the Agent, and each director of the Company, each officer of the Company who signed the Registration Statement, and each person, if
any, who controls the Company within the meaning of the Securities Act and the Exchange Act shall have the same rights to contribution
as the Company.
Section 7. TERMINATION &
SURVIVAL
(a) Term.
Subject to the provisions of this Section 7, the term of this Agreement shall continue from the date of this Agreement
until the end of the Agency Period, unless earlier terminated by the parties to this Agreement pursuant to this Section 7.
(b) Termination;
Survival Following Termination.
(i) Either
party may terminate this Agreement prior to the end of the Agency Period, by giving written notice as required by this Agreement, upon
ten (10) Trading Days’ notice to the other party; provided that, (A) if the Company terminates this Agreement after the
Agent confirms to the Company any sale of Shares, the Company shall remain obligated to comply with Section 3(b)(v) with
respect to such Shares and (B) Section 2, Section 3(d) (solely to the extent any costs, fees
and expenses payable by the Company are owed but unpaid as of the termination of this Agreement), Section 6, Section 7
and Section 8 shall survive termination of this Agreement. If termination shall occur prior to the Settlement Date for any
sale of Shares, such sale shall nevertheless settle in accordance with the terms of this Agreement.
(ii) In
addition to the survival provision of Section 7(b)(i), the respective indemnities, agreements, representations, warranties
and other statements of the Company, of its officers and of the Agent set forth in or made pursuant to this Agreement will remain in full
force and effect, regardless of any investigation made by or on behalf of the Agent or the Company or any of its or their partners, officers
or directors or any controlling person, as the case may be, and, anything herein to the contrary notwithstanding, will survive delivery
of and payment for the Shares sold hereunder and any termination of this Agreement.
Section 8. MISCELLANEOUS
(a) Press
Releases and Disclosure. The Company may issue a press release describing the material terms of the transactions contemplated hereby
as soon as practicable following the date of this Agreement, and may file with the Commission a Report on Form 6-K, with this Agreement
attached as an exhibit thereto, describing the material terms of the transactions contemplated hereby, and the Company shall consult with
the Agent prior to making such disclosures, and the parties hereto shall use all commercially reasonable efforts, acting in good faith,
to agree upon a text for such disclosures that is reasonably satisfactory to all parties hereto. No party hereto shall issue thereafter
any press release or like public statement (including, without limitation, any disclosure required in reports filed with the Commission
pursuant to the Exchange Act) related to this Agreement or any of the transactions contemplated hereby without the prior written approval
of the other party hereto, except as may be necessary or appropriate in the reasonable opinion of the party seeking to make disclosure
to comply with the requirements of applicable law or stock exchange rules. If any such press release or like public statement is so required
(other than disclosures regarding sales of Shares pursuant to this Agreement on Form 6-K or Annual Reports on Form 20-F), the
party making such disclosure shall consult with the other party prior to making such disclosure, and the parties shall use all commercially
reasonable efforts, acting in good faith, to agree upon a text for such disclosure that is reasonably satisfactory to all parties hereto.
(b) No
Advisory or Fiduciary Relationship. The Company acknowledges and agrees that (i) the transactions contemplated by this Agreement,
including the determination of any fees, are arm’s-length commercial transactions between the Company and the Agent, (ii) when
acting as a principal under this Agreement, the Agent is and has been acting solely as a principal is not the agent or fiduciary of the
Company, or its stockholders, creditors, employees or any other party, (iii) the Agent has not assumed nor will assume an advisory
or fiduciary responsibility in favor of the Company with respect to the transactions contemplated hereby or the process leading thereto
(irrespective of whether the Agent has advised or is currently advising the Company on other matters) and the Agent does not have any
obligation to the Company with respect to the transactions contemplated hereby except the obligations expressly set forth in this Agreement,
(iv) the Agent and its Affiliates may be engaged in a broad range of transactions that involve interests that differ from those of
the Company, and (v) the Agent has not provided any legal, accounting, regulatory or tax advice with respect to the transactions
contemplated hereby and the Company has consulted its own legal, accounting, regulatory and tax advisors to the extent it deemed appropriate.
(c) Research
Analyst Independence. The Company acknowledges that the Agent’s research analysts and research departments are required to and
should be independent from their respective investment banking divisions and are subject to certain regulations and internal policies,
and as such the Agent’s research analysts may hold views and make statements or investment recommendations and/or publish research
reports with respect to the Company or the offering that differ from the views of their respective investment banking divisions. The Company
understands that the Agent is a full service securities firm and as such from time to time, subject to applicable securities laws, may
effect transactions for its own account or the account of its customers and hold long or short positions in debt or equity securities
of the companies that may be the subject of the transactions contemplated by this Agreement.
(d) Notices.
All communications hereunder shall be in writing and shall be mailed, hand delivered, sent via email (if applicable) or telecopied and
confirmed to the parties hereto as follows:
If to the Agent:
Jefferies LLC
520 Madison Avenue
New York, NY 10022
Facsimile: (646) 786-5719
Attention: General Counsel
with a copy (which shall not constitute notice)
to:
Cooley LLP
55 Hudson Yards
New York, NY 10001
Attention: Daniel I. Goldberg, Esq.
Email: *
If to the Company:
CASI Pharmaceuticals, Inc.
9620 Medical Center Drive, Suite 300
Rockville, Maryland 20850
Attention: Daniel Lang, M.D., Chief Financial
Officer
Email: *
with a copy (which shall not constitute notice)
to:
Harter Secrest & Emery LLP
Attention: C. Christopher Murillo
1600 Bausch & Lomb Pl
Rochester, New York 14604
Email: *
Any party hereto may change the address for receipt
of communications by giving written notice to the others in accordance with this Section 8(d).
(e) Successors.
This Agreement will inure to the benefit of and be binding upon the parties hereto, and to the benefit of the employees, officers and
directors and controlling persons referred to in Section 6, and in each case their respective successors, and no other person
will have any right or obligation hereunder. The term “successors” shall not include any purchaser of the Shares as such from
the Agent merely by reason of such purchase.
(f) Partial
Unenforceability. The invalidity or unenforceability of any Article, Section, paragraph or provision of this Agreement shall not affect
the validity or enforceability of any other Article, Section, paragraph or provision hereof. If any Article, Section, paragraph or provision
of this Agreement is for any reason determined to be invalid or unenforceable, there shall be deemed to be made such minor changes (and
only such minor changes) as are necessary to make it valid and enforceable.
(g) Governing
Law Provisions. This Agreement shall be governed by and construed in accordance with the internal laws of the State of New York applicable
to agreements made and to be performed in such state. Any legal suit, action or proceeding arising out of or based upon this Agreement
or the transactions contemplated hereby may be instituted in the federal courts of the United States of America located in the Borough
of Manhattan in the City of New York or the courts of the State of New York in each case located in the Borough of Manhattan in the City
of New York (collectively, the “Specified Courts”), and each party irrevocably submits to the exclusive jurisdiction
(except for proceedings instituted in regard to the enforcement of a judgment of any such court, as to which such jurisdiction is non-exclusive)
of such courts in any such suit, action or proceeding. Service of any process, summons, notice or document by mail to such party’s
address set forth above shall be effective service of process for any suit, action or other proceeding brought in any such court. The
parties irrevocably and unconditionally waive any objection to the laying of venue of any suit, action or other proceeding in the Specified
Courts and irrevocably and unconditionally waive and agree not to plead or claim in any such court that any such suit, action or other
proceeding brought in any such court has been brought in an inconvenient forum.
(h) Appointment
of Agents for Service. The Company hereby irrevocably appoints Rui Zhang as its agent for service of process in any legal suit, action
or proceeding arising out of or based upon this Agreement or the transactions contemplated hereby (each, a “Related Proceeding”)
and agrees that service of process in any such Related Proceeding may be made upon it at the office of such agent. The Company waives,
to the fullest extent permitted by law, any other requirements of or objections to personal jurisdiction with respect thereto. The Company
represents and warrants that such agent has agreed to act as the Company’s agent for service of process, and the Company agrees
to take any and all action, including the filing of any and all documents and instruments, that may be necessary to continue such appointment
in full force and effect.
(i) Judgment
Currency. If for the purposes of obtaining judgment in any court it is necessary to convert a sum due hereunder into any currency
other than United States dollars, the parties hereto agree, to the fullest extent permitted by law, that the rate of exchange used shall
be the rate at which in accordance with normal banking procedures the Agent could purchase United States dollars with such other currency
in The City of New York on the business day preceding that on which final judgment is given. The obligation of the Company with respect
to any sum due from it to the Agent or any person controlling the Agent shall, notwithstanding any judgment in a currency other than United
States dollars, not be discharged until the first business day following receipt by the Agent or controlling person of any sum in such
other currency, and only to the extent that the Agent or controlling person may in accordance with normal banking procedures purchase
United States dollars with such other currency. If the United States dollars so purchased are less than the sum originally due to the
Agent or controlling person hereunder, the Company agrees as a separate obligation and notwithstanding any such judgment, to indemnify
the Agent or controlling person against such loss. If the United States dollars so purchased are greater than the sum originally due to
the Agent or controlling person hereunder, the Agent or controlling person agrees to pay to the Company an amount equal to the excess
of the dollars so purchased over the sum originally due to the Agent or controlling person hereunder.
(j) General
Provisions. This Agreement constitutes the entire agreement of the parties to this Agreement and supersedes all prior written or oral
and all contemporaneous oral agreements, understandings and negotiations with respect to the subject matter hereof. This Agreement may
be executed in two or more counterparts, each one of which shall be an original, with the same effect as if the signatures thereto and
hereto were upon the same instrument, and may be delivered by facsimile transmission or by electronic delivery of a portable document
format (PDF) file (including any electronic signature covered by the U.S. federal ESIGN Act of 2000, Uniform Electronic Transactions Act,
the Electronic Signatures and Records Act or other applicable law, e.g., www.docusign.com). This Agreement may not be amended or modified
unless in writing by all of the parties hereto, and no condition herein (express or implied) may be waived unless waived in writing by
each party whom the condition is meant to benefit. The Article and Section headings herein are for the convenience of the parties
only and shall not affect the construction or interpretation of this Agreement.
[Signature Page Immediately Follows]
If the foregoing is in accordance
with your understanding of our agreement, kindly sign and return to the Company the enclosed copies hereof, whereupon this instrument,
along with all counterparts hereof, shall become a binding agreement in accordance with its terms.
| |
Very truly yours, |
| |
|
| |
CASI PHARMACEUTICALS, INC. |
| |
|
| |
By: |
/s/ Dan Lang |
| |
|
Name: Dan Lang |
| |
|
Title: Chief Financial Officer |
The foregoing Agreement is
hereby confirmed and accepted by the Agent in New York, New York as of the date first above written.
JEFFERIES LLC
| By: |
/s/
Michael Brinkman |
|
| |
Name: Michael Brinkman |
|
| |
Title: Managing Director |
|
EXHIBIT A
ISSUANCE NOTICE
[Date]
Jefferies LLC
520 Madison Avenue
New York, New York 10022
Attn: [__________]
Reference is made to the Open Market Sale AgreementSM
between CASI Pharmaceuticals, Inc.(the “Company”) and Jefferies LLC (the “Agent”) dated as
of ________ ___, 20__. The Company confirms that all conditions to the delivery of this Issuance Notice are satisfied as of the date hereof.
Date of Delivery of Issuance Notice (determined pursuant to Section 3(b)(i)):
_______________________
Issuance Amount (equal to the total Sales Price for such Shares):
| |
$ |
| |
|
| Number of days in selling period: |
|
| |
|
| First date of selling period: |
|
| |
|
| Last date of selling period: |
|
Settlement Date(s) if other than standard T+1 settlement:
Floor Price Limitation (in no event less than $1.00 without the prior
written consent of the Agent, which consent may be withheld in the Agent’s sole discretion): $ ____ per share
Schedule A
Notice Parties
The Company
The Agent
Form of Officer’s Certificate Pursuant
to Section 4(o)
Form of
Company’s Confirmation and Undertaking Pursuant to Section 4(w)
Exhibit 5.1
| Our ref | RDS/815438-000001/30983982v2 |
CASI Pharmaceuticals, Inc.
PO Box 309
Ugland House
Grand Cayman, KY1-1104
Cayman Islands
20 December 2024
Dear Sir or Madam
CASI Pharmaceuticals, Inc.
We have acted as counsel as to Cayman Islands
law to CASI Pharmaceuticals, Inc., an exempted company incorporated in the Cayman Islands with limited liability (the "Company"),
in connection with the filing with the Securities and Exchange Commission under the U.S. Securities Act of 1933, as amended, of the Company's
registration statement on Form F-3 dated 20 December 2024 (the "Registration Statement") relating to the following
securities to be issued and sold by the Company on a delayed or continuous basis from time to time in one or more offerings (the "Securities"):
| (a) | ordinary shares of the Company of par value US$0.0001 per share (the "Ordinary Shares"); |
| (b) | preferred shares of the Company of par value US$0.0001 per share (the "Preferred Shares"); |
| (c) | warrants (the "Warrants") to purchase Ordinary Shares ("Warrant Shares"),
to be issued under warrant agreements to be entered into between the Company and the warrant agent for such Warrants thereunder (the "Warrant
Agreements"); |
| (d) | subscription rights (the "Rights") to purchase Ordinary Shares or Preferred Shares (the
"Rights Shares"), to be issued under subscription rights agreements to be entered into among the Company and one or more
underwriters or other purchasers for such Rights (the "Subscription Rights Agreements"); and |
| (e) | units consisting of one or more of the Securities in any combination (the "Units"), to
be issued under unit agreements to be entered into between the Company and a unit agent for such Units (the "Unit Agreements"). |
We are furnishing
this opinion as Exhibits 5.1 and 23.2 to the Registration Statement.
We have reviewed originals, copies, drafts or
conformed copies of the following documents:
| 1.1 | The Certificate of Incorporation dated 10 January 2023 and the Certificate of Incorporation on Change
of Name dated 21 March 2023. |
| 1.2 | The Amended and Restated Memorandum and Articles of Association of the Company as adopted by a special
resolution of the Company dated 20 March 2023 and effective on 21 March 2023 (the "Memorandum and Articles"). |
| 1.3 | The written resolutions of the directors of
the Company dated 13 December 2024 (the "Directors' Resolutions"). |
| 1.4 | A certificate of good standing with respect to the Company issued by the Registrar of Companies in the
Cayman Islands dated 7 November 2024 (the "Certificate of Good Standing"). |
| 1.5 | The Registration Statement. |
The following opinions are given only as to, and
based on, circumstances and matters of fact existing and known to us on the date of this opinion letter. These opinions only relate to
the laws of the Cayman Islands which are in force on the date of this opinion letter. In giving the following opinions, we have relied
(without further verification) upon the completeness and accuracy, as of the date of this opinion letter, of the Certificate of Good Standing.
We have also relied upon the following assumptions, which we have not independently verified:
| 2.1 | The Warrant Agreements, Subscription Rights Agreements and Unit Agreements (together, the "Transaction
Documents"), and the Securities (other than the Ordinary Shares and Preferred Shares), have been, or will be, authorised and
duly executed and unconditionally delivered by or on behalf of all relevant parties in accordance with all relevant laws (other than,
with respect to the Company, the laws of the Cayman Islands). |
| 2.2 | The Transaction Documents and the Securities (other than the Ordinary Shares and Preferred Shares) are,
or will be, legal, valid, binding and enforceable against all relevant parties in accordance with their terms under the laws of the State
of New York and all other relevant laws (other than, with respect to the Company, the laws of the Cayman Islands). |
| 2.3 | The choice of the laws of the State of New York as the governing law of the Transaction Documents and
the Securities (other than the Ordinary Shares and Preferred Shares) has been, or will be, made in good faith and would be regarded as
a valid and binding selection which will be upheld by the courts of the State of New York and any other relevant jurisdiction (other than
the Cayman Islands) as a matter of the laws of the State of New York and all other relevant laws (other than the laws of the Cayman Islands). |
| 2.4 | Copies of documents, conformed copies or drafts of documents provided to us are true and complete copies
of, or in the final forms of, the originals, and translations of documents provided to us are complete and accurate. |
| 2.5 | All signatures, initials and seals are genuine. |
| 2.6 | The capacity, power, authority and legal right of all parties under all relevant laws and regulations
(other than, with respect to the Company, the laws and regulations of the Cayman Islands) to enter into, execute, unconditionally deliver
and perform their respective obligations under the Transaction Documents and the Securities. |
| 2.7 | There is no contractual or other prohibition or restriction (other than as arising under Cayman Islands
law) binding on the Company prohibiting or restricting it from issuing the Ordinary Shares or the Preferred Shares or entering into and
performing its obligations under the Transaction Documents and the Securities. |
| 2.8 | No monies paid to or for the account of any party under the Transaction Documents or the Securities or
any property received or disposed of by any party to the Transaction Documents or the Securities in each case in connection with the Transaction
Documents or the Securities, or the consummation of the transactions contemplated thereby, represent or will represent proceeds of criminal
conduct or criminal property or terrorist property (as defined in the Proceeds of Crime Act (As Revised) and the Terrorism Act (As Revised),
respectively). |
| 2.9 | There is nothing contained in the minute book or corporate records of the Company (which, other than the
records set out in section 1 of this opinion letter, we have not inspected) which would or might affect the opinions set out below. |
| 2.10 | There is nothing under any law (other than the laws of the Cayman Islands) which would or might affect
the opinions set out below. Specifically, we have made no independent investigation of the laws of the State of New York. |
| 2.11 | The Company will receive money or money's worth in consideration for the issue of the Ordinary Shares
and the Preferred Shares, and none of the Ordinary Shares or the Preferred Shares will be issued for less than their par value. |
| 2.12 | There will be sufficient Ordinary Shares and Preferred Shares authorised for issue under the Memorandum
and Articles at the time of issuance. |
| 2.13 | The Memorandum and Articles remain in full force and effect and are unamended. |
| 2.14 | The Directors' Resolutions were duly passed in the manner prescribed in the Memorandum and Articles (including,
without limitation, with respect to the disclosure of interests (if any) by directors of the Company) and have not been amended, varied
or revoked in any respect. |
| 2.15 | The shareholders of the Company have not restricted or limited the powers of the directors of the Company
in any way. |
| 2.16 | The authorised share capital of the Company is US$50,000 divided into 500,000,000 shares of a par value
of US$0.0001 each. |
| 2.17 | Each director of the Company considers the transactions contemplated by the Registration Statement, the
execution and delivery of the Transaction Documents and the issue of the Securities will be of commercial benefit to the Company and has
acted bona fide in the best interests of the Company, and for a proper purpose of the Company in relation to the transactions the subject
of the Opinion. |
| 2.18 | The Warrants, Rights, and Units will respectively be issued and authenticated or duly authorised, executed
and delivered as required in accordance with the provisions of the Warrant Agreement, Subscription Rights Agreement, and Unit Agreement
(as the case may be). |
| 2.19 | The Transaction Documents and the Securities (other than the Ordinary Shares and Preferred Shares) will
be, or have been, duly executed and delivered by an authorised person of the parties thereto. |
| 2.20 | No invitation has been or will be made by or on behalf of the Company to the public in the Cayman Islands
to subscribe for any of the Securities. |
Based upon, and subject to, the foregoing assumptions
and the qualifications set out below, and having regard to such legal considerations as we deem relevant, we are of the opinion that:
| 3.1 | The Company has been duly incorporated as an exempted company with limited liability and is validly existing
and in good standing with the Registrar of Companies under the laws of the Cayman Islands. |
| 3.2 | With respect to the Ordinary Shares, when (i) the board of directors of the Company (the "Board")
has taken all necessary corporate action to approve the issue thereof, the terms of the offering thereof and related matters; (ii) the
issue of such Ordinary Shares has been recorded in the Company's register of members (shareholders); and (iii) the subscription price
of such Ordinary Shares (being not less than the par value of the Ordinary Shares) has been fully paid in cash or other consideration
approved by the Board, the Ordinary Shares will be duly authorised, validly issued, fully paid and non-assessable. |
| 3.3 | With respect to the Preferred Shares, when (i) the Board has taken all necessary corporate action
to approve the issue thereof, the terms of the offering thereof and related matters; (ii) the issue of such Preferred Shares has
been recorded in the Company's register of members (shareholders); and (iii) the subscription price of such Preferred Shares (being
not less than the par value of the Preferred Shares) has been fully paid in cash or other consideration approved by the Board, the Preferred
Shares will be duly authorised, validly issued, fully paid and non-assessable. |
| 3.4 | With respect to each issue of the Warrants, when (i) the Board has taken all necessary corporate
action to approve the creation and terms of the Warrants and to approve the issue thereof, the terms of the offering thereof and related
matters; (ii) a Warrant Agreement relating to the Warrants shall have been duly authorised and validly executed and delivered by
the Company and the warrant agent thereunder; and (iii) the certificates representing the Warrants have been duly executed, countersigned,
registered and delivered in accordance with the Warrant Agreement relating to the Warrants and the applicable definitive purchase, underwriting
or similar agreement approved by the Board upon payment of the consideration therefor provided therein, (A) such Warrants will be
duly authorised, legal and binding obligations of the Company, and (B) the issue and allotment of the Warrant Shares upon exercise
of the Warrants will be duly authorised and when allotted, issued and paid for in accordance with the relevant Warrants, such Warrant
Shares will be legally issued and allotted, fully paid and non-assessable. As a matter of Cayman law, a share is only issued when it has
been entered in the register of members (shareholders). |
| 3.5 | With respect to each issue of the Rights, when (i) the Board has taken all necessary corporate action
to approve the creation and terms of the Rights and to approve the issue thereof, the terms of the offering thereof and related matters;
(ii) a Subscription Rights Agreement relating to the Rights shall have been duly authorised and duly executed and delivered by the
Company and all the relevant parties thereunder; and (iii) the certificates representing the Rights have been duly executed, countersigned,
registered and delivered in accordance with the Subscription Rights Agreement relating to the Rights and the applicable definitive purchase,
underwriting or similar agreement approved by the Board upon payment of the consideration therefor provided therein, (A) such Rights
will be duly authorised, legal and binding obligations of the Company, and (B) the issue and allotment of the Rights Shares upon
exercise of the Rights will be duly authorised and when allotted, issued and paid for in accordance with the relevant Subscription Rights
Agreements, such Rights Shares will be legally issued and allotted, fully paid and non-assessable. As a matter of Cayman law, a share
is only issued when it has been entered in the register of members (shareholders). |
| 3.6 | With respect to each issue of the Units, when (i) the Board has taken all necessary corporate action
to approve the creation and terms of the Units and to approve the issue thereof and the Securities comprised in such Units, the terms
of the offering thereof and related matters; (ii) a Unit Agreement relating to the Units shall have been duly authorised and
duly executed and delivered by and on behalf of the Company and all the relevant parties thereunder; (iii) in respect of any
Ordinary Shares which are components of the Units, the issue of such Ordinary Shares has been recorded in the Company’s register
of members (shareholders); (iv) in respect of any Preferred Shares which are components of the Units, the issue of such Preferred
Shares has been recorded in the Company’s register of members (shareholders); (v) in respect of any Warrants which are
components of the Units, a Warrant Agreement relating to the Warrants shall have been duly authorised and validly executed and delivered
by and on behalf of the Company and the warrant agent thereunder in accordance with all relevant laws; (vi) in respect of any Rights
which are components of the Units, a Subscription Rights Agreement relating to the Rights shall have been duly authorised and validly
executed and delivered by and on behalf of the Company and all the relevant parties thereunder in accordance with all relevant laws; and
(vii) the certificates representing the Units, the Units and any Securities which are components of the Units shall have been duly
executed, countersigned, registered, authenticated, issued and delivered (in each case, as and when applicable), in accordance with (A) the
applicable Unit Agreement relating to the Units, (B) the applicable Warrant Agreement relating to any Warrants which are components
of the Units, (C) the applicable Subscription Rights Agreement relating to any Rights which are components of the Units and (D) the
applicable definitive purchase, underwriting or similar agreement approved by the Board and any relevant prospectus supplement, and upon
payment of the consideration therefor provided therein, (AA) such Units will be duly authorised, legal and binding obligations of the
Company and (BB) the issue and allotment of any Ordinary Shares or Preferred Shares which are components of the Units will be duly authorised
and when allotted, issued and paid for in accordance with the relevant Unit Agreements, such Ordinary Shares or Preferred Shares will
be legally issued and allotted, fully paid and non-assessable. As a matter of Cayman law, a share is only issued when it has been entered
in the register of members (shareholders). |
The opinions expressed above are subject to the
following qualifications:
| 4.1 | To maintain the Company in good standing under the laws of the Cayman Islands, annual filing fees must
be paid and returns made to the Registrar of Companies within the time frame prescribed by law. |
| 4.2 | The obligations assumed by the Company under the Transaction Documents and the Securities (other than
the Ordinary Shares and Preferred Shares) will not necessarily be enforceable in all circumstances in accordance with their terms. In
particular: |
| (a) | enforcement may be limited by bankruptcy, insolvency, liquidation, reorganisation, readjustment of debts
or moratorium or other laws of general application relating to, protecting or affecting the rights of creditors and/or contributories; |
| (b) | enforcement may be limited by general principles of equity. For example, equitable remedies such as specific
performance may not be available, inter alia, where damages are considered to be an adequate remedy; |
| (c) | some claims may become barred under relevant statutes of limitation or may be or become subject to defences
of set off, counterclaim, estoppel and similar defences; |
| (d) | where obligations are to be performed in a jurisdiction outside the Cayman Islands, they may not be enforceable
in the Cayman Islands to the extent that performance would be illegal under the laws of that jurisdiction; |
| (e) | the courts of the Cayman Islands have jurisdiction to give judgment in the currency of the relevant obligation
and statutory rates of interest payable upon judgments will vary according to the currency of the judgment. If the Company becomes insolvent
and is made subject to a liquidation proceeding, the courts of the Cayman Islands will require all debts to be proved in a common currency,
which is likely to be the "functional currency" of the Company determined in accordance with applicable accounting principles.
Currency indemnity provisions have not been tested, so far as we are aware, in the courts of the Cayman Islands; |
| (f) | arrangements that constitute penalties will not be enforceable; |
| (g) | enforcement may be prevented by reason of fraud, coercion, duress, undue influence, misrepresentation,
public policy or mistake or limited by the doctrine of frustration of contracts; |
| (h) | provisions imposing confidentiality obligations may be overridden by compulsion of applicable law or the
requirements of legal and/or regulatory process; |
| (i) | the courts of the Cayman Islands may decline to exercise jurisdiction in relation to substantive proceedings
brought under or in relation to the Transaction Documents or Securities (other than Ordinary Shares and Preferred Shares) in matters where
they determine that such proceedings may be tried in a more appropriate forum; |
| (j) | we reserve our opinion as to the enforceability of the relevant provisions of the Transaction Documents
or Securities (other than the Ordinary Shares and Preferred Shares) to the extent that they purport to grant exclusive jurisdiction as
there may be circumstances in which the courts of the Cayman Islands would accept jurisdiction notwithstanding such provisions; and |
| (k) | a company cannot, by agreement or in its articles of association, restrict
the exercise of a statutory power and there is doubt as to the enforceability of any provision in the Transaction Documents or Securities
(other than the Ordinary Shares and Preferred Shares) whereby the Company covenants to restrict the exercise of powers specifically given
to it under the Companies Act (As Revised) of the Cayman Islands (the "Companies Act"),
including, without limitation, the power to increase its authorised share capital, amend its memorandum and articles of association or
present a petition to a Cayman Islands court for an order to wind up the Company. |
| 4.3 | We express no opinion as to the meaning, validity or effect of any references to foreign (i.e. non-Cayman
Islands) statutes, rules, regulations, codes, judicial authority or any other promulgations and any references to them in the Transaction
Documents or Securities (other than the Ordinary Shares and Preferred Shares). |
| 4.4 | We have not reviewed the final form of any of the Warrant Agreements or the Warrants to be issued thereunder,
the Subscription Rights Agreements or the Rights to be issued thereunder, or the Unit Agreements or the Units to be issued thereunder,
and our opinions are qualified accordingly. |
| 4.5 | We reserve our opinion as to the extent to which the courts of the Cayman Islands would, in the event
of any relevant illegality or invalidity, sever the relevant provisions of the Transaction Documents or Securities (other than the Ordinary
Shares and Preferred Shares) and enforce the remainder of the Transaction Documents or Securities (other than the Ordinary Shares and
Preferred Shares), or the transaction of which such provisions form a part, notwithstanding any express provisions in this regard. |
| 4.6 | Under the Companies Act, the register of members of a Cayman Islands company is by statute regarded as
prima facie evidence of any matters which the Companies Act directs or authorises to be inserted therein. A third party interest in the
shares in question would not appear. An entry in the register of members may yield to a court order for rectification (for example, in
the event of fraud or manifest error). |
| 4.7 | In this opinion the phrase "non-assessable" means, with respect to the issuance of shares in
the Company, that a shareholder shall not, in respect of the relevant shares and in the absence of a contractual arrangement, or an obligation
pursuant to the memorandum and articles of association, to the contrary, have any obligation to make further contributions to the Company's
assets (except in exceptional circumstances, such as involving fraud, the establishment of an agency relationship or an illegal or improper
purpose or other circumstances in which a court may be prepared to pierce or lift the corporate veil). |
We hereby consent to the filing of this opinion
as an exhibit to the Registration Statement and to the references to our name under the headings "Enforceability of Civil Liabilities"
and "Legal Matters" and elsewhere included in the Registration Statement. In giving such consent, we do not thereby admit that
we come within the category of persons whose consent is required under Section 7 of the U.S. Securities Act of 1933, as amended,
or the Rules and Regulations of the Commission thereunder.
We express no view as to the commercial terms
of the Transaction Documents or the Securities or whether such terms represent the intentions of the parties and make no comment with
regard to warranties or representations that may be made by the Company.
The opinions in this opinion letter are strictly
limited to the matters contained in the opinions section above and do not extend to any other matters. We have not been asked to review
and we therefore have not reviewed any of the ancillary documents relating to the Transaction Documents or Securities and express no opinion
or observation upon the terms of any such document.
This opinion letter may be relied upon by US counsel
to the Company for the purposes solely of any legal opinion that they may be required to give with respect to the Registration Statement.
Yours faithfully
Maples and Calder (Hong Kong) LLP
/s/ Maples
and Calder (Hong Kong) LLP
Exhibit 5.2

December 20, 2024
CASI Pharmaceuticals, Inc.
1701-1702, China Central Office Tower 1
No. 81 Jianguo Road Chaoyang District
Beijing, 100025
People’s Republic of China
| Re: | Registration
Statement on Form F-3 |
Ladies and Gentlemen:
We have acted as special United States counsel to CASI Pharmaceuticals,
Inc., an exempted company incorporated under the laws of the Cayman Islands (the “Company”), in connection with
its filing of a Registration Statement on Form F-3 (the “Registration Statement”) with the Securities and Exchange
Commission (the “SEC”) pursuant to the Securities Act of 1933, as amended (the “Securities Act”).
The Registration Statement includes a prospectus (the "Base Prospectus"), that provides it will be supplemented
in the future by one or more prospectus supplements (each, a "Prospectus Supplement"). The Registration Statement
including the Base Prospectus (as supplemented from time to time by one or more Prospectus Supplements), will provide for the registration
and public offering by the Company, from time to time, of up to $200,000,000 in the aggregate amount of any combination of the following:
| (i) | ordinary shares of common stock, par value $0.0001 per share, of the Company (“Ordinary Shares”); |
| (ii) | preferred shares, par value $0.0001 per share, of the Company (“Preferred Shares”),
which may be issued in one or more series; |
| (iii) | warrants to purchase Ordinary Shares of the Company (“Warrants”); |
| (iv) | subscription rights to purchase Ordinary Shares or Preferred Shares (the “Subscription Rights”);
and |
| (v) | units comprised of one or more of the securities described above in any combination (the “Units”). |
The Ordinary Shares and Preferred Shares are collectively referred
to herein as the “Shares”. The Warrants, Subscription Rights and Units are collectively referred to herein
as the “Securities.”
| 1600
BAUSCH & LOMB PLACE ROCHESTER, NY 14604-2711 PHONE: 585.232.6500 FAX: 585.232.2152 |
| rochester,
ny • buffalo, ny • albany, ny • corning, ny • new
york, ny |

CASI Pharmaceuticals, Inc.
December 20, 2024
Page 2
As such counsel, and for
purposes of our opinion set forth below, we have reviewed such documents and made such examination of law as we have deemed appropriate
to give the opinions set forth below. We have relied, without independent verification, on certificates of public officials and, as to
matters of fact material to the opinions set forth below, on certificates of officers of the Company.
We are not hereby rendering any opinion with respect to any Shares
issuable in combination with or upon the conversion or exercise, as applicable, of any of the Securities or any Securities that are components
of Units. For purposes of the opinion set forth below, without limiting and other exceptions or qualifications set forth herein, we have
assumed that (i) at the time the Securities are issued the Company is validly existing under the laws of the Cayman Islands and in good
standing, has the corporate power to enter into and perform its obligations under the Securities in accordance with their respective terms,
(ii) upon issuance, the Company will have duly authorized, executed and delivered the Securities in accordance with its organizational
documents and the laws of the Cayman Islands, (iii) any Shares issued in combination with or upon conversion of the Securities will be
duly authorized, validly issued, fully paid and non-assessable, and (iv) the execution, delivery and performance by the Company of its
obligations under the Securities will not violate the laws of the Cayman Islands or any other applicable laws (excepting from such assumption
the laws of the State of New York).
For purposes of the opinions set forth below, the “Future
Authorization and Issuance” means (a) the Registration Statement and any required post-effective amendment thereto have
become effective under the Securities Act and the Base Prospectus and any and all Prospectus Supplement(s) required by applicable laws
have been delivered and filed as required by such laws; (b) the Securities have been duly authorized by the Company by all necessary corporate
action; (c) the terms of the Securities and their issuance and sale, including as to any Shares to be issued in combination therewith
or upon the conversion thereof, have been duly authorized by the Company by all necessary corporate action (together with (b) above the
“Authorization”); (d) any securities issuable upon conversion, exchange, redemption or exercise of such Securities
being offered will be duly authorized, created and, if appropriate, reserved for issuance upon such conversion, exchange, redemption,
or exercise; (e) the terms of the Securities and of their issuance and sale have been duly established in conformity with the applicable
definitive purchase, underwriting, subscription rights, warrant or similar agreement under which such Securities are to be issued (the
“Applicable Agreements”), and as described in the Registration Statement, the Base Prospectus and the related
Prospectus Supplement(s), so as not to violate any applicable law or result in a default under or breach of any agreement or instrument
binding upon the Company, so as to be in conformity with the Company’s Amended and Restated Memorandum of Association, and so as
to comply with any requirement or restriction imposed by any court or governmental body having jurisdiction over the Company; (f) the
Applicable Agreements with respect to any Securities offered will have been validly executed and delivered by the Company and the other
party or parties thereto, in conformity with the Applicable Agreement and applicable law; (g) if applicable, certificates representing
such Securities have been duly executed, countersigned, registered and delivered either (i) in accordance with the Applicable Agreements,
or (ii) upon conversion or exercise of any other Security, in accordance with the terms of such Security or the instrument governing such
Security providing for such conversion or exercise; (h) any such Securities and any such Applicable Agreements will be governed by New
York law and will not include any provision that is unenforceable; and (i) the Securities have been duly executed and delivered by the
Company pursuant to the Applicable Agreement and delivered against payment therefor in accordance with the Authorization.
Based upon the foregoing,
and subject to the additional qualifications set forth below, we are of the opinion that:
| 1. | Upon the Future Authorization and Issuance of Warrants, such Warrants will be valid and binding obligations
of the Company. |

CASI Pharmaceuticals, Inc.
December 20, 2024
Page 3
| 2. | Upon the Future Authorization and Issuance of Subscription Rights, such Subscription Rights will be valid
and binding obligations of the Company. |
| 3. | Upon the Future Authorization and Issuance of Units, such Units will be valid and binding obligations
of the Company. |
The opinions expressed above
are subject to bankruptcy, insolvency, fraudulent transfer, reorganization, moratorium and other similar laws of general application affecting
the rights and remedies of creditors and to general principles of equity.
We express no opinion as to the law of any jurisdiction other than
the law of the State of New York. Our opinion is based on these laws as in effect on the date hereof. We express no opinion to the extent
that any other laws are applicable to the subject matter hereof and express no opinion and provide no assurance as to compliance with
any federal or state securities law, rule or regulation.
This opinion letter has been
prepared in accordance with the customary practice of lawyers who regularly give, and lawyers who regularly advise opinion recipients
concerning, opinions of the type contained herein.
This opinion letter deals
only with the specified legal issues expressly addressed herein, and you should not infer any opinion that is not explicitly addressed
herein from any matter stated in this letter.
We consent to the use of this
opinion as an exhibit to the Registration Statement. In giving such consent, we do not hereby admit that we are within the category of
persons whose consent is required under Section 7 of the Securities Act and the rules and regulations thereunder. This opinion
is rendered to you as of the date hereof and we assume no obligation to advise you or any other person hereafter with regard to any change
after the date hereof in the circumstances or the law that may bear on the matters set forth herein even though the changes may affect
the legal analysis or legal conclusion or other matters in this letter.
| |
Very truly yours, |
| |
|
| |
/s/ Harter Secrest & Emery LLP |
Exhibit 5.3
| Our ref | JLH/815438-000001/31050658v1 |
CASI Pharmaceuticals, Inc.
PO Box 309
Ugland House
Grand Cayman, KY1-1104
Cayman Islands
20 December 2024
Dear Sirs and/or Madams
CASI Pharmaceuticals, Inc.
We have acted as Cayman Islands legal advisers to CASI Pharmaceuticals, Inc.
(the "Company") in connection with the Company’s registration statement on Form F-3, including all amendments
or supplements thereto (the "Registration Statement"), filed with the Securities and Exchange Commission (the "SEC")
under the U.S. Securities Act of 1933, as amended to date, on 20 December 2024 and the prospectus supplement dated 20 December 2024
(the "Prospectus Supplement"), relating to the Company's at-the-market offering (the "Offering") to
issue and sell by the Company of certain ordinary shares of par value US$0.0001 each (the "Shares") in accordance with
the open market sale agreement dated 20 December 2024 (the "Sales Agreement") entered into between the Company and
Jefferies LLC.
We are furnishing this opinion as Exhibits 5.3
and 23.4 to the Registration Statement.
For the purposes of this opinion, we have reviewed
only originals, copies or final drafts of the following documents:
| 1.1 | The Certificate of Incorporation dated 10 January 2023 and the Certificate of Incorporation on Change
of Name dated 21 March 2023. |
| 1.2 | The Amended and Restated Memorandum and Articles of Association of the Company as adopted by a special
resolution of the Company dated 20 March 2023 and effective on 21 March 2023 (the "Memorandum and Articles"). |
| 1.3 | The written resolutions of the directors of the Company dated 13 December 2024 (the "Directors'
Resolutions"). |
| 1.4 | A certificate from a director of the Company, a copy of which is attached hereto (the "Director's
Certificate"). |
| 1.5 | A certificate of good standing with respect to the Company issued by the Registrar of Companies in the
Cayman Islands dated 7 November 2024 (the "Certificate of Good Standing"). |
| 1.6 | The Registration Statement. |
| 1.7 | The Prospectus Supplement. |
The following opinions are given only as to, and
based on, circumstances and matters of fact existing and known to us on the date of this opinion letter. These opinions only relate to
the laws of the Cayman Islands which are in force on the date of this opinion letter. In giving these opinions we have relied (without
further verification) upon the completeness and accuracy, as at the date of this opinion letter, of the Director's Certificate and the
Certificate of Good Standing. We have also relied upon the following assumptions, which we have not independently verified:
| 2.1 | Copies of documents, conformed copies or drafts of documents provided to us are true and complete copies
of, or in the final forms of, the originals, and translations of documents provided to us are complete and accurate. |
| 2.2 | All signatures, initials and seals are genuine. |
| 2.3 | There is nothing under any law (other than the law of the Cayman Islands), which would or might affect
the opinions set out below. |
| 2.4 | There is nothing contained in the minute book or corporate records of the Company (which, other than the
records set out in paragraph 1 of this opinion letter, we have not inspected) which would or might affect the opinions set out below. |
| 2.5 | The Company will receive money or money's worth in consideration for the issue of the Shares and none
of the Shares will be issued for less than par value. |
| 2.6 | The issue of the Shares will be of commercial benefit to the Company. |
| 2.7 | No invitation has been or will be made by or on behalf of the Company to the public in the Cayman Islands
to subscribe for any of the Shares. |
Based upon the foregoing and subject to the qualifications set out
below and having regard to such legal considerations as we deem relevant, we are of the opinion that:
| 3.1 | The Company has been duly incorporated as an exempted company with limited liability and is validly existing
and in good standing with the Registrar of Companies under the laws of the Cayman Islands. |
| 3.2 | The authorised share capital of the Company is US$50,000 divided into 500,000,000 ordinary shares of a
par value of US$0.0001 each. |
| 3.3 | The issue and allotment of the Shares have been duly authorised and when allotted, issued and paid for
as contemplated in the Registration Statement, the Prospectus Supplement and the Sales Agreement, the Shares will be legally issued and
allotted, fully paid and non-assessable. As a matter of Cayman Islands law, a share is only issued when it has been entered in the register
of members (shareholders). |
The opinions expressed above are subject to the
following qualifications:
| 4.1 | To maintain the Company in good standing with the Registrar of Companies under the laws of the Cayman
Islands, annual filing fees must be paid and returns made to the Registrar of Companies within the time frame prescribed by law. |
| 4.2 | In this opinion the phrase "non-assessable" means, with respect to shares in the Company, that
a shareholder shall not, solely by virtue of its status as a shareholder and in absence of a contractual arrangement, or an obligation
pursuant to the memorandum and articles of association, to the contrary, have any obligation to make further contributions to the Company's
assets (except in exceptional circumstances, such as involving fraud, the establishment of an agency relationship or an illegal or improper
purpose or other circumstances in which a court may be prepared to pierce or lift the corporate veil). |
4.3 The
obligations of the Company may be subject to restrictions pursuant to:
| (a) | United Nations and United Kingdom sanctions extended to the Cayman Islands by Orders in Council; and |
| (b) | sanctions imposed by Cayman Islands authorities under Cayman Islands legislation. |
| 4.4 | We express no opinion as to the meaning, validity or effect of any references to foreign (i.e. non-Cayman
Islands) statutes, rules, regulations, codes, judicial authority or any other promulgations and any references to them in the Sales Agreement. |
Except as specifically stated herein, we make
no comment with respect to any representations and warranties which may be made by or with respect to the Company in any of the documents
or instruments cited in this opinion or otherwise with respect to the commercial terms of the transactions, which are the subject of this
opinion.
We hereby consent to the filing of this opinion
as an exhibit to the Registration Statement and to the reference to our name under the headings "Enforceability of Civil Liabilities"
and "Legal Matters" and elsewhere in the Registration Statement and the Prospectus Supplement. In giving such consent, we do
not thereby admit that we come within the category of persons whose consent is required under Section 7 of the U.S. Securities Act
of 1933, as amended, or the Rules and Regulations of the SEC thereunder.
Yours faithfully
Maples and Calder (Hong Kong) LLP
/s/ Maples and Calder (Hong Kong) LLP
Exhibit 23.1
Consent of Independent Registered Public Accounting
Firm
We consent to the use of our report dated March 28, 2024, with
respect to the consolidated financial statements of CASI Pharmaceuticals, Inc., incorporated herein by reference and to the reference
to our firm under the heading “Experts” in the prospectus.
/s/ KPMG Huazhen LLP
Beijing, China
December 20, 2024
Exhibit 107
Calculation of Filing Fee Table
Form F-3
(Form Type)
CASI Pharmaceuticals, Inc.
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered Securities
| |
Security Type |
Security Class Title |
Fee
Calculation
Rule |
Amount
Registered (1) (2) |
Proposed Maximum
Offering Price Per
Unit (3) |
Maximum
Aggregate
Offering Price(3) |
Fee Rate |
Amount of
Registration Fee |
| Fees to be Paid |
Equity |
Ordinary Shares |
457(o) |
— |
— |
— |
— |
— |
| |
Equity |
Preferred Shares |
457(o) |
— |
— |
— |
— |
— |
| |
Other |
Warrants |
457(o) |
— |
— |
— |
— |
— |
| |
Other |
Subscription Rights |
457(o) |
— |
— |
— |
— |
— |
| |
Other |
Units |
457(o) |
— |
— |
— |
— |
— |
| |
Unallocated (Universal) Shelf |
Unallocated (Universal) Shelf |
457(o) |
— |
— |
$150,000,000(4) |
0.00015310 |
$22,965 |
| |
|
|
|
|
|
| |
Total Offering Amounts |
|
$150,000,000 |
|
$22,965 |
| |
Total Fees Previously Paid |
|
|
|
— |
| |
Total Fee Offsets |
|
|
|
— |
| |
Net Fee Due |
|
|
|
$22,965 |
| (1) | On May 3, 2024, CASI Pharmaceuticals, Inc. (the “Company”) filed a registration
statement (the “Prior Registration Statement”) on Form F-3 (File No. 333-279096) with the U.S. Securities
and Exchange Commission related to the offer and sale of up to an aggregate of $50.0 million of any combination of the securities described
in the prospectus relating to the Prior Registration Statement (the “Prior Securities”). The Prior Registration Statement
was subsequently declared effective on May 10, 2024. Pursuant to Rule 429 under the Securities Act of 1933, as amended (the
“Securities Act”), the registration statement with which this Calculation of Filing Fee Table is being filed, (the
“New Registration Statement” and together with the Prior Registration Statement, the “Combined Registration
Statement”), combines the Prior Securities from the Prior Registration Statement, all of which remain unissued as of the date
hereof, with the additional securities registered by the New Registration Statement for offer and sale by the Company, to enable the offer
and sale of up to an aggregate of $200.0 million of the Company’s ordinary shares, preferred shares, warrants, subscription rights,
and/or units, from time to time in one or more offerings, pursuant to a combined base prospectus. |
| (2) | There are being registered under the New Registration Statement such indeterminate number of ordinary
shares, preferred shares, warrants, subscription rights, and units as shall have an aggregate initial offering price not to exceed $150
million. Any securities registered under the New Registration Statement may be sold separately or in combination with other securities
registered under the Combined Registration Statement. In addition, pursuant to Rule 416 under the Securities Act, the shares being
registered under the New Registration Statement also include such indeterminate number of shares as may be issuable with respect to the
shares being registered hereunder as a result of stock splits, stock dividends or similar transactions. |
| (3) | The Proposed Maximum Offering Price Per Unit and Maximum Aggregate Offering Price per class of securities
will be determined from time to time by the registrant in connection with the issuance by the registrant of the securities registered
under the Combined Registration Statement and is not specified as to each class of security pursuant to General Instruction II.C. of Form F-3
under the Securities Act. Separate consideration may or may not be received for securities that are issuable on exercise, conversion or
exchange of other securities, or that are issued in units. |
| (4) | Estimated solely for the purpose of calculating the registration fee of the New Registration Statement.
Subject to Rule 462(b) under the Securities Act, the aggregate maximum offering price of all securities sold by the registrant
from time to time pursuant to the New Registration Statement will not exceed $150 million. The aggregate maximum offering price of all
securities sold by the registrant from time to time pursuant to the Combined Registration Statement will not exceed $200.0 million. |
Table 3: Combined Prospectuses
| Security Type |
Security Class Title |
Amount of
Securities Previously
Registered (1) (2) |
Maximum Aggregate
Offering Price of
Securities Previously
Registered (3) |
Form Type |
File Number |
Initial Effective
Date |
| Equity |
Ordinary Shares |
— |
— |
F-3 |
333-279096 |
May 10, 2024 |
| Equity |
Preferred Shares |
— |
— |
F-3 |
333-279096 |
May 10, 2024 |
| Other |
Warrants |
— |
— |
F-3 |
333-279096 |
May 10, 2024 |
| Other |
Subscription Rights |
— |
— |
F-3 |
333-279096 |
May 10, 2024 |
| Other |
Units |
— |
— |
F-3 |
333-279096 |
May 10, 2024 |
|
Unallocated
(Universal) Shelf |
Unallocated
(Universal) Shelf |
— |
$50,000,000 (4) |
F-3 |
333-279096 |
May 10, 2024 |
| (1) | See Note 1 to Table 1 above. |
| (2) | The Prior Registration Statement includes such indeterminate number of ordinary shares, preferred shares,
warrants, subscription rights, and units as shall have an aggregate initial offering price not to exceed $50 million. Any securities registered
thereunder may be sold separately or in combination with other securities registered under the Combined Registration Statement, pursuant
to Rule 429 of the Securities Act. In addition, pursuant to Rule 416 under the Securities Act, the shares registered under the
Prior Registration Statement also include such indeterminate number of shares as may be issuable with respect to the shares being registered
thereunder as a result of stock splits, stock dividends or similar transactions. |
| (3) | The Proposed Maximum Offering Price Per Unit and Maximum Aggregate Offering Price per class of securities
will be determined from time to time by the registrant in connection with the issuance by the registrant of the securities registered
under the Combined Registration Statement and is not specified as to each class of security pursuant to General Instruction II.C. of Form F-3
under the Securities Act. Separate consideration may or may not be received for securities that are issuable on exercise, conversion or
exchange of other securities, or that are issued in units. |
| (4) | Estimated in the Prior Registration Statement solely for the purpose of calculating the registration fee
previously paid in connection therewith. Subject to Rule 462(b) under the Securities Act, the aggregate maximum offering price
of all securities sold by the registrant from time to time pursuant to the Prior Registration Statement will not exceed $50 million. The
aggregate maximum offering price of all securities sold by the registrant from time to time pursuant to the Combined Registration Statement
will not exceed $200.0 million. |
CASI Pharmaceuticals (NASDAQ:CASI)
Historical Stock Chart
From Nov 2024 to Dec 2024
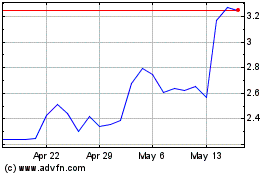
CASI Pharmaceuticals (NASDAQ:CASI)
Historical Stock Chart
From Dec 2023 to Dec 2024