| PROSPECTUS |
Filed
pursuant to Rule 424(b)(4) |
Registration
No. 333-266745
Up
to 958,903 Units
Each Consisting of One Ordinary Share (representing an aggregate of 958,903 Ordinary Shares)
and Three Warrants, each to Purchase One Ordinary Share (representing an aggregate of 2,876,709
Ordinary Shares issuable upon the exercise of such warrants)

Polyrizon Ltd.
This
is the initial public offering of Polyrizon Ltd. We are offering 958,903 units, or Units,
each consisting of one of our ordinary shares, no par value per share, or Ordinary Shares,
and three warrants, each to purchase one of our Ordinary Shares, or each, a Warrant. The
initial public offering price per Unit is $4.38 and the exercise price of each Warrant included
in the Unit is $4.38 per Ordinary Share (with such exercise price equal to the public offering
price per Unit). The Units have no stand-alone rights and will not be certificated or issued
as stand-alone securities. The Ordinary Shares and Warrants are immediately separable and
will be issued separately in this offering. The Warrants offered hereby will be immediately
exercisable on the date of issuance and will expire five years from the date of issuance.
We
have been approved to list our Ordinary Shares on The Nasdaq Capital Market, or Nasdaq, under
the symbol “PLRZ”. We do not intend to apply to list the Warrants on any securities
exchange or other nationally recognized trading system.
We
are both an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (or the JOBS Act), and a “foreign
private issuer,” as defined under the U.S. federal securities law and are subject to reduced public company reporting requirements.
See “Prospectus Summary – Implications of Being an Emerging Growth Company and Foreign Private Issuer” for additional
information.
In
addition, we have registered an aggregate of 2,801,330 Ordinary Shares for resale by certain shareholders, or the Selling Shareholders,
by means of a separate prospectus, or the Resale Prospectus. Other than certain affiliates of the registrant that have entered into lock-up
agreements with the underwriter, the Selling Shareholders are not subject to any lock-up or leakage agreements and have the right to
sell the shares being registered hereby at any time following the closing of our initial public offering. We will pay the expenses associated
with the sale of the Ordinary Shares by the Selling Shareholders pursuant to this prospectus. Our registration of the Ordinary Shares
by the Selling Shareholders covered by this prospectus does not mean that the Selling Shareholders will issue, offer or sell, as applicable,
any of the Ordinary Shares. The Selling Shareholders may offer and sell the Ordinary Shares covered by this prospectus in a number of
different ways and at varying prices. We provide more information in the section entitled “Plan of Distribution” in the Resale
Prospectus.
Investing
in our securities involves a high degree of risk. See “Risk Factors” beginning on page 13.
Neither
the Securities and Exchange Commission (or the SEC) nor any state or other foreign securities commission has approved nor disapproved
these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| | |
Per
Unit | | |
Total | |
| Public offering price | |
$ | 4.38 | | |
$ | 4,199,995 | |
| Underwriting discounts and commissions (1) | |
$ | 0.35 | | |
$ | 336,000 | |
| Proceeds to us (before expenses) (2) | |
$ | 4.03 | | |
$ | 3,863,995 | |
| (1) |
Does not include a non-accountable
expense allowance equal to 1.0% of the initial public offering price payable to the underwriter. See the section titled “Underwriting”
beginning on page 148 of this prospectus for additional disclosure regarding underwriter compensation and offering expenses. |
| (2) |
Does not include proceeds from
the exercise of the Warrants in cash, if any. |
Certain
of our existing shareholders, including beneficial owners of greater than 5% of our share capital, have indicated an interest in purchasing
up to an aggregate of $1 million of Units in this offering, which represents approximately 24% of the total Units in this offering, based
on the public offering price of $4.38 per Unit. However, because indications of interest are not binding agreements or commitments to
purchase, the underwriters may determine to sell more, less or no Units in this offering to any of these shareholders, or any of these
shareholders may determine to purchase more, less or no Units in this offering. The underwriters will receive the same underwriting discount
on any Units purchased by these shareholders as they will on any other Units sold to the public in this offering. See the sections titled
“Principal Shareholders” on page 125, “Selling Shareholders” on page 138, and “Risk Factors” on page
13 in this prospectus for additional information regarding the shareholders that have expressed an interest in purchasing Units in this
offering.
We
have granted the underwriter an option to purchase from us, up to an additional 143,835 Ordinary
Shares and/or up to an additional 431,505 Warrants within 45 days from the date of this prospectus
to cover over-allotments, if any. The purchase price to be paid per additional Ordinary Share
will be equal to the public offering price of one Unit (less the $0.01 purchase price allocated
to each Warrant), as applicable, less the underwriting discounts and commissions. The underwriter
may exercise the over-allotment option with respect to the Ordinary Shares or Warrants only,
or any combination thereof. If the underwriter exercises the option in full, the total
underwriting discounts and commissions and management fees payable will be $583,850 and the
total proceeds to us, before expenses, will be $4,246,150.
The
underwriter expects to deliver the Ordinary Shares on or about October 30, 2024.
Sole
Book-Running Manager
Aegis
Capital Corp.
The
date of this prospectus is October 28, 2024.
TABLE
OF CONTENTS
Neither
we, the Selling Shareholders nor the underwriter have authorized anyone to provide you with information that is different from that contained
in this prospectus, any amendment or supplement to this prospectus, or in any free writing prospectus we may authorize to be delivered
or made available to you. Neither we, the Selling Shareholders nor the underwriter take responsibility for, and can provide no assurance
as to the reliability of, any other information that others may give you. We, the Selling Shareholders and the underwriter are offering
to sell Ordinary Shares and seeking offers to purchase Ordinary Shares only in jurisdictions where offers and sales are permitted. The
information contained in this prospectus is accurate only as of the date on the front of this prospectus, regardless of the time of delivery
of this prospectus or any sale of Ordinary Shares. Our business, financial condition, results of operations and prospects may have changed
since the date on the front cover of this prospectus.
Through
and including November 22, 2024 (the 25th day after the date of this prospectus), all dealers effecting transactions in these
securities, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition
to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
Neither
we, the Selling Shareholders nor any of the underwriter have taken any action to permit this offering or possession or distribution of
this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform
yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
“Capture
and Contain” and “Trap and Target” are trademarks of ours that we use in this prospectus. This prospectus also includes
trademarks, tradenames and service marks that are the property of other organizations. Solely for convenience, our trademarks and tradenames
referred to in this prospectus often appear without the ® or ™ symbols, but those references are not intended
to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the right of the applicable
licensor to our trademark and tradenames.
The
terms “shekel,” “Israeli shekel” and “NIS” refer to New Israeli Shekels, the lawful currency of the
State of Israel, and the terms “dollar,” “U.S. dollar” or “$” refer to United States dollars, the
lawful currency of the United States of America. All references to “shares” in this prospectus refer to Ordinary Shares of
Polyrizon Ltd., no par value per share.
On
September 29, 2022, the Company effected (i) a reverse stock split of our issued and outstanding shares at a ratio of 1-for-8.80, pursuant
to which holders of our shares received one Ordinary Share for every 8.80 Ordinary Shares held, and (ii) cancelled the par value
of our Ordinary Shares and Preferred Shares, or collectively, the Initial Reverse Share Split.
On
December 19, 2022, the Company effected the issuance of an aggregate of 858,148 bonus shares to the holders of our Ordinary Shares on
a basis of 1.25 bonus shares for each Ordinary Share outstanding (equivalent to a forward share split at a ratio of 1.25-for-1), or the
Forward Share Split.
On
June 18, 2023, the Company effected a reverse stock split of our issued and outstanding shares at a ratio of 1-for-1.7, pursuant to which
holders of our shares received one Ordinary Share for every 1.7 Ordinary Shares held, or the Second Reverse Share Split.
On
May 12, 2024, the Company effected a reverse stock split of our issued and outstanding shares at a ratio of 1-for-2, pursuant to which
holders of our shares received one (1) Ordinary Share for every two (2) Ordinary Shares held, or the Third Reverse Share Split.
On
August 16, 2024, the Company effected the issuance of an aggregate of 420,618 bonus shares to the holders of our Ordinary Shares on a
basis of 0.1494 bonus shares for each Ordinary Share outstanding (equivalent to a forward share split at a ratio of 0.1494-for-1), or
the Second Forward Share Split.
Unless
the context expressly dictates otherwise, all references to share and per share amounts referred to herein reflect the foregoing share
splits, or collectively, the Share Splits.
MARKET,
INDUSTRY AND OTHER DATA
This
prospectus contains estimates, projections and other information concerning our industry, our business, and the markets for our product
candidates. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject
to uncertainties, and actual events or circumstances may differ materially from events and circumstances that are assumed in this information.
Unless otherwise expressly stated, we obtained this industry, business, market and other data from our own internal estimates and research
as well as from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry,
medical and general publications, government data and similar sources. None of the reports or studies cited in this prospectus were commissioned
by the Company.
In
addition, assumptions and estimates of our and our industry’s future performance are necessarily subject to a high degree of uncertainty
and risk due to a variety of factors, including those described in “Risk Factors.” These and other factors could cause our
future performance to differ materially from our assumptions and estimates. See “Special Note Regarding Forward-Looking Statements.”
PROSPECTUS
SUMMARY
This
summary highlights selected information contained elsewhere in this prospectus and does not contain all of the information that you should
consider in making your investment decision. Before deciding to invest in our securities, you should read this entire prospectus carefully,
including the sections of this prospectus entitled “Risk Factors” and “Management’s Discussion and Analysis of
Financial Condition and Results of Operations” and our financial statements and the related notes included elsewhere in this prospectus.
Unless the context otherwise requires, references in this prospectus to the “company,” “Polyrizon,” “we,”
“us,” “our” and other similar designations refer to Polyrizon Ltd.
Company
Overview
We
are a development stage biotech company specializing in the development of innovative medical device hydrogels delivered in the form
of nasal sprays, which form a thin hydrogel-based shield containment barrier in the nasal cavity that can provide a barrier against viruses
and allergens from contacting the nasal epithelial tissue. Our proprietary Capture and Contain TM, or C&C, hydrogel technology,
comprised of a mixture of naturally occurring building blocks, is delivered in the form of nasal sprays, and potentially functions as
a “biological mask” with a thin shield containment barrier in the nasal cavity. We are further developing certain aspects
of our C&C hydrogel technology such as the bioadhesion and prolonged retention at the nasal deposition site for intranasal delivery
of drugs. We refer to our additional technology, which is in an earlier stage of pre-clinical development, that is focused on nasal delivery
of active pharmaceutical ingredients, or APIs, as Trap and Target ™, or T&T.
Our
Product Candidates
Our
nasal hydrogels have been designed to serve as a non-invasive and fast-acting system. The hydrogels are formulated as an innovative mixture
of mucoadhesive polymers (e.g., sodium alginate) which are Generally Recognized as Safe, or GRAS, by the Federal Drug Administration,
or the FDA. Our mucoadhesive polymers derived from seaweed polysaccharides possess promising features as they are renewable, biodegradable,
biocompatible, and environment friendly. The formulated hydrogel is sprayed into the nose to create a physical barrier with long-lasting
adhesion to the mucosal membranes. Our polymers have an atomic mass much higher than the upper cell penetration limit, the polymers will
simply lay on top of the cells and act as a physical barrier to viruses and allergens from contacting the nasal epithelial tissue, as
opposed to penetrating the cells and causing a chemical reaction. Therefore, the C&C product candidates are not expected to be considered
as drugs by the FDA but as medical devices.
Our
leading technologies are C&C and T&T. The C&C technology is a containment barrier against a wide range of allergen particulates
and viruses.
PL-14
– Nasal Allergies Blocker
| o | We
expect our PL-14 C&C technology medical device product candidate, or our PL-14 product
candidate, to be regulated as a Class II medical device by the FDA under its 510(k) pathway. |
| |
o |
Our PL-14 product candidate
is scheduled to initiate preclinical safety trials in the second quarter of 2025. In addition, we expect pivotal clinical trial on
our PL-14 product candidate to commence in the fourth quarter of 2025, following which we plan to submit a 510(k) application for
FDA clearance. |
| o | For
our PL-14 technology product candidate, we will pursue the 510(k) pathway which requires
a manufacturer to demonstrate substantial equivalence to an FDA-cleared device (i.e., predicate
device) to a subject device (i.e., our product candidate). This process for clearing our
device with the FDA entails performing a medical device analysis of the product candidates
(e.g., PL-14 product candidate) description, operational principle, potential accessories
and proposed intended use, for the purpose of identifying a predicate device that has already
been cleared by the FDA. Through this review, we found three possible predicate devices for
establishing substantial equivalence, Alzair Allergy Blocker (510(k) Number: K170848), or
Alzair, NASAL EASE ALLERGY BLOCKER (510(k) Number: K132520), or Nasalease, and Bentrio Allergy
Blocker (510(k) Number: K213114), or Bentrio. There is no guarantee that our PL-14 product
candidate will advance in the FDA 510(k) process at the same rate as the aforementioned predicate
devices or will reach commercialization. |
| |
o |
The estimated
timeline for obtaining 510(k) clearance for our PL-14 product candidate is based on the estimated time needed for the following activities:
(i) GMP manufacturing of our clinical trial materials, which usually requires 9-12 months; (ii) biocompatibility preclinical studies,
which usually requires 3-6 months (although these studies may be performed concurrently with the GMP manufacturing mentioned above);
(iii) clinical trials, which usually requires 6-12 months; and (iv) FDA submission and clearance, which usually requires 3-12 months.
Regarding FDA submission and clearance, generally 510(k) applicants can expect submission acceptance review decisions within 15 calendar
days, substantive review decisions within 60 days, and final decisions within 90 days. However, the FDA’s time of review does
not include time on “hold”, which includes any time spent by us responding to any FDA information requests, meaning that
the total timeframe of the review process could take longer than anticipated. In the case of our predicate devices for our PL-14
product candidate, Alzai, Nasalese, and Bentrio, the FDA submission and clearance process took 86 and 140 days, respectively. For
additional information, please see “Business – FDA clearance plan for our C&C product candidates.” |
PL-15
– COVID-19 and PL-16 – Influenza Blockers
| o | We
expect our PL-15 C&C technology medical device product candidate, or our PL-15 product
candidate, and our PL-16 C&C technology medical device product candidate, or our PL-16
product candidate, which provide a barrier to COVID-19 and influenza from contacting the
nasal epithelial tissue, respectively, to be regulated as a Class II medical device under
a De Novo Classification request. For the clinical studies planned for PL-15 & PL-16
which will include human subjects; the Investigational Device Exemptions, or IDE, regulation
describes three types of device studies: significant risk, nonsignificant risk, and exempt
studies. During the first quarter following the closing of this offering, the company intends
to schedule a pre-submission meeting with the FDA to determine the IDE regulation type of
device studies for PL-15 & PL-16. Our proposed 12-month interval from the scheduled FDA
pre-sub meeting to the planned IDE clinical trial initiation should provide ample time to
fulfill the necessary tasks for the IDE filing, such as 1) reporting previous studies to
support the IDE, 2) preparing IDE required design and manufacturing control documentation,
3) conducting bench and biocompatibility tests to support safety of the device prior to starting
the a human study, and 4) obtaining clinical protocol and ethics committee approvals as well
as FDA IDE approval to start the clinical trial. Once IDE has been initiated, Polyrizon will
comply with FDA Guidance “Changes or Modifications During the Conduct of a Clinical
Investigation”, 2001. |
| |
o |
Our PL-15 product candidate
is scheduled to initiate preclinical safety trials in the second quarter of 2025, and subject to securing additional financing, we
intend to initiate feasibility clinical trials in the third quarter of 2026 and pivotal clinical trials in the second quarter of
2027. Following these trials, we plan to submit De Novo Classification requests for each product candidate. Our PL-16 product candidate
is scheduled to initiate preclinical safety trials in the second quarter of 2025, and subject to securing additional financing, we
intend to initiate feasibility clinical trials in the first quarter of 2026 and pivotal clinical trials in the third quarter of 2026.
Following these trials, we plan to submit De Novo Classification requests for each product candidate. |
| |
o |
Upon a review similar
to the one performed for our PL-14 product candidate, we found that there were no potential predicate devices in the FDA’s
database matching the proposed intended uses of our PL-15 and PL-16 product candidates. Because of this, we will pursue a De Novo
Classification request for each product candidate. This pathway involves demonstrating that the product candidates provide a reasonable
assurance of safety and effectiveness. During the first quarter following the closing of this offering, we intend to submit a Q-submission
(Pre-submission) for each product candidate and request a pre-submission meeting with FDA’s Center for Devices and Radiological
Health, or CDRH, to confirm the potential for this regulatory path. For more information, please see “Business – Our
Product Candidates – The determination process for the C&C product candidates as a Class II medical devices.” |
| |
o |
The
estimated timeline for marketing authorization via De Novo Classification grant for our PL-15 and PL-16 product candidates is based
on taking similar steps as the steps described above for obtaining 510(k) clearance for our PL-14 product candidate. We estimate
a longer period of time for the entire grant process for each of these product candidates due to possibly extended clinical trials
requested by the FDA and also due to a longer review timeframe. For additional information, please see “Business – FDA
clearance plan for our C&C product candidates.” |
In
the event the FDA does not agree with our regulatory assessments regarding the C&C product candidates (510(k) for our PL-14 product
candidate, and Class II De Novo pathway for our PL-15 and PL-16 product candidates), it may require us to go through a lengthier, more
rigorous examination than we had expected (such as Premarket approval, or PMA, which is the FDA process of scientific and regulatory
review to evaluate the safety and effectiveness of Class III medical devices. If we are required to pursue a PMA, the introduction of
our product candidates into the market could be delayed significantly. For more information, please see “Risks Related to the Discovery,
Development and Clinical Testing of Product Candidates.”
Trap
and Target ™ Product Candidates
In
contrast to our C&C product candidates, the hydrogel in our T&T product candidates is formulated differently in order to provide
for sustained release of the API. The content of the hydrogel (quantity and quality) in the T&T product candidates is formulated
differently than the content of C&C product candidates, and therefore enable different functions: physical barrier for the C&C
product candidates and API sustained release for the T&T product candidates. It is through these differences that we rationalize
the different regulatory treatment of our C&C and T&T product candidates.
The
T&T platform technology is designed to allow a long residence time and an intimate contact with the mucosal tissue for a targeted
delivery of medicines. We expect that our T&T platform product candidates will be regulated as a combination-product consisting of
a nasal sprayer and formulation consisting of a hydrogel and a generic API, which we intend to pursue under the FDA’s 505(b)(2)
pathway. We aim to conduct feasibility studies for our T&T platform product candidates with corticosteroids, benzodiazepines and
naloxone, beginning in the fourth quarter of 2024, through the first quarter of 2026. Pre-clinical studies will follow and are expected
to begin in the second quarter of 2026. Phase I clinical trials for the leading T&T technology product candidate are planned for
the fourth quarter of 2027. Both pre-clinical studies and Phase I clinical trials are subject to securing additional financing. In addition,
we plan to start an initial testing to explore the potential of our SCI-160 platform when combined with the T&T technology, in the
first quarter of 2025.
People
Our
leadership team has a vast industry experience. Each of our executive management team has over 15 years (on average) of experience in
life science companies, which includes extensive work history in the area of research and development of medical devices, pharmaceutical
and other drug compounds. Our board of directors have similar experience in the life sciences industry as well as strong financial background
that is derived from their roles as executives of publicly-traded companies and having educational backgrounds in finance, business,
tax and accounting. We believe that these aforementioned practical and educational experiences of our group will strongly contribute
to a successful path from clinical development, regulatory approvals and commercialization of our product candidates. In addition, our
management is supported by our Scientific Advisory Board which is an advisory panel of professors with expertise in drug delivery systems,
chemistry and pharmaceuticals.
Market
Opportunities
We
believe that our technologies have the potential to provide solutions to a broad range of unmet needs in the healthcare market. With
our C&C technology, we aim to introduce solutions to address common medical and public health challenges, such as allergic rhinitis
and nasal viral infections, including COVID-19.
With
our T&T technology, we aim to address challenges in the markets of allergic and non-allergic rhinitis by local intranasal delivery
of corticosteroids; for systemic delivery of central nervous system, or CNS, related drugs for the growing markets of combatting opioid
overdose using intranasal naloxone, and benzodiazepines for seizure clusters.
Recent
Developments
SciSparc
License Agreement
On
August 13, 2024, we entered into an exclusive patent license agreement with SciSparc, or the SciSparc License Agreement, pursuant to
which SciSparc granted us an exclusive, worldwide, royalty-bearing, sublicensable license with respect to intellectual property rights
associated with SciSparc’s SCI-160 platform, or the Licensed Patent Rights, in order to research, develop and commercialize the
Licensed Patent Rights in connection with the diagnosis, prevention, and treatment of pain in humans.
Pursuant
to the terms of the SciSparc License Agreement, SciSparc is entitled to up to $3.32 million based on the achievement of certain milestones,
including (i) $50,000 upon a successful preclinical safety test, (ii) $100,000 upon first patient enrolled in phase I clinical trial,
(iii) $120,000 upon first patient enrolled in Phase 2a clinical trial, (iv) $150,000 upon first patient enrolled in Phase 2b clinical
trial, (v) $500,000 upon first patient enrolled in Phase 3 clinical trials, (vi) $800,000 upon approval by the FDA, (vii) $800,000 upon
approval by an EU regulatory body, and (viii) $800,000 upon regulatory approval in any additional jurisdiction. Additionally, SciSparc
is eligible to receive royalties, on a country-by-country and product-by-product basis, at a rate of 5%, on aggregate net sales of a
product that comprises, contains and/or incorporates and/or is based on the Licensed Patent Rights, or a Licensed Product, and sublicense
income that we may receive until the longer of (i) fifteen years from the date of the first sale of a Licensed Product (on a country-by-country
basis), and (ii) the last to expire valid claim of any licensed patents with respect to a Licensed Product in such country.
Under
the SciSparc License Agreement, we have the right to grant sublicenses for the Licensed Patent Rights, to any sublicense that meets the
following criteria: (i) not being involved in legal proceedings against the Licensor and (ii) having equity of at least $5.0 million
(as shown in the most recent audited financial statements or other applicable financial reports if audited statements are not required
and no such financial statements are available). In such cases, we must pay SciSparc 25% of any proceeds generated from such sublicenses,
including proceeds from granting the sublicense. The other terms of the sublicense agreement must be consistent with the SciSparc License
Agreement.
We
may terminate the SciSparc License Agreement for cause upon six months’ prior written notice to SciSparc and payment of all amounts
owed to SciSparc through the effective date of such termination. SciSparc may terminate the SciSparc License Agreement if we (or the
sublicensee) (i) cease to invest sufficient resources in the development efforts of the Licensed Products or our T&T technology at
any time during the term of the SciSparc License Agreement, or (ii) fail to invest at least $300,000 in such development efforts of the
Licensed Products or our T&T technology during the first three years of the term of the SciSparc License Agreement. If SciSparc is
entitled to terminate the SciSparc License Agreement because we did not cure a breach of the SciSparc License Agreement to SciSparc 's
satisfaction, we will be given an additional six months to sell the license on to a buyer who will step into our shoes. If we find a
buyer, SciSparc will be given the right to purchase the license back under the same conditions. After this period, if we failed to sell
the license, the SciSparc License Agreement will be terminated.
In
addition, pursuant to the SciSparc License Agreement, we issued to SciSparc 320,000 Ordinary Shares, or the Company's Shares, and are
subject to the issuance of additional securities and adjustments, equal to an aggregate amount of $3,000,000, or the SciSparc License
Purchase Amount. In the event of a trigger event, including the listing of our Ordinary Shares on a stock exchange, pursuant to which
our Ordinary Shares are issued at a price per share, less or more than the price per share calculated by dividing the SciSparc License
Purchase Amount by the Company's Shares, then immediately prior to the closing of such trigger event, we will adjust the number of Company's
Shares issued to SciSparc, In addition, SciSparc will be entitled to receive, for no additional consideration, upon the occurrence of
a trigger event, the same composition of securities offered in such trigger event, or pre-funded warrants in lieu of such shares offered
in such trigger event. Upon the closing of this offering, based on the public offering price of $4.38 per Ordinary Share, SciSparc will
receive pre-funded warrants to purchase 364,931 Ordinary Shares and 2,054,793 warrants to purchase 2,054,793 Ordinary Shares upon the
same terms as the warrants to be issued in this offering.
Simultaneously,
on the date of the SciSparc License Agreement, the parties terminated their collaboration agreement, dated May 30, 2022.
NurExone
Collaboration
On
July 18, 2022, we signed a collaboration agreement with NurExone Biologic Inc., or NurExone, pursuant to which we will use our T&T
platform technology to develop formulations, conduct analytical development and produce technical
batches of a tailored intranasal delivery system. The intranasal system is being designed for delivery of NurExone’s ExoTherapy
to patients with traumatic spinal cord injuries and may also be relevant to other indications through intranasal exosome delivery.
The collaboration shall be in effect for an unlimited period of time until terminated by either party. The agreement may be terminated
at any time by either party upon delivering prior written notice of at least 90 days, and may also be terminated by either party within
7 days as a result of certain breaches of the agreement. Certain clauses under the agreement, including the royalty clause and the license
clause shall withstand the termination of the agreement.
Pursuant
to the collaboration agreement, NurExone will pay the costs of the formulation development in an estimated amount of $220,000 in three
installments, subject to certain development milestones. We expect to be able to perform a biological efficacy study of the intranasal
system within three quarters. NurExone shall pay development fees to us of up to a total of $3,350,000 subject to the completion of certain
milestones, including the payment of an aggregate of: (i) $500,000 upon successful completion of a Phase 2 clinical trial, (ii) $600,000
upon successful completion of Phase III clinical trial, (iii) $1,125,000 upon approval by the FDA, and (iv) $1,125,000 upon approval
by an EU regulatory body. To date, we have received approximately $78,000 pursuant to the collaboration agreement. Moreover, NurExone
shall pay the following royalties based on any product sales resulting from the collaboration agreement: (i) a 2.25% royalty for sales
of products by NurExone that generate an income between $50,000 and $2,500,000, (ii) a 2.75% royalty for sales of products by Nurexone
that generate an income between $2,500,000 and $10,000,000, and (iii) a 3.25% royalty for sales of products by NurExone that generate
an income greater than $10,000,000. Any products sold by a sublicensee of Nurexone will be subject to a 35% royalty fee. In advanced
stages of the collaboration, we may assist NurExone with regulatory submissions for the United States and Europe. Manufacturing and marketing
rights for formulations under the collaboration agreement are exclusive to NurExone.
Recent
Financings
In
January 2022, June 2022 and August 2022, we signed Simple Agreements for Future Equity, or the 2022 Convertible Notes, with several existing
investors of the Company, or the 2022 Investors, for an aggregate amount of approximately $719,000. Pursuant to the 2022 Convertible
Notes, upon consummation by the Company of capital raise transaction with aggregate proceeds to the Company of at least $300,000, or
a Qualified Equity Financing, the Company agreed to issue to each 2022 Investor the number of most senior class of shares issued in the
Qualified Equity Financing equal to the Investment Amount divided by the discount price (which is defined as the lowest price per convertible
note share sold in the Qualified Equity Financing discounted by to 20%). Following an investment agreement in June 2023, the 2022 Convertible
Notes were converted into an aggregate of 792,830 Ordinary Shares.
On
February 4, 2023, the Company signed a convertible loan agreement with certain shareholders,
or the 2023 Lenders, in the principal amount of up to $180,000, or the 2023 Loan. The 2023
Loan bears simple interest at an annual rate of 4%. In the event that the Company issues
securities in a bona fide transaction or series of transactions with the principal purpose
of raising capital, pursuant to which the Company issues and sells shares at a fixed pre-money
valuation, in which the aggregate proceeds to the Company are at least $500,000, or a Qualified
Financing, then the entire 2023 Loan and all interest accrued thereon shall automatically
convert into such number of shares of the Company, of the same class as shall be issued in
such transaction at a price per share equal to the price per share paid in the Qualified
Financing minus the discount of 20%, rounded up to the nearest whole number, and the lenders,
or the 2023 Lenders, will receive the same rights and protections granted to the investors
in such Qualified Financing. The 2023 Lenders have an optional conversion right upon consummation
by the Company of a non-Qualified Financing. If the 2023 Loan (and the accrued interest thereon)
has not been repaid or converted prior to the lapse of six (6) months following the effective
date, at the request of a Lender, made at Lender’s sole discretion, the entire 2023
Loan and the accrued interest thereon shall be converted into such number of the most senior
class of shares of the Company then outstanding, equal to the 2023 Loan and any interest
accrued thereon, divided by the lowest price per share actually paid to the Company since
August 1, 2021, for such most senior class of shares of the Company then outstanding, discounted
by 20%, rounded up to the nearest whole number. In the event that either (a) a consolidation,
merger or reorganization of the Company with or into, or a sale of all or substantially all
of the Company's assets, or a majority of the Company's issued and outstanding share capital,
to, any person or entity, other than a wholly-owned subsidiary of the Company, excluding
a transaction in which shareholders of the Company prior to the transaction will maintain
voting control of the resulting entity after the transaction; or (b) any transaction resulting
in all or substantially all of the Company's assets being sold, transferred or exclusively
licensed (c) an initial public offering of the Company's shares, or collectively, an Exit
Event, should occur prior to the conversion of the 2023 Loan, then immediately following
the closing of the Exit Event, the Company shall repay each Lender an amount equal to the
2023 Loan amount extended by such Lender, together with any and all interest accrued thereon.
On May 12, 2024, the 2023 Loan converted into 198,489 Ordinary Shares pursuant to a non-Qualified
Financing.
In
June 2023, December 2023 and May 2024, we entered into a series of securities purchase agreements
with certain investors, pursuant to which we issued an aggregate of 513,878 Ordinary Shares
for gross proceeds of $582,000.
In
April 2024, we and L.I.A Pure Capital Ltd., entered into a convertible loan agreement, or the April 2024 CLA, pursuant to which we may
draw down an amount of up to $250,000, or the April 2024 CLA Amount. The April 2024 CLA bears interest at an annual rate of 4% and has
a maturity date of April 2026. In accordance with the terms of the April 2024 CLA, in the event that we have an initial public offering,
the then outstanding April 2024 CLA Amount shall be repaid from the proceeds received in such initial public offering. In the event we
do not succeed in offering our shares in an initial public offering, upon the elapse of 24 months from the effective date of the April
2024 CLA, the outstanding April 2024 CLA Amount shall be converted into the most senior class of shares issued in the most recent financing
round.
In
August 2024, we and L.I.A Pure Capital Ltd. and Reuven Srugo Construction Company Ltd., entered into a convertible loan agreement, or
the August 2024 CLA, pursuant to which we may draw down an amount of up to $60,000, or the August 2024 CLA Amount. The August 2024 CLA
bears interest at an annual rate of 4% and has a maturity date of August 2026. In accordance with the terms of the August 2024 CLA, in
the event that we have an initial public offering, the then outstanding August 2024 CLA Amount shall be repaid from the proceeds received
in such initial public offering. In the event we do not succeed in offering our shares in an initial public offering, upon the elapse
of 24 months from the effective date of the August 2024 CLA, the outstanding August 2024 CLA Amount shall be converted into the most
senior class of shares issued in the most recent financing round.
Corporate
Information
We
are an Israeli corporation, incorporated in January 2005. Our principal executive offices are located at 5 Ha-Tidhar Street, Raanana,
4366507, Israel. Our telephone number is +972-55-433-5665. Our website address is www.polyrizon-biotech.com. The information
contained on our website and available through our website is not incorporated by reference into and should not be considered a part
of this prospectus, and the reference to our website in this prospectus is an inactive textual reference only.
Summary
of Risks Associated with our Business
Our
business is subject to a number of risks of which you should be aware before a decision to invest in our Ordinary Shares. You should
carefully consider all the information set forth in this prospectus and, in particular, should evaluate the specific factors set forth
in the sections titled “Risk Factors” before deciding whether to invest in our Ordinary Shares. Among these important risks
are, but not limited to, the following:
Risks
Related to Our Financial Condition and Capital Requirements
| |
● |
We have incurred significant
losses since our inception. We anticipate that we will continue to incur significant losses for the foreseeable future. We have never
generated any revenue from product candidates sales and may never be profitable. |
| |
|
|
| |
● |
We expect that we will need
to raise substantial additional funding, which may not be available on acceptable terms, or at all. Failure to obtain funding on
acceptable terms and on a timely basis may require us to curtail, delay or discontinue our product candidates’ development
efforts or other operations. |
Risks
Related to the Discovery, Development and Clinical Testing of Product Candidates
| |
● |
We depend on enrollment of
patients in our upcoming clinical trials in order to continue development of our product candidates. |
| |
|
|
| |
● |
We
may not receive, or may be delayed in receiving, the necessary clearances or approvals for our product
candidates, failure to timely obtain necessary clearances or approvals would adversely affect our ability
to grow our business. |
| |
● |
We
do not plan to conduct a pre-submission meeting with the FDA’s CDRH to confirm the potential for
the Class II medical device path under a de novo classification request for our PL-15 and PL-16 products
until after the completion of this initial public offering. If we are denied submission under the de
novo pathway, it may require us to go through a different pathway, such as a PMA pathway, which may result
in a lengthier approval process for our devices. |
| |
● |
Legislative
or regulatory reforms in the United States or the European Union may make it more difficult and costly
for us to obtain regulatory clearances or approvals for our product candidates or to manufacture, market
or distribute our product candidates after clearance or approval is obtained. |
| |
|
|
| |
● |
We are heavily dependent on
the success of our C&C product candidates. |
| |
● |
Regulatory
approval processes of the FDA, EMA and comparable foreign regulatory authorities are lengthy, time-consuming and unpredictable,
if we are unable to obtain regulatory clearances, grants and approvals, our business may fail. |
| |
● |
If the FDA does not conclude that our T&T platform
product candidate satisfies the requirements under Section 505(b)(2) of the Federal Food, Drug and Cosmetic Act, or FFDCA,
or Section 505(b)(2), or if we are unable to utilize the hybrid application pathway in the European Union, or if the requirements
are not as we expect, the approval pathway for our T&T platform product candidates will likely take significantly longer,
cost significantly more and entail significantly greater complications and risks than anticipated, and in either case may not be
successful. |
| |
● |
Our C&C and T&T technologies are novel technologies,
which makes it difficult to accurately and reliably predict the time and cost of development and regulatory approval. |
| |
|
|
| |
● |
As an organization, we have not previously conducted
pivotal clinical trials, and we may be unable to do so for any product candidates we may develop, including our T&T platform
product candidates. |
| |
● |
We
may find it difficult to enroll patients in our clinical trials due to various reasons, including possible disruption due to
any resurgence of the COVID-19 pandemic, which could delay or prevent us from proceeding with such trials. |
| |
|
|
| |
● |
Our
product candidates and the administration of our product candidates may cause undesirable side effects or have other properties
that could delay or prevent their regulatory approval. |
| |
|
|
| |
● |
We
may be subject, directly or indirectly, to U.S. federal and state healthcare fraud and abuse laws, false claims laws, physician
payment transparency laws and health information privacy and security laws. If we are unable to comply, or have not fully complied,
with such laws, we could face substantial penalties. |
| |
|
|
| |
● |
We
face intense competition in an environment of rapid technological change, which may adversely affect our financial condition
and our ability to successfully market or commercialize our product candidates. |
| |
|
|
| |
● |
The
misuse or off-label use of our product candidates may harm our reputation in the marketplace, result in injuries that lead to
product candidates liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed
to have engaged in the promotion of these uses, any of which could be costly to our business. |
Risks
Related to our Reliance on Third Parties
| |
● |
We
will rely on third parties to conduct certain elements of our preclinical studies and clinical trials
and perform other tasks for us. If these third parties do not successfully carry out their contractual
duties, meet expected deadlines or comply with regulatory requirements, we may not be able to obtain
regulatory approval for or commercialize our product candidates. |
| ● | Independent
clinical investigators and contract research organizations, or CROs, that we will engage
to conduct our clinical trials may not devote sufficient time or attention to our clinical
trials or be able to repeat their past success. |
| |
● |
We
rely on third parties to manufacture the raw materials that we use to create our product candidates.
Our business could be harmed if existing and prospective third parties fail to provide us with sufficient
quantities of these materials and product candidates or fail to do so at acceptable quality levels or
prices. |
Risks
Related to Our Intellectual Property
| |
● |
If
we are unable to obtain and maintain effective patent rights for our product candidates or any future
product candidates, we may not be able to compete effectively in our markets. If we are unable to protect
the confidentiality of our trade secrets or know-how, such proprietary information may be used by others
to compete against us. |
| |
● |
Changes
in patent policy and national intellectual property laws could increase the uncertainties and costs surrounding
the prosecution of our patent applications and the enforcement or defense of any issued patents. |
| |
● |
We
may be involved in lawsuits to protect or enforce our intellectual property, which could be expensive,
time consuming and unsuccessful. |
| |
● |
We may be subject to claims
that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties
or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers, and we may be subject to
claims challenging the inventorship of our intellectual property. |
Risks
Related to Our Business Operations
| |
● |
We will need to expand our
organization, and we may experience difficulties in managing this growth, which could disrupt our operations. |
| |
● |
Due to our limited resources
and access to capital, we must, and have in the past decided to, prioritize development of certain product candidates over other
potential candidates. These decisions may prove to have been wrong and may adversely affect our revenues. |
| |
● |
We may not be successful in
our efforts to identify, discover or license additional product candidates. |
| |
● |
Any resurgence
of the COVID-19 pandemic could adversely affect our business, financial condition and results of operations. |
| |
● |
Our employees and independent
contractors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements. |
| |
● |
Under applicable employment
laws, we may not be able to enforce covenants not to compete and therefore may be unable to prevent our competitors from benefiting
from the expertise of some of our former employees. |
Risks
Related to Commercialization of Our Product Candidates
| |
● |
We
currently have no marketing and sales organization. If we are unable to establish marketing and sales
capabilities, or enter into agreements with third parties to market and sell our product candidates,
we may be unable to generate any product candidates revenue. |
| |
● |
We
are subject to significant regulatory oversight with respect to manufacturing our product candidates.
Delays in establishing and obtaining regulatory approval of our manufacturing process may delay or disrupt
our product candidates’ development and commercialization efforts. |
| ● | If
we receive marketing approval for our product candidates, sales will be limited unless the
product candidates achieves broad market acceptance. |
| ● | It
may be difficult for us to profitably sell our product candidates if coverage and reimbursement
for these product candidates is limited by government authorities and/or third-party payor
policies. |
| |
● |
Our
business entails a significant risk of clinical trial and/or product candidates liability and our ability
to obtain sufficient insurance coverage could have a material effect on our business, financial condition,
results of operations or prospects. |
Risks
Related to this Offering and Ownership of Our Securities
| ● | Our
executive officers, directors and principal shareholders will maintain the ability to exert
significant control over matters submitted to our shareholders for approval. |
| |
● |
If we are determined to be
a passive foreign investment company, or PFIC, U.S. taxpayers would be subject to certain adverse U.S. federal income tax rules. |
| ● | If
a United States person is treated as owning at least 10% of our shares, such holder may be
subject to adverse U.S. federal income tax consequences. |
| ● | As
a foreign private issuer, we intend to follow certain home country corporate practices instead
of Nasdaq requirements, and we will not be subject to certain U.S. securities laws. |
| ● | We
are an emerging growth company and the reduced disclosure requirements applicable to emerging
growth companies may make our Ordinary Shares less attractive to investors. |
| |
● |
Certain recent initial public
offerings of companies with public floats comparable to the anticipated public float of Polyrizon have experienced extreme volatility
that was seemingly unrelated to the underlying performance of the respective company. |
Risks
Related to Israeli Law and Our Operations in Israel
| |
● |
Our
headquarters and other significant operations are located in Israel, and, therefore, our results may
be adversely affected by political, economic and military instability in Israel, including the recent
attack by Hamas and other terrorist organizations from the Gaza Strip and Israel’s war against
them. |
| ● | It
may be difficult to enforce a judgment of a U.S. court against us and our executive officers
and directors in Israel, to assert U.S. securities laws claims in Israel or to serve process
on us. |
| |
● |
Your
rights and responsibilities as a shareholder will be governed in key respects by Israeli laws, which
differs from U.S. companies. |
Implications
of Being an “Emerging Growth Company” and a Foreign Private Issuer
Emerging
Growth Company
As
a company with less than $1.235 billion in revenue during our last fiscal year, we qualify as an “emerging growth company”
as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of specified
reduced reporting and other burdens that are otherwise applicable generally to public companies. In particular, as an emerging growth
company, we:
| ● | may
present only two years of audited financial statements and only two years of related Management’s
Discussion and Analysis of Financial Condition and Results of Operations disclosure in our
initial registration statement; |
| ● | are
not required to provide a detailed narrative disclosure discussing our compensation principles,
objectives and elements and analyzing how those elements fit with our principles and objectives,
which is commonly referred to as “compensation discussion and analysis”; |
| ● | are
not required to obtain a non-binding advisory vote from our shareholders on executive compensation
or golden parachute arrangements (commonly referred to as the “say-on-pay,” “say-on
frequency” and “say-on-golden-parachute” votes); |
| ● | will
not be required to conduct an evaluation of our internal control over financial reporting; |
| ● | are
exempt from certain executive compensation disclosure provisions requiring a pay-for-performance
graph and chief executive officer pay ratio disclosure; and |
| ● | an
exemption from the auditor attestation requirement in the assessment of our internal control
over financial reporting pursuant to the Sarbanes-Oxley Act of 2002. |
We
may take advantage of these provisions for up to five years or such earlier time that we are no longer an emerging growth company. We
would cease to be an emerging growth company upon the earlier to occur of: (1) the last day of the fiscal year in which we have
total annual gross revenues of $1.235 billion or more; (2) the date on which we have issued more than $1.0 billion in
nonconvertible debt during the previous three years; or (3) the date on which we are deemed to be a large accelerated filer under
the rules of the Securities and Exchange Commission, or the SEC. We may choose to take advantage of some but not all of these reduced
burdens, and therefore the information that we provide holders of our Ordinary Shares may be different than the information you might
receive from other public companies in which you hold equity. In addition, Section 107 of the JOBS Act also provides that an emerging
growth company can take advantage of an extended transition period for complying with new or revised accounting standards applicable
to public companies. We have elected to take advantage of the extended transition period to comply with new or revised accounting standards
and to adopt certain of the reduced disclosure requirements available to emerging growth companies. As a result of the accounting standards
election, we will not be subject to the same implementation timing for new or revised accounting standards as other public companies
that are not emerging growth companies which may make comparison of our financials to those of other public companies more difficult.
In addition, the information that we provide in this prospectus may be different than the information you may receive from other public
companies in which you hold equity interests.
Foreign
Private Issuer
Upon
consummation of this offering, we will report under the Securities Exchange Act of 1934, as amended, or the Exchange Act, as a non-U.S.
company with foreign private issuer status. Even after we no longer qualify as an emerging growth company, as long as we continue to
qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable
to U.S. domestic public companies, including:
| ● | the
sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations
with respect to a security registered under the Exchange Act; |
| ● | the
sections of the Exchange Act requiring insiders to file public reports of their share ownership
and trading activities and liability for insiders who profit from trades made in a short
period of time; and |
| ● | the
rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q
containing unaudited financial statements and other specified information, and current reports
on Form 8-K upon the occurrence of specified significant events. |
We
will be required to file an annual report on Form 20-F within four months of the end of each fiscal year. In addition, we intend to publish
our results on a quarterly basis through press releases, distributed pursuant to the rules and regulations of the Nasdaq Stock Exchange.
Press releases relating to financial results and material events will also be furnished to the SEC on Form 6-K. However, the information
we are required to file with or furnish to the SEC will be less extensive and less timely compared to that required to be filed with
the SEC by U.S. domestic issuers. As a result, you may not be afforded the same protections or information, which would be made available
to you, were you investing in a U.S. domestic issuer.
We
may take advantage of these exemptions until such time as we are no longer a foreign private issuer. We would cease to be a foreign private
issuer at such time as more than 50% of our outstanding voting securities are held by U.S. residents and any of the following three circumstances
applies: (i) the majority of our executive officers or directors are U.S. citizens or residents; (ii) more than 50% of our assets are
located in the United States; or (iii) our business is administered principally in the United States.
Both
foreign private issuers and emerging growth companies are also exempt from certain more stringent executive compensation disclosure rules.
Thus, even if we no longer qualify as an emerging growth company, but remain a foreign private issuer, we will continue to be exempt
from the more stringent compensation disclosures required of companies that are neither an emerging growth company nor a foreign private
issuer.
THE
OFFERING
| Ordinary Shares currently issued
and outstanding |
|
3,235,542 Ordinary Shares |
| |
|
|
| Units offered by us |
|
958,903 Units (based on the public offering
price of $4.38 per Unit, each consisting of one Ordinary Share (representing an aggregate of 958,903 Ordinary Shares) and three
Warrants, each to purchase one Ordinary Share (representing an aggregate of 2,876,709 Ordinary Shares issuable upon the exercise
of such warrants). The Units have no stand-alone rights and will not be certificated or issued as stand-alone securities. The
Ordinary Shares and Warrants are immediately separable and will be issued separately in this offering. |
| |
|
|
| Warrants |
|
Each Warrant
has an exercise price of $4.38 per Ordinary Share (with such exercise price equal to the public offering price per Unit), will be
immediately exercisable and will expire five years from the date of issuance. |
| |
|
|
| |
|
To
better understand the terms of the Warrants, you should carefully read the “Description of
the Securities We are Offering” section of this prospectus. You should also read the form
of Warrant, which is filed as an exhibit to the registration statement of which this prospectus
forms a part. |
| Ordinary Shares offered by the
Selling Shareholders |
|
An aggregate of 2,801,330 Ordinary
Shares. |
| |
|
|
| Ordinary Shares to be issued and outstanding after
this offering |
|
4,194,445 Ordinary Shares
(excluding 2,876,709 Ordinary Shares issuable upon exercise of the Warrants), or 4,338,280 Ordinary Shares if the underwriter exercises
in full the over-allotment option to purchase additional Ordinary Shares. |
| |
|
|
| Over-allotment option |
|
We have granted the underwriter
an option to purchase from us, up to an additional 143,835 Ordinary Shares and/or up to an additional 431,505 Warrants, within 45
days from the date of this prospectus to cover over-allotments, if any. The purchase price to be paid per additional Ordinary Share
will be equal to the public offering price of one Unit (less the $0.01 purchase price allocated to each Warrant), as applicable,
less the underwriting discounts and commissions. The underwriter may exercise the over-allotment option with respect to the Ordinary
Shares or Warrants only, or any combination thereof. |
| Lock-Up
Agreements |
|
Our directors,
executive officers, employees and any other holder(s) of ten percent (10%) or more of the outstanding shares have agreed with the
underwriter not to offer for sale, issue, sell, contract to sell, pledge or otherwise dispose of any of our Ordinary Shares or securities
convertible into Ordinary Shares for a period of 180 days from the closing of this offering. |
| Use of proceeds |
|
We
expect to receive approximately $3.4 million in net proceeds from the sale of Units offered by us in this
offering (approximately $4.0 million if the underwriter exercises its over-allotment option in full), based
upon the public offering price of $4.38 per Unit, and after deducting the underwriting discounts and commissions
and estimated offering expenses payable by us.
We currently
expect to use the net proceeds from this offering for the following purposes:
●
approximately $450,000 to complete the preclinical development for our PL-14, PL-15 and PL-16 product candidates (approximately
$150,000 per product candidate);
●
approximately $600,000 to complete the clinical development for our PL-14 product candidate;
●
approximately $300,000 to complete in vitro feasibility studies of corticosteroid, benzodiazepine, naloxone for our T&T
platform technology product candidates;
●
approximately $310,000 to repay the April 2024 CLA and August 2024 CLA. The April 2024 CLA and August 2024 CLA bear interest at
an annual rate of 4% and has a maturity date of April 2026 and August 2026, respectively. In accordance with the terms of the April 2024
CLA and August 2024 CLA, upon the closing of this offering we will repay the April 2024 CLA Amount and August 2024 CLA Amount out of
the proceedings received in this offering; and
●
the remainder for working capital and general corporate purposes and possible future acquisitions.
The amounts
and schedule of our actual expenditures will depend on multiple factors. As a result, our management will have broad discretion in
the application of the net proceeds of this offering.
We will not receive any proceeds from the sale of Ordinary
Shares by the Selling Shareholders.
|
| |
|
|
| Risk factors |
|
Investing in our securities
involves a high degree of risk. You should read the “Risk Factors” section starting on page 13 of this prospectus
for a discussion of factors to consider carefully before deciding to invest in the Ordinary Shares. |
| |
|
|
| Nasdaq Capital Market symbol: |
|
We have been approved
to list the Ordinary Shares on the Nasdaq under the symbol “PLRZ”. |
The
number of the Ordinary Shares to be issued and outstanding immediately after this offering as shown above assumes that all of the Ordinary
Shares offered hereby are sold, and is based on 3,235,542 Ordinary Shares issued and outstanding as of the date of this prospectus. This
number excludes:
| |
●
|
246,129 Ordinary Shares issuable
upon the exercise of options issued to directors, employees and consultants under our incentive option plan outstanding as of such
date, with exercise prices at a range of $0.02 - $1.1335 per share, of which options to purchase 146,064 Ordinary Shares were vested
as of such date (including options to purchase 42,744 Ordinary Shares that vest upon the completion of this offering); |
| |
● |
such number
of Ordinary Shares issuable upon the exercise of options representing 2.5% of the Company’s post-initial public offering issued
and outstanding shares which shall vest and become exercisable over a total period of three years commencing on the grant date on
a monthly basis in equal installments which will be granted to Company’s Chief Executive Officer subsequent to the completion
of this offering; and |
| |
● |
58,132 Ordinary Shares reserved
for future issuance under our equity incentive plan, or the 2021 Equity Incentive Plan. |
Unless
otherwise indicated, all information in this prospectus assumes or gives effect to:
| |
● |
no exercise of the underwriter’s over-allotment
option; |
| |
● |
no
exercise of the Warrants; |
| ● | no
exercise of the 364,931 pre-funded warrants and 2,054,793 warrants to purchase 2,054,793
Ordinary Shares upon the same terms as the warrants to be issued in this offering, issuable
to SciSparc in connection with the SciSparc License Agreement; |
| |
● |
the
issuance of 104,711 Ordinary Shares upon the automatic conversion of the 104,711 Preferred Shares issued and outstanding as of
the date hereof, which will automatically convert upon the completion of this offering; and |
| |
|
|
| |
● |
the
Initial Reverse Share Split, the Forward Share Split, the Second Reverse Share Split, the Third Reverse Share Split, and the
Second Forward Share Split. |
See
“Description of Share Capital and Governing Documents” for additional information.
Certain
of our existing shareholders, including beneficial owners of greater than 5% of our share
capital, have indicated an interest in purchasing up to an aggregate of $1 million of Units
in this offering, which represents approximately 24% of the total Units in this offering,
based on the public offering price of $4.38 per Unit. However, because indications of interest
are not binding agreements or commitments to purchase, the underwriters may determine to
sell more, less or no Units in this offering to any of these shareholders, or any of these
shareholders may determine to purchase more, less or no Units in this offering. The underwriters
will receive the same underwriting discount on any Units purchased by these shareholders
as they will on any other Units sold to the public in this offering.
SUMMARY
FINANCIAL DATA
The
following table summarizes our financial data. We have derived the following statements of comprehensive loss data for the years ended
December 31, 2023 and 2022 and the balance sheet data as of December 31, 2023 from our audited financial statements as of December
31, 2023 included elsewhere in this prospectus. We have also derived the statements of comprehensive loss data for the six months ended
June 30, 2024 and 2023 and the balance sheet data as of June 30, 2024 from our unaudited interim financial statements included elsewhere
in this prospectus. Such financial statements have been prepared in accordance with U.S. GAAP. Our historical results are not necessarily
indicative of the results that may be expected in the future, and our results for any interim period are not necessarily indicative of
results that may be expected for any full year. The following summary financial data should be read in conjunction with “Selected
Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and
our audited financial statements and related notes included elsewhere in this prospectus.
| | |
For
the Years Ended
December 31, | | |
Six
Months Ended
June 30, | |
| (U.S. dollars
in thousands except share and per share data) | |
2023 | | |
2022 | | |
2024 | | |
2023 | |
| Statement of Comprehensive Loss: | |
| | |
| | |
| | |
| |
| Research and development expenses | |
$ | 332 | | |
| 347 | | |
| 137 | | |
| 172 | |
| General and administrative
expenses | |
| 303 | | |
| 548 | | |
| 210 | | |
| 153 | |
| Operating loss | |
| 635 | | |
| 895 | | |
| 347 | | |
| 325 | |
| Financing expense (income),
net | |
| (35 | ) | |
| (116 | ) | |
| 241 | | |
| (134 | ) |
| Net loss and comprehensive
loss | |
| 600 | | |
| 779 | | |
| 588 | | |
| 191 | |
| Basic and diluted net loss per share | |
$ | 0.3 | | |
| 0.6 | | |
| 0.2 | | |
| 0.1 | |
| Weighted
average number of shares outstanding used in computing basic and diluted net loss per share | |
| 2,030,327 | | |
| 1,305,635 | | |
| 2,604,325 | | |
| 1,587,012 | |
| |
As
of June 30, 2024 | |
| | |
| | |
| | |
Pro Forma | |
| (in
thousands of USD) | |
Actual | | |
Pro
Forma(1) | | |
As
Adjusted(2) | |
| Balance Sheet: | |
| | |
| | |
| |
| Cash and cash equivalents | |
$ | 23 | | |
$ | (39 | ) | |
$ | 3,412 | |
| Other current assets | |
$ | 20 | | |
$ | 20 | | |
$ | 20 | |
| Deferred offering costs | |
$ | 532 | | |
$ | 532 | | |
$ | - | |
| Property and equipment, net | |
$ | 11 | | |
$ | 11 | | |
$ | 11 | |
| Patent rights | |
$ | - | | |
$ | 3,000 | | |
$ | 3,000 | |
| Total assets | |
$ | 586 | | |
$ | 3,524 | | |
$ | 6,443 | |
| | |
| | | |
| | | |
| | |
| Employees and payroll related liabilities | |
$ | 22 | | |
$ | 22 | | |
$ | 22 | |
| Accrued expenses | |
$ | 191 | | |
$ | 191 | | |
$ | 161 | |
| Convertible notes | |
$ | 151 | | |
$ | - | | |
$ | - | |
| Total current liabilities | |
$ | 364 | | |
$ | 213 | | |
$ | 183 | |
| | |
| | | |
| | | |
| | |
| Temporary equity | |
$ | 248 | | |
$ | - | | |
$ | - | |
| | |
| | | |
| | | |
| | |
| Ordinary shares | |
$ | - | | |
$ | - | | |
$ | - | |
| Additional paid-in capital | |
$ | 4,102 | | |
$ | 7,437 | | |
$ | 10,386 | |
| Receivables on account of shares | |
$ | (19 | ) | |
$ | - | | |
$ | - | |
| Accumulated deficit | |
$ | (4,109 | ) | |
$ | (4,126 | ) | |
$ | (4,126 | ) |
| Total shareholders’ equity | |
$ | (26 | ) | |
$ | 3,311 | | |
$ | 6,260 | |
| | |
| | | |
| | | |
| | |
| Total liabilities, temporary
equity and shareholders’ deficit | |
$ | 586 | | |
$ | 3,524 | | |
$ | 6,443 | |
| (1) |
Pro Forma data gives effect
to the following events as if each event had occurred on or before June 30, 2024: (i) the conversion of 104,711 preferred shares
into 104,711 Ordinary Shares; (ii) the repayment of $151,000 pursuant to the 2024 convertible loan agreement; (iii) the issuance
of 61,751 Ordinary Shares pursuant to a $70,000 investment (according to a contractual agreement with some of the investors); and
(iv) the issuance, as consideration for the purchase of patent rights, of 320,000 ordinary shares in August 2024 pursuant to the
SciSparc License Agreement and the additional issuance, as consideration for such purchase, of 364,931 pre-funded warrants and 2,054,793
warrants to purchase 2,054,793 ordinary shares upon the same terms as the warrants to be issued in this offering, which will be issued
upon the consummation of this offering, calculated based on the initial public offering price of $4.38 per Ordinary Share. |
| (2) |
Pro Forma as Adjusted data
gives additional effect to the sale of 958,903 Units in this offering at the initial public offering price of $4.38 per Unit, after
deducting underwriting discounts and commissions and estimated offering expenses payable by us, as if the sale had occurred on June
30, 2024. |
RISK
FACTORS
Investing
in our securities involves a high degree of risk. You should carefully consider the risks and uncertainties described below, in addition
to the other information set forth in this prospectus, including the financial statements and the related notes included elsewhere in
this prospectus, before purchasing our securities. Our business, financial condition, cash flows and results of operations could be negatively
impacted as a result of these risks. In that case, the trading price of our Ordinary Shares would likely decline and you might lose all
or part of your investment.
Risks
Related to Our Financial Condition and Capital Requirements
We
have incurred significant losses since our inception. We anticipate that we will continue to incur significant losses for the foreseeable
future, and we may never achieve or maintain profitability.
We
are a development stage biotech company. We have incurred operating losses since our inception,
including operating losses of $635,000 and $895,000 for the years ended December 31, 2023
and December 31, 2022, respectively, and operating losses of $347,000 and $325,000 for the
six months ended June 30, 2024 and June 30, 2023, respectively. As of December 31, 2023 and
June 30, 2024, we had an accumulated deficit of approximately $3.5 million and $4.1 million,
respectively. We have devoted substantially all of our financial resources to designing and
developing our C&C product candidates, including preclinical studies and clinical development
and providing general and administrative support for these operations. We expect that our
expenses and operating losses will increase for the foreseeable future as we continue clinical
development of our C&C product candidates to provide a barrier against allergens, influenza
and COVID-19 from contacting the nasal epithelial tissue and develop other product candidates
using our T&T platform technology for nasal delivery of APIs. Our ability to ultimately
achieve revenues and profitability is dependent upon our ability to successfully complete
the development of our C&C product candidates and any future product candidates, obtain
necessary regulatory approvals for and successfully manufacture, market and commercialize
our product candidates.
We
anticipate that our expenses will increase substantially based on a number of factors, including to the extent that we:
| |
● |
Begin
our planned clinical trial of our C&C product candidates in the fourth quarter of 2025; |
| |
|
|
| |
● |
seek regulatory and marketing approvals for any product
candidates that successfully complete clinical trials; |
| |
|
|
| |
● |
advance our preclinical and research and development
programs; |
| |
|
|
| |
● |
identify, assess, acquire, license and/or develop other
product candidates; |
| |
|
|
| |
● |
manufacture current good manufacturing practices, or
cGMP, material for clinical trials or potential commercial sales; |
| |
|
|
| |
● |
establish a sales, marketing and distribution infrastructure
to commercialize any product candidates for which we may obtain marketing approval; |
| |
|
|
| |
● |
hire personnel and invest in additional infrastructure
to support our operations as a public company and expand our product candidates’ development; |
| |
|
|
| |
● |
enter into agreements to license intellectual property
from third parties; |
| |
|
|
| |
● |
develop, maintain, protect and expand our intellectual
property portfolio; and |
| |
|
|
| |
● |
experience any delays or encounter issues with respect
to any of the above, including, but not limited to, failed trials, complex results, safety issues or other regulatory challenges
that require longer follow-up of existing clinical trials, additional major clinical trials or additional supportive studies in order
to pursue marketing approval. |
To
date, we have financed our operations primarily through the sale of equity securities, convertible loans made by certain of our shareholders,
royalty-bearing and non-royalty bearing grants that we received from the Israeli Innovation Authority, or the IIA. The amount of any
future operating losses will depend, in part, on the rate of our future expenditures and our ability to obtain funding through equity
or debt financings, strategic collaborations or grants. Even if we obtain regulatory approval to market one or more product candidates,
our future revenue will depend upon the size of any markets in which such product candidates receive approval and our ability to achieve
sufficient market acceptance, pricing, reimbursement from third-party payors for such product candidates. Further, the operating losses
that we incur may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of our results
of operations may not be a good indication of our future performance. Other unanticipated costs may also arise.
We
have never generated any revenue from product candidates sales and may never be profitable.
We
have no product approved for marketing in any jurisdiction and we have never generated any revenue from product candidates sales. Our
ability to generate revenue and achieve profitability depends on our ability, alone or with strategic collaboration partners, to successfully
complete the development of, and obtain the regulatory and marketing approvals necessary to commercialize, our product candidates or
any future product candidates. We do not anticipate generating revenue from product candidates sales for at least the next year. Our
ability to generate future revenue from product candidates sales will depend heavily on our ability to:
| ● | complete
research and preclinical and clinical development of our product candidates and any future
product candidates in a timely and successful manner, including our C&C product candidates
to provide a barrier against viruses and allergens from contacting the nasal epithelial tissue. |
| ● | obtain
regulatory and marketing approval for any product candidates for which we complete clinical
trials; |
| ● | maintain
and enhance a commercially viable, sustainable, scalable, reproducible and transferable manufacturing
process for our technology product candidates and any future product candidates that is compliant
with cGMPs; |
| ● | establish
and maintain supply and, if applicable, manufacturing relationships with third parties that
can provide, in both amount and quality, adequate product candidates to support clinical
development and the market demand for our technology product candidates and any future product
candidates, if and when approved; |
| ● | launch
and commercialize any product candidates for which we obtain regulatory and marketing approval,
either directly by establishing a sales force, marketing and distribution infrastructure,
and/or with collaborators or distributors; |
| ● | expose
and educate physicians and other medical professionals to use our product candidates; |
| ● | obtain
market acceptance, if and when approved, of our product candidates and any future product
candidates from the medical community and third-party payors; |
| ● | ensure
our product candidates are approved for reimbursement from governmental agencies, healthcare
providers and insurers in jurisdictions where they have been approved for marketing; |
| ● | address
any competing technological and market developments that impact our product candidates and
any future product candidates or their prospective usage by medical professionals; |
| ● | identify,
assess, acquire and/or develop new product candidates; |
| ● | negotiate
favorable terms in any collaboration, licensing or other arrangements into which we may enter
and perform our obligations under such collaborations; |
| ● | maintain,
protect and expand our portfolio of intellectual property rights, including patents, patent
applications, trade secrets and know-how; |
| ● | avoid
and defend against third-party interference or infringement claims; |
| ● | attract,
hire and retain qualified personnel; and |
| ● | locate
and lease or acquire suitable facilities to support our clinical development, manufacturing
facilities and commercial expansion. |
Even
if our product candidates or any future product candidates are approved for marketing and sale, we anticipate incurring significant incremental
costs associated with commercializing such product candidates. Our expenses could increase beyond expectations if we are required by
the FDA, the EMA or other regulatory agencies, domestic or foreign, or ethical committees in medical centers, to change our manufacturing
processes or assays or to perform clinical, nonclinical or other types of studies in addition to those that we currently anticipate.
Even if we are successful in obtaining regulatory approvals to market our technology product candidates or any future product candidates,
our revenue earned from such product candidates will be dependent in part upon the breadth of the product candidates label, the size
of the markets in the territories for which we gain regulatory approval for such product candidates, the accepted price for such product
candidates, our ability to obtain reimbursement for such product candidates at any price, whether we own the commercial rights for that
territory in which such product candidates have been approved and the expenses associated with manufacturing and marketing such product
candidates for such markets. Therefore, we may not generate significant revenue from the sale of such product candidates, even if approved.
Further, if we are not able to generate significant revenue from the sale of our approved product candidates, we may be forced to curtail
or cease our operations. Due to the numerous risks and uncertainties involved in product candidates’ development, it is difficult
to predict the timing or amount of increased expenses, or when, or if, we will be able to achieve or maintain profitability.
We
expect that we will need to raise substantial additional funding, which may not be available on acceptable terms, or at all. Failure
to obtain funding on acceptable terms and on a timely basis may require us to curtail, delay or discontinue our product candidates’
development efforts or other operations.
We
are currently advancing our C&C product candidates through pre-clinical and clinical development in multiple indications, in order
to obtain regulatory approvals. Developing product candidates is expensive, and we expect our research and development expenses to increase
substantially in connection with our ongoing activities, particularly as we advance product candidates through clinical trials and regulatory
approvals. Furthermore, we expect to incur additional ongoing costs associated with operating as a public company.
To
date, we have financed our operations primarily through the sale of equity securities. As of June 30, 2024, we had cash, cash equivalents
of $23,000. We will require significant additional financing to fund our operations. Our future funding requirements will depend on many
factors, including but not limited to:
| ● | the
progress, results and costs of our anticipated clinical trials of our C&C product candidate
and any future product candidates; |
| ● | the
cost, timing and outcomes of regulatory review of our product candidates and any future product
candidates; |
| ● | the
scope, progress, results and costs of product candidates’ development, laboratory testing,
manufacturing, preclinical development and clinical trials for any other product candidates
that we may develop or otherwise obtain in the future; |
| ● | the
cost of our future activities, including establishing sales, marketing and distribution capabilities
for any product candidates in any particular geography where we receive marketing approval
for such product candidates; |
| ● | the
terms and timing of any collaborative, licensing and other arrangements that we may establish; |
| ● | the
costs of preparing, filing and prosecuting patent applications, maintaining and enforcing
our intellectual property rights and defending intellectual property-related claims; and |
| ● | the
level of revenue, if any, received from commercial sales of any product candidates for which
we receive marketing approval. |
Identifying
potential product candidates and conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process
that takes years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve
product candidates sales. In addition, our product candidates, if and when approved, may not achieve commercial success. Our product
candidates revenues, if any, will be derived from or based on sales of product candidates that may not be commercially available for
many years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Any
additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to
develop and commercialize our product candidates.
We
cannot guarantee that financing will be available in sufficient amounts or on terms acceptable to us, if at all, and the terms of any
financing may adversely affect the interests or rights of our shareholders. Even if we believe that we have sufficient funds for our
current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic
considerations. The issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause
the market price of our shares to decline. Further, our ability to raise additional capital may be adversely impacted by potential worsening
global economic conditions and the recent disruptions to and volatility in the credit and financial markets in the United States and
worldwide resulting from the resurgence of the COVID-19 pandemic.
To
the extent that we raise capital through the sale of equity or convertible debt securities, your ownership interest will be diluted,
and the terms of such securities may include liquidation or other preferences that adversely affect your rights as a shareholder. Debt
financing, if available, may involve covenants restricting our operations or our ability to incur additional debt. If we raise funds
through collaboration and licensing arrangements with third parties, it may be necessary to relinquish certain rights to our technologies
or our product candidates, or to grant licenses on terms that are not favorable to us.
If
we are unable to obtain funding on acceptable terms and on a timely basis, we may be required to significantly curtail, delay or discontinue
one or more of our research, development or manufacturing programs or the commercialization of any approved product candidates, or be
unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our
business, financial condition and results of operations.
Our
financial statements contain an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern, which
could prevent us from obtaining new financing on reasonable terms or at all.
Our
audited financial statements for the year ended December 31, 2023, contain an explanatory paragraph regarding substantial doubt about
our ability to continue as a going concern. We have net losses in each year since our inception, including a net loss of approximately
$600,000 for the year ended December 31, 2023 and $779,000 for the year ended December 31, 2022 and a net loss of approximately
$588,000 for the six months ended June 30, 2024 and $191,000 for the six months ended June 30, 2023. These events and conditions, along
with other matters, indicate that a material uncertainty exists that may cast significant doubt on our ability to continue as a going
concern. The financial statements for 2023 do not include any adjustments that might result from the outcome of this uncertainty. This
going concern opinion could materially limit our ability to raise additional funds through the issuance of equity or debt securities
or otherwise. Further financial statements may include an explanatory paragraph with respect to our ability to continue as a going concern.
Until we can generate significant recurring revenues, we expect to satisfy our future cash needs through debt or equity financing. We
cannot be certain that additional funding will be available to us on acceptable terms, if at all. If funds are not available, we may
be required to delay, reduce the scope of, or eliminate research or development plans for, or commercialization efforts with respect
to our product candidates. This may raise substantial doubts about our ability to continue as a going concern.
Risks
Related to the Discovery, Development and Clinical Testing of Product Candidates
We
depend on enrollment of patients in our upcoming clinical trials in order to continue development of our product candidates.
We
intend conduct clinical trials as part of the development of our product candidates. Our anticipated time to data in these trials is
subject to our ability to recruit sufficient eligible patients and the number and size of cohorts that will need to be enrolled prior
to observing activity, if achieved at all for the dose escalation and expansion arms of the relevant trials. There can be no assurance
that we will complete enrollment or have data from the trials when we anticipate or at all. The timely completion of clinical trials
in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients that are in
line with our inclusions and exclusion criteria and our ability to monitor these patients as required.
We
may experience difficulties in patient enrollment in our clinical trials for a variety of reasons. Patient enrollment is affected by
many factors including the size and nature of the patient population, the eligibility criteria for the trial, the design of the clinical
trial, the size of the patient population required for analysis of the trial’s primary endpoints, the proximity of patients to
study sites, our ability to recruit clinical trial investigators with the appropriate competencies and experience, the number of enrolling
clinical sites, our ability to obtain and maintain patient consents, the risk that patients enrolled in clinical trials will drop out
of the trials before completion, and competing clinical trials (including other clinical trials that we are conducting or will conduct
in the future) and clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation
to other available therapies, or competing drugs against the same target as well as any new drugs that may be approved for the indications
we are investigating.
Additionally,
we must compete for clinical sites, clinicians and the limited number of patients who fulfill the stringent requirements for participation
in clinical trials. Also, due to the confidential nature of clinical trials, we do not know how many of the eligible patients may be
enrolled in competing studies and who are consequently not available to us for our clinical trials. Our clinical trials may be delayed
or terminated due to the inability to enroll enough patients. The delay or inability to meet planned patient enrollment may result in
increased costs and delay or termination of our trials, which could have a harmful effect on our ability to develop product candidates.
While we are only in the early stages of pre-clinical development for our T&T platform, the foregoing and similar regulatory risks
may impact our business, results of operations and prospects as we progress with the development of our T&T platform.
We
may not receive, or may be delayed in receiving, the necessary clearances or approvals for our C&C product candidates or future product
candidates, and failure to timely obtain necessary clearances or approvals for our C&C product candidates or future product candidates
would adversely affect our ability to grow our business.
In
the United States, before we can market a new medical device, or a new use of, new claim for or significant modification to existing
product candidates, we must first receive either clearance under Section 510(k) of the Federal Food, Drug and Cosmetic Act, or FFDCA,
or approval of a pre-market approval application, or a PMA, from the FDA, unless an exemption applies. In the clearance process for Section
510(k) of the FFDCA, or Section 510(k), before a device may be marketed, the FDA must determine that a proposed device is “substantially
equivalent” to a legally-marketed “predicate” device, which is defined as a device that has been previously cleared
through the 510(k) process, a device that was legally marketed prior to May 28, 1976 (pre-amendments device), a device that was originally
on the U.S. market pursuant to an approved PMA and later down-classified, or a 510(k)-exempt device. To be “substantially equivalent,”
the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as
the predicate device or have different technological characteristics that do not raise different questions of safety or effectiveness
than the predicate device. Clinical data are sometimes required to support substantial equivalence. In the process of obtaining PMA approval,
the FDA must determine that a proposed device is safe and effective for its intended use based, in part, on extensive data, including,
but not limited to, technical, pre-clinical, clinical trial, manufacturing and labeling data. For a PMA, the FDA is making a determination
that the probable benefits must be determined to outweigh the probable risk of the device for the labeled indication, and there must
be a reasonable assurance of safety and effectiveness.
Modifications
to product candidates that are approved through a PMA application generally require FDA approval. Similarly, certain modifications made
to product candidates cleared through a 510(k) may require a new 510(k) clearance. Both the PMA approval and the 510(k) clearance process
can be expensive, lengthy and uncertain. The FDA’s 510(k) clearance process usually takes from three to 12 months, but can last
longer. The process of obtaining a PMA is much more costly and uncertain than the 510(k) clearance process and generally takes from one
to three years, or even longer, from the time the application is submitted to the FDA. In addition, a PMA generally requires the performance
of one or more clinical trials. Despite the time, effort and cost, a device may not be approved or cleared by the FDA. Any delay or failure
to obtain necessary regulatory clearances or approvals could harm our business. Furthermore, even if we are granted regulatory clearances
or approvals, they may include significant limitations on the indications for use of the device or other restrictions or requirements,
which may limit the market for the device.
In
the United States, we expect to take a multi-step approach to the regulatory clearance process. The review process is an iterative process
and may be more costly and time consuming than we expect and we may not ultimately be successful in completing the review process and
our 510 (k) application may not be cleared by the FDA in a timely manner or at all. If cleared, any modification to our C&C product
candidate that has not been previously cleared may require us to submit a new 510(k) application and obtain clearance, or submit a PMA
and obtain FDA approval prior to implementing the change. Specifically, any modification to a 510(k)-cleared device that could significantly
affect its safety or effectiveness, or that would constitute a major change in its intended use, design or manufacture, requires a new
510(k) clearance or, possibly, approval of a PMA. The FDA requires every manufacturer to make this determination in the first instance,
but the FDA may review any manufacturer’s decision. The FDA may not agree with our decisions regarding whether new clearances or
approvals are necessary. We may make modifications or add additional features in the future that we believe do not require a new 510(k)
clearance or approval of a PMA. If the FDA disagrees with our determination and requires us to submit new 510(k) notifications or PMA
applications for modifications to our previously cleared product candidates for which we have concluded that new clearances or approvals
are unnecessary, we may be required to cease marketing or to recall the modified product candidates until we obtain clearance or approval,
and we may be subject to significant regulatory fines or penalties. If the FDA requires us to go through a lengthier, more rigorous examination
for future product candidates or modifications to existing product candidates than we had expected, product candidates introductions
or modifications could be delayed or canceled, which could adversely affect our ability to grow our business.
The
FDA can delay, limit or deny clearance or approval of a medical device for many reasons, including:
| |
● |
our inability to demonstrate to the satisfaction of
the FDA or the applicable regulatory entity or notified body that our product candidates are safe or effective for their intended
uses; |
| |
|
|
| ● | the
disagreement of the FDA or the applicable foreign regulatory body with the design or the
interpretation of data from pre- clinical studies or clinical trials; |
| | | |
| |
● |
serious and unexpected adverse effects experienced
by participants in our clinical trials; |
| |
|
|
| |
● |
the data from our pre-clinical studies and clinical
trials may be insufficient to support clearance or approval, where required; |
| |
|
|
| |
● |
our inability to demonstrate that the clinical and
other benefits of the device outweigh the risks; |
| |
|
|
| |
● |
the manufacturing process or facilities we use may
not meet applicable requirements; and |
| |
|
|
| |
● |
the potential for approval policies or regulations
of the FDA or applicable foreign regulatory bodies to change significantly in a manner rendering our clinical data or regulatory
filings insufficient for clearance or approval. |
In
order to sell our product candidates in member states of the European Union, or the EU, our product candidates must comply with the general
safety and performance requirements of the EU Medical Devices Regulation (Regulation (EU) No 2017/745), which repeals and replaces the
EU Medical Devices Directive (Council Directive 93/42/EEC). Compliance with these requirements is a prerequisite to be able to affix
the European Conformity, or CE, mark to our product candidates, without which they cannot be sold or marketed in the EU. All medical
devices placed on the market in the EU must meet the general safety and performance requirements laid down in Annex I to the EU Medical
Devices Regulation including the requirement that a medical device must be designed and manufactured in such a way that, during normal
conditions of use, it is suitable for its intended purpose. Medical devices must be safe and effective and must not compromise the clinical
condition or safety of patients, or the safety and health of users and – where applicable – other persons, provided that
any risks which may be associated with their use constitute acceptable risks when weighed against the benefits to the patient and are
compatible with a high level of protection of health and safety, taking into account the generally acknowledged state of the art. The
European Commission has adopted various standards applicable to medical devices. These include standards governing common requirements,
such as sterilization and safety of medical electrical equipment and product standards for certain types of medical devices. There are
also harmonized standards relating to design and manufacture. While not mandatory, compliance with these standards is viewed as the easiest
way to satisfy the general safety and performance requirements as a practical matter, as it creates a rebuttable presumption that the
device satisfies the general safety and performance requirements.
To
demonstrate compliance with the general safety and performance requirements we must undergo a conformity assessment procedure, which
varies according to the type of medical device and its (risk) classification. As a general rule, demonstration of conformity of medical
devices and their manufacturers with the general safety and performance requirements must be based, among other things, on the evaluation
of clinical data supporting the safety and performance of the product candidates during normal conditions of use. Specifically, a manufacturer
must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks,
and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims
made about the performance and safety of the device are supported by suitable evidence. Except for low-risk medical devices (Class I),
where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment of the conformity of its product candidates
with the general safety and performance requirements (except for any parts which relate to sterility, metrology or reuse aspects), a
conformity assessment procedure requires the intervention of an organization accredited or designated by a member state of the EU to
conduct conformity assessments, or a notified body. Depending on the relevant conformity assessment procedure, the notified body would
typically audit and examine the technical file and the quality system for the manufacture, design and final inspection of our devices.
If satisfied that the relevant product candidates conforms to the relevant essential requirements, the notified body issues a certificate
of conformity, which the manufacturer uses as a basis for its own declaration of conformity. The manufacturer may then apply the CE Mark
to the device, which allows the device to be placed on the market throughout the EU. If we fail to comply with applicable EU laws and
regulations, and corresponding EU member state laws, we would be unable to affix the CE mark to our product candidates, which would prevent
us from selling them within the EU.
The
aforementioned EU rules are generally applicable in the European Economic Area, or EEA, which consists of the 27 EU member states plus
Norway, Liechtenstein and Iceland. Non-compliance with the above requirements would also prevent us from selling our product candidates
in these three countries.
We
do not plan to conduct a pre-submission meeting with the FDA’s CDRH to confirm the potential for the Class II medical device path
under a de novo classification request for our PL-15 and PL-16 product candidates until after the completion of this initial public offering.
If we are denied submission under the de novo pathway, it may require us to go through a different pathway, such as a PMA pathway,
which may result in a lengthier approval process for our devices.
We
do not plan to conduct a pre-submission meeting with the FDA’s CDRH to confirm the potential for the Class II medical device path
under a de novo classification request for our PL-15 and PL-16 product candidates until after the completion of the initial public offering.
In the event the FDA does not agree with our regulatory assessments for our PL-15 and PL-16 product candidates under a Class II De Novo
pathway, the FDA may require us to go through a lengthier, more rigorous examination than we had expected. This examination could involve
pursuing a PMA, which is the FDA process of scientific and regulatory review to evaluate the safety and effectiveness of Class III medical
devices. PMA submissions must comply with far more rigorous standards compared to 510k or De Novo to prove device safety and effectiveness.
Typically, Class III devices require both laboratory testing and clinical trials that include human participants. PMA is the most rigorous
type of device marketing application required by the FDA. PMA approval is based on a determination by the FDA that the PMA contains sufficient
valid scientific evidence to ensure that the device is safe and effective for its intended use(s). A PMA application generally includes
extensive information about the device including the results of clinical testing conducted on the device and a detailed description of
the manufacturing process. If we are required to pursue a PMA, the introduction of our product candidates into the market could be delayed
significantly.
Legislative
or regulatory reforms in the United States or the European Union may make it more difficult and costly for us to obtain regulatory clearances
or approvals for our product candidates or to manufacture, market or distribute our product candidates after clearance or approval is
obtained.
From
time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the
regulation of medical devices. In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise
existing regulations, or take other actions, which may prevent or delay approval or clearance of our future product candidates under
development or impact our ability to modify our currently cleared product candidates on a timely basis. Over the last several years,
the FDA has proposed reforms to its 510(k) clearance process, and such proposals could include increased requirements for clinical data
and a longer review period, or could make it more difficult for manufacturers to utilize the 510(k) clearance process for their product
candidates. For example, in November 2018, FDA officials announced forthcoming steps that the FDA intends to take to modernize the premarket
notification pathway under Section 510(k) of the FDCA. Among other things, the FDA announced that it planned to develop proposals to
drive manufacturers utilizing the 510(k) pathway toward the use of newer predicates devices. These proposals included plans to potentially
sunset certain older devices that were used as predicates devices under the 510(k) clearance pathway, and to potentially publish a list
of devices that have been cleared on the basis of demonstrated substantial equivalence to predicate devices that are more than 10 years
old. In May 2019, the FDA solicited public feedback on these proposals. The FDA requested public feedback on whether it should consider
certain actions that might require new authority, such as whether to sunset certain older devices that were used as predicates devices
under the 510 (k) clearance pathway. These proposals have not yet been finalized or adopted, and the FDA may work with Congress to implement
such proposals through legislation. Accordingly, it is unclear the extent to which any proposals, if adopted, could impose additional
regulatory requirements on us that could delay our ability to obtain new 510(k) clearances, increase the costs of compliance, or restrict
our ability to maintain our current clearances, or otherwise create competition that may negatively affect our business.
More
recently, in September 2019, the FDA finalized guidance describing an optional “safety and performance based” premarket review
pathway for manufacturers of “certain, well-understood device types” to demonstrate substantial equivalence under the 510(k)
clearance pathway by showing that such device meets objective safety and performance criteria established by the FDA, thereby obviating
the need for manufacturers to compare the safety and performance of their medical devices to specific predicate devices in the clearance
process. The FDA intends to develop and maintain a list device types appropriate for the “safety and performance based” pathway
and will continue to develop product -specific guidance documents that identify the performance criteria for each such device type, as
well as the testing methods recommended in the guidance documents, where feasible. The FDA may establish performance criteria for classes
of devices for which we or our competitors seek or currently have received clearance, and it is unclear the extent to which such performance
standards, if established, could impact our ability to obtain new 510(k) clearances or otherwise create competition that may negatively
affect our business.
In
addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business
and our product candidates. Any new statutes, regulations or revisions or reinterpretations of existing regulations may impose additional
costs or lengthen review times of any future product candidates or make it more difficult to obtain clearance or approval for, manufacture,
market or distribute our product candidates. We cannot determine what effect changes in regulations, statutes, legal interpretation or
policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things,
require: additional testing prior to obtaining clearance or approval; changes to manufacturing methods; recall, replacement or discontinuance
of our product candidates; or additional record keeping.
The
FDA’s and other regulatory authorities’ policies may change and additional government regulations may be promulgated that
could prevent, limit or delay regulatory clearance or approval of our future product candidates. We cannot predict the likelihood, nature
or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad.
If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not
able to maintain regulatory compliance, we may lose any marketing approval or clearance that we may have obtained and we may not achieve
or sustain profitability.
On
April 5, 2017, the European Parliament passed the Medical Devices Regulation (Regulation 2017/745), which repeals and replaces the
EU Medical Devices Directive. Unlike directives, which must be implemented into the national laws of the EEA member states, the regulations
would be directly applicable, i.e., without the need for adoption of EEA member state laws implementing them, in all EEA member states
and are intended to eliminate current differences in the regulation of medical devices among EEA member States. The Medical Devices Regulation,
among other things, is intended to establish a uniform, transparent, predictable and sustainable regulatory framework across the EEA
for medical devices and ensure a high level of safety and health while supporting innovation. The Medical Devices Regulation has become
applicable as of May 26, 2021. Among other things, the new Medical Devices Regulation:
| |
● |
strengthens the rules on placing devices on the market
and reinforce surveillance once they are available; |
| |
|
|
| |
● |
establishes explicit provisions on manufacturers’
responsibilities for follow-up regarding the quality, performance and safety of devices placed on the market; |
| |
|
|
| |
● |
improves the traceability of medical devices throughout
the supply chain to the end-user or patient through a unique identification number; |
| |
|
|
| |
● |
sets up a central database to provide patients, healthcare
professionals and the public with comprehensive information on product available in the EU; and |
| |
|
|
| |
● |
Provides
strengthened rules for the assessment of certain high-risk devices, which may have to undergo an additional check by experts
before they are placed on the market. |
These
modifications may have an effect on the way we conduct our business in the EEA.
We
are heavily dependent on the success of our C&C product candidates, including obtaining regulatory approval to market our C&C
product candidates in the United States and the European Union.
To
date, we have invested substantially all of our efforts and financial resources to research and develop our C&C technology, including
conducting preclinical studies, developing and securing our intellectual property portfolio for our product candidates and technology.
Our future success is dependent on our ability to successfully develop, obtain regulatory approval for and commercialize one or more
of our current and future product candidates. Our product candidates’ marketability is subject to significant risks associated
with successfully completing current and future clinical trials, including:
| |
● |
our ability to initiate our clinical trials; |
| |
|
|
| |
● |
Success
in in-vitro cell culture assays or other non-clinical experiments or animal studies does not ensure that later, clinical trials
will be successful nor does it predict final results. |
| |
|
|
| |
● |
acceptance by the FDA, EMA or other regulatory agencies
of our strategies for seeking regulatory approvals for our C&C product candidates and any future product candidates, including
our proposed indications, primary and secondary endpoint assessments and measurements, safety evaluations and regulatory pathways; |
| |
|
|
| |
● |
the acceptance by the FDA, EMA or other regulatory
agencies of the number, design, size, conduct and implementation of our clinical trials, our trial protocols and the interpretation
of data from preclinical studies or clinical trials; |
| |
● |
our ability to successfully initiate and complete clinical
trials of our C&C product candidates and any future product candidates, including timely patient enrollment and acceptable
safety and efficacy data and our ability to demonstrate the safety and efficacy of the product candidates undergoing such clinical
trials; |
| |
|
|
| |
● |
the willingness of the FDA, EMA or other regulatory
agencies to schedule an advisory committee meeting in a timely manner in connection with our regulatory submissions, if such advisory
committee meetings are required; |
| |
|
|
| |
● |
the recommendation of the FDA’s advisory committee
to approve our applications to market our C&C product candidates and any future product candidates in the United States,
and the EMA’s approval to market our C&C product candidates in the European Union, if such advisory committee reviews
are scheduled, without limiting the approved labeling, specifications, distribution or use of the product candidates, or imposing
other restrictions; |
| |
|
|
| |
● |
the
satisfaction of the FDA, EMA or other regulatory agencies with the safety and efficacy of C&C product candidates and any
future product candidates; |
| |
|
|
| |
● |
the
prevalence and severity of adverse events associated with C&C product candidates and any future product candidates; |
| |
|
|
| |
● |
the timely and satisfactory performance by third-party
contractors, trial sites and principal investigators of their obligations in relation to our clinical trials; |
| |
|
|
| |
● |
our
success in educating medical professionals and patients about the benefits, administration and use of our C&C product candidates
and any future product candidates, if approved; |
| |
|
|
| |
● |
the
availability, perceived advantages, relative cost, safety and efficacy of alternative and competing product for the indications
addressed by our C&C product candidates and any future product candidates; |
| |
|
|
| |
● |
the effectiveness of our marketing, sales and distribution
strategy, and operations, as well as that of any current and future licensees; |
| |
|
|
| |
● |
our ability to scale, validate and maintain a commercially
viable manufacturing process that is cGMP-compliant; |
| |
|
|
| |
● |
our
ability to obtain, protect and enforce our intellectual property rights with respect to our C&C product candidates, any future
product candidates and our C&C technology; and |
| |
|
|
| |
● |
changes to regulatory guidelines. |
Many
of these clinical, regulatory and commercial risks are beyond our control. Accordingly, we cannot assure you that we will be able to
advance our C&C product candidates and any future product candidates through clinical development, or to obtain regulatory approval
of or commercialize any product candidates. If we fail to achieve these objectives or overcome the challenges presented above, we could
experience significant delays or an inability to successfully commercialize our C&C product candidates and any future product candidates.
Accordingly, we may not be able to generate sufficient revenues through the sale of our product candidates to enable us to continue our
business.
Additionally,
approval of a product candidate in the United States by the FDA does not ensure approval of such product candidate by regulatory authorities
in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities
in other foreign countries or by the FDA. We may never obtain approval outside of the United States, which would limit our market opportunities
and adversely affect our business.
Regulatory
approval processes of the FDA, EMA and comparable foreign regulatory authorities are lengthy, time-consuming and unpredictable, and if
we are ultimately unable to obtain regulatory approval for our current product candidates or any future product candidates, our
business may fail.
The
research, development, testing, manufacturing, labeling, packaging, approval, promotion, advertising, storage, recordkeeping, marketing,
distribution, post-approval monitoring and reporting and export and import of drug product candidates are subject to extensive regulation
by the FDA, the EMA and by foreign regulatory authorities in other countries. These regulations differ from country to country. To gain
approval to market our T&T platform product candidates and future product candidates, we must provide data from well-controlled clinical
trials that adequately demonstrate the safety and efficacy of the product candidates for the intended indication to the satisfaction
of the FDA, EMA or other regulatory authority. We have not yet obtained regulatory approval to market any product in the United States
or any other jurisdiction. The FDA, EMA or other regulatory agencies can delay, limit or deny approval of our T&T platform product
candidates or any future product candidate for many reasons, including:
| |
● |
regulatory requests for additional analyses, reports,
data, non-clinical and preclinical studies and clinical trials; |
| |
|
|
| |
● |
our inability to demonstrate that a product candidate
is safe and effective for the target indication to the satisfaction of the FDA, EMA or other regulatory agencies; |
| |
|
|
| |
● |
the FDA’s, EMA’s, or other regulatory agencies’
disagreement with our trial protocol, the interpretation of data from preclinical studies or clinical trials, or adequacy of the
conduct and control of clinical trials; |
| |
|
|
| |
● |
clinical holds, other regulatory objections to commencing
or continuing a clinical trial or the inability to obtain regulatory approval to commence a clinical trial in countries that require
such approvals; |
| |
|
|
| |
● |
the population studied in the clinical trial may not
be sufficiently broad or representative to assess safety in the patient population for which we seek approval; |
| |
|
|
| |
● |
unfavorable or inconclusive results of clinical trials
and supportive non-clinical studies, including unfavorable results regarding safety or efficacy of a product candidate observed in
clinical trials; |
| |
|
|
| |
● |
our inability to demonstrate that clinical or other
benefits of a product candidate outweighs any safety or other perceived risks; |
| |
|
|
| |
● |
any determination that a clinical trial presents unacceptable
health risks to subjects; |
| |
|
|
| |
● |
our inability to obtain approval from institutional
review boards, or IRBs, to conduct clinical trials at their respective sites; |
| |
|
|
| |
● |
the FDA’s determination that the regulatory pathways
under FFDCA Section 505(b)(2), or Section 505(b)(2), or Section 510(k) are not available for a product candidate; |
| |
|
|
| |
● |
the non-approval of the formulation, labeling or the
specifications of a product candidate; |
| |
|
|
| |
● |
the failure to accept the manufacturing processes or
facilities at of third-party manufacturers with which we contract; |
| |
|
|
| |
● |
the potential for approval policies or regulations
of the FDA, EMA or other regulatory agencies to significantly change in a manner rendering our clinical data insufficient for approval;
or |
| |
|
|
| |
● |
resistance to approval from the advisory committees
of the FDA, EMA or other regulatory agencies for any reason including safety or efficacy concerns. |
In
the United States, we will be required to submit an NDA to obtain FDA approval before marketing our T&T platform product candidate.
An NDA must include extensive preclinical and clinical data and supporting information to establish the product candidate’s safety
and efficacy for each desired indication. In the case of an NDA covered by Section 505(b)(2), we may rely in part on data not developed
by us and for which we have not obtained a right of reference or use, including published scientific literature or the FDA’s findings
of safety and/or effectiveness for a previously approved drug. The NDA must also include significant information regarding the chemistry,
manufacturing and controls for the product candidates. The FDA may further inspect our manufacturing facilities to ensure that the facilities
can manufacture any product candidate and any product candidates, if and when approved, in compliance with the applicable regulatory
requirements, as well as inspect our clinical trial sites to ensure that our trials are properly conducted. Obtaining approval of an
NDA is a lengthy, expensive and uncertain process, and approval may not be obtained. Upon submission of an NDA, the FDA must make an
initial determination that the application is sufficiently complete to accept the submission for filing. We cannot be certain that any
submissions will be accepted for filing and review by the FDA, or ultimately be approved. If the application is not accepted for review
or approval, the FDA may require that we conduct additional clinical or preclinical trials, or take other actions before it will reconsider
our application. If the FDA requires additional trials or data, we would incur increased costs and delays in the marketing approval process,
which may require us to expend more resources than we have available.
Regulatory
authorities outside of the United States, such as in the European Union, also have requirements for approval of drugs for commercial
sale with which we must comply prior to marketing in those areas. Regulatory requirements can vary widely from country to country and
could delay or prevent the introduction of a product candidate. Clinical trials conducted in one country may not be accepted by regulatory
authorities in other countries, and obtaining regulatory approval in one country does not mean that regulatory approval will be obtained
in any other country. However, the failure to obtain regulatory approval in one jurisdiction could have a negative impact on our ability
to obtain approval in a different jurisdiction. Approval processes vary among countries and can involve additional product candidate
testing and validation and additional administrative review periods. Seeking foreign regulatory approval could require additional non-clinical
studies or clinical trials, which could be costly and time consuming. Foreign regulatory approval may include all of the risks associated
with obtaining FDA approval. For all of these reasons, if we seek foreign regulatory approval for any product candidate, we may not obtain
such approvals on a timely basis, if at all.
Even
if we eventually complete clinical testing and receive approval of any regulatory filing for a product candidate, the FDA may grant approval
contingent on the performance of costly and potentially time-consuming additional post-approval clinical trials or subject to contraindications,
black box warnings, restrictive surveillance or Risk Evaluation and Mitigation Strategies, or REMS. Further, the FDA, EMA or other foreign
regulatory authorities may also approve a product candidate for a more limited indication or a narrower patient population than we originally
requested, and these regulatory authorities may not approve the labeling that we believe is necessary or desirable for the successful
commercialization of any product candidate. Following any approval for commercial sale of a product candidate, certain changes to the
product candidates, such as changes in manufacturing processes and additional labeling claims, as well as new safety information, will
be subject to additional FDA notification, or review and approval. Also, regulatory approval for any product candidate may be withdrawn.
To the extent we seek regulatory approval in foreign countries, we may face challenges similar to those described above with regulatory
authorities in applicable jurisdictions. Any delay in obtaining, or inability to obtain, applicable regulatory approval for our T&T
platform product candidates or any future product candidate would delay or prevent commercialization of such product candidate and would
thus negatively impact our business, results of operations and prospects. While we are only in the early stages of pre-clinical development
for our T&T platform, the foregoing and similar regulatory risks may impact our business, results of operations and prospects as
we progress with the development of our T&T platform.
Clinical
drug development is difficult to design and implement and involves a lengthy and expensive process with uncertain outcomes.
Clinical
testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Additionally, we are only in the early
stages of pre-clinical development for our T&T platform. A failure of one or more of our clinical trials can occur at any time during
the clinical trial process. We do not know whether future clinical trials, if any, will begin on time, need to be redesigned, enroll
an adequate number of patients on time or be completed on schedule, if at all. Clinical trials can be delayed, suspended or terminated
for a variety of reasons, including failure to:
| |
● |
generate sufficient preclinical, toxicology, or other in
vivo or in vitro data to support the initiation or continuation of clinical trials; |
| |
|
|
| |
● |
obtain regulatory approval, or feedback on trial design,
in order to commence a trial; |
| |
|
|
| |
● |
identify, recruit and train suitable clinical investigators; |
| |
|
|
| |
● |
reach agreement on acceptable terms with prospective
CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among CROs and
clinical trial sites, and have such CROs and sites effect the proper and timely conduct of our clinical trials; |
| |
|
|
| |
● |
obtain and maintain IRB approval at each clinical trial
site; |
| |
|
|
| |
● |
identify, recruit and enroll suitable patients to participate
in a trial; |
| |
|
|
| |
● |
have a sufficient number of patients complete a trial
or return for post-treatment follow-up; |
| |
|
|
| |
● |
ensure clinical investigators and clinical trial sites
observe trial protocol or continue to participate in a trial; |
| |
|
|
| |
● |
address any patient safety concerns that arise during
the course of a trial; |
| |
|
|
| |
● |
address any conflicts with new or existing laws or
regulations; |
| |
|
|
| |
● |
add a sufficient number of clinical trial sites; |
| |
|
|
| |
● |
manufacture sufficient quantities at the required quality
of product candidate for use in clinical trials; or |
| |
|
|
| |
● |
raise sufficient capital to fund a trial. |
We
may also experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to
receive marketing approval or commercialize any product candidate, including:
| |
● |
we may receive feedback from regulatory authorities
that requires us to modify the design of our clinical trials; |
| |
|
|
| |
● |
clinical trials of a product candidate may produce
negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon
drug development programs; |
| |
|
|
| |
● |
the number of patients required for clinical trials
of a product candidate may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate or
participants may drop out of these clinical trials at a higher rate than we anticipate; |
| |
|
|
| |
● |
our third-party contractors may fail to comply with
regulatory requirements or meet their contractual obligations to us in a timely manner, or at all; |
| |
|
|
| |
● |
regulators or IRBs may not authorize us or our investigators
to commence a clinical trial or conduct a clinical trial at a prospective trial site or amend a trial protocol; |
| |
|
|
| |
● |
we may have delays in reaching or fail to reach agreement
on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites and CROs; |
| |
|
|
| |
● |
we
or our investigators might have to suspend or terminate clinical trials of a product candidate for various reasons, including
non- compliance with regulatory requirements, a finding that a product candidate have undesirable side effects or other unexpected
characteristics, or a finding that the participants are being exposed to unacceptable health risks; |
| |
|
|
| |
● |
the cost of clinical trials of a product candidate
may be greater than we anticipate; |
| |
|
|
| |
● |
the supply or quality of a product candidate or other
materials necessary to conduct clinical trials of such product candidate may be insufficient or inadequate; |
| |
|
|
| |
● |
there may be changes in government regulations or administrative
actions; |
| |
|
|
| |
● |
a product candidate may have undesirable adverse effects
or other unexpected characteristics; |
| |
|
|
| |
● |
we may not be able to demonstrate that a produce candidate’s
clinical and other benefits outweigh its safety risks; |
| |
|
|
| |
● |
we may not be able to demonstrate that a product candidate
provides an advantage over current standards of care of future competitive therapies in development; |
| |
|
|
| |
● |
regulators may revise the requirements for approving
a product candidate, or such requirements may not be as we anticipate; and |
| |
|
|
| |
● |
any future collaborators that conduct clinical trials
may face any of the above issues, and may conduct clinical trials in ways they view as advantageous to them but that are suboptimal
for us. |
In
addition, disruptions caused by any resurgence of the COVID-19 pandemic may increase the likelihood that we encounter such difficulties
in initiating, enrolling, conducting or completing our planned and ongoing clinical trials. We may also encounter delays if a clinical
trial is suspended or terminated by us, by the IRBs of the institutions in which such trials are being conducted, by the trial’s
data safety monitoring board, by the FDA, EMA or other regulatory agencies. Such authorities may suspend or terminate one or more of
our clinical trials due to a number of factors, including our failure to conduct the clinical trial in accordance with relevant regulatory
requirements or clinical protocols, inspection of the clinical trial operations or site by the FDA, EMA or other regulatory agencies
resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from
using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial.
Further,
conducting clinical trials outside of the United States, as we plan to do for our product candidates and any future product candidates,
presents additional risks that may delay completion of our clinical trials. These risks include the failure of enrolled patients in the
countries outside of the United States to adhere to clinical protocol as a result of differences in healthcare services or cultural customs,
managing additional administrative burdens associated with foreign regulatory schemes, as well as political and economic risks relevant
to such foreign countries.
If
we experience delays in completing any clinical trial of a product candidate or successfully obtaining regulatory approval, the commercial
prospects of such product candidate may be harmed, and our ability to generate product candidates revenues from such product candidate
will be delayed. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development
and approval process and jeopardize our ability to commence product candidates sales and generate revenues. Any of these occurrences
may significantly harm our business and financial condition. In addition, many of the factors that cause, or lead to, a delay in the
commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
If
the FDA does not conclude that our T&T platform product candidate satisfies the requirements under Section 505(b)(2) of
the FFDCA, or Section 505(b)(2), or if we are unable to utilize the hybrid application pathway in the European Union, or if the
requirements are not as we expect, the approval pathway for our T&T platform product candidates will likely take significantly
longer, cost significantly more and entail significantly greater complications and risks than anticipated, and in either case may not
be successful.
While
we are only in the early stages of pre-clinical development for our T&T platform product candidates, we intend to utilize the FDA’s
Section 505(b)(2) regulatory pathway, and the hybrid application pathway in the European Union, which is analogous to the Section 505(b)(2)
pathway, to seek approval of our T&T platform product candidates. The Drug Price Competition and Patent Term Restoration Act of 1984,
also known as the Hatch-Waxman Act, added Section 505(b)(2) to the FFDCA. Section 505(b)(2) permits the filing of an NDA where
at least some of the information required for approval comes from trials or studies that were not conducted by or for the applicant,
and for which the applicant has not received a right of reference or use from the person by or for whom the investigations were conducted,
which we believe could expedite the development program for our T&T platform product candidates by potentially decreasing the
amount of preclinical and clinical data that we would need to generate in order to obtain FDA approval. However, while we believe that
our T&T platform product candidates is a reformulation of an already-approved drug and, therefore, will be eligible for submission
of an NDA under Section 505(b)(2), the FDA may disagree and determine that our T&T platform product candidates is not eligible
for review under such regulatory pathway.
If
we are unable to pursue these regulatory pathways as anticipated, we may need to conduct additional preclinical experiments and clinical
trials, provide additional data and information and meet additional standards for regulatory approval. If this were to occur, the time
and financial resources required to obtain FDA approval for T&T platform product candidates, and complications and risks associated
with T&T platform product candidates, would likely increase significantly. Moreover, inability to pursue the Section 505(b)(2) or
similar regulatory pathway could result in new competitive products reaching the market more quickly than T&T platform product candidates
or any future product candidates, which would likely harm our competitive position and prospects. Even if we are allowed to pursue the
Section 505(b)(2) or similar regulatory pathway, T&T platform product candidates may not receive the requisite approvals for commercialization,
and there is no guarantee the 505(b)(2) or similar pathway would ultimately lead to faster product candidates’ development or earlier
approval.
In
addition, notwithstanding the approval of a number of products by the FDA under Section 505(b)(2) over the last few years, certain competitors
and others have objected to the FDA’s interpretation of Section 505(b)(2). If the FDA’s interpretation of Section 505(b)(2)
is successfully challenged, the FDA may be required to change its 505(b)(2) policies and practices, which could delay or even prevent
the FDA from approving any NDA that we submit under Section 505(b)(2). In addition, the pharmaceutical industry is highly competitive,
and Section 505(b)(2) NDAs are subject to special requirements designed to protect the patent rights of sponsors of previously approved
drugs that are referenced in a Section 505(b)(2) NDA. These requirements may give rise to patent litigation and mandatory delays in approval
of our potential future NDAs for up to 30 months depending on the outcome of any litigation. It is also not uncommon for a manufacturer
of an approved product to file a citizen petition with the FDA seeking to delay approval of, or impose additional approval requirements
for, pending competing product candidates. If successful, such petitions can significantly delay, or even prevent, the approval of the
new product candidates. However, even if the FDA ultimately denies such a petition, the FDA may substantially delay approval while it
considers and responds to the petition.
Moreover,
even if our T&T platform product candidates or any future product candidates are approved under the Section 505(b)(2) pathway, as
the case may be, the approval may be subject to limitations on the indicated uses for which the product candidates may be marketed or
to other conditions of approval, or may contain requirements for costly post-marketing testing and surveillance to monitor the safety
or efficacy of the product candidates.
Our
C&C and T&T technologies are novel technologies, which makes it difficult to accurately and reliably predict the time and cost
of development and of subsequently obtaining regulatory approval of our C&C and T&T technologies product candidates or any future
product candidates.
We
have concentrated our efforts and product candidates research on our technologies, and our future success depends on the successful development
of these technologies and product candidates based on them. There can be no assurance that any development problems we experience in
the future related to our product candidates will not cause significant delays or unanticipated costs, or that such development problems
can be solved. We may be unable to maintain and further develop sustainable, reproducible and scalable manufacturing processes, or transfer
these processes to collaborators, which may prevent us from completing our clinical studies or commercializing our product candidates
on a timely or profitable basis, if at all. To our knowledge, no regulatory authority has granted approval to any person or entity, including
us, to market and commercialize therapeutics using our novel delivery system. We may never receive approval to market and commercialize
any product candidates that utilize our technologies.
As
an organization, we have not previously conducted pivotal clinical trials, and we may be unable to do so for any product candidates we
may develop, including our T&T platform product candidates.
We
will need to successfully complete pivotal clinical trials in order to obtain the approval of the FDA, EMA or other regulatory agencies
to market our T&T platform product candidates or any future product candidates. Carrying out later-stage clinical trials and the
submission of a successful NDA is a complicated process. As an organization, we have not previously conducted any later stage or pivotal
clinical trials and have limited experience in preparing, submitting and prosecuting regulatory filings. Consequently, we may be unable
to successfully and efficiently execute and complete necessary clinical trials, including our ongoing Phase 3 trials, in a way that
leads to marketing approval of our T&T platform product candidates. We may require more time and incur greater costs than our competitors
and may not succeed in obtaining regulatory approvals of product candidates that we develop. Failure to commence or complete, or delays
in, our planned clinical trials, could prevent us from or delay us in commercializing our T&T platform product candidates, which
are in early stages of pre-clinical development. See “Risks Related to Our Reliance on Third Parties.” We rely on third parties
to conduct certain elements of our preclinical and clinical trials and perform other tasks for us. If these third parties do not successfully
carry out their contractual duties, meet expected deadlines or comply with regulatory requirements, we may not be able to obtain regulatory
approval for or commercialize our product candidates.
We may
find it difficult to enroll patients in our clinical trials due to various reasons, including possible disruption due to any resurgence
of the COVID-19 pandemic, which could delay or prevent us from proceeding with such trials.
Identifying
and qualifying patients to participate in our clinical trials is critical to our success. The timing of our clinical trials depends in
part on the speed at which we can recruit patients to participate in testing our product candidates, and we may experience delays in
our clinical trials if we encounter difficulties in enrollment. Patient enrollment and retention in clinical trials depends on many factors,
including the size of the patient population, the nature of the trial protocol, our ability to recruit clinical trial investigators with
the appropriate competencies and experience, the existing body of safety and efficacy data with respect to the study drug, the number
and nature of competing product candidates and ongoing clinical trials of competing drugs for the same indication, the proximity of patients
to clinical sites, clinicians’ and patients’ perceptions as to the potential advantages of the product candidate being studied
in relation to other available therapies, including any drugs that may be approved for the indications we are investigating, the eligibility
criteria for the trials, our ability to obtain and maintain patient consents and the risk that patients enrolled in clinical trials will
drop out of the trials before completion.
We
may not be able to identify, recruit and enroll a sufficient number of patients to complete our clinical trials because of the perceived
risks and benefits of the product candidate under study, the availability and efficacy of competing therapies and clinical trials, the
proximity and availability of clinical trial sites for prospective patients and the patient referral practices of physicians. We may
also face challenges in identifying trial sites and enrolling patients in global trials such as our ongoing and planned clinical trials
in the fourth quarter of 2025 for our C&C product candidates. If patients are unwilling to participate in our trials for any reason,
the timeline for recruiting patients, conducting trials and obtaining regulatory approval of potential product candidates will be delayed.
Our
product candidates and the administration of our product candidates may cause undesirable side effects or have other properties that
could delay or prevent their regulatory approval, limit the commercial profile of an approved label or result in significant negative
consequences following marketing approval, if any.
Undesirable
side effects, including toxicology, caused by product candidates or any future product candidates, or the drugs encapsulated by such
product candidates, could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive
label or the delay or denial of regulatory approval by the FDA, EMA or other regulatory agencies. Results of our trials could reveal
a high and unacceptable severity and prevalence of these or other side effects. In such an event, our clinical studies could be suspended
or terminated, and the FDA, EMA or other regulatory agencies could order us to cease further development of or deny or withdraw approval
of our product candidates for any or all targeted indications. Moreover, during the conduct of clinical trials, patients report changes
in their health, including illnesses, injuries and discomforts, to their study doctor. Often, it is not possible to determine whether
or not the product candidate being studied caused these conditions.
Drug-related,
formulation-related and administration-related side effects could affect patient recruitment, the ability of enrolled patients to complete
the clinical trials or result in potential product candidates liability claims, which could exceed our clinical trial insurance coverage.
We do not currently have product candidates liability insurance and do not anticipate obtaining product candidates liability insurance
until such time as we have received FDA, EMA or other comparable foreign authority marketing approval for one of our product candidates
and such product candidates is being provided to patients outside of clinical trials.
Additionally,
if one or more of our product candidates receives marketing approval, and we or others later identify undesirable side effects caused
by such product candidates, a number of potentially significant negative consequences could result, including but not limited to:
| |
● |
regulatory
authorities may suspend or withdraw approvals of such product candidates; |
| |
|
|
| |
● |
regulatory authorities may require additional warnings
on the label, such as a “black box” warning or contraindication; |
| |
|
|
| |
● |
additional restrictions may be imposed on the marketing
of the particular product candidates or the manufacturing processes for the product candidates or any component thereof; |
| |
|
|
| |
● |
we may be required to create a REMS, which could include
a medication guide outlining the risks of such side effects for distribution to patients, a communication plan for healthcare providers
and/or other elements to assure safe use; |
| |
|
|
| |
● |
we
may be required to recall a product candidates, change the way a product candidate is administered or conduct additional clinical
trials; |
| |
|
|
| |
● |
we could be sued and held liable for harm caused to
patients; |
| |
|
|
| |
● |
the
product candidates may become less competitive; and |
| |
|
|
| |
● |
our reputation may suffer. |
While
we are only in the early stages of pre-clinical development for our T&T platform, any of these events could prevent us from achieving
or maintaining market acceptance of the particular product candidate, if approved, and could significantly harm our business, results
of operations and prospects.
Even
if we complete the necessary clinical trials, we cannot predict when, or if, we will obtain regulatory approval to commercialize any
of our product candidates, and the approval may be for a more narrow indication than we seek or be subject to other limitations or restrictions
that limit its commercial profile.
We
cannot commercialize a product candidate until the appropriate regulatory authorities have reviewed and approved the product candidate.
Even if our current or future product candidates meet safety and efficacy endpoints in pivotal clinical trials, the regulatory authorities
may not complete their review processes in a timely manner, or we may not be able to obtain regulatory approval. Additional delays may
result if an FDA Advisory Committee or other regulatory authority recommends non-approval or restrictions on approval. This may include
approval of a product candidate for more limited indications than requested or they may impose significant limitations in the form of
warnings. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or
administrative action, or changes in regulatory authority policy during the period of product candidates’ development, clinical
trials and the review process.
Regulatory
authorities also may approve a product candidate for more limited indications than requested or they may impose significant limitations
in the form of warnings or a REMS. These regulatory authorities may require precautions or contra-indications with respect to conditions
of use or they may grant approval subject to the performance of costly post-marketing clinical trials. In addition, regulatory authorities
may not approve the labeling claims that are necessary or desirable for the successful commercialization of any of our product candidates.
While we are only in the early stages of pre-clinical development for our T&T platform, the foregoing and similar regulatory risks
may impact our business, results of operations and prospects as we progress with the development of our T&T platform.
Even
if we obtain regulatory approval for a product candidate, our product candidates and business will remain subject to ongoing regulatory
obligations and review.
While
we are only in the early stages of pre-clinical development for our T&T platform, if our product candidates are approved, they will
be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping,
conduct of post-marketing studies and submission of safety, efficacy and other post- market information, including both federal and state
requirements in the United States and comparable requirements outside of the United States. Accordingly, we and others with whom we work
must continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production and quality
control.
Any
regulatory approvals that we receive for our product candidates may also be subject to limitations on the cleared intended use(s) for
which the product candidates may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing
testing, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of the product candidate. The FDA may
also require a REMS as a condition of approval of our product candidates, which could include requirements for a medication guide, physician
communication plans or additional elements to ensure safe use, such as restricted distribution methods, patient registries and other
risk minimization tools. We will also be required to report certain adverse reactions and production problems, if any, to the FDA, EMA
or other regulatory agencies and to comply with requirements concerning advertising and promotion for our product candidates. Promotional
communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent
with the information in the product candidates’ approved label. As such, we may not promote our product candidates for indications
or uses for which they do not have FDA, EMA or other regulatory agency approval. The holder of an approved NDA must also submit new or
supplemental applications and obtain FDA approval for certain changes to the approved product candidates, product candidates labeling,
or manufacturing process. We could also be asked to conduct post-marketing clinical trials to verify the safety and efficacy of our product
candidates in general or in specific patient subsets. An unsuccessful post-marketing trial or failure to complete such a clinical trial
could result in the withdrawal of marketing approval. Furthermore, any new legislation addressing drug safety issues could result in
delays in product candidates’ development or commercialization or increased costs to assure compliance. Foreign regulatory authorities
impose similar requirements. If a regulatory agency discovers previously unknown problems with a product candidate, such as adverse events
of unanticipated severity or frequency, or disagrees with the promotion, marketing or labeling of product candidates, such regulatory
agency may impose restrictions on that product candidates or us, including requiring withdrawal of the product candidates from the market.
If we fail to comply with applicable regulatory requirements, a regulatory agency or enforcement authority may, among other things:
| |
● |
issue warning letters asserting that we are in violation
of the law; |
| |
● |
seek an injunction or impose civil or criminal penalties
or monetary fines; |
| |
● |
suspend or withdraw regulatory approval; |
| |
|
|
| |
● |
suspend any of our ongoing clinical trials; |
| |
|
|
| |
● |
refuse to approve pending applications or supplements
to approved applications submitted by us or our strategic partners; |
| |
|
|
| |
● |
restrict
the marketing or manufacturing of our product candidates; |
| |
|
|
| |
● |
seize
or detain product candidates, or require a product candidates recall; |
| |
|
|
| |
● |
refuse to permit the import or export of our product
candidates; or |
| |
|
|
| |
● |
refuse to allow us to enter into government contracts. |
Any
government investigation of alleged violations of law could require us to expend significant time and resources in response and could
generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability
to commercialize and generate revenue from our product candidates. If regulatory sanctions are applied or if regulatory approval is withdrawn,
the value of our company and our operating results will be adversely affected.
The
FDA’s and other regulatory authorities’ policies may change and additional government regulations may be enacted that could
prevent, limit or delay regulatory approval of our product candidates. If we are slow or unable to adapt to changes in existing requirements
or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval
that we may have obtained and we may not achieve or sustain profitability, which would adversely affect our business, prospects, financial
condition and results of operations.
We
also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative
or executive action, either in the United States or abroad.
Disruptions
at the FDA and other government agencies caused by funding shortages or global health concerns could hinder their ability to hire, retain
or deploy key leadership and other personnel, or otherwise prevent new or modified product candidates from being developed, approved
or commercialized in a timely manner or at all, which could negatively impact our business.
The
ability of the FDA to review and approve new product candidates can be affected by a variety of factors, including government budget
and funding levels, statutory, regulatory, and policy changes, the FDA’s ability to hire and retain key personnel and accept the
payment of user fees, and other events that may otherwise affect the FDA’s ability to perform routine functions. Average review
times at the agency have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund
research and development activities is subject to the political process, which is inherently fluid and unpredictable. Disruptions at
the FDA and other agencies may also slow the time necessary for new drugs or modifications to approved drugs to be reviewed and/or approved
by necessary government agencies, which would adversely affect our business. For example, over the last several years, including for
35 days beginning on December 22, 2018, the U.S. government has shut down several times and certain regulatory agencies, such as the
FDA, have had to furlough critical FDA employees and stop critical activities.
Separately,
in response to the COVID-19 pandemic, in March 2020, the FDA announced the postponement of most foreign and routine surveillance inspections
of domestic manufacturing facilities, and only restarted domestic inspections on a risk-based basis in July 2020. Regulatory authorities
outside the United States have adopted similar restrictions or other policy measures in response to the COVID-19 pandemic.
If
a prolonged government shutdown occurs, or if global health concerns continue to prevent the FDA or other regulatory authorities from
conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of the FDA or
other regulatory authorities to timely review and process our regulatory submissions, which could have a material adverse effect on our
business.
We
may be subject, directly or indirectly, to U.S. federal and state healthcare fraud and abuse laws, false claims laws, physician payment
transparency laws and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such
laws, we could face substantial penalties.
Our
current and future operations may be directly or indirectly through our relationships with U.S. healthcare providers, patients and other
persons and entities, subject to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain our
business or financial arrangements and relationships through which we research, market, sell and distribute our product candidates in
the United States. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which
we conduct our business. The laws that may affect our ability to operate include:
| |
● |
The U.S. Anti-Kickback Statute prohibits, among other
things, any person or entity from knowingly and willfully offering, paying, soliciting, receiving or providing any remuneration,
directly or indirectly, overtly or covertly, to induce or in return for purchasing, leasing, ordering or arranging for or recommending
the purchase, lease or order of any item or service reimbursable, in whole or in part, under Medicare, Medicaid or other U.S. federal
healthcare programs. The U.S. Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers
on the one hand and prescribers, purchasers, and formulary managers, among others, on the other. There are a number of statutory
exceptions and regulatory safe harbors protecting some common activities from prosecution, but the exceptions and safe harbors are
drawn narrowly and require strict compliance in order to offer protection. |
| ● | The
U.S. federal false claims laws, including the False Claims Act, or FCA, and civil monetary
penalties laws, which prohibit any person or entity from, among other things, knowingly presenting,
or causing to be presented, a false, fictitious or fraudulent claim for payment to, or approval
by, the U.S. federal government or knowingly making, using or causing to be made or used
a false record or statement material to a false or fraudulent claim to the U.S. federal government.
As a result of a modification made by the Fraud Enforcement and Recovery Act of 2009, a claim
includes “any request or demand” for money or property presented to the U.S.
government. In addition, manufacturers can be held liable under the FCA even when they do
not submit claims directly to government third-party payors if they are deemed to “cause”
the submission of false or fraudulent claims. The FCA also permits a private individual acting
as a “whistleblower” to bring actions on behalf of the federal government alleging
violations of the FCA and to share in any monetary recovery. FCA liability is potentially
significant in the healthcare industry because the statute provides for treble damages and
mandatory penalties per false claim or statement. Government enforcement agencies and private
whistleblowers have investigated pharmaceutical companies for or asserted liability under
the FCA for a variety of alleged promotional and marketing activities, such as providing
free products to customers with the expectation that the customers would bill federal programs
for the products; providing consulting fees and other benefits to physicians to induce them
to prescribe products; engaging in promotion for “off-label” uses; and submitting
inflated best price information to the Medicaid Rebate Program. |
| | | |
| |
● |
The U.S. Health Insurance Portability and Accountability
Act of 1996, or HIPAA, prohibits, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud
any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare
benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying,
concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the
delivery of or payment for healthcare benefits, items or services. |
| |
|
|
| |
● |
The Physician Payments Sunshine Act, enacted as part
of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively,
the ACA, imposes, among other things, annual reporting requirements for covered manufacturers for certain payments and “transfers
of value” provided to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching
hospitals, as well as ownership and investment interests held by the physicians described above and their immediate family members.
Beginning in 2022, applicable manufacturers also will be required to report such information regarding payments and transfers of
value provided during the previous year to physician assistants, nurse practitioners, clinical nurse specialists, anesthesiologist
assistants, certified nurse anesthetists and certified nurse midwives. |
| |
● |
HIPAA, as amended by the Health Information Technology
for Economic and Clinical Health Act, or HITECH, and their respective implementing regulations, impose, among other things, specified
requirements relating to the privacy, security and transmission of individually identifiable health information held by covered entities,
which include certain healthcare providers, health plans and healthcare clearinghouses, and their business associates, which include
individuals or entities that perform services for covered entities that involve the creation, use, maintenance or disclosure of,
individually identifiable health information as well as their covered subcontractors. HITECH also created new tiers of civil monetary
penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys
general new authority to file civil actions for damages or injunctions in U.S. federal courts to enforce the federal HIPAA laws and
seek attorneys’ fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and
security of health information in specified circumstances, many of which differ from each other in significant ways and may not have
the same effect, thus complicating compliance efforts. |
| |
|
|
| |
● |
European and other foreign law equivalents of each
of the laws, including reporting requirements detailing interactions with and payments to healthcare providers. |
Many
states have analogous state laws and regulations, such as state anti-kickback and false claims laws, that may apply to our business practices,
including but not limited to, research, distribution, sales or marketing arrangements and claims involving healthcare items or services
reimbursed by non-governmental third-party payors, including private insurers. In addition, certain states require pharmaceutical companies
to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by
the U.S. federal government. Certain states and local jurisdictions require drug manufacturers to report information related to payments
and other transfers of value to physicians and other healthcare providers and register pharmaceutical sales representatives. Additionally,
certain states also require pharmaceutical companies to file reports relating to pricing information or marketing expenditures and have
laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant
ways and may not have the same effect, thus complicating compliance efforts.
Because
of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our
business activities could be subject to challenge under one or more of such laws. In addition, the ACA has strengthened these laws. For
example, health care reform legislation, has among other things, amended the intent requirement of the U.S. Anti-Kickback statute and
certain criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent
to violate it. Moreover, the ACA provides that the government may assert that a claim including items or services resulting from a violation
of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
Ensuring
that our internal operations and business arrangements with third parties comply with applicable healthcare laws and regulations will
likely be costly. It is possible that governmental authorities will conclude that our business practices, including arrangements we may
have with physicians and other healthcare providers, some of whom may receive stock options as compensation for services provided, do
not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and
regulations. If our operations were found to be in violation of any of these laws or any other governmental regulations that may apply
to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, possible
exclusion from government funded healthcare programs, contractual damages, reputational harm, diminished profits and future earnings,
additional reporting obligations and oversight if we become subject to a corporate integrity agreement or other agreement to resolve
allegations of non-compliance with these laws, and curtailment of our operations, any of which could substantially disrupt our operations.
If the physicians or other providers or entities with whom we expect to do business are found not to be in compliance with applicable
laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Legislative
or regulatory healthcare reforms in the United States may make it more difficult and costly for us to obtain regulatory clearance or
approval of our product candidates and to produce, market and distribute our product candidates after clearance or approval is obtained.
From
time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the
regulatory clearance or approval, manufacture and marketing of regulated products or the reimbursement thereof. In addition, FDA regulations
and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our product candidates.
Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of
our product candidates. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and
if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require:
| ● | changes
to manufacturing methods; |
| ● | change
in protocol design; |
| ● | additional
treatment arm (control); |
| ● | recall,
replacement, or discontinuance of one or more of our product candidates; and |
| ● | additional
recordkeeping. |
In
addition, in the United States, there have been a number of legislative and regulatory proposals to change the health care system in
ways that could affect our ability to sell our products profitably. The pharmaceutical industry in the United States, as an example,
has been affected by the passage of the ACA, which, among other things, imposed new fees on entities that manufacture or import certain
branded prescription drugs and expanded pharmaceutical manufacturer obligations to provide discounts and rebates to certain government
programs. There have been executive, judicial and Congressional challenges to certain aspects of the ACA. For example, on June 17, 2021,
the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the ACA is unconstitutional in its entirety because the
“individual mandate” was repealed by Congress. On August 16, 2022, President Biden signed the Inflation Reduction Act of
2022, or IRA, into law, which among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in
ACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning
in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and creating a new manufacturer discount program. It is
possible that the ACA will be subject to judicial or Congressional challenges in the future. It is unclear how any such challenges and
the healthcare reform measures of the Biden administration will impact the ACA and our business.
Other
legislative changes have been proposed and adopted in the United States since the ACA was enacted. These changes include, among others,
aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect in 2013 and, due to subsequent
legislative amendments to the statute, will remain in effect until 2032 unless additional Congressional action is taken. Congress is
considering additional health reform measures.
Further,
there has been particular and increasing legislative and enforcement interest in the United States with respect to drug pricing practices
in recent years, particularly with respect to drugs that have been subject to relatively large price increases over relatively short
time periods. There have been several recent U.S. Presidential executive orders, Congressional inquiries and proposed bills designed
to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship
between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs.
For
example, in July 2021, the Biden administration released an executive order, “Promoting Competition in the American Economy,”
with multiple provisions aimed at prescription drugs. In response to President Biden’s executive order, on September 9, 2021, the
Department of Health and Human Services, or HHS, released a Comprehensive Plan for Addressing High Drug Prices that outlines principles
for drug pricing reform and sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative
actions HHS can take to advance these principles. Further, the IRA, among other things (i) directs HHS to negotiate the price of certain
high-expenditure, single-source drugs and biologics covered under Medicare and (ii) imposes rebates under Medicare Part B and Medicare
Part D to penalize price increases that outpace inflation. These provisions will take effect progressively starting in fiscal year 2023.
On August 29, 2023, HHS announced the list of the first ten drugs that will be subject to price negotiations, although the Medicare drug
price negotiation program is currently subject to legal challenges. It is currently unclear how the IRA will be implemented but is likely
to have a significant impact on the pharmaceutical industry. Further in response to the Biden administration’s October 2022 executive
order, on February 14, 2023, HHS released a report outlining three new models for testing by the Centers for Medicare & Medicare
Services, or CMS, Innovation Center which will be evaluated on their ability to lower the cost of drugs, promote accessibility, and improve
quality of care. It is unclear whether the models will be utilized in any health reform measures in the future. Further, on December
7, 2023, the Biden administration announced an initiative to control the price of prescription drugs through the use of march-in rights
under the Bayh-Dole Act. On December 8, 2023, the National Institute of Standards and Technology published for comment a Draft Interagency
Guidance Framework for Considering the Exercise of March-In Rights which for the first time includes the price of a product as one factor
an agency can use when deciding to exercise march-in rights. While march-in rights have not previously been exercised, it is uncertain
if that will continue under the new framework. Individual states in the United States have also become increasingly active in passing
legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement
constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and in some cases,
designed to encourage importation from other countries and bulk purchasing. For example, on January 5, 2024, the FDA approved Florida’s
Section 804 Importation Program (SIP) proposal to import certain drugs from Canada for specific state healthcare programs. It is unclear
how this program will be implemented, including which drugs will be chosen, and whether it will be subject to legal challenges in the
United States or Canada. Other states have also submitted SIP proposals that are pending review by the FDA. Any such approved importation
plans, when implemented, may result in lower drug prices for products covered by those programs. In the future, there will likely continue
to be proposals relating to the reform of the U.S. healthcare system, some of which could further limit coverage and reimbursement of
drug products, including our product candidates. Any reduction in reimbursement from Medicare or other government programs may result
in a similar reduction in payments from private payors. Our results of operations could be adversely affected by the ACA and by other
health care reforms that may be enacted or adopted in the future.
We
face intense competition in an environment of rapid technological change and the possibility that our competitors may develop products
and drug delivery systems that are similar, more advanced or more effective than ours, which may adversely affect our financial condition
and our ability to successfully market or commercialize our product candidates.
The
medical device and pharmaceutical industry in which we operate is intensely competitive and subject to rapid and significant technological
change. We are currently aware of various existing therapies in the market and in development that may in the future compete with our
product candidates, for drug delivery mechanisms that T&T technology to deliver APIs at the local level. Other approaches may also
emerge for the prevention or treatment of any of the indications on which we focus, and new technologies may emerge in localized drug
delivery.
We
have competitors both in the United States and internationally, including major multinational medical device and pharmaceutical companies.
Our competitors may succeed in developing, acquiring or licensing on an exclusive basis products that are more effective or less costly
than any product candidate that we may develop, or achieve earlier patent protection, regulatory approval, product candidates commercialization
and market penetration than we do. Additionally, technologies developed by our competitors may render our potential product candidates
uneconomical or obsolete, and we may not be successful in marketing our product candidates against competitors.
Even
if we obtain and maintain approval for our T&T platform product candidates or our other product candidates from the FDA, we
may never obtain approval outside of the United States, which would limit our market opportunities and adversely affect our business.
Approval
of a product candidate in the United States by the FDA does not ensure approval of such product candidate by regulatory authorities in
other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities
in other foreign countries or by the FDA. However, the failure to obtain approval from the FDA or other regulatory authorities may negatively
impact our ability to obtain approval in other foreign countries. Sales of our T&T platform product candidates or our other
product candidates outside of the United States will be subject to foreign regulatory requirements governing clinical trials and marketing
approval. Even if the FDA grants marketing approval for a product candidate, comparable regulatory authorities of foreign countries also
must approve the manufacturing and marketing of the product candidate in those countries. Approval procedures vary among jurisdictions
and can involve requirements and administrative review periods different from, and more onerous than, those in the United States, including
additional preclinical studies or clinical trials. In many countries outside the United States, a product candidate must be approved
for reimbursement before it can be approved for sale in that country. In some cases, the price that we intend to charge for our product
candidates, if approved, is also subject to approval.
We
intend to submit a marketing authorization application to the EMA for approval of our C&C product candidates in the European Union,
but obtaining such approval from the European Commission following the opinion of the EMA is a lengthy and expensive process. Even if
a product candidate is approved, the applicable regulatory agency may limit the indications for which the product candidates may be marketed,
require extensive warnings on the product candidates labeling or require expensive and time-consuming additional clinical trials or reporting
as conditions of approval. Regulatory authorities in countries outside of the United States and the European Union also have requirements
for approval of product candidates with which we must comply prior to marketing in those countries. Obtaining foreign regulatory approvals
and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay
or prevent the introduction of our product candidates in certain countries.
Further,
clinical trials conducted in one country may not be accepted by regulatory authorities in other countries. Also, regulatory approval
for a product candidate may be withdrawn. If we fail to comply with the regulatory requirements, our target market will be reduced and
our ability to realize the full market potential of our T&T platform product candidates or our other product candidates will
be harmed and our business, financial condition, results of operations and prospects will be adversely affected.
The misuse
or off-label use of our product candidates may harm our reputation in the marketplace, result in injuries that lead to product candidates’
liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed to have engaged in the promotion
of these uses, any of which could be costly to our business.
Prescription
drugs may be promoted only for the approved indications in accordance with the approved label. The FDA and other agencies actively enforce
the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off- label
uses may be subject to significant liability. We will train our marketing and sales personnel to not promote our product candidates,
if approved, for any off-label uses. We cannot, however, prevent a physician from using our product candidates off-label, when in the
physician’s independent professional medical judgment he or she deems it appropriate.
If
the FDA, EMA or any foreign regulatory body determines that our promotional materials or training constitute promotion of an off-label
use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including
the issuance or imposition of an untitled letter, which is used for violators that do not necessitate a warning letter, injunction, seizure,
civil fine or criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action
under other regulatory authority, such as false claims laws, if they consider our business activities to constitute promotion of an off-label
use, which could result in significant penalties, including, but not limited to, criminal, civil and administrative penalties, damages,
fines, disgorgement, exclusion from participation in government healthcare programs and the curtailment of our operations.
Risks
Related to our Reliance on Third Parties
We
will rely on third parties to conduct certain elements of our preclinical studies and clinical trials and perform other tasks for us.
If these third parties do not successfully carry out their contractual duties, meet expected deadlines or comply with regulatory requirements,
we may not be able to obtain regulatory approval for or commercialize our product candidates.
We
will rely upon third-party vendors, including CROs, to monitor and manage data for our ongoing preclinical studies and clinical trials.
If our CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced
or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols,
regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain
regulatory approval for or successfully commercialize our product candidates. We will rely on these CROs for execution of our preclinical
studies and clinical trials, and we control only certain aspects of their activities. Nevertheless, we will be responsible for ensuring
that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our
reliance on the vendors and CROs does not relieve us of our regulatory responsibilities. We and our CROs and other vendors will be required
to comply with good clinical practice, cGMP, the Helsinki Declaration, the International Conference on Harmonization Guideline for Good
Clinical Practice, applicable European Commission Directives on Clinical Trials, laws and regulations applicable to clinical trials conducted
in other territories, and good laboratory practices, or GLP, which are regulations and guidelines enforced by the FDA, the Competent
Authorities of the Member States of the EEA, and comparable foreign regulatory authorities for all of our product candidates in clinical
development. Regulatory authorities enforce these regulations through periodic inspections of study sponsors, principal investigators,
study sites and other contractors. If we or any of our CROs or vendors fail to comply with applicable regulations, including Good Clinical
Practice, or GCP, and cGMP regulations, the clinical data generated in our clinical studies may be deemed unreliable and the FDA, EMA
or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications.
Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process.
If
any of our relationships with these third-party CROs or vendors terminate, we may not be able to enter into arrangements with alternative
CROs or vendors or do so on commercially reasonable terms. In addition, our CROs are not our employees, and, except for remedies available
to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our ongoing
clinical, nonclinical and preclinical programs. If CROs do not successfully carry out their contractual duties or obligations or meet
expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to
the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed
or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. CROs may
also generate higher costs than anticipated, which could adversely affect our results of operations and the commercial prospects for
our product candidates, increase our costs and delay our ability to generate revenue.
Replacing
or adding additional CROs involves additional cost and requires management time and focus. In addition, there is a natural transition
period when a new CRO commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical
development timelines. Though we carefully manage our relationships with our CROs, we may encounter similar challenges or delays in the
future, which could have a material adverse impact on our business, financial condition and prospects.
Independent
clinical investigators and CROs that we will engage to conduct our clinical trials may not devote sufficient time or attention to our
clinical trials or be able to repeat their past success.
We
will depend on third parties, including independent clinical investigators and CROs, to conduct our clinical trials. CROs may also assist
us in the collection and analysis of data. There is a limited number of third-party service providers and vendors that specialize or
have the expertise required to achieve our business objectives. Identifying, qualifying and managing performance of third-party service
providers can be difficult, time consuming and cause delays in our development programs.
These
investigators and CROs will not be our employees and we will not be able to control, other than through contract, the amount of resources,
including time, which they devote to our product candidates and clinical trials. If independent investigators or CROs fail to devote
sufficient resources to the development of our product candidates, or if their performance is substandard, it may delay or compromise
the prospects for approval and commercialization of any product candidates that we develop. Further, the performance of our CROs may
also be interrupted by any resurgence of the COVID-19 pandemic, including due to travel or quarantine policies, heightened exposure of
CRO staff who are healthcare providers to COVID-19 patients or prioritization of resources toward any resurgence of the COVID-19 pandemic.
Investigators
for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection
with such services. Under certain circumstances, we may be required to report some of these relationships to the FDA or other regulatory
authorities. The FDA or other regulatory authorities may conclude that a financial relationship between us and an investigator has created
a conflict of interest or otherwise affected interpretation of the trial. The FDA or other regulatory authorities may therefore question
the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized.
This could result in a delay in approval or rejection of our marketing applications by the FDA or other regulatory authorities, as the
case may be, and may ultimately lead to the denial of marketing approval of one or more of our product candidates.
In
addition, the use of third-party service providers requires us to disclose our proprietary information to these parties, which could
increase the risk that this information will be misappropriated. Further, the FDA and other regulatory authorities require that we comply
with standards, commonly referred to as GCP, for conducting, recording and reporting clinical trials to assure that data and reported
results are credible and accurate and that the rights, integrity and confidentiality of trial subjects are protected. Failure of clinical
investigators or CROs to meet their obligations to us or comply with GCP procedures could adversely affect the clinical data, the outcome
of the clinical studies or the development of our product candidates and harm our business.
We
rely on third parties to manufacture the raw materials that we use to create our product candidates. Our business could be harmed if
existing and prospective third parties fail to provide us with sufficient quantities of these materials and product candidates or fail
to do so at acceptable quality levels or prices.
We
rely on third party suppliers for certain raw materials necessary to manufacture our product candidates for our preclinical studies and
clinical trials. We do not have any control over the availability of raw materials. If we or our manufacturers are unable to purchase
these raw materials on acceptable terms, at sufficient quality levels, or in adequate quantities, if at all, the development and commercialization
of our product candidates or any future product candidates, would be delayed or there would be a shortage in supply, which would impair
our ability to meet our development objectives for our product candidates or generate revenues from the sale of any approved product
candidates.
Even
following our establishment of our own cGMP-compliant manufacturing capabilities, we intend to continue to rely on third party suppliers
for these raw materials, which will continue to expose us to manufacturing risks including:
| |
● |
reduced control for certain aspects of manufacturing
activities; |
| |
|
|
| |
● |
termination or nonrenewal of manufacturing and service
agreements with third parties in a manner or at a time that is costly or damaging to us; and |
| |
|
|
| |
● |
disruptions to the operations of our third-party manufacturers
and service providers caused by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer
or service provider. |
Certain
of our raw material suppliers will be required to become cGMP-compliant and establish a drug master file for the applicable ingredient
before we can submit an NDA for our T&T platform product candidates. If these suppliers do not successfully carry out their contractual
duties or manufacture our raw materials in accordance with regulatory requirements, we will not be able to submit our NDA as planned
or complete, or may be delayed in completing, the clinical trials required for approval of our T&T platform product candidates. In
such instances, we may need to locate an appropriate replacement third-party relationship, which may not be readily available or on acceptable
terms, which would cause additional delay or increased expense prior to the approval of our C&C product candidates and would thereby
have a material adverse effect on our business, financial condition, results of operations and prospects.
Additionally,
we have not yet entered into binding agreements with certain third-party manufacturers to produce the raw materials and products that
we use to manufacture our product candidates. Although we intend to rely on third-party manufacturers for the raw materials and products
to support the manufacturing of our product candidates for commercialization, we have not yet entered into agreements with certain manufacturers.
We may be unable to negotiate binding agreements with the manufacturers to support our commercialization activities at commercially reasonable
terms.
Our
reliance on third parties requires us to share our intellectual property, including trade secrets, which increases the possibility that
a competitor will discover them or that our intellectual property will be misappropriated or disclosed.
Because
we rely on third parties to provide us with the materials that we use to develop and, if appropriate in the future, manufacture our product
candidates or approved product candidates, we may, at times, share trade secrets and intellectual property with such third parties. We
seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer
agreements, collaborative research agreements, consulting agreements, or other similar agreements with our collaborators, advisors, employees
and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the
third parties to use or disclose our confidential information, such as trade secrets and intellectual property. Despite the contractual
provisions employed when working with third parties, the need to share trade secrets and other confidential information increases the
risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed
or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a
competitor’s discovery of our trade secrets or other unauthorized use or disclosure would impair our competitive position and may
have a material adverse effect on our business.
Despite
our efforts to protect our trade secrets and knowhow, our competitors may discover our trade secrets, either through breach of these
agreements, independent development or publication of information including our trade secrets by third parties. A competitor’s
discovery of our trade secrets and knowhow would impair our competitive position and have an adverse impact on our business, financial
condition, results of operations and prospects.
Risks
Related to Our Intellectual Property
If
we are unable to obtain and maintain effective patent rights for our product candidates or any future product candidates, we may not
be able to compete effectively in our markets. If we are unable to protect the confidentiality of our trade secrets or know-how, such
proprietary information may be used by others to compete against us.
We
intend to rely upon a combination of patents, trade secret protection and confidentiality agreements to protect the intellectual property
related to our technologies and product candidates. Our success depends in large part on our ability to obtain and maintain patent and
other intellectual property protection in the United States and in other countries with respect to our proprietary technology and product
candidates. This ability depends to a large extent on identifying aspects of the technology that are patentable, that their exposure
via patent applications would not provide a third party of engineering around capabilities and on the ability to generate and identify
superior data that would present a comparative edge vis-à-vis third party technologies.
We
have sought to protect our proprietary position by filing patent applications in the United States, with respect to our novel technologies
and product candidates, which are important to our business. These patent applications will base international and national patent filings
in countries of interest to our business, such as the United States, the European Union and Israel. Patent prosecution is expensive and
time consuming, and we may not be able to file and prosecute our applications in the United States, the European Union and Israel, at
a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development
output before it is too late to obtain patent protection.
As
of October 2, 2024, our growing portfolio of patent applications consists of two families that disclose our technology. We cannot offer
any assurances about which, if any, patents will issue in due time, the breadth of any such patent or whether any issued patents will
be found invalid and unenforceable or will be threatened by third parties. Any successful opposition to these patents or any other patents
owned by or licensed to us after patent issuance could deprive us of rights necessary for the successful commercialization of any product
candidates that we may develop. Further, if we encounter delays in regulatory approvals, the period of time during which we could market
a product candidate under patent protection could be reduced.
Further,
the patent position of medical device and pharmaceutical companies generally is highly uncertain and involves complex legal and factual
questions for which legal principles remain unsolved. This renders the patent prosecution process particularly expensive and time-consuming.
There is no assurance that all potentially relevant prior art relating to our patent applications has been found, which can invalidate
a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue, and even if such
patents cover our product candidates, third parties may challenge their validity, enforceability, or scope, which may result in such
patents being narrowed, found unenforceable or invalidated. Furthermore, even if they are unchallenged, our patent applications and any
future patents may not adequately protect our intellectual property, provide exclusivity for our product candidates, or prevent others
from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties, which may
have an adverse impact on our business.
If
we cannot obtain and maintain effective patent rights for our product candidates, we may not be able to compete effectively, and our
business and results of operations would be harmed.
We
may not have sufficient patent lifespan to effectively protect our product candidates and business.
Patents
have a limited lifespan. The natural expiration of a patent is generally 20 years counted from its filing date (or PCT filing date in
case it is derived from an international application). Although various extensions may be available, they are uncommon and the protection
they afford, is limited. Even if any of our patent applications matures into issued patents, if we do not have sufficient patent terms
or regulatory exclusivity to protect our product candidates, our business and results of operations will be adversely affected.
Changes
in patent policy and national intellectual property laws could increase the uncertainties and costs surrounding the prosecution of our
patent applications and the enforcement or defense of any issued patents.
Changes
in patent laws or interpretation of patent laws in the United States and other countries may diminish the value of any patents that may
issue from our patent applications, or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights
to the same extent as the laws of the United States. Publications of discoveries in the scientific literature often lag behind the actual
discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after
filing. We therefore cannot be certain that we were the first to make the invention claimed in our owned patent or pending applications,
or that we were the first to file for patent protection of such inventions.
If
our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest
and our business may be adversely affected.
Our
registered or unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to
be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name
recognition by potential partners or customers in our markets of interest. Over the long term, if we are unable to establish name recognition
based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. If
other entities use trademarks similar to ours in different jurisdictions, or have senior rights to ours, it could interfere with our
use of our current trademarks throughout the world.
If
we are unable to maintain effective proprietary rights for our product candidates or any future product candidates, we may not be able
to compete effectively in our markets.
In
addition to the protection afforded by any patents that may be granted, we rely on trade secret protection and confidentiality agreements
to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce
and any other elements of our product candidate discovery and development processes that involve proprietary know-how, information or
technology that is not covered by patents. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology
and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors and contractors.
We also seek to preserve the integrity and confidentiality of our data, trade secrets and intellectual property by maintaining the physical
security of our premises and physical and electronic security of our information technology systems. While we have confidence in these
individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any
breach. In addition, our trade secrets and intellectual property may otherwise become known or be independently discovered by competitors.
Although
we expect all of our employees and consultants to assign their inventions to us, and all of our employees, consultants, advisors and
any third parties who have access to our proprietary know-how, information, or technology to enter into confidentiality agreements, we
cannot provide any assurances that all such agreements have been duly executed or that our trade secrets and other confidential proprietary
information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially
equivalent information and techniques. Misappropriation or unauthorized disclosure of our trade secrets and intellectual property could
impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain
our trade secrets and intellectual property are deemed inadequate, we may have insufficient recourse against third parties for misappropriating
the trade secret.
Intellectual
property rights of third parties could adversely affect our ability to commercialize our product candidates, and we might be required
to litigate or obtain licenses from third parties in order to develop or market our product candidate. Such litigation or licenses could
be costly or not available on commercially reasonable terms.
Our
competitive position may suffer if patents issued to third parties or other third party intellectual property rights cover our product
candidates or elements thereof, or our manufacturing or uses relevant to our development plans. In such cases, we may not be in a position
to develop or commercialize our product candidates unless we successfully pursue litigation to nullify or invalidate the third party
intellectual property right concerned, or enter into a license agreement with the intellectual property right holder, if available on
commercially reasonable terms. There may also be pending patent applications that if they result in issued patents, could be alleged
to be infringed by our product candidates. If such an infringement claim should be brought and be successful, we may be required to pay
substantial damages, be forced to abandon our product candidates or seek a license from any patent holders. No assurances can be given
that a license will be available on commercially reasonable terms, if at all.
It
is also possible that we have failed to identify relevant third party patents or applications. Patent applications in the U.S. and elsewhere
are published approximately 18 months after the earliest filing to which priority is claimed, with such earliest filing date being commonly
referred to as the priority date. Therefore, patent applications covering our product candidates or technology could have been filed
by others without our knowledge. Additionally, pending patent applications which have been published can, subject to certain limitations,
be later amended in a manner that could cover our technologies, our product candidates or the use of our product candidates. Third party
intellectual property right holders may also actively bring infringement claims against us. We cannot guarantee that we will be able
to successfully settle or otherwise resolve such infringement claims. If we are unable to successfully settle future claims on terms
acceptable to us, we may be required to engage in or continue costly, unpredictable and time-consuming litigation and may be prevented
from or experience substantial delays in pursuing the development of and/or marketing of our product candidates. If we fail in any such
dispute, in addition to being forced to pay damages, we may be temporarily or permanently prohibited from commercializing our product
candidates that are held to be infringing. We might, if possible, also be forced to redesign our product candidates so that we no longer
infringe the third party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to
divert substantial financial and management resources that we would otherwise be able to devote to our business.
Third-party
claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our
commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. There have been
many lawsuits and other proceedings involving patent and other intellectual property rights in the biotechnology, medical device and
pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions and reexamination proceedings before the
USPTO and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned
by third parties, exist in the fields in which we are developing product candidates. As the medical device and pharmaceutical industry
expands and more patents are issued, the risk increases that our product candidates may be subject to claims of infringement of the patent
rights of third parties.
Third
parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent
applications with claims to materials, formulations, methods of manufacture, or methods for treatment related to the use or manufacture
of our product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications
that may later result in issued patents that our product candidates may infringe. In addition, third parties may obtain patents in the
future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent
jurisdiction to cover the manufacturing process of any of our product candidates, any materials formed during the manufacturing process
or any final product candidates itself, the holders of any such patents may be able to block our ability to commercialize such product
candidates unless we obtain a license under the applicable patents, or until such patents expire or are finally determined to be invalid
or unenforceable.
Similarly,
if any third-party patents were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture,
or methods of use, the holders of any such patents may be able to block our ability to develop and commercialize the applicable product
candidate unless we obtain a license or until such patent expires or is finally determined to be invalid or unenforceable. In either
case, such a license may not be available on commercially reasonable terms or at all.
Parties
making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop
and commercialize one or more of our product candidates. Defense of these claims, regardless of their merit, would involve substantial
litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of
infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement,
pay royalties, redesign our infringing product candidates or obtain one or more licenses from third parties, which may be impossible
or require substantial time and monetary expenditure.
We
may not be successful in obtaining or maintaining necessary rights to our product candidates through acquisitions and in-licenses.
Because
our technology may require the use of proprietary rights held by third parties, the growth of our business will likely depend in part
on our ability to acquire, in-license, or use these proprietary rights. In addition, our product candidates may require specific formulations
to work effectively and efficiently and the rights to these formulations may be held by others. We may be unable to acquire or in-license
any compositions, methods of use, processes, or other third-party intellectual property rights from third parties that we identify as
necessary for our product candidates. The licensing and acquisition of third-party intellectual property rights is a competitive area,
and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights
that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources,
and greater clinical development and commercialization capabilities.
For
example, we sometimes collaborate with academic institutions to accelerate our preclinical research or development under written agreements
with these institutions. Typically, these institutions provide us with an option to negotiate a license to any of the institution’s
rights in technology resulting from the collaboration. Regardless of such option, we may be unable to negotiate a license within the
specified timeframe or under terms that are acceptable to us. If we are unable to do so, the institution may offer the intellectual property
rights to other parties, potentially blocking our ability to pursue our program.
In
addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to
license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment.
If we are unable to successfully obtain rights to required third-party intellectual property rights, we may have to abandon development
of that program and our business and financial condition could suffer.
We
may be involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time consuming and unsuccessful.
Competitors
may infringe our intellectual property or that of our licensors that we may acquire in the future. If we or a future licensing partner
were to initiate legal proceedings against a third party to enforce a patent covering one of our product candidates, the defendant could
counterclaim that the patent covering our product candidate is invalid and/or unenforceable. In patent litigation in the United States,
defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged
failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability
assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO,
or made a misleading statement, during prosecution. Under the AIA, the validity of U.S. patents may also be challenged in post-grant
proceedings before the USPTO. The outcome following legal assertions of invalidity and unenforceability is unpredictable.
Interference
proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions
with respect to our patent or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the
related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party
does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even
if successful, may result in substantial costs and distract our management and other employees. In addition, the uncertainties associated
with litigation could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue
our research programs, license necessary technology from third parties, or enter into development partnerships that would help us bring
our product candidates to market.
Furthermore,
because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some
of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements
of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these
results to be negative, it could have a material adverse effect on the price of our Ordinary Shares.
We
may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information
of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
We
employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors
or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary
information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants, or independent
contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information,
of any of our employees’ former employers or other third parties. Litigation may be necessary to defend against these claims. If
we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel,
which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial
costs and be a distraction to management and other employees.
We
may be subject to claims challenging the inventorship of our intellectual property.
We
may be subject to claims that former employees, collaborators or other third parties have an interest in or right to compensation with
respect to our patent applications, future patents or other intellectual property as an inventor or co-inventor. For example, we may
have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our product candidates.
Litigation may be necessary to defend against these and other claims challenging inventorship or claiming the right to compensation.
If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such
as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our
business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction
to management and other employees. To the extent that our employees have not effectively waived the right to compensation with respect
to inventions that they helped create, they may be able to assert claims for compensation with respect to our future revenue. As a result,
we may receive less revenue from future product candidates if such claims are successful which in turn could impact our future profitability.
Changes
in United States and international patent law could diminish the value of patents in general, thereby impairing our ability to protect
our product candidates.
Our
success is heavily dependent on intellectual property. Obtaining and enforcing patents in the medical device and pharmaceutical industries
involve both technological and legal complexity. Therefore, obtaining and enforcing these patents is costly, time consuming and inherently
uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation.
Recent United States Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened
the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents
in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on future
actions by the United States Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable
ways that would weaken our ability to obtain patents or to enforce patents that we might obtain in the future.
We
may not be able to protect our intellectual property rights throughout the world.
Filing,
prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our
intellectual property rights in some countries outside the United States may be less extensive than those in the United States. In addition,
the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United
States.
Competitors
may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and may also export
otherwise infringing product candidates to territories where we have patent protection, but enforcement is not as strong as that in the
United States. These products may compete with our product candidates. Future patents or other intellectual property rights may not be
effective or sufficient to prevent them from competing.
Many
companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The
legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets
and other intellectual property protection, particularly those relating to biotechnology products or methods of treatment, which could
make it difficult for us to stop the marketing of competing products in violation of our proprietary rights generally. Proceedings to
enforce our future patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our
efforts and attention from other aspects of our business, could put our future patents at risk of being invalidated or interpreted narrowly
and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in
any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our
efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from
the intellectual property that we develop or license.
Risks
Related to Our Business Operations
We
will need to expand our organization, and we may experience difficulties in managing this growth, which could disrupt our operations.
Our
future financial performance and our ability to commercialize product candidates and compete effectively will depend, in part, on our
ability to effectively manage any future growth. As our development and commercialization plans and strategies develop, we expect to
need additional managerial, operational, sales, marketing, financial and legal personnel. Our management may need to divert a disproportionate
amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities.
We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational
mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could
require significant capital expenditures and may divert financial resources from other projects, such as the development of additional
product candidates. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability
to generate and/or grow revenue could be reduced, and we may not be able to implement our business strategy.
Due
to our limited resources and access to capital, we must, and have in the past decided to, prioritize development of certain product candidates
over other potential candidates. These decisions may prove to have been wrong and may adversely affect our revenues.
Because
we have limited resources and access to capital to fund our operations, we must decide which product candidates to pursue and the amount
of resources to allocate to each. Our decisions concerning the allocation of research, collaboration, management and financial resources
toward particular product candidates may not lead to the development of viable commercial product candidates and may divert resources
away from better opportunities. Similarly, our decisions to delay, terminate or collaborate with third parties in respect of certain
product candidates’ development programs may also prove not to be optimal and could cause us to miss valuable opportunities. If
we make incorrect determinations regarding the market potential of our product candidates or misread trends in the medical device and
pharmaceutical industries, in particular for our lead product candidate, our business, financial condition and results of operations
could be materially adversely affected.
We
may not be successful in our efforts to identify, discover or license additional product candidates.
Although
a substantial amount of our effort will focus on the continued clinical testing, potential approval and commercialization of our product
candidates, the success of our business also depends upon our ability to identify, discover or license additional product candidates.
Our research programs or licensing efforts may fail to yield additional product candidates for clinical development for a number of reasons,
including: lack of financial or personnel resources to acquire or discover additional product candidates; new product candidates may
not succeed in preclinical or clinical testing, or may be shown to have harmful side effects or may have other characteristics that may
make them unmarketable or unlikely to receive marketing approval; our competitors may develop alternatives that render our product candidates
obsolete or less attractive; the market for a product candidate may change during our development program so that such product candidates
may become unprofitable to continue to develop; new product candidates may not be capable of being produced in commercial quantities
at an acceptable cost, or at all; and new product candidates may not be accepted as safe and effective by patients, the medical community,
or third-party payors.
We
may be forced to abandon our development efforts for a program or programs that are unsuccessful, or we may not be able to identify,
license, or discover additional product candidates, which would have a material adverse effect on our business and could potentially
cause us to cease operations. Further, research programs to identify new product candidates require substantial technical, financial
and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful.
European
data collection is governed by restrictive regulations governing the collection, use, processing and cross-border transfer of personal
information.
We
may collect, process, use or transfer personal information from individuals located in the European Union in connection with our business,
including in connection with conducting clinical trials in the European Union. Additionally, we intend to commercialize our product candidates,
and any of our product candidates that receive marketing approval, in the European Union. The collection and use of personal health data
in the European Union is governed by the provisions of the General Data Protection Regulation ((EU) 2016/679), or the GDPR, along with
other European Union and country-specific laws and regulations. The United Kingdom and Switzerland have also adopted data protection
laws and regulations. These legislative acts (together with regulations and guidelines) impose requirements relating to having legal
bases for processing personal information relating to identifiable individuals and transferring such information outside of the EEA,
including to the United States, providing details to those individuals regarding the processing of their personal information, keeping
personal information secure, having data processing agreements with third parties who process personal information, responding to individuals’
requests to exercise their rights in respect of their personal information, reporting security breaches involving personal data to the
competent national data protection authority and affected individuals, appointing data protection officers, conducting data protection
impact assessments and record-keeping. The GDPR imposes additional responsibilities and liabilities in relation to personal data that
we process and we may be required to put in place additional mechanisms ensuring compliance with the new data protection rules. Failure
to comply with the requirements of the GDPR and related national data protection laws of the member states of the European Union may
result in substantial fines, other administrative penalties and civil claims being brought against us, which could have a material adverse
effect on our business, prospects, financial condition and results of operations.
If
we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur
costs that could have a material adverse effect on the success of our business.
Our
research, development and manufacturing activities and our third party manufacturers’ and suppliers’ activities involve the
controlled storage, use and disposal of hazardous materials, including the components of our product candidates and other hazardous compounds.
We and our manufacturers and suppliers are subject to laws and regulations governing the use, manufacture, storage, handling and disposal
of these hazardous materials. In some cases, these hazardous materials and various wastes resulting from their use are stored at our
and our manufacturers’ facilities pending their use and disposal. We cannot eliminate the risk of contamination, which could cause
an interruption of our commercialization efforts, research and development efforts and business operations, environmental damage resulting
in costly clean-up and liabilities under applicable laws and regulations governing the use, storage, handling and disposal of these materials
and specified waste product candidates. Although we believe that the safety procedures utilized by our third-party manufacturers for
handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, we cannot guarantee
that this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held
liable for any resulting damages, such liability could exceed our resources, and state or federal or other applicable authorities may
curtail our use of certain materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex,
change frequently and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our
future compliance. We do not currently carry biological or hazardous waste insurance coverage.
Our
employees and independent contractors may engage in misconduct or other improper activities, including noncompliance with regulatory
standards and requirements.
We
are exposed to the risk of fraud or other misconduct by our employees and independent contractors. Misconduct by these parties could
include intentional failures to comply with FDA regulations, provide accurate information to the FDA, comply with manufacturing standards
we may establish, comply with federal and state healthcare fraud and abuse laws and regulations, report financial information or data
accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry
are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices.
These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission,
customer incentive programs and other business arrangements. Employee and independent contractor misconduct could also involve the improper
use of information obtained in the course of clinical trials, including individually identifiable information, creating fraudulent data
in our preclinical studies or clinical trials or illegal misappropriation of product candidates, which could result in regulatory sanctions
and serious harm to our reputation. It is not always possible to identify and deter misconduct by employees and other third parties,
and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses
or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such
laws or regulations. Additionally, we are subject to the risk that a person or government could allege such fraud or other misconduct,
even if none occurred. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our
rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
Under
applicable employment laws, we may not be able to enforce covenants not to compete and therefore may be unable to prevent our competitors
from benefiting from the expertise of some of our former employees.
We
generally enter into non-competition agreements with our employees and certain key consultants. These agreements prohibit our employees
and certain key consultants, if they cease working for us, from competing directly with us or working for our competitors or clients
for a limited period of time. We may be unable to enforce these agreements under the laws of the jurisdictions in which our employees
work and it may be difficult for us to restrict our competitors from benefitting from the expertise our former employees or consultants
developed while working for us.
For
example, Israeli courts have required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that
the competitive activities of the former employee will harm one of a limited number of material interests of the employer which have
been recognized by the courts, such as the secrecy of a company’s confidential commercial information or the protection of its
intellectual property. If we cannot demonstrate that such interests will be harmed, we may be unable to prevent our competitors from
benefiting from the expertise of our former employees or consultants and our ability to remain competitive may be diminished.
Increasing
scrutiny of, and evolving expectations for, sustainability and environmental, social, and governance, or ESG, initiatives could increase
our costs or otherwise adversely impact our business.
Public
companies are facing increasing scrutiny related to ESG practices and disclosures from certain investors, capital providers,
shareholder advocacy groups, other market participants and other stakeholder groups. With this increased focus, public reporting regarding ESG practices
is becoming more broadly expected. Such increased scrutiny may result in increased costs, enhanced compliance or disclosure obligations,
or other adverse impacts on our business, financial condition or results of operations. If our ESG practices and reporting
do not meet investor or other stakeholder expectations, which continue to evolve, we may be subject to investor or regulator engagement
regarding such matters. In addition, new sustainability rules and regulations have been adopted and may continue to be introduced in
various states and other jurisdictions. For example, the SEC has adopted rules that require companies to provide expanded climate-related
disclosures in their periodic reporting, which may require us to incur significant additional costs to comply and impose increased oversight
obligations on our management and board of directors. Our failure to comply with any applicable rules or regulations could lead to penalties
and adversely impact our reputation, access to capital and employee retention. Such ESG matters may also impact our third-party
contract manufacturers and other third parties on which we rely, which may augment or cause additional impacts on our business, financial
condition, or results of operations.
Any
resurgence of the COVID-19 pandemic could adversely affect our business, financial condition and results of operations.
In
late 2019, a novel strain of COVID-19, also known as coronavirus, was reported in Wuhan, China. While initially the outbreak was largely
concentrated in China, it spread to countries across the globe, including in Israel and the United States. Many countries around the
world, including in Canada, Israel and the United States, implemented significant governmental measures to control the spread of the
virus, including temporary closure of businesses, severe restrictions on travel and the movement of people, and other material limitations
on the conduct of business.
While
the potential economic impact brought by, and the duration of, the COVID-19 pandemic may be difficult to assess or predict, it has already
caused, and could result in further, significant disruption of global financial markets, reducing our ability to access capital, which
could in the future negatively affect our liquidity and financial position. In addition, the trading prices for other companies have
been highly volatile as a result of the COVID-19 pandemic. As a result, we may face difficulties raising capital through sales of our
Ordinary Shares or other securities and such sales may be on unfavorable terms. To the extent that future waves of COVID-19 disrupt normal
business operations, we may face operational challenges with our services, and we likely will have to adopt remote working and workplace
protocols for employees in accordance with government requirements and other measures to minimize such impact.
The
COVID-19 pandemic and its impacts continue to evolve. We cannot predict the scope and severity of any further disruptions as a result
of COVID-19 or their impacts on us, but business disruptions for us or any of the third parties with whom we engage, including the manufacturers,
suppliers, customers, regulators and other third parties with whom we conduct business could materially and negatively impact our ability
to conduct our business in the manner and on the timelines presently planned. The extent to which the COVID-19 pandemic may continue
to impact our business and financial performance will depend on future developments, which are highly uncertain and cannot be predicted
with confidence, including the scope and duration of the pandemic, the extent and effectiveness of government restrictions and other
actions, including relief measures, implemented to address the impact of the pandemic, and resulting economic impacts. We are unable
to determine the extent of the impact of the pandemic on our operations and financial condition going forward. These developments are
highly uncertain and unpredictable and may materially adversely affect our financial position and results of operations.
Our
business, operating results and growth rates may be adversely affected by current or future unfavorable economic and market conditions
and adverse developments with respect to financial institutions and associated liquidity risk.
Our
business depends on the economic health of the global economies. If the conditions in the global economies remain uncertain or continue
to be volatile, or if they deteriorate, including as a result of the impact of military conflict, terrorism or other geopolitical events,
our business, operating results and financial condition may be materially adversely affected. Economic weakness, inflation and increases
in interest rates, limited availability of credit, liquidity shortages and constrained capital spending have at times in the past resulted,
and may in the future result, in challenging and delayed sales cycles, slower adoption of new technologies and increased price competition,
and could negatively affect our ability to forecast future periods, which could result in an inability to satisfy demand for our products
and a loss of market share.
In
addition, increases in inflation raise our costs for commodities, labor, materials and services and other costs required to grow and
operate our business, and failure to secure these on reasonable terms may adversely impact our financial condition. Additionally, increases
in inflation, along with the uncertainties surrounding any resurgence of COVID-19, geopolitical developments and global supply chain
disruptions, have caused, and may in the future cause, global economic uncertainty and uncertainty about the interest rate environment,
which may make it more difficult, costly or dilutive for us to secure additional financing. A failure to adequately respond to these
risks could have a material adverse impact on our financial condition, results of operations or cash flows.
There
can be no assurance that future credit and financial market instability and a deterioration in confidence in economic conditions will
not occur. Our general business strategy may be adversely affected by any such economic downturn, liquidity shortages, volatile business
environment or continued unpredictable and unstable market conditions. If equity and credit markets deteriorate, or if adverse developments
are experienced by financial institutions, it may cause short-term liquidity risk and also make any necessary debt or equity financing
more difficult, more costly, more onerous with respect to financial and operating covenants and more dilutive. Failure to secure any
necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial
performance and stock price and could require us to alter our operating plans. In addition, there is a risk that one or more of our service
providers, financial institutions, manufacturers, suppliers and other partners may be adversely affected by the foregoing risks, which
could directly affect our ability to attain our operating goals on schedule and on budget.
International
expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing
business outside of the United States or Israel.
Other
than our headquarters and other operations which are located in Israel (as further described below), we currently have limited international
operations, but our business strategy incorporates potentially significant international expansion, particularly in anticipation of approval
of our product candidates. We plan to retain sales representatives and third party distributors and conduct physician, ENT specialist,
hospital pharmacist and patient association outreach activities, as well as clinical trials, outside of the United States, European Union
and Israel. Doing business internationally involves a number of risks, including but not limited to:
| |
● |
multiple, conflicting and changing laws and regulations
such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental
approvals, permits, and licenses; |
| |
● |
failure by us to obtain regulatory approvals for the
use of our product candidates in various countries; |
| |
● |
additional potentially relevant third-party patent
rights; |
| |
|
|
| |
● |
complexities and difficulties in obtaining protection
and enforcing our intellectual property; |
| |
|
|
| |
● |
difficulties in staffing and managing foreign operations; |
| |
|
|
| |
● |
complexities associated with managing multiple payor
reimbursement regimes, government payors, prince controls or patient self-pay systems; |
| |
|
|
| |
● |
limits in our ability to penetrate international markets; |
| |
|
|
| |
● |
financial risks, such as longer payment cycles, difficulty
collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our product candidates
and exposure to foreign currency exchange rate fluctuations; |
| |
|
|
| |
● |
an outbreak of a contagious
disease, including any resurgence of COVID-19, which may cause us or our distributors, third party vendors and manufacturers and/or
customers to temporarily suspend our or their respective operations in the affected city or country; |
| |
|
|
| |
● |
natural disasters, political and economic instability,
including wars, terrorism, and political unrest, boycotts, curtailment of trade, and other business restrictions; |
| |
|
|
| |
● |
certain expenses including, among others, expenses
for travel, translation and insurance; and |
| |
|
|
| |
● |
regulatory and compliance risks that relate to maintaining
accurate information and control over sales and activities that may fall within the purview of the U.S. Foreign Corrupt Practices
Act its books and records provisions, or its anti-bribery provisions. |
Any
of these factors could significantly harm our future international expansion and operations and, consequently, our results of operations.
Risks
Related to Commercialization of Our Product Candidates
We
currently have no marketing and sales organization. If we are unable to establish marketing and sales capabilities, or enter into agreements
with third parties to market and sell our product candidates, we may be unable to generate any product candidates revenue.
We
have no experience selling and marketing our product candidates, and we currently have no marketing or sales organization. To successfully
commercialize any product candidates that may result from our development programs, we will need to develop these capabilities, either
on our own or with others. If our product candidates receive regulatory approval, we intend to establish a sales and marketing organization
independently or by utilizing experienced third parties with technical expertise and supporting distribution capabilities to commercialize
our product candidates in major markets, all of which will be expensive, difficult and time consuming. Any failure or delay in the development
of our internal sales, marketing and distribution capabilities would adversely impact our ability to commercialize our product candidates.
Further,
given our lack of prior experience in marketing and selling medical device and pharmaceutical products, our initial estimate of the size
of the required sales force may be materially more or less than the size of the sales force actually required to effectively commercialize
our product candidates. As such, we may be required to hire sales representatives and third party distributors to adequately support
the commercialization of our product candidates, or we may incur excess costs if we hire more sales representatives than necessary. With
respect to certain geographical markets, we may enter into collaborations with other entities to utilize their local marketing and distribution
capabilities, but we may be unable to enter into such agreements on favorable terms, if at all. We also may enter into collaborations
with large medical device and pharmaceutical companies to develop and commercialize product candidates. If our future collaborators do
not commit sufficient resources to develop and commercialize our future product candidates, if any, and we are unable to develop the
necessary marketing capabilities on our own, we will be unable to generate sufficient product candidates revenue to sustain our business.
We may compete with companies that currently have extensive and well-funded marketing and sales operations. Without an internal team
or the support of a third party to perform marketing and sales functions, we may be unable to compete successfully against these more
established companies.
Our
efforts to educate the medical community, including physicians, hospital pharmacists and third-party payors on the benefits of our product
candidates may require significant resources and may never be successful. If any of our product candidates are approved but fail to achieve
market acceptance among physicians, patients or third-party payors, we will not be able to generate significant revenues from such product
candidates, which could have a material adverse effect on our business, financial condition, results of operations and prospects.
We
are subject to significant regulatory oversight with respect to manufacturing our product candidates. Delays in establishing and obtaining
regulatory approval of our manufacturing process may delay or disrupt our product candidates’ development and commercialization
efforts.
The
preparation of drug products for clinical trials or commercial sale is subject to extensive regulation. Before we can begin to commercially
manufacture our product candidates or any product candidate, we must obtain regulatory approvals from the Israeli Ministry of Health,
or MOH, the FDA and similar regulatory agencies, as applicable for our manufacturing process and facility. A manufacturing authorization
must also be obtained from the appropriate regulatory authorities in the European Union and worldwide. In addition, we must pass a pre-approval
inspection of our manufacturing facility by the FDA before our C&C product candidates or any product candidate can obtain marketing
approval. In order to obtain approval, we will need to ensure that all of our processes, methods and equipment are compliant with cGMP,
and perform extensive audits of vendors, contract laboratories and suppliers. If any of our vendors, contract laboratories or suppliers
is found to be out of compliance with cGMP, we may experience delays or disruptions in manufacturing while we work with these third parties
to remedy the violation or while we work to identify suitable replacement vendors. For example, in the past, a cGMP audit by the MOH
of the manufacturing process in the facility of our contract manufacturer for our C&C product candidates resulted in certain critical
observations, which have since been resolved. There can be no guarantee, however, that future inspections by regulatory authorities of
our manufacturing facilities or those of our contract manufacturers will result in MOH’s agreement that these critical observations
have been resolved or that similar inspectional observations will not be identified. If we do not demonstrate to the satisfaction of
the applicable regulator that our manufacturing facilities, or those of our contract manufacturers, are in compliance with applicable
requirements, we may be materially delayed in the development of our product candidates, which would materially harm our business. The
cGMP requirements govern quality control of the manufacturing process and documentation policies and procedures. In complying with cGMP,
we will be obligated to expend time, money and effort in production, record keeping and quality control to assure that the product candidates
meets applicable specifications and other requirements. If we fail to comply with these requirements, we would be subject to possible
regulatory action and may not be permitted to sell any product candidate that we may develop.
Our
failure to comply with applicable regulations could result in sanctions being imposed on us, including clinical holds, fines, injunctions,
civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of products or product candidates,
operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of any approved products
and our product candidates.
If
we receive marketing approval for our product candidates, sales will be limited unless the product candidates achieves broad market acceptance
by physicians, patients, third-party payors, hospital pharmacists, infectious disease specialists and others in the medical community.
The
commercial success of our product candidates will depend upon the acceptance of the product candidates by the medical community, including
physicians, patients, healthcare payors, hospital pharmacists and infectious disease specialists. The degree of market acceptance of
any approved product candidates will depend on a number of factors, including:
| |
● |
the demonstration of clinical safety and efficacy of
our product candidates in clinical trials; |
| |
● |
the efficacy, potential and perceived advantages of
our product candidates over alternative treatments; |
| |
● |
the cost of treatment relative to alternative treatments; |
| |
|
|
| |
● |
the prevalence and severity of any adverse side effects; |
| |
|
|
| |
● |
product candidates labeling or product candidates insert
requirements of the FDA or other regulatory authorities, including any limitations or warnings contained in a product candidate’s
approved labeling; |
| |
|
|
| |
● |
distribution and use restrictions imposed by the FDA
or agreed to by us as part of a mandatory or voluntary risk management plan; |
| |
|
|
| |
● |
our ability to obtain third-party coverage and adequate
reimbursement; |
| |
|
|
| |
● |
the willingness of patients to pay for drugs out of
pocket in the absence of third-party coverage; |
| |
|
|
| |
● |
the demonstration of the effectiveness of our product
candidates in reducing the cost of treatment; |
| |
|
|
| |
● |
the strength of marketing and distribution support; |
| |
|
|
| |
● |
the timing of market introduction of competitive products; |
| |
|
|
| |
● |
the availability of product candidates and their ability
to meet market demand; and |
| |
|
|
| |
● |
publicity concerning our product candidates or competing
products and treatments. |
If
our product candidates are approved but do not achieve an adequate level of acceptance by physicians, patients, healthcare payors, hospital
pharmacists and infectious disease specialists, we may not generate sufficient revenue from the product candidates, and we may not become
or remain profitable. In addition, our efforts to educate the medical community and third-party payors on the benefits of our product
candidates may require significant resources and may never be successful.
It
may be difficult for us to profitably sell our product candidates if coverage and reimbursement for these product candidates, or the
procedures in which they are used, is limited by government authorities and/or third-party payor policies.
In
addition to any healthcare reform measures which may affect reimbursement, market acceptance and sales of our product candidates, if
approved, will depend on, in part, the extent to which the procedures utilizing our product candidates, performed by health care providers,
will be covered by third party payors, such as government health care programs, commercial insurance and managed care organizations.
Our product candidates will be purchased or provided by health care providers for utilization in certain surgical procedures. In the
event health care providers and patients accept our product candidates as medically useful, cost effective and safe, there is uncertainty
regarding whether our product candidates will be directly reimbursed, reimbursed through a bundled payment or if the product candidates
will be included in another type of value-based reimbursement program. Third party payors determine the extent to which new product candidates
or procedures will be covered as a benefit under their plans and the level of reimbursement for any covered product candidates or procedure
which may utilize a covered product candidates.
When
used in connection with certain procedures, our product candidates may not be reimbursed separately but their cost may instead be bundled
as part of the payment received by the provider for the procedure only. Treating physicians are unlikely to use and order our product
candidates unless coverage is provided and the reimbursement is adequate to cover all or a significant portion of the cost of the procedures
which utilize our product candidates. A decision by a third-party payor not to cover or adequately reimburse for our product candidates
or procedures using our product candidates, could reduce physician utilization of our product candidates once approved. Therefore, coverage
and adequate reimbursement for procedures which utilize new product candidates is critical to the acceptance of such new product candidates.
A
primary trend in the U.S. healthcare industry and elsewhere has been cost containment, including price controls, restrictions on coverage
and reimbursement and requirements for substitution of less expensive products and procedures. Third party payors decide which products
and procedures they will pay for and establish reimbursement and co-payment levels. Government and other third-party payors are increasingly
challenging the prices charged for healthcare products and procedures, examining the cost effectiveness of procedures, and the products
used in such procedures, in addition to their safety and efficacy, and limiting or attempting to limit both coverage and the level of
reimbursement. We cannot be sure that coverage will be available for our product candidates, if approved, or, if coverage is available,
the level of direct or indirect reimbursement.
We
expect to experience pricing pressures in connection with the sale of any of our product candidates due to the trend toward managed healthcare,
the increasing influence of health maintenance organizations, and additional legislative changes. The downward pressure on healthcare
costs in general, particularly prescription drugs and surgical procedures and other treatments, has become very intense. As a result,
increasingly high barriers are being erected to the successful commercialization of new product candidates. Further, the adoption and
implementation of any future governmental cost containment or other health reform initiative may result in additional downward pressure
on the price that we may receive for any approved product candidates.
Reimbursement
by a third-party payor may depend upon a number of factors including the third-party payor’s determination that use of a product
candidates is:
| |
● |
a covered benefit or part of a covered benefit under
its health plan; |
| |
|
|
| |
● |
safe, effective and medically necessary; |
| |
|
|
| |
● |
appropriate for the specific patient; |
| |
|
|
| |
● |
cost-effective; and |
| |
|
|
| |
● |
neither experimental nor investigational. |
In
the United States, third-party payors, including private and governmental payors such as the Medicare and Medicaid programs, play an
important role in determining the extent to which procedures using new product candidates will be covered and reimbursed. The Medicare
and Medicaid programs are increasingly used as models for how private payors and other governmental payors develop their coverage and
reimbursement policies. It is difficult to predict at this time what third-party payors will decide with respect to reimbursement for
fundamentally novel product candidates such as ours, as there is no body of established practices and precedents for these new product
candidates.
Obtaining
coverage and reimbursement approval for a products from a government or other third-party payor is a time-consuming and costly process
that could require us to provide supporting scientific, clinical and cost effectiveness data for the use of our product candidates to
the payor.
Additionally,
we may not be able to provide data sufficient to gain acceptance with respect to coverage and reimbursement. We cannot be sure that coverage
or adequate reimbursement will be available for our product candidates, if approved. Also, we cannot be sure that reimbursement amounts
will not reduce the demand for, or the price of, our future product candidates. If reimbursement is not available, or is available only
to limited levels, we may not be able to commercialize our product candidates, or achieve profitably at all, even if approved.
Our
business entails a significant risk of clinical trial and/or product candidates liability and our ability to obtain sufficient insurance
coverage could have a material effect on our business, financial condition, results of operations or prospects.
Our
business exposes us to significant clinical trial and/or product candidates liability risks inherent in the development, testing, manufacturing
and marketing of therapeutic treatments. Clinical trial and/or product candidates liability claims could delay or prevent completion
of our development programs. If we succeed in marketing product candidates, product candidates liability claims could result in an FDA
investigation of the safety and effectiveness of our product candidates, our manufacturing processes and facilities or our marketing
programs and potentially a recall of our product candidates or more serious enforcement action, limitations on the approved indications
for which they may be used or suspension or withdrawal of approvals. Regardless of the merits or eventual outcome, liability claims may
also result in decreased demand for our product candidates, injury to our reputation, costs to defend the related litigation, a diversion
of management’s time and our resources, substantial monetary awards to trial participants or patients and a decline in our company
valuation. While we currently have clinical trial liability insurance, we do not have product candidates liability insurance and do not
anticipate obtaining product candidates liability insurance until such time as we have received FDA or other comparable foreign authority
approval for a product candidates and there is a product candidates that is being provided to patients outside of clinical trials. Any
insurance we have or may obtain may not provide sufficient coverage against potential liabilities. In some countries, the institution
or the doctors involved do not have sufficient insurance to cover their activities. Furthermore, clinical trial and product candidates
liability insurance are becoming increasingly expensive. As a result, we may be unable to obtain sufficient insurance at a reasonable
cost to protect us against losses caused by clinical trial and product candidates liability claims that could have a material adverse
effect on our business.
Risks
Related to this Offering and Ownership of Our Securities
The market
price of our securities may be highly volatile, and you may not be able to resell your Ordinary Shares at or above the initial public
offering price.
Prior
to this offering, there has not been a public market for our securities. Although we have
been approved to list our Ordinary Shares on Nasdaq, an active trading market for the Ordinary
Shares may not develop or be sustained. If an active trading market for our Ordinary Shares
does not develop following this offering, you may not be able to sell your Ordinary Shares
quickly or at the market price. The initial public offering price for the Units was determined
by negotiations between us and the underwriter and may not be indicative of prices that will
prevail in the trading market.
The
trading price of each of our Ordinary Shares is likely to be volatile. The following factors, in addition to other risk factors described
in this section, may have a significant impact on the market price of such securities:
| |
● |
inability to obtain the approvals
necessary to commence clinical trials; |
| |
|
|
| |
● |
unsatisfactory results of clinical trials; |
| |
|
|
| |
● |
announcements of regulatory approval or the failure
to obtain it, or specific label indications or patient populations for its use, or changes or delays in the regulatory review process; |
| |
|
|
| |
● |
announcements of innovations or new product candidates
by us or our competitors; |
| |
|
|
| |
● |
adverse actions taken by regulatory authorities with
respect to our clinical trials, manufacturing supply chain or sales and marketing activities; |
| |
|
|
| |
● |
any adverse changes to our relationship with manufacturers
or suppliers; |
| |
|
|
| |
● |
any intellectual property infringement actions in which
we may become involved; |
| |
|
|
| |
● |
achievement of expected product candidates sales and
profitability or our failure to meet expectations; |
| |
|
|
| |
● |
our commencement of, or involvement in, litigation; |
| |
|
|
| |
● |
any major changes in our board of directors or management; |
| |
|
|
| |
● |
our ability to recruit and retain qualified regulatory,
research and development personnel; |
| |
|
|
| |
● |
legislation in the United States relating to the sale
or pricing of medical devices; |
| |
|
|
| |
● |
the depth of the trading market in our securities; |
| |
● |
termination of the lock-up agreements or other restrictions
limiting our ability or that of any of our existing shareholders to sell our Ordinary Shares (or any other securities that we may
issue, if any) after this offering; |
| |
|
|
| |
● |
economic weakness, including inflation, or political
instability in particular foreign economies and markets; |
| |
|
|
| |
● |
business interruptions resulting from a local or worldwide
pandemic, such as any resurgence of COVID-19, geopolitical actions, including war and terrorism, or natural disasters; |
| |
|
|
| |
● |
the granting or exercise of employee stock options
or other equity awards; and |
| |
|
|
| |
● |
changes in investors’ and securities analysts’
perception of the business risks and conditions of our business. |
In
addition, the stock market in general, and the Nasdaq in particular, have experienced extreme price and volume fluctuations that have
often been unrelated or disproportionate to the operating performance of small companies. Broad market and industry factors may negatively
affect the market price of our securities, regardless of our actual operating performance. Further, a systemic decline in the financial
markets and related factors beyond our control may cause our share price to decline rapidly and unexpectedly.
The
Warrants are speculative in nature.
Except
as otherwise set forth therein, the Warrants offered in this offering do not confer any rights
of Ordinary Share ownership on their holders, such as voting rights, but rather merely represent
the right to acquire Ordinary Shares at a fixed price for a limited period of time. Specifically,
commencing on the date of issuance, holders of the Warrants may exercise their right to acquire
Ordinary Shares and pay an exercise price of (subject to certain adjustments, see “Description
of Securities We are Offering”) $4.38 per Ordinary Share (with such exercise price
equal to the public offering price per Unit), prior to five years from the date of issuance,
after which date any unexercised Warrants will expire and have no further value. There can
be no assurance that the market price of our Ordinary Shares will ever equal or exceed the
exercise price of the Warrants offered by this prospectus. In the event that our Ordinary
Shares price does not exceed the exercise price of such Warrants during the period when such
Warrants are exercisable, the Warrants may not have any value.
There
is no established market for the Warrants being offered in this offering and we do not intend to apply to list the Warrantson any securities
exchange or other nationally recognized trading system.
There
is no established trading market for the Warrants offered in this offering. We do not intend
to apply to list the Warrants on any securities exchange or other nationally recognized trading
system. Without an active trading market, the liquidity of the Warrants will be limited.
Future
sales of our Ordinary Shares could reduce the market price of our Ordinary Shares.
Substantial
sales of our Ordinary Shares on Nasdaq, including following this offering, may cause the market price of our Ordinary Shares to decline.
Sales by us or our security holders of substantial amounts of our Ordinary Shares, or the perception that these sales may occur in the
future, could cause a reduction in the market price of our Ordinary Shares.
The
issuance of any additional Ordinary Shares or any securities that are exercisable for or convertible into Ordinary Shares, may have an
adverse effect on the market price of our Ordinary Shares and will have a dilutive effect on our existing shareholders and holders of
Ordinary Shares.
Participation
in this offering by certain of our existing shareholders, including beneficial owners of greater than 5% of our share capital, could
reduce the public float for our shares.
Prior
to this offering, there has been no public market for our Ordinary Shares. Although our Ordinary
Shares have been approved for listing on Nasdaq, we cannot guarantee an active public market
for our Ordinary Shares will develop or be sustained after this offering. If an active and
liquid trading market does not develop, you may have difficulty selling or may not be able
to sell any of the Ordinary Shares that you purchase.
Certain
of our existing shareholders, including beneficial owners of greater than 5% of our share capital, have indicated an interest in purchasing
up to an aggregate of approximately $1 million of Units in this offering, which represents approximately 24% of the total Units in this
offering, based on the public offering price of $4.38 per Unit. However, because indications of interest are not binding agreements or
commitments to purchase, the underwriters may determine to sell more, less or no Units in this offering to any of these shareholders,
or any of these shareholders may determine to purchase more, less or no Units in this offering. If these shareholders are allocated all
or a portion of the Units in which each has indicated an interest in this offering or are allocated more Units than each has indicated
an interest in this offering, and these shareholders purchase any such Units, such purchase could reduce the available public float for
our shares.
Our executive
officers, directors and principal shareholders will maintain the ability to exert significant control over matters submitted to our shareholders
for approval.
As
of October 2, 2024, our executive officers, directors and principal shareholders who own more than 5% of our outstanding Ordinary Shares,
in the aggregate, beneficially own shares representing approximately 87% of our share capital. Upon completion of this offering, our
executive officers, directors and principal shareholders who own more than 5% of our outstanding Ordinary Shares, in the aggregate, will
beneficially own approximately 67% of our outstanding ordinary shares. As a result, if these shareholders were to act together, they
would be able to exert significant influence over all matters submitted to our shareholders for approval (including a prospective acquisition
or other change of control of our company), as well as our management and affairs.
Our Selling
Shareholders have purchased their shares at a price that is lower than the purchase price of the shares in this offering and will be
able to sell their shares after completion of this offering subject to restrictions under the lock-up requirement.
Our
Selling Shareholders have purchased their Ordinary Shares at an average price of approximately
$2.15 per share, which is substantially lower than the initial public offering price of $4.38
per Unit. The Ordinary Shares issued to the Selling Shareholders are “restricted”
securities under applicable U.S. federal and state securities laws and are being registered
to provide the Selling Shareholders the opportunity to sell those Ordinary Shares. Because
these shareholders have paid a lower price per Ordinary Share than participants in this offering,
they may be more willing to accept a lower sales price than the IPO price. This fact could
impact the trading price of the Ordinary Shares following completion of the offering to the
detriment of participants in this offering. Other than certain affiliates holding Ordinary
Shares, the remaining Selling Shareholders are not subject to any lock-up or leakage agreements
and have the right to sell the shares being registered hereby at any time. See “Shares
Eligible for Future Sale—Eligibility of Restricted Shares for Sale in the Public Market”
for additional information.
Management
will have broad discretion as to the use of the proceeds from this offering.
Our
management will have broad discretion in the allocation of the net proceeds and could use them for purposes other than those contemplated
at the time of this offering and as described in the section titled “Use of Proceeds.” Our shareholders may not agree
with the manner in which our management chooses to allocate and spend the net proceeds.
U.S. shareholders
may suffer adverse tax consequences if we are characterized as a passive foreign investment company, or PFIC, for U.S. federal income
tax purposes.
Based
on the projected composition of our income and valuation of our assets, we do not believe
that we were a PFIC for 2023, and do not expect to be a PFIC for 2024 and in the future,
although there can be no assurance in this regard. The determination of whether we are a
PFIC is made on an annual basis and will depend on the composition of our income and assets
from time to time. We will be treated as a PFIC for U.S. federal income tax purposes in any
taxable year in which either (1) at least 75% of our gross income is “passive income”
or (2) on quarterly average at least 50% of our assets by value produce passive income or
are held for the production of passive income. Passive income for this purpose generally
includes, among other things, certain dividends, interest, royalties, rents and gains from
commodities and securities transactions and from the sale or exchange of property that gives
rise to passive income. Passive income also includes amounts derived by reason of the temporary
investment of funds, including those raised in a public offering. In determining whether
a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each
corporation in which it owns, directly or indirectly, at least a 25% interest (by value)
is taken into account. The tests for determining PFIC status are applied annually and it
is difficult to make accurate projections of future income and assets which are relevant
to this determination. In addition, our PFIC status may depend in part on the market value
of our Ordinary Shares. Accordingly, there can be no assurance that we currently are not
or will not become a PFIC in subsequent years. If we were a PFIC in 2023, or are a PFIC in
any subsequent taxable year during which a U.S. taxpayer holds our Ordinary Shares (and Warrants,
which are treated as PFIC stock), such U.S. taxpayer would be subject to certain adverse
U.S. federal income tax rules. In particular, if the U.S. taxpayer did not make an election
to treat us as a “qualified electing fund”, or QEF, or make a “mark-to-market”
election, then “excess distributions” to the U.S. taxpayer, and any gain realized
on the sale or other disposition of our Ordinary Shares by the U.S. taxpayer: (1) would be
allocated ratably over the U.S. taxpayer’s holding period for our Ordinary Shares;
(2) the amount allocated to the current taxable year and any period prior to the first day
of the first taxable year in which we were a PFIC would be taxed as ordinary income; and
(3) the amount allocated to each of the other taxable years would be subject to tax at the
highest rate of tax in effect for the applicable class of taxpayer for that year, and an
interest charge for the deemed deferral benefit would be imposed with respect to the resulting
tax attributable to each such other taxable year. In addition, if the U.S. Internal Revenue
Service, or the IRS, determines that we are a PFIC for a year with respect to which we have
determined that we were not a PFIC, it may be too late for a U.S. taxpayer to make a timely
QEF or mark-to-market election. A U.S. taxpayer that has held our Ordinary Shares during
a period when we were a PFIC will generally be subject to the foregoing rules, unless we
cease to be a PFIC and such U.S. taxpayer makes a “deemed sale” election with
respect to our ADSs or our ordinary shares. If we are a PFIC in any year, U.S. taxpayers
may be subject to additional IRS filing requirements, including the filing of IRS Form 8621,
as a result of directly or indirectly owning stock of a PFIC. We do not intend to notify
U.S. taxpayers that hold our Ordinary Shares if we believe we will be treated as a PFIC for
any taxable year in order to enable U.S. taxpayers to consider whether to make a QEF election.
In addition, we do not intend to furnish such U.S. taxpayers annually with information needed
in order to complete IRS Form 8621 and to make and maintain a valid QEF election for any
year in which we are a PFIC. U.S. taxpayers that hold our Ordinary Shares are strongly urged
to consult their tax advisors about the PFIC rules, including tax return filing requirements
and the eligibility, manner, and consequences to them of making a QEF or mark-to-market election
with respect to our Ordinary Shares in the event that we are a PFIC. See “Taxation—Certain
Material U.S. Federal Income Tax Considerations—Passive Foreign Investment Companies”
for additional information.
If a United
States person is treated as owning at least 10% of our Ordinary Shares, such holder may be subject to adverse U.S. federal income tax
consequences.
If
a United States person is treated as owning (directly, indirectly or constructively) at least 10% of the value or voting power of our
Ordinary Shares, such person may be treated as a “United States shareholder” with respect to each “controlled foreign
corporation” in our Company (if any). A United States shareholder of a controlled foreign corporation may be required to annually
report and include in its U.S. taxable income its pro rata share of “Subpart F income,” “global intangible low-taxed
income” and investments in U.S. property by controlled foreign corporations, whether or not we make any distributions, and may
be subject to tax reporting obligations. An individual that is a United States shareholder with respect to a controlled foreign corporation
generally would not be allowed certain tax deductions or foreign tax credits that would be allowed to a United States shareholder that
is a U.S. corporation. A failure to comply with these reporting obligations may subject you to significant monetary penalties and may
prevent the statute of limitations with respect to your U.S. federal income tax return for the year for which reporting was due from
starting. We cannot provide any assurances that we will assist any holder of our Ordinary Shares in determining whether such investors
are treated as a United States shareholder with respect to any “controlled foreign corporations” in our group (if any) or
furnish to any United States holders of our Ordinary Shares information that may be necessary to comply with the aforementioned reporting
and tax paying obligations. A United States holder of our Ordinary Shares should consult its tax advisors regarding the potential application
of these rules to its investment in the shares.
As
a foreign private issuer, we are permitted, and intend, to follow certain home country corporate governance practices instead of otherwise
applicable Nasdaq requirements, and we will not be subject to certain U.S. securities laws including, but not limited to, U.S. proxy
rules and the filing of certain Exchange Act reports.
As
a foreign private issuer, we will be permitted, and intend, to follow certain home country corporate governance practices instead of
those otherwise required by the Nasdaq Stock Market for domestic U.S. issuers. Following our home country governance practices as opposed
to the requirements that would otherwise apply to a U.S. company listed on The Nasdaq Global Market may provide less protection to you
than what is accorded to investors under the listing rules of Nasdaq applicable to domestic U.S. issuers.
As
a foreign private issuer, we will be exempt from the rules and regulations under the Securities Exchange Act of 1934, or the Exchange
Act, related to the furnishing and content of proxy statements, including the applicable compensation disclosure requirements. Nevertheless,
pursuant to regulations promulgated under the Israeli Companies Law, 5759-1999, or the Israeli Companies Law, we are required to disclose
the annual compensation of our five most highly compensated office holders on an individual basis. Such disclosure will not be as extensive
as that required of a U.S. domestic issuer. Our officers, directors and principal shareholders will also be exempt from the reporting
and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under
the Exchange Act to file reports and financial statements with the SEC as frequently or as promptly as U.S. domestic companies whose
securities are registered under the Exchange Act and we will be exempt from filing quarterly reports with the SEC under the Exchange
Act. Moreover, we will not be required to comply with Regulation FD, which restricts the selective disclosure of material information,
although we intend to voluntarily adopt a corporate disclosure policy substantially similar to Regulation FD. These exemptions and
leniencies will reduce the frequency and scope of information and protections to which you may otherwise have been eligible in relation
to a U.S. domestic issuer.
We
would lose our foreign private issuer status if a majority of our shares are owned by U.S. residents and a majority of our directors
or executive officers are U.S. citizens or residents or we fail to meet additional requirements necessary to avoid loss of foreign private
issuer status. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly higher.
If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer
forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. We may also be required
to modify certain of our policies to comply with accepted governance practices associated with U.S. domestic issuers. Such conversion
and modifications will involve additional costs. In addition, we would lose our ability to rely upon exemptions from certain corporate
governance requirements on U.S. stock exchanges that are available to foreign private issuers.
We
are an emerging growth company and the reduced disclosure requirements applicable to emerging growth companies may make our Ordinary
Shares less attractive to investors.
We
are an emerging growth company, as defined in the JOBS Act, and we may take advantage of certain exemptions from various requirements
that are applicable to other public companies that are not emerging growth companies.
For
as long as we remain an emerging growth company we are permitted and intend to rely on exemptions from certain disclosure requirements
that are applicable to other public companies that are not “emerging growth companies.” These exemptions include:
| |
● |
not being required to comply with the auditor attestation
requirements in the assessment of our internal control over financial reporting; |
| |
|
|
| |
● |
Section 107 of the JOBS Act, which provides that an
“emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the
Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. This means that
an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise
apply to private companies. We have elected to delay such adoption of new or revised accounting standards. As a result of this adoption,
our financial statements may not be comparable to companies that comply with the public company effective date; |
| |
|
|
| |
● |
not being required to comply with any requirement that
may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s
report providing additional information about the audit and the financial statements; |
| |
|
|
| |
● |
reduced disclosure obligations regarding executive
compensation; and |
| |
|
|
| |
● |
exemptions from the requirements of holding a nonbinding
advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. |
We
will remain an emerging growth company until the earliest of: (i) the last day of the fiscal year in which we have total annual
gross revenues of $1.235 billion or more; (ii) the date on which we have issued more than $1.0 billion in nonconvertible
debt during the previous three years; (iii) the date on which we are deemed to be a large accelerated filer under the rules of the
SEC; or (iv) the last day of the fiscal year following the fifth anniversary of this offering. We have opted out of the extended
transition period made available to emerging growth companies to comply with newly adopted public company accounting requirements.
When
we are no longer deemed to be an emerging growth company, we will not be entitled to the exemptions provided in the JOBS Act discussed
above. We cannot predict if investors will find our Ordinary Shares less attractive as a result of our reliance on exemptions under the
JOBS Act. If some investors find our Ordinary Shares less attractive as a result, there may be a less active trading market for our Ordinary
Shares and our share price may be more volatile.
If
you purchase securities in this offering, you will incur immediate and substantial dilution in the book value of your shares.
The
offering price of the Ordinary Shares is substantially higher than the net tangible book
value per share of our Ordinary Shares. Therefore, if you purchase securities in this offering,
you will pay a price per Ordinary Share that substantially exceeds our net tangible book
value per Ordinary Share after this offering. Based on the public offering price of $4.38
per Unit, you will experience immediate dilution of $3.60 per Ordinary Share, representing
the difference between our pro forma net tangible book value per Ordinary Share after giving
effect to this offering and the offering price. See “Dilution” for further information.
Certain
recent initial public offerings of companies with public floats comparable to the anticipated public float of Polyrizon have experienced
extreme volatility that was seemingly unrelated to the underlying performance of the respective company.
Our
Ordinary Shares may be subject to extreme volatility that is seemingly unrelated to the underlying performance of our business. Recently,
companies with comparable anticipated public floats and initial public offering sizes have experienced instances of extreme stock price
run-ups followed by rapid price declines, and such stock price volatility was seemingly unrelated to the respective company’s underlying
performance. Although the specific cause of such volatility is unclear, our anticipated public float may amplify the impact of the actions
taken by a few shareholders on the price of our Ordinary Shares, which may cause our share price to deviate, potentially significantly,
from a price that better reflects the underlying performance of our business. Should our Ordinary Shares experience run-ups and declines
that are seemingly unrelated to our actual or expected operating performance and financial condition or prospects, prospective investors
may have difficulty assessing the rapidly changing value of our Ordinary Shares. In addition, investors of shares of our Ordinary Shares
may experience losses, which may be material, if the price of our Ordinary Shares declines after this offering or if such investors purchase
shares of our Ordinary Shares prior to any price decline.
Risks
Related to Israeli Law and Our Operations in Israel
Our headquarters
and other significant operations are located in Israel, and, therefore, our results may be adversely affected by political, economic
and military instability in Israel, including the recent attack by Hamas and other terrorist organizations from the Gaza Strip and Israel’s
war against them.
Our
executive offices, research and development laboratories are located in Ra’anana, Israel. In addition, the majority of our key
employees, officers and directors are residents of Israel. Accordingly, political, geopolitical, economic and military conditions in
Israel may directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken
place between Israel and groups in its neighboring countries, and between Israel and the Hamas (an Islamist militia and political group
in the Gaza Strip), Hezbollah (an Islamist militia and political group in Lebanon) and other terrorist organizations active in the region.
In
particular, in October 2023, Hamas terrorists infiltrated Israel’s southern border from the Gaza Strip and conducted a series of
attacks on civilian and military targets. Hamas also launched extensive rocket attacks on the Israeli population and industrial centers
located along Israel’s border with the Gaza Strip and in other areas within the State of Israel. These attacks resulted in thousands
of deaths and injuries, and kidnapping of civilians and soldiers. Following the attack, Israel’s security cabinet declared war
against Hamas and commenced a military campaign against Hamas and these terrorist organizations in parallel continued rocket and terror
attacks. As a result of the events of October 7, 2023 whereby Hamas terrorists invaded southern Israel and launched thousands of rockets
in a widespread terrorist attack on Israel, the Israeli government declared that the country was at war and the Israeli military began
to call-up reservists for active duty. None of our employees were called up for active duty; however military service call ups that result
in absences of personnel from us for an extended period of time may materially and adversely affect our business, prospects, financial
condition and results of operations. As of the date hereof, we currently have two full-time and three part-time employees, with four
employees located in Israel and 1 employee located outside of Israel.
Since
the war broke out on October 7, 2023, our operations have not been adversely affected by this situation, and we have not experienced
disruptions to our development. Our commercial operations, including Product Development, Regulatory Compliance, Market Research, Commercialization
Strategy, Partnerships and Collaborations, Intellectual Property Management take place in the Tel Aviv, Israel area and remain unaffected
by the war against Hamas. However, the intensity and duration of Israel’s current war against Hamas is difficult to predict at
this stage, as are such war’s economic implications on the Company’s business and operations and on Israel’s economy
in general. If the war extends for a long period of time or expands to other fronts, such as Lebanon, Syria and the West Bank, our operations
may be adversely affected.
In
addition, since the commencement of these events, there have been continued hostilities along Israel’s northern border with Lebanon
(with the Hezbollah terror organization) and southern border (with the Houthi movement in Yemen). It is possible that hostilities with
Hezbollah in Lebanon will escalate, and that other terrorist organizations, including Palestinian military organizations in the West
Bank as well as other hostile countries will join the hostilities. Such clashes may escalate in the future into a greater regional
conflict. In addition, Iran recently launched a direct attack on Israel involving hundreds of drones and missiles and has threatened
to continue to attack Israel and is widely believed to be developing nuclear weapons. Iran is also believed to have a strong influence
among extremist groups in the region, such as Hamas in Gaza, Hezbollah in Lebanon, the Houthi movement in Yemen and various rebel militia
groups in Syria. These situations may potentially escalate in the future to more violent events which may affect Israel and us. Any armed
conflicts, terrorist activities or political instability in the region could adversely affect business conditions, could harm our results
of operations and could make it more difficult for us to raise capital. Parties with whom we do business may decline to travel to Israel
during periods of heightened unrest or tension, forcing us to make alternative arrangements when necessary in order to meet our business
partners face to face. In addition, the political and security situation in Israel may result in parties with whom we have agreements
involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to
force majeure provisions in such agreements. Further, in the past, the State of Israel and Israeli companies have been subjected to economic
boycotts. Several countries still restrict business with the State of Israel and with Israeli companies. These restrictive laws and policies
may have an adverse impact on our operating results, financial condition or the expansion of our business. Any hostilities involving
Israel or the interruption or curtailment of trade between Israel and its trading partners could adversely affect our operations and
results of operations.
Our
commercial insurance does not cover losses that may occur as a result of events associated with the security situation in the Middle
East. Although the Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks
or acts of war, we cannot assure you that this government coverage will be maintained or, if maintained, will be sufficient to compensate
us fully for damages incurred. Any losses or damages incurred by us could have a material adverse effect on our business, financial condition
and results of operations. Any armed conflicts or political instability in the region would likely negatively affect business conditions
and could harm our results of operations.
Finally,
political conditions within Israel may affect our operations. Israel has held five general elections between 2019 and 2022, and prior
to October 2023, the Israeli government pursued extensive changes to Israel’s judicial system, which sparked extensive political
debate and unrest. To date, these initiatives have been substantially put on hold. Actual or perceived political instability in Israel,
or any negative changes in the political environment, may individually or in the aggregate adversely affect the Israeli economy and,
in turn, our business, financial condition, results of operations and growth prospects.
Our
operations are subject to currency and interest rate fluctuations.
Although
our functional currency is the U.S. dollar, and our financial records are maintained in U.S. dollars, we also incur expenses in Euros
and New Israeli Shekels. In the future, we expect that a substantial portion of our revenues will be generated in U.S. dollars, Euros
and other foreign currencies, although we currently incur a significant portion of our expenses in currencies other than U.S. dollars,
mainly New Israeli Shekels. As a result, we are affected by foreign currency exchange fluctuations through both translation risk and
transaction risk, and our financial results may be affected by fluctuations in the exchange rates of currencies in the countries in which
our prospective product candidates may be sold.
We
received Israeli government grants for certain of our research and development activities, the terms of which may require us to pay royalties
and to satisfy specified conditions in order to manufacture product candidates and transfer technologies outside of Israel. If we fail
to satisfy these conditions, we may be required to pay penalties and refund grants previously received.
Our
research and development efforts have been financed in part through royalty-bearing grants in an aggregate amount of $620,000 that we
received from the IIA during 2007-2010. The last IIA-approved research and development grants ended on December 31, 2018. With respect
to the royalty-bearing grants we are committed to pay royalties at a rate between 3% and 4.5% on sales proceeds from our product candidates
that were developed under IIA programs up to the total amount of grants received, linked to the U.S. dollar and bearing interest at an
annual rate of SOFR applicable to U.S. dollar deposits. As of October 2, 2024, our contingent liabilities regarding IIA grants received
by us were in an aggregate amount of $756,000 (including accumulated interest). We are further required to comply with the requirements
of the Israeli Encouragement of Industrial Research, Development and Technological Innovation Law, 5744-1984, as amended, and related
regulations, or the Research Law, with respect to those past grants. When a company develops know-how, technology or product candidates
using IIA grants, the terms of these grants and the Research Law restrict the transfer or license of such know-how, and the transfer
of manufacturing or manufacturing rights of such product candidates, technologies or know-how outside of Israel, without the prior approval
of the IIA. Therefore, the discretionary approval of an IIA committee would be required for any transfer or license to third parties
inside or outside of Israel of know how or for the transfer outside of Israel of manufacturing or manufacturing rights related to those
aspects of such technologies. We may not receive those approvals. Furthermore, the IIA may impose certain conditions on any arrangement
under which it permits us to transfer technology or development.
The
transfer or license of IIA-supported technology or know-how outside of Israel and the transfer
of manufacturing of IIA-supported product candidates, technology or know-how outside of Israel
may involve the payment of significant amounts, depending upon the value of the transferred
or licensed technology or know-how, our research and development expenses, the amount of
IIA support, the time of completion of the IIA-supported research project and other factors.
These restrictions and requirements for payment may impair our ability to sell, license or
otherwise transfer our technology assets outside of Israel or to outsource or transfer development
or manufacturing activities with respect to any product candidates or technology outside
of Israel. It should be noted that an event of change of control may be considered a transfer
of our technology or assets and therefore require the prior approval of the IIA before completing
such a transaction. The IIA may also require potential acquirers to execute undertakings
to procure our continued adherence to the terms of the IIA grants and/or impose other restrictions
on such transactions. Furthermore, the consideration available to our shareholders in a transaction
involving the transfer outside of Israel of technology or know-how developed with IIA funding
(such as a merger or similar transaction) may be reduced by any amounts that we are required
to pay to the IIA.
Provisions
of Israeli law and our amended and restated articles of association, or the Articles, may delay, prevent or otherwise impede a merger
with, or an acquisition of, us, which could prevent a change of control, even when the terms of such a transaction are favorable to us
and our shareholders.
Israeli
corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals
for transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to such types
of transactions. For example, a merger may not be consummated unless at least 50 days have passed from the date on which a merger
proposal is filed by each merging company with the Israel Registrar of Companies and at least 30 days have passed from the date
on which the shareholders of both merging companies have approved the merger. In addition, a majority of each class of securities of
the target company must approve a merger. Moreover, a tender offer for all of a company’s issued and outstanding shares can only
be completed if the acquirer receives positive responses from the holders of at least 95% of the issued share capital. Completion of
the tender offer also requires approval of a majority of the offerees that do not have a personal interest in the tender offer, unless,
following consummation of the tender offer, the acquirer would hold at least 98% of the Company’s outstanding shares. Furthermore,
the shareholders, including those who indicated their acceptance of the tender offer, may, at any time within six months following the
completion of the tender offer, claim that the consideration for the acquisition of the shares does not reflect their fair market value,
and petition an Israeli court to alter the consideration for the acquisition accordingly, unless the acquirer stipulated in its tender
offer that a shareholder that accepts the offer may not seek such appraisal rights, and the acquirer or the company published all required
information with respect to the tender offer prior to the tender offer’s response date.
Furthermore,
Israeli tax considerations may make potential transactions unappealing to us or to our shareholders whose country of residence does not
have a tax treaty with Israel exempting such shareholders from Israeli tax. For example, Israeli tax law does not recognize tax-free
share exchanges to the same extent as U.S. tax law. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances
but makes the deferral contingent on the fulfillment of a number of conditions, including, in some cases, a holding period of two years
from the date of the transaction during which sales and dispositions of shares of the participating companies are subject to certain
restrictions. Moreover, with respect to certain share swap transactions, the tax deferral is limited in time, and when such time expires,
the tax becomes payable even if no disposition of the shares has occurred. These provisions could delay, prevent or impede an acquisition
of us or our merger with another company, even if such an acquisition or merger would be beneficial to us or to our shareholders.
It
may be difficult to enforce a judgment of a U.S. court against us and our executive officers and directors in Israel or the United States,
to assert U.S. securities laws claims in Israel or to serve process on our executive officers and directors and these experts.
We
were incorporated in Israel. Substantially all of our executive officers and directors reside outside of the United States, and all of
our assets and most of the assets of these persons are located outside of the United States. Therefore, a judgment obtained against us,
or any of these persons, including a judgment based on the civil liability provisions of the U.S. federal securities laws, may not be
collectible in the United States and may not be enforced by an Israeli court. It also may be difficult for you to effect service of process
on these persons in the United States or to assert U.S. securities law claims in original actions instituted in Israel. Additionally,
it may be difficult for an investor, or any other person or entity, to initiate an action with respect to U.S. securities laws in Israel.
Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most
appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that
Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must
be proven as a fact by expert witnesses, which can be a time consuming and costly process. Certain matters of procedure will also be
governed by Israeli law. There is little binding case law in Israel that addresses the matters described above. As a result of the difficulty
associated with enforcing a judgment against us in Israel, you may not be able to collect any damages awarded by either a U.S. or foreign
court.
Our Articles
shall be effective upon the closing this offering will provide that unless the Company consents otherwise, the competent courts of Tel
Aviv, Israel shall be the sole and exclusive forum for substantially all disputes between the Company and its shareholders under the
Companies Law and the Israeli Securities Law.
The
competent courts of Tel Aviv, Israel shall be the exclusive forum for (i) any derivative action or proceeding brought on behalf of the
Company, (ii) any action asserting a claim of breach of fiduciary duty owed by any director, officer or other employee of the Company
to the Company or the Company’s shareholders, or (iii) any action asserting a claim arising pursuant to any provision of the Companies
Law or the Israeli Securities Law, 5728-1968 (the “Israeli Securities Law”). This exclusive forum provisions is intended
to apply to claims arising under Israeli Law and would not apply to claims brought pursuant to the Securities Act or the Exchange Act
or any other claim for which United States federal courts would have exclusive or concurrent jurisdiction. Such exclusive forum provision
in our Articles will not relieve the Company of its duties to comply with federal securities laws and the rules and regulations thereunder,
and shareholders of the Company will not be deemed to have waived the Company’s compliance with these laws, rules and regulations.
This exclusive forum provision may limit a shareholders ability to bring a claim in a judicial forum of its choosing for disputes with
the Company or its directors or other employees which may discourage lawsuits against the Company, its directors, officers and employees
and may result in increased costs for claims required to be brought before Israeli courts.
Your
rights and responsibilities as a shareholder will be governed in key respects by Israeli laws, which differs in some material respects
from the rights and responsibilities of shareholders of U.S. companies.
The
rights and responsibilities of the holders of our Ordinary Shares are governed by our Articles and by Israeli law. These rights and responsibilities
differ in some material respects from the rights and responsibilities of shareholders in U.S. companies. In particular, a shareholder
of an Israeli company has a duty to act in good faith and in a customary manner in exercising its rights and performing its obligations
towards the company and other shareholders, and to refrain from abusing its power in such company, including, among other things, in
voting at a general meeting of shareholders on matters such as amendments to a company’s articles of association, increases in
a company’s authorized share capital, mergers and acquisitions and related party transactions requiring shareholder approval, as
well as a general duty to refrain from discriminating against other shareholders. In addition, a shareholder who is aware that it possesses
the power to determine the outcome of a vote at a meeting of the shareholders or to appoint or prevent the appointment of a director
or executive officer in the company has a duty of fairness toward the company. However, Israeli law does not define the substance of
this duty of fairness. There is limited case law available to assist us in understanding the nature of these duties or the implications
of these provisions. These provisions may be interpreted to impose additional obligations and liabilities on holders of our Ordinary
Shares that are not typically imposed on shareholders of U.S. companies.
General
Risk Factors
Our
business and operations would suffer in the event of computer system failures, cyber attacks or a deficiency in our cybersecurity.
Despite
the implementation of security measures intended to secure our data against impermissible access and to preserve the integrity and confidentiality
of our data, our internal computer systems, and those of third parties on which we rely, are vulnerable to damage from computer viruses,
malware, natural disasters, terrorism, war, telecommunication and electrical failures, cyber-attacks or cyber-intrusions over the Internet,
attachments to emails, persons inside our organization, or persons with access to systems inside our organization. The risk of a security
breach or disruption, particularly through cyber attacks or cyber intrusion, including by computer hackers, foreign governments, and
cyber terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around
the world have increased. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption
of our drug development programs. For example, the loss of clinical trial data from completed or ongoing or planned clinical trials could
result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent
that any disruption or security breach was to result in a loss of or damage to our data or applications, or inappropriate disclosure
of confidential or proprietary information, we could incur material legal claims and liability, including under data privacy laws such
as the GDPR, damage to our reputation, and the further development of our drug candidates could be delayed.
Our
future success depends in part on our ability to retain our senior management team and to attract, retain and motivate other qualified
personnel.
We
are highly dependent on the members of our senior management team. The loss of their services without a proper replacement may adversely
impact the achievement of our objectives. Our employees may leave our employment at any time. Recruiting and retaining other qualified
employees, consultants and advisors for our business, including scientific and technical personnel, will also be critical to our success.
There is currently a shortage of skilled personnel in our industry, which is likely to continue for the foreseeable future. As a result,
competition for skilled personnel is intense, and the turnover rate can be high. We may not be able to attract and retain personnel on
acceptable terms given the competition among numerous medical device and pharmaceutical companies for individuals with similar skill
sets. In addition, failure to succeed in preclinical studies or clinical trials may make it more challenging to recruit and retain qualified
personnel. The inability to recruit and retain qualified personnel, or the loss of the services of any members of our senior management
team without proper replacement, may impede the progress of our research, development and commercialization objectives. We do not maintain
key man insurance for our senior management team.
We
will continue to incur significant increased costs as a result of operating as a public company in the United States, and our management
will be required to devote substantial time to new compliance initiatives.
As
a public company whose Ordinary Shares are listed in the United States, we are subject to an extensive regulatory regime, requiring us,
among other things, to maintain various internal controls and facilities and to prepare and file periodic and current reports and statements,
including reports on the effectiveness of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley
Act of 2002. Complying with these requirements will be costly and time consuming. We will need to retain additional employees to supplement
our current finance staff, and we may not be able to do so in a timely manner, or at all. In the event that we are unable to demonstrate
compliance with our obligations as a public company in a timely manner, or are unable to produce timely or accurate financial statements,
we may be subject to sanctions or investigations by regulatory authorities, such as the SEC or The Nasdaq Global Market, and investors
may lose confidence in our operating results and the price of our Ordinary Shares could decline.
Our
independent registered public accounting firm was not engaged to perform an audit of our internal control over financial reporting, and
as long as we remain an emerging growth company, as such term is defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS
Act, we will be exempt from the requirement to have an independent registered public accounting firm perform such audit. Accordingly,
no such opinion was expressed or will be expressed any during any such period. Once we cease to qualify as an emerging growth company
our independent registered public accounting firm will be required to attest to our management’s annual assessment of the effectiveness
of our internal controls over financial reporting, which will entail additional costs and expenses.
Furthermore,
we are only in the early stages of determining formally whether our existing internal control over financial reporting systems are compliant
with Section 404 and whether there are any material weaknesses or significant deficiencies in our existing internal controls. These
controls and other procedures are designed to ensure that information required to be disclosed by us in the reports that we file with
the SEC is disclosed accurately and is recorded, processed, summarized and reported within the time periods specified in SEC rules and
forms.
We
have never paid cash dividends on our share capital, and we do not anticipate paying any cash dividends in the foreseeable future.
We
have never declared or paid cash dividends on our Ordinary Shares. We currently anticipate that we will retain future earnings for the
development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends in the foreseeable
future. As a result, capital appreciation, if any, of our Ordinary Shares will be investors’ sole source of gain for the foreseeable
future. In addition, Israeli law limits our ability to declare and pay dividends, and may subject our dividends to Israeli withholding
taxes.
If
securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they
adversely change their recommendations or publish negative reports regarding our business or our shares, our share price and trading
volume could decline.
The
trading market for our Ordinary Shares will be influenced by the research and reports that industry or securities analysts may publish
about us, our business, our market or our competitors. We do not have any control over these analysts and we cannot provide any assurance
that analysts will cover us or provide favorable coverage. If any of the analysts who may cover us adversely change their recommendation
regarding our shares, or provide more favorable relative recommendations about our competitors, our share price would likely decline.
If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility
in the financial markets, which in turn could cause our share price or trading volume to decline.
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus contains forward-looking statements concerning our business, operations and financial performance and condition, as well as
our plans, objectives and expectations for our business operations and financial performance and condition. Any statements contained
herein that are not statements of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking
statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,”
“continue,” “could,” “due,” “estimate,” “expect,” “goal,” “intend,”
“may,” “objective,” “plan,” “predict,” “potential,” “positioned,”
“seek,” “should,” “target,” “will,” “would,” and other similar expressions
that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology.
These forward-looking statements include, but are not limited to, statements about:
| ● | the
ability of our clinical trials to demonstrate safety and efficacy of our future product candidates,
and other positive results; |
| | | |
| ● | the
timing and focus of our future preclinical studies and clinical trials, and the reporting
of data from those studies and trials; |
| | | |
| ● | the
size of the market opportunity for our future product candidates, including our estimates
of the number of patients who suffer from the diseases we are targeting; |
| | | |
| ● | the
success of competing therapies that are or may become available; |
| | | |
| ● | the
beneficial characteristics, safety, efficacy and therapeutic effects of our future product
candidates; |
| | | |
| ● | our
ability to obtain and maintain regulatory approval of our future product candidates; |
| | | |
| ● | our
plans relating to the further development of our future product candidates, including additional
disease states or indications we may pursue; |
| | | |
| ● | existing
regulations and regulatory developments in the United States and other jurisdictions; |
| | | |
| ● | our
plans and ability to obtain or protect intellectual property rights, including extensions
of patent terms where available and our ability to avoid infringing the intellectual property
rights of others; |
| | | |
| ● | the
need to hire additional personnel and our ability to attract and retain such personnel; |
| | | |
| ● | our
estimates regarding expenses, future revenue, capital requirements and needs for additional
financing; |
| | | |
| ● | our
dependence on third parties; |
| | | |
| ● | our
financial performance; |
| | | |
| ● | the
period over which we estimate our existing cash and cash equivalents will be sufficient to
fund our future operating expenses and capital expenditure requirements; |
| | | |
| ● | our
ability to generate revenue and profit margin under our anticipated contracts which is subject
to certain risks; |
| | | |
| ● | difficulties
in our and our partners’ ability to recruit and retain qualified physicians and other
healthcare professionals, and enforce our non-compete agreements with our physicians; and |
| | | |
| ● | our
ability to restructure our operations to comply with future changes in government regulation. |
Forward-looking
statements are based on our management’s current expectations, estimates, forecasts and projections about our business and the
industry in which we operate and our management’s beliefs and assumptions, and are not guarantees of future performance or development
and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control. As a result, any or all
of our forward-looking statements in this prospectus may turn out to be inaccurate. Important factors that may cause actual results to
differ materially from current expectations include, among other things, those listed under “Risk Factors” and elsewhere
in this prospectus. Potential investors are urged to consider these factors carefully in evaluating the forward-looking statements.
The
forward-looking statements included in this prospectus speak only as of the date of this prospectus. Although we believe that the expectations
reflected in the forward-looking statements are reasonable, we cannot guarantee that future results, levels of activity, performance
and events and circumstances reflected in the forward-looking statements will be achieved or will occur. Except as required by law, we
assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in
the future. You should, however, review the factors and risks we describe in the reports we will file from time to time with the SEC
after the date of this prospectus. See “Where You Can Find More Information.”
USE
OF PROCEEDS
We
estimate that the net proceeds from the sale of Securities in this offering will be approximately $3.4 million, after deducting the estimated
underwriting discounts and commissions and estimated offering expenses payable by us. We will not receive any proceeds from the sale
of the Ordinary Shares by the Selling Shareholders. If the underwriter exercises its option to purchase up to an additional 143,835 Ordinary
Shares, and/or an additional 2,876,709 Warrants in full, we estimate that the net proceeds to us from this offering will be approximately
$4.5 million, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We
currently expect to use the net proceeds from this offering for the following purposes:
| ● | approximately
$450,000 to complete the preclinical development for our PL-14, PL-15 and PL-16 product candidates
(approximately $150,000 per product candidate); |
| ● | approximately
$600,000 to complete the clinical development for our PL-14 product candidate; |
| ● | approximately
$300,000 to complete in vitro feasibility studies of corticosteroid, benzodiazepine, naloxone
for our T&T platform technology product candidates; |
| ● | approximately
$310,000 to repay the April 2024 CLA and August 2024 CLA. The April 2024 CLA and August 2024
CLA bear interest at an annual rate of 4% and has a maturity date of April 2026 and August
2026, respectively. In accordance with the terms of the April 2024 CLA and August 2024 CLA,
upon the closing of this offering we will repay the April 2024 CLA Amount and August 2024
CLA Amount out of the proceedings received in this offering; and |
| ● | the remainder
for working capital and general corporate purposes and possible future acquisitions. |
Although
we currently anticipate that we will use the net proceeds from this offering as described above, there may be circumstances where a reallocation
of funds is necessary. Due to the uncertainties inherent in the clinical development and regulatory approval process, it is difficult
to estimate with certainty the exact amounts of the net proceeds from this offering that may be used for any of the above purposes on
a stand-alone basis. Amounts and timing of our actual expenditures will depend upon a number of factors, including our sales, marketing
and commercialization efforts, regulatory approval and demand for our product candidates, operating costs and other factors described
under “Risk Factors” in this prospectus. Accordingly, our management will have flexibility in applying the net proceeds from
this offering. An investor will not have the opportunity to evaluate the economic, financial or other information on which we base our
decisions on how to use the proceeds.
Based
on our current plans, we believe that our existing cash and cash equivalents will be sufficient to enable us to fund our operating expenses
and capital expenditure requirements through October 2024. We anticipate that these funds, together with the net proceeds of this offering,
will be sufficient to fund our operating expenses and capital expenditure requirements through 18 months after the completion of this
offering. We have based this estimate on assumptions that may prove to be incorrect, and we could use our available capital resources
sooner than we currently expect. See “Management’s Discussion and Analysis of Financial Condition and Results of Operation
– Liquidity and Capital Resources – Financings Requirements”.
Pending
our application of the net proceeds from this offering, we plan to invest such proceeds in short-term, investment-grade, interest-bearing
securities and depositary institutions.
DIVIDEND
POLICY
We
have never declared or paid any cash dividends to our shareholders of our Ordinary Shares, and we do not anticipate or intend to pay
cash dividends in the foreseeable future. Payment of cash dividends, if any, in the future will be at the discretion of our board of
directors in compliance with applicable legal requirements and will depend on a number of factors, including future earnings, our financial
condition, operating results, contractual restrictions, capital requirements, business prospects, our strategic goals and plans to expand
our business, applicable law and other factors that our board of directors may deem relevant.
The
Israeli Companies Law imposes further restrictions on our ability to declare and pay dividends. See “Description of Share Capital—Dividend
and Liquidation Rights” for additional information.
Payment
of dividends may be subject to Israeli withholding taxes. See “Taxation—Material Israeli Tax Considerations” for additional
information.
CAPITALIZATION
The
following table sets forth our cash and cash equivalents and our capitalization as of June 30, 2024:
| |
● |
On
a pro forma basis to give effect to the following events as if each event had occurred on or before June
30, 2024: (i) the conversion of 104,711 preferred shares into 104,711 Ordinary Shares; (ii) the repayment
of $151,000 pursuant to the 2024 convertible loan agreement; (iii) the issuance of 61,751 Ordinary Shares
pursuant to a $70,000 investment (according to a contractual agreement with some of the investors); and
(iv) the issuance, as consideration for the purchase of patent rights, of 320,000 ordinary shares in
August 2024 pursuant to the SciSparc License Agreement and the additional issuance as consideration for
such purchase, of 364,931 pre-funded warrants and 2,054,793 warrants to purchase ordinary shares upon
the same terms as the warrants to be issued in this offering, which will be issued upon the consummation
of this offering, based on the initial public offering price of $4.38 per Ordinary Share,. |
| |
● |
on a pro forma as adjusted
basis to give effect to the additional issuance of 958,903 Units in this offering, at the public offering price of $4.38 per Unit,
and after deducting underwriting discounts and commissions and estimated offering expenses, as if the sale of the securities had
occurred on June 30, 2024. |
You
should read this table in conjunction with the sections titled “Selected Financial Data” and “Management’s Discussion
and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere
in this prospectus.
| | |
As
of June 30, 2024 | |
| U.S.
dollars in thousands | |
Actual | | |
Pro
Forma | | |
Pro
Forma
As Adjusted (1) | |
| | |
(Unaudited) | |
| | |
| | |
| | |
| |
| Temporary
equity, no par value per share; 104,711 shares authorized, 104,711 shares issued and outstanding, actual; and no shares authorized,
issued and outstanding pro forma; and pro forma as adjusted | |
$ | 248 | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | |
| Shareholders’
equity: | |
| | | |
| | | |
| | |
| Ordinary
shares, no par value per share; 19,895,289 shares authorized, 2,749,077 shares issued and outstanding; 20,000,000 shares authorized
and 3,235,542 shares issued and outstanding, pro forma; 20,000,000 shares authorized and 4,194,445 shares issued and outstanding,
pro forma as adjusted | |
$ | - | | |
| - | | |
| - | |
| Additional
paid-in capital | |
$ | 4,102 | | |
| 7,437 | | |
| 10,386 | |
| Receivables
on account of shares | |
$ | (19 | ) | |
| - | | |
| - | |
| Accumulated
deficit | |
$ | (4,109 | ) | |
| (4,126 | ) | |
| (4,126 | ) |
| Total
shareholders’ equity (deficit) | |
$ | (26 | ) | |
| 3,311 | | |
| 6,260 | |
| | |
| | | |
| | | |
| | |
| Total
capitalization | |
$ | 222 | | |
| 3,311 | | |
| 6,260 | |
The
number of the Ordinary Shares to be issued and outstanding immediately after this offering
as shown above assumes that all of the Ordinary Shares offered hereby are sold, and is based
on 3,235,542, Ordinary Shares issued and outstanding as of the date of this prospectus. This
number excludes:
| |
● |
246,129 Ordinary Shares issuable
upon the exercise of options issued to directors, employees and consultants under our incentive option plan outstanding as of such
date, with exercise prices at a range of $0.02 -$1.1335 per share, of which options to purchase 146,064 Ordinary Shares were vested
as of such date (including options to purchase 42,744 Ordinary Shares that vest upon the completion of this offering); |
| |
● |
such number of Ordinary Shares
issuable upon the exercise of options representing 2.5% of the Company’s post-initial public offering issued and outstanding
shares which shall vest and become exercisable over a total period of three years commencing on the grant date on a monthly basis
in equal installments which will be granted to Company’s Chief Executive Officer subsequent to the completion of this offering;
and |
| |
● |
58,132 Ordinary Shares reserved for future issuance
under our 2021 Equity Incentive Plan. |
DILUTION
If
you invest in our securities, your interest will be immediately diluted to the extent of
the difference between the initial public offering price per Ordinary Share in this offering
and the pro forma as adjusted net tangible book value per Ordinary Share after this offering.
Dilution results from the fact that the initial public offering price per Ordinary Share
is substantially in excess of the net tangible book value per Ordinary Share. As of June
30, 2024, we had a historical net tangible book value of $(0.026) million, or $(0.01) per
Ordinary Share. Our net tangible book value per share represents total tangible assets less
total liabilities, divided by the number of Ordinary Shares outstanding on June 30, 2024.
Our
pro forma net tangible book value as of June 30, 2024 was $0.3 million, or $0.10 per Ordinary
Share. Pro forma net tangible book value per share represents total tangible assets less
total liabilities, divided by the number of Ordinary Shares outstanding as of June 30, 2024,
after giving effect to (i) the conversion of 104,711 preferred shares into 104,711 Ordinary
Shares; (ii) the repayment of $151,000 pursuant to the 2024 convertible loan agreement; (iii)
the issuance of 61,751 Ordinary Shares pursuant to a $70,000 investment; and (iv) the issuance,
as consideration for the purchase of patent rights, of 320,000 ordinary shares in August
2024 pursuant to the SciSparc License Agreement, and the additional issuance as consideration
for such purchase, of 364,931 pre-funded warrants and warrants to purchase 2.054,793 ordinary
shares upon the same terms as the warrants to be issued in this offering, which will be issued
upon the consummation of this offering, based on the initial public offering price of $4.38
per Ordinary Share.
After
giving additional effect to the sale of 958,903 Units in this offering at the initial public
offering price of $4.38 per Unit, after deducting the estimated underwriting discounts and
commissions and estimated offering expenses, our pro forma as adjusted net tangible book
value at June 30, 2024 would have been $0.78 per Ordinary Share. This represents an immediate
increase in pro forma as adjusted net tangible book value of $0.79 per share to existing
shareholders and immediate dilution of $3.60 per Ordinary Share to new investors.
The
following table illustrates this dilution per Ordinary Share:
| Initial public offering price per Unit |
|
|
|
|
$ |
4.38 |
|
| Historical
net tangible book value per share as of June 30, 2024 |
|
|
$ |
(0.01 |
) |
|
|
|
|
| Increase
in net tangible book value per share attributable to the pro forma adjustments described above |
|
|
|
0.11 |
|
|
|
|
|
| Pro
forma net tangible book value per share |
|
|
|
0.10 |
|
|
|
|
|
| Increase
in net tangible book value per share attributable to new investors participating in this offering |
|
|
$ |
0.68 |
|
|
|
|
|
| Pro forma
as adjusted net tangible book value per share after this offering |
|
|
|
|
|
|
$ |
0.78 |
|
| Dilution
per share to new investors participating in this offering |
|
|
|
|
|
|
$ |
3.60 |
|
If
the underwriter exercises in full its option to purchase additional Ordinary Shares and/or Warrants the pro forma as adjusted net tangible
book value will increase to $0.89 per Ordinary Share, representing an immediate increase in pro forma as adjusted net tangible book value
to existing shareholders of $0.89 per Ordinary Share and an immediate dilution of $3.49 per Ordinary Share to new investors participating
in this offering.
The
following table shows, as of the date of this prospectus, on a pro forma as adjusted basis,
the number of Ordinary Shares purchased from us as part of the Units, the total consideration
paid to us and the average price paid per share by existing shareholders and by new investors
purchasing Units in this offering at the initial public offering price of $4.38 per Unit,
before deducting the estimated underwriting discounts and commissions and estimated offering
expenses payable by us:
| |
|
Shares |
|
|
Total
Consideration |
|
|
Average
Price
Per Ordinary |
|
| |
|
Number |
|
|
Percent
|
|
|
Amount |
|
|
Percent
|
|
|
Share
|
|
| Existing shareholders |
|
|
2,655,302 |
|
|
|
63.3 |
% |
|
$ |
3,709,000 |
|
|
|
33.3 |
% |
|
$ |
0.72 |
|
| 2023 convertible loan agreement
shareholders |
|
|
198,489 |
|
|
|
4.7 |
% |
|
|
180,000 |
|
|
|
1.6 |
% |
|
$ |
1.10 |
|
| Additional funding from existing
shareholder |
|
|
61,751 |
|
|
|
1.5 |
% |
|
|
70,000 |
|
|
|
0.6 |
% |
|
|
1.13 |
|
| SciSparc |
|
|
320,000 |
|
|
|
7.6 |
% |
|
|
3,000,000 |
|
|
|
26.9 |
|
|
|
9.375 |
|
| New investors |
|
|
958,903 |
|
|
|
22.9 |
% |
|
$ |
4,199,995 |
|
|
|
37.6 |
% |
|
$ |
4.38 |
|
| Total |
|
|
4,194,445 |
|
|
|
100 |
% |
|
$ |
11,158,995 |
|
|
|
100 |
% |
|
$ |
|
|
The
number of the Ordinary Shares to be issued and outstanding immediately after this offering
as shown above assumes that all of the Ordinary Shares offered hereby are sold, and is based
on 3,235,542 Ordinary Shares issued and outstanding as of the date of this prospectus. This
number excludes:
| |
● |
246,129 Ordinary Shares issuable
upon the exercise of options to directors, employees and consultants under our incentive option plan outstanding as of such date,
with exercise prices at a range of $0.02 - $1.1335 per share, of which options to purchase 146,064 Ordinary Shares were vested as
of such date (including options to purchase 42,744 Ordinary Shares that vest upon the completion of this offering); |
| |
● |
such number of Ordinary Shares issuable upon the exercise
of options representing 2.5% of the Company’s post-initial public offering issued and outstanding shares which shall vest and
become exercisable over a total period of three years commencing on the grant date on a monthly basis in equal installments which
will be granted to Company’s Chief Executive Officer subsequent to the completion of this offering; and |
| |
● |
58,132 Ordinary Shares reserved
for future issuance under our 2021 Equity Incentive Plan. |
To
the extent that outstanding options are exercised, new options or warrants are issued or we issue additional Ordinary Shares in the future,
there will be further dilution to new investors. We may choose to raise additional capital due to market conditions or strategic considerations
even if we believe we have sufficient funds for our current or future operating plans. To the extent that we raise additional capital
through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our equity
holders.
Certain
of our existing shareholders, including beneficial owners of greater than 5% of our share capital, have indicated an interest in purchasing
up to an aggregate of $1 million of Units in this offering, which represents approximately 24%% of the total Units in this offering,
based on the public offering price of $4.38 per Unit. However, because indications of interest are not binding agreements or commitments
to purchase, the underwriters may determine to sell more, less or no Units in this offering to any of these shareholders, or any of these
shareholders may determine to purchase more, less or no Units in this offering. The underwriters will receive the same underwriting discount
on any Units purchased by these shareholders as they will on any other Units sold to the public in this offering.
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATIONS
You
should read the following discussion in conjunction with our audited financial statements including the related notes thereto, beginning
on page F-1 of this prospectus. In addition to historical information, this discussion contains forward-looking statements that
involve risks and uncertainties. You should read the sections of this prospectus titled “Risk Factors” and “Special
Note Regarding Forward-Looking Statements” for a discussion of the factors that could cause our actual results to differ materially
from our expectations.
Overview
We
are a clinical development stage biotech company specializing in the development of innovative hydrogels delivered in the form of nasal
sprays, and form a thin hydrogel-based shield containment barrier in the nasal cavity that can provide a barrier against viruses and
allergens from contacting the nasal epithelial tissue. Our proprietary C&C hydrogel technology, comprised of a mixture of naturally
occurring building blocks, is delivered in the form of nasal sprays, and potentially functions as a “biological mask” with
a thin shield containment barrier in the nasal cavity. We are further developing certain aspects of our C&C hydrogel technology such
as the bioadhesion and prolonged retention at the nasal deposition site for intranasal delivery of drugs. We refer to our separate platform
technology that is focused on nasal delivery of APIs as T&T.
We
have experienced net losses since our inception in 2005. We incurred net losses of $600,000 and $779,000 for the years ended December 31,
2023 and December 31, 2022, respectively, net losses of $588,000 and $191,000 for the six months ended June 30, 2024 and June 30, 2023,
respectively. As of December 31, 2023 and June 30, 2024, we had an accumulated deficit of approximately $3.5 million and $4.1 million,
respectively. We anticipate that we will continue to incur significant losses for the foreseeable future as our operating expenses and
capital expenditures increase substantially due to our continued investment in our research and development activities and as we hire
additional employees over the coming years. Furthermore, upon closing of this offering, we expect to incur additional expenses associated
with operating as a U.S. public company, including significant legal, accounting, investor relations and other expenses.
For
further information regarding our business and operations, see “Business” below.
Components
of Operating Results
Revenues
We
have not recognized any revenue to date and we do not expect to generate revenue from the sale of product candidates in the near future.
Research
and Development Expenses
Research
and development activities related to our product candidates are our primary focus. We do not believe that it is possible at this time
to accurately project total expenses required for us to reach the point at which we will be ready to out-license our technologies. Development
timelines, the probability of success and development costs can differ materially from expectations. In addition, we cannot forecast
whether and when collaboration arrangements will be entered into, if at all, and to what degree such arrangements would affect our development
plans and capital requirements. We expect our research and development expenses to increase over the next several years as our development
program progresses. We would also expect to incur increased research and development expenses if we were to identify and develop additional
technologies.
Research
and development expenses include the following:
| ● | employee-related
expenses, such as salaries and share-based compensation; |
| ● | expenses
relating to outsourced and contracted services, such as consulting, research and advisory
services; |
| ● | supply
and development costs; |
| ● | expenses
incurred in operating our small-scale equipment; and |
| ● | costs
associated with regulatory compliance. |
We
recognize research and development expenses as we incur them.
General
and Administrative Expenses
General
and administrative expenses consist primarily of personnel costs, related to directors, executive, finance, and human resource functions,
facility costs and external professional service costs, including legal, accounting, marketing and audit services and other consulting
fees.
We
anticipate that our general and administrative expenses will increase in the future as we increase our administrative headcount and infrastructure
to support our continued research and development programs and the potential commercialization of our product candidates. We also anticipate
that we will incur increased expenses related to audit, legal, regulatory and tax-related services associated with maintaining compliance
with Nasdaq and SEC requirements, director and officer insurance premiums, director compensation, and other costs associated with being
a public company.
Finance
Income, Net
Our
net financing income consists primarily of exchange rate differences and net changes in fair value of financial instruments.
Income
Taxes
We
have yet to generate taxable income in Israel. As of December 31, 2023, our operating tax
loss carryforwards were approximately NIS 10 million ($2.8 million). We anticipate that we
will continue to generate tax losses for the foreseeable future and that we will be able
to carry forward these tax losses indefinitely to future taxable years. Accordingly, we do
not expect to pay taxes in Israel until we have taxable income after the full utilization
of our carry forward tax losses.
Results
of Operations
Our
results of operations have varied in the past and can be expected to vary in the future due to numerous factors. We believe that period-to-period
comparisons of our operating results should not be relied upon as indications of future performance.
Six
Months Ended June 30, 2024 Compared to Six Months Ended June 30, 2023
Our
results of operations for the six months ended June 30, 2024 and 2023 were as follows:
Results
of Operations
Our
results of operations have varied in the past and can be expected to vary in the future due to numerous factors. We believe that period-to-period
comparisons of our operating results are not necessarily meaningful and should not be relied upon as indications of future performance.
Our
results of operations for the six months ended June 30, 2024 and 2023 were as follows:
| | |
| |
Six
months ended
June 30, | |
| | |
Note | |
2024 | | |
2023 | |
| | |
| |
| | |
| |
| Operating
expenses: | |
| |
| | | |
| | |
| Research
and development expenses | |
| |
$ | 137 | | |
$ | 172 | |
| General
and administrative expenses | |
| |
| 210 | | |
| 153 | |
| | |
| |
| | | |
| | |
| Operating
loss | |
| |
| 347 | | |
| 325 | |
| | |
| |
| | | |
| | |
| Financial
expense (income), net | |
| |
| 241 | | |
| (134 | ) |
| | |
| |
| | | |
| | |
| Net
loss and comprehensive loss | |
| |
$ | 588 | | |
$ | 191 | |
| | |
| |
| | | |
| | |
| Basic
and diluted net loss per share (*) | |
8 | |
$ | 0.2 | | |
$ | 0.1 | |
| | |
| |
| | | |
| | |
| Weighted
average number of shares of ordinary share used in computing basic and diluted net loss per share | |
| |
| 2,604,325 | | |
| 1,587,012 | |
Research
and Development Expenses
The
following table describes the breakdown of our research and development expenses for the indicated periods:
| | |
For
the Six Months Ended
June 30, | |
| (U.S.
dollars in thousands except share and per share data) | |
2024 | | |
2023 | |
| Subcontractors
and consultants | |
$ | 6 | | |
| 49 | |
| Payroll
and related expenses | |
| 85 | | |
| 104 | |
| Share based payment | |
| 28 | | |
| 19 | |
| Patents | |
| 18 | | |
| - | |
| Total
research and development expenses | |
$ | 137 | | |
| 172 | |
Six Months
Ended June 30, 2024 Compared to Six Months Ended June 30, 2023
Our
research and development expenses for the six months ended June 30, 2024 and 2023 were $137,000 and $172,000, respectively. The decrease
of $35,000, or 20.3%, is mainly attributed to a decrease of $43,000 in subcontractors and consultants’ expenses and a $19,000 decrease
in payroll and related expenses, offset substantially by $18,000 increase patents costs due to national phase submission made in February
2024. The decrease in Company’s expenses was due to decrease in Company’s research and development activity.
General
and Administrative Expenses
The
following table describes the breakdown of our general and administrative expenses for the indicated periods:
| | |
For
the Six Months Ended
June 30, | |
| (U.S.
dollars in thousands) | |
2024 | | |
2023 | |
| Payroll
and related expenses | |
$ | 76 | | |
| 77 | |
| Professional
services | |
| 117 | | |
| 64 | |
| Others | |
| 17 | | |
| 12 | |
| | |
$ | 210 | | |
| 153 | |
Our
general and administrative expenses for the six months ended June 30, 2024 and 2023 were $210,000 and $153,000, respectively. The increase
of $57,000, or 37.2%, is primarily attributable to an increase in professional services related to the company's effort with the IPO.
Financing
Expenses (income), net
Our
financing expenses (income), net for the six months ended June 30, 2024 and 2023 were $241,000 and ($134,000), respectively. The increase
of $375,000, or 279.8%, is primarily attributable to a reduction in the fair value of the derivative warrant liability in H1 2023, as
a result of the decrease in the estimate of probability of successful completion of an IPO during H1 2023, and as a result of financing
expenses incurred due to the 20% discount in the 2023 convertible notes that were converted in May 2024.
Year
Ended December 31, 2023 Compared to Year Ended December 31, 2022
Our
results of operations for the years ended December 31, 2023 and 2022 were as follows:
| | |
For the Years Ended
December 31, | |
| (U.S. dollars in thousands except share and
per share data) | |
2023 | | |
2022 | |
| Statement of Comprehensive
Loss: | |
| | |
| |
| Research
and development expenses | |
$ | 332 | | |
| 347 | |
| General
and administrative expenses | |
| 303 | | |
| 548 | |
| Operating
loss | |
| 635 | | |
| 895 | |
| Financing
income, net | |
| (35 | ) | |
| (116 | ) |
| Net
loss and comprehensive loss | |
| 600 | | |
| 779 | |
| Basic
and diluted net loss per share | |
$ | 0.3 | | |
| 0.6 | |
| Weighted
average number of shares outstanding used in computing basic and diluted net loss per share | |
| 2,030,327 | | |
| 1,305,635 | |
Research
and Development Expenses
The
following table describes the breakdown of our research and development expenses for the indicated periods:
| | |
For the Years Ended
December 31, | |
| (U.S. dollars
in thousands except share and per share data) | |
2023 | | |
2022 | |
| Subcontractors and consultants | |
$ | 52 | | |
$ | 66 | |
| Payroll and related expenses | |
| 196 | | |
| 231 | |
| Share based payment | |
| 83 | | |
| 111 | |
| Patents | |
| 1 | | |
| 17 | |
| Research and development expenses participation | |
| - | | |
$ | (78 | ) |
| Total research and development expenses | |
| 332 | | |
| 347 | |
Our
research and development expenses for the years ended December 31, 2023 and 2022 were $332,000
and $347,000, respectively. The decrease of $15,000, or 4.3%, is mainly attributed to the
decrease in the NIS/USD exchange rate which caused the payroll and related expenses denominated
in NIS to be smaller in USD terms.
General
and Administrative Expenses
The
following table describes the breakdown of our general and administrative expenses for the indicated periods:
| | |
For
the year ended
December 31, | |
| (U.S. dollars
in thousands) | |
2023 | | |
2022 | |
| | |
| | |
| |
| Payroll and related expenses | |
$ | 151 | | |
$ | 171 | |
| Professional services | |
| 107 | | |
| 289 | |
| Share based payment | |
| 17 | | |
| 19 | |
| Marketing | |
| - | | |
| 15 | |
| Others | |
| 28 | | |
| 54 | |
| | |
$ | 303 | | |
| 548 | |
Our
general and administrative expenses for the years ended December 31, 2023 and 2022 were $303,000
and $548,000, respectively. The decrease of $245,000, or 44.7%, is primarily attributable
to lower fees of professional services providers as a result of IPO related fees we incurred
during 2022.
Financing
Expenses
Our
financing expenses for the years ended December 31, 2023 and 2022 were immaterial.
Critical
Accounting Policies and Estimates
We
describe our significant accounting policies and estimates in Note 2 to our annual financial statements contained elsewhere in this
prospectus.
We
prepare our financial statements in accordance with U.S. GAAP.
In
preparing these financial statements, management has made judgments, estimates and assumptions that affect the application of our accounting
policies and the reported amounts recognized in the financial statements. On a periodic basis, we evaluate our estimates, including those
related to share-based compensation and a derivative warrant lability using an option pricing model. The option-pricing model requires
a number of assumptions, including the expected share price, share price volatility, free risk interest rate, dividends and expected
option term. Expected volatility was calculated based on comparison companies. We estimate the primarily based on recent financing rounds.
We base our estimates on historical experience, authoritative pronouncements and various other assumptions that we believe to be reasonable
under the circumstances. Actual results may differ from these estimates.
Additionally,
we classified convertible notes that may not be repaid in cash as liabilities measured fair value as we estimated that under the predominant
scenario these notes will be settled by issuing a variable number of shares that in the aggregate provide a fixed monetary value. We
estimated the fair value based on such fixed monetary value, as repressed by the par value of the notes and interest accrued thereon
(as appliable) and the contractual discount rate to be used at conversion into shares.
Other
than as described above, for the periods included in the financial statements, we do not believe there are critical accounting estimates
that are subject to uncertainty or that have significantly changed during the relevant periods.
Recently-Issued
Accounting Pronouncements
Certain
recently-issued accounting pronouncements are discussed in Note 2, Significant Accounting Policies, to the financial statements
included in elsewhere in this registration statement, regarding the impact of the U.S. GAAP standards as issued by the FASB that we will
adopt in future periods in our financial statements.
Emerging
Growth Company Status
We
qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An
emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to
public companies. These provisions include:
| ● | a
requirement to present only two years of audited financial statements in addition to any
required interim financial statements and correspondingly reduced Management’s Discussion
and Analysis of Financial Condition and Results of Operations disclosure; |
| ● | to
the extent that we no longer qualify as a foreign private issuer, (i) reduced disclosure
obligations regarding executive compensation in our periodic reports and proxy statements
and (ii) exemptions from the requirement to hold a non-binding advisory vote on executive
compensation, including golden parachute compensation; |
| ● | an
exemption from the auditor attestation requirement in the assessment of our internal control
over financial reporting pursuant to the Sarbanes-Oxley Act of 2002; and |
| ● | an
exemption from compliance with the requirement that the Public Company Accounting Oversight
Board has adopted regarding a supplement to the auditor’s report providing additional
information about the audit and the financial statements. |
We
may take advantage of these exemptions for up to five years or until such earlier time that we are no longer an emerging growth company.
We would cease to be an emerging growth company upon the earliest to occur of: (i) the last day of the fiscal year in which we have
total annual gross revenues of $1.235 billion or more; (ii) the date on which we have issued more than $1.0 billion in
nonconvertible debt during the previous three years; (iii) the date on which we are deemed to be a large accelerated filer under
the rules of the SEC; or (iv) the last day of the fiscal year following the fifth anniversary of this offering. We may choose to
take advantage of some but not all of these exemptions. Section 107 of the JOBS Act provides that an “emerging growth company”
can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new
or revised accounting standards. This means that an “emerging growth company” can delay the adoption of certain accounting
standards until those standards would otherwise apply to private companies. We have elected to take advantage of the extended transition
period to comply with new or revised accounting standards and to adopt certain of the reduced disclosure requirements available to emerging
growth companies. As a result of the accounting standards election, we will not be subject to the same implementation timing for new
or revised accounting standards as other public companies that are not emerging growth companies which may make comparison of our financials
to those of other public companies more difficult.
Liquidity
and Capital Resources
Since
our inception, we have incurred losses and negative cash flows from our operations. For the year ended December 31, 2023, we incurred
a net loss of $600,000 and used net cash of $537,000 in our operating activities. For the six months ended June 30, 2024, we incurred
a net loss of $588,000 and used net cash of $337,000 in our operating activities. As of December 31, 2023 and June 30, 2024, we had a
negative working capital of $448,000 and $321,000, respectively, and an accumulated deficit of approximately $3.5 million and $4.1 million,
respectively. As of June 30, 2024, our cash and cash equivalents totaled approximately $23,000. We believe that after the completion
of this offering our cash and cash equivalents will enable us to fund our operations through 18 months following the completion of this
offering.
Through
June 30, 2024, we have financed our operations primarily through private placements. Total invested capital as of June 30, 2024 was $4.0
million, which included Ordinary Shares, preferred shares, option to purchase Ordinary Shares and convertible note agreements.
Six
Months Ended June 30, 2024 Compared to Six Months Ended June 30, 2023
The
following table summarizes our statement of cash flows for the six months ended June 30, 2024 and 2023:
| | |
For the 6
Months Ended
June 30, | |
| (U.S. dollars in thousands except share and
per share data) | |
2024 | | |
2023 | |
| Net cash used in operating activities | |
$ | (337 | ) | |
| (335 | ) |
| Net cash used in investing activities | |
| - | | |
| - | |
| Net cash provided by
financing activities | |
| 356 | | |
| 320 | |
| Increase (decrease) in
cash and cash equivalents | |
$ | 19 | | |
| (15 | ) |
Net
cash used in operating activities
Net
cash used in operating activities was $337,000 and $335,000 for the six months ended June 30, 2024, and 2023 respectively. While we incurred
IPO related expenses during the six months period ended June 30, 2024, no significant increase was noted in negative operating cash flows
in relation to the six months ended June 30, 2023, since we have incurred IPO related expenses in H1 2023 as well, prior to cessation
of the IPO in April 2023.
| Cash
flows from financing activities | |
| | | |
| | |
| | |
| | | |
| | |
| Proceeds
from sale of investment in shares | |
| 28 | | |
| - | |
| Proceeds
from issuance of convertible notes | |
| 151 | | |
| 200 | |
| Proceeds
from issuance of ordinary shares | |
| 177 | | |
| 120 | |
| | |
| | | |
| | |
| Net
cash provided by financing activities | |
| 356 | | |
| 320 | |
Year
Ended December 31, 2023 Compared to Year Ended December 31, 2022
The
following table summarizes our statement of cash flows for the years ended December 31, 2023 and 2022:
| | |
For the Years Ended
December 31, | |
| (U.S. dollars in thousands except share and
per share data) | |
2023 | | |
2022 | |
| Net cash used in operating activities | |
$ | (537 | ) | |
| (1,132 | ) |
| Net cash used in investing activities | |
| - | | |
| (3 | ) |
| Net cash provided by
financing activities | |
| 505 | | |
| 648 | |
| Decrease in cash and cash
equivalents | |
$ | (32 | ) | |
| (487 | ) |
Net cash
used in operating activities
Net
cash used in operating activities was $537,000 and $1,132,000 for the years ended December 31, 2023 and 2022, respectively. The $595,000
decrease was attributable primarily to a decrease in our net loss due to lower fees of professional services providers, as a result of
IPO related expenses we incurred in 2022.
Net
cash used in investing activities
Net
cash used in investing activities was nil and $3,000 for the years ended December 31, 2023 and 2022, respectively.
Net
cash provided by financing activities
Net
cash provided by financing activities was $505,000 and $648,000 for the years ended December 31, 2023 and 2022, respectively. The decrease
was due to a decrease in financing activity related to share purchase agreements (in 2022 we issued a higher amount of convertible notes).
Funding
Requirements
We
have incurred losses and cash flow deficits from operations since the inception, resulting in an accumulated deficit at December 31,
2023 of approximately $3.5 million and at June 30, 2024 of approximately $4.1 million. We anticipate that we will continue to incur net
losses for the foreseeable future. We believe that our existing cash and cash equivalents will be sufficient to fund our projected cash
needs until the end of October 2024. To meet future capital needs, we would need to raise additional capital through equity or debt financing
or other strategic transactions. However, any such financing may not be on favorable terms or even available to us. Our failure to obtain
sufficient funds on commercially acceptable terms when needed would have a material adverse effect on our business, results of operations
and financial condition. Our forecast of the period of time through which our financial resources will be adequate to support our operations
is a forward-looking statement that involves risks and uncertainties, and the actual amount of our expenses could vary materially and
adversely as a result of a number of factors. We have based our estimates on assumptions that may prove to be wrong, and our expenses
could prove to be significantly higher than we currently anticipate.
Our
future capital requirements will depend on many factors, including, but not limited to:
| ● | the
progress and costs of our research and development activities; |
| ● | the
costs of development and expansion of our operational infrastructure; |
| ● | our
ability, or that of our collaborators, to achieve development milestones and other events
or developments under potential future licensing agreements; |
| ● | the
amount of revenues and contributions we receive under future licensing, collaboration, development
and commercialization arrangements with respect to our technologies; |
| ● | the
costs of filing, prosecuting, enforcing and defending patent claims and other intellectual
property rights; |
| ● | the
costs of contracting with third parties to provide sales and marketing capabilities for us
or establishing such capabilities ourselves, once our technologies are developed and ready
for commercialization; |
| ● | the
costs of acquiring or undertaking development and commercialization efforts for any future
product candidates or technology; |
| ● | the
magnitude of our general and administrative expenses; and |
| ● | any
additional costs that we may incur under future in- and out-licensing arrangements relating
to our technologies and futures product candidates. |
Until
we generate significant recurring revenues, we expect to satisfy our future cash needs through capital raising or by out-licensing and/or
co-developing applications of one or more of our product candidates. We cannot be certain that additional funding will be available to
us on acceptable terms, if at all. If funds are not available on favorable terms, or at all, we may be required to delay, reduce the
scope of or eliminate research or development efforts or plans for commercialization with respect to our technologies and make necessary
change to our operations to reduce the level of our expenditures in line with available resources. This may raise substantial doubts
about our ability to continue as a going concern.
We
are a development-stage biotech company and it is not possible for us to predict with any degree of accuracy the outcome of our research
and development efforts. As such, it is not possible for us to predict with any degree of accuracy any significant trends, uncertainties,
demands, commitments or events that are reasonably likely to have a material effect on our net loss, liquidity or capital resources,
or that would cause financial information to not necessarily be indicative of future operating results or financial condition. However,
to the extent possible, certain trends, uncertainties, demands, commitments and events are described herein.
Contractual
Obligations
As
of June 30, 2024, we had the following contractual obligations:
During
2007-2010 we received funding from the IIA (previously known as Officer of Chief Scientist) for its participation in research and development
costs, based on budgets approved by the IIA, subject to the fulfillment of specified milestones. The Company is committed to pay royalties
to the IIA on proceeds from sale of product candidates in the research and development of which the IIA participates by way of grants.
Royalties between 3% and 4.5% are payable on sales of developed product candidates funded, up to 100% of the funding received by the
Company, linked to US dollar and bearing SOFR interest rate. In the case of failure of a financed project, the Company is not obligated
to pay any such royalties to the IIA. The total grant amount received from the IIA, including interest, as of June 30, 2024 was $758,000.
See
“Management – Compensation” below for information regarding our contractual obligations to certain of our officers.
2023
Loan
On
February 4, 2023, the Company signed the 2023 Loan, a convertible loan agreement with the 2023 Lenders, in the principal amount of up
to $180,000. The 2023 Loan bears simple interest at an annual rate of 4%. In the event that the Company issues securities in a bona fide
transaction or series of transactions with the principal purpose of raising capital, pursuant to which the Company issues and sells shares
at a fixed pre-money valuation, in which the aggregate proceeds to the Company are at least $500,000, or a Qualified Financing, then
the entire 2023 Loan and all interest accrued thereon shall automatically convert into such number of shares of the Company, of the same
class as shall be issued in such transaction at a price per share equal to the price per share paid in the Qualified Financing minus
the discount of 20%, rounded up to the nearest whole number, and the lenders, or the 2023 Lenders, will receive the same rights and protections
granted to the investors in such Qualified Financing. The 2023 Lenders have an optional conversion right upon consummation by the Company
of a non-Qualified Financing. If the 2023 Loan (and the accrued interest thereon) has not been repaid or converted prior to the lapse
of six (6) months following the effective date, at the request of a Lender, made at Lender’s sole discretion, the entire 2023 Loan
and the accrued interest thereon shall be converted into such number of the most senior class of shares of the Company then outstanding,
equal to the 2023 Loan and any interest accrued thereon, divided by the lowest price per share actually paid to the Company since August
1, 2021, for such most senior class of shares of the Company then outstanding, discounted by 20%, rounded up to the nearest whole number.
In the event that either (a) a consolidation, merger or reorganization of the Company with or into, or a sale of all or substantially
all of the Company's assets, or a majority of the Company's issued and outstanding share capital, to, any person or entity, other than
a wholly-owned subsidiary of the Company, excluding a transaction in which shareholders of the Company prior to the transaction will
maintain voting control of the resulting entity after the transaction; or (b) any transaction resulting in all or substantially all of
the Company's assets being sold, transferred or exclusively licensed (c) an initial public offering of the Company's shares, or collectively,
an Exit Event, should occur prior to the conversion of the 2023 Loan, then immediately following the closing of the Exit Event, the Company
shall repay each Lender an amount equal to the 2023 Loan amount extended by such Lender, together with any and all interest accrued thereon.
On May 12, 2024, the 2023 Loan converted into 198,486 Ordinary Shares pursuant to a non-Qualified Financing.
April
2024 CLA
In
April 2024, we and L.I.A Pure Capital Ltd., entered into a convertible loan agreement, or the April 2024 CLA, pursuant to which we may
draw down an amount of up to $250,000, or the April 2024 CLA Amount. The 2024 CLA bears interest at an annual rate of 4% and has a maturity
date of April 2026. In accordance with the terms of the April 2024 CLA, in the event that we have an initial public offering, the then
outstanding April 2024 CLA Amount shall be repaid from the proceedings received in such initial public offering. In the event we do not
succeed in offering our shares in an initial public offering, upon the elapse of 24 months from the effective date of the April 2024
CLA, the outstanding April 2024 CLA Amount shall be converted into the most senior class of shares issued in the most recent financing
round.
August
2024 CLA
In
August 2024, we and L.I.A Pure Capital Ltd. and Reuven Srugo Construction Company Ltd., entered into a convertible loan agreement, or
the August 2024 CLA, pursuant to which we may draw down an amount of up to $60,000, or the August 2024 CLA Amount. The August 2024 CLA
bears interest at an annual rate of 4% and has a maturity date of August 2026. In accordance with the terms of the August 2024 CLA, in
the event that we have an initial public offering, the then outstanding August 2024 CLA Amount shall be repaid from the proceedings received
in such initial public offering. In the event we do not succeed in offering our shares in an initial public offering, upon the elapse
of 24 months from the effective date of the August 2024 CLA, the outstanding August 2024 CLA Amount shall be converted into the most
senior class of shares issued in the most recent financing round.
Quantitative
and Qualitative Disclosures About Market Risk
Liquidity
Risk
Liquidity
risk is the risk that we will encounter difficulty in meeting the obligations associated with our financial liabilities that are settled
in cash. Cash flow forecasting is performed in our operating entity level. We monitor forecasts of our liquidity requirements to ensure
we have sufficient cash to meet operational needs. We may be reliant on our ability to raise additional investment capital from the issuance
of both debt and equity securities to fund our business operating plans and future obligations.
Credit
risk
Credit
risk is the risk of financial loss to us if a debtor or counterparty to a financial instrument fails to meet its contractual obligations,
and arises mainly from our receivables.
We
restrict exposure to credit risk in the course of our operations by investing only in bank deposits.
Equity
price risk
As
we have not invested substantial amounts in securities riskier than short-term bank deposits, we do not believe that changes in equity
prices pose a material risk to our holdings. However, decreases in the market price of our Ordinary Shares could make it more difficult
for us to raise additional funds in the future or require us to raise funds at terms unfavorable to us.
Inflation
risk
We
do not believe that inflation has had a material effect on our business, financial condition or results of operations in the reporting
period. If our costs were to become subject to significant inflationary pressures, we may not be able to fully offset such higher costs
through hedging transactions. Our inability or failure to do so could harm our business, financial condition and results of operations.
Foreign
Currency Exchange Risk
Currency
fluctuations could affect us through increased or decreased costs, mainly for goods and services acquired outside of Israel. Currency
fluctuations did not have a material effect on our results of operations during the years ended December 31, 2023 and 2022 or the
six months ended June 30, 2024 and 2023.
BUSINESS
Overview
We
are a development stage biotech company specializing in the development of innovative medical device hydrogels delivered in the form
of nasal sprays, which form a thin hydrogel-based shield containment barrier in the nasal cavity that can provide a barrier against viruses
and allergens from contacting the nasal epithelial tissue. Our C&C, hydrogel technology, comprised of a mixture of naturally occurring
building blocks, is delivered in the form of nasal sprays, and potentially functions as a “biological mask” with a thin shield
containment barrier in the nasal cavity. We are further developing certain aspects of our proprietary C&C hydrogel technology such
as the bioadhesion and prolonged retention at the nasal deposition site for intranasal delivery of drugs. We refer to our separate platform
technology that is focused on nasal delivery of APIs, as T&T.
Our
Product Candidates
Our
nasal hydrogels have been designed to serve as a non-invasive and fast-acting system. The hydrogels are formulated as an innovative mixture
of mucoadhesive polymers (e.g., sodium alginate) which are GRAS by the FDA. Our mucoadhesive polymers derived from seaweed polysaccharides
possess promising features as they are renewable, biodegradable, biocompatible, and environment friendly. The formulated hydrogel is
sprayed into the nose to create a physical barrier with long-lasting adhesion to the mucosal membranes. Our polymers have an atomic mass
much higher than the upper cell penetration limit, the polymers will simply lay on top of the cells and act as a physical barrier to
viruses and allergens from contacting the nasal epithelial tissue, as opposed to penetrating the cells and causing a chemical reaction.
Therefore, the C&C product candidates are not expected to be considered as drugs by the FDA but as medical devices.
Our
leading technologies are C&C and T&T. The C&C provides a barrier against a wide range of allergen particulates and viruses.
PL-14
– Nasal Allergies Blocker
| o | We
expect our PL-14 product candidate to be regulated as a Class II medical device by the FDA
under its 510(k) pathway. |
| |
o |
Our
PL-14 product candidate is scheduled to initiate preclinical safety trials in the second quarter of 2025. In addition, we expect
pivotal clinical trial on our PL-14 product candidate to commence in the fourth quarter of 2025, following which we plan to submit
a 510(k) application for FDA clearance. |
| |
o |
For our PL-14 product candidate,
we will pursue the 510(k) pathway which requires a manufacturer to demonstrate substantial equivalence to an FDA-cleared device (i.e.,
predicate device) to a subject device (i.e., our product candidate). This process for clearing our device with the FDA entails performing
a medical device analysis of the product candidates (e.g., PL-14 product candidate) description, operational principle, potential
accessories and proposed intended use, for the purpose of identifying a predicate device that has already been cleared by the FDA.
Through this review, we found three possible predicate devices for establishing substantial equivalence, Alzair, Nasalease and Bentrio.
There is no guarantee that PL-14 product candidate will advance in the FDA 510(k) process at the same rate as the aforementioned
predicate devices or will reach commercialization. |
| o | The
estimated timeline for obtaining 510(k) clearance for our PL-14 product candidate is based
on the estimated time needed for the following activities: (i) GMP manufacturing of our clinical
trial materials, which usually requires 9-12 months; (ii) Biocompatibility preclinical studies,
which usually requires 3-6 months (although these studies may be performed concurrently with
the GMP manufacturing mentioned above); (iii) Clinical trials, which usually requires 6-12
months; and (iv) FDA submission and clearance, which usually requires 3-12 months. Regarding
FDA submission and clearance, generally 510(k) applicants can expect submission acceptance
review decisions within 15 calendar days, substantive review decisions within 60 days, and
final decisions within 90 days. In the case of our predicate devices for our PL-14 product
candidate, Alzair, Nasalese and Bentrio, the FDA submission and clearance process took 86
and 140 days, respectively. For additional information, please see “Business –
FDA clearance plan for our C&C product candidates.” |
PL-15
– COVID-19 and PL-16 – Influenza Blockers
| o | We
expect our PL-15 and PL-16 product candidates, which provide a barrier against COVID-19 and
influenza from contacting the nasal epithelial tissue, respectively, to be regulated as a
Class II medical device under a De Novo Classification request. For the clinical studies
planned for PL-15 & PL-16 which will include human subjects; the Investigational Device
Exemptions regulation describes three types of device studies: significant risk, nonsignificant
risk, and exempt studies. During the first quarter following the closing of this offering,
the company intends to schedule a pre-submission meeting with the FDA to determine the IDE
regulation type of device studies for PL-15 & PL-16. Our proposed 12-month interval from
the scheduled FDA pre-sub meeting to the planned IDE clinical trial initiation should provide
ample time to fulfill the necessary tasks for the IDE filing, such as 1) reporting previous
studies to support the IDE, 2) preparing IDE required design and manufacturing control documentation,
3) conducting bench and biocompatibility tests to support safety of the device prior to starting
the a human study, and 4) obtaining clinical protocol and ethics committee approvals as well
as FDA IDE approval to start the clinical trial. Once IDE has been initiated, Polyrizon will
comply with FDA Guidance “Changes or Modifications During the Conduct of a Clinical
Investigation”, 2001. |
| |
o |
Our PL-15 product candidate
is scheduled to initiate preclinical safety trials in the second quarter of 2025, and subject to securing additional financing, we
intend to initiate feasibility clinical trials in the third quarter of 2026 and pivotal clinical trials in the second quarter of
2027. Following these trials, we plan to submit De Novo Classification requests for each product candidate. Our PL-16 product candidate
is scheduled to initiate preclinical safety trials in the second quarter of 2025, and subject to securing additional financing, we
intend to initiate feasibility clinical trials in the first quarter of 2026 and pivotal clinical trials in the third quarter of 2026.
Following these trials, we plan to submit De Novo Classification requests for each product candidate. |
| |
o |
Upon
a review similar to the one performed for our PL-14 product candidate, we found that there were no potential predicate devices in
the FDA’s database matching the proposed intended uses of our PL-15 and PL-16 product candidates. Because of this, we will
pursue a De Novo Classification request for each product candidate. This pathway involves demonstrating that the product candidates
provide a reasonable assurance of safety and effectiveness. During the first quarter following the closing of this offering, we intend
to submit a Q-submission (Pre-submission) for each product candidate and request a pre-submission meeting with FDA’s CDRH to
confirm the potential for this regulatory path. For more information, please see “Business – Our Product Candidates –
The determination process for the C&C product candidates as a Class II medical devices.” |
| |
o |
The estimated timeline
for marketing authorization via De Novo Classification grant for our PL-15 & PL-16 product candidates is based on taking similar
steps as the steps described above for obtaining 510(k) clearance for our PL-14 product candidate. We estimate a longer period of
time for the entire grant process for each of these product candidates due to possibly extended clinical trials requested by the
FDA and also due to a longer review timeframe. For additional information, please see “Business – FDA clearance plan
for our C&C product candidates.” |
In
the event the FDA does not agree with our regulatory assessments regarding the C&C product candidates 510(k) for our PL-14 product
candidate, and Class II De Novo pathway for our PL-15& PL-16 product candidates), the FDA may require us to go through a lengthier,
more rigorous examination than we had expected (such as PMA, which is the FDA process of scientific and regulatory review to evaluate
the safety and effectiveness of Class III medical devices. If we are required to pursue a PMA, the introduction of our product candidates
into the market could be delayed. For more information, please see “Risks Related to the Discovery, Development and Clinical Testing
of Product Candidates.”
Trap
and Target ™ Product Candidates
In
contrast to our C&C product candidates, the hydrogel in the T&T product candidates is formulated differently in order to provide
for sustained release of the API. The content of the hydrogel (quantity and quality) in the T&T product candidates is formulated
differently than the content of C&C product candidates, and therefore enable different functions: physical barrier for the C&C
product candidates and API sustained release for the T&T product candidates. It is through these differences that we rationalize
the different regulatory treatment of our C&C and T&T product candidates.
The
T&T platform technology is designed to allow a long residence time and an intimate contact
with the mucosal tissue for a targeted delivery of medicines. We expect that our T&T
platform product candidates will be regulated as a combination-product consisting of a nasal
sprayer and formulation consisting of a hydrogel and a generic API, which we intend to pursue
under the FDA’s 505(b)(2) pathway. We aim to conduct feasibility studies for our T&T
platform product candidates with corticosteroids, benzodiazepines and naloxone, beginning
in the fourth quarter of 2024 through the first quarter of 2026. Pre-clinical studies will
follow and are expected to begin in the second quarter of 2026. Phase I clinical trials for
the leading T&T technology product candidate are planned for the fourth quarter of 2027.
Both pre-clinical studies and Phase I clinical trials are subject to securing additional
financing. In addition, we plan to start an initial testing to explore the potential of our
SCI-160 platform when combined with the T&T technology, in the first quarter of 2025.
The
determination process for the C&C product candidates as a Class II medical devices
According
to the FDA, a product will be considered as a medical device, and subject to FDA regulation, if it meets the following criteria: 1) recognized
in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them and 2) intended for use in the diagnosis
of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals; or 3) intended
to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes
through chemical action within or on the body of man or other animals.
Because
the intended use for each of our C&C product candidates is creating a physical barrier and its primary mode of action, or PMOA, is
physical, we believe that our C&C product candidates will be regulated as Class II medical devices. We believe that our PL-14 product
candidate can utilize the 510(k) pathway for alleviating allergic symptoms, and that our PL-15 and PL-16 product candidates can utilize
the De Novo Classification pathway for reducing the risk of nasal infections caused by COVID-19 and influenza, respectively.
Because
our C&C product candidates’ mode of action is based on creating a physical barrier that is not associated to chemical action
within or on the body, we believe that our C&C product candidates will be classified by the FDA and similar regulatory bodies as
a medical device.
Unless
an exemption applies, any medical device that is to be marketed in the United States be cleared
via submission of a premarket notification (i.e., 510(k)), for class II devices, or a PMA
for class III devices. Alternatively, the device can be marketed following the granting of
a De Novo Classification request for devices that do not have a legally marketed predicate
device. We performed an FDA medical device analysis based on the PL-14 product candidate
description along with potential accessories and the proposed intended use. Based on the
abovementioned criteria we believe that our PL-14 product candidate’s regulatory classification
is: 21 C.F.R. § 880.5045 “Medical recirculating air cleaner” (under the
product code: NUP-Cream, Nasal, Topical, Mechanical Allergen Particle Barrier) which is FDA
Class II requiring a 510(k) submission. This means a 510(k) submission for FDA review is
required for clearance allowing it to be marketed. To provide the best possible predicate
device to establish substantial equivalency within the 510(k) submission, a review of FDA’s
510(k) database for Product Code NUP was performed, which includes several possibilities
for a potential predicate device, such as Alzair, Nasalese and Bentrio with intended uses
of “promoting alleviation of mild allergic symptoms triggered by the inhalation of
various airborne allergens.”
Our
PL-15 and PL-16 product candidates are intended to provide a barrier against COVID-19 and influenza from contacting the nasal epithelial
tissue, respectively. We performed a regulatory assessment review for our PL-15 and PL-16 product candidates where the intended use includes
a “nasal mechanical virus blocker” and found that there are no valid predicate devices found in the FDA’s databases
matching this intended use. The lack of available predicate devices, combined with the fact that our PL-15 and PL-16 product candidates
have similar risk profile as our PL-14 product candidate (due to three product candidates using the same ingredients and method of use),
we believe that our PL-15 and PL-16 product candidates may be regulated as a Class II medical device if the FDA agrees and grants a De
Novo Classification request. In order to assess the likelihood of approval under a De Novo pathway, during the first quarter following
the closing of this offering we intend to schedule a pre-submission meeting with the FDA, but have not yet communicated directly with
the FDA regarding any of its C&C product candidates.
The
estimated timeline for obtaining 510(k) clearance for our C&C product candidates, is
based on the estimated time needed for the following activities: (i) GMP manufacturing of
our clinical trial materials, which usually requires 9-12 months; (ii) Biocompatibility preclinical
studies, which usually requires 3-6 months (although these studies are performed concurrently
with the GMP manufacturing mentioned above); (iii) Clinical trials, which usually requires
6-12 months; and (iv) FDA submission and clearance, which usually requires 3-12 months. Regarding
FDA submission and clearance, generally 510(k) applicants can expect submission acceptance
review decisions within 15 calendar days, substantive review decisions within 60 days, and
final decisions within 90 days. Applicants with outstanding review issues will be notified
within 100 days. However, the FDA’s time of review does not include time on “hold”,
which includes any time spent by us responding to any FDA information requests, meaning that
the total timeframe of the review process could take longer than anticipated. In the case
of our predicate devices for our PL-14 product candidate, Alzair, Nasalese and Bentrio, the
FDA submission and clearance process took 86 and 140 days, respectively. For additional information,
please see “Business FDA clearance plan for our C&C product candidates.”
Furthermore,
we believe that our PL-15 and PL-16 product candidates would nevertheless still be classified as medical devices (rather than drugs),
even in the event the FDA does not agree with our regulatory assessments regarding the Class II De Novo pathway based on our review of
the FDA’s September 2017 guidance document entitled “Classification of Products as Drugs and Devices & Additional Product
Classification Issues: Guidance for Industry and FDA Staff”. This guidance document notes that a key difference between the statutory
definition of drugs and devices is that a device “does not achieve its primary intended purposes through chemical action within
or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of its primary intended
purposes”. The guidance further clarifies that the term “chemical action” is consistent with “pharmaceutical
action” and also provides examples of products which have been determined to be devices, one of which is a polymer that provides
a physical barrier (abdominal adhesion barrier).
We
believe that our PL-15 and PL-16 product candidates will be regulated as medical device due to the following: (i) the mode of action
by which each of the PL-15 and PL-16 product candidates is creating a physical barrier, and achieving this barrier is not dependent on
chemical action nor is it dependent on the product being metabolized; and (ii) the polymer used in the PL-15 and PL-16 product candidates
have molecular mass that is much higher than the upper cell penetration limit. Therefore, the polymers will simply lay on top of the
cells and function as a physical barrier to viruses and allergens.
The
determination process for the T&T product candidates as a drug-device combination product
In
contrast to our C&C product candidates, the hydrogel in our T&T product candidates is formulated differently in order to provide
for sustained release of the API. The content of the hydrogel (quantity and quality) in the T&T product candidates is formulated
differently than to the content of C&C product candidates, and therefore enable different functions: physical barrier for the C&C
product candidates and API sustained release for the T&T product candidates. It is through these differences that we rationalize
the different regulatory treatment of our C&C and T&T product candidates.
The
T&T platform technology is designed to allow a long residence time for the API, and an intimate contact with the mucosal tissue for
a targeted delivery of medicines. We expect that our T&T platform product candidates will be regulated as a drug-device combination
product consisting of a nasal sprayer (the medical device) and a formulation that consists of a hydrogel and a generic API (the drug),
which we intend to pursue under the FDA’s 505(b)(2) pathway. We aim to conduct feasibility studies for our T&T platform product
candidates with corticosteroids, benzodiazepines and naloxone, beginning in the fourth quarter of 2024 through the first quarter of 2026.
Pre-clinical studies will follow and are expected to begin in the second quarter of 2026. Phase I clinical trials for the leading T&T
technology product candidate are planned for the fourth quarter of 2027, both pre-clinical studies and Phase I clinical trials are subject
to securing additional financing. In addition, we plan to start an initial testing to explore the potential of our SCI-160 platform when
combined with the T&T technology, in the first quarter of 2025.
Background
related to the C&C and T&T technologies
The
nasal cavity is a large, air-filled space above and behind the nose in the middle of the face. Each cavity is the continuation of one
of the two nostrils. The nasal cavity is the uppermost part of the respiratory system and provides the nasal passage for inhaled air
from the nostrils to the nasopharynx and rest of the respiratory tract. The nasal mucosa, also called respiratory mucosa, lines the entire
nasal cavity, from the nostrils to the pharynx. A dynamic layer of mucus overlies the nasal epithelium (the outermost layer of
cells of the nasal mucosa).
The
nasal sub-mucosa underlies the basement membrane. This layer is made up of glands which secrete watery substances and mucus, nerves,
an extensive network of blood vessels and cellular elements like blood plasma. The entire mucosa is highly concentrated with blood vessels
and contains large venous-like spaces; bodies which have a vein-like appearance and swell and congest in response to allergy or infection.
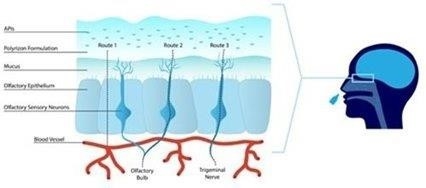
Schematic
illustrations of the mucosal tissue (left) and nasal cavity anatomy (right)
The
nasal mucosa plays an important role in regulating the immune responses to allergens and other airborne pathogens, which enter the nose.
Normally, it prevents allergens and pathogens from penetrating the nasal cavity and deleterious infections or allergic reactions. Healthy
intact mucus is covering the nasal cavity and provides a physical barrier against biological assaults due to its viscous and adhesive
properties. Upon extensive exposure to biological assaults the mucus may fail to provide the necessary defense which results in infection
or allergic reaction. For this manner, mucoadhesive polymers can contribute to a better functionality in defense from biological assaults.
The
term ‘mucoadhesion’ refers to the adhesion of specific polymers to the surface of the mucosal layer. The mucosal layer is
made up of mucus, a viscoelastic fluid, which is secreted by the epithelial cells. A mucoadhesion polymer helps to promote the adhering
of a given formulation to the nasal mucosa by physically interacting with the mucosa. Various properties impact the mucoadhesive of polymers,
such as: (i) molecular weight; (ii) chain length; (iii) viscosity; (iv) degree of cross-linking; (v) spatial conformation; (vi) flexibility
of polymer chains; (vii) concentration; (viii) charge and degree of ionization – anion>cation>non-ionic; (ix) degree of hydration;
and (x) pH.
The
mechanism of mucoadhesion is characterized by to two steps: contact stage and consolidation stage. The first contact stage is characterized
by the initial contact between the polymers and the mucous membrane, with spreading and initiating a deep contact with the mucus layer.
In the second consolidation step, the polymers are activated by the presence of moisture and as they hydrate they become mucoadhesive.
Our innovative technologies consist of a unique mixture of mucoadhesive polysaccharides polymers creating a 3-dimentional network structure
to better interact with mucosal tissues.
Moreover,
the highly vascularized nature of the nasal cavity enabled an alternative administration route of drugs and has gained interest among
pharmaceutical companies as a means of advanced method to increase residence time in the nasal cavity.
Capture
and Contain TM, or C&C
We
are constantly exposed to airborne contaminations, and as we breathe, these pathogens and allergens may cause serious health issues.
Our proprietary C&C is based on natural 3-D polymeric network tailored to optimally adhere to the nasal mucosal surface. The polymeric
network creates a physical barrier that captures and contains biological assaults such as particulate allergen or viruses from interacting
with the nasal epithelial tissue.
The
C&C technology consist of mucoadhesive polymers mixture optimized for the purposes of coverage and adherence to the nasal cavity
as well as capturing and containing the biological assaults based on physical interactions.

Our
product candidate is presented in a liquid form, which allows a rapid interaction between the polymers and the mucosa and avoid the irritating
effect of powdered based formulations. In addition, our product candidate is negatively charged to allow a high degree of mucoadhesivness.
Moreover, its unique structure allows a high degree of capturing and containing of variable types of biological assaults. The product
candidates’ pH was adjusted to further decrease the viral viability without damaging the nasal mucosal tissue.
The
innovative formulation is expected to provide a barrier against viruses and allergens from contacting the nasal epithelial tissue for
approximately six hours after each nasal administration without any adverse side effects. The potential blocking of initial colonization
can reduce the viral load, which helps the immune system to better control the infection. The same concept applies for blocking allergens
from interacting with epithelial tissue.
As
a physical barrier, our lead product candidates have the potential advantages:
| ● | optimal
coverage of nasal cavity |
| |
● |
up to 6 hours of protection after each application |
With
respect to COVID-19, the virus tends to become firmly established in the nasal cavity. Then, in some cases, the virus is aspirated into
the lungs where it may cause more serious disease, including potentially fatal pneumonia.
With
respect to COVID-19, our C&C product candidates is designed to protect the upper respiratory tract in conjunction with additional
preventative measures such as vaccination, wearing masks, keeping social distance and maintaining proper hygiene to decrease the probability
of serious disease.
FDA
clearance plan for our C&C product candidates
We
performed a regulatory assessment review for our PL-15 and PL-16 product candidates where the intended use includes a “nasal mechanical
virus blocker”. There are no regulatory classifications or predicate devices found in the FDA’s databases matching this intended
use. We believe the regulatory pathway will be considered a Class II medical device without substantial equivalence, which would require
a De Novo Classification request. During the first quarter following the closing of this offering we intend to schedule an FDA Pre-submission
meeting for both our PL-15 and PL-16 product candidates to discuss whether both would be considered as Class II medical device and to
ensure FDA agreement with the proposed testing plan.
PL-14
– Nasal Allergies
Our
PL-14 product candidate is scheduled to initiate its preclinical safety trials in the second quarter of 2025. We expect pivotal clinical
trial to commence a few months afterward, in the fourth quarter 2025, following which we plan to submit a 510(k) application for blocking
allergens from contacting the nasal epithelial tissue to the FDA.
PL-15
– COVID-19
Our
PL-15 product candidate is scheduled to undergo preclinical safety trials in the second quarter of 2025. Subject to securing additional
financing in an estimated amount of $2 million, we intend to initiate feasibility clinical trial to commence in the third quarter 2026
and pivotal clinical trials in the second quarter 2027. Following these trials, we plan to submit a De Novo Classification request for
blocking SARS-CoV-2 from contacting the nasal epithelial tissue to the FDA.
PL-16
- Influenza
Our
PL-16 product candidate is scheduled to undergo preclinical safety trials in the second quarter of 2025. Subject to securing additional
financing in an estimated amount of $2 million, we intend to initiate feasibility clinical trial to commence in the first quarter 2026,
and pivotal clinical trials in the third quarter 2026. Following these trials, we plan to submit a De Novo Classification request for
blocking Influenza from contacting the nasal epithelial tissue to the FDA.
Study
Results
Over
the last 24 months our formulations have been tested for their efficacy to block different types of biological assaults from interacting
with host cells. For this purpose, we used a validated and well accepted cultured cells in vitro blocking assays, to evaluate
the potential of the formulation to block the human coronavirus 229E and Influenza H1N1 virus, as well as the house dust mite Der P1
and the timothy grass Phl P1 allergens. The studies have been conducted mainly by our service partner PharmaSeed Ltd., Israel’s
largest GLP-certified pre-clinical CRDO specializing in translational and regenerative studies. The main goal of these studies was to
evaluate our formulations for their potential to prevent cell mortality as a consequence of viral infection, or to decrease the anti-inflammatory
cytokines secretion following allergens encounter. Together with the biological effect of the formulations, we evaluated their cytotoxicity
effect using cytotoxic cells assay on variable cell lines (MRC5, MDCK and A549).
The
viral or allergen blocking assay was performed by using specific host cells, susceptible to viral infection or allergen interaction.
The host cells were treated with our formulation and challenged by viral infection or allergens. The viability of non-treated or treated
cells was monitored using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) to determine the formulations’ effective
concentrations.
While
determinations of safety and efficacy are solely within the authority of the FDA and comparable regulatory bodies, we believe that based
on the results of the aforementioned studies, our formulations presented a consistent and significant (p-value < 0.05) barrier effect,
as expressed in the viral blocking assays with the preservation of 100% of cell viability compare to significantly lower cell viability
of cells infected with human coronavirus 229E and Influenza H1N1 (31% and 5%, respectively). In addition, our formulation presented barrier
activity against the house dust mite allergen Der P1 and the timothy grass allergen Phl P1 from interacting with the host cells. This
barrier effect was expressed with the inhibition of IL-8 secretion, an important protein related to inflammation, where it plays a key
role in the recruitment of neutrophils and other immune cells to the site of infection.
In
all tested cell lines (MRC5, MDCK and A549), no significant cytotoxicity was observed when compared to the untreated cells.
The
charts below represent six, unique studies performed to demonstrate the reduction of inflammation in various cell lines after application
with our C&C product candidates’ polymers. Our polymers have a mass of around 100,000 Daltons, or 100,000 Da. According to
the Scientific Journal of Medicine, molecules above 1,000 Da, cannot penetrate cell lines. Because our polymers have an atomic mass much
higher than the upper cell penetration limit, the polymers will simply sit on top of the cells and reduce exposure to allergens and viruses,
as opposed to actually penetrating the cells and causing a chemical reaction.
UT
– Untreated cells
UT+A
– Untreated cells challenged with allergen
 |
 |
Figure
1 - The effect of the C&C technology (PL-14) in reduction the IL-8 secretion in response
to dust mite allergen Der P1. A lower bar indicates lower inflammation (lowercase letters
are significantly different from each other (P< 0.05)). |
Figure
2 - The effect of the C&C technology (PL-14) in reduction the IL-8 secretion in response
to timothy grass allergen Phl P1. A lower bar indicates lower inflammation (lowercase letters
are significantly different from each other (P< 0.05)) |
|
|
 |
 |
Figure
3 - The effect of the C&C technology (PL-14) in reduction the IL-8 secretion in response
to European hornbeam allergen Car B1. A lower bar indicates lower inflammation (lowercase
letters are significantly different from each other (P< 0.05) |
Figure
4 - The effect of the C&C technology (PL-14) in reduction the IL-8 secretion in response
to European dust mite allergen Der F1. A lower bar indicates lower inflammation (lowercase
letters are significantly different from each other (P< 0.05)) |
 |
 |
Figure
5 - The effect of the C&C technology (PL-15) in protection of the cells against human
corona virus. A higher bar indicates higher degree of protection (lowercase letters are significantly
different from each other (P< 0.05))
|
Figure
6 - The effect of the C&C technology (PL-16) in protection of the cells against influenza
virus. A higher bar indicates higher degree of protection (lowercase letters are significantly
different from each other (P< 0.05))
|
|
|
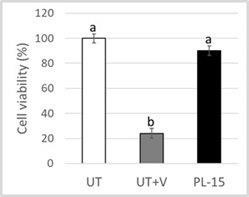 |
|
Figure
7 - The effect of the C&C technology (PL-15) in protection of the cells against SARS-CoV-2
Omicron BA.1 virus. A higher bar indicates higher degree of protection (lowercase letters
are significantly different from each other (P< 0.05)) |
|
Trap
and Target ™ (T&T)
Novel
delivery systems are required to address unmet clinical needs. In circumstances where local or systemic administration may not be the
best approach, nasal drug delivery may be a viable option. Intranasal administration is an attractive option for local and systemic delivery
of many therapeutic agents.
Advantages
of intranasal drugs delivery
The
nasal cavity is an important target for local and systemic drug administration as well as targeting the central nervous system. Due to
highly vascularization of the nasal mucosa, liquids or particles that attach to this surface can act either locally or be absorbed into
the bloodstream. To treat localized diseases including congestion, sinusitis, and allergic reactions, a variety of drugs such as corticosteroids
and antihistamines often are administered to the nasal cavity. The first cranial nerve, or olfactory nerve, is the only point where the
central nervous system is exposed to the body’s mucosa, and it is one of six nerves that branch into the nose cavity. This means
that medications can be absorbed directly into the brain, bypassing the blood-brain barrier.
Although
there are many advantages for delivering medicines intranasally, there are also a few drawbacks, such as quick evacuation from the nasal
canal, limited bioavailability, and difficulty getting a big enough dosage due to the limited absorption area. Our T&T technology
is developed to address the abovementioned drawbacks to further improve the efficiency of intranasal administration. Based on our C&C
hydrogel technology, we adjusted specific characteristic parameters and established the T&T drug delivery system for intranasal targeted
drugs.
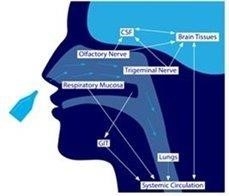
While
determinations of safety and efficacy are solely within the authority of the FDA and comparable regulatory bodies, the T&T platform
delivery technology consist of a mucoadhesive polymers mixture designed to allow a long residence time and an intimate contact with the
mucosal tissue for what we believe to be an effective delivery of medicines. The T&T platform can be tailored for different molecules
to address their specific challenges thus believed to induce improved therapeutic effect. The T&T technology has been designed to
enable mucoadhesion and prolonged retention at the deposition site by tailoring the physicochemical properties through composition, concentration
and crosslinking of the key polymers of the formulation appears pivotal for the potential development as nasal medicinal product candidates.
The
T&T platform is optimized into two main applications: local and systemic delivery.
Locally
acting nasal product candidates
Several
nasal products are present on the market for the treatment of local ailments of the nose. In general, they are nasal sprays and the principal
APIs contained in such products are antihistamines, corticosteroids and vasoconstrictors.
Corticosteroids
Among
the locally acting nasal products, intranasal corticosteroids, or INCS, are particularly interesting for their clinical and commercial
value, being indicated as primary or adjunct therapy for treating nasal congestion, allergic/non-allergic rhinitis, acute rhinosinusitis,
chronic rhinosinusitis with or without nasal polyposis and adenoid hypertrophy.
The
efficacy and safety profiles of INCS for adults and pediatric patients is well established. The bioavailability of the CS can be increased
due to the bioadhesion and viscosity of our formulations. Patents related to INCS products on the market expired recently in addition
to the usage of CS as a promising treatment to COVID-19 symptoms create an opportunity for market penetration.
We
aim to conduct feasibility studies for the corticosteroids product candidate of the T&T technology beginning in the fourth quarter
of 2024 through the first quarter of 2026. The feasibility studies may include drug loading capacity, release kinetics profile of the
APIs, stability and local toxicity. Based on these feasibility studies we will identify the leading candidates that will be further optimized
to be tested in preclinical studies for safety and efficacy evaluation.
Systemically
acting nasal product candidates
The
high vascularization and high permeability of the nasal mucosa enabling systemic distribution of drugs. Nasal drug products on the market
now include several indications such as hormone replacement therapy, osteoporosis, migraine, prostate cancer and vaccination against
influenza. Because nasal administration avoids first-pass metabolism and the gastrointestinal tract, it is used for the delivery of APIs
with low bioavailability, including peptides and proteins.
We
believe that our T&T hydrogel technology can be compatible with drugs related to the central nervous system and significantly improve
their bioavailability. We identify a need for improved delivery system for benzodiazepines drugs as well as for the opioid antagonist
naloxone. We are currently evaluating the feasibility of these candidates in collaboration with one of the members of our Scientific
Advisory Board, Prof. Fabio Sonvico (Pharmaceutical Technology, University of Parma, Italy), and will select our lead candidate to proceed
to preclinical and clinical evaluations.
Benzodiazepines
Benzodiazepines,
or BZDs, are widely recommended drugs in many countries around the world, as they are used to lessen anxiety, seizures, relax muscles,
induce rest, or as sedatives for general anesthesia or sedation before medical procedure. Intranasal administration of benzodiazepines)
is advantageous for home treatment of prolonged seizures, for pre-hospital treatment of seizures by emergency medical technicians, and
for care of severely cognitively and behaviorally impaired patients when patient cooperation may be restricted.
We
are planning to conduct feasibility studies for the BZD product candidates utilizing the T&T technology beginning in the fourth quarter
of 2024 through the first quarter 2026. We will explore the effect of drug loading, release kinetics profile, stability, and local toxicity.
Based on these feasibility studies we will identify two or three leading candidates that will be further optimized and be tested in preclinical
studies for safety and efficacy. Based on the pre-clinical studies results we will proceed to conduct Phase I clinical trial.
Naloxone
– an Opioid Antagonist
The
current opioid overdose epidemic can be attributed to fentanyl and other super-potent opioids found in substantial numbers of recent
overdoses. Providing Naloxone immediately after a person experiences respiratory depression can reverse opioid toxicity. It is possible
to administer Naloxone by injection or intranasally. Ampoules and prefilled syringes of Naloxone injectables are available. In comparison
with naloxone injectables, naloxone nasal spray provides several advantages to community usage, including ease of administration, minimal
training requirements, and no risk of needlestick injuries. In the event of an overdose of opioids in the community, Naloxone nasal spray
might be used for emergency rescue treatment. Patients who may witness an opioid overdose or are at risk of opioid overdose are advised
to carry naloxone nasal spray for use in the event of an opioid overdose emergency.
We
believe that the characteristic of our T&T derived formulations may provide an improved uptake profile and bioavailability of naloxone
through intranasal administration due to its mucoadhesive and viscous properties. We aim to conduct feasibility studies for the naloxone
product candidate of the T&T technology beginning in the fourth quarter of 2024 through the first quarter of 2026. The feasibility
studies may include drug loading capacity, release kinetics profile of the APIs, stability and local toxicity. Based on these feasibility
studies we will identify the leading candidates that will be further optimized to be tested in preclinical studies for safety and efficacy
evaluation. Based on the pre-clinical study results we plan to proceed to Phase I clinical trial.
SCI-160
– Pain solution
Pain
is the most common reason for physician consultation in most developed countries. It is a major symptom in many medical conditions and
can interfere with a person’s quality of life and general functioning. Opioid medications can provide short, intermediate or long
acting analgesia depending upon the specific properties of the medication and whether it is formulated as an extended release drug. Opioids
are efficacious analgesics in chronic malignant pain and modestly effective in nonmalignant pain management. However, there are associated
adverse effects, especially during the commencement or change in dose. Prolonged opioid use may cause drug tolerance, chemical dependency,
diversion and addiction. The potency and availability of these substances, despite their high risk of addiction and overdose, have made
them popular both as formal medical treatments and as recreational drugs. Due to their sedative effects on the medulla oblongata, opioids
in high doses present the potential for respiratory depression, and may cause respiratory failure and death. In a 2013 review study published
in Fundamental & Clinical Pharmacology, various studies were cited demonstrating that cannabinoids exhibit comparable effectiveness
to opioids in models of acute pain and even greater effectiveness in models of chronic pain. Cannabis produces several compounds with
various known activities known together as cannabinoids, such as THC and CBD. In general, cannabinoids bind and act through the two characterized
CRP: CB1 and CB2. However, activation of the CB1 receptor (as for example in the case of THC) leads to unwanted psychoactive “high”
and other adverse events, whereas activation of CB2 does not lead to any psychoactivity. In addition, and unrelated to the above sentence,
the affinity of the cannabis derived cannabinoids to these receptors is limited and partial.
Currently
available treatments are antidepressant and anticonvulsant analgesics, opioids and nonsteroidal anti-inflammatory drugs. These are inadequate
to control all pain or are associated with limiting side effects (e.g., most problematic being sedation with the antidepressant and anticonvulsant
group, constipation with the opioids and gastrointestinal and cardiovascular effects with the Non-steroidal anti-inflammatory drugs).
We
plan to start an initial testing to explore the potential of our SCI-160 platform when combined with the T&T technology, in the first
quarter of 2025.
Our
Strengths
We
believe that our experienced results-oriented management team, promising IP portfolio, scalable robust business model and multiple product
candidates validating our technologies gives us a distinct advantage in the marketplace.
| |
● |
People:
Our
leadership team has a vast industry experience. Our management team has over 15 years (on average) of experience in life science
companies. Our board of directors have vast experience in the life sciences industry as well as strong financial background. We believe
that the holistic knowhow of our group will strongly contribute to a successful path from clinical development, regulatory approvals
and commercialization of our product candidates. In addition, our management is supported by our Scientific Advisory Board which
is an advisory panel of world-renowned academics and thought leaders with expertise in drug delivery systems, Chemistry and Pharmaceuticals. |
| |
● |
Process:
We
are developing and optimizing set of business processes including pre-clinical and clinical development, quality and regulatory processes.
These processes can contribute shortening the time to market of our future product candidates position us with a competitive value
in the competition landscape. The regulatory path for the C&C product candidates will be Class II 510 (k). With regards to the
T&T platform technology development process our feasibility set of studies is well defined and accepted in the intranasal delivery
industry. We focus on already approved APIs (corticosteroids, benzodiazepines and naloxone) to shorten the clinical and regulatory
processes time towards 505(b)(2) approval. |
| |
● |
Adaptable
Technology:
Our
C&C hydrogel technology is tunable and can provide a solution against a wide range of biological assaults based on its versatile
morphological properties. Our T&T drug delivery platform is designed to allow a long residence time and an intimate contact with
the mucosal tissue for a targeted delivery of medicines. The T&T platform can be tailored for different drugs to address their
specific challenges thus believed to induce improved therapeutic effect. Both technologies are relatively easily adjusted and can
potentially provide solutions in a rapid manner to new medicinal challenges. |
Market
Opportunities
We
believe that our technologies have the potential to provide solutions to a broad range of unmet needs in the healthcare market. With
our C&C technology, we aim to introduce solutions to address common medical and public health challenges, such as allergic rhinitis
and nasal viral infections, including COVID-19. Looking towards the future, the COVID-19 pandemic highlighted the need for action at
the global level to invest in technologies, tools and solutions that will help overcome the next world health crisis. We believe our
technology can play an important role in aiding nations and global organizations to combat viral outbreaks. While people across the world
have become accustomed to preventative measures such as vaccination, wearing masks, keeping social distance and maintaining proper hygiene,
we believe that there is an obvious need for a broader arsenal of more technologically advanced tools to help protect people as they
return to normal routine.
With
our T&T technology, we aim to address challenges in the markets of: allergic and non-allergic rhinitis by local intranasal delivery
of corticosteroids; for systemic delivery of CNS related drugs for the growing markets of combatting opioid overdose using intranasal
naloxone, and benzodiazepines for seizure clusters.
C&C
Technology Market Opportunities
The
human body is under constant external threats. Allergens, viruses and other toxins commonly penetrate our body’s defenses, first
through its mucosal membranes, such as the lining of the mouth, nose, and eyes. While our immune system is typically well-equipped to
fend-off or manage such threats, even common colds, influenza, and allergies continue to affect hundreds of millions of people every
year.
Nasal
gels and nasal sprays have emerged as a promising approach to block viruses and allergens from contacting the nasal epithelial tissue.
According to an August 2024 Fortune Business Insights report, titled Nasal Spray Market Size, Share and COVID-19 Impact Analysis, the
nasal spray market is expected to gain market growth in the forecast period of 2023 to 2030. Fortune Business Insights analysts valued
the total market size to account to $49.54 billion by 2030 growing at a compound annual growth rate, or CAGR, of 8.8% in the above-mentioned
forecast period. Rising infections, as well as allergic conditions are major growth-driver of the market.
Additionally,
certain advantages offered by these products, such as efficient and painless drug delivery, ease of availability, and convenience to
patients are expected to drive market growth during the forecast period.
Other
trends that are impacting the market are an increasing prevalence of respiratory disorders and significant pipeline of potential product
candidates and their expected launches. Both are anticipated to drive market growth in the forecast period.
Market
for SARS-CoV-2/COVID-19
As
of August 2024, according to the World Health Organization Covid-19 Dashboard, more than 7 million COVID-19 fatalities have been reported
worldwide. While global vaccination efforts have curbed new infections, even complete vaccination is not a guarantee against further
spreading and contracting of the disease. Certain variants of COVID-19 are also considered to cause more infections and spread faster
than the original strain of the virus, and the CDC expects that additional variants of the virus will continue to occur. Furthermore,
the efficacy of vaccinations is limited by time. For example, with respect to the Pfizer-BioNTech COVID-19 vaccines, booster doses are
recommended for certain adults after six months post vaccination. Also, vaccination efforts in emerging and developing countries are
lagging significantly, posing the risk of causing new growth of infections and deaths.
According
to a January 2023 Transparency Market Research report, titled COVID-19 Therapeutics Market, the global COVID-19 therapeutics market is
forecasted to reach USD 16.2 billion in 2031.
Market
for Influenza and Common Cold Viruses
In
March 2019, the World Health Organization introduced guidelines, titled Global Influenza Strategy 2019-2030, to combat the 1 billion
cases worldwide, three to five million of which are typically severe, and 290,000-650,000 influenza-related respiratory deaths. According
to a March 2024 Statista report, titled Cold and Cough Remedies Report, the global cold and cough remedies market is valued at $42.65
billion in 2024 and is expected to grow at a CAGR of 6.18% from 2024 to 2029.
Market
for Allergic Rhinitis
Allergic
rhinitis is another vexing health problem and troubling factor in global health care. It causes discomfort, illness, and even disability
to hundreds of millions of people worldwide, with an estimated prevalence ranging from 10% to 20% in the United States and Europe. Accordingly,
global per capita healthcare spending has increased exponentially due to the myriads of potential treatments and diagnostic methods.
This has resulted in a call for more effective, better quality, and more rapid diagnostic methods, which, in turn, creates significant
market and growth opportunities for players offering new effective treatment options. According to a January 2023 Global Market Insights
report, titled Allergic Rhinitis Drugs Market, the global therapeutic market for allergic rhinitis is projected to reach $16 billion
by 2032, growing at a CAGR of 2.5% from 2023 to 2032.
One
of the fastest-growing products in the nasal gel sector are allergen blockers. According to an October 2023 Infinium Global Research
report, titled Allergen Blocker Market, the global allergen blocker market will reach $214.5 million in 2030, growing at a CAGR of 3.64%
during 2023-2030. The global allergen blocker market is primarily driven by the increasing prevalence of allergies worldwide, which drives
the demand for products aimed at alleviating allergy symptoms.
The
C&C technology serviceable available market is the segments of nasal blockers derived from COVID-19, influenza, common cold and nasal
allergies markets. It can be estimated that the nasal blockers market including viral blocking blockers is significantly higher than
the allergen nasal blocker market.
Prospective
studies published between 2019 and 2021, including a January 2021 Journal of Pain Management report, titled Clinical Pharmacokinetics
and Pharmacodynamics of Nasal Sprays for Acute Migraine Treatment, a March 2020 Journal of Pharmaceutical Sciences and Research report,
titled Advantages of Intranasal Drug Delivery System, and an August 2019 Patient Preference and Adherence report, titled Patient Preferences
in Medication Administration: A Study of Nasal Sprays vs. Other Delivery Methods, have demonstrated that a key driver for patients preferring
a nasal spray is speed of onset. The ease of administration of the intranasal products plays a vital role in improving their compliance.
Several factors driving the growth of intranasal markets. Some of which related to the general intranasal markets while other related
to more specific intranasal markets such as the nasal blockers and drug delivery.
Drivers
for the general intranasal market
| ● | Ongoing
R&D activities as well as private and government investments in the healthcare industry
to introduce novel strategies to treat complex allergies and to deliver drug alternatively
are expected to positively impact the intranasal market’s revenue growth. |
| ● | In
recent years, the disparities in healthcare, medical remedies, and treatments have led to
an increasing number of people choosing to self-medicate with over-the-counter drugs rather
than see a doctor. |
| ● | According
to a Health Awareness report titled, Prevalence of Pain and Fear as Barriers to Vaccination
in Children – Systematic Review and Meta-Analysis, published in December 2022, the
fear of needles in children and the safe injection of vaccinations into the body by nasal
spray stimulate market growth of other intranasal products. For the population of people
with a phobia for needles, a gel or sprays is a massive relief for them. Most people prefer
other forms of medication or drug delivery rather than injecting. |
| ● | Rising
adoption of nasal sprays due to ease of administration. |
Drivers
for the Capture and Contain TM related markets
| ● | Rising
prevalence of allergies, particularly those caused by airborne allergens, is driving the
demand for allergic rhinitis treatment devices, according to an October 2024 market research
survey conducted by Fact.MR, titled Allergic Rhinitis Treatment Devices. |
| |
● |
The increasing adoption rate
of OTC products is leading to high market penetration and is boosting the growth of the global nasal market. An increasing number
of pharmaceuticals companies, supermarkets, drug stores, and retail stores offer many opportunities for the market’s growth.
According to the Australian publication, Health Direct, in a report last reviewed by the publisher in April 2024, more than 200 viruses
worldwide can cause cold, and adults are susceptible to these viruses about 2 to 4 times a year. The same source also suggests that
cold and influenza symptoms are generally mild to moderate and self-limiting, which propels the demand for prevention approaches,
fueling the growth of the nasal barriers market. |
| ● | The
debilitating effects of influenza and the effect of the global pandemic have been the major
growth factors for effective blockers. The coronavirus pandemic infested fear due to its
viral nature, which encouraged people to get vaccinated against influenza to improve their
immune system and to seek multiple solutions while facing the challenge of the COVID-19 pandemic. |
| ● | Growing
awareness regarding allergy immunotherapy, increased public awareness regarding regular medical
check-ups, and increased healthcare expenditure are other key factors contributing to the
market’s revenue growth. |
T&T
technology market opportunities
According
to a December 2023 report by The Brainy Insights, titled Nasal Drug Delivery Market Size by Container, the global market for intranasal
drug delivery is projected to reach $136.46 billion by 2032, growing at a CAGR of 7.18%. Factors such as increasing patient inclination
for nasal drug delivery and growing acceptance of self-administration of drugs are driving the progress of nasal drug delivery market.
The easy administration and enhanced efficacy have improved patient inclination for the nasal drug transfer which subsequently has boosted
the demand for global market for the nasal drug delivery. Intranasal drug delivery is one of the most preferred drug delivery routes
among patients and healthcare providers. This can be majorly attributed to the non-invasive nature of this route of delivery and the
fact that drug absorbability is higher through the nasal route. Intranasal drug delivery is one of the most preferred drug delivery routes
among patients and healthcare providers. This can majorly be attributed to the non-invasive nature of this route of delivery and the
fact that drug absorbability is higher through the nasal route. In addition, the nasal route offers a less hostile environment as compared
to the gastro-intestinal route; this enables better absorption of drugs. Moreover, nasal drug delivery, unlike some other routes of drug
delivery, does not require any sterile method for administering drugs into the body. The easy administration of these drugs plays a crucial
role in improving compliance to drug therapies among patients, which in turn drives patient outcomes. Considering these factors, the
preference for nasal drug delivery is increasing among patients and healthcare providers.
The
nasal cavity is mainly used for treatment of local diseases of the upper respiratory tract such as nasal congestion, nasal infections
and nasal allergic diseases e.g., allergic rhinitis. However, in the last decades the nasal cavity has also been exploited for systemic
delivery of small molecular weight drugs, especially where a rapid onset of action is required. Examples of such marketed nasal products
are drugs for treatment of migraine such as, zolmatriptan (Zomig®), sumatriptan (Imitrex®) and butorphanol tartrate (Stadol NS),
treatment of severe pain such as fentanyl (PecFent®; Instanyl®), for smoking cessation (Nicorette®) and for treatment of
menopausal symptoms (Aerodiol®).
Intranasal
corticosteroids market opportunities
According
to a June 2023 Future Market Insight research, titled Global Intranasal Corticosteroids Market, the INCS products market is estimated
to reach $11.2 billion by 2033. INCS remain the most effective treatment option for nasal symptoms associated with moderate to severe
allergic rhinitis.
The
global market for INCS experienced growth over past few decades driven by the increased prevalence of condition like allergic rhinitis
and Chronic Obstructive Pulmonary Disease. This increased prevalence has impacted infants, young children, and the elderly population
suffering from these conditions. Most corticosteroids used are for treatment of seasonal allergic rhinitis caused due to aeroallergens.
Our
serviceable available market for local intranasal delivery of corticosteroids is the segments of intranasal gels and nasal corticosteroids
markets which we believe to be approximately $700 million.
Intranasal
Benzodiazepines market opportunities
According
to a 2024 Polaris Market Research report, titled Benzodiazepine Drugs Market, the benzodiazepines drug market is expected to reach $4.2
billion by 2032, which reflects a CAGR of 3.7% during 2024-2032. The demand for benzodiazepines is being driven by an increase in the
number of people who are suffering from medical disorders such as muscle spasms, insomnia, anxiety, seizure and other mental health conditions.
Moreover, a high preference for benzodiazepine drugs over other psychoactive medications is expected to increase the prescription volume,
thereby fueling the overall market growth. Furthermore, the high prevalence of epilepsy worldwide will increase the need for benzodiazepine
drugs during the forecasted timeframe. According to a February 2024 report published by the World Health Organization, titled Epilepsy
Key Facts, around 50 million people worldwide have epilepsy, making it one of the most common neurological diseases globally. Nearly
80% of people with epilepsy live in low- and middle-income countries. 30% of uncontrolled epilepsy patients also experience seizure clusters.
The market for high income countries can be estimated around 10 million patients. According to a September 2022 market research survey
conducted by Fact.MR, titled Benzodiazepine Drugs Market Outlook 2022-2032, the market for Benzodiazepines for seizure clusters is estimated
to be around $700 million.
Intranasal
Naloxone market opportunities
Opioid
abuse is one of the leading causes of drug overdose and death globally. Naloxone is an opioid antagonist designed to rapidly reverse
opioid overdose. The growing need for an effective treatment and rising number of opioid dependent patients driving the growth of naloxone
market in the United States. According to an August 2023 report published by the World Health Organization, titled Opioid Overdose, more
than 25 million people are being affected annually making it a serious global concern.
According
to Custom Market Insight report published in December 2023, titled Global Naloxone Market 2024–2033, the global naloxone market
is expected to grow to $2.47 billion in 2032, at a CAGR of 11% between 2023 and 2032. The naloxone market is propelled by several growth
factors including the urgent need to mitigate the opioid crisis, with naloxone being a crucial life-saving intervention for opioid overdoses,
the increasing cases of opioid abuse and overdoses across various demographics underscore the demand for effective naloxone solutions,
efforts to enhance naloxone accessibility through distribution programs and community initiatives contribute to market growth. In addition,
supportive legislative measures, including the expansion of naloxone access and Good Samaritan laws, foster a conducive environment for
market development.
According
to a Vantage Market Research report published in February 2022, titled Naloxone Spray Market Global Industry Assessment and Forecast,
the naloxone intranasal spray market is projected to attain a value of $1.4 billion by 2030. Increased launches and approvals of novel
naloxone spray products are expected to be a major factor in the market’s growth in the future years.
Intranasal
SCI-160 pain solution market opportunities
According
to an August 2024 report by Precedence Research, titled Pain Management Therapeutics Market Size, the global pain management drugs market
size was estimated at $78.14 billion in 2022 and is projected to hit around $115 billion by 2032, growing at a CAGR of 3.94% during the
forecast period 2023 to 2032. North America dominated the global market and captured more than 45% revenue share in 2022.
In
a July 2020 International Journal of Surgery article, titled Trauma of Major Surgery: A Global Problem That Is Not Going Away, it was
reported that according to the U.S. National Center for Health Statistics, over 310 million major procedures are performed annually,
of which approximately 40 to 50 million are in the U.S. and 20 million in Europe. Moreover, there is a rise in the rate of surgeries
worldwide which is the primary reason for the growing consumption of pain management drugs. Subsequently, the increased cancer therapies
associated with pain incidence and a rising geriatric population having various therapies drive the market. Consumer preference for pain
management therapies is influenced by their high availability, ease of access, heightened awareness, cost-effectiveness, and rapid relief.
Furthermore,
rising road accidents due to the increasing number of vehicles and traffic violations lead to related trauma injuries, which create demand
for pain management post surgeries. According to a December 2023 World Health Organization report, titled Global Status Report on Road
Safety 2023, 1.19 million people die yearly from road traffic crashes. Between 20 and 50 million people suffer from non-fatal injuries,
with many incurring a disability due to their injury.
Drivers
for the Trap and Target TM related markets
| |
● |
Surging interest in controlled
and/or sustained release drug delivery systems across many therapeutic areas is a key factor expected to contribute to the intranasal
drug delivery market growth. |
| ● | Recent
technological developments contribute to the growth of the U.S. intranasal drug delivery
market. |
| ● | During
the past few years there is a trend towards an increased approvals of intranasal treatment
for various disorders, this trend assumed to boost this market growth. |
Drivers
for the corticosteroids intranasal market
| ● | Increasing
number of people suffering from allergic rhinitis together with inflammation of the nose
is a major factor driving the global INCS market. |
| ● | Prevalence
of allergic rhinitis in children found to be increased in several regions and is projected
to drive the INCS market. |
Drivers
for the benzodiazepines intranasal market
| ● | Rise
in prevalence of anxiety and seizures is a major driver of the global benzodiazepine drugs
market. Current lifestyle and urban life have made peoples’ lives more stressful, which
leads to mental disorders such as depression, anxiety, panic, and maniac conditions |
| ● | Increased
adoption of generic drugs and comparatively higher prescriptions for benzodiazepines in general,
as compared to other psychoactive drugs, also drive the intranasal BZD market. |
Drivers
for the naloxone intranasal market
| ● | Growing
prevalence of opioid overdoses results in increased number of deaths involving synthetic
opioids in recent years. |
| ● | Government
campaigns in the U.S. regarding the awareness of opioid dependence and related risks increased
during the past years which create a driven force to the intranasal market of naloxone. |
Competition
The
pharmaceutical and medical device industries are characterized by constantly introduced new technologies, strong competition and various
innovative products that may be similar to ours being developed by several pharmaceutical and medical device companies, public and private
universities and research organizations.
Our
competitors, either alone or through their strategic partners, have substantially greater name recognition and financial, technical,
manufacturing, marketing and human resources than we do and significantly greater experience and infrastructure in the research and development
of medical devices, obtaining the FDA and other regulatory clearances of those devices and commercializing those devices around the world.
Competition
related to the C&C TM technology
We
face competition at various levels and from various competitors. This includes competition based on different forms of treatment, including
vaccination or allergen immunotherapy, protective gear, such as face masks, and remedies that merely treat symptoms. Our main competitors
are companies promoting nasal barrier products, which are described in the chart below:
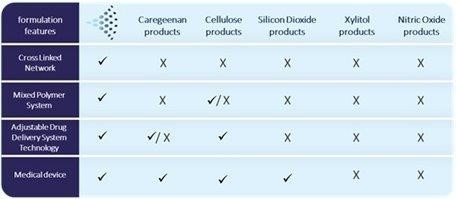
The
features described above translate to product candidate characteristics of non-drip, non-irritate, optimal coverage of the nasal cavity
and relatively prolonged retention of the nasal epithelial tissue. We believe that a product candidate with these characteristics will
make a user-friendly product candidate with significant market opportunities.
Competition
related to the T&T TM technology
Intranasal
corticosteroids for allergic/non-allergic rhinitis
The
most preferred treatment for allergic rhinitis is the INCS approach. Although all INCSs are considered safe and effective for this indication,
different products differ in formulation form (e.g., powder, gel or liquid solution), potency, molecular structure features and physicochemical
and pharmacokinetic properties that may result in differences in clinical efficacy and safety. We believe that the T&T technology
can bring a value proposition to the selected drugs by improving its bioavailability profile.
Some
of the dominant players in the global INCS market include Sanofi, GlaxoSmithKline plc. Merck Sharp Dohme, McNeil Consumer Healthcare,
Sunovion Pharmaceuticals Inc, Teva Branded Pharm, Ivax Pharmaceuticals Incorporated, AstraZeneca and more. In addition, presence of small
and local manufacturers across the countries will account for competitiveness in intranasal corticosteroids market.
Intranasal
benzodiazepines for seizure clusters
The
first intranasal BZD first product Nayzilam® (midazolam) nasal spray by UCB Biopharma SPRL was approved by the FDA in November 2019
for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive
seizures) that are distinct from a patient’s usual seizure pattern in patients with epilepsy aged 12 years or older. Net sales
in the United States in the first six months of 2020 were $12.6 million, $17.25 in second quarter of 2020 and $24.15 in first quarter
of 2021, representing a growth of ~ 40% between each quarter.
Intranasal
naloxone for opioid overdose
The
FDA approved on April 2019 the first generic naloxone hydrochloride nasal spray, commonly known as Narcan®,
a life-saving medication that can stop or reverse the effects of an opioid overdose. Narcan sales in the US in 2020 was $311 million.
In the third quarter of 2021 Narcan® nasal spray sales reached $133 million, 50% increase
with regards to the equivalent quarter of 2020.
In
April 2021, the FDA approved an 8 mg dose naloxone hydrochloride nasal spray (Kloxxado®;
Hikma Pharmaceuticals) for the emergency treatment of known or suspected opioid overdose in adult and pediatric patients. This product
is a higher dosage of naloxone hydrochloride than the 2 mg and 4 mg dosage product previously approved by the FDA (Narcan®).
Bioavailability
and rapid onset are among the desirable features for intranasal naloxone and several companies around the globe have nasal naloxone as
part of its development pipeline: Emergent BioSolutions, Pfizer, Teva Pharmaceutical Industries Ltd., Opiant Pharmaceuticals, Hikma Pharmaceuticals,
Nasus Pharma, Amphastar Pharmaceuticals, Indivior PLC, Samarth Pharma Pvt. Ltd., Troikaa Pharmaceuticals Ltd., and Neon Laboratories
Limited.
Manufacturing
We
currently rely on and expect to continue to rely on third parties for the supply of raw materials and to manufacture supplies for clinical
trials of our product candidates. For the foreseeable future, we expect to continue to rely on such third parties for the manufacture
of our product candidates on a clinical and thereafter on commercial scale, if any of our product candidates receive regulatory approval
or clearance.
Regulation
Government
Regulation and Approval Process
According
to FDA guidelines, a product will be considered as a medical device, and subject to FDA regulation, if it meets the definition of a medical
device per Section 201(h) of the FFDCA
Per
Section 201(h) of the FFDCA, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,
or other similar or related article, including a component part, or accessory which is:
| ● | recognized
in the official National Formulary, or the United States Pharmacopoeia, or any supplement
to them; |
| ● | intended
for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment,
or prevention of disease, in man or other animals; or |
| ● | intended
to affect the structure or any function of the body of man or other animals, and which does
not achieve its primary intended purposes through chemical action within or on the body of
man or other animals. |
In
addition, it must not achieve its primary intended purpose(s) through chemical action within or on the body of humans or other animals.
Further, it cannot be dependent upon being metabolized for the achievement of its primary intended purposes.
Because
the intended use for each of our C&C product candidates is creating a physical barrier and its PMOA is physical, we believe that
our C&C product candidates will be regulated as Class II medical devices. We believe that our PL-14 product candidate can utilize
the 510(k) pathway for alleviating allergic symptoms, and that our PL-15 and PL-16 product candidates can utilize the De Novo Classification
pathway for reducing the risk of nasal infections caused by COVID-19 and influenza, respectively.
Device
Approval Process
Unless
an exemption applies, any medical device that is to be marketed in the United States be cleared
via submission of a premarket notification (i.e., 510(k)) for Class II devices, or a PMA
for Class III devices. Alternatively, the device can be marketed following the granting of
a De Novo Classification request for devices that do not have a legally marketed predicate
device. We performed an FDA medical device analysis based on our PL-14 product candidate’s
description, along with potential accessories and the proposed intended use. We believe our
PL-14 product candidate’s classification is: 21 C.F.R. § 880.5045 “Medical
recirculating air cleaner” (under the product code: NUP-Cream, Nasal, Topical, Mechanical
Allergen Particle Barrier) which is FDA Class II requiring a 510(k) submission. This means
a 510(k) submission for FDA review is required for clearance allowing it to be marketed.
To provide the best possible predicate device to establish substantial equivalency within
the 510(k) submission, a review of FDA’s 510(k) database for Product Code NUP was performed,
which includes several possibilities for a potential predicate device, such as Alzair, Nasalese
and Bentrio with intended uses of “promoting alleviation of mild allergic symptoms
triggered by the inhalation of various airborne allergens”.
To
obtain 510(k) clearance, a company must submit a premarket notification demonstrating substantial equivalence between the proposed device,
and a legally marketed “predicate” device, which is defined as a legally marketed device, that (i) was legally marketed prior
to May 28, 1976, for which the FDA has not yet called for submission of a PMA application; (ii) has been reclassified from Class III
to Class II or Class I; (iii) has been cleared through the 510(k) premarket notification process; or (iv) has been previously determined
to be exempt from the 510(k) process.
Substantial
equivalence means that the proposed device has the same intended use and the same technological
characteristics as the predicate device, or if the new device has different technological
characteristics, that the device is as safe and effective as the predicate device and does
not raise different questions of safety and effectiveness. We have identified three such
predicate devices, Alzair, Nasalese and Bentrio, and plan to reference them in our planned
510(k) submission.
Our
PL-15 and PL-16 product candidates are intended to provide a barrier against COVID-19 and influenza from contacting the nasal epithelial
tissue, respectively. We performed a regulatory assessment review for our PL-15 and PL-16 product candidates where the intended use includes
a “nasal mechanical virus blocker” and found that there are no valid predicate devices found in the FDA’s databases
matching this intended use. The lack of available predicate devices, combined with the fact that our PL-15 and PL-16 product candidates
have similar risk profile as our PL-14 product candidate (due to three product candidates using the same ingredients and method of use),
we believe that our PL-15 and PL-16 product candidates may be regulated as a Class II medical device if FDA agrees and grants a De Novo
Classification request. In order to assess the likelihood of approval under a De Novo pathway, during the first quarter following the
closing of this offering we intend to schedule a pre-submission meeting with the FDA, but have not yet communicated directly with the
FDA regarding any of its C&C product candidates.
For
the clinical studies planned for PL-15 & PL-16 which will include human subjects; the Investigational Device Exemptions regulation
describes three types of device studies: significant risk, nonsignificant risk, and exempt studies. During the first quarter following
the closing of this offering, the company intends to schedule a pre-submission meeting with the FDA to determine the IDE regulation type
of device studies for PL-15 & PL-16.
We
believe the time frame of 15 months between the planned pre-sub meeting and the planned initiation of clinical trials is sufficient for
the completion of IDE-enabling preclinical studies, preparation of clinical study protocol(s), design control documentation and manufacturing
documentation to enable an IDE filing.
The
estimated timeline for obtaining 510(k) clearance for our C&C product candidates, is
based on the estimated time needed for the following activities: (i) GMP manufacturing of
our clinical trial materials, which usually requires 9-12 months; (ii) Biocompatibility preclinical
studies, which usually requires 3-6 months (although these studies are performed concurrently
with the GMP manufacturing mentioned above); (iii) Clinical trials, which usually requires
6-12 months; and (iv) FDA submission and clearance, which usually requires 3-12 months. Regarding
FDA submission and clearance, generally 510(k) applicants can expect submission acceptance
review decisions within 15 calendar days, substantive review decisions within 60 days, and
final decisions within 90 days. Applicants with outstanding review issues will be notified
within 100 days. However, the FDA’s time of review does not include time on “hold”,
which includes any time spent by us responding to any FDA information requests, meaning that
the total timeframe of the review process could take longer than anticipated. In the case
of our predicate devices for our PL-14 product candidate, Alzair, Nasalese and Bentrio, the
FDA submission and clearance process took 86 and 140 days, respectively. For additional information,
please see “Business – FDA clearance plan for our C&C product candidates.”
Many
foreign countries in which we intend to market our PL-14 product candidate have regulatory bodies and restrictions similar to those of
the FDA. International sales are subject to foreign government regulation, the requirements of which vary substantially from country
to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA clearance and
the requirements may differ.
In
order to sell our product candidates in member states of the European Union, or the EU, our product candidates must comply with the general
safety and performance requirements of the EU Medical Devices Regulation, or Regulation (EU) No 2017/745), which repeals and replaces
the EU Medical Devices Directive (Council Directive 93/42/EEC).
Compliance
with these requirements is a prerequisite to be able to affix the CE mark to our product candidates, without which they cannot be sold
or marketed in the EU. All medical devices placed on the market in the EU must meet the general safety and performance requirements laid
down in Annex I to the EU Medical Devices Regulation including the requirement that a medical device must be designed and manufactured
in such a way that, during normal conditions of use, it is suitable for its intended purpose. Medical devices must be safe and effective
and must not compromise the clinical condition or safety of patients, or the safety and health of users and – where applicable
– other persons, provided that any risks which may be associated with their use constitute acceptable risks when weighed against
the benefits to the patient and are compatible with a high level of protection of health and safety, taking into account the generally
acknowledged state of the art. The European Commission has adopted various standards applicable to medical devices. These include standards
governing common requirements, such as sterilization and safety of medical electrical equipment and product candidates standards for
certain types of medical devices. There are also harmonized standards relating to design and manufacture. While not mandatory, compliance
with these standards is viewed as the easiest way to satisfy the general safety and performance requirements as a practical matter, as
it creates a rebuttable presumption that the device satisfies the general safety and performance requirements.
To
demonstrate compliance with the general safety and performance requirements we must undergo a conformity assessment procedure, which
varies according to the type of medical device and its (risk) classification. As a general rule, demonstration of conformity of medical
devices and their manufacturers with the general safety and performance requirements must be based, among other things, on the evaluation
of clinical data supporting the safety and performance of the product candidates during normal conditions of use. Specifically, a manufacturer
must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks,
and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims
made about the performance and safety of the device are supported by suitable evidence. Except for low-risk medical devices (Class I),
where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment of the conformity of its product candidates
with the general safety and performance requirements (except for any parts which relate to sterility, metrology or reuse aspects), a
conformity assessment procedure requires the intervention of an organization accredited or designated by a member state of the EU to
conduct conformity assessments, or a notified body. Depending on the relevant conformity assessment procedure, the notified body would
typically audit and examine the technical file and the quality system for the manufacture, design and final inspection of our devices.
If satisfied that the relevant product candidates conforms to the relevant essential requirements, the notified body issues a certificate
of conformity, which the manufacturer uses as a basis for its own declaration of conformity. The manufacturer may then apply the CE Mark
to the device, which allows the device to be placed on the market throughout the EU. If we fail to comply with applicable EU laws and
regulations, and corresponding EU member state laws, we would be unable to affix the CE mark to our product candidates, which would prevent
us from selling them within the EU.
The
aforementioned EU rules are generally applicable in the EEA, which consists of the 27 EU member states plus Norway, Liechtenstein and
Iceland. Non-compliance with the above requirements would also prevent us from selling our product candidates in these three countries.
Manufacturers
must demonstrate that their devices conform to the relevant essential requirements through a conformity assessment procedure. The nature
of the assessment depends upon the classification of the device. The classification rules are mainly based on three criteria: the length
of time the device is in contact with the body, the degree of invasiveness, and the extent to which the device affects the anatomy. Conformity
assessment procedures for all but the lowest risk classification of devices involve a notified body. Notified bodies are often private
entities and are authorized or licensed to perform such assessments by government authorities. Manufacturers usually have some flexibility
to select a notified body for the conformity assessment procedures for a particular class of device and to reflect their circumstances,
e.g., the likelihood that the manufacturer will make frequent modifications to its product candidates. Conformity assessment procedures
require an assessment of available clinical evidence, literature data for the product candidates, and post-market experience in respect
of similar product candidates already marketed. Notified bodies also may review the manufacturer’s quality systems. If satisfied
that the product candidates conforms to the relevant essential requirements, the notified body issues a certificate of conformity, which
the manufacturer uses as a basis for its own declaration of conformity and application of the CE Mark. Application of the CE Mark allows
the general commercializing of a product candidates in the EU. The product candidates can also be subjected to local registration requirements,
depending on the country.
In
May 2017, the EU adopted a new Medical Devices Regulation (EU) 2017/745 (MDR), which will repeal and replace the MDD with effect from
May 26, 2021. The MDR clearly envisages, among other things, stricter controls of medical devices, including strengthening of the conformity
assessment procedures, increased expectations with respect to clinical data for devices and pre-market regulatory review of high-risk
devices. The MDR also envisages greater control over notified bodies and their standards, increased transparency, more robust device
vigilance requirements, and clarification of the rules for clinical investigations. Under transitional provisions, medical devices with
notified body certificates issued under the MDD prior to May 26, 2021, may continue to be placed on the market for the remaining validity
of the certificate, until May 27, 2024, at the latest. After the expiry of any applicable transitional period, only devices that have
been CE marked under the MDR may be placed on the market in the EU.
Device
Clinical Trials
Clinical
trials are almost always required to support a PMA and are sometimes required to support a 510(k) submission. All clinical investigations
of investigational devices to determine safety and effectiveness must be conducted in accordance with the FDA’s Investigational
Device Exemption, or IDE, regulations which govern investigational device labeling, prohibit promotion of the investigational device,
and specify an array of recordkeeping, reporting and monitoring responsibilities of study sponsors and study investigators. If the device
presents a “significant risk” to human health, as defined by the FDA, the FDA requires the device sponsor to submit an IDE
application to the FDA, which must become effective prior to commencing human clinical trials. A significant risk device is one that
presents a potential for serious risk to the health, safety or welfare of a patient and either is implanted, used in supporting or sustaining
human life, substantially important in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human
health, or otherwise presents a potential for serious risk to a subject. An IDE application must be supported by appropriate data, such
as animal and laboratory test results, showing that it is safe to test the device in humans and that the testing protocol is scientifically
sound. The IDE will automatically become effective 30 days after receipt by the FDA unless the FDA notifies the company that the
investigation may not begin. If the FDA determines that there are deficiencies or other concerns with an IDE for which it requires modification,
the FDA may permit a clinical trial to proceed under a conditional approval.
In
addition, the study must be approved by, and conducted under the oversight of, an Institutional Review Board, or IRB. The IRB is responsible
for the initial and continuing review of the study and may pose additional requirements for the conduct of the study. If an IDE application
is allowed to go into effect by the FDA and the study approved by the reviewing IRB(s), human clinical trials may begin at a specific
number of investigational sites with a specific number of subjects as set forth in the study protocol. If the device presents a non-significant
risk to the patient, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate
review from the FDA, but must still follow abbreviated IDE requirements, such as monitoring the investigation, ensuring that the investigators
obtain informed consent, and labeling and record-keeping requirements. Acceptance of an IDE application for review does not guarantee
that the FDA will allow the IDE to become effective and, if it does become effective, the FDA may or may not determine that the data
derived from the trials support the safety and effectiveness of the device or warrant the continuation of clinical trials. An IDE supplement
must be submitted to, and allowed to go into effect by, the FDA before a sponsor or investigator may make a change to the investigational
plan that may affect its scientific soundness, study plan or the rights, safety or welfare of human subjects.
During
a study, the sponsor is required to comply with the applicable FDA requirements, including, for example, trial monitoring, selecting
clinical investigators and providing them with the investigational plan, ensuring IRB review, adverse event reporting, record keeping
and prohibitions on the promotion of investigational devices or on making safety or effectiveness claims for them. The clinical investigators
in the clinical study are also subject to FDA regulations and must obtain patient informed consent, follow the investigational plan and
study protocol, control the disposition of the investigational device, and comply with all reporting and recordkeeping requirements.
Additionally, after a trial begins, we, the FDA or the IRB could suspend or terminate a clinical trial at any time for various reasons,
including a belief that the risks to study subjects outweigh the anticipated benefits.
Pharmaceutical
Approval Process (nasal delivery)
The
clinical testing, manufacturing, labeling, storage, distribution, record keeping, advertising, promotion, import, export, and marketing,
among other things, of our product candidates are subject to extensive regulation by governmental authorities in the United States and
other countries. The FDA, under the FFDCA, regulates pharmaceutical products and medical devices in the United States.
The
steps required before a drug may be approved for marketing in the United States generally include:
| ● | the
completion of pre-clinical laboratory tests and animal tests conducted under GLP regulations; |
| ● | the
submission to the FDA of an Investigational New Drug, or IND application for human clinical
testing, which must become effective before human clinical trials commence; |
| ● | the
performance of adequate and well-controlled human clinical trials to establish the safety
and efficacy of the product candidate for each proposed indication and conducted in accordance
with current GCPs; |
| ● | the
submission to the FDA of a NDA; |
| ● | the
FDA’s acceptance of the NDA; |
| ● | satisfactory
completion of an FDA inspection of the manufacturing facilities at which the product candidates
is made to assess compliance with cGMPs; and |
| ● | the
FDA’s review and approval of an NDA prior to any commercial marketing or sale of the
drug in the United States. |
The
testing and approval process requires substantial time, effort, and financial resources, and the receipt and timing of any approval are
uncertain.
Pre-clinical
studies include laboratory evaluations of the product candidate, as well as animal studies
to assess the potential safety and efficacy of the product candidate. The results of the
pre-clinical studies, together with manufacturing information and analytical data, are submitted
to the FDA as part of the IND, which must become effective before clinical trials may be
commenced. The IND will become effective automatically 30 days after receipt by the FDA,
unless the FDA raises concerns or questions about the conduct of the trials as outlined in
the IND prior to that time. In this case, the IND sponsor and the FDA must resolve any outstanding
concerns before clinical trials can proceed.
Clinical
trials involve the administration of the product candidates to healthy volunteers or patients with the disease to be treated under the
supervision of a qualified principal investigator. Clinical trials are conducted under protocols detailing, among other things, the objectives
of the study, the parameters to be used in monitoring safety, and the efficacy criteria to be evaluated. A protocol for each clinical
trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. Further, each clinical trial must be reviewed
and approved by an independent IRB, either centrally or individually at each institution at which the clinical trial will be conducted.
The IRB will consider, among other things, ethical factors, the safety of human subjects, and the possible liability of the institution.
There are also requirements governing the reporting of ongoing clinical trials and clinical trial results to public registries. The FDA,
the IRB, or the clinical trial sponsor may suspend or terminate clinical trials at any time on various grounds, including a finding that
the subjects or patients are being exposed to an unacceptable health risk. Additionally, some clinical trials are overseen by an independent
group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group
provides authorization for whether or not a trial may move forward at designated checkpoints based on access to certain data from the
study. We may also suspend or terminate a clinical trial based on evolving business objectives and/or competitive climate.
Clinical
trials are typically conducted in three sequential phases prior to approval, but the phases may overlap. These phases generally include
the following:
| ● | Phase
1. Phase 1 clinical trials represent the initial introduction of a product candidate
into human subjects, frequently healthy volunteers. In Phase 1, the product candidate is
usually tested for safety, including adverse effects, dosage tolerance, absorption, distribution,
metabolism, excretion, and pharmacodynamics. |
| ● | Phase
2. Phase 2 clinical trials usually involve studies in a limited patient population to
(1) evaluate the efficacy of the product candidate for specific indications, (2) determine
dosage tolerance and optimal dosage, and (3) identify possible adverse effects and safety
risks. |
| ● | Phase
3. If a product candidate is found to be potentially effective and to have an acceptable
safety profile in Phase 2 studies, the clinical trial program will be expanded to Phase 3
clinical trials to further demonstrate clinical efficacy, optimal dosage, and safety within
an expanded patient population at geographically dispersed clinical study sites. |
Phase
4 clinical trials are conducted after approval to gain additional experience from the treatment of patients in the intended therapeutic
indication and to document a clinical benefit in the case of drugs approved under accelerated approval regulations, or when otherwise
requested by the FDA in the form of post-market requirements or commitments. Failure to promptly conduct any required Phase 4 clinical
trials could result in withdrawal of approval.
The
results of pre-clinical studies and clinical trials, including negative or ambiguous results as well as positive findings, together with
detailed information on the manufacture, composition, and quality of the product candidates, are submitted to the FDA in the form of
an NDA requesting approval to market the product candidates. The NDA must be accompanied by a significant user fee payment. The FDA has
substantial discretion in the approval process and may refuse to accept any application or decide that the data is insufficient for approval
and require additional pre-clinical, clinical, or other studies.
In
addition, under the Pediatric Research Equity Act, or PREA, an NDA or supplement to an NDA must contain data to assess the safety and
effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration
for each pediatric subpopulation for which the product candidates is safe and effective. The FDA may grant deferrals for submission of
data or full or partial waivers. Unless otherwise required by regulation, PREA does not apply to any drug for an indication for which
orphan designation has been granted. However, if only one indication for a product candidates has orphan designation, a pediatric assessment
may still be required for any applications to market that same product candidates for the non-orphan indication(s).
Once
the NDA submission has been submitted, the FDA has 60 days after submission of the NDA to conduct an initial review to determine whether
it is sufficient to accept for filing. Under the Prescription Drug User Fee Act, the FDA sets a goal date by which it plans to complete
its review. This is typically 12 months from the date of submission of the NDA application. The review process is often extended by FDA
requests for additional information or clarification. Before approving an NDA, the FDA will inspect the facilities at which the product
candidates is manufactured and will not approve the product candidates unless the manufacturing facility complies with cGMPs and may
also inspect clinical trial sites for the integrity of data supporting safety and efficacy. The FDA may also convene an advisory committee
of external experts to provide input on certain review issues relating to risk, benefit, and interpretation of clinical trial data. The
FDA is not bound by the recommendations of an advisory committee, but generally follows such recommendations in making its decisions.
The FDA may delay approval of an NDA if applicable regulatory criteria are not satisfied and/or the FDA requires additional testing or
information. The FDA may require post-marketing testing and surveillance to monitor the safety or efficacy of a product candidates.
After
the FDA evaluates the NDA and conducts inspections of manufacturing facilities where the drug product candidates and/or its API will
be produced, it may issue an approval letter or a Complete Response Letter. An approval letter authorizes commercial marketing of the
drug with specific prescribing information for specific indications. A Complete Response Letter indicates that the review cycle of the
application is complete and the application is not ready for approval. A Complete Response Letter may require additional clinical data
and/or an additional pivotal Phase 3 clinical trial(s), and/or other significant, expensive and time-consuming requirements related to
clinical trials, pre-clinical studies, or manufacturing. Even if such additional information is submitted, the FDA may ultimately decide
that the NDA does not satisfy the criteria for approval. The FDA could also approve the NDA with a Risk Evaluation and Mitigation Strategy,
or REMS, plan to mitigate risks, which could include medication guides, physician communication plans, or elements to assure safe use,
such as restricted distribution methods, patient registries, and other risk minimization tools. The FDA also may condition approval on,
among other things, changes to proposed labeling, development of adequate controls and specifications, or a commitment to conduct one
or more post-market studies or clinical trials. Such post-market testing may include Phase 4 clinical trials and surveillance to further
assess and monitor the product candidate’s safety and effectiveness after commercialization.
FDA
Regulation of Combination Product Candidates
The
FDA has specified a definition for the term “combination product,” which includes: (1) a product comprised of two or more
regulated components, e.g., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are physically, chemically, or
otherwise combined or mixed and produced as a single entity; (2) two or more separate product packaged together in a single package or
as a unit and comprised of drug and device product, device and biological product, or biological and drug product; (3) a drug, device,
or biological product candidates packaged separately that according to its investigational plan or proposed labeling is intended for
use only with an approved individually specified drug, device, or biological product where both are required to achieve the intended
use, indication, or effect and where upon approval of the proposed product the labeling of the approved product would need to be changed,
e.g., to reflect a change in intended use, dosage form, strength, route of administration, or significant change in dose; or (4) any
investigational drug, device, or biological product packaged separately that according to its proposed labeling is for use only with
another individually specified investigational drug, device, or biological product where both are required to achieve the intended use,
indication, or effect.
The
FDA is divided into various “Centers” by product type such as the Center for Drug Evaluation and Research, or CDER, the Center
for Biologics Evaluation and Research, or CBER, or the CDRH. Different Centers review drug, biologic, or device applications.
The
FDA is charged with assigning a Center with primary jurisdiction, or a lead Center, for review of a combination product. That determination
is based on the primary mode of action, or PMOA, of the combination product. Thus, if the PMOA of a device-biologic combination product
is attributable to the biologic product, CBER, which is responsible for premarket review of the biologic product, would have primary
jurisdiction for the combination product. If there are two independent modes of action, neither of which is subordinate to the other,
the FDA makes a determination as to which center to assign the product based on consistency with other combination product raising similar
types of safety and effectiveness questions or to the center with the most expertise in evaluating the most significant safety and effectiveness
questions raised by the combination product.
The
FDA has also established an Office of Combination Product to address issues surrounding combination product and provide more certainty
to the regulatory review process. That office serves as a focal point for combination product issues for agency reviewers and industry.
It is also responsible for developing guidance and regulations to clarify the regulation of combination product, and for assignment of
the FDA center that has primary jurisdiction for review of combination product where the jurisdiction is unclear or in dispute.
After
formally establishing the PMOA through an applicant’s Request for Designation, the Center that regulates that portion of the product
that generates the PMOA becomes the lead evaluator. When evaluating an application, a lead Center may consult other centers but still
retain complete reviewing authority, or it may collaborate with another Center, wherein the lead Center assigns concurrent review of
a specific section of the application to another Center, delegating its review authority for that section.
Typically,
the FDA requires a single marketing application submitted to the Center selected to be the lead evaluator, although the agency has the
discretion to require separate applications to more than one Center. One reason to submit multiple evaluations is if the applicant wishes
to receive some benefit that accrues only from approval under a particular type of application, like new drug product or orphan drug
exclusivity. If multiple applications are submitted, each may be evaluated by a different lead Center. When submitting multiple applications,
the applicant may be subject to the payment of two user fees, but a waiver of such fees may be obtained under certain limited circumstances.
The
FDA may subject a combination product to two or more sets of legal authorities, e.g., drug/device, biologic/device, drug/biologic drug,
but it has the authority to deem one set of legal authorities sufficient. FDA’s standard of review for a combination product application
and the applicable legal authority or authorities will depend on a case-by-case basis evaluation of the scientific and technical issues
and risk profile relevant to a combination product and its constituent parts. Because of the breadth and complexity of this analysis
in each case, no single regulatory paradigm is appropriate for all combination product.
After
receiving FDA approval or clearance, an approved or cleared product must comply with post-market safety reporting requirements applicable
to the product based on the application type under which it received marketing authorization. In the case of current good manufacturing
practices, or cGMP, the applicant may take one of two approaches: (1) complying with cGMP for each constituent part, or (2) a streamlined
approach specific to combination product, subject to certain limitations.
We
believe the FDA will classify the nasal hydrogels used as a drug delivery platform as a combination product subject to the primary jurisdiction
of the CDER.
The
Hatch-Waxman Amendments
505(b)(2)
NDAs
The
FDA is authorized to approve an alternative type of NDA under Section 505(b)(2) of the FDCA. Section 505(b)(2) permits the filing of
an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for
which the applicant has not obtained a right of reference from the data owner. The applicant may rely upon the FDA’s findings of
safety and efficacy for an approved product that acts as the “listed drug.” The FDA may also require 505(b)(2) applicants
to perform additional studies or measurements to support the change from the listed drug. The FDA may then approve the new product for
all, or some, of the conditions of use for which the branded reference drug has been approved, or for a new condition of use sought by
the 505(b)(2) applicant.
Abbreviated
New Drug Applications, or ANDAs
The
Hatch-Waxman amendments to the FDCA established a statutory procedure for submission and FDA review and approval of ANDAs for generic
versions of listed drugs. An ANDA is a comprehensive submission that contains, among other things, data and information pertaining to
the active pharmaceutical ingredient, drug product formulation, specifications and stability of the generic drug, as well as analytical
methods, manufacturing process validation data, and quality control procedures. Premarket applications for generic drugs are termed abbreviated
because they generally do not include clinical data to demonstrate safety and effectiveness. However, a generic manufacturer is typically
required to conduct bioequivalence studies of its test product against the listed drug. Bioequivalence is established when there is an
absence of a significant difference in the rate and extent for absorption of the generic product and the reference listed drug. For some
drugs, other means of demonstrating bioequivalence may be required by the FDA, especially where the rate or extent of absorption is difficult
or impossible to measure. The FDA will approve an ANDA application if it finds that the generic product does not raise new questions
of safety and effectiveness as compared to the reference listed drug. A product is not eligible for ANDA approval if the FDA determines
that it is not bioequivalent to the reference listed drug if it is intended for a different use or if it is not subject to, and requires
an approved Suitability Petition.
Patent
Exclusivity and Orange Book Listing
In
seeking approval for a drug through an NDA, including a 505(b)(2) NDA, applicants are required to list with the FDA certain patents whose
claims cover the applicant’s product. Upon approval of an NDA, each of the patents listed in the application for the drug is then
published in the Orange Book. Any applicant who files an ANDA seeking approval of a generic equivalent version of a drug listed in the
Orange Book or a 505(b)(2) NDA referencing a drug listed in the Orange Book must certify to the FDA (i) that there is no patent
listed with the FDA as covering the relevant branded product, (ii) that any patent listed as covering the branded product has expired,
(iii) that the patent listed as covering.
The
branded product will expire prior to the marketing of the generic product, in which case the ANDA will not be finally approved by the
FDA until the expiration of such patent or (iv) that any patent listed as covering the branded drug is invalid or will not be infringed
by the manufacture, sale or use of the generic product for which the ANDA is submitted. A notice of the Paragraph IV certification must
be provided to each owner of the patent that is the subject of the certification and to the holder of the approved NDA to which the ANDA
or 505(b)(2) application refers. The applicant may also elect to submit a “section viii” statement certifying that its proposed
label does not contain (or carves out) any language regarding the patented method-of-use rather than certify to a listed method-of-use patent.
If
the reference NDA holder and patent owners assert a patent challenge directed to one of the Orange Book listed patents within 45 days
of the receipt of the Paragraph IV certification notice, the FDA is prohibited from approving the application until the earlier of 30 months
from the receipt of the Paragraph IV certification, expiration of the patent, settlement of the lawsuit or a decision in the infringement
case that is favorable to the applicant. The ANDA or 505(b)(2) application also will not be approved until any applicable non-patent exclusivity
listed in the Orange Book for the branded reference drug has expired, as described in further detail below.
Non-Patent
Exclusivity
In
addition to patent exclusivity, the holder of the NDA for the listed drug may be entitled to a period of non-patent exclusivity,
during which the FDA cannot approve an ANDA or 505(b)(2) application that relies on the listed drug.
For
example, a drug that is considered a new chemical entity (NCE) at the time of approval may be awarded a five-year period of marketing
exclusivity, starting at the time of product approval. An ANDA or 505(b)(2) application referencing that drug may not be approved until
the five-year period expires. Also, an ANDA or 505(b)(2) application referencing that drug may not be filed with the FDA until the
expiration of five years, unless the submission is accompanied by a Paragraph IV certification, in which case the applicant may submit
its application four years following the original product approval.
A
drug, including one approved under Section 505(b)(2), may obtain a three-year period of exclusivity for a particular condition
of approval, or change to a marketed product, such as a new formulation for a previously approved product, if one or more new clinical
studies (other than bioavailability or bioequivalence studies) was essential to the approval of the application and was conducted/sponsored
by the applicant.
Pricing
and Reimbursement
Successful
commercialization of our product candidates depends, in part, on the availability of governmental and third-party payor reimbursement
for the cost of our product candidates. Government authorities and third-party payors increasingly are challenging the price of medical
products and services. On the government side, there is a heightened focus, at both the federal and state levels, on decreasing costs
and reimbursement rates for Medicaid, Medicare, and other government insurance programs. This has led to an increase in federal and state
legislative initiatives related to drug prices, which could significantly influence the purchase of pharmaceutical products, resulting
in lower prices and changes in products demand. If enacted, these changes could lead to reduced payments to pharmaceutical manufacturers.
Many states have also created preferred drug lists and include drugs on those lists only when the manufacturers agree to pay a supplemental
rebate. If our current product candidates or future drug candidates are not included on these preferred drug lists, physicians may not
be inclined to prescribe them to their Medicaid patients, thereby diminishing the potential market for our product candidates.
In
addition, third-party payors have been imposing additional requirements and restrictions on coverage and limiting reimbursement
levels for pharmaceutical products. Third-party payors may require manufacturers to provide them with predetermined discounts from
list prices and limit coverage to specific pharmaceutical products on an approved list, or formulary, which might not include all of
the FDA-approved pharmaceutical products for particular indications. Third-party payors may challenge the price and examine
the medical necessity and cost-effectiveness of pharmaceutical products in addition to their safety and efficacy. Manufacturers
may need to conduct expensive pharmaco-economic studies in order to demonstrate the medical necessity and cost-effectiveness of
pharmaceutical products in addition to the costs required to obtain the FDA approvals. Adequate third-party reimbursement may not
be available to enable manufacturers to maintain price levels sufficient to realize an appropriate return on their investment in drug
development.
Healthcare
Reform
In
the United States, there have been several federal and state proposals during the last several years regarding the pricing of pharmaceutical
products, government control, and other changes to the healthcare system of the United States. It is uncertain what other legislative
proposals may be adopted or what actions federal, state, or private payors may take in response to any healthcare reform proposals or
legislation. We cannot predict the effect such reforms may have on our business, and no assurance can be given that any such reforms
will not have a material adverse effect.
By
way of example, in March 2010, the ACA was signed into law, which, among other things, includes changes to the coverage and payment
for drug products under government health care programs. The law includes measures that (i) significantly increase Medicaid rebates
through both the expansion of the program and significant increases in rebates, (ii) substantially expand the Public Health System
(340B) program to allow other entities to purchase prescription drugs at substantial discounts, (iii) extend the Medicaid rebate
rate to a significant portion of Managed Medicaid enrollees, (iv) assess a rebate on Medicaid Part D spending in the coverage gap
for branded and authorized generic prescription drugs, and (v) levy a significant excise tax on the industry to fund the healthcare
reform.
In
addition to the changes brought about by the ACA, other legislative changes have been proposed and adopted, including aggregate reductions
of Medicare payments to providers of 2% per fiscal year and reduced payments to several types of Medicare providers. Moreover, there
has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which
has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things,
bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs and reform government
program reimbursement methodologies for drug products. At the state level, legislatures have increasingly passed legislation and implemented
regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions
on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation
from other countries and bulk purchasing.
Healthcare
Regulations
Pharmaceutical
companies are subject to various federal and state laws that are intended to combat health care fraud and abuse and that govern certain
of our business practices, especially our interactions with third-party payors, healthcare providers, patients, customers and potential
customers through sales and marketing or research and development activities. These include anti-kickback laws, false claims laws,
sunshine laws, privacy laws, and FDA regulation of advertising and promotion of pharmaceutical products.
Anti-kickback
laws, including the federal Anti-Kickback Statute, make it a criminal offense knowingly and willfully to offer, pay, solicit, or receive
any remuneration to induce or reward the referral of an individual for, or the purchase, order or recommendation of, any good or service
reimbursable by, a federal health care program (including our product candidates). The federal Anti-Kickback Statute has been interpreted
to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers, and formulary managers on
the other. Although there are several statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution,
the exceptions, and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchasing,
or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor. In addition, a person or entity does
not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation. Moreover, the government
may assert that a claim, including items or services resulting from a violation of the federal Anti-Kickback Statute, constitutes a false
or fraudulent claim for purposes of the False Claims Act. The penalties for violating the federal Anti-Kickback Statute include administrative
civil money penalties, imprisonment for up to five years, fines of up to $25,000 per violation, and possible exclusion from federal healthcare
programs such as Medicare and Medicaid. The federal civil and criminal false claims laws, including the civil False Claims Act, prohibit
knowingly presenting, or causing to be presented, claims for payment to the federal government (including Medicare and Medicaid) that
are false or fraudulent (and, under the Federal False Claims Act, a claim is deemed false or fraudulent if it is made pursuant to an
illegal kickback). Manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false
or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label.
Actions under the False Claims Act may be brought by the Attorney General or as a qui tam action by a private individual in the name
of the government. Violations of the False Claims Act can result in significant monetary penalties, including fines ranging from $11,665
to $22,331 for each false claim assessed after June 19, 2020, and treble damages. The federal government is using the False Claims Act,
and the accompanying threat of significant liability, in its investigation and prosecution of pharmaceutical companies throughout the
country, for example, in connection with the promotion of products for unapproved uses and other improper sales and marketing practices.
The government has obtained multi-million and multi-billion dollar settlements under the False Claims Act, in addition to individual
criminal convictions under applicable criminal statutes. In addition, companies have been forced to implement extensive corrective action
plans and have often become subject to consent decrees or corporate integrity agreements, severely restricting the manner in which they
conduct their business. Given the significant size of actual and potential settlements, it is expected that the government will continue
to devote substantial resources to investigating healthcare providers’ and manufacturers’ compliance with applicable fraud
and abuse laws.
The
Federal Civil Monetary Penalties Law prohibits, among other things, the offering or transferring of remuneration to a Medicare or Medicaid
beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of
Medicare or Medicaid payable items or services. Noncompliance can result in civil money penalties of up to $20,866 for each wrongful
act, assessment of three times the amount claimed for each item or service, and exclusion from the federal healthcare programs.
Federal
criminal statutes prohibit, among other actions, knowingly and willfully executing or attempting to execute a scheme to defraud any healthcare
benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit
program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or
covering up a material fact or making any materially fictitious or fraudulent statement in connection with the delivery of or payment
for healthcare benefits, items or services. Like the federal Anti-Kickback Statute, the ACA amended the intent standard for certain
healthcare fraud statutes under HIPAA such that a person or entity no longer needs to have actual knowledge of the statute or specific
intent to violate it in order to have committed a violation.
Analogous
state and foreign laws and regulations, including state anti-kickback and false claims laws, may apply to products and services
reimbursed by non-governmental third-party payors, including commercial payors. Additionally, there are state laws that require
pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance
guidance promulgated by the federal government or that otherwise restrict payments that may be made to healthcare providers as well as
state and foreign laws that require drug manufacturers to report marketing expenditures or pricing information.
Sunshine
laws, including the Federal Open Payments law enacted as part of the ACA, require pharmaceutical manufacturers to disclose payments and
other transfers of value to physicians and certain other health care providers or professionals, and in the case of some state sunshine
laws, restrict or prohibit certain such payments. Pharmaceutical manufacturers are required to submit reports to the government by the
90th day of each calendar year. Failure to submit the required information may result in civil monetary penalties of up to an aggregate
of not less than $10,000, but not more than $100,000 per year (or up to an aggregate of $1.150 million per year for “knowing
failures”) for all payments, transfers of value or ownership, or investment interests not reported in an annual submission, and
may result in liability under other federal laws or regulations. Certain states and foreign governments require the tracking and reporting
of gifts, compensation, and other remuneration to physicians.
Privacy
laws, such as the privacy regulations implemented under HIPAA, restrict covered entities from using or disclosing protected health information.
Covered entities commonly include physicians, hospitals, and health insurers from which we may seek to acquire data to aid in our research,
development, sales and marketing activities. Although pharmaceutical manufacturers are not covered entities under HIPAA, our ability
to acquire or use protected health information from covered entities may be affected by privacy laws. Specifically, HIPAA, as amended
by HITECH, and their respective implementing regulations, including the final omnibus rule published on January 25, 2013, imposes
specified requirements relating to the privacy, security, and transmission of individually identifiable health information.
Among
other things, HITECH makes HIPAA’s privacy and security standards directly applicable to “business associates,” defined
as independent contractors or agents of covered entities that create, receive, maintain or transmit protected health information in connection
with providing a service for or on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed
against covered entities, business associates, and possibly other persons, and gave state attorneys general new authority to file civil
actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated
with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in certain circumstances,
many of which differ from each other in significant ways, thus complicating compliance efforts.
The
FDA regulates the sale and marketing of prescription drug products and, among other things, prohibits pharmaceutical manufacturers from
making false or misleading statements and from promoting products for unapproved uses. There has been an increase in government enforcement
efforts at both the federal and state level. Numerous cases have been brought against pharmaceutical manufacturers under the Federal
False Claims Act, alleging, among other things, that certain sales or marketing-related practices violate the Anti-Kickback Statute or
the FDA’s regulations, and many of these cases have resulted in settlement agreements under which the companies were required to
change certain practices, pay substantial fines and operate under the supervision of a federally appointed monitor for a period of years.
Due to the breadth of these laws and their implementing regulations and the absence of guidance in some cases, it is possible that our
practices might be challenged by government authorities. Violations of fraud and abuse laws may be punishable by civil and criminal sanctions,
including fines, civil monetary penalties, as well as the possibility of exclusion of our product candidates from payment by federal
health care programs.
Government
Price Reporting
Government
regulations regarding reporting and payment obligations are complex, and we are continually evaluating the methods we use to calculate
and report the amounts owed with respect to Medicaid and other government pricing programs. Our calculations are subject to review and
challenge by various government agencies and authorities, and it is possible that any such review could result either in material changes
to the method used for calculating the amounts owed to such agency or the amounts themselves. Because the process for making these calculations,
and our judgments supporting these calculations, involve subjective decisions, these calculations are subject to audit. In the event
that a government authority challenges or finds ambiguity with regard to our report of payments, such authority may impose civil and
criminal sanctions, which could have a material adverse effect on our business. From time to time, we conduct routine reviews of our
government pricing calculations. These reviews may have an impact on government price reporting and rebate calculations used to comply
with various government regulations regarding reporting and payment obligations.
Many
governments and third-party payors reimburse the purchase of certain prescription drugs based on a drug’s AWP. In the past
several years, state and federal government agencies have conducted ongoing investigations of manufacturers’ reporting practices
with respect to AWP, which they have suggested have led to excessive payments by state and federal government agencies for prescription
drugs. We and numerous other pharmaceutical companies have been named as defendants in various state and federal court actions alleging
improper or fraudulent practices related to the reporting of AWP.
Drug
Pedigree Laws
State
and federal governments have proposed or passed various drug pedigree laws which can require the tracking of all transactions involving
prescription drugs from the manufacturer to the pharmacy (or other dispensing) level. Companies are required to maintain records documenting
the chain of custody of prescription drug products, beginning with the purchase of such products from the manufacturer. Compliance with
these pedigree laws requires the implementation of extensive tracking systems as well as heightened documentation and coordination with
customers and manufacturers. While we fully intend to comply with these laws, there is uncertainty about future changes in legislation
and government enforcement of these laws. Failure to comply could result in fines or penalties, as well as loss of business that could
have a material adverse effect on our financial results.
Federal
Regulation of Patent Litigation Settlements and Authorized Generic Arrangements
As
part of the Medicare Prescription Drug Improvement and Modernization Act of 2003, companies are required to file with the U.S. Federal
Trade Commission, or FTC, and the U.S. Department of Justice certain types of agreements entered into between brand and generic pharmaceutical
companies related to the settlement of patent litigation or manufacture, marketing and sale of generic versions of branded drugs. This
requirement could affect the manner in which generic drug manufacturers resolve intellectual property litigation and other disputes with
brand pharmaceutical companies and could result generally in an increase in private-party litigation against pharmaceutical companies
or additional investigations or proceedings by the FTC or other governmental authorities.
Other
The
U.S. federal government, various states and localities have laws regulating the manufacture and distribution of pharmaceuticals, as well
as regulations dealing with the substitution of generic drugs for branded drugs. Our operations are also subject to regulation, licensing
requirements, and inspection by the states and localities in which our operations are located or in which we conduct business.
Certain
of our activities are also subject to FTC enforcement actions. The FTC also enforces a variety of antitrust and consumer protection laws
designed to ensure that the nation’s markets function competitively, are vigorous, efficient, and free of undue restrictions. Federal,
state, local and foreign laws of general applicability, such as laws regulating working conditions, also govern us.
In
addition, we are subject to numerous and increasingly stringent federal, state and local environmental laws and regulations concerning,
among other things, the generation, handling, storage, transportation, treatment and disposal of toxic and hazardous substances, the
discharge of pollutants into the air and water and the cleanup of contamination. We are required to maintain and comply with environmental
permits and controls for some of our operations, and these permits are subject to modification, renewal, and revocation by the issuing
authorities. Our environmental capital expenditures and costs for environmental compliance may increase in the future as a result of
changes in environmental laws and regulations or increased manufacturing activities at any of our facilities. We could incur significant
costs or liabilities as a result of any failure to comply with environmental laws, including fines, penalties, third-party claims,
and the costs of undertaking a clean-up at a current or former site or at a site to which our wastes were transported. In addition,
we have grown in part by acquisition, and our diligence may not have identified environmental impacts from historical operations at sites
we have acquired in the past or may acquire in the future.
Intellectual
Property
We
rely on a combination of intellectual property law and contractual restrictions to establish and protect proprietary technology and data
used in the development and realization of our product candidates. Provisional patent applications were filed in the United States and
are intended to protect and support current and future developments of our technologies and corresponding product candidates. The existing
applications as well as the new applications that will be filed aim to pursue patent protection for formulations, unique properties,
modes of administration as well as specific indications that will be developed in corporation with other partners.
A
list of our filed patent applications is described in the table below:
| Filing
Date |
|
Application
No. |
|
Status |
|
Title
|
|
Type
|
| 02/26/2024 |
|
311113 |
|
Application Filed |
|
Mucoadhesive Polymers for Nasal Drug Delivery |
|
Israeli National Phase |
| 03/21/2024 |
|
22768986.6 |
|
Application Filed |
|
Mucoadhesive Polymers for Nasal Drug Delivery |
|
European National Phase |
| 02/26/2024 |
|
Not Yet Available |
|
Application Filed |
|
Mucoadhesive Polymers for Nasal Drug Delivery |
|
Japanese National Phase |
| 02/29/2024 |
|
11202401392V |
|
Application Filed |
|
Mucoadhesive Polymers for Nasal Drug Delivery |
|
Singapore National Phase |
| 02/29/2024 |
|
18/687,950 |
|
Application Filed |
|
Mucoadhesive Polymers for Nasal Drug Delivery |
|
U.S. National Phase |
Our
success and ability to compete successfully depend on our ability to effectively protect, maintain, and defend our intellectual property
and operate without infringing on the proprietary rights of others. As an additional defense mechanism, we seek to limit disclosures
about our intellectual property strictly to a need-to-know basis and then, only to the minimum extent possible. In addition, before disclosing
any proprietary information to employees, partners, contractors, and regulators, we insist on executing stringent confidentiality and
non-disclosure agreements. Employees, who work in research and product candidates’ development are also required to waive all rights
to their work products and execute non-compete agreements.
Grants
from the Israeli Innovation Authority
Tax
Benefits and Grants for Research and Development
Under
the Israeli Encouragement of Research, Development and Industrial Initiative Technology Law, 5744-1984, as amended, and related regulations,
or the Research Law, research and development programs which meet specified criteria and are approved by the IIA are eligible for grants
of up to 50% of the project’s expenditure, as determined by the research committee, in exchange for the payment of royalties from
the revenues generated from the sale of product candidates and related services developed, in whole or in part pursuant to, or as a result
of, a research and development program funded by the IIA. The royalties are generally at a range of 3.0% to 5.0% of revenues until the
entire IIA grant is repaid, together with an annual interest generally equal to the 12 months Secured Overnight Financing Rate (SOFR)
that is published on the first business day of each calendar year by CME Group or any other body authorized by the Federal Reserve to
publish the rate (or by a succeeding publication issues by the Bank of Israel which determines such rate).
The
terms of the Research Law also require that the manufacture of product candidates developed with government grants be performed in Israel.
The transfer of manufacturing activity outside Israel may be subject to the prior approval of the IIA. Under the regulations of the Research
Law, assuming we receive approval from the IIA to manufacture our IIA-funded product candidates outside Israel, we may be required to
pay increased royalties. The increase in royalties depends upon the manufacturing volume that is performed outside of Israel as follows:
Manufacturing
Volume Outside of Israel Royalties to the IIA as a Percentage of Grant
| Under 25% | |
| 100 | % |
| Between 25% and 50% | |
| 120 | % |
| 50% and more | |
| 150 | % |
If
the manufacturing is performed outside of Israel by us, the rate of royalties payable by us on revenues from the sale of product candidates
manufactured outside of Israel will increase by 1% over the regular rates. If the manufacturing is performed outside of Israel by a third
party, the rate of royalties payable by us on those revenues will be equal to the ratio obtained by dividing the amount of the grants
received from the IIA and our total investment in the project that was funded by these grants. The transfer of no more than 10% of the
manufacturing capacity in the aggregate outside of Israel is exempt under the Research Law from obtaining the prior approval of the IIA
(however, does require a notice to the IIA). A company requesting funds from the IIA also has the option of declaring in its IIA grant
application an intention to perform part of its manufacturing outside Israel, thus avoiding the need to obtain additional approval. the
Research Law was amended to clarify that the potential increased royalties specified in the table above will apply even in those cases
where the IIA approval for transfer of manufacturing outside of Israel is not required, namely when the volume of the transferred manufacturing
capacity is less than 10% of total capacity or when the company received an advance approval to manufacture abroad in the framework of
its IIA grant application.
The
know-how developed within the framework of the IIA plan may not be transferred to third parties outside Israel without the prior approval
of a governmental committee charted under the Research Law. The approval, however, is not required for the export of any product candidates
developed using grants received from the IIA. The IIA approval to transfer know-how created, in whole or in part, in connection with
an IIA-funded project to a third party outside Israel where the transferring company remains an operating Israeli entity is subject to
payment of a redemption fee to the IIA calculated according to a formula provided under the Research Law that is based, in general, on
the ratio between the aggregate IIA grants to the company’s aggregate investments in the project that was funded by these IIA grants,
multiplied by the transaction consideration. The transfer of such know-how to a party outside Israel where the transferring company ceases
to exist as an Israeli entity is subject to a redemption fee formula provided under the Innovation Law. According to regulations promulgated
following the 2011 amendment, the maximum amount payable to the IIA in case of transfer of know-how outside Israel shall not exceed 6
times the value of the grants received plus interest, and in the event that the receiver of the grants ceases to be an Israeli corporation
such payment shall not exceed six times the value of the grants received plus interest, with a possibility to reduce such payment to
up to three times the value of the grants received plus interest if the R&D activity remains in Israel for a period of three years
after payment to the IIA.
Transfer
of know-how within Israel is subject to an undertaking of the recipient Israeli entity to comply with the provisions of the Research
Law and related regulations, including the restrictions on the transfer of know-how and the obligation to pay royalties, as further described
in the Research Law and related regulations.
These
restrictions may impair our ability to outsource manufacturing, engage in change of control transactions or otherwise transfer our know-how
outside Israel and may require us to obtain the approval of the IIA for certain actions and transactions and pay additional royalties
to the IIA. In particular, any change of control and any change of ownership of our Ordinary Shares that would make a non-Israeli citizen
or resident an “interested party,” as defined in the Research Law, requires a prior written notice to the IIA in addition
to any payment that may be required of us for transfer of manufacturing or know-how outside Israel. If we fail to comply with the Research
Law, we may be subject to criminal charges.
Other
Tax Benefits
Tax
Benefits on Capital Expenditures for Research and Development
Israeli
tax law allows, under certain conditions, a tax deduction for expenditures, including capital expenditures, for the year in which they
are incurred. Expenditures are deemed related to scientific research and development projects, if:
| ● | The
expenditures are approved by the relevant Israeli government ministry, determined by the
field of research; |
| ● | The
research and development must be for the promotion of the company; and |
| ● | The
research and development is carried out by or on behalf of the company seeking such tax deduction. |
The
amount of such deductible expenses is reduced by the sum of any funds received through government grants for the finance of such scientific
research and development projects. No deduction under these research and development deduction rules is allowed if such deduction is
related to an expense invested in an asset depreciable under the general depreciation rules of the Income Tax Ordinance, 1961. Expenditures
not so approved are deductible in equal amounts over three years.
From
time to time, we may apply to the IIA for approval to allow a tax deduction for all research and development expenses during the year
incurred. There can be no assurance that such an application will be accepted.
Law
for the Encouragement of Capital Investments, 5719-1959
The
Law for the Encouragement of Capital Investments, 5719-1959, generally referred to as the Investment Law, provides certain incentives
for capital investments in production facilities (or other eligible assets).
Tax
Benefits for Preferred Companies
The
Investment Law grants tax benefits for income generated by a “Preferred Company” through its “Preferred Enterprise”
(as such terms are defined in the Investment Law) The definition of a Preferred Company includes a company incorporated in Israel that
is not fully owned by a governmental entity, and that has, among other things, Preferred Enterprise status and is controlled and managed
from Israel. A Preferred Company is entitled to a reduced corporate tax rate of 16% with respect to its income derived by its Preferred
Enterprise, unless the Preferred Enterprise is located in a specified development zone, in which case the rate will be 7.5%.
Dividends
paid out of income attributed to a Preferred Enterprise are generally subject to withholding tax at source at the rate of 20% or such
lower rate as may be provided in an applicable tax treaty. However, if such dividends are paid to an Israeli company, no tax is required
to be withheld.
Property
and Facilities
Our
main business activities are conducted in Israel. Our offices, research and development are located at 5 Ha-Tidhar Street, Raanana, Israel,
where we occupy approximately 35 square meters (approximately 378 square feet). Our lease is a month-to-month lease with no current expiration
date. Our monthly rent payment as of January 2024, was approximately NIS 4,200 (approximately $1,100).
We
consider that our current office space is sufficient to meet our anticipated needs for the foreseeable future and is suitable for the
conduct of our business.
Employees
As
of October 2, 2024, we had five members of senior management (including our Chief Executive Officer), of which one is a full-time employee,
three are part-time contractors and one is a full-time contractor (our Chief Executive Officer). None of our employees located in Israel
are represented by labor unions or covered by collective bargaining agreements. However, in Israel, we are subject to certain Israeli
labor laws, regulations and national labor court precedent rulings, as well as certain provisions of collective bargaining agreements
applicable to us by virtue of extension orders issued in accordance with relevant labor laws by the Israeli and Industry of Economy and
which apply such agreement provisions to our employees even though they are not part of a union that has signed a collective bargaining
agreement.
All
of our employment and consulting agreements include employees’ and consultants’ undertakings with respect to non-competition
and assignment to us of intellectual property rights developed in the course of employment and confidentiality. The enforceability of
such provisions is limited by Israeli law.
Legal
Proceedings
We
are not currently subject to any legal proceedings.
MANAGEMENT
Executive
Officers and Directors
The
following table sets forth information regarding our executive officers and directors:
| Name |
|
Age |
|
Position |
| Tomer Izraeli |
|
46 |
|
Chief Executive Officer, Director |
| Nir Ben Yosef |
|
49 |
|
Chief Financial Officer |
| Dr. Eyal S. Ron |
|
69 |
|
Chief Technology Officer |
| Dr. Tidhar Turgeman |
|
48 |
|
Chief R&D Officer |
| Daphna Avital |
|
58 |
|
Chief People Officer |
| Mr. Asaf Itzhaik |
|
51 |
|
Director |
| Oz Adler |
|
38 |
|
Director |
| Omer Srugo |
|
29 |
|
Director Nominee |
| Liat Sidi |
|
50 |
|
Director Nominee |
| Yehonatan Zalman Vinokur |
|
46 |
|
Director Nominee |
Tomer
Izraeli, Chief Executive Officer, Director
Mr.
Tomer Izraeli has served as our Chief Executive Officer and a member of our board of directors since March 2020 and also served as
our chief executive officer and director from 2005 to 2013. Mr. Izraeli also served as Department Manager in Lapidot Medical Ltd. Mr.
Izraeli has served as a director in LMF Medical Ltd. since 2023. Mr. Izraeli has a BSc in chemical engineering and an MBA from the Ben
Gurion University of the Negev.
Nir
Ben Yosef, Chief Financial Officer
Nir
Ben Yosef, has been our Chief Financial Officer since December 2021. Mr. Ben Yosef serves
as our Chief Financial Officer pursuant to an agreement that we have with Shimony & Co
– CPA and Financial consultants (Isr.), with whom Mr. Ben Yosef is a partner since
2011. Mr. Ben Yosef has served as the Chief Financial Officer of Envizion Medical Ltd. (TASE:
ENVM.TA) from January to October 2021. Mr. Ben Yosef also holds a B.A. degree in Accounting
and Business Management from The College of Management, Israel.
Dr.
Eyal S. Ron, Chief Technology Officer
Dr.
Eyal S. Ron has served as our Chief Technology Officer since March 2020. Dr. Ron has also served as the Chief Technical Officer and
Co-Founder of Rich PSC since 2020. Dr. Ron has extensive experience in executive roles for various biomedical companies. This experience
includes serving as: Chief Technical Officer and Co-founder for Gelesis Inc. from 2006 to 2019, Chief Technical Officer for Combinent
Biomedical Systems from 1994 to 2015, Chief Operating Officer for Oxford Pharmaceutical Services from 2003 to 2007, Chief Technical Officer
for Palmetto Pharmaceuticals from 1999 to 2017, Chief Technical Officer and Co-founder for Flo from 2015 to 2019, and Chief Technical
Officer and Co-founder for GelMed / Gel Sciences from 1994 to 1997. Dr. Ron has served as a director of Pharmedica Ltd since 2008. Previously,
has also served as a director of Acuity Bio from 2009 until 2021, and of GelMed / Gel Sciences from 1994 to 1997. Dr. Ron has a BSc from
Tel Aviv University and a Ph.D. from Brandeis University and a post doctorate from MIT.
Dr.
Tidhar Turgeman, Chief R&D Officer
Dr.
Tidhar Turgeman has served as our Chief R&D Officer since December 2020. Prior to that, Mr. Turgeman served as an Innovative
drug delivery technologies products development manager at ADAMA from 2017 to 2020 and as a research leader at Evogene from 2014 to 2017.
Mr. Turgeman has served as a director in LMF Medical Ltd. since 2023. Mr. Turgeman has a Ph.D degree from Ben-Gurion University of the
Negev.
Daphna
Avital, Chief People Officer
Ms.
Daphna Avital has served as our Chief People Officer since August 2021. Since 2019, Ms. Avital has served as an independent consultant
to Allergan and Astellas among other biotechnology companies. Prior to that, Ms. Avital served as Regional Human Resources and Learning&
Development Manager in Allergan from 2016 to 2018. From 2008 to 2014, Ms. Avital served as Human Resources Director of AstraZeneca and
Allegran. Ms. Avital holds an MA in Organizational Consulting from the College of Management Academic Studies, is a certified Group Facilitator
from IDC and a Logotherapy Associate.
Asaf Itzhaik,
Director
Mr.
Itzhaik is a seasoned international businessman in retail, BTC, BTB and real estate. He has served as a director of the Company since
May 2024. He has served as a director in Clearmind Ltd (NASDAQ) since 2022, GIX Internet (Israel) since 2021, Save Foods Ltd. (Nasdaq)
since 2024, Rani Zim (Israel) since 2022 and Plentify Ltd. (Canadian SE) since 2023. He has 28 years of experience running an optic brand
specializing in athletes.
Oz
Adler, Director
Mr.
Oz Adler has served as our director since September 2021. Mr. Adler has served as the Chief Financial Officer of SciSparc Ltd. since
April 2018, and the Chief Executive Officer of SciSparc, and prior to that, from September 2017, he served as Vice President of Finance
at SciSparc. Additionally, Mr. Adler currently serves on the board of directors of Elbit Imaging Ltd (TASE: EMITF.TA, OTC: EMITF), Clearmind
Medicine Inc. (CSE: CMND, FSE: CWYO, OTC: CMNDF), Jeff’s Brands Ltd. And Charging Robotics Ltd. Previously, Mr. Adler served as
the Chief Financial Officer of Xylo Technologies Ltd. from December 2012 and August 2017. Before this, Mr. Adler worked in the audit
department of Kost Forer Gabbay & Kasierer, a member of Ernst & Young Global, from 2012 to 2017. Mr. Adler is a certified public
accountant in Israel and holds a B.A. degree in Accounting and Business Management from The College of Management, Israel.
Omer
Srugo, Director Nominee
Mr.
Omer Srugo has agreed to serve on our board of directors subject to the consummation of this offering. Mr. Srugo currently serves
as the Head of Debt at Electra Real Estate Ltd., where he manages debt funds totaling $620 million, focusing on multi-family home properties
across the Southeastern region of the United States. Prior to that, between 2021 to 2024, Mr. Srugo was a Senior Financial Advisor at
Somekh Chaikin, a member firm of KPMG, where he led teams in financial due diligence and transaction support in various industries, including
biotech, hi-tech and real estate. Mr. Srugo holds a B.A. in Accounting and Business Management, with honors, from Reichman University.
Mr.
Srugo is a certified public accountant in Israel.
Liat
Sidi, Director Nominee
Ms.
Liat Sidi will join our Board of Directors upon the consummation of this offering. Ms. Sidi has served as a director at Scisparc
Ltd. since June 2020. Ms. Sidi has served as the accountant of Foresight Autonomous Holdings Ltd. (NASDAQ and TASE: FRSX) since September
2016. In addition, Ms. Sidi additionally provides accounting services for various public and private companies such as: Panaxia israel
laboratories ltd, Soho real estate ltd, Pure food ltd and others. Ms. Sidi holds a B.A. degree in Tax, finance and accounting studies
from the Ramat Gan College of Accounting
Yehonatan
Zalman Vinokur, Director Nominee
Mr.
Yehonatan Zalman Vinokur will join our Board of Directors upon the consummation of this offering. Mr. Vinokur has served as the Chief
Executive Officer and Owner of Danny Zeevi Insurance Agency, LTD. since 2017. In addition, since 2002, Mr. Vinokur has worked as a financial
adviser for Topick Finance. Mr. Vinokur has a B.A. in Business Management from The College of Management Academic Studies in Rishon LeTsiyon.
Scientific
Advisory Board
Prof.
Smadar Cohen has served as a member of our scientific advisory board since October 2005. Prof. Cohen currently serves as the Claire
and Harold Oshry Professor Chair in Biotechnology at Ben-Gurion University, as Founder of the Avram and Stella Goldstein-Goren Department
of Biotechnology Engineering at Ben-Gurion University, and as a founder and director of the regenerative medicine and stem cell Research
Center of Ben-Gurion University. Professor Cohen’s research focuses on the advancement and development of novel bio-inspired materials
as nano-sized delivery systems for therapeutics and as scaffolds in regenerative medicine. Professor Cohen has been invited to given
over 100 lectures and seminars, as well as chaired 30 sessions of international scientific conferences in the field of biomaterials and
drug delivery systems, has 29 issued U.S. patents, is the author of over 120 papers in peer-reviewed scientific journals, co-authored
a book on Cardiac Tissue Engineering and edited two books. She serves as the Associate Editor of the Annals of Biomedical Engineering
Journal, is an executive board member of the biomedical journal Tissue Engineering, and is a member of the editorial board of the biomedical
journal Biomatter. She is on the scientific advisory board of several bio-nano-technology companies and serves as a member of the Magnet/Magneton/Nofar
Committee of the Israel Ministry of Economics.
Prof.
Avi Schroeder, has served as a member of our scientific advisory board since July 2021. Prof. Schroeder is a tenured Associate Professor
of Chemical Engineering at the Technion – Israel Institute of Technology, he heads the Laboratory for Targeted Drug Delivery and
Personalized Medicine Technologies. Prof. Schroeder has years of experience in nanotechnology and personalized medicine, has translational
experience with the development of liposome-based clinical systems. He is an author of over 50 research papers, an inventor of 19 patents
a co-founder of multiple Technion spin-out startup companies, including PEEL Therapeutics, Barcode Diagnostics and ViAqua Therapeutics,
and the recipient of 20 national and international innovation awards. Prof. Schroeder is a member of Israel Young National Academy of
Sciences, President of the Israel Institute of Chemical Engineers, and an appointed member of the Israel National Council for Civilian
Research and Development.
Prof.
Fabio Sonvico has served as a member of our scientific advisory board since November 2021. Prof. Sonvico is an Associate Professor
in the Food and Drug Department of the University of Parma, Italy. Prof. Sonvico is highly experienced in the development of intranasal
and pulmonary routes and products. He has published more than 75 papers in international journals on advanced drug delivery topics and
he is also author of 6 book chapters and 5 patents focusing on innovative drug delivery systems. Prof. Sonvico is appointed as
an Invited Professor at the Faculty of Medicine and Pharmacy of the University of Lyon.
Prof.
Nancy Agmon-Levin has served as a member of our scientific advisory board since March 2022. Professor Agmon-Levin serves as the Head
of the Clinical Immunology, Angioedema and Allergy Unit, at the Lupus and Autoimmune Diseases Clinic. Professor Agmon is a graduate of
the Hadassah Medical School at Hebrew University, and completed her fellowship in Clinical Immunology at the German Cancer Research Center
(DKFZ), in Heidelberg, Germany. Prof. Agmon- Levin’s major field of interest are autoimmune diseases (lupus, antiphospholipid syndrome),
immune system diseases (urticaria, angioedema, immune deficiency, Granulomatous Disease), allergies in children and adults, immunotherapy.
Professor Agmon-Levin is the author of 123 published works.
Family
Relationships
There
are no family relationships between any members of our executive management and our directors.
Arrangements
for Election of Directors and Members of Management
Our
board of directors consists of directors, each of whom will continue to serve pursuant to their appointment until the annual general
meeting of our shareholders in which his or her term expires. We are not a party to, and are not aware of, any voting agreements among
our shareholders. In addition, there are no family relationships among our executive officers and directors. See “Related Party
Transactions” for additional information.
Compensation
The
following table presents in the aggregate all compensation we paid to all of our directors and senior management as a group for the year
ended December 31, 2023. The table does not include any amounts we paid to reimburse any of such persons for costs incurred in providing
us with services during this period.
All
amounts reported in the table below reflect our cost, in thousands of U.S. dollars. Amounts paid in NIS are translated into U.S. dollars
at the rate of NIS 3.69 = U.S. $1.00, based on the average representative rate of exchange between the NIS and the U.S. dollar as reported
by the Bank of Israel during such period of time.
| | |
Salary,
bonuses and Related Benefits | | |
Pension, Retirement and
Other Similar Benefits | | |
Share
Based
Compensation | |
| All
directors and senior management as a group, consisting of four persons as of December 31, 2023. | |
$ | 426,000 | | |
$ | 27,000 | | |
$ | 90,000 | |
As
of December 31, 2023, 229,255 options were granted to our directors and executive officers.
On
September 1, 2021, we entered into a consulting agreement with Tomer Izraeli, pursuant which
he serves as our Chief Executive Officer. According to the agreement, Mr. Izraeli is entitled
to receive, among other things: (i) a one-time NIS 150,000 (approximately $48,000) bonus
upon completion of the Company’s initial public offering, and (ii) options representing
2.5% of our post IPO issued and outstanding shares which shall vest and become exercisable
over a total period of three years commencing on the grant date on a monthly basis in equal
installments.
On
June 22, 2022, we entered into an employment agreement with Tidhar Turgeman, pursuant to which Mr. Turgeman is engaged as the Company’s
Chief R&D Officer. Pursuant to his employment agreement and subject to a successful completion of the Company’s initial public
offering, Mr. Turgeman will be entitled to receive: (i) a one-time bonus of $40,000, and
(ii) options to purchase Ordinary Shares of the Company representing up to 2% of the Company’s post IPO issued and outstanding
share-capital. These options will vest and become exercisable over a period of three years commencing on the grant date, on a quarterly
basis in equal instalments.
For
so long as we qualify as a foreign private issuer, we will not be required to comply with the proxy rules applicable to U.S. domestic
companies regarding disclosure of the compensation of certain executive officers on an individual basis. Pursuant to the Companies Law,
we will be required, after we become a public company, to disclose the annual compensation of our five most highly compensated officers
on an individual basis. This disclosure will not be as extensive as that required of a U.S. domestic issuer. We intend to commence providing
such disclosure, at the latest, in the annual proxy statement for our first annual meeting of shareholders following the closing of this
offering, which will be filed under cover of a report on Form 6-K.
Employment
and Consulting Agreements with Executive Officers
We
have entered into written employment and consulting agreements with each of our executive officers. All of these agreements contain customary
provisions regarding noncompetition, confidentiality of information and assignment of inventions. However, the enforceability of the
noncompetition provisions may be limited under applicable law. In addition, we intend to enter into indemnification agreements, subject
to the listing of our securities on Nasdaq, with each executive officer and director pursuant to which we will indemnify each of them
up to a certain amount and to the extent that these liabilities are not covered by directors and officers insurance.
For
a description of the terms of our options and option plans, see “Management—Equity Incentive Plan” below.
Directors’
Service Contracts
Other
than with respect to our directors that are also executive officers, we do not have written agreements with any director providing for
benefits upon the termination of his employment with our company.
Differences
between the Companies Law and Nasdaq Requirements
Corporations
incorporated under the laws of the State of Israel whose shares are publicly traded, including companies with shares listed on Nasdaq,
are considered public companies under Israeli law and are required to comply with various corporate governance requirement under Israeli
law relating to such matters as the composition and responsibilities of the audit committee and the compensation committee (subject to
certain exceptions we intend to utilize), and a requirement to have an internal auditor. These requirements are in addition to the corporate
governance requirements imposed by the rules of the Nasdaq Stock Market and other applicable provisions of the US Securities laws to
which we will become subject (as a foreign private issuer) upon the closing of this offering and the listing of our Ordinary Shares and
Warrants on Nasdaq. Under those rules, we may elect to follow certain corporate governance practices permitted under the Companies Law
in lieu of compliance with corresponding corporate governance requirements otherwise imposed by the Nasdaq Stock Market rules for U.S.
domestic issuers.
In
accordance with Israeli law and practice and subject to the exemption set forth in Rule 5615 of the Nasdaq Stock Market rules, we
have elected to follow the provisions of the Companies Law, rather than the Nasdaq Stock Market rules, with respect to the following
requirements:
| ● | Quorum.
While the Nasdaq Stock Market rules require that the
quorum for purposes of any meeting of the holders of a listed company’s ordinary voting
stock, as specified in the company’s bylaws, be no less than 33 1/3% of the company’s
outstanding ordinary voting stock, under Israeli law, a company is entitled to determine
in its articles of association the number of shareholders and percentage of holdings required
for a quorum at a shareholders meeting. Our Articles provide that a quorum of two or more
shareholders holding at least 25% of the voting rights in person or by proxy is required
for commencement of business at a general meeting. However, the quorum set forth in our Articles
with respect to an adjourned meeting consists of at least one shareholders present in person
or by proxy. |
| ● | Compensation
of officers. Israeli law and our Articles do not require that the independent members
of our board of directors (or a compensation committee composed solely of independent members
of our board of directors) determine an executive officer’s compensation, as is generally
required under the Nasdaq Stock Market rules with respect to the chief executive officer
and all other executive officers. Instead, compensation of executive officers is determined
and approved by our compensation committee and our board of directors, and in certain circumstances
by our shareholders, either in consistency with our office holder compensation policy or,
in special circumstances in deviation therefrom, taking into account certain considerations
stated in the Companies Law. See “Management—Board Practices—Approval of
Related Party Transactions under Israeli Law” for additional information. |
| ● | Shareholder
approval. We will seek shareholder approval for all corporate actions requiring
such approval under the requirements of the Companies Law, rather than seeking approval for
corporation actions in accordance with Nasdaq Listing Rule 5635. In particular, under
this Nasdaq Stock Market rule, shareholder approval is generally required for: (i) an
acquisition of shares/assets of another company that involves the issuance of 20% or more
of the acquirer’s shares or voting rights or if a director, officer or 5% shareholder
has greater than a 5% interest in the target company or the consideration to be received;
(ii) the issuance of shares leading to a change of control; (iii) adoption/amendment
of equity compensation arrangements (although under the provisions of the Companies Law there
is no requirement for shareholder approval for the adoption/amendment of the equity compensation
plan); and (iv) issuances of 20% or more of the shares or voting rights (including securities
convertible into, or exercisable for, equity) of a listed company via a private placement
(and/or via sales by directors/officers/5% shareholders) if such equity is issued (or sold)
at below the greater of the book or market value of shares. By contrast, under the Companies
Law, shareholder approval is required for, among other things: (i) transactions with
directors concerning the terms of their service or indemnification, exemption and insurance
for their service (or for any other position that they may hold at a company), for which
approvals of the compensation committee, board of directors and shareholders are all required,
(ii) extraordinary transactions with controlling shareholders of publicly held companies,
which require the special approval, and (iii) terms of employment or other engagement
of the controlling shareholder of us or such controlling shareholder’s relative, which
require special approval. In addition, under the Companies Law, a merger requires approval
of the shareholders of each of the merging companies. Further, we intend to adopt and approve
equity incentive plans and, to the extent required, material changes thereto in accordance
with the Companies Law, which does not impose a requirement of shareholder approval for such
actions. In addition, we intend to follow Israeli corporate governance practice instead of
the Nasdaq corporate governance rule which requires shareholder approval prior to an issuance
of securities in connection with equity-based compensation of officers, directors, employees
or consultants. |
| ● | Approval
of Related Party Transactions. All related party transactions are approved in accordance
with the requirements and procedures for approval of interested party acts and transaction
as set forth in the Companies Law, which requires the approval of the audit committee, or
the compensation committee, as the case may be, the board of directors and shareholders,
as may be applicable, for specified transactions, rather than approval by the audit committee
or other independent body of our board of directors as required under the Nasdaq Stock Market
rules. See “Management—Board Practices—Approval of Related Party Transactions
under Israeli Law” for additional information. |
| ● | Annual
Shareholders Meeting. As opposed to the Nasdaq Stock Market Rule 5620(a),
which mandates that a listed company hold its annual shareholders meeting within one year
of the company’s fiscal year-end, we are required, under the Companies Law, to hold
an annual shareholders meeting each calendar year and within 15 months of the last annual
shareholders meeting. |
| |
● |
Distribution of periodic
reports to shareholders; proxy solicitation. As opposed to the Nasdaq Stock Market rules, which require listed issuers to
make such reports available to shareholders in one of a number of specific manners, Israeli law does not require us to distribute
periodic reports directly to shareholders, and the generally accepted business practice in Israel is not to distribute such reports
to shareholders but to make such reports available through a public website. In addition to making such reports available on a public
website, we currently make our audited financial statements available to our shareholders at our offices and will only mail such
reports to shareholders upon request. As a foreign private issuer, we are generally exempt from the SEC’s proxy solicitation
rules. |
Board
Practices
Introduction
Upon
the consummation of this offering, our board of directors will consist of seven members. We believe that Asaf Itzhaik, Oz Adler, Liat
Sidi and Yehonatan Zalman Vinokur are “independent” for purposes of the Nasdaq Stock Market rules. Our Articles provide that
the number of board of directors’ members shall be set by the general meeting of the shareholders provided that it will consist
of not less than three and not more than nine. Pursuant to the Companies Law, the management of our business is vested in our board of
directors. Our board of directors may exercise all powers and may take all actions that are not specifically granted to our shareholders
or to management. Our executive officers are responsible for our day-to-day management and have individual responsibilities established
by our board of directors. Our Chief Executive Officer is appointed by, and serves at the discretion of, our board of directors, subject
to the consulting agreement that we have entered into with him. All other executive officers are appointed by our Chief Executive Officer.
Their terms of employment are subject to the approval of the board of directors’ compensation committee and of the board of directors,
and are subject to the terms of any applicable employment or consulting agreements that we may enter into with them.
Each
director, except external directors that may be required to be appointed under the Companies Law under certain circumstances, will hold
office until the next annual general meeting of our shareholders following his or her appointment, or until he or she resigns or unless
he or she is removed by a majority vote of our shareholders at a general meeting of our shareholders or upon the occurrence of certain
events, in accordance with the Companies Law and our Articles.
In
addition, under certain circumstances, our Articles allow our board of directors to appoint directors to fill vacancies on our board
of directors or in addition to the acting directors (subject to the limitation on the number of directors), until the next annual general
meeting or special general meeting in which directors may be appointed or terminated. External directors may be elected for up to two
additional three-year terms after their initial three-year term under the circumstances described below, with certain exceptions as described
in “External Directors” below. External directors may be removed from office only under the limited circumstances set forth
in the Companies Law.
Under
the Companies Law, any shareholder holding at least one percent of our outstanding voting power may nominate a director. However, any
such shareholder may make such a nomination only if a written notice of such shareholder’s intent to make such nomination has been
given to our board of directors. Any such notice must include certain information, including the consent of the proposed director nominee
to serve as our director if elected, and a declaration that the nominee signed declaring that he or she possesses the requisite skills
and has the availability to carry out his or her duties. Additionally, the nominee must provide details of such skills, and demonstrate
an absence of any limitation under the Companies Law that may prevent his or her election, and affirm that all of the required election-information
is provided to us, pursuant to the Companies Law.
Under
the Companies Law, our board of directors must determine the minimum number of directors who are required to have accounting and financial
expertise. In determining the number of directors required to have such expertise, our board of directors must consider, among other
things, the type and size of the company and the scope and complexity of its operations. Our board of directors has determined that the
minimum number of directors of our company who are required to have accounting and financial expertise is two.
The
board of directors must elect one director to serve as the chairman of the board of directors to preside at the meetings of the board
of directors, and may also remove that director as chairman. Pursuant to the Companies Law, neither the chief executive officer nor any
of his or her relatives is permitted to serve as the chairman of the board of directors, and a company may not vest the chairman or any
of his or her relatives with the chief executive officer’s authorities. In addition, a person who reports, directly or indirectly,
to the chief executive officer may not serve as the chairman of the board of directors; the chairman may not be vested with authorities
of a person who reports, directly or indirectly, to the chief executive officer; and the chairman may not serve in any other position
in the company or a controlled company, but he or she may serve as a director or chairman of a controlled company. However, the Companies
Law permits a company’s shareholders to determine, for a period not exceeding three years from each such determination, that the
chairman or his or her relative may serve as chief executive officer or be vested with the chief executive officer’s authorities,
and that the chief executive officer or his or her relative may serve as chairman or be vested with the chairman’s authorities.
Such determination of a company’s shareholders requires either: (1) the approval of at least a majority of the shares of those
shareholders present and voting on the matter (other than controlling shareholders and those having a personal interest in the determination)
(shares held by abstaining shareholders shall not be considered); or (2) that the total number of shares opposing such determination
does not exceed 2% of the total voting power in the company. Currently, we have a separate chairman and chief executive officer.
The
board of directors may, subject to the provisions of the Companies Law, delegate any or all of its powers to committees of the board,
and it may, from time to time, revoke such delegation or alter the composition of any such committees, subject to certain limitations.
Unless otherwise expressly provided by the board of directors, the committees shall not be empowered to further delegate such powers.
The composition and duties of our audit committee, financial statement examination committee and compensation committee are described
below.
The
board of directors oversees how management monitors compliance with our risk management policies and procedures, and reviews the adequacy
of the risk management framework in relation to the risks faced by us. The board of directors is assisted in its oversight role by an
internal auditor. The internal auditor undertakes both regular and ad hoc reviews of risk management controls and procedures, the results
of which are reported to our audit committee.
External
Directors
Under
the Companies Law, except as provided below, companies incorporated under the laws of the State of Israel that are publicly traded, including
Israeli companies with shares listed on the Nasdaq, are required to appoint at least two external directors who meet the qualification
requirements set forth in the Companies Law. The definitions of an external director under the Companies Law and independent director
under Nasdaq Stock Market rules are similar such that it would generally be expected that our two external directors will also comply
with the independence requirement under Nasdaq Stock Market rules.
Pursuant
to regulations under the Companies Law, the board of directors of a company such as us is not required to have external directors if:
(i) the company does not have a controlling shareholder (as such term is defined in the Companies Law); (ii) a majority of the directors
serving on the board of directors are “independent,” as defined under Nasdaq Rule 5605(a)(2); and (iii) the company follows
Nasdaq Rule 5605(e)(1), which requires that the nomination of directors be made, or recommended to the board of directors, by a Nominating
Committee of the board of directors consisting solely of independent directors, or by a majority of independent directors. The Company
meets all these requirements. Our board of directors has resolved to adopt the corporate governance exemption set forth above, and accordingly
we will not have external directors as members of our board of directors.
Independent
Directors Under the Companies Law
An
“independent director” is either an external director or a director who meets the same non-affiliation criteria as an external
director (except for (i) the requirement that the director be an Israeli resident (which does not apply to companies such as ours whose
securities have been offered outside of Israel or are listed outside of Israel) and (ii) the requirement for accounting and financial
expertise or professional qualifications), as determined by the audit committee, and who has not served as a director of the company
for more than nine consecutive years. For these purposes, ceasing to serve as a director for a period of two years or less would not
be deemed to sever the consecutive nature of such director’s service.
Regulations
promulgated pursuant to the Companies Law provide that a director in a public company whose shares are listed for trading on specified
exchanges outside of Israel, including Nasdaq, who qualifies as an independent director under the relevant non-Israeli rules and who
meets certain non-affiliation criteria, which are less stringent than those applicable to independent directors as set forth above, would
be deemed an “independent” director pursuant to the Companies Law provided: (i) he or she has not served as a director for
more than nine consecutive years; (ii) he or she has been approved as such by the audit committee; and (iii) his or her remuneration
shall be in accordance with the Companies Law and the regulations promulgated thereunder. For these purposes, ceasing to serve as a director
for a period of two years or less would not be deemed to sever the consecutive nature of such director’s service.
Furthermore,
pursuant to these regulations, such company may reappoint a person as an independent director for additional terms, beyond nine years,
which do not exceed three years each, if each of the audit committee and the board of directors determine, in that order, that in light
of the independent director’s expertise and special contribution to the board of directors and its committees, the reappointment
for an additional term is in the company’s best interest.
Alternate
Directors
Our
Articles provide, as allowed by the Companies Law, that any director may, subject to the conditions set thereto including approval of
the nominee by our board of directors, appoint a person as an alternate to act in his place, to remove the alternate and appoint another
in his place and to appoint an alternate in place of an alternate whose office is vacated for any reason whatsoever. Under the Companies
Law, a person who is not qualified to be appointed as a director, a person who is already serving as a director or a person who is already
serving as an alternate director for another director, may not be appointed as an alternate director. Nevertheless, a director who is
already serving as a director may be appointed as an alternate director for a member of a committee of the board of directors so long
as he or she is not already serving as a member of such committee, and if the alternate director is to replace an external director,
he or she is required to be an external director and to have either “financial and accounting expertise” or “professional
expertise,” depending on the qualifications of the external director he or she is replacing. A person who does not have the requisite
“financial and accounting experience” or the “professional expertise,” depending on the qualifications of the
external director he or she is replacing, may not be appointed as an alternate director for an external director. A person who is not
qualified to be appointed as an independent director, pursuant to the Companies Law, may not be appointed as an alternate director of
an independent director qualified as such under the Companies Law. Unless the appointing director limits the time or scope of the appointment,
the appointment is effective for all purposes until the appointing director ceases to be a director or terminates the appointment.
Committees
of the Board of Directors
Our
board of directors has established two standing committees, the audit committee and the compensation committee.
Audit
Committee
Under
the Companies Law, we will be required to appoint an audit committee subject to the listing of our Ordinary Shares and Warrants on Nasdaq.
The audit committee must be comprised of at least three directors, including all of the external directors, if applicable, (one of whom
must serve as chair of the committee). The audit committee may not include the chairman of the board; a controlling shareholder of the
company or a relative of a controlling shareholder; a director employed by or providing services on a regular basis to the company, to
a controlling shareholder or to an entity controlled by a controlling shareholder; or a director who derives most of his or her income
from a controlling shareholder.
In
addition, a majority of the members of the audit committee of a publicly traded company must be independent directors under the Companies
Law. Our audit committee will be comprised of Mr. Yehonatan Zalman Vinokur, Mr. Oz Adler, and Ms. Liat Sidi.
Under
the Companies Law, our audit committee is responsible for:
| |
(i) |
determining
whether there are deficiencies in the business management practices of our company, and making recommendations to the board of directors
to improve such practices; |
| |
|
|
| |
(ii) |
determining
whether to approve certain related party transactions (including transactions in which an office holder has a personal interest and
whether such transaction is extraordinary or material under Companies Law) and establishing the approval process for certain transactions
with a controlling shareholder or in which a controlling shareholder has a personal interest (see “Management—Board Practices—Approval
of Related Party Transactions under Israeli law”); |
| |
|
|
| |
(iii) |
determining
the approval process for transactions that are “non-negligible” (i.e., transactions with a controlling shareholder that
are classified by the audit committee as non-negligible, even though they are not deemed extraordinary transactions), as well as
determining which types of transactions would require the approval of the audit committee, optionally based on criteria which may
be determined annually in advance by the audit committee; |
| |
|
|
| |
(iv) |
examining
our internal controls and internal auditor’s performance, including whether the internal auditor has sufficient resources and
tools to dispose of its responsibilities; |
| |
|
|
| |
(v) |
examining
the scope of our auditor’s work and compensation and submitting a recommendation with respect thereto to our board of directors
or shareholders, depending on which of them is considering the appointment of our auditor; |
| |
|
|
| |
(vi) |
establishing
procedures for the handling of employees’ complaints as to deficiencies in the management of our business and the protection
to be provided to such employees; and |
| |
|
|
| |
(vii) |
where
the board of directors approves the working plan of the internal auditor, examining such working plan before its submission to the
board of directors and proposing amendments thereto. |
Our
audit committee may not conduct any discussions or approve any actions requiring its approval (see “Management—Board Practices—Approval
of Related Party Transactions under Israeli law”), unless at the time of the approval a majority of the committee’s members
are present, which majority consists of independent directors under the Companies Law.
Our
board of directors intends to adopt an audit committee charter to be effective upon the listing of our Ordinary Shares and Warrants on
Nasdaq setting forth, among others, the responsibilities of the audit committee consistent with the rules of the SEC and Nasdaq Listing
Rules (in addition to the requirements for such committee under the Companies Law), including, among others, the following:
| |
● |
oversight
of our independent registered public accounting firm and recommending the engagement, compensation or termination of engagement of
our independent registered public accounting firm to the board of directors in accordance with Israeli law; |
| |
● |
recommending
the engagement or termination of the person filling the office of our internal auditor, reviewing the services provided by our internal
auditor and reviewing effectiveness of our system of internal control over financial reporting; |
| |
|
|
| |
● |
recommending
the terms of audit and non-audit services provided by the independent registered public accounting firm for pre-approval by our board
of directors; and |
| |
|
|
| |
● |
reviewing
and monitoring, if applicable, legal matters with significant impact, finding of regulatory authorities’ findings, receive
reports regarding irregularities and legal compliance, acting according to “whistleblower policy” and recommend to our
board of directors if so required. |
Nasdaq
Stock Market Requirements for Audit Committee
Under
the Nasdaq Stock Market rules, we are required to maintain an audit committee consisting of at least three members, all of whom are independent
and are financially literate and one of whom has accounting or related financial management expertise.
As
noted above, the members of our audit committee will include, Mr. Oz Adler, Mr. Yehonatan Zalman Vinokur, and Ms. Liat Sidi. Mr. Oz Adler
will serve as the chairman of our audit committee. All members of our audit committee meet the requirements for financial literacy under
the Nasdaq Stock Market rules. Our board of directors has determined that each member of our audit committee is an audit committee financial
expert as defined by the SEC rules and has the requisite financial experience as defined by the Nasdaq Stock Market rules.
Under
the Companies Law, our audit committee also carries out the duties of a financial statement examination committee. As such, the audit
committee is responsible for: (i) estimations and assessments made in connection with the preparation of financial statements; (ii) internal
controls related to the financial statements; (iii) completeness and propriety of the disclosure in the financial statements; (iv) the
accounting policies adopted and the accounting treatments implemented in material matters of the company; and (v) value evaluations,
including the assumptions and assessments on which evaluations are based and the supporting data in the financial statements.
Compensation
Committee
Under
the Companies Law, the board of directors of any public company must establish a compensation committee. The compensation committee must
be comprised of at least three directors, including all of the external directors (if any). The compensation committee is subject to
the same Companies Law restrictions as the audit committee as to: (a) who may not be a member of the committee; and (b) who may
not be present during committee deliberations as described above.
Our
compensation committee, acting pursuant to a written charter, will consist of Mr. Asaf Itzhaik, Mr. Oz Adler, and Ms. Liat Sidi, who
will serve as chairwoman of our compensation committee. Our compensation committee complies with the provisions of the Companies Law,
the regulations promulgated thereunder, and our Articles, on all aspects referring to its independence, authorities and practice. Our
compensation committee follows home country practice as opposed to complying with the compensation committee membership and charter requirements
prescribed under the Nasdaq Stock Market rules.
Our
compensation committee reviews and recommends to our board of directors: with respect to our executive officers’ and directors’:
(1) annual base compensation (2) annual incentive bonus, including the specific goals and amounts; (3) equity compensation; (4) employment
agreements, severance arrangements, and change in control agreements and provisions; (5) retirement grants and/or retirement bonuses;
and (6) any other benefits, compensation, compensation policies or arrangements.
The
duties of the compensation committee include the recommendation to the company’s board of directors of a policy regarding the terms
of engagement of office holders, to which we refer as a compensation policy. Such policy must be adopted by the company’s board
of directors, after considering the recommendations of the compensation committee. The compensation policy is then brought for approval
by our shareholders, which requires a special majority (see “Management—Board Practices—Approval of Related Party Transactions
under Israeli law”). Under the Companies Law, the board of directors may adopt the compensation policy if it is not approved by
the shareholders, provided that after the shareholders oppose the approval of such policy, the compensation committee and the board of
directors revisit the matter and determine that adopting the compensation policy would be in the best interests of the company. Under
the Companies Law, we are required to adopt an office holder compensation policy no later than 9 months from the consummation of this
offering.
The
compensation policy must serve as the basis for decisions concerning the financial terms of employment or engagement of executive officers
and directors, including exculpation, insurance, indemnification or any monetary payment or obligation of payment in respect of employment
or engagement. The compensation policy must relate to certain factors, including advancement of the company’s objectives, the company’s
business and its long-term strategy, and creation of appropriate incentives for executives. It must also consider, among other things,
the company’s risk management, size and the nature of its operations. The compensation policy must furthermore consider the following
additional factors:
| |
● |
the
education, skills, expertise and accomplishments of the relevant director or executive; |
| |
|
|
| |
● |
the
director’s or executive’s roles and responsibilities and prior compensation agreements with him or her; |
| |
|
|
| |
● |
the
relationship between the cost of the terms of service of an office holder and the average median compensation of the other employees
of the company (including those employed through manpower companies), including the impact of disparities in salary upon work relationships
in the company; |
| |
|
|
| |
● |
the
possibility of reducing variable compensation at the discretion of the board of directors; and the possibility of setting a limit
on the exercise value of non-cash variable compensation; and |
| |
|
|
| |
● |
as
to severance compensation, the period of service of the director or executive, the terms of his or her compensation during such service
period, the company’s performance during that period of service, the person’s contribution towards the company’s
achievement of its goals and the maximization of its profits, and the circumstances under which the person is leaving the company. |
The
compensation policy must also include the following principles:
| |
● |
with
the exception of office holders who report directly to the chief executive officer, the link between variable compensation and long-term
performance and measurable criteria; |
| |
|
|
| |
● |
the
relationship between variable and fixed compensation, and the ceiling for the value of variable compensation at the time of its grant; |
| |
|
|
| |
● |
the
conditions under which a director or executive would be required to repay compensation paid to him or her if it was later shown that
the data upon which such compensation was based was inaccurate and was required to be restated in the company’s financial statements; |
| |
|
|
| |
● |
the
minimum holding or vesting period for variable, equity-based compensation; and |
| |
|
|
| |
● |
maximum
limits for severance compensation. |
The
compensation policy must also consider appropriate incentives from a long-term perspective.
The
compensation committee is responsible for: (1) recommending the compensation policy to a company’s board of directors for its approval
(and subsequent approval by the shareholders); and (2) duties related to the compensation policy and to the compensation of a company’s
office holders, including:
| |
● |
recommending
whether a compensation policy should continue in effect, if the then-current policy has a term of greater than three years (approval
of either a new compensation policy or the continuation of an existing compensation policy must in any case occur every three years); |
| |
|
|
| |
● |
recommending
to the board of directors periodic updates to the compensation policy; |
| |
|
|
| |
● |
assessing
implementation of the compensation policy; |
| |
|
|
| |
● |
determining
whether the terms of compensation of certain office holders of the company need not be brought to approval of the shareholders; and |
| |
|
|
| |
● |
determining
whether to approve the terms of compensation of office holders that require the committee’s approval. |
Our
compensation policy will be designed to promote our long-term goals, work plan and policy, retain, motivate and incentivize our directors
and executive officers, while considering the risks that our activities involve, our size, the nature and scope of our activities and
the contribution of an officer to the achievement of our goals and maximization of profits, and align the interests of our directors
and executive officers with our long-term performance. To that end, a portion of an executive officer compensation package is targeted
to reflect our short and long-term goals, as well as the executive officer’s individual performance. On the other hand, our compensation
policy will include measures designed to reduce the executive officer’s incentives to take excessive risks that may harm us in
the long-term, such as limits on the value of cash bonuses and equity-based compensation, limitations on the ratio between the variable
and the total compensation of an executive officer and minimum vesting periods for equity-based compensation.
Our
compensation policy will also address our executive officer’s individual characteristics (such as his or her respective position,
education, scope of responsibilities and contribution to the attainment of our goals) as the basis for compensation variation among our
executive officers, and considers the internal ratios between compensation of our executive officers and directors and other employees.
For example, the compensation that may be granted to an executive officer may include: base salary, annual bonuses, equity-based compensation,
benefits and retirement and termination of service arrangements. All cash bonuses are limited to a maximum amount linked to the executive
officer’s base salary. In addition, our compensation policy will provide for maximum permitted ratios between the total variable
(cash bonuses and equity-based compensation) and non-variable (base salary) compensation components, in accordance with an officer’s
respective position with the company.
An
annual cash bonus may be awarded to executive officers upon the attainment of pre-set periodic objectives and individual targets. The
annual cash bonus that may be granted to executive officers other than our chairman or Chief Executive Officer may be based entirely
on a discretionary evaluation. Our Chief Executive Officer will be entitled to recommend performance objectives to such executive officers,
and such performance objectives will be approved by our compensation committee (and, if required by law, by our board of directors).
The
performance measurable objectives of our chairman and Chief Executive Officer will be determined annually by our compensation committee
and board of directors. A less significant portion of the chairman’s and/or the Chief Executive Officer’s annual cash bonus
may be based on a discretionary evaluation of the chairman’s or the Chief Executive Officer’s respective overall performance
by the compensation committee and the board of directors based on quantitative and qualitative criteria.
The
equity-based compensation under our compensation policy for our executive officers (including members of our board of directors) will
be designed in a manner consistent with the underlying objectives in determining the base salary and the annual cash bonus, with its
main objectives being to enhance the alignment between the executive officers’ interests with our long-term interests and those
of our shareholders and to strengthen the retention and the motivation of executive officers in the long term. Our compensation policy
will provide for executive officer compensation in the form of share options or other equity-based awards, such as restricted shares
and phantom, options, in accordance with our equity incentive plan then in place. Share options granted to executive officers shall be
subject to vesting periods in order to promote long-term retention of the awarded executive officers. The equity-based compensation shall
be granted from time to time and be individually determined and awarded according to the performance, educational background, prior business
experience, qualifications, role and the personal responsibilities of the executive officer.
In
addition, our compensation policy will contain compensation recovery provisions which allows us under certain conditions to recover bonuses
paid in excess, will enable our Chief Executive Officer to approve an immaterial change in the terms of employment of an executive officer
(provided that the changes of the terms of employment are in accordance our compensation policy) and will allow us to exculpate, indemnify
and insure our executive officers and directors subject to certain limitations set forth thereto.
Our
compensation policy will also provide for compensation to the members of our board of directors either: (i) in accordance with the amounts
provided in the Companies Regulations (Rules Regarding the Compensation and Expenses of an External Director) of 2000, as amended by
the Companies Regulations (Relief for Public Companies Traded in Stock Exchange Outside of Israel) of 2000, as such regulations may be
amended from time to time; or (ii) in accordance with the amounts determined in our compensation policy.
Our
compensation policy, which will be approved by our board of directors and shareholders prior to the closing of this offering, will become
effective immediately prior to the closing of this offering and will be filed as an exhibit to the registration statement of which this
prospectus forms a part.
Internal
Auditor
Under
the Companies Law, the board of directors of an Israeli public company must appoint an internal auditor nominated by the audit committee.
We intend to appoint our internal auditor within 90 days following the consummation of this offering. The role of the internal auditor
is to examine, among other things, whether a company’s actions comply with the law and proper business procedure. The audit committee
is required to oversee the activities, and to assess the performance of the internal auditor as well as to review the internal auditor’s
work plan. An internal auditor may not be an interested party or office holder, or a relative of any interested party or office holder,
and may not be a member of the company’s independent accounting firm or its representative. The Companies Law defines an interested
party as a holder of 5% or more of the outstanding shares or voting rights of a company, any person or entity that has the right to appoint
at least one director or the general manager of the company or any person who serves as a director or as the general manager of a company.
Remuneration
of Directors
Under
the Companies Law, remuneration of directors is subject to the approval of the compensation committee, thereafter by the board of directors
and thereafter, unless exempted under the regulations promulgated under the Companies Law, by the general meeting of the shareholders.
In case the remuneration of the directors is in accordance with regulations applicable to remuneration of the external directors then
such remuneration shall be exempt from the approval of the general meeting. Where the director is also a controlling shareholder, the
requirements for approval of transactions with controlling shareholders apply.
Fiduciary
Duties of Office Holders
The
Companies Law imposes a duty of care and a duty of loyalty on all office holders of a company.
The
duty of care requires an office holder to act with the level of care with which a reasonable office holder in the same position would
have acted under the same circumstances. The duty of care of an office holder includes a duty to use reasonable means to obtain:
| |
● |
information
on the advisability of a given action brought for his approval or performed by him by virtue of his position; and |
| |
|
|
| |
● |
all
other important information pertaining to these actions. |
The
duty of loyalty of an office holder requires an office holder to act in good faith and for the benefit of the company, and includes a
duty to:
| |
● |
refrain
from any conflict of interest between the performance of his duties in the company and his performance of his other duties or personal
affairs; |
| |
|
|
| |
● |
refrain
from any action that is competitive with the company’s business; |
| |
|
|
| |
● |
refrain
from exploiting any business opportunity of the company to receive a personal gain for himself or others; and |
| |
|
|
| |
● |
disclose
to the company any information or documents relating to the company’s affairs which
the office holder has received due to his position as an office holder.
Under
the Companies Law, a company may approve an act specified above which would otherwise constitute
a breach of the office holder’s duty of loyalty, provided that the office holder acted
in good faith, neither the act nor its approval harms the company, and the office holder
discloses his, her or its personal interest a sufficient time before the approval of such
act. Any such approval is subject to the terms of the Companies Law setting forth, among
other things, the appropriate bodies of the company required to provide such approval and
the methods of obtaining such approval. |
Insurance
Under
the Companies Law, a company may obtain insurance for any of its office holders against the following liabilities incurred due to acts
he or she performed as an office holder, if and to the extent provided for in the company’s articles of association:
| |
● |
breach
of his or her duty of care to the company or to another person, to the extent such a breach arises out of the negligent conduct of
the office holder; |
| |
● |
a
breach of his or her duty of loyalty to the company, provided that the office holder acted in good faith and had reasonable cause
to assume that his or her act would not prejudice the company’s interests; and |
| |
|
|
| |
● |
a
financial liability imposed upon him or her in favor of another person.
An
Israeli Company may also insure an office holder against expenses, including reasonable litigation
expenses and legal fees, incurred by the office holder as a result of an administrative proceeding
instituted against him or her, pursuant to certain provisions of the Israeli Securities Law, 5728-1968,
or the Securities Law. |
Prior
to the consummation of this offering, we intend to purchase directors’ and officers’ liability insurance, providing total
coverage of $10 million for the benefit of all of our directors and officers.
Indemnification
The
Companies Law and the Israeli Securities Law, 5728-1968, or the Securities Law, provide that a company may indemnify an office holder
against the following liabilities and expenses incurred for acts performed by him or her as an office holder, either pursuant to an undertaking
made in advance of an event or following an event, provided its articles of association include a provision authorizing such indemnification:
| |
● |
a
financial liability imposed on him or her in favor of another person by any judgment concerning an act performed in his or her capacity
as an office holder, including a settlement or arbitrator’s award approved by a court; However, if an undertaking to indemnify
an office holder with respect to such liability is provided in advance, then such an undertaking must be limited to events which,
in the opinion of the board of directors, can be foreseen based on the company’s activities when the undertaking to indemnify
is given, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances, and
such undertaking shall detail the abovementioned events and amount or criteria; |
| |
|
|
| |
● |
reasonable
litigation expenses, including attorneys’ fees, expended by the office holder (a) as a result of an investigation or proceeding
instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (1) no indictment
(as defined in the Companies Law) was filed against such office holder as a result of such investigation or proceeding; and (2) no
financial liability as a substitute for the criminal proceeding (as defined in the Companies Law) was imposed upon him or her as
a result of such investigation or proceeding, or, if such financial liability was imposed, it was imposed with respect to an offense
that does not require proof of criminal intent; or (b) in connection with a monetary sanction; |
| |
|
|
| |
● |
reasonable
litigation expenses, including attorneys’ fees, expended by the office holder or imposed on him or her by a court: (1) in proceedings
that the company institutes, or that another person institutes on the company’s behalf, against him or her; (2) in a criminal
proceeding of which he or she was acquitted; or (3) as a result of a conviction for a crime that does not require proof of criminal
intent; and |
| |
|
|
| |
● |
expenses
incurred by an office holder in connection with an Administrative Procedure under the Securities Law, including reasonable litigation
expenses and reasonable attorneys’ fees. An “Administrative Procedure” is defined as a procedure pursuant to chapters
H3 (Monetary Sanction by the Israeli Securities Authority), H4 (Administrative Enforcement Procedures of the Administrative Enforcement
Committee) or I1 (Arrangement to prevent Procedures or Interruption of procedures subject to conditions) to the Securities Law. |
We
have entered into indemnification agreements with all of our directors and with all members of our senior management. Each such indemnification
agreement provides the office holder with indemnification permitted under applicable law and up to a certain amount, and to the extent
that these liabilities are not covered by directors and officers insurance.
Exculpation
Under
the Companies Law, an Israeli company may not exculpate an office holder from liability for a breach of his or her duty of loyalty, but
may exculpate in advance an office holder from his or her liability to the company, in whole or in part, for damages caused to the company
as a result of a breach of his or her duty of care (other than in relation to distributions), but only if a provision authorizing such
exculpation is included in its articles of association. Our Articles provide that we may exculpate, in whole or in part, any office holder
from liability to us for damages caused to the company as a result of a breach of his or her duty of care, but prohibit an exculpation
from liability arising from a company’s transaction in which our controlling shareholder or officer has a personal interest. Subject
to the aforesaid limitations, under the indemnification agreements, we exculpate and release our office holders from any and all liability
to us related to any breach by them of their duty of care to us to the fullest extent permitted by law.
Limitations
The
Companies Law provides that we may not exculpate or indemnify an office holder nor enter into an insurance contract that would provide
coverage for any liability incurred as a result of any of the following: (1) a breach by the office holder of his or her duty of loyalty
unless (in the case of indemnity or insurance only, but not exculpation) the office holder acted in good faith and had a reasonable basis
to believe that the act would not prejudice us; (2) a breach by the office holder of his or her duty of care if the breach was carried
out intentionally or recklessly (as opposed to merely negligently); (3) any act or omission committed with the intent to derive an illegal
personal benefit; or (4) any fine, monetary sanction, penalty or forfeit levied against the office holder.
Under
the Companies Law, exculpation, indemnification and insurance of office holders in a public company must be approved by the compensation
committee and the board of directors and, with respect to certain office holders or under certain circumstances, also by the shareholders.
Our
Articles permit us to exculpate (subject to the aforesaid limitation), indemnify and insure our office holders to the fullest extent
permitted or to be permitted by the Companies Law.
The
foregoing descriptions summarize the material aspects and practices of our board of directors. For additional details, we also refer
you to the full text of the Companies Law, as well as of our Articles, which are exhibits to this registration statement of which this
prospectus forms a part, and are incorporated herein by reference.
There
are no service contracts between us, on the one hand, and our directors in their capacity as directors, on the other hand, providing
for benefits upon termination of service.
Approval
of Related Party Transactions under Israeli Law
General
Under
the Companies Law, we may approve an action by an office holder from which the office holder would otherwise have to refrain, as described
above, if:
| |
● |
the
office holder acts in good faith and the act or its approval does not cause harm to the company; and |
| |
|
|
| |
● |
the
office holder disclosed the nature of his or her interest in the transaction (including any significant fact or document) to the
company at a reasonable time before the company’s approval of such matter. |
Disclosure
of Personal Interests of an Office Holder
The
Companies Law requires that an office holder disclose to the company, promptly, and, in any event, not later than the board meeting at
which the transaction is first discussed, any direct or indirect personal interest that he or she may have and all related material information
known to him or her relating to any existing or proposed transaction by the company. If the transaction is an extraordinary transaction,
the office holder must also disclose any personal interest held by:
| |
● |
the
office holder’s relatives; or |
| |
|
|
| |
● |
any
corporation in which the office holder or his or her relatives holds 5% or more of the shares or voting rights, serves as a director
or general manager or has the right to appoint at least one director or the general manager. |
An
office holder is not, however, obliged to disclose a personal interest if it derives solely from the personal interest of his or her
relative in a transaction that is not considered an extraordinary transaction. Under the Companies Law, an extraordinary transaction
is a transaction:
| |
● |
not
in the ordinary course of business; |
| |
|
|
| |
● |
not
on market terms; or |
| |
|
|
| |
● |
that
is likely to have a material effect on the company’s profitability, assets or liabilities. |
The
Companies Law does not specify to whom within us nor the manner in which required disclosures are to be made. We require our office holders
to make such disclosures to our board of directors.
Under
the Companies Law, once an office holder complies with the above disclosure requirement, the board of directors may approve a transaction
between the company and an office holder, or a third party in which an office holder has a personal interest, unless the articles of
association provide otherwise and provided that the transaction is in the company’s interest. If the transaction is an extraordinary
transaction in which an office holder has a personal interest, first the audit committee and then the board of directors, in that order,
must approve the transaction. Under specific circumstances, shareholder approval may also be required. Generally, a person who has a
personal interest in a matter which is considered at a meeting of the board of directors or the audit committee may not be present at
such a meeting unless the chairman of the audit committee or board of directors (as applicable) determines that he or she should be present
in order to present the transaction that is subject to approval. A director who has a personal interest in a transaction, which is considered
at a meeting of the board of directors or the audit committee, may not be present at this meeting or vote on this matter, unless a majority
of members of the board of directors or the audit committee, as the case may be, has a personal interest. If a majority of the board
of directors has a personal interest, then shareholder approval is generally also required.
Disclosure
of Personal Interests of a Controlling Shareholder
Under
the Companies Law, the disclosure requirements that apply to an office holder also apply to a controlling shareholder of a public company.
Extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, including a
private placement in which a controlling shareholder has a personal interest, as well as transactions for the provision of services whether
directly or indirectly by a controlling shareholder or his or her relative, or a company such controlling shareholder controls, and transactions
concerning the terms of engagement and compensation of a controlling shareholder or a controlling shareholder’s relative, whether
as an office holder or an employee, require the approval of the audit committee or the compensation committee, as the case may be, the
board of directors and a majority of the shares voted by the shareholders of the company participating and voting on the matter in a
shareholders’ meeting. In addition, the shareholder approval must fulfill one of the following requirements or a special majority:
| |
● |
at
least a majority of the shares held by shareholders who are not controlling shareholders and have no personal interest in the transaction
and are voting at the meeting must be voted in favor of approving the transaction, excluding abstentions; or |
| |
|
|
| |
● |
the
shares voted by shareholders who have no personal interest in the transaction who vote against the transaction represent no more
than 2% of the voting rights in the company. |
In
addition, any extraordinary transaction with a controlling shareholder or in which a controlling shareholder has a personal interest
with a term of more than three years requires the abovementioned approval every three years; however, such transactions not involving
the receipt of services or compensation can be approved for a longer term, provided that the audit committee determines that such longer
term is reasonable under the circumstances.
The
Companies Law requires that every shareholder that participates, in person, by proxy or by voting instrument, in a vote regarding a transaction
with a controlling shareholder, must indicate in advance or in the ballot whether or not that shareholder has a personal interest in
the vote in question. Failure to so indicate will result in the invalidation of that shareholder’s vote.
The
term “controlling shareholder” is defined in the Companies Law as a shareholder with the ability to direct the activities
of the company, other than by virtue of being an office holder. A shareholder is presumed to be a controlling shareholder if the shareholder
holds 50% or more of the voting rights in a company or has the right to appoint 50% or more of the directors of the company or its general
manager. In the context of a transaction involving a shareholder of the company, a controlling shareholder also includes a shareholder
who holds 25% or more of the voting rights in the company if no other shareholder holds more than 50% of the voting rights in the company.
For this purpose, the holdings of all shareholders who have a personal interest in the same transaction will be aggregated.
Approval
of the Compensation of Directors and Executive Officers
The
compensation of, or an undertaking to indemnify, insure or exculpate, an office holder who is not a director requires the approval of
the company’s compensation committee, followed by the approval of the company’s board of directors, and, if such compensation
arrangement or an undertaking to indemnify, insure or exculpate is inconsistent with the company’s stated compensation policy,
or if the said office holder is the chief executive officer of the company (subject to a number of specific exceptions), then such arrangement
is subject to the approval of our shareholders, subject to a special majority requirement.
Directors.
Under the Companies Law, the compensation of our directors requires the approval of our compensation committee, the subsequent approval
of the board of directors and, unless exempted under the regulations promulgated under the Companies Law, the approval of the general
meeting of our shareholders. If the compensation of our directors is inconsistent with our stated compensation policy, then, provided
that those provisions that must be included in the compensation policy according to the Companies Law have been considered by the compensation
committee and board of directors, shareholder approval by a special majority will be required.
Executive
officers other than the chief executive officer. The Companies Law requires the approval of the compensation of a public company’s
executive officers (other than the chief executive officer) in the following order: (i) the compensation committee, (ii) the company’s
board of directors, and (iii) only if such compensation arrangement is inconsistent with the company’s stated compensation policy,
the company’s shareholders by a special majority. However, if the shareholders of the company do not approve a compensation arrangement
with an executive officer that is inconsistent with the company’s stated compensation policy, the compensation committee and board
of directors may override the shareholders’ decision if each of the compensation committee and the board of directors provide detailed
reasons for their decision.
Chief
executive officer. Under the Companies Law, the compensation of a public company’s chief executive officer is required
to be approved by: (i) the company’s compensation committee; (ii) the company’s board of directors, and (iii) the company’s
shareholders by a special majority. However, if the shareholders of the company do not approve the compensation arrangement with the
chief executive officer, the compensation committee and board of directors may override the shareholders’ decision if each of the
compensation committee and the board of directors provides detailed reasons for their decision. In addition, the compensation committee
may exempt the engagement terms of a candidate to serve as the chief executive officer from shareholders’ approval, if the compensation
committee determines that the compensation arrangement is consistent with the company’s stated compensation policy, that the chief
executive officer did not have a prior business relationship with the company or a controlling shareholder of the company, and that subjecting
the approval to a shareholder vote would impede the company’s ability to attain the candidate to serve as the company’s chief
executive officer (and provide detailed reasons for the latter).
The
approval of each of the compensation committee and the board of directors, with regard to the office holders and directors above, must
be in accordance with the company’s stated compensation policy; however, under special circumstances, the compensation committee
and the board of directors may approve compensation terms of a chief executive officer that are inconsistent with the company’s
compensation policy provided that they have considered those provisions that must be included in the compensation policy according to
the Companies Law and that shareholder approval was obtained by a special majority requirement.
Duties
of Shareholders
Under
the Companies Law, a shareholder has a duty to refrain from abusing his power in the company and to act in good faith and in an acceptable
manner in exercising his rights and performing his obligations toward the company and other shareholders, including, among other things,
in voting at general meetings of shareholders (and at shareholder class meetings) on the following matters:
| |
● |
amendment
of the articles of association; |
| |
|
|
| |
● |
increase
in the company’s authorized share capital; |
| |
|
|
| |
● |
merger;
and |
| |
|
|
| |
● |
the
approval of related party transactions and acts of office holders that require shareholder approval. |
A
shareholder also has a general duty to refrain from oppressing other shareholders. The remedies generally available upon a breach of
contract will also apply to a breach of the above mentioned duties, and in the event of oppression of other shareholders, additional
remedies are available to the injured shareholder.
In
addition, any controlling shareholder, any shareholder that knows that its vote can determine the outcome of a shareholder vote and any
shareholder that, under a company’s articles of association, has the power to appoint or prevent the appointment of an office holder,
or has another power with respect to a company, is under a duty to act with fairness towards the company. The Companies Law does not
describe the substance of this duty except to state that the remedies generally available upon a breach of contract will also apply in
the event of a breach of the duty to act with fairness, taking the shareholder’s position in the company into account.
Equity
Incentive Plan - 2021 Equity Incentive Plan
We
maintain one equity incentive plan – the 2021 Incentive Plan. As of September 30, 2024, the number of options allotted is 246,129.
In addition. The number of options that have vested and have not yet been exercised or expired is 146,064 (including 42,744 option which
vests upon this offering).
Our
2021 Incentive Plan was adopted by our board of directors in February 2021 and expires on February 2031. Our employees, directors, officer,
consultants, advisors, suppliers and any other person or entity whose services are considered valuable to us are eligible to participate
in this plan.
Our
2021 Incentive Plan is administered by our board of directors, regarding the granting of options and the terms of option grants, including
exercise price, method of payment, vesting schedule, acceleration of vesting and the other matters necessary in the administration of
these plan. Eligible Israeli employees, officers and directors, would qualify for provisions of Section 102(b)(2) of the Israeli Income
Tax Ordinance (New Version), 5721 –1961, or the Tax Ordinance. Pursuant to such Section 102(b)(2), qualifying options and shares
issued upon exercise of such options are held in trust and registered in the name of a trustee selected by the board of directors. The
trustee may not release these options or shares to the holders thereof for two years from the date of the registration of the options
in the name of the trustee. Under Section 102, any tax payable by an employee from the grant or exercise of the options is deferred until
the transfer of the options or Ordinary Shares by the trustee to the employee or upon the sale of the options or Ordinary Shares, and
gains may qualify to be taxed as capital gains at a rate equal to 25%, subject to compliance with specified conditions (which rate may
be increased by an additional 3% surtax depending on the individual income amounts). Our Israeli non-employee service providers and controlling
shareholders may only be granted options under Section 3(9) of the Tax Ordinance, which does not provide for similar tax benefits. The
2021 Incentive Plan also permits the grant to Israeli grantees of options that do not qualify under Section 102(b)(2).
Upon
termination of employment without Cause, as defined in the 2021 Incentive Plan, all unvested options will expire, and all vested options
will generally be exercisable for three (3) months following termination, or such other period as determined by the plan administrator,
subject to the terms of the 2021 Incentive Plan and the governing option agreement.
Upon
termination of employment due to death, retirement or absolute disability, all the vested options at the time of termination will be
exercisable for 12 months following termination, or such other period as determined by the plan administrator, subject to the terms of
the 2021 Incentive Plan and the governing option agreement.
PRINCIPAL
SHAREHOLDERS
The
following table sets forth information with respect to the beneficial ownership of our Ordinary Shares as October 2, 2024 by:
| ● | each
person or entity known by us to own beneficially 5% or more of our outstanding Ordinary Shares; |
| | | |
| ● | each
of our directors and executive officers individually; and |
| | | |
| ● | all
of our directors and executive officers as a group. |
The
beneficial ownership of our Ordinary Shares is determined in accordance with the rules of
the SEC and generally includes any shares over which a person exercises sole or shared voting
or investment power, or the right to receive the economic benefit of ownership. For purposes
of the table below, we deem Ordinary Shares issuable pursuant to options that are currently
exercisable or exercisable within 60 days of October 2, 2024 to be outstanding and to be
beneficially owned by the person holding the options for the purposes of computing the percentage
ownership of that person, but we do not treat them as outstanding for the purpose of computing
the percentage ownership of any other person. Percentage of shares beneficially owned before
this offering is based on 3,235,542 Shares issued and outstanding as of October 2, 2024.
The number of Ordinary Shares deemed issued and outstanding after this offering is based
on Ordinary Shares as part of the Units which includes the Ordinary Shares offered hereby
but assumes no exercise of the underwriter’s over-allotment option.
As
of October 2, 2024 and based on their reported registered office, none of our shareholders were U.S. persons. We have also set forth
below information known to us regarding any significant change in the percentage ownership of our Ordinary Shares by any major shareholders
during the past three years. Except where otherwise indicated, we believe, based on information furnished to us by such owners, that
the beneficial owners of the Ordinary Shares listed below have sole investment and voting power with respect to such shares.
Certain
of our existing shareholders, including beneficial owners of greater than 5% of our share capital, have indicated an interest in purchasing
up to an aggregate of $1 million of Units in this offering, which represents approximately 24% of the total Units in this offering, based
on the public offering price of $4.38 per Unit. However, because indications of interest are not binding agreements or commitments to
purchase, the underwriters may determine to sell more, less or no Units in this offering to any of these shareholders, or any of these
shareholders may determine to purchase more, less or no Units in this offering. The underwriters will receive the same underwriting discount
on any Units purchased by these shareholders as they will on any other Units sold to the public in this offering.
Following
the closing of this offering, all of our shareholders, including the shareholders listed below, will have the same voting rights attached
to their Ordinary Shares, and neither our principal shareholders nor our directors and executive officers will have different or special
voting rights with respect to their Ordinary Shares. See “Description of Share Capital—Voting Rights.” A description
of any material relationship that our principal shareholders have had with us or any of our predecessors or affiliates within the past
three years is included under “Certain Relationships and Related Party Transactions.”
Unless
otherwise noted below, the address of each shareholder, director and executive officer is c/o Polyrizon Ltd., 5 Ha-Tidhar Street, Raanana,
4366507 Israel.
| |
|
Ordinary
Shares |
|
|
Percentage
of
Ordinary Shares
beneficially owned |
|
| 5% or greater
shareholders: |
|
beneficially
owned |
|
|
Before
offering |
|
|
After
offering |
|
| |
|
|
|
|
|
|
|
|
|
| Xylo Technologies Ltd. (1) |
|
|
315,521 |
|
|
|
9.8 |
% |
|
|
7.5 |
% |
| L.I.A. Pure Capital LTD (2)(3)** |
|
|
247,521 |
|
|
|
7.7 |
% |
|
|
5.9 |
% |
| E.G. Europe Property Ltd. (2)(4)** |
|
|
291,886 |
|
|
|
9.0 |
% |
|
|
7.0 |
% |
| Reuven Srugo Construction Company Ltd. (5) |
|
|
168,136 |
|
|
|
5.2 |
% |
|
|
4.0 |
% |
| Yoav Srugo (2)(6)** |
|
|
351,678 |
|
|
|
10.9 |
% |
|
|
8.4 |
% |
| Itzhak Srugo (2)(7)** |
|
|
351,678 |
|
|
|
10.9 |
% |
|
|
8.4 |
% |
| Sofi Yochelman Srugo (2) |
|
|
287,249 |
|
|
|
8.9 |
% |
|
|
6.8 |
% |
| Raul Srugo (2)(8)** |
|
|
351,678 |
|
|
|
10.9 |
% |
|
|
8.4 |
% |
| SciSparc Ltd. (9)** |
|
|
320,000 |
|
|
|
9.9 |
% |
|
|
7.6 |
% |
| Directors and executive officers: |
|
|
|
|
|
|
|
|
|
|
|
|
| Omer Srugo (2) |
|
|
- |
|
|
|
- |
% |
|
|
- |
% |
| Tomer Izraeli (10) |
|
|
192,991 |
|
|
|
5.8 |
% |
|
|
4.5 |
% |
| Eyal S. Ron (11) |
|
|
32,730 |
|
|
|
1.0 |
% |
|
|
0.8 |
% |
| Tidhar Turgeman (12) |
|
|
61,654 |
|
|
|
1.9 |
% |
|
|
1.4 |
% |
| Daphna Avital |
|
|
- |
|
|
|
- |
% |
|
|
- |
% |
| Oz Adler |
|
|
- |
|
|
|
- |
% |
|
|
- |
% |
| Asaf Itzhaik |
|
|
- |
|
|
|
- |
% |
|
|
- |
% |
| Nir Ben Yosef |
|
|
1,592 |
|
|
|
* |
% |
|
|
* |
% |
| Liat Sidi |
|
|
- |
|
|
|
- |
% |
|
|
- |
% |
| Yehonatan Zalman Vinokur |
|
|
- |
|
|
|
- |
% |
|
|
- |
% |
| All directors and executive officers as a group (11
persons) |
|
|
288,967 |
|
|
|
8.7 |
% |
|
|
6.7 |
% |
| * | Indicates
beneficial ownership of less than 1% of the total Ordinary Shares outstanding. |
| ** |
Has indicated
an interest in purchasing Units in this offering. Certain of our existing shareholders, including beneficial owners of greater than
5% of our share capital, have indicated an interest in purchasing up to an aggregate of approximately $1 million of Units in this
offering, which represents approximately 24% of the total Units in this offering, based on the public offering price of $4.38 per
Unit. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters may determine
to sell more, less or no Units in this offering to any of these shareholders, or any of these shareholders may determine to purchase
more, less or no Units in this offering. The underwriters will receive the same underwriting discount on any Units purchased by these
shareholders as they will on any other Units sold to the public in this offering. |
| (1) |
Consists of Ordinary Shares
only. The address of Xylo Technologies Ltd. is Omer Industrial Park No. 7A, P.O. Box 3030, 8496500, Israel. Xylo Technologies Ltd.
is a publicly traded company. To the best of our knowledge, Xylo Technologies Ltd. does not have any controlling shareholders. The
chief executive officer of Xylo Technologies Ltd. is Liron Carmel. Xylo Technologies Ltd. has expressed an interest in acquiring
up to $300,000 of Units in this offering, or 68,493 Units (based on the public offering price of $4.38 per Unit). If Xylo Technologies
Ltd. acquires 68,493 Units in this offering, which is approximately 7.1% of the Units offered by us in this offering, they will beneficially
own approximately 9.1% of our Ordinary Shares after this offering. |
| (2) |
Consists of Ordinary Shares
only. |
| (3) |
Kfir
Silberman is the controlling shareholder of L.I.A. Pure Capital Ltd., and its address is 20 Raoul Wallenberg
Street, Tel Aviv, Israel 6971916. L.I.A Pure Capital Ltd. has expressed an interest in acquiring up to
$106,000 of Units in this offering, or 24,201 Units (based on the public offering price of $4.38 per
Unit). If L.I.A Pure Capital Ltd. acquires 24,201 Units in this offering, which is approximately 2.5%
of the Units offered by us in this offering, they will beneficially own approximately 6.5% of our Ordinary
Shares after this offering. |
| (4) |
Eyal Gohar is the sole shareholder
and director of E. G. Europe Property Ltd. (“E. G. Europe Property”), and its address is 44 Hamitnadev St., Tel-Aviv
– Jaffa, Israel 6969024. E. G. Europe Property has expressed an interest in acquiring up to $125,000 of Units in this offering,
or 28,539 Units (based on the public offering price of $4.38 per Unit). If E. G. Europe Property acquires 28,539 Units in this offering,
which is approximately 3.0% of the Units offered by us in this offering, they will beneficially own approximately 7.7% of our Ordinary
Shares after this offering. |
| (5) |
Consists of Ordinary Shares
only. Reuven Srugo Construction Company Ltd. is an Israeli company owned by Raul Srugo and his family. The address of Reuven Srugo
Construction Company Ltd. is 10 Ein Hakore, Rishon Lezion, Israel, 7528910. |
| (6) |
Yoav Srugo has expressed an
interest in acquiring up to $100,000 of Units in this offering, or 22,831 Units (based on the public offering price of $4.38 per
Unit). If Yoav Srugo acquires 22,831 Units in this offering, which is approximately 2.4% of the Units offered by us in this offering,
they will beneficially own approximately 8.9% of our Ordinary Shares after this offering. |
| (7) |
Itzhak Srugo has expressed
an interest in acquiring up to $100,000 of Units in this offering, or 22,831 Units (based on the public offering price of $4.38 per
Uni). If Itzhak Srugo acquires 22,831 Units in this offering, which is approximately 2.4% of the Units offered by us in this offering,
they will beneficially own approximately 8.9% of our Ordinary Shares after this offering. |
| (8) |
Raul Srugo has expressed an
interest in acquiring up to $100,000 of Units in this offering, or 22,831 Units (based on the public offering price of $4.38 per
Unit). If Raul Srugo acquires 22,831 Units in this offering, which is approximately 2.4% of the Units offered by us in this offering,
they will beneficially own approximately 8.9% of our Ordinary Shares after this offering. |
| (9) |
Consists of 320,000 Ordinary
Shares. In addition, SciSparc will hold pre-funded warrants with a nominal exercise price, to purchase 364,931 Ordinary Shares, based
on the public offering price of $4.38 per Ordinary Share. The pre-funded warrants are subject to a beneficial ownership limitation
of 9.99%, which such limitation restricts the holder from exercising that portion of the warrants that would result in the holder
and its affiliates owning, after exercise, a number of ordinary shares in excess of the beneficial ownership limitation. In addition,
SciSparc will hold 2,054,793 warrants to purchase 2,054,793 Ordinary Shares upon the same terms as the warrants to be issued in this
offering. The address of SciSparc Ltd. is 20 Raul Wallenberg Street, Tower A, Tel Aviv 6971916, Israel. SciSparc Ltd. is a publicly
traded company. To the best of our knowledge, SciSparc Ltd. does not have any controlling shareholders. The chief executive officer
of SciSparc Ltd. is Oz Adler. SciSparc Ltd. has expressed an interest in acquiring up to $200,000 of Units in this offering, or 45,662
Units (based on the public offering price of $4.38 per Unit). If SciSparc Ltd. acquires 45,662 Units in this offering, which is approximately
4.8% of the Units offered by us in this offering, they will beneficially own approximately 8.7% of our Ordinary Shares after this
offering. |
| (10) |
Consists of (i) 118,691 Ordinary
Shares; and (ii) 74,300 options exercisable to Ordinary Shares that are exercisable within 60 days from the date of this prospectus. |
| (11) |
Consists of 32,730 options
exercisable to Ordinary Shares that are exercisable within 60 days from the date of this prospectus. |
| (12) |
Consists of 61,654 options
exercisable to Ordinary Shares that are exercisable within 60 days from the date of this prospectus. |
CERTAIN
RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The
following is a description of the material terms of those transactions with related parties to which we, or our subsidiaries, are party.
Participation
in this Offering
Certain
of our existing shareholders, including beneficial owners of greater than 5% of our share capital, have indicated an interest in purchasing
up to an aggregate of $1 million of Units in this offering, which represents approximately 24% of the total Units in this offering, based
on the public offering price of $4.38 per Unit. However, because indications of interest are not binding agreements or commitments to
purchase, the underwriters may determine to sell more, less or no Units in this offering to any of these shareholders, or any of these
shareholders may determine to purchase more, less or no shares in this offering. The underwriters will receive the same underwriting
discount on any Units purchased by these shareholders as they will on any other Units sold to the public in this offering.
Private
Placements of our Securities
The
Company believes that each of the issuances set forth below was exempt from registration under the Securities Act in reliance on Section
4(a)(2) of the Securities Act and/or Regulation S under the Securities Act.
Medigus
Ltd. Share Purchase Agreements
On
July 15, 2020, we entered into a share purchase agreement, or the XYLO Purchase Agreement,
with Medigus Ltd. (which subsequently changed its name to Xylo
Technologies Ltd.), or XYLO, pursuant to which we issued a total of 43,964 of our Ordinary Shares, at a price per share of $2.36
for aggregate gross proceeds of $104,000.
As
part of the XYLO Purchase Agreement, XYLO was granted with an option to purchase 510,217 Ordinary Shares for $1,000,000, or the Original
Option. The Original Option expired by its terms on April 23, 2023.
The
Company and XYLO subsequently entered into (i) a first amendment to the XYLO Purchase Agreement on December 15, 2021, which among other
things, granted an option to XYLO, instead of the Original Option, to purchase 459,770 Ordinary Shares for an aggregate purchase price
of $2,000,000, or the Second Option; (ii) a second amendment to the XYLO Purchase Agreement on December 23, 2021, which changed certain
terms of the Original Option; (iii) a third amendment to the XYLO Purchase Agreement on November 21, 2023, which terminated the Original
Option and extended the term of the Second Option to December 31, 2024; and (iv) a fourth amendment to the XYLO Purchase Agreement on
May 7, 2024, which changed certain terms with respect to the exercise of the Second Option, including among other things, (a) that in
the event of Listing (such as an IPO), the exercise price of the Second Option will be $5.00 per Ordinary Share for an investment of
$2,000,000 and (b) that the exercise price of the Second Option shall be subject to adjustments in certain events with respect to its
Ordinary Shares such as a subdivision, combination and issuance of Ordinary Shares (other than pursuant to this Offering).
Concurrently
with the execution of the XYLO Purchase Agreement, the Company and XYLO entered into an exclusive reseller agreement on July 15, 2020,
whereby XYLO was granted an exclusive, worldwide, sub-licensable right to promote, market and resell certain product candidates of the
Company to customers. The license commencement date would be the date upon which the Company receives FDA approvals with regards to those
product candidates and would continue for a period of four years. In consideration of such license, the Company would be entitled to
receive annual royalty payments equal to 10% of (i) the proceeds from actual sales (excluding rebates and returns) of the product candidates
by XYLO in any calendar year, less (ii) the aggregate price paid for such product candidates and less (iii) XYLO’
operating expenses relating to the sale of such product candidates, as determined in good faith by XYLO. On May 7, 2024, the Company
and XYLO terminated the reseller agreement.
On
March 9, 2021, we entered into a private placement with certain investors, including XYLO,
pursuant to which we issued an aggregate of 368,257 Ordinary Shares, at a price per share of $0.68, for aggregate gross proceeds of $250,000.
One
of our former directors, Liron Carmel, is the Chief Executive Officer of XYLO. XYLO currently holds 315,521 or 9.8% of our Ordinary Shares.
August
2021 Share Purchase Agreement
On
August 25, 2021, we entered into a Share Purchase Agreement with certain investors, including Tomer Izraeli, our Chief Executive Officer,
XYLO, Sara Srugo and Reuven Srugo Construction Company Ltd., a Company owned by Raul Srugo, pursuant to which we issued 688,060 Ordinary
Shares, at a price per share of $1.13 for aggregate net proceeds of $779,971.
June
2023, December 2023 and May 2024 Share Purchase Agreements
In
June 2023, December 2023 and May 2024, we entered into a series of securities purchase agreements
with certain of our principal shareholders, including XYLO, Raul Srugo, Itzhak Srugo, Yoav
Srugo and Sofi Yochelman Srugo, pursuant to which we issued an aggregate of 513,878 Ordinary
Shares for gross proceeds of $582,000. The price per share in the June 2023 securities purchase
agreement was $1.1322, and the price per share in the December 2023 and May 2024 securities
purchase agreement was $1.1336. Raul Srugo was a member of our Board of Directors at the
time of the transaction. Itzhak Srugo, Yoav Srugo and Sofi Yochelman Srugo are members of
Raul Srugo’s immediate family. XYLO paid a portion of the consideration pursuant to
the June 2023 securities purchase agreement by issuing us 11,328 American Depositary Shares
representing 169,920 ordinary shares of XYLO, representing aggregate consideration in an
amount equal to $60,000, reflecting a price per American Depositary Shares equal to a 30-day
volume-weighted average price of XYLO American Depositary Shares on the Nasdaq Capital Market.
Loans
with Related Parties
Convertible
Loan with XYLO
On
February 4, 2023, we entered into a convertible loan agreement with Medigus Ltd. (which subsequently changed its name to Xylo Technologies
Ltd.), pursuant to which XYLO extended a loan to the Company in the principal amount of $80,000, with an annual interest rate
of approximately 4%. The convertible loan agreement included (i) an automatic conversion mechanism in the event of a qualified financing
of at least $500,000, or (ii) optional conversion, both at a conversion price equal to the price per share in such financing minus a
20% discount. If the loan (and the accrued interest thereon) has not been repaid or converted prior to the lapse of six months, then
at the request of a lender the entire loan and the accrued interest thereon shall be converted into such number of the most senior class
of shares of the Company then outstanding, at a conversion price equal to the lowest price per share actually paid to the Company since
August 1, 2021. Pursuant to the terms of the loan agreement, on May 12, 2024 the entire loan was converted into 88,216 Ordinary Shares.
Convertible
Loan with Reuven Srugo Construction Company Ltd.
On
February 4, 2023, we entered into a convertible loan agreement with Reuven Srugo Construction
Company Ltd., or Reuven Construction, pursuant to which Reuven Construction extended a loan
the Company in the principal amount of $100,000, with an annual interest rate of approximately
4%. The convertible loan agreement included (i) an automatic conversion mechanism in the
event of a qualified financing of at least $500,000, or (ii) optional conversion, both at
a conversion price equal to the price per share in such financing minus a 20% discount. If
the loan (and the accrued interest thereon) has not been repaid or converted prior to the
lapse of six months, then at the request of a lender the entire loan and the accrued interest
thereon shall be converted into such number of the most senior class of shares of the Company
then outstanding, at a conversion price equal to the lowest price per share actually paid
to the Company since August 1, 2021. Pursuant to the terms of that loan agreement, on May
12, 2024 the entire loan was converted into 110,270 Ordinary Shares.
Reuven
Construction is owned by Raul Srugo, who at the time of the transaction was a member of our
Board of Directors, and Yoav Srugo, Itzhak Srugo and Sofi Yochelman Srugo
Agreements
and Arrangements With, and Compensation of, Directors and Executive Officers
We
have entered into written employment and consulting agreements with each of our executive officers. All of these agreements contain customary
provisions regarding noncompetition, confidentiality of information and assignment of inventions. However, the enforceability of the
noncompetition provisions may be limited under applicable law. In addition, we have entered into agreements with each executive officer
and director pursuant to which we have agreed to indemnify each of them up to a certain amount and to the extent that these liabilities
are not covered by directors and officers insurance.
Options
Since
our inception, we have granted options to purchase our Ordinary Shares to our officers and certain of our directors. Such option agreements
may contain acceleration provisions upon certain merger, acquisition, or change of control transactions. We describe our option plans
under “Management—Equity Incentive Plan.” If the relationship between us and an executive officer or a director is
terminated, except for cause (as defined in the various option plan agreements), options that are vested will generally remain exercisable
for three months after such termination.
DESCRIPTION
OF SHARE CAPITAL
The
following descriptions of our share capital and provisions of our Articles which will be effective upon the closing of this offering
are summaries and do not purport to be complete. A form of our Articles will be filed with the SEC as an exhibit to our registration
statement, of which this prospectus forms a part. The description of the Ordinary Shares reflects changes to our capital structure
that will occur upon the closing of this offering.
On
September 29, 2022, the Company effected the Initial Reverse Share Split, as follows: (i) a reverse stock split of our issued and outstanding
shares at a ratio of 1-for-8.80, pursuant to which holders of our shares received one Ordinary Share for every 8.80 Ordinary Shares
held, and (ii) cancelled the par value of our Ordinary Shares and Preferred Shares.
On
December 19, 2022, the Company effected the Forward Share Split, as follows: the issuance of an aggregate of 858,148 bonus shares to
the holders of our Ordinary Shares on a basis of 1.25 bonus shares for each Ordinary Share outstanding (equivalent to a forward share
split at a ratio of 1.25-for-1).
On
June 18, 2023, the Company effected the Second Reverse Share Split, as follows: a reverse stock split of our issued and outstanding shares
at a ratio of 1-for-1.7, pursuant to which holders of our shares received one Ordinary Share for every 1.7 Ordinary Shares held.
On
May 12, 2024, the Company effected the Third Reverse Share Split, as follows: a reverse stock
split of our issued and outstanding shares at a ratio of 1-for-2, pursuant to which holders
of our shares received one Ordinary Share for every two Ordinary Shares held.
On
August 16, 2024, the Company effected the Second Forward Share Split as follows: the issuance of an aggregate of 420,618 bonus shares
to the holders of our Ordinary Shares on a basis of 0.1494 bonus shares for each Ordinary Share outstanding (equivalent to a forward
share split at a ratio of 0.1494-for-1).
Unless
the context expressly dictates otherwise, all references to share and per share amounts referred to herein reflect the foregoing Share
Splits.
General
As
of the date of this prospectus, our authorized share capital consisted of 20,000,000 shares divided into 19,895,289 Ordinary Shares and
104,711 Preferred Shares, no par value per share, of which 3,130,831 Ordinary Shares were issued and outstanding as of such date. All
of our outstanding Ordinary Shares have been validly issued, fully paid and non-assessable. Our Ordinary Shares are not redeemable and
are not subject to any preemptive right.
Our
registration number with the Israeli Registrar of Companies is 513637025.
Since
January 2019, we have issued an aggregate of 2,738,564 Ordinary Shares.
In
addition to Ordinary Shares, in the last three years, we have granted options to purchase an aggregate of 246,129 Ordinary Shares to
directors, officers and employees with exercise prices ranging between $0.02 and $1.1335 per share under our 2021 Plan. As of the date
of this prospectus, the total outstanding amount of options under the 2021 Plan is 246,129.
In
addition, we currently have 104,711 Preferred Shares in our registered share capital, of which 104,711 Preferred Shares are issued and
outstanding. All Preferred Shares will automatically convert into Ordinary Shares on a one-to-one basis upon the completion of this offering.
In addition, following the conversion of the foregoing Preferred Shares into Ordinary Shares, and upon the adoption of our Articles to
be effective upon the closing of this offering, we will no longer have any authorized preferred shares available for issuance.
All
of our outstanding Ordinary Shares are validly issued, fully paid and non-assessable. Our Ordinary Shares are not redeemable and do not
have any preemptive rights.
All
Ordinary Shares have identical voting and other rights in all respects.
The
Powers of the Directors
Our
Board of Directors shall direct our policy and shall supervise the performance of our Chief Executive Officer and his actions. Our Board
of Directors may exercise all powers that are not required under the Companies Law or under our Articles to be exercised or taken by our
shareholders.
Rights
Attached to Shares
Our
Ordinary Shares shall confer upon the holders thereof:
| |
● |
equal
right to attend and to vote at all of our general meetings, whether regular or special, with each Ordinary Share entitling the holder
thereof, which attend the meeting and participate at the voting, either in person or by a proxy or by a written ballot, to one vote; |
| |
● |
equal
right to participate in distribution of dividends, if any, whether payable in cash or in bonus shares, in distribution of assets
or in any other distribution, on a per share pro rata basis; and |
| |
|
|
| |
● |
equal
right to participate, upon our dissolution, in the distribution of our assets legally available for distribution, on a per share
pro rata basis. |
Election
of Directors
Pursuant
to our Articles, our directors are divided into three classes with staggered three-year terms. Each class of directors consists, as nearly
as possible, of one-third of the total number of directors constituting the entire board of directors (other than the external directors,
to the extent applicable). At each annual general meeting of our shareholders, the election or re-election of directors following the
expiration of the term of office of the directors of that class of directors is for a term of office that expires on the third annual
general meeting following such election or re-election, such that at each annual general meeting the term of office of only one class
of directors expires. Each director will hold office until the annual general meeting of our shareholders in which his or her term expires,
unless they are removed by a vote of 65% of the total voting power of our shareholders at a general meeting of our shareholders or upon
the occurrence of certain events, in accordance with the Companies Law and our articles. External directors, if applicable, are elected
for an initial term of three years, may be elected for additional terms of three years each under certain circumstances, and may be removed
from office pursuant to the terms of the Companies Law. See “Management—Board Practices—External Directors.”
Annual
and Special Meetings
Under
the Israeli law, we are required to hold an annual general meeting of our shareholders once every calendar year, at such time and place
which shall be determined by our Board of Directors, that must be no later than 15 months after the date of the previous annual general
meeting. All meetings other than the annual general meeting of shareholders are referred to as special general meetings. Our Board of
Directors may call special meetings whenever it sees fit and upon the request (i) any two of our directors or such number of directions
equal to ¼ of the directors then at office (ii) any
shareholder or shareholders holding at least five percent (5%) or a higher percent of our outstanding and issued shares and 1% of our
outstanding voting rights or (iii) any shareholder or shareholders holding at least five percent (5%) of our outstanding voting rights.
Subject
to the provisions of the Companies Law and the regulations promulgated thereunder, shareholders entitled to participate and vote at general
meetings are the shareholders of record on a date to be decided by the board of directors, which may be between four and twenty-one days
prior to the date of the meeting. Resolutions regarding the following matters must be passed at a general meeting of our shareholders:
| |
● |
amendments to our Articles; |
| |
|
|
| |
● |
the
exercise of our Board of Director’s powers by a general meeting if our Board of Directors is unable to exercise its powers
and the exercise of any of its powers is required for our proper management; |
| |
|
|
| |
● |
appointment
or termination of our auditors; |
| |
|
|
| |
● |
appointment
of directors, including external directors; |
| |
|
|
| |
● |
approval
of acts and transactions requiring general meeting approval pursuant to the provisions of the Companies Law (mainly certain related
party transactions) and any other applicable law; |
| |
|
|
| |
● |
increases
or reductions of our authorized share capital; |
| |
|
|
| |
● |
a
merger (as such term is defined in the Companies Law); and |
| |
|
|
| |
● |
a
dissolution of the company by its shareholders (as such term is defined in the Company’s Law). |
Notices
The
Companies Law and our Articles require that a notice of any annual or special shareholders meeting be published in at least two widely
circulated newspapers, in addition to the company’s internet website, at least 21 days prior to the meeting, and if the agenda
of the meeting includes the appointment or removal of directors, the approval of transactions with office holders or interested or related
parties, approval of the company’s general manager to serve as the chairman of the board of directors or an approval of a merger,
notice must be provided at least 35 days prior to the meeting.
Quorum
As
permitted under the Companies Law, the quorum required for our general meetings consists of at least two shareholders present in person,
by proxy, written ballot or voting by means of electronic voting system, who hold or represent between them at least 25% of the total
outstanding voting rights. If within half an hour of the time set forth for the general meeting a quorum is not present, the general
meeting shall stand adjourned the same day of the following week, at the same hour and in the same place, or to such other date, time
and place as prescribed in the notice to the shareholders and in such adjourned meeting, if no quorum is present within half an hour
of the time arranged, any number of shareholders participating in the meeting, shall constitute a quorum.
If
a special general meeting was summoned following the request of a shareholder, and within half an hour a legal quorum shall not have
been formed, the meeting shall be canceled.
Adoption
of Resolutions
Our
Articles provide that all resolutions of our shareholders require a simple majority vote, unless otherwise required under the Companies
Law or our Articles. A shareholder may vote in a general meeting in person, by proxy, by a written ballot.
Changing
Rights Attached to Shares
Unless
otherwise provided by the terms of the shares and subject to any applicable law, any modification of rights attached to any class of
shares must be adopted by the holders of a majority of the shares of that class present a general meeting of the affected class or by
a written consent of all the shareholders of the affected class.
The
enlargement of an existing class of shares or the issuance of additional shares thereof, shall not be deemed to modify the rights attached
to the previously issued shares of such class or of any other class, unless otherwise provided by the terms of the shares.
Limitations
on the Right to Own Securities in Our Company
There
are no limitations on the right to own our securities. In certain circumstances the Warrants being offered hereby have restrictions upon
the exercise of such warrants if such exercise would result in the holders thereof owning more than 4.99% or 9.99% of our Ordinary Shares
upon such exercise, as further described below.
Provisions
Restricting Change in Control of Our Company
There
are no specific provisions of our Articles that would have an effect of delaying, deferring or preventing a change in control of our
company or that would operate only with respect to a merger, acquisition or corporate restructuring involving us (or our Subsidiary).
However, as described below, certain provisions of the Companies Law may have such effect.
The
Companies Law includes provisions that allow a merger transaction and requires that each company that is a party to the merger have the
transaction approved by its board of directors and, unless certain requirements described under the Companies Law are met, a vote of
the majority of shareholders, and, in the case of the target company, also a majority vote of each class of its shares. For
purposes of the shareholder vote of each party, unless a court rules otherwise, the merger will not be deemed approved if shares representing
a majority of the voting power present at the shareholders meeting and which are not held by the other party to the merger (or by any
person or group of persons acting in concert who holds 25% or more of the voting power or the right to appoint 25% or more of the directors
of the other party) vote against the merger. If, however, the merger involves a merger with a company’s own controlling shareholder
or if the controlling shareholder has a personal interest in the merger, then the merger is instead subject to the same Special Majority
approval that governs all extraordinary transactions with controlling shareholders. Upon the request of a creditor of either party
to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that as a result
of the merger the surviving company will be unable to satisfy the obligations of any of the parties to the merger, and may further give
instructions to secure the rights of creditors. If the transaction would have been approved by the shareholders of a merging company
but for the separate approval of each class or the exclusion of the votes of certain shareholders as provided above, a court may still
approve the merger upon the petition of holders of at least 25% of the voting rights of a company. For such petition to be granted, the
court must find that the merger is fair and reasonable, taking into account the value of the parties to the merger and the consideration
offered to the shareholders. In addition, a merger may not be completed unless at least (1) 50 days have passed from the time that
the requisite proposals for approval of the merger were filed with the Israeli Registrar of Companies by each merging company and (2)
30 days have passed since the merger was approved by the shareholders of each merging company.
The
Companies Law also provides that, subject to certain exceptions, an acquisition of shares in an Israeli public company must be made by
means of a “special” tender offer if as a result of the acquisition (1) the purchaser would become a controlling shareholder
if there is no controlling shareholder in the company or (2) the purchaser would become a holder of 45% or more of the voting rights
in the company, unless there is already a holder of more than 45% of the voting rights in the company. These requirements do not apply
if, in general, the acquisition (1) was made in a private placement that received shareholders’ approval, subject to certain conditions,
(2) was from a controlling shareholder in the company which resulted in the acquirer becoming a controlling shareholder in the company,
or (3) was from a holder of more than 45% of the voting rights in the company which resulted in the acquirer becoming a holder of more
than 45% of the voting rights in the company. A “special” tender offer must be extended to all shareholders. In general,
a “special” tender offer may be consummated only if (1) at least 5% of the voting power attached to the company’s outstanding
shares will be acquired by the offeror and (2) the offer is accepted by a majority of the offerees who notified the company of their
position in connection with such offer (excluding the offeror, controlling shareholders, holders of 25% or more of the voting rights
in the company or anyone on their behalf, or any person having a personal interest in the acceptance of the tender offer). If a special
tender offer is accepted, then the purchaser or any person or entity controlling it or under common control with the purchaser or such
controlling person or entity may not make a subsequent tender offer for the purchase of shares of the target company and may not enter
into a merger with the target company for a period of one year from the date of the offer, unless the purchaser or such person or entity
undertook to effect such an offer or merger in the initial special tender offer.
If,
as a result of an acquisition of shares, the acquirer will hold more than 90% of an Israeli company’s outstanding shares or of
certain class of shares, the acquisition must be made by means of a tender offer for all of the outstanding shares, or for all of the
outstanding shares of such class, as applicable. In general, if less than 5% of the outstanding shares, or of applicable class, are not
tendered in the tender offer and more than half of the offerees who have no personal interest in the offer tendered their shares, all
the shares that the acquirer offered to purchase will be transferred to it by operation of law. However, a tender offer will also be
accepted if the shareholders who do not accept the offer hold less than 2% of the issued and outstanding share capital of the company
or of the applicable class of shares. Any shareholders that was an offeree in such tender offer, whether such shareholder accepted the
tender offer or not, may request, by petition to an Israeli court, (i) appraisal rights in connection with a full tender offer, and (ii)
that the fair value should be paid as determined by the court, for a period of six months following the acceptance thereof. However,
the acquirer is entitled to stipulate, under certain conditions, that tendering shareholders will forfeit such appraisal rights.
Lastly,
Israeli tax law treats some acquisitions, such as stock-for-stock exchanges between an Israeli company and a foreign company, less favorably
than U.S. tax laws. For example, Israeli tax law may, under certain circumstances, subject a shareholder who exchanges his Ordinary Shares
for shares in another corporation to taxation prior to the sale of the shares received in such stock-for-stock swap.
Exclusive
Forum
Our
articles of association provide that unless we consent in writing to the selection of an alternative forum, the federal district courts
of the United States of America shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising
under the Securities Act. Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all such
Securities Act actions, and accordingly, both state and federal courts have jurisdiction to entertain such claims. While the federal
forum provision in our articles of association does not restrict the ability of our shareholders to bring claims under the Securities
Act, we recognize that it may limit shareholders’ ability to bring a claim in the judicial forum that they find favorable and may
increase certain litigation costs, which may discourage the filing of claims under the Securities Act against the Company, its directors
and officers. However, the enforceability of similar forum provisions (including exclusive federal forum provisions for actions, suits
or proceedings asserting a cause of action arising under the Securities Act) in other companies’ organizational documents has been
challenged in legal proceedings, and there is uncertainty as to whether courts would enforce the exclusive forum provisions in our articles
of association. Any person or entity purchasing or otherwise acquiring any interest in our share capital shall be deemed to have notice
of and to have consented to the choice of forum provision of our articles of association described above. It is clarified that the federal
district courts of the United States of America shall be the exclusive forum for suits brought to enforce a duty or liability created
by the Exchange Act or the rules and regulations thereunder.
Our
Articles to be effective upon the closing of this offering also provide that unless we consent in writing to the selection of an alternative
forum, the competent courts in Tel Aviv, Israel shall be the exclusive forum for any derivative action or proceeding brought on behalf
of the Company, any action asserting a breach of a fiduciary duty owed by any of our directors, officers or other employees to the Company
or our shareholders or any action asserting a claim arising pursuant to any provision of the Companies Law or the Israeli Securities
Law.
Changes
in Our Capital
The
general meeting may, by a simple majority vote of the shareholders attending the general meeting:
| |
● |
increase
our registered share capital by the creation of new shares from the existing class or a new class, as determined by the general meeting; |
| |
|
|
| |
● |
cancel
any registered share capital which have not been taken or agreed to be taken by any person; |
| |
|
|
| |
● |
consolidate
and divide all or any of our share capital into shares of larger nominal value than our existing shares; |
| |
|
|
| |
● |
subdivide
our existing shares or any of them, our share capital or any of it, into shares of smaller nominal value than is fixed; and |
| |
|
|
| |
● |
reduce
our share capital and any fund reserved for capital redemption in any manner, and with and subject to any incident authorized, and
consent required, by the Companies Law. |
DESCRIPTION
OF SECURITIES WE ARE OFFERING
Units
We
are offering the Units at the initial public offering price of $4.38 per Unit. Each Unit consists of one Ordinary Share and three Warrants,
each to purchase one Ordinary Share at an exercise price equal to $4.38 (based on the public offering price of $4.38 per Unit) per Ordinary
Share (with such exercise price equal to the public offering price per Unit). The Ordinary Shares and Warrants may be transferred separately
immediately upon issuance.
Ordinary
Shares
The
material terms and provisions of our Ordinary Shares are described under the caption “Description of Share Capital” in this
prospectus.
Warrants
Included in the Units
The
following summary of certain terms and provisions of the Warrants offered hereby is not complete and is subject to, and qualified in
its entirety by, the provisions of the warrant agent agreement between us and VStock Transfer, LLC, as warrant agent, and the form of
Warrant, both of which are filed as exhibits to the registration statement of which this prospectus is a part. Prospective investors
should carefully review the terms and provisions set forth in the warrant agent agreement, including the annexes thereto, and form of
Warrant.
Exercisability.
The Warrants are exercisable at any time after their original issuance and at any time up to the date that is five years after their
original issuance. The Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed
exercise notice and, at any time a registration statement registering the issuance of the Ordinary Shares underlying the warrants under
the Securities Act is effective and available for the issuance of such shares, by payment in full in immediately available funds for
the number of Ordinary Shares purchased upon such exercise. If a registration statement registering the issuance of the Ordinary Shares
underlying the Warrants under the Securities Act is not effective or available the holder may, in its sole discretion, elect to exercise
the Warrant through a cashless exercise, in which case the holder would receive upon such exercise the net number of Ordinary Shares
determined according to the formula set forth in the Warrant. No fractional shares will be issued in connection with the exercise of
a Warrant. In lieu of fractional shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise
price.
Exercise
Limitation. A holder will not have the right to exercise any portion of the warrant if the holder (together with its affiliates)
would beneficially own in excess of 4.99% of the number of Ordinary Shares outstanding immediately after giving effect to the exercise,
as such percentage ownership is determined in accordance with the terms of the Warrants. However, any holder may increase or decrease
such percentage to any other percentage not in excess of 9.99%, provided that any increase in such percentage shall not be effective
until 61 days following notice from the holder to us.
Exercise
Price. The exercise price per whole Ordinary Share purchasable upon exercise of the Warrants is $4.38 per share, which is equal to
the price per Unit. The exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions,
stock splits, stock combinations, reclassifications or similar events affecting our Ordinary Shares and also upon any distributions of
assets, including cash, stock or other property to our stockholders.
Transferability.
Subject to applicable laws, the Warrants may be offered for sale, sold, transferred or assigned without our consent.
Warrant
Agent. The Warrants will be issued in registered form under a warrant agent agreement between VStock Transfer, LLC, as warrant agent,
and us. The Warrants shall initially be represented only by one or more global warrants deposited with the warrant agent, as custodian
on behalf of The Depository Trust Company, or DTC, and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed
by DTC.
Exchange
Listing. We do not intend to apply to list the Warrants on any securities exchange or other trading system.
Fundamental
Transactions. In the event of a fundamental transaction, as described in the Warrants
and generally including any reorganization, recapitalization or reclassification of our Ordinary
Shares, the sale, transfer or other disposition of all or substantially all of our properties
or assets, our consolidation or merger with or into another person, the acquisition of more
than 50% of our outstanding Ordinary Shares, or any person or group becoming the beneficial
owner of more than 50% of the voting power represented by our outstanding Ordinary Shares,
the holders of the Warrants will be entitled to receive upon exercise of the Warrants the
kind and amount of securities, cash or other property that the holders would have received
had they exercised the Warrants immediately prior to such fundamental transaction without
regard to any limitations on exercised contained in the Warrants.
Rights
as a Stockholder. Except as otherwise provided in the Warrants or by virtue of such holder’s ownership of our Ordinary Shares,
the holder of a Warrant does not have the rights or privileges of a holder of our Ordinary Shares, including any voting rights, until
the holder exercises the Warrant.
Governing
Law. The Warrants and the warrant agent agreement are governed by New York law.
SHARES
ELIGIBLE FOR FUTURE SALE
Prior
to this offering, no public market existed for our Ordinary Shares. Sales of substantial
amounts of our Ordinary Shares following this offering, or the perception that these sales
could occur, could adversely affect prevailing market prices of our Ordinary Shares and could
impair our future ability to obtain capital, especially through an offering of equity securities.
Assuming that the underwriter does not exercise in full its option to purchase additional
Ordinary Shares and/or Warrants with respect to this offering and assuming no exercise of
options outstanding following this offering, we will have an aggregate of 4,194,445
Ordinary Shares outstanding upon the closing of this offering. Of these shares, the Ordinary
Shares sold in this offering by the Company and Ordinary Shares sold by the Selling Shareholders
will be freely tradable without restriction or further registration under the Securities
Act, unless purchased by “affiliates” (as that term is defined under Rule 144
of the Securities Act, or Rule 144), who may sell only the volume of shares described
below and whose sales would be subject to additional restrictions described below.
The
remaining Ordinary Shares will be held by our existing shareholders and will be deemed to be “restricted securities” under
Rule 144. Subject to certain contractual restrictions, including the lock-up agreements described below, restricted securities may
only be sold in the public market pursuant to an effective registration statement under the Securities Act or pursuant to an exemption
from registration under Rule 144, Rule 701 or Rule 904 under the Securities Act. These rules are summarized below. Sales
of these shares in the public market after the restrictions under the lock-up agreements lapse, or the perception that those sales may
occur, could cause the prevailing market price of our Ordinary Shares to decrease or to be lower than it might be in the absence of those
sales or perceptions.
Eligibility
of Restricted Shares for Sale in the Public Market
We
have registered an aggregate of 2,801,330 Ordinary Shares for resale by the Selling Shareholders
by means of a separate prospectus. Other than certain affiliates that hold an aggregate of
118,691 Ordinary Shares, the remaining Selling Shareholders are not subject to any lock-up
or leakage agreements and have the right to sell the shares being registered hereby at any
time. Our directors, executive officers, and any other holder(s) of 10 percent or more of
the outstanding shares, representing 118,691 Ordinary Shares or 2.83% of our Ordinary Shares,
will be subject to the contractual restrictions arising under the lock-up agreements described
below.
The
Selling Shareholders may also sell their Ordinary Shares under Rule 144 or any other exemption from registration under the Securities
Act, if available.
All
of the Ordinary Shares sold in this offering will be eligible for immediate sale upon the closing of this offering.
Certain
of our existing shareholders, including beneficial owners of greater than 5% of our share
capital, have indicated an interest in purchasing up to an aggregate of $1 million of Units
in this offering, which represents approximately 24% of the total Units in this offering,
based on the public offering price of $4.38 per Unit. However, because indications of interest
are not binding agreements or commitments to purchase, the underwriters may determine to
sell more, less or no shares in this offering to any of these shareholders, or any of these
shareholders may determine to purchase more, less or no Units in this offering. The underwriters
will receive the same underwriting discount on any shares purchased by these shareholders
as they will on any other Units sold to the public in this offering.
Lock-Up
Agreements
We,
all of our directors, employees and executive officers and holders of 10% or more of our outstanding Ordinary Shares and our Ordinary
Shares issuable upon the exercise of options and warrants have signed lock-up agreements. Pursuant to such lock-up agreements, such persons
have agreed, subject to certain exceptions, not to sell or otherwise dispose of Ordinary Shares or any securities convertible into or
exchangeable for Ordinary Shares for a period of 180 days after the date of this prospectus without the prior written consent of
the underwriter in this offering, which may, in its sole discretion, at any time without prior notice, release all or any portion of
the Ordinary Shares from the restrictions in any such agreement.
Rule
144
Shares
Held for Six Months
In
general, under Rule 144 as currently in effect, and subject to the terms of any lock-up agreement, commencing 90 days after
the closing of this offering, a person (or persons whose shares are aggregated), including an affiliate, who has beneficially owned our
Ordinary Shares for six months or more, including the holding period of any prior owner other than one of our affiliates (i.e., commencing
when the shares were acquired from our company or from an affiliate of our company as restricted securities), is entitled to sell our
shares, subject to the availability of current public information about us. In the case of an affiliate shareholder, the right to sell
is also subject to the fulfillment of certain additional conditions, including manner of sale provisions and notice requirements, and
to a volume limitation that limits the number of shares to be sold thereby, within any three-month period, to the greater of:
| ● | 1%
of the number of Ordinary Shares then outstanding; or |
| ● | the
average weekly trading volume of our Ordinary Shares on Nasdaq during the four calendar weeks
preceding the filing of a notice on Form 144 with respect to the sale. |
The
six month holding period of Rule 144 does not apply to sales of unrestricted securities. Accordingly, persons who hold unrestricted
securities may sell them under the requirements of Rule 144 described above without regard to the six-month holding period, even
if they were considered our affiliates at the time of the sale or at any time during the ninety days preceding such date.
Shares
Held by Non-Affiliates for One Year
Under
Rule 144 as currently in effect, a person (or persons whose shares are aggregated) who is not considered to have been one of our
affiliates at any time during the 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at
least one year, including the holding period of any prior owner other than one of our affiliates, is entitled to sell his, her or its
shares under Rule 144 without complying with the provisions relating to the availability of current public information or with any
other conditions under Rule 144. Therefore, unless subject to a lock-up agreement or otherwise restricted, such shares may be sold
immediately upon the closing of this offering.
Rule 701
In
general, under Rule 701, any of our employees, directors, officers, consultants or advisors who received or purchased Ordinary Shares
from us under our incentive option plan or other written agreement before the closing of this offering is entitled to resell these shares.
The
SEC has indicated that Rule 701 will apply to typical share options granted by an issuer before it becomes subject to the reporting
requirements of the Exchange Act, along with the shares acquired upon exercise of these options, including exercises after the closing
of this offering. Securities issued in reliance on Rule 701 are restricted securities and, subject to the contractual restrictions
described above (see “Lock-Up Agreements”), may be sold beginning 90 days after the closing of this offering in reliance
on Rule 144 by:
| ● | persons
other than affiliates, without restriction; and |
| ● | affiliates,
subject to the manner-of-sale, current public information and filing requirements of Rule 144,
in each case, without compliance with the six-month holding period requirement of Rule 144. |
Selling
Shareholders Resale Prospectus
As
described in the Explanatory Note to the registration statement of which this prospectus forms a part, the registration statement also
contains the Resale Prospectus to be used in connection with the potential resale by the Selling Shareholders of our Ordinary Shares
held by them. These Ordinary Shares have been registered to permit public resale of such shares, and the Selling Shareholders may offer
the shares for resale from time to time pursuant to the Resale Prospectus. The Selling Shareholders may also sell, transfer or otherwise
dispose of all or a portion of their shares in transactions exempt from the registration requirements of the Securities Act or pursuant
to another effective registration statement covering those shares. No sales of the shares covered by the Resale Prospectus shall occur
until the Ordinary Shares sold in our initial public offering begin trading on The Nasdaq Capital Market. Thereafter, any sales will
occur at prevailing market prices or in privately negotiated prices.
SELLING
SHAREHOLDERS
The
Resale Prospectus covers the offering for resale of 2,801,330 Ordinary Shares by the Selling Shareholders. This prospectus and any prospectus
supplement will only permit the Selling Shareholders to sell the number of Ordinary Shares identified in the column “Maximum Number
of Ordinary Shares to be Sold Pursuant to the Resale Prospectus.” The Ordinary Shares issued to the Selling Shareholders
are “restricted” securities under applicable U.S. federal and state securities laws and are being registered to provide
the Selling Shareholders the opportunity to sell those Ordinary Shares.
The
following table sets forth the name of the Selling Shareholders who are offering the Ordinary Shares for resale by the Resale Prospectus,
the number and percentage of Ordinary Shares beneficially owned by each such Selling Shareholder, the maximum number of Ordinary Shares
that may be offered for resale by the Resale Prospectus and the number of Ordinary Shares each such Selling Shareholder will own after
the offering, assuming each such Selling Shareholder sells the maximum number of Ordinary Shares to be sold by it pursuant to the Resale
Prospectus. The information appearing in the table below is based on information provided by or on behalf of the named Selling Shareholders.
We will not receive any proceeds from the resale of the Ordinary Shares by the Selling Shareholders. The Selling Shareholders may sell
all, some or none of their shares in this offering. See “Plan of Distribution” in the Resale Prospectus for additional
information.
| Name of
Selling Shareholder |
|
Ordinary
Shares Beneficially
Owned Prior to Offering |
|
|
Percentage
Ownership Prior to Offering(1) |
|
|
Maximum
Number of
Ordinary
Shares to
be Sold
Pursuant to
this
Prospectus |
|
|
Ordinary Shares
Owned
Immediately
After Sale of
Maximum
Number of
Shares in this
Offering |
|
| Reuven Srugo Construction Company
Ltd.(2) |
|
|
168,136 |
|
|
|
5.20 |
% |
|
|
168,136 |
|
|
|
— |
|
| Raul Srugo(2)(3)** |
|
|
351,678 |
|
|
|
10.87 |
% |
|
|
351,678 |
|
|
|
— |
|
| Itzhak Srugo(2)(4)** |
|
|
351,678 |
|
|
|
10.87 |
% |
|
|
351,678 |
|
|
|
— |
|
| Yoav Srugo(2)(5)** |
|
|
351,678 |
|
|
|
10.87 |
% |
|
|
351,678 |
|
|
|
— |
|
| Sofi Yochelman Srugo(2) |
|
|
287,249 |
|
|
|
8.88 |
% |
|
|
287,249 |
|
|
|
— |
|
| Ofakim Hi-Tech Ventures Ltd.(2) |
|
|
88,487 |
|
|
|
2.73 |
% |
|
|
88,487 |
|
|
|
— |
|
| West East Pte Ltd.(2)(6)** |
|
|
40,194 |
|
|
|
1.24 |
% |
|
|
40,194 |
|
|
|
— |
|
| Capitallink Ltd. (2)(7)** |
|
|
93,403 |
|
|
|
2.89 |
% |
|
|
93,403 |
|
|
|
— |
|
| Gabriel Kabazo (2)(8)** |
|
|
46,702 |
|
|
|
1.44 |
% |
|
|
46,702 |
|
|
|
— |
|
| L.I.A Pure Capital Ltd.(2)(9)** |
|
|
247,521 |
|
|
|
7.65 |
% |
|
|
247,521 |
|
|
|
— |
|
| Ronen Fatal (2)(10)** |
|
|
35,026 |
|
|
|
1.08 |
% |
|
|
35,026 |
|
|
|
— |
|
| E. G. Europe Property(2)(11)** |
|
|
291,886 |
|
|
|
9.02 |
% |
|
|
291,886 |
|
|
|
— |
|
| Itamar David(2)(12)** |
|
|
46,702 |
|
|
|
1.44 |
% |
|
|
46,702 |
|
|
|
— |
|
| SciSparc Ltd.(13)(14)** |
|
|
320,000 |
|
|
|
9.90 |
% |
|
|
320,000 |
|
|
|
— |
|
| B.G. Negev Technologies and Applications Ltd.(2) |
|
|
16,786 |
|
|
|
* |
% |
|
|
16,786 |
|
|
|
— |
|
| Anthony Bishop(2) |
|
|
14,732 |
|
|
|
* |
% |
|
|
14,732 |
|
|
|
— |
|
| CFH Beteiligungsgesellschaft mbH.(2) |
|
|
11,355 |
|
|
|
* |
% |
|
|
11,355 |
|
|
|
— |
|
| Erez Paran.(2) |
|
|
7,870 |
|
|
|
* |
% |
|
|
7,870 |
|
|
|
— |
|
| Capital Point Ltd.(2) |
|
|
4,869 |
|
|
|
* |
% |
|
|
4,869 |
|
|
|
— |
|
| Amnon Zatorsky(2) |
|
|
4,585 |
|
|
|
* |
% |
|
|
4,585 |
|
|
|
— |
|
| Yossi Geva.(2) |
|
|
3,689 |
|
|
|
* |
% |
|
|
3,689 |
|
|
|
— |
|
| Hajime Machikawa(2) |
|
|
2,523 |
|
|
|
* |
% |
|
|
2,523 |
|
|
|
— |
|
| Michal Almog(2) |
|
|
1,977 |
|
|
|
* |
% |
|
|
1,977 |
|
|
|
— |
|
| Smadar Cohen(15) |
|
|
14,921 |
|
|
|
* |
% |
|
|
1,822 |
|
|
|
13,099 |
|
| Roi Shefts(2) |
|
|
2,715 |
|
|
|
* |
% |
|
|
2,715 |
|
|
|
— |
|
| Zvi Haimovitch.(2) |
|
|
1,534 |
|
|
|
* |
% |
|
|
1,534 |
|
|
|
— |
|
| Decker Holdings 1997 Ltd.(2) |
|
|
1,408 |
|
|
|
* |
% |
|
|
1,408 |
|
|
|
— |
|
| Irit Ran Cohen.(2) |
|
|
2,933 |
|
|
|
* |
% |
|
|
2,933 |
|
|
|
— |
|
| Meir Wilchek.(2) |
|
|
808 |
|
|
|
* |
% |
|
|
808 |
|
|
|
— |
|
| Vladislav Papper(2) |
|
|
643 |
|
|
|
* |
% |
|
|
643 |
|
|
|
— |
|
| Yehuda Shoenfeld(2) |
|
|
430 |
|
|
|
* |
% |
|
|
430 |
|
|
|
— |
|
| Avi Schroeder (16) |
|
|
3,928 |
|
|
|
* |
% |
|
|
311 |
|
|
|
3,617 |
|
| * | Indicates
beneficial ownership of less than 1% of the total Ordinary Shares outstanding. |
| ** |
Has indicated an interest in
purchasing Units in this offering. Certain of our existing shareholders, including beneficial owners of greater than 5% of our share
capital, have indicated an interest in purchasing up to an aggregate of approximately $1 million of Units in this offering, which
represents approximately 24% of the total Units in this offering, based on the public offering price of $4.38 per Unit. However,
because indications of interest are not binding agreements or commitments to purchase, the underwriters may determine to sell more,
less or no Units in this offering to any of these shareholders, or any of these shareholders may determine to purchase more, less
or no Units in this offering. The underwriters will receive the same underwriting discount on any Units purchased by these shareholders
as they will on any other Units sold to the public in this offering. |
| (1) |
Based on 3,235,542 Ordinary Shares issued and
outstanding immediately prior to the offering and not including any Ordinary Shares issued in our IPO. |
| (2) |
Consists of Ordinary Shares only. |
| |
|
| (3) |
Raul Srugo has expressed an interest in acquiring up
to $100,000 of Units in this offering, or 22,831 Units (based on the public offering price of $4.38 per Unit). If Raul Srugo acquires
22,831 Units in this offering, which is approximately 2.4% of the Units offered by us in this offering, they will beneficially own
approximately 8.9% of our Ordinary Shares after this offering. |
| |
|
| (4) |
Itzhak Srugo has expressed an interest in acquiring
up to $100,000 of Units in this offering, or 22,831 Units (based on the public offering price of $4.38 per Unit). If Itzhak Srugo
acquires 22,831 Units in this offering, which is approximately 2.4% of the Units offered by us in this offering, they will beneficially
own approximately 8.9% of our Ordinary Shares after this offering. |
| |
|
| (5) |
Yoav Srugo has expressed an interest in acquiring up
to $100,000 of Units in this offering, or 22,831 Units (based on the public offering price of $4.38 per Unit). If Yoav Srugo acquires
22,831 Units in this offering, which is approximately 2.4% of the Units offered by us in this offering, they will beneficially own
approximately 8.9% of our Ordinary Shares after this offering. |
| |
|
| (6) |
West East Pte Ltd. has expressed an interest in acquiring
up to $20,000 of Units in this offering, or 4,566 Units (based on the public offering price of $4.38 per Unit). If West East Pte
Ltd. acquires 4,566 Units in this offering, which is approximately 0.5% of the Units offered by us in this offering, they will beneficially
own approximately 1.1% of our Ordinary Shares after this offering. |
| |
|
| (7) |
Capitallink Ltd. has expressed an interest in acquiring
up to $32,000 of Units in this offering, or 7,306 Units (based on the public offering price of $4.38 per Unit). If Capitallink Ltd.
acquires 7,306 Units in this offering, which is approximately 0.8% of the Units offered by us in this offering, they will beneficially
own approximately 2.4% of our Ordinary Shares after this offering. |
| |
|
| (8) |
Gabriel Kabazo has expressed an interest in acquiring
up to $20,000 of Units in this offering, or 4,566 Units (based on the public offering price of $4.38 per Unit). If Gabriel Kabazo
acquires 4,566 Units in this offering, which is approximately 0.5% of the Units offered by us in this offering, they will beneficially
own approximately 1.2_% of our Ordinary Shares after this offering. |
| |
|
| (9) |
L.I.A Pure Capital Ltd. has expressed an interest in
acquiring up to $106,000 of Units in this offering, or 24,201 Units (based on the public offering price of $4.38 per Unit). If L.I.A
Pure Capital Ltd. acquires 24,201 Units in this offering, which is approximately 2.5% of the Units offered by us in this offering,
they will beneficially own approximately 6.5% of our Ordinary Shares after this offering. |
| |
|
| (10) |
Ronen Fatal has expressed an interest in acquiring
up to $15,000 of Units in this offering, or 3,425 Units (based on the public offering price of $4.38 per Unit). If Ronen Fatal acquires
3,425 Units in this offering, which is approximately 0.4% of the Units offered by us in this offering, they will beneficially own
approximately 0.9% of our Ordinary Shares after this offering. |
| |
|
| (11) |
E. G. Europe Property has expressed an interest in
acquiring up to $125,000 of Units in this offering, or 28,539 Units (based on the public offering price of $4.38 per Unit). If E.
G. Europe Property acquires 28,539 Units in this offering, which is approximately 3.0% of the Units offered by us in this offering,
they will beneficially own approximately 7.7% of our Ordinary Shares after this offering. |
| |
|
| (12) |
Itamar David has expressed an interest in acquiring
up to $20,000 of Units in this offering, or 4,566 Units (based on the public offering price of $4.38 per Unit). If Gabriel Kabazo
acquires 4,566 Units in this offering, which is approximately 0.5% of the Units offered by us in this offering, they will beneficially
own approximately 1.2% of our Ordinary Shares after this offering. |
| |
|
| (13) |
SciSparc Ltd. has expressed an interest in acquiring
up to $200,000 of Units in this offering, or 45,662 Units (based on the public offering price of $4.38 per Unit). If SciSparc Ltd.
acquires 45,662 Units in this offering, which is approximately 4.8% of the Units offered by us in this offering, they will beneficially
own approximately 8.7% of our Ordinary Shares after this offering. |
| |
|
| (14) |
Consists of 320,000 Ordinary Shares. In addition, SciSparc
will hold pre-funded warrants with a nominal exercise price, to purchase 364,931 Ordinary Shares, based on the public offering price
of $4.38 per Ordinary Share. The pre-funded warrants are subject to a beneficial ownership limitation of 9.99%, which such limitation
restricts the holder from exercising that portion of the warrants that would result in the holder and its affiliates owning, after
exercise, a number of Ordinary Shares in excess of the beneficial ownership limitation. In addition, SciSparc will hold 2,054,793
warrants to purchase 2,054,793 Ordinary Shares upon the same terms as the warrants to be issued in this offering. |
| |
|
| (15) |
Consists of (i) 1,822 Ordinary
Shares; and (ii) 13,099 options exercisable to Ordinary Shares that are exercisable within 60 days from the date of this prospectus. |
| (16) |
Consists of (i) 311 Ordinary Shares; and (ii) 3,617
options exercisable to Ordinary Shares that are exercisable within 60 days from the date of this prospectus. |
TAXATION
The
following description is not intended to constitute a complete analysis of all tax consequences relating to the ownership or disposition
of our Ordinary Shares. You should consult your own tax advisor concerning the tax consequences of your particular situation, as well
as any tax consequences that may arise under the laws of any state, local, foreign, including Israeli, or other taxing jurisdiction.
ISRAELI
TAX CONSIDERATIONS AND GOVERNMENT PROGRAMS
The
following is a description of the material Israeli income tax consequences of the ownership of our Ordinary Shares. The following also
contains a description of material relevant provisions of the current Israeli income tax structure applicable to companies in Israel,
with reference to its effect on us. To the extent that the discussion is based on new tax legislation which has not been subject to judicial
or administrative interpretation, there can be no assurance that the tax authorities will accept the views expressed in the discussion
in question. The discussion is not intended, and should not be taken, as legal or professional tax advice and is not exhaustive of all
possible tax considerations.
The
following description is not intended to constitute a complete analysis of all tax consequences relating to the ownership or disposition
of our Ordinary Shares. Shareholders should consult their own tax advisors concerning the tax consequences of their particular situation,
as well as any tax consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
General
Corporate Tax Structure in Israel
The
Company is subject to a corporate tax rate is 23%. However, the effective tax rate payable by a company that derives income from a Preferred
Enterprise (as discussed below) may be considerably less. Capital gains derived by an Israeli company are generally subject to the prevailing
corporate tax rate.
Capital
gains derived by an Israeli resident company are subject to tax at the prevailing corporate tax rate. Under Israeli tax legislation,
a corporation will be considered as an “Israeli resident company” if it meets one of the following: (i) it was incorporated
in Israel; or (ii) the control and management of its business are exercised in Israel.
Law
for the Encouragement of Industry (Taxes), 5729-1969
The
Law for the Encouragement of Industry (Taxes), 5729-1969, generally referred to as the Industry Encouragement Law, provides several tax
benefits for “Industrial Companies.”
The
Industry Encouragement Law defines an “Industrial Company” as an Israeli resident-company, of which 90% or more of its income
in any tax year, other than income from defense loans, is derived from an “Industrial Enterprise” owned by it. An “Industrial
Enterprise” is defined as an enterprise whose principal activity in a given tax year is industrial production.
The
following corporate tax benefits, among others, are available to Industrial Companies:
| |
● |
amortization
of the cost of purchased a patent, rights to use a patent, and know-how, which are used for the development or advancement of the
company, over an eight-year period, commencing on the year in which such rights were first exercised; |
| |
|
|
| |
● |
under limited conditions, an election to file consolidated
tax returns with related Israeli Industrial Companies; and |
| |
|
|
| |
● |
expenses related to a public offering are deductible
in equal amounts over three years. |
Eligibility
for benefits under the Industry Encouragement Law is not contingent upon approval of any governmental authority.
Tax
Benefits and Grants for Research and Development
Under
the Israeli Encouragement of Research, Development and Industrial Initiative Technology Law, 5744-1984, as amended, and related regulations,
or the Research Law, research and development programs which meet specified criteria and are approved by the IIA are eligible for grants
of up to 50% of the project’s expenditure, as determined by the research committee, in exchange for the payment of royalties from
the revenues generated from the sale of products and related services developed, in whole or in part pursuant to, or as a result of,
a research and development program funded by the IIA. The royalties are generally at a range of 3.0% to 5.0% of revenues until the entire
IIA grant is repaid, together with an annual interest generally equal to the 12 months London Interbank Offered Rate applicable to dollar
deposits that is published on the first business day of each calendar year.
The
terms of the Research Law also require that the manufacture of products developed with government grants be performed in Israel. The
transfer of manufacturing activity outside Israel may be subject to the prior approval of the IIA. Under the regulations of the Research
Law, assuming we receive approval from the IIA to manufacture our IIA-funded products outside Israel, we may be required to pay increased
royalties. The increase in royalties depends upon the manufacturing volume that is performed outside of Israel as follows:
| Manufacturing
Volume Outside of Israel | |
Royalties
to
the Chief
Scientist as a
Percentage
of Grant | |
| | |
| |
| Up to 50% | |
| 120 | % |
| Between 50% and 90% | |
| 150 | % |
| 90% and more | |
| 300 | % |
If
the manufacturing is performed outside of Israel by us, the rate of royalties payable by us on revenues from the sale of product candidates
manufactured outside of Israel will increase by 1% over the regular rates. If the manufacturing is performed outside of Israel by a third
party, the rate of royalties payable by us on those revenues will be equal to the ratio obtained by dividing the amount of the grants
received from the IIA and our total investment in the project that was funded by these grants. The transfer of no more than 10% of the
manufacturing capacity in the aggregate outside of Israel is exempt under the Research Law from obtaining the prior approval of the IIA.
A company requesting funds from the IIA also has the option of declaring in its IIA grant application an intention to perform part of
its manufacturing outside Israel, thus avoiding the need to obtain additional approval. On January 6, 2011, the Research Law was amended
to clarify that the potential increased royalties specified in the table above will apply even in those cases where the IIA approval
for transfer of manufacturing outside of Israel is not required, namely when the volume of the transferred manufacturing capacity is
less than 10% of total capacity or when the company received an advance approval to manufacture abroad in the framework of its IIA grant
application.
The
know-how developed within the framework of the IIA plan may not be transferred to third parties outside Israel without the prior approval
of a governmental committee charted under the Research Law. The approval, however, is not required for the export of any product candidates
developed using grants received from the IIA. The IIA approval to transfer know-how created, in whole or in part, in connection with
an IIA-funded project to third party outside Israel where the transferring company remains an operating Israeli entity is subject to
payment of a redemption fee to the IIA calculated according to a formula provided under the Research Law that is based, in general, on
the ratio between the aggregate IIA grants to the company’s aggregate investments in the project that was funded by these IIA grants,
multiplied by the transaction consideration. The transfer of such know-how to a party outside Israel where the transferring company ceases
to exist as an Israeli entity is subject to a redemption fee formula that is based, in general, on the ratio between the aggregate IIA
grants to the total financial investments in the company, multiplied by the transaction consideration. According to the January 2011
amendment, the redemption fee in case of transfer of know-how to a party outside Israel will be based on the ratio between the aggregate
IIA grants received by the company and the company’s aggregate R&D expenses, multiplied by the transaction consideration. According
to regulations promulgated following the 2011 amendment, the maximum amount payable to the IIA in case of transfer of know how outside
Israel shall not exceed 6 times the value of the grants received plus interest, and in the event that the receiver of the grants ceases
to be an Israeli corporation such payment shall not exceed six times the value of the grants received plus interest, with a possibility
to reduce such payment to up to three times the value of the grants received plus interest if the R&D activity remains in Israel
for a period of three years after payment to the IIA.
Transfer
of know-how within Israel is subject to an undertaking of the recipient Israeli entity to comply with the provisions of the Research
Law and related regulations, including the restrictions on the transfer of know-how and the obligation to pay royalties, as further described
in the Research Law and related regulations.
These
restrictions may impair our ability to outsource manufacturing, engage in change of control transactions or otherwise transfer our know-how
outside Israel and may require us to obtain the approval of the IIA for certain actions and transactions and pay additional royalties
to the IIA. In particular, any change of control and any change of ownership of our Ordinary Shares that would make a non-Israeli citizen
or resident an “interested party,” as defined in the Research Law, requires a prior written notice to the IIA in addition
to any payment that may be required of us for transfer of manufacturing or know-how outside Israel. If we fail to comply with the Research
Law, we may be subject to criminal charges.
Tax
Benefits for Research and Development
Israeli
tax law allows, under certain conditions, a tax deduction for expenditures, including capital expenditures, for the year in which they
are incurred. Expenditures are deemed related to scientific research and development projects, if:
| |
● |
The expenditures are approved by the relevant Israeli
government ministry, determined by the field of research; |
| |
|
|
| |
● |
The research and development must be for the promotion
of the company; and |
| |
|
|
| |
● |
The research and development is carried out by or on
behalf of the company seeking such tax deduction. |
The
amount of such deductible expenses is reduced by the sum of any funds received through government grants for the finance of such scientific
research and development projects. No deduction under these research and development deduction rules is allowed if such deduction is
related to an expense invested in an asset depreciable under the general depreciation rules of the income Tax Ordinance, 1961. Expenditures
not so approved are deductible in equal amounts over three years.
From
time to time we may apply to the IIA for approval to allow a tax deduction for all research and development expenses during the year
incurred. There can be no assurance that such application will be accepted.
Law
for the Encouragement of Capital Investments, 5719-1959
The
Law for the Encouragement of Capital Investments, 5719-1959, generally referred to as the Investment Law, provides certain incentives
for capital investments in production facilities (or other eligible assets).
Tax
Benefits
The
Investment Law grants tax benefits for income generated by a “Preferred Company” through its “Preferred Enterprise”
(as such terms are defined in the Investment Law) The definition of a Preferred Company includes a company incorporated in Israel that
is not fully owned by a governmental entity, and that has, among other things, Preferred Enterprise status and is controlled and managed
from Israel. A Preferred Company is entitled to a reduced corporate tax rate of 16% with respect to its income derived by its Preferred
Enterprise, unless the Preferred Enterprise is located in a specified development zone, in which case the rate will be 7.5%.
Dividends
paid out of income attributed to a Preferred Enterprise are generally subject to withholding tax at source at the rate of 20% or such
lower rate as may be provided in an applicable tax treaty. However, if such dividends are paid to an Israeli company, no tax is required
to be withheld.
Taxation
of our Shareholders
Capital
Gains Taxes Applicable to Non-Israeli Resident Shareholders. A non-Israeli resident who derives capital gains from the sale of shares
in an Israeli resident company will be exempt from Israeli tax so long as the shares were not held through a permanent establishment
that the non-resident maintains in Israel. However, non-Israeli corporations will not be entitled to the foregoing exemption if Israeli
residents: (i) have a controlling interest of 25% or more in such non-Israeli corporation or (ii) are the beneficiaries of, or are entitled
to, 25% or more of the revenues or profits of such non-Israeli corporation, whether directly or indirectly.
Additionally,
a sale of securities by a non-Israeli resident may be exempt from Israeli capital gains tax under the provisions of an applicable tax
treaty. For example, under Convention Between the Government of the United States of America and the Government of the State of Israel
with respect to Taxes on Income, as amended, or the United States-Israel Tax Treaty, the sale, exchange or other disposition of shares
by a shareholder who is a United States resident (for purposes of the treaty) holding the shares as a capital asset and is entitled to
claim the benefits afforded to such a resident by the U.S.-Israel Tax Treaty, or a Treaty U.S. Resident, is generally exempt from Israeli
capital gains tax unless: (i) the capital gain arising from such sale, exchange or disposition is attributed to real estate located in
Israel; (ii) the capital gain arising from such sale, exchange or disposition is attributed to royalties; (iii) the capital gain arising
from the such sale, exchange or disposition is attributed to a permanent establishment in Israel, under certain terms; (iv) such Treaty
U.S. Resident holds, directly or indirectly, shares representing 10% or more of the voting capital during any part of the 12-month period
preceding the disposition, subject to certain conditions; or (v) such Treaty U.S. Resident is an individual and was present in Israel
for 183 days or more during the relevant taxable year.
In
some instances where our shareholders may be liable for Israeli tax on the sale of their Ordinary Shares, the payment of the consideration
may be subject to the withholding of Israeli tax at source. Shareholders may be required to demonstrate that they are exempt from tax
on their capital gains in order to avoid withholding at source at the time of sale.
Taxation
of Non-Israeli Shareholders on Receipt of Dividends. Non-Israeli residents are generally subject to Israeli income tax on the receipt
of dividends paid on our Ordinary Shares at the rate of 25%, which tax will be withheld at source, unless relief is provided in a treaty
between Israel and the shareholder’s country of residence. With respect to a person who is a “substantial shareholder”
at the time of receiving the dividend or on any time during the preceding twelve months, the applicable tax rate is 30%. A “substantial
shareholder” is generally a person who alone or together with such person’s relative or another person who collaborates with
such person on a permanent basis, holds, directly or indirectly, at least 10% of any of the “means of control” of the corporation.
“Means of control” generally include the right to vote, receive profits, nominate a director or an executive officer, receive
assets upon liquidation, or order someone who holds any of the aforesaid rights how to act, regardless of the source of such right. However,
a distribution of dividends to non-Israeli residents is subject to withholding tax at source at a rate of 20% if the dividend is distributed
from income attributed to a Preferred Enterprise, unless a reduced tax rate is provided under an applicable tax treaty. For example,
under the United States-Israel Tax Treaty, the maximum rate of tax withheld at source in Israel on dividends paid to a holder of our
Ordinary Shares who is a Treaty U.S. Resident is 25%. However, generally, the maximum rate of withholding tax on dividends, not generated
by a Preferred Enterprise, that are paid to a United States corporation holding 10% or more of the outstanding voting capital throughout
the tax year in which the dividend is distributed as well as during the previous tax year, is 12.5%, provided that not more than 25%
of the gross income for such preceding year consists of certain types of dividends and interest. Notwithstanding the foregoing, dividends
distributed from income attributed to an Preferred Enterprise are not entitled to such reduction under the tax treaty but are subject
to a withholding tax rate of 15% for a shareholder that is a U.S. corporation, provided that the condition related to our gross income
for the previous year (as set forth in the previous sentence) is met. If the dividend is attributable partly to income derived from a
Preferred Enterprise, and partly to other sources of income, the withholding rate will be a blended rate reflecting the relative portions
of the two types of income. We cannot assure you that we will designate the profits that we may distribute in a way that will reduce
shareholders’ tax liability.
CERTAIN
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS
THE
FOLLOWING SUMMARY IS NOT INTENDED TO BE, AND SHOULD NOT BE CONSIDERED TO BE, LEGAL OR TAX ADVICE TO ANY PARTICULAR INVESTOR. EACH PROSPECTIVE
INVESTOR SHOULD CONSULT WITH HIS OR HER OWN TAX ADVISOR AS TO THE PERSONAL U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP
AND SALE OF THE EQUITY SECURITIES OFFERED BY THIS PROSPECTUS, INCLUDING THE EFFECTS OF APPLICABLE STATE, LOCAL, FOREIGN OR OTHER TAX
LAWS AND POSSIBLE CHANGES IN THE TAX LAWS.
Subject
to the limitations described in the next two paragraphs, the following discussion summarizes
the material U.S. federal income tax consequences to a “U.S. Holder” arising
from the purchase, ownership and sale of the Ordinary Shares and Warrants being offered by
this prospectus, which we collectively refer to as “Equity Securities”.
For this purpose, a “U.S. Holder” is a holder of Equity Securities that is: (1)
an individual citizen or resident of the United States, including an alien individual who
is a lawful permanent resident of the United States or meets the substantial presence residency
test under U.S. federal income tax laws; (2) a corporation (or entity treated as a corporation
for U.S. federal income tax purposes) or a partnership (other than a partnership that is
not treated as a U.S. person under any applicable U.S. Treasury regulations) created or organized
under the laws of the United States or the District of Columbia or any political subdivision
thereof; (3) an estate, the income of which is includable in gross income for U.S. federal
income tax purposes regardless of its source; (4) a trust if a court within the United States
is able to exercise primary supervision over the administration of the trust and one or more
U.S. persons have authority to control all substantial decisions of the trust; or (5) a trust
that has a valid election in effect to be treated as a U.S. person to the extent provided
in U.S. Treasury regulations.
This
summary does not purport to be a comprehensive description of all of the U.S. federal income tax considerations that may be relevant
to a decision to purchase our Equity Securities. This summary generally considers only U.S. Holders that will own our Equity Securities
as capital assets. Except to the limited extent discussed below, this summary does not consider the U.S. federal tax consequences to
a person that is not a U.S. Holder, nor does it describe the rules applicable to determine a taxpayer’s status as a U.S. Holder.
This summary is based on the provisions of the Internal Revenue Code of 1986, as amended, or the Code, final, temporary and proposed
U.S. Treasury regulations promulgated thereunder, administrative and judicial interpretations thereof, and the United
States-Israel Tax Treaty, all as in effect as of the date hereof and all of which are subject to change, possibly on a retroactive
basis, and all of which are open to differing interpretations. This summary does not discuss the potential effects, whether adverse or
beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive basis. We will not seek a ruling from the
Internal Revenue Service, or the IRS, with regard to the U.S. federal income tax treatment of an investment in our Equity Securities
by U.S. Holders and, therefore, can provide no assurances that the IRS will agree with the conclusions set forth below.
This
discussion does not address all of the aspects of U.S. federal income taxation that may be relevant to a particular U.S. holder based
on such holder’s particular circumstances and in particular does not discuss any estate, gift, generation-skipping transfer, state,
local, excise or non-U.S. tax considerations. In addition, this discussion does not address the U.S. federal income tax treatment of
a U.S. Holder who is: (1) a bank, life insurance company, regulated investment company, or other financial institution or “financial
services entity;” (2) a broker or dealer in securities or foreign currency; (3) a person who acquired our Equity Securities in
connection with employment or other performance of services; (4) a U.S. Holder that is subject to the U.S. alternative minimum tax; (5)
a U.S. Holder that holds our Equity Securities as a hedge or as part of a hedging, straddle, conversion or constructive sale transaction
or other risk-reduction transaction for U.S. federal income tax purposes; (6) a tax-exempt entity; (7) real estate investment trusts
or grantor trusts; (8) a U.S. Holder that expatriates out of the United States or a former long-term resident of the United States; or
(9) a person having a functional currency other than the U.S. dollar. This discussion does not address the U.S. federal income tax treatment
of a U.S. Holder that owns, directly, indirectly or constructively, at any time, Ordinary Shares representing 10% or more of the stock
(by vote or value) of our Company. Additionally, the U.S. federal income tax treatment of partnerships (or other pass-through entities)
or persons who hold ordinary shares through a partnership or other pass-through entity are not addressed.
Each
prospective investor is advised to consult his or her own tax adviser for the specific tax consequences to that investor of purchasing,
holding or disposing of our EQUITY securities, including the effects of applicable state, local, NON-U.S. or other tax laws and possible
changes in the tax laws.
Allocation
of Purchase Price Between Ordinary Shares and Accompanying Warrants
For
U.S. federal income tax purposes, with respect to each Unit, the Ordinary Shares and Warrants acquired in this prospectus will be treated
as an “investment unit” consisting of one Ordinary Share and three Warrants, with each Warrant exercisable into one Ordinary
Share. The purchase price for each investment unit will be allocated between these two components in proportion to their relative fair
market values at the time the Unit is purchased by the U.S. Holder. This allocation of the purchase price for each Unit will establish
the U.S. Holder’s initial tax basis for U.S. federal income tax purposes in each security included in each Unit. The separation
of components of each Unit should not be a taxable event for U.S. federal income tax purposes.
Exercise
and Expiration of Warrants
In
general, a U.S. Holder will not recognize gain or loss for U.S. federal income tax purposes upon the exercise of Warrants into Ordinary
Shares. The U.S. federal income tax treatment of a cashless exercise of Warrants into our Ordinary Shares is unclear. U.S. Holders should
consult their tax advisors regarding the U.S. federal income tax consequences of a cashless exercise of Warrants.
The
expiration of a Warrant will be treated as if the U.S. Holder sold or exchanged the Warrant and recognized a capital loss equal to the
U.S. Holder’s tax basis in the Warrant.
Certain
Adjustments to the Warrants
Under
Section 305 of the Code, an adjustment to the number of Ordinary Shares issued on the exercise of the Warrants, or an adjustment to the
exercise price of the Warrants, may be treated as a constructive distribution to a U.S. Holder of the Warrants if, and to the extent
that, such adjustment has the effect of increasing such U.S. Holder’s proportionate interest in our “earnings and profits”
or assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of
cash or other property to our shareholders). An adjustment to the Warrants that could result in a constructive distribution to a U.S.
Holder would be treated as described under “Taxation of Dividends Paid on Ordinary Shares” below, and the tax treatment of
distributions on the Warrants is unclear. Any resulting withholding tax attributable to deemed dividends would be collected from other
amounts payable or distributable to the U.S. Holder.
Taxation
of Dividends Paid on Ordinary Shares
We
do not intend to pay dividends in the foreseeable future. In the event that we do pay dividends, and subject to the discussion under
the heading “Passive Foreign Investment Companies” below and the discussion of “qualified dividend income” below,
a U.S. Holder, other than certain U.S. Holders that are U.S. corporations, will be required to include in gross income as ordinary income
the amount of any distribution paid on the Ordinary Shares (including the amount of any Israeli tax withheld on the date of the distribution),
to the extent that such distribution does not exceed our current and accumulated earnings and profits, as determined for U.S. federal
income tax purposes. The amount of a distribution that exceeds our earnings and profits will be treated first as a non-taxable return
of capital, reducing the U.S. Holder’s tax basis for the Ordinary Shares to the extent thereof, and then capital gain. We do not
expect to maintain calculations of our earnings and profits under U.S. federal income tax principles and, therefore, U.S. Holders should
expect that the entire amount of any distribution generally will be reported as dividend income.
In
general, preferential tax rates for “qualified dividend income” and long-term capital gains are applicable for U.S. Holders
that are individuals, estates or trusts. For this purpose, “qualified dividend income” means, inter alia, dividends received
from a “qualified foreign corporation.” A “qualified foreign corporation” is a corporation that is entitled to
the benefits of a comprehensive tax treaty with the United States that includes an exchange of information program. The IRS has stated
that the United States-Israel Tax Treaty satisfies this requirement and we believe we are
eligible for the benefits of that treaty. We generally will be treated as a qualified foreign corporation if we are not a PFIC in the
taxable year in which such dividends are paid or in the preceding taxable year (see discussion below), and (i) we are eligible for benefits
under the United States-Israel income tax treaty or (ii) our Ordinary Shares are listed on an established securities market in the United
States (which includes the Nasdaq Global Market).
In
addition, our dividends will be qualified dividend income if our Ordinary Shares are readily tradable on Nasdaq or another established
securities market in the United States. Dividends will not qualify for the preferential rate if we are treated, in the year the dividend
is paid or in the prior year, as a PFIC, as described below under “Passive Foreign Investment Companies.” A U.S. Holder will
not be entitled to the preferential rate: (1) if the U.S. Holder has not held our Ordinary Shares for at least 61 days of the 121 day
period beginning on the date which is 60 days before the ex-dividend date, or (2) to the extent the U.S. Holder is under an obligation
to make related payments with respect to positions in substantially similar or related property. Any days during which the U.S. Holder
has diminished its risk of loss on our Ordinary Shares are not counted towards meeting the 61-day holding period. Finally, U.S. Holders
who elect to treat the dividend income as “investment income” pursuant to Section 163(d)(4) of the Code will not be eligible
for the preferential rate of taxation.
The
amount of a distribution with respect to our Ordinary Shares will be measured by the amount of the fair market value of any property
distributed, and for U.S. federal income tax purposes, the amount of any Israeli taxes withheld therefrom. Cash distributions paid by
us in NIS, if any, will be included in the income of U.S. Holders at a U.S. dollar amount based upon the spot rate of exchange in effect
on the date the dividend is includible in the income of the U.S. Holder, and U.S. Holders will have a tax basis in such NIS for U.S.
federal income tax purposes equal to such U.S. dollar value. If the U.S. Holder subsequently converts the NIS into U.S. dollars or otherwise
disposes of them, any subsequent gain or loss in respect of such NIS arising from exchange rate fluctuations will be U.S. source ordinary
exchange gain or loss.
Subject
to certain significant conditions and limitations, any Israeli taxes paid on or withheld from distributions from us and not refundable
to a U.S. Holder may be credited against the U.S. Holder’s U.S. federal income tax liability or, alternatively, may be deducted
from the U.S. Holder’s taxable income. However, as a result of recent changes to the U.S. foreign tax credit rules, a withholding
tax generally may need to satisfy certain additional requirements in order to be considered a creditable tax for a U.S. Holder. We have
not determined whether these requirements have been met and, accordingly, no assurance can be given that any withholding tax on dividends
paid by us will be creditable. The election to deduct, rather than credit, foreign taxes, is made on a year-by-year basis and applies
to all foreign taxes paid by a U.S. Holder or withheld from a U.S. Holder that year Dividends paid with respect to our Ordinary Shares
will be treated as foreign source income, which may be relevant in calculating the holder’s foreign tax credit limitation. The
limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose,
dividends that we distribute generally should constitute “passive category income,” or, in the case of certain U.S. Holders,
“general category income.” The rules relating to the determination of the foreign tax credit are complex, and U.S. Holders
should consult their tax advisor to determine whether and to what extent such holder will be entitled to this credit. Dividends paid
with respect to our ordinary shares will not be eligible for the “dividends-received” deduction generally allowed to corporate
U.S. Holders with respect to dividends received from U.S. corporations.
Taxation
of the Sale, Exchange or other Disposition of Equity Securities
Except
as provided under the PFIC rules described below under “Passive Foreign Investment Companies,” upon the sale, exchange or
other disposition of our Equity Securities, a U.S. Holder will recognize capital gain or loss in an amount equal to the difference between
such U.S. Holder’s tax basis for the Equity Securities, determined in U.S. dollars, and the U.S. dollar value of the amount realized
on the disposition (or its U.S. dollar equivalent determined by reference to the spot rate of exchange on the date of disposition, if
the amount realized is denominated in a foreign currency). The gain or loss realized on the sale, exchange or other disposition of Equity
Securities will be long-term capital gain or loss if the U.S. Holder has a holding period of more than one year at the time of the disposition.
Individuals who recognize long-term capital gains may be taxed on such gains at reduced rates of tax. The deduction of capital losses
is subject to various limitations. U.S. Holders should consult their own tax advisors regarding the U.S. federal income tax consequences
of receiving currency other than U.S. dollars upon the disposition of their ordinary shares.
Passive
Foreign Investment Companies
Generally,
a non-U.S. corporation will be a PFIC for U.S. federal income tax purposes in any taxable year in which either (i) 75% or more of
its gross income for such year consists of certain types of “passive” income or (ii) 50% or more of the average fair
market value of its assets during such year (based on quarterly valuations) produce or are held for the production of passive income.
Passive income for this purpose generally includes dividends, interest, rents, royalties, annuities, income from certain commodities
transactions and from notional principal contracts, and the excess of gains over losses from the disposition of assets that produce passive
income. Passive income also includes amounts derived by reason of the temporary investment of funds, including those raised in a public
offering. Assets that produce or are held for the production of passive income may include cash, even if held as working capital or raised
in a public offering, as well as marketable securities, and other assets that may produce passive income. In determining whether a non-U.S.
corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at
least a 25% interest (by value) is taken into account.
A
foreign corporation’s PFIC status is an annual determination that is based on tests that are factual in nature, and our PFIC status
for any year will depend on the composition of our income, fair market value of our assets, and our activities for such year. Based on
the projected composition of our income and valuation of our assets, we do not believe that we were a PFIC for 2023, and do not expect
to be a PFIC for 2024 and in the future, although there can be no assurance in this regard. Because PFIC status is based on our
income, assets and activities for the entire taxable year, and our market capitalization, it is not possible to determine whether we
will be characterized as a PFIC for the December 31, 2024 taxable year until after the close of the taxable year.
If
we were a PFIC for any taxable year during which a U.S. holder held Ordinary Shares, then unless an election has been made by a U.S.
holder to be taxed under one of the alternative regimes discussed below, gain recognized by a U.S. holder on a sale or other disposition
(including certain pledges) of our Ordinary Shares would be allocated ratably over the U.S. holder’s holding period for our Ordinary
Shares. The amounts allocated to the taxable year of the sale or other disposition and to any year before we became a PFIC would be taxed
as ordinary income. The amount allocated to each other taxable year would be subject to tax at the highest rate in effect for individuals
or corporations, as appropriate, for that taxable year, and an interest charge would be imposed on the amount allocated to that taxable
year. Similar rules would apply to any distribution with respect to our Ordinary Shares in excess of 125% of the average of the annual
distributions received by a U.S. holder during the preceding three years or such U.S. holder’s holding period, whichever is shorter.
In addition, non-corporate U.S. holders will not be eligible for reduced rates of taxation on any dividends received from us if we are
a PFIC in the taxable year in which such dividends are paid or in the preceding taxable year.
If
we are treated as a PFIC for any taxable year during the holding period of a non-electing U.S. holder (i.e., a U.S. holder that
does not elect to be taxed under one of the alternative regimes discussed below), we will continue to be treated as a PFIC for all succeeding
years during which such non-electing U.S. holder is treated as a direct or indirect holder even if we are not a PFIC for such years.
A U.S. holder is encouraged to consult its tax advisor with respect to any available elections that may be applicable in such a situation,
including the “deemed sale” election of Section 1298(b)(1) of the Code.
Notwithstanding
the default PFIC rules described in the preceding paragraphs, certain elections may be available that would result in alternative tax
consequences; i.e., the “qualified electing fund” or “QEF” election and the “mark to market”
election. If a U.S. holder makes a timely and valid mark-to-market election, the U.S. holder generally will recognize as ordinary income
any excess of the fair market value of our Ordinary Shares at the end of each taxable year over their adjusted tax basis, and will recognize
an ordinary loss in respect of any excess of the adjusted tax basis of our Ordinary Shares over their fair market value at the end of
the taxable year (but only to the extent of the net amount of income previously included as a result of the mark-to-market election).
The U.S. holder’s tax basis in our Ordinary Shares will be adjusted to reflect the income or loss resulting from the mark-to-market
election. Any gain recognized on the sale or other disposition of Ordinary Shares in a year when we are a PFIC will be treated as ordinary
income and any loss will be treated as an ordinary loss (but only to the extent of the net amount of income previously included as a
result of the mark-to-market election and any loss in excess of such amount will be treated as capital loss). The mark-to-market election
is available only if we are a PFIC and our Ordinary Shares are “regularly traded” on a “qualified exchange” within
the meaning of applicable U.S. Treasury regulations. Our Ordinary Shares will be treated as “regularly traded” in any calendar
year in which more than a de minimis quantity of our Ordinary Shares are traded on a qualified exchange on at least 15 days during
each calendar quarter. Although the IRS has not published any authority identifying specific exchanges that may constitute “qualified
exchanges,” Treasury Regulations provide that a qualified exchange is (i) a U.S. securities exchange that is registered with
the Securities and Exchange Commission, (ii) the U.S. market system established pursuant to Section 11A of the Securities and
Exchange Act of 1934, or (iii) a non-U.S. securities exchange that is regulated or supervised by a governmental authority of the
country in which the market is located, provided that: (a) such non-U.S. exchange has trading volume, listing, financial disclosure,
surveillance, and other requirements designed to prevent fraudulent and manipulative acts and practices, to remove impediments to and
perfect the mechanism of a free and open, fair and orderly, market, and to protect investors, and the laws of the country in which such
non-U.S. exchange is located and the rules of such non-U.S. exchange ensure that such requirements are actually enforced; and (b) the
rules of such non-U.S. exchange effectively promote active trading of listed shares. The Nasdaq Global Market is a qualified exchange
for this purpose, but there can be no assurance that the trading in our Ordinary Shares will be sufficiently regular to qualify our Ordinary
Shares as marketable stock. A mark-to-market election will not apply to Ordinary Shares held by a U.S. holder for any taxable year during
which we are not a PFIC, but will remain in effect with respect to any subsequent taxable year in which we become a PFIC unless our Ordinary
Shares are no longer regularly traded on a qualified exchange or the IRS consents to the revocation of the election. Such election will
not apply to any PFIC subsidiary that we own. Each U.S. holder is encouraged to consult its own tax advisor with respect to the availability
and tax consequences of a mark-to-market election with respect to our Ordinary Shares.
Another
way in which certain of the adverse consequences of PFIC status can be mitigated is for a U.S. holder to make a QEF election. Generally,
a shareholder making the QEF election is required for each taxable year to include in income a pro rata share of our ordinary earnings
and net capital gain of the QEF, subject to a separate election to defer payment of taxes, which deferral is subject to an interest charge.
An election to treat us as a QEF will not be available if we do not provide the information necessary to make such an election. We are
not obligated and do not currently intend to provide the information necessary to make a QEF election and thus it is not expected that
a QEF election will be available for U.S. holders of our Ordinary Shares if we were a PFIC in any prior year, the current year or any
future year.
U.S.
holders should consult their tax advisors to determine under what circumstances these elections would be available and, if available,
what the consequences of the alternative treatments would be in their particular circumstances.
If
a U.S. holder holds Ordinary Shares in any year in which we are treated as a PFIC, the U.S. holder will be required to file IRS Form 8621
and may be subject to certain other information reporting requirements.
The
U.S. federal income tax rules relating to PFICs are complex. U.S. holders are urged to consult their own tax advisors with respect to
the consequences to them of an investment in a PFIC, any elections available with respect to our Ordinary Shares and the IRS information
reporting obligations with respect to the purchase, ownership, and disposition of our Ordinary Shares in the event we are determined
to be a PFIC.
Tax
on Net Investment Income
U.S.
Holders who are individuals, estates or trusts will generally be required to pay a 3.8% Medicare tax on their net investment income (including
dividends on and gains from the sale or other disposition of our Equity Securities), or in the case of estates and trusts on their net
investment income that is not distributed to beneficiaries of the estate or trust. In each case, the 3.8% Medicare tax applies only to
the extent the U.S. Holder’s total adjusted income exceeds applicable thresholds.
Information
Reporting and Withholding
A
U.S. Holder may be subject to backup withholding at a rate of 24% with respect to cash dividends and proceeds from a disposition of our
Equity Securities. In general, backup withholding will apply only if a U.S. Holder fails to comply with specified identification procedures.
Backup withholding will not apply with respect to payments made to designated exempt recipients, such as corporations and tax-exempt
organizations. Backup withholding is not an additional tax and may be claimed as a credit against the U.S. federal income tax liability
of a U.S. Holder, provided that the required information is timely furnished to the IRS.
Certain
U.S. Holders with interests in “specified foreign financial assets” (including, among other assets, our Equity Securities,
unless such Equity Securities are held on such U.S. Holder’s behalf through a financial institution) may be required to file an
information report with the IRS if the aggregate value of all such assets exceeds $50,000 on the last day of the taxable year or $75,000
at any time during the taxable year (or such higher dollar amount as may be prescribed by applicable IRS guidance). You should consult
your own tax advisor as to the possible obligation to file such information report.
THE
DISCUSSION ABOVE IS A GENERAL SUMMARY AND IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL TAX CONSEQUENCES RELATING TO THE PURCHASE,
OWNERSHIP AND DISPOSITION OF OUR ORDINARY SHARES. IT DOES NOT COVER ALL TAX MATTERS THAT MAY BE OF IMPORTANCE TO A PROSPECTIVE INVESTOR.
EACH PROSPECTIVE INVESTOR IS URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT RELATING TO THE PURCHASE, OWNERSHIP,
AND DISPOSITION OF ORDINARY SHARES IN LIGHT OF THE INVESTOR’S OWN CIRCUMSTANCES.
UNDERWRITING
Aegis
Capital Corp., or Aegis, is acting as the underwriter and the book-running manager of this
offering. Under the terms of an underwriting agreement, which is filed as an exhibit to the
registration statement, the underwriter named below has agreed to purchase from us the number
of Units shown opposite its name below:
| Underwriter | |
Number
of
Units | |
| Aegis Capital Corp. | |
| 958,903 | |
The
underwriting agreement provides that the underwriter’s obligation to purchase Units
depends on the satisfaction of the conditions contained in the underwriting agreement including:
| |
● |
the representations and warranties
made by us to the underwriter are true; |
| |
|
|
| |
● |
there is no material change
in our business or the financial markets; and |
| |
|
|
| |
● |
we deliver customary closing
documents to the underwriter. |
Underwriting
Commissions and Discounts and Expenses
The
following table shows the per Unit and total underwriting discounts and commissions we will pay to Aegis. These amounts are shown assuming
both no exercise and full exercise of the underwriter’s option to purchase additional securities.
| | |
| | |
Total | |
| | |
Per
Unit | | |
No Exercise | | |
Full
Exercise | |
| Public offering
price | |
$ | 4.38 | | |
$ | 4,199,995 | | |
$ | 4,829,994 | |
| Underwriting
discounts and commissions to be paid by us (8%): | |
$ | 0.35 | | |
$ | 336,000 | | |
$ | 386,400 | |
| Non-accountable
expense allowance (1%)(1) | |
$ | 0.04 | | |
$ | 42,000 | | |
$ | 48,300 | |
| Proceeds,
before expenses, to us | |
$ | 3.99 | | |
$ | 3,821,995 | | |
$ | 4,395,294 | |
| (1) | We
have agreed to pay a non-accountable expense allowance to the underwriter equal to 1% of
the gross proceeds we received in this offering. |
We
have also agreed to reimburse the underwriter for certain of their expenses, including “roadshow”, diligence, and reasonable
legal fees and disbursements, in an amount not to exceed $125,000 in the aggregate.
We
have also agreed to pay a banking management fee to the underwriter equal to 0.5% of the gross proceeds we receive in this offering.
We
estimate that the total expenses of the offering payable by us, excluding underwriting discounts and commissions, will be approximately
$225,000.
Over-Allotment
Option
We
have granted the underwriter an option to purchase from us, up to an additional 143,835 Ordinary
Shares, and/or up to an additional 431,505 Warrants, within 45 days from the date of this
prospectus to cover over-allotments, if any. The underwriter may exercise the over-allotment
option with respect to Ordinary Shares only, Warrants only, or any combination thereof. The
purchase price to be paid per additional Ordinary Share will be equal to the public offering
price of one Unit (less $0.01 allocated to the Warrants), as applicable, less the underwriting
discount, and the purchase price to be paid per additional over-allotment Warrant will be
$0.01. We will be obligated, pursuant to the option, to sell these additional Ordinary Shares
or Warrants to the underwriter to the extent the option is exercised. If any additional Ordinary
Shares or Warrants are purchased, the underwriter will offer the additional Ordinary Shares
and Warrants on the same terms as those on which the other Ordinary Shares and Warrants are
being offered hereunder. If the underwriter exercises the option in full, our total proceeds,
before expenses except underwriter fees, will be approximately $4.246 million. The total
additional proceeds to us, before expenses, if the underwriter exercises the option in full
for Warrants only, will be $45,433 (based upon the public offering price of $4.38 per Unit).
Discretionary
Accounts
The
underwriter does not intend to confirm sales of the securities offered hereby to any accounts over which it has discretionary authority.
Lock-Up
Agreements
Pursuant
to certain “lock-up” agreements, the Company, its executive officers, employees, directors and holders of at least 10% of
the Company’s outstanding Ordinary Shares and securities exercisable for or convertible into its Ordinary Shares outstanding immediately
prior to the closing of this offering, have agreed, subject to certain exceptions, not to offer, sell, assign, transfer, pledge, contract
to sell, or otherwise dispose of or announce the intention to otherwise dispose of, or enter into any swap, hedge or similar agreement
or arrangement that transfers, in whole or in part, the economic risk of ownership of, directly or indirectly, engage in any short selling
of any Ordinary Shares or securities convertible into or exchangeable or exercisable for any Ordinary Shares, whether currently owned
or subsequently acquired, without the prior written consent of the underwriter, for a period of 180 days from the closing date of the
offering.
Security
Issuance Standstill
For
18 months after the closing date of the Offering, the Company shall not effect or enter into an agreement to effect any issuances of
Ordinary Shares or securities convertible or exercisable into Ordinary Shares involving an at-the-market offering or variable rate transaction.
In no event shall any transaction within these 18 months period result in the sale of securities at an effective offering price less
than that of the public offering price of the Units.
Right
of First Refusal
We
have granted the underwriter a right of first refusal, for a period of three years from the consummation of this offering, to act as
sole book-runner, sole manager, sole placement agent, sole agent, sole book-runner, sole book-running manger and/or sole underwriter,
at the underwriter’s sole discretion, for each and every future public and private equity or debt offering or debt refinancing,
including all equity linked financings, or each, a Subject Transaction, during such three year period, of the Company, or any successor
to or subsidiary of the Company, on terms and conditions customary to the underwriter for such Subject Transactions.
Electronic
Offer, Sale and Distribution of Shares
A
prospectus in electronic format may be made available on the websites maintained by the underwriter, if any, participating in this offering
and the underwriter participating in this offering may distribute prospectuses electronically. The underwriter may agree to allocate
a number of shares for sale to its online brokerage account holders. Internet distributions will be allocated by the underwriter that
will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information
on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus
forms a part, has not been approved or endorsed by us or the underwriter in its capacity as underwriter, and should not be relied upon
by investors.
Stabilization
In
connection with this offering, the underwriter may engage in stabilizing transactions, over-allotment transactions, syndicate-covering
transactions, penalty bids and purchases to cover positions created by short sales.
| |
● |
Stabilizing transactions permit bids to purchase shares
so long as the stabilizing bids do not exceed a specified maximum and are engaged in for the purpose of preventing or retarding a
decline in the market price of the shares while the offering is in progress. |
| |
|
|
| |
● |
Over-allotment
transactions involve sales by the underwriter of shares in excess of the number of shares the underwriter is obligated to purchase.
This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered
short position, the number of shares over-allotted by the underwriter is not greater than the number of shares that it may purchase
in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in
the over-allotment option. The underwriter may close out any short position by exercising its over-allotment option and/or purchasing
shares in the open market. |
| |
|
|
| |
● |
Syndicate
covering transactions involve purchases of shares in the open market after the distribution has been completed in order to cover
syndicate short positions. In determining the source of shares to close out the short position, the underwriter will consider,
among other things, the price of shares available for purchase in the open market as compared with the price at which it may
purchase shares through exercise of the over- allotment option. If the underwriter sells more shares than could be covered by
exercise of the over-allotment option and, therefore, have a naked short position, the position can be closed out only by buying
shares in the open market. A naked short position is more likely to be created if the underwriter is concerned that after pricing
there could be downward pressure on the price of the shares in the open market that could adversely affect investors who purchase
in the offering. |
| |
● |
Penalty bids permits the underwriter to reclaim a selling
concession from a syndicate member when the shares originally sold by that syndicate member are purchased in stabilizing or syndicate
covering transactions to cover syndicate short positions. |
These
stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price
of the Company’s Ordinary Shares or preventing or retarding a decline in the market price of its Ordinary Shares. As a result,
the price of the Company’s Ordinary Shares or warrants in the open market may be higher than it would otherwise be in the absence
of these transactions. Neither the Company nor the underwriter make any representation or prediction as to the effect that the transactions
described above may have on the price of the Company’s Ordinary Shares. These transactions may be effected on Nasdaq, in the over-the-counter
market or otherwise and, if commenced, may be discontinued at any time.
Passive
Market Making
In
connection with this offering, the underwriter may engage in passive market making transactions in the Company’s Ordinary Shares
on Nasdaq in accordance with Rule 103 of Regulation M under the Exchange Act, during a period before the commencement of offers or sales
of the shares and extending through the completion of the distribution. A passive market maker must display its bid at a price not in
excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s
bid, then that bid must then be lowered when specified purchase limits are exceeded.
Other
Relationships
The
underwriter and its respective affiliates may, in the future provide various investment banking, commercial banking and other financial
services for the Company and its affiliates for which they have received, and may in the future receive, customary fees. However, except
as disclosed in this prospectus, the Company has no present arrangements with the underwriter for any further services.
Offer
Restrictions Outside the United States
Other
than in the United States, no action has been taken by us or the underwriter that would permit a public offering of the securities offered
by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be
offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with
the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result
in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are
advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus.
This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in
any jurisdiction in which such an offer or a solicitation is unlawful.
EXPENSES
Set
forth below is an itemization of the total expenses, excluding underwriting discounts, expected to be incurred in connection with the
offer and sale of the securities offered by us and the Selling Shareholders. With the exception of the SEC registration fee and
the FINRA filing fee, all amounts are estimates:
| SEC registration fee | |
$ | 5,452 | |
| Nasdaq listing fee | |
$ | 50,000 | |
| FINRA filing fee | |
$ | 10,000 | |
| Printer fees and expenses | |
$ | 20,000 | |
| Legal fees and expenses | |
$ | 150,000 | |
| Accounting fees and expenses | |
$ | 30,000 | |
| Miscellaneous | |
$ | 147,548 | |
| Total | |
$ | 413,000 | |
LEGAL
MATTERS
The
validity of the issuance of our Ordinary Shares offered in this prospectus and certain other matters of Israeli law will be passed upon
for us by Keren Law Firm. Certain matters of U.S. federal law will be passed upon for us by Greenberg Traurig, P.A., Tel Aviv, Israel.
Certain legal matters in connection with this offering will be passed upon for the underwriter by Sichenzia Ross Ference Carmel LLP,
New York, New York, with respect to U.S. federal law.
EXPERTS
The
financial statements of Polyrizon Ltd. as of December 31, 2023 and 2022 and for each of the
two years in the period ended December 31, 2023, included in this prospectus have been audited
by Brightman Almagor Zohar & Co., a member firm in the Deloitte global network, an independent
registered public accounting firm, as stated in their report. Such financial statements are
included in reliance upon the report of such firm given their authority as experts in accounting
and auditing.
ENFORCEMENT
OF CIVIL LIABILITIES
We
are incorporated under the laws of the State of Israel. Service of process upon us and upon our directors and officers and the Israeli
experts named in this registration statement, most of whom reside outside of the United States, may be difficult to obtain within the
United States. Furthermore, because substantially all of our assets and substantially all of our directors and officers are located outside
of the United States, any judgment obtained in the United States against us or any of our directors and officers may not be collectible
within the United States.
We
have been informed by our legal counsel in Israel, Keren Law Firm, that it may be difficult to assert U.S. securities law claims in original
actions instituted in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning
that Israel is not the most appropriate forum to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it
may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable
U.S. law must be proved as a fact which can be a time-consuming and costly process. Certain matters of procedure will also be governed
by Israeli law.
We
have irrevocably appointed Puglisi & Associates as our agent to receive service of process in any action against us in any U.S. federal
or state court arising out of this offering or any purchase or sale of securities in connection with this offering.
Subject
to specified time limitations and legal procedures, Israeli courts may enforce a U.S. judgment in a civil matter which, subject to certain
exceptions, is non-appealable, including a judgment based upon the civil liability provisions of the Securities Act and the Exchange
Act and including a monetary or compensatory judgment in a non-civil matter, provided that among other things:
| |
● |
the judgment was rendered by a court which was,
according to the laws of the state of the court, competent to render the judgment; |
| |
● |
the obligation imposed by the judgment is enforceable
according to the rules relating to the enforceability of judgments in Israel and the substance of the judgment is not contrary to
public policy; and |
| ● | the
judgment is executory in the state in which it was given Even if these conditions are met,
an Israeli court may not declare a foreign civil judgment enforceable if:
|
| | | |
| ● | the
judgment was given in a state whose laws do not provide for the enforcement of judgments
of Israeli courts (subject to exceptional cases); |
| | | |
| ● | the
enforcement of the judgment is likely to prejudice the sovereignty or security of the State
of Israel; |
| | | |
| ● | the
judgment was obtained by fraud; |
| | | |
| ● | the
opportunity given to the defendant to bring its arguments and evidence before the court was
not reasonable in the opinion of the Israeli court; |
| | | |
| ● | the
judgment was rendered by a court not competent to render it according to the laws of private
international law as they apply in Israel; |
| |
● |
the judgment is contradictory
to another judgment that was given in the same matter between the same parties and that is still valid; or |
| |
● |
at the time the action was
brought in the foreign court, a lawsuit in the same matter and between the same parties was pending before a court or tribunal in
Israel. |
If
a foreign judgment is enforced by an Israeli court, it generally will be payable in Israeli currency, which can then be converted into
non- Israeli currency and transferred out of Israel. The usual practice in an action before an Israeli court to recover an amount in
a non-Israeli currency is for the Israeli court to issue a judgment for the equivalent amount in Israeli currency at the rate of exchange
in force on the date of the judgment, but the judgment debtor may make payment in foreign currency. Pending collection, the amount of
the judgment of an Israeli court stated in Israeli currency ordinarily will be linked to the Israeli consumer price index plus interest
at the annual statutory rate set by Israeli regulations prevailing at the time. Judgment creditors must bear the risk of unfavorable
exchange rates.
WHERE
YOU CAN FIND MORE INFORMATION
We
have filed with the SEC a registration statement on Form F-1 under the Securities Act relating to this offering of our securities.
This prospectus does not contain all of the information contained in the registration statement. The rules and regulations of the SEC
allow us to omit certain information from this prospectus that is included in the registration statement. Statements made in this prospectus
concerning the contents of any contract, agreement or other document are summaries of all material information about the documents summarized,
but are not complete descriptions of all terms of these documents. If we filed any of these documents as an exhibit to the registration
statement, you may read the document itself for a complete description of its terms.
Our
SEC filings are available to the public at the SEC’s website at http://www.sec.gov. Upon completion of this offering,
we will be subject to the information reporting requirements of the Exchange Act that are applicable to foreign private issuers, and
under those requirements will file reports with the SEC.
Upon
completion of this offering, we will be subject to the information reporting requirements of the Exchange Act that are applicable to
foreign private issuers, and under those requirements are filing reports with the SEC. Those other reports or other information may be
inspected without charge at the locations described above. As a foreign private issuer, we will be exempt from the rules under the Exchange
Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders will be exempt
from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will
not be required under the Exchange Act to file reports and financial statements with the SEC as frequently or as promptly as U.S. companies
whose securities are registered under the Exchange Act. However, we will file with the SEC, within 120 days after the end of each fiscal
year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent
registered public accounting firm, and will submit to the SEC, on Form 6-K, unaudited quarterly financial information.
We
maintain a corporate website at www.polyrizon-biotech.com. Information contained on, or that can be accessed through, our website
does not constitute a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual
reference. We will post on our website any materials required to be so posted on such website under applicable corporate or securities
laws and regulations, including, posting any XBRL interactive financial data required to be filed with the SEC and any notices of general
meetings of our shareholders.
POLYRIZON
LTD. FINANCIAL STATEMENTS
AS
OF DECEMBER 31, 2023
U.S.
DOLLARS IN THOUSANDS
INDEX

REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the Shareholders and Board of Directors of Polyrizon Ltd.
Opinion
on the Financial Statements
We
have audited the accompanying balance sheets of Polyrizon Ltd. (the “Company”) as of December 31, 2023 and 2022, the related
statements of comprehensive loss, changes in temporary equity and shareholders’ deficit and cash flows for each of the two years
in the period ended December 31, 2023, and the related notes (collectively referred to as the “financial statements”). In
our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December
31, 2023 and 2022 and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2023,
in conformity with accounting principles generally accepted in the United States of America.
Going
Concern
The
accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note
1B to the financial statements, the Company’s lack of revenues and accumulated operating losses raise substantial doubt about its
ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1B. The financial
statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis
for Opinion
These
financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s
financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board
(United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities
laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We
conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company
is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits,
we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion
on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our
audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error
or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding
the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant
estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits
provide a reasonable basis for our opinion.
/s/
Brightman Almagor Zohar & Co.
Certified
Public Accountants
A
Firm in the Deloitte Global Network
Tel
Aviv, Israel
May
20, 2024 (September 3, 2024, as to the subsequent events described in Note 13e and 13f and the effects of the forward share split described
in Note 7b(5) and 13g)
We
have served as the Company’s auditor since 2022.
POLYRIZON
LTD.
BALANCE
SHEETS
U.S. dollars
in thousands (except share and per share data)
| | |
| | |
As
of December 31, | |
| | |
Note | | |
2023 | | |
2022 | |
| Assets | |
| | |
| | |
| |
| Current assets: | |
| | |
| | |
| |
| Cash and cash equivalents | |
| | | |
$ | 4 | | |
$ | 36 | |
| Investment in shares- at fair value | |
| 3 | | |
| 37 | | |
| - | |
| Other current assets | |
| | | |
| 13 | | |
| 15 | |
| | |
| | | |
| | | |
| | |
| Total current assets | |
| | | |
| 54 | | |
| 51 | |
| | |
| | | |
| | | |
| | |
| Property and equipment, net | |
| | | |
| 12 | | |
| 16 | |
| | |
| | | |
| | | |
| | |
| Deferred offering costs | |
| 4 | | |
| 493 | | |
| 479 | |
| | |
| | | |
| | | |
| | |
| Total assets | |
| | | |
$ | 559 | | |
$ | 546 | |
| | |
| | | |
| | | |
| | |
| Liabilities, temporary equity and shareholders’
deficit | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | | |
| | |
| Employees and payroll-related liabilities | |
| | | |
$ | 39 | | |
$ | 37 | |
| Accrued expenses | |
| | | |
| 158 | | |
| 141 | |
| Derivative warrant liability | |
| 9 | | |
| 105 | | |
| 398 | |
| Convertible notes | |
| 10 | | |
| 200 | | |
| 622 | |
| | |
| | | |
| | | |
| | |
| Total current liabilities | |
| | | |
| 502 | | |
| 1,198 | |
| | |
| | | |
| | | |
| | |
| Temporary equity: | |
| 6 | | |
| | | |
| | |
| Preferred
shares, no par value per share; Authorized: 104,711 shares; Issued and outstanding: 104,711 shares; (*) | |
| | | |
| 248 | | |
| 248 | |
| | |
| | | |
| | | |
| | |
| Shareholders’ deficit: | |
| 7 | | |
| | | |
| | |
| Ordinary
shares, no par value per share; Authorized: 19,895,289 shares; Issued and outstanding: 2,550,591 and 1,305,635 shares as of December
31, 2023 and 2022, respectively; (*) | |
| | | |
| - | | |
| - | |
| Additional paid-in capital | |
| | | |
| 3,526 | | |
| 2,021 | |
| Receivables on account of shares | |
| | | |
| (196 | ) | |
| | |
| Accumulated deficit | |
| | | |
| (3,521 | ) | |
| (2,921 | ) |
| | |
| | | |
| | | |
| | |
| Total shareholders’
deficit | |
| | | |
| (191 | ) | |
| (900 | ) |
| | |
| | | |
| | | |
| | |
| Total liabilities, temporary
equity and shareholders’ deficit | |
| | | |
$ | 559 | | |
$ | 546 | |
| (*) | After
giving effect to the reverse and forward share splits, see also note 7b. |
The accompanying
notes are an integral part of the financial statements.
POLYRIZON
LTD.
STATEMENTS
OF COMPREHENSIVE LOSS
U.S. dollars
in thousands (except share and per share data)
| | |
| | |
For
the Year Ended
December 31, | |
| | |
Note | | |
2023 | | |
2022 | |
| | |
| | |
| | |
| |
| Operating expenses: | |
| | |
| | |
| |
| Research
and development expenses, net | |
| 11a | | |
$ | 332 | | |
$ | 347 | |
| General
and administrative expenses | |
| 11b | | |
| 303 | | |
| 548 | |
| | |
| | | |
| | | |
| | |
| Operating loss | |
| | | |
| 635 | | |
| 895 | |
| | |
| | | |
| | | |
| | |
| Financial income, net | |
| 11c | | |
| (35 | ) | |
| (116 | ) |
| | |
| | | |
| | | |
| | |
| Net loss and comprehensive loss | |
| | | |
$ | 600 | | |
$ | 779 | |
| | |
| | | |
| | | |
| | |
| Basic and diluted net
loss per ordinary share (*) | |
| 12 | | |
$ | 0.3 | | |
$ | 0.6 | |
| | |
| | | |
| | | |
| | |
| Weighted
average number of shares of ordinary share used in computing basic and diluted net loss per share (*) | |
| | | |
| 2,030,327 | | |
| 1,305,635 | |
| (*) | After
giving effect to the reverse and forward share splits, see also note 7b. |
The accompanying
notes are an integral part of the financial statements.
POLYRIZON
LTD.
STATEMENTS
OF CHANGES IN TEMPORARY EQUITY AND SHAREHOLDERS’ DEFICIT
U.S. dollars
in thousands (except share data)
| | |
Preferred
shares | | |
Ordinary
shares | | |
Additional
paid-in | | |
Receivables
on account | | |
Accumulated | | |
Total
shareholders’ | |
| | |
Number (*) | | |
Amount | | |
Number (*) | | |
Amount | | |
capital | | |
of
shares | | |
deficit | | |
deficit | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance as
of December 31, 2021 | |
| 104,711 | | |
$ | 248 | | |
| 1,305,635 | | |
$ | - | | |
$ | 1,891 | | |
$ | - | | |
| (2,142 | ) | |
$ | (251 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Share based payment | |
| - | | |
| - | | |
| - | | |
| - | | |
| 130 | | |
| - | | |
| - | | |
| 130 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (779 | ) | |
| (779 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance as of December
31, 2022 | |
| 104,711 | | |
| 248 | | |
| 1,305,635 | | |
| - | | |
| 2,021 | | |
| - | | |
| (2,921 | ) | |
| (900 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Issuance
of shares (see notes 7a2 and 7a3) | |
| - | | |
| - | | |
| 452,126 | | |
| - | | |
| 506 | | |
| (196 | ) | |
| - | | |
| 310 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Conversion
of convertible notes into shares (see note 10) | |
| - | | |
| - | | |
| 792,830 | | |
| - | | |
| 899 | | |
| - | | |
| - | | |
| 899 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Share based payment | |
| - | | |
| - | | |
| - | | |
| - | | |
| 100 | | |
| - | | |
| - | | |
| 100 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (600 | ) | |
| (600 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
as of December 31, 2023 | |
| 104,711 | | |
| 248 | | |
| 2,550,591 | | |
| - | | |
| 3,526 | | |
| (196 | ) | |
| (3,521 | ) | |
| (191 | ) |
| (*) | After
giving effect to the reverse and forward share splits, see also note 7b. |
The accompanying
notes are an integral part of the financial statements.
POLYRIZON
LTD.
STATEMENTS
OF CASH FLOWS
U.S. dollars
in thousands
| | |
For
the Year Ended
December 31, | |
| | |
2023 | | |
2022 | |
| Cash flows from operating activities | |
| | |
| |
| | |
| | |
| |
| Net loss | |
$ | (600 | ) | |
$ | (779 | ) |
| Adjustments to reconcile net loss to net cash
used in operating activities: | |
| | | |
| | |
| Depreciation | |
| 4 | | |
| 5 | |
| Share based payment | |
| 100 | | |
| 130 | |
| Fair value revaluation in investment in shares | |
| 17 | | |
| - | |
| Fair value revaluation in derivative warrant
liability | |
| (293 | ) | |
| (118 | ) |
| Fair value revaluation in convertible notes | |
| 228 | | |
| (26 | ) |
| Change in: | |
| | | |
| | |
| Other current assets | |
| 2 | | |
| 6 | |
| Deferred offering costs | |
| (14 | ) | |
| (354 | ) |
| Employees and payroll-related
liabilities | |
| 2 | | |
| 19 | |
| Accrued
expenses | |
| 17 | | |
| (15 | ) |
| | |
| | | |
| | |
| Net cash used in operating
activities | |
| (537 | ) | |
| (1,132 | ) |
| | |
| | | |
| | |
| Cash flows from
investing activities | |
| | | |
| | |
| | |
| | | |
| | |
| Purchase of property
and equipment | |
| - | | |
| (3 | ) |
| | |
| | | |
| | |
| Net cash used in investing
activities | |
| - | | |
| (3 | ) |
| | |
| | | |
| | |
| Cash flows from
financing activities | |
| | | |
| | |
| | |
| | | |
| | |
| Proceeds from issuance of convertible notes | |
| 249 | | |
| 648 | |
| Proceeds from issuance
of ordinary shares | |
| 256 | | |
| - | |
| | |
| | | |
| | |
| Net cash provided by
financing activities | |
| 505 | | |
| 648 | |
| | |
| | | |
| | |
| Change in cash and cash equivalents | |
| (32 | ) | |
| (487 | ) |
| Cash and cash equivalents
at the beginning of the year | |
| 36 | | |
| 523 | |
| | |
| | | |
| | |
| Cash and cash equivalents
at the end of the year | |
$ | 4 | | |
$ | 36 | |
| | |
| | | |
| | |
| Non-cash financing
activities: | |
| | | |
| | |
| Conversion
of convertible notes into ordinary shares (see Note 10) | |
$ | 899 | | |
$ | - | |
| Receivables
on account of shares (see Notes 7a2 and 7a3) | |
| 196 | | |
| - | |
| Ordinary
shares issued in exchange for securities (see Note 7a2) | |
$ | 54 | | |
$ | - | |
The accompanying
notes are an integral part of the financial statements.
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| |
a. |
Polyrizon
Ltd. (the “Company”) was incorporated and commenced its business operations in January 2005. The Company is a clinical
development stage biotech company specializing on the development of nasal gels to provide preventative treatment to protect
against a wide cross section of viruses, including certain variants of COVID-19 that are also considered to cause more infections
and spread faster than the original strain of the virus (the CDC expects that additional variants of the virus will continue
to occur), influenza, allergens, and other toxins. The Company’s proprietary Capture and Contain (“C&C”)
hydrogel platform is delivered in the form of nasal sprays, and form a thin gel-based protective shield containment barrier in
the nasal cavity that prevents viruses, bacteria, allergens, and other toxins from penetrating the nasal epithelial tissue.
Due
to lack of resources, the Company suspended its operations in 2016. In connection with the COVID-19 pandemic, the Company resumed
its operations in 2020. |
| |
b. |
Going concern and management
plans |
The
accompanying financial statements are prepared assuming the Company will continue as a going concern, which contemplates the realization
of assets and liquidation of liabilities in the normal course of business. To date, the Company has not generated revenues from its activities
and has incurred substantial operating losses. Management expects the Company to continue to generate substantial operating losses for
the foreseeable future until it completes development of its products and obtains regulatory approvals to market such products.
Such
conditions raise substantial doubts about the Company’s ability to continue as a going concern for at least a year after the issuance
date of the accompanying financial statements. Management plans to address these conditions by raising funds from its current investors
as well as outside potential investors. However, there is no assurance that such funding will be available to the Company or that it
will be obtained on terms favorable to the Company or will provide the Company with sufficient funds to meet its objectives. The accompanying
financial statements do not include any adjustments relating to the recoverability and classification of assets, carrying amounts or
the amount or classification of liabilities that may be required should the Company be unable to continue as a going concern.
| |
c. |
The Company’s headquarters
and other significant operations are located in Israel, and, therefore, our results may be adversely affected by political, economic
and military instability in Israel, including the recent attack by Hamas that started a war on October 7, 2023. Since the war broke
out, our operations have not been adversely affected by this situation, and we have not experienced disruptions to our development.
However, the intensity and duration of Israel’s current war against Hamas is difficult to predict at this stage, as are such
war’s economic implications on the Company’s business and operations and on Israel’s economy in general. If the
war extends for a long period of time or expands to other fronts, such as Lebanon, Syria and the West Bank, our operations may be
adversely affected. |
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 2: |
SIGNIFICANT ACCOUNTING POLICIES |
| |
a. |
Basis of presentation: |
The
financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“U.S.
GAAP”).
| |
b. |
Use of estimate in preparation
of financial statements: |
The
preparation of financial statements in conformity with U.S. GAAP requires management to make estimates, judgments and assumptions
that affect the amounts reported in the financial statements and accompanying notes. The Company evaluates on an ongoing basis its assumptions.
The Company’s management believes that the estimates, judgments and assumptions used are reasonable based upon information available
at the time they are made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities and disclosure
of contingent assets and liabilities at the dates of the financial statements, and the reported amounts of expenses during the reporting
periods. Actual results could differ from those estimates.
| |
c. |
Financial statements in United
States dollars: |
The
Company’s functional currency is the U.S. dollar (“dollar” or “$”) since the dollar is the currency of
the primary economic environment in which the Company has operated and expects to continue to operate in the foreseeable future. Transactions
and balances denominated in dollars are presented at their original amounts. Transactions and balances denominated in currencies other
than dollars have been re-measured to dollars and the differences were recorded as foreign exchange gain or loss.
| |
d. |
Cash and cash equivalents: |
Cash
equivalents are short-term highly liquid investments that are readily convertible to cash with original maturities of three months or
less at acquisition.
| |
e. |
Property and equipment, net: |
Property
and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the
estimated useful lives of the assets at the following rates:
| | |
% | |
| Computers and electronic equipment | |
| 33 | |
In
accordance with ASC 480-10, “Accounting for Certain Financial instruments with Characteristics of both Liabilities and Equity”,
financial instruments that have characteristics of both liabilities and equity are classified as liabilities and are initially and subsequent
to issuance measured at fair value if at inception such instruments, in the predominant scenario, may be settled either by issuing a
variable number of shares that in the aggregate provide a fixed monetary value, may be settled by issuing a variable number of shares
that is inversely related to changes in the fair value of the Company’s share or may be settled based on variations in an observable
market or index (other than variations based on the fair value of the Company’s share).
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 2: |
SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| |
|
| |
g. |
Impairment of long-lived assets: |
Property
and equipment are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may
not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the
future undiscounted cash flows expected to be generated by the assets. If such assets are considered to be impaired, the impairment to
be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. As of December
31, 2023, no impairment indicators have been identified.
| |
h. |
Research and development expenses: |
Research
and development expenses are charged to the statements of comprehensive loss as incurred. Research and development participation income
is recognized at the time the Company is entitled to such participation fees and is applied as a deduction from research and development
expenses. Royalty-bearing grants from the IIA are recognized at the time the Company is entitled to such grants, on the basis of the
costs incurred and are applied as a deduction from research and development expenses.
| |
i. |
Fair Value Measurements: |
Fair
value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction
between market participants at the reporting date. Assets and liabilities recorded at fair value in the financial statements are categorized
based upon the level of judgment associated with the inputs used to measure their fair value. Hierarchical levels are directly related
to the amount of subjectivity with the inputs to the valuation of these assets or liabilities as follows:
Level
1 – Observable inputs such as unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement
date;
Level
2 – Inputs (other than quoted prices included in Level 1) are either directly or indirectly observable inputs for similar assets
or liabilities. These include quoted prices for identical or similar assets or liabilities in active markets and quoted prices for identical
or similar assets of liabilities in markets that are not active;
Level
3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the
assets or liabilities.
The
Company’s derivative warrant liability is measured at fair value at each reporting date and is classified within Level 3 of the
fair value hierarchy because its fair values is estimated by utilizing valuation models and significant unobservable inputs. The Company’s
convertible notes are measured at fair value at each reporting date and are classified within Level 2 of the fair value hierarchy because
their fair values are estimated by utilizing observable inputs. The Company’s investment in shares is measured at fair value at
each reporting date and is classified within Level 1 of the fair value hierarchy because its fair value is estimated based on the shares’
quoted price.
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 2: |
SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
The
carrying values of Company’s financial assets and liabilities, including cash and cash equivalents, other current assets, employees
and payroll-related liabilities and accrued expenses approximate their fair value due to the short-term maturity of these instruments.
The
Company accounts for income taxes using the liability method whereby deferred tax asset and liability account balances are determined
based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates
and laws that will be in effect when the differences are expected to reverse.
The
Company provides a valuation allowance, if necessary, to reduce deferred tax assets to their estimated realizable value. As of December
31, 2023, and 2022, a full valuation allowance was provided by the Company.
The
recognition and measurement of a liability for uncertain tax positions contains a two-step approach. The first step is to evaluate the
tax position taken or expected to be taken in a tax return by determining if the weight of available evidence indicates that it is more
likely than not that, on an evaluation of the technical merits, the tax position will be sustained on audit, including resolution of
any related appeals or litigation processes. The second step is to measure the tax benefit as the largest amount that is more than 50%
likely to be realized upon ultimate settlement. As of December 31, 2023 and 2022, no liability for unrecognized tax benefits was recorded.
The
Company’s liability for severance pay is pursuant to Section 14 of the Israeli Severance Compensation Act, 1963 (“Section
14”), pursuant to which all the Company’s employees are included under Section 14, and are entitled only to monthly deposits,
at a rate of 8.33% of their monthly salary, made in the employee’s name with insurance companies. Under Israeli employment law,
payments in accordance with Section 14 release the Company from any future severance payments in respect of those employees. The fund
is made available to the employee at the time the employer-employee relationship is terminated, regardless of cause of termination. The
severance pay liabilities and deposits under Section 14 are not reflected in the balance sheets as the severance pay risks have been
irrevocably transferred to the severance funds.
| |
l. |
Share-based payment transactions: |
The
Company accounts for share-based compensation in accordance with ASC 718, “Compensation – Stock Compensation” (“ASC
718”), which requires companies to estimate the fair value of equity-based payment awards on the date of grant. The value of the
portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service periods in the statements
of comprehensive loss.
The
Company recognizes compensation expenses for the value of its awards granted based on the vesting attribution approach over the requisite
service period of each of the awards, net of estimated forfeitures. Forfeitures are accounted for as they occur.
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 2: |
SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
The
Company estimates the fair value of share options granted using the Black-Scholes option pricing model. The option-pricing model requires
a number of assumptions, including the expected share price volatility, free risk interest rate, dividends and the expected option term.
Expected volatility was calculated based upon historical volatility of the Company. The expected option term represents the period that
the Company’s share options are expected to be outstanding and is determined based on the simplified method until sufficient historical
exercise data will support using expected life assumptions. The risk-free interest rate is based on the yield from U.S. treasury bonds
with an equivalent term. The Company has historically not paid dividends and has no foreseeable plans to pay dividends. As a result,
the dividend rate was zero.
| |
m. |
Basic and diluted loss per
ordinary share: |
Basic
loss per share is computed by dividing the net loss by the weighted average number of ordinary shares outstanding during the period.
Diluted loss per share is computed by dividing the net loss by the weighted average number of ordinary shares outstanding together with
the number of additional ordinary shares that would have been outstanding if all potentially dilutive ordinary shares had been issued.
Since the Company was in a loss position for the period presented, basic net loss per share is the same as diluted net loss per share
since the effects of potentially dilutive securities are antidilutive.
| |
n. |
Recently accounting pronouncements: |
As
an “emerging growth company,” the Jumpstart Our Business Startups Act (“JOBS Act”) allows the Company to delay
adoption of new or revised accounting pronouncements applicable to public companies until such pronouncements are made applicable to
private companies. The Company has elected to use this extended transition period under the JOBS Act.
The
following are accounting pronouncements that are not yet effective for the Company:
In
August 2020, the FASB issued ASU 2020-06, “Debt-Debt with Conversion and Other Options Subtopic 470-20) and Derivatives and Hedging-
Contracts in Entity’s Own Equity (Subtopic 815-40).” The amendments in this update affect entities that issue convertible
instruments and/or contracts in an entity’s own equity. For convertible instruments, the instruments primarily affected are those
issued with beneficial conversion features or cash conversion features because the accounting models for those specific features are
removed. This ASU is effective for the Company starting on January 1, 2024. The Company does not believe that the adoption of this standard
will have a material impact on the Company’s financial statements.
In
November 2023, the FASB issued ASU 2023-07, “Segment Reporting (Topic 280)”, Improvements to Reportable Segment Disclosures
to improve reportable segment disclosure requirements through enhanced disclosures about significant segment expenses on an interim and
annual basis. All disclosure requirements of ASU 2023-07 are required for entities with a single reportable segment. ASU 2023-07 is effective
starting January 1, 2024, and should be applied on a retrospective basis to all periods presented. Early adoption is permitted. The Company
does not believe that the adoption of this standard will have a material impact on the Company’s financial statements.
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 2: |
SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
In
December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740) – “Improvements to Income Tax Disclosures”. The
ASU requires that an entity disclose specific categories in the effective tax rate reconciliation as well as provide additional information
for reconciling items that meet a quantitative threshold. Further, the ASU requires certain disclosures of state versus federal income
tax expense and taxes paid. The amendments in this ASU are required to be adopted starting January 1, 2025. Early adoption is permitted,
and the amendments should be applied on a prospective basis. The Company is currently evaluating the effect of adopting the ASU on its
disclosures.
| NOTE 3: |
INVESTMENT IN SHARES- at
Fair Value |
On
June 6, 2023, the Company entered into a securities purchase agreement with certain shareholders pursuant to which the Company issued
359,498 ordinary shares for gross proceeds of $407 (out of which the Company received from one of the investors shares in the value of
$54). See also note 7a2. The shares are held as a short-term investment in trading securities and are accordingly classified in current
assets.
The
Company’s investment in shares is measured at fair value at each reporting date, based on the shares’ quoted prices (Level
1 measurement). The following table presents changes in the level 1 fair value measurement of Investments in shares:
| Balance as of December 31, 2022 | |
$ | - | |
| | |
| | |
| Investment in shares | |
| 54 | |
| Changes
in fair value | |
| (17 | ) |
| | |
| | |
| Balance as of December 31, 2023 | |
$ | 37 | |
| NOTE 4: |
DEFERRED OFFERING COSTS |
The
Company capitalizes incremental legal and other third-party fees that are directly related to the Company’s in-process equity financings
until such financings are consummated, including the Company’s IPO. After the consummation of the equity financing, these costs
will be recorded as a reduction of the gross proceeds. As of December 31, 2023, and 2022, there were $493 and $479, respectively, of
deferred offering costs on the balance sheet.
| |
a. |
Tax
rates applicable to the Company: |
Taxable
income of the Company is subject to the Israeli Corporate tax rate which was 23% for the years ended December 31, 2023 and 2022.
| |
b. |
Net operating loss carry forward: |
As
of December 31, 2023, and 2022, the Company had net operating loss carry forwards for Israeli income tax purposes of approximately $2,751
and $2,313, respectively. Such net operating loss carry forwards may be carried forward indefinitely and offset against future taxable
income.
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 5: |
TAXES ON INCOME (Cont.) |
| |
c. |
Deferred
income taxes: |
Deferred
income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial
reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets are
as follows:
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Deferred tax assets: | |
| | |
| |
| Net operating loss carry forward | |
$ | 633 | | |
$ | 532 | |
| Other temporary differences | |
| 55 | | |
| 42 | |
| | |
| | | |
| | |
| Deferred tax asset before valuation allowance | |
| 688 | | |
| 574 | |
| Valuation allowance | |
| (688 | ) | |
| (574 | ) |
| | |
| | | |
| | |
| Net deferred tax asset | |
$ | - | | |
$ | - | |
In
assessing the realization of deferred tax assets, management considers whether it is more likely than not that all or some portion of
the deferred tax assets will not be realized.
The
ultimate realization of the deferred tax assets is dependent upon the generation of future taxable income during the periods in which
temporary differences are deductible and net operating losses are utilized. Based on consideration of these factors, the Company recorded
a full valuation allowance as of December 31, 2023 and 2022.
| |
d. |
Reconciliation
of income tax expenses: |
The
reconciliation of income tax benefit computed at statutory tax rate to income tax expense is as follows:
| | |
Year
Ended
December 31, | |
| | |
2023 | | |
2022 | |
| Income tax benefit computed at
statutory tax rate | |
| (138 | ) | |
| (179 | ) |
| Change in valuation allowance | |
| 114 | | |
| 54 | |
| Exchange rate differences
on carry forward losses | |
| 24 | | |
| 125 | |
| | |
| | | |
| | |
| Income tax expense | |
$ | - | | |
$ | - | |
| |
e. |
As
of December 31, 2023, the Company had final tax assessments for tax years prior to and including the tax year ended December 31,
2018. |
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
In
September 2012, the Company’s Article of Association was amended to reflect the agreement reached between the Company and certain
holders of preferred shares issued in November 2008 (the “2008 Preferred Shares”) following an investment of NIS 2,780 (approximately
$894) (the “Original Issue Price”), to issue 104,711 shares of a new class of preferred shares (the “Preferred Shares”)
in exchange for the 2008 Preferred Shares.
Holders
of the Preferred Shares are entitled in the event of: (i) any liquidation, dissolution or winding up of the Company, whether voluntary
or involuntary; or (ii) any Deemed Liquidation Event (as defined in Company’s Article of Association) to receive out of the assets
of the Company available for distribution to its shareholders (the “Distributable Assets”), prior to the holders of any class
or series of shares of the Company, an amount (in cash, cash equivalents or, if applicable, securities) equal to 15% of the Distributable
Assets. Afterwards, the holders of the Preferred Shares are also entitled to participate with all other ordinary shareholders and receive
a proportionate amount from the remainder of aforementioned assets available for distribution, provided that the holders of preferred
shares are not entitled to receive in the aggregate more than 2.75 times the Original Issue Price (adjusted for certain recapitalization
events) plus annual interest on the Original Issue Price, at an annual rate of 4%, compounded annually from the date of the issuance
of the 2008 Preferred Shares.
Additionally,
prior to and in preference to the distribution of any dividends to the holders of any class or series of shares of the Company, each
of the holders of the Preferred Shares are entitled to receive for each preferred share held by them an amount equal to the Original
Issue Price, plus annual interest at an annual rate of 6% of the Original Issue Price (adjusted for certain recapitalization events)
calculated from the date of issuance of the 2008 Preferred Shares (the “Dividend Preference”). Afterwards, the holders of
the Preferred Shares are also entitled to participate with all other holders of ordinary shares in the receipt of any additional dividends
distributed pro rata in accordance with their respective shareholdings in the Company. No dividends is to be paid on any other class
of shares, unless the Dividend Preference has been paid in full.
These
rights attached to the Preferred Shares expire upon completion of an Initial Public Offering (“IPO”), or earlier, in the
event holders of the Preferred Shares have received, in the aggregate, through any distribution (including dividend distribution), amounts
equal to the Original Issue Price, adjusted to changes in the Israeli consumer index, plus annual interest at a rate of 5%, compounded
annually from the date of the issuance of the 2008 Preferred Shares. Upon successful completion of an IPO, the Preferred Shares are to
be converted to 104,711 ordinary shares. Once so converted, the Preferred Shares are no longer available for issuance.
The
Preferred Shares only confer upon their holders the rights specified above and do not confer upon their holders the right to participate
and vote in general shareholder meetings of the Company.
A
Deemed Liquidation Event, as defined in Company’s Article of Association, includes change of controls events in which shareholders
of the Company, immediately prior to the event, hold less than 50% of the voting power in the Company (or the surviving entity) after
the event, as well as events in which the Company sells substantially all of its assets. In the occurrence of a Deemed Liquidation Event,
all of the Distributable Assets legally available for distribution among the shareholders of the Company would be distributed in
accordance with the preference described above.
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 6: |
TEMPORARY EQUITY (Cont.) |
Although
the Preferred Shares are not redeemable, in the occurrence of a Deemed Liquidation Event, which may occur not solely within the Company’s
control, the holders of the Preferred Shares are entitled to the preference amounts before distribution to other shareholders (as provided
above) and hence effectively redeem the preference amount.
As
a result of these in-substance contingent redemption rights, the Preferred Shares are classified outside of Shareholders’ deficit.
The
Preferred Shares were recorded at their fair value as of their issuance date (September 2012). The Company did not subsequently adjust
the carrying values of the Preferred Shares to the deemed liquidation value of such shares since a Deemed Liquidation Event was not probable
of occurring.
| NOTE 7: |
SHAREHOLDERS’ DEFICIT
|
Ordinary
shares confer upon their holders the right to participate and vote in general shareholder meetings of the Company and the right to receive
dividends, if any, declared by the Company, and rights to receive a distribution of assets upon liquidation in the preference order described
above.
Shares
Issuances:
| 1. | As
part of an investment agreement from July 15, 2020, a warrant was granted to an investor
(the “Original Warrant” and the “July 2020 Investor”, respectively),
which investor became a related party of the Company following the July 2020 investment agreement
given its equity interest in the Company. The Original warrant expired on April 23, 2023,
pursuant to its original terms. On December 15, 2021, the Company and the July 2020 Investor
entered into an addendum to the agreement pursuant to which the July 2020 Investor agreed
to change the terms of the Original Warrant and in return was granted a new warrant (the
“New Warrant”), which in the event of successful completion of an IPO replaces
the Original Warrant and is only exercisable subject to the successful completion of an IPO,
to invest an aggregate amount of up to $2,000 at an exercise price to be determined at 125%
of the price per share in an IPO. The New Warrant expires 3 years after the date of a successful
completion of an IPO. In accordance with a third addendum to the July 2020 investment agreement
signed in November 2023, such right to receive New Warrants upon successful completion of
an IPO expires on December 31, 2024. See also Note 9. |
| 2. | On
June 6, 2023, the Company entered into a securities purchase agreement with certain shareholders
pursuant to which the Company issued 359,498 ordinary shares for gross proceeds of $407 (out
of which the Company received from one of the investors shares in the value of $54). As of
December 31, 2023, proceeds of $91 have not yet been received and accordingly were presented
as a deduction of equity in the Company’s statement of changes in temporary equity
and shareholder’s deficit. The remaining proceeds have been received by April 2024. |
As
a result of this financing, the Company’s 2022 convertible notes were converted into 792,830 ordinary shares, pursuant to their
original conversion terms (see also Note 10).
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 7: |
SHAREHOLDERS’ DEFICIT (Cont.) |
| 3. | On
December 19, 2023, the Company entered into a securities purchase agreement with a shareholder
pursuant to which the Company issued 92,628 ordinary shares for gross proceeds of $105. As
of December 31, 2023, the proceeds have not yet been received and accordingly were presented
as a deduction of equity in the Company’s statement of changes in temporary equity
and shareholder’s deficit. $21 of the proceeds have been received by April 2024. |
| 1. | On
September 29, 2022, Company’s shareholders effected a reverse share split of the issued
and outstanding shares at a ratio of 8.8-for-one, pursuant to which holders of Company’s
Shares received one Share for every 8.8 Shares held and cancelled the par value of Company’s
Shares. In addition, the shareholders of the Company effected an increase to the authorized
share capital of its ordinary shares to 19,844,707. |
| 2. | On
December 19, 2022, Company’s shareholders effected forward share split at ratio of
1.25-for-one by means of issuance of bonus shares to the holders of Company’s ordinary
shares on a basis of 1.25 bonus shares for each ordinary share. |
| 3. | On
June 18, 2023, Company’s shareholders effected a reverse share split of the issued
and outstanding shares at a ratio of 1.7-for-one, pursuant to which holders of Company’s
Shares received one Share for every 1.7 Shares held. |
| 4. | On
May 12, 2024 (after the balance sheet date), Company’s shareholders effected a reverse
share split of the issued and outstanding shares at a ratio of 2-for-one, pursuant to which
holders of Company’s Shares received one Share for every 2 Shares held. See also note
13b. |
| | | |
| | 5. | On
August 16, 2024, the Company effected the issuance of an aggregate of 420,618 bonus shares
to the holders of our Ordinary Shares on a basis of 0.1494 bonus shares for each Ordinary
Share outstanding (equivalent to a forward share split at a ratio of 0.1494-for-1) (the “Second
Forward Share Split”). |
For
accounting purposes, all share and per share amounts for ordinary share, Preferred shares, warrants, options and loss per share amounts
have been adjusted to give retroactive effect to the reverse share splits for all periods presented in these financial statements. Any
fractional shares that resulted from the reverse share split have been rounded up to the nearest whole share.
On
February 19, 2021, the Board of Directors approved the adoption of the 2021 Share Option Plan (the “2021 Plan”). Under the
2021 Plan, the Company may grant share options to its officers, directors, employees and consultants. Each share option granted shall
be exercisable at such times and terms and conditions as the Board of Directors may specify in the applicable option agreement (each
an “Option Agreement”). Upon the adoption of the 2021 Plan, the Company reserved for issuance 112,780 ordinary shares. On
November 14, 2021, the Board of Directors approved an increase to the Company’s reserved shares for issuance under the 2021 Plan
so that the current number of ordinary shares reserved for issuance under the 2021 Plan increased to 304,261 ordinary shares.
Between
August and December 2021, the Company’s Board of Directors approved a grant of 70,365 options to purchase 70,365 ordinary shares
to Company’s employee and officers with an exercise price ranging between $0.68 and $1.13 per share. The vesting period for the
options granted range from six months to four years.
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 7: |
SHAREHOLDERS’ DEFICIT (Cont.) |
On
June 6, 2023, the Company granted options to purchase 165,899 ordinary shares of the Company in consideration for $1.11 per share option.
The share options will vest and become exercisable over a period of two (2) years commencing on the grant date on a monthly basis in
equal instalments.
On
November 30, 2023, the Company granted options to purchase 9,865 ordinary shares of the Company in consideration for NIS 0.07 (0.02 $)
per option. The options will vest and become exercisable over a period of twelve (12) months commencing on the grant date on a monthly
basis in equal instalments.
In
these grants, certain officers and employees have a clause under their Option Agreement according to which all of the unvested share
options become fully vested upon successful completion of an IPO and additionally, upon the successful completion of a merger, acquisition
or reorganization of the Company with other entity, change in the ownership of more 50%, involuntary termination by the Company, or any
other similar event. As such, 42,744 share options with exercise prices ranging between $0.02 and $1.1335 per share will become fully
vested upon successful completion of an IPO.
Expenses
recognized in the financial statements:
| | |
Year
Ended
December 31, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Research and development expenses | |
$ | 83 | | |
$ | 111 | |
| General and administrative
expenses | |
| 17 | | |
| 19 | |
| Total | |
| 100 | | |
| 130 | |
The
fair value of the Company’s share options granted was estimated using the Black-Scholes option pricing model using the following
range assumptions:
| Description | |
2023 | |
| | |
| |
| Risk-free interest rate | |
| 4.00%
- 4.22% | |
| Expected volatility | |
| 90.02%-94.24% | |
| Dividend yield | |
| 0 | |
| Contractual life | |
| 5-7 | |
| Exercise price | |
| 0.02
– 1.133 | |
No
share options were granted in 2022.
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 7: |
SHAREHOLDERS’ DEFICIT (Cont.) |
A
summary of the Company’s share options outstanding and exercisable as of December 31, 2022 and changes during the year then ended
are presented below:
| | |
2022 | |
| | |
Number
of
share
option | | |
Weighted
average
exercise
price | |
| | |
| | |
| |
| Share options
outstanding at beginning of year | |
| 70,365 | | |
| 0.84 | |
| | |
| | | |
| | |
| Share options outstanding
at year end | |
| 70,365 | | |
| 0.84 | |
| | |
| | | |
| | |
| Share options exercisable
at year end | |
| 45,652 | | |
| 0.84 | |
A
summary of the Company’s share options outstanding and exercisable as of December 31, 2023 and changes during the year then ended
are presented below:
| | |
2023 | |
| | |
Number
of
share
option | | |
Weighted
average
exercise
price | |
| | |
| | |
| |
| Share options outstanding at beginning
of year | |
| 70,365 | | |
| 0.84 | |
| Granted | |
| 175,764 | | |
| 1.07 | |
| | |
| | | |
| | |
| Share options outstanding
at year end | |
| 246,129 | | |
| 1.01 | |
| | |
| | | |
| | |
| Share options exercisable
at year end | |
| 103,349 | | |
| 0.93 | |
| NOTE 8: |
COMMITMENTS AND CONTINGENT LIABILITIES |
| a. | From
2007 through 2010, the Company received funding from the Israeli Innovation Authority (“IIA”,
previously known as Officer of Chief Scientist) for its participation in research and development
costs, based on budgets approved by the IIA, subject to the fulfillment of specified milestones.
The Company is committed to pay royalties to the IIA on proceeds from sale of products in
the research and development of which the IIA participates by way of grants. According to
the IIA’s fundings terms, royalties between 3% and 4.5% are payable on sales of developed
products funded, up to 100% of the funding received by the Company, linked to US dollar and
bearing 12 months SOFR interest rate. In the case of failure of a financed project, the Company
is not obligated to pay any such royalties to the IIA. The total funding received from the
IIA, including interest, as of December 31, 2023 is $753. |
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 8: |
COMMITMENTS AND CONTINGENT LIABILITIES (Cont.) |
| b. | On
September 1, 2021, the Company signed a consulting agreement with its CEO. According to the
agreement, the CEO is entitled to receive (i) a monthly salary, (ii) a one-time NIS 150 thousand
(approximately $48) bonus upon completion of the Company’s IPO, (iii) share options
representing 0.5% of the Company’s pre-IPO issued and outstanding ordinary shares which
will vest upon the completion of the Company’s IPO and become exercisable for a period
of 5 years, and (iv) share options representing 2.5% of the Company’s post-IPO issued
and outstanding ordinary shares which shall vest and become exercisable over a total period
of three years commencing on the grant date on a monthly basis in equal installments. |
| c. | On
May 29, 2022, the Company and the Company’s Chief R&D Officer (“CRDO”)
entered into a new employment agreement. Under the new employment agreement and subject to
a successful completion of the Company’s IPO, the Company (1) shall grant the CRDO
options to purchase 7,577 ordinary shares of the Company in consideration for NIS 1.084 per
share option with these share options vesting and become exercisable over a period of eleven
(11) months commencing on the grant date (in accordance with the Board’s approval),
on a monthly basis in equal instalments; and (2) may grant the CRDO options to purchase ordinary
shares of the Company representing up to 2% of the Company’s post IPO issued and outstanding
share-capital with these share options vesting and become exercisable over a period of three
years commencing on the grant date, on a quarterly basis in equal instalments. In addition,
the CRDO’s monthly salary increased to $8 starting July 2022. |
| d. | On
May 30, 2022, the Company entered into a collaboration agreement with SciSparc Ltd.(“SciSparc”)
(Nasdaq: SPRC), a specialty clinical-stage pharmaceutical company focusing on the development
of therapies to treat disorders of the central nervous system. Under the collaboration agreement,
the Company will work with SciSparc to develop technology for the treatment of pain, based
on SciSparc’s SCI-160 platform and Company’s trap and target (“T&T”)
platform technology. In accordance with the collaboration agreement, SciSparc will pay development
fees to the Company upon successful completion of clinical trials, of $80 by successful completion
of preclinical safety tests, $120 by successful completion of Phase 1 clinical trial, $150
by upon successful completion of Phase 2a clinical trial, $200 upon successful completion
of Phase 2b clinical trial, $500 upon successful completion of Phase 3 clinicals, $750 upon
approval by the U.S. Food and Drug Administration and $750 upon approval by an EU regulatory
body. Additionally, SciSparc will pay royalties of 3.25% on any product sales by SciSparc
resulting from the collaboration agreement. No development fees or royalties have been paid
as of the date of issuance of these financial statements. On August 13, 2024, with the execution
of the license purchase agreement with SciSparc, the collaboration agreement was terminated
(see also Note 13e). |
| e. | On
July 18, 2022, the Company signed a collaboration agreement with NurExone Biologic Inc. (“NurExone”)
pursuant to which the Company, as part of its R&D activity, will use its T&T platform
technology to develop formulations of an intranasal delivery system tailored for NurExone’s
drug candidate. In accordance with the collaboration agreement, NurExone will cover the costs
of the formulation development in an estimated amount of $220 in three installments based
on meeting development milestones, of which $0 and $78 were paid in 2023 and 2022, respectively.
The collaboration agreement further provides that NurExone will pay additional development
fees to the Company upon successful completion of clinical trials, of $500 by successful
completion of Phase 2 clinical trial, $600 upon successful completion of Phase III clinical
trial, $1,125 upon approval by the U.S. Food and Drug Administration and $1,125 upon approval
by an EU regulatory body. Additionally, NurExone will pay royalties of 2.25-3.25% depending
on volume of sales based on any product sales by NurExone resulting from the collaboration
agreement. Manufacturing and marketing rights for formulations developed under the collaboration
agreement are exclusive to NurExone. |
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 9: |
DERIVATIVE WARRANT LIABILITY |
As
discussed in Note 7, on December 15, 2021, the Company and the July 2020 Investor amended the terms of the warrant issued in July 2020.
The
amended warrant did not meet the US GAAP criteria for equity classification as the number of shares to be issued upon exercise of the
warrant and the exercise price of the warrant depend on the share price in the IPO, as well as on whether the IPO is successfully completed.
Accordingly, the amended warrant was initially recognized as a liability at fair value, as of December 15, 2021, with a corresponding
reduction in additional paid-in capital. The amended warrant is subsequently measured at fair value at each reporting date with changes
in fair value recognized as financial income (loss) in the statements of comprehensive loss.
A
summary of significant unobservable inputs (Level 3 inputs) used in measuring the warrant issued are as follows:
| | |
As
of
December 31,
2023 | | |
As
of
December 31,
2022 | |
| Expected volatility | |
| 96.45 | % | |
| 81.93 | % |
| Risk free rate | |
| 4.51 | % | |
| 4.00 | % |
| Expected life (years) | |
| 4 | | |
| 2.5 | |
| Dividend yield | |
| 0 | % | |
| 0 | % |
The
following table presents changes in the fair value of the derivative warrant liability recorded in respect of the warrants:
| Balance as of January 1, 2022 | |
$ | 516 | |
| | |
| | |
| Changes
in fair value | |
| (118 | ) |
| | |
| | |
| Balance as of December 31, 2022 | |
$ | 398 | |
| | |
| | |
| Changes
in fair value | |
| (293 | ) |
| | |
| | |
| Balance as of December 31, 2023 | |
$ | 105 | |
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 10: |
CONVERTIBLE
NOTES |
| a. | 2023
Convertible Notes: |
On
February 4, 2023, the Company completed an additional financing by means of issuance of convertible notes in the amount of $180 from
two existing investors who were related parties of the Company at time of the financing given their equity interests. The convertible
notes have a par value of $180, bear interest at an annual rate of 4% and have an optional maturity date of August 4, 2023, as described
below.
The
terms of the convertible notes provide that in the event a financing in the amount of $500 or more is completed, the par value and interest
accrued thereon are automatically converted into shares based on the share price in such financing, discounted by 20%. In the event a
financing in the amount of less than $500 is completed, the notes and interest accrued thereon are convertible at the option of the noteholders
into shares based on the share price in such financing, discounted by 20%.
In
the event no conversion occurred prior to maturity, the note holder has the option, at its sole discretion, to convert the notes into
shares based on the lowest price per share actually paid to the Company since August 1, 2021 in equity fundings, discounted by 20%. As
of December 31, 2023, as no conversion has occurred, the convertible notes and interest accrued thereon are convertible only in conjunction
with a financing as aforesaid.
In
the occurrence of an exit event, including certain consolidations, mergers or reorganizations that result in a change of control, or
the sale of substantially all of the Company’s assets, as such terms are defined in the financing agreement, prior to conversion
of the notes, the par value and interest accrued thereon are repayable in cash.
Other
than as indicated above, the convertible notes may not be repaid in cash.
The
notes are classified as a liability and are measured at fair value, pursuant to ASC 480-10, “Accounting for Certain Financial instruments
with Characteristics of both Liabilities and Equity”. The fair value was determined based on the fixed monetary amount of the variable
number of shares to be issued upon conversion of the notes, as represented by the 20% discount on the Company’s share value. The
significant input used in the fair value measurement of the notes was the notes’ par value and the contractual 20% discount rate,
as they determine the fixed monetary value of the variable number of shares to be issued upon conversion of the notes in all predominant
scenarios. As the notes’ par value and discount rate are observable inputs, the notes’ fair value is classified as a level
2 measurement in accordance with fair value hierarchy per ASC 820-10, “Fair Value Measurement”.
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 10: |
CONVERTIBLE NOTES (Cont.) |
The
following table presents changes in the fair value of the 2023 Convertible Notes:
| Balance as of December 31, 2022 | |
$ | - | |
| | |
| | |
| Proceeds
from issuance of Convertible Notes | |
| 180 | |
| Changes
in fair value | |
| 20 | |
| | |
| | |
| Balance as of December 31, 2023 | |
$ | 200 | |
| b. | 2022
Convertible notes: |
In
January, June and August 2022, the Company completed a bridge financing by means of issuance of convertible notes in the amount of $718.
The convertible notes have a par value of $718, do not bear interest and have a maturity date of July 31, 2023.
The
terms of the convertible notes provide that in the event an IPO is successfully completed prior to the maturity date, or in the event
a financing in the amount of $300 or more is completed, the notes are automatically converted into shares (or other securities sold in
such IPO) based on the IPO share or unit price.
In
all other instances, including equity fundings, liquidation of the Company or the occurrence of a deemed liquidation transaction, as
well as upon maturity of the notes if no such events occurred prior to the convertible notes maturity date, the convertible notes are
automatically converted into shares of the Company, based on the fair value of the share discounted by 20%. The share price fair value
to be used in the determination of the number of shares to be issued is the share price in such equity fundings or deemed liquidation
transactions, the liquidation value of the Company in a liquidation event, or the share price of the Company in its most recent funding
at the date of issuance of the convertible notes in the event of automatic conversion upon maturity.
The
convertible notes may not be repaid in cash.
The
Company received proceeds of $648 in 2022 and additional $70 in 2023.
The
convertible notes were classified as a liability and were measured at fair value, pursuant to ASC 480-10, “Accounting for Certain
Financial instruments with Characteristics of both Liabilities and Equity”. The fair value was determined based on the fixed monetary
amount of the variable number of shares to be issued upon automatic conversion of the notes, as represented by the 0% discount on the
Company’s share price in an IPO scenario and as represented by the contractual 20% discount on the Company’s share value
in all other scenarios that trigger automatic conversion, adjusted to weigh in the probabilities assigned to each scenario. As the non-IPO
scenarios resulted in an immaterial impact on the notes’ fair value, the significant input used in the fair value measurement was
the notes’ par value, as it represents the fixed monetary value of the variable number of shares to be issued upon automatic conversion
of the notes in an IPO. As the convertible notes’ par value is an observable input, the convertible notes’ fair value is
classified as a level 2 measurement in accordance with fair value hierarchy per ASC 820-10, “Fair Value Measurement”.
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 10: |
CONVERTIBLE NOTES (Cont.) |
On
June 6, 2023, the Company entered into a securities purchase agreement with certain shareholders pursuant to which the Company issued
359,498 ordinary shares for gross proceeds of $407 (see also note 7a2). Following consummation of the securities purchase agreement,
the convertible notes were converted into 792,830 ordinary shares pursuant to their original terms.
The
following table presents changes in the fair value of the 2022 Convertible Notes:
| Balance as of January
1, 2022 | |
$ | - | |
| | |
| | |
| Proceeds from
issuance of 2022 Convertible Notes | |
| 648 | |
| Changes
in fair value | |
| (26 | ) |
| | |
| | |
| Balance
as of December 31, 2022 | |
$ | 622 | |
| Proceeds from
issuance of 2022 Convertible Notes | |
| 70 | |
| Changes in fair value | |
| 206 | |
| Conversion
into ordinary shares | |
| (898 | ) |
| | |
| | |
| Balance
as of December 31, 2023 | |
$ | - | |
| NOTE 11: |
SELECTED STATEMENTS OF COMPREHENSIVE LOSS DATA |
| |
a. |
Research and development expenses: |
| | |
Year
ended
December 31, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Subcontractors and consultants | |
$ | 52 | | |
$ | 66 | |
| Share based payment | |
| 83 | | |
| 111 | |
| Payroll and payroll related | |
| 196 | | |
| 231 | |
| Other | |
| 1 | | |
| 17 | |
| Total expenses | |
| 332 | | |
| 425 | |
| | |
| | | |
| | |
| Research and development
expenses participation (see note 8e) | |
| - | | |
| (78 | ) |
| | |
| | | |
| | |
| | |
$ | 332 | | |
$ | 347 | |
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 11: |
SELECTED STATEMENTS OF COMPREHENSIVE LOSS DATA (Cont.) |
| |
b. |
General and administrative
expenses: |
| | |
Year
ended
December 31, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Payroll and payroll related | |
$ | 151 | | |
$ | 171 | |
| Professional services | |
| 107 | | |
| 289 | |
| Share based payment | |
| 17 | | |
| 19 | |
| Marketing | |
| - | | |
| 15 | |
| Others | |
| 28 | | |
| 54 | |
| | |
| | | |
| | |
| | |
$ | 303 | | |
$ | 548 | |
| |
c. |
Financial income, net: |
| | |
Year
ended
December 31, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Exchange rate differences | |
$ | 11 | | |
$ | 26 | |
| Fair value revaluation in investment in shares | |
| 17 | | |
| - | |
| Fair value revaluation in derivative warrant
liability | |
| (293 | ) | |
| (118 | ) |
| Fair value revaluation in convertible notes | |
| 228 | | |
| (26 | ) |
| Bank fees | |
| 2 | | |
| 2 | |
| | |
| | | |
| | |
| | |
$ | (35 | ) | |
$ | (116 | ) |
The
loss and the weighted average number of ordinary shares used in computing basic and diluted net loss per share is as follows:
| | |
Year
ended
December 31, | |
| | |
2023 | | |
2022 | |
| Numerator: | |
| | |
| |
| Net
loss applicable to shareholders of ordinary shares | |
$ | 600 | | |
$ | 779 | |
| Interest
accrued on Preferred Shares | |
| 69 | | |
| 44 | |
| Total
loss attributed to ordinary shares | |
| 669 | | |
| 823 | |
| | |
| | | |
| | |
| Denominator: | |
| | | |
| | |
| Number
of ordinary shares used in computing basic and diluted net loss per share | |
| 2,030,327 | | |
| 1,305,635 | |
| Net loss
per share of ordinary share, basic and diluted | |
$ | 0.3 | | |
$ | 0.6 | |
POLYRIZON
LTD.
NOTES
TO FINANCIAL STATEMENTS
U.S. dollars
in thousands, except share and per share data
| NOTE 13: |
SUBSEQUENT EVENTS |
The
Company evaluated subsequent events through September 3, 2024, the date these financial statements were available to be issued.
| a. | In
April 2024 the Company completed a bridge financing by means of issuance of a convertible
loan in the amount of $250. The convertible loan has a par value of $250, a maturity date
of April 10, 2026 and bears interest at an annual rate of 4% accrued to maturity. On the
maturity date, the par value and interest accrued thereon are automatically convertible into
shares of the Company based on the price per share actually paid to the Company in the most
recent funding prior to the maturity date. If an IPO is completed prior to the maturity date,
the par value and interest accrued thereon are repayable in cash. Other than as indicated
above and other than in a liquidation event the convertible loan may not be repaid in cash. |
| b. | On
May 7, 2024, the Company and the July 2020 Investor (see Note 7a1) signed an amendment to
the New Warrant according to which the New Warrant will be exercisable, upon successful completion
of an IPO, into 459,770 ordinary shares at an exercise price of $4.35 per share. The amendment
further includes a customary down round protection feature for the exercise price. The other
material terms of the agreement, including the 3 -year term of the New Warrant from the date
of completions of an IPO, did not change as a result of the amendment. |
| c. | On
May 12, 2024, the Company effected a reverse split at a ratio of 2-for-one, pursuant to which
holders of Company’s ordinary shares received one Share for every 2 Shares held. All
shares and per share data in these financial statements have been retrospectively adjusted
to reflect this reverse split and the forward split described in Note 13(g). |
| d. | On
May 12, 2024, the Company entered into a securities purchase agreement with an existing shareholder
pursuant to which the Company will issue 61,751 ordinary shares for gross proceeds of $70. As
of the date of issuance these financial statements, the shares have not been issued and no
proceeds have been received. |
| e. | On
August 13, 2024, the Company entered into an agreement with SciSparc for the purchase of
an exclusive, worldwide, royalty-bearing license with respect to intellectual property rights
associated with SciSparc’s SCI-160 platform (the “Licensed Patent Rights”),
in order to research, develop and commercialize the Licensed Patent Rights in connection
with the diagnosis, prevention, and treatment of pain in humans. |
Pursuant
to the terms of the agreement, SciSparc is entitled to up to $3.32 million based on the achievement of certain milestones, including
(i) $50,000 upon a successful preclinical safety test, (ii) $100,000 upon first patient enrolled in phase I clinical trial, (iii) $120,000
upon first patient enrolled in Phase 2a clinical trial, (iv) $150,000 upon first patient enrolled in Phase 2b clinical trial, (v) $500,000
upon first patient enrolled in Phase 3 clinical trials, (vi) $800,000 upon approval by the FDA, (vii) $800,000 upon approval by an EU
regulatory body, and (viii) $800,000 upon regulatory approval in any additional jurisdiction.
Additionally,
SciSparc is eligible to receive royalties, on a country-by-country and product-by-product basis, at a rate of 5%, on aggregate net sales
of a product that is based on the Licensed Patent Rights for a period of fifteen years from the date of the first sale of a Licensed
Product, on a country-by-country basis, or through the date of expiration of valid claims of any licensed patents with respect to a Licensed
Product in such country, if longer.
Furthermore,
the Company has the right to sell sublicenses for the Licensed Patent Rights, at any point in time, to any sublicensee that is not involved
in legal proceedings against Scisparc and that has equity of at least $5.0 million as per its most recent audited financial statements.
In the event of such sublicensing, the Company is required to pay SciSparc 25% of any proceeds generated from such sublicenses (including
proceeds from the sale of the sublicense). The other material terms of the sublicense agreement, including with respect to payments to
Scisparc by the sublicensee upon the achievement of the aforementioned pre-clinical, clinical trial and regulatory milestones, are required
to be consistent with the August 13, 2024 agreement.
In
consideration for purchase of the license, the Company issued to SciSparc 320,000 ordinary shares and additionally committed to issue
to Sciparc additional securities in the occurrence of certain events, including the listing of the Company’s shares on a public
exchange pursuant to an initial public offering, for a period of two years, such that the value of the aggregate amount of shares and
other securities, as applicable, to be issued to Scisparc will equal $3,000 based on the price at which such securities are to be offered
at such initial public offering.
Simultaneously,
on the date of this Agreement, the parties terminated their collaboration agreement, dated May 30, 2022 (see also note 8d).
| |
f. |
On August
13, 2024, the Company completed a bridge financing by means of issuance of a convertible loan in the amount of $60. The convertible
loan has a par value of $60, a maturity date of August 13, 2026 and bears interest at an annual rate of 4% accrued to maturity. On
the maturity date, the par value and interest accrued thereon are automatically convertible into shares of the Company based on the
price per share actually paid to the Company in the most recent funding prior to the maturity date. If an IPO is completed prior
to the maturity date, the par value and interest accrued thereon are repayable in cash. Other than as indicated above and other than
in a liquidation event the convertible loan may not be repaid in cash. |
| |
|
|
| |
g. |
On August
16, 2024, the Company effected the Second Forward Share Split as follows: the issuance of an aggregate of 420,618 bonus shares to
the holders of our Ordinary Shares on a basis of 0.1494 bonus shares for each Ordinary Share outstanding (equivalent to a forward
share split at a ratio of 0.1494-for-1).1 All shares and per share data in these financial statements have been retrospectively adjusted
to reflect this forward split and the reverse split described in Note 13(c). |
POLYRIZON
LTD.
CONDENSED
FINANCIAL STATEMENTS
AS
OF JUNE 30, 2024
UNAUDITED
U.S.
DOLLARS IN THOUSANDS
INDEX
-
- - - - - - - - - - - - - -
POLYRIZON
LTD.
CONDENSED
BALANCE SHEETS (UNAUDITED)
U.S. dollars
in thousands (except share and per share data)
| | |
| |
As
of
June 30, | | |
As
of
December 31, | |
| | |
Note | |
2024 | | |
2023 | |
| Assets | |
| |
| | | |
| | |
| Current
assets: | |
| |
| | | |
| | |
| Cash
and cash equivalents | |
| |
$ | 23 | | |
$ | 4 | |
| Investment
in shares - at fair value | |
3 | |
| - | | |
| 37 | |
| Other
current assets | |
| |
| 20 | | |
| 13 | |
| | |
| |
| | | |
| | |
| Total
current assets | |
| |
| 43 | | |
| 54 | |
| | |
| |
| | | |
| | |
| Property
and equipment, net | |
| |
| 11 | | |
| 12 | |
| | |
| |
| | | |
| | |
| Deferred
offering costs | |
4 | |
| 532 | | |
| 493 | |
| | |
| |
| | | |
| | |
| Total
assets | |
| |
$ | 586 | | |
$ | 559 | |
| | |
| |
| | | |
| | |
| Liabilities,
temporary equity and shareholders’ deficit | |
| |
| | | |
| | |
| | |
| |
| | | |
| | |
| Current
liabilities: | |
| |
| | | |
| | |
| Employees
and payroll-related liabilities | |
| |
$ | 22 | | |
$ | 39 | |
| Accrued
expenses | |
| |
| 191 | | |
| 158 | |
| Derivative
warrant liability | |
6 | |
| - | | |
| 105 | |
| Convertible
notes | |
7 | |
| - | | |
| 200 | |
| Convertible
loan | |
7 | |
| 151 | | |
| - | |
| | |
| |
| | | |
| | |
| Total
current liabilities | |
| |
| 364 | | |
| 502 | |
| | |
| |
| | | |
| | |
| Temporary
equity: | |
| |
| | | |
| | |
| Preferred shares,
no par value; Authorized: 104,711 shares; Issued and outstanding: 104,711 shares; (*) | |
| |
| 248 | | |
| 248 | |
| | |
| |
| | | |
| | |
| Shareholders’
deficit: | |
5 | |
| | | |
| | |
| Ordinary shares,
no par value; Authorized: 19,895,289 shares; Issued and outstanding: 2,749,077 and 2,550,591 shares as of June 30, 2024
and December 31, 2023, respectively; (*) | |
| |
| - | | |
| - | |
| Additional
paid-in capital | |
| |
| 4,102 | | |
| 3,526 | |
| Receivables on
account of shares | |
| |
| (19 | ) | |
| (196 | ) |
| Accumulated
deficit | |
| |
| (4,109 | ) | |
| (3,521 | ) |
| | |
| |
| | | |
| | |
| Total
shareholders’ deficit | |
| |
| (26 | ) | |
| (191 | ) |
| | |
| |
| | | |
| | |
| Total
liabilities, temporary equity and shareholders’ deficit | |
| |
$ | 586 | | |
$ | 559 | |
| (*) | After
giving effect to the reverse share splits, see also note 5. |
The accompanying
notes are an integral part of the condensed financial statements.
POLYRIZON
LTD.
CONDENSED
STATEMENTS OF COMPREHENSIVE LOSS (UNAUDITED)
U.S. dollars
in thousands (except share and per share data)
| | |
| |
Six
Months Ended
June 30, | |
| | |
Note | |
2024 | | |
2023 | |
| | |
| |
| | |
| |
| Operating expenses: | |
| |
| | |
| |
| Research
and development expenses | |
| |
$ | 137 | | |
$ | 172 | |
| General
and administrative expenses | |
| |
| 210 | | |
| 153 | |
| | |
| |
| | | |
| | |
| Operating loss | |
| |
| 347 | | |
| 325 | |
| | |
| |
| | | |
| | |
| Financial expense (income), net | |
| |
| 241 | | |
| (134 | ) |
| | |
| |
| | | |
| | |
| Net loss and comprehensive
loss | |
| |
$ | 588 | | |
$ | 191 | |
| | |
| |
| | | |
| | |
| Basic and diluted net
loss per share (*) | |
8 | |
$ | 0.2 | | |
$ | 0.1 | |
| | |
| |
| | | |
| | |
| Weighted
average number of shares of ordinary share used in computing basic and diluted net loss per share (*) | |
| |
| 2,604,325 | | |
| 1,587,012 | |
| (*) | After
giving effect to the reverse share splits, see also note 5. |
The accompanying
notes are an integral part of the condensed financial statements.
POLYRIZON
LTD.
CONDENSED
STATEMENT OF CHANGES IN TEMPORARY EQUITY AND SHAREHOLDERS’ DEFICIT (UNAUDITED)
U.S. dollars
in thousands (except share data)
| | |
Preferred
shares | | |
Ordinary
shares | | |
Additional
paid-in | | |
Receivables
on account | | |
Accumulated | | |
Total
shareholders’ | |
| | |
Number (*) | | |
Amount | | |
Number (*) | | |
Amount | | |
capital | | |
of
shares | | |
deficit | | |
deficit | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance as
of December 31, 2023 | |
| 104,711 | | |
| 248 | | |
| 2,550,591 | | |
| - | | |
| 3,526 | | |
| (196 | ) | |
| (3,521 | ) | |
| (191 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Share based payment | |
| - | | |
| - | | |
| - | | |
| - | | |
| 35 | | |
| - | | |
| - | | |
| 35 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Conversion
of convertible note (see Note 5) | |
| - | | |
| - | | |
| 198,486 | | |
| | | |
| 225 | | |
| - | | |
| - | | |
| 225 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Issuance of shares (see
Note 5) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 177 | | |
| - | | |
| 177 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Reclassification
of warrant liability to equity (see Note 6) | |
| - | | |
| - | | |
| - | | |
| - | | |
| 316 | | |
| -- | | |
| | | |
| 316 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (588 | ) | |
| (588 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
as of June 30, 2024 | |
| 104,711 | | |
| 248 | | |
| 2,749,077 | | |
| - | | |
| 4,102 | | |
| (19 | ) | |
| (4,109 | ) | |
| (26 | ) |
POLYRIZON
LTD.
CONDENSED
STATEMENT OF CHANGES IN TEMPORERY EQUITY AND SHAREHOLDERS’ DEFICIT (UNAUDITED)
U.S. dollars
in thousands (except share data)
| | |
Preferred
shares | | |
Ordinary
shares | | |
Additional
paid-in | | |
Receivables
on account | | |
Accumulated | | |
Total
shareholders’ | |
| | |
Number (*) | | |
Amount | | |
Number (*) | | |
Amount | | |
capital | | |
of
shares | | |
deficit | | |
deficit | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance as
of December 31, 2022 | |
| 104,711 | | |
| 248 | | |
| 1,305,635 | | |
| - | | |
| 2,021 | | |
| - | | |
| (2,921 | ) | |
| (900 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Issuance of shares | |
| - | | |
| - | | |
| 359,498 | | |
| - | | |
| 175 | | |
| - | | |
| - | | |
| 175 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Conversion
of convertible notes into shares | |
| - | | |
| - | | |
| 792,830 | | |
| - | | |
| 899 | | |
| - | | |
| - | | |
| 899 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Share based payment | |
| - | | |
| - | | |
| - | | |
| - | | |
| 19 | | |
| - | | |
| - | | |
| 19 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (191 | ) | |
| (191 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
as of June 30, 2023 | |
| 104,711 | | |
| 248 | | |
| 2,457,963 | | |
| - | | |
| 3,114 | | |
| - | | |
| (3,112 | ) | |
| 2 | |
| (*) | After
giving effect to the reverse share splits, see also note 5. |
The accompanying
notes are an integral part of the condensed financial statements.
POLYRIZON
LTD.
CONDENSED
STATEMENTS OF CASH FLOWS (UNAUDITED)
U.S. dollars
in thousands
| | |
Six
Months Ended
June 30, | |
| | |
2024 | | |
2023 | |
| Cash flows from operating activities | |
| | |
| |
| | |
| | |
| |
| Net loss | |
$ | (588 | ) | |
$ | (191 | ) |
| Adjustments to reconcile net loss to net cash
used in operating activities: | |
| | | |
| | |
| Depreciation | |
| 1 | | |
| 2 | |
| Share based payment | |
| 35 | | |
| 19 | |
| Fair value revaluation of investment in shares | |
| 8 | | |
| - | |
| Fair value revaluation in derivative warrant
liability | |
| 211 | | |
| (338 | ) |
| Fair value revaluation in convertible notes | |
| 25 | | |
| 206 | |
| Change in: | |
| | | |
| | |
| Other current assets | |
| (7 | ) | |
| - | |
| Deferred offering costs | |
| (39 | ) | |
| (20 | ) |
| Employees and payroll-related
liabilities | |
| (16 | ) | |
| (1 | ) |
| Accrued
expenses | |
| 33 | | |
| (12 | ) |
| | |
| | | |
| | |
| Net cash used in operating
activities | |
| (337 | ) | |
| (335 | ) |
| | |
| | | |
| | |
| Cash flows from
investing activities | |
| | | |
| | |
| | |
| | | |
| | |
| Purchase of property
and equipment | |
| - | | |
| - | |
| | |
| | | |
| | |
| Net cash used in investing
activities | |
| - | | |
| - | |
| | |
| | | |
| | |
| Cash flows from financing activities | |
| | | |
| | |
| | |
| | | |
| | |
| Proceeds from sale of investment in shares | |
| 28 | | |
| - | |
| Proceeds from issuance of convertible notes | |
| 151 | | |
| 200 | |
| Proceeds from issuance
of ordinary shares | |
| 177 | | |
| 120 | |
| | |
| | | |
| | |
| Net cash provided by
financing activities | |
| 356 | | |
| 320 | |
| | |
| | | |
| | |
| Change in cash and cash equivalents | |
| 19 | | |
| (15 | ) |
| Cash and cash equivalents
at the beginning of the year | |
| 4 | | |
| 36 | |
| | |
| | | |
| | |
| Cash and cash equivalents
at the end of the year | |
$ | 23 | | |
$ | 21 | |
| | |
| | | |
| | |
| Non-cash financing activities: | |
| | | |
| | |
| Conversion
of convertible notes into ordinary shares (see Note 5) | |
$ | 225 | | |
$ | 899 | |
| Classification
of warrant liability into Additional paid-in capital (see Note 6) | |
| 316 | | |
| - | |
| Ordinary
shares issued in exchange for securities | |
$ | - | | |
$ | 54 | |
The accompanying
notes are an integral part of the condensed unaudited financial statements.
POLYRIZON
LTD.
NOTES
TO CONDENSED FINANCIAL STATEMENTS (UNAUDITED)
U.S.
dollars in thousands, except share and per share data
| |
a. |
Polyrizon
Ltd. (the “Company”) was incorporated and commenced its business operations in January 2005. The Company is a clinical
development stage biotech company specializing on the development of nasal gels to provide preventative treatment to protect
against a wide cross section of viruses, including certain variants of COVID-19 that are also considered to cause more infections
and spread faster than the original strain of the virus (the CDC expects that additional variants of the virus will continue
to occur), influenza, allergens, and other toxins. The Company’s proprietary Capture and Contain (“C&C”)
hydrogel platform is delivered in the form of nasal sprays, and form a thin gel-based protective shield containment barrier in
the nasal cavity that prevents viruses, bacteria, allergens, and other toxins from penetrating the nasal epithelial tissue.
Due
to lack of resources, the Company suspended its operations in 2016. In connection with the COVID-19 pandemic, the Company resumed
its operations in 2020. |
| |
b. |
Going concern and management
plans |
The
accompanying financial statements are prepared assuming the Company will continue as a going concern, which contemplates the realization
of assets and liquidation of liabilities in the normal course of business. To date, the Company has not generated revenues from its activities
and has incurred substantial operating losses. Management expects the Company to continue to generate substantial operating losses for
the foreseeable future until it completes development of its products and obtains regulatory approvals to market such products.
Such
conditions raise substantial doubts about the Company’s ability to continue as a going concern for at least a year after the issuance
date of the accompanying financial statements. Management plans to address these conditions by raising funds from its current investors
as well as outside potential investors. However, there is no assurance that such funding will be available to the Company or that it
will be obtained on terms favorable to the Company or will provide the Company with sufficient funds to meet its objectives. The accompanying
financial statements do not include any adjustments relating to the recoverability and classification of assets, carrying amounts or
the amount or classification of liabilities that may be required should the Company be unable to continue as a going concern.
| |
c. |
The Company’s headquarters
and other significant operations are located in Israel, and, therefore, our results may be adversely affected by political, economic
and military instability in Israel, including the recent attack by Hamas that started a war on October 7, 2023. Since the war broke
out, our operations have not been adversely affected by this situation, and we have not experienced disruptions to our development.
However, the intensity and duration of Israel’s current war against Hamas is difficult to predict at this stage, as are such
war’s economic implications on the Company’s business and operations and on Israel’s economy in general. If the
war extends for a long period of time or expands to other fronts, such as Lebanon, Syria and the West Bank, our operations may be
adversely affected. |
POLYRIZON
LTD.
NOTES
TO CONDENSED FINANCIAL STATEMENTS (UNAUDITED)
U.S. dollars
in thousands, except share and per share data
| NOTE 2:
|
SIGNIFICANT ACCOUNTING POLICIES |
| |
a. |
Unaudited
financial statements: |
The
accompanying interim condensed financial statements are unaudited. These unaudited interim condensed financial statements have been prepared
in accordance with generally accepted accounting principles in the United States (the “U.S. GAAP”) and applicable rules and
regulations of the Securities and Exchange Commission regarding interim financial reporting. In management’s opinion, the unaudited
interim condensed financial statements include all adjustments of a normal recurring nature necessary for the fair presentation of the
Company’s financial position as of June 30, 2024, as well as its results of operations and cash flows for the six months ended
June 30, 2024 and 2023. The results of operations for the six months ended June 30, 2024, are not necessarily indicative of the results
to be expected for the year ending December 31, 2024, or for other interim periods or for future years.
These
unaudited interim condensed financial statements should be read in conjunction with the audited financial statements and accompanying
notes and of and for the years ended December 31, 2023 and 2022 (“audited financial statements”). The significant accounting
policies applied in the audited financial statements are applied consistently in these interim condensed financial statements. There
have been no changes to the significant accounting policies described in the audited financial statements that have had a material impact
on the unaudited interim financial statements and related notes.
| |
b. |
Significant
Accounting Policies: |
The
significant accounting policies followed in the preparation of these unaudited interim condensed financial statements are identical to
those applied in the preparation of the audited financial statements.
| |
c. |
Recently
accounting pronouncements: |
In
August 2020, the FASB issued ASU 2020-06, “Debt-Debt with Conversion and Other Options Subtopic 470-20) and Derivatives and Hedging-
Contracts in Entity’s Own Equity (Subtopic 815-40).” The amendments in this update affect entities that issue convertible
instruments and/or contracts in an entity’s own equity. For convertible instruments, the instruments primarily affected are those
issued with beneficial conversion features or cash conversion features because the accounting models for those specific features are
removed. This ASU is effective for the Company starting on January 1, 2024. This standard did not have a material impact on the Company’s
financial statements.
The
following are accounting pronouncements that are not yet effective for the Company:
In
November 2023, the FASB issued ASU 2023-07, “Segment Reporting (Topic 280)”, Improvements to Reportable Segment Disclosures
to improve reportable segment disclosure requirements through enhanced disclosures about significant segment expenses on an interim and
annual basis. All disclosure requirements of ASU 2023-07 are required for entities with a single reportable segment. ASU 2023-07 is effective
starting January 1, 2024, and should be applied on a retrospective basis to all periods presented. Early adoption is permitted. The Company
does not believe that the adoption of this standard will have a material impact on the Company’s financial statements.
POLYRIZON
LTD.
NOTES
TO CONDENSED FINANCIAL STATEMENTS (UNAUDITED)
U.S.
dollars in thousands, except share and per share data
| NOTE 3: |
INVESTMENT IN SHARES - AT
FAIR VALUE |
On
June 6, 2023, the Company entered into a securities purchase agreement with certain shareholders pursuant to which the Company issued
359,498 ordinary shares for gross proceeds of $407 (out of which the Company received from one of the investors shares in the value of
$54, all of which were sold during March and April 2024). The shares are held as a short-term investment in trading securities and are
accordingly classified in current assets.
The
Company’s investment in shares is measured at fair value at each reporting date, based on the shares’ quoted prices (Level
1 measurement). The following table presents changes in the level 1 fair value measurement of Investments in shares:
| | |
U.S.
dollars in
thousands | |
| Balance as of December 31, 2023 | |
$ | 37 | |
| | |
| | |
| Changes in fair value | |
| (8 | ) |
| Sale of investment | |
| (29 | ) |
| | |
| | |
| Balance as of June 30, 2024 | |
$ | - | |
| NOTE 4: |
DEFERRED OFFERING COSTS |
The
Company capitalizes incremental legal and other third-party fees that are directly related to the Company’s in-process equity financings
until such financings are consummated, including the Company’s IPO. After the consummation of the equity financing, these costs
will be recorded as a reduction of the gross proceeds. As of June 30, 2024 and December 31, 2023, there were $532 and $493, respectively,
of deferred offering costs on the balance sheets.
| NOTE 5: |
SHAREHOLDERS’ DEFICIT
|
On
June 6, 2023, the Company entered into a securities purchase agreement with certain shareholders pursuant to which the Company issued
359,498 ordinary shares for gross proceeds of $407 (out of which the Company received from one of the investors shares in the value of
$54). As of December 31, 2023, proceeds of $91 have not yet been received and accordingly were presented as a deduction of equity in
the Company’s statement of changes in temporary equity and shareholder’s deficit. The remaining proceeds have been received
by April 2024.
On
December 19, 2023, the Company entered into a securities purchase agreement with a shareholder pursuant to which the Company issued 80,586
ordinary shares for gross proceeds of $105 thousands. As of December 31, 2023, the proceeds have not yet been received and, accordingly,
were presented as a deduction of equity in the Company’s statement of changes in temporary equity and shareholder’s deficit.
$86 thousands of the proceeds have been received by June 30, 2024.
POLYRIZON
LTD.
NOTES
TO CONDENSED FINANCIAL STATEMENTS (UNAUDITED)
U.S.
dollars in thousands, except share and per share data
| NOTE 5: |
SHAREHOLDERS’ DEFICIT (Cont.) |
On
February 4, 2023, the Company completed an additional financing by means of issuance of convertible notes in the amount of $180 (the
“2023 Convertible notes”). On May 12, 2024, the 2023 Convertible notes were converted into 198,486 ordinary shares (see also
note 7a).
On
May 12, 2024, Company’s shareholders effected a reverse share split of the issued and outstanding shares at a ratio of 2-for-one,
pursuant to which holders of Company’s shares received one share for every 2 shares held.
On
August 16, 2024, the Company effected the issuance of an aggregate of 420,618 bonus shares to the holders of our ordinary shares on a
basis of 0.1494 bonus shares for each ordinary share outstanding (equivalent to a forward share split at a ratio of 0.1494-for-1) (the
“Second Forward Share Split”).
For
accounting purposes, all share and per share amounts for ordinary share, Preferred shares, warrants, options and loss per share amounts
have been adjusted to give retroactive effect to the forward and reverse share splits for all periods presented in these financial statements.
Any fractional shares that resulted from the forward and reverse share splits have been rounded up to the nearest whole share.
| NOTE 6: |
DERIVATIVE WARRANT LIABILITY |
As
part of an investment agreement from July 15, 2020, a warrant was granted to an investor (the “Original Warrant” and the
“July 2020 Investor”, respectively). On December 15, 2021, the Company and the July 2020 Investor amended the terms of the
Original Warrant and in return the July 2020 Investor was granted a new warrant (the “New Warrant”).
The
New Warrant did not meet the US GAAP criteria for equity classification as the number of shares to be issued upon exercise of the New
Warrant and the exercise price of the New Warrant depend on the share price in the IPO, as well as on whether the IPO is successfully
completed. Accordingly, the New Warrant was initially recognized as a liability at fair value, as of December 15, 2021, with a corresponding
reduction in additional paid-in capital. The New Warrant was subsequently measured at fair value at each reporting date with changes
in fair value recognized as financial income (loss) in the statements of comprehensive loss.
On
May 7, 2024, the Company and the July 2020 Investor signed an amendment to the New Warrant according to which the amended New Warrant
will be exercisable, upon successful completion of an IPO, into 459,770 ordinary shares at an exercise price of $4.35 per
share. The amendment further includes a customary down round protection feature for the exercise price. The other material terms of the
agreement, including the 3 -year term of the amended New Warrant from the date of completion of an IPO, did not change.
As
the exercise price and the number of shares to be issued pursuant to exercise became fixed, the derivative warrant liability was classified
to equity on May 7, 2024, the effective date of the amendment.
POLYRIZON
LTD.
NOTES
TO CONDENSED FINANCIAL STATEMENTS (UNAUDITED)
U.S. dollars
in thousands, except share and per share data
| NOTE 6: |
DERIVATIVE WARRANT LIABILITY (Cont.) |
The
following table presents changes in the fair value of the derivative warrant liability recorded in respect of the warrants:
| | |
U.S.
dollars in
thousands | |
| | |
| |
| Balance as of December 31, 2023 | |
| 105 | |
| | |
| | |
| Changes in fair value | |
| 211 | |
| | |
| | |
| Reclassification
to equity | |
| (316 | ) |
| | |
| | |
| Balance as of June 30, 2024 | |
$ | - | |
| NOTE 7: |
CONVERTIBLE NOTES |
| |
a. |
2023 Convertible Notes: |
On
February 4, 2023, the Company completed an additional financing by means of issuance of convertible notes in the amount of $180 from
two existing investors who were related parties of the Company at time of the financing given their equity interests. The convertible
notes have a par value of $180, bear interest at an annual rate of 4% and have an optional maturity date of August 4, 2023, as described
below.
The
terms of the convertible notes provide that in the event a financing in the amount of $500 or more is completed, the par value and interest
accrued thereon are automatically converted into ordinary shares based on the share price in such financing, discounted by 20%. In the
event a financing in the amount of less than $500 is completed, the notes and interest accrued thereon are convertible at the option
of the noteholders into ordinary shares based on the share price in such financing, discounted by 20%.
In
the event no conversion occurred prior to maturity, the note holder has the option, at its sole discretion, to convert the notes into
ordinary shares based on the lowest price per share actually paid to the Company since August 1, 2021 in equity fundings, discounted
by 20%. As of December 31, 2023, as no conversion has occurred, the convertible notes and interest accrued thereon were convertible only
in conjunction with a financing as aforesaid.
In
the occurrence of an exit event, including certain consolidations, mergers or reorganizations that result in a change of control, or
the sale of substantially all of the Company’s assets, as such terms are defined in the financing agreement, prior to conversion
of the notes, the par value and interest accrued thereon were repayable in cash. Other than as indicated above, the convertible notes
may not have been repaid in cash.
On
May 12, 2024, pursuant to issuance of the April 2024 funding (see note 7b) the 2023 convertible notes were converted into 198,486 ordinary
shares.
POLYRIZON
LTD.
NOTES
TO CONDENSED FINANCIAL STATEMENTS (UNAUDITED)
U.S. dollars
in thousands, except share and per share data
| NOTE 7: |
CONVERTIBLE NOTES (Cont.) |
The
convertible notes were classified as a liability and were measured at fair value pursuant to ASC 480-10, “Accounting for Certain
Financial instruments with Characteristics of both Liabilities and Equity.” The fair value was determined based on the fixed monetary
amount of the variable number of ordinary shares to be issued upon conversion of the notes, as represented by the 20% discount on the
Company’s share value. The significant input used in the fair value measurement of the notes was the notes’ par value and
the contractual 20% discount rate, as they determined the fixed monetary value of the variable number of ordinary shares to be issued
upon conversion of the notes in all predominant scenarios. As the notes’ par value and discount rate are observable inputs, the
notes’ fair value was classified as a level 2 measurement in accordance with fair value hierarchy per ASC 820-10, “Fair Value
Measurement”.
The
following table presents changes in the fair value of the 2023 Convertible Notes:
| | |
U.S.
dollars
in
thousands | |
| | |
| |
| Balance as of December 31,
2023 | |
| 200 | |
| | |
| | |
| Changes in fair value | |
| 25 | |
| Conversion into
ordinary shares | |
| (225 | ) |
| | |
| | |
| Balance as of June 30, 2024 | |
$ | - | |
| |
b. |
April 2024 Convertible
Loan: |
In
April 2024, the Company completed a bridge financing by means of issuance of a convertible loan in the amount of $250. The convertible
loan has a principal amount of $250, a maturity date of April 10, 2026 and bears interest at an annual rate of 4%. On the maturity date,
the principal amount and interest accrued thereon are automatically convertible into ordinary shares of the Company based on the price
per share actually paid to the Company in the most recent funding prior to the maturity date. If an IPO is completed prior to the maturity
date, the par value and interest accrued thereon are repayable in cash. Other than as indicated above and other than in a liquidation
event, the convertible loan may not be repaid in cash.
POLYRIZON
LTD.
NOTES
TO CONDENSED FINANCIAL STATEMENTS (UNAUDITED)
U.S. dollars
in thousands, except share and per share data
| NOTE 7: |
CONVERTIBLE NOTES (Cont.) |
The
Company elected to measure the convertible loan at fair value in accordance with ASC 825-10, “Recognition and Measurement of Financial
Assets and Financial Liabilities.” The fair value was determined based on the loan repayment amount in the event an IPO is completed
prior to the maturity date and based on the fixed monetary amount of the variable number of ordinary shares to be issued at the maturity
date if an IPO is not completed by such date. The fair value measurement was substantially identical under both scenarios. The significant
input used in the fair value measurement of the convertible loan is the loan’s principal amount and contractual interest, as these
contractual terms determine the variable number of ordinary shares to be issued at maturity and the cash repayment amount in the event
an IPO is completed prior to maturity. As the loan’s principal amount and contractual interest are observable inputs, the loan’s
fair value is classified as level 2 measurement in accordance with the fair value hierarchy per ASC 820-10, “Fair Value Measurement”.
As
of June 30, 2024 $151 of the proceeds were received. The remaining $99 were received subsequent to the balance sheet date.
The
following table presents changes in the fair value of the April 2024 Convertible Loan:
| | |
U.S.
dollars in
thousands | |
| | |
| |
| Balance as of December 31, 2023 | |
$ | - | |
| | |
| | |
| Proceeds from
issuance of Convertible Loan | |
| 151 | |
| Changes
in fair value | |
| * | |
| | |
| | |
| Balance as of June 30, 2024 | |
| 151 | |
| * | Represents
a value lower than 1 |
The
loss and the weighted average number of ordinary shares used in computing basic and diluted net loss per share is as follows:
| |
|
Six
Months ended
June 30, |
|
| |
|
2024 |
|
|
2023 |
|
| Numerator: |
|
|
|
|
|
|
| Net
loss applicable to holders of ordinary shares |
|
$ |
588 |
|
|
$ |
191 |
|
| Interest
accrued on Preferred Shares |
|
|
36 |
|
|
|
34 |
|
| Total
loss attributed to ordinary shares |
|
|
624 |
|
|
|
225 |
|
| |
|
|
|
|
|
|
|
|
| Denominator: |
|
|
|
|
|
|
|
|
| Number
of ordinary shares used in computing basic and diluted net loss per share |
|
|
2,604,325 |
|
|
|
1,587,012 |
|
| Net loss
per share of ordinary share, basic and diluted |
|
$ |
0.2 |
|
|
$ |
0.1 |
|
POLYRIZON
LTD.
NOTES
TO CONDENSED FINANCIAL STATEMENTS (UNAUDITED)
U.S.
dollars in thousands, except share and per share data
| NOTE 9: |
SUBSEQUENT EVENTS |
The
Company evaluated subsequent events through September 30, 2024, the date these financial statements were available to be issued.
| |
a. |
On August 13, 2024, the Company
entered into an agreement with SciSparc Ltd. (the “SciSparc”) for the purchase of an exclusive, worldwide, royalty-bearing
license with respect to intellectual property rights associated with SciSparc’s SCI-160 platform (the “Licensed Patent
Rights”), in order to research, develop and commercialize the Licensed Patent Rights in connection with the diagnosis, prevention,
and treatment of pain in humans. |
Pursuant
to the terms of the August 13, 2024 agreement, SciSparc is entitled to up to $3.32 million based on the achievement of certain milestones,
including (i) $50,000 upon a successful preclinical safety test, (ii) $100,000 upon first patient enrolled in phase I clinical trial,
(iii) $120,000 upon first patient enrolled in Phase 2a clinical trial, (iv) $150,000 upon first patient enrolled in Phase 2b clinical
trial, (v) $500,000 upon first patient enrolled in Phase 3 clinical trials, (vi) $800,000 upon approval by the FDA, (vii) $800,000 upon
approval by an EU regulatory body, and (viii) $800,000 upon regulatory approval in any additional jurisdiction.
Additionally,
SciSparc is eligible to receive royalties, on a country-by-country and product-by-product basis, at a rate of 5%, on aggregate net sales
of a product that is based on the Licensed Patent Rights for a period of fifteen years from the date of the first sale of a Licensed
Product, on a country-by-country basis, or through the date of expiration of valid claims of any licensed patents with respect to a Licensed
Product in such country, if longer.
Furthermore,
the Company has the right to sell sublicenses for the Licensed Patent Rights, at any point in time, to any sublicensee that is not involved
in legal proceedings against Scisparc and that has equity of at least $5.0 million as per its most recent audited financial statements.
In the event of such sublicensing, the Company is required to pay SciSparc 25% of any proceeds generated from such sublicenses (including
proceeds from the sale of the sublicense). The other material terms of the sublicense agreement, including with respect to payments to
Scisparc by the sublicensee upon the achievement of the aforementioned pre-clinical, clinical trial and regulatory milestones, are required
to be consistent with the August 13, 2024 agreement.
In
consideration for purchase of the license, the Company issued to SciSparc 320,000 ordinary shares and additionally committed to issue
to Sciparc additional securities in the occurrence of certain events, including the listing of the Company’s shares on a public
exchange pursuant to an initial public offering, for a period of two years, such that the value of the aggregate amount of shares and
other securities, as applicable, to be issued to Scisparc will equal $3,000 based on the price at which such securities are to be offered
at such initial public offering.
Simultaneously,
on the date of the August 13, 2024 agreement, the parties terminated their collaboration agreement, dated May 30, 2022.
| |
b. |
On August
13, 2024, the Company completed a bridge financing by means of issuance of a convertible loan in the amount of $60. The convertible
loan has a par value of $60, a maturity date of August 13, 2026 and bears interest at an annual rate of 4% accrued to maturity. On
the maturity date, the par value and interest accrued thereon are automatically convertible into ordinary shares of the Company based
on the price per share actually paid to the Company in the most recent funding prior to the maturity date. If an IPO is completed
prior to the maturity date, the par value and interest accrued thereon are repayable in cash. Other than as indicated above and other
than in a liquidation event the convertible loan may not be repaid in cash. |
| c. | On
August 16, 2024, the Company effected the Second Forward Share Split as follows: the issuance
of an aggregate of 420,618 bonus shares to the holders of our ordinary Shares on a basis
of 0.1494 bonus shares for each Ordinary Share outstanding (equivalent to a forward share
split at a ratio of 0.1494-for-1). All ordinary shares and per share data in these financial
statements have been retrospectively adjusted to reflect this forward split and the reverse
split described in Note 5. |
| d. | On
May 12, 2024, the Company entered into a securities purchase agreement with an existing shareholder
pursuant to which the Company will issue 53,274 ordinary shares for gross proceeds of $70.
The shares have been issued and the proceeds have been received subsequent to the balance
sheet date during July through September 2024. |
Up
to 958,903 Units
Each
Consisting of One Ordinary Share (representing an aggregate of 958,903 Ordinary Shares)
and
Three Warrants, each to Purchase One Ordinary Share (representing an aggregate of 2,876,709 Ordinary Shares issuable upon the exercise
of such warrants)
Polyrizon Ltd.
PROSPECTUS
October
28, 2024
Sole
Book-Running Manager
Aegis
Capital Corp.
Until
and including November 22, 2024, 25 days after the date of this prospectus, all dealers that buy, sell or trade our securities,
whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation
to deliver a prospectus when acting as an underwriter and with respect to unsold allotments or subscriptions.
Polyrizon (NASDAQ:PLRZ)
Historical Stock Chart
From Oct 2024 to Nov 2024

Polyrizon (NASDAQ:PLRZ)
Historical Stock Chart
From Nov 2023 to Nov 2024
