As filed with the Securities and Exchange
Commission on December 18, 2024
Registration
No. 333-283734
UNITED
STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
QUOIN
PHARMACEUTICALS LTD.
(Exact name of Registrant as specified in its
charter)
| State of Israel |
|
2834 |
|
92-2593104 |
| (State or other jurisdiction of |
|
(Primary Standard Industrial |
|
(I.R.S. Employer |
| incorporation or organization) |
|
Classification Code Number) |
|
Identification No.) |
42127
Pleasant Forest Court
Ashburn, VA 20148-7349
(703) 980-4182
(Address, including zip code and telephone number, including area code, of Registrant’s principal executive offices)
Dr. Michael
Myers
Chief Executive Officer
Quoin Pharmaceuticals Ltd.
42127 Pleasant Forest Ct
Ashburn, VA 20148
Tel: 703-980-4182
(Name, address, including zip code and telephone number, including area code of agent for service)
Copies to:
Peter I. Tsoflias
Melissa Palat Murawsky
Blank Rome LLP |
Jonathan Irom, Adv.
Matthew Rudolph, Adv.
Meitar | Law Offices |
Barry I. Grossman
Matthew Bernstein
Ellenoff Grossman & Schole LLP |
One Logan Square
130 North 18th Street
Philadelphia, PA 19103
Tel: (215) 569-5500 |
16 Abba
Hillel Silver Rd.
Ramat Gan 5250608, Israel
Tel: +972-3-610-3100 |
1345 Avenue of the Americas, 11th Floor
New York, NY 10105
Tel: (212) 370-1300 |
Approximate date of commencement of proposed sale to the public: From
time to time after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to
be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, or Securities Act, check the following
box. x
If this Form is filed to register additional securities for an
offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration
statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under
the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration
statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under
the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration
statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated
filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions
of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging
growth company” in Rule 12b-2 of the Securities Exchange Act of 1934, or the Exchange Act.
| Large accelerated filer |
¨ |
|
Accelerated filer |
¨ |
| Non-accelerated filer |
x |
|
Smaller reporting company |
x |
| |
|
|
Emerging growth company |
¨ |
If an emerging growth company, indicate by check mark if the registrant
has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant
to Section 7(a)(2)(B) of the Securities Act. ¨
The Registrant hereby amends this Registration Statement on such
date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states
that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of
1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting
pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus
is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange
Commission is effective. This preliminary prospectus is not an offer to sell these securities and is not soliciting an offer to buy these
securities in any jurisdiction where the offer or sale is not permitted.
| Preliminary
Prospectus |
Subject
to Completion, Dated DECEMBER 18, 2024 |
|

Up
to 19,230,769 Ordinary Shares Represented by American Depositary Shares
Up
to 19,230,769 Pre-Funded Warrants to Purchase Up to 19,230,769 Ordinary Shares Represented by American Depositary Shares
Up
to 19,230,769 Series F Warrants to Purchase Up to 19,230,769 Ordinary Shares Represented by American Depositary Shares
Up
to 19,230,769 Series G Warrants to Purchase Up to 19,230,769 Ordinary Shares Represented by American Depositary Shares
Up
to 57,692,307 Ordinary Shares Represented by American Depositary Shares Issuable Upon Exercise of the Pre-Funded Warrants,
Series F Warrants and Series G Warrants
We
are offering on a “reasonable best efforts” basis up to 19,230,769 ordinary shares of Quoin Pharmaceuticals Ltd. represented
by American Depositary Shares (“ADSs”) together with Series F warrants to purchase an aggregate of up to19,230,769 ordinary
shares represented by ADSs (the “Series F Warrants”) and Series G warrants to purchase an aggregate of up to 19,230,769
ordinary shares represented by ADSs (the “Series G Warrants,” and together with the Series F Warrants, the “Warrants”).The
assumed combined public offering price for each ADS and accompanying Warrants is $0.78, which was the closing
price of our ADSs as reported by The Nasdaq Capital Market (“Nasdaq”) on December 9, 2024. Each ADS represents one ordinary
share. Each Series F Warrant will have an exercise price equal to 100% of the public offering price per ADS and accompanying Warrants,
will be exercisable upon issuance, and will expire two years from the date of issuance. Each Series G Warrant will have an exercise
price of equal to 100% of the public offering price per ADS and accompanying Warrants, will be exercisable upon issuance, and will expire
five years from the date of issuance. The ADSs and the Warrants will be issued separately and will be immediately separable upon issuance
but will be sold together in this offering. This prospectus also relates to the ADSs issuable upon exercise of the Warrants sold in this
offering.
We are also offering to each purchaser, if any,
whose purchase of ADSs in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties,
beneficially owning more than 4.99% of our outstanding ordinary shares immediately following the consummation of this offering, the opportunity
to purchase, if the purchaser so chooses, pre-funded warrants (the “Pre-Funded Warrants”) in lieu of ADSs that would otherwise
result in the purchaser’s beneficial ownership exceeding 4.99% of our outstanding ordinary shares. Each Pre-Funded Warrant will
be immediately exercisable for one ADS and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. The
purchase price of each Pre-Funded Warrant and accompanying Warrants will equal the price at which each ADS and accompanying Warrants are
being sold to the public in this offering, minus $0.0001, and the exercise price of each Pre-Funded Warrant will be $0.0001 per ADS. The
Pre-Funded Warrants and Warrants will be issued separately and will be immediately separable upon issuance but will be purchased together
in this offering. For each Pre-Funded Warrant we sell, the number of ADSs we are offering will be decreased on a one-for-one basis. This
offering also relates to the ADSs issuable upon exercise of any Pre-Funded Warrants sold in this offering. We refer to the ADSs, Warrants
and Pre-Funded Warrants to be sold in this offering collectively as the “Securities.”
For purposes of clarity, each ADS or Pre-Funded Warrant to purchase
one ADS is being sold together with a Series F Warrant to purchase one ADS and a Series G Warrant to purchase one ADS.
Our
ADSs are listed on Nasdaq under the symbol “QNRX.” We have assumed a public offering price of $0.78 per ADS and
accompanying Warrants, which was the closing price of our ADSs on Nasdaq on December 9, 2024. The actual offering price
per ADS and accompanying Warrants or Pre-Funded Warrant and accompanying Warrants, will be negotiated between us and the investors,
in consultation with the placement agent based on, among other things, our history and our prospects, our past and present operating
results, the general condition of the securities markets at the time of this offering and the trading price of our ADS prior to the
offering and may be at a discount to the current market price. Therefore, the assumed public offering price used throughout this
prospectus may not be indicative of the final offering price. In addition, there is no established public trading market for the
Warrants and Pre-Funded Warrants and we do not expect a market to develop. We do not intend to apply for a listing of the Warrants
or Pre-Funded Warrants on any national securities exchange.
We
have engaged Maxim Group LLC to act as our exclusive placement agent (the “Placement Agent” or Maxim”) in connection
with this offering. The Placement Agent has agreed to use its reasonable best efforts to arrange for the sale of the Securities offered
by this prospectus. The Placement Agent is not purchasing or selling any of the Securities we are offering, and the Placement Agent is
not required to arrange the purchase or sale of any specific number of Securities or dollar amount. We have agreed to pay to the Placement
Agent the placement agent fees set forth in the table below, which assumes that we sell all of the Securities offered by this prospectus.
The Securities are expected to be issued in a single closing and the
public offering price per ADS or Pre-Funded Warrant and accompanying Warrants will be fixed for the duration of this offering. We will
deliver all Securities to be issued in connection with this offering delivery versus payment (“DVP”)/receipt versus payment
(“RVP”) upon receipt of investor funds received by us. Accordingly, neither we nor the Placement Agent have made any arrangements
to place investor funds in an escrow account or trust account since the Placement Agent will not receive investor funds in connection
with the sale of the Securities offered hereunder. There is no minimum offering requirement as a condition of closing of this offering.
Because there is no minimum offering amount required as a condition to closing this offering, we may sell fewer than all of the Securities
offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering will not receive
a refund in the event that we do not sell an amount of Securities sufficient to pursue our business goals described in this prospectus.
Any proceeds from the sale of Securities offered by us will be available for our immediate use, despite uncertainty about whether we would
be able to use such funds to effectively implement our business plan. See the section entitled “Risk Factors” on page 11
of this prospectus for more information.
This
offering will terminate on January 11, 2025, unless the offering is fully subscribed
before that date or we decide to terminate the offering (which we may do at any time in our discretion) prior to that date. We will bear
all costs associated with the offering.
One or more of our officers and directors
have indicated interest in participating in this offering at the public offering price and on the same terms as the other purchasers
in this offering. However, because indications of interest are not binding, we cannot guarantee
if any officer or director will participate in this offering.
The
Securities offered in this prospectus involve a high degree of risk. Before deciding whether to invest in our Securities, you should
consider carefully the risks and uncertainties under the heading “Risk Factors” beginning on page 11 of this
prospectus.
| |
|
Per ADS and
Accompanying
Warrants |
|
Per Pre-Funded Warrant
and Accompanying
Warrants |
|
Total(1) |
| Public offering price |
|
$ |
|
|
$ |
|
|
$ |
|
| Placement Agent’s fees (2) |
|
$ |
|
|
$ |
|
|
$ |
|
| Proceeds to us, before expenses (3) |
|
$ |
|
|
$ |
|
|
$ |
|
| (1) | Assumes the sale of the maximum amount of Securities offered hereunder. Because there is no minimum number of Securities or amount
of proceeds required as a condition to closing in this offering, the actual public offering amount, placement agent fees, and proceeds
to us, if any, are not presently determinable and may be substantially less than the total maximum offering amounts set forth above. |
| (2) | We
have agreed to pay the Placement Agent a cash fee equal to 7.0% of the aggregate gross proceeds raised in this offering. We have also
agreed to reimburse the Placement Agent for certain of its offering-related expenses. See “Plan of Distribution” beginning
on page 51 of this prospectus for a description of the compensation to be received by the Placement Agent. |
| (3) | The amount of the proceeds to us presented in this table does not give effect to any exercise of the Warrants or Pre-Funded Warrants. |
Neither the U.S. Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these Securities or passed upon the adequacy or accuracy of this prospectus.
Any representation to the contrary is a criminal offense.
Delivery of the Securities is expected to be made on or about ,
2024, subject to satisfaction of customary closing conditions.
Maxim Group LLC
The date of this prospectus is , 2024.
Table
of Contents
The registration statement containing this prospectus, including
the exhibits to the registration statement, provides additional information about us and the Securities offered under this prospectus.
The registration statement, including the exhibits, can be read on our website and the website of the Securities and Exchange Commission.
See “Where You Can Find More Information.”
Information
contained in, and that can be accessed through our web site, www.quoinpharma.com, shall not be deemed to be part
of this prospectus or incorporated herein by reference and should not be relied upon by any prospective investors for the purposes of
determining whether to purchase the Securities offered hereunder.
Unless
otherwise indicated or the context otherwise requires, all references in this prospectus to the terms “Quoin,” “Quoin
Ltd.,” the “Company,” “us,” “we”, “our” and the “Registrant” refer to
Quoin Pharmaceuticals Ltd., an Israeli company, and its consolidated subsidiaries and “this offering” refers to the
offering contemplated in this prospectus.
About
this Prospectus
We and the Placement Agent have not authorized anyone to provide any
information or to make any representations other than those contained, or incorporated by reference, in this prospectus or in any free
writing prospectuses prepared by us or on our behalf or to which we have referred you. We take no responsibility for, and can provide
no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the Securities
offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. We are not making an offer to sell these
Securities in any jurisdiction where the offer or sale is not permitted or where the person making the offer or sale is not qualified
to do so or to any person to whom it is not permitted to make such offer or sale. You should not assume that the information contained
in this prospectus (as supplemented or amended) is accurate on any date subsequent to the date set forth on the front of the document,
even though this prospectus (as supplemented or amended) is delivered, or Securities are sold, on a later date.
We may also file a prospectus supplement or post-effective amendment
to the registration statement of which this prospectus forms a part that may contain material information relating to this offering. The
prospectus supplement or post-effective amendment may also add, update or change information contained in this prospectus. If there
is any inconsistency between the information in this prospectus and the applicable prospectus supplement or post-effective amendment,
you should rely on the prospectus supplement or post-effective amendment, as applicable. Before purchasing any Securities, you should
carefully read this prospectus, any post-effective amendment, and any applicable prospectus supplement, together with the additional
information described under the heading “Where You Can Find More Information” and “Incorporation of Certain Information
by Reference.”
For
investors outside the United States: We have not done anything that would permit the offering or possession or distribution of this prospectus
in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who
come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of
the Securities described herein and the distribution of this prospectus outside the United States.
This prospectus contains summaries of certain provisions contained
in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries
are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed
or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain
copies of those documents as described below under the section entitled “Where You Can Find More Information.”
The Quoin logo and other trademarks or service marks of Quoin Ltd.
appearing in this prospectus are the property of Quoin Ltd. or Quoin Inc., as applicable. This prospectus contains references to our trademarks
and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including
logos, artwork and other visual displays, may appear without the ™ symbols, but such references are not intended to indicate, in
any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these
trademarks and trade names.
PROSPECTUS SUMMARY
The following summary highlights information contained elsewhere
in this prospectus or incorporated by reference herein and does not contain all the information that may be important to purchasers of
our Securities. Prospective purchasers of our Securities should carefully read the entire prospectus and any applicable prospectus supplement,
including the risks of investing in our Securities discussed under the heading “Risk Factors” contained in this
prospectus, the applicable prospectus supplement and under similar headings in the other documents that are incorporated by reference
into this prospectus. Prospective purchasers of our Securities should also carefully read the information incorporated by reference into
this prospectus, including our financial statements, and the exhibits to the registration statement of which this prospectus is a part.
Company Overview
We are a clinical stage specialty pharmaceutical company dedicated
to the development and commercialization of therapeutic products that treat rare and orphan diseases for which there are currently no
approved treatments or cures. Our initial focus is on the development of products, using our proprietary owned and in-licensed drug delivery
technologies, that could help address rare skin diseases. Our first lead product is QRX003, a once daily, topical lotion comprised of
a broad-spectrum serine protease inhibitor, formulated with the proprietary in-licensed Invisicare® technology, is under development
as a potential treatment for Netherton Syndrome (“NS”), a rare hereditary genetic disease. QRX003 is currently being tested
in two clinical studies in five clinical sites in the United States (“U.S.”) under an open Investigational New Drug (“IND”)
application with the Food and Drug Administration (“FDA”). An Investigator study in a pediatric NS patient has been initiated
in Ireland. We are also developing QRX004 as a potential treatment for Recessive Dystrophic Epidermolysis Bullosa (“RDEB”).
In addition, we entered into two separate Research Agreements with the Queensland University of Technology (“QUT”), under
which we have obtained an option for global licenses to QRX007 for the potential treatment of NS and QRX008 for the potential treatment
of scleroderma, as well as a Research Agreement with the University College Cork (“UCC”) for the development of novel topical
formulations of Rapamycin (sirolimus) as potential treatments for a number of rare and orphan diseases for which there are currently no
approved therapies or cures.
Quoin’s mission is to develop and commercialize proprietary therapeutic
drug products that treat rare and orphan diseases, particularly those where none currently exists. To achieve this, we plan to:
● complete the late-stage
clinical testing of QRX003 in NS and, if successful, file for marketing approval in the United States and other territories;
● prepare to
commercialize QRX003 by establishing our own sales infrastructure in the U.S. and Europe and entering into distribution partnerships
in other territories such as those currently established for Canada, Australia/New Zealand, the Middle East, China, Hong Kong,
Taiwan, Latin America, Central and Eastern Europe, Turkey and Singapore; and
● pursue
business development activities by seeking partnering, licensing, merger and acquisition
opportunities or other transactions to further expand our pipeline and drug-development
capabilities.
Our Current Product Pipeline

*3 Clinical studies underway
**Clinical trial initiated
***Clinical testing to commence Q12025
Netherton Syndrome
NS is a rare autosomal recessive genetic disease affecting approximately
6,000 – 8,000 patients in the U.S. and Europe which is caused by a mutation in the SPINK5 gene and has an incidence of approximately
1/200,000 births. The SPINK5 gene encodes a protein, called lympho-epithelial kazal type related inhibitor (“LEKTI”) that
serves as a brake system on the activity of certain proteases (enzymes that digest proteins) in the skin called Kallikreins. The absence
of the LEKTI protein, as a result of the genetic defect that causes NS, leads to unregulated protease activity in the skin by the Kallikreins,
resulting in too few layers of the outer skin (stratum corneum), thereby leading to a highly defective and compromised skin barrier. As
a result, patients with NS suffer from a variety of medical issues including regular, severe infections, skin cancer, chronic pruritis,
asthma, and allergies among others.
There are currently no approved therapies to treat NS. In the absence
of an approved therapeutic product, patients can only obtain minor symptomatic relief, generally by the regular use of emollients and
moisturizing creams and lotions. Other topical agents must be used with caution because the highly compromised skin in NS patients may
allow ingredients from some topically applied medications to be excessively absorbed into the bloodstream, which may pose a danger to
the patient. Use of topical keratolytic agents, such as urea or lactic acid derivatives, may be limited by skin irritation and is generally
reserved for older children or adults. Base line treatment may also include oral antihistamines, which can help to control the itchy,
eczematous component, and topical or systemic antibiotics as needed. Oral and topical steroids and systemic biologics may be beneficial
in reducing inflammation and the eczematous component of the disease. However, the well-documented side effects of long-term steroid use
need to be carefully considered. There is a critical need for a new and effective treatment for NS.
Regulatory Status of QRX003 for the Treatment of NS
Our lead asset, QRX003, is currently in late-stage clinical development
in the U.S. under an open IND application with the FDA. We submitted an IND in March 2022 to the FDA to initiate a clinical study
of QRX003 in adult NS patients. We received a ‘Study May Proceed’ notification from the FDA on June 13, 2022, which
cleared us to initiate clinical testing of QRX003 in NS patients. This study is fully up and running and five clinical sites in the U.S.
have been opened and are actively recruiting and dosing patients. This study originally was designed as a randomized, double blinded assessment
of two different doses of QRX003 versus a placebo vehicle in 18 adult NS patients. The test materials are applied once daily, over a twelve-week
period, to pre-selected areas of the patient’s body, primarily the arms and legs. Based on discussions with the FDA, a number of
different clinical endpoints are being assessed in the study, including but not limited to, an Investigators Global Assessment (IGA),
Patient’s Global Assessment (PaGA) and Pruritis.
In November 2022, we submitted a protocol for our second clinical
study in NS patients to the FDA under our currently open IND (the “Open Label Study”). This study was cleared by the FDA
to initiate in December 2022. This study originally was designed to be conducted in ten adult NS patients who are currently receiving,
and will continue to do so throughout the study, off-label systemic therapy, primarily systemic biologic therapy. This is an open-label
study with no placebo control. Both of these NS clinical studies are running concurrently and utilize the same clinical trial sites and
investigators.
On October 24, 2023, we released positive initial clinical results
obtained from the first six evaluable subjects in the Open Label Study. Five of the six subjects reported absence of or negligible pruritis,
with one subject reporting no change; three subjects demonstrated improvement with respect to skin appearance on completion of the study
and three showing such improvements during the study though not necessarily on completion of the study. In addition, all six subjects
reported a favorable impression of QRX003 across a number of key metrics, including: ease of use, time to start working, overall satisfaction,
lack of side effects.
As a result of this positive initial data and the absence of any safety
concerns from both studies, on November 8, 2023 we submitted a number of protocol amendments to the FDA, under our open IND, with
a view to optimizing both studies and potentially leading to even better clinical outcomes and a more rapid regulatory approval. These
protocol amendments included eliminating the lower dose from the double-blinded study, modifying the dosing frequency from once-daily
to twice-daily and increasing the number of subjects from 18 to 30. For the Open Label Study, the number of subjects was increased from
10 to 20 and dosing was modified from once-daily to twice-daily. On December 13, 2023, we announced that we were cleared by the FDA
to implement these protocol amendments. In February 2024 we submitted a further protocol amendment to the FDA requesting permission
to lower the age of eligibility for participation in both studies to 14 years and older from 18 years and older. On March 4,
2024 we announced that we were cleared to implement this protocol amendment. All protocol amendments have now been implemented and going
forward participants in both regulatory studies will be dosed twice-daily with those enrolled in the blinded study receiving either a
4% dose of QRX003 or a placebo, while subjects in the Open Label Study will receive a 4% dose of QRX003 only.In March 2022, we submitted
a briefing document to the European Medicines Agency (“EMA”) seeking guidance regarding the clinical and regulatory development
of QRX003 for the European Union (“EU”), to which we received comprehensive and constructive feedback. We also intend to apply
for Orphan Drug status in the U.S. and Europe as well as Rare Pediatric Disease designation in the U.S. for QRX003.On June 27, 2024
we announced that we will expand our ongoing Netherton Syndrome clinical studies to include international sites. The first international
site will be opened at a research hospital in Saudi Arabia. This hospital is currently treating a number of NS patients who will now become
eligible for recruitment into our studies. An experienced Clinical Research Organization has been engaged to manage the study locally.
On August 6, 2024, we announced the planned initiation of an investigator-led
clinical study in New Zealand for QRX003 in pediatric patients with Peeling Skin Syndrome. This rare genetic condition currently has no
approved treatments or cures. The first clinical site and patient have been identified, and Quoin is actively evaluating additional clinical
sites in other countries.
On October 22, 2024 we announced the further expansion of our
ongoing Netherton Syndrome clinical studies to include to include two additional international sites in the United Kingdom (UK). Both
of these sites, Great Ormond Street Hospital and St. Thomas’ Hospital, which are located in London, are highly qualified centers
of excellence for treating Netherton Syndrome patients in the UK. Both sites have available cohorts of patients potentially eligible
to participate in Quoin’s studies. A globally recognized expert in the treatment of NS patients has been appointed as Principal
Investigator for the UK studies and a Clinical Research Organization has been engaged.
The UK and Saudi Arabia sites will operate under the auspices of Quoin’s
open IND application with the FDA. Quoin is also in advanced stage of preparation for the opening of additional sites in several other
Western European countries and is concluding a feasibility study in multiple Eastern European countries with both territories having available
cohorts of patients with Netherton Syndrome.
On November 5, 2024, we announced that QRX003 is being tested
in a pediatric NS patient at the Childrens Hospital in Dublin, Ireland and that we intend to expand this study to include up to three
additional pediatric patients with NS in Spain and up to six additional pediatric patients in the UK. The test materials will be applied
twice daily to pre-selected areas of the patient’s body.
Recent Developments
On December 18, 2024, the Company provided
data from the first subject being dosed twice-daily in Quoin’s ongoing open label study after six weeks of dosing with QRX003,
which is the midpoint of testing. At baseline, prior to dosing, the subject’s Modified Ichthyosis Area of Severity Index (MIASI)
was 18. Following six weeks of dosing with QRX003, the subject’s MIASI had been reduced to 4. In addition, the Investigator’s
Global Assessment (IGA) of disease severity prior to dosing classified the subject as ‘moderate’. After six weeks of dosing
with QRX003, the IGA for the subject was classified as ‘mild’. The subject’s pruritus or itch assessment at baseline
was 7 out of a maximum of 11 based on the Worst Itch Numeric Rating Scale (WINRS) and was reduced to 4 at the treatment midpoint. Finally,
the patient satisfaction scores across assessed metrics were highly positive. No safety concerns were reported for the subject during
this initial testing period.
In addition, after the initial 12 days of
dosing in Quoin’s ongoing 12-week Investigator Pediatric Study, a significant improvement was observed in the skin area treated
with QRX003 versus the non-treated area. Specifically, at baseline prior to dosing with QRX003, the IGA assessment of the subject’s
skin was classified as ‘severe’. After 12 days of treatment with QRX003, this was improved to ‘mild-moderate’,
representing a very rapid improvement in skin appearance. There have been no adverse events or safety concerns reported to date.
Smaller Reporting Company
We are a “smaller reporting company” as defined in the
Securities Exchange Act of 1934, as amended (the “Exchange Act”). As a result, we may take advantage of certain reduced disclosure
obligations available to smaller reporting companies, including the exemption from compliance with the auditor attestation requirements
pursuant to the Sarbanes-Oxley Act of 2022, reduced disclosure about our executive compensation arrangements and the requirements to provide
only two years of audited financial statements in our annual reports and registration statements. We will continue to be a “smaller
reporting company” as long as (1) we have a public float (i.e., the market value of our ADSs held by non-affiliates) less than
$250 million calculated as of the last business day of our most recently completed second fiscal quarter, or (2) our annual revenues
are less than $100 million for our previous fiscal year and we have either no public float or a public float of less than $700 million
as of the end of that fiscal year’s second fiscal quarter. Decreased disclosures in our SEC filings due to our status as a “smaller
reporting company” may make it harder for investors to analyze our results of operations and financial prospects.
Company Information
We were incorporated under the laws of the State of Israel in 1986
under the name Montiger Ltd. Between 1986 and 2021, we underwent several name changes, including the name change to Cellect Biotechnology
Ltd. (“Cellect”). On October 28, 2021, Cellect completed the business combination with Quoin Pharmaceuticals, Inc.,
a Delaware corporation (“Quoin Inc.”), in accordance with the terms of the Agreement and Plan of Merger and Reorganization,
dated as of March 24, 2021 (the “Merger Agreement”), by and among Cellect, Quoin Inc. and CellMSC, Inc., a
Delaware corporation and wholly-owned subsidiary of Cellect (“Merger Sub”), pursuant to which Merger Sub merged with and into
Quoin Inc., with Quoin Inc. surviving as a wholly-owned subsidiary of Cellect (the “Merger”). Immediately after completion
of the Merger, Cellect changed its name to “Quoin Pharmaceuticals Ltd.”
Prior to January 1, 2023, we qualified as a “foreign private
issuer” as such term is defined in Rule 405 under the Securities Act. Since January 1, 2023, we have been obligated to
file or furnish reports, proxy statements, and other information on U.S. domestic issuer forms with the Securities and Exchange Commission
(the “SEC”), which are more detailed and extensive in certain respects, and which must be filed more promptly, than the forms
available to a foreign private issuer.
The address of our executive corporate offices is 42127 Pleasant Forest
Ct., Ashburn, VA 20148, and our telephone number is (703) 980-4182. Our website is www.quoinpharma.com. Information contained on
or accessible through this website is not incorporated by reference in, or otherwise a part of, this prospectus, and any references to
this website are intended to be inactive textual references only.
THE
OFFERING
| Issuer |
|
Quoin Pharmaceuticals Ltd. |
| |
|
|
| ADSs offered by us |
|
Up to 19,230,769 ADSs at an assumed public offering price of $0.78 per ADS and accompanying Warrants, which represents the closing price of our ADSs on Nasdaq on December 9, 2024. |
| |
|
|
| Pre-Funded Warrants offered by us |
|
We are also offering up to 19,230,769 Pre-Funded Warrants to purchase
up to 19,230,769ADSs in lieu of ADSs to any purchaser whose purchase of shares of ADSs in this offering would otherwise result in such
purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% of our outstanding
ordinary shares immediately following the consummation of this offering. Each Pre-Funded Warrant will be exercisable for one ADS, will
have an exercise price of $0.0001 per ADS, will be immediately exercisable, and will not expire until exercised in full. This prospectus
also relates to the offering of the ADSs issuable upon exercise of the Pre-Funded Warrants. For each Pre-Funded Warrant that we sell,
the number of ADSs that we are selling will be decreased on a one-for-one basis. |
| |
|
|
| Warrants offered by us |
|
We are also offering (i) up to 19,230,769 Series F Warrants to purchase up to 19,230,769 ADSs and (ii) up to 19,230,769 Series G Warrants to purchase up to 19,230,769 ADSs. Each Series F Warrant will be exercisable for one ADS, will have an exercise price equal to 100% of the combined public offering price per share of ADS and accompanying Warrants, will be exercisable upon issuance, and will expire two years from the date of issuance. Each Series G Warrant will be exercisable for one ADS, will have an exercise price equal to 100% of the combined public offering price per share of ADSs and accompanying Warrants, will be exercisable upon issuance, and will expire five years from the date of issuance. This prospectus also relates to the offering of the ADSs issuable upon exercise of the Warrants. Because we will issue a Series F Warrant and a Series G Warrant for each ADS and for each Pre-Funded Warrant sold in this offering, the number of Warrants sold in this offering will not change as a result of a change in the mix of ADSs and Pre-Funded Warrants sold. |
| |
|
|
| ADSs outstanding prior to this offering |
|
5,049,720 ADSs (assuming all ordinary shares are represented by ADSs) |
| |
|
|
| ADSs to be outstanding after this offering |
|
24,280,489 ADSs (assuming all ordinary shares are represented by ADSs, all of the ADSs we are offering under this prospectus are sold, and assuming no sale of Pre-Funded Warrants, which, if sold, would reduce the number of ADSs that we are offering on a one-for-one basis, and no exercise of the Warrants issued in this offering) |
| Reasonable best efforts offering |
|
We have agreed
to offer and sell the Securities offered hereby to the purchasers through the Placement Agent. The Placement Agent has agreed to use
its reasonable best-efforts to arrange for the sale of the Securities offered by this prospectus. The Placement Agent is not purchasing
or selling any of the Securities we are offering and the Placement Agent is not required to arrange the purchase or sale of any specific
number or dollar amount of Securities. See “Plan of Distribution” on page 51 of this prospectus. |
| |
|
|
| Use of proceeds |
|
Assuming 19,230,769ADSs are sold in this offering at an assumed combined public offering price of $0.78 per ADS and accompanying Warrants, which represents the closing price of our ADS on Nasdaq on December 9, 2024, and assuming no issuance of Pre-Funded Warrants and no exercise of Warrants issued in connection with this offering, we estimate that our net proceeds from the this offering will be approximately 13.4 million, after deducting placement agent fees and estimated offering expenses payable by us. However, this is a best efforts offering with no minimum number of Securities or amount of proceeds as a condition to closing, and we may not sell all or any of these Securities offered pursuant to this prospectus; as a result, we may receive significantly less in net proceeds. |
| |
|
|
| |
|
We
currently intend to use the net proceeds from the sale of the Securities under this prospectus for general corporate purposes, which
may include operating expenses, research and development, including clinical and pre-clinical testing of our product candidates, working
capital, future acquisitions and general capital expenditures. See “Use of Proceeds” on page 16 of this prospectus. |
| |
|
|
Insider participation |
|
One
or more of our officers and directors have indicated interest in participating in this offering at
the public offering price and on the same terms as the other purchasers in this offering. However,
because indications of interest are not binding, we cannot guarantee if any officer or director will
participate in this offering.
|
| |
|
|
| Depositary |
|
The Bank of New York Mellon |
| |
|
|
| Transfer Agent and Registrar |
|
Computershare Trust Company, N.A. |
| |
|
|
| Risk factors |
|
See “Risk Factors”
beginning on page 11 of this prospectus and the documents incorporated by reference herein for a discussion of factors you
should carefully consider before deciding to invest in our Securities. |
| |
|
|
| Listing |
|
Our ADSs are listed on The Nasdaq Capital Market under the symbol “QNRX.” We do not intend to apply for a listing of the Pre-Funded Warrants or the Warrants on any national securities exchange or other nationally recognized trading system. |
The
information above is based on 5,049,720 ordinary shares represented by 5,049,720 ADSs outstanding as of December 9, 2024
(assuming all ordinary shares are represented by ADSs), and excludes the following:
| · | 1,943,787 ordinary shares represented by 1,943,787 ADSs issuable upon the exercise of outstanding options at a weighted-average exercise
price of $4.29 per ADS; and |
| · | 8,988,346 ordinary shares represented by 8,988,346 ADSs issuable upon the exercise of outstanding warrants at a weighted-average exercise
price of $2.10 per ADS. |
Unless
otherwise indicated, all information in this prospectus assumes no exercise of the outstanding options or warrants described above after
December 9, 2024, including, for the avoidance of doubt, any Pre-Funded Warrants or Warrants.
We may enter into privately negotiated agreements with the holders
of certain existing outstanding warrants to purchase up to 8,988,346 ADSs (the “Prior Warrants”) to, among other things,
reduce the exercise price of such Prior Warrants and to extend the current expiration date of the Prior Warrants. There can be no assurance
that we will amend the Prior Warrants or as to the final terms of any amendments to the Prior Warrants.
CAUTIONARY STATEMENT
REGARDING FORWARD-LOOKING STATEMENTS
Certain information contained, or incorporated by reference, in this
prospectus may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of
1995 and other securities laws. Forward-looking statements are often characterized by the use of forward-looking terminology such as “may,”
“will,” “expect,” “anticipate,” “estimate,” “continue,” “believe,”
“should,” “intend,” “project” or other similar words, but are not the only way these statements are
identified.
These forward-looking statements may include, but are not limited to,
statements relating to our objectives, plans and strategies, statements that contain projections of results of operations or of financial
condition, expected capital needs and expenses, statements relating to the research, development, completion and use of our products,
and all statements (other than statements of historical facts) that address activities, events or developments that we intend, expect,
project, believe or anticipate will or may occur in the future.
Forward-looking statements are not guarantees of future performance
and are subject to risks and uncertainties. We have based these forward-looking statements on assumptions and assessments made by our
management in light of their experience and their perception of historical trends, current conditions, expected future developments and
other factors they believe to be appropriate.
Important factors that could cause actual results, developments and
business decisions to differ materially from those anticipated in these forward-looking statements include, among other things:
| · | our limited operating history and the difficulties encountered by a small developing company; |
| · | our history of losses and need for additional capital to fund our operations and our inability to obtain additional capital on acceptable
terms, or at all; |
| · | our lack of revenue generated from product sales since inception, and potential inability to be profitable; |
| · | uncertainties of cash flows and inability to meet working capital needs; |
| · | our ability to obtain regulatory approvals; |
| · | our ability to generate favorable pre-clinical and clinical trial results; |
| · | our ability to identify and develop potential product candidates; |
| · | additional costs or delays associated with unsuccessful clinical trials; |
| · | the inability to predict the timing of revenue from sales of a future product; |
| · | the extensive regulatory requirements and future developmental and regulatory challenges we will still face even if we obtain approval
for a product candidate; |
| · | our ability to obtain or maintain orphan drug designation or data exclusivity for our product candidates; |
| · | our ability to obtain Orphan Disease and Rare Pediatric Disease designations for our product candidates; |
| · | the potential oversight of programs or product candidates that may be more profitable or more successful; |
| · | our manufacturing processes may not be validated and our methodology
may not be accepted by the scientific community; |
| · | the ability to conduct clinical trials, because of difficulties enrolling patients or other reasons; |
| · | the requirements of being a publicly traded company may strain our resources; |
| · | potential adverse effects resulting from failure to maintain effective internal controls; |
| · | our ability to comply with the applicable continued listing requirements of Nasdaq; |
| · | the potential negative impact on our securities price and trading volume if securities or industry analysts do not publish reports
about us or if they adversely change their recommendations about our business; |
| · | failure to meet the continued listing requirements of the Nasdaq Capital Market could result in a delisting of our ADSs; |
| · | the potential volatility of the market price for our ADSs; |
| · | the potential dilution of our shareholders’ potential ownership due to future issuances of share capital; |
| · | the requirement for holders of ADSs to act through the depositary to exercise their rights; |
| · | the potential limitations on ADS holders with respect to the transfer of their ADSs; and |
| · | the risks of securities class action litigation. |
The
risks and uncertainties included here are not exhaustive or necessarily in order of importance. Other sections of this prospectus, including
“Risk Factors” beginning on page 11, our Annual Report on Form 10-K for the year ended
December 31, 2023, and other reports that we file with the SEC include additional factors that could affect our businesses and financial
performance. New risk factors emerge from time to time, and it is not possible for management to predict all such risk factors. You are
urged to carefully review and consider the various disclosures made throughout this prospectus which are designed to advise interested
parties of the risks and factors that may affect our business, financial condition, results of operations and prospects.
You
should not put undue reliance on any forward-looking statements. Although the forward-looking statements in this prospectus are
based on our beliefs, assumptions and expectations, taking into account all information currently available to us, we cannot guarantee
future transactions, results, performance, achievements or outcomes. No assurance can be made to any investor by anyone that the expectations
reflected in our forward-looking statements will be attained, or that deviations from them will not be material and adverse. We undertake
no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise,
except as required by law.
In addition, certain sections of this prospectus contain information
obtained from independent industry sources and other sources that we have not independently verified.
RISK FACTORS
Investing in our Securities involves a
high degree of risk. Before making an investment in our Securities, you should carefully consider the risk factors discussed below
as well as other information we include or incorporate by reference in this prospectus, including the risks and uncertainties
discussed under “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2023 and
our Quarterly
Report on Form 10-Q for the nine months ended September 30, 2024, each of which has been filed with the SEC and is
incorporated by reference in this prospectus, as well as any updates thereto contained in subsequent filings with the SEC or any
free writing prospectus. All of these risk factors are incorporated herein in their entirety. If any of the following risks occur,
our business, financial condition, results of operations and prospects could be materially and adversely affected. In that case, the
market price of our Securities could decline and you could lose all or part of your investment. Additional risks and uncertainties
not presently known to us or that we currently deem immaterial may also materially harm our business, operating results and
financial condition and could result in a complete loss of your investment.
Risks Related to this Offering
This is a reasonable best efforts offering, in which no minimum
number or dollar amount of Securities is required to be sold, and we may not raise the amount of capital we believe is required for our
business plans.
The Placement Agent has agreed to use its reasonable best efforts to
solicit offers to purchase the Securities in this offering. The Placement Agent has no obligation to buy any of the Securities from us
or to arrange for the purchase or sale of any specific number or dollar amount of the Securities. There is no required minimum number
of Securities that must be sold as a condition to completion of this offering, and there can be no assurance that the offering contemplated
hereby will ultimately be consummated. Even if we sell Securities offered hereby, because there is no minimum offering amount required
as a condition to the closing of this offering, the actual offering amount is not presently determinable and may be substantially less
than the maximum amount set forth above. We may sell fewer than all of the Securities offered hereby, which may significantly reduce the
amount of proceeds received by us. Thus, we may not raise the amount of capital we believe is required for our operations in the short-term
and may need to raise additional funds, which may not be available or available on terms acceptable to us.
Because there is no minimum required for the offering to close,
investors in this offering will not receive a refund in the event that we do not sell an amount of Securities sufficient to pursue the
business goals outlined in this prospectus.
We have not specified a minimum offering amount nor have or will we
establish an escrow account in connection with this offering. Because there is no escrow account and no minimum offering amount, investors
could be in a position where they have invested in our company, but we are unable to fulfill our objectives due to a lack of interest
in this offering. Further, because there is no escrow account in operation and no minimum investment amount, any proceeds from the sale
of Securities offered by us will be available for our immediate use, despite uncertainty about whether we would be able to use such funds
to effectively implement our business plan. Investor funds will not be returned under any circumstances whether during or after the offering.
You may experience immediate and substantial dilution
in the net tangible book value of the shares you purchase in this offering and may experience additional dilution in the future.
The public offering price per ADS and accompanying Warrants and the
public offering price of each Pre-Funded Warrant and accompanying Warrants may be substantially higher than the as adjusted net tangible
book value per ADS after giving effect to this offering. Assuming the sale of 19,230,769 ADSs at an assumed public offering price of
$0.78 per ADS and accompanying Warrants (which is based on the closing price of our ADSs on Nasdaq on December 9, 2024), assuming
no sale of any Pre-Funded Warrants in this offering, and after deducting the Placement Agent fees and commissions and estimated offering
expenses payable by us, you will incur immediate dilution in as adjusted net tangible book value of approximately $0.03 per ADS.
As a result of the dilution to investors purchasing Securities in this offering, investors may receive significantly less than the purchase
price paid in this offering, if anything, in the event of the liquidation of our company. See the section entitled “Dilution”
below for a more detailed discussion of the dilution you will incur if you participate in this offering. To the extent shares are issued
under outstanding options and warrants at exercise prices lower than the public offering price of our ADSs or Pre-Funded Warrants in
this offering, you will incur further dilution.
This offering may cause the trading price of our ADSs to decrease.
The price per ADS, together with the number of ADSs we propose
to issue and ultimately will issue if this offering is completed, may result in an immediate decrease in the market price of our ADSs.
This decrease may continue after the completion of this offering. Sales of substantial amounts of our ADSs in the public market, or the
perception that such sales might occur, could adversely affect the market price of our ADSs.
You may experience future dilution as a result of future equity
offerings.
In order to raise additional capital, we may in the future offer additional
ADSs or other securities convertible into or exchangeable for our ADSs that could result in further dilution to investors purchasing our
ordinary shares represented by ADSs in this offering or result in downward pressure on the price of our ADSs. We may sell ADSs or other
securities in any other offering at prices that are higher or lower than the prices paid by investors in this offering, and investors
purchasing shares or other securities in the future could have rights superior to existing shareholders.
In
addition, we may enter into privately negotiated agreements with the holders of certain existing outstanding warrants to purchase up to
8,988,346 ADSs to, among other things, reduce the exercise price of such Prior Warrants and to extend the current expiration
date of the Prior Warrants. There can be no assurance that we will amend the Prior Warrants or as to the final terms of any amendments
to the Prior Warrants.
Our management will have broad discretion over the use of the
net proceeds from this offering, you may not agree with how we use the proceeds, and the proceeds may not be invested successfully.
We have not designated any portion of the net proceeds from this offering
to be used for any particular purpose. Accordingly, our management will have broad discretion as to the use of the net proceeds from this
offering and could use them for purposes other than those contemplated at the time of commencement of this offering. Accordingly, you
will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity,
as part of your investment decision, to assess whether the proceeds are being used appropriately. It is possible that, pending their use,
we may invest the net proceeds in a way that does not yield a favorable, or any, return for our company. Our management’s judgment
may not result in positive returns on your investment and you will not have the opportunity to evaluate the economic, financial or other
information upon which our management bases its decisions.
We will need to raise additional funding to fund our working
capital needs or consummate potential future acquisitions. Additional financing may not be available on acceptable terms, or at all. Failure
to obtain additional capital may force us to limit or terminate our operations.
Even if we sell all Securities offered hereby, the expected net proceeds
of this offering will not be sufficient for us to fund the working capital needs of our business or potential strategic acquisitions we
may pursue in the future. We will continue to seek funds through equity or debt financings, collaborative or other arrangements with corporate
sources, or through other sources of financing. Additional funding may not be available to us on acceptable terms, or at all. Any failure
to raise capital as and when needed could have a negative impact on our financial condition and on our ability to pursue our business
plans and strategies.
There is no public market for the Pre-Funded Warrants or the
Warrants being offered in this offering.
There is no established public trading market for the Pre-Funded
Warrants or the Warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply
to list the Pre-Funded Warrants or the Warrants on any securities exchange or nationally recognized trading system. Without an active
market, the liquidity of the Pre-Funded Warrants or the Warrants will be limited.
Holders of our Pre-Funded Warrants or the Warrants will have
no rights as holders of ordinary shares represented by ADSs until such warrants are exercised.
Until you acquire ordinary shares represented by ADSs upon exercise
of your Pre-Funded Warrants or Warrants, you will have no rights with respect to ADSs issuable upon exercise of your Pre-Funded Warrants
or Warrants. Upon exercise of your Pre-Funded Warrants or Warrants, you will be entitled to exercise the rights of a holder of ordinary
shares represented by ADSs only as to matters for which the record date occurs after the exercise date.
The Warrants may not have any value.
Each Warrant has an exercise price equal to 100% of the public offering
price per ADS and accompanying Warrants in this offering. In the event the market price per our ADS does not exceed the exercise price
of the Warrants during the period when the Warrants are exercisable, the Warrants may not have any value.
Provisions of the Warrants offered by this prospectus could discourage
an acquisition of us by a third party.
Certain provisions of the Warrants offered by this prospectus could
make it more difficult or expensive for a third party to acquire us. The Warrants prohibit us from engaging in certain transactions constituting
“fundamental transactions” unless, among other things, the surviving entity assumes our obligations under the Warrants. Further,
the Warrants provide that, in the event of certain transactions constituting “fundamental transactions,” with some exceptions,
holders of such warrants will have the right, at their option, to require us to purchase such Warrants from the holders for consideration
of the same type as that offered to the holders of ordinary shares in such transaction in an amount determined pursuant to a formula set
forth in such warrants. These and other provisions of the Warrants offered by this prospectus could prevent or deter a third party from
acquiring us even where the acquisition could be beneficial to you.
Purchasers who purchase our Securities in this offering pursuant
to a securities purchase agreement may have rights not available to purchasers that purchase without the benefit of a securities purchase
agreement.
In
addition to rights and remedies available to all purchasers in this offering under federal securities and state law, the purchasers that
enter into a securities purchase agreement will also be able to bring claims of breach of contract against us. The ability to pursue a
claim for breach of contract provides those investors with the means to enforce the covenants uniquely available to them under the securities
purchase agreement including, but not limited to: (i) a covenant to not enter into variable rate financings for a period of 180
days following the closing of the offering, subject to certain exceptions; (ii) a covenant to not enter into any equity financings
for 90 days from closing of the offering, subject to certain exceptions.
Risks Related to Ownership of Our ADSs and Ordinary Shares
Our failure to meet the continued listing requirements
of The Nasdaq Capital Market could result in a delisting of our ADSs.
Our ADSs are listed on the Nasdaq Capital Market,
which imposes, among other requirements, a minimum bid requirement.
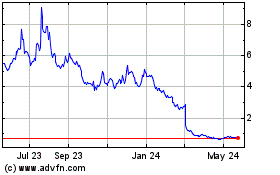
On April 29, 2024, we received a deficiency
letter from the Listing Qualifications Department of Nasdaq notifying us that for the preceding 31 consecutive business days (March 14,
2024 through April 26, 2024), our ADSs did not maintain a minimum closing bid price of $1.00 (“Minimum Bid Price Requirement”)
per ADS as required by Nasdaq Listing Rule 5550(a)(2). In accordance with Nasdaq Listing Rule 5810(c)(3)(A), we had a compliance
period of 180 calendar days, or until October 28, 2024, to regain compliance with Nasdaq Listing Rule 5550(a)(2). On October 16,
2024, the Company submitted a letter to Nasdaq requesting an additional 180-day grace period to regain compliance with the Minimum Bid
Price Requirement. On October 29, 2024, the Company received a letter from the Listing Qualifications Department of Nasdaq granting
the Company an additional 180 calendar day grace period, or until April 28, 2025, to regain compliance. The Staff’s determination
in granting the Company the extension was based on the Company meeting the continued listing requirement for market value of publicly
held shares and all other applicable requirements for initial listing on the Nasdaq Capital Market with the exception of the Minimum Bid
Price Requirement, and the Company’s written notice of its intention to cure the deficiency during the second compliance period
by effecting a reverse split, if necessary. Compliance may be achieved without further action if the closing bid price of the Company’s
ADS is at or above $1.00 for a minimum of ten consecutive business days at any time during the second compliance period, in which case
Nasdaq will notify the Company if it determines the Company is in compliance and the matter will be closed; however Nasdaq could require
the closing bid price to equal or to exceed the $1.00 minimum bid price requirement for more than 10 consecutive business days before
determining that the Company complies. If compliance cannot be demonstrated by April 28, 2025, the Staff will provide written notification
that the Company’s securities will be delisted. At that time, the Company may appeal the Staff’s determination to a Hearings
Panel.
If we cannot regain compliance with the Minimum
Bid Price Requirement or if we otherwise fail to meet any of Nasdaq’s listing standards, our ADSs will be subject to delisting.
If that were to occur, our ADSs would be subject to rules that impose additional sales practice requirements on broker-dealers who
sell our securities. The additional burdens imposed upon broker-dealers by these requirements could discourage broker-dealers from effecting
transactions in our ADSs. This would adversely affect the ability of investors to trade our ADSs and would adversely affect the value
of our ADSs. Delisting from Nasdaq would cause us to pursue eligibility for trading of our ADSs on other markets or exchanges, or on an
over-the-counter market. In such case, our stockholders’ ability to trade or obtain quotations of the market value of our ADSs would
be severely limited because of lower trading volumes and transaction delays. These factors could contribute to lower prices and larger
spreads in the bid and ask prices of these securities. There can be no assurance that our ADSs, if delisted from the Nasdaq, would be
listed on a national securities exchange, a national quotation service or the over-the-counter markets. Delisting from the Nasdaq could
also result in negative publicity, adversely affect the market liquidity of our ADSs, decrease securities analysts’ coverage of
us or diminish investor, supplier and employee confidence.
The delisting of our ADSs from Nasdaq may make
it more difficult for us to raise capital on favorable terms in the future, or at all. Such a delisting would likely have a negative effect
on the price of our ADSs and would impair your ability to sell or purchase our ADSs when you wish to do so. Further, if our ADSs were
to be delisted from Nasdaq, our ADSs would cease to be recognized as a covered security, and we would be subject to additional regulation
in each state in which we offer our securities. Moreover, there is no assurance that any actions that we take to restore our compliance
with the Nasdaq Minimum Bid Price Requirement would stabilize the market price or improve the liquidity of our ADSs, prevent our ADSs
from falling below the Nasdaq minimum bid price required for continued listing again or prevent future non-compliance with other applicable
Nasdaq listing requirements, including maintaining minimum levels of stockholders’ equity or market values of our ADSs, our ADSs
could be delisted.
USE OF PROCEEDS
We estimate that we will receive net proceeds
from this offering of approximately $13.4 million (assuming the sale of the maximum number of Securities offered hereby), based upon an
assumed public offering price of $0.78 per ADS and accompanying Warrants (which is the closing price of our ADS on Nasdaq on December 9,
2024), after deducting the estimated placement agent fees and estimated offering expenses payable by us and assuming no issuance of any
Pre-Funded Warrants and no exercise of the Warrants. However, because this is a reasonable best efforts offering with no minimum number
of Securities or amount of proceeds as a condition to closing, the actual offering amount, placement agent fees, and net proceeds to us
are not presently determinable and may be substantially less than the maximum amounts set forth on the cover page of this prospectus,
and we may not sell all or any of the Securities we are offering. As a result, we may receive significantly less in net proceeds. Based
on the assumed offering price set forth above, we estimate that our net proceeds from the sale of 75%, 50%, and 25% of the Securities
offered in this offering would be approximately $9.9 million, $6.4 million, and $2.9 million, respectively, after deducting
the estimated placement agent fees and estimated offering expenses payable by us, and assuming no issuance of any Pre-Funded Warrants
and assuming no exercise of the Warrants. We will only receive additional proceeds from the exercise of the Warrants we are selling in
this offering if the Warrants are exercised for cash. We cannot predict when or if these Warrants will be exercised. It is possible that
these Warrants may expire and may never be exercised.
These estimates exclude the proceeds, if any,
from the exercise of the Warrants offered hereby. If all of the Warrants offered hereby were to be exercised in cash, we would receive
additional proceeds of approximately $30.0 million. We cannot predict when or if these Warrants will be exercised. It is possible that
these Warrants may expire and may never be exercised. Additionally, these Warrants contain a cashless exercise provision that permit
exercise of such Warrants on a cashless basis at any time when there is no effective registration statement under the Securities Act
covering the issuance of the underlying shares.
We currently intend to use these net proceeds for general corporate
purposes, which may include operating expenses, research and development, including clinical and pre-clinical testing of our product candidates,
working capital, future acquisitions and general capital expenditures. We have not determined the amount of net proceeds to be used specifically
for any of such purposes.
The expected use of net proceeds from this offering represents our
intentions based upon our current plans and business conditions, which could change in the future as our plans and business conditions
evolve and change. The amounts and timing of our actual expenditures, specifically with respect to working capital, may vary significantly
depending on numerous factors. As a result, our management will retain broad discretion over the allocation of the net proceeds from this
offering. We have no current agreements, commitments or understandings for any material acquisitions or licenses of any products, businesses
or technologies that are definitive or probable to close. Unforeseen events or changed business conditions may result in application of
the proceeds of the offering in a manner other than as described in this prospectus. Our shareholders may not agree with the manner in
which our management chooses to allocate and spend the net proceeds. Moreover, our management may use the net proceeds for corporate purposes
that may not result in our being profitable or increase our market value.
Pending our use of the net proceeds from this offering, we intend to
invest the net proceeds in a variety of capital preservation investments, including short-term, investment-grade, interest-bearing instruments
and U.S. government securities.
CAPITALIZATION
The following table sets forth our cash and capitalization as
of September 30, 2024 on:
| · | an as adjusted basis, assuming our sale of the maximum number of Securities
offered hereby in this offering at an assumed public offering price of $0.78 per ADS and accompanying Warrants, based on the closing price
of our ADSs on Nasdaq on December 9, 2024, resulting in net proceeds to us of approximately $13.4 million, after deducting the placement
agent fees and estimated offering expenses payable by us, and assuming no sale of Pre-Funded Warrants and no exercise of Warrants. |
The as adjusted information set forth in the
table below is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering as
determined at pricing. You should read the information in this table together with our audited financial statements and related
notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in the
Company’s Annual
Report on Form 10-K for the fiscal year ended December 31, 2023 and Quarterly Report on Form 10-Q for the quarter ended September 30, 2024, incorporated by reference in this prospectus.
| | |
As of September 30, 2024 | |
| | |
Actual | | |
As Adjusted | |
| | |
(Unaudited) | |
| Cash and Short-Term Investments | |
$ | 10,306,888 | | |
$ | 23,671,888 | |
| Liabilities: | |
| | | |
| | |
| Non-Current Liabilities | |
| 2,473,733 | | |
| 2,473,733 | |
| Shareholders’ Equity: | |
| | | |
| | |
| Ordinary shares, no par value per share, 100,000,000 ordinary shares authorized and 5,049,720 ordinary shares (represented by 5,049,720 ADSs) issued and outstanding actual and 24,280,489 ordinary shares (represented by 24,280,489 ADSs) issued and outstanding as adjusted | |
| — | | |
| - | |
| Additional paid-in capital | |
| 58,296,199 | | |
| 71,661,199 | |
| Accumulated deficit | |
| (52,854,518 | ) | |
| (52,854,518 | ) |
| Total shareholders’ equity | |
$ | 5,441,681 | | |
$ | 18,806,681 | |
| Total capitalization | |
$ | 7,915,414 | | |
$ | 21,280,414 | |
The
information above is based on 5,049,720 ordinary shares, represented by 5,049,720 ADSs outstanding as of September 30,
2024 (assuming all ordinary shares are represented by ADSs) and excludes the following:
| · | 278,011 ordinary shares represented by 278,011 ADSs issuable upon the exercise of outstanding options at a weighted-average exercise
price of $25.34 per ADS; and |
| · | 8,988,346 ordinary shares represented by 8,988,346 ADSs issuable upon the exercise of outstanding warrants at a weighted-average exercise
price of $2.10 per ADS. |
Dilution
If you invest in our Securities in this offering, your interest will
be diluted immediately to the extent of the difference between the public offering price paid by the purchasers of the ADSs or Pre-Funded
Warrants sold in this offering and the pro forma as adjusted net tangible book value per ADS after this offering.
Our historical net tangible book value of our ADSs as of September 30,
2024, was approximately $4.9 million, or approximately $0.98 per ADS. Net tangible book value per share represents the amount of our total
tangible assets less total liabilities divided by the total number of our ADS outstanding as of September 30, 2024.
After giving
effect to the sale by us in this offering of 19,230,769 ADSs at a price of $0.78 per ADS and accompanying Warrants, based on the
closing price of our ADSs on Nasdaq on December 9, 2024, after deducting the Placement Agent fees and estimated offering expenses payable
by us, and assuming no sale of any Pre-Funded Warrants in this offering and no exercise of any Warrants, our as adjusted net tangible
book value as of September 30, 2024, would have been approximately $18.3 million, or approximately $0.75 per ADS. This represents
an immediate decrease in net tangible book value of approximately $0.23 per ADS to our existing security holders and an immediate dilution
in as adjusted net tangible book value of approximately $0.03 per ADS to purchasers of ADSs in this offering. The final public offering
price will be determined through negotiation between us, the Placement Agent, and prospective investors in the offering and may be at
a discount to the current market price. Therefore, the assumed public offering price used throughout this prospectus may not be indicative
of the final public offering price. The following table illustrates this per share dilution:
| Assumed public offering price per ADS | |
| | | |
$ | 0.78 | |
| Historical net tangible book value per ADS as of September 30, 2024 | |
$ | 0.98 | | |
| | |
| Decrease in as adjusted net tangible book value per ADS attributable to new investors participating in this offering | |
$ | (0.23 | ) | |
| | |
| As adjusted net tangible book value per ADS after this offering | |
| | | |
$ | 0.75 | |
| Dilution per ADS to new investors purchasing ADSs in this offering | |
| | | |
$ | 0.03 | |
A $0.10 increase in the assumed public offering price to $0.88 per
ADS and accompanying Warrants, based on the closing price of our ADSs on Nasdaq on December 9, 2024, would increase our as adjusted net
tangible book value as of September 30, 2024 after this offering to approximately $20.1 million, or approximately $0.83 per ADS, and would
change the dilution to investors in this offering to approximately $0.05 per ADS, assuming the sale by us of 19,230,769 ADSs and accompanying
Warrants, assuming no sale of any Pre-Funded Warrants and no exercise of any Warrants, and after deducting the Placement Agent
fees and estimated offering expenses payable by us. A $0.10 decrease in the assumed initial public offering price to $0.68 per ADS and
accompanying Warrants, based on the closing price of our ADSs on Nasdaq on December 9, 2024, would decease our as adjusted net tangible
book value as of September 30, 2024 after this offering to approximately $16.5 million, or approximately $0.68 per ADS, and would not
be dilutive to investors in this offering, assuming the sale by us of 19,230,769 ADSs and accompanying Warrants, assuming no sale of any Pre-Funded Warrants
and no exercise of any Warrants, and after deducting the Placement Agent fees and estimated offering expenses payable by us.
We may also increase or decrease the number of securities we are offering.
A 2,000,000 increase in the number of ADSs sold by us in the offering would increase our as adjusted net tangible book value as of September
30, 2024 after this offering to approximately $19.8 million, or approximately $0.75 per ADS, and would be dilutive to investors in this
offering by approximately $0.03 per ADS, assuming the assumed public offering price remains the same, assuming no sale of any Pre-Funded Warrants
and no exercise of any Warrants, and after deducting the Placement Agent fees and estimated offering expenses payable by us. A 2,000,000
decrease in the number of ADSs sold by us in the offering would decrease our as adjusted net tangible book value as of September 30, 2024
after this offering to approximately $16.8 million, or approximately $0.76 per ADS, and would change the dilution to investors in this
offering to approximately $0.02 per ADS, assuming the assumed public offering price remains the same, assuming no sale of any Pre-Funded Warrants
and no exercise of any Warrants, and after deducting the Placement Agent fees and estimated offering expenses payable by us.
The table and discussion above are based on 5,049,720 ordinary
shares, represented by 5,049,720 ADSs outstanding (assuming all ordinary shares are represented by ADSs) as of September 30, 2024
and excludes the following:
| · | 278,011 ordinary shares represented by 278,011 ADSs issuable upon the exercise of outstanding options at a weighted-average exercise
price of $25.34 per ADS; and |
| · | 8,988,346 ordinary shares represented by 8,988,346 ADSs issuable upon the exercise of outstanding warrants at a weighted-average exercise
price of $2.10 per ADS. |
The information discussed above is illustrative only and will adjust
based on the actual public offering price, the actual number of ADSs that we offer in this offering, and other terms of this offering
determined at pricing. Except as indicated otherwise, the discussion and table above assumes no sale of Pre-Funded Warrants, which, if
sold, would reduce the number of ADSs that we are offering on a one-for-one basis.
DESCRIPTION
OF SHARE CAPITAL
The following are summaries of material provisions of our articles
of association and the Israeli Companies Law, 5759-1999 (the “Companies Law”), insofar as they relate to the material terms
of our ordinary shares.
Purposes and Objects of the Company
Our purpose is set forth in Section 2 of our articles of association
and includes every lawful purpose.
Voting Rights
Holders of our ordinary shares have one vote for each ordinary share
held on all matters submitted to a vote of shareholders at a shareholders meeting. Shareholders may vote at shareholders meetings either
in person, by proxy or by written ballot. Israeli law does not allow public companies to adopt shareholder resolutions by means of written
consent in lieu of a shareholders meeting. The board of directors shall determine and provide a record date for each shareholders meeting
and all shareholders at such record date may vote. Unless stipulated differently in the Companies Law or in the articles of association,
all shareholders’ resolutions shall be approved by a simple majority vote. As a general rule, an amendment to our articles of association
requires the prior approval of a simple majority of our shares represented and voting at a general meeting.
Transfer of Shares
Our ordinary shares that are fully paid for are issued in registered
form and may be freely transferred under our articles of association, unless the transfer is restricted or prohibited by applicable law
or the rules of a stock exchange on which the shares are traded. The ownership or voting of our ordinary shares by non-residents
of Israel is not restricted in any way by our articles of association or Israeli law, except for ownership by nationals of some countries
that are, or have been, in a state of war with Israel.
Amendment of Share Capital
Our articles of association enable us to increase or reduce our share
capital. Any such changes are subject to the provisions of the Companies Law and must be approved by a resolution duly passed by our shareholders
at a annual or special general meeting by voting on such change in the capital. In addition, transactions that have the effect of reducing
capital, such as the declaration and payment of dividends in the absence of sufficient retained earnings and profits, or an issuance of
shares for less than their nominal value (which would be applicable to our company should our articles be changed so as to permit the
issue of shares having a nominal value, however our shares currently have no nominal value), require a resolution of our board of directors
and court approval.
Dividends and Liquidation Rights
We may declare a dividend to be paid to the holders of our ordinary
shares in proportion to their respective shareholdings. Under the Companies Law, dividend distributions are determined by the board of
directors and do not require the approval of the shareholders of a company unless the company’s articles of association provide
otherwise. The Company’s Articles of Association do not require shareholder approval of a dividend distribution and provide that
dividend distributions may be determined by the Board.
Pursuant to the Companies Law, the distribution amount is limited
to the greater of retained earnings or earnings generated over the previous two years, according to our then last reviewed or audited
financial statements (less the amount of previously distributed dividends, if not reduced from the earnings), provided that the end of
the period to which the financial statements relate is not more than six months prior to the date of the distribution. If we do not meet
such criteria, then we may distribute dividends only with court approval; as a company listed on an exchange outside of Israel, however,
court approval is not required if the proposed distribution is in the form of an equity repurchase, provided that we notify our creditors
of the proposed equity repurchase and allow such creditors an opportunity to initiate a court proceeding to review the repurchase. If
within 30 days such creditors do not file an objection, then we may proceed with the repurchase without obtaining court approval. In
each case, we are only permitted to distribute a dividend if the Board and, if applicable, the court determines that there is no reasonable
concern that payment of the dividend will prevent us from satisfying our existing and foreseeable obligations as they become due.
In the event of our liquidation, after satisfaction of liabilities
to creditors, our assets will be distributed to the holders of our ordinary shares in proportion to their shareholdings. This right, as
well as the right to receive dividends, may be affected by the grant of preferential dividend or distribution rights to the holders of
a class of shares with preferential rights that may be authorized in the future.
Exchange Controls
There are currently no Israeli currency control restrictions on payments
of dividends or other distributions with respect to our ordinary shares or the proceeds from the sale of the shares, except, under certain
circumstances, for shareholders who are subjects of countries that are, or have been, in a state of war with Israel. Israeli residents
have an obligation to file reports with the Bank of Israel regarding certain transactions. However, legislation remains in effect pursuant
to which currency controls can be imposed by administrative action at any time.
Shareholders Meetings
Under Israeli law, we are required to hold an annual general meeting
of our shareholders once every calendar year and in any event no later than 15 months after the date of the previous annual general meeting.
All meetings other than the annual general meeting of shareholders are referred to as special meetings. The Board may call special meetings
whenever it sees fit, at such time and place, within or outside of Israel, as it may determine. In addition, the Companies Law provides
that a board of directors is required to convene a special meeting upon the written request of (1) any two or more of our directors,
(2) one quarter of the directors then in office; or (3) as a company listed on an exchange in the U.S., one or more shareholders
holding, in the aggregate either (a) 10% or more of our issued and outstanding share capital and 1% of our outstanding voting rights,
or (b) 10% or more of our outstanding voting rights.
Under
Israeli law, one or more shareholders holding at least 1% of the voting rights at the general meeting of the shareholders may request
that the board of directors include a matter in the agenda of a general meeting of the shareholders to be convened in the future, provided
that it is appropriate to discuss such a matter at the general meeting. Notwithstanding the foregoing, as a company listed on an exchange
outside of Israel, a matter relating to the appointment or removal of a director may only be requested by one or more shareholders holding
at least 5% of the voting rights at the general meeting of the shareholders.
Subject to the provisions of the Companies Law and the regulations
promulgated thereunder, shareholders entitled to participate and vote at general meetings of a company are the shareholders of record
on a date to be decided by the board of directors which for us, as a company listed on an exchange outside Israel, may be between four
and sixty days prior to the date of the meeting.
The Companies Law and our articles of association require that resolutions
regarding the following matters must be passed at a general meeting of our shareholders:
| · | amendments to our articles of association; |
| · | appointment, terms of service or termination of service of our auditors; |
| · | appointment and dismissal of external directors, if and to the extent any are required to be appointed under the Companies Law; |
| · | approval of acts and transactions requiring general meeting approval pursuant to the Companies Law; |
| · | increases or reductions of our authorized share capital; |
| · | the exercise of our board of directors’ powers by a general meeting, if our board of directors is unable to exercise its powers
and the exercise of any of its powers is required for our proper management. |
The Companies Law requires that a notice of any annual general meeting
or special general meeting be provided to shareholders at least 21 days prior to the meeting and if the agenda of the meeting includes,
among other things, the appointment or removal of directors, the approval of transactions with office holders or interested or related
parties, or an approval of a merger, notice must be provided at least 35 days prior to the meeting. Under the Companies Law and our amended
and restated articles of association, shareholders are not permitted to take action by way of written consent in lieu of a meeting.
Quorum
Our articles of association provide that the quorum required for our
general meetings of shareholders consists of two or more shareholders present in person, by proxy or by other voting instrument in accordance
with the Companies Law and our articles of association, who hold or represent, in the aggregate, at least 33 1/3% (of the total outstanding
voting rights (the quorum requirement for domestic filing companies under Nasdaq current corporate governance rules) unless the
Company qualifies as a “foreign private issuer” under U.S. federal securities laws and the general meeting is convened pursuant
to a resolution of the Board, in which case the requisite quorum will be 25% of the Company’s shares entitled to vote at a general
meeting of the shareholders.
Vote Requirements
Our articles of association provide that all resolutions of our shareholders
require a simple majority vote, unless otherwise required by the Companies Law or by our articles of association. Under the Companies
Law, certain actions require the approval of a special majority, including: (i) an extraordinary transaction with a controlling shareholder
or in which the controlling shareholder has a personal interest, (ii) the terms of employment or other engagement of a controlling
shareholder of the company or a controlling shareholder’s relative (even if such terms are not extraordinary) and (iii) certain
compensation-related matters described above under “Management—Compensation Committee—Compensation Policy under the
Companies Law.” Under our articles of association, the alteration of the rights, privileges, preferences or obligations of any class
of our shares (to the extent there are classes other than ordinary shares) requires the approval of a simple majority of the class so
affected (or such other percentage of the relevant class that may be set forth in the governing documents relevant to such class), in
addition to a majority of all classes of shares voting together as a single class at a shareholder meeting.
Dissolution
Generally under Israeli law, a resolution for the voluntary winding
up of a company requires the approval of holders of 75% of the voting rights represented at the meeting, in person, by proxy or by written
ballot and voting on the resolution.
In the event of our liquidation, after satisfaction of liabilities
to creditors, our assets will be distributed to the holders of our ordinary shares (including holders of entitlements to shares, after
deducting the nominal value (if any) of such shares and the price which would have been paid in order to exercise the right to such shares),
in proportion to their shareholdings. This right, as well as the right to receive dividends, may be affected by the grant of preferential
dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized in the future.
Access to Corporate Records
Under the Companies Law, all shareholders of a company generally have
the right to review minutes of the company’s general meetings, its register of shareholders and material shareholders, articles
of association, financial statements and any document it is required by law to file publicly with the Israeli Companies Registrar. Any
of our shareholders may request to review any document in our possession that relates to any action or transaction with a related party,
interested party, or office holder that requires shareholder approval under the Companies Law. We may deny a request to review a document
if we determine that the request was not made in good faith, that the document contains a trade secret or patent, or that the document’s
disclosure may otherwise prejudice our interests.
Acquisitions under Israeli Law
Full Tender Offer
A person wishing to acquire shares of a public Israeli company, and
who would as a result hold over 90% of the target company’s issued and outstanding share capital, is required by the Companies Law
to make a tender offer to all of the company’s shareholders for the purchase of all of the issued and outstanding shares of the
company. A person wishing to acquire shares of a public Israeli company, and who would as a result hold over 90% of the issued and outstanding
share capital of a certain class of shares, is required to make a tender offer to all of the shareholders who hold shares of that class
for the purchase of all of the issued and outstanding shares of that class. If the shareholders who do not accept the offer hold less
than 5% of the issued and outstanding share capital of the company or of the applicable class, all of the shares that the acquirer offered
to purchase will be transferred to the acquirer by operation of law, provided that a majority of the offerees that do not have a personal
interest in such tender offer, have accepted the tender offer. Alternatively, if shareholders who do not accept the tender offer represent
less than 2% of the company’s issued and outstanding share capital (or less than 2% of the applicable class of shares), approval
by a majority of the offerees that do not have a personal interest in such tender offer is not required to complete the tender offer.
A shareholder whose shares are so transferred may petition the court regarding the fair value to be paid in consideration of such shares,
within six months from the date of acceptance of the full tender offer; this right of petition applies to all offeree shareholders,
unless the acquirer stipulated in the tender offer that a shareholder accepting the offer may not seek appraisal rights, and prior to
the acceptance of the full tender offer, the acquirer and the company disclosed the information required by law in connection with a full
tender offer. To the extent a court so petitioned determines that the offered value was less than the fair value per share, the court
may order the difference to be paid.
Special Tender Offer
The Companies Law provides that an acquisition of shares of an Israeli
public company must be made by means of a “special tender offer” complying with the relevant provisions of the Companies Law
if, as a result of the acquisition, the purchaser would become a holder of 25% or more of the voting rights in the company, if there did
not previously exist a holder of 25% or more of the voting rights in the company, or if, as a result of the acquisition, the purchaser
would become a holder of more than 45% of the voting rights in the company, if there did not previously exist a holder of more than 45%
of the voting rights in the company. This requirement does not apply if the acquisition: (a) occurs in the context of a private placement
by the company that received shareholder approval as a private placement giving the offeree 25% or 45% of the company’s voting rights
(as the case may be); (b) is from a holder of 25% or more of the voting rights in the company and results in the acquirer becoming
a holder of 25% or more of the voting rights in the company; or (c) is from a holder of more than 45% of the voting rights in the
company and results in the acquirer becoming a holder of more than 45% of the voting rights in the company.
In the event that a special tender offer is made, the target company’s
board of directors is required to express its opinion on the advisability of the offer, or may abstain from expressing any opinion if
it is unable to do so, provided that it gives the reasons for its abstention.
A special tender offer must be directed to all offerees, and the offerees
may give notice of their agreement or opposition to the special tender offer. The special tender offer will be consummated only if: (a) at
least 5% of the voting rights attached to the company’s outstanding shares will be acquired by the offeror, and (b) among those
shareholders who gave notice of their position (excluding any controlling shareholders of the offeror, holders of 25% or more of the voting
rights in the target company, and any person having a personal interest in the acceptance of the tender offer, including relatives or
corporations under the control of any of the above), the number of shares whose holders agreed to the offer exceeds the number of shares
whose holders objected to the offer.
If a special tender offer is accepted by the procedure described above,
then shareholders who did not respond to or who objected the offer may accept the offer within four days of the last day set for
the acceptance of the offer.
An office holder in a company which is the target of a special tender
offer who, in his or her capacity as an office holder, performs an act or omits to act for in order to cause the failure of an existing
or foreseeable special tender offer, or to impair the likelihood of its acceptance, is liable to the offeror and offerees for damages,
unless such office holder acted in good faith and had reasonable grounds to believe that such act or omission was beneficial to the company.
As a safe harbor, office holders of the target company may negotiate with a potential purchaser in order to improve the terms of a special
tender offer, or negotiate with third parties in order to obtain a competing offer.
In
the event that a special tender offer is accepted, the purchaser, any person or entity controlling or controlled by the purchaser, or
under common control with the purchaser, may not make a subsequent tender offer for the purchase of shares of the target company, and
may not enter into a merger with the target company, for a period of one year from the date of the offer, unless the purchaser or
such person or entity undertakes to effect such an offer or merger as a special tender offer in compliance with the Companies Law requirements.
Merger
The Companies Law permits merger transactions if approved by each
party’s board of directors and, unless certain conditions described under the Companies Law are met, by each party’s shareholders
by a majority vote as described below.
For purposes of the shareholder vote, unless a court rules otherwise,
the merger will not be deemed approved if a majority of the shares voted at the shareholders meeting held by shareholders who are not
the other party to the merger, or held by any person who holds 25% or more of the outstanding shares or the right to appoint 25% or more
of the directors of the other party to the merger (including relatives or entities in control of the above), vote against the merger.
If the transaction would have been approved but for the separate approval of each class or the exclusion of the votes of certain shareholders
as provided above, a court may still approve the merger upon the request of holders of at least 25% of the voting rights of a company,
if the court holds that the merger is fair and reasonable, taking into account the relative value of the merger parties and the consideration
offered to the shareholders. If the non-surviving entity of the merger has more than one class of shares, the merger must be approved
by each class of shareholders. If a merger is with a company’s controlling shareholder, or if a controlling shareholder has a personal
interest in the merger, then the merger will be subject to the special majority approval required for an extraordinary transaction with
a controlling shareholder. In the context of mergers (as well as other related party transactions), a “controlling shareholder”
under Israeli law is deemed to include any shareholder holding 25% or more of the voting rights in the company if no other shareholder
owns more than 50% of the voting rights in the company, and two or more shareholders with a personal interest in the approval of the
same transaction are deemed to be one shareholder for such purpose.
The Companies Law requires the board of directors of a merging company
to discuss and determine whether, in its view, there exists a reasonable concern that as a result of the proposed merger, the surviving
company will not be able to satisfy its obligations towards its creditors, and if not, the board of directors may not approve the merger.
The Companies Law requires each merging company to inform its secured creditors of the proposed merger plan. Upon the request of a creditor
of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern
that, as a result of the merger, the surviving company will be unable to satisfy the obligations of any of the parties to the merger,
and may further give instructions to secure the rights of creditors.
A merger may not be completed unless at least 50 days have passed
from the date that a proposal for approval of the merger is filed with the Israeli Registrar of Companies, and 30 days have passed
from the date the merger was approved by the shareholders of each merging company.
Antitakeover Measures
The
Companies Law allows us to create and issue shares having rights different from those attached to our ordinary shares, including shares
providing certain preferred rights, distributions or other matters, and shares having preemptive rights. As of the date of the registration
statement of which this prospectus forms a part, we do not have any authorized or issued classes of shares other than our ordinary shares.
In the future, if we do create and issue a class of shares other than ordinary shares, such class of shares, depending on the specific
rights that may be attached to them, may delay or prevent a takeover or otherwise prevent our shareholders from realizing a potential
premium over the market value of their ordinary shares. The authorization of a new class of shares will require an amendment to our articles
of association which requires the prior approval of the holders of a majority of our shares at a general meeting. Shareholders voting
in such meeting will be subject to the restrictions provided in the Companies Law as described above.
DESCRIPTION
OF AMERICAN DEPOSITARY SHARES
The
Bank of New York Mellon (the “Depositary”), as depositary, has registered and delivered American Depositary Shares, also
referred to as ADSs. Each ADS represents one ordinary share (or a right to receive one ordinary share) deposited with The Bank of New
York Mellon in Manchester, United Kingdom, as custodian for the Depositary. The Depositary’s corporate trust office at which the
ADSs will be administered is located at 240 Greenwich Street, New York, New York 10286. The Bank of New York Mellon’s principal
executive office is located at 240 Greenwich Street, New York, New York 10286.
ADSs may be held either (a) directly (1) by having an American
Depositary Receipt, also referred to as an ADR, which is a certificate evidencing a specific number of ADSs or (2) by having uncertificated
ADSs, or (b) indirectly by holding a security entitlement in ADSs through a broker or other financial institution that is a direct
or indirect participant in The Depository Trust Company, also called DTC. If ADSs are held directly by the holder, then that holder is
registered as such, and is referred to in our description here an ADS holder. An indirect holder of ADSs indirectly must rely on the
procedures of the holder’s broker or other financial institution to assert the rights of ADS holder described in this Exhibit.
Registered holders of uncertificated ADSs will receive statements
from the depositary confirming their holdings.
We will not treat registered ADS holders as one of our shareholders,
and they will not have shareholder rights. Israeli law governs shareholder rights. The depositary will be the holder of the ordinary
shares underlying ADSs. A registered holder of ADSs will have ADS holder rights. A deposit agreement among us, the depositary, ADS holders
and all other persons indirectly or beneficially holding ADSs sets out ADS holder rights as well as the rights and obligations of the
depositary. New York law governs the deposit agreement and the ADSs.
The following is a summary of the material provisions of the deposit
agreement. For more complete information, you should read the entire deposit agreement and the form of ADR.
Dividends and Other Distributions
How will you receive dividends and other distributions on the
shares?
The depositary has agreed to pay or distribute to ADS holders the
cash dividends or other distributions it or the custodian receives on ordinary shares or other deposited securities, upon payment or
deduction of its fees and expenses. You will receive these distributions in proportion to the number of ordinary shares your ADSs represent.
Cash. The
depositary will convert any cash dividend or other cash distribution we pay in non-U.S. currency on the ordinary shares into U.S. dollars,
if it can do so on a reasonable basis and can transfer the U.S. dollars to the United States. If that is not possible or if any government
approval is needed and cannot be obtained, the deposit agreement allows the depositary to distribute the foreign currency only to those
ADS holders to whom it is possible to do so. It will hold the foreign currency it cannot convert for the account of the ADS holders who
have not been paid. It will not invest the foreign currency, and it will not be liable for any interest.
Before making a distribution, the depositary will deduct any withholding
taxes, or other required governmental charges. See “Certain Material U.S. Federal Income Tax Considerations” below. The depositary
will distribute only whole U.S. dollars and cents and will round fractional cents to the nearest whole cent. If the exchange rates fluctuate
during a time when the depositary cannot convert the foreign currency, you may lose some or all of the value of the distribution.
Shares. The
depositary may distribute additional ADSs representing any ordinary shares we distribute as a dividend or free distribution. The depositary
will only distribute whole ADSs. It will sell ordinary shares which would require it to deliver a fraction of an ADS (or ADSs representing
those shares) and distribute the net proceeds in the same way as it does with cash. If the depositary does not distribute additional
ADSs, the outstanding ADSs will also represent the new shares. The depositary may sell a portion of the distributed ordinary shares (or
ADSs representing those shares) sufficient to pay its fees and expenses in connection with that distribution.
Rights
to purchase additional shares. If we offer holders of our securities any rights to subscribe for additional ordinary
shares or any other rights, the depositary may (1) exercise those rights on behalf of ADS holders, (2) distribute those rights
to ADS holders or (3) sell those rights and distribute the net proceeds to ADS holders, in each case after deduction or upon payment
of its fees and expenses. To the extent the depositary does not do any of those things, it will allow the rights to lapse. In that case,
you will receive no value for them. The depositary will exercise or distribute rights only if we ask it to and provide satisfactory assurances
to the depositary that it is legal to do so. If the depositary will exercise rights, it will purchase the securities to which the rights
relate and distribute those securities or, in the case of ordinary shares, new ADSs representing the new ordinary shares, to subscribing
ADS holders, but only if ADS holders have paid the exercise price to the depositary. U.S. securities laws may restrict the ability of
the depositary to distribute rights or ADSs or other securities issued on exercise of rights to all or certain ADS holders, and the securities
distributed may be subject to restrictions on transfer.
Other
Distributions. The depositary will send to ADS holders anything else we distribute on deposited securities by any
means it thinks is legal, fair and practical. If it cannot make the distribution in that way, the depositary has a choice. It may decide
to sell what we distributed and distribute the net proceeds, in the same way as it does with non-U.S. currency. Alternatively, it may
decide to hold what we distributed, in which case ADSs will also represent the newly distributed property. However, the depositary is
not required to distribute any securities (other than ADSs) to ADS holders unless it receives satisfactory evidence from us that it is
legal to make that distribution. The depositary may sell a portion of the distributed securities or property sufficient to pay its fees
and expenses in connection with that distribution. U.S. securities laws may restrict the ability of the depositary to distribute securities
to all or certain ADS holders, and the securities distributed may be subject to restrictions on transfer.
The depositary is not responsible if it decides that it is unlawful
or impractical to make a distribution available to any ADS holders. We have no obligation to register ADSs, shares, rights or other securities
under the Securities Act. We also have no obligation to take any other action to permit the distribution of ADSs, shares, rights
or anything else to ADS holders. This means that you may not receive the distributions we make on our ordinary shares or any value for
them if it is illegal or impractical for us to make them available to you.
Deposit, Withdrawal and Cancellation
How
are ADSs issued?
The depositary will deliver ADSs upon deposits of ordinary shares
or evidence of rights to receive ordinary shares with the custodian. Upon payment of its fees and expenses and of any taxes or charges,
such as stamp taxes or stock transfer taxes or fees, the depositary will register the appropriate number of ADSs and will deliver the
ADSs to or upon the order of the person or persons that made the deposit.
How can ADS holders withdraw the deposited securities?
ADS holders may surrender ADSs for the purpose of withdrawal at the
Depositary’s account at DTCC (BNYM’s DTC participant #2504). Upon payment of its cancellation fees and expenses and of any
taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will deliver the ordinary shares and any other
deposited securities underlying the ADSs to the ADS holder or a person the ADS holder designates in accordance with the Cancellation
Instruction provided to The Bank of New York Mellon.
How do ADS holders interchange between certificated ADSs and
uncertificated ADSs?
ADS holders may surrender ADS to the depositary for the purpose of
exchanging ADS for uncertificated ADSs. The depositary will cancel that ADS and will send to the ADS holder a statement confirming that
the ADS holder is the registered holder of uncertificated ADSs. Upon receipt by the depositary of a proper instruction from a registered
holder of uncertificated ADSs requesting the exchange of uncertificated ADSs for certificated ADSs, the depositary will execute and deliver
to the ADS holder an ADS evidencing those ADSs.
Voting Rights
ADS holders may instruct the depositary how to vote the number of
deposited ordinary shares their ADSs represent. If we request the depositary to solicit your voting instructions (and we are not required
to do so), the depositary will notify you of a shareholders’ meeting and send or make voting materials available to you. Those
materials will describe the matters to be voted on and explain how ADS holders may instruct the depositary how to vote. For instructions
to be valid, they must reach the depositary by a date set by the depositary.
The depositary will try, as far as practical, subject to the laws
of Israel and the provisions of our articles of association or similar documents, to vote or to have its agents vote the ordinary shares
or other deposited securities as instructed by ADS holders. If we do not request the depositary to solicit your voting instructions,
you can still send voting instructions, and, in that case, the depositary may try to vote as you instruct, but it is not required to
do so.
Except by instructing the depositary as described above, ADS holders
will not be able to exercise voting rights, unless they surrender your ADSs and withdraw the ordinary shares. However, ADS holders may
not know about the meeting sufficiently in advance to withdraw the ordinary shares. In any event, the depositary will not exercise any
discretion in voting deposited securities and it will only vote or attempt to vote as instructed.
We cannot assure that ADS holders will receive the voting materials
in time to ensure that they can instruct the depositary to vote ordinary shares. In addition, the depositary and its agents are not responsible
for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that ADS holders may not
be able to exercise voting rights and there may be nothing they can do if your ordinary shares are not voted as requested.
In
order to give ADS holders a reasonable opportunity to instruct the depositary as to the exercise of voting rights relating to deposited
securities, if we request the Depositary to act, we agree to give the depositary notice of any such meeting and details concerning the
matters to be voted upon at least thirty days in advance of the meeting date.
Fees and Expenses
Persons depositing or withdrawing shares or ADS
holders must pay: |
|
For: |
| $5.00 (or less) per ADS (or portion of ADS) |
|
Issuance of ADSs, including issuances resulting from a distribution of ordinary shares or rights or other property Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates |
| |
|
|
| $0.05 (or less) per ADS |
|
Any cash distribution to ADS holders |
| |
|
|
| A fee equivalent to the fee that would be payable if securities distributed to you had been ordinary shares and the ordinary shares had been deposited for issuance of ADSs |
|
Distribution of securities distributed to holders of deposited securities (including rights) that are distributed by the depositary to ADS holders |
| |
|
|
| $0.05 (or less) per ADSs per calendar year |
|
Depositary services |
| |
|
|
| Registration or transfer fees |
|
Transfer and registration of ordinary shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw ordinary shares |
| |
|
|
| Expenses of the Depositary |
|
Cable, telex and facsimile transmissions (when expressly provided in the deposit agreement); converting foreign currency to U.S. dollars |
| |
|
|
| Taxes and other governmental charges the Depositary or the custodian have to pay on any ADS or share underlying an ADS, for example, stock transfer taxes, stamp duty or withholding taxes |
|
As necessary |
| |
|
|
| Any charges incurred by the Depositary or its agents for servicing the deposited securities |
|
As necessary |
The depositary collects its fees for delivery and surrender of ADSs
directly from investors depositing ordinary shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for
them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling
a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from
cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The
depositary may collect any of its fees by deduction from any cash distribution payable (or by selling a portion of securities or other
property distributable) to ADS holders that are obligated to pay those fees. The depositary may generally refuse to provide fee-attracting
services until its fees for those services are paid.
From
time to time, the depositary may make payments to us to reimburse us for costs and expenses generally arising out of establishment and
maintenance of the ADS program, waive fees and expenses for services provided to us by the depositary or share revenue from the fees
collected from ADS holders. In performing its duties under the deposit agreement, the depositary may use brokers, dealers, foreign currency
dealers or other service providers that are owned by or affiliated with the depositary and that may earn or share fees, spreads or commissions.
The depositary may convert currency itself or through any of its affiliates
and, in those cases, acts as principal for its own account and not as agent, advisor, broker or fiduciary on behalf of any other person
and earns revenue, including, without limitation, transaction spreads, that it will retain for its own account. The revenue is based
on, among other things, the difference between the exchange rate assigned to the currency conversion made under the deposit agreement
and the rate that the depositary or its affiliate receives when buying or selling foreign currency for its own account. The depositary
makes no representation that the exchange rate used or obtained in any currency conversion under the deposit agreement will be the most
favorable rate that could be obtained at the time or that the method by which that rate will be determined will be the most favorable
to ADS holders, subject to the depositary’s obligations under the deposit agreement. The methodology used to determine exchange
rates used in currency conversions is available upon request.
Payment of Taxes
ADS holders are responsible for any taxes or other governmental charges
payable on their ADSs or on the deposited securities represented by any of their ADSs. The depositary may refuse to register any transfer
of ADSs or allow a withdrawal of the deposited securities represented by your ADSs, until such taxes or other charges are paid. It may
apply payments owed to the ADS holder or sell deposited securities represented by the ADSs to pay any taxes owed and the ADS holder will
remain liable for any deficiency. If the depositary sells deposited securities, it will, if appropriate, reduce the number of ADSs to
reflect the sale and pay to ADS holders any proceeds, or send to ADS holders any property, remaining after it has paid the taxes.
Tender and Exchange Offers; Redemption, Replacement or Cancellation
of Deposited Securities
The depositary will not tender deposited securities in any voluntary
tender or exchange offer unless instructed to do by an ADS holder surrendering ADSs and subject to any conditions or procedures the depositary
may establish.
If deposited securities are redeemed for cash in a transaction that
is mandatory for the depositary as a holder of deposited securities, the depositary will call for surrender of a corresponding number
of ADSs and distribute the net redemption money to the holders of called ADSs upon surrender of those ADSs.
If there is any change in the deposited securities such as a sub-division,
combination or other reclassification, or any merger, consolidation, recapitalization or reorganization affecting the issuer of deposited
securities in which the depositary receives new securities in exchange for or in lieu of the old deposited securities, the depositary
will hold those replacement securities as deposited securities under the deposit agreement. However, if the depositary decides it would
not be lawful and to hold the replacement securities because those securities could not be distributed to ADS holders or for any other
reason, the depositary may instead sell the replacement securities and distribute the net proceeds upon surrender of the ADSs.
If
there is a replacement of the deposited securities and the depositary will continue to hold the replacement securities, the depositary
may distribute new ADSs representing the new deposited securities or ask you to surrender your outstanding ADRs in exchange for new ADSs
identifying the new deposited securities.
If there are no deposited securities underlying ADSs, including if
the deposited securities are cancelled, or if the deposited securities underlying ADSs have become apparently worthless, the depositary
may call for surrender or of those ADSs or cancel those ADSs upon notice to the ADS holders.
Amendment and Termination
How may the deposit agreement be amended?
We may agree with the depositary to amend the deposit agreement and
the ADSs without consent of the ADS holders for any reason. If an amendment adds or increases fees or charges, except for taxes and other
governmental charges or expenses of the depositary for registration fees, facsimile costs, delivery charges or similar items, or prejudices
a substantial right of ADS holders, it will not become effective for outstanding ADSs until 30 days after the depositary notifies
ADS holders of the amendment. At the time an amendment becomes effective, ADS holders are considered, by continuing to hold your ADSs,
to agree to the amendment and to be bound by the ADRs and the deposit agreement as amended.
How may the deposit agreement be terminated?
The depositary will initiate termination of the deposit agreement
if we instruct it to do so. The depositary may initiate termination of the deposit agreement if
| · | 60 days
have passed since the depositary told us it wants to resign but a successor depositary has
not been appointed and accepted its appointment; |
| · | we
delist our ordinary shares from an exchange on which they were listed and do not list the
ordinary shares on another exchange; |
| · | we
appear to be insolvent or enter insolvency proceedings all or substantially all the value
of the deposited securities has been distributed either in cash or in the form of securities; |
| · | there
are no deposited securities underlying the ADSs or the underlying deposited securities have
become apparently worthless; or |
| · | there
has been a replacement of deposited securities. |
If the deposit agreement will terminate, the depositary will notify
ADS holders at least 90 days before the termination date. At any time after the termination date, the depositary may sell the deposited
securities. After that, the depositary will hold the money it received on the sale, as well as any other cash it is holding under the
deposit agreement, unsegregated and without liability for interest, for the pro rata benefit of the ADS holders that have not surrendered
their ADSs. Normally, the depositary will sell as soon as practicable after the termination date.
After
the termination date and before the depositary sells, ADS holders can still surrender their ADSs and receive delivery of deposited securities,
except that the depositary may refuse to accept a surrender for the purpose of withdrawing deposited securities if it would interfere
with the selling process. The depositary may refuse to accept a surrender for the purpose of withdrawing sale proceeds until all the
deposited securities have been sold. The depositary will continue to collect distributions on deposited securities, but, after the termination
date, the depositary is not required to register any transfer of ADSs or distribute any dividends or other distributions on deposited
securities to the ADSs holder (until they surrender their ADSs) or give any notices or perform any other duties under the deposit agreement
except as described in this paragraph.
Limitations on Obligations and Liability
Limits on our Obligations and the Obligations of the Depositary;
Limits on Liability to Holders of ADSs
The deposit agreement expressly limits our obligations and the obligations
of the depositary. It also limits our liability and the liability of the depositary. We and the depositary:
| · | are
only obligated to take the actions specifically set forth in the deposit agreement without
negligence or bad faith; |
| · | are
not liable if we are or it is prevented or delayed by law or circumstances beyond our or
its control from performing our or its obligations under the deposit agreement; |
| · | are
not liable if we or it exercises discretion permitted under the deposit agreement; |
| · | are
not liable for the inability of any holder of ADSs to benefit from any distribution on deposited
securities that is not made available to holders of ADSs under the terms of the deposit agreement,
or for any special, consequential or punitive damages for any breach of the terms of the
deposit agreement; |
| · | have
no obligation to become involved in a lawsuit or other proceeding related to the ADSs or
the deposit agreement on your behalf or on behalf of any other person; |
| · | are
not liable for the acts or omissions of any securities depository, clearing agency or settlement
system; and |
| · | may
rely upon any documents we believe or it believes in good faith to be genuine and to have
been signed or presented by the proper person. |
In the deposit agreement, we and the depositary agree to indemnify
each other under certain circumstances.
Requirements for Depositary Actions
Before the depositary will deliver or register a transfer of ADSs,
make a distribution on ADSs, or permit withdrawal of shares, the depositary may require:
| · | payment
of stock transfer or other taxes or other governmental charges and transfer or registration
fees charged by third parties for the transfer of any ordinary shares or other deposited
securities; |
| · | satisfactory proof of the identity and genuineness of any signature
or other information it deems necessary; and |
| · | compliance
with regulations it may establish, from time to time, consistent with the deposit agreement,
including presentation of transfer documents. |
The depositary may refuse to deliver ADSs or register transfers of
ADSs when the transfer books of the depositary or our transfer books are closed or at any time if the depositary or we think it advisable
to do so.
Right to Receive the Ordinary Shares Underlying ADSs
ADS holders have the right to cancel their ADSs and withdraw the underlying
ordinary shares at any time except:
| · | when
temporary delays arise because: (1) the depositary has closed its transfer books or
we have closed our transfer books; (2) the transfer of ordinary shares is blocked to
permit voting at a shareholders meeting; or (3) we are paying a dividend on our shares; |
| · | when
you owe money to pay fees, taxes and similar charges; or |
| · | when
it is necessary to prohibit withdrawals in order to comply with any laws or governmental
regulations that apply to ADSs or to the withdrawal of ordinary shares or other deposited
securities. |
This right of withdrawal may not be limited by any other provision
of the deposit agreement.
Pre-release of ADSs
The
deposit agreement permits the depositary to deliver ADSs before deposit of the underlying shares. This is called a pre-release of the
ADSs. The depositary may also deliver ordinary shares upon cancellation of pre-released ADSs (even if the ADSs are canceled before the
pre-release transaction has been closed out). A pre-release is closed out as soon as the underlying ordinary shares are delivered to
the depositary. The depositary may receive ADSs instead of ordinary shares to close out a pre-release. The depositary may pre-release
ADSs only under the following conditions: (1) before or at the time of the pre-release, the person to whom the pre-release is being
made represents to the depositary in writing that it or its customer owns the ordinary shares or ADSs to be deposited; (2) the
pre-release is fully collateralized with cash or other collateral that the depositary considers appropriate; and (3) the depositary
must be able to close out the pre-release on not more than five business days’ notice. In addition, the depositary will limit
the number of ADSs that may be outstanding at any time as a result of pre-release, although the depositary may disregard the limit from
time to time if it thinks it is appropriate to do so.
Direct Registration System
In the deposit agreement, all parties to the deposit agreement acknowledge
that the Direct Registration System, or DRS, and Profile Modification System, or Profile, will apply to the ADSs. DRS is a system administered
by DTC that facilitates interchange between registered holdings of uncertificated ADSs and holdings of security entitlements in ADSs
through DTC and a DTC participant. Profile is a feature of DRS that allows a DTC participant, claiming to act on behalf of a registered
holder of ADSs, to direct the depositary to register a transfer of those ADSs to DTC or its nominee and to deliver those ADSs to the
DTC account of that DTC participant without receipt by the depositary of prior authorization from the ADS holder to register that transfer.
In
connection with and in accordance with the arrangements and procedures relating to DRS/Profile, the parties to the deposit agreement
understand that the depositary will not determine whether the DTC participant that is claiming to be acting on behalf of an ADS holder
in requesting registration of transfer and delivery as described in the paragraph above has the actual authority to act on behalf of
the ADS holder (notwithstanding any requirements under the Uniform Commercial Code). In the deposit agreement, the parties agree that
the depositary’s reliance on and compliance with instructions received by the depositary through the DRS/Profile system and in
accordance with the deposit agreement will not constitute negligence or bad faith on the part of the depositary.
Shareholder communications; inspection of register of holders of
ADSs
The depositary will make available for your inspection at its office
all communications from us that we make generally available to holders of deposited securities. The depositary will send you copies of
those communications or otherwise make those communications available to you upon our request. You have a right to inspect the register
of holders of ADSs, but not for the purpose of contacting those holders about a matter unrelated to our business or the ADSs.
DESCRIPTION
OF Warrants WE ARE OFFERING
The
following summary of certain terms and provisions of Pre-Funded Warrants and Warrants that are being offered hereby is not complete and
is subject to, and qualified in its entirety by, the provisions of the forms of such warrants, which are filed as exhibits to the registration
statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in such
forms of warrants for a complete description of the terms and conditions of the Pre-Funded Warrants and Warrants. The Pre-Funded Warrants
will be issued separately from the accompanying Warrants, and may be transferred separately immediately thereafter.
Pre-Funded Warrants Being Offered in this Offering
Duration and Exercise Price
Each Pre-Funded Warrant offered hereby will have an initial exercise
price equal to $0.0001 per ADS. The Pre-Funded Warrants will be immediately exercisable and may be exercised at any time until the Pre-Funded
Warrants are exercised in full. The exercise price and number of ADSs issuable upon exercise is subject to appropriate proportional adjustment
in the event of share dividends, share splits, reorganizations or similar events affecting our ADSs and the exercise price.
Exercisability
The Pre-Funded Warrants will be exercisable, at
the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice and, within the earlier of (i) one
trading day and (ii) the number of trading days comprising the standard settlement period with respect to the ADSs as in
effect on the date of delivery of the notice of exercise thereafter, payment in full for the number of ADSs purchased upon such exercise
(except in the case of a cashless exercise as discussed below). A holder may not exercise any portion of the Pre-Funded Warrant to the
extent that the holder, together with its affiliates and any other persons acting as a group together with any such persons, would beneficially
own more than 4.99% of the number of ordinary shares outstanding immediately after exercise (the “Beneficial Ownership Limitation”).
Cashless Exercise
The Pre-Funded Warrants may also be exercised, in whole or in part,
at such time by means of a “cashless exercise” in which the holder shall be entitled to receive upon such exercise (either
in whole or in part) the net number of ADSs determined according to a formula set forth in the Pre-Funded Warrants.
Fractional Shares
No fractional ADSs will be issued upon the exercise of the Pre-Funded
Warrants. Rather, we will, at our election, either pay a cash adjustment in respect of such final fraction in an amount equal to such
fraction multiplied by the exercise price or round up to the next whole ADS.
Transferability
Subject
to applicable laws, a Pre-Funded Warrant may be transferred at the option of the holder upon surrender of the Pre-Funded Warrant to us
together with the appropriate instruments of transfer and funds sufficient to pay any transfer taxes payable upon such transfer.
Trading Market
There is no trading market available for the Pre-Funded Warrants on
any securities exchange or nationally recognized trading system. We do not intend to list the Pre-Funded Warrants on any securities exchange
or nationally recognized trading system. The ADSs issuable upon exercise of the Pre-Funded Warrants are currently listed on The Nasdaq
Capital Market under the symbol “QNRX.”
Rights as a Shareholder
Except as otherwise provided in the Pre-Funded Warrants or by virtue
of such holder’s ownership of the underlying ordinary shares represented by ADSs, the holders of the Pre-Funded Warrants do not
have the rights or privileges of holders of our ordinary shares represented by ADSs, including any voting rights, until they exercise
their Pre-Funded Warrants.
Fundamental Transaction
In the event of a fundamental transaction, as described in the Pre-Funded
Warrants and generally including any reorganization, recapitalization or reclassification of our Ordinary Shares, the sale, transfer
or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person,
the acquisition of more than 50% of our outstanding Ordinary Shares, the holders of the Pre-Funded Warrants will be entitled to receive
upon exercise of the Pre-Funded Warrants the kind and amount of securities, cash or other property that the holders would have received
had they exercised the Pre-Funded Warrants immediately prior to such fundamental transaction.
Warrants Being Offered in this Offering
Duration and Exercise Price
Each Warrant offered hereby will be a warrant to purchase one ADS
and will be immediately exercisable. The Series F Warrants will have an initial exercise price equal to $0.78 per ADS and will expire
on the second anniversary of the original issuance date. The Series G Warrants will have an initial exercise price equal to $0.78
per ADS and will expire on the fifth anniversary of the original issuance date. The exercise price and number of ADSs issuable upon exercise
is subject to appropriate proportional adjustment in the event of share dividends, share splits, reorganizations or similar events affecting
our ADSs and the exercise price.
Exercisability
The Warrants will be exercisable, at the option
of each holder, in whole or in part, by delivering to us a duly executed exercise notice and, within the earlier of (i) one trading day
and (ii) the number of trading days comprising the standard settlement period with respect to the ADSs as in effect on the date
of delivery of the notice of exercise thereafter, payment in full for the number of ADSs purchased upon such exercise (except in the case
of a cashless exercise as discussed below). A holder may not exercise any portion of the Warrant to the extent that the holder, together
with its affiliates and any other persons acting as a group together with any such persons, would beneficially own more than 4.99% of
the number of ordinary shares outstanding immediately after exercise (the “Beneficial Ownership Limitation”).
Cashless Exercise
If, at the time a holder exercises its Warrants, a registration statement
registering the issuance of the ADSs underlying the Warrants under the Securities Act is not then effective or available for the issuance
of such shares, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the
aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of ADSs
determined according to a formula set forth in the Warrants.
Fractional Shares
No fractional ADSs will be issued upon the exercise of the Warrants.
Rather, we will, at our election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction
multiplied by the exercise price or round up to the next whole ADS.
Transferability
Subject to applicable laws, a Warrant may be transferred at the option
of the holder upon surrender of the Warrant to us together with the appropriate instruments of transfer and funds sufficient to pay any
transfer taxes payable upon such transfer.
Trading Market
There is no trading market available for the Warrants on any securities
exchange or nationally recognized trading system. We do not intend to list the Warrants on any securities exchange or nationally recognized
trading system. The ADSs issuable upon exercise of the Warrants are currently listed on The Nasdaq Capital Market under the symbol “QNRX.”
Rights as a Shareholder
Except
as otherwise provided in the Warrants or by virtue of such holder’s ownership of the underlying ordinary shares represented by
ADSs, the holders of the Warrants do not have the rights or privileges of holders of our ordinary shares represented by ADSs, including
any voting rights, until they exercise their Warrants.
Fundamental Transaction
In the event of a fundamental transaction, as described in the Warrants
and generally including any reorganization, recapitalization or reclassification of our Ordinary Shares, the sale, transfer or other
disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition
of more than 50% of our outstanding Ordinary Shares, the holders of the Warrants will be entitled to receive upon exercise of the Warrants
the kind and amount of securities, cash or other property that the holders would have received had they exercised the Warrants immediately
prior to such fundamental transaction. Additionally, in the event of a fundamental transaction, we or any successor entity will, at the
option of the holder of a Warrant exercisable at any time concurrently with or within 30 days after the consummation of the fundamental
transaction (or, if later, the date of the public announcement thereof), purchase the Warrant from the holder by paying to the holder
an amount of consideration equal to the value of the remaining unexercised portion of such Warrant on the date of consummation of the
fundamental transaction based on the Black-Scholes option pricing model, determined pursuant to a formula set forth in the Warrants.
The consideration paid to the holder will be the same type or form of consideration that was offered and paid to the holders of ordinary
shares in connection with the fundamental transaction; provided that if no such consideration was offered or paid, the holders of ordinary
shares will be deemed to have received ordinary shares of the successor entity in such fundamental transaction for purposes of this provision
of the Warrants.
CERTAIN
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS
The
following summary is included herein for general information and is not intended to be, and should not be considered to be, legal or
tax advice. Each holder should consult with his or her own tax advisor as to the particular u.s. federal income tax consequences of the
purchase, ownership and sale of ordinary shares, american depository shares and warrants, including the effects of applicable state,
local, foreign or other tax laws and possible changes in the tax laws.
Subject to the limitations described in the next paragraph, the following
discussion summarizes the material U.S. federal income tax consequences to a “U.S. Holder” arising from the purchase, ownership
and disposition of the ordinary shares, ADSs and warrants. For this purpose, a “U.S. Holder” is a beneficial owner of ordinary
shares or ADSs or warrants that is: (1) an individual citizen or resident of the United States, including an alien individual who
is a lawful permanent resident of the United States or meets the substantial presence residency test under U.S. federal income tax laws;
(2) a corporation (or entity treated as a corporation for U.S. federal income tax purposes) created or organized under the laws
of the United States, any state therein, or the District of Columbia; (3) an estate, the income of which is includable in gross
income for U.S. federal income tax purposes regardless of source; (4) a trust if a court within the United States is able to exercise
primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions
of the trust; and (5) a trust that has a valid election in effect to be treated as a U.S. person to the extent provided in U.S.
Treasury regulations. A “non-U.S. Holder” is a beneficial owner of ordinary shares or ADSs or warrants that is not a U.S.
Holder.
This summary is for general information purposes only and does not
purport to be a comprehensive description of all of the U.S. federal income tax considerations that may be relevant to a decision to
purchase our ordinary shares or ADSs or warrants. This summary generally considers only U.S. Holders that will own our ordinary shares
or ADSs or warrants as capital assets (generally, property held for investment). Except to the limited extent discussed below, this summary
does not consider the U.S. federal tax consequences to a person that is a non-U.S. Holder, nor does it describe the rules applicable
to determine a taxpayer’s status as a U.S. Holder. This summary is based on the provisions of the Code, final, temporary and proposed
U.S. Treasury regulations promulgated thereunder, administrative and judicial interpretations thereof, and the Convention Between the
Government of the United States of America and the Government of the State of Israel with Respect to Taxes on Income (the “U.S.-Israel
Double Tax Treaty”), all as in effect as of the date hereof and all of which are subject to change, possibly on a retroactive basis,
and all of which are open to differing interpretations. We will not seek a ruling from the Internal Revenue Service, or IRS, with regard
to the U.S. federal income tax treatment of an investment in our ordinary shares or ADSs or warrants and, therefore, can provide no assurances
that the IRS will agree with the conclusions set forth below.
This discussion does not address all of the tax considerations that
may be relevant to a particular U.S. Holder based on such holder’s particular circumstances, or to U.S. Holders that are subject
to special treatment under U.S. federal income tax law, including: (1) banks, life insurance companies, regulated investment companies,
or other financial institutions or “financial services entities”; (2) brokers or dealers in securities or foreign currency;
(3) persons who acquired our ordinary shares or ADSs or warrants in connection with employment or other performance of services;
(4) U.S. Holders that are subject to the U.S. alternative minimum tax; (5) U.S. Holders that hold our ordinary shares or ADSs
or warrants as a hedge or as part of a hedging, straddle, conversion or constructive sale transaction or other risk-reduction transaction
for U.S. federal income tax purposes; (6) tax-exempt entities; (7) real estate investment trusts; (8) U.S. Holders that
expatriate out of the United States or former long-term residents of the United States; or (9) U.S. Holders having a functional
currency other than the U.S. dollar. This discussion does not address the U.S. federal income tax treatment of a U.S. Holder that owns,
directly, indirectly or constructively, at any time, ordinary shares or ADSs or warrants representing 10% or more of our voting power
or value. This discussion also does not address any U.S. state or local or non-U.S. tax considerations, any U.S. federal estate, gift,
generation-skipping, transfer, or alternative minimum tax considerations, or any U.S. federal tax consequences other than U.S. federal
income tax consequences.
If
an entity or arrangement treated as a partnership for U.S. federal income tax purposes holds our ordinary shares or ADSs or warrants,
the tax treatment of such entity or arrangement treated as a partnership and each person treated as a partner thereof generally will
depend upon the status and activities of the entity and such person. A holder that is treated as a partnership for U.S. federal income
tax purposes and partners thereof should consult their own tax advisors regarding the U.S. federal income tax considerations applicable
to the purchase, ownership and disposition of our ordinary shares or ADSs or warrants.
Each prospective investor is advised to consult his or her own tax
adviser for the specific tax consequences to that investor of purchasing, holding or disposing of our ordinary shares or ADSs or warrants,
including the effects of applicable state, local, foreign or other tax laws and possible changes in the tax laws.
U.S. Tax Status of the Company
Although the Company is incorporated under Israeli law, as a result
of the consummation of the Merger, the Company should be treated, pursuant to Section 7874 of the Code, as a U.S. corporation for
all purposes under the Code. As a result, since the Company is and will be treated as a U.S. corporation for U.S. federal income tax
purposes and, we do not intend to treat the Company as a “passive foreign investment company,” as such rules apply only
to non-U.S. corporations that are treated as such for U.S. federal income tax purposes. Since the Company is a taxable corporation in
Israel, it would likely be subject to income taxation in both the United States and Israel on the same income, which could reduce the
amount of income available for distribution to shareholders. The ability of the Company to take foreign tax credits against its U.S.
tax liability in respect of taxes paid in Israel may be limited.
The remainder of this discussion assumes that the Company is treated
as a U.S. corporation for all U.S. federal income tax purposes. If, for some reason (e.g., future repeal of Section 7874 of the
Code), we were no longer treated as a U.S. corporation under the Code, the U.S. federal income tax consequences described herein could
be materially and adversely affected.
Taxation of Pre-Funded Warrants
The position of the IRS is that when the holder of an option to purchase
property is economically compelled to exercise the option based on all the facts and circumstances, the option holder is treated as the
beneficial owner of the underlying property for U.S. federal income tax purposes. Economic compulsion to exercise an option or warrant
to acquire stock can result when the exercise price of the option or warrant is nominal in relation to the value of the stock subject
to the option or warrant at the time of issuance of such option or warrant.
The purchase price of each Pre-Funded Warrant will comprise substantially
all of the value of the ADSs representing our ordinary shares underlying the Pre-Funded Warrant at the time the Pre-Funded Warrants are
sold. As a result, the discussion of the U.S. federal income taxation of warrants in this prospectus treats holders of Pre-Funded Warrants
as economically compelled to exercise the Pre-Funded Warrants and receive ordinary shares represented by ADSs. Accordingly, references
to ordinary shares or ADSs in this section, “Certain Material U.S. Federal Income Tax Considerations”, include Pre-Funded
Warrants as if the Pre-Funded Warrant holders receive ordinary shares represented by ADSs at the time such Pre-Funded Warrant holders
purchase the Pre-Funded Warrants. Additionally, references to warrants in this section, “Certain Material U.S. Federal Income Tax
Considerations”, means the Warrants only and not the Pre-Funded Warrants. There can be no assurance that the IRS or a court will
not take a contrary position on the U.S. federal income taxation of the Pre-Funded Warrants. Each prospective investor in Pre-Funded
Warrants is urged to consult his or her own tax advisor regarding the U.S. federal income tax consequences of the Pre-Funded Warrants.
Taxation
of Dividends Paid on Ordinary Shares or ADSs
We do not intend to pay dividends in the foreseeable future. In the
event that we do pay dividends, a U.S. Holder will be required to include in gross income as ordinary income the amount of any distribution
paid on ordinary shares or ADSs (including the amount of any Israeli tax withheld on the date of the distribution), to the extent that
such distribution does not exceed our current or accumulated earnings and profits, as determined for U.S. federal income tax purposes.
The amount of a distribution which exceeds our current and accumulated earnings and profits will be treated first as a non-taxable return
of capital, reducing the U.S. Holder’s tax basis for the ordinary shares or ADSs to the extent thereof, and then as capital gain.
Corporate holders generally will not be allowed a deduction for dividends received.
In general, preferential tax rates for “qualified dividend income”
and long-term capital gains are applicable for U.S. Holders that are individuals, estates or trusts. For this purpose, “qualified
dividend income” means, inter alia, dividends received from a “domestic corporation.” As indicated above, we believe
we should be treated as a domestic corporation and our dividends will therefore be qualified dividend income. A U.S. Holder will not
be entitled to the preferential rate: (1) if the U.S. Holder has not held our ordinary shares or ADSs for at least 61 days
of the 121-day period beginning on the date which is 60 days before the ex-dividend date, or (2) to the extent the U.S. Holder
is under an obligation to make related payments on substantially similar property. Any days during which the U.S. Holder has diminished
its risk of loss on our ordinary shares or ADSs are not counted towards meeting the 61-day holding period. Finally, U.S. Holders who
elect to treat the dividend income as “investment income” pursuant to Code section 163(d)(4) will not be eligible for
the preferential rate of taxation.
The amount of a distribution with respect to our ordinary shares or
ADSs will be measured by the amount of the fair market value of any property distributed, and for U.S. federal income tax purposes, the
amount of any Israeli taxes withheld therefrom. Cash distributions paid by us in NIS will be included in the income of U.S. Holders at
a U.S. dollar amount based upon the spot rate of exchange in effect on the date the dividend is includible in the income of the U.S.
Holder, and U.S. Holders will have a tax basis in such NIS for U.S. federal income tax purposes equal to such U.S. dollar value. If the
U.S. Holder subsequently converts the NIS into U.S. dollars or otherwise disposes of it, any subsequent gain or loss in respect of such
NIS arising from exchange rate fluctuations will be U.S. source ordinary exchange gain or loss.
U.S. Holders’ eligibility to claim a foreign tax credit with
respect to any Israeli withholding tax imposed on dividends paid by us may be limited. The foreign tax credit rules are complex,
and their application in connection with Section 7874 of the Code in the presence of the U.S.-Israel Double Tax Treaty, are not
entirely clear at this time. U.S. Holders should consult their own tax advisors with respect to any benefits they may be entitled to
under the foreign tax credit rules and the U.S.-Israel Double Tax Treaty, and to determine whether, and to what extent, they are
entitled to such credits.
Taxation of the Disposition of Ordinary Shares or ADSs or Warrants
Upon
the sale, exchange or other taxable disposition of our ordinary shares or ADSs or warrants, a U.S. Holder generally will recognize capital
gain or loss in an amount equal to the difference between such U.S. Holder’s tax basis for the ordinary shares or ADSs or warrants
in U.S. dollars and the amount realized on the disposition in U.S. dollars (or its U.S. dollar equivalent determined by reference to
the spot rate of exchange on the date of disposition, if the amount realized is denominated in a foreign currency). The gain or loss
realized on the sale, exchange or other disposition of ordinary shares or ADSs or warrants will be long-term capital gain or loss if
the U.S. Holder has a holding period of more than one year at the time of the disposition. U.S. Holders should consult their own
tax advisors regarding the U.S. federal income tax consequences of receiving currency other than U.S. dollars upon the disposition of
their ordinary shares.
Gain realized by a U.S. Holder on a sale, exchange or other disposition
of ordinary shares or ADSs or warrants will generally be treated as U.S. source income for U.S. foreign tax credit purposes. A loss realized
by a U.S. Holder on the sale, exchange or other disposition of ordinary shares or ADSs or warrants is generally allocated to U.S. source
income. The deductibility of a loss realized on the sale, exchange or other disposition of ordinary shares or ADSs or warrants is subject
to limitations.
A U.S. Holder’s eligibility to claim a foreign tax credit with
respect to any Israeli withholding tax imposed on gain from the sale or other disposition of our ordinary shares or ADSs or warrants
may be limited. The foreign tax credit rules are complex, and their application in connection with Section 7874 of the Code
in the presence of the U.S.-Israel Double Tax Treaty are not entirely clear at this time. U.S. Holders should consult their own tax advisors
with respect to any benefits they may be entitled to under the foreign tax credit rules and the U.S.-Israel Double Tax Treaty.
Exercise or Lapse of a Warrant
Except as discussed below with respect to a cashless exercise of a
warrant, a U.S. Holder generally will not recognize gain or loss upon the exercise of a warrant for cash. An ordinary share or ADS acquired
pursuant to the exercise of a warrant for cash generally will have a tax basis equal to the U.S. Holder’s tax basis in the warrant,
increased by the amount paid to exercise the warrant. The holding period of such share or ADS generally begins on the day after the date
of exercise of the warrant and will not include the period during which the U.S. Holder held the warrant.
The tax consequences of a cashless exercise of a warrant are not clear
under current tax law. A cashless exercise may be tax-free, either because the exercise is not a gain realization event or because the
exercise is treated as a recapitalization for U.S. federal income tax purposes. In either tax-free situation, a U.S. Holder’s basis
in the ordinary shares or ADSs received upon exercise of a warrant would equal the holder’s basis in the warrant. If the cashless
exercise were not treated as a gain realization event, a U.S. Holder’s holding period in the ordinary shares or ADSs received upon
exercise of a warrant would be treated as commencing on the date following the date of exercise (or possibly the date of exercise) of
the warrant. If the cashless exercise were treated as a recapitalization, the holding period of the ordinary shares or ADSs received
upon exercise of a warrant would include the holding period of the warrant.
It is also possible that a cashless exercise could be treated in part
as a taxable exchange in which gain or loss would be recognized. In such event, a U.S. Holder would recognize gain or loss with respect
to the portion of the exercised warrants treated as surrendered to pay the exercise price of the warrants (the “surrendered warrants”).
The U.S. Holder would recognize capital gain or loss in an amount equal to the difference between the fair market value of the surrendered
warrants and the U.S. Holder’s tax basis in such warrants. In this case, a U.S. Holder’s tax basis in the ordinary shares
or ADSs received upon exercise of a warrant would equal the sum of the fair market value of the surrendered warrants and the U.S. Holder’s
tax basis in the warrants exercised (except for any such tax basis allocable to the surrendered warrants). A U.S. Holder’s holding
period for the ordinary shares or ADSs received upon exercise of a warrant would commence on the date following the date of exercise
(or possibly the date of exercise) of the warrant.
Due
to the absence of authority on the U.S. federal income tax treatment of a cashless exercise, there can be no assurance which, if any,
of the alternative tax consequences and holding periods described above would be adopted by the IRS or a court. Accordingly, U.S. Holders
should consult their tax advisors regarding the tax consequences of a cashless exercise.
If a warrant is allowed to lapse unexercised, a U.S. Holder generally
will recognize a capital loss equal to such holder’s tax basis in the warrant. U.S. Holders should consult their own tax advisors
regarding the U.S. federal income tax consequences of the exercise of a warrant, including with respect to whether the exercise is a
taxable event, and their holding period and tax basis in the ordinary shares or ADSs received.
Tax on Investment Income
U.S. Holders who are individuals, estates or trusts will generally
be required to pay a 3.8% Medicare tax on their net investment income (including dividends on and gains from the sale or other disposition
of our ordinary shares and ADSs or warrants), or in the case of estates and trusts on their net investment income that is not distributed.
In each case, the 3.8% Medicare tax applies only to the extent the U.S. Holder’s total adjusted income exceeds applicable thresholds.
Tax Consequences for Non-U.S. Holders of Ordinary Shares or ADSs
or Warrants
Taxation of Dividends Paid on Ordinary Shares or ADSs
In general, any distributions we make to a non-U.S. Holder on ordinary
shares or ADSs, to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax
principles), will constitute dividends for U.S. federal income tax purposes and, provided such dividends are not effectively connected
with the non-U.S. Holder’s conduct of a trade or business within the United States, we will be required to withhold tax from the
gross amount of the dividend at a rate of 30%, unless such non-U.S. Holder is eligible for a reduced rate of withholding tax under an
applicable income tax treaty and provides proper certification of its eligibility for such reduced rate (usually on an IRS Form W-8BEN
or W-8BEN-E, as applicable). Any distribution on our ordinary shares or ADSs not constituting a dividend for U.S. federal income tax
purposes will be treated first as reducing (but not below zero) the non-U.S. Holder’s adjusted tax basis in its shares of such
stock and, to the extent such distribution exceeds the non-U.S. Holder’s adjusted tax basis in such stock, as gain realized from
the sale or other disposition of such stock, which will be treated as described under “Gain on Sale, Exchange or Other Taxable
Disposition of Ordinary Shares, ADSs, and Warrants” below. The full amount of any distributions to you may, however, be subject
to U.S. withholding tax unless the applicable withholding agent elects to withhold a lesser amount based on a reasonable estimate of
the amount of the distribution that would be treated as a dividend for U.S. federal income tax purposes. In addition, if we determine
that we are classified as a “United States real property holding corporation” (see “-Gain on Sale, Exchange or Other
Taxable Disposition of Ordinary Shares, ADSs, and Warrants” below), we will withhold 15% of any distribution that exceeds our current
and accumulated earnings and profits.
Dividends we pay to a non-U.S. Holder that are effectively connected
with such non-U.S. Holder’s conduct of a trade or business within the United States (and if a tax treaty applies are attributable
to a U.S. permanent establishment or fixed base maintained by the non-U.S. Holder) will generally not be subject to U.S. withholding
tax, provided such non-U.S. Holder complies with certain certification and disclosure requirements (usually by providing an IRS Form W-8ECI).
Instead, such dividends will generally be subject to U.S. federal income tax, net of certain deductions, at the same graduated individual
or corporate rates applicable to U.S. Holders. If the non-U.S. Holder is a corporation, dividends that are effectively connected income
may also be subject to a “branch profits tax” at a rate of 30% (or such lower rate as may be specified by an applicable income
tax treaty).
Exercise
or Lapse of a Warrant
The U.S. federal income tax treatment of a non-U.S. Holder’s
exercise of a warrant or the lapse of a warrant held by a non-U.S. Holder generally will correspond to the U.S. federal income tax treatment
of the exercise or lapse of a warrant by a U.S. Holder, as described under “Exercise of a Warrant” above. Accordingly, a
non-U.S. Holder generally will not be subject to U.S. federal income tax on the exercise of a warrant in exchange for ordinary shares
or ADSs. However, if a cashless exercise of warrants results in a taxable exchange, as described above in “Exercise of a Warrant”
above,” the rules described below under “- Gain on Sale, Exchange or Other Taxable Disposition of Ordinary Shares, ADSs,
and Warrants” would apply.
Gain on Sale, Exchange or Other Taxable Disposition of Ordinary
Shares, ADSs, and Warrants
A non-U.S. Holder generally will not be subject to U.S. federal income
or withholding tax on the proceeds from the disposition of, our ordinary shares or ADSs or warrants, unless:
| · | the
gain is effectively connected with the conduct of a trade or business by the non-U.S. Holder
within the United States (and, if an applicable tax treaty so requires, is attributable to
a U.S. permanent establishment or fixed base maintained by the non-U.S. Holder); |
| · | the
non-U.S. Holder is an individual who is present in the United States for 183 days or
more in the taxable year of disposition and certain other conditions are met; or |
| · | we
are or have been a “United States real property holding corporation” for U.S.
federal income tax purposes at any time during the shorter of the five-year period ending
on the date of disposition or the period that the non-U.S. Holder held our ordinary shares
or ADSs, and, in the case where our ordinary shares or ADSs are regularly traded on an established
securities market, the non-U.S. Holder has owned, directly or constructively, more than 5%
of our regularly-traded stock at any time within the shorter of the five-year period preceding
the disposition or such non-U.S. Holder’s holding period for the stock disposed of
by the non-U.S. holder. There can be no assurance that our ordinary shares or ADSs will be
treated as regularly traded on an established securities market for this purpose. |
Gain described in the first bullet point above will be subject to
tax at generally applicable U.S. federal income tax rates. Any gains described in the first bullet point above of a non-U.S. Holder that
is a foreign corporation may also be subject to an additional “branch profits tax” at a 30% rate (or lower applicable treaty
rate). Gain described in the second bullet point above will generally be subject to a flat 30% U.S. federal income tax, although the
gain may be offset by some United States source capital losses realized during the same taxable year. Non-U.S. Holders are urged
to consult their tax advisors regarding possible eligibility for benefits under income tax treaties.
If
the third bullet point above applies to a non-U.S. Holder, gain recognized by such holder on the sale, exchange or other disposition
of our ordinary shares, ADSs, or warrants will be subject to tax at generally applicable U.S. federal income tax rates. In addition,
a buyer of our ordinary shares, ADSs, or warrants from such holder may be required to withhold U.S. income tax at a rate of 15% of the
amount realized upon such disposition. We will be classified as a United States real property holding corporation if the fair market
value of our “United States real property interests” equals or exceeds 50% of the sum of the fair market value of our worldwide
real property interests plus our other assets used or held for use in a trade or business, as determined for U.S. federal income tax
purposes. Non-U.S. Holders are urged to consult their own tax advisors regarding the application of these rules.
Payments to Foreign Financial Institutions
The Foreign Account Tax Compliance Act (“FATCA”) generally
provides that a 30% withholding tax may be imposed on payments of U.S. source income, such as U.S. source dividends, to certain non-U.S.
entities unless such entities enter into an agreement with the IRS to disclose the name, address and taxpayer identification number of
certain U.S. persons that own, directly or indirectly, interests in such entities, as well as certain other information relating to such
interests. Non-U.S. Holders are encouraged to consult with their own tax advisors regarding the possible implications and obligations
of FATCA. While withholding under FATCA would have applied also to payments of gross proceeds from the sale or other disposition of ordinary
shares or ADSs on or after January 1, 2019, proposed Treasury Regulations eliminate FATCA withholding on payments of gross proceeds
entirely. Taxpayers generally may rely on these proposed Treasury Regulations until final Treasury Regulations are issued.
Information Reporting and Withholding
A U.S. Holder may be subject to backup withholding at a rate of 24%
with respect to dividends and proceeds from a disposition of ordinary shares or ADSs or warrants. In general, backup withholding will
apply only if a U.S. Holder fails to comply with specified identification procedures. Backup withholding will not apply with respect
to payments made to designated exempt recipients, such as corporations and tax-exempt organizations.
In general, non-U.S. Holders will not be subject to backup withholding
with respect to the payment of dividends and proceeds from a disposition of ordinary shares or ADSs or warrants, provided that the applicable
withholding agent does not have actual knowledge or reason to know the holder is a United States person, and the holder either certifies
its non-U.S. status, such as by furnishing a valid IRS Form W-8BEN, W-8BEN-E or W-8ECI, or otherwise establishes an exemption. However,
information returns are required to be filed with the IRS in connection with any payments of dividends on our ordinary shares or ADSs
paid to the non-U.S. holder, regardless of whether any tax was actually withheld. In addition, proceeds of the sale or other taxable
disposition of our ordinary shares or ADSs or warrants within the United States or conducted through certain U.S.-related brokers generally
will not be subject to backup withholding or information reporting if the applicable withholding agent receives the certification described
above and does not have actual knowledge or reason to know that such holder is a United States person, or the holder otherwise establishes
an exemption. Proceeds of a disposition of our ordinary shares or ADSs or warrants conducted through a non-U.S. office of a non-U.S.
broker generally will not be subject to backup withholding or information reporting.
Backup withholding is not an additional tax and may be claimed as
a credit against the U.S. federal income tax liability of a holder, provided that the required information is timely furnished to the
IRS.
CERTAIN
MATERIAL ISRAELI TAX CONSIDERATIONS
The
following description is not intended to constitute a complete analysis of all tax consequences relating to the ownership or disposition
of our ordinary shares or ADSs or warrants (all referred to in this section as “the Shares”). You should consult your own
tax advisor concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws
of any state, local, foreign, including Israeli, or other taxing jurisdiction.
The following is a summary of the material tax consequences under
Israeli law concerning the purchase, ownership and disposition of our Shares.
This discussion does not purport to constitute a complete analysis
of all potential tax consequences applicable to investors upon purchasing, owning or disposing of our Shares. In particular, this discussion
does not take into account the specific circumstances of any particular investor (such as traders in securities, not for profit organizations,
pension funds and other tax-exempt entities, financial institutions, certain financial companies, broker-dealers, partnerships and other
transparent entities, investors that own, directly or indirectly, 10% or more of our outstanding voting rights, all of whom are subject
to special tax regimes not covered under this discussion). To the extent that issues discussed herein are based on legislation that has
yet to be subject to judicial or administrative interpretation, there can be no assurance that the views expressed herein will accord
with any such interpretation in the future. The discussion below is subject to change, including due to amendments under Israeli law
or changes to the applicable judicial or administrative interpretations of Israeli law, which change could affect the tax consequences
described below. The discussion should not be construed as legal or professional tax advice and does not cover all possible tax considerations.
You are urged to consult your own tax advisors as to the Israeli or
other tax consequences of the purchase, ownership, and disposition of the Shares, including, in particular, the effect of any foreign,
state or local taxes.
General Corporate Tax Structure in Israel
Israeli resident companies are generally subject to corporate tax
on their taxable income at the rate of 23% for the 2024 tax year. Capital gains derived by an Israeli resident company are generally
subject to the prevailing corporate tax rate.
Taxation of Shareholders
Capital Gains
Capital gains tax is imposed on the disposition of capital assets
by an Israeli resident and on the disposition of such assets by a non-Israeli resident if those assets are either (i) located in
Israel; (ii) are shares or a right to a share in an Israeli company, or (iii) represent, directly or indirectly, rights to
assets located in Israel, unless an exemption is available or unless an applicable double tax treaty between Israel and the seller’s
country of residence provides otherwise. The Israeli Income Tax Ordinance distinguishes between “Real Gain” and the “Inflationary
Surplus”. “Real Gain” is the excess of the total capital gain over Inflationary Surplus generally computed on the basis
of the increase in the Israeli Consumer Price Index between the date of purchase and the date of disposition. Inflationary Surplus is
not subject to tax.
Taxable capital gain accrued by individuals on the sale of the Shares
are taxed at the rate of 25%. However, if the individual shareholder is a “Substantial Shareholder” at the time of sale or
at any time during the preceding 12-month period, such gain will be taxed at the rate of 30%. In this regard, broadly, a “Substantial
Shareholder” is considered to be a person who alone, or together with his relative or another person who collaborates with him
on a regular basis based on a contract, holds, directly or indirectly, at least 10% of any our means of control. In this context “means
of control” generally includes the right to vote, receive profits, nominate a director or an officer, receive assets upon liquidation,
or instruct someone who holds any of these rights regarding the manner in which he or she is to exercise such right(s), and all regardless
of the source of such rights.
The
term “Israeli resident” is generally defined under Israeli tax legislation with respect to individuals as a person whose
center of life is in Israel. The Israeli tax legislation provides that in order to determine the center of life of an individual, account
will be taken of the individual’s family, economic and social connections, including: (a) place of permanent home; (b) place
of residential dwelling of the individual and the individual’s immediate family; (c) place of the individual’s regular
or permanent occupation or the place of his permanent employment; (d) place of the individual’s active and substantial economic
interests; and (e) place of the individual’s activities in organizations, associations and other institutions. The center
of life of an individual will be presumed to be in Israel if: (a) the individual was present in Israel for 183 days or more
in the tax year; or (b) the individual was present in Israel for 30 days or more in the tax year, and the total period
of the individual’s presence in Israel in that tax year and the two previous tax years is 425 days or more. The
presumption in this paragraph may be rebutted either by the individual or by the assessing officer.
Capital gains derived by corporations are generally subject to tax
at the ordinary corporate tax rate (currently 23%). Under Israeli tax legislation, a corporation will be considered as an “Israeli
Resident” if it meets one of the following criteria: (a) it was incorporated in Israel; or (b) the control and management
of its business are exercised in Israel.
Despite the above, capital gains generated from the sale of our Shares
by a non-Israeli shareholder may be exempt from Israeli tax under the Israeli tax legislation provided that the following cumulative
conditions are met: (i) the Shares were purchased by the shareholder upon or after the registration of the Shares on the non-Israeli
stock exchange (i.e., July 29, 2016); (ii) the shareholder does not have a permanent establishment maintained in Israel to
which the generated capital gain is attributed; and (iii) so long as neither the shareholder nor the particular capital gain is
otherwise subject to the Israeli Income Tax Law (Inflationary Adjustments) 5745-1985. However, a seller of our Shares that is a non-Israeli
resident corporation will not be entitled to this exemption if Israeli residents: (i) have a controlling interest, directly or indirectly,
alone or together with another (i.e., together with a relative, or together with someone who is not a relative but with whom, according
to an agreement, there is regular cooperation in material matters of the company, directly or indirectly), or together with another Israeli
resident, of more than 25% in any of the means of control of such non-Israeli corporation or (ii) are the beneficiaries of, or are
entitled to, 25% or more of the income or profits of such non-Israeli corporation, whether directly or indirectly. In addition, this
exemption would not be available to a person whose gains from selling or otherwise disposing of our Shares are deemed to be business
income.
Likewise, capital gains generated from the sale of our Shares by a
non-Israeli shareholder who purchased the Shares before the registration of the Shares on the non-Israeli stock exchange may also be
exempt from Israeli tax under the Israeli tax legislation provided that the following cumulative conditions are met: (i) the Shares
were purchased on January 1, 2009 or afterwards; (ii) the Shares were not purchased from a related party (as defined for this
purpose) or as part of an exempted reorganization for Israeli tax purposes; (iii) the Shares are not registered for trade on an
Israeli stock exchange at the date of the sale; (iv) on the day of the purchase of the Shares and in the two preceding years
- most of the value of the assets held by the Israeli company, directly or indirectly, are not rights in, or attached or related to,
or in connection with the use of or proceeds from, real estate rights or a real estate corporations, as defined under the Real Estate
Taxation Law 5723-1963, and any other rights to real estate, rights to use state natural resources such as minerals or rights to use
benefits derived from the real estate in Israel; and (v) the capital gain is not allocated to a permanent establishment that the
non-Israeli resident maintains in Israel.
In
addition, the sale of the Shares may be also exempt from Israeli capital gains tax under the provisions of an applicable double tax treaty.
For example, the tax treaty between the Government of the United States of America and the Government of the State of Israel with respect
to Taxes on Income, as amended, (the “U.S.-Israel Double Tax Treaty”) generally exempts a shareholder who is a United States
resident (for purposes of the treaty) holding the shares as a capital asset and is entitled to claim the benefits afforded to such a
resident by the U.S.-Israel Double Tax Treaty (“Treaty U.S. Resident”) from Israeli capital gain tax in connection with the
sale of our Shares, provided that: (i) the Treaty U.S. Resident owns, directly or indirectly, less than 10% of our voting power
at any time within the 12-month period preceding such sale, subject to certain conditions; (ii) the Treaty U.S. Resident, if an
individual, was present in Israel for a period or periods of less than 183 days in the aggregate during the relevant taxable year;
(iii) the capital gain from the sale was not derived through a permanent establishment of the Treaty U.S. Resident which is maintained
in Israel, under certain terms; (iv) the capital gain from the sale is not attributed to royalties; and (v) the capital gain
from the sale is not attributed to real estate located in Israel. A Treaty U.S. Resident not exempt from Israeli capital gains tax may
be limited under U.S. law in its ability to claim a credit for such taxes against the U.S. federal income tax imposed with respect to
such sale, exchange or disposition even if such Treaty U.S. Resident is eligible for benefits under the U.S.-Israel Double Tax Treaty.
The U.S.-Israel Double Tax Treaty does not relate to U.S. state or local taxes.
There may be some other circumstances in which exemptions (or partial
exemptions) may apply, so that any non-Israeli shareholder who does not meet the aforementioned exemption criteria (whether under the
Israeli internal tax law or the relevant tax treaty) should consult their own tax advisors.
Regardless of whether non-Israeli shareholders may be liable for Israeli
capital gains tax on the sale of our Shares, the payment of the consideration for such sale may be subject to withholding of Israeli
tax at source and holders of our Shares may be required to demonstrate that they are exempt from tax on their capital gains in order
to avoid withholding at source at the time of sale. Specifically, in transactions involving a sale of all of the shares of an Israeli
resident company, in the form of a merger or otherwise, the Israel Tax Authority may require shareholders who are not liable for Israeli
capital gains tax on such a sale to sign declarations on forms specified by the Israel Tax Authority, provide documents (including, for
example, a certificate of residency) or obtain a specific exemption from the Israel Tax Authority to confirm their status as non-Israeli
residents and, in the absence of such declarations or exemptions, the Israel Tax Authority may require the purchaser of the shares to
withhold tax at source.
The
purchaser, the Israeli stockbroker or the financial institution through which the Shares are held, is obligated, subject to the
abovementioned exemptions, to withhold tax on the amount of consideration paid upon the sale of Shares at a rate of 25% (for individuals)
or 23% (for corporations).
Upon
the sale of traded securities, a detailed return, including a computation of the tax due, generally need to be filed and an advance payment
must be paid to the Israel Tax Authority on January 31 and July 31 of every calendar year in respect of sales of traded
securities made within the previous six months. This will apply to the sale of our Shares. However, if all tax due was withheld
at source according to applicable provisions of the Israeli Income Tax Ordinance and regulations promulgated thereunder, such return
need not be filed, and no advance payment must be paid. Capital gains are also reportable on annual income tax returns.
Dividends
Dividends distributed by an Israeli company to a shareholder who is
an Israeli resident individual will generally be subject to income tax at a rate of 25%. However, a 30% tax rate will apply if the dividend
recipient is a Substantial Shareholder, as defined above, at the time of distribution or at any time during the preceding 12-month period.
If the recipient of the dividend is an Israeli resident corporation, dividends will generally be exempted from Israeli income tax provided
that the income from which such dividend is distributed was derived or accrued within Israel.
Dividends
distributed by an Israeli company to a non-Israeli resident (either an individual or a corporation) are generally subject to Israeli
withholding tax at the rate of 25% (30% if the dividend recipient is a Substantial Shareholder at the time of distribution or at any
time during the preceding 12-month period). Dividends paid on publicly traded shares, to non-Israeli residents or Israeli resident
individual, are generally subject to Israeli withholding tax at a rate of 25%, so long as the shares are registered with a nominee company
(whether or not the recipient is a substantial shareholder), unless a lower rate is provided under an applicable tax treaty (provided
that a certificate from the ITA allowing for a reduced withholding tax rate is obtained in advance). However, a distribution of dividends
to non-Israeli residents is generally subject to a withholding tax at the source at a rate of 15%, or such lower rate as may be provided
in an applicable tax treaty, if the dividend is distributed from income attributed to a "Benefited or Approved Enterprise,"
and 20% if the dividend is distributed from income attributed to "Preferred Enterprise" as such term is defined in the Law
for the Encouragement of Capital Investments, 5719-1959, or such lower rate as may be provided in an applicable tax treaty (subject to
the receipt in advance of a valid certificate from the ITA allowing for a reduced withholding tax rate). For example, under the U.S.-Israel
Double Tax Treaty, the following tax rates will apply in respect of dividends distributed by an Israeli resident company to a Treaty
U.S. Resident: (i) if the Treaty U.S. Resident is a corporation that holds during that portion of the taxable year which precedes
the date of payment of the dividend and during the whole of its prior taxable year (if any), at least 10% of the outstanding shares
of the voting stock of the Israeli company paying the dividend and not more than 25% of the gross income of such Israeli company for
such prior taxable year (if any) consists of certain types of interest or dividends, the maximum tax rate is 12.5%; (ii) if
both the conditions mentioned in clause (i) above are met and the dividend is paid from an Israeli resident company’s income
which was entitled to a reduced tax rate under the Law for the Encouragement of Capital Investments, 1959, the tax rate is 15%, if a
certificate for a reduced withholding tax rate would be provided in advance from the Israel Tax Authority; and (iii) in most other
cases, the tax rate is 25%. The aforementioned lower rates under the U.S.-Israel Double Tax Treaty will not apply if the dividend income
is attributed to a permanent establishment of the Treaty U.S. Resident maintained in Israel.
U.S.
residents who are subject to Israeli withholding tax on a dividend may be entitled to a credit or deduction for United States federal
income tax purposes in the amount of the taxes withheld, subject to detailed rules contained in the Code.A non-Israeli resident
who receives dividends from which tax was withheld is generally exempt from the obligation to file tax returns in Israel with respect
to such income, provided that (i) such income was not generated from business conducted in Israel by the taxpayer, (ii) in
the case of individuals, the non-Israeli resident is not subject to Surtax in Israel, and; (iii) the taxpayer has no other taxable
sources of income in Israel with respect to which a tax return is required to be filed.
Surtax
Individual
who are subject to tax in Israel (whether any such individual is an Israeli resident or non-Israeli resident) and who have taxable income
that exceeds a certain threshold in a tax year (NIS 721,560 for 2024, linked annually to the Israeli Consumer Price Index) will
be subject to an additional tax at the rate of 3% on his or her taxable income for such tax year that is in excess of such amount.
For this purpose, taxable income includes taxable capital gains and taxable income from interest and dividends, subject to the provisions
of an applicable double tax treaty.
Estate and Gift Tax
Israeli tax law presently does not impose estate or gift taxes.
You should consult your own
tax advisor regarding the particular israeli tax consequences of purchasing, holding, and disposing of our shares, including the consequences
of any proposed change in applicable laws.
PLAN
OF DISTRIBUTION
Maxim Group LLC (the “Placement Agent”)
has agreed to act as our exclusive Placement Agent in connection with this offering subject to the terms and conditions of the placement
agency agreement dated , 2024. The Placement Agent is not purchasing or selling any of the Securities offered by this prospectus, nor
is it required to arrange the purchase or sale of any specific number or dollar amount of Securities, but has agreed to use its reasonable
best efforts to arrange for the sale of the Securities offered hereby. Therefore, we may not sell the entire amount of Securities offered
pursuant to this prospectus. We will enter into a securities purchase agreement directly with certain investors, at the investor’s
option, who purchase our Securities in this offering. Investors who do not enter into a securities purchase agreement shall rely solely
on this prospectus and the documents incorporated by reference herein in connection with the purchase of our Securities in this offering.
We will deliver all Securities to be issued in connection with this
offering delivery versus payment (“DVP”)/receipt versus payment (“RVP”) upon receipt of investor funds. We expect
to deliver the Securities being offered pursuant to this prospectus on or about ,
2024, subject to satisfaction or waiver of customary closing conditions.
We have agreed to indemnify the Placement Agent and specified other
persons against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the Placement Agent
may be required to make in respect thereof.
Fees and Expenses
This offering is being conducted on a “reasonable best efforts”
basis and the Placement Agent has no obligation to buy any of the Securities from us or to arrange for the purchase or sale of any specific
number or dollar amount of Securities. We have agreed to pay the Placement Agent a fee based on the aggregate proceeds as set forth in
the table below (assuming the sale of all of the Securities we are offering):
| | |
Per ADS
and Accompanying
Warrants | | |
Per Pre-Funded Warrant
and Accompanying
Warrants | | |
Total | |
| Public offering price | |
$ | | | |
$ | | | |
$ | | |
| Placement Agent’s fees | |
$ | | | |
$ | | | |
$ | | |
| Proceeds to us, before expenses (1) | |
$ | | | |
$ | | | |
$ | | |
| (1) | The
amount of the proceeds to us presented in this table does not give effect to any exercise
of the Warrants or Pre-Funded Warrants. |
We
have agreed to pay to the Placement Agent a cash fee equal to 7% of the aggregate gross proceeds raised in this offering. Because
there is no minimum offering amount required as a condition to closing in this offering, the actual aggregate cash placement fee, if
any, is not presently determinable and may be substantially less than the maximum amount set forth above. The Placement Agent may retain
other brokers or dealers to act as sub-agents on their behalf in connection with this offering and may pay any sub-agent a fee with respect
to any Securities placed by such placement agent.
In addition, we will bear the costs in connection with the offering
and have also agreed to reimburse the Placement Agent for its expenses relating to this offering, including, but not limited to, fees,
expenses and disbursements relating to background checks of our officers and directors and the fees and expenses of counsel for the Placement
Agent, up to a maximum reimbursement allowance of $125,000. We have agreed to the payment of $25,000 to be applied against the Placement
Agent’s expenses (the “Advance”). Upon acceptance of the engagement by the Placement Agent, the Company delivered to
Maxim the Advance. Such Advance will be applied against the Placement Agent’s expenses in connection with the offering, and to
the extent not actually incurred, such Advance shall be reimbursed to us.
Regulation
M Compliance
The Placement Agent may be deemed to be an underwriter within the
meaning of Section 2(a)(11) of the Securities Act, and any commissions received by them and any profit realized on the resale of
the shares sold by them while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act.
As an underwriter, a placement agent would be required to comply with the requirements of the Securities Act and the Exchange Act, including,
without limitation, Rule 415(a)(4) under the Securities Act and Rule 10b-5 and Regulation M under the Exchange Act. These
rules and regulations may limit the timing of purchases and sales of Securities by the placement agents acting as principal. Under
these rules and regulations, the placement agents:
| · | may
not engage in any stabilization activity in connection with our securities; and |
| · | may
not bid for or purchase any of our securities or attempt to induce any person to purchase
any of our securities, other than as permitted under the Exchange Act, until it has completed
its participation in the distribution. |
Lock-Up Agreements
Our
directors and officers have entered into lock-up agreements. Under these agreements, these individuals have agreed, subject to specified
exceptions, not to sell or transfer any ADSs or securities convertible into, or exchangeable or exercisable for, our ADSs during a period
ending six (6) months after the completion of this offering, without first obtaining the written consent of the placement
agent, subject to certain exceptions. Specifically, these individuals have agreed, in part, not to:
| · | offer,
pledge, sell, contract to sell or otherwise dispose of Quoin’s securities or any securities
convertible into or exercisable or exchangeable for ordinary shares; |
| · | enter
into any swap or other arrangement that transfers to another, in whole or in part, any of
the economic consequences of ownership of our securities, whether any such transaction is
to be settled by delivery of our securities, in cash or otherwise; |
| · | make
any demand for or exercise any right with respect to the registration of any of our securities;
and |
| · | publicly
disclose the intention to make any offer, sale, pledge or disposition of, or to enter into
any transaction, swap, hedge, or other arrangement relating to any of our securities. |
Notwithstanding these limitations, our securities may be transferred
under limited circumstances, including, without limitation, by gift, will or intestate succession.
We have agreed with the Placement Agent to be subject to a lock-up
period of 90 days following the date of closing of the offering pursuant to this prospectus. This means that, during the applicable lock-up
period, subject to certain limited exceptions, we may not: (i) issue, enter into any agreement to issue or announce the issuance
or proposed issuance of any ADSs, ordinary shares or ordinary share equivalents, or (ii) file or cause to be filed any registration
statement or amendment or supplement thereto, other than the preliminary prospectus or the prospectus related to this offering, a registration
statement on Form S-8 in connection with any employee benefit plan, or the filing of any amendment or supplement to any existing
registration statement solely for the purpose of revising any required disclosure in such registration statement and not for the purpose
of increasing the offering size pursuant to any such registration statement. In addition, subject to certain exceptions, we have agreed
not to effect or enter into an agreement to effect any issuance of securities in a Variable Rate Transaction (as defined in the placement
agency agreement) for a period of 180 days following the closing date of this offering, subject to certain exceptions.
Determination
of Offering Price
The public offering price of the Securities we are offering, including
the exercise price of the Warrants, was negotiated between us and the investors, in consultation with the Placement Agent based on the
trading of our ADSs prior to the offering, among other things. Other factors considered in determining the public offering price of the
Securities we are offering include our history and prospects, the industry in which we operate, our past and present operating results,
the stage of development of our business, our business plans for the future and the extent to which they have been implemented, the previous
experience of our executive officers, general conditions of the securities markets at the time of the offering and such other factors
as were deemed relevant.
Listing
Our ADSs are listed on The Nasdaq Capital Market under the trading
symbol “QNRX.” We do not plan to list the Pre-Funded Warrants or the Warrants on The Nasdaq Capital Market or any other securities
exchange or trading market.
Insider Participation
One or more of our officers and directors
have indicated interest in participating in this offering at the public offering price and on the same terms as the other purchasers
in this offering. However, because indications of interest are not binding, we cannot guarantee if any officer or
director will participate in this offering.
Other Activities and Relationships
The Placement Agent and certain of its affiliates are full service
financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial
advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The Placement
Agent and certain of its affiliates have, from time to time, performed, and may in the future perform, various commercial and investment
banking and financial advisory services for us and our affiliates, for which they received or will receive customary fees and expenses.
In the ordinary course of their various business activities, the Placement
Agent and certain of its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related
derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers,
and such investment and securities activities may involve securities and/or instruments issued by us and our affiliates. If the Placement
Agent or its affiliates enter into a lending relationship with us, they will routinely hedge their credit exposure to us consistent with
their customary risk management policies. The Placement Agent and its affiliates may hedge such exposure by entering into transactions
that consist of either the purchase of credit default swaps or the creation of short positions in our securities or the securities of
our affiliates, including potentially the ADSs offered hereby. Any such short positions could adversely affect future trading prices
of the ADSs offered hereby. The Placement Agent and certain of its affiliates may also communicate independent investment recommendations,
market color or trading ideas and/or publish or express independent research views in respect of such securities or instruments and may
at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
This
prospectus in electronic format may be made available on a website maintained by the Placement Agent, and the Placement Agent may distribute
this prospectus electronically.
The foregoing does not purport to be a complete statement of the terms
and conditions of the placement agency agreement or the securities purchase agreement entered into in connection with this offering,
copies of which have been filed as exhibits to the registration statement of which this prospectus is a part. See “Where You Can
Find More Information.”
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or
the Placement Agent that would permit a public offering of the securities offered by us in any jurisdiction where action for that purpose
is required. The securities offered by us in this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus
or any other offering material or advertisements in connection with the offering of the securities offered by us under this prospectus
be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and
regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe
any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell
or a solicitation of an offer to buy any securities offered by us under this prospectus in any jurisdiction in which such an offer or
a solicitation is unlawful.
Notice to Prospective Investors in Israel
This document does not constitute a prospectus under the Israeli Securities
Law and has not been filed with or approved by the Israel Securities Authority. In the State of Israel, this document is being distributed
only to, and is directed only at, and any offer of the securities is directed only at, investors listed in the first addendum, or the
Addendum, to the Israeli Securities Law, consisting primarily of joint investment in trust funds, provident funds, insurance companies,
banks, portfolio managers, investment advisors, members of the Tel Aviv Stock Exchange, underwriters, venture capital funds, entities
with equity in excess of NIS 50 million and “qualified individuals”, each as defined in the Addendum (as it may be amended
from time to time), collectively referred to as qualified investors (in each case purchasing for their own account or, where permitted
under the Addendum, for the accounts of their clients who are investors listed in the Addendum). Qualified investors will be required
to submit written confirmation that they fall within the scope of the Addendum, are aware of the meaning of same and agree to it.
Notice to Prospective Investors in Canada
The securities may be sold in Canada only to
purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus
Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument
31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made
in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada
may provide a purchaser with remedies for rescission or damages if this prospectus supplement (including any amendment thereto) contains
a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed
by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions
of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal
advisor.
Pursuant to section 3A.3 of National Instrument
33-105 Underwriting Conflicts (NI 33-105), the placement agents are not required to comply with the disclosure requirements
of NI 33-105 regarding placement agents conflicts of interest in connection with this offering.
Notice to Prospective Investors in European
Economic Area
In relation to each Member State of the European
Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”) an offer to the public of
any securities may not be made in that Relevant Member State, except that an offer to the public in that Relevant Member State of any
securities may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that
Relevant Member State:
| |
· |
to any legal
entity which is a qualified investor as defined in the Prospectus Directive; |
| |
· |
to fewer than
100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal
persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject
to obtaining the prior consent of the representatives for any such offer; or |
| |
· |
in any other
circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall
result in a requirement for the publication by us or any placement agents of a prospectus pursuant to Article 3 of the Prospectus
Directive. |
For the purposes of this provision, the expression
an “offer to the public” in relation to any securities in any Relevant Member State means the communication in any form and
by any means of sufficient information on the terms of the offer and any securities to be offered so as to enable an investor to decide
to purchase any securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that
Member State, the expression “Prospectus Directive” means Directive 2003/71/EC (and amendments thereto, including the 2010
PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in the
Relevant Member State, and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
Notice to Prospective Investors in United
Kingdom
Each placement agent has represented and agreed
that:
| |
· |
it has only
communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage
in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000 (the FSMA) received
by it in connection with the issue or sale of the securities in circumstances in which Section 21(1) of the FSMA does not
apply to us; and |
| |
· |
it has complied
and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the securities in,
from or otherwise involving the United Kingdom. |
Notice to Prospective Investors in Switzerland
The securities may not be publicly offered in
Switzerland and will not be listed on the SIX Swiss Exchange (the SIX) or on any other stock exchange or regulated trading facility in
Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or
art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing
Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document
nor any other offering or marketing material relating to the securities or the offering may be publicly distributed or otherwise made
publicly available in Switzerland.
Neither this document nor any other offering
or marketing material relating to the offering, or the securities have been or will be filed with or approved by any Swiss regulatory
authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial
Market Supervisory Authority FINMA, and the offer of securities has not been and will not be authorized under the Swiss Federal Act on
Collective Investment Schemes (CISA). Accordingly, no public distribution, offering or advertising, as defined in CISA, its implementing
ordinances and notices, and no distribution to any non-qualified investor, as defined in CISA, its implementing ordinances and notices,
shall be undertaken in or from Switzerland, and the investor protection afforded to acquirers of interests in collective investment schemes
under CISA does not extend to acquirers of securities.
Notice to Prospective Investors in Australia
No placement document, prospectus, product disclosure
statement or other disclosure document has been lodged with the Australian Securities and Investments Commission (ASIC), in relation
to the offering.
This prospectus does not constitute a prospectus,
product disclosure statement or other disclosure document under the Corporations Act 2001 (the Corporations Act) and does not purport
to include the information required for a prospectus, product disclosure statement or other disclosure document under the Corporations
Act.
Any offer in Australia of the securities may
only be made to persons (the Exempt Investors) who are “sophisticated investors” (within the meaning of section 708(8) of
the Corporations Act), “professional investors” (within the meaning of section 708(11) of the Corporations Act) or otherwise
pursuant to one or more exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the securities without
disclosure to investors under Chapter 6D of the Corporations Act.
The securities applied for by Exempt Investors
in Australia must not be offered for sale in Australia in the period of 12 months after the date of allotment under the offering, except
in circumstances where disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption
under section 708 of the Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter
6D of the Corporations Act. Any person acquiring securities must observe such Australian on-sale restrictions.
This prospectus contains general information
only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does
not contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider
whether the information in this prospectus is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert
advice on those matters.
Notice to Prospective Investors in the Cayman
Islands
No invitation, whether directly or indirectly,
may be made to the public in the Cayman Islands to subscribe for our securities.
Notice to Prospective Investors in Taiwan
The securities have not been and will not be
registered with the Financial Supervisory Commission of Taiwan pursuant to relevant securities laws and regulations and may not be sold,
issued or offered within Taiwan through a public offering or in circumstances which constitutes an offer within the meaning of the Securities
and Exchange Act of Taiwan that requires a registration or approval of the Financial Supervisory Commission of Taiwan. No person or entity
in Taiwan has been authorized to offer, sell, give advice regarding or otherwise intermediate the offering and sale of the securities
in Taiwan.
Notice to Prospective Investors in Hong Kong
The contents of this prospectus have not been
reviewed by any regulatory authority in Hong Kong. You are advised to exercise caution in relation to the offer. If you are in any doubt
about any of the contents of this prospectus, you should obtain independent professional advice. Please note that (i) our shares
may not be offered or sold in Hong Kong, by means of this prospectus or any document other than to “professional investors”
within the meaning of Part I of Schedule 1 of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) (SFO) and any rules made
thereunder, or in other circumstances which do not result in the document being a “prospectus” within the meaning of the
Companies Ordinance (Cap.32, Laws of Hong Kong) (CO) or which do not constitute an offer or invitation to the public for the purpose
of the CO or the SFO, and (ii) no advertisement, invitation or document relating to our shares may be issued or may be in the possession
of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere) which is directed at, or the contents of which
are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other
than with respect to the shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional
investors” within the meaning of the SFO and any rules made thereunder.
Notice to Prospective Investors in the People’s
Republic of China
This prospectus may not be circulated or distributed
in the PRC and the shares may not be offered or sold, and will not offer or sell to any person for re-offering or resale directly or
indirectly to any resident of the PRC except pursuant to applicable laws, rules and regulations of the PRC. For the purpose of this
paragraph only, the PRC does not include Taiwan and the special administrative regions of Hong Kong and Macau.
LEGAL
MATTERS
Certain
legal matters as to United States law in connection with this offering will be passed upon for us by Blank Rome LLP. The validity
of the securities and other matters of Israeli law will be passed upon for us by Meitar | Law Offices, Ramat Gan, Israel. Certain
legal matters related to the offering will be passed upon for the Placement Agent by Ellenoff Grossman & Schole LLP.
EXPERTS
The consolidated financial statements as of and
for the years ended December 31, 2023 and 2022 incorporated by reference in this registration statement have been audited by Marcum
LLP, an independent registered public accounting firm, as stated in their report. Such financial statements are incorporated in reliance
upon the report of such firm given upon their authority as experts in accounting and auditing.
ENFORCEABILITY
OF CIVIL LIABILITIES
To the extent any of our shareholders may seek
to enforce a U.S. judgment in Israel against us or our executive officers and directors, or to assert U.S. securities law claims in Israel,
shareholders may have difficulties enforcing such a U.S. judgment, including judgments based upon the civil liability provisions of the
U.S. federal securities laws, in Israel.
We have been informed by our legal counsel in
Israel that it may be difficult to assert U.S. securities laws claims in original actions instituted in Israel. Israeli courts may refuse
to hear a claim based on a violation of U.S. securities laws because Israel is not the most appropriate forum in which to bring such
a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable
to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proven as a fact which can be a time-consuming
and costly process. Matters of procedure will also be governed by Israeli law.
We have irrevocably appointed Quoin Pharmaceuticals, Inc.,
as our agent to receive service of process in any action against us in any U.S. federal or state court arising out of this offering or
any purchase or sale of securities in connection with this offering. Subject to specified time limitations and legal procedures, Israeli
courts may enforce a U.S. judgment in a civil matter which is non-appealable, including a judgment based upon the civil liability provisions
of the Securities Act or the Exchange Act and including a monetary or compensatory judgment in a non-civil matter, provided that, among
other things:
| · | the judgment was rendered by a court of competent jurisdiction,
according to the laws of the state in which the judgment is given; |
| · | the judgment is enforceable according to the laws of Israel
and according to the law of the foreign state in which the relief was granted; and |
| · | the judgment is not contrary to public policy of Israel. |
Even if such conditions are met, an Israeli court
may not declare a foreign civil judgment enforceable if:
| · | the prevailing law of the foreign state in which the judgment
is rendered does not allow for the enforcement of judgments of Israeli courts (subject to exceptional cases); |
| · | the defendant did not have a reasonable opportunity to be heard
and to present his or her evidence, in the opinion of the Israeli court; |
| · | the enforcement of the civil liabilities set forth in the judgment
is likely to impair the security or sovereignty of Israel; |
| · | the judgment was obtained by fraud; |
| · | the judgment was rendered by a court not competent to render
it according to the rules of private international law prevailing in Israel; |
| · | the judgment conflicts with any other valid judgment in the
same matter between the same parties; or |
| · | an action between the same parties in the same matter was pending
in any Israeli court or tribunal at the time at which the lawsuit was instituted in the foreign court. |
If a foreign judgment is enforced by an Israeli court, it generally
will be payable in Israeli currency, which can then be converted into non-Israeli currency and transferred out of Israel. The usual practice
in an action before an Israeli court to recover an amount in a non-Israeli currency is for the Israeli court to issue a judgment for
the equivalent amount in Israeli currency at the rate of exchange in force on the date of the judgment, but the judgment debtor may make
payment in foreign currency. Pending collection, the amount of the judgment of an Israeli court stated in Israeli currency ordinarily
will be linked to the Israeli consumer price index plus interest at the annual statutory rate set by Israeli regulations prevailing at
the time. Judgment creditors must bear the risk of unfavorable exchange rates.”
WHERE
YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under
the Securities Act with respect to the securities offered hereby. This prospectus, which constitutes a part of the registration statement,
does not contain all of the information set forth in the registration statement or the exhibits and schedules filed with the registration
statement. For further information about us and the securities offered hereby, we refer you to the registration statement and the exhibits
filed with the registration statement. Statements contained in this prospectus regarding the contents of any contract or any other document
that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects
by reference to the full text of such contract or other document filed as an exhibit to the registration statement. The SEC also maintains
an internet website that contains reports, proxy statements and other information about registrants, like us, that file electronically
with the SEC. The address of that website is www.sec.gov.
We are required to file periodic reports, proxy statements, and other
information with the SEC pursuant to the Exchange Act. These reports, proxy statements, and other information will be available
on the website of the SEC referred to above.
We also maintain a website at www.quoinpharma.com, through
which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished
to, the SEC. Information contained on or accessed through our website is not a part of this prospectus and the inclusion of our
website address in this prospectus is an inactive textual reference only.
Incorporation
of Certain Information By Reference
The SEC allows us to “incorporate by reference” information
from other documents that we file with it, which means that we can disclose important information to you by referring you to those documents.
The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information
incorporated by reference that we filed with the SEC prior to the date of this prospectus.
We incorporate by reference into this prospectus and the registration
statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC (Commission File
No. 001-37846):
| · | Our
Quarterly Report on Form 10-Q for the quarter ended March 31, 2024 filed with the
SEC on May 9, 2024, our Quarterly Report on Form 10-Q for the quarter ended June 30,
2024 filed with the SEC on August 8, 2024, and our Quarterly Report on Form 10-Q
for the quarter ended September 30, 2024 filed with the SEC on November 8, 2024; |
| · | Our
Current Reports on Form 8-K filed with the SEC on January 30, 2024, March 6, 2024, March 8, 2024, April 9, 2024, May 3, 2024, September 30, 2024,
October 31, 2024 and December 10, 2024; |
We also incorporate by reference any future filings (other than current
reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items
unless such Form 8-K expressly provides to the contrary) made with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of
the Exchange Act, including those made (i) on or after the date of the initial filing of the registration statement of which this
prospectus forms a part and prior to effectiveness of such registration statement, and (ii) on or after the date of this prospectus
but prior to the termination of the offering (i.e., until the earlier of the date on which all of the securities registered hereunder
have been sold or the registration statement of which this prospectus forms a part has been withdrawn). Information in such future filings
updates and supplements the information provided in this prospectus. Any statements in any such future filings will automatically be
deemed to modify and supersede any information in any document we previously filed with the SEC that is incorporated or deemed to be
incorporated herein by reference to the extent that statements in the later filed document modify or replace such earlier statements.
We will furnish without charge to each person, including any beneficial
owner, to whom a prospectus is delivered, upon written or oral request, a copy of any or all of the documents incorporated by reference
into this prospectus but not delivered with the prospectus, including exhibits that are specifically incorporated by reference into such
documents. You should direct any requests for documents to:
Quoin
Pharmaceuticals Ltd.
42127 Pleasant Forest Ct.
Ashburn, VA 20148
Telephone: (703) 980-4182
Attention: Corporate Secretary
You may also access these documents, free of charge, on the SEC’s
website at www.sec.gov or on our website at https://investors.quoinpharma.com/financial-information/sec-filings. The information
contained in, or that can be accessed through, our website is not incorporated by reference in, and is not part of, this prospectus or
any accompanying prospectus supplement.
In accordance with Rule 412 of the Securities Act, any statement
contained in a document incorporated by reference herein shall be deemed modified or superseded to the extent that a statement contained
herein or in any other subsequently filed document which also is or is deemed to be incorporated by reference herein modifies or supersedes
such statement.
You should rely only on information contained in, or incorporated
by reference into, this prospectus and any prospectus supplement. We have not authorized anyone to provide you with information different
from that contained in this prospectus or incorporated by reference into this prospectus. We are not making offers to sell the securities
in any jurisdiction in which such an offer or solicitation is not authorized or in which the person making such offer or solicitation
is not qualified to do so or to anyone to whom it is unlawful to make such an offer or solicitation.

Up
to 19,230,769 Ordinary Shares Represented by American Depositary Shares
Up
to 19,230,769 Pre-Funded Warrants to Purchase Up to19,230,769 Ordinary Shares Represented by American Depositary Shares
Up
to 19,230,769 Series F Warrants to Purchase Up to 19,230,769 Ordinary Shares Represented by American Depositary Shares
Up
to 19,230,769 Series G Warrants to Purchase Up to 19,230,769 Ordinary Shares Represented by American Depositary Shares
Up
to 57,692,307 Ordinary Shares Represented by American Depositary Shares Issuable Upon Exercise of the Pre-Funded Warrants,
Series F Warrants and Series G Warrants
________________________________________________________________________
Preliminary Prospectus
________________________________________________________________________
Sole Placement Agent
Maxim Group LLC
The date of this prospectus is ,
2024.
PART II
INFORMATION NOT REQUIRED
IN PROSPECTUS
| Item 13. | Other Expenses of Issuance and Distribution |
The following table sets forth the various costs
and expenses, other than the placement agent’s fees and expenses, to be paid in connection with the offering of securities described
in this registration statement. All the amounts shown are estimates except the SEC registration fee.
| SEC registration fee | |
$ | 6,890 | |
| Printing and mailing expenses | |
$ | 50,000 | |
| Legal fees and expenses | |
$ | 200,000 | |
| Accounting fees and expenses | |
$ | 57,500 | |
| Transfer agent and registrar fees | |
$ | 80,000 | |
| Miscellaneous | |
$ | 65,610 | |
| Total | |
$ | 460,000 | |
| Item 14. | Indemnification of Directors and Officers |
Under the Companies Law, a company may not exculpate an Office Holder
from liability for a breach of the duty of loyalty. An Israeli company may exculpate an Office Holder in advance from liability to the
company, in whole or in part, for damages caused to the company as a result of a breach of duty of care but only if a provision authorizing
such exculpation is included in its articles of association. Our articles of association include such a provision. An Israeli company
may not exculpate in advance a director from liability arising out of a prohibited dividend or distribution to shareholders.
Under the Companies Law, an Israeli company may indemnify an Office
Holder in respect of the following liabilities and expenses incurred for acts performed by him or her as an Office Holder, either pursuant
to an undertaking made in advance of an event or following an event, provided its articles of association include a provision authorizing
such indemnification, which ours do:
| · | financial
liability imposed on him or her in favor of another person pursuant to a judgment, including
a settlement or arbitrator’s award approved by a court. However, if an undertaking
to indemnify an Office Holder with respect to such liability is provided in advance, then
such an undertaking must be limited to events which, in the opinion of the board of directors,
can be reasonably foreseen based on the company’s activities when the undertaking to
indemnify is given, and to an amount or according to criteria determined by the board of
directors as reasonable under the circumstances, and such undertaking shall detail the abovementioned
foreseen events and amount or criteria; |
| · | reasonable
litigation expenses, including attorneys’ fees, incurred by the Office Holder (1) as
a result of an investigation or proceeding instituted against him or her by an authority
authorized to conduct such investigation or proceeding, provided that (a) no indictment
was filed against such Office Holder as a result of such investigation or proceeding; and
(b) no financial liability, such as a criminal penalty, was imposed upon him or her
as a substitute for the criminal proceeding as a result of such investigation or proceeding
or, if such financial liability was imposed, it was imposed with respect to an offense that
does not require proof of criminal intent; and (2) in connection with a monetary sanction; |
| · | reasonable litigation expenses, including attorneys’ fees,
incurred by the Office Holder or imposed by a court in proceedings instituted against him or her by the company, on its behalf, or by
a third party, or in connection with criminal proceedings in which the Office Holder was acquitted, or as a result of a conviction for
an offense that does not require proof of criminal intent; and |
| · | expenses,
including reasonable litigation expenses and legal fees, incurred by an Office Holder in
relation to an administrative proceeding instituted against such Office Holder, or certain
compensation payments made to an injured party imposed on an Office Holder by an administrative
proceeding, pursuant to certain provisions of the Israeli Securities Law, 1968 (the “Israeli
Securities Law”). |
Under the Companies Law and the Israeli Securities Law, a company
may insure an Office Holder against the following liabilities incurred for acts performed by him or her as an Office Holder if and to
the extent provided in the company’s articles of association:
| · | a
breach of the duty of loyalty to the company, provided that the Office Holder acted in good
faith and had a reasonable basis to believe that the act would not harm the company; |
| · | a
breach of duty of care to the company or to a third party, including a breach arising out
of the negligent conduct of the Office Holder; and |
| · | a
financial liability imposed on the Office Holder in favor of a third party. |
Under our articles of association, we may insure an Office Holder
against the aforementioned liabilities as well as the following liabilities:
| · | a
breach of duty of care to the company or to a third party; |
| · | any
other action against which we are permitted by law to insure an Office Holder; |
| · | expenses
incurred and/or paid by the Office Holder in connection with an administrative enforcement
procedure under any applicable law including Parts 8(3), 8(4) and 9(1) of the Israeli
Securities Law, and a proceeding according to Section D of Chapter 4 in Part 9
of the Companies Law, including reasonable litigation expenses and attorney fees; |
| · | a
payment to a person injured by a violation of Section 52BBB(a)(1)(a) of the Israeli
Securities Law; and |
| · | expenses
incurred in connection with a proceeding under the Economic Competition Law 5748-1988, including
reasonable litigation expenses and attorney fees. |
Under the Companies Law, an Israeli company may not indemnify, exculpate
or insure an Office Holder against any of the following:
| · | a breach of the duty of loyalty, except for indemnification
and insurance for a breach of the duty of loyalty to the company to the extent that the Office Holder acted in good faith and had a reasonable
basis to believe that the act would not harm the company; |
| · | a
breach of duty of care committed intentionally or recklessly, excluding a breach arising
solely out of the negligent conduct of the Office Holder; |
| · | an
act or omission committed with intent to derive illegal personal benefit; or |
| · | a
fine, civil fine, or other financial sanction levied against the Office Holder. |
Under the Companies Law, exculpation, indemnification and insurance
of Office Holders in a public company must be approved by the compensation committee and the board of directors and, with respect to
directors and the Chief Executive Officers or under certain circumstances, also by the shareholders. However, under regulations promulgated
under the Companies Law, the insurance of Office Holders does not require shareholder approval and may be approved by only the compensation
committee, if the engagement terms are determined in accordance with the limitations set forth in the company’s compensation policy,
which was approved by the shareholders by the requisite special majority, provided that the insurance policy is on market terms and the
insurance policy is not likely to materially impact the company’s profitability, assets or obligations.
Our articles of association permit us to exculpate, indemnify and
insure our Office Holders to the fullest extent permitted or to be permitted by the Companies Law and the Israeli Securities Law.
Upon the recommendation of our compensation committee, our board of
directors has approved, and our shareholders have approved, at the annual general meeting held on April 12, 2022, the form of indemnification
and release agreements to be entered into with each of our current and future directors and executive officers exculpating them, to the
fullest extent permitted by law and our articles of association, and undertaking to indemnify them to the fullest extent permitted by
law and our articles of association. This indemnification will be limited to events determined as foreseeable by the board of directors
based on our activities, and to an amount or according to criteria determined by the board of directors and our compensation committee
as reasonable under the circumstances.
The maximum indemnification amount set forth in our indemnification
and release agreements during any period of three years in the aggregate for all of the covered directors and executive officers, is
limited to an amount equal to the higher of: (i) $35,000,000 and (ii) 25% of our total shareholders’ equity as reflected
in our most recent financial statements as of the time of the actual payment of indemnification is made.
In the opinion of the SEC, indemnification of directors and other
Office Holders for liabilities arising under the Securities Act, however, is against public policy and therefore unenforceable.
We have obtained directors’ and officers’ liability insurance
for the benefit of our Office Holders and intend to continue to maintain such coverage and pay all premiums thereunder to the fullest
extent permitted by the Companies Law.
| Item 15. | Recent Sales of Unregistered Securities |
Agreements with Altium Growth Fund, LP and Warrant Exercises
On October 28, 2021, the Company completed a private placement
transaction with Altium Growth Fund, LP (“Altium”or “Investor”) for an aggregate purchase price of approximately
$17.0 million (comprised of a set off of approximately $5.0 million of bridge notes from bridge financing earlier in 2021 (the “Bridge
Notes”), and approximately $12.0 million in cash) (the “Primary Financing”), which resulted in net proceeds of approximately
$10.1 million. The Company issued 28,508 ADSs to Altium.
The Company also issued to Altium, effective as of March 13,
2022 (i) a Series A Warrant to purchase 28,508 ADSs (the “Series A Warrant”) (ii) a Series B Warrant
to purchase 28,508 ADSs (the “Series B Warrant”) and (iii) a Series C Warrant to purchase 15,931 ADSs (the
“Series C Warrant” and, together with the Series A Warrant and the Series B Warrant, the “Investor Warrants”).
The exercise price for the Investor Warrants was $597 per ADS, with the Series A Warrant having a five-year maturity, and the Series B
Warrant and the Series C Warrant having a two-year maturity.
The Company had the right to require the mandatory exercise of the
Series C Warrant, subject to an effective registration statement being in place for the resale of the shares underlying such warrant
and the satisfaction of equity market conditions, as defined in the Series C Warrant. In the period from April 22, 2022 to
June 30, 2022, the Investor exercised the Series B Warrant in full pursuant to the alternate cashless exercise rights of such
warrant, resulting in the issuance of a total of 28,508 ADSs to the Investor. The market related conditions to require the mandatory
exercise of the Series C Warrant were not met during the period up to July 14, 2022.
On July 14, 2022, the Company entered into an agreement with
Quoin Inc. and Altium (the “Altium Agreement”), pursuant to which the parties agreed to, among other things, (i) amend
certain terms of the Series A Warrant and the Investor Exchange Warrants previously issued to Altium to reduce the exercise price
to $0.00 per ADS with respect to a total of 33,333 ADSs, (ii) cancel the Series C Warrant and the remaining portion of the
Series A Warrant previously issued to Altium, and (iii) terminate the Purchase Agreements, pursuant to which the warrants were
previously issued to Altium. The incremental fair value of the modified warrants was approximately $491,000, which was charged against
the gross proceeds of the 2022 Offering (see below) as the modification was done in contemplation of the offering. As of August 2,
2022, Altium exercised all of its warrants to purchase ADSs at $0.00 per ADS exercise price, and the Company issued a total of 33,333
ADSs to Altium.
Noteholder Warrant Exercises
Commencing in October 2020, Quoin Inc. issued promissory notes
(the “2020 Notes”) to five noteholders, including our directors, Messrs. Langer and Culverwell (collectively, the “2020
Noteholders”). The 2020 Notes were issued at a 25% original issue discount with an aggregate face value of $1,213,313 with interest
at a rate of 20% per annum. The 2020 Notes were mandatorily convertible into ADSs based on the valuation negotiated in the Primary Financing.
The 2020 Noteholders also received warrants exercisable at any time after the issuance date for a number of shares of Quoin Inc.’s
common stock equal to 100% of the “as if converted” shares as if the 2020 Notes principal and interest were convertible at
the lowest price any securities are sold, convertible, or exercisable into in the Primary Financing or the next round of financing (whichever
is lower). At the closing of the Cellect/Quoin merger, ADSs were issued to the 2020 Noteholders upon the conversion of the principal
of the 2020 Notes. In addition, effective as of March 13, 2022, Quoin Ltd. exchanged Quoin Inc. warrants held by the 2020 Noteholders
for warrants on substantially the same terms as the Investor Exchange Warrants, exercisable for 2,449 ADSs, in the aggregate, at the
exercise price of $597 per ADS (the “Noteholder Warrants”). The Noteholder Warrants became exercisable immediately upon issuance
and were to expire five years from March 13, 2022. The exercise price of the warrants held by the 2020 Noteholders was also reduced
to $0.00 as of July 14, 2022 as a result of the Altium Agreement. The change in the exercise price of the Noteholder Warrants resulted
in a deemed dividend of approximately $65,000. From July to September 2022, the 2020 Noteholders exercised all their warrants
to purchase ADSs at $0.00 per ADS exercise price, and a total of 2,449 ADSs were issued to such noteholders.
Axella
In addition, in August 2022, we issued 3,683 ADSs to one of the
principals of Axella Research LLC (“Axella”), a provider of regulatory and pre- clinical/clinical services to us with respect
to QRX003 and QRX004, to settle in full the outstanding liability to Axella for accrued fees under our consulting agreements with Axella.
We believe that each of such issuances was exempt from registration
under the Securities Act in reliance on Section 4(a)(2) of the Securities Act or Rule 506 of Regulation D promulgated
under the Securities Act. No underwriter or underwriting discount or commission was involved in any such transaction.
| Item 16. | Exhibits and Financial Statement Schedules |
(a) Exhibits.
The exhibits to the registration statement are listed in
the Exhibit Index to this registration statement and are incorporated herein by reference.
(b) Financial Statement Schedules
All schedules have been omitted because either they are
not required, are not applicable or the information is otherwise set forth in the consolidated financial statements and related notes
thereto.
The Registrant hereby undertakes that:
(1) To file, during any period
in which offers or sales are being made, a post-effective amendment to this registration statement:
| (i) | To include any prospectus required by Section 10(a)(3) of
the Securities Act of 1933, as amended (the “Securities Act”); |
| (ii) | To reflect in the prospectus any facts or events arising after
the effective date of the registration statement (or the most recent post-effective amendment
thereof) which, individually or in the aggregate, represent a fundamental change in the information
set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease
in the volume of securities offered (if the total dollar value of securities offered would
not exceed that which was registered) and any deviation from the low or high end of the estimated
maximum offering range may be reflected in the form of prospectus filed with the Commission
pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent
no more than 20% change in the maximum aggregate offering price set forth in the “Calculation
of Registration Fee” table in the effective registration statement; and |
| (iii) | To include any material information with respect to the plan
of distribution not previously disclosed in the registration statement or any material change to such information in the registration
statement. |
provided, however, that paragraphs (1)(i), (1)(ii) and
(1)(iii) do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained
in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or Section 15(d) of the
Securities Exchange Act of 1934, as amended (the “Exchange Act”), that are incorporated by reference in the registration
statement.
| (2) | That, for the purpose of determining any liability under the Securities
Act, each such post-effective amendment shall be deemed to be a new registration statement
relating to the securities offered therein, and the offering of such securities at that time
shall be deemed to be the initial bona fide offering thereof. |
| (3) | To remove from registration by means of a post-effective amendment
any of the securities being registered which remain unsold at the termination of the offering. |
| (4) | That, for the purpose of determining liability under the Securities
Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as
part of a registration statement relating to an offering, other than registration statements
relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A,
shall be deemed to be part of and included in the registration statement as of the date it
is first used after effectiveness. Provided, however, that no statement made in a registration
statement or prospectus that is part of the registration statement or made in a document
incorporated or deemed incorporated by reference into the registration statement or prospectus
that is part of the registration statement will, as to a purchaser with a time of contract
of sale prior to such first use, supersede or modify any statement that was made in the registration
statement or prospectus that was part of the registration statement or made in any such document
immediately prior to such date of first use. |
| (5) | That, for the purpose of determining liability of the registrant
under the Securities Act to any purchaser in the initial distribution of the securities,
the undersigned registrant undertakes that in a primary offering of securities of the undersigned
registrant pursuant to this registration statement, regardless of the underwriting method
used to sell the securities to the purchaser, if the securities are offered or sold to such
purchaser by means of any of the following communications, the undersigned registrant will
be a seller to the purchaser and will be considered to offer or sell such securities to such
purchaser: |
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant
relating to the offering required to be filed pursuant to Rule 424; |
| (ii) | Any free writing prospectus relating to the offering prepared by
or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | The portion of any other free writing prospectus relating to the
offering containing material information about the undersigned registrant or its securities
provided by or on behalf of the undersigned registrant; and |
| (iv) | Any other communication that is an offer in the offering made
by the undersigned registrant to the purchaser. |
| (6) | That, for purposes of determining any liability under the Securities
Act: |
| (i) | the information omitted from the form of prospectus filed as part
of the registration statement in reliance upon Rule 430A and contained in the form of
prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under
the Securities Act shall be deemed to be part of the registration statement as of the time
it was declared effective; and |
| (ii) | each post-effective amendment that contains a form of prospectus
shall be deemed to be a new registration statement relating to the securities offered therein,
and the offering of such securities at that time shall be deemed to be the initial bona fide
offering thereof. |
| (7) | For purposes of determining any liability under the Securities Act
of 1933, each filing of the registrant’s annual report pursuant to section 13(a) or
section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing
of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities
Exchange Act of 1934) that is incorporated by reference in the registration statement shall
be deemed to be a new registration statement relating to the securities offered therein,
and the offering of such securities at that time shall be deemed to be the initial bona fide
offering thereof. |
| (8) | Insofar as indemnification for liabilities arising under the Securities
Act may be permitted to directors, officers and controlling persons of the registrant pursuant
to the indemnification provisions described herein, or otherwise, the registrant has been
advised that in the opinion of the SEC such indemnification is against public policy as expressed
in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification
against such liabilities (other than the payment by the registrant of expenses incurred or
paid by a director, officer or controlling person of the registrant in the successful defense
of any action, suit or proceeding) is asserted by such director, officer or controlling person
in connection with the securities being registered, the registrant will, unless in the opinion
of its counsel the matter has been settled by controlling precedent, submit to a court of
appropriate jurisdiction the question whether such indemnification by it is against public
policy as expressed in the Securities Act and will be governed by the final adjudication
of such issue. |
EXHIBIT INDEX
Exhibit
No. |
|
Exhibit Description |
| 1.1* |
|
Form of Placement Agency Agreement. |
| |
|
|
| 2.1 |
|
Agreement and Plan of Merger and Reorganization, dated as of March 24, 2021, by and among Cellect Biotechnology Ltd., CellMSC, Inc. and Quoin Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.1 of the Form 6-K filed with the SEC on March 24, 2021). |
| |
|
|
| 2.2 |
|
Amendment made as of September 24, 2021, to the Agreement and Plan of Merger and Reorganization, dated as of March 24, 2021, by and among Cellect Biotechnology Ltd., CellMSC, Inc., and Quoin Pharmaceuticals, Inc. (incorporated by reference to Exhibit 99.2 to Form 6-K filed with the SEC on September 27, 2021). |
| |
|
|
| 2.3 |
|
Amended and Restated Share Transfer Agreement, dated May 27, 2021 by and between Cellect Biotechnology Ltd. and EnCellX Inc. (incorporated by reference to Exhibit 2.2 to Registration Statement on Form F-4 filed with the SEC on June 16, 2021). |
| |
|
|
| 2.4 |
|
Amendment made as of September 26, 2021, to the Amended and Restated Share Transfer Agreement dated as of May 27, 2021, by and between EnCellX, Inc. and Cellect Biotechnology Ltd. (incorporated by reference to Exhibit 99.3 to Form 6-K filed with the SEC on September 27, 2021). |
| |
|
|
| 2.5 |
|
Securities Purchase Agreement, dated as of March 24, 2021, by and among Cellect Biotechnology Ltd., Quoin Pharmaceuticals, Inc. and the investors named on the Schedule of Buyers attached thereto (incorporated by reference to Exhibit 10.4 of the Form 6-K filed with the SEC on March 24, 2021). |
| |
|
|
| 2.6 |
|
Securities Purchase Agreement, dated as of March 24, 2021, by and among Quoin Pharmaceuticals, Inc. and the investors listed on the Schedule of Buyers attached thereto (incorporated by reference to Exhibit 10.6 of the Form 6-K filed with the SEC on March 24, 2021). |
| |
|
|
| 2.7 |
|
Amendment Agreement, dated as of September 17, 2021, by and among Quoin Pharmaceuticals, Inc., Cellect Biotechnology, Ltd., and Altium Growth Fund, L.P. (incorporated by reference to Exhibit 99.1 of the Form 6-K filed with the SEC on September 17, 2021). |
| |
|
|
| 2.8 |
|
Letter Agreement, dated September 17, 2021, between Quoin Pharmaceuticals, Inc. and Cellect Biotechnology, Ltd. (incorporated by reference to Exhibit 99.2 of the Form 6-K filed with the SEC on September 17, 2021). |
| |
|
|
| 2.9 |
|
Second Amendment Agreement, dated as of March 13, 2022, by and among Quoin Pharmaceuticals, Inc., Quoin Pharmaceuticals Ltd., and Altium Growth Fund, L.P. (incorporated by reference to Exhibit 4.1 to Form 6-K filed with the SEC on March 28, 2022). |
| |
|
|
| 2.10 |
|
Waiver Agreement, dated June 6, 2022, by and among Quoin Pharmaceuticals Ltd., Quoin Pharmaceuticals, Inc. and Altium Growth Fund, LP (incorporated by reference to Exhibit 10.2 to Form 6-K filed with the SEC on June 6, 2022). |
| |
|
|
| 2.11 |
|
Agreement, dated July 14,
2022, by and among Quoin Pharmaceuticals, Inc., Quoin Pharmaceuticals Ltd. and Altium Growth Fund, LP (incorporated by reference
to Exhibit 10.1 to Form 6-K filed with the SEC on July 15, 2022). |
| |
|
|
| 2.12 |
|
Letter of Agreement among
Cellect Biotechnology Ltd, Dr. Shai Yarkoni and EnCellX, Inc. (incorporated by reference to Exhibit 2.5 to Registration
Statement on Form F-4 filed with the SEC on July 16, 2021). |
| 2.13 |
|
Form of Representative Agreement among Cellect Biotechnology Ltd, Eyal Leibovitz, as Representative, and EnCellX, Inc. (incorporated by reference to Exhibit 2.6 to Registration Statement on Form F-4 filed with the SEC on August 6, 2021). |
| |
|
|
| 3.1 |
|
Amended and Restated Articles of Association of Quoin Pharmaceuticals Ltd., adopted on February 28, 2022 (incorporated by reference to Annex A included in Exhibit 99.1 to Form 6-K filed with the SEC on February 8, 2022). |
| |
|
|
| 3.2 |
|
Amendment to the Amended and Restated Articles of Association of Quoin Pharmaceuticals Ltd., adopted on April 12, 2022 (incorporated by reference to Annex A included in Exhibit 99.1 to Form 6-K filed with the SEC on March 8, 2022). |
| |
|
|
| 3.3 |
|
Amendment to the Amended and Restated Articles of Association of Quoin Pharmaceuticals Ltd., adopted on November 3, 2022 (incorporated by reference to Annex A included in Exhibit 99.1 to Form 6-K filed with the SEC on September 21, 2022). |
| |
|
|
| 3.4 |
|
Amendment to the Amended and Restated Articles of Association of Quoin Pharmaceuticals Ltd., adopted on October 26, 2023 (incorporated by reference to Form 8-K filed with the SEC on November 1, 2023). |
| |
|
|
| 3.5 |
|
Amendment to the Amended and Restated Articles of Association of Quoin Pharmaceuticals Ltd., adopted on December 5, 2024 (incorporated by reference to Form 8-K filed with the SEC on December 10, 2024). |
| |
|
|
| 4.1 |
|
Form of Deposit Agreement between Cellect Biotechnology Ltd. (n/k/a Quoin Pharmaceuticals Ltd.), The Bank of New York Mellon as Depositary, and owners and holders from time to time of ADSs issued thereunder (incorporated by reference to Exhibit 4.1 to Registration Statement on Form F-1/A as filed with the SEC on July 26, 2016). |
| |
|
|
| 4.2 |
|
Specimen American Depositary Receipt (included in Exhibit 2.1). |
| |
|
|
| 4.3 |
|
Form of Amendment No. 1 to Warrant to Purchase Ordinary Shares Represented by American Depositary Shares (incorporated by reference to Exhibit 4.3 to the Current Report on Form 8-K filed with the SEC on February 28, 2023). |
| |
|
|
| 4.4 |
|
Form of Pre-Funded Warrant (incorporated by reference to Exhibit 4.1 to the Current Report on Form 8-K filed with the SEC on February 28, 2023). |
| |
|
|
| 4.5 |
|
Form of Common Warrant (incorporated by reference to Exhibit 4.2 to the Current Report on Form 8-K filed with the SEC on February 28, 2023). |
| |
|
|
| 4.6 |
|
Form of Pre-Funded Warrant issued in the February 2024 Offering (incorporated by reference to Exhibit 4.1 to the Current Report on Form 8-K filed with the SEC on March 8, 2024). |
| 4.7 |
|
Form of Series D Warrant (incorporated by reference to Exhibit 4.2 to the Current Report on Form 8-K filed with the SEC on March 8, 2024). |
| |
|
|
| 4.8 |
|
Form of Series E Warrant (incorporated by reference to Exhibit 4.3 to the Current Report on Form 8-K filed with the SEC on March 8, 2024). |
| |
|
|
| 4.9 |
|
Form of Amendment to Warrants to Purchase Ordinary Shares Represented by American Depositary Shares (incorporated by reference to Exhibit 4.4 to the Current Report on Form 8-K filed with the SEC on March 8, 2024). |
| |
|
|
| 4.10* |
|
Form of Pre-Funded Warrant. |
| |
|
|
| 4.11* |
|
Form of Series F Warrant. |
| |
|
|
| 4.12* |
|
Form of Series G Warrant. |
| |
|
|
| 5.1* |
|
Legal Opinion of Meitar | Law Offices, Israeli legal counsel to Quoin Pharmaceuticals Ltd. |
| |
|
|
| 5.2* |
|
Legal Opinion of Blank Rome LLP, US legal counsel to Quoin Pharmaceuticals Ltd. |
| |
|
|
| 10.1# |
|
Compensation Policy for Executives and Directors of Quoin Pharmaceuticals Ltd., adopted on April 12, 2022 (incorporated by reference to Annex B included in Exhibit 99.1 to Form 6-K filed with the SEC on March 8, 2022). |
| |
|
|
| 10.2# |
|
Amended and Restated Equity Incentive Plan of Quoin Pharmaceuticals Ltd., effective as of April 12, 2022 (incorporated by reference to Annex C included in Exhibit 99.1 to Form 6-K filed with the SEC on March 8, 2022). |
| |
|
|
| 10.3# |
|
Form of Indemnification and Release Agreement, entered into by and between Quoin Pharmaceuticals Ltd. and each of the officers and directors of Quoin Pharmaceuticals Ltd. as of April 12, 2022 (incorporated by reference to Annex D included in Exhibit 99.1 to Form 6-K filed with the SEC on March 8, 2022). |
| |
|
|
| 10.4# |
|
Executive Employment Agreement, dated March 9, 2018, by and between Quoin Pharmaceuticals, Inc. and Dr. Michael Myers (incorporated by reference to Exhibit 10.1 to Form 6-K filed with the SEC on October 29, 2021). |
| |
|
|
| 10.5# |
|
Executive Employment Agreement, dated March 9, 2018, by and between Quoin Pharmaceuticals, Inc. and Denise Carter (incorporated by reference to Exhibit 10.2 to Form 6-K filed with the SEC on October 29, 2021). |
| |
|
|
| 10.6# |
|
Service Agreement, dated November 1, 2021, by and between Quoin Pharmaceuticals, Inc. and Gordon Dunn (incorporated by reference to Exhibit 10.1 to Form 6-K filed with the SEC on November 23, 2021). |
| 10.7 |
|
Research Agreement, dated November 1, 2021, by and between Quoin Pharmaceuticals, Inc. and Queensland University of Technology (incorporated by reference to Exhibit 10.2 to Form 6-K filed with the SEC on November 23, 2021). |
| |
|
|
| 10.8 |
|
License and Distribution Agreement, dated November 5, 2021, by and between Quoin Pharmaceuticals, Inc. and AFT Pharmaceuticals Ltd. (incorporated by reference to Exhibit 10.3 to Form 6-K filed with the SEC on November 23, 2021). |
| |
|
|
| 10.9 |
|
Supply Agreement, dated September 15, 2021, by and between Quoin Pharmaceuticals, Inc. and AFT Pharmaceuticals Ltd. (incorporated by reference to Exhibit 10.4 to Form 6-K filed with the SEC on November 23, 2021). |
| |
|
|
| 10.10 |
|
License and Distribution Agreement, dated November 7, 2021, by and between Quoin Pharmaceuticals, Inc. and GenPharm Services FZ LLC (incorporated by reference to Exhibit 10.5 to Form 6-K filed with the SEC on November 23, 2021). |
| |
|
|
| 10.11 |
|
Supply Agreement, dated November 7, 2021, by and between Quoin Pharmaceuticals, Inc. and GenPharm Services FZ LLC (incorporated by reference to Exhibit 10.6 to Form 6-K filed with the SEC on November 23, 2021). |
| |
|
|
| 10.12 |
|
Distribution Agreement, dated December 15, 2021, by and between Quoin Pharmaceuticals, Inc. and Orpharm LLC (certain provisions of this exhibit have been omitted pursuant to Instruction No. 4 to Exhibits in Form 20-F) (incorporated by reference to Exhibit 10.1 to Form 6-K filed with the SEC on December 20, 2021). |
| |
|
|
| 10.13 |
|
License and Distribution Agreement, dated as of January 24, 2022 between the Company and E-Log Logistica LTDA (certain provisions of this exhibit have been omitted pursuant to Instruction No. 4 to Exhibits in Form 20-F) (incorporated by reference to Exhibit 10.1 to Form 6-K filed with the SEC on January 31, 2022). |
| |
|
|
| 10.14 |
|
License and Distribution Agreement, dated as of February 1, 2022, by and between Quoin Pharmaceuticals Ltd. and Er-Kim İlaç Sanayi ve Ticaret A.Ş, and the First Amendment to the License and Distribution Agreement, dated as of February 17, 2022, by and between Quoin Pharmaceuticals, Inc. and Er-Kim İlaç Sanayi ve Ticaret A.Ş (certain provisions of this exhibit have been omitted pursuant to Instruction No. 4 to Exhibits in Form 20-F) (incorporated by reference to Exhibit 10.4 to Form 6-K filed with the SEC on March 8, 2022). |
| |
|
|
| 10.15 |
|
License and Distribution Agreement, dated as of February 11, 2022, by and between Quoin Pharmaceuticals Ltd. and Neopharm (Israel) 1996 Ltd. (certain provisions of this exhibit have been omitted pursuant to Instruction No. 4 to Exhibits in Form 20-F) (incorporated by reference to Exhibit 10.5 to Form 6-K filed with the SEC on March 8, 2022). |
| |
|
|
| 10.16 |
|
Supply Agreement, dated as of February 11, 2022, by and between Quoin Pharmaceuticals Ltd. and Neopharm (Israel) 1996 Ltd. (incorporated by reference to Exhibit 10.6 to Form 6-K filed with the SEC on March 8, 2022). |
| 10.17 |
|
License Agreement, dated June 14, 2022, by and between Quoin Pharmaceuticals, Inc. and WinHealth Investment (HK) Limited (certain provisions of this exhibit have been omitted pursuant to Instruction No. 4 to Exhibits in Form 20-F) (incorporated by reference to Exhibit 10.1 to Form 6-K filed with the SEC on June 17, 2022). |
| |
|
|
| 10.18 |
|
License and Distribution Agreement, dated July 14, 2022, by and between Quoin Pharmaceuticals, Inc. and Endo Ventures Limited (certain provisions of this exhibit have been omitted pursuant to Instruction No. 4 to Exhibits in Form 20-F) (incorporated by reference to Exhibit 10.2 to Form 6-K filed with the SEC on July 15, 2022). |
| |
|
|
| 10.19 |
|
Supply Agreement, dated July 14, 2022, by and between Quoin Pharmaceuticals, Inc. and Endo Ventures Limited (certain provisions of this exhibit have been omitted pursuant to Instruction No. 4 to Exhibits in Form 20-F) (incorporated by reference to Exhibit 10.3 to Form 6-K filed with the SEC on July 15, 2022). |
| |
|
|
| 10.20 |
|
Research Agreement, dated May 20, 2022, by and between Quoin Pharmaceuticals, Inc. and Queensland University of Technology, Australia (certain provisions of this exhibit have been omitted pursuant to Instruction No. 4 to Exhibits in Form 20-F) (incorporated by reference to Exhibit 10.1 to Form 6-K filed with the SEC on June 6, 2022). |
| |
|
|
| 10.21 |
|
Exclusive License Agreement, dated October 17, 2019, by and between Quoin Pharmaceuticals, Inc. and Skinvisible Inc. (incorporated by reference to Exhibit 4.30 to Form 20-F filed with the SEC on April 13, 2022). |
| |
|
|
| 10.22 |
|
Exclusive License Agreement Renewal, dated May 8, 2020, by and between Quoin Pharmaceuticals, Inc. and Skinvisible Inc. (incorporated by reference to Exhibit 4.31 to Form 20-F filed with the SEC on April 13, 2022). |
| |
|
|
| 10.23 |
|
First Amendment to the Exclusive License Agreement, dated July 31, 2020, by and between Quoin Pharmaceuticals, Inc. and Skinvisible Inc. (incorporated by reference to Exhibit 4.32 to Form 20-F filed with the SEC on April 13, 2022). |
| |
|
|
| 10.24 |
|
Second Amendment to the Exclusive License Agreement, dated September 30, 2020, by and between Quoin Pharmaceuticals, Inc. and Skinvisible Inc. (incorporated by reference to Exhibit 4.33 to Form 20-F filed with the SEC on April 13, 2022). |
| |
|
|
| 10.25 |
|
Third Amendment to the Exclusive License Agreement, dated January 27, 2021, by and between Quoin Pharmaceuticals, Inc. and Skinvisible Inc. (incorporated by reference to Exhibit 4.34 to Form 20-F filed with the SEC on April 13, 2022). |
| |
|
|
| 10.26 |
|
Fourth Amendment to the Exclusive License Agreement, dated April 19, 2021, by and between Quoin Pharmaceuticals, Inc. and Skinvisible Inc. (incorporated by reference to Exhibit 4.35 to Form 20-F filed with the SEC on April 13, 2022). |
| |
|
|
| 10.27 |
|
Fifth Amendment to the Exclusive License Agreement, dated June 14, 2021, by and between Quoin Pharmaceuticals, Inc. and Skinvisible Inc. (incorporated by reference to Exhibit 4.36 to Form 20-F filed with the SEC on April 13, 2022). |
| 10.28 |
|
Quotation - Tech Transfer and Clinical Manufacture for QRX003 Topical Lotion, dated April 8, 2021, by Ferndale Contract Manufacturing to Quoin Pharmaceuticals, Inc. (incorporated by reference to Exhibit 4.37 to Form 20-F filed with the SEC on April 13, 2022). |
| |
|
|
| 10.29 |
|
Development and Supply Agreement, dated January 13, 2021, by and between TopChem Pharmaceuticals Limited and Quoin Pharmaceuticals Limited (incorporated by reference to Exhibit 4.38 to Form 20-F filed with the SEC on April 13, 2022). |
| |
|
|
| 10.30 |
|
Master Services Agreement, dated November 2, 2020, by and between Therapeutics, Inc. and Quoin Pharmaceuticals, Inc. (incorporated by reference to Exhibit 4.39 to Form 20-F filed with the SEC on April 13, 2022). |
| |
|
|
| 10.31 |
|
Term Sheet for Agreement, dated October 29, 2019, by and between Axella Research, LLC and Quoin Pharmaceuticals, Inc. (incorporated by reference to Exhibit 4.40 to Form 20-F filed with the SEC on April 13, 2022). |
| |
|
|
| 10.32 |
|
Term Sheet for Agreement, dated January 11, 2020, by and between Axella Research, LLC and Quoin Pharmaceuticals, Inc. (re: QRX003) (incorporated by reference to Exhibit 4.41 to Form 20-F filed with the SEC on April 13, 2022). |
| |
|
|
| 10.33 |
|
Term Sheet for Agreement, dated January 11, 2020, by and between Axella Research, LLC and Quoin Pharmaceuticals, Inc. (re: QRX004) (incorporated by reference to Exhibit 4.42 to Form 20-F filed with the SEC on April 13, 2022). |
| |
|
|
| 10.34# |
|
Form of Non-Qualified Stock Option Award Agreement for directors (incorporated by reference to Exhibit 10.34 to Form F-1 filed with the SEC on August 3, 2022) |
| |
|
|
| 10.35# |
|
Form of Non-Qualified Stock Option Award Agreement for officers (incorporated by reference to Exhibit 10.35 to Form F-1 filed with the SEC on August 3, 2022) |
| |
|
|
| 10.36 |
|
Form of Securities Purchase Agreement, dated February 22, 2023 (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed with the SEC on February 28, 2023). |
| |
|
|
| 10.37# |
|
Code of Ethics and Business Conduct (incorporated by reference to Exhibit 14.1 to Form 10-K filed with the SEC on March 15, 2023) |
| |
|
|
| 10.38 |
|
License and Distribution Agreement, by and between Quoin Pharmaceuticals Inc. and Farma Mondo (incorporated by reference to Exhibit 10.1 to Form 8-K filed with the SEC on September 13, 2023) |
| |
|
|
| 10.39 |
|
Purchase Agreement, dated January 25, 2024, by and between Quoin Pharmaceuticals Ltd. and Alumni Capital LP (incorporated by reference to Exhibit 10.1 to Form 8-K filed with the SEC on January 30, 2024) |
| * | Previously
filed. |
| | ** | Filed herewith
|
| # | Indicates management contract or compensatory plan or arrangement. |
| + | Exhibits have been omitted pursuant to Item 601(a)(5) of Regulation
S-K. The Company agrees to furnish supplementally a copy of any omitted exhibit to the SEC upon request. |
SIGNATURES
Pursuant to the requirements of the Securities
Act of 1933, as amended, the registrant has duly caused this Amendment No. 1 to registration statement on Form S-1 to be signed on its
behalf by the undersigned, thereunto duly authorized, in the City of Ashburn, Commonwealth of Virginia on December 18, 2024.
| |
Quoin
Pharmaceuticals Ltd. |
| |
|
| |
By: |
/s/
Michael Myers |
| |
|
Name: |
Dr. Michael
Myers |
| |
|
Title: |
Chief
Executive Officer |
Pursuant to the requirements of the Securities
Act of 1933, as amended, this Amendment No. 1 to registration statement on Form S-1 has been signed by the following persons in
the capacities and on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/
Michael Myers |
|
Chief
Executive Officer |
|
December 18,
2024 |
| Dr. Michael
Myers |
|
(Principal Executive Officer) |
|
|
| |
|
|
|
|
| /s/
Gordon Dunn |
|
Chief
Financial Officer |
|
December 18,
2024 |
| Gordon
Dunn |
|
(Principal Financial Officer and
Principal Accounting Officer) |
|
|
| |
|
|
|
|
| /s/
Denise Carter |
|
|
|
|
| Denise
Carter |
|
Director
and Chief Operating Officer |
|
December 18,
2024 |
| |
|
|
|
|
| * |
|
|
|
|
| Joseph
Cooper |
|
Director |
|
December 18,
2024 |
| |
|
|
|
|
| * |
|
|
|
|
| James
Culverwell |
|
Director |
|
December 18,
2024 |
| |
|
|
|
|
| * |
|
|
|
|
| Dennis
Langer |
|
Director |
|
December 18,
2024 |
| |
|
|
|
|
| * |
|
|
|
|
| Natalie
Leong |
|
Director |
|
December 18,
2024 |
| |
|
|
|
|
| * |
|
|
|
|
| Mike
Sember |
|
Director |
|
December 18,
2024 |
*
By: |
/s/
Michael Myers |
|
|
|
|
Dr. Michael
Myers, Attorney-In-Fact |
|
|
|
|
SIGNATURE OF AUTHORIZED U.S. REPRESENTATIVE
Pursuant to the requirements of the Securities
Act of 1933, as amended, the undersigned, the duly authorized representative in the United States of Quoin Pharmaceuticals Ltd.,
has signed this registration statement in the City of Ashburn, Commonwealth of Virginia on December 18, 2024.
| |
Authorized
U.S. Representative |
| |
Dr. Michael
Myers |
| |
|
| |
By: |
/s/
Michael Myers |
| |
Name: |
Dr. Michael
Myers |
| |
Title: |
Chief
Executive Officer |
Exhibit 23.1
Independent
Registered Public Accounting Firm’s Consent
We consent to the incorporation by reference
in this Registration Statement of Quoin Pharmaceuticals Ltd. on Amendment No. 1 to Form S-1 (File No. 333-283734) of our
report dated March 14, 2024, with respect to our audits of the consolidated financial statements of Quoin Pharmaceuticals Ltd. as
of and for the years ended December 31, 2023 and 2022 appearing in the Annual Report on Form 10-K of Quoin Pharmaceuticals
Ltd. for the year ended December 31, 2023. We also consent to the reference to our firm under the heading “Experts”
in the Prospectus, which is part of this Registration Statement.
| /s/ Marcum llp | | |
| | | |
| Marcum llp | | |
| Morristown, New Jersey | | |
| December 18, 2024 | | |
Quoin Pharmaceuticals (NASDAQ:QNRX)
Historical Stock Chart
From Nov 2024 to Dec 2024
Quoin Pharmaceuticals (NASDAQ:QNRX)
Historical Stock Chart
From Dec 2023 to Dec 2024