0001575295false--08-31FY2023false500000000.000100003000000009396759410159291400000.08035000350000.10.10.10.11435601500001500001.251.251.251.251.251.251.251.250.402022-06-2310000000P2YP2YP1Y01000001000000.200.201.251.251.250.751.250.755000031200300000244792521759337500006375004250000.10.100015752952022-09-012023-08-310001575295alid:ColombiaMember2022-09-012023-08-310001575295us-gaap:CanadaRevenueAgencyMember2022-09-012023-08-310001575295alid:ColombiaMember2023-08-310001575295us-gaap:CanadaRevenueAgencyMember2023-08-310001575295alid:AlliedColombiaMember2023-08-310001575295alid:AlliedMember2023-08-310001575295alid:AlliedColombiaMember2022-09-012023-08-310001575295alid:AlliedMember2022-09-012023-08-310001575295alid:NonCashActivitiesMember2022-09-012023-08-310001575295alid:NonCashActivitiesMember2021-09-012022-08-310001575295srt:MaximumMember2023-01-012023-01-310001575295srt:MinimumMember2023-01-012023-01-310001575295alid:DirectorsAndOfficersMember2023-01-012023-01-310001575295alid:ConsultantsAndAmbassadorsMember2023-01-012023-01-3100015752952023-01-012023-01-3100015752952023-05-012023-05-160001575295alid:WarrantThirteenMember2023-08-310001575295alid:WarrantTwelveMember2023-08-310001575295alid:WarrantElevenMember2023-08-310001575295alid:WarrantTenMember2023-08-310001575295alid:WarrantNineMember2023-08-310001575295alid:WarrantEightMember2023-08-310001575295alid:WarrantSevenMember2023-08-310001575295alid:WarrantSixMember2023-08-310001575295alid:WarrantFiveMember2023-08-310001575295alid:WarrantFourMember2023-08-310001575295alid:WarrantThreeMember2023-08-310001575295alid:WarrantTwoMember2023-08-310001575295alid:WarrantOneMember2023-08-310001575295alid:WarrantsMember2022-09-012023-08-310001575295alid:ColombianPesoExchangeRateMember2021-09-012022-08-310001575295alid:ColombianPesoExchangeRateMember2022-09-012023-08-310001575295alid:CanadianDollarExchangeRateMember2021-09-012022-08-310001575295alid:CanadianDollarExchangeRateMember2022-09-012023-08-310001575295alid:ColombianPesoExchangeRateMember2023-08-310001575295alid:CanadianDollarExchangeRateMember2023-08-310001575295alid:COOandDirectorMember2021-09-012021-09-020001575295alid:CFOMember2021-09-012021-09-020001575295alid:CFOANDCOOMember2021-09-012022-08-310001575295srt:DirectorMember2022-08-310001575295srt:DirectorMember2023-08-310001575295alid:AnEntityControlledByADirectorMember2022-08-310001575295alid:AnEntityControlledByADirectorMember2023-08-310001575295alid:CFOMember2022-08-310001575295alid:CFOMember2023-08-310001575295alid:COOandDirectorMember2022-08-310001575295alid:COOandDirectorMember2023-08-310001575295alid:CEOandDirectorMember2022-08-310001575295alid:CEOandDirectorMember2023-08-310001575295alid:AugustThirtyOneTwoThousandTwentyTwoOneMember2022-08-310001575295alid:OnOctoberTwentyTwoThousandTwentyOneMember2022-09-012023-08-310001575295alid:SeptemberTwoTwoThousandTwentyOneTwoMember2022-09-012023-08-310001575295alid:SeptemberTwoTwoThousandTwentyOneOneMember2022-09-012023-08-310001575295alid:SeptemberTwoTwoThousandTwentyOneTwoMember2021-09-020001575295alid:AugustThirtyOneTwoThousandTwentyTwoOneMember2021-09-020001575295alid:SeptemberTwoTwoThousandTwentyOneOneMember2021-09-020001575295alid:MayTwentySevenTwoThousandTwentyOneMember2022-09-012023-08-310001575295alid:OctoberSevenTwoThousandTwentyTwoMember2022-10-070001575295alid:OctoberSevenTwoThousandTwentyTwoMember2023-08-310001575295alid:MayTwentySixTwoThousandTwentyTwoTwoMember2022-05-012022-05-260001575295alid:MayTwentySixTwoThousandTwentyTwoOneMember2022-05-012022-05-260001575295alid:FebruaryTwnetyFourTwoThousandTwentyTwoOneMember2022-01-272022-02-240001575295alid:FebruaryTenTwoThousandTwentyTwoTwoMember2022-02-022022-02-100001575295alid:FebruaryTenTwothousandTwentyTwoOneMember2022-02-022022-02-100001575295alid:JanuaryTwentyEightThousandTwentyTwoMember2022-02-012022-02-280001575295alid:JanuaryTwentyTwoThousandTwentyTwoOneMember2022-01-012022-01-200001575295alid:OnNovemberTwentyFourTwoThousandTwentyOneMember2021-11-012021-11-240001575295alid:OnNovemberFiveTwoThousandTwentyOneMember2021-10-062021-11-050001575295alid:OnOctoberTwentyTwoThousandTwentyOneMember2020-09-012021-08-310001575295alid:OnOctoberTwentyTwoThousandTwentyOneMember2021-10-012021-10-200001575295alid:SeptemberTwoTwoThousandTwentyOneTwoMember2021-09-012021-09-020001575295alid:AugustThirtyOneTwoThousandTwentyTwoOneMember2022-09-012023-08-310001575295alid:AugustThirtyOneTwoThousandTwentyTwoOneMember2021-09-012021-09-020001575295alid:SeptemberTwoTwoThousandTwentyOneOneMember2021-09-012021-09-020001575295alid:AugustTwoOneThousandTwentyThreeMember2022-09-012023-08-310001575295alid:OctoberSevenTwoThousandTwentyTwoMember2022-10-012022-10-070001575295alid:OctoberSevenTwoThousandTwentyTwoMember2022-09-012023-08-310001575295alid:SeptemberTwentyOneTwoThousandTwentyTwoMember2022-09-012023-08-310001575295alid:FebruaryTwnetyFourTwoThousandTwentyTwoTwoMember2022-01-272022-02-240001575295alid:OnNovemberFiveTwoThousandTwentyOneMember2022-09-012023-08-3100015752952021-11-3000015752952022-08-022022-08-2300015752952022-02-082022-03-0700015752952022-02-012022-02-2800015752952022-01-282022-02-2500015752952022-02-032022-02-2200015752952022-02-042022-02-2100015752952022-01-222022-02-2000015752952022-01-082022-02-0700015752952022-08-012022-08-2300015752952022-01-272022-02-2400015752952022-02-012022-02-070001575295alid:AugustThirtyOneTwoThousandTwentyTwoOneMember2022-05-260001575295alid:AugustThirtyOneTwoThousandTwentyTwoOneMember2023-08-310001575295alid:MayTwentySixTwoThousandTwentyTwoOneMember2023-08-310001575295alid:MayTwentySixTwoThousandTwentyTwoTwoMember2023-08-310001575295alid:MayTwentySixTwoThousandTwentyTwoTwoMember2022-05-260001575295alid:MayTwentySixTwoThousandTwentyTwoOneMember2022-05-260001575295alid:FebruaryTwnetyFourTwoThousandTwentyTwoTwoMember2023-08-310001575295alid:FebruaryTwnetyFourTwoThousandTwentyTwoTwoMember2022-02-240001575295alid:FebruaryTwnetyFourTwoThousandTwentyTwoOneMember2023-08-310001575295alid:FebruaryTwnetyFourTwoThousandTwentyTwoOneMember2022-02-240001575295alid:FebruaryTenTwoThousandTwentyTwoTwoMember2023-08-310001575295alid:FebruaryTenTwoThousandTwentyTwoTwoMember2022-02-100001575295alid:MayTwentySevenTwoThousandTwentyOneMember2022-02-100001575295alid:MayTwentySevenTwoThousandTwentyOneMember2023-08-310001575295alid:FebruaryTenTwothousandTwentyTwoOneMember2023-08-310001575295alid:FebruaryTenTwothousandTwentyTwoOneMember2022-02-100001575295alid:JanuaryTwentyEightThousandTwentyTwoMember2023-08-310001575295alid:JanuaryTwentyTwoThousandTwentyTwoOneMember2022-01-280001575295alid:JanuaryTwentyEightThousandTwentyTwoMember2022-01-280001575295alid:JanuaryTwentyTwoThousandTwentyTwoOneMember2023-08-310001575295alid:OnNovemberTwentyFourTwoThousandTwentyOneMember2022-01-200001575295alid:JanuaryTwentyTwoThousandTwentyTwoOneMember2022-01-200001575295alid:OnNovemberTwentyFourTwoThousandTwentyOneMember2023-08-310001575295alid:OnNovemberTwentyFourTwoThousandTwentyOneMember2021-11-240001575295alid:OnNovemberFiveTwoThousandTwentyOneMember2023-08-310001575295alid:OnNovemberFiveTwoThousandTwentyOneMember2021-11-050001575295alid:OnOctoberTwentyTwoThousandTwentyOneMember2023-08-310001575295alid:OnOctoberTwentyTwoThousandTwentyOneMember2021-10-200001575295alid:AugustTwoOneThousandTwentyThreeMember2023-08-310001575295alid:SeptemberTwentyOneTwoThousandTwentyTwoMember2022-10-070001575295alid:SeptemberTwentyOneTwoThousandTwentyTwoMember2023-08-310001575295alid:SeptemberTwentyOneTwoThousandTwentyTwoMember2022-08-3100015752952022-08-2300015752952022-10-0700015752952021-08-012021-08-310001575295alid:SeptemberTwoTwoThousandTwentyOneTwoMember2020-09-012021-08-310001575295alid:TenConvertibleNotesMember2021-10-310001575295alid:ElevenConvertibleNotesMember2022-08-310001575295alid:ElevenConvertibleNotesMember2023-08-310001575295alid:TenConvertibleNotesMember2022-08-310001575295alid:TenConvertibleNotesMember2023-08-310001575295alid:EaightConvertibleNotesMember2022-08-310001575295alid:SevenConvertibleNotesMember2022-08-310001575295alid:FiveConvertibleNotesMember2022-08-310001575295alid:FourConvertibleNotesMember2022-08-310001575295alid:ThreeConvertibleNotesMember2022-08-310001575295alid:ThreeConvertibleNotesMember2023-08-310001575295alid:TwoConvertibleNotesMember2022-09-012023-08-310001575295alid:TwoConvertibleNotesMember2021-09-012022-08-310001575295alid:SixConvertibleNotesMember2020-11-110001575295alid:OnJulyOneTwoThousandTwentyMemberalid:TwoConvertibleNotesMember2023-08-3100015752952021-03-022021-03-2600015752952022-02-012022-02-2200015752952022-10-012022-10-0700015752952022-03-012022-05-3100015752952023-03-012023-05-310001575295alid:SixConvertibleNotesMember2021-04-300001575295alid:SixConvertibleNotesMember2020-12-020001575295alid:OnNovemberOneTwoThousandTwentyMemberalid:TwoConvertibleNotesMember2022-09-012023-08-310001575295alid:FiveConvertibleNotesMember2020-11-012020-11-110001575295alid:FourConvertibleNotesMember2020-10-012020-10-260001575295alid:ThreeConvertibleNotesMember2020-09-012020-09-2900015752952020-06-012020-06-300001575295alid:JulyTwentyFiveTwoThousandTwentyTwoMemberalid:OneConvertibleNoteMember2021-11-0100015752952021-11-0100015752952022-03-0700015752952022-02-2800015752952022-02-2500015752952022-02-2200015752952022-02-2100015752952022-02-2000015752952022-02-070001575295alid:NineConvertibleNotesMember2021-09-260001575295alid:NineConvertibleNotesMember2021-01-070001575295alid:JulyTwentyFiveTwoThousandTwentyTwoMemberalid:OneConvertibleNoteMember2021-07-250001575295alid:EaightConvertibleNotesMember2021-01-070001575295alid:FiveConvertibleNotesMember2020-11-110001575295alid:FourConvertibleNotesMember2020-10-260001575295alid:ThreeConvertibleNotesMember2020-09-290001575295alid:TenConvertibleNotesMember2021-04-300001575295alid:NineConvertibleNotesMember2021-03-260001575295alid:EaightConvertibleNotesMember2021-03-260001575295alid:ElevenConvertibleNotesMember2020-12-0200015752952020-11-1100015752952020-10-2600015752952020-09-2900015752952021-12-012021-12-2300015752952021-10-032021-10-2500015752952021-10-012021-10-020001575295alid:DecemberTwentyThreeMember2022-08-310001575295alid:TwoConvertibleNotesMember2022-08-310001575295alid:DecemberTwentyThreeMember2023-08-310001575295alid:EaightConvertibleNotesMember2023-08-310001575295alid:SevenConvertibleNotesMember2023-08-310001575295alid:FiveConvertibleNotesMember2023-08-310001575295alid:FourConvertibleNotesMember2023-08-310001575295alid:TwoConvertibleNotesMember2023-08-310001575295alid:JulyTwentyFiveTwoThousandTwentyTwoMemberalid:OneConvertibleNoteMember2021-10-052021-11-0200015752952021-07-022021-07-250001575295alid:EaightConvertibleNotesMember2021-03-022021-03-2600015752952022-12-052022-12-0700015752952022-11-272022-12-0200015752952022-11-012022-11-2800015752952022-06-022022-06-1600015752952022-03-022022-03-2900015752952022-01-032022-01-310001575295alid:DecemberTwentyThreeMember2021-12-012021-12-230001575295alid:JulyTwentyFiveTwoThousandTwentyTwoMemberalid:OneConvertibleNoteMember2021-07-022021-07-250001575295alid:ElevenConvertibleNotesMember2021-04-012021-04-290001575295alid:TenConvertibleNotesMember2021-04-012021-04-300001575295alid:NineConvertibleNotesMember2021-03-022021-03-260001575295alid:OnMarchThirtyOneTwoThousandTwentyTwoMemberalid:NineConvertibleNotesMember2022-09-012023-08-310001575295alid:SixConvertibleNotesMember2020-12-012020-12-020001575295alid:OnMarchThirtyOneTwoThousandTwentyTwoMemberalid:TenConvertibleNotesMember2022-09-012023-08-310001575295alid:OnNovemberOneTwoThousandTwentyOneMemberalid:ElevenConvertibleNotesMember2022-09-012023-08-310001575295alid:OnMarchThirtyOneTwoThousandTwentytwoMemberalid:ThreeConvertibleNotesMember2022-09-012023-08-310001575295alid:OnJuneOneTwoThousandTwentyOneMemberalid:ThreeConvertibleNotesMember2022-09-012023-08-310001575295alid:OnMarchThirtyOneTwoThousandTwentyTwoMemberalid:TwoConvertibleNotesMember2022-09-012023-08-3100015752952022-01-022022-01-110001575295alid:SevenConvertibleNotesMember2021-01-012021-01-070001575295alid:SevenConvertibleNotesMember2022-09-012023-08-3100015752952023-05-012023-05-1700015752952023-02-012023-02-2800015752952021-10-052021-11-020001575295alid:OnMarchThirtyOneTwoThousandTwentyOneMemberalid:TwoConvertibleNotesMember2022-09-012023-08-310001575295alid:OnMarchThirtyOneTwoThousandTwentyTwoMemberalid:ElevenConvertibleNotesMember2022-09-012023-08-310001575295alid:OnOctoberOneTwoThousandTwentyOneMemberalid:TwoConvertibleNotesMember2022-09-012023-08-310001575295alid:OnMarchThirtyOneTwoThousandTwentyOneMemberalid:ThreeConvertibleNotesMember2022-09-012023-08-310001575295alid:OnMarchThirtyOneTwoThousandTwentyTwoMemberalid:EightConvertibleNotesMember2022-09-012023-08-310001575295alid:OnJulyOneTwoThousandTwentyMemberalid:TwoConvertibleNotesMember2022-09-012023-08-310001575295alid:TwoConvertibleNotesMember2019-12-312020-01-2300015752952022-12-0700015752952022-12-0100015752952022-11-2800015752952022-06-1600015752952022-03-2900015752952022-01-3100015752952022-01-110001575295alid:DecemberTwentyThreeMember2021-12-2300015752952021-12-2300015752952021-10-2500015752952021-10-010001575295alid:NineConvertibleNotesMember2021-07-250001575295alid:NineConvertibleNotesMember2022-08-310001575295alid:NineConvertibleNotesMember2023-08-310001575295alid:ElevenConvertibleNotesMember2021-04-2900015752952023-05-1700015752952023-02-2800015752952020-06-300001575295alid:TwoConvertibleNotesMember2020-01-230001575295alid:OneConvertibleNoteMember2020-01-2300015752952020-11-012020-11-1100015752952020-10-012020-10-2600015752952020-09-012020-09-290001575295alid:DecemberSeventeenTwoThousandTwentyOneMember2021-09-012022-08-310001575295alid:DecemberSeventeenTwoThousandTwentyOneMember2022-09-012023-08-310001575295alid:DecemberSeventeenTwoThousandTwentyOneMember2022-08-310001575295alid:DecemberSeventeenTwoThousandTwentyOneMember2023-08-310001575295alid:JuneTwoThousandTwentyMember2021-09-012022-08-310001575295alid:JuneTwoThousandTwentyMember2022-08-310001575295alid:JuneTwoThousandTwentyMember2023-08-310001575295alid:JuneTwoThousandTwentyMember2022-09-012023-08-3100015752952021-12-012021-12-2700015752952020-11-012020-11-2000015752952020-06-012020-06-2000015752952020-05-2900015752952021-08-1000015752952021-08-012021-08-100001575295alid:AlliedColombiaMember2020-02-170001575295alid:AlliedColombiaMember2020-09-012021-08-3100015752952020-09-012021-08-310001575295alid:CannabisLicensesMember2022-09-012023-08-310001575295alid:CannabisLicensesMember2022-08-310001575295alid:CannabisLicensesMember2023-08-310001575295alid:LandequipmentMember2023-08-310001575295alid:OfficeAndComputerEquipmentMember2023-08-310001575295alid:FarmFacilityAndEquipmentMember2023-08-310001575295us-gaap:ConstructionInProgressMember2023-08-310001575295alid:OfficeAndComputerEquipmentMember2022-09-012023-08-310001575295alid:FarmFacilityAndEquipmentMember2022-09-012023-08-310001575295us-gaap:ConstructionInProgressMember2022-09-012023-08-310001575295alid:LandequipmentMember2022-08-310001575295alid:OfficeAndComputerEquipmentMember2022-08-310001575295alid:FarmFacilityAndEquipmentMember2022-08-310001575295us-gaap:ConstructionInProgressMember2022-08-310001575295alid:DepositTowardsALicenseAcquisitionMember2021-03-300001575295alid:TowardsPurchaseOfPrefabricatedBuildingsMember2021-09-012022-08-310001575295alid:PrepaymentsForConstructionFacilityInColombiaMember2023-08-310001575295alid:PrepaymentsForConstructionFacilityInColombiaMember2022-08-310001575295alid:DepositTowardsALicenseAcquisitionMember2023-08-310001575295alid:DepositTowardsALicenseAcquisitionMember2022-08-310001575295alid:TowardsPurchaseOfPrefabricatedBuildingsMember2023-08-310001575295alid:TowardsPurchaseOfPrefabricatedBuildingsMember2022-08-310001575295alid:LandequipmentMember2022-09-012023-08-310001575295alid:OfficeAndComputerEquipmentMembersrt:MaximumMember2022-09-012023-08-310001575295alid:OfficeAndComputerEquipmentMembersrt:MinimumMember2022-09-012023-08-310001575295alid:FarmFacilityAndEquipmentMembersrt:MaximumMember2022-09-012023-08-310001575295alid:FarmFacilityAndEquipmentMembersrt:MinimumMember2022-09-012023-08-310001575295us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-08-310001575295us-gaap:RetainedEarningsMember2023-08-310001575295alid:StockIssuableMember2023-08-310001575295us-gaap:AdditionalPaidInCapitalMember2023-08-310001575295us-gaap:CommonStockMember2023-08-310001575295us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-09-012023-08-310001575295us-gaap:RetainedEarningsMember2022-09-012023-08-310001575295alid:StockIssuableMember2022-09-012023-08-310001575295us-gaap:AdditionalPaidInCapitalMember2022-09-012023-08-310001575295us-gaap:CommonStockMember2022-09-012023-08-310001575295us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-08-310001575295us-gaap:RetainedEarningsMember2022-08-310001575295alid:StockIssuableMember2022-08-310001575295us-gaap:AdditionalPaidInCapitalMember2022-08-310001575295us-gaap:TreasuryStockCommonMember2022-08-310001575295us-gaap:CommonStockMember2022-08-310001575295us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-09-012022-08-310001575295us-gaap:RetainedEarningsMember2021-09-012022-08-310001575295alid:StockIssuableMember2021-09-012022-08-310001575295us-gaap:AdditionalPaidInCapitalMember2021-09-012022-08-310001575295us-gaap:TreasuryStockCommonMember2021-09-012022-08-310001575295us-gaap:CommonStockMember2021-09-012022-08-3100015752952021-08-310001575295us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-08-310001575295us-gaap:RetainedEarningsMember2021-08-310001575295alid:StockIssuableMember2021-08-310001575295us-gaap:AdditionalPaidInCapitalMember2021-08-310001575295us-gaap:TreasuryStockCommonMember2021-08-310001575295us-gaap:CommonStockMember2021-08-3100015752952021-09-012022-08-3100015752952022-08-3100015752952023-08-31iso4217:USDxbrli:sharesiso4217:USDxbrli:sharesxbrli:pure
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ | ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
☐ | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE EXCHANGE ACT |
For the fiscal year ended August 31, 2023
000-56002
(Commission file number)
Allied Corp. |
(Exact name of registrant as specified in its charter) |
Nevada | | 33-1227173 |
(State or other jurisdiction of incorporation or organization) | | (IRS Employer Identification No.) |
1405 St. Paul St., Suite 201
Kelowna, BC Canada V1Y 9N2
877-255-4337
(Address and telephone number of principal executive offices)
____________________________________________________________
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act: Common Stock, $0.001 par value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ☐ No ☒
Indicate by check mark whether the issuer (1) filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the past 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or such shorter period that the registrant was required to submit such files. Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in the definitive proxy or information statement incorporated by reference in Part III of this Form 10-K or amendment to Form 10-K. Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a small reporting company. See definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer | ☐ | Accelerated filer | ☐ |
Non-accelerated filer | ☐ | Smaller reporting company | ☒ |
(Do not check if a smaller reporting company) | Emerging growth company | ☒ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates as of August 31, 2023, was $14,251,900.
(At August 31, 2023, the registrant had 101,592,914 shares of common stock issued and outstanding, of which 40,946,531 shares of common stock issued and outstanding were held by officers and directors. Market value has been computed based upon trading price per OTC markets at or about such date.)
TABLE OF CONTENTS
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS AND INFORMATION
This Annual Report on Form 10-K, the other reports, statements, and information that we have previously filed or that we may subsequently file with the Securities and Exchange Commission, or SEC, and public announcements that we have previously made or may subsequently make include, may include, incorporate by reference or may incorporate by reference certain statements that may be deemed to be “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 and are intended to enjoy the benefits of that act. Unless the context is otherwise, the forward-looking statements included or incorporated by reference in this Form 10-K and those reports, statements, information and announcements address activities, events or developments that Allied Corp. (hereinafter referred to as “we,” “us,” “our,” “our Company” or “Allied”) expects or anticipates, will or may occur in the future. Any statements in this document about expectations, beliefs, plans, objectives, assumptions or future events or performance are not historical facts and are forward-looking statements. These statements are often, but not always, made through the use of words or phrases such as “may,” “should,” “could,” “predict,” “potential,” “believe,” “will likely result,” “expect,” “will continue,” “anticipate,” “seek,” “estimate,” “intend,” “plan,” “projection,” “would” and “outlook,” and similar expressions. Accordingly, these statements involve estimates, assumptions and uncertainties, which could cause actual results to differ materially from those expressed in them. Any forward-looking statements are qualified in their entirety by reference to the factors discussed throughout this document. All forward-looking statements concerning economic conditions, rates of growth, rates of income or values as may be included in this document are based on information available to us on the dates noted, and we assume no obligation to update any such forward-looking statements. It is important to note that our actual results may differ materially from those in such forward-looking statements due to fluctuations in interest rates, inflation, government regulations, economic conditions and competitive product and pricing pressures in the geographic and business areas in which we conduct operations, including our plans, objectives, expectations and intentions and other factors discussed elsewhere in this Report.
Certain risk factors could materially and adversely affect our business, financial conditions and results of operations and cause actual results or outcomes to differ materially from those expressed in any forward-looking statements made by us, and you should not place undue reliance on any such forward-looking statements. Any forward-looking statement speaks only as of the date on which it is made and we do not undertake any obligation to update any forward-looking statement or statements to reflect events or circumstances after the date on which such statement is made or to reflect the occurrence of unanticipated events. The risks and uncertainties we currently face are not the only ones we face. New factors emerge from time to time, and it is not possible for us to predict which will arise. There may be additional risks not presently known to us or that we currently believe are immaterial to our business. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. If any such risks occur, our business, operating results, liquidity and financial condition could be materially affected in an adverse manner. Under such circumstances, you may lose all or part of your investment.
The industry and market data contained in this report are based either on our management’s own estimates or, where indicated, independent industry publications, reports by governmental agencies or market research firms or other published independent sources and, in each case, are believed by our management to be reasonable estimates. However, industry and market data is subject to change and cannot always be verified with complete certainty due to limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties inherent in any statistical survey of market shares. We have not independently verified market and industry data from third-party sources. In addition, consumption patterns and customer preferences can and do change. As a result, you should be aware that market share, ranking and other similar data set forth herein, and estimates and beliefs based on such data, may not be verifiable or reliable.
Item 1. Description of Business
Our Company
Allied Corp. is an international cannabis company with its main production center in Colombia and is one of the few companies that has exported from Colombia internationally and was the first company to export commercial cannabis flower from Colombia. By leveraging the Colombian advantages and its Canadian cannabis cultivation expertise, Allied offers consistent supply of premium cannabis product at scale and attractive prices, while meeting high quality standards, thus significantly de-risking its partners supply chain.
Our mission is to be the most reliable wholesale medical cannabis supplier. We seek to help people around the world by creating and providing targeted cannabinoid health solutions.
Historical Company Information
We were incorporated in the State of Nevada on February 3, 2013 under the name “Cosmo Ventures, Inc.” On July 1, 2019 the Company filed a Certificate of Amendment with Nevada changing the name to Allied Corp.
Effective July 25, 2019 the Company entered into a Stock Purchase and Reorganization Agreement with AM (Advanced Micro) Biosciences, Inc., a British Columbia Canada corporation. Upon completion of the closing effective September 10, 2019, AM (Advanced Micro) Biosciences became a wholly-owned operating subsidiary of the Company.
References in this Offering Circular to “Allied” or the “Company” may include references to the operations of our subsidiaries AM (Advanced Micro) Biosciences, Inc., Allied Corp Colombia S.A.S., Allied US Products, LLC and Tactical Relief, LLC. All of these entities are 100% wholly owned subsidiaries of Allied.
The Company’s principal corporate office is located at 1405 St. Paul St., Suite 201, Kelowna, BC Canada V1Y 9N2. Our telephone number is (877) 255-4337. Our email is ir@allied.health.
Our Opportunity
We focus on the development of Colombian produced medicinal cannabis for patients with conditions potentially suitable for treatment therewith. Such conditions include anxiety, insomnia, anorexia, chronic pain, epilepsy, chemotherapy-induced nausea and vomiting, post-traumatic stress disorder (PTSD), Parkinson’s disease, Tourette syndrome, irritable bowel syndrome (IBS) and spasticity associated with multiple sclerosis (MS) and spinal cord injury (SCI).
Our objective is to be a company that controls its own international vertically integrated supply chain, in order to maximize cash flow and profit margins. Our management team believes that having control over our supply chain should enable us to provide a consistent, rolling-harvest supply to the global cannabis community.
Given the average cost of production in North America being approximately $1.00 to $2.00 per gram, we believe our anticipated direct agricultural cost of raw flower to be in the range of $0.05 – $0.075 per gram based on historical production of ours and other companies in the same region of cannabis production afforded by our Colombian production and cultivation should provide us a competitive advantage.
In addition to what we consider as our demonstrated ability to cultivate low-cost and high margin cannabis in Colombia primarily for international distribution, we have received commercial approval for sale of medical cannabis being produced in Colombia for export internationally. Allied Corp’s commitment to building and maintaining strong relationships with its partners and customers around the world has been a driving force behind its recent successes. The company is dedicated to meeting the evolving needs of patients and healthcare professionals with its high-quality medical cannabis products, underpinned by rigorous quality standards and ethical cultivation & processing practices.
We intend for our clients to access superior cannabis-related products developed with a high level of quality control. The Company believes that it is fortunate to have assembled a team of industry veterans and management professionals with the background and experience to enable the Company to build, manage and grow such systems.
Key Strategic Objectives
Scale cannabis production in Colombia
We believe our Bucaramanga, Colombia development and cultivation facility gives us a significant competitive advantage in our production of cannabis. In fact, Allied has established a track-record of producing high-quality product with consistent supply year-round at scale, while meeting European quality assurance. Most importantly, Allied has successfully executed on several shipments from Colombia. Notable achievements include:
| · | We have constructed a one-hectare greenhouse, with 5 hectares of space under production. Of these five hectares, we have approximately 3 in production and 2 hectares for working and cannabis processing space and storage. We have the ability to expand to an additional 20 hectares if and when it is needed. |
| | |
| · | We are licensed to produce, extract, import and export psychoactive and non-psychoactive strains of cannabis. |
| | |
| · | Each of our ten non-psychoactive CBD seed strains, and our ten THC seed strains submitted to ICA have been approved over a course of a process that began in October 2019. |
| | |
| · | We obtained commercial approval for production of non-psychoactive cannabis in October 2020, and commercial approval for production of psychoactive cannabis in February 2021. |
| | |
| · | We completed our first harvest in July 2020 for the Colombian Ministry of Agriculture (“ICA”). |
| | |
| · | We were approved to export cannabis derivatives by the Colombian government in April 2021. |
| | |
| · | In April 2022, Colombia opened the opportunity for companies to export dried cannabis flower. Up until this date, only cannabis derivatives were allowed. Allied immediately applied for the first export. We only sell and ship Colombia produced cannabis flower to countries where it is legal to do so. |
| | |
| · | In September 2022, we harvested our first psychoactive THC harvest of 2,200 plants. In October 2023, we harvested an additional 6,100 plants. These have been dried and cured and are to be tested before being offered to sale and export. The production pipeline has additional work packages of approximately 6,000 plants every three weeks. |
| | |
| · | We applied for a psychoactive quota for the equivalent of 8,000 kgs of psychoactive flower in 2021. Non psychoactive production and sales are limitless under current Colombian law. |
| | |
| · | Allied has demonstrated its ability to harvest up to 1000 kgs per month of high-quality cannabis flower. Since June 2023, it has scaled down production to 650 flowers per month as it was reaching the end of its quota. |
| | |
| · | In late August 2023, Allied was granted an increase of its THC cannabis flower quota, expanding from 8,000kgs to an impressive 50,000 kgs of THC cannabis flower; of which 5,000kgs shall be used for THC derivatives production. |
| | |
| · | Allied has access to scalable land holdings in what it considers to be one of Colombia’s best suited climates for Cannabis production in the order of 200 acres. |
Since we received cultivation approval from ICA, we have developed what we consider to be competitive advantages of Yield, Cannabinoid Potency, Quality, Cost, Frequency of Harvests, and, notably have:
| ● | Yield-Continually increased our yields per hectare. |
| | |
| ● | Potency-Increased and replicated the THC and CBD yields of our plant/seed strains. |
| | |
| ● | Cost-Reduced our direct agricultural cost of raw flower production to as little as $0.05 - $0.075 per gram based on historical production of ours and other companies in the same region. This is favorably compared to the estimated North American production costs of between $1.00 and $2.00 per gram that we have observed. |
| | |
| ● | Quality-Met or Exceeded European Pharmacopeia Standards. |
| | |
| ● | Frequency of Harvest-We currently harvest six to seven times per year in order maximize our production footprint. Consequently, each hectare at our Colombian cultivation site is expected to produce the equivalent of six to seven hectares of cannabis on a mono-crop yearly cycle. (See-Cannabis Production-Colombia). |
Allied’s recent successes are underpinned by what we consider to be one of Colombia’s leading agricultural genetics teams for cannabis production, combined with a strong pharmaceutical background. In fact, Allied’s production team is led by Canadian cultivation and genetics experts with over 30 years of production expertise. The Canadian cultivation team is joined by an existing Colombian agronomical science team who managed 8,000 hectares of rice plantations in Colombia, bringing an unmatched advantage and experience in understanding local growing conditions and best agronomy practices. Moreover, the cultivation team is supported by a strong pharmaceutical background, with Allied’s CEO having over 10 years of experience managing hospital quality assurance programs. The aim is to bring the hospital and pharma background to the standard operating procedures and quality assurance of its Colombian based production.
Allied has now executed on Part 1 (site identification and setup) and Part 2 (cultivation and export) of its plan. With this strong foundation, it is now implementing Part 3 of its journey: while continuing to leverage its Colombian cost advantages, cultivation know-how and pharma expertise, Allied is now focusing on distribution and commercialization to become the most reliable global wholesale cannabis supplier.
Establish United States Production of Cannabis if Federally legalized
At Allied Corp, our strategic vision extends far beyond geographical boundaries, as we proudly direct our focus towards the international burgeoning cannabis industry. While the United States cannabis market undoubtedly holds immense potential, the Board of Directors have strategically made the decision to allocate all our financial and human resources to the expansive fields of Colombia, where we are dedicated to the large-scale production of premium cannabis for international markets. By leveraging the favorable legal landscape in Colombia, we position ourselves as key players in the global cannabis trade, ensuring that our products reach regions where their sale is legally sanctioned. Our commitment to excellence in cultivation, coupled with our dedication to navigating international legal frameworks, defines Allied Corp as a trailblazer in the ever-evolving landscape of the cannabis industry.
Our wholly owned subsidiary, Allied US Products LLC, a Nevada limited liability company, intends to lease land and acquire licenses for production upon on the occurrence or waiver of the changes in U.S. federal law to permit the general cultivation, distribution and possession of marijuana or to remove the regulation of such activities from the federal laws of the United States. We will only conduct business activities related to growing or processing cannabis in jurisdictions, including the United States, when it is federally permissible to do so. While we have several arrangements with United States based companies that may themselves participate in the United States cannabis market, these relationships do not violate the federal laws of the United States respecting cannabis and in no manner involve us in any activities in the United States respecting cannabis.
The company is now focusing its efforts on its cultivation in Colombia and as such, United States production is currently not an area of priority to the business and little progress has been made on this front.
Generate Revenues from Natural Health Products containing Hemp-based CBD
In a decisive move aimed at maximizing our global impact, the Board of Directors at Allied Corp has strategically chosen to channel all available resources into the vibrant landscape of Colombia. This pivotal decision reflects our commitment to positioning Allied Corp as a major player in the international cannabis market. By concentrating our financial and human resources on large-scale commercial cannabis production in Colombia, we align ourselves with a jurisdiction that not only offers a conducive environment for cultivation but also presents opportunities to cater to the growing demand for cannabis products in regions where legality supports its trade. This strategic reallocation underscores our dedication to fostering sustainable growth and global prominence in the dynamic cannabis industry.
Previously, with the launch of our health hemp-based CBD brands in the United States, we expect from product rollout and expanded distribution. We have conducted focus groups, brand creation and test marketing over the past year. We have now commercially launched three brands in the US: Tactical Relief™, Equilibrium Bio™, MaXXa ©. The Buds Pure Naturals brand is intended to be licensed to a Canadian License holder to sell into the Canadian market. (See-Our CBD Brands and Products).
The company is now focusing its efforts on its cultivation in Colombia and as such, it is not an area of priority to the business and little progress has been made on this front.
Complete trials of ALID 11, 11 and Psilonex™ Prescription Medications
In response to fiscal constraints, Allied Corp has taken a proactive approach by temporarily pausing our ongoing clinical trials for ALID-11, 11 and Psilonex. This strategic decision allows us to reallocate all available resources towards the large-scale production of cannabis in Colombia. While clinical trials are invaluable for advancing our understanding of cannabis's medicinal potential, we recognize the importance of adapting to current financial circumstances. By focusing on the cultivation and production of high-quality cannabis in Colombia, we aim to capitalize on the robust international market demand while addressing fiscal challenges. This temporary shift underscores our commitment to flexibility and resilience, ensuring that Allied Corp remains at the forefront of the global cannabis industry despite current constraints on clinical trial endeavors.
We completed our clinical Phase 1 pharma trials in 2021, eventually leading to a drug indication for PTSD. We plan to pursue a licensing deal with a larger developer, producer, and distributor of pharmaceuticals. (See-Pharmacologic Products).
The company is now focusing its efforts on its cultivation in Colombia and as such, these clinical trials are not an area of priority to the business and little progress has been made on this front.
Distribution
Our goal is to become a market leader and reliable partner in the cultivation and processing of medicinal-grade cannabis oil and high quality cannabis derived medical and well-being products for large channel distributors. The Board of Directors has made a conscious decision to prioritize and direct all available resources toward the success of our Colombian production and operations, as well as commercialization of our high-quality Colombian cannabis. Once profitability is realized and our position in the market is strengthened, Allied Corp, at the discretion of the Board of Directors, will seamlessly transition to capitalize on the previously developed brands and products, expand its geographic footprint, continue to explore strategic partnerships and pursue accretive acquisitions to supplement our organic growth as set forth below.
Allied has already successfully executed on Part 1 (Site Set up) and Part 2 (Cultivation / Export) of its plan. On this strong foundation, we are entering Part 3: Distribution to accelerate market entry and become a global leader in the supply of wholesale cannabis. In 2022 and throughout 2023, the company has been focusing its efforts on building the baseline partnerships and infrastructure to enable its growth by focusing on the following segments:
| · | Partnerships: Secure partnerships across the supply chain (cultivators, manufacturers and distributors) to accelerate product sale and market entry, as well as enable Allied’s product lines in key jurisdictions. For instance, Allied announced in April 2023, a joint venture with Blueberries Medical in Colombia to expand its product offering into extracts and enable sale of derivative products in new markets. Similarly, in May 2023, the company announced a new partnership with a GMP manufacturer in Switzerland allowing for the sale of both flower and extracts into countries like Germany and UK, among others. |
| | |
| · | Certification: Allied has identified the need to secure CUMCS and IMC GAP Audits and licensing to enter countries such as Israel. Throughout 2023, the company has begun pre-audit work and is in the process of planning an audit date for the near future. |
| | |
| · | Commercial team expansion: In September 2023, the Company has made strategic additions to its commercial team with the appointment of a new Chief Business Development Officer and a VP of Global Sales. Allied Corp has also Contracted a Cannabis Sales and Go-To-Market Consulting Group set to equip and guide Allied Corp’s sales efforts while validating and extending its go-to-market strategy for an appropriate targeted approach. |
| | |
| · | Sales Pipeline: Allied has continued to develop its sales pipeline in key markets of Germany, UK, Australia, Latam. |
These efforts have culminated in the announcement of two commercial shipments of THC based medical cannabis from Colombia to Australia in November 2023. This achievement is a testament to the company’s ability to comply with one of the world’s most stringent quality standards set by the Australian Therapeutics Goods Association (TGA) and unwavering commitment to providing high-quality medical cannabis products worldwide.
Allied is poised to scale its operations, leveraging the groundwork laid in 2022 and early 2023 to make a lasting impact on the global cannabis market. The successful passing of audits and the commencement of commercial shipments reflect Allied's commitment to excellence, compliance, and reliability in meeting the demands of its international clientele.
Eventually explore strategic partnerships
Following the attainment of company profitability through the successful production and sales of Colombian-grown cannabis, Allied Corp is poised to strategically resume its focus on natural health products and brands. This phased approach reflects our commitment to diversification and innovation within the evolving landscape of the cannabis industry. Having established a strong foundation through the lucrative Colombian cannabis market, we plan to integrate natural health products seamlessly into our portfolio, leveraging the knowledge and expertise gained from our initial successes. This complementary strategy aligns with our vision to not only contribute significantly to the international cannabis market but also to broaden our offerings to cater to a broader spectrum of health and wellness needs. As Allied Corp continues to navigate and thrive in dynamic market conditions, our dedication to excellence and adaptability remains unwavering.
Because we offer a variety of cannabis related products, including beauty and skincare products, foods and beverages, we believe that we can create a competitive advantage by partnering with national and multinational companies, across various product categories to jointly develop and market branded cannabis offerings.
Pursue accretive acquisitions
We believe that our deal-making capabilities and experience will allow us to successfully identify, consummate and integrate acquisitions. The cannabis industry is highly fragmented and as it continues to evolve, we expect significant industry consolidation in existing and new markets. Our deal-making capabilities and experience may allow us to successfully identify, consummate and integrate acquisitions. As a public company, we could have greater ability to finance acquisitions, including through using our equity as consideration and accessing the capital markets.
Due to the competitive and dynamic nature of the emerging cannabis products market and rapid changes in the regulatory environment, we recognize the need to remain flexible, so we can react to opportunities and risks as they develop. We will continue to re-evaluate and re-prioritize our strategies to respond to these developments. We are actively fostering a culture of continued agility and exploration since the ability to pivot depending on market dynamics will deliver competitive advantage.
Our CBD Brands and Products
While the extensive brand and product development initiatives undertaken by Allied Corp remain invaluable, the company is strategically deferring the pursuit of these opportunities until after achieving profitability from the sale of its Colombia-produced cannabis. The Board of Directors has made a conscious decision to prioritize and direct all available resources toward the success of our Colombian operations. This focused approach underscores our commitment to establishing a solid foundation in the global cannabis market through the large-scale production and sale of high-quality Colombian cannabis. Once profitability is realized and our position in the market is strengthened, Allied Corp will seamlessly transition to capitalize on the previously developed brands and products, leveraging the groundwork laid during this strategic phase. This approach reflects our dedication to a methodical and phased growth strategy, ensuring sustained success and longevity in the ever-evolving cannabis industry.
Over the past years we have developed, manufactured and test marketed all of our hemp derived CBD products for sale in the United States. Under the Farm Bill of December 2018, the use and sale of hemp derived CBD products in the United States is legal.
We have three hemp derived CBD product brands to market in the United States all with a catalog of products. We are selling these through e-commerce portal at www.alliedcorpbrands.com, and we are also actively seeking physical retail store distribution throughout many states.
Our core hemp derived CBD brands are:
Tactical Relief™ Brand www.tacticalrelief.com
Tactical Relief™ is intended to be considered a patriotic brand under which health and wellness products are brought to market to serve veterans and first responders. The flagship product “Liberty” is a hemp derived CBD tincture for sale in the United States. Additional products include Tactical Hydration, a CBD infused electrolyte replacement drink and many others (described below). The Tactical Relief™. Novel formulations are created to help those living with PTSD across the United States.
Some of the Tactical Relief ™ products currently offered under this brand:
| ● | Tactical Relief ™ Tactical Hydration – a CBD infused electrolyte replacement drink with sodium citrated electrolyte replacement in three refreshing flavors. |
| | |
| ● | Tactical Relief ™ Liberty – A mint and vanilla flavored 1000mg CBD tincture fortified with specific terpene profile. |
| | |
| ● | Tactical Relief ™ FIZZ Tablets – 35mg CBD fizz tablets for adding to your water bottle. |
| | |
| ● | Tactical Relief ™ Battle Balm – CBD roll on activity rub for aches and pains. |
| | |
| ● | Tactical Relief ™ Quick Hit – CBD gummies. |
Equilibrium Bio™ Brand www.equilibriumbiomed.com
Equilibrium Bio is a lifestyle brand that is focused on everything athletic. From Crossfit workouts to Ironman races to general athletic consumers, Equilibrium Bio products have been formulated to assist the athlete along the competitive journey. Hydration and recovery are the primary areas of focus for Equilibrium Bio products. We built a sales and distribution infrastructure for this brand in 2020. We signed contracts with two major athletes (Camille Leblanc and Dave Lipson) who have 2,000,000 followers on Instagram to endorse the products. On December 10, 2020, we announced the completion of the first manufacturing run of Hydro Sport CBD-infused rehydration drinks from our latest brand, Equilibrium Bio. These drinks have also been produced for the Tactical Relief™ branding as Tactical Hydration electrolyte replacement and rehydration drink products.
The first Equilibrium Bio™ products known as Hydro Sport are available for purchase today on e-commerce platforms and have also been shipped to national retail buyers throughout the United States. The first manufactured batch encompasses six unique product SKUs (stock-keeping units). The CBD-infused drink pouches come in three flavors: Lemon Lime, Berry Fresh and Orange Burst. The all-natural flavoring and ingredients in the drinks include: Filtered Water, CBD 20 (mg), CBD, CBG, Sodium Citrate, Citric Acid, Sea Salt, Natural Flavor, Tripotassium Citrate, Vitamin B Complex, Ascorbic Acid (Vitamin C), Sucralose, Acesulfame Potassium and Beta Carotene for color.
Some of the Equilibrium Bio™ products that are currently offered:
| ● | Equilibrium Bio™ - Hydrosport CBD Drink – a CBD infused electrolyte replacement drink with sodium citrate electrolyte replacement in three refreshing flavors. |
| | |
| ● | Equilibrium Bio™ Hydrosport Tincture – 1000mg CBD tincture fortified with specific terpene profile. |
| | |
| ● | Equilibrium Bio™ FIZZ Tablets – 35mg CBD fizz tab for adding to a water bottle. |
| | |
| ● | Equilibrium Bio™ Sport Rub – CBD post activity rub for aches and pains. |
| | |
| ● | Equilibrium Bio™ Quick Hit – CBD gummies with vitamin B12. |
MaXXa© Brand www.maxxabrand.com
MaXXa is a brand designed to be athletically sleek and focused on developing natural CBD infused cosmetics and beauty products. There are currently six unique products in the catalogue. These products are targeted with beauty and anti-aging in mind. There are MaXXa products focusing on anti-aging and cosmetics. MaXXa’s first shipment of products to Asia was completed on January 28, 2020. This shipment included a total of 120 total units encompassing 20 units for every six unique product SKUs (stock-keeping units). We then temporarily paused sales activities due to COVID. We intend to resume sales expansion efforts of MaXXa into the Asian marketplace following the decrease in restrictions due to COVID.
Some of the MaXXa™ products currently offered include:
| ● | MaXXa™ Skin Structure - Skin Structure is a facial formula topically applied to improve skin’s radiance and youthfulness. |
| | |
| ● | MaXXa™ Eye Recover - Eye Recover is based on antioxidant power of low molecular weight hyaluronic acid. Ultra-hydrating eye serum helps the delicate eye skin ward off free radicals as well as helping to protect against sun damage. |
| | |
| ● | MaXXa™ Glossy Lip Recover - Glossy Lip Recover moisturizes and nourishes the lips and gives them a silky smoothness. Enriched with rich fruit extracts - beeswax as well as oils and butter - this lip gloss moisturizes, beautifies and revitalizes your lips. |
| | |
| ● | MaXXa™ Skin Designer – skin designer uplifts your skin with the power of CBD extracts and hyaluronic acid. This product stimulates cell regeneration while minimizing age spots. |
| | |
| ● | MaXXa™ Vitamin Absolute - Vitamin Absolute is a soothing, calming and all-around moisturizing facial oil. |
| | |
| ● | MaXXa™ Absolute Recover - this specialized formulation allows the infused bioactive compounds to be delivered directly to the desired areas of inflammation, pain and other skin ailments. |
The company is now focusing its efforts on its cultivation in Colombia and as such, the development and commercialization of Allied’s brands described above is not an area of priority and little progress has been made on this front.
Pharmacologic Products
While the development and licensing of pharmaceutical products presents a compelling avenue with significant future revenue potential for Allied Corp, the company has made a strategic decision to temporarily direct all resources toward the production and sales of Colombian cannabis. This focused approach is aimed at capitalizing on the immediate opportunities in the rapidly growing cannabis market, particularly in Colombia. Once Allied achieves profitability from its Colombian cannabis operations, the company intends to transition its attention back to advancing its work in pharmaceutical development. This strategic sequencing reflects Allied Corp's commitment to a phased and adaptable business strategy, ensuring that resources are deployed efficiently to address immediate market demands while maintaining a long-term vision for diversified revenue streams through pharmaceutical ventures. The temporary shift in focus underscores our dedication to strategic agility and the overarching goal of establishing Allied Corp as a formidable player in both the cannabis and pharmaceutical sectors. As such, this is no longer an area of focus to the business and very little progress has been made.
Allied was organized because our founders believed veterans and first responders suffering from Post-Traumatic Stress Disorder (“PTSD”) could have their underlying symptoms better addressed with targeted cannabinoid pharmacologic solutions. We entered into an agreement to purchase all common shares of Pacific Sun Fungi Inc. on January 24, 2021 which was subsequently amended and the acquisition later closed in March 2021. The primary business plan for acquiring Pacific Sun Fungi Inc. is to develop psilocybin-based solutions, including both potential pharmaceutical and natural health products, for those suffering from PTSD. Accordingly, we have expanded our pharmacologic research to find better treatment options for those suffering from depression, anxiety and general mental health issues. Accordingly, we believe we are in a unique position to develop a full-scope, closed-loop therapy protocol, which contrasts with psilocybin therapies that support the patient for an episodic micro-dosing treatment regime following a specific dosing and wash out cycle. We believe our existing cannabinoid treatment solutions may alleviate the effects of the “treatment gaps” between psilocybin dosing periods, providing what we call the “Allied Full Scope Treatment”.
Our proprietary “Allied Full Scope Treatment” offers the patient continual therapy with treatment options both before and after the psilocybin treatment period. This is accomplished by offering our cannabinoid and natural health products combined with the psilocybin products and protocols under legally mandated licenses with physician supervision.
Products in Potential Development
Psilonex™
On March 25, 2021 we announced that we filed for trademark protection with the United States Patent Office for our pharmaceutical product Psilonex™. Psilonex™ is a proprietary formulation prescribed within a treatment path that involves both a pharmaceutical psilocybin-based formulation, followed by a daily cannabinoid maintenance period. Psilonex™ is intended to be prescribed by a physician, followed by the cannabinoid daily therapy that can be purchased by the consumer in regions where it is legal to do so as “Psilonex™ Daily,” Both of these products are currently being researched, developed and tested by our pharmaceutical development team.
The filed trademark protects our intellectual property for Psilonex™ covering: chemical preparations for pharmaceutical or medical purposes, namely, for treating depression, anxiety, and PTSD, and for improving mental health; nutraceuticals for the treatment of mental health disorders and conditions, including depression, anxiety, and PTSD; nutraceuticals for use as a dietary supplement; Nutraceuticals for use as a dietary supplement for improving mental health; pharmaceutical preparations and substances for the treatment of psychiatric diseases and disorders; pharmaceutical preparations for the treatment and prevention of mental health disorders; Pharmaceuticals, namely, psychotropics; dietary and nutritional supplements; fungal extracts sold as a component ingredient of nutritional supplements and vitamins, and; related websites.
ALID 10 and ALID 11
ALID 10 and ALID 11 target the treatment of Post-Traumatic Stress Disorder and related mental health conditions through the targeted function of the 5-HT2 receptor and physiological pathways. Following the completion of the pre-clinical research phase, we intend to bring ALID 10 and ALID 11 through human clinical trials through its relationships with the University of Haifa, Israel and MGC Pharma in Europe.
In 2020, we filed a United States provisional patent application entitled “Compositions and Methods for Modulating Endocannabinoid Systems Activity and Treating Mental Health Disorders”. The patent application covers ALID-10 and ALID-11, as well as other cannabis derived compounds designed for superior pharmaceutical properties for treatment of PTSD and other mental disorders.
In October 2019, we signed a Master Services Agreement with the University of Haifa, Israel. This academic research center gives us access to a proprietary pre-clinical animal model that is owned by Carmel. Carmel is the business unit that manages the University of Haifa center for Research in Israel. Specifically, we will use this proprietary animal model for the purposes of specialized pharmaceutical cannabinoid research and pharmaceutical product development. Through our agreement, we have access to the University of Haifa’s leading-edge facilities, proprietary animal model, pharmaceutical development, academic laboratory and associated scientists and investigators. This should enable us to conduct the pre-clinical phase of the development of a proprietary pharmaceutical product for ALID-10 and ALID-11.
In the second quarter of 2020, we expanded our ALID-10 and ALID-11 research activities, as well as entered into a manufacturing and sales agreement with MGC Pharmaceuticals of Australia (“MGC”). Under the terms of these agreements, MGC will manufacture our pharmaceutical products within their GMP (Good Manufacturing Practices) certified factory in Slovenia (which is in the European Union, or EU), which should enable us to have our pharmacological products produced under procedures and methods that meet those high-quality standards. In addition, the MGC sales and distribution agreement, should provide us a channel to sell our products following the completion of a PHASE I clinical trial. MGC currently has cannabis-based pharmaceutical products for sale in Europe, and. Moreover, we believe it has demonstrated a proven ability to commercialize cannabis based pharmaceutical products. We also announced the initiation of a pharmaceutical human clinical PHASE I research trial with MGC, but COVID-19 had an operational impact on the progression of this research trial. We intend to complete this trial as a part of the use of proceeds from this financing.
Under the terms of our agreements with MGC, it will provide what we consider to be a comprehensive suite of pharmaceutical services to advance our pharmaceutical products into human clinical PHASE 1. This trial is expected to test the efficacy and pharmacodynamics of our pipeline of proprietary cannabis derived drug candidates targeting PTSD. The scope of MGC Pharma’s services are to cover clinical research, Investigational Medicinal Product (IMP) registration, manufacturing of lab volumes for the research project, drug stability testing, GMP manufacturing and regulatory assistance for obtaining an IMP numbers that are needed in order for pharmaceutical products to be sold.
The company is now focusing its efforts on its cultivation in Colombia and as such, the products in development described above are not an area of priority to the business and little progress has been made on this front.
Cannabis Production
Colombia
We believe our Bucaramanga, Colombia development and cultivation facility gives us a significant competitive advantage in our production and sales of cannabis. Allied has established a track-record of producing high-quality product with consistent supply year-round at scale, while meeting European quality assurance. Most importantly, the company has successfully executed on several shipments from Colombia. Notable achievements include:
| ● | We are licensed to produce, extract, as well as both import and export psychoactive and non-psychoactive strains of cannabis. |
| ● | We were approved to export cannabis by the Colombian government in April 2021. |
| ● | We have constructed a one-hectare greenhouse, with 5 hectares of space under production. Of these five hectares, we have approximately 3 in production and 2 hectares for working and cannabis processing space and storage. We have the ability to expand to an additional 20 hectares if and when it is needed. |
| ● | We completed our first harvest in July 2020 as part of the approval process for the Colombian Ministry of Agriculture (“ICA”). |
| ● | We obtained commercial approval for production of non-psychoactive cannabis in October 2020, and commercial approval for production of psychoactive cannabis in February 2021. |
| ● | Each of our ten non-psychoactive CBD seed strains, and our ten THC seed strains submitted to ICA have been approved over a course of a process that began in October 2019. |
| ● | Lower production cost: Our anticipated direct agricultural cost of raw flower production is approximately $0.05 – $0.075 per gram based on historical production of ours and other companies in the same region, as favorably compared to the North American production costs of as opposed to most production between $1.00 and $2.00 per gram that we have observed. |
| ● | Access to scalable land holdings in what we consider to be one of Colombia’s best suited climates for cannabis production in the order of 200 acres. |
| ● | We have applied for a psychoactive quota for the equivalent of 8,000 kgs of psychoactive flower for 2021. Non psychoactive production and sales are limitless under current Colombian law. |
| ● | We have engaged what we consider to be one of Colombia’s leading agricultural genetics teams for cannabis production. |
| ● | In September 2022 we harvested our first psychoactive THC harvest of 2,200 plants. In October 2022, we harvested an additional 6,100 plants. These have been dried, cured and tested before being offered to sale and export. The production pipeline has additional work packages of approximately 6,000 plants every two to three weeks. We only sell and ship Colombia produced cannabis flower to countries where it is legal to do so. |
| ● | Allied has demonstrated its ability to harvest up to 1000 kgs per month of high-quality cannabis flower. Since June 2023, it has scaled down production to 650 flowers per month as it was reaching the end of its quota. |
| ● | In late August 2023, Allied was granted an increase of its THC cannabis flower quota, expanding from 8,000kgs to an impressive 50,000 kgs of THC cannabis flower; of which 5,000kgs shall be used for THC derivatives production. |
In the 2020 fiscal year, we activated our Colombian licenses that are required to produce, extract, import and export psychoactive and non-psychoactive cannabis. This has been a year-long process that began with the registration of our ten (10) novel strains with the national cultivar registry. Each strain was then germinated into plantlets in our cultivation center in Colombia. Following germination, the plants entered into five (5) months of field trials that included rigorous data collection, analysis and phenotyping of the strains while in the vegetation life cycle. During this time, the strains were provided with proprietary nutrients, handled with standard operating procedures and guided towards the plant flowering phase. Proprietary nutrients and procedures were also adhered to during the flowering phase and detailed batch record audit data were diligently collected during every day of the plant life cycle. Following flowering, the plants were harvested and tested for cannabinoid profiles and quality assurance testing parameters. The harvested material was then sent to several accredited testing laboratories and tested by high performance liquid chromatography testing methods. Testing was carried in several labs for validation of the results regarding the accuracy of the cannabinoid profiles.
The lab results from the testing of the cannabis showed a higher cannabinoid profile from being grown in the Colombian climate when compared to the same strains grown in North American climate. The lab results, batch records and procedural archives were also submitted to the Colombian Institute of Agriculture and a day-long presentation was provided by our team. As a result of this process, we received approval for twenty novel strains (ten psychoactive, ten non-psychoactive) from the technical directorate of the Colombian Ministry of Agriculture, representing a diverse range of chemotypes with novel cannabinoid profiles.
Our production approach is designed according to the European Pharmacopeia and Good Agricultural Collection Practices (GACP) and Buenos Practicales Agrinomicales (BPA) standards. These standards meet South American, European, US and Canadian quality assurance standards for production of commercialization of cannabis.
In addition, in order to adhere to Canadian production regulations with respect to seed approval and strain-acclimatization, in Canada we have created research and development environments (under personal production licenses approved by the Canada Regulators) that mimic the micro-climates in Suesca, Ibague and Bucaramanga. We found Bucaramanga to have the best micro climate for production of our plant strains, and accordingly located our Colombian production there. We have stressed the strains with high temperatures and humidity levels to mimic the natural weather patterns in the areas in which we will be cultivating. This is designed to mitigate the acclimatization risk that we believe many peer companies are experiencing in Colombia.
Our Colombian Cultivation Results
On April 15, 2021 we shared an analysis of our Colombian production harvests. Over this period, we continued to harvest every 6-7 weeks, while adding to our inventory and expanding our analytic data set.
We have now cultivated several successive Colombian harvests with what we consider to be consistent results. The results showed increased production yield, higher cannabinoid percentages, high quality as measured by cannabinoid potency, as well and as low cost per gram production cost relative to North American production. Our lab results showed a higher cannabinoid profile from being grown in the Colombian climate when compared to similar genotypes of strains grown in North American climate. These lab results, batch records and procedural archives, were submitted to the Colombian Ministry of Agriculture during a day-long presentation by our team, which, in turn, resulted in the approval by the technical directorate of the Colombian Ministry of Agriculture for the ten THC (i.e., psychoactive) strains tested. We believe these THC strains represent a diverse range of chemotypes with novel psychoactive cannabinoid profiles.
In addition to the increase in grams produced per square meter, the cannabinoid percentages showed an increase when produced in Colombia when compared to North America. We have now cultivated several successive harvests with repeatable results. We believe these results suggest we have competitive advantages that we describe as Yield, Cannabinoid Potency, Quality, Cost, Frequency of Harvests.
Increased yield – Approaching One kilogram per Square Meter
We believe we have a proprietary approach to cultivation which fosters high yield production. Our first Colombian harvest yielded 424 grams per square meter of production space. The second harvest, 612 grams per square meter and third and fourth harvest resulted in yields of 684 grams and 893 grams per square meter respectively. The fifth and sixth harvest yielded 894 and 905 grams per square meter respectively. This has continued with this trend throughout 2022. We have started to enter into our production pipeline new genetics that have been registered and brought forth to first harvest in 2024. We believe this data shows that our team, is approaching its goal of one kilogram per square meter goal of production output.
Potency-Cannabinoid Production
In Colombia, per our internal testing we have achieved repeatable THC cannabinoid percentages of 29.08% in the psychoactive varieties and CBD percentages of 24.64%. With our continuous quality improvement program, these percentages have continued to increase. The data collected showed that these strain profiles showed a significant increase in cannabinoid content when comparing the output from our Colombian cultivation to our North American cultivation environments.
Direct Agricultural Costs – Approximately $0.05 – $0.075 per Gram
Our direct agricultural cost of raw flower production is anticipated to be $0.05 - $0.075 cents per gram based on historical production of ours and other companies in the same region. This presents a potential increased margin advantage to multi-state operators within the United States if and when we are able to sell cannabis to the United States, and potentially for wholesale buyers internationally. We believe suggests we can reduce the cost of supply and improve margin for distributors in markets with costs of production between $1.00 - $2.00.
Quality – Meeting European Pharmacopeia (International) Standards
Our cultivation and production processes meet European Pharmacopeia standards and Good Agricultural Collection Practices (GACP) standards with zero pesticide use, as they were designed from inception. These standards meet South American, European, US and Canadian quality assurance standards for production of commercialization of cannabis. Every cannabis plant is counted and audited during every day of its life cycle. Data collected includes temperature, humidity, pest and disease management indicators. All harvested material is tested for physical identity, aflatoxins, foreign body count, total aerobic plate count, total yeast, e coli, salmonella, bile tolerant gram-negative bacteria, cannabinoids, cadmium, lead, arsenic, mercury, terpenes and pesticides. All product is analyzed and approved based upon meeting thresholds for each of the testing parameters.
Frequency of Harvests: We Have Maintained Consistent Production with Consecutive Harvests every 6-7 weeks
Given the advantages that the equatorial climate of Colombia offers, we have been harvesting every 6-7 weeks, suggesting we should be able to offer reliably consistent supplies in large volumes to multiple markets simultaneously.
Success at Quality Assurance and Customer Vendor Qualification
Allied Corp takes pride in its proven track record of success, having successfully navigated the vendor qualification process with approximately a dozen different international cannabis and Pharmaceutical companies. This rigorous process has involved thorough desktop audits of our Standard Operating Procedures (SOPs), virtual farm tours, in-person third-party neutral audits, and various other assessments—all of which Allied has passed. The meticulous scrutiny of our operations has been instrumental in establishing a high level of trust and credibility in the industry. The successful completion of these qualification processes play a preliminary role prior to discussing sales contracts. Allied Corp's commitment to transparency, quality, and compliance is reflected in these achievements, underscoring our dedication to maintaining the highest standards in the cannabis industry and fostering long-term partnerships with discerning companies.
Our Focused Cultivation and Cannabis Plant Development Approach
We received approval from the Colombian Ministry of Agriculture (“ICA”) for 100% compliance, or 20 out of 20 plant strains (10 CBD and 10 THC). ICA reported to us in February 2021 that we were the first company to do so. Our strains have both non-psychoactive and psychoactive varieties that have been legally cultivated both in North America and now in the South America. As a result, we have activated our Colombian licenses for production, extraction, importation and exportation of psychoactive and non-psychoactive cannabis.
This is the culmination of a year-long process that began with the registration of what, in our opinion, are our novel strains with the Colombian national cultivar registry. Each strain was then germinated into plantlets in our cultivation center in Colombia. Following germination, the plants were tested in field trials over five months of field trials that included data collection, analysis and phenotyping of the strains during the vegetation life cycle. These strains were given proprietary nutrients, handled with standard operating procedures guided towards the plant flowering phase, while detailed batch record audit data was collected every day of the plant life cycle. Following flowering, the plants were harvested and tested for cannabinoid profiles and quality assurance testing parameters. The harvested material was then sent to several (accredited testing laboratories and tested by liquid chromatography testing methods for validation of the accuracy of the cannabinoid profiles.
It should be noted that production is divided into two key types of products: (i) “non-psychoactive” means the strains and genetics with THC levels of less than 1% by weight (ii) “psychoactive” means the strains that have more than 1% THC by weight. Approval of psychoactive cultivation is much more complex than the non-psychoactive approvals in Colombia. In order for a Colombian licensed producer to gain approval to sell and export, the producer must first register their genetics, then go forth and cultivate a limited number of plants as a test harvest. Following the harvest, drying and extraction processing, the product is analyzed and presented to the Colombian Ministry of Agriculture; we have received the requisite approvals from the Colombian Government for each phase of its cannabis development and cultivation.
Our Path from Testing to Approved Cannabis Exports from Colombia
In October 2020 we were granted from the Colombian Ministry of Agriculture approval to begin the seed evaluation process for our ten non-psychoactive CBD strains. In addition, on April 29, 2021, the Ministry of Justice signed a resolution authorizing us to evaluate our Company’s proprietary strains in order that we could select our best pheno-typed THC psychoactive cannabis strains with the best commercial production characteristics. Along with our previous approval for non-psychoactive evaluation, our subsidiary Allied Colombia S.A.S. could then evaluate all twenty of our psychoactive and non-psychoactive strains.
On October 15, 2020, we received approval from the Colombian Institute of Agriculture (“ICA”) for the cultivation and commercialization of each of our ten proprietary CBD non-psychoactive strains submitted within seed evaluation process which commenced a year earlier. Such enabled us to process and sell our Colombian CBD non-psychoactive production.
On March 9, 2021, we announced that we received ICA approval for the commercialization of its our proprietary THC psychoactive cannabis strains, which was the culmination of the evaluation process for which permission was granted in late April 2020. Each of our ten psychoactive strains were approved and we have passed all of the ICA requirements to be able to cultivate the resultant plants. As a result, we have has applied for a psychoactive export quota for both 2021 and 2022 under the Colombian regulatory regime. In late August 2023, Allied was granted an increase of its THC cannabis flower quota, expanding from 8,000kgs to an impressive 50,000 kgs of THC cannabis flower; of which 5,000kgs shall be used for THC derivatives production.
On April 20, 2021 we received clearance from the Colombian authorities to export our derivative based non psychoactive cannabis-based products from our first harvest. This milestone was achieved after having undergone the testing and cultivation activities described immediately above herein, and having received the ICA and Colombian Ministry of Justice approvals and licenses described below. Moreover, on April 26, 2021, we received approval to export cannabis-based products to a second international market.
In a groundbreaking development in April 2022, Colombia announced its authorization for the export of locally produced cannabis flower, opening new horizons for the industry. Allied Corp promptly submitted an application, aiming to be at the forefront of this significant milestone. Allied emerged as the pioneer in June 2022 by successfully executing the first-ever export of dried Colombian cannabis flower. This accomplishment not only positions Allied as an industry trailblazer but also underscores our commitment to capitalizing on emerging opportunities in the dynamic global cannabis market.
Throughout the entirety of 2022, Allied Corp maintained a steadfast focus on cultivating and nurturing customer relationships, embarking on long international sales cycles to establish a strong market presence. This dedication extended into the early part of 2023, during which Allied underwent several audits and customer due diligence visits. Demonstrating the robustness of our operations, Allied successfully passed these assessments, garnering approvals that further solidify our credibility in the industry.
Building on these accomplishments, Allied has successfully exported and shipped the first commercial shipments. This marks a pivotal moment as the company transitions from relationship-building and due diligence through to the ability to export and ship, and now will position itself to scale and enter into sales agreements. With these initial commercial shipments, Allied is poised to scale its operations, leveraging the groundwork laid in 2022 and early 2023 to make a lasting impact on the global cannabis market. The successful passing of audits and the commencement of shipments reflect Allied's commitment to excellence, compliance, and reliability in meeting the demands of its international clientele.
Regulatory Matters
Colombia
Our cultivation operations are in Colombia and are conducted by Allied Colombia S.A.S., a wholly-owned subsidiary. As a cultivator of cannabis, the Company is substantially dependent on the licenses for cultivation, production and other regulatory activities, granted to Allied Colombia by Colombian governmental and regulatory bodies.
The following table summarizes regulations applicable to the cultivation, fabrication, import, export and use of cannabis in Colombia.
Regulation: | Regulates: |
Law 1787 of 2016 | Legalizes the use of Cannabis for medical and scientific purposes |
Decree 613 of 2017 modifies Decree 780 of 2016 | Regulates law 1787 establishing a licensing system and process. Defines psychoactive and non-psychoactive cannabis and the quota system for psychoactive cannabis in accordance with Single Convention of Narcotics of 1961 and amendments |
Resolution 577 of 2017 from the Ministry of Justice | Regulates the evaluation and control of the following licenses: a. Seed Use b. Cultivation of psychoactive plants (High-THC cultivation license) c. Cultivation of non-psychoactive plants (Low-THC cultivation license) Creates requirement for security protocol |
Resolution 578 of 2017 from the Ministry of Justice | Resolution 578 of 2017 from the Ministry of Justice Regulates the cost of the following licenses: a. Seed Use b. Cultivation of psychoactive plants (High-THC cultivation license) c. Cultivation of non-psychoactive plants (Low-THC cultivation license) |
Resolution 579 of 2017 from the Ministry of Justice | Establishes that growers that cultivate on a half a hectare area (5,000 square meters) or less are considered small and medium growers and, therefore, may access technical advice, priority allocation of quotas and purchase of their production by the processor and requires that 10 percent of the total production of the processor must come from small and medium producers. |
Resolution 2892 of 2017 from the Ministry of Health | Regulates the evaluation and control of the Fabrication of Cannabis derivatives (High-THC Production License) Provides guidelines for appropriate security protocols for manufacturing cannabis derivatives including physical security, monitoring, detection, and incident reporting to authorities. |
Resolution 2891 of 2017 from the Ministry of Health | Regulates the cost of the High-THC production License |
Resolution 1478 of 2006 from the Ministry of Health modified by Resolution 315 of 2020. | Regulation of the control, monitoring and surveillance of the import, export, processing, synthesis, manufacture, distribution, dispensing, purchase, sale, destruction and use of controlled substances, medicines or products containing them and on those which are State Monopoly |
Decree 2200 of 2005 from the Ministry of Health | Regulates pharmaceutical services including the Magistral Preparations |
Guidelines for the GEP certification for Magistral Preparations with Cannabis issued the 25 of October 2019 by INVIMA | Establishes the requirements for labs to obtain the GEP certification for the fabrication of Magistral Preparations with Cannabis derivatives |
Licenses
The Ministries of Health, Justice and Agriculture issued Decree 613 of 2017 to define the licenses that can be granted with respect to permissible activities related to medical cannabis that include: (i) the production of cannabis derivatives; (ii) use of seeds for sowing; (iii) planting of psychoactive cannabis plants; and (iv) planting non-psychoactive cannabis plants.
Apart from a psychoactive cannabis license, Allied Colombia has obtained licenses in each of the above categories, necessary to carry out its operations. Licenses are not transferable, interchangeable, or assignable and are valid for five years and can be renewed for additional five-year periods upon request. Each of the Licenses is current and has not expired. None of the Licenses are subject to current, pending, or compromised regulatory action.
In addition to its efforts in customer relationships and international sales cycles, Allied Corp has proactively engaged with regulatory bodies and authorities by hosting a series of both announced and unannounced audits. These comprehensive evaluations, conducted by regulators and authorities, have consistently resulted in "Full Conformance" acknowledgments. This signifies that Allied Corp meets the regulatory standards and requirements governing the cannabis industry.
The commitment to transparency and compliance demonstrated during these audits reinforces Allied's dedication to operating at the highest industry standards. Successfully navigating these regulatory assessments positions the company as a trustworthy and responsible player in the cannabis sector. As Allied continues to uphold rigorous compliance measures, it establishes a solid foundation for sustained success and growth, ensuring that the company's operations align seamlessly with regulatory expectations.
Strains Registration
Allied Colombia has varieties of cannabis in various stages of the registration process. Each strain, whether high or low in THC, must undergo an agronomic evaluation by the agricultural health entity - Instituto Colombiano Agropecuario (ICA). For the strains to be included in the National Registry of Cultivars, the following steps must be completed: (i) Genetic Stabilization; (ii) Agronomic Test; (iii) Phase 1 strain registry (legal document that allows the license to enter a strain in the registry); and (iv) Strain registration phase 2 (registration that allows the licensee to market any cannabis product derived from the specific strain in the registry). The harvest is also in the process of agronomic evaluation of additional strains.
Based on the yields of each strain, as determined by agronomic testing, Allied Colombia may decide to register fewer strains than available. The decision to complete the registration process of a strain depends on several factors, such as biomass yields, cannabinoid profile, average cannabinoid content, resistance to pests among others as determined by agronomic tests, and the intended uses by the company.
In 2021, Allied Colombia has twenty varieties of medicinal cannabis registered strains with the ICA and has the registration as a producer of selected seeds granted by the ICA. Upon receiving the ICA Permit, Allied Colombia commenced commercial cultivation.
In compliance with its international obligations, Colombia establishes an annual limit for the production volume of cannabis plants and derivatives, which is monitored by the International Narcotics Control Board. Based on this limit, the Colombian government established a quota system, in order to control the amount of psychoactive cannabis production per license. This means that for the Psychoactive Cannabis License, licensees must first apply for a specific crop or manufacturing quota, before beginning production.
Protection of Environment and Human Health and Safety
We are subject to various Colombian federal, state and local and regulations relating to the protection of the environment and human health and safety, including those governing the management and disposal of hazardous substances and wastes, the cleanup of contaminated sites and the maintenance of a safe workplace. Some of our operations include the use, generation and disposal of hazardous materials. We also plan to acquire ownership in new facilities and properties, some of which may have had a history of commercial or other operations. We may, in the future, incur liability under environmental statutes and regulations with respect to contamination of sites we own or operate, including contamination caused by prior owners or operators of such sites, abutters or other persons, and the off-site disposal of hazardous substances. Violations of these laws and regulations may result in substantial civil penalties or fines.
United States
Allied is ready to supply the US market from its facility in Colombia if and when the United States federally legalizes the use and sale of cannabinoids. The United States health care industry is subject to extensive regulation by federal, state and local governments. Government regulation affects our businesses in several ways, including requiring licensure or certification of facilities. Our ability to conduct our business and to operate profitability depends in part upon obtaining and maintaining all necessary licenses and other approvals; and complying with applicable healthcare laws and regulations.
Legalization of CBD (Medical Cannabidiol Product)
The United States Agricultural Improvement Act of 2018 (the “2018 Farm Act”) removed hemp, defined as cannabis with less than 0.3% of THC, from Schedule 1 status under the Controlled Substances Act (“CSA”), and Federally legalized the cultivation and sale of hemp, subject to compliance with certain federal requirements and state laws. THC is the psychoactive component of plants in the cannabis family generally identified as marihuana or marijuana. Our medical CBD products are Federally legal in the United States in that they contain less than 0.3% THC in compliance with the 2018 Farm Act guidelines and have no psychoactive effects on our patients and customers. However, there can be no assurance that the 2018 Farm Act will not be repealed or amended such that our products containing hemp-derived CBD would once again be deemed illegal under Federal law in the United States.
The 2018 Farm Act also shifted regulatory authority from the Drug Enforcement Administration to the Department of Agriculture. The 2018 Farm Act did not change the United States Food and Drug Administration’s (“FDA”) oversight authority over CBD products. The 2018 Farm Act delegated authority to regulate and limit the production of hemp and hemp derived products to the state governments. Although many states have adopted laws and regulations that allow for the production and sale of hemp and hemp derived products under certain circumstances, no assurance can be given that such laws of the several states will not be repealed or amended such that our intended products containing hemp-derived CBD would once again be deemed illegal, which in turn would render such intended products illegal in those states under federal law even if the federal law is unchanged. In the event of either repeal of Federal or State laws and regulations, or amendments thereto that are adverse to our intended medical CBD products, we may be restricted or limited with respect to those products that we may sell or distribute, which could adversely impact our intended business plan with respect to such intended products.
Additionally, the FDA has indicated its view that certain types of products containing CBD may not be permissible under the United States Federal Food, Drug and Cosmetic Act (“FDCA”). The FDA’s position is related to its approval of Epidiolex, a marijuana-derived prescription medicine to be available in the United States. The active ingredient in Epidiolex is CBD. On December 20, 2018, after the passage of the 2018 Farm Bill, FDA Commissioner Scott Gottlieb issued a statement in which he reiterated the FDA’s position that, among other things, the FDA requires a cannabis product (hemp-derived or otherwise) that is marketed with a claim of therapeutic benefit, or with any other disease claim, to be approved by the FDA for its intended use before it may be introduced into interstate commerce and that the FDCA prohibits introducing into interstate commerce food products containing added CBD, and marketing products containing CBD as a dietary supplement, regardless of whether the substances are hemp-derived. Although we believe our existing and planned CBD product offerings comply with applicable federal and state laws and regulations, legal proceedings alleging violations of such laws could have a material adverse effect on our business, financial condition and results of operations.
Cannabis
In the United States cannabis is regulated at the both the Federal and State levels. Notwithstanding the permissive regulatory environment of cannabis in some states, cannabis continues to be categorized as a Schedule I controlled substance under the Controlled Substances Act (“CSA”), making it illegal under Federal law in the United States to cultivate, distribute, or possess cannabis. Certain states may have enacted statutes and regulations take a permissive approach to medical and/or recreational use of cannabis, the CSA may still be enforced by U.S. Federal law enforcement officials against citizens and businesses of those states for activity that is legal under state law.
As a result of the conflicting views between state legislatures and the U.S. federal government regarding cannabis, investments in cannabis businesses in the United States are subject to inconsistent legislation and regulation. The response to this inconsistency was first addressed in August 2013 when then Deputy Attorney General James Cole authored a memorandum (the “Cole Memorandum”), addressed to all U.S. district attorneys acknowledging that notwithstanding the designation of cannabis as a controlled substance at the federal level in the United States, several U.S. states have enacted laws relating to cannabis.
The Cole Memorandum outlined certain priorities for the Department of Justice relating to the prosecution of cannabis offenses. In particular, the Cole Memorandum noted that in jurisdictions that have enacted laws legalizing cannabis in some form and that have also implemented strong and effective regulatory and enforcement systems to control the cultivation, distribution, sale and possession of cannabis, conduct in compliance with those laws and regulations is less likely to be a priority at the federal level. Notably, however, the Department of Justice has never provided specific guidelines for what regulatory and enforcement systems it deems sufficient under the Cole Memorandum standard.
In light of limited investigative and prosecutorial resources, the Cole Memorandum concluded that the Department of Justice should be focused on addressing only the most significant threats related to cannabis. States where medical cannabis had been legalized were not characterized as a high priority. On January 4, 2018, then U.S. Attorney General Jeff Sessions issued a memorandum (the “Sessions Memorandum”) that rescinded the Cole Memorandum. The Sessions Memorandum rescinded previous nationwide guidance specific to the prosecutorial authority of U.S. attorneys relative to cannabis enforcement on the basis that they are unnecessary, given the well-established principles governing federal prosecution that are already in place. Those principles require federal prosecutors deciding which cases to prosecute to weigh all relevant considerations, including federal law enforcement priorities set by the Attorney General, the seriousness of the crime, the deterrent effect of criminal prosecution and the cumulative impact of particular crimes on the community.
As a result of the Sessions Memorandum, federal prosecutors are now free to utilize their prosecutorial discretion to decide whether to prosecute cannabis activities despite the existence of state-level laws that may be inconsistent with federal prohibitions. No direction was given to federal prosecutors in the Sessions Memorandum as to the priority they should ascribe to such cannabis activities, and therefore it is uncertain how active federal prosecutors will be in relation to such activities. Due to the ambiguity of the Sessions Memorandum, there can be no assurance that the federal government will not seek to prosecute cases involving cannabis businesses that are otherwise compliant with state law.
On January 15, 2019, U.S. Attorney General nominee William P. Barr suggested a different approach to cannabis regulation than Sessions (his predecessor) during his confirmation hearing before the Senate Judiciary Committee. Mr. Barr stated that his approach to cannabis regulation would be not to upset settled expectations that have arisen as a result of the Cole Memorandum, that it would be inappropriate to upset the current situation as there has been reliance on the Cole Memorandum and that he would not be targeting companies that have relied on the Cole Memorandum and are complying with state laws with respect to the distribution and production of cannabis. While he did not offer support for cannabis legalization, Mr. Barr did emphasize the need for the U.S. Congress to clarify federal laws to address the untenable current situation which has resulted in a backdoor nullification of federal law.
Additionally, under U.S. federal law it may, under certain circumstances, be a violation of federal money laundering statutes for financial institutions to accept any proceeds from cannabis sales or any other Schedule I controlled substances. Certain Canadian banks are similarly reluctant to transact business with U.S. cannabis companies, due to the uncertain legal and regulatory framework characterizing the industry at present. Banks and other financial institutions could be prosecuted and possibly convicted of money laundering for providing services to U.S. cannabis businesses. Under U.S. federal law, banks or other financial institutions that provide a cannabis business with a checking account, debit or credit card, small business loan or any other service could be found guilty of money laundering or conspiracy. Despite these laws, in February 2014, the Financial Crimes Enforcement Network (“FCEN”) of the U.S. Treasury Department issued a memorandum (the “FCEN Memo”) providing instructions to banks seeking to provide services to cannabis-related businesses. The FCEN Memo states that in some circumstances, it is permissible for banks to provide services to cannabis-related businesses without risking prosecution for violation of federal money laundering laws. It refers to supplementary guidance that Deputy Attorney General Cole issued to federal prosecutors relating to the prosecution of money laundering offenses predicated on cannabis-related violations of the CSA. It is unclear at this time whether the Biden administration will follow the guidelines of the FCEN Memo.
As previously discussed herein, we plan on expanding our activities to include cannabis product sales and distribution in the United States if and when such activities are legalized by the Federal Government of the United States.
If federally legalized in the United States, we expect that our cannabis products will be specifically focused on cannabis for use (i) as a treatment aid; (ii) to provide relief for a large array of neurological and musculoskeletal system disorders; and (iii) as an alternative option for healthcare providers in place of prescribing opioids to patients. Offering our patients access to non-hallucinogenic and non-addictive natural remedies, under required clinical oversight policies and procedures as they relate to medicinal cannabis, combined with our existing clinic-based treatment protocols may allow us to provide a unique model that we do not believe is readily available in the United States.
Other Medical Practice Regulation
The United States healthcare industry is heavily regulated and closely scrutinized by federal, state and local governments. Comprehensive statutes and regulations govern the manner in which we may provide cannabis products, our contractual relationships with our providers, vendors and clients, our marketing activities, and other aspects of our planned operations in the event such activities become Federally legal in the United States, of which there can be no assurance. Of particular importance are:
| ● | The Federal Anti-Kickback Statute that prohibits the knowing and willful offer, payment, solicitation or receipt of any bribe, kickback, rebate or other remuneration for referring an individual, in return for ordering, leasing, purchasing or recommending or arranging for, or to induce the referral of an individual or the ordering, purchasing or leasing of items or services covered, in whole or in part, by any federal healthcare program, such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act. |
| ● | The criminal healthcare fraud provisions of the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and their implementing regulations (collectively, “HIPAA”), and related rules which prohibit knowingly and willfully executing a scheme or artifice to defraud any healthcare benefit program or falsifying, concealing or covering up a material fact or making any material false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent, to violate it to have committed a violation. |
| | |
| ● | Similar state law provisions pertaining to anti-kickback, and false claims issues. |
| | |
| ● | State laws that prohibit general business corporations, such as us, from practicing medicine, controlling physicians’ medical decisions, or engaging in certain practices such as splitting fees with physicians. |
| | |
| ● | Laws that regulate debt collection practices as applied to our debt collection practices. |
Failure to comply with these laws and other laws can result in civil and criminal penalties such as fines, damages, overpayment, recoupment, imprisonment, and loss of status. The risk of our being found in violation of these laws and regulations is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are sometimes open to a variety of interpretations. Our failure to accurately anticipate the application of these laws and regulations to our business or any other failure to comply with applicable regulatory requirements could impose liability on us and negatively affect our business. Any action against us for violation of these laws or regulations could cause us to incur significant legal expenses, divert our management’s attention from business operations, and result in adverse publicity.
Packaging, Labeling and Advertising
The processing, formulation, manufacturing, packaging, labeling, advertising and distribution of our products are subject to federal laws and regulation by one or more federal agencies, including the FDA, the FTC, HHS, the USDA and the United States Environmental Protection Agency (the “EPA”). These activities are also regulated by various state, local and international laws and agencies of the states and localities in which our products are sold. Regulations may prevent or delay the introduction, or require the reformulation, of our products, which could result in lost sales and increased costs to the Company. A regulatory agency may not accept the evidence of safety for any new ingredients that we may want to market, or may determine that a particular product or product ingredient presents an unacceptable health risk. Regulatory agencies may also determine that a particular statement of nutritional support on our products, or a statement that we want to use on our products, is an unacceptable drug claim or an unauthorized version of a food “health claim,” or that particular claims are not adequately supported by available scientific evidence. Any such regulatory determination could prevent us from marketing particular products or using certain statements on those products, which could adversely affect our sales and results of operations.
Canada
On October 17, 2018 marijuana in Canada became Federally legal.
According to Canadian Federal law, adults are legally permitted to purchase, use, possess, and grow recreational cannabis. The legal age and cultivation laws vary depending on the province. The minimum legal age is 19 in Canada except in the provinces of Alberta and Quebec where it is 18.
The maximum amount one is allowed to possess from a recreational source is 30 grams in all Canadian provinces, but in most locations, one is allowed to have larger amounts at home. In British Columbia for example, a person may possess up to 1,000 grams of marijuana at home. There is no limit to home possession in Manitoba. Those with a prescription are authorized to possess up to a five-day amount of their prescribed amount of medical cannabis.
Peru
We have been authorized to export our cannabis products from Colombia to Peru. Medical marijuana possession is legal, although recreational possession is not. In October 2017, Peru’s Congress passed a bill to legalize medical marijuana allowing cannabis oil to be produced, imported and commercialized. Additionally, although the cultivation and sale of cannabis remains illegal, the possession of small amounts of cannabis for personal usage has been decriminalized in Peru. According to Article 299 of the Peruvian Penal Code, one may legally carry eight grams of marijuana or two grams of its derivatives, for “your own and immediate consumption”.
Europe
The state of cannabis legalization in Europe varies widely among countries, with different nations adopting diverse approaches ranging from strict prohibition to varying degrees of decriminalization and medical cannabis legalization. It's important to note that the legal landscape is constantly changing.
Several European countries have legalized medical cannabis to some extent. Countries such as Germany, the United Kingdom, Italy, and the Netherlands have established medical cannabis programs, allowing patients access to cannabis-based medicines under certain conditions. Some European countries have decriminalized the possession of small amounts of cannabis for personal use. This means that while it remains illegal, the focus is often on treatment or education rather than criminal penalties. Portugal is often cited as a notable example of a country that decriminalized the possession of drugs, including cannabis, for personal use. Generally speaking, recreational cannabis use is generally illegal in most European countries. However, attitudes toward recreational use are evolving, and several countries are engaged in discussions about potential reforms. Luxembourg, for example, has announced plans to legalize recreational cannabis, becoming the first European country to do so. Hemp cultivation is legal in many European countries, with some nations having a long history of hemp cultivation for industrial purposes. Hemp-derived products, such as CBD (cannabidiol), have gained popularity and are widely available. Cannabis laws and regulations can vary significantly from one European country to another. Some countries have taken a more progressive stance, while others maintain strict prohibition. It's crucial to stay updated on the latest developments as attitudes and regulations regarding cannabis are subject to change. Countries across Europe are continually reevaluating their policies, and some may have made significant changes since my last update in January 2022. It is also interesting to note that most countries in Europe have now followed the German footsteps in requiring EU-GMP and EU-GACP certifications standards to allow for imports.
Australia
Australia has a legal medical marijuana program, and while recreational cannabis remains illegal on the federal level, the enforcement of cannabis laws and the penalties handed out can differ widely from one territory to another.
Furthermore, regardless of the legal status of the plant in Australia, it is by far the most popular illicit drug, and past year use in Australia (10.6 percent) remains far higher than the world average (3.9 percent), according to the United Nations World Drug Report for 2020.In addition, a survey published in July 2020 found that 41% of Australians support cannabis legalization, and 37% are opposed. This represents nearly double the level of support found in a 2007 survey (21%).
The country legalized medical cannabis in 2016, but patients in the country have long criticized the program as too restrictive, and access to cannabis-based medicines remains behind that of many other legal cannabis jurisdictions.
A major move in the direction of legalization took place in September 2019, when the Australian Capital Territory legalized cannabis possession (up to 50 grams) and the cultivation of up to two cannabis plants. And while voters in nearby New Zealand voted down a legalization initiative in late 2020, the cause of cannabis reform in the region is clearly gaining prominence.
Legal Proceedings
In 2020, Allied signed a purchase agreement to acquire a Nevada state license and had a 9800 sq ft. modular building built in Nevada as well. Allied signed a 25 year lease with a publicly listed company called FIORE cannabis. In December 2022, FIORE cannabis went insolvent so the business assets were seized by the debt holders of that company. The Allied building was on that land. Needless to say the lease was cancelled and Allied submitted an application to gain possession of the building back and have it be excluded from the bankruptcy proceedings of FIORE. This is in process with the state of Nevada. We are not aware of any other legal proceedings contemplated by any governmental authority or any other party involving us or our properties.
Employees
As of the date of this Offering Circular, the Company has approximately 15 contracted employees. Our wholly-owned subsidiary Allied Colombia, S.A.S. has a fluctuating casual labor compliment in Colombia depending on harvest cycles. This can be anywhere from 12-150 employees who farm and produce our cannabis products. The Company does not intend to pay a full salary to any of its executives or management at this time, but has entered into certain consulting agreements. (See “Executive Compensation”). The Company believes that its relations with its employees are good.
Item 1A. Risk Factors.
An investment in our securities involves a high degree of risk. Before making an investment decision, you should carefully consider the risks described below. Our business, financial condition, results of operations and cash flows could be materially adversely affected by any of these risks, and the market or trading price of our securities could decline due to any of these risks. In addition, please read “Disclosure Regarding Forward-Looking Statements” in this Annual Report, where we describe additional uncertainties associated with our business and the forward-looking statements included or incorporated by reference in this Annual Report. Please note that additional risks not presently known to us or that we currently deem immaterial may also impair our business and operations. In this Section, the terms the “Company,” “we”, “our” and “us” refer to Allied Corp. as well as its subsidiaries AM (Advanced Micro) Biosciences, Inc., Allied Colombia S.A.S., Allied U.S., Products, LLC and Tactical Relief LLC.
Below is a summary of material risks, uncertainties and other factors that could have a material effect on the Company and its operations:
| · | We have a history of operating losses; |
| | |
| · | Public health epidemics or outbreaks (such as the novel strain of coronavirus (COVID-19)) could adversely impact our business; |
| | |
| · | We may not be able to obtain sufficient additional capital to continue our operations; |
| | |
| · | We recently launched our cannabis products and have limited sales and marketing experience; |
| | |
| · | The cannabis industry is heavily regulated in the United States, Canada and Colombia, and if we fail to comply with these laws and governmental regulations, we could incur penalties or be required to make significant changes to our operations or even face criminal or civil sanctions; |
| | |
| · | There are conflicts in the United States between Federal and State regulations related to marijuana; |
| | |
| · | There are also provincial variations in cannabis regulation in Canada that could restrict certain of our operations; |
| | |
| · | Past and future cannabis reform legislation and other changes in the health care industry could adversely affect our business, financial condition and results of operations; |
| | |
| · | We are subject to the Canada Health Act, Canada’s National Health Insurance Program and Food and Drugs Act and analogous provisions of applicable federal, provincial, state and local laws and could face substantial penalties if we fail to comply with such laws; |
| | |
| · | Our products may not be accepted in the marketplace or achieve sales sufficient to provide sufficient profitability; |
| | |
| · | In the United States in particular and also elsewhere we are required to rely on third parties to perform many necessary services for our products, including services related to cultivation, distribution, invoicing, storage and transportation of our products; |
| | |
| · | We may be unable to consistently retain or hire third-party manufacturers, suppliers or other service providers to produce our products; |
| | |
| · | We will depend on a limited number of customers for the majority of our revenue; |
| | |
| · | There may be unanticipated delays in the development and introduction of our current and future products and/or our inability to control costs; |
| · | Significant competition; |
| | |
| · | Potential third party infringement claims; |
| | |
| · | Risks associated with our current and potential acquisitions related to costs and integration into our product line; |
| | |
| · | We are subject to significant regulatory requirements in the United States, Canada and Colombia; |
| | |
| · | Our stock is subject to dilution through future sale of shares or conversion of existing convertible securities; |
| | |
| · | A significant portion of our stock is held by our officers and directors; |
| | |
| · | We depend on our management and key personnel for our success; |
| | |
| · | The market price for our common stock has and may continue to be volatile; |
| | |
| · | Adequate protection of confidential information; |
| | |
| · | Potential litigation from competitors and claims from customers; |
| | |
| · | Our ability to adequately protect the intellectual property used to produce our products; and |
| | |
| · | Our ability to stay abreast of modified or new laws and regulations applying to our business. |
Risks Related to Our Operations
We have a history of operating losses and our management has concluded that factors raise substantial doubt about our ability to continue as a going concern and our auditor has included an explanatory paragraph relating to its ability to continue as a going concern in their audit report for the years ended August 31, 2023 and 2022.
We are a relatively newly formed company focused on cultivating, processing and supplying natural, medicinal-grade cannabis well being products to large channel distributors, and have limited operating history. We have limited financial resources and minimal operating cash flow. In addition, we do not currently have significant revenues and for the year ended August 31, 2023, had a loss of $7,924,604, and an accumulated deficit of $42,857,269. There is no guarantee that we will ever become profitable. The costs for research, product development, machinery adaptation to create our product candidates and cannabis products and/or technologies, along with marketing and selling expenses, and the general and administrative expenses, will be principal causes of our costs and/or potential losses. We may never become profitable and if we do not become profitable your investment could be harmed or lost completely.
We anticipate that we will continue to report losses and negative cash flow for the foreseeable future. We have concluded that our historical recurring losses from operations and negative cash flows from operations as well as our dependence on private equity and other financings raise substantial doubt about our ability to continue as a going concern and our auditor has included an explanatory paragraph relating to our ability to continue as a going concern in its audit report for the years ended August 31, 2023 and 2022.
The consolidated financial statements of the Company do not include any adjustments that might result from the outcome of this uncertainty. These adjustments would likely include substantial impairment of the carrying amount of our assets and potential contingent liabilities that may arise if we are unable to fulfill various operational commitments. In addition, the value of our securities, including common stock issued in this offering, would be greatly impaired. Our ability to continue as a going concern is dependent upon generating sufficient cash flow from operations and obtaining additional capital and financing, including funds to be raised in this offering. If our ability to generate cash flow from operations is delayed or reduced and we are unable to raise additional funding from other sources, we may be unable to continue in business even if this offering is successful. For further discussion about our ability to continue as a going concern and our plan for future liquidity, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Going Concern.”
We may need additional capital in the future in order to continue our operations.
We conducted private placements totaling $11,995,918 since 2019 of shares of common stock and/or warrants to fund acquisitions and operations in transactions that are exempt from the registration requirements of the Securities Act pursuant to Regulation 506(c) of common stock, which we are using for acquisitions and operations. However, if in the future we do not turn profitable or generate cash from operations and additional capital is needed to support operations, economic and market conditions may make it difficult or impossible to raise additional funds through debt or equity financings. If funds are not sufficient to support operations, we may need to pursue a financing or reduce expenditures to meet our cash requirements. If we do obtain such financing, we cannot assure that the amount or the terms of such financing will be as attractive as we may desire, and your equity interest in the company may be diluted considerably. If we are unable to obtain such financing when needed, or if the amount of such financing is not sufficient, it may be necessary for us to take significant cost saving measures or generate funding in ways that may negatively affect our business in the future. To reduce expenses, we may be forced to make personnel reductions or curtail or discontinue development programs. To generate funds, it may be necessary to monetize future royalty streams, sell intellectual property, divest of technology platforms or liquidate assets. However, there is no assurance that, if required, we will be able to generate sufficient funds or reduce spending to provide the required liquidity. Long-term capital requirements will depend on numerous factors, including, but not limited to, the status of collaborative arrangements, the progress of research and development programs and the receipt of revenues from sales of products. Our ability to achieve and/or sustain profitable operations depends on a number of factors, many of which are beyond our control, including:
| · | our ability to successfully sell our cannabis products; |
| · | Government legislation changes |
| · | our ability to successfully develop and obtain necessary national, federal, provincial, and/or state regulatory approval(s) for our own product candidates such as hemp cigarettes; |
| · | the success of our partners and distributors in selling our products; |
| · | our ability to successfully sell future products if we choose not to partner the product; |
| · | our ability to manufacture, or have manufactured, products efficiently, at the appropriate commercial scale, and with the required quality, and our ability to source and purchase from third party hemp cultivators that supplies required to produce our products; |
| · | timing of our partners’ development, regulatory and commercialization plans; |
| · | the demand for our products from current and future distribution and/or wholesale and/or retails partners; |
| · | our ability to increase and continue to outsource our raw materials, quality cannabis, and our manufacturing capacity to allow for new product introductions; |
| · | the level of product competition and of price competition; |
| · | consumer acceptance of our current and future products; |
| · | our ability to obtain reimbursement for our products from third-party payers; |
| · | our ability to develop additional commercial applications for our products; |
| · | our ability to attract the right personnel to execute our plans; |
| · | our ability to develop, maintain or acquire patent positions; |
| · | our ability to procure quality industrial hemp flowers and control these costs; and |
| · | general economic conditions in the USA and the hemp industry. |
The Coronavirus (“COVID-19”) outbreak or similar pandemics could adversely affect our operations.
The Company’s operations could be significantly adversely affected by the effects of a widespread global outbreak of a contagious disease and other unforeseen events, including the outbreak a of respiratory illness caused by COVID-19 and the related economic repercussions. We cannot accurately predict what, if any further, effects COVID-19 has had or will have on our operations and the ability of others to meet their obligations with the Company, including uncertainties relating to the ultimate geographic spread of the virus, the severity of the disease, the duration of the outbreak, and the length of travel and quarantine restrictions imposed by governments of affected countries. We were required to postpone distribution of our MaXXa© product as a direct result of COVID-19. In addition, a significant outbreak of contagious diseases in the human population could result in a widespread health crisis that could adversely affect the economies and financial markets of many countries, resulting in an economic downturn that could further affect the Company’s operations and ability to finance its operations.
We launched our cannabis products in 2019 in an initially limited fashion and as a company, we have limited sales and marketing experience.
We launched marketing for our cannabis products in 2019, and although we have hired highly qualified personnel with specialized expertise, as a company, we have limited experience commercializing cannabis products on our own. In order to commercialize the cannabis products business, we have to build our sales, marketing, distribution, managerial and other non-technical capabilities and make arrangements with third parties to perform these services when needed. We may have to hire sales representatives and district managers to fill sales territories. To the extent we are relying on third parties to commercialize our business, we may receive less revenues or incur more expenses than if we had commercialized the products ourselves. In addition, we may have limited control over the sales efforts of any third parties involved in our commercialization efforts. If we are unable to successfully implement our commercial plans and drive adoption by patients and physicians of our products through our sales, marketing and commercialization efforts, or if our partners fail to successfully commercialize our products, then we may not be able to generate sustainable revenues from product sales which will have a material adverse effect on our business and future product opportunities. Similarly, we may not be successful in establishing the necessary commercial infrastructure, including sales representatives, wholesale distributors, legal and regulatory affairs teams. The establishment and development of commercialization capabilities to market our products has been and will continue to be expensive and time-consuming. As we continue to develop these capabilities, we will have to compete with other hemp companies to recruit, hire, train and retain sales and marketing personnel. If we have underestimated the necessary sales and marketing capabilities or have not established the necessary infrastructure to support successful commercialization, or if our efforts to do so take more time and expense than anticipated, our ability to market and sell our products may be adversely affected.
Recent and future acquisitions and strategic investments could be difficult to integrate, divert the attention of key management personnel, disrupt our business, dilute shareholder value, and harm our results of operations and financial condition.
We acquired Medicolombia S.A.S. (renamed Allied Colombia S.A.S.), and we may in the future seek to acquire or invest in, businesses, products, or technologies that we believe could complement our operations or expand our breadth, enhance our capabilities, or otherwise offer growth opportunities. Our diversity of product offerings may not be successful. While our growth strategy includes broadening our service and product offerings, implementing an aggressive marketing plan and employing product diversification, there can be no assurance that our systems, procedures and controls will be adequate to support our operations as they expand. We cannot assure you that our existing personnel, systems, procedures or controls will be adequate to support our operations in the future or that we will be able to successfully implement appropriate measures consistent with our growth strategy. As part of our planned growth and diversified product offerings, we may have to implement new operational and financial systems, procedures and controls to expand, train and manage our employee base, and maintain close coordination among our staff. We cannot guarantee that we will be able to do so, or that if we are able to do so, we will be able to effectively integrate them into our existing staff and systems. Additionally, the integration of our acquisitions and pursuit of potential future acquisitions may divert the attention of management and cause us to incur various expenses in identifying, investigating, and pursuing suitable acquisitions, whether or not they are consummated. Any acquisition, investment or business relationship may result in unforeseen operating difficulties and expenditures. In addition, we have limited experience in acquiring other businesses. Specifically, we may not successfully evaluate or utilize the acquired products, assets or personnel, or accurately forecast the financial impact of an acquisition transaction, including accounting charges. Moreover, the anticipated benefits of any acquisition, investment, or business relationship may not be realized, or we may be exposed to unknown risks or liabilities of our acquisitions.
We may not be able to find and identify desirable acquisition targets or we may not be successful in entering into an agreement with any one target. Acquisitions could also result in dilutive issuances of equity securities or the incurrence of debt, which could harm our results of operations. In addition, if an acquired business fails to meet our expectations, our business, results of operations, and financial condition may suffer. In some cases, minority shareholders may exist in certain of our non-wholly-owned acquisitions (for businesses we do not purchase as an 100% owned subsidiary) and may retain minority shareholder rights which could make a future change of control or corporate approvals for actions more difficult to achieve and/or more costly.
We also make strategic investments in early-stage companies developing products or technologies that we believe could complement our business or expand our breadth, enhance our technical capabilities, or otherwise offer growth opportunities. These investments may be in early-stage private companies for restricted stock. Such investments are generally illiquid and may never generate value. Further, the companies in which we invest may not succeed, and our investments would lose their value.
Certain conditions or events could disrupt the Company’s supply chains, disrupt operations, and increase operating expenses.
Conditions or events including, but not limited to, the following could disrupt the Company’s supply chains and in particular its ability to deliver its products, interrupt operations at its facilities, increase operating expenses, resulting in loss of sales, delayed performance of contractual obligations or require additional expenditures to be incurred: (i) extraordinary weather conditions or natural disasters such as hurricanes, tornadoes, floods, fires, extreme heat, earthquakes, etc.; (ii) a local, regional, national or international outbreak of a contagious disease, including the COVID-19 coronavirus, Middle East Respiratory Syndrome, Severe Acute Respiratory Syndrome, H1N1 influenza virus, avian flu, or any other similar illness could result in a general or acute decline in economic activity; (iii) political instability, social and labor unrest, war or terrorism; or (iv) interruptions in the availability of basic commercial and social services and infrastructure including power and water shortages, and shipping and freight forwarding services including via air, sea, rail and road.
We will depend on a limited number of customers for the majority of our revenue, and the loss of any one of these customers could substantially reduce our revenue and impact our liquidity.
The loss of any significant customers or partners or reduction in our business activities could cause our revenues to decrease significantly and increase our continuing losses from operations. If our cannabis products are not successful and we cannot broaden our customer base, we will continue to depend on a few customers for the majority of our revenues. Additionally, if we are unable to negotiate favorable business terms with these customers in the future, our revenues and gross profits may be insufficient to allow us to achieve and/or sustain profitability or continue operations.
Demand for cannabis and derivative products could be adversely affected and significantly influenced by scientific research or findings, regulatory proceedings, litigation, media attention or other research findings.
Consumer perceptions regarding legality, morality, consumption, safety, efficacy and quality of medicinal cannabis are mixed and evolving and can be significantly influenced by scientific research or findings, regulatory investigations, litigation, media attention and other publicity regarding the consumption of medicinal cannabis products. There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favorable to the medicinal cannabis market or any particular product, or consistent with earlier publicity. Future research reports, findings, regulatory proceedings, litigation, media attention or other publicity that are perceived as less favorable than, or that question, earlier research reports, findings or publicity, could have a material adverse effect on the demand for medicinal cannabis and on our business, results of operations, financial condition and cash flows. Further, adverse publicity reports or other media attention regarding cannabis in general or associating the consumption of medicinal cannabis with illness or other negative effects or events, could have such a material adverse effect. Public opinion and support for medicinal cannabis use has traditionally been inconsistent and varies from jurisdiction to jurisdiction. Our ability to gain and increase market acceptance of our business may require substantial expenditures on investor relations, strategic relationships and marketing initiatives. There can be no assurance that such initiatives will be successful and their failure to materialize into significant demand may have an adverse effect on our financial condition.
Damage to the Company’s reputation can be the result of the actual or perceived occurrence of any number of events, and could include any negative publicity, whether such publicity is accurate or not.
The increased usage of social media and other web-based tools used to generate, publish and discuss user-generated content and to connect with other users has made it increasingly easier for individuals and groups to communicate and share opinions and views regarding the Company and its activities, whether true or not. Although the Company believes that it operates in a manner that is respectful to all stakeholders and that it takes pride in protecting its image and reputation, it does not ultimately have direct control over how it is perceived by others. Reputational loss may result in decreased ability to enter into new customer, distributor or supplier relationships, retain existing customers, distributors or suppliers, reduced investor confidence and access to capital, increased challenges in developing and maintaining community relations and an impediment to our overall ability to advance our projects, thereby having a material adverse effect on our financial performance, financial condition, cash flows and growth prospects.
We are subject to the inherent risks involved with product recalls.
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labelling disclosure. If any of our products are recalled due to an alleged product defect or for any other reason, we could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall. We may lose a significant amount of sales and may not be able to replace those sales at an acceptable margin, or at all. In addition, a product recall may require significant management attention. There can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. Additionally, if our products are subject to recall, our reputation could be harmed. A recall for any of the foregoing reasons could lead to decreased demand for our products and could have a material adverse effect on our results of operations and financial condition. Additionally, product recalls may lead to increased scrutiny of our operations by regulatory agencies, requiring further management attention, potential loss of applicable licenses, and potential legal fees and other expenses.
The Company’s products could have unknown side effects.
If the products the Company sells are not perceived to have the effects intended by the end user, its business may suffer and the business may be subject to products liability or other legal actions. Many of the Company’s products contain innovative ingredients or combinations of ingredients. There is little long-term data available with respect to efficacy, unknown side effects and/or interaction with individual human biochemistry, or interaction with other drugs. Moreover, there is little long-term data available with respect to efficacy, unknown side effects and/or its interaction with individual animal biochemistry. As a result, the Company’s products could have certain side effects if not taken as directed or if taken by an end user that has certain known or unknown medical conditions.
The Company may be unable to anticipate changes in its potential client requirements that could make the Company’s existing products and services obsolete. The Company’s success will depend, in part, on its ability to continue to enhance its product and service offerings so as to address the increasing sophistication and varied needs of the market and respond to technological and regulatory changes and emerging industry standards and practices on a timely and cost-effective basis.
Research regarding the medical benefits, viability, safety, efficacy, use and social acceptance of cannabis remains in early stages.
There have been relatively few clinical trials on the benefits of cannabis. Although the Company believes that the articles, reports and studies support its beliefs regarding the medical benefits, viability, safety, efficacy, dosing and social acceptance of cannabis, future research and clinical trials may prove such statements to be incorrect, or could raise concerns regarding, and perceptions relating to, cannabis. Given these risks, uncertainties and assumptions, investors should not place undue reliance on such articles and reports. Future research studies and clinical trials may draw opposing conclusions to those stated herein or reach negative conclusions related to medical cannabis, which could have a material adverse effect on the demand for the Company’s products, which could result in a material adverse effect on our business, financial condition and results of operations or prospects.
The seasonal trends in our business create variability in our financial and operating results.
Our financial and operating results are subject to seasonal and quarterly variations in our net revenue and operating income and, as a result, our quarterly results may fluctuate and could be below expectations.
We anticipate that our business will realize a disproportionate amount of net revenue and earnings in the third and fourth quarter as a result of the holiday season. If we experience lower than expected net revenue during any third or fourth quarter, it may have disproportionately large effects on our operating results and financial condition for that year. Any factors that harm our third or fourth quarter operating results, including disruptions in our brands or our supply chains or unfavorable economic conditions, could have a disproportionate effect on our results of operations and our financial condition for our entire fiscal year.
The Company may not be able to maintain effective quality control systems.
The Company may not be able to maintain an effective quality control system. The Company ascribes its early successes, in part, on its commitment to product quality and its effective quality control system. The effectiveness of the Company’s quality control system and its ability to obtain or maintain regulatory certification with respect to its manufacturing, processing and testing facilities depend on a number of factors, including the design of its quality control procedures, training programs, and its ability to ensure that its employees adhere to the Company’s policies and procedures. The Company also depends on service providers such as toll manufacturers and contract laboratories to manufacture, process or test its products, that are subject to regulatory certification requirements.
We expect that regulatory agencies will periodically inspect our and our service providers’ facilities to evaluate compliance with applicable requirements. Failure to comply with these requirements may subject us or our service providers to possible regulatory enforcement actions. Any failure or deterioration of the Company’s or its service providers’ quality control systems, including loss of any required certification, may have a material adverse effect on the Company’s business, results of operations and financial condition.
Energy prices and supply may be subject to change or curtailment due to new laws or regulations, imposition of new taxes or tariffs, interruptions in production by suppliers, imposition of restrictions on energy supply by government, worldwide price levels and market conditions.
The Company requires diesel and electric energy and other resources for its cultivation and harvest activities and for transportation of cannabis. the Company relies upon third parties for its supply of energy resources used in its operations. The prices for and availability of energy resources may be subject to change or curtailment, respectively, due to, among other things, new laws or regulations, imposition of new taxes or tariffs, interruptions in production by suppliers, imposition of restrictions on energy supply by government, worldwide price levels and market conditions. Although the Company attempts to mitigate the effects of fuel shortages, electricity outages and cost increases, the Company’s operations will continue to depend on external suppliers of fuel and electricity. If energy supply is cut for an extended period and the Company is unable to find replacement sources at comparable prices, or at all, the Company’s business, financial condition and results of operations could be materially and adversely affected.
There are risks associated with the regulatory regime and permitting requirements of our operations.
Achievement of our business objectives is contingent, in part, upon compliance with regulatory requirements enacted by governmental authorities and obtaining all regulatory approvals, where necessary, for the sale of our products. We may not be able to obtain or maintain the necessary licenses, permits, quotas, authorizations or accreditations to operate our business, or may only be able to do so at great cost. We cannot predict the time required to secure all appropriate regulatory approvals for our products, or the extent of testing and documentation that may be required by local governmental authorities.
Our officers and directors must rely, to a great extent, on Colombian legal counsel and local consultants retained in order to keep abreast of material legal, regulatory and governmental developments as they pertain to and affect our business operations, and to assist us with governmental relations. We must rely, to some extent, on those members of management and the Board who have previous experience working and conducting business in Colombia in order to enhance our understanding of and appreciation for the local business culture and practices in Colombia.
We also rely on the advice of local experts and professionals in connection with any current and new regulations that develop in respect of banking, financing and tax matters in Colombia. Any developments or changes in such legal, regulatory or governmental requirements or in local business practices in Colombia are beyond our control and may adversely affect our business.
We will incur ongoing costs and obligations related to regulatory compliance. Failure to comply with applicable laws, regulations and permitting requirements may result in enforcement actions thereunder, including orders issued by regulatory or judicial authorities causing operations to cease or be curtailed, and may include corrective measures requiring capital expenditures, installation of additional equipment, or remedial actions. We may be required to compensate those suffering loss or damage by reason of our operations and may have civil or criminal fines or penalties imposed for violations of applicable laws or regulations. In addition, changes in regulations, more vigorous enforcement thereof or other unanticipated events could require extensive changes to our operations, increased compliance costs or give rise to material liabilities, which could have a material adverse effect on our business, results of operations and financial condition.
The cannabinoid industry faces strong opposition and may face similar opposition in other jurisdictions in which we operate.
Many political and social organizations oppose hemp and cannabis and their legalization, and many people, even those who support legalization, oppose the sale of hemp and cannabis in their geographies. Our business will need support from local governments, industry participants, consumers and residents to be successful. Additionally, there are large, well-funded businesses that may have a strong opposition to the cannabis industry. For example, the pharmaceutical and alcohol industries have traditionally opposed cannabis legalization. Any efforts by these or other industries opposed to cannabis would make in halting or impeding the cannabis industry could have detrimental effects on our business.
We are subject to the risks inherent in an agricultural business.
Our business involves the growing of cannabis, which is an agricultural product. The occurrence of severe adverse weather conditions, especially droughts, fires, storms or floods is unpredictable and may have a potentially devastating impact on agricultural production and may otherwise adversely affect the supply of cannabis. Adverse weather conditions may be exacerbated by the effects of climate change and may result in the introduction and increased frequency of pests and diseases. The effects of severe adverse weather conditions may reduce our yields or require us to increase our level of investment to maintain yields. Additionally, higher than average temperatures and rainfall can contribute to an increased presence of insects and pests, which could negatively affect cannabis crops. Future droughts could reduce the yield and quality of our cannabis production, which could materially and adversely affect our business, financial condition and results of operations.
The occurrence and effects of plant disease, insects and pests can be unpredictable and devastating to agricultural production, potentially rendering all or a substantial portion of the affected harvests unsuitable for sale. Even when only a portion of the production is damaged, our results of operations could be adversely affected because all or a substantial portion of the production costs may have been incurred. Although some plant diseases are treatable, the cost of treatment can be high and such events could adversely affect our operating results and financial condition. Furthermore, if we fail to control a given plant disease and the production is threatened, we may be unable to adequately supply our customers, which could adversely affect our business, financial condition and results of operations. There can be no assurance that natural elements will not have a material adverse effect on production.
Our operations could be materially and adversely affected if the supply of cannabis seeds is ceased or delayed and we do not find replacement suppliers and obtain all necessary authorizations.
If for any reason the supply of cannabis seeds is ceased or delayed, we would have to seek alternate suppliers and obtain all necessary authorization for the new seeds. If replacement seeds cannot be obtained at comparable prices, or at all, or if the necessary authorizations are not obtained, our business, financial condition and results of operations would be materially and adversely affected.
Many of our competitors have greater resources that may enable them to compete more effectively than us in the cannabis industry.
The industry in which we operate is subject to intense and increasing competition. Some of our competitors have a longer operating history and greater capital resources and facilities, which may enable them to compete more effectively in this market. We expect to face additional competition from existing licensees and new market entrants who are granted licenses in Colombia, who are not yet active in the industry. If a significant number of new licenses are granted in the near term, we may experience increased competition for market share and may experience downward pricing pressure on our products as new entrants increase production. Such competition may cause us to encounter difficulties in generating revenues and market share, and in positioning our products in the market. If we are unable to successfully compete with existing companies and new entrants to the market, our lack of competitive advantage will have a negative effect on our business and financial condition.
The legalization of adult-use, recreational cannabis may reduce sales of medical cannabis.
Legalization of the sale to adults of recreational, non-medical cannabis in any country may increase competition in the medical cannabis market. We may not be able to achieve our business plan in a highly competitive market where recreational, adult-use cannabis is legal, or the market may experience a drop in the price of cannabis and cannabis products over time, decreasing our profit margins.
The Company is reliant on third party transportation services and importation services to deliver its products to customers.
The Company relies on third party transportation services and importation services to deliver its products to its customers. The Company is exposed to the inherent risks associated with relying on third party transportation service-providers, including logistical problems, delays, loss or theft of product and increased shipping and insurance costs. Any delay in transporting the product, breach of security or loss of product, could have a material adverse effect on the Company’s business, financial performance and results of operations. Further, any breach of security and loss of product during transport could affect the Company’s status as a licensed producer in Colombia.
The Company is dependent on suppliers to supply equipment, parts and components for the operation of its business.
The Company’s ability to compete and grow will be dependent upon having access, at a reasonable cost and in a timely manner, to equipment, parts and components. No assurances can be given that the Company will be successful in maintaining the required supply of equipment, parts and components. It is also possible that the final costs of the major equipment contemplated by capital expenditure programs may be significantly greater than anticipated or available, in which circumstance there could be a materially adverse effect on the Company’s financial results.
We may not be able to establish and maintain bank accounts in certain countries.
There is a risk that banking institutions in countries where we operate will not open accounts for us or will not accept payments or deposits from proceeds related to the cannabis industry. Such risks could increase our costs or prevent us from expanding into certain jurisdictions. Banks in the United States frequently refuse to do business with cannabis related companies because of the current federal law prohibiting cannabis sales.
The Company may be subject to cyber-security and privacy risks that could disrupt its operations and expose the Company to financial losses, contractual losses, liability, reputational damage and additional expense.
The Company may be subject to risks related to our information technology systems, including cyber-attacks, malware, ransomware and phishing attacks that could target our intellectual property, trade secrets, financial information, personal information of our employees, customers and patients, including sensitive personal health information. The occurrence of such an attack could disrupt our operations and expose the Company to financial losses, contractual damages, liability under labor and privacy laws, reputational damage and additional expenses. We have implemented security measures to protect our data and information technology systems; however, such measures may not be effective in preventing cyber-attacks. We may be required to allocate additional resources to implement additional preventative measures including significant investments in information technology systems. A serious cyber-security breach could have a material adverse effect on our business, financial condition and results of operations.
The Company may collect and store certain personal information about customers and is responsible for protecting such information from privacy breaches. A privacy breach may occur through procedural or process failure, information technology malfunction, or deliberate unauthorized intrusions. In addition, theft of data is an ongoing risk whether perpetrated via employee collusion or negligence or through deliberate cyber-attack. Any such privacy breach or theft could have a material adverse effect on the Company’s business, financial condition and results of operations. If the Company were found to be in violation of privacy or security rules or other laws protecting the confidentiality of information, the Company could be subject to sanctions and civil or criminal penalties, which could increase its liabilities, harm its reputation and have a material adverse effect on the Company’s business, financial condition and results of operations.
The Company may incur significant costs to defend its intellectual property and other proprietary rights.
The ownership and protection of trademarks, patents, trade secrets and intellectual property rights are significant aspects of the Company’s future success. Unauthorized parties may attempt to replicate or otherwise obtain and use the Company’s products and technology. Policing the unauthorized use of the Company’s current or future trademarks, patents, trade secrets or intellectual property rights could be difficult, expensive, time-consuming and unpredictable, as may be enforcing these rights against unauthorized use by others.
In addition, other parties may claim that the Company’s products infringe on their proprietary and perhaps patent protected rights. Such claims, regardless of their merit, may result in the expenditure of significant financial and managerial resources, legal fees, injunctions, temporary restraining orders and/or require the payment of damages. As well, the Company may need to obtain licenses from third parties who allege that the Company has infringed on their lawful rights. Such licenses may not be available on terms acceptable to the Company or at all. In addition, the Company may not be able to obtain or utilize on terms that are favorable to it, or at all, licenses or other rights with respect to intellectual property that it does not own.
Risks Related to Operations in Colombia
We are reliant on certain licenses and authorizations to operate in Colombia.
Our ability to grow, store and sell cannabis in Colombia is dependent on our ability to sustain and/or obtain the necessary licenses and authorizations by certain authorities in Colombia. To date, we have received the licenses we need to operate our facilities in Colombia. The effects of the compliance regime, any delays in obtaining, or failure to obtain or keep the regulatory approvals may significantly delay or impair the development of markets, products and sales initiatives and could have a material adverse effect on our business, results of operations and financial condition.
The licenses and authorizations are subject to ongoing compliance and reporting requirements and our ability to obtain, sustain or renew any such licenses and authorizations on acceptable terms is subject to changes in regulations and policies and to the discretion of the applicable authorities or other governmental agencies in Colombia and potentially in other foreign jurisdictions. Failure to comply with the requirements of the licenses or authorizations or any failure to maintain the licenses or authorizations would have a material adverse effect on our business, financial condition and operating results.
Although we believe that we will meet the requirements to obtain, sustain or renew the necessary licenses and authorizations, there can be no guarantee that the applicable authorities will issue these licenses or authorizations. Should the authorities fail to issue the necessary licenses or authorizations, we may be curtailed or prohibited from the production and/or distribution of cannabis or from proceeding with the development of our operations as currently proposed and our business, results of operations and financial condition may be materially adversely affected.
Restrictions or regulations concerning changes in corporate structure may discourage transactions that otherwise could involve payment of a premium over prevailing market process for our securities.
Colombian cannabis licenses are granted on a non-transferable, non-exchangeable and non-assignable basis. Any breach of this restriction may result in the revocation of the license. While there are no specific regulations or restrictions regarding the effects of a change in control, modification of the corporate structure, issuance of shares, or any changes in holders or final beneficiaries on the cannabis licenses, these restrictions may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for our securities.
Our cultivation operations are located in Colombia, which may make it more difficult for investors to understand and predict how changing market and economic conditions will affect our financial results.
Our cultivation operations are located in Colombia and, consequently, are subject to the economic, political and tax conditions prevalent in that country. The economic conditions in Colombia are subject to different growth expectations, market weaknesses and business practices than economic conditions in other markets. We may not be able to predict how changing market conditions in Colombia will affect our financial results.
Currently Colombia’s long-term foreign currency sovereign credit ratings were affirmed “BB+” by S&P and “BB+” by Fitch, two of the main rating agencies worldwide. The Colombian economy is expected to experience some modest recovery in growth, along with a decrease in the current account deficit and a marginal increase in debt in the coming years. The stable outlook reflects their expectation that Colombia’s established political institutions and track record of consensus on key economic policies will contribute to economic stability and continuity over the coming two to three years.
Colombia’s economy, like most Latin-American countries, continues to suffer from the effects of lower commodity prices, mainly oil, reflected in its elevated level of external debt. Even though the country has taken measures to stabilize the economy, it is uncertain how these measures will be perceived and if the intended goal of increasing investor’s confidence will be achieved.
Economic and political conditions in Colombia may have an adverse effect on our financial condition and results of operations.
Our cultivation operations are located in Colombia. Consequently, our financial condition and results of operations depend significantly on macroeconomic and political conditions prevailing in Colombia. Decreases in the growth rate, periods of negative growth, increases in inflation, changes in law, regulation, policy, or future judicial rulings and interpretations of policies involving exchange controls and other matters such as (but not limited to) currency depreciation, inflation, interest rates, taxation, banking laws and regulations and other political or economic developments in or affecting Colombia may affect the overall business environment and may, in turn, adversely affect our financial condition and results of operations in the future. The Colombian government frequently intervenes in Colombia’s economy and from time to time makes significant changes in monetary, fiscal and regulatory policy. Our business and results of operations or financial condition may be adversely affected by changes in government or fiscal policies, and other political, diplomatic, social and economic developments that may affect Colombia. We cannot predict what policies the Colombian government will adopt and whether those policies would have a negative effect on the Colombian economy or on our business and financial performance in the future.
We cannot assure you whether current stability in the Colombian economy will be sustained. If the condition of the Colombian economy were to deteriorate, we would likely be adversely affected.
Colombia is experiencing substantial inflation resulting in the Company’s costs in the Colombian peso increasing significantly.
Colombia has in the past experienced double-digit rates of inflation. Colombia’s inflation rate is currently 10.42% and is expected to be 9.5% by the end of calendar 2023. If Colombia experiences substantial inflation in the future, the Company’s costs in Colombian peso terms will increase significantly, subject to movements in applicable exchange rates. Inflationary pressures may also curtail the Company’s ability to access global financial markets in the longer term and its ability to fund planned capital expenditures, and could materially adversely affect the Company’s business, financial condition and results of operations. The Colombian government’s response to inflation or other significant macro-economic pressures may include the introduction of policies or other measures that could increase the Company’s costs, reduce operating margins and materially adversely affect its business, financial condition and results of operations.
Colombia has experienced and continues to experience internal security issues that have had or could have a negative effect on the Colombian economy and our financial condition.
Colombia is subject to sustained internal security issues, primarily due to the activities of guerrilla groups, such as dissidents from the former Revolutionary Armed Forces of Colombia (Fuerzas Armadas Revolucionarias de Colombia), or “FARC,” the National Liberation Army (Ejército de Liberación Nacional), or “ELN,” paramilitary groups, drug cartels and criminal gangs (Bacrim). In remote regions of the country with minimal governmental presence, these groups have exerted influence over the local population and funded their activities by protecting and rendering services to drug traffickers and participating in drug trafficking activities. Even though the Colombian government’s policies have reduced guerilla presence and criminal activity, particularly in the form of terrorist attacks, homicides, kidnappings and extortion, such activity persists in Colombia, and possible escalation of such activity and the effects associated with them have had and may have in the future a negative effect on the Colombian economy and on us, including on our employees, results of operations and financial condition. The Colombian government commenced peace talks with the FARC in August 2012, and peace negotiations with the ELN began in November 2016. The Colombian government and the FARC signed a peace deal on September 26, 2016, which was amended after voters rejected it in the referendum held on October 2, 2016. The new agreement was signed on November 24, 2016 and was ratified by the Colombian Congress on November 30, 2016 and is being implemented after four years of negotiations. Pursuant to the peace agreements negotiated between the FARC and the Colombian government in 2016, the FARC occupies five seats in the Colombian Senate and five seats in the Colombian House of Representatives. The deal clarifies protection to private property, is expected to increase the government’s presence in rural areas and bans former rebels from running for office in certain newly created congressional districts in post-conflict zones. Colombia may experience an increase in internal security issues, drug-related crime and guerilla and paramilitary activities, which may have a negative effect on the Colombian economy. Our business or financial condition could be adversely affected by rapidly changing economic or social conditions, including the Colombian government’s response to implementation of the agreement with FARC and ongoing peace negotiations, if any, which may result in legislation that increases the tax burden of Colombian companies.
Despite efforts by the Colombian government, drug-related crime, guerrilla paramilitary activity and criminal bands continue to exist in Colombia, and allegations have surfaced regarding members of the Colombian congress and other government officials having ties to guerilla and paramilitary groups. On January 17, 2019, a car with explosives burst through the gates at a police academy in Bogotá resulting in 21 people dead and many injured. The Colombian Defense Minister confirmed that the terrorist attack was perpetrated by the ELN. Any possible escalation in the violence associated with this terrorist attack and/or these activities may have a negative effect on the Colombian economy. In addition, the current administration has not honored the peace protocols to be applied in the event of a suspension of peace negotiations entered into by the prior administration, on the grounds that these protocols are only binding to the administration that agreed to them. This situation could result in escalated violence by the ELN and may have a negative effect on the credibility of the Colombian government which could in turn have a negative effect on the Colombian economy. Any terrorist activity in Colombia generally may disrupt supply chains and discourage qualified individuals from being involved with our operations.
Political and economic instability in the region may affect the Colombian economy and, consequently, our results of operations and financial condition.
Some of Colombia’s neighboring countries, particularly Venezuela, have experienced and continue to experience periods of political and economic instability. According to figures from the United Nations, more than two million Venezuelans have emigrated amid food and medicine shortages and profound political divisions in their country. Approximately half of those migrants have opted to live in Colombia, and many have arrived with only what they could carry. Providing migrants with access to healthcare, utilities and education may have a negative effect on Colombia’s economy if the Colombian government is not able to respond adequately to legalize migrants, generate programs to help them find formal jobs, and increase tax revenue and consumption.
Moreover, diplomatic relations with Venezuela and Ecuador have from time to time been tense and affected by events surrounding the Colombian military forces’ confrontations with guerilla groups, particularly on Colombia’s borders with each of Venezuela and Ecuador. More recently, the Colombian government joined an international campaign against Nicolás Maduro asking him to relinquish power, which has further increased diplomatic tensions with Venezuela.
On November 19, 2012, the International Court of Justice placed a sizeable area of the Caribbean Sea within Nicaragua’s exclusive economic zone, which until then had been deemed by Colombia as part of its own exclusive economic zone. A worsening of diplomatic relations between Colombia and Nicaragua involving the disputed waters could result in the Nicaraguan government taking measures, or a reaction among the Nicaraguan public, which would be detrimental to Colombian-owned interests in that country.
Further economic and political instability in Colombia’s neighboring countries or any future deterioration in relations with Venezuela, Ecuador, Nicaragua and other countries in the region may result in the closing of borders, the imposition of trade barriers and a breakdown of diplomatic ties, or a negative effect on Colombia’s trade balance, economy and general security situation, which may adversely affect our results of operations and financial condition.
Finally, political conditions such as changes in the United States policies related to immigration and remittances could affect the regions in which we operate. Economic conditions in the United States and the region generally may be affected by the new United States-Mexico-Canada Agreement. This could have an indirect effect on the Colombian economy and other countries in which we may operate.
Any additional taxes resulting from changes to tax regulations or the interpretation thereof in Colombia or other countries where we operate, could adversely affect our consolidated results.
Uncertainty relating to tax legislation poses a constant risk to us. Colombian national authorities have levied new taxes in recent years. Changes in legislation, regulation and jurisprudence can affect tax burdens by increasing tax rates and fees, creating new taxes, limiting stated expenses and deductions, and eliminating incentives and non-taxed income.
Additional tax regulations could be implemented that could require us to make additional tax payments, negatively affecting our financial condition, results of operation, and cash flow. In addition, either national or local taxing authorities may not interpret tax regulations in the same way that we do. Differing interpretations could result in future tax litigation and associated costs.
Risks Related to Our Regulatory Framework
United States Federal regulation and enforcement may adversely affect the implementation of medical Cannabis and/or Cannabis adult use laws and regulations may negatively impact our revenues and profits.
Legislation in the United States continues to evolve throughout 2023 relative to laws regulating marijuana. Subsequent to the 2023 elections, there are currently 46 states in the United States, plus the District of Columbia that have laws and/or regulations that recognize in one form or another legitimate medical uses for cannabis and consumer use of cannabis in connection with medical treatment. More states are considering permitting cannabis use. Although it has not been passed, Congress recently presented legislation which would decriminalize marijuana use federally and relegate legalization to state law.
Around the world there are countries who are enacting medical and/or adult use cannabis regulations almost weekly. Conversely, currently under the Controlled Substance Act (the “CSA”), the policy and regulations of the Federal government and its agencies is that cannabis has no medical benefit and a range of activities including cultivation and use of cannabis for personal use is prohibited. Until Congress amends the CSA with respect to medical marijuana or there is an outcome of the current lawsuit against the un-constitutional application of cannabis in the CSA, there is a risk that federal authorities may enforce current federal law, and we may be deemed to be facilitating the selling or distribution of drug paraphernalia in violation of federal law. Active enforcement of the current federal regulatory position on cannabis may thus indirectly and adversely affect revenues and profits of the Company. The risk of strict enforcement of the CSA in light of congressional activity, judicial holdings and stated federal policy remains uncertain.
The DOJ (Department of Justice) has not historically devoted resources to prosecuting individuals whose conduct is limited to possession of small amounts of marijuana for use on private property but relied on state and local law enforcement to address marijuana activity. In the event the DOJ reverses stated policy and begins strict enforcement of the CSA in states that have laws legalizing medical marijuana and recreational marijuana in small amounts, there may be a direct and adverse impact to our revenue and profits.
There are conflicts in the United States between Federal and State regulations related to marijuana.
Federal regulation and enforcement may adversely affect the implementation of adult use/medical Cannabis laws and regulations may negatively impact our revenues and profits. As of the date of this Annual Report, 46 states and the District of Columbia allow its citizens to use medical marijuana. The state laws are in conflict with the federal Controlled Substances Act, which makes marijuana use and possession illegal on a national level. Each of the Obama, Trump and Biden administrations effectively stated that it is not an efficient use of resources to direct law federal law enforcement agencies to prosecute those lawfully abiding by state designated laws allowing the use and distribution of medical cannabis. In October 2022, Biden pardoned all persons convicted of minor marijuana infractions. In May 2017, Congress unveiled their new budget bill and as such, lawmakers included a provision, known as the Rohrabacher-Farr amendment, that allows states to carry on with crafting their own medical marijuana policies without fear of federal intervention. This bill was passed and as a result, no federal monies have been approved or appropriated to be used to enforce federal law in these cannabis program participating states.
In 2020, the Democrats through President Biden called for decriminalizing and rescheduling marijuana through executive action, with support for legalizing medical marijuana and expunging past criminal convictions for cannabis-related offenses all on the table.
Investors should understand that there is no guarantee that current or future administrations will not attempt to change this again in the future. Additionally, any new administration that follows could change this policy and decide to enforce the federal laws strongly. Any such change in the federal government’s enforcement of current federal laws could cause significant financial damage to the Company and its shareholders. While we will not harvest, distribute, or sell cannabis directly, we may be irreparably harmed by a change in enforcement by the Federal or state governments.
Cannabis remains illegal under United States federal law. It is a schedule-I controlled substance. Even in those jurisdictions in which the use of medical marijuana has been legalized at the state level, its prescription is a violation of federal law. The United States Supreme Court has ruled in United States v. Oakland Cannabis Buyers’ Coop. and Gonzales v. Raich that it is the federal government that has the right to regulate and criminalize cannabis, even for medical purposes. Therefore, federal law criminalizing the use of marijuana trumps state laws that legalize its use for medicinal purposes. At present, the states are standing tall against the federal government, maintaining existing laws and passing new ones in this area. A change in the federal attitude towards enforcement could have a negative effect on the industry, potentially ending it entirely. While we are not insulated from economic risk if such a change were to occur, we believe that by virtue of the fact that we do not sell, or produce Cannabis or Cannabis related products, our shareholders and we should be insulated from federal prosecution or harassment. However, the growers and sellers of adult use and medical cannabis are our primary customers, and if this industry were unable to operate, we would lose substantially all of our potential clients, which would have a negative impact on our business, operations, and financial condition.
Laws and regulations affecting the Cannabis industry are constantly changing, which could detrimentally affect our proposed operations. Local, state, and federal Cannabis laws and regulations are broad in scope and subject to evolving interpretations, which could require us to incur substantial costs associated with compliance or alter our business plan. In addition, violations of these laws, or allegations of such violations, could disrupt our business and result in a material adverse effect on our operations. In addition, it is possible that regulations may be enacted in the future that will be directly applicable to our business. We cannot predict the nature of any future laws, regulations, interpretations, or applications, nor can we determine what effect additional governmental regulations or administrative policies and procedures, when and if promulgated, could have on our business.
Any failure on our part to comply with applicable regulations could prevent us from being able to carry on our business, and there may be additional costs associated with any such failure.
Our business activities are heavily regulated in all jurisdictions where we do business. Our operations are subject to various laws, regulations and guidelines by governmental authorities relating to the cultivation, processing, manufacture, marketing, management, distribution, transportation, storage, sale, packaging, labelling, pricing and disposal of cannabis and cannabis products. In addition, we are subject to laws and regulations relating to employee health and safety, insurance coverage and the environment. Laws and regulations, applied generally, grant government agencies and self-regulatory bodies broad administrative discretion over our activities, including the power to limit or restrict business activities as well as impose additional disclosure requirements on our products and services.
Any failure by us to comply with the applicable regulatory requirements could
| · | require extensive changes to our operations; |
| · | result in regulatory or agency proceedings or investigations; |
| · | result in the revocation of our licenses and permits, increased compliance costs; |
| · | result in damage awards, civil or criminal fines or penalties; |
| · | result in restrictions on our operations; |
| · | harm our reputation; or |
| · | give rise to material liabilities. |
There can be no assurance that any future regulatory or agency proceedings, investigations or audits will not result in substantial costs, a diversion of management’s attention and resources or other adverse consequences to our business.
Achievement of our business objectives is contingent, in part, upon compliance with regulatory requirements enacted by governmental authorities and obtaining all necessary regulatory approvals for the cultivation, processing, production, storage, distribution, transportation, sale, import and export, as applicable, of our products. Any failure to comply with the regulatory requirements applicable to our operations may lead to possible sanctions, including:
| · | the revocation or imposition of additional conditions on licenses to operate our business; |
| · | the suspension or expulsion from a particular market or jurisdiction or of our key personnel; |
| · | the imposition of additional or more stringent inspection, testing and reporting requirements; |
| · | product recalls or seizures; and |
| · | the imposition of fines and censures. |
In addition, changes in regulations, government or judicial interpretation of regulations, or more vigorous enforcement thereof or other unanticipated events could require extensive changes to our operations, increase compliance costs or give rise to material liabilities or a revocation of our licenses and other permits. Furthermore, governmental authorities may change their administration, application or enforcement procedures at any time, which may adversely affect our ongoing regulatory compliance costs. There is no assurance that we will be able to comply or continue to comply with applicable regulations.
The legal cannabis market is a relatively new industry. As a result, the size of our target market is difficult to quantify, and investors will be reliant on their own estimates on the accuracy of market data.
Because the cannabis industry remains in a relatively nascent stage, there is a lack of information about comparable companies available for potential investors to review in deciding about whether to invest in us and, few, if any, established companies whose business model we can follow or upon whose success we can build. Accordingly, investors should rely on their own estimates regarding the potential size, economics and risks of the cannabis market in deciding whether to invest in our Common Shares. We are an early-stage company that has not generated net income. There can be no assurance that our growth estimates are accurate or that the cannabis market will be large enough for our business to grow as projected.
Although we are committed to researching and developing new markets and products and improving existing products, there can be no assurances that such research and market development activities will prove profitable or that the resulting markets or products, if any, will be commercially viable or successfully produced and marketed. We must rely largely on our own market research to forecast sales and design products as detailed forecasts and consumer research are not generally obtainable from reliable third-party sources in Canada and in other international jurisdictions.
In addition, there is no assurance that the industry and market will continue to exist and grow as currently estimated or anticipated or function and evolve in the manner consistent with management’s expectations and assumptions. We could also be subject to other events or circumstances that that adversely affect the cannabis industry, such as the imposition of further restrictions on sales and marketing or further restrictions on sales in certain areas and markets.
We could be adversely affected by violations of the U.S. Foreign Corrupt Practices Act and similar international anti-bribery and anti-kickback laws with respect to our activities outside the United States.
We anticipate distributing our products to locations in Canada and United States as well as operate our business in Canada and United States. The U.S. Foreign Corrupt Practices Act, and other similar anti-bribery and anti-kickback laws and regulations, generally prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. We cannot assure you that we will be successful in preventing our agents from taking actions in violation of these laws or regulations. Such violations, or allegations of such violations, could disrupt our business and result in a material adverse effect on our financial condition, results of operations and cash flows.
Risks Related to Financials and Accounting
Assumptions, estimates and judgments related to critical accounting matters could significantly affect our reported financial results or financial condition.
The preparation of financial statements requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, as provided in the notes to our financial statements, the results of which form the basis for making judgments about the carrying values of assets, liabilities, equity, revenue and expenses that are not readily apparent from other sources. Our operating results may be adversely affected if the assumptions change or if actual circumstances differ from those in the assumptions, which could cause our operating results to fall below the expectations of securities analysts and investors, resulting in a decline in the price of our Common Shares. Significant assumptions and estimates used in preparing the financial statements include those related to the credit quality of accounts receivable, income tax credits receivable, share based payments, impairment of non-financial assets, fair value of biological assets, as well as revenue and cost recognition.
There are tax risks the Company may be subject to in carrying on business in multiple jurisdictions.
We and our subsidiaries will operate and, accordingly, will be subject to income tax and other forms of taxation in multiple jurisdictions. We may be subject to income taxes and non-income taxes in a variety of jurisdictions and our tax structure may be subject to review by both domestic and foreign taxation authorities. Those tax authorities may disagree with our interpretation and/or application of relevant tax rules. A challenge by a tax authority in these circumstances might require us to incur costs in connection with litigation against the relevant tax authority or reaching a settlement with the tax authority and, if the tax authority’s challenge is successful, could result in additional taxes (perhaps together with interest and penalties) being assessed on us, and as a result an increase in the amount of tax payable by us. In addition, we may be subject to different taxes imposed by the Colombian government, and changes within such tax, legal and regulatory framework may have an adverse effect on our financial results.
Taxation laws and rates which determine taxation expenses may vary significantly in different jurisdictions, and legislation governing taxation laws and rates are also subject to change. Therefore, our earnings may be affected by changes in the proportion of earnings taxed in different jurisdictions, changes in taxation rates, changes in estimates of liabilities and changes in the amount of other forms of taxation. The determination of our provision for income taxes and other tax liabilities will require significant judgment (including based on external advice) as to the interpretation and application of these rules. We may have exposure to greater than anticipated tax liabilities or expenses.
Additionally, dividends and other intra-group payments made by our subsidiaries or international branches may expose the recipients of such payments to taxes in their jurisdictions of organization and operation and such dividends and other intra-group payments may also be subject to withholding taxes imposed by the jurisdiction in which the entity making the payment is organized or tax resident. Unless such withholding taxes are fully creditable or refundable, dividends and other intra-group payments may increase the amount of tax paid by us. Although the Company and its subsidiaries arrange themselves and their affairs with a view to minimizing the incurrence of such taxes, there can be no assurance that we will succeed.
Restrictions on Deduction of Certain Expenses for U.S. Federal Income Tax Purposes
Section 280E of the Internal Revenue Code of 1986, as amended (the “Code”), prohibits businesses from deducting certain expenses associated with trafficking controlled substances for United States federal income tax purposes. The IRS has invoked Code Section 280E in tax audits against various cannabis businesses in the U.S. that are permitted under applicable state laws. Section 280E of the Code prohibits cannabis businesses that are deemed to be trafficking in controlled substances from deducting certain ordinary and necessary business expenses, forcing them to pay higher effective federal tax rates than similar companies in other industries. The effective tax rate on a cannabis business depends on how large its ratio of non-deductible expenses is to its total revenues. Therefore, businesses in the legal cannabis industry may be less profitable than they would otherwise be.
Although the IRS issued a clarification allowing the deduction of certain expenses, the scope of such items is interpreted very narrowly and the bulk of operating costs and general administrative costs are not permitted to be deducted. While there are currently several pending cases before various administrative bodies and federal courts challenging these restrictions, there is no guarantee that these authorities will issue an interpretation of Code Section 280E favorable to cannabis businesses.
Failure to develop our internal controls over financial reporting as we grow could have an adverse effect on our operations.
As our Company matures we will need to continue to develop and improve our current internal control systems and procedures to manage our growth. We are required to establish and maintain appropriate internal controls over financial reporting. Failure to establish appropriate controls, or any failure of those controls once established, could adversely affect our public disclosures regarding our business, financial condition or results of operations. In addition, management’s assessment of internal controls over financial reporting may identify weaknesses and conditions that need to be addressed in our internal controls over financial reporting or other matters that may raise concerns for investors. Any actual or perceived weaknesses and conditions that need to be addressed in our internal control over financial reporting, disclosure of management’s assessment of our internal controls over financial reporting or disclosure of our public accounting firm’s attestation to or report on management’s assessment of our internal controls over financial reporting may have an adverse effect on the price of our Common Shares.
Risks Related to Our Common Shares
We will need, but may be unable to, obtain additional funding on satisfactory terms, which could dilute our shareholders or impose burdensome financial restrictions on our business.
In the future, we hope to rely on revenues generated from operations to fund all of the cash requirements of our activities. However, there can be no assurance that we will be able to generate any significant cash from our operating activities in the future. Future financings may not be available on a timely basis, in sufficient amounts or on terms acceptable to us, if at all. Any debt financing or other financing of securities senior to the Common Shares will likely include financial and other covenants that will restrict our flexibility. Any failure to comply with these covenants would have a material adverse effect on our business, prospects, financial condition and results of operations because we could lose our existing sources of funding and impair our ability to secure new sources of funding. There can be no assurance that we will be able to generate any investor interest in our securities. If we do not obtain additional financing, our business may never commence, in which case you would likely lose the entirety of your investment in the Company.
Holders of our Common Shares are subject to dilution resulting from the issuance of equity-based compensation by us.
We award warrants or restricted stock units to management to incentivize their performance and retention. Any additional equity grants and any exercise of existing warrants or restricted stock units will cause our shareholders to be diluted and may negatively affect the price of the Common Shares.
Ownership of our Common Shares may be considered unlawful in some jurisdictions and holders of our Common Shares may consequently be subject to liability in such jurisdictions.
Cannabis-related financial transactions, including investment in the securities of cannabis companies and receipt of any associated benefits, such as dividends, are currently subject to anti-money laundering and a variety of other laws that vary by jurisdiction, many of which are unsettled and still developing. While the interpretation of these laws is unclear, in some jurisdictions, financial benefit directly or indirectly arising from conduct that would be considered unlawful in such jurisdiction may be viewed to be within the purview of these laws, and persons receiving any such benefit, including investors in an applicable jurisdiction, may be subject to liability under such laws. Each prospective investor should therefore contact his, her or its own legal advisor regarding the ownership of our Common Shares and any related potential liability.
Our executive officers and directors and their respective affiliates may continue to exercise significant control over our Company after this offering, which will limit your ability to influence corporate matters and could delay or prevent a change in corporate control.
As of the date of this 10-K, our executive officers and directors currently represent beneficial ownership, in the aggregate, of approximately 40.5% of our outstanding Common Shares. As a result, these shareholders may be able to influence our management and affairs and control the outcome of matters submitted to our shareholders for approval, including the election of directors and any sale, merger, consolidation, or sale of all or substantially all of our assets. These shareholders may have interests, with respect to their Common Shares, that are different from other shareholders, and the concentration of voting power among one or more of these shareholders may have an adverse effect on the price of our Common Shares. In addition, this concentration of ownership might adversely affect the market price of our Common Shares by:
| · | delaying, deferring or preventing a change of control of the Company; |
| · | impeding a merger, consolidation, takeover or other business combination involving the Company; or |
| · | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of the Company. |
The Company’s directors and officers may have a conflicts of interest in conducting their duties.
We may be subject to various potential conflicts of interest because of the fact that some of our officers and directors may be engaged in a range of business activities. In addition, our executive officers and directors may devote time to their outside business interests, so long as such activities do not materially or adversely interfere with their duties to the Company. In some cases, our executive officers and directors may have fiduciary obligations associated with these business interests that interfere with their ability to devote time to our business and affairs and that could adversely affect our operations. These business interests could require significant time and attention of our executive officers and directors.
The regulated nature of our business may impede or discourage a takeover, which could reduce the market price of our Common Shares.
We require and hold various government licenses to operate our business. These licensing requirements could impede a merger, amalgamation, takeover or other business combination involving us or discourage a potential acquiror from making a tender offer for our Common Shares, which, under certain circumstances, could reduce the market price of our Common Shares.
We do not intend to pay dividends on our Common Shares in the near future, and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our Common Shares.
We have never declared or paid any cash dividend on our Common Shares and do not currently intend to do so in the foreseeable future. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends in the foreseeable future. Therefore, the success of an investment in the Common Shares will depend upon any future appreciation in their value. There is no guarantee that the Common Shares will appreciate in value or even maintain the price at which you purchased them.
Future issuances of debt securities, which would rank senior to our Common Shares upon our bankruptcy or liquidation, and future issuances of preferred stock, which could rank senior to our Common Shares for the purposes of dividends and liquidating distributions, may adversely affect the level of return you may be able to achieve from an investment in our Common Shares.
In the future, we may attempt to increase our capital resources by offering debt securities. Upon bankruptcy or liquidation, holders of our debt securities, and lenders with respect to other borrowings we may make, would receive distributions of our available assets prior to any distributions being made to holders of our Common Shares. Moreover, if we issue preferred stock, the holders of such preferred stock could be entitled to preferences over holders of Common Shares in respect of the payment of dividends and the payment of liquidating distributions. Because our decision to issue debt or preferred stock in any future offering, or borrow money from lenders, will depend in part on market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of any such future offerings or borrowings. Holders of our Common Shares must bear the risk that any future offerings we conduct or borrowings we make may adversely affect the level of return, if any, they may be able to achieve from an investment in our Common Shares.
General Risk Factors
The Company may become involved in legal proceedings from time to time, which could adversely affect the Company.
From time to time, we may be a party to legal and regulatory proceedings, including matters involving governmental agencies, entities with whom it does business and other proceedings arising in the ordinary course of business. We will evaluate our exposure to these legal and regulatory proceedings and establish reserves for the estimated liabilities in accordance with generally accepted accounting principles. Assessing and predicting the outcome of these matters involves substantial uncertainties. Unexpected outcomes in these legal proceedings, or changes in management’s evaluations or predictions and accompanying changes in established reserves, could have an adverse impact on our financial results.
Our participation in the cannabis industry may lead to litigation, formal or informal complaints, enforcement actions, and inquiries by third parties, other companies and/or various governmental authorities against us. Litigation, complaints, and enforcement actions involving us could consume considerable amounts of financial and other corporate resources, which could have an adverse effect on our future cash flows, earnings, results of operations and financial condition.
The Company’s success will depend, in part, on its ability to continue to enhance its product and service offerings to respond to technological and regulatory changes and emerging industry standards and practices.
Rapidly changing markets, technology, emerging industry and regulatory standards and frequent introduction of new products characterize the Company’s business. The introduction of new products embodying new technologies and regulatory developments may render the Company’s equipment obsolete and its products and services less competitive or less marketable. The process of developing the Company’s products and services is complex and requires significant continuing costs, development efforts, third-party commitments and regulatory approvals. The Company may not be successful in developing or effectively commercializing such new products and services, or obtaining any required regulatory approvals, which, together with any capital expenditures made in the course of developing such products and services, may have a material adverse effect on the Company’s business, financial condition and operating results.
We are dependent upon our management and key employees, and the loss of any member of our management team or key employees could have a material adverse effect on our operations.
The Company’s success is dependent upon the ability, expertise, judgment, discretion and good faith of its senior management and key employees. The loss of any member of our management team or key employees could have a material adverse effect on our business and results of operations. While employment agreements and incentive programs are customarily used as primary methods of retaining the services of key employees, these agreements and incentive programs cannot assure the continued services of such employees. Any loss of the services of such individuals, or an inability to attract other suitably qualified persons when needed, could have a material adverse effect on the Company’s business, operating results or financial condition. We do not currently maintain key-person insurance on the lives of any of our key employees. Competition for qualified technical, sales and marketing staff, as well as officers and directors can be intense, and no assurance can be provided that the Company will be able to attract or retain key employees in the future, which may adversely affect the Company’s operations.
Our inability to retain and acquire skilled personnel could impair our business and operations.
The loss of any member of our management team could have a material adverse effect on our business and results of operations. In addition, the inability to hire or the increased costs of hiring new personnel, including members of executive management, could have a material adverse effect on our business and operating results. The expansion of marketing and sales of our products will require us to find, hire and retain additional capable employees who can understand, explain, market and sell our products. There is intense competition for capable personnel in all of these areas and we may not be successful in attracting, training, integrating, motivating, or retaining new personnel, vendors, or subcontractors for these required functions. New employees often require significant training and, in many cases, take a significant amount of time before they achieve full productivity. As a result, we may incur significant costs to attract and retain employees, including significant expenditures related to salaries and benefits and compensation expenses issued in connection to equity awards, and we may lose new employees to our competitors or other companies before we realize the benefit of our investment in recruiting and training them. In addition, as we move into new jurisdictions, we will need to attract and recruit skilled employees in those new areas.
We will need to grow the size of our organization, and we may experience difficulties in managing any growth we may achieve.
As our development and commercialization plans and strategies develop, we expect to need additional research, development, managerial, operational, sales, marketing, financial, accounting, legal and other resources. Future growth would impose significant added responsibilities on members of management. In order to manage growth and changes in strategy effectively, the Company must: (a) maintain adequate systems to meet customer demand; (b) expand sales and marketing, distribution capabilities, and administrative functions; (c) expand the skills and capabilities of its current management team; and (d) attract and retain qualified employees. Our management may not be able to accommodate those added responsibilities, and our failure to do so could prevent us from effectively managing future growth and successfully growing our Company.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about us, our share price and trading volume could decline.
The trading market for our Common Shares will depend, in part, on the research and reports that securities or industry analysts publish about us or our operations. We do not have any control over these analysts and their research and reports. Securities and industry analysts do not currently, and may never, publish research on our business. If no security or industry analysts commence coverage of our Company, the trading price for our Common Shares would likely be negatively affected. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover us downgrade our shares or publish inaccurate or unfavorable research about our business, our share price would likely decline. In addition, if our operating results fail to meet the forecast of analysts, our share price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, demand for our shares could decrease, which might cause our share price and trading volume to decline.
We expect to incur significant ongoing costs and obligations related to our investment in infrastructure, growth, regulatory compliance and operations.
We expect to incur significant ongoing costs and obligations related to our investment in infrastructure and growth and regulatory compliance, which could have a material adverse effect on our results of operations, financial condition and cash flows. In addition, future changes in regulations, more vigorous enforcement thereof or other unanticipated events could require extensive changes to our operations, increased compliance costs or give rise to material liabilities, which could have a material adverse effect on our business, results of operations and financial condition. Our efforts to grow our business may be costlier than we expect, and we may not be able to generate sufficient revenue to offset such higher operating expenses. We may incur significant losses in the future for a number of reasons, including unforeseen expenses, difficulties, complications and delays, and other unknown events.
There is no assurance that the Company’s will have insurance coverage sufficient to cover all claims to which the Company may become subject.
Our production is, in general, subject to different risks and hazards, including adverse weather conditions, fires, plant diseases and pest infestations, other natural phenomena, industrial accidents, labor disputes, changes in the legal and regulatory framework applicable to us and environmental contingencies.
We currently do not maintain insurance coverage over our production and facilities. We may not be able to maintain insurance of the type and amount desired at a reasonable cost. If we were to incur significant liability for which we were not fully insured, it could have an adverse effect on our business, financial condition and results of operations.
We do not currently maintain key-person insurance on the lives of any of our key employees.
We may be unable to implement our business strategy, which could have negative financial and reputational effects on our business.
The growth and expansion of our business is heavily dependent upon the successful implementation of our business strategy as described under the heading “Our Business.” There can be no assurance that we will be successful in the implementation of our business strategy. A failure to do so could have negative financial and reputational effects on us. Future clinical research studies may lead to conclusions that dispute or conflict with our understanding and belief regarding the medical benefits, viability, safety, efficacy, dosing and social acceptance of cannabis.
The Company could be subject to a security breach that could result in significant damage or theft of products and equipment.
Breaches of security at our facilities may occur and could result in damage to or theft of products and equipment. A security breach at our facilities could result in a significant loss of inventory or work in process, expose us to liability under applicable regulations and increase expenses relating to the investigation of the breach and implementation of additional preventative security measures, any of which could have an adverse effect on our business, financial condition and results of operations.
Item 1B. Unresolved Staff Comments
Not applicable.
Item 2. Properties.
Bucamaranga, Colombia
The Company’s 100% owned subsidiary, Allied Colombia, S.A.S., has secured vast agricultural land extension in Bucamaranga, a key production farming region of Colombia. Both areas have the benefit of having 12 hours of sunlight year-round, temperatures oscillating between 20-30 degrees celsius, with constant humidity. This environment and situation ideal to growing cannabis at low cost. The Ibague land has an area of 1,400 hectares (about 3,450 acres), all with water rights, an electrical substation, and great paved access to the property. This is situated in close proximity to the free trade zone. The Company currently has lease arrangement for 5 hectares and a cost of $1,600 per month with an option to purchase. Further, the Company has the ability to expand its leased land footprint to the adjacent 24 hectare property.
Kelowna, British Columbia, Canada
Our corporate headquarters are located at 1405 St. Paul Street, #201, Kelowna, BC V1Y 2E4 Canada. At that location Allied leases an office space within which Allied Executives can host virtual meetings via zoom, host team days and operate general corporate affairs. There is working space for Allied corporate personnel and several meeting rooms that can be rented from $10.00 per hour to a large hall that can be rented for $50.00 per hour.
In order to adhere to Canadian production regulations with respect to seed approval and strain-acclimatization, in our facility in Kelowna Canada we have created research and development environments (under personal production licenses approved by the Canada Regulators) that mimic the micro-climates in Suesca, Ibague and Bucaramanga.
In June 2019, AM Biosciences signed the production and manufacturing contract to begin the manufacturing of the full building for what was originally anticipated to be a Canada extraction and production facility. The Company made an upfront payment of $230,000 in June 2019, an additional payment of $903,385 in August 2019 and an additional payment of $92,000 in March 2020. At August 31, 2022, Company had deposits of $2,656,695 to purchase prefabricated buildings. During the year ended August 31, 2023, the Company recorded a loss on impairment of deposit of $2,656,695 due to the legal proceedings described under “Business”.
Recently the Board of directors made a strategic decision to allocate all financial resources to the production of Colombian cannabis destined for international markets. After the company reaches profitability, the Company may revisit the use of this building.
Item 3. Legal Proceedings.
Other than the legal proceedings in Nevada involving retrieving our asset from the bankruptcy proceedings of another company as described under “Business”, we are not aware of any legal proceedings contemplated by any governmental authority or any other party involving us or our properties.
As of the date of this report, no director, officer or affiliate is (i) a party adverse to us in any legal proceeding, or (ii) has an adverse interest to us in any legal proceedings. We are not aware of any other legal proceedings pending or that have been threatened against us or our properties.
From time to time the Company may be named in claims arising in the ordinary course of business. Currently, no legal proceedings or claims, other than those disclosed above, are pending against or involve the Company that, in the opinion of management, could reasonably be expected to have a material adverse effect on its business and financial condition.
PART II
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Market for Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Our common stock is quoted on the OTC Bulletin Board under the symbol “ALID”. Our symbol was changed to ALID effective August 3, 2019 upon notice to and review by FINRA. Prior to such date the Company had minimal trading under its previous symbol. Such quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not represent actual transactions.
Below is a table indicating the range of high and low closing price information for the common stock as reported by the OTC Markets Group for the periods listed. These prices do not necessarily reflect actual transactions.
| | Low | | | High | |
August 31, 2023 to date | | $ | 0.06 | | | $ | 0.24 | |
Year ended August 31, 2023 | | $ | 0.14 | | | $ | 0.65 | |
Quarter ended May 31, 2023 | | $ | 0.14 | | | $ | 0.35 | |
Quarter ended February 28, 2023 | | $ | 0.20 | | | $ | 0.41 | |
Quarter ended November 30, 2022 | | $ | 0.23 | | | $ | 0.65 | |
Year ended August 31, 2022 | | $ | 0.43 | | | $ | 0.75 | |
Quarter ended May 31, 2022 | | $ | 0.72 | | | $ | 1.76 | |
Quarter ended February 28, 2022 | | $ | 1.75 | | | $ | 1.89 | |
Quarter ended November 30, 2021 | | $ | 1.05 | | | $ | 2.11 | |
Holders
As of August 31, 2023, there were approximately 144 record holders of our common stock. This does not include the holders of our common stock who held their shares in street name as of that date. Based on reports we have reviewed relative to such holders, we currently have at least 3,062 beneficial stockholders.
Dividends
We have never paid or declared any cash dividends on our common stock and do not anticipate paying cash dividends in the foreseeable future but rather intend to retain future earnings, if any, for reinvestment in our future business. Any future determination to pay cash dividends will be in compliance with our contractual obligations and otherwise at the discretion of the board of directors and based upon our financial condition, results of operations, capital requirements and such other factors as the board of directors deems relevant.
Transfer Agent
Our registrar and transfer agent is ClearTrust LLC., 16540 Pointe Village Dr., Suite 205, Lutz, FL 33558.
Recent Sales of Unregistered Securities
In July 2022 the Company commenced a private placement pursuant to Rule 506(c) promulgated under Regulation D of the Securities Exchange Act of 1934, as amended. The private placement sought to raise funds through the sale of shares at $0.40 per share. Through August 31, 2022 we obtained gross proceeds of $1,260,000 from this offering and an additional $210,000 subsequent to August 31, 2022.
During the year ended August 31, 2023, the Company obtained gross proceeds of $990,952 through the sale of shares at $0.20 per share. Subsequent to the year ended August 31, 2023, the Company obtained an additional $346,000 for subscriptions at $0.20 per share.
Item 6. Selected Financial Data.
Not applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion relates to the historical operations and financial statements of Allied Corp. for the fiscal years ending August 31, 2023 and 2022.
Forward-Looking Statements
The following Management’s Discussion and Analysis should be read in conjunction with our financial statements and the related notes thereto included elsewhere in this Annual Report. The Management’s Discussion and Analysis contains forward-looking statements that involve risks and uncertainties, such as statements of our plans, objectives, expectations and intentions. Any statements that are not statements of historical fact are forward-looking statements. When used, the words “believe,” “plan,” “intend,” “anticipate,” “target,” “estimate,” “expect,” and the like, and/or future-tense or conditional constructions (“will,” “may,” “could,” “should,” etc.), or similar expressions, identify certain of these forward-looking statements. These forward-looking statements are subject to risks and uncertainties that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements in this Annual Report. Our actual results and the timing of events could differ materially from those anticipated in these forward-looking statements. Factors that could cause or contribute to such differences in results and outcomes include, without limitation, those specifically addressed under the heading “Risks Factors” in our various filings with the Securities and Exchange Commission. We do not undertake any obligation to update forward-looking statements to reflect events or circumstances occurring after the date of this Annual Report.
As a result of the Reorganization Agreement and the change in business and operations of the Company, a discussion of the past financial results of the Company, formally known as Cosmo Ventures, Inc., is not pertinent, and, under generally accepted accounting principles in the United States the historical financial results of AM Biosciences, the acquirer for accounting purposes, prior to the Reorganization Agreement are considered the historical financial results of the Company.
The following discussion highlights the Company’s results of operations and the principal factors that have affected its consolidated financial condition as well as its liquidity and capital resources for the periods described, and provides information that management believes is relevant for an assessment and understanding of the Company’s consolidated financial condition and results of operations presented herein. The following discussion and analysis are based Allied Corp’s audited and unaudited financial statements contained in this Current Report, which have been prepared in accordance with generally accepted accounting principles in the United States. You should read the discussion and analysis together with such financial statements and the related notes thereto.
Critical Accounting Policies
Business presentation
The consolidated financial statements and related notes are presented in accordance with accounting principles generally accepted in the United States of America (“US GAAP”), and are expressed in United States dollars. The Company’s fiscal year end is August 31. The significant accounting policies followed are:
Principles of consolidation
The consolidated financial statements include accounts of Allied Corp. and its majority owned subsidiaries. Subsidiaries are consolidated from the date of acquisition and control and continue to be consolidated until the date that such control ceases. Control is achieved when the Company is exposed, or has rights, to variable returns from its involvement with the investee and has the ability to affect these returns through its power over the investee. All intercompany balances, income, expenses, and unrealized gains and losses resulting from intercompany transactions are eliminated on consolidation.
Cash and cash equivalents
Cash is comprised of cash on hand, cash held in trust accounts and demand deposits. Cash equivalents are short-term, highly liquid investments with maturities within three months when acquired. The Company did not have any cash equivalents as of August 31, 2023 and 2022.
Property and equipment
Property and equipment are stated at cost. The Company depreciates the cost of property and equipment over their estimated useful lives at the following annual rates:
| Farm facility and equipment | 1-10 years straight-line basis |
| Office and computer equipment | 5-10 years straight-line basis |
| Land equipment | 10 years straight-line basis |
Inventory
Inventory is comprised of raw materials, supplies, vegetative and flowering plants, dried flower, diluted crude and CBD isolates available for sale, and purchased cannabis products.
Inventory is stated at the lower of cost or net realizable value, determined using weighted average cost. Net realizable value is defined as the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal and transportation. At the end of each reporting period, the Company performs an assessment of inventory and records write-downs for excess and obsolete inventories based on the Company’s estimated forecast of product demand, production requirements, market conditions, regulatory environment, and spoilage. Actual inventory losses may differ from management’s estimates and such differences could be material to the Company’s consolidated balance sheets, statements of net loss and comprehensive loss and statements of cash flows.
Intangible assets
Intangible assets include licenses which are being amortized over their estimated useful lives of 10 years. The Company’s licenses are amortized over their economic or legal life on a straight-line basis, whichever is shorter. The licenses have been amortized from the date of acquisition.
The Company periodically evaluates the reasonableness of the useful lives of these assets. Once these assets are fully amortized, they are removed from the accounts. These assets are reviewed for impairment or obsolescence when events or changes in circumstances indicate that the carrying amount may not be recoverable. If impaired, intangible assets are written down to fair value based on discounted cash flows or other valuation techniques. The Company has no intangibles with indefinite lives.
For long-lived assets, impairment losses are only recorded if the asset’s carrying amount is not recoverable through its undiscounted, probability-weighted future cash flows. The Company measures the impairment loss based on the difference between the carrying amount and the estimated fair value. When an impairment exists, the related assets are written down to fair value.
Long-lived assets
In accordance with ASC 360, Property, Plant and Equipment, the Company tests long-lived assets or asset groups for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable. Circumstances which could trigger a review include, but are not limited to: significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction of the asset; current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset; and current expectation that the asset will more likely than not be sold or disposed significantly before the end of its estimated useful life. Recoverability is assessed based on the carrying amount of the asset and its fair value, which is generally determined based on the sum of the undiscounted cash flows expected to result from the use and the eventual disposal of the asset, as well as specific appraisal in certain instances. An impairment loss is recognized when the carrying amount is not recoverable and exceeds fair value.
Foreign currency translation and functional currency conversion
Items included in these consolidated financial statements of each of the Company’s entities are measured using the currency of the primary economic environment in which the entities operate (the “functional currency”).
Prior to September 10, 2019, the Company’s functional currency was the Canadian dollar. Translation gains and losses from the application of the U.S. dollar as the reporting currency during the period that the Canadian dollar was the functional currency are included as part of cumulative currency translation adjustment, which is reported as a component of shareholders’ equity under accumulated other comprehensive loss.
The Company re-assessed its functional currency and determined as at September 10, 2019, its functional currency changed from the Canadian dollar to the U.S. dollar based on management’s analysis of changes in our organization. The change in functional currency was accounted for prospectively from September 10, 2019 and prior period financial statements were not restated for the change in functional currency.
For periods commencing September 10, 2019, monetary assets and liabilities denominated in foreign currencies are translated into U.S. dollars using exchange rates in effect at the balance sheet date. Opening balances related to non-monetary assets and liabilities are based on prior period translated amounts, and non-monetary assets and non-monetary liabilities incurred after September 10, 2019 are translated at the approximate exchange rate prevailing at the date of the transaction. Revenue and expense transactions are translated at the approximate exchange rate in effect at the time of the transaction. Foreign exchange gains and losses are included in the statement of operations and comprehensive loss as foreign exchange gains.
The Company assessed the functional currency for Allied Colombia S.A.S. to be the Colombian Peso. The functional currency for all other subsidiaries is the U.S. dollar.
Share issuance costs
Costs directly attributable to the raising of capital are charged against the related share capital. Costs related to shares not yet issued are recorded as deferred share issuance costs. These costs are deferred until the issuance of the shares to which the costs relate, at which time the costs will be charged against the related share capital or charged to operations if the shares are not issued.
Research and development costs
Research and development costs are expensed as incurred.
Advertising costs
Advertising costs are expensed as incurred.
Revenue recognition
The Company’s revenue is comprised of sales of cannabis products.
The Company’s revenue-generating activities have a single performance obligation and revenue is recognized at the point in time when control of the product transfers and the Company’s obligations have been fulfilled. This generally occurs when the product is shipped or delivered to the customer, depending upon the method of distribution and shipping terms set forth in the customer contract. Revenue is measured as the amount of consideration the Company expects to receive in exchange for the sale of the Company’s product. Certain of the Company’s customer contracts may provide the customer with a right of return. In certain circumstances the Company may also provide a retrospective price adjustment to a customer. These items give rise to variable consideration, which is recognized as a reduction of the transaction price based upon the expected amounts of the product returns and price adjustments at the time revenue for the corresponding product sale is recognized. The determination of the reduction of the transaction price for variable consideration requires that the Company make certain estimates and assumptions that affect the timing and amounts of revenue recognized.
Sales of products are for cash or otherwise agreed-upon credit terms. The Company’s payment terms vary by location and customer; however, the time period between when revenue is recognized and when payment is due is not significant. The Company estimates and reserves for its bad debt exposure based on its experience with past due accounts and collectability, write-off history, the aging of accounts receivable and an analysis of customer data.
Net income (loss) per common share
Net income (loss) per share is calculated in accordance with ASC 260, Earnings per Share. The weighted-average number of common shares outstanding during each period is used to compute basic earning or loss per share. Diluted earnings or loss per share is computed using the weighted average number of shares and diluted potential common shares outstanding to the extent the effect would not be antidilutive. Dilutive potential common shares are additional common shares assumed to be exercised.
Basic net income (loss) per common share is based on the weighted average number of shares of common stock outstanding.
Income taxes
The Company accounts for income taxes under ASC 740, Income Taxes. Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Under ASC 740, the effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period the enactment occurs. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax assets through future operations.
Related party transactions
Related parties are any entities or individuals that, through employment, ownership or other means, possess the ability to direct or cause the direction of the management and policies of the Company. The Company discloses related party transactions that are outside of normal compensatory agreements, such as salaries. Related party transactions are measured at the exchange amounts.
Significant accounting estimates and judgments
The preparation of the financial statements in conformity with US GAAP requires management to make judgments, estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Although management uses historical experience and its best knowledge of the amount, events or actions to for the basis for judgments and estimates, actual results may differ from these estimates.
The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and further periods if the review affects both current and future periods.
Significant estimates and assumptions included in these financial statements relate to the valuation assumptions related to the estimated useful lives and recoverability of long-lived assets, stock-based compensation, and deferred income tax assets and liabilities. Judgments are required in the assessment of the Company’s ability to continue to as going concern.
Financial instruments
ASC 825, “Financial Instruments,” requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 825 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 825 prioritizes the inputs into three levels that may be used to measure fair value:
Level 1
Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
Level 2
Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
Level 3
Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
The financial instruments consist principally of cash, due from related parties, accounts payable, note payable, and a loan payable to Allied. The fair value of cash when applicable is determined based on “Level 1” inputs, which consist of quoted prices in active markets for identical assets. The Company believes that the recorded values of all other financial instruments which are categorized as loans and receivables approximate their current fair values because of their nature and respective relatively short maturity dates or current market rates of interest for similar instruments.
For certain of the Company’s financial instruments, including cash and accounts payable and accrued liabilities, the carrying amounts approximate their fair values due to the short maturities.
The Company does not have any assets or liabilities measured at fair value on a recurring basis presented on the Company’s balance sheet as of August 31, 2023 and 2022.
Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash. The Company limits its exposure to credit loss by placing its cash with high credit quality financial institutions.
Leases
The Company determines if an arrangement contains a lease in whole or in part at the inception of the contract. Right-of-use (“ROU”) assets represent the Company’s right to use an underlying asset for the lease term while lease liabilities represent our obligation to make lease payments arising from the lease. All leases with terms greater than twelve months result in the recognition of a ROU asset and a liability at the lease commencement date based on the present value of the lease payments over the lease term. Unless a lease provides all of the information required to determine the implicit interest rate, the Company uses its incremental borrowing rate based on the information available at the commencement date in determining the present value of the lease payments. The Company uses the implicit interest rate in the lease when readily determinable.
Our lease terms include all non-cancelable periods and may include options to extend (or to not terminate) the lease when it is reasonably certain that we will exercise that option. Leases with terms of twelve months or less at the commencement date are expensed on a straight-line basis over the lease term and do not result in the recognition of an asset or liability.
Recent accounting pronouncements
The Company does not expect that recent accounting pronouncements or changes in accounting pronouncements during the year ended August 31, 2023, are of significance or potential significance to the Company.
Financial Condition and Results of Operations
We have incurred recurring losses to date. Our financial statements have been prepared assuming that we will continue as a going concern and, accordingly, do not include adjustments relating to the recoverability and realization of assets and classification of liabilities that might be necessary should we be unable to continue in operation.
We expect we will require additional capital to meet our long-term operating requirements. We expect to raise additional capital through, among other things, the sale of equity or debt securities.
Results of Operations
Comparison of Results for the Fiscal Year ended August 31, 2023 compared to the Fiscal Year Ended August 31, 2022
Net Revenues
For the fiscal year ended August 31, 2023 the Company had $72,096 in revenues. For the fiscal year ended August 31, 2022, the Company had $165,596 in revenues. We have a very limited operating history upon which to base an evaluation of our business and prospects. Our short operating history may hinder our ability to successfully meet our objectives and makes it difficult for potential investors to evaluate our business or prospective operations.
Gross Margin
For the fiscal year ended August 31, 2023 the Company had a negative gross margin of $1,461,767 compared to a gross margin of $74,120 for the fiscal year ended August 31, 2022. The negative gross margin was due to an inventory write-off of $1,507,018 for the fiscal year ended August 31, 2023, compared to inventory write-off of $31,737 for the fiscal year ended August 31, 2022.
Expenses
For the fiscal year ended August 31, 2023 we had total operating expenses of $9,213,904, compared to total expenses of $15,612,973 for the fiscal year ended August 31, 2022. Of the operating expense for 2023, a total of $5,994,713 (2022 – $14,849,714) was operating expense and the remainder was non-recurring expenses which included interest expense of $385,453 (2022 – $219,307), loss on deposit impairment of $2,656,695 (2022 – $nil), loss on license acquisition advance of $150,000 (2022 – $nil), and accretion of $nil (2022 – $547,598). The operating expenses consisted principally of consulting fees in the development of the Company’s cannabis business, general office expenses, professional fees and rent.
Net Loss
For the fiscal year ended August 31, 2023, the Company had a net operating loss of $10,675,671 compared to a net loss of $15,538,853 for the fiscal year ended August 31, 2022. This was principally related to the expenses referenced in the previous paragraph.
Liquidity and Capital Resources
The following table sets forth the major components of our statements and consolidated statements of cash flows for the periods presented.
| | Year Ended August 31, 2023 | | | Year Ended August 31, 2022 | |
Cash used in operating activities | | $ | (1,956,779 | ) | | $ | (4,284,266 | ) |
Cash from financing activities | | $ | 2,068,160 | | | $ | 5,329,884 | |
Cash used in investing activities | | $ | (87,795 | ) | | $ | (1,205,369 | ) |
Effect of exchange rate change | | $ | 90,107 | | | $ | (164,031 | ) |
Change in cash during the year | | $ | 23,586 | | | $ | (159,751 | ) |
Cash, beginning of the year | | $ | 96,043 | | | $ | 419,825 | |
Cash, end of the year | | $ | 209,736 | | | $ | 96,043 | |
As at August 31, 2023, we had working deficit of $8,379,055, compared to working deficit of $5,665,758 for the fiscal year ended August 31, 2022. Our primary cash flow needs are for the development of our cannabis products, operating costs, administrative expenses and for general working capital.
As of August 31, 2023, the Company had $470,580 in current assets, consisting principally of $209,736 in cash and $107,510 in inventory, as compared to $1,241,646 in current assets, consisting principally of $96,043 in cash and $998,988 in inventory as of August 31, 2022. As of August 31, 2023, other assets mainly include property plant and equipment of $1,417,192, as compared to deposits and advances of $2,869,825, and property plant and equipment of $1,444,038 as of August 31, 2022.
To date, the Company has financed its operations through equity sales and through the sale of convertible notes.
Secured Convertible Notes
The Company has granted each and every of the secured convertible note holders a continuing security interest in, a general lien upon, and a right of set-off against all existing and future assets and property under the terms of a security agreement. The following table summarizes the Company’s secured convertible notes payable as of August 31, 2023 and 2022.
Issuance date | | Maturity date as amended | | | Interest rate | | | Conversion price per share | | | Principal as at August 31, 2023 | | | Principal as at August 31, 2022 | | | Accrued interest as at August 31, 2023 | | | Accrued interest as at August 31, 2022 | |
1/23/2020 | | 9/30/2022 | | | | 10 | % | | $ | 1.25 | | | | 200,000 | | | | 200,000 | | | | 58,575 | | | | 38,575 | |
1/23/2020 | | 9/30/2022 | | | | 10 | % | | $ | 1.25 | | | | 200,000 | | | | 200,000 | | | | 38,630 | | | | 18,630 | |
9/29/2020 | | 9/30/2022 | | | | 10 | % | | $ | 1.25 | | | | 63,341 | | | | 63,341 | | | | 33,540 | | | | 27,206 | |
10/26/2020 | | 9/30/2022 | | | | 10 | % | | $ | 1.25 | | | | 37,613 | | | | 37,613 | | | | 10,706 | | | | 6,945 | |
11/11/2020 | | 9/30/2022 | | | | 10 | % | | $ | 1.25 | | | | 85,937 | | | | 85,937 | | | | 24,087 | | | | 15,493 | |
12/2/2020 | | 9/30/2022 | | | | 10 | % | | $ | 1.25 | | | | 600,000 | | | | 600,000 | | | | 164,712 | | | | 104,712 | |
1/7/2021 | | 9/30/2022 | | | | 10 | % | | $ | 1.25 | | | | 300,000 | | | | 300,000 | | | | 52,521 | | | | 22,521 | |
3/26/2021 | | 9/30/2022 | | | | 10 | % | | $ | 1.25 | | | | 18,000 | | | | 18,000 | | | | 4,379 | | | | 2,579 | |
3/26/2021 | | 9/30/2022 | | | | 10 | % | | $ | 1.25 | | | | 100,000 | | | | 100,000 | | | | 24,274 | | | | 14,274 | |
4/30/2021 | | 9/30/2022 | | | | 10 | % | | $ | 1.00 | | | | 100,000 | | | | 100,000 | | | | 23,370 | | | | 13,370 | |
4/29/2021 | | 9/30/2022 | | | | 10 | % | | $ | 1.00 | | | | 180,000 | | | | 180,000 | | | | 42,016 | | | | 24,016 | |
7/25/2021 | | 9/30/2022 | | | | 10 | % | | $ | 1.25 | | | | 35,000 | | | | 35,000 | | | | 7,336 | | | | 3,836 | |
7/25/2021 | | 9/30/2022 | | | | 10 | % | | $ | 1.25 | | | | 15,000 | | | | 15,000 | | | | 3,144 | | | | 1,644 | |
10/1/2021 | | 9/30/2022 | | | | 10 | % | | $ | 1.00 | | | | 100,000 | | | | 100,000 | | | | 19,123 | | | | 9,123 | |
10/25/2021 | | 9/30/2022 | | | | 10 | % | | $ | 1.00 | | | | 100,000 | | | | 100,000 | | | | 18,466 | | | | 8,466 | |
12/23/2021 | | 6/23/2022 | | | | 10 | % | | $ | 1.25 | | | | 100,000 | | | | 100,000 | | | | 16,877 | | | | 6,877 | |
12/23/2021 | | 6/23/2022 | | | | 10 | % | | $ | 1.25 | | | | 100,000 | | | | 100,000 | | | | 16,877 | | | | 6,877 | |
1/11/2022 | | 7/10/2022 | | | | 10 | % | | $ | 1.25 | | | | 150,000 | | | | 150,000 | | | | 24,534 | | | | 9,534 | |
1/31/2022 | | 7/31/2022 | | | | 10 | % | | $ | 1.25 | | | | 100,000 | | | | 100,000 | | | | 15,808 | | | | 5,808 | |
3/29/2022 | | 9/30/2022 | | | | 10 | % | | $ | 1.25 | | | | 500,000 | | | | 500,000 | | | | 71,233 | | | | 21,233 | |
6/16/2022 | | 12/16/2022 | | | | 10 | % | | $ | 1.25 | | | | 250,000 | | | | 250,000 | | | | 30,206 | | | | 5,206 | |
11/28/2022 | | 6/30/2023 | | | | 10 | % | | | * | | | | 100,000 | | | | - | | | | 7,616 | | | | - | |
12/1/2022 | | 6/30/2023 | | | | 10 | % | | | * | | | | 70,000 | | | | - | | | | 5,236 | | | | - | |
12/7/2022 | | 6/30/2023 | | | | 10 | % | | | * | | | | 60,000 | | | | - | | | | 4,389 | | | | - | |
2/28/2023 | | 4/30/2023 | | | | 10 | % | | | * | | | | 150,000 | | | | - | | | | 7,562 | | | | - | |
5/17/2023 | | 9/17/2023 | | | | 10 | % | | | * | | | | 10,000 | | | | - | | | | 504 | | | | - | |
Total | | | | | | | | | | | | | | 3,724,891 | | | | 3,334,891 | | | | 725,721 | | | | 366,925 | |
*Conversion price is the lesser of 80% of the twenty-day average closing price per share (but not less than $0.20 per share) or $0.50 per share.
The Company defaulted on all of the convertible notes above and is in process of amending the maturity dates and conversion prices. Refer to the consolidated financial statements for the history of modifications on each convertible note.
Equity Transactions
During the year ended August 31, 2022:
On September 2, 2021, the Company issued 2,175,933 common shares at fair value of $ 2,447,925 on issuance date from treasury to the CFO and COO and 1,900,000 common shares at fair value of $2,137,500 to certain employees of the Company as bonuses for past services, which is expensed as a total of $4,585,425 for consulting fees.
On September 2, 2021, the Company issued 2,997,237 common shares measured at fair value on issuance date of $3,371,892 from treasury for consulting services related to business development for a 12-month period from the issuance date. As the future benefit of the consulting services to be performed cannot be determined, the entire amount was expensed during the year ended August 31, 2022. The total $3,584,392 stock-based compensation – consulting services is comprised of this $3,371,892 share issuance plus the $212,500 described in the next paragraph below.
During the year ended August 31, 2021, the Company re-issued 750,000 shares of common stock with total fair value of $637,500 for consulting services, out of which, $425,000 was expensed as consulting fees during the prior year and $212,500 was expensed during the year ended August 31, 2022.
On October 20, 2021, the Company issued 3,699,955 units at $0.75 per unit for proceeds of $2,774,966, of which $865,467 was received during the year ended August 31, 2021. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred brokerage commission fees and other share issuance costs of $210,736.
On November 5, 2021, the Company issued 905,000 units at $0.75 per unit for proceeds of $678,750. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company issued 8,000 shares of common stock with a fair value of $6,000 as a finder’s fee and incurred other finders’ fees and other share issuance costs of $42,654.
On November 24, 2021, the Company issued 36,000 units at $0.75 per unit for proceeds of $27,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years.
On November 30, 2021, the Company issued 8,268 shares as consideration for extending the maturity date of a convertible note.
On January 20, 2022, the Company issued 75,000 units at $0.75 per unit for proceeds of $56,250 which was received during the year ended August 31, 2021. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years.
On January 28, 2022, the Company issued 66,667 units at $0.75 per unit for proceeds of $50,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years.
On February 7, 2022, the Company issued 66,667 units at $0.75 per unit for proceeds of $50,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years.
On February 10, 2022, the Company issued 33,334 units at $0.75 per unit for proceeds of $25,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years.
On February 10, 2022, the Company issued 80,000 units at $1.25 per unit for proceeds of $100,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $8,000.
On February 20, 2022, the Company issued 40,000 units at $1.25 per unit for proceeds of $50,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $4,000.
On February 21, 2022, the Company issued 300,000 units at $1.25 per unit for proceeds of $375,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $30,000.
On February 22, 2022, the Company issued 80,000 units at $1.25 per unit for proceeds of $100,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $8,000.
On February 24, 2022, the Company issued 41,600 units at $0.75 per unit for proceeds of $31,200. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years.
On February 24, 2022, the Company issued 40,000 units at $1.25 per unit for proceeds of $50,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $4,000.
On February 25, 2022, the Company issued 96,000 units at $1.25 per unit for proceeds of $120,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $9,600.
On February 28, 2022, the Company issued 120,000 units at $1.25 per unit for proceeds of $150,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $12,000.
On March 7, 2022, the Company issued 20,000 units at $1.25 per unit for proceeds of $25,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $4,000.
On May 26, 2022, the Company issued 133,333 shares at $0.75 per share for proceeds of $100,000.
On May 26, 2022, the Company issued 67,733 units at $0.75 per unit for proceeds of $50,800. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years.
On August 23, 2022, the Company issued 750,000 shares at $0.40 per share for proceeds of $300,000.
During the year ended August 31, 2022, the Company issued 368,000 common shares to certain investors for no consideration by error. The Company is in the process of retracting the shares.
At August 31, 2022, the Company had received $540,000 in cash for share subscriptions.
During the year ended August 31, 2023:
On September 21, 2022, the Company issued 1,350,000 shares of common stock at $0.40 per share for $540,000 subscriptions received during the year ended August 31, 2022.
On October 7, 2022, the Company issued 1,575,000 shares of common stock at $0.40 per share for proceeds of $630,000. In connection with the financing, the Company incurred finder’s fee of $10,000 and share issuance costs of $2,792.
On October 7, 2022, the Company issued 75,000 shares of common stock with a fair value of $44,606 to settle related party accounts payable of $30,000, resulting in a loss on settlement of $14,606.
On October 7, 2022, the Company issued 70,560 shares of common stock with a fair value of $41,966 to settle $29,529 in debt, resulting in a loss on settlement of $12,437.
On August 21, 2023, the Company issued 1,525,000 shares of common stock at $0.20 per share for proceeds of $305,000.
On August 31, 2023, the Company issued 3,029,760 shares of common stock at $0.20 per share for proceeds of $605,952. In connection with the financing, the Company issued 1,000,000 warrants with a fair value of $204,452 as finder’s fees. Each warrant entitles the holder to acquire one common share at a price of $0.20 per share until July 30, 2025. The fair value of the finder’s warrants was calculated using the Black-Scholes option pricing model assuming the following weighted-average assumptions: a risk-free rate of 4.87%, no expected dividends or forfeiture rate, volatility of 160.87% and an expected life of 2 years.
At August 31, 2023, the Company had received $80,000 in cash for shares subscriptions at $0.20 per share. Subsequent to the year ended August 31, 2023, the Company received additional $346,000 in cash for share subscriptions at $0.20 per share and issued 250,000 shares for subscriptions received.
Future Financing
In connection with its proposed business plan and currently ongoing and proposed acquisitions, in addition to the possible proceeds from this offering the Company will be required to complete substantial and significant additional capital formation. Such formation could be through additional equity offerings, debt, bank financings or a combination of any source of financing. There can be no assurance that the Company will be successful in completion of such financings.
Plan of Operations
As noted above, the continuation of our current plan of operations requires us to raise significant additional capital. If we are successful in raising capital through the sale of Common Shares offered for sale in this offering, we believe that we will have sufficient cash resources to fund our plan of operations through the 4th quarter of 2024. If we are unable to do so, we may have to curtail and possibly cease some operations. We intend to use the net proceeds from the offering for operating capacity in Colombia and Canada, regulatory compliance, intellectual property, working capital and general corporate purposes.
We continually evaluate our plan of operations to determine the manner in which we can most effectively utilize our limited cash resources. The timing of completion of any aspect of our plan of operations is highly dependent upon the availability of cash to implement that aspect of the plan and other factors beyond our control. There is no assurance that we will successfully obtain the required capital or revenues, or, if obtained, that the amounts will be sufficient to fund our ongoing operations.
Capital Expenditures
As of August 31, 2023 the company had purchased property plant and equipment of $1,417,192 and paid net cash of $2,833,049 in deposits for an asset acquisition. As of August 31, 2022, the Company purchased property plant and equipment of $1,444,038 and paid net cash of $2,869,825 in deposits and advances for an asset acquisition.
MediColombias Acquisition (Colombia Licensed Producer)
On August 29, 2019, the Company entered into a Share Purchase Agreement (“Purchase Agreement”) with Dorson Commercial Corp. (“Dorson”) as the sole owner of Baleno Ltd. to purchase all of the issued and outstanding shares of Baleno Ltd., the sole owner of Medicolombia Cannabis S.A.S. (“Medicolombia”). Medicolombia is based in Colombia with a full set of licenses and a lease agreement in place to begin production on a 5 hectare parcel of land. We have the ability to scale production to over hundreds of hectares. This is located in the area of Bucamaranga, Colombia.
Pursuant to the agreement the Company acquired all of the issued and outstanding shares of Medicolombia in exchange for $700,000 and 4,500,000 shares of Allied. The Company closed and completed the acquisition on February 17, 2020. Medicolombia has subsequently changed its name to Allied Colombia S.A.S.
Natural Health Products Acquisition
In May 2019 the management team of AM Biosciences were able to negotiate the inclusion of a natural health products catalogue of products. This includes 50 products in the natural health vertical market. Three of these products are of particular interest as they have Natural Health Products registration numbers with Health Canada. AM Biosciences can add these to the product offerings both in Canada and the United States.
Since the Board of Directors of the Company has determined to currently focus on the cannabis product, this project is currently on hold.
Xtreme Cubes Building
In June 2019, AM Biosciences signed the production and manufacturing contract to begin the manufacturing of the full building for the Canada extraction and production facility. The Company made an upfront payment of $230,000 USD in June 2019, an additional payment of $903,385 in August 2019 and an additional payment of $92,000 in March 2020. At August 31, 2022, Company had deposits of $2,656,695 to purchase prefabricated buildings. During the year ended August 31, 2023, the Company recorded a loss on impairment of deposit of $2,656,695 due to the legal proceedings described below.
In 2020, Allied signed a purchase agreement to acquire a Nevada state license and had the 9800 sq ft. modular building built in Nevada as well. Allied signed a 25 year lease with a publicly listed company called FIORE cannabis. In December 2022, FIORE cannabis went insolvent so the business assets were seized by the debt holders of that company. The Allied building was on that land. Needless to say the lease was cancelled and Allied submitted an application to gain possession of the building back and have it be excluded from the bankruptcy proceedings of FIORE. This is in process with the state of Nevada. We are not aware of any other legal proceedings contemplated by any governmental authority or any other party involving us or our properties.
Commitments and Contractual Obligations
As a “smaller reporting company” as defined by Item 10 of Regulation S-K, the Company is not required to provide this information.
Off-balance Sheet Arrangements
The Company has no off-balance sheet arrangements.
Going Concern
As reflected in the accompanying financial statements, the Company had an accumulated deficit of $45,608,336 at August 31, 2023 and a net loss of $10,675,671 for the fiscal year ended August 31, 2023.
The Company does not yet have a history of financial stability. Historically, the principal source of liquidity has been the issuance of convertible notes and equity securities. In addition, the Company has generated no revenues since inception. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
The ability of the Company to continue operations is dependent on the success of Management’s plans, which include the raising of capital through the issuance of equity securities, until such time that funds provided by operations are sufficient to fund working capital requirements.
The Company will require additional funding to finance the growth of its current and expected future operations as well as to achieve its strategic objectives. The Company believes its current available cash will be sufficient to meet its cash needs for the near future. There can be no assurance that financing will be available in amounts or terms acceptable to the Company, if at all.
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. These financial statements do not include any adjustments relating to the recovery of the recorded assets or the classification of the liabilities that might be necessary should the Company be unable to continue as a going concern.
Item 8. Financial Statements and Supplementary Data.
ALLIED CORP.
CONSOLIDATED FINANCIAL STATEMENTS

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Allied Corp.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Allied Corp. (the Company) as of August 31, 2023 and 2022, and the related consolidated statements of operations and comprehensive loss, stockholders’ deficit, and cash flows for each of the two years in the period ended August 31, 2023, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of August 31, 2023 and 2022, and the results of its operations and its cash flows for each of the two years in the period ended August 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered net losses from operations, has a net capital deficiency, and has minimal revenue which raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are discussed in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Black Scholes Calculations
As discussed in Note 16 to the Financial Statements, the Company utilizes Black Scholes calculations to determine fair value of the Company’s stock options.
Auditing management’s calculations of fair value of stock options involves significant judgements and estimates to determine the proper value. Volatility and term are the major assumptions used by management in determining the value of the stock options.
To evaluate the appropriateness of fair value calculation, we evaluated management’s significant judgements and estimates in what inputs were utilized within the Black Scholes calculations.
/s/M&K CPAS, PLLC |
| |
We have served as the Company’s auditor since 2022. |
| |
Houston, TX |
| |
December 14, 2023 | |
ALLIED CORP. Consolidated Balance Sheets (Expressed in US Dollars) |
| | August 31, 2023 | | | August 31, 2022 | |
| | | | | | |
Assets | | | | | | |
Current assets | | | | | | |
Cash | | $ | 209,736 | | | $ | 96,043 | |
Inventory (Note 3) | | | 107,510 | | | | 998,988 | |
Other receivables | | | 134,482 | | | | 121,428 | |
Prepaid expenses | | | 18,852 | | | | 25,187 | |
Total current assets | | | 470,580 | | | | 1,241,646 | |
| | | | | | | | |
Deposits and advances (Note 4) | | | 26,354 | | | | 2,869,825 | |
Right-of-use assets (Note 7) | | | 109,301 | | | | 189,325 | |
Property, plant and equipment (Note 5) | | | 1,417,192 | | | | 1,444,038 | |
Intangible assets (Note 6) | | | 40,301 | | | | 44,773 | |
Total assets | | $ | 2,063,728 | | | $ | 5,789,607 | |
| | | | | | | | |
Liabilities | | | | | | | | |
Current liabilities | | | | | | | | |
Accounts payable and accrued liabilities | | $ | 2,393,359 | | | $ | 1,607,862 | |
Due to related parties (Note 11) | | | 693,292 | | | | 384,272 | |
Current portion of lease liabilities (Note 7) | | | 10,745 | | | | 24,725 | |
Loans payable (Note 8) | | | 2,027,348 | | | | 1,555,654 | |
Secured convertible notes payable (Note 9) | | | 3,724,891 | | | | 3,334,891 | |
Total current liabilities | | | 8,849,635 | | | | 6,907,404 | |
| | | | | | | | |
Lease liabilities, net of current portion (Note 7) | | | 98,556 | | | | 164,600 | |
Total liabilities | | $ | 8,948,191 | | | $ | 7,072,004 | |
| | | | | | | | |
Stockholders’ deficit | | | | | | | | |
Preferred stock – 50,000,000 shares authorized, $0.0001 par value Nil shares issued and outstanding | | $ | - | | | $ | - | |
Common stock – 300,000,000 shares authorized, $0.0001 par value; 101,592,914 shares issued and outstanding (93,967,594 – par value $0.0001 – August 31, 2022) | | | 10,160 | | | | 9,397 | |
Additional paid in capital | | | 39,404,667 | | | | 33,999,090 | |
Common stock issuable | | | 80,000 | | | | 540,000 | |
Accumulated deficit | | | (45,608,336 | ) | | | (34,932,665 | ) |
Accumulated other comprehensive loss | | | (770,954 | ) | | | (898,219 | ) |
| | | | | | | | |
Total stockholders’ deficit | | | (6,884,463 | ) | | | (1,282,397 | ) |
Total liabilities and stockholders’ deficit | | $ | 2,063,728 | | | $ | 5,789,607 | |
Nature of operations and going concern (Note 1)
Commitments (Note 14)
Subsequent events (Note 20)
The accompanying notes form an integral part of these consolidated financial statements.
ALLIED CORP. Consolidated Statements of Operations and Comprehensive Loss (Expressed in US dollars) |
| | For the Year Ended August 31, 2023 | | | For the Year Ended August 31, 2022 | |
Revenues | | | | | | |
Sales | | $ | 72,096 | | | $ | 165,596 | |
Cost of sales (Note 3) | | | (1,533,863 | ) | | | (91,476 | ) |
Gross margin | | | (1,461,767 | ) | | | 74,120 | |
| | | | | | | | |
Expenses | | | | | | | | |
Amortization and depreciation | | | 137,046 | | | | 82,589 | |
Consulting fees (Note 10 and 11) | | | 4,189,953 | | | | 12,080,684 | |
Foreign exchange loss (gain) | | | 147,722 | | | | (1,393 | ) |
Interest expense and bank charges | | | 477,750 | | | | 467,451 | |
Office and miscellaneous | | | 590,374 | | | | 1,133,560 | |
Professional fees | | | 439,603 | | | | 1,031,091 | |
Research and development | | | - | | | | 12,062 | |
Travel | | | 12,265 | | | | 43,670 | |
Loss on deposit impairment (Note 4) | | | (2,656,695 | ) | | | - | |
Loss on license acquisition advance (Note 4) | | | (150,000 | ) | | | - | |
Operating expenses | | | 8,801,408 | | | | 14,849,714 | |
Loss before other items | | | (10,263,175 | ) | | | (14,775,594 | ) |
Other income and expenses | | | | | | | | |
Accretion | | | - | | | | (547,598 | ) |
Bad debt recovery | | | - | | | | 3,646 | |
Interest expense | | | (385,453 | ) | | | (219,307 | ) |
Loss on debt extinguishment | | | (27,043 | ) | | | - | |
Total other expenses | | | (412,496 | ) | | | (763,259 | ) |
Net loss | | | (10,675,671 | ) | | | (15,538,853 | ) |
| | | | | | | | |
Other comprehensive income (loss) | | | | | | | | |
Foreign currency translation adjustments | | | 127,265 | | | | (283,346 | ) |
Comprehensive loss | | $ | (10,548,406 | ) | | $ | (15,822,199 | ) |
| | | | | | | | |
Basic and diluted loss per share | | $ | (0.11 | ) | | $ | (0.17 | ) |
| | | | | | | | |
Weighted average number of common shares outstanding | | | 96,797,714 | | | | 92,098,050 | |
The accompanying notes form an integral part of these consolidated financial statements.
ALLIED CORP. Consolidated Statements of Stockholders’ Deficit (Expressed in US dollars) |
| | Common stock | | | Treasury stock | | | Additional | | | | | | | | | Accumulated other | | | | |
| | Number of shares | | | Amount | | | Number of shares | | | Amount | | | paid in capital | | | Stock issuable | | | Accumulated deficit | | | comprehensive loss | | | Total | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, August 31, 2021 | | | 79,858,867 | | | $ | 7,986 | | | | 7,073,170 | | | $ | 707 | | | $ | 18,099,226 | | | $ | 929,985 | | | $ | (19,393,812 | ) | | $ | (614,873 | ) | | $ | (970,781 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Shares issued from treasury | | | 7,073,170 | | | | 707 | | | | (7,073,170 | ) | | | (707 | ) | | | 7,957,316 | | | | - | | | | - | | | | - | | | | 7,957,316 | |
Shares issued for cash | | | 6,659,557 | | | | 666 | | | | - | | | | - | | | | 5,141,571 | | | | (929,985 | ) | | | - | | | | - | | | | 4,212,252 | |
Share issuance costs | | | - | | | | - | | | | - | | | | - | | | | (340,616 | ) | | | - | | | | - | | | | - | | | | (340,616 | ) |
Shares subscribed | | | - | | | | - | | | | - | | | | - | | | | - | | | | 540,000 | | | | - | | | | - | | | | 540,000 | |
Shares issued for finders fees | | | 8,000 | | | | 1 | | | | - | | | | - | | | | 5,999 | | | | - | | | | - | | | | - | | | | 6,000 | |
Shares issued in error not yet cancelled | | | 368,000 | | | | 37 | | | | - | | | | - | | | | (37 | ) | | | - | | | | - | | | | - | | | | - | |
Detachable warrants issued with convertible notes payable | | | - | | | | - | | | | - | | | | - | | | | 120,310 | | | | - | | | | - | | | | - | | | | 120,310 | |
Beneficial conversion feature | | | - | | | | - | | | | - | | | | - | | | | 281,447 | | | | - | | | | - | | | | - | | | | 281,447 | |
Stock-based compensation | | | - | | | | - | | | | - | | | | - | | | | 2,733,874 | | | | - | | | | - | | | | - | | | | 2,733,874 | |
Comprehensive loss for the year | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (15,538,853 | ) | | | (283,346 | ) | | | (15,822,199 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, August 31, 2022 | | | 93,967,594 | | | | 9,397 | | | | - | | | | - | | | | 33,999,090 | | | | 540,000 | | | | (34,932,665 | ) | | | (898,219 | ) | | | (1,282,397 | ) |
The accompanying notes form an integral part of these consolidated financial statements.
ALLIED CORP. Consolidated Statements of Stockholders’ Deficit (Expressed in US dollars) |
| | Common stock | | | Additional | | | | | | | | | Accumulated other comprehensive | | | | |
| | Number of shares | | | Amount | | | paid in capital | | | Stock issuable | | | Accumulated deficit | | | income (loss) | | | Total | |
| | | | | | | | | | | | | | | | | | | | | |
Balance, August 31, 2022 | | | 93,967,594 | | | $ | 9,397 | | | $ | 33,999,090 | | | $ | 540,000 | | | $ | (34,932,665 | ) | | $ | (898,219 | ) | | $ | (1,282,397 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Shares issued for cash | | | 7,479,760 | | | | 748 | | | | 2,080,204 | | | | (540,000 | ) | | | - | | | | - | | | | 1,540,952 | |
Share issuance costs | | | - | | | | - | | | | 191,660 | | | | - | | | | - | | | | - | | | | 191,660 | |
Shares subscribed | | | - | | | | - | | | | - | | | | 80,000 | | | | - | | | | - | | | | 80,000 | |
Shares issued to settle debts | | | 145,560 | | | | 15 | | | | 86,557 | | | | - | | | | - | | | | - | | | | 86,572 | |
Stock-based compensation | | | - | | | | - | | | | 3,047,156 | | | | - | | | | - | | | | - | | | | 3,047,156 | |
Comprehensive loss for the year | | | - | | | | - | | | | - | | | | - | | | | (10,675,671 | ) | | | 127,265 | | | | (10,548,406 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, August 31, 2023 | | | 101,592,914 | | | | 10,160 | | | | 39,404,667 | | | | 80,000 | | | | (45,608,336 | ) | | | (770,954 | ) | | | (6,884,463 | ) |
The accompanying notes form an integral part of these consolidated financial statements.
ALLIED CORP. Consolidated Statements of Cash Flows (Expressed in US dollars) |
| | For the Year Ended August 31, 2023 | | | For the Year Ended August 31, 2022 | |
Cash provided by (used in): | | | | | | |
| | | | | | |
Operating activities | | | | | | |
Net loss for the year | | $ | (10,675,671 | ) | | $ | (15,538,853 | ) |
Adjustment to net loss for the period for non-cash items | | | | | | | | |
Accretion | | | - | | | | 547,598 | |
Accrued interest | | | 857,146 | | | | 238,646 | |
Amortization and depreciation | | | 137,046 | | | | 82,589 | |
Inventory write-down to net realizable value | | | 1,507,018 | | | | 31,737 | |
Fair value of finder’s warrants | | | 204,452 | | | | - | |
Loss on debt extinguishment | | | 27,043 | | | | - | |
Loss on deposit impairment | | | 2,656,695 | | | | - | |
Loss on license acquisition advance | | | 150,000 | | | | - | |
Stock-based compensation - consulting services | | | - | | | | 3,584,392 | |
Stock-based compensation - bonus shares | | | - | | | | 4,585,425 | |
Stock-based compensation - options | | | 3,047,156 | | | | 2,733,874 | |
Changes in non-cash working capital balance: | | | | | | | | |
Increase in other receivables | | | (15,692 | ) | | | (38,591 | ) |
Decrease in prepaid expenses | | | 6,335 | | | | 39,842 | |
Decrease in deposits and advances | | | 37,162 | | | | 315,450 | |
Increase (decrease) in accounts payable and accrued liabilities | | | 212,686 | | | | (215,583 | ) |
Increase in due to related parties | | | 339,020 | | | | 136,752 | |
Increase in inventory | | | (447,175 | ) | | | (787,544 | ) |
| | | (1,956,779 | ) | | | (4,284,266 | ) |
Investing activities | | | | | | | | |
Purchase of intangible assets | | | (4,625 | ) | | | (12,111 | ) |
Purchase of property, plant, and equipment | | | (83,170 | ) | | | (1,193,258 | ) |
| | | (87,795 | ) | | | (1,205,369 | ) |
Financing activities | | | | | | | | |
Advance from related party | | | 70,000 | | | | - | |
Proceeds of convertible notes payable | | | 390,000 | | | | 1,400,000 | |
Repayment of loans payable | | | - | | | | (387,750 | ) |
Repayment of convertible notes payable | | | - | | | | (100,000 | ) |
Proceeds from the issuance of common stock | | | 1,528,160 | | | | 3,877,634 | |
Proceeds for subscriptions of stock issuable | | | 80,000 | | | | 540,000 | |
| | | 2,068,160 | | | | 5,329,884 | |
| | | | | | | | |
Increase (decrease) in cash | | | 23,586 | | | | (159,751 | ) |
Effect of exchange rate on changes of cash | | | 90,107 | | | | (164,031 | ) |
Cash, beginning of year | | | 96,043 | | | | 419,825 | |
Cash, end of year | | $ | 209,736 | | | $ | 96,043 | |
| | | | | | | | |
Supplemental cash flow disclosures: | | | | | | | | |
Income taxes paid | | | - | | | | - | |
Interest paid | | | - | | | | 401,990 | |
Non-cash activities: See Note 17 | | | | | | | | |
The accompanying notes form an integral part of these consolidated financial statements.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
1. Nature of operations, reverse take-over transaction and going concern
a) Nature of operations
Allied Corp. (the “Company or Allied”) was incorporated in the State of Nevada on February 3, 2013. On July 1, 2019, the Company changed its name to Allied Corp. The head office and the registered office of the Company are located at 1405 St. Paul Street, Kelowna BC V1Y 2E4.
The Company’s business plan is to discover new medical technologies some of which are cannabis derived to target full scope therapy and support for trauma survivors, military veterans and first responders, however the Company has not begun such operations nor obtained the required permits to begin such operations.
On September 10, 2019, the Company was acquired in a reverse takeover (“RTO”) transaction and the RTO is considered a purchase of the Company’s net assets by AM (Advanced Micro) Biosciences, Inc. (“AM Biosciences”). For accounting purposes, the legal subsidiary, AM Biosciences has been treated as the acquirer and Allied Corp., the legal parent, has been treated as the acquiree. Accordingly, these consolidated financial statements reflect a continuation of the financial position, operating results, and cash flow of the Company’s legal subsidiary, AM Biosciences from the date of incorporation on September 13, 2018.
On February 18, 2020, the Company acquired all the issued and outstanding share capital of a Colombian company, Allied Colombia S.A.S (“Allied Colombia”). The assets, liabilities and results of Allied Colombia are consolidated in these financial statements beginning from the February 18, 2020 acquisition date. As at August 31, 2023, Allied Colombia has a licensed cannabis farm in Colombia.
b) Going concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. The Company incurred a net loss for the year ended August 31, 2023 of $10,675,671, has generated minimal revenue and as at August 31, 2023 has a working capital deficit of $8,379,055. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern. The consolidated financial statements of the Company do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classifications of liabilities that might be necessary should the Company be unable to continue as a going concern. The Company’s ability to continue as a going concern is dependent upon the Company’s ability to raise sufficient financing to acquire or develop a profitable business. Management intends on financing its operations and future development activities largely from the sale of equity securities with some additional funding from other traditional financing sources, including related party loans until such time that funds provided by future planned operations are sufficient to fund working capital requirements.
c) Business Risks
While some states in the United States have authorized the use and sale of cannabis, it remains illegal under federal law and the approach to enforcement of U.S. federal laws against cannabis is subject to change. The Company plans to engage in cannabis-related activities in the United States, only if and when cannabis operations are federally legalized.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
On January 4, 2018, the then United States Attorney General Jeff Sessions issued a memorandum to United States district attorneys (the “Sessions Memorandum”) which rescinded previous guidance from the United States Department of Justice specific to cannabis enforcement in the United States, including the Cole Memorandum. With the Cole Memorandum rescinded, United States federal prosecutors no longer have guidance relating to the exercise of their discretion in determining whether to prosecute cannabis related violations of United States federal law. Since that time, United States district attorneys have taken no legal action against state law compliant entities, and the Biden administration is generally anticipated to seek federal decriminalization of state legal cannabis activity. Nevertheless, a significant change in the federal government’s enforcement policy with respect to current federal laws applicable to cannabis could cause significant financial damage to the Company. The Company may be irreparably harmed by a change in enforcement policies of the federal government depending on the nature of such change.
Given the current illegality of cannabis under United States federal law, the Company’s ability to access both public and private capital may be hindered by the fact that certain financial institutions are regulated by the United States federal government and are thus prohibited from providing financing to companies engaged in cannabis related activities. The Company’s ability to access public capital markets in the United States is directly hindered as a result. The Company may, however, be able to access public and private capital markets in the United States through institutions which are not regulated by the United States federal government, in Canada, and in many other countries in order to support continuing operations.
2. Significant accounting policies
Business Presentation
These consolidated financial statements and related notes are presented in accordance with accounting principles generally accepted in the United States of America (“US GAAP”), and are expressed in United States dollars. The Company’s fiscal year end is August 31.
The significant accounting policies followed are:
a) Principles of consolidation
The consolidated financial statements include accounts of Allied Corp. and its wholly-owned subsidiaries, including AM Biosciences, Allied US Products LLC, Tactical Relief LLC, Baleno Ltd. and Allied Colombia. Subsidiaries are consolidated from the date of acquisition and control and continue to be consolidated until the date that such control ceases. Control is achieved when the Company is exposed, or has rights, to variable returns from its involvement with the investee and has the ability to affect these returns through its power over the investee. All intercompany balances, income, expenses, and unrealized gains and losses resulting from intercompany transactions are eliminated on consolidation.
b) Cash and cash equivalents
Cash is comprised of cash on hand, cash held in trust accounts and demand deposits. Cash equivalents are short-term, highly liquid investments with maturities within three months when acquired. The Company did not have any cash equivalents as of August 31, 2023 and August 31, 2022.
c) Property, plant and equipment
Property, plant and equipment are stated at cost. The Company depreciates the cost of property, plant and equipment over their estimated useful lives at the following annual rates and methods:
Farm facility and equipment | 1 - 10 years straight-line basis |
Office and computer equipment | 5 - 10 years straight-line basis |
Land equipment | 10 years straight-line basis |
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
d) Inventory
Inventory is comprised of raw materials, supplies, vegetative and flowering plants, dried flower, diluted crude and CBD isolates available for sale, and purchased cannabis products.
Inventory is stated at the lower of cost or net realizable value, determined using weighted average cost. Net realizable value is defined as the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal and transportation. At the end of each reporting period, the Company performs an assessment of inventory and records write-downs for excess and obsolete inventories based on the Company’s estimated forecast of product demand, production requirements, market conditions, regulatory environment, and spoilage. Actual inventory losses may differ from management’s estimates and such differences could be material to the Company’s consolidated balance sheets, statements of net loss and comprehensive loss and statements of cash flows.
e) Intangible assets
Intangible assets include licenses which are being amortized over their estimated useful lives of 10 years. The Company’s licenses are amortized over their economic or legal life on a straight-line basis, whichever is shorter. The licenses have been amortized from the date of acquisition.
The Company periodically evaluates the reasonableness of the useful lives of these assets. Once these assets are fully amortized, they are removed from the accounts. These assets are reviewed for impairment or obsolescence when events or changes in circumstances indicate that the carrying amount may not be recoverable. If impaired, intangible assets are written down to fair value based on discounted cash flows or other valuation techniques. The Company has no intangibles with indefinite lives.
For long-lived assets, impairment losses are only recorded if the asset’s carrying amount is not recoverable through its undiscounted, probability-weighted future cash flows. The Company measures the impairment loss based on the difference between the carrying amount and the estimated fair value. When an impairment exists, the related assets are written down to fair value.
f) Long-lived assets
In accordance with ASC 360, Property, Plant and Equipment, the Company tests long-lived assets or asset groups for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable. Circumstances which could trigger a review include, but are not limited to: significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction of the asset; current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset; and current expectation that the asset will more likely than not be sold or disposed significantly before the end of its estimated useful life. Recoverability is assessed based on the carrying amount of the asset and its fair value, which is generally determined based on the sum of the undiscounted cash flows expected to result from the use and the eventual disposal of the asset, as well as specific appraisal in certain instances. An impairment loss is recognized when the carrying amount is not recoverable and exceeds fair value.
g) Foreign currency translation and functional currency conversion
Items included in these consolidated financial statements of each of the Company’s entities are measured using the currency of the primary economic environment in which the entities operate (the “functional currency”).
Prior to September 10, 2019, the Company’s functional currency was the Canadian dollar. Translation gains and losses from the application of the U.S. dollar as the reporting currency during the period that the Canadian dollar was the functional currency are included as part of cumulative currency translation adjustment, which is reported as a component of shareholders’ equity under accumulated other comprehensive loss.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
The Company re-assessed its functional currency and determined as at September 10, 2019, its functional currency changed from the Canadian dollar to the U.S. dollar based on management’s analysis of changes in our organization. The change in functional currency was accounted for prospectively from September 10, 2019 and prior period financial statements were not restated for the change in functional currency.
For periods commencing September 10, 2019, monetary assets and liabilities denominated in foreign currencies are translated into U.S. dollars using exchange rates in effect at the balance sheet date. Opening balances related to non-monetary assets and liabilities are based on prior period translated amounts, and non-monetary assets and non-monetary liabilities incurred after September 10, 2019 are translated at the approximate exchange rate prevailing at the date of the transaction. Revenue and expense transactions are translated at the approximate exchange rate in effect at the time of the transactions. Foreign exchange gains and losses are included in the statement of operations and comprehensive loss as foreign exchange gains.
The Company assessed the functional currency for Allied Colombia to be the Colombian peso. The functional currency for all other subsidiaries is the U.S. dollar.
h) Share issuance costs
Costs directly attributable to the raising of capital are charged against the related share capital. Costs related to shares not yet issued are recorded as deferred share issuance costs. These costs are deferred until the issuance of the shares to which the costs relate, at which time the costs will be charged against the related share capital or charged to operations if the shares are not issued.
i) Research and development costs
Research and development costs are expensed as incurred. During the year ended August 31, 2023, the Company incurred research and development costs of $nil (August 31, 2022 – $12,062).
j) Advertising costs
Advertising costs are expensed as incurred. During the year ended August 31, 2023, the Company incurred advertising costs of $11,425 (August 31, 2022 – $391,740).
k) Revenue recognition
The Company’s revenue is comprised of sales of cannabis products.
The Company’s revenue-generating activities have a single performance obligation and revenue is recognized at the point in time when control of the product transfers and the Company’s obligations have been fulfilled. This generally occurs when the product is shipped or delivered to the customer, depending upon the method of distribution and shipping terms set forth in the customer contract. Revenue is measured as the amount of consideration the Company expects to receive in exchange for the sale of the Company’s product. Certain of the Company’s customer contracts may provide the customer with a right of return. In certain circumstances the Company may also provide a retrospective price adjustment to a customer. These items give rise to variable consideration, which is recognized as a reduction of the transaction price based upon the expected amounts of the product returns and price adjustments at the time revenue for the corresponding product sale is recognized. The determination of the reduction of the transaction price for variable consideration requires that the Company make certain estimates and assumptions that affect the timing and amounts of revenue recognized.
Sales of products are for cash or otherwise agreed-upon credit terms. The Company’s payment terms vary by location and customer; however, the time period between when revenue is recognized and when payment is due is not significant. The Company estimates and reserves for its bad debt exposure based on its experience with past due accounts and collectability, write-off history, the aging of accounts receivable and an analysis of customer data.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
l) Net income (loss) per common share
Net income (loss) per share is calculated in accordance with ASC 260, Earnings per Share. The weighted-average number of common shares outstanding during each period is used to compute basic earning or loss per share. Diluted earnings or loss per share is computed using the weighted average number of shares and diluted potential common shares outstanding to the extent the effect would not be antidilutive. Dilutive potential common shares are additional common shares assumed to be exercised.
Basic net income (loss) per common share is based on the weighted average number of shares of common stock outstanding.
m) Income taxes
The Company accounts for income taxes under ASC 740, Income Taxes. Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Under ASC 740, the effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period the enactment occurs. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax assets through future operations.
n) Related party transactions
Related parties are any entities or individuals that, through employment, ownership or other means, possess the ability to direct or cause the direction of the management and policies of the Company. The Company discloses related party transactions that are outside of normal compensatory agreements, such as salaries. Related party transactions are measured at the exchange amounts.
o) Significant accounting estimates and judgments
The preparation of the financial statements in conformity with US GAAP requires management to make judgments, estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Although management uses historical experience and its best knowledge of the amount, events or actions to for the basis for judgments and estimates, actual results may differ from these estimates.
The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and further periods if the review affects both current and future periods.
Significant estimates and assumptions included in these financial statements relate to the valuation assumptions related to the estimated useful lives and recoverability of long-lived assets, stock-based compensation, and deferred income tax assets and liabilities. Judgments are required in the assessment of the Company’s ability to continue to as going concern as described in Note 1.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
p) Financial instruments
ASC 825, Financial Instruments, requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 825 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 825 prioritizes the inputs into three levels that may be used to measure fair value:
Level 1
Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
Level 2
Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
Level 3
Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
The financial instruments consist principally of cash, due from related parties, accounts payable, note payable, and convertible notes payable. The fair value of cash when applicable is determined based on “Level 1” inputs, which consist of quoted prices in active markets for identical assets. The Company believes that the recorded values of all other financial instruments which are categorized as loans and receivables approximate their current fair values because of their nature and respective relatively short maturity dates or current market rates of interest for similar instruments.
For certain of the Company’s financial instruments, including accounts payable, due from related parties, notes and loans payable, the carrying amounts approximate their fair values due to the short maturities.
The Company does not have any assets or liabilities measured at fair value on a recurring basis presented on the Company’s balance sheet as of August 31, 2023 and August 31, 2022 other than cash.
Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash. The Company limits its exposure to credit loss by placing its cash with high credit quality financial institutions.
q) Leases
The Company determines if an arrangement contains a lease in whole or in part at the inception of the contract. Right-of-use (“ROU”) assets represent the Company’s right to use an underlying asset for the lease term while lease liabilities represent our obligation to make lease payments arising from the lease. All leases with terms greater than twelve months result in the recognition of a ROU asset and a liability at the lease commencement date based on the present value of the lease payments over the lease term. Unless a lease provides all of the information required to determine the implicit interest rate, the Company uses its incremental borrowing rate based on the information available at the commencement date in determining the present value of the lease payments. The Company uses the implicit interest rate in the lease when readily determinable.
Our lease terms include all non-cancelable periods and may include options to extend (or to not terminate) the lease when it is reasonably certain that we will exercise that option. Leases with terms of twelve months or less at the commencement date are expensed on a straight-line basis over the lease term and do not result in the recognition of an asset or liability. See Note 7 – Leases.
r) Recent accounting pronouncements
In August 2020, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2020-06, Debt — Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40) (“ASU 2020-06”) to simplify accounting for certain financial instruments. ASU 2020-06 eliminates the current models that require separation of beneficial conversion and cash conversion features from convertible instruments and simplifies the derivative scope exception guidance pertaining to equity classification of contracts in an entity’s own equity. The new standard also introduces additional disclosures for convertible debt and freestanding instruments that are indexed to and settled in an entity’s own equity. ASU 2020-06 amends the diluted earnings per share guidance, including the requirement to use the if-converted method for all convertible instruments. ASU 2020-06 is effective for fiscal years beginning after December 15, 2023, and should be applied on a full or modified retrospective basis, with early adoption permitted beginning on January 1, 2021. The Company adopted ASU 2020-06 effective January 1, 2022. The adoption of ASU 2020-06 did not have an impact on the Company’s financial statements.
The Company does not expect that recent accounting pronouncements or changes in accounting pronouncements during the year ended August 31, 2023, are of significance or potential significance to the Company.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
3. Inventory
Inventory is comprised of the following items:
| | August 31, 2023 | | | August 31, 2022 | |
| | | | | | |
Work in progress | | $ | 80,094 | | | $ | 326,556 | |
Finished goods | | | 27,416 | | | | 383,459 | |
Inventory in transit | | | - | | | | 288,973 | |
Total inventory | | $ | 107,510 | | | $ | 998,988 | |
The costs of inventory include but are not limited to labor, utilities, nutrition and irrigation, overhead and the depreciation of manufacturing equipment and production facilities, and amortization of licenses determined at normal capacity. Manufacturing overhead and related expenses include salaries, wages, employee benefits, utilities, maintenance and rent of grow facility. The Company began production in Colombia in late 2020, when the Company obtained approval for its strains of products. During the current period, certain costs were determined based on the actual usage of production space as compared to the normal predetermined operational production of the facility based on capacity as the Company gradually started to grow products and prepared the facility ready.
During the year ended August 31, 2023, the Company recorded a $43,861 write-off of inventory costs for CBD isolates and other derivative products to reduce to its net realizable value of $nil. In addition, the Company recorded additional $1,463,157 write-off of inventory costs to its net realizable value of $107,510. During the year ended August 31, 2022, the Company recorded a $31,737 write-off of inventory costs for products purchased for resale to reduce to its net realizable value of $nil.
4. Deposits and advances
| | August 31, 2023 | | | August 31, 2022 | |
| | | | | | |
a) Towards the purchase of prefabricated buildings | | $ | - | | | $ | 2,656,695 | |
b) Deposit towards a license acquisition | | | - | | | | 150,000 | |
c) Prepayments for construction facility in Colombia | | | 26,354 | | | | 63,130 | |
Total deposits and advances | | $ | 26,354 | | | $ | 2,869,825 | |
a) In June 2019, the Company’s wholly owned subsidiary, AM Biosciences, signed a production and manufacturing contract to begin the manufacturing of the full building initially intended for the Canada extraction and production facility. The Company had determined to utilize these building assets in connection with potential United States operations in the State of Nevada upon on the occurrence or waiver of the changes in U.S. federal law to permit the general cultivation, distribution, and possession of marijuana or to remove the regulation of such activities from the federal laws of the United States. In connection with that determination, on March 30, 2021, the Company’s wholly owned subsidiary, Allied US Products LLC, entered into a contingent asset purchase agreement (“Agreement”) to acquire two privileged licenses issued by the Nevada Department of Taxation purposed for the cultivation of cannabis (the “Licenses”). In consideration for the licenses, the Company agreed to pay $150,000, issue a $1,350,000 promissory note and assume certain liabilities. Concurrent with the execution of the Agreement, the Company’s wholly owned subsidiary, Tactical Relief LLC, agreed to enter into a 25-year land lease with the Fiore Subsidiary.
At August 31, 2022, Company had paid deposits of $2,656,695 to purchase the prefabricated buildings and $150,000 to purchase the licenses. At August 31, 2022, the Company had not yet received the buildings and the amounts have been recorded as deposits. In addition, the Agreement has not been closed and the payment of $150,00 has been recorded as a deposit.
As the Company made payments towards the purchase of the prefabricated buildings, various parts of the building were delivered to the property on the land lease. In December 2022, the Company was advised that the Fiore Subsidiary had become insolvent and that a trustee’s sale had been scheduled for the property which the Company had agreed to lease. In order to protect the Company’s interests, the Company filed a complaint in the Eighth Judicial District Court in Clark County, Nevada seeking injunctive relief to terminate the trustee’s sale or alternatively money damages. The Company filed a lis pendens against the property to reflect this complaint and to protect its interests in the building. However, the trustee’s sale went forward and the successful bidder claimed that the buildings were part of the real property and that it became the owner of the buildings. As a result, the Company filed suit, seeking a declaration of the Company’s ownership of the buildings as the buildings were personal property and not a part of the real property. A status conference was held for December 13, 2023 and the Company is currently waiting for the next court date.
Due to the uncertainties of collectability, the Company recorded a loss on impairment of deposits during the year ended August 31, 2023. In addition, the Company recorded a loss on license acquisition advance of $150,000 during the year ended August 31, 2023 as the Agreement has effectively been terminated.
b) The Company paid certain vendors for the construction of farm facilities in Colombia in advance. At August 31, 2023, the prepayments totaled $26,354 (August 31, 2022 – $63,130).
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
5. Property, plant and equipment
At August 31, 2023, property, plant and equipment consisted of:
| | Construction in process | | | Farm facility and equipment | | | Office and computer equipment | | | Land equipment | | | Total | |
Cost | | | | | | | | | | | | | | | |
August 31, 2022 | | $ | 460,648 | | | $ | 1,152,822 | | | $ | 21,348 | | | $ | 27,150 | | | $ | 1,661,968 | |
Additions | | | 31,528 | | | | 45,344 | | | | 6,167 | | | | 132 | | | | 83,171 | |
Transfer | | | (93,363 | ) | | | 93,363 | | | | - | | | | - | | | | - | |
Foreign exchange | | | 30,936 | | | | 54,844 | | | | 2,437 | | | | 2,243 | | | | 90,460 | |
August 31, 2023 | | $ | 429,749 | | | $ | 1,346,373 | | | $ | 29,952 | | | $ | 29,525 | | | $ | 1,835,599 | |
| | | | | | | | | | | | | | | | | | | | |
Accumulated depreciation | | | | | | | | | | | | | | | | | | | | |
August 31, 2022 | | $ | - | | | $ | 210,899 | | | $ | 4,084 | | | $ | 2,947 | | | $ | 217,930 | |
Additions | | | - | | | | 167,747 | | | | 2,953 | | | | 2,663 | | | | 173,363 | |
Foreign exchange | | | - | | | | 25,913 | | | | 663 | | | | 538 | | | | 27,114 | |
August 31, 2023 | | $ | - | | | $ | 404,559 | | | $ | 7,700 | | | $ | 6,148 | | | $ | 418,407 | |
| | | | | | | | | | | | | | | | | | | | |
Net book value | | | | | | | | | | | | | | | | | | | | |
August 31, 2022 | | $ | 460,648 | | | $ | 941,923 | | | $ | 17,264 | | | $ | 24,203 | | | $ | 1,444,038 | |
August 31, 2023 | | $ | 429,749 | | | $ | 941,814 | | | $ | 22,252 | | | $ | 23,377 | | | $ | 1,417,192 | |
As of August 31, 2023 and August 31, 2022, the construction in process has not been in use.
6. Intangible assets
At August 31, 2023, intangible assets consisted of:
| | Cost $ | | | Foreign exchange $ | | | Accumulated amortization $ | | | Accumulated impairment $ | | | August 31, 2023 Net carrying value $ | | | August 31, 2022 Net carrying value $ | |
| | | | | | | | | | | | | | | | | | |
Cannabis licenses | | | 5,451,409 | | | | (468,910 | ) | | | (1,140,531 | ) | | | (3,801,667 | ) | | | 40,301 | | | | 44,773 | |
| | | 5,451,409 | | | | (468,910 | ) | | | (1,140,531 | ) | | | (3,801,667 | ) | | | 40,301 | | | | 44,773 | |
On February 17, 2020, the Company acquired $5,435,334 of licenses as part of the acquisition of Allied Colombia. The licenses acquired are issued by the Republic of Colombia and include the use of seeds for growing Cannabis, production of derivatives from Cannabis for medicinal and scientific use, cultivation of Cannabis plants, and producer of seeds. The Company recorded amortization of these licenses of $658,836 for the year ended August 31, 2021, of which $158,121 was included in cost of inventory.
Management acquired the licenses with a plan to operate in Colombia and believed the amounts paid for the licenses would be recovered from future operations. The Company has only recently started its operations in Colombia and has limited history on which to base future outcomes from operations including cash flows. Cannabis and hemp are considered to be an emerging industry and Colombia does not yet have a sufficiently established observable legal market in which the Company could sell its Cannabis or hemp flower and CBD or THC extracts. Laws and regulations in Colombia are evolving and there is uncertainty in what will be legally permissible in a future market and what prices and demand there would be in Colombia for the Company’s products. At the present, the Company’s export activities are regulated and restricted by Colombian law and it is uncertain whether future changes in law would favorably impact the Company’s operations. Due to the uncertainty in the timing and amount of future cash flows from operations the Company has written down its licenses to the estimated recoverable amount. During the year ended August 31, 2021, the Company recorded impairment of intangible assets in the amount of $2,687,695.
During the year ended August 31, 2023, the Company acquired $3,964 (COP 19,254,199) of licenses and Company recorded amortization of $9,097 (COP 41,315,205).
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
7. Leases
The Company accounts for leases under ASC 842, Leases, which establishes a right-of-use (“ROU”) model that requires a lessee to record an ROU asset and a lease liability, measured on a discounted basis, on the balance sheet for all leases with terms longer than 12 months. The Company also elected to keep all leases with an initial term of 12 months or less off the balance sheet.
The Company did not have any leases until the acquisition of Allied Colombia during the year ended August 31, 2020. The acquisition resulted in the addition of $82,398 of operating lease assets and liabilities. During the year ended August 31, 2021, the Company re-measured its lease liabilities under this lease as the criteria was met for additional monthly payment when the Company started production as outlined in the lease agreement, resulting in additional lease liabilities of $70,705.
On August 10, 2021, the Company entered into another lease for additional land in Colombia with monthly payment of $2,647 (COP9,970,675) for 12.5 hectares which is intended for use of outdoor cultivation, resulting in additional lease liabilities of $104,902. The lease is for 5 years, and expires in July 2026. Management has assessed the lease as an operating lease. Effective August 31, 2023, the Company terminated the lease. Pursuant to ASC 842-20 upon the termination of the lease, the Company derecognized the lease related asset and liability and included any consideration paid or received upon termination that was not already included in the lease payments in the gain or loss on termination of the lease.
ROU assets represent the Company's right to use an underlying asset for the lease term and lease liabilities represent the Company's obligation to make lease payments arising from the lease calculated by discounting fixed lease payments over the lease term at the rate implicit in the lease or the Company’s incremental borrowing rate. Lease liabilities are increased by interest and reduced by payments each period, and the ROU asset is amortized over the lease term. For operating leases, interest on the lease liability and the amortization of the ROU asset result in straight-line rent expense over the lease term. For finance leases, interest on the lease liability and the amortization of the ROU asset results in front-loaded expense over the lease term. ROU assets and liabilities are recognized at the commencement date of the lease based on the present value of lease payments over the lease term. At August 31, 2023, the Company did not have any finance leases.
At August 31, 2023, the weighted average remaining operating lease term was 6 years and the weighted average discount rate associated with operating leases was 15%.
The components of lease expenses were as follows:
| | $ | |
Operating lease cost: | | | |
Amortization of right-of-use assets | | | 24,079 | |
Interest on lease liabilities | | | 26,046 | |
| | | | |
Total operating lease cost | | | 50,125 | |
The following table provides supplemental cash flow and other information related to leases for the year ended August 31, 2023:
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
Supplemental balance sheet information related to leases as of August 31, 2023 are as below:
| | | $ | |
Cost | | | 282,273 | |
Accumulated amortization | | | (55,220 | ) |
Lease termination | | | (61,928 | ) |
Foreign exchange | | | (55,824 | ) |
| | | | |
Net carrying value at August 31, 2023 | | | 109,301 | |
Future minimum lease payments related to lease obligations are as follows:
| | $ | |
2024 | | | 26,422 | |
2025 | | | 26,422 | |
2026 | | | 26,422 | |
2027 | | | 26,422 | |
2028 | | | 26,422 | |
2029 | | | 26,422 | |
2030 | | | 13,210 | |
| | | | |
Total minimum lease payments | | | 171,742 | |
| | | | |
Less: amount of lease payments representing effects of discounting | | | (62,441 | ) |
| | | | |
Present value of future minimum lease payments | | | 109,301 | |
| | | | |
Less: current obligations under leases | | | (10,745 | ) |
| | | | |
Lease liabilities, net of current portion | | | 98,556 | |
8. Loans payable
a) In June 2020, the Company entered into a financing agreement to finance the buildings described in Note 4(a). Pursuant to the agreement, the Company financed $1,253,772 of the purchase price. The Company paid $71,023 at commencement date on May 29, 2020 and would make six monthly interest payments of $37,613 commencing June 20, 2020 and repay the principal of $1,253,772 on November 20, 2020. The loan was extended after the initial maturity date. On December 27, 2021, the Company entered into an amendment agreement. Pursuant to the amendment, the Company will make 27 monthly payments starting on January 20, 2022. For the first 3 months, the Company will make monthly payments of $37,613. For the remaining 24 months, the Company will make monthly payments of $66,288. If the first 6 monthly payments are made on time, the Company may prepay the unamortized loan balance with a 2% penalty of the remaining balance. During the year ended August 31, 2023, the Company paid interest in the amount of $nil (2022 - $363,337). As of August 31, 2023, the Company has missed thirteen monthly payments and the balance owing is $1,742,648 (August 31, 2022 - $1,291,290).
b) On December 17, 2021, the Company entered into a financing agreement to finance an equipment purchase. On March 31, 2022, the agreement was amended to remove shipping charges. Pursuant to the amended agreement, the Company financed $295,543 of the purchase price with an interest rate of 8% per annum. The Company will make 21 monthly principal and interest payments of $15,000 and a final payment of $633 in September 2023. During the year ended August 31, 2023, the Company paid interest in the amount of $nil (2022 - $3,653). As of August 31, 2023, the Company has missed eighteen monthly payments and the balance owing is $284,700 (August 31, 2022 - $264,364).
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
9. Secured convertible notes payable
The Company has granted each and every of the secured convertible note holders a continuing security interest in, a general lien upon, and aright of set-off against all existing and future assets and property under the terms of a security agreement.
a) On January 23, 2020, the Company issued two convertible notes with principal amounts of $400,000 and $200,000, respectively, with a total face value of $600,000 (the “Notes”) and warrants to purchase 240,000 shares of the Company’s common stock at $1.25 per share for 1 year. The Notes were issued with an original discount of $12,000, and bear interest at 10% per annum compounded monthly. The notes mature on July 20, 2020 and are convertible into shares of the Company’s common stock at any time prior to maturity at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Notes or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion features are indexed to the Company’s own stock and are classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion features and warrants do not meet derivative classification.
The relative fair values of the convertible notes and the warrants were $470,467 and $117,533 respectively. The effective conversion price was then determined to be $0.98. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $115,383 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $108,100 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $364,517. The beneficial conversion feature of $115,383, the original issue discount of $12,000 and the relative fair value of the warrants of $108,100 discounted the carrying value of the convertible debt on the date of issue. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. On June 30, 2020, the Company repaid $200,000 of the $600,000 note which left $200,000 outstanding on each note.
i. First Modification:
On July 1, 2020, the Company entered into amendments to the convertible notes. Pursuant to the amendments, the convertible notes bear simple interest at 5% per annum. The maturity date of the convertible notes was amended to due on demand on or before October 31, 2020. In consideration for extending the maturity date, the Company issued to the convertible note holders 16,000 common shares of the Company and warrants to purchase additional 320,000 common shares of the Company at $1.25 per share expiring October 31, 2021. Each note holder received 8,000 common shares and 160,000 warrants.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was not granted. As the creditor has not granted a concession, the guidance contained in ASC 470-60 was applied. As present value of the cash flows under the new debt instrument differed by more than 10% from the present value of the remaining cash flows under the terms of the original debt instrument, it was determined that the debt was substantially different which resulted in extinguishment accounting.
The extended convertible notes had a total carrying value of $400,000. As the common shares and warrants were issued as consideration for extending the convertible notes, the fair value of the common share and warrants of $218,397 were expensed under extinguishment accounting. The fair value of these costs were included in the calculation of the loss on extinguishment of $220,065.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
ii. Second Modification:
On November 1, 2020, the Company entered into amendments to the convertible notes. Pursuant to the amendments, the maturity date of the convertible notes was amended to due on demand on or before March 31, 2021. In consideration for extending the maturity date, the Company agreed to issue to the convertible note holders 100,000 common shares of the Company. Each note holder received 50,000 common shares.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was not granted. As the creditor has not granted a concession, the guidance contained in ASC 470-60 was applied. As present value of the cash flows under the new debt instrument differed by more than 10% from the present value of the remaining cash flows under the terms of the original debt instrument, it was determined that the debt was substantially different which resulted in extinguishment accounting.
The extended convertible notes had a total carrying value of $400,000. As the common shares were issued as consideration for extending the convertible notes, the fair value of the common share of $110,000 were expensed under extinguishment accounting. The fair value of these costs were included in the calculation of the loss on extinguishment of $110,000.
iii. Third Modification:
On March 31, 2021, the Company entered into amendments to the convertible notes. Pursuant to the amendments, the convertible notes bear simple interest at 10% per annum. Pursuant to the amendments, the maturity date of the convertible notes was amended to due on demand on or before September 30, 2021. In consideration for extending the maturity date, the Company agreed to issue to the convertible note holders 20,000 common shares of the Company. Each note holder received 10,000 common shares.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was not granted. As the creditor has not granted a concession, the guidance contained in ASC 470-60 was applied. As present value of the cash flows under the new debt instrument differed by more than 10% from the present value of the remaining cash flows under the terms of the original debt instrument, it was determined that the debt was substantially different which resulted in extinguishment accounting.
The extended convertible notes had a total carrying value of $400,000. As the common shares were issued as consideration for extending the convertible notes, the fair value of the common share of $20,000 were expensed under extinguishment accounting. The fair value of these costs were included in the calculation of the loss on extinguishment of $20,000.
iv. Fourth Modification:
On October 1, 2021, the Company entered into amendments to the convertible notes. Pursuant to the amendments, the convertible notes bear simple interest at 10% per annum. Pursuant to the amendments, the maturity date of the convertible notes was amended to due on demand on or after March 31, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
v. Fifth Modification:
On March 31, 2022, the Company entered into amendments to the convertible notes. Pursuant to the amendments, the convertible notes bear simple interest at 10% per annum. Pursuant to the amendments, the maturity date of the convertible notes was amended to due on demand on or after September 30, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
During the year ended August 31, 2023, the Company made interest payment of $nil (2022 - $20,000). As at August 31, 2023, the Company has recorded accrued interest of $97,205, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $400,000 (August 31, 2022 - $400,000).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
b) On September 29, 2020, the Company issued a convertible note with a fair value of $163,341 (the “Note”) and warrants to purchase 130,673 shares of the Company’s common stock at $1.25 per share for 2 years. The Note bears interest at 10% per annum. The Note is due on demand after March 27, 2021. The Note was convertible into shares of the Company’s common stock at any time prior to March 27, 2021 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
The relative fair values of the convertible note and the warrants were $85,330 and $78,011 respectively. The effective conversion price was then determined to be $0.65. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $85,330 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $78,011 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $Nil. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method.
i. First Modification:
On March 31, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before September 30, 2021. In consideration for extending the maturity date, the Company issued to the convertible note holder 8,268 common shares of the Company.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was not granted. As the creditor has not granted a concession, the guidance contained in ASC 470-60 was applied. As present value of the cash flows under the new debt instrument differed by more than 10% from the present value of the remaining cash flows under the terms of the original debt instrument, it was determined that the debt was substantially different which resulted in extinguishment accounting.
The extended convertible note had a total carrying value of $163,341. As the common shares were issued as consideration for extending the convertible note, the fair value of the common shares of $8,268 was expensed under extinguishment accounting. The fair value of these costs were included in the calculation of the loss on extinguishment of $8,268.
ii. Second Modification:
On June 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before November 30, 2021 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
iii. Third Modification:
On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
iv. Fourth Modification:
On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
As at August 31, 2023, the Company has recorded accrued interest of $33,540, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $63,341 (August 31, 2022 - $63,341).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
c) On October 26, 2020, the Company issued a convertible note with a face value of $37,613 (the “Note”) and warrants to purchase 30,090 shares of the Company’s common stock at $1.25 per share for 2 years. The Note bears interest at 10% per annum. The Note is due on demand after April 23, 2021. The Note was convertible into shares of the Company’s common stock at any time prior to April 23, 2021 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
The relative fair values of the convertible note and the warrants were $20,176 and $17,437 respectively. The effective conversion price was then determined to be $0.65. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $20,176 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $17,437 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $Nil. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method.
i. First Modification:
On June 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before November 30, 2021 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
ii. Second Modification:
On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
iii. Third Modification:
On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
As at August 31, 2023, the Company has recorded accrued interest of $10,706, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $37,613 (August 31, 2022 - $37,613).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
d) On November 11, 2020, the Company issued a convertible note with a face value of $85,937 (the “Note”) and warrants to purchase 68,750 shares of the Company’s common stock at $1.25 per share for 2 years. The Note bears interest at 10% per annum. The Note is due on demand after May 9, 2021. The Note was convertible into shares of the Company’s common stock at any time prior to April 23, 2021 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification.
The relative fair values of the convertible note and the warrants were $48,258 and $37,679 respectively. The effective conversion price was then determined to be $0.70. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $48,258 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $37,679 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $Nil. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method.
i. First Modification:
On June 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before November 30, 2021 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
ii. Second Modification:
On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
iii. Third Modification:
On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
As at August 31, 2023, the Company has recorded accrued interest of $24,087, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $85,937 (August 31, 2022 - $85,937).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
e) On December 2, 2020, the Company issued a convertible note with a face value of $600,000 (the “Note”) and warrants to purchase 240,000 shares of the Company’s common stock at $1.25 per share for 2 years. The Note bears interest at 10% per annum. The Note is due on demand after November 27, 2021. The Note was convertible into shares of the Company’s common stock at any time prior to November 27, 2021 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification.
The relative fair values of the convertible note and the warrants were $457,436 and $142,564 respectively. The effective conversion price was then determined to be $0.95. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $457,436 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $22,564 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $Nil. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method.
i. First Modification:
On October 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
ii. Second Modification:
On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
As at August 31, 2023, the Company has recorded accrued interest of $164,712, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $600,000 (August 31, 2022 - $600,000).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
f) On January 7, 2021, the Company issued a convertible note with a face value of $300,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after November 27, 2021. The Note was convertible into shares of the Company’s common stock at any time prior to April 23, 2021 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification. In addition, it was determined that there was no beneficial conversion feature (“BCF”) as the stock price at the issuance date was less than the conversion price.
i. First Modification:
On October 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
ii. Second Modification:
On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
As at August 31, 2023, the Company has recorded accrued interest of $52,521, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $300,000 (August 31, 2022 - $300,000).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
g) On March 26, 2021, the Company issued a convertible note with a face value of $18,000 (the “Note”) and warrants to purchase 18,000 shares of the Company’s common stock at $0.50 per share for 1 year. The Note bears interest at 10% per annum. The Note is due on demand after September 26, 2021. The Note was convertible into shares of the Company’s common stock at any time prior to September 26, 2021 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification.
The relative fair values of the convertible note and the warrants were $10,096 and $7,904 respectively. The effective conversion price was then determined to be $0.70. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $4,016 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $7,904 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $6,080. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method.
i. First Modification:
On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
ii. Second Modification:
On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
As at August 31, 2023, the Company has recorded accrued interest of $4,379, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $18,000 (August 31, 2022 - $18,000).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
h) On March 26, 2021, the Company issued a convertible note with a face value of $100,000 (the “Note”) and warrants to purchase 100,000 shares of the Company’s common stock at $0.50 per share for 1 year. The Note bears interest at 10% per annum. The Note is due on demand after September 26, 2021. The Note is convertible into shares of the Company’s common stock at any time prior to September 26, 2021 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
The relative fair values of the convertible note and the warrants were $56,086 and $43,914 respectively. The effective conversion price was then determined to be $0.70. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $22,314 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $43,914 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $33,772. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method.
i. First Modification:
On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
ii. Second Modification:
On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
As at August 31, 2023, the Company has recorded accrued interest of $24,274, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $100,000).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
i) On April 30, 2021, the Company issued a convertible note with a face value of $100,000 (the “Note”) and warrants to purchase 100,000 shares of the Company’s common stock at $1.00 per share for 1 year. The Note bears interest at 10% per annum. The Note is due on demand after October 31, 2021. The Note is convertible into shares of the Company’s common stock at any time prior to October 31, 2021 at a conversion price of $1.00 per share.
The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification.
The relative fair values of the convertible note and the warrants were $61,493 and $38,507 respectively. The effective conversion price was then determined to be $0.61. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $27,272 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $38,507 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $34,221. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method.
i. First Modification:
On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
ii. Second Modification:
On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
As at August 31, 2023, the Company has recorded accrued interest of $23,370, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $100,000).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
j) On April 29, 2021, the Company issued a convertible note with a face value of $180,000 (the “Note”) and warrants to purchase 180,000 shares of the Company’s common stock at $1.00 per share for 1 year. The Note bears interest at 10% per annum. The Note is due on demand after October 29, 2021. The Note was convertible into shares of the Company’s common stock at any time prior to October 29, 2021 at a conversion price of $1.00 per share.
The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification.
The relative fair values of the convertible note and the warrants were $111,422 and $68,578 respectively. The effective conversion price was then determined to be $0.62. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $46,078 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $68,578 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $65,344. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method.
i. First Modification:
On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
ii. Second Modification:
On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
As at August 31, 2023, the Company has recorded accrued interest of $42,016, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $180,000 (August 31, 2022 - $180,000).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
k) On July 25, 2021, the Company issued a convertible note with a face value of $35,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after January 25, 2022. The Note was convertible into shares of the Company’s common stock at any time prior to January 25, 2022 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification. In addition, it was determined that there was no beneficial conversion feature (“BCF”) as the stock price at the issuance date was less than the conversion price.
i. First Modification:
On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after March 31, 2022 for no additional consideration.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was not granted. As the creditor has not granted a concession, the guidance contained in ASC 470-50 was applied. As present value of the cash flows under the new debt instrument differed by less than 10% from the present value of the remaining cash flows under the terms of the original debt instrument, it was determined that the debt was not substantially different which resulted in modification accounting.
If a convertible debt instrument is modified or exchanged in a transaction that is not accounted for as an extinguishment, an increase in the fair value of the embedded conversion option (calculated as the difference between the fair value of the embedded conversion option immediately before and after the modification or exchange) shall reduce the carrying amount of the debt instrument (increasing a debt discount or reducing a debt premium) with a corresponding increase in additional paid-in capital.
The Company recognized the increase in the fair value of the embedded beneficial conversion feature of $1,090 as additional-paid-in capital and an equivalent discount that reduced the carrying value of the convertible debt to $33,910 at November 1, 2021.
ii. Second Modification:
On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
As at August 31, 2023, the Company has recorded accrued interest of $7,336, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $35,000 (August 31, 2022 - $35,000).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
l) On July 25, 2021, the Company issued a convertible note with a face value of $15,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after January 25, 2022. The Note was convertible into shares of the Company’s common stock at any time prior to January 25, 2022 at a conversion price of $1.25 per share.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification. In addition, it was determined that there was no beneficial conversion feature (“BCF”) as the stock price at the issuance date was less than the conversion price.
i. First Modification:
On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after March 31, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was not granted. As the creditor has not granted a concession, the guidance contained in ASC 470-50 was applied. As present value of the cash flows under the new debt instrument differed by less than 10% from the present value of the remaining cash flows under the terms of the original debt instrument, it was determined that the debt was not substantially different which resulted in modification accounting.
If a convertible debt instrument is modified or exchanged in a transaction that is not accounted for as an extinguishment, an increase in the fair value of the embedded conversion option (calculated as the difference between the fair value of the embedded conversion option immediately before and after the modification or exchange) shall reduce the carrying amount of the debt instrument (increasing a debt discount or reducing a debt premium) with a corresponding increase in additional paid-in capital.
The Company recognized the increase in the fair value of the embedded beneficial conversion feature of $467 as additional-paid-in capital and an equivalent discount that reduced the carrying value of the convertible debt to $14,533 at November 1, 2021.
ii. Second Modification:
On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
As at August 31, 2023, the Company has recorded accrued interest of $3,144, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $15,000 (August 31, 2022 - $15,000).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
m) On October 1, 2021, the Company issued a convertible note with a face value of $100,000 (the “Note”) and warrants to purchase 100,000 shares of the Company’s common stock at $1.25 per share for 1 year. The Note bears interest at 10% per annum. The Note is due on demand after March 31, 2022. The Note was convertible into shares of the Company’s common stock at any time prior to March 31, 2022 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification.
The relative fair values of the convertible note and the warrants were $40,117 and $59,883 respectively. The effective conversion price was then determined to be $0.40. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $40,117 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $59,883 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $Nil. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method.
i. First Modification:
On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after March 31, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was not granted. As the creditor has not granted a concession, the guidance contained in ASC 470-50 was applied. As present value of the cash flows under the new debt instrument differed by less than 10% from the present value of the remaining cash flows under the terms of the original debt instrument, it was determined that the debt was not substantially different which resulted in modification accounting.
If a convertible debt instrument is modified or exchanged in a transaction that is not accounted for as an extinguishment, an increase in the fair value of the embedded conversion option (calculated as the difference between the fair value of the embedded conversion option immediately before and after the modification or exchange) shall reduce the carrying amount of the debt instrument (increasing a debt discount or reducing a debt premium) with a corresponding increase in additional paid-in capital.
There was no change in the fair value of the embedded beneficial conversion feature immediately before and after the modification. As a result, the Company did not adjust the carrying amount of $1,546 of the convertible debt at November 1, 2021.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
ii. Second Modification:
On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
As at August 31, 2023, the Company has recorded accrued interest of $19,123, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $100,000).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
n) On October 25, 2021, the Company issued a convertible note with a face value of $100,000 (the “Note”) and warrants to purchase 100,000 shares of the Company’s common stock at $1.25 per share for 1 year. The Note bears interest at 10% per annum. The Note is due on demand after March 31, 2022. The Note was convertible into shares of the Company’s common stock at any time prior to March 31, 2022 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification.
The relative fair values of the convertible note and the warrants were $39,573 and $60,427 respectively. The effective conversion price was then determined to be $0.40. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $39,573 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $60,427 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $Nil. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
Modification:
On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration.
The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied.
As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method.
As at August 31, 2023, the Company has recorded accrued interest of $18,466, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $100,000).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
o) On December 23, 2021, the Company issued a convertible note with a face value of $100,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after June 23, 2022. The Note is convertible into shares of the Company’s common stock at any time prior to June 23, 2022 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification.
It was determined that there was a beneficial conversion feature (“BCF”) as the stock price at the issuance date was greater than the effective conversion price. The Company recognized the relative fair value of the BCF of $40,800 as additional-paid-in capital and an equivalent discount that reduced the carrying value of the convertible debt to $59,200. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method.
As at August 31, 2023, the Company has recorded accrued interest of $16,877, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $100,000).
On June 23, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
p) On December 23, 2021, the Company issued a convertible note with a face value of $100,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after June 23, 2022. The Note is convertible into shares of the Company’s common stock at any time prior to June 23, 2022 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification.
It was determined that there was a beneficial conversion feature (“BCF”) as the stock price at the issuance date was greater than the effective conversion price. The Company recognized the relative fair value of the BCF of $40,800 as additional-paid-in capital and an equivalent discount that reduced the carrying value of the convertible debt to $59,200. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method.
As at August 31, 2023, the Company has recorded accrued interest of $16,877, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $100,000).
On June 23, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
q) On January 11, 2022, the Company issued a convertible note with a face value of $150,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after July 10, 2022. The Note is convertible into shares of the Company’s common stock at any time prior to July 10, 2022 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification.
It was determined that there was a beneficial conversion feature (“BCF”) as the stock price at the issuance date was greater than the effective conversion price. The Company recognized the relative fair value of the BCF of $75,600 as additional-paid-in capital and an equivalent discount that reduced the carrying value of the convertible debt to $74,400. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method.
As at August 31, 2023, the Company has recorded accrued interest of $24,534, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $150,000 (August 31, 2022 - $150,000).
On July 10, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
r) On January 31, 2022, the Company issued a convertible note with a face value of $100,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after July 31, 2022. The Note is convertible into shares of the Company’s common stock at any time prior to July 31, 2022 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification.
It was determined that there was a beneficial conversion feature (“BCF”) as the stock price at the issuance date was greater than the effective conversion price. The Company recognized the relative fair value of the BCF of $75,600 as additional-paid-in capital and an equivalent discount that reduced the carrying value of the convertible debt to $74,400. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method.
As at August 31, 2023, the Company has recorded accrued interest of $15,808, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $100,000).
On July 31, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
s) On March 29, 2022, the Company issued a convertible note with a face value of $500,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after September 30, 2022. The Note is convertible into shares of the Company’s common stock at any time prior to September 30, 2022 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification. In addition, it was determined that there was no beneficial conversion feature (“BCF”) as the stock price at the issuance date was less than the conversion price.
As at August 31, 2023, the Company has recorded accrued interest of $71,233, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $500,000 (August 31, 2022 - $500,000).
On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
t) On June 16, 2022, the Company issued a convertible note with a face value of $250,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after December 16, 2022. The Note is convertible into shares of the Company’s common stock at any time prior to December 16, 2022 at a conversion price of $1.25 per share.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification. In addition, it was determined that there was no beneficial conversion feature (“BCF”) as the stock price at the issuance date was less than the conversion price.
As at August 31, 2023, the Company has recorded accrued interest of $30,206, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $250,000 (August 31, 2022 - $250,000).
On December 16, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
u) On November 28, 2022, the Company issued a convertible note with a face value of $100,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after June 30, 2023. The Note is convertible into shares of the Company’s common stock at any time prior to June 30, 2023 at a conversion price equal to the lesser of 80% of the twenty-day average closing price per share (but not less than $0.20 per share) or $0.50 per share.
As at August 31, 2023, the Company has recorded accrued interest of $7,616, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $nil).
On June 30, 2023, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
v) On December 1, 2022, the Company issued a convertible note with a face value of $70,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after June 30, 2023. The Note is convertible into shares of the Company’s common stock at any time prior to June 30, 2023 at a conversion price equal to the lesser of 80% of the twenty-day average closing price per share (but not less than $0.20 per share) or $0.50 per share.
As at August 31, 2023, the Company has recorded accrued interest of $5,236, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $70,000 (August 31, 2022 - $nil).
On June 30, 2023, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
w) On December 7, 2022, the Company issued a convertible note with a face value of $60,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after June 30, 2023. The Note is convertible into shares of the Company’s common stock at any time prior to June 30, 2023 at a conversion price equal to the lesser of 80% of the twenty-day average closing price per share (but not less than $0.20 per share) or $0.50 per share.
As at August 31, 2023, the Company has recorded accrued interest of $4,389, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $60,000 (August 31, 2022 - $nil).
On June 30, 2023, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
x) On February 28, 2023, the Company issued a convertible note with a face value of $150,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after April 30, 2023. The Note is convertible into shares of the Company’s common stock at any time prior to April 30, 2023 at a conversion price equal to the lesser of 80% of the twenty-day average closing price per share (but not less than $0.20 per share) or $0.50 per share.
As at August 31, 2023, the Company has recorded accrued interest of $7,562, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $150,000 (August 31, 2022 - $nil).
On April 30, 2023, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
y) On May 17, 2023, the Company issued a convertible note with a face value of $10,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after September 17, 2023. The Note is convertible into shares of the Company’s common stock at any time prior to September 17 at a conversion price equal to the lesser of 80% of the twenty-day average closing price per share (but not less than $0.20 per share) or $0.50 per share.
As at August 31, 2023, the Company has recorded accrued interest of $504, which is included in accounts payable and accrued liabilities on the consolidated balance sheets.
As of August 31, 2023, the outstanding principal owing is $10,000 (August 31, 2022 - $nil).
On September 17, 2023, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
10. Equity
During the year ended August 31, 2022:
On September 2, 2021, the Company issued 2,175,933 common shares at fair value of $ 2,447,925 on issuance date from treasury to the CFO and COO (Note 11) and 1,900,000 common shares at fair value of $2,137,500 to certain employees of the Company as bonuses for past services, which is expensed as a total of $4,585,425 for consulting fees.
On September 2, 2021, the Company issued 2,997,237 common shares measured at fair value on issuance date of $3,371,892 from treasury for consulting services related to business development for a 12-month period from the issuance date. As the future benefit of the consulting services to be performed cannot be determined, the entire amount was expensed during the year ended August 31, 2022. The total $3,584,392 stock-based compensation – consulting services is comprised of this $3,371,892 share issuance plus the $212,500 described in the next paragraph below.
During the year ended August 31, 2021, the Company re-issued 750,000 shares of common stock with total fair value of $637,500 for consulting services, out of which, $425,000 was expensed as consulting fees during the prior year and $212,500 was expensed during the year ended August 31, 2022.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
On October 20, 2021, the Company issued 3,699,955 units at $0.75 per unit for proceeds of $2,774,966, of which $865,467 was received during the year ended August 31, 2021. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred brokerage commission fees and other share issuance costs of $210,736.
On November 5, 2021, the Company issued 905,000 units at $0.75 per unit for proceeds of $678,750. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company issued 8,000 shares of common stock with a fair value of $6,000 as a finder’s fee and incurred other finders’ fees and other share issuance costs of $42,654.
On November 24, 2021, the Company issued 36,000 units at $0.75 per unit for proceeds of $27,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years.
On November 30, 2021, the Company issued 8,268 shares as consideration for extending the maturity date of a convertible note (Note 9(b)).
On January 20, 2022, the Company issued 75,000 units at $0.75 per unit for proceeds of $56,250 which was received during the year ended August 31, 2021. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years.
On January 28, 2022, the Company issued 66,667 units at $0.75 per unit for proceeds of $50,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years.
On February 7, 2022, the Company issued 66,667 units at $0.75 per unit for proceeds of $50,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years.
On February 10, 2022, the Company issued 33,334 units at $0.75 per unit for proceeds of $25,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years.
On February 10, 2022, the Company issued 80,000 units at $1.25 per unit for proceeds of $100,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $8,000.
On February 20, 2022, the Company issued 40,000 units at $1.25 per unit for proceeds of $50,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $4,000.
On February 21, 2022, the Company issued 300,000 units at $1.25 per unit for proceeds of $375,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $30,000.
On February 22, 2022, the Company issued 80,000 units at $1.25 per unit for proceeds of $100,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $8,000.
On February 24, 2022, the Company issued 41,600 units at $0.75 per unit for proceeds of $31,200. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
On February 24, 2022, the Company issued 40,000 units at $1.25 per unit for proceeds of $50,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $4,000.
On February 25, 2022, the Company issued 96,000 units at $1.25 per unit for proceeds of $120,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $9,600.
On February 28, 2022, the Company issued 120,000 units at $1.25 per unit for proceeds of $150,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $12,000.
On March 7, 2022, the Company issued 20,000 units at $1.25 per unit for proceeds of $25,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $4,000.
On May 26, 2022, the Company issued 133,333 shares at $0.75 per share for proceeds of $100,000.
On May 26, 2022, the Company issued 67,733 units at $0.75 per unit for proceeds of $50,800. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years.
On August 23, 2022, the Company issued 750,000 shares at $0.40 per share for proceeds of $300,000.
During the year ended August 31, 2022, the Company issued 368,000 common shares to certain investors for no consideration by error. The Company is in the process of retracting the shares.
At August 31, 2022, the Company had received $540,000 in cash for share subscriptions at $0.40 per share.
During the year ended August 31, 2023:
On September 21, 2022, the Company issued 1,350,000 shares of common stock at $0.40 per share for $540,000 subscriptions received during the year ended August 31, 2022.
On October 7, 2022, the Company issued 1,575,000 shares of common stock at $0.40 per share for proceeds of $630,000. In connection with the financing, the Company incurred finder’s fee of $10,000 and share issuance costs of $2,792.
On October 7, 2022, the Company issued 75,000 shares of common stock with a fair value of $44,606 to settle related party accounts payable of $30,000, resulting in a loss on settlement of $14,606.
On October 7, 2022, the Company issued 70,560 shares of common stock with a fair value of $41,966 to settle $29,529 in debt, resulting in a loss on settlement of $12,437.
On August 21, 2023, the Company issued 1,525,000 shares of common stock at $0.20 per share for proceeds of $305,000.
On August 31, 2023, the Company issued 3,029,760 shares of common stock at $0.20 per share for proceeds of $605,952. In connection with the financing, the Company issued 1,000,000 warrants with a fair value of $204,452 as finder’s fees. Each warrant entitles the holder to acquire one common share at a price of $0.20 per share until July 30, 2025. The fair value of the finder’s warrants was calculated using the Black-Scholes option pricing model assuming the following weighted-average assumptions: a risk-free rate of 4.87%, no expected dividends or forfeiture rate, volatility of 160.87% and an expected life of 2 years.
At August 31, 2023, the Company had received $80,000 in cash for shares subscriptions at $0.20 per share.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
11. Related party transactions and balances
All transactions with related parties have occurred in the normal course of operations and are recorded at the exchange amount which is the amount agreed to by the Company and the related party.
a) Key management compensation and related party transactions
The Company has identified its directors and certain senior officers as its key management personnel. The compensation costs for key management personnel were as follows:
| | August 31, 2023 | | | August 31, 2022 | |
Consulting fees and benefits | | $ | 396,052 | | | $ | 369,079 | |
b) Amounts due to related parties
In the normal course of operations, the company shares certain administrative resources with companies related by common management and directors. The administrative resources and services, which were provided in the normal course of operations, were measured at the exchange. All amounts payable and receivable are non-interest bearing, unsecured and due on demand. The following table summarizes the amounts due to related parties:
| | August 31, 2023 | | | August 31, 2022 | |
| | | | | | |
CEO and Director | | $ | 156,028 | | | $ | 80,892 | |
COO and Director | | | 201,612 | | | | 143,816 | |
An entity controlled by the CFO | | | 84,962 | | | | 46,387 | |
An entity controlled by a Director | | | 159,690 | | | | 60,677 | |
Director | | | 91,000 | | | | 52,500 | |
| | $ | 693,292 | | | $ | 384,272 | |
As of August 31, 2023, the Company had $693,292 (August 31, 2022 - $384,272) payable to related parties. From this amount, $623,292 (August 31, 2022 - $384,272) was for expenses incurred or expensed paid on behalf of the Company by the parties and $70,000 (August 31, 2022 - $nil) was for advances received from the parties.
c) Stock-based compensation
A $2,447,925 portion of the stock-based compensation – bonus shares (See Note 10) recorded in the year ended August 31, 2022 as consulting fees was for bonus shares issued to the CFO and COO described as follows:
On September 2, 2021, the Company issued 400,000 common shares from treasury at fair value of $450,000 to an entity controlled by the CFO as a bonus for past services provided.
On September 2, 2021, the Company issued 1,775,933 common shares from treasury at fair value of $1,997,925 to entities controlled by the COO and Director for as a bonus for past services provided.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
12. Financial risk factors
The Company is exposed in varying degrees to a variety of financial instrument related risks. The Board of Directors approves and monitors the risk management processes, inclusive of documented investment policies, counterparty limits, and controlling and reporting structures. The type of risk exposure and the way in which such exposure is managed is provided as follows:
a) Credit risk:
Credit risk is the risk that one party to a financial instrument will fail to discharge an obligation and cause the other party to incur a financial loss. The Company’s primary exposure to credit risk is on its cash account. Cash accounts are held with major banks in Canada. The Company has deposited its cash with a bank from which management believes the risk of loss is low.
b) Liquidity risk:
Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they become due. The Company’s approach to managing liquidity is to ensure that it will have sufficient liquidity to meet liabilities when due. Accounts payable are due within the current operating period. The Company has a working capital deficit and requires additional financing to meet its current obligations (see Note 1).
c) Market risk:
Market risk is the risk that changes in market prices, such as foreign exchange rates, interest rates and equity prices will affect the Company’s income or the value of its holdings of financial instruments. The objective of market risk management is to manage and control market risk exposures within acceptable parameters, while optimizing the return. The Company is not exposed to market risk.
d) Interest rate risk:
Interest rate risk is the risk that the fair value of future cash flows of a financial instrument will fluctuate because of changes in market interest rates. The Company is exposed to interest rate risk, from time to time, on its cash balances. Surplus cash, if any, is placed on call with financial institutions and management actively negotiates favorable market related interest rates.
e) Foreign exchange risk:
Foreign currency risk is limited to the portion of the Company’s business transactions denominated in currencies other than the Canadian dollar. The Company has not entered into any foreign currency contracts to mitigate risk, but manages the risk my minimizing the value of financial instruments denominated in foreign currency. The Company is exposed to foreign currency risk to the extent that the following monetary assets and liabilities are denominated in Canadian dollars:
| | August 31, 2023 | |
Balance in Canadian dollars: | | | |
Accounts payable | | $ | (947,214 | ) |
Net exposure | | | (947,214 | ) |
Balance in US dollars: | | $ | (700,032 | ) |
A 10% change in the US dollar to the Canadian dollar exchange rate would impact the Company’s net loss by approximately $70,003 for the year ended August 31, 2023 (August 31, 2022 – $53,595).
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
The Company is exposed to foreign currency risk to the extent that the following monetary assets and liabilities are denominated in Colombian Pesos:
| | August 31, 2023 | |
Balance in Colombian Pesos dollars: | | | |
Cash and cash equivalents | | $ | 415,100,982 | |
Other receivables | | | 539,704,008 | |
Accounts payable | | | (3,669,893,803 | ) |
Net exposure | | | (2,715,088,813 | ) |
Balance in US dollars: | | $ | (664,234 | ) |
A 10% change in the US dollar to the Colombian Peso exchange rate would impact the Company’s net loss by approximately $66,423 for the year ended August 31, 2023 (August 31, 2022 - $49,281).
13. Capital management
The Company manages its capital structure and makes adjustments to it, based on the funds available to the Company, in order to support the business and continue as a going concern. The Company considers capital to be all accounts in equity. The Board of Directors does not establish quantitative return on capital criteria for management, but rather relies on the expertise of the Company’s management to sustain future development of the business. The Company has a working capital deficit and requires additional capital to finance is future business plans. The Company is not subject to any externally imposed capital requirements.
14. Commitments
a) As of August 31, 2023, the Company recorded a contingent liability of $547,190 (CAD$700,000) for expenses in connection with Allied Colombia acquisition, which is included in the balance of accounts payable and accrued liabilities on the consolidated balance sheet.
b) The Company has entered into leases for farm land in Colombia. See Note 7 for details.
c) The Company initiated litigation in the Judicial District Court in Clark County, Nevada in connection with ownership of prefabricated buildings. See Note 4 a) for details.
15. Share purchase warrants
The following table summarizes the continuity of share purchase warrants:
| | Number of warrants | | | Weighted average exercise price $ | |
| | | | | | |
Balance, August 31, 2022 | | | 7,704,135 | | | | 1.23 | |
| | | | | | | | |
Issued | | | 1,000,000 | | | | 0.20 | |
Expired | | | (3,245,135 | ) | | | 1.25 | |
Balance, August 31, 2023 | | | 5,459,000 | | | | 1.06 | |
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
As at August 31, 2023, the following share purchase warrants were outstanding:
| Number of warrants | | | Exercise price $ | | | Expiry date | |
| | | | | | | | |
| | 2,545,999 | | | | 1.25 | | | October 20, 2023* | |
| | 905,000 | | | | 1.25 | | | November 6, 2023* | |
| | 36,000 | | | | 1.25 | | | November 24, 2023* | |
| | 66,667 | | | | 1.25 | | | January 29, 2024 | |
| | 66,667 | | | | 1.25 | | | February 7, 2024 | |
| | 113,334 | | | | 1.25 | | | February 12, 2024 | |
| | 40,000 | | | | 1.25 | | | February 20, 2024 | |
| | 300,000 | | | | 1.25 | | | February 21, 2024 | |
| | 177,600 | | | | 1.25 | | | February 26, 2024 | |
| | 120,000 | | | | 1.25 | | | February 28, 2024 | |
| | 20,000 | | | | 1.25 | | | March 7, 2024 | |
| | 67,733 | | | | 1.25 | | | May 27, 2024 | |
| | 1,000,000 | | | | 0.20 | | | July 30, 2025 | |
| | 5,459,000 | | | | | | | | |
* Expired subsequently
16. Stock options
A summary of the Company’s stock option activity is as follows:
| | Number of Options | | | Weighted Average Exercise Price $ | | | Weighted Average Remaining Contractual Term | | | Aggregate Intrinsic Value $ | |
| | | | | | | | | | | | |
Balance, August 31, 2022 | | | 6,000,000 | | | | 0.44 | | | | 3.85 | | | | 144,000 | |
| | | | | | | | | | | | | | |
Granted | | | 6,140,000 | | | | 0.24 | | | | 4.63 | | | | |
| | | | | | | | | | | | | | |
Outstanding, August 31, 2023 | | | 12,140,000 | | | | 0.25 | | | | 3.70 | | | | 55,900 | |
Exercisable, August 31, 2023 | | | 11,456,667 | | | | 0.25 | | | | 3.70 | | | | 48,400 | |
In January 2023, the Company modified the exercise prices for previously granted and unexercised options. The options held by current consultants and ambassadors were repriced to $0.22 per share and the options held by current directors and officers were repriced to $0.25 per share. There was no other modification to the vesting schedule of the previously issued options. As a result, 4,900,000 unexercised options originally granted to purchase common stock at prices ranging from $0.75 to $0.825 per share were repriced. The repricing was treated as a modification of the original awards and the additional compensation costs for the difference between the fair value of the modified award and the fair value of the original award on the modification date was calculated. The repricing resulted in incremental stock-based compensation expense of $80,739. Expense related to vested shares was expensed on the repricing date and expense related to unvested shares will be amortized over the remaining vesting period.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
On May 16, 2023, the Company modified the exercise price of the 6,000,000 options to $0.24 per share. The repricing was treated as a modification of the original awards and the additional compensation costs for the difference between the fair value of the modified award and the fair value of the original award on the modification date was calculated. The repricing resulted in incremental stock-based compensation expense of $14,506. Expense related to vested shares was expensed on the repricing date.
During the year ended August 31, 2023, the Company recorded stock-based compensation of $3,047,156 for options granted on the consolidated statement of operations. The fair value of each option granted was estimated on the date of grant using the Black-Scholes option pricing model with the following weighted average assumptions:
| | Year Ended August 31, 2023 | | | Year Ended August 31, 2022 | |
| | | | | | |
Expected dividend yield | | | 0 | % | | | 0 | % |
Expected volatility | | | 162 | % | | | 150 | % |
Expected life (in years) | | | 3.45 | | | | 4.05 | |
Risk-free interest rate | | | 4.41 | % | | | 3.38 | % |
At August 31, 2023, there was $373,838 of unrecognized compensation costs related to non-vested stock-based compensation arrangements granted under the Plan.
17. Non-cash activities
| | For the Year Ended August 31, 2023 | | | For the Year Ended August 31, 2022 | |
Non-cash activities: | | | | | | |
Shares issued from treasury for services | | | | | | 7,957,316 | |
Fair value of warrants issued as finder’s fee | | | 204,452 | | | | - | |
Beneficial conversion feature | | | - | | | | 281,447 | |
Relative fair value of warrants issuable with convertible note | | | - | | | | 120,310 | |
Fair value of shares issued on modification of convertible note | | | - | | | | 129,952 | |
Fair value of shares issuable on modification of debt | | | - | | | | 8,268 | |
18. Segment disclosure
The Company has two operating segments including:
a) Allied Colombia, a Colombian based company through which the Company intends to commence commercial production in Colombia. (Allied Colombia)
b) Allied Corp. which consists of the rest of the Company’s operations. (Allied)
Factors used to identify the Company’s reportable segments include the organizational structure of the Company and the financial information available for evaluation by the chief operating decision-maker in making decisions about how to allocate resources and assess performance. The Company’s operating segments have been broken out based on similar economic and other qualitative criteria. The Company operates the Allied reporting segment in one geographical area (Canada), and the Allied Colombia reporting segment in one geographical area (Colombia).
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
Financial statement information by operating segment for the year ended August 31, 2023 is presented below:
| | Allied $ | | | Allied Colombia $ | | | Total $ | |
Gross margin | | | 3,799 | | | | (1,465,566 | ) | | | (1,461,767 | ) |
Net loss | | | (8,123,159 | ) | | | (2,552,512 | ) | | | (10,675,671 | ) |
Depreciation and amortization | | | 66,484 | | | | 70,562 | | | | 137,046 | |
Total assets as of August 31, 2023 | | | 719,015 | | | | 1,344,713 | | | | 2,063,728 | |
Geographic information for the year ended and as at August 31, 2023 is presented below:
| | Gross Margin $ | | | Total Assets $ | |
| | | | | | |
Canada | | | 3,799 | | | | 719,015 | |
Colombia | | | (1,465,566 | ) | | | 1,344,713 | |
Total | | | (1,461,767 | ) | | | 2,063,728 | |
19. Income taxes
At August 31, 2023, the Company has a net operating loss carryforward of approximately $20,588,000. The significant components of deferred income tax assets at August 31, 2023 and 2022 were as follows:
| | August 31, 2023 | | | August 31, 2022 | |
Deferred tax asset: | | | | | | |
Net operating loss carryforward | | $ | 5,095,000 | | | $ | 3,125,000 | |
Less: valuation allowance | | | (5,095,000 | ) | | | (3,125,000 | ) |
Net deferred income tax asset | | $ | - | | | $ | - | |
The amount taken into income as deferred income tax assets must reflect that portion of the income tax loss carry forwards that is more likely-than-not to be realized from future operations. The Company has chosen to provide a full valuation allowance against all available income tax loss carry forwards. The Company has recognized a valuation allowance for the deferred income tax asset since the Company cannot be assured that it is more likely than not that such benefit will be utilized in future years. The valuation allowance is reviewed annually. When circumstances change and which cause a change in management’s judgment about the realizability of deferred income tax assets, the impact of the change on the valuation allowance is generally reflected in current income.
As of August 31, 2023 and 2022, the Company has no recognized income tax benefits. The Company’s policy for classifying interest and penalties associated with unrecognized income tax benefits is to include such items as tax expense. No interest or penalties have been recorded during the year ended August 31, 2023 or 2022. No interest or penalties have been accrued as of August 31, 2023. As of August 31, 2023 and 2022, the Company did not have any amounts recorded pertaining to uncertain tax positions.
ALLIED CORP. Notes to the consolidated financial statements For the year ended August 31, 2023 and 2022 (Expressed in US dollars) |
A reconciliation of the provision for income taxes at the combined statutory rate for the year ended August 31, 2023 and 2022 is as follows:
| | August 31, 2023 | | | August 31, 2022 | |
Income tax benefit | | $ | 1,970,000 | | | $ | 925,000 | |
Change in valuation allowance | | | (1,970,000 | ) | | | (925,000 | ) |
Provision for income taxes | | $ | - | | | $ | - | |
As of August 31, 2023, the Company had approximately $20,588,000 of federal net operating losses that may be available to offset future taxable income. The net operating loss carryforwards will begin to expire in 2034 unless utilized. In accordance with Section 382 of the Internal Revenue Code, deductibility of the Company’s U.S. net operating carryovers may be subject to an annual limitation in the event of a change of control as defined the regulations. A Section 382 analysis has not been prepared and the Company’s NOLs could be subject to limitation.
20. Subsequent events
a) Subsequent to the year ended August 31, 2023, the Company issued 250,000 shares for subscriptions received.
b) Subsequent to the year ended August 31, 2023, the Company the Company received $346,000 in cash for share subscriptions at $0.20 per share.
Item 9. Change in and Disagreement with Accountants on Accounting and Financial Disclosure
Effective August 1, 2022, we engaged M&K CPAS, LLLC (“M&K”) to serve as our independent registered public accounting firm for our fiscal years ending August 31, 2023 and 2022. During our most recent fiscal year ending August 31, 2023 and 2022 we did not consult with M&K regarding (i) the application of accounting principles to a specific transaction, either completed or contemplated, or the type of audit opinion that might be rendered on our financial statements, and no written report or oral advice was provided to us that was an important factor to be considered by us in reaching a decision as to an accounting, auditing or financial reporting issue; or (ii) any matter that was either the subject of a disagreement (as that term is defined in Item 304(a)(1)(iv) of Regulation S-K) or a reportable event (as that term is defined in Item 304(a)(1)(v) of Regulation S-K).
Item 9A. Controls and Procedures
Management’s Annual Report on Internal Control over Financial Reporting.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes of accounting principles generally accepted in the United States.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance of achieving their control objectives.
Our management evaluated the effectiveness of the Company’s internal control over financial reporting as of August 31, 2023. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control — Integrated Framework. While our initial assessment, completed on August 31, 2023 deemed internal controls effective, based upon a further evaluation of legal proceedings during our annual audit, which was conducted subsequent to August 31, 2023, we modified management’s initial estimates used in our asset impairment in a manner that caused audit adjustments. Accordingly, management concluded there was a material weakness in our internal control over financial reporting at August 31, 2023, based on the COSO framework criteria, since management lacked a formal policy of testing for impairment resulting in adjusting journal entries.
This annual report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to rules of the SEC that permits us to provide only management’s report in this annual report.
Changes in Internal Control over Financial Reporting.
There were no changes in our internal control over financial reporting that occurred during the fiscal year ended August 31, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
Item 10. Directors, Executive Officers and Corporate Governance
The table below reflects the Company’s executive officers and directors. There is no agreement or understanding between the Company and each current or proposed director or executive officer pursuant to which he was selected as an officer or director. The address for each such officer and director is 1405 St. Paul St., Suite 201, Kelowna, BC Canada V1Y 9N2.
Name | | Intended Positions and Offices |
Calum Hughes | | Chairman of the Board, Chief Executive Officer and Director |
Michael Moses | | Chief Business Development Officer |
Paul Bullock | | Chief Operating Officer and Director |
Jim Smeeding | | Vice President of Pharmaceuticals and Director |
Santiago Ribero | | Vice President Corporate Development and Director |
Ryan Maarschalk | | Chief Financial Officer |
The Directors and Officers named above will serve until the next annual meeting of the stockholders or until their respective resignation or removal from office. Thereafter, Directors are anticipated to be elected for one-year terms at the annual stockholders’ meeting. Officers will hold their positions at the pleasure of the Board of Directors, absent any employment agreement, of which none currently exists or is contemplated.
Calum Hughes, Chairman of the Board, Chief Executive Officer, Director
Calum Hughes has been responsible for leading activities relating to large scale Quality Assurance and Evaluation of Health Programs. He is skilled in the Project Management Institute’s Methodology© for managing projects through mitigating risk, maximizing communication and ensuring project success.
In the past, Calum has created and presented at top level educational presentations for Healthcare Executives, Business Professionals, Psychiatrists, Nursing staff, General Practitioners, Internal Medicine, ObGyn, and Cardiologists. He has worked as an Adjunct Professor for the UBC Faculty of Health and Social Development, as a Consultant to several large for-profit and non-profit Health Organizations and Nutraceutical companies.
Calum, has held an appointment of Adjunct Professor for the University of British Columbia Faculty of Health and Social Development, and is a Registered Kinesiologist with the British Columbia Association of Kinesiologists. He has also completed a Post Baccalaureate Diploma in Gerontology. Calum holds certification in designation of Project Management Professional (PMP) from the Project Management Institute (Pennsylvania, USA). He achieved a 98% (certified with Gold Standing) in his studies towards a LEAN Greenbelt in 2009, and is also working towards his Doctorate Degree with Royal Roads University.
Calum’s past clinical experience includes functioning as a health care provider for patients with co-morbid chronic diseases, acute disability and workplace health & wellness. Working as a clinician, Calum has created Physical & Functional Diagnostic Tools and Chronic Disease Management Protocols for Cardiovascular, Orthopedic, Obesity, Respiratory, Brain Injury, and Cerebrovascular disease. He has created teaching resources and presented at top-level educational presentations regarding Change Management, Quality Improvement, Personality & Communication, Project Management and Lean Manufacturing Method for Healthcare.
Michael Moses, Chief Business Development Officer
Entrepreneur with executional expertise in go to market, business strategy and partnership building with some of the largest philanthropy organizations in the UK. With experience in working with Engineering, Investment and Risk Management firms from across three continents, Michael brings a strong understanding of both the business needs to taking products to market. Masters in Electronic Engineering with Management from Imperial College London & Guest Lecturer at Imperial College Business School. His expertise, along with his network in the Cannabis industry, will be instrumental in identifying new opportunities and driving growth for the company.
Paul Bullock, Chief Operating Officer and Director
Born and raised in Kelowna, BC, Canada, Jim Bullock comes from one of Kelowna’s oldest pioneer families, which possesses a rich history in both agricultural innovation and entrepreneurial spirit. Jim brings 10 years of experience to the Allied team from MMAR, MMPR, and ACMPR cannabis cultivation, genetics, compliance and facility design.
Previous to joining Allied, Jim owned and operated a company specializing in both personal and corporate finance for clients, which offered exportable services in overseas and offshore markets. Earlier, Jim worked in the oil and gas industry specializing in offshore construction. Jim also owned and operated an agricultural management and consulting firm, which renovated and replanted over 2,500 acres of orchard and vineyard in the Okanagan Valley. He was also the Kelowna site operations manager for Kettle Mountain Ginseng, one of the pioneers in the British Colombia ginseng industry.
Jim Smeeding, Vice President of Pharmaceuticals and Director
Jim Smeeding is a Registered Pharmacist (RPh) from the State University of New York, and also holds a Master’s degree in Business Administration (MBA) from the University of Texas (with an emphasis in pharmaceutical marketing, organizational strategies and pharma sales management). He is the Executive Vice President of CP Pharmaceutical International: a division of CP Global Health (Hong Kong and Texas).
Some of Mr. Smeeding’s past experience has included working as the Executive Director of the National Association of Specialty Pharmacy (NASP), a founder of the Center for Pharmacoeconomic Studies at the University of Texas College of Pharmacy and a founder and President of the International Society of Pharmacoeconomics and Outcomes Research (ISPOR).
Within Mr. Smeeding’s specialty pharmacy consultancy practice, Project Rx, Mr. Smeeding has offered management services to hospitals and pharmaceutical companies throughout the US. Mr. Smeeding has also been active in other Cannabis Based Medicines (CBM) research as a founder of CannaPharma Rx and has authored more than 85 peer-reviewed publications and has given hundreds of presentations.
Mr. Smeeding has worked with many major pharmaceutical companies and is aa founder and president of Indication Biosciences; an early-stage drug discovery company that is examining the use of CBD with statin agents to safely lower lipid anomalies. Mr. Smeeding also was the Executive VP and founder of Engaged Media; a technology driven patient engagement solution used in multiple pharmaceutical co-pay programs that was acquired in May of 2018.
As President of the National Payer Roundtable, Mr. Smeeding is in regular contact with the Chief Medical Officers and Chief Pharmacy Officers of national and regional health insurers, as well as, pharmaceutical executives.
Santiago Ribero, Vice President of Corporate Development and Director
Santiago Ribero has a 20-year track record of success improving financial stability, reducing risk, and achieving strategic growth for public and private companies. He previously served as the Columbian Manager at Banco de Credito e Inversiones and BCI Miami, improving the asset value of the institution from $10 million to $430 million in two years. Prior to this Ribero was director of corporate finance and banking at Citi Columbia where he executed structured loans and loan syndications, debt capital market transactions, ECA financings, mergers and acquisitions. He holds a MA in Finance Management from Macquarie University and a BA in Industrial Engineering from the University of Los Andes.
Ryan Maarschalk, BSc, CPA, Chief Financial Officer
Mr. Maarschalk is a driven leader with multifaceted communication skills, and a proven track record of successfully working with senior leadership to achieve growth. Mr. Maarschalk uses his entrepreneurship experience to communicate financial information for real life decision making. Some of Mr. Maarschalk’s past experience includes merger and acquisition activities to successfully close the acquisition of IMPACT Radio Accessories to private equity for $23,000,000, building extraction and cultivation facilities in Las Vegas with 1933 Industries (TGIF.CSE). Mr. Maarschalk has worked with variety of companies since becoming a consulting CFO, including micro- mobility, wineries, cannabis and real estate. Mr Maarschalk has also worked as a Business Valuation Associate with MVI Valuations & Planning, Senior Accountant with Crowe MacKay LLP (accounting firm) and has been a Co-Founder / Board Member of various companies over the years.
Mr. Maarschalk is a Chartered Professional Accountant (CPA) Institute of Chartered Professional Accountants. He has a Bachelor of Biomedical Science BSc. (Hons) and is a Chartered Business Valuator (CBV) candidate (CICBV Canadian Institute of Business Valuators).
Dr. Terry Johnston, Medical Advisor to the Board
Occupational Physician Doing Occupational medicals for Transport Canada Aviation & Marine, WorkSafeBC, Commerical Dive Medicals as well as DCBC, OGUK off shore Medicals, FAA class 1 examiner as well as fitness to work and pre-placement medicals. Audiometry, Resting and Stress EKGs, NIOSH Drug testing, Chester fitness test, Farnsworth and Titmus visual screening, Visia Facial Skin assessment. 10 years’ experience in Botox, fillers and skin rejuvenation.
Involvement in Certain Legal Proceedings
No director, executive officer, promoter or control person of Allied has, during the last ten years: (i) been convicted in or is currently subject to a pending a criminal proceeding (excluding traffic violations and other minor offenses); (ii) been a party to a civil proceeding of a judicial or administrative body of competent jurisdiction and as a result of such proceeding was or is subject to a judgment, decree or final order enjoining future violations of, or prohibiting or mandating activities subject to any federal or state securities or banking or commodities laws including, without limitation, in any way limiting involvement in any business activity, or finding any violation with respect to such law, nor (iii) any bankruptcy petition been filed by or against the business of which such person was an executive officer or a general partner, whether at the time of the bankruptcy or for the two years prior thereto.
Committees of the Board
Decisions of the Board of Directors are generally taken by written unanimous resolutions. The current Board comprises four members and is intending to hold regularly scheduled meetings. The entire board provides the functions of Audit, Compensation and Governance committees until such time as charters for these committees can be adopted and they can be populated by independent directors.
Family Relationships
No family relationships exist between any of our present directors and officers.
Compliance with Section 16(A) of The Exchange Act
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires that our directors and executive officers and persons who beneficially own more than 10% of our common stock (referred to herein as the “reporting persons”) file with the SEC various reports as to their ownership of and activities relating to our common stock. Such reporting persons are required by the SEC regulations to furnish us with copies of all Section 16(a) reports they file. Based solely upon a review of copies of Section 16(a) reports and representations received by us from reporting persons, and without conducting any independent investigation of our own during the fiscal year ended August 31, 2023, all forms required, if any, were filed with the SEC by such reporting persons.
Changes in Nominating Procedures
None
Item 11. Executive Compensation
Summary Compensation Table
The following table sets forth the total compensation paid to, or accrued by, the Named Executive Officers and any other employees earning over $100,000 per year from August 31, 2020 to date. No restricted stock awards, long-term incentive plan payout or other types of compensation were paid to these executive officers during those fiscal years.
Name | | Year | | Fees earned or paid in cash ($) | | | Stock awards ($) | | | Number of stock options granted | | | Non-equity incentive plan compensation ($) | | | Change in pension value and nonqualifed deferred compensation earnings ($) | | | All other compensation ($) | | | Total ($) | |
Calum Hughes | | 2023 | | $ | 112,311 | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 112,311 | |
| | 2022 | | $ | 119,205 | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 119,205 | |
| | 2021 | | $ | 118,756 | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 118,756 | |
| | 2020 | | $ | 169,460 | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 169,460 | |
Paul Bullock | | 2023 | | $ | 93,592 | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 93,592 | |
| | 2022 | | $ | 99,337 | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 99,337 | |
| | 2021 | | $ | 101,563 | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 101,563 | |
| | 2020 | | $ | 159,193 | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 159,193 | |
Jim Smeeding | | 2023 | | $ | 60,000 | | | | - | | | | 140,000 | | | | - | | | | - | | | | - | | | $ | 60,000 | |
| | 2022 | | $ | 60,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 60,000 | |
| | 2021 | | $ | 75,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 75,000 | |
| | 2020 | | $ | 20,000 | | | | - | | | | 600,000 | | | | - | | | | - | | | | - | | | $ | 20,000 | |
Santiago Ribero | | 2023 | | $ | 45,900 | | | | - | | | | 1,900,000 | | | | - | | | | - | | | | - | | | $ | 45,900 | |
| | 2022 | | $ | 42,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 42,000 | |
| | 2021 | | $ | 42,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 42,000 | |
| | 2020 | | $ | 48,569 | | | | - | | | | 300,000 | | | | - | | | | - | | | | - | | | $ | 48,569 | |
Ryan Maarschalk | | 2023 | | $ | 84,249 | | | | - | | | | 1,900,000 | | | | - | | | | - | | | | - | | | $ | 84,249 | |
| | 2022 | | $ | 69,536 | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 69,536 | |
| | 2021 | | $ | 96,530 | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 96,530 | |
| | 2020 | | $ | 36,768 | | | | - | | | | 600,000 | | | | - | | | | - | | | | - | | | $ | 36,768 | |
The following table summarizes the stock options granted during the year ended August 31, 2023.
Name | | Number of options granted | | | Number of options exercisable | | | Exercise price | | | Expiry date | |
Jim Smeeding | | | 140,000 | | | | 140,000 | | | $ | 0.20 | | | June 3, 2028 | |
Ryan Maarschalk | | | 1,900,000 | | | | 1,900,000 | | | $ | 0.24 | | | March 8, 2028 | |
Santiago Ribero | | | 1,900,000 | | | | 1,900,000 | | | $ | 0.24 | | | March 8, 2028 | |
Although compensation has been paid to our officers and directors on behalf of Advanced Biosciences, no compensation has been paid to date from Allied Corp. to any of our existing officers and directors.
Employment Agreements
The Company is not a party to any employment agreement with its Officers or its Directors at this time. At present, the Company has entered into consulting agreements with entities controlled by Calum Hughes (Director & CEO), Paul Bullock (Director & COO), Jim Smeeding (Director & VP Pharmaceutical), and Ryan Maarschalk (CFO). At present these individuals are paid on a monthly basis in the amounts of CA$10,000, CA$10,000, US$5,000 and CA$10,000 respectively. Total balances paid during the course of the year included amounts paid to previous directors and officers of the Company.
1.1. Effective September 1, 2023 the Company entered into an Interim Operating Agreement with Michael Moses pursuant to which he became Chief Business Development Officer. Pursuant to that agreement, the Company shall work to continue to achieve the revenue objectives of the Company through the sale of its product line including without limitation, the following two matters (together the “Triggering Event”): (i) obtain funding for the Company in the amount of $1,500,000, which aggregate sum must be raised within 60 days following the date on which the first amount is advanced (the “Finance Event”) and (ii) enter into a sales agreement(s) with a projected revenue stream for the first twelve months of a minimum of $500,000 aggregate sales (the “Contract Event”). Upon that Triggering Event, Moses has the option to enter into a Consulting Agreement pursuant to which he would assume the role of Chief Executive Officer.
Equity Compensation Plans
During 2020, the Company’s board of directors approved the Allied Corp. 2020 Incentive Plan (“Plan”) which authorized the issuance of options, restricted stock units or other equity compensation to its employees directors, and consultants.
On February 1, 2021, the Company granted 4,900,000 stock options to directors, officers and employees of the Company. The options expire five years after the grant date and 1,200,000 options are exercisable at $0.825 per share and 3,700,000 options are exercisable at $0.75 per share. The options vest one third on the grant date and one third on the first and second years after the grant date. The weighted average grant date fair value of stock options granted was $0.77 per share. During the year ended August 31, 2021, the Company recorded stock-based compensation of $2,666,077 on the consolidated statement of operations.
During the year ended August 31, 2022, the Company granted 2,150,000 stock options to directors, officers and employees of the Company. The options expire five years after the grant date and 900,000 options are exercisable at $0.465 per share and 1,550,000 options are exercisable at $0.42 per share. The options vest one third on the grant date and one third on the first and second years after the grant date. The weighted average grant date fair value of stock options granted was $0.44 per share. During the year ended August 31, 2022, the Company recorded stock-based compensation of $2,733,874 on the consolidated statement of operations.
During the year ended August 31, 2023, the Company granted 6,140,000 stock options to directors, officers and employees of the Company. The options expire five years after the grant date and 6,000,000 options are exercisable at $0.24 per share and 140,000 options are exercisable at $0.20 per share. All options vested immediately on the grant date. The weighted average grant date fair value of stock options granted was $0.23 per share. During the year ended August 31, 2023, the Company recorded stock-based compensation of $3,047,156 on the consolidated statement of operations.
In January 2023, the Company modified the exercise prices for previously granted and unexercised options. The options held by current consultants and ambassadors were repriced to $0.22 per share and the options held by current directors and officers were repriced to $0.25 per share. As a result, 4,900,000 unexercised options originally granted to purchase common stock at prices ranging from $0.75 to $0.825 per share were repriced. The repricing resulted in incremental stock-based compensation expense of $80,739. Expense related to vested shares was expensed on the repricing date and expense related to unvested shares will be amortized over the remaining vesting period.
On May 16, 2023, the Company modified the exercise price of the 6,000,000 options to $0.24 per share. The repricing resulted in incremental stock-based compensation expense of $14,506. Expense related to vested shares was expensed on the repricing date.
During the year ended August 31, 2023, the Company granted 1,000,000 warrants as finder’s fees to the Chief Business Development Officer. The warrants expire five years after the grant date and are exercisable at $0.20 per share.
All compensation and stock option plans for executives and employees will be governed by the Compensation and Governance Committee.
Expense Reimbursement
We will reimburse our officers and directors for reasonable expenses incurred during the course of their performance.
Retirement Plans and Benefits
None.
Director Compensation
We do not have a standard compensation arrangement for directors.
Indemnification and Limitation on Liability of Directors
The Nevada Revised Statutes allows indemnification of directors, officers, employees and agents of a company against liabilities incurred in any proceeding in which an individual is made a party because he or she was a director, officer, employee or agent of the company if such person conducted himself in good faith and reasonably believed his actions were in, or not opposed to, the best interests of the company, and with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. A person must be found to be entitled to indemnification under this statutory standard by procedures designed to assure that disinterested members of the board of directors have approved indemnification or that, absent the ability to obtain sufficient numbers of disinterested directors, independent counsel or shareholders have approved the indemnification based on a finding that the person has met the standard. Indemnification is limited to reasonable expenses.
We do not have indemnification provisions in our articles or bylaws.
Insofar as indemnification for liabilities arising under the 1933 Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the 1933 Act and is, therefore, unenforceable.
Outstanding Stock Options or other Equity Awards
The following table summarizes the stock options and warrants we have issued during the fiscal year ended August 31, 2023.
Name | | Issuance Date | | Expiration Date | | Exercise Price per Share ($) | | | Number of Stock Options or Warrants Granted | |
Jim Smeeding | | June 3, 2023 | | June 3, 2028 | | $ | 0.20 | | | | 140,000 | |
Michael Moses | | July 30, 2023 | | July 30, 2025 | | $ | 0.20 | | | | 1,000,000 | |
Ryan Maarschalk | | March 8, 2023 | | March 8, 2023 | | $ | 0.24 | | | | 1,900,000 | |
Santiago Ribero | | March 8, 2023 | | March 8, 2023 | | $ | 0.24 | | | | 1,900,000 | |
The fair value of each option and warrant granted was estimated on the date of grant using the Black-Scholes option pricing model with the following weighted average assumptions: a risk-free rate of 4.65%, no expected dividends or forfeiture rate, volatility of 167.41% and an expected life of 3.01 years.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth as of August 31, 2023 the number of shares of common stock beneficially owned by (i) those persons or groups known to beneficially own more than 10% of the Company’s common stock, (ii) each current director and executive officer of the Company, and (iii) all the current executive officers and directors as a group.
Pursuant to Rule 13d-3 under the Exchange Act, a beneficial owner of securities is a person who directly or indirectly, through any contract, arrangement, understanding, relationship or otherwise has, or shares, voting power and/or investment power with respect to the securities, and any person who has the right to acquire beneficial ownership of the security within 60 days through any means, including the exercise of any option, warrant or right or the conversion of a security. Any shares that are not outstanding that a person has the right to acquire are deemed to be outstanding for the purpose of calculating the percentage of beneficial ownership of such person, but are not deemed to be outstanding for the purpose of calculating the percentage of beneficial ownership of any other person.
Title of Class | | Name of Beneficial Owner | | Amount of Beneficial Ownership (1) | | | Percentage | |
| | | | | | | | |
Common Stock | | Calum Hughes, Chief Executive Officer and Director | | | 20,490,028 | | | | 20.2 | % |
Common Stock | | Michael Moses, Chief Business Development Officer (2) | | | 1,300,000 | | | | 0.3 | % |
Common Stock | | Paul Bullock, Chief Operating Officer and Director (3) | | | 9,967,969 | | | | 9.8 | % |
Common Stock | | Jim Smeeding, Director (4) | | | 831,667 | | | | 0.1 | % |
Common Stock | | Oceanus Contracting Ltd (3) | | | 9,114,652 | | | | 9.0 | % |
Common Stock | | Ryan Maarschalk, Chief Financial Officer (5) | | | 2,919,898 | | | | 0.4 | % |
Common Stock | | 0994091 BC, Ltd. (3) | | | 862,317 | | | | 0.8 | % |
Common Stock | | Santiago Ribero, Vice President Corporate Development and Director (6) | | | 2,500,000 | | | | 0.0 | % |
Common Stock | | All officers and directors (6 persons) | | | 47,986,531 | | | | 40.6 | % |
____________
(1) | Calculated pursuant to Rule 13d-3 under the Exchange Act, a beneficial owner of securities is a person who directly or indirectly, through any contract, arrangement, understanding, relationship or otherwise has, or shares, voting power and/or investment power with respect to the securities, and any person who has the right to acquire beneficial ownership of the security within 60 days through any means, including the exercise of any option, warrant or right or the conversion of a security. Any shares that are not outstanding that a person has the right to acquire are deemed to be outstanding for the purpose of calculating the percentage of beneficial ownership of such person, but are not deemed to be outstanding for the purpose of calculating the percentage of beneficial ownership of any other person. All of the foregoing shares are legally held of record by SECFAC Exchange Corp., which has an agreement with the shareholders to exchange shares of SECFAC Exchange Corp. for Allied Corp. shares on a one for one basis at the request of any such shareholder. |
(2) | Mr. Moses holds 300,000 shares and an additional 1,000,000 shares pursuant to warrants. |
(3) | All shares are held through Oceanus Consulting Ltd and 0994091 BC Ltd. Mr. Paul James Bullock is the principal of Oceanus Contracting Ltd. and 09940901 BC Ltd., and consequently may be deemed to be the beneficial owner of shares held by such entities. |
(4) | Mr. Smeeding holds 740,000 shares pursuant to options and an additional 91,667 shares are held by Adjudicate LLC, a company beneficially owned by Mr. Smeeding. |
(5) | Mr. Maarschalk holds 2,500,000 shares pursuant to options and an additional 419,898 shares are held by Maarschalk Capital, Inc., a company beneficially owned by Mr. Maarschalk. |
(6) | Mr. Ribero holds 2,500,000 shares pursuant to options. |
Holders of Common Stock
As of August 31, 2023, there were approximately 144 record holders of our common stock. This does not include the holders of our common stock who held their shares in street name as of that date. Based on reports we have reviewed relative to such holders, we currently have at least 3,062 beneficial stockholders.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
All transactions with related parties have occurred in the normal course of operations and are recorded at the exchange amount which is the amount agreed to by the Company and the related party.
Key management compensation and related party transactions
The Company has identified its directors and certain senior officers as its key management personnel. The compensation costs for key management personnel during the nine months ended were as follows:
| | August 31, 2023 | | | August 31, 2022 | |
Consulting fees and benefits | | $ | 396,052 | | | $ | 369,079 | |
Amounts due to/from related parties
In the normal course of operations, the company shares certain administrative resources with companies related by common management and directors. The administrative resources and services, which were provided in the normal course of operations, were measured at the exchange. All amounts payable and receivable are noninterest bearing, unsecured and due on demand. The following table summarizes the amounts were due from related parties:
| | August 31, 2023 | | | August 31, 2022 | |
CEO and Director | | $ | 156,028 | | | $ | 80,892 | |
COO and Director | | | 201,612 | | | | 143,816 | |
An entity controlled by the CFO | | | 84,962 | | | | 46,387 | |
An entity controlled by a director | | | 159,690 | | | | 60,677 | |
Director | | | 91,000 | | | | 52,500 | |
| | $ | 693,292 | | | $ | 384,272 | |
As of August 31, 2023, the Company had $693,292 (August 31, 2022 - $384,272) payable to related parties for fees or expenses incurred or expensed paid on behalf of the Company by the parties which has been presented in accounts payable and accrued liabilities.
Director Independence
The Company is not currently listed on any national exchange, or quoted on any inter-dealer quotation service, that imposes independence requirements on any committee of the Company’s directors, such as an audit, nominating or compensation committee. The Company currently does not currently have any independent directors on its Board.
Item 14. Principal Accounting Fees and Services
Audit Fees
The aggregate fees billed for the fiscal year ended August 31, for professional services rendered by the principal accountants for the audit of the registrant's annual financial statements and review of financial statements included in the registrant's Form 10-K or services that are normally provided by the accountant in connection with statutory and regulatory filings or engagements were: $92,000.
Audit-Related Fees
$nil was billed in the fiscal year ended August 31, 2023 for assurance and related services by the principal accountant that are reasonably related to the performance of the audit or review of the registrant's financial statements.
Tax Fees
$nil was billed for professional services rendered by the principal accountant for tax compliance, tax advice, and tax planning for the year ended August 31, 2023.
All Other Fees
Other than the services reported above, no other fees billed for professional services provided by the principal accountant.
Audit Committee Pre-Approval Policies
Our Board of Directors performing as the Audit Committee by their Chair has approved the principal accountant’s performance of services for the audit of the registrant’s annual financial statements and review of financial statements included in our Report on Form 10-K or services that are normally provided by the accountant in connection with statutory and regulatory filings or engagements for the fiscal year ending August 31, 2023. Audit-related fees, tax fees, and all other fees, if any, were approved by the Board of Directors.
Item 15. Exhibits, Financial Statement Schedules
The following exhibits are filed as part of this Annual Report.
Exhibit | | Description |
3.1 | | Articles of Incorporation of Registrant (incorporated by reference to Exhibit 3.1(i) of the Company’s Registration Statement filing on Form S-1 filed with the Securities and Exchange Commission on May 28, 2013) |
3.2 | | Bylaws of Registrant (incorporated by reference to Exhibit 3.1(ii) of the Company’s Registration Statement filing on Form S-1 filed with the Securities and Exchange Commission on May 28, 2013) |
3.3 | | Certificate of Amendment of Articles of Incorporation dated July 1, 2019 (incorporated by reference to Exhibit 3.3 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2019) |
3.4 | | Form of Underwriter Warrant (incorporated by reference to Exhibit 3.1 of the Company’s Offering Statement filing on Form 1-A filed with the Securities and Exchange Commission on June 11, 2021) |
10.1 | | Reorganization Agreement among Allied Corp., Pacific Capital Investment Group, Inc., SECFAC Exchange Corp., AM (Advanced Micro) Biosciences, Inc. and shareholders of AM (Advanced Micro) Biosciences, Inc. Dated as of July 25, 2019 and as amended effective August 27, 2019 (incorporated by reference to Exhibit 10.1 of the Company’s Registration Statement filing on Form 8-K filed with the Securities and Exchange Commission on September 10, 2019) |
10.2 | | Assumption of contract of purchase and sale of 8999 Jim Bailey Rd. between the Company and 1185710 B.C. Ltd. Dated November 6, 2018 (incorporated by reference to Exhibit 10.2 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2019) |
10.3 | | Share Purchase Agreement between AM (Advanced Micro) Biosciences, Inc. and Maryls Wolfe and Grant Wolfe dated May 24, 2019. (incorporated by reference to Exhibit 10.3 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2019) |
10.4 | | Escrow Agreement between AM (Advanced Micro) Biosciences, Inc., and Maryls Wolfe dated May 31, 2019 (incorporated by reference to Exhibit 10.4 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2019) |
10.5 | | Xtreme Cubes – Purchase Proposal dated May 14, 2019 (incorporated by reference to Exhibit 10.5 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2019) |
10.6 | | Vitalis Extraction Technology Sales Order dated August 30, 2019 (incorporated by reference to Exhibit 10.6 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2019) |
10.7 | | Purchase Agreement between AM (Advanced Micro) Biosciences, Inc. and 1150641 BC Ltd., doing business as Activated Nano, dated May 22, 2019 (incorporated by reference to Exhibit 10.7 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2019) |
10.8 | | Asset Purchase Agreement between AM (Advanced Micro) Biosciences, Inc. and Clifford Wade Lackie and Robin Dale Lackie for Bud’s Naturals dated February 13, 2019 (incorporated by reference to Exhibit 10.8 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2019) |
10.9 | | Consulting Agreement between AM (Advanced Micro) Biosciences, Inc. and John Saric dated May 31, 2019 (incorporated by reference to Exhibit 10.9 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2019) |
10.10 | | Convertible Promissory Note issued to CA Indosuez (Switzerland) S.A. January 23, 2020 (incorporated by reference to Exhibit 10.10 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.11 | | Series A Warrant issued to CA Indosuez (Switzerland) S.A. January 23, 2020 (incorporated by reference to Exhibit 10.11 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.12 | | Security Agreement dated as of January 23, 2020 between the Company and CA Indosuez (Switzerland) S.A. (incorporated by reference to Exhibit 10.12 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.13 | | Convertible Promissory Note issued to Parkward Holding Ltd. January 23, 2020 (incorporated by reference to Exhibit 10.13 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.14 | | Series A Warrant issued to Parkward Holding Ltd. January 23, 2020 (incorporated by reference to Exhibit 10.14 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.15 | | Security Agreement dated as of January 23, 2020 between the Company and Parkward Holding Ltd. (incorporated by reference to Exhibit 10.15 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.16 | | Convertible Promissory Note issued to Allied Special Opportunities Limited February 25, 2020 (incorporated by reference to Exhibit 10.16 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.17 | | Series A Warrant issued to Allied Special Opportunities Limited February 25, 2020 (incorporated by reference to Exhibit 10.17 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.18 | | Security Agreement dated as of January 23, 2020 between the Company and Allied Special Opportunities Lit.. (incorporated by reference to Exhibit 10.18 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.18 | | Loan and Security Agreement dated May 14, 2020 between the Company and SLCI1, LLC. (incorporated by reference to Exhibit 10.19 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.19 | | Promissory Note issued May 14, 2020 from the Company to SLCI1, LLC. (incorporated by reference to Exhibit 10.20 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.20 | | 2020 Long term Incentive Plan (incorporated by reference to Exhibit 10.21 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.21 | | Share Purchase Agreement between Dorson Commercial Corp. and AD (Advanced Micro) Biosciences, Inc. dated August 29, 2019 (incorporated by reference to Exhibit 10.1 of the Company’s Report filing on Form 8-K filed with the Securities and Exchange Commission on October 7, 2020) |
10.22 | | License for Medicolombia Cannabis A.S. from Republic of Columbia, Ministry of Justice and Law, dated August 3, 2018 (incorporated by reference to Exhibit 10.2 of the Company’s Report filing on Form 8-K filed with the Securities and Exchange Commission on October 7, 2020) |
10.23 | | License for Medicolombia Cannabis A.S. from Republic of Columbia, Ministry of Health and Social Protection, dated December 4, 2018 (incorporated by reference to Exhibit 10.3 of the Company’s Report filing on Form 8-K filed with the Securities and Exchange Commission on October 7, 2020) |
10.24 | | License for Medicolombia Cannabis A.S. from Republic of Columbia, Ministry of Justice and Law, dated July 31, 2019 (incorporated by reference to Exhibit 10.4 of the Company’s Report filing on Form 8-K filed with the Securities and Exchange Commission on October 7, 2020) |
10.25 | | License for Medicolombia Cannabis A.S. from Republic of Columbia, Ministry of Justice and Law, dated February 20, 2019 (incorporated by reference to Exhibit 10.5 of the Company’s Report filing on Form 8-K filed with the Securities and Exchange Commission on October 7, 2020) |
10.26 | | License for Medicolombia Cannabis A.S. from Republic of Columbia, Ministry of Health and Social Protection, dated November 29, 2019 (incorporated by reference to Exhibit 10.6 of the Company’s Report filing on Form 8-K filed with the Securities and Exchange Commission on October 7, 2020) |
10.27 | | License for Medicolombia Cannabis A.S. from Republic of Columbia, National Narcotics Fund U.A.E., Ministry of Health and Social Protection dated January 13, 2020 (incorporated by reference to Exhibit 10.7 of the Company’s Report filing on Form 8-K filed with the Securities and Exchange Commission on October 7, 2020) |
10.28 | | Settlement and Release Agreement between the Company and Anthony Zelen dated September 17, 2020 (incorporated by reference to Exhibit 10.29 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.29 | | Settlement and Release Agreement between the Company and David Weinkauf dated September 21, 2020 (incorporated by reference to Exhibit 10.30 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.30 | | Settlement and Release Agreement between the Company and Malcolm Davidson dated September 16, 2020 (incorporated by reference to Exhibit 10.31 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.31 | | Convertible Promissory Note issued to Sawasawa Inc. September 30, 2020 (incorporated by reference to Exhibit 10.32 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.32 | | Convertible Promissory Note issued to Sawasawa Inc. November 7, 2020 (incorporated by reference to Exhibit 10.33 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.33 | | Convertible Promissory Note issued to Sawasawa, Inc. November 30, 2020 (incorporated by reference to Exhibit 10.34 of the Company’s Report filing on Form 10-K filed with the Securities and Exchange Commission on August 31, 2020) |
10.34 | | Placement Agent Agreement between the Company and Boustead Securities, LLC dated as of May 7, 2021 (incorporated by reference to Exhibit 3.1 of the Company’s filing on Form 1-A filed with the Securities and Exchange Commission on August 23, 2021) |
10.35 | | Amendment No. 1 to Placement Agent Agreement between the Company and Boustead Securities, LLC dated as of August 17, 2021 (incorporated by reference to Exhibit 3.2 of the Company’s filing on Form 1-A filed with the Securities and Exchange Commission on August 23, 2021) |
10.36 | | Convertible Promissory Note issued to Steve Moses on March 26, 2021 (incorporated by reference to Exhibit 10.36 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.37 | | Convertible Promissory Note issued to Tulip Enterprises LLC on March 26, 2021 (incorporated by reference to Exhibit 10.37 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.38 | | Convertible Promissory Note issued to Tulip Enterprises LLC on April 21, 2021 (incorporated by reference to Exhibit 10.38 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.39 | | Convertible Promissory Note issued to Steve Moses on April 30, 2021 (incorporated by reference to Exhibit 10.39 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.40 | | Convertible Promissory Note issued to Tulip Enterprises LLC on July 25, 2021 (incorporated by reference to Exhibit 10.40 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.41 | | Convertible Promissory Note issued to Steve Moses on July 25, 2021 (incorporated by reference to Exhibit 10.41 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.42 | | Convertible Promissory Note issued to Tulip Enterprises LLC on October 1, 2021 (incorporated by reference to Exhibit 10.42 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.43 | | Convertible Promissory Note issued to Tulip Enterprises LLC on October 25, 2021 (incorporated by reference to Exhibit 10.43 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.44 | | Convertible Promissory Note issued to Tulip Enterprises LLC on December 23, 2021 (incorporated by reference to Exhibit 10.44 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.45 | | Convertible Promissory Note issued to Steve Moses on December 23, 2021 (incorporated by reference to Exhibit 10.45 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.46 | | Convertible Promissory Note issued to Tulip Enterprises LLC on January 11, 2022 (incorporated by reference to Exhibit 10.46 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.47 | | Convertible Promissory Note issued to Tulip Enterprises LLC on January 31, 2022 (incorporated by reference to Exhibit 10.47 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.48 | | Convertible Promissory Note issued to 0994091 BC Ltd. on March 29, 2022 (incorporated by reference to Exhibit 10.48 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.49 | | Convertible Promissory Note issued to Mulsane Ltd. on June 16, 2022 (incorporated by reference to Exhibit 10.49 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.50 | | Form of Amendment for all outstanding convertible promissory notes September 30, 2022 (incorporated by reference to Exhibit 10.50 of the Company’s filing on Form 10-K filed with the Securities and Exchange Commission on December 14, 2022). |
10.51 | | Interim Operating Agreement between the Company and Michael Moses dated as of September 1, 2023. |
10.52 | | Consulting Agreement between the Company and Michael Moses |
10.53 | | SOL NDA Forward Purchase Agreement dated February 8, 2023. (incorporated by reference to Exhibit 10.1 of the Company’s filing on Form 8-K filed with the Securities and Exchange Commission on December 5, 2023) |
10.54 | | Australian Cannabis Group Forward Purchase Agreement dated May 30, 2023. (incorporated by reference to Exhibit 10.3 of the Company’s filing on Form 8-K filed with the Securities and Exchange Commission on December 5, 2023) |
10.55 | | One Life Labs Pty Ldt Forward Purchase Agreement dated November 13, 2023. (incorporated by reference to Exhibit 10.2 of the Company’s filing on Form 8-K filed with the Securities and Exchange Commission on December 5, 2023) |
10.56 | | Term Sheet for Contract Manufacturing Services with Blossom Genetics Unipessoal Lda dated October 27, 2023. (incorporated by reference to Exhibit 10.1 of the Company’s filing on Form 8-K filed with the Securities and Exchange Commission on December 5, 2023) |
21 | | List of Subsidiaries. |
31.1 | | Certification of Chief Executive Officer pursuant to Rule 13a-14 of the Securities Exchange Act of 1934 |
31.2 | | Certification of Chief Financial Officer pursuant to Rule 13a-14 of the Securities Exchange Act of 1934 |
32.1 | | Certification of Chief Executive Officer pursuant to Section 1350 |
32.2 | | Certification of Chief Financial Officer pursuant to Section 1350 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this transition report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Allied Corp. | |
| | | |
December 14, 2023 | By: | /s/ Calum Hughes | |
| | Calum Hughes | |
| | Chief Executive Officer | |
| | (Principal Executive Officer) | |
| | | |
December 14, 2023 | By: | /s/ Ryan Maarschalk | |
| | Ryan Maarschalk | |
| | Chief Financial Officer | |
| | (Principal Financial and Accounting Officer) | |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Date: December 14, 2023 | /s/ Calum Hughes | |
| Calum Hughes, Director | |
| and Chief Executive Officer | |
| | |
Date: December 14, 2023 | /s/ Paul Bullock | |
| Paul Bullock, Director | |
| | |
Date: December 14, 2023 | /s/ Jim Smeeding | |
| Jim Smeeding, Director | |
nullnullnullnullnullnullnull
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPCAOB issued Audit Firm Identifier Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorFirmId |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:nonemptySequenceNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorLocation |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an annual report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentAnnualReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter.
| Name: |
dei_EntityPublicFloat |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
| Name: |
dei_EntityVoluntaryFilers |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Is used on Form Type: 10-K, 10-Q, 8-K, 20-F, 6-K, 10-K/A, 10-Q/A, 20-F/A, 6-K/A, N-CSR, N-Q, N-1A. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 405
| Name: |
dei_EntityWellKnownSeasonedIssuer |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Consolidated Balance Sheets - USD ($)
|
Aug. 31, 2023 |
Aug. 31, 2022 |
| Current assets |
|
|
| Cash |
$ 209,736
|
$ 96,043
|
| Inventory (Note 3) |
107,510
|
998,988
|
| Other receivables |
134,482
|
121,428
|
| Prepaid expenses |
18,852
|
25,187
|
| Total current assets |
470,580
|
1,241,646
|
| Deposits and advances (Note 4) |
26,354
|
2,869,825
|
| Right-of-use assets (Note 7) |
109,301
|
189,325
|
| Property, plant and equipment (Note 5) |
1,417,192
|
1,444,038
|
| Intangible assets (Note 6) |
40,301
|
44,773
|
| Total assets |
2,063,728
|
5,789,607
|
| Current liabilities |
|
|
| Accounts payable and accrued liabilities |
2,393,359
|
1,607,862
|
| Due to related parties (Note 11) |
693,292
|
384,272
|
| Current portion of lease liabilities (Note 7) |
10,745
|
24,725
|
| Loans payable (Note 8) |
2,027,348
|
1,555,654
|
| Secured convertible notes payable (Note 9) |
3,724,891
|
3,334,891
|
| Total current liabilities |
8,849,635
|
6,907,404
|
| Lease liabilities, net of current portion (Note 7) |
98,556
|
164,600
|
| Total liabilities |
8,948,191
|
7,072,004
|
| Preferred stock - 50,000,000 shares authorized, $0.0001 par value Nil shares issued and outstanding |
0
|
0
|
| Common stock - 300,000,000 shares authorized, $0.0001 par value; 101,592,914 shares issued and outstanding (93,967,594 - par value $0.0001 - August 31, 2022) |
10,160
|
9,397
|
| Additional paid in capital |
39,404,667
|
33,999,090
|
| Common stock issuable |
80,000
|
540,000
|
| Accumulated deficit |
(45,608,336)
|
(34,932,665)
|
| Accumulated other comprehensive loss |
(770,954)
|
(898,219)
|
| Total stockholders' deficit |
(6,884,463)
|
(1,282,397)
|
| Total liabilities and stockholders' deficit |
$ 2,063,728
|
$ 5,789,607
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionStated value of common units of ownership issued by a limited liability company (LLC).
| Name: |
us-gaap_CommonUnitIssuanceValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of the portion of long-term debt due within one year or the operating cycle if longer identified as Convertible Notes Payable. Convertible Notes Payable is a written promise to pay a note which can be exchanged for a specified amount of another, related security, at the option of the issuer and the holder. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_ConvertibleNotesPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value of amounts transferred to third parties for security purposes that are expected to be returned or applied towards payment after one year or beyond the operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DepositsAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483154/926-20-50-5
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after valuation and LIFO reserves of inventory expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date of portion of long-term loans payable due within one year or the operating cycle if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LoansPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount due from parties in nontrade transactions, classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(5)(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(3)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherReceivables |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482955/340-10-05-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483032/340-10-45-1
| Name: |
us-gaap_PrepaidExpenseCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
Consolidated Balance Sheets (Parenthetical) - $ / shares
|
Aug. 31, 2023 |
Aug. 31, 2022 |
| Stockholders' equity (deficit) |
|
|
| Preferred stock, shares authorized |
50,000,000
|
50,000,000
|
| Preferred stock, shares par value |
$ 0.0001
|
$ 0.0001
|
| Preferred stock, shares issued |
0
|
0
|
| Preferred stock, shares outstanding |
0
|
0
|
| Common stock, shares authorized |
300,000,000
|
300,000,000
|
| Common stock, shares par value |
$ 0.0001
|
$ 0.0001
|
| Common stock, shares issued |
101,592,914
|
93,967,594
|
| Common stock, shares outstanding |
101,592,914
|
93,967,594
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) issued to shareholders (includes related preferred shares that were issued, repurchased, and remain in the treasury). May be all or portion of the number of preferred shares authorized. Excludes preferred shares that are classified as debt. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Consolidated Statements of Operations and Comprehensive Loss - USD ($)
|
12 Months Ended |
Aug. 31, 2023 |
Aug. 31, 2022 |
| Revenues |
|
|
| Sales |
$ 72,096
|
$ 165,596
|
| Cost of sales (Note 3) |
(1,533,863)
|
(91,476)
|
| Gross margin |
(1,461,767)
|
74,120
|
| Expenses |
|
|
| Amortization and depreciation |
137,046
|
82,589
|
| Consulting fees (Note 10 and 11) |
4,189,953
|
12,080,684
|
| Foreign exchange loss (gain) |
147,722
|
(1,393)
|
| Interest expense and bank charges |
477,750
|
467,451
|
| Office and miscellaneous |
590,374
|
1,133,560
|
| Professional fees |
439,603
|
1,031,091
|
| Research and development |
0
|
12,062
|
| Travel |
12,265
|
43,670
|
| Loss on deposit impairment (Note 4) |
(2,656,695)
|
0
|
| Loss on license acquisition advance (Note 4) |
(150,000)
|
0
|
| Operating expenses |
8,801,408
|
14,849,714
|
| Loss before other items |
(10,263,175)
|
(14,775,594)
|
| Other income and expenses |
|
|
| Accretion |
0
|
(547,598)
|
| Bad debt recovery |
0
|
3,646
|
| Interest expense |
(385,453)
|
(219,307)
|
| Loss on debt extinguishment |
(27,043)
|
0
|
| Total other expenses |
(412,496)
|
(763,259)
|
| Net loss |
(10,675,671)
|
(15,538,853)
|
| Foreign currency translation adjustments |
127,265
|
(283,346)
|
| Comprehensive loss |
$ (10,548,406)
|
$ (15,822,199)
|
| Basic and diluted loss per share |
$ (0.11)
|
$ (0.17)
|
| Weighted average number of common shares outstanding |
96,797,714
|
92,098,050
|
| X |
- References
+ Details
| Name: |
alid_BasicAndDilutedLossPerShare |
| Namespace Prefix: |
alid_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_GainLossOnLicenseAcquisitionAdvance |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_WeightedAverageNumberOfCommonSharesOutstanding |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount recognized for the passage of time, typically for liabilities, that have been discounted to their net present values. Excludes accretion associated with asset retirement obligations. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481639/420-10-35-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
| Name: |
us-gaap_AccretionExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of recurring noncash expense charged against earnings in the period to allocate the cost of assets over their estimated remaining economic lives. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_AdjustmentForAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate cost of goods produced and sold and services rendered during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_CostOfRevenue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, before tax, of realized gain (loss) from foreign currency transaction. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-6
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481956/830-20-45-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481926/830-20-50-1
| Name: |
us-gaap_ForeignCurrencyTransactionGainLossRealized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionDifference between the fair value of payments made and the carrying amount of debt which is extinguished prior to maturity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481303/470-50-40-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481303/470-50-40-4
| Name: |
us-gaap_GainsLossesOnExtinguishmentOfDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of loss from the write-down of an asset representing the future economic benefits arising from other assets acquired in a business combination that are not individually identified and separately recognized. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482548/350-20-55-24
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482598/350-20-45-2
| Name: |
us-gaap_GoodwillImpairmentLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate revenue less cost of goods and services sold or operating expenses directly attributable to the revenue generation activity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.1,2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GrossProfit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionInterest and debt related expenses associated with nonoperating financing activities of the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 20
-Topic 835
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestAndDebtExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of the cost of borrowed funds accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (210.5-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of expense provided in the period for legal costs incurred on or before the balance sheet date pertaining to resolved, pending or threatened litigation, including arbitration and mediation proceedings. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_LegalFees |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature, attributable to parent entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(3)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_OtherComprehensiveIncomeForeignCurrencyTransactionAndTranslationAdjustmentNetOfTaxPortionAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of income (expense) related to nonoperating activities, classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherNonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherNonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA fee charged for services from professionals such as doctors, lawyers and accountants. The term is often expanded to include other professions, for example, pharmacists charging to maintain a medicinal profile of a client or customer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (k)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-3
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
| Name: |
us-gaap_ProfessionalFees |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (reversal of expense) for expected credit loss on accounts receivable. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_ProvisionForDoubtfulAccounts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue recognized from goods sold, services rendered, insurance premiums, or other activities that constitute an earning process. Includes, but is not limited to, investment and interest income before deduction of interest expense when recognized as a component of revenue, and sales and trading gain (loss). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
| Name: |
us-gaap_Revenues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_RevenuesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpenses incurred for travel and entertainment during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_TravelAndEntertainmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
Consolidated Statements of Stockholders Deficit - USD ($)
|
Total |
Common Stock [Member] |
Treasury Stock [Member] |
Additional Paid-in Capital [Member] |
Stock Issuable [Member] |
Accumulated Deficit [Member] |
Accumulated Other Comprehensive Loss [Member] |
| Balance, shares at Aug. 31, 2021 |
|
79,858,867
|
7,073,170
|
|
|
|
|
| Balance, amount at Aug. 31, 2021 |
$ (970,781)
|
$ 7,986
|
$ 707
|
$ 18,099,226
|
$ 929,985
|
$ (19,393,812)
|
$ (614,873)
|
| Shares issued from treasury, shares |
|
7,073,170
|
(7,073,170)
|
|
|
|
|
| Shares issued from treasury, amount |
7,957,316
|
$ 707
|
$ (707)
|
7,957,316
|
0
|
0
|
0
|
| Shares issued for cash, shares |
|
6,659,557
|
|
|
|
|
|
| Shares issued for cash, amount |
4,212,252
|
$ 666
|
0
|
5,141,571
|
(929,985)
|
0
|
0
|
| Share issuance costs |
(340,616)
|
0
|
0
|
(340,616)
|
0
|
0
|
0
|
| Shares subscribed |
540,000
|
$ 0
|
0
|
0
|
540,000
|
0
|
0
|
| Shares issued for finders fees, shares |
|
8,000
|
|
|
|
|
|
| Shares issued for finders fees, amount |
6,000
|
$ 1
|
0
|
5,999
|
0
|
0
|
0
|
| Shares issued in error not yet cancelled, shares |
|
368,000
|
|
|
|
|
|
| Shares issued in error not yet cancelled, amount |
0
|
$ 37
|
0
|
(37)
|
0
|
0
|
0
|
| Detachable warrants issued with convertible notes payable |
120,310
|
0
|
0
|
120,310
|
0
|
0
|
0
|
| Beneficial conversion feature |
281,447
|
0
|
0
|
281,447
|
0
|
0
|
0
|
| Stock-based compensation |
2,733,874
|
0
|
0
|
2,733,874
|
0
|
0
|
0
|
| Comprehensive loss for the year |
(15,822,199)
|
$ 0
|
0
|
0
|
0
|
(15,538,853)
|
(283,346)
|
| Balance, shares at Aug. 31, 2022 |
|
93,967,594
|
|
|
|
|
|
| Balance, amount at Aug. 31, 2022 |
(1,282,397)
|
$ 9,397
|
$ 0
|
33,999,090
|
540,000
|
(34,932,665)
|
(898,219)
|
| Shares issued for cash, shares |
|
7,479,760
|
|
|
|
|
|
| Shares issued for cash, amount |
1,540,952
|
$ 748
|
|
2,080,204
|
(540,000)
|
0
|
0
|
| Share issuance costs |
191,660
|
0
|
|
191,660
|
0
|
0
|
0
|
| Shares subscribed |
80,000
|
0
|
|
0
|
80,000
|
0
|
0
|
| Stock-based compensation |
3,047,156
|
0
|
|
3,047,156
|
0
|
0
|
0
|
| Comprehensive loss for the year |
(10,548,406)
|
$ 0
|
|
0
|
0
|
(10,675,671)
|
127,265
|
| Shares issued to settle debts, shares |
|
145,560
|
|
|
|
|
|
| Shares issued to settle debts, amount |
86,572
|
$ 15
|
|
86,557
|
0
|
0
|
0
|
| Balance, shares at Aug. 31, 2023 |
|
101,592,914
|
|
|
|
|
|
| Balance, amount at Aug. 31, 2023 |
$ (6,884,463)
|
$ 10,160
|
|
$ 39,404,667
|
$ 80,000
|
$ (45,608,336)
|
$ (770,954)
|
| X |
- References
+ Details
| Name: |
alid_BeneficialConversionFeature |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_DetachableWarrantsIssuedWithConvertibleNotesPayable |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_ShareIssuanceCosts |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_SharesIssuedForCashAmount |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_SharesIssuedForCashShares |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_SharesIssuedForFindersFeesAmount |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_SharesIssuedForFindersFeesShares |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_SharesIssuedFromTreasuryAmount |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_SharesIssuedFromTreasuryShares |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_SharesIssuedInErrorNotYetCancelledAmount |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_SharesIssuedInErrorNotYetCancelledShares |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_SharesIssuedToSettleDebtsAmount |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_SharesIssuedToSettleDebtsShares |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_SharesSubscribed |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of other comprehensive income (loss) attributable to parent entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(3)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
| Name: |
us-gaap_OtherComprehensiveIncomeLossNetOfTaxPortionAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued as of the balance sheet date, including shares that had been issued and were previously outstanding but which are now held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
| Name: |
us-gaap_SharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
Consolidated Statements of Cash Flows - USD ($)
|
12 Months Ended |
Aug. 31, 2023 |
Aug. 31, 2022 |
| Cash provided by (used in):Operating activities |
|
|
| Net loss for the year |
$ (10,675,671)
|
$ (15,538,853)
|
| Adjustment to net loss for the period for non-cash items |
|
|
| Accretion |
0
|
547,598
|
| Accrued interest |
857,146
|
238,646
|
| Amortization and depreciation |
137,046
|
82,589
|
| Inventory write-down to net realizable value |
1,507,018
|
31,737
|
| Fair value of finder's warrants |
204,452
|
0
|
| Loss on debt extinguishment |
27,043
|
0
|
| Loss on deposit impairment |
2,656,695
|
0
|
| Loss on license acquisition advance |
150,000
|
0
|
| Stock-based compensation - consulting services |
0
|
3,584,392
|
| Stock-based compensation - bonus shares |
0
|
4,585,425
|
| Stock-based compensation - options |
3,047,156
|
2,733,874
|
| Changes in non-cash working capital balance: |
|
|
| Increase in other receivables |
(15,692)
|
(38,591)
|
| Decrease in prepaid expenses |
6,335
|
39,842
|
| Decrease in deposits and advances |
37,162
|
315,450
|
| Increase (decrease) in accounts payable and accrued liabilities |
212,686
|
(215,583)
|
| Increase in due to related parties |
339,020
|
136,752
|
| Increase in inventory |
(447,175)
|
(787,544)
|
| Net cash provided by (Used in) Operating Activities |
(1,956,779)
|
(4,284,266)
|
| Investing activities |
|
|
| Purchase of intangible assets |
(4,625)
|
(12,111)
|
| Purchase of property, plant, and equipment |
(83,170)
|
(1,193,258)
|
| Net cash provided by (Used in) Investing Activities |
(87,795)
|
(1,205,369)
|
| Financing activities |
|
|
| Advance from related party |
70,000
|
0
|
| Proceeds of convertible notes payable |
390,000
|
1,400,000
|
| Repayment of loans payable |
0
|
(387,750)
|
| Repayment of convertible notes payable |
0
|
(100,000)
|
| Proceeds from the issuance of common stock |
1,528,160
|
3,877,634
|
| Proceeds for subscriptions of stock issuable |
80,000
|
540,000
|
| Net cash provided by (Used in) Financing Activities |
2,068,160
|
5,329,884
|
| Increase (decrease) in cash |
(23,586)
|
(159,751)
|
| Effect of exchange rate on changes of cash |
90,107
|
(164,031)
|
| Cash, beginning of year |
96,043
|
419,825
|
| Cash, end of year |
209,736
|
96,043
|
| Supplemental cash flow disclosures: |
|
|
| Income taxes paid |
0
|
0
|
| Interest paid |
$ 0
|
$ 401,990
|
| X |
- References
+ Details
| Name: |
alid_EffectOfExchangeRateOnChangeOfCash |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_GainLossOnLicenseAcquisitionAdvance |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_InventoryWriteOffToNetRealizableValue |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_LossOnDepositImpairment |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_ProceedsFromSubscriptionsReceived |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_RepaymentOfLoansPayable |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_StockBasedCompensationBonusShares |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_StockBasedCompensationConsultingServices |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount recognized for the passage of time, typically for liabilities, that have been discounted to their net present values. Excludes accretion associated with asset retirement obligations. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481639/420-10-35-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
| Name: |
us-gaap_AccretionExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of recurring noncash expense charged against earnings in the period to allocate the cost of assets over their estimated remaining economic lives. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_AdjustmentForAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsNoncashItemsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionIncrease for accrued, but unpaid interest on the debt instrument for the period. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(f))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
| Name: |
us-gaap_DebtInstrumentIncreaseAccruedInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionDifference between the fair value of payments made and the carrying amount of debt which is extinguished prior to maturity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481303/470-50-40-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481303/470-50-40-4
| Name: |
us-gaap_GainsLossesOnExtinguishmentOfDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amounts payable to vendors for goods and services received and the amount of obligations and expenses incurred but not paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amount of (a) prepayments by customers for goods or services to be provided at a later date, (b) the amount of customer money held in customer accounts, including security deposits, collateral for a current or future transactions, initial payment of the cost of acquisition or for the right to enter into a contract or agreement, or (c) a combination of (a) and (b). Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInCustomerAdvancesAndDeposits |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate value of all inventory held by the reporting entity, associated with underlying transactions that are classified as operating activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInInventories |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in receivables classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInOtherReceivables |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amount of outstanding money paid in advance for goods or services that bring economic benefits for future periods. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash paid for interest, excluding capitalized interest, classified as operating activity. Includes, but is not limited to, payment to settle zero-coupon bond for accreted interest of debt discount and debt instrument with insignificant coupon interest rate in relation to effective interest rate of borrowing attributable to accreted interest of debt discount. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-2
| Name: |
us-gaap_InterestPaidNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow to acquire asset without physical form usually arising from contractual or other legal rights, excluding goodwill. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow from the acquisition of or improvements to long-lived, physical assets used to produce goods and services and not intended for resale, classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireOtherPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the issuance of a long-term debt instrument which can be exchanged for a specified amount of another security, typically the entity's common stock, at the option of the issuer or the holder. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromConvertibleDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow from the repayment of a long-term debt instrument which can be exchanged for a specified amount of another security, typically the entity's common stock, at the option of the issuer or the holder. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_RepaymentsOfConvertibleDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for option under share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_StockOptionPlanExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
Nature of operations reverse takeover transaction and going concern
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Nature of operations reverse takeover transaction and going concern |
|
| Nature of operations, reverse take-over transaction and going concern |
1. Nature of operations, reverse take-over transaction and going concern a) Nature of operations Allied Corp. (the “Company or Allied”) was incorporated in the State of Nevada on February 3, 2013. On July 1, 2019, the Company changed its name to Allied Corp. The head office and the registered office of the Company are located at 1405 St. Paul Street, Kelowna BC V1Y 2E4. The Company’s business plan is to discover new medical technologies some of which are cannabis derived to target full scope therapy and support for trauma survivors, military veterans and first responders, however the Company has not begun such operations nor obtained the required permits to begin such operations. On September 10, 2019, the Company was acquired in a reverse takeover (“RTO”) transaction and the RTO is considered a purchase of the Company’s net assets by AM (Advanced Micro) Biosciences, Inc. (“AM Biosciences”). For accounting purposes, the legal subsidiary, AM Biosciences has been treated as the acquirer and Allied Corp., the legal parent, has been treated as the acquiree. Accordingly, these consolidated financial statements reflect a continuation of the financial position, operating results, and cash flow of the Company’s legal subsidiary, AM Biosciences from the date of incorporation on September 13, 2018. On February 18, 2020, the Company acquired all the issued and outstanding share capital of a Colombian company, Allied Colombia S.A.S (“Allied Colombia”). The assets, liabilities and results of Allied Colombia are consolidated in these financial statements beginning from the February 18, 2020 acquisition date. As at August 31, 2023, Allied Colombia has a licensed cannabis farm in Colombia. b) Going concern The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. The Company incurred a net loss for the year ended August 31, 2023 of $10,675,671, has generated minimal revenue and as at August 31, 2023 has a working capital deficit of $8,379,055. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern. The consolidated financial statements of the Company do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classifications of liabilities that might be necessary should the Company be unable to continue as a going concern. The Company’s ability to continue as a going concern is dependent upon the Company’s ability to raise sufficient financing to acquire or develop a profitable business. Management intends on financing its operations and future development activities largely from the sale of equity securities with some additional funding from other traditional financing sources, including related party loans until such time that funds provided by future planned operations are sufficient to fund working capital requirements. c) Business Risks While some states in the United States have authorized the use and sale of cannabis, it remains illegal under federal law and the approach to enforcement of U.S. federal laws against cannabis is subject to change. The Company plans to engage in cannabis-related activities in the United States, only if and when cannabis operations are federally legalized. On January 4, 2018, the then United States Attorney General Jeff Sessions issued a memorandum to United States district attorneys (the “Sessions Memorandum”) which rescinded previous guidance from the United States Department of Justice specific to cannabis enforcement in the United States, including the Cole Memorandum. With the Cole Memorandum rescinded, United States federal prosecutors no longer have guidance relating to the exercise of their discretion in determining whether to prosecute cannabis related violations of United States federal law. Since that time, United States district attorneys have taken no legal action against state law compliant entities, and the Biden administration is generally anticipated to seek federal decriminalization of state legal cannabis activity. Nevertheless, a significant change in the federal government’s enforcement policy with respect to current federal laws applicable to cannabis could cause significant financial damage to the Company. The Company may be irreparably harmed by a change in enforcement policies of the federal government depending on the nature of such change. Given the current illegality of cannabis under United States federal law, the Company’s ability to access both public and private capital may be hindered by the fact that certain financial institutions are regulated by the United States federal government and are thus prohibited from providing financing to companies engaged in cannabis related activities. The Company’s ability to access public capital markets in the United States is directly hindered as a result. The Company may, however, be able to access public and private capital markets in the United States through institutions which are not regulated by the United States federal government, in Canada, and in many other countries in order to support continuing operations.
|
| X |
- DefinitionThe entire disclosure for the business description and basis of presentation concepts. Business description describes the nature and type of organization including but not limited to organizational structure as may be applicable to holding companies, parent and subsidiary relationships, business divisions, business units, business segments, affiliates and information about significant ownership of the reporting entity. Basis of presentation describes the underlying basis used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205/tableOfContent
| Name: |
us-gaap_BusinessDescriptionAndBasisOfPresentationTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Significant accounting policies
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Significant accounting policies |
|
| Significant accounting policies |
2. Significant accounting policies Business Presentation These consolidated financial statements and related notes are presented in accordance with accounting principles generally accepted in the United States of America (“US GAAP”), and are expressed in United States dollars. The Company’s fiscal year end is August 31. The significant accounting policies followed are: a) Principles of consolidation The consolidated financial statements include accounts of Allied Corp. and its wholly-owned subsidiaries, including AM Biosciences, Allied US Products LLC, Tactical Relief LLC, Baleno Ltd. and Allied Colombia. Subsidiaries are consolidated from the date of acquisition and control and continue to be consolidated until the date that such control ceases. Control is achieved when the Company is exposed, or has rights, to variable returns from its involvement with the investee and has the ability to affect these returns through its power over the investee. All intercompany balances, income, expenses, and unrealized gains and losses resulting from intercompany transactions are eliminated on consolidation. b) Cash and cash equivalents Cash is comprised of cash on hand, cash held in trust accounts and demand deposits. Cash equivalents are short-term, highly liquid investments with maturities within three months when acquired. The Company did not have any cash equivalents as of August 31, 2023 and August 31, 2022. c) Property, plant and equipment Property, plant and equipment are stated at cost. The Company depreciates the cost of property, plant and equipment over their estimated useful lives at the following annual rates and methods: Farm facility and equipment | 1 - 10 years straight-line basis | Office and computer equipment | 5 - 10 years straight-line basis | Land equipment | 10 years straight-line basis |
d) Inventory Inventory is comprised of raw materials, supplies, vegetative and flowering plants, dried flower, diluted crude and CBD isolates available for sale, and purchased cannabis products. Inventory is stated at the lower of cost or net realizable value, determined using weighted average cost. Net realizable value is defined as the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal and transportation. At the end of each reporting period, the Company performs an assessment of inventory and records write-downs for excess and obsolete inventories based on the Company’s estimated forecast of product demand, production requirements, market conditions, regulatory environment, and spoilage. Actual inventory losses may differ from management’s estimates and such differences could be material to the Company’s consolidated balance sheets, statements of net loss and comprehensive loss and statements of cash flows. e) Intangible assets Intangible assets include licenses which are being amortized over their estimated useful lives of 10 years. The Company’s licenses are amortized over their economic or legal life on a straight-line basis, whichever is shorter. The licenses have been amortized from the date of acquisition. The Company periodically evaluates the reasonableness of the useful lives of these assets. Once these assets are fully amortized, they are removed from the accounts. These assets are reviewed for impairment or obsolescence when events or changes in circumstances indicate that the carrying amount may not be recoverable. If impaired, intangible assets are written down to fair value based on discounted cash flows or other valuation techniques. The Company has no intangibles with indefinite lives. For long-lived assets, impairment losses are only recorded if the asset’s carrying amount is not recoverable through its undiscounted, probability-weighted future cash flows. The Company measures the impairment loss based on the difference between the carrying amount and the estimated fair value. When an impairment exists, the related assets are written down to fair value. f) Long-lived assets In accordance with ASC 360, Property, Plant and Equipment, the Company tests long-lived assets or asset groups for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable. Circumstances which could trigger a review include, but are not limited to: significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction of the asset; current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset; and current expectation that the asset will more likely than not be sold or disposed significantly before the end of its estimated useful life. Recoverability is assessed based on the carrying amount of the asset and its fair value, which is generally determined based on the sum of the undiscounted cash flows expected to result from the use and the eventual disposal of the asset, as well as specific appraisal in certain instances. An impairment loss is recognized when the carrying amount is not recoverable and exceeds fair value. g) Foreign currency translation and functional currency conversion Items included in these consolidated financial statements of each of the Company’s entities are measured using the currency of the primary economic environment in which the entities operate (the “functional currency”). Prior to September 10, 2019, the Company’s functional currency was the Canadian dollar. Translation gains and losses from the application of the U.S. dollar as the reporting currency during the period that the Canadian dollar was the functional currency are included as part of cumulative currency translation adjustment, which is reported as a component of shareholders’ equity under accumulated other comprehensive loss. The Company re-assessed its functional currency and determined as at September 10, 2019, its functional currency changed from the Canadian dollar to the U.S. dollar based on management’s analysis of changes in our organization. The change in functional currency was accounted for prospectively from September 10, 2019 and prior period financial statements were not restated for the change in functional currency. For periods commencing September 10, 2019, monetary assets and liabilities denominated in foreign currencies are translated into U.S. dollars using exchange rates in effect at the balance sheet date. Opening balances related to non-monetary assets and liabilities are based on prior period translated amounts, and non-monetary assets and non-monetary liabilities incurred after September 10, 2019 are translated at the approximate exchange rate prevailing at the date of the transaction. Revenue and expense transactions are translated at the approximate exchange rate in effect at the time of the transactions. Foreign exchange gains and losses are included in the statement of operations and comprehensive loss as foreign exchange gains. The Company assessed the functional currency for Allied Colombia to be the Colombian peso. The functional currency for all other subsidiaries is the U.S. dollar. h) Share issuance costs Costs directly attributable to the raising of capital are charged against the related share capital. Costs related to shares not yet issued are recorded as deferred share issuance costs. These costs are deferred until the issuance of the shares to which the costs relate, at which time the costs will be charged against the related share capital or charged to operations if the shares are not issued. i) Research and development costs Research and development costs are expensed as incurred. During the year ended August 31, 2023, the Company incurred research and development costs of $nil (August 31, 2022 – $12,062). j) Advertising costs Advertising costs are expensed as incurred. During the year ended August 31, 2023, the Company incurred advertising costs of $11,425 (August 31, 2022 – $391,740). k) Revenue recognition The Company’s revenue is comprised of sales of cannabis products. The Company’s revenue-generating activities have a single performance obligation and revenue is recognized at the point in time when control of the product transfers and the Company’s obligations have been fulfilled. This generally occurs when the product is shipped or delivered to the customer, depending upon the method of distribution and shipping terms set forth in the customer contract. Revenue is measured as the amount of consideration the Company expects to receive in exchange for the sale of the Company’s product. Certain of the Company’s customer contracts may provide the customer with a right of return. In certain circumstances the Company may also provide a retrospective price adjustment to a customer. These items give rise to variable consideration, which is recognized as a reduction of the transaction price based upon the expected amounts of the product returns and price adjustments at the time revenue for the corresponding product sale is recognized. The determination of the reduction of the transaction price for variable consideration requires that the Company make certain estimates and assumptions that affect the timing and amounts of revenue recognized. Sales of products are for cash or otherwise agreed-upon credit terms. The Company’s payment terms vary by location and customer; however, the time period between when revenue is recognized and when payment is due is not significant. The Company estimates and reserves for its bad debt exposure based on its experience with past due accounts and collectability, write-off history, the aging of accounts receivable and an analysis of customer data. l) Net income (loss) per common share Net income (loss) per share is calculated in accordance with ASC 260, Earnings per Share. The weighted-average number of common shares outstanding during each period is used to compute basic earning or loss per share. Diluted earnings or loss per share is computed using the weighted average number of shares and diluted potential common shares outstanding to the extent the effect would not be antidilutive. Dilutive potential common shares are additional common shares assumed to be exercised. Basic net income (loss) per common share is based on the weighted average number of shares of common stock outstanding. m) Income taxes The Company accounts for income taxes under ASC 740, Income Taxes. Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Under ASC 740, the effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period the enactment occurs. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax assets through future operations. n) Related party transactions Related parties are any entities or individuals that, through employment, ownership or other means, possess the ability to direct or cause the direction of the management and policies of the Company. The Company discloses related party transactions that are outside of normal compensatory agreements, such as salaries. Related party transactions are measured at the exchange amounts. o) Significant accounting estimates and judgments The preparation of the financial statements in conformity with US GAAP requires management to make judgments, estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Although management uses historical experience and its best knowledge of the amount, events or actions to for the basis for judgments and estimates, actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and further periods if the review affects both current and future periods. Significant estimates and assumptions included in these financial statements relate to the valuation assumptions related to the estimated useful lives and recoverability of long-lived assets, stock-based compensation, and deferred income tax assets and liabilities. Judgments are required in the assessment of the Company’s ability to continue to as going concern as described in Note 1. p) Financial instruments ASC 825, Financial Instruments, requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 825 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 825 prioritizes the inputs into three levels that may be used to measure fair value: Level 1 Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities. Level 2 Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data. Level 3 Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities. The financial instruments consist principally of cash, due from related parties, accounts payable, note payable, and convertible notes payable. The fair value of cash when applicable is determined based on “Level 1” inputs, which consist of quoted prices in active markets for identical assets. The Company believes that the recorded values of all other financial instruments which are categorized as loans and receivables approximate their current fair values because of their nature and respective relatively short maturity dates or current market rates of interest for similar instruments. For certain of the Company’s financial instruments, including accounts payable, due from related parties, notes and loans payable, the carrying amounts approximate their fair values due to the short maturities. The Company does not have any assets or liabilities measured at fair value on a recurring basis presented on the Company’s balance sheet as of August 31, 2023 and August 31, 2022 other than cash. Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash. The Company limits its exposure to credit loss by placing its cash with high credit quality financial institutions. q) Leases The Company determines if an arrangement contains a lease in whole or in part at the inception of the contract. Right-of-use (“ROU”) assets represent the Company’s right to use an underlying asset for the lease term while lease liabilities represent our obligation to make lease payments arising from the lease. All leases with terms greater than twelve months result in the recognition of a ROU asset and a liability at the lease commencement date based on the present value of the lease payments over the lease term. Unless a lease provides all of the information required to determine the implicit interest rate, the Company uses its incremental borrowing rate based on the information available at the commencement date in determining the present value of the lease payments. The Company uses the implicit interest rate in the lease when readily determinable. Our lease terms include all non-cancelable periods and may include options to extend (or to not terminate) the lease when it is reasonably certain that we will exercise that option. Leases with terms of twelve months or less at the commencement date are expensed on a straight-line basis over the lease term and do not result in the recognition of an asset or liability. See Note 7 – Leases. r) Recent accounting pronouncements In August 2020, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2020-06, Debt — Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40) (“ASU 2020-06”) to simplify accounting for certain financial instruments. ASU 2020-06 eliminates the current models that require separation of beneficial conversion and cash conversion features from convertible instruments and simplifies the derivative scope exception guidance pertaining to equity classification of contracts in an entity’s own equity. The new standard also introduces additional disclosures for convertible debt and freestanding instruments that are indexed to and settled in an entity’s own equity. ASU 2020-06 amends the diluted earnings per share guidance, including the requirement to use the if-converted method for all convertible instruments. ASU 2020-06 is effective for fiscal years beginning after December 15, 2023, and should be applied on a full or modified retrospective basis, with early adoption permitted beginning on January 1, 2021. The Company adopted ASU 2020-06 effective January 1, 2022. The adoption of ASU 2020-06 did not have an impact on the Company’s financial statements. The Company does not expect that recent accounting pronouncements or changes in accounting pronouncements during the year ended August 31, 2023, are of significance or potential significance to the Company.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Inventory
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Inventory |
|
| Inventory |
3. Inventory Inventory is comprised of the following items: | | August 31, 2023 | | | August 31, 2022 | | | | | | | | | Work in progress | | $ | 80,094 | | | $ | 326,556 | | Finished goods | | | 27,416 | | | | 383,459 | | Inventory in transit | | | - | | | | 288,973 | | Total inventory | | $ | 107,510 | | | $ | 998,988 | |
The costs of inventory include but are not limited to labor, utilities, nutrition and irrigation, overhead and the depreciation of manufacturing equipment and production facilities, and amortization of licenses determined at normal capacity. Manufacturing overhead and related expenses include salaries, wages, employee benefits, utilities, maintenance and rent of grow facility. The Company began production in Colombia in late 2020, when the Company obtained approval for its strains of products. During the current period, certain costs were determined based on the actual usage of production space as compared to the normal predetermined operational production of the facility based on capacity as the Company gradually started to grow products and prepared the facility ready. During the year ended August 31, 2023, the Company recorded a $43,861 write-off of inventory costs for CBD isolates and other derivative products to reduce to its net realizable value of $nil. In addition, the Company recorded additional $1,463,157 write-off of inventory costs to its net realizable value of $107,510. During the year ended August 31, 2022, the Company recorded a $31,737 write-off of inventory costs for products purchased for resale to reduce to its net realizable value of $nil.
|
| X |
- References
+ Details
| Name: |
us-gaap_InventoryDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for inventory. Includes, but is not limited to, the basis of stating inventory, the method of determining inventory cost, the classes of inventory, and the nature of the cost elements included in inventory. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//330/tableOfContent
| Name: |
us-gaap_InventoryDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Deposits and advances
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Deposits and advances |
|
| Deposits and advances |
4. Deposits and advances | | August 31, 2023 | | | August 31, 2022 | | | | | | | | | a) Towards the purchase of prefabricated buildings | | $ | - | | | $ | 2,656,695 | | b) Deposit towards a license acquisition | | | - | | | | 150,000 | | c) Prepayments for construction facility in Colombia | | | 26,354 | | | | 63,130 | | Total deposits and advances | | $ | 26,354 | | | $ | 2,869,825 | |
a) In June 2019, the Company’s wholly owned subsidiary, AM Biosciences, signed a production and manufacturing contract to begin the manufacturing of the full building initially intended for the Canada extraction and production facility. The Company had determined to utilize these building assets in connection with potential United States operations in the State of Nevada upon on the occurrence or waiver of the changes in U.S. federal law to permit the general cultivation, distribution, and possession of marijuana or to remove the regulation of such activities from the federal laws of the United States. In connection with that determination, on March 30, 2021, the Company’s wholly owned subsidiary, Allied US Products LLC, entered into a contingent asset purchase agreement (“Agreement”) to acquire two privileged licenses issued by the Nevada Department of Taxation purposed for the cultivation of cannabis (the “Licenses”). In consideration for the licenses, the Company agreed to pay $150,000, issue a $1,350,000 promissory note and assume certain liabilities. Concurrent with the execution of the Agreement, the Company’s wholly owned subsidiary, Tactical Relief LLC, agreed to enter into a 25-year land lease with the Fiore Subsidiary. At August 31, 2022, Company had paid deposits of $2,656,695 to purchase the prefabricated buildings and $150,000 to purchase the licenses. At August 31, 2022, the Company had not yet received the buildings and the amounts have been recorded as deposits. In addition, the Agreement has not been closed and the payment of $150,00 has been recorded as a deposit. As the Company made payments towards the purchase of the prefabricated buildings, various parts of the building were delivered to the property on the land lease. In December 2022, the Company was advised that the Fiore Subsidiary had become insolvent and that a trustee’s sale had been scheduled for the property which the Company had agreed to lease. In order to protect the Company’s interests, the Company filed a complaint in the Eighth Judicial District Court in Clark County, Nevada seeking injunctive relief to terminate the trustee’s sale or alternatively money damages. The Company filed a lis pendens against the property to reflect this complaint and to protect its interests in the building. However, the trustee’s sale went forward and the successful bidder claimed that the buildings were part of the real property and that it became the owner of the buildings. As a result, the Company filed suit, seeking a declaration of the Company’s ownership of the buildings as the buildings were personal property and not a part of the real property. A status conference was held for December 13, 2023 and the Company is currently waiting for the next court date. Due to the uncertainties of collectability, the Company recorded a loss on impairment of deposits during the year ended August 31, 2023. In addition, the Company recorded a loss on license acquisition advance of $150,000 during the year ended August 31, 2023 as the Agreement has effectively been terminated. b) The Company paid certain vendors for the construction of farm facilities in Colombia in advance. At August 31, 2023, the prepayments totaled $26,354 (August 31, 2022 – $63,130).
|
| X |
- References
+ Details
| Name: |
alid_DepositsAndAdvanceTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_ReceivablesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Property plant and equipment
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Property plant and equipment |
|
| Property plant and equipment |
5. Property, plant and equipment At August 31, 2023, property, plant and equipment consisted of: | | Construction in process | | | Farm facility and equipment | | | Office and computer equipment | | | Land equipment | | | Total | | Cost | | | | | | | | | | | | | | | | August 31, 2022 | | $ | 460,648 | | | $ | 1,152,822 | | | $ | 21,348 | | | $ | 27,150 | | | $ | 1,661,968 | | Additions | | | 31,528 | | | | 45,344 | | | | 6,167 | | | | 132 | | | | 83,171 | | Transfer | | | (93,363 | ) | | | 93,363 | | | | - | | | | - | | | | - | | Foreign exchange | | | 30,936 | | | | 54,844 | | | | 2,437 | | | | 2,243 | | | | 90,460 | | August 31, 2023 | | $ | 429,749 | | | $ | 1,346,373 | | | $ | 29,952 | | | $ | 29,525 | | | $ | 1,835,599 | | | | | | | | | | | | | | | | | | | | | | | Accumulated depreciation | | | | | | | | | | | | | | | | | | | | | August 31, 2022 | | $ | - | | | $ | 210,899 | | | $ | 4,084 | | | $ | 2,947 | | | $ | 217,930 | | Additions | | | - | | | | 167,747 | | | | 2,953 | | | | 2,663 | | | | 173,363 | | Foreign exchange | | | - | | | | 25,913 | | | | 663 | | | | 538 | | | | 27,114 | | August 31, 2023 | | $ | - | | | $ | 404,559 | | | $ | 7,700 | | | $ | 6,148 | | | $ | 418,407 | | | | | | | | | | | | | | | | | | | | | | | Net book value | | | | | | | | | | | | | | | | | | | | | August 31, 2022 | | $ | 460,648 | | | $ | 941,923 | | | $ | 17,264 | | | $ | 24,203 | | | $ | 1,444,038 | | August 31, 2023 | | $ | 429,749 | | | $ | 941,814 | | | $ | 22,252 | | | $ | 23,377 | | | $ | 1,417,192 | |
As of August 31, 2023 and August 31, 2022, the construction in process has not been in use.
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//360/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-7
| Name: |
us-gaap_PropertyPlantAndEquipmentDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Intangible assets
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Intangible assets |
|
| Intangible assets |
6. Intangible assets At August 31, 2023, intangible assets consisted of: | | Cost $ | | | Foreign exchange $ | | | Accumulated amortization $ | | | Accumulated impairment $ | | | August 31, 2023 Net carrying value $ | | | August 31, 2022 Net carrying value $ | | | | | | | | | | | | | | | | | | | | | Cannabis licenses | | | 5,451,409 | | | | (468,910 | ) | | | (1,140,531 | ) | | | (3,801,667 | ) | | | 40,301 | | | | 44,773 | | | | | 5,451,409 | | | | (468,910 | ) | | | (1,140,531 | ) | | | (3,801,667 | ) | | | 40,301 | | | | 44,773 | |
On February 17, 2020, the Company acquired $5,435,334 of licenses as part of the acquisition of Allied Colombia. The licenses acquired are issued by the Republic of Colombia and include the use of seeds for growing Cannabis, production of derivatives from Cannabis for medicinal and scientific use, cultivation of Cannabis plants, and producer of seeds. The Company recorded amortization of these licenses of $658,836 for the year ended August 31, 2021, of which $158,121 was included in cost of inventory. Management acquired the licenses with a plan to operate in Colombia and believed the amounts paid for the licenses would be recovered from future operations. The Company has only recently started its operations in Colombia and has limited history on which to base future outcomes from operations including cash flows. Cannabis and hemp are considered to be an emerging industry and Colombia does not yet have a sufficiently established observable legal market in which the Company could sell its Cannabis or hemp flower and CBD or THC extracts. Laws and regulations in Colombia are evolving and there is uncertainty in what will be legally permissible in a future market and what prices and demand there would be in Colombia for the Company’s products. At the present, the Company’s export activities are regulated and restricted by Colombian law and it is uncertain whether future changes in law would favorably impact the Company’s operations. Due to the uncertainty in the timing and amount of future cash flows from operations the Company has written down its licenses to the estimated recoverable amount. During the year ended August 31, 2021, the Company recorded impairment of intangible assets in the amount of $2,687,695. During the year ended August 31, 2023, the Company acquired $3,964 (COP 19,254,199) of licenses and Company recorded amortization of $9,097 (COP 41,315,205).
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all or part of the information related to intangible assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//350-30/tableOfContent
| Name: |
us-gaap_IntangibleAssetsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Leases
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Leases |
|
| Leases |
7. Leases The Company accounts for leases under ASC 842, Leases, which establishes a right-of-use (“ROU”) model that requires a lessee to record an ROU asset and a lease liability, measured on a discounted basis, on the balance sheet for all leases with terms longer than 12 months. The Company also elected to keep all leases with an initial term of 12 months or less off the balance sheet. The Company did not have any leases until the acquisition of Allied Colombia during the year ended August 31, 2020. The acquisition resulted in the addition of $82,398 of operating lease assets and liabilities. During the year ended August 31, 2021, the Company re-measured its lease liabilities under this lease as the criteria was met for additional monthly payment when the Company started production as outlined in the lease agreement, resulting in additional lease liabilities of $70,705. On August 10, 2021, the Company entered into another lease for additional land in Colombia with monthly payment of $2,647 (COP9,970,675) for 12.5 hectares which is intended for use of outdoor cultivation, resulting in additional lease liabilities of $104,902. The lease is for 5 years, and expires in July 2026. Management has assessed the lease as an operating lease. Effective August 31, 2023, the Company terminated the lease. Pursuant to ASC 842-20 upon the termination of the lease, the Company derecognized the lease related asset and liability and included any consideration paid or received upon termination that was not already included in the lease payments in the gain or loss on termination of the lease. ROU assets represent the Company's right to use an underlying asset for the lease term and lease liabilities represent the Company's obligation to make lease payments arising from the lease calculated by discounting fixed lease payments over the lease term at the rate implicit in the lease or the Company’s incremental borrowing rate. Lease liabilities are increased by interest and reduced by payments each period, and the ROU asset is amortized over the lease term. For operating leases, interest on the lease liability and the amortization of the ROU asset result in straight-line rent expense over the lease term. For finance leases, interest on the lease liability and the amortization of the ROU asset results in front-loaded expense over the lease term. ROU assets and liabilities are recognized at the commencement date of the lease based on the present value of lease payments over the lease term. At August 31, 2023, the Company did not have any finance leases. At August 31, 2023, the weighted average remaining operating lease term was 6 years and the weighted average discount rate associated with operating leases was 15%. The components of lease expenses were as follows: | | $ | | Operating lease cost: | | | | Amortization of right-of-use assets | | | 24,079 | | Interest on lease liabilities | | | 26,046 | | | | | | | Total operating lease cost | | | 50,125 | |
The following table provides supplemental cash flow and other information related to leases for the year ended August 31, 2023: Supplemental balance sheet information related to leases as of August 31, 2023 are as below: | | | $ | | Cost | | | 282,273 | | Accumulated amortization | | | (55,220 | ) | Lease termination | | | (61,928 | ) | Foreign exchange | | | (55,824 | ) | | | | | | Net carrying value at August 31, 2023 | | | 109,301 | |
Future minimum lease payments related to lease obligations are as follows: | | $ | | 2024 | | | 26,422 | | 2025 | | | 26,422 | | 2026 | | | 26,422 | | 2027 | | | 26,422 | | 2028 | | | 26,422 | | 2029 | | | 26,422 | | 2030 | | | 13,210 | | | | | | | Total minimum lease payments | | | 171,742 | | | | | | | Less: amount of lease payments representing effects of discounting | | | (62,441 | ) | | | | | | Present value of future minimum lease payments | | | 109,301 | | | | | | | Less: current obligations under leases | | | (10,745 | ) | | | | | | Lease liabilities, net of current portion | | | 98,556 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for lessee entity's leasing arrangements including, but not limited to, all of the following: (a.) The basis on which contingent rental payments are determined, (b.) The existence and terms of renewal or purchase options and escalation clauses, (c.) Restrictions imposed by lease agreements, such as those concerning dividends, additional debt, and further leasing. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//840/tableOfContent
| Name: |
us-gaap_LeasesOfLesseeDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Loans payable
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Loans payable |
|
| Loans payable |
8. Loans payable a) In June 2020, the Company entered into a financing agreement to finance the buildings described in Note 4(a). Pursuant to the agreement, the Company financed $1,253,772 of the purchase price. The Company paid $71,023 at commencement date on May 29, 2020 and would make six monthly interest payments of $37,613 commencing June 20, 2020 and repay the principal of $1,253,772 on November 20, 2020. The loan was extended after the initial maturity date. On December 27, 2021, the Company entered into an amendment agreement. Pursuant to the amendment, the Company will make 27 monthly payments starting on January 20, 2022. For the first 3 months, the Company will make monthly payments of $37,613. For the remaining 24 months, the Company will make monthly payments of $66,288. If the first 6 monthly payments are made on time, the Company may prepay the unamortized loan balance with a 2% penalty of the remaining balance. During the year ended August 31, 2023, the Company paid interest in the amount of $nil (2022 - $363,337). As of August 31, 2023, the Company has missed thirteen monthly payments and the balance owing is $1,742,648 (August 31, 2022 - $1,291,290). b) On December 17, 2021, the Company entered into a financing agreement to finance an equipment purchase. On March 31, 2022, the agreement was amended to remove shipping charges. Pursuant to the amended agreement, the Company financed $295,543 of the purchase price with an interest rate of 8% per annum. The Company will make 21 monthly principal and interest payments of $15,000 and a final payment of $633 in September 2023. During the year ended August 31, 2023, the Company paid interest in the amount of $nil (2022 - $3,653). As of August 31, 2023, the Company has missed eighteen monthly payments and the balance owing is $284,700 (August 31, 2022 - $264,364).
|
| X |
- DefinitionThe entire disclosure for claims held for amounts due a entity, excluding financing receivables. Examples include, but are not limited to, trade accounts receivables, notes receivables, loans receivables. Includes disclosure for allowance for credit losses. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//310-10/tableOfContent
| Name: |
us-gaap_LoansNotesTradeAndOtherReceivablesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Secured convertible notes payable
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Secured convertible notes payable |
|
| Secured convertible notes payable |
9. Secured convertible notes payable The Company has granted each and every of the secured convertible note holders a continuing security interest in, a general lien upon, and aright of set-off against all existing and future assets and property under the terms of a security agreement. a) On January 23, 2020, the Company issued two convertible notes with principal amounts of $400,000 and $200,000, respectively, with a total face value of $600,000 (the “Notes”) and warrants to purchase 240,000 shares of the Company’s common stock at $1.25 per share for 1 year. The Notes were issued with an original discount of $12,000, and bear interest at 10% per annum compounded monthly. The notes mature on July 20, 2020 and are convertible into shares of the Company’s common stock at any time prior to maturity at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Notes or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion features are indexed to the Company’s own stock and are classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion features and warrants do not meet derivative classification. The relative fair values of the convertible notes and the warrants were $470,467 and $117,533 respectively. The effective conversion price was then determined to be $0.98. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $115,383 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $108,100 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $364,517. The beneficial conversion feature of $115,383, the original issue discount of $12,000 and the relative fair value of the warrants of $108,100 discounted the carrying value of the convertible debt on the date of issue. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. On June 30, 2020, the Company repaid $200,000 of the $600,000 note which left $200,000 outstanding on each note. i. First Modification: On July 1, 2020, the Company entered into amendments to the convertible notes. Pursuant to the amendments, the convertible notes bear simple interest at 5% per annum. The maturity date of the convertible notes was amended to due on demand on or before October 31, 2020. In consideration for extending the maturity date, the Company issued to the convertible note holders 16,000 common shares of the Company and warrants to purchase additional 320,000 common shares of the Company at $1.25 per share expiring October 31, 2021. Each note holder received 8,000 common shares and 160,000 warrants. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was not granted. As the creditor has not granted a concession, the guidance contained in ASC 470-60 was applied. As present value of the cash flows under the new debt instrument differed by more than 10% from the present value of the remaining cash flows under the terms of the original debt instrument, it was determined that the debt was substantially different which resulted in extinguishment accounting. The extended convertible notes had a total carrying value of $400,000. As the common shares and warrants were issued as consideration for extending the convertible notes, the fair value of the common share and warrants of $218,397 were expensed under extinguishment accounting. The fair value of these costs were included in the calculation of the loss on extinguishment of $220,065. ii. Second Modification: On November 1, 2020, the Company entered into amendments to the convertible notes. Pursuant to the amendments, the maturity date of the convertible notes was amended to due on demand on or before March 31, 2021. In consideration for extending the maturity date, the Company agreed to issue to the convertible note holders 100,000 common shares of the Company. Each note holder received 50,000 common shares. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was not granted. As the creditor has not granted a concession, the guidance contained in ASC 470-60 was applied. As present value of the cash flows under the new debt instrument differed by more than 10% from the present value of the remaining cash flows under the terms of the original debt instrument, it was determined that the debt was substantially different which resulted in extinguishment accounting. The extended convertible notes had a total carrying value of $400,000. As the common shares were issued as consideration for extending the convertible notes, the fair value of the common share of $110,000 were expensed under extinguishment accounting. The fair value of these costs were included in the calculation of the loss on extinguishment of $110,000. iii. Third Modification: On March 31, 2021, the Company entered into amendments to the convertible notes. Pursuant to the amendments, the convertible notes bear simple interest at 10% per annum. Pursuant to the amendments, the maturity date of the convertible notes was amended to due on demand on or before September 30, 2021. In consideration for extending the maturity date, the Company agreed to issue to the convertible note holders 20,000 common shares of the Company. Each note holder received 10,000 common shares. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was not granted. As the creditor has not granted a concession, the guidance contained in ASC 470-60 was applied. As present value of the cash flows under the new debt instrument differed by more than 10% from the present value of the remaining cash flows under the terms of the original debt instrument, it was determined that the debt was substantially different which resulted in extinguishment accounting. The extended convertible notes had a total carrying value of $400,000. As the common shares were issued as consideration for extending the convertible notes, the fair value of the common share of $20,000 were expensed under extinguishment accounting. The fair value of these costs were included in the calculation of the loss on extinguishment of $20,000. iv. Fourth Modification: On October 1, 2021, the Company entered into amendments to the convertible notes. Pursuant to the amendments, the convertible notes bear simple interest at 10% per annum. Pursuant to the amendments, the maturity date of the convertible notes was amended to due on demand on or after March 31, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. v. Fifth Modification: On March 31, 2022, the Company entered into amendments to the convertible notes. Pursuant to the amendments, the convertible notes bear simple interest at 10% per annum. Pursuant to the amendments, the maturity date of the convertible notes was amended to due on demand on or after September 30, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. During the year ended August 31, 2023, the Company made interest payment of $nil (2022 - $20,000). As at August 31, 2023, the Company has recorded accrued interest of $97,205, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $400,000 (August 31, 2022 - $400,000). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. b) On September 29, 2020, the Company issued a convertible note with a fair value of $163,341 (the “Note”) and warrants to purchase 130,673 shares of the Company’s common stock at $1.25 per share for 2 years. The Note bears interest at 10% per annum. The Note is due on demand after March 27, 2021. The Note was convertible into shares of the Company’s common stock at any time prior to March 27, 2021 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification. The relative fair values of the convertible note and the warrants were $85,330 and $78,011 respectively. The effective conversion price was then determined to be $0.65. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $85,330 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $78,011 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $Nil. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. i. First Modification: On March 31, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before September 30, 2021. In consideration for extending the maturity date, the Company issued to the convertible note holder 8,268 common shares of the Company. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was not granted. As the creditor has not granted a concession, the guidance contained in ASC 470-60 was applied. As present value of the cash flows under the new debt instrument differed by more than 10% from the present value of the remaining cash flows under the terms of the original debt instrument, it was determined that the debt was substantially different which resulted in extinguishment accounting. The extended convertible note had a total carrying value of $163,341. As the common shares were issued as consideration for extending the convertible note, the fair value of the common shares of $8,268 was expensed under extinguishment accounting. The fair value of these costs were included in the calculation of the loss on extinguishment of $8,268. ii. Second Modification: On June 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before November 30, 2021 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. iii. Third Modification: On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. iv. Fourth Modification: On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. As at August 31, 2023, the Company has recorded accrued interest of $33,540, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $63,341 (August 31, 2022 - $63,341). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. c) On October 26, 2020, the Company issued a convertible note with a face value of $37,613 (the “Note”) and warrants to purchase 30,090 shares of the Company’s common stock at $1.25 per share for 2 years. The Note bears interest at 10% per annum. The Note is due on demand after April 23, 2021. The Note was convertible into shares of the Company’s common stock at any time prior to April 23, 2021 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification. The relative fair values of the convertible note and the warrants were $20,176 and $17,437 respectively. The effective conversion price was then determined to be $0.65. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $20,176 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $17,437 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $Nil. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. i. First Modification: On June 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before November 30, 2021 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. ii. Second Modification: On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. iii. Third Modification: On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. As at August 31, 2023, the Company has recorded accrued interest of $10,706, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $37,613 (August 31, 2022 - $37,613). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. d) On November 11, 2020, the Company issued a convertible note with a face value of $85,937 (the “Note”) and warrants to purchase 68,750 shares of the Company’s common stock at $1.25 per share for 2 years. The Note bears interest at 10% per annum. The Note is due on demand after May 9, 2021. The Note was convertible into shares of the Company’s common stock at any time prior to April 23, 2021 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification. The relative fair values of the convertible note and the warrants were $48,258 and $37,679 respectively. The effective conversion price was then determined to be $0.70. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $48,258 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $37,679 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $Nil. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. i. First Modification: On June 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before November 30, 2021 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. ii. Second Modification: On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. iii. Third Modification: On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. As at August 31, 2023, the Company has recorded accrued interest of $24,087, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $85,937 (August 31, 2022 - $85,937). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
e) On December 2, 2020, the Company issued a convertible note with a face value of $600,000 (the “Note”) and warrants to purchase 240,000 shares of the Company’s common stock at $1.25 per share for 2 years. The Note bears interest at 10% per annum. The Note is due on demand after November 27, 2021. The Note was convertible into shares of the Company’s common stock at any time prior to November 27, 2021 at a conversion price of $1.25 per share.
The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification. The relative fair values of the convertible note and the warrants were $457,436 and $142,564 respectively. The effective conversion price was then determined to be $0.95. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $457,436 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $22,564 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $Nil. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. i. First Modification: On October 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. ii. Second Modification: On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. As at August 31, 2023, the Company has recorded accrued interest of $164,712, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $600,000 (August 31, 2022 - $600,000). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. f) On January 7, 2021, the Company issued a convertible note with a face value of $300,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after November 27, 2021. The Note was convertible into shares of the Company’s common stock at any time prior to April 23, 2021 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification. In addition, it was determined that there was no beneficial conversion feature (“BCF”) as the stock price at the issuance date was less than the conversion price. i. First Modification: On October 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. ii. Second Modification: On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. As at August 31, 2023, the Company has recorded accrued interest of $52,521, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $300,000 (August 31, 2022 - $300,000). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. g) On March 26, 2021, the Company issued a convertible note with a face value of $18,000 (the “Note”) and warrants to purchase 18,000 shares of the Company’s common stock at $0.50 per share for 1 year. The Note bears interest at 10% per annum. The Note is due on demand after September 26, 2021. The Note was convertible into shares of the Company’s common stock at any time prior to September 26, 2021 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification. The relative fair values of the convertible note and the warrants were $10,096 and $7,904 respectively. The effective conversion price was then determined to be $0.70. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $4,016 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $7,904 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $6,080. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. i. First Modification: On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. ii. Second Modification: On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. As at August 31, 2023, the Company has recorded accrued interest of $4,379, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $18,000 (August 31, 2022 - $18,000). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. h) On March 26, 2021, the Company issued a convertible note with a face value of $100,000 (the “Note”) and warrants to purchase 100,000 shares of the Company’s common stock at $0.50 per share for 1 year. The Note bears interest at 10% per annum. The Note is due on demand after September 26, 2021. The Note is convertible into shares of the Company’s common stock at any time prior to September 26, 2021 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification. The relative fair values of the convertible note and the warrants were $56,086 and $43,914 respectively. The effective conversion price was then determined to be $0.70. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $22,314 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $43,914 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $33,772. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. i. First Modification: On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. ii. Second Modification: On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. As at August 31, 2023, the Company has recorded accrued interest of $24,274, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $100,000). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. i) On April 30, 2021, the Company issued a convertible note with a face value of $100,000 (the “Note”) and warrants to purchase 100,000 shares of the Company’s common stock at $1.00 per share for 1 year. The Note bears interest at 10% per annum. The Note is due on demand after October 31, 2021. The Note is convertible into shares of the Company’s common stock at any time prior to October 31, 2021 at a conversion price of $1.00 per share. The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification. The relative fair values of the convertible note and the warrants were $61,493 and $38,507 respectively. The effective conversion price was then determined to be $0.61. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $27,272 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $38,507 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $34,221. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. i. First Modification: On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. ii. Second Modification: On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. As at August 31, 2023, the Company has recorded accrued interest of $23,370, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $100,000). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. j) On April 29, 2021, the Company issued a convertible note with a face value of $180,000 (the “Note”) and warrants to purchase 180,000 shares of the Company’s common stock at $1.00 per share for 1 year. The Note bears interest at 10% per annum. The Note is due on demand after October 29, 2021. The Note was convertible into shares of the Company’s common stock at any time prior to October 29, 2021 at a conversion price of $1.00 per share. The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification. The relative fair values of the convertible note and the warrants were $111,422 and $68,578 respectively. The effective conversion price was then determined to be $0.62. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $46,078 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $68,578 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $65,344. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. i. First Modification: On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. The maturity date of the convertible note was amended to due on demand on or before March 31, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. ii. Second Modification: On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. As at August 31, 2023, the Company has recorded accrued interest of $42,016, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $180,000 (August 31, 2022 - $180,000). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. k) On July 25, 2021, the Company issued a convertible note with a face value of $35,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after January 25, 2022. The Note was convertible into shares of the Company’s common stock at any time prior to January 25, 2022 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification. In addition, it was determined that there was no beneficial conversion feature (“BCF”) as the stock price at the issuance date was less than the conversion price. i. First Modification: On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after March 31, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was not granted. As the creditor has not granted a concession, the guidance contained in ASC 470-50 was applied. As present value of the cash flows under the new debt instrument differed by less than 10% from the present value of the remaining cash flows under the terms of the original debt instrument, it was determined that the debt was not substantially different which resulted in modification accounting. If a convertible debt instrument is modified or exchanged in a transaction that is not accounted for as an extinguishment, an increase in the fair value of the embedded conversion option (calculated as the difference between the fair value of the embedded conversion option immediately before and after the modification or exchange) shall reduce the carrying amount of the debt instrument (increasing a debt discount or reducing a debt premium) with a corresponding increase in additional paid-in capital. The Company recognized the increase in the fair value of the embedded beneficial conversion feature of $1,090 as additional-paid-in capital and an equivalent discount that reduced the carrying value of the convertible debt to $33,910 at November 1, 2021. ii. Second Modification: On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. As at August 31, 2023, the Company has recorded accrued interest of $7,336, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $35,000 (August 31, 2022 - $35,000). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. l) On July 25, 2021, the Company issued a convertible note with a face value of $15,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after January 25, 2022. The Note was convertible into shares of the Company’s common stock at any time prior to January 25, 2022 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification. In addition, it was determined that there was no beneficial conversion feature (“BCF”) as the stock price at the issuance date was less than the conversion price. i. First Modification: On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after March 31, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was not granted. As the creditor has not granted a concession, the guidance contained in ASC 470-50 was applied. As present value of the cash flows under the new debt instrument differed by less than 10% from the present value of the remaining cash flows under the terms of the original debt instrument, it was determined that the debt was not substantially different which resulted in modification accounting. If a convertible debt instrument is modified or exchanged in a transaction that is not accounted for as an extinguishment, an increase in the fair value of the embedded conversion option (calculated as the difference between the fair value of the embedded conversion option immediately before and after the modification or exchange) shall reduce the carrying amount of the debt instrument (increasing a debt discount or reducing a debt premium) with a corresponding increase in additional paid-in capital. The Company recognized the increase in the fair value of the embedded beneficial conversion feature of $467 as additional-paid-in capital and an equivalent discount that reduced the carrying value of the convertible debt to $14,533 at November 1, 2021. ii. Second Modification: On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. As at August 31, 2023, the Company has recorded accrued interest of $3,144, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $15,000 (August 31, 2022 - $15,000). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. m) On October 1, 2021, the Company issued a convertible note with a face value of $100,000 (the “Note”) and warrants to purchase 100,000 shares of the Company’s common stock at $1.25 per share for 1 year. The Note bears interest at 10% per annum. The Note is due on demand after March 31, 2022. The Note was convertible into shares of the Company’s common stock at any time prior to March 31, 2022 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification. The relative fair values of the convertible note and the warrants were $40,117 and $59,883 respectively. The effective conversion price was then determined to be $0.40. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $40,117 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $59,883 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $Nil. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. i. First Modification: On November 1, 2021, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bears simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after March 31, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was not granted. As the creditor has not granted a concession, the guidance contained in ASC 470-50 was applied. As present value of the cash flows under the new debt instrument differed by less than 10% from the present value of the remaining cash flows under the terms of the original debt instrument, it was determined that the debt was not substantially different which resulted in modification accounting. If a convertible debt instrument is modified or exchanged in a transaction that is not accounted for as an extinguishment, an increase in the fair value of the embedded conversion option (calculated as the difference between the fair value of the embedded conversion option immediately before and after the modification or exchange) shall reduce the carrying amount of the debt instrument (increasing a debt discount or reducing a debt premium) with a corresponding increase in additional paid-in capital. There was no change in the fair value of the embedded beneficial conversion feature immediately before and after the modification. As a result, the Company did not adjust the carrying amount of $1,546 of the convertible debt at November 1, 2021. ii. Second Modification: On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. As at August 31, 2023, the Company has recorded accrued interest of $19,123, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $100,000). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. n) On October 25, 2021, the Company issued a convertible note with a face value of $100,000 (the “Note”) and warrants to purchase 100,000 shares of the Company’s common stock at $1.25 per share for 1 year. The Note bears interest at 10% per annum. The Note is due on demand after March 31, 2022. The Note was convertible into shares of the Company’s common stock at any time prior to March 31, 2022 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note or warrants under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion feature would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion feature is not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature and warrants do not meet derivative classification. The relative fair values of the convertible note and the warrants were $39,573 and $60,427 respectively. The effective conversion price was then determined to be $0.40. As the stock price at the issuance date was greater than the effective conversion price, it was determined that there was a beneficial conversion feature (“BCF”). The Company recognized the relative fair value of the BCF of $39,573 and an equivalent discount. The Company then recognized the relative fair value of the warrants of $60,427 as additional-paid-in capital and an equivalent discount that further reduced the carrying value of the convertible debt to $Nil. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. Modification: On March 31, 2022, the Company entered into amendment to the convertible note. Pursuant to the amendment, the convertible note bear simple interest at 10% per annum. Pursuant to the amendment, the maturity date of the convertible note was amended to due on demand on or after September 30, 2022 for no additional consideration. The Company evaluated the transaction under the guidance found in ASC 470-50 Modification and Extinguishment. The Company concluded that the Company is experiencing financial difficulty and that a concession was granted. As the creditor has granted a concession, the guidance contained in ASC 470-60-35 was applied. As the future undiscounted cash flows are greater than or equal to the net carrying value of the original debt, the carrying amount of the debt at the time of the restructuring was not changed and a new effective interest rate was calculated as the discount rate that equates the present value of the future cash payments specified by the new terms with the carrying amount of the debt. Interest expense will be recognized prospectively such that a constant effective interest rate is applied to the carrying amount of the debt at the beginning of each period between restructuring and maturity, consistent with the interest method. As at August 31, 2023, the Company has recorded accrued interest of $18,466, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $100,000). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. o) On December 23, 2021, the Company issued a convertible note with a face value of $100,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after June 23, 2022. The Note is convertible into shares of the Company’s common stock at any time prior to June 23, 2022 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification. It was determined that there was a beneficial conversion feature (“BCF”) as the stock price at the issuance date was greater than the effective conversion price. The Company recognized the relative fair value of the BCF of $40,800 as additional-paid-in capital and an equivalent discount that reduced the carrying value of the convertible debt to $59,200. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. As at August 31, 2023, the Company has recorded accrued interest of $16,877, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $100,000). On June 23, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. p) On December 23, 2021, the Company issued a convertible note with a face value of $100,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after June 23, 2022. The Note is convertible into shares of the Company’s common stock at any time prior to June 23, 2022 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification. It was determined that there was a beneficial conversion feature (“BCF”) as the stock price at the issuance date was greater than the effective conversion price. The Company recognized the relative fair value of the BCF of $40,800 as additional-paid-in capital and an equivalent discount that reduced the carrying value of the convertible debt to $59,200. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. As at August 31, 2023, the Company has recorded accrued interest of $16,877, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $100,000). On June 23, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. q) On January 11, 2022, the Company issued a convertible note with a face value of $150,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after July 10, 2022. The Note is convertible into shares of the Company’s common stock at any time prior to July 10, 2022 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification. It was determined that there was a beneficial conversion feature (“BCF”) as the stock price at the issuance date was greater than the effective conversion price. The Company recognized the relative fair value of the BCF of $75,600 as additional-paid-in capital and an equivalent discount that reduced the carrying value of the convertible debt to $74,400. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. As at August 31, 2023, the Company has recorded accrued interest of $24,534, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $150,000 (August 31, 2022 - $150,000). On July 10, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. r) On January 31, 2022, the Company issued a convertible note with a face value of $100,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after July 31, 2022. The Note is convertible into shares of the Company’s common stock at any time prior to July 31, 2022 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification. It was determined that there was a beneficial conversion feature (“BCF”) as the stock price at the issuance date was greater than the effective conversion price. The Company recognized the relative fair value of the BCF of $75,600 as additional-paid-in capital and an equivalent discount that reduced the carrying value of the convertible debt to $74,400. The discount is being expensed over the term of the loan to increase the carrying value to the face value of the loan using effective interest rate method. As at August 31, 2023, the Company has recorded accrued interest of $15,808, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $100,000). On July 31, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. s) On March 29, 2022, the Company issued a convertible note with a face value of $500,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after September 30, 2022. The Note is convertible into shares of the Company’s common stock at any time prior to September 30, 2022 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification. In addition, it was determined that there was no beneficial conversion feature (“BCF”) as the stock price at the issuance date was less than the conversion price. As at August 31, 2023, the Company has recorded accrued interest of $71,233, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $500,000 (August 31, 2022 - $500,000). On September 30, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. t) On June 16, 2022, the Company issued a convertible note with a face value of $250,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after December 16, 2022. The Note is convertible into shares of the Company’s common stock at any time prior to December 16, 2022 at a conversion price of $1.25 per share. The Company determined that there was no derivative liability associated with the Note under ASC 815-15, Derivatives and Hedging. Per ASC 815-10-15-74(a), contracts that are both indexed to its own stock and classified in stockholders’ equity in its statement of financial position are not considered to be derivative instruments. The conversion feature is indexed to the Company’s own stock and is classified in stockholders’ equity in the Company’s statement of financial position. As the conversion features would meet the scope exception as described in paragraphs ASC 815-15-74(a), the conversion features are not required to be separated from the host instrument and accounted for separately. As a result, at August 31, 2023, the conversion feature does not meet derivative classification. In addition, it was determined that there was no beneficial conversion feature (“BCF”) as the stock price at the issuance date was less than the conversion price. As at August 31, 2023, the Company has recorded accrued interest of $30,206, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $250,000 (August 31, 2022 - $250,000). On December 16, 2022, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. u) On November 28, 2022, the Company issued a convertible note with a face value of $100,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after June 30, 2023. The Note is convertible into shares of the Company’s common stock at any time prior to June 30, 2023 at a conversion price equal to the lesser of 80% of the twenty-day average closing price per share (but not less than $0.20 per share) or $0.50 per share. As at August 31, 2023, the Company has recorded accrued interest of $7,616, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $100,000 (August 31, 2022 - $nil). On June 30, 2023, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. v) On December 1, 2022, the Company issued a convertible note with a face value of $70,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after June 30, 2023. The Note is convertible into shares of the Company’s common stock at any time prior to June 30, 2023 at a conversion price equal to the lesser of 80% of the twenty-day average closing price per share (but not less than $0.20 per share) or $0.50 per share. As at August 31, 2023, the Company has recorded accrued interest of $5,236, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $70,000 (August 31, 2022 - $nil). On June 30, 2023, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. w) On December 7, 2022, the Company issued a convertible note with a face value of $60,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after June 30, 2023. The Note is convertible into shares of the Company’s common stock at any time prior to June 30, 2023 at a conversion price equal to the lesser of 80% of the twenty-day average closing price per share (but not less than $0.20 per share) or $0.50 per share. As at August 31, 2023, the Company has recorded accrued interest of $4,389, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $60,000 (August 31, 2022 - $nil). On June 30, 2023, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. x) On February 28, 2023, the Company issued a convertible note with a face value of $150,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after April 30, 2023. The Note is convertible into shares of the Company’s common stock at any time prior to April 30, 2023 at a conversion price equal to the lesser of 80% of the twenty-day average closing price per share (but not less than $0.20 per share) or $0.50 per share. As at August 31, 2023, the Company has recorded accrued interest of $7,562, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $150,000 (August 31, 2022 - $nil). On April 30, 2023, the Company defaulted on the Note and is in process of amending the maturity date and conversion price. y) On May 17, 2023, the Company issued a convertible note with a face value of $10,000 (the “Note). The Note bears interest at 10% per annum and is due on demand after September 17, 2023. The Note is convertible into shares of the Company’s common stock at any time prior to September 17 at a conversion price equal to the lesser of 80% of the twenty-day average closing price per share (but not less than $0.20 per share) or $0.50 per share. As at August 31, 2023, the Company has recorded accrued interest of $504, which is included in accounts payable and accrued liabilities on the consolidated balance sheets. As of August 31, 2023, the outstanding principal owing is $10,000 (August 31, 2022 - $nil). On September 17, 2023, the Company defaulted on the Note and is in process of amending the maturity date and conversion price.
|
| X |
- References
+ Details
| Name: |
alid_SecuredConvertibleNotesPayableDisclosureTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DebtDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Equity
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Equity |
|
| Equity |
10. Equity During the year ended August 31, 2022: On September 2, 2021, the Company issued 2,175,933 common shares at fair value of $ 2,447,925 on issuance date from treasury to the CFO and COO (Note 11) and 1,900,000 common shares at fair value of $2,137,500 to certain employees of the Company as bonuses for past services, which is expensed as a total of $4,585,425 for consulting fees. On September 2, 2021, the Company issued 2,997,237 common shares measured at fair value on issuance date of $3,371,892 from treasury for consulting services related to business development for a 12-month period from the issuance date. As the future benefit of the consulting services to be performed cannot be determined, the entire amount was expensed during the year ended August 31, 2022. The total $3,584,392 stock-based compensation – consulting services is comprised of this $3,371,892 share issuance plus the $212,500 described in the next paragraph below. During the year ended August 31, 2021, the Company re-issued 750,000 shares of common stock with total fair value of $637,500 for consulting services, out of which, $425,000 was expensed as consulting fees during the prior year and $212,500 was expensed during the year ended August 31, 2022. On October 20, 2021, the Company issued 3,699,955 units at $0.75 per unit for proceeds of $2,774,966, of which $865,467 was received during the year ended August 31, 2021. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred brokerage commission fees and other share issuance costs of $210,736. On November 5, 2021, the Company issued 905,000 units at $0.75 per unit for proceeds of $678,750. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company issued 8,000 shares of common stock with a fair value of $6,000 as a finder’s fee and incurred other finders’ fees and other share issuance costs of $42,654. On November 24, 2021, the Company issued 36,000 units at $0.75 per unit for proceeds of $27,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. On November 30, 2021, the Company issued 8,268 shares as consideration for extending the maturity date of a convertible note (Note 9(b)). On January 20, 2022, the Company issued 75,000 units at $0.75 per unit for proceeds of $56,250 which was received during the year ended August 31, 2021. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. On January 28, 2022, the Company issued 66,667 units at $0.75 per unit for proceeds of $50,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. On February 7, 2022, the Company issued 66,667 units at $0.75 per unit for proceeds of $50,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. On February 10, 2022, the Company issued 33,334 units at $0.75 per unit for proceeds of $25,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. On February 10, 2022, the Company issued 80,000 units at $1.25 per unit for proceeds of $100,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $8,000. On February 20, 2022, the Company issued 40,000 units at $1.25 per unit for proceeds of $50,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $4,000. On February 21, 2022, the Company issued 300,000 units at $1.25 per unit for proceeds of $375,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $30,000. On February 22, 2022, the Company issued 80,000 units at $1.25 per unit for proceeds of $100,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $8,000. On February 24, 2022, the Company issued 41,600 units at $0.75 per unit for proceeds of $31,200. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. On February 24, 2022, the Company issued 40,000 units at $1.25 per unit for proceeds of $50,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $4,000. On February 25, 2022, the Company issued 96,000 units at $1.25 per unit for proceeds of $120,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $9,600. On February 28, 2022, the Company issued 120,000 units at $1.25 per unit for proceeds of $150,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $12,000. On March 7, 2022, the Company issued 20,000 units at $1.25 per unit for proceeds of $25,000. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. In connection with the financing, the Company incurred finder’s fee of $4,000. On May 26, 2022, the Company issued 133,333 shares at $0.75 per share for proceeds of $100,000. On May 26, 2022, the Company issued 67,733 units at $0.75 per unit for proceeds of $50,800. Each unit consists of one common share of the Company and one warrant to purchase the Company’s one common shares at $1.25 for a period of two years. On August 23, 2022, the Company issued 750,000 shares at $0.40 per share for proceeds of $300,000. During the year ended August 31, 2022, the Company issued 368,000 common shares to certain investors for no consideration by error. The Company is in the process of retracting the shares. At August 31, 2022, the Company had received $540,000 in cash for share subscriptions at $0.40 per share. During the year ended August 31, 2023: On September 21, 2022, the Company issued 1,350,000 shares of common stock at $0.40 per share for $540,000 subscriptions received during the year ended August 31, 2022. On October 7, 2022, the Company issued 1,575,000 shares of common stock at $0.40 per share for proceeds of $630,000. In connection with the financing, the Company incurred finder’s fee of $10,000 and share issuance costs of $2,792. On October 7, 2022, the Company issued 75,000 shares of common stock with a fair value of $44,606 to settle related party accounts payable of $30,000, resulting in a loss on settlement of $14,606. On October 7, 2022, the Company issued 70,560 shares of common stock with a fair value of $41,966 to settle $29,529 in debt, resulting in a loss on settlement of $12,437. On August 21, 2023, the Company issued 1,525,000 shares of common stock at $0.20 per share for proceeds of $305,000. On August 31, 2023, the Company issued 3,029,760 shares of common stock at $0.20 per share for proceeds of $605,952. In connection with the financing, the Company issued 1,000,000 warrants with a fair value of $204,452 as finder’s fees. Each warrant entitles the holder to acquire one common share at a price of $0.20 per share until July 30, 2025. The fair value of the finder’s warrants was calculated using the Black-Scholes option pricing model assuming the following weighted-average assumptions: a risk-free rate of 4.87%, no expected dividends or forfeiture rate, volatility of 160.87% and an expected life of 2 years. At August 31, 2023, the Company had received $80,000 in cash for shares subscriptions at $0.20 per share.
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Related party transactions and balances
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Related party transactions and balances |
|
| Related party transactions and balances |
11. Related party transactions and balances All transactions with related parties have occurred in the normal course of operations and are recorded at the exchange amount which is the amount agreed to by the Company and the related party. a) Key management compensation and related party transactions The Company has identified its directors and certain senior officers as its key management personnel. The compensation costs for key management personnel were as follows: | | August 31, 2023 | | | August 31, 2022 | | Consulting fees and benefits | | $ | 396,052 | | | $ | 369,079 | |
b) Amounts due to related parties In the normal course of operations, the company shares certain administrative resources with companies related by common management and directors. The administrative resources and services, which were provided in the normal course of operations, were measured at the exchange. All amounts payable and receivable are non-interest bearing, unsecured and due on demand. The following table summarizes the amounts due to related parties: | | August 31, 2023 | | | August 31, 2022 | | | | | | | | | CEO and Director | | $ | 156,028 | | | $ | 80,892 | | COO and Director | | | 201,612 | | | | 143,816 | | An entity controlled by the CFO | | | 84,962 | | | | 46,387 | | An entity controlled by a Director | | | 159,690 | | | | 60,677 | | Director | | | 91,000 | | | | 52,500 | | | | $ | 693,292 | | | $ | 384,272 | |
As of August 31, 2023, the Company had $693,292 (August 31, 2022 - $384,272) payable to related parties. From this amount, $623,292 (August 31, 2022 - $384,272) was for expenses incurred or expensed paid on behalf of the Company by the parties and $70,000 (August 31, 2022 - $nil) was for advances received from the parties. c) Stock-based compensation A $2,447,925 portion of the stock-based compensation – bonus shares (See Note 10) recorded in the year ended August 31, 2022 as consulting fees was for bonus shares issued to the CFO and COO described as follows: On September 2, 2021, the Company issued 400,000 common shares from treasury at fair value of $450,000 to an entity controlled by the CFO as a bonus for past services provided. On September 2, 2021, the Company issued 1,775,933 common shares from treasury at fair value of $1,997,925 to entities controlled by the COO and Director for as a bonus for past services provided.
|
| X |
- DefinitionThe entire disclosure for related party transactions. Examples of related party transactions include transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners; and (d) affiliates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(g)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//850/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
| Name: |
us-gaap_RelatedPartyTransactionsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Financial risk factors
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Financial risk factors |
|
| Financial risk factors |
12. Financial risk factors The Company is exposed in varying degrees to a variety of financial instrument related risks. The Board of Directors approves and monitors the risk management processes, inclusive of documented investment policies, counterparty limits, and controlling and reporting structures. The type of risk exposure and the way in which such exposure is managed is provided as follows: a) Credit risk: Credit risk is the risk that one party to a financial instrument will fail to discharge an obligation and cause the other party to incur a financial loss. The Company’s primary exposure to credit risk is on its cash account. Cash accounts are held with major banks in Canada. The Company has deposited its cash with a bank from which management believes the risk of loss is low. b) Liquidity risk: Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they become due. The Company’s approach to managing liquidity is to ensure that it will have sufficient liquidity to meet liabilities when due. Accounts payable are due within the current operating period. The Company has a working capital deficit and requires additional financing to meet its current obligations (see Note 1). c) Market risk: Market risk is the risk that changes in market prices, such as foreign exchange rates, interest rates and equity prices will affect the Company’s income or the value of its holdings of financial instruments. The objective of market risk management is to manage and control market risk exposures within acceptable parameters, while optimizing the return. The Company is not exposed to market risk. d) Interest rate risk: Interest rate risk is the risk that the fair value of future cash flows of a financial instrument will fluctuate because of changes in market interest rates. The Company is exposed to interest rate risk, from time to time, on its cash balances. Surplus cash, if any, is placed on call with financial institutions and management actively negotiates favorable market related interest rates. e) Foreign exchange risk: Foreign currency risk is limited to the portion of the Company’s business transactions denominated in currencies other than the Canadian dollar. The Company has not entered into any foreign currency contracts to mitigate risk, but manages the risk my minimizing the value of financial instruments denominated in foreign currency. The Company is exposed to foreign currency risk to the extent that the following monetary assets and liabilities are denominated in Canadian dollars: | | August 31, 2023 | | Balance in Canadian dollars: | | | | Accounts payable | | $ | (947,214 | ) | Net exposure | | | (947,214 | ) | Balance in US dollars: | | $ | (700,032 | ) |
A 10% change in the US dollar to the Canadian dollar exchange rate would impact the Company’s net loss by approximately $70,003 for the year ended August 31, 2023 (August 31, 2022 – $53,595). The Company is exposed to foreign currency risk to the extent that the following monetary assets and liabilities are denominated in Colombian Pesos: | | August 31, 2023 | | Balance in Colombian Pesos dollars: | | | | Cash and cash equivalents | | $ | 415,100,982 | | Other receivables | | | 539,704,008 | | Accounts payable | | | (3,669,893,803 | ) | Net exposure | | | (2,715,088,813 | ) | Balance in US dollars: | | $ | (664,234 | ) |
A 10% change in the US dollar to the Colombian Peso exchange rate would impact the Company’s net loss by approximately $66,423 for the year ended August 31, 2023 (August 31, 2022 - $49,281).
|
| X |
- References
+ Details
| Name: |
alid_FinancialRiskFactorsDisclosureTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PriceRiskFairValueHedgesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Capital management
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Capital management |
|
| Capital management |
13. Capital management The Company manages its capital structure and makes adjustments to it, based on the funds available to the Company, in order to support the business and continue as a going concern. The Company considers capital to be all accounts in equity. The Board of Directors does not establish quantitative return on capital criteria for management, but rather relies on the expertise of the Company’s management to sustain future development of the business. The Company has a working capital deficit and requires additional capital to finance is future business plans. The Company is not subject to any externally imposed capital requirements.
|
| X |
- References
+ Details
| Name: |
alid_CapitalManagementDisclosureTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Commitments
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Commitments |
|
| Commitments |
14. Commitments a) As of August 31, 2023, the Company recorded a contingent liability of $547,190 (CAD$700,000) for expenses in connection with Allied Colombia acquisition, which is included in the balance of accounts payable and accrued liabilities on the consolidated balance sheet. b) The Company has entered into leases for farm land in Colombia. See Note 7 for details. c) The Company initiated litigation in the Judicial District Court in Clark County, Nevada in connection with ownership of prefabricated buildings. See Note 4 a) for details.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//450/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480327/954-440-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Share purchase warrants
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Share purchase warrants |
|
| Share purchase warrants |
15. Share purchase warrants The following table summarizes the continuity of share purchase warrants: | | Number of warrants | | | Weighted average exercise price $ | | | | | | | | | Balance, August 31, 2022 | | | 7,704,135 | | | | 1.23 | | | | | | | | | | | Issued | | | 1,000,000 | | | | 0.20 | | Expired | | | (3,245,135 | ) | | | 1.25 | | Balance, August 31, 2023 | | | 5,459,000 | | | | 1.06 | |
As at August 31, 2023, the following share purchase warrants were outstanding: | Number of warrants | | | Exercise price $ | | | Expiry date | | | | | | | | | | | | | 2,545,999 | | | | 1.25 | | | October 20, 2023* | | | | 905,000 | | | | 1.25 | | | November 6, 2023* | | | | 36,000 | | | | 1.25 | | | November 24, 2023* | | | | 66,667 | | | | 1.25 | | | January 29, 2024 | | | | 66,667 | | | | 1.25 | | | February 7, 2024 | | | | 113,334 | | | | 1.25 | | | February 12, 2024 | | | | 40,000 | | | | 1.25 | | | February 20, 2024 | | | | 300,000 | | | | 1.25 | | | February 21, 2024 | | | | 177,600 | | | | 1.25 | | | February 26, 2024 | | | | 120,000 | | | | 1.25 | | | February 28, 2024 | | | | 20,000 | | | | 1.25 | | | March 7, 2024 | | | | 67,733 | | | | 1.25 | | | May 27, 2024 | | | | 1,000,000 | | | | 0.20 | | | July 30, 2025 | | | | 5,459,000 | | | | | | | | |
* Expired subsequently
|
| X |
- References
+ Details
| Name: |
alid_SharePurchaseWarrantsTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LineOfCreditFacilityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Stock Options
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Stock Options |
|
| Stock Options |
16. Stock options A summary of the Company’s stock option activity is as follows: | | Number of Options | | | Weighted Average Exercise Price $ | | | Weighted Average Remaining Contractual Term | | | Aggregate Intrinsic Value $ | | | | | | | | | | | | | | | Balance, August 31, 2022 | | | 6,000,000 | | | | 0.44 | | | | 3.85 | | | | 144,000 | | | | | | | | | | | | | | | | | Granted | | | 6,140,000 | | | | 0.24 | | | | 4.63 | | | | | | | | | | | | | | | | | | | | Outstanding, August 31, 2023 | | | 12,140,000 | | | | 0.25 | | | | 3.70 | | | | 55,900 | | Exercisable, August 31, 2023 | | | 11,456,667 | | | | 0.25 | | | | 3.70 | | | | 48,400 | |
In January 2023, the Company modified the exercise prices for previously granted and unexercised options. The options held by current consultants and ambassadors were repriced to $0.22 per share and the options held by current directors and officers were repriced to $0.25 per share. There was no other modification to the vesting schedule of the previously issued options. As a result, 4,900,000 unexercised options originally granted to purchase common stock at prices ranging from $0.75 to $0.825 per share were repriced. The repricing was treated as a modification of the original awards and the additional compensation costs for the difference between the fair value of the modified award and the fair value of the original award on the modification date was calculated. The repricing resulted in incremental stock-based compensation expense of $80,739. Expense related to vested shares was expensed on the repricing date and expense related to unvested shares will be amortized over the remaining vesting period. On May 16, 2023, the Company modified the exercise price of the 6,000,000 options to $0.24 per share. The repricing was treated as a modification of the original awards and the additional compensation costs for the difference between the fair value of the modified award and the fair value of the original award on the modification date was calculated. The repricing resulted in incremental stock-based compensation expense of $14,506. Expense related to vested shares was expensed on the repricing date. During the year ended August 31, 2023, the Company recorded stock-based compensation of $3,047,156 for options granted on the consolidated statement of operations. The fair value of each option granted was estimated on the date of grant using the Black-Scholes option pricing model with the following weighted average assumptions: | | Year Ended August 31, 2023 | | | Year Ended August 31, 2022 | | | | | | | | | Expected dividend yield | | | 0 | % | | | 0 | % | Expected volatility | | | 162 | % | | | 150 | % | Expected life (in years) | | | 3.45 | | | | 4.05 | | Risk-free interest rate | | | 4.41 | % | | | 3.38 | % |
At August 31, 2023, there was $373,838 of unrecognized compensation costs related to non-vested stock-based compensation arrangements granted under the Plan.
|
| X |
- DefinitionThe entire disclosure for shareholders' equity comprised of portions attributable to the parent entity and noncontrolling interest, including other comprehensive income. Includes, but is not limited to, balances of common stock, preferred stock, additiona
| Name: |
alid_StockOptionsTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Non cash activities
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Non cash activities |
|
| Non-cash activities |
17. Non-cash activities | | For the Year Ended August 31, 2023 | | | For the Year Ended August 31, 2022 | | Non-cash activities: | | | | | | | Shares issued from treasury for services | | | | | | 7,957,316 | | Fair value of warrants issued as finder’s fee | | | 204,452 | | | | - | | Beneficial conversion feature | | | - | | | | 281,447 | | Relative fair value of warrants issuable with convertible note | | | - | | | | 120,310 | | Fair value of shares issued on modification of convertible note | | | - | | | | 129,952 | | Fair value of shares issuable on modification of debt | | | - | | | | 8,268 | |
|
| X |
- References
+ Details
| Name: |
alid_NonCashActivitiesAbstract |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_NonCashActivitiesDislosureTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Segment disclosure
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Segment disclosure |
|
| Segment disclosure |
18. Segment disclosure The Company has two operating segments including: a) Allied Colombia, a Colombian based company through which the Company intends to commence commercial production in Colombia. (Allied Colombia) b) Allied Corp. which consists of the rest of the Company’s operations. (Allied) Factors used to identify the Company’s reportable segments include the organizational structure of the Company and the financial information available for evaluation by the chief operating decision-maker in making decisions about how to allocate resources and assess performance. The Company’s operating segments have been broken out based on similar economic and other qualitative criteria. The Company operates the Allied reporting segment in one geographical area (Canada), and the Allied Colombia reporting segment in one geographical area (Colombia). Financial statement information by operating segment for the year ended August 31, 2023 is presented below: | | Allied $ | | | Allied Colombia $ | | | Total $ | | Gross margin | | | 3,799 | | | | (1,465,566 | ) | | | (1,461,767 | ) | Net loss | | | (8,123,159 | ) | | | (2,552,512 | ) | | | (10,675,671 | ) | Depreciation and amortization | | | 66,484 | | | | 70,562 | | | | 137,046 | | Total assets as of August 31, 2023 | | | 719,015 | | | | 1,344,713 | | | | 2,063,728 | |
Geographic information for the year ended and as at August 31, 2023 is presented below: | | Gross Margin $ | | | Total Assets $ | | | | | | | | | Canada | | | 3,799 | | | | 719,015 | | Colombia | | | (1,465,566 | ) | | | 1,344,713 | | Total | | | (1,461,767 | ) | | | 2,063,728 | |
|
| X |
- References
+ Details
| Name: |
alid_SegmentDisclosureAbstract |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for reporting segments including data and tables. Reportable segments include those that meet any of the following quantitative thresholds a) it's reported revenue, including sales to external customers and intersegment sales or transfers is 10 percent or more of the combined revenue, internal and external, of all operating segments b) the absolute amount of its reported profit or loss is 10 percent or more of the greater, in absolute amount of 1) the combined reported profit of all operating segments that did not report a loss or 2) the combined reported loss of all operating segments that did report a loss c) its assets are 10 percent or more of the combined assets of all operating segments. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//280/tableOfContent
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 26
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-26
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 34
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-34
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-21
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-21
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_SegmentReportingDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Income taxes
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Income taxes |
|
| Income taxes |
19. Income taxes At August 31, 2023, the Company has a net operating loss carryforward of approximately $20,588,000. The significant components of deferred income tax assets at August 31, 2023 and 2022 were as follows: | | August 31, 2023 | | | August 31, 2022 | | Deferred tax asset: | | | | | | | Net operating loss carryforward | | $ | 5,095,000 | | | $ | 3,125,000 | | Less: valuation allowance | | | (5,095,000 | ) | | | (3,125,000 | ) | Net deferred income tax asset | | $ | - | | | $ | - | |
The amount taken into income as deferred income tax assets must reflect that portion of the income tax loss carry forwards that is more likely-than-not to be realized from future operations. The Company has chosen to provide a full valuation allowance against all available income tax loss carry forwards. The Company has recognized a valuation allowance for the deferred income tax asset since the Company cannot be assured that it is more likely than not that such benefit will be utilized in future years. The valuation allowance is reviewed annually. When circumstances change and which cause a change in management’s judgment about the realizability of deferred income tax assets, the impact of the change on the valuation allowance is generally reflected in current income. As of August 31, 2023 and 2022, the Company has no recognized income tax benefits. The Company’s policy for classifying interest and penalties associated with unrecognized income tax benefits is to include such items as tax expense. No interest or penalties have been recorded during the year ended August 31, 2023 or 2022. No interest or penalties have been accrued as of August 31, 2023. As of August 31, 2023 and 2022, the Company did not have any amounts recorded pertaining to uncertain tax positions. A reconciliation of the provision for income taxes at the combined statutory rate for the year ended August 31, 2023 and 2022 is as follows: | | August 31, 2023 | | | August 31, 2022 | | Income tax benefit | | $ | 1,970,000 | | | $ | 925,000 | | Change in valuation allowance | | | (1,970,000 | ) | | | (925,000 | ) | Provision for income taxes | | $ | - | | | $ | - | |
As of August 31, 2023, the Company had approximately $20,588,000 of federal net operating losses that may be available to offset future taxable income. The net operating loss carryforwards will begin to expire in 2034 unless utilized. In accordance with Section 382 of the Internal Revenue Code, deductibility of the Company’s U.S. net operating carryovers may be subject to an annual limitation in the event of a change of control as defined the regulations. A Section 382 analysis has not been prepared and the Company’s NOLs could be subject to limitation.
|
| X |
- DefinitionThe entire disclosure for income taxes. Disclosures may include net deferred tax liability or asset recognized in an enterprise's statement of financial position, net change during the year in the total valuation allowance, approximate tax effect of each type of temporary difference and carryforward that gives rise to a significant portion of deferred tax liabilities and deferred tax assets, utilization of a tax carryback, and tax uncertainties information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//740/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-14
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-21
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482526/740-270-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB TOPIC 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Subsequent events
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Subsequent events |
|
| Subsequent events |
20. Subsequent events a) Subsequent to the year ended August 31, 2023, the Company issued 250,000 shares for subscriptions received. b) Subsequent to the year ended August 31, 2023, the Company the Company received $346,000 in cash for share subscriptions at $0.20 per share.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Significant accounting policies (Policies)
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Significant accounting policies |
|
| Principles of consolidation |
The consolidated financial statements include accounts of Allied Corp. and its wholly-owned subsidiaries, including AM Biosciences, Allied US Products LLC, Tactical Relief LLC, Baleno Ltd. and Allied Colombia. Subsidiaries are consolidated from the date of acquisition and control and continue to be consolidated until the date that such control ceases. Control is achieved when the Company is exposed, or has rights, to variable returns from its involvement with the investee and has the ability to affect these returns through its power over the investee. All intercompany balances, income, expenses, and unrealized gains and losses resulting from intercompany transactions are eliminated on consolidation.
|
| Cash and cash equivalents |
Cash is comprised of cash on hand, cash held in trust accounts and demand deposits. Cash equivalents are short-term, highly liquid investments with maturities within three months when acquired. The Company did not have any cash equivalents as of August 31, 2023 and August 31, 2022.
|
| Property, plant and equipment |
Property, plant and equipment are stated at cost. The Company depreciates the cost of property, plant and equipment over their estimated useful lives at the following annual rates and methods: Farm facility and equipment | 1 - 10 years straight-line basis | Office and computer equipment | 5 - 10 years straight-line basis | Land equipment | 10 years straight-line basis |
|
| Inventory |
Inventory is comprised of raw materials, supplies, vegetative and flowering plants, dried flower, diluted crude and CBD isolates available for sale, and purchased cannabis products. Inventory is stated at the lower of cost or net realizable value, determined using weighted average cost. Net realizable value is defined as the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal and transportation. At the end of each reporting period, the Company performs an assessment of inventory and records write-downs for excess and obsolete inventories based on the Company’s estimated forecast of product demand, production requirements, market conditions, regulatory environment, and spoilage. Actual inventory losses may differ from management’s estimates and such differences could be material to the Company’s consolidated balance sheets, statements of net loss and comprehensive loss and statements of cash flows.
|
| Intangible assets |
Intangible assets include licenses which are being amortized over their estimated useful lives of 10 years. The Company’s licenses are amortized over their economic or legal life on a straight-line basis, whichever is shorter. The licenses have been amortized from the date of acquisition. The Company periodically evaluates the reasonableness of the useful lives of these assets. Once these assets are fully amortized, they are removed from the accounts. These assets are reviewed for impairment or obsolescence when events or changes in circumstances indicate that the carrying amount may not be recoverable. If impaired, intangible assets are written down to fair value based on discounted cash flows or other valuation techniques. The Company has no intangibles with indefinite lives. For long-lived assets, impairment losses are only recorded if the asset’s carrying amount is not recoverable through its undiscounted, probability-weighted future cash flows. The Company measures the impairment loss based on the difference between the carrying amount and the estimated fair value. When an impairment exists, the related assets are written down to fair value.
|
| Long-lived assets |
In accordance with ASC 360, Property, Plant and Equipment, the Company tests long-lived assets or asset groups for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable. Circumstances which could trigger a review include, but are not limited to: significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction of the asset; current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset; and current expectation that the asset will more likely than not be sold or disposed significantly before the end of its estimated useful life. Recoverability is assessed based on the carrying amount of the asset and its fair value, which is generally determined based on the sum of the undiscounted cash flows expected to result from the use and the eventual disposal of the asset, as well as specific appraisal in certain instances. An impairment loss is recognized when the carrying amount is not recoverable and exceeds fair value.
|
| Foreign currency translation and functional currency conversion |
Items included in these consolidated financial statements of each of the Company’s entities are measured using the currency of the primary economic environment in which the entities operate (the “functional currency”). Prior to September 10, 2019, the Company’s functional currency was the Canadian dollar. Translation gains and losses from the application of the U.S. dollar as the reporting currency during the period that the Canadian dollar was the functional currency are included as part of cumulative currency translation adjustment, which is reported as a component of shareholders’ equity under accumulated other comprehensive loss. The Company re-assessed its functional currency and determined as at September 10, 2019, its functional currency changed from the Canadian dollar to the U.S. dollar based on management’s analysis of changes in our organization. The change in functional currency was accounted for prospectively from September 10, 2019 and prior period financial statements were not restated for the change in functional currency. For periods commencing September 10, 2019, monetary assets and liabilities denominated in foreign currencies are translated into U.S. dollars using exchange rates in effect at the balance sheet date. Opening balances related to non-monetary assets and liabilities are based on prior period translated amounts, and non-monetary assets and non-monetary liabilities incurred after September 10, 2019 are translated at the approximate exchange rate prevailing at the date of the transaction. Revenue and expense transactions are translated at the approximate exchange rate in effect at the time of the transactions. Foreign exchange gains and losses are included in the statement of operations and comprehensive loss as foreign exchange gains. The Company assessed the functional currency for Allied Colombia to be the Colombian peso. The functional currency for all other subsidiaries is the U.S. dollar.
|
| Share issuance costs |
Costs directly attributable to the raising of capital are charged against the related share capital. Costs related to shares not yet issued are recorded as deferred share issuance costs. These costs are deferred until the issuance of the shares to which the costs relate, at which time the costs will be charged against the related share capital or charged to operations if the shares are not issued.
|
| Research and development costs |
Research and development costs are expensed as incurred. During the year ended August 31, 2023, the Company incurred research and development costs of $nil (August 31, 2022 – $12,062).
|
| Advertising costs |
Advertising costs are expensed as incurred. During the year ended August 31, 2023, the Company incurred advertising costs of $11,425 (August 31, 2022 – $391,740).
|
| Revenue Recognition |
The Company’s revenue is comprised of sales of cannabis products. The Company’s revenue-generating activities have a single performance obligation and revenue is recognized at the point in time when control of the product transfers and the Company’s obligations have been fulfilled. This generally occurs when the product is shipped or delivered to the customer, depending upon the method of distribution and shipping terms set forth in the customer contract. Revenue is measured as the amount of consideration the Company expects to receive in exchange for the sale of the Company’s product. Certain of the Company’s customer contracts may provide the customer with a right of return. In certain circumstances the Company may also provide a retrospective price adjustment to a customer. These items give rise to variable consideration, which is recognized as a reduction of the transaction price based upon the expected amounts of the product returns and price adjustments at the time revenue for the corresponding product sale is recognized. The determination of the reduction of the transaction price for variable consideration requires that the Company make certain estimates and assumptions that affect the timing and amounts of revenue recognized. Sales of products are for cash or otherwise agreed-upon credit terms. The Company’s payment terms vary by location and customer; however, the time period between when revenue is recognized and when payment is due is not significant. The Company estimates and reserves for its bad debt exposure based on its experience with past due accounts and collectability, write-off history, the aging of accounts receivable and an analysis of customer data.
|
| Net income (loss) per common share |
Net income (loss) per share is calculated in accordance with ASC 260, Earnings per Share. The weighted-average number of common shares outstanding during each period is used to compute basic earning or loss per share. Diluted earnings or loss per share is computed using the weighted average number of shares and diluted potential common shares outstanding to the extent the effect would not be antidilutive. Dilutive potential common shares are additional common shares assumed to be exercised. Basic net income (loss) per common share is based on the weighted average number of shares of common stock outstanding.
|
| Income taxes |
The Company accounts for income taxes under ASC 740, Income Taxes. Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Under ASC 740, the effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period the enactment occurs. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax assets through future operations.
|
| Related party transactions |
Related parties are any entities or individuals that, through employment, ownership or other means, possess the ability to direct or cause the direction of the management and policies of the Company. The Company discloses related party transactions that are outside of normal compensatory agreements, such as salaries. Related party transactions are measured at the exchange amounts.
|
| Significant accounting estimates and judgments |
The preparation of the financial statements in conformity with US GAAP requires management to make judgments, estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Although management uses historical experience and its best knowledge of the amount, events or actions to for the basis for judgments and estimates, actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and further periods if the review affects both current and future periods. Significant estimates and assumptions included in these financial statements relate to the valuation assumptions related to the estimated useful lives and recoverability of long-lived assets, stock-based compensation, and deferred income tax assets and liabilities. Judgments are required in the assessment of the Company’s ability to continue to as going concern as described in Note 1.
|
| Financial instruments |
ASC 825, Financial Instruments, requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 825 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 825 prioritizes the inputs into three levels that may be used to measure fair value: Level 1 Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities. Level 2 Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data. Level 3 Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities. The financial instruments consist principally of cash, due from related parties, accounts payable, note payable, and convertible notes payable. The fair value of cash when applicable is determined based on “Level 1” inputs, which consist of quoted prices in active markets for identical assets. The Company believes that the recorded values of all other financial instruments which are categorized as loans and receivables approximate their current fair values because of their nature and respective relatively short maturity dates or current market rates of interest for similar instruments. For certain of the Company’s financial instruments, including accounts payable, due from related parties, notes and loans payable, the carrying amounts approximate their fair values due to the short maturities. The Company does not have any assets or liabilities measured at fair value on a recurring basis presented on the Company’s balance sheet as of August 31, 2023 and August 31, 2022 other than cash. Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash. The Company limits its exposure to credit loss by placing its cash with high credit quality financial institutions.
|
| Leases |
The Company determines if an arrangement contains a lease in whole or in part at the inception of the contract. Right-of-use (“ROU”) assets represent the Company’s right to use an underlying asset for the lease term while lease liabilities represent our obligation to make lease payments arising from the lease. All leases with terms greater than twelve months result in the recognition of a ROU asset and a liability at the lease commencement date based on the present value of the lease payments over the lease term. Unless a lease provides all of the information required to determine the implicit interest rate, the Company uses its incremental borrowing rate based on the information available at the commencement date in determining the present value of the lease payments. The Company uses the implicit interest rate in the lease when readily determinable. Our lease terms include all non-cancelable periods and may include options to extend (or to not terminate) the lease when it is reasonably certain that we will exercise that option. Leases with terms of twelve months or less at the commencement date are expensed on a straight-line basis over the lease term and do not result in the recognition of an asset or liability. See Note 7 – Leases.
|
| Recent accounting pronouncements |
In August 2020, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2020-06, Debt — Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40) (“ASU 2020-06”) to simplify accounting for certain financial instruments. ASU 2020-06 eliminates the current models that require separation of beneficial conversion and cash conversion features from convertible instruments and simplifies the derivative scope exception guidance pertaining to equity classification of contracts in an entity’s own equity. The new standard also introduces additional disclosures for convertible debt and freestanding instruments that are indexed to and settled in an entity’s own equity. ASU 2020-06 amends the diluted earnings per share guidance, including the requirement to use the if-converted method for all convertible instruments. ASU 2020-06 is effective for fiscal years beginning after December 15, 2023, and should be applied on a full or modified retrospective basis, with early adoption permitted beginning on January 1, 2021. The Company adopted ASU 2020-06 effective January 1, 2022. The adoption of ASU 2020-06 did not have an impact on the Company’s financial statements. The Company does not expect that recent accounting pronouncements or changes in accounting pronouncements during the year ended August 31, 2023, are of significance or potential significance to the Company.
|
| X |
- References
+ Details
| Name: |
alid_RelatedPartyTransactionPolicyTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_ShareIssuanceCostsPolicyTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for advertising cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-SubTopic 35
-Topic 720
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483406/720-35-50-1
| Name: |
us-gaap_AdvertisingCostsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy regarding (1) the principles it follows in consolidating or combining the separate financial statements, including the principles followed in determining the inclusion or exclusion of subsidiaries or other entities in the consolidated or combined financial statements and (2) its treatment of interests (for example, common stock, a partnership interest or other means of exerting influence) in other entities, for example consolidation or use of the equity or cost methods of accounting. The accounting policy may also address the accounting treatment for intercompany accounts and transactions, noncontrolling interest, and the income statement treatment in consolidation for issuances of stock by a subsidiary. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1
| Name: |
us-gaap_ConsolidationPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for determining the fair value of financial instruments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 60
-Paragraph 1
-SubTopic 10
-Topic 820
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482053/820-10-60-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 825
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-1
| Name: |
us-gaap_FairValueOfFinancialInstrumentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for (1) transactions denominated in a currency other than the reporting enterprise's functional currency, (2) translating foreign currency financial statements that are incorporated into the financial statements of the reporting enterprise by consolidation, combination, or the equity method of accounting, and (3) remeasurement of the financial statements of a foreign reporting enterprise in a hyperinflationary economy. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//830/tableOfContent
| Name: |
us-gaap_ForeignCurrencyTransactionsAndTranslationsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for recognizing and measuring the impairment of long-lived assets. An entity also may disclose its accounting policy for long-lived assets to be sold. This policy excludes goodwill and intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.CC)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480091/360-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 05
-Paragraph 4
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482338/360-10-05-4
| Name: |
us-gaap_ImpairmentOrDisposalOfLongLivedAssetsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-25
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-28
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-19
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-20
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for finite-lived intangible assets. This accounting policy also might address: (1) the amortization method used; (2) the useful lives of such assets; and (3) how the entity assesses and measures impairment of such assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483154/926-20-50-5
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 920
-SubTopic 350
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483256/920-350-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 920
-SubTopic 350
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483256/920-350-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 920
-SubTopic 350
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483256/920-350-50-4
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_IntangibleAssetsFiniteLivedPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of inventory accounting policy for inventory classes, including, but not limited to, basis for determining inventory amounts, methods by which amounts are added and removed from inventory classes, loss recognition on impairment of inventories, and situations in which inventories are stated above cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483489/210-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 912
-SubTopic 330
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482105/912-330-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//330/tableOfContent
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483080/330-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483080/330-10-50-4
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Subparagraph (a)
-SubTopic 10
-Topic 270
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482989/270-10-45-6
| Name: |
us-gaap_InventoryPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for leasing arrangements entered into by lessor. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479773/842-30-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 3A
-Subparagraph (a)
-SubTopic 30
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479773/842-30-50-3A
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 3A
-Subparagraph (b)
-SubTopic 30
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479773/842-30-50-3A
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-SubTopic 30
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479773/842-30-50-14
| Name: |
us-gaap_LessorLeasesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for costs it has incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483044/730-10-05-1
| Name: |
us-gaap_ResearchAndDevelopmentExpensePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for revenue. Includes revenue from contract with customer and from other sources. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (e)
-SubTopic 10
-Topic 235
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
| Name: |
us-gaap_RevenueRecognitionPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of public utility physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation expense and method used, including composite depreciation, and accumulated depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 980
-SubTopic 20
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481834/980-20-45-1
| Name: |
us-gaap_ScheduleOfPublicUtilityPropertyPlantAndEquipmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Inventory (Tables)
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Inventory |
|
| Schedule of Inventory |
| | August 31, 2023 | | | August 31, 2022 | | | | | | | | | Work in progress | | $ | 80,094 | | | $ | 326,556 | | Finished goods | | | 27,416 | | | | 383,459 | | Inventory in transit | | | - | | | | 288,973 | | Total inventory | | $ | 107,510 | | | $ | 998,988 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_InventoryDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the carrying amount as of the balance sheet date of merchandise, goods, commodities, or supplies held for future sale or to be used in manufacturing, servicing or production process. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483489/210-10-50-1
| Name: |
us-gaap_ScheduleOfInventoryCurrentTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Deposits and advances (Tables)
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Deposits and advances |
|
| Schedule of deposits and advances |
| | August 31, 2023 | | | August 31, 2022 | | | | | | | | | a) Towards the purchase of prefabricated buildings | | $ | - | | | $ | 2,656,695 | | b) Deposit towards a license acquisition | | | - | | | | 150,000 | | c) Prepayments for construction facility in Colombia | | | 26,354 | | | | 63,130 | | Total deposits and advances | | $ | 26,354 | | | $ | 2,869,825 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_ReceivablesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA tabular presentation of the information summarizing investments in and advances to majority-owned subsidiaries, other controlled companies, and other affiliates, as prescribed by the SEC. It reflects specified information about ownership, financial results from, and financial position in such entities. Includes the tabular presentations that disaggregate investments in and advances to majority-owned subsidiaries, other controlled companies, and other affiliates.
| Name: |
us-gaap_ScheduleOfInvestmentsInAndAdvancesToAffiliatesScheduleOfInvestmentsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Property plant and equipment (Tables)
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Property plant and equipment |
|
| Schedule of property plant and equipment |
| | Construction in process | | | Farm facility and equipment | | | Office and computer equipment | | | Land equipment | | | Total | | Cost | | | | | | | | | | | | | | | | August 31, 2022 | | $ | 460,648 | | | $ | 1,152,822 | | | $ | 21,348 | | | $ | 27,150 | | | $ | 1,661,968 | | Additions | | | 31,528 | | | | 45,344 | | | | 6,167 | | | | 132 | | | | 83,171 | | Transfer | | | (93,363 | ) | | | 93,363 | | | | - | | | | - | | | | - | | Foreign exchange | | | 30,936 | | | | 54,844 | | | | 2,437 | | | | 2,243 | | | | 90,460 | | August 31, 2023 | | $ | 429,749 | | | $ | 1,346,373 | | | $ | 29,952 | | | $ | 29,525 | | | $ | 1,835,599 | | | | | | | | | | | | | | | | | | | | | | | Accumulated depreciation | | | | | | | | | | | | | | | | | | | | | August 31, 2022 | | $ | - | | | $ | 210,899 | | | $ | 4,084 | | | $ | 2,947 | | | $ | 217,930 | | Additions | | | - | | | | 167,747 | | | | 2,953 | | | | 2,663 | | | | 173,363 | | Foreign exchange | | | - | | | | 25,913 | | | | 663 | | | | 538 | | | | 27,114 | | August 31, 2023 | | $ | - | | | $ | 404,559 | | | $ | 7,700 | | | $ | 6,148 | | | $ | 418,407 | | | | | | | | | | | | | | | | | | | | | | | Net book value | | | | | | | | | | | | | | | | | | | | | August 31, 2022 | | $ | 460,648 | | | $ | 941,923 | | | $ | 17,264 | | | $ | 24,203 | | | $ | 1,444,038 | | August 31, 2023 | | $ | 429,749 | | | $ | 941,814 | | | $ | 22,252 | | | $ | 23,377 | | | $ | 1,417,192 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation and depletion expense and method used, including composite depreciation, and accumulated deprecation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Intangible assets (Tables)
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Intangible assets |
|
| Schedule of intangible assets |
| | Cost $ | | | Foreign exchange $ | | | Accumulated amortization $ | | | Accumulated impairment $ | | | August 31, 2023 Net carrying value $ | | | August 31, 2022 Net carrying value $ | | | | | | | | | | | | | | | | | | | | | Cannabis licenses | | | 5,451,409 | | | | (468,910 | ) | | | (1,140,531 | ) | | | (3,801,667 | ) | | | 40,301 | | | | 44,773 | | | | | 5,451,409 | | | | (468,910 | ) | | | (1,140,531 | ) | | | (3,801,667 | ) | | | 40,301 | | | | 44,773 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of assets, excluding financial assets and goodwill, lacking physical substance with a finite life, by either major class or business segment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_ScheduleOfFiniteLivedIntangibleAssetsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Leases (Tables)
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Leases |
|
| Schedule of components of lease expenses |
| | $ | | Operating lease cost: | | | | Amortization of right-of-use assets | | | 24,079 | | Interest on lease liabilities | | | 26,046 | | | | | | | Total operating lease cost | | | 50,125 | |
|
| Schedule of supplemental cash flow and other information related to leases |
|
| Schedule of supplemental balance sheet information related to leases |
| | | $ | | Cost | | | 282,273 | | Accumulated amortization | | | (55,220 | ) | Lease termination | | | (61,928 | ) | Foreign exchange | | | (55,824 | ) | | | | | | Net carrying value at August 31, 2023 | | | 109,301 | |
|
| Schedule of future minimum lease payments |
| | $ | | 2024 | | | 26,422 | | 2025 | | | 26,422 | | 2026 | | | 26,422 | | 2027 | | | 26,422 | | 2028 | | | 26,422 | | 2029 | | | 26,422 | | 2030 | | | 13,210 | | | | | | | Total minimum lease payments | | | 171,742 | | | | | | | Less: amount of lease payments representing effects of discounting | | | (62,441 | ) | | | | | | Present value of future minimum lease payments | | | 109,301 | | | | | | | Less: current obligations under leases | | | (10,745 | ) | | | | | | Lease liabilities, net of current portion | | | 98,556 | |
|
| X |
- References
+ Details
| Name: |
alid_ScheduleOfComponentsOfLeaseExpensesTableTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_ScheduleOfSupplementalBalanceSheetInformationRelatedToLeasesTableTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_ScheduleOfSupplementalCashFlowAndOtherInformationRelatedToLeasesTableTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of future minimum lease payments as of the date of the latest balance sheet presented, in aggregate and for each of the five years succeeding fiscal years, with separate deductions from the total for the amount representing executor costs, including any profit thereon, included in the minimum lease payments and for the amount of the imputed interest necessary to reduce the net minimum lease payments to present value. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481161/840-30-50-1
| Name: |
us-gaap_ScheduleOfFutureMinimumLeasePaymentsForCapitalLeasesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Related party transactions and balances (Tables)
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Related party transactions and balances |
|
| Schedule of related party compensation costs |
| | August 31, 2023 | | | August 31, 2022 | | Consulting fees and benefits | | $ | 396,052 | | | $ | 369,079 | |
|
| Schedule of due from related party |
| | August 31, 2023 | | | August 31, 2022 | | | | | | | | | CEO and Director | | $ | 156,028 | | | $ | 80,892 | | COO and Director | | | 201,612 | | | | 143,816 | | An entity controlled by the CFO | | | 84,962 | | | | 46,387 | | An entity controlled by a Director | | | 159,690 | | | | 60,677 | | Director | | | 91,000 | | | | 52,500 | | | | $ | 693,292 | | | $ | 384,272 | |
|
| X |
- References
+ Details
| Name: |
alid_ScheduleOfRelatedPartyCompensationCostsTableTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of related party transactions. Examples of related party transactions include, but are not limited to, transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners and (d) affiliates.
| Name: |
us-gaap_ScheduleOfRelatedPartyTransactionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Financial risk factors (Tables)
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Financial risk factors |
|
| Schedule of foreign currency translation |
| | August 31, 2023 | | Balance in Canadian dollars: | | | | Accounts payable | | $ | (947,214 | ) | Net exposure | | | (947,214 | ) | Balance in US dollars: | | $ | (700,032 | ) |
| | August 31, 2023 | | Balance in Colombian Pesos dollars: | | | | Cash and cash equivalents | | $ | 415,100,982 | | Other receivables | | | 539,704,008 | | Accounts payable | | | (3,669,893,803 | ) | Net exposure | | | (2,715,088,813 | ) | Balance in US dollars: | | $ | (664,234 | ) |
|
| X |
- References
+ Details
| Name: |
us-gaap_PriceRiskFairValueHedgesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of long-term intercompany foreign balances, including related intercompany entity, underlying foreign currencies and amounts of intercompany foreign currency transactions that are of a long-term investment nature (that is settlement is not planned or anticipated in the foreseeable future), as of the balance sheet date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 830
-SubTopic 30
-Section 45
-Paragraph 20
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
| Name: |
us-gaap_ScheduleOfIntercompanyForeignCurrencyBalancesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Share purchase warrants (Tables)
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Share purchase warrants |
|
| Schedule of share purchase warrants |
| | Number of warrants | | | Weighted average exercise price $ | | | | | | | | | Balance, August 31, 2022 | | | 7,704,135 | | | | 1.23 | | | | | | | | | | | Issued | | | 1,000,000 | | | | 0.20 | | Expired | | | (3,245,135 | ) | | | 1.25 | | Balance, August 31, 2023 | | | 5,459,000 | | | | 1.06 | |
| Number of warrants | | | Exercise price $ | | | Expiry date | | | | | | | | | | | | | 2,545,999 | | | | 1.25 | | | October 20, 2023* | | | | 905,000 | | | | 1.25 | | | November 6, 2023* | | | | 36,000 | | | | 1.25 | | | November 24, 2023* | | | | 66,667 | | | | 1.25 | | | January 29, 2024 | | | | 66,667 | | | | 1.25 | | | February 7, 2024 | | | | 113,334 | | | | 1.25 | | | February 12, 2024 | | | | 40,000 | | | | 1.25 | | | February 20, 2024 | | | | 300,000 | | | | 1.25 | | | February 21, 2024 | | | | 177,600 | | | | 1.25 | | | February 26, 2024 | | | | 120,000 | | | | 1.25 | | | February 28, 2024 | | | | 20,000 | | | | 1.25 | | | March 7, 2024 | | | | 67,733 | | | | 1.25 | | | May 27, 2024 | | | | 1,000,000 | | | | 0.20 | | | July 30, 2025 | | | | 5,459,000 | | | | | | | | |
|
| X |
- References
+ Details
| Name: |
alid_ScheduleOfSharePurchaseWarrantsTableTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LineOfCreditFacilityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Stock Options (Tables)
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Stock Options |
|
| Schedule of Stock Options activity |
| | Number of Options | | | Weighted Average Exercise Price $ | | | Weighted Average Remaining Contractual Term | | | Aggregate Intrinsic Value $ | | | | | | | | | | | | | | | Balance, August 31, 2022 | | | 6,000,000 | | | | 0.44 | | | | 3.85 | | | | 144,000 | | | | | | | | | | | | | | | | | Granted | | | 6,140,000 | | | | 0.24 | | | | 4.63 | | | | | | | | | | | | | | | | | | | | Outstanding, August 31, 2023 | | | 12,140,000 | | | | 0.25 | | | | 3.70 | | | | 55,900 | | Exercisable, August 31, 2023 | | | 11,456,667 | | | | 0.25 | | | | 3.70 | | | | 48,400 | |
|
| Schedule of weighted average assumptions |
| | Year Ended August 31, 2023 | | | Year Ended August 31, 2022 | | | | | | | | | Expected dividend yield | | | 0 | % | | | 0 | % | Expected volatility | | | 162 | % | | | 150 | % | Expected life (in years) | | | 3.45 | | | | 4.05 | | Risk-free interest rate | | | 4.41 | % | | | 3.38 | % |
|
| X |
- DefinitionTabular disclosure of assumption used to determine benefit obligation and net periodic benefit cost of defined benefit plan. Includes, but is not limited to, discount rate, rate of compensation increase, expected long-term rate of return on plan assets and interest crediting rate. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (k)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-1
| Name: |
us-gaap_ScheduleOfAssumptionsUsedTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Non cash activities (Tables)
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Non cash activities |
|
| Schedule of Non-cash activities |
| | For the Year Ended August 31, 2023 | | | For the Year Ended August 31, 2022 | | Non-cash activities: | | | | | | | Shares issued from treasury for services | | | | | | 7,957,316 | | Fair value of warrants issued as finder’s fee | | | 204,452 | | | | - | | Beneficial conversion feature | | | - | | | | 281,447 | | Relative fair value of warrants issuable with convertible note | | | - | | | | 120,310 | | Fair value of shares issued on modification of convertible note | | | - | | | | 129,952 | | Fair value of shares issuable on modification of debt | | | - | | | | 8,268 | |
|
| X |
- References
+ Details
| Name: |
alid_NonCashActivitiesAbstract |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_ScheduleOfNonCashActivitiesTableTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Segment disclosure (Tables)
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Segment disclosure |
|
| Schedule of operating segment |
| | Allied $ | | | Allied Colombia $ | | | Total $ | | Gross margin | | | 3,799 | | | | (1,465,566 | ) | | | (1,461,767 | ) | Net loss | | | (8,123,159 | ) | | | (2,552,512 | ) | | | (10,675,671 | ) | Depreciation and amortization | | | 66,484 | | | | 70,562 | | | | 137,046 | | Total assets as of August 31, 2023 | | | 719,015 | | | | 1,344,713 | | | | 2,063,728 | |
|
| Schedule of geographical information |
| | Gross Margin $ | | | Total Assets $ | | | | | | | | | Canada | | | 3,799 | | | | 719,015 | | Colombia | | | (1,465,566 | ) | | | 1,344,713 | | Total | | | (1,461,767 | ) | | | 2,063,728 | |
|
| X |
- References
+ Details
| Name: |
alid_ScheduleOfGeographicalInformationTableTextBlock |
| Namespace Prefix: |
alid_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_SegmentDisclosureAbstract |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the profit or loss and total assets for each reportable segment. An entity discloses certain information on each reportable segment if the amounts (a) are included in the measure of segment profit or loss reviewed by the chief operating decision maker or (b) are otherwise regularly provided to the chief operating decision maker, even if not included in that measure of segment profit or loss. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-25
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 30
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
| Name: |
us-gaap_ScheduleOfSegmentReportingInformationBySegmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Income Taxes (Tables)
|
12 Months Ended |
Aug. 31, 2023 |
|---|
| Income taxes |
|
| Schedule of deferred income tax assets |
| | August 31, 2023 | | | August 31, 2022 | | Deferred tax asset: | | | | | | | Net operating loss carryforward | | $ | 5,095,000 | | | $ | 3,125,000 | | Less: valuation allowance | | | (5,095,000 | ) | | | (3,125,000 | ) | Net deferred income tax asset | | $ | - | | | $ | - | |
|
| Schedule of Effective Income Tax Rate Reconciliation |
| | August 31, 2023 | | | August 31, 2022 | | Income tax benefit | | $ | 1,970,000 | | | $ | 925,000 | | Change in valuation allowance | | | (1,970,000 | ) | | | (925,000 | ) | Provision for income taxes | | $ | - | | | $ | - | |
|
| X |
- DefinitionTabular disclosure of the components of income tax expense attributable to continuing operations for each year presented including, but not limited to: current tax expense (benefit), deferred tax expense (benefit), investment tax credits, government grants, the benefits of operating loss carryforwards, tax expense that results from allocating certain tax benefits either directly to contributed capital or to reduce goodwill or other noncurrent intangible assets of an acquired entity, adjustments of a deferred tax liability or asset for enacted changes in tax laws or rates or a change in the tax status of the entity, and adjustments of the beginning-of-the-year balances of a valuation allowance because of a change in circumstances that causes a change in judgment about the realizability of the related deferred tax asset in future years. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 9
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
| Name: |
us-gaap_ScheduleOfComponentsOfIncomeTaxExpenseBenefitTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the reconciliation using percentage or dollar amounts of the reported amount of income tax expense attributable to continuing operations for the year to the amount of income tax expense that would result from applying domestic federal statutory tax rates to pretax income from continuing operations. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 12
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-12
| Name: |
us-gaap_ScheduleOfEffectiveIncomeTaxRateReconciliationTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
| X |
- References
+ Details
| Name: |
alid_WorkingCapitalDeficit |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
| X |
- References
+ Details
| Name: |
alid_PropertyPlantAndEquipmentsUsefulLife |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=alid_FarmFacilityAndEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=alid_OfficeAndComputerEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=alid_LandequipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- References
+ Details
| Name: |
alid_IntangibleAssetUsefulLife |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount charged to advertising expense for the period, which are expenses incurred with the objective of increasing revenue for a specified brand, product or product line. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 720
-SubTopic 35
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483406/720-35-50-1
| Name: |
us-gaap_AdvertisingExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of other research and development expense. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
| Name: |
us-gaap_OtherResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
| X |
- References
+ Details
| Name: |
us-gaap_InventoryDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying amount, net of valuation reserves and adjustments, as of the balance sheet date of merchandise or goods held by the company that are readily available for sale. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.BB)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480581/330-10-S99-2
| Name: |
us-gaap_InventoryFinishedGoodsNetOfReserves |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after valuation and LIFO reserves of inventory expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before valuation and LIFO reserves of merchandise or goods in the production process expected to be completed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryWorkInProcess |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionGross amount of merchandise or supplies to which the entity holds the title but does not hold physical possession because the goods are currently being transported. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherInventoryInTransit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.3
| X |
- References
+ Details
| Name: |
alid_AdditionalInventoryWriteDown |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_OtherDepreciationAndAmortizationAdditional |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_InventoryDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of loss from reductions in inventory due to subsequent measurement adjustments, including, but not limited to, physical deterioration, obsolescence, or changes in price levels. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483080/330-10-50-2
| Name: |
us-gaap_InventoryWriteDown |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense charged against earnings to allocate the cost of tangible and intangible assets over their remaining economic lives, classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherDepreciationAndAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
Deposits and advances (Details) - USD ($)
|
Aug. 31, 2023 |
Aug. 31, 2022 |
Mar. 30, 2021 |
| Total deposits and advances |
$ 26,354
|
$ 2,869,825
|
|
| Towards the purchase of prefabricated buildings [Member] |
|
|
|
| Total deposits and advances |
0
|
2,656,695
|
|
| Deposit towards a license acquisition [Member] |
|
|
|
| Total deposits and advances |
0
|
150,000
|
$ 150,000
|
| Prepayments for construction facility in Colombia [Member] |
|
|
|
| Total deposits and advances |
$ 26,354
|
$ 63,130
|
|
| X |
- DefinitionCarrying value of amounts transferred to third parties for security purposes that are expected to be returned or applied towards payment within one year or during the operating cycle, if shorter. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DepositsAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=alid_TowardsPurchaseOfPrefabricatedBuildingsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=alid_DepositTowardsALicenseAcquisitionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=alid_PrepaymentsForConstructionFacilityInColombiaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Deposits and advances (Details Narrative) - USD ($)
|
12 Months Ended |
|
Aug. 31, 2023 |
Aug. 31, 2022 |
Mar. 30, 2021 |
| Total deposits and advances |
$ 26,354
|
$ 2,869,825
|
|
| Payment to purchase licenses |
4,625
|
12,111
|
|
| Loss on license acquisition advance |
(150,000)
|
0
|
|
| Promissory note and certain liabilities |
1,350,000
|
|
|
| Towards the purchase of prefabricated buildings [Member] |
|
|
|
| Total deposits and advances |
0
|
2,656,695
|
|
| Payment to purchase licenses |
|
150,000
|
|
| Deposit towards a license acquisition [Member] |
|
|
|
| Total deposits and advances |
0
|
150,000
|
$ 150,000
|
| Prepayments for construction facility in Colombia [Member] |
|
|
|
| Total deposits and advances |
$ 26,354
|
$ 63,130
|
|
| X |
- References
+ Details
| Name: |
alid_CertainLiabilitiesAndPromissoryNote |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_GainLossOnLicenseAcquisitionAdvance |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value of amounts transferred to third parties for security purposes that are expected to be returned or applied towards payment within one year or during the operating cycle, if shorter. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DepositsAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash outflow to acquire asset without physical form usually arising from contractual or other legal rights, excluding goodwill. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=alid_TowardsPurchaseOfPrefabricatedBuildingsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=alid_DepositTowardsALicenseAcquisitionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=alid_PrepaymentsForConstructionFacilityInColombiaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Property plant and equipment (Details)
|
12 Months Ended |
|
Aug. 31, 2023
USD ($)
|
|---|
| Cost of asset, Beginning balance |
$ 1,661,968
|
| Cost of asset, Additions |
83,171
|
| Cost of asset, Transfer |
0
|
| Cost of asset, Foreign exchange |
90,460
|
| Cost of asset, Ending balance |
1,835,599
|
| Accumulated depreciation, Beginning balances |
217,930
|
| Accumulated depreciation, Additions |
173,363
|
| Accumulated depreciation, Foreign exchange |
27,114
|
| Accumulated depreciation, Ending balance |
418,407
|
| Net book value, Beginning balance |
1,444,038
|
| Net book value, Ending balance |
1,417,192
|
| Land Equipment [Member] |
|
| Cost of asset, Beginning balance |
27,150
|
| Cost of asset, Additions |
132
|
| Cost of asset, Transfer |
0
|
| Cost of asset, Foreign exchange |
2,243
|
| Cost of asset, Ending balance |
29,525
|
| Accumulated depreciation, Beginning balances |
2,947
|
| Accumulated depreciation, Additions |
2,663
|
| Accumulated depreciation, Foreign exchange |
538
|
| Accumulated depreciation, Ending balance |
6,148
|
| Net book value, Beginning balance |
24,203
|
| Net book value, Ending balance |
23,377
|
| Construction in progress [Member] |
|
| Cost of asset, Beginning balance |
460,648
|
| Cost of asset, Additions |
31,528
|
| Cost of asset, Transfer |
(93,363)
|
| Cost of asset, Foreign exchange |
30,936
|
| Cost of asset, Ending balance |
429,749
|
| Accumulated depreciation, Beginning balances |
0
|
| Accumulated depreciation, Additions |
0
|
| Accumulated depreciation, Foreign exchange |
0
|
| Accumulated depreciation, Ending balance |
0
|
| Net book value, Beginning balance |
460,648
|
| Net book value, Ending balance |
429,749
|
| Farm facility and equipment [Member] |
|
| Cost of asset, Beginning balance |
1,152,822
|
| Cost of asset, Additions |
45,344
|
| Cost of asset, Transfer |
93,363
|
| Cost of asset, Foreign exchange |
54,844
|
| Cost of asset, Ending balance |
1,346,373
|
| Accumulated depreciation, Beginning balances |
210,899
|
| Accumulated depreciation, Additions |
167,747
|
| Accumulated depreciation, Foreign exchange |
25,913
|
| Accumulated depreciation, Ending balance |
404,559
|
| Net book value, Beginning balance |
941,923
|
| Net book value, Ending balance |
941,814
|
| Office and Computer Equipment [Member] |
|
| Cost of asset, Beginning balance |
21,348
|
| Cost of asset, Additions |
6,167
|
| Cost of asset, Transfer |
0
|
| Cost of asset, Foreign exchange |
2,437
|
| Cost of asset, Ending balance |
29,952
|
| Accumulated depreciation, Beginning balances |
4,084
|
| Accumulated depreciation, Additions |
2,953
|
| Accumulated depreciation, Foreign exchange |
663
|
| Accumulated depreciation, Ending balance |
7,700
|
| Net book value, Beginning balance |
17,264
|
| Net book value, Ending balance |
$ 22,252
|
| X |
- References
+ Details
| Name: |
alid_AccumulatedDepreciationDepletionAndAmortizationPropertyPlantAndEquipmentAdditions |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_AccumulatedDepreciationDepletionAndAmortizationPropertyPlantAndEquipmentForeignExchange |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_PropertyPlantAndEquipmentForeignExchange |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of accumulated depreciation, depletion and amortization for physical assets used in the normal conduct of business to produce goods and services. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_AccumulatedDepreciationDepletionAndAmortizationPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of acquisition of long-lived, physical assets used in the normal conduct of business and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, furniture and fixtures, and computer equipment.
| Name: |
us-gaap_PropertyPlantAndEquipmentAdditions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(13))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) of physical assets used in the normal conduct of business and not intended for resale, from reclassification, impairment, donation, or changes classified as other. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-2
| Name: |
us-gaap_PropertyPlantAndEquipmentTransfersAndChanges |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=alid_LandequipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_ConstructionInProgressMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=alid_FarmFacilityAndEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=alid_OfficeAndComputerEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Intangible assets (Details) - USD ($)
|
12 Months Ended |
|
Aug. 31, 2023 |
Aug. 31, 2022 |
| Cost |
$ 5,451,409
|
|
| Foreign Exchange |
(468,910)
|
|
| Accumulated amortization |
(1,140,531)
|
|
| Accumulated impairment |
(3,801,667)
|
|
| Net carrying value |
40,301
|
$ 44,773
|
| Cannabis Licenses [Member] |
|
|
| Cost |
5,451,409
|
|
| Foreign Exchange |
(468,910)
|
|
| Accumulated amortization |
(1,140,531)
|
|
| Accumulated impairment |
(3,801,667)
|
|
| Net carrying value |
$ 40,301
|
$ 44,773
|
| X |
- References
+ Details
| Name: |
alid_CostIntangibleAssets |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_FiniteLivedIntangibleAssetsAccumulatedImpairment |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAccumulated amount of amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAccumulatedAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of foreign currency translation gain (loss) which increases (decreases) assets, excluding financial assets and goodwill, lacking physical substance with a finite life.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsForeignCurrencyTranslationGainLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483154/926-20-50-5
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=alid_CannabisLicensesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Intangible assets (Details Narrative) - USD ($)
|
12 Months Ended |
|
Aug. 31, 2023 |
Aug. 31, 2021 |
Feb. 17, 2020 |
| Impairment of intangible assets |
|
$ 2,687,695
|
|
| Amortization of licenses |
$ 9,097
|
|
|
| Acquisition of licenses |
$ 3,964
|
|
|
| Allied Colombia [Member] |
|
|
|
| Amortization of licenses |
|
658,836
|
|
| Acquisition of licenses |
|
|
$ 5,435,334
|
| Amortization included in inventory |
|
$ 158,121
|
|
| X |
- References
+ Details
| Name: |
alid_AcquisitionOfLicenses |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_AmortizationIncludedInInventory |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_ImpairmentOfIntangibleAssets |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate expense charged against earnings to allocate the cost of intangible assets (nonphysical assets not used in production) in a systematic and rational manner to the periods expected to benefit from such assets. As a noncash expense, this element is added back to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482686/350-30-45-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_AmortizationOfIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsegmentsAxis=alid_AlliedColombiaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- References
+ Details
| Name: |
alid_InterestOnLeaseLiabilities |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of amortization expense attributable to right-of-use asset from finance lease. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_FinanceLeaseRightOfUseAssetAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingCostsAndExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow from operating lease, excluding payments to bring another asset to condition and location necessary for its intended use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-5
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeasePayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.3
| X |
- References
+ Details
| Name: |
alid_CostLease |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_ForeignExchange |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_GroundLeaseAccumulatedAmortization |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_LeaseTermination |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_NetCarryingValue |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Leases (Details 3)
|
Aug. 31, 2023
USD ($)
|
| Leases |
|
| 2024 |
$ 26,422
|
| 2025 |
26,422
|
| 2026 |
26,422
|
| 2027 |
26,422
|
| 2028 |
26,422
|
| 2029 |
26,422
|
| 2030 |
13,210
|
| Total minimum lease payments |
171,742
|
| Less: amount of lease payments representing effects of discounting |
(62,441)
|
| Present value of future minimum lease payments |
109,301
|
| Less current obligations under leases |
(10,745)
|
| Lease liabilities, net of current portion |
$ 98,556
|
| X |
- References
+ Details
| Name: |
alid_LessAmountOfLeasePaymentsRepresentingEffectsOfDiscounting |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_LessCurrentObligationsUnderLeases |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_LesseeOperatingLeaseLiabilityPaymentsDueYearSeven |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_LesseeOperatingLeaseLiabilityPaymentsDueYearSix |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_PresentValueOfFutureMinimumLeasePayments |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_TotalMinimumLeasePayments |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of long-term debt and lease obligation, classified as current. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermDebtAndCapitalLeaseObligationsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
| X |
- References
+ Details
| Name: |
alid_LeaseTerm |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_OperatingLeasesLiability |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_WeightedAverageRemainingOperatingLeaseTerm |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of the required periodic payments including both interest and principal payments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 470
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480848/942-470-50-3
| Name: |
us-gaap_DebtInstrumentPeriodicPayment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.23.3
Loans payable (Details Narrative) - USD ($)
|
1 Months Ended |
12 Months Ended |
|
Dec. 27, 2021 |
Nov. 20, 2020 |
Jun. 20, 2020 |
Aug. 31, 2023 |
Aug. 31, 2022 |
May 29, 2020 |
| Initialy payment for building |
|
|
|
|
|
$ 71,023
|
| Monthly interest payments |
|
|
$ 37,613
|
|
|
|
| Repayment of Principal Amount |
|
$ 1,253,772
|
|
|
|
|
| Debt repayment description |
the Company will make 27 monthly payments starting on January 20, 2022. For the first 3 months, the Company will make monthly payments of $37,613. For the remaining 24 months, the Company will make monthly payments of $66,288. If the first 6 monthly payments are made on time, the Company may prepay the unamortized loan balance with a 2% penalty of the remaining balance
|
|
|
|
|
|
| Compensation cost fair value |
|
|
|
$ 633
|
|
|
| June 2020 [Member] |
|
|
|
|
|
|
| Purchase price of Building |
|
|
|
1,253,772
|
|
|
| Balance owing amount |
|
|
|
1,742,648
|
$ 1,291,290
|
|
| Interest paid on loans for purchase of buildings |
|
|
|
0
|
$ 363,337
|
|
| December 17, 2021 [Member] |
|
|
|
|
|
|
| Final payment on promissory notes |
|
|
|
$ 295,543
|
|
|
| Interest rate |
|
|
|
8.00%
|
8.00%
|
|
| Interest payment |
|
|
|
$ 15,000
|
|
|
| Balance owing |
|
|
|
284,700
|
$ 264,364
|
|
| Interest paid |
|
|
|
$ 0
|
$ 3,653
|
|
| X |
- References
+ Details
| Name: |
alid_BalanceOwing |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_BalanceOwingAmount |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_CompensationCostFairValue |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_DebtRepaymentDescription |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_FinalPaymentOnPromissoryNotes |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_InitialyPaymentForBuilding |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_InterestPaidOnLoansForPurchaseOfBuildings |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_InterestPayment |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_MonthlyInterestPayments |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of the required periodic payments applied to principal. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DebtInstrumentPeriodicPaymentPrincipal |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash paid for interest, including, but not limited to, capitalized interest and payment to settle zero-coupon bond attributable to accreted interest of debt discount and debt instrument with insignificant coupon interest rate in relation to effective interest rate of borrowing attributable to accreted interest of debt discount; classified as operating and investing activities. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-2
| Name: |
us-gaap_InterestPaid |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of business during the period. The cash portion only of the acquisition price. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479581/805-30-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireBusinessesGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of the carrying amount of short-term borrowings outstanding as of the balance sheet date which accrues interest at a set, unchanging rate.
| Name: |
us-gaap_ShortTermDebtPercentageBearingFixedInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_JuneTwoThousandTwentyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_DecemberSeventeenTwoThousandTwentyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Secured convertible notes payable (Details Narrative) - USD ($)
|
|
|
|
|
|
|
|
|
1 Months Ended |
3 Months Ended |
12 Months Ended |
|
|
|
|
|
|
|
|
|
|
|
Dec. 07, 2022 |
Dec. 02, 2022 |
Oct. 07, 2022 |
Jan. 11, 2022 |
Oct. 02, 2021 |
Jan. 07, 2021 |
Dec. 02, 2020 |
Nov. 11, 2020 |
May 17, 2023 |
Feb. 28, 2023 |
Nov. 28, 2022 |
Jun. 16, 2022 |
Mar. 29, 2022 |
Feb. 22, 2022 |
Jan. 31, 2022 |
Dec. 23, 2021 |
Nov. 02, 2021 |
Oct. 25, 2021 |
Jul. 25, 2021 |
Apr. 30, 2021 |
Apr. 29, 2021 |
Mar. 26, 2021 |
Oct. 26, 2020 |
Sep. 29, 2020 |
Jun. 30, 2020 |
Jan. 23, 2020 |
May 31, 2023 |
May 31, 2022 |
Aug. 31, 2023 |
Aug. 31, 2022 |
Dec. 01, 2022 |
Mar. 07, 2022 |
Feb. 28, 2022 |
Feb. 25, 2022 |
Feb. 21, 2022 |
Feb. 20, 2022 |
Feb. 07, 2022 |
Nov. 01, 2021 |
Oct. 31, 2021 |
Oct. 01, 2021 |
Sep. 26, 2021 |
| Relative fair value of convertible notes |
|
|
|
|
|
|
|
$ 48,258
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 20,176
|
$ 85,330
|
|
|
|
|
$ 470,467
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible notes payable |
$ 60,000
|
|
|
$ 150,000
|
|
|
|
|
$ 10,000
|
$ 150,000
|
$ 100,000
|
$ 250,000
|
$ 500,000
|
|
$ 100,000
|
$ 100,000
|
|
$ 100,000
|
|
|
|
|
|
|
$ 600,000
|
|
|
|
|
|
$ 70,000
|
|
|
|
|
|
|
|
|
$ 100,000
|
|
| Simple interest rate |
10.00%
|
10.00%
|
|
10.00%
|
|
|
|
|
10.00%
|
10.00%
|
10.00%
|
10.00%
|
10.00%
|
|
10.00%
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Beneficial conversion feature |
|
|
|
|
|
|
|
48,258
|
|
|
|
|
|
|
|
|
|
|
$ 1,090
|
|
|
|
20,176
|
115,383
|
|
|
|
|
115,383
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accounts payable and accrued liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
15,808
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accounts payable and accrued interest |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
504
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accrued interest |
$ 5,236
|
|
|
|
|
|
|
|
$ 7,562
|
$ 4,389
|
$ 30,206
|
|
$ 71,233
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
24,534
|
|
$ 7,616
|
|
|
|
|
|
|
|
|
|
|
| Accrued interest included in accounts payable and accrued liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
33,540
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accrued interest |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3,144
|
|
|
|
|
|
|
|
|
|
|
|
|
| Principal owing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
15,000
|
$ 15,000
|
|
|
|
|
|
|
|
|
|
|
|
| Principal owing one |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100,000
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
| Principal owing two |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
500,000
|
500,000
|
|
|
|
|
|
|
|
|
|
|
|
| Principal owing three |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
250,000
|
250,000
|
|
|
|
|
|
|
|
|
|
|
|
| Principal owing four |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100,000
|
0
|
|
|
|
|
|
|
|
|
|
|
|
| Principal owing five |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
70,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Principal owing six |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
60,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Principal owing seven |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
150,000
|
150,000
|
|
|
|
|
|
|
|
|
|
|
|
| Principal owing eight |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
150,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Principal owing nine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrants to purchase |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100,000
|
|
| Convertible notes, Fair value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 39,573
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 40,117
|
|
| Warrants, Fair value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 59,883
|
|
| Relative fair value of warrants |
|
|
|
|
$ 59,883
|
|
|
37,679
|
|
|
|
|
|
|
|
|
|
60,427
|
|
|
|
|
17,437
|
78,011
|
|
|
|
|
117,533
|
|
|
|
|
|
|
|
|
|
|
|
|
| Relative fair value of warrants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
204,452
|
0
|
|
|
|
|
|
|
|
|
|
|
|
| Relative fair value |
|
|
|
$ 75,600
|
$ 40,117
|
|
|
|
|
|
|
|
|
|
|
$ 40,800
|
|
39,573
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accrued interests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
16,877
|
|
|
|
|
|
|
|
|
|
|
|
|
| Additional paid in capital, fair value of warrants |
|
|
|
|
|
|
|
$ 37,679
|
|
|
|
|
|
|
$ 75,600
|
|
|
$ 60,427
|
|
|
|
|
$ 17,437
|
$ 78,011
|
|
|
|
|
$ 108,100
|
|
|
|
|
|
|
|
|
|
|
|
|
| Conversion price |
$ 0.50
|
|
|
$ 1.25
|
|
|
|
|
$ 0.50
|
$ 0.50
|
$ 0.50
|
$ 1.25
|
$ 1.25
|
$ 1.25
|
$ 1.25
|
$ 1.25
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
$ 0.98
|
|
$ 0.50
|
$ 1.25
|
$ 1.25
|
$ 1.25
|
$ 1.25
|
$ 1.25
|
$ 1.25
|
|
|
$ 1.25
|
|
| Stock price |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 0.40
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 0.40
|
|
| Convertible debt |
|
|
|
$ 74,400
|
|
|
|
|
|
|
|
|
|
|
$ 74,400
|
$ 59,200
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 364,517
|
|
|
|
|
|
|
|
|
$ 33,910
|
|
|
|
| Convertible debts |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,546
|
|
|
|
| Original issue discount on beneficial conversion feature |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible debt discounted |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
108,100
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
200,000
|
|
|
|
0
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding note payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 200,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Maturity date |
|
|
|
Jul. 10, 2022
|
Mar. 31, 2022
|
|
|
|
|
|
|
|
|
|
Jul. 31, 2022
|
Jun. 23, 2022
|
|
Mar. 31, 2022
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3,724,891
|
3,334,891
|
|
|
|
|
|
|
|
|
|
|
|
| Gain/Loss on extinguishment |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 0
|
$ 0
|
(27,043)
|
0
|
|
|
|
|
|
|
|
|
|
|
|
| Debt instrument, maturity term |
|
|
2 years
|
|
|
|
|
|
|
|
|
|
|
2 years
|
|
|
|
|
|
|
|
1 year
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal owing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100,000
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
| December 23, 2021 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accrued interest |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 14,356
|
|
|
|
|
|
|
|
|
|
|
|
|
19,123
|
|
|
|
|
|
|
|
|
|
|
|
|
| Principal owing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100,000
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
| Relative fair value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 40,800
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Conversion price |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible debt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 59,200
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal owing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100,000
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
| One Convertible Notes [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 400,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| One Convertible Notes [Member] | July 25, 2021 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Beneficial conversion feature |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 467
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Conversion price |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible debt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 14,533
|
|
|
|
| Maturity date |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Jan. 25, 2022
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 15,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Two Convertible Notes [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 200,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accrued interest |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23,370
|
|
|
|
|
|
|
|
|
|
|
|
|
| Principal owing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100,000
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
| Accrued interests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
97,205
|
|
|
|
|
|
|
|
|
|
|
|
|
| Conversion price |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 600,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Debt instrument, maturity term |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 year
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrant exercise price |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest payments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0
|
20,000
|
|
|
|
|
|
|
|
|
|
|
|
| Warrants issued to purchase common shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
240,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal owing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 400,000
|
400,000
|
|
|
|
|
|
|
|
|
|
|
|
| Debt discount |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 12,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Two Convertible Notes [Member] | On July 1, 2020 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding note payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 400,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Description of debt instrument |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As the creditor has not granted a concession, the guidance contained in ASC 470-60 was applied. As present value of the cash flows under the new debt instrument differed by more than 10% from the present value of the remaining cash flows under the terms of the original debt instrument
|
|
|
|
|
|
|
|
|
|
|
|
|
| Gain/Loss on extinguishment |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 220,065
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrant exercise price |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shares received by each noteholder |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Fair value of debt amount extingushed |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 218,397
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common shares issued upon debt conversion |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
16,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Purchase additional common shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
320,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrants issued upon debt conversion |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
160,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Two Convertible Notes [Member] | On March 31, 2022 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Two Convertible Notes [Member] | On March 31, 2021 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding note payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 400,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Description of debt instrument |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As present value of the cash flows under the new debt instrument differed by more than 10% from the present value of the remaining cash flows under the terms of the original debt instrument
|
|
|
|
|
|
|
|
|
|
|
|
|
| Gain/Loss on extinguishment |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 20,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shares received by each noteholder |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Fair value of debt amount extingushed |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 20,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common shares issued upon debt conversion |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Two Convertible Notes [Member] | On October 1, 2021 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Two Convertible Notes [Member] | On November 1, 2020 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding note payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 400,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Description of debt instrument |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As present value of the cash flows under the new debt instrument differed by more than 10% from the present value of the remaining cash flows under the terms of the original debt instrument
|
|
|
|
|
|
|
|
|
|
|
|
|
| Gain/Loss on extinguishment |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 110,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shares received by each noteholder |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
50,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Fair value of debt amount extingushed |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 110,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common shares issued upon debt conversion |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Note J Convertible Notes [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 180,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Beneficial conversion feature |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 65,344
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Relative fair value of warrants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
68,578
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Additional paid in capital, fair value of warrants |
|
|
|
|
|
|
$ 22,564
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 68,578
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Conversion price |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 0.62
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Original issue discount on beneficial conversion feature |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 46,078
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 111,422
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Debt instrument, maturity term |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 year
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal owing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 180,000
|
180,000
|
|
|
|
|
|
|
|
|
|
|
|
| Accounts payable and accrued liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 42,016
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrants to purchase |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 180,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Note J Convertible Notes [Member] | On March 31, 2022 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Note J Convertible Notes [Member] | On November 1, 2021 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Note H Convertible Notes [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 35,000
|
|
|
|
|
|
|
|
|
|
$ 35,000
|
35,000
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Beneficial conversion feature |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 33,772
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Relative fair value of warrants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
43,914
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Relative fair value of convertible note |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
56,086
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Additional paid in capital, fair value of warrants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 43,914
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Conversion price |
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
| Original issue discount on beneficial conversion feature |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 22,314
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Debt instrument, maturity term |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 year
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal owing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100,000
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
| Accounts payable and accrued liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 7,336
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrants to purchase |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Note H Convertible Notes [Member] | On March 31, 2022 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Eight Convertible Notes [Member] | On March 31, 2022 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Three Convertible Notes [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Conversion price |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 163,341
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Debt instrument, maturity term |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 years
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrant exercise price |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrants issued to purchase common shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
130,673
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal owing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 63,341
|
63,341
|
|
|
|
|
|
|
|
|
|
|
|
| Interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Three Convertible Notes [Member] | On March 31, 2021 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding note payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 163,341
|
|
|
|
|
|
|
|
|
|
|
|
|
| Description of debt instrument |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As present value of the cash flows under the new debt instrument differed by more than 10% from the present value of the remaining cash flows under the terms of the original debt instrument
|
|
|
|
|
|
|
|
|
|
|
|
|
| Gain/Loss on extinguishment |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 8,268
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shares received by each noteholder |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8,268
|
|
|
|
|
|
|
|
|
|
|
|
|
| Fair value of debt amount extingushed |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 8,268
|
|
|
|
|
|
|
|
|
|
|
|
|
| Three Convertible Notes [Member] | On June 1, 2021 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Three Convertible Notes [Member] | On March 31, 2022 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Seven Convertible Notes [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accrued interest |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 164,712
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment Convertible notes payable |
|
|
|
|
|
$ 300,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal owing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
600,000
|
600,000
|
|
|
|
|
|
|
|
|
|
|
|
| Note I Convertible Notes [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Beneficial conversion feature |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 34,221
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Relative fair value of warrants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
38,507
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Additional paid in capital, fair value of warrants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 38,507
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Conversion price |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 0.61
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Original issue discount on beneficial conversion feature |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 27,272
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
61,493
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Debt instrument, maturity term |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 year
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal owing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100,000
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
| Accounts payable and accrued liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 24,274
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrants to purchase |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| common stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.00
|
|
|
| Note I Convertible Notes [Member] | On March 31, 2022 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Six Convertible Notes [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Simple interest rate |
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Beneficial conversion feature |
|
|
|
|
|
|
$ 457,436
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Relative fair value of warrants |
|
|
|
|
|
|
240,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment Convertible notes payable |
|
|
|
|
|
|
457,436
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Convertible notes payable |
|
|
|
|
|
|
$ 600,000
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Debt instrument, maturity term |
|
|
|
|
|
|
2 years
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrant exercise price |
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Relative fair values of convertible warrants |
|
|
|
|
|
|
$ 142,564
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Eaight Convertible Notes [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Beneficial conversion feature |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6,080
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accrued interest |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 52,521
|
|
|
|
|
|
|
|
|
|
|
|
|
| Relative fair value of warrants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7,904
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Relative fair value of convertible note |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10,096
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Additional paid in capital, fair value of warrants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 7,904
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Conversion price |
|
|
|
|
|
$ 0.70
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 18,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Debt instrument, maturity term |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 year
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal owing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
300,000
|
300,000
|
|
|
|
|
|
|
|
|
|
|
|
| Accounts payable and accrued liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,379
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal owing 1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
18,000
|
18,000
|
|
|
|
|
|
|
|
|
|
|
|
| Warrants to purchase |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 18,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Four Convertible Notes [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accrued interest |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10,706
|
|
|
|
|
|
|
|
|
|
|
|
|
| Conversion price |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment Convertible notes payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 37,613
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Debt instrument, maturity term |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 years
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrant exercise price |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrants issued to purchase common shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
30,090
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal owing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
37,613
|
37,613
|
|
|
|
|
|
|
|
|
|
|
|
| Interest rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Five Convertible Notes [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accrued interest |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
24,087
|
|
|
|
|
|
|
|
|
|
|
|
|
| Conversion price |
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repayment Convertible notes payable |
|
|
|
|
|
|
|
$ 85,937
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Debt instrument, maturity term |
|
|
|
|
|
|
|
2 years
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Warrants issued to purchase common shares |
|
|
|
|
|
|
|
68,750
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding principal owing |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 85,937
|
$ 85,937
|
|
|
|
|
|
|
|
|
|
|
|
| Interest rate |
|
|
|
|
|
|
|
10.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| X |
- References
+ Details
| Name: |
alid_AccountsPayableAndAccruedInterest |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_AccruedInterest |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_AccruedInterestIncludedInAccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_AdditionalPaidIn |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_ConvertibleDebts |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_ConvertibleNotesPayables |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_FairValueAdjustment |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_FairValueAdjustmentOfConvertibleNotes |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_FairValuesOfConvertibleNotesPayables |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_FairValuesOfWarrants |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_InterestPayments |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_InterestsPayable |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_OriginalIssueDiscountOnBeneficialConversionFeature |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_OutstandingPrincipalAmount |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_OutstandingPrincipalAmountone |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_OutstandingPrincipalOwing |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_PrincipalOwingEight |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_PrincipalOwingFive |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_PrincipalOwingFour |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_PrincipalOwingNine |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_PrincipalOwingOne |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_PrincipalOwingSeven |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_PrincipalOwingSix |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_PrincipalOwingThree |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_PrincipalOwingTwo |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_PurchaseAdditionalShares |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_RelativeFairValueOfConvertibleNote |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_RelativeFairValueOfWarrants |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_RelativeFairValuesOfConvertibleWarrants |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_StockPricePerShare |
| Namespace Prefix: |
alid_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_WarrantsIssuedToPurchaseCommonShares |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_WarrantsToPurchase |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities incurred and payable to vendors for goods and services received, and accrued liabilities classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(10)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
| Name: |
us-gaap_AccountsPayableAndOtherAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount per share of no-par value common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockNoParValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of new shares issued in the conversion of stock in a noncash (or part noncash) transaction. Noncash is defined as transactions during a period that do not result in cash receipts or cash payments in the period. "Part noncash" refers to that portion of the transaction not resulting in cash receipts or cash payments in the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_ConversionOfStockSharesIssued1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIncluding the current and noncurrent portions, carrying amount of debt identified as being convertible into another form of financial instrument (typically the entity's common stock) as of the balance sheet date, which originally required full repayment more than twelve months after issuance or greater than the normal operating cycle of the company. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_ConvertibleDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionIncluding the current and noncurrent portions, carrying value as of the balance sheet date of a written promise to pay a note, initially due after one year or beyond the operating cycle if longer, which can be exchanged for a specified amount of one or more securities (typically common stock), at the option of the issuer or the holder. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_ConvertibleNotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of the portion of long-term debt due within one year or the operating cycle if longer identified as Convertible Notes Payable. Convertible Notes Payable is a written promise to pay a note which can be exchanged for a specified amount of another, related security, at the option of the issuer and the holder. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_ConvertibleNotesPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionDividend or interest rate associated with the financial instrument issued in exchange for the original debt being converted in a noncash or part noncash transaction. Noncash are transactions that affect recognized assets or liabilities but that do not result in cash receipts or cash payments. Part noncash refers to that portion of the transaction not resulting in cash receipts or cash payments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_DebtConversionConvertedInstrumentRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of a favorable spread to a debt holder between the amount of debt being converted and the value of the securities received upon conversion. This is an embedded conversion feature of convertible debt issued that is in-the-money at the commitment date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-8
| Name: |
us-gaap_DebtInstrumentConvertibleBeneficialConversionFeature |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe price per share of the conversion feature embedded in the debt instrument. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-5
| Name: |
us-gaap_DebtInstrumentConvertibleConversionPrice1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIdentification of the lender and information about a contractual promise to repay a short-term or long-term obligation, which includes borrowings under lines of credit, notes payable, commercial paper, bonds payable, debentures, and other contractual obligations for payment. This may include rationale for entering into the arrangement, significant terms of the arrangement, which may include amount, repayment terms, priority, collateral required, debt covenants, borrowing capacity, call features, participation rights, conversion provisions, sinking-fund requirements, voting rights, basis for conversion if convertible and remarketing provisions. The description may be provided for individual debt instruments, rational groupings of debt instruments, or by debt in total. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(13))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 470
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480848/942-470-50-3
| Name: |
us-gaap_DebtInstrumentDescription |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFace (par) amount of debt instrument at time of issuance. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482900/835-30-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69B
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69C
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-2
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482949/835-30-55-8
| Name: |
us-gaap_DebtInstrumentFaceAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionEffective interest rate for the funds borrowed under the debt agreement considering interest compounding and original issue discount or premium. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482900/835-30-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-6
| Name: |
us-gaap_DebtInstrumentInterestRateEffectivePercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDate when the debt instrument is scheduled to be fully repaid, in YYYY-MM-DD format. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22(a)(2))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DebtInstrumentMaturityDate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod of time between issuance and maturity of debt instrument, in PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days.
| Name: |
us-gaap_DebtInstrumentTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after accumulated amortization, of debt discount. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 1A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-1A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482949/835-30-55-8
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1D
| Name: |
us-gaap_DebtInstrumentUnamortizedDiscount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionGross amount of debt extinguished.
| Name: |
us-gaap_ExtinguishmentOfDebtAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionDifference between the fair value of payments made and the carrying amount of debt which is extinguished prior to maturity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481303/470-50-40-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 50
-Section 40
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481303/470-50-40-4
| Name: |
us-gaap_GainsLossesOnExtinguishmentOfDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date of [accrued] interest payable on all forms of debt, including trade payables, that has been incurred and is unpaid. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InterestPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities incurred and payable to vendors for goods and services received classified as other, and expenses incurred but not yet paid, payable within one year or the operating cycle, if longer.
| Name: |
us-gaap_OtherAccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe aggregate amount paid by the entity to reacquire the right to purchase equity shares at a predetermined price, usually issued together with corporate debt. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsForRepurchaseOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow from the repayment of a long-term debt instrument which can be exchanged for a specified amount of another security, typically the entity's common stock, at the option of the issuer or the holder. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_RepaymentsOfConvertibleDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the form of debt having an initial term of less than one year or less than the normal operating cycle, if longer, average borrowings during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Regulation S-K (SK)
-Number 229
-Section 1402
-Paragraph (a)
-Publisher SEC
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Regulation S-K (SK)
-Number 229
-Section 1402
-Paragraph (b)
-Subparagraph (1)
-Publisher SEC
| Name: |
us-gaap_ShorttermDebtAverageOutstandingAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued attributable to transactions classified as other.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_DecemberTwentyThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShortTermDebtTypeAxis=alid_OneConvertibleNoteMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_JulyTwentyFiveTwoThousandTwentyTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShortTermDebtTypeAxis=alid_TwoConvertibleNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_OnJulyOneTwoThousandTwentyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_OnMarchThirtyOneTwoThousandTwentyTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_OnMarchThirtyOneTwoThousandTwentyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_OnOctoberOneTwoThousandTwentyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_OnNovemberOneTwoThousandTwentyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShortTermDebtTypeAxis=alid_ElevenConvertibleNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_OnNovemberOneTwoThousandTwentyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShortTermDebtTypeAxis=alid_NineConvertibleNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShortTermDebtTypeAxis=alid_EightConvertibleNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShortTermDebtTypeAxis=alid_ThreeConvertibleNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_OnJuneOneTwoThousandTwentyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_OnMarchThirtyOneTwoThousandTwentytwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShortTermDebtTypeAxis=alid_SevenConvertibleNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShortTermDebtTypeAxis=alid_TenConvertibleNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShortTermDebtTypeAxis=alid_SixConvertibleNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShortTermDebtTypeAxis=alid_EaightConvertibleNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShortTermDebtTypeAxis=alid_FourConvertibleNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShortTermDebtTypeAxis=alid_FiveConvertibleNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Equity (Detail Narrative) - USD ($)
|
|
|
|
|
1 Months Ended |
12 Months Ended |
|
|
Oct. 07, 2022 |
Feb. 10, 2022 |
Feb. 07, 2022 |
Sep. 02, 2021 |
May 16, 2023 |
Jan. 31, 2023 |
Aug. 23, 2022 |
Aug. 23, 2022 |
May 26, 2022 |
Mar. 07, 2022 |
Feb. 28, 2022 |
Feb. 25, 2022 |
Feb. 24, 2022 |
Feb. 22, 2022 |
Feb. 22, 2022 |
Feb. 21, 2022 |
Feb. 20, 2022 |
Feb. 07, 2022 |
Jan. 20, 2022 |
Nov. 24, 2021 |
Nov. 05, 2021 |
Oct. 20, 2021 |
Aug. 31, 2021 |
Mar. 26, 2021 |
Aug. 31, 2023 |
Aug. 31, 2022 |
Aug. 31, 2021 |
Jan. 28, 2022 |
Nov. 30, 2021 |
| Expense consulting fees |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 425,000
|
|
|
|
|
|
|
| Common stocks shares issued |
1,575,000
|
|
66,667
|
|
|
|
750,000
|
750,000
|
|
20,000
|
120,000
|
96,000
|
|
80,000
|
80,000
|
300,000
|
40,000
|
66,667
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
$ 0.40
|
|
$ 0.75
|
|
|
|
$ 0.40
|
$ 0.40
|
|
$ 1.25
|
$ 1.25
|
$ 1.25
|
|
$ 1.25
|
$ 1.25
|
$ 1.25
|
$ 1.25
|
$ 0.75
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
| Common stock shares reissued |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
750,000
|
|
|
| fair value -consulting services |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 637,500
|
|
|
|
|
|
|
| Proceed from issuance of common shares |
$ 630,000
|
|
$ 50,000
|
|
|
|
$ 300,000
|
$ 300,000
|
|
$ 25,000
|
$ 150,000
|
$ 120,000
|
$ 31,200
|
|
$ 100,000
|
$ 375,000
|
$ 50,000
|
$ 50,000
|
|
|
|
|
|
|
|
$ 368,000
|
|
|
|
| Stock issued for extending the maturity date of convertible note |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8,268
|
| Finders fee |
10,000
|
|
|
|
|
|
|
|
|
$ 4,000
|
12,000
|
$ 9,600
|
|
|
$ 8,000
|
$ 30,000
|
$ 4,000
|
|
|
|
|
|
|
|
|
|
|
|
|
| Issuance costs |
$ 2,792
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Proceed from issuance of common shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1,528,160
|
$ 3,877,634
|
|
|
|
| Common stock shares issued |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101,592,914
|
93,967,594
|
|
|
|
| Stock-based compensation |
|
|
|
|
$ 14,506
|
$ 80,739
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 3,047,156
|
$ 2,733,874
|
|
|
|
| Debt instrument, maturity term |
2 years
|
|
|
|
|
|
|
|
|
|
|
|
|
2 years
|
|
|
|
|
|
|
|
|
|
1 year
|
|
|
|
|
|
| On November 5, 2021 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 0.75
|
|
|
|
$ 1.25
|
|
|
|
|
| Finders fee |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 6,000
|
|
|
|
|
| Proceed from issuance of common shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 678,750
|
|
|
|
|
|
|
|
|
| Common stock shares issued |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
905,000
|
|
|
|
8,000
|
|
|
|
|
| Other's finder fee |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 42,654
|
|
|
|
|
| On November 24, 2021 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
$ 0.75
|
|
|
|
|
$ 1.25
|
|
|
|
|
| Proceed from issuance of common shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 27,000
|
|
|
|
|
|
|
|
|
|
| Common stock shares issued |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
36,000
|
|
|
|
|
|
|
|
|
|
| September 2, 2021 Two [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Expense consulting fees |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 212,500
|
|
|
| Proceed from issuance of common shares |
|
|
|
$ 3,371,892
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock shares issued |
|
|
|
2,997,237
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock-based compensation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 3,584,392
|
|
|
|
|
| Stock-based compensation consulting service |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 3,371,892
|
|
|
|
|
| September 21, 2022 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
$ 0.20
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 0.40
|
$ 0.20
|
|
|
|
| Proceed from issuance of common shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 540,000
|
|
|
|
|
| Common stock shares issued |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,350,000
|
80,000
|
|
|
|
| On October 20, 2021 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 0.75
|
|
|
$ 1.25
|
|
|
|
|
| Proceed from issuance of common shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 2,774,966
|
|
|
|
|
$ 865,467
|
|
|
| Common stock shares issued |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3,699,955
|
|
|
|
|
|
|
|
| Commission fees |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 210,736
|
|
|
|
|
| January 20, 2022 One [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 0.75
|
|
|
|
|
|
$ 1.25
|
|
|
$ 1.25
|
|
| Proceed from issuance of common shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 56,250
|
|
|
|
|
|
|
|
|
|
|
| Common stock shares issued |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
75,000
|
|
|
|
|
|
|
|
|
|
|
| January 28, 2022 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1.25
|
|
|
$ 0.75
|
|
| Proceed from issuance of common shares |
|
|
|
|
|
|
|
|
|
|
$ 50,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock shares issued |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
66,667
|
|
| February 10, 2022 One [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
|
$ 0.75
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1.25
|
|
|
|
|
| Proceed from issuance of common shares |
|
$ 25,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock shares issued |
|
33,334
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| February 10, 2022 Two [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1.25
|
|
|
|
|
| Proceed from issuance of common shares |
|
$ 100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock shares issued |
|
80,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Fnder fee |
|
$ 8,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| February 24, 2022 One [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
|
|
|
|
|
|
|
|
|
|
|
|
$ 0.75
|
|
|
|
|
|
|
|
|
|
|
|
1.25
|
|
|
|
|
| Proceed from issuance of common shares |
|
|
|
|
|
|
|
|
|
|
|
|
$ 31,200
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock shares issued |
|
|
|
|
|
|
|
|
|
|
|
|
41,600
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| February 24, 2022 Two [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
|
|
|
|
|
|
|
1.25
|
|
|
|
|
| Finders fee |
|
|
|
|
|
|
|
|
|
|
|
|
$ 4,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Proceed from issuance of common shares |
|
|
|
|
|
|
|
|
|
|
|
|
$ 50,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock shares issued |
|
|
|
|
|
|
|
|
|
|
|
|
40,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| May 26, 2022 One [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
|
|
|
|
|
|
|
|
$ 0.75
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
| Proceed from issuance of common shares |
|
|
|
|
|
|
|
|
$ 100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock shares issued |
|
|
|
|
|
|
|
|
133,333
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| On October 7, 2022 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Proceed from issuance of common shares |
$ 41,966
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 44,606
|
|
|
|
|
| Common stock shares issued |
70,560
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
75,000
|
|
|
|
|
| Accounts payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 30,000
|
|
|
|
|
| Loss on settlement |
$ 12,437
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 14,606
|
|
|
|
|
| Debt |
$ 29,529
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| August 21, 2023 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 0.20
|
|
|
|
|
| Proceed from issuance of common shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 305,000
|
|
|
|
|
| Common stock shares issued |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,525,000
|
|
|
|
|
| September 2, 2021 One [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Proceed from issuance of common shares |
|
|
|
$ 2,447,925
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock shares issued |
|
|
|
2,175,933
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock-based compensation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 4,585,425
|
|
|
|
|
| Fair value of common share |
|
|
|
$ 2,137,500
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| May 26, 2022 Two [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
|
|
|
|
|
|
|
|
$ 0.75
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 1.25
|
|
|
|
|
| Proceed from issuance of common shares |
|
|
|
|
|
|
|
|
$ 50,800
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock shares issued |
|
|
|
|
|
|
|
|
67,733
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| August 31, 2023 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
|
|
|
|
|
|
|
|
$ 0.75
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 0.20
|
|
|
|
|
| Proceed from issuance of common shares |
|
|
|
$ 2,447,925
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 605,952
|
|
|
|
|
| Common stock shares issued |
|
|
|
2,175,933
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3,029,760
|
|
|
|
|
| Reissued shares of common stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 750,000
|
|
|
|
| Fair value of consulting services |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
637,500
|
|
|
|
| Expensed as consulting fee |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
425,000
|
|
|
|
| Deferred compensation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
212,500
|
|
|
|
| Share subscriptions |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 540,000
|
|
|
|
| May 27, 2021 [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Price per share |
|
$ 0.75
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 0.20
|
|
|
|
|
| Common stock shares issued |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,000,000
|
|
|
|
|
| Risk-free rate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.87%
|
|
|
|
|
| Fair value of in connection with modifications of convertible note payable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 204,452
|
|
|
|
|
| Volatility |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
160.87%
|
|
|
|
|
| Debt instrument, maturity term |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 years
|
|
|
|
|
| X |
- References
+ Details
| Name: |
alid_CommissionFees |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_CommonStockSharesReissued |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_CommonStocksSharesIssued1 |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_ConsultingfeesExpense |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_ExpensedAsConsultingFee |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_FairValueConsultingServices |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_FairValueOfCommonShare |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_FairValueOfConsultingServices |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_FairValueOfInConnectionWithModificationsOfConvertibleNotePayable |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_FindersFee |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_FnderFee |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_OthersFinderFee |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_ProceedFromIssuanceOfCommonShares |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_ReissuedSharesOfCommonStock |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_StockBasedCompensationConsultingService |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_StockIssuedForExtendingTheMaturityDateOfConvertibleNote |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_Volatility |
| Namespace Prefix: |
alid_ |
| Data Type: |
num:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionMonetary value of common stock allocated to investors to buy shares of a new issue of common stock before they are offered to the public. When stock is sold on a subscription basis, the issuer does not initially receive the total proceeds. In general, the issuer does not issue the shares to the investor until it receives the entire proceeds. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481142/505-10-45-2
| Name: |
us-gaap_CommonStockSharesSubscriptions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionFor an entity that discloses a concentration risk in relation to quantitative amount, which serves as the "benchmark" (or denominator) in the equation, this concept represents the concentration percentage derived from the division. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 21
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-21
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-20
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-18
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-20
| Name: |
us-gaap_ConcentrationRiskPercentage1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of debt and lease obligation, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DebtCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPeriod of time between issuance and maturity of debt instrument, in PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days.
| Name: |
us-gaap_DebtInstrumentTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued under share-based plans to employees or officers which is the unearned portion, accounted for under the fair value method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 35
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.30)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DeferredCompensationEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of litigation expense, including but not limited to legal, forensic, accounting, and investigative fees.
| Name: |
us-gaap_LitigationSettlementExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow paid to third parties in connection with debt origination, which will be amortized over the remaining maturity period of the associated long-term debt. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsOfDebtIssuanceCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or per unit amount of equity securities issued.
| Name: |
us-gaap_SharesIssuedPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=alid_OnNovemberFiveTwoThousandTwentyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=alid_OnNovemberTwentyFourTwoThousandTwentyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_SeptemberTwoTwoThousandTwentyOneTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_SeptemberTwentyOneTwoThousandTwentyTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_OnOctoberTwentyTwoThousandTwentyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_JanuaryTwentyTwoThousandTwentyTwoOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_JanuaryTwentyEightThousandTwentyTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_FebruaryTenTwothousandTwentyTwoOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_FebruaryTenTwoThousandTwentyTwoTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_FebruaryTwnetyFourTwoThousandTwentyTwoOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_FebruaryTwnetyFourTwoThousandTwentyTwoTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_MayTwentySixTwoThousandTwentyTwoOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_OctoberSevenTwoThousandTwentyTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_AugustTwoOneThousandTwentyThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_SeptemberTwoTwoThousandTwentyOneOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_MayTwentySixTwoThousandTwentyTwoTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_AugustThirtyOneTwoThousandTwentyTwoOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=alid_MayTwentySevenTwoThousandTwentyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- References
+ Details
| Name: |
alid_ConsultingFeesAndBenefits |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.3
Related party transactions and balances (Details 1) - USD ($)
|
Aug. 31, 2023 |
Aug. 31, 2022 |
| Amounts due from related party |
$ 693,292
|
$ 384,272
|
| CEO and Director [Member] |
|
|
| Amounts due from related party |
156,028
|
80,892
|
| COO and Director [Member] |
|
|
| Amounts due from related party |
201,612
|
143,816
|
| CFO [Member] |
|
|
| Amounts due from related party |
84,962
|
46,387
|
| An EntityC ontrolled By A Director [Member] |
|
|
| Amounts due from related party |
159,690
|
60,677
|
| Director [Member] |
|
|
| Amounts due from related party |
$ 91,000
|
$ 52,500
|
v3.23.3
Related party transactions and balances (Details Narrative) - USD ($)
|
|
1 Months Ended |
12 Months Ended |
Sep. 02, 2021 |
May 16, 2023 |
Jan. 31, 2023 |
Aug. 31, 2023 |
Aug. 31, 2022 |
| Due to related party |
|
|
|
$ 693,292
|
$ 384,272
|
| Bonus Shares |
|
$ 14,506
|
$ 80,739
|
$ 3,047,156
|
2,733,874
|
| COO and Director [Member] |
|
|
|
|
|
| Issue of common share treasury fair value |
$ 1,997,925
|
|
|
|
|
| Issue of common shares |
1,775,933
|
|
|
|
|
| CFO [Member] |
|
|
|
|
|
| Issue of common share treasury fair value |
$ 450,000
|
|
|
|
|
| Issue of common shares |
400,000
|
|
|
|
|
| CFO and COO [Member] |
|
|
|
|
|
| Bonus Shares |
|
|
|
|
$ 2,447,925
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of treasury shares or units reissued. Excludes reissuance of shares or units in treasury for award under share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesTreasuryStockReissued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the cost of common and preferred stock that were repurchased during the period. Recorded using the cost method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481549/505-30-45-1
| Name: |
us-gaap_TreasuryStockValueAcquiredCostMethod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
| X |
- References
+ Details
| Name: |
alid_BalanceinUSdollars |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_NetExposure |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_BalanceSheetLocationAxis=alid_CanadianDollarExchangeRateMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Financial risk factors (Details 1) - USD ($)
|
Aug. 31, 2023 |
Aug. 31, 2022 |
| Accounts payable |
$ (2,393,359)
|
$ (1,607,862)
|
| Colombian Peso exchange rate [Member] |
|
|
| Cash and cash equivalents |
415,100,982
|
|
| Other receivables |
539,704,008
|
|
| Accounts payable |
(3,669,893,803)
|
|
| Net exposure |
(2,715,088,813)
|
|
| Balance in US dollars |
$ (664,234)
|
|
| X |
- References
+ Details
| Name: |
alid_BalancesInUsDollars |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_NetExposures |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance, of receivables classified as other, due within one year or the operating cycle, if longer.
| Name: |
us-gaap_OtherReceivablesNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_BalanceSheetLocationAxis=alid_ColombianPesoExchangeRateMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_BalanceSheetLocationAxis=alid_CanadianDollarExchangeRateMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BalanceSheetLocationAxis=alid_ColombianPesoExchangeRateMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionAmount of liability recognized arising from contingent consideration in a business combination. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479581/805-30-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 30
-Section 25
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479668/805-30-25-6
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 35
-Paragraph 1
-Subparagraph (b)
-SubTopic 30
-Topic 805
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479613/805-30-35-1
| Name: |
us-gaap_BusinessCombinationContingentConsiderationLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Share purchase warrants (Details) - Warrants [Member]
|
12 Months Ended |
|
Aug. 31, 2023
$ / shares
shares
|
|---|
| Number of warrants, Beginning balance | shares |
7,704,135
|
| Number of warrants, issued | shares |
1,000,000
|
| Number of warrants, expired | shares |
(3,245,135)
|
| Ending balance | shares |
5,459,000
|
| Weighted average exercise price, Beginning balance | $ / shares |
$ 1.23
|
| Weighted average exercise price, issued | $ / shares |
0.20
|
| Weighted average exercise price, expired | $ / shares |
1.25
|
| Weighted average exercise price, Ending balance | $ / shares |
$ 1.06
|
| X |
- References
+ Details
| Name: |
alid_EndingBalance |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_NumberOfWarrantsBeginningBalance |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionExpirationsInPeriod |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionIssuedInPeriod |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_WeightedAverageExercisePriceBeginningBalance |
| Namespace Prefix: |
alid_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_WeightedAverageExercisePriceEndingBalance |
| Namespace Prefix: |
alid_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_WeightedAverageExercisePriceexpired |
| Namespace Prefix: |
alid_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_WeightedAverageExercisePriceissued |
| Namespace Prefix: |
alid_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=alid_WarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Share purchase warrants (Details 1)
|
Aug. 31, 2023
$ / shares
shares
|
| Number of warrant |
5,459,000
|
| Warrant 1 [Member] |
|
| Number of warrant |
2,545,999
|
| Weighted average exercise price | $ / shares |
$ 1.25
|
| Expiry date |
Oct. 20, 2023
|
| Warrant 2 [Member] |
|
| Number of warrant |
905,000
|
| Weighted average exercise price | $ / shares |
$ 1.25
|
| Expiry date |
Nov. 06, 2023
|
| Warrant 3 [Member] |
|
| Number of warrant |
36,000
|
| Weighted average exercise price | $ / shares |
$ 1.25
|
| Expiry date |
Nov. 24, 2023
|
| Warrant 4 [Member] |
|
| Number of warrant |
66,667
|
| Weighted average exercise price | $ / shares |
$ 1.25
|
| Expiry date |
Jan. 29, 2024
|
| Warrant 5 [Member] |
|
| Number of warrant |
66,667
|
| Weighted average exercise price | $ / shares |
$ 1.25
|
| Expiry date |
Feb. 07, 2024
|
| Warrant Six [Member] |
|
| Number of warrant |
113,334
|
| Weighted average exercise price | $ / shares |
$ 1.25
|
| Expiry date |
Feb. 12, 2024
|
| Warrant 7 [Member] |
|
| Number of warrant |
40,000
|
| Weighted average exercise price | $ / shares |
$ 1.25
|
| Expiry date |
Feb. 20, 2024
|
| Warrant 8 [Member] |
|
| Number of warrant |
300,000
|
| Weighted average exercise price | $ / shares |
$ 1.25
|
| Expiry date |
Feb. 21, 2024
|
| Warrant 9 [Member] |
|
| Number of warrant |
177,600
|
| Weighted average exercise price | $ / shares |
$ 1.25
|
| Expiry date |
Feb. 26, 2024
|
| Warrant 10 [Member] |
|
| Number of warrant |
120,000
|
| Weighted average exercise price | $ / shares |
$ 1.25
|
| Expiry date |
Feb. 28, 2024
|
| Warrant 11 [Member] |
|
| Number of warrant |
20,000
|
| Weighted average exercise price | $ / shares |
$ 1.25
|
| Expiry date |
Mar. 07, 2024
|
| Warrant 12 [Member] |
|
| Number of warrant |
67,733
|
| Weighted average exercise price | $ / shares |
$ 1.25
|
| Expiry date |
May 27, 2024
|
| Warrant 13 [Member] |
|
| Number of warrant |
1,000,000
|
| Weighted average exercise price | $ / shares |
$ 0.20
|
| Expiry date |
Jul. 30, 2025
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionExpiration date of outstanding warrant and right embodying unconditional obligation requiring redemption by transferring asset at specified or determinable date or upon event certain to occur, in YYYY-MM-DD format. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_WarrantsAndRightsOutstandingMaturityDate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=alid_WarrantOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=alid_WarrantTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=alid_WarrantThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=alid_WarrantFourMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=alid_WarrantFiveMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=alid_WarrantSixMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=alid_WarrantSevenMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=alid_WarrantEightMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=alid_WarrantNineMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=alid_WarrantTenMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=alid_WarrantElevenMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=alid_WarrantTwelveMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=alid_WarrantThirteenMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Stock options (Details)
|
12 Months Ended |
|
Aug. 31, 2023
$ / shares
shares
|
|---|
| Stock Options |
|
| Number of warrants, Beginning balance |
6,000,000
|
| Number of options, granted |
6,140,000
|
| Ending balance |
12,140,000
|
| Number of options, Exercisable balance |
11,456,667
|
| Weighted Average exercise price, Beginnning balance | $ / shares |
$ 0.44
|
| Weighted Average exercise price, granted | $ / shares |
0.24
|
| Weighted Average exercise price, exercisable balance | $ / shares |
0.25
|
| Weighted average exercise price, Ending balance | $ / shares |
$ 0.25
|
| Weighted average contractual term, beginning |
3 years 10 months 6 days
|
| Weighted average contractual term, granted |
4 years 7 months 17 days
|
| Weighted average contractual term, outstanding |
3 years 8 months 12 days
|
| Weighted average contractual term, exercisable |
3 years 8 months 12 days
|
| Aggregate Intrinsic value, Beginning |
144,000
|
| Aggregate Intrinsic value, outstanding balance |
55,900
|
| Aggregate Intrinsic value, exercisable balance |
48,400
|
| X |
- References
+ Details
| Name: |
alid_AggregateIntrinsicValueBeginning |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_AggregateIntrinsicValueExercisableBalance |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_AggregateIntrinsicValueOutstandingBalance |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_WeightedAverageContractualTermBeginning |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_WeightedAverageContractualTermExercisable |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_WeightedAverageContractualTermOutstanding |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_WeightedAverageExercisePriceBeginnningBalance |
| Namespace Prefix: |
alid_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_vcygfjfj |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNet number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average grant-date fair value of options granted during the reporting period as calculated by applying the disclosed option pricing methodology. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options that were terminated. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Stock options (Details Narrative) - USD ($)
|
1 Months Ended |
12 Months Ended |
May 16, 2023 |
Jan. 31, 2023 |
Aug. 31, 2023 |
Aug. 31, 2022 |
| Unrecognized compensation costs- related to non-vested stock-based compensation |
|
|
$ 373,838
|
|
| Bonus Shares |
$ 14,506
|
$ 80,739
|
$ 3,047,156
|
$ 2,733,874
|
| Options unexercised |
|
4,900,000
|
6,000,000
|
|
| Options repriced |
|
|
$ 0.24
|
|
| Minimum [Member] |
|
|
|
|
| Options unexercised repriced |
|
$ 0.75
|
|
|
| Maximum [Member] |
|
|
|
|
| Options unexercised repriced |
|
0.825
|
|
|
| Consultants and ambassadors [Member] |
|
|
|
|
| Options repriced |
|
0.22
|
|
|
| Directors and officers [Member] |
|
|
|
|
| Options repriced |
|
$ 0.25
|
|
|
| X |
- References
+ Details
| Name: |
alid_OptionsRepriced |
| Namespace Prefix: |
alid_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_OptionsUnexercised |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_OptionsUnexercisedRepriced |
| Namespace Prefix: |
alid_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cost to be recognized for option under share-based payment arrangement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedStockOptions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Noncash activities (Details) - USD ($)
|
12 Months Ended |
Aug. 31, 2023 |
Aug. 31, 2022 |
| Relative fair value of warrants issuable with convertible note |
$ 204,452
|
$ 0
|
| Non-Cash Activities [Member] |
|
|
| Shares issued from treasury for services |
|
7,957,316
|
| Fair value of warrants issued as finder's fee |
204,452
|
$ 0
|
| Beneficial conversion feature |
0
|
281,447
|
| Relative fair value of warrants issuable with convertible note |
0
|
120,310
|
| Fair value of shares issued on modification of convertible note |
0
|
129,952
|
| Fair value of shares issuable on modification of debt |
$ 0
|
$ 8,268
|
| X |
- References
+ Details
| Name: |
alid_DebtInstrumentNonCashConvertibleBeneficialConversionFeature |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_FairValueOfSharesIssuableOnModificationOfDebt |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_FairValueOfSharesIssuedOnModificationOfConvertibleNote |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_FairValueOfWarrantsIssuedAsFinderSFee |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
alid_SharesIssuedFromTreasuryForServices |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementOperatingActivitiesSegmentAxis=alid_NonCashActivitiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Segment disclosure (Details) - USD ($)
|
12 Months Ended |
Aug. 31, 2023 |
Aug. 31, 2022 |
| Gross sales |
$ (1,461,767)
|
|
| Depreciation and amortization |
137,046
|
|
| Total assets |
2,063,728
|
$ 5,789,607
|
| Net losses |
(10,675,671)
|
$ (15,538,853)
|
| Allied Colombia [Member] |
|
|
| Gross sales |
(1,465,566)
|
|
| Depreciation and amortization |
70,562
|
|
| Total assets |
1,344,713
|
|
| Net losses |
(2,552,512)
|
|
| Allied [Member] |
|
|
| Gross sales |
3,799
|
|
| Depreciation and amortization |
66,484
|
|
| Total assets |
719,015
|
|
| Net losses |
$ (8,123,159)
|
|
| X |
- References
+ Details
| Name: |
alid_GrossSales |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe current period expense charged against earnings on long-lived, physical assets not used in production, and which are not intended for resale, to allocate or recognize the cost of such assets over their useful lives; or to record the reduction in book value of an intangible asset over the benefit period of such asset; or to reflect consumption during the period of an asset that is not used in production. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_DepreciationAndAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsegmentsAxis=alid_AlliedColombiaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsegmentsAxis=alid_AlliedMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Segment disclosure (Details 1)
|
12 Months Ended |
|
Aug. 31, 2023
USD ($)
|
|---|
| Total long lived assets |
$ (1,461,767)
|
| Revenue |
2,063,728
|
| Canada [Member] |
|
| Total long lived assets |
719,015
|
| Revenue |
3,799
|
| Colombia [Member] |
|
| Total long lived assets |
1,344,713
|
| Revenue |
$ (1,465,566)
|
| X |
- References
+ Details
| Name: |
alid_LongLivedAssets |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
alid_Revenue |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.3
Income Taxes (Details) - USD ($)
|
Aug. 31, 2023 |
Aug. 31, 2022 |
| Deferred tax asset: |
|
|
| Net operating loss carry-forward |
$ 5,095,000
|
$ 3,125,000
|
| Less: valuation allowance |
(5,095,000)
|
(3,125,000)
|
| Net deferred income tax asset |
$ 0
|
$ 0
|
| X |
- DefinitionAmount after allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible operating loss carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsOperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax assets for which it is more likely than not that a tax benefit will not be realized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsValuationAllowance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
v3.23.3
| X |
- References
+ Details
| Name: |
alid_NetOperatingLossCarryforwardExpiryDate1 |
| Namespace Prefix: |
alid_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of operating loss carryforward, before tax effects, available to reduce future taxable income under enacted tax laws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_OperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.3
| X |
- DefinitionPrice of a single share of a number of saleable stocks of a company.
| Name: |
us-gaap_SharePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued in lieu of cash for services contributed to the entity. Number of shares includes, but is not limited to, shares issued for services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued in lieu of cash for services contributed to the entity. Value of the stock issued includes, but is not limited to, services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodValueIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Allied (QB) (USOTC:ALID)
Historical Stock Chart
From Jan 2025 to Feb 2025
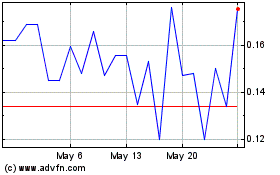
Allied (QB) (USOTC:ALID)
Historical Stock Chart
From Feb 2024 to Feb 2025