false
0001860657
0001860657
2024-02-27
2024-02-27
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE
COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section
13 OR 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of
earliest event reported): February 27, 2024
ALLARITY THERAPEUTICS,
INC.
(Exact name of registrant
as specified in our charter)
| Delaware |
|
001-41160 |
|
87-2147982 |
(State or other jurisdiction
of incorporation) |
|
(Commission File Number) |
|
(IRS Employer
Identification No.) |
|
24 School Street, 2nd Floor
Boston, MA |
|
02108 |
| (Address of principal executive offices) |
|
(Zip Code) |
(401) 426-4664
(Registrant’s telephone
number, including area code)
Check the appropriate
box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following
provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities
Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange
Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under
the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under
the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered
pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share |
|
ALLR |
|
The Nasdaq Stock Market LLC |
Indicate by check mark
whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter)
or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
☒
If an emerging growth
company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or
revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 1.01 Entry into
a Material Definitive Agreement.
Amendment to Senior
Convertible Notes
On
February 27, 2024, Allarity Therapeutics, Inc., a Delaware corporation (“we,” “our,” or the “Company”)
and 3i, LP, a Delaware limited partnership, (the “Holder” and together with us, the “Parties”) entered into an
Amendment to Senior Convertible Notes (the “Amendment”) to two senior convertible notes dated as of January 18, 2024 and February
13, 2024 (each a “Note” and collectively the “Notes”).
The
Parties amended Section 4(c)(vi) of the Notes to clarify that the Holder cannot convert, nor can we issue our shares of common stock,
if such issuance would necessitate stockholder approval by our trading market or exceed 19.9% of the shares of common stock. Except as
so amended, all of the terms relating to the Notes continue in full force and effect.
The description of the Amendment
is qualified in its entirety by the full text of the Amendment, a copy of which is filed herewith as Exhibit 10.1, and which is incorporated
herein by reference.
Item 7.01 Regulation FD Disclosure.
On
March 1, 2024, we provided updated corporate presentation slides about the Company (the “Corporate Presentation”) on the Company’s
website. The Corporate Presentation may be used in presentations to investors, analysts and others. A copy of the Corporate Presentation
is attached as Exhibit 99.1 to this Current Report on Form 8-K.
The
information reported under Item 7.01 in this Current Report on Form 8-K, and Exhibit 99.1 attached hereto are being “furnished”
and shall not be deemed filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)
or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities
Act of 1933, as amended, or the Exchange Act, regardless of any general incorporation language in such filing. This Current Report on
Form 8-K will not be deemed an admission as to the materiality of any information contained herein.
Item 9.01 Financial Statements and Exhibits.
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on our behalf by the
undersigned hereunto duly authorized.
| |
Allarity Therapeutics, Inc. |
| Date: March 1, 2024 |
By: |
/s/ Thomas Jensen |
| |
|
Thomas Jensen |
| |
|
Chief Executive Officer |
2
Exhibit 10.1
AMENDMENT TO SENIOR CONVERTIBLE NOTES
This Amendment to Senior
Convertible Notes (this “Amendment”) is made and entered into as of February 27, 2024 (“Effective Date”),
by and between Allarity Therapeutics, Inc., a Delaware corporation (the “Company”), and 3i, LP, a Delaware
limited partnership (the “Holder”). The Company and the Holder are sometimes hereinafter referred to as the “Parties.”
The Holder and the Company
are parties to a Securities Purchase Agreement, dated as of January 18, 2024 (the “Purchase Agreement”). Pursuant to
the Purchase Agreement, the Holder has paid an aggregate of $800,000, as evidenced by (a) the Senior Convertible Note, dated January
18, 2024 (the “January Note”), in the principal amount of $440,000 and (b) the Senior Convertible Note, dated
February 13, 2024, in the principal amount of $440,000 (the “February Note” and, together with the January Note, the
“Notes”). The purpose of this Amendment is to set forth the Parties mutual agreements and understandings with respect
to the amendments and modifications to Section 4(c)(vi) of the Notes.
NOW, THEREFORE, in consideration
of the mutual covenants and agreements hereinafter set forth and for other good and valuable consideration, the receipt and sufficiency
of which are hereby acknowledged, the Parties agree as follows:
A. Section 4(c)(vi) of the
Notes is hereby amended by deleting it in its entirety and inserting in lieu thereof the following:
“vi. Reservation
of Shares Issuable Upon Conversion. The Company covenants that it will at all times reserve and keep available out of its
authorized and unissued shares of Common Stock for the sole purpose of issuance upon conversion of this Note and payment of interest
on this Note, each as herein provided, free from preemptive rights or any other actual contingent purchase rights of Persons other
than the Holder (and the other holders of the Notes), not less than such aggregate number of shares of the Common Stock as shall
(subject to the terms and conditions set forth in the Purchase Agreement) be issuable (taking into account the adjustments and
restrictions of Section 5) upon the conversion of the then outstanding principal amount of this Note and payment of interest
hereunder. Notwithstanding the foregoing, the Holder may not effectuate any Conversion and the Company may not issue any shares of
Common Stock in connection therewith that would trigger any Trading Market requirement to obtain stockholder approval prior to a
Conversion or any issuance of shares of Common Stock in connection therewith that would be in excess of that number of shares of
Common Stock equivalent to 19.9% of the number of shares of Common Stock as of the Effective Date; provided, however,
that the Holder may effectuate any Conversion and the Company shall be obligated to issue shares of Common Stock in connection
therewith that would not trigger such a requirement. This restriction shall be of no further force or effect upon the approval of
the stockholders in compliance with stockholder voting requirements of the applicable Trading Market rules. The Company covenants
that all shares of Common Stock that shall be so issuable shall, upon issue, be duly authorized, validly issued, fully paid and
nonassessable and, if the Registration Statement is then effective under the Securities Act, shall be registered for public resale
in accordance with such Registration Statement.”
B. This Amendment shall be
construed and enforced in accordance with the laws of the State of Delaware, without regard to its principles of conflict of laws.
C. This Amendment may be
executed in any number of counterparts (including facsimile or PDF counterparts), each of which will be deemed an original, but all of
which together will constitute one and the same instrument.
D. Except as modified and
amended hereby, the Notes shall remain in full force and effect.
[Signature Page Follows]
IN WITNESS WHEREOF, the
Parties have caused this Amendment to be executed as of the date first written above by their respective officers thereunto duly authorized.
| |
ALLARITY THERAPEUTICS, INC. |
| |
|
|
| |
By: |
/s/ Thomas Jensen |
| |
Name: |
Thomas Jensen |
| |
Title: |
Chief Executive Officer |
| |
|
|
| |
By: |
3i Management, LLC |
| |
Its: |
General Partner |
| |
|
|
| |
By: |
/s/ Maier J. Tarlow |
| |
Name: |
Maier J. Tarlow |
| |
Title: |
Manager |
Exhibit 99.1

Copyright © 2023 Allarity Therapeutics. All rights reserved. Nasdaq: ALLR A Gene Expression Based Biomarker For Predicting Response To Treatment Thomas Jensen, CEO Feb 2024

2 Allarity Therapeutics Legal Statement This presentation is provided for informational purposes only and is subject to change. The information contained herein does not purport to be all - inclusive. The data contained herein is derived from various internal and external sources, and may rely upon assumptions, stated or otherwise, and forward - looking statements discussed below. No representation is made as to the reasonableness of the assumptions made within or the accuracy or completeness of any forward - looking statements, modeling or any other information contained herein. Allarity Therapeutics, Inc. (collectively “Allarity Therapeutics,” “Allarity,” or the “Company”) assume no obligation to update the information in this presentation. This material is not for the benefit of, and does not convey any rights or remedies for the benefit of, any holder of securities or any other person. This material is not intended to provide the basis for evaluation of any transaction and does not purport to contain all information that may be required and should not be considered a recommendation or opinion of any kind with respect to any transaction. No Offer or Solicitation. This material shall not constitute an offer to sell or a solicitation of an offer to buy any securities, nor shall there be any sale of such securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of such state or jurisdiction. No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended. Forward - Looking Statements. This presentation contains “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward - looking statements provide Allarity’s current expectations or forecasts of future events. The words “anticipates,” “believe,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predicts,” “project,” “should,” “would” and similar expressions may identify forward - looking statements, but the absence of these words does not mean that a statement is not forward - looking. These forward - looking statements include, but are not limited to, statements relating to estimated time periods for interim data read outs for our ongoing and prospective clinical trials and value inflection points, any resubmission of the NDA for dovitinib and PMA for the drug - specific DRP ® companion diagnostic for dovitinib, and ongoing clinical trials for stenoparib and IXEMPRA ® . Any forward - looking statements in this presentation are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward - looking statements. These risks and uncertainties include, but are not limited to, the risk that that the Company is unable to raise sufficient capital to fund its ongoing and prospective clinical trials and operations, the risk that results of a clinical study do not necessarily predict final results and that one or more of the clinical outcomes may materially change following more comprehensive reviews of the data, and as more patient data become available, the risk that results of a clinical study are subject to interpretation and additional analyses may be needed and/or may contradict such results, the receipt of regulatory approval for dovitinib, the drug - specific DRP ® companion diagnostic for dovitinib, or any of our other therapeutic candidates or, if approved, the successful commercialization of such products, the risk of cessation or delay of any of the ongoing or planned clinical trials and/or our development of our product candidates, the risk that the results of previously conducted studies will not be repeated or observed in ongoing or future studies involving our therapeutic candidates, and the risk that the current COVID - 19 pandemic will impact the Company’s current and future clinical trials and the timing of the Company’s preclinical studies and other operations. For a discussion of other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward - looking statements, see the section entitled “Risk Factors” in our annual report on Form 10 - K on file with the Securities and Exchange Commission, available at the Securities and Exchange Commission’s website at www.sec.gov , and as well as discussions of potential risks, uncertainties and other important factors in the Company’s subsequent filings with the Securities and Exchange Commission. All information in this presentation is as of the date of the presentation , and the Company undertakes no duty to update this information unless required by law. Any financial projections in this presentation are forward - looking statements that are based on assumptions that are inherently subject to significant uncertainties and contingencies, many of which are beyond Allarity’s control. While all projections are necessarily speculative, Allarity believes that the preparation of prospective financial information involves increasingly higher levels of uncertainty the further out the projection extends from the date of preparation. The assumptions and estimates underlying the projected results are inherently uncertain and are subject to a wide variety of significant business, economic and competitive risks and uncertainties that could cause actual results to differ materially from those contained in the projections. The inclusion of projections in this communication should not be regarded as an indication that Allarity or their representatives, considered or consider the projections to be a reliable prediction of future events.

Our DRP ® Companion Diagnostics Platform

DRP ® Supplements Conventional Biomarker Technology Conventional Biomarker Approaches work for some, but not all oncology drugs Gene Mutation Sequencing Some drugs can be predicted with a single or a few mutations Drug Target Analysis Focus on drug with single target Artificial Intelligence or Machine Learning Approaches Lacks clinical validation Allarity’s DRP ® develops predictive biomarkers based upon complex gene expression analysis Broadly Applicable Extensively Published First - In - Class Retrospectively & Prospectively Validated Regulatory Acceptable Trusted By Clinical collaborators * DRP® is based on comprehensive, tumor cell transcriptome data from actual cancer patients. * DRP® is not based on data mining, AI, or database analysis. Allarity Therapeutics 4
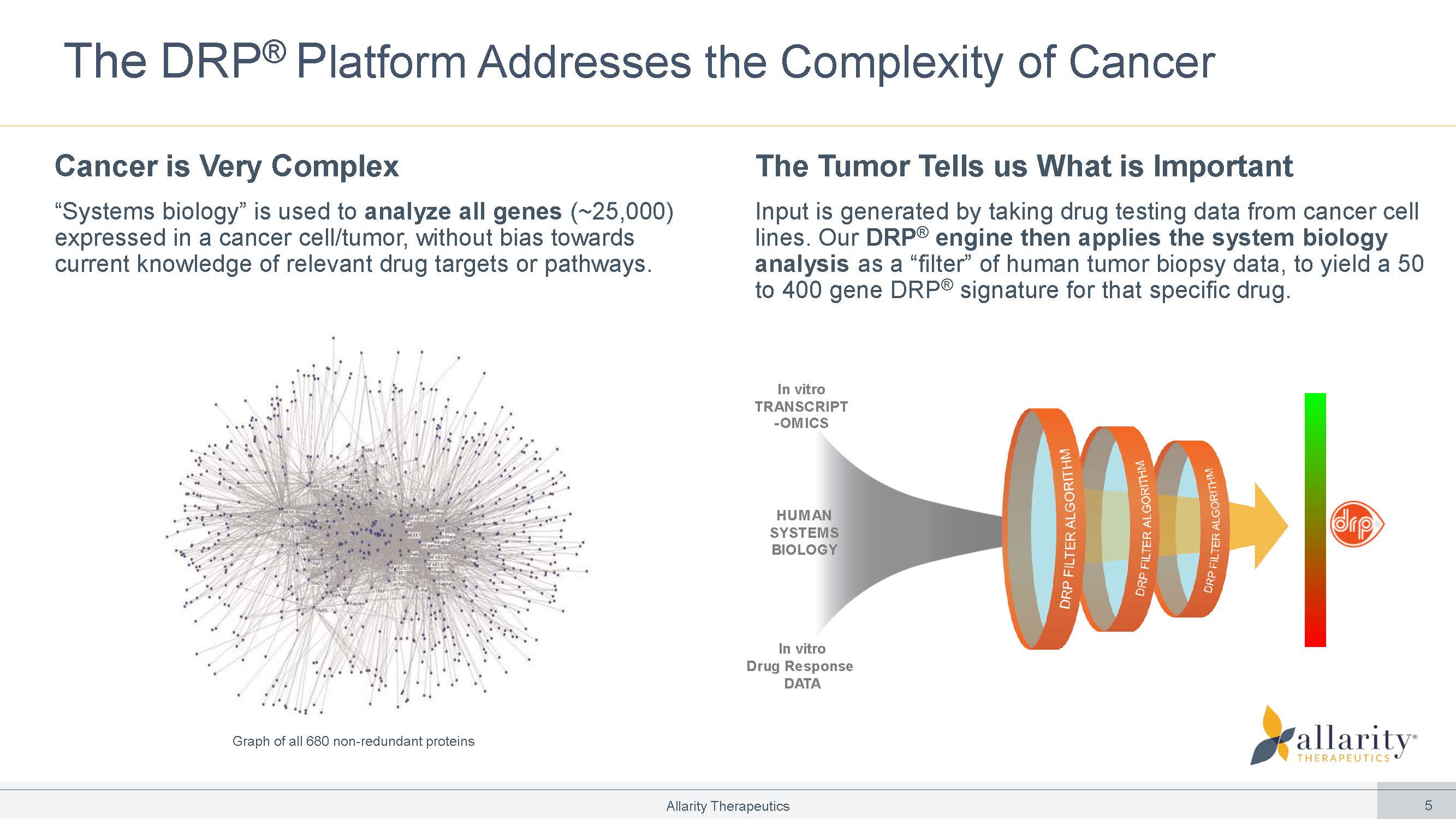
In vitro TRANSCRIPT - OMICS HUM AN SYSTEM S BIOLOGY In vitro Drug Response DATA The DRP ® P latform Addresses the Complexity of Cancer Cancer is Very Complex “Systems biology” is used to analyze all genes (~25,000) expressed in a cancer cell/tumor, without bias towards current knowledge of relevant drug targets or pathways. The Tumor Tells us What is Important Input is generated by taking drug testing data from cancer cell lines. Our DRP ® engine then applies the system biology analysis as a “filter” of human tumor biopsy data, to yield a 50 to 400 gene DRP ® signature for that specific drug. Graph of all 680 non - redundant proteins Allarity Therapeutics 5

Compare patients’ tumor gene expression to DRP ® signature PATIENTS’ TUMORS DRP ® CDx: Predicting a Cancer Patient’s Drug Response STRONG WEAK Patients’ biopsy samples PATIENTS’ TUMORS Step 1 Step 2 Step 3 WEAK MODERATE STRONG Identify patients with high DRP ® score for a given drug DRP + Allarity Therapeutics 6

DRP ® Platform: Extensively Proven in 47 Clinical Trials Cisplatin/LiPlaCis® Stenoparib IXEMPRA® (+ dozens of other validations*) PROSPECTIVE CLINICAL TRIALS – PHASE 2 DRP® Clinical Impact More than 50% increase in response or time to progression between predicted sensitive and predicted resistant RETROSPECTIVE (BLINDED) CLINICAL TRIALS – PHASE 2/3 Allarity Therapeutics 7 More than 50% increase in response or time to progression between Lung Lung – NSCLC Ovarian Renal RCC – metastatic AML Breast Breast – Metastatic Dovitinib Fulvestrant Belinostat 5 - FU predicted sensitive and predicted resistant Solid Tumors Breast – Neoadjuvant Colon Epirubicin Exemestane Phase 2 study (n=37) completed – late - stage metastatic BC Phase 2 study (n=30) underway – 3 rd line ovarian cancer Phase 2 study (n=60) underway – 2 nd line metastatic BC

s
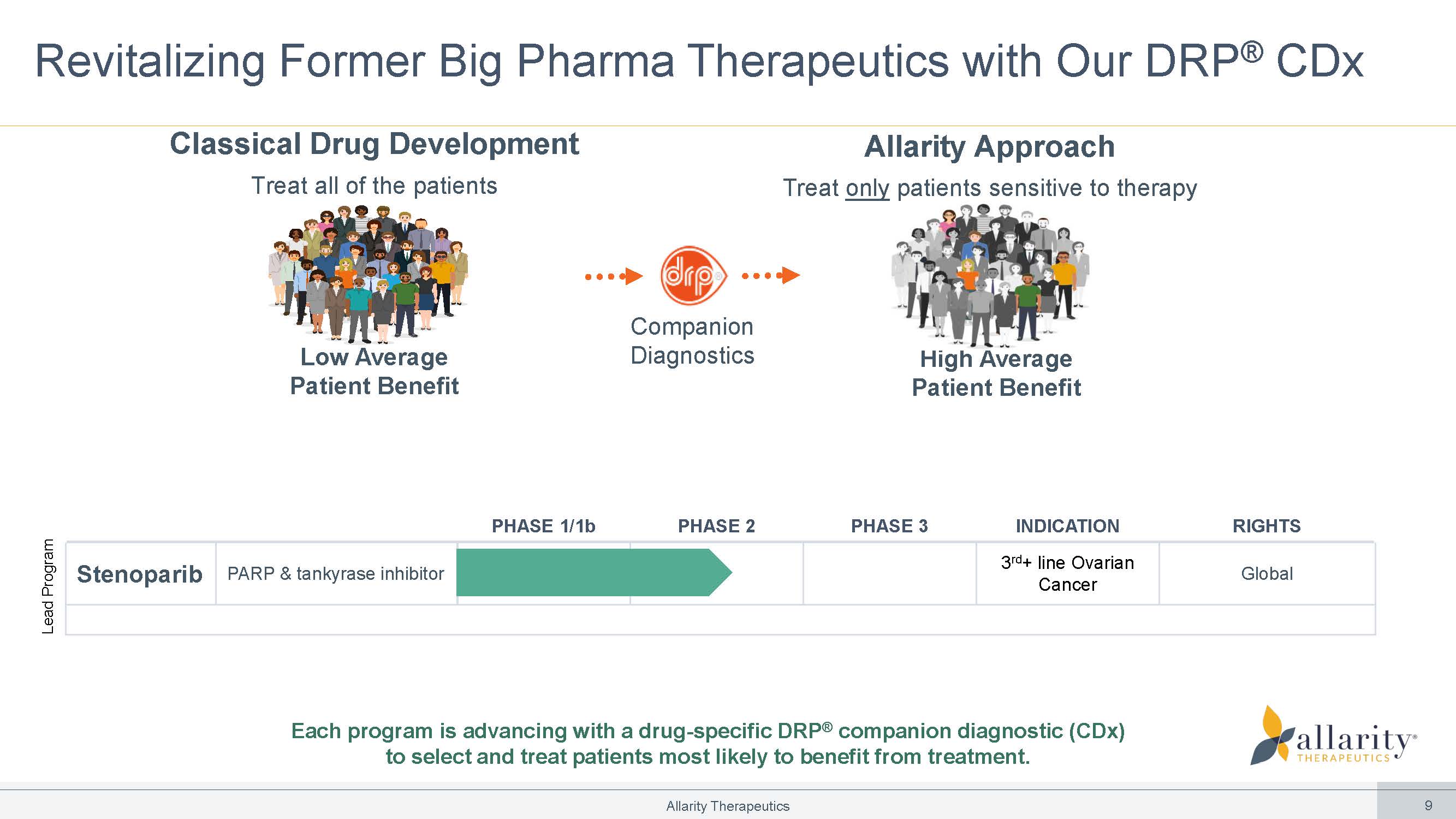
Revitalizing Former Big Pharma Therapeutics with Our DRP ® CDx Companion Diagnostics Low Average Patient Benefit High Average Patient Benefit Classical Drug Development Treat all of the patients Allarity Approach Treat only patients sensitive to therapy RIGHTS INDICATION PHASE 3 PHASE 2 PHASE 1/1b Global UG OLQH 2YDULDQ&DQFHU PARP & tankyrase inhibitor Stenoparib Lead Program Each program is advancing with a drug - specific DRP ® companion diagnostic (CDx) to select and treat patients most likely to benefit from treatment. Allarity Therapeutics 9

Prior validation example: Dovitinib Knudsen S, et al. PLOS ONE . 18(8): 2023

Dovitinib Has Shown Therapeutic Equivalence to Sorafenib in Prior Phase 3 mRCC Study. It is no longer in clinical development, but was used to validate DRP • Pan - Tyrosine Kinase Inhibitor (TKI), small molecule, targeted inhibitor of FGFR, VEGFR, and other RTKs . Unique, inhibitory profile. • Clinical activity demonstrated in monotherapy Phase 3 Renal Cell Carcinoma (mRCC) trial 2 , and in Phase 2 studies for gastrointestinal stromal tumors (GIST) 3 , liver (HCC) 4 , breast 5 , and endometrial cancers 6 • Dovitinib - DRP ® CDx evaluated in 5 different cancers including mRCC, GIST, liver, metastatic breast, and endometrial. Allarity Therapeutics 11 1) 2. NCT01223027 3. NCT01478373 4. NCT01232296 5. NCT01528345 and NCT00958971 6. NCT01379534 Dovitinib: A Unique Pan - Tyrosine Kinase Inhibitor (TKI)

1. Knudsen S, et al. A novel drug specific mRNA biomarker predictor for selection of patients responding to dovitinib treatment of advanced renal cell carcinoma and other solid tumors . PLOS ONE . 18(8): e0290681 Dovitinib - DRP ® CDx Identifies Dovitinib - Responsive Patients Dovitinib - DRP ® CDx (58 mRNAs) Identified Patients with Overall Survival (OS) Benefit in Novartis Phase 3 in 3 rd line RCC 1 DRP selects survivors in dovitinib arm but not in sorafenib arm • Dovitinib - DRP ® positive: • median 15.0 mo . OS (95%CI 12.9 - 26.3) 0 5 10 15 20 25 30 35 0.0 0.2 0.4 0.6 0.8 1.0 Months Survival probability Dov arm DRP+ (N=49, median 15.0 mo) Dov arm DRP− (N=86, median 9.1 mo) Sor arm DRP+ (N=36, median 9.7 mo) Sor arm DRP− (N=68, median 12.8 mo) • Dovitinib - DRP ® negative: • median 9.1 mo . OS (95% CI 7.6 - 13,2) Allarity Therapeutics 12 • Dovitinib - DRP ® CDx does not select Sorafenib responders.

Ongoing Phase 2 Prospective trial: Stenoparib

• First - in - class small molecule targeted inhibitor of DNA damage repair enzymes (PARP) with dual Tankyrase inhibitor activity: • Tankyrases are involved in telomerase maintenance during tumor cell division and are active in the Wnt signaling pathway in tumor cells. • Dual inhibition of PARP & Tankyrase potentially yields improved anti - tumor activity , including in tumors that develop PARPi resistance . • Stenoparib has shown promising monotherapy activity against OC and pancreatic cancer in prior Phase 1 clinical trial. • Stenoparib - DRP ® companion diagnostic showed ability to identify patients that benefited in Phase I study. • Global rights, exclusively in - licensed from Eisai . GMP drug manufacturing contract in place with LONZA. • PARPi’s approved for use in ovarian, breast, prostate, pancreatic, fallopian tube and peritoneal cancers. 1. McGonicle et al, OncoTarget vol 6 no 38, 2016. Stenoparib: A Unique Dual PARP and Tankyrase Inhibitor Ongoing Phase 2 Monotherapy Study in 3 rd Line Ovarian Cancer (OC) All patients screened with Stenoparib DRP to identify patients for inclusion Stenoparib (E7449) targets Wnt/ - catenin related genes like selective tankyrase inhibitor XAV939 1 0 2.000 4.000 6.000 8.000 10.000 12.000 2019A 2026E Ovarian cancer Pancreatic cancer Allarity Therapeutics 14

Unpublished preliminary data. Not yet fully source data verified. At first interim analysis, all patients selected by DRP showed benefit (i.e. reduction in tumor size. This is better than what other PARP inhibitors have shown without DRP) −8 −8 −11 −19 −100 Best percent change from baseline (target lesions) −60 −40 −20 0 20 Best overall response CR* SD>16weeks SD Second interim analysis planned for H1 2024 at N=18 Allarity Therapeutics 15

Thomas Jensen CEO tjensen@allarity.com allarity.com
v3.24.0.1
Cover
|
Feb. 27, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Feb. 27, 2024
|
| Entity File Number |
001-41160
|
| Entity Registrant Name |
ALLARITY THERAPEUTICS,
INC.
|
| Entity Central Index Key |
0001860657
|
| Entity Tax Identification Number |
87-2147982
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
24 School Street
|
| Entity Address, Address Line Two |
2nd Floor
|
| Entity Address, City or Town |
Boston
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02108
|
| City Area Code |
401
|
| Local Phone Number |
426-4664
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.0001 per share
|
| Trading Symbol |
ALLR
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Allarity Therapeutics (NASDAQ:ALLR)
Historical Stock Chart
From Jan 2025 to Feb 2025
Allarity Therapeutics (NASDAQ:ALLR)
Historical Stock Chart
From Feb 2024 to Feb 2025