MD Anderson and Myriad Genetics form strategic alliance to evaluate clinical utility of Myriad’s molecular residual disease assay
January 07 2025 - 7:00AM

The University of Texas MD Anderson Cancer Center and Myriad
Genetics, Inc. today announced a five-year strategic alliance to
accelerate the clinical evaluation and development of Myriad’s
molecular residual disease (MRD) assay.
This strategic alliance brings together the longstanding
oncology diagnostic experience of Myriad Genetics and the clinical
and translational research expertise of MD Anderson to create a
portfolio of studies to evaluate the clinical validity and utility
of Myriad’s Precise MRD.
“We look forward to working with MD Anderson to evaluate Precise
MRD’s utility in cancer care,” said Dale Muzzey, PhD, Chief
Scientific Officer at Myriad Genetics. “Our collaborative studies
will explore how our ultrasensitive Precise MRD test can enhance
treatment strategies for providers and improve patient outcomes. We
are optimistic that our MRD test’s ability to quantitatively detect
tumor-derived DNA at very low levels — far lower than is possible
with first-generation MRD tests — will open new opportunities for
therapy-response and recurrence monitoring.”
Under the terms of the agreement, MD Anderson and Myriad
Genetics will collaborate to design both retrospective and
prospective studies to investigate the test’s utility in breast,
gastrointestinal, genitourinary, and gynecological cancers. The
goal of these studies is to generate evidence that supports
national guideline inclusion and healthcare provider adoption. MD
Anderson investigators will lead patient enrollment, sample
collection, clinical data analysis and manuscript writing. Myriad
will provide funding, MRD testing and scientific research support
as well as potential milestone and royalty payments under the
collaboration.
“This strategic alliance brings together cutting-edge technology
and expertise from Myriad with disease-focused expertise and
clinical trials excellence from MD Anderson,” said Christopher
Flowers, M.D., division head of Cancer Medicine at MD Anderson. “We
aim to explore numerous applications for MRD testing, including
monitoring patients for relapse after treatment, identifying
high-risk patients in need of clinical trials, and potentially
intervention approaches.”
The alliance expands upon existing collaborations between MD
Anderson researchers and Myriad, including ongoing studies in
breast cancer and renal cell carcinoma.
About Myriad GeneticsMyriad Genetics is a
leading genetic testing and precision medicine company dedicated to
advancing health and well-being for all. Myriad develops and offers
genetic tests that help assess the risk of developing disease or
disease progression and guide treatment decisions across medical
specialties where genetic insights can significantly improve
patient care and lower healthcare costs. For more information,
visit Myriad.com.
About MD AndersonThe University of Texas MD
Anderson Cancer Center in Houston ranks as one of the world's most
respected centers focused on cancer patient care, research,
education and prevention. The institution’s sole mission is to end
cancer for patients and their families around the world, and, in
1971, it became one of the nation’s first National Cancer Institute
(NCI)-designated comprehensive cancer centers. MD Anderson is
No. 1 for cancer in U.S. News & World Report’s “Best Hospitals”
rankings and has been named one of the nation’s top two hospitals
for cancer since the rankings began in 1990. MD Anderson
receives a cancer center support grant from the NCI of the National
Institutes of Health (P30 CA016672).
Safe Harbor StatementThis press release
contains “forward-looking statements” within the meaning of the
Private Securities Litigation Reform Act of 1995, including that
Myriad will work with MD Anderson to evaluate Precise MRD’s utility
in cancer care, MD Anderson and Myriad Genetics will collaborate to
design both retrospective and prospective studies to investigate
the test’s utility in breast, gastrointestinal, genitourinary, and
gynecological cancers, the collaborative studies will explore how
the Precise MRD test can enhance treatment strategies for providers
and improve patient outcomes, and Myriad’s belief that its MRD
test’s ability to quantitatively detect tumor-derived DNA at very
low levels will open new opportunities for therapy-response and
recurrence monitoring. These “forward-looking statements” are
management’s expectations of future events as of the date hereof
and are subject to known and unknown risks and uncertainties that
could cause actual results, conditions, and events to differ
materially and adversely from those anticipated. Such factors
include those risks described in the company’s filings with the
U.S. Securities and Exchange Commission, including the company’s
Annual Report on Form 10-K filed on February 28, 2024, as well as
any updates to those risk factors filed from time to time in the
company’s Quarterly Reports on Form 10-Q or Current Reports on Form
8-K. Myriad is not under any obligation, and it expressly disclaims
any obligation, to update or alter any forward-looking statements,
whether as a result of new information, future events or otherwise
except as required by law.
Investor Contact Matt Scalo (801)
584-3532 IR@myriad.com
Media Contact Glenn Farrell (385)
318-3718 PR@myriad.com
Myriad Genetics (NASDAQ:MYGN)
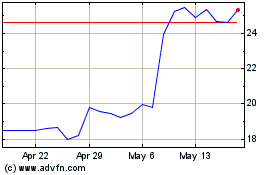
Historical Stock Chart
From Dec 2024 to Jan 2025
Myriad Genetics (NASDAQ:MYGN)
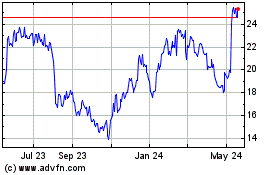
Historical Stock Chart
From Jan 2024 to Jan 2025