Arix Bioscience PLC (ARIX) Financial Results for the Year Ended
31 December 2022, Publication of Annual Report & Notice of AGM
25-Apr-2023 / 07:00 GMT/BST
-----------------------------------------------------------------------------------------------------------------------
Arix Bioscience plc
Financial Results for the Year Ended 31 December 2022,
Publication of Annual Report and Notice of Annual General
Meeting
LONDON, UK, 25 April 2023: Arix Bioscience plc ("Arix" or the
"Company") (LSE: ARIX), a global venture capital company focused on
investing in breakthrough biotechnology companies, today announces
its financial results for the full year ended 31 December 2022 (the
"Period") alongside the Annual Report and Accounts together with
its Notice of 2023 Annual General Meeting (the "AGM").
Financial highlights
-- Net Asset Value at Period end of GBP226 million (December
2021: GBP255 million); 175p per share (December2021: 198p)
-- Gross Portfolio Value (realised and unrealised) of
GBP99.6million (December 2021: GBP118.2 million)
-- Cash as at 31 December 2022 of GBP122.8 million (December
2021: GBP134.2 million)
-- GBP11.1 million of capital deployed into new and existing
Core Portfolio companies
Corporate, strategic and operational progress
-- Strengthened the Board of Directors with the appointment of
seasoned biotech executives Debra Barker MDas Senior Independent
Director and Andrew Smith as Non-Executive Director
-- Demonstrated agile approach to capital deployment in highly
volatile market conditions, by limitingcapital deployment in
unlisted companies and conserving cash
-- Diversified investment strategy with capital deployment
pivoted towards public opportunities and a focuson the Public
Opportunities Portfolio for value
-- Tassos Konstantinou will join Arix's investment team as
Managing Director following the departure of MarkChin, who has left
the Company to pursue other business opportunities
-- Continued disciplined approach to portfolio management with
exits from legacy public positions Autolus,LogicBio, and Pyxis,
generating aggregate proceeds of GBP7.7 million
Portfolio highlights
-- Portfolio companies are collectively running 11 clinical
trials and conducting 9 pre-clinical studies,providing Arix with
multiple opportunities for value creation? Disc started Phase 2
studies of bitopertin in patients with erythropoietic
protoporphyria andX-linked protoporphyria respectively ? Artios
initiated a Phase 2 clinical trial with Pol0 inhibitor, ART4215, in
combination with Pfizer'sPARP inhibitor talazoparib in patients
with BRCA deficient breast cancer ? Aura Biosciences presented
positive interim data from its Phase 2 trial evaluating
suprachoroidaladministration of AU-011 for the treatment of
early-stage choroidal melanoma, showing encouraging efficacy
andsafety ? Harpoon Therapeutics presented interim data from the
ongoing clinical trial of HPN328 demonstratingclinical activity and
a favourable safety profile in solid tumour patients
-- Well-funded portfolio entering into 2023, with Aura and Disc
Medicine collectively raising USD133.9 millionon the public markets
in the second half of 2022
-- Disc completed a merger with Gemini Therapeutics to create a
NASDAQ-listed company with sufficientfinancing to take it through
upcoming clinical data readouts
Post-Period end
-- USD85 million financing co-led by Arix for Ensoma along with
acquisition of Twelve Bio to createbest-in-class engineered cell
therapy platform
-- Disc Medicine announced USD62.5 million financing led by Bain
Capital to advance its portfolio of novelhaematology programmes
-- Arix participated in a USD25m private placement round with a
GBP2.8 million (USD3.5m) investment in redeemablepreferred stock to
continue to support Harpoon with its ongoing clinical trials
Outlook
-- The Company maintains a selective approach to capital
deployment as it works on achieving significantexits in the
portfolio
-- Careful portfolio adjustments to continue while
bio-pharmaceutical sector headwinds persist, with manyconstituent
companies remaining well-funded through 2023
-- With signs that M&A activity is returning to the sector,
Arix is well-placed to generate superior returnsthrough its diverse
portfolio
Robert Lyne, CEO of Arix, commented:
"As for many, 2022 proved more challenging than we had hoped at
the start of the year. Markets were performing worse in the fourth
quarter than in the same period in 2021 when life sciences stocks
first began a steep fall. In the ensuing market correction, we are
seeing a more conservative environment and a flight to quality,
with the market oriented more towards value.
"Despite these uncertain times, the factors driving growth in
the pharmaceutical industry remain unchanged: an ageing population,
a rising prevalence of chronic conditions, and an increasing per
capita spend on healthcare in developed and emerging markets
underscore the inherent value that the sector has to offer.
"For investors such as Arix, unlocking this value requires
recovery in the biotechnology sector markets and an increase in
licensing and M&A activity. We are confident that the
fundamentals will play through when the macro challenges are no
longer weighing as heavily on the markets."
Analyst Briefing: 10:00am BST today, Tuesday 25 April 2023
Management will host a virtual briefing for Analysts at 10:00am
BST today. Analysts wishing to join should register their interest
by contacting Powerscourt on arix@powerscourt-group.com or on +44
(0)20 7290 1050.
Investor Presentation: 4:00pm BST today, Tuesday 25 April
2023
Management will be hosting a live presentation and Q&A
session via the online platform, Investor Meet Company, at 4:00pm
BST today.
The presentation is open to analysts and all existing and
potential shareholders. Questions can be submitted pre-event via
the Investor Meet Company dashboard or at any time during the live
presentation via the "Ask a Question" function.
Investors can sign up to Investor Meet Company for free via:
https://www.investormeetcompany.com/arix-bioscience-plc
/register-investor
Investors who already follow Arix on the Investor Meet Company
platform will automatically receive an invitation to the event.
Annual General Meeting
The following documents have today been posted or otherwise made
available to shareholders by the Company: 1. Form of Proxy 2.
Notice of 2023 Annual General Meeting
3. Annual Report and Accounts 2022
The Form of Proxy is publicly available on the Company's
website:
https://arixbioscience.com/investor-relations/document-library
The Annual Report and Notice of AGM is also publicly available
on the Company's website:
https://arixbioscience.com/investor-relations/document-library
Copies of the above documents have been submitted to the UK
National Storage Mechanism. The documents will therefore shortly be
available for inspection at
https://data.fca.org.uk/#/nsm/nationalstoragemechanism and at the
following address:
Arix Bioscience Plc
Duke Street House,
50 Duke Street,
London,
W1K 6JL,
United Kingdom
[S]
Enquiries:
For more information on Arix, please contact:
Registrar and Receiving Agent:
Equiniti Limited
+44 (0) 371 384 2030
Company Secretary:
Kin Company Secretarial Limited
+44 20 8819 6486
Arix Bioscience plc
+44 (0) 20 7290 1050
ir@arixbioscience.com
Powerscourt Group
Sarah MacLeod, Ibrahim Khalil, Nick Johnson
+44 (0)20 7250 1446
arix@powerscourt-group.com
About Arix Bioscience plc
Arix Bioscience plc is a global venture capital company focused
on investing in breakthrough biotechnology companies around
cutting-edge advances in life sciences.
We collaborate with exceptional entrepreneurs and provide the
capital, expertise, and global networks to help accelerate their
ideas into important new treatments for patients. As a listed
company, we are able to bring this exciting growth phase of our
industry to a broader range of investors. www.arixbioscience.com
ARIX BIOSCIENCE PLC
INVESTING IN LIFE CHANGING SCIENCE
Annual report and accounts 2022
Arix Bioscience plc is a global venture capital company focused
on investing in breakthrough biotechnology companies to deliver
superior risk-adjusted returns to shareholders.
OUR PURPOSE To generate superior returns for our investors and
to make a tangible difference to patients' lives, by investing in a
focused portfolio of innovative biotechnology companies addressing
areas of high unmet need in healthcare
OUR GOAL Delivery of double digit NAV growth through a
diversified portfolio of biotechnology investments
OUR VALUES AND EXPECTATIONS Our values and expectations are at
the heart of everything we do and form an important part of our
culture.
? Integrity
? Respect
? Transparency
? Collaboration
? Discipline
? Accountability
CONTENTS
Strategic Report
. Highlights
. At a Glance
. Investment Proposition
. Chairman's Statement
. Chief Executive Officer's Review
. Market Insight
. Our Investment Strategy
. Business Model
. Our Strategic Objectives
. Key Performance Indicators
. Portfolio Review
. Broad and Rich Clinical Pipeline
. Core Portfolio
. Public Opportunities Portfolio
. Financial Review
. Risk Management
. Our Stakeholders
. Sustainability
Corporate Governance
. Corporate Governance Report
. Board of Directors
. Report of the Nomination Committee
. Report of the Audit and Risk Committee
. Directors' Remuneration Report
. Directors' Report
Financial Statements
. Independent Auditors' Report
. Financial Statements
Other information
. Shareholder Information
. Glossary
STRATEGIC REPORT
HIGHLIGHTS
Performance snapshot Net Asset Value (NAV) GBP226m 2021:
GBP255m
NAV per share 175p 2021: 198p
Gross Portfolio net revaluation* (GBP19m) 2021: (GBP54m)
Business highlights Realised capital GBP21m 2021: GBP39m
Capital pool GBP123m 2021: GBP134m
Capital raised by portfolio companies in 2022 USD134m 2021:
USD776m
Operational highlights
> Agreement to acquire Twelve Bio by Ensoma in an all share
transaction with concurrent financing which was completed in
February 2023
> Reverse merger of Disc Medicine onto Nasdaq completed in
December 2022, 15 months after first Arix investment
> Cost run rate below 2% of Net Asset Value
* Year on year net movement includes investments, FX, and
impairment.
AT A GLANCE
Who we are: Arix Bioscience plc is a global venture capital
company focused on investing in breakthrough biotechnology
companies.
We collaborate with experienced entrepreneurs and provide the
capital, expertise and global networks to help accelerate the
science they have developed into important new treatments for
patients. As a listed company, we are able to bring this exciting
growth phase of our industry to a broader range of investors.
We are here for two key reasons. To generate superior returns
for our investors and to make a tangible difference to patients'
lives.
Investment strategy providing resilience through market cycles
We focus on innovation and partner with highly experienced
entrepreneurs to create companies that can significantly improve
patients' lives.
Diverse portfolio Geographic split
Therapeutic split
Development stage split
NAV per share 175p 2021: 198p
Capital Pool GBP123m 2021: GBP134m
Rolling 36 month goals
Double Digit NAV per share Growth
-9% annualised in 2020-2022 BELOW TARGET (11% 2019-2021)
2 x Successful exits ON TARGET
2 x IPOs ON TARGET
Maintain cost base within 2% of NAV ON TARGET
Read more in the Chairman's statement in this report.
STRONG CLINICAL TRIALS PIPELINE Collectively our portfolio
companies were running 11 clinical trials at 31 December 2022, with
a further 9 in preclinical development.
Clinical trials 11 (2021: 22)
Read more on our Pipeline below.
* NAV Per Share = NAV / Total Number of Issued Shares less those
held in treasury.
Investment proposition Public market access to ground-breaking
medical innovation
1 Large, high-growth industry Biotech's core fundamentals are
strong: long-term, sustainable growth drivers, resilient,
attractive M&A environment.
Arix provides unique exposure to a portfolio of high growth
global biotech companies, both private and public, through a listed
vehicle.
Read more in Market Insight below.
2 High impact and value creation potential Diverse portfolio of
companies addressing significant unmet needs in healthcare, with
the potential to deliver breakthrough treatments to patients.
Multiple near to mid-term milestones anticipated with the
potential to deliver significant returns, including: new data
readouts, initiation of new trials, further funding rounds and
potential for IPOs and M&A.
Read more in the Portfolio Review below.
3 Expertise and networks Expert team with deep scientific,
commercial and transactional expertise and a proven track record of
success to drive growth in portfolio value.
Arix's global networks and transatlantic team provide access to
a large pool of opportunities across the full spectrum of biotech
disciplines and a deep understanding of the industries and markets
in which we invest.
4 Active, disciplined capital management Initial investments are
typically tranched to pre-agreed milestones supportive of the
original investment thesis.
Active management of public portfolio positions to manage risk
and optimise returns.
Transparent valuation policy; valuations adhere to IPEV
Guidelines.
Read more in the Financial Review below.
To see how our investment case works in practice, please see our
Business Model below.
5 Uncorrelated returns The healthcare and pharma sector is
generally uncorrelated with other industries and the wider
macroeconomic environment. As big pharma are a key driver of the
biotech sector through M&A, the potential for cash exits from
our portfolio is less impacted by the broader economic cycle.
Our core purpose is to help translate scientific innovation into
new medicines for patients. Through the portfolio of companies that
we back and build, we aim to address significant challenges in
healthcare in the areas of oncology, genetic diseases, immunology
and anti-infectives.
At Arix we focus on outcomes beyond financial performance and
through our portfolio companies we hope to make a tangible
difference to patients' lives. To date, we have invested more than
GBP200m into innovative biotech companies in our Gross Portfolio,
which, in turn, have since raised more than USD3bn of funding.
CHAIRMAN'S STATEMENT
Introduction At the start of 2022, we had grave concerns about
financial market conditions. I am pleased to report that, post year
end, the outlook is more positive. We have always held the view
that our particular area of the market is not as subject to the
cyclical impact of consumer demand as other areas of the economy.
As such, we believe that as the cost of capital starts to decline,
the sector will recover. We are already seeing signs of funds
coming back into the market and a pick-up in M&A, albeit
modest, by large pharmaceutical companies.
Our NAV during 2022, continued to be affected by the protracted
weakness in public markets that began in late 2021. Our response to
those challenging conditions was to adopt a judicious approach to
the deployment of cash and pause our core investing activity of
taking large positions in unlisted companies.
Instead, with a significant disconnect between depressed public
valuations and elevated private valuations, we created a "public
opportunities" portfolio of between 5% and 10% of NAV to invest in
undervalued listed companies, many of which we had assessed when
they were still private. Across the entirety of the portfolio, we
took decisive action to exit positions that we felt no longer
offered compelling prospects, either for their growth potential or
as M&A targets.
We also strengthened the Board with new additions which, as well
as improving corporate governance, brought deep and complementary
life sciences sector expertise from which we continue to
benefit.
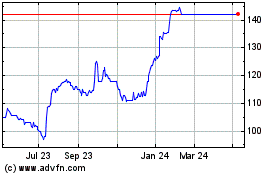
Performance Compared to the prior year end, the net asset value
fell from GBP255 million to GBP226 million (from 198p to 175p per
share). The reduction was predominantly driven by share price
declines in some of our legacy Nasdaq-listed holdings.
Our decision to conserve cash has served us well. While the
market price and discount to NAV of our shares reflect considerable
risk aversion with regards to venture investments in
biopharmaceutical companies, our strategy has helped to protect the
downside on our shares. We ended the year with GBP122.8 million in
cash compared to GBP134.2 million in the prior year.
In view of the considerable market uncertainties, we continued
to focus on the optimised management of our existing portfolio.
This was borne out by our participation in the Disc Medicine
fundraising in support of its merger with Gemini Therapeutics, and
the merger of Imara with Enliven which we continue to hold. The
maturation of the portfolio enables us to demonstrate our strategy
in action, along with our ability to identify and support
businesses with products and technologies that are attractive to
large pharmaceutical company buyers.
Corporate Governance We continue to benefit from improved
governance on the Board, having introduced new skills and balance
with the additions of Dr Debra Barker and Andrew Smith as Senior
Independent Director and Non-Executive Director respectively. Debra
is a seasoned international life sciences executive with more than
25 years' senior and board experience from start-up biotech to big
pharma companies, while Andrew is an internationally experienced
CFO and COO with strong financial and operational experience in US,
Swiss and UK-based biotech and pharmaceutical companies.
The appointments were coincidental with the resignation of Sir
Michael Bunbury. I am thankful to Sir Michael for the stewardship
he provided and for his contribution to improving corporate
governance at Arix during his tenure.
Together with Maureen O'Connell and Isaac Kohlberg,
Non-Executive directors proposed by Acacia Research Corporation, we
have a Board that encompasses a breadth and depth of investment and
sector expertise, and a collegiate group of highly experienced and
competent individuals who are all aligned on achieving success for
Arix.
Progress on key targets Our key performance indicators exist to
ensure that our core value-creating portfolio companies receive the
appropriate level of strategic and financial support to maximise
the company's risk-adjusted investment return. While they are
helpful markers of success, the dynamic environment in which we
operate necessitates an agile approach, which is why we take a
long-term view with targets set over a rolling 36-month period.
? Following IPOs from Aura and Pyxis Oncology in 2021, Disc
Medicine completed a reverse merger in 2022, exceeding our target
of achieving two IPOs over a 36-month period.
? We have maintained costs to within 2% of NAV (under normal
market conditions) and anticipate that we will continue to do
so.
? Having already achieved two strategic exits ahead of our 2023
target, there were no exits in 2022 beyond the sale of legacy
positions in some of our listed company holdings.
We are attuned to the vagaries of market sentiment and its
impact on sector activity, and we will not seek to achieve exits
where they compromise our ability to generate a return in order to
achieve an arbitrary, short-term target. As ever, we are focused on
one over-arching objective: to deliver significant returns to
shareholders through double-digit Net Asset Value growth over the
long term. We believe that this can be achieved through a range of
different portfolio events, the timing of which may vary.
Market overview The market correction that began in 2021
continued in 2022. The major sell-off in small and micro-cap
biotech stocks took the XBI, an equal-weighted biotechnology index
and indicator of the general health of the sector, from USD112 on
31 December 2021 to USD83 per share at the end of 2022, a 26%
decline after bottoming out at around 63% twice in May. A
confluence of macro-economic and political events drove up the cost
of capital creating a challenging environment for the biotech
sector. Investors took flight to safety as capital dried up,
financing costs soared and equity markets declined. The sell off
has been broad, with many new and non-specialist investors reducing
their exposure to the biotech sector.
Inevitably smaller biotech companies felt the effects more
acutely. Even those companies with positive clinical trial data
often failed to impress investors. The number of biotech companies
trading below cash remains far in excess of the pre-COVID normal.
It is indicative of how the industry has reset valuations and is
positioned for a recovery.
The IPO window remained mainly shut, with Evaluate Vantage
reporting only 19 flotations over the year. The closing of the IPO
window also hit the biotech venture world, with sums deployed
markedly lower than in 2021. While many private companies seized
the opportunity to raise capital prior to the slow-down, ongoing
economic and geopolitical concerns will impact the timing of a
recovery in 2023, affecting both private and public financings.
This has created an environment of 'haves' and 'have nots'; those
companies with clinical data will have an easier time raising
capital and doing strategic deals while those without such data
will find fundraising increasingly difficult, requiring other
avenues such as mergers to stay afloat.
The result has been a forced consolidation in the market, where
companies with cash combine with those in need of capital. In
recent months we have seen some signs of interest in those
companies at the larger, late-stage development end of the scale,
notably Amgen's USD28.3 billion acquisition of Horizon Therapeutics
and Pfizer's agreement to acquire Seagen for USD43 billion.
Inevitably, the proceeds from these large deals will be recycled in
the market, and we anticipate a trickle-down effect in time. We
have already seen two meaningful mid-cap M&A deals in CinCor
Pharma (acquired by AstraZeneca) and Amryt (acquired by Chiesi)
since the start of 2023 and expect more to come.
Following the banking crisis that resulted in the collapse of
Silicon Valley Bank, and the subsequent Federel Reserve
announcements about interest rates, it seems likely that we will
have a sustained period of stable interest rates without further,
major, increases which will allow valuations to settle and some
confidence to return to parts of the equity market during this
year. After the incontinence of monetary policy during the years
2019 through 2021, the Fed has for historic reasons been reluctant
to halt rate increases while US employment statistics remain
strong, in spite of declines in money supply measures M1 and M2
over the past 15 months. With total US bank deposits continuing to
fall week by week, it is likely that inflation will drop further,
creating improved market conditions for our sector.
Applying our flexible investment strategy One of our competitive
advantages is our ability to be nimble in responding to changing
market conditions and the opportunities that they present. In terms
of our policy last year, the Board considered the importance of
conserving cash to ensure that our investors were protected against
the downside during a period of significant market uncertainty. We
anticipated a correction in private company valuations, which tend
to lag the public market and pivoted towards public company
investing.
While we continue to see value in the public markets, we are
beginning to see some attractive valuations for high quality
companies in the private market and expect to add to this part of
our portfolio very selectively. Our support of Ensoma at the
beginning of 2023 is one such example. While in previous years, we
have sought to take a lead or co-lead position on private company
financings, we now prefer to take smaller positions to increase our
number of potential successful outcomes and overall portfolio
liquidity. This strategy has become more viable now that the market
has become increasingly selective and that we are being given
access to a wide-ranging number of opportunities.
While we anticipate that 2023 will be a more propitious
environment for exits, we also note that unlisted company
valuations have come down. We are seeing a number of compelling
opportunities to make smaller private equity investments, with
smaller follow-on commitments, where we will have the ability to
exit in a relatively short time period without having to commit a
substantial portion of NAV. The Board considers the importance of
diversifying risk by ensuring we have a greater spread of
opportunities within the portfolio, giving us more 'shots on goal'
and introducing more liquidity into the Core Portfolio.
We will also continue to invest in listed companies through our
Public Opportunities Portfolio, being approximately 5-10% of NAV.
While initially we looked at this strategy as being defensive in
case of a speedier reversion to more normal valuation metrics, our
positioning has become more aggressive in seeking stakes in
companies with attractive M&A potential in the near term.
Overall, we will take a very balanced view of capital
deployment. We will be led by our perception not only of pharma
interest in general but also of the level of pharma interest in our
holdings in particular.
Share Repurchases Consistent with our strategy of creating and
delivering value for all stakeholders, the Board is seeking to
renew the authority to purchase up to 10% of its issued share
capital, to be cancelled or held in treasury for future reissuance.
The Board may not necessarily use the authority in 2023 but
considers that buybacks are an attractive mechanism to improve
liquidity for sellers while potentially generating a substantial
uplift in NAV for ongoing shareholders.
Outlook The current discount to NAV which our share price
currently trades at is partly a consequence of increased investor
risk aversion, as well as a reflection of the widening in the
bid-ask spread, particularly in unlisted securities. While
"risk-off" sentiment in response to macroeconomic and geopolitical
drivers is likely to persist for some time, we are seeing a
broadening of our shareholder base, which should be of benefit when
there is a change in the cost of capital cycle. We are, however,
currently maintaining a cautious approach to capital deployment,
with significant cash and short-term treasury balances continuing
to provide some downside protection, while we work on achieving
significant exits.
We are navigating the headwinds facing the bio-pharmaceutical
sector with careful adjustments to our portfolio, based on expected
risk and return. With signs that M&A activity is returning to
the sector, we are well placed to achieve our goal of generating
superior returns with our portfolio of diverse companies developing
innovative treatments which will make a tangible difference to
patients' lives.
PEREGRINE MONCREIFFE Chairman 24 April 2023
Chief Executive Officer's Review
Introduction As it was for many, 2022 proved more challenging
than we had hoped at the start of the year. Markets were performing
worse in the fourth quarter than in the same period in 2021 when
life sciences stocks first began a steep fall. In the ensuing
market correction, we are seeing a more conservative environment
and a flight to quality, with the market oriented more towards
value, from which Arix has been a beneficiary. Through testing
times our Listed Portfolio navigated tough conditions, with some
having taken the opportunity to raise capital in 2020-2021 when
markets were more receptive, leaving them well-funded through
2023.
Our decision to be selective with capital deployment proved
well-judged. During the Period, we committed GBP11.1 million to the
Core Portfolio and ended the year with a GBP13.5 million exposure
in our Public Opportunities Portfolio. We began 2023 in a strong
position to be able to support our existing portfolio and invest in
new opportunities that can improve outcomes for millions of
patients and achieve healthy returns for shareholders.
We welcomed the Government's intervention in the rapidly
evolving situation at Silicon Valley Bank (SVB) in the UK in March
2023 which resulted in its rescue by HSBC and demonstrated the
strategic importance of the life sciences sector to the UK
economy.
At Arix, we had a de minimis exposure to SVB, through brokerage
accounts at SVB Leerink. While we have no direct exposure to SVB,
the swift action by the US authorities ensured that none of our
investee companies encountered any banking problems. Our portfolio
companies have a range of banking relationships, some of which
include SVB. We worked closely with affected portfolio companies
through the period to support them in their efforts to mitigate any
risk from SVB. We were pleased that the situation was resolved with
no disruption to the trading or prospects of Arix or our portfolio
companies.
Performance After a modest fall early in the year, NAV remained
steady during 2022 with small fluctuations led by developments in
the portfolio including market movements for listed investments,
continued cost control and movement in foreign exchange rates.
At the year end, we reported a decline in NAV to GBP226 million
(31 December 2021: GBP255 million), or 175p per share (31 December
2021: 198p per share) representing a reduction of 11%. The decline
was driven by a reduction in the valuation of the Gross Portfolio
of GBP18.6 million to GBP99.6 million predominantly as a result of
downward movement in our Core Portfolio public company
holdings.
In common with many of our listed peers, risk aversion in the
market affected our share price, which also came under pressure.
While the discount to NAV was stable during the year, this is an
area of focus for us as we seek to ensure that shareholders benefit
from the value of our assets.
In a year when public markets were markedly subdued it is
unsurprising that both the NAV and the share price did not perform
as hoped. Weak public markets have limited the funding
opportunities for many biotech companies and in turn reduced the
competitive tension which drives the M&A market. This
combination impacted the performance of the Core Portfolio.
Within the Core Portfolio, the most significant movement was
Harpoon, where the value of our holding reduced from GBP12.2m at
the start of the year to GBP1.3m at year end. Clearly this was a
very disappointing performance reflecting a significant downrating
of the company by public investors. Harpoon began significant
action in November 2022 to restructure and refocus the business,
which we expect to bear fruit in 2023 as important data read-outs
are announced.
Aura is another public holding which suffered from the
challenges of the public biotech markets in 2022, despite the
impressive clinical progress it made. The valuation of our holding
in Aura fell over the period from GBP20.0m to GBP13.2m. We feel
that this downrating is unwarranted and look forward to an improved
appreciation of Aura's progress as confidence returns to the
sector.
By contrast, Imara saw a doubling of valuation during 2022,
rising from GBP3.9m to GBP7.8m at year-end. Although this holding
value is still below the original cost, the improvement over the
period is testament to the management team's handling of the
clinical failures suffered in April 2022. After these setbacks, and
to preserve capital, Imara diligently cut costs and positioned
itself as a prime merger candidate, executing a successful reverse
merger with Enliven at the end of 2022. This has been well received
by the market and provides us with an interesting new prospect for
value creation and realisation in the Core Portfolio.
In the unlisted portfolio, a write down of STipe Therapeutics of
GBP3.7 million was partially offset by a write up of our holding in
Twelve Bio of GBP1.2 million following its acquisition by
Ensoma.
We began our Public Opportunities Portfolio in February 2022,
investing in a range of undervalued public biotech companies. This
is a dynamic portfolio of liquid positions which we actively manage
in response to market conditions and individual company
performance. By the end of 2022, the value of the Public
Opportunities Portfolio was broadly flat at GBP13.5m against a cost
of GBP13.6m. This compares favourably with the XBI which had fallen
9.6% from when we began the Public Opportunities Portfolio, to 2022
year-end. As of 13 April 2023, we had maintained this out
performance with the Public Opportunities Portfolio recording only
a marginal fall in value of 0.4% against a drop in the XBI of 13.5%
over the same period. The Public Opportunities Portfolio is well
placed to benefit from the individual progress of the companies we
have selected and a future change in sentiment towards these public
stocks more generally.
Since the beginning of 2023, NAV performance has decreased by
approximately 2%, to GBP222m, primarily as a result of weak public
equity markets impacting our listed investments.
At year end we held cash of GBP122.8 million, slightly down from
GBP134.2 million at the end of 2021, with new investments of
GBP11.1 million deployed into new and existing portfolio companies
during the year. This healthy cash balance, which can be attributed
to previous realisations, leaves us well placed to make new
investments to refresh the portfolio while we separately exit
legacy positions.
Portfolio Overview Throughout 2022 we continued our efforts to
generate value for shareholders through important portfolio
developments. We ended the year with nine companies across various
therapeutic areas in the Core Portfolio, five of which were private
and four of which were public, all having floated following
previous private investment by Arix.
While we adopted a highly selective approach to capital
deployment during the period, we demonstrated our commitment to
developing the portfolio with our investment in Ensoma at the end
of the year, alongside two important public mergers in the
portfolio, Disc Medicine and Imara Inc. The former of these two was
a validation of our capital commitment, occurring within 15 months
of our initial investment. Disc now holds enough cash to advance
its clinical programmes well into 2025 and beyond. Our USD9 million
investment in Ensoma as part of a financing we co-led with 5AM
Ventures coincided with the acquisition of portfolio company Twelve
Bio for a modest uplift. These types of transactions demonstrate
our readiness to pursue the right deals at the right time.
Portfolio progress Overall, the portfolio made good progress in
2022, with several companies reaching important clinical milestones
and securing financing.
Disc Medicine announced positive Phase 1 data of DISC-0974 in
healthy volunteers, thus de-risking further clinical development
and paving the way for Phase 2 studies. Based on the successful
Phase 1 trial, Disc initiated a Phase 1b/2 clinical trial of
DISC-0974 in myelofibrosis patients with severe anaemia. In
addition, Disc started two Phase 2 studies of bitopertin in
patients with erythropoietic protoporphyria and X-linked
protoporphyria, respectively. Disc completed a merger with Gemini
Therapeutics to create a NASDAQ-listed company with sufficient
financing through all upcoming clinical data readouts and well into
2025.
Artios Pharma advanced its two clinical-stage assets through
clinical trials and entered Phase 2 clinical development. For the
first time Artios announced clinical data from human studies,
highlighting encouraging safety and early signs of clinical
activity. Notably, one of the initiated clinical trials is a Phase
2 study with PolØ inhibitor, ART4215, in combination with Pfizer's
PARP inhibitor talazoparib in patients with BRCA deficient breast
cancer.
Aura Biosciences presented positive interim data from its
ongoing Phase 2 trial evaluating suprachoroidal administration of
AU-011 for the first-line treatment of patients with early-stage
choroidal melanoma. The data showed encouraging efficacy as well as
safety. Based on this data, Aura aligned with regulatory agencies
and finalised the design of the planned global Phase 3 trial. The
trial will evaluate the efficacy and safety of AU-011 with
suprachoroidal administration, for the first-line treatment of
early-stage choroidal melanoma. In addition, Aura received FDA Fast
Track Designation for AU-011 for the treatment of non-muscle
invasive bladder cancer and announced the first patient dosed in
its Phase 1 study. To support the ongoing clinical development of
AU-011 in bladder cancer as well as the pivotal Phase 3 study, Aura
raised USD80 million through a public offering at USD12 a share, to
finance the company through these clinical milestones. Arix chose
not participate given our significant existing position.
Harpoon Therapeutics presented interim data from the ongoing
clinical trial of HPN328 demonstrating clinical activity and a
favourable safety profile in patients with solid tumours. Harpoon
also announced revised strategic priorities for its pipeline,
shifting focus to ongoing clinical programs, HPN217, HPN328, and
HPN601, to reduce operating expense and to extend cash runway
through the end of 2023. Post-period end, we participated in a
USD25m private placement round with a USD3.5m (GBP2.8m) investment
to continue to support Harpoon with its ongoing clinical
trials.
Following its acquisition by Ensoma, Twelve Bio's novel gene
editing technology is now well placed to progress as part of the
wider Ensoma platform. This is a testament for the outstanding
efforts by the Twelve Bio team. We are excited to welcome Ensoma to
the Arix portfolio and looking forward to working together with the
Ensoma team and investors.
Depixus has made good progress since raising the Series A in
2021 and the technology for developing their platform for fast,
accurate and straightforward extraction of multiomic information
from DNA, RNA and proteins, continues to progress well as they
approach significant milestones, including prototype development
and early preparation for commercialisation.
Sorriso continued to advance its pipeline of anti-inflammatory
therapies through preclinical development and is on track to start
firstin-human trials over the course of 2023.
Post period end we have been pleased to add another company to
the Core Portfolio with an USD8.1m investment into Evommune, a
clinical stage company developing treatments for chronic
inflammatory disease. As I outlined in my statement last year, we
deliberately took a selective approach to new private investments
in 2022. We have been following the Evommune story since inception
and were impressed at the progress the company made to reach the
high bar we set for new investments. Together with the USD3.5m
(GBP2.8m) we invested in Harpoon's March 2023 financing, this
investment demonstrates our commitment to continuing to support and
develop the portfolio where we see risk-adjusted opportunities for
significant capital growth.
The year ahead will be significant for a number of our portfolio
companies as they reach important clinical and development
milestones throughout 2023. Our portfolio companies are
collectively running 11 clinical trials, a number of which are
expected to read out over the next 12 months with the potential for
value inflection if the results are positive. There is already
significant value in these companies and with multiple clinical
milestones on the horizon, we see substantial growth potential
across this portfolio in the near future. In addition to clinical
milestones, there is potential for M&A activity, strategic
partnerships as well as other financing events across the
portfolio, which could greatly increase the value of our companies,
and in turn our NAV.
Active management of the portfolio We actively manage our listed
holdings and reduce our positions where appropriate whilst
retaining and in certain cases, building our positions, where we
have conviction that we will see greater value in the future. We
have been reducing our exposure to legacy companies that have
become less compelling. During the period under review, we sold our
positions in Pyxis Oncology, LogicBio and Autolus, generating
GBP7.7m of aggregate proceeds. Outside of the Core Portfolio, we
also closed our position in GenSight.
Investment expertise New additions to the Board in 2022 brought
strength and depth to our investment expertise, and we are
fortunate to benefit from a highly skilled investment committee to
complement our investment team. As we continue with the resumption
of our core investing activities, we will continue to evolve our
human resources to fit the needs of the business. As part of this,
I am delighted to welcome Tassos Konstantinou to the Arix
investment team as Managing Director, following the departure of
Mark Chin.
Outlook As we move through 2023, we remain cautious about the
public markets and their appetite to support listed biotech
valuations and further fundraisings. This is likely to be
determined by the broader macro-outlook this year and will require
markets to see the peak of inflation and interest rates before
generalist investors will be comfortable pricing risk for assets in
our sector.
Despite these uncertain times, the factors driving growth in the
pharmaceutical industry remain unchanged: an ageing population, a
rising prevalence of chronic conditions, and an increasing per
capita spend on healthcare in developed and emerging markets
underscore the inherent value that the sector has to offer. All of
these factors serve to support our founding purpose: to provide
public market investors with access to the abundant opportunities
that exist within the healthcare and life sciences industries
through a liquid, evergreen vehicle. For investors such as Arix,
unlocking this value requires recovery in the biotechnology sector
markets and an increase in licensing and M&A activity. We are
confident that the fundamentals will play through when the macro
challenges are no longer weighing as heavily on the markets.
ROBERT LYNE Chief Executive Officer 24 April 2023
Market insight A vibrant sector, transforming lives
worldwide
The coming years are rich with opportunity for companies in life
sciences, as a series of key drivers combine with the continuing
realities of the pandemic to demonstrate the enormous value of the
sector - to investors, to economies and ultimately to the health
and wellbeing of every person on our planet.
1 Scientific discovery continues at pace Entrepreneurial
scientists have changed the life science landscape forever. Biotech
innovation is shaping a new understanding of the causes and
dynamics of disease at a molecular level - and this is driving an
acceleration in discovery. For example, the number of new clinical
trials added per year has increased from fewer than 11,000 in 2006
to more than 38,000 new trials initiated in 2022. Therapeutic
approaches are making new treatments possible and transforming
lives across therapeutic areas, from oncology and inflammatory to
infectious diseases.
2 The increased number of ongoing clinical trials is likely to
result in the development of breakthrough medicines addressing high
unmet needs and underserved disease areas. Amongst new 2022 drug
approvals was UK-based ImmunoCore's Kimmtrak, a bispecific T cell
engager protein, that was approved for unresectable or metastatic
uveal melanoma. Moreover, in August 2022 bluebird bio's Zynteglo
became the first FDA-approved stem cell-based gene therapy for
patients with ß-thalassemia. The year 2022 also saw emerging
developments in the radiopharmaceutical space. Novartis's Pluvicto
was approved in March for prostate-specific membrane
antigen-positive metastatic castration-resistant prostate cancer.
2022 also saw a number of approvals in the metabolic space, an area
of increasingly high medical need. The first new drug to slow the
onset of type 1 diabetes, Provention Bio's Tzield, was approved in
November 2022. This anti-CD3 monoclonal antibody binds to T
lymphocytes and dampens their attack on insulin-producing
pancreatic beta cells.
3 Demographics are driving demand The world's population is
growing older and living longer - and with that trend comes an
inevitable increase in the prevalence of chronic diseases.
Cardiovascular, cancer and neurological conditions are the biggest
killers on the planet, and all three are diseases of ageing. In the
US, EU and Japan the number of people aged over 65 is expected to
double from 200 million to 400 million in the next decade. The
pattern repeats in the emerging markets, where increased longevity
is matched by a growing middle class able to afford medical care.
In China, Brazil, India and Russia, the average total number of
prescriptions filled per year has doubled since 2009 and continues
to rise. While economies go through cycles, demand for treatments
increases inexorably - this is a long-term defensive sector, with
great resilience to other factors.
4 The regulatory environment is increasingly favourable
Scientists are now more effective at evaluating targets and
selecting the appropriate patients than ever before - and this has
led to more products successfully navigating the approvals process,
to the benefit of companies, investors and patients alike. For
example, in 2007 only 18 new drugs were approved by the FDA, the US
approval authority. However, between 2012 and 2021 the FDA approved
on average 44 drugs per year. In 2022, 37 new drugs were approved,
which marks a 26% decrease from the 50 drug approvals in 2021, but
notably this temporary decrease may be a result of trial delays as
well as trials discontinuations that were a result of the Covid-19
pandemic in 2020 and 2021. Notably, taking into account a number of
impactful FDA approvals post period end in early 2023, it becomes
apparent that the biotech industry remains strong to deliver new
medicines and we remain highly optimistic that the number of drug
approvals will increase again.
5 The route to exit is clear Our role is to invest in young
companies, position them for growth and reap the rewards for our
investors when these bright, successful companies are acquired,
often by Big Pharma. In the last ten years, the average amount
invested by venture capital companies in biotech businesses has
remained broadly flat at around USD50 million per company. However,
the average total exit value has risen from approximately USD200
million to USD561 million in the same period, demonstrating
significant and increasing returns on investment. There is also a
trend for pharmaceutical companies to compete with each other and
agree deals at an earlier stage - and with smaller and younger
companies. In the recent past, larger pharmaceutical companies
focused primarily on products in phase two or three of clinical
trials. Today, they are acquiring companies involved in phase one
or even those still working in the pre-clinical stage. It is
interesting to note that companies acquired at the early stages of
clinical development often generate higher return multiples than
later stage companies.
37 New drugs approved by the FDA in 2022
55% Novel drug approvals originated by smaller biopharma
companies between 2011-2021
38,035 Increase in the number of clinical trials in 2022
USD78bn Value of Biotech companies acquired in 2022
Sources: FDA, clinicaltrials.gov, SVB Leerink, Nature
Publishing.
Our Investment strategy Investing in life changing science
Our focus: We focus on true innovation and partner with the most
experienced entrepreneurs, management teams and investors to
develop treatments that can significantly improve patients'
lives.
High impact innovation Invest in breakthrough therapies which
have the potential to revolutionise patient outcomes.
Focused Geographies Source primarily from the USA and Europe,
areas with world-leading science and biotech ecosystems in which we
have strong networks.
Therapeutics focus Novel therapeutics with first or
best-in-class approach, focusing on therapeutic areas with high
unmet need, significant market opportunity and a disease and
mechanism of action which are well understood.
Clinical and late pre-clinical opportunities Investing into
assets which are partially de-risked with near-term value inflexion
points of clinical progress with the potential for valuation
uplifts and exits.
Our approach We focus purely on life sciences, with a team that
is highly experienced in this sector. We aim to remain at the
forefront of new exciting therapeutic areas by anticipating hot
areas across the biotech and life science sectors and by
identifying the most promising investment opportunities early. We
invest in true innovation and disease areas where in our belief,
the most opportunity exists to advance new treatment options for
patients.
We have a global network across top tier biotech investors and
world-leading management teams with a proven track record of
success in biotech. This network of excellence ensures that we have
access to top tier syndicates and premier deals across Europe and
the US. We have a renowned group of advisors, including serial drug
developers and biotech executives, who aid in the sourcing and
assessment of potential investment opportunities. We take a
proactive approach when we invest, frequently either leading or
co-leading financing rounds and joining the board of portfolio
companies. We can help secure funding, develop business strategy,
make connections and recruit experienced and talented management
teams.
How we allocate capital and manage risk Private venture To
minimise risk, we focus on investments into later stage private
companies which are already conducting clinical trials. The
majority of these companies are clinical stage and have begun
testing their treatments in patients. These companies will have at
least one live clinical trial, in either Phase 1, Phase 2 or Phase
3 and have raised significant capital, supported by a strong
syndicate of leading venture investors.
We also retain the flexibility to invest in companies which are
late pre-clinical. These companies would have the goal of advancing
their lead asset into clinical development within 12 to 18 months
of investing. As these companies are not yet assessing their drug
candidates in patients, these opportunities are of higher
translational risk and therefore we allocate a minority of our
capital to such opportunities. However, the increased risk is
typically offset by greater return multiples than late-stage
investments. We minimise that risk by investing into clinically
de-risked programmes based upon well-understood biology. Sorriso
Pharmaceuticals is a recent example of such an investment.
PIPE (Private Investment in Public Equity) Whilst the majority
of our investments are into private biotech companies, the public
markets can offer investment opportunities at attractive
valuations. By investing through structured PIPE transactions, we
can act as a catalyst to provide funding for and unblock
opportunities in clinical stage public companies with depressed
valuations. These businesses will typically have clinical readouts
within 18 months of our investment. Their public status provides a
mechanism for the re-rating of our investment in short order when
clinical read-outs are published, as well as liquidity to return
funds to our balance sheet.
Types of companies we invest in New investments are
predominantly made into private biotech companies. However, we do
have the flexibility to invest in public companies, if we believe
there is the potential to make significant investment returns.
Business model How we create sustainable value
Key strengths and resources
1. Discover We source globally and review hundreds of companies
each year
2. Evaluate Rigorous due diligence for new and follow-on
investments
3. Invest Invest in innovation with a clear commercial pathway,
approx 1 out of every 90 seen
4. Develop We typically take a board seat and play an active
role to help our companies grow
5. Exit We take a long-term view and seek to exit when the
optimum value is reached
6. Reinvest Capital is recycled onto the balance sheet for
reinvestment
Value created and shared For portfolio companies
? Flexible, long-term capital.
? Deep industry and capital markets expertise.
? Access to a broad range of co-investment opportunities.
? Introduction to potential acquisition targets.
? Due diligence and company building support.
For society
? We invest in companies that address serious unmet needs in
healthcare and have the potential to transform patient
outcomes.
? New company creation and job creation.
For shareholders
? Investing in a business that has a meaningful impact on
society.
? A diverse portfolio of opportunities and exposure to
disruptive, high-growth biotech companies.
? Financial returns.
? Balanced portfolio.
For employees
? Employee engagement.
? Talent development.
? Working for a business that helps create companies which
address serious unmet needs in healthcare.
Diversity, equity and inclusion At Arix we take great pride in
our small team of employees and our Board who work tirelessly to
deliver value for our shareholders. As we expand the team, we
continue to deepen our commitment to Diversity & Inclusion by
ensuring we have a diverse selection of candidates to choose from
with a focus on building a diverse culture and gender balance. At
the end of the reporting period, two of our Board members were
female and five members were male, and the Board keep our Board
Diversity policy under regular review. The Company had nine
employees (including contractors) at the end of the reporting
period, of which four were female and five were male. One male
holds a managerial position within the Company.
Underpinned by our values
? Integrity.
? Respect.
? Transparency.
? Discipline.
? Collaboration.
? Accountability.
Our strategic objectives
To generate superior returns for investors and to make a
tangible difference to patients' lives by investing in a focused
portfolio of innovative biotechnology companies addressing areas of
high unmet need in healthcare.
Discover Develop
high impact innovation in areas of unmet need, and build the value of these Deliver
with the potential to deliver transformative companies through hands-on support double digit NAV growth
treatments to patients
Performance in 2022
Performance in 2022
Performance in 2022 ? USD134m of capital raised by
portfolio companies. ? NAV decreased by 11% to GBP226m
? Provided GBP11.1m new capital to Core (175p per share).
Portfolio companies. ? New clinical trials initiated by
Artios and Disc Medicine in the ? GBP30.8m cash realised from Core
? Up to GBP22.5m available for a new strategy of period. Portfolio during the year.
diversifed investments in undervalued public
companies. ? Twelve Bio acquired by Ensoma ? Share price decreased by 11.9%.
alongside concurrent financing in
GBP33.6m which Arix participated. ? Cost base run-rate below 2% NAV.
capital deployed
USD134m GBP20.8m
capital raised by portfolio capital realised
companies
Priorities going forward
Increase value of portfolio
companies through hands-on support
including: Priorities going forward
Priorities going forward ? Raising capital. ? Targeting a cost run rate within
2% of Net Asset Value under normal
? Maintain exposure to quality life science ? Clinical development. market conditions.
opportunities across the globe.
? Management search. ? Targeting double digit NAV
growth.
? Business strategy.
? Developing strategic interest.
Link to KPIs
Link to KPIs Link to KPIs
? NAV growth.
? Diverse portfolio. ? NAV growth.
? TSR.
? Active clinical pipeline. ? Capital pool.
? Capital pool.
Key performance indicators
Performance Link
Financial KPI Description/rationale 2022 Links to strategic goals to
risks
DISCOVER high impact innovation in
areas of unmet need, with the
potential to deliver transformative
1 ? 11% decline treatments to patients
Includes performance of portfolio companies in NAV in 2022 1 2 3
NAV growth and capital pool. (22% decline in DEVELOP and build the value of these 4 5 6
2021) companies through hands-on support
DELIVER attractive returns to
shareholders
? Share price
2 Measures performance of delivering value to decreased from
shareholders. As no dividend were declared 122p to 107.5p DELIVER attractive returns to 1 2 3
Total during the period, TSR is calculated solely in 2022 shareholders 4 5 6
Shareholder on change in share price.
Return ? TSR decrease
of 11.9%
DISCOVER high impact innovation in
areas of unmet need, with the
potential to deliver transformative
3 Maintain sufficient capital to support growth ? GBP122.8m of treatments to patients
of portfolio companies and take advantage of cash and cash 1 2 3
Capital pool new investment opportunities. equivalents DEVELOP and build the value of these 4 5 6
companies through hands-on support
DELIVER attractive returns to
shareholders
Non-financial Performance Link
KPI Description/rationale 2022 Links to strategic goals to
risks
4 DISCOVER high impact innovation in
areas of unmet need, with the
Robust and Measures number of clinical trials across the ? 11 clinical potential to deliver transformative
active portfolio, with the potential to deliver programmes treatments to patients 1 6
clinical important new treatments to patients.
pipeline DEVELOP and build the value of these
companies through hands-on support
5 Measures Arix's commitment to invest in the DISCOVER high impact innovation in
best opportunities worldwide, across ? 9 companies areas of unmet need, with the 1 2 4
Diverse and different stages of development and in Arix's Core potential to deliver transformative 6
broad therapeutic areas. Portfolio treatments to patients
portfolio
KEY
1 Clinical trial risks
2 Unlisted investments
3 Taxation
4 Personnel
5 Macroeconomic conditions
6 Legislation and Regulation
Portfolio review
At year end, our portfolio companies were collectively running
11 clinical trials and conducting 9 pre-clinical studies, providing
Arix with multiple shots on goal for value creation.
Overall, the portfolio made good progress in 2022, with several
companies reaching important clinical milestones and securing
financing, as detailed below.
Operationally, there was continued progress across the
portfolio. Disc Medicine announced positive Phase 1 data of
DISC-0974 in healthy volunteers, thus de-risking further clinical
development and paving the way for Phase 2 studies. Based on the
successful Phase 1 trial, Disc initiated a Phase 1b/2 clinical
trial of DISC-0974 in myelofibrosis patients with severe anemia. In
addition, Disc started two Phase 2 studies of bitopertin in
patients with erythropoietic protoporphyria and X-linked
protoporphyria, respectively.
Disc completed a merger with Gemini Therapeutics to create a
NASDAQ-listed company with sufficient financing through all
upcoming clinical data readouts.
Our portfolio company Artios Pharma advanced its two
clinical-stage assets through clinical trials and entered Phase 2
clinical development. For the first time Artios announced clinical
data from human studies, highlighting encouraging safety and early
signs of clinical activity. Notably, one of the initiated clinical
trials is a Phase 2 study with Pol0 inhibitor, ART4215, in
combination with Pfizer's PARP inhibitor talazoparib in patients
with BRCA deficient breast cancer.
Aura Biosciences presented positive interim data from its
ongoing Phase 2 trial evaluating suprachoroidal administration of
AU-011 for the first-line treatment of patients with early-stage
choroidal melanoma. The data showed encouraging efficacy as well as
safety. Based on this data, Aura aligned with regulatory agencies
and finalized the design of the planned global Phase 3 trial. The
trial will evaluate the efficacy and safety of AU-011 with
suprachoroidal administration, for the first-line treatment of
early-stage choroidal melanoma. In addition, Aura received FDA Fast
Track Designation for AU-011 for the treatment of non-muscle
invasive bladder cancer and announced the first patient dosed in
its Phase 1 study. To support the ongoing clinical development of
AU-011 in bladder cancer as well as the pivotal Phase 3 study Aura
did a public offering to finance the company through these clinical
milestones.
Harpoon Therapeutics presented interim data from the ongoing
clinical trial of HPN328 demonstrating clinical activity and a
favourable safety profile in patients with solid tumours. Harpoon
announced revised strategic priorities for its pipeline, shifting
focus to ongoing clinical programs, HPN217, HPN328, and HPN601, to
reduce operating expense and to extend cash runway through the end
of 2023. Post period end, Arix participated in a USD25m private
placement round with a GBP2.8m (USD3.5m) investment in redeemable
preferred stock to continue to support Harpoon with its ongoing
clinical trials within the current subdued markets.
Following period end, Twelve Bio announced that it entered into
a definitive agreement to be acquired by Ensoma, a Boston-based
genomic medicines company developing one-time in vivo treatments
that precisely engineer any cell of the hematopoietic system. A
concurrent Series B financing for Ensoma was announced on the same
day and included Arix Bioscience as a co-lead investor. The Twelve
Bio team had made continuous progress to advance its novel gene
editing technology and the acquisition by Ensoma is a testament for
the outstanding efforts by the Twelve Bio team. We are also excited
to welcome Ensoma to the Arix portfolio and looking forward to
working together with the Ensoma team and investors.
Another deal completed post period end was Evommune, a clinical
stage company developing therapeutics for the treatment of chronic
inflammatory disease. Evommune has four programmes under
development, one of which is already in the clinic and targeting
patients with atopic dermatitis, a common form of eczema with a
large potential patient opulation. The company is lead by Luis
Pena, who cofounded Dermira and served as Chief Development Officer
before it was acquired by Eli Lily. Arix contributed GBP6.6m
(USD8.1m) to the round which will see the funds progress their
programs through the clinic.
The year ahead will be significant for a number of our portfolio
companies as they reach important clinical and development
milestones throughout 2023. Our portfolio companies are
collectively running 11 clinical trials, a number of which are
expected to read out over the next 12 months with the potential for
value inflection if the results are positive. There is already
significant value in these companies and with multiple clinical
milestones on the horizon, we see significant growth potential
across this portfolio in the near future. In addition to clinical
milestones, there is potential for M&A activity, strategic
partnerships, and other financing events across the portfolio,
which could greatly increase the value of our companies, and in
turn our NAV.
AN OVERVIEW OF 2022 IN NUMBERS
USD134m raised by portfolio companies in the year
11 clinical trials across the portfolio at year end
1 new addition to the portfolio
1 portfolio company acquisition
1 Reverse merger by a portfolio company on NASDAQ
Broad and rich clinical pipeline
Across our portfolio at year-end we have 11 studies in the
clinic, focusing on areas of high unmet medical need.
Expected next
Company Programme Indication Clinical Stage steps in 2023
Aura AU-011 Choroidal melanoma (IVT*) Phase 2 Completion
Aura AU-011 Choroidal melanoma (SC**) Phase 2 Phase 3 initiation
Artios ART4215 + talazoparib Breast cancer Phase 2 Ph2 data in 2024
Disc Medicine Bitopertin Erythropoietic porphyria Phase 2 Phase 2 data
Disc Medicine Bitopertin X-linked Protoporphyria Phase 2 Phase 2 data
Harpoon HPN328 Small cell lung cancer Phase 1 Phase 1/2 data
Harpoon HPN217 Multiple myeloma Phase 1 Phase 1/2 data
Artios ART0380 Advanced or metastatic solid tumours Phase 1 Phase 1 data
Artios ART4215 Advanced or metastatic solid tumours Phase 1 Phase 1 data
Disc Medicine DISC-0974 Myelofibrosis Phase 1 Phase 1/2 data
Aura AU-011 Non-muscle invasive bladder cancer Phase 1 Phase 1 data
Multiple undisclosed preclinical programmes Preclinical - At
this stage, the focus is on researching the feasibility and safety
of a treatment before commencing clinical trials.
Phase 1 - This is the first time a product is tested in humans.
The focus at this stage is testing the side effects and safety.
Phase 2 - Phase 2 involves further trials testing the efficacy
and safety and different dosing levels.
Phase 3 - This is the final stage of testing before
registration. Phase 3 trials focus on testing the effectiveness of
the new product compared to existing treatments or to a
placebo.
* Intravitreal
** Suprachoroidal
Core Portfolio
Artios Pharma Limited Artios is a leading independent DNA Damage
Response (DDR) company with a strong pipeline of novel cancer
therapies in development with first-in-class potential.
Therapeutic area: Oncology
Original cost: GBP20.2m Phase
2
Holding value: GBP24.9m
% of Gross Portfolio: 24.9%
The year of 2022 was a successful year for Artios as it advanced
its two clinical-stage assets through clinical trials and entered
Phase 2 clinical development. Artios announced initial positive
data from the Phase 1a study with its small molecule ATR inhibitor,
ART0380, in patients with advanced or metastatic solid tumours in
April 2022. The Phase 1a data demonstrated a predictable safety
profile, preliminary clinical activity, and supported the
initiation of a Phase 1b dose expansion study targeting ATM
deficient tumours.
Based on the encouraging initial Phase 1b data, Artios
announced, post period end, in February 2023 the initiation of a
Phase 2 randomised trial for ART0380 in combination with
gemcitabine in patients with platinum resistant ovarian cancer.
Preliminary Phase 2 data will become available in 1H 2025.
The company initiated a Phase 2 study of its Pol0 inhibitor,
ART4215, in combination with the PARP inhibitor talazoparib in
patients with BRCA deficient breast cancer. Phase 1 safety and
tolerability data for ART4215 in advanced solid tumours is expected
in 1H 2023 and Phase 2 data in BRCA deficient breast cancer
patients is expected in 2024.
Artios further expanded its board with the appointment of
Samantha Truex, a seasoned biotechnology executive, in June
2022.
Aura Biosciences (NASDAQ: AURA) Aura is a biopharmaceutical
company developing a new class of oncology therapies based on the
combination of a viral like particle with high affinity to tumour
cells coupled to a laser-activatable dye.
Therapeutic area: Oncology
Original cost: GBP11.5m
Phase
Holding value: GBP13.1m 2
Realised to date: GBP2.7m
% of Gross Portfolio: 13.2%
Aura's drug binds to malignant tumour cells with high
specificity and once the dye is activated by a short laser
treatment there is an acute tumour cell necrosis. AU-11, Aura's
lead asset, is being developed for the first line treatment of
indeterminate lesions and small choroidal melanoma, a life and
vision threatening and rare disease with no approved therapies
besides radiation.
During the period, Aura presented positive interim data from its
ongoing Phase 2 trial evaluating suprachoroidal administration of
AU-011 for the first-line treatment of patients with early-stage
choroidal melanoma. The data showed encouraging efficacy as well as
safety. Based on this data, Aura aligned with regulatory agencies
and finalised the design of the planned global Phase 3 trial. The
trial will evaluate the efficacy and safety of AU-011 with
suprachoroidal administration, for the first-line treatment of
early-stage choroidal melanoma.
The company also reported topline data from a retrospective
study of AU-011 versus plaque radiotherapy supporting the value of
a vision preserving therapy for the treatment of patients with
early-stage choroidal melanoma. AU-011 demonstrated statistically
significant vision preservation compared to the current standard of
care, which highlights the potential clinical benefit of Aura's
therapy for patients. Aura also announced that the European
Commission granted Orphan Drug Designation to AU-011 for the
treatment of uveal melanoma, which includes choroidal melanoma.
In addition, Aura received FDA Fast Track Designation for AU-011
for the treatment of non-muscle invasive bladder cancer and
announced the first patient dosed in its Phase 1 study.
Notably, Aura announced the pricing of a public offering of
Common Stock in November 2022, sufficient to finance the company
through the global Phase 3 study in choroidal melanoma.
Disc Medicine Disc Medicine is a clinical-stage
biopharmaceutical company that is dedicated to transforming the
lives of patients with hematologic disorders.
Therapeutic area: Hematology
Original cost: GBP8.1m Phase
2
Holding value: GBP9.0m
% of Gross Portfolio: 9.0%
In mid-2022, Disc announced positive Phase 1 data of DISC-0974
in healthy volunteers. The clinical trial demonstrated good safety,
pharmacokinetics and encouraging effects on hepcidin levels as well
as iron metabolism. Importantly, DISC-0974 achieved robust
increases in serum iron and hemoglobin. Based on the successful
Phase 1 trial, Disc initiated a Phase 1b/2 clinical trial of
DISC-0974 in myelofibrosis patients with severe anemia in June
2022. In August 2022, Disc initiated two Phase 2 studies of
bitopertin in patients with erythropoietic protoporphyria (EPP) and
X-linked protoporphyria (XLP), respectively.
During the period, Disc announced a merger agreement with Gemini
Therapeutics to create a NASDAQ-listed company focused on advancing
Disc's clinical pipeline. A concurrent financing of USD53.5m
restricted to Disc's existing investors was announced alongside the
merger. The combined cash provides Disc with financial runway into
2025. The completion of the merger was announced in December 2022,
when Disc started to trade on the Nasdaq Global Marker under the
ticker symbol IRON.
Disc appointed Jay Backstrom to its board of directors and Rahul
Khara as General Counsel.
Depixus Depixus is developing technology for the fast, accurate,
and inexpensive extraction of genetic and epigenetic information
from single molecules of DNA and RNA.
Therapeutic area: Genetic diseases
Original cost: GBP4.6m
Holding value: GBP8.2m
% of Gross Portfolio: 8.3%
Having closed the Series A financing in December 2021, during
the period, the company has continued to make good progress and
plans to provide further updates in 2023.
Imara (NASDAQ: IMRA) In April 2022, Imara announced interim
results of IMR-687 Phase 2b clinical trials in SCD and
beta-thalassemia. Interim results in the Ardent trial for SCD
showed no significant difference in median annualized rate of
vaso-occlusive crises in high-dose groups versus placebo in an
intent-to-treat population. Interim results in the Forte trial for
beta-thalassemia demonstrated no significant benefit in transfusion
burden or improvement in most disease-related biomarkers. IMR-687
was generally well tolerated across studies. Based on results from
both Phase 2b clinical trials, further development of IMR-687 in
SCD and beta-thalassemia was discontinued. To preserve cash, the
company reduced staff by >80% and a minimal core management team
decided to evaluate, together with the Board of Directors, the best
way forward to maximise returns for existing shareholders.
Therapeutic area: Genetic diseases
Original cost: GBP19.6m
Phase
Holding value: GBP7.8m 2
Realised to date: GBP7.3m
% of Gross Portfolio: 7.8%
In October 2022, Imara and Enliven Therapeutics announced a
merger agreement to create a Nasdaq-listed, clinical-stage
biopharmaceutical company focused on advancing Enliven's portfolio
of precision oncology programs. Enliven is advancing two parallel
lead product candidates: ELVN-001, a highly selective small
molecule BCR-ABL inhibitor for the treatment of chronic myeloid
leukemia, and ELVN-002, a potent, selective and irreversible HER2
and pan-HER2 mutant kinase inhibitor for the treatment of HER2
mutant lung cancer and other HER2-driven tumor types. Upon
completion of the merger, which is subject to approval by Imara's
and Enliven's stockholders, the combined company is expected to
operate under the name Enliven Therapeutics, Inc. and trade on the
Nasdaq Global Select Market under the ticker symbol ELVN. In
support of the merger, Enliven also intends to raise approximately
USD165m in a concurrent private financing. The combined company is
expected to have a cash balance of approximately USD300m at close,
which is expected to provide cash runway through multiple clinical
milestones and into early 2026.
Sorriso Pharmaceuticals Sorriso is a biotechnology company
advancing a pipeline of disease-modifying antibodies for the
treatment of inflammatory diseases, including Crohn's disease and
ulcerative colitis.
Therapeutic area: Immunology
Original cost: GBP6.0m
Preclinical
Holding value: GBP6.6m
% of Gross Portfolio: 6.62%
During the period the company has made good progress preparing
for initiation of Phase 1 clinical development in 2023. The company
remains on track to enter clinical trials in 2023.
Sorriso has made key additions to its team to strengthen its
leadership. The company appointed Jeffrey W. Sherman, Chief Medical
Officer and Executive Vice President at Horizon Therapeutics, to
its board of directors. In addition, Jackie Benson, former Vice
President of Immunology Scientific Innovation at Johnson &
Johnson, joined Sorriso as Chief Scientific Officer.
Twelve Bio Twelve Bio is developing a gene editing platform
based on CRISPR-Cas12a variants for the treatment of genetic
diseases with high unmet medical need.
Therapeutic area: Genetic diseases
Original cost: GBP3.5m
Discovery
Holding value: GBP5.0m
% of Gross Portfolio: 4.8%
During the period, Twelve Bio has made continuous progress to
advance its CRISPR-Cas12a toolbox. Following period end, on 5
January 2023, Twelve Bio announced that it entered into a
definitive agreement to be acquired by Ensoma, a Boston-based a
genomic medicines company developing one-time in vivo treatments
that precisely engineer any cell of the hematopoietic system. A
concurrent Series B financing for Ensoma was announced on the same
day, and included Arix Bioscience as a co-lead investor. Closing of
the acquisition of Twelve Bio by Ensoma was announced on 9 February
2023.
STipe Therapeutics STipe is developing first-in-class drugs that
sensitize the STING pathway, a major driver of innate immunity, to
enable a patient's immune system to overcome the immune suppression
often observed within solid tumours.
Therapeutic area: Oncology
Original cost: GBP5.0m
Preclinical
Holding value: GBP1.3m
% of Gross Portfolio: 1.3%
STipe has a differentiated approach from other programmes
targeting the STING pathway since it does not rely on direct
overstimulation and therefore has the potential to be a
systemically delivered therapy with broader applications.
STipe continues its preclinical work on the lead program,
however based upon the challenging funding environment for
preclinical companies such as STipe, we have taken a view to
further adjust the holding of value of STipe following the write
down at the half year, as at year-end the holding value is
GBP1.3m.
Harpoon Therapeutics (NASDAQ: HARP) Harpoon is a clinical-stage
immunotherapy company developing a novel class of T cell engagers
that harness the power of the body's immune system to treat
patients suffering from cancer and other diseases.
Therapeutic area: Oncology
Original cost: GBP20.5m
Phase
Holding value: GBP1.3m 1/2
Realised to date: GBP12.5m
% of Gross Portfolio: 1.33%
During the period, Harpoon received FDA Fast Track Designation
for HPN217 for the treatment of patients with relapsed, refractory
multiple myeloma. In November and December 2022, Harpoon presented
interim results from the Phase 1 trial of HPN217 in heavily
pre-treated patients with relapsed/refractory multiple myeloma.
This data established a clinical proof of concept, demonstrating
strong as well as durable efficacy and favourable safety.
Moreover, Harpoon was granted Orphan Drug Designation for HPN328
for the treatment of small cell lung cancer. Notably, Harpoon and
Roche announced a collaboration on clinical trials to study HPN328
in combination with the anti-PD-L1 antibody atezolizumab in
patients with small cell lung cancer. In May 2022 Harpoon presented
interim data from the ongoing dose escalation of HPN328. The data
showed clinical activity and a favourable safety profile in
patients with solid tumours.
In November 2022, Harpoon announced revised strategic priorities
for its pipeline. The company noted its strategic realignment to
focus resources on ongoing clinical programs, HPN217, HPN328, and
HPN601. This step involved a restructuring in workforce to support
prioritized clinical development and resulted in reduced operating
expense, and an extended cash runway through the end of 2023.
On a corporate level, Harpoon announced the departure of Chief
Medical Officer Natalie Sacks and later in the period announced the
expansion of its leadership team with the appointment of Wendy
Chang to SVP HR, Banmeet Anand to SVP Translational Medicine, and
Luke Walker to Chief Medical Officer. In addition, Harpoon
appointed Lauren Silvernail, a highly experienced industry veteran,
to its board of directors.
Pyxis Oncology (NASDAQ: PYXS) Pyxis Oncology is building a
differentiated portfolio of biologics, including antibody-drug
conjugates and immunotherapies, to improve the lives of patients
with difficult-to-treat cancers.
Therapeutic area: Oncology
Original cost: GBP14.5m
Holding value: GBP0.0m
% of Gross Portfolio: 0%
Arix fully exited its position in Pyxis Oncology in 2022 at a
loss of GBP10.3m against original cost.
LogicBio Therapeutics (NASDAQ: LOGC) LogicBio Therapeutics is a
genome editing company, dedicated to extending the reach of genetic
medicine with pioneering targeted delivery platforms.
Therapeutic area: Genetic diseases
Original cost: GBP13.3m
Phase
Holding value: GBP0.0m 1
Realised to date: GBP2.1m
% of Gross Portfolio: 0.0%
Arix fully exited its position in LogicBio in 2022.
Autolus (NASDAQ: AUTL) Autolus is developing next-generation
programmed T cell therapies for the treatment of cancer.
Therapeutic area: Oncology
Original cost: GBP24.6m
Holding value: GBP0.0m
Realised to date: GBP1.6m
% of Gross Portfolio: 0.0%
Arix fully exited its position in Autolus in 2022.
Public Opportunities Portfolio
Public Opportunities Portfolio 2022 continued to see a
significant disconnect between public and private valuations for
biotechnology companies. This led Arix to deploy capital into a
range of public market investments which met the same criteria as
our private investments, but offered significant value given the
unwarranted shift in sentiment against these companies.
Original cost: GBP13.6m
Holding value: GBP13.5m
% of Gross Portfolio: 13.6%
Each of the companies selected for the Public Opportunities
Portfolio (POP) is a clinical stage drug development company with
near-term clinical trial read-outs and the potential for
acquisition by big pharma. Whilst this is consistent with our
investment philosophy for private companies, the liquid nature of
public market investments allows for dynamic trading of these
positions which are deliberately kept at a size which allows for
rapid liquidation. By comparison, our Core Portfolio public
holdings are inevitably prone to illiquidity due to the significant
percentage holdings we have as a legacy of being a pre-IPO
investor. Whilst these Core Portfolio positions provide significant
exposure to potential big wins, the Public Opportunities Portfolio
provides a more balanced opportunity for us to generate value from
volatile markets.
A successful example of one of our POP selections has been
Ventyx Biosciences which we first invested in during July 2022. At
the time of first investing, Ventyx's pipeline included three
clinical-stage programs targeting TYK2, S1P1R and NLRP3, all of
which are clinically validated targets that have strong prospect of
becoming impactful oral therapies in the immunology space. It was
the TYK2 program as well as the strong cash position and
experienced Ventyx team that attracted Arix to this investment. We
viewed the wholly owned TYK2 inhibitor as a potential best-in-class
agent that could differentiate from Bristol-Myers Squibb (BMY) and
others. Therefore, we viewed BMY's imminently expected
deucravacitinib approval by the FDA as well as Ventyx's near-term
Phase 1 safety/tolerability and PK/PD data in early Q3 2022 as
potential positive catalysts. In August 2022, Ventyx presented
highly encouraging Phase 1 data for its TYK2 inhibitor and in
September 2022, the FDA approved BMY's deucravacitinib without a
black box warning. Given the significant return that resulted from
this positive news, we decided to partially sell down our position
in Q4 2022, however, we remain excited about the return prospect of
the stock.
Financial review YEARED 31 DECEMBER 2022
2022 highlights
Net Asset Value (NAV) GBP226m 2021: GBP255m
At year end, NAV totalled GBP226 million, a decrease of GBP29.5
million, or 11%, compared to 2021's GBP255.4 million. The loss for
the year was GBP27.6 million (2021: loss of GBP61.1 million), while
cash decreased by 8.5% to GBP122.8 million (2021: GBP134.2 million)
per note 16, following net investments made of GBP12.8 million, and
operational cash movements.
2022 saw a more challenging macro-economic environment than we
had hoped for at the start of the year. The war in Ukraine has
exacerbated inflationary pressures leading central banks to tighten
monetary policy and increase the cost of capital. This has
contributed to the challenges facing biotech companies seeking
funding during the period and has kept pressure on public
valuations. Nonetheless, the portfolio is well funded as we enter
2023, with Core Portfolio companies Aura Bioscience and Disc
Medicine having collectively raised USD133.9m on the public markets
in the second half of 2022.
Portfolio revaluations The value of Gross Portfolio (see table
below), including investments and realisations in the year, reduced
by GBP18.6 million to GBP99.6 million predominantly as a result of
downward movement in the public assets. This was driven by a
further decrease in the value of Harpoon by GBP10.9 million.
In the private portfolio, a further write down of STipe
Therapeutics (GBP1.3 million) was partially offset by a 20%
increase in our holding in Twelve Bio to GBP1.2 million. This was
as a result of Twelve Bio's all share transaction-acquisition by
Ensoma and the resultant conversion of an outstanding Twelve Bio
convertible loan to 20%.
Portfolio realisations During the year we continued to exit
certain legacy public positions. This resulted in the complete exit
from Autolus, LogicBio and Pyxis generating GBP7.7 million of
aggregate proceeds. There was also a modest realisation from Aura
in the first half of 2022 when the stock was trading well,
generating GBP1.2m of proceeds and leaving a significant remaining
position for Arix.
Portfolio investment As outlined at the start of 2022, we
expected capital deployment to be reduced and refocused on the
Public Opportunities Portfolio. This was reflected in only one new
company being added to the portfolio with the financing of GBP7.5m
into Ensoma alongside its acquisition of existing portfolio company
Twelve Bio.
The focus of new capital deployment was into the Public
Opportunities Portfolio which began in February 2022. This is a
dynamic portfolio which adjusts to conditions in the market as well
as individual opportunities. At its peak there was GBP22.5 million
invested in this portfolio. By year end, the value of the holdings
in the Public Opportunities Portfolio was GBP13.5 million.
Foreign exchange The GBP/USD exchange rate fell during 2022.
Starting at USD1.35 it declined to USD1.11 in September 2022,
before recovering to USD1.21 by the year end. This resulted in a
modest net negative impact of GBP3.45 million on portfolio
valuations with the majority of Arix's investments being
denominated in US dollars.
Arix continues to expect that the majority of future investment
cash flows, both in and out, will be in US dollars and as such,
does not consider hedging strategies to be appropriate at this
time, particularly given the uncertainty over the quantum and
timing of these movements.
Cash and deposits With a cash balance of GBP122.8 million per
note 16 at 31 December 2022, Arix has a very strong capital pool to
support both the current portfolio and new biotech
opportunities.
Counterparty risk is managed by holding cash across financial
institutions, all of which have a credit rating of at least F1,
according to Fitch ratings. Returns on cash have been historically
low but Arix continues to target yield where possible, weighed
against the anticipated timing and quantum of the needs of the
portfolio. The Company's Treasury Policy is overseen by the Audit
and Risk Committee.
Net operating costs 2022 saw continued control of costs which
have been reduced significantly in recent years. Net operating
costs were GBP8m in 2019, GBP6.9m in 2020 and GBP6.6m in 2021. Cost
reduction began in 2021 following management changes, with GBP1.49m
of exceptional changes taken that year in relation to shareholder
engagement and restructuring. For 2022, net operating costs were
GBP3.5 million, representing 1.5% of 2022 NAV, below the 2%
target.
Fund management fee income of GBP0.01 million, (2021: GBP0.3m)
received from managing The Wales Life Sciences Investment Fund,
continues to reduce in line with expectation as the fund reached
the end of its tenure in February 2023.
Finance income of GBP1.6 million was generated in the year
reflecting the significantly increased interest rate environment
compared to GBP0.1m last year when interest rates were considerably
lower.
The share-based payment was a charge in the year of GBP0.3m
compared to the credit in the previous year (GBP0.3 million).
Taxation As a UK operating group, Arix is subject to UK
corporation tax on the majority of its activities, which can
include the gains arising on investments. However, wherever
possible we aim to take advantage of the UK's Substantial
Shareholding Exemption (SSE), which exempts taxable gains or losses
arising from the disposal of shares, where certain conditions are
met. This is a nuanced exemption and is always dependent on
individual investment fact patterns.
Where investment gains are unrealised and are not expected to
qualify for SSE, the anticipated tax due based on the current
valuation of the underlying investment is reflected in a deferred
tax balance.
These factors, combined with the ability to utilise certain
brought forward losses, reduce the Group's tax expense in any given
year. The tax expense for 2022 was GBPnil (2021: GBPnil).
Valuation policy Arix's investments are valued in accordance
with International Private Equity and Venture Capital Valuation
Guidelines December 2018 (IPEV Guidelines). Listed investments are
marked-to-market at the period end. Unlisted investments are valued
with reference to the most recent funding round, milestones, and
comparable valuations. The Group uses valuation techniques that
management consider appropriate in the circumstances and for which
sufficient data are available to measure fair value, maximising the
use of relevant observable inputs and minimising the use of
unobservable inputs taking into account any discounts required for
non-marketability and other risks inherent in early-stage
businesses.
Further information is available in Note 2 to the financial
statements.
Post Year End In March 2023, Arix contributed GBP2.8m to
Harpoon's private placement financing. In April 2023, Arix invested
GBP6.6m in Evommune Inc.. For further details after the reporting
date please see note 24 of the financial statements.
Investment summary
1 Jan Investments Realisations Change in FX 31 Dec Equity Not Fully
2022 in period in period valuation movement 2022 interest invested funded
Investment GBPm GBPm GBPm GBPm GBPm GBPm % GBPm %
Core
portfolio
Listed on
NASDAQ
Aura 20.0 0.0 (1.2) (4.2) (1.5) 13.1 4.1% - 4.1%
Pyxis 14.1 0.0 (4.2) (9.9) 0.0 0.0 0.0% - 0.0%
Oncology
Disc Medicine 8.1 1.7 0.0 0.0 (0.8) 9.0 4.2% - 4.2%
Harpoon 12.2 0.0 0.0 (14.6) 3.7 1.3 6.7% - 6.7%
LogicBio 4.9 0.0 (1.9) (3.1) 0.1 0.0 - 0.0%
Imara 3.9 0.0 0.0 3.9 0.0 7.8 6.1% - 6.1%
Autolus 1.9 0.0 (1.6) (0.3) 0.0 0.0 0.0% - 0.0%
-------------- -------------- -------------- -------------- -------------- --------------
Nasdaq listed 65.1 1.7 (8.8) (28.3) 1.5 31.2
total
========= ========= ========= ========= ========= =========
Unlisted
Artios 24.9 0.0 0.0 0.0 0.0 24.9 9.9% - 9.9%
Depixus 7.8 0.0 0.0 0.1 0.3 8.2 21.4% - 21.4%
Sorriso 5.9 0.0 0.0 0.0 0.7 6.6 26.0% 3.7 26.0%
Twelve Bio 3.8 0.0 0.0 1.0 0.2 5.0 49.0% - 49.0%
STipe 2.9 2.0 0.0 (3.7) 0.2 1.3 19.8% 19.8%
Amplyx 1.2 0.0 0.0 0.0 0.1 1.3 - - -
Ensoma 0.0 7.5 0.0 0.0 0.0 7.5
-------------- -------------- -------------- -------------- -------------- --------------
Unlisted 46.4 9.4 0.0 (2.6) 1.6 54.8
total
========= ========= ========= ========= ========= =========
Public
opportunities 0.0 22.5 (7.8) (0.8) (0.4) 13.5
portfolio
total
========= ========= ========= ========= ========= =========
Other public
market
investments
GenSight 6.3 0.0 (4.2) (2.6) 0.5 0.0 0.0% - 0.0%
Legacy assets 0.4 0.0 0.0 (0.3) 0.0 0.1 -
Gross 118.2 33.6 (20.8) (34.5) 3.1 99.6
Portfolio
Other 2.4 0.0 0.0 (0.7) 1.4 3.0 -
interests
-------------- -------------- -------------- -------------- -------------- --------------
Total 120.6 33.6 (20.8) (35.3) 4.5 102.6
investments
========= ========= ========= ========= ========= =========
Risk Management
The Group monitors a number of principal risks and uncertainties
that may impact the business. These include financial,
non-financial, internal and external concerns.
Risk management framework The Directors are able to manage the
business, and achieve its strategic objectives, due to an effective
risk management framework which features multiple layers.
Board Managing risk is a key responsibility of the Board, which
sets a strong tone, in line with best practice corporate
governance.
Key committees The Audit and Risk Committee oversees the
effectiveness of the risk management processes.
The Remuneration Committee ensures incentives and reward are
balanced and appropriate for achieving the strategy.
The Nomination Committee addresses the need for continuing
strength at the senior levels of the Company and is responsible for
succession planning.
The Strategy & Investment Committee reviews investment and
divestment proposals for management and recommends actions to the
Board for approval. It also formulates strategy for the Group for
ratification by the Board.
Executive management The management team is responsible for
identifying, assessing and mitigating the day-to-day operational
risks. Emerging risks are monitored by the management team with the
support of the Board. These risks are considered in the context of
the Company's business and stakeholders. Where potentially
significant new emerging risks are identified, these are reported
to the Board for consideration and mitigation. No new emerging
risks have been identified during the period.
Portfolio company boards and independent assurance The boards of
our portfolio companies are responsible for ensuring they meet key
commercial objectives, and in this they are typically supported by
Arix appointee directors.
Independent assurance is provided by industry experts when
required. For example, external advisers are engaged to provide
regulatory compliance support to the board of Arix Capital
Management, Arix Bioscience's FCA-regulated fund management
subsidiary.
The Board
Sets the tone for corporate governance
Internal knowledge Key committees
Four committees oversee the effectiveness; they ensure balance and are responsible for succession
Audit & Risk Committee Nomination Committee Remuneration Committee Strategy & Investment Committee
Executive management
External assurance
Portfolio company boards and independent assurance
Risks and mitigants The key risks to Arix have been assessed in
light of the current environment; these, along with the steps taken
by Arix to manage such risks, are detailed below. The Covid-19
pandemic was an area of significant risk for all businesses during
2020 and into 2021. However its impact on Arix and our portfolio
companies has diminished to such an extent that is it no longer
seen as a key risk area going forward. Post year-end the solvency
concerns at Silicon Valley Bank (SVB) became a potential risk to
those portfolio companies with deposits at the bank. Although Arix
had no direct relationship with SVB, it did utilize SVB Leerink, a
subsidiary of SVB, as a broker for US listed securities. Whilst SVB
Leerink has continued to operate unaffected by actions at SVB, and
the public securities were not at risk, instructions were given to
transfer all holdings from SVB Leerink before FDIC intervened with
SVB. Given the actions taken by the US Government we do not foresee
a liquidity risk from SVB to any of our portfolio companies. There
have been no other changes to Arix's risk profile during the year,
although Arix continues to consider and reflect on emerging risks,
currently with particular regard to cyber security and climate
change and their potential impact on the business and its
stakeholders.
Area Risk Impact Mitigation
Negative clinical trial read outs may
reduce the value of the portfolio Arix has an experienced team responsible
company, potentially to nil. This for identifying and developing portfolio
would therefore result in a decrease companies, resulting in a high standard
in Arix's profitability, and reduce of due diligence before the commitment of
Arix's ability to generate positive any capital. Post-investment, Arix
cash flows from future realisations. typically has representatives on the
company's board of directors, ensuring it
Arix's portfolio Inconclusive read outs may both is fully aware of business developments,
typically comprises reduce the value of the portfolio and allowing for mitigation of possible
companies that are company, impacting Arix's issues as they arise.
1 engaged in clinical profitability, and require further
trials. capital to fund additional trials to Arix's commitments to investments are
Clinical seek further clarity in the results, typically tranched, such that capital is
trial risks There is a risk that the adversely impacting Arix's cash flow. not overly committed to a company at a
trials may produce single stage, with further funds only
negative or inconclusive Portfolio companies are usually not invested once pre-agreed milestones have
results. revenue-generating and are typically been reached.
only funded through to an anticipated
subsequent clinical milestone. Arix funds a range of portfolio companies
Negative or inconclusive clinical and continues to develop its portfolio
trial readouts might impact the across a range of therapeutic areas. Its
portfolio company's ability to diverse portfolio means that Arix's
attract further capital and therefore financial performance is not overly
may be unable to continue in reliant on any one business.
operation.
Arix has an experienced team responsible
for identifying and developing portfolio
companies, resulting in a high standard
of due diligence before the commitment of
Arix's portfolio any capital. Post-investment, Arix
comprises certain Arix may be unable to realise returns typically has representatives on the
investments which are not on its unlisted investments at company's board of directors, ensuring it
listed on a recognised prevailing fair values. This may is fully aware of business developments,
stock exchange, making result in a reduction in the carrying and allowing for mitigation of possible
2 them both harder to value value of investments, reducing Arix's issues as they arise.
and more difficult to profitability.
Unlisted liquidate. This should therefore improve the
investments If investments cannot ultimately be likelihood of the investment being a
There is a risk that realised, this will reduce Arix's desirable acquisition target, and
ultimate cash proceeds ability to generate positive cash therefore Arix's investment being
from an investment may be flows and reduce Arix's ability to monetised.
significantly below an continue to fund new investment
investment's current fair opportunities. Arix funds a range of portfolio companies
value. and continues to develop its portfolio
across a range of therapeutic areas. Its
diverse portfolio means that Arix's
financial performance is not overly
reliant on any one business.
Arix aims to generate Where Arix believes it has met the Arix's finance team comprises chartered
significant gains on its appropriate qualifying criteria, Arix accountants who are experienced with the
investments, which can claims the UK's Substantial tax treatments and exemptions.
result in potentially Shareholding Exemption which reduces
large corporation tax the tax on such gains and losses to Arix employs the use of a 'Big Four'
charges. nil. professional services firm to assist with
3 all tax disclosures, returns and
Where possible, Arix aims If the use of this exemption were regulatory correspondence.
Taxation to take advantage of rejected by HMRC, this would increase
available exemptions to Arix's tax liabilities, reducing For areas of significant judgement in
reduce its tax Arix's profit after tax. It would relation to tax, Arix seeks further
liabilities. also reduce Arix's cash reserves, advice from eminent professionals in the
resulting in fewer funds being field, such as a Queen's Counsel
There is a risk that tax available to fund Arix's operations Barrister, to support the tax treatment
authorities challenge the and future investment opportunities. adopted.
use of these exemptions.
Arix's Board and investment team have
Arix's success is The financial performance of Arix strong scientific and commercial
predicated on the quality depends on its ability to identify backgrounds.
of its investment and develop outstanding portfolio
decisions, which in turn companies and, as such, is reliant on Arix is implementing competitive
4 is a product of the its key personnel. long-term incentives for its investment
calibre of its investment team. Corporate staff and executive
Personnel team. Loss of key individuals could reduce directors are incentivised with a share
the quality of Arix's investment option scheme which aligns with Arix's
There is a risk of Arix decision-making and therefore performance and shareholder experience.
being unable to attract negatively affect Arix's financial
or retain staff of performance and future prospects. Arix's Nomination Committee is
sufficient calibre. responsible for appropriate succession
planning.
Arix's strategy is to deploy capital into
innovative businesses which, if
successful, will have unique, high impact
Adverse geopolitical and/or outcomes on patients' lives. Arix
Adverse macroeconomic and macroeconomic conditions may reduce believes that the inherent value of such
geopolitical market opportunities for Arix to realise businesses to patients and therefore
5 conditions may impact capital from portfolio companies, acquirers, is less susceptible to
Arix's operational model. affecting cash flow and financial macroeconomic cycles and disruptive
Macroeconomic performance if portfolio valuations geopolitical events.
and In particular, the are reduced.
geopolitical current conflict in Arix has generally well-funded portfolio
conditions Ukraine, including The same conditions may also companies across the USA and Europe. As
sanctions imposed on negatively impact the availability of such, it is not overly reliant on capital
Russia. capital for any external fundraising markets in the short term.
by Arix or its portfolio companies.
Arix monitors its availability of capital
closely, ensuring sufficient funds are
available for the investment and
operational needs of the business.
A change in government regulation Arix's portfolio is diversified by
Changes to government (for example CFIUS in the United geography, with exposure to the USA and
policy or regulation in States) may adversely affect the Europe, protecting the company from the
6 the research, healthcare profitability of the healthcare and adverse actions of any one government.
or life sciences life sciences industry, resulting in
Legislation & industries could impact a reduction in the number of Arix's corporate team actively monitors
regulation Arix or its portfolio investment opportunities, changes to laws and regulation, and where
companies. availability of external funding or necessary enlists the advice of relevant
potential exit opportunities for experts to consider any company or
portfolio companies. portfolio impacts.
Viability statement The Board has assessed the prospects of Arix
over a period greater than 12 months. We have considered a period
of three years from the balance sheet date, as the Board expects
the majority of Arix's current commitments and new proceeds raised
to be committed over the next three years, and therefore reflects
the period over which the Group's cash flows are assessed
internally.
A robust assessment of the principal and emerging risks and
their mitigants has been carried out. The Board assessed Arix's
business model, particularly its approach to capital which is
legally committed to, and provisionally reserved for further
investment into existing portfolio companies. Key judgements
reflected how future cash requirements may change from restrictive
regulations and how the availability of capital may be impacted by
ability to raise further capital on the public markets.
Having initially started with a base case scenario considering
Arix's finances over the assessment period, the estimated impacts
on the Group's cash flow, as described above, are modelled,
creating a range of adverse scenarios. An extreme downside case is
then considered, reflecting the estimated cash flow impact of all
considered risks occurring concurrently. Finally, the analysis
considers the mitigating actions the Group could take to reduce the
financial impact of the noted risks.
Based on its review, and the consideration of any changes that
had occurred post year-end, including the risk of additional market
volatility due to the emerging conflict in Ukraine (note 24), the
Board has a reasonable expectation that Arix will be able to
continue in operation and meet its liabilities as they fall due
over a three-year period from the date of this report and confirm
that preparing the financial statements on a going concern basis is
appropriate.
Our stakeholders Engagement and Decision-Making
The views and interests of our Stakeholders remain of paramount
importance to the Board and continue to inform Board discussions
and decision-making.
Stakeholder Engagement The Board maintains an open and
productive dialogue with all our Stakeholders throughout the year
as discussed below and is satisfied, through the careful tracking
of the outcomes of these discussions and decisions, that the
approach it takes to stakeholder engagement is proportionate to our
business and remains effective.
Statement by the Board in accordance with s172 Companies Act
2006 We believe that considering our stakeholders in key business
decisions is not only the right thing to do, but is fundamental to
our ability to drive value creation over the longer term. During
this financial year, in the midst of the War in Ukraine, balancing
the needs and expectations of our stakeholders has never been a
more important or challenging task.
The Directors of the Board are cognisant of their duties under
s172 of the Companies Act and consider that they have acted in a
way they each consider, in good faith, would be most likely to
promote the success of the Company for the benefit of its members
as a whole. In doing so they have had regard to those stakeholders
identified under s172, as well as the additional stakeholders set
out here.
Shareholders Securing our shareholders' trust through continuous
engagement ensures their ongoing investment and support and the
Board is focused upon delivering long-term value for their benefit.
This purpose is evident throughout the Board's decision-making and
is a constant consideration when addressing the interests of our
other stakeholders.
The Company engages with its shareholders on a regular basis
with significant engagement in 2022. Whilst physical meetings were
restricted for the first half of the year, virtual meetings have
continued to be held and with a return to physical meetings there
has been the opportunity to engage with shareholders throughout the
year. The results of this Investor Engagement are reported to the
Board to help inform our communications and strategy.
Colleagues We cannot operate and achieve our strategic goals
without an engaged colleague base that feels appreciated and is
motivated to deliver for our investors and the business' success.
Key priorities for the Board in respect to employee engagement
include promoting an inclusive and diverse place to work with a
respectful corporate culture where colleagues are encouraged to
share their views and have their voices heard in decision-making
and we strive to make our colleagues feel valued and appropriately
rewarded.
The Board are proud of the small team of employees which work
collaboratively and continuously to deliver for our investors. As a
result of the culture at Arix, and the small number of employees,
each colleague has open and continuous access to engage with our
Board of Directors and they meet with the Board throughout the year
both virtually and face to face to discuss any matters arising.
2022 saw further evolution of the management team which enabled
internal progression for some employees, whilst strengthening the
Company's ability to deliver on its strategy. The Board, in
collaboration with the Remuneration Committee, have had particular
regard to employees as they have considered the Company's long-term
incentive arrangements as part of its strategy to attract, retain
and motivate colleagues in order to deliver value for shareholders.
These actions were consistent with the Board's commitment to
investing in and responsibility rewarding employees as they deliver
on the Company's strategy.
Service Providers The Board regularly evaluates the performance
of its key panel of third-party professional service providers
which includes the Company's external accounting team, remuneration
advisors and company secretary.
Portfolio companies Our portfolio companies are at the heart of
our business as it is their operational and clinical progress which
will ultimately deliver value for our shareholders. However, whilst
the Board is naturally focused on their development and what it
will mean for the growth in our NAV, we are also conscious of the
benefit we can bring to those companies as an engaged and
supportive shareholder. Arix is closely involved with its portfolio
companies and the members of the investment team provide a direct
connection from the Board to the portfolio companies, ensuring that
the Board is regularly updated on their progress and can support
their development.
Decisions Investment: The Board has supported the continued
deployment of capital into the portfolio, including supporting Disc
Medicine's reverse merger with Gemini Therapeutics and new
investment into Ensoma alongside its acquisition of existing
portfolio company Twelve Bio. This continued investment not only
supports our portfolio companies as they work to deliver new
treatments for patients, but also provides the opportunity for
significant financial returns to shareholders.
Cost control: 2022 saw a continued focus on managing the
run-rate net operating costs of the business. In ensuring that
costs are kept low, the Board had particular regard to Arix's
shareholders but also the interests of its employees and portfolio
companies in building a sustainable business which can deliver
superior returns over the longer term.
Imara: The Board was engaged and supportive of Imara's merger
with Enliven Therapeutics. Following the disappointing clinical
failure at Imara in early 2022, the successful reverse merger with
Enliven will not only provide liquidity for Arix as in investor in
due course, but also allows Imara's capital to support Enliven's
important clinical programs. This transaction is consistent with
Arix's goal of maximizing return on investment, even after a
disappointing clinical outcome, and delivering new treatments for
patients.
Environment, Employees, Human Rights and Social Matters Due to
Arix's status as a life sciences investment company with minimal
full time employees the direct impact on environmental matters,
social, community and human rights issues is minimal. No further
disclosures are to be made in respect of these matters beyond those
set out in the Streamlined Energy & Carbon Reporting
section.
Sustainability Streamlined Energy & Carbon Reporting
The section below includes our mandatory Streamlined Energy and
Carbon Reporting requirements. The reporting period is the same as
the Group's financial year, 1 January 2022 to 31 December 2022.
Emissions We have reported on all of the emission sources
required under the Companies (Directors' Report) and Limited
Liability Partnerships (Energy and Carbon Report) Regulations 2018.
These sources fall within the Group's consolidated financial
statement.
An operational control approach has been used in order to define
our organisational boundary. This is the basis for determining the
Scope 1 and 2 emissions for which the Group is responsible.
The emissions sources that constitute our boundary for the year
to 31 December 2022 are:
? Scope 1: natural gas combustion within boilers and refrigerant
gas losses; and
? Scope 2: purchased electricity for our own use.
All carbon dioxide emissions and energy consumption figures
relate to emissions in the United Kingdom (UK), accounting for 73%
of total emissions, and the United States of America (USA),
accounting for 27% of total emissions.
Methodology For the Group's reporting, the Group has employed
the services of a specialist adviser, Verco Limited, to quantify
and verify the Greenhouse Gas (GHG) emissions associated with the
Group's operations.
The following methodology was applied by Verco in the
preparation and presentation of this data:
? The Greenhouse Gas Protocol published by the World Business
Council for Sustainable Development and the World Resources
Institute (the "WBCSD/WRI GHG Protocol");
? application of appropriate emission factors to the Group's
activities to calculate GHG emissions;
? application of 2 reporting methods, location-based and
market-based emission factors, for electricity supplies;
? inclusion of all the applicable Kyoto gases, expressed in
carbon dioxide equivalents, or CO2e;
? presentation of gross emissions as the Group does not purchase
carbon credits (or equivalents);
? presentation of global annual energy use;
? where data was missing, values were estimated using an
extrapolation of available data or available benchmarks; and
? calculation of electricity and gas consumption using CIBSE
energy benchmarks where data was not available.
Absolute Emissions The total Scope 1 and 2 GHG emissions from
the Group's operations in the year ending 31 December 2022
were:
? 8.1 tonnes of CO2 equivalent (tCO2e) using a 'location-based'
emission factor methodology for Scope 2 emissions; and
? 8.9 tonnes of CO2 equivalent (tCO2e) using a 'market-based'
emission factor methodology for Scope 2 emissions.
The Group's GHG emissions have been separated by those generated
inside of the UK and those generated outside of the UK - the USA in
this case. The breakdown is as follows:
. 61% of emissions were generated in the UK and 39% were
generated in the USA using the 'location-based' emissions factor
methodology for Scope 2 emissions;
. 71% of emissions were generated in the UK and 29% were
generated in the USA using the 'market-based' emissions factor
methodology for Scope 2 emissions.
Total Energy Use The total energy use for the Group for FY2022
was 36,334 kWh.
Electricity/Fuel Total
Energy Use
Electricity Gas (kWh)
(kWh) (kWh)
2022 16,142 20,192 36,334
2021 12,524 26,565 39,089
2020 29,006 27,331 56,337
========= ========= =========
Intensity Ratio As well as reporting the absolute emissions, the
Group's GHG emissions are reported on the metrics of tonnes of CO2
equivalent per employee and tonnes of CO2 equivalent per square
foot of the occupied areas. These are the most appropriate metrics
given that the majority of emissions result from the operation of
the Group's offices and the day-to-day activities of the
employees.
The intensity metrics for FY2022 were as follows:
? 1.01 tonnes of CO2e per employee (location-based method) and
1.11 tonnes of CO2e per employee (market-based method); and
? 0.004 tonnes of CO2e per square foot of occupied space
(location-based method) and 0.004 tonnes of CO2e per square foot of
occupied space (market-based method).
Change in Emissions There has been an increase in emissions for
both location-based of 8.0% and an increase in emissions
market-based methods of 14%. The market based emissions in 2022
have increased due to increased office occupancy and resultant
energy and facilities usage per head in comparison to that of 2021
when post Covid-19 events and working from home arrangements were
more frequent.
Sustainability continued
Key Figures
Arix Bioscience plc - Breakdown of emissions by scope
GHG emissions 2022 2021 2020
Tonnes tCO2e / tCO2e / Tonnes tCO2e / tCO2e / Tonnes tCO2e / tCO2e /
CO2e emp.4 sq. ft.5 CO2e emp.4 sq. ft.5 CO2e emp.4 sq. ft.5
Scope 11 3.68 0.46 0.002 4.86 - - 5.02 0.55 0.002
Scope 22 4.41 0.55 0.002 2.67 - - 6.77 0.74 0.002
Scope 23 5.21 0.65 0.003 2.94 - - 0.09 0.00 0.00
Total GHG emissions
(Location-based Scope 8.10 1.01 0.004 7.53 - - 11.80 1.29 0.004
2)
Total GHG emissions
(Market-based Scope 2) 8.89 1.11 0.004 7.80 - - 5.11 0.55 0.002
========= ========= ========= ========= ========= ========= ========= ========= =========
1 Scope 1 being emissions from the Group's combustion of fuel
and operation of facilities.
2 Scope 2 being emissions from electricity (from location-based
calculations), heat, steam and cooling purchased for the Group's
own use.
3 Scope 2 being emissions from electricity (from market-based
calculations), heat, steam and cooling purchased for the Group's
own use.
4 Employee numbers: 8 (FY2022); varies throughout FY2021: 9 9
(FY2020). Intensity ratio not calculated for 2021 due to an office
move and absolute figures not being obtained.
5 Occupied office space: 2,077 ft2 (FY2022; varies throughout
FY2021 due to office move; 2,844 ft2 (FY2020). Intensity ratio not
calculated for FY2021 due to an office move and absolute figures
not being obtained.
6 Location based emissions are UK only.
Energy Efficiency Actions The Group offers fully remote working
twice a week to all employees, with the aim of reducing emissions
related to commuting.
Corporate Governance
Corporate Governance Report
Chairman's Governance Overview Dear Shareholder, I am pleased to
present this year's Report on Corporate Governance. This report
forms part of the Directors' Report which follows. Since its
listing on the London Stock Exchange in February of 2017, the
Company has voluntarily applied the UK Corporate Governance Code
(the Code) as an integral part of its approach to governance. This
report includes a description of how the Company has applied the
Code in the context of the Company's governance structures.
Turbulent Times The Board has delivered a heightened degree of
oversight and scrutiny throughout 2022 to ensure the long-term
sustainable success of the Company in light of the impacts of
unstable markets and the War in Ukraine on our business and on the
markets more generally.
New Leadership There were a number of changes to the Arix Board
this year. Shareholders will have noted our announcement last
August confirming the appointments of Dr Debra Barker, Dr Benny
Soffer, and Andrew Smith as independent, Non-Executive Directors.
At the same time, Sir Michael Bunbury resigned from the Board as
Senior Independent Director ("SID"). Debra Barker was subsequently
appointed as SID and Chair of the Remuneration Committee and we are
very pleased that Andrew Smith has since accepted the role of Chair
of the Audit and Risk Committee. Benny Soffer resigned from the
Board on 31 January 2023, post year-end.
On behalf of the Board, I would like to thank Sir Michael
Bunbury personally for his contributions to the Board and
Committees of the Company and his significant contribution to
improving corporate governance at Arix throughout 2022.
Peregrine Moncreiffe Chairman 24 April 2023
UK Corporate Governance Code Compliance Statement As a company
admitted to the standard segment of the Official List, the Company
is not required to adopt the UK Corporate Governance Code but it
has voluntarily chosen to observe the requirements of the Code, a
copy of which is available at www.frc.org.uk. For the year ended 31
December 2022, the Board considers it has made valiant efforts to
comply in full with all applicable principles and provisions of the
Code. Where the Company has deviated from the Code, an explanation
is set out below: During the year the Company has applied all of
the main principles of the Code and provides below explanations of
its non-compliance with the Code provisions:
Provision 5 - The Company operates a lean business model
employing only 9 employees across Europe and the USA (at 31
December 2022); this scale means that the Board has not felt it
necessary to designate a Non-Executive Director to specially engage
with the workforce, as the Board has regular and Direct contact
with its employees as discussed later in this report.
Provision 11 - During the year, the Non-Executive Director
Appointments of Debra Barker, Benny Soffer and Andrew meant that
the Company were compliant with this provision as over 50% of the
NEDs were independent upon appointment (excluding the Chair),
however Benny Soffer's departure on 31 January this year meant that
only one third of the Board is currently comprised of independent
non-executive directors. The Board is looking to add a further
independent non-executive director in 2023 to address this
balance.
Key areas of Board Focus and Consideration during the year under
review are set out in the table below
Focus Operation
? Providing leadership and setting values and standards.
Leadership, strategy
and Management ? Approving the Company's strategic aims and objectives.
? Overseeing operations.
? Changes to the Group's capital or corporate structure.
Structure and capital
? Changes to the Group's management and control structure.
? Approval of financial statements.
? Review of the dividend policy.
Financial reporting
? Approval of material changes in accounting policies.
? Approval of major capital expenditure.
? Ensuring maintenance of a sound system of internal control and risk management.
Risk management and
internal controls ? Determining the principal risks of the Company and how they are managed and mitigated.
? Reviewing the effectiveness of the risk and controls processes.
? Changes to the structure, size and composition of the Board.
Board membership ? Ensuring adequate succession planning.
? Appointment or removal of the Chairman, CEO, SID and Company Secretary.
? Review of Group's overall governance framework.
? Determining the independence of Directors.
Corporate governance
? Considering the balance of interests between shareholders and other stakeholders.
? Authorising any conflicts of interest.
? Determining the policy for remuneration of the Chairman, the Chief Executive Officer (CEO),
the Company Secretary and senior investment team members.
? Ensuring that the pension contribution rates for the CEO, or payments in lieu, are aligned
with those available to the workforce.
? Ensuring that workforce remuneration and related policies are taken into account when
Remuneration setting Directors' remuneration.
? Ensuring that employee engagement has taken place to explain how executive remuneration
aligns with wider company pay policy.
? Determining the remuneration of the Non-Executive Directors.
? Introducing new share incentive plans or major changes to existing plans.
? Approval and monitoring of the share dealing code.
? Keeping the Company's ESG Strategy and mandatory reporting obligations under review.
Other
? Approving policies and political and charitable donations.
? Approval of the overall levels of insurance for the Group.
Board leadership and company purpose Our governance framework
facilitates responsive and effective decision-making, ensuring that
the Board and its Committees, senior management and the Company's
professional service providers are able to collaborate proactively,
consider issues and respond accordingly.
An effective Board The role of the Board is to define the
company's purpose and provide entrepreneurial leadership to the
Group to set a strategy underpinned by the values and behaviours
that shape its culture. An effective board monitor's performance
and ensures that the necessary financial and human resources are in
place to enable the Group to meet its objectives. It will be able
to explain the main trends and factors affecting the long-term
success and future viability of the company and how these and the
company's principal risks and uncertainties have been addressed. In
addition, the Board ensures the appropriate financial and business
systems and controls are in place to safeguard shareholders'
interests and maintain effective corporate governance.
The Board operates in accordance with the Company's Articles of
Association and its own written schedule of matters reserved for
Board decision-making. The Board has established a number of
committees, each with its own defined terms of reference, which are
reviewed at least annually and are publicly available on the
Company's website at: www.arixbioscience.com/investrorelations
Assessing and monitoring culture The Board is keen to ensure
that the culture of the Company is aligned to Arix's Purpose, Goal
and Values (as set out on the inside front cover to this report).
Individual Board members have regular, direct contact with the
business and are confident that the culture of the Company and its
employees is consistent with what it expects in order to maintain a
high standard of business conduct and deliver the Company's
strategy. This is consistent with the Board's duties under s172 of
the Companies Act.
Stakeholder and employee engagement The Board has actively
engaged with stakeholders, including employees, throughout the
period and has taken their interests into account when making
decisions. Due to the low employee numbers it is possible for the
Board to have regular and direct interaction with a large
proportion of the Company's employees, and management team, many of
whom participate at meetings of the Board and its Committees.
A full description of the Company's engagement with its
stakeholders is set out further below with specific description of
engagement with employees on remuneration in the Remuneration
Report. As described further below, the Company has kept its
Whistleblowing Policy and arrangements under review during
2022.
External relationships Due to the nature of our business, we
have very few suppliers and direct customers, however the Board
recognises that to deliver our strategy requires strong, mutual and
beneficial relationships with our professional service providers.
The Board carefully considers the need to foster its business
relationships with its key stakeholders of which an explanation is
set out further below outlining how this is achieved.
Conflicts of interest Each Director has a duty under the
Companies Act 2006 to avoid a situation where they have, or can
have, a direct or indirect interest that conflicts, or possibly may
conflict with the company's interests. The Company's Articles of
Association set out the policy for dealing with Directors'
conflicts of interest, in line with the Companies Act 2006. The
Articles permit the Board to authorise conflicts and potential
conflicts, as long as the potentially conflicted Director is not
counted in the quorum and does not vote on the authorising
resolution. All Directors declare any potential conflicts of
interest before their appointment, such that the Board can consider
how to address any pre-existing potential conflicts before an
appointment is confirmed. A record of Directors' interests is kept
by the company secretary and Directors are reminded at the
beginning of each Board meeting to notify the Board of any further
conflicts of interest, in accordance with Sections 175, 177 and 182
of the Companies Act 2006.
Board attendance
Board Audit Remuneration Nomination Strategy &
& Risk Investment
Peregrine Moncreiffe 8/8 3/3 2/2 3/3
Debra Baker* 3/3 2/2 1/1
Isaac Kohlberg 7/8 1/2 3/3
Maureen O'Connell** 7/8 3/3 1/1 1/1 3/3
Robert Lyne 8/8 3/3
Andrew Smith* 3/3 1/1 1/1
Benny Soffer* 3/3 1/1 2/2
Sir Michael Bunbury* 5/5 5/5 1/1
========= ========= ========= ========= =========
* Sir Michael Bunbury resigned from the Board of Directors on 9
August 2022. Debra Barker, Andrew Smith and Benny Soffer were
appointed to the Company's Board and its Committees on 10 August
2022.
** Maureen O'Connell stepped down as Chair of the Remuneration
Committee and resigned from the Company's Board Committees during
the year.
Attendance is expressed as the number of scheduled meetings
attended out of the number of such meetings possible or applicable
for the Director to attend.
Board independence Non-Independent Current directors Robert Lyne
(being an executive director), appointed 29 April 2021
Isaac Kohlberg (having been proposed by and a non-executive
director of the Company's largest shareholder, Acacia), appointed
29 April 2021
Maureen O'Connell (having been proposed by and the non-executive
Chair of the Company's largest shareholder, Acacia), appointed 29
April 2021
Independent Current directors Peregrine Moncreiffe, appointed 29
April 2021
Dr. Debra Barker, appointed 10 August 2022
Andrew Smith, appointed 10 August 2022
Dr. Benny Soffer, appointed 10 August 2022 and resigned on 31
January 2023, after the reporting date.
Sir Michael Bunbury, appointed 6 October 2021 and resigned on 9
August 2022.
Board Process The Board meets formally at least once per quarter
with ad hoc meetings called as and when circumstances require at
short notice. The table above shows the attendance of each Director
at formal meetings of the Board and the Code governed committees of
which they are a member.
All Directors are expected to attend all meetings of the Board,
and any committees of which they have been appointed and to devote
sufficient time to the Company's affairs to fulfil their duties as
Directors. Where Directors are unable to attend a meeting, they
will be encouraged to submit to the Chairman any comments on papers
to be considered at the meeting in advance, to ensure their views
are recorded and taken into account.
The Non-Executive Directors meet at least once per year without
the Chairman present and on such additional times as are
required.
Training and development of Board Directors The Company
Secretary regularly provides the Board with updates on Corporate
Governance and regulatory matters at Board meetings. All Directors
are provided with an introduction to the Company and relevant
background materials. All Directors have access to the advice of
the Company Secretary who is responsible for advising the Board on
all governance matters.
Information and support An agenda and accompanying detailed
papers are circulated to the Board in advance of each Board
meeting. These include reports from the Chairman and other members
of senior management, and all Directors have direct access to
senior management should they require additional information on any
of the items to be discussed. In December 2022, the Board
transitioned to utilising a reputable Board portal to securely
disseminate and review all confidential Board and Committee meeting
materials.
The information supplied to the Board and its committees will be
kept under review to ensure it is fit and proper for purpose, and
that it enables sound decision-making.
The Company has adopted a formal procedure through which
Directors may obtain independent professional advice at the
Company's expense. The Directors also have unrestricted access to
the services of the Company Secretary.
Division of responsibilities Key Board roles and
responsibilities The Board is ultimately responsible to
shareholders for the direction, management, performance and
long-term sustainable success of the Company. It sets the Group's
strategy and objectives and oversees and monitors internal
controls, risk management, principal risks, governance and
viability of the Company. In doing so, the Directors comply with
their duties under section 172 of the Companies Act 2006.
The Board has established certain principal committees to assist
it in fulfilling its oversight responsibilities, providing
dedicated focus on particular areas as described in the reports of
the Nomination, Remuneration, Strategy & Investment and Audit
& Risk Committee reports incorporated in this Annual Report.
The Chair of each committee reports to the Board on the committee's
activities after each meeting.
Role of the Chairman The Chairman is responsible for the
leadership and effectiveness of the Board:
? In particular, the formulation of strategy and its alignment
with culture, governance (having regard to best practice); Board
changes and succession planning.
? Ensuring constructive relations between Non-Executive
Directors and the Chief Executive Officer and senior
management.
? Ensuring that new Directors receive a full, formal and
tailored induction on joining the Board.
? Monitoring and actively participating in stakeholder
engagement.
? Ensuring that the Company Secretary is effective and
supported.
? Chairing the Company's AGM and all other formal shareholder
meetings.
Role of the Senior Independent Director Dr. Debra Barker was
appointed as Senior Independent Director (SID) on 10 August 2022
following the resignation of Sir Michael Bunbury on 9 August 2022.
The role as SID is to act as a sounding board for the Chairman and
serve as an intermediary for the other Directors when necessary. In
order to fulfil this role:
? The SID will meet other Non-Executive Directors without the
Chairman present at least once a year, to appraise the Chairman's
performance, taking into account the views of any Executive
Directors, plus on such other occasions as are deemed
appropriate.
? The SID is also available to shareholders should they wish to
discuss concerns they have failed to resolve through the normal
channels of the Chairman or any Executive Director or for which
such contact is inappropriate.
Role of the Chief Executive Officer The Chief Executive role is
primarily responsible for the running of the Group and for
executing strategy as agreed by the Board. This involves:
? Driving the execution of the Company's strategy.
? Chairing the Strategy and Investment Committee.
? Ensuring implementation of the Board's decisions.
? Ensuring the timely communication of information to the Board
in sufficient detail to allow it to monitor the performance of the
Group's business as a whole.
? Communicating to the Board their own views and those of the
management team, on business issues facing the Group such that the
Board may have a full and balanced view of the issues and factors
it should consider when making decisions.
? Managing their direct reports and ensuring that the overall
team is motivated and develops in order to deliver on the Group's
strategy.
? Ensuring the effective implementation of the Company's wider
stakeholder engagement programmes.
Board Composition The Board currently consists of six Directors
(including the Chief Executive Officer), three of whom are
considered to be independent (including the Chair). We clearly
define the roles of the Chair and the Chief Executive and fully
support the separation of the two roles. Furthermore, the Board
believes it operates effectively with the appropriate balance of
independent Non-Executive Directors and Executive Directors.
Commitment The Board regularly considers the time commitments of
our Directors. The Board expects Non-Executive Directors to commit
sufficient time to allow them to meet their obligations to the
Company. The Non-Executive Directors are required to confirm, on
acceptance of the role, that they have sufficient time to meet the
expectations of their role. Non-Executive Directors will need to
attend scheduled and emergency Board meetings and committees as
well as the AGM, as well as allowing appropriate preparation time
ahead of each meeting.
The quality of information and resources available to the Board
has enabled us to operate effectively and efficiently throughout
the year.
Peregrine Moncreiffe Chairman 24 April 2023
Board of Directors
Peregrine Moncreiffe Peregrine Moncreiffe has extensive
experience in investment management and banking. In his previous
career, Peregrine held various corporate finance and trading
positions in New York, London and East Asia within the Credit
Suisse First Boston (CSFB) group over a ten-year period, ending up
as an Executive Director of CSFB in London in 1982. Peregrine also
held a number of trading management roles at Lehman Brothers as a
Managing Director in New York and London until 1986, when he joined
E F Hutton as Managing Director of International Capital Markets
before it was acquired by Shearson Lehman in 1988. From 1990 to
2000 he was Chief Executive Officer of Buchanan Partners Ltd, a
proprietary investment company which he co-founded. In 1998 he
became Chairman of UA Group, the agricultural services and property
investment business sold to Elphinstone in 2005. He remains a
director of North Atlantic Investment Trust plc after stepping down
from the Chair after more than 9 years in the role. Peregrine
remains a director of Metage Funds Limited, a Jersey holding
company through which Acacia, alongside other shareholders, hold an
interest in Viamet Pharmaceuticals, Inc.
Peregrine received an undergraduate degree from the University
of Oxford.
Peregrine is a member of the Strategy & Investment, Audit,
Nomination and Remuneration Committees for Arix.
Debra Barker, MD Dr Debra Barker is a seasoned International
Life Sciences executive with more than 25 years' senior experience
in major pharmaceutical companies Roche, SmithKline Beecham and
most extensively Novartis where she held several senior scientific,
operational and commercial roles over 17 years, including
Development Head for Infectious Diseases, Immunology and
Transplantation, Global Programme Head in Oncology and Head of
Clinical and Medical Services. Biotech experience was gained in
Chief Medical Officer & Head of Development roles and Debra was
also the medical lead for the Swiss-based Biotech Polyphor's highly
successful IPO on the SIX Swiss Exchange.
Debra is currently serving as a Non-Executive Director for three
public Biotechnology companies listed in UK, EU & USA namely
Destiny Pharma PLC, BergenBio ASA and CureVac NV.
Debra has a Diploma in Pharmaceutical Medicine and received a
MSc in Immunology from the King's College in London and a Medical
Degree from the Queens' College, Cambridge, UK.
Debra joined the Arix Board in August 2022 as Senior Independent
Director and is Chair of the Remuneration Committee, a member of
the Audit & Risk Committee, the Nomination Committee and the
Strategy & Investment Committee.
Robert Lyne Robert Lyne has 15 years' experience working with
high growth technology companies having spent several years working
in listed venture capital. He joined Arix in 2017 as General
Counsel and Company Secretary before being promoted to Chief
Operating Officer in 2019. He joined the Board in April 2021 and
was appointed Interim Chief Executive Officer before being
subsequently appointed to the role on a permanent basis. He began
his career as a lawyer at international law firm Bird & Bird
LLP in London. He has worked on over 80 venture capital financings
in Europe and North America as well as multiple trade exits and
IPOs, working with both company boards and investors to
successfully execute complex cross-border transactions. As an
experienced plc Company Secretary, Robert has broad experience of
public company governance.
Robert has a BA from the University of Oxford and an LLB from
Oxford Brookes University.
Isaac Kohlberg Isaac Kohlberg has had a distinguished career
protecting and commercializing IP for leading universities and
research institutions. He currently is a Senior Associate Provost
and Chief Technology Development Officer at Harvard University,
where he is responsible for the strategic management and commercial
development of all technologies and intellectual property (IP)
arising from Harvard's research enterprise. Isaac's role at Harvard
University includes industry liaising and outreach, IP management,
business development, technology commercialization and the
formation of startup companies and new ventures around Harvard
technology platforms. In tandem, he is also responsible for
generating, structuring, and negotiating research alliances and
collaborations with industry and generating industry-sponsored
research funding for Harvard faculty.
Isaac currently serves as a non-executive director of Acacia,
Arix's largest shareholder, and received his M.B.A. from INSEAD and
LL.B. from Tel Aviv University.
Isaac joined the Arix Board in April 2021 and is a member of the
Strategy & Investment Committee.
Maureen O'Connell Maureen O'Connell is a global business
executive, Chief Financial Officer and corporate director
recognized for significant value creation through strategic
initiatives in a variety of industries including media, education,
digital, retail, technology, professional services, biotech,
pharma, homebuilding, real estate and insurance.
From 2007 to 2017, Maureen served as the Chief Financial Officer
of Scholastic Corporation, the world's largest publisher and
distributor of children's books. In her role as Chief Financial
Officer, where she had significant experience licensing rights,
partnering with trademark and copyright owners, and overseeing the
protection and assertion of rights on a world basis. Earlier in her
career, Maureen served as President and Chief Operating Officer of
the Gartner Group the world's leading research and advisory company
which has developed more than 300,000 business case studies of
intellectual property since 1979.
Maureen has received numerous and diverse awards including CFO
Studio's CFO World Class Award in 2017, Treasury and Risk
magazine's 30 Outstanding Women in Business in 2012 and Irish
Voice's Top 75 Influential Women in 2009.
Maureen also served as an independent director, audit committee
chair and transaction committee chair at Sucampo Pharmaceuticals, a
biopharmaceutical company focused on the development and
commercialization of highly specialized medicines, from 2013 to
2018 when it was acquired by Mallinckrodt in a USD1.2 billion
transaction. At Sucampo, Maureen played a key role in evaluating
the acquisition of highly specialized medicines in development
resulting in the acquisition of two companies. She was previously
an independent director at Harte-Hanks Inc. and previously served
on the board of directors of Beazer Homes USA Inc. Maureen
currently serves as a Director of Acacia, Arix's largest
shareholder.
Maureen graduated Magna Cum Laude with a B.S. in Accounting and
Economics (dual major) from New York University Stern School of
Business in 1985 and is a Certified Public Accountant.
Maureen joined the Arix Board in April 2021 and is a member of
the Strategy & Investment Committee.
Andrew Smith Andrew Smith is an accomplished executive leader
with over 30 years' international experience within the medical
device and bio-pharma sectors. He currently serves as Chief
Financial Officer for Santhera Pharmaceuticals AG, a public company
listed on SIX under SANN, a global specialty pharmaceutical company
focused on developing medicines for rare diseases. Before joining
Santhera he held the roles of CFO and COO at Allecra Therapeutics
GmbH and CFO at Sucampo Pharmaceuticals Inc, listed on NASDAQ under
SCMP, until 2017, it was subsequently acquired by Mallinckrodt in
February 2018.
Together with previous senior financial and operations roles
focusing on business improvement and transformation he has gained
significant exposure to European and US markets working in public
and private organizations with a focus on start-ups, spinoffs and
growth and has been involved in a number of capital market
transactions in excess of USD800 million.
Andrew is a Fellow of the Chartered Institute of Management
Accountants and a Chartered Global Management Accountant. He
studied Business and Accounting at Liverpool John Moores University
and Durham University Business School.
Andrew joined the Arix Board in August 2022 and is Chair of the
Audit Committee and a member of the Nomination Committee.
Sir Michael Bunbury Sir Michael Bunbury stepped down from the
Board of Directors and associated Board Committees on 9 August
2022.
Benny Soffer Benny Soffer resigned from the Board of Directors
and associated Board Committees on 31 January 2023, after the
reporting date.
Report of the Nomination Committee
Peregrine Moncreiffe Chairman of the Nomination Committee
Composition Peregrine Moncreiffe (Chairman) Dr. Debra Barker
Andrew Smith
Dear Shareholders, On behalf of the Board, I am pleased to
present the Nomination Committee report for the year ended 31
December 2022.
Key areas of focus for the committee in 2022 included:
? Non-Executive Director appointments.
? Executive Team succession planning.
? Diversity & inclusion.
Key areas of focus for 2023
? Diversity & inclusion.
? Management Team diversity and succession planning.
Role and responsibilities The role of the Nomination Committee
is set out in its terms of reference, available on the Company's
website www.arixbioscience.com/investrorrelations.
The Nomination Committee assists the Board in discharging its
responsibilities relating to the composition and make-up of the
Board and its committees.
Specific duties of the Nomination Committee include:
? Reviewing the composition of the Board and the Board's
committees.
? Reviewing the balance of skills required by the Board and its
committees and the business as a whole.
? Considering the need for succession planning within the Board
and the Board's committees.
? Setting and managing the process for the search for new
Non-Executive Directors (NEDs).
Key areas of focus for the committee in 2022 included:
Effectiveness and succession planning
? The committee reviewed the results of the Board performance
evaluation process and agreed actionable items arising to take
forward to 2023.
? The committee reviewed the time commitments of all
Non-Executive Directors to ensure all members of the Board are
devoting sufficient time to fulfil their duties.
? The committee met to discuss and assist with succession
planning, advised by the CEO about any strategic and commercial
changes affecting the Company and satisfied itself that appropriate
processes and plans are in place for succession planning.
Appointments
? The committee actively participated in the recruitment
process, and actively contributed to the onboarding and induction
of each of the newly appointed Non-Executive Directors assisted by
the Company Secretary.
? The committee made recommendations to the Board regarding the
re-election of Directors by shareholders at the company's Annual
General Meeting.
Board and Committee composition
? The committee reviewed the structure, size and composition of
the Board and its committees and made recommendations for changes
to the membership of the committees.
? The committee evaluated the balance of skills, knowledge,
experience and diversity of the Board.
? The committee reviewed suitable candidates for the role of the
Senior Independent Director and recommended the appointment of Dr.
Debra Barker as SID with her appointment effective as at 10 August
2022.
? The committee reviewed the appropriateness of the Board's
policy on diversity and expanded it accordingly.
Meetings The number of meetings of the Nomination Committee and
attendance is set out in the Corporate Governance Report.
Only members of the Nomination Committee have the right to
attend meetings, but we may invite other Directors, executives or
advisers to attend all or part of any meeting as appropriate.
Board changes There were a number of Board changes during the
year as explained in the Corporate Governance Report. Three new
independent, Non-Executive Directors were appointed in August 2022
which coincided with the resignation and retirement of Sir Michael
Bunbury as Senior Independent Director.
Debra Barker is a seasoned international life sciences executive
with more than 25 years' senior and board experience from start-up
biotech to big pharma companies, having held a number of senior
drug development, strategic and operational roles in Novartis,
Roche, SmithKline Beecham and Knoll. Debra is currently
Non-Executive Director of three publicly listed biotechnology
companies: Destiny Pharma plc, BergenBio in Norway and most
recently, CureVac AG in Germany. Debra has a Diploma in
Pharmaceutical Medicine and received a MSc in Immunology from the
King's College in London and a Medical Degree from the Queens'
College, Cambridge, UK.
Benny Soffer, MD is Co-Founder and Chief Investment Officer of
Consonance Capital Management, a healthcare-focused public equity
investment management firm. He previously completed a residency in
internal medicine at Yale and served in a variety of managerial
roles at Yale-New Haven Hospital. Dr Soffer is a Clinical Assistant
Professor of Medicine at Weill Cornell Medicine, receiving his MD
at Emory University and his MBA at Yale University. Benny Soffer
resigned from the Board on 31 January 2023 in order to spend more
time on his other business interests.
Andrew Smith is an internationally experienced chief financial
officer with strong financial and operational experience in US,
Swiss and UK-based biotech and pharmaceutical companies. Andrew is
currently Chief Financial Officer of Santhera Pharmaceuticals, the
Swiss-based speciality pharmaceutical company, having previously
held a number of senior operational and financial roles in
companies including Allecra Therapeutics, Sucampo Pharmaceuticals
Inc., and Retroscreen Virology (Now HVIVO Ltd). He is a Fellow of
the Chartered Institute of Management Accountants and a Chartered
Global Management Accountant. He studied business and accounting at
Liverpool John Moores University and Durham University Business
School.
The range of skills and experience of the newly appointed NEDs
are a welcomed change to the Arix Board and serve to further
reinforce and focus our investment strategy, particularly following
the creation of the Public Opportunities Portfolio earlier this
year, and we look forward to benefitting from their international
industry, financial and operational expertise.
The Nomination Committee has worked throughout 2022 to ensure
that the Board and its committees continue to have the necessary
balance of skills and appropriate succession plans in place to
ensure its continued effectiveness. The Nomination Committee in
conjunction with the rest of the Board also keeps the necessary
balance of skills amongst management under regular review to inform
succession plans for executive roles.
In accordance with past practice, and the Code, all Directors
will be subject to re-election at each AGM.
Diversity Policy A comprehensive review of the Board's Diversity
Policy was reviewed in December 2022. The Policy acknowledges the
benefits of greater diversity, including gender diversity and
states that the Company remains committed to ensuring that the
Company's Directors bring a wide range of skills, knowledge,
experience, backgrounds and perspectives. All appointments will,
however, continue to be on merit against objective criteria, in the
context of the overall balance of skills and backgrounds that the
Board needs to maintain in order to remain effective. The
objectives of the Policy set out the process to be followed by the
Nomination Committee during the recruitment process in order to
ensure that an appropriately diverse pool of candidates is
considered to enhance the balance of skills and backgrounds on the
Board. The Board is satisfied that the Policy is in line with its
strategic priorities, and is looking to improve the Board's
diversity with future appointments.
During the period under review, The Committee consulted with a
number of reputable external search consultants before selecting
Nurole to carry out a search for new Non-Executive Directors. This
process resulted in the appointment of Debra Baker as a
Non-executive director and Senior independent Director of the
Company.
Annual Effectiveness Evaluation The performance of the Board,
its Committees, the Chairman and individual Directors were
internally evaluated in 2022, the outcomes of which are set out in
more detail below.
Peregrine Moncreiffe Chairman of the Nomination Committee 24
Aprill 2023
BOARD EFFECTIVENESS Provision 21 of the Financial Reporting
Council's UK Corporate Governance Code provides that Boards should
conduct a formal and rigorous annual evaluation of the performance
of the board, its committees, the chair and individual directors.
Provision 22 of the Code provides that the Chair should act on the
results of the evaluation by recognising the strengths and
addressing any weaknesses of the board. Accordingly, in 2022, the
Board conducted an annual evaluation to consider its composition,
diversity and how effectively members work together to achieve
objectives.
Review of Board and Committees This year we decided to carry out
an internal review of our Board's effectiveness, supported by Kin
Company Secretarial (Kin) which provided the platform and some
input for the basis of our questionnaire-based approach.
Having onboarded three new Non-Executive Directors in the latter
half of 2022, we decided that it would be appropriate to carry out
an internal review to set a new baseline.
The effectiveness of each of the Board's committees were taken
into account as part of the evaluation and the output will assist
the Nomination Committee in its thinking as it develops and keeps
under review the composition of the Board Committees in 2023. The
review took the form of an online questionnaire, designed with
assistance from Kin with input from the Chairman and Senior
Independent Director. Kin provided an anonymised report following
the evaluation and the Chairman, in discussion with the Board, have
agreed an action plan for 2023.
In addition to this review, during a private meeting of the
Non-Executive Directors, the Senior Independent Director led an
initial review of the Chairman's performance, by way of an
additional questionnaire. Assisted by the company secretary, the
NEDS together with the SID discussed and agreed the Chairman's
objectives for 2023.
Each Committee chair considers feedback for the Committees for
which they are responsible. Each reviewed the effectiveness and
processes to support their Committees and introduced a number of
changes for how those Committees operate.
Stages of the Board Effectiveness Evaluation
August 2022
? Appointment of new NEDS
December 2022
? Decision reached to undertake an internal Board effectiveness
evaluation and a plan of action was established to support the
process.
December 2022
? Both the Board Effectiveness Review and the Chairman's
Evaluation questionnaires were drafted and circulated to the
Board
January 2023
? Responses received and reviewed by the Company Secretary and
draft action plan for 2023 prepared and shared with the Chairman
and SID.
March 2023
? Board reviewed and approved the proposed action plan for the
year ahead
Key outcomes The evaluation reviewed key areas of Board
performance which included:
? boardroom dynamics and processes;
? leadership Succession;
? strategy and Purpose; and
? board Diversity and Committee Composition.
Overall, the Board have a collaborative working relationship and
the recently appointed Directors have been contributing positively
to the overall effectiveness of the Board. The key emerging themes
from the evaluation have highlighted a few key areas of focus which
require the Board's attention and which the Board, together with
the Committee Chairs have agreed an action plan for 2023.
The broard areas in which the Board will continue to focus on
during 2023 will include:
? Determining the Company's core purpose and setting and
agreeing an appropriate strategy to align with these values.
? Determining the Company's Investment Strategy and rationale
for Board-Level decision-making on Investments and communicating
this strategy to the Investment Team.
? With input from the Nomination Committee, establishing a
sufficient succession plan for the Board, Executive and senior
management team.
? Keeping the Board's Diversity Policy under review to ensure it
remains appropriate and evolves with the legislation in this
area.
Report of the Audit and Risk Committee
Andrew Smith Chairman of the Audit and Risk Committee
Composition Andrew Smith (Chair) Peregrine Moncreiffe Debra
Barker
Dear Shareholders, On behalf of the Board, I am pleased to
present the Audit and Risk Committee report for the year ended 31
December 2022.
The Committee is chaired by an independent non-executive
director and including the Committee chair, it is comprised solely
of independent non-executive directors.
The Board considers that I have recent and relevant financial
experience as recommended under Provision 24 of the UK Corporate
Governance Code (the Code) as it applies to the Company. In line
with the Code, the Audit and Risk Committee as a whole is deemed to
have competence relevant to the sector in which the Company
operates.
The Committee's role is to assist the Board with the discharge
of its responsibilities in relation to internal and external audits
and controls, including reviewing the Group's annual financial
statements, considering the scope of the annual audit and the
extent of the non-audit work undertaken by external auditors,
advising on the appointment of external auditors and reviewing the
effectiveness of the internal control systems in place within the
Group.
During the year in review the Committee has met once during my
chairmanship and five times in 2022 prior to my appointment on 10
August. Further details on the activities of the Committee during
the year and how it has discharged its responsibilities are
provided in the report below.
Andrew Smith Chairman of the Audit and Risk Committee 24 April
2023
Duties and responsibilities The Audit and Risk Committee's
duties and responsibilities are set out in its terms of reference
which are available on the Company's website.
Internal controls and risk management
? Monitor and review the adequacy and effectiveness of the
Company's internal financial controls and risk management
systems.
? Review and recommend to the Board the disclosures in the
Annual Report concerning internal controls and risk management.
? Promote sound risk management and internal control
systems.
? Monitor and keep under review the policies and overall process
for identifying and assessing business risk.
Whistleblowing, fraud, bribery and other compliance
? Review the Company's arrangements for its employees and
contractors to raise concerns in confidence.
? Review procedures for detecting fraud and preventing
bribery.
? Review the Company's code of corporate conduct/ business
ethics.
Financial and narrative reporting
? Monitor the integrity of the financial statements.
? Review and report to the Board on significant financial issues
and judgements.
? Review and challenge accounting policies, methods used to
account for significant or unusual transactions, clarity and
completeness of disclosure.
External audit
? Recommend the appointment, reappointment or removal of the
auditors, including conducting a tender for new appointments.
? Oversee the relationship, make recommendations on their
remuneration, approve terms of engagement and review independence
and objectivity.
? Meet regularly without management present.
? Develop policy on the supply of non-audit services.
? Ensure the audit contract is tendered at least every ten
years.
? Review and approve the audit plan.
? Review the findings of the audit.
Internal audit To review the need for an internal audit
function
? If an internal audit function is appointed:
- Approve the appointment or termination of the head of internal
audit.
- Consider and approve the Terms of Reference for the internal
audit.
- Monitor and review the operation and the effectiveness.
- Review and assess the internal audit plan and reports.
Meetings and attendees The Audit and Risk Committee has met six
times during the year. The Audit and Risk Committee will normally
meet no fewer than three times a year with further meetings being
called as required.
The external auditors are invited to attend the majority of the
meetings. Outside of the formal meeting programme, the Audit and
Risk Committee chairman maintains a dialogue with key individuals
involved in the Company's governance, including the Chairman, the
Group Head of Finance and the external audit lead partner.
Activity during the year Matters discussed during the year have
included:
? Reviewing the Committee's terms of reference and recommending
changes to the Board.
? Reviewing the Company's internal controls and risk management
processes and procedures and assessing and keeping under review
their appropriateness.
? Reviewing the Company's Whistleblowing Policy.
? Reviewing the Company's Treasury Policy for recommendation to
the Board.
? Considering the Group's policy on the provision of non-audit
services by the external auditors.
? Reviewing the going concern and viability assessment of the
Company and the Group.
? Reviewing the external auditor's audit plan, process and
scope.
? Reviewing the independence of the external auditor.
? Reviewing the significant issues in the external audit
report.
? Reviewing the Annual Report and Accounts and recommending
their approval by the Board.
? Reviewing the need for an internal audit function and
concluding that it is not required.
Significant issues considered in relation to the financial
statements Significant issues and accounting judgements are
identified by the finance team and are considered and reviewed by
the Audit and Risk Committee. The significant issues considered by
the Committee in respect of the year ended 31 December 2022 are set
out in the table below:
Significant
issues How the issues were addressed
and judgements
Valuation of The Audit and Risk Committee reviewed management's determination of the valuations of the unlisted
unlisted investments, including the valuation methodology applied. The Committee concluded that the valuations
Investments of the unlisted investments were properly prepared in accordance with the stated accounting policy and
the evidence available.
Calculation of The Audit and Risk Committee considered management's calculation of the share-based payment expense
share-based relating to management options and the Executive Incentive Plan, including the assumptions made
payment expense regarding volatility and the risk-free interest rate. The Committee was satisfied that the expense had
been calculated appropriately.
The Audit and Risk Committee reviewed management's presentation of the Annual Report. The Committee
Presentation of noted that the inputs into, and disclosures and accounting policies included, in the Annual Report are
the Annual reviewed by people with relevant financial experience and knowledge of the business, up to and
Report including the Audit and Risk Committee. The Committee concluded that management has presented the
report in a suitable manner, and that it is fair, balanced and understandable.
Risk management and internal control The Board has overall
responsibility for setting the Group's risk appetite and ensuring
there is an effective risk management framework to maintain levels
of risk within this risk appetite. The Board has, however,
delegated responsibility for reviewing the risk management
methodology and effectiveness of internal control to the Audit and
Risk Committee. The Audit and Risk Committee provides oversight and
advice to the Board on current risk exposures and future risk
strategy. Further details of the Group's risk management approach,
structure and principal risks are set out in the Strategic
Report.
The Group's system of internal control comprises entity-wide
high level controls, controls over business processes and centre
level controls. Policies and procedures are clearly defined. Levels
of delegated authority have been communicated across the Group and
management has identified the key operational and financial
processes which exist within the business and implemented internal
controls over these processes, in addition to the higher level
review and authorisation based controls. Policies cover defined
lines of accountability and delegation of authority; financial
reporting procedures; and preparation of monthly management
accounts; these facilitate the accuracy and reliability of
financial reporting and govern the preparation of financial
statements.
The Board is ultimately responsible for the Group's system of
internal controls and risk management. It has discharged its duties
in this area by:
? holding regular Board meetings to consider the matters
reserved for its consideration;
? receiving regular management reports which provide an
assessment of key risks and controls;
? scheduling annual Board reviews of strategy, including reviews
of the material risks and uncertainties facing the business;
? ensuring there is a clear organisational structure, with
defined responsibilities and levels of authority;
? ensuring there are documented policies and procedures in
place; and
? reviewing regular reports containing detailed information
regarding financial performance, rolling forecasts, actual and
forecast covenant compliance and financial and non-financial
KPIs.
In reviewing the effectiveness of the system of internal
controls, the Audit and Risk Committee:
? reviews the risk register compiled and maintained by senior
management within the Group and questions and challenges where
necessary;
? reviews the system of financial and accounting controls
regularly; and
? reports to the Board on the risk and control culture within
the Group.
No significant failings or weaknesses were identified.
Internal audit The Group does not have an internal audit
function. The Audit and Risk Committee reviews the need for an
internal audit function at least annually and following the most
recent review, the Committee has determined that due to the small
size of the Group, it does not warrant having an internal Audit
function at this time.
External auditors The Audit and Risk Committee is responsible
for overseeing the Group's relationship with its external
auditors.
This includes the ongoing assessment of the auditors'
independence and the effectiveness of the external audit process,
by regular meetings and assessment of non-audit engagements. The
results of this inform the Committee's recommendation to the Board
as to the auditors' appointment (subject to shareholder approval)
or otherwise.
Appointment and tenure The Board appointed BDO LLP (BDO) as the
Company's external auditors in June 2020. BDO was recommended to
the Board for appointment by the Audit and Risk Committee following
a competitive tender process conducted in accordance with the
Financial Reporting Council's best practice for Audit Tenders. The
current statutory audit partner for BDO is therefore in their third
year.
Regulations require the rotation of the lead audit partner every
five years for a listed client. Therefore, we expect a new lead
audit partner to be selected for the 2025 audit. In accordance with
retained EU legislation, the Committee intends to put the external
audit out to tender at least every ten years.
Non-audit services The engagement of the external audit firm to
provide non-audit services to the Group can affect the independence
assessment, and the Group has therefore adopted a policy which
conforms to the Revised Ethical Standard 2016 published by the
Financial Reporting Council. Under the policy the engagement of the
external auditors to provide statutory audit services, certain
assurance, taxation and certain advisory services with fees of less
than GBP5,000 is pre-approved. Any engagement of the external
auditors to provide permitted services above GBP5,000 is subject to
the specific approval of the Audit and Risk Committee.
The policy recognises that certain non-audit services may not be
carried out by the external auditors (in accordance with the EU
Statutory Audit regime).
During the year ended 31 December 2022, BDO provided non-audit
services in relation to reviewing the Group's Interim financial
statements (GBP18,400); and performing an FCA CASS Audit of Arix
Capital Management Limited, a 100% subsidiary of Arix Bioscience
plc (GBP5,050). The fees paid to BDO for non-audit services during
the year totalled GBP23,400 representing 14% of the total audit
fee.
Whistleblowing The Group has adopted procedures where employees
may, in confidence, raise concerns relating to possible
improprieties in matters of financial reporting, financial control
or any other matter. The whistleblowing policy applies to all Group
employees. The Audit and Risk Committee is responsible for
monitoring the Group's whistleblowing arrangements and the Board
reviews the policy periodically. The Audit and Risk Committee, on
behalf of the Board, reviewed the Group's whistleblowing
arrangements in December 2022 - and it was considered that they
were still appropriate in their current form.
Andrew Smith Chairman of the Audit and Risk Committee 24 April
2023
Directors' Remuneration Report
Debra Barker Chair of the Remuneration Committee
Composition Debra Barker (Chair from 10 August 2022) Peregrine
Moncreiffe Benny Soffer (from 10 August 2022) Maureen O'Connell
(Chair up to 10 August 2022) Sir Michael Bunbury (member up to 10
August 2022)
Annual Statement by the Chair of the Remuneration Committee Dear
Shareholders, As the Chair of the Remuneration Committee (the
"Committee") I am delighted to introduce our 2022 Directors'
Remuneration Report.
This is my first report as Chair of the Committee having joined
the Board as Senior Independent Director and succeeding Maureen
O'Connell on 10 August 2022. I would like to thank Maureen for her
support over the course of the year.
Board changes During the year, there were a number of
Non-Executive Director changes, as set out below:
? Andrew Smith and Benny Soffer joined the Board as
Non-Executive Directors on 10 August 2022. Benny Soffer
subsequently stepped down effective 31 January 2023.
? I joined the Board as the Senior Independent Director on 10
August 2022.
? Sir Michael Bunbury stepped down from the Board and from the
role of Senior Independent Director on 9 August 2022.
No payments for loss of office were made to Non-Executive
Directors that stepped down from the board.
Pay for performance 2022 saw a decline in NAV from GBP255
million at the end of 2021 to GBP226 million at 31 December 2022.
The Gross Portfolio declined in value to GBP99.6 million, driven
largely by a decline in valuation in our listed holdings. Whilst
recognizing this disappointing financial performance, the year saw
continued progress on an operational basis with the aquisition of
TwelveBio by Ensoma, concurrent with Ensoma's Series B financing,
the reverse merger of Disc Medicine with Gemini Therapeutics and
Imara's reverse merger with Enliven Therapeutics.
2022 Annual bonus Bonus awards for 2022 reflect performance
delivered in the year as outlined above, with the Remuneration
Committee determining that no bonus pay-out was due in respect of
financial performance of the year (weighted at 50% of the bonus
opportunity), with a bonus of 80% of maximum due in respect of the
non-financial element, reflecting the continued progress being made
on an operational basis, as noted above. This resulted in an
overall bonus of 40% of maximum for the CEO, with further details
shown below. The Committee considers the level of pay-out is
reflective of the individual performance of Robert Lyne and the
overall performance of the Company in the year and therefore
determined that no discretion should be exercised to reduce the
formulaic outcome.
2020 EIP awards The 2020 EIP award was based on compound share
price growth (60%) and NAV per share growth (40%) targets to 31
December 2022. Performance against the original targets set, which
were set under the leadership of the previous Executive Chair and a
significantly different point in the economic cycle, resulted in a
formulaic outcome of 23.2% of maximum.
Whilst conscious of the formulaic out-turn of the 2020 EIP
awards, following extensive discussion with the Board (excluding
Robert Lyne), which unanimously supported the approach, the
Committee determined that it would be appropriate to exercise its
discretion to increase the vesting under this award to 43.2% of
maximum, based on the following three key principles:
1. To reward the CEO for his performance in the role to
date.
2. To increase the alignment of the CEO with shareholders.
3. To support in the retention of the CEO.
Further details of the Committee's considerations under each of
the three principles noted above are set out below.
1. To reward the CEO for his performance in the role to date
Robert Lyne joined Arix in 2017 and was appointed to the Board in
April 2021. Since stepping up as CEO, Robert has been a constant
stabilizing force for the Company delivering strong operational
performance through a period of significant change for the company,
with a material change in the shareholder base, and a rapidly
evolving macro-economic environment. Highlights of Robert's key
achievements since his appointment to the Board include:
? Strengthening the reputation of the Company with key
stakeholders, including through a re-organisation of key internal
functions related to investor and public relations.
? Leading a successful investor outreach, resulting in two new
significant investors into the business over the course of
FY22.
? Completing a successful cost reduction exercise following
appointment, resulting in the cost base being lowered by 25%.
? Playing a pivotal role in ensuring the retention and
successful recruitment of key personnel to the business, including
(i) a pivotal role in the recruitment and retention of members of
the investment team that are considered key talent for the future;
and (ii) developing staff in the back office and multi-skilling
them to cover wider responsibilities, including investor
relations.
? Managing the company through a period of significant change in
Director composition, with 10 changes in Directors over the past 22
months.
2. To increase the alignment of the CEO with shareholders Since
joining Arix in 2017, there has not been any vesting under EIP
awards granted to Robert Lyne, with historic awards severely
impacted by the share price growth targets being set by reference
to the IPO / fund raising prices. Whilst there has been no vesting
under the long-term incentive scheme, Robert as CEO acknowledges
the importance of alignment with shareholders and has personally
purchased approximately 65,000 shares in the Company.
The Committee believes alignment to shareholders, through the
building of a shareholding, is an important factor in successfully
motivating management and as such has sought to reward the CEO's
performance in a way that further aligns the CEO with shareholders,
both on the upside and on the downside.
In order to further support this principle, the Committee has
determined that a two-year holding period will apply to any shares
vesting under the 2020 EIP, a feature not originally attached to
the award made to Robert on account of it having been granted prior
to his appointment to the Board. Robert will therefore not be able
to sell any shares vesting from the award (subject to settling any
taxes due) until 30 June 2025.
3. To support in the retention of the CEO At the time Robert
Lyne was appointed to the Board, his salary was set below that of
his predecessor in recognition that this was his first CEO role.
Over the course of the last 22 months, the CEO has enhanced his
reputation both with shareholders and across industry.
Whilst the Board is confident that Robert is both committed to
and is best placed to deliver Arix's long-term strategic goals, it
is acutely aware that Robert has gained invaluable experience as
the CEO of Arix and if the remuneration arrangements in place do
not continue to motivate and align Robert with shareholders over
the longer-term, there is a risk that he could move to a role
within an alternative asset management firm that would benefit from
significantly increased upside potential through mechanisms such as
carried interest that are not generally accepted in the listed
market, but are a common feature of competitor firms.
Reflecting on the above principles, the Committee considered
that increasing the vesting under the EIP was the most effective
route to recognising the CEO's performance since appointment while
still ensuring alignment with shareholders on the basis the EIP is
paid in Company shares.
The Committee has been in dialogue with the Company's largest
shareholders, who have expressed support for the proposal set out
above. I would like to place on record my thanks to shareholders
for engaging proactively with the Committee on this issue.
2022 EIP awards As set out in the 2021 Annual Report and
Accounts, reflecting external uncertainty at the time of writing
that report, the Remuneration Committee were still in the process
of discussing the performance measures and targets to be attached
to the 2022 EIP award. During the year the Committee conducted a
thorough review of the performance measures used for the EIP and
determined that TSR and NAV growth continue to be the best metrics
available to measure long-term performance at Arix. However,
reflecting the current significant cash balance of the total
portfolio, the Committee split the NAV metric, such that 40% of the
metric is based on invested NAV per share growth (driving
performance across the invested portfolio), with the balance, 20%,
based on overall NAV per share growth (which measures NAV
performance across the portfolio, including incentivising the right
investments in the future portfolio). The table below sets out the
performance measures and targets that apply to the 2022 EIP
award:
Threshold Maximum
Performance measure Weighting (25% vesting) (100% vesting)
Absolute TSR growth 40% 5% p.a. 15% p.a.
NAV per share growth 20% 5% p.a. 12% p.a.
Invested NAV per share growth 40% 7% p.a. 15% p.a.
======== ======== ========
In determining the performance targets, the Committee sought to
ensure that the award would be both stretching and achievable to
ensure that participants are appropriately incentivised to achieve
the Company's strategic goals.
The Committee is mindful of windfall gains and as such, the
Committee will review the 2022 EIP award on vesting to ensure
against windfall gains. The Committee will disclose the factors
considered in determining whether any windfall gains have arisen at
the time of vesting.
Implementation in 2023 For 2023, Robert Lyne's salary will be
increased by 5% to GBP315,000. The increase is below the average
increase for the wider workforce, albeit noting the relative
limited size of the workforce at Arix.
He will remain eligible for an annual bonus, with the maximum
opportunity unchanged at 125% of salary. The 2023 annual bonus will
be based on the achievement of specific financial, strategic and
personal goals, which will be disclosed retrospectively as part of
the 2023 annual report due to targets being considered commercially
sensitive.
An award of up to 225% of salary will be made under the EIP. In
line with the Directors' Remuneration Policy, a two-year
post-vesting holding period will apply to the EIP award. Details of
the performance targets attached to the 2023 EIP award is set out
further below.
Alignment of executive and employee pay Consistent with best
practice and the 2018 UK Corporate Governance Code, the Committee
considers pay and employment conditions across the Company when
reviewing the remuneration of the CEO and other senior
employees.
Arix has a small number of employees and as a result is not
required to publish the ratio of the CEO remuneration relative to
that of employees more widely. The Committee is confident that
there is considerable alignment between the structure of the CEO
pay and the arrangements in place for other employees, with all
employees participating in a similar bonus structure.
While the Company does not directly consult with employees as
part of the process of reviewing executive pay and formulating the
Remuneration Policy, the Committee receives updates from the CEO on
his discussions and reviews with senior management and
employees.
Looking ahead Our new Remuneration Policy ("Policy") was
approved by shareholders at the 2022 Annual General Meeting ("AGM")
with 99.9% votes cast in favour. While the Policy may apply for a
period of up to three years, the Committee regularly reviews the
appropriateness of the pay arrangements to ensure it continues to
support the Company in meeting its key strategic goals.
Given the uniqueness of Arix's business, where we compete with
firms that are typically private and operate a very different
remuneration structure (often driven through carried interest
arrangements), over the course of the year, the Committee intends
to review the current Policy, and in particular the use of an EIP
which requires setting of long-term performance targets which are
not fully aligned to the Group's investment life cycle, to ensure
that it remains fit for purpose. Where any changes are considered
appropriate, the Committee will consult with key shareholders prior
to implementing the proposed approach.
I hope that you find the information contained in this report
helpful, thoughtful and clear. The Committee continues to welcome
dialogue with shareholders on remuneration matters and any
questions or feedback you have would be gratefully received.
At the forthcoming AGM, shareholders will be asked to approve an
advisory resolution on the contents of the Annual Report on
Remuneration. I hope the Committee can count on your continued
support.
Debra Barker Chair of the Remuneration Committee 24 April
2023
Remuneration snapshot The following table sets out how the
Remuneration Policy will be implemented in 2023 for the CEO.
Element of pay Implementation for the year ending 31 December 2023
Base salary GBP315,000 (5% increase from prior year, below the average increase for the wider workforce)
Benefits Eligible to receive private health cover, life assurance, income protection and a company car or car
allowance
Pension 7.5% of salary, in line with the wider workforce
contribution
Maximum annual bonus opportunity of 125% of salary.
Bonus for 2023 will be based on a range of challenging financial, strategic and personal measures
aligned with the Company's KPIs, as set out in the table below. Specific targets will not be
disclosed upfront because the Remuneration Committee consider forward looking targets to be
commercially sensitive. However, the Committee intends to disclose these retrospectively in next
year's Remuneration Report to the extent that they do not remain commercially sensitive.
Annual bonus
Performance measure Weighting
Financial (NAV per share and share price growth) 50%
Strategic/personal measures 50%
Up to 50% of the annual bonus will be deferred and invested into shares which must be held for a
period of three years.
Maximum EIP opportunity of 225% of salary.
EIP awards for 2023 will be based upon share price growth, NAV growth and Invested NAV per share
growth, as set out below.
Threshold Maximum
Long-term Performance measure Weighting (25% of maximum) (100% of maximum)
incentive
Absolute TSR growth 40% 5% p.a. growth 15% p.a. growth
NAV per share growth 20% 5% p.a. growth 12% p.a. growth
Invested NAV per share growth 40% 7% p.a. growth 15% p.a. growth
Any shares which vest will be subject to a two-year post-vesting holding period.
The CEO is required to build a shareholding equivalent to 225% of basic salary.
Shareholding
requirements A post-cessation shareholding requirement also applies such that the CEO will normally be required to
hold shares with a value of 225% of basic salary (or actual shareholding, excluding personal
investment, on cessation if lower) for two years post cessation.
Fees for 2023 will be as follows (no change):
? Non-Executive Chairman: GBP150,000.
? Senior Independent Director: GBP10,000.
NED Fees ? Non-Executive Director: GBP65,000.
? Chairs of Audit and Risk, Remuneration and Nomination Committees: GBP10,000.
? Chair of Board Investment Committee: GBP20,000.
? Member of the Audit Committee: GBP10,000.
Annual Report on Remuneration This section sets out details of
the remuneration of the Executive and Non-Executive Directors
received during the financial year ended 31 December 2022 and also
describes the operation of the Remuneration Committee.
Remuneration Committee Membership Debra Barker is currently the
Chair of the Committee, succeeding Maureen O'Connell on 10 August
2022. Debra has several years' experience of serving on
remuneration committees. The other members of the Committee are
Peregrine Moncreiffe and Benny Soffer (from 10 August 2022). During
the year, Sir Michael Bunbury and Maureen O'Connell (both until 10
August 2022) also served on the Remuneration Committee.
The Committee met 3 times during the year under review. Meeting
attendance is shown in the Corporate Governance Report.
The Board considers a majority of the members of the Committee
to be independent when considered against the UK Corporate
Governance Code ("the Code"). The CEO attended meetings of the
Committee by invitation, but was not present when matters relating
to his remuneration were discussed.
Role of the Remuneration Committee The Remuneration Committee's
responsibilities are set out in its Terms of Reference which are
available on request to shareholders and on the Company's
website.
The Committee's role includes:
? Setting the Remuneration Policy for all Executive Directors of
the Company, the Chairman of the Board and key management (being
the Executive Committee (including the Company Secretary) and all
personnel receiving an annual basic salary of GBP250,000 or
more).
? Within the terms of the Remuneration Policy and in
consultation with the Chairman of the Board and/or the CEO, where
appropriate, determining the total individual remuneration package
of the CEO, the Chairman and other designated senior executives
including bonuses, incentive payments and share option or other
share awards.
? Approving the design of, and determining targets for, any
performance-related pay schemes operated by the Company and
approving total annual payments made under such schemes.
? Ensuring that contractual terms on termination, and any
payments made, are fair to the individual and the Company, that
failure is not rewarded, that the duty to mitigate loss is fully
recognised and that any payments are consistent with the
shareholder-approved Remuneration Policy.
In carrying out its duties the Remuneration Committee takes into
account any legal and regulatory requirements, including the Code
and the UK Listing Rules, as well as good practice guidance issued
by investors and investor representative bodies.
The Committee believes that its approach to Executive Director
remuneration is consistent with the factors set out in Provision 40
of the Code:
? Clarity: the Remuneration Policy and its implementation are
set out in extensive detail in this report;
? Simplicity: Remuneration is based on a mix of fixed and
variable pay, and is well understood by both participants and
shareholders. Incentives involve an annual bonus scheme based on
the achievement of key corporate objectives, and a long-term plan
which rewards the generation of long-term value for
shareholders;
? Risk: Performance targets for incentive schemes are calibrated
carefully to ensure that the ultimate rewards will correspond
closely with an appropriate level of performance. For example, EIP
awards will only vest if a certain level of share price; invested
NAV per share growth; and NAV per share growth is achieved. Malus
and Clawback provisions apply to both the Annual Bonus and EIP;
? Predictability: annual participation in the bonus scheme and
the EIP is capped (as a percentage of basic salary), and awards
cannot exceed these levels. Our Remuneration Policy contains
details of threshold, target and maximum opportunity levels under
the Annual Bonus and EIP, with potential outcomes in different
performance scenarios illustrated by the charts in the 2021
Directors' Remuneration Report. The ultimate value of any vested
EIP award will depend on the share price at the time which cannot
be predicted but is simple to calculate;
? Proportionality: there is a clear link between the delivery of
strategy and individual awards through the annual bonus scheme. The
EIP rewards the successful delivery of long-term outperformance. If
there is little or no growth in share price or NAV, awards will not
vest; and
? Alignment to culture: Arix's high performance culture and the
awareness within the Company of what ultimately drives shareholder
value are reflected in the incentive schemes operated and the
choice of performance metrics.
Key matters considered by the Remuneration Committee Key issues
reviewed and discussed by the Remuneration Committee during 2022
included:
? Review and approval of the 2022 Directors' Remuneration
Report.
? Review of Executive Director and senior manager salaries for
2023.
? Review of Executive Director and senior manager bonuses and
equity incentive awards for 2022.
? Pay benchmarking for key roles within the organisation and a
review of alternative incentive structures.
Advisers to the Committee Following a competitive tender to
advise on all aspects of the Directors' Remuneration Policy and its
implementation, Deloitte were appointed as advisers to the
Remuneration Committee on 30 June 2020.
The Committee is satisfied that the advice received during the
year was objective and independent. Deloitte is a founding member
of the Remuneration Consultants Group. Deloitte received fees of
GBP85,700 for its advice during the year (fees charged on a costs
incurred basis). Deloitte also provided Group tax advice during the
year.
Single figure table - Executive Directors (audited)
Total Total
Basic salary Benefits Annual bonus EIP Pension Other Total fixed pay variable pay
2022 2021 2022 2021 2022 2021 2022 2021 2022 2021 2022 2021 2022 2021 2022 2021 2022 2021
GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000
Robert Lyne1 300 188 11 7 150 143 218 - 22 14 - - 702 352 333 209 368 143
====== ====== ====== ====== ====== ====== ====== ====== ====== ====== ====== ====== ====== ====== ====== ====== ====== ======
== == == == == == == == == == == == == == == == == ==
1 Joined the Board on 29 April 2021 as Interim CEO and was
appointed as Chief Executive Officer on 6 October 2021. 2021 values
shown above relate to remuneration received since appointment to
the Board.
? Basic salary: amount earned for the year.
? Benefits: the taxable value of benefits received in the year,
including life assurance, long-term sickness insurance, private
healthcare and company car cash allowance.
? Pension: the value of the Company's contribution in the year:
7.5%.
? Annual Bonus: see separate section below for explanation of
determination of bonus amounts.
? Subject to Board approval, the Company allows its Executive
Directors to hold non-executive positions outside of the Company
that complement and enhance their current role. Any fees may be
retained by the Director. Robert Lyne did not hold any external
directorships during the year.
Single figure table - Non-Executive Directors (audited)
Fees Benefits Other Total Total fixed pay Total
variable pay
2022 2021 2022 2021 2022 2021 2022 2021 2022 2021 2022 2021
GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000
Current Non-Executive
Directors
Peregrine Moncreiffe1 150 101 - - 150 101 150 101 -
Isaac Kohlberg1 85 44 - - 85 44 85 44 -
Maureen O'Connell1 85 44 - - 85 44 85 44 -
Debra Barker2 38 - - - 38 - 38 - -
Andrew Smith2 33 - - - 33 - 33 - -
Benny Soffer2 30 - - - 30 - 30 - -
Former Non-Executive
Directors
Sir Michael Bunbury3 82 20 - - - 1 82 21 82 21 - -
======= ======= ======= ======= ======= ======= ======= ======= ======= ======= ======= =======
= = = = = = = = = = = =
1 Joined the Board on 29 April 2021. 2021 remuneration relates
to fees received between 29 April 2021 and 31 December 2021.
2 Joined the Board on 10 August 2022. 2022 remuneration relates
to fees received between 10 August 2022 and 31 December 2022.
3 Joined the Board on 6 October 2021 and stepped down from the
Board on 10 August 2022. 2021 remuneration relates to fees received
between 6 October 2021 and 31 December 2021 and 2022 remuneration
relates to fees received between 1 January 2022 to 10 August 2022.
Other relates to use of office.
Annual bonus payout table (audited) The CEO was eligible for a
bonus of up to 125% of base salary. The annual bonus for 2022 was
based on a range of challenging financial, strategic and personal
measures aligned with the Company's KPIs. The table below sets out
performance against the targets set by the Committee.
Key factors / achievements considered by the
Metric Remuneration Committee Outcome
In considering the financial element of the bonus
(weighted at 50%) the Committee took into account:
Notwithstanding the operational costs being
Financial ? Net Asset Value per share performance; delivered within the approved target, the Committee
(50%) agreed that due to the 9.5% decline in NAV and
? Total shareholder returns and share price over the 11.9% decline in share price over the year, this
year; element of the bonus should lapse in full.
? Operational costs.
? Key role, as the sole full-time executive on the
Board, in managing the Company's relationship with
shareholders and corporate partners during the course
of extensive shareholder and wider stakeholder
engagement. The Committee considers that Robert Lyne has been
instrumental in ensuring operational stability
? Pivotal in ensuring the retention and continued within the business and continuing to develop the
Non-financial development of key personnel. relationship of the Company with external
(50%) stakeholders.
? Undertook successful investor outreach, further
developing the reputation of the Company with both The Committee considered that achievement against
new and existing shareholders. Key achievements the non-financial element of the business should be
include bringing new shareholders into the Company 80% of maximum.
who have been strong buyers in the market.
? Supporting the successful IPO of Disc Medicine in
December 2022.
Outcome (percentage of maximum annual bonus) 40%
The Committee considered the appropriateness of the annual bonus
outcome for Robert Lyne in the context of the overall Company
performance and his individual performance and determined that the
bonus outcome was appropriate.
LTIPs vesting in the year (audited) Awards were made to previous
executive directors in 2020 under the EIP. Robert Lyne received a
grant as part of the 2020 EIP award, although he was not an
executive director at that time. The performance against the
targets is set out in the table below.
Level of vesting
Metric Weighting Threshold Maximum Actual (% of elements)
Compound share price growth 60% 7% p.a. growth 21% p.a. growth 9 38.6%
NAV per share growth 40% 7% p.a. growth 17% p.a. growth Below threshold 0%
Formulaic outcome 100% 23.2%
========= =========
As set out in further detail in the Chair's statement, the
Committee, with the full Board's support, determined that it would
be appropriate to exercise its discretion as provided for in the
Directors' Remuneration Policy to increase the vesting under this
award to 43.2% of maximum, based on the following three
principles:
1. To reward the CEO for his performance in the role to
date.
2. To increase the alignment of the CEO with shareholders.
3. To support in the retention of the CEO.
As the award was granted to Robert Lyne prior to being appointed
to the Board, the 2020 EIP would ordinarily not be subject to a
post-vesting holding period. However, in order to further support
the alignment and retention principles noted above, the Committee
determined that a two year holding period will apply to vested
awards.
Further details in relation to the rationale are set out in the
Chair's statement.
The award value shown in the single figure is based on the
average share price over the last three months of the financial
year ended 31 December 2022 of GBP1.07. 24% of the value of this
award is attributable to the growth in share price since grant.
Scheme interests awarded in 2022 (audited) During the year ended
31 December 2022, the following Directors were awarded nil-cost
options under the EIP, details of which are summarised below.
Number of Award
Name Date of grant Basis of award shares price1 Vesting date
Robert Lyne 30 November 2022 225% of salary 636,792 GBP1.27 1 December 2025
========= =========
Threshold Maximum
Performance measure Weighting (25% of maximum) (100% of maximum) Performance period
Absolute TSR growth 40% 5% p.a. growth 15% p.a. growth
1 January 2022 to
NAV per share growth 20% 5% p.a. growth 12% p.a. growth 31 December 2024
Invested NAV per share growth 40% 7% p.a. growth 15% p.a. growth
=========
1 Starting price based on the 30 day rolling average to the
start of the performance period.
The Committee is mindful of windfall gains and as such, the
Committee will review the 2022 EIP award on vesting to ensure
against windfall gains. The Committee will disclose the factors
considered in determining whether any windfall gains have arisen at
the time of vesting.
Any awards vesting will be subject to a two-year holding
period.
Payments for loss of office/payments to past Directors (audited)
During the year no payments for loss of office or payments to past
Directors were made.
Executive Directors' shareholdings and share interests (audited)
The interests of Executive Directors' in the Company as at 31
December 2022 (or, if earlier, the date of stepping down from the
Board) are shown in the table below. Robert Lyne is required to
build a shareholding equivalent to 225% of basic salary. This
shareholding requirement was not met at the end of the financial
year given his recent appointment to the Board.
Executive Directors will normally be required to hold shares
with a value of 100% of their incumbent shareholding requirement
(or their actual shareholding, excluding personal investment, on
cessation if lower) for two years post cessation as an Executive
Director. The Committee retains the discretion to operate this
policy flexibly and waive part or all of the policy, for example in
compassionate circumstances. No options were exercised during the
year.
2020 EIP
Awards
(no longer
subject to 2021 EIP 2022 EIP Shareholding
Ordinary shares performance Awards Awards as % of basic
Name held conditions) (unvested)1 (unvested)1 salary2
Robert Lyne 65,221 203,764 352,335 636,792 62.1%
========= ========= ========= ========= =========
1 Awards are nil-cost options. The 2021 and 2022 EIP Awards
include performance conditions which must be met prior to vesting.
Details of the specific performance targets in place for each grant
are included in the relevant year's Annual Report on
Remuneration.
2 Reflects value of ordinary shares, as well as value of 2020
EIP awards no longer subject to performance conditions on a net of
tax basis, calculated at closing share price on 31 December 2022
(GBP1.075).
There has been no change in the Executive Directors'
shareholdings since the balance sheet date.
NON-EXECUTIVE DIRECTORS' SHAREHOLDINGS (AUDITED) Non-Executive
Directors are not subject to a shareholding requirement. Details of
their interests in Ordinary Shares in the Company are set out
below:
Shareholding as at
Name 31 December 2022
Peregrine Moncreiffe 259,000
Isaac Kohlberg 0
Maureen O'Connell 0
Debra Barker 0
Andrew Smith 0
Benny Soffer 0
Sir Michael Bunbury1 40,000
=========
1 Reflects position as at the date of his departure from the
Board (10 August 2022).
Since the Balance Sheet Date, Peregrine Moncreiffe's holdings
have increase from 259,000 to 459.000 following the purchase of
200,000 shares on 6 February 2023.
Comparison of overall performance and pay The graph below shows
the value of GBP100 invested in the Company's shares since listing
in February 2017 compared to the FTSE SmallCap index (excluding
investment trusts) and the XBI. Although Arix is not a member of
the FTSE SmallCap index, the index has been chosen as a broad
equity market index, the constituents of which include companies of
a similar size and scale to Arix. The XBI has been chosen as this
is reflective of the industry in which we operate.
Comparison of overall performance and pay
Source: Datastream (Thomson Reuters)
CEO - historic remuneration information (audited)
Robert Robert Naseem Naseem Joe
Lyne Lyne Amin Amin Anderson
2022 2021 2021 2020 2020 2019 2018 2017
GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000
Single figure total 701 352 97 266 417 737 633 1,726
Annual variable against maximum 40% 40% N/A 100% 0% 50% 75% 80%
opportunity
EIP vesting rates against maximum 43% 0% N/A N/A N/A N/A N/A N/A
opportunity
========= ========= ========= ========= ========= ========= ========= =========
Comparison of Directors' and employees' pay The table below sets
out the annual percentage change in each Director's salary/fees,
benefits and annual bonus between the year ended 31 December 2022
and the year ended 31 December 2020, and the average percentage
change in the same remuneration over the same periods in respect of
the employees of the Company on a full-time equivalent basis.
The average employee change has been calculated by reference to
the median of employee pay.
Debra Barker, Andrew Smith and Benny Soffer were appointed to
the Board and Sir Michael Bunbury stepped down from the Board
during the year ended 31 December 2022. Accordingly, they have been
excluded from the table below.
Peregrine Moncreiffe, Sir Michael Bunbury, Isaac Kohlberg and
Maureen O'Connell were appointed in 2021.
Average Robert Peregrine Isaac Maureen
employee Lyne1 Moncreiffe2 Kohlberg2 O'Connell2
Salary / fees 2022 13% 6% 0% 29% 29%
2021 4% N/A N/A N/A N/A
2020 5% N/A N/A N/A N/A
Benefits 2022 59% 5% N/A N/A N/A
2021 3% N/A N/A N/A N/A
2020 2% N/A N/A N/A N/A
Annual incentive 2022 48% 5% N/A N/A N/A
2021 - N/A N/A N/A N/A
2020 32% N/A N/A N/A N/A
========= ========= ========= ========= ========= =========
1 Robert Lyne was appointed to the Board on 29 April 2021 as
interim CEO and was appointed as Chief Executive Officer on 6
October 2021. To enable comparison and to provide meaningful
reflection of the annual percentage change, his fees for the year
ended 31 December 2021 have been annualised.
2 Peregrine Moncreiffe, Isaac Kphlberg and Maureen O'Connell
were appointed to the Board on 29 April 2021. To enable comparison
and to provide meaningful reflection of the annual percentage
change, their fees for the year ended 31 December 2021 have been
annualised.
Relative importance of spend on pay The table below shows the
relative importance of total spend on pay in the 2022 and 2021
financial years compared with distributions to shareholders. The
Company did not pay a dividend in either 2022 or 2021 and only
undertook a share buyback programme in 2021 and not 2022.
Underlying operating (loss)/profit is considered the most
appropriate metric given the current stage of the Group.
Total Group spend on remuneration decreased by 30% compared to
the previous year:
2022 2021
GBP'000 GBP'000 % change
Underlying operating (loss)/profit (29,160) (58,647) (50%)
Dividends/share buybacks - 11,593 N/A
Total Company spend on remuneration 2,482 3,529 (30%)
Total Company spend on remuneration excluding exceptional costs 2,482 3,070 (19%)
========= ========= =========
Statement of voting on remuneration The results of the voting on
the Directors' Remuneration Policy and the Annual Report on
Remuneration at the AGM held on 7 June 2022 are set out below:
Votes Votes Votes
Votes for Votes for against against withheld
# % # % #
To approve the Directors' Remuneration Policy 63,589,770 99.90% 65,188 0.10% 31,126
To approve the Annual Report on Remuneration 60,232,861 94.62% 3,422,747 5.83% 30,476
========= ========= ========= ========= =========
DIRECTORS' REMUNERATION POLICY (SUMMARY) Introduction The
Directors' Remuneration Policy ("Policy") was approved by
shareholders at the AGM held on 7 June 2022 and applies for a
period of up to three years from the date of approval. A summary of
the Policy is set out on the following pages. The full Policy is
included on pages 55 to 63 within the 2021 Annual Report, available
in the Investor Relations section of Arix's website,
www.arixbioscience.com.
REMUNERATION POLICY TABLE (EXECUTIVE DIRECTORS)
How it supports the
Company's
Element of short and long-term Opportunity and performance
remuneration strategic Operation metrics
objectives
An Executive Director's basic salary
is set on appointment and reviewed The Committee ensures that maximum
annually or when there is a change in salary levels are positioned with
position or responsibility. consideration for:
When determining an appropriate level ? the need to acquire and retain
of salary, the Committee considers: Executives with the skills and
experience to develop and implement
? individual degree of responsibility; the Company's strategy;
Provide salaries that ? the general operational performance ? companies that are comparable in
support the Company to of the Group and individual terms of business activities,
acquire and retain the performance (if applicable); complexity and size to Arix, which we
Salary highly qualified Executive would compete for talent against.
Directors who are needed to ? the economic environment and the
develop and implement the sustainable development of the Group; In general, increases for Executive
Group's strategy Directors will be in line with the
? remuneration structures in companies increase for employees. Increases
that are comparable in terms of above this level may be considered in
business activities, complexity and certain circumstances such as where
size; there is a significant change in
responsibility or where the salary for
? any change in scope, role and a new hire is deliberately set below
responsibilities; and market levels with the intention to
implement larger increases in
? remuneration practices within the subsequent years.
Group.
The Executive Directors are eligible
to receive private health cover, life
assurance, income protection and a
company car or car allowance.
Provides a benefits package
in line with standard The Committee recognises the need to
market practice to enable maintain suitable flexibility in the
Benefits the Group to recruit and benefits provided to ensure it is able The maximum will be set at the cost of
retain Executive Directors to support the objective of attracting providing the benefits described.
with the experience and and retaining personnel in order to
expertise to deliver the deliver the Group strategy.
Group's strategy.
Additional benefits may therefore be
offered, such as relocation allowances
on recruitment and reasonable tax
advice and filing support.
Provides a pension The Group contributes to defined
provision in line with contribution (DC) pensions schemes for
standard market practice to UK employees and US employees The maximum contribution will be in
enable the Company to contribute into the Arix 401(k) line with the rates available to the
Pensions recruit and retain pension scheme (which is open to all workforce in the Executive Director's
Executive Directors with employees) with a contribution made by local jurisdiction. The maximum
the experience and Arix alongside an employee's contribution for UK employees is
expertise to deliver the contribution. An equivalent cash currently 7.5% of salary.
Group's strategy. allowance may be paid where
appropriate.
The Committee will normally determine
the bonus to be delivered following
the end of the relevant financial
year, taking into account all relevant
factors including performance against The maximum bonus deliverable in
any targets set and the underlying respect of any financial year under
performance of the business. the plan will not exceed 125% of a
The bonus plan provides an participant's annual basic salary.
incentive to the Executive The Committee retains the discretion
Directors linked to to adjust the formulaic outcome if Bonus targets and weightings are set
achievement in delivering considered appropriate in the context each year and will take into account
goals that are closely of overall company and individual the strategic priorities of the
aligned with the Company's performance. business at the time.
strategy and the creation
of value for shareholders. The Committee can require part of any Details of the performance conditions,
bonus (up to 50% of the maximum bonus targets and their level of
Annual bonus The plan supports the earned) to be deferred on a post-tax satisfaction for the year being
Company's objectives, basis and invested into shares. These reported on will be set out in the
allowing the setting of shares must be held for a minimum Annual Report on Remuneration.
annual targets based on the period, normally, three years.
business strategy at the Percentage of bonus maximum earned for
time, meaning that a wider The Group will set out in the levels of performance (with straight
range of performance Remuneration Report in the following linepay-outs between these points):
metrics can be used that financial year the decisions taken
are relevant and around any requirement to invest in Threshold: 0%
achievable. shares.
On target: 50%
The bonus plan includes malus and
clawback provisions. Maximum: 100%
The Remuneration Committee may adjust
and amend awards in accordance with
the relevant plan rules.
Normal maximum value of 225% of salary
in respect of any financial year,
based on the market value at the date
of grant set in accordance with the
Awards are granted annually to rules of the Plan.
Executive Directors in the form of a
conditional share award or nil cost In exceptional circumstances the
option. Awards are normally subject to Committee may grant an award with a
The purpose of the EIP is a three-year performance period, with maximum of 300% of salary.
to incentivise and reward a subsequent two-year holding period.
Executive Directors in The amount payable for threshold
relation to long-term Performance targets are normally set performance is up to 25% of maximum of
performance and achievement annually for each three-year cycle by the award.
of Group strategy. the Remuneration Committee.
EIP awards will be subject to the
This will better align The Committee retains the discretion achievement of challenging performance
Executive Directors' to adjust the formulaic outcome, if conditions set by the Remuneration
Long-Term interests with the considered appropriate, taking into Committee prior to each grant linked
Incentive long-term interests of the account all relevant factors including to the achievement of the Company's
Plan("EIP") Group and will also act as the underlying performance of the long-term strategic priorities and the
a retention mechanism. business. creation of long-term shareholder
value.
The award is designed to The Committee may award dividend
incentivise Executive equivalents on awards in either shares The Remuneration Committee retains
Directors to grow the or cash to the extent that these vest. discretion to change performance
investment portfolio and measures and targets and the
value creation by The Remuneration Committee may adjust weightings attached to performance
successfully delivering the and amend awards in accordance with measures part way through a
Group's strategy. the EIP rules. performance period if there is a
significant and material event which
Awards are subject to malus and causes the Remuneration Committee to
clawback. believe the original measures,
weightings and targets are no longer
appropriate. Any changes made and the
circumstances for such a change will
be clearly disclosed to shareholders
in the Annual Report on Remuneration.
The Committee has adopted formal shareholding guidelines that will encourage the Executive Directors to
build up and then subsequently hold a shareholding equivalent to a percentage of base salary. Adherence
to these guidelines is a condition of continued participation in the equity incentive arrangements. This
policy ensures that the interests of Executive Directors and those of shareholders are closely aligned.
Minimum
shareholding The Committee will determine the relevant shareholding guideline on an annual basis.
requirement
Post-cessation of employment, Executive Directors are also expected to remain aligned with the interests
of shareholders for an extended period after leaving the Company, other than in exceptional
circumstances. Details of the application of this policy are set out in the Annual Report on
Remuneration.
The Board as a whole is responsible
for setting the remuneration of the
Non-Executive Directors. Non-Executive Non-Executive Directors (including a
Directors are paid an annual fee and Non-Executive Chairman) are not
additional fees for additional eligible to participate in any
responsibilities, such as chairmanship incentive arrangements.
and membership of committees or being
Provide a level of fees to appointed the Senior Independent The Chairman and Non-Executive
Director fees support Director. Additional fees may be paid Directors may be eligible for benefits
recruitment and retention for other responsibilities or time such as use of secretarial support or
of high-calibre commitments (including committee other benefits which may be
Non-Executive Non-Executive Directors membership or for substantial appropriate for performing their
Director fees with the necessary additional time requirements above duties including travel allowances, if
experience to advise and those expected for the role). considered appropriate.
assist with establishing
and monitoring the Group's The Board is responsible for setting The Company will pay reasonable
strategic objectives. the pay of the Chairman. The Chairman business-related expenses incurred by
receives an all-inclusive fee. the Non-Executive Directors and may
settle any tax incurred in relation to
Fees are normally paid in cash. these.
In general, the level of fee increase
for the Non-Executive Directors will
be set taking account of any change in
responsibility and workload.
SERVICE AGREEMENTS AND LETTERS OF APPOINTMENT The Executive
Director's service agreement is on a rolling term basis. The
details of the Executive Director's term is set out below:
Notice periods by Notice periods by
Name Date of appointment Company (months) Director (months)
Robert Lyne 29 April 2021 12 6
The Non-Executive Directors of the Company do not have service
contracts but are appointed by letters of appointment. Each
Non-Executive Director's term of office runs for an initial term of
up to and including the next AGM after their appointment unless
terminated earlier upon written notice or upon their resignations.
The terms of the Non-Executive Directors' appointments are subject
to their re-election by the Company's shareholders at the 2022 AGM
and to re-election at any subsequent AGM at which the Non-Executive
Directors stand for re-election. The details of each Non-Executive
Director's term are set out below:
Notice periods Notice periods
Current term by Company by Director
Name Date of appointment (full years) (months) (months)
Peregrine Moncreiffe 29 April 2021 1 3 3
Isaac Kohlberg 29 April 2021 1 3 3
Maureen O'Connell 29 April 2021 1 3 3
Debra Barker 10 August 2022 0 3 3
Andrew Smith 10 August 2022 0 3 3
Benny Soffer 10 August 2022 0 3 3
DIRECTOR'S REPORT FOR THE YEARED 31 DECEMBER 2022
The Directors present their report for the year ended 31
December 2022. Additional information which is incorporated by
reference into this Directors' Report, including information
required in accordance with the Companies Act 2006, can be found as
follows:
Disclosure Location
Important events affecting the Company since the year- end, likely future business ? Strategic Report
developments and research and development activities
Financial risk management objectives and policies (including hedging policy and use of ? Notes to the financial
financial instruments) statements
Going concern ? Strategic Report
Statement of Directors' responsibilities ? Report of the
Nomination Committee
Diversity Policy ? Report of the
Nomination Committee
Details of long-term incentive schemes ? Notes to the financial
statements
Significant Interests ? Directors' Report
Waiver of emoluments by a Director ? Directors' Remuneration
Report
Compensation for loss of office arrangements ? Directors' Remuneration
Report
For the purposes of LR 9.8.4CR, the information required to be
disclosed by LR 9.8.4R can be found in the following locations:
Disclosure Location
Interest capitalised Not applicable
Publication of unaudited financial information Not applicable
Details of long-term incentive schemes ? Directors' Remuneration Report
Waiver of emoluments by a Director ? Directors' Remuneration Report
Waiver of future emoluments by a Director ? Directors' Remuneration Report
Non pre-emptive issues of equity for cash Not applicable
Non pre-emptive issues of equity for cash in relation to major subsidiary undertakings Not applicable
Parent participation in a placing by a listed subsidiary Not applicable
Contract of significance in which a Director is interested Not applicable
Contract of significance with a controlling shareholder Not applicable
Provision of services by a controlling shareholder Not applicable
Shareholder waiver of dividends Not applicable
Shareholder waiver of future dividends Not applicable
Agreements with controlling shareholder Not applicable
Compensation for loss of office arrangements ? Directors' Remuneration Report
The Strategic Report and this Directors' Report have been drawn
up and presented in accordance with, and in reliance upon,
applicable English company law and any liability of the Directors
in connection with these reports shall be subject to the
limitations and restrictions provided by such law.
DIRECTORS The Directors of the Company who held office during
the year are:
Peregrine Moncreiffe Appointed 29 April 2021
Sir Michael Bunbury Appointed 6 October 2021 Resigned 9 August
2022
Isaac Kohlberg Appointed 29 April 2021
Robert Lyne Appointed 29 April 2021
Maureen O'Connell Appointed 29 April 2021
Dr Debra Barker Appointed 10 August 2022
Dr. Benny Soffer Appointed 10 August 2022 Resigned 31 January
2023
Andrew Smith Appointed 10 August 2022
Results and dividend The results for the year ended 31 December
2022 are set out in the Consolidated Statement of Comprehensive
Income
The Board is not recommending a dividend for the year ended 31
December 2022.
Articles of Association The rules governing the appointment and
replacement of Directors are set out in the Company's Articles of
Association. The Articles of Association may be amended by a
special resolution of the Company's shareholders.
Share capital Details of the Company's share issued capital,
including changes during the year, are set out in Note . to the
financial statements. As at 31 December 2022, the Company's share
capital consisted of:
? 135,609,653 Ordinary Shares of GBP0.00001 each of which
6,428,853 are held in Treasury; and
? 49,671 C Shares of GBP1.00 each.
Ordinary shareholders are entitled to receive notice of, and to
attend and speak at, any general meeting of the Company.
On a show of hands every shareholder present in person or by
proxy (or being a corporation represented by a duly authorised
representative) shall have one vote, and on a poll every
shareholder who is present in person or by proxy shall have one
vote for every share they hold. The Notice of Annual General
Meeting specifies deadlines for exercising voting rights and
appointing a proxy or proxies. Ordinary Shares held as Restricted
Shares pursuant to the Restrictive Share Agreement are
disenfranchised and, accordingly, holders of such Restricted Shares
are not entitled to vote, attend the meetings of the Company or
receive dividends or other distributions made or paid on the
Ordinary Share capital of the Company.
No voting rights attach to the C Shares and their holders are
not entitled to receive notice of, or to attend and speak at, any
general meeting of the Company. Holders of C Shares are not
entitled to receive any dividend or distributions made or paid on
the Ordinary Share capital of the Company.
Other than the general provisions of the Articles of Association
(and prevailing legislation), there are no specific restrictions of
the size of a holding or on the transfer of any class of shares in
the Company except as follows:
? Prior consent of the Directors is required for the transfer of
C Shares.
? Holders of Restricted Shares may not dispose of Restricted
Shares until and unless the relevant Restricted Shares are released
from their respective undertakings pursuant to the Restrictive
Share Agreement.
Other than as set out above, the Directors are not aware of any
other agreements between holders of the Company's shares that may
result in the restriction of the transfer of securities or on
voting rights. No shareholder holds securities carrying any special
rights or control over the Company's share capital.
Authority for the Company to purchase its own shares Subject to
authorisation by shareholder resolution, the Company may purchase
its own shares in accordance with the Act. Any shares which have
been bought back may be held as treasury shares or cancelled
immediately upon completion of the purchase.
At the AGM on 7 June 2022, the Company was generally and
unconditionally authorised by its shareholders to make market
purchases (within the meaning of section 693 of the Companies Act
2006) of up to a maximum of 12,918,080 of its Ordinary Shares. The
Company repurchased 6,428,853 of its Ordinary Shares under this
authority before the programme was suspended on 18 October 2021.
The authority is due to expire on the earlier of the date of this
year's AGM or 30 June 2023.
Directors' interests The number of Ordinary Shares of the
Company in which the Directors were beneficially interested at 31
December 2022, is set out in the Directors' Remuneration
Report.
Directors' indemnities The Company's Articles of Association
(the "Articles") provide, subject to the provisions of UK
legislation, an indemnity for Directors and officers of the Company
and the Group in respect of liabilities they may incur in the
discharge of their duties or in the exercise of their powers. The
Company has made qualifying third party indemnity provisions for
the benefit of its Directors during the period and these remain in
force at the date of this report.
The Company maintains Directors' and Officers' liability
insurance cover and this is in place for all the Company's
Directors at the date of this report. The Company will review its
level of cover annually.
Overseas offices Arix Bioscience, Inc. has an office in New
York, USA.
Political donations The Group did not make any political
donations during the year.
Change of control - significant agreements There are a number of
agreements that may take effect, alter or terminate on a change of
control of the Company, such as commercial contracts and property
lease agreements.
None of these are considered to be significant in their likely
impact on the business as a whole.
Audit information Each Director has taken all the steps that
they ought to have taken as a director in order to make themselves
aware of any relevant audit information and to establish that the
company's auditors are aware of that information. The auditors have
been provided with:
? access to all information of which the Directors are aware
that is relevant to the preparation of the financial statements
such as records, documentation and other matters;
? additional information that has been requested for the purpose
of the audit; and
? unrestricted access to persons within the Group from whom it
was determined necessary to obtain audit evidence.
Significant interests The table below shows the interests in
shares notified to the Company in accordance with the Disclosure
Guidance and Transparency Rules:
As at 5 April 2023 As at 31 December 2022
Number of Percentage Number of Percentage
Ordinary of total Ordinary of total
Shares of 0.001 voting rights Shares of 0.001 voting rights
Name of Shareholder pence each held held pence each held held
Acacia Research Corporation 33,023,210 25.6 27,182,317 20.05%
Fosun International 11,309,849 8.7 11,189,403 8.25%
Christopher Chipperton (including restricted shares) 6,862,889 5.3 6,862,889 5.06%
Ruffer 6,815,000 5.3 9,163,000 6.76%
Ipsen 6,666,666 5.2 6,666,666 4.92%
Hargreaves Lansdown, stockbrokers (EO) 5,678,780 4.4 5,718,958 4.07%
UCB 5,647,679 4.4 5,647,679 4.17%
Polygon Investment Partners 4,500,000 3.5 5,511,258 3.32%
========= ========= ========= =========
So far as each Director is aware, there is no relevant audit
information of which the auditors are unaware.
The confirmation is given and should be interpreted in
accordance with the provisions of section 418 of the Companies Act
2006.
Independent auditors BDO LLP have indicated their willingness to
continue in office and a resolution seeking to reappoint them will
be proposed at the forthcoming Annual General Meeting.
Annual General Meeting The Annual General Meeting will be held
on Tuesday 23 May 2023 at 13.00 BST at the offices of Brown Rudnick
LLP, 8 Clifford Street, London W1S 2LQ. Further details can be
found in the Notice of Annual General meeting which will be
published shortly.
Statement of Directors' Responsibilities The directors are
responsible for preparing the annual report and the financial
statements in accordance with UK adopted international accounting
standards and applicable law and regulations.
Company law requires the directors to prepare financial
statements for each financial year. Under that law the directors
are required to prepare the group financial statements and have
elected to prepare the company financial statements in accordance
with UK adopted international accounting standards. Under company
law the directors must not approve the financial statements unless
they are satisfied that they give a true and fair view of the state
of affairs of the group and company and of the profit or loss for
the group for that period.
In preparing these financial statements, the directors are
required to:
? select suitable accounting policies and then apply them
consistently;
? make judgements and accounting estimates that are reasonable
and prudent;
? state whether they have been prepared in accordance with UK
adopted international accounting standards, subject to any material
departures disclosed and explained in the financial statements;
? prepare the financial statements on the going concern basis,
unless it is inappropriate to presume that the group and the
company will continue in business; and
? prepare a directors' report, a strategic report and directors'
remuneration report which comply with the requirements of the
Companies Act 2006.
The directors are responsible for keeping adequate accounting
records that are sufficient to show and explain the company's
transactions and disclose with reasonable accuracy at any time the
financial position of the company and enable them to ensure that
the financial statements comply with the Companies Act 2006.
They are also responsible for safeguarding the assets of the
company and hence for taking reasonable steps for the prevention
and detection of fraud and other irregularities. The Directors are
responsible for ensuring that the annual report and accounts, taken
as a whole, are fair, balanced, and understandable and provides the
information necessary for shareholders to assess the group's
performance, business model and strategy.
Website publication The directors are responsible for ensuring
the annual report and the financial statements are made available
on a website. Financial statements are published on the company's
website in accordance with legislation in the United Kingdom
governing the preparation and dissemination of financial
statements, which may vary from legislation in other jurisdictions.
The maintenance and integrity of the company's website is the
responsibility of the directors. The directors' responsibility also
extends to the ongoing integrity of the financial statements
contained therein.
Directors' responsibilities pursuant to DTR4 The directors
confirm to the best of their knowledge:
The financial statements have been prepared in accordance with
the applicable set of accounting standards, give a true and fair
view of the assets, liabilities, financial position and profit and
loss of the group.
The annual report includes a fair review of the development and
performance of the business and the financial position of the group
and company, together with a description of the principal risks and
uncertainties that they face.
BY ORDER OF THE BOARD KIN COMPANY SECRETARIAL LIMITED Company
Secretary 24 April 2023
FINANCIAL STATEMENTS
INDEPENT AUDITORS' REPORT to the members of Arix Bioscience
plc
OPINION ON THE FINANCIAL STATEMENTS In our opinion:
? the financial statements give a true and fair view of the
state of the Group's and of the Parent Company's affairs as at 31
December 2022 and of the Group's loss for the year then ended;
? the Group financial statements have been properly prepared in
accordance with UK adopted international accounting standards;
? the Parent Company financial statements have been properly
prepared in accordance with UK adopted international accounting
standards and as applied in accordance with the provisions of the
Companies Act 2006; and
? the financial statements have been prepared in accordance with
the requirements of the Companies Act 2006.
We have audited the financial statements of Arix Bioscience plc
(the 'Parent Company') and its subsidiaries (the 'Group') for the
year ended 31 December 2022 which comprise the Consolidated
Statement of Comprehensive Income, the Consolidated Statement of
Financial Position, the Consolidated Statement of Changes in
Equity, the Consolidated Statement of Cash Flows, the Company
Statement of Financial Position, the Company Statement of Changes
in Equity and notes to the financial statements, including a
summary of significant accounting policies. The financial reporting
framework that has been applied in their preparation is applicable
law and UK adopted international accounting standards and as
regards the Parent Company financial statements, as applied in
accordance with the provisions of the Companies Act 2006.
Basis for opinion We conducted our audit in accordance with
International Standards on Auditing (UK) (ISAs (UK)) and applicable
law. Our responsibilities under those standards are further
described in the Auditor's responsibilities for the audit of the
financial statements section of our report. We believe that the
audit evidence we have obtained is sufficient and appropriate to
provide a basis for our opinion. Our audit opinion is consistent
with the additional report to the audit committee.
Independence Following the recommendation of the audit
committee, we were appointed by the board in May 2020 to audit the
financial statements for the year ending 31 December 2020 and
subsequent financial periods. The period of total uninterrupted
engagement including retenders and reappointments is 3 years,
covering the years ending 2020 to 2022. We remain independent of
the Group and the Parent Company in accordance with the ethical
requirements that are relevant to our audit of the financial
statements in the UK, including the FRC's Ethical Standard as
applied to listed public interest entities, and we have fulfilled
our other ethical responsibilities in accordance with these
requirements. The non-audit services prohibited by that standard
were not provided to the Group or the Parent Company.
Conclusions relating to going concern In auditing the financial
statements, we have concluded that the Directors' use of the going
concern basis of accounting in the preparation of the financial
statements is appropriate. Our evaluation of the Directors'
assessment of the Group and the Parent Company's ability to
continue to adopt the going concern basis of accounting
included:
? Reviewing the latest Board approved forecasts covering 3 years
from the year-end date of the financial statements.
? Considering the appropriateness and accuracy of these
forecasts, corroborating the key inputs such as cash inflows to our
knowledge of the entity and evidence obtained from our work on
other areas of the financial statements, as well as reviewing the
Board's stress test to ascertain the likelihood of the Group and
Parent Company not having the ability to meet their obligations as
they fall due.
? Challenging the Board about the implications of the ongoing
conflict in Ukraine, current economic climate and inflation on the
business.
Based on the work we have performed, we have not identified any
material uncertainties relating to events or conditions that,
individually or collectively, may cast significant doubt on the
Group and the Parent Company's ability to continue as a going
concern for a period of at least twelve months from when the
financial statements are authorised for issue.
Our responsibilities and the responsibilities of the Directors
with respect to going concern are described in the relevant
sections of this report.
Overview
100% (2021: 100%) of Group revenue
Coverage
100% (2021: 100%) of Group investments
Key audit matters 2022 2021
Valuation of Unquoted Investments Yes Yes
Group financial statements as a whole
Materiality
GBP3.5m (2021: GBP3.8m) based on 1.5% (2021: 1.5%) of net assets.
An overview of the scope of our audit Our Group audit was scoped
by obtaining an understanding of the Group and its environment,
including the Group's system of internal control, and assessing the
risks of material misstatement in the financial statements. We also
addressed the risk of management override of internal controls,
including assessing whether there was evidence of bias by the
Directors that may have represented a risk of material
misstatement.
We identified six components in the Group, three of which are
significant, five operate in the United Kingdom ('UK') and one in
the United States ('US'). All five UK components were subject to
full scope audits by the Group Engagement Team to our component
materiality. The material balances and transactions of the US
component were audited to our component materiality by the Group
Engagement Team for group purposes.
Climate change Our work on the assessment of potential impacts
on climate-related risks on the Group's operations and financial
statements included:
? Enquiries and challenge of management to understand the
actions they have taken to identify climate-related risks and their
potential impacts on the financial statements and adequately
disclose climate-related risks within the annual report;
? Our own qualitative risk assessment taking into consideration
the sector in which the Group operates and how climate change
affects this particular sector; and
? Review of the minutes of Board and Audit Committee meeting and
other papers related to climate change and performed a risk
assessment as to how the impact of the Group's commitment as set
out in Note 21 may affect the financial statements and our
audit.
We challenged the extent to which climate-related
considerations, including the expected cash flows from the
initiatives and commitments have been reflected, where appropriate,
in management's going concern assessment and viability
assessment.
We also assessed the consistency of managements disclosures
included as 'Other Information' within the financial statements and
with our knowledge obtained from the audit.
Based on our risk assessment procedures, we did not identify
there to be any Key Audit Matters materially impacted by
climate-related risks and related commitments.
Key audit matters Key audit matters are those matters that, in
our professional judgement, were of most significance in our audit
of the financial statements of the current period and include the
most significant assessed risks of material misstatement (whether
or not due to fraud) that we identified, including those which had
the greatest effect on: the overall audit strategy, the allocation
of resources in the audit, and directing the efforts of the
engagement team. These matters were addressed in the context of our
audit of the financial statements as a whole, and in forming our
opinion thereon, and we do not provide a separate opinion on these
matters.
How the scope of our audit addressed
Key audit the key audit matter
matter
We tested the valuations of a sample of unquoted
investments.
For all investments in our sample we:
? Considered whether the valuation methodology chosen was
in accordance with accounting standards and was the most
appropriate in the circumstances under the International
Private Equity and Venture Capital (IPEV) Guidelines;
? Held meetings with management to understand the recent
performance of the investee companies in the context of
their "milestones", and corroborated information obtained
There is a high level of estimation in these meetings to board papers, management information
Valuation of uncertainty involved in determining the and publicly available industry articles, reports and
Unquoted valuation of the unquoted investments in the press releases;
Investments portfolio.
? Where a valuation had been amended based on the price of
Refer Investments are also the most significant a recent funding round, we obtained associated Sale
Accounting balance contributing to the Net Asset Value Purchase Agreements for the funding round in order to
Policies and (NAV) of the fund, and therefore may be corroborate the price of the round, and considered whether
Note 12. subject to management bias. We therefore the funding round had been carried out on an arm's length
determined the valuation of investments to be basis;
a key audit matter.
? Where a valuation had been amended based on an investee
company achieving or failing to meet certain key
milestones, we challenged the basis of the change in value
and obtained third party evidence. We assessed this by
enquiries with management and corroborated this by
inspecting board packs and financial performance of the
investee companies.
Key observations:
Based on the procedures performed, we consider the
estimates made by management in valuing the unquoted
investments to be reasonable.
Our application of materiality We apply the concept of
materiality both in planning and performing our audit, and in
evaluating the effect of misstatements. We consider materiality to
be the magnitude by which misstatements, including omissions, could
influence the economic decisions of reasonable users that are taken
on the basis of the financial statements.
In order to reduce to an appropriately low level the probability
that any misstatements exceed materiality, we use a lower
materiality level, performance materiality, to determine the extent
of testing needed. Importantly, misstatements below these levels
will not necessarily be evaluated as immaterial as we also take
account of the nature of identified misstatements, and the
particular circumstances of their occurrence, when evaluating their
effect on the financial statements as a whole.
Based on our professional judgement, we determined materiality
for the financial statements as a whole and performance materiality
as follows:
Group financial statements Parent Company financial statements
2022 2021 2022 2021
GBPm GBPm GBPm GBPm
Materiality 3.5 3.8 2.8 2.8
Basis for
determining 1.5% net assets 1.5% net assets 1.5% net assets 1.5% net assets
materiality
Given the activities of the Group as a venture capital The nature of the parent company as a
Rationale for the group and the needs of the users of the financial holding company and therefore being an
benchmark applied statements, we determined that Net Assets was the most asset-based entity.
appropriate benchmark.
Performance 2.7 2.8 2.1 2.1
materiality
Basis for 75% materiality 75% materiality 75% materiality 75% materiality
determining
performance On the basis of our risk assessment together with our assessment of the overall control environment
materiality and expected total value of known and likely misstatements, based on past experience, our judgement
was that overall performance materiality for the Group and Parent should be 75% of materiality.
Component materiality We set materiality for each component of
the Group based on a percentage of 62% (2021: 49%) of Group
materiality dependent on the size and our assessment of the risk of
material misstatement of that component. Component materiality
ranged from GBP2.1m (2021: GBP1.7m) to GBP2.2m (2021: GBP2.8m). In
the audit of each component, we further applied performance
materiality levels of 75% (2021: 75%) of the component materiality
to our testing to ensure that the risk of errors exceeding
component materiality was appropriately mitigated.
Reporting threshold We agreed with the Audit Committee that we
would report to them all individual audit differences in excess of
GBP71k (2021: GBP76k). We also agreed to report differences below
this threshold that, in our view, warranted reporting on
qualitative grounds.
Other information The directors are responsible for the other
information. The other information comprises the information
included in the Annual Report and Accounts other than the financial
statements and our auditor's report thereon. Our opinion on the
financial statements does not cover the other information and,
except to the extent otherwise explicitly stated in our report, we
do not express any form of assurance conclusion thereon. Our
responsibility is to read the other information and, in doing so,
consider whether the other information is materially inconsistent
with the financial statements or our knowledge obtained in the
course of the audit, or otherwise appears to be materially
misstated. If we identify such material inconsistencies or apparent
material misstatements, we are required to determine whether this
gives rise to a material misstatement in the financial statements
themselves. If, based on the work we have performed, we conclude
that there is a material misstatement of this other information, we
are required to report that fact.
We have nothing to report in this regard.
Corporate governance statement As the Group has voluntarily
adopted the UK Corporate Governance Code 2018, we are required to
review the Directors' statement in relation to going concern,
longer-term viability and that part of the Corporate Governance
Statement relating to the parent company's compliance with the
provisions of the UK Corporate Governance Code specified for our
review.
Based on the work undertaken as part of our audit, we have
concluded that each of the following elements of the Corporate
Governance Statement is materially consistent with the financial
statements or our knowledge obtained during the audit.
? The Directors' statement with regards to the appropriateness of adopting the going concern
Going concern and basis of accounting and any material uncertainties identified set out within the report; and
longer-term viability
? The Directors' explanation as to their assessment of the Group's prospects, the period this
assessment covers and why the period is appropriate is set out within the report
? Directors' statement on fair, balanced and understandable set within the report
? Board's confirmation that it has carried out a robust assessment of the emerging and principal
risks
Other Code provisions
? The section of the annual report that describes the review of effectiveness of risk management
and internal control systems set out above; and
? The section describing the work of the audit committee set out in the Audit Report
Other Companies Act 2006 reporting Based on the responsibilities
described below and our work performed during the course of the
audit, we are required by the Companies Act 2006 and ISAs (UK) to
report on certain opinions and matters as described below.
In our opinion, based on the work undertaken in the course of the audit:
? the information given in the Strategic report and the Directors' report for the financial
year for which the financial statements are prepared is consistent with the financial
statements; and
Strategic report and
Directors' report ? the Strategic report and the Directors' report have been prepared in accordance with
applicable legal requirements.
In the light of the knowledge and understanding of the Group and Parent Company and its
environment obtained in the course of the audit, we have not identified material
misstatements in the strategic report or the Directors' report.
Directors' remuneration In our opinion, the part of the Directors' remuneration report to be audited has been
properly prepared in accordance with the Companies Act 2006.
We have nothing to report in respect of the following matters in relation to which the
Companies Act 2006 requires us to report to you if, in our opinion:
? adequate accounting records have not been kept by the Parent Company, or returns adequate
Matters on which we are for our audit have not been received from branches not visited by us; or
required to report by
exception ? the Parent Company financial statements and the part of the Directors' remuneration report
to be audited are not in agreement with the accounting records and returns; or
? certain disclosures of Directors' remuneration specified by law are not made; or
? we have not received all the information and explanations we require for our audit.
Responsibilities of Directors As explained more fully in the
Statement of Directors' Responsibilities, the Directors are
responsible for the preparation of the financial statements and for
being satisfied that they give a true and fair view, and for such
internal control as the Directors determine is necessary to enable
the preparation of financial statements that are free from material
misstatement, whether due to fraud or error.
In preparing the financial statements, the Directors are
responsible for assessing the Group's and the Parent Company's
ability to continue as a going concern, disclosing, as applicable,
matters related to going concern and using the going concern basis
of accounting unless the Directors either intend to liquidate the
Group or the Parent Company or to cease operations, or have no
realistic alternative but to do so.
Auditor's responsibilities for the audit of the financial
statements Our objectives are to obtain reasonable assurance about
whether the financial statements as a whole are free from material
misstatement, whether due to fraud or error, and to issue an
auditor's report that includes our opinion. Reasonable assurance is
a high level of assurance, but is not a guarantee that an audit
conducted in accordance with ISAs (UK) will always detect a
material misstatement when it exists. Misstatements can arise from
fraud or error and are considered material if, individually or in
the aggregate, they could reasonably be expected to influence the
economic decisions of users taken on the basis of these financial
statements.
Extent to which the audit was capable of detecting
irregularities, including fraud Irregularities, including fraud,
are instances of non-compliance with laws and regulations. We
design procedures in line with our responsibilities, outlined
above, to detect material misstatements in respect of
irregularities, including fraud. The extent to which our procedures
are capable of detecting irregularities, including fraud is
detailed below:
Non-compliance with laws and regulations Based on:
? Our understanding of the Group and the industry in which it
operates;
? Discussion with management and those charged with
governance;
? Obtaining and understanding of the Group's policies and
procedures regarding compliance with laws and regulations; and
we considered the significant laws and regulations to be
Companies Act 2006, the FCA listing and DTR rules, the principles
of the UK Corporate Governance Code, requirements of PAYE and VAT
legislation and IFRS.
Our procedures in respect of the above included:
? Review of minutes of meeting of those charged with governance
for any instances of non-compliance with laws and regulations;
? Review of correspondence with regulatory and tax authorities
for any instances of non-compliance with laws and regulations;
? Review of financial statement disclosures and agreeing to
supporting documentation;
? Involvement of tax specialists in the audit; and
? Review of legal expenditure accounts to understand the nature
of expenditure incurred.
Fraud We assessed the susceptibility of the financial statements
to material misstatement, including fraud. Our risk assessment
procedures included:
? Enquiry with management and those charged with governance
regarding any known or suspected instances of fraud;
? Obtaining an understanding of the Group's policies and
procedures relating to:
- Detecting and responding to the risks of fraud; and
- Internal controls established to mitigate risks related to
fraud.
? Review of minutes of meeting of those charged with governance
for any known or suspected instances of fraud;
? Discussion amongst the engagement team as to how and where
fraud might occur in the financial statements;
? Performing analytical procedures to identify any unusual or
unexpected relationships that may indicate risks of material
misstatement due to fraud; and
? Considering remuneration incentive schemes and performance
targets and the related financial statement areas impacted by
these.
Based on our risk assessment, we considered the areas most
susceptible to fraud to be valuation of unquoted investments and
management override of controls.
Our procedures in respect of the above included:
? Testing a sample of journal entries throughout the year, which
met a defined risk criteria, by agreeing to supporting
documentation;
? Assessing significant estimates made by management for bias in
relation to valuation of unquoted investments. The key audit
matters section of our report explains this matter in more detail
and also describes the specific procedures we performed in response
to that key audit matter; and
? Agreement of the financial statements disclosures to
underlying supporting documentation.
We also communicated relevant identified laws and regulations
and potential fraud risks to all engagement team members who were
all deemed to have appropriate competence and capabilities and
remained alert to any indications of fraud or non-compliance with
laws and regulations throughout the audit.
Our audit procedures were designed to respond to risks of
material misstatement in the financial statements, recognising that
the risk of not detecting a material misstatement due to fraud is
higher than the risk of not detecting one resulting from error, as
fraud may involve deliberate concealment by, for example, forgery,
misrepresentations or through collusion. There are inherent
limitations in the audit procedures performed and the further
removed non-compliance with laws and regulations is from the events
and transactions reflected in the financial statements, the less
likely we are to become aware of it.
A further description of our responsibilities is available on
the Financial Reporting Council's website at:
www.frc.org.uk/auditorsresponsibilities. This description forms
part of our auditor's report.
Use of our report This report is made solely to the Parent
Company's members, as a body, in accordance with Chapter 3 of Part
16 of the Companies Act 2006. Our audit work has been undertaken so
that we might state to the Parent Company's members those matters
we are required to state to them in an auditor's report and for no
other purpose. To the fullest extent permitted by law, we do not
accept or assume responsibility to anyone other than the Parent
Company and the Parent Company's members as a body, for our audit
work, for this report, or for the opinions we have formed.
VANESSA-JAYNE BRADLEY (Senior Statutory Auditor) FOR AND ON
BEHALF OF BDO LLP, Statutory Auditor London, United Kingdom Date:
24 April 2023
BDO LLP is a limited liability partnership registered in England
and Wales (with registered number OC305127).
CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME FOR THE YEARED 31
DECEMBER 2022
2022 2021
Note GBP'000 GBP'000
Change in fair value of investments 12 (30,768) (47,975)
Impairment of investments 12 - (5,943)
Revenue 3 112 340
Administrative expenses 6, 19 (5,405) (5,069)
Foreign exchange gain/(loss) 6,901 -
--------------- ---------------
Operating loss before exceptional costs (29,160) (58,647)
Exceptional costs 8 - (1,490)
--------------- ---------------
Operating loss after exceptional costs (29,160) (60,137)
Finance income 7 1,581 156
Foreign exchange loss* - (1,369)
Share-based payment* - 266
--------------- ---------------
Loss before taxation (27,579) (61,084)
Taxation 10 4 -
--------------- ---------------
Loss for the year (27,575) (61,084)
========= =========
Other comprehensive income
Items that have been/may be reclassified subsequently to profit or loss:
Exchange differences on translating foreign operations (2,050) 91
Taxation 10 - -
--------------- ---------------
Total comprehensive expense for the year (29,625) (60,993)
========= =========
Attributable to
Owners of Arix Bioscience plc (29,625) (60,993)
--------------- ---------------
Loss per share
Basic loss per share (p) 11 (23.9) (48.0)
========= =========
The above consolidated statement of comprehensive income should
be read in conjunction with the accompanying notes.
* In the current year the foreign exchange gain has been moved
to be included within operating expenses and the share-based
payment is now included within Administrative expenses.
CONSOLIDATED STATEMENT OF FINANCIAL POSITION AS AT 31 DECEMBER
2022
2022 2021
Note GBP'000 GBP'000
ASSETS
Non-current assets
Investments held at fair value 12 102,694 120,635
Intangible assets 13 24 168
Property, plant and equipment 14A 57 85
Right of use asset 14B 72 121
--------------- ---------------
102,847 121,009
Current assets
Cash and cash equivalents 16 122,782 134,230
Other assets 15 2,218 1,839
--------------- ---------------
125,000 136,069
========= =========
TOTAL ASSETS 227,847 257,078
========= =========
LIABILITIES
Current liabilities
Trade and other payables 17 (1,864) (1,600)
Lease Liability (59) -
--------------- ---------------
(1,923) (1,600)
Non-current liabilities
Lease liability (11) (121)
TOTAL LIABILITIES (1,934) (1,721)
--------------- ---------------
NET ASSETS 225,913 255,357
========= =========
EQUITY
Share capital and share premium 18 188,585 188,585
Retained earnings 51,250 80,694
Other reserves (13,922) (13,922)
--------------- ---------------
TOTAL EQUITY 225,913 255,357
========= =========
The accompanying notes form an integral part of the financial
statements. The financial statements were approved by the Board of
Directors and authorised for issue on 24 April 2023, and were
signed on its behalf by
PEREGRINE MONCREIFFE Chairman
CONSOLIDATED STATEMENT OF CHANGES IN EQUITY FOR THE YEAR 31
DECEMBER 2022
Share Treasury
Capital and Other Other Share Retained
Premium Equity Reserves Reserve Earnings Total
GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000
As at 1 January 188,585 (1,216) (1,113) (11,593) 80,694 255,357
2022
Loss for the year - - - - (27,575) (27,575)
Other
comprehensive - - - - (2,050) (2,050)
income
Share-based - - - - 181 181
payment charge
--------------- --------------- --------------- --------------- --------------- ---------------
As at 31 December 188,585 (1,216) (1,113) (11,593) 51,250 225,913
2022
========= ========= ========= ========= ========= =========
FOR THE YEAR 31 DECEMBER 2021
Share Treasury
Capital and Other Other Share Retained
Premium Equity Reserves Reserve Earnings Total
GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000
As at 1 January 188,585 (1,240) (1,180) - 142,044 328,209
2021
Loss for the year - - - - (61,084) (61,084)
Other
comprehensive - - 91 - - 91
income
Share-based - - - - (266) (266)
payment credit
Acquisition of - - - (11,593) - (11,593)
own shares
Issue of own
shares to - 24 (24) - - -
employees
--------------- --------------- --------------- --------------- --------------- ---------------
As at 31 December 188,585 (1,216) (1,113) (11,593) 80,694 255,357
2021
========= ========= ========= ========= ========= =========
CONSOLIDATED STATEMENT OF CASH FLOWS FOR THE YEARED 31 DECEMBER
2022
2022 2021
Note GBP'000 GBP'000
Cash flows from operating activities 20 (6,221) (7,294)
Finance income 1,582 156
Finance expenses (1) -
Tax received 4 -
--------------- ---------------
Net cash used in operating activities (4,636) (7,138)
========= =========
Cash flows from investing activities
Purchase of equity investments 12 (33,623) (59,221)
Disposal of equity and loan investments 12 20,796 39,084
Purchase of property, plant and equipment 14A (8) (101)
Cash received from/(placed on) long-term deposit - 62,276
--------------- ---------------
Net cash (used in)/from investing activities (12,835) 42,038
========= =========
Cash flows from financing activities
Purchase of own shares - (11,593)
Principal elements of lease payments (63) -
--------------- ---------------
Net cash used in financing activities (63) (11,593)
========= =========
Net (decrease)/increase in cash and cash equivalents (17,534) 23,307
========= =========
Cash and cash equivalents at start of year 134,230 112,085
Effect of exchange rate changes 6,086 (1,162)
--------------- ---------------
Cash and cash equivalents at end of year 122,782 134,230
========= =========
NOTES TO THE FINANCIAL STATEMENTS
1. GENERAL INFORMATION The principal activity of Arix Bioscience
plc (the "Company") and its subsidiaries (together the "Arix Group"
or "the Group" or "Arix") is to invest in breakthrough
biotechnology companies to deliver superior risk-adjusted returns
to shareholders.
The Company is incorporated and domiciled in the United Kingdom.
Arix Bioscience plc was incorporated on 15 September 2015 as
Perceptive Bioscience Investments Limited and changed its name to
Arix Bioscience Limited. It subsequently re-registered as a public
limited company and changed its name to Arix Bioscience plc. The
address of its registered office is Duke Street House, 50 Duke
Street, London, W1K 6JL. The registered number is 09777975. The
Company is the ultimate parent company into which the results of
all subsidiaries are consolidated.
2. ACCOUNTING POLICIES A. Basis of preparation The consolidated
financial statements of the Arix Group have been prepared in
accordance with international accounting standards in conformity
with the requirements of the Companies Act 2006 and prepared in
accordance with UK adopted international accounting standards.
The financial statements have been prepared on a historical cost
basis, except for certain financial assets which have been measured
at fair value through profit or loss. The financial statements are
presented in British pounds sterling, which is the functional and
presentational currency of the Company, and the presentational
currency of the Group; balances are presented in thousands of
British pounds sterling unless otherwise stated.
The Arix Group has applied all standards and interpretations
issued by the IASB and endorsed by the UKEB that were effective at
the period end date. The accounting policies set out below have,
unless otherwise stated, been applied consistently to all periods
presented.
Use of judgements and estimates In preparing these financial
statements, management has made judgements, estimates and
assumptions that affect the application of the Arix Group's
accounting policies and reported amounts of assets, liabilities,
income and expenses. Actual results may differ from those
estimates.
Estimates and underlying assumptions are reviewed on an ongoing
basis. Revisions to estimates are recognised prospectively.
Significant estimates are made by the Arix Group when applying
the appropriate methodology for valuing investments (see Note
2(I)), share-based payments (see Note 2(P) and Note 19).
Sensitivity of the investment portfolio is disclosed in Note
12.
In preparing these financial statements, the Directors have
concluded that the Company meets the definition of an investment
entity as per IFRS 10, as it has the typical characteristics set
out in the standard, including holding more than one investment and
having more than one investor which is not a related party of the
entity.
Going concern The financial information presented within these
financial statements has been prepared on a going concern basis.
The Directors have made an assessment of going concern over a
period of greater than 12 months, taking into account the Group's
current performance and outlook, which considered the risks the
business is exposed to. These risks include an increase in
operating costs of 50%, a reduction in or absence of any
realisations, and an increase in new investment commitments. Given
the substantial free cash available to the business and the
proportionately small cost base and legal funding commitments of
the group, the Directors have concluded that no material
uncertainty exists around the Company or the Group's ability to
continue as a going concern. As disclosed in the Corporate
Governance statement the Board considers it appropriate to adopt
the going concern basis.
B. Basis of consolidation Subsidiaries The Directors have
concluded that the Group has all the elements of control as
prescribed by IFRS 10 "Consolidated financial statements" in
relation to all its subsidiaries and that the Company satisfies
three essential criteria to be regarded as an investment entity as
defined in IFRS 10, IFRS 12 "Disclosure of Interests in other
entities" and IAS 27 "Separate Financial Statements". The three
essential criteria are such that the entity must: obtain funds from
more than one investor for the purpose of providing these investors
with professional investment management services; commit to its
investors that its business purpose is to invest its funds solely
for returns from capital appreciation, investment income or both;
and measure and evaluate the performance.
Subsidiaries are therefore measured at Fair Value through profit
or loss in accordance with IFRS 13 "Fair Value measurement" and
IFRS 9 "Financial Instruments".
Notwithstanding this, IFRS 10 requires subsidiaries that provide
services that relate to the investment entity's investment
activities to be consolidated. Accordingly, the financial
statements consolidate the results of the entities listed in the
table below. This table contains the disclosures required by
Section 409 of the Companies Act 2006 for subsidiaries:
Entity Country of Registered Address Ownership
Incorporation
Arix Bioscience Holdings Limited England and Wales Duke Street House, 50 Duke Street, London, W1K 100%
6JL
Arix Bioscience, Inc United States 401 Park Avenue South, New 100% York, NY 10016 100%
Arix Capital Management Limited England and Wales Sophia House, 28 Cathedral Road, Cardiff, CF11 100%
9LJ
Arthurian Life Sciences GP Limited Scotland 101 Rose Street South Lane, Edinburgh, 100%
Scotland, EH2 3JG
ALS SPV Limited England and Wales Duke Street House, 50 Duke Street, London, W1K 100%
6JL
Arthurian Life Sciences SPV GP Limited England and Wales Sophia House, 28 Cathedral Road, Cardiff, CF11 100%
9LJ
Arix Bioscience plc Employee Benefit Trust Jersey 26 New Street, St Helier, Jersey, JE2 3RA 100%
Arthurian Life Sciences Carried Interest Scotland 101 Rose Street South Lane, Edinburgh, 100%
Partner LP Scotland, EH2 3JG
Subsidiaries are fully consolidated from the date on which
control is transferred. They are deconsolidated from the date that
control ceases. The acquisition method of accounting is used to
account for business. All companies are involved in investing in
and building breakthrough biotech companies around cutting edge
advances in life sciences, other than Arix Capital Management and
the Arthurian Life Sciences companies, which are engaged in fund
management activity, and Arthurian Life Sciences Carried Interest
Partner LP, which holds a financial interest in a limited
partnership. Intercompany transactions, balances and unrealised
gains on transactions between Group companies are eliminated.
Unrealised losses are also eliminated unless the transaction
provides evidence of an impairment of the transferred asset.
Associates The Group has taken the exemption permitted by IAS 28
"Investments in Associates and Joint Ventures" and IFRS 11 "Joint
Arrangements" for entities similar to investment entities and
measures its investments in associates and joint ventures at fair
value. The Directors consider an Associate to be an entity over
which the Group has significant influence, but does not control,
generally accompanied by a shareholding of between 20% and 50% of
the voting rights.
No associates are presented on the Statement of Financial
Position as the Group elects to hold such investments at fair value
through profit and loss. This treatment is permitted by IAS 28
Investment in Associates and Joint Ventures, which permits
investments held by entities that are akin to venture capital
organisations to be excluded from its measurement methodology
requirements where those investments are designated, upon initial
recognition, at fair value through profit or loss and accounted for
in accordance with IFRS 9 Financial Instruments. Changes in fair
value of associates are recognised in the Statement of
Comprehensive Income in the period in which the change occurs. The
Group has no interests in associates through which it carries on
its business.
The disclosures required by Section 409 of the Companies Act
2006 for associated undertakings are included in Note 12 to the
financial statements. Similarly, those investments which may not
have qualified as an associate but fall within the wider scope of
significant holdings and so are subject to Section 409 disclosure
acts are also included in Note 12 to the financial statements.
WLSIF is considered neither a subsidiary (as detailed in Note
2(A)) nor an associate, as the Group does not have a 20-50%
interest in the entity nor is considered to have significant
influence.
In preparing these financial statements, the Directors have
considered the relationship that the Group has with The Wales Life
Sciences Investment Fund LP (the "WLSIF") and specifically as to
whether the Group controls WLSIF. The Directors note that while
Arix Capital Management Limited (a 100% subsidiary of Arix
Bioscience plc), in its role as fund manager to WLSIF, and
Arthurian Life Sciences SPV GP Limited (a 100% subsidiary of Arix
Bioscience plc) in its role as general partner of the WLSIF, both
exercise power over the activities of WLSIF, they do not have
sufficient exposure to variability of returns from WLSIF to meet
the definition of control. Accordingly, WLSIF has not been
consolidated into these financial statements.
C. Adoption of new and revised standards Management have
considered changes/amendments to the standards and concluded no
impact to the figures.
D. Revenue recognition Revenue is generated from fund management
fees, and from board adviser fees. Fund management fees are earned
as a percentage of funds managed and are recognised in the period
in which these services are provided. Board adviser fees are
recognised at a point of time.
E. Foreign currency translation The assets and liabilities of
foreign operations are translated to the Group's presentational
currency (British pounds sterling) at foreign exchange rates ruling
at the period-end date. The revenues and expenses of foreign
operations are translated at an average rate for the period where
this rate approximates to the foreign exchange rates ruling at the
dates of the transactions. Exchange differences arising from this
translation of foreign operations are reported as an item of other
comprehensive income and accumulated in the translation reserve.
Foreign exchange movements on Investments held at fair value are
reported within the Change in Fair Value of Investments on the face
of the Consolidated Statement of Comprehensive Income. Foreign
exchange differences arising from other items are disclosed
separately on face of the Consolidated Statement of Comprehensive
Income.
F. Leases Lessees are required to recognise lease obligations as
a liability and a right-of-use asset. The cost of the lease is
subsequently recognised in the consolidated statement of
comprehensive income as interest charged on the liability and as
depreciation charged on the right-of-use asset.
G. Exceptional items Items that are material in size and unusual
in nature are disclosed separately to provide a more accurate
indication of underlying performance.
H. Property, plant and equipment Property, plant and equipment
is stated at cost less accumulated depreciation and any accumulated
impairment losses. Cost includes expenditure that is directly
attributable to the acquisition of the asset. Depreciation is
calculated using the straight-line method over the estimated useful
lives of the related assets:
Office equipment Three years
Fixtures and fittings Five years
I. Right-of-use asset Right-of-use asset is stated at cost less
accumulated depreciation and any accumulated impairment losses and
is depreciated over the lower of the lease and the useful life of
the right-of-use asset. The depreciation period is 3 years.
J. Financial assets The Arix Group classifies its financial
assets as either at fair value through profit or loss or amortised
cost. The classification depends on the purpose for which the
financial assets have been acquired and is determined on initial
recognition.
Amortised cost assets are non-derivative financial assets with
fixed or determinable payments that are not listed in an active
market. They are included in current assets, except for maturities
greater than 12 months after the end of the reporting period, which
are classified as non-current assets. The Arix Group's loans and
receivables comprise trade and other receivables and cash and cash
equivalents in the Consolidated Statement of Financial
Position.
Regular purchases and sales of financial assets are recognised
on the trade date - the date on which the Arix Group commits to
purchase or sell the asset. Financial assets are derecognised when
the rights to receive cash flows from the investments have expired
or have been transferred and the Arix Group has transferred
substantially all risks and rewards of ownership.
Equity investments Those investments in the Arix Group that are
held with a view to the ultimate realisation of capital gains are
recognised as equity investments within the scope of IFRS 9 and are
classified as financial assets at fair value through profit or
loss. This includes investments in associated undertakings, as per
Note 12, and investment subsidiaries. When financial assets are
initially recognised they are measured at fair value. They are
subsequently remeasured periodically at board meetings or if a
valuation event occurs, such as merger, reverse merger, or fund
raise.
Valuation of investments The fair value of the Group's
investments is determined using International Private Equity and
Venture Capital Valuation Guidelines December 2018 ("IPEV
Guidelines"), which comply with IFRS.
The fair value of listed investments is based on bid prices at
the period end date.
Upon investment, the fair value of unlisted securities is
recognised at cost. Similarly, following a further funding round
with participation by at least one third party, the price paid by
the external investor is generally considered to represent the
investment's fair value at the transaction date, although the
specific terms and circumstances of each funding round must always
be considered.
Following the transaction date, each investment is observed for
objective evidence of an increase or impairment in its value. This
reflects the fact that investments made in seed, start-up and early
stage biotech companies often have no current and no short-term
future revenues or positive cash flows; in such circumstances, it
can be difficult to gauge the probability and financial impact of
the success or failure of development or research activities and to
make reliable cash flow forecasts. As such, the Group carries out
an enhanced assessment based on milestone analysis, which seeks to
determine whether there is an indication of a change in fair value
based on changes to the company's prospects. A milestone event may
include, but is not limited to, technical measures, such as
clinical trial progress; financial measures, such as a company's
availability of cash; and market measures, such as licensing
agreements agreed by the company. Indicators of impairment might
include significant delays to clinical progress, technical
complications or financial difficulties. Often qualitative
milestones provide a directional indication of the movement of fair
value. Calibrating such milestones may result in a fair value equal
to the transaction value. Any ultimate change in valuation reflects
the assessed impact of the progress against milestones and the
consequential impact on a potential future external valuation
point, such as a future funding round or initial public
offering.
When forming a view of the fair value of its investment, the
Arix Group takes into account circumstances where an investment's
equity structure involves different class rights on a sale or
liquidity event.
The valuation metrics used in these financial statements are
discussed in Note 12.
Although the Directors use their best judgement, there are
inherent limitations in any valuation techniques. Whilst fair value
estimates presented herein attempt to present the amount the Arix
Group could realise in a current transaction, the final realisation
may be different, as future events will also affect the current
estimates of fair value. The effects of such events on the
estimates of fair value, including the ultimate realisation of
investments, could be material to the financial statements.
Treatment of gains and losses arising on fair value Realised and
unrealised gains and losses on financial assets at fair value
through profit and loss are included in the Statement of
Comprehensive Income in the period in which they arise.
Recognition of financial assets Purchases and sales of financial
assets are recognised on trade-date, the date on which the Group
commits to purchase or sell the asset. Financial assets are
derecognised when the rights to receive cash flows from the
financial assets have expired or have been transferred and the
Group has transferred substantially all the risks and rewards of
ownership.
Loans and receivables are subsequently carried at amortised cost
using the effective interest method.
Impairment of financial assets At the end of each reporting
period the Group assesses whether there is objective evidence that
its loans and other receivables are impaired. The amount of the
loss is measured as the difference between the asset's carrying
amount and the present value of estimated future cash flows
discounted at the financial asset's original effective interest
rate. The asset's carrying amount is reduced through the use of an
allowance account and the amount of the loss is recognised in the
Statement of Comprehensive Income as a separate line item. If, in a
subsequent period, the amount of the impairment loss decreases and
the decrease can be related objectively to an event occurring after
the impairment was recognised, the reversal of the previously
recognised impairment loss is recognised in the Statement of
Comprehensive Income within administrative expenses. The Group's
financial assets that are subject to IFRS 9's expected credit loss
model are its loans and receivables, cash and cash equivalents and
cash on long-term deposit. The identified impairment loss is
considered immaterial.
Financial assets and liabilities are offset when there is a
legally enforceable right to offset the recognised amounts and
there is an intention to settle on a net basis or realise the asset
and settle the liability simultaneously. The legally enforceable
right must not be contingent on future events and must be
enforceable in the normal course of business and in the event of
default, insolvency or bankruptcy of the Arix Group or the
counterparty. Where these conditions are met, the net amount is
reported in the Statement of Financial Position.
K. Cash and cash equivalents Cash and cash equivalents comprise
cash at bank and in hand and call deposits.
L. Goodwill and intangible assets Intangibles were acquired by
the Arix Group as part of the acquisition of Arix Capital
Management Limited and Arthurian Life Sciences SPV GP Limited.
It is the policy of the Arix Group to amortise these fair values
over the period in which the Arix Group is expected to obtain
economic benefit from the related intangible assets. The excess of
consideration transferred over the fair value of net identifiable
assets acquired is recorded as goodwill. If those amounts are less
than the fair value of the net identifiable assets of the business
acquired, the difference is recognised directly in the Statement of
Comprehensive Income as a bargain purchase. The asset is assessed
for impairment periodically and marked down appropriately if an
indication of impairment is noted.
M. Share capital Ordinary Shares and Series C Shares are
classified as equity. Equity instruments issued by the Arix Group
are recorded at the proceeds received, net of direct issue
costs.
Own shares represent shares of Arix Bioscience plc that are held
by an employee share trust for the purpose of fulfilling
obligations in respect of various employee share plans. Own shares
are treated as a deduction from equity until the shares are
cancelled, reissued or disposed of. When they vest, they are
transferred from own shares to retained earnings at their weighted
average cost.
N. Trade payables Trade payables are obligations to pay for
goods or services that have been acquired in the ordinary course of
business from suppliers. Accounts payable are classified as current
liabilities if payment is due within one year or less (or in the
normal operating cycle of the business if longer).
If not, they are presented as non-current liabilities.
Trade payables are initially recognised at fair value, generally
being the invoiced amount and are subsequently measured at
amortised cost, using the effective interest method.
O. Current and deferred taxation The tax expense for the year
comprises current tax and deferred tax. Tax is recognised in the
Statement of Comprehensive Income, except to the extent that it
relates to items recognised directly in equity.
The current income tax charge is calculated on the basis of the
tax laws enacted or substantively enacted at the balance sheet date
in the countries where the Arix Group operates and generates
taxable income. Management periodically evaluates positions taken
in tax returns with respect to situations in which applicable tax
regulation is subject to interpretation. It establishes provisions
where appropriate on the basis of amounts expected to be paid to
the tax authorities.
Deferred income tax is recognised on temporary differences
arising between the tax bases of assets and liabilities and their
carrying amounts in the balance sheets, using the liability method.
However, the deferred income tax is not accounted for if it arises
from initial recognition of an asset or liability in a transaction
other than a business combination that at the time of the
transaction affects neither accounting nor taxable profit or loss.
Deferred income tax is determined using tax rates (and laws) that
have been enacted or substantially enacted by the Statement of
Financial Position date and are expected to apply when the related
deferred income tax asset is realised or the deferred income tax
liability is settled.
Deferred income tax assets are recognised only to the extent
that it is probable that future taxable profit will be available
against which the temporary differences can be utilised.
Deferred income tax assets and liabilities are offset when there
is a legally enforceable right to offset current tax assets against
current tax liabilities and when the deferred income tax assets and
liabilities relate to income taxes levied by the same taxation
authority on either the taxable entity or different taxable
entities where there is an intention to settle the balances on a
net basis.
The Group primarily seeks to generate capital gains from its
portfolio company investments, which would ordinarily be subject to
UK corporation tax. However, where the Group holds or has held in
excess of 10% of the share capital of a portfolio company, and
those companies are themselves trading or preparing to carry on a
trade, the Directors continue to believe that these holdings will
qualify for the UK's Substantial Shareholdings Exemption ("SSE"),
which exempts taxable gains or losses from corporation tax. For
unrealised gains and losses that are expected to meet the
qualifying criteria, no deferred tax provision will be made in the
Group's financial statements. Where investment gains or losses are
unrealised and are not expected to qualify for SSE, the anticipated
tax due based on the current valuation of the underlying investment
is reflected in a deferred tax balance, to the extent that these
exceed the Group's historical operating losses from time to time.
SSE has not been applied to any realised gains in the year (2021:
GBPnil). The Directors have taken what they consider to be all
necessary steps to support the determination that the gains in 2022
in the Arix portfolio qualify for SSE, including close
collaboration with their appointed tax advisers and further
consultation and receipt of written opinion from a Queen's Counsel
Barrister at a leading tax chambers.
P. Share-based payments The Arix Group operates an equity
incentive plan and an executive share option plan in which the
Group's founders also participate. Share options must be measured
at fair value and recognised as an expense in the Statement of
Comprehensive Income with a corresponding increase in equity. The
fair value of the option is estimated at the date of grant using a
Black-Scholes Model or Monte Carlo simulation and is charged as an
expense in the Statement of Comprehensive Income over the vesting
period. Where relevant, the charge is adjusted each year to reflect
the expected and actual level of vesting. Further detail on
Share-based Payments is available in Note 19.
Q. Other reserves Other reserves relate to a Translation
Reserve, for foreign exchange differences which arise on the
translation of foreign operations; and a reserve relating to the
issue of shares by the Company's Employee Benefit Trust upon
vesting of employee share schemes.
R. Treasury share reserve The Treasury Share Reserve comprises
the cost of the Company's shares bought under the share buyback
programme.
S. Financial risk management The Arix Group is exposed to market
risk, interest rate risk, credit risk and liquidity risk. The
senior management oversees the management of these risks and
ensures that the financial risk taking is governed by appropriate
policies and procedures and that financial risks are identified,
measured and managed in accordance with the Arix Group's policies
and risk appetite.
The Board of Directors review and agree the policies for
managing each of these risks, which are summarised below:
Market risk Foreign exchange risk - the Arix Group operates
internationally and is exposed to foreign exchange risk arising
from various currency exposures, primarily with respect to the US
dollar and euros. Foreign exchange risk arises from future
commercial transactions, recognised assets and liabilities and net
investments in foreign operations. The Arix Group has certain
investments whose net assets are exposed to foreign currency
translation risk; at period-end the Arix Group held US
dollar-denominated assets valued at USD108.7m (2021: USD131.0m) and
euro-denominated assets valued at EUR18.0m (2021: EUR24.7m). A 10%
appreciation in each currency would have a GBP9.6m positive impact
(2021: GBP11.8m positive impact) on Arix's Income Statement; a 10%
depreciation would have a GBP9.6m negative impact (2021: GBP11.8m
negative impact) on Arix's income statement. The impact of foreign
exchange on these holdings is closely monitored.
Price risk - the Arix Group is exposed to equity securities
price risk because investments are held at fair value through
profit or loss.
The Group's strategy is to deploy long-term capital into
innovative companies which have novel, high-impact outcomes; Arix
believes that such companies are less susceptible to macroeconomic
cycles. The Group monitors the availability of its capital closely,
ensuring sufficient balances are available for the continuing
operation of the business throughout the period assessed in the
viability statement.
Interest rate risk Cash flow interest rate risk is the risk that
the future cash flows of a financial instrument will fluctuate
because of changes in market interest rates. Fair value interest
rate risk is the risk that the fair value of a financial instrument
will fluctuate due to changes in market interest rates.
The Arix Group's income is substantially independent of changes
in market interest rates. Interest-bearing assets include only cash
and cash equivalents and cash on long-term deposit, which earn
interest at variable rates. The Arix Group has a treasury policy to
manage cash and cash equivalents. In the year ended 31 December
2022, a 10% change in underlying interest rates would have impacted
Arix's finance income by GBP158k (2021: GBP16k).
Credit risk Credit risk refers to the risk that a counterparty
will default on its contractual obligations resulting in financial
loss to the Arix Group. The major classes of financial assets of
the Arix Group are cash and cash equivalents (GBP122.8m (2021:
GBP134m)); and trade and other receivables (GBP2.0m (2021:
GBP1.6m)).
Risk of counterparty default arising on cash and cash
equivalents is controlled within a framework of dealing with high
quality institutions.
As at 31 December 2022, 100% of cash and cash equivalents was
deposited with institutions that have a short-term credit rating of
at least F1, according to Fitch Ratings.
No counterparty has failed to meet its obligations over the
period. The maximum exposure to credit risk is represented by the
carrying amount of each asset. Management does not expect any
significant counterparty to fail to meet its obligations.
Liquidity risk The Arix Group manages liquidity risk by
maintaining sufficient cash to enable it to meet its operational
requirements. The following table details the Group's remaining
contractual maturity for its financial liabilities based on
undiscounted contractual payments:
2022
Within 2022
one year Total
GBP'000 GBP'000
Trade, Other Payables and Accruals (excluding non-financial liabilities) 1,864 1,864
========= =========
2022
Within 2022
one year Total
GBP'000 GBP'000
Trade, Other Payables and Accruals (excluding non-financial liabilities) 1,600 1,600
========= =========
Capital risk management The Arix Group manages its capital to
ensure that it will be able to continue as a going concern, whilst
also maximising the operating potential of the business. The
capital structure of the Arix Group consists of equity attributable
to equity holders of the Arix Group, comprising issued capital and
retained earnings as disclosed in the Consolidated Statement of
Changes in Equity. The Arix Group is not subject to externally
imposed capital requirements.
3. REVENUE
2022 2021
GBP'000 GBP'000
Fund management fee income 96 321
Other income 16 19
--------------- ---------------
112 340
========= =========
The total revenue for the Arix Group has been derived from its
principal activity of investing in breakthrough biotechnology
companies. All of this revenue relates to trading undertaken in the
United Kingdom.
4. SEGMENTAL INFORMATION Information for the purposes of
resource allocation and assessment of performance is reported to
the Arix Group's Chief Executive Officer, who is considered to be
the chief operating decision-maker, based wholly on the overall
activities of the Arix Group. Although Arix makes investments
globally, these are considered by one Investment Committee and
reported internally as a single portfolio. It has therefore been
determined that the Arix Group has only one reportable segment
under IFRS 8 ("Operating Segments"), which is that of sourcing,
financing and developing healthcare and life science businesses
globally. The Arix Group's revenue, results and assets for this one
reportable segment can be determined by reference to the
Consolidated Statement of Comprehensive Income and Consolidated
Statement of Financial Position. The geographic split of the
portfolio is shown above in this report.
5. LOSS BEFORE TAXATION
2022 2021
GBP'000 GBP'000
Amortisation (144) (144)
Depreciation (36) (65)
Right of Use Depreciation (49) -
--------------- ---------------
Auditors' remuneration
Statutory audit services
Fees payable for the audit of the Arix Group accounts 112 97
Fees payable for the audit of the accounts of subsidiaries of the Arix Group 50 43
--------------- ---------------
Non-audit services
Other assurance and advisory services 23 20
--------------- ---------------
Total auditors' remuneration 185 160
========= =========
Non-audit services in the year relate to the Arix Bioscience plc
interim review (GBP18k) and an FCA Client Asset Report (GBP5k)
(2021: interim review GBP16k; FCA Client Asset Report GBP4k).
6. ADMINISTRATIVE EXPENSES The administrative expenses charge
broken down by nature is as follows:
2022 2021
GBP'000 GBP'000
Employment costs 2,482 3,070
Recruitment costs 114 169
Consultancy fees 233 25
Other expenses 2,576 1,805
--------------- ---------------
5,405 5,069
========= =========
7. FINANCE INCOME
2022 2021
GBP'000 GBP'000
Bank interest 1,581 156
--------------- ---------------
1,581 156
========= =========
8. EXCEPTIONAL COSTS
2022 2021
GBP'000 GBP'000
Shareholder engagement costs - 1,032
Restructuring costs - 458
--------------- ---------------
Total exceptional costs - 1,490
========= =========
Items that are of exceptional size or material in size and
unusual in nature are included in administrative expenses and
disclosed separately to provide a more accurate indication of
underlying performance.
The shareholder engagement process resulted in a change to the
composition of the Board. Restructuring costs include the costs of
separation pay and payments in lieu of notice.
9. EMPLOYEE COSTS Employee costs (including Directors)
comprise:
2022 2021
GBP'000 GBP'000
Salary and bonus 2,186 2,735
Social security costs 184 200
Pension and benefits costs 112 135
--------------- ---------------
Employee costs excluding share-based payments 2,482 3,070
--------------- ---------------
Share-based payments (Note 19) 181 (266)
--------------- ---------------
2,663 2,804
========= =========
The average number of employees during the year was 7 (2021: 11)
(investment team: 3 (2021: 5); non-investment team: 4 (2021:
6)).
10. INCOME TAX
2022 2021
GBP'000 GBP'000
Current year tax charge
Current tax (4) -
Deferred tax - current year - -
Deferred tax - effect of change in tax rates - -
Adjustment in respect of previous periods - -
--------------- ---------------
Total tax charge (4) -
========= =========
Reconciliation of tax charge
Loss before tax (27,579) (61,084)
Expected tax based on 19.00% (2021: 19.00%) (5,240) (11,606)
--------------- ---------------
Effects of:
Expenses not deductible for tax purposes 6,142 10,765
Adjustment made in respect of prior years (4) -
Income not taxable (47) (434)
Employee share options - (51)
Deferred tax not recognised 856 1,326
--------------- ---------------
Total tax charge (4) -
========= =========
Recognised deferred tax provisions
Brought forward - -
Adjustments in respect of prior year - -
Relating to profit and loss - -
--------------- ---------------
Carried forward - -
========= =========
Represented by:
Unutilised tax losses (1,941) (3,461)
ACAs - -
Intangibles - -
Employee benefits - -
Investments 1,941 3,462
Other timing differences - (1)
--------------- ---------------
- -
========= =========
Unrecognised deferred tax provisions
Unutilised tax losses (15,459) (11,390)
Priority profit share outstanding (9,249) 363
Other timing differences (565) (8,565)
--------------- ---------------
(25,273) (19,592)
========= =========
The corporation tax rate for the year was 19%. The UK
corporation tax rate will increase from 19% to 25% from 1 April
2023 for companies with annual profits exceeding GBP250k but remain
at 19% for annual profits below GBP50k whilst companies with
profits between GBP50k and GBP250k will pay tax at the main rate of
25% reduced by marginal relief. This change has been enacted at the
balance sheet date and therefore the deferred tax assets and
liabilities as at 31 December 2022 have been measured using the
rates that would be expected to apply in the periods when the
underlying timing differences, on which deferred tax is recognised,
are expected to unwind. The Group is subject to UK corporation tax
on the majority of its activities, which can include gains arising
on investments. However, where possible the Group aims to take
advantage of the UK's Substantial Shareholding Exemption ("SSE"),
which exempts taxable gains or losses arising from the disposal of
shares, where certain conditions are met. Where SSE has been
applied in prior years, the Directors have taken what they consider
to be all necessary steps to support the determination that these
gains and losses in the Arix portfolio qualify for SSE, including
close collaboration with their appointed tax advisers and further
consultation and receipt of written opinion from a Queen's Counsel
Barrister at a leading tax chambers. The Directors continue to
believe that the application of SSE to the tax computation remains
appropriate.
11. Loss per Share During 2022, the Group did not undertake a
share buyback programme and there remains 6,428,853 shares being
held in treasury. In the prior year 2021, the Group undertook a
share buyback programme and this resulted in 6,428,853 shares being
held in treasury at the time that the programme was suspended, on
18 October 2021. As at 31 December 2022 the Group had 129,180,800
ordinary shares in issue (2021: 129,180,800).
Basic earnings per share is calculated by dividing the profit
attributable to equity holders of Arix Bioscience plc by the
weighted average number of enfranchised shares (as adjusted for
capital subscription in accordance with the terms of the
restrictive share agreement) in issue during the period.
Potentially dilutive ordinary shares include options and
conditional share awards issued under the Company's long-term
incentive plans. At the year end date, the weighted average number
of shares in relation to: (i) options and conditional share awards
was 3,181,859; and (ii) ordinary shares subject to restrictions was
5,080,582. Restricted ordinary shares are not entitled to vote,
attend meetings or to receive dividends or other distributions.
Consequently, they have been excluded from the calculation of the
weighted average number of shares in issue.
As at As at
31 December 31 December
2022 2021
GBP'000 GBP'000
Loss attributable to equity holders of Arix Bioscience plc (29,625) (60,993)
Weighted average number of shares in issue for the purposes of basic earnings per share 124,100,217 126,950,904
Weighted average number of shares in issue for the purposes of diluted earnings per share 132,362,659 136,430,200
Basic loss per share (23.9)p (48.0)p
Diluted loss per share n/a* n/a*
========= =========
* n/a as anti-dilutive.
12. INVESTMENTS HELD AT FAIR VALUE
Equity Investments 2022
Level 1 - Level 3 -
Listed Unlisted
Investments Investments Total
GBP'000 GBP'000 GBP'000
At 1 January 2022 63,698 56,937 120,635
Additions 22,525 11,098 33,623
Disposals (20,796) - (20,796)
Transfers* 8,964 (8,964) -
Change in fair value (32,036) (3,301) (35,337)
Foreign exchange (losses)/profits 2,416 2,153 4,569
--------------- --------------- ---------------
At 31 December 2022 44,771 57,923 102,694
========= ========= =========
* Disc Medicine listed on 29 December 2022
Level 3 investments are valued with reference to either the most
recent funding round (GBP47.2m, 2021: GBP53.3m); net asset value
(GBP3.1m, 2021: GBP1.0m); market-based write-up (GBP5.0m, 2021:
GBPnil); discretionary write-down (GBP1.3m, 2021: GBP1.4m) or
deferred consideration (GBP1.3m, 2021: GBP1.2m). See Note 2(I) for
further details on the valuation of Level 3 investments.
The Group's milestone valuation approach cannot be readily
sensitised and therefore the Group has not disclosed sensitivity
analysis for Level 3 inputs. A 10% movement in the share price of
Level 1 inputs would result in a GBP4.5m (2021: GBP6.4m) movement
in the investment portfolio value.
Equity investments - 2021
Level 1 - Level 3 -
Listed Unlisted
Investments Investments Total
GBP'000 GBP'000 GBP'000
At 1 January 2021 95,712 58,704 154,416
Additions 15,277 43,944 59,221
Disposals (30,530) (8,554) (39,084)
Transfers 26,908 (26,908) -
Change in fair value (43,210) (9,251) (52,461)
Foreign exchange losses (459) (998) (1,457)
--------------- --------------- ---------------
At 31 December 2021 63,698 56,937 120,635
========= ========= =========
As permitted by IAS 28 'Investment in Associates' and in
accordance with the Arix Group accounting policy, investments are
held at fair value even though the Arix Group may have significant
influence over the companies. Significant influence is determined
to exist when the Group holds more than 20% of the holding or when
less than 20% is held but in combination with a certain level of
board representation is deemed to be able to exert significant
influence. As at 31 December 2022, the Arix Group is deemed to have
significant influence over the following entities:
% of
Issued Net Profit/ Date of
Country of Registered Share Assets (Loss) Financial
Company Incorporation Address Capital of of Information
Held Company Company
Depixus SAS (EUR) France 3-5 Impasse Reille, 75014 Paris 21.4% EUR25,216k EUR(5,409)k 31 December
2021
Sorriso 9295 S 1300 Unit 3 Sandy, UT 84092 Not publicly
Pharmaceuticals Inc United States USA 26.0% N/A N/A available
(USD)
STipe Therapeutics Denmark Inge Lehmans Gade 10, Aarhus Centrum, 19.8% EUR1,356k EUR(8,903)k 31 December
Aps (EUR) 8000 Aarhus, Denmark 2022
Twelve Bio ApS (EUR) Denmark Ole Maaloes Vej 3, 2200 Copenhagen, 49.0% EUR2,379k EUR(1,784)k 31 December
Denmark 2021
========= ========= =========
In addition, at 31 December 2022, the Group held the following
investments in companies where it is not considered to have
significant influence:
% of Issued
Contractural Arix executive Share Capital
Company Board seat on Board Held
Artios Pharma Limited Y Y 9.9%
Aura Biosciences, Inc. N N 4.1%
Disc Medicine Inc. N Y 4.2%
Ensoma Y Y 4.1%
Harpoon Therapeutics, Inc. N Y 6.7%
Imara, Inc. N Y 6.1%
Iterum Therapeutics Limited N Y 0.1%
=========
The Arix Group has an interest in one structured entity, The
Wales Life Sciences Investment Fund (registered address: Sophia
House, 28 Cathedral Road, Cardiff, Wales, CF10 4PL). The fund has
interests in Welsh life sciences opportunities. A structured entity
is an entity that is structured in such a way that voting or
similar rights are not the dominant factor in deciding who controls
the entity. The Arix Group is not deemed to have control over this
fund for the reasons disclosed in Note 2(A). The Group's interest
is GBP0.2m (2021: GBP1.0m).
13. INTANGIBLE ASSETS
Year ended Year ended
31 December 31 December
2022 2021
GBP'000 GBP'000
Cost
As at 1 January and 31 December 1,534 1,534
--------------- ---------------
Amortisation
As at 1 January (1,366) (1,222)
Amortisation in the year (144) (144)
--------------- ---------------
Impairment in the year - -
As at 31 December (1,510) (1,366)
--------------- ---------------
Net book value 1 January 168 312
--------------- ---------------
Net book value 31 December 24 168
========= =========
An intangible asset arose on Arix Bioscience plc's acquisition
of Arthurian Life Sciences entities, relating to management fees
due to Arix Capital Management Limited as a result of managing The
Wales Life Sciences Investment Fund. At the date of acquisition,
the fees for the remaining life of the fund were calculated and
then amortised over the remaining life of the fund.
14. Property, Plant and Equipment Year ended 31 December
2022
Fixtures and Leasehold Office
Fittings Improvements Equipment Total
GBP'000 GBP'000 GBP'000 GBP'000
Cost
As at 1 January 2022 166 - 39 205
Additions - - 8 8
Disposals - - - -
--------------- --------------- --------------- ---------------
At 31 December 2022 166 - 47 213
========= ========= ========= =========
Accumulated Depreciation
As at 1 January 2022 (89) - (31) (120)
Depreciation charge (32) - (4) (36)
Disposals - - - -
Foreign Exchange differences - - - -
--------------- --------------- --------------- ---------------
At 31 December 2022 (121) - (35) (156)
========= ========= ========= =========
Net Book Value
As at 1 January 2022 77 - 8 85
--------------- --------------- --------------- ---------------
At 31 December 2022 45 - 12 57
========= ========= ========= =========
Year ended 31 December 2021
Fixtures and Leasehold Office
Fittings Improvements Equipment Total
GBP'000 GBP'000 GBP'000 GBP'000
Cost
As at 1 January 2021 504 50 77 631
Additions 97 - 8 105
Disposals (434) (50) (46) (530)
Foreign Exchange differences (1) - - (1)
--------------- --------------- --------------- ---------------
At 31 December 2021 166 - 39 205
========= ========= ========= =========
Accumulated Depreciation
As at 1 January 2021 (468) (45) (69) (582)
Disposals 433 49 43 525
Depreciation charge (54) (4) (6) (64)
Foreign Exchange differences - - 1 1
--------------- --------------- --------------- ---------------
At 31 December 2021 (89) - (31) (120)
========= ========= ========= =========
Net Book Value
As at 1 January 2021 36 5 8 49
--------------- --------------- --------------- ---------------
At 31 December 2021 77 - 8 85
========= ========= ========= =========
14B. RIGHT OF USE ASSET
Right of use Right of use
Asset Asset
2022 2021
GBP'000 GBP'000
Cost
As at 1 January 160 -
Additions - 160
--------------- ---------------
At 31 December 160 160
========= =========
Accumulated Depreciation
As at 1 January (39) -
Depreciation charge (10) (39)
--------------- ---------------
At 31 December (49) (39)
========= =========
Net Book Value
As at 31 December 72 121
========= =========
15. OTHER ASSETS
As at As at
31 December 31 December
2022 2021
GBP'000 GBP'000
Trade receivables 1,980 1,656
Prepayments 130 148
VAT receivable 108 35
--------------- ---------------
2,218 1,839
========= =========
Trade and other receivables are recognised at amortised cost in
accordance with IFRS 9, which includes the requirement to calculate
expected credit losses. The maximum exposure to credit risk at the
reporting date is the carrying value of each asset class listed
above and the fair value is akin to book value. The Arix Group does
not hold any collateral as security.
16. Cash and Cash Equivalents
As at As at
31 December 31 December
2022 2021
GBP'000 GBP'000
Cash at bank and in hand 122,782 134,230
--------------- ---------------
The carrying value of cash and cash equivalents approximates to
its fair value.
17. Trade and Other Payables The carrying values of trade and
other payables approximates their fair value.
As at As at
31 December 31 December
2022 2021
GBP'000 GBP'000
Trade payables 173 77
Accruals and other payables 1,691 1,523
--------------- ---------------
1,864 1,600
========= =========
18. Share Capital and Share Premium
As at As at
31 December 31 December
2022 2021
GBP'000 GBP'000
Allotted and called up
135,609,653 Ordinary Shares of GBP0.00001 each (2021: 135, 609, 653 shares) 1 1
49,671 Series C Shares of GBP1 each (2021: 49,671 shares) 50 50
Share Premium 188,534 188,534
========= =========
6,428,853 shares were held in Treasury at 31 December 2022
(2021: 6,428,853 shares).
The maximum number of Treasury Shares in the year was 6,428,853
and this represents 4.7% of Ordinary Share Capital.
19. SHARE OPTIONS During 2022, share-based payment
(credits)/expenses have been recognised relating to a range of
share schemes operated by the Arix Group.
Year Ended Year Ended
31 December 31 December
2022 2021
GBP'000 GBP'000
Executive Incentive Plan 2018 - (186)
Executive Incentive Plan 2019 - (108)
Executive Incentive Plan 2020 97 (14)
Executive Incentive Plan 2021 68 42
Executive Incentive Plan 2022 16 -
Executive Share Option Plan - -
Non-Executive Director Awards - -
--------------- ---------------
181 (266)
========= =========
Executive Incentive Plan The Arix Group operates an Executive
Incentive Plan for Executive Directors and certain employees of the
Company.
In May 2018, the former Executive Directors and certain
employees were awarded options or conditional awards which, in case
of options, will become exercisable at nil cost and, in the case of
the conditional share awards, were scheduled to vest at nil cost on
the third anniversary of their grant, on 17 May 2021, subject to
performance criteria. This required the share price to have grown
by a set percentage over the assessment period, with the quantum of
shares vesting dependent on the level of share price growth; all
options lapsed during 2021. In the year ended 31 December 2022,
GBPnil (2021: credit GBP186k) was recognised in relation to the
Executive Incentive Plan.
In May 2019, the former Executive Directors and certain
employees were awarded options or conditional awards which, in case
of options, will become exercisable at nil cost and, in the case of
the conditional share awards, will vest at nil cost at the end of
the three year performance period, subject to performance criteria.
This required the net asset value and the share price to have grown
a minimum of 7% pa compound over the assessment period to 1 January
2022, and up to 15% pa compound to achieve 100% of the award. All
options lapsed during the year due to performance conditions not
being met. In the year ended 31 December 2022, GBPnil (2021: credit
GBP108k) was recognised in relation to the Executive Incentive
Plan.
In June 2020, the Executive Directors and certain employees were
awarded options or conditional awards which, in case of options,
will become exercisable at nil cost and, in the case of the
conditional share awards, will vest at nil cost at the end of the
three year performance period, subject to performance criteria.
This requires the net asset value and the share price to have grown
by a minimum of 7% pa compound over the assessment period to 1
January 2023, up to 21% pa compound to achieve 100% of the award.
1,658,441 are unvested at year end (2021: unvested 1,658,441)
GBP97k (2021: GBP14k) was recognised in relation to the Executive
Incentive Plan.
In August 2021, the Executive Directors and certain employees
were awarded options or conditional awards which, in case of
options, will become exercisable at nil cost and, in the case of
the conditional share awards, will vest at nil cost at the end of
the three year performance period, subject to performance criteria.
This requires the net asset value and the share price to have grown
by a minimum of 7% pa compound over the assessment period to 1
January 2024, and up to 15% pa compound to achieve 100% of the
award. 408,460 options were issued in 2021, all of which are
unvested at year-end. In the year ended 31 December 2022, a
share-based payment charge of GBP68k (2021: GBP42k) was recognised
in relation to the Executive Incentive Plan. The charge relating to
net asset value growth was calculated based upon the share price at
grant of GBP1.82, and the assessed likelihood of vesting 50%
reducing to 10% from July 2022 onwards (2021: 50%). The charge
relating to share price growth was calculated using a Monte Carlo
simulation model, using assumptions relating to share price at
grant (GBP1.82); risk-free interest rate (-0.08%); time to vesting
(2 years and 6 months); and expected volatility based on comparable
listed investments 23.5%).
In November 2022, the Executive Director and certain employees
were awarded options which will become exercisable at nil cost at
the end of the three-year performance period, subject to
performance criteria. The scheme in three part relates to growth of
net asset value, invested net asset value and share price
growth.
Net asset value and separately the invested net asset value must
grow by a minimum of 5% pa (for Nav) and 7% pa (for invested NAV)
compound over the assessment period to 1 January 2024, and up to
12% pa (NAV) , 15% pa (invested NAV) compound to achieve 100% of
the award. Additionally, a third element relating to share price
growth from start point of GBP1.27 must grow by minimum of 5% pa
compound over the performance period and up to 15% pa compound to
achieve 100% of the award.
648,584 options were issued in 2022, all of which are unvested
at year-end. In the year ended 31 December 2022, a share-based
payment charge of GBP16k was recognised in relation to the
Executive Incentive Plan. The charge relating to net asset value
growth was calculated based upon the share price at grant of
GBP1.27, with an assessed likelihood of vesting of 50%. The charge
relating to share price growth was calculated using a Monte Carlo
simulation model, using assumptions relating to share price at
grant (GBP1.27); risk-free interest rate (-2.4%); time to vesting
(2 years and 4 months); and expected volatility based on comparable
listed investments 23.5%).
Executive Share Option Plan and Founder Incentive Shares At the
Arix Group's inception, an Executive Share Option Plan was in
operation, in which two Directors participated. Options were
granted on 8 February 2016 with an original exercise price of
GBP1.80 per ordinary share. This was subsequently amended for one
Director, with the exercise price reducing by GBP0.18. The number
of ordinary shares subject to the options totals 5,520,559. The
options vested in four equal proportions on 8 February of 2017,
2018, 2019 and 2020. The options may not be exercised after the
tenth anniversary of the grant date and it will lapse on that date
if it has not lapsed or been exercised in full before then. All
options vest at the end of the vesting period relating to that
option or on the occurrence of a contingent event; these include a
change of control or cessation of employment in accordance with
'good leaver' provisions.
No options have been exercised to date. In the year ended 31
December 2022, a share-based payment charge of GBPnil (2021:
GBPnil) was recognised in relation to the Executive Share Option
Plan, calculated using the Black-Scholes model. Assumptions used in
the model relating to the risk-free interest rate and expected
volatility were unchanged from those used in the prior period.
Restricted shares with identical terms, including a GBP1.80
price for the lifting of restrictions, were offered to the founders
of the Company, totalling 5,080,582 shares. A charge of GBPnil was
recognised in the year ended 31 December 2022 (2021: GBPnil). The
charge was calculated using the Black-Scholes model. Assumptions
used in the model relating to the risk-free interest rate and
expected volatility were unchanged from those used in the prior
period.
Non-Executive Director Awards In the current year and prior year
no awards were given. A share-based payment charge of GBPnil (2021:
GBPnil) was recognised during the period.
20. NET CASH FROM OPERATING ACTIVITIES
Year Ended Year Ended
31 December 31 December
2022 2021
GBP'000 GBP'000
Loss before income tax (27,579) (61,084)
--------------- ---------------
Adjustments for:
Change in fair value of investments 30,768 47,975
Impairment of investments - 5,943
Foreign exchange (gains)/losses (8,120) 1,328
Share-based payment charge/(credit) 181 (266)
Depreciation and amortisation 229 209
Impairment of assets - -
Finance expenses 1 -
Finance income (1,582) (156)
Income Tax (4) -
--------------- ---------------
Changes in working capital
Increase in trade and other receivables (379) (461)
Decrease in trade and other payables 264 (782)
--------------- ---------------
Cash used in operations (6,221) (7,294)
========= =========
21. FINANCIAL COMMITMENTS The Group has amounts committed to
portfolio companies but not yet invested; at 31 December 2022 these
totalled GBP4.1m (2021: GBP5.6m).
22. FINANCIAL INSTRUMENTS Financial Assets The Arix Group has
other receivables and cash that derive directly from its
operations. Financial assets at fair value through profit or loss
are measured as either Level 1 or Level 3 under the fair value
hierarchy, as described in Note 2 (i) and disclosed in Note 12.
As at As at
31 December 31 December
2022 2021
GBP'000 GBP'000
Financial assets at fair value through profit or loss
Equity investments 102,694 120,635
Other receivables (excluding prepayments) 1,980 1,656
Cash and cash equivalents 122,782 134,230
========= =========
The credit quality of financial assets that are neither past due
nor impaired can be assessed by reference to external credit
ratings (if available) or to historical information about
counterparty default rates. The Arix Group's cash and cash
equivalents are deposited with F1 or above rated institutions.
Investments and other receivables do not have a credit rating.
However, the Group does not believe these to be past due nor
impaired.
Financial Liabilities The Arix Group's principal financial
liabilities comprise trade and other payables. The primary purpose
of these financial liabilities is to finance the operations.
As at As at
31 December 31 December
2022 2021
GBP'000 GBP'000
Trade, other payables and accruals (excluding non-financial liabilities) 1,864 1,600
Lease liability 70 121
========= =========
23. RELATED PARTY TRANSACTIONS During the period, key management
has comprised Executive Directors, whose remuneration is disclosed
in the Directors' Remuneration Report. Remuneration of
Non-Executive Directors is also disclosed in the Directors'
Remuneration Report.
24. Events After the Reporting Date On Friday 10 March 2023, the
US Federal Deposit Insurance Corporation (FDIC) took control of the
deposits of Silicon Valley Bank (SVB), later announcing on Monday
13 March 2023 that it would guarantee all insured and uninsured
deposits at the bank. Whilst Arix had no deposits at SVB, the
group-maintained brokerage accounts at SVB Leerink, which is part
of SVB Financial Group. Whilst the integrity of these securities
was not at risk, given the dynamic situation, instructions were
given to move all securities at SVB Leerink to other brokerage
accounts out of an abundance of caution. Arix also worked with
those portfolio companies which had deposits at SVB to ensure that
they had access to their capital. Following the announcement of
FDIC's guarantee of all SVB deposits, we do not foresee any
disruption to the trading of capital reserves of any portfolio
companies.
On 8 February 2023, Ensoma completed its acquisition of Twelve
Bio. This had been agreed prior to year-end but was subject to
routine foreign direct investment clearance in Denmark. The
acquisition resulted in a GBP1.2m uplift in our previous holding
value of Twelve Bio, which was exchanged for shares in Ensoma at
the Series B round price. 90% of this uplift was reflected in the
31 December 2022 valuation of Twelve Bio.
On 28 March 2023, Harpoon announced it closed a private
placement to raise USD25 million from redeemable preferred stock
and warrants for the purchase of common stock. Arix participated
with a USD3.5 million (GBP2.8m) investment in redeemable preferred
stock which is not convertible into common stock but is redeemable
on the occurrence of certain events, including the receipt of
proceeds in connection with certain strategic transactions.
On 28 March 2023, Arix invested GBP6.6m (USD8.1m) into Evommune
Inc. as part of an equity transaction.
Company statement of financial position As at 31 December
2022
2022 2021
Note GBP'000 GBP'000
ASSETS
Non-current assets
Investments in subsidiary undertakings 2 891 891
Amounts due from subsidiary undertakings 4 79,543 57,164
--------------- ---------------
80,434 58,055
========= =========
Current assets
Cash and cash equivalents 3 112,147 130,232
Trade and other receivables 383 72
--------------- ---------------
112,530 130,304
========= =========
TOTAL ASSETS 192,964 188,359
========= =========
LIABILITIES
Current liabilities
Trade and other payables (472) (566)
--------------- ---------------
TOTAL LIABILITIES (472) (566)
========= =========
NET ASSETS 192,492 187,793
========= =========
EQUITY
Share capital and share premium 188,585 188,585
Profit/(Loss) for the period 4,518 (3,586)
Retained earnings 13,179 16,584
Other reserves (13,790) (13,790)
--------------- ---------------
TOTAL EQUITY 192,492 187,793
========= =========
Company statement of changes in equity For the year 31 December
2022
Share Treasury
Capital and Other Other Share Retained
Premium Equity Reserves Reserve Earnings Total
GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000
As at 1 January 188,585 (1,216) (981) (11,593) 12,998 187,793
2022
Profit for the - - - - 4,518 4,518
year
Share-based - - - - 181 181
payment charge
--------------- --------------- --------------- --------------- --------------- ---------------
As at 31 December 188,585 (1,216) (981) (11,593) 17,697 192,492
2022
========= ========= ========= ========= ========= =========
FOR THE YEAR 31 DECEMBER 2021
Share Treasury
Capital and Other Other Share Retained
Premium Equity Reserves Reserve Earnings Total
GBP'000 GBP'000 GBP'000 GBP'000 GBP'000 GBP'000
As at 1 January 188,585 (1,240) (957) - 16,850 203,238
2021
Loss for the year - - - - (3,586) (3,586)
Share-based - - - - (266) (266)
payment credit
Acquisition of - - - (11,593) - (11,593)
own shares
Issue of own
shares to - 24 (24) - - -
employees
--------------- --------------- --------------- --------------- --------------- ---------------
As at 31 December 188,585 (1,216) (981) (11,593) 12,998 187,793
2021
========= ========= ========= ========= ========= =========
NOTES TO THE COMPANY FINANCIAL STATEMENTS
1. ACCOUNTING POLICIES These financial statements have been
prepared in accordance with Financial Reporting Standard 101
Reduced Disclosure Framework ("FRS 101").
In preparing these financial statements, the Company applies the
recognition, measurement and disclosure requirements of
International Financial Reporting Standards in conformity with the
requirements of the Companies Act 2006 and prepared in accordance
with FRS 101. The Company has set out below where advantage of the
FRS 101 disclosure exemptions has been taken.
Under section 408 of the Companies Act 2006 the Company is
exempt from the requirement to present its own profit and loss
account. In these financial statements, the Company has applied the
exemptions available under FRS 101 in respect of the following
disclosures:
Statement of Cash Flows and related notes; disclosures in
respect of transactions with wholly owned subsidiaries; disclosures
in respect of capital management; the effects of new but not yet
effective IFRSs; and disclosures of transactions with a management
entity that provides key management personnel services to the
Company.
As the consolidated financial statements include the equivalent
disclosures, the Company has also taken the exemptions under FRS
101 available in respect of the following disclosures: IFRS 2 Share
Based Payments; certain disclosures required by IFRS 13 Fair Value
Measurement; and the disclosures required by IFRS 7 Financial
Instrument Disclosures.
The Company proposes to continue to adopt the reduced disclosure
framework of FRS 101 in its next financial statements. The
accounting policies set out below have been applied consistently.
Where relevant, the accounting policies of the Arix Group have been
applied to the Company.
Investments in subsidiary undertakings Unlisted investments are
held at cost less any provision for impairment.
Amounts due from subsidiary undertakings All amounts due from
subsidiary undertakings are initially recognised at fair value and
subsequently measured at amortised cost. Amounts provided to
subsidiaries are intended for use on a continuing basis in the
Company's activities, with no intention of their settlement in the
foreseeable future; as such, they are presented as non-current
fixed assets.
2. Investments in Subsidiary Undertakings
2022 2021
GBP'000 GBP'000
Opening balance 891 891
Additions - -
Disposals - -
--------------- ---------------
At 31 December 891 891
========= =========
The Company's subsidiary undertakings are detailed in Note 2(B)
to the Group financial statements.
3. Cash and Cash Equivalents
As at As at
31 December 31 December
2022 2021
GBP'000 GBP'000
Cash at bank and in hand 112,147 130,232
========= =========
The carrying value of cash and cash equivalents approximates to
its fair value.
4. Amounts Due from Subsidiary Undertakings
As at As at
31 December 31 December
2022 2021
GBP'000 GBP'000
Opening balance 57,164 29,927
Net additions/(repayments) during the year 22,379 27,237
Impairments during the year - -
--------------- ---------------
At 31 December 79,543 57,164
========= =========
The amounts due from subsidiary undertakings are interest free
and unsecured. Arix Bioscience plc currently has no intention to
request repayment of any amounts due.
5. EMPLOYEES The average number of employees during the year was
one (2021: 1).
OTHER INFORMATION
SHAREHOLDER INFORMATION
WARNING ABOUT UNSOLICITED APPROACHES TO SHAREHOLDERS AND 'BOILER
ROOM' SCAMS In recent years, many companies have become aware that
their shareholders have received unsolicited phone calls or
correspondence concerning investment matters. These are typically
from overseas-based 'brokers' who target UK shareholders, offering
to sell them what often turn out to be worthless or high risk
shares in UK investments. These operations are commonly known as
'boiler rooms'.
These 'brokers' can be very persistent and persuasive. Arix
Bioscience plc shareholders are advised to be extremely wary of
such approaches and are advised to only deal with firms authorised
by the FCA. You can check whether an enquirer is properly
authorised and report scam approaches by contacting the FCA on
www.fca.org.uk/scams (where you can also review the latest scams)
or by calling the FCA Consumer Helpline: 0800 111 6768.
If you have already paid money to share fraudsters then contact
Action Fraud on 0300 123 2040.
REGISTRAR The Company's register of shareholders is maintained
by our Registrar, Equiniti Limited. All enquiries regarding
shareholder administration, including lost share certificates or
changes of address, should be communicated in writing or by calling
+44 (0)371 384 2030 (lines are open 8.30am to 5.30pm Mondays to
Fridays, excluding Bank Holidays in England and Wales). Please use
the country code when calling from outside the UK.
Shareholders can also view and manage their shareholdings online
by registering at www.shareview.co.uk.
FORWARD-LOOKING STATEMENTS This Annual Report has been prepared
for, and only for, the members of Arix Bioscience plc ("the
Company") as a body, and for no other persons. The Company, its
Directors, employees, agents or advisers do not accept or assume
responsibility to any other person to whom this document is shown
or into whose hands it may come and any such responsibility or
liability is expressly disclaimed.
By their nature, the statements concerning the risks and
uncertainties facing the Group in this Annual Report involve
uncertainty since future events and circumstances can cause results
and developments to differ materially from those anticipated. The
forward-looking statements reflect knowledge and information
available at the date of preparation of this Annual Report and the
Company undertakes no obligation to update these forward-looking
statements. Nothing in this Annual Report should be construed as a
profit forecast.
DIRECTORS Peregrine Moncreiffe Isaac Kohlberg Robert Lyne
Maureen O'Connell Debra Barker Andrew Smith
COMPANY SECRETARY Kin Company Secretarial Limited Hyde Park
House 5 Manfred Road London SW15 2RS
REGISTERED OFFICE Duke Street House 50 Duke Street London W1K
6JL United Kingdom
COMPANY NUMBER 09777975
ADVISORS Joint Broker Jefferies International Limited 100
Bishopsgate London EC2N 4JL
Joint Broker Peel Hunt LLP 100 Liverpool Street London EC2M
2AT
LEGAL ADVISERS Brown Rudnick LLP 8 Clifford Street London W1S
2LQ United Kingdom
One Financial Center Boston MA 02111 United States
INDEPENT AUDITORS BDO LLP 55 Baker Street London W1U 7EU United
Kingdom
REGISTRAR Equiniti Limited Aspect House Spencer Road Lancing
BN99 6DA United Kingdom
GLOSSARY
ANTI-PD-L1 ANTIBODY Monoclonal antibody directed against
programmed cell death-1 ligand 1 (PD-L1). The antibody binds to
PD-L1, blocking its binding to and activation of its receptor
programmed death 1 (PD-1), the activation of which normally dampens
anticancer immune responses.
ATR INHIBITOR A molecule that inhibits ataxia telangiectasia and
Rad3 related (ATR) kinase, an enzyme frequently overexpressed in
cancer contributing to cancer cell survival and growth by
contributing towards DNA repair and cell cycle progression.
BCR-ABL A gene formed due to the fusion of the BCR and ABL genes
from different chromosomes which is often a driver of certain types
of leukaemia.
BLA Biologics License Application is submitted after an
investigational new drug has been approved. The biologics license
application is a request for permission to introduce, or deliver
for introduction, a biologic product into interstate commerce.
CORE PORTFOLIO Arix's core portfolio comprises investments in
companies that are developing novel therapeutics with first or
best-in-class approach in a range of therapeutics areas with high
unmet need and significant market opportunity. These companies have
raised significant capital, supported by a strong syndicate of
leading venture investors, and have reached validating
milestones.
CRISPR-CAS12A RNA-guided endonuclease which can be used to make
highly specific modifications to DNA or RNA.
GROSS PORTFOLIO Arix's Core Portfolio, Listed Portfolio and
Public Opportunities Portfolio.
HEMATOLOGY The branch of medicine concerned with the study of
the cause, prognosis, treatment, and prevention of diseases related
to blood.
HEMATOPOIETIC SYSTEM The system in the body that produces blood
cells.
HER2 Acronym for human epidermal growth factor receptor 2. This
is a receptor found on the surface of all breast cells and is
involved in the control of cell growth.
MYELOMA A type of blood cancer arising from plasma cells found
in the bone marrow.
NET ASSET VALUE (NAV) A company's assets less its
liabilities.
NET ASSET VALUE PER SHARE A company's net asset value divided by
the number of shares in issue.
NSTI Necrotising Soft Tissue Infections; serious bacterial
infections that cause inflammation and damage to the soft tissue
layers underneath the surface of the skin.
PARP INHIBITOR A molecule that inhibits the enzyme poly ADP
ribose polymerase (PARP) which is important in repairing DNA
single-strand breaks.
PHASE 1 A clinical study testing a therapy in humans (healthy
volunteers or in some cases in patients) for the first time to
establish the safety of a range of doses.
PHASE 2 A clinical study testing a therapy in patients to
establish the safety and efficacy of one or more doses. Intended to
provide 'Proof of Concept' and to influence design of one or more
Phase 3 studies.
PHASE 3 A clinical study testing a therapy in a larger group of
patients (vs. Phase 2) to establish efficacy and safety with
statistical significance in order to support registration and
approval by a regulatory agency (e.g. FDA, EMA).
PRECLINICAL Testing of drug in non-human subjects, to gather
efficacy, toxicity and pharmacokinetic information.
Pol? INHIBITOR A molecule that inhibits DNA polymerase theta
(Pol?) which is a DNA repair enzyme. Pol? has limited expression in
normal tissues but is frequently over expressed in cancer
cells.
PUBLIC OPPORTUNITIES PORTFOLIO Arix taking advantage of low
public valuations in publicly listed clinical-stage biotech
companies, focusing on de-risked opportunities that are well
funded, trading at negative enterprise value or close to an
equivalent of cash reserves, with expected clinical milestones over
the next 6 to 18 months.
SCD Sickle Cell Disease - an inherited health condition that
affects the red blood cells.
SOLID TUMOUR A cancer comprising solid tissue (i.e. not a blood
cancer).
TRITAC Tri-specific T cell Activating Construct - Harpoon's
approach for targeted penetration and destruction of solid tumours
and haematologic malignancies.
Printed on FSC® certified paper.
100% of the inks used are vegetable oil based 95% of press
chemicals are recycled for further use and on average 99% of any
waste associated with this production will be recycled.
The FSC® logo identifies products which contain wood from
well-managed forests certified in accordance with the rules of the
Forest Stewardship Council®.
This document is printed on Cocoon Silk; a paper made using 50%
recycled fibre from genuine waste paper and 50% virgin fibre.
The unavoidable carbon emissions generated during the
manufacture and delivery of this document, have been reduced to net
zero through a verified, carbon offsetting project.
Duke Street House 50 Duke Street London W1K 6JL United
Kingdom
+44 (0)20 7290 1050
info@arixbioscience.com
-----------------------------------------------------------------------------------------------------------------------
Dissemination of a Regulatory Announcement, transmitted by EQS
Group. The issuer is solely responsible for the content of this
announcement.
-----------------------------------------------------------------------------------------------------------------------
ISIN: GB00BD045071
Category Code: FR
TIDM: ARIX
LEI Code: 213800OVT3AHQCXNIX43
OAM Categories: 1.1. Annual financial and audit reports
3.1. Additional regulated information required to be disclosed under the laws of a Member State
Sequence No.: 239154
EQS News ID: 1615697
End of Announcement EQS News Service
=------------------------------------------------------------------------------------
Image link:
https://eqs-cockpit.com/cgi-bin/fncls.ssp?fn=show_t_gif&application_id=1615697&application_name=news
(END) Dow Jones Newswires
April 25, 2023 02:01 ET (06:01 GMT)
Arix Bioscience (LSE:ARIX)
Historical Stock Chart
From Dec 2024 to Jan 2025
Arix Bioscience (LSE:ARIX)
Historical Stock Chart
From Jan 2024 to Jan 2025